Abstract
Background:
Chronic pain is common, disabling, and costly. Few clinical trials have examined cognitive behavioral therapy (CBT) interventions embedded in primary care settings to improve chronic pain among those receiving long-term opioid therapy.
Objective:
To determine the effectiveness of a group-based CBT intervention for chronic pain.
Design:
Pragmatic, cluster randomized controlled trial. (ClinicalTrials.gov: NCT02113592)
Setting:
Kaiser Permanente health care systems in Georgia, Hawaii, and the Northwest.
Participants:
Adults (aged ≥18 years) with mixed chronic pain conditions receiving long-term opioid therapy.
Intervention:
A CBT intervention teaching pain self-management skills in 12 weekly, 90-minute groups delivered by an interdisciplinary team (behaviorist, nurse, physical therapist, and pharmacist) versus usual care.
Measurements:
Self-reported pain impact (primary outcome, as measured by the PEGS scale [pain intensity and interference with enjoyment of life, general activity, and sleep]) was assessed quarterly over 12 months. Pain-related disability, satisfaction with care, and opioid and benzodiazepine use based on electronic health care data were secondary outcomes.
Results:
A total of 850 patients participated, representing 106 clusters of primary care providers (mean age, 60.3 years; 67.4% women); 816 (96.0%) completed follow-up assessments. Intervention patients sustained larger reductions on all self-reported outcomes from baseline to 12-month follow-up; the change in PEGS score was −0.434 point (95% CI, −0.690 to −0.178 point) for pain impact, and the change in pain-related disability was −0.060 point (CI, −0.084 to −0.035 point). At 6 months, intervention patients reported higher satisfaction with primary care (difference, 0.230 point [CI, 0.053 to 0.406 point]) and pain services (difference, 0.336 point [CI, 0.129 to 0.543 point]). Benzodiazepine use decreased more in the intervention group (absolute risk difference, −0.055 [CI, −0.099 to −0.011]), but opioid use did not differ significantly between groups.
Limitation:
The inclusion of only patients with insurance in large integrated health care systems limited generalizability, and the clinical effect of change in scores is unclear.
Conclusion:
Primary care–based CBT, using frontline clinicians, produced modest but sustained reductions in measures of pain and pain-related disability compared with usual care but did not reduce use of opioid medication.
Primary Funding Source:
National Institutes of Health.
Chronic pain is one of the most common, debilitating, and refractory conditions facing primary care providers (PCPs) (1–3). Many patients with chronic pain have multisite pain and comorbid medical and mental health disorders, which increase management challenges (4–6). Opioids have historically been touted as a solution to long-term management, despite the lack of rigorous evidence. This approach created a host of patient and societal adverse effects (7–9). Consequently, viable nonopioid options for long-term management of chronic pain in primary care are needed (2, 10).
Although studies suggest that multimodal approaches to chronic pain care are safe and effective in reducing pain and improving function (11–15), most studies have focused on patients with specific types of chronic pain rather than those with broader chronic pain conditions seen in primary care, have relied on specialty-trained clinicians, and have lacked geographic diversity. To our knowledge, none exclusively targeted patients with chronic pain who are receiving long-term opioid therapy or encouraged PCPs to prioritize their most challenging patients—often perceived to be those with numerous medical or mental health comorbid conditions, high health care use, benzodiazepine use, and resistance to opioid tapering (16).
The purpose of this study was to compare a cognitive behavioral therapy (CBT) intervention embedded in primary care versus usual care for treating chronic pain among patients receiving long-term opioid therapy. The primary hypothesis was that patients who received the intervention would have greater reduction in pain impact—a composite of pain intensity and interference—at 12 months of follow-up (approximately 8 months after treatment) than those receiving usual care. Our secondary hypotheses were that patients who received the intervention would have greater reductions in pain-related disability, improved satisfaction with primary care and pain-related services, and reduced opioid and benzodiazepine use. In addition, the study assessed the intervention’s cost-effectiveness and effect on health care use; these secondary outcomes will be reported elsewhere.
Methods
The PPACT (Pain Program for Active Coping and Training) study was a pragmatic, cluster randomized trial comparing a primary care–embedded, interdisciplinary, behavioral intervention versus usual care for treating chronic pain among patients receiving long-term opioid therapy (ClinicalTrials.gov: NCT02113592). This pragmatic trial was designed to suit primary care needs (16, 17). Patients with a range of pain conditions were eligible, and exclusions were minimized. Existing staff in participating health care systems delivered the intervention, and use of nonstudy therapies was not restricted. Outcomes included patient-reported assessments typically used in primary care and health care use data available in electronic health records. Participants were contacted for follow-up regardless of intervention adherence or continued insurance coverage.
Setting and Participants
The PPACT study was done in 3 Kaiser Permanente (KP) health care regions (Georgia, Hawaii, and Northwest). Institutional review boards at all sites retained oversight and approved trial procedures. The Supplement (available at Annals.org) contains the trial protocol, including a description of modifications.
Primary care clinics from the 3 KP regions were invited to participate from 2014 through 2016. The methods for recruiting and enrolling PCPs from these clinics are described elsewhere (16). Potentially eligible patients were paneled to a participating PCP and met the following criteria: age 18 years or older, at least 180 days of KP coverage, at least 2 dispensings of long-acting opioids during the prior 6 months or a cumulative supply of short-acting opioids of at least 90 days during 4 months in the same period, and any pain diagnosis during the previous year (18). Exclusion criteria were 2 or more cancer diagnoses and an oncology encounter during the prior 60 days, hospice or end-of-life palliative care within the past year, current addiction treatment or substance dependence diagnosis, and cognitive impairment precluding participation. Recruitment prioritized the following patients, whom the health care systems identified as high-need: those receiving higher-dose opioids (≥120 morphine milligram equivalents [MME]), concurrent recipients of benzodiazepines, and high users of primary care (16). Potentially eligible patients were mailed a letter describing the study, followed by telephone contact to assess study interest and eligibility (pain interference with general activity ≥4 on an 11-point scale). Verbal consent was obtained by telephone (16). No compensation was provided for study participation.
Randomization and Blinding
Participating PCPs were grouped into clinic-based clusters of 1 to 6 providers, according to patient panel size, and were randomly assigned before the start of patient recruitment for the cluster. Randomization and recruitment occurred in 20 waves between March 2014 and February 2017 (16). The study team was blinded to group assignment until after recruitment for the wave was complete. Study outcomes were collected by blinded, trained assessors.
Intervention
The 3-month, primary care–based, CBT intervention was designed for delivery by an interdisciplinary team of a behavioral health specialist, nurse care manager, physical therapist, and pharmacist, working in collaboration with PCPs. In practice, staffing varied across settings for behavioral health and nursing, depending on clinical staff availability and system operations. The PPACT intervention had 4 components, described briefly in the following sections. The design paper (16) and protocol (Section 4 of Part 2 of the Supplement) provide details.
Intake Evaluation
The intake evaluation included 2 in-person sessions with the behavioral health specialist or nurse case manager, 1 in-person session with the physical therapist, and a pharmacist’s medical record review of medications. The evaluation aimed to identify factors contributing to pain and functional impairment and tailor intervention goals to patients’ circumstances and preferences.
Core Skills
Twelve weekly, 90-minute group sessions made up the core skills training, which included progressive muscle relaxation and brief applied relaxation techniques, activity–rest cycling, pleasant activity scheduling, guided imagery and other distraction techniques, emotional regulation skills, cognitive restructuring, problem solving, and relapse prevention and maintenance. Each group session included instruction and practice in a yoga-based, adapted movement approach (19–22) (Appendix Table 1, available at Annals.org).
PCP Consultation
Interventionists met with PCPs to review the intake summaries after completing the intake evaluations but before starting group sessions, and they met again after group sessions ended. Further contacts were made as needed, at the discretion of interventionists and PCPs. When feasible, PCPs did telephone outreach calls with their patients to discuss patients’ self-identified goals and reinforce self-management efforts.
Patient Monitoring
At approximately the middle of treatment (week 6), the physical therapist checked in with patients to assess progress and encourage increased physical activity. At the posttreatment assessment, participants were asked to repeat the intake assessment to evaluate progress and aid in developing a maintenance plan.
Usual Care
Clusters of PCPs randomly assigned to usual care continued to provide pharmacologic and nonpharmacologic treatments to their patients without restriction.
Primary Outcome
The primary outcome was patient-reported pain impact at 12 months, as measured by the 4-item PEGS scale (23, 24), a composite of pain intensity and interference with enjoyment of life, general activity, and sleep (range, 0 to 10; higher score indicates worse pain impact). Participants were assessed at baseline (pretreatment) and 3 (posttreatment), 6, 9, and 12 months of follow-up.
Secondary Outcomes
Secondary patient-reported outcomes were pain-related disability, assessed using the 24-item Roland Morris Disability Questionnaire (RMDQ; range, 0 to 1; higher score indicates worse function) (25–29) at 12 months, and patient satisfaction with primary care and pain services at 6 months, assessed using a 6-point Likert scale (0 [very dissatisfied] to 5 [very satisfied]). Data on RMDQ were collected at baseline (pretreatment) and 3 (posttreatment), 6, 9, and 12 months; satisfaction data were collected at baseline (pretreatment) and 6 months (posttreatment).
