Key Points
Hyperkalemia is frequently observed in patients with CKD, and its frequency and severity increase as CKD progresses.
Patiromer is an effective and well-tolerated treatment option for hyperkalemia in patients with advanced or mild/ moderate CKD on RAASi.
Keywords: chronic kidney disease, hyperkalemia, patiromer, RAASi
Visual Abstract
Abstract
Background
Hyperkalemia is a common electrolyte abnormality in patients with CKD, which is associated with worse outcomes and limits use of renin–angiotensin–aldosterone system inhibitors (RAASi). This post hoc subgroup analysis of three clinical trials evaluated the efficacy and safety of the sodium-free, potassium-binding polymer, patiromer, for the treatment of hyperkalemia in adults with nondialysis CKD.
Methods
Data from the 4-week treatment periods of AMETHYST-DN, OPAL-HK, and TOURMALINE studies were combined. Patients had baseline diagnosis of CKD, hyperkalemia (serum potassium >5.0 mEq/L), and received patiromer 8.4–33.6 g/day. Patients were stratified by baseline eGFR into two subgroups: severe/end-stage CKD (stage 3b–5; eGFR <45 ml/min per 1.73 m2) and mild/moderate CKD (stage 1–3a; eGFR ≥45 ml/min per 1.73 m2). Efficacy was assessed by the change in serum potassium (mean±SE) from baseline to week 4. Safety assessments included incidence and severity of adverse events (AEs).
Results
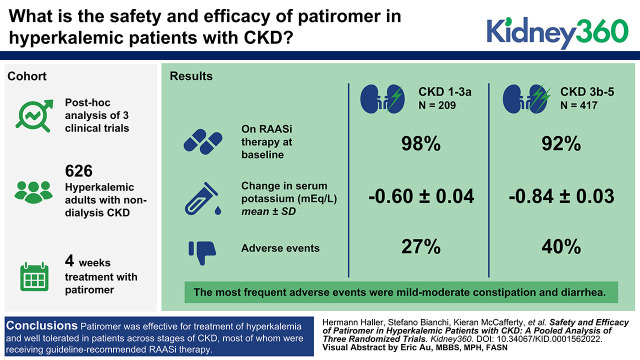
Efficacy analyses (n=626; 62% male, mean age 66 years) included 417 (67%) patients with severe/end-stage CKD and 209 (33%) with mild/moderate CKD. Most patients were receiving RAASi therapy at baseline (severe/end-stage CKD 92%; mild/moderate CKD 98%). The mean±SE change in serum potassium (baseline to week 4) was −0.84±0.03 in the severe/end-stage CKD subgroup, and −0.60±0.04 mEq/L in the mild/moderate CKD subgroup. AEs were reported for 40% and 27% patients in the severe/end-stage and mild/moderate CKD subgroups, respectively, with 16% and 12% reporting AEs considered related to patiromer. The most frequent AEs were mild-to-moderate constipation (8% and 3%) and diarrhea (4% and 2%). AEs leading to patiromer discontinuation occurred in 6% and 2% of patients with severe/end-stage CKD, and mild/moderate CKD, respectively.
Conclusions
Patiromer was effective for treatment of hyperkalemia and well tolerated in patients across stages of CKD, most of whom were receiving guideline-recommended RAASi therapy.
Introduction
Hyperkalemia (serum potassium >5.0 mEq/L) is a frequently observed electrolyte abnormality in patients with CKD, and its frequency and severity increases as CKD progresses (1). Chronic hyperkalemia increases secretion of aldosterone, which can lead to deleterious effects on the kidney and cardiovascular system, including vascular inflammation, renal and myocardial fibrosis, and ventricular hypertrophy (2). Hyperkalemia has consistently been linked to increased hospitalization and worse clinical outcomes in patients with heart failure (3).
The Kidney Disease: Improving Global Outcomes clinical practice guidelines recommend that treatment with an angiotensin-converting enzyme inhibitor (ACEi) or angiotensin II receptor blocker (ARB) should be initiated in patients with nondialysis CKD with or without diabetes who have hypertension, and either severely or moderately increased albuminuria, and these medications should be titrated to the highest approved tolerated dose (4,5). These recommendations are on the basis of several pivotal trials reporting benefits of renin–angiotensin–aldosterone inhibitors (RAASi) in reducing BP, decreasing albuminuria, delaying kidney disease progression, and reducing cardiovascular complications (6–11).
RAASi increases the risk of hyperkalemia, which frequently leads to downtitration or discontinuation of guideline-recommended RAASi therapy in patients with CKD (12–14). Observational studies have shown that dose reduction or cessation of RAASi therapy is associated with increased morbidity and mortality in this population (15). The Kidney Disease: Improving Global Outcomes clinical practice guidelines recommend that the dose of an ACEi or ARB should be reduced or discontinued only as a last resort in patients with CKD and with hyperkalemia (4), and to consider the use of potassium binders for the treatment of hyperkalemia.
Patients with reduced kidney function can be susceptible to further kidney injury and increased metabolic derangements, with effect and sensitivity to specific risk factors linked to remaining levels of kidney function (16,17). It is therefore particularly important to assess the safety and efficacy of medications in patient subpopulations with both early and late-stage disease.
Patiromer is a nonabsorbed, sodium-free, potassium-binding polymer shown to reduce serum potassium to normal levels in clinical trials in patients with hyperkalemia, and thereby enables RAASi therapy (18–20). The vast majority of patients enrolled in these studies had CKD; however, the effect of patiromer has not previously been evaluated between patients across different stages of kidney disease. We conducted a post hoc subgroup analysis of three clinical trials (AMETHYST-DN, OPAL-HK, and TOURMALINE) (18–20) to evaluate the efficacy and safety of patiromer for the treatment of hyperkalemia in adult patients with stage 3b–5 CKD and those with stage 1−3a CKD over a 4-week treatment period.
