Abstract
Standard clinical care of neonates and the ventilation status of human patients affected with coronavirus disease involves continuous CO2 monitoring. However, existing noninvasive methods are inadequate owing to the rigidity of hard-wired devices, insubstantial gas permeability and high operating temperature. Here, we report a cost-effective transcutaneous CO2 sensing device comprising elastomeric sponges impregnated with oxidized single-walled carbon nanotubes (oxSWCNTs)-based composites. The proposed device features a highly selective CO2 sensing response (detection limit 155 ± 15 ppb), excellent permeability and reliability under a large deformation. A follow-up prospective study not only offers measurement equivalency to existing clinical standards of CO2 monitoring but also provides important additional features. This new modality allowed for skin-to-skin care in neonates and room-temperature CO2 monitoring as compared with clinical standard monitoring system operating at high temperature to substantially enhance the quality for futuristic applications.
Keywords: Permeable, Transcutaneous, Stretchable sensor, Single wall carbon nanotube, Polyaniline, CO2 sensor
An emerging coronavirus (COVID-19) swept through the entire world within three months and shuttered the global economy [1], [2]. COVID-19 affects the respiratory system, and promotes progression to acute respiratory distress syndrome (ARDS) in some patients, leading to grave hypoxemia [3], [4]. Clinicians often diagnose it from arterial oxygen saturation (SpO2) by using pulse oximetry, without knowing the accompanying partial arterial pressure of carbon dioxide (pCO2) [5], [6] . SpO2 reflects gas transfer in the lungs, pCO2 reflects ventilation, and they should be evaluated independently in cases of respiratory failure. Also, ventilated CO2 provides significant information regarding bronchospasms, the neonatal intensive care unit, and the effectiveness of therapy in the operation theatre. They provide information regarding hypo or hyperventilation to evaluate potential respiratory drive depression in patients affected with chronic obstructive pulmonary disease. pCO2 can be estimated from arterial or central venous blood samples; however, these invasive approaches are limited to laboratory settings, as they must be analysed promptly upon collection and required trained staff for analysis. Additionally, repeated diagnostic blood drawing involves a risk of infection, thrombosis, and iatrogenic anemia [7]. Therefore, noninvasive monitoring of CO2 is highly desirable for continuous monitoring of lung and airway disorders in COVID-19 patients to reduce the patient load [8]. Commonly used end-tidal capnometry is used for monitoring CO2 gas in exhaled breath for patients with stable haemodynamic conditions. There is poor correlation in the data obtained with capnometry and arterial partial pressure of CO2 in case of ventilation-perfusion mismatch (8,9). Noninvasive transcutaneous monitoring of CO2 can be vital for early detection of respiratory deterioration as CO2 diffuses through tissues approximately 20 times more rapidly than oxygen (O2) [5]. Additionally, it can be used to reflect health conditions related to hypercapnic respiratory failure and other pulmonary and cardiac disorders. Notably, noninvasive continuous real-time measurement of CO2 can be a vital aid in ventilatory supervision of critically ill newborn infants [9], [10], [11], [12]. Although gas emitted through the skin has very low concentrations of these biomarkers compared to the gas emitted through breath, analysis is advantageous owing to unconscious and continuous emission from the skin surface. More importantly, transcutaneous gas analysis requires no intentional action, such as deep exhalation, and could achieve precise quantification [13]. This will further alleviate the requirement for high-level care settings by providing opportunities for home registration. A highly sensitive, selective, and stretchable gas sensor that can accommodate large strain, even when conformally attached at muscles and joints, would be indispensable for achieving improvements in health care.
However, the devices typically used for noninvasive CO2 monitoring are often rigid, incompatible with integration to soft interfaces and based on either a nondispersive infrared (NDIR) absorption detector or a pH electrode in a bicarbonate buffer solution. In addition to the questionable reliability, these devices have limitations related to size, response time and calibration. Furthermore, to achieve accuracy, the sensing probe must be kept at 43–44 °C [14], which has a risk of burning/damaging skin during prolonged use [15]. Another probe involves gas chromatography (GC) and mass spectrometry (MS), which employ skin gas collection bags or trapping filters that can be used only once [13]. This nonreusability leads to challenging situations for health professionals in hospitals with heavy patient loads requiring resources such as intensive care units and ventilators [16], [17].
Recently, a few carbon nanotube composites [18], [19], [20], metal oxide derivatives [21], [22] and conductive polymer-based [23], [24] flexible CO2 sensors using resistive [25], [26] or capacitive [27], [28] transducers have been fabricated for environmental gas monitoring and air quality control. However, the associated methodology relies on either a nonporous nature or limited gas/vapour permeability, which adversely affects the physiological conditions and limit long-term feasibility. In addition, such devices incorporate nanostructural sensing thin films prepared by sophisticated techniques, and these films undergo structural alterations (delamination) during mechanical stretching or physical movement of body parts [29]. Therefore, concurrently endowing CO2 sensing devices with stretchability, sensitivity, reusability, permeability and low-cost along with real-time direct detection capability under ambient conditions and is still extremely challenging.
Recent reports have successfully addressed this challenge by using graphene, carbon nanotubes (CNTs) and especially single-walled carbon nanotubes (SWCNTs), which have large effective surface-to-volume ratios, hollow geometries, and one-dimensional nanoscale morphologies. SWCNTs are reported to be ideal candidates for target gas physical adsorption or chemical reactions with fast and efficient signal transformation during sensing [30], [31], [32]. Pristine SWCNTs are sensitive to the presence of strong charge donors (NH3) or acceptors (NO2) but, are less selective and sensitive towards weak Lewis acids or bases, such as CO2, CH4 or H2 [33]. Modification of the surface chemistry of SWCNTs with Lewis bases (pyrrolic and amine nitrogen functionalities) can significantly alter selective adsorption of CO2, which is a weak Lewis acid [34]. Furthermore, adsorption capacity has been reported to be sacrificed by blocking of channels to the active sites and amine degradation [35]. Based on these findings, we oxidized SWCNTs before functionalization with the Lewis base polyaniline (PANI) to achieve charge transfer interactions with CO2, which led to an efficient cyclic process involving highly selective CO2 capture and release.
Here, we report a permeable and stretchable, yet interference-insensitive CO2 sensor, to address the unmet clinical needs. It is based on a highly porous sugar-templated polydimethylsiloxane (PDMS) elastomer sponge (PP) consisting of in situ polymerized PANI over an oxidized SWCNT composite (oxSWCNT-PANI). The fabrication method involves dip-coating PP into a sol–gel dispersant (Zn-Al) infused oxSWCNT-PANI ink, thereby providing an easy, economical, and highly efficient manufacturing approach for developing transcutaneous gas sensors. As CO2 recognition layers, many amines and imine functional groups of PANI and the hygroscopic dispersant act as anchoring sites to attract more water molecules, facilitating an enhanced reaction between the PANI and CO2. This interaction consequently increases the local CO2 concentration, and hence high sensitivity can be achieved. The fabricated CO2 gas sensor exhibits fast response/recovery, high gas and vapour permeability and sensitive detection of transcutaneous CO2 without skin heating irrespective of the location, unlike that of clinical grade commercial devices. This newly fabricated sensing platform can withstand a tensile strain of 60 %, thereby accommodating the maximum deformation induced by human body parts to open new opportunities for health care monitoring.
1. Results
1.1. Fabricated stretchable and porous sensor
The fabrication method of the stretchable, highly porous gas sensor is shown in Fig. 1 a. The process began with preparation of the PP by capillary action and sugar leaching techniques using commercial sugar cubes (1 cm × 1 cm × 1 cm) and silicone elastomer precursor mixtures (10:1 ratio by weight of PDMS and the curing agent). The PP (1 cm × 1 cm × 0.2 cm) was dipped in the oxSWCNT-PANI ink prepared by using a Zn-Al sol–gel dispersant and dried at 70 °C. Finally, 20 nm Au was thermally deposited to form an electrical contact with the oxSWCNT-PANI/PP based multifunctional on-skin CO2 sensing electrode. The detailed process can be seen in Fig. S1. The prepared sensing electrode can be easily mounted onto an infant or adult chest, forearm, face, neck, or finger to monitor various physiological activities, and no allergic reaction was observed when it was placed in contact with the human body for two days.
Fig. 1.
Fabrication and structure of the stretchable CO2 gas sensor. (a) Photograph of the porous PDMS sponge (PP), oxSWCNT-PANI ink and fabricated sensor (oxSWCNT-PANI/PP). (b) oxSWCNT-PANI/PP SEM micrographs under 0% and 60% strain. (c) ADF-STEM micrograph of oxSWCNT-PANI with EELS mapping of the region defined by the blue box. (d) ADF-STEM micrograph of the oxSWCNT-PANI ink with EELS mapping of particular region in blue box. (e) Raman spectra of SWCNT, oxSWCNT, oxSWCNT-PANI and oxSWCNT-PANI ink. The insets showed enlarged views of the RBM peaks (left) and quantitative shifts in the G-peaks in all the samples (right). (f) Raman spectra of the sensor at 0%, 30% and 60% strain. The inset shows the enlarged G-peak region.
