Abstract
Background
Ustekinumab has been recently approved for the treatment of moderately to severely active ulcerative colitis (UC). The registry trials for ustekinumab in UC demonstrated efficacy and safety, but data on real-world outcomes are limited. We describe the effectiveness and safety of ustekinumab in patients with UC from 2 US tertiary inflammatory bowel disease centers.
Methods
Patients with moderately to severely active UC treated with ustekinumab at NYU Langone Health (New York, New York) and University of Chicago Medical Center (Chicago, Illinois) between January 2016 and March 2020 were retrospectively included. The primary outcome was clinical remission at 3 and 12 months, defined as a partial Mayo score of ≤2, with a combined rectal bleeding and stool frequency subscore of ≤1.
Results
Sixty-six UC patients were included. Ninety-two percent of patients had prior exposure to biologics or tofacitinib. Forty-three percent and 45% of patients achieved clinical remission by 3 and 12 months, respectively. Anti-TNF nonresponse and endoscopic Mayo score of 3 were negative predictors of clinical remission. Thirty-three percent of those followed for a year achieved concurrent endoscopic and histologic healing, which was significantly associated with lower partial Mayo score (P < 0.01) and lower stool frequency (P = 0.02). Serious adverse events occurred in 4 (6%) patients (3 UC exacerbations, 1 vasculitis).
Conclusions
In this cohort of mostly biologic-refractory UC patients, treatment with ustekinumab achieved remission in nearly half of them at 12 months, and was associated with an overall favorable safety profile. These results are modestly better than the pivotal trials.
Keywords: ulcerative colitis, ustekinumab, real-world experience, clinical remission
INTRODUCTION
Ulcerative colitis (UC) is an inflammatory bowel disease (IBD) affecting nearly 1.5 million Americans1 and patients with moderately to severely active UC have increased morbidity, rates of hospitalizations, and need for surgeries.2,3 Numerous biologic and small molecule therapies are available for the treatment of moderately to severely active UC, but with limited efficacy and primary nonresponse rates in pivotal trials as high as 52%–79%.4–9 Additionally, among patients who initially respond to therapy, a high percentage will lose response over time.10–12
Ustekinumab, a human monoclonal antibody that targets the p40 subunit of interleukin (IL)-12 and IL-23, was FDA approved for the treatment of Crohn disease in September 2016 and for the treatment of moderately to severely active UC in October 2019.13 The pivotal UNIFI (Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis) trial demonstrated significant efficacy and safety with clinical remission rates of 15% at week 8 and 40% at week 44, both of which were significantly higher than placebo (24%).14 However, it is well recognized that study populations in clinical trials may not accurately represent the general IBD population, which may limit the generalizability of their results.15 Therefore, real-world assessment of both effectiveness and safety is needed.
We sought to describe the experience of ustekinumab in UC from 2 tertiary IBD centers in the United States.
METHODS
Study Population
This is a retrospective cohort study of all patients with moderately to severely active UC who received ustekinumab at NYU Langone Health (New York, New York) or University of Chicago Medical Center (Chicago, Illinois) between January 2016 and March 2020. The study period includes patients who received ustekinumab prior to FDA approval for UC in October 2019, when its use was considered “off-label” and limited to patients who had exhausted other treatment options. This study did not include any patients enrolled in the registry trials for ustekinumab in UC.
Baseline Characteristics
We recorded patient demographics, disease characteristics, and medical treatment history. Data included age, sex, duration of disease, extent of disease using the Montreal classification, history of prior hospitalizations, and presence of comorbidities such as UC-related arthropathy, psoriasis, and psoriatic arthritis. Baseline laboratory values of white blood cell count, hemoglobin, albumin, C-reactive protein (CRP), and fecal calprotectin were collected. Prior and current medication exposures were recorded. For those with prior anti-TNF exposure, reason for cessation was recorded including primary nonresponse, secondary nonresponse (loss of response), and drug intolerance due to adverse side effects based on narrative chart review. Baseline and follow-up total Mayo scores and Mayo component subscores were recorded.
