Summary
Across multiple disciplines undertaking airway management globally, preventable episodes of unrecognised oesophageal intubation result in profound hypoxaemia, brain injury and death. These events occur in the hands of both inexperienced and experienced practitioners. Current evidence shows that unrecognised oesophageal intubation occurs sufficiently frequently to be a major concern and to merit a co‐ordinated approach to address it. Harm from unrecognised oesophageal intubation is avoidable through reducing the rate of oesophageal intubation, combined with prompt detection and immediate action when it occurs. The detection of ‘sustained exhaled carbon dioxide’ using waveform capnography is the mainstay for excluding oesophageal placement of an intended tracheal tube. Tube removal should be the default response when sustained exhaled carbon dioxide cannot be detected. If default tube removal is considered dangerous, urgent exclusion of oesophageal intubation using valid alternative techniques is indicated, in parallel with evaluation of other causes of inability to detect carbon dioxide. The tube should be removed if timely restoration of sustained exhaled carbon dioxide cannot be achieved. In addition to technical interventions, strategies are required to address cognitive biases and the deterioration of individual and team performance in stressful situations, to which all practitioners are vulnerable. These guidelines provide recommendations for preventing unrecognised oesophageal intubation that are relevant to all airway practitioners independent of geography, clinical location, discipline or patient type.
Keywords: airway management, capnography, human factors, oesophageal intubation, tracheal intubation
Key recommendations
Exhaled carbon dioxide monitoring and pulse oximetry should be available and used for all episodes of airway management.
Routine use of a videolaryngoscope is recommended whenever feasible.
At each attempt at laryngoscopy, the airway operator is encouraged to verbalise the view obtained.
The airway operator and assistant should each verbalise whether ‘sustained exhaled carbon dioxide’ and adequate oxygen saturation are present.
Inability to detect sustained exhaled carbon dioxide requires oesophageal intubation to be actively excluded.
The default response to the failure to satisfy the criteria for sustained exhaled carbon dioxide should be to remove the tube and attempt ventilation using a facemask or supraglottic airway.
If immediate tube removal is not undertaken, actively exclude oesophageal intubation: repeat laryngoscopy, flexible bronchoscopy, ultrasound and use of an oesophageal detector device are valid techniques.
Clinical examination should not be used to exclude oesophageal intubation.
- Tube removal should be undertaken if any of the following are true:
- Oesophageal placement cannot be excluded
- Sustained exhaled carbon dioxide cannot be restored
- Oxygen saturation deteriorates at any point before restoring sustained exhaled carbon dioxide
Actions should be taken to standardise and improve the distinctiveness of variables on monitor displays.
Interprofessional education programmes addressing the technical and team aspects of task performance should be undertaken to implement these guidelines.
What other guidelines are available on this topic?
Many professional groups worldwide have produced airway management guidelines directed at a particular practitioner group, category of patients or context for care, that refer to techniques to confirm tracheal intubation [1, 2, 3, 4, 5, 6, 7]. However, none of these focus specifically on preventing unrecognised oesophageal intubation.
Why was this guideline developed?
Preventable mortality and serious morbidity from unrecognised oesophageal intubation continue to occur worldwide [8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30], despite existing guideline recommendations and widely publicised education programmes [31] .
How does this differ from existing guidelines?
This is the first guideline providing comprehensive recommendations for preventing unrecognised oesophageal intubation. In addition to providing clear guidance on the technical aspects of task performance and decision‐making, recommendations also address the critical role of human factors in preventing unrecognised oesophageal intubation.
Disclaimer
This guideline is intended for use by appropriately trained airway practitioners [32]. It does not represent international standards but consensus guidance. Recommendations regarding the use of specific equipment, monitoring or additional staff apply where accessing the relevant resources is feasible. Where this is not the case, these recommendations should be regarded as aspirational.
Introduction
The term unrecognised, or undetected, oesophageal intubation is widely used but not defined. This guideline defines unrecognised oesophageal intubation as unintended placement or migration of a tracheal tube into the oesophagus, that is not promptly identified and addressed.
Oesophageal intubation is more common than many clinicians appreciate. Its incidence was reported as 1 in 18 cases in a recent international study of emergency intubations of the critically ill [33], but is lower in an elective surgical setting [34]. Oesophageal intubation occurs after both straightforward and challenging intubations and whether undertaken by experienced or inexperienced airway practitioners [35]. Even when promptly identified, oesophageal intubation is associated with increased rates of severe hypoxaemia, pulmonary aspiration, cardiac arrest and rarely with rupture of the stomach or oesophagus [35, 36, 37, 38, 39, 40].
Failure to recognise oesophageal intubation is a much less frequent complication of airway management, but rapidly results in irreversible brain injury or death from hypoxaemia. No discipline, context or level of operator experience is exempt. Cases commonly involve experienced airway practitioners both during airway management for surgical anaesthesia and that undertaken for other indications [30, 41]. There is no structured method for estimating the frequency of unrecognised oesophageal intubation. Improved collection and accessibility of data relating to episodes of unrecognised oesophageal intubation would facilitate understanding of the scope of the issue and its causes. Occurrences may only be highlighted by coronial or media reports, and it is likely not all cases are reported. The number of these reports in recent years clearly indicates that preventable events occur regularly and are an international problem [8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30]. In the 4th National Audit Project (NAP4) of the Royal College of Anaesthetists and Difficult Airway Society, nine cases of unrecognised oesophageal intubation were reported [42, 43]: three during anaesthesia for surgery, four in the intensive care unit (ICU) and two in emergency departments. Of the nine patients, six died and one suffered brain damage. Based on the denominator data in NAP4 [42, 44], unrecognised oesophageal intubation causing severe harm occurred in approximately 1 in 1,000,000 tracheal intubations for anaesthesia, 1 in 15,000 in ICU and 1 in 10,000 in the emergency department. These estimates, however, are prone to major statistical and clinical uncertainty. After two deaths due to unrecognised oesophageal intubation in 2017–2018, the slogan "no trace = wrong place" was promoted by the Royal College of Anaesthetists and the Difficult Airway Society in the UK and disseminated internationally [31], but deaths from oesophageal intubation continue to be reported [9, 10, 11].
This consensus guideline aims to provide a consistent approach to preventing the occurrence of, and harm from, unrecognised oesophageal intubation. It is intended for use by all airway practitioners independent of geography, clinical location, discipline or patient type.
The focus of this guideline is on unrecognised oesophageal intubation during attempted tracheal intubation. Subsequent displacement of a correctly placed tube into the oesophagus [10, 26, 27, 28], or misplacement of a tracheal or tracheostomy tube during a front‐of‐neck approach, may also cause harm. Many of the principles described here apply equally to those settings.
Methods
This consensus guideline was derived from recommendations developed for the Project for Universal Management of Airways (PUMA) guidelines. The method for developing these recommendations was recently published [32]. An updated literature search has since been undertaken including articles published up until the end of April 2022. No structured mechanism exists to identify instances of unrecognised oesophageal intubation. Cases were sourced in an ad‐hoc fashion from news media publications and coroner's reports known to the authors.
The Difficult Airway Society, Society for Airway Management, European Airway Management Society, All India Difficult Airway Association, Canadian Airway Focus Group, Safe Airway Society and International Airway Management Society each appointed affiliated existing members of the PUMA working group as their representatives for the purpose of developing this guideline. As members of the PUMA advisory group familiar with specific aspects of the subject matter, TC and SM were invited to contribute as co‐authors during drafting of the manuscript.
The recommendations in this guideline are categorised according to the American Heart Association recommendation classification system in online Supporting Information (Appendix S1) [45]. A summary of this system and the clinical implications of each category of recommendation as used in this guideline are outlined in online Supporting Information (Appendix S2).
Aetiology of unrecognised oesophageal intubation
Preventing harm from unrecognised oesophageal intubation involves the following: reducing the occurrence of oesophageal intubation, recognising oesophageal intubation promptly when it occurs and managing oesophageal intubation efficiently.
The extent to which potential aetiological factors contribute to occurrence and recognition has not been studied, but it is likely that multiple factors act in combination (Table 1).
Table 1.
Potential factors contributing to unrecognised oesophageal intubation. An expanded version of this table is available at https://www.UniversalAirway.org/puoi.
| Occurrence of oesophageal intubation |
Misidentification of larynx
|
Delivery issue
|
Movement after successful tracheal placement
|
| Failure to recognise oesophageal intubation * |
| CO 2 detection not available/used/functioning |
| Failure to confirm CO 2 detection |
Spurious CO
2
detection
|
Misinterpretation of monitoring display:
|
Failure to acknowledge the potential for absence of sustained exhaled CO
2
to indicate oesophageal intubation.
|
Many of these may be a consequence of, or aggravated by, stress and suboptimal teamwork.
Technical issues may interfere with the diagnosis of oesophageal intubation but other factors often contribute to failure to recognise it as a possibility or take action in a timely manner [46]. Serious harm from oesophageal intubation requires that airway teams not only overlook or misinterpret the absence of exhaled carbon dioxide, but also the presence of profound or prolonged hypoxaemia. Breakdown of perception, cognition and team interaction feature regularly in reports of unrecognised oesophageal intubation. These commonly occur in association with underlying flaws in the clinical environment or system design [9, 10, 11, 28, 46].
A variety of cognitive biases contribute to failure to recognise oesophageal intubation and may mislead even the most skilled clinician [30, 47]. Confirmation bias involves prioritising information supporting an expected or desired diagnosis (e.g. relying on auscultation of breath sounds despite a flat capnograph and hypoxaemia) [47]. Anchoring refers to fixation on one issue with reluctance to consider alternative diagnoses (e.g. erroneously concluding that lack of exhaled carbon dioxide must be due to severe bronchospasm or cardiac arrest) [47]. Omission bias describes the tendency to avoid performing potentially harmful actions in favour of condoning equally or more harmful omissions (e.g. reluctance to remove a tube because of uncertainty about whether it is in the trachea or oesophagus) [47]. Decisions during airway management are also strongly influenced by past experience which may lead practitioners to deprioritise infrequently encountered events as options when troubleshooting a problem [48]. A practitioner, who has found the absence of exhaled carbon dioxide to be most commonly (or exclusively) explained by causes other than oesophageal intubation, may be conditioned to doubt this diagnosis. Paradoxically, this may make the most experienced practitioners particularly vulnerable to being reluctant to accept that they have intubated the oesophagus, especially when coupled with perceptual errors such as ‘glottic impersonation’ that might convince them that the tube is in the trachea (Fig. 1) [49].
Figure 1.
Glottic impersonation. Blanching of the lateral aspects of the oesophageal opening (below) due to forceful laryngoscopy may lead it to be mistaken for the glottis (above). Careful attention by an experienced practitioner should enable the oesophageal and glottic openings to be distinguished. However, confusion may be more likely when intubation is challenging, with the potential combination of one or more of excessively deep blade insertion, aggressive laryngoscopy, time pressure, expectation of seeing the larynx, impaired decision‐making and a restricted view that does not reveal the larynx above (see box). Photo reproduced from original publication in Canadian Journal of Anesthesia [49] with permission. White box was added for this guideline.
As an airway crisis evolves, the effects of stress on both individual and team function may lead to a significant deterioration in decision‐making, compromised long‐term memory, loss of situation awareness, impaired communication and decreased fine motor skills, in both inexperienced and experienced practitioners [50, 51]. Hierarchies between team members (e.g. operator and assistant, consultant and trainee) may deter individuals from speaking up or hinder acknowledgement of raised concerns [11, 46]. Conversely, practitioners at an equivalent level (e.g. one consultant arriving as help for another) may be reluctant to challenge one another so as not to appear disrespectful (‘malignant politeness’). Communication problems between the initial airway team and responding help are common [30]. In some cases, airway operators have refused to allow the tube to be replaced or have its position checked by another practitioner, despite multiple clear signs of oesophageal intubation, indicating breakdown of individual objectivity and team function [30].
