Abstract
Childhood obesity is, according to the WHO, one of the most serious challenges of the 21st century. More than 100 million children have obesity today. Already during childhood, almost all organs are at risk of being affected by obesity. In this review, we present the current knowledge about diseases associated with childhood obesity and how they are affected by weight loss. One major causative factor is obesity‐induced low‐grade chronic inflammation, which can be observed already in preschool children. This inflammation—together with endocrine, paracrine, and metabolic effects of obesity—increases the long‐term risk for several severe diseases. Type 2 diabetes is increasingly prevalent in adolescents and young adults who have had obesity during childhood. When it is diagnosed in young individuals, the morbidity and mortality rate is higher than when it occurs later in life, and more dangerous than type 1 diabetes. Childhood obesity also increases the risk for several autoimmune diseases such as multiple sclerosis, Crohn's disease, arthritis, and type 1 diabetes and it is well established that childhood obesity also increases the risk for cardiovascular disease. Consequently, childhood obesity increases the risk for premature mortality, and the mortality rate is three times higher already before 30 years of age compared with the normal population. The risks associated with childhood obesity are modified by weight loss. However, the risk reduction is affected by the age at which weight loss occurs. In general, early weight loss—that is, before puberty—is more beneficial, but there are marked disease‐specific differences.
Keywords: cardiometabolic disease, inflammation, obesity comorbidities, pediatric obesity, weight loss

Introduction
Childhood obesity is a condition which, throughout the last 100 years, has transformed from a sign of wealth and health to a disease that according to the WHO is “one of the most serious challenges of the 21st century” [1]. There are several reasons for the WHO statement. According to the Global Burden of Disease Study [2], health hazards—such as global exposure to unsafe sanitation, household air pollution, childhood underweight, childhood stunting, and smoking—decreased by more than 25% between 1990 and 2015. During the same period, risks associated with high body‐mass index (BMI) increased by more than 25%. The prevalence of obesity has doubled in more than 70 countries since 1980. A total of 107.7 million children had obesity in 2015 [2].
Childhood and adolescent obesity is associated with increased risk of premature death [3, 4]. Many factors contribute, such as cardiovascular disease, [5] type 2 diabetes (T2D) [6], and several endocrine disorders, [7] as well as depression and cognitive disturbances that may contribute to poor school results [8]. Almost all organ systems are affected by obesity in childhood (Fig. 1).
Fig. 1.
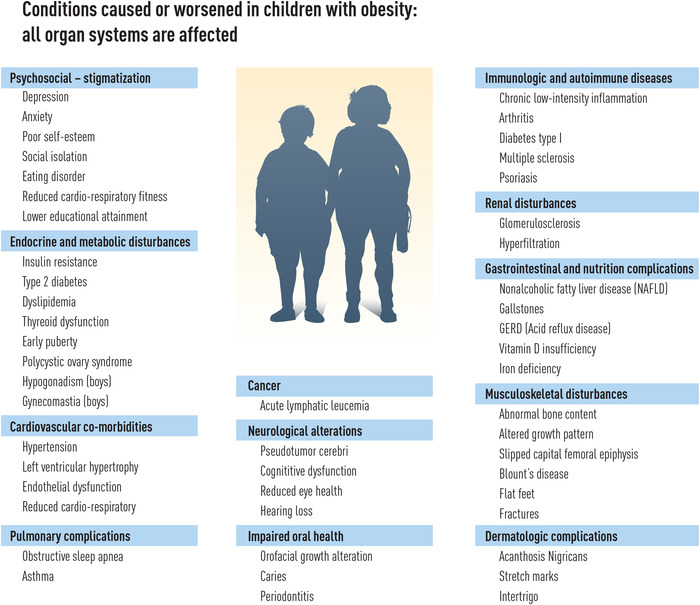
Diseases and conditions caused or aggravated by obesity during childhood and adolescence. Childhood obesity is a systemic disease and almost all organs in the body are potentially affected.
An additional concern for children with obesity is the negative effects obesity may have on social interaction. Bullying is common, and the negative attitudes toward children with obesity may increase the risks for eating disorders, social isolation, and reduced physical activity. Childhood obesity is today associated with stigma to such a degree that, despite it being considered a disease, the health care system is requested to not call the disease “obesity” but to use nonbiased language such as “weight” and “body mass index” [9]. There is also a powerful “body activism” movement claiming that obesity is not a disease and that the “medicalization” of someone's body is harmful [10]. Consequently, children with obesity should be left alone and treatment postponed. Although such a statement will be misleading, it is important to carefully evaluate the psychosocial risks of stigmatization associated with childhood obesity treatment and compare these risks with the potential health benefits of successful treatment.
In this article, we will go through the long‐term medical and psychosocial consequences of childhood obesity and discuss to what extent comorbidity markers during childhood are associated with disease in adulthood. We will demonstrate how much can be gained if effective treatment of obesity is initiated already during childhood.
Immunological consequences
As mentioned, almost all organ systems are affected by childhood obesity. Many different factors contribute and interact. Together they create complex vicious circles, which makes it difficult to evaluate the specific importance of one single factor (Fig. 2). One factor of major importance is that obesity—directly due to immunological effects in the adipose tissue and indirectly via endocrinological alterations—affects the immune system. The altered immune system contributes to all long‐term obesity‐associated comorbidities such as T2D and other metabolic diseases, cardiovascular disease, cancer, and autoimmune diseases.
Fig. 2.
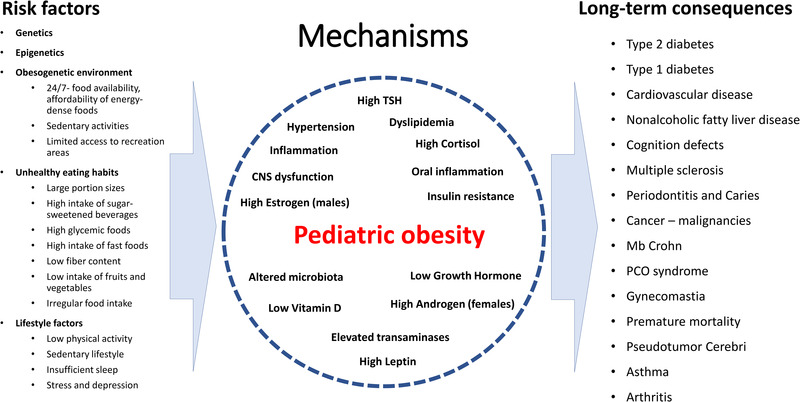
Many different factors contribute to the long‐term effects of childhood obesity. They interact in a complex pattern and it is difficult to identify one specific mechanism responsible for one long‐term consequence. The low‐intensity inflammation caused by obesity in young children already contributes to many obesity‐associated diseases.
Obesity and the immune system
The effects of obesity on the immune system have been described previously [11, 12]. Briefly, when pathological amounts of fat accumulate in the adipose tissue, the fat cells become larger, and a pathological immune activation takes place, resulting in low‐grade chronic inflammation. Both cytokines secreted from the adipocytes and an increased number of macrophages, lymphocytes, and other immunologically active cells contribute. This condition, which is not due to infection or tissue damage, is sometimes called metabolic inflammation or metinflammation. Also, during normal conditions, the adipose tissue—both adipocytes and immune cells—secretes several endocrine and immunologically active adipokines, but the secretion pattern is altered in children with obesity [12]. Thus, the secretion of pro‐inflammatory cytokines—such as tumor necrosis factor–alpha (TNF‐α), resistin, and retinol binding protein‐4 (RBP‐4)—increases, whereas the secretion of anti‐inflammatory adipokines such as adiponectin is reduced. Leptin is an adipokine that is mainly secreted from adipose tissue and circulating leptin levels increase with the growth of the adipose tissue [13]. Leptin is involved in appetite regulation in the central nervous system and puberty development, but also has multiple pro‐inflammatory effects such as stimulation of the release of IL‐6 and TNF‐α. The adipokines also interact with cytokines from the liver and muscle tissues, which further increases the complexity [14].
In addition to these direct effects of adipose tissue alterations on the immune system, endocrine alterations secondary to obesity also affect the immune system. Growth hormone (GH) is downregulated in childhood obesity [15], whereas IGF‐1 levels are normal. Both GH and IGF‐1 have effects on proinflammatory cytokines. GH deficiency has been associated with high TNF‐α, but conflicting results are found, probably due to a different disease background and varying balance between GH and IGF‐1 [16, 17]. Another important key player is cortisone, which is converted to cortisol by means of the enzyme 11‐β‐HSD in several tissues. Increased total body adipose tissue will result in enhanced conversion and thereby higher bioavailability of cortisol, which also affects the immune system [18, 19]. This association is bidirectional as high cortisol levels may predict later development of childhood obesity [20].
Sleep disturbances may also affect the immune system [21], and there is an association between disturbed sleep and obesity. The causality is unclear, but disturbed sleep in children 2–10 years of age may precede later obesity [22, 23], and it is well established in adults that sleep disturbances induce a different and more unhealthy eating pattern [24]. Again, there is a bidirectional association as obesity also may negatively affect sleep quality [25].
The gastrointestinal microbiome may also contribute to an altered immunological response. An obesogenic food intake with saturated fat and low fiber content may alter the microbiome and induce an inflammatory response [26]. Further, low physical activity—a common problem among individuals with obesity—also contributes to an altered inflammatory response [27], and may also affect the microbiome [28].
Child obesity comorbidities due to an activated immune system
Increased C‐reactive protein levels and other signs of chronic inflammation can be detected already in preschool children with overweight and obesity [29, 30], and several diseases are probably affected by the low‐grade inflammation. Some of them—nonalcoholic fatty liver disease (NAFLD)/metabolic dysregulation to metabolic (dysfunction)–associated fatty liver disease (MAFLD), insulin resistance and T2D, atherosclerosis, and hypertension—are described elsewhere in this review. The prevalence of asthma is higher among children with obesity compared with normal‐weight children [31]. Most probably both mechanical and immunological factors contribute, as two types of asthma phenotypes associated with obesity are described—one early‐onset allergic and one late‐onset nonatopic asthma type [32].
To which extent the altered immune response among children with obesity will negatively affect the outcome of infections remains unclear. This has been a concern during the COVID‐19 pandemic, and it is plausible that obesity and obesity‐associated chronic inflammation and other obesity‐related factors such as oxidative stress and insulin resistance contribute to the worse outcomes observed among critically ill children with obesity [33, 34].
Osteoarthritis is a well‐established comorbidity to obesity in adults [35]. However, there is also an association between pediatric obesity and osteoarthritis and knee pain in adulthood, independent of weight status in adulthood [36]. This may indicate that although mechanical factors and bone growth alterations may contribute, chronic inflammation is probably a major contributing factor.
Child obesity and autoimmune diseases
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system leading to demyelination and neurodegeneration. Chronic inflammation is probably the most important factor behind the association between childhood obesity and MS [37, 38]. As there is a strong association between early development of obesity and later MS, and also mechanistic data supporting a causal relationship, it is likely that early prevention and treatment of obesity can reduce the MS prevalence [39]. On the other hand, if childhood obesity is not treated, given the high childhood obesity prevalence, childhood and adolescent obesity is projected to contribute to up to 14% of overall risk of MS in 2035 [40].
Type 1 diabetes (T1D) is also an autoimmune disease with increasing prevalence worldwide [41]. It is a disease where genetics and environmental factors contribute, but it is unclear why the prevalence varies markedly between countries [42]. It has been proposed that obesity is involved in the increased incidence of T1D primarily via obesity‐induced insulin resistance. The so‐called “accelerator hypothesis” proposes that individuals with autoimmune destruction of insulin‐producing cells who are more insulin resistant will be more prone to develop clinically manifested diabetes [43]. However, the association between obesity and T1D is complex, and the genetic HLA pattern affects the association between BMI and T1D incidence [44]. Obesity‐induced inflammation also seems to increase the risk of developing clinical T1D with only one type of autoantibody present [45]. Furthermore, already long before the T1D is clinically apparent, obesity increases the shift from one to two autoantibodies in childhood and adolescence, but only in specific genetic groups [46].
An association has also been observed between obesity, higher prevalence, and worsened prognosis of several other autoimmune conditions such as rheumatoid arthritis, systemic lupus erythematosus, inflammatory bowel diseases, and psoriasis [26, 47]. This has also been observed in children, but whether diet contributes to the risk is still unclear [48].
In conclusion, obesity‐induced low‐grade inflammation is of importance for the development of almost all obesity‐related comorbidities and increases the prevalence and severity of several other immunological and autoimmune diseases. The associations between obesity and disease are found already before puberty, which may indicate that early treatment of obesity is required to reduce the incidence of immunologically related diseases.