The secondary health services outcomes of opioid use, measured as average daily dose of opioids in MME per 90-day period (calculation methods in protocol), and benzodiazepine receipt (yes or no) over 90-day periods for 12 months were assessed using electronic health record data.
Adverse Outcomes
The only adverse events monitored were serious adverse events, which were defined as hospitalizations and deaths, assessed systematically from electronic health record data every 6 months, and reviewed by an independent monitor. For deaths, medical record abstraction determined relatedness.
Post Hoc Outcomes
Post hoc pain-related outcomes were the 3-item PEG scale (composite of pain intensity and interference with enjoyment of life and general activity) and meeting criteria for clinically meaningful improvement (yes or no), defined as 30% or greater reduction in score from baseline on pain-related outcomes (PEGS, PEG, and RMDQ). These measures are widely used and recommended as pain outcomes, enabling comparability with other studies (30, 31). In addition, selected opioid use metrics (average daily MME ≥90 and continued long-term receipt of opioids [≥70 days’ supply per 90-day period]) were identified as post hoc outcomes. These 2 opioid use outcomes are included here because of recruitment prioritization for patients with these characteristics (16). However, use of other prescription medications (one of the prespecified secondary outcomes for health service use) will be reported elsewhere with cost analyses.
Statistical Analysis
We calculated our needed sample (power = 0.88) as 106 PCP clusters (average cluster size of 8 patients) to enable detection of a small but clinically meaningful group difference (31–33) (standardized effect size, 0.22) in the primary study outcome at 12 months, using an intraclass correlation coefficient of 0.002 based on preliminary pilot study data (16). All analyses were intention-to-treat: All participants with at least 1 follow-up data point were included and analyzed according to original group assignment. Missing data were handled using direct maximum likelihood (34, 35). We used a 2-tailed α level of 0.05 for all inferential tests, and all analyses were done using Stata, version 15.1 (StataCorp) (36).
For our primary outcome (PEGS) and continuous secondary outcomes (PEG, RMDQ, and average opioid dose per day), a 3-level linear mixed model was used to account for the nesting of repeated observations within patients nested within provider clusters (37–39). A linear trajectory was originally proposed to describe the change in pain scores over time (16). However, because we found a nonlinear effect across time, we used segmented or piecewise regression models (40–42) to more appropriately model the pattern of these findings. The first level included 2 predictors for time: slope from baseline to the posttreatment assessment (3 months; treatment effect) and slope from the posttreatment assessment to 12-month follow-up (maintenance effect). The second level included random effects for the level-1 intercept and 2 time-variable coefficients. The third level included study group as the predictor of the patient-level intercept and coefficients for time and random effect of the PCP cluster–level intercept. From this model, we estimated the marginal means and associated 95% CIs by group and time, as well as the differences in change between groups for the treatment period (baseline to 3 months), maintenance period (3 to 12 months), and overall study period (baseline to 12 months). Analysis of patient satisfaction with primary care and pain services was based on the same analytic framework but used a linear model because these variables were measured only before treatment and at 6 months of follow-up.
Analyses of binary outcomes included benzodiazepine receipt (secondary outcome), post hoc analyses of clinically meaningful improvement (≥30%) (33) in pain outcomes (PEGS, PEG, and RMDQ), and additional opioid use metrics (continued long-term opioid therapy and ≥90-MME opioid dose) using mixed-effects logistic regression. Marginal risks (that is, proportions) and associated 95% CIs were calculated by group and time, as were differences in change in absolute risks and relative risks between groups over the treatment, maintenance, and overall study periods.
Role of the Funding Source
The National Institutes of Health had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Results
Participants
We identified 10 196 members of KP health plans who were paneled with study PCPs and met preliminary eligibility criteria. Of these, 4934 were eligible and 850 participated. The Appendix Figure (available at Annals.org) describes the flow of participants from recruitment to enrollment. Baseline characteristics of intervention and usual care participants were similar (Table 1). The mean age was 60.3 years (SD, 12.2), 67% of participants were women, and 77% were White. One quarter were receiving disability payments at the time of enrollment, and the median number of pain conditions was 4.0 (interquartile range, 3 to 5 conditions). The most common pain diagnoses were limb or extremity pain, joint pain, and arthritic disorders (81%); back and neck pain (74%); and general and widespread pain (70%). The median average daily dose of opioids among enrollees was 29.6 MME (interquartile range, 16 to 62 MME), 18% of enrollees were receiving opioid dosages of 90 MME/day or higher, and 27% had received benzodiazepines during the past year. In addition, 33% had 2 or more chronic medical conditions, average body mass index was in the obese range (mean, 32.8 kg/m2 [SD, 8.9]), and almost half (44%) had a mental health disorder diagnosis in the past year.
Table 1.
Baseline Characteristics of Patients in the PPACT Study
| Characteristic | All Patients (n = 850) | Intervention (n = 433) | Usual Care (n = 417) |
|---|---|---|---|
|
| |||
| Mean age (SD), y | 60.3 (12.2) | 61.4 (11.9) | 59.2 (12.4) |
| Sex, n (%) | |||
| Women | 573 (67.4) | 287 (66.3) | 286 (68.6) |
| Men | 277 (32.6) | 146 (33.7) | 131 (31.4) |
| Race, n (%) | |||
| White | 651 (76.6) | 334 (77.1) | 317 (76.0) |
| Black or African American | 110 (12.9) | 55 (12.7) | 55 (13.2) |
| Other | 89 (10.5) | 44 (10.2) | 45 (10.8) |
| Hispanic ethnicity, n (%) | 28 (3.3) | 16 (3.7) | 12 (2.9) |
| Receives disability benefits, n (%)* | 215 (25.3) | 105 (24.3) | 110 (26.4) |
| Current smoking, n (%)* | 137 (16.2) | 62 (14.4) | 75 (18.1) |
| Mean BMI (SD), kg/m2† | 32.8 (8.9) | 32.9 (9.1) | 32.7 (8.6) |
| Alcohol misuse (history and/or current), n (%)* | 37 (4.4) | 19 (4.4) | 18 (4.3) |
| Drug misuse (history and/or current), n (%)* | 44 (5.2) | 25 (5.8) | 19 (4.6) |
| Chronic comorbid conditions, n (%)* | |||
| Diabetes | 202 (23.8) | 110 (25.4) | 92 (22.1) |
| Cardiovascular disorder | 198 (23.3) | 102 (23.6) | 96 (23.0) |
| Hypertension | 414 (48.7) | 220 (50.8) | 194 (46.5) |
| Chronic pulmonary disease | 175 (20.6) | 84 (19.4) | 91 (21.8) |
| ≥2 of above chronic comorbid conditions | 304 (35.8) | 148 (34.2) | 156 (37.4) |
| Mental health comorbid conditions, n (%)* | |||
| Any mental health diagnosis | 374 (44.0) | 183 (42.3) | 191 (45.8) |
| Anxiety | 189 (22.2) | 86 (19.9) | 103 (24.7) |
| Depression | 306 (36.0) | 157 (36.3) | 149 (35.7) |
| Other mental health diagnoses | 42 (4.9) | 19 (4.4) | 23 (5.5) |
| Median nonmalignantchronic pain types (IQR), n‡ | 4.0 (3.0–5.0) | 4.0 (3.0–5.0) | 4.0 (3.0–5.0) |
| Nonmalignant chronic pain types, n (%)‡ | |||
| Limb/extremity pain, joint pain, and arthritic disorders | 689 (81.1) | 348 (80.4) | 341 (81.8) |
| Back and neck pain | 627 (73.8) | 318 (73.4) | 309 (74.1) |
| General and widespread pain | 593 (69.8) | 307 (70.9) | 286 (68.6) |
| Abdominal and bowel pain | 237 (27.9) | 116 (26.8) | 121 (29.0) |
| Neuropathy | 209 (24.6) | 118 (27.3) | 91 (21.8) |
| Other painful conditions | 207 (24.4) | 104 (24.0) | 103 (24.7) |
| Fibromyalgia | 188 (22.1) | 90 (20.8) | 98 (23.5) |
| Musculoskeletal chest pain | 173 (20.4) | 79 (18.2) | 94 (22.5) |
| Headache | 184 (21.7) | 94 (21.7) | 90 (21.6) |
| Urogenital, pelvic, and menstrual pain | 65 (7.7) | 30 (6.9) | 35 (8.4) |
| Orofacial, ear, and temporomandibular disorder pain | 34 (4.0) | 18 (4.2) | 16 (3.8) |
| Median average daily dose of opioids (IQR), MME* | 29.6 (16.0–62.0) | 28.5 (16.0–53.2) | 30.8 (16.0–75.1) |
| Average daily dose of opioids ≥90 MME, n (%)* | 155 (18.2) | 67 (15.5) | 88 (21.1) |
| Benzodiazepine receipt, n (%)* | 227 (26.7) | 114 (26.3) | 113 (27.1) |
| High user of primary care services (≥12 contacts in 3-mo period), n (%)§ | 42 (4.9) | 20 (4.6) | 22 (5.3) |
BMI = body mass index; IQR = interquartile range; MME = morphine milligram equivalents; PPACT = Pain Program for Active Coping and Training.
