Abstract
Myelin is a specialized cell membrane indispensable for rapid nerve conduction. The high abundance of membrane lipids is one of myelin’s salient features that contribute to its unique role as an insulator that electrically isolates nerve fibers across their myelinated surface. The most abundant lipids in myelin include cholesterol, glycosphingolipids, and plasmalogens, each playing critical roles in myelin development as well as function. This review serves to summarize the role of lipid metabolism in myelination and myelin maintenance, as well as the molecular determinants of myelin lipid homeostasis, with an emphasis on findings from genetic models. In addition, the implications of myelin lipid dysmetabolism in human diseases are highlighted in the context of hereditary leukodystrophies and neuropathies as well as acquired disorders such as Alzheimer’s disease.
Graphical abstract
Public summary
-
•
Myelin is an electrical insulator required for rapid nerve conduction.
-
•
Myelin is lipid-rich and has a unique lipid composition.
-
•
Disruption of lipid metabolism adversely affects myelin homeostasis.
-
•
Targeting lipid dysmetabolism could help address disease-associated myelin loss.
Introduction
Myelin is a lipid-rich, multilamellar membrane that insulates axons and enables an ion channel configuration necessary for saltatory action potentials that markedly enhance nerve conduction velocity (Figures 1A and 1B).1,2 In the central nervous system (CNS), myelin primarily forms postnatally and is produced by multiprocessed, neuroepithelium-derived oligodendrocytes (OLs). OL numbers peak in early life and are highly stable during the human lifespan, yet new OLs continually arise and initiate adaptive myelination throughout adulthood.3,4,5 In the peripheral nervous system (PNS), large-caliber (>1 μm) axons are myelinated by neural crest-derived Schwann cells (SCs), whereas small-caliber axons are enwrapped by non-myelinated SCs, which form the bundles of Remak.6 Similar to OLs, SC-mediated myelination occurs postnatally; moreover, once established, SC numbers are stable with minimal cell turnover.7,8
Figure 1.
Representative images of myelinated fibers and myelin sheath from mouse nerve
(A) Left: transmission electron micrograph of myelinated and non-myelinated nerve fibers from cross-sectioned mouse sciatic nerve. Right: pseudo-colored overlay of the electron micrograph on the left. Red regions denote the myelin sheath, and yellow regions represent axon fibers.
(B) High-magnification micrograph of mouse sciatic nerve to illustrate the multiple layers of myelin membrane that together compose the myelin sheath.
(C) Pie chart delineating myelin lipid composition as molar percentage of total lipids as reported for bovine myelin from the CNS.11,12 Chol, cholesterol; Galc, galactocerebrosides; Sulf, sulfatides; Plas, plasmalogens; PC, phosphatidylcholine phospholipids; SM, sphingomyelin.
Among the biochemical properties unique to myelin compared with other cell membranes is the relatively high ratio of lipids to proteins, as more than 70% of myelin consists of lipids.9,10,11 In contrast to myelin proteins, many of which are myelin specific, myelin lipid species are non-specific; however, the myelin lipid composition is distinctive, with a characteristic overrepresentation of cholesterol, galactocerebrosides (GalC), sulfatides, and plasmalogens (Figure 1C).12,13 Great strides have been made in deciphering the role of myelin-specific proteins and their contribution to health and disease. Yet various questions involving myelin lipids remain unaddressed, particularly regarding the contribution of lipid metabolism to myelin maintenance, as well as the underlying molecular determinants of myelin lipid homeostasis. This review aims to help bridge this gap of knowledge by addressing past and recent discoveries associated with the function of myelin lipid metabolism in both the CNS and the PNS, summarizing their known molecular determinants, and addressing the relevance to human disease.
Myelin lipids: Composition and respective roles
Myelin lipids are paramount to myelin sheath generation and maintenance. Beyond the structural need inherent in synthesizing immense volumes of myelin membrane, myelin lipids provide the intermolecular forces necessary for myelin anchorage against the axonal membrane and afford a platform for myelin proteins in the form of specialized lipid rafts or microdomains.14,15,16 The three lipid classes most abundant in myelin are cholesterol, GalC, and plasmalogens. Here we review their respective roles in myelination as elucidated through genetically engineered mouse models.
Cholesterol
Cholesterol is highly enriched in myelin compared with other cell membranes and is the most abundant lipid in both the CNS and the PNS.9,10,11,17 Cholesterol resides within the phospholipid bilayer, where it stabilizes membrane proteins and helps establish the fluidity and permeability of plasma membranes.14,18 Importantly, cholesterol synthesis and trafficking in myelinating glia have critical functions in myelin development and homeostasis.
Cholesterol synthesis and import by myelinating glia are necessary for myelination
The significance of cholesterol’s role in myelination was first appreciated in seminal studies wherein the rate-limiting enzyme for cholesterol biosynthesis, farnesyl-diphosphate farnesyltransferase 1 (FDFT1) (Figure 2A), was conditionally depleted in myelinating glia using Cre-lox recombination.19 Despite expressing normal levels of myelin proteins, mice harboring Cnp-Cre, Fdft1L/L alleles were hypomyelinated, with a substantial deficit in brain cholesterol levels.19 Moreover, the diminished myelination that occurred was attributed to an associated reduction in non-sterol lipid classes, which preserved the cholesterol-to-non-cholesterol lipid ratio in myelin.19 Interestingly, myelination and motor function in Fdft1-knockout mice improved with age, likely through the uptake of exogenous cholesterol from neighboring cells. This robust capability to maintain myelin cholesterol stoichiometry by attenuating non-sterol lipid synthesis and scavenging for extracellular cholesterol highlights the importance of sterols in myelin.20 As the Cnp promoter is active in both OLs and SCs, Fdft1-null mutants also displayed significant PNS hypomyelination. In the PNS, cholesterol is required for shuttling the myelin structural protein, P0, from the endoplasmic reticulum (ER) to the myelin membrane.21,22 Impaired cholesterol synthesis in SCs accompanied a compensatory upregulation of genes responsible for exogenous cholesterol uptake, including ApoE and ApoD.22 These findings established a significant role for cholesterol in developmental myelination of both the CNS and the PNS and demonstrated that, although de novo cholesterol synthesis is critical, it can be compensated for, at least partially, by environmental sterol uptake.16,20
Figure 2.
Cholesterol, sterol-response-element-binding protein (SREBP), and mammalian target of rapamycin (mTOR) pathways necessary in myelination
(A) Farnesyl-diphosphate farnesyltransferase 1 (FDFT1) synthesizes squalene, a key intermediate in cholesterol synthesis.
(B) Low-density lipoprotein receptor-related protein 1 (LRP1) enables endocytosis of lipoprotein particles, which deliver exogenous cholesterol.
(C) Sterol cleavage-activating protein (SCAP), in response to decreased ER cholesterol, shuttles the SREBPs to the Golgi apparatus for cleavage-mediated activation by site-1 and site-2 proteases (S1P and S2P).
(D) Elevated ER cholesterol induces SCAP to associate with and be inhibited by insulin-induced gene protein (Insig), reducing SCAP-mediated SREBP2 activation.
(E) Quaking (QKI) is a critical co-activator for SREBP2 in OLs that is necessary for transcription of cholesterol-synthesizing genes.
(F) mTOR, regulatory-associated protein of mTOR (Raptor), and mTOR-associated protein, LST8 homolog (mLst8) are subunits of the mTORC1 complex that increase SREBP activity through multiple mechanisms. One reported mechanism includes inhibiting the flux of autophagolysosome-derived cholesterol to the ER and preventing cholesterol-mediated inhibition on SREBP activation. OL, oligodendrocyte.
Studies targeting lipid transporters further illuminated the impact of lipid influx on myelination and myelin repair. In adult mice with inducible whole-body depletion of low-density lipoprotein receptor-related protein 1 (LRP1), a cholesterol importer, remyelination was significantly impaired after toxin-induced demyelination (Figure 2B).23 Inducible and conditional knockout of Lrp1 in OL precursor cells (OPCs) replicated the remyelination deficits; moreover, depletion of Lrp1 across the OL lineage led to hypomyelination, indicating that cholesterol uptake specifically by OLs is important to myelin development and repair.23 Mechanistically, Lrp1 depletion in OPCs reduced cellular cholesterol and hindered their differentiation to myelinating OLs. Of interest, myelination was partially rescued by dietary cholesterol supplementation.23
In addition to lipid transporters, structural myelin proteins have been shown to play a critical role in maintaining myelin cholesterol levels.16,24 Loss of proteolipid protein (PLP) alone or concomitant with knockout of a homolog glycoprotein, M6B, reduced myelin cholesterol, with the double knockout also causing hypomyelination.25,26 More recently, one of the major peripheral myelin proteins, peripheral myelin protein 22 (PMP22), was shown to be critical for proper cholesterol homeostasis in myelin via its interaction with and regulation of the cholesterol efflux transporter ATP-binding cassette family protein 1 (ABCA1) in SCs.27
Complementary approaches reinforcing cholesterol’s role in myelination have included pharmaceutical and genetic targeting of upstream regulators of cholesterol synthesis. For instance, challenging adult mice with simvastatin, a cholesterol-lowering drug that inhibits sterol synthesis, induced demyelination, reduced mature OL numbers, and decreased OPC differentiation.28 After cuprizone-mediated demyelination in adult mice, simvastatin treatment also impaired remyelination by lowering mature OL density, possibly by disrupting OPC differentiation or migration.28 Targeting the master transcriptional regulators of cholesterol metabolism, namely the sterol-response-element-binding proteins (SREBPs), has also been very informative, although a limitation to this approach is the significant cross talk with fatty acid metabolism regulated by SREBPs.29 SREBP transcription factors were first linked to the PNS when Verheijen et al. demonstrated that myelination onset correlated with upregulation of Srebp1 and Srebp2.30 Shortly thereafter, Leblanc et al. confirmed that both Srebp1 and Srebp2—as well as their critical regulator, SREBP cleavage-activating protein (SCAP)—were induced during PNS myelination (Figure 2C).31 Furthermore, PNS myelination was found to be dependent in part on the cooperation between SC differentiation factor EGR2 and SREBPs, which together activated downstream fatty acid and cholesterol biosynthesis gene expression.31 These findings prompted the use of Scap knockouts to interrogate the role of SREBP-mediated sterol metabolism in myelination. Although germline knockout of Scap is embryonically lethal, haploinsufficient Scap-mutant mice were viable and had up to 30% reduction in brain cholesterol, which was accompanied by behavioral and cognitive defects.32 Conditional depletion of Scap in SCs using Mpz-Cre-driven recombination further emphasized the importance of SREBP-mediated sterol and fatty acid metabolism during myelination.33 In Mpz-Cre, ScapL/L mice, the sciatic nerve was hypomyelinated, and although the myelinated axon percentage recovered with age, reduced myelin thickness persisted.33 As expected, myelin lipid composition in mutant mice was altered, with an estimated 50% reduction in lipids, accompanied by downregulation of SREBP targets such as Hmgcr and Fasn.33 Finally, studies using explanted dorsal root ganglia (DRG) showed that SCAP-mutant DRG neurons had reduced myelination in lipid-free media, which was partially rescued by lipid supplementation.33 In line with previous work, these findings indicated that extracellular lipid uptake may have supported myelination in Scap-mutant nerves.22
Astrocyte-derived cholesterol complements myelin development and integrity
In search of the major cell types responsible for brain cholesterol levels, Camargo et al. depleted SCAP in astrocytes using Gfap-Cre, ScapL/L alleles.34 The knockout mice died prematurely with significant motor deficits and substantially reduced brain cholesterol levels. Of interest, however, they exhibited a compensatory uptake of dietary lipids.34 High-fat diet (HFD) enriched with monounsaturated fatty acids (MUFAs), cholesterol, and saturated fatty acids improved motor function and survival in contrast to either standard diet or HFD enriched for polyunsaturated fatty acids (PUFAs). Furthermore, deletion of Scap at P20 in astrocytes using Glast-CreERT, ScapL/L mice also induced hypomyelination, further supporting the hypothesis that astrocyte-derived lipids are critical for myelination and myelin maintenance.35 OL-specific depletion of Scap using Cnp-Cre, ScapL/L mutants also caused hypomyelination, although, in contrast to Gfap-Cre, ScapL/L mice, the myelin deficits in OL-specific knockouts improved with age, becoming comparable to controls by late adulthood.19,34 Finally, relative to knockouts restricted to single lineages, mutants with Scap depleted in both astrocytes and OLs (Cnp-Cre, Gfap-Cre, ScapL/L) displayed greater hypomyelination and worse survival and were refractory to HFD supplementation.35
In summary, myelin is highly dependent on both astrocyte- and OL-derived cholesterol in the CNS; however, whether other astrocyte-derived lipids contribute to myelination and myelin maintenance remains elusive.
Galactocerebrosides and sulfatides
Glycosphingolipids (GSLs) are a major glycolipid subtype that includes GalC and their sulfated form, sulfatides. GSLs are derivatives of ceramide lipids, which consist of a fatty acid attached to a sphingosine backbone and are synthesized in the ER. Galactose is added to a ceramide lipid base to produce GalC, whereas the addition of sulfate to GalC in the Golgi apparatus generates sulfatides (Figures 3A and 3B).36,37 GalC and sulfatides are abundantly present in myelin38 and enhance myelin compaction stability through trans interactions between apposing lipid bilayers.39,40,41,42 Although not essential for de novo myelination, GalC and sulfatides are indispensable for normal myelin structure and long-term myelin stability.43,44,45,46
Figure 3.
Fatty acid, galactocerebroside, and sulfatide synthesis pathways implicated in myelination and myelin maintenance
(A) Condensation of serine and palmitoyl-CoA by serine palmitoyltransferase (SPT) initiates Cer synthesis, which is then completed through the activity of ceramide synthase (Cers) and dihydroceramide desaturase (DEGS).
(B) Ceramide galactosyl-transferase (CGT)-mediated addition of galactose to Cer generates GalC. Following translocation to the Golgi, addition of sulfate to GalC by cerebroside sulfotransferase (CST) forms Sulf. Conversely, Cer taken to the Golgi can be glycosylated to GlcC by UDP-glucose ceramide glucosyltransferase (UGCG), which can be further glycosylated into complex glycosphingolipids including globosides and gangliosides.
(C) Fatty acid 2-hydroxylase (FA2H) catalyzes FA hydroxylation. Hydroxylated FA (2OH-FA) are used as substrates by sphingolipid-synthesizing enzymes to generate hydroxylated sphingolipids.
(D) The rich heterogeneity of sphingolipids stems from the multiple species of FA used as substrates for dihydrosphingosine acylation, including saturated (SFA), monounsaturated (MUFA), and polyunsaturated (PUFA) FA. Elongation of endogenous or diet-derived FA requires fatty acid elongases (ELOVL) and hydroxyacyl dehydratases (HACD). FA desaturation depends on FA desaturases, including FADS and SCD.
(E) Fatty acid synthase (FASN) generates palmitate, a principal source for endogenous SFA and MUFA.
(F) The nuclear receptors peroxisome proliferator-activated receptor β (PPARβ) and retinoic X receptor α (RXRα), following co-activation by quaking (QKI), drive transcription of the FA biosynthesis pathway. FA, fatty acid; Cer, ceramide; GalC, galactocerebroside; Sulf, sulfatides; GlcC, glucocerebroside.