The morphological features of the fabricated sensor were characterized by scanning electron microscopy (SEM) and scanning transmission electron microscopy (STEM). Elemental analysis was simultaneously carried out by electron energy loss spectroscopy (EELS) and energy dispersive X-ray spectroscopy (EDX). The PP displayed a continuous network of well-defined, interconnected open macropores (Fig. S1a). After grafting the oxSWCNT-PANI composite onto the PP surface (oxSWCNT-PANI/PP), the open porous structure remained intact (Fig. S2a, b). This engineered 3D open-pore structure free from clogged sites enhances the stretchability and minimizes the local strain on the sensor, as can be observed from the oxSWCNT-PANI/PP sensor micrograph under 60 % strain (Fig. 1b). The open pores seemed to be stretched by 10 % along the stretching axis; however, the microporous entangled network of oxSWCNT-PANI remained unperturbed (Fig. S2c, d). This framework enabled in-depth penetration and easy diffusion of the test gas. Interaction of the test gas with the oxSWCNT-PANI was further indicated by analysing the assembly of PANI-wrapped oxSWCNTs. The STEM-EELS mapping image of the oxSWCNT-PANI clearly showed wrapping of the oxSWCNT perimeter with PANI as shown in the schematic. The annular dark-field (ADF) image showed an amorphous layer with a uniform thickness, which was attributed to the PANI surrounding the CNT core (Fig. 1c). EELS mapping images taken from the region in the blue box in the ADF image clearly illustrated the uniform distribution of C and N, as indicated by the bright contrast on CNTs in the ADF image. Furthermore, STEM was utilized to examine the metal atoms (Zn-Al of dispersant) present in the oxSWCNT-PANI (ink). The micrograph (Fig. 1d) revealed fine dispersion of Zn-Al atoms over the surface of oxSWCNT-PANI as bright dots due to their higher atomic numbers. Further, elemental mapping showed clear distribution of Zn and Al along with C and N (Fig. 1d), which are anticipated to enhance the sensing response by providing a hydrophilic environment.
The fabricated samples were further analysed with Raman spectroscopy to understand the local strain on the oxSWCNT bonding networks. Fig. 1e and 1f depicts Raman spectra for the samples used in the preparation of the oxSWCNT-PANI composite. The radial breathing mode (RBM) peaks of the oxSWCNTs showed no considerable shift (left inset of Fig. 1e) compared to those of pristine SWCNTs, confirming that no changes occurred in the tubular frame structure occurred [36]. The oxSWCNTs were then uniformly wrapped with PANI during in situ polymerization, which resulted in the appearance of signature PANI peaks with an upshift of the G-peak (right inset of Fig. 1e). The G-peak shift indicated a stronger interaction between the oxSWCNTs and PANI, which was remarkably enhanced during ink preparation with the Zn-Al sol gel dispersant, as reported in previous articles [37], [38]. Furthermore, the decreased intensities of the PDMS peaks observed in the Raman spectra of the sensor during the fabrication process (Fig. S3) confirmed uniform coverage of the PP network by the oxSWCNT-PANI composite. The fabricated sensor was examined unstretched and under different stretching conditions. Under stretching conditions involving 30 % and 60 % strain, the G-peak demonstrated downshifts (Fig. 1f) owing to the stronger interaction of oxSWCNT-PANI with PP, this reflected the augmented sensing response, as examined in the following section.
1.2. Gas sensing performance of the sensor
The fabricated sensor was tested for CO2 gas sensing at room temperature (25 °C) and with a RH of 50–60 %. The experimental setup adopted for sensor performance evaluation includes a gas flow controller with a sample holder that allowed the test gas to permeate through the sensor as explained in Fig. S4. The gas injection system was additionally equipped with a Keithley multimeter and dedicated data acquisition software. Initially, the response and recovery saturation time are monitored in presence of 5 ppm CO2. Noticeably, the response saturated after ≈180 sec and remained constant. The response recovered over 96 % in ≈160 sec to attain constancy. The response and recovery saturation conditions are shown in Fig. S5a. Under similar conditions, sensor responses were recorded for different CO2 concentration (Fig. S5b), depicting no dependence on exposure time ascertaining the practicability of the sensor. Following this, to maintain the consistency the response was recorded for 180 s in all experiments. Before new experiment, the system was flushed with synthetic air (CO2-free) to reduce the CO2 concentration to a typical environmental value, and resistance evolution with changing concentrations of test gas was observed under atmospheric conditions for real-time application. Further, the response of the sensor was also observed in a temperature range from 25 °C to 40 °C, considering its practical application for skin sensing with no significant change was observed (Fig. S5c). Fig. 2 a shows the response of the sensors to exposure of 5 ppm CO2 gas. As the CO2 gas passed through the sensor, the response increased and then recovered after flushing the CO2 gas by introducing synthetic air. Compared with the pristine oxSWCNT-based sensor, the oxSWCNT-PANI composite-based sensor showed an almost twofold higher response towards 5 ppm CO2 gas. This result was attributed to the augmented gas adsorption and desorption efficiency owing to stronger interfacial interactions between CO2 and PANI on the surface of the oxSWCNTs. Under ambient conditions, CO2 was easily adsorbed on the surface (oxSWCNTs uniformly grafted with PANI) and partially extracted electrons via charge transfer interactions, thereby increasing the response of the sensor, as shown in the schematic in Fig. 2b. Afterward, the response recovered to the initial value when CO2 was desorbed as the system was flushed with synthetic air. Interestingly, this phenomenon was highly reversible and corresponded to changes in the resistance during subsequent CO2 gas cycles; this behaviour formed the basis of the room temperature reversible sensing mechanism.
Fig. 2.
CO2 gas sensing performance of the fabricated sensor (oxSWCNT-PANI/PP). (a) CO2 gas sensing response of oxSWCNT-PANI/PP and oxSWCNT/PP to 5 ppm CO2 gas. (b) Schematic representation of the CO2 sensing mechanism of the sensor. (c) Response and recovery time of the oxSWCNT-PANI/PP sensor in CO2 gas sensing (5 ppm). (d) Concentration-dependent CO2 gas sensing response of the sensor (oxSWCNT-PANI/PP). (e) Comparative CO2 gas sensing responses of oxSWCNT-PANI/PP and oxSWCNT/PP. The inset shows the changes in the responses of the sensor (oxSWCNT-PANI/PP) at lower and higher CO2 concentrations. (f) CO2 gas sensing performance of the oxSWCNT-PANI/PP sensor at 0, 30 and 60 % strain. (g) Response and recovery time of the sensor (oxSWCNT-PANI/PP) for consecutive CO2 sensing cycles at different strains. (h) Strain-dependent CO2 sensing response of the sensor at different CO2 concentrations. (i) Langmuir and Freundlich equation fits of the concentration-dependent CO2 sensing response at different strains.
Additionally, the quick adsorption–desorption phenomenon might be related to the lower response and recovery times of 120 and 122 s respectively (Fig. 2c), which were even lower than those of NO2 gas reported in the literature [39], [40], [41]. In addition, the sensitivity of the response to CO2 gas was also estimated from the time-dependent CO2 flow (Fig. S5d). The sensor showed a noticeable response even during CO2 gas flow for 10 s, revealing the sensitiveness of the sensor. Hence, the fabricated sensor enabled a quick response to CO2 gas even at room temperature. The response to various CO2 concentrations from 1 ppm to 50 ppm were further tested under similar conditions (Fig. 2d). Importantly, the response magnitudes steadily increased with increasing CO2 concentrations. This can be attributed to the unsaturated state, even at higher concentrations of CO2, which cause the response magnitude to keep increasing. Additionally, the composite showed higher sensitivity than ox-SWCNTs over the entire measured concentration range (Fig. 2e). Remarkably, our sensor detected CO2 at a very low concentration, 1 ppm at room temperature and showed a monotonic increase in response to an increase in gas concentration. The response exhibited two linear regions (inset of Fig. 2e), one at lower concentrations below 5 ppm and the other above 5 ppm, which indicated the stabilizing nature of the response. This phenomenon may have arisen because the CO2 concentration on the exposed area of the fabricated sensor stabilized. Considering the lower concentration region, the theoretical detection limit can be calculated. Detection limit is calculated from standard error (SE) and slope of calibration curve (S) as follows [42]:
| (1) |
For calculating the limit of detection, we used STEYX function of excel to calculate the standard error, which was found to be 0.041. The slope from the allometric equation fitting was 0.8 (described in later section). So, the detection limit was calculated to be 155 ± 15 ppb, which is astonishing as compared to the recently reported article [43]. Furthermore, for the lower CO2 concentration region, the corresponding sensing response can be described by an allometric equation given by
| (2) |
where [CO2] is the concentration of CO2 in the lower region (Fig. S6).