Outcomes
The primary outcome was clinical remission at 3 and 12 months after ustekinumab induction. Clinical remission was defined as a partial Mayo score of ≤2, with a combined rectal bleeding and stool frequency subscore of ≤1. Secondary endpoints included clinical response at 3 and 12 months, defined as a decrease in partial Mayo score of at least 3 point and 30% from baseline with at least a 1 point decrease in the rectal bleeding subscore or a rectal bleeding subscore of 0 or 1; other secondary endpoints include corticosteroid-free remission, endoscopic remission defined as a Mayo endoscopic subscore of 0 or 1, mucosal healing defined as histologic quiescence, histo-endoscopic healing defined as a combined outcome of Mayo endoscopic subscore of 0 and histologic quiescence, persistence on ustekinumab and increase in frequency of ustekinumab dosing. Outcomes at 3 and 12 months were analyzed based on patients with available follow-up at those time points. Total Mayo scores at 3 and 12 months were recorded when data were available. All adverse events occurring during follow-up were recorded, and serious adverse events were defined as any adverse event leading to treatment discontinuation, hospitalization, disability, colectomy, or death.
Statistical Analysis
Continuous variables were reported as means and standard deviations or medians with interquartile ranges (IQRs) and were compared using Student t test or Wilcoxon rank-sum test. Categorical values were reported as percentages and were compared using chi-square analysis or Fisher exact test for frequencies less than 5. Predictors of clinical remission at 3 and 12 months were identified using univariate logistic regression. Multivariate logistic regression was then performed, adjusting for any variables with P < 0.10 in the univariate analysis. Odds ratios (ORs) with 95% confidence intervals (CIs) are reported. A Kaplan–Meier analysis was performed to assess rates of treatment discontinuation over time. A P-value of <0.05 was considered statistically significant for all tests. Statistical analysis was performed using SPSS v25 (IBM, Armonk, NY).
Ethical Considerations
This study was approved by the Institutional Review Boards at NYU Langone Health (New York, New York) and the University of Chicago Medical Center (Chicago, Illinois).
RESULTS
Patient Demographics, Disease Characteristics, and Treatment History
A total of 66 patients with UC were treated with ustekinumab during the study period (Table 1). All patients received the standard weight-based IV induction dose (260, 390, or 520 mg) followed by 90 mg subcutaneous (SC) injection every 8 weeks thereafter. Of these patients, 49% were men; median age was 40 years (IQR 28–54). The median duration of UC was 6.0 (IQR 3.0–15.0) years and the majority of patients had extensive colitis (60.6%) or left-sided colitis (36.4%). The mean baseline partial Mayo score was 4.1 ± 2.0. Baseline endoscopies were available in 64 patients (96.9%), for whom the mean baseline total Mayo score was 6.5 ± 2.4.
Table 1.
Patient and Disease Characteristics and Baseline Laboratory Parameters
| All Patients (n = 66) | |
|---|---|
| Patient characteristics | |
| Male | 32 (48.5) |
| Age (y) | 39.5 (28.0, 54.0) |
| BMI | 26.6 ± 5.1 |
| Smoking | |
| Former | 23 (34.8) |
| Current | 4 (6.1) |
| Prior UC-related hospitalization | 27 (40.9) |
| UC-related arthropathy | 14 (12.1) |
| Psoriatic arthritis | 1 (1.5) |
| Psoriasis | 11 (16.7) |
| Disease characteristics | |
| Duration of disease (y) | 6.0 (3.0, 15.0) |
| Extent of UC | |
| Extensive colitis | 40 (60.6) |
| Left-sided colitis | 24 (36.4) |
| Proctitis | 2 (3.0) |
| Baseline total Mayo Score | 6.5 ± 2.4 |
| No. with score ≥6 (moderate to severe disease) | 45 (68.2) |
| Baseline partial Mayo score | 4.1 ± 2.0 |
| Baseline endoscopic subscore (n = 64) | 2.3 ± 0.9 |
| Laboratory parameters | |
| WBC (103/µL) | 8.7 ± 3.2 |
| Hemoglobin (g/dL) | 12.4 ± 1.7 |
| Albumin (g/dL) | 4.1 (3.9, 4.3) |
| CRP (mg/L) (n = 48) | 4.8 (2.1, 15.3) |
| Fecal calprotectin (µg/g) (n = 9) | 1000.0 (500.0, 1251.0) |
| Prior medications | |
| Aminosalicylate | 63 (95.5) |
| Corticosteroid | 64 (97.0) |
| Immunomodulator (IMM) | 37 (56.1) |
| TNF-antagonist | 56 (84.8) |
| Prior use of ≥2 TNF antagonists | 16 (24.2) |
| Combination therapy with IMM | 17/56 (30.4) |
| Primary nonresponse | 31/56 (55.3) |
| Secondary loss of response | 17/56 (30.4) |
| History of anti-TNF antibodies | 10/39 (25.6) |
| Vedolizumab | 50 (75.8) |
| Tofacitinib | 22 (33.3) |
| Prior use of any biologic or novel small molecule | 61 (92.4) |
| Prior use of ≥2 biologics or novel small molecules | 49 (74.2) |
| Mean no. prior biologics/novel small molecules | 2.2 ± 1.2 |
| Current medications | |
| Aminosalicylate | 16 (24.2) |
| Corticosteroid | 23 (34.8) |
| Duration ≥3 months | 12/23 (52.2) |
| Immunomodulator | 8 (12.1) |
| TNF-antagonist | 9 (13.6) |
| Vedolizumab | 4 (6.1) |
| Tofacitinib | 6 (9.1) |
Proportions reported as no. (%). Continuous variables reported as mean ± SD, or median (IQR) when data not distributed normally.
