Abstract
Scrub typhus, caused by Orientia tsutsugamushi, is characterized by local as well as systemic inflammatory manifestations. The main pathologic change is focal or disseminated multiorgan vasculitis, which is caused by the destruction of endothelial cells and perivascular infiltration of leukocytes. We investigated the regulation of chemokine induction in transformed human dermal microvascular endothelial cells (HMEC-1) in response to O. tsutsugamushi infection. The monocyte chemoattractant protein-1 (MCP-1) and interleukin 8 (IL-8) mRNAs were induced, and their levels showed a transitory peak at 3 and 6 h, respectively. The RANTES transcript was detected at 6 h after infection, with increased levels evident by 48 h. The induction of the MCP-1 and IL-8 genes was not blocked by cycloheximide, suggesting that de novo protein synthesis of host cell proteins is not required for their transcriptional activation. Heat- or UV-inactivated O. tsutsugamushi induced a similar extent of MCP-1 and IL-8 responses. The induction of MCP-1 and IL-8 transcripts in the endothelial cells by O. tsutsugamushi was not blocked by the inhibitors of NF-κB. Furthermore, the activation of NF-κB was not detected in HMEC-1 stimulated with O. tsutsugamushi. These results demonstrate that heat-stable molecules of O. tsutsugamushi induce the MCP-1 and IL-8 genes and the induction of the chemokine genes may be mediated by an NF-κB independent mechanism. We also showed that another major transcription factor, activator protein-1 (AP-1), was up-regulated in HMEC-1 after O. tsutsugamushi infection. This suggests the possible involvement of AP-1 in the chemokine gene expression.
O. tsutsugamushi, an obligate intracellular bacterium, is the causative agent of scrub typhus (26). The disease is characterized by fever, rash, eschar, pneumonitis, meningitis, and disseminated intravascular coagulation, which leads to severe multiorgan failure (2, 12, 54) in untreated cases. Although the extent of the pathological changes of vasculature is less severe than those of other rickettsia diseases (2), it has been reported that vascular endothelial cells are one of the major target cells of O. tsutsugamushi infection (28, 39, 55). Although the precise mechanism of vascular damage caused by O. tsutsugamushi infections remains unclear, the primary cause of the pathophysiological consequences might be the destruction of endothelial cells lining small blood vessels and the accompanying inflammatory responses (2, 31). In the histological study of eschar and rashes, dense collections of mononuclear cells, including lymphocytes, plasma cells, and macrophages, were found around the dermal vasculatures (2). The extent of infiltrating leukocytes around the small blood vessels is closely related with the clinical manifestation of scrub typhus (2, 12, 54).
Endothelial cells are critical elements in the evolution of inflammation (33, 38, 47). Through the expression of surface proteins and the secretion of soluble mediators, the endothelium controls vascular tone and permeability, regulates coagulation and thrombosis, and directs the passage of leukocytes into areas of inflammation (33, 47). In the process of inflammation, endothelial cells are known to produce proinflammatory cytokines, such as interleukin 1 (IL-1), IL-6, and IL-8 as well as adhesion molecules (33, 47). In particular, the chemotactic cytokines (chemokines) and adhesion molecules expressed by endothelial cells are known to be key players in regulating the recruitment of leukocytes to the sites of inflammation (22). The chemokine genes are induced in vascular endothelial cells either by proinflammatory cytokines such as IL-1 and tumor necrosis factor or by interaction with microbial pathogens (19, 27, 33, 34, 47). The interactions of different chemokines with specific leukocyte receptors enable activation and chemotaxis of neutrophils, lymphocytes, or monocytes necessary for migration to sites of evolving inflammation. The cellular influx into inflamed tissue is provoked by chemokine gradients that contribute to the adhesion of leukocytes to the endothelium, transendothelial migration, and movement through the extracellular matrix (22). Infections caused by different microbial pathogens elicit distinct patterns of chemokine responses (42). In endothelial cells activated by different microbial pathogens, distinct chemokine genes are expressed with different kinetics and magnitude (4, 19, 27, 34). The mechanisms responsible for differences in host responses are incompletely understood, but these differences partly explain the presence of different inflammatory cell types and the magnitude in immune responses that are associated with characteristic pathologic findings and clinical manifestations of disease. Previously, we have reported that a subset of chemokine genes are expressed in macrophages, which are known to be a primary source of chemokines and play a critical role in the immunity against rickettsia infection (13, 26). Macrophages and endothelial cells are the primary targets for the rickettsia infection and seem to be important cell types in rickettsia disease, not only pathologically but also immunologically (26). Therefore, these cells might play significant roles in determining the magnitude and profile of the host inflammatory response to local or systemic infection with O. tsutsugamushi. Furthermore, the type of T-cell response is also affected by the kind of chemokine present since different sets of chemokine receptors are exposed on different T-cell subsets (41). Protective immunity against O. tsutsugamushi is largely due to cell-mediated immune responses, particularly those provided by macrophages and T cells (26, 44). Among the chemokines expressed by endothelial cells, monocyte chemoattractant protein-1 (MCP-1) and RANTES belong to the CC chemokine subfamily and preferentially attract monocytes and lymphocytes (5). It has been reported that selective diapedesis of Th1 cells is induced by RANTES produced by endothelial cells (25). For these reasons, it is important to understand the regulatory components that determine the quality and magnitude of inflammatory responses in the rickettsia infection. To date, however, proinflammatory mediators and chemokine responses to the O. tsutsugamushi infection have been poorly elucidated (13, 23).
The transcription factor NF-κB is known to play important roles in the regulation of inflammatory mediators, such as cytokines, acute proteins, and adhesion molecules (6, 21). The promoters of many chemokine genes, including IL-8, RANTES, and MCP-1, also contain binding sites for NF-κB (9, 35, 49). In addition to NF-κB, other transcription factors such as activator protein-1 (AP-1) are also implicated in the stimulus-specific regulation of chemokine expression (40). Recently, it has also been shown that AP-1 could induce a chemokine gene through a mechanism independent of NF-κB (53). In this study, we analyzed the transcriptional activation of a subset of chemokine genes of human microvascular endothelial cell line (HMEC-1) in response to O. tsutsugamushi infection. We also investigated whether the expression of the chemokine genes is dependent on NF-κB activation and whether AP-1 is activated in O. tsutsugamushi-infected endothelial cells.
