Abstract
Purpose
Information is limited about adherence to practice guidelines in patients with hepatitis B virus (HBV), hepatitis C virus (HCV), or HIV infection receiving anticancer treatment.
Methods
Newly diagnosed adult cancer patients were enrolled in a multicenter, prospective cohort study (SWOG S1204) during 2013–2017 to evaluate the prevalence of HBV, HCV, or HIV in patients initiating anticancer treatment. At 6 months, records of virus-positive patients were reviewed for antiviral therapy use; anticancer treatment dose reduction; and HBV reactivation (elevated viral load). Categorical variables were compared using chi-square or Fisher’s exact test.
Results
Of 3055 enrolled patients with viral testing, 230 had chronic or past HBV, HCV, or HIV with 6-month follow-up data (chronic HBV, 15 patients; past HBV, 158; HCV, 49; HIV, 30). Twenty percent (3/15) of chronic HBV and 11% (17/158) of past HBV patients were co-infected with HCV and/or HIV. Rates of antiviral therapy use by 6 months were as follows: chronic HBV, 85% (11/13); past HBV receiving anti-B cell therapy, 60% (3/5); past HBV receiving systemic anticancer therapy without anti-B cell therapy, 8% (8/105); HCV, 6% (2/35); and HIV, 90% (19/21). Among patients with available data, anticancer treatment dose was reduced in 1 of 145 patients with past HBV and 1 of 42 with HCV. HBV reactivation occurred in 1 of 15 patients with chronic HBV; this patient was not receiving antiviral therapy.
Conclusion
Many patients with cancer and viral infections either do not receive guideline-recommended antiviral treatment or receive antiviral treatment that is not recommended in guidelines. Further education is needed to improve adherence to guidelines.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00520-022-07525-1.
Keywords: Hepatitis B virus, Hepatitis C virus, HIV, Latent infection, Infection reactivation, Antineoplastic agents
Introduction
National organizations in the USA have published clinical practice guidelines for the care of patients with cancer and viral infections. Regarding hepatitis B virus (HBV) infection, according to the American Society of Clinical Oncology (ASCO), National Comprehensive Cancer Network (NCCN), and American Association for the Study of Liver Diseases (AASLD), patients with a hematologic malignancy and chronic HBV infection (ASCO, NCCN, and AASLD) and patients with a hematologic malignancy and past HBV infection who are anticipating stem cell transplant or anti-CD20 monoclonal antibody therapy (ASCO and AASLD) should start on anti-HBV therapy at the beginning of anticancer treatment to prevent reactivation and adverse liver outcomes [1–3]. The NCCN [2] recommends that patients with cancer and chronic hepatitis C virus (HCV) infection be treated with direct-acting antiviral therapy to prevent hepatitis flare and avoid delays in anticancer treatment, although not concomitantly with anticancer therapy [2], and that patients with cancer and HIV infection be started on antiretroviral therapy to improve immune function and clinical outcomes [4]. The extent to which these clinical practice guidelines are followed is unknown. To address this knowledge gap, we examined the patterns of antiviral therapy use in patients with cancer and HBV, HCV, or HIV infection enrolled in a large, multicenter, prospective cohort study of academic and community oncology practices across the USA and compared these patterns with the guideline recommendations [5]. We also examined the incidence of adverse liver outcomes, chemotherapy interruptions, and antiviral therapy initiation by 6 months after the enrollment date.
Methods
SWOG 1204 was a prospective observational study of patients at least 18 years of age who presented for initial treatment of a new malignancy at one of 18 participating academic or community oncology institutions during 2013–2017. The primary study findings were previously published [5]. Institutional review board approval for the primary study was provided by the Protocol Review Committee of the Cancer Therapy Evaluation Program on August 5, 2013. In brief, in the study, patients were categorized as positive or negative for chronic HBV, past HBV, HCV, or HIV infection based on standard diagnostic criteria for each virus, in order to establish the prevalence of these infections in cancer patients. Patients were categorized as positive for chronic HBV infection if they tested positive for hepatitis B surface antigen; positive for past HBV infection if they tested negative for hepatitis B surface antigen and positive for hepatitis B core antibody; positive for HCV infection if they had detectable HCV RNA; and positive for HIV if they had a documented history of HIV, positive HIV screening or confirmatory test, or detectable HIV RNA. The study estimated cancer population prevalence rates for past HBV, chronic HBV, HCV, and HIV infection in cancer patients at 5.3%, 0.4%, 1.9%, and 1.0%, respectively, and showed that most patients with past HBV and many with HCV infection were unaware of their positive viral status at cancer diagnosis. For all patients, HBV, HCV, and HIV status was determined by viral testing done within 12 months prior to registration; most patients had viral status determined just prior to registration, meaning that the viral status reflected their current viral status. Patients infected with more than 1 virus are included in the analysis for each infection, and no adjustments were made for co-infection.
Baseline data collected included cancer descriptors, planned treatment modalities, and laboratory values. For patients with HBV, HCV, or HIV infection, the participating study sites reviewed the medical records approximately 6 months after registration for the following post-registration events: most recent viral load; antiviral therapy received; type(s) of anticancer treatment received; changes in anticancer treatment due to viral infection (reduction in anticancer treatment dose or omission or suspension of anticancer treatment); and adverse liver outcomes due to viral infection (HBV reactivation [increase in HBV DNA], hepatitis flare [doubling of alanine transaminase or aspartate aminotransferase], increase in international normalized ratio [INR], or jaundice). Event dates were not collected. A patient could have more than 1 anticancer treatment modality, anticancer drug, change in cancer treatment, or adverse liver outcome reported.
This study extends the initial findings by examining antiviral therapy use, changes in anticancer treatment due to viral infection, and adverse liver outcomes due to viral infection. We compared observed antiviral management to guideline recommendations [1–3, 6]. The rates of antiviral therapy use, changes in anticancer treatment due to viral infection, and adverse liver outcomes by type of viral infection were estimated among patients with known (non-missing) data. Descriptive statistics were used. The p-values for comparisons between categorical variables were obtained by using the chi-square test or Fisher’s exact test, a t-test for continuous variables, and a Cochran-Armitage trend test for ordered variables. A 2-sided alpha = 0.05 test was considered statistically significant. No adjustments were made for multiple comparisons.