CRP, C-reactive protein; WBC, white blood cell.
Overall, 92.4% of patients had any prior exposure to biologics or tofacitinib. A history of anti-TNF agents, vedolizumab or tofacitinib exposure was noted in 84.8%, 75.8%, and 33.3% of patients, respectively. A majority of patients (74.2%) had exposure to 2 or more biologic/small molecules and 24.2% had a history of 2 or more anti-TNF agents. Concurrent steroids at the time of ustekinumab induction were present in 34.8% of patients; 52.2% (12/23) of whom were on steroids for longer than 3 months.
Clinical Outcomes
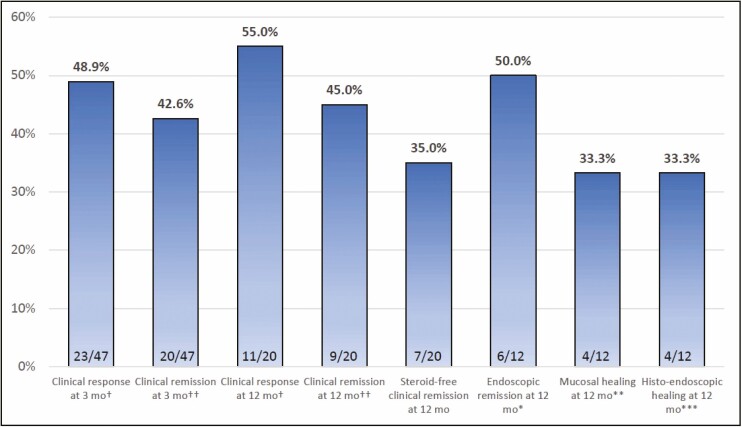
Patients had a total median duration of follow-up of 178 (IQR 57–482) days, with partial Mayo scores available at 3 and 12 months in 47 (71.3%) and 20 (30.3%) patients, respectively. 20/47 patients (42.6%) achieved the primary endpoint of clinical remission at 3 months and 9/20 patients (45.0%) at 12 months (Fig. 1). Corticosteroid-free remission rates were 15/47 (31.9%) and 7/20 (35.0%) at 3 and 12 months, respectively. 23/47 patients (48.9%) achieved clinical response at 3 months and 11/20 patients (55.0%) achieved response at 12 months. Of the 12 patients with colonoscopies available at 12 months, 6 (50.0%) were in endoscopic remission and 4 (33.3%) had mucosal healing. At 12 months, there was a significant decrease in mean partial Mayo score (1.8 ± 2.6; P < 0.01) and a nonsignificant decrease in median fecal calprotectin [1000 (IQR 500–51) vs 366 (IQR 115–978); P = 0.06] in those with fecal calprotectin levels available (N = 5). There was no significant decrease in median CRP at 12 months [4.8 (IQR 2.1–15.3) vs 4.6 (IQR 1.3–17.4); P = 0.06] in those with levels at 12 months (N = 17). A subgroup analysis of patients with total Mayo scores available at 3 months (n = 11) and 12 months (n = 12), showed no differences in clinical remission based on partial vs total Mayo scores at 3 months (36.4% vs 41.3%; P = 0.76) or 12 months (41.7% vs 45.0%; P = 0.77).
Figure 1.
Clinical outcomes at 3 and 12 months. †Clinical response defined as decrease in partial Mayo score of at least 30% or 3 points from baseline, with an accompanying decrease of at least 1 point on the Mayo rectal bleeding subscore or a rectal bleeding subscore of 0 or 1. ††Clinical remission defined as partial Mayo score of 2 or less with no subscore greater than 1. *Endoscopic remission defined as a Mayo endoscopic subscore of 0 or 1. **Mucosal healing defined as histologic quiescence. ***Histo-endoscopic healing defined as Mayo endoscopic subscore of 0 and histologic quiescence.
