Abstract
This study aimed to profile the clinical progression, demographics, and oxidative status of COVID-19 patients, correlating with disease severity. The study included 143 participants: 93 patients with COVID-19 (28 outpatients, 65 inpatients), and 50 control participants. Thiobarbituric acid reactive substance (TBARS) was used as an oxidative damage marker. Antioxidant activity was assessed via quantification of Vitamin C, sulfhydryl groups, ferric reduction ability of plasma (FRAP), Uric acid (UA), and evaluation of delta-aminolevulinate dehydratase (δ-ALA-D) enzymatic activity. Geriatric patients, especially men, with comorbidities such as obesity and/or chronic diseases were more likely to develop the most severe form of COVID-19. The activity of the δ-ALA-D was lower in inpatients, and there was no significant difference with the outpatient. Antioxidants decreased in COVID-19 groups, while lipid peroxidation increased. FRAP and Vitamin C decreased with evolution of the disease. Oxidative stress could be used as a predictor of worsening clinical condition.
Keywords: SARS-CoV-2, Oxidative stress, Antioxidant, δ-ALA-D, COVID-19
Abbreviations: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; COVID-19, coronavirus disease; ACE2, angiotensin-II converting enzyme; RT-PCR, real-time polymerase chain reaction; COPD, chronic obstructive pulmonary disease; ROS, reactive oxygen species; HUSM, Santa Maria University Hospital; ICU, intensive care unit; CEP, Research Ethics Committee; TBARS, thiobarbituric acid reactive substance; P-SH, Protein thiol groups; NP-SH, Non protein thiol groups; FRAP, ferric reduction ability of plasma; UA, acid uric; δ-ALA-D, delta-aminolevulinate dehydratase enzyme
1. Introduction
Wuhan, capital of the Hubei Province, China, experienced an outbreak of pneumonia cases of unknown etiology in December 2019 [1]. The epidemic later evolved into a global pandemic [1,2]. The etiological agent was named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), owing to its phylogenetic similarity to SARS—causative agent of a viral outbreak in 2022; the clinical condition was referred to as coronavirus disease (COVID-19) [3].
SARS-CoV-2 binds to the receptor angiotensin-II converting enzyme (ACE2) within the lung parenchyma; thus, ACE2 is the main mediator of virus entry into human host cells [4], [5], [6]. SARS-CoV-2 is transmitted via direct contact with infected patients or respiratory droplets and is detected through real-time polymerase chain reaction (RT-PCR) [[1], [2], [3],5]. Patients present with a wide range of symptoms, from mild to severe; the most common symptoms are fever, dry cough, fatigue, and shortness of breath. However, symptoms such as sputum production, headache, hemoptysis, diarrhea, and lymphopenia have also been reported [1,5]. The symptoms of COVID-19 are more severe in older age groups with comorbidities; allergies, asthma, and chronic obstructive pulmonary disease (COPD) are also risk factors [1,2].
Oxidative stress occurs due to an imbalance between the production of oxidizing compounds and the antioxidant defense capacity, generating many free radicals [7,8]. This increases reactive oxygen species (ROS), eventually resulting in oxidative damage, cell degeneration, and functional decline, which can further worsen respiratory diseases, including COVID-19 [7,9,10]. Viruses disrupt the homeostasis of infected cells and increase ROS production in phagocytes. Patients with SARS-CoV-2 who present with comorbidities have an increased state of oxidative stress due to chronic diseases and viral diseases, and assessment of oxidative stress is important to determine the severity of COVID-19 [11].
Oxidative stress markers can be used as a tool for decision making, and the correlation between oxidative stress and disease severity should be further studied to identify predictors of disease severity and associated complications. Therefore, this study aimed to profile the clinical condition, demographics, and oxidative status of patients with COVID-19 and correlate them with disease severity.