Knowledge deficits, exacerbated by inability to recall and apply information under stress, also play a significant role. Coroners' reports reveal that practitioners commonly make diagnoses that are typically incompatible with the absence of sustained exhaled carbon dioxide (especially cardiac arrest and bronchospasm) [8, 9, 10, 13, 14, 29, 30], or rely on clinical signs that are unable to exclude oesophageal intubation [9, 10, 15, 16, 17, 29, 30].
Design, familiarity, availability and use of monitoring may impact the ability of airway practitioners to recognise important cues. Lack of access to carbon dioxide detection devices, as well as failure to use them or check their readings, has contributed to delays in recognising oesophageal intubation [8, 11, 15, 28, 29, 30, 52]. Variable configuration of monitor displays may predispose to misinterpretation of capnography readings [8, 11].
These multiple factors may lead the entire airway team to fail to identify or remove a tube that is potentially placed in the oesophagus. Inquest reports of fatal oesophageal intubation are typified by these behaviours, lacking a structured or logical approach to crisis management. A recurrent term used in coroners' reports is ‘chaos’ [9, 11, 15], reflecting a deterioration in team function during the emergency.
Preventing oesophageal intubation
Routine use of a videolaryngoscope
Routine use of a videolaryngoscope is recommended whenever feasible [34, 53, 54]. Videolaryngoscopy improves glottic view and reduces the incidence of oesophageal intubation [34, 53, 54, 55, 56, 57, 58, 59, 60, 61]. By enabling other team members to view the intubation, it also provides the opportunity for them to confirm or query correct tube placement. It is recognised that access to videolaryngoscopes may impede immediate implementation of this recommendation in some settings. In these contexts, the recommendation should be viewed as aspirational and a plan developed to ensure implementation at the earliest opportunity. Strategies, that improve the ability of the videolaryngoscope screen to be easily viewed by the whole team, are likely to further augment teamwork and are encouraged [62].
Laryngoscopy technique
Deliberate, sequential exposure of anatomy during laryngoscope blade insertion may help avoid ‘overshooting’ the larynx in situations of stress.
Stating the view at laryngoscopy
At each attempt at laryngoscopy, the airway operator is encouraged to verbalise the view obtained by describing the anatomical structures seen or using a classification understood by all members of the airway team [63]. An example might be: "I can see the epiglottis, the back of the vocal cords and the arytenoids:". Verbalisation requires the airway operator to acknowledge the view obtained, promotes attention to glottic anatomy and encourages the whole airway team to be part of the tracheal intubation process. Communicating the view to the rest of the team creates a shared mental model of the challenges associated with the task of tracheal intubation [63, 64, 65], which is particularly important if videolaryngoscopy is not available. When videolaryngoscopy is used, verbalisation provides the opportunity for other members of the team to confirm or query the stated view. Verbalisation of actions is a well‐described technique used in other safety critical industries and such behaviours are recognised to reduce the rate of perceptual error [65, 66].
Sharing the view with the rest of the team also empowers them to prompt the airway operator to take swift, appropriate actions if sustained, exhaled carbon dioxide is subsequently absent. When a good view at laryngoscopy is verbalised, removal of the tube may be encouraged on the basis that re‐intubation of the trachea should be straightforward. When a compromised view is verbalised, removal may be encouraged on the basis that oesophageal intubation is more likely. Thus, this sharing of information may help the team overcome the reluctance to remove the tube which often features in cases of unrecognised oesophageal intubation.
Recognising oesophageal intubation
Observing tube placement
Following intubation, the ability to see the tube between the vocal cords and anterior to the arytenoids should be assessed prior to withdrawal of the laryngoscope blade.
Exhaled carbon dioxide detection
The most valuable role of detection of exhaled carbon dioxide during airway management is providing breath‐by‐breath confirmation that alveolar ventilation is occurring. Equipment for monitoring exhaled carbon dioxide and pulse oximetry should be available in all locations where airway management is undertaken and should be used for all episodes of airway management [8, 30, 33, 67]. This applies to adults and children in the prehospital setting, emergency department, ICU, post‐anaesthesia care unit and locations where anaesthesia is administered (in and out of the operating theatre) or unexpected emergency intubation may be performed (e.g. cardiac arrest on wards). Institutions should ensure timely access to these monitors for unexpected emergency airway management events [8]. In low‐resource settings where accessing these monitors is currently unfeasible, this recommendation should be viewed as aspirational and consideration given to how its implementation can be facilitated. Supporting initiatives such as the Global Pulse Oximetry Initiative and the Global Capnography Project is crucial to improving their availability in these contexts [52, 68, 69, 70]. In the interim, in settings where accessing carbon dioxide monitoring is currently unfeasible, airway practitioners should continue to adhere to the other principles of this guideline whenever the question of potential oesophageal intubation arises.
Continuous waveform capnography is recommended to detect exhaled carbon dioxide [71, 72, 73, 74, 75, 76, 77]. If this is unavailable, continuous capnometry without a waveform display may be used [73, 74, 78]. Colorimetric exhaled carbon dioxide detection may be used where continuous electronic carbon dioxide measurement is unfeasible but is not advocated as it is less accurate [79, 80, 81, 82, 83, 84, 85, 86].
Outside anaesthetic practice, the role of waveform capnography in neonates is debated and requires further research and consensus [87]. While the concerns raised are acknowledged, they have limited applicability to the peri‐intubation setting and supportive data are lacking [88, 89].
Functioning of the carbon dioxide detection device should be confirmed before induction of unconsciousness [67]. Capnography should be commenced during pre‐oxygenation when a facemask is used, to embed this opportunity to check its function into routine practice [67, 90].
‘Sustained exhaled carbon dioxide’
Sustained exhaled carbon dioxide should be used to confirm alveolar ventilation following passage of a tracheal tube [74, 91]. This applies whether the patient is intubated while awake or unconscious, including when tracheal intubation is performed with a flexible bronchoscope [92]. While oesophageal intubation is typically associated with the absence of any detected carbon dioxide (i.e. ‘no trace’), many fatal cases involve confusion over deteriorating, inadequate or grossly abnormal carbon dioxide detection [9, 10, 12, 30]. This guideline therefore provides unambiguous criteria for sustained exhaled carbon dioxide that if not met, empower teams to initiate the actions outlined below. Verifying the presence of sustained exhaled carbon dioxide requires all the following criteria to be met (Fig. 2; [93]):
Amplitude rises during exhalation and falls during inspiration.
Consistent or increasing amplitude over at least seven breaths [74, 91].
Peak amplitude more than 1 kPa (7.5 mmHg) above baseline [74, 94].
Reading is clinically appropriate.
Figure 2.
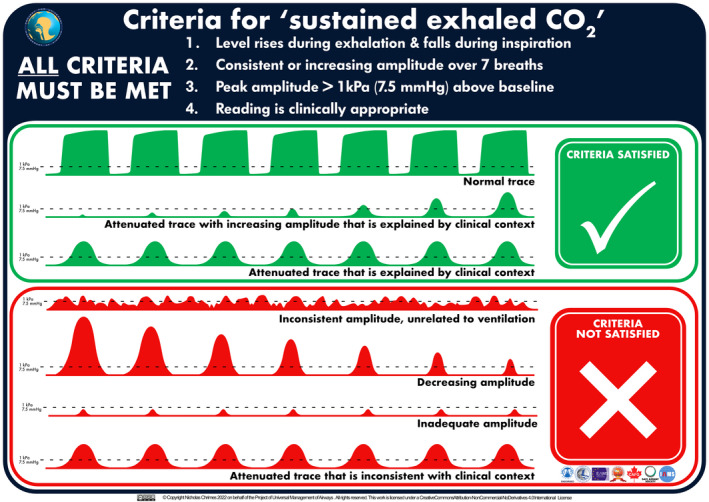
Criteria for ‘sustained exhaled carbon dioxide’. This graphic has been designed to be used as both a foundation tool to be reviewed in advance of clinical use and an implementation tool to be referred to in real time during clinical practice [93]. A high‐resolution version of this graphic is available for download at https://www.UniversalAirway.org/downloads. Printing and laminating this at A3 size is recommended.
Inability to satisfy these criteria does not preclude tracheal placement but indicates that the risk of oesophageal intubation is unacceptably high. Failure to satisfy the criteria for sustained exhaled carbon dioxide following passage of a tracheal tube therefore requires oesophageal intubation to be actively excluded [8, 71, 72, 73, 74, 75, 76, 77]. Although in some circumstances exhaled carbon dioxide may be detected despite oesophageal intubation (Table 2 [12, 91, 95, 96, 97]), even these false positives will not typically result in sustained exhaled carbon dioxide. In rare situations, the first three criteria may be satisfied despite the tube being misplaced (e.g. tracheo‐oesophageal fistula or high oesophageal intubation with an uncuffed tube in children). As such, for the criteria for sustained exhaled carbon dioxide to be met, the carbon dioxide reading must also be consistent with the clinical situation.
Table 2.
| No alveolar ventilation occurring |
|
| Some alveolar ventilation potentially occurring |
|
Cardiac arrest and bronchospasm as confounders
An exhaled carbon dioxide partial pressure below 1 kPa (7.5 mmHg) is highly unlikely after tracheal intubation in a patient who has a spontaneous cardiac output. While the carbon dioxide level may be attenuated in a patient in cardiac arrest receiving chest compressions, a level below 1 kPa (7.5 mmHg) would generally reflect either an incorrectly placed tube or a very high likelihood of a poor outcome from resuscitation [74, 94, 98]. The first response to a peak carbon dioxide amplitude below 1 kPa (7.5 mmHg) should be to check for the presence of spontaneous cardiac output and if this is absent, commence high‐quality chest compressions [74]. If the amplitude remains below this threshold following these interventions, then the criteria for sustained exhaled carbon dioxide should be considered not to have been met. In the presence of high‐quality chest compressions, cardiac arrest cannot be assumed to be the cause of inability to satisfy the criteria for sustained exhaled carbon dioxide [99], and certainly does not explain a ‘flat trace’ (https://youtu.be/t97G65bignQ). Rather, failure to satisfy the criteria for sustained exhaled carbon dioxide in association with cardiac arrest should prompt exclusion of oesophageal intubation as its cause [11, 13, 14, 15, 16, 18, 21, 23, 29, 30].
Severe bronchospasm (including during anaphylaxis) has anecdotally been described as a cause of absent exhaled carbon dioxide and often features as a presumptive alternative cause for the absence of sustained exhaled carbon dioxide in cases of unrecognised oesophageal intubation [9, 10, 30]. Anchoring bias in this context has contributed to severe patient morbidity or death [9, 10, 30]. However, in the presence of adequate airway pressures and a prolonged expiratory time, bronchospasm is extremely unlikely to result in inability to satisfy the criteria for sustained exhaled carbon dioxide. If bronchospasm is suspected, then ventilation with adequate inspiratory pressure and expiratory time should be ensured. If sustained exhaled carbon dioxide is not obtained, bronchospasm should not be assumed to be the cause and oesophageal intubation must be excluded.
Sustained exhaled carbon dioxide only confirms that alveolar ventilation is occurring at that point in time, so vigilance, including continuous carbon dioxide monitoring, is required throughout the period the patient's trachea remains intubated [8]. Migration of the tube out of the trachea can occur, particularly in children or during chest compressions [10, 26, 27, 28, 100]. Reconfirmation of sustained exhaled carbon dioxide should be undertaken if the capnograph waveform morphology changes markedly, following changes in patient position, if movement of the tracheal tube occurs, following sudden changes in airway pressure, if oxygen saturation falls or in any other situation where the presence of ongoing alveolar ventilation may be called into question [10, 26, 27, 28].