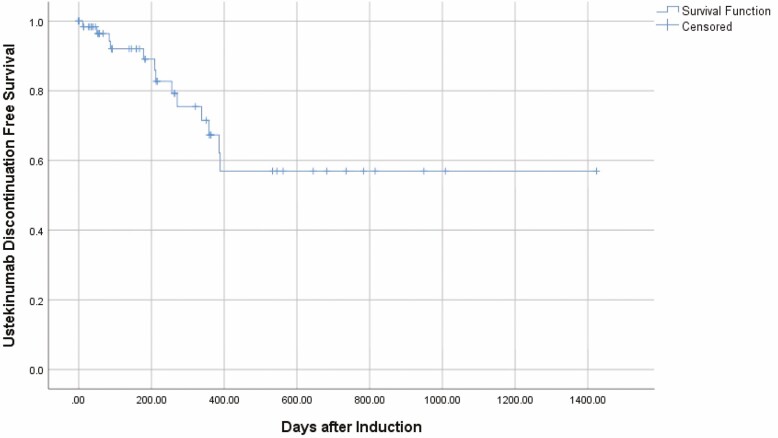
When clinical outcomes were analyzed based on the patient’s most recent follow-up, 47 patients (71.2%) remained on ustekinumab, and 29 patients (43.9%) were receiving monthly dosing (Table 2). For those patients with partial Mayo scores recorded at their most recent visit, 20/53 patients (37.3%) were in clinical remission. Thirteen patients (19.6%) had follow-up longer than 12 months, with a median follow-up time of 682 days (IQR 539–882) and 6/13 of these patients (46.2%) were in clinical remission at their most recent follow-up. The majority of ustekinumab discontinuation events occurred within the first year after induction (Fig. 2).
Table 2.
Clinical Outcomes at Most Recent Follow-Up
| All Patients (n = 66) | |
|---|---|
| Follow-up duration median days (minimum, maximum) | 178 (12, 1424) |
| >12 months follow-up | 13 (19.6) |
| Still on ustekinumab at most recent follow-up | 47 (71.2) |
| Median days to ustekinumab cessation (IQR) | 209 (56, 358) |
| Increased ustekinumab to q4 week dosing | 29 (43.9) |
| Median days to q4 week dose escalation (IQR) | 114 (63, 147) |
| Clinical remission achieved after dose escalation†† | 11/27 (40.7) |
| Ustekinumab discontinued after dose escalation | 8/27 (29.6) |
| Clinical response at most recent follow-up† | 30/53 (56.6) |
| Clinical remission at most recent follow-up†† | 20/53 (37.3) |
| Endoscopic remission at most recent endoscopy* | 9/25 (36.0) |
*Endoscopic remission defined as a Mayo endoscopic subscore of 0 or 1.
†Clinical response defined as decrease in partial Mayo score of at least 30% or 3 points from baseline, with an accompanying decrease of at least 1 point on the Mayo rectal bleeding subscore or a rectal bleeding subscore of 0 or 1.
††Clinical remission defined as partial Mayo score of 2 or less with no subscore greater than 1.
Figure 2.
Rate of ustekinumab discontinuation over time. (Represents confirmed ustekinumab discontinuation events over time. All other patients censored at time of last visit when ustekinumab use was recorded as active.)
Predictors of Clinical Remission at 3 and 12 Months
In univariate analysis, a history of TNF-antagonist primary nonresponse (P = 0.05), CRP >5 mg/L (P = 0.03), baseline total Mayo score ≥6 (P = 0.01), and baseline Mayo endoscopic subscore = 3 (P < 0.01) were significant negative predictors of clinical remission at 3 months, while prior vedolizumab was not a statistically significant negative predictor (P = 0.09) (Table 3). In multivariate analysis, history of TNF-antagonist primary nonresponse [OR = 0.03, 95% CI (0.01–0.82); P = 0.04] and baseline Mayo endoscopic score of 3 [OR = 0.04, 95% CI (0.01–0.73); P = 0.03] remained significant predictors, while CRP >5 mg/L was not a statistically significant predictor [OR = 0.06, 95% CI (0.04–1.08); P = 0.06].
Table 3.