Results
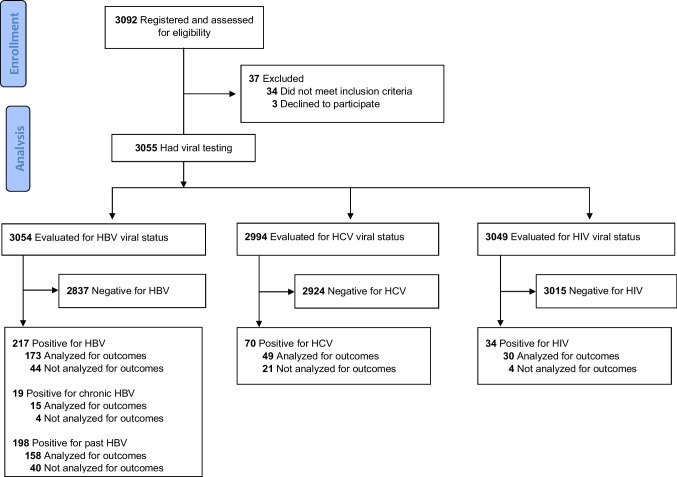
A total of 3055 newly diagnosed cancer patients who presented for treatment at one of the participating centers during the study period were registered to the study and had viral testing. Of these, 289 patients had viral infection; among these, 230 (80%) had 6-month follow-up data available. There were no clinically significant differences in baseline patient characteristics between infected patients with and without 6-month data (summarized in Online Appendix Table). Among the patients with 6-month follow-up data available, 15 patients had chronic HBV, 158 had past HBV, 49 had HCV, and 30 had HIV infection (Fig. 1). We found that 18 patients were infected with 2 viruses, and 2 patients were infected with 3 viruses. Among those with chronic HBV infection, 20% (3/15) were co-infected (2 with HCV and 1 with HIV). Among patients with past HBV infection, 11% (17/158) were co-infected (7 with HCV, 8 with HIV, and 2 with both HCV and HIV). No patients were co-infected with only HCV and HIV. Baseline characteristics of the patients with viral infection and 6-month follow-up data are presented in Table 1.
Fig. 1.
Modified CONSORT flow diagram. HBV, hepatitis B virus; HCV, hepatitis C virus
Table 1.
Demographic and clinical characteristics overall and by antiviral therapy use by 6 months in patients with HBV, HCV, or HIV infectiona,b
| Characteristic | Chronic HBV | Past HBV | HCV | HIV | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-HBV therapy use at 6 monthsc | Anti-HBV therapy use at 6 monthsc | Anti-HCV therapy use at 6 monthsc | Anti-HIV therapy use at 6 monthsc | |||||||||
| All patients | Yes | No | All patients | Yes | No | All patients | Yes | No | All patients | Yes | No | |
| All patients | 15 (100) | 11 (85) | 2 (15) | 158 (100) | 11 (10) | 100 (90) | 49 (100) | 2 (6) | 33 (94) | 30 (100) | 19 (90) | 2 (10) |
| Age, median (range), years | 57.2 (31.6–68.0) | 57.3 (31.6–68.0) | 46.3 (45.1–47.5) | 61.0 (27.9–83.7) | 60.7 (27.9–73.0) | 61.0 (29.9–83.7) | 57.0 (46.8–77.5) | 63.8 (54.4–73.2) | 57.0 (47.6–77.5) | 52.8 (21.8–75.7) | 52.9 (21.8–69.4) | 40.0 (34.8–45.1) |
| Sex | ||||||||||||
| Female | 4 (27) | 3 (27) | 0 (0) | 91 (58) | 4 (36) | 59 (59) | 19 (39) | 1 (50) | 16 (48) | 6 (20) | 3 (16) | 1 (50) |
| Male | 11 (73) | 8 (73) | 2 (100) | 67 (42) | 7 (64) | 41 (41) | 30 (61) | 1 (50) | 17 (52) | 24 (80) | 16 (84) | 1 (50) |
| Race | ||||||||||||
| White | 3 (20) | 1 (9) | 1 (50) | 59 (37) | 7 (64) | 32 (32) | 28 (57) | 2 (100) | 19 (58) | 16 (53) | 11 (58) | 1 (50) |
| Black | 5 (33) | 3 (27) | 1 (50) | 58 (37) | 0 (0) | 36 (36) | 17 (35) | 0 (0) | 11 (33) | 13 (43) | 7 (37) | 1 (50) |
| Asian | 6 (40) | 6 (55) | 0 (0) | 25 (16) | 4 (36) | 19 (19) | 1 (2) | 0 (0) | 1 (3) | 1 (3) | 1 (5) | 0 (0) |
| Other/multi-racial | 1 (7) | 1 (9) | 0 (0) | 16 (10) | 0 (0) | 13 (13) | 3 (6) | 0 (0) | 2 (6) | 0 (0) | 0 (0) | 0 (0) |
| Ethnicity | ||||||||||||
| Hispanic | 4 (27) | 3 (27) | 1 (50) | 28 (18) | 1 (9) | 19 (19) | 6 (13) | 0 (0) | 5 (15) | 6 (20) | 4 (21) | 1 (50) |
| Not Hispanic | 11 (73) | 8 (73) | 1 (50) | 129 (82) | 10 (91) | 80 (81) | 42 (88) | 1 (100) | 28 (85) | 24 (80) | 15 (79) | 1 (50) |
| Unknown/missing | 1 | 1 | 1 | 1 | ||||||||
| Education | ||||||||||||
| Less than high school diploma | 1 (7) | 1 (10) | 0 (0) | 28 (19) | 0 (0) | 19 (20) | 15 (31) | 0 (0) | 10 (30) | 7 (23) | 3 (16) | 1 (50) |
| High school diploma | 6 (43) | 3 (30) | 1 (50) | 40 (27) | 3 (27) | 23 (24) | 14 (29) | 1 (50) | 7 (21) | 8 (27) | 6 (32) | 0 (0) |
| College | 7 (50) | 6 (60) | 1 (50) | 68 (45) | 7 (64) | 44 (47) | 16 (33) | 0 (0) | 15 (45) | 11 (37) | 7 (37) | 1 (50) |
| Graduate school | 0 (0) | 0 (0) | 0 (0) | 14 (9) | 1 (9) | 8 (9) | 3 (6) | 1 (50) | 1 (3) | 4 (13) | 3 (16) | 0 (0) |
| Missing | 1 | 1 | 8 | 6 | 1 | |||||||
| Employment | ||||||||||||
| Employed | 6 (43) | 5 (50) | 0 (0) | 48 (31) | 3 (27) | 29 (30) | 12 (25) | 0 (0) | 8 (24) | 8 (28) | 7 (37) | 0 (0) |
| Retired | 5 (36) | 4 (40) | 0 (0) | 55 (36) | 2 (18) | 36 (37) | 11 (23) | 1 (50) | 8 (24) | 7 (24) | 4 (21) | 0 (0) |
| Unemployed | 1 (7) | 1 (10) | 0 (0) | 24 (16) | 4 (36) | 13 (13) | 12 (25) | 1 (50) | 10 (30) | 9 (31) | 6 (32) | 1 (50) |
| Disabled or other | 2 (14) | 0 (0) | 2 (100) | 27 (18) | 2 (18) | 19 (20) | 13 (27) | 0 (0) | 7 (21) | 5 (17) | 2 (11) | 1 (50) |
