Abstract
Background
Latitude and lactase digestion status influence incidence and prevalence rates of some noncommunicable diseases. Latitudinal correlations helped define beneficial roles of vitamin D in many diseases like inflammatory bowel disease (IBD). In view of recent global expansion of IBD and population migrations, we reexamine relations with these markers. As these changes also paralleled the pandemic of obesity, we explore possible interactions with IBD.
Methods
We undertook a literature review to compare rates of obesity, Crohn’s disease and ulcerative colitis with the geographic markers of lactase digestion status, average population-weighted national latitude, and national yearly sunshine exposure. Pearson correlations were used throughout to determine r correlation factors. Statistical significance was accepted at P <0.05 using 2-tailed tests.
Results
Forty-seven countries were matched with various data sets that could be analyzed (range of availability was 49%–85%). While global correlations of IBD with latitude and lactase status remain similar to previous analyses, in Europe and Asia, outcomes were different. Global outcome contains a statistical paradox related to combining countries from Europe and Asia. Obesity showed moderate global correlations with IBD but weak and negligible correlations in Europe and Asia. There was also a weak global correlation with latitude.
Conclusions
It is suggested that global correlations point to parallel geographic spread of IBD and obesity. The lack of latitudinal relations with obesity suggests reduced vitamin D effect. The paradox supports epidemiological differences in western and eastern IBD. Obesity combined with IBD may contribute to different relations, partly due to variable vitamin D effects.
Keywords: inflammatory bowel diseases, obesity, latitude, epidemiology
INTRODUCTION
On a global level, two ecological geographic markers or modifiers were found to correlate inversely with “western”-type diseases. Latitude’s impact was attributed to reduction of sunshine exposure in northerly regions. In turn, reduced sunshine focused research on the possible role of vitamin D as an immune modifier1, 2 and anti-carcinogen.3–6 The second marker which also varies inversely with similar diseases as latitude is the population distributions of adult lactase digestion status.7 Lactase distributions (lactase persistence [LP] and lactase nonpersistence [LNP]), also vary inversely with latitude but extend laterally to include many other regions (Asia South America, Pacifica, and parts of Africa).8 Modern distributions of LP/LNP populations which divided the human race along genetic lines, depended on both ancient and more recent population migrations.9 The explanations for LNP disease relationships are less clear, but may be multifactorial.
The IBDs (consisting of Crohn’s disease [CD] and ulcerative colitis [UC]) have been used as examples where incidence and prevalence are modified by latitude10–13 and national LNP status.7 However, by the end of the 20th century, the sharp distinction of the north–south gradient effect was questioned in Europe.14 In addition, more precise information emerged on national IBD rates in the last 2–3 decades which showed increases in world regions with previously low or nonexistent rates.15, 16 Second more precise information of population lactase distributions emerged during the same time frame.17 Importing of western industrialization and adoption of new lifestyles have been blamed for such disease expansion into previously low incidence areas.16, 18
Accompanying the increased spread of IBD, a more common condition of obesity has become a pandemic in the last few decades.19 The World Health Organization defines obesity by the formula, body mass index (BMI) as weight in kilograms per (height in meters)2, >30 kg/m2.20 Obesity was predicted to expand into less developed countries with large LNP populations.21
Intriguingly, pathogenic features of obesity seem to coincide with those attributed to IBD. The general features of obesity include promotion of a pro-inflammatory state by insulin resistance, oxidative stress and effects of adipokines. In addition, dysbiosis in the microbiome of obese persons superficially approximates some features found in IBD.22, 23 Complications of the metabolic syndrome such as fatty liver,24 cardiovascular disease,25 and in UC, type 2 diabetes26 have been described. Obesity has controversial effects on IBD clinical course, which include response to biologic agents27–29 and variable alterations on severity of IBD.30 Furthermore, IBD and obesity may merge with time as patients with IBD gain weight.31 These observations raise a hypothesis that the interactions of obesity with IBD may have impacted on previously found relationships of the latter with geographic markers.
In this analytical review, we re-examine relationships between both forms of IBD and two geographic markers, incorporating new available data on IBD rates and national LP/LNP distributions. We also evaluate relationships between obesity and the same geographic markers to examine possible impact on IBD epidemiology.
METHODS
For this focused review and analysis, PubMed, Google Scholar, several web sites (where noted) as well as individual articles were searched for data on designated target variables. Population statistics obtained from the Internet, for the year 2014 which approximate updated IBD, obesity, and LNP rates were used for adjusting for national rates as needed. The literature was searched to recover national incidence and prevalence rates for Crohn`s disease and ulcerative colitis, national percent frequency of obesity and for national percent frequency of lactase nonpersistence (LNP). Average national latitudes and national Ultraviolet-B exposure per year were calculated as described briefly and as published previously.32
The most recent national rates (incidence and prevalence as per 100,000) of IBD are included from references.15, 16 If national rates were unavailable, estimates were calculated based on population statistics using previously reported methods.32 In these cases, disease rates (D) were based on regional data using the following formula:
| (1) |
where Xi is the number of patients with new or ongoing disease (CD or UC) in region or city “i,” Ai is the population of region/city, Pi is the populations of the N population centers considered, and N is the number of cities and/or regions.