GalC and sulfatides promote myelin maintenance and nodal organization
Despite the relative abundance of GalC and sulfatides in myelin, myelination follows unimpaired, albeit defectively, in mice lacking the GalC-synthesizing enzyme cerebroside galactosyl-transferase (CGT), loss of which blocks GalC and sulfatide production.44,46 However, by 2 months of age, myelin vacuolization and demyelination occurred in mutants.43,44 Nerve conductivity in Cgt-null mice diminished due to paranodal defects, accompanied by tremors and ataxia.44 Interestingly, targeted loss of sulfatides through knockout of ceramide sulfotransferase (CST), which impaired sulfatide synthesis while leaving GalC levels intact, evinced a milder phenotype relative to mutants lacking both GalC and sulfatides, possibly indicating a greater role for GalC in myelin sheath compactness and stability.47,48 Nevertheless, sulfatide reduction in Cst-null mice precipitated mild demyelination that progressed with age, and by 8 months of age, Cst-null mutants demonstrated significant paranodal defects accompanied by hindlimb weakness and tremor. In addition, myelin in Cst-null mice demonstrated vacuolar degeneration and aberrant paranodal loops that were aggravated with age. These findings, in line with previous myelin ultrastructural analyses, suggest that sulfatides are necessary for maintaining paranodal junctions.45,47,48,49 GalC and sulfatides also contribute to myelin protein expression.50 In adult Cst-null mice, myelin-associated glycoprotein (MAG) and PLP were downregulated, along with neurofascin-155 (NF155), a critical paranodal junction protein.51,52 Thus, myelin stability and compactness, nodal and paranodal organization, and myelin protein regulation depend on GalC and sulfatide metabolism.45,47,48,53,54 Finally, whereas animal studies investigating myelin sphingolipids typically used whole-body knockout, the few available conditional models indicated that myelinating glia are the main producers of myelin sphingolipids, as OL-specific overexpression of Cgt was sufficient to rescue late-onset demyelination in Cgt-null mice.55
2-Hydroxylated sphingolipids contribute to myelin stability
2-Hydroxylated fatty acids (2-HF) are common components of both GalC and sulfatides.56 Fatty acid 2-hydroxylase (FA2H) catalyzes fatty acid hydroxylation for 2-hydroxylated glucosylceramides (HFA-GlcC) and 2-hydroxylated sphingomyelin (HFA-SM) (Figure 3C).57,58,59 Of interest, HFA-SM levels were significantly diminished in Cgt-null mutants,43,44 whereas in Cst-null mice, HFA-SM levels were unaffected, coinciding with the less severe phenotype relative to Cgt-null mutants.47 To explore the role of 2-hydroxylated sphingolipid species in myelin, Fa2h was targeted. Fa2h-null mice experienced no impairments during developmental myelination despite notable depletion of 2-hydroxylated sphingolipids in both CNS and PNS myelin.60 By 18 months of age, however, Fa2h knockouts demonstrated severe myelin degeneration and demyelination in both CNS and PNS, accompanied by hindlimb paralysis.60 Myelinating glia-specific knockout of Fa2h using Cnp-Cre led to similar late-onset demyelination at age 12 months, specific to the CNS.61 This model also recapitulated the cerebellar degeneration phenotype observed in the germline Fa2h deletion, demonstrating that 2-HF sphingolipids are essential for long-term myelin stability and subsequent motor function.61 Moreover, these results identified OLs as the main producers of the 2-HF sphingolipids in CNS myelin61 and emphasized the overall stronger phenotype relative to the PNS, which developed findings only at a later time (18 months).60
Initial studies depleting GSL species also noted a subsequent increase in other lipids, such as HFA-GlcC, alluding to potential compensatory mechanisms by other sphingolipid counterparts.43,44,47 This hypothesis was disputed, however, with the Cnp-Cre, UgcgL/L knockout model, which depleted glucosylceramides in the OL lineage by deleting UDP-glucose ceramide glucosyltransferase (UGCG) (Figure 3B). Loss of glucosylceramides in CNS myelin led to no observable phenotypes, even in aged mice (age 1.5 years). Of interest, double knockout of Ucgc and Cgt using Cnp-Cre, UgcgL/L, CgtL/L mutants did not precipitate more severe demyelination despite the absence of HFA-GlcC in myelin.62 In addition, germline Fa2h−/−, Cgt−/− double-knockout mice in which sulfatides, GalC, HFA-GlcC, and HFA-SM, were reduced by 80%, still formed compact myelin.63 These mice did not display any dysmyelination by 4 weeks of age, demonstrating that non-hydroxylated sphingolipids are resilient in forming intact myelin despite missing their 2-hydroxylated counterparts.63
For future studies, cell-autonomous roles of GSL synthesis enzymes in the context of myelination and myelin maintenance require further elucidation. Moreover, the difference in the strength of observed hypomyelination phenotypes between the CNS and the PNS upon loss of specific GSLs, despite similar overall myelin lipid composition, necessitates detailed comparative analyses of myelin GSL homeostasis between the CNS and the PNS.
Plasmalogens
Most phospholipids are formed through the esterification of fatty acids to a glycerol backbone, forming a diacylglycerol lipid moiety with an attached polar head group. However, an estimated 10%–20% of phospholipids possess only one ester-linked acyl chain together with an ether-linked alkyl chain forming ether phospholipids.64,65,66,67 Ether phospholipids with a vinyl bond are classified as plasmenyl phospholipids or, more commonly, as plasmalogens and constitute the vast majority of ether phospholipids.65
Plasmalogens are enriched in the myelin phospholipid pool
Plasmalogens are partially synthesized in peroxisomes and are particularly abundant in myelin. In myelin-rich white matter, plasmalogens make up more than 30% of total phospholipids, a higher proportion relative to that in gray matter, which tends to be less than 20%.9,66,68 Plasmalogens exist primarily as phosphatidylethanolamine (PE) phospholipids,65,68,69 which have an ethanolamine head group and constitute the third most abundant myelin lipid class.9,10,70 In myelin, more than 80% of PE lipids are constituted by plasmalogens, making them a significant contributor to myelin lipid composition.9,70 Moreover, brain plasmalogen levels increase postnatally in parallel with myelination, likely reflecting a rise in myelin-associated plasmalogens.69,71 Given their ether-linked alkyl chain, plasmalogens possess a hydrophobic tail with a narrower cross-sectional area, which increases liquid order in a cell membrane.72,73 Other roles attributed to plasmalogens include serving as a reservoir for arachidonic acid, modulating intracellular radical oxygen species propagation, regulating cell death, and contributing to lipid raft formation.74,75,76,77,78
Plasmalogens contribute to myelination and myelin integrity
Plasmalogen deficiency through knockout of peroxisome-related genes, such as Pex7 and Gnpat, is detrimental to myelination and myelin stability.79,80 Up to 70% of Pex7-null neonates do not survive past weaning, and although remaining survivors can live more than 18 months without serious morbidity, they experience reduced body weight, impaired ossification, congenital cataracts, testicular atrophy, and infertility.81 Gnpat-null mice similarly have reduced survival after birth, with 40% not surviving more than 6 weeks and experiencing reduced body weight and size, infertility, cataracts, and optic nerve hypoplasia.82 Both adult Pex7-null and Gnpat-null mice experience hypomyelination with delayed myelination in PNS, followed by demyelination after a year of life.79 In Gnpat-null mice, a similar pattern of hypomyelination, delayed myelination, and demyelination was also reported in the CNS, which adversely affected action potential conductivity.80,83 In both knockout models, plasmalogens were significantly reduced in the nervous tissue. These genetic models substantiate that plasmalogens are imperative for normal myelination during development and myelin maintenance later in life. Of interest, upregulation of PE with diacylglycerol moieties can compensate for plasmalogen deficiency and likely support some degree of myelination84; however, it is insufficient for long-term myelin stability. In summary, plasmalogens contribute to multiple processes, including the genesis and integrity of myelin, likely in part due to their ability to increase lipid membrane packing and order.
De novo fatty acid synthesis in myelinating glia is required for normal myelination
Fatty acids are the essential building blocks for the various major myelin lipid species detailed above, and loss of fatty acid metabolism-related gene products has further elucidated the importance of lipid metabolism in myelin homeostasis (Figure 3D). First, whole-body knockout of ELOVL fatty acid elongase 5 (ELOVL5), an enzyme essential for PUFA elongation, induced significant reduction of PUFAs longer than 18 carbons, accompanied by increased myelin layer periodicity, paranodal junction gaps in the PNS, and motor deficits.85 Elsewhere, knockout of fatty acid synthase (Fasn) in SCs using Dhh-Cre induced severe and persistent hypomyelination in the PNS, with motor neuropathy (Figure 3E).86 Lipidomic profiles of the peripheral nerves revealed depletion of myelin complex lipids such as ceramides and cerebrosides.86 Of interest, dietary supplementation did not ameliorate the mutant phenotype, suggesting that de novo fatty acid synthesis by SCs is indispensable. Conversely, this study demonstrated that peroxisome proliferator-activated receptor γ (PPARγ)-dependent activity contributed to fatty acid synthesis in SCs, as treatment with PPARγ agonists partially rescued PNS hypomyelination.86 These findings were later recapitulated in the CNS, as Fasn conditional knockouts using an OL-specific Olig2-Cre system also developed hypomyelination.87 In contrast to the PNS, however, HFD supplementation partially ameliorated CNS hypomyelination in Olig2-Cre, FasnL/L knockouts.87 The mechanisms for these contrasting outcomes between the CNS and the PNS remain undefined but are possibly related to differences in exogenous lipid use between SCs and OLs or through contributions of dietary lipids mediated by other cell types not shared between the CNS and the PNS, such as astrocytes.35,87
Molecular determinants of myelin lipid metabolism
Myelin lipid metabolism is tightly regulated by various molecular determinants, the most prominent of which include SREBPs, mammalian target of rapamycin (mTOR) complexes, PPARs, and quaking (QKI). Below, we summarize currently described mechanisms by which these regulators contribute to myelin and myelin lipid homeostasis.
PPARβ is a regulator of myelin lipid metabolism
The PPARs comprise a family of three closely related ligand-activated transcription factors, namely PPARα, PPARβ, and PPARγ.88,89 Lipids, including eicosanoids and phospholipids, serve as endogenous ligands for PPARs. Heterodimerization with retinoic X receptor (RXR), binding to PPAR-response elements, and ligand-dependent activation are requisite for PPAR-mediated gene transcription.88,89,90 PPARs regulate multiple pathways that contribute to various biological processes, including mitochondrial homeostasis, adipogenesis, and inflammation.90,91,92,93 Within the PPAR family, PPARβ has been particularly associated with myelination and myelin homeostasis. PPARβ is abundantly expressed in the brain, with differential expression in neurons and OLs.94,95,96,97,98 PPARβ activation in vitro can increase OL sheet deposition, myelin protein expression, and OL differentiation.99,100,101 In addition, PPARβ expression is upregulated in OLs during tissue repair following spinal cord injury.102 Interrogating the effects of whole-body Pparβ deficiency using a hypomorphic Pparβ allele revealed partial hypomyelination in the corpus callosum, indicating a role for PPARβ in myelination.103 More recently, inactivation of Pparβ in adult OLs was shown to disrupt myelin maintenance in the CNS through dysregulation of fatty acid biosynthesis, findings which were replicated when Pparβ was conditionally depleted in OLs.104 Pparβ and its heterodimerization partner, Rxrα, interact and functionally cooperate with the KH-domain RNA-binding protein QKI, which is necessary for the transcriptional activation of fatty acid biosynthesis genes (Figure 3F). Loss of Qki in adult OLs using Plp-CreERT2, QkL/L mice severely affected the ability of the Pparβ-Rxrα complex to transcribe fatty acid biosynthesis pathways, leading to morbid demyelination.104 Both the PPARβ agonist KD3010 and the RXR agonist bexarotene ameliorated the lipid loss and consequent demyelination in Qki-null mutants.104
Although less described than PPARβ, other PPAR members may also affect myelination. For instance, an abnormal lipid profile in SCs secondary to loss of SREBP1c leads to dysregulated PPARα activation, elevated fatty acid oxidation, and aberrant hypermyelination, highlighting the need for a balance between lipogenic and oxidative processes in myelin-forming glia, in which distinctive PPARs may have opposing roles.105
SREBPs are versatile regulators of myelin lipids
SREBPs are basic helix-leucine-zipper proteins that initially reside in the ER and depend on SCAP-mediated cleavage for activation.29 The SCAP-SREBP complex is hindered by insulin-induced gene (Insig), which inhibits SREBP activation by preventing its translocation to the Golgi, where SREBP cleavage occurs (Figure 2D).106 Once in the nucleus, SREBPs activate transcription of cholesterol and fatty acid biosynthesis genes that are critical for myelination. While SREBP1 has been shown to regulate both fatty acid and cholesterol metabolism, SREBP2 is regarded as the primary regulator for cholesterol metabolism.106 During cellular lipid homeostasis, both family members can act as lipid sensors, providing feedback for intracellular cholesterol levels in order to fine-tune expression of lipid biosynthesis genes in response to lipid supply and demand.107
As described above, both Srebp and Scap expression correlated with myelin formation during development.30 Moreover, loss of Scap in SCs disrupted myelination, downregulated SREBP targets, and impaired lipid synthesis.33,108 In vitro inhibition of SREBPs impaired OL differentiation and reduced expression of myelin lipid metabolism targets, similar to earlier findings in SCs in vivo.33 Finally, myelin cholesterol and lipid homeostasis was dependent on Scap-mediated activation of Srebp in both OLs and astrocytes.34,35 Additional findings have further highlighted the significance of the SCAP-SREBP axis to myelin lipid homeostasis. For instance, loss of Srebp1c is accompanied by peripheral neuropathy, secondary to an imbalance in SC fatty acid utilization.105 Likewise, loss of Srebp2 activity significantly hinders CNS myelination. As similarly seen with Pparβ, Qki was recently identified as a co-activator for Srebp2 in OLs.109 Loss of Qki-dependent co-activation in OLs significantly disrupted Srebp2-mediated transcription of cholesterol biosynthesis pathways, which blunted myelination and caused severe congenital hypomyelination accompanied by motor deficits and high morbidity (Figure 2E).110
In summary, SREBP1 and SREBP2 are critical to myelin lipid synthesis owing to their transcriptional downstream targets. To date, our knowledge of SREBP proteins’ involvement in myelination has been limited to developmental mouse models; however, understanding how SREBPs work in the adult nervous system could provide significant implications in the clinical setting.
mTOR is an upstream regulator of lipid metabolism
mTOR is a serine/threonine protein kinase that controls cellular metabolism and growth. mTOR constitutes the catalytic kinase domain for two distinct complexes,111 mTORC1 and mTORC2. Together, mTOR, regulatory-associated protein of mTOR (Raptor), and mTOR-associated protein, LST8 homolog (mLST8 or GβL) form the mTORC1 complex, which regulates autophagy and lipid, nucleotide, and protein synthesis. mTORC2 is composed of mTOR, rapamycin-insensitive companion of mTOR (Rictor), and regulatory subunits mSin1 and Protor1/2.111,112 mTORC2 influences cellular proliferation and survival. Of note, mTORC1 has been shown to activate the lipid master regulators SREBP1a, SREBP1c, and SREBP2, leading to increased transcription of fatty acid and cholesterol biosynthesis genes.111,112 The proposed mechanisms of mTORC1-mediated activation of SREBPs include modulating the availability of cholesterol in the ER by regulating autophagic and lysosomal pathways (Figure 2F).113 Owing to its intricate relationship with cell growth and lipid metabolism, as well as its position upstream of other myelin regulators such as SREBPs and PPAR, mTOR function has been investigated extensively in glia and myelin.114 For instance, Cnp-Cre-driven depletion of mTOR in myelinating glia, which disrupts both mTORC1 and mTORC2, causes hypomyelination in the PNS and CNS that persists into adulthood.115,116 Of interest, Dhh-Cre-driven ablation of Raptor or Rictor in SCs demonstrated that mTORC1, but not mTORC2, is necessary for proper PNS myelination.117 Although mTOR activity correlates with myelination onset in SCs, mTOR was not found to be essential for SC survival.115 However, loss of mTORC1 function led to a severe reduction in fatty acids and cholesterol in peripheral myelin through downregulation of SREBP family proteins.115
Unlike the PNS, CNS myelination is regulated by both mTORC1 and mTORC2.118,119 Loss of Raptor alone or in combination with Rictor induced hypomyelination in the CNS in both congenital and adult-onset experimental settings using Cnp-Cre and Plp-CreERT2, respectively.118,119 Of interest, the spinal cord demonstrated greater myelin defects relative to the corpus callosum after mTORC1 signaling was blocked.112,118,119,120,121 Although Raptor was found to be more critical for CNS myelination relative to Rictor,117,118,119,120,122 double knockout of both Raptor and Rictor induced greater demyelination in adult animals relative to single knockouts,118 as well as loss of mature OLs in the spinal cord, a striking difference from PNS findings.120,122 These observations suggest that both mTORC complexes critically contribute to myelin maintenance in the spinal cord.
