Abstract
Herein, we review evidence that targeting mitochondrial dysfunction with ‘mitoceuticals’ is an effective neuroprotective strategy following neurotrauma, and that isolated exogenous mitochondria can be effectively transplanted into host spinal cord parenchyma to increase overall cellular metabolism. We further discuss control measures to ensure greatest potential for mitochondrial transfer, notably using erodible thermogelling hydrogels to deliver respiratory competent mitochondria to the injured spinal cord.
Keywords: Mitochondria, Transplantation, Metabolism, Hydrogel, Spinal cord, Bioenergetics, Oxidative phosphorylation
1. Mitoceuticals for neurotrauma
Emerging neurotrauma therapeutics are evaluating, in particular, pharmacological or transgenic or delivery approaches that target more directly the mitochondrion, the origin(s) of oxidative damage in the injured central nervous system (CNS). In our research investigations, we have characterized certain mitoceuticals which target mitochondrial dysfunction in various ways to promote neuroprotection in models of both traumatic brain and spinal cord injury (TBI and SCI) (Rabchevsky et al., 2020; Semple, 2014). More recently, we have been developing novel approaches to maintain compromised bioenergetics after neurotrauma by transplanting healthy mitochondria into the penumbra of the injured spinal cord (Gollihue and Rabchevsky, 2017). Herein, we summarize mitoceuticals that we have tested in neurotrauma models before introducing novel strategies to deliver respiratory competent mitochondria to the spinal cord.
Cyclosporine A (CsA) was one of the first mitoceuticals tested that that we found to be very neuroprotective following models of TBI (Sullivan et al., 2000; Sullivan et al., 2011), and the mechanism appears to be mediated by its ability to selectively block the mitochondrial permeability transition pore (mPTP) (Readnower et al., 2021). However, early on it was found that similar intraperitoneal CsA treatment is not neuroprotective after contusion SCI (Rabchevsky et al., 2001), which likely reflects both anatomical and physiological differences documented among distinct regions of the CNS even under normal conditions (Sullivan et al., 2004). Critically, all investigations employ oxygen flux analysis respirometry (Clark type electrode or Seahorse XF extracellular flux analyzer) to establish oxygen consumption ratios (OCR) as validation of mitochondrial integrity (see Fig. 1).
Fig. 1.
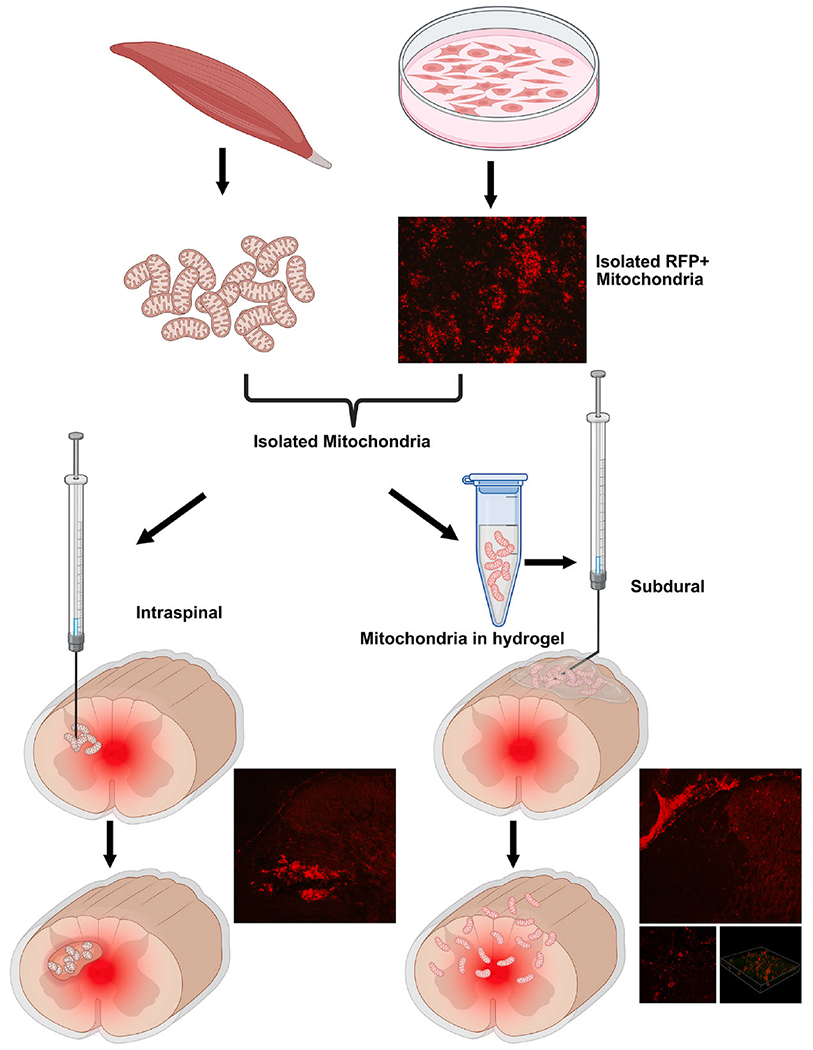
Schematic showing targeted delivery of tissue- or cell line-derived mitochondria into injured spinal cords via Intraspinal (left) or Subdural (right) injection routes, both of which effectively transfer isolated mitochondria at and around the injury. Mitochondria genetically tagged with red fluorescent protein (RFP) and injected into the spinal cord accumulate as a bolus with tissue perturbation. Alternatively, subdural delivery of RFP+ mitochondria via erodible thermo hydrogels obviates parenchymal damage and allows their widespread distribution and graded uptake into host parenchymal cells. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
