Abstract
T lymphocytes are the key protective contributors in chronic infection and tumor, but experience exhaustion by persistent antigen stimulation. As an unconventional lineage of T cells, γδ T cells can rapidly response to varied infectious and tumor challenges in a non‐MHC‐restricted manner and play key roles in immune surveillance via pleiotropic effector functions, showing promising as candidates for cellular tumor immunotherapy. Activated γδ T cells can also acquire exhaustion signature with elevated expression of immune checkpoints, such as PD‐1, decreased cytokine production, and functional impairment. However, the exhaustion features of γδ T cells are distinct from conventional αβ T cells. Here, we review the researches regarding the characteristics, heterogeneity, and mechanisms of γδ T cell exhaustion. These studies provide insights into the combined strategies to overcome the exhaustion of γδ T cells and enhance antitumor immunity.
Summary sentence: Review of the characteristics, heterogeneity, and mechanisms of γδ T cell exhaustion provides insights into the combined strategies to enhance γδ T cell‐based antitumor immunotherapy.
Keywords: exhaustion, immune checkpoints, immunotherapy, PD‐1, γδ T cells
Graphical Abstract
Review of the characteristics, heterogeneity and mechanisms of γδ T cell exhaustion provides insights into the combined strategies to enhance γδ T cell‐based anti‐tumor immunotherapy.
Abbreviations
- MHC
major histocompatibility complex
- TCR
T cell receptor
- TLRs
Toll‐like receptors
- CMV
Cytomegalovirus
- HIV
human immunodeficiency virus
- EBV
Epstein‐Barr virus
- HBV
hepatitis B virus
- HMB‐PP
(E)‐ 4‐Hydroxy‐3‐methyl‐but‐2‐enyl pyrophosphate
1. INTRODUCTION
Continuously activated T cells displayed decreased capability of cytokine production and this functional state of T cells is defined as exhaustion, which was originally described during chronic lymphocytic choriomeningitis virus infection in mice. 1 Subsequently, T cell exhaustion has been widely demonstrated during chronic infection and tumor microenvironment both in various animal and human research models. 2 Exhausted T cells are functionally distinct from effector and memory T cells. The hallmarks of T cell exhaustion are progressive loss of effector function and proliferative ability, sustained high expression of inhibitory receptors (IRs), reduced responsiveness to homeostatic cytokines, altered epigenetic and transcriptional landscape, and specific metabolic program. 3 Among these features, the up‐regulation of programmed cell death‐1 (PD‐1) has emerged as a major marker of T cell exhaustion. 4 Current insights into the mechanisms of exhaustion suggests that T cell exhaustion is driven by continuous viral or tumor antigen stimulation, the negative regulatory signals of IRs and chronic inflammation. 5 Coexpression of multiple distinct IRs was associated with T cell exhaustion severity. 6 Growing evidence demonstrates that T cell exhaustion primarily contributes to immune imbalance during chronic infection and tumor progression. 2 Therefore, reversing T cell exhaustion is paramount to antitumor immunity. Blockade of PD‐1 or its ligand PD‐L1 to inhibit the PD‐1/PD‐L1 axis in T cells has been recently shown to be effective for tumor therapy. 2 While most extensive studies focus on T cell exhaustion, an unconventional lineage of T cells expressing the γδ TCR sharing certain cellular characteristics with αβ T cells is reported to exhibit exhaustion feature. 7 This review will focus on recent investigation regarding γδ T cell exhaustion and the clinical implications for tumor therapy involving the reinvigoration of γδ T cell exhaustion.
2. γδ T LYMPHOCYTES
2.1. The distribution and repertoire of γδ T cells
γδ T cells, characterized by TCRs composed of γ and δ chains, display tropism for mucosal epithelial tissues, providing a first line of defense against foreign pathogens. 8 , 9 γδ T cells arise early in the thymus during fetal thymic ontogeny and make up a minor fraction of rodent and human thymocytes. 8 Similar to αβ TCR, γδ TCR is also formed by the rearrangement of V (variable), D (diversity), and J (joining) gene segments. Structural diversity of γδ TCR is less than that of traditional αβ TCR due to the Vγ and Vδ chain pairing requirements. 8 In mouse, structural diversity of γδ T cells in particular tissue locations depends on the biased use of certain TCR γ chain. 10 Seven distinct Vγ subsets (Vγ1–7) derived from early “waves” of fetal γδ thymocytes that vary in localization, paired Vδ chain, effector function, and contribution to homeostasis and disease. 11 , 12 , 13 For instance, the well‐studied innate‐like γδ T subsets in mouse were skin epidermal Vγ5+ and intestinal Vγ7+ cells, which coincides with their tissue localization and function. 14 Individual γδ T cell subsets in particular tissue locations are summarized in Table 1.
TABLE 1.