MATERIALS AND METHODS
Cell culture.
HMEC-1, derived from human dermal microvascular endothelial cells (1), was kindly provided by the Centers for Disease Control and Prevention (Atlanta, Ga.). The cells were propagated in MCDB 131 medium (Life Technologies, Grand Island, N.Y.) supplemented with 15% fetal bovine serum (Life Technologies), hydrocortisone (1 μg/ml; Sigma Chemical Co., St. Louis, Mo.), epidermal growth factor (10 ng/ml; Life Technologies), penicillin (100 U/ml; Life Technologies), and streptomycin (100 μg/ml; Life Technologies). Endothelial cells were seeded onto 100-mm-diameter dishes (Becton Dickinson Labware, Franklin Lakes, N.J.) for the preparation of total RNA or for the preparation of nuclear extract. O. tsutsugamushi Karp (American Type Culture Collection, Manassas, Va.) was propagated in monolayers of L929 cells (13). The titer of infectivity of the inoculum was determined as described previously (13, 48). An O. tsutsugamushi infected-cell counting unit of 1.4 × 107 (48) was used to infect endothelial cells for the preparation of total RNA and nuclear extracts. The L929 cell lysate was prepared as described previously (13) and was used in the infection of endothelial cells for the control experiments. Lipopolysaccharide (LPS) (Sigma Chemical Co.) derived from Escherichia coli was used as a positive control for each experiment. In the inhibition assays, endothelial cells were preincubated with 50 μM pyrrolidinedithiocarbamate (PDTC) (Sigma Chemical Co.), 100 μM N-tosyl-l-phenylalanine chloromethyl ketone (TPCK) (Sigma Chemical Co.), or 10 μg of cycloheximide (CHX) (Sigma Chemical Co.) per ml for 1 h before O. tsutsugamushi was inoculated. Inhibitors were maintained during the course of inhibition assays. In one experimental set, 30 μg of polymyxin B sulfate (Sigma Chemical Co.) per ml was added to the cell culture to neutralize the LPS for the exclusion of the possibility of LPS contamination in the medium or the inoculum. Inactivation of O. tsutsugamushi was accomplished by heat treatment (100°C for 10 min) or by exposure to a 30-W UV lamp for 30 min at a distance of 20 cm with gentle shaking.
RNase protection assay.
Total RNA was prepared with an RNeasy kit (Qiagen GmbH, Hilden, Germany) as specified by the manufacturer and was quantified spectrophotometrically. Detection and semiquantification of various murine chemokine mRNAs was performed with a multiprobe RNase protection assay system from Pharmingen (San Diego, Calif.). In brief, a mixture of [32P]CTP-labeled antisense riboprobes were generated from chemokine template DNAs including lymphotactin (Ltn), RANTES, I-309, macrophage inhibitory protein-1α (MIP-1α), MIP-1β, IL-8, gamma interferon-inducible protein 10 (IP-10), and MCP-1. The template DNAs for the human housekeeping genes encoding glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and a human ribosomal protein (L32) were also included to ensure equal loading of total RNA onto the gels. Total RNAs from each sample (10 μg each) were hybridized overnight at 56°C with 2 × 105 cpm of the 32P-labeled antisense riboprobe mixture. After hybridization, the samples were digested with a mixture of RNases A and T1. Nuclease-protected RNA fragments were precipitated with ethanol. The samples were resolved on a 5% polyacrylamide sequencing gel (13). The bands were observed after autoradiography. The specific chemokine bands were identified on the basis of their individual mobilities compared with labeled standard probes. The band intensities shown in autoradiography were digitized by scanning the images and analyzed with TINA software (Raytest Isotopenmeßgeräte GmbH, Straubenhardt, Germany). The densitometric intensity was normalized with respect to the average intensities of the bands for the housekeeping genes, GAPDH and L32.
Nuclear extraction and EMSA.
Electrophoretic mobility shift assay (EMSA) was performed as described previously with some modifications (13). Following infection with O. tsutsugamushi, endothelial cells were washed with cold phosphate-buffered saline and collected by centrifugation at 500 × g for 5 min. The cells were resuspended in 200 μl of buffer I (50 mM Tris-HCl [pH 7.9], 10 mM KCl, 1 mM EDTA, 0.2% Nonidet P-40, 10% glycerol, antiprotease cocktail [Roche Diagnostic GmbH, Mannheim, Germany]) and incubated for 4 min at 4°C. The nuclei were pelleted by centrifugation (3,000 × g for 3 min at 4°C) and resuspended for 20 min on ice in 50 μl of cold buffer II (20 mM HEPES, 20% glycerol, 400 mM NaCl, 1 mM EDTA, 10 mM KCl, antiprotease cocktail). Nuclear debris were removed by centrifugation (13,000 × g for 10 min) at 4°C, and supernatants were used as nuclear extracts. Protein concentrations were determined with the bicinchoninic acid protein assay reagent (Pierce Chemical Co. Rockford, Ill.). Aliquots of the supernatant were frozen in liquid nitrogen and stored at −70°C until use. Equal amounts of nuclear extracts (10 μg of protein) from each sample were incubated for 30 min at 25°C in 20 μl of binding buffer [10 mM Tris-HCl (pH 7.5), 50 mM NaCl, 0.5 mM dithiothreitol, 0.5 mM EDTA, 4% glycerol, 1 mM MgCl2, 1 μg of poly(dI-dC)] containing 30,000 cpm of an NF-κB-specific or AP-1-specific oligonucleotide probe. Probes were radiolabeled with [γ-32P]ATP (Amersham Ltd., Little Chalfont, England). The sequence of the NF-κB-specific probe was 5′-AGT TGA GGG GAC TTT CCC AGG C-3′, and the sequence of the AP-1-specific probe was 5′-TGC TTG ATG AGT CAG CCG GAA-3′. To ascertain the specific binding of nuclear extracts with the NF-κB or AP-1 probe, a competition assay was performed with a 50-fold molar excess of unlabeled oligonucleotides. Nuclear translocation of the NF-κB heterodimer was analyzed by a supershift assay with the anti-p65 antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.). The nuclear extracts were mixed with 2 μg of the anti-p65 antibody and were hybridized with the NF-κB-specific oligonucleotide probe. The supershifted bands were analyzed after separation on 5% nondenaturing polyacrylamide gels and autoradiography.