| Missing | 1 | 1 | 4 | 3 | 1 | 1 | ||||||
| Cancer type | ||||||||||||
| Hematologic (any) | 1 (7) | 1 (9) | 0 (0) | 13 (8) | 4 (36) | 7 (7) | 8 (16) | 0 (0) | 6 (18) | 7 (23) | 5 (26) | 1 (50) |
| Leukemia | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 1 (5) | 0 (0) |
| Lymphoma | 1 (7) | 1 (9) | 0 (0) | 2 (1) | 0 (0) | 2 (2) | 2 (4) | 0 (0) | 2 (6) | 0 (0) | 0 (0) | 0 (0) |
| Myeloma | 0 (0) | 0 (0) | 0 (0) | 10 (6) | 4 (36) | 4 (4) | 6 (12) | 0 (0) | 4 (12) | 6 (20) | 4 (21) | 1 (50) |
| Solid (any) | 14 (93) | 10 (91) | 2 (100) | 145 (92) | 7 (64) | 93 (93) | 41 (84) | 2 (100) | 27 (82) | 23 (77) | 14 (74) | 1 (50) |
| Breast | 3 (20) | 2 (18) | 0 (0) | 61 (39) | 1 (9) | 39 (39) | 9 (18) | 1 (50) | 7 (21) | 2 (7) | 2 (11) | 0 (0) |
| GI, colorectal | 1 (7) | 1 (9) | 0 (0) | 14 (9) | 1 (9) | 9 (9) | 6 (12) | 0 (0) | 4 (12) | 5 (17) | 4 (21) | 0 (0) |
| GI, liver | 3 (20) | 2 (18) | 1 (50) | 3 (2) | 0 (0) | 3 (3) | 4 (8) | 0 (0) | 4 (12) | 0 (0) | 0 (0) | 0 (0) |
| GI, other | 3 (20) | 2 (18) | 1 (50) | 17 (11) | 1 (9) | 12 (12) | 2 (4) | 0 (0) | 1 (3) | 5 (17) | 2 (11) | 1 (50) |
| Head and neck | 1 (7) | 1 (9) | 0 (0) | 8 (5) | 0 (0) | 6 (6) | 5 (10) | 0 (0) | 4 (12) | 1 (3) | 1 (5) | 0 (0) |
| Lung | 2 (13) | 1 (9) | 0 (0) | 19 (12) | 2 (18) | 12 (12) | 8 (16) | 0 (0) | 2 (6) | 1 (3) | 1 (5) | 0 (0) |
| Prostate | 0 (0) | 0 (0) | 0 (0) | 9 (6) | 0 (0) | 3 (3) | 3 (6) | 1 (50) | 1 (3) | 1 (3) | 0 (0) | 0 (0) |
| Other | 1 (7) | 1 (9) | 0 (0) | 14 (9) | 2 (18) | 9 (9) | 4 (8) | 0 (0) | 4 (12) | 8 (27) | 4 (21) | 0 (0) |
| Performance status (Zubrod) | ||||||||||||
| 0 | 5 (42) | 3 (38) | 1 (50) | 66 (52) | 4 (44) | 42 (52) | 23 (51) | 1 (50) | 15 (52) | 12 (50%) | 8 (57) | 1 (50) |
| 1 | 6 (50) | 4 (50) | 1 (50) | 54 (42) | 4 (44) | 33 (41) | 16 (36) | 1 (50) | 10 (34) | 10 (42) | 5 (36) | 1 (50) |
| 2 | 1 (8) | 1 (13) | 0 (0) | 6 (5) | 1 (11) | 5 (6) | 4 (9) | 0 (0) | 3 (10) | 0 (0) | 0 (0) | 0 (0) |
| 3 | 0 (0) | 0 (0) | 0 (0) | 2 (2) | 0 (0) | 1 (1) | 2 (4) | 0 (0) | 1 (3) | 2 (8) | 1 (7) | 0 (0) |
| Missing | 3 | 3 | 30 | 2 | 19 | 4 | 4 | 6 | 5 | |||
| Prior viral diagnosisd | ||||||||||||
| Yes | 9 (60) | 8 (73) | 1 (50) | 22 (14) | 4 (36) | 16 (16) | 34 (69) | 2 (100) | 24 (73) | 29 (97) | 19 (100) | 1 (50) |
| No | 4 (27) | 2 (18) | 1 (50) | 110 (70) | 4 (36) | 69 (69) | 12 (24) | 0 (0) | 8 (24) | 1 (3) | 0 (0) | 1 (50) |
| Don't know | 2 (13) | 1 (9) | 0 (0) | 26 (16) | 3 (27) | 15 (15) | 3 (6) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 0 (0) |
| Viral loadd | ||||||||||||
| Detectable | 4 (27) | 4 (36) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 49 (100) | 2 (100) | 33 (100) | 11 (37) | 7 (37) | 1 (50) |
| Undetectable | 2 (13) | 2 (18) | 0 (0) | 16 (10) | 2 (18) | 10 (10) | 0 (0) | 0 (0) | 0 (0) | 14 (47) | 10 (53) | 1 (50) |
| Not done/reported | 9 (60) | 5 (45) | 2 (100) | 142 (90) | 9 (82) | 90 (90) | 0 (0) | 0 (0) | 0 (0) | 5 (17) | 2 (11) | 0 (0) |
| Cancer treatmente | ||||||||||||
| Cytotoxic chemotherapy | 11 (79) | 9 (82) | 1 (50) | 113 (73) | 9 (82) | 72 (72) | 36 (77) | 1 (50) | 26 (79) | 20 (74) | 15 (79) | 1 (50) |
| Immunotherapy | 0 (0) | 0 (0) | 0 (0) | 4 (3) | 0 (0) | 4 (4) | 2 (4) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 0 (0) |
| Targeted biologic drug | 1 (7) | 1 (9) | 0 (0) | 10 (6) | 4 (36) | 4 (4) | 1 (2) | 0 (0) | 1 (3) | 1 (4) | 1 (5) | 0 (0) |
| Bone marrow transplant | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 0 (0) |
| Anti-B cell therapy, including rituximab | 0 (0) | 0 (0) | 0 (0) | 6 (4) | 3 (27) | 2 (2) | 5 (11) | 0 (0) | 5 (15) | 2 (7) | 2 (11) | 0 (0) |
| Radiation therapy | 5 (36) | 3 (27) | 1 (50) | 67 (43) | 1 (9) | 47 (47) | 14 (30) | 1 (50) | 10 (30) | 9 (33) | 5 (26) | 1 (50) |
| Surgery | 2 (14) | 2 (18) | 0 (0) | 48 (31) | 2 (18) | 37 (37) | 5 (11) | 0 (0) | 4 (12) | 4 (15) | 3 (16) | 0 (0) |
| Treatment unknown | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 1 (1) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Missing treatment data | 1 | 3 | 2 | 3 | ||||||||
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; GI, gastrointestinal
aValues in table are number of patients (percentage) unless otherwise indicated
bValues are measured at baseline except as noted