2. Materials and methods
2.1. Study population
One hundred and forty-three participants with COVID-19 were selected, between August 2020 and March 2021, from the University Hospital of Santa Maria (HUSM), RS, Brazil. The samples were divided into three groups: i) outpatients (n = 28): patients with mild COVID-19, who were in isolation at home; ii) inpatients (n = 65): patients with moderate or severe COVID-19, who were admitted to the HUSM intensive care unit (ICU), following the COVID-19 Treatment Guidelines Panel by the National Institutes of Health [12] and the Guidelines for New Clinical Manifestations of Coronavirus by the World Health Organization [13], and iii) control (n = 50): community volunteers, without COVID-19, collected before the emergence of the pandemic, with sex, age, and comorbidities (such as hypertension and diabetes mellitus) similar to participants with COVID-19. Informed consent was obtained from all patients and participants. This study was approved by the Research Ethics Committee (CEP) of the Federal University of Santa Maria after receiving the certificate of presentation for ethical appreciation (CAAE: 13897319.0.0000.5346). Patients with neoplasms were excluded from the study.
2.2. Sample collection
Venous blood samples were collected in vacuum tubes as follows: 8 mL in a tube with heparin and 2 mL in a tube without any anticoagulant. The whole blood sample in the heparin tube was centrifuged at 3000 rpm for 10 minutes to separate the plasma from the erythrocytes, which were then rinsed three times with a 0.9% NaCl solution. Plasma aliquots were separated and stored at −80◦C as soon as they were obtained. This was done for performing the ferric reducing ability of plasma (FRAP) tests at a later stage, while the other tests were performed on the same day. Medical history of patients pertinent to the study was obtained verbally from patients or from electronic medical records. Body mass index (BMI) was calculated by dividing weight by height squared (kg/m2).
2.3. Oxidative profile
2.3.1. Oxidants
To evaluate lipid peroxidation in erythrocytes and plasma, thiobarbituric acid reactive substance (TBARS) was measured spectrophotometrically at 532 nm using the method described by Lapenna et al. [14] and expressed as nmol MDA/mL of plasma and nmol MDA/mL of erythrocytes.
2.3.2. Antioxidants
Protein thiol groups (P-SH) in plasma and nonprotein thiol groups (NP-SH) in erythrocytes were represented as nmol P-SH/mL of plasma and nmol N-PSH/ mL of erythrocytes and were evaluated according to the Ellman and Boyne [15] method modified by Jacques-Silva et al. [16]. Estimation of Vitamin C levels in plasma was performed as described by Galley et al. [17] with some modifications by Jacques-Silva et al. [16], and the results are expressed in µg vitamin C/ mL of plasma. The ferric reducing ability of plasma (FRAP), technique described by Benzie and Strain (1996) [18] was applied on a Mindray BS 380 automated analyzer, Shenzhen, China, and the results were expressed in µmol/L. The uric acid (UA), measured in serum were assessed by standard method with a commercial kit (Labtest).
2.3.3. Enzymatic activity
The Berlin and Schaller [19] technique was used to determine the activity of δ-ALA-D. The results are expressed as U/l (U = nanomole of PBG/hour/milligram of Hb).
2.4. Statistical analysis
The software Graph Pad Prism 8.0.2 software (San Diego, California) was used to analyze the data. The normality of the distribution was tested with the Shapiro-Wilk test. For the analysis of non-parametric data, the Kruskal-Wallis test was used and the results were expressed as median (interquartile range) and for the parametric data, the One-Way analysis of variance (ANOVA) followed by the Tukey post hoc test to compare groups and data were expressed as mean standard deviation (SD). Categorical variables analyzed by the Pearson chi-square test. For correlation, Spearman's correlation coefficient was used for nonparametric data. Differences in the probability of rejecting the null hypothesis of 5% (P < 0.05) were considered statistically significant.
3. Results
Ninety-three COVID-19 patients confirmed using the RT-PCR test were included in the study; 28 patients had mild symptoms and were assigned to the outpatient group, 65 patients had moderate/severe symptoms, and were assigned to the inpatient group, and 50 volunteers were assigned to the control group. Table 1 shows the clinical and demographic details of the study participants.
Table 1.
