Abstract
Preventive measures to reduce infection are needed to combat the COVID-19 pandemic and prepare for a possible endemic phase. Current prophylactic vaccines are highly effective to prevent disease but lose their ability to reduce viral transmission as viral evolution leads to increasing immune escape. Long-term proactive public health policies must therefore complement vaccination with available nonpharmaceutical interventions aiming to reduce the viral transmission risk in public spaces. Here, we revisit the quantitative assessment of airborne transmission risk, considering asymptotic limits that considerably simplify its expression. We show that the aerosol transmission risk is the product of three factors: a biological factor that depends on the viral strain, a hydrodynamical factor defined as the ratio of concentration in viral particles between inhaled and exhaled air, and a face mask filtering factor. The short-range contribution to the risk, present both indoors and outdoors, is related to the turbulent dispersion of exhaled aerosols by air drafts and by convection (indoors), or by the wind (outdoors). We show experimentally that airborne droplets and CO2 molecules present the same dispersion. As a consequence, the dilution factor, and therefore the risk, can be measured quantitatively using the CO2 concentration, regardless of the room volume, the flow rate of fresh air, and the occupancy. We show that the dispersion cone leads to a concentration in viral particles, and therefore a short-range transmission risk, inversely proportional to the squared distance to an infected person and to the flow velocity. The aerosolization criterion derived as an intermediate result, which compares the Stokes relaxation time to the Lagrangian time-scale, may find application for a broad class of aerosol-borne pathogens and pollutants.
Keywords: COVID-19, SARS-CoV-2, carbon dioxide, infection risk
Significance Statement.
Making rational public health policy decisions to prevent the dominant routes of viral transmission requires a simple but quantitative assessment of the airborne transmission risk in public places, both indoors and outdoors. Here, we show that CO2 and aerosol viral particles disperse following the same law, which allows us to relate this aerosol transmission risk to the CO2 concentration. The results provide quantitative guidance useful to complement vaccination, treatment for the vulnerable patient population, and the test–trace–isolate strategy by a national ventilation plan, and gradual mandates of FFP2 respirators in indoor places, when the epidemic circulates above a threshold.
Introduction
The SARS-CoV-2 pandemic enters its third year despite the design of highly effective vaccines, which induce circulating antibodies and systemic T- and B-cell responses that block viral spread and disease. Besides the lack of vaccination at the global scale, they do not establish immunity at mucosal surfaces against infection by variants such as Omicron (B.1.1.529), which present both increased intrinsic transmissibility and viral immune evasion. Widespread transmission therefore contributes to a degree of unpredictability in the evolution of the pandemic. As a consequence, vaccination must be complemented in the long term by effective public health policies contributing to suppress transmission at a low economic and social cost. To help making rational public health policy decisions, we propose here a method to measure the risk for long- and short-range airborne transmission of SARS-CoV-2, both indoors and outdoors.
Respiratory viruses and bacteria can be transported by droplets emitted by coughing or sneezing, which may cause symptomatic transmission, and during expiratory human activities such as breathing, speaking, or laughing, which may cause asymptomatic and presymptomatic transmission. Pathogens responsible for illnesses such as influenza, tuberculosis, measles, or SARS, initially carried by these droplets, can form an aerosol phase (1, 2) and cause airborne transmission by inhalation. The silent spread of SARS-CoV-2 by asymptomatic infected individuals, who do not cough nor sneeze, has been hypothesized as early as January 2020. Since June 2020, there is ample evidence that this virus is primarily transmitted through aerosols (3–6). This is particularly obvious in public places where face mask wearing has been mandatory (7) as the heavier, millimeter-sized droplets have a ballistic trajectory that is relatively insensitive to the presence of air and are stopped by all types of masks.
Many misconceptions regarding aerosols can be found in the medical and scientific literature. Clinicians often use an incorrect definition, still reported by the World Health Organization: aerosols would be particles smaller than 5 μm that settle slowly enough to be transported over a few meters (6, 8). As a consequence, problematic criteria are introduced, such as the distance after which a particle launched with an initial velocity in a fluid at rest stops (9), or the settling time of a single particle dropped from head height in still air (10). An aerosol is a locally homogeneous phase constituted of solid or liquid particles suspended in a gas, either by thermal fluctuations (for very small particles) or by turbulent fluctuations. The aerosol phase tends to homogenize and to diffuse over the whole available space by turbulent dispersion. The “slow settling” misconception therefore omits the fundamental constitutive mechanism of aerosols of any particle size: turbulence.
The infection risk has been modeled in a series of papers, starting from the seminal works of Wells and Riley (11, 12), which parametrize the infection risk as a function of the global air dilution and the disease infectiousness. The authors work upon the well-mixed hypothesis, in which air inside a room is instantly mixed by turbulence so that all people inside breathe the same air. Beyond this hypothesis, fluid dynamics models of indoor air circulation (13–17) and heterogeneous airflows produced by respiratory activity have been introduced (18–20). The Wells–Riley model has been extended to unsteady conditions by Rudnick and Milton (21), who have pointed out that CO2 could be used as a risk proxy (22). Regarding SARS-CoV-2, most models extend the Wells–Riley equation to account for unsteady conditions in ventilation or occupancy (23, 24) and to different viral emission rates depending on the respiratory activity (25), face coverings, and particle removal and inactivation (26–28). All of these models use well-characterized “super-spreading” events to derive estimates of the viral emission rate, or closed microsocieties such as cruise ships (29, 30).
These models have left three problems open up to now. (i) Can an analytical closed formula be derived, simple enough to be used to produce regulatory ventilation standards? (ii) Is the dispersion of CO2 and airborne viral particles governed by the same law? (iii) What is the law governing the short-range contribution to the transmission, both indoor sand outdoor?s Here, we propose a definition of the environmental risk of viral transmission in public spaces such as schools, offices, university lecture halls, museums, or shopping centers, but also outdoors. We report experimental and theoretical results showing that exhaled CO2 and airborne viral particles diffuse at the same rate. We finally derive a quantitative analytical model that relates this risk to the CO2 concentration.
Airborne transmission mechanism
The main entry of SARS-CoV-2 virus is through the upper respiratory epithelium. To colonize a cell, an inhaled viral particle interacts through the spike protein—which is cleaved by a host cell protease, mostly the TMPRSS2 protease for the wild Wuhan-1 strain—with a host cell membrane protein, the ACE2 receptor. Cleavage of the spike protein is necessary for a conformational change so that it can effectively interact with the ACE2 receptor. This interaction leads to the formation of a virus–ACE2 complex that triggers the internalization of the virus inside the cell. It replicates its RNA molecule and produces the proteins required for self-assembly of new viral particles, which are released, leading to the colonization of neighboring cells (33).
An organism may become infected if a sufficient amount of viral particles interact with cells expressing both the TMPRSS2 protease and the ACE2 receptor and if the virus is able to hack into cellular mechanisms to produce and disseminate new virions. From the upper respiratory epithelium, which is the first tissue to be infected (nasal epithelium for viral strains before Omicron, but also throat epithelium for Omicron, due to a weaker dependency to the TMPRSS2 protease), the virus, embedded in mucus, is carried to the trachea, to the lungs or the esophagus, and finally to deeper organs. It has also the possibility to reach the brain and to colonize certain cells of the cortex.
When the virus is concentrated in the nasal cavity, it is disseminated via a mist of fine droplets of mucus or saliva dispersed by breathing, talking or singing. A sneeze or cough produces larger droplets containing viral particles (Figure S3). The evaporation of mucosalivary liquid droplets in the air is controlled by the ambient relative humidity RH and by its content in surfactants, proteins, and electrolytes. Initially, transport of water molecules from the droplets to the surrounding air is diffusive so that the drop squared radius decreases linearly in time. After a very short time, the droplet stabilizes at an equilibrium radius at which the viral particle is surrounded by proteins and water (10, 34–37). The influence of droplet chemical composition on the equilibrium radius is still poorly understood (38–40). The radius shrinks by a factor two to five (see Supplementary Material): droplets emitted during breathing, below 20 μm, are therefore submicronic or micronic and stay suspended in the air.
The transmission risk increases with the intake viral dose d, defined as the amount of infectious viral particles inhaled by a person, cumulated over time. d increases with the time of exposure to the virus, with the inhalation rate qe, i.e. the product of the breathing rate by the tidal volume (for light exercise, qe ≃ 0.5 m3/h), and with the concentration Ci of infectious viral particles in the inhaled air. As a dose is a quantity of virus, it can be measured using quantitative RT-PCR and is then expressed in genome units (GU). However, it is better adapted to measure a dose by infecting a culture cell monolayer, in plaque-forming units (PFU). PEU measure the ability of viral particles to replicate and to be secreted by the chosen cell type, while qRT-PCR measures the number of RNA molecules and is not sensitive to replication potential. On generic Vero cells, the amount of virus needed on average to form one cell lysis has decreased from 1400 GU/PFU for the wild strain to 240 GU/PFU for the Delta variant (41).
The inhaled dose d is the product of two factors:
a purely biological factor reflecting the exhalation flux of viral particles and the ability of the virus to infect a person;
a purely physical factor ϵ reflecting the dilution of viral particles between exhalation and inhalation.
We will investigate these two factors independently in the next sections.