Cardiovascular health
It is well established that there is an association between long‐term cardiovascular health and obesity in general. Cardiovascular diseases and atherosclerosis develop slowly over time, and it is therefore reasonable that the associated mortality rate is higher if obesity develops in young adults compared with later in life [4]. This is of course dependent on the obesity remaining, which unfortunately is regularly the case. Weight loss reduces the cardiovascular risks in adults [49].
Cardiovascular disease in adulthood is associated with childhood and adolescent BMI [50]. In Denmark, it was found that the risk of any cardiovascular event was positively associated with BMI at 7–13 years of age for boys and 10–13 years of age for girls. The risk increased across the entire BMI distribution [5]. However, to what extent individuals with childhood and adolescent obesity have higher cardiovascular risk than individuals who develop obesity as young adults is unclear.
Cardiovascular risk markers with a clear‐cut association to cardiovascular morbidity in adults can be found in prepubertal children. Already in 7–8‐year‐old children there is an association between early signs of atherosclerosis and BMI [51]. Also, endothelial cell dysfunction is associated with obesity in young children [52].
Blood pressure—another factor of importance for later cardiovascular disease and stroke—is frequently increased in children with obesity already before puberty [53]. It has been estimated that 37% of all hypertension in children is attributed to obesity [54]. Night‐time systolic blood pressure normally drops more than 10% from daytime blood pressure, so‐called nocturnal dipping [55]. Nocturnal reduction of less than 10% is defined as nondipping, and studies have consistently shown an association between nondipping and increased incidence of fatal and nonfatal cardiovascular events in adults [56]. Among both prepubertal and pubertal children, nondipping occurs frequently [57, 58].
Several factors act in concert to increase the cardiovascular risk (Fig. 2). In addition to the effects of low‐grade inflammation discussed above, long‐term hypercholesterolemia is probably of major importance for the development of atherosclerosis [59]. Atherosclerosis is thought to develop early in childhood with fatty streaks, and it has been demonstrated in a series of Brazilian studies that cholesterol levels in adolescence are of major importance for fatal heart disease in adulthood [59].
Weight loss in childhood seems to be even more beneficial for long‐term cardiovascular health than weight loss in adulthood. A weight loss of 0.2 BMI Z‐score units (corresponds to approximately 5–6 kg weight loss for an adult) is associated with reduced cardiometabolic risk markers [60]. Hypertension is effectively reduced provided that marked weight loss is achieved [53, 61]. Moreover, obesity in childhood that does not remain till adulthood does not seem to be associated with increased long‐term morbidity compared with children who have had normal weight during childhood [62]. The importance of pubertal BMI is also supported by the finding that a BMI increase from 8 years of age to 20 years is associated with increased cardiovascular risk [63]. Weight loss from adolescence to adulthood effectively reduces diabetes risk to some extent, but the association with coronary heart disease remains [64].
In conclusion, there is a strong association between childhood obesity and cardiovascular morbidity in adulthood. However, normalization of weight status before puberty seems to eliminate these risks. The earlier the treatment is initiated, the better the effect on long‐term health, provided that the treatment leads to a normalized weight. As previously mentioned, risk markers for cardiovascular disease are with an alarming frequency found in prepubertal children. The epidemiological data may indicate that these cardiovascular risk markers are reversible and of limited prognostic value before puberty. Furthermore, it is still unclear when these comorbidity markers and high blood pressure should be pharmacologically treated in children if the goal is to maintain long‐term cardiovascular health.
Cardiorespiratory fitness
Low levels of cardiorespiratory fitness (CRF) are associated with a high risk of cardiovascular disease and all‐cause mortality [65]. It is to some extent an integrated measure of several factors with negative effects on cardiovascular health such as obesity, T2D, smoking, and sedentary activity, but there are genetic factors that contribute to individual variations of CRF. These familiar and genetic factors are also of major importance for the association between CRF and disease in young males [66].
There is a modest correlation between BMI and CRF in young males, indicating that individuals with obesity may have a normal CRF [67]. A low CRF increases the risk of future cardiovascular disease in young males with obesity [67]. There is also an association between cognition and physical fitness in children [68].
Indirect evaluation of oxygen consumption during a bicycle test is often used as a measure of CRF. In children with obesity, the relative oxygen consumption per kilogram of body weight is invariably low compared with normal‐weight children whereas the total maximum oxygen consumption is relatively normal [69, 70], and similar results are found with direct measurement of maximum oxygen uptake [71].
In adolescents, weight loss after bariatric surgery results, as expected, in improved maximal oxygen consumption per kilogram body mass. In addition, pain associated with movement was reduced. More surprising, despite a marked reduction of fat‐free mass, absolute oxygen consumption increased. These results suggest that bariatric surgery in adolescence might add specific benefits of importance for future health [72]. The lungs are expanded after weight loss (Fig. 3) but it is unclear whether this improvement is due to changes in the heart, microcirculation, or the lungs.
Fig. 3.
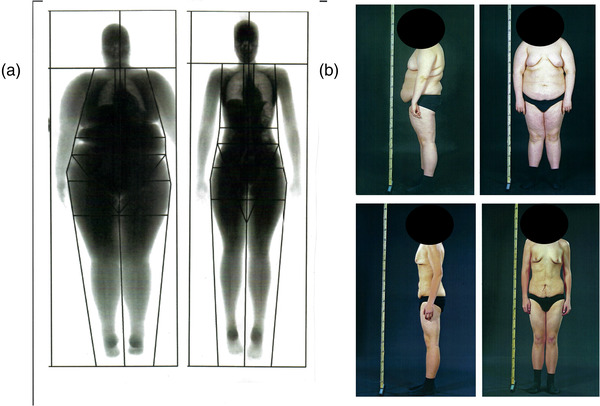
Effect of severe adolescent obesity and weight loss on lung volume, posture, and gynecomastia in adolescents. (Panel a) Adolescent before and 2 years after gastric bypass surgery and 40 kg of weight loss. The lung volume is markedly restored. (Panel b) One year with 30 kg weight loss as an effect of successful lifestyle support. The posture is normalized and the lipomastia is reduced but the gynecomastia remains despite weight loss. Written approval obtained from the patient to use the photos of him before and after weight loss presented in Fig. 3.
Glucose homeostasis
Hyperinsulinemia and a disturbed glucose homeostasis are key features of obesity. Glucose homeostasis depends on the balance between β‐cell function, that is, insulin release, and target organ insulin sensitivity. Insulin has several biological effects, and the sensitivity may differ in different organs. As the effect of obesity on insulin sensitivity is also organ specific, it is difficult to predict the pathophysiological picture in hyperinsulinemic conditions.
Insulin resistance usually refers to reduced glucose uptake in the presence of insulin. When the insulin release can no longer compensate for the insulin resistance, T2D occurs. Hyperinsulinemia in obesity is usually considered a compensatory elevation of insulin to achieve the required biological effect on glucose uptake so normal blood glucose is maintained.
Insulin resistance is problematic to measure. The key peripheral tissues affected by insulin include the skeletal muscle mass (the largest insulin‐responsive organ that modulates glucose metabolism in the postabsorptive state), the liver (which has the capacity to produce glucose in the fasting state), and the adipose tissue (the source of free fatty acids in fasting and a storage depot during the postabsorptive state). Resistance to the effects of insulin results in reduced glucose uptake in skeletal muscle and adipocytes and reduced suppression of hepatic glucose production. Insulin resistance also results in increased adipose tissue lipolysis. Taken together, the results are elevated circulating insulin, glucose, and free fatty acids, the typical markers of the insulin resistant state.
Obesity in childhood drives insulin resistance together with several other factors. Genetic and epigenetic factors are of crucial importance [73, 74]. Almost all children who develop T2D have family members with the same disease [75]. Several lifestyle factors also contribute, such as physical inactivity, eating habits, stress, and psychosocial problems such as depression [75].
T2D is preceded by a range of glucose‐related phenotypes characterized by a progressive decline in β‐cell function and an increase in insulin resistance. These conditions are defined as intermediate hyperglycemia or prediabetes. Common markers of dysregulated glucose homeostasis include acanthosis nigricans (isolated hyperpigmentation of the skin), elevated HbA1c, impaired fasting glycemia, and impaired glucose tolerance measured with the oral glucose tolerance test. In adults, intermediate hyperglycemia has been associated with increased risk for cardiovascular disease, cognition, cancer, and premature death even in the absence of the development of T2D [76, 77, 78, 79, 80].
T2D in children and adolescents was extremely rare before the present obesity epidemic, that is, only a few decades ago. The prevalence of prediabetes and diabetes in the pediatric obesity population varies greatly across countries and ethnicities but an increase is generally observed [81, 82, 83, 84, 85].
Among children and adolescents with obesity, impaired fasting glycemia and elevated HbA1c increase the risk for adult T2D with a hazard ratio of 3.7 and 3.1, respectively [6]. However, the degree of obesity is a major risk factor for future T2D, independent of the presence of intermediate hyperglycemia [6, 62, 86]. Not only obesity, but also overweight in adolescents without any apparent metabolic disturbances, confers a sixfold increase in the risk of T2DM more than 20 years later, compared with normal‐weight controls [87].
The complications of T2D are like the ones of T1D. However, the early onset of T2D, that is, among adolescents and young adults, is fiercer with a much faster progression of the disease compared with T2D with middle‐age onset [88]. Moreover, early‐onset T2D, that is, in adolescence and young adulthood, is a much more severe disease than T1D diagnosed in the same age range. Diabetes complications such as nephropathy, retinopathy, and neuropathy come earlier and more frequently and together this results in a much higher mortality rate [85, 89–91]. Further, youths with T2D have a more deteriorated fluid cognition than youths with T1D, but the association seems to be mediated by obesity and depression [92].
In conclusion, it is imperative to prevent early development of T2D. While increased BMI in childhood is associated with high risk for future T2D [93], normalization of childhood weight by late adolescence/adulthood eliminates the increased risk to develop T2D in adulthood [62, 64, 94]. Further, successful obesity treatment in childhood and adolescence improves insulin sensitivity, fades acanthosis nigricans, and lowers the risk for future T2D [6, 95].
Nonalcoholic fatty liver disease
Pediatric NAFLD is a chronic disease and is defined as a condition where ≥5% of hepatocytes have vesicular fatty infiltration upon examination of liver histology, provided that it is not secondary to infections, metabolic or genetic disorders, alcohol consumption, or medications [96, 97]. Insulin resistance is a key factor for fatty liver development and visceral adipose tissue is the dominant source of fat accumulation in the liver. Inflammation, diet, and gut microbiota are also involved in the clinical progression and manifestation of NAFLD [98].
Due to its association with obesity, it has become the most common liver disease in children [96], with an estimated prevalence of 28%–41% among children and adolescents with obesity [99]. Among pediatric obesity population, NAFLD is more prevalent among males, those with severe obesity, and those having impaired glucose metabolism [100, 101, 102, 103]. Recently, it has been suggested by an international expert group that the condition should be renamed and redefined. The definition, “nonalcoholic,” is primarily problematic for children, for whom alcohol consumption is usually not a concern. MAFLD is therefore suggested with age‐appropriate definitions based on sex and age percentiles [104].
It remains unknown whether or to what extent children with fatty liver disease are more likely to have progressive fibrosis or increased risk of cardiovascular disease as adults [97, 104]. It has recently been demonstrated that children and young adults with NAFLD in Sweden have a higher mortality rate compared with age‐matched individuals from the normal population [105]. However, children with obesity in Sweden also have higher mortality rate compared with the normal population [3]. Thus, as the children with NAFLD were not BMI matched, it is impossible to estimate whether or how much pediatric NAFLD contributes to increased mortality.
NAFLD is associated with increased risk to develop T2D in adults. The liver is essential in glucose regulation and a disturbed liver function in combination with inflammatory factors associated with NAFLD increases the diabetes risk [106]. However, the association is bidirectional as they so frequently are in this review. T2D is also associated with impaired liver function with increased progression of NAFLD to fibrosis and nonalcoholic steatohepatitis [106].
In the pediatric obesity population, NAFLD is not rarely accompanied by T2D [107], but may also predict future T2D. Two recent studies show that NAFLD and elevated ALT markedly increase the risk for later development of T2D in children and adolescents, respectively, with obesity. After adjustments, the risk for later T2D increased with a hazard ratio of 2.6 in both studies [108, 109]. This is an increase only slightly lower than that observed for children and adolescents with impaired fasting glycemia [6]. The mechanism for the progression to T2D is complex, but hepatic fat infiltration, independent of visceral fat and intramyocellular lipid content, reduces the insulin‐induced suppression of endogenous glucose production, which is of major importance for insulin resistance in adolescents with obesity [110].