Observing oximetry
In parallel with assessment of sustained exhaled carbon dioxide, the adequacy of the oxygen saturation relative to that before intubation should be assessed using pulse oximetry [101]. Pulse oximetry cannot be used to exclude oesophageal intubation, but early recognition of desaturation may aid in its diagnosis [102]. Maintenance of normal oxygen saturation, particularly after effective pre‐oxygenation, can be misleading and should not influence decision‐making when sustained exhaled carbon dioxide is not detected [102, 103]. Rarely, other causes (Table 3) may also lead to oxygen saturation being at least partially preserved, despite oesophageal intubation [9, 28].
Table 3.
|
The combined use of carbon dioxide detection and pulse oximetry may facilitate earlier recognition and resolution of issues resulting in failure to meet the criteria for sustained exhaled carbon dioxide. Falling or inadequate oxygen saturation following tube placement should be an indication for re‐evaluating whether the tube is correctly sited [9, 11, 30]. While patients may be hypoxaemic for other reasons, a deterioration in oxygen saturation following tube placement, in association with the inability to detect sustained exhaled carbon dioxide, provides further support for the diagnosis of oesophageal intubation. Hypoxaemia is the mechanism by which unrecognised oesophageal intubation causes harm and indicates an urgent need to address the situation. An absent pulse oximetry trace also prompts the need to exclude unrecognised cardiac arrest as a cause of failure to meet the criteria for sustained exhaled carbon dioxide.
Sustained exhaled carbon dioxide following passage of a tracheal tube excludes oesophageal intubation but does not confirm correct tracheal placement, as the tube may still be positioned in a bronchus or above the vocal cords [104]. This should be considered if oxygen saturation is low despite detecting sustained exhaled carbon dioxide.
Verbalising checks for sustained exhaled carbon dioxide and adequate oxygen saturation
Following passage of the tube, the airway operator and assistant should each independently assess for the presence of sustained exhaled carbon dioxide and adequate oxygen saturation, then verbalise the outcome of these checks [63, 64, 65, 105]. Ideally, this should be phrased in a manner that both encourages the other practitioner to perform the check and empowers them to disagree if required, such as ‘I can see sustained exhaled carbon dioxide and adequate oxygen saturation. Do you agree?’ Making this statement has potential benefits. It encourages the airway operator and assistant to perform the checks and acknowledge their outcome, potentially allowing for early recognition of issues with alveolar ventilation (including but not limited to oesophageal intubation). It also confirms that the checks have been performed, providing an opportunity for members of the airway team to prompt for their performance if they are not verbalised. Finally, it communicates the outcomes of the checks, providing the opportunity for the airway team to confirm or query the conclusions reached, facilitating a shared mental model.
Default removal of tube
If the criteria for sustained exhaled carbon dioxide are not met or the oxygen saturation is inadequate, urgent interventions to resolve this are required [88, 102]. An algorithm outlining the recommended approach to failure to satisfy the criteria for sustained exhaled carbon dioxide following passage of a tracheal tube is presented in Fig. 3 [93]. An interactive digital version of the algorithm will be available in the PUMA app (https://www.UniversalAirway.org/app).
Figure 3.
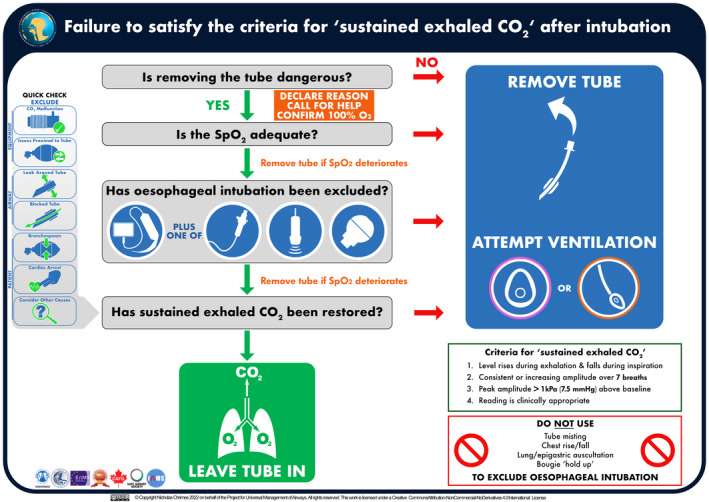
Algorithm for approaching failure to satisfy the criteria for ‘sustained exhaled carbon dioxide’ following passage of a tracheal tube. This algorithm has been designed to be used as an implementation tool [93], to be referred to in real time during clinical practice. Optimal use during clinical practice requires prior familiarity with the algorithm and guideline text. A high‐resolution version of the algorithm is available for download at https://www.UniversalAirway.org/downloads. Printing and laminating this at A3 size is recommended.
In the absence of an obvious, immediately remediable cause (e.g. zeroing/disconnection of the carbon dioxide detection device, system leaks, etc.), the default response to failure to meet the criteria for sustained exhaled carbon dioxide should be to remove the tube and attempt lung ventilation with 100% oxygen via a facemask or supraglottic airway [9, 10]. This applies even when tracheal intubation seemed uneventful and the airway operator is confident of tracheal placement [9, 11, 30]. The key question to be asked is ‘is removing the tube dangerous?’. Tube removal is the most rapid and definitive method for excluding oesophageal intubation when the criteria for sustained exhaled carbon dioxide are not met. While there are other common causes for failure to meet the criteria for sustained exhaled carbon dioxide (Table 4) [96, 97, 106, 107], unrecognised oesophageal intubation has catastrophic consequences, whereas there is usually low potential for harm if a tracheal tube is unnecessarily removed, particularly if airway management was straightforward. Furthermore, many causes of inability to meet the criteria for sustained exhaled carbon dioxide that occur despite tracheal placement still indicate absent alveolar ventilation (e.g. blocked tube, obstruction distal to the tube) making harm from removal of the tube unlikely (Fig. 2). Thus, the presumption that an inadequate trace reflects oesophageal intubation should be acted upon, unless doing so poses a higher risk than not doing so [31].
Table 4.
Potential causes of failure to detect sustained exhaled carbon dioxide (CO2) despite tracheal placement of tube [96, 97, 106, 107]. Many of these will often be associated with additional clinical cues.
| Sampling error |
| Mainstream (In‐line) sensor: not in circuit; inappropriately placed |
| Side‐stream sampling: low flow rate; loose, disconnected, leaking; not in circuit; inappropriately placed; obstructed |
| Excessive equipment dead space (esp. in association with significant positive end expiratory pressure) |
| Excessive fresh gas flow rate in some circuits |
| Leak around tube |
| Massive tracheo‐oesophageal fistula or traumatic tracheal disruption with tube proximal to tracheal defect |
| Massive broncho‐pleural fistula |
| Monitoring failure |
| Faulty monitor |
| CO2 detection module/cable disconnected from monitor |
| CO2 detection device warming up/calibrating |
| Equipment problem proximal to tracheal tube |
| Absence of driving pressure for positive pressure ventilation: circuit leak or disconnection; error in ventilation device function, assembly or settings; inadequate fresh gas flow |
| Obstructed circuit: stuck valve, incorrect circuit assembly, obstructed filter/heat and moisture exchanger or other circuit components |
| Airway obstruction |
| Tracheal tube obstruction: foreign body; secretions; blood; vomitus; pus; severe pulmonary oedema; kinking |
| Obstruction distal to tip of tracheal tube: foreign body; secretions; blood; vomitus; pus; severe pulmonary oedema; intraluminal tracheal mass; anterior mediastinal mass causing tracheal compression |
| Severely reduced pulmonary compliance with insufficient inspiratory pressures |
|
Severe bronchospasm (incl. anaphylaxis) Tension pneumothorax |
| Inadequate pulmonary blood flow |
|
Unrecognised cardiac arrest Pulmonary embolus Cardiopulmonary bypass |
For removal of the tube to be considered dangerous, there must be a reasonable basis for believing the tube is in the trachea despite the criteria for sustained exhaled carbon dioxide not being met, combined with a likelihood of serious harm if it is removed. The requirement for an additional laryngoscopy to re‐intubate if a correctly placed tube is removed does not in itself constitute a significant risk. While repeated attempts at laryngoscopy are associated with (though not necessarily causative of) increased airway complications [108, 109], when sustained exhaled carbon dioxide is not obtained, the risk–benefit ratio favours removal. Potential reasons to defer tube removal include high aspiration risk or a lack of confidence in the ability to rapidly and reliably ventilate the lungs with a facemask or supraglottic airway. Not every indication for rapid sequence intubation would necessarily be expected to justify leaving the tube in. Similarly, while challenges experienced with intubation may understandably increase reluctance to remove the tube, they may also have contributed to oesophageal placement. Therefore, provided ventilation with a facemask or supraglottic airway is expected to be straightforward, challenges with intubation are generally less concerning. Falling or inadequate oxygen saturation further supports the likelihood of oesophageal intubation, making the argument to remove the tube even more compelling, as does cardiac arrest.
Attempting ventilation before re‐intubation
Re‐intubation should only be attempted once the presence of sustained exhaled carbon dioxide has been assessed using a facemask or supraglottic airway. Ventilating the lungs before re‐intubating may provide important information that might improve situation awareness. Detecting sustained exhaled carbon dioxide with a facemask or supraglottic airway confirms a problem with the tube (oesophageal intubation, blocked tube or leak around tube). It also reinforces that if the criteria for sustained exhaled carbon dioxide are not met on replacement of the tracheal tube, it must again be removed. Success with a facemask or supraglottic airway also reduces time pressure, decreasing stress. This provides an opportunity to restore oxygen saturation if needed, re‐oxygenate the functional residual capacity, evaluate the reasons sustained exhaled carbon dioxide was not previously detected and optimise intubating conditions (including sourcing a videolaryngoscope if not immediately available) before re‐intubation is re‐attempted [93, 101, 110]. If lung ventilation via a facemask or supraglottic airway is not initially successful, airway rescue should be implemented using optimised attempts at one or more of facemask, supraglottic airway, tracheal tube or a front‐of‐neck approach as appropriate [1, 2, 6, 93].
Without access to an infallible test, preventing serious harm or death from oesophageal intubation mandates a conservative approach that accepts removal of some correctly placed tubes. Provided the above recommendations are adhered to, this is unlikely to cause harm.
Alternative techniques to exclude oesophageal intubation
When immediate tube removal is not undertaken, the justification for this (specifying both the reason for believing the tube is correctly placed and the serious harm likely to occur if it were removed) should be declared to the team, help summoned, administration of 100% oxygen confirmed and oesophageal placement actively excluded using valid alternative techniques [8] (Fig. 4; [93, 111]). Repeat laryngoscopy is recommended initially, supplemented by another valid alternative technique whenever feasible to reduce the risk of error [9, 15, 28, 30]. Other valid alternative techniques are flexible bronchoscopy, ultrasound and oesophageal detector device.
Figure 4.