Materials and Methods
Study Designs and Patients
This post hoc analysis was performed using pooled data from the initial 4-week treatment phases of the AMETHYST-DN and OPAL-HK studies (18,19), and the complete 4-week treatment period of the TOURMALINE study (20). All three trials evaluated the efficacy and safety of patiromer for the treatment of hyperkalemia and enrolled adult patients with a baseline serum potassium value >5.0 mEq/L, as evaluated by local laboratory measurement. None of the trials used a placebo arm for the initial 4-week treatment phases. The methods and results of AMETHYST-DN, OPAL-HK, and TOURMALINE have been described previously (18–20). A brief overview of the design and key inclusion criteria for each study is provided below and in Supplemental Table 1. This study was exempt from Institutional Review Board review due to the use of public, deidentified data.
AMETHYST-DN (NCT01371747) was a 52-week, phase 2, open-label, randomized, dose-ranging study that evaluated patiromer in 304 adults with type 2 diabetes mellitus, CKD (mean±SD eGFR 40.6±50.7 ml/min per 1.73 m2) and hyperkalemia who were receiving RAASi therapy (18). Patients were stratified according to their baseline serum potassium levels and assigned into two separate groups (mild hyperkalemia: serum potassium >5.0–5.5 mEq/L or moderate hyperkalemia: >5.5 to <6.0 mEq/L). Patients with mild hyperkalemia received patiromer at starting doses of 8.4 g to 25.2 g/day, whereas those with moderate hyperkalemia received 16.8–33.6 g/day. Patiromer was titrated during the treatment period to reach and maintain serum potassium ≤5.0 mEq/L.
OPAL-HK (NCT01810939) was a 12-week, phase 3, two-phase, single-blind, randomized withdrawal trial that evaluated patiromer in 243 patients with CKD (mean±SD eGFR 35.4±16.2 ml/min per 1.73 m2) and hyperkalemia who were receiving RAASi (19). During the initial 4-week treatment phase, patiromer was administered at a starting dose of either 8.4 g/day for patients with mild hyperkalemia at baseline (serum potassium 5.1 to <5.5 mEq/L), or 16.8 g/day for those with moderate-to-severe hyperkalemia (≥5.5 to <6.5 mEq/L). Patiromer was titrated to achieve and maintain serum potassium within the target range (3.8 to <5.1 mEq/L). After the initial 4-week treatment phase, eligible patients entered a randomized withdrawal phase, and continued patiromer treatment or were switched to placebo for 8 weeks.
TOURMALINE (NCT02694744) was a 4-week, phase 4, open-label, randomized trial that evaluated patiromer administered without food versus with food in 112 patients with hyperkalemia. Most patients who participated in the study (n=85, 76%) had a baseline diagnosis of CKD (mean±SD eGFR 31.8±18.3 ml/min per 1.73 m2). Patiromer was administered at a starting dose of 8.4 g/day and titrated in increments of 8.4 g/day (to a maximum of 25.2 g/day) to reach and maintain serum potassium levels in the target range of 3.8–5.0 mEq/L. In contrast with AMETHYST-DN and OPAL-HK, patients in TOURMALINE were not required to be taking RAASi therapy before and during the study.
CKD Subgroups and Analysis Populations
In the present pooled analysis, patients with an investigator determined diagnosis of CKD at screening (defined as eGFR 15 ml/min per 1.73 m2 to <60 ml/min per 1.73 m2 at screening) from the AMETHYST-DN, OPAL-HK, and TOURMALINE studies were stratified according to central laboratory-measured baseline eGFR into two subgroups: (1) stage 3b–5 CKD (nondialysis; eGFR <45 ml/min per 1.73 m2) or (2) stage 1–3a CKD (eGFR ≥45 ml/min per 1.73 m2).
All safety assessments were performed on the safety population (n=632), which comprised all randomized patients with CKD from AMETHYST-DN, OPAL-HK, and TOURMALINE who received ≥1 dose of patiromer. Evaluation of baseline characteristics and efficacy assessments was performed on the efficacy population (n=626), comprising all randomized patients with CKD from the three trials who received ≥1 dose patiromer and had ≥1 post-baseline weekly serum potassium assessment available (AMETHYST-DN: n=304/304; OPAL-HK: n=237/237; TOURMALINE: n=85/112). The efficacy population excluded six patients from the OPAL-HK study without a weekly post-baseline serum potassium measurement available. Six patients from the AMETHYST-DN study did not have a baseline eGFR value available; therefore, for the purposes of this analysis, the earliest available assessment of eGFR was used to impute their CKD stage.
Efficacy and Safety Assessments
Efficacy was evaluated as the mean±SE change in central laboratory serum potassium from baseline to week 4, which was the primary endpoint of the AMETHYST-DN and OPAL-HK studies, and a secondary endpoint of the TOURMALINE study. Changes in serum potassium during the 4-week treatment period and the proportion of patients who achieved target serum potassium levels (≥1 serum potassium measurement 3.8–5.0 mEq/L) were also evaluated.
Safety outcomes included the frequency and severity of adverse events (AEs) with onset during the 4-week treatment period. All AEs were recorded on the basis of on reports from the study investigator and separately on the basis of prespecified laboratory values of interest.
All efficacy and safety results are presented separately for the stage 3b–5 and stage 1–3a CKD subgroups.