National obesity frequencies were sought while rates of overweight were excluded due to less clear effects of the graded spectrum of weight on complications of obesity. The definition was based on the BMI which is defined by the World Health Organization as BMI ≥ 30 kg/m220 and most reports used this definition. The main source for obesity frequency was from Ng et al.33
This article evaluated the rates of overweight and obesity from world data for four periods between 1980 and 2013 and estimated national rates for 2013 using 188 countries. Frequencies were based on measured and self-reported values categorized by age, greater or less than 20 years and by gender. Those greater than 20 years of age were used and tabulated as percent frequencies (which were listed with 2.5%–97.5% uncertainty intervals). National male and female frequencies were added and averaged for facilitating comparisons. We made the assumption that the national populations were approximately made up of men and women in a ratio of 1:1. We also supplemented some national obesity data, not listed in Ng et al33 from other references (Taiwan,34 Thailand,35 Indonesia,36 Kuwait,37 and Saudi-Arabia38). Hong Kong rates were derived from an information web page by the Chinese University of Hong Kong.39
For comparison and consistency over time, rates of obesity were also sought from the Organization of Economic Co-operation and Development (OECD) for two nonsequential years and which includes data limited to 36 member nations.40, 41
National rates of lactase digestion status were derived from Storhaug et al.17 However, several other sources were also used because these were not available in one reference.42–45 Determination of lactase status was based on duodenal biopsies, indirect tests of lactose maldigestion such as blood glucose or breath hydrogen response to lactose loads and direct genetic testing.
National average latitudes are presented in terms of single values for each country as previously published.32 The method is reiterated here. A population-weighted latitude is calculated for the country based on the latitudes of the most heavily populated cities within the country of interest. So, Pi is the population of the N population centers considered. The number of population centers (N) included in the calculation of a national average varied from 1 for small countries to typically 10 or more for the larger countries with many large population centers.
| (2) |
where LATi is the latitude of population center i.
National yearly ultraviolet-B (280–315 nm) exposures (UVB/kJ/m2/year) were deduced from the data of Lee-Taylor and Madronich46 and have also been described previously.32 Briefly, monthly surface-level radiation based on a radiative transfer model driven by satellite-measured variables was used. Annual averages from the sum of monthly averages for the period 1990–2000 were computed. To obtain a single representative value for each of the countries, population-weighted averages for ultraviolet B surface radiation were calculated for the locations of the largest population centers in each country. A single population-weighted latitude was calculated for each country using the same population weighting as used for calculation of the population-weighted latitude.
The national annual average ultraviolet-B exposure is calculated as
| (3) |
where UVBi is the annual ultraviolet-B exposure at population center i.
Statistical and Data Analysis
Pearson correlation coefficients were used to compare relationships among the different variables. For national rates of Crohn’s disease and ulcerative colitis, log-transformations were used due to skewness of data. Statistical significance was accepted for a 2-tailed P value at P < 0.05. The primary objectives were to explore relationships between IBD and LNP, latitude or UVB, and then between obesity and LNP, latitude, or UVB. A correlation between obesity and CD or UC incidence was also evaluated. The strength of correlations was qualitatively and arbitrarily defined as strong; r ≥ 0.7, moderate r ≥ 0.5, weak ≤ 0. 49, and negligible ≤ 0.3. This subjective classification is based on Mukaka.47
Missing information for countries was not included. The number of countries linked to each variable is shown in Supplementary Table A. SAS statistical analysis package (version 9.3; SAS Institute Inc., Cary, NC, USA) was used for calculations.
The rationale of the strategy for analysis includes the following assumptions. Latitude is a fixed variable which is insensitive to the calculation of national average latitudes. The relationship of annual national sunshine exposure is also stable with a minimal variation over decades and is strongly and inversely correlated with latitude.8, 32 The world distributions of lactase digestion are dependent on population migrations. The most recent compilation of national LNP rates shows considerable changes of increased LNP populations in North America and Europe.17, 45 However, Asian, African, and South American rates have changed little. Pearson’s was chosen over Spearman’s correlations because previous observations have linked IBD with a more linear relationship with latitude.32 We explored possible relations among target variables first at the global level. Then we also evaluated these relationships in Europe and Asia.
RESULTS
A total of 47 countries supplied frequencies of obesity.33–39 Comparison of data from Ng et al33 with those from the OECD for 201440 and 201541 were r = 0.88 and r = 0.87, respectively. These suggest that reports from Ng et al were consistent with other reports. Similarly, we were able to derive LNP rates reported from the same 47 countries.17, 42, 45 Calculated average national latitudes were derived for 46/47 (98%) and average national yearly UVB kilo Joules per year exposure for 36/47 (77%).
As a control for small numbers of available data, the outcome of comparisons of latitude and annual UVB exposure was carried out. Results were as expected in all 3 domains. These ranged from r = −0.85 in Asia to r = −0.98 in Europe. Comparisons of latitude and annual UVB exposure with national LNP rates were also as expected globally and in Europe.10, 26 In Asia, the majority of the populations are LNP, which is largely independent of latitude (data not shown).