Assessed for 180 d before randomization.
All patients, n = 841; intervention, n = 429; usual care, n = 412.
Assessed for 360 d before randomization. Pain types include limb/extremity pain, joint pain, and arthritic disorders; back and neck pain; general and widespread pain; abdominal and bowel pain; neuropathy; other painful conditions; fibromyalgia; musculoskeletal chest pain; headache; urogenital, pelvic, and menstrual pain; and orofacial, ear, and temporomandibular disorder pain.
Assessed for 90 d before randomization; primary care services include in-person, telephone, and e-mail encounters.
Those randomly assigned to the CBT intervention completed an average of 2.9 of 4 possible in-person sessions and 6.2 of 12 possible group sessions. We categorized 63% of intervention participants as “intervention completers” (that is, finished ≥2 in-person sessions and ≥6 group sessions). Therapist adherence to the protocol was high (mean, 4.4 on a scale of 0 to 5) but with a fair degree of variability (SD, 0.51; range, 3 to 5).
Pain Impact
Compared with the usual care group, intervention participants sustained larger reductions in the primary outcome of self-reported pain on the PEGS scale at 12 months of follow-up (difference, −0.434 point [95% CI, −0.690 to −0.178 point]) (Table 2), with an overall standardized effect size of −0.21 (Supplement Table 1, available at Annals.org). Intervention participants also had a greater reduction in PEGS score immediately after treatment (difference, −0.565 point [CI, −0.796 to −0.333 point]; overall standardized effect size, −0.28). Supplement Table 2 (available at Annals.org) shows the unadjusted means, and Table 3 and the Figure present estimated, model-based means showing the trajectory of PEGS scores from baseline over the follow-up time points. A larger proportion of intervention participants than usual care participants reported clinically meaningful improvement at the posttreatment assessment (proportion, 0.272 [CI, 0.231 to 0.312] vs. 0.134 [CI, 0.103 to 0.164]; absolute risk difference, 0.138 [CI, 0.088 to 0.188]) and over the 12-month follow-up (proportion, 0.266 [CI, 0.225 to 0.306] vs. 0.186 [CI, 0.150 to 0.222]; absolute risk difference, 0.079 [CI, 0.025 to 0.133]) (Table 4; Appendix Table 2, available at Annals.org). Findings for the 3-item PEG mirrored those for the 4-item PEGS (Tables 2 to 4). Appendix Table 2 presents the adjusted proportions of participants whose reduction in pain-related measures met thresholds for clinically meaningful improvement; unadjusted proportions are presented in Supplement Table 3 (available at Annals.org).
Table 2.
Estimated Differences in Change in Continuous Outcomes for CBT Versus Usual Care From Baseline to Posttreatment Assessment and Posttreatment Assessment to 12-Month Follow-up*
| Outcome | Baseline to Posttreatment Assessment† |
Posttreatment Assessment to 12 Months |
Baseline to 12 Months |
|||
|---|---|---|---|---|---|---|
| Within-Group Change (95% CI) | Relative Difference Between Groups (95% CI) | Within-Group Change (95% CI) | Relative Difference Between Groups (95% CI) | Within-Group Change (95% CI) | Relative Difference Between Groups (95% CI) | |
|
| ||||||
| PEGS score (n = 106 clusters, 850 participants, 3768 observations) | ||||||
| Usual care | −0.435 (−0.600 to −0.271) | - | −0.040 (−0.228 to 0.149) | - | −0.475 (−0.657 to −0.293) | - |
| CBT | −1.000 (−1.162 to −0.837) | −0.565 (−0.796 to −0.333) | 0.090 (−0.096 to 0.277) | 0.130 (−0.135 to 0.395) | −0.909 (−1.089 to −0.730) | −0.434 (−0.690 to −0.178) |
| PEG score (n = 106 clusters, 850 participants, 3768 observations) | ||||||
| Usual care | −0.455 (−0.625 to −0.285) | - | −0.054 (−0.247 to 0.139) | - | −0.509 (−0.695 to −0.322) | - |
| CBT | −1.062 (−1.230 to −0.895) | −0.607 (−0.846 to −0.369) | 0.120 (−0.071 to 0.310) | 0.174 (−0.098 to 0.445) | −0.942 (−1.126 to −0.759) | −0.434 (−0.695 to −0.172) |
| RMDQ score (n = 106 clusters, 849 participants, 3372 observations) | ||||||
| Usual care | −0.017 (−0.032 to −0.001) | - | 0.008 (−0.009 to 0.026) | - | −0.008 (−0.026 to 0.009) | - |
| CBT | −0.059 (−0.074 to −0.044) | −0.043 (−0.064 to −0.021) | −0.009 (−0.026 to 0.009) | −0.017 (−0.042 to 0.007) | −0.068 (−0.085 to −0.051) | −0.060 (−0.084 to −0.035) |
| Satisfaction with primary care services (n = 106 clusters, 848 participants, 1578 observations) | ||||||
| Usual care | −0.253 (−0.379 to −0.127) | - | NA | - | NA | - |
| CBT | −0.023 (−0.146 to 0.100) | 0.230 (0.053 to 0.406) | NA | NA | NA | NA |
| Satisfaction with pain services (n = 106 clusters, 849 participants, 1575 observations) | ||||||
| Usual care | 0.086 (−0.062 to 0.234) | - | NA | - | N/A | - |
| CBT | 0.422 (0.277 to 0.567) | 0.336 (0.129 to 0.543) | NA | N/A | N/A | N/A |
| Average daily dose of opioids, MME‡ (n = 106 clusters, 848 participants, 4081 observations) | ||||||
| Usual care | 1.443 (−0.868 to 3.755) | - | −3.397 (−6.468 to −0.325) | - | −1.954 (−5.377 to 1.470) | - |
| CBT | −0.817 (−3.100 to 1.466) | −2.260 (−5.509 to 0.989) | −3.106 (−6.111 to −0.100) | 0.291 (−4.006 to 4.588) | −3.922 (−7.281 to −0.564) | −1.969 (−6.765 to 2.827) |
CBT = cognitive behavioral therapy; MME = morphine milligram equivalents; PEG = pain intensity and interference with enjoyment of life and general activity; PEGS = pain intensity and interference with enjoyment of life, general activity, and sleep; RMDQ = Roland Morris Disability Questionnaire.
Analyses based on 3-level, piecewise linear mixed model. Negative values (i.e., lower or decreased scores) indicate improvement or advantage on the specified variable.
Posttreatment assessment is at 3-mo follow-up for PEGS, PEG, RMDQ, and MME and 6-mo follow-up for satisfaction with primary care services and satisfaction with pain services.
Winsorized.
Table 3.
Estimated Means and Associated 95% CIs, by Treatment Group and Time Point, for Primary and Secondary Continuous Outcomes
| Outcome | Estimated Mean (95% CI)* |
||||
|---|---|---|---|---|---|
| Baseline | 3-Month Follow-up | 6-Month Follow-up | 9-Month Follow-up | 12-Month Follow-up | |
|
| |||||
| PEGS score (0–10) | |||||
| Usual care | 6.645 (6.447–6.843) | 6.210 (5.994–6.426) | 6.197 (5.994–6.399) | 6.183 (5.976–6.390) | 6.170 (5.940–6.400) |
| CBT | 6.485 (6.290–6.681) | 5.486 (5.272–5.699) | 5.516 (5.316–5.715) | 5.546 (5.342–5.750) | 5.576 (5.349–5.803) |
| PEG score (0–10) | |||||
| Usual care | 6.750 (6.550–6.950) | 6.295 (6.077–6.514) | 6.277 (6.074–6.481) | 6.259 (6.051–6.468) | 6.242 (6.010–6.473) |
| CBT | 6.643 (6.446–6.840) | 5.581 (5.365–5.796) | 5.621 (5.420–5.821) | 5.660 (5.455–5.866) | 5.700 (5.472–5.929) |
| RMDQ score (0–1) | |||||
| Usual care | 0.692 (0.672–0.711) | 0.675 (0.654–0.696) | 0.678 (0.658–0.698) | 0.681 (0.660–0.701) | 0.683 (0.661–0.706) |
| CBT | 0.672 (0.652–0.691) | 0.612 (0.591 –0.633) | 0.609 (0.590–0.629) | 0.607 (0.586–0.627) | 0.604 (0.581–0.626) |
| Satisfaction with primary care services (0–10) | |||||
| Usual care | 4.237 (4.134–4.339) | - | 3.984 (3.856–4.112) | - | - |
| CBT | 4.263 (4.162–4.365) | - | 4.240 (4.115–4.365) | - | - |
| Satisfaction with pain services (0–10) | |||||
| Usual care | 3.581 (3.461 –3.700) | - | 3.667 (3.538–3.796) | - | - |
| CBT | 3.658 (3.540–3.776) | - | 4.080 (3.954–4.205) | - | - |
| Average daily dose of opioids, MME † | |||||
| Usual care | 55.252 (48.333–62.171) | 56.695 (49.556–63.834) | 55.563 (48.421–62.704) | 54.430 (47.142–61.719) | 53.298 (45.725–60.871) |
| CBT | 47.458 (40.647–54.269) | 46.641 (39.610–53.627) | 45.606 (38.574–52.638) | 44.571 (37.396–51.745) | 43.536 (36.085–50.986) |
MME = morphine milligram equivalents; PEG = pain intensity and interference with enjoyment of life and general activity; PEGS = pain intensity and interference with enjoyment of life, general activity, and sleep; RMDQ = Roland Morris Disability Questionnaire.