Dispersion and transport of CO2 and viral particles
Average concentration
In a public space where many people are gathered, let us single out two people: one is infectious and exhales viral particles and the second one inhales the air. The air exhaled by the infectious person is concentrated in viral particles, which are gradually diluted in the ambient air, in a way similar to the smoke of a cigarette. Outdoors, far from an infected person, the viral particle concentration vanishes. However, indoors, viral particles accumulate: the concentration in the wake of an infected person decays from its exhalation concentration Ce, which is high, to a constant concentration 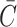 far away. The dilution factor ϵ is defined as the ratio of the viral concentration Ci in the inhaled air and the viral concentration in exhaled air Ce (Fig. 1). First, consider a closed room of volume V where there is a single person exhaling viral particles at a concentration Ce. A ventilation of flow rate Q replaces exhaust air at the average concentration
far away. The dilution factor ϵ is defined as the ratio of the viral concentration Ci in the inhaled air and the viral concentration in exhaled air Ce (Fig. 1). First, consider a closed room of volume V where there is a single person exhaling viral particles at a concentration Ce. A ventilation of flow rate Q replaces exhaust air at the average concentration 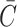 by fresh air. The average concentration
by fresh air. The average concentration 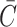 obeys the conservation equation:
obeys the conservation equation: 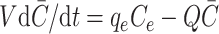 . It is a linear relaxation equation whose exponential relaxation time is V/Q. The average dilution factor tends towards the steady-state solution
. It is a linear relaxation equation whose exponential relaxation time is V/Q. The average dilution factor tends towards the steady-state solution 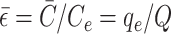 (21).
(21).
Fig. 1.
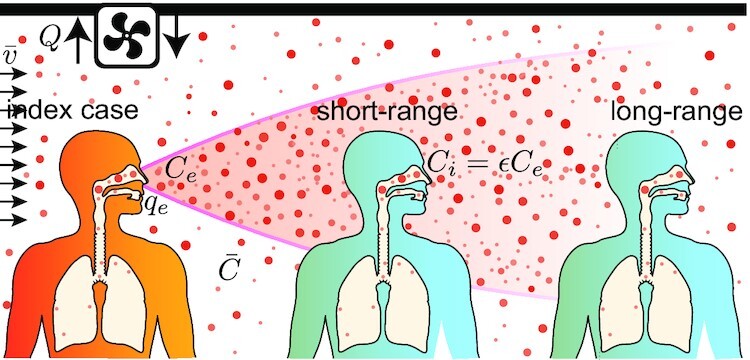
Viral particles are exhaled by an infected person at a concentration Ce. They are dispersed by drafts (velocity  ), which lead to a decrease of their concentration. The dispersion cone must not be confused with the conical shape of high-speed jets in a fluid at rest (19, 31, 32). In an indoor space, viral particles are stored, which translates into a background homogeneous concentration
), which lead to a decrease of their concentration. The dispersion cone must not be confused with the conical shape of high-speed jets in a fluid at rest (19, 31, 32). In an indoor space, viral particles are stored, which translates into a background homogeneous concentration  , controlled by the ventilation rate Q.
, controlled by the ventilation rate Q.
Dispersion
We have investigated experimentally and theoretically the turbulent dispersion of viral particles and CO2 in a turbulent flow of mean velocity 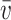 , characterized by a root mean square velocity σV. This corresponds to a generic situation where horizontal drafts (indoors) or wind (outdoors) dominate over thermal plumes, so that natural convection can be neglected. We have, for instance, shown in a recent paper that this was the case in the corridors of commercial malls (42). The dispersion of CO2 and of oil droplets of typical size 10 μm is studied separately, but in the same conditions, in the wind tunnel schematized in Fig. 2B. The smoke concentration field is measured using high-resolution pictures (7380 × 4920) with a 2 s exposure time (Fig. S1). The CO2 concentration profile
, characterized by a root mean square velocity σV. This corresponds to a generic situation where horizontal drafts (indoors) or wind (outdoors) dominate over thermal plumes, so that natural convection can be neglected. We have, for instance, shown in a recent paper that this was the case in the corridors of commercial malls (42). The dispersion of CO2 and of oil droplets of typical size 10 μm is studied separately, but in the same conditions, in the wind tunnel schematized in Fig. 2B. The smoke concentration field is measured using high-resolution pictures (7380 × 4920) with a 2 s exposure time (Fig. S1). The CO2 concentration profile 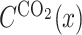 along the axis is measured after careful sampling of air using a syringe: the air is transferred into a chamber where a vacuum has been set, equipped with a CO2 nondispersive infrared sensor (Fig. S2).
along the axis is measured after careful sampling of air using a syringe: the air is transferred into a chamber where a vacuum has been set, equipped with a CO2 nondispersive infrared sensor (Fig. S2).
Fig. 2.
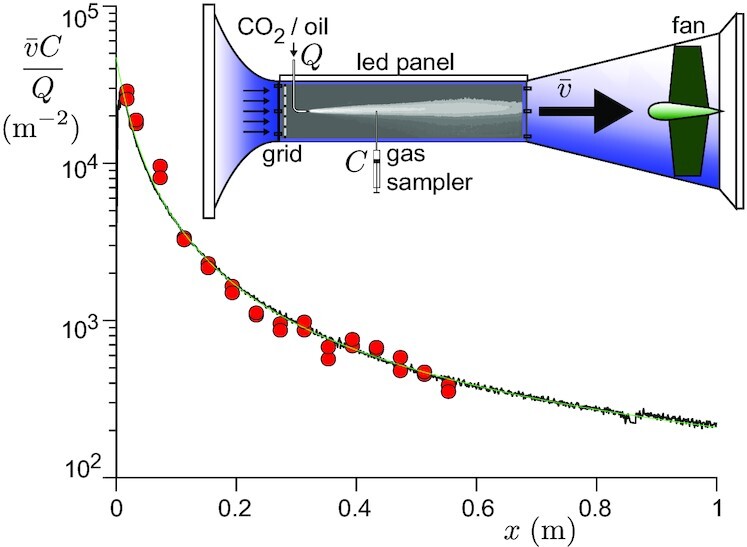
Rescaled concentration profile of 10-μm-size oil droplets (black solid line) and CO2 (red circles) in a wind tunnel experiment. The CO2 concentration along the axis is measured after sampling the gaz with a syringe. The oil droplet concentration is measured using the optical absorption of light. CO2 and oil aerosol are introduced successively at a rate Q, 20 cm downstream of the turbulence generating grid. The working velocity is 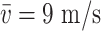 . The green line is the best fit by Eqs. (1) and (2).
. The green line is the best fit by Eqs. (1) and (2).
As dispersion is slow compared to the axial convection, the axial coordinate x is equivalent to time 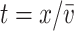 . Transverse diffusion is controlled by random motion and leads, according to the central limit theorem, to a quasi-Gaussian radial profile of concentration (42). As the flux across a transverse section is equal to the source emission rate qeCe, the concentration field takes the form
. Transverse diffusion is controlled by random motion and leads, according to the central limit theorem, to a quasi-Gaussian radial profile of concentration (42). As the flux across a transverse section is equal to the source emission rate qeCe, the concentration field takes the form
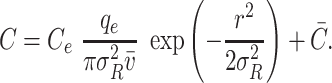 |
(1) |
The measurement of the dispersion radius σR as a function of x gives the law of spatial decay of the concentration.
Because of turbulence, the velocity of an elementary volume of fluid is correlated along its Lagrangian trajectory. This drives transported particle velocities to be correlated too. We model the dispersion as a Langevin process characterized by an exponentially decaying Lagrangian correlation function: 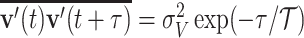 , where
, where 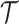 is the Lagrangian integral time-scale. The dispersion of fluid particles injected at a source point at time t = 0 is given by Taylor’s theorem, which gives at time
is the Lagrangian integral time-scale. The dispersion of fluid particles injected at a source point at time t = 0 is given by Taylor’s theorem, which gives at time 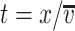 (42)
(42)
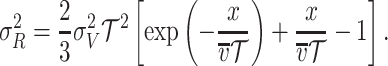 |
(2) |
At large distances 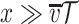 , it predicts a diffusive regime
, it predicts a diffusive regime 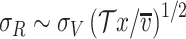 , the concentration along the axis (r = 0) decaying as 1/x. The most important dispersion takes place at short distances
, the concentration along the axis (r = 0) decaying as 1/x. The most important dispersion takes place at short distances 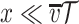 . Transport obeys a ballistic-like regime of the form
. Transport obeys a ballistic-like regime of the form 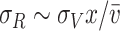 , which corresponds to a fast decay of the concentration along the axis (r = 0) as
, which corresponds to a fast decay of the concentration along the axis (r = 0) as
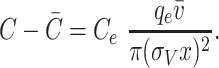 |
(3) |
Face masks and respirators
In public places where face masks are mandatory, the aerodynamical contribution to ϵ must be multiplied by the product of the exhalation and inhalation mask filtration factors. All types of masks totally filter droplets of a fraction of millimeter. However, they have very different efficiencies between 0.1 and 0.5 μm, which is the range of equilibrium sizes of the smallest mucus droplets after evaporation. Mask efficiency presents a minimum around 0.3 μm, where the particles are large enough to make Brownian collection inefficient, and tiny enough to have small inertia, insufficient to make them leave the streamlines wrapping around the fibers (43). Filtration factors are determined by the material properties and the respiratory activity at play, but most importantly by proper mask fit. Cloth and surgical masks tend to be much looser fitted than respirators, thereby greatly reducing their filtration factor compared to what their fabric could achieve alone (44). Cloth masks have an effective filtration factor around λ = 0.70, on average (45, 46). Assuming they are well-fitted, surgical masks have an effective filtration factor λ = 0.28 (47, 48) and N95/FFP2 respirators λ = 0.10 (49, 50).