We matched 40/47 (85%) countries for incident rates of CD i and 39/47 (83%) for UC i.15, 16 National disease rates for 26 countries were estimated (calculated as described) from ref. 15 and relevant national populations listed on the internet (Supplemental Table A). There were fewer available prevalence rates; CD p 23/47 (49%) and UC p 25/47 (53%). The correlation between incidence of CD and incidence of UC was r = 0.87. The correlations between CD incidence and CD prevalence were 0.82 based on 22 countries. Similarly, the correlation between UC incidence and UC prevalence was 0.76 based on 24 countries. All of these correlations were statistically significant P < 0.001.
We then examined relationships among target variables to include a global assessment and then in Europe and Asia. Table 1 shows the correlations among incidence rates for national Crohn’s disease, ulcerative colitis, latitude, UVB, and LNP in three regions of the world.
Table 1.
Correlations of Crohn’s Disease Incidence (CD) and Ulcerative Colitis Incidence (UC) with National Lactase Nonpersistence Frequencies (LNP) [Panel A], Calculated Mean National Latitude Latitude [Panel B] or Average Calculated National Yearly Ultra Violet B Exposure [UVB/kJ/m2/year] [Panel C] on a Global, European or Asian Division
| Global | P | Europe | P | Asia | P | |
|---|---|---|---|---|---|---|
| Panel A | ||||||
| LNP | ||||||
| CD | −0.68 [40] | <0.0001 | −0.55 [20] | 0.012 | −0.13 [12] | NS |
| UC | −0.61 [39] | <0.0001 | −0.33 [20] | NS | −0.09 [12] | NS |
| Panel B | ||||||
| Latitude | ||||||
| CD | 0.75 [39] | <0.0001 | 0.4 [19] | NS (0.09) | 0.65 [12] | 0.02 |
| UC | 0.75 [38] | <0.0001 | 0.42 [19] | NS (0.07) | 0.71 [12] | 0.009 |
| Panel C | ||||||
| UVB | ||||||
| CD | −0.48 [33] | 0.005 | −0.38 [19] | NS | −0.27 [6] | NS |
| UC | −0.42 [32] | 0.015 | −0.34 [19] | NS | 0.19 [6] | NS |
NS, not significant.
The main findings shown are the modest-to-moderate correlations of Crohn’s and UC incidence with geographic markers globally. However, when the same relationships are examined in Europe or Asia only the correlation of CD incidence with LNP in Europe and the correlation of CD and UC incidence with latitude in Asia remain moderate or strong.
When prevalence data on IBD are assessed the outcomes with the geographic markers are similar globally but less clear in the other two regions where UC prevalence remains moderately associated with LNP and strongly associated with latitude in Asia (Supplemental Table B).
The same analysis of obesity and IBD shown in Table 2 reveals weak global correlations between obesity and LNP or latitude and a negligible correlation with UVB. The relationships are consistently negligible in Europe and Asia.
Table 2.
Correlations of National Obesity Frequency (Ob) with National Lactase Nonpersistence Frequencies (LNP) [Panel A], Calculated Mean National Latitude [Panel B] or Average Calculated National Yearly Ultra Violet B Exposure [UVB/kJ/m2/year] [Panel C] on a Global, European or Asian Division
| Global | P | Europe | P | Asia | P | |
|---|---|---|---|---|---|---|
| Panel A | ||||||
| LNP | ||||||
| Ob | −0.47 [47] | 0.0009 | −0.05 [21] | NS | 0.16 [14] | NS |
| Panel B | ||||||
| Latitude | ||||||
| Ob | 0.36 [46] | 0.015 | 0.11 [20] | NS | −0.11 [14] | NS |
| Panel C | ||||||
| UVB | ||||||
| Ob | 0.16 [36] | NS | −0.1 [20] | NS | 0.39 [6] | NS |
NS, not significant.
Correlations between obesity and the two forms of IBD show global moderate relations, but these are lost and are negligible in Europe and Asia (Table 3).
Table 3.
Correlation of National Obesity Frequency with Either Crohn’s Disease or Ulcerative Colitis Incidence Rates Are Shown
| Obesity | Global | P | Europe | P | Asia | P |
|---|---|---|---|---|---|---|
| CD | 0.50 [40] | 0.0009 | 0.06 [20] | NS | −0.23 [12] | NS |
| UC | 0.51 [39] | 0.0008 | 0.26 [20] | NS | 0.07 [12] | NS |
NS, not significant.