Predictors of Clinical Remission at 3 and 12 Months
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Predictors at 3 months (n = 47) | ||||
| Prior TNFa primary nonresponse* | 0.28 (0.08–1.02) | 0.05 | 0.03 (0.01–0.82) | 0.04 |
| Prior vedolizumab use | 0.27 (0.06–1.27) | 0.09 | — | NS |
| CRP >5 mg/L | 0.17 (0.04–0.81) | 0.03 | 0.06 (0.04–1.08) | 0.06 |
| Baseline total Mayo score ≥6 | 0.09 (0.02–0.51) | <0.01 | — | NS |
| Baseline Mayo endoscopic score = 3 | 0.18 (0.05–0.66) | 0.01 | 0.04 (0.01–0.73) | 0.03 |
| Predictors at 12 months (n = 20) | ||||
| Disease duration ≥5 years | 0.10 (0.01–0.90) | 0.04 | — | NS |
| CRP >5 mg/L | 0.09 (0.01–1.08) | 0.06 | — | NS |
| Prior TNFa primary nonresponse* | 0.40 (0.05–3.42) | 0.40 | Not included | |
| Baseline Mayo endoscopic score = 3 | 0.17 (0.02–1.38) | 0.10 | Not included | |
*History of TNFa primary nonresponse, in contrast to those with secondary nonresponse (loss of response) or intolerance in TNFa-exposed patients.
In univariate analysis of predictors of clinical remission at 12 months, only disease duration ≥5 years (P = 0.04) and CRP ≥5 mg/L (P = 0.06) were significant, but neither were significant in multivariate analysis (Table 3).
Subgroup Analysis: Histologic–Endoscopic Healing
Of patients with endoscopies available at 12 months, 4/12 (33.3%) achieved histologic–endoscopic healing. The achievement of this endpoint was significantly associated with a lower mean partial Mayo score (0.5 ± 0.6 vs 3.5 ± 1.7; P < 0.01) and stool frequency score (0.3 ± 0.5 vs 1.4 ± 0.7; P = 0.02), and there was a nonsignificant decrease in CRP levels (1.2 ± 0.9 vs 10.5 ± 10.1; P = 0.11). There was a numerical difference in mean fecal calprotectin levels (190.8 ± 247.9 vs 690.0 ± 671.8; P = 0.43) which did not reach statistical significance in patients with fecal calprotectin levels available (N = 5).
Adverse Events
Adverse events occurred in 8 patients (12.1%), of whom 3 had UC exacerbations, 4 had infectious complications, and 1 had a case of leukocytoclastic vasculitis which was attributed to ustekinumab. Four patients (6.0%) had serious adverse events leading to hospitalization (3 UC exacerbations, 1 vasculitis), of whom 3 (4.5%) required colectomy. No malignancies or deaths were reported during follow-up (Table 4).
Table 4.
Adverse Events
| All Patients (n = 66) | |
|---|---|
| Total adverse events | 8 (12.1) |
| UC exacerbation | 3 |
| Upper respiratory tract infection | 2 |
| E. coli colitis | 1 |
| Shigellosis | 1 |
| Leukocytoclastic vasculitis | 1 |
| Any serious adverse event* | 4 (6.0) |
| Colectomy | 3 (4.5) |
| Any cancer | 0 |
*Defined as any adverse event leading to treatment discontinuation, hospitalization, disability, colectomy, or death.
DISCUSSION
To our knowledge, this is the first US study to assess the efficacy and safety of ustekinumab in UC. The rates of clinical remission at 3 and 12 months were 43% and 45%, respectively. Steroid-free clinical remission rate was 35% (total N = 20), along with a 50% rate of endoscopic remission and a 33% rate of mucosal and histo-endoscopic healing (total N = 12) at 12 months. In median follow-up of 178 days (IQR 57–483), ustekinumab was associated with an overall favorable safety profile.
The efficacy and safety of ustekinumab for UC have been demonstrated in the previously published randomized, placebo-controlled UNIFI pivotal trials.14 These phase III trials included 961 patients with moderately to severely active UC. Patients were assessed for clinical remission using a total Mayo score at week 8 postinduction and at week 44 of maintenance. At week 8 postinduction with IV ustekinumab, patients demonstrated a 15% clinical remission rate compared with 5% in the placebo arm (P < 0.0001). Those who responded to induction were further randomized to maintenance therapy with SC ustekinumab injections every 12 or 8 weeks with subsequent clinical response rates of 38% and 44%, respectively (compared with placebo, P = 0.002 and P < 0.001, respectively). Fifty-one percent of UNIFI’s cohort had prior exposure to biologics, compared with 93% of our cohort. Despite a more refractory cohort of patients in our real-world experience, we demonstrated a higher rate of remission postinduction with comparable results at 1 year. However, it is important to emphasize that in UNIFI, only patients who responded to induction were randomized to maintenance and therefore the true long-term remission rate of all patients initiated on ustekinumab is presumably lower than that reported at 44 weeks in the maintenance phase of the UNIFI trial.