Modeling the transmission risk
Intake viral dose
Transmission risk assessment requires the estimate of the probability of infection under a given intake viral dose d. The simplest hypothesis is to assume an independent action of all inhaled replicable viral particles, which means that a single virus can initiate the infection. However, more than one is statistically needed, as the probability that a single infectious viral particle overwhelms the host immunity defences successfully is small, typically between 10−3 and 10−2 (41). For a person having inhaled an intake dose d, the probability law of infection p(d) takes the form
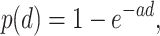 |
(4) |
where the susceptibility a is the inverse of the infection dose defined, for each individual, as the intake dose for which the probability of infection is 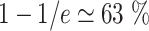 . a is widely distributed across individuals, according to a probability distribution f(a). We denote by
. a is widely distributed across individuals, according to a probability distribution f(a). We denote by 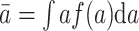 the population average of a.
the population average of a. 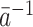 is the infectious dose, called the quantum of infection when used as a convenient unit for a quantity of infectious viral particles; the product
is the infectious dose, called the quantum of infection when used as a convenient unit for a quantity of infectious viral particles; the product 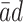 is then the dose expressed in “quanta.” For the wild strain Wuhan-1, the infectious quantum has been estimated around 5.6 × 105GU and 400 PFU (41). Importantly, the distribution f(a) includes the effect of vaccinal or infection induced immunization. The infectious quantum is indeed a population average over different immunity conditions.
is then the dose expressed in “quanta.” For the wild strain Wuhan-1, the infectious quantum has been estimated around 5.6 × 105GU and 400 PFU (41). Importantly, the distribution f(a) includes the effect of vaccinal or infection induced immunization. The infectious quantum is indeed a population average over different immunity conditions.
Relation between inhaled dose and viral exhalation rate
In the literature (15, 25, 26, 35, 51–53) devoted to airborne transmission of SARS-CoV-2, most authors have considered that the viral emission rate, i.e. the number of virus exhaled per unit time, is the product of the volume of mucus droplets emitted per unit time by the viral load in the nasal cavity for mucus droplets, in the throat for saliva droplets (28) or deeper in the lungs for respiratory aerosols. This excludes the possibility of a viral enrichment at the interface between mucosalivary fluid and air, which could lead to a viral content of droplets, which is not proportional to their initial volume.
Infectivity is proportional to the concentration of infectious viral particles in the exhaled air, noted Ce. It is assumed here to follow the same kinetics as the infectious viral load. It also depends on biological factors, which differ from one viral strain to the other. The viral kinetics results from the competition between viral replication and immune response. For simplicity, Ce can be assumed to present the same temporal profile amongst patients:
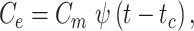 |
(5) |
where Cm is a characteristic concentration and tc the infection time. Both Ce and Cm are expressed in PFU/m3. The rescaled viral load curve ψ(t) is dimensionless and can be considered as the average over the subpopulation considered. Typically, ψ increases exponentially in the presymptomatic period and decreases exponentially at long times, as shown in Fig. 3 (54–56). We choose here the following normalization for ψ(t), which provides an unambiguous definition of the mean contagious time T
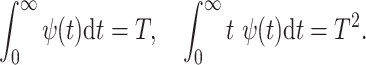 |
(6) |
The characteristic viral concentration Cm is highly variable among infected people. Moreover, it depends on the vaccination status, on former infections, on the quality of the immune system, etc. In order to consider an average over all statistical realizations, we introduce the probability density function g(Cm), which is typically log-normal. Depending on the hypotheses used to extract Cm from sparse RT-qPCR measurements, its dispersion is found between 0.1 to 1 log10 copies/mL above and below average (55, 57, 58).
Fig. 3.
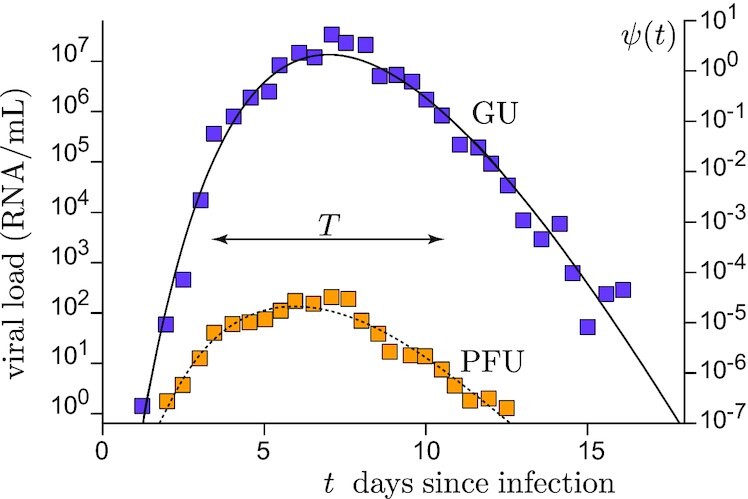
Viral kinetics of SARS-CoV-2. Average viral load in the nasal cavity as a function of time since infection from Killingley, et al. (59), using a strain close to Wuhan-1. Blue squares are obtained by quantitative RT-PCR and expressed in GU. Orange squares are obtained by counting lysis plaques and expressed PFU. Right axis: model dimensionless viral load ψ(t) as a function of time t, in days, after infection.
The inhaled dose can be expressed as
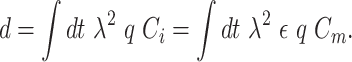 |
(7) |
The key quantity is the mean integrated viral emission 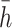 , which accounts for all the biological part of the risk. By definition, it is the dose in “quanta” exhaled by a patient during the entire infectious period:
, which accounts for all the biological part of the risk. By definition, it is the dose in “quanta” exhaled by a patient during the entire infectious period:
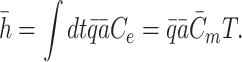 |
(8) |
It is defined as an average over the subpopulation attending the public space considered and may depend on the particular activity taking place in the public space through the mean inhalation rate 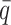 . The integrated viral emission
. The integrated viral emission 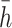 is between 450 and 500 quanta for Wuhan-1, 800 for Alpha, 1500 for Delta, 2,800 for Omicron BA.1, and 4,700 for Omicron BA.2 (41).
is between 450 and 500 quanta for Wuhan-1, 800 for Alpha, 1500 for Delta, 2,800 for Omicron BA.1, and 4,700 for Omicron BA.2 (41).
Environmental risk
We define the transmission risk of a public space as the average number of infections r that an infected person staying in for an infinitely long time would cause, on average. This public space contributes to epidemic decay if r < 1 and to its growth otherwise. r characterizes the environmental conditions and behaviors in the public space. We consider a public place hosting N people during a given period of time, among which M are infectious. The epidemic incidence I(t) is the fraction of a given population infected per unit time. In practice, the fraction of the population I(t)δt infected between t and δt is measured with δt = 1 day, although conceptually δt is infinitesimal. The prevalence P is the fraction of this population currently infectious and is related to I by the equation
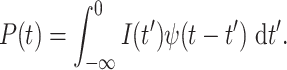 |
(9) |
On average, the number M of infectious people among N is 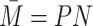 . Under the Poissonian hypothesis, the mean number rM of people infected reads
. Under the Poissonian hypothesis, the mean number rM of people infected reads
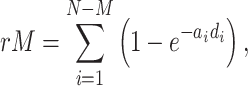 |
(10) |
where di is the intake dose of the individual labeled i while 1/ai is their infection dose. In the case where all N people are statistically subjected to the same intake dose di = d, the risk r reads
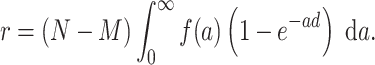 |
(11) |
The quantity ρ(d) = ∫f(a)(1 − e−ad) da is the probability to get infected when an intake dose d is inhaled and is called the dose response function. We consider now the limit where the intake dose, expressed in quanta 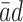 , has a very low probability of being larger than 1. This excludes super-spreading events, which occur when one infectious person (or several) with a large exhaled concentration Cm attends an under-ventilated place, leading to multiple simultaneous infections. Then, performing the linearization 1 − exp ( − ad) ≃ ad, the equation simplifies into
, has a very low probability of being larger than 1. This excludes super-spreading events, which occur when one infectious person (or several) with a large exhaled concentration Cm attends an under-ventilated place, leading to multiple simultaneous infections. Then, performing the linearization 1 − exp ( − ad) ≃ ad, the equation simplifies into 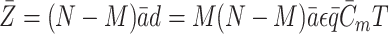 . The average number of secondary infections
. The average number of secondary infections 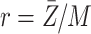 is therefore proportional to the biological factor
is therefore proportional to the biological factor  , to the dilution ratio ϵ:
, to the dilution ratio ϵ:
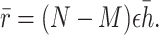 |
(12) |
For the same concentration of viral particles in the air, the transmission risk increases with the number of people (N − M) susceptible to be infected as they all have an equal individual risk of being infected.