Treatment options for pediatric NAFLD are limited. There are some studies demonstrating promising effects of probiotics both in adults and children [111, 112] but no long‐term studies are available. There is currently no other accredited treatment for NAFLD but decreasing the degree of obesity improves NAFLD [97]. Several studies with a duration of up to 15 months suggest that weight loss is efficacious in reducing NAFLD prevalence [113, 114, 115, 116]. Among teenagers who have undergone gastric bypass, transaminases are normalized 5 years past surgery [61]. One recent study has shown that a weight loss of 0.25 BMI standard deviation score or z‐score (SDS) units almost eliminates the long‐term risk for future NAFLD in pediatric years [117]. Similarly, despite childhood obesity being associated with increased risk for adult NAFLD, the increased risk can be largely reduced by obtaining a normal adult BMI [118].
In conclusion, pediatric NAFLD/MAFLD is associated with increased risk for later development of T2D and effective weight loss seems to eliminate that risk. It remains to be established whether pediatric NAFLD/MAFLD is associated with liver disease in the adult.
Other endocrine alterations
Thyroid
Elevated thyroid stimulating hormone (TSH) levels as a sign of disturbed thyroid function are regularly observed in individuals with obesity. Usually, thyroid hormone levels, T3 and T4 are within the normal range [119] and weight loss results in normalization of the TSH levels. The situation is similar in children [120]. The mechanisms are unclear but a modest and reversible obesity‐induced thyroid gland dysfunction or an increased turnover of thyroid hormones might contribute. Elevated TSH among children with obesity is associated with cardiometabolic markers in childhood and later cardiovascular disease [120, 121]. TSH may have other effects in the body beyond the stimulation of thyroid hormone production [121, 122, 123] but based on the present knowledge, there are no signs of a causal association between increased TSH levels in childhood and later disease.
Vitamin D
Low vitamin D levels are often found in children with obesity. Vitamin D deficiency in childhood has been associated with the development of several diseases such as MS [124] and prediabetes [125, 126], that is, two conditions also associated with obesity. Whether supplementation of vitamin D affects T2D, prediabetes, and the metabolic syndrome has been investigated, but with contradictory results [127] and the pathophysiological significance of vitamin D deficiency for the development of T2D is unclear. A large double‐blind intervention study in adults failed to identify any beneficial effect on glucose metabolism of vitamin D substitution [128] whereas a modest effect has been noted in children and adolescents [129]. Yet, most likely the association between vitamin D insufficiency and disturbed glucose metabolism in obesity is primarily due to that similar factors contribute to both low vitamin D levels and insulin resistance, that is, sedentary indoor life and unhealthy food intake [130, 131].
The mechanisms behind low vitamin D levels are unknown. Both increased storage in the adipose tissue and increased metabolism have been proposed [130] and it has been suggested that there is no true deficiency since the levels are low due to increased accumulation in the adipose tissue [132]. It has also been suggested that the metabolism of vitamin D is disturbed [133]. Vitamin D can be metabolized in the adipose tissue and the expression of 25‐hydroxylase CYP2J2 and the 1a‐hydroxylase CYP27B1 are decreased in subcutaneous adipose tissue from obese subject [134]. But as mentioned, both indoor activities and poor eating habits probably contribute as well.
GH, IGF‐1, and longitudinal growth
GH has many effects on bone growth, metabolism, immune responses, and the central nervous system [16, 135–137]. Many functions are mediated via IGF‐1, but some organs, such as the liver and adipocytes, have only GH receptors and no IGF1‐receptors. GH levels are markedly downregulated in obesity to levels usually found in severe GH deficiency conditions. Despite that, IGF‐1 levels are normal. Most probably, adipose tissue is the main source of circulating IGF‐1 in children with obesity and GH levels are downregulated due to negative feedback [15]. Weight loss normalizes GH secretion.
Children with obesity grow faster than normal‐weight children despite low GH levels. During puberty, when normal‐weight children have a growth spurt, children with obesity have a lower growth velocity and they end up with a similar final height as normal‐weight children [138].
The relatively low GH levels in obesity may contribute to the psychosocial effects of obesity. GH deficiency is associated with shyness, inhibition, and memory dysfunction, and memory dysfunction has also been observed in adult females [139, 140]. Given the effects of GH on metabolism and immune response the low GH levels may also contribute to the association between cardiovascular dysfunction and obesity.
Bone health
Osteoporosis is a skeletal disorder characterized by low bone mass, micro‐architectural deterioration of bone tissue leading to bone fragility, and increased fracture risk [141]. This is a disease associated with malnutrition and ageing and not with childhood obesity. The bone mass is positively correlated with body weight both in children and adults probably due to greater excess weight activation of bone growth. However, endocrine or paracrine factors associated with the metabolic syndrome and obesity may negatively influence bone mass. They are released from the adipose tissue and they may have both systemic and local effects within the bone marrow [142, 143, 144].
Contrary to expectations, children with obesity have a higher fracture rate than normal‐weight children [145, 146]. Mechanistic factors such as a heavier body in combination with altered body proportions may contribute to increased risks for children with obesity when specific fractures are studied [147] in combination with other obesity‐related factors such as reduced motor skills and balance problems, as previously suggested [146]. However, it is also possible that bone density is negatively affected in children with obesity [148]. Several factors may contribute to disturbed bone synthesis such as low levels of vitamin D, low‐grade inflammation, reduced physical activity, poor eating habits, and insulin resistance [149].
Adolescents with severe obesity who undergo gastric bypass surgery may constitute a risk group for later osteoporosis especially if bone quality is reduced and dual energy X‐ray absorptiometry (DEXA) measurements are somewhat misleading. Peak bone mass is usually reached between 22 and 26 years of age [150]. Thus, these individuals have not reached their peak bone mass before surgery. They have a bone mass above normal before surgery but the weight loss in combination with low vitamin D levels results in a very rapid loss of bone mineral content, which may be a future concern [151].
Puberty, polycystic ovary syndrome, gynecomastia, and fertility
Puberty often starts early in females with obesity whereas it is somewhat delayed in males [152]. In females, the menstruations are often irregular, and the insulin resistance seems to trigger a hyperandrogenic state, which may trigger the development of a polycystic ovary syndrome with higher androgen levels and infertility [152]. This is reversible and weight loss during adolescence, causing unexpected increased fertility, may result in unwanted pregnancies [61].
Among pubertal boys with obesity, gynecomastia is relatively common (Fig. 3b). As this occurs together with lipomastia, the breast enlargement may affect the psychosocial well‐being. The gynecomastia is primarily thought to be due to aromatization of testosterone to estrogen in the adipose tissue—a process that is increased in individuals with obesity [153]. Mild gynecomastia is reversible, but often surgical treatment is required [153].
Fertility is reduced both among males and females with obesity. Female fertility is markedly improved after weight loss [154, 155, 156]. Couples with an obese male partner have a significantly higher risk of infertility than couples with normal‐weight male partners and male obesity negatively affects the success of assisted reproductive technology [157, 158]. To which extent pediatric obesity is of importance for the reproductive health disturbances is unclear, but the factors involved are complex and probably long‐term duration of obesity will have more detrimental effects. It is not clarified whether weight loss improves male fertility.
Oral health
The association between poor oral health and cardiometabolic disease in adults is well established [159]. Hypertension, ischemic heart disease, cerebrovascular disease, prediabetes, and T2D are all associated with periodontal disease [159, 160]. Furthermore, improved oral health may improve cardiometabolic health [161]. The local oral chronic inflammation and microbiome alterations are probably of major importance for these associations. The periodontal inflammation triggers a systemic inflammatory response partly due to periodontal pathogens entering the circulation [159].
Oral morbidity is increased in children and adolescents with obesity. Children with obesity have higher occurrence of caries in some studies [162], but the results are inconsistent [163, 164, 165]. Adolescents with obesity seems to have markedly lower saliva production compared with normal‐weight adolescents, which might contribute to increased caries risk [166]. Periodontal risk markers and cytokine concentration and oral microbiota are also altered in the gingival fluid in adolescents with obesity [167, 168, 169]. There is also an association between pathological periodontal pockets and blood pressure among adolescents with obesity [170].
In conclusion, oral health is markedly disturbed among adolescents with obesity. Data from adults may indicate that this will add to the long‐term cardiometabolic risk, but to what extent poor oral health among adolescents with obesity will compromise long‐term health remains to be established.
The central nervous system (CNS), cognition, neuropsychology, and psychosocial consequences
School achievement and cognition
Children and adolescents with obesity face several issues in the school environment. Children with obesity more frequently have lower school grades and reach a lower level of education compared with normal‐weight peers [171, 172]. Several factors may interplay, for example, resourcefulness, parental education, intelligence, or ability to conform. Also psychosocial aspects such as stigma [173] and increased risk for anxiety and depression contribute [174]. The risk of bullying and social isolation has been shown to increase with the severity of obesity. It has been reported that more than 50% of adolescent boys with obesity and 45% of the girls experience frequent bullying [175].
However, the effects of obesity on school performance remained even when adjusted for socioeconomic status (SES) and neurodevelopmental disorders [8]. Yet, another important aspect is that childhood obesity negatively affects cognitive functions, such as memory, executive functions, and processing speed [176, 177, 178]. Animal studies confirm that obesity affects cognition [179, 180], and studies in adult females found that the effects may be reversible [181], but it has not been observed in all studies [182]. One contributing factor may be the obesity‐induced downregulated GH levels discussed elsewhere in this article. GH deficiency is associated with similar reversible cognitive effects on memory, as has been found among females with obesity [183, 184].
The negative impact of childhood obesity on educational level may partly be reversed by successful obesity treatment in childhood [8], and in adults, cognitive deficits may be improved after weight loss [181, 185]. Regardless of mechanisms, it is of great importance to increase awareness of the association between obesity and school achievement.
ADHD
Neurodevelopmental disorders, such as attention deficit disorder with (ADHD) or without hyperactivity (ADD), are (at least) 2–3 times more common among children and adolescents with obesity compared to normal‐weight peers. The prevalence of ADHD/ADD in clinical samples of pediatric obesity has been reported to be 10%–11% among females and 17%–31% among males [174, 186]. The direction of the causal associations between ADHD/ADD and obesity is not clear, even though most studies suggest ADHD/ADD to precede obesity [187, 188].
ADHD/ADD is characterized by altered executive functions, such as impulsivity and impaired ability to retain concentration, attention, as well as self‐monitoring. Further, individuals with ADHD exhibit dysfunctional eating patterns to a greater extent than non‐ADHD individuals and may even experience a greater reward sensitivity to food through dopaminergic activation in key brain reward areas [187]. Thus, it is likely that ADHD/ADD is a trigger for obesity development in an obesogenic environment. In addition, the psychosocial effects of ADHD/ADD on children with obesity may contribute to an increase in the obesity‐related stigmatization. When ADHD/ADD remains undiagnosed among children with obesity, ADHD/ADD‐related behavior will incorrectly be associated with obesity.
Depression and anxiety
The association between pediatric obesity and psychiatric problems, such as depression and anxiety, is complex since neurodevelopmental disorders and low SES are known risk factors for anxiety and depression [189, 190] and prevalent in the pediatric obesity population [174, 187, 191]. Nevertheless, there is an increasing body of literature supporting the link between obesity and depression. The results are mixed, and the link seems to be bidirectional [174, 192, 193]. Hence, obesity increases the risk for depression and vice versa. Successful obesity treatment has been associated with lower risk of depression, but not anxiety, in males but not females [174]. However, long term follow‐up of adolescents after bariatric surgery indicates that, despite good short‐term effects [194], the positive effects of weight loss on depression and anxiety are very limited. These results indicate that depression and anxiety, at least among adolescents with severe obesity, are associated with but not caused by obesity [195, 196, 197].
In conclusion, obesity in childhood affects the likelihood to reach the same level of education as that for their normal‐weighted peers. Despite that, the full mechanisms of the mediating factors for this are yet to be determined and the association seems to be partly reversible by weight reduction. Further, it is important to identify children with altered executive functions since ADHD/ADD is common in the obesity population. An attention deficit disorder can worsen the obesity‐related psychosocial consequences and increase the risk for stigmatization. Further, depression is prevalent in the pediatric obesity population and needs to be addressed to enable realistic conditions for obesity treatment.
Pseudotumor cerebri
Pseudotumor cerebri, also called idiopathic intracranial hypertension or benign intracranial hypertension, is a neurologic syndrome consisting of headache caused by elevated intracranial pressure. Severe cases may also have signs of vision loss and cranial nerve symptoms, which can be permanent [198, 199]. When it occurs, it is often present together with obesity, but the underlying mechanisms remain unknown. The diagnosis is often delayed as the initial symptom, headache, occurs frequently among individuals with obesity. A substantial weight loss—10% or more—is an effective treatment [200].
Cancer
It is well established that increased BMI in adults is associated with several types of cancer, including the upper intestinal tract, stomach, lower intestinal tract, and parenchymal and endocrine organs [201]. A study from the UK showed that obesity is responsible for nearly 5% of all cancer types, and thereby the second largest modifiable cancer risk factor after smoking that accounts for 17% of the cancer incidents [202]. In a viewpoint review, they found 13 cancer types associated with severe obesity with relative risks that varied from 7.1 for corpus uteri cancer down to 1.1 for thyroid cancer. For eleven other cancer types, the evidence was limited or inadequate [201].