Based on 3-level, piecewise linear mixed model.
Winsorized.
Figure.
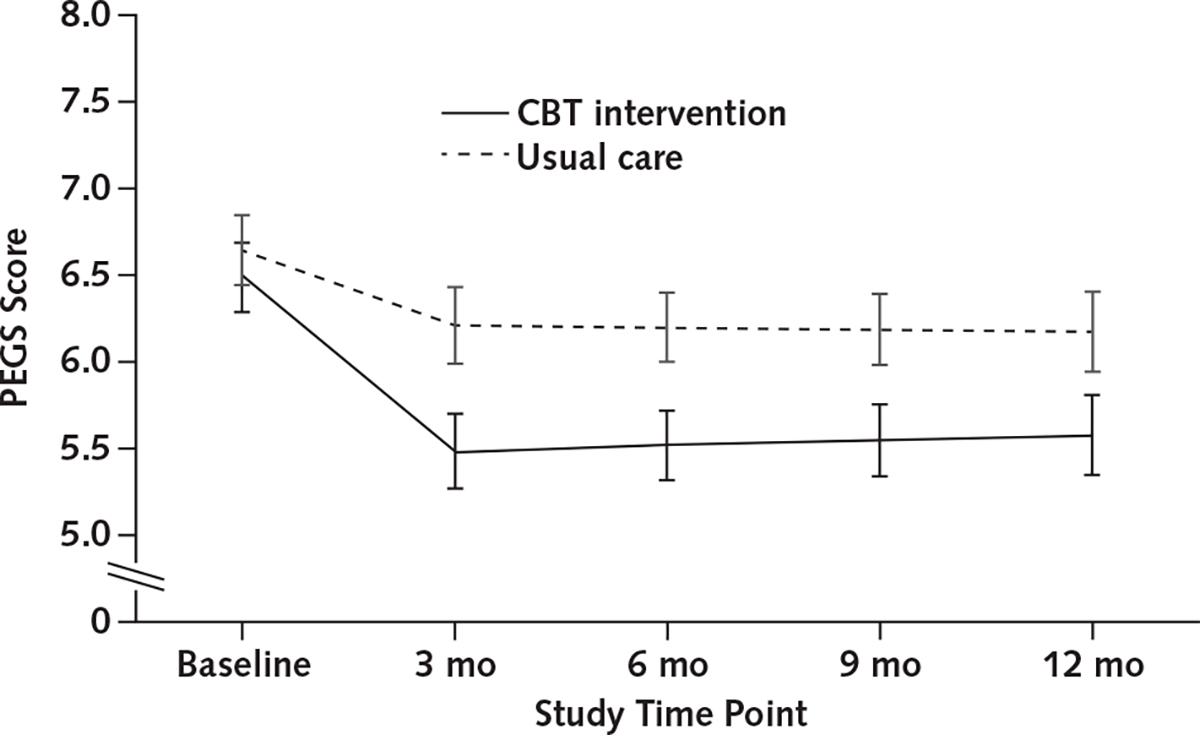
Estimated mean PEGS score, by treatment group and time point, from piecewise linear mixed model.
CBT = cognitive behavioral therapy; PEGS = pain intensity and interference with enjoyment of life, general activity, and sleep.
Table 4.
Estimated Risk Difference and Relative Risk for Minimal Clinically Important Differences in Pain-Related Outcomes, by Treatment Group*
| Outcome | Posttreatment Assessment† |
12-Month Follow-up |
||
|---|---|---|---|---|
| Risk Difference Between Groups (95% CI) | Relative Risk Between Groups (95% CI) | Risk Difference Between Groups (95% CI) | Relative Risk Between Groups (95% CI) | |
|
| ||||
| PEGS score change exceeding minimal clinically important difference threshold (n = 106 clusters, 814 participants, 2916 observations) | 0.138 (0.088–0.188) | 2.03 (1.55–2.67) | 0.079 (0.025–0.133) | 1.43 (1.12–1.82) |
| PEG score change exceeding minimal clinically important difference threshold (n = 106 clusters, 814 participants, 2916 observations) | 0.132 (0.081–0.183) | 1.92 (1.48–2.50) | 0.076 (0.023–0.129) | 1.42 (1.11–1.81) |
| RMDQ score change exceeding minimal clinically important difference threshold (n = 106 clusters, 779 participants, 2475 observations) | 0.071 (0.018–0.124) | 1.81 (1.22–2.68) | 0.125 (0.074–0.176) | 2.43 (1.67–3.51) |
PEG = pain intensity and interference with enjoyment of life and general activity; PEGS = pain intensity and interference with enjoyment of life, general activity, and sleep; RMDQ = Roland Morris Disability Questionnaire.
Minimal clinically important difference is defined as ≥30% decrease from baseline value.
3-mo follow-up.
Pain-Related Disability
The intervention had a significant effect on pain-related disability as measured using the RMDQ score at the posttreatment assessment (difference, −0.043 point [CI, −0.064 to −0.021 point]) and at 12 months (difference, −0.060 point [CI, −0.084 to −0.035 point]) (Table 2), with an overall standardized effect size of −0.28 at 12 months (Supplement Table 1). Table 3 presents the adjusted mean scores over time, and Supplement Table 2 presents the unadjusted scores. After treatment, proportions reporting clinically meaningful improvement were similar for the intervention and usual care groups (proportion, 0.159 [CI, 0.123 to 0.195] vs. 0.134 [CI, 0.103 to 0.164]; absolute risk difference, 0.071 [CI, 0.018 to 0.124]), with a modestly greater separation between groups at 12 months (proportion, 0.212 [CI, 0.168 to 0.255] vs. 0.087 [CI, 0.060 to 0.114]; absolute risk difference, 0.125 [CI, 0.074 to 0.176]) (Table 4; Supplement Table 3). Appendix Table 2 reports adjusted proportions for participants exceeding the minimal clinically important difference for RMDQ; unadjusted proportions are presented in Supplement Table 3.
Satisfaction
Change in satisfaction with primary care at 6 months was also different for participants in the intervention and usual care groups (difference, 0.230 point [CI, 0.053 to 0.406 point]) (Table 2). Intervention participants’ satisfaction remained relatively stable from baseline to the posttreatment assessment, whereas those in usual care reported a decline in satisfaction over that period (Table 3). Satisfaction with pain services showed similar differential change across groups (difference, 0.336 point [CI, 0.129 to 0.543 point]) (Table 2). Usual care participants had stable scores from baseline to the posttreatment assessment, whereas intervention participants had an increase in mean scores (Table 3); Supplement Table 2 presents unadjusted mean scores.
Medication-Related Outcomes
No intervention-related differences were seen in average daily opioid dose (in MME) at the posttreatment assessment (difference, −2.260 points [CI, −5.509 to 0.989 points]) or at 12 months (difference, −1.969 points [CI, −6.765 to 2.827 points]) (Table 2). Similarly, we found no intervention-related differences for the post hoc, opioid-related outcomes of continued long-term opioid therapy (12-month absolute risk difference, −0.010 [CI, −0.072 to 0.051]) or average daily dose of opioids of 90 MME or greater (12-month absolute risk difference, −0.018 [CI, −0.053 to 0.017]) (Table 5). An intervention effect was found for benzodiazepine receipt at 12 months, with greater reductions among intervention than usual care participants (absolute risk difference, −0.055 [CI, −0.099 to −0.011]), but not at the posttreatment assessment (absolute risk difference, −0.026 [CI, −0.065 to 0.012]) (Table 5). Appendix Table 3 (available at Annals.org) presents the estimated differences in relative risks for these outcomes, and Appendix Table 4 (available at Annals.org) includes the adjusted proportions.
Table 5.
Estimated Differences in Change in Binary Outcomes for CBT Versus Usual Care From Baseline to Posttreatment Assessment and Posttreatment Assessment to 12-Month Follow-up*
| Outcome | Baseline to Posttreatment Assessment† |
Posttreatment Assessmentto 12 Months |
Baseline to 12 Months |
|||
|---|---|---|---|---|---|---|
| Within-Group Change (95% CI) | Relative Difference Between Groups (95% CI) | Within-Group Change (95% CI) | Relative Difference Between Groups (95% CI) | Within-Group Change (95% CI) | Relative Difference Between Groups (95% CI) | |
|
| ||||||
| Benzodiazepine receipt (n = 106 clusters, 848 participants, 4081 observations) | ||||||
| Usual Care | 0.009 (−0.018 to 0.036) | - | −0.001<0 (−0.031 to 0.031) | - | 0.009 (−0.023 to 0.040) | - |
| CBT | −0.017 (−0.044 to 0.010) | −0.026 (−0.065 to 0.012) | −0.029 (−0.057 to 0>−0.001) | −0.028 (−0.070 to 0.014) | −0.046 (−0.077 to −0.015) | −0.055 (−0.099 to −0.011) |
| Continued long-term opioid therapy (n = 106 clusters, 848 participants, 4081 observations) | ||||||
| Usual Care | −0.045 (−0.083 to −0.007) | - | −0.039 (−0.081 to 0.003) | - | −0.085 (−0.129 to −0.041) | - |
| CBT | −0.067 (−0.106 to −0.029) | −0.022 (−0.075 to 0.031) | −0.028 (−0.070 to 0.014) | 0.012 (−0.047 to 0.071) | −0.095 (−0.139 to −0.052) | −0.010 (−0.072 to 0.051) |
| Average daily dose of opioids ≥90 MME (n = 106 clusters, 848 participants, 4081 observations) | ||||||
| Usual Care | 0.013 (−0.008 to 0.034) | - | −0.025 (−0.050 to <0.001) | - | −0.012 (−0.039 to 0.014) | - |
| CBT | 0.003 (−0.016 to 0.022) | −0.010 (−0.038 to 0.018) | −0.033 (−0.055 to −0.010) | −0.008 (−0.041 to 0.026) | −0.030 (−0.053 to −0.007) | −0.018 (−0.053 to 0.017) |
CBT = cognitive behavioral therapy; MME = morphine milligram equivalents.