Results
Transport of respiratory aerosols
Does the dispersion of CO2 and virus laden droplets obey the same law? To answer this question, we compare experimentally and theoretically the turbulent dispersion of an aerosol of oil droplets and of CO2 in a controlled turbulent flow. Fig. 2A shows that the concentration decays of the gaz and of the aerosol superimpose without any adjustable parameter. This remarkable result is highly nontrivial. Inertial particles disperse at the same rate as massless particles if the particle velocity correlation is close enough to the fluid velocity correlation function. Inertia acts as a low-pass filter of the fluid velocity, with a cut-off time controlled by the Stokes times τS = ρpd2/18η, which is the particle response time to a change in the fluid velocity (60).
The quantitative criterion for the aerosolization of particles of different sizes must compare the particle inertia to the dispersion by turbulent fluctuations. The stopping distance after which a droplet exiting the upper airways with a large initial velocity stops in a fluid at rest (43) includes inertia but ignores turbulent particle diffusion. Inertia acts all along the particle trajectory: the effect of particle size on turbulent dispersion is to make large enough particles slow-paced at changing their velocities, so that they leave the gas streamlines and decorrelate from the flow. We have shown here that the relevant turbulent property to which inertia must be compared is the Lagrangian time-scale 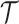 . This experimental result is in agreement with correlations functions found from direct numerical simulations of particles in turbulence (61). Particles form an aerosol phase whenever turbulence can homogenize their concentration. Settling gradually depletes the phase, but does not determine whether droplets should be regarded as individual ballistic particles or as an Eulerian phase. Settling times incorrectly assume that the air is still. Our experiments show that gravity is not the relevant physical mechanism that separates small particles from large particles, but the balance of inertia and turbulence is, and the dimensionless ratio
. This experimental result is in agreement with correlations functions found from direct numerical simulations of particles in turbulence (61). Particles form an aerosol phase whenever turbulence can homogenize their concentration. Settling gradually depletes the phase, but does not determine whether droplets should be regarded as individual ballistic particles or as an Eulerian phase. Settling times incorrectly assume that the air is still. Our experiments show that gravity is not the relevant physical mechanism that separates small particles from large particles, but the balance of inertia and turbulence is, and the dimensionless ratio 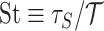 is the correct way of defining the Stokes number for the dispersion problem (see Supplementary Material for a derivation). If the Stokes number St is much smaller than 1, particles presents a negligible inertia, and are therefore aerosolized and dispersed according to Eq. (2).
is the correct way of defining the Stokes number for the dispersion problem (see Supplementary Material for a derivation). If the Stokes number St is much smaller than 1, particles presents a negligible inertia, and are therefore aerosolized and dispersed according to Eq. (2).
In the wind tunnel used in our experiments, we measured 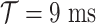 (42). The cross-over above which inertia becomes important (
(42). The cross-over above which inertia becomes important (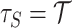 ) is therefore expected for a droplet diameter ∼50 μm much larger than the oil droplet diameter. For a meter scale ventilated room, the Lagrangian time-scale
) is therefore expected for a droplet diameter ∼50 μm much larger than the oil droplet diameter. For a meter scale ventilated room, the Lagrangian time-scale 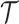 is in the range of 102 ms and the cross-over droplet diameter is larger than ∼100 μm. To conclude, CO2 and viral particles are dispersed at the same rate so that the dimensionless factor ϵ can indeed be determined quantitatively from the measurement of the CO2 concentration.
is in the range of 102 ms and the cross-over droplet diameter is larger than ∼100 μm. To conclude, CO2 and viral particles are dispersed at the same rate so that the dimensionless factor ϵ can indeed be determined quantitatively from the measurement of the CO2 concentration.
Fig. 2A shows that Eqs. (1) and (2), with a single relaxation time-scale 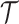 , perfectly fit the data. This result is particularly subtle and surprising. At such high Reynold numbers (between 105 and 106), turbulence is fully developed in space. According to the energy cascade picture of turbulence, kinetic energy is injected at large scale and dissipated by viscosity at small scale. At intermediate length scales, called the inertial range, energy is transferred by inertial effects (62), which cause nontrivial velocity correlations between two points separated in space. The typical flow evolution time-scale at the dissipation scale is called the Kolmogorov time τK and is used in the turbulence community to define a different particle Stokes number St (61). Indeed, τS/τK is relevant for the pair dispersion of particles (63, 64). The transport of a single particle is a Lagrangian problem (42), and therefore does not involve the Kolmogorov time, but rather the Lagrangian decorrelation time
, perfectly fit the data. This result is particularly subtle and surprising. At such high Reynold numbers (between 105 and 106), turbulence is fully developed in space. According to the energy cascade picture of turbulence, kinetic energy is injected at large scale and dissipated by viscosity at small scale. At intermediate length scales, called the inertial range, energy is transferred by inertial effects (62), which cause nontrivial velocity correlations between two points separated in space. The typical flow evolution time-scale at the dissipation scale is called the Kolmogorov time τK and is used in the turbulence community to define a different particle Stokes number St (61). Indeed, τS/τK is relevant for the pair dispersion of particles (63, 64). The transport of a single particle is a Lagrangian problem (42), and therefore does not involve the Kolmogorov time, but rather the Lagrangian decorrelation time 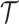 . Surprisingly, for the Reynolds numbers accessible numerically and experimentally, Lagrangian two-points correlation functions in time do not feature any inertial range, while Eulerian two-points space correlation functions do (61). Fig. 2 shows that we do not find any signature of an inertial range on the Lagrangian correlation function in time either. We do not observe any signature of a power-law regime, but instead, a simple ballistic-like regime. Simple arguments à la Kolmogorov would rather predict a much faster power-law decay of the velocity correlation function, leading quickly to a diffusive law and therefore of a decay of
. Surprisingly, for the Reynolds numbers accessible numerically and experimentally, Lagrangian two-points correlation functions in time do not feature any inertial range, while Eulerian two-points space correlation functions do (61). Fig. 2 shows that we do not find any signature of an inertial range on the Lagrangian correlation function in time either. We do not observe any signature of a power-law regime, but instead, a simple ballistic-like regime. Simple arguments à la Kolmogorov would rather predict a much faster power-law decay of the velocity correlation function, leading quickly to a diffusive law and therefore of a decay of  as x−1.
as x−1.
Experiments with a volunteer breathing through the mouth (42) have shown that viral particles are injected at a length scale a ≈ 0.3 m, set by the head size, which is significantly smaller than the turbulent integral scale. Eq. (3) can directly be used for exhalations, by changing x to x + a, so that
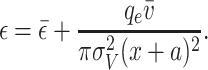 |
(13) |
The aerosol concentration therefore present a fast decay as the inverse squared distance x to the infected person. It is inversely proportional to the wind speed, at constant fluctuation rate 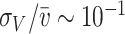 . Eq. (13) constitutes a central result of this paper, as it controls the spatial structure of the transmission risk outdoors.
. Eq. (13) constitutes a central result of this paper, as it controls the spatial structure of the transmission risk outdoors.
Transmission risk and CO2 concentration
The N people present exhale CO2 while only the M infected people emit viral particles. As a consequence, the expression of ϵ in terms of the average CO2 concentration 〈C〉 also contains a factor N:
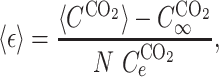 |
(14) |
where Ce ≃ 37,500 ppm is the average CO2 concentration in exhaled air. It must be emphasized that Eq. (14) does not assume any relationship between CO2 and virus emission rates and is equally valid for breathing, talking, or singing. It only hypothesizes that airborne viral particles are inhaled. The two factors balance each others in the transmission risk, which reads, after averaging over M: 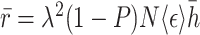 . The prevalence is in general much smaller than 1 so that we obtain the final formula relating the viral transmission risk to the CO2 concentration:
. The prevalence is in general much smaller than 1 so that we obtain the final formula relating the viral transmission risk to the CO2 concentration:
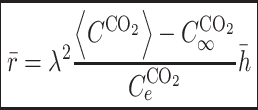 |
(15) |
This equation is remarkable: the dependence of the environmental risk, which characterizes the creation of transmission chains, with respect to the volume of the room, the ventilation, and the occupancy number are all encoded in the space-averaged CO2 concentration 〈C〉. The result is more subtle than it seems at first sight. Indeed, the N occupants of a public space all exhale CO2 but only the M infected ones exhale virions. Let us compare the risk of a well-ventilated lecture hall (say, at 750 ppm of CO2), with 50 students, to the risk if the same students are spread out in two conventional rooms with the same CO2 concentration. Obviously, all other things being equal, the probability of a student being infectious is twice lower when 25 students are grouped together, rather than 50. However, since the ventilation must be proportional to the room occupancy to keep the CO2 level, the viral particles are twice more concentrated in the small room and therefore the inhaled dose doubles. Under the above assumption, the average number of people infected is the same, although the risk is distributed differently.