Statistical Analyses
Descriptive statistics were summarized as mean±SD or SE for continuous variables, or as proportions for categorical variables. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline Demographics and Clinical Characteristics
Baseline serum potassium (mean±SD) was comparable in all three trials: in OPAL-HK, it was 5.6±0.5 mmol/L; in AMETHYST-DN it was 5.3±0.4 mmol/L; and in TOURMALINE it was 5.4±0.4 and 5.3±0.4 mmol/L in the with and without food groups, respectively. Of the 626 patients included in the efficacy population, 417 patients (67%) had stage 3b–5 CKD (including 27 patients [6%] with stage 5 CKD) and 209 patients (33%) had stage 1–3a CKD. Baseline characteristics were generally balanced between the subgroups, with some notable differences (Table 1). Mean±SD serum potassium was higher among patients with stage 3b–5 CKD than in those with stage 1−3a CKD (5.47±0.41 and 5.32±0.42 mEq/L, respectively). Patients in the stage 3b–5 CKD subgroup also had a higher mean spot urine albumin-creatinine ratio than patients in the stage 1–3a CKD subgroup. Most patients in the stage 3b–5 CKD and stage 1–3a subgroups had comorbid hypertension (99% and 98%, respectively) and comorbid diabetes (80% and 88%, respectively). Approximately one third of patients with stage 3b–5 and stage 1–3a CKD had heart failure (34% and 35%, respectively), predominantly New York Heart Association class II. The mean age was 66 years in both subgroups.
Table 1.
Baseline demographics and clinical characteristics (efficacy population; n=626)
| Characteristics | Patients with Stage 3b–5 CKD (n=417) | Patients with Stage 1–3a CKD (n=209) |
|---|---|---|
| Male, n (%) | 253 (61) | 134 (64) |
| Age (yr), mean (SD) | 66 (10) | 66 (9) |
| White, n (%) | 398 (95) | 207 (99) |
| Body weight (kg) mean (SD) | 84.8 (15.0) | 85.6 (14.1) |
| History of diabetes mellitus, n (%) | 332 (80) | 183 (88) |
| Hypertension, n (%) | 411 (99) | 205 (98) |
| Ejection fraction (%)a, mean (SD) | 49.8 (8.9) | 50.0 (8.9) |
| Previous myocardial infarction, n (%) | 72 (17) | 47 (22) |
| History of heart failure | 141 (34) | 73 (35) |
| NYHA heart failure classb, n (%) | ||
| I | 36 (9) | 14 (7) |
| II | 92 (22) | 54 (26) |
| III | 13 (3) | 5 (2) |
| eGFR (ml/min per 1.73 m2)c, mean (SD) | 27.9 (8.9) | 58.0 (12.8) |
| CKD stage (eGFR) n (%) | ||
| Stage 1 (≥90) | 0 | 7 (3) |
| Stage 2 (60–89) | 0 | 61 (29) |
| Stage 3a (45–59) | 0 | 141 (67) |
| Stage 3b (30–44) | 178 (43) | 0 |
| Stage 4 (15–29) | 212 (51) | 0 |
| Stage 5 (<15) | 27 (6) | 0 |
| Spot urine ACR (mg/g),d median (Q1, Q3) | 412.0 (41.7, 1103.3) | 599. (16.5, 545.3) |
| Serum potassium (mEq/L), mean (SD) | 5.47 (0.41) | 5.32 (0.42) |
SD, standard deviation; NYHA, New York Heart Association; ACR, albumin-creatinine ratio.
Ejection fraction data were collected only in AMETHYST-DN (n=304).
Patients with NYHA class IV heart failure were excluded.
Central laboratory baseline eGFR was on the basis of the Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) formula. Seven subjects in AMETHYST-DN did not have a baseline value; for the purpose of this analysis, the earliest available assessment of eGFR was used to impute CKD stage at baseline.
Spot urine ACR data were collected only in AMETHYST-DN (n=304) and OPAL-HK (n=243).
Overall, 92% of patients with stage 3b−5 CKD and 98% of patients with stage 1−3a CKD were receiving RAASi therapy at baseline (Table 2). In the stage 3b–5 CKD subgroup, the most frequently prescribed RAASi medications were ACEi (60%) and ARB (41%). Approximately one third of patients with stage 3b–5 CKD (37%) were receiving a beta blocker, and 41% a loop diuretic.
Table 2.
Antihypertensive medications received by patients at baseline (efficacy population; n=626)
| Medications | Patients with Stage 3b–5 CKD (n=417) | Patients with Stage 1–3a CKD (n=209) |
|---|---|---|
| Any RAASi, n (%) | 383 (92) | 204 (98) |
| ACE inhibitor | 252 (60) | 138 (66) |
| ARB | 171 (41) | 81 (39) |
| MRA | 31 (7) | 18 (9) |
| Renin inhibitor | 1 (0.2) | 1 (0.5) |
| Dual RAASi blockadea | 67 (16) | 31 (15) |
| ACE inhibitor + ARB | 37 (9) | 14 (7) |
| ACE inhibitor + MRA | 10 (2) | 5 (2) |
| ACE inhibitor + renin inhibitor | 1 (0.2) | (0) |
| ARB + MRA | 14 (3) | 10 (5) |
| ACE inhibitor + ARB + MRA | 5 (1) | 1 (0.5) |
| ACE inhibitor + ARB + MRA + renin inhibitor | 0 (0) | 1 (0.5) |
| Beta blocker, n (%) | 155 (37) | 85 (41) |
| Diuretic, n (%) | ||
| Thiazide | 33 (8) | 15 (7) |
| Loop | 172 (41) | 52 (25) |
RAASi, renin–angiotensin–aldosterone system inhibitor; ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; MRA, mineralocorticoid receptor antagonist.
Any combination of ≥2 of the following: ACE inhibitor, ARB, MRA, renin inhibitor.