RESULTS
Induction of chemokine gene expression.
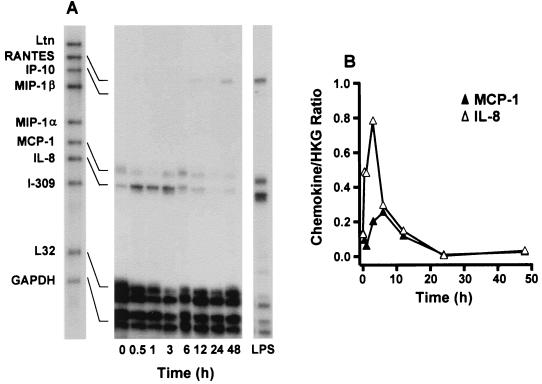
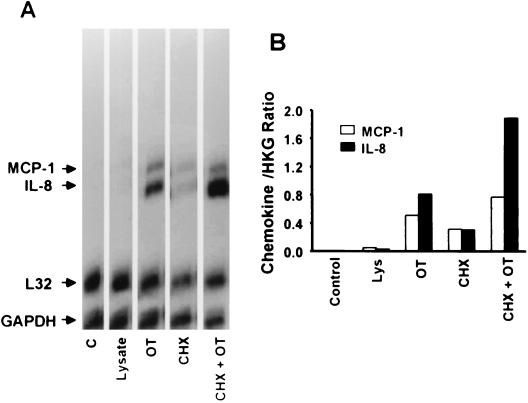
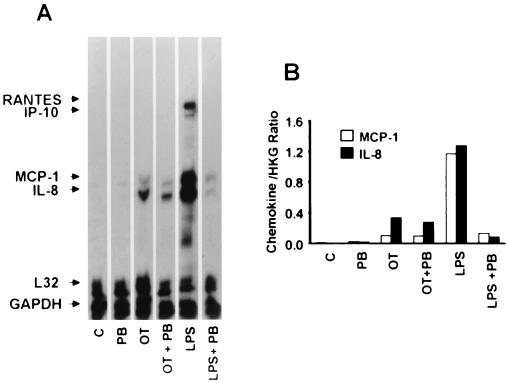
Before and after exposure of endothelial cells to O. tsutsugamushi, the chemokine transcript levels were assayed at various time points by an RNase protection assay (Fig. 1). The MCP-1 and IL-8 mRNAs were constitutively expressed at low levels in HMEC-1 (Fig. 1). The MCP-1 RNA messages were up-regulated after infection and peaked at 6 h. The IL-8 messages were increased as early as 30 min after infection and persisted for 6 h. Both of the two chemokine mRNA levels were reduced to uninfected cell levels by 12 h. The RANTES transcript was detected at 6 h after infection, with increased levels present by 48 h. No mRNA was detected during the infection using the RNase protection assay for Ltn, IP-10, MIP-1β, MIP-1α, and I-309. When the cells were treated with uninfected L929 cell lysate, the chemokine gene mRNAs were barely detectable (Fig 2.). The chemokine genes have also been reported to be induced by proinflammatory cytokines, such as IL-1 (51). To investigate whether the induction of the chemokine genes was a consequence of the host cytokine expression, cells were incubated for an hour with CHX, a eukaryotic protein synthesis inhibitor. Cells treated only with CHX expressed low levels of the chemokine gene mRNAs (18). CHX increased rather than inhibited chemokine expression in O. tsutsugamushi-infected endothelial cells (Fig. 2). Four chemokine genes, those encoding RANTES, IP-10, MCP-1, and IL-8 were expressed when HMEC-1 was stimulated with LPS for 3 h. MCP-1 and IL-8 mRNA levels did not differ significantly, whether cells were treated with polymyxin B and O. tsutsugamushi or with O. tsutsugamushi alone, whereas LPS-mediated chemokine induction was significantly reduced (Fig. 3).
FIG. 1.
Time course of O. tsutsugamushi-stimulated chemokine induction in HMEC-1. (A) Before and after HMEC-1 incubation with O. tsutsugamushi, the levels of chemokine mRNAs at each time point were assayed by RNase protection. (B) The band densities were determined with TINA software, and the mRNA expression level for each chemokine was normalized with respect to the average intensities of the bands of L32 and GAPDH. HKG, housekeeping genes (L32 and GAPDH).
FIG. 2.
(A) Induction of chemokine genes in HMEC-1 incubated for 3 h with medium alone (C), L929 cell lysate (Lysate), O. tsutsugamushi (OT), CHX, or CHX and OT. (B) Normalized mRNA expression level for each chemokine as described in the legend to Fig. 1. HKG, housekeeping genes (L32 and GAPDH).
FIG. 3.
(A) Induction of chemokine genes in HMEC-1 incubated for 3 h with medium (C), polymyxin B (PB) (30 μg/ml), O. tsutsugamushi (OT), and LPS derived from E. coli (LPS) in the absence or presence of polymyxin B. (B) Normalized expression level for each chemokine mRNA as described in the legend to Fig. 1. HKG, housekeeping genes (L32 and GAPDH).
Heat stability of stimulating molecule.
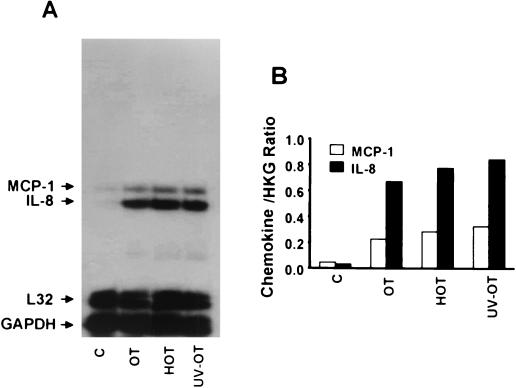
To evaluate whether active rickettsia invasion is required for chemokine induction, we exposed endothelial cells for 3 h to heat- or UV-inactivated O. tsutsugamushi. It has been reported that both heat- and UV-inactivated O. tsutsugamushi can bind host cell surfaces but cannot penetrate the cells through induced phagocytosis (50). As shown in Fig. 4, MCP-1 and IL-8 mRNA levels in cells treated with heat- or UV-inactivated O. tsutsugamushi were comparable to those that had been treated with live rickettsia.
FIG. 4.