cPatients missing antiviral therapy status at 6 months are excluded; numbers do not add up across rows
dSpecific to the virus indicated in the column heading, e.g., HBV for “chronic HBV.”
eCancer treatment received by 6 months; may have received more than 1 type of treatment
Chronic HBV infection
Of 3054 newly diagnosed cancer patients evaluated for HBV infection, 19 (1%) had chronic HBV infection (Fig. 1). Of these 19 patients, 15 (79%) had 6-month follow-up data available (Online-Only Appendix Table).
Among patients with known chronic HBV infection whose medication status was available, 15% (2/13) had not received anti-HBV medication by 6 months (Table 1). Patients who received anti-HBV medication received entecavir, tenofovir disoproxil fumarate, or lamivudine. One patient with chronic HBV infection had an adverse liver outcome, HBV reactivation; this patient had not received anti-HBV therapy by 6 months (Table 2). One patient with chronic HBV infection had a hematologic malignancy (Table 1); this patient received antiviral therapy. There were no cases of change in anticancer treatment due to viral infection in patients with chronic HBV infection (Table 3).
Table 2.
Antiviral therapy use among patients with known adverse liver outcomes by 6 months in patients with HBV, HCV, or HIV infectiona
| Chronic HBV | Past HBV | HCV | HIV | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-HBV therapy use at 6 monthsb | Anti-HBV therapy use at 6 monthsb | Anti-HCV therapy use at 6 monthsb | Anti-HIV therapy use at 6 monthsb | |||||||||
| All patients | Yes | No | All patients | Yes | No | All patients | Yes | No | All patients | Yes | No | |
| Any adverse liver outcome | 1 (100) | 0/1 (0) | 1/1 (100) | 29 (100) | 5/24 (21) | 19/24 (79) | 10 (100) | 1/8 (13) | 7/8 (87) | 5 (100) | 4/4 (100) | 0/4 (0) |
| Specific adverse liver outcomesc | ||||||||||||
| HBV reactivation | 1 (100) | — | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | — |
| Hepatitis flare | 0 (0) | — | 0 (0) | 23 (79) | 5 (100) | 14 (74) | 7 (70) | 1 (100) | 5 (71) | 5 (100) | 4 (100) | — |
| Increase in INR | 0 (0) | — | 0 (0) | 12 (41) | 1 (20) | 9 (47) | 5 (50) | 0 (0) | 4 (57) | 1 (20) | 1 (25) | — |
| Developed jaundice | 0 (0) | — | 0 (0) | 1 (3) | 0 (0) | 1 (5) | 3 (30) | 1 (100) | 1 (14) | 0 (0) | 0 (0) | — |
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; INR, international normalized ratio
aValues in table are number of patients (percentage)
bPatients missing antiviral therapy status at 6 months are excluded; the denominators show the numbers of patients for whom antiviral status was available
cPatients may have experienced more than 1 adverse liver outcome
Table 3.
Viral-related changes in treatment by 6 months in patients with HBV, HCV, or HIV infectiona
| Chronic HBV (n = 15) | Past HBV (n = 158) | HCV (n = 49) | HIV (n = 30) | |
|---|---|---|---|---|
| Change in treatment due to viral infection | ||||
| Yes | 2 (14) | 12 (8) | 3 (7) | 5 (21) |
| No | 12 (86) | 134 (92) | 42 (93) | 19 (79) |
| Unknown/missing | 1 | 12 | 4 | 6 |
| Type of changeb | ||||
| Reduction of chemotherapy dose | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Omission of anticancer treatment due to viral infection | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Addition of antiviral/antiretroviral therapy | 2 (14) | 8 (5) | 1 (2) | 4 (17) |
| Other change | 0 (0) | 6 (4) | 2 (4) | 1 (4) |
| Change in treatment due to end organ effects of viral infection | ||||
| Yes | 1 (7) | 4 (3) | 2 (5) | 1 (4) |
| No | 13 (93) | 141 (97) | 40 (95) | 25 (96) |
| Unknown/missing | 1 | 13 | 7 | 4 |
| Type of change | ||||
| Reduction of chemotherapy dose | 0 (0) | 1 (1) | 1 (2) | 0 (0) |
| Omission of anticancer treatment due to viral infection | 0 (0) | 0 (0) | 1 (2) | 0 (0) |
| Addition of antiviral/antiretroviral therapy | 1 (7) | 2 (1) | 0 (0) | 1 (4) |
| Other change | 0 (0) | 1 (1) | 0 (0) | 0 (0) |
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus
aValues in table are number of patients (percentage)
bPatients may have had more than 1 change in treatment
Past HBV infection
Of 3054 newly diagnosed cancer patients evaluated for HBV infection, 198 (6%) had past HBV infection (Fig. 1). Of these 198 patients, 158 (80%) had 6-month follow-up data available (Online-Only Appendix Table).