Efficacy
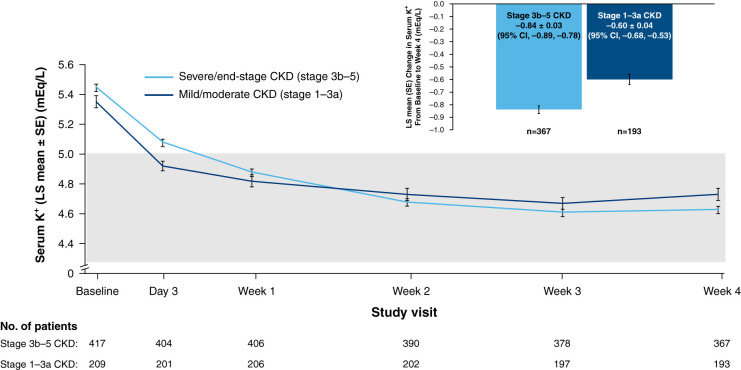
Treatment with patiromer induced early reductions in serum potassium in both subgroups, with mean levels decreasing from baseline to <5.0 mEq/L by week 1 or day 3 in the stage 3b–5 and stage 1–3a CKD subgroups, respectively (Figure 1). The mean±SD change in serum potassium from baseline to week 4 was −0.84±0.03 and −0.60±0.04 mEq/L among patients with stage 3b–5 and stage 1–3a CKD, respectively. The proportion of patients who achieved normokalemia (at least one serum potassium value in the range 3.8–5.0 mEq/L) at any time during the 4-week treatment period was 96% in the stage 3b–5 CKD subgroup (86% at week 4) and 99% in the CKD stage 1–3a subgroup (88% at week 4).
Figure 1.
Least squares mean (SE) serum potassium levels over time by study visit and change in serum potassium from baseline to week 4 in patients with stage 3b–5 CKD or stage 1–3a CKD (efficacy population; n=626). Shaded area denotes target serum K+ range: 3.8–5.0 mEq/L. 95% CI, 95% confidence interval; K+, potassium; LS, least squares; SE, standard error of the mean.
The mean±SD change in systolic BP from baseline to week 4 was −6.2±19.2 and −10.7±17.5 mm Hg (−4.0±12.0 and −6.5±12.2 for diastolic BP) among patients with stage 3b–5 and stage 1–3a CKD, respectively.
In patients with hyperkalemic, RAASi medication use at week 4 was reported in 85% and 95% of patients in the stage 3b–5 and stage 1–3a CKD groups, respectively.
Safety and Tolerability
In total, 421 patients with stage 3b–5 CKD and 211 patients with stage 1–3a CKD were included in the safety population. During the 4-week treatment period, 16% of patients with stage 3b–5 CKD and 12% of patients with stage 1–3a CKD reported ≥1 treatment-emergent AE related to patiromer (Table 3). The most frequent treatment-emergent AE related to patiromer in the stage 3b–5 CKD and stage 1–3a CKD subgroups, respectively, were constipation (7% and 3%) and diarrhea (3% and 2%), none of which were severe.
Table 3.
Treatment-emergent adverse events related to patiromer (safety population; n=632)
| No. of Patients (%) | Severity | Patients with Stage 3b–5 CKD (n=421) | Patients with Stage 1–3a CKD (n=211) |
|---|---|---|---|
| ≥1 adverse event | Any severity | 67 (15.9) | 25 (11.8) |
| Mild | 45 (10.7) | 18 (8.5) | |
| Moderate | 22 (5.2) | 7 (3.3) | |
| Severe | 0 (0.0) | 0 (0.0) | |
| Gastrointestinal disorders | Any severity | 54 (12.8) | 13 (6.2) |
| Mild | 36 (8.6) | 10 (4.7) | |
| Moderate | 18 (4.3) | 3 (1.4) | |
| Severe | 0 (0.0) | 0 (0.0) | |
| Constipation | Any severity | 30 (7.1) | 6 (2.8) |
| Mild | 21 (5.0) | 6 (2.8) | |
| Moderate | 9 (2.1) | 0 (0.0) | |
| Severe | 0 (0.0) | 0 (0.0) | |
| Diarrhea | Any severity | 12 (2.9) | 4 (1.9) |
| Mild | 8 (1.9) | 4 (1.9) | |
| Moderate | 4 (1.0) | 0 (0.0) | |
| Severe | 0 (0.0) | 0 (0.0) | |
| Metabolism and nutrition disorders | Any severity | 16 (3.8) | 7 (3.3) |
| Mild | 13 (3.1) | 4 (1.9) | |
| Moderate | 3 (0.7) | 3 (1.4) | |
| Severe | 0 (0.0) | 0 (0.0) | |
| Hypomagnesemia | Any severity | 9 (2.1) | 5 (2.4) |
| Mild | 8 (1.9) | 3 (1.4) | |
| Moderate | 1 (0.2) | 2 (0.9) | |
| Severe | 0 (0.0) | 0 (0.0) |
This table summarizes adverse events during the first 4 weeks after the start of patiromer treatment (defined as onset on or before study day 32), regardless of the date of treatment discontinuation. Adverse event that occurred in ≥2% of patients in either subgroup.
AEs leading to discontinuation of patiromer were reported for 24 (6%) patients with stage 3b–5 CKD and five (2%) of patients with stage 1–3a CKD (Supplemental Table 2).
Overall, 19 patients had ≥1 serious AE (16 in the stage 3b–5 CKD subgroup and three in the stage 1–3a CKD subgroup); none of these were related to patiromer, on the basis of investigator assessment (Supplemental Table 3).
Mean serum magnesium, calcium, and phosphate levels remained unchanged, and within the normal range in both subgroups during the 4-week treatment period (Supplemental Table 4).