We first documented mitochondrial dysfunction in a rat model of contusion SCI (Sullivan et al., 2007) and reported significant reductions in the activities of mitochondrial enzyme complexes, but in particular pyruvate dehydrogenase complex (PDHC) (Patel et al., 2010). As it can bypass PDHC to provide acetyl CoA for the Kreb’s cycle, we found that acetyl-l-carnitine (ALC) administered intraperitoneally acutely after SCI significantly maintains respiratory capacity of isolated mitochondria associated with tissue sparing (Patel et al., 2010). When administered 1-hour after injury, ALC maintains mitochondrial function after 24 h and daily administration promotes long-term tissue sparing and hindlimb locomotor recovery (Patel et al., 2012). Then, based on glutathione depletion after injury, we found treatment with a modified glutathione (GSH) precursor, N-acetylcysteine amide (NACA) maintains glutathione levels and mitochondrial function 24hr after SCI, and continuous intraperitoneal delivery leads to chronic functional neuroprotection (Patel et al., 2014). We similarly reported NACA’s functional neuroprotection in TBI models (Pandya et al., 2014), further validating our targeting of mitochondrial dysfunction following neurotrauma (Semple, 2014). As discussed later, these mitoceuticals are the foundation upon which we are designing hydrogels to protect the integrity of mitochondria in vivo.
After we reported that the PPAR-γ agonist, pioglitazone, maintains mitochondrial integrity following TBI (Sauerbeck et al., 2011), SCI mice treated acutely with pioglitazone intraperitoneally showed greater functional neuroprotection, but not attributed to its reported immunomodulatory effects (Patel et al., 2017). Instead, we posited that pioglitazone confers neuroprotection via its interaction with the mitoNEET protein, referred to as CDGSH iron sulfur domain 1 (Geldenhuys et al., 2014), which regulates the voltage-dependent anion channel (VDAC) in a redox-dependent manner, thus closing the mPTP (Lipper et al., 2019). When pioglitazone is administered 15 min or 3 h after SCI it maintains mitochondrial respiration 24 h post-injury in wild-type mice, but similar treatment of mitoNEET knockout (KO) (−/−) mice does not maintain mitochondrial function because pioglitazone is prevented from interacting with mitoNEET (Rabchevsky et al., 2017). Critically, our research group recently reported that pioglitazone indeed targets mitoNEET following TBI (Yonutas et al., 2020), and is effective at elevating bioenergetics even with delayed treatment (Hubbard et al., 2021). We posit that mitoNEET can be targeted with specific ligands to modulate redox and afford therapeutic neuroprotection after neurotrauma.