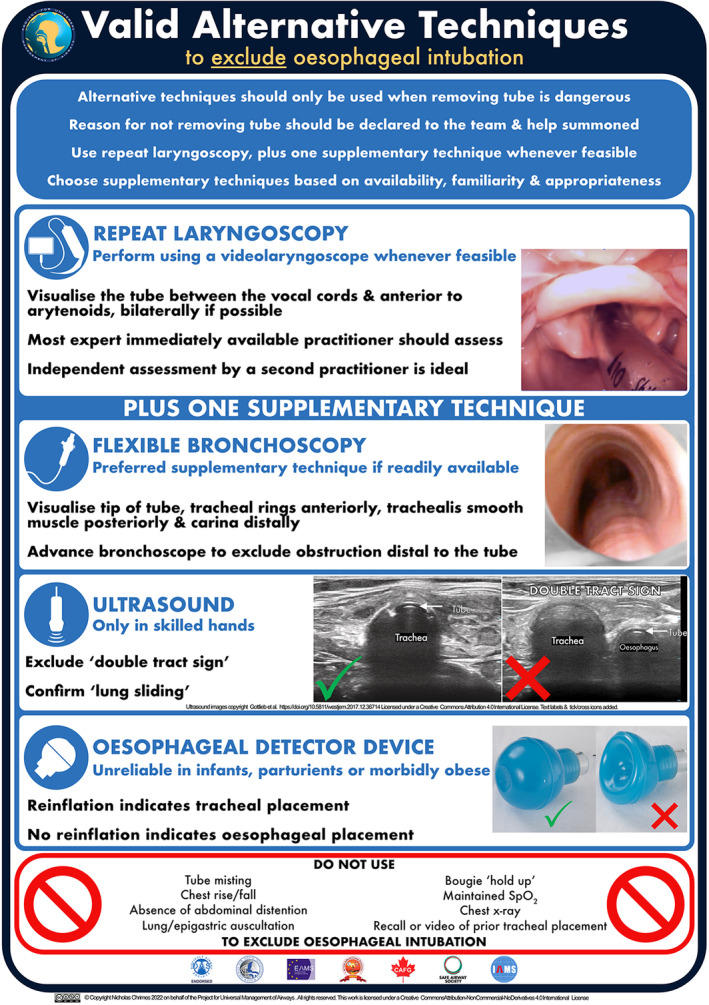
Valid alternative techniques to exclude oesophageal intubation. This graphic has been designed to be used as both a foundation tool to be reviewed in advance of a clinical use and an implementation tool to be used as a real‐time prompt during clinical practice [93]. A high‐resolution version of this graphic is available for download at https://www.UniversalAirway.org/downloads. Printing and laminating this at A3 size is recommended. The photos of the glottis and trachea are clinical cadaver images from Dalhousie University's Human Body Donation Program. Used with permission. Ultrasound images are copyright Gottlieb et al. https://doi.org/10.5811/westjem.2017.12.36714 [111] and licensed under a Creative Commons Attribution 4.0 International License. Text labels and tick/cross‐icons were added for this guideline.
The chosen supplementary technique should be familiar to the operator, appropriate to the situation and able to be rapidly implemented. Retrieving and preparing videolaryngoscopes, flexible bronchoscopes or ultrasound devices should not delay attempts to exclude unrecognised oesophageal intubation when the tube is left in. The tube should be removed immediately if any technique confirms oesophageal placement. When using alternative techniques to exclude oesophageal intubation, airway practitioners should be mindful of factors that might produce unreliable results. Inability to exclude oesophageal intubation by at least one valid alternative technique within a reasonable timeframe demands that incorrect placement be assumed and the tube removed [31].
Relying on previous laryngoscopic or bronchoscopic views obtained during attempted tracheal intubation is inappropriate. Perceptual errors may make recollections inaccurate, or the tube may have moved [10, 26, 27, 28, 49]. Apparently, straightforward initial laryngoscopy has contributed to delays in re‐evaluating tube position in fatal cases of oesophageal intubation [9, 10, 30]. If other staff attend to assist, it should be explicitly highlighted that the criteria for sustained exhaled carbon dioxide have not been met [29].
Repeat laryngoscopy
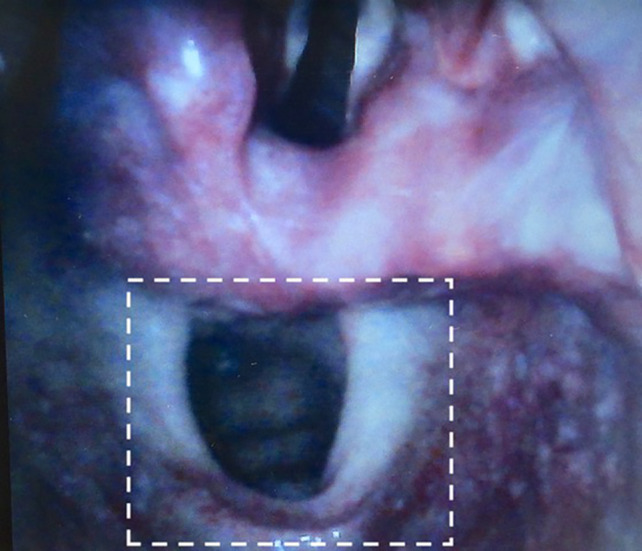
When undertaking repeat laryngoscopy to exclude oesophageal intubation, videolaryngoscopy is recommended whenever feasible [34, 53, 54]. The most expert immediately available clinician should undertake this assessment [15]. If a videolaryngoscope is not immediately available, repeat direct laryngoscopy should be performed and subsequently verified with videolaryngoscopy if possible. Meticulous attention to identifying glottic anatomy is emphasised. Exclusion of oesophageal intubation requires deliberate visualisation of the tube entering the glottis between the vocal cords and anterior to the arytenoids, bilaterally if possible (Fig. 4). Ideally, placement should be independently assessed by a second appropriately trained practitioner [15, 28, 30]. Without adherence to these criteria, tubes placed in the oesophagus (Fig. 5) may be misidentified as tracheal, especially when the operator is time pressured or expecting the tube to be correctly placed. Such misidentification has occurred in fatal cases of unrecognised oesophageal intubation [9, 15, 28, 30]. Furthermore, oesophageal intubation was not detected when repeat laryngoscopy was performed by the practitioner who performed the initial intubation, in any of the cases reviewed for this guideline [15, 28, 30]. In contrast, repeat laryngoscopy by a different operator often detected oesophageal intubation [10, 12, 15, 28, 30].
Figure 5.
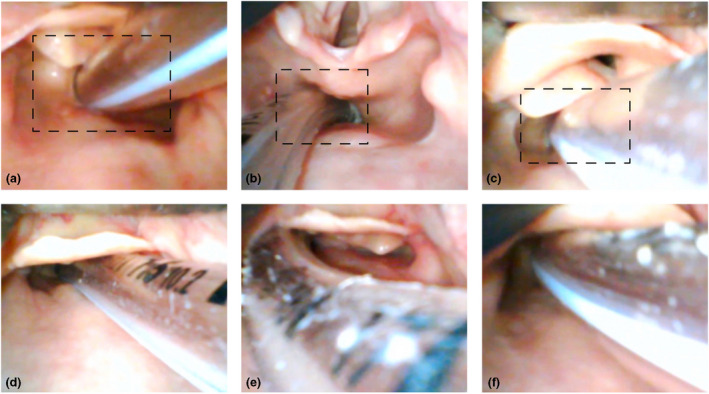
Repeat laryngoscopy in the presence of oesophageal intubation. All tubes pictured are placed in the oesophagus. None meet the visual criteria for excluding oesophageal intubation on repeat laryngoscopy. The boxes (upper row) illustrate how a more restricted view, as might occur with challenging anatomy or use of direct laryngoscopy, could contribute to misinterpretation of the site of tube placement. This is particularly true if the practitioner is time pressured or at risk of confirmation bias. The arytenoids may be mistaken for the epiglottis (a and c), blanching of the lateral aspects of the oesophageal opening (c and f) or the cuff (b) may be mistaken for the vocal cords. The epiglottis may conceal the larynx entirely (D and F). In (e), the right arytenoid is visible lateral to the tube but cannot be confirmed to be passing posterior to it. All photos are clinical cadaver images from Dalhousie University's Human Body Donation Program. Used with permission.
Meeting the criteria for excluding oesophageal intubation by repeat laryngoscopy may not be possible, especially when intubation was initially challenging. Even if initial laryngoscopy appeared straightforward, repeat laryngoscopy may be challenging due to the presence of the tube impeding placement of the laryngoscope blade and obscuring the view. When obtaining an adequate view is challenging, advanced techniques can be used to help confirm tracheal intubation via laryngoscopy. Examples include withdrawal of the tracheal tube over a bougie or airway exchange catheter to confirm that they are passing through the glottis. However, time‐consuming or unfamiliar manoeuvres should be avoided [112].
Flexible bronchoscopy
Confirmation of tracheal placement by passing a flexible bronchoscope down the lumen of the tube is likely to be both more reliable and simpler than repeat laryngoscopy in many circumstances. The tracheal rings anteriorly, trachealis smooth muscle posteriorly and tip of the tube above the carina should be visualised [8, 14, 29] (Fig. 4). Blood, secretions, aspirated material or fogging may sometimes impede identification of these structures.
Ultrasound
Using ultrasound to exclude the ‘double tract sign’ and see ‘lung sliding’ can exclude oesophageal intubation (Fig. 4). The use of ultrasound is promising but should only be relied upon when performed by an operator trained in its use for these purposes [113, 114, 115, 116, 117].
Oesophageal detector device
The oesophageal detector device generates a negative pressure which results in return of gas if the attached tube is in the (non‐collapsible) trachea, or failure of gas return if it is in the (collapsible) oesophagus (Fig. 4). Its use and limitations are described in more detail elsewhere [118, 119, 120]. Provided the connection with the tube is airtight, both bulb and syringe design devices are able to distinguish between tracheal and oesophageal placement with >97% accuracy when used in an appropriate patient group, but may yield unreliable results in infants, parturients, morbidly obese patients and some specific contexts [118, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139].
While the oesophageal detector device can be assembled from commonly available equipment [118], this may inadvertently introduce hazards and commercially made devices are preferred [140]. Unfortunately, at the time of writing, commercially manufactured oesophageal detector devices are not easily accessible in many parts of the world.
Inability of clinical examination to exclude oesophageal placement
When sustained, exhaled carbon dioxide cannot be detected, clinical examination has traditionally been used to help assess whether the tube is placed in the oesophagus (Fig. 4). However, examination findings are frequently reassuring even when the tube is in the oesophagus [72, 73, 74, 75, 121, 141, 142, 143]. Misplaced reliance on clinical examination is common in cases of fatal unrecognised oesophageal intubation [9, 10, 15, 16, 17, 30]. In every case of unrecognised oesophageal intubation reviewed for this guideline in which lung auscultation was undertaken, breath sounds were reported to be heard [9, 10, 15, 16, 28, 30, 144]. Misting of the tube on exhalation, chest rise, absence of abdominal distension, lung/epigastric auscultation, bougie ‘hold up’ and chest x‐ray should therefore not be used to exclude oesophageal intubation [72, 73, 74, 75, 121, 141, 142, 143]. While a minority of these findings can reliably confirm oesophageal intubation [121, 141, 142], this is of limited practical value as failure to confirm oesophageal intubation does not equate with excluding it, so the tube must be removed regardless of examination findings.
Evaluating other causes of failure to meet the criteria for sustained exhaled carbon dioxide
While prompt exclusion of oesophageal intubation is the priority, other potential equipment, airway or patient causes of inability to satisfy the criteria for sustained exhaled carbon dioxide may also require time‐critical action to avoid patient harm. Furthermore, rapidly correcting other causes may enable sustained exhaled carbon dioxide to be restored, avoiding unnecessary tube removal when this is considered dangerous. The ‘quick check’ outlined in Fig. 6 [93] prioritises other common causes that can be rapidly evaluated. It should be performed in parallel with steps to exclude oesophageal intubation, when the tube is not removed. It is not sufficient merely to identify a plausible alternative explanation for the absence of sustained exhaled carbon dioxide, restoration of sustained exhaled carbon dioxide is necessary to halt progression through the algorithm and avoid removing the tube. The quick check should also be performed in parallel with airway rescue, whenever the tube is removed and initial ventilation with a facemask or supraglottic airway does not restore adequate exhaled carbon dioxide. Very rarely following tube removal, addressing the quick check items may not restore adequate exhaled carbon dioxide despite clearly unobstructed ventilation with a facemask or supraglottic airway (e.g. cardiac arrest due to massive pulmonary embolus). In this situation, repeated tube removal is not required to address ongoing inability to satisfy the criteria for sustained exhaled carbon dioxide following re‐intubation, provided tracheal placement is confirmed using valid alternative techniques. The focus should instead be on identifying and treating patient causes other than oesophageal intubation (Table 4).
Figure 6.

‘Quick Check’ to identify the causes of failure to satisfy the criteria for ‘sustained exhaled carbon dioxide’ other than oesophageal intubation: This graphic is designed to be used as a foundation tool [93], to be reviewed in advance of clinical use, to enable subsequent prompting by the icons on the algorithm (Fig. 3). It is not intended to be referred to in real time during clinical practice. A high‐resolution version of this graphic is available for download at https://www.UniversalAirway.org/downloads.