This pattern of different outcomes, when global outcomes are compared with separate included regions, is consistent with Simpson’s paradox.48, 49 A re-analysis of the exact same data combined or separately is shown in Supplementary Table C. Eight countries are excluded from the total in this table, thus the same data are used for combined and separate analysis, meeting the definition of the paradox.48, 49
As a test, we also calculated Spearman’s coefficients for geographic variables as well as interaction of latitude, LNP and CD and UC incidence. With some variations, the statistical outcomes and patterns of the paradox persisted (data not shown). This was done to facilitate comparison with the last analysis which was carried out with Spearman’s coefficients.32
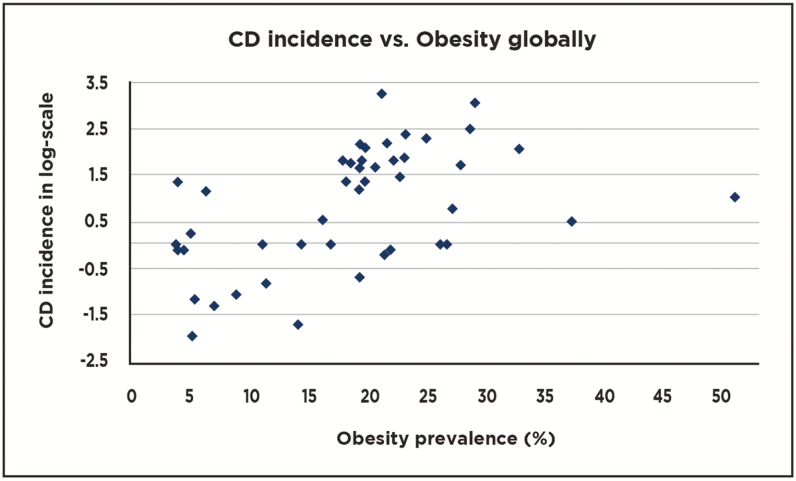
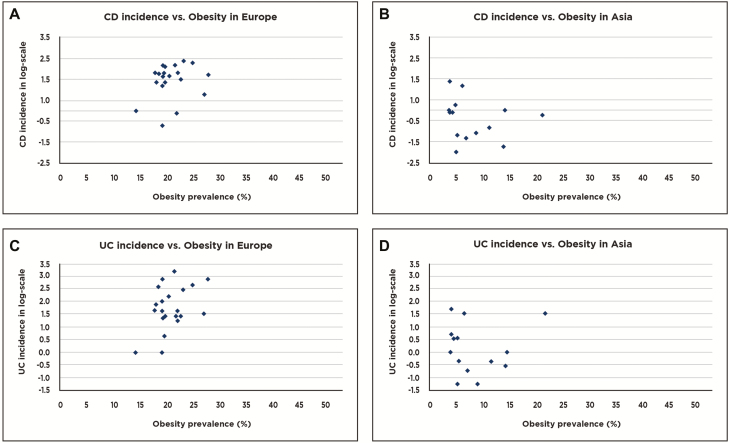
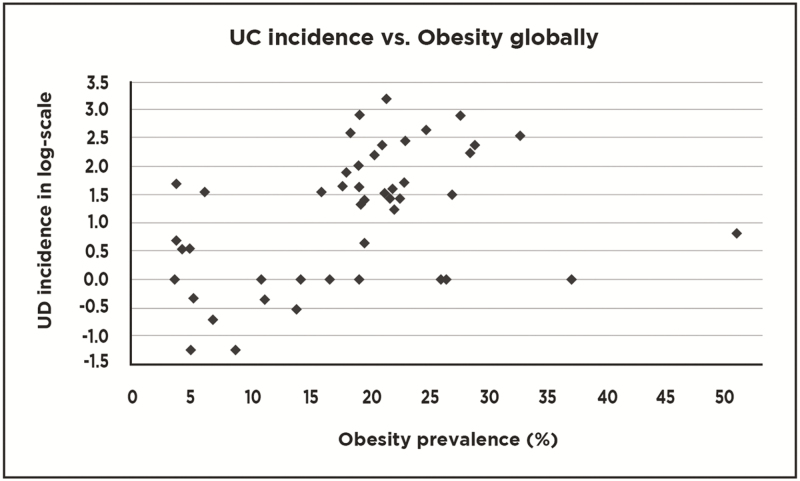
An exemplary scattergram of CD or UC incidence vs obesity globally is compared with a similar scattergram of CD or UC incidence vs obesity frequency in either Europe or Asia (Figs. 1–3a–d). These figure help to demonstrate how the combination of data from Europe and Asia form a reasonably linear graph while evaluation of data points within each region fail to form linear plots. These findings suggest that obesity seems not to correlate with CD or UC in Europe or Asia.
Figure 1.
Scattergram showing relationships between national Crohn’s disease (CD) incidences and frequencies of obesity on a global level (40 countries represented).
Figure 3.
Scattergram showing relationships between (a) national Crohn’s disease (CD) incidences and frequencies of obesity in Europe (20 countries represented). (b) National Crohn’s disease incidences and frequencies of obesity in Asia (12 countries represented). (c) Ulcerative colitis incidences and frequencies of obesity in Europe (20 countries represented). (d) Ulcerative colitis incidences and frequencies of obesity in Asia (12 countries represented).
Figure 2.
Scattergram showing relationships between national ulcerative colitis (UC) incidences and frequencies of obesity on a global level (39 countries represented).