(A) Chemokine response to heat-inactivated (HOT), UV-inactivated (UV-OT), or active O. tsutsugamushi (OT) in HMEC-1. (B) Normalized expression level for each chemokine mRNA as described in the legend to Fig. 1. HKG, housekeeping genes (L32 and GAPDH).
Effect of NF-κB activation inhibitors on chemokine expression.
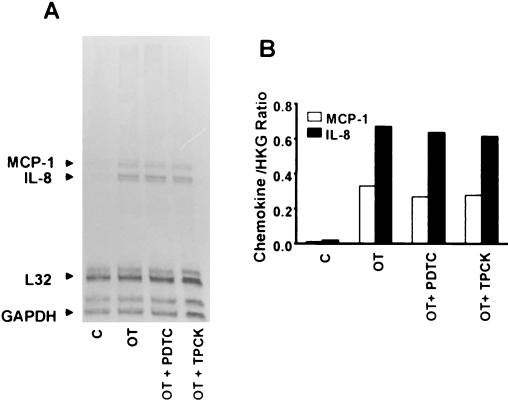
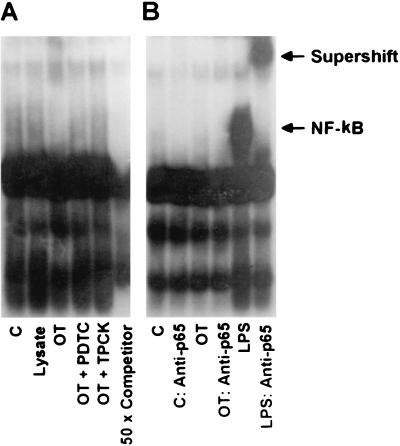
In order to examine whether NF-κB activation is involved in the chemokine induction of O. tsutsugamushi-exposed endothelial cells, we used two inhibitors of NF-κB activation, the antioxidant PDTC (36, 45) and the proteasome inhibitor TPCK (16, 37, 45). Interestingly, when the endothelial cells were incubated with O. tsutsugamushi in the presence of 50 μM PDTC or 100 μM TPCK, induction of MCP-1 and IL-8 was not affected in HMEC-1 (Fig. 5). The results were reproduced in three separate trials. We, therefore, investigated whether O. tsutsugamushi-infected endothelial cells could induce NF-κB activation (Fig. 6). At 2 h after HMEC-1 was infected with O. tsutsugamushi, no NF-κB complexed with oligonucleotide was detected (Fig. 6). However, in the cells treated with LPS, a high level of activated NF-κB was detected, which was confirmed by a supershift assay using anti- NF-κB p65 antibody (Fig. 6B). This result was reproduced in three separate experimental attempts. The activation of NF-κB was not detected at any point during the time when HMEC-1 was infected with O. tsutsugamushi for up to 8 h (data not shown).
FIG. 5.
(A) Effect of PDTC and TPCK on O. tsutsugamushi-induced chemokine mRNA levels in HMEC-1. Chemokine mRNA levels were analyzed using an RNase protection assay in total RNA samples that were prepared from uninfected cells (C), cells infected with O. tsutsugamushi for 3 h (OT), and infected cells in the presence of PDTC (OT + PDTC) or TPCK (OT + TPCK). (B) Normalized expression level for each chemokine mRNA as described in the legend to Fig. 1. HKG, housekeeping genes (L32 and GAPDH).
FIG. 6.
(A) EMSA was performed to analyze the activation of NF-κB using nuclear extracts prepared from HMEC-1 treated for 2 h with medium (C), L929 cell lysate (Lysate), O. tsutsugamushi (OT), or LPS. Nuclear extracts from cells pretreated with PDTC (OT + PDTC) or TPCK (OT + TPCK) for 1 h before infection with O. tsutsugamushi were also assayed. A competitive inhibition assay was performed with nuclear extracts that were preincubated with the unlabeled NF-κB consensus oligonucleotide (50 × Competitor). (B) In the supershift assay, nuclear extracts from HMEC-1 were preincubated with antibodies against the p65 subunit of NF-κB
Activation of AP-1 in O. tsutsugamushi-infected endothelial cells.
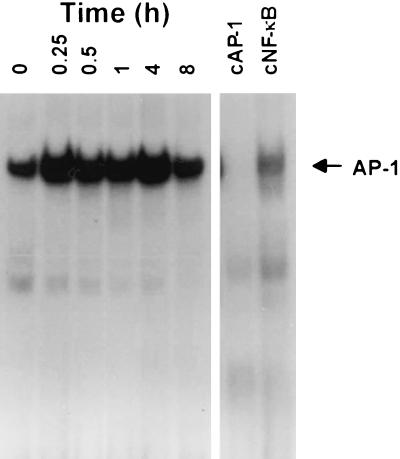
We also investigated whether another inducible transcription factor, AP-1, was activated when endothelial cells were infected with O. tsutsugamushi (Fig. 7). Nuclear protein extracts were prepared from O. tsutsugamushi-infected HMEC-1 after 0.25, 0.5, 1, 4, or 8 h. AP-1 binding activity was determined by EMSA. A basal level of AP-1 binding activity was detected in nuclear extracts of HMEC-1 (29). When the cells were stimulated with O. tsutsugamushi, AP-1 binding activity increased as early as 15 min and persisted for at least 4 h. At 8 h after infection, the levels of AP-1 binding activity decreased to levels similar to those in cells incubated with medium alone. The specificity of the mobility-shifted complex was confirmed by a competition assay using 50 times the molar excess of the binding sequence for either unlabeled AP-1 or NF-κB as shown in Fig. 7. The AP-1 binding complex was successfully competed by the 50-fold molar excess of the consensus AP-1 binding sequence. This indicates that O. tsutsugamushi activates the DNA-binding activity of AP-1 in the human endothelial cell, which is another major transcriptional regulator of chemokine genes.
FIG. 7.
Activation of transcription factor AP-1 in O. tsutsugamshi-infected HMEC-1 at each indicated time point. A competitive inhibition assay was performed with nuclear extracts that were preincubated with either the unlabeled NF-κB (cNF-κB) or the AP-1 (cAP-1) consensus oligonucleotide.
DISCUSSION
In this study, we showed that the expression of MCP-1 and IL-8 is induced in response to O. tsutsugamushi infection in the human endothelial cell line HMEC-1. Low levels of MCP-1 and IL-8 RNA messages are expressed constitutively in HMEC-1 (19). After infection, the MCP-1 and IL-8 gene messages were induced and peaked transiently between 3 and 6 h. In endothelial cells, the RANTES mRNAs were expressed at 6 h and gradually increased by 48 h after infection.