The distribution and repertoire of γδ T cells
| Species | Distribution | Predominant V gene segment usage | Paired V gene segment usage | Refs |
|---|---|---|---|---|
| Mouse | Liver, lung, intestine | Vγ1 | Vδ2/4/5/6 | 11 , 12 , 13 , 40 |
| Lung | Vγ2 | Vδ5 | 12 , 13 | |
| Epidermis | Vγ3 | Vδ1 | 12 , 13 | |
| Liver, lung, intestine, dermis and lymph nodes | Vγ4 | Vδ1/2/4/5 | 11 , 12 , 13 , 40 | |
| Epidermis, lung | Vγ5 | Vδ1 | 11 , 12 , 13 , 14 | |
| Tongue, dermis, lung, intestine, uterus, testis, peritoneal Cavity, adipose tissue and brain meninges | Vγ6 | Vδ1 | 11 , 12 , 13 , 40 | |
| Intestine | Vγ7 | Vδ2/4/5/6 | 11 , 12 , 13 , 14 , 40 | |
| Human | PB, skin, gut, spleen, liver | Vδ1 | Vγ2/3/4/5/8/9 | 12 , 17 |
| PB | Vδ2 | Vγ9 | 12 , 16 | |
| PB, liver | Vδ3 | Vγ2/3/8 | 12 , 15 , 18 | |
| PB | Vδ5 | Vγ4 | 12 , 19 | |
| PB | Vδ4/6/7/8 | Unknown | 12 , 20 |
No obvious homologies between mouse and human γδ TCR genes was observed. In human, γδ T cells are primarily divided into Vδ1, Vδ2, and Vδ3 subsets according to their TCR δ chain usage. 15 Vδ2 subsets are predominant in human peripheral blood (PB) with almost exclusively paired with Vγ9 chain (also termed Vγ9Vδ2 γδ T cell), while Vδ1 and Vδ3subsets constitute less than 30% of γδT cells in PB and are enriched in mucosal epithelial tissues and liver, respectively, with diverse paired Vγ chains. 16 Novel structural subsets and the Vγ and Vδ chain pairing are gradually discovered (Table 2). Recently, Vδ1 T cells were reported to pair with Vγ9 chain, displaying γδ TCR‐dependent adaptive immune surveillance. 17 Another novel PB Vδ3Vγ8 subset was demonstrated to recognize MHC class I‐related protein (MR1) independent of the presented antigen. 18 A rare Vγ4Vδ5 clone in human PB was reported to directly bind endothelial protein C receptor (EPCR). 19 In addition, PB Vδ4, Vδ6, Vδ7, and Vδ8 subsets have been detected in lymphoma patients. 20 The tissue distribution and γδ TCR repertoire of different γδ T cell subsets may determine their strikingly different activation modes and response to varied infectious and tumor challenges.
TABLE 2.
The exhaustion phenotype and dysfunction of γδ T cells
| Species | Disease model | Subset | Exhaustion phenotype | Dysfunction | Refs |
|---|---|---|---|---|---|
| Mouse | Plasmodium infection | Vγ1 | TIM‐3, LAG‐3, and PD‐1 | Decreased IFN‐γ‐producing | 32 |
| Colon cancer | Vγ6 | PD‐1 | Elevated IL‐17 expression and decreased cytotoxicity | 40 | |
| Human | Plasmodium vivax | No report | PD‐1, CTLA‐4, Tim‐3, and LAG‐3 | No report | 34 |
| HIV infection | Vδ1 | CD95 and PD1 | Decreased IFN‐γ response | 35 | |
| HIV infection | CD3εlo Vδ1 | PD‐1 but not LAG‐3 | Unable to produce IL‐17 | 36 | |
| HIV infection | Vδ1 | TIGIT | Impairment of cytokine production | 47 | |
| Tuberculosis | Vδ2 | PD‐1 | Decreased response to IL‐23 | 37 | |
| Common variable immunodeficiency (CVID) | Vδ2 | PD‐1 | Decrease of Vδ2 frequency | 39 | |
| Acute dengue infection | Vδ2 | High TIM‐3 but not PD‐1 | Impairment of IFN‐γ production | 46 | |
| Acute myeloid leukemia (AML) | Vδ2 | PD‐1 | Decreased IFN‐γ secretion and increased IL‐17 secretion | 41 | |
| Acute myeloid leukemia (AML) | Vδ2 | PD‐1 and Tim‐3 | Decreased TNF‐α and IFN‐γ expression | 50 | |
| Non‐M3 AML | Vδ2 | TIGIT | Associated with poor prognosis | 51 | |
| Breast cancer | Vδ2 | PD‐1 | Associated with tumor‐draining lymph node invasion | 42 | |
| Ovarian cancer | Vδ1 | Coexpression of PD‐1, Tim‐3, CD39 with TIGIT | Increased TEMRA (terminally differentiated effector memory) differentiation | 43 |
2.2. The activation and function of γδ T cells
γδ T cells rapidly recognize conserved peptide and nonpeptide antigens that are up‐regulated by stressed cells in a MHC‐unrestricted manner, which distinguishes them from of αβ T cells. 18 , 21 Phosphoantigens (PAgs) produced by microbes and transformed cells are known to uniquely activate human Vδ2 T cells via TCR‐dependent manner, enabling them to rapidly respond to extracellular and intracellular pathogens. 22 γδ T cells can also be activated following recognition of distress signals by TLRs and NK receptors (NKRs). 23 , 24 Activated γδ T cells exhibit varied effector functions, including lysis of infected or stressed cells, cytokine and chemokine production, B cell help and IgE production, priming of αβ T cells via antigen presentation, dendritic cell maturation, and regulation of stromal cell function via growth factor production, maintaining the functional integrity of epithelial barriers and provide immunosurveillance by modulation of innate and adaptive immune responses. 25 Functionally pleiotropic γδ T cells play nonredundant roles in various physiopathologic processes including infection, allergy, autoimmunity, and cancer. 26 , 27 , 28 γδ T cells can fight against common pathogen infections, such as Mycobacterium tuberculosis (Mtb), Listeria monocytogenes, influenza viruses, HIV, EBV, and HBV. 29
A large number of studies have proved that γδ T cells are widely involved in the immune response of a variety of malignant tumors and are one of the most effective early antitumor effector cells. 25 Tumor‐infiltrating γδ T cells have been demonstrated to be the most significant predictor of favorable prognosis in a variety of tumors. 30 In our previous study, we have found that high cytotoxic human PB Vδ1 T cells can directly kill colon cancer cells via cytolytic receptor–ligand interactions. 31 Taken together, γδ T cells are activated in a non‐MHC‐restricted manner and play key roles in immune surveillance via pleiotropic effector function, making them to be the promising candidates for cellular tumor immunotherapy.