Hypomagnesemia was reported for nine patients (2%) in the stage 3b–5 CKD subgroup and for five patients (3%) in the stage 1–3a CKD subgroup. During the treatment period, 19 patients (5%) in the stage CKD 3b−5 subgroup and 18 patients (9%) in the stage 1–3 CKD subgroup had serum magnesium <1.4 mg/dl (Table 4). One patient from the stage 3b–5 CKD subgroup had a serum magnesium value <1.2 mg/dl (1.05 mg/dl).
Table 4.
Prespecified laboratory values of interest during 4-week treatment period (safety population; n=632)
| No. of Patients (%)a | Patients with Stage 3b–5 CKD (n=421) | Patients with Stage 1–3a CKD (n=211) |
|---|---|---|
| Any serum K+ value in target range (3.8–5.0 mEq/L) | 403/418 (96) | 209/211 (99) |
| Any serum K+ value <3.5 mEq/L | 8/418 (2) | 3/211 (1) |
| Any serum value Mg2+ | ||
| <1.4 mg/dl | 19/412 (5) | 18/209 (9) |
| <1.2 mg/dl | 1/412 (0.2) | 0/209 (0.0) |
| Any serum Ca2+ value >ULNb | 21/412 (5) | 13/209 (6) |
| Any serum phosphate value >ULNc | 121/411 (29) | 24/209 (11) |
K+, potassium; Mg2+, magnesium; Ca2+, calcium; ULN, upper limit of normal.
Data summary is on the basis of observed patients; for some patients, laboratory values were missing during the first 4 weeks of the study.
Serum Ca2+ ULN >10.3 mg/dl for AMETHYST-DN, and >10.5 mg/dl for studies OPAL-HK and TOURMALINE.
Serum phosphate ULN >4.5 mg/dL in subjects aged <65 years, and >4.3 mg/dl in subjects aged ≥65 years for AMETHYST-DN and >4.8 mg/dL for OPAL-HK and TOURMALINE.
AEs of hypokalemia were reported for seven patients (2%) with stage 3b–5 CKD (serum potassium 3.0–4.9 mEq/L) and one patient (0.5%) with stage 1–3a CKD. The proportion of patients with serum potassium <3.5 mEq/L during the 4-week treatment period was similarly low in both the stage 3b–5 CKD and stage 1–3a CKD subgroups (2% and 1%, respectively) (Table 4).
No AEs of hypercalcemia were reported for patients in either subgroup and no clinically relevant increases in serum calcium or postbaseline values >12.0 mg/dl were observed. Serum calcium values above the upper limit of normal (ULN) were recorded for 21 patients (5%) with stage 3b–5 CKD and 13 patients (6%) with stage 1–3a CKD (Table 4). One patient had an AE of hypocalcemia in the stage 3b–5 CKD subgroup. The highest postbaseline calcium value was 11.9 mg/dl, recorded for a patient with elevated serum calcium at study baseline (12.7 mg/dl).
No AEs of hyperphosphatemia were reported for patients in either subgroup. Any serum phosphate values >ULN was reported for 29% of patients in the stage 3b–5 CKD subgroup and 11% in the stage 1–3a subgroup (Table 4).
Overall, there were no clinically meaningful changes from baseline in mean eGFR during the 4-week treatment period in either subgroup (Supplemental Table 5). A reduction from baseline to week 4 in mean spot urine albumin-creatinine was observed in the stage 3b–5 CKD subgroup, whereas levels remained unchanged in the stage 1–3a CKD subgroup (Supplemental Table 5).
Discussion
Patiromer, a sodium-free potassium binder, may be an appropriate treatment option to treat hyperkalemia in patients with CKD on RAASi. Patients with CKD may be at risk of volume overload and are often on sodium-restricted diets to help control hypertension and albuminuria (21). Sodium may also blunt the response to RAAS blockade (21).
Given the potential variability in both efficacy and safety of treatments in patients with early versus advanced CKD, it is important to assess the safety and efficacy of medications in both these subpopulations (16,17).
This subgroup analysis demonstrated that patiromer provided effective reduction of serum potassium in patients who are hyperkalemic, with either mild/moderate or advanced/end-stage CKD, >92% of whom were receiving RAASi therapy at baseline. Most patients responded to the 8.4 g starting dose, with reductions in mean serum potassium occurring early during treatment and reaching normal levels in both the mild/moderate and advanced/end-stage CKD patient subgroups, regardless of initial serum potassium levels.
This analysis showed that patiromer was generally well tolerated by patients with either mild/moderate or advanced/end-stage CKD over the 4-week treatment period, with a minimal risk of hypokalemia. Consistent with previous reports for the overall patient populations from these three studies (18–20), the most frequent AEs in both subgroups were gastrointestinal in nature, comprising mainly mild-to-moderate constipation and diarrhea.
Overall, the proportion of patients with AEs and serious AEs was numerically higher among patients with advanced/end-stage CKD versus patients with mild/moderate CKD. However, this finding was not unexpected, given that a higher incidence of AEs among patients with advanced versus earlier stages of CKD has been reported in several other studies (22–26); indicating that patients with advanced CKD typically have a greater burden of comorbidities than those with earlier stages of CKD. None of the serious AEs recorded for patients in either subgroup was considered by investigators to be related to treatment with patiromer. The profile of the serious AEs that occurred more frequently in patients with stage 3b–5 CKD (renal and urinary disorders, cardiac disorders, and vascular disorders), was consistent with the profile expected for patients with lower eGFR, and appeared to be related to underlying disease, rather than to any specific treatment effects with patiromer
In this subgroup analysis, mean serum magnesium levels remained within the target range during the 4-week treatment period in both the stage 3b–5 CKD and stage 1–3a CKD subgroups, which is consistent with serum magnesium levels observed in the AMBER study (27). Reported AEs of hypomagnesemia occurred in approximately 2% of patients in each subgroup. However, at least one serum magnesium level <1.4 mg/dl was reported for fewer patients in the stage 3b–5 CKD subgroup (5%) than in the stage 1–3a CKD subgroup (9%), similar to findings reported in previous trials (28,29). AEs of hypokalemia were reported for more patients in the stage 3b–5 CKD subgroup (2%) than in the stage 1–3a CKD subgroup (0.5%), but the overall incidence was low. In the 12-week, placebo-controlled AMBER trial, rates of serum magnesium were 0.7% in patients who are placebo-treated versus 2% in patiromer treated patients (data on file). The lower rates of low serum magnesium in this advanced CKD population may be due to the lower starting dose of 8.4 g of patiromer in all patients in the AMBER study.