Clinical and demographic characteristics of participants.
| Control group | Hospitalization | ||||
|---|---|---|---|---|---|
| Characteristics | (n = 50) | No (n = 28) | Yes (n = 65) | P value | |
| Age (y) | 51.6 (21–81) | 38.7 (19–62)a | 59.7 (14–91)a,b | <0.001 | |
| Weight (Kg) | 74.8 (58–110) | 72.0 (45–105) | 87.7 (52–157)a,b | 0.001 | |
| Height (m) | 1.7 (1.55–1.90) | 1.7 (1.53–1.89) | 1.7 (1.48–1.85) | 0.625 | |
| BMI (Kg/m2) | 26.2 (20.98–37.20) | 25.6 (19.22–36.10) | 30.9 (20.06–48.50)a,b | 0.001 | |
| Sex (n, %) | |||||
| Male | 30 (60.00) | 11 (39.29) | 40 (61.54) | ||
| 0.071 | |||||
| Female | 20 (40.00) | 17 (60.71) | 25 (38.46) | ||
| Death n (%) | No | - | 28 (100)a | 32 (49.23)a,b | 0.001 |
| Yes,Male | - | - | 20 (30.77)a,b | ||
| Yes, Female | - | - | 13 (20.0)a,b | ||
| Comorbidity, n (%) | |||||
| Systemic hypertension | No | 32 (64.00) | 26 (92.90)a | 26 (40.00)a,b | <0.001 |
| Yes | 18 (36.00) | 2 (7.10)a | 39 (60.00)a,b | ||
| Congestive heart failure | No | - | 28 (100) | 52 (80.00)a,b | 0.011 |
| Yes | - | - | 13 (20.00)a,b | ||
| Diabetes mellitus | No | 43 (85.00) | 28 (100) | 44 (67.70)a,b | 0.001 |
| Yes | 7 (15.00) | - | 21 (32.30)a,b | ||
| Obesity | No | - | 28 (100) | 53 (81.50)a,b | 0.015 |
| Yes | - | - | 12 (18.50)a,b | ||
| COPD | No | - | 28 (100) | 54 (83.10)a,b | 0.02 |
| Yes | - | - | 11 (16.90)a,b | ||
Values represented as median (interquartile range) or n (%). Results analyzed by Kruskal-Wallis or Pearson's chi-square test. Statistical significance was set at P < 0.05. BMI, body mass index; COPD, chronic obstructive pulmonary disease.
P < 0.05, compared to control group.
P < 0.05, comparing the out and inpatient groups.
The mean age of the outpatients was lower than that of inpatients and the control group. The inpatient group had the highest weight and BMI, corresponding to grade I obesity. There was no significant difference between the heights of the three analyzed groups. Inpatients had a higher mortality rate, predominantly among men than other groups. The outpatients and the control group experienced zero fatalities. Hypertension (39/65) and diabetes mellitus (21/65) were the most common comorbidities among inpatients, and arterial hypertension was the only observed comorbidity (2/28) among outpatients. In the control group, hypertension (18/50) and diabetes mellitus (7/50) were the observed comorbidities.
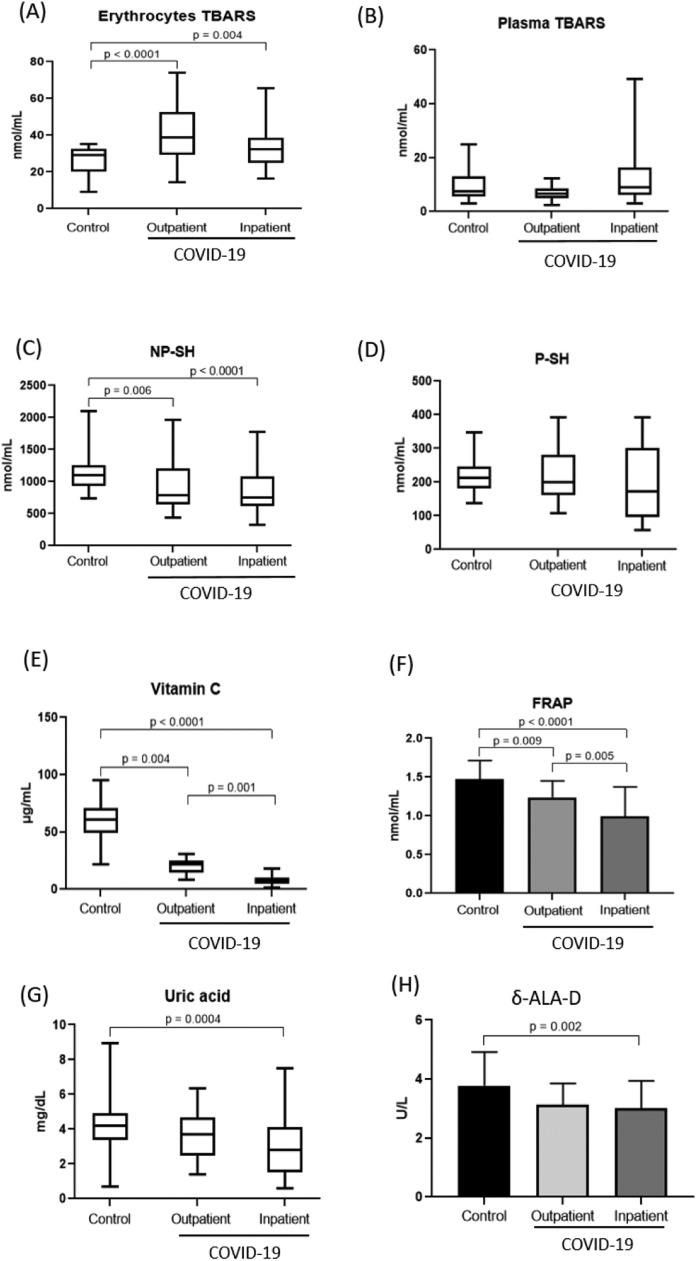