The average CO2 concentration 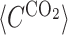 can be defined to take into account both the long-range dilution
can be defined to take into account both the long-range dilution 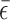 and the short-range contribution, present both indoor and outdoor, due to the higher aerosol concentration in the wake of infectious people. To achieve this, the CO2 concentration must be averaged over the surface where one more person would stand in the public place. The reasoning is the same as before. Each time a CO2 molecule reaches the measurement point, there is a probability M/N that it has been exhaled by one of the M infectious people. In that case, viral particles follow the same path as CO2 molecules. The dilution between inhaled and exhaled viral particle concentrations is still valid, but only if averaged over the possible permutations of the M infectious people amongst the N people. Again, the transmission risk is distributed very differently in the wake of infectious and noninfected people but the average remains correct. The quantitative determination of the aerosol transmission risk is directly applicable to outdoor spaces, where it is limited to this dispersion cone in the wake of infected people. The short-range transmission risk outdoors is generally smaller than indoors, due to larger air flow velocities.
and the short-range contribution, present both indoor and outdoor, due to the higher aerosol concentration in the wake of infectious people. To achieve this, the CO2 concentration must be averaged over the surface where one more person would stand in the public place. The reasoning is the same as before. Each time a CO2 molecule reaches the measurement point, there is a probability M/N that it has been exhaled by one of the M infectious people. In that case, viral particles follow the same path as CO2 molecules. The dilution between inhaled and exhaled viral particle concentrations is still valid, but only if averaged over the possible permutations of the M infectious people amongst the N people. Again, the transmission risk is distributed very differently in the wake of infectious and noninfected people but the average remains correct. The quantitative determination of the aerosol transmission risk is directly applicable to outdoor spaces, where it is limited to this dispersion cone in the wake of infected people. The short-range transmission risk outdoors is generally smaller than indoors, due to larger air flow velocities.
Let us illustrate on two examples the consequences of Eqs. (3) and (13). Consider a shopping mall corridor in which we have measured the air draft velocity to be typically 0.2 m/s. This corresponds to an extra CO2 concentration of 160 ppm at 1 m, of 50 ppm at 2 m, of 10 ppm at 5 m. Consider now an outdoor situation with a 1 m/s wind. This corresponds to an extra CO2 concentration of 30 ppm at 1 m, of 10 ppm at 2 m, and of 2 ppm at 5 m (Fig. S7).
Fig. 4 shows the linear relationship between the aerosol transmission risk and the CO2 average concentration for different face masks and viral strains. The natural risk threshold is r < 1, below which an infected person infects on average less than one other person, making the epidemic recede. With the current level of mask-wearing in public (Figs. S4 and S5), which leads to a small filtration factor λ2 = 0.2, the maximum excess CO2 concentration should be 70 ppm. This corresponds to a CO2 concentration of at most C = 490 ppm. If the risk is large but ventilation cannot be changed, N95/FFP2 respirators could be mandated. Social acceptability of such mandates could be managed by targeting first high-risk spaces, such as public transportation (65), then gradually expanding the scope of nonpharmaceutical interventions based on reproduction number and prevalence thresholds, following transparent and planned rules. If respirators were mandated, the factor λ2 would be decreased by 50 and the maximum excess CO2 concentration should be 670 ppm. The risk becomes smaller than 1 for standard ventilation conditions. Without mandatory face masks, the maximum excess CO2 concentration should be C = 13 ppm. This is far too small to be achievable in practice. Ventilation with fresh air is energy consuming, both in winter and during heat waves. It is therefore important to complement ventilation with HEPA filters or UV-C neon lights air purifiers (Fig. S6), which are already used reliably and regularly (66), are cost-effective techniques for destroying nucleic acids, DNA or RNA from bacteria, viruses, or other microorganisms present in the air.
Fig. 4.
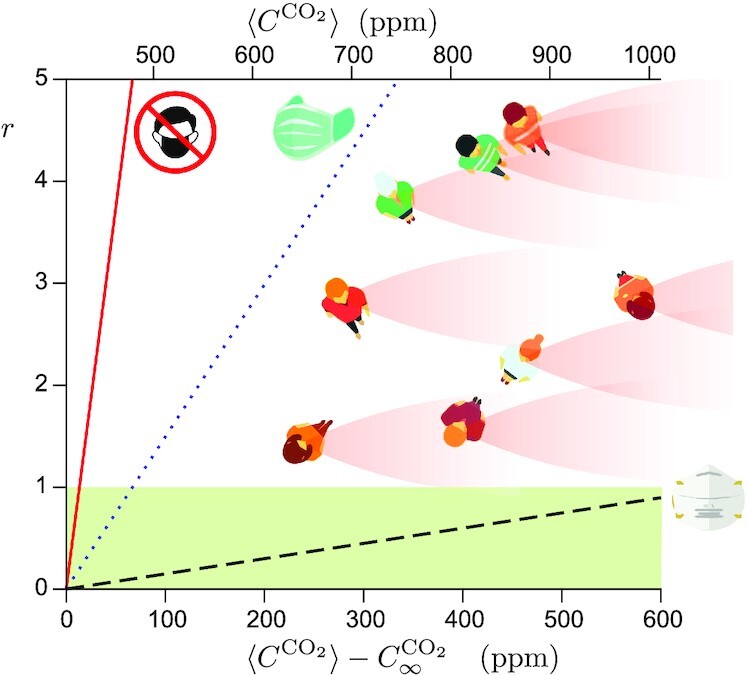
Airborne transmission risk r as a function of the concentration excess 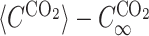 for the Omicron BA.1 strain (
for the Omicron BA.1 strain (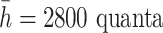 ). Solid line: no face mask. Dotted line: current level of mask-wearing in public, when mandatory (λ2 = 0.2, Fig. S4). Dashed line: FFP2 respirator (λ2 = 0.02). Inset: schematic showing the spatial distribution of short-range transmission risk in a public space.
). Solid line: no face mask. Dotted line: current level of mask-wearing in public, when mandatory (λ2 = 0.2, Fig. S4). Dashed line: FFP2 respirator (λ2 = 0.02). Inset: schematic showing the spatial distribution of short-range transmission risk in a public space. 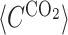 is by definition the space average of the CO2 concentration over all places where a further person would spontaneously stand.
is by definition the space average of the CO2 concentration over all places where a further person would spontaneously stand.
Discussion
We have obtained, using controlled approximations, a very simple expression showing a linear relationship between airborne transmission risk and CO2 concentration. Several effects have been neglected, which we discuss here.
The effect of gravity is to transport droplets vertically at the settling velocity. It can be neglected when the latter is small in front of turbulent velocities. Typically, the 10 μm oil droplets settle at 3 mm/s. For the wind tunnel used here, the fluctuating velocity is typically σV ∼ 0.7 m/s, which corresponds to a cross-over droplet diameter, above which settling is dominant, of 150 μm. For a meter scale ventilated room, the cross-over diameter remains of order 102 μm. For an aerosol of viral particles, deposition is therefore negligible, as can be checked from models taking it into account (23, 25, 26). As evaporation is very fast, the effect of gravity is at worst transient and limited to the largest droplets.
We assume that dispersion is controlled only by the ambient air flow, and not by expiratory air flows. Unmasked coughs and sneezes can create large velocities that dominate over ambient transport, in which aerosols are exhaled in a buoyant jet that gets mixed at its boundaries (18, 67). Face masks greatly reduce expiratory velocities, so that transport by the ambient flow is recovered 20 cm away from the source (68–70). Our work is particularly applicable outdoor, where there is no mixing but rather a horizontal turbulent flow, and wind velocities easily dominate over exhalation (Fig. S7). Measurements of crowds taking into account head orientation, crowd density and walking velocity show that horizontal velocities completely control the transmission risk (71, 72).
Virions transported in an aerosol phase are gradually degraded by the damage done by antiviral proteins in mucosalivary fluid (73) and by UV-B radiation in sunlight or by UV-C. The inactivation rate of enveloped, airborne viruses such as SARS-CoV-2 depends on the physicochemical composition of the aerosol droplets, relative humidity RH, and temperature in ways that are still unclear. Relative humidity plays an important role: the inactivation rate has a maximum at high RH, reaches a minimum at intermediate RH then increases again for lower RH (38, 74–76). This suggests that virions can associate with proteins which protects them both from dessication and antivirals (39). Ignoring viral inactivation is our main simplifying assumption outdoors, as sunlight may make it much faster. Inactivation can be modeled as a relaxation process of time-scale τ, once C is defined as the concentration of replicable viral particles: 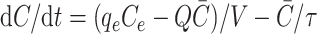 . The dilution factor ϵ and the transmission risk are therefore divided by 1 + V/(Qτ). The assumption made before corresponds to the regime where Q ≫ V/τ. As τ is on the order of an hour, this condition requires at least several “air changes per hour” (ACH) to be satisfied, which is the case in correctly ventilated public spaces. In steady state, measuring ϵ is therefore equivalent to measure both occupancy and ventilation rate. In transient conditions, often found in large public spaces, this approximation fails.
. The dilution factor ϵ and the transmission risk are therefore divided by 1 + V/(Qτ). The assumption made before corresponds to the regime where Q ≫ V/τ. As τ is on the order of an hour, this condition requires at least several “air changes per hour” (ACH) to be satisfied, which is the case in correctly ventilated public spaces. In steady state, measuring ϵ is therefore equivalent to measure both occupancy and ventilation rate. In transient conditions, often found in large public spaces, this approximation fails.