3. ACTIVATED γδ T CELLS EXHIBIT EXHAUSTION SIGNATURE
3.1. The exhaustion phenotype of γδ T cells in infection
Similar to CD8+ T cells, persistent antigen stimulation affects the composition of γδ T cell subsets and immune exhaustion. 7 Pathogen infection is the major driver of peripheral γδ T cell activation. In a mouse model of Plasmodium infection, Vγ1+ γδ T cells highly expressed markers of T‐cell exhaustion (TIM‐3, LAG‐3, and PD‐1) and the IFN‐γ‐producing ability of Vγ1+ γδ T cells is reduced in late‐phase due to γδ T‐cell dysfunction. 32 The number of γδ T cells dramatically increased in the spleen of metformin treated mice during the later phase of Plasmodium infection with high expression of IRs and severe defects in cytokine production, suggesting a state of exhaustion. 33
In human, continuous exposure to Plasmodium vivax induces up‐regulation of the exhaustion markers including PD‐1, CTLA‐4, Tim‐3, and LAG‐3 on γδ T cells. 34 HIV infection is associated with a rapid and sustained inversion of the Vδ1:Vδ2 T‐cell ratio in PB. Activated Vδ1 subset exhibited significant expression of exhaustion markers CD95 and PD1 in HIV patients, suggesting persistent activation and altered function of Vδ1 T cells. 35 In another study, CD3εlo Vδ1 T cells were reported to frequently express terminally differentiated phenotypes and the immune checkpoint PD−1 but not LAG‐3, suggesting these cells are in a state of exhaustion and are unable to produce IL‐17 in HIV infection. 36 CD3ε can be transiently down‐regulated by Vδ1 T cell activation and is restored in the presence of exogenous IL‐2, indicating that the exhaustion phenotype of Vδ1 T cells is transient and could be reversed by in vitro conditional culture. 36 Tuberculosis destroy the effects of IL‐2 and IL‐23 signaling and induce the HMBPP‐specific Vδ2 T‐cell subpopulation at the cytokine level. 37 Recently, single‐cell RNAseq profiling of human γδ T lymphocytes showed that the exhaustion signature was up‐regulated on γδ T cells in lung lesions from acute COVID‐19‐infected patients. 38 Elevated PD‐1 expression on Vδ2 T cells and was also reported to be associated with immune activation and exhaustion in common variable immunodeficiency (CVID). 39
3.2. The exhaustion phenotype of γδ T cells in tumor
Although widely characterized in chronic infection, the exhaustion of γδ T cells has been investigated in tumor in recent years. In a colon cancer mouse model, tumor‐infiltrating CD8αα− PD‐1+ γδ T cells showed decreased expression of cytotoxic‐related genes, whereas displayed increased expression of genes associated with IL‐17 and protumor activity. 40 Analogous to the observations in mice, PD‐1 expression was elevated on γδ T cells with decreased IFN‐γ secretion and increased IL‐17 secretion in acute myeloid leukemia (AML), while TNF‐α and IL‐2 secretion level was similar to their PD‐1– γδ T counterparts. 41 Furthermore, PD‐1 expression on the surface of γδ T cells was down‐regulated in patients with complete remission after chemotherapy. These findings suggest PD‐1+ γδ T cells were highly activated or immune exhausted with unique cytokine secretion profile distinct from αβ T cells. 41 Peripheral terminally differentiated Vδ2 T cells of breast cancer patients were demonstrated to display exhaustion phenotype with elevated PD‐1 expression, which was significantly associated with tumor‐draining lymph node invasion. 42 Recently, coexpression of PD‐1, Tim‐3, CD39 with TIGIT was reported on tumor‐infiltrating Vδ1 T cells in ovarian cancer, implying an increased state of exhaustion. 43 A majority of tumor‐infiltrating Vγ9‐ γδ T cells were detected in both lymphomas and solid tumors, which is quantitatively correlated with tissue residency and exhaustion and response to immune checkpoint therapy. 44 However, tissue resident memory γδ T cells with PD‐1 high expression were reported to maintain the capacity to produce IFN‐γ upon stimulation, 45 suggesting the expression of IRs does not always mark exhausted γδ T cells and can also be tightly linked to γδ T cell activation and differentiation. Recently, we have identified a γδ T subset characterized by high expression of PD‐1 that were significantly increased in colorectal cancer tissues. These tumor‐infiltrating PD‐1+γδ T cells express tissue resident, activation, and exhaustion markers, and maintain the capacity of a certain level of GzmB and Perforin secretion (unpublished data). The above evidence has demonstrated that activated γδ T cells can also exhibit exhaustion features both in chronic infection and tumor as described in CD8+ T cells, characterized but not restricted by simultaneous and progressive high expression of immune checkpoints.