Although we did not perform assays to confirm the secretion of active chemokine proteins following gene induction, several recent studies have shown a correlation between mRNA expression and chemokine protein secretion (4, 7). The chemokine genes were induced specifically in response to O. tsutsugamushi infection. In the cells treated with L929 cell lysate, chemokine genes were not induced. Previous studies had already demonstrated that various cytokines stimulate chemokine expression in vitro (51). We, therefore, examined the role of newly synthesized proteins in chemokine gene expression. When the eukaryotic protein synthesis inhibitor (CHX) and O. tsutsugamushi were added simultaneously to endothelial cell cultures, MCP-1 and IL-8 mRNA levels were higher than those of the cells infected with O. tsutsugamushi alone. This indicates that chemokine induction is not an indirect effect due to prior induction of proinflammatory cytokines, such as IL-1, which are known to induce chemokine production in endothelial cells (7). The superinduction of chemokine genes by CHX treatment may be mediated by preventing the degradation of otherwise labile mRNA or by inhibiting the synthesis of inhibitory proteins for the induction of chemokine genes (8). Contamination by LPS during the preparation of O. tsutsugamushi was also examined. It has been previously reported that the O. tsutsugamushi cell wall component is deficient in LPS (3). In O. tsutsugamushi-infected endothelial cells, blocking LPS activity with polymyxin B did not decrease chemokine responses. It shows that exogenous sources of LPS are not responsible for the induction of the chemokine genes.
In addition, we tried to investigate rickettsia components eliciting chemokine responses in endothelial cells. The physicochemical characteristics of the components were analyzed after O. tsutsugamushi was subjected to heat or UV treatment. MCP-1 and IL-8 transcript levels did not change significantly whether cells were treated with live O. tsutsugamushi or with heat- or UV-treated O. tsutsugamushi. It suggests that the rickettsia components are heat stable, and that O. tsutsugamushi binding to the endothelial cell surface leads to the activation of transcription factors. It clearly shows that O. tsutsugamushi proliferation within infected endothelial cells is not a prerequisite for expression of the chemokines. We have previously reported a similar result in macrophages that were stimulated either by heat-inactivated or live O. tsutsugamushi (13). The expression of MCP-1, MIP-2, and MIP-1β in macrophages was not affected by heat-inactivated O. tustusgamushi. The stimulatory molecules of O. tsutsugamushi may be heat-resistant molecules, such as polysaccharides, lipids, or heat-stable proteins, that are in the outer membrane of the bacterium. It has been previously reported that the Staphylococcus aureus capsular polysaccharide and the outer surface lipoprotein A of Borrelia burgdorferi were sufficient to induce the chemokine genes IL-8 and MCP-1 in both human endothelial cells and monocytes (18, 46). Although the signal transduction pathways involved in chemokine expression during infection have been poorly elucidated, it is probable that a tyrosine kinase and MAP kinase pathway play critical roles in the induction of the IL-8 gene in diverse cells in response to pathogenic microorganisms (10, 17, 30). In this study, the chemokine responses of endothelial cells were elicited by the heat-stable components of O. tsutsugamushi, but the identity of these components was not precisely elucidated. Further studies on O. tsutsugamushi stimulatory components and signal transduction pathways in host cells during rickettsia infection will provide valuable insight into the mechanisms controlling the inflammatory responses during O. tsutsugamushi infection.
The transcription factor NF-κB/Rel family plays a central role in the regulation of a variety of genes involved in host innate immunity, including the chemokines (21). For the chemokine genes tested in this study, regulation by NF-κB has either been demonstrated or is suggested by the presence of the NF-κB consensus motif in the promoter (9, 35, 49, 51). Furthermore, we have previously reported that O. tsutsugamushi induces an increase in active NF-κB in the nucleus of macrophages, particularly the p65-p50 heterodimer (13). Diverse NF-κB activation inhibitors, such as antioxdants and proteasome inhibitors, have been used to investigate whether the activation of NF-κB is involved in the transcriptional activation of inflammatory genes (6). PDTC, an antioxidant, inhibits the phosphorylation of IκB (36, 43), a prerequisite for its subsequent proteolytic degradation. TPCK, an inhibitor of chymotryptic activity associated with the proteasome, blocks NF-κB activation by inhibiting proteasome-dependent degradation of inhibitory peptides (32). In this study, however, the induction levels of MCP-1 and IL-8 transcripts in endothelial cells upon O. tsutsugamushi infection were not affected by NF-κB activation inhibitors. According to previous data, the inhibitor concentrations used for this study were sufficient to repress NF-κB activation in endothelial cells (16, 36, 37, 45). The result suggests that induction of the chemokine genes is not mediated by NF-κB activation. Furthermore, we investigated whether O. tsutsugamushi infection activates NF-κB in endothelial cells. To confirm our ability to detect the presence of NF-κB activation and nuclear translocation, HMEC-1 was stimulated with LPS. A high level of the LPS-activated NF-κB complex was observed, which was confirmed by using a supershift assay that hybridized to anti-NF-κB p65 antibody (Fig. 6B). Taken together, these results indicate that expression of the MCP-1 and IL-8 in human endothelial cells upon O. tsutsugamushi infection is mediated by an NF-κB independent mechanism.
It has been reported that NF-κB activation in human umbilical endothelial cells by Rickettsia rickettsii inhibits apoptosis of endothelial cells and that this provides a possible mechanism that enables host cells to remain as a site for rickettsia replication (15). However, apoptosis of lymphocytes and an endothelial cell line infected with O. tsutsugamushi has been previously reported (24, 28). Additionally, NF-κB activation in macrophages infected with O. tsutsugamushi was not correlated with immediate-early apoptotic responses of cells (13, 14). In this study, activation of NF-κB was not observed in HMEC-1 infected with O. tsutsugamushi.