Interestingly, mTOR activity requires tight control during myelination, as both suppression and hyperactivation disturb myelin homeostasis. For instance, loss of tuberous sclerosis complex subunits 1 and 2 (TSC1/2), which inhibit mTORC1, precipitated severe hypomyelination in the CNS and PNS.123,124 These findings are consistent with clinical observations in tuberous sclerosis patients and mouse models, which experience white matter abnormalities.125,126,127,128 However, more studies are needed to uncover the intricate mechanisms behind the regulation of CNS and PNS myelination by mTOR complexes.
QKI is both a transcriptional and a posttranscriptional regulator of myelin pathways
QKI belongs to the KH-domain family of proteins characterized by their ability to bind single-stranded RNA and DNA.129,130 Qki was first identified in quaking viable (qkv) mutant mice in which Qki is downregulated secondary to loss of regulatory elements in a 1 Mb deletion upstream of the Qk gene.131,132 Qkv mice demonstrate hindlimb paralysis and tremors due to severe hypomyelination, primarily in the CNS.131 Qk knockout is embryonically lethal; however, OL-specific depletion of Qki using Olig2-Cre phenocopied the motor deficits and histopathology exhibited in qkv mutants, highlighting that loss of OL-specific Qki accounted for the myelin deficits.133
Mechanistically, earlier findings described a regulatory role for Qki in alternative splicing of transcripts for myelin-related proteins, including myelin basic protein (MBP), MAG, PLP, and NF155.133,134,135 In addition, our laboratory uncovered a novel transcriptional function for Qki in the regulation of myelin lipid synthesis.104,110 Depletion of Qki in OLs in adult mice induced severe demyelination in the CNS. Although myelin protein expression remained stable, lipidomic profiling revealed a substantial loss of myelin lipids, with a differential reduction in MUFAs and very-long-chain fatty acids (VLCFAs).104 In OLs, Qki served as a critical co-activator for the Pparβ-Rxrα heterodimer, as target gene promotor occupancy and transcriptional activation by Pparβ-Rxrα were Qki dependent (Figure 3F).104 Importantly, HFD supplementation or PPARβ agonist treatment provided a substantial rescue effect, supporting the hypothesis that QKI regulates myelin maintenance through PPARβ-mediated lipid biosynthesis.104 Beyond myelin stability, the role of Qki in lipid metabolism is also critical for de novo myelination.110 Induced knockout of Qk in either neural/glial precursor cells or OPCs during postnatal myelination onset led to severe hindlimb paralysis, significant CNS hypomyelination, and poor survival after weaning. Of interest, loss of Qki during developmental myelination significantly reduced cholesterol in myelin as well as downregulating sterol synthesis genes.110 Similar to its interaction with Pparβ, which drives expression of fatty acid metabolism-related gene targets, Qki functionally cooperated with cholesterol master regulator Srebp2 during developmental myelination to enable expression of cholesterol synthesis pathway enzymes (Figure 2E).110 Thus, Qki is a transcriptional co-regulator for fatty acid and cholesterol metabolic pathways essential for myelin maintenance and myelination.
Beyond its physiological roles, QKI is associated with a variety of neurological diseases, including 6-q terminal deletion syndrome, schizophrenia, multiple sclerosis, and glioma.136,137,138 Given QKI’s importance to myelin synthesis and maintenance as well as its correlation with human diseases, further investigation into upstream regulators of QKI, as well as cross talk with other molecular pathways, is warranted.
Contribution of lipid metabolism to myelin maintenance
As alluded to above, lipids play diverse roles in myelin homeostasis and are regulated by various key molecular determinants. However, one aspect less appreciated but no less vital is the role of lipid metabolism in supporting myelin maintenance specifically, which merits special focus given the growing attention to myelin maintenance and plasticity in adults.
Myelin maintenance is an active process
Although myelin has been traditionally regarded as highly stable and metabolically inert, its maintenance is now more appreciated as a dynamic and active process, wherein myelin membrane turnover represents a significant component. Myelin sheath renewal rate independent of cell turnover was best appreciated through carbon-dating studies in postmortem tissues and cell lineage tracing.5,139 14C levels in OL nuclei isolated from human brains revealed that the majority of OLs are formed within the first 5 years of life, with an annual turnover of 0.32% in adult life.5 Similarly, OLs are very stable in mice, with an estimated half-life of up to 10 years, depending on the CNS region, with up to 90% of OLs surviving 20 months in the corpus callosum.139 In the PNS, SC stability in the sciatic nerve is even more robust than that of OLs, since in vivo proliferation assays revealed minimal turnover in myelinating SCs and an estimated turnover rate of more than 70 months for non-myelinating SCs.8 In contrast to the slow turnover of myelinating glia, myelin membrane undergoes a more frequent renewal, with 14C measurements in humans indicating a continual turnover of myelin on a yearly basis.5 Interrogation of myelin-specific proteins and lipids has revealed that continual synthesis is required for myelin maintenance, albeit at significantly varied degrees.
Myelin proteins exhibit robust stability with durable lifespans
Within the myelin sheath, myelin-specific proteins enjoy a high degree of stability with a relatively low exchange rate. For instance, Plp protein expression can remain stable months after inducible knockout in adult mice.140 Likewise, the half-life for Mbp was approximately 11 weeks following induced knockout in adult mice, with Mbp protein expression remaining at 26% more than 6 months after injection.141 Moreover, in rats ante- and postnatally exposed to 15N, 18.5% of 15N-labeled Plp protein persisted 6 months after eliminating 15N exposure.142 Similarly, 20.2% of 15N-labeled Mbp protein remained intact during the same time course.142 In a complementary 15N pulse study, Plp and Mbp exhibited turnover rates of 0.003 day−1, which corresponded to half-lives that exceeded well beyond 6 months.143 Furthermore, the use of SILAC mouse labeling with [13C]lysine further substantiated that Plp and Mbp are long-lived, both surpassing the 99th percentile of measured protein lifetimes in the brain.144 Blocking protein translation in adult OLs minimally affects myelin stability in the short term,145 although longer-term impairment can induce late-onset demyelination.140 Moreover, continual myelin protein synthesis, such as for Mbp, is necessary for adult myelin renewal and maintenance.141 Therefore, although the estimated half-life for myelin proteins can vary between experimental approaches, myelin-specific proteins are robustly stable with durable lifetimes.
Continual and frequent myelin lipid self-renewal is necessary for myelin maintenance
In contrast to proteins, myelin lipids experience faster exchange rates, and myelin maintenance is more vulnerable to lipid dysmetabolism. For instance, isotope tracing demonstrated continual turnover of myelin lipids, although at different rates across lipid species.146 By measuring deuterium incorporation rates in myelin lipids in mice, half-replacement times for myelin-specific cholesterol, phosphatidylcholine, PE, and GalC were estimated to be 359, 20, 25, and 94 days, respectively.146 Thus, although cholesterol has a fairly stable half-life, phospholipids experience more rapid turnover, with a half-life approaching 3 weeks.146 In addition to lipid tracing, investigation of Qki demonstrated a critical link between lipid turnover and myelin maintenance for the first time through genetic approaches. As discussed above, QKI functions as a transcriptional co-activator that regulates lipid synthesis in OLs through activation of PPARβ.104 Metabolic disruption in OLs secondary to Qki loss decreased fatty acid elongation and desaturation, impairing lipid synthesis necessary for myelin turnover.104 The altered myelin lipid metabolism arrested membrane turnover, impaired myelin maintenance without affecting OL survival, and induced significant and morbid demyelination, illuminating a greater need for lipid renewal relative to proteins in myelin homeostasis, at least in the short term.104 Of note, OL-specific Qki depletion resulted in rapid demyelination within 1 week, highlighting the fast turnover rate of the myelin lipids that are regulated by Qki.104 Thus, in myelinating OLs, PPARβ with QKI coactivation is critical for driving myelin lipid renewal and myelin maintenance.
Beyond the CNS, it remains unclear whether lipid metabolism has similar importance in peripheral myelin stability, although some recent studies have helped address this question. For example, excessive production of sphingolipids, including ceramide and sphingosine, through elevated serine palmitoyltransferase activity in adult SCs caused significant neuropathy accompanied by loss of myelinated fibers and formation of excessive or redundant myelin membrane.147 Thus, excess of simple sphingolipids appears deleterious to peripheral myelin homeostasis, indicating a possible dependency on a balanced lipid turnover. Supporting this notion, albeit indirectly, mitochondrial dysfunction through knockout of mitochondrial transcription factor A (Tfam) in SCs led to peripheral demyelination coupled with attenuated expression of lipid synthesis genes, including Srebp1, Fasn, and Hmgcr, as well as depressed cerebroside and sulfatide levels in nerves.148 Conversely, impaired oxidative phosphorylation secondary to Cox10 deficiency in SC induced significant neuropathy and PNS dysmyelination similar to Tfam knockout; however, here insufficient energy production was the ascribed mechanism.149 Separately, loss of Lkb1 in SCs induced peripheral neuropathy and axonal degeneration without causing demyelination, despite decreases in important myelin lipid species such as cholesterol, cerebrosides, and phospholipids within nerves.150 Thus, further investigation is warranted to resolve these contrasting observations and to further define how lipid metabolism and myelin maintenance relate in SCs.
Lipid dysmetabolism contributes to myelin disorders
To summarize the relevance of myelin lipid physiology to human diseases, a brief discussion regarding the interrelationship between myelin-related disorders and lipid metabolism is next provided.
Leukodystrophies highlight an intersection between lipid dysmetabolism and myelin pathology
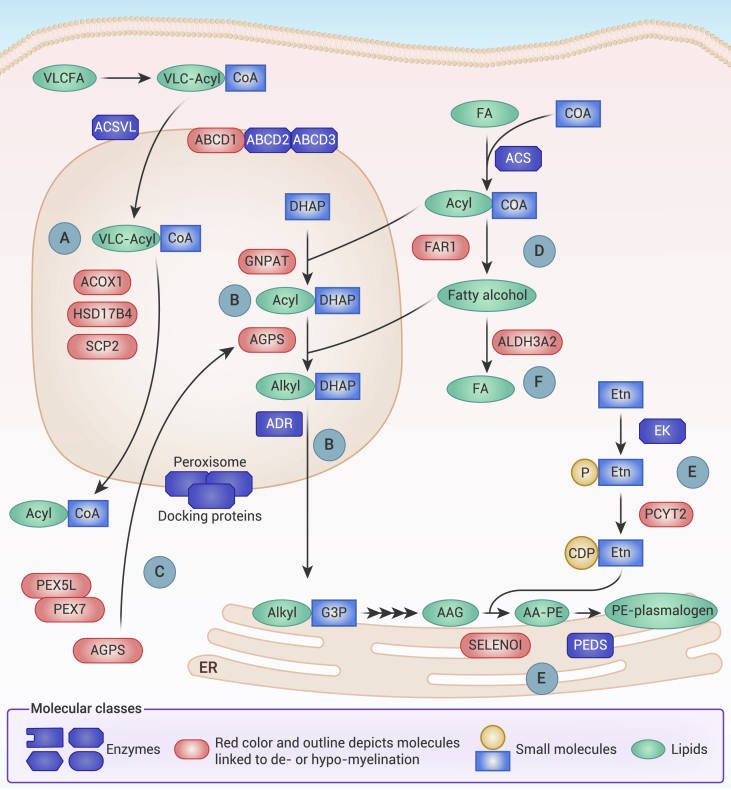
Hypomyelinating and demyelinating disorders known as leukodystrophies provide a genetic link between lipid metabolism and myelin maintenance, as their underlying mutations commonly occur in genes directly involved in lipid metabolic pathways (Table 1). For instance, mutations affecting fatty acid oxidation in peroxisomes can lead to leukodystrophy. Loss of function of ATP-binding cassette subfamily D member 1 (ABCD1) causes X-linked adrenoleukodystrophy (ALD), one of the most common leukodystrophies. ABCD1 is a peroxisome lipid transporter for VLCFAs, which require ABCD1 for import and subsequent degradation in peroxisomes (Figure 4A).151,152 VLCFA accumulation in the CNS following ABCD1 loss is considered detrimental for white matter integrity and possibly causative for ALD. Beyond transport of VLCFA, mutations in the peroxisome fatty acid oxidizing enzymes ACOX1, HSD17B4, and SCP2 also contribute to leukodystrophies that, similar to those in ALD, are ostensibly secondary to the deleterious accumulation of lipid substrates in myelin and brain tissue.153,154,155 Animal models of peroxisome-related leukodystrophies, which include dACOX1-mutant fruit flies156 and Hsd17b4- and Pex5-mutant mice,157,158 further reinforce the association of perturbed peroxisome lipid oxidation and glial and myelin homeostasis (Figure 4A).
Table 1.