3.3. The heterogeneity of exhausted γδ T cells
Distinct from αβ T cells, high PD‐1 expression is not necessary for defining γδ T cell exhaustion. In acute dengue infection, high TIM‐3 but not PD‐1 expression contributes to the impairment of IFN‐γ production by circulating Vδ2 T cells. 46 Healthy aging and HIV infection independently drive TIGIT and multi‐IR expression on γδ T cells. CD160+ γδ T cells are a potential resting/precursor population to TIGIT+, TIGIT+CD160+, and PD‐1+TIGIT+CD160+ subsets and such IR expression is suggestive of an activated or exhausted state. 47
In addition to the role in infection, the capability to provide an early source of TNF‐α and IFN‐γ early is critical to γδ T cell‐mediated antitumor response. 48 , 49 In AML patients, the PD‐1+TIM‐3– Vδ2 subset presented higher TNF‐α and IFN‐γ expression than the PD‐1+TIM‐3+ subset, implicating the up‐regulation of PD‐1 alone was insufficient to indicate functional impairment of γδ T cells. 50 γδ T cells display an exhaustion phenotype defined by increased TIGIT expression and TIGIT+CD226− γδ T cells may predict poor prognosis in non‐M3 AML patients. 51 These evidence demonstrates that γδ T cells display a vast heterogeneity according to the IR expression and functional impairment (Figure 1). The existence of progenitor cells and whether the effector function of γδ T cells could be enhanced by stimulating progenitor cell proliferation and differentiation to terminal exhaustion state needs further study.
FIGURE 1.
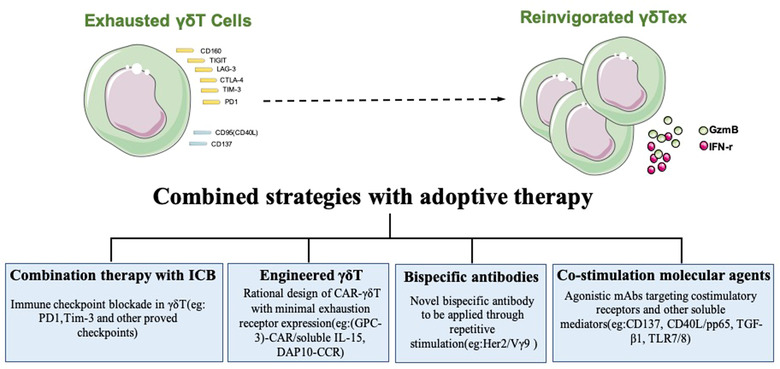
Expression of immune checkpoints on exhausted γδ T cells and strategies to improve γδ T cell‐based immunotherapy.
3.4. Molecular insights into γδ T cell exhaustion
The transcription factors including TOX, PTPN2, TCF‐1, and Eomes are involved in the regulation of T cell exhaustion. 2 , 52 , 53 , 54 EOMEShiPD1hi T cells represent a terminal progeny subsets of exhausted T cells with higher coexpression of other IRs and limited proliferative capacity. 55 Similarly, mouse Eomeshi γδ T cells coexpressed Th1 lineage‐related factors such as CD27, T‐bet, and Ly6C, displayed an exhausted phenotype with high levels of PD‐1 and CD160, and were less capable of IFN‐γ production, highlighting Eomes as a marker for the differentiation exhaustion of Th1‐like effector γδ T cells. 56
In human, it has been reported that the impairment of IL‐23‐induced expansion of Vδ2 T cells driven by tuberculosis appears to be different from T‐cell exhaustion linked to PD‐1 signaling. 37 Tuberculosis might inhibit the STAT3/JAK2 signaling pathway in Vδ2 T cells, leading to γδ T cell exhaustion in response to IL‐23/HMBPP costimulation. While the expansion of Vδ2 T cells induced by IL‐23 cannot be restored via blockade of the PD‐1 signaling. 37 The molecular mechanisms of γδ T cell exhaustion are poorly explored, and a clearer molecular understanding of γδ T cell exhaustion could help reveal new therapeutic targets for persistent infection and cancer.
3.5. The reinvigoration potential of exhausted γδ T cells
The functional activity of γδ T cells is strikingly modulated by their activation level and activation pathway. 57 The functional remodeling of exhausted γδ T cells is rarely studied, and most studies focus on PB γδ T cells. Zoledronic acid (Zol)+IL‐2 activates human PB γδ T cells in vitro to induce up‐regulation of PD‐1 expression, and anti‐PD‐1 monoclonal antibody can enhance IFN‐γ secretion of PD‐1+ γδ T cells. 58 Blockage of PD‐1 can enhance the secretion of GzmB and lysosomal‐associated membrane protein from PB PD‐1+ γδ T cells induced by histone deacetylase inhibitors, thus enhancing the antitumor effect of PD‐1+ γδ T cells. 59 Anti‐PD‐L1 monoclonal antibodies reversed the antigen‐activated killing activity of PB PD‐1+ γδ T cells against PD‐L1+ lymphoma cells, 60 demonstrating that PD‐1 delivered a coinhibitory signal and blocking the PD‐1 signal enhanced γδ T cell function. TGF‐β1 potentiates Vγ9Vδ2 T cell adoptive immunotherapy of cancer. 61 TGF‐β has been shown to repress mammalian target of mTOR signaling to promote a less exhausted T cell metabolic state, indicating that TGF‐β1 may contribute to rescue Vδ2 T cell exhaustion. 61 TLR7/8 activation decreased the potential exhaustion of Vδ2 T cells in the Zol+IL‐2 culture. 62 The only study on γδ T cells in tumor tissues has shown that blocking PD‐1 enhances the antitumor cytotoxic activity of follicular lymphoma infiltrating PD‐1+CD16+ γδ T cells and the antibody‐dependent cellular cytotoxicity. 63 Recently, PD‐1+ γδ T cells were reported to accumulate in tumor tissue of colorectal cancer with mismatch repair gene defects, and highly expressed activation‐related markers such as CD69, CD38, and HLA‐DR. These PD‐1+ γδ T cells can produce IFN‐γ, GzmB, and perforin stimulated by PMA, implicating that fully activated PD‐1+ γδ T cells displayed potentially cytotoxic activity in the antitumor immune response and are the potential target cells treated by immunocheckpoint blockade (ICB). 64 These evidence demonstrates that exhausted γδ T cells have the reinvigoration potential, providing opportunities for antitumor intervention.