Adolescence cancers
In a large study on 2.3 million Israeli adolescents who were followed for almost 30 million person‐years, obesity in adolescence increased the incidence of several cancers [203]. Compared with normal weight, the hazard ratios for overall cancer were 1.26 in males with obesity and 1.27 for females when cervix and breast cancer were excluded. However, when including all cancer types, female adolescent obesity was not associated with future cancer. In addition, cancer was diagnosed at an earlier age and with a higher mortality rate for both males and females who had obesity in adolescence [203].
Childhood cancers
Children who gain more in weight than average have an increased risk of several cancers [204, 205]. The duration of obesity, and whether the weight gain is in childhood or adulthood, may affect the risk of cancer. However, it is difficult to distinguish as childhood and adolescent obesity often remains till adulthood. BMI in childhood and during puberty has been positively associated with risks of bladder, colon, endometrial, kidney, liver, esophageal, ovarian, pancreatic, prostate, and thyroid cancer in adulthood, whereas there seems to be an inverse association between breast cancer before menopause and BMI [204]. The associations become stronger with the child's age; for example, the hazard ratios for overall cancer ranges from 1.05 to 1.68 per BMI Z‐score at ages 7 and 13 years. Further, childhood BMI is directly associated with the risk of some adult hematologic malignancies, such as non‐Hodgkin's lymphoma and diffuse large B‐cell lymphoma [205].
A recent study, pooling data from seven international longitudinal cohorts, found that high childhood BMI was associated with subsequent overall cancer mortality, independent of other examined childhood risk factors and more importantly, independent of adult BMI [206]. Further, in individuals who had obesity in childhood, cancer seems to be the most common endogenous cause of death in young adulthood [3]. Thus, childhood and adolescence, periods essential for determining exposures over the life‐course, may be an optimal period to intervene to lower subsequent cancer risk.
There is evidence suggesting that high BMI in childhood followed by an increase in BMI during puberty increase the risk for colon cancer [207, 208]. Yet, another study shows that overweight in childhood that persists into early adulthood is associated with an increased risk of colon cancer whereas overweight that disappears before early adulthood or is developed after childhood is not [209]. Two studies found no association between childhood BMI and later rectal cancer, suggesting different etiologies of colon and rectal cancer [207, 208].
There are several mechanisms involved in the association between childhood obesity and cancer [210]. The research is complicated as there may go several decades between the effects of the obesity‐associated cancerogenic factors on the organs and the development of the cancer. The chronic inflammation, as discussed above, the altered endocrine pattern and alterations in the growth factor secretion, and the metabolic derangements resulting in oxidative stress are most likely contributory factors. Also, the gut microbiome may contribute [211]. More detailed longitudinal long‐term studies are required to disentangle the specific factors and their interaction with genetic and epigenetic factors for the development of the different cancer forms.
In summary, epidemiological studies have shown a strong association between obesity in childhood and adolescence and increased risk for several malignancies in adulthood. The underlying mechanisms are not completely understood, but several adipocytokines and inflammatory markers seem to be key players in this process. Normalization of body weight before the onset of puberty seems to reduce the risk for cancer later in life.
Conclusion
It is well established that childhood obesity is associated with long‐term risks to develop cardiometabolic diseases and colon cancer, leading to markedly reduced life expectancy [212, 213]. However, the effects of early childhood obesity on immunological processes and the development of autoimmune diseases and several other cancers have been clarified relatively recently and our understanding of mechanisms and how to prevent these diseases are still limited.
The effect of weight loss on future disease risk seems to be disease and age dependent and early normalization of weight appears to be required for cancers and probably also for autoimmune diseases (Table 1). However, it has to be emphasized that the associations between weight loss and long‐term comorbidities have to be handled cautiously. There are decades between the weight loss and some of the comorbidities studied, and the obesity treatment offered 20–40 years ago was of limited efficiency and often nonexistent. During such conditions, genetic and social factors contributed considerably to weight loss, factors that may also affect later comorbidity risks. Thus, the associations between specific diseases and the timing of weight loss have to be confirmed in studies performed when effective weight‐loss treatment methods are available.
Table 1.
Risk reduction of future disease depends on when weight loss is achieved
| Cardiovascular | Type II diabetes | Cancers | Autoimmune disease | |
|---|---|---|---|---|
| Prepubertal children | ↓↓↓ | ↓↓↓ | ↓↓ | Unknown |
| Adolescents | ↓↓ | ↓↓ | ↓ | Unknown |
| Adults | ↓ | ↓↓ | ↓ | Unknown |
Note: Schematic summary of available evidence of risk reductions associated with weight loss. Note that other factors contribute to the development of these diseases, such as genetic and environmental factors. ↓↓↓, total or almost total risk elimination; ↓↓, marked risk reduction; ↓, modest risk reduction.
The societal costs for childhood obesity are obvious, but difficult to estimate. Many diseases associated with childhood obesity may first become apparent in adulthood and costs associated with these diseases, such as health care utilization, productivity loss, disability retirement, and premature mortality, are possible to identify first after several decades [3, 214–216]. Consequently, the estimated costs vary considerably. In Sweden, the extra lifetime costs for a 4‐year‐old child with obesity have been estimated to be approximately 75,000 Euro [217, 218] but lower estimations have been published from other countries and with other methods [214].
The efficacy of childhood obesity treatment is still rather limited [60, 219]. A reduction in calorie intake is always the goal, but this is difficult to achieve in our society. Intensive lifestyle support with frequent visits every other week is required to obtain a consistent effect [220], but this is rarely an option for families or health care systems. However, digital supported interaction between family and health care staff may markedly reduce the need for frequent physical visits and thereby increase the feasibility, effectivity, and cost effectiveness of lifestyle support [221, 222].
A new generation of pharmacological treatments are currently being tested in children also, and the first of them, liraglutide, a GLP‐1 agonist, is approved from 12 years of age both in Europe and the United States. Despite a good effect on adolescents [223], liraglutide is not yet reimbursed in Europe. The second generation of GLP‐1 agonists, semaglutide, which requires weekly injections instead of daily, is currently being tested in adolescents, and tirzepatide, a combined GLP‐1 and GIP agonist with marked weight‐loss effects, has recently been approved by the FDA for the treatment of T2D.
Also, bariatric surgery is an option for adolescents with severe obesity. Gastric bypass has been shown to be effective with a marked reduction of cardiometabolic risk markers [61] but one concern is that the long‐term outcome is limited for up to 25% of individuals after surgery [224]. Given the life‐long perspective, the long‐term outcome must be improved for adolescents after bariatric surgery, most probably via intensified post‐surgical lifestyle support and pharmacological treatment. Nevertheless, as summarized in Table 2, the pediatric obesity treatment toolbox is far better equipped today than only 5 years ago.
Table 2.
Treatment options for childhood obesity
| Type of treatment | Age group | Average treatment outcome (body‐mass index Z‐score) | Adverse effects | Comments | Reference |
|---|---|---|---|---|---|
| Lifestyle modification support, 4–6 h/year | 4–18 years | −0.13 to −0.15 | None | Limited effect for adolescents | 60, 219 |
| Intensive lifestyle support, 26–52 h/year | 4–18 years | −0.17 to −0.30 | None |
Low adherence a Time consuming |
220, 225, 226 |
| Parental group sessions, parenting training | 4–6 years | −0.30 | None |
Low adherence a Limited experience |
227 |
| Digital support for lifestyle modification | 4–18 years | −0.30 | None | Limited experience | 221 |
| Liraglutide | 12–18 years | −0.22 | Primarily gastrointestinal | For adolescents with insufficient effect of lifestyle support | 223 |
| Orlistat | 12–16 years | −0.10 b | Frequent steatorrheas | Low adherence a | 228 |
| Gastric bypass surgery | 14–18 years | −1.6 | Several c | Only for adolescents with severe obesity | 229 |
Note: This table provides an overview of treatment options with key references without any ambition to provide a complete list of treatments and supporting references. The outcomes presented are after a treatment period of at least 12 months; for surgery it is 5 years post surgery.
Includes both unwillingness to participate and/or high dropout rate.
Calculated from average body‐mass index and age provided in the article.
Abdominal surgery for intestinal obstruction and gallstones, nutrition problems, gastrointestinal problems, and more.
Conflict of interest
No conflict of interest was declared.
Acknowledgments
The study was partially funded by the Swedish Heart and Lung Foundation.
Open Access funding was provided by Karolinska Institutet.
Marcus C, Danielsson P, Hagman E. Pediatric obesity—Long‐term consequences and effect of weight loss. J Intern Med. 2022;292:870–891.
References
- 1. World Obesity Federation . Taking action on childhood obesity. World Health Organization, 2018. www.worldobesity.org
- 2. GBDRF Collaborators . Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1659–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lindberg L, Danielsson P, Persson M, Marcus C, Hagman E. Association of childhood obesity with risk of early all‐cause and cause‐specific mortality: a Swedish prospective cohort study. PLoS Med. 2020;17:e1003078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;289:187–93. [DOI] [PubMed] [Google Scholar]
- 5. Baker JL, Olsen LW, Sorensen TI. Childhood body‐mass index and the risk of coronary heart disease in adulthood. N Engl J Med. 2007;357:2329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hagman E, Danielsson P, Brandt L, Ekbom A, Marcus C. Association between impaired fasting glycaemia in pediatric obesity and type 2 diabetes in young adulthood. Nutr Diabetes. 2016;6:e227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Han JC, Lawlor DA, Kimm SY. Childhood obesity. Lancet. 2010;375:1737–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lindberg L, Persson M, Danielsson P, Hagman E, Marcus C. Obesity in childhood, socioeconomic status, and completion of 12 or more school years: a prospective cohort study. BMJ Open. 2021;11:e040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pont SJ, Puhl R, Cook SR, Slusser W, Section On Obesity; Obesity Society . Stigma experienced by children and adolescents with obesity. Pediatrics. 2017;140:e20173034. [DOI] [PubMed] [Google Scholar]
- 10. Ellison J. Weighing in: the “evidence of experience” and Canadian fat women's activism. Can Bull Med Hist. 2013;30:55–75. [DOI] [PubMed] [Google Scholar]
- 11. Fang X, Henao‐Mejia J, Henrickson SE. Obesity and immune status in children. Curr Opin Pediatr. 2020;32:805–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Umano GR, Pistone C, Tondina E, Moiraghi A, Lauretta D, Miraglia Del Giudice E, Brambilla I. Pediatric obesity and the immune system. Front Pediatr. 2019;7:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Francisco V, Pino J, Campos‐Cabaleiro V, Ruiz‐Fernã¡Ndez C, Mera A, Gonzalez‐Gay MA, et al. Obesity, fat mass and immune system: role for leptin. Front Physiol. 2018;9:640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Oliveira Dos Santos AR, de Oliveira Zanuso B, Miola VFB, Barbalho SM, Santos Bueno PCC, Flato UAP, et al. Adipokines, myokines, and hepatokines: crosstalk and metabolic repercussions. Int J Mol Sci. 2021;22:2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nam SY, Marcus C. Growth hormone and adipocyte function in obesity. Horm Res. 2000;53(1):87–97. [DOI] [PubMed] [Google Scholar]