Fig. 1 shows the oxidative stress markers and the activity of the δ-ALA-D enzyme and their relationships with the three groups. TBARS in erythrocytes, an oxidative damage marker, were significantly higher in the outpatient and inpatient groups than in the control group and did not differ between the outpatient and inpatient groups. Regarding antioxidant defenses, NP-SH, Vitamin C, FRAP and Uric acid were significantly lower in the infected patients than in the control group, and Vitamin C and FRAP are significantly reduced with disease worsening in the inpatient group compared with the outpatient group. The activity of the δ-ALA-D enzyme was lower in patients with moderate/severe COVID-19 (3.00 ± 0.93), but not in the outpatients (3.13 ± 0.70), when compared to the control group (3.77 ± 1.14), and there was no significant difference between the outpatient and inpatient groups. No significant differences were found in plasma TBARS and P-SH between the groups.
Fig. 1.
Oxidative stress markers in the groups: Erythrocyte TBARS (A), Plasma TBARS (B), NP-SH (C), P-SH (D), Vitamin C (E), FRAP (F), Uric acid (G) and Enzyme activity of δ-ALA-D (H) and their relationships with the three groups. Nonparametric results were determined by Kruskal-Wallis and represented as median (interquartile range). Parametric results were determined by ANOVA followed by Tukey's test and represented as mean standard deviation (SD). Values with P < 0.05 were considered statistically significant. P-SH: protein thiol groups; NP-SH: non-protein thiol groups; FRAP: ferric reduction ability of plasma; TBARS: thiobarbituric acid reactive substance and δ -ALA-D: delta-aminolevulinate dehydratase.
Table 2 shows a negative Spearman's correlation between Vitamin C and FRAP with COVID-19 and a positive correlation between FRAP and Vitamin C, indicating that antioxidant defenses decrease as disease severity increases.
Table 2.
Correlations of oxidative stress parameters and disease severity in COVID-19 patients.
| Correlations | Spearman r | P value |
|---|---|---|
| TBARS plasma X COVID-19 | 249 | 0.020 |
| Vitamin C X COVID-19 | -648 | 0.00 |
| FRAP X COVID-19 | -370 | 0.001 |
| FRAP X Vitamin C | 315 | 0.005 |
Correlation of data performed using Spearman correlation coefficient (n = 93). Values of P < 0.05 were considered statistically significant.
FRAP = ferric reduction ability of plasma; TBARS = thiobarbituric acid reactive substance.
4. Discussion
The carried out study detected, in an unprecedented way, a decrease in the activity of the δ-ALA-D enzyme in patients with moderate/severe COVID-19 when compared to the control group. Antioxidant defenses (NP-SH, Vitamin C, FRAP e Uric acid) also showed a decrease in out and in patients groups, while lipid peroxidation was shown to be increased, representing a data of greater oxidative stress in patients with COVID-19. FRAP and Vitamin C decreased with evolution of the disease, thus, oxidative stress in clinical practice is an indicator of deterioration of clinical condition.