The few phosphate measurements more than ULN observed were likely due to the underlying CKD, and not to patiromer treatment. There is no mechanistic reason to expect patiromer treatment to result in increases in phosphate; patiromer has been shown to lower phosphate in previous studies (30,31).
This study had several limitations. It was a post hoc analysis, so the findings should be considered exploratory in nature. Additionally, duration of follow-up was limited to the 4-week treatment periods of the three trials; however, data from the overall AMETHYST-DN study have demonstrated that patiromer was well tolerated, long term, and can control serum potassium levels among patients with CKD over a 1-year treatment period (18). Furthermore, a recent global pharmacovigilance database study of patiromer, which reviewed >17,000 individual case reports over a 4-year period, confirmed that the safety and tolerability profile of patiromer in the real-world setting is consistent with the clinical trial data (32). The three trials that were the subject of the present analysis had no active comparator or placebo group; however, placebo-controlled data for patiromer in patients with advanced CKD were provided in the AMBER study, which showed similar rates of AEs in the patiromer and placebo groups (56% and 53%, respectively) over a 12-week treatment period (27).
In conclusion, this pooled analysis of three clinical trials showed that patiromer provided an effective treatment for patients with hyperkalemia across the spectrum of both early and advanced nondialysis CKD, most of whom were receiving guideline-recommended RAASi. Patiromer was also well tolerated in both subpopulations, with mild-to-moderate gastrointestinal events being reported in a small proportion of patients, and relatively few treatment discontinuations.
Disclosures
C. M. Quinn reports having employment with Vifor Pharma; reports having an ownership interest in AstraZeneca and Vifor Pharma; reports receiving research funding from CARE-HK in the Heart Failure registry; reports having patents or royalties with AstraZeneca; and reports having an advisory or leadership role with the Journal of Precision Medicine editorial board. H. Haller reports having consultancy agreements with Alexion, AstraZeneca, Bayer Pharma, Boehringer, MedWiss, Phenos, and Vifor-Fresenius; reports receiving honoraria from Alexion, AstraZeneca, Bayer Pharma, Boehringer, MedWiss, Novartis, Phenos, and Vifor-Fresenius; reports advisory or leadership role with Alexion, Bayer Pharma, Der Internist, and Der Nephrologe; and speakers bureau Amgen, Alexion, AstraZeneca, Bayer Pharma, Boehringer, Novartis, MedWiss, Phenos, and Vifor-Fresenius. J. Budden and S. Arthur report having employment with, and an ownership interest in, Vifor Pharma. K. Mccafferty reports having employment with the National Health Service; reports having consultancy agreements with Oncacare; reports receiving research funding from AstraZeneca; reports receiving honoraria from AstraZeneca, Bayer, Vifor Fresenius, Napp, and Pharmacosmos; and speakers bureau from AstraZeneca and Bayer. M. Weir reports having consultancy agreements with Akebia, AstraZeneca, Bayer, Boehringer-Ingelheim, CareDx, Janssen, Merck, NovoNordisk, and Vifor Pharma, all are modest (<$10,000); reports receiving honoraria and having an advisory or leadership role with Akebia, AstraZeneca, Bayer, Boehringer-Ingelheim, CareDx, Janssen, Merck, NovoNordisk, and Vifor Pharma for ad hoc advisory boards. S. Bianchi reports consultancy and lectures fees from AstraZeneca, Bayer Pharma, Boehringer, Lilly, Novo Nordisk, Pfizer, and Vifor Pharma; and reports being a grant holder for The Italian Ministry of Health.
Funding
The analyses presented in this manuscript and the original clinical studies were funded by Vifor Pharma.
Acknowledgments
Editorial assistance for this manuscript was provided by AXON Communications (London, UK) and funded by Vifor Pharma.
Author Contributions
S. Arthur, J. Budden, and M. Weir conceptualized the study; S. Arthur was responsible for the data curation, formal analysis, methodology, validation, and visualization, and provided supervision; S. Arthur, J. Budden, and M. Weir wrote the original draft; S. Arthur, S. Bianchi, J. Budden, H. Haller, K. McCafferty, C. Quinn, and M. Weir reviewed and edited the manuscript.