The higher percentage of biological exposure in a real-world experience is not unexpected, given that new drugs are often positioned at the end of treatment algorithms until physicians and patients are more comfortable with the drug’s safety and efficacy profile. However, our higher rates of remission postinduction are contrary to what one might expect. There are a number of possible reasons for our higher rates of remission. One, this experience was open label, which allowed physicians to select patients who may have been better candidates for ustekinumab, and allowed patients to be aware of their exposure to this therapy, thereby increasing the risk of a possible placebo effect.16 It is possible that physicians and patients may have overestimated their response to the therapy since, given the refractory nature of these patients, surgery would be the next step and they were optimistically reporting enhanced improvements. Additionally, our study includes less precise retrospectively determined endpoint measurements due to limited endoscopic data at follow-up, therefore our primary outcome was limited to partial Mayo score assessment as opposed to a both clinical and endoscopic assessment by the total Mayo score as done in the UNIFI trial.
Recently reported real-world experiences from Europe have demonstrated induction and maintenance remission rates similar to our findings.17,18 Amiot et al, who published the GETAID experience, performed a retrospective short-term efficacy and safety study of ustekinumab in patients with moderately to severely active UC. Clinical remission rates at weeks 12–16 and steroids-free clinical remission were 40% and 35%, respectively. Interestingly, Amiot et al’s cohort was also highly refractory to biologic therapy, and similarly demonstrated higher rates of response postinduction than those reported in the UNIFI trial.17 This may be explained by their use of partial Mayo score as the primary outcome measure as well, and the open-label assignment of treatments in their study. Another smaller study published by Ochsenkühn et al assessed ustekinumab as rescue therapy in UC patients who were refractory or intolerant of steroids, purine analogues, anti-TNF, and vedolizumab therapy. The study was limited to 19 patients, all of whom received 6 mg/kg induction followed by 90 mg SC injections every 8 weeks. Similar to our findings, they demonstrated a 53% clinical remission rate at 1 year.
Dose escalation in our study was comparable to prior experiences with ustekinumab in Crohn disease with 44% of patients requiring increase in dosing frequency at anytime during follow-up and of those in clinical remission at 12 months, 5/9 (56%) were on every 4 week dosing.19 This is a higher rate than that reported by Amiot et al, likely due to longer duration of follow-up in our study. Encouragingly, of patients who required dose escalation, 11/27 (38%) were able to recapture response with a dose of 90 mg SC injection every 4 weeks, suggesting that this may be an effective strategy for the subset of patients with incomplete response to ustekinumab. Histologic and endoscopic healing have emerged as important treatment targets and are associated with better long-term outcomes.20–22 In the UNIFI trial, a novel outcome of histo-endoscopic mucosal healing was achieved in 18%–20% of patients at 8 weeks after induction In the long-term extension of UNIFI, achievement of this endpoint was associated with a 20%–30% greater likelihood of favorable clinical outcomes at weeks 44 and 92, including lower disease activity defined as lower partial Mayo scores and decreased stool frequency and decreased inflammatory burden defined by CRP and fecal calprotectin, compared with those who did not achieve histologic–endoscopic mucosal healing.23 In our cohort, 4 of 12 (33.3%) patients achieved histologic–endoscopic healing at 12 months. As seen in UNIFI, patients who achieved this endpoint had lower partial Mayo scores (0.5 ± 0.6 vs 3.5 ± 1.7, P < 0.01) and lower stool frequency scores (0.25 ± 0.5 vs 1.4 ± 0.7, P = 0.02) than those who did not. We acknowledge that this analysis was based on a small sample size (N = 12) possibly confounded by recruitment bias, which limits our ability to draw strong conclusions about the combined histologic–endoscopic healing endpoint.
Under multivariate analysis, we identified a baseline endoscopic Mayo score of 3 and a history of anti-TNF nonresponse as negative predictors of clinical remission at 3 months [OR = 0.04; 95% CI (0.01–0.78) and OR 0.03; 95% CI (0.01–0.82), respectively]. This is consistent with prior reported experiences17 and perhaps suggests that earlier use of ustekinumab in the treatment algorithm may result in even higher rates of success. We were not able to identify significant multivariate predictors of remission at 12 months, although this analysis was limited by small sample size (N = 20).