COVID-19 is a new disease associated with high morbidity and mortality rates [20]. COVID-19 patients often present with mild symptoms such as cough, sore throat, and fever; however, patients also present with severe forms of the disease known as severe acute respiratory syndrome (SARS), which is associated with a high mortality rate [21]. The geriatric population, pregnant women, indigenous populations, and patients with comorbidities are further predisposed to the infection due to lowered immune defenses and are thus considered a high-risk group [22,23]. In this context, our study showed (Table 1) that older adults with high BMI, especially obese patients and those with chronic diseases such as hypertension, diabetes mellitus, heart failure, and chronic lung disease, develop more severe forms of the disease and often require admission to the ICU.
Our study also demonstrated that patients in the inpatient group had a high mortality rate of approximately 50%, and the majority were men. Bayrak et al. [20] also found high mortality rates of patients in the ICU (62.8%) and the predominance of deaths among men.
The oxidative stress relationship, which is the loss of balance between pro-oxidants and antioxidants, has been linked to several diseases such as cancer, diabetes, hypertension, and viral infections, including respiratory diseases [24], [25], [26], [27]. Some studies have already demonstrated the association between oxidative stress and SARS-CoV-2 infection and its complications [21,23].
TBARS was used to evaluate the peroxidation of lipids present in cell membranes of erythrocytes. This technique is based on the reaction between malondialdehyde (MDA) with thiobarbituric acid and is considered a good marker of oxidative stress in clinical conditions [28,29]. Our results showed that TBARS levels in erythrocytes were higher in COVID-19 patients than in the control group, showing the existence of an oxidative imbalance in these patients. There was no significant difference in erythrocyte TBARS levels between outpatients and inpatients. In Muhammad et al.’s 2020 study, higher levels of MDA were found in COVID-19 patients than in the control group, indicating overproduction of free radicals.
The continuous generation of free radicals leads to the development of antioxidant defense mechanisms to limit the intracellular levels of such reactive species and control the occurrence of resulting damage [7]. The thiol groups (-SH or sulfhydryl) plays an important role because of its potential for chelation, reduction, and protection against damage caused by oxidative stress [30]. In our study, there was a decrease of (NP-SH) in patients with COVID-19. Vitamin C donates electrons to ROS, acting as a reducing agent, thus destroying them before they react with lipoproteins and membranes and, in turn, preventing lipid peroxidation and reducing oxidative stress [31]. The FRAP technique tests the antioxidant strength of a sample in reducing ferric to ferrous ions [32]. The main advantage of the test is that it represent the antioxidant capacity of all compounds present in a biological sample, whether endogenous or exogenous, and not an isolated compound [32]. Our study showed a decrease in the antioxidant defenses of COVID-19 patients compared with the control group, with the lowest levels of Vitamin C and FRAP, associated with the severity of the disease, showing statistical significance between the inpatient and outpatient group, and both with the control group. These data are confirmed by the correlation study presented in Table 2, where a negative correlation is observed between Vitamin C and COVID-19 as well as between FRAP and COVID-19, proving that as the severity of the disease increases, there is a decrease in antioxidant defenses. A positive correlation between FRAP and Vitamin C was observed, both markers decrease as disease severity increases. In a pilot study conducted by Arvinte et al. [33], among critically ill COVID-19 patients hospitalized in Colorado, older age and low Vitamin C levels appeared to be co-dependent risk factors for COVID-19-associated mortality.
Uric acid (AU) is a hydrophilic antioxidant, being generated during the metabolism of purine nucleotides, being frequently associated with renal dysfunction, however, it is also considered a marker of tissue damage and oxidative stress [34,32]. Its antioxidant effect is due to the fact that AU is able to inhibit the action of free radicals on organic molecules, creating stable complexes with iron ions, inhibiting the Fenton and Haber-Weiss reaction, thus preventing the formation of the hydroxyl radical. This is considered the most important antioxidant effect of AU, however, it is also related to the improvement of endothelial function, by preventing the loss of NO in endothelial cells exposed to peroxynitrite, a potentially harmful oxidant [35], [36], [37]. In the study by Lucca et al. [32]. AU was related to tissue damage and oxidative stress in women with preeclampsia. Our study shows a higher AU value in the control group, and a reduction in antioxidant defenses was observed as the severity of the disease progresses, with the lowest AU value in patients admitted to the COVID-19 ICU.