The strain-dependent performance of the sensor when the sensor was in a stretched state was further examined for real-time practical applications; the stretched state is the harshest condition the sensor would experience. The sensor can actually bear a large strain, but for gas sensing, we limited the strain load to 60 % as human body motions usually result in strains of less than 55 % [44]. The response curve (Fig. 2f) showed the response at no strain, along with the responses at 30 % and 60 % strain. Interestingly, the curves obtained with no strain and 30 % strain demonstrated inconsiderable difference, while a significant change was observed at 60 % strain; the performance was almost 40 % higher than that at no strain. This result may be attributed to the varied pores in the PP channel and efficient exposure of the oxSWCNT-PANI composite at higher strain, which correspondingly modifies the interfacial interactions of CO2 gas with the oxSWCNT-PANI/PP surface. The response time decreased with increasing strain loading, whereas the recovery time did not explicitly depend on strain loading (Fig. 2g). These observations were likely due to the varying interactions of CO2 gas with the fabricated sensor under different loaded strains, and the results were in agreement with the Raman spectroscopic analysis discussed earlier. The response time decreased with larger strain; the strain produced more active sites for CO2 adsorption on the sensor’s surface, thereby facilitating adsorption of more gas molecules compared to the no strain condition. The 30 % strain enabled limited exposure of oxSWCNT-PANI in less populated zones, and there was no significant change in response. In contrast, some of these active sites for oxSWCNT-PANI were effectively exposed to CO2 at higher strain and they offered efficient binding and increased recovery time of the device. This can also be explained by analysing the CO2 concentration-dependent response at higher strains (Fig. 2h). At higher CO2 concentrations (larger than 5 ppm), the response behaviour was different from the behaviour previously observed with 5 ppm CO2. The response increased with increasing strain, even at 30 % strain loading. A much larger enhancement in response (∼65 %) was observed for a 50 ppm CO2 concentration at 60 % strain. The responses observed at different CO2 concentrations under unstrained and strained conditions could be explained by using the Langmuir and Freundlich adsorption models. The Langmuir model assumes that monolayer adsorption occurs on homogeneous adsorbent surfaces with identical specific adsorption sites, while the Freundlich model assumes heterogeneous adsorbent surfaces. Fig. 2i shows plots of the response (%) vs CO2 concentration (ppm) for sensors strained at 0, 30 and 60 %; these plots resembled apparent adsorption isotherms and were fitted to Langmuir and Freundlich isotherms (the fitting curves are not shown here). The responses (%) with increasing CO2 concentrations for the 0 and 30 % strained sensors were correlated very well with the Langmuir model based on the R2 values (0.99 and 0.98), indicating monolayer adsorption on homogeneously distributed energetically equivalent adsorption sites. The results can be understood by considering that adsorption of CO2 molecules on the surface followed the Langmuir isotherm. At lower concentrations, the sensor response was linearly related to the CO2 concentration, whereas at higher concentrations, the surface underwent saturation, leading to a saturation response. In contrast, the response (%) of the sensor strained at 60 % to varying CO2 concentrations (ppm) was adequately fitted with the Freundlich model (R2 0.97). This was in agreement with our hypothesis that there are different energetic active sites available at 60 % strain that offer specific binding interactions with CO2 gas [45], [46]. Additionally, the fabricated sensor also displayed high selectivity for CO2 sensing (Fig. S7a). The change in resistance for the oxSWCNT-PANI/PP sensor in the presence of 5 ppm CO2 was very high compared to those seen in the presence of 5 ppm N2, O2, NH3 and acetone. Although the sensitivity to NH3 was approximately half that for CO2, the concentration of skin NH3 gas is four order less than that of skin CO2 gas [47], [48], which endow the oxSWCNT-PANI/PP sensor with high selectivity for real applications. Additionally, there was no considerable effect of humidity on the sensing response of the sensor (oxSWCNT-PANI/PP), as demonstrated in Fig. S7b. The sensing response seen in the presence of 1 ppm CO2 gas was almost similar for different humidity levels except for the response and recovery time. With an increase in humidity, more electrons were available on the surface of the sensor for uptake by CO2 gas molecules leading to shorter response and recovery times. This indicated that no further calibration was required under humid working conditions. In order to analyze the effect at low humidity, the sensor was placed in vacuum initially. Afterward, it was placed in the chamber with 30 % humidity and the response of the sensor was analyzed immediately. Notably, the results showed lesser response at 30 % humidity in the initial stage as compared to 50 % humidity. However, when sensing response was determined after 15 min of placing in the humidity chamber, it showed no significant change in the response compared to 50 % response. This may be associated with the time taken to saturate the surface with water molecules at lower humidity (from vacuum), which is consistent with the proposed mechanism reported in the manuscript.
1.3. Microscopic understanding of the interfacial interactions of CO2 with the present sensor
The interfacial sensing properties of the sensor were investigated at 25 °C and 50 % RH by performing in situ Raman spectroscopic studies on the sensor under unstretched and stretched (60 %) conditions with and without CO2 gas flow (20 ml min−1). The sensor exhibited redshifts in the G-band peak at 1595 cm−1 (Fig. 3 a) and the peak corresponding to PANI in the 1320–1360 cm−1 region upon exposure to CO2 flow (inset of Fig. 3a). This redshift upon exposure to CO2 were attributed to charge transfer from CO2 to the oxSWCNT-PANI/PP sensor. After CO2 was removed from the sensor holder via air flow, both bands shifted back to their original positions (shown as CO2 OFF). Moreover, the band shifts were highly reversible for multiple cycles of exposure to the CO2 atmosphere (Fig. S8). Under 60 % stretching and upon CO2 exposure (Fig. 3b), the sensor exhibited a redshift of 3.8 cm−1 for the band corresponding to PANI, which reverted to its original position upon CO2 removal (shown by CO2 OFF). This result suggested that upon stretching the sensor, PANI functional groups became more accessible for CO2 interactions, resulting in quicker diffusion through the polymer SWCNT matrix (oxSWCNT-PANI) and an efficient response, as discussed earlier.
Fig. 3.
In situ Raman and DRIFT spectroscopic studies and adsorption interaction energetics for the sensor (oxSWCNT-PANI/PP). In-situ Raman spectra of the sensor under unstretched (a) and stretched (60%) (b) conditions in the presence (ON) and absence (OFF) of CO2 gas. The insets show an enlarged region of the PANI peaks (left) and G peak (right). (c) In-situ DRIFT spectra of the sensor before and after exposure to a continuous CO2 flow. (d) Enlarged region of in-situ DRIFT spectra corresponding to C-NH-C of PANI. (e) CO2 adsorption–desorption isotherms for oxSWCNT-PANI and oxSWCNT-PANI with Zn-Al dispersant. (f) Isosteric heats (Qiso) of CO2 adsorption for PANI, oxSWCNTs, oxSWCNT-PANI and the sensor (oxSWCNT-PANI/PP).