Persistent inability to meet the criteria for sustained exhaled carbon dioxide
As confirmation of tracheal placement does not confirm alveolar ventilation, if sustained exhaled carbon dioxide cannot be restored, ultimately tube removal is still required. Viscid secretions, vomit or blood clots may allow passage of a suction catheter or flexible bronchoscope, while being resistant to removal, and obstructing material may not always be visible during passage of a flexible bronchoscope [145], so these actions cannot exclude tube obstruction. Similarly, if oxygen saturation becomes inadequate at any stage before restoring sustained exhaled carbon dioxide, the tube should be removed, even if tracheal position has been confirmed. If tracheal placement has been confirmed, then removal of the tube over an airway exchange catheter (or bougie if an airway exchange catheter is not immediately available) may be considered if there are concerns about the ability to replace the tube, provided this does not delay taking action.
Education and culture
The responsibility to ensure appropriate levels of training is shared by regulatory bodies, healthcare organisations, clinical departments and individual practitioners. Tracheal intubation should only be performed by individuals who have undergone appropriate training. Airway assistants should receive training specific to this role [146]. Successful implementation of the recommendations in this guideline requires interprofessional education programmes. Prior familiarity and training with the algorithm are essential to its effective use in clinical practice [147]. As well as reinforcing the recommendations in this guideline, training should include education addressing laryngoscopy and tracheal intubation technique; identification of laryngeal anatomy and interpretation of capnography waveforms [8]. During clinical practice, engaging the airway team in the tracheal intubation process through videolaryngoscopy, verbalisation of the view and two‐person verification of the presence of sustained exhaled carbon dioxide represents a valuable educational opportunity that consolidates non‐clinical training.
In addition to technical aspects of task performance and decision‐making, the need for human factors training to optimise individual and team performance cannot be overstated [8, 46, 148]. Education programmes should encourage behaviours that acknowledge individual fallibility, flatten hierarchies in the workplace and empower all team members to speak up if concerned (including the use of graded assertiveness) and to seek help if concerns are not being addressed [8]. Training teams to allocate a member to observe monitors and declare key variables may reduce the likelihood that recognition of abnormalities is delayed or overlooked [64, 65]. Interdisciplinary simulation training, including practice drills based on the accompanying algorithm could usefully form part of education programmes [8, 46, 148]. Review of real‐life cases of unrecognised oesophageal intubation is also likely to be of value. A catalogue of such cases is available at https://www.UniversalAirway.org/puoi/cases. In addition to technical proficiency, the goals of training include creating a culture of mutual respect, shared responsibility, flattened hierarchies and collaborative practice. Free educator and learner resources to facilitate translation of these guidelines into clinical practice will progressively be made available at https://www.UniversalAirway.org/training/puoi.
While this document focuses on strategies specifically directed at avoiding unrecognised oesophageal intubation, more general non‐technical skills training is required to improve team performance during the crisis that develops when unrecognised oesophageal intubation occurs. Training in processes directed at enhancing team preparation and performance (e.g. pre‐briefs, use of checklists, verbalisation, communication and handover, role allocation, use of cognitive aids, etc.) avoiding loss of time perception and minimising distractions during airway management is recommended [8]. Although addressing these broader issues is beyond the scope of this guideline, such training is an essential component of optimising the response to such events and improving patient outcomes.
Equipment and monitoring
Organisations in which airway management is undertaken should prioritise improving access to videolaryngoscopes where feasible, to facilitate routine use by their staff [53, 54].
Monitor displays should be positioned and oriented to be clearly visible to the airway team whenever possible. Alarms for absent capnography waveform and inadequate oxygen saturation should be set to appropriate limits and audible to the team [67, 149]. Modulation of the pulse oximetry tone with a change in oxygen saturation is a desirable feature in monitors and when available should be routinely enabled and audible to the team during airway management [150]. To minimise the risk of misidentification of other variables as the carbon dioxide waveform (e.g. pressure waveforms), efforts should be made to standardise the layout, format and colours of the variables displayed on monitors [8, 11, 151].
Manufacturers of airway monitors are encouraged to support the ability to standardise displays in this manner and to take other steps to increase the distinctiveness of the carbon dioxide waveform (e.g. using a ‘shaded in’ trace while leaving other waveforms as ‘line only’) [46, 151]. The potential for enhanced displays or smart monitors [152], able to use information from gas measurements, pulse oximetry, volume and pressure readings to make a verbal declaration of the need to exclude oesophageal intubation or of the time elapsed since the oxygen saturation has dropped below a specified level should be explored [46, 67, 153, 154].
The continued occurrence of death and serious harm from unrecognised oesophageal intubation worldwide suggests that an approach to prevention solely focused on stressing removal of the tube if no carbon dioxide is detected is not a complete solution. This guideline emphasises this point but also provides a more comprehensive approach that addresses both technical‐ and human factors‐based contributions to the occurrence of unrecognised oesophageal intubation. The emphasis is on the trigger for tube removal being identification of an unacceptable risk rather than a definitive diagnosis, that it is misplaced, accepting that some correctly placed tubes will be removed. Recommendations directed at creating a shared mental model seek to promote cue recognition, while provision of clearly defined triggers linked to specific actions and promotion of shared decision‐making, aims to avoid fixation on inappropriate diagnoses and overcome barriers to tube removal.
Supporting information
Appendix S1. Class of recommendation and levels of evidence for recommendations according to American Heart Association recommendation classification system.
Appendix S2. Clinical practice implications of the American Heart Association class of recommendation classifications as applied to this guideline.
Acknowledgements
This guideline has been endorsed by the Difficult Airway Society, Society for Airway Management, European Airway Management Society, All India Difficult Airway Society, Canadian Airway Focus Group, Safe Airway Society and the International Airway Management Society. A copy of the guideline has been distributed to the working group currently establishing a pan‐African airway society. A complete list of organisations supporting this guideline is available at https://www.UniversalAirway.org/puoi/support.
Additional information on the Project for Universal Management of Airways is available at https://www.UniversalAirway.org. The authors wish to thank J. Nolan for his advice during preparation of this manuscript as well as the following airway society representatives and members of our international multidisciplinary advisory group (https://www.UniversalAirway.org/advisorygroup) for completing expert surveys and/or reviewing the manuscript: I. Ahmad, Z. Ali, T. Asai, M. Aziz, R. Bhagrath, D. Brewster, M. Bromiley, C. Brown, S. Carley, P. Charco‐Mora, T. Do, K. El‐Boghdadly, T. Engelhardt, J. C. Flores‐Carillo, P. Diemunsch, L. Duggan, F. Mir, K. Fraser, J. Fiadjoe, J. Gatward, P. Gardiner, R. Garg, D. Gilby, C. Groombridge, R. Hodgson, R. Hofmeyr, R. Horowitz, M. Humar, M. Huntington, S. Jaber, N. Jagannathan, P. M. Jones, F. Kelly, M. Kristensen, J. Lioy, D. Lockey, A. M. López, W. Ma, B. McGuire, A. McNarry, G. Navarro, C. Nickson, D. Olvera, M. Parotto, A. I. Pereira, K. Quintero, K. Rivett, S. Sahu, G. Sachdev, R. Schnittker, T. St Clair, A. Timmermann, F. Urdaneta, R. Urtubia, O. V. Orgaz, B. von Ungern‐Sternberg, H. Wei, S. Weingart, G. Zhou and the other anonymous reviewers from the international airway societies.
NC is the creator of the Vortex Approach but has no financial interest in this material which is licensed under a Creative Commons Attribution‐NonCommercial‐NoDerivatives 4.0 International License. NC has undertaken unpaid consultancy work for Verathon Inc. NC's partner is employed by Verathon Inc. and he has accompanied her to a corporate retreat funded by Verathon Inc. NC is a director of the healthcare education provider Simpact Pty Ltd. AH is immediate past Treasurer of the Difficult Airway Society. CAH has received research support from Ambu, Fisher and Paykel Health Limited, Karl Storz Endoscopy, Teleflex and Vyaire Medical. CAH has received honoraria from UpToDate and Elsevier. PAB is the owner of Airway Simulation Ltd which manufactures the ORSIM® bronchoscopy simulator. RG is treasurer of the European Airway Management Society and has received research support from Karl Storz Endoscopy. GK and JAL are Co‐Directors of Airway Interventions and Management in Emergencies (AIME) Educational Programs. SDM is a Councillor of the Australian and New Zealand College of Anaesthetists and an Associate Editor of Anaesthesia. CHR is an independent contractor for Teleflex Inc. and also assists in the development and teaching of procedural skills laboratories. JCS is faculty on The Difficult Airway Course, Associate Editor of Walls Manual of Emergency Airway Management, Author UpToDate Chapter: RSI for Adults Outside the Operating Room and Author UpToDate Chapter: Emergency Cricothyrotomy. MS is the current president of the European Airway Management Society. He has received paid consultancy from Teleflex Medical, Verathon Medical and DEAS Italia, is a patent co‐owner (no royalties) of DEAS Italia and has received lecture grants and travel reimbursements from MSD Italia and MSD USA. TC's department has received devices for evaluation or research, either free or at cost, from several manufacturers. No other competing interests or external funding declared.
International airway societies: Difficult Airway Society; Society for Airway Management; European Airway Management Society; All India Difficult Airway Society; Canadian Airway Focus Group; Safe Airway Society; and International Airway Management Society
This article is accompanied by an editorial by I. Ahmad et al., Anaesthesia 2022; 77: 1321–5.
Contributor Information
N. Chrimes, Email: nicholaschrimes@gmail.com, @NicholasChrimes.
A. Higgs, @AndyHiggsGAA.
C. A. Hagberg, @CarinHagberg.
P. A. Baker, @PaulBakerORSIM.
R. M. Cooper, @gaspasser.
J. A. Law, @jadamlaw.
S. D. Marshall, @hypoxicchicken.
S. N. Myatra, @SheilaMyatra.
E. P. O'Sullivan, @ProfEllenO.
W. H. Rosenblatt, @AirwayOnDemand.
C. H. Ross, @crossermed.
J. C. Sakles, @JohnCSakles.
M. Sorbello, @SorbelloMax.
T. M. Cook, @doctimcook.
References
- 1. Law JA, Duggan LV, Asselin M, et al. Canadian Airway Focus Group updated consensus‐based recommendations for management of the difficult airway: part 1. Difficult airway management encountered in an unconscious patient. Canadian Journal of Anesthesia 2021; 68: 1373–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Apfelbaum JL, Hagberg CA, Connis RT, et al. 2022 American Society of Anesthesiologists Practice Guidelines for Management of the Difficult Airway. Anesthesiology 2022; 136: 31–81. [DOI] [PubMed] [Google Scholar]
- 3. Japanese Society of Anesthesiologists . JSA airway management guideline 2014: to improve the safety of induction of anesthesia. Journal of Anesthesia 2014; 28: 482–93. [DOI] [PubMed] [Google Scholar]
- 4. British Association of Perinatal Medicine . Managing the difficult airway in the neonate. https://www.bapm.org/resources/199‐managing‐the‐difficult‐airway‐in‐the‐neonate (accessed 28/12/2021).
- 5. Rehn M, Hyldmo PK, Magnusson V, et al. Scandinavian SSAI clinical practice guideline on pre‐hospital airway management. Acta Anaesthesiologica Scandinavica 2016; 60: 852–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Higgs A, McGrath BA, Goddard C, et al. Guidelines for the management of tracheal intubation in critically ill adults. British Journal of Anaesthesia 2018; 120: 323–52. [DOI] [PubMed] [Google Scholar]
- 7. Ramkumar V, Dinesh E, Shetty SR, et al. All India Difficult Airway Association 2016 guidelines for the management of unanticipated difficult tracheal intubation in obstetrics. Indian Journal of Anaesthesia 2016; 60: 899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cook TM, Woodall N, Frerk C. 4th National Audit Project of The Royal College of Anaesthetists and The Difficult Airway Society. Major complications of airway management in the United Kingdom, report and findings. 2011. https://www.nationalauditprojects.org.uk/downloads/NAP4%20Full%20Report.pdf (accessed 24/06/2022). [DOI] [PubMed]
- 9. Lee D. Inquest into the death of Emiliana Obusan, 2021. https://coroners.nsw.gov.au/documents/findings/2021/OBUSAN_Emiliana_‐_Findings.pdf (accessed 18/02/2022).