DISCUSSION
In this report, we re-examine relationships among CD, UC, and the geographic modifiers of disease rates of latitude, UVB, and LNP national distributions. This reanalysis is prompted by recent expansion of IBD into previously low incidence areas. Also recent population migrations largely into traditional “western”-type countries in Europe, North America, and Australia could influence previous geographic associations. A third possible influencing variable is the coincidental pandemic of obesity, which has been hypothesized to share some pathogenic similarities with IBD and includes similar expanding trajectories. While correlations cannot prove causation, the results can lead to hypotheses to explain patterns.
Correlations among these variables on a global level are similar to findings from an earlier study.26 These correlations grossly reflect national disease parameters but do not imply homogeneity within countries. However, the global outcomes consist of an amalgamation of a paradox formed by combining data from Europe and Asia, as well as, other various world regions. As such, it appears that the regional disruptions from globally homogenous correlations, result from differences in Europe and Asia. There are too few countries from other regions to analyze these separately.
Although the etiology of IBD is thought to be homogenous throughout the world,50 there are epidemiological differences described between IBD in the west and the east. Some of these examples include the following. Originally in the west, UC was more frequent and was followed by CD about 10 years later.51 Disease rates in the west appear to be leveling off50, 51 with some variation between adult and pediatric CD in countries such as Canada.52 In Asia, UC and CD tend to develop at more similar rates and CD tends to be more clinically severe. Rates of both diseases are increasing in previously low incidence areas. While industrialization and western lifestyle adoptions are thought to be causative, the differences in disease rates are not completely paralleled by industrial growth (eg, Japan, earlier and China, later).50 Epidemiological parameters such as smoking and appendectomy have variable relations with CD and UC between east and west. Genetic differences between populations exist. One example is the mutations in nucleotide-binding oligomerization domain 2 (NOD2). This was the first gene described to be related to CD but is largely limited to Caucasians. Other genes such as a new and different mutation in IL-23R was found to protect against CD in Asians. These and other regional differences are reviewed by Mak et al.53
In addition, there are likely microbiome differences between western and eastern populations,54 although these differences need further studies in patients with IBD.55 These different epidemiological attributes could account for paradoxical relationships noted for evaluated variables.
Second population changes have occurred which disproportionately changed western compared with eastern countries. Hence, populations from previously low IBD incidence regions (which include majority of LNP phenotypes) have migrated north and west. However, the previously large LP population residing in largely north and western Europe may have been diluted leading to decrease in the sharp north-south gradient of LP and LNP populations previously noted.
An east–west gradient in IBD distributions has also been noted in Europe more recently.56 These population shifts in Europe but not Asia (where the large majority of the population are LNP phenotype) could also have contributed to changing ecological relationships.
It is of note that some aspects of IBD (CD incidence and UC prevalence) retain correlations with LNP in Europe. However, both forms of IBD in Asia retain national relationships with latitude. This latter pattern is reminiscent of early IBD relations with latitude in the west.
How might obesity contribute to the observed patterns? Obesity largely began in North America and extended to less-developed nations.19 The path of the obesity pandemic was predicted to involve large areas inhabited by LNP populations.21 This pattern could be reflected in the global moderate negative correlations of obesity with LNP and also with the weak correlation with latitude. In addition, both forms of IBD correlate with obesity possibly reflecting similar trajectories of these conditions.
The most consistent findings are that obesity has weak to negligible correlations with UVB and weak correlation with latitude. Although latitudinal effects may work through other factors such as temperature57 and changes in intestinal microbiome,58 the north–south gradient effect on diseases was hypothesized to be the lack of sunshine and the lower availability of vitamin D. As a result, the present observations raise the suggestion that obesity may respond poorly to vitamin D.
Indeed, obesity is associated with low vitamin D levels,59 the outcome of supplemental replacement appears to be controversial. Indeed, in vitro and small animal studies report conflicting outcomes in studies on obesity, with vitamin D. While animal studies suggest that vitamin D inhibits adipogenesis, in vitro studies suggest that the vitamin is pro-adipogenic.60 In humans, a recent meta-analysis of randomized controlled trials of supplementary vitamin D ingestion failed to impact on weight loss.61 Also, a recent controlled trial of vitamin D supplementation disclosed that in healthy men with low serum vitamin D levels (<50 nmol L) there was an increase in central obesity while with less severe insufficiency supplemental vitamin D had a negative effect on insulin sensitivity.62
In the case of IBD, the initially observed inverse link with low latitude and sunshine suggested that vitamin D has an important immune modulatory effect.63 Subsequently, both observational and some interventional trials supported beneficial effects of vitamin D on IBD outcome.11–13, 64, 65
If the global association with IBD is correct, we might not be surprised by the variable relations of obesity found in regional analyses with IBD. Such disparity in findings may be due to interactions of obesity with IBD, which lose geographic correlations in Europe and Asia for the reasons outlined above.
The coexistence of obesity with IBD may occur for reasons outlined in Introduction. The contribution of interactions of two conditions with possible diverging effects of vitamin D (favorable in IBD and possibly neutral or negative in obesity) is not possible to predict. In Asia where IBD is more recent, obesity may or may not have a protective effect for prevalence of ulcerative colitis.