Together with modulating fusion/fission processes pharmacologically, promoting mitochondrial biogenesis is another strategy used to restore mitochondrial numbers in the injured CNS. Acute and delayed intraperitoneal treatment of the FDA-approved beta2-adrenoreceptor agonist, formoterol, promotes mitochondrial biogenesis after severe mouse SCI, and maintains expression of PGC-1α correlated with improved tissue sparing and hind-limb locomotion (Scholpa et al., 2019). Following experimental TBI formoterol therapy restores mtDNA levels, promotes bioenergetics and increases cognitive function; interestingly, without sparing of cortical tissue (Vekaria et al., 2020).
2. Evolution of mitochondrial transplantation
A burgeoning concept is that mitochondria released into extracellular space after acute neurotrauma can be transferred among cells to support oxidative phosphorylation in recipient cells (Hayakawa et al., 2018; Hayakawa et al., 2016; Nakamura et al., 2020). The evolution of techniques for mitochondrial isolation and transplantation has led to strategies for replacing damaged or dysfunctional ones with exogenous healthy mitochondria (see (Gollihue et al., 2018b; Gollihue and Rabchevsky, 2017)). The mode of transplantation in vivo, however, has been approached in different ways, by 1) direct injection of exogenous mitochondria into injured tissues (Cowan et al., 2016; Gollihue et al., 2018a; Gollihue et al., 2017; Hayakawa et al., 2016; Masuzawa et al., 2013; McCully et al., 2009), 2) utilizing cell-to-cell contact and transfer (Cabrera et al., 2019; Islam et al., 2012), or 3) vesicle mediated cell–cell donation (Hayakawa et al., 2018; Hayakawa et al., 2016). This concept has been extended to explain how transplanted cells (e.g. stem-cell therapy) may manifest their effects by “donating” mitochondria to compromised host cells, especially in light of generally poor cell graft survival/integration (Vignais et al., 2017).
Mitochondrial transfer, itself, is not a new concept. In the early 1980s, it was shown that mitochondria could be transferred into carcinoma cells in vitro (Clark and Shay, 1982), and by using antibiotic-resistant mitochondria they were able to confer resistance to host cells upon successful mitochondrial incorporation. Conferred resistance (King and Attardi, 1988), donation of a desired attribute (Rebbeck et al., 2011), or mtDNA replacement (Katrangi et al., 2007; Yang and Koob, 2012) are outcome measures researchers have used to verify successful mitochondrial transplantation. More recent transplantation studies utilize fluorescent tagging of mitochondria to visualize them in host cells - though caveats exist with various voltage-sensitive dyes (Baek et al., 2016). While researchers have validated that mitochondria can be taken into host cells, there are discrepancies on the exact mechanism(s) by which mitochondria are taken across the plasma membrane and incorporated into the cell. This is an important distinction as the mitochondria are thought be incorporated functionally to effect physiological benefits, not just endocytosed within a vesicle. Whether they do or must fuse with host mitochondrial network is a matter of uncertainty.
There are two clinical trials investigating mitochondrial transplant as a therapeutic for irreparable cardiac ischemia (Emani et al., 2017) (Emani (NCT02851758)) and Pearson bone marrow syndrome (Minovia, NCT03384420), both with promising initial results. While there is debate about the ability of, and/or mechanisms by which transplanted mitochondria confer cardioprotection (Bertero et al., 2018), the clinical results are compelling (Emani and McCully, 2018). Moreover, the cited equivocations have been categorically addressed and rebutted (McCully et al., 2020a, b), with supporting evidence for mitochondrial transplantation to mitigate ischemia reperfusion injuries (Doulamis and McCully, 2021).
3. Mitochondrial transplantation for spinal cord injury
Our rationale and approach to transplant exogenous, well-coupled mitochondria into tissues surrounding contusion SCI sites (Gollihue and Rabchevsky, 2017) theorized that supplementation of endogenous antioxidant systems via mitochondrial transplantation will increase overall Ca2+ buffering capacity and improve energy production of host tissues. Using transgenic-labeled (tGFP) mitochondria for tracking purposes, we found that transplanted mitochondria become co-localized within microglia/brain macrophages, endothelial cells, pericytes, astrocytes and oligodendrocytes, with most abundant internalization in pericytes and brain macrophages; but no evidence of uptake in neurons (Gollihue and Rabchevsky, 2017). While we found that acute mitochondrial transplantation into a contusion injury penumbra significantly maintains bioenergetics after 24 h, such early preservation does not manifest in long-term tissue sparing or functional improvements (Gollihue et al., 2018a).