- 16. Szalecki M, Malinowska A, Prokop‐Piotrkowska M, Janas R. Interactions between the growth hormone and cytokines—a review. Adv Med Sci. 2018;63:285–9. [DOI] [PubMed] [Google Scholar]
- 17. Witkowska‐Sedek E, Pyrzak B. Chronic inflammation and the growth hormone/insulin‐like growth factor‐1 axis. Cent Eur J Immunol. 2020;45:469–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stimson RH, Walker BR. The role and regulation of 11beta‐hydroxysteroid dehydrogenase type 1 in obesity and the metabolic syndrome. Horm Mol Biol Clin Investig. 2013;15:37–48. [DOI] [PubMed] [Google Scholar]
- 19. Slominski RM, Tuckey RC, Manna PR, Jetten AM, Postlethwaite A, Raman C, et al. Extra‐adrenal glucocorticoid biosynthesis: implications for autoimmune and inflammatory disorders. Genes Immun. 2020;21:150–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vehmeijer FOL, Santos S, Gaillard R, De Rijke YB, Voortman T, Van Den Akker ELT, et al. Associations of hair cortisol concentrations with general and organ fat measures in childhood. J Clin Endocrinol Metab. 2021;106:e551–61. [DOI] [PubMed] [Google Scholar]
- 21. Lange T, Dimitrov S, Born J. Effects of sleep and circadian rhythm on the human immune system. Ann NY Acad Sci. 2010;1193:48–59. [DOI] [PubMed] [Google Scholar]
- 22. Xiu L, Ekstedt M, Hagstromer M, Bruni O, Bergqvist‐Noren L, Marcus C. Sleep and adiposity in children from 2 to 6 years of age. Pediatrics. 2020;145:e20191420. [DOI] [PubMed] [Google Scholar]
- 23. Ekstedt M, Nyberg G, Ingre M, Ekblom O, Marcus C. Sleep, physical activity and BMI in six to ten‐year‐old children measured by accelerometry: a cross‐sectional study. Int J Behav Nutr Phys Act. 2013;10:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jarvela‐Reijonen E, Jarvinen S, Karhunen L, Sairanen E, Lindroos S, Peuhkuri K, et al. Sleep‐time physiological recovery is associated with eating habits in distressed working‐age Finns with overweight: secondary analysis of a randomised controlled trial. J Occup Med Toxicol. 2021;16:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. MacLean JE, DeHaan K, Chowdhury T, Nehme J, Bendiak GN, Hoey L, et al. The scope of sleep problems in Canadian children and adolescents with obesity. Sleep Med. 2018;47:44–50. [DOI] [PubMed] [Google Scholar]
- 26. Versini M, Jeandel PY, Rosenthal E, Shoenfeld Y. Obesity in autoimmune diseases: not a passive bystander. Autoimmun Rev. 2014;13:981–1000. [DOI] [PubMed] [Google Scholar]
- 27. Metsios GS, Moe RH, Kitas GD. Exercise and inflammation. Best Pract Res Clin Rheumatol. 2020;34:101504. [DOI] [PubMed] [Google Scholar]
- 28. Sharma M, Li Y, Stoll ML, Tollefsbol TO. The epigenetic connection between the gut microbiome in obesity and diabetes. Front Genet. 2019;10:1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Merrill RD, Burke RM, Northrop‐Clewes CA, Rayco‐Solon P, Flores‐Ayala R, Namaste SM, et al. Factors associated with inflammation in preschool children and women of reproductive age: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;106:348S–58S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Collier F, Chau C, Mansell T, Faye‐Chauhan K, Vuillermin P, Ponsonby A‐L, et al. Innate immune activation and circulating inflammatory markers in preschool children. Front Immunol. 2021;12:830049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yiallouros PK, Lamnisos D, Kolokotroni O, Moustaki M, Middleton N. Associations of body fat percent and body mass index with childhood asthma by age and gender. Obesity (Silver Spring). 2013;21:E474–82. [DOI] [PubMed] [Google Scholar]
- 32. Gomez‐Llorente MA, Romero R, Chueca N, Martinez‐Canavate A, Gomez‐Llorente C. Obesity and asthma: a missing link. Int J Mol Sci. 2017;18:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Srinivasan V, Nadkarni VM, Helfaer MA, Carey SM, Berg RA, American Heart Association National Registry of Cardiopulmonary Resuscitation Investigators . Childhood obesity and survival after in‐hospital pediatric cardiopulmonary resuscitation. Pediatrics. 2010;125:e481–8. [DOI] [PubMed] [Google Scholar]
- 34. Radman M, McGuire J, Zimmerman J. Childhood obesity, endothelial cell activation, and critical illness. Front Pediatr. 2020;8:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ells LJ, Lang R, Shield JP, Wilkinson JR, Lidstone JS, Coulton S, et al. Obesity and disability—a short review. Obes Rev. 2006;7:341–5. [DOI] [PubMed] [Google Scholar]
- 36. Antony B, Jones G, Venn A, Cicuttini F, March L, Blizzard L, et al. Association between childhood overweight measures and adulthood knee pain, stiffness and dysfunction: a 25‐year cohort study. Ann Rheum Dis. 2015;74:711–7. [DOI] [PubMed] [Google Scholar]
- 37. Ji Z, Wu S, Xu Y, Qi J, Su X, Shen L. Obesity promotes EAE through IL‐6 and CCL‐2‐mediated T cells infiltration. Front Immunol. 2019;10:1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marrodan M, Farez MF, Balbuena Aguirre ME, Correale J. Obesity and the risk of multiple sclerosis. The role of leptin. Ann Clin Transl Neurol. 2021;8:406–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schreiner TG, Genes TM. Obesity and multiple sclerosis—a multifaceted association. J Clin Med. 2021;10:2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pakpoor J, Schmierer K, Cuzick J, Giovannoni G, Dobson R. Estimated and projected burden of multiple sclerosis attributable to smoking and childhood and adolescent high body‐mass index: a comparative risk assessment. Int J Epidemiol. 2021;49:2051–7. [DOI] [PubMed] [Google Scholar]
- 41. Mobasseri M, Shirmohammadi M, Amiri T, Vahed N, Hosseini Fard H, Ghojazadeh M. Prevalence and incidence of type 1 diabetes in the world: a systematic review and meta‐analysis. Health Promot Perspect. 2020;10:98–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Delli AJ, Lindblad B, Carlsson A, Forsander G, Ivarsson S‐A, Ludvigsson J, et al. Type 1 diabetes patients born to immigrants to Sweden increase their native diabetes risk and differ from Swedish patients in HLA types and islet autoantibodies. Pediatr Diabetes. 2010;11:513–20. [DOI] [PubMed] [Google Scholar]
- 43. Fourlanos S, Harrison LC, Colman PG. The accelerator hypothesis and increasing incidence of type 1 diabetes. Curr Opin Endocrinol Diabetes Obes. 2008;15:321–5. [DOI] [PubMed] [Google Scholar]
- 44. Carlsson A, Kockum I, Lindblad B, Engleson L, Nilsson A, Forsander G, et al. Low risk HLA‐DQ and increased body mass index in newly diagnosed type 1 diabetes children in the Better Diabetes Diagnosis study in Sweden. Int J Obes (Lond). 2012;36:718–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Redondo MJ, Sosenko J, Libman I, McVean JJF, Tosur M, Atkinson MA, et al. Single islet autoantibody at diagnosis of clinical type 1 diabetes is associated with older age and insulin resistance. J Clin Endocrinol Metab. 2020;105:1629–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ferrara‐Cook C, Geyer SM, Evans‐Molina C, Libman IM, Becker DJ, Gitelman SE, et al. Excess BMI accelerates islet autoimmunity in older children and adolescents. Diabetes Care. 2020;43:580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shreberk‐Hassidim R, Galili E, Hassidim A, Ramot Y, Merdler I, Baum S, et al. Epidemiology and comorbidities of psoriasis among Israeli adolescents: a large cross‐sectional Study. Dermatology. 2019;235:488–94. [DOI] [PubMed] [Google Scholar]
- 48. Raisanen L, Lommi S, Engberg E, Kolho KL, Viljakainen H. Central obesity in school‐aged children increases the likelihood of developing paediatric autoimmune diseases. Pediatr Obes. 2022;17:e12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52. [DOI] [PubMed] [Google Scholar]
- 50. Sommer A, Twig G. The impact of childhood and adolescent obesity on cardiovascular risk in adulthood: a systematic review. Curr Diab Rep. 2018;18:91. [DOI] [PubMed] [Google Scholar]
- 51. Tounian P, Aggoun Y, Dubern B, Varille V, Guy‐Grand B, Sidi D, et al. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet. 2001;358:1400–4. [DOI] [PubMed] [Google Scholar]
- 52. Hedvall Kallerman P, Hagman E, Edstedt Bonamy AK, Zemack H, Marcus C, Norman M, et al. Obese children without comorbidities have impaired microvascular endothelial function. Acta Paediatr. 2014;103:411–7. [DOI] [PubMed] [Google Scholar]
- 53. Hagman E, Danielsson P, Elimam A, Marcus C. The effect of weight loss and weight gain on blood pressure in children and adolescents with obesity. Int J Obes (Lond). 2019;43:1988–94. [DOI] [PubMed] [Google Scholar]
- 54. Chiolero A, Cachat F, Burnier M, Paccaud F, Bovet P. Prevalence of hypertension in schoolchildren based on repeated measurements and association with overweight. J Hypertens. 2007;25:2209–17. [DOI] [PubMed] [Google Scholar]
- 55. Soergel M, Kirschstein M, Busch C, Danne T, Gellermann J, Holl R, et al. Oscillometric twenty‐four‐hour ambulatory blood pressure values in healthy children and adolescents: a multicenter trial including 1141 subjects. J Pediatr. 1997;130:178–84. [DOI] [PubMed] [Google Scholar]
- 56. Hermida RC, Ayala DE, Fernandez JR, Portaluppi F, Fabbian F, Smolensky MH. Circadian rhythms in blood pressure regulation and optimization of hypertension treatment with ACE inhibitor and ARB medications. Am J Hypertens. 2011;24:383–91. [DOI] [PubMed] [Google Scholar]
- 57. Westerstahl M, Hedvall Kallerman P, Hagman E, Ek AE, Rossner SM, Marcus C. Nocturnal blood pressure non‐dipping is prevalent in severely obese, prepubertal and early pubertal children. Acta Paediatr. 2014;103:225–30. [DOI] [PubMed] [Google Scholar]
- 58. Westerstahl M, Marcus C. Association between nocturnal blood pressure dipping and insulin metabolism in obese adolescents. Int J Obes (Lond). 2010;34:472–7. [DOI] [PubMed] [Google Scholar]
- 59. Rodrigues AN, Abreu GR, Resende RS, Goncalves WL, Gouvea SA. Cardiovascular risk factor investigation: a pediatric issue. Int J Gen Med. 2013;6:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ells LJ, Rees K, Brown T, Mead E, Al‐Khudairy L, Azevedo L, et al. Interventions for treating children and adolescents with overweight and obesity: an overview of Cochrane reviews. Int J Obes (Lond). 2018;42:1823–33. [DOI] [PubMed] [Google Scholar]
- 61. Olbers T, Beamish AJ, Gronowitz E, Flodmark C‐E, Dahlgren J, Bruze G, et al. Laparoscopic Roux‐en‐Y gastric bypass in adolescents with severe obesity (AMOS): a prospective, 5‐year, Swedish nationwide study. Lancet Diabetes Endocrinol. 2017;5:174–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365:1876–85. [DOI] [PubMed] [Google Scholar]
- 63. Kindblom JM, Bygdell M, Hjelmgren O, Martikainen J, Rosengren A, Bergstrom G, et al. Pubertal body mass index change is associated with adult coronary atherosclerosis and acute coronary events in men. Arterioscler Thromb Vasc Biol. 2021;41:2318–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tirosh A, Shai I, Afek A, Dubnov‐Raz G, Ayalon N, Gordon B, et al. Adolescent BMI trajectory and risk of diabetes versus coronary disease. N Engl J Med. 2011;364:1315–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ross R, Blair SN, Arena R, Church TS, Despres J‐P, Franklin BA, et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134:e653–99. [DOI] [PubMed] [Google Scholar]
- 66. Ballin M, Nordstrom A, Nordstrom P. Cardiovascular disease and all‐cause mortality in male twins with discordant cardiorespiratory fitness: a nationwide cohort study. Am J Epidemiol. 2020;189:1114–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Henriksson H, Henriksson P, Tynelius P, Ekstedt M, Berglind D, Labayen I, et al. Cardiorespiratory fitness, muscular strength, and obesity in adolescence and later chronic disability due to cardiovascular disease: a cohort study of 1 million men. Eur Heart J. 2020;41:1503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mora‐Gonzalez J, Esteban‐Cornejo I, Cadenas‐Sanchez C, Migueles JH, Molina‐Garcia P, Rodriguez‐Ayllon MA, et al. Physical fitness, physical activity, and the executive function in children with overweight and obesity. J Pediatr. 2019;208:50–6.e1. [DOI] [PubMed] [Google Scholar]