The transcription factor AP-1 is a dimer composed of the Fos and Jun members (20). Recently, it has been suggested that this inducible transcription factor is important in immune response regulation including cytokine gene expression (20). AP-1 has also been implicated in the transcriptional regulation of chemokine genes and adhesion molecules in endothelial cells (29, 36, 40, 53), particularly in the stimulus-specific and cell type-specific regulation of chemokine gene transcription. The differential activation and binding of inducible transcription factors, such as AP-1 and NF-κB, seem to provide a critical regulatory mechanism (29, 40). Although the regulatory effects of AP-1 in human endothelial cells is poorly understood, a recent work demonstrated that the overexpression of AP-1 in endothelial cells is sufficient to induce adhesion molecules and chemokine genes through an NF-κB-independent mechanism (53). They suggested that AP-1 could play a key regulatory role, whereby a variety of stimuli activate endothelial cells in a specific pattern of gene expression and subsequently contribute to the development of vascular pathological processes. In this study, we demonstrate that in HMEC-1 infected with O. tsutsugamushi, the binding activity of the AP-1 was increased as early as 15 min after infection and returned to basal levels after 8 h. These results suggest the possibility that AP-1 regulatory proteins mediate the activation of endothelial cells infected by O. tsutsugamushi through the cognate sequence in the promoter regions of target genes (36, 40, 53). Further studies with site-directed mutagenesis experiments will help determine if the induction of chemokine genes depends on the proximal AP-1 site and its flanking sequences and help identify potential interactions with other sites.
In this work, we report that O. tsutsugamushi induces the expression of three chemokine genes, those encoding MCP-1, IL-8, and RANTES, in human endothelial cells. Both RANTES and MCP-1 are known to recruit monocytes and lymphocytes preferentially (51). We have previously reported that RANTES, MIP-1α, MIP-1β, MCP-1, and MIP-2 genes are induced in O. tsutsugamushi-infected macrophages (13). Besides MIP-2, other chemokines also belong to the CC chemokine subfamily that are potent attractants for monocytes and lymphocytes (51). These data correlate well with previous results showing that the infiltration of mononuclear cells is frequently observed in eschar, rashes, and organs that have vascular inflammation (2). The induction of a specific subset of chemokine genes has been correlated to the subsequent disease pathogenesis in other pathogenic microorganisms (11, 18, 34, 52). The levels and kinetics of chemokine gene expression are slightly different from each other in cells infected with specific pathogenic microorganisms (4, 19). The differences in chemokine gene induction can partly explain differences in the population of leukocytes recruited to the site of inflammation and the pathological changes around the vasculatures (42). Our results have shown that a specific subset of chemokine genes are induced in O. tsutsugamushi-infected cells and suggest that the heterogeneous expression of chemokines may be due to differential activation of inducible transcription factors such as NF-κB and AP-1. The differential activation of inducible transcription factors could critically influence the site-specific recruitment of distinct leukocyte subsets to sites of inflammation and partly explains differences in pathology due to the infecting organism in vivo.
ACKNOWLEDGMENT
This work was supported by the Korea Research Foundation of the Republic of Korea (grant 97110814).
REFERENCES
- 1.Ades E W, Candal F J, Swerlick R A, George V G, Summers S, Bosse D C, Lawley T J. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Investig Dermatol. 1992;99:683–690. doi: 10.1111/1523-1747.ep12613748. [DOI] [PubMed] [Google Scholar]
- 2.Allen A C, Spitz S. A comparative study of the pathology of scrub typhus (tsutsugamushi disease) and other rickettsial disease. Am J Pathol. 1945;21:603–681. [PMC free article] [PubMed] [Google Scholar]
- 3.Amano K, Tamura A, Ohashi N, Urakami H, Kaya S, Fukushi K. Deficiency of peptidoglycan and lipopolysaccharide components in Rickettsia tsutsugamushi. Infect Immun. 1987;55:2290–2292. doi: 10.1128/iai.55.9.2290-2292.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avirutnan P, Malasit P, Seliger B, Bhakdi S, Husmann M. Dengue virus infection of human endothelial cells leads to chemokine production, complement activation, and apoptosis. J Immunol. 1998;161:6338–6346. [PubMed] [Google Scholar]
- 5.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 6.Baldwin A S., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 7.Beck G C, Yard B A, Breedijk A J, Van Ackern K, Van Der Woude F J. Release of CXC-chemokines by human lung microvascular endothelial cells (LMVEC) compared with macrovascular umbilical vein endothelial cells. Clin Exp Immunol. 1999;118:298–303. doi: 10.1046/j.1365-2249.1999.01052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blease K, Chen Y, Hellewell P G, Burke-Gaffney A. Lipoteichoic acid inhibits lipopolysaccharide-induced adhesion molecule expression and IL-8 release in human lung microvascular endothelial cells. J Immunol. 1999;163:6139–6147. [PubMed] [Google Scholar]
- 9.Brasier A R, Jamaluddin M, Casola A, Duan W, Shen Q, Garofalo R P. A promoter recruitment mechanism for tumor necrosis factor-alpha-induced interleukin-8 transcription in type II pulmonary epithelial cells. Dependence on nuclear abundance of Rel A, NF-kappaB1, and c-Rel transcription factors. J Biol Chem. 1998;273:3551–3561. doi: 10.1074/jbc.273.6.3551. [DOI] [PubMed] [Google Scholar]