4. THE STRATEGIES TO OVERCOME EXHAUSTION AND IMPROVE γδ T CELL‐BASED IMMUNOTHERAPY
In view of the direct recognition characteristics, cytolytic activity, and interaction with other immune cells, γδ T cells have irreplaceable advantages in immunotherapy. Many clinical trials have been conducted to evaluate the antitumor role of γδ T cells via in vivo activation, adoptive cell transfer, and genetic engineering for cancer treatment. 65 The MHC‐independent antitumor activity endows γδ T cells potent promising for allogeneic adoptive immunotherapy.
Recently, a phase I clinical trial in 132 late‐stage lung or liver cancer patients validated the clinical safety and survival benefit of allogeneic Vγ9Vδ2 T‐cell immunotherapy. 66 Despite of the therapeutic efficiency, the loss of the γδ T cell persistent response is likely due to activation‐induced exhaustion and cell death due to repeated treatments with PAg. In avoid of Vδ2 T cell exhaustion through repetitive PAg stimulation, novel bispecific antibody Her2/Vγ9 were designed and applied after initially activated by PAg and IL‐2 in vivo. 67 This attempt provides a tool to further increase γδ T cytotoxicity when PAg failed because of exhaustion. Therefore, it is urgent and necessary to develop combination strategies for γδ T cell‐based cancer treatment.
Different strategies have been developed to improve the antitumor effect of γδ T cell immunotherapy for clinical application (Figure 1). 68 Activated γδ T cells highly expressed PD‐1, suggesting the potential for a combination therapy harnessing adoptive γδ T cell therapy and ICB. 69 PD‐1 checkpoint blockade has been demonstrated to enhance the effectiveness of adoptive immunotherapy with human γδ T cells in treating prostate tumors in a preclinical model. 70 Expanded and activated polyclonal Vδ1 cells costimulated by CD40L and CMV antigen‐pp65 maintains the memory phenotype without inducing overexpression of exhaustion markers, representing an attractive antitumor therapeutic option. 71 In influenza virus infection, the expression of CD137 was inducible in Vγ9Vδ2 T cells following continuous antigen stimulation. CD137+ Vγ9Vδ2 T cells displayed more potent antiviral activity than their CD137‐ counterparts both in vitro and in vivo. 72 In this infectious model, the efficiency of CD137 costimulation for Vγ9Vδ2 T cell activation, proliferation, survival, and effector function, provides a novel strategy of combination targeting CD137 with γδ T cell‐based immunotherapy to improve the antitumor therapeutic efficacy. 72
The expression of a second‐generation CD19‐CAR (chimeric antigen receptor) on Vδ2 T cells was reported to be associated with significant increase in exhaustion markers. 73 While DAP10‐CCR (chimeric costimulatory receptor) was designed based on the important role of NKG2D in γδ T cell activation, which had no effect on γδ T cell exhaustion profile. 73 Expanded glypican‐3 (GPC‐3)‐CAR/soluble IL‐15 Vδ1 T cells were reported to display robust antitumor efficacy against hepatocellular carcinoma (HCC) with minimal exhaustion receptor expression. 74 These studies demonstrated that rational design of CAR‐γδ T could overcome functional exhaustion. Further clinical studies examining the combination therapy with ICB, costimulation molecular agents, bispecific antibodies, and engineered γδ T cell immunotherapy are needed to ensure successful clinical application of γδ T cell‐based antitumor immunotherapy.
5. CONCLUDING REMARKS
T cell exhaustion is the core of immune imbalance and γδ T cells are the critical participants in this process but are rarely studied compared to αβ T cells. γδ T cell exhaustion is characterized by the high expression of IRs and decreased cytokine production distinct from conventional αβ T cells. High expression of PD‐1 is not always associated with functional impairment and is even not necessary for defining γδ T cell exhaustion. γδ T cell exhaustion is more complex and unique, and there might be more accurate molecules in defining exhausted γδ T cells. In summary, γδ T cells show promising therapeutic potential in antitumor treatment, but the study of γδ T cell exhaustion is still in its infancy. Therefore, fully deciphering the characteristics, heterogeneity, and mechanisms of γδ T cell exhaustion will pave the way for combined strategies to overcome exhaustion and enhance antitumor immunity.
AUTHORSHIP
Di Chen, Yinglu Guo, and Jiahuan Jiang contributed in literature collection and manuscript writing. Pin Wu and Ting Zhang contributed in review discussion and language editing. Dang Wu, Jian Huang, and Qichun Wei participated in the design and review of the manuscript. Di Chen, Yinglu Guo, and Jiahuan Jiang contributed equally to this work.