- 10. English C. Amended version of Finding into Death Without Inquest regarding XH. 2021. https://www.coronerscourt.vic.gov.au/sites/default/files/COR%202019%20001998%20XH%20Finding%20Amended.pdf (accessed 18/02/2022).
- 11. Cummings S. Glenda Logsdail: prevention of future deaths report. 2021. https://www.judiciary.uk/publications/glenda‐logsdail‐prevention‐of‐future‐deaths‐report/ (accessed 18/02/2022).
- 12. McCabe EB, Lukins M. Gastric carbon dioxide insufflation can lead to misleading capnography trace during esophageal intubation. A&A Practice 2018; 11: 328. [DOI] [PubMed] [Google Scholar]
- 13. Horstead S. Peter Saint: prevention of future deaths report. 2018. https://www.judiciary.uk/publications/peter‐saint/ (accessed 18/02/2022).
- 14. Roberts D. Sharon Grierson: prevention of future deaths report. 2018. https://www.judiciary.uk/publications/sharon‐grierson/ (accessed 19/02/2022).
- 15. Walker LA. Findings into the death of Sukanya Thurairajah. 2014. https://courts.act.gov.au/__data/assets/pdf_file/0003/961824/thurairajah_.pdf (accessed 19/02/2022).
- 16. Vicker EF. Finding upon inquest into the death of Richard Christopher Jankowski. 2003. https://emcrit.org/wp‐content/uploads/jankowski‐finding.pdf (accessed 19/02/2022).
- 17. Sharpe M. Shot man died because hospital staff put oxygen tube into his oesophagus instead of his airway. 2020. https://www.stuff.co.nz/national/health/119824317/shot‐man‐died‐because‐hospital‐staff‐put‐oxygen‐tube‐into‐his‐oesophagus‐instead‐of‐his‐airway (accessed 19/02/2022).
- 18. Cheston P. IVF mother died in caesarean surgery without seeing baby. 2012. https://www.standard.co.uk/hp/front/ivf‐mother‐died‐in‐caesarean‐surgery‐without‐seeing‐baby‐6892151.html (accessed 19/02/2022).
- 19. Inquest into Mia Atkins choking death: misadventure ruling. 2019. https://www.bbc.com/news/uk‐england‐kent‐50213251 (accessed 19/02/2022).
- 20. Dinham P. Police investigate the deaths of 15 babies in a single year at the Countess of Chester hospital. 2017. https://www.dailymail.co.uk/news/article‐4518212/Baby‐deaths‐Countess‐Chester‐Hospital‐probed.html (accessed 19/02/2022).
- 21. Lydall R. Botched routine op at children's hospital led to death of baby. 2019. https://www.standard.co.uk/news/health/botched‐routine‐op‐at‐children‐s‐hospital‐led‐to‐death‐of‐baby‐a4318776.html (accessed 19/02/2022).
- 22. Summer . Parents awarded 11 million dollars after botched birth leaves daughter brain damaged. 2010. https://www.growingyourbaby.com/parents‐awarded‐11‐million‐dollars‐after‐botched‐birth‐leaves‐daughter‐brain‐damaged/ (accessed 19/02/2022).
- 23. Mayberry H, Burgart AM, Kanaris C. Intubated, awake, and paralysed: a never event. Intensive Care Research 2021; 1: 60–4. [Google Scholar]
- 24. Bates D. Unqualified plastic surgeon guilty of killing three patients in botched liposuction operations. 2011. https://www.dailymail.co.uk/news/article‐2016553/Unqualified‐plastic‐surgeon‐Peter‐Normann‐guilty‐killing‐3‐botched‐liposuction.html?ito=feeds‐newsxml (accessed 19/02/2022).
- 25. Narain J. Mother died after ‘starved of oxygen during dental surgery’. 2007. https://www.dailymail.co.uk/health/article‐475130/Mother‐died‐starved‐oxygen‐dental‐surgery.html (accessed 19/02/2022).
- 26. Edwards A. Newborn baby died from ‘severe brain injury’ after breathing tube became dislodged while she recovered in hospital. 2012. https://www.dailymail.co.uk/news/article‐2203121/Newborn‐baby‐died‐severe‐brain‐injury‐breathing‐tube‐dislodged‐recovered‐hospital.html (accessed 19/02/2022).
- 27. Halle‐Richards S. Newborn baby died days after twin brother when hospital staff ‘missed opportunity’ to save him. 2018. https://www.manchestereveningnews.co.uk/news/greater‐manchester‐news/newborn‐baby‐died‐days‐after‐15548454 (accessed 19/02/2022).
- 28. Schapel AE. Finding of Inquest into the death of Matthew John Lynn. 2009. https://studylib.net/doc/6975387/lynn‐matthew‐john‐‐‐courts‐administration‐authority (accessed 19/02/2022).
- 29. Parkinson KMW. Inquest into the death of Ruben Chand. 2012. https://www.coronerscourt.vic.gov.au/sites/default/files/2018‐12/rubenchand_167909.pdf (accessed 19/02/2022).
- 30. Honardar MR, Posner KL, Domino KB. Delayed detection of esophageal intubation in anesthesia malpractice claims: brief report of a case series. Anesthesia and Analgesia 2017; 125: 1948–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Royal College of Anaesthetists . Capnography: no trace = wrong place. https://rcoa.ac.uk/safety‐standards‐quality/guidance‐resources/capnography‐no‐trace‐wrong‐place (accessed 17/02/2022).
- 32. Chrimes N, Higgs A, Law JA, et al. Project for Universal Management of Airways ‐ part 1: concept and methods. Anaesthesia 2020; 75: 1671–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Russotto V, Myatra SN, Laffey JG, et al. Intubation practices and adverse peri‐intubation events in critically ill patients from 29 countries. Journal of American Medical Association 2021; 325: 1164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hansel J, Rogers AM, Lewis SR, Cook TM, Smith AF. Videolaryngoscopy versus direct laryngoscopy for adults undergoing tracheal intubation. Cochrane Database of Systematic Reviews 2022; 4: CD011136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Holland R, Webb RK, Runciman WB. Oesophageal intubation: an analysis of 2000 incident reports. Anaesthesia and Intensive Care 1993; 21: 608–10. [DOI] [PubMed] [Google Scholar]
- 36. Chen PN, Shih CK, Li YH, Cheng WC, Hsu HT, Cheng KI. Gastric perforation after accidental esophageal intubation in a patient with deep neck infection. Acta Anaesthesiologica Taiwanica 2014; 52: 143–5. [DOI] [PubMed] [Google Scholar]
- 37. Schvadron E, Moses Y, Weissberg D. Gastric rupture complicating inadvertent intubation of the esophagus. Canadian Journal of Surgery 1996; 39: 487–9. [PMC free article] [PubMed] [Google Scholar]
- 38. Jougon J, Cantini O, Delcambre F, Minniti A, Velly JF. Esophageal perforation: life threatening complication of endotracheal intubation. European Journal of Cardio‐Thoracic Surgery 2001; 20: 7–11. [DOI] [PubMed] [Google Scholar]
- 39. Dubost C, Kaswin D, Duranteau A, Jehanno C, Kaswin R. Esophageal perforation during attempted endotracheal intubation. Journal of Thoracic and Cardiovascular Surgery 1979; 78: 44–51. [PubMed] [Google Scholar]
- 40. Mort TC. Esophageal intubation with indirect clinical tests during emergency tracheal intubation: a report on patient morbidity. Journal of Clinical Anesthesia 2005; 17: 255–62. [DOI] [PubMed] [Google Scholar]
- 41. Gannon K. Mortality associated with anaesthesia: a case review study. Anaesthesia 1991; 46: 962–6. [DOI] [PubMed] [Google Scholar]
- 42. Woodall NM, Cook TM. National census of airway management techniques used for anaesthesia in the UK: first phase of the Fourth National Audit Project at the Royal College of Anaesthetists. British Journal of Anaesthesia 2011; 106: 266–71. [DOI] [PubMed] [Google Scholar]
- 43. Cook TM, Woodall N, Harper J, Benger J. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 2: intensive care and emergency departments. British Journal of Anaesthesia 2011; 106: 632–42. [DOI] [PubMed] [Google Scholar]
- 44. Benger J, Hopkinson S. Rapid sequence induction of anaesthesia in UK emergency departments: a national census. Emergency Medicine Journal 2011; 28: 217–20. [DOI] [PubMed] [Google Scholar]
- 45. Halperin JL, Levine GN, Al‐Khatib SM, et al. Further evolution of the ACC/AHA clinical practice guideline recommendation classification system: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology 2016; 67: 1572–4. [DOI] [PubMed] [Google Scholar]
- 46. Pandit JJ, Young P, Davies M. Why does oesophageal intubation still go unrecognised? Lessons for prevention from the coroner's court. Anaesthesia 2022; 77: 123–8. [DOI] [PubMed] [Google Scholar]
- 47. Stiegler MP, Neelankavil JP, Canales C, Dhillon A. Cognitive errors detected in anaesthesiology: a literature review and pilot study. British Journal of Anaesthesia 2012; 108: 229–35. [DOI] [PubMed] [Google Scholar]
- 48. Schnittker R, Marshall S, Horberry T, Young K, Lintern G. Exploring decision pathways in challenging airway management episodes. Journal of Cognitive Engineering and Decision Making 2017; 11: 353–70. [Google Scholar]
- 49. Kovacs G, Duggan LV, Brindley PG. Glottic impersonation. Canadian Journal of Anesthesia 2017; 64: 320. [DOI] [PubMed] [Google Scholar]
- 50. LeBlanc V. The effects of acute stress on performance: implications for health professions education. Academic Medicine 2009; 84: S25–33. [DOI] [PubMed] [Google Scholar]
- 51. Schnittker R, Marshall S, Horberry T, Young KL. Human factors enablers and barriers for successful airway management – an in‐depth interview study. Anaesthesia 2018; 73: 980–9. [DOI] [PubMed] [Google Scholar]
- 52. Russotto V, Cook TM. Capnography use in the critical care setting: why do clinicians fail to implement this safety measure? British Journal of Anaesthesia 2021; 127: 661–4. [DOI] [PubMed] [Google Scholar]
- 53. Lewis SR, Butler AR, Parker J, Cook TM, Smith AF. Videolaryngoscopy versus direct laryngoscopy for adult patients requiring tracheal intubation. Cochrane Database of Systematic Reviews 2016; 11: CD011136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bhattacharjee S, Maitra S, Baidya DK. A comparison between video laryngoscopy and direct laryngoscopy for endotracheal intubation in the emergency department: a meta‐analysis of randomized controlled trials. Journal of Clinical Anesthesia 2018; 47: 21–6. [DOI] [PubMed] [Google Scholar]