Among patients with known past HBV infection whose medication status was available, 10% (11/111) received antiviral therapy by 6 months (Table 1). Patients who received anti-HBV medication received entecavir, tenofovir disoproxil fumarate, or lamivudine. Compared to patients who had not received anti-HBV medication by 6 months, patients who had received anti-HBV medication by 6 months had higher rates of white race, non-Hispanic ethnicity, male sex, unemployed status, high education level, and hematologic cancers (Table 1). Among patients with past HBV infection receiving anti-B cell therapy, 40% (2/5) had not received antiviral therapy by 6 months. Taking into account that combinations of anticancer drugs were possible, 50% (4/8) of patients with past HBV infection receiving targeted biologic therapy, 11% (9/81) receiving cytotoxic therapy, and 8% (8/105) receiving systemic anticancer therapy without an anti-B cell drug received antiviral therapy by 6 months.
Twenty-nine patients with past HBV infection had at least 1 adverse liver outcome: 23 (79%) had a hepatitis flare, 12 (41%) had an increase in INR, and 1 (3%) had jaundice (Table 2). Fourteen patients with past HBV infection and hepatitis flare had not received anti-HBV therapy by 6 months (Table 2). One patient (1%) with past HBV infection required a lowering of chemotherapy dose (Table 3).
HCV infection
Of 2994 newly diagnosed cancer patients evaluated for HCV infection, 70 (2%) were infected with HCV (Fig. 1). Of these 70 patients, 49 (70%) had 6-month follow-up data available (Online-Only Appendix Table).
Among patients with known HCV infection whose medication status was available, 94% (33/35) had not received anti-HCV medication by 6 months (Table 1). Patients who received anti-HCV medications received ledipasvir/sofosbuvir combination. Ten patients with HCV infection had at least 1 adverse liver outcome: 7 (70%) had a hepatitis flare, 5 (50%) had an increase in INR, and 3 (30%) had jaundice (Table 2). Five patients with HCV infection and hepatitis flare had not received anti-HCV therapy by 6 months (Table 2). Three patients (3/33, 9%) had changes in treatment possibly due to viral infection (Table 3). Two patients (2/42, 5%) had a change in anticancer treatment due to end organ effects associated with viral infection: 1 had the chemotherapy dose lowered, and 1 had therapy omitted that would have been recommended had the patient’s viral status been negative (Table 3).
HIV infection
Of 3049 newly diagnosed cancer patients evaluated for HIV infection, 34 (1%) were infected with HIV (Fig. 1). Of these 34 patients, 30 (88%) had 6-month follow-up data available (Online-Only Appendix Table).
Among patients with known HIV infection whose medication status was available, 10% (2/21) had not received anti-HIV medications by 6 months (Table 1). Patient who were treated for HIV received nucleoside reverse transcriptase inhibitors (abacavir, emtricitabine, lamivudine, tenofovir disoproxil fumarate, or zidovudine); non-nucleoside reverse transcriptase inhibitors (efavirenz, etravirine, or nevirapine); protease inhibitors (ritonavir or darunavir); integrase strand transfer inhibitors (dolutegravir or raltegravir); or a fixed combination therapy (efavirenz, emtricitabine, and tenofovir disoproxil fumarate; lamivudine and zidovudine; abacavir and lamivudine; emtricitabine and tenofovir disoproxil fumarate; darunavir and cobicistat; or abacavir, dolutegravir, and lamivudine). Five patients with HIV infection had an adverse liver outcome: 5 (100%) had a hepatitis flare, and 1 (20%) had an increase in INR (Table 2). All 4 patients with HIV infection and hepatitis flare for whom data were available had received anti-HIV therapy by 6 months (Table 2). No patient with HIV infection had a lowered dose of chemotherapy or omission of an anticancer drug (Table 3).
Discussion
In this study, we examined the patterns of use of antiviral therapy, the rates of changes in anticancer treatment due to viral infection, and adverse liver outcomes at 6 months in patients with cancer and HBV, HCV, or HIV infection. We compared the patterns of antiviral therapy use with guidelines in force during the study period, which are outlined in Table 4 (along with current guidelines).
Table 4.