Hereditary myelin disorders secondary to mutations in lipid metabolic pathway-related genes
| Name | OMIM | Gene | Gene product; function | Description | Reference |
|---|---|---|---|---|---|
| Peroxisomal acyl-CoA oxidase deficiency | 264470 | ACOX1 | Peroxisomal acyl-coenzyme A oxidase 1; catalyzes first reaction in peroxisomal fatty acid β-oxidation | Leukodystrophy, hypotonia, seizures, developmental regression, mean survival of 5 years | Ferdinandusse et al.,153 Watkins et al.216 |
| D-bifunctional protein deficiency | 261515 | HSD17B4 | D-bifunctional protein; catalyzes second and third reactions in peroxisomal fatty acid β-oxidation | Leukodystrophy, seizures, hypotonia, delayed development, neuronal migration defects, mean survival of <2 years | Ferdinandusse et al.154,217 |
| X-linked adrenoleukodystrophy | 300100 | ABCD1 | ATP-binding cassette subfamily D member 1; importation of very-long-chain fatty acids into peroxisomes | Leukodystrophy, dementia, adrenal insufficiency, paralysis, audiovisual impairment | Moser et al.,151,152, Schaumburg et al.218 |
| Fatty acid hydroxylase-associated neurodegeneration | 612319 | FA2H | Fatty acid 2-hydroxylase; hydroxylation at the 2 position of N-acyl chain of ceramide moieties | Leukodystrophy, spastic paraplegia, cognitive impairment | Edvardson et al.,171 Kruer et al.,172 Dick et al.219 |
| Sjogren-Larsson syndrome | 270200 | ALDH3A2 | Aldehyde dehydrogenase family-3 member A2; oxidation of lipid-derived aldehydes | Leukoencephalopathy, intellectual disability, macular dystrophy, spastic paresis | Lossos et al.,220 Sjogren et al.,221 Willemsen et al.220,221,222 |
| Metachromatic leukodystrophy | 250100, 249900 | ARSA/PSAP | Arylsulfatase A; catalyzes hydrolyzation of sulfatides | Leukodystrophy, late infantile: seizures, hypotonia, developmental regression | Mahmood et al.,177 MacFaul et al.,223 Zafeiriou et al.224 |
| Prosaposin; precursor of saposins A–D, catalyzes hydrolysis of glycosphingolipids | Leukodystrophy, juvenile/adult: behavioral disturbances, dementia, ataxia, pyramidal signs, neuropathy | ||||
| Globoid cell leukodystrophy | 245200 | GALC | Galactosylceramidase; catalyzes hydrolysis of galactoceramide | Leukodystrophy, infantile: developmental delay, hypotonia, quadriparesis, seizures, survival of <2 years | Komatsuzaki et al.,178 Wenger et al.179 |
| Leukodystrophy, juvenile/adult: behavioral disturbances, motor-sensory neuropathy, cognitive decline | |||||
| Hypomyelinating leukodystrophy-18 | 618404 | DEGS1 | Dihydroceramide desaturase 1; catalyzes final step of ceramide de novo synthesis | Leukodystrophy, failure to thrive, poor psychomotor development, severe intellectual disability | Karsai et al.,175 Pant et al.,176 Dolgin et al.225 |
| Smith-Lemli-Opitz | 270400 | DHCR7 | 7-Dehydrocholesterol reductase; catalyzes reduction and conversion of 7-dehydrocholesterol into cholesterol | Congenital malformations, photosensitivity, intellectual disability, abnormal brain MRI (corpus callosum, white matter lesions) | Anstey et al.,226 Irons et al.,227 Lee et al.228,229 |
| Rhizomelic chondrodysplasia punctata (types 1, 2, 3, and 5) | 215100, 222765, 600121, 616716 | PEX7, GNPAT, AGPS, PEX5-L | Peroxin 7; import of PTS2 peroxisome matrix proteins.Dihydroxyacetone phosphate acyltransferase; catalyzes synthesis of plasmalogens | Abnormal facies, rhizomelia, congenital cataracts, intellectual disability, developmental delay, joint deformities, myelination abnormalities | Bams-Mengerink et al.,159 Sztriha et al.,160 Barøy et al.163 |
| Alkyl dihydroxyacetone phosphate synthase; catalyzes synthesis of plasmalogens. Peroxin 5, long isoform; co-transporter for PTS2 peroxisome matrix proteins | |||||
| Peroxisomal fatty acyl-CoA reductase 1 disorder (PFCRD) | 616154 | FAR1 | Fatty acyl-CoA reductase 1; catalyzes reduction of long-chain fatty acyl-CoA to fatty alcohols | Intellectual disability, epilepsy, growth retardation, spastic paresis, cataracts | Buchert et al.,164 Ferdinandusse et al.165 |
| Spastic paraplegia 81 (SPG81) | 618768 | SELENOI | Ethanolamine phosphotransferase 1; catalyzes transfer of ethanolamine phosphate group to AAG or DAG | Hypomyelination, spastic paraplegia, epilepsy, sensorineural hearing loss, blindness | Ahmed et al.,166 Horibata et al.167 |
| Spastic paraplegia 82 (SPG82) | 618770 | PCYT2 | Ethanolamine-phosphate cytidylyltransferase; required for CDP-ethanolamine synthesis | Brain atrophy, spastic paresis, epilepsy, intellectual disability | Vaz et al.168 |
| Leukodystrophy, progressive, early childhood onset (PLDECO) | 617762 | ACER3 | Alkaline ceramidase 3; hydrolysis of ceramides into sphingosine | Developmental regression, spastic paresis, dystonia, abnormal facies | Edvardson et al.230 |
A non-exhaustive list of inherited disorders with white matter involvement (leukodystrophy or leukoencephalopathy) that are attributed to underlying mutations in genes involved in lipid metabolism is presented.
OMIM, Online Mendelian Inheritance in Man; DAG, 1,2-diacylglycerol; AAG, 1-alkyl-2-acylglycerol.
Figure 4.
Elements of peroxisome, plasmalogen, and phospholipid metabolism required for myelin integrity
(A) Oxidation of very-long-chain fatty acids (VLCFAs) occurs within peroxisomes. VLC-acyl-CoA are imported by ATP-binding cassette transporter subfamily D (ABCD) transporters, principally ABCD1. VLC-acyl-CoA are then oxidized into shorter-chain acyl-CoA by a series of reactions requiring acyl-CoA oxidase (ACOX1), 17-β-hydroxysteroid dehydrogenase IV (HSD17B4), and sterol carrier protein 2 (SCP2).
(B) Initial steps for plasmalogen synthesis require peroxisomal enzymes. Glycerone phosphate-O-acyltransferase (GNPAT) synthesizes 1-acyl-DHAP, which is converted to 1-alkyl-O-DHAP by alkylglycerone phosphate synthase (AGPS). 1-Alkyl-O-DHAP is reduced by alkyl DHAP reductase (ADR) to alkyl-G3P, which passes to the ER to complete plasmalogen synthesis.
(C) Peroxisome matrix proteins, including AGPS, require carrier proteins, such as PEX7 and the PEX5L isoform, to enter peroxisomes.
(D) Fatty alcohols derived from fatty acyl-CoA reductase 1 (FAR1) are utilized for plasmalogen synthesis.
(E) Most plasmalogens in myelin exist as phosphatidylethanolamine lipids (PE-plasmalogens). Ethanolamine (Etn) is phosphorylated by ethanolamine kinase (EK) and coupled with CDP by ethanolamine phosphate cytidylyltransferase (PCYT2). Selenoprotein I (SELENOI) converts 1-O-alkyl-2-acylglycerol (AAG) into 1-O-alkyl-2-acyl-GPE (AA-PE) using PCYT2-derived CDP-Etn. AA-PE is then converted to PE-plasmalogen by plasmanylethanolamine desaturase (PEDS).
(F) Balance of fatty acids and fatty alcohols is maintained in part by members of the aldehyde dehydrogenase (ALDH) family, including ALDH3A2. CDP, cytidine diphosphate; DHAP, dihydroxyacetone phosphate; ACSVL, very-long-chain acyl-CoA-synthase; ACS, acyl-CoA synthase.
Myelin integrity is also sensitive to changes in phospholipid and plasmalogen synthesis. Rhizomelic chondrodysplasia punctata (RCDP) is a disorder characterized by abnormal facies, impaired growth of the proximal long bones (rhizomelia), and myelination abnormalities.159,160 RCDP is secondary to mutations in PEX7, GNPAT, and AGPS, proteins critical for the plasmalogen biosynthesis pathway in peroxisomes,161,162 as well as to mutations in PEX5, which indirectly perturb PEX7 function (Figures 4B and 4C).163 Severely decreased levels of plasmalogens are characteristic of RCDP and may contribute to myelin anomalies, given that most myelin PE phospholipids are derived from plasmalogens. Another RCDP-like disorder with significant neurological impairments follows from plasmalogen deficiency due to loss of FAR1, a critical ether phospholipid biosynthetic enzyme (Figure 4D).164 Of interest, separate gain-of-function mutations in FAR1 that aberrantly increase plasmalogens also correlate with disrupted neurodevelopment,165 suggesting that neurological and, likely, myelin homeostasis rest on delicately balanced plasmalogen levels. Conversely, individuals with mutations undermining the generation of PE phospholipids present with severe hereditary spastic paraplegias and white matter pathology, as seen with loss-of-function mutations in SELENOI166,167 and PCYT2,168 which are necessary for de novo synthesis of PE phospholipids (Figure 4E).169,170 Of note, these mutations lead to substantial changes in plasmalogens that may be major contributors to disease.
Additional lipid and fatty acid metabolic pathways can affect myelin homeostasis and development. For instance, mutations of FA2H, which catalyzes the synthesis of 2-HFs, cause a leukodystrophy accompanied by iron accumulation and neurodegeneration.57,171,172 Sjogren-Larsson syndrome (SLS) is caused by mutations in fatty aldehyde dehydrogenase, which converts fatty aldehydes into fatty acids and is encoded by ALDH3A2, disruption of which causes an imbalance of fatty aldehydes and ether lipids in the brain (Figure 4F).173,174 SLS presents as a childhood-onset leukodystrophy accompanied by ichthyosis and macular dystrophy. Impaired sphingolipid metabolism is also associated with myelin abnormalities. Dihydroceramide desaturase (DEGS1) contributes to the synthesis of ceramide, the simplest form of sphingolipid. DEGS1 mutations cause hypomyelinating leukodystrophy-18, which is characterized by accumulations in the enzyme substrate dihydroceramide and possibly toxic ceramide isoform ceramide-14Z.175,176 Metachromatic leukodystrophy (MLD) is secondary to mutations of either ARSA or PSAP, which encode lysosomal enzyme aryl sulfatase A and prosaposin, respectively.177 Loss of function of ARSA, or impaired activation secondary to mutant prosaposin, arrests the degradation of sulfatides. MLD manifests as a sulfatide lipidosis marked by both CNS and PNS demyelination.177 Finally, globoid cell leukodystrophy or Krabbe disease is another form of sphingolipidosis in which demyelination occurs and is caused by mutations in GALC, which encodes galactosylceramidase, the loss of which leads to the accumulation of GalC as well as psychosine, a toxic metabolite of sphingosine.178,179 Altogether, this non-exhaustive enumeration of various leukodystrophies supports an interdependence between myelin formation and stability and lipid homeostasis (Figure 5A).
Figure 5.
Myelin disruption in leukodystrophies and Alzheimer’s disease
(A) Leukodystrophy-associated lipid dysregulation occurs from mutations in genes that contribute to lipid synthesis or catabolism (see Table 1).
(B) With aging, there is a decline with brain lipids enriched in myelin and a decrease in myelin renewal.
(C) APP/PS1 mice experience demyelination, which exacerbates AD-like memory deficits. Clemastine treatment or conditional knockout (cKO) of M1R in OPCs restored myelin renewal and ameliorated cognitive decline.207
Hereditary demyelinating neuropathies and lipid homeostasis
Charcot-Marie-Tooth disease (CMT) represents a genetically diverse spectrum of hereditary neuropathies with a population prevalence estimated to be 1 in 2,500.180,181 CMT can occur as a primary axonopathy or, more frequently, as a demyelinating neuropathy, with the latter representing more than 80% of CMT cases in Sweden and Iceland.182 Demyelinating forms of CMT include CMT1, CMT4, and CMTX1 and are most often caused by mutations in proteins expressed in SCs and related to myelination, such as PMP22, GJB1, MPZ, and EGR2.183,184
Of note, some studies have associated these common variants of CMT with disturbances in lipid metabolism in SCs that could impair myelin maintenance. First, a rat model of CMT1A, the most frequent subtype of CMT1, revealed altered lipid metabolism in sciatic nerves of animals with severe neuropathy relative to moderately affected animals.185 A phospholipid-enriched diet was able to rescue the motor neuropathy in CMT1A rats when given either postnatally or during adulthood.186 Although major myelin proteins remained unaltered, multiple lipid species were significantly reduced in peripheral myelin from mutant rats; however, the high-phospholipid diet partially improved the myelin lipid profile, which was associated with attenuated loss of myelinated fibers and rescue of the disrupted myelin ultrastructure.186
Separately, lipidomics of CMT1A rat nerve, matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) of nerve endoneurium, and targeted lipidomics of isolated myelin all demonstrated significant dysregulation of sphingolipid and phospholipid levels.187 Moreover, lipid profiling of sera from patients with CMT1A reflected similar and pronounced changes in phospholipid and sphingolipid metabolism.187 In a separate mouse model of CMT1, supplementation with HFD in adult animals improved myelinated fiber density and myelin thickness and mitigated inflammatory infiltrates.188 Of interest, CMT1A is most frequently caused by duplication of PMP22, which was recently demonstrated to contribute to lipid raft formation and cholesterol homeostasis in SCs27,189; moreover, overexpression of PMP22 in SCs induced cholesterol sequestration in lysosomes.190 Similar observations were reported for PLP, the most abundant myelin protein in the CNS, which is likewise associated with lipid raft formation and lipid trafficking.191,192 Overexpression of Plp, which occurs in the hypomyelinating leukodystrophy called Pelizaeus-Merzbacher disease (PMD), dysregulates trafficking of cholesterol and sphingolipids to the plasma membrane, leading to intracellular lipid accumulation.191 Retention of cholesterol in the ER depresses de novo cholesterol synthesis and arrests myelination.193 Thus, the lipid dysmetabolism and demyelination related to PMP22 duplication in CMT1A may in part be attributed to interrupted lipid trafficking and lipid raft formation, as similarly ascribed to aberrant PLP expression in PMD.
Myelin instability and lipid dysregulation are featured in Alzheimer’s disease
Brain lipids, including those enriched in myelin, decrease with age. Moreover, with aging there is a concomitant decline in myelin stability (Figure 5B).194,195,196 Beyond age-related changes, loss of myelin integrity is further compounded in Alzheimer’s disease (AD), one of the most common causes of dementia. Two observations related to myelin are noteworthy in AD. First, AD is accompanied by significant perturbations in brain lipid metabolism, particularly for lipids abundant in myelin. Second, myelin instability contributes to AD progression.
In early-stage AD, sulfatides were decreased in postmortem brain tissues, including in specimens from mildly affected individuals.197 Sulfatide depletion was also detected in the cortex of two transgenic AD mouse models after 10 months of age.198 In severely affected AD brain samples, plasmalogens were reduced, and VLCFAs accumulated199; moreover, in the cortices of transgenic AD mice, plasmalogens were likewise reduced.200 In the 5×FAD AD mouse model, MALDI-MS revealed decreases in myelin lipids, including sulfatides and plasmalogens, which spatially correlated with amyloid-β plaque deposition and myelin loss.201
Myelin stability deficits accompany brain lipidome dysregulation in AD
MRI studies of healthy controls and AD patients demonstrated accelerated decline in white matter integrity in AD patients versus age-matched controls.202 In addition, imaging studies revealed white matter perturbations in presymptomatic familial AD cases203 and intracortical demyelination in preclinical AD.204 Focal demyelination associated with amyloid-β plaque deposition occurred in both AD patients and animal models.205 Moreover, transcriptomic changes in the OL lineage from patients and 5×FAD mice have been reported.206,207 Myelination in adult mice significantly declines with age, which can contribute to age-related deficits in memory.195 In aged APP/PS1-mutant mice, however, myelin membrane turnover was augmented, with increased myelin degradation partially compensated for by new myelin from newly differentiated OPCs.208 Nonetheless, by age 8 months, AD mice exhibited significant cortical and hippocampal demyelination that exacerbated memory-related deficits (Figure 5C).208 Augmenting myelin renewal by repressing muscarinic signaling, either by providing clemastine (a muscarinic receptor antagonist) or by conditional knockout of the muscarinic receptor M1R in OPCs, restored myelin renewal and ameliorated cognitive deficits in AD mice (Figure 5C). The correlation between lipid dysregulated and demyelination observed in AD remains largely unexplored and merits deeper investigation because such studies could both improve our understanding of lipid-dependent myelin homeostasis and uncover new therapeutic approaches to AD-related myelin instability.