- 55. Mosier JM, Whitmore SP, Bloom JW, Snyder LS, Graham LA, Carr GE, Sakles JC. Video laryngoscopy improves intubation success and reduces esophageal intubations compared to direct laryngoscopy in the medical intensive care unit. Critical Care 2013; 17: R237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Goksu E, Kilic T, Yildiz G, Unal A, Kartal M. Comparison of the C‐MAC video laryngoscope to the Macintosh laryngoscope for intubation of blunt trauma patients in the ED. Turkish Journal of Emergency Medicine 2016; 16: 53–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. De Jong A, Molinari N, Conseil M, et al. Video laryngoscopy versus direct laryngoscopy for orotracheal intubation in the intensive care unit: a systematic review and meta‐analysis. Intensive Care Medicine 2014; 40: 629–39. [DOI] [PubMed] [Google Scholar]
- 58. Arulkumaran N, Lowe J, Ions R, Mendoza M, Bennett V, Dunser MW. Videolaryngoscopy versus direct laryngoscopy for emergency orotracheal intubation outside the operating room: a systematic review and meta‐analysis. British Journal of Anaesthesia 2018; 120: 712–24. [DOI] [PubMed] [Google Scholar]
- 59. Brown CA, Kaji AH, Fantegrossi A, et al. Video laryngoscopy compared to augmented direct laryngoscopy in adult emergency department tracheal intubations: a National Emergency Airway Registry (NEAR) Study. Academic Emergency Medicine 2020; 27: 100–8. [DOI] [PubMed] [Google Scholar]
- 60. Hypes CD, Stolz U, Sakles JC, et al. Video laryngoscopy improves odds of first‐attempt success at intubation in the intensive care unit. A propensity‐matched analysis. Annals of the American Thoracic Society 2016; 13: 382–90. [DOI] [PubMed] [Google Scholar]
- 61. Lakticova V, Koenig SJ, Narasimhan M, Mayo PH. Video laryngoscopy is associated with increased first pass success and decreased rate of esophageal intubations during urgent endotracheal intubation in a medical intensive care unit when compared to direct laryngoscopy. Journal of Intensive Care Medicine 2013; 30: 44–8. [DOI] [PubMed] [Google Scholar]
- 62. Paolini J‐B, Donati F, Drolet P. Review article: video‐laryngoscopy: another tool for difficult intubation or a new paradigm in airway management? Canadian Journal of Anesthesia 2013; 60: 184–91. [DOI] [PubMed] [Google Scholar]
- 63. Nourallah B, Levy N. Utilisation of ‘verbalisation’ to reduce the complications of tracheal intubation. Anaesthesia 2020; 75: 556–7. [DOI] [PubMed] [Google Scholar]
- 64. Shinohara K, Naito H, Matsui Y, Hikono M. The effects of “finger pointing and calling” on cognitive control processes in the task‐switching paradigm. International Journal of Industrial Ergonomics 2013; 43: 129–36. [Google Scholar]
- 65. Tsang LF, Tsang WY, Yiu KC, Tang SK, Sham SYA. Using the PDSA cycle for the evaluation of pointing and calling implementation to reduce the rate of high‐alert medication administration incidents in the United Christian Hospital of Hong Kong, China. Patient Safety and Quality Improvement Journal 2017; 5: 577–83. [Google Scholar]
- 66. Flin R, O'Connor P, Crichton M. Safety at the Sharp End: A Guide to Non‐Technical Skills. Hampshire, England: Ashgate Publishing, 2008. [Google Scholar]
- 67. Klein AA, Meek T, Allcock E, et al. Recommendations for standards of monitoring during anaesthesia and recovery 2021. Anaesthesia 2021; 76: 1212–23. [DOI] [PubMed] [Google Scholar]
- 68. Thoms GMM, McHugh GA, O'Sullivan EP. The global oximetry initiative. Anaesthesia 2007; 62: 75–7. [DOI] [PubMed] [Google Scholar]
- 69. Jooste R, Roberts F, Mndolo S, Mabedi D, Chikumbanje S, Whitaker DK, O'Sullivan EP. Global Capnography Project (GCAP): implementation of capnography in Malawi ‐ an international anaesthesia quality improvement project. Anaesthesia 2019; 74: 158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wollner E, Nourian MM, Booth W, et al. Impact of capnography on patient safety in high‐ and low‐income settings: a scoping review. British Journal of Anaesthesia 2020; 125: e88–e103. [DOI] [PubMed] [Google Scholar]
- 71. Linko K, Paloheimo M, Tammisto T. Capnography for detection of accidental oesophageal intubation. Acta Anaesthesiologica Scandinavica 1983; 27: 199–202. [DOI] [PubMed] [Google Scholar]
- 72. Takeda T, Tanigawa K, Tanaka H, Hayashi Y, Goto E, Tanaka K. The assessment of three methods to verify tracheal tube placement in the emergency setting. Resuscitation 2003; 56: 153–7. [DOI] [PubMed] [Google Scholar]
- 73. Grmec S, Mally S. Prehospital determination of tracheal tube placement in severe head injury. Emergency Medicine Journal 2004; 21: 518–20. [PMC free article] [PubMed] [Google Scholar]
- 74. Grmec S. Comparison of three different methods to confirm tracheal tube placement in emergency intubation. Intensive Care Medicine 2002; 28: 701–4. [DOI] [PubMed] [Google Scholar]
- 75. Knapp S, Kofler J, Stoiser B, et al. The assessment of four different methods to verify tracheal tube placement in the critical care setting. Anesthesia and Analgesia 1999; 88: 766–70. [DOI] [PubMed] [Google Scholar]
- 76. Silvestri S, Ralls GA, Krauss B, et al. The effectiveness of out‐of‐hospital use of continuous end‐tidal carbon dioxide monitoring on the rate of unrecognized misplaced intubation within a regional emergency medical services system. Annals of Emergency Medicine 2005; 45: 497–503. [DOI] [PubMed] [Google Scholar]
- 77. Sum‐Ping ST, Mehta MP, Anderton JM. A comparative study of methods of detection of esophageal intubation. Anesthesia and Analgesia 1989; 69: 627–32. [PubMed] [Google Scholar]
- 78. Vukmir RB, Heller MB, Stein KL. Confirmation of endotracheal tube placement: a miniaturized infrared qualitative CO2 detector. Annals of Emergency Medicine 1991; 20: 726–9. [DOI] [PubMed] [Google Scholar]
- 79. Goldberg JS, Rawle PR, Zehnder JL, Sladen RN. Colorimetric end‐tidal carbon dioxide monitoring for tracheal intubation. Anesthesia and Analgesia 1990; 70: 191–4. [DOI] [PubMed] [Google Scholar]
- 80. Kelly JS, Wilhoit RD, Brown RE, James R. Efficacy of the FEF colorimetric end‐tidal carbon dioxide detector in children. Anesthesia and Analgesia 1992; 75: 45–50. [PubMed] [Google Scholar]
- 81. Chow LH, Lui PW, Cheung EL, Jong HR, Yang TC, Chen YC. Verification of endotracheal tube misplacement with the colorimetric carbon dioxide detector during anesthesia. Zhonghua Yi Xue Za Zhi 1993; 51: 415–8. [PubMed] [Google Scholar]
- 82. Anton WR, Gordon RW, Jordan TM, Posner KL, Cheney FW. A disposable end‐tidal CO2 detector to verify endotracheal intubation. Annals of Emergency Medicine 1991; 20: 271–5. [DOI] [PubMed] [Google Scholar]
- 83. Ornato JP, Shipley JB, Racht EM, et al. Multicenter study of a portable, hand‐size, colorimetric end‐tidal carbon dioxide detection device. Annals of Emergency Medicine 1992; 21: 518–23. [DOI] [PubMed] [Google Scholar]
- 84. MacLeod BA, Heller MB, Gerard J, Yealy DM, Menegazzi JJ. Verification of endotracheal tube placement with colorimetric end‐tidal CO2 detection. Annals of Emergency Medicine 1991; 20: 267–70. [DOI] [PubMed] [Google Scholar]
- 85. Denman WT, Hayes M, Higgins D, Wilkinson DJ. The Fenem CO2 detector device. An apparatus to prevent unnoticed oesophageal intubation. Anaesthesia 1990; 45: 465–7. [DOI] [PubMed] [Google Scholar]
- 86. Bhende MS, Thompson AE. Evaluation of an end‐tidal CO2 detector during pediatric cardiopulmonary resuscitation. Pediatrics 1995; 95: 395–9. [PubMed] [Google Scholar]
- 87. Foy KE, Mew E, Cook TM, et al. Paediatric intensive care and neonatal intensive care airway management in the United Kingdom: the PIC‐NIC survey. Anaesthesia 2018; 73: 1337–44. [DOI] [PubMed] [Google Scholar]
- 88. Cook TM, Harrop‐Griffiths W. Capnography prevents avoidable deaths. British Medical Journal 2019; 364: l439. [DOI] [PubMed] [Google Scholar]
- 89. Cook TM, Kelly FE, Foy K, et al. The PIC‐NIC survey: capnography and neonatal intensive care ‐ a reply. Anaesthesia 2019; 74: 118–20. [DOI] [PubMed] [Google Scholar]
- 90. Chrimes N. The vortex approach to airway management preoxygenation. 2016. http://vortexapproach.org/preox (accessed 21/02/2022).
- 91. Kramer‐Johansen J, Dorph E, Steen PA. Detection of carbon dioxide in expired air after oesophageal intubation; the role of bystander mouth‐to‐mouth ventilation. Acta Anaesthesiologica Scandinavica 2008; 52: 155–7. [DOI] [PubMed] [Google Scholar]
- 92. Ahmad I, El‐Boghdadly K, Bhagrath R, et al. Difficult Airway Society guidelines for awake tracheal intubation (ATI) in adults. Anaesthesia 2020; 75: 509–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chrimes N. The Vortex: a universal ‘high‐acuity implementation tool’ for emergency airway management. British Journal of Anaesthesia 2016; 117(Suppl 1): i20–i7. [DOI] [PubMed] [Google Scholar]
- 94. Sandroni C, De Santis P, D'Arrigo S. Capnography during cardiac arrest. Resuscitation 2018; 132: 73–7. [DOI] [PubMed] [Google Scholar]
- 95. Sum Ping ST, Mehta MP, Symreng T. Reliability of capnography in identifying esophageal intubation with carbonated beverage or antacid in the stomach. Anesthesia and Analgesia 1991; 73: 333–7. [DOI] [PubMed] [Google Scholar]
- 96. Keller WR, Biehler J, Linares MY‐R, Garcia‐Pena BM. False‐positive colorimetric capnometry after ingestion of carbonated beverages. Pediatric Emergency Care 2009; 25: 69–73. [DOI] [PubMed] [Google Scholar]
- 97. Pandit JJ. ‘No trace, wrong place’ does not mean ‘positive trace, right place’. Identifying and managing misplaced or displaced tracheal tubes in cardiopulmonary resuscitation. Anaesthesia 2022; 77: 16–21. [DOI] [PubMed] [Google Scholar]
- 98. Carlson JN, Colella MR, Daya MR, J. de Maio V, Nawrocki P, Nikolla DA, Bosson N. Prehospital cardiac arrest airway management: an NAEMSP position statement and resource document. Prehospital Emergency Care 2022; 26: 54–63. [DOI] [PubMed] [Google Scholar]
- 99. Cook TM, Nolan JP. Use of capnography to confirm correct tracheal intubation during cardiac arrest. Anaesthesia 2011; 66: 1183–4. [DOI] [PubMed] [Google Scholar]
- 100. Komasawa N, Fujiwara S, Miyazaki S, Ohchi F, Minami T. Shifts in endotracheal tube position due to chest compressions: a simulation comparison by fixation method. Journal of Emergency Medicine 2015; 48: 241–6. [DOI] [PubMed] [Google Scholar]
- 101. Chrimes N. The vortex approach to airway management green zone. 2016. http://vortexapproach.org/greenzone/ (accessed 21/02/2022).