DISCLOSURE
None of the authors have any conflict of interests.
ACKNOWLEDGMENTS
This work was supported by the grant from the National Natural Science Foundation of China (82073142, D. W.; 82073141, P. W.; 81930079, J. H.; 82073332, Q. W.; and 82173089, T. Z.), Natural Science Foundation of Zhejiang Province (LY19H160050, D.W.;LR22H160006, P. W. and LY21H100004, T. Z.), and Immunoradiotherapy Research Fund of Chinese Society of Radiation Oncology (Z‐2017‐24‐2108, D. W.).
Chen D, Guo Y, Jiang J, et al. γδ T cell exhaustion: Opportunities for intervention. J Leukoc Biol. 2022;112:1669–1676. 10.1002/JLB.5MR0722-777R
REFERENCES
- 1. Gallimore A, Glithero A, Godkin A, et al. Induction and exhaustion of lymphocytic choriomeningitis virus‐specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I‐peptide complexes. J Exp Med. 1998;187:1383‐1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36:265‐276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492‐499. [DOI] [PubMed] [Google Scholar]
- 4. Thommen DS, Schumacher TNT. Cell dysfunction in cancer. Cancer Cell. 2018;33:547‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McLane LM, Abdel‐Hakeem MS, Wherry EJ. CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol. 2019. [DOI] [PubMed] [Google Scholar]
- 6. Blackburn SD, Shin H, Haining WN, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kallemeijn MJ, Boots AMH, van der Klift MY, et al. Ageing and latent CMV infection impact on maturation, differentiation and exhaustion profiles of T‐cell receptor gammadelta T‐cells. Sci Rep. 2017;7:5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chien YH, Meyer C, Bonneville M. gammadelta T cells: first line of defense and beyond. Annu Rev Immunol. 2014;32:121‐155. [DOI] [PubMed] [Google Scholar]
- 9. Nielsen MM, Witherden DA, Havran WL. γδ T cells in homeostasis and host defence of epithelial barrier tissues. Nat Rev Immunol. 2017;17:733‐745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bonneville M, O'Brien RL, Born WK. γδ T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010;10:467‐478. [DOI] [PubMed] [Google Scholar]
- 11. Pellicci DG, Koay HF, Berzins SP. Thymic development of unconventional T cells: how NKT cells, MAIT cells and gammadelta T cells emerge. Nat Rev Immunol. 2020;20:756‐770. [DOI] [PubMed] [Google Scholar]
- 12. Godfrey DI, Uldrich AP, McCluskey J, et al. The burgeoning family of unconventional T cells. Nat Immunol. 2015;16:1114‐1123. [DOI] [PubMed] [Google Scholar]
- 13. Boucontet L, Grana M, Alzari PM, et al. Mechanisms determining cell membrane expression of different gammadelta TCR chain pairings. Eur J Immunol. 2009;39:1937‐1946. [DOI] [PubMed] [Google Scholar]
- 14. Ribot JC, Lopes N. Silva‐Santos B. gammadelta T cells in tissue physiology and surveillance. Nat Rev Immunol. 2021;21:221‐232. [DOI] [PubMed] [Google Scholar]
- 15. Bottino C, Tambussi G, Ferrini S, et al. Two subsets of human T lymphocytes expressing gamma/delta antigen receptor are identifiable by monoclonal antibodies directed to two distinct molecular forms of the receptor. J Exp Med. 1988;168:491‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kenna T, Golden‐Mason L, Norris S, et al. Distinct subpopulations of gamma delta T cells are present in normal and tumor‐bearing human liver. Clin Immunol. 2004;113:56‐63. [DOI] [PubMed] [Google Scholar]
- 17. Davey MS, Willcox CR, Joyce SP, et al. Clonal selection in the human Vdelta1 T cell repertoire indicates gammadelta TCR‐dependent adaptive immune surveillance. Nat Commun. 2017;8:14760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rice MT, von Borstel A, Chevour P, et al. Recognition of the antigen‐presenting molecule MR1 by a Vdelta3(+) gammadelta T cell receptor. Proc Natl Acad Sci USA. 2021;118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Willcox CR, Pitard V, Netzer S, et al. Cytomegalovirus and tumor stress surveillance by binding of a human gammadelta T cell antigen receptor to endothelial protein C receptor. Nat Immunol. 2012;13:872‐879. [DOI] [PubMed] [Google Scholar]
- 20. Wang L, Xu M, Wang C, et al. The feature of distribution and clonality of TCR gamma/delta subfamilies T cells in patients with B‐cell non‐Hodgkin lymphoma. J Immunol Res. 2014;2014:241246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu D, Wu P, Qiu F, et al. Human gammadeltaT‐cell subsets and their involvement in tumor immunity. Cell Mol Immunol. 2017;14:245‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Willcox BE, Willcox CR. γδ TCR ligands: the quest to solve a 500‐million‐year‐old mystery. Nat Immunol. 2019;20:121‐128. [DOI] [PubMed] [Google Scholar]
- 23. Pietschmann K, Beetz S, Welte S, et al. Toll‐like receptor expression and function in subsets of human gammadelta T lymphocytes. Scand J Immunol. 2009;70:245‐255. [DOI] [PubMed] [Google Scholar]
- 24. Rincon‐Orozco B, Kunzmann V, Wrobel P, et al. Activation of V gamma 9 V delta 2 T cells by NKG2D. J Immunol. 2005;175:2144‐2151. [DOI] [PubMed] [Google Scholar]
- 25. Vantourout P, Hayday A. Six‐of‐the‐best: unique contributions of gammadelta T cells to immunology. Nat Rev Immunol. 2013;13:88‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Paul S, Shilpi LalG. Role of gamma‐delta (gammadelta) T cells in autoimmunity. J Leukoc Biol. 2015;97:259‐271. [DOI] [PubMed] [Google Scholar]