The in-situ Raman spectroscopic observations described above suggested that the response of the sensor is governed by specific molecular-level interactions of CO2 with PANI and oxSWCNTs. We also investigated the influence of CO2 and analysed different functional groups present on the sensor by taking advantage of in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFT). The DRIFT analysis chamber allowed us to control the temperature, pressure, atmospheric composition, and other key variables in-situ to maintain the desired conditions for gas sensor analyses. Fig. 3c shows in situ DRIFT spectra of the sensor in the range 1000–4000 cm−1 before and after exposure to a continuous CO2 flow at 20 ml min−1. As expected, the intensity of the gaseous CO2 peak (∼2300 cm−1) [49] quickly rose with increasing CO2 concentration in the DRIFT chamber. In addition, the functional group bands of the sensor exhibited blue shift with simultaneous increases in intensity in response to increases in CO2 concentration. The sensor exhibited functional group bands in five primary regions: C-NH-C at 1138 cm−1, C—N/COO− at 1258 cm−1, benzenoid/quinonoid at 1418 and 1447 cm−1, C C/C O at 1596 and 1759 cm−1 and N—H/O—H at 3590 and 3686 cm−1 (black solid line) [50]. Upon CO2 exposure, the band shifts varied with the nature of the functional groups and increased with increasing CO2 concentration, saturation was attained after 3 min of CO2 exposure (red line). The band at 1138 cm−1 corresponding to the secondary amine (C-NH-C) of PANI demonstrated a maximum upshift of 5 cm−1 (Fig. 3d). After that, the CO2 atmosphere in the DRIFT chamber was evacuated and atmospheric air was allowed to fill the chamber. Notably, the band reversed to the original wavenumber (black dotted line). Other functional groups showed smaller band shifts (Fig. S9) than the secondary amine C-NH-C, suggesting that this amine provided the most favourable interaction site [51], [52]. In the hydroxyl region, the bands at 3590 and 3686 cm−1 corresponded to isolated OH groups [53]; these groups were quite dramatically influenced by the presence of CO2 and the intensities increased with increasing CO2 concentration (Fig. S9). Previously, such species were considered active sites in detection of gases with metal oxide-based sensors [53]. Remarkably, the intensities in this region were significantly decreased after the sensor was washed with acid (3 M HNO3) (Fig. S9). This suggested the role of the Zn-Al dispersant in the oxSWCNT-PANI matrix, which enhanced water adsorption and hence provided CO2 reactive sites and greater sensing response. Once the amount of this Zn-Al dispersant was decreased through acid washing (Fig. S10), the sensing response also decreased (Fig. S10). These results are in agreement with the well-established promoting effect of humidity for CO2 adsorption on amine-based adsorbents [52]. Water molecules alter the response mechanism, as both secondary amines present in the PANI backbone and CO2 interact first with water before interacting with each other and this result in energy-efficient CO2 capture and release [52]. Reversibility is further confirmed by the CO2 adsorption–desorption isotherms (Fig. 3e) for the oxSWCNT-PANI composite and the oxSWCNT-PANI composite with Zn-Al dispersant (oxSWCNT-PANI (Zn-Al)). Although the CO2 adsorption capacity of oxSWCNT-PANI (Zn-Al) decreased due to pore blocking, it exhibited complete reversibility in the adsorption and desorption branches. In contrast, oxSWCNT-PANI displayed an evident hysteresis loop, signifying irreversibility in the uptake and release of CO2. This can be further understood from the corresponding isosteric heat of adsorption (∼30 kJ mol−1) for oxSWCNT-PANI, which was calculated as reported in our previous article [54]; the heat of adsorption decreased with increasing CO2 adsorption (Fig. 3f). The isosteric heat of adsorption for the oxSWCNT-PANI (Zn-Al) sensor was approximately-one-half (≈ 16 kJ mol−1), of that for the composite without the Zn-Al dispersant. The corresponding heat of CO2 adsorption was comparable with the lowest measured value reported for a solid adsorbent [51], [55], [56]. The small heat of CO2 adsorption by oxSWCNT-PANI (Zn-Al), which is based primarily on secondary amine wrapped SWCNTs, leads to reversible cycling of CO2 uptake and release. Additionally, the presence of secondary amines and the Zn-Al dispersant as spacers could assist in reducing the regeneration energy [51].
1.4. Electromechanical performance and permeability of the sensor
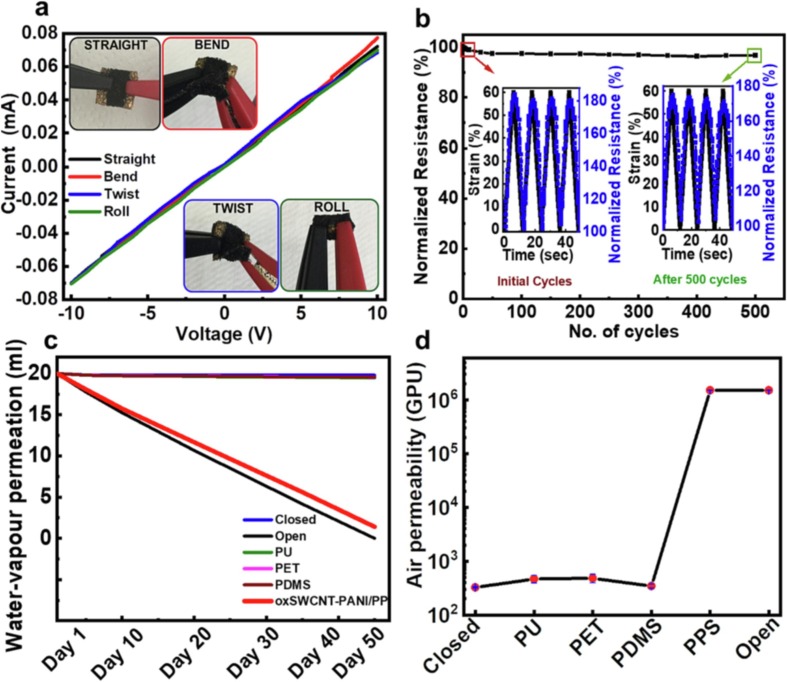
Flexibility and stretchability are highly desired properties for transcutaneous sensors, since the elasticity of human skin varies considerably with location, age, hydration and typical deformations ranging up to 30 %–60 % [57], [58]. To investigate the effects of mechanical deformations on the electrical resistance of the oxSWCNT-PANI/PP sensor, its current–voltage (I-V) characteristics were recorded (Fig. 4 a). Under different degrees of deformity, such as with bending, twisting and rolling, the I-V characteristics, which showed ohmic behaviour, remained invariable. Furthermore, the sensor could bear a maximum tensile strain of up to 140 %, which is comparable to that of pristine PP (Fig. S11). The developed sensor was then subjected to 60 % strain for 500 cycles by using a stretching machine operating at a rate of 1 mm s−1 and the changes in resistance were monitored. The changes in resistance with strain during loading and unloading cycles exhibited a monotonic trend and linearly increased as strain was applied (inset of Fig. 4b). This one-to-one correspondence between the resistance output and the applied strain facilitates sensor software development for effective detection of transcutaneous CO2 gas monitoring. Remarkably, even after 500 strain loading and unloading cycles, the resistance output showed no considerable change, as demonstrated by the resistance stability observed during cycling (Fig. 4b). Previous research suggested that weak adhesion between the thin film and flexible substrate of a sensor leads to fracture, slip, or delamination of the thin film, even with a tensile strain of ∼1% [57]. However, the present sensor morphology and integrity remained similar after 500 loading and unloading cycles at 60 % tensile strain, as seen from SEM micrographs of the sensor taken before and after cycling (Fig. S12). This result suggested efficient tensile load sharing and transfer ability for the present oxSWCNT-PANI/PP architecture, which accommodated deformations and maintained mechanical robustness due to strain delocalization. Such robustness of the active sensing material on the substrate should significantly improve the long-term stability and reliability of the sensor.
Fig. 4.
Electromechanical properties, deformation stability, and permeability of the sensor (oxSWCNT-PANI/PP). (a) Current (I)-voltage (V) characteristics of the sensor under different conditions. The insets show photographs of the sensor during bending, twisting, and rolling of the sensor. (b) Cycling stability of the sensor during 500 cycles at 60 % strain. The insets show time vs strain curves and the corresponding resistance changes of the sensor before and after cycling. (c) Time-dependent quantitative water-vapour permeation for different substrates attached to a vial cap (10 mm dia.) along with closed and open conditions for 50 days: polyurethane (PU), polyethylene terephthalate (PET), polydimethylsiloxane (PDMS) and the sensor (oxSWCNT-PANI/PP, PPS). (d) Air permeabilities of different substrates and our sensor along with closed and open conditions.
The present sensor also demonstrated high permeability for water vapour and air; this can be advantageous because it prevents sweat or gas accumulation at the skin-sensor interface, which alleviates discomfort and inflammation [59], [60]. Here, we determined water vapour permeation rates under physiological conditions of 37 °C and 50–60 % RH by quantifying the volume of water lost with test samples attached to the 10 mm (dia.) opening of a vial cap on a vial containing 20 ml of water (Fig. S13). In addition, we tested the most extensively used flexible substrates, including polyurethane (PU), polyethylene terephthalate (PET), nonporous PDMS, and compared them with the closed, open and oxSWCNT-PANI/PP systems. Notably, the water vapour permeability of oxSWCNT-PANI/PP was ≈ 0.13 ± 0.004 ml cm−2 day−1 (Fig. 4c), which is approximately 50 times higher than those of PU, PET and nonporous PDMS (≈ 0.003 ± 0.001 ml cm−2 day−1). In addition, the sensor also exhibited an excellent air permeability of 1.5 × 106 GPU (Fig. 4d), determined by using a laboratory designed air permeation measurement instrument (described in Fig. S13 text) [61] and was as good as the open system; this result was determined from the drop in pressure as a function of time (Fig. S13d). Other substrate permeabilities remained in the range of 102 GPU and were similar to that of the closed system. This excellent water vapour and air permeability confirmed the high respirability of the fabricated oxSWCNT-PANI/PP sensor, which was similar to that of the open system. Based on the above analyses, a real-time sensor (oxSWCNT-PANI/PP) has been realized and validated to substantiate the applicability of the fabricated sensor in a real environment.