- 10.Bruder J T, Kovesdi I. Adenovirus infection stimulates the Raf/MAPK signaling pathway and induces interleukin-8 expression. J Virol. 1997;71:398–404. doi: 10.1128/jvi.71.1.398-404.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charles P C, Chen X, Horwitz M S, Brosnan C F. Differential chemokine induction by the mouse adenovirus type-1 in the central nervous system of susceptible and resistant strains of mice. J Neurovirol. 1999;5:55–64. doi: 10.3109/13550289909029746. [DOI] [PubMed] [Google Scholar]
- 12.Chi W C, Huang J J, Sung J M, Lan R R, Ko W C, Chen F F. Scrub typhus associated with multiorgan failure: a case report. Scand J Infect Dis. 1997;29:634–635. doi: 10.3109/00365549709035911. [DOI] [PubMed] [Google Scholar]
- 13.Cho N H, Seong S Y, Huh M S, Han T H, Koh Y S, Choi M S, Kim I S. Expression of chemokine genes in murine macrophages infected with Orientia tsutsugamushi. Infect Immun. 2000;68:594–602. doi: 10.1128/iai.68.2.594-602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi N J, Kim M K, Park H J, Lim B U, Kang J S. Apoptosis of murine macrophage-like cells infected with Orientia tsutsugamushi. J Korean Soc Microbiol. 1998;33:399–406. [Google Scholar]
- 15.Clifton D R, Goss R A, Sahni S K, van Antwerp D, Baggs R B, Marder V J, Silverman D J, Sporn L A. NF-kappa B-dependent inhibition of apoptosis is essential for host cellsurvival during Rickettsia rickettsii infection. Proc Natl Acad Sci USA. 1998;95:4646–4651. doi: 10.1073/pnas.95.8.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cobb R R, Felts K A, Parry G C, Mackman N. Proteasome inhibitors block VCAM-1 and ICAM-1 gene expression in endothelial cells without affecting nuclear translocation of nuclear factor-kappa B. Eur J Immunol. 1996;26:839–845. doi: 10.1002/eji.1830260417. [DOI] [PubMed] [Google Scholar]
- 17.Ding S Z, Cho C H, Lam S K. Helicobacter pylori induces interleukin-8 expression in endothelial cells and the signal pathway is protein tyrosine kinase dependent. Biochem Biophys Res Commun. 1997;240:561–565. doi: 10.1006/bbrc.1997.7699. [DOI] [PubMed] [Google Scholar]
- 18.Ebnet K, Brown K D, Siebenlist U K, Simon M M, Shaw S. Borrelia burgdorferi activates nuclear factor-kappa B and is a potent inducer of chemokine and adhesion molecule gene expression in endothelial cells and fibroblasts. J Immunol. 1997;158:3285–3292. [PubMed] [Google Scholar]
- 19.Ebnet K, Simon M M, Shaw S. Regulation of chemokine gene expression in human endothelial cells by proinflammatory cytokines and Borrelia burgdorferi. Ann N Y Acad Sci. 1996;797:107–117. doi: 10.1111/j.1749-6632.1996.tb52953.x. [DOI] [PubMed] [Google Scholar]
- 20.Foletta V C, Segal D H, Cohen D R. Transcriptional regulation in the immune system: all roads lead to AP-1. J Leukoc Biol. 1998;63:139–152. doi: 10.1002/jlb.63.2.139. [DOI] [PubMed] [Google Scholar]
- 21.Ghosh S, May M J, Kopp E B. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 22.Imhof B A, Dunon D. Leukocyte migration and adhesion. Adv Immunol. 1995;58:345–416. doi: 10.1016/s0065-2776(08)60623-9. [DOI] [PubMed] [Google Scholar]
- 23.Iwasaki H, Takada N, Nakamura T, Ueda T. Increased levels of macrophage colony-stimulating factor, gamma interferon, and tumor necrosis factor alpha in sera of patients with Orientia tsutsugamushi infection. J Clin Microbiol. 1997;35:3320–3322. doi: 10.1128/jcm.35.12.3320-3322.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasuya S, Nagano I, Ikeda T, Goto C, Shimokawa K, Takahashi Y. Apoptosis of lymphocytes in mice induced by infection with Rickettsia tsutsugamushi. Infect Immun. 1996;64:3937–3941. doi: 10.1128/iai.64.9.3937-3941.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawai T, Seki M, Hiromatsu K, Eastcott J W, Watts G F, Sugai M, Smith D J, Porcelli S A, Taubman M A. Selective diapedesis of Th1 cells induced by endothelial cell RANTES. J Immunol. 1999;163:3269–3278. [PubMed] [Google Scholar]
- 26.Kawamura J A, Tanaka H, Tamura A. Tsutsugamushi disease. Tokyo, Japan: University of Tokyo Press; 1995. [Google Scholar]
- 27.Kayal S, Lilienbaum A, Poyart C, Memet S, Israel A, Berche P. Listeriolysin O-dependent activation of endothelial cells during infection with Listeria monocytogenes: activation of NF-kappa B and upregulation of adhesion molecules and chemokines. Mol Microbiol. 1999;31:1709–1722. doi: 10.1046/j.1365-2958.1999.01305.x. [DOI] [PubMed] [Google Scholar]
- 28.Kim M K, Kee S H, Cho K A, Chung M H, Lim B U, Chang W H, Kang J S. Apoptosis of endothelial cell line ECV304 persistently infected with Orientia tsutsugamushi. Microbiol Immunol. 1999;43:751–757. doi: 10.1111/j.1348-0421.1999.tb02466.x. [DOI] [PubMed] [Google Scholar]
- 29.Lakshminarayanan V, Drab-Weiss E A, Roebuck K A. H2O2 and tumor necrosis factor-alpha induce differential binding of the redox-responsive transcription factors AP-1 and NF-kappaB to the interleukin-8 promoter in endothelial and epithelial cells. J Biol Chem. 1998;273:32670–32678. doi: 10.1074/jbc.273.49.32670. [DOI] [PubMed] [Google Scholar]
- 30.Legastelois I, Levrey H, Greenland T, Mornex J F, Cordier G. Visna-maedi virus induces interleukin-8 in sheep alveolar macrophages through a tyrosine-kinase signaling pathway. Am J Respir Cell Mol Biol. 1998;18:532–537. doi: 10.1165/ajrcmb.18.4.2812. [DOI] [PubMed] [Google Scholar]
- 31.Leving D H. Pathologic study of thirty-one cases of scrub typhus fever with special reference to the cardiovascular system. Am Heart J. 1946;31:314–328. doi: 10.1016/0002-8703(46)90313-4. [DOI] [PubMed] [Google Scholar]
- 32.Mackman N. Protease inhibitors block lipopolysaccharide induction of tissue factor gene expression in human monocytic cells by preventing activation of c-Rel/p65 heterodimers. J Biol Chem. 1994;269:26363–26367. [PubMed] [Google Scholar]
- 33.McGill S N, Ahmed N A, Christou N V. Endothelial cells: role in infection and inflammation. World J Surg. 1998;22:171–178. doi: 10.1007/s002689900366. [DOI] [PubMed] [Google Scholar]