Regarding the study of the activity of δ-ALA-D, which is an enzyme that contains sulfhydryl groups in its structure, it is sensitive to pro-oxidant elements, causing changes in the synthesis of the heme group of hemoglobin, thus being inhibited in situations of oxidative stress; therefore, the enzyme can be suggested as an indirect marker of oxidative stress [38,39]. To our knowledge, the activity of the δ-ALA-D enzyme has not been studied in COVID-19 patients. In our study, the activity of the δ-ALA-D enzyme was lower in patients with moderate/severe COVID-19, but not in outpatients, when compared with the control group and there was no significant difference between the outpatient and inpatient groups. In the control group the enzyme was more active, and the oxidative stress in this group was lower. A decrease in enzyme activity was observed as the disease severity advanced, with the lowest enzyme value found in patients who were hospitalized in the ICU with moderate/severe COVID-19, proving the presence of more free radicals in this group, which were able to decrease the enzyme activity.
Our study had some limitations, such as the low number of participants, mainly in the nonhospitalized group and the variation in the diet of participants.
5. Conclusion
Geriatric male patients with comorbidities were more likely to develop severe COVID-19, requiring hospital intervention. The mortality rates among this group were also relatively higher. Furthermore, the activity of the δ-ALA-D enzyme was lower in patients with moderate/severe COVID-19, than in the control group. Antioxidant defenses also showed a decrease in out and inpatients groups, while lipid peroxidation was shown to be increased. FRAP and Vitamin C decrease with worsening of the disease, thus, oxidative stress in clinical practice is an indicator of deterioration of clinical condition.
Author Contributions
Protocol and Project Development: Andressa de Azambuja Pias Weber and Thissiane de Lima Gonçalves. Data collection or Management: Andressa de Azambuja Pias Weber, Jovana Simonetti Bulegon, Manoela Dias de Souza, Silmara Ana Vendrame and Larissa Venturini. Data Analysis: Andressa de Azambuja Pias Weber, Jovana Simonetti Bulegon, Wendel Mombaque dos Santos and Thissiane de Lima Gonçalves. Manuscript Editing and Writing: Andressa de Azambuja Pias Weber, Jovana Simonetti Bulegon, Manoela Dias de Souza, Silmara Ana Vendrame, Larissa Venturini, Wendel Mombaque dos Santos and Thissiane de Lima Gonçalves.
Acknowledgments
Acknowledgments
The authors thank all of the volunteers who participated in this study. Additionally, we thank the University Hospital of Santa Maria (HUSM), Rio Grande do Sul, Brazil, for support in this study.
Funding
This work was supported by the Coordination of Improvement of Higher Level Personnel (CAPES) and the Graduate Support Program (PROAP).
Disclosures
The authors reported no potential conflict of interest.