There are limitations to the findings and interpretation of this study. These stem from the need to approximate information to similar time frames and to obtain data at national levels in order to allow more homogenous comparisons of different variables. However, such national-level information is not uniformly available in the literature. As a result significant numbers of IBD incidence and prevalence data were calculated from regional information that was available. These still do not give necessarily accurate national data because intra-country rates often vary. The effort to match data from different countries resulted in restriction of available data which reduces the power of comparisons. As such a type 2 error is a possibility with low correlations. Secondly, average national latitudes and annual sunshine exposure were estimates also based on calculations. While small countries are less affected, large regions such as Canada, United States, and China, the average estimates may be less accurate. In this regard, however, the finding of the previously expected relationships between geographic markers is somewhat reassuring. Furthermore, despite limited data available in Asia, confirmation of the published relationship of IBD with latitude15 supports the findings in this analysis. Finally, we reiterate that the interpretations are largely hypotheses. However, the strengths are based on the fact that information on various aspects was obtained from independent sources which should reduce the bias of associations.
In conclusion, re-evaluation of correlations of IBD incidence and prevalence with geographic modifiers of IBD show continued similarities on global level. However, comparison of these ecological markers as they relate to IBD are divergent in Europe and Asia but support epidemiological studies which suggest differences in IBD between east and west.
In Europe, influx of immigrants from low incidence areas possibly reflects retention of the correlations of IBD with LNP phenotype. In Asia, the more homogenous LNP population re-enacts early observations of correlations with latitude. These different regional patterns support independent effects of latitude and LNP phenotype on these conditions.
Moderate positive global correlations of obesity disappear in regional analyses. However, loss of correlations between geographic markers and obesity, especially, in Europe may be explained by similar factors that affect IBD, but retain mutual disease associations.
Similarly, geographic patterns observed suggest possible weaker effects of vitamin D on obesity. As such, with the coexistence of both conditions, the effect of vitamin D on IBD may vary in ways that are not predictable. The impact of obesity on vitamin D effects in IBD and other diseases linked with dependent incidence on latitude could benefit from further evaluation.
Supplementary Material
DISCLOSURE STATEMENT
None of the authors have any financial conflicts to declare. There was no financial support for this work.
DATA AVAILABILITY
Supplemental Table A contains raw data used in this manuscript and is included with manuscript submission.
References
- 1. Simpson S Jr, Blizzard L, Otahal P, et al. . Latitude is significantly associated with the prevalence of multiple sclerosis: a meta-analysis. J Neurol Neurosurg Psychiatry. 2011;82:1132–1141. [DOI] [PubMed] [Google Scholar]
- 2. Peyrin-Biroulet L, Oussalah A, Bigard MA. Crohn’s disease: the hot hypothesis. Med Hypotheses. 2009;73:94–96. [DOI] [PubMed] [Google Scholar]
- 3. Grant WB. A meta-analysis of second cancers after a diagnosis of nonmelanoma skin cancer: additional evidence that solar ultraviolet-B irradiance reduces the risk of internal cancers. J Steroid Biochem Mol Biol. 2007;103:668–674. [DOI] [PubMed] [Google Scholar]
- 4. Grant WB. How strong is the evidence that solar ultraviolet B and vitamin D reduce the risk of cancer?: an examination using Hill’s criteria for causality. Dermatoendocrinol. 2009;1:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Leeuwen MT, Turner JJ, Falster MO, et al. . Latitude gradients for lymphoid neoplasm subtypes in Australia support an association with ultraviolet radiation exposure. Int J Cancer. 2013;133:944–951. [DOI] [PubMed] [Google Scholar]
- 6. Mohr SB, Garland CF, Gorham ED, et al. . Ultraviolet B and incidence rates of leukemia worldwide. Am J Prev Med. 2011;41:68–74. [DOI] [PubMed] [Google Scholar]
- 7. Shrier I, Szilagyi A, Correa JA. Impact of lactose containing foods and the genetics of lactase on diseases: an analytical review of population data. Nutr Cancer. 2008;60:292–300. [DOI] [PubMed] [Google Scholar]
- 8. Szilagyi A, Leighton H, Burstein B, Shrier I. Significant positive correlation between sunshine and lactase nonpersistence in Europe may implicate both in similarly altering risks for some diseases. Nutr Cancer. 2011;63:991–999. [DOI] [PubMed] [Google Scholar]
- 9. Walker C, Thomas MG. The evolution of lactose digestion. In: Paques M, Lindner C. Lactose Evolutionary Role, Health Effects, and Applications. London 201: Academic Press, Elsevier; 2019: 1–35. [Google Scholar]
- 10. Nerich V, Jantchou P, Boutron-Ruault MC, et al. . Low exposure to sunlight is a risk factor for Crohn’s disease. Aliment Pharmacol Ther. 2011;33:940–945. [DOI] [PubMed] [Google Scholar]