- 34.Molestina R E, Miller R D, Ramirez J A, Summersgill J T. Infection of human endothelial cells with Chlamydia pneumoniae stimulates transendothelial migration of neutrophils and monocytes. Infect Immun. 1999;67:1323–1330. doi: 10.1128/iai.67.3.1323-1330.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moriuchi H, Moriuchi M, Fauci A S. Nuclear factor-kappa B potently up-regulates the promoter activity of RANTES, a chemokine that blocks HIV infection. J Immunol. 1997;158:3483–3491. [PubMed] [Google Scholar]
- 36.Munoz C, Pascual-Salcedo D, Castellanos M C, Alfranca A, Aragones J, Vara A, Redondo M J, de Landazuri M O. Pyrrolidine dithiocarbamate inhibits the production of interleukin-6, interleukin-8, and granulocyte-macrophage colony-stimulating factor by human endothelial cells in response to inflammatory mediators: modulation of NF-kappa B and AP-1 transcription factors activity. Blood. 1996;88:3482–3490. [PubMed] [Google Scholar]
- 37.Parry G C, Martin T, Felts K A, Cobb R R. IL-1beta-induced monocyte chemoattractant protein-1 gene expression in endothelial cells is blocked by proteasome inhibitors. Arterioscler Thromb Vasc Biol. 1998;18:934–940. doi: 10.1161/01.atv.18.6.934. [DOI] [PubMed] [Google Scholar]
- 38.Pober J S, Cotran R S. The role of endothelial cells in inflammation. Transplantation. 1990;50:537–544. doi: 10.1097/00007890-199010000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Pongponratn E, Maneerat Y, Chaisri U, Wilairatana P, Punpoowong B, Viriyavejakul P, Riganti M. Electron-microscopic examination of Rickettsia tsutsugamushi-infected human liver. Trop Med Int Health. 1998;3:242–248. doi: 10.1046/j.1365-3156.1998.00231.x. [DOI] [PubMed] [Google Scholar]
- 40.Roebuck K A, Carpenter L R, Lakshminarayanan V, Page S M, Moy J N, Thomas L L. Stimulus-specific regulation of chemokine expression involves differential activation of the redox-responsive transcription factors AP-1 and NF-kappaB. J Leukoc Biol. 1999;65:291–298. doi: 10.1002/jlb.65.3.291. [DOI] [PubMed] [Google Scholar]
- 41.Sallusto F, Lanzavecchia A, Mackay C R. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol Today. 1998;19:568–574. doi: 10.1016/s0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]
- 42.Schluger N W, Rom W N. Early responses to infection: chemokines as mediators of inflammation. Curr Opin Immunol. 1997;9:504–508. doi: 10.1016/s0952-7915(97)80102-1. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt K N, Traenckner E B, Meier B, Baeuerle P A. Induction of oxidative stress by okadaic acid is required for activation of transcription factor NF-kappa B. J Biol Chem. 1995;270:27136–27142. doi: 10.1074/jbc.270.45.27136. [DOI] [PubMed] [Google Scholar]
- 44.Seong S Y, Huh M S, Jang W J, Park S G, Kim J G, Woo S G, Choi M S, Kim I S, Chang W H. Induction of homologous immune response to Rickettsia tsutsugamushi Boryong with a partial 56-kilodalton recombinant antigen fused with the maltose-binding protein MBP-Bor56. Infect Immun. 1997;65:1541–1545. doi: 10.1128/iai.65.4.1541-1545.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi R J, Simpson-Haidaris P J, Lerner N B, Marder V J, Silverman D J, Sporn L A. Transcriptional regulation of endothelial cell tissue factor expression during Rickettsia rickettsii infection: involvement of the transcription factor NF-κB. Infect Immun. 1998;66:1070–1075. doi: 10.1128/iai.66.3.1070-1075.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soell M, Diab M, Haan-Archipoff G, Beretz A, Herbelin C, Poutrel B, Klein J P. Capsular polysaccharide types 5 and 8 of Staphylococcus aureus bind specifically to human epithelial (KB) cells, endothelial cells, and monocytes and induce release of cytokines. Infect Immun. 1995;63:1380–1386. doi: 10.1128/iai.63.4.1380-1386.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swerlick R A, Lawley T J. Role of microvascular endothelial cells in inflammation. J Investig Dermatol. 1993;100:111S–115S. doi: 10.1111/1523-1747.ep12356595. [DOI] [PubMed] [Google Scholar]
- 48.Tamura A, Urakami H. Easy method for infectivity titration of Rickettsia tsutsugamushi by infected cell counting. Nippon Saikingaku Zasshi. 1981;36:783–785. [PubMed] [Google Scholar]
- 49.Ueda A, Ishigatsubo Y, Okubo T, Yoshimura T. Transcriptional regulation of the human monocyte chemoattractant protein-1 gene. Cooperation of two NF-kappaB sites and NF-kappaB/Rel subunit specificity. J Biol Chem. 1997;272:31092–31099. doi: 10.1074/jbc.272.49.31092. [DOI] [PubMed] [Google Scholar]
- 50.Urakami H, Tsuruhara T, Tamura A. Penetration of Rickettsia tsutsugamushi into cultured mouse fibroblasts (L cells): an electron microscopic observation. Microbiol Immunol. 1983;27:251–263. doi: 10.1111/j.1348-0421.1983.tb03587.x. [DOI] [PubMed] [Google Scholar]
- 51.Vaddi K, Keller M, Newton R C. The chemokine FactsBook. New York, N.Y: Academic Press, Inc.; 1997. [Google Scholar]
- 52.Vester B, Muller K, Solbach W, Laskay T. Early gene expression of NK cell-activating chemokines in mice resistant to Leishmania major. Infect Immun. 1999;67:3155–3159. doi: 10.1128/iai.67.6.3155-3159.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang N, Verna L, Hardy S, Forsayeth J, Zhu Y, Stemerman M B. Adenovirus-mediated overexpression of c-Jun and c-Fos induces intercellular adhesion molecule-1 and monocyte chemoattractant protein-1 in human endothelial cells. Arterioscler Thromb Vasc Biol. 1999;19:2078–2084. doi: 10.1161/01.atv.19.9.2078. [DOI] [PubMed] [Google Scholar]
- 54.Watt G, Strickman D. Life-threatening scrub typhus in a traveler returning from Thailand. Clin Infect Dis. 1994;18:624–626. doi: 10.1093/clinids/18.4.624. [DOI] [PubMed] [Google Scholar]
- 55.Yotsukura M, Aoki N, Fukuzumi N, Ishikawa K. Review of a case of tsutsugamushi disease showing myocarditis and confirmation of Rickettsia by endomyocardial biopsy. Jpn Circ J. 1991;55:149–153. doi: 10.1253/jcj.55.149. [DOI] [PubMed] [Google Scholar]