National guidelines in force during the study period for patients with cancer and viral infections
| Virus | Guidelines during study period | Contemporary guidelines | ||||
|---|---|---|---|---|---|---|
| ASCO | NCCN | AASLD | ASCO | NCCN | AASLD | |
| HBV |
Chronic HBV [7]: Start anti-HBV therapy before and continue during anticancer therapy, continue after anticancer therapy is completed. Past HBV [7]: For patients receiving anti-CD20 monoclonal antibody therapy, start anti-HBV therapy (or monitor) before and continue during anticancer therapy, continue after anticancer therapy is completed. For all other anticancer therapies, monitor and start anti-HBV therapy only if HBV reactivation occurs. |
Start anti-HBV therapy before and continue during anticancer therapy, continue after anticancer therapy is completed. For patients receiving anti-CD20 monoclonal antibody therapy, start anti-HBV therapy (or monitor) before and continue during anticancer therapy, continue after anticancer therapy is completed. |
Chronic HBV [8]: Start anti-HBV therapy before and continue during anticancer therapy, continue after anticancer therapy is completed. |
Chronic HBV [1]: Same as guidelines during the study period. Optimal timing for anti-HBV therapy is not clear for patients with a solid tumor due to lack of strong data. Past HBV [1]: Same as guidelines during the study period. |
Chronic HBV [2]: Same as guidelines during the study period. Past HBV [2]: Same as guidelines during the study period. |
Chronic HBV [3]: Same as guidelines during the study period. Past HBV [3]: For patients receiving anti-CD20 monoclonal antibody therapy, start anti-HBV therapy (or monitor) before and continue during anticancer therapy, continue after anticancer therapy is completed. For all other anticancer therapies, monitor and start anti-HBV therapy only if HBV reactivation occurs. |
| HCV | Not available. | Start and complete DAA therapy before cancer therapy or after cancer therapy, not during cancer therapy. [2] | Not available. | Not applicable. | Same as guidelines during the study period. [2] | Not applicable. |
| HIV | Not available. | Select ART cautiously due to potential drug-drug interactions, start and continue during anticancer therapy. [2] | Not applicable. | Not applicable. | Same as guidelines during the study period. [4] | Not applicable. |
AASLD, American Association for the Study of Liver Diseases; ART, antiretroviral therapy; ASCO, American Society of Clinical Oncology; DAA, direct-acting antiviral; HBV, hepatitis B virus; HCV, hepatitis C virus; NCCN, National Comprehensive Cancer Network
Though guidelines in force during the study period as well as contemporary guidelines from ASCO [1, 7], AASLD [3, 8], and NCCN [2, 9] recommend that patients with chronic HBV infection receiving anticancer treatment receive concomitant antiviral therapy, we found that 15% of cancer patients with chronic HBV infection had not received antivirals by 6 months. Similarly, while guidelines in force during the study period as well as contemporary guidelines from NCCN [2, 4] recommend that patients with HIV infection receiving anticancer treatment receive concomitant antiviral therapy, we found that 10% of cancer patients with HIV infection had not received antivirals by 6 months. Further, while ASCO guidelines in force during the study period as well as current ASCO guidelines [1, 7] recommend that patients with past HBV infection treated with anti-CD20 drugs receive concomitant antiviral prophylaxis, 40% (2/5) of our patients with past HBV infection treated with anti-CD20 therapy had not received antiviral therapy by 6 months. On the other hand, 8% of patients with past HBV infection who received anticancer treatment that did not include an anti-CD20 drug—a group for which monitoring without antiviral prophylaxis is recommended by ASCO guidelines in force during the study and current ASCO guidelines [1, 7] and current AASLD guidelines [3]—actually had received antiviral therapy by 6 months. Although guidelines in force during the study period recommended antiviral therapy for cancer patients with HCV infection [2], only 6% of our patients with HCV infection had received antiviral therapy by 6 months.
For patients with chronic HBV infection and a hematologic malignancy, in whom the risk of HBV reactivation is nearly 50% [10], guidelines in force during the study period as well as contemporary guidelines from ASCO [1, 7], NCCN [2, 9], and AASLD [3, 8] recommend antiviral prophylaxis prior to anticancer treatment. In this study, the sole patient with a hematologic malignancy and chronic HBV infection did receive antiviral prophylaxis and had no adverse liver outcomes or changes in anticancer treatment. It is estimated that the risk of reactivation for patients with solid tumors and chronic HBV infection is 25% [11]. However, because the risk of reactivation during treatment with individual anticancer drugs or combinations of anticancer drugs is unclear for patients with solid tumors, the optimal timing for antiviral therapy for patients with solid tumors and chronic HBV infection has not been determined [1]. Treatment options may include antiviral prophylaxis at the initiation of anticancer treatment or close monitoring with initiation of antiviral therapy at the earliest sign of HBV reactivation. With the increasing use of checkpoint blockade, challenges include the known risks of immune-related hepatitis and further immune suppression and the risk of HBV reactivation if patients receive high-dose steroids for immune-related adverse events. HBV DNA contains a transcriptional regulatory element that has been shown to be activated by glucocorticoids [12]. Reactivation has been reported among HBV patients who received PD-1 blockade therapy [13].
Patients with past HBV infection receiving anti-B cell therapy, such as anti-CD20 monoclonal antibody, are at high risk of HBV reactivation and should receive antiviral prophylaxis, as recommended by ASCO during the study period [7] and afterwards [1] and AASLD after the study period [3]. Patients with past HBV infection have covalently closed circular DNA capable of replication remaining in the hepatocytes. Replication is normally inhibited by a healthy host’s strong immune system but may spiral out of control and lead to HBV reactivation with potent immunosuppression. In our study, we found that 40% (2/5) of the patients with past HBV infection receiving anti-B cell therapy had not received antiviral prophylaxis by 6 months; none of these patients had developed HBV reactivation by 6 months.
On the other hand, the risk of HBV reactivation is low for patients with a solid tumor and past HBV infection receiving anticancer treatment that does not include anti-CD20 monoclonal antibody therapy or stem cell transplant [11], and thus, antiviral prophylaxis has never been recommended for such patients [1, 3, 7]. We found that a small proportion (8%) of patients with past HBV infection receiving anticancer treatment that did not include anti-B cell therapy received antiviral therapy. Starting antiviral therapy without clear indications may subject patients to the burden of long-term therapy and financial costs without adding clinical benefit. Per current ASCO guidelines [1], AASLD guidelines [3], and expert opinion [14], these patients could have close monitoring during cancer treatment, with antiviral therapy started at the earliest sign of HBV reactivation.
NCCN consensus in force at the time of the study [2] along with later guidance from ASCO [6] and oncology experts [15] recommends that patients with cancer and HCV infection receive direct-acting antiviral therapy to eradicate HCV and decrease the risk of HCV-associated complications of anticancer treatment, including enhanced HCV replication and hepatitis flare, as well as the risk of secondary malignancies [6, 15]. Direct-acting antiviral therapy also prevents chronic hepatitis and progression to liver fibrosis or cirrhosis. Concomitant administration of anti-HCV therapy with anticancer therapy is generally not recommended by NCCN consensus [2], although oncology experts offer alternative concomitant treatment strategies if patients can be closely monitored by a multidisciplinary team [15]. In our study, we found that only 6% of patients with HCV infection had received antivirals by 6 months.
NCCN consensus during our study period recommended that patients with cancer and HIV receive antiretroviral therapy before and during anticancer treatment [2], and a more recent NCCN consensus supports this in order to decrease the risk of infections and complications [4]. In our study, 10% of patients with HIV had not received antiretroviral therapy by 6 months. We found high rates (up to 20%) of co-infection with other viruses, supporting a comprehensive approach to screening for HBV, HCV, and HIV prior to the initiation of anticancer treatment.