- 69. Berndtsson G, Mattsson E, Marcus C, Larsson UE. Age and gender differences in VO2max in Swedish obese children and adolescents. Acta Paediatr. 2007;96:567–71. [DOI] [PubMed] [Google Scholar]
- 70. Johansson L, Brissman M, Morinder G, Westerstahl M, Marcus C. Reference values and secular trends for cardiorespiratory fitness in children and adolescents with obesity. Acta Paediatr. 2020;109:1665–71. [DOI] [PubMed] [Google Scholar]
- 71. Bhammar DM, Adams‐Huet B, Babb TG. Quantification of cardiorespiratory fitness in children with obesity. Med Sci Sports Exerc. 2019;51:2243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Brissman M, Ekbom K, Hagman E, Marild S, Gronowitz E, Flodmark C‐E, et al. Physical fitness and body composition two years after Roux‐En‐Y gastric bypass in adolescents. Obes Surg. 2017;27:330–7. [DOI] [PubMed] [Google Scholar]
- 73. Brown AE, Walker M. Genetics of insulin resistance and the metabolic syndrome. Curr Cardiol Rep. 2016;18:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Beltrand J, Levy‐Marchal C. Pathophysiology of insulin resistance in subjects born small for gestational age. Best Pract Res Clin Endocrinol Metab. 2008;22:503–15. [DOI] [PubMed] [Google Scholar]
- 75. Barclay AW, Augustin LSA, Brighenti F, Delport E, Henry CJ, Sievenpiper JL, et al. Dietary glycaemic index labelling: a global perspective. Nutrients. 2021;13:3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Emerging Risk Factors Collaboration , Sarwar N, Gao P, Kondapally Seshasai SR, Gobin R, Kaptoge S, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta‐analysis of 102 prospective studies. Lancet. 2010;375:2215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Huang Y, Cai X, Chen P, Mai W, Tang H, Huang Y, et al. Associations of prediabetes with all‐cause and cardiovascular mortality: a meta‐analysis. Ann Med. 2014;46:684–92. [DOI] [PubMed] [Google Scholar]
- 78. Huang Y, Cai X, Qiu M, Chen P, Tang H, Hu Y, et al. Prediabetes and the risk of cancer: a meta‐analysis. Diabetologia. 2014;57:2261–9. [DOI] [PubMed] [Google Scholar]
- 79. Levitan EB, Song Y, Ford ES, Liu S. Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? A meta‐analysis of prospective studies. Arch Intern Med. 2004;164:2147–55. [DOI] [PubMed] [Google Scholar]
- 80. Weinstein G, Maillard P, Himali JJ, Beiser AS, Au R, Wolf PA, et al. Glucose indices are associated with cognitive and structural brain measures in young adults. Neurology. 2015;84:2329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Baranowski T, Cooper DM, Harrell J, Hirst K, Kaufman FR, Goran M, et al. Presence of diabetes risk factors in a large U.S. eighth‐grade cohort. Diabetes Care. 2006;29:212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hagman E, Reinehr T, Kowalski J, Ekbom A, Marcus C, Holl RW. Impaired fasting glucose prevalence in two nationwide cohorts of obese children and adolescents. Int J Obes (Lond). 2014;38:40–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Brufani C, Ciampalini P, Grossi A, Fiori R, Fintini D, Tozzi A, et al. Glucose tolerance status in 510 children and adolescents attending an obesity clinic in Central Italy. Pediatr Diabetes. 2010;11:47–54. [DOI] [PubMed] [Google Scholar]
- 84. Yamamoto‐Kimura L, Posadas‐Romero C, Posadas‐Sanchez R, Zamora‐Gonzalez J, Cardoso‐Saldana G, Mendez Ramirez I. Prevalence and interrelations of cardiovascular risk factors in urban and rural Mexican adolescents. J Adolesc Health. 2006;38:591–8. [DOI] [PubMed] [Google Scholar]
- 85. Ek AE, Samuelsson U, Janson A, Carlsson A, Elimam A, Marcus C. Microalbuminuria and retinopathy in adolescents and young adults with type 1 and type 2 diabetes. Pediatr Diabetes. 2020;21:1310–21. [DOI] [PubMed] [Google Scholar]
- 86. Weiss R, Taksali SE, Tamborlane WV, Burgert TS, Savoye M, Caprio S. Predictors of changes in glucose tolerance status in obese youth. Diabetes Care. 2005;28:902–9. [DOI] [PubMed] [Google Scholar]
- 87. Koskinen J, Magnussen CG, Sabin MA, Laitinen T, Taittonen L, Jokinen E, et al. Youth overweight and metabolic disturbances in predicting carotid intima‐media thickness, type 2 diabetes, and metabolic syndrome in adulthood: the Cardiovascular Risk in Young Finns study. Diabetes Care. 2014;37:1870–7. [DOI] [PubMed] [Google Scholar]
- 88. Sattar N, Rawshani A, Franzen S, Rawshani A, Svensson A‐M, Rosengren A, et al. Age at diagnosis of type 2 diabetes mellitus and associations with cardiovascular and mortality risks. Circulation. 2019;139:2228–37. [DOI] [PubMed] [Google Scholar]
- 89. Constantino MI, Molyneaux L, Limacher‐Gisler F, Al‐Saeed A, Luo C, Wu T, et al. Long‐term complications and mortality in young‐onset diabetes: type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care. 2013;36:3863–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dabelea D, Stafford JM, Mayer‐Davis EJ, Dolan L, Imperatore G, Linder B, et al. Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA. 2017;317:825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Svensson M, Sundkvist G, Arnqvist HJ, Bjoìk E, Blohmeì GR, Bolinder J, et al. Signs of nephropathy may occur early in young adults with diabetes despite modern diabetes management: results from the nationwide population‐based Diabetes Incidence Study in Sweden (DISS). Diabetes Care. 2003;26:2903–9. [DOI] [PubMed] [Google Scholar]
- 92. Shapiro ALB, Dabelea D, Stafford JM, Dâgostino R, Pihoker C, Liese AD, et al. Cognitive function in adolescents and young adults with youth‐onset type 1 versus type 2 diabetes: the SEARCH for Diabetes in Youth study. Diabetes Care. 2021;44:1273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Geng T, Smith CE, Li C, Huang T. Childhood BMI and adult type 2 diabetes, coronary artery diseases, chronic kidney disease, and cardiometabolic traits: a Mendelian randomization analysis. Diabetes Care. 2018;41:1089–96. [DOI] [PubMed] [Google Scholar]
- 94. Ohlsson C, Bygdell M, Nethander M, Rosengren A, Kindblom JM. BMI change during puberty is an important determinant of adult type 2 diabetes risk in men. J Clin Endocrinol Metab. 2019;104:1823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Reinehr T, Kiess W, Kapellen T, Andler W. Insulin sensitivity among obese children and adolescents, according to degree of weight loss. Pediatrics. 2004;114:1569–73. [DOI] [PubMed] [Google Scholar]
- 96. Vajro P, Lenta S, Socha P, Dhawan A, Mckiernan P, Baumann U, et al. Diagnosis of nonalcoholic fatty liver disease in children and adolescents: position paper of the ESPGHAN Hepatology Committee. J Pediatr Gastroenterol Nutr. 2012;54:700–13. [DOI] [PubMed] [Google Scholar]
- 97. Vos MB, Abrams SH, Barlow SE, Caprio S, Daniels SR, Kohli R et al. NASPGHAN clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children: recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr. 2017;64:319–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sayin O, Tokgoz Y, Arslan N. Investigation of adropin and leptin levels in pediatric obesity‐related nonalcoholic fatty liver disease. J Pediatr Endocrinol Metab. 2014;27:479–84. [DOI] [PubMed] [Google Scholar]
- 99. Anderson EL, Howe LD, Jones HE, Higgins JP, Lawlor DA, Fraser A. The prevalence of non‐alcoholic fatty liver disease in children and adolescents: a systematic review and meta‐analysis. PLoS One. 2015;10:e0140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Cali AM, De Oliveira AM, Kim H, Chen S, Reyes‐Mugica M, Escalera S, et al. Glucose dysregulation and hepatic steatosis in obese adolescents: is there a link? Hepatology. 2009;49:1896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Greber‐Platzer S, Thajer A, Bohn S, Brunert A, Boerner F, Siegfried W, et al. Increased liver echogenicity and liver enzymes are associated with extreme obesity, adolescent age and male gender: analysis from the German/Austrian/Swiss obesity registry APV. BMC Pediatr. 2019;19:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Koutny F, Weghuber D, Bollow E, Greberâlatzer S, Hartmann K, Kaner A, et al. Prevalence of prediabetes and type 2 diabetes in children with obesity and increased transaminases in European German‐speaking countries. Analysis of the APV initiative. Pediatr Obes. 2020;15:e12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Putri RR, Casswall T, Hagman E. Prevalence of increased transaminases and its association with sex, age, and metabolic parameters in children and adolescents with obesity—a nationwide cross‐sectional cohort study. BMC Pediatr. 2021;21:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Eslam M, Alkhouri N, Vajro P, Baumann U, Weiss R, Socha P, et al. Defining paediatric metabolic (dysfunction)‐associated fatty liver disease: an international expert consensus statement. Lancet Gastroenterol Hepatol. 2021;6:864–73. [DOI] [PubMed] [Google Scholar]
- 105. Simon TG, Roelstraete B, Hartjes K, Shah U, Khalili H, Arnell H, et al. Non‐alcoholic fatty liver disease in children and young adults is associated with increased long‐term mortality. J Hepatol. 2021;75:1034–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Tomah S, Alkhouri N, Hamdy O. Nonalcoholic fatty liver disease and type 2 diabetes: where do diabetologists stand? Clin Diabetes Endocrinol. 2020;6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Newton KP, Hou J, Crimmins NA, Lavine JE, Barlow SE, Xanthakos SA, et al. Prevalence of prediabetes and type 2 diabetes in children with nonalcoholic fatty liver disease. JAMA Pediatr. 2016;170:e161971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Bardugo A, Bendor CD, Zucker I, Lutski M, Cukierman‐Yaffe T, Derazne E, et al. Adolescent nonalcoholic fatty liver disease and type 2 diabetes in young adulthood. J Clin Endocrinol Metab. 2021;106:e34–44. [DOI] [PubMed] [Google Scholar]
- 109. Koutny F, Stein R, Kiess W, Weghuber D, Korner A. Elevated transaminases potentiate the risk for emerging dysglycemia in children with overweight and obesity. Pediatr Obes. 2021;16:e12822. [DOI] [PubMed] [Google Scholar]
- 110. D'Adamo E, Cali AM, Weiss R, Santoro N, Pierpont B, Northrup V, et al. Central role of fatty liver in the pathogenesis of insulin resistance in obese adolescents. Diabetes Care. 2010;33:1817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Arellano‐Garcia L, Portillo MP, Martinez JA, Milton‐Laskibar I. Usefulness of probiotics in the management of NAFLD: evidence and involved mechanisms of action from preclinical and human models. Int J Mol Sci. 2022;23:3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Guimber D, Debray D, Bocquet A, Briend A, Chouraqui J‐P, Darmaun D, et al. The role of nutrition in non‐alcoholic fatty liver disease treatment in obese children. Arch Pediatr. 2022;29:1–11. [DOI] [PubMed] [Google Scholar]
- 113. Gronbaek H, Lange A, Birkebaek NH, Holland‐Fischer P, Solvig J, Halyck A, et al. Effect of a 10‐week weight loss camp on fatty liver disease and insulin sensitivity in obese Danish children. J Pediatr Gastroenterol Nutr. 2012;54:223–8. [DOI] [PubMed] [Google Scholar]
- 114. Nobili V, Marcellini M, Devito R, Ciampalini P, Piemonte F, Comparcola D, et al. NAFLD in children: a prospective clinical‐pathological study and effect of lifestyle advice. Hepatology. 2006;44:458–65. [DOI] [PubMed] [Google Scholar]
- 115. Reinehr T, Kleber M, Toschke AM. Lifestyle intervention in obese children is associated with a decrease of the metabolic syndrome prevalence. Atherosclerosis. 2009;207:174–80. [DOI] [PubMed] [Google Scholar]
- 116. Wang CL, Liang L, Fu JF, Zou C‐C, Hong F, Xue J‐Z, et al. Effect of lifestyle intervention on non‐alcoholic fatty liver disease in Chinese obese children. World J Gastroenterol. 2008;14:1598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Putri RR, Casswall T, Hagman E. Risk and protective factors of non‐alcoholic fatty liver disease in paediatric obesity: a nationwide nested case‐control study. Clin Obes. 2022;12:e12502. [DOI] [PubMed] [Google Scholar]
- 118. Cuthbertson DJ, Brown E, Koskinen J, Magnussen CG, Sabin M, Tossavainen P, et al. Longitudinal analysis of risk of non‐alcoholic fatty liver disease in adulthood. Liver Int. 2019;39:1147–54. [DOI] [PubMed] [Google Scholar]
- 119. Vigone MC, Capalbo D, Weber G, Salerno M. Mild hypothyroidism in childhood: who, when, and how should be treated? J Endocr Soc. 2018;2:1024–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Lundback V, Ekbom K, Hagman E, Dahlman I, Marcus C. Thyroid‐stimulating hormone, degree of obesity, and metabolic risk markers in a cohort of Swedish children with obesity. Horm Res Paediatr. 2017;88:140–6. [DOI] [PubMed] [Google Scholar]