The approach taken was somewhat naive and based on the seminal studies directly transplanting autologous mitochondrial to mitigate ischemic reperfusion injuries in both pre-clinical and clinical studies (Doulamis and McCully, 2021), in which 7.7 × 106 to 1.3 × 107 mitochondria/ml are injected into myocardium in 50–100 μL injection volumes (Kaza et al., 2017; McCully et al., 2009). Because we found dose-dependent increases in OCR with increased concentrations of mitochondria injected (Gollihue et al., 2018a)), we injected 50 to 150 μg mitochondria totaling 3.0 μL in isolation buffer into four sites of intact tissue. There are slight differences in isolation and respiration buffer constituencies used, but similarities allow extrapolations based on calculations that the liver, kidney and heart have ~8 × 109 mitochondria/mg protein, whereas for the brain it is ~19.5 × 109 (Schmitt et al., 2014). So, in our in vivo transplantation studies, we injected between ~3.6 × 108 (50 ug) and ~18.3 × 108 (150 μg) mitochondria in a total of 3 μL in 4 injection sites compared to ~65 × 107 in a total of 50–100 μL in multiple injection sites. Such enormous differences in concentration make any resolution of whether focal circumferential injections around contusion sites are adequate to spare compromised tissues very difficult, notably because the boluses cause localized inflammation, although not compromising functional recovery (Gollihue et al., 2018a). While cardio-protective effects of direct mitochondrial injections into ischemic myocardium is unequivocal, the extent of ischemia in contused spinal cords is quite varied in incidence and severity, and we were concerned that injected mitochondria could not disperse. Moving forward, we began developing thermogelling erodible hydrogels designed to protect isolated mitochondria in the extracellular environment while being delivered in a controlled manner into the subdural space at the injury site to avert tissue perturbation.
4. Control measures for rigor
It is important to note that the delivery of mitochondria via systemic blood administration has been attempted and reported with limited success. While voltage-dependent dyes suggest the presence of cell-free mitochondria in human blood samples (Stephens et al., 2020), with indications of respiratory competence (Al Amir Dache et al., 2020), high-resolution respirometry shows that circulating cell-free mitochondria are unlikely to be functional in vivo since they display no potential for oxidative phosphorylation (Stier, 2021). Notably, the presence of cell-free mitochondria in human plasma samples is detected using MitoTracker Green fluorescence and complex IV activity, which are not indicators of respiratory capacity. Much is to be explored to explain whether cell-free mitochondria are taken up by platelets or blood immune cells or encapsulated by circulating vesicles to form “mitovesicles” (D’Acunzo et al., 2021).
We employ OCR measurements routinely for validation of mitochondrial integrity prior to transplantation both in vitro or in vivo to ensure that mitochondria are respiratory competent. Once they encounter the spinal cord environment in vivo, however, the physiological concentration of mitochondria that we deliver cannot be determined, empirically, to be sufficiently beneficial or, alternatively, even toxic in vivo. The spinal cord cellular parenchyma is neuroanatomically and regionally heterogenous, unlike the larger myocardium. Controls for labeling mitochondria for the purposes of indelible tracking in host tissues are critical, and in recent studies we note the lack of specificity of certain routinely used membrane potential-sensitive dyes (Patel et al., 2022). Indelible labeling for in vivo studies may require genetically-labeled mitochondria to avoid misinterpretations (Gollihue et al., 2018a; Gollihue et al., 2017), but since other evidence for exists cellular uptake such as detecting mtDNA by qPCR, we do not label mitochondria for short or long-term studies in vivo.