Analyses based on 3-level, piecewise logistic mixed models. Negative values (i.e., lower or decreased) indicate improvement or advantage on the specified variable.
Posttreatment assessment is at 3 mo for all medication outcomes.
Adverse Outcomes
Twelve participants died; all deaths were unrelated to study participation. Hospitalizations totaled 287, with 116 for intervention participants and 171 for usual care.
Discussion
This pragmatic clinical trial tested whether a CBT intervention delivered in primary care by frontline clinicians improved measures of pain-related outcomes among patients with chronic pain receiving long-term opioid therapy. As hypothesized, those receiving CBT had greater reductions in pain impact (measured as a composite of pain intensity and interference) than those receiving usual care. Modest effects were seen at the posttreatment assessment (3 months) and sustained through 12 months, with standardized effect sizes of −0.28 and −0.21, respectively. Further, 1 in 4 participants receiving CBT reported clinically meaningful improvements in self-reported pain at 12 months, compared with 1 in 6 participants receiving usual care. Pain-related disability showed treatment effects that were similar in overall pattern and magnitude. In addition, the intervention group reported greater satisfaction than the usual care group with both primary care and pain-related health services. Our analyses suggested no intervention-related reductions in opioid use and inconsistent effects on benzodiazepine use.
Although the treatment-related effect on pain outcomes was small, it was similar to that found in recent systematic reviews of CBT and nonpharmacologic treatments for chronic pain (standardized effect sizes, 0.20 to 0.32) (43, 44). The magnitude of our intervention effects was also similar to that in trials of yoga for low back pain (43), which is relevant given the yoga-based exercise component of our intervention. Further, the magnitude of effects we found was similar to that for nonopioid pharmacotherapy over the short term (≤6 months) (45). Comparing the longer-term effects of our CBT intervention with those of either nonopioid or opioid pharmacotherapy is not feasible because evidence of their intermediate-term (≥6 months and <12 months) effects has not been established and long-term outcomes (≥12 months) have not been assessed (45, 46). The impact of the effects we saw on work productivity or disability are unclear, although productivity costs (absenteeism and presenteeism) have been associated with self-reported pain severity, suggesting the potential benefit of even moderate pain reductions (47).
The intensity of our intervention was similar to that in other CBT trials. However, our patients were on average older, more likely to be receiving disability benefits, and modestly higher in disease burden (that is, number and types of pain conditions and comorbid conditions). All were receiving long-term opioid therapy. We identified only 1 study in which participants were more likely to be receiving disability benefits than in our trial: Thorn and colleagues’ study (48) of a literacy-adapted CBT intervention for low-income persons at community health centers in the rural Southeast. Except for an opioid-taper pilot study that found no difference in opioid dose for a 17-session CBT intervention compared with usual care but significantly greater reductions in pain-related interference at 4 months (49), we are unaware of other trials of nonpharmacologic treatment restricted to patients receiving long-term opioid therapy. Others have described patients with chronic pain and long-term receipt of opioids as potentially a “lost generation” (50), given that opioid use may change their physiology and render them less responsive to behavioral treatments (45). However, our findings and those of Sullivan and colleagues (49, 51) suggest that behaviorally based interventions can be effective for such patients.
An important strength of our study was its focus on patients with chronic pain deemed at highest need by their medical providers: those receiving long-term opioid treatment with substantive medical and mental health comorbid conditions. We also had the advantage of a large and geographically diverse patient sample. Other strengths arose from pragmatic design features, including use of frontline clinicians, many without previous training in using evidence-based, nondrug treatment of chronic pain. Further, by embedding the treatment directly in primary care clinics and using clinical infrastructure to collect pain-related outcomes relevant to our health care providers, we enhanced the likelihood of sustainability in everyday clinical workflow (52).
Study limitations included that all participants were insured and were receiving care in large, integrated health care systems. Although advantageous for delivering this multidisciplinary, team-led intervention, this restriction limited generalizability. Further, although we sought to enroll patients prioritized by the health system due to high doses of opioids, concurrent benzodiazepine receipt, or high health care use, only a modest proportion met these criteria. This may have been partly because the narrow (4-week) window in which a PCP’s eligible patients could elect to participate (dictated by our cluster randomized approach) may have inadvertently eliminated patients who were initially hesitant about behavior-based treatments. Other limitations imposed by the pragmatic trial design include restricted patient-reported measures, including adverse events (16, 53). Identifying hospitalizations and deaths from electronic health records was feasible, but many less severe adverse events are not systematically documented. Finally, our power estimates were based on the sample needed to detect small effect sizes rather than minimal clinically important differences across study groups, although we included the latter in post hoc analyses.
Despite limitations, this study shows the potential for skill-based, CBT interventions delivered by frontline clinicians to reduce pain impact and improve function among patients with chronic pain receiving long-term opioid treatment. Although effects were modest, they persisted after treatment through final 12-month follow-up. Given the limited efficacy (45) and safety of long-term opioid treatment of chronic pain and increasing demand for nonpharmacologic treatment (54), this type of intervention may be an attractive option.
Supplementary Material
Acknowledgment:
The authors thank the KP patients who participated in the trial; their colleagues in the KP Georgia, Hawaii, and Northwest health care systems; and members of the KP Georgia, Hawaii, and Northwest institutional review boards. They thank Dr. Sarah Duffy, Associate Director for Economics Research, Division of Epidemiology, Services and Prevention Research for the National Institute on Drug Abuse, for serving as Project Scientist, and Dr. Stacey Honda, who served as the initial site principal investigator for KP Hawaii. The authors also thank the members of the research team who served as programmers and analysts for each region: Alexandra Varga, Matthew Slaughter, Lee Cromwell, and Carmen Wong; they received compensation for their contribution.
Financial Support:
By the National Institutes of Health Common Fund within the Health Care Systems Research Collaboratory through cooperative agreement U24AT009676 from the Office of Strategic Coordination within the Office of the Director and cooperative agreements UH2AT007788 and UH3NS088731 from the National Institute of Neurological Disorders and Stroke.
Appendix Table 1.
CBT-Based Pain Coping Skills Training Sessions
| Session | Skill Focus | Description |
|---|---|---|
|
| ||
| Session 1: Understanding pain/pain education and role of pain coping skills | Adaptation model/neuromatrix model of pain | Simple diagrams, including the neuromatrix and persistent pain cycle, are used to illustrate the pain cycle along with the role of the brain and other parts of the central nervous system in influencing the pain experience. The group explores pain’s effect on patients’ activities, feelings, and thoughts and how these changes similarly affect the pain they experience. The menu of coping skills modules is discussed, as well as the fact that these skills can be used not only for managing pain but also for managing stressors related to pain. Patients are taught how to use a brief relaxation method (PMR) that enables them to apply relaxation during daily activities that may increase their pain (e.g., walking, transferring from 1 position to another, and prolonged sitting). |
| Session 2: Applying PMR and adaptation model | PMR | Using the information presented in session 1, experiential activities encourage the group to envision how application of the program’s coping skills can change their pain, stress, and adaptation to challenging situations. Time is spent breaking down the PMR activity to promote a successful experience of this important skill and an overall understanding of how this directly affects the perception of pain and stress in the brain. |
| Session 3: Activity-rest cycle | Activity-rest cycling | Patients are taught to use a quota system to pace their activities and increase activity level. The quota system involves targeting a daily activity that the patient tends to overdo and learning to split this activity into periods of moderate activity (e.g., 10 min of walking) followed by limited rest (e.g., 5 min of rest). The patient will build up the activity quota over time. A range of activity options are discussed, along with benefits of gradually increasing activity. Barriers and obstacles to using this quota system are identified and solutions for overcoming them are formulated. |
| Session 4: Pleasant activity scheduling | Pleasant activity scheduling | Pleasant activity scheduling is used to help patients identify and incorporate a variety of enjoyable and realistic activities in their day-to-day life that help them overcome the deactivation common for patients with pain and to address mood-related impairments common among patients with chronic pain. |
| Session 5: Relaxation mini practices | Mini practices | Patients are taught to use, and then practice as a group, these brief relaxation techniques that are designed for use in the midst of various daily activities. These mini-practices provide an alternative to longer relaxation methods, such as the full PMR, but still provide the mental and physiological benefits necessary to overcome instances of pain, tension, and stress. |
| Session 6: Pleasant imagery | Pleasant imagery | Patients are assisted in identifying an imaginary, personal scene and are then guided through pleasant imagery sessions that focus attention on pleasant experiences in the midst of pain, stress, or negative thoughts. The group then strategizes about building these imagery sessions into their day to promote relaxation. |
| Session 7: Emotional regulation: leaning in | Leaning in: emotional regulation | Mood modulation skills, mindfulness, and the role of acceptance are taught and practiced to assist patients in working with strong emotions. |
| Session 8: Emotional regulation: leaning out | Leaning out: distraction | In working with patients to counterbalance leaning into and away from challenging emotions, distraction techniques using physical or auditory stimuli are discussed and practiced as helpful tools in managing pain. |
| Session 9: Cognitive restructuring | Cognitive restructuring | Cognitive restructuring is used to help patients recognize overly negative thoughts that occur in response to pain. Effects of such thoughts on feelings and behaviors are discussed. |
| Session 10: Use of calming self-statements | Positive self-statements | Patients develop alternative, calming/coping thoughts and self-statements that are more helpful/useful in coping with pain. |
| Session 11: Problem solving | Problem solving/reinforcing the application of learned skills | Following patient-stated reviews of the coping skills used throughout the program,the group works through several problem-solving scenarios to gain experience applying learned coping skills in the context of challenges faced. |
| Session 12: Relapse prevention and maintenance enhancement training | Relapse prevention/maintenance plan | Patients are taught strategies to enhance maintenance of learned coping skills. To pinpoint situational factors affecting maintenance, each patient is taught to identify high-risk situations that are likely to interfere with coping efforts. A rationale for anticipating and coping with setbacks is discussed. Cognitive strategies for recognizing early warning signs of pain and symptom flares and coping with setbacks are emphasized. |
CBT = cognitive behavioral therapy; PMR = progressive muscle relaxation.