- 102. McShane AJ, Martin JL. Preoxygenation and pulse oximetry may delay detection of esophageal intubation. Journal of the National Medical Association 1987; 79: 991–2. [PMC free article] [PubMed] [Google Scholar]
- 103. Nimmagadda U, Salem MR, Crystal GJ. Preoxygenation: physiologic basis, benefits, and potential risks. Anesthesia and Analgesia 2017; 124: 507–17. [DOI] [PubMed] [Google Scholar]
- 104. Werman HA, Falcone RE. Glottic positioning of the endotracheal tube tip: a diagnostic dilemma. Annals of Emergency Medicine 1998; 31: 643–6. [DOI] [PubMed] [Google Scholar]
- 105. Jafferji D, Morris R, Levy N. Reducing the risk of confirmation bias in unrecognised oesophageal intubation. British Journal of Anaesthesia 2019; 122: e66–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Higgs A, Tham S‐M. Attenuated post intubation capnograph trace: haemodynamic collapse or technical error? Trends in Anaesthesia and Critical Care 2022; 44: 34–5. [Google Scholar]
- 107. Collins J, Ní Eochagáin A, O'Sullivan EP. A recurring case of ‘no trace, right place’ during emergency tracheal intubations in the critical care setting. Anaesthesia 2021; 76: 1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Mort TC. Emergency tracheal intubation: complications associated with repeated laryngoscopic attempts. Anesthesia and Analgesia 2004; 99: 607–13. [DOI] [PubMed] [Google Scholar]
- 109. Sakles JC, Chiu S, Mosier J, Walker C, Stolz U. The importance of first pass success when performing orotracheal intubation in the emergency department. Academic Emergency Medicine 2013; 20: 71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Frerk C, Mitchell VS, McNarry AF, et al. Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. British Journal of Anaesthesia 2015; 115: 827–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Gottlieb M, Nakitende D, Sundaram T, Serici A, Shah S, Bailitz J. Comparison of static versus dynamic ultrasound for the detection of endotracheal intubation. Western Journal of Emergency Medicine 2018; 19: 412–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Baker PA, Feinleib J, O'Sullivan EP. Is it time for airway management education to be mandatory? British Journal of Anaesthesia 2016; 117(Suppl 1): i13–i6. [DOI] [PubMed] [Google Scholar]
- 113. Weaver B, Lyon M, Blaivas M. Confirmation of endotracheal tube placement after intubation using the ultrasound sliding lung sign. Academic Emergency Medicine 2006; 13: 239–44. [DOI] [PubMed] [Google Scholar]
- 114. Gottlieb M, Holladay D, Peksa GD. Ultrasonography for the confirmation of endotracheal tube intubation: a systematic review and meta‐analysis. Annals of Emergency Medicine 2018; 72: 627–36. [DOI] [PubMed] [Google Scholar]
- 115. Lin MJ, Gurley K, Hoffmann B. Bedside ultrasound for tracheal tube verification in pediatric emergency department and ICU patients: a systematic review. Pediatric Critical Care Medicine 2016; 17: e469–e76. [DOI] [PubMed] [Google Scholar]
- 116. Das SK, Choupoo NS, Haldar R, Lahkar A. Transtracheal ultrasound for verification of endotracheal tube placement: a systematic review and meta‐analysis. Canadian Journal of Anesthesia 2015; 62: 413–23. [DOI] [PubMed] [Google Scholar]
- 117. Chou EH, Dickman E, Tsou P‐Y, et al. Ultrasonography for confirmation of endotracheal tube placement: a systematic review and meta‐analysis. Resuscitation 2015; 90: 97–103. [DOI] [PubMed] [Google Scholar]
- 118. Wee MYK. The oesophageal detector device: assessment of a new method to distinguish oesophageal from tracheal intubation. Anaesthesia 1988; 43: 27–9. [DOI] [PubMed] [Google Scholar]
- 119. Wee MY. Comments on the oesophageal detector device. Anaesthesia 1989; 44: 930–1. [DOI] [PubMed] [Google Scholar]
- 120. Wafai Y, Salem MR, Joseph NJ, Baraka A. The self‐inflating bulb for confirmation of tracheal intubation: incidence and demography of false negatives. Anesthesiology 1994; 81: A1303. [Google Scholar]
- 121. Andersen KH, Hald A. Assessing the position of the tracheal tube: the reliability of different methods. Anaesthesia 1989; 44: 984–5. [DOI] [PubMed] [Google Scholar]
- 122. Donahue PL. The oesophageal detector device. An assessment of accuracy and ease of use by paramedics. Anaesthesia 1994; 49: 863–5. [DOI] [PubMed] [Google Scholar]
- 123. O'Leary JJ, Pollard BJ, Ryan MJ. A method of detecting oesophageal intubation or confirming tracheal intubation. Anaesthesia and Intensive Care 1988; 16: 299–301. [DOI] [PubMed] [Google Scholar]
- 124. Wee MYK, Walker AKY. The oesophageal detector device: an assessment with uncuffed tubes in children. Anaesthesia 1991; 46: 869–71. [DOI] [PubMed] [Google Scholar]
- 125. Haynes SR, Morton NS. Use of the oesophageal detector device in children under one year of age. Anaesthesia 1990; 45: 1067–9. [DOI] [PubMed] [Google Scholar]
- 126. Williams KN, Nunn JF. The oesophageal detector device: a prospective trial on 100 patients. Anaesthesia 1989; 44: 412–4. [DOI] [PubMed] [Google Scholar]
- 127. Baraka A, Khoury PJ, Siddik SS, Ramez Salem M, Joseph NJ. Efficacy of the self‐inflating bulb in differentiating esophageal from tracheal intubation in the parturient undergoing cesarean section. Anesthesia and Analgesia 1997; 84: 533–7. [DOI] [PubMed] [Google Scholar]
- 128. Lang DJ, Wafai Y, Salem MR, Czinn EA, Halim AA, Baraka A. Efficacy of the self‐inflating bulb in confirming tracheal intubation in the morbidly obese. Anesthesiology 1996; 85: 246–53. [DOI] [PubMed] [Google Scholar]
- 129. Zaleski L, Abello D, Gold MI. The esophageal detector device. Does it work? Anesthesiology 1993; 79: 244–7. [DOI] [PubMed] [Google Scholar]
- 130. Pelucio M, Halligan L, Dhindsa H. Out‐of‐hospital experience with the syringe esophageal detector device. Academic Emergency Medicine 1997; 4: 563–8. [DOI] [PubMed] [Google Scholar]
- 131. Bozeman WP, Hexter D, Liang HK, Kelen GD. Esophageal detector device versus detection of end‐tidal carbon dioxide level in emergency intubation. Annals of Emergency Medicine 1996; 27: 595–9. [DOI] [PubMed] [Google Scholar]
- 132. Salem MR, Wafai Y, Baraka A, Taimorrazy B, Joseph NJ, Nimmagadda U. Use of the self‐inflating bulb for detecting esophageal intubation after “esophageal ventilation”. Anesthesia and Analgesia 1993; 77: 1227–31. [DOI] [PubMed] [Google Scholar]
- 133. Salem MR, Wafai Y, Joseph NJ, Baraka A, Czinn EA. Efficacy of the self‐inflating bulb in detecting esophageal intubation. Does the presence of a nasogastric tube or cuff deflation make a difference? Anesthesiology 1994; 80: 42–8. [DOI] [PubMed] [Google Scholar]
- 134. Kasper CL, Deem S. The self‐inflating bulb to detect esophageal intubation during emergency airway management. Anesthesiology 1998; 88: 898–902. [DOI] [PubMed] [Google Scholar]
- 135. Jenkins WA, Verdile VP, Paris PM. The syringe aspiration technique to verify endotracheal tube position. American Journal of Emergency Medicine 1994; 12: 413–6. [DOI] [PubMed] [Google Scholar]
- 136. Marley CD, Eitel DR, Anderson TE, Mum AJ, Patterson GA. Evaluation of a prototype esophageal detection device. Academic Emergency Medicine 1995; 2: 503–7. [DOI] [PubMed] [Google Scholar]
- 137. Sharieff GQ, Rodarte A, Wilton N, Silva PD, Bleyle D. The self‐inflating bulb as an esophageal detector device in children weighing more than twenty kilograms: a comparison of two techniques. Annals of Emergency Medicine 2003; 41: 623–9. [DOI] [PubMed] [Google Scholar]
- 138. Sharieff GQ, Rodarte A, Wilton N, Bleyle D. The self‐inflating bulb as an airway adjunct: is it reliable in children weighing less than 20 kilograms? Academic Emergency Medicine 2003; 10: 303–8. [DOI] [PubMed] [Google Scholar]
- 139. Ghabash M, Choueiry P, Matta M. Aspirated air capnography with esophageal detector device to confirm tracheal intubation in rapid sequence induction. Middle East Journal of Anaesthesiology 1998; 14: 381–6. [PubMed] [Google Scholar]
- 140. Duggan LV, Marshall SD, Scott J, Brindley PG, Grocott HP. The MacGyver bias and attraction of homemade devices in healthcare. Canadian Journal of Anesthesia 2019; 66: 757–61. [DOI] [PubMed] [Google Scholar]
- 141. Gillespie John H, Knight Robert G, Middaugh Robert E, Menk Emil J, Baysinger CL. Efficacy of endotracheal tube cuff palpation and humidity in distinguishing endotracheal from esophageal intubation. Anesthesiology 1988; 69: A265. [Google Scholar]
- 142. Haridas RP. Condensation on tracheal tubes is commonly seen with oesophageal intubation. British Journal of Anaesthesia 1995; 75: 115–6. [PubMed] [Google Scholar]
- 143. Bair AE, Laurin EG, Schmitt BJ. An assessment of a tracheal tube introducer as an endotracheal tube placement confirmation device. American Journal of Emergency Medicine 2005; 23: 754–8. [DOI] [PubMed] [Google Scholar]
- 144. Project for universal management of airways: preventing unrecognised oesophageal intubation cases: Australia. 2022. https://www.universalairway.org/puoi/cases/australia (accessed 11/06/2022). [DOI] [PMC free article] [PubMed]
- 145. Law JA, Mariotti C, Mullen T. Failure of both suction catheter passage and bronchoscopy to diagnose an obstructing tracheal mucus plug. Canadian Journal of Anesthesia 2012; 59: 911–2. [DOI] [PubMed] [Google Scholar]
- 146. Bantug K, Groombridge CJ, Maini A. The airway ally. 2021. https://www.simpact.com.au/airwayally (accessed 24/05/2022).
- 147. Marshall S. The use of cognitive aids during emergencies in anesthesia: a review of the literature. Anesthesia and Analgesia 2013; 117: 1162–71. [DOI] [PubMed] [Google Scholar]
- 148. Leonard M. The human factor: the critical importance of effective teamwork and communication in providing safe care. Quality and Safety in Health Care 2004; 13: i85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Ruskin KJ, Hueske‐Kraus D. Alarm fatigue: impacts on patient safety. Current Opinion in Anesthesiology 2015; 28: 685–90. [DOI] [PubMed] [Google Scholar]
- 150. Craven RM, McIndoe AK. Continuous auditory monitoring—how much information do we register? British Journal of Anaesthesia 1999; 83: 747–9. [DOI] [PubMed] [Google Scholar]
- 151. Kelly FE, Cook TM. Unrecognised oesophageal intubation: additional human factors and ergonomics solutions. Anaesthesia 2022; 77: 718–9. [DOI] [PubMed] [Google Scholar]
- 152. Paterson E, Sanderson PM, Salisbury IS, Burgmann FP, Mohamed I, Loeb RG, Paterson NAB. Evaluation of an enhanced pulse oximeter auditory display: a simulation study. British Journal of Anaesthesia 2020; 125: 826–34. [DOI] [PubMed] [Google Scholar]
- 153. Chrimes N, Higgs A, Rehak A. Lost in transition: the challenges of getting airway clinicians to move from the upper airway to the neck during an airway crisis. British Journal of Anaesthesia 2020; 125: e38–46. [DOI] [PubMed] [Google Scholar]
- 154. Roche TR, Braun J, Ganter MT, et al. Voice alerting as a medical alarm modality for next‐generation patient monitoring: a randomised international multicentre trial. British Journal of Anaesthesia 2021; 127: 769–77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Class of recommendation and levels of evidence for recommendations according to American Heart Association recommendation classification system.
Appendix S2. Clinical practice implications of the American Heart Association class of recommendation classifications as applied to this guideline.