Our study had several limitations. We examined outcomes in an observational cohort of patients from community-based oncology practices with a primary focus on seroprevalence of HBV, HCV, and HIV. Because this study examined only whether events had occurred by 6 months, we do not know if antiviral therapy was started to prevent or in response to hepatitis flare or other adverse liver outcomes, and we are unable to determine the cause of liver outcomes. Antiviral therapy and clinical management were under the discretion of the providers; this approach mirrors actual practice, increasing the generalizability of our findings, but also resulted in a variety of viral management strategies. Also, since chronic HBV infection and HIV infection are relatively uncommon, our study included small numbers of patients with these viral infections, limiting our ability to draw strong conclusions about patterns of care for these patients. However, this is one of the largest US studies of patients with cancer and HBV, HCV, or HIV infection. We are hopeful that evolution of oncology practice toward adoption of universal screening for viral infections, along with future development of longitudinal cohort studies, will permit larger studies of patients with cancer and viral infections. Another limitation is that study participants with co-infection were not treated separately in our analysis. Patients may have received more than 1 anticancer drug, and we did not separately analyze the myriad combinations. Anticancer treatments have evolved in recent years, and thus clinical outcomes could be different if the current study were repeated with the newer agents. Patients with conditions other than hepatitis that may confer a risk of adverse liver outcomes were not excluded from or accounted for in our analyses. Chemotherapy dose reductions potentially influenced by conditions other than viral infections were not explored. Our results may not be generalizable to the resource-limited populations where viral screening may not be available. Future collaborative efforts are needed to prioritize viral screening for all cancer patients.
Although the clinical study completed enrollment in 2017, the findings from this analysis remain highly relevant. Patients with viral infections receiving systemic anticancer therapy remain at risk for adverse clinical outcomes if antiviral therapy and management guidance are not followed. For example, recent reports show that reactivation is still occurring in HBV patients receiving chemoradiation for cervical cancer [16]; that anti-CD19 chimeric antigen receptor (CAR) T-cell therapy can cause HBV reactivation in patients with B-cell malignancies and chronic [17] or past HBV infection [18], and the administration of this therapy may require heightened awareness on the part of providers who care for patients coinfected with HBV and HIV since CAR T-cell therapy may be a novel treatment for HIV [19]; and that dexamethasone and tocilizumab for treatment of COVID-19 may increase the risk of HBV reactivation [20].
In summary, we found that antiviral therapy use often was not in agreement with national treatment guidelines. Substantial proportions of patients with cancer and viral infections either do not receive guideline-recommended antiviral treatment or receive antiviral treatment that is not recommended in guidelines. Our results suggest that providers caring for patients with viral infections receiving cancer treatment may need additional education and support. Future efforts focused on increasing awareness of guideline-recommended management strategies among oncology providers and increasing implementation of these strategies in clinical care could be warranted. These efforts could reduce the risk of adverse liver outcomes in patients with hepatitis infections, which would contribute to optimizing their clinical outcomes. Ongoing research priorities include ascertaining reactivation risk by specific anticancer drug, repeating this study in high-risk populations, developing longitudinal cohorts of cancer patients with viral infections, and prospective surveillance for hepatitis flares and their causes. Collaboration between medical providers who screen for viruses and those who manage the viral infections would be beneficial for patients with cancer. Such providers include specialists in oncology, infectious disease, hepatology, primary care, dissemination and implementation, decision support, clinical informatics, and electronic health records.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Stephanie Deming of the Research Medical Library at MD Anderson Cancer Center for editorial assistance. We are grateful to the study patients who generously gave their time and effort to this project.
Author contribution
Jessica P. Hwang: conceptualization, writing—original draft, writing—review and editing; Kathryn B. Arnold: formal analysis, conceptualization, writing—original draft, writing—review and editing; Joseph M. Unger: formal analysis, supervision, writing—review and editing; Rashmi Chugh: formal analysis, writing—review and editing; Monica A. Tincopa: formal analysis, writing—review and editing; Rohit Loomba: formal analysis, writing—review and editing; Dawn Hershman: formal analysis, writing—review and editing; Scott D. Ramsey: conceptualization, formal analysis, writing—review and editing, funding acquisition.
Funding
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award number UG1CA189974. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data availability
Data may be requested from the SWOG Cancer Research Network.
Declarations
Ethics approval
Institutional review board approval for the primary study, SWOG S1204, was provided by the Protocol Review Committee of the Cancer Therapy Evaluation Program on August 5, 2013.
Consent to participate
Informed consent was obtained from all participants in SWOG S1204.
Consent for publication
Not applicable.