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0001562022/-/DCSupplemental
Comparison of trial design for AMETHYST-DN, OPAL-HK, and TOURMALINE. Download Supplemental Table 1, PDF file, 178 KB (177.9KB, pdf)
Nonfatal treatment-emergent adverse events leading to treatment discontinuation, with onset during the first 4 weeks, by system organ class and preferred term. Download Supplemental Table 2, PDF file, 178 KB (177.9KB, pdf)
Serious treatment-emergent adverse events with onset during the first 4 weeks, by system organ class and preferred term. Download Supplemental Table 3, PDF file, 178 KB (177.9KB, pdf)
Mean (SD) serum calcium, phosphate, and magnesium at baseline, week 4 and change from baseline to week 4 (safety population; n=632). Download Supplemental Table 4, PDF file, 178 KB (177.9KB, pdf)
Mean (SD) eGFR and spot urine ACR at baseline, week 4, and change from baseline to week 4 (safety population; n=632). Download Supplemental Table 5, PDF file, 178 KB (177.9KB, pdf)
References
- 1.Kovesdy CP, Matsushita K, Sang Y, Brunskill NJ, Carrero JJ, Chodick G, Hasegawa T, Heerspink HL, Hirayama A, Landman GWD, Levin A, Nitsch D, Wheeler DC, Coresh J, Hallan SI, Shalev V, Grams ME; CKD Prognosis Consortium : Serum potassium and adverse outcomes across the range of kidney function: A CKD Prognosis Consortium meta-analysis. Eur Heart J 39: 1535–1542, 2018. 10.1093/eurheartj/ehy100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunter RW, Bailey MA: Hyperkalemia: Pathophysiology, risk factors and consequences. Nephrol Dial Transplant 34[Suppl 3]: iii2–iii11, 2019. 10.1093/ndt/gfz206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossignol P, Dobre D, McMurray JJV, Swedberg K, Krum H, Veldhuisen DJv, Shi H, Messig M, Vincent J, Girerd N, Bakris G, Pitt B, Zannad F: Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy. Circ Heart Fail 7: 51–58, 2014. 10.1161/CIRCHEARTFAILURE.113.000792 [DOI] [PubMed] [Google Scholar]
- 4.Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group: KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int 99: S1–S87, 2021. 10.1016/j.kint.2020.11.003 [DOI] [PubMed] [Google Scholar]
- 5.Cheung AK, Chang TI, Cushman WC, Furth SL, Hou FF, Ix JH, Knoll GA, Muntner P, Pecoits-Filho R, Sarnak MJ, Tobe SW, Tomson CRV, Lytvyn L, Craig JC, Tunnicliffe DJ, Howell M, Tonelli M, Cheung M, Earley A, Mann JFE: Executive summary of the KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int 99: 559–569, 2021. 10.1016/j.kint.2020.10.026 [DOI] [PubMed] [Google Scholar]
- 6.Maschio G, Alberti D, Janin G, Locatelli F, Mann JF, Motolese M, Ponticelli C, Ritz E, Zucchelli P; The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group : Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. N Engl J Med 334: 939–945, 1996. 10.1056/NEJM199604113341502 [DOI] [PubMed] [Google Scholar]
- 7.Hou FF, Zhang X, Zhang GH, Xie D, Chen PY, Zhang WR, Jiang JP, Liang M, Wang GB, Liu ZR, Geng RW: Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med 354: 131–140, 2006. 10.1056/NEJMoa053107 [DOI] [PubMed] [Google Scholar]
- 8.Ruggenenti P, Perna A, Gherardi G, Garini G, Zoccali C, Salvadori M, Scolari F, Schena FP, Remuzzi G: Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 354: 359–364, 1999. 10.1016/S0140-6736(98)10363-X [DOI] [PubMed] [Google Scholar]
- 9.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S; RENAAL Study Investigators : Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001. 10.1056/NEJMoa011161 [DOI] [PubMed] [Google Scholar]
- 10.Parving HH, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P; Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group : The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 345: 870–878, 2001. 10.1056/NEJMoa011489 [DOI] [PubMed] [Google Scholar]
- 11.Keane WF, Brenner BM, de Zeeuw D, Grunfeld JP, McGill J, Mitch WE, Ribeiro AB, Shahinfar S, Simpson RL, Snapinn SM, Toto R; RENAAL Study Investigators : The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: The RENAAL study. Kidney Int 63: 1499–1507, 2003. 10.1046/j.1523-1755.2003.00885.x [DOI] [PubMed] [Google Scholar]
- 12.Einhorn LM, Zhan M, Hsu VD, Walker LD, Moen MF, Seliger SL, Weir MR, Fink JC: The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med 169: 1156–1162, 2009. 10.1001/archinternmed.2009.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vijayakumar S, Butler J, Bakris GL: Barriers to guideline mandated renin-angiotensin inhibitor use: Focus on hyperkalaemia. Eur Heart J Suppl 21[Suppl A]: A20–A27, 2019. 10.1093/eurheartj/suy030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Lullo L, Ronco C, Granata A, Paoletti E, Barbera V, Cozzolino M, Ravera M, Fusaro M, Bellasi A: Chronic hyperkalemia in cardiorenal patients: Risk factors, diagnosis, and new treatment options. Cardiorenal Med 9: 8–21, 2019. 10.1159/000493395 [DOI] [PubMed] [Google Scholar]
- 15.Epstein M, Reaven NL, Funk SE, McGaughey KJ, Oestreicher N, Knispel J: Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care 21[Suppl]: S212–S220, 2015 [PubMed] [Google Scholar]
- 16.Whittaker CF, Miklich MA, Patel RS, Fink JC: Medication safety principles and practice in CKD. Clin J Am Soc Nephrol 13: 1738–1746, 2018. 10.2215/CJN.00580118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramaswamy R, Wee SN, George K, Ghosh A, Sarkar J, Burghaus R, Lippert J: CKD subpopulations defined by risk-factors: A longitudinal analysis of electronic health records. CPT Pharmacometrics Syst Pharmacol 10: 1343–1356, 2021. 10.1002/psp4.12695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bakris GL, Pitt B, Weir MR, Freeman MW, Mayo MR, Garza D, Stasiv Y, Zawadzki R, Berman L, Bushinsky DA; AMETHYST-DN Investigators : Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: The AMETHYST-DN randomized clinical trial. JAMA 314: 151–161, 2015. 10.1001/jama.2015.7446 [DOI] [PubMed] [Google Scholar]