5. Potential
There are many questions that lie ahead regarding the mode and timing of mitochondrial transplantation to mitigate the complex pathophysiology following neurotrauma, notably to target neuroprotection of vulnerable tissues susceptible to ischemia. For instance, how long after transplantation could the mitochondria still have effects, do they fuse with the host cellular network, must we consider the timeline of mitochondrial turnover, are the transplant effects short-lived? In order to deliver to the CNS, intracerebroventricular administration of mitochondria is used as a CNS-specific route in a preclinical model of cerebral ischemia (Zhang et al., 2019), and intravenous administration of mitochondria can target the brain in a preclinical model of Parkinson disease (Shi et al., 2017). However, the mere presence of intravenously injected mitochondria in various tissues, including the brain, was visualized with Mitotracker Red CMXRos staining, but the putative routes of entry are not considered. Also, extracellular mitochondria are procoagulant (Zhao et al., 2020) and there is no evidence that functional mitochondrial can enter the CNS after administered intravenously. As we formulate our hydrogels to incorporate mitochondrial protectants, we aim to maximize their integrity extracellularly during their controlled release, and testing whether this may be accomplished by coadministrating ALC and/or NACA to maintain both host and transplanted mitochondria. One of our biggest limitations from a translational standpoint is the lack of rapid isolation procedures for spinal cord mitochondria that have been developed for heart studies.
Acknowledgements
Financial Support: DoD W81XWH2010347 (AGR); and NIH R01 NS119337 (AGR & SPP); Spinal Cord & Brain Injury Research Center Chair #1 and #3 Endowments, University of Kentucky (AGR & PGS).
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Al Amir Dache Z, Otandault A, Tanos R, Pastor B, Meddeb R, Sanchez C, Arena G, Lasorsa L, Bennett A, Grange T, El Messaoudi S, Mazard T, Prevostel C, Thierry AR, 2020. Blood contains circulating cell-free respiratory competent mitochondria. FASEB J 34, 3616–3630. [DOI] [PubMed] [Google Scholar]
- Baek Y, Park SJ, Zhou X, Kim G, Kim HM, Yoon J, 2016. A viscosity sensitive fluorescent dye for real-time monitoring of mitochondria transport in neurons. Biosens. Bioelectron 86, 885–891. [DOI] [PubMed] [Google Scholar]
- Bertero E, Maack C, O’Rourke B, 2018. Mitochondrial transplantation in humans: “magical” cure or cause for concern? J. Clin. Invest 128, 5191–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera F, Ortega M, Velarde F, Parra E, Gallardo S, Barba D, Soto L, Pena G, Pedroza LA, Jorgensen C, Khoury M, Caicedo A, 2019. Primary allogeneic mitochondrial mix (PAMM) transfer/transplant by MitoCeption to address damage in PBMCs caused by ultraviolet radiation. BMC Biotech. 19, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MA, Shay JW, 1982. Mitochondrial transformation of mammalian cells. Nature 295, 605–607. [DOI] [PubMed] [Google Scholar]
- Cowan DB, Yao R, Akurathi V, Snay ER, Thedsanamoorthy JK, Zurakowski D, Ericsson M, Friehs I, Wu Y, Levitsky S, Del Nido PJ, Packard AB, McCully JD, 2016. Intracoronary Delivery of Mitochondria to the Ischemic Heart for Cardioprotection. PLoS ONE 11, e0160889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Acunzo P, Pérez-González R, Kim Y, Hargash T, Miller C, Alldred MJ, Erdjument-Bromage H, Penikalapati SC, Pawlik M, Saito M, Saito M, Ginsberg SD, Neubert TA, Goulbourne CN, Levy E, 2021. Mitovesicles are a novel population of extracellular vesicles of mitochondrial origin altered in Down syndrome. Sci. Adv 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doulamis IP, McCully JD, 2021. Mitochondrial Transplantation for Ischemia Reperfusion Injury. Methods Mol. Biol 2277, 15–37. [DOI] [PubMed] [Google Scholar]