1.5. Real-Time application
As a proof of concept, the potential of the sensor for continuous noninvasive transcutaneous monitoring of CO2 was analysed. In the real-time experiment, the sensor was affixed in a Teflon cell with a circular opening (dia. 1 cm) in the middle (Fig. 5 a 1) and connected to a Keithley multimeter to record changes in the resistance of the sensor. The CO2 released from the fingertip was precisely monitored by recording the corresponding resistance change. During the initial 60 s, the sensor displayed stable resistance, as evident from the plot (Fig. 5a). The resistance changed sharply when a finger was lightly placed blocking the hole of Teflon cell (Fig. 5a 2); this resulted in an increased sensing response, as shown in the orange region in the plot (Fig. 5a). The resistance eventually returned to the initial level when the finger was removed, implying that the response of the sensor was reversible and sensitive. Furthermore, we shielded the sensor with a metal block (Fig. 5a 3) to analyse the effects of masking the sensor with a non-CO2 releasing material. As expected, there was no change in the resistance of the sensor during metal shielding, as shown by the green-coloured region in the plot (Fig. 5a). Remarkably, a precise and repeatable response was obtained (except for some disturbances caused by finger fatigue) during consecutive application cycles.
Fig. 5.
Real-time application of the sensor. (a) Photograph of the sensor in the Teflon cell only (1) with a finger (2) and with a metal shield (3) during CO2 gas monitoring of the finger. Transcutaneous sensing of CO2 released from the first finger of the right hand corresponding to settings 1, 2 and 3 (shown in the coloured regions). (b) Response of the sensor to CO2 gas released from the wrist when the sensor is placed directly on the skin. (c) Response when the sensor is kept in a Teflon cell with a circular opening in the middle (wrist band). The inset shows the arrangement of the sensor on the wrist.
Similar monitoring was also observed when the CO2 sensor was placed directly on the wrist (Fig. 5b). To prevent the mounted sensor from slipping, it was attached to surgical tape and connected to the Keithley multimeter and a temperature sensor (inset of Fig. 5b). Initially, the sensor was placed in an open environment and monitored for extended time, as shown in Fig. S14. In the presence of environmental CO2, the sensor displayed an almost stable resistance, as shown in the inset images (Fig. S14). Furthermore, after a few minutes, the response stabilized when the sensor was in direct contact with the skin and showed slight variations owing to uncomfortable fatigue of the wrist. Remarkably, the sensor was used continuously for approximately 30 min with stable behaviour, revealing the reliability of the fabricated sensor. These results indicate that our sensor can detect transcutaneous CO2 gas under physiological conditions with an efficient on-skin interface.
Under certain circumstances, when the sensor cannot be placed in direct contact with the skin, it will work reliably when placed in the vicinity of the desired CO2 monitoring region. The results obtained in Fig. 5c demonstrate a situation in which the sensor was placed in a Teflon cell with a circular opening (dia. 1 cm) as shown in the inset of Fig. 5c. Initially, the sensing signal increased quickly as CO2 diffused from the skin to the sensor, and then the signal stabilized as concentration equilibrium was established. The stabilized sensing response to CO2 gas released from the wrist was observed in ∼ 1 min and remained stable for 30 min of continuous detection.
1.6. Clinical transcutaneous CO2 measurements
For practical validation, we conducted a prospective observational study on cohort of patients. Here we showed the data observed for two volunteers, a clinically stable patient (normal volunteer) and another volunteer diagnosed with obstructive sleep apnea. Their transcutaneous CO2 measurements were made simultaneously with the TCM TOSCA monitor (commercially available) attached to an ear lobe and our sensor tied on a wrist, as shown in Fig. 6 . The data observed by our sensor obtained after background correction using the stable response of the sensor in ambient environment for an extended period, were processed through a smoothing filter to obtain noise-free output. The normal volunteer was subjected to a steady exercise load with a treadmill (Fig. 6a), and the corresponding measurements were recorded. The data obtained by TCM were in pCO2 (mm Hg), and our sensor output was in terms of the sensor’s response. To compare the results, the sensor response can be converted to the concentration of CO2 (ppm) with a calibration equation described in a later section. The pattern for pCO2 in mm Hg (TCM) and ppm (our sensor) can be compared as a common factor will be computed. Further work is intended to characterize this association during use of the sensor for transcutaneous CO2 gas measurement in future testing. In the near term, the data will offer a method for comparing our sensor and a commercially available TCM TOSCA monitor to define probable consequences. The TCM data showed a sudden rise occurring 10 s after an increase in exercise on the treadmill (Fig. 6b). After 1 min of continuous exercise (region A marked with green colour), the volunteer relaxed for 1 min (region B). Subsequently, the gradual increases in exercise followed a staircase pattern. Consistent with the TCM data, our sensor exhibited an initial small peak and later, detected increment in the sensor response (peaks in region A). Even in the almost relaxed state of the volunteer, when the TCM data were responding steadily (region B), our sensor depicted the minute perturbations in form of small peaks, indicating the high sensitivity of our sensor. Additionally, the presence of supplemental oxygen during the exercise load (Fig. 6c) did not affect the performance of our sensor, as observed in Fig. 6d. The volunteer began exercise after 50 s (green coloured region, A) during which TCM data and our sensor showed almost no change followed by increments in the responses of both. It is important to note that stabilization in the TCM data (region B) corresponding to the exercise load for the volunteer appeared as desaturation in the sensor response. Subsequently, our sensor illustrated faster resaturation with an increase in TCM data during further exercise load. Importantly, the sensor was also used by a patient diagnosed with obstructive sleep apnea where interrupted breathing is more apparent in night. Notably, our sensor can be used for unsupervised continuous measurement without any discomfort (Fig. 6e). Here, strong concurrence was also noticed in the data produced by TCM and our sensor, as shown in Fig. 6f. TCM data peaks were observed in response to irregular breathing (green coloured, region A), whereas our sensor showed a sensitive response with a small peak increment followed by a sharp decline corresponding to decreasing TCM data (region B). This stepwise increment in the room temperature response of our sensor (green coloured, region A) unlike that of TCM (operating at high temperature) illustrates the potential of our sensor, as relevant information can be extracted from these peaks representing bodily health status in future applications. Concerns regarding the heating of the probes during transcutaneous CO2 monitoring (which may cause cutaneous blistering when used for prolonged periods) no longer apply. We encountered no safety issues associated with the use of our sensor for continuous monitoring over an extended time. Furthermore, the advantages of our sensor over existing transcutaneous CO2 monitoring methods are depicted in Fig. 6g. Although a comparison to CO2 measurement techniques is difficult owing to incomplete referenced works, we tried our best to provide the related information.
Fig. 6.
Comparative performance of our sensor with a commercially available TCM TOSCA monitor. (a) Schematic showing a normal volunteer subjected to a steady exercise load on a treadmill. (b) Corresponding measurements of transcutaneous CO2 recorded by the TCM TOSCA monitor (black colour) and our sensor (red colour). (c) Schematic showing a normal volunteer with a steady exercise load on a treadmill in the presence of supplemental oxygen. (d) Corresponding measurements of CO2 concentration with a TCM TOSCA monitor (black colour) and our sensor (red colour). (e) Schematic showing observation of a volunteer with obstructive sleep apnea. (f) Measurements of CO2 concentration by a TCM TOSCA monitor (black colour) and our sensor (red colour). (g) Table depicting the advantages of our sensor compared with the TCM TOSCA monitor. *End-tidal measurements are used for monitoring CO2 from the breath unlike TOSCA monitor and our sensor (transcutaneous CO2 monitoring). It is compared here since commonly used in medical field.
2. Discussion
The trial test on the volunteers demonstrates the feasibility of our transcutaneous CO2 gas sensor for quantitative indication of ventilation status of the patients and neonates. The fabricated CO2 sensing device comprising silicone elastomer sponges impregnated with a PANI-wrapped oxSWCNT-based composite (oxSWCNT-PANI) offers the potential for noninvasive transcutaneous CO2 monitoring with simplicity of user interface. The accessibility to continuous and high-quality data expands the utility range to unsupervised monitoring leading to high possibility of futuristic remote monitoring. The reversible sensing response and resistance to interference of other gases (N2, O2, NH3 and acetone) endow the potential to provide ventilation indicators. Another important outcome demonstrated the capability of the device under stretching conditions, which has been elucidated for the first time. The stable and reliable response under a large strain of 60 % reveal the outstanding ability of the fabricated sensor, minimizing the disturbance to the patients, especially neonates. Additionally, its excellent gas and water-vapour permeabilities of 1.5 × 106 GPU and 0.13 ml cm−2 day−1 indicate high respirability leading to long-term durability. Hence, the sensor can be applicable to monitoring of the CO2 levels in expiration gas. More importantly, we believe our findings of transcutaneous CO2 observed by our sensor operating at room temperature to be similar to that of commercially available TCM TOSCA monitor (operated at elevated temperature), enable reliable estimates at user-defined location. Nevertheless, despite this small cohort of patients, trends observed by our sensor were found to be significant. This portable, user-operable and noninvasive transcutaneous CO2 sensor will open new horizons for clinical use and broad scalability.