Competing interests
Jessica P. Hwang reports research funding to her institution from Merck. Rashmi Chugh reports research funding to her institution from AADI, Advenchen, Novartis, Epizyme, Lilly, Medivation, Mundipahrma, Glaxo Smith Kline, Janssen, Pfizer, Plexxikonn, Springworks, Qilu Puget Sound, and AstraZeneca. Rohit Loomba reports research funding to his institution from Arrowhead Pharmaceuticals, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Gilead, Intercept, Hanmi, Intercept, Inventiva, Ionis, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Novo Nordisk, Merck, Pfizer, Sonic Incytes, and Terns Pharmaceuticals; reports consulting roles at Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen Inc., Madrigal, Metacrine, Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 bio, Terns Pharmaceuticals, and Viking Therapeutics; and is co-founder of LipoNexus. Scott D. Ramsey reports research funding to his institution from Bayer, Bristol-Myers Squibb, and Microsoft Corp and consulting roles at Bayer, Bristol-Myers Squibb, AstraZeneca, Merck, GRAIL, Pfizer, Seattle Genetics, Biovica, and Genentech. All other authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hwang JP, Feld JJ, Hammond SP, Wang SH, Alston-Johnson DE, Cryer DR, Hershman DL, Loehrer AP, Sabichi AL, Symington BE, Terrault N, Wong ML, Somerfield MR, Artz AS (2020) Hepatitis B virus screening and management for patients with cancer prior to therapy: ASCO provisional clinical opinion update. J Clin Oncol [DOI] [PMC free article] [PubMed]
- 2.Baden LR, Swaminathan S, Angarone M, Blouin G, Camins BC, Casper C, Cooper B, Dubberke ER, Engemann AM, Freifeld AG, Greene JN, Ito JI, Kaul DR, Lustberg ME, Montoya JG, Rolston K, Satyanarayana G, Segal B, Seo SK, Shoham S, Taplitz R, Topal J, Wilson JW, Hoffmann KG, Smith C. Prevention and treatment of cancer-related infections, Version 2.2016. NCCN Clin Pract Guidelines Oncol J Natl Compr Canc Netw. 2016;14:882–913. doi: 10.6004/jnccn.2016.0093. [DOI] [PubMed] [Google Scholar]
- 3.Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS, Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reid E, Suneja G, Ambinder RF, Ard K, Baiocchi R, Barta SK, Carchman E, Cohen A, Gupta N, Johung KL, Klopp A, LaCasce AS, Lin C, Makarova-Rusher OV, Mehta A, Menon MP, Morgan D, Nathwani N, Noy A, Palella F, Ratner L, Rizza S, Rudek MA, Taylor J, Tomlinson B, Wang CJ, Dwyer MA, Freedman-Cass DA. Cancer in people living with HIV, Version 1.2018. NCCN Cli Pract Guidelines Oncol J Natl Compr Canc Netw. 2018;16:986–1017. doi: 10.6004/jnccn.2018.0066. [DOI] [PubMed] [Google Scholar]
- 5.Ramsey SD, Unger JM, Baker LH, Little RF, Loomba R, Hwang JP, Chugh R, Konerman MA, Arnold K, Menter AR, Thomas E, Michels RM, Jorgensen CW, Burton GV, Bhadkamkar NA, Hershman DL. Prevalence of hepatitis B virus, hepatitis C virus, and HIV infection among patients with newly diagnosed cancer from academic and community oncology practices. JAMA Oncol. 2019;5:497–505. doi: 10.1001/jamaoncol.2018.6437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hwang JP, LoConte NK, Rice JP, Foxhall LE, Sturgis EM, Merrill JK, Torres HA, Bailey HH. Oncologic implications of chronic hepatitis C virus infection. J Oncol Pract. 2019;15:629–637. doi: 10.1200/JOP.19.00370. [DOI] [PubMed] [Google Scholar]
- 7.Hwang JP, Somerfield MR, Alston-Johnson DE, Cryer DR, Feld JJ, Kramer BS, Sabichi AL, Wong SL, Artz AS. Hepatitis B virus screening for patients with cancer before therapy: American Society of Clinical Oncology provisional clinical opinion update. J Clin Oncol. 2015;33:2212–2220. doi: 10.1200/JCO.2015.61.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lok ASF, McMahon BJ (2009) AASLD practice guideline chronic Hepatitis B: Update 2009. Hepatology
- 9.Baden LR, Bensinger W, Angarone M, Casper C, Dubberke ER, Freifeld AG, Garzon R, Greene JN, Greer JP, Ito JI, Karp JE, Kaul DR, King E, Mackler E, Marr KA, Montoya JG, Morris-Engemann A, Pappas PG, Rolston K, Segal B, Seo SK, Swaminathan S, Naganuma M, Shead DA. Prevention and treatment of cancer-related infections. J Natl Compr Canc Netw. 2012;10:1412–1445. doi: 10.6004/jnccn.2012.0146. [DOI] [PubMed] [Google Scholar]
- 10.Loomba R, Rowley A, Wesley R, Liang TJ, Hoofnagle JH, Pucino F, Csako G. Systematic review: the effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Ann Intern Med. 2008;148:519–528. doi: 10.7326/0003-4819-148-7-200804010-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paul S, Saxena A, Terrin N, Viveiros K, Balk EM, Wong JB. Hepatitis B virus reactivation and prophylaxis during solid tumor chemotherapy: a systematic review and meta-analysis. Ann Intern Med. 2016;164:30–40. doi: 10.7326/M15-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tur-Kaspa R, Burk RD, Shaul Y, Shafritz DA. Hepatitis B virus DNA contains a glucocorticoid-responsive element. Proc Natl Acad Sci U S A. 1986;83:1627–1631. doi: 10.1073/pnas.83.6.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang HC, Luo W, Wang Y. Acute liver injury in the context of immune checkpoint inhibitor-related colitis treated with infliximab J Immunother. Cancer. 2019;7:47. doi: 10.1186/s40425-019-0532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loomba R, Liang TJ (2017) Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies and future directions. Gastroenterology [DOI] [PMC free article] [PubMed]
- 15.Torres HA, Shigle TL, Hammoudi N, Link JT, Samaniego F, Kaseb A, Mallet V. The oncologic burden of hepatitis C virus infection: a clinical perspective. CA Cancer J Clin. 2017;67:411–431. doi: 10.3322/caac.21403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dimond C, Negroiu A, Huge DM, Patel J. Fatal hepatitis B reactivation in a patient receiving chemoradiation for cervical cancer. J Oncol Pharm Pract. 2021;27:1296–1301. doi: 10.1177/1078155220964256. [DOI] [PubMed] [Google Scholar]
- 17.Yang C, Xie M, Zhang K, Liu H, Liang A, Young KH, Qian W. Risk of HBV reactivation post CD19-CAR-T cell therapy in DLBCL patients with concomitant chronic HBV infection. Leukemia. 2020;34:3055–3059. doi: 10.1038/s41375-020-0913-y. [DOI] [PubMed] [Google Scholar]
- 18.Li P, Zhou L, Ye S, Zhang W, Wang J, Tang X, Liu J, Xu Y, Qian W, Liang A. Risk of HBV reactivation in patients with resolved HBV infection receiving anti-CD19 chimeric antigen receptor T cell therapy without antiviral prophylaxis. Front Immunol. 2021;12:638678. doi: 10.3389/fimmu.2021.638678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rust BJ, Kiem HP, Uldrick TS. CAR T-cell therapy for cancer and HIV through novel approaches to HIV-associated haematological malignancies. Lancet Haematol. 2020;7:e690–e696. doi: 10.1016/S2352-3026(20)30142-3. [DOI] [PubMed] [Google Scholar]
- 20.Sagnelli C, Montella L, Grimaldi P, Pisaturo M, Alessio L, De Pascalis S, Sagnelli E, Coppola N (2022) COVID-19 as another trigger for HBV reactivation: clinical case and review of literature pathogens [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data may be requested from the SWOG Cancer Research Network.