- Emani SM, McCully JD, 2018. Mitochondrial transplantation: applications for pediatric patients with congenital heart disease. Transl Pediatr 7, 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emani SM, Piekarski BL, Harrild D, Del Nido PJ, McCully JD, 2017. Autologous mitochondrial transplantation for dysfunction after ischemia-reperfusion injury. J. Thorac. Cardiovasc. Surg 154, 286–289. [DOI] [PubMed] [Google Scholar]
- Geldenhuys WJ, Leeper TC, Carroll RT, 2014. mitoNEET as a novel drug target for mitochondrial dysfunction. Drug Discov Today 19, 1601–1606. [DOI] [PubMed] [Google Scholar]
- Gollihue JL, Patel SP, Mashburn C, Eldahan KC, Sullivan PG, Rabchevsky AG, 2017. Optimization of mitochondrial isolation techniques for intraspinal transplantation procedures. J. Neurosci. Methods 287, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollihue JL, Patel SP, Eldahan KC, Cox DH, Donahue RR, Taylor BK, Sullivan PG, Rabchevsky AG, 2018. Effects of Mitochondrial Transplantation on Bioenergetics, Cellular Incorporation, and Functional Recovery after Spinal Cord Injury. J. Neurotrauma 35, 1800–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollihue JL, Patel SP, Rabchevsky AG, 2018. Mitochondrial transplantation strategies as potential therapeutics for central nervous system trauma. Neural Regen Res 13, 194–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollihue JL, Rabchevsky AG, 2017. Prospects for therapeutic mitochondrial transplantation. Mitochondrion 35, 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, Ji X, Lo EH, 2016. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535, 551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Bruzzese M, Chou SH, Ning M, Ji X, Lo EH, 2018. Extracellular Mitochondria for Therapy and Diagnosis in Acute Central Nervous System Injury. JAMA Neurol 75, 119–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard WB, Spry ML, Gooch JL, Cloud AL, Vekaria HJ, Burden S, Powell DK , Berkowitz BA, Geldenhuys WJ, Harris NG, Sullivan PG, 2021. Clinically relevant mitochondrial-targeted therapy improves chronic outcomes after traumatic brain injury. Brain 144, 3788–3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J, 2012. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med 18, 759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katrangi E, D’Souza G, Boddapati SV, Kulawiec M, Singh KK, Bigger B, Weissig V, 2007. Xenogenic transfer of isolated murine mitochondria into human rho0 cells can improve respiratory function. Rejuvenation Res. 10, 561–570. [DOI] [PubMed] [Google Scholar]
- Kaza AK, Wamala I, Friehs I, Kuebler JD, Rathod RH, Berra I, Ericsson M, Yao R, Thedsanamoorthy JK, Zurakowski D, Levitsky S, Del Nido PJ, Cowan DB, McCully JD, 2017. Myocardial rescue with autologous mitochondrial transplantation in a porcine model of ischemia/reperfusion. J. Thorac. Cardiovasc. Surg 153, 934–943. [DOI] [PubMed] [Google Scholar]
- King MP, Attardi G, 1988. Injection of mitochondria into human cells leads to a rapid replacement of the endogenous mitochondrial DNA. Cell 52, 811–819. [DOI] [PubMed] [Google Scholar]
- Lipper CH, Stofleth JT, Bai F, Sohn YS, Roy S, Mittler R, Nechushtai R, Onuchic JN, Jennings PA, 2019. Redox-dependent gating of VDAC by mitoNEET. Proc Natl Acad Sci U S A 116, 19924–19929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuzawa A, Black KM, Pacak CA, Ericsson M, Barnett RJ, Drumm C, Seth P, Bloch DB, Levitsky S, Cowan DB, McCully JD, 2013. Transplantation of autologously derived mitochondria protects the heart from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 304, H966–H982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCully JD, Cowan DB, Pacak CA, Toumpoulis IK, Dayalan H, Levitsky S, 2009. Injection of isolated mitochondria during early reperfusion for cardioprotection. Am J Physiol Heart Circ Physiol 296, H94–H105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCully JD, Emani SM, Del Nido PJ, 2020. Letter by McCully et al Regarding Article, “Mitochondria Do Not Survive Calcium Overload”. Circ. Res 126, e56–e57. [DOI] [PubMed] [Google Scholar]