- 121. Pacifico L, Anania C, Ferraro F, Andreoli GM, Chiesa C. Thyroid function in childhood obesity and metabolic comorbidity. Clin Chim Acta. 2012;413:396–405. [DOI] [PubMed] [Google Scholar]
- 122. Janson A, Rawet H, Perbeck L, Marcus C. Presence of thyrotropin receptor in infant adipocytes. Pediatr Res. 1998;43:555–8. [DOI] [PubMed] [Google Scholar]
- 123. Drvota V, Janson A, Norman C, Sylven C, Haggblad J, Bronnegard M, et al. Evidence for the presence of functional thyrotropin receptor in cardiac muscle. Biochem Biophys Res Commun. 1995;211:426–31. [DOI] [PubMed] [Google Scholar]
- 124. Gianfrancesco MA, Stridh P, Rhead B, Shao X, Xu E, Graves JS, et al. Evidence for a causal relationship between low vitamin D, high BMI, and pediatric‐onset MS. Neurology. 2017;88:1623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ekbom K, Marcus C. Vitamin D deficiency is associated with prediabetes in obese Swedish children. Acta Paediatr. 2016;105:1192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Szternel L, Krintus M, Bergmann K, Derezinski T, Sypniewska G. Association between fasting glucose concentration, lipid profile and 25(OH)D status in children aged 9(‐)11. Nutrients. 2018;10:1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Contreras‐Bolivar V, Garcia‐Fontana B, Garcia‐Fontana C, Munoz‐Torres M. Mechanisms involved in the relationship between vitamin D and insulin resistance: impact on clinical practice. Nutrients. 2021;13:3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Pittas AG, Dawson‐Hughes B, Sheehan P, Ware JH, Knowler WC, Aroda VR, et al. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med. 2019;381:520–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Rajakumar K, Moore CG, Khalid AT, Vallejo AN, Virji MA, Holick MF, et al. Effect of vitamin D3 supplementation on vascular and metabolic health of vitamin D‐deficient overweight and obese children: a randomized clinical trial. Am J Clin Nutr. 2020;111:757–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Migliaccio S, Di Nisio A, Mele C, Scappaticcio L, Savastano S, Colao A. Obesity and hypovitaminosis D: causality or casualty? Int J Obes Suppl. 2019;9:20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Ekbom K, Lundback V, Marcus C. Follow‐up study found that vitamin D deficiency and weight gain increased the risk of impaired fasting glycaemia. Acta Paediatr. 2020;109:847–8. [DOI] [PubMed] [Google Scholar]
- 132. Lagunova Z, Porojnicu AC, Lindberg FA, Aksnes L, Moan J. Vitamin D status in Norwegian children and adolescents with excess body weight. Pediatr Diabetes. 2011;12:120–6. [DOI] [PubMed] [Google Scholar]
- 133. Alemzadeh R, Kichler J, Babar G, Calhoun M. Hypovitaminosis D in obese children and adolescents: relationship with adiposity, insulin sensitivity, ethnicity, and season. Metabolism. 2008;57:183–91. [DOI] [PubMed] [Google Scholar]
- 134. Wamberg L, Christiansen T, Paulsen SK, Fisker S, Rask P, Rejnmark L, et al. Expression of vitamin D‐metabolizing enzymes in human adipose tissue—the effect of obesity and diet‐induced weight loss. Int J Obes (Lond). 2013;37:651–7. [DOI] [PubMed] [Google Scholar]
- 135. Takahashi Y. The role of growth hormone and insulin‐like growth factor‐I in the liver. Int J Mol Sci. 2017;18:1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Dehkhoda F, Lee CMM, Medina J, Brooks AJ. The growth hormone receptor: mechanism of receptor activation, cell signaling, and physiological aspects. Front Endocrinol (Lausanne). 2018;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Martinez‐Moreno CG, Aramburo C. Growth hormone (GH) and synaptogenesis. Vitam Horm. 2020;114:91–123. [DOI] [PubMed] [Google Scholar]
- 138. Shalitin S, Kiess W. Putative effects of obesity on linear growth and puberty. Horm Res Paediatr. 2017;88:101–10. [DOI] [PubMed] [Google Scholar]
- 139. Stabler B, Clopper RR, Siegel PT, Nicholas LM, Silva SG, Tancer ME, et al. Links between growth hormone deficiency, adaptation and social phobia. Horm Res. 1996;45:30–3. [DOI] [PubMed] [Google Scholar]
- 140. Szarka N, Szellar D, Kiss S, Farkas N, Szakacs Z, Czigler A, et al. Effect of growth hormone on neuropsychological outcomes and quality of life of patients with traumatic brain injury: a systematic review. J Neurotrauma. 2021;38:1467–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Glaser DL, Kaplan FS. Osteoporosis. Definition and clinical presentation. Spine (Phila Pa 1976). 1997;22:12S–6S. [DOI] [PubMed] [Google Scholar]
- 142. Wang L, Zhang H, Wang S, Chen X, Su J. Bone marrow adipocytes: a critical player in the bone marrow microenvironment. Front Cell Dev Biol. 2021;9:770705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Pollock NK, Bernard PJ, Wenger K, Misra S, Gower BA, Allison JD, et al. Lower bone mass in prepubertal overweight children with prediabetes. J Bone Miner Res. 2010;25:2760–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Pollock NK, Bernard PJ, Gutin B, Davis CL, Zhu H, Dong Y. Adolescent obesity, bone mass, and cardiometabolic risk factors. J Pediatr. 2011;158:727–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Kessler J, Koebnick C, Smith N, Adams A. Childhood obesity is associated with increased risk of most lower extremity fractures. Clin Orthop Relat Res. 2013;471:1199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Lane JC, Butler KL, Poveda‐Marina JL, Martinezâaguna D, Reyes C, Bont J, et al. Preschool obesity is associated with an increased risk of childhood fracture: a longitudinal cohort study of 466,997 children and up to 11 years of follow‐up in Catalonia, Spain. J Bone Miner Res. 2020;35:1022–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kim JE, Hsieh MH, Soni BK, Zayzafoon M, Allison DB. Childhood obesity as a risk factor for bone fracture: a mechanistic study. Obesity (Silver Spring). 2013;21:1459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Cole ZA, Harvey NC, Kim M, Ntani G, Robinson SM, Inskip HM, et al. Increased fat mass is associated with increased bone size but reduced volumetric density in pre pubertal children. Bone. 2012;50:562–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Pollock NK. Childhood obesity, bone development, and cardiometabolic risk factors. Mol Cell Endocrinol. 2015;410:52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Lu J, Shin Y, Yen MS, Sun SS. Peak bone mass and patterns of change in total bone mineral density and bone mineral contents from childhood into young adulthood. J Clin Densitom. 2016;19:180–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Beamish AJ, Gronowitz E, Olbers T, Flodmark CE, Marcus C, Dahlgren J. Body composition and bone health in adolescents after Roux‐en‐Y gastric bypass for severe obesity. Pediatr Obes. 2017;12:239–46. [DOI] [PubMed] [Google Scholar]
- 152. Burt Solorzano CM, McCartney CR. Obesity and the pubertal transition in girls and boys. Reproduction. 2010;140:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Sansone A, Romanelli F, Sansone M, Lenzi A, Di Luigi L. Gynecomastia and hormones. Endocrine. 2017;55:37–44. [DOI] [PubMed] [Google Scholar]
- 154. Best D, Avenell A, Bhattacharya S. How effective are weight‐loss interventions for improving fertility in women and men who are overweight or obese? A systematic review and meta‐analysis of the evidence. Hum Reprod Update. 2017;23:681–705. [DOI] [PubMed] [Google Scholar]
- 155. Sliwowska JH, Fergani C, Gawalek M, Skowronska B, Fichna P, Lehman MN. Insulin: its role in the central control of reproduction. Physiol Behav. 2014;133:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Chandrasekaran S, Neal‐Perry G. Long‐term consequences of obesity on female fertility and the health of the offspring. Curr Opin Obstet Gynecol. 2017;29:180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Mushtaq R, Pundir J, Achilli C, Naji O, Khalaf Y, El‐Toukhy T. Effect of male body mass index on assisted reproduction treatment outcome: an updated systematic review and meta‐analysis. Reprod Biomed Online. 2018;36:459–71. [DOI] [PubMed] [Google Scholar]
- 158. Barbagallo F, Condorelli RA, Mongioi LM, Cannarella R, Cimino L, Magagnini MC, et al. Molecular mechanisms underlying the relationship between obesity and male infertility. Metabolites. 2021;11:840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Carrizales‐Sepulveda EF, Ordaz‐Farias A, Vera‐Pineda R, Flores‐Ramirez R. Periodontal disease, systemic inflammation and the risk of cardiovascular disease. Heart Lung Circ. 2018;27:1327–34. [DOI] [PubMed] [Google Scholar]
- 160. Choi YH, McKeown RE, Mayer‐Davis EJ, Liese AD, Song KB, Merchant AT. Association between periodontitis and impaired fasting glucose and diabetes. Diabetes Care. 2011;34:381–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Koromantzos PA, Makrilakis K, Dereka X, Katsilambros N, Vrotsos IA, Madianos PN. A randomized, controlled trial on the effect of non‐surgical periodontal therapy in patients with type 2 diabetes. Part I: effect on periodontal status and glycaemic control. J Clin Periodontol. 2011;38:142–7. [DOI] [PubMed] [Google Scholar]
- 162. Sharma A, Hegde AM. Relationship between body mass index, caries experience and dietary preferences in children. J Clin Pediatr Dent. 2009;34:49–52. [DOI] [PubMed] [Google Scholar]
- 163. Hayden C, Bowler JO, Chambers S, Freeman R, Humphris G, Richards D, et al. Obesity and dental caries in children: a systematic review and meta‐analysis. Community Dent Oral Epidemiol. 2013;41:289–308. [DOI] [PubMed] [Google Scholar]
- 164. Guare RO, Perez MM, Novaes TF, Ciamponi AL, Gorjao R, Diniz MB. Overweight/obese children are associated with lower caries experience than normal‐weight children/adolescents. Int J Paediatr Dent. 2019;29:756–64. [DOI] [PubMed] [Google Scholar]
- 165. Garcia Perez A, Barrera Ortega CC, Gonzalez‐Aragon Pineda AE, Villanueva Gutierrez T, Perez Perez NG, Calderon Uriostegui D. An inverse relationship between obesity and dental caries in Mexican schoolchildren: a cross‐sectional study. Public Health. 2020;180:163–7. [DOI] [PubMed] [Google Scholar]
- 166. Modeer T, Blomberg CC, Wondimu B, Julihn A, Marcus C. Association between obesity, flow rate of whole saliva, and dental caries in adolescents. Obesity (Silver Spring). 2010;18:2367–73. [DOI] [PubMed] [Google Scholar]
- 167. Khosravi R, Ka K, Huang T, Khalili S, Nguyen BH, Nicolau B, et al. Tumor necrosis factor–alpha and interleukin‐6: potential interorgan inflammatory mediators contributing to destructive periodontal disease in obesity or metabolic syndrome. Mediators Inflamm. 2013;2013:728987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Lundin M, Yucel‐Lindberg T, Dahllof G, Marcus C, Modeer T. Correlation between TNFalpha in gingival crevicular fluid and body mass index in obese subjects. Acta Odontol Scand. 2004;62:273–7. [DOI] [PubMed] [Google Scholar]
- 169. Zeigler CC, Persson GR, Wondimu B, Marcus C, Sobko T, Modeer T. Microbiota in the oral subgingival biofilm is associated with obesity in adolescence. Obesity (Silver Spring). 2012;20:157–64. [DOI] [PubMed] [Google Scholar]
- 170. Zeigler CC, Wondimu B, Marcus C, Modeer T. Pathological periodontal pockets are associated with raised diastolic blood pressure in obese adolescents. BMC Oral Health. 2015;15:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Laitinen J, Power C, Ek E, Sovio U, Jarvelin MR. Unemployment and obesity among young adults in a northern Finland 1966 birth cohort. Int J Obes Relat Metab Disord. 2002;26:1329–38. [DOI] [PubMed] [Google Scholar]
- 172. Karnehed N, Rasmussen F, Hemmingsson T, Tynelius P. Obesity and attained education: cohort study of more than 700,000 Swedish men. OBES. 2006;14:1421–8. [DOI] [PubMed] [Google Scholar]
- 173. Finn KE, Seymour CM, Phillips AE. Weight bias and grading among middle and high school teachers. Br J Educ Psychol. 2020;90:635–47. [DOI] [PubMed] [Google Scholar]
- 174. Lindberg L, Hagman E, Danielsson P, Marcus C, Persson M. Anxiety and depression in children and adolescents with obesity: a nationwide study in Sweden. BMC Med. 2020;18:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Neumark‐Sztainer D, Falkner N, Story M, Perry C, Hannan PJ, Mulert S. Weight‐teasing among adolescents: correlations with weight status and disordered eating behaviors. Int J Obes Relat Metab Disord. 2002;26:123–31. [DOI] [PubMed] [Google Scholar]