References
- 1.Ciotti M, Angeletti S, Minieri M, Giovannetti M, Benvenuto D, Pascarella S, et al. COVID-19 outbreak: an overview. Chemotherapy. 2019;64(5–6):215–223. doi: 10.1159/000507423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn DG, Shin HJ, Kim MH, Lee S, Kim HS, Myoung J, et al. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19) J Microbiol Biotechnol. 2020;30(3):313–324. doi: 10.4014/jmb.2003.03011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shanmugaraj B, Siriwattananon K, Wangkanont K, Phoolcharoen W. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for coronavirus disease-19 (COVID-19) Asian Pac J Allergy Immunol. 2020;38(1):10–18. doi: 10.12932/AP-200220-0773. [DOI] [PubMed] [Google Scholar]
- 4.Artal CFJ. Neurological complications of coronavirus and COVID-19. Rev Neurol. 2020;70(9):311–322. doi: 10.33588/rn.7009.2020179. [DOI] [PubMed] [Google Scholar]
- 5.Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sales-Peres SHC, de Azevedo-Silva LJ, Bonato RCS, Sales-Peres MC, Pinto ACS, Santiago Junior JF. Coronavirus (SARS-CoV-2) and the risk of obesity for critically illness and ICU admitted: meta-analysis of the epidemiological evidence. Obes Res Clin Pract. 2020;14(5):389–397. doi: 10.1016/j.orcp.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012;10:49. doi: 10.1186/1477-7827-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halliwell B, Gutteridge JMC. Vol. 3. Oxford University Press, Oxford; 1999. (Free Radical in Biology & Medicine). [Google Scholar]
- 9.Salim S. Oxidative stress and the central nervous system. J Pharmacol Exp Ther. 2017;360(1):201–205. doi: 10.1124/jpet.116.237503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muhammad Y, Kani YA, Iliya S, Muhammad JB, Binji A, Ahmad AE, et al. Deficiency of antioxidants and increased oxidative stress in COVID-19 patients: a cross-sectional comparative study in Jigawa, Northwestern Nigeria. SAGE Open Med. 2021:9. doi: 10.1177/2050312121991246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zendelovska D, Atanasovska E, Petrushevska M, Spasovska K, Stevanovikj M, Demiri J et-al. Evaluation of oxidative stress markers in hospitalized patients with moderate and severe COVID-19. Rom J Intern Med. 2021;59(4):375–383. doi: 10.2478/rjim-2021-0014. [DOI] [PubMed] [Google Scholar]
- 12.NHI. National Institutes of Health. COVID-19 treatment guidelines panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available at: https://www.covid19treatmentguidelines.nih.gov/. Accessed 1 Aug 2022.
- 13.WHO. World Health Organization (2021). Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: Interim guidance. 13 March 2020. Reference number: WHO/2019-nCoV/Clinical/2020.
- 14.Lapenna D, Ciofani G, Pierdomenico SD, Giamberardino MA, Cuccurulho F. Reaction conditions affecting the relationship between thiobarbituric acid reactivity and lipid peroxides in human plasma. Free Radic Biol Med. 2001;31(3):331–335. doi: 10.1016/s0891-5849(01)00584-6. [DOI] [PubMed] [Google Scholar]
- 15.Boyne AF, Ellman GL. A methodology for analysis of tissue sulfhydryl components. Anal Biochem. 1972;46(2):639–653. doi: 10.1016/0003-2697(72)90335-1. [DOI] [PubMed] [Google Scholar]
- 16.Jacques-Silva MC, Nogueira CW, Broch LC, Flores EM, Rocha JBT. Diphenyl diselenide and ascorbic acid changes deposition of selenium and ascorbic acid in liver and brain of mice. Pharmacol Toxicol. 2001;88(3):119–125. doi: 10.1034/j.1600-0773.2001.d01-92.x. [DOI] [PubMed] [Google Scholar]
- 17.Galley HF, Davies MJ, Webster NR. Ascorbyl radical formation in patients with sepsis: effect of ascorbate loading. Free Radic Biol Med. 1996;20(1):139–143. doi: 10.1016/0891-5849(95)02022-5. [DOI] [PubMed] [Google Scholar]
- 18.Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 19.Berlin A, Schaller KH. European standardized method for the determination of delta-aminolevulinic acid dehydratase activity in blood. Z Klin Chem Klin Biochem. 1974;12(8):389–390. [PubMed] [Google Scholar]