- 27. Zheng R, Yang Q. The role of the gamma delta T cell in allergic diseases. J Immunol Res. 2014;2014:963484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Riganti C, Massaia M, Davey MS, et al. Human gammadelta T‐cell responses in infection and immunotherapy: common mechanisms, common mediators? Eur J Immunol. 2012;42:1668‐1676. [DOI] [PubMed] [Google Scholar]
- 29. Zhao Y, Lin L, Xiao Z, et al. Protective role of gammadelta T cells in different pathogen infections and its potential clinical application. J Immunol Res. 2018;2018:5081634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gentles AJ, Newman AM, Liu CL, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21:938‐945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu D, Wu P, Wu X, et al. Ex vivo expanded human circulating Vdelta1 gammadeltaT cells exhibit favorable therapeutic potential for colon cancer. Oncoimmunology. 2015;4:e992749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Inoue SI, Niikura M, Asahi H, et al. Preferentially expanding Vgamma1(+) gammadelta T cells are associated with protective immunity against Plasmodium infection in mice. Eur J Immunol. 2017;47:685‐691. [DOI] [PubMed] [Google Scholar]
- 33. Miyakoda M, Bayarsaikhan G, Kimura D, et al. Metformin promotes the protection of mice infected with Plasmodium yoelii independently of gammadelta T cell expansion. Front Immunol. 2018;9:2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gogoi D, Biswas D, Borkakoty B, et al. Exposure to Plasmodium vivax is associated with the increased expression of exhaustion markers on gammadelta T lymphocytes. Parasite Immunol. 2018;40:e12594. [DOI] [PubMed] [Google Scholar]
- 35. Negash M, Tsegaye A, Wassie L, et al. Phenotypic and functional heterogeneity of peripheral gammadelta T cells in pulmonary TB and HIV patients in Addis Ababa. Ethiopia BMC Infect Dis. 2018;18:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dunne PJ, Maher CO, Freeley M, et al. CD3epsilon expression defines functionally distinct subsets of Vdelta1 T cells in patients with human immunodeficiency virus infection. Front Immunol. 2018;9:940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shen H, Gu J, Xiao H, et al. Selective destruction of interleukin 23‐induced expansion of a major antigen‐specific gammadelta T‐cell subset in patients with tuberculosis. J Infect Dis. 2017;215:420‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cerapio JP, Perrier M, Pont F, et al. Single‐cell RNAseq profiling of human gammadelta T lymphocytes in virus‐related cancers and COVID‐19 disease. Viruses. 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Paquin‐Proulx D, Barsotti NS, Santos BAN, et al. Inversion of the Vdelta1 to Vdelta2 gammadelta T cell ratio in CVID is not restored by IVIg and is associated with immune activation and exhaustion. Medicine (Baltimore). 2016;95:e4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reis BS, Darcy PW, Khan IZ, et al. TCR‐Vgammadelta usage distinguishes protumor from antitumor intestinal gammadelta T cell subsets. Science. 2022;377:276‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tang L, Wu J, Li CG, et al. Characterization of immune dysfunction and identification of prognostic immune‐related risk factors in acute myeloid leukemia. Clin Cancer Res. 2020;26:1763‐1772. [DOI] [PubMed] [Google Scholar]
- 42. Fattori S, Gorvel L, Granjeaud S, et al. Quantification of immune variables from liquid biopsy in breast cancer patients links Vdelta2(+) gammadelta T cell alterations with lymph node invasion. Cancers (Basel). 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weimer P, Wellbrock J, Sturmheit T, et al. Tissue‐Specific Expression of TIGIT, PD‐1, TIM‐3, and CD39 by gammadelta T Cells in ovarian cancer. Cells. 2022;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cerapio JP, Perrier M, Balanca CC, et al. Phased differentiation of gammadelta T and T CD8 tumor‐infiltrating lymphocytes revealed by single‐cell transcriptomics of human cancers. Oncoimmunology. 2021;10:1939518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zakeri N, Hall A, Swadling L, et al. Characterisation and induction of tissue‐resident gamma delta T‐cells to target hepatocellular carcinoma. Nature Communications. 2022;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cimini E, Grassi G, Beccacece A, et al. In acute dengue infection, high TIM‐3 expression may contribute to the impairment of IFNgamma production by circulating Vdelta2 T cells. Viruses. 2022;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Belkina AC, Starchenko A, Drake KA, et al. Multivariate computational analysis of gamma delta T cell inhibitory receptor signatures reveals the divergence of healthy and ART‐suppressed HIV+ aging. Front Immunol. 2018;9:2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gao Y, Yang W, Pan M, et al. Gamma delta T cells provide an early source of interferon gamma in tumor immunity. J Exp Med. 2003;198:433‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ramstead AG, Jutila MA. Complex role of gammadelta T‐cell‐derived cytokines and growth factors in cancer. J Interferon Cytokine Res. 2012;32:563‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wu K, Feng J, Xiu Y, et al. Vdelta2 T cell subsets, defined by PD‐1 and TIM‐3 expression, present varied cytokine responses in acute myeloid leukemia patients. Int Immunopharmacol. 2020;80:106122. [DOI] [PubMed] [Google Scholar]