- 19.Weir MR, Bakris GL, Bushinsky DA, Mayo MR, Garza D, Stasiv Y, Wittes J, Christ-Schmidt H, Berman L, Pitt B; OPAL-HK Investigators : Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med 372: 211–221, 2015. 10.1056/NEJMoa1410853 [DOI] [PubMed] [Google Scholar]
- 20.Pergola PE, Spiegel DM, Warren S, Yuan J, Weir MR: Patiromer lowers serum potassium when taken without food: Comparison to dosing with food from an open-label, randomized, parallel group hyperkalemia study. Am J Nephrol 46: 323–332, 2017. 10.1159/000481270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muntinghe F, Navis G: Sodium status and the response to blockade of the renin-angiotensin system. In, 2007, pp 417–432
- 22.Wanner C, Inzucchi SE, Zinman B: Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 375: 1801–1802, 2016. 10.1056/NEJMoa1515920 [DOI] [PubMed] [Google Scholar]
- 23.Roger SD, Lavin PT, Lerma EV, McCullough PA, Butler J, Spinowitz BS, von Haehling S, Kosiborod M, Zhao J, Fishbane S, Packham DK: Long-term safety and efficacy of sodium zirconium cyclosilicate for hyperkalaemia in patients with mild/moderate versus severe/end-stage chronic kidney disease: Comparative results from an open-label, Phase 3 study. Nephrol Dial Transplant 36: 137–150, 2021. 10.1093/ndt/gfz285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perkovic V, Toto R, Cooper ME, Mann JFE, Rosenstock J, McGuire DK, Kahn SE, Marx N, Alexander JH, Zinman B, Pfarr E, Schnaidt S, Meinicke T, von Eynatten M, George JT, Johansen OE, Wanner C; CARMELINA investigators : Effects of linagliptin on cardiovascular and kidney outcomes in people with normal and reduced kidney function: Secondary analysis of the CARMELINA randomized trial. Diabetes Care 43: 1803–1812, 2020. 10.2337/dc20-0279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bakris G, Oshima M, Mahaffey KW, Agarwal R, Cannon CP, Capuano G, Charytan DM, de Zeeuw D, Edwards R, Greene T, Heerspink HJL, Levin A, Neal B, Oh R, Pollock C, Rosenthal N, Wheeler DC, Zhang H, Zinman B, Jardine MJ, Perkovic V: Effects of canagliflozin in patients with baseline eGFR <30 ml/min per 1.73 m2: Subgroup analysis of the randomized CREDENCE trial. Clin J Am Soc Nephrol 15: 1705–1714, 2020. 10.2215/CJN.10140620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jhund PS, Solomon SD, Docherty KF, Heerspink HJL, Anand IS, Böhm M, Chopra V, de Boer RA, Desai AS, Ge J, Kitakaze M, Merkley B, O’Meara E, Shou M, Tereshchenko S, Verma S, Vinh PN, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Bengtsson O, Langkilde AM, Sjöstrand M, McMurray JJV: Efficacy of dapagliflozin on renal function and outcomes in patients with heart failure with reduced ejection fraction: Results of DAPA-HF. Circulation 143: 298–309, 2021. 10.1161/CIRCULATIONAHA.120.050391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agarwal R, Rossignol P, Romero A, Garza D, Mayo MR, Warren S, Ma J, White WB, Williams B: Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): A phase 2, randomised, double-blind, placebo-controlled trial. Lancet 394: 1540–1550, 2019. 10.1016/S0140-6736(19)32135-X [DOI] [PubMed] [Google Scholar]
- 28.Bushinsky DA, Spiegel DM, Gross C, Benton WW, Fogli J, Hill Gallant KM, Du Mond C, Block GA, Weir MR, Pitt B: Effect of patiromer on urinary ion excretion in healthy adults. Clin J Am Soc Nephrol 11: 1769–1776, 2016. 10.2215/CJN.01170216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pitt B, Anker SD, Bushinsky DA, Kitzman DW, Zannad F, Huang IZ; PEARL-HF Investigators : Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J 32: 820–828, 2011. 10.1093/eurheartj/ehq502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bushinsky DA, Spiegel DM, Yuan J, Warren S, Fogli J, Pergola PE: Effects of the potassium-binding polymer patiromer on markers of mineral metabolism. Clin J Am Soc Nephrol 14: 103–110, 2019. 10.2215/CJN.04500418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bushinsky DA, Rossignol P, Spiegel DM, Benton WW, Yuan J, Block GA, Wilcox CS, Agarwal R: Patiromer decreases serum potassium and phosphate levels in patients on hemodialysis. Am J Nephrol 44: 404–410, 2016. 10.1159/000451067 [DOI] [PubMed] [Google Scholar]
- 32.Rossignol P, David L, Chan C, Conrad A, Weir MR: Safety and tolerability of the potassium binder patiromer from a global pharmacovigilance database collected over 4 years compared with data from the clinical trial program. Drugs Real World Outcomes 8: 315–323, 2021. 10.1007/s40801-021-00254-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of trial design for AMETHYST-DN, OPAL-HK, and TOURMALINE. Download Supplemental Table 1, PDF file, 178 KB (177.9KB, pdf)
Nonfatal treatment-emergent adverse events leading to treatment discontinuation, with onset during the first 4 weeks, by system organ class and preferred term. Download Supplemental Table 2, PDF file, 178 KB (177.9KB, pdf)
Serious treatment-emergent adverse events with onset during the first 4 weeks, by system organ class and preferred term. Download Supplemental Table 3, PDF file, 178 KB (177.9KB, pdf)
Mean (SD) serum calcium, phosphate, and magnesium at baseline, week 4 and change from baseline to week 4 (safety population; n=632). Download Supplemental Table 4, PDF file, 178 KB (177.9KB, pdf)
Mean (SD) eGFR and spot urine ACR at baseline, week 4, and change from baseline to week 4 (safety population; n=632). Download Supplemental Table 5, PDF file, 178 KB (177.9KB, pdf)