3. Materials and methods
3.1. Porous PDMS (PP)
A porous PDMS sponge (PP) was prepared by using a sugar cube (1 cm × 1 cm × 1 cm) as the template [62]. First, the elastomer, PDMS and crosslinker (Dow Corning Toray) were mixed (10:1) and vacuum treated to remove trapped air bubbles. Then the mixture was placed in a petridish and sugar was added to the mixture, which was set aside for 2 h to allow the mixture to infiltrate the sugar by capillary force. The samples were further cured at 60 °C for 2 h to obtain PDMS-infiltrated sugar. The obtained sample was then scraped on all sides to remove excess PDMS and was then subjected to ultrasonic treatment in hot water to dissolve the sugar. The resulting PP was dried overnight in an oven at 80 °C and cut into thin pieces with dimensions of 1 cm × 1 cm × 0.2 cm.
3.2. oxSWCNT-PANI composite
Air-oxidized SWCNTs (oxSWCNTs) were synthesized by following a previously reported procedure [36]. oxSWCNT (45 mg) was placed in a conical flask along with solution of protonated aniline (10 mM) in 50 ml deionized water and ultrasonicated for 30 min. Then, 20 mM ammonium persulfate (APS) was added to the above solution and continuously stirred at 0–5 °C. Afterwards, stirring was continued for an additional 3 h under the same conditions. The precipitated product (oxSWCNT-PANI) was then filtered and dried at 80 °C in an oven for 2 h.
3.3. oxSWCNT-PANI ink
The oxSWCNT-PANI composite was mixed with Zn-Al dispersant to formulate the ink in deionized water as described in a previous report [63]. Briefly, 20 mg of the composite was mixed with 100 mg of the Zn-Al dispersant in 20 ml of DI water followed by ultrasonication for 30 min using a homogenizer tip (SONIC vS 505). The resulting ink was then utilized as an active coating for the stretchable matrix (PP). oxSWCNT ink was also prepared for comparison.
3.4. Sensor fabrication (oxSWCNT-PANI/PP)
The obtained PP (1 cm × 1 cm × 0.2 cm) was dipped in oxSWCNT-PANI ink for 10 min and dried at 70 °C for 1 h. The same procedure was followed four times to obtain uniform oxSWCNT-PANI coated PP with a resistivity of 620 ± 110 O cm. The total weight of the oxSWCNT-PANI coated on PP and the corresponding resistivity can be controlled by simply adjusting the number of dipping-drying cycles. The sensor was also fabricated with oxSWCNT ink (oxSWCNT/PP) to compare the gas sensing results.
3.5. Characterization
The surface morphologies of the prepared samples were characterized with Hitachi SU8000 cold field emission scanning electron microscope (FE-SEM). Scanning transmission electron microscopy (STEM) was performed on a JEOL ltd. microscope (ARM-200CF) with an accelerating voltage of 120 kV. The samples were dispersed by sonication in isopropanol and deposited on a holey carbon TEM grid with drying prior to the examination. Diffuse reflectance infrared Fourier transform spectroscopy (DRIFT) analyses were performed with JASCO FT/IR-6700 spectrometer equipped with a DLaTGS (with Peltier temperature control) detector with a maximum resolution of 0.25 cm−1 and operated under N2 purging. The sensing electrode samples were placed in a sample cup, and the data were collected. In situ Raman spectroscopy (Jasco Laser Raman Spectrometer, NRS-4100) via optical microscopy was used to record Raman spectra in the absence and presence of CO2 flow under relaxed and stretched conditions. X-ray photoelectron spectroscopy (XPS) analyses were conducted by using a JPS-9200, JEOL spectrometer equipped with a polychromatic Mg Kα X-ray source. The water vapour permeation rates under physiological conditions, 37 °C and 50–60 % RH were evaluated by quantifying the volume of water lost, which was done by attaching the samples to vial caps with openings measuring 10 mm in diameter on vials containing 20 ml of water. The air permeability was measured by using a lab designed gas permeation instrument. All tested samples had similar thicknesses. The adsorption isotherms of the samples were measured using a Microtrac MRB apparatus (BELSORB MAX) after pretreatment at 60 °C for 3 h. The mechanical properties and deformation of the fabricated sensor were measured with an automated instrument (EZ Test, EZ-SX, Shimadzu), and the corresponding changes in the electrical resistance of the sensor were measured using a Keithley multimeter (DMM7510 7 ½ Digit Multimeter) attached to the automated instrument. A lab assembled measurement system was used to evaluate the gas-sensing performance of the fabricated sensor. Test gases (CO2, N2, O2, NH3 and acetone) with controlled concentrations were fed into the sample cell for specific time periods, and the corresponding changes in electrical resistance were recorded with a Keithley multimeter. The response of the sensor was calculated with the following equation:
| (3) |
where Rair and Rtest gas are the electrical resistances values of the fabricated sensor in the presence of air and the test gas, respectively. For practical validation, the responses observed by the sensors were compared with those of a commercially available TCM TOSCA monitor for CO2 measurements.
Different parameters (which affected the CO2 gas sensing performance) such as temperature, pressure, exposure duration and operating conditions, were held constant during this study, unless stated.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by Japan Science Technology Agency (JST) Open Innovation Platform with Enterprise, Research Institute and Academia (OPERA)- (JPMJOP1722), TAKAGI Co., Ltd. and NIH grant #1 R56EB033801-01. The research was approved by the Office for Research Protections and Compliance of University of Maryland Baltimore County (Protocol #: Y16XG05263). The experimental subjects were informed about the test and its characteristics and gave consent for use of the data.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cej.2022.141260.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- 1.Verschuur J., Koks E.E., Hall J.W. Observed impacts of the COVID-19 pandemic on global trade. Nat. Hum. Behav. 2021;5:305–307. doi: 10.1038/s41562-021-01060-5. [DOI] [PubMed] [Google Scholar]
- 2.Galanakis C.M., Rizou M., Aldawoud T.M.S., Ucak I., Rowan N.J. Innovations and technology disruptions in the food sector within the COVID-19 pandemic and post-lockdown era. Trends Food Sci. Technol. 2021;110:193–200. doi: 10.1016/j.tifs.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blot M., Jacquier M., Manoha C., Piroth L., Charles P.-E. Immunomodulation in coronavirus disease, (COVID-19): strategic considerations for personalized therapeutic intervention. Clin. Infect. Dis. 2019;72(2021):e446–e447. doi: 10.1093/cid/ciaa904. [DOI] [Google Scholar]
- 4.Montenegro F., Unigarro L., Paredes G., Moya T., Romero A., Torres L., López J.C., González F.E.J., Del Pozo G., López-Cortés A., Diaz A.M., Vasconez E., Cevallos-Robalino D., Lister A., Ortiz-Prado E. Acute respiratory distress syndrome (ARDS) caused by the novel coronavirus disease (COVID-19): a practical comprehensive literature review. Expert Rev. Respir. Med. 2021;15:183–195. doi: 10.1080/17476348.2020.1820329. [DOI] [PubMed] [Google Scholar]
- 5.Ottestad W., Søvik S. COVID-19 patients with respiratory failure: what can we learn from aviation medicine? Br. J. Anaesth. 2020;125:e280–e281. doi: 10.1016/j.bja.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubortone S.A., De Carolis M.P., Lacerenza S., Bersani I., Occhipinti F., Romagnoli C. Use of a combined SpO2/PtcCO2 sensor in the delivery room. Sensors. 2012;12:10980–10989. doi: 10.3390/s120810980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernet-Buettiker V., Ugarte M.J., Frey B., Hug M.I., Baenziger O., Weiss M. Evaluation of a new combined transcutaneous measurement of PCO2/pulse oximetry oxygen saturation ear sensor in newborn patients. Pediatrics. 2005;115:e64–e68. doi: 10.1542/peds.2004-0946. [DOI] [PubMed] [Google Scholar]