- 11. Ananthakrishnan AN, Khalili H, Higuchi LM, et al. . Higher predicted vitamin D status is associated with reduced risk of Crohn’s disease. Gastroenterology. 2012;142:482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khalili H, Huang ES, Ananthakrishnan AN, et al. . Geographical variation and incidence of inflammatory bowel disease among US women. Gut. 2012;61:1686–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holmes EA, Ponsonby AL, Pezic A, et al. ; PAID Consortium . Higher Sun exposure is associated with lower risk of pediatric inflammatory bowel disease: a matched case-control study. J Pediatr Gastroenterol Nutr. 2019;69:182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shivananda S, Lennard-Jones J, Logan R, et al. . Incidence of inflammatory bowel disease across Europe: is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD). Gut. 1996;39:690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ng SC, Kaplan GG, Tang W, et al. . Population density and risk of inflammatory bowel disease: a prospective population-based study in 13 countries or regions in Asia-Pacific. Am J Gastroenterol. 2019;114:107–115. [DOI] [PubMed] [Google Scholar]
- 16. Ng SC, Shi HY, Hamidi N, et al. . Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390:2769–2778. [DOI] [PubMed] [Google Scholar]
- 17. Storhaug CL, Fosse SK, Fadnes LT. Country, regional, and global estimates for lactose malabsorption in adults: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2:738–746. [DOI] [PubMed] [Google Scholar]
- 18. Ramos GP, Papadakis KA. Mechanisms of disease: inflammatory bowel diseases. Mayo Clin Proc. 2019;94:155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev. 2012;70:3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Onis M, Onyango AW, Borghi E, et al. . Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world–a growing challenge. N Engl J Med. 2007;356:213–215. [DOI] [PubMed] [Google Scholar]
- 22. Mendall MA, Gunasekera AV, John BJ, Kumar D. Is obesity a risk factor for Crohn’s disease? Dig Dis Sci. 2011;56:837–844. [DOI] [PubMed] [Google Scholar]
- 23. Szilagyi A. Relationship(s) between obesity and inflammatory bowel diseases: possible intertwined pathogenic mechanisms. Clin J Gastroenterol. 2019;13:139–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gizard E, Ford AC, Bronowicki JP, Peyrin-Biroulet L. Systematic review: the epidemiology of the hepatobiliary manifestations in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2014;40:3–15. [DOI] [PubMed] [Google Scholar]
- 25. Feng W, Chen G, Cai D, et al. . Inflammatory Bowel disease and risk of ischemic heart disease: an updated meta-analysis of cohort studies. J Am Heart Assoc. 2017;6. pii:e005892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dregan A, Charlton J, Chowienczyk P, Gulliford MC. Chronic inflammatory disorders and risk of type 2 diabetes mellitus, coronary heart disease, and stroke: a population-based cohort study. Circulation. 2014;130:837–844. [DOI] [PubMed] [Google Scholar]
- 27. Harper JW, Sinanan MN, Zisman TL. Increased body mass index is associated with earlier time to loss of response to infliximab in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2118–2124. [DOI] [PubMed] [Google Scholar]
- 28. Bultman E, de Haar C, van Liere-Baron A, et al. . Predictors of dose escalation of adalimumab in a prospective cohort of Crohn’s disease patients. Aliment Pharmacol Ther. 2012;35:335–341. [DOI] [PubMed] [Google Scholar]
- 29. Dreesen E, Verstockt B, Bian S, et al. . Evidence to support monitoring of vedolizumab trough concentrations in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2018;16:1937–1946.e8. [DOI] [PubMed] [Google Scholar]
- 30. Hu Q, Ren J, Li G, et al. . The impact of obesity on the clinical course of inflammatory bowel disease: a meta-analysis. Med Sci Monit. 2017;23:2599–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moran GW, Dubeau MF, Kaplan GG, et al. . The increasing weight of Crohn’s disease subjects in clinical trials: a hypothesis-generatings time-trend analysis. Inflamm Bowel Dis. 2013;19:2949–2956. [DOI] [PubMed] [Google Scholar]
- 32. Szilagyi A, Leighton H, Burstein B, Xue X. Latitude, sunshine, and human lactase phenotype distributions may contribute to geographic patterns of modern disease: the inflammatory bowel disease model. Clin Epidemiol. 2014;6:183–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ng M, Fleming T, Robinson M, et al. . Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chu NF. Prevalence of obesity in Taiwan. Obes Rev. 2005;6:271–274. [DOI] [PubMed] [Google Scholar]
- 35. Jitnarin N, Kosulwat V, Rojroongwasinkul N, et al. . Prevalence of overweight and obesity in Thai population: results of the National Thai Food Consumption Survey. Eat Weight Disord. 2011;16:e242–e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roemling C, Qaim M. Obesity trends and determinants in Indonesia. Appetite. 2012;58:1005–1013. [DOI] [PubMed] [Google Scholar]
- 37. Karageorgi S, Alsmadi O, Behbehani KA. Review of adult obesity prevalence, trends, risk factors, and epidemiologic methods in Kuwait. J Obes. 2013;2013:378650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Memish ZA, El Bcheraoui C, Tuffaha M, et al. . Obesity and associated factors — Kingdom of Saudi Arabia, 2013. Prev Chronic Dis 2014;11:140236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Prevalence data from Hong Kong for 2013, 2014. http://www.activehealthykidshongkong.com.hk/en/report_card/2018/Obesity.asp (26 April 2020, date last accessed).