The mean integrated viral emission 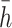 has three sources of variability. The most important one is the variation of this biological factor between different public spaces, depending on the type of respiratory activity taking place there and on the characteristics of the population attending it. For breathing, the respiratory rate qe can vary by a factor 2 to 3, depending on activity level and physiological characteristics. Here, it should be understood as the average value for the level of activity inside the public space, e.g. its typical value at rest for a theater, light exercise for a shopping mall where people walk, and exercise for a sport facility.
has three sources of variability. The most important one is the variation of this biological factor between different public spaces, depending on the type of respiratory activity taking place there and on the characteristics of the population attending it. For breathing, the respiratory rate qe can vary by a factor 2 to 3, depending on activity level and physiological characteristics. Here, it should be understood as the average value for the level of activity inside the public space, e.g. its typical value at rest for a theater, light exercise for a shopping mall where people walk, and exercise for a sport facility.
The viral exhalation rate dependence on respiratory activity, talking, and singing is scarcely known. It has been estimated that talking increases it by a factor ∼10 (77, 78). However, few speech samples were culture-positive, either due to protocol limitations or because the virus may have lost some of its ability to replicate when exiting through the mouth (77, 79). It is therefore difficult to define 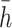 for each public space, and it must be averaged over the whole society. Individual exhalation rates, peak viral load, and individual infection doses are also broadly distributed, which creates another source of variability. There is also a variability originating from the number of contacts between individuals. Individuals who have many contacts get infected earlier and are thus removed from the pool of susceptible individuals, which decreases
for each public space, and it must be averaged over the whole society. Individual exhalation rates, peak viral load, and individual infection doses are also broadly distributed, which creates another source of variability. There is also a variability originating from the number of contacts between individuals. Individuals who have many contacts get infected earlier and are thus removed from the pool of susceptible individuals, which decreases 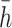 over time.
over time.
The last simplifying assumption is a small intake dose 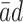 (in quanta), which corresponds to a small dilution factor ϵ. Eq. (14) shows that this hypothesis corresponds to a situation, which is either well ventilated or with a large number of people. This is generally the case for public spaces. By contrast, domestic spaces in winter generically gather few people in bad ventilation conditions, during long periods of time. This assumption excludes super-spreading events in which almost everyone gets infected because of these large deviations and poor ventilation.
(in quanta), which corresponds to a small dilution factor ϵ. Eq. (14) shows that this hypothesis corresponds to a situation, which is either well ventilated or with a large number of people. This is generally the case for public spaces. By contrast, domestic spaces in winter generically gather few people in bad ventilation conditions, during long periods of time. This assumption excludes super-spreading events in which almost everyone gets infected because of these large deviations and poor ventilation.
Conclusion
In this article, we have introduced an effective definition of the airborne transmission risk r associated with a public space, defined as the average secondary infections per initially infected person. Under the commonly accepted hypothesis of no cooperation between virions, the risk is computed in the limit where it is low. It is related to the integrated quantum emission 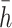 , to the mask filtration factor λ2, and to the dilution factor ϵ between exhaled and inhaled air.
, to the mask filtration factor λ2, and to the dilution factor ϵ between exhaled and inhaled air. 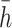 accounts for all biological aspects and depends on the viral strain considered but also on the characteristics of the subpopulation attending the public space considered. In that sense,
accounts for all biological aspects and depends on the viral strain considered but also on the characteristics of the subpopulation attending the public space considered. In that sense, 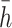 takes into account the average respiratory activity in the public space in question. ϵ accounts for all hydrodynamical aspects and is decomposed into an average contribution controlled by ventilation and a spatially dependent contribution, localized in the dispersion cone of infected people, controlled by turbulent air flows. Indoor and outdoor spaces both present a risk of airborne transmission at short range, in the dilution cone of the exhaled breath. However, indoor, enclosed spaces only present a risk of airborne transmission at long range. Eq. (13) provides the answer to our initial question (iii) as it provides the law governing the short-range contribution to the transmission, both indoors and outdoors.
takes into account the average respiratory activity in the public space in question. ϵ accounts for all hydrodynamical aspects and is decomposed into an average contribution controlled by ventilation and a spatially dependent contribution, localized in the dispersion cone of infected people, controlled by turbulent air flows. Indoor and outdoor spaces both present a risk of airborne transmission at short range, in the dilution cone of the exhaled breath. However, indoor, enclosed spaces only present a risk of airborne transmission at long range. Eq. (13) provides the answer to our initial question (iii) as it provides the law governing the short-range contribution to the transmission, both indoors and outdoors.
Eq. (15) constitutes a central result of the article, as it provides an answer to our initial question (i). Indeed, it incorporates the ventilation flow rate, the room volume, and the occupancy number into a single measurable quantity: the CO2 concentration. The disappearance of the occupancy N from Eq. (15) at large N is nontrivial and comes from two factors balancing each other: on the one hand, CO2 is exhaled by all individuals present, and not only by people infected by the virus; on the other hand, the transmission risk increases linearly with the number of people susceptible to be infected. As the risk is proportional to the CO2 concentration, the acceptable CO2 concentration below which r < 1 can be readily adjusted as new variants with higher 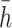 keep on appearing, based on a feedback using the measured reproduction number.
keep on appearing, based on a feedback using the measured reproduction number.
The simplicity of Eq. (15) is based on the third important result derived here, both experimentally and theoretically: the dispersion of CO2 and airborne viral particles is governed by the same law, for usual Reynolds numbers (question ii). The quantitative criterion is a small enough Stokes number 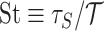 defined as the ratio of the Stokes time to the Lagrangian integral time. This result, albeit simple in appearance, results from a subtle effect of Lagrangian turbulence. This has important implications in determining infectivity times: as settling is not the relevant physical mechanism for aerosol transport, the infectivity of particles between 20 and 100 μm could be underestimated by models in which the air is still (10). We are not aware of previous simultaneous measurements of gas and particles in the same controlled flow.
defined as the ratio of the Stokes time to the Lagrangian integral time. This result, albeit simple in appearance, results from a subtle effect of Lagrangian turbulence. This has important implications in determining infectivity times: as settling is not the relevant physical mechanism for aerosol transport, the infectivity of particles between 20 and 100 μm could be underestimated by models in which the air is still (10). We are not aware of previous simultaneous measurements of gas and particles in the same controlled flow.
The transmission of SARS-CoV-2 in public spaces is predominantly airborne. In complement to vaccination, treatments for the vulnerable patient population and the test–trace–isolate strategy, the infection risk can be reduced by a combination of four collective actions:
ventilation with a sufficient fresh air flow rate per person to reduce the long-range risk;
monitoring CO2 to measure the risk and adjust practices;
air purification to complement ventilation where needed;
turbulent dispersion, distancing, and reduction of static crowds to reduce the short-range risk, both indoors and outdoors.
Ventilation guidelines or regulations in public spaces are centered around balancing thermal comfort with energy consumption and perceived air quality, not infection prevention (80), in part because the physical basis of airborne transmission was poorly understood (6, 8). The approach presented here defines an unambiguous transmission risk, encoding most hydrodynamical aspects into a single measurable quantity. It is applicable to all airborne pathogens and to many flow configurations. It also defines a maximum acceptable risk: r = 1 is the threshold above which the epidemic continues to propagate. In that sense, it is a step toward ventilation regulation for infection prevention.
The transmission risk model outdoors is extremely easy to implement in practice. Combining Eqs. (12) and (13), we find
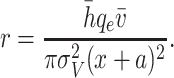 |
(16) |
At the request of municipal services, we have applied it to the “Canal Saint-Martin” in Paris, France, the popular banks of a canal along which many people eat and drink. Measuring the mean distance between visitors (typically 1 m), we find that when the wind is aligned with the banks, r is below 1 for the Wuhan-1 strain and for wind speeds above 1.5 m/s. However, the wind is not always perfectly aligned, and fluctuations in the wind direction lead to dispersion over the canal, and therefore reduced risk. Recommendation to local policymakers was to emphasize the lower risk outdoors but simultaneously to mandate face masks during the very few days where there is not enough wind to disperse viral particles. Using a slightly revised dispersion formula, adapted to a cycling group, we have similarly been able to establish that the transmission risk is low in the Tour de France peloton, but not indoors, in the hotels hosting the cyclists.
We propose that the paradigm of airborne infection prevention policies could shift from a set of independent nonpharmaceutical interventions (mask mandates, occupancy limits, social distancing) to a standard of risk, translated to a maximum acceptable CO2 concentration. Each public place has several options to meet this imposed standard, itself depending on the local community transmission, by lowering the maximum occupancy or by investing in better ventilation, by mandating masks or respirators, or by purifying the air with HEPA filters or UV-C flash lights (Fig. S6). Restrictions can be gradually added at different reproduction rates and prevalence thresholds by targeting high-risk spaces first to balance social acceptability. Education on good quality masks and their wider availability could greatly reduce risk in public transportation (65) and shopping malls. If, after targeting first high-risk spaces, the global reproduction rate is still above 1, restrictions can be expanded to lower-risk spaces, until the epidemic recedes. It would also be possible, for example, to mandate that mechanical ventilation in public buildings have two flow rates: one that allows for ordinary air renewal, keeping the CO2 concentration below, say, 1,000 ppm, and another much more powerful one designed for a drastic lowering of pathogen concentrations in the air, despite energy or comfort costs. Based on the results presented here, French shopping malls have successfully used smoke extractor fans, renewing the air in 5 min (12 ACH), to that effect since May 2021. Insofar as smoke extractors are compulsory in this type of public spaces, this has not resulted in any additional costs.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Joël Pothier and Alice Lebreton for the fruitful discussions.