Appendix Table 2.
Participants Exceeding MCID Thresholds for Pain-Related Outcomes: Model-Based Proportions and Associated 95% CIs, by Treatment Group and Time Point*
| Outcome | Usual Care |
CBT |
||||
|---|---|---|---|---|---|---|
| Proportion | 95% CI |
Proportion | 95% CI |
|||
| LB | UB | LB | UB | |||
|
| ||||||
| Participants exceeding MCID for PEGS | ||||||
| 3-mo follow-up | 0.134 | 0.103 | 0.164 | 0.272 | 0.231 | 0.312 |
| 6-mo follow-up | 0.149 | 0.124 | 0.175 | 0.267 | 0.235 | 0.299 |
| 9-mo follow-up | 0.167 | 0.139 | 0.195 | 0.265 | 0.232 | 0.299 |
| 12-mo follow-up | 0.186 | 0.150 | 0.222 | 0.266 | 0.225 | 0.306 |
| Participants exceeding MCID for PEG | ||||||
| 3-mo follow-up | 0.143 | 0.112 | 0.175 | 0.276 | 0.236 | 0.316 |
| 6-mo follow-up | 0.156 | 0.130 | 0.182 | 0.268 | 0.237 | 0.300 |
| 9-mo follow-up | 0.169 | 0.142 | 0.197 | 0.263 | 0.230 | 0.296 |
| 12-mo follow-up | 0.183 | 0.148 | 0.218 | 0.259 | 0.220 | 0.299 |
| Participants exceeding MCID for RMDQ | ||||||
| 3-mo follow-up | 0.088 | 0.060 | 0.116 | 0.159 | 0.123 | 0.195 |
| 6-mo follow-up | 0.083 | 0.062 | 0.104 | 0.177 | 0.146 | 0.208 |
| 9-mo follow-up | 0.084 | 0.061 | 0.106 | 0.196 | 0.162 | 0.231 |
| 12-mo follow-up | 0.087 | 0.060 | 0.114 | 0.212 | 0.168 | 0.255 |
CBT = cognitive behavioral therapy; LB = lower bound; MCID = minimal clinically important difference; PEG = pain, enjoyment of life, and general activity; PEGS = pain intensity and interference with enjoyment of life, general activity, and sleep; RMDQ = Roland Morris Disability Questionnaire; UB = upper bound.
MCID is defined as ≥30% decrease in score from baseline. Proportions are based on mixed model logistic regression equation.
Appendix Table 3.
Estimated RR Differences for CBT Versus Usual Care From Baseline to Posttreatment Assessment and Posttreatment Assessment to 12-Month Follow-up for Binary Outcomes*
| Outcome | Baseline to Posttreatment Assessment |
Posttreatment Assessment to 12 Months |
Baseline to 12 Months |
|||
|---|---|---|---|---|---|---|
| Within-Group RR (95% CI) | Between Group Ratio of RR (95% CI) | Within-Group RR (95% CI) | Between Group Ratio of RR (95% CI) | Within-Group RR (95% CI) | Between Group Ratio of RR (95% CI) | |
|
| ||||||
| Benzodiazepine receipt (n = 106 clusters, 848 participants, 4081 observations) | ||||||
| Usual care | 1.04 (0.92–1.18) | - | 1.00 (0.87–1.14) | - | 1.04 (0.90–1.20) | - |
| CBT | 0.93 (0.82–1.04) | 0.89 (0.75–1.05) | 0.87 (0.75–1.00) | 0.87 (0.71–1.06) | 0.80 (0.70–0.93) | 0.77 (0.63–0.95) |
| Continued long-term opioid therapy (n = 106 clusters, 848 participants, 4081 observations) | ||||||
| Usual care | 0.94 (0.89–0.99) | - | 0.95 (0.89–1.00) | - | 0.89 (0.84–0.95) | - |
| CBT | 0.91 (0.86–0.96) | 0.97 (0.90–1.04) | 0.96 (0.90–1.02) | 1.02 (0.93–1.11) | 0.88 (0.82–0.93) | 0.98 (0.90–1.07) |
| Average daily dose of opioids ≥90 MME (n = 106 clusters, 848 participants, 4081 observations) | ||||||
| Usual care | 1.05 (0.97–1.14) | - | 0.91 (0.82–1.00) | - | 0.95 (0.85–1.06) | - |
| CBT | 1.02 (0.91–1.13) | 0.97 (0.84–1.11) | 0.81 (0.70–0.94) | 0.90 (0.75–1.07) | 0.83 (0.71 –0.96) | 0.87 (0.72–1.04) |
CBT = cognitive behavioral therapy; MME = morphine milligram equivalents; RR = relative risk.
Analyses based on logistic mixed models.
Appendix Table 4.
Secondary Binary Study Outcomes: Model-Based Proportions and Associated 95% CIs, by Treatment Group and Time Point*
| Outcome | Usual Care |
CBT |
||||
|---|---|---|---|---|---|---|
| Proportion | 95% CI |
Proportion | 95% CI |
|||
| LB | UB | LB | UB | |||
|
| ||||||
| Benzodiazepine receipt | ||||||
| Baseline | 0.220 | 0.184 | 0.255 | 0.236 | 0.199 | 0.273 |
| 3-mo follow-up | 0.229 | 0.195 | 0.263 | 0.218 | 0.185 | 0.251 |
| 6-mo follow-up | 0.226 | 0.195 | 0.256 | 0.206 | 0.178 | 0.234 |
| 9-mo follow-up | 0.227 | 0.195 | 0.258 | 0.197 | 0.168 | 0.227 |
| 12-mo follow-up | 0.229 | 0.193 | 0.264 | 0.190 | 0.157 | 0.222 |
| Continued long-term opioid therapy | ||||||
| Baseline | 0.767 | 0.722 | 0.813 | 0.763 | 0.719 | 0.806 |
| 3-mo follow-up | 0.722 | 0.677 | 0.767 | 0.696 | 0.651 | 0.740 |
| 6-mo follow-up | 0.715 | 0.672 | 0.757 | 0.692 | 0.650 | 0.733 |
| 9-mo follow-up | 0.700 | 0.657 | 0.744 | 0.681 | 0.638 | 0.724 |
| 12-mo follow-up | 0.683 | 0.636 | 0.729 | 0.668 | 0.622 | 0.713 |
| Average daily dose of opioids ≥90 MME | ||||||
| Baseline | 0.253 | 0.234 | 0.273 | 0.173 | 0.153 | 0.192 |
| 3-mo follow-up | 0.266 | 0.252 | 0.280 | 0.175 | 0.160 | 0.191 |
| 6-mo follow-up | 0.256 | 0.241 | 0.270 | 0.162 | 0.147 | 0.178 |
| 9-mo follow-up | 0.247 | 0.230 | 0.265 | 0.151 | 0.134 | 0.168 |
| 12-mo follow-up | 0.241 | 0.218 | 0.264 | 0.143 | 0.122 | 0.164 |
CBT = cognitive behavioral therapy; LB = lower bound; MME = morphine milligram equivalents; UB = upper bound.
Proportions are based on mixed model logistic regression equation.
Appendix Figure.