Perspective
Myelin requires enrichment of diverse lipids, including cholesterol, GalC, and plasmalogens. Disruption of lipid metabolism is detrimental to myelin formation and stability, an observation reinforced by mouse and human genetic studies. Various mutations associated with demyelination suggest that myelin health requires intact lipid pathways with a steady equilibrium between lipid synthesis and degradation. Recent findings indicate that myelin loss contributes to AD evolution and age-related memory deficits. As AD and aging are also associated with myelin lipid dysmetabolism, the relationship between myelin lipids and demyelination in these contexts warrants further investigation.
Multiple studies have addressed the interrelationship between myelin and myelin lipids; however, various questions remain unaddressed. For instance, the functions of diverse lipid classes in myelin maintenance remain poorly understood. In addition, the source of these lipids is not always clear. Myelin-producing glia have naturally been the focus of this question, but this could leave underappreciated the contributions of supporting cells and circulating lipids. Moreover, how lipids are exchanged between myelin and cytosolic compartments within glia is unknown. Also unclear is the heterogeneity of myelin lipid dynamics between the PNS and the CNS and among the different subsets of myelinating glia present in both compartments.
Exciting prospects derived from the expanding knowledge of myelin lipid metabolism include the development of novel therapeutic approaches. For instance, dietary supplementation with neutral lipids was beneficial in a mouse model of CMT,188 while cholesterol-enriched diets were beneficial in separate models of acute demyelination and PMD-associated leukodystrophy.193,209 High-fat, ketogenic diets also ameliorated PMD in a preclinical model.210 Targeted modifications of lipid metabolism in the context of lipid dysregulation have also shown positive signs. In a fly model of ACOX1 loss of function, improving oxidation of VLCFAs with the PPARα agonist bezafibrate ameliorated the mutant phenotype.156 Conversely, in models of ALD, induction of the fatty acid desaturase SCD1 significantly corrected the disease-associated lipid profiles.211 Finally, the discovery of the QKI-PPARβ-RXRα axis and its role in maintaining myelin lipid homeostasis revealed that PPAR and RXR agonists could be favorable in cases of progressive multiple sclerosis that exhibit reduced QKI-PPARβ-RXRα activity.104
Investigating myelin lipid metabolism could also help predict adverse effects on myelin health. For instance, given cholesterol’s importance in providing new myelin, further studies may be warranted to investigate the effects of widely used statins on cognition and learning, which can be myelin dependent. This is especially relevant for individuals of advanced age or with dementia in whom myelin renewal is already impaired.195,212,213 Although mTOR inhibition has received positive attention for its anti-aging effects,214,215 caution should be exercised in inhibiting this axis, especially in patients with compromised myelin maintenance, given how mTOR supports myelin development.
In conclusion, mechanistic research in the field of myelin lipid homeostasis has promising potential for harnessing new approaches to reinforcing myelin health and redressing disease-related myelin loss.
Acknowledgments
We thank Q. Zhang for her contributions to the public description and reporting for the manuscript. We also thank the Central Academy of Fine Arts for its assistance in figure editing and design. Editorial support was provided by Bryan Tutt, Scientific Editor, Research Medical Library at the MD Anderson Cancer Center.
Author contributions
J.A.B.-V. and F.B.A.Y. contributed equally to the literature search, conceptual design, drafting and editing of the manuscript, and figure design. J.H. contributed to manuscript editing, conceptual design, and scientific discussion.
Declaration of interests
The authors declare no competing interests.
Published Online: December 7, 2022
Lead contact website
https://www.mdanderson.org/research/departments-labs-institutes/labs/hu-laboratory.html
References
- 1.Tasaki I. The electro-saltatory transmission of the nerve impulse and the effect of narcosis upon the nerve fiber. American Journal of Physiology-Legacy Content. 1939;127:211–227. [Google Scholar]
- 2.Huxley A.F., Stämpfli R. Evidence for saltatory conduction in peripheral myelinated nerve fibres. J. Physiol. 1949;108:315–339. [PubMed] [Google Scholar]
- 3.Pease-Raissi S.E., Chan J.R. Building a (w)rapport between neurons and oligodendroglia: reciprocal interactions underlying adaptive myelination. Neuron. 2021;109:1258–1273. doi: 10.1016/j.neuron.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xin W., Chan J.R. Myelin plasticity: sculpting circuits in learning and memory. Nat. Rev. Neurosci. 2020;21:682–694. doi: 10.1038/s41583-020-00379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeung M.S.Y., Zdunek S., Frisén J. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell. 2014;159:766–774. doi: 10.1016/j.cell.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Harty B.L., Monk K.R. Unwrapping the unappreciated: recent progress in Remak Schwann cell biology. Curr. Opin. Neurobiol. 2017;47:131–137. doi: 10.1016/j.conb.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stierli S., Imperatore V., Lloyd A.C. Schwann cell plasticity-roles in tissue homeostasis, regeneration, and disease. Glia. 2019;67:2203–2215. doi: 10.1002/glia.23643. [DOI] [PubMed] [Google Scholar]
- 8.Stierli S., Napoli I., Lloyd A.C. The regulation of the homeostasis and regeneration of peripheral nerve is distinct from the CNS and independent of a stem cell population. Development. 2018;145:dev170316. doi: 10.1242/dev.170316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norton W.T., Poduslo S.E. Myelination in rat brain: changes in myelin composition during brain maturation. J. Neurochem. 1973;21:759–773. doi: 10.1111/j.1471-4159.1973.tb07520.x. [DOI] [PubMed] [Google Scholar]
- 10.O'Brien J.S., Sampson E.L. Lipid composition of the normal human brain: gray matter, white matter, and myelin. J. Lipid Res. 1965;6:537–544. [PubMed] [Google Scholar]
- 11.O'Brien J.S., Sampson E.L., Stern M.B. Lipid composition of myelin from the peripheral nervous system. Intradural spinal roots. J. Neurochem. 1967;14:357–365. doi: 10.1111/j.1471-4159.1967.tb09532.x. [DOI] [PubMed] [Google Scholar]
- 12.Poitelon Y., Kopec A.M., Belin S. Myelin fat facts: an overview of lipids and fatty acid metabolism. Cells. 2020;9:812. doi: 10.3390/cells9040812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitt S., Castelvetri L.C., Simons M. Metabolism and functions of lipids in myelin. Biochim. Biophys. Acta. 2015;1851:999–1005. doi: 10.1016/j.bbalip.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Lingwood D., Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 15.Korade Z., Kenworthy A.K. Lipid rafts, cholesterol, and the brain. Neuropharmacology. 2008;55:1265–1273. doi: 10.1016/j.neuropharm.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saher G., Quintes S., Nave K.A. Cholesterol: a novel regulatory role in myelin formation. Neuroscientist. 2011;17:79–93. doi: 10.1177/1073858410373835. [DOI] [PubMed] [Google Scholar]
- 17.Woelk H., Borri P. Lipid and fatty acid composition of myelin purified from normal and MS brains. Eur. Neurol. 1973;10:250–260. doi: 10.1159/000114281. [DOI] [PubMed] [Google Scholar]
- 18.Yang S.T., Kreutzberger A.J.B., Lee J., Kiessling V., Tamm L.K. The role of cholesterol in membrane fusion. Chem. Phys. Lipids. 2016;199:136–143. doi: 10.1016/j.chemphyslip.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saher G., Brügger B., Nave K.A. High cholesterol level is essential for myelin membrane growth. Nat. Neurosci. 2005;8:468–475. doi: 10.1038/nn1426. [DOI] [PubMed] [Google Scholar]
- 20.Saher G., Simons M. Cholesterol and myelin biogenesis. Subcell. Biochem. 2010;51:489–508. doi: 10.1007/978-90-481-8622-8_18. [DOI] [PubMed] [Google Scholar]
- 21.Lappe-Siefke C., Goebbels S., Nave K.A. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat. Genet. 2003;33:366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- 22.Saher G., Quintes S., Nave K.A. Cholesterol regulates the endoplasmic reticulum exit of the major membrane protein P0 required for peripheral myelin compaction. J. Neurosci. 2009;29:6094–6104. doi: 10.1523/JNEUROSCI.0686-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin J.P., Mironova Y.A., Shrager P., Giger R.J. LRP1 regulates peroxisome biogenesis and cholesterol homeostasis in oligodendrocytes and is required for proper CNS myelin development and repair. Elife. 2017;6 doi: 10.7554/eLife.30498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saher G., Stumpf S.K. Cholesterol in myelin biogenesis and hypomyelinating disorders. Biochim. Biophys. Acta. 2015;1851:1083–1094. doi: 10.1016/j.bbalip.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Klugmann M., Schwab M.H., Nave K.A. Assembly of CNS myelin in the absence of proteolipid protein. Neuron. 1997;18:59–70. doi: 10.1016/s0896-6273(01)80046-5. [DOI] [PubMed] [Google Scholar]
- 26.Werner H.B., Krämer-Albers E.M., Nave K.A. A critical role for the cholesterol-associated proteolipids PLP and M6B in myelination of the central nervous system. Glia. 2013;61:567–586. doi: 10.1002/glia.22456. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Y., Miles J.R., Notterpek L. PMP22 regulates cholesterol trafficking and ABCA1-mediated cholesterol efflux. J. Neurosci. 2019;39:5404–5418. doi: 10.1523/JNEUROSCI.2942-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miron V.E., Zehntner S.P., Antel J.P. Statin therapy inhibits remyelination in the central nervous system. Am. J. Pathol. 2009;174:1880–1890. doi: 10.2353/ajpath.2009.080947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Camargo N., Smit A.B., Verheijen M.H.G. SREBPs: SREBP function in glia-neuron interactions. FEBS J. 2009;276:628–636. doi: 10.1111/j.1742-4658.2008.06808.x. [DOI] [PubMed] [Google Scholar]
- 30.Verheijen M.H.G., Chrast R., Burrola P., Lemke G. Local regulation of fat metabolism in peripheral nerves. Genes Dev. 2003;17:2450–2464. doi: 10.1101/gad.1116203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leblanc S.E., Srinivasan R., Svaren J. Regulation of cholesterol/lipid biosynthetic genes by Egr2/Krox20 during peripheral nerve myelination. J. Neurochem. 2005;93:737–748. doi: 10.1111/j.1471-4159.2005.03056.x. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki R., Ferris H.A., Chee M.J., Maratos-Flier E., Kahn C.R. Reduction of the cholesterol sensor SCAP in the brains of mice causes impaired synaptic transmission and altered cognitive function. PLoS Biol. 2013;11 doi: 10.1371/journal.pbio.1001532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verheijen M.H.G., Camargo N., Smit A.B. SCAP is required for timely and proper myelin membrane synthesis. Proc. Natl. Acad. Sci. USA. 2009;106:21383–21388. doi: 10.1073/pnas.0905633106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Camargo N., Brouwers J.F., Verheijen M.H.G. High-fat diet ameliorates neurological deficits caused by defective astrocyte lipid metabolism. Faseb. J. 2012;26:4302–4315. doi: 10.1096/fj.12-205807. [DOI] [PubMed] [Google Scholar]
- 35.Camargo N., Goudriaan A., Verheijen M.H.G. Oligodendroglial myelination requires astrocyte-derived lipids. PLoS Biol. 2017;15 doi: 10.1371/journal.pbio.1002605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wattenberg B.W. Intra- and intercellular trafficking in sphingolipid metabolism in myelination. Adv. Biol. Regul. 2019;71:97–103. doi: 10.1016/j.jbior.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamaji T., Hanada K. Sphingolipid metabolism and interorganellar transport: localization of sphingolipid enzymes and lipid transfer proteins. Traffic. 2015;16:101–122. doi: 10.1111/tra.12239. [DOI] [PubMed] [Google Scholar]
- 38.O'Brien J.S., Rouser G. The fatty acid composition of brain sphingolipids: sphingomyelin, ceramide, cerebroside, and cerebroside sulfate. J. Lipid Res. 1964;5:339–342. [PubMed] [Google Scholar]
- 39.Bakhti M., Aggarwal S., Simons M. Myelin architecture: zippering membranes tightly together. Cell. Mol. Life Sci. 2014;71:1265–1277. doi: 10.1007/s00018-013-1492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boggs J.M., Gao W., Basu A. Participation of galactosylceramide and sulfatide in glycosynapses between oligodendrocyte or myelin membranes. FEBS Lett. 2010;584:1771–1778. doi: 10.1016/j.febslet.2009.11.074. [DOI] [PubMed] [Google Scholar]
- 41.Grassi S., Prioni S., Prinetti A. The role of 3-O-sulfogalactosylceramide, sulfatide, in the lateral organization of myelin membrane. Neurochem. Res. 2016;41:130–143. doi: 10.1007/s11064-015-1747-2. [DOI] [PubMed] [Google Scholar]
- 42.Kulkarni K., Snyder D.S., McIntosh T.J. Adhesion between cerebroside bilayers. Biochemistry. 1999;38:15264–15271. doi: 10.1021/bi991725m. [DOI] [PubMed] [Google Scholar]
- 43.Bosio A., Binczek E., Stoffel W. Functional breakdown of the lipid bilayer of the myelin membrane in central and peripheral nervous system by disrupted galactocerebroside synthesis. Proc. Natl. Acad. Sci. USA. 1996;93:13280–13285. doi: 10.1073/pnas.93.23.13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coetzee T., Fujita N., Popko B. Myelination in the absence of galactocerebroside and sulfatide: normal structure with abnormal function and regional instability. Cell. 1996;86:209–219. doi: 10.1016/s0092-8674(00)80093-8. [DOI] [PubMed] [Google Scholar]
- 45.Marcus J., Honigbaum S., Dupree J.L. Sulfatide is essential for the maintenance of CNS myelin and axon structure. Glia. 2006;53:372–381. doi: 10.1002/glia.20292. [DOI] [PubMed] [Google Scholar]
- 46.Dupree J.L., Coetzee T., Suzuki K., Popko B. Myelin abnormalities in mice deficient in galactocerebroside and sulfatide. J. Neurocytol. 1998;27:649–659. doi: 10.1023/a:1006908013972. [DOI] [PubMed] [Google Scholar]