- McCully JD, Emani SM, Del Nido PJ, 2020. Reply: Intracoronary Delivery of Mitochondria to Prevent Ischemia-Reperfusion Injury: Challenging Pathway From Bench to Bedside. JACC Basic Transl. Sci 5, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Park JH, Hayakawa K, 2020. Therapeutic use of extracellular mitochondria in CNS injury and disease. Exp. Neurol 324, 113114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya JD, Readnower RD, Patel SP, Yonutas HM, Pauly JR, Goldstein GA, Rabchevsky AG, Sullivan PG, 2014. N-acetylcysteine amide confers neuroprotection, improves bioenergetics and behavioral outcome following TBI. Exp. Neurol 257, 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SP, Cox DH, Gollihue JL, Bailey WM, Geldenhuys WJ, Gensel JC, Sullivan PG, Rabchevsky AG, 2017. Pioglitazone treatment following spinal cord injury maintains acute mitochondrial integrity and increases chronic tissue sparing and functional recovery. Exp. Neurol 293, 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SP, Sullivan PG, Lyttle TS, Rabchevsky AG, 2010. Acetyl-L-carnitine ameliorates mitochondrial dysfunction following contusion spinal cord injury. J. Neurochem 114, 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SP, Sullivan PG, Lyttle TS, Magnuson DS, Rabchevsky AG, 2012. Acetyl-L-carnitine treatment following spinal cord injury improves mitochondrial function correlated with remarkable tissue sparing and functional recovery. Neuroscience 210, 296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SP, Sullivan PG, Pandya JD, Goldstein GA, VanRooyen JL, Yonutas HM, Eldahan KC, Morehouse J, Magnuson DS, Rabchevsky AG, 2014. N-acetylcysteine amide preserves mitochondrial bioenergetics and improves functional recovery following spinal trauma. Exp. Neurol 257, 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SP, Michael FM, Arif Khan M, Duggan B, Wyse S, Darby DR, Chaudhuri K, Pham JT, Gollihue J, DeRouchey JE, Sullivan PG, Dziubla TD, Rabchevsky AG, 2022. Erodible thermogelling hydrogels for localized mitochondrial transplantation to the spinal cord. Mitochondrion 64, 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabchevsky AG, Fugaccia I, Sullivan PG, Scheff SW, 2001. Cyclosporin A treatment following spinal cord injury to the rat: behavioral effects and stereological assessment of tissue sparing. J. Neurotrauma 18, 513–522. [DOI] [PubMed] [Google Scholar]
- Rabchevsky AG, Patel SP, Sullivan PG, 2017. Targeting mitoNEET with pioglitazone for therapeutic neuroprotection after spinal cord injury. Neural Regen Res 12, 1807–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabchevsky AG, Michael FM, Patel SP, 2020. Mitochondria focused neurotherapeutics for spinal cord injury. Exp. Neurol 330, 113332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Readnower RD, Hubbard WB, Kalimon OJ, Geddes JW, Sullivan PG, 2021a. Genetic Approach to Elucidate the Role of Cyclophilin D in Traumatic Brain Injury Pathology. Cells 10, 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Readnower RD, Hubbard WB, Kalimon OJ, Geddes JW, Sullivan PG, 2021b. Genetic Approach to Elucidate the Role of Cyclophilin D in Traumatic Brain Injury Pathology. Cells 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebbeck CA, Leroi AM, Burt A, 2011. Mitochondrial capture by a transmissible cancer. Science 331, 303. [DOI] [PubMed] [Google Scholar]
- Sauerbeck A, Gao J, Readnower R, Liu M, Pauly JR, Bing G, Sullivan PG, 2011. Pioglitazone attenuates mitochondrial dysfunction, cognitive impairment, cortical tissue loss, and inflammation following traumatic brain injury. Exp. Neurol 227, 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt S, Schulz S, Schropp EM, Eberhagen C, Simmons A, Beisker W, Aiehler M, Zischka H, 2014. Why to compare absolute numbers of mitochondria. Mitochondrion 19 Pt A, 113–123. [DOI] [PubMed] [Google Scholar]