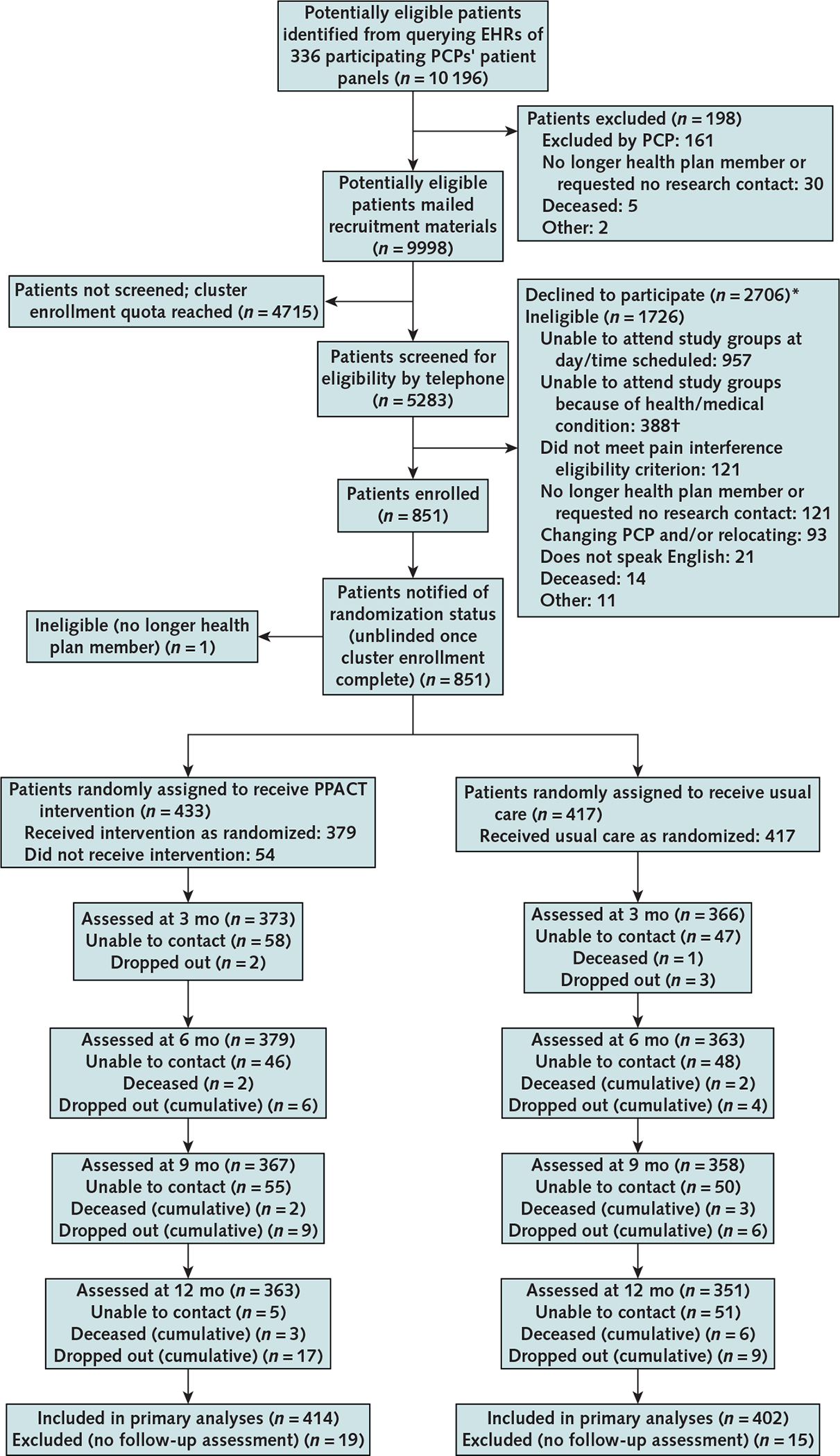
Study flow diagram.
EHR = electronic health record; PCP = primary care provider; PPACT = Pain Program for Active Coping and Training.
* Patients could decline to participate at any point in the screening process, including before the telephone eligibility interview; therefore, participants who declined to participate were not necessarily eligible.
† Too physically impaired to attend or unavailable because of condition (e.g., hospitalization or dialysis) or planned medical procedure (e.g., surgery).
Footnotes
Disclosures: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M21-1436.
For author, article, and disclosure information, see end of text.
Note: Dr. DeBar had access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Trinacty was affiliated with KP Hawaii during the time of the study.
Contributor Information
Lynn DeBar, Kaiser Permanente Washington Health Research Institute, Seattle, Washington.
Meghan Mayhew, Kaiser Permanente Center for Health Research, Portland, Oregon.
Lindsay Benes, Kaiser Permanente Center for Health Research, Portland, Oregon, and Montana State University College of Nursing, Missoula, Montana.
Allison Bonifay, Kaiser Permanente Center for Health Research, Portland, Oregon.
Richard A. Deyo, Oregon Health & Science University School of Medicine, Portland, Oregon.
Charles R. Elder, Kaiser Permanente Center for Health Research, Portland, Oregon.
Francis J. Keefe, Duke University School of Medicine, Durham, North Carolina.
Michael C. Leo, Kaiser Permanente Center for Health Research, Portland, Oregon.
Carmit McMullen, Kaiser Permanente Center for Health Research, Portland, Oregon.
Ashli Owen-Smith, Georgia State University School of Public Health and Kaiser Permanente Center for Clinical and Outcomes Research, Atlanta, Georgia.
David H. Smith, Kaiser Permanente Center for Health Research, Portland, Oregon.
Connie M. Trinacty, The Queen’s Medical Center, Honolulu, Hawaii.
William M. Vollmer, Kaiser Permanente Center for Health Research, Portland, Oregon.
Data Sharing Statement:
The following data will be made available with publication: deidentified participant data and data dictionary (NIH Collaboratory Living Textbook of Pragmatic Clinical Trials website: https://rethinkingclinicaltrials.org/data-and-resource-sharing). The following supporting documents will be made available with publication: As part of the data release, the authors will provide the following: 1) a detailed data dictionary listing all of the variables, their format, and, as appropriate, value labels; 2) for computed variables, actual code and/or descriptions of how these variables were created from the original source data; 3) summary statistics on all variables; and 4) summary tables describing, by treatment group, selected patient- and cluster-level characteristics, including data on patient race and PCP characteristics. These data will be made available on the public website https://rethinkingclinicaltrials.org/data-and-resource-sharing (restrictions: none).
References
- 1.Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education and Research. National Academies Pr; 2011. [PubMed] [Google Scholar]
- 2.Kroenke K, Cheville A. Management of chronic pain in the after-math of the opioid backlash. JAMA. 2017;317:2365–2366. doi: 10.1001/jama.2017.4884 [DOI] [PubMed] [Google Scholar]
- 3.Schneiderhan J, Clauw D, Schwenk TL. Primary care of patients with chronic pain. JAMA. 2017;317:2367–2368. doi: 10.1001/jama.2017.5787 [DOI] [PubMed] [Google Scholar]
- 4.Barry DT, Cutter CJ, Beitel M, et al. Psychiatric disorders among patients seeking treatment for co-occurring chronic pain and opioid use disorder. J Clin Psychiatry. 2016;77:1413–1419. doi: 10.4088/JCP.15m09963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maixner W, Fillingim RB, Williams DA, et al. Overlapping chronic pain conditions: implications for diagnosis and classification. J Pain. 2016;17:T93–T107. doi: 10.1016/j.jpain.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edmond SN, Heapy AA, Kerns RD. Engaging mental health professionals in addressing pain. JAMA Psychiatry. 2019;76:565–566. doi: 10.1001/jamapsychiatry.2019.0254 [DOI] [PubMed] [Google Scholar]
- 7.Manchikanti L, Singh A. Therapeutic opioids: a ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Physician. 2008;11:S63–88. [PubMed] [Google Scholar]
- 8.Von Korff M, Kolodny A, Deyo RA, et al. Long-term opioid therapy reconsidered. Ann Intern Med. 2011;155:325–8. doi: 10.7326/0003-4819-155-5-201109060-00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scholl L, Seth P, Kariisa M, et al. Drug and opioid-involved overdose deaths — United States, 2013–2017. MMWR Morb Mortal Wkly Rep. 2018;67:1419–1427. doi: 10.15585/mmwr.mm675152e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315:1624–45. doi: 10.1001/jama.2016.1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bair MJ, Ang D, Wu J, et al. Evaluation of stepped care for chronic pain (ESCAPE) in veterans of the Iraq and Afghanistan conflicts: a randomized clinical trial. JAMA Intern Med. 2015;175:682–9. doi: 10.1001/jamainternmed.2015.97 [DOI] [PubMed] [Google Scholar]
- 12.Kroenke K, Krebs E, Wu J, et al. Stepped Care to Optimize Pain care Effectiveness (SCOPE) trial study design and sample characteristics. Contemp Clin Trials. 2013;34:270–81. doi: 10.1016/j.cct.2012.11.008 [DOI] [PubMed] [Google Scholar]
- 13.Kroenke K, Krebs EE, Wu J, et al. Telecare collaborative management of chronic pain in primary care: a randomized clinical trial. JAMA. 2014;312:240–8. doi: 10.1001/jama.2014.7689 [DOI] [PubMed] [Google Scholar]
- 14.Dobscha SK, Corson K, Perrin NA, et al. Collaborative care for chronic pain in primary care: a cluster randomized trial. JAMA. 2009;301:1242–52. doi: 10.1001/jama.2009.377 [DOI] [PubMed] [Google Scholar]
- 15.Von Korff M, Balderson BHK, Saunders K, et al. A trial of an activating intervention for chronic back pain in primary care and physical therapy settings. Pain. 2005;113:323–330. doi: 10.1016/j.pain.2004.11.007 [DOI] [PubMed] [Google Scholar]
- 16.DeBar L, Benes L, Bonifay A, et al. Interdisciplinary team-based care for patients with chronic pain on long-term opioid treatment in primary care (PPACT) – protocol for a pragmatic cluster randomized trial. Contemp Clin Trials. 2018;67:91–99. doi: 10.1016/j.cct.2018.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loudon K, Treweek S, Sullivan F, et al. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015;350:h2147. doi: 10.1136/bmj.h2147 [DOI] [PubMed] [Google Scholar]
- 18.Mayhew M, DeBar LL, Deyo RA, et al. Development and assessment of a crosswalk between ICD-9-CM and ICD-10-CM to identify patients with common pain conditions. J Pain. 2019;20:1429–1445. doi: 10.1016/j.jpain.2019.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wren AA, Wright MA, Carson JW, et al. Yoga for persistent pain: new findings and directions for an ancient practice. Pain. 2011;152:477–480. doi: 10.1016/j.pain.2010.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carson JW, Carson KM, Jones KD, et al. A pilot randomized controlled trial of the Yoga of Awareness program in the management of fibromyalgia. Pain. 2010;151:530–539. doi: 10.1016/j.pain.2010.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carson JW, Carson KM, Porter LS, et al. Yoga of Awareness program for menopausal symptoms in breast cancer survivors: results from a randomized trial. Support Care Cancer. 2009;17:1301–9. doi: 10.1007/s00520-009-0587-5 [DOI] [PubMed] [Google Scholar]