Notes
Competing Interest: The authors declare no competing interest.
Contributor Information
Florian Poydenot, Laboratoire de Physique de l’Ecole Normale Supérieure (LPENS), CNRS UMR 8023, Ecole Normale Supérieure, Université PSL, Sorbonne Université, and Université Paris Cité, 24 rue Lhomond, 75005 Paris, France.
Ismael Abdourahamane, Laboratoire de Physique de l’Ecole Normale Supérieure (LPENS), CNRS UMR 8023, Ecole Normale Supérieure, Université PSL, Sorbonne Université, and Université Paris Cité, 24 rue Lhomond, 75005 Paris, France.
Elsa Caplain, Laboratoire de Physique de l’Ecole Normale Supérieure (LPENS), CNRS UMR 8023, Ecole Normale Supérieure, Université PSL, Sorbonne Université, and Université Paris Cité, 24 rue Lhomond, 75005 Paris, France.
Samuel Der, Laboratoire de Physique de l’Ecole Normale Supérieure (LPENS), CNRS UMR 8023, Ecole Normale Supérieure, Université PSL, Sorbonne Université, and Université Paris Cité, 24 rue Lhomond, 75005 Paris, France.
Jacques Haiech, Cogitamus Laboratory and CNRS UMR 7242 BSC, 300 Bd Sébastien Brant, CS 10413, 67412 Illkirch Cedex, France.
Antoine Jallon, Laboratoire de Physique de l’Ecole Normale Supérieure (LPENS), CNRS UMR 8023, Ecole Normale Supérieure, Université PSL, Sorbonne Université, and Université Paris Cité, 24 rue Lhomond, 75005 Paris, France.
Inés Khoutami, Laboratoire de Physique de l’Ecole Normale Supérieure (LPENS), CNRS UMR 8023, Ecole Normale Supérieure, Université PSL, Sorbonne Université, and Université Paris Cité, 24 rue Lhomond, 75005 Paris, France.
Amir Loucif, Laboratoire de Physique de l’Ecole Normale Supérieure (LPENS), CNRS UMR 8023, Ecole Normale Supérieure, Université PSL, Sorbonne Université, and Université Paris Cité, 24 rue Lhomond, 75005 Paris, France.
Emil Marinov, Laboratoire de Physique de l’Ecole Normale Supérieure (LPENS), CNRS UMR 8023, Ecole Normale Supérieure, Université PSL, Sorbonne Université, and Université Paris Cité, 24 rue Lhomond, 75005 Paris, France.
Bruno Andreotti, Laboratoire de Physique de l’Ecole Normale Supérieure (LPENS), CNRS UMR 8023, Ecole Normale Supérieure, Université PSL, Sorbonne Université, and Université Paris Cité, 24 rue Lhomond, 75005 Paris, France.
Funding
Unibail-Rodamco-Westfield on behalf of the Conseil National des Centres Commerciaux (CNCC) has funded this work under the Centre National de la Recherche Scientifique (CNRS) contract 217977.
Authors' Contributions
F.P., I.A., E.C., S.D., A.J., I.K., A.L., E.M., and B.A. conceived and conducted the experiments, and all authors analyzed the results. B.A., J.H., and F.P. wrote and reviewed the manuscript.
Data Availability
The data supporting the findings of this study are available within the article and its supplementary material.
Conflict of Interest
This work was funded by Unibail-Rodamco-Westfield on behalf of the Conseil National des Centres Commerciaux (CNCC), who asked the authors to make recommendations for a health protocol aiming to reduce and quantify the transmission risk in shopping centers. The conclusions of the present article are therefore of direct interest for the funding company. The funding company had no such involvement in study design, in the collection, analysis, and interpretation of data, nor in the writing of the article. The authors had the full responsibility in the decision to submit it for publication.
References
- 1. Zhou J, et al. 2018. Defining the sizes of airborne particles that mediate influenza transmission in ferrets. Proc Natl Acad Sci. 115(10):E2386–E2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fennelly KP. 2020. Particle sizes of infectious aerosols: implications for infection control. Lancet Respir Med. 8(9):914–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morawska L, Milton DK. 2020. It is time to address airborne transmission of coronavirus disease 2019 (COVID-19). Clin Infect Dis. 71(9):2311–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang R, Li Y, Zhang AL, Wang Y, Molina MJ. 2020. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc Natl Acad Sci. 117(26):14857–14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Greenhalgh T, et al. 2021. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. Lancet. 397(10285):1603–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jimenez JL, et al. 2022. What were the historical reasons for the resistance to recognizing airborne transmission during the COVID-19 pandemic?. Indoor Air. 32(8):e13070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng Y, et al. 2021. Face masks effectively limit the probability of SARS-CoV-2 transmission. Science. 372(6549):1439–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Randall K, Ewing ET, Marr LC, Jimenez JL, Bourouiba L. 2021. How did we get here: what are droplets and aerosols and how far do they go? A historical perspective on the transmission of respiratory infectious diseases. Interface Focus. 11(6):20210049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nazaroff WW. 2022. Indoor aerosol science aspects of SARS-CoV-2 transmission. Indoor Air. 32(1):e12970. [DOI] [PubMed] [Google Scholar]
- 10. Merhi T, Atasi O, Coetsier C, Lalanne B, Roger K. 2022. Assessing suspension and infectivity times of virus-loaded aerosols involved in airborne transmission. Proc Natl Acad Sci. 119(32):e2204593119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wells WF. 1955. Airborne contagion and air hygiene. An ecological study of droplet infections. Cambridge (MA): Harvard University Press. [Google Scholar]
- 12. Riley EC, Murphy G, Riley RL. 1978. Airborne spread of measles in a suburban elementary school. Am J Epidemiol. 107(5):421–432. [DOI] [PubMed] [Google Scholar]
- 13. Ho CK. 2021. Modeling airborne pathogen transport and transmission risks of SARS-CoV-2. Appl Math Model. 95:297–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li Y, et al. 2021. Probable airborne transmission of SARS-CoV-2 in a poorly ventilated restaurant. Build Environ. 196:107788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vuorinen V, et al. 2020. Modelling aerosol transport and virus exposure with numerical simulations in relation to SARS-CoV-2 transmission by inhalation indoors. Safety Sci. 130:104866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu H, He S, Shen L, Hong J. 2021. Simulation-based study of COVID-19 outbreak associated with air-conditioning in a restaurant. Phys Fluids. 33:023301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang J, et al. 2021. Short-range exposure to airborne virus transmission and current guidelines. Proc Natl Acad Sci. 118(37):e2105279118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bourouiba L. 2021. The fluid dynamics of disease transmission. Ann Rev Fluid Mech. 53(1):473–508. [Google Scholar]
- 19. Yang F, Pahlavan AA, Mendez S, Abkarian M, Stone HA. 2020. Towards improved social distancing guidelines: space and time dependence of virus transmission from speech-driven aerosol transport between two individuals. Phys Rev Fluids. 5(12):122501. [Google Scholar]
- 20. Giri A, et al. 2022. Colliding respiratory jets as a mechanism of air exchange and pathogen transport during conversations. J Fluid Mech. 930:R1–1-R1-14. [Google Scholar]
- 21. Rudnick SN, Milton DK. 2003. Risk of indoor airborne infection transmission estimated from carbon dioxide concentration. Indoor Air. 13(3):237–245. [DOI] [PubMed] [Google Scholar]
- 22. Bivolarova M, Ondráv̆ek J, Melikov A, Ždímal V. 2017. A comparison between tracer gas and aerosol particles distribution indoors: the impact of ventilation rate, interaction of airflows, and presence of objects. Indoor Air. 27(6):1201–1212. [DOI] [PubMed] [Google Scholar]
- 23. Bazant MZ, et al. 2021. Monitoring carbon dioxide to quantify the risk of indoor airborne transmission of COVID-19. Flow. 1:e10. [Google Scholar]
- 24. Burridge HC, Fan S, Jones RL, Noakes CJ, Linden PF. 2021. Predictive and retrospective modelling of airborne infection risk using monitored carbon dioxide. Indoor Built Environ. 31(5):1363–1380. [Google Scholar]
- 25. Bazant MZ, Bush JWM. 2021. A guideline to limit indoor airborne transmission of COVID-19. Proc Natl Acad Sci. 118(17):e2018995118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peng Z, Jimenez JL. 2021. Exhaled CO2 as a COVID-19 infection risk proxy for different indoor environments and activities. Environ Sci Technol Lett.8: 392–397. [DOI] [PubMed] [Google Scholar]
- 27. Peng Z, et al. 2022. Practical indicators for risk of airborne transmission in shared indoor environments and their application to COVID-19 outbreaks. Environ Sci Tech. 56(2):1125–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Buonanno G, Stabile L, Morawska L. 2020. Estimation of airborne viral emission: quanta emission rate of SARS-CoV-2 for infection risk assessment. Environ Int. 141:105794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Azimi P, Keshavarz Z, Laurent JGC, Stephens B, Allen JG. 2021. Mechanistic transmission modeling of COVID-19 on the Diamond Princess cruise ship demonstrates the importance of aerosol transmission. Proc Natl Acad Sci. 118(8);e2015482118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Parhizkar H, Van Den Wymelenberg KG, Haas CN, Corsi RL. 2021. A quantitative risk estimation platform for indoor aerosol transmission of COVID-19. Risk Anal. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Abkarian M, Stone HA. 2020. Stretching and break-up of saliva filaments during speech: a route for pathogen aerosolization and its potential mitigation. Phys Rev Fluids. 5(10):102301. [Google Scholar]