- 51. Jin Z, Lan T, Zhao Y, et al. Higher TIGIT(+)CD226(‐) gammadelta T cells in patients with acute myeloid leukemia. Immunol Invest. 2022;51:40‐50. [DOI] [PubMed] [Google Scholar]
- 52. LaFleur MW, Nguyen TH, Coxe MA, et al. PTPN2 regulates the generation of exhausted CD8(+) T cell subpopulations and restrains tumor immunity. Nat Immunol. 2019;20:1335‐1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Khan O, Giles JR, McDonald S, et al. TOX transcriptionally and epigenetically programs CD8(+) T cell exhaustion. Nature. 2019;571:211‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chen Z, Ji Z, Ngiow SF, et al. TCF‐1‐centered transcriptional network drives an effector versus exhausted CD8 T cell‐fate decision. Immunity. 2019;51:840‐855e845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lino CNR, Barros‐Martins J, Oberdorfer L, et al. Eomes expression reports the progressive differentiation of IFN‐gamma‐producing Th1‐like gammadelta T cells. Eur J Immunol. 2017;47:970‐981. [DOI] [PubMed] [Google Scholar]
- 57. Sun D, Chan N, Shao H, et al. gammadelta T cells activated in different inflammatory environments are functionally distinct. J Immunol. 2022;208:1224‐1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hoeres T, Holzmann E, Smetak M, et al. PD‐1 signaling modulates interferon‐gamma production by gamma delta (gammadelta) T‐Cells in response to leukemia. Oncoimmunology. 2019;8:1550618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bhat SA, Vedpathak DM, Chiplunkar SV. Checkpoint blockade rescues the repressive effect of histone deacetylases inhibitors on gammadelta T cell function. Front Immunol. 2018;9:1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Iwasaki M, Tanaka Y, Kobayashi H, et al. Expression and function of PD‐1 in human gammadelta T cells that recognize phosphoantigens. Eur J Immunol. 2011;41:345‐355. [DOI] [PubMed] [Google Scholar]
- 61. Gabriel SS, Tsui C, Chisanga D, et al. Transforming growth factor‐beta‐regulated mTOR activity preserves cellular metabolism to maintain long‐term T cell responses in chronic infection. Immunity. 2021;54:1698‐1714e1695. [DOI] [PubMed] [Google Scholar]
- 62. Wang H, Chen H, Liu S, et al. Costimulation of gammadeltaTCR and TLR7/8 promotes Vdelta2 T‐cell antitumor activity by modulating mTOR pathway and APC function. J Immunother Cancer. 2021;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rossi C, Gravelle P, Decaup E, et al. Boosting gammadelta T cell‐mediated antibody‐dependent cellular cytotoxicity by PD‐1 blockade in follicular lymphoma. Oncoimmunology. 2019;8:1554175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. de Vries NL, van Unen V, Ijsselsteijn ME, et al. High‐dimensional cytometric analysis of colorectal cancer reveals novel mediators of antitumour immunity. Gut. 2020;69:691‐703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hannani D, Ma Y, Yamazaki T, et al. Harnessing gammadelta T cells in anticancer immunotherapy. Trends Immunol. 2012;33:199‐206. [DOI] [PubMed] [Google Scholar]
- 66. Xu Y, Xiang Z, Alnaggar M, et al. Allogeneic Vgamma9Vdelta2 T‐cell immunotherapy exhibits promising clinical safety and prolongs the survival of patients with late‐stage lung or liver cancer. Cell Mol Immunol. 2021;18:427‐439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Oberg HH, Peipp M, Kellner C, et al. Novel bispecific antibodies increase gammadelta T‐cell cytotoxicity against pancreatic cancer cells. Cancer Res. 2014;74:1349‐1360. [DOI] [PubMed] [Google Scholar]
- 68. Miyashita M, Shimizu T, Ashihara E, et al. Strategies to improve the antitumor effect of gammadelta T cell immunotherapy for clinical application. Int J Mol Sci. 2021;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tanaka Y. Cancer immunotherapy harnessing gammadelta T cells and programmed death‐1. Immunol Rev. 2020. [DOI] [PubMed] [Google Scholar]
- 70. Nada MH, Wang H, Hussein AJ, et al. PD‐1 checkpoint blockade enhances adoptive immunotherapy by human Vgamma2Vdelta2 T cells against human prostate cancer. Oncoimmunology. 2021;10:1989789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Polito VA, Cristantielli R, Weber G, et al. Universal ready‐to‐use immunotherapeutic approach for the treatment of cancer: expanded and activated polyclonal gammadelta. Memory T Cells Front Immunol. 2019;10:2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pei Y, Wen K, Xiang Z, et al. CD137 costimulation enhances the antiviral activity of Vgamma9Vdelta2‐T cells against influenza virus. Signal Transduct Target Ther. 2020;5:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fisher J, Sharma R, Don DW, et al. Engineering gammadeltaT cells limits tonic signaling associated with chimeric antigen receptors. Sci Signal. 2019;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Makkouk A, Yang XC, Barca T, et al. Off‐the‐shelf Vdelta1 gamma delta T cells engineered with glypican‐3 (GPC‐3)‐specific chimeric antigen receptor (CAR) and soluble IL‐15 display robust antitumor efficacy against hepatocellular carcinoma. J Immunother Cancer. 2021;9. [DOI] [PMC free article] [PubMed] [Google Scholar]