- 176. Sweat V, Yates KF, Migliaccio R, Convit A. Obese adolescents show reduced cognitive processing speed compared with healthy weight peers. Child Obes. 2017;13:190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Tsai CL, Chen FC, Pan CY, Tseng YT. The neurocognitive performance of visuospatial attention in children with obesity. Front Psychol. 2016;7:1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Laurent JS, Watts R, Adise S, Allgaier N, Chaarani B, Garavan H, et al. Associations among body mass index, cortical thickness, and executive function in children. JAMA Pediatr. 2020;174:170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Kothari V, Luo Y, Tornabene T, O'Neill AM, Greene MW, Geetha T, et al. High fat diet induces brain insulin resistance and cognitive impairment in mice. Biochim Biophys Acta Mol Basis Dis. 2017;1863:499–508. [DOI] [PubMed] [Google Scholar]
- 180. Shi H, Yu Y, Lin D, Zheng P, Zhang P, Hu M, et al. Beta‐glucan attenuates cognitive impairment via the gut‐brain axis in diet‐induced obese mice. Microbiome. 2020;8:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Alosco ML, Spitznagel MB, Strain G, Devlin M, Cohen R, Paul R, et al. Improved memory function two years after bariatric surgery. Obesity (Silver Spring). 2014;22:32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Baskaran C, Animashaun A, Rickard F, Toth AT, Eddy KT, Plessow F, et al. Memory and executive function in adolescent and young adult females with moderate to severe obesity before and after weight loss surgery. Obes Surg. 2021;31:3372–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. van Dam PS. Neurocognitive function in adults with growth hormone deficiency. Horm Res. 2005;64(3):109–14. [DOI] [PubMed] [Google Scholar]
- 184. van Nieuwpoort IC, Drent ML. Cognition in the adult with childhood‐onset GH deficiency. Eur J Endocrinol. 2008;159(1):S53–7. [DOI] [PubMed] [Google Scholar]
- 185. Spitznagel MB, Alosco M, Inge TH, Rochette A, Strain G, Devlin M, et al. Adolescent weight history and adult cognition: before and after bariatric surgery. Surg Obes Relat Dis. 2016;12:1057–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Wentz E, Bjork A, Dahlgren J. Neurodevelopmental disorders are highly over‐represented in children with obesity: a cross‐sectional study. Obesity (Silver Spring). 2017;25:178–84. [DOI] [PubMed] [Google Scholar]
- 187. Cortese S. The association between ADHD and obesity: intriguing, progressively more investigated, but still puzzling. Brain Sci. 2019;9:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Barker ED, Ing A, Biondo F, Jia T, Pingault J‐B, Du Rietz E, et al. Do ADHD‐impulsivity and BMI have shared polygenic and neural correlates? Mol Psychiatry. 2021;26:1019–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Emerson E. Prevalence of psychiatric disorders in children and adolescents with and without intellectual disability. J Intellect Disabil Res. 2003;47:51–8. [DOI] [PubMed] [Google Scholar]
- 190. Meinzer MC, Pettit JW, Viswesvaran C. The co‐occurrence of attention‐deficit/hyperactivity disorder and unipolar depression in children and adolescents: a meta‐analytic review. Clin Psychol Rev. 2014;34:595–607. [DOI] [PubMed] [Google Scholar]
- 191. Shrewsbury V, Wardle J. Socioeconomic status and adiposity in childhood: a systematic review of cross‐sectional studies 1990–2005. Obesity (Silver Spring). 2008;16:275–84. [DOI] [PubMed] [Google Scholar]
- 192. Muhlig Y, Antel J, Focker M, Hebebrand J. Are bidirectional associations of obesity and depression already apparent in childhood and adolescence as based on high‐quality studies? A systematic review. Obes Rev. 2016;17:235–49. [DOI] [PubMed] [Google Scholar]
- 193. Geoffroy MC, Li L, Power C. Depressive symptoms and body mass index: co‐morbidity and direction of association in a British birth cohort followed over 50 years. Psychol Med. 2014;44:2641–52. [DOI] [PubMed] [Google Scholar]
- 194. Jarvholm K, Karlsson J, Olbers T, Peltonen M, Marcus C, Dahlgren J, et al. Two‐year trends in psychological outcomes after gastric bypass in adolescents with severe obesity. Obesity (Silver Spring). 2015;23:1966–72. [DOI] [PubMed] [Google Scholar]
- 195. Jarvholm K, Olbers T, Peltonen M, Marcus C, Flodmark C‐E, Gronowitz E, et al. Depression, anxiety, and suicidal ideation in young adults 5 years after undergoing bariatric surgery as adolescents. Eat Weight Disord. 2021;26:1211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Jarvholm K, Bruze G, Peltonen M, Marcus C, Flodmark C‐E, Henfridsson P, et al. 5‐year mental health and eating pattern outcomes following bariatric surgery in adolescents: a prospective cohort study. Lancet Child Adolesc Health. 2020;4:210–9. [DOI] [PubMed] [Google Scholar]
- 197. Jarvholm K, Olbers T, Peltonen M, Marcus C, Dahlgren J, Flodmark C‐E, et al. Binge eating and other eating‐related problems in adolescents undergoing gastric bypass: results from a Swedish nationwide study (AMOS). Appetite. 2018;127:349–55. [DOI] [PubMed] [Google Scholar]
- 198. Rook BS, Phillips PH. Pediatric pseudotumor cerebri. Curr Opin Ophthalmol. 2016;27:416–9. [DOI] [PubMed] [Google Scholar]
- 199. Burkett JG, Ailani J. An up to date review of pseudotumor cerebri syndrome. Curr Neurol Neurosci Rep. 2018;18:33. [DOI] [PubMed] [Google Scholar]
- 200. Handley JD, Baruah BP, Williams DM, Horner M, Barry J, Stephens JW. Bariatric surgery as a treatment for idiopathic intracranial hypertension: a systematic review. Surg Obes Relat Dis. 2015;11:1396–403. [DOI] [PubMed] [Google Scholar]
- 201. Lauby‐Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, International Agency for Research on Cancer Handbook Working Group . Body fatness and cancer—viewpoint of the IARC Working Group. N Engl J Med. 2016;375:794–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Brown KF, Rumgay H, Dunlop C, Ryan M, Quartly F, Cox A, et al. The fraction of cancer attributable to modifiable risk factors in England, Wales, Scotland, Northern Ireland, and the United Kingdom in 2015. Br J Cancer. 2018;118:1130–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Furer A, Afek A, Sommer A, Keinan‐Boker L, Derazne E, Levi Z, et al. Adolescent obesity and midlife cancer risk: a population‐based cohort study of 2.3 million adolescents in Israel. Lancet Diabetes Endocrinol. 2020;8:216–25. [DOI] [PubMed] [Google Scholar]
- 204. Aarestrup J, Bjerregaard LG, Meyle KD, Pedersen DC, Gjaerde LK, Jensen BW, et al. Birthweight, childhood overweight, height and growth and adult cancer risks: a review of studies using the Copenhagen School Health Records Register. Int J Obes (Lond). 2020;44:1546–60. [DOI] [PubMed] [Google Scholar]
- 205. Celind J, Ohlsson C, Bygdell M, Martikainen J, Lewerin C, Kindblom JM. Childhood body mass index is associated with the risk of adult hematologic malignancies in men—the best Gothenburg cohort. Int J Cancer. 2020;147:2355–62. [DOI] [PubMed] [Google Scholar]
- 206. Nuotio J, Laitinen TT, Sinaiko AR, Woo JG, Urbina EM, Jacobs DR Jr, et al. Obesity during childhood is associated with higher cancer mortality rate during adulthood: the i3C Consortium. Int J Obes (Lond). 2022;46:393–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Celind J, Ohlsson C, Bygdell M, Nethander M, Kindblom JM. Childhood body mass index is associated with risk of adult colon cancer in men: an association modulated by pubertal change in body mass index. Cancer Epidemiol Biomarkers Prev. 2019;28:974–9. [DOI] [PubMed] [Google Scholar]
- 208. Jensen BW, Gamborg M, Gogenur I, Renehan AG, Sorensen TIA, Baker JL. Childhood body mass index and height in relation to site‐specific risks of colorectal cancers in adult life. Eur J Epidemiol. 2017;32:1097–106. [DOI] [PubMed] [Google Scholar]
- 209. Jensen BW, Bjerregaard LG, Angquist L, Gaenur I, Renehan AG, Osler M, et al. Change in weight status from childhood to early adulthood and late adulthood risk of colon cancer in men: a population‐based cohort study. Int J Obes (Lond). 2018;42:1797–803. [DOI] [PubMed] [Google Scholar]
- 210. Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3:565–74. [DOI] [PubMed] [Google Scholar]
- 211. Gopalakrishnan V, Helmink BA, Spencer CN, Reuben A, Wargo JA. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell. 2018;33:570–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Mossberg HO. 40‐year follow‐up of overweight children. Lancet. 1989;2:491–3. [DOI] [PubMed] [Google Scholar]
- 213. Dietz WH. Childhood weight affects adult morbidity and mortality. J Nutr. 1998;128:411S–4S. [DOI] [PubMed] [Google Scholar]
- 214. Sonntag D, Ali S, Lehnert T, Konnopka A, Riedel‐Heller S, Konig HH. Estimating the lifetime cost of childhood obesity in Germany: results of a Markov model. Pediatr Obes. 2015;10:416–22. [DOI] [PubMed] [Google Scholar]
- 215. Sonntag D, Ali S, De Bock F. Lifetime indirect cost of childhood overweight and obesity: a decision analytic model. Obesity (Silver Spring). 2016;24:200–6. [DOI] [PubMed] [Google Scholar]
- 216. Hamilton D, Dee A, Perry IJ. The lifetime costs of overweight and obesity in childhood and adolescence: a systematic review. Obes Rev. 2018;19:452–63. [DOI] [PubMed] [Google Scholar]
- 217. Neovius K, Rehnberg C, Rasmussen F, Neovius M. Lifetime productivity losses associated with obesity status in early adulthood: a population‐based study of Swedish men. Appl Health Econ Health Policy. 2012;10:309–17. [DOI] [PubMed] [Google Scholar]
- 218. Odegaard K, Borg S, Persson U, Svensson M. The Swedish cost burden of overweight and obesity—evaluated with the PAR approach and a statistical modelling approach. Int J Pediatr Obes. 2008;3:51–7. [DOI] [PubMed] [Google Scholar]
- 219. Hagman E, Danielsson P, Lindberg L, Marcus C, Committee BS. Paediatric obesity treatment during 14 years in Sweden: lessons from the Swedish Childhood Obesity Treatment Register—BORIS. Pediatr Obes. 2020;15:e12626. [DOI] [PubMed] [Google Scholar]
- 220. O'Connor EA, Evans CV, Burda BU, Walsh ES, Eder M, Lozano P. Screening for obesity and intervention for weight management in children and adolescents: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317:2427–44. [DOI] [PubMed] [Google Scholar]
- 221. Hagman E, Johansson L, Kollin C, Marcus E, Drangel A, Marcus L, et al. Effect of an interactive mobile health support system and daily weight measurements for pediatric obesity treatment, a 1‐year pragmatical clinical trial. Int J Obes (Lond). 2022;46:1527–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Azevedo LB, Stephenson J, Ells L, Aduâtiamoah S, Desmet A, Giles EL, et al. The effectiveness of e‐health interventions for the treatment of overweight or obesity in children and adolescents: a systematic review and meta‐analysis. Obes Rev. 2022;23:e13373. [DOI] [PubMed] [Google Scholar]
- 223. Kelly AS, Auerbach P, Barrientos‐Perez M, Gies I, Hale PM, Marcus C, et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N Engl J Med. 2020;382:2117–28. [DOI] [PubMed] [Google Scholar]
- 224. Brissman M, Beamish AJ, Olbers T, Marcus C. Prevalence of insufficient weight loss 5 years after Roux‐en‐Y gastric bypass: metabolic consequences and prediction estimates: a prospective registry study. BMJ Open. 2021;11:e046407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Knop C, Singer V, Uysal Y, Schaefer A, Wolters B, Reinehr T. Extremely obese children respond better than extremely obese adolescents to lifestyle interventions. Pediatr Obes. 2015;10:7–14. [DOI] [PubMed] [Google Scholar]
- 226. Wilfley DE, Saelens BE, Stein RI, Best JR, Kolko RP, Schechtman KB, et al. Dose, content, and mediators of family‐based treatment for childhood obesity: a multisite randomized clinical trial. JAMA Pediatr. 2017;171:1151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Ek A, Lewis Chamberlain K, Sorjonen K, Hammar U, Etminan Malek M, Sandvik P, et al. A parent treatment program for preschoolers with obesity: a randomized controlled trial. Pediatrics. 2019;144:e20183457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Chanoine JP, Hampl S, Jensen C, Boldrin M, Hauptman J. Effect of orlistat on weight and body composition in obese adolescents: a randomized controlled trial. JAMA. 2005;293:2873–83. [DOI] [PubMed] [Google Scholar]
- 229. Olbers T, Gronowitz E, Werling M, Malid S, Flodmark C‐E, Peltonen M, et al. Two‐year outcome of laparoscopic Roux‐en‐Y gastric bypass in adolescents with severe obesity: results from a Swedish nationwide study (AMOS). Int J Obes (Lond). 2012;36:1388–95. [DOI] [PubMed] [Google Scholar]