- 32. Abkarian M, Mendez S, Xue N, Yang F, Stone HA. 2020. Speech can produce jet-like transport relevant to asymptomatic spreading of virus. Proc Natl Acad Sci. 117(41):25237–25245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Snijder EJ, et al. 2020. A unifying structural and functional model of the coronavirus replication organelle: tracking down RNA synthesis. PLoS Biol. 18(6):e3000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mikhailov E, Vlasenko S, Niessner R, Poschl U. 2004. Interaction of aerosol particles composed of protein and salts with water vapor: hygroscopic growth and microstructural rearrangement. Atmos Chem Phys. 4:323–350. [Google Scholar]
- 35. Pöhlker ML, et al. 2021. Respiratory aerosols and droplets in the transmission of infectious diseases. preprint, arXiv:210301188. [physics.med-ph]. [Google Scholar]
- 36. Seyfert C, Rodríguez-Rodríguez J, Lohse D, Marin A. 2022. Stability of respiratory-like droplets under evaporation. Phys Rev Fluids. 7(2):023603. [Google Scholar]
- 37. Chong KL, et al. 2021. Extended lifetime of respiratory droplets in a turbulent vapor puff and its implications on airborne disease transmission. Phys Rev Lett. 126(3):034502. [DOI] [PubMed] [Google Scholar]
- 38. Huynh E, et al. 2022. Evidence for a semisolid phase state of aerosols and droplets relevant to the airborne and surface survival of pathogens. Proc Natl Acad Sci. 119(4):e2109750119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vejerano EP, Marr LC. 2018. Physico-chemical characteristics of evaporating respiratory fluid droplets. J Roy Soc Inter. 15(139):20170939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Oswin HP, et al. 2022. The Dynamics of SARS-CoV-2 infectivity with changes in aerosol microenvironment. Proc Natl Acad Sci. 119(27):e2200109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Poydenot F, Noûs C, Haiech J, Andreotti B. 2022. At the crossroads of epidemiology and biology: bridging the gap between SARS-CoV-2 viral strain properties and epidemic wave characteristics. submitted to Biochimie. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Poydenot F, et al. 2021. Turbulent dispersion of breath by the wind. Am. J. Phys. arXiv:211206501. [physics.flu-dyn]. [Google Scholar]
- 43. Hinds WC. 1999. Aerosol technology: properties, behavior, and measurement of airborne particles. New York (NY): John Wiley & Sons. [Google Scholar]
- 44. Cappa CD, et al. 2021. Expiratory aerosol particle escape from surgical masks due to imperfect sealing. Sci Rep. 11(1):12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pan J, Harb C, Leng W, Marr LC. 2021. Inward and outward effectiveness of cloth masks, a surgical mask, and a face shield. Aerosol Sci Tech. 55(6):718–733. [Google Scholar]
- 46. Hill WC, Hull MS, MacCuspie RI. 2020. Testing of commercial masks and respirators and cotton mask insert materials using SARS-CoV-2 virion-sized particulates: comparison of ideal aerosol filtration efficiency versus fitted filtration efficiency. Nano Lett. 20(10):7642–7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lindsley WG, et al. 2021. A comparison of performance metrics for cloth masks as source control devices for simulated cough and exhalation aerosols. Aerosol Sci Tech. 55(10):1125–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Oberg T, Brosseau LM. 2008. Surgical mask filter and fit performance. Am J Infect Control. 36(4):276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Qian Y, Willeke K, Grinshpun SA, Donnelly J, Coffey CC. 1998. Performance of N95 respirators: filtration efficiency for airborne microbial and inert particles. Am Ind Hyg Assoc J. 59(2):128–132. [DOI] [PubMed] [Google Scholar]
- 50. Asadi S, et al. 2020. Efficacy of masks and face coverings in controlling outward aerosol particle emission from expiratory activities. Sci Rep. 10(1):15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vouriot CVM, Burridge HC, Noakes CJ, Linden PF. 2021. Seasonal variation in airborne infection risk in schools due to changes in ventilation inferred from monitored carbon dioxide. Indoor Air. 31:1154–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kriegel M, et al. 2020. Predicted infection risk for aerosol transmission of SARS-CoV-2. medRxiv:2020100820209106. Available at: 10.1101/2020.10.08.20209106. [DOI] [Google Scholar]
- 53. Lelieveld J, et al. 2020. Model calculations of aerosol transmission and infection risk of COVID-19 in indoor environments. Int J Environ Res Pub Health. 17(21):8114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jang S, Rhee JY, Wi YM, Jung BK. 2021. Viral kinetics of SARS-CoV-2 over the preclinical, clinical, and postclinical period. Int J Infect Dis. 102:561–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jones TC, et al. 2021. Estimating infectiousness throughout SARS-CoV-2 infection course. Science. 373(6551):1116–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sethuraman N, Jeremiah SS, Ryo A. 2020. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 323(22):2249–2251. [DOI] [PubMed] [Google Scholar]
- 57. Monel B, et al. 2021. Release of infectious virus and cytokines in nasopharyngeal swabs from individuals infected with non-Alpha or Alpha SARS-CoV-2 variants: an observational retrospective study. EBioMedicine. 73:103637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Elie B, et al. 2021. Inferring SARS-CoV-2 variant within-host kinetics. medRxiv:2021052621257835. [Google Scholar]
- 59. Killingley B, et al. 2022. Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults. Nat Med. 28(5):1031–1041. [DOI] [PubMed] [Google Scholar]
- 60. Bec J, Biferale L, Lanotte AS, Scagliarini A, Toschi F. 2010. Turbulent pair dispersion of inertial particles. J Fluid Mech. 645:497–528. [Google Scholar]
- 61. Biferale L, et al. 2008. Lagrangian structure functions in turbulence: a quantitative comparison between experiment and direct numerical simulation. Phys Fluids. 20(6):065103. [Google Scholar]
- 62. Lesieur M. 2008. Turbulence in fluids. Dordrecht: Springer. [Google Scholar]
- 63. Richardson LF, Walker GT. 1926. Atmospheric diffusion shown on a distance-neighbour graph. Proc R Soc Lond A. 110(756):709–737. [Google Scholar]
- 64. Bourgoin M. 2015. Turbulent pair dispersion as a ballistic cascade phenomenology. J Fluid Mech. 772:678–704. [Google Scholar]
- 65. Bertone M, et al. 2022. Assessment of SARS-CoV-2 airborne infection transmission risk in public buses. Geosci Front. 13:101398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Raeiszadeh M, Adeli B. 2020. A critical review on ultraviolet disinfection systems against COVID-19 outbreak: applicability, validation, and safety considerations. ACS Photon. 7(11):2941–2951. [DOI] [PubMed] [Google Scholar]
- 67. Bourouiba L, Dehandschoewercker E, Bush JM. 2014. Violent expiratory events: on coughing and sneezing. J Fluid Mech. 745:537–563. [Google Scholar]
- 68. Deng Z, Chen Q. 2021. What is suitable social distancing for people wearing face masks during the COVID-19 pandemic?. Indoor Air. 32(1):e12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Verma S, Dhanak M, Frankenfield J. 2020. Visualizing the effectiveness of face masks in obstructing respiratory jets. Phys Fluids. 32(6):061708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Simha PP, Rao PSM. 2020. Universal trends in human cough airflows at large distances. Phys Fluids. 32(8):081905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Garcia W, Mendez S, Fray B, Nicolas A. 2021. Model-based assessment of the risks of viral transmission in non-confined crowds. Safety Sci. 144:105453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mendez S, Garcia W, Nicolas A. 2022. From microscopic droplets to macroscopic crowds: crossing the scales in models of short-range respiratory disease transmission, with application to COVID-19. preprint, arXiv:220803147. [physics.bio-ph]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Malamud D, et al. 2011. Antiviral activities in human saliva. Adv Dental Res. 23(1):34–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yang W, Marr LC. 2011. Dynamics of airborne influenza a viruses indoors and dependence on humidity. PLoS One. 6(6):e21481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tang JW. 2009. The effect of environmental parameters on the survival of airborne infectious agents. J Roy Soc Interface. 6:S737–S746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Smither SJ, Eastaugh LS, Findlay JS, Lever MS. 2020. Experimental aerosol survival of SARS-CoV-2 in artificial saliva and tissue culture media at medium and high humidity. Emerg Microbes Infect. 9(1):1415–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Coleman KK, et al. 2022. Viral load of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in respiratory aerosols emitted by patients with coronavirus disease 2019 (COVID-19) while breathing, talking, and Singing. Clin Inf Dis. 74(10):1722–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Shen Y, Courtney JM, Anfinrud P, Bax A. 2022. Hybrid measurement of respiratory aerosol reveals a dominant coarse fraction resulting from speech that remains airborne for minutes. Proc Natl Acad Sci. 119(26):e2203086119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Adenaiye OO, et al. 2021. Infectious severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in exhaled aerosols and efficacy of masks during early mild infection. Clin Inf Dis. 75(1):e241–e248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Morawska L, et al. 2021. A paradigm shift to combat indoor respiratory infection. Science. 372(6543):689–691. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available within the article and its supplementary material.