Adverse events occurred in 12% of our patients, with 6% experiencing serious adverse events leading to hospitalizations (3 UC exacerbations and 1 vasculitis) and 4.5% undergoing colectomies. The case of vasculitis was in a patient with recurrent episodes of acute abdominal pain 2 and 5 months after ustekinumab initation, with CT findings of acute duodenojejunitis. Endoscopic evaluation revealed only nonspecific duodenitis, but skin biopsies of concurrent diffuse pruritic lower extremity rashes confirmed leukocytoclastic vasculitis. Three cases of ustekinumab-related leukocytoclastic vasculitis have been reported in the literature, and it is hypothesized that use of newer biologics increases autoantibodies which lead to immune complex deposition causing small vessel vasculitis.24,25 Regarding cancer or deaths, in our cohort we report no instances, in comparison to7 cancer cases and 3 deaths reported in UNIFI’s ustekinumab arm. Our lower rate of adverse events, although similar to other real-world experiences,17 is likely due to our significantly smaller cohort of patients compared to the UNIFI trial. Additionally, less severe adverse events such as headaches or nasopharyngitis are less likely to be documented, and therefore may not have been captured in this real-world experience.
CONCLUSIONS
We found in this real-world experience from 2 US tertiary centers that ustekinumab is effective in inducing and maintaining clinical remission in a highly refractory cohort of UC patients along with a favorable safety profile.
Funding: Noa Krugliak Cleveland is funded in part by University of Chicago National Institute of Diabetes and Digestive and Kidney Diseases T32. Shintaro Akiyama is funded in part by the Gastrointestinal Research Foundation of Chicago, IL.
Conflict of Interest: Simon J. Hong, Shintaro Akiyama, Samantha Zullow, Yangtian Yi, and Seth R. Shaffer report no conflicts of interest. Noa Krugliak Cleveland is a consultant to Takeda and reports personal fee from Vindico. Lisa B. Malter has received educational or IBD fellowship education grants from AbbVie, Gilead, Janssen Pharmaceuticals, Merck & Co., Inc., Pfizer, Prometheus, Laboratories, Takeda, and UCB. Jordan E. Axelrad has received research support from BioFire Diagnostics, and has served as a consultant for BioFire Diagnostics and Janssen Pharmaceuticals. Shannon Chang has served as a consultant for Pfizer, Shire, and Oshi Health. David P. Hudesman has received research support from Pfizer, and has served as a consult for AbbVie, BMS, Janssen, Takeda, Pfizer, and Samsung. David T. Rubin has received research funding from Takeda, and has served as a consultant to AbbVie, Abgenomics, Allergan, Inc., Arena Pharmaceuticals, Biomica, Bristol-Myers Squibb, Dizal Pharmaceuticals, Ferring Pharmaceuticals, Inc., Genentech/Roche, Janssen Pharmaceuticals, Lilly, Mahana Therapeutics, Medtronic, Merck & Co., Inc., Napo Pharmaceuticals, Pfizer, Prometheus Laboratories, Shire, Takeda, Target Pharma Solutions, Inc. and is a co-founder of Cornerstones Health, Inc.
Author Contribution: Simon J. Hong and Noa Krugliak Cleveland contributed to study concept and design, data acquisition, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis, material support, and study supervision. Shintaro Akiyama, Samantha Zullow, Yangtian Yi, and Seth R. Shaffer helped in data acquisition. Lisa B. Malter and Jordan E. Axelrad contributed to analysis and interpretation of data and helped in critical revision of the manuscript for important intellectual content. Shannon Chang contributed to study concept and design, analysis and interpretation of data, and critical revision of the manuscript for important intellectual content. David P. Hudesman contributed to study concept and design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, and study supervision. David T. Rubin contributed to study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis, material support, and study supervision.