- 22.Carson JW, Carson KM, Porter LS, et al. Yoga for women with metastatic breast cancer: results from a pilot study. J Pain Symptom Manage. 2007;33:331–41. [DOI] [PubMed] [Google Scholar]
- 23.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap. 1994;23:129–38. [PubMed] [Google Scholar]
- 24.Keller S, Bann CM, Dodd SL, et al. Validity of the Brief Pain Inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20:309–18. [DOI] [PubMed] [Google Scholar]
- 25.Roland M, Morris R. A study of the natural history of back pain. Part I: development of a reliable and sensitive measure of disability in low-back pain. Spine (Phila Pa 1976). 1983;8:141–4. [DOI] [PubMed] [Google Scholar]
- 26.Roland M, Fairbank J. The Roland-Morris Disability Questionnaire and the Oswestry Disability Questionnaire. Spine (Phila Pa 1976). 2000;25:3115–24. [DOI] [PubMed] [Google Scholar]
- 27.Davidson M, Keating JL. A comparison of five low back disability questionnaires: reliability and responsiveness. Phys Ther. 2002;82:8–24. [DOI] [PubMed] [Google Scholar]
- 28.Jordan K, Dunn KM, Lewis M, et al. A minimal clinically important difference was derived for the Roland-Morris Disability Questionnaire for low back pain. J Clin Epidemiol. 2006;59:45–52. [DOI] [PubMed] [Google Scholar]
- 29.Stratford PW, Binkley J, Solomon P, et al. Defining the minimum level of detectable change for the Roland-Morris questionnaire. Phys Ther. 1996;76:359–65; discussion 366–8. [DOI] [PubMed] [Google Scholar]
- 30.Kroenke K, Krebs EE, Turk D, et al. Core outcome measures for chronic musculoskeletal pain research: recommendations from a Veterans Health Administration work group. Pain Med. 2019;20:1500–1508. doi: 10.1093/pm/pny279 [DOI] [PubMed] [Google Scholar]
- 31.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–21. [DOI] [PubMed] [Google Scholar]
- 32.Krebs EE, Bair MJ, Damush TM, et al. Comparative responsiveness of pain outcome measures among primary care patients with musculoskeletal pain. Med Care. 2010;48:1007–14. doi: 10.1097/MLR.0b013e3181eaf835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dworkin RH, Turk DC, McDermott MP, et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain. 2009;146:238–244. doi: 10.1016/j.pain.2009.08.019 [DOI] [PubMed] [Google Scholar]
- 34.Allison PD. Missing Data. Sage; 2002. doi: 10.4135/9781412985079 [DOI] [Google Scholar]
- 35.Enders CK. Applied Missing Data Analysis. Guilford Pr; 2010. [Google Scholar]
- 36.Stata 15. Version 15. StataCorp; 2017. [Google Scholar]
- 37.Hox JJ. Multilevel Analysis: Techniques and Applications. 2nd ed. Routledge; 2010. [Google Scholar]
- 38.Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. 2nd ed. Sage; 2002. [Google Scholar]
- 39.Snijders TAB, Bosker RJ. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. Sage; 1999. [Google Scholar]
- 40.Wagner AK, Soumerai SB, Zhang F, et al. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299–309. [DOI] [PubMed] [Google Scholar]
- 41.Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13:S38–44. doi: 10.1016/j.acap.2013.08.002 [DOI] [PubMed] [Google Scholar]
- 42.Garson GD. Hierarchical Linear Modeling: Guide and Applications. Sage; 2013. doi: 10.4135/9781483384450 [DOI] [Google Scholar]
- 43.Skelly AC, Chou R, Dettori JR, et al. Noninvasive Nonpharmacological Treatment for Chronic Pain: A Systematic Review Update. AHRQ publication no. 20-EHC009. Agency for Healthcare Research and Quality; 2020. doi: 10.23970/AHRQEPCCER227 [DOI] [PubMed] [Google Scholar]
- 44.Williams ACC, Fisher E, Hearn L, et al. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2020;8:CD007407. doi: 10.1002/14651858.CD007407.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McDonagh MS, Selph SS, Buckley DI, et al. AHRQ Comparative Effectiveness Reviews. Nonopioid Pharmacologic Treatments for Chronic Pain. AHRQ publication no. 20-EHC010. Agency for Healthcare Research and Quality; 2020. doi: 10.23970/AHRQEPCCER228 [DOI] [PubMed] [Google Scholar]
- 46.Busse JW, Wang L, Kamaleldin M, et al. Opioids for chronic noncancer pain: a systematic review and meta-analysis. JAMA. 2018;320:2448–2460. doi: 10.1001/jama.2018.18472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lalonde L, Choinière M, Martin E, et al. Costs of moderate to severe chronic pain in primary care patients - a study of the ACCORD Program. J Pain Res. 2014;7:389–403. doi: 10.2147/JPR.S55388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thorn BE, Eyer JC, Van Dyke BP, et al. Literacy-adapted cognitive behavioral therapy versus education for chronic pain at low-income clinics: a randomized controlled trial. Ann Intern Med. 2018;168:471–480. doi: 10.7326/M17-0972 [DOI] [PubMed] [Google Scholar]
- 49.Sullivan MD, Turner JA, DiLodovico C, et al. Prescription opioid taper support for outpatients with chronic pain: a randomized controlled trial. J Pain. 2017;18:308–318. doi: 10.1016/j.jpain.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ballantyne JC. Opioid analgesia: perspectives on right use and utility. Pain Physician. 2007;10:479–91. [PubMed] [Google Scholar]
- 51.Ballantyne JC, Sullivan MD. Discovery of endogenous opioid systems: what it has meant for the clinician’s understanding of pain and its treatment. Pain. 2017;158:2290–2300. doi: 10.1097/j.pain.0000000000001043 [DOI] [PubMed] [Google Scholar]
- 52.Owen-Smith A, Mayhew M, Leo MC, et al. Automating collection of pain-related patient-reported outcomes to enhance clinical care and research. J Gen Intern Med. 2018;33:31–37. doi: 10.1007/s11606-018-4326-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DeBar LL, Kindler L, Keefe FJ, et al. A primary care-based interdisciplinary team approach to the treatment of chronic pain utilizing a pragmatic clinical trials framework. Transl Behav Med. 2012;2:523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fritz JM, Davis AF, Burgess DJ, et al. Pivoting to virtual delivery for managing chronic pain with nonpharmacological treatments: implications for pragmatic research. Pain. 2021;162:1591–1596. doi: 10.1097/j.pain.0000000000002139 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following data will be made available with publication: deidentified participant data and data dictionary (NIH Collaboratory Living Textbook of Pragmatic Clinical Trials website: https://rethinkingclinicaltrials.org/data-and-resource-sharing). The following supporting documents will be made available with publication: As part of the data release, the authors will provide the following: 1) a detailed data dictionary listing all of the variables, their format, and, as appropriate, value labels; 2) for computed variables, actual code and/or descriptions of how these variables were created from the original source data; 3) summary statistics on all variables; and 4) summary tables describing, by treatment group, selected patient- and cluster-level characteristics, including data on patient race and PCP characteristics. These data will be made available on the public website https://rethinkingclinicaltrials.org/data-and-resource-sharing (restrictions: none).