- 8.Hu D., Li J., Gao R., Wang S., Li Q., Chen S., Huang J., Huang Y., Li M., Long W., Liu Z., Guo L., Wu X. Decreased CO2 levels as indicators of possible mechanical ventilation-induced hyperventilation in COVID-19 patients: a retrospective analysis. Front. Public Heal. 2021;8:1–9. doi: 10.3389/fpubh.2020.596168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uslu S., Bulbul A., Dursun M., Zubarioglu U., Turkoglu E., Guran O. Agreement of mixed venous carbon dioxide tension (PvCO2) and transcutaneous carbon dioxide (PtCO2) measurements in ventilated infants. Iran. J. Pediatr. 2015;25:e184. doi: 10.5812/ijp.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merritt T.A., Liyamasawad S., Boettrich C., Brooks J.G. Skin-surface CO2 and term infants in sick preterm. J. Pediatr. 1981;99:782–786. doi: 10.1016/S0022-3476(81)80411-8. [DOI] [PubMed] [Google Scholar]
- 11.Cao W., Duan Y. Breath analysis: potential for clinical diagnosis and exposure assessment. Clin. Chem. 2006;52:800–811. doi: 10.1373/clinchem.2005.063545. [DOI] [PubMed] [Google Scholar]
- 12.Pavlou A.K., Turner A.P.F. Sniffing out the truth: clinical diagnosis using the electronic nose. Clin. Chem. Lab. Med. 2000;38:99–112. doi: 10.1515/CCLM.2000.016. [DOI] [PubMed] [Google Scholar]
- 13.Arakawa T., Suzuki T., Tsujii M., Iitani K., Chien P.J., Ye M., Toma K., Iwasaki Y., Mitsubayashi K. Real-time monitoring of skin ethanol gas by a high-sensitivity gas phase biosensor (bio-sniffer) for the non-invasive evaluation of volatile blood compounds. Biosens. Bioelectron. 2019;129:245–253. doi: 10.1016/j.bios.2018.09.070. [DOI] [PubMed] [Google Scholar]
- 14.Emmanuel D., Michael T., Uhring W. Carbon dioxide sensing-biomedical applications to human subjects. Sensors. 2022;22:188. doi: 10.1017/cbo9781139174176.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mulmi S., Thangadurai V. Solid-state electrochemical carbon dioxide sensors: fundamentals, materials and applications. J. Electrochem. Soc. 2020;167 doi: 10.1149/1945-7111/ab67a9. [DOI] [Google Scholar]
- 16.Rossman H., Meir T., Somer J., Shilo S., Gutman R., Ben Arie A., Segal E., Shalit U., Gorfine M. Hospital load and increased COVID-19 related mortality in Israel. Nat. Commun. 2021;12:1–7. doi: 10.1038/s41467-021-22214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asch D.A., Sheils N.E., Islam M.N., Chen Y., Werner R.M., Buresh J., Doshi J.A. Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern. Med. 2021;181:471–478. doi: 10.1001/jamainternmed.2020.8193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans G.P., Powell M.J., Johnson I.D., Howard D.P., Bauer D., Darr J.A., Parkin I.P. Room temperature vanadium dioxide-carbon nanotube gas sensors made via continuous hydrothermal flow synthesis. Sensors Actuators B Chem. 2018;255:1119–1129. doi: 10.1016/j.snb.2017.07.152. [DOI] [Google Scholar]
- 19.Rahimabady M., Tan C.Y., Tan S.Y., Chen S., Zhang L., Chen Y.F., Yao K., Zang K., Humbert A., Soccol D., Bolt M. Sensors and actuators B: chemical dielectric nanocomposite of diphenylethylenediamine and P-type multi-walled carbon nanotube for capacitive carbon dioxide sensors. Sensors Actuators B Chem. 2017;243:596–601. doi: 10.1016/j.snb.2016.12.023. [DOI] [Google Scholar]
- 20.Kazemi Y., Kakroodi A.R., Wang S., Ameli A., Filleter T., Petra P., Park C.B. Conductive network formation and destruction in polypropylene/carbon nanotube composites via crystal control using supercritical carbon dioxide. Polymer. 2017;129:179–188. doi: 10.1016/j.polymer.2017.09.056. [DOI] [Google Scholar]
- 21.Korotcenkov G., Cho B.K. Metal oxide composites in conductometric gas sensors: achievements and challenges. Sensors Actuators B Chem. 2017;244:182–210. doi: 10.1016/j.snb.2016.12.117. [DOI] [Google Scholar]
- 22.Yu J., Liu H., Song S., Wang Y., Tsiakaras P. Electrochemical reduction of carbon dioxide at nanostructured SnO2/carbon aerogels: the effect of tin oxide content on the catalytic activity and formate selectivity. Appl. Catal. A, Gen. 2017;545:159–166. doi: 10.1016/j.apcata.2017.07.043. [DOI] [Google Scholar]
- 23.Bhadra J., Al-Thani N.J., Madi N.K., Al-maadeed M.A. Preparation and characterization of chemically synthesized polyaniline-polystyrene blends as a carbon dioxide gas sensor. Synth. Met. 2013;181:27–36. doi: 10.1016/j.synthmet.2013.07.026. [DOI] [Google Scholar]
- 24.Irimia-vladu M., Fergus J.W. Suitability of emeraldine base polyaniline-PVA composite film for carbon dioxide sensing. Synth. Met. 2006;156:1401–1407. doi: 10.1016/j.synthmet.2006.11.005. [DOI] [Google Scholar]
- 25.Lin Z., Young S., Chang S. CO2 gas sensors based on carbon nanotube thin films using a simple transfer method on flexible substrate. IEEE Sens. J. 2015;15:7017–7020. doi: 10.1109/JSEN.2015.2472968. [DOI] [Google Scholar]
- 26.Abdellah A., Abdelhalim A., Loghin F., Köhler P., Ahmad Z., Scarpa G., Lugli P. Flexible carbon nanotube based gas sensors fabricated by large-scale spray deposition. IEEE Sens. 2013;13:4014–4021. doi: 10.1109/JSEN.2013.2265775. [DOI] [Google Scholar]
- 27.Ong K.G., Grimes C.A. A carbon nanotube-based sensor for CO2 monitoring. Sensors. 2001;1:193–205. doi: 10.3390/s10600193. [DOI] [Google Scholar]
- 28.Ishihara T., Kometani K., Nishi Y., Takita Y. Improved sensitivity of CuO-BaTiO3 capacitive-type by additives. Sensors Actuators B Chem. 1995;28:49–54. doi: 10.1016/0925-4005(94)01539-T. [DOI] [Google Scholar]
- 29.Liu Y., Chen Y., Fan W., Cao P., Yan J., Zhao X., Dong W., Huang W. Mechanical distension induces serotonin release from intestine as revealed by stretchable electrochemical sensing. Angew. Chemie. Int. Ed. 2020;59:4075–4081. doi: 10.1002/anie.201913953. [DOI] [PubMed] [Google Scholar]
- 30.Tit N., Al Ezzi M.M., Abdullah H.M., Yusupov M., Kouser S., Bahlouli H., Yamani Z.H. Detection of CO2 using CNT-based sensors: Role of Fe catalyst on sensitivity and selectivity. Mater. Chem. Phys. 2017;186:353–364. doi: 10.1016/j.matchemphys.2016.11.006. [DOI] [Google Scholar]
- 31.Meyyappan M. Carbon nanotube-based chemical sensors. Small. 2016;12:2118–2129. doi: 10.1002/smll.201502555. [DOI] [PubMed] [Google Scholar]
- 32.Schroeder V., Savagatrup S., He M., Lin S., Swager T.M. Carbon nanotube chemical sensors. Chem. Rev. 2019;119:599–663. doi: 10.1021/acs.chemrev.8b00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Star A., Han T.-R., Joshi V., Gabriel J.P., Grüner G. Nanoelectronic carbon dioxide sensors. Adv. Mater. 2004;16:2049–2052. doi: 10.1002/adma.200400322. [DOI] [Google Scholar]
- 34.Peyravi M. Synthesis of nitrogen doped activated carbon/polyaniline material for CO2 adsorption. Polym. Adv. Technol. 2018;29:319–328. doi: 10.1002/pat.4117. [DOI] [Google Scholar]
- 35.Kutorglo E.M., Hassouna F., Beltzung A., Kopecký D., Sedlářová I., Šoóš M. Nitrogen-rich hierarchically porous polyaniline-based adsorbents for carbon dioxide (CO2) capture. Chem. Eng. J. 2019;360:1199–1212. doi: 10.1016/j.cej.2018.10.133. [DOI] [Google Scholar]
- 36.Ahuja P., Ujjain S.K., Urita K., Furuse A., Moriguchi I., Kaneko K. Chemically and mechanically robust SWCNT based strain sensor with monotonous piezoresistive response for infrastructure monitoring. Chem. Eng. J. 2020;388 doi: 10.1016/j.cej.2020.124174. [DOI] [Google Scholar]