- 20.Bayrak V, Durukan NS, Aydemir FD, Ergan B, Gezer NS, Kutsoylu OOE, et al. Risk factors associated with mortality in intensive care COVID-19 patients: the importance of chest CT score and intubation timing as risk factors. Turk J Med Sci. 2021;51(4):1665–1674. doi: 10.3906/sag-2101-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chernyak BV, Popova EN, Prikhodko AS, Grebenchikov OA, Zinovkina LA. Zinovkin RA. COVID-19 and oxidative stress. Biochemistry (Mosc) 2020;85(12):1543–1553. doi: 10.1134/S0006297920120068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laforge M, Elbim C, Frère C, Hémadi M, Massaad C, Nussud P, et al. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat Rev Immunol. 2020;20(9):515–516. doi: 10.1038/s41577-020-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonçalves TL, Erthal F, Corte CL, Müller LG, Piovezan CM, Nogueira CW, et al. Involvement of oxidative stress in the pre-malignant and malignant states of cervical cancer in women. Clin Biochem. 2005;38(12):1071–1075. doi: 10.1016/j.clinbiochem.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Rodrigues F, de Lucca L, Neme WS, Gonçalves TL. Influence of gestational diabetes on the activity of δ-aminolevulinate dehydratase and oxidative stress biomarkers. Redox Rep. 2018;23(1):63–67. doi: 10.1080/13510002.2017.1402981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonfanti G, Ceolin RB, Valcorte T, Bona KD, Lucca L, Gonçalves TL, et al. δ-aminolevulinate dehydratase activity in type 2 diabetic patients and its association with lipid profile and oxidative stress. Clin Biochem. 2011;44(13):1105–1109. doi: 10.1016/j.clinbiochem.2011.06.980. [DOI] [PubMed] [Google Scholar]
- 27.Khomich OA, Kochetkov SN, Bartosch B, Ivanov AV. Redox biology of respiratory viral infections. Viruses 2018;10(8):392. 10.3390/v10080392. [DOI] [PMC free article] [PubMed]
- 28.Ayala A, Munoz MF, Arguelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014 doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahal A, Kumar A, Singh V, Yadav B, Tiwari R, Chakraborty S, et al. Oxidative stress, prooxidants, and antioxidants: the interplay. BioMed Res Int. 2014;2014:761264. doi: 10.1155/2014/761264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Guan X. Rapid and thiol-specific high-throughput assay for simultaneous relative quantification of total thiols, protein thiols, and nonproteinthiols in cells. Anal Chem. 2015;87(1):649–655. doi: 10.1021/ac503411p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boni A, Pugliese C, Cláudio CC, Patin RV, Oliveira FLC, et al. Vitaminas antioxidantes e prevenção da arteriosclerose na infância. Rev Paul Pediatr. 2010;28(4):373–380. doi: 10.1590/S0103-05822010000400014. [DOI] [Google Scholar]
- 32.Lucca L, Jantsch LB, Vendrame SA, Stein CS, Klein VCG, Soares KB et al. Longitudinal study of delta-aminolevulinate dehydratase activity and oxidative stress profile in healthy pregnant women. Biomolecules. 2019;9(1):18 doi: 10.3390/biom9010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arvinte C, Singh M, Marik PE. Serum levels of vitamin C and vitamin D in a cohort of critically Ill COVID-19 patients of a North American community hospital intensive care unit in May 2020: a pilot study. Med Drug Discov. 2020;8 doi: 10.1016/j.medidd.2020.100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moussa Z, Zaher MA, Saleh A. Nonenzymatic exogenous and endogenous antioxidants. Free Radic Med Biol. 2019;1:11–22. doi: 10.5772/intechopen.87778. [DOI] [Google Scholar]
- 35.Lobo V., Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 2010;4(8):118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soltani Z, Rasheed K, Kapusta DR, Reisin E. Potential role of uric acid in metabolic syndrome, hypertension, kidney injury, and cardiovascular diseases: is it time for reappraisal? Curr Hypertens Rep. 2013;15(3):175–181. doi: 10.1007/s11906-013-0344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanďár R, Štramová X, Drábková P, Krenková J. A monitoring of allantoin, uric acid, and malondialdehyde levels in plasma and erythrocytes after ten minutes of running activity. Physiol Res. 2014;63(6):753–762. doi: 10.33549/physiolres.932696. [DOI] [PubMed] [Google Scholar]
- 38.Valentini J, Grotto D, Paniz C, Roehrs M, Burg G, Garcia SC. The influence of the hemodialysis treatment time under oxidative stress biomarkers in chronic renal failure patients. Biomed Pharmacother. 2008;62(6):378–382. doi: 10.1016/j.biopha.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 39.Rocha JBT, Saraiva RA, Garcia SC, Gravina FS. Nogueira CW Aminolevulinate dehydratase (δ-ALA-D) as marker protein of intoxication with metals and other pro-oxidant situations. Toxicol Res. 2012;1(2):85–102. doi: 10.1039/C2TX20014G. [DOI] [Google Scholar]