- 47.Honke K., Hirahara Y., Taniguchi N. Paranodal junction formation and spermatogenesis require sulfoglycolipids. Proc. Natl. Acad. Sci. USA. 2002;99:4227–4232. doi: 10.1073/pnas.032068299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishibashi T., Dupree J.L., Baba H. A myelin galactolipid, sulfatide, is essential for maintenance of ion channels on myelinated axon but not essential for initial cluster formation. J. Neurosci. 2002;22:6507–6514. doi: 10.1523/JNEUROSCI.22-15-06507.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayashi A., Kaneko N., Tomihira C., Baba H. Sulfatide decrease in myelin influences formation of the paranodal axo-glial junction and conduction velocity in the sciatic nerve. Glia. 2013;61:466–474. doi: 10.1002/glia.22447. [DOI] [PubMed] [Google Scholar]
- 50.Ozgen H., Schrimpf W., Baron W. The lateral membrane organization and dynamics of myelin proteins PLP and MBP are dictated by distinct galactolipids and the extracellular matrix. PLoS One. 2014;9 doi: 10.1371/journal.pone.0101834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palavicini J.P., Wang C., Han X. Novel molecular insights into the critical role of sulfatide in myelin maintenance/function. J. Neurochem. 2016;139:40–54. doi: 10.1111/jnc.13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pomicter A.D., Deloyht J.M., Dupree J.L. Nfasc155H and MAG are specifically susceptible to detergent extraction in the absence of the myelin sphingolipid sulfatide. Neurochem. Res. 2013;38:2490–2502. doi: 10.1007/s11064-013-1162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki A., Hoshi T., Baba H. Paranodal axoglial junction is required for the maintenance of the Nav1.6-type sodium channel in the node of Ranvier in the optic nerves but not in peripheral nerve fibers in the sulfatide-deficient mice. Glia. 2004;46:274–283. doi: 10.1002/glia.20008. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki K., Vanier M.T., Coetzee T., Popko B. Drastically abnormal gluco- and galactosylceramide composition does not affect ganglioside metabolism in the brain of mice deficient in galactosylceramide synthase. Neurochem. Res. 1999;24:471–474. doi: 10.1023/a:1022571410445. [DOI] [PubMed] [Google Scholar]
- 55.Zöller I., Büssow H., Gieselmann V., Eckhardt M. Oligodendrocyte-specific ceramide galactosyltransferase (CGT) expression phenotypically rescues CGT-deficient mice and demonstrates that CGT activity does not limit brain galactosylceramide level. Glia. 2005;52:190–198. doi: 10.1002/glia.20230. [DOI] [PubMed] [Google Scholar]
- 56.Hama H. Fatty acid 2-Hydroxylation in mammalian sphingolipid biology. Biochim. Biophys. Acta. 2010;1801:405–414. doi: 10.1016/j.bbalip.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alderson N.L., Maldonado E.N., Kern M.J., Bhat N.R., Hama H. FA2H-dependent fatty acid 2-hydroxylation in postnatal mouse brain. J. Lipid Res. 2006;47:2772–2780. doi: 10.1194/jlr.M600362-JLR200. [DOI] [PubMed] [Google Scholar]
- 58.Eckhardt M., Yaghootfam A., Fewou S.N., Zöller I., Gieselmann V. A mammalian fatty acid hydroxylase responsible for the formation of alpha-hydroxylated galactosylceramide in myelin. Biochem. J. 2005;388:245–254. doi: 10.1042/BJ20041451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maldonado E.N., Alderson N.L., Monje P.V., Wood P.M., Hama H. FA2H is responsible for the formation of 2-hydroxy galactolipids in peripheral nervous system myelin. J. Lipid Res. 2008;49:153–161. doi: 10.1194/jlr.M700400-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zöller I., Meixner M., Eckhardt M. Absence of 2-hydroxylated sphingolipids is compatible with normal neural development but causes late-onset axon and myelin sheath degeneration. J. Neurosci. 2008;28:9741–9754. doi: 10.1523/JNEUROSCI.0458-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Potter K.A., Kern M.J., Hama H. Central nervous system dysfunction in a mouse model of FA2H deficiency. Glia. 2011;59:1009–1021. doi: 10.1002/glia.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saadat L., Dupree J.L., Popko B. Absence of oligodendroglial glucosylceramide synthesis does not result in CNS myelin abnormalities or alter the dysmyelinating phenotype of CGT-deficient mice. Glia. 2010;58:391–398. doi: 10.1002/glia.20930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meixner M., Jungnickel J., Grothe C., Gieselmann V., Eckhardt M. Myelination in the absence of UDP-galactose:ceramide galactosyl-transferase and fatty acid 2 -hydroxylase. BMC Neurosci. 2011;12:22. doi: 10.1186/1471-2202-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Braverman N.E., Moser A.B. Functions of plasmalogen lipids in health and disease. Biochim. Biophys. Acta. 2012;1822:1442–1452. doi: 10.1016/j.bbadis.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 65.Nagan N., Zoeller R.A. Plasmalogens: biosynthesis and functions. Prog. Lipid Res. 2001;40:199–229. doi: 10.1016/s0163-7827(01)00003-0. [DOI] [PubMed] [Google Scholar]
- 66.Rapport M.M., Lerner B. The structure of plasmalogens. IV. Lipids in normal and neoplastic tissues of man and in normal tissues of rabbit and rat. Biochim. Biophys. Acta. 1959;33:319–325. doi: 10.1016/0006-3002(59)90119-2. [DOI] [PubMed] [Google Scholar]
- 67.Heymans H.S., Schutgens R.B., Tan R., van den Bosch H., Borst P. Severe plasmalogen deficiency in tissues of infants without peroxisomes (Zellweger syndrome) Nature. 1983;306:69–70. doi: 10.1038/306069a0. [DOI] [PubMed] [Google Scholar]
- 68.Webster G.R. Studies on the plasmalogens of nervous tissue. Biochim. Biophys. Acta. 1960;44:109–116. doi: 10.1016/0006-3002(60)91529-8. [DOI] [PubMed] [Google Scholar]
- 69.Balakrishnan S., Goodwin H., Cumings J.N. The distribution of phosphorus-containing lipid compounds in the human brain. J. Neurochem. 1961;8:276–284. doi: 10.1111/j.1471-4159.1961.tb13553.x. [DOI] [PubMed] [Google Scholar]
- 70.Norton W.T., Autilio L.A. The lipid composition of purified bovine brain myelin. J. Neurochem. 1966;13:213–222. doi: 10.1111/j.1471-4159.1966.tb06794.x. [DOI] [PubMed] [Google Scholar]
- 71.Cumings J.N., Goodwin H., Woodward E.M., Curzon G. Lipids in the brains of infants and children. J. Neurochem. 1958;2:289–294. doi: 10.1111/j.1471-4159.1958.tb12377.x. [DOI] [PubMed] [Google Scholar]
- 72.Han X.L., Gross R.W. Plasmenylcholine and phosphatidylcholine membrane bilayers possess distinct conformational motifs. Biochemistry. 1990;29:4992–4996. doi: 10.1021/bi00472a032. [DOI] [PubMed] [Google Scholar]
- 73.Rubio J.M., Astudillo A.M., Casas J., Balboa M.A., Balsinde J. Regulation of phagocytosis in macrophages by membrane ethanolamine plasmalogens. Front. Immunol. 2018;9:1723. doi: 10.3389/fimmu.2018.01723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dean J.M., Lodhi I.J. Structural and functional roles of ether lipids. Protein Cell. 2018;9:196–206. doi: 10.1007/s13238-017-0423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luoma A.M., Kuo F., Kirschner D.A. Plasmalogen phospholipids protect internodal myelin from oxidative damage. Free Radic. Biol. Med. 2015;84:296–310. doi: 10.1016/j.freeradbiomed.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 76.Sindelar P.J., Guan Z., Dallner G., Ernster L. The protective role of plasmalogens in iron-induced lipid peroxidation. Free Radic. Biol. Med. 1999;26:318–324. doi: 10.1016/s0891-5849(98)00221-4. [DOI] [PubMed] [Google Scholar]
- 77.Zou Y., Henry W.S., Schreiber S.L. Plasticity of ether lipids promotes ferroptosis susceptibility and evasion. Nature. 2020;585:603–608. doi: 10.1038/s41586-020-2732-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gross R.W. High plasmalogen and arachidonic acid content of canine myocardial sarcolemma: a fast atom bombardment mass spectroscopic and gas chromatography-mass spectroscopic characterization. Biochemistry. 1984;23:158–165. doi: 10.1021/bi00296a026. [DOI] [PubMed] [Google Scholar]
- 79.da Silva T.F., Eira J., Brites P. Peripheral nervous system plasmalogens regulate Schwann cell differentiation and myelination. J. Clin. Invest. 2014;124:2560–2570. doi: 10.1172/JCI72063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Malheiro A.R., Correia B., Brites P. Leukodystrophy caused by plasmalogen deficiency rescued by glyceryl 1-myristyl ether treatment. Brain Pathol. 2019;29:622–639. doi: 10.1111/bpa.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brites P., Motley A.M., Baes M. Impaired neuronal migration and endochondral ossification in Pex7 knockout mice: a model for rhizomelic chondrodysplasia punctata. Hum. Mol. Genet. 2003;12:2255–2267. doi: 10.1093/hmg/ddg236. [DOI] [PubMed] [Google Scholar]
- 82.Rodemer C., Thai T.P., Just W.W. Inactivation of ether lipid biosynthesis causes male infertility, defects in eye development and optic nerve hypoplasia in mice. Hum. Mol. Genet. 2003;12:1881–1895. doi: 10.1093/hmg/ddg191. [DOI] [PubMed] [Google Scholar]
- 83.Teigler A., Komljenovic D., Draguhn A., Gorgas K., Just W.W. Defects in myelination, paranode organization and Purkinje cell innervation in the ether lipid-deficient mouse cerebellum. Hum. Mol. Genet. 2009;18:1897–1908. doi: 10.1093/hmg/ddp110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dorninger F., Brodde A., Berger J. Homeostasis of phospholipids - the level of phosphatidylethanolamine tightly adapts to changes in ethanolamine plasmalogens. Biochim. Biophys. Acta. 2015;1851:117–128. doi: 10.1016/j.bbalip.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hoxha E., Balbo I., Tempia F. Elovl5 is required for proper action potential conduction along peripheral myelinated fibers. Glia. 2021;69:2419–2428. doi: 10.1002/glia.24048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Montani L., Pereira J.A., Suter U. De novo fatty acid synthesis by Schwann cells is essential for peripheral nervous system myelination. J. Cell Biol. 2018;217:1353–1368. doi: 10.1083/jcb.201706010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dimas P., Montani L., Suter U. CNS myelination and remyelination depend on fatty acid synthesis by oligodendrocytes. Elife. 2019;8 doi: 10.7554/eLife.44702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Berger J., Moller D.E. The mechanisms of action of PPARs. Annu. Rev. Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 89.Krey G., Keller H., Wahli W. Xenopus peroxisome proliferator activated receptors: genomic organization, response element recognition, heterodimer formation with retinoid X receptor and activation by fatty acids. J. Steroid Biochem. Mol. Biol. 1993;47:65–73. doi: 10.1016/0960-0760(93)90058-5. [DOI] [PubMed] [Google Scholar]
- 90.Berger J., Wagner J.A. Physiological and therapeutic roles of peroxisome proliferator-activated receptors. Diabetes Technol. Therapeut. 2002;4:163–174. doi: 10.1089/15209150260007381. [DOI] [PubMed] [Google Scholar]
- 91.Mandrekar-Colucci S., Sauerbeck A., Popovich P.G., McTigue D.M. PPAR agonists as therapeutics for CNS trauma and neurological diseases. ASN Neuro. 2013;5 doi: 10.1042/AN20130030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zolezzi J.M., Santos M.J., Inestrosa N.C. PPARs in the central nervous system: roles in neurodegeneration and neuroinflammation. Biol. Rev. Camb. Phil. Soc. 2017;92:2046–2069. doi: 10.1111/brv.12320. [DOI] [PubMed] [Google Scholar]
- 93.Agarwal S., Yadav A., Chaturvedi R.K. Peroxisome proliferator-activated receptors (PPARs) as therapeutic target in neurodegenerative disorders. Biochem. Biophys. Res. Commun. 2017;483:1166–1177. doi: 10.1016/j.bbrc.2016.08.043. [DOI] [PubMed] [Google Scholar]
- 94.Braissant O., Foufelle F., Scotto C., Dauça M., Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology. 1996;137:354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- 95.Braissant O., Wahli W. Differential expression of peroxisome proliferator-activated receptor-alpha, -beta, and -gamma during rat embryonic development. Endocrinology. 1998;139:2748–2754. doi: 10.1210/endo.139.6.6049. [DOI] [PubMed] [Google Scholar]
- 96.Granneman J., Skoff R., Yang X. Member of the peroxisome proliferator-activated receptor family of transcription factors is differentially expressed by oligodendrocytes. J. Neurosci. Res. 1998;51:563–573. doi: 10.1002/(SICI)1097-4547(19980301)51:5<563::AID-JNR3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 97.Higashiyama H., Kinoshita M., Asano S. Expression profiling of liver receptor homologue 1 (LRH-1) in mouse tissues using tissue microarray. J. Mol. Histol. 2007;38:45–52. doi: 10.1007/s10735-007-9077-6. [DOI] [PubMed] [Google Scholar]
- 98.Woods J.W., Tanen M., Berger J.P. Localization of PPARdelta in murine central nervous system: expression in oligodendrocytes and neurons. Brain Res. 2003;975:10–21. doi: 10.1016/s0006-8993(03)02515-0. [DOI] [PubMed] [Google Scholar]
- 99.Saluja I., Granneman J.G., Skoff R.P. PPAR delta agonists stimulate oligodendrocyte differentiation in tissue culture. Glia. 2001;33:191–204. [PubMed] [Google Scholar]