- Scholpa NE, Williams H, Wang W, Corum D, Narang A, Tomlinson S, Sullivan PG, Rabchevsky AG, Schnellmann RG, 2019. Pharmacological Stimulation of Mitochondrial Biogenesis Using the Food and Drug Administration-Approved beta2-Adrenoreceptor Agonist Formoterol for the Treatment of Spinal Cord Injury. J. Neurotrauma 36, 962–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, 2014. Early preservation of mitochondrial bioenergetics supports both structural and functional recovery after neurotrauma. Exp. Neurol 261, 291–297. [DOI] [PubMed] [Google Scholar]
- Shi X, Zhao M, Fu C, Fu A, 2017. Intravenous administration of mitochondria for treating experimental Parkinson’s disease. Mitochondrion 34, 91–100. [DOI] [PubMed] [Google Scholar]
- Stephens OR, Grant D, Frimel M, Wanner N, Yin M, Willard B, Erzurum SC, Asosingh K, 2020. Characterization and origins of cell-free mitochondria in healthy murine and human blood. Mitochondrion 54, 102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stier A, 2021. Human blood contains circulating cell-free mitochondria, but are they really functional? Am. J. Physiol. Endocrinol. Metab 320, E859–E863. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Thompson M, Scheff SW, 2000a. Continuous Infusion of Cyclosporin A Postinjury Significantly Ameliorates Cortical Damage Following Traumatic Brain Injury. Exp. Neurol 161, 631–637. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Rabchevsky AG, Hicks RR, Gibson TR, Fletcher-Turner A, Scheff SW, 2000b. Dose-response curve and optimal dosing regimen of cyclosporin A after traumatic brain injury in rats. Neuroscience 101, 289–295. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Rabchevsky AG, Keller JN, Lovell M, Sodhi A, Hart RP, Scheff SW, 2004. Intrinsic differences in brain and spinal cord mitochondria: Implication for therapeutic interventions. J. Comp. Neurol 474, 524–534. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Krishnamurthy S, Patel SP, Pandya JD, Rabchevsky AG, 2007. Temporal characterization of mitochondrial bioenergetics after spinal cord injury. J. Neurotrauma 24, 991–999. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Sebastian AH, Hall ED, 2011. Therapeutic window analysis of the neuroprotective effects of cyclosporine A after traumatic brain injury. J. Neurotrauma 28, 311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vekaria HJ, Hubbard WB, Scholpa NE, Spry ML, Gooch JL, Prince SJ, Schnellmann RG, Sullivan PG, 2020. Formoterol, a β(2)-adrenoreceptor agonist, induces mitochondrial biogenesis and promotes cognitive recovery after traumatic brain injury. Neurobiol. Dis 140, 104866. [DOI] [PubMed] [Google Scholar]
- Vignais ML, Caicedo A, Brondello JM, Jorgensen C, 2017. Cell Connections by Tunneling Nanotubes: Effects of Mitochondrial Trafficking on Target Cell Metabolism, Homeostasis, and Response to Therapy. Stem Cells Int 2017, 6917941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YW, Koob MD, 2012. Transferring isolated mitochondria into tissue culture cells. Nucleic Acids Res. 40, el48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonutas HM, Hubbard WB, Pandya JD, Vekaria HJ, Geldenhuys WJ, Sullivan PG, 2020. Bioenergetic restoration and neuroprotection after therapeutic targeting of mitoNEET: New mechanism of pioglitazone following traumatic brain injury. Exp. Neurol 327, 113243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Ma Z, Yan C, Pu K, Wu M, Bai J, Li Y, Wang Q, 2019. Muscle-derived autologous mitochondrial transplantation: A novel strategy for treating cerebral ischemic injury. Behav. Brain Res 356, 322–331. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Zhou Y, Hilton T, Li F, Han C, Liu L, Yuan H, Li Y, Xu X, Wu X, Zhang F, Thiagarajan P, Cap A, Shi FD, Zhang J, Dong JF, 2020a. Extracellular mitochondria released from traumatized brains induced platelet procoagulant activity. Haematologica 105, 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Zhou Y, Li M, Zhang J, Dong JF, 2020b. Extracellular Mitochondria in Traumatic Brain Injury Induced Coagulopathy. Semin. Thromb. Hemost 46, 167–175. [DOI] [PubMed] [Google Scholar]