- 37.Kukobat R., Hayashi T., Matsuda T., Sunaga M., Sakai T., Futamura R., Kaneko K. Zn/Al complex-SWCNT ink for transparent and conducting homogeneous films by scalable bar coating method. Chem. Phys. Lett. 2016;650:113–118. doi: 10.1016/j.cplett.2016.02.049. [DOI] [Google Scholar]
- 38.Ahuja P., Akiyama S., Ujjain S.K., Kukobat R., Vallejos-Burgos F., Futamura R., Hayashi T., Kimura M., Tomanek D., Kaneko K. A water-resilient carbon nanotube based strain sensor for monitoring structural integrity. J. Mater. Chem. A. 2019;7:19996–20005. doi: 10.1039/c9ta06810d. [DOI] [Google Scholar]
- 39.Yun Y.J., Kim D.Y., Hong W.G., Ha D.H., Jun Y., Lee H.K. Highly stretchable, mechanically stable, and weavable reduced graphene oxide yarn with high NO2 sensitivity for wearable gas sensors. RSC Adv. 2018;8:7615–7621. doi: 10.1039/c7ra12760j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li W., Chen R., Qi W., Cai L., Sun Y., Sun M., Li C., Yang X., Xiang L., Xie D., Ren T. Reduced graphene oxide/mesoporous ZnO NSs hybrid fibers for flexible, stretchable, twisted, and wearable NO2 E - textile gas sensor. ACS Sensors. 2019;4:2809–2818. doi: 10.1021/acssensors.9b01509. [DOI] [PubMed] [Google Scholar]
- 41.Luan Y., Zhang S., Nguyen T.H., Yang W., Noh J.-S. Chemical Polyurethane sponges decorated with reduced graphene oxide and silver nanowires for highly stretchable gas sensors. Sensors Actuators B Chem. 2018;265:609–616. doi: 10.1016/j.snb.2018.03.114. [DOI] [Google Scholar]
- 42.Mu J., Yu L.L., Wellems T.E. Sensitive immunoassay detection of plasmodium lactate dehydrogenase by inductively coupled plasma mass spectrometry. Front. Cell. Infect. Microbiol. 2021;10:1–7. doi: 10.3389/fcimb.2020.620419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Escobedo P., Fernandez-Ramos M.D., Lopez-Ruiz N., Moyano-Rodriguez O., Martinez-Olmos A., Pérez de Vargas-Sansalvador I.M., Carvajal M.A., Capitan-Vallvey L.F., Palma A.J. Smart facemask for wireless CO2 monitoring. Nat. Commun. 2022;13:1–12. doi: 10.1038/s41467-021-27733-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liao X., Zhang Z., Kang Z., Gao F., Liao Q., Zhang Y. Ultrasensitive and stretchable resistive strain sensors designed for wearable electronics. Mater. Horiz. 2017;4:502–510. doi: 10.1039/C7MH00071E. [DOI] [Google Scholar]
- 45.Avila M., Burks T., Akhtar F., Göthelid M., Lansåker P.C., Toprak M.S., Muhammed M., Uheida A. Surface functionalized nanofibers for the removal of chromium (VI) from aqueous solutions. Chem. Eng. J. 2014;245:201–209. doi: 10.1016/j.cej.2014.02.034. [DOI] [Google Scholar]
- 46.Soares J.C., Soares A.C., Pereira P.A.R., Rodrigues V.d.C., Shimizu F.M., Melendez M.E., Scapulatempo Neto C., Carvalho A.L., Leite F.L., Machado S.A.S., Oliveira O.N. Adsorption according to the Langmuir – Freundlich model is the detection mechanism of the antigen p53 for early diagnosis of cancer. Phys. Chem. Chem. Phys. 2016;18(12):8412–8418. doi: 10.1039/c5cp07121f. [DOI] [PubMed] [Google Scholar]
- 47.Li M., Weschler C.J., Bekö G., Wargocki P., Lucic G., Williams J. Human ammonia emission rates under various indoor environmental conditions. Environ. Sci. Technol. 2020;54:5419–5428. doi: 10.1021/acs.est.0c00094. [DOI] [PubMed] [Google Scholar]
- 48.Carlson D.A., Schreck C.E., Brenner R.J. Carbon dioxide released from human skin: effect of temperature and insect repellents. J. Med. Entomol. 1992;29:165–170. doi: 10.1093/jmedent/29.2.165. [DOI] [PubMed] [Google Scholar]
- 49.Veyrié D., Lellouchi D., Roux J.L., Pressecq F., Tetelin A., Pellet C. FTIR spectroscopy for the hermeticity assessment of micro-cavities. Microelectron. Reliab. 2005;45:1764–1769. doi: 10.1016/j.microrel.2005.07.091. [DOI] [Google Scholar]
- 50.Konyushenko E.N., Stejskal J., Trchova M., Hradil J., Kovarova J., Prokes J., Cieslar M., Hwang J.-Y., Chen K.-H., Sapurina I. Multi-wall carbon nanotubes coated with polyaniline. Polymer. 2006;47:5715–5723. doi: 10.1016/j.polymer.2006.05.059. [DOI] [Google Scholar]
- 51.Deng M., Park H.G. Spacer-assisted amine-coiled carbon nanotubes for CO2 capture. Langmuir. 2019;35:4453–4459. doi: 10.1021/acs.langmuir.8b03980. [DOI] [PubMed] [Google Scholar]
- 52.Liu S., Gao H., He C., Liang Z. Experimental evaluation of highly efficient primary and secondary amines with lower energy by a novel method for post-combustion CO2 capture. Appl. Energy. 2019;233–234:443–452. doi: 10.1016/j.apenergy.2018.10.031. [DOI] [Google Scholar]
- 53.Emiroglu S., Bârsan N., Weimar U., Hoffmann V. In situ diffuse reflectance infrared spectroscopy study of CO adsorption on SnO2. Thin Solid Films. 2001;391(2):176–185. [Google Scholar]
- 54.Ujjain S.K., Bagusetty A., Matsuda Y., Tanaka H., Ahuja P., De Tomas C., Sakai M., Vallejos-Burgos F., Futamura R., Suarez-Martinez I., Matsukata M., Kodama A., Garberoglio G., Gogotsi Y., Johnson J.K., Kaneko K. Adsorption separtion of heavier isotope gases in subnanometer carbon pores. Nat. Commun. 2021;12:546. doi: 10.1038/s41467-020-20744-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu F.-Q., Wang L., Huang Z.-G., Li C.-Q., Li W., Li R.-X., Li W.-H. Amine-tethered adsorbents based on three-dimensional macroporous silica for CO2 capture from simulated flue gas and air. ACS Appl. Mater. Interfaces. 2014;6:4371–4381. doi: 10.1021/am500089g. [DOI] [PubMed] [Google Scholar]
- 56.Su F., Lu C., Chen H.-S. Adsorption, desorption, and thermodynamic studies of CO2 with high-amine-loaded multiwalled carbon nanotubes. Langmuir. 2011;27:8090–8098. doi: 10.1021/la201745y. [DOI] [PubMed] [Google Scholar]
- 57.Heikenfeld J., Jajack A., Rogers J., Gutruf P., Tian L., Pan T., Li R., Khine M., Kim J., Wang J., Kim J. Lab on a Chip. Lab Chip. 2018;18:217–248. doi: 10.1039/C7LC00914C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun B., McCay R.N., Goswami S., Xu Y., Zhang C., Ling Y., Lin J., Yan Z. Gas-permeable, multifunctional on-skin electronics based on laser-induced porous graphene and sugar-templated elastomer sponges. Adv. Mater. 2018;30(50):1804327. doi: 10.1002/adma.201804327. [DOI] [PubMed] [Google Scholar]
- 59.Miyamoto A., Lee S., Cooray N.F., Lee S., Mori M., Matsuhisa N., Jin H., Yoda L., Yokota T., Itoh A., Sekino M., Kawasaki H., Ebihara T., Amagai M., Someya T. Inflammation-free, gas-permeable, lightweight, stretchable on-skin electronics with nanomeshes. Nat. Nanotechnol. 2017;12:907–913. doi: 10.1038/nnano.2017.125. [DOI] [PubMed] [Google Scholar]
- 60.Peng Y., Chen J., Song A.Y., Catrysse P.B., Hsu P., Cai L., Liu B., Zhu Y., Zhou G., Wu D.S., Lee H.R., Fan S., Cui Y. large-scale radiative cooling fabric. Nat. Sustain. 2018;1:105–112. doi: 10.1038/s41893-018-0023-2. [DOI] [Google Scholar]
- 61.Kukobat R., Sakai M., Tanaka H., Otsuka H., Vallejos-Burgos F., Lastoskie C., Matsukata M., Sasaki Y., Yoshida K., Hayashi T., Kaneko K. Ultrapermeable 2D-channeled graphene-wrapped zeolite molecular sieving membranes for hydrogen separation. Sci. Adv. 2022;8:1–12. doi: 10.1126/sciadv.abl3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.González-Rivera J., Iglio R., Barillaro G., Duce C., Tinè M.R. Structural and thermoanalytical characterization of 3D porous PDMS foam materials: The effect of impurities derived from a sugar templating process. Polymers. 2018;10:1–13. doi: 10.3390/polym10060616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kukobat R., Minami D., Hayashi T., Hattori Y., Matsuda T., Sunaga M., Bharti B., Asakura K., Kaneko K. Sol-gel chemistry mediated Zn/Al-based complex dispersant for SWCNT in water without foam formation. Carbon. 2015;94:518–523. doi: 10.1016/j.carbon.2015.07.025. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.