- 100.Jana M., Mondal S., Gonzalez F.J., Pahan K. Gemfibrozil, a lipid-lowering drug, increases myelin genes in human oligodendrocytes via peroxisome proliferator-activated receptor-beta. J. Biol. Chem. 2012;287:34134–34148. doi: 10.1074/jbc.M112.398552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vittoria Simonini M., Polak P.E., Feinstein D.L. Regulation of oligodendrocyte progenitor cell maturation by PPARdelta: effects on bone morphogenetic proteins. ASN Neuro. 2010;2 doi: 10.1042/AN20090033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Almad A., McTigue D.M. Chronic expression of PPAR-delta by oligodendrocyte lineage cells in the injured rat spinal cord. J. Comp. Neurol. 2010;518:785–799. doi: 10.1002/cne.22242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Peters J.M., Lee S.S., Gonzalez F.J. Growth, adipose, brain, and skin alterations resulting from targeted disruption of the mouse peroxisome proliferator-activated receptor beta(delta) Mol. Cell Biol. 2000;20:5119–5128. doi: 10.1128/mcb.20.14.5119-5128.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhou X., He C., Hu J. Mature myelin maintenance requires Qki to coactivate PPARbeta-RXRalpha-mediated lipid metabolism. J. Clin. Invest. 2020;130:2220–2236. doi: 10.1172/JCI131800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cermenati G., Audano M., Mitro N. Lack of sterol regulatory element binding factor-1c imposes glial Fatty Acid utilization leading to peripheral neuropathy. Cell Metabol. 2015;21:571–583. doi: 10.1016/j.cmet.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 106.DeBose-Boyd R.A., Ye J. SREBPs in lipid metabolism, insulin signaling, and beyond. Trends Biochem. Sci. 2018;43:358–368. doi: 10.1016/j.tibs.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Horton J.D., Goldstein J.L., Brown M.S. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Monnerie H., Romer M., Grinspan J.B. Reduced sterol regulatory element-binding protein (SREBP) processing through site-1 protease (S1P) inhibition alters oligodendrocyte differentiation in vitro. J. Neurochem. 2017;140:53–67. doi: 10.1111/jnc.13721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shin S., Zhou H., Hu J. Qki activates Srebp2-mediated cholesterol biosynthesis for maintenance of eye lens transparency. Nat. Commun. 2021;12:3005. doi: 10.1038/s41467-021-22782-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhou X., Shin S., Hu J. Qki regulates myelinogenesis through Srebp2-dependent cholesterol biosynthesis. Elife. 2021;10:e60467. doi: 10.7554/eLife.60467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu G.Y., Sabatini D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020;21:183–203. doi: 10.1038/s41580-019-0199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Saxton R.A., Sabatini D.M. mTOR signaling in growth, metabolism, and disease. Cell. 2017;169:361–371. doi: 10.1016/j.cell.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 113.Eid W., Dauner K., Zha X. mTORC1 activates SREBP-2 by suppressing cholesterol trafficking to lysosomes in mammalian cells. Proc. Natl. Acad. Sci. USA. 2017;114:7999–8004. doi: 10.1073/pnas.1705304114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Figlia G., Gerber D., Suter U. Myelination and mTOR. Glia. 2018;66:693–707. doi: 10.1002/glia.23273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sherman D.L., Krols M., Brophy P.J. Arrest of myelination and reduced axon growth when Schwann cells lack mTOR. J. Neurosci. 2012;32:1817–1825. doi: 10.1523/JNEUROSCI.4814-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wahl S.E., McLane L.E., Bercury K.K., Macklin W.B., Wood T.L. Mammalian target of rapamycin promotes oligodendrocyte differentiation, initiation and extent of CNS myelination. J. Neurosci. 2014;34:4453–4465. doi: 10.1523/JNEUROSCI.4311-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Norrmén C., Figlia G., Suter U. mTORC1 controls PNS myelination along the mTORC1-RXRgamma-SREBP-lipid biosynthesis axis in Schwann cells. Cell Rep. 2014;9:646–660. doi: 10.1016/j.celrep.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 118.Bercury K.K., Dai J., Macklin W.B. Conditional ablation of raptor or rictor has differential impact on oligodendrocyte differentiation and CNS myelination. J. Neurosci. 2014;34:4466–4480. doi: 10.1523/JNEUROSCI.4314-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lebrun-Julien F., Bachmann L., Suter U. Balanced mTORC1 activity in oligodendrocytes is required for accurate CNS myelination. J. Neurosci. 2014;34:8432–8448. doi: 10.1523/JNEUROSCI.1105-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dai J., Bercury K.K., Macklin W.B. Interaction of mTOR and Erk1/2 signaling to regulate oligodendrocyte differentiation. Glia. 2014;62:2096–2109. doi: 10.1002/glia.22729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Khandker L., Jeffries M.A., Wood T.L. Cholesterol biosynthesis defines oligodendrocyte precursor heterogeneity between brain and spinal cord. Cell Rep. 2022;38 doi: 10.1016/j.celrep.2022.110423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zou Y., Jiang W., Xiao B. Oligodendrocyte precursor cell-intrinsic effect of Rheb1 controls differentiation and mediates mTORC1-dependent myelination in brain. J. Neurosci. 2014;34:15764–15778. doi: 10.1523/JNEUROSCI.2267-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Carson R.P., Kelm N.D., Ess K.C. Hypomyelination following deletion of Tsc2 in oligodendrocyte precursors. Ann. Clin. Transl. Neurol. 2015;2:1041–1054. doi: 10.1002/acn3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shi Q., Saifetiarova J., Taylor A.M., Bhat M.A. mTORC1 activation by loss of Tsc1 in myelinating glia causes downregulation of quaking and neurofascin 155 leading to paranodal domain disorganization. Front. Cell. Neurosci. 2018;12:201. doi: 10.3389/fncel.2018.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ercan E., Han J.M., Sahin M. Neuronal CTGF/CCN2 negatively regulates myelination in a mouse model of tuberous sclerosis complex. J. Exp. Med. 2017;214:681–697. doi: 10.1084/jem.20160446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lewis W.W., Sahin M., Warfield S.K. Impaired language pathways in tuberous sclerosis complex patients with autism spectrum disorders. Cerebr. Cortex. 2013;23:1526–1532. doi: 10.1093/cercor/bhs135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Peters J.M., Taquet M., Warfield S.K. Diffusion tensor imaging and related techniques in tuberous sclerosis complex: review and future directions. Future Neurol. 2013;8:583–597. doi: 10.2217/fnl.13.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ruppe V., Dilsiz P., Talos D.M. Developmental brain abnormalities in tuberous sclerosis complex: a comparative tissue analysis of cortical tubers and perituberal cortex. Epilepsia. 2014;55:539–550. doi: 10.1111/epi.12545. [DOI] [PubMed] [Google Scholar]
- 129.Feng Y., Bankston A. The star family member QKI and cell signaling. Adv. Exp. Med. Biol. 2010;693:25–36. [PubMed] [Google Scholar]
- 130.Larocque D., Richard S. QUAKING KH domain proteins as regulators of glial cell fate and myelination. RNA Biol. 2005;2:37–40. doi: 10.4161/rna.2.2.1603. [DOI] [PubMed] [Google Scholar]
- 131.Sidman R.L., Dickie M.M., Appel S.H. Mutant mice (quaking and jimpy) with deficient myelination in the central nervous system. Science. 1964;144:309–311. doi: 10.1126/science.144.3616.309. [DOI] [PubMed] [Google Scholar]
- 132.Ebersole T.A., Chen Q., Justice M.J., Artzt K. The quaking gene product necessary in embryogenesis and myelination combines features of RNA binding and signal transduction proteins. Nat. Genet. 1996;12:260–265. doi: 10.1038/ng0396-260. [DOI] [PubMed] [Google Scholar]
- 133.Darbelli L., Vogel G., Almazan G., Richard S. Quaking regulates neurofascin 155 expression for myelin and axoglial junction maintenance. J. Neurosci. 2016;36:4106–4120. doi: 10.1523/JNEUROSCI.3529-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Larocque D., Pilotte J., Richard S. Nuclear retention of MBP mRNAs in the quaking viable mice. Neuron. 2002;36:815–829. doi: 10.1016/s0896-6273(02)01055-3. [DOI] [PubMed] [Google Scholar]
- 135.Li Z., Zhang Y., Li D., Feng Y. Destabilization and mislocalization of myelin basic protein mRNAs in quaking dysmyelination lacking the QKI RNA-binding proteins. J. Neurosci. 2000;20:4944–4953. doi: 10.1523/JNEUROSCI.20-13-04944.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Aberg K., Saetre P., Jareborg N., Jazin E. Human QKI, a potential regulator of mRNA expression of human oligodendrocyte-related genes involved in schizophrenia. Proc. Natl. Acad. Sci. USA. 2006;103:7482–7487. doi: 10.1073/pnas.0601213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lavon I., Leykin I., Vaknin-Dembinsky A. QKI-V5 is downregulated in CNS inflammatory demyelinating diseases. Mult. Scler. Relat. Disord. 2019;39 doi: 10.1016/j.msard.2019.101881. [DOI] [PubMed] [Google Scholar]
- 138.Richard S. Reaching for the stars: linking RNA binding proteins to diseases. Adv. Exp. Med. Biol. 2010;693:142–157. [PubMed] [Google Scholar]
- 139.Tripathi R.B., Jackiewicz M., Richardson W.D. Remarkable stability of myelinating oligodendrocytes in mice. Cell Rep. 2017;21:316–323. doi: 10.1016/j.celrep.2017.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lüders K.A., Nessler S., Werner H.B. Maintenance of high proteolipid protein level in adult central nervous system myelin is required to preserve the integrity of myelin and axons. Glia. 2019;67:634–649. doi: 10.1002/glia.23549. [DOI] [PubMed] [Google Scholar]
- 141.Meschkat M., Steyer A.M., Möbius W. White matter integrity in mice requires continuous myelin synthesis at the inner tongue. Nat. Commun. 2022;13:1163. doi: 10.1038/s41467-022-28720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Toyama B.H., Savas J.N., Hetzer M.W. Identification of long-lived proteins reveals exceptional stability of essential cellular structures. Cell. 2013;154:971–982. doi: 10.1016/j.cell.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Price J.C., Guan S., Burlingame A., Prusiner S.B., Ghaemmaghami S. Analysis of proteome dynamics in the mouse brain. Proc. Natl. Acad. Sci. USA. 2010;107:14508–14513. doi: 10.1073/pnas.1006551107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fornasiero E.F., Mandad S., Rizzoli S.O. Precisely measured protein lifetimes in the mouse brain reveal differences across tissues and subcellular fractions. Nat. Commun. 2018;9:4230. doi: 10.1038/s41467-018-06519-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lin Y., Pang X., Lin W. Impaired eukaryotic translation initiation factor 2B activity specifically in oligodendrocytes reproduces the pathology of vanishing white matter disease in mice. J. Neurosci. 2014;34:12182–12191. doi: 10.1523/JNEUROSCI.1373-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ando S., Tanaka Y., Toyoda Y., Kon K. Turnover of myelin lipids in aging brain. Neurochem. Res. 2003;28:5–13. doi: 10.1023/a:1021635826032. [DOI] [PubMed] [Google Scholar]
- 147.Clarke B.A., Majumder S., Proia R.L. The Ormdl genes regulate the sphingolipid synthesis pathway to ensure proper myelination and neurologic function in mice. Elife. 2019;8 doi: 10.7554/eLife.51067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Viader A., Sasaki Y., Milbrandt J. Aberrant Schwann cell lipid metabolism linked to mitochondrial deficits leads to axon degeneration and neuropathy. Neuron. 2013;77:886–898. doi: 10.1016/j.neuron.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fünfschilling U., Supplie L.M., Nave K.A. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–521. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Beirowski B., Babetto E., Milbrandt J. Metabolic regulator LKB1 is crucial for Schwann cell-mediated axon maintenance. Nat. Neurosci. 2014;17:1351–1361. doi: 10.1038/nn.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Moser H.W., Loes D.J., Lu S.E. X-Linked adrenoleukodystrophy: overview and prognosis as a function of age and brain magnetic resonance imaging abnormality. A study involving 372 patients. Neuropediatrics. 2000;31:227–239. doi: 10.1055/s-2000-9236. [DOI] [PubMed] [Google Scholar]
- 152.Moser H.W., Raymond G.V., Dubey P. Adrenoleukodystrophy: new approaches to a neurodegenerative disease. JAMA. 2005;294:3131–3134. doi: 10.1001/jama.294.24.3131. [DOI] [PubMed] [Google Scholar]
- 153.Ferdinandusse S., Denis S., Waterham H.R. Clinical, biochemical, and mutational spectrum of peroxisomal acyl-coenzyme A oxidase deficiency. Hum. Mutat. 2007;28:904–912. doi: 10.1002/humu.20535. [DOI] [PubMed] [Google Scholar]
- 154.Ferdinandusse S., Denis S., Poll-The B.T. Clinical and biochemical spectrum of D-bifunctional protein deficiency. Ann. Neurol. 2006;59:92–104. doi: 10.1002/ana.20702. [DOI] [PubMed] [Google Scholar]
- 155.Horvath R., Lewis-Smith D., Chinnery P.F. SCP2 mutations and neurodegeneration with brain iron accumulation. Neurology. 2015;85:1909–1911. doi: 10.1212/WNL.0000000000002157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chung H.L., Wangler M.F., Bellen H.J. Loss- or gain-of-function mutations in ACOX1 cause axonal loss via different mechanisms. Neuron. 2020;106:589–606.e6. doi: 10.1016/j.neuron.2020.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Huyghe S., Schmalbruch H., Hartmann D. Peroxisomal multifunctional protein-2 deficiency causes motor deficits and glial lesions in the adult central nervous system. Am. J. Pathol. 2006;168:1321–1334. doi: 10.2353/ajpath.2006.041220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kassmann C.M., Lappe-Siefke C., Nave K.A. Axonal loss and neuroinflammation caused by peroxisome-deficient oligodendrocytes. Nat. Genet. 2007;39:969–976. doi: 10.1038/ng2070. [DOI] [PubMed] [Google Scholar]
- 159.Bams-Mengerink A.M., Majoie C.B.L.M., Poll-The B.T. MRI of the brain and cervical spinal cord in rhizomelic chondrodysplasia punctata. Neurology. 2006;66:798–803. doi: 10.1212/01.wnl.0000205594.34647.d0. discussion 789. [DOI] [PubMed] [Google Scholar]
- 160.Sztriha L., Al-Gazali L.I., Lestringant G.G. Abnormal myelin formation in rhizomelic chondrodysplasia punctata type 2 (DHAPAT-deficiency) Dev. Med. Child Neurol. 2000;42:492–495. doi: 10.1017/s0012162200000918. [DOI] [PubMed] [Google Scholar]
- 161.Wanders R.J.A., Waterham H.R. Peroxisomal disorders I: biochemistry and genetics of peroxisome biogenesis disorders. Clin. Genet. 2005;67:107–133. doi: 10.1111/j.1399-0004.2004.00329.x. [DOI] [PubMed] [Google Scholar]
- 162.Waterham H.R., Ebberink M.S. Genetics and molecular basis of human peroxisome biogenesis disorders. Biochim. Biophys. Acta. 2012;1822:1430–1441. doi: 10.1016/j.bbadis.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 163.Barøy T., Koster J., Frengen E. A novel type of rhizomelic chondrodysplasia punctata, RCDP5, is caused by loss of the PEX5 long isoform. Hum. Mol. Genet. 2015;24:5845–5854. doi: 10.1093/hmg/ddv305. [DOI] [PubMed] [Google Scholar]
- 164.Buchert R., Tawamie H., Abou Jamra R. A peroxisomal disorder of severe intellectual disability, epilepsy, and cataracts due to fatty acyl-CoA reductase 1 deficiency. Am. J. Hum. Genet. 2014;95:602–610. doi: 10.1016/j.ajhg.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ferdinandusse S., McWalter K., Vaz F.M. An autosomal dominant neurological disorder caused by de novo variants in FAR1 resulting in uncontrolled synthesis of ether lipids. Genet. Med. 2021;23:740–750. doi: 10.1038/s41436-020-01027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ahmed M.Y., Al-Khayat A., Crosby A.H. A mutation of EPT1 (SELENOI) underlies a new disorder of Kennedy pathway phospholipid biosynthesis. Brain. 2017;140:547–554. doi: 10.1093/brain/aww318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Horibata Y., Elpeleg O., Sugimoto H. EPT1 (selenoprotein I) is critical for the neural development and maintenance of plasmalogen in humans. J. Lipid Res. 2018;59:1015–1026. doi: 10.1194/jlr.P081620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Vaz F.M., McDermott J.H., Banka S. Mutations in PCYT2 disrupt etherlipid biosynthesis and cause a complex hereditary spastic paraplegia. Brain. 2019;142:3382–3397. doi: 10.1093/brain/awz291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kryukov G.V., Castellano S., Gladyshev V.N. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- 170.Nakashima A., Hosaka K., Nikawa J. Cloning of a human cDNA for CTP-phosphoethanolamine cytidylyltransferase by complementation in vivo of a yeast mutant. J. Biol. Chem. 1997;272:9567–9572. doi: 10.1074/jbc.272.14.9567. [DOI] [PubMed] [Google Scholar]