- 40. OECD, Obesity Update 2014.https://www.oecd.org/els/health-systems/Obesity-Update-2014.pdf (26 April 2020, date last accessed)
- 41. OECD, Obesity Update 2017.: https://www.oecd.org/els/health-systems/Obesity-Update-2017.pdf (26 April 2020, date last accessed).
- 42. Itan Y, Jones BL, Ingram CJ, et al. . A worldwide correlation of lactase persistence phenotype and genotypes. BMC Evol Biol. 2010;10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Al-Sanae H, Saldanha W, Sugathan TN, Majid Molla A. Comparison of lactose intolerance in healthy Kuwaiti and Asian volunteers. Med Princ Pract. 2003;12:160–163. [DOI] [PubMed] [Google Scholar]
- 44. San Diego PC, Iskandar A. Lactose intolerance in an indonesian closed community. Paediratrica Indonesi·1974;3111:a1492-105. [Google Scholar]
- 45. Fung M, Xue X, Szilagyi A. Estimating lactase nonpersistence distributions in the multi-ethnic Canadian demographic: a population-based study. J Can Assoc Gastroenterol, 2018;3:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee-Taylor J, Madronich S.. Climatology of UV-A, UV-B, and Erythemal Radiation at the Earth’s Surface, 1979–2000. Technical Note TN- 474+STR. Boulder, CO, USA: National Center for Atmospheric Research; 2007. [Google Scholar]
- 47. Mukaka MM. Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012;24:69–71. [PMC free article] [PubMed] [Google Scholar]
- 48. Simpson EH. The interpretation of interaction in contingency tables. J R Stat Soc Ser B. 1951;13:238–241 [Google Scholar]
- 49. Yule GU. Notes on the theory of association of attributes in statistics. Biometrika 1903;2:121–134. [Google Scholar]
- 50. Bernstein CN. Review article: changes in the epidemiology of inflammatory bowel disease-clues for aetiology. Aliment Pharmacol Ther. 2017;46:911–919. [DOI] [PubMed] [Google Scholar]
- 51. Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794. [DOI] [PubMed] [Google Scholar]
- 52. Kaplan GG, Bernstein CN, Coward S, et al. . The impact of inflammatory bowel disease in Canada 2018: epidemiology. J Can Assoc Gastroenterol. 2019;2:S6–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mak WY, Zhao M, Ng SC, Burisch J. The epidemiology of inflammatory bowel disease: East meets West. J Gastroenterol Hepatol. 2020;35:380–389. [DOI] [PubMed] [Google Scholar]
- 54. Costea PI, Hildebrand F, Arumugam M, et al. . Enterotypes in the landscape of gut microbial community composition. Nat Microbiol. 2018;3:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pittayanon R, Lau JT, Leontiadis GI, et al. . Differences in gut microbiota in patients with vs without inflammatory Bowel diseases: a systematic review. Gastroenterology. 2020;158:930–946.e1. [DOI] [PubMed] [Google Scholar]
- 56. Burisch J, Pedersen N, Čuković-Čavka S, et al. ; EpiCom-group . East-West gradient in the incidence of inflammatory bowel disease in Europe: the ECCO-EpiCom inception cohort. Gut. 2014;63:588–597. [DOI] [PubMed] [Google Scholar]
- 57. Aamodt G, Bengtson MB, Vatn MH. Can temperature explain the latitudinal gradient of ulcerative colitis? Cohort of Norway. BMC Public Health. 2013;13:530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dikongué E, Ségurel L. Latitude as a co-driver of human gut microbial diversity? Bioessays 2017;39:1600145. [DOI] [PubMed] [Google Scholar]
- 59. Pereira-Santos M, Costa PR, Assis AM, et al. . Obesity and vitamin D deficiency: a systematic review and meta-analysis. Obes Rev. 2015;16:341–349. [DOI] [PubMed] [Google Scholar]
- 60. Dix CF, Barcley JL, Wright ORL. The role of vitamin D in adipogenesis. Nutr Rev. 2018;76:47–59. [DOI] [PubMed] [Google Scholar]
- 61. Bassatne A, Chakhtoura M, Saad R, Fuleihan GE. Vitamin D supplementation in obesity and during weight loss: a review of randomized controlled trials. Metabolism. 2019;92:193–205. [DOI] [PubMed] [Google Scholar]
- 62. Lerchbaum E, Trummer C, Theiler-Schwetz V, et al. . Effects of Vitamin D supplementation on body composition and metabolic risk factors in men: a randomized controlled trial. Nutrients 2019;11. pii:E1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med (Maywood). 2004;229:1136–1142. [DOI] [PubMed] [Google Scholar]
- 64. Hlavaty T, Krajcovicova A, Payer J. Vitamin D therapy in inflammatory bowel diseases: who, in what form, and how much? J Crohns Colitis. 2015;9:198–209. [DOI] [PubMed] [Google Scholar]
- 65. Gubatan J, Chou ND, Nielsen OH, Moss AC. Systematic review with meta-analysis: association of vitamin D status with clinical outcomes in adult patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2019;50:1146–1158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supplemental Table A contains raw data used in this manuscript and is included with manuscript submission.