DATA AVAILABILITY
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- 1. Dahlhamer JM, Zammitti EP, Ward BW, et al. . Prevalence of inflammatory bowel disease among adults aged ≥18 years—United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:1166–1169. [DOI] [PubMed] [Google Scholar]
- 2. Bernstein CN, Ng SC, Lakatos PL, et al. ; Epidemiology and Natural History Task Force of the International Organization of the Study of Inflammatory Bowel Disease . A review of mortality and surgery in ulcerative colitis: milestones of the seriousness of the disease. Inflamm Bowel Dis. 2013;19:2001–2010. [DOI] [PubMed] [Google Scholar]
- 3. Samuel S, Ingle SB, Dhillon S, et al. . Cumulative incidence and risk factors for hospitalization and surgery in a population-based cohort of ulcerative colitis. Inflamm Bowel Dis. 2013;19:1858–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rutgeerts P, D’Haens G, Targan S, et al. . Efficacy and safety of retreatment with anti-tumor necrosis factor antibody (infliximab) to maintain remission in Crohn’s disease. Gastroenterology. 1999;117:761–769. [DOI] [PubMed] [Google Scholar]
- 5. Targan SR, Hanauer SB, van Deventer SJ, et al. . A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med. 1997;337:1029–1035. [DOI] [PubMed] [Google Scholar]
- 6. Hanauer SB, Sandborn WJ, Rutgeerts P, et al. . Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323–333; quiz 591. [DOI] [PubMed] [Google Scholar]
- 7. Schreiber S, Khaliq-Kareemi M, Lawrance IC, et al. ; PRECISE 2 Study Investigators . Maintenance therapy with certolizumab pegol for Crohn’s disease. N Engl J Med. 2007;357:239–250. [DOI] [PubMed] [Google Scholar]
- 8. Schreiber S, Sandborn WJ. CLASSIC-I study the efficacy of adalimumab. Gastroenterology. 2006;130:1929–1930. [DOI] [PubMed] [Google Scholar]
- 9. Sandborn WJ, Feagan BG, Stoinov S, et al. ; PRECISE 1 Study Investigators . Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med. 2007;357:228–238. [DOI] [PubMed] [Google Scholar]
- 10. Rutgeerts P, Sandborn WJ, Feagan BG, et al. . Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. [DOI] [PubMed] [Google Scholar]
- 11. Sandborn WJ, van Assche G, Reinisch W, et al. . Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142:257–265.e1. [DOI] [PubMed] [Google Scholar]
- 12. Sandborn WJ, Feagan BG, Marano C, et al. . Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146:85–95; quiz e14. [DOI] [PubMed] [Google Scholar]
- 13. Korvick J. Biologics License Application (BLA) 761044 Approval Letter. 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/761044Orig1s000Approv.pdf [Google Scholar]
- 14. Sands BE, Sandborn WJ, Panaccione R, et al. ; UNIFI Study Group . Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019;381:1201–1214. [DOI] [PubMed] [Google Scholar]
- 15. Ha C, Ullman TA, Siegel CA, et al. . Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin Gastroenterol Hepatol. 2012;10:1002–1007; quiz e78. [DOI] [PubMed] [Google Scholar]
- 16. Peck C, Coleman G. Implications of placebo theory for clinical research and practice in pain management. Theor Med. 1991;12:247–270. [DOI] [PubMed] [Google Scholar]
- 17. Amiot A, Filippi J, Abitbol V, et al. ; UC-USK-GETAID Study Group . Effectiveness and safety of ustekinumab induction therapy for 103 patients with ulcerative colitis: a GETAID multicentre real-world cohort study. Aliment Pharmacol Ther. 2020;51:1039–1046. [DOI] [PubMed] [Google Scholar]
- 18. Ochsenkühn T, Tillack C, Szokodi D, et al. . Clinical outcomes with ustekinumab as rescue treatment in therapy-refractory or therapy-intolerant ulcerative colitis. United European Gastroenterol J. 2020;8:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ma C, Fedorak RN, Kaplan GG, et al. . Long-term maintenance of clinical, endoscopic, and radiographic response to ustekinumab in moderate-to-severe Crohn’s disease: real-world experience from a multicenter cohort study. Inflamm Bowel Dis. 2017;23:833–839. [DOI] [PubMed] [Google Scholar]
- 20. Christensen B, Hanauer SB, Erlich J, et al. . Histologic normalization occurs in ulcerative colitis and is associated with improved clinical outcomes. Clin Gastroenterol Hepatol. 2017;15:1557–1564.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Colombel JF, Rutgeerts P, Reinisch W, et al. . Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011;141:1194–1201. [DOI] [PubMed] [Google Scholar]
- 22. Peyrin-Biroulet L, Bressenot A, Kampman W. Histologic remission: the ultimate therapeutic goal in ulcerative colitis? Clin Gastroenterol Hepatol. 2014;12: 929–934.e2. [DOI] [PubMed] [Google Scholar]
- 23. Li K, Marano C, Zhang H, et al. . Relationship between combined histologic and endoscopic endpoints and efficacy of ustekinumab treatment in patients with ulcerative colitis. Gastroenterology. 2020;159:2052–2064. [DOI] [PubMed] [Google Scholar]
- 24. Costa-Moreira P, Lopes S, Santos AL, et al. . Leukocytoclastic vasculitis related to ustekinumab in a Crohn’s disease patient: first case report and literature review. J Crohns Colitis. 2020;14:274–276. [DOI] [PubMed] [Google Scholar]
- 25. Chugh R, Proctor D, Little A, et al. . P099 Leukocytoclastic vasculitis after ustekinumab induction in Crohn’s disease: a case series and systematic review. Gastroenterology. 2020;159:S3. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.