- 171.Edvardson S., Hama H., Elpeleg O. Mutations in the fatty acid 2-hydroxylase gene are associated with leukodystrophy with spastic paraparesis and dystonia. Am. J. Hum. Genet. 2008;83:643–648. doi: 10.1016/j.ajhg.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kruer M.C., Paisán-Ruiz C., Hayflick S.J. Defective FA2H leads to a novel form of neurodegeneration with brain iron accumulation (NBIA) Ann. Neurol. 2010;68:611–618. doi: 10.1002/ana.22122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kitamura T., Seki N., Kihara A. Phytosphingosine degradation pathway includes fatty acid alpha-oxidation reactions in the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA. 2017;114:E2616–E2623. doi: 10.1073/pnas.1700138114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Staps P., Rizzo W.B., Vaz F.M., et al. Disturbed brain ether lipid metabolism and histology in Sjogren-Larsson syndrome. J. Inherit. Metab. Dis. 2020;43:1265–1278. doi: 10.1002/jimd.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Karsai G., Kraft F., Kurth I. DEGS1-associated aberrant sphingolipid metabolism impairs nervous system function in humans. J. Clin. Invest. 2019;129:1229–1239. doi: 10.1172/JCI124159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pant D.C., Dorboz I., Pujol A. Loss of the sphingolipid desaturase DEGS1 causes hypomyelinating leukodystrophy. J. Clin. Invest. 2019;129:1240–1256. doi: 10.1172/JCI123959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mahmood A., Berry J., Eichler F.S. Metachromatic leukodystrophy: a case of triplets with the late infantile variant and a systematic review of the literature. J. Child Neurol. 2010;25:572–580. doi: 10.1177/0883073809341669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Komatsuzaki S., Zielonka M., Ries M. Clinical characteristics of 248 patients with Krabbe disease: quantitative natural history modeling based on published cases. Genet. Med. 2019;21:2208–2215. doi: 10.1038/s41436-019-0480-7. [DOI] [PubMed] [Google Scholar]
- 179.Wenger D.A., Rafi M.A., Luzi P., Datto J., Costantino-Ceccarini E. Krabbe disease: genetic aspects and progress toward therapy. Mol. Genet. Metabol. 2000;70:1–9. doi: 10.1006/mgme.2000.2990. [DOI] [PubMed] [Google Scholar]
- 180.Pareyson D., Saveri P., Pisciotta C. New developments in Charcot-Marie-Tooth neuropathy and related diseases. Curr. Opin. Neurol. 2017;30:471–480. doi: 10.1097/WCO.0000000000000474. [DOI] [PubMed] [Google Scholar]
- 181.Rossor A.M., Polke J.M., Houlden H., Reilly M.M. Clinical implications of genetic advances in Charcot-Marie-Tooth disease. Nat. Rev. Neurol. 2013;9:562–571. doi: 10.1038/nrneurol.2013.179. [DOI] [PubMed] [Google Scholar]
- 182.Barreto L.C.L.S., Oliveira F.S., de Souza Araújo A.A. Epidemiologic study of charcot-marie-tooth disease: a systematic review. Neuroepidemiology. 2016;46:157–165. doi: 10.1159/000443706. [DOI] [PubMed] [Google Scholar]
- 183.Berger P., Niemann A., Suter U. Schwann cells and the pathogenesis of inherited motor and sensory neuropathies (Charcot-Marie-Tooth disease) Glia. 2006;54:243–257. doi: 10.1002/glia.20386. [DOI] [PubMed] [Google Scholar]
- 184.Murakami T., Sunada Y. Schwann cell and the pathogenesis of charcot-marie-tooth disease. Adv. Exp. Med. Biol. 2019;1190:301–321. doi: 10.1007/978-981-32-9636-7_19. [DOI] [PubMed] [Google Scholar]
- 185.Fledrich R., Schlotter-Weigel B., Sereda M.W. A rat model of Charcot-Marie-Tooth disease 1A recapitulates disease variability and supplies biomarkers of axonal loss in patients. Brain. 2012;135:72–87. doi: 10.1093/brain/awr322. [DOI] [PubMed] [Google Scholar]
- 186.Fledrich R., Abdelaal T., Sereda M.W. Targeting myelin lipid metabolism as a potential therapeutic strategy in a model of CMT1A neuropathy. Nat. Commun. 2018;9:3025. doi: 10.1038/s41467-018-05420-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Visigalli D., Capodivento G., Nobbio L. Exploiting sphingo- and glycerophospholipid impairment to select effective drugs and biomarkers for CMT1A. Front. Neurol. 2020;11:903. doi: 10.3389/fneur.2020.00903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhou Y., Bazick H., Notterpek L. A neutral lipid-enriched diet improves myelination and alleviates peripheral nerve pathology in neuropathic mice. Exp. Neurol. 2019;321 doi: 10.1016/j.expneurol.2019.113031. [DOI] [PubMed] [Google Scholar]
- 189.Lee S., Amici S., Notterpek L. PMP22 is critical for actin-mediated cellular functions and for establishing lipid rafts. J. Neurosci. 2014;34:16140–16152. doi: 10.1523/JNEUROSCI.1908-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhou Y., Borchelt D., Notterpek L. Subcellular diversion of cholesterol by gain- and loss-of-function mutations in PMP22. Glia. 2020;68:2300–2315. doi: 10.1002/glia.23840. [DOI] [PubMed] [Google Scholar]
- 191.Simons M., Kramer E.M., Schulz J.B. Overexpression of the myelin proteolipid protein leads to accumulation of cholesterol and proteolipid protein in endosomes/lysosomes: implications for Pelizaeus-Merzbacher disease. J. Cell Biol. 2002;157:327–336. doi: 10.1083/jcb.200110138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Simons M., Krämer E.M., Thiele C., Stoffel W., Trotter J. Assembly of myelin by association of proteolipid protein with cholesterol- and galactosylceramide-rich membrane domains. J. Cell Biol. 2000;151:143–154. doi: 10.1083/jcb.151.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Saher G., Rudolphi F., Nave K.A. Therapy of Pelizaeus-Merzbacher disease in mice by feeding a cholesterol-enriched diet. Nat. Med. 2012;18:1130–1135. doi: 10.1038/nm.2833. [DOI] [PubMed] [Google Scholar]
- 194.de Faria O., Jr., Pivonkova H., Káradóttir R.T. Periods of synchronized myelin changes shape brain function and plasticity. Nat. Neurosci. 2021;24:1508–1521. doi: 10.1038/s41593-021-00917-2. [DOI] [PubMed] [Google Scholar]
- 195.Wang F., Ren S.Y., Mei F. Myelin degeneration and diminished myelin renewal contribute to age-related deficits in memory. Nat. Neurosci. 2020;23:481–486. doi: 10.1038/s41593-020-0588-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Svennerholm L., Boström K., Helander C.G., Jungbjer B. Membrane lipids in the aging human brain. J. Neurochem. 1991;56:2051–2059. doi: 10.1111/j.1471-4159.1991.tb03466.x. [DOI] [PubMed] [Google Scholar]
- 197.Han X., M Holtzman D., McKeel D.W., Kelley J., Morris J.C. Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer's disease: potential role in disease pathogenesis. J. Neurochem. 2002;82:809–818. doi: 10.1046/j.1471-4159.2002.00997.x. [DOI] [PubMed] [Google Scholar]
- 198.Cheng H., Zhou Y., Holtzman D.M., Han X. Apolipoprotein E mediates sulfatide depletion in animal models of Alzheimer's disease. Neurobiol. Aging. 2010;31:1188–1196. doi: 10.1016/j.neurobiolaging.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kou J., Kovacs G.G., Berger J. Peroxisomal alterations in Alzheimer's disease. Acta Neuropathol. 2011;122:271–283. doi: 10.1007/s00401-011-0836-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Han X., Holtzman D.M., McKeel D.W. Plasmalogen deficiency in early Alzheimer's disease subjects and in animal models: molecular characterization using electrospray ionization mass spectrometry. J. Neurochem. 2001;77:1168–1180. doi: 10.1046/j.1471-4159.2001.00332.x. [DOI] [PubMed] [Google Scholar]
- 201.Kaya I., Jennische E., Fletcher J.S. Brain region-specific amyloid plaque-associated myelin lipid loss, APOE deposition and disruption of the myelin sheath in familial Alzheimer's disease mice. J. Neurochem. 2020;154:84–98. doi: 10.1111/jnc.14999. [DOI] [PubMed] [Google Scholar]
- 202.Bartzokis G., Cummings J.L., Mintz J. White matter structural integrity in healthy aging adults and patients with Alzheimer disease: a magnetic resonance imaging study. Arch. Neurol. 2003;60:393–398. doi: 10.1001/archneur.60.3.393. [DOI] [PubMed] [Google Scholar]
- 203.Ringman J.M., O'Neill J., Bartzokis G. Diffusion tensor imaging in preclinical and presymptomatic carriers of familial Alzheimer's disease mutations. Brain. 2007;130:1767–1776. doi: 10.1093/brain/awm102. [DOI] [PubMed] [Google Scholar]
- 204.Luo X., Li K., Zhang M. Application of T1-/T2-weighted ratio mapping to elucidate intracortical demyelination process in the alzheimer's disease continuum. Front. Neurosci. 2019;13:904. doi: 10.3389/fnins.2019.00904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mitew S., Kirkcaldie M.T.K., Dickson T.C. Focal demyelination in Alzheimer's disease and transgenic mouse models. Acta Neuropathol. 2010;119:567–577. doi: 10.1007/s00401-010-0657-2. [DOI] [PubMed] [Google Scholar]
- 206.Mathys H., Davila-Velderrain J., Tsai L.H. Single-cell transcriptomic analysis of Alzheimer's disease. Nature. 2019;570:332–337. doi: 10.1038/s41586-019-1195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhou Y., Song W.M., Colonna M. Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer's disease. Nat. Med. 2020;26:131–142. doi: 10.1038/s41591-019-0695-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chen J.F., Liu K., Mei F. Enhancing myelin renewal reverses cognitive dysfunction in a murine model of Alzheimer's disease. Neuron. 2021;109:2292–2307.e5. doi: 10.1016/j.neuron.2021.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Berghoff S.A., Gerndt N., Saher G. Dietary cholesterol promotes repair of demyelinated lesions in the adult brain. Nat. Commun. 2017;8 doi: 10.1038/ncomms14241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Stumpf S.K., Berghoff S.A., Saher G. Ketogenic diet ameliorates axonal defects and promotes myelination in Pelizaeus-Merzbacher disease. Acta Neuropathol. 2019;138:147–161. doi: 10.1007/s00401-019-01985-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Raas Q., van de Beek M.C., Kemp S. Metabolic rerouting via SCD1 induction impacts X-linked adrenoleukodystrophy. J. Clin. Invest. 2021;131 doi: 10.1172/JCI142500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hill R.A., Li A.M., Grutzendler J. Lifelong cortical myelin plasticity and age-related degeneration in the live mammalian brain. Nat. Neurosci. 2018;21:683–695. doi: 10.1038/s41593-018-0120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Safaiyan S., Kannaiyan N., Simons M. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat. Neurosci. 2016;19:995–998. doi: 10.1038/nn.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lamming D.W., Ye L., Sabatini D.M., Baur J.A. Rapalogs and mTOR inhibitors as anti-aging therapeutics. J. Clin. Invest. 2013;123:980–989. doi: 10.1172/JCI64099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Johnson S.C., Rabinovitch P.S., Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493:338–345. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Watkins P.A., McGuinness M.C., Moser H.W. Distinction between peroxisomal bifunctional enzyme and acyl-CoA oxidase deficiencies. Ann. Neurol. 1995;38:472–477. doi: 10.1002/ana.410380322. [DOI] [PubMed] [Google Scholar]
- 217.Ferdinandusse S., Ylianttila M.S., Glumoff T. Mutational spectrum of D-bifunctional protein deficiency and structure-based genotype-phenotype analysis. Am. J. Hum. Genet. 2006;78:112–124. doi: 10.1086/498880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Schaumburg H.H., Powers J.M., Raine C.S., Suzuki K., Richardson E.P., Jr. Adrenoleukodystrophy. A clinical and pathological study of 17 cases. Arch. Neurol. 1975;32:577–591. doi: 10.1001/archneur.1975.00490510033001. [DOI] [PubMed] [Google Scholar]
- 219.Dick K.J., Al-Mjeni R., Crosby A.H. A novel locus for an autosomal recessive hereditary spastic paraplegia (SPG35) maps to 16q21-q23. Neurology. 2008;71:248–252. doi: 10.1212/01.wnl.0000319610.29522.8a. [DOI] [PubMed] [Google Scholar]
- 220.Lossos A., Khoury M., Rosenmann H. Phenotypic variability among adult siblings with Sjogren-Larsson syndrome. Arch. Neurol. 2006;63:278–280. doi: 10.1001/archneur.63.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sjogren T., Larsson T. Oligophrenia in combination with congenital ichthyosis and spastic disorders; a clinical and genetic study. Acta Psychiatr. Neurol. Scand. Suppl. 1957;113:1–112. [PubMed] [Google Scholar]
- 222.Willemsen M.A., Cruysberg J.R., Deutman A.F. Juvenile macular dystrophy associated with deficient activity of fatty aldehyde dehydrogenase in Sjogren-Larsson syndrome. Am. J. Ophthalmol. 2000;130:782–789. doi: 10.1016/s0002-9394(00)00576-6. [DOI] [PubMed] [Google Scholar]
- 223.MacFaul R., Cavanagh N., Lake B.D., Stephens R., Whitfield A.E. Metachromatic leucodystrophy: review of 38 cases. Arch. Dis. Child. 1982;57:168–175. doi: 10.1136/adc.57.3.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zafeiriou D.I., Kontopoulos E.E., Michelakakis H.M., Anastasiou A.L., Gombakis N.P. Neurophysiology and MRI in late-infantile metachromatic leukodystrophy. Pediatr. Neurol. 1999;21:843–846. doi: 10.1016/s0887-8994(99)00093-4. [DOI] [PubMed] [Google Scholar]
- 225.Dolgin V., Straussberg R., Birk O.S. DEGS1 variant causes neurological disorder. Eur. J. Hum. Genet. 2019;27:1668–1676. doi: 10.1038/s41431-019-0444-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Anstey A.V., Ryan A., Pearse A.D. Characterization of photosensitivity in the Smith-Lemli-Opitz syndrome: a new congenital photosensitivity syndrome. Br. J. Dermatol. 1999;141:406–414. doi: 10.1046/j.1365-2133.1999.03032.x. [DOI] [PubMed] [Google Scholar]
- 227.Irons M., Elias E.R., Salen G. Treatment of Smith-Lemli-Opitz syndrome: results of a multicenter trial. Am. J. Med. Genet. 1997;68:311–314. [PubMed] [Google Scholar]
- 228.Lee R.W.Y., Conley S.K., Gropman A., Porter F.D., Baker E.H. Brain magnetic resonance imaging findings in Smith-Lemli-Opitz syndrome. Am. J. Med. Genet. 2013;161A:2407–2419. doi: 10.1002/ajmg.a.36096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lee R.W.Y., Yoshida S., Porter F.D. Corpus callosum measurements correlate with developmental delay in Smith-Lemli-Opitz syndrome. Pediatr. Neurol. 2013;49:107–112. doi: 10.1016/j.pediatrneurol.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Edvardson S., Yi J.K., Elpeleg O. Deficiency of the alkaline ceramidase ACER3 manifests in early childhood by progressive leukodystrophy. J. Med. Genet. 2016;53:389–396. doi: 10.1136/jmedgenet-2015-103457. [DOI] [PMC free article] [PubMed] [Google Scholar]