Abstract
Inflammation-mediated alterations in glutamate neurotransmission constitute the most important pathway in the pathophysiology of various brain disorders. The excessive signalling of glutamate results in excitotoxicity, neuronal degeneration, and neuronal cell death. In the present study, we investigated the relative efficacy of black cumin (Nigella sativa) oil with high (5 % w/w) and low (2 % w/w) thymoquinone content (BCO-5 and BCO-2, respectively) in alleviating ibotenic acid-induced excitotoxicity and neuroinflammation in Wistar rats. It was found that BCO-5 reversed the abnormal behavioural patterns and the key inflammatory mediators (TNF-α and NF-κB) when treated at 5 mg/kg body weight. Immunohistochemical studies showed the potential of BCO-5 to attenuate the glutamate receptor subunits NMDA and GluR-2 along with increased glutamate decarboxylase levels in the brain tissues. Histopathological studies revealed the neuroprotection of BCO-5 against the inflammatory lesions, as evidenced by the normal cerebellum, astrocytes, and glial cells. BCO-2 on the other hand showed either a poor protective effect or no effect even at a 4-fold higher concentration of 20 mg/kg body weight indicating a very significant role of thymoquinone content on the neuroprotective effect of black cumin oil and its plausible clinical efficacy in counteracting the anxiety and stress-related neurological disorders under conditions such as depression and Alzheimer's disease.
Keywords: Black cumin, Excitotoxicity, Ibotenic acid, Neuroinflammation, Nigella sativa, Thymoquinone
1. Introduction
Neuroinflammation, an inflammatory response within the brain due to factors such as stress, infection, toxins, autoimmunity or injury, is interceded by the generation of cytokines, reactive oxygen species and secondary messengers produced by the glial cells in the central nervous system (CNS) (Di Sabato et al., 2016). Inflammation-mediated alteration in the glutamate neurotransmission constitutes the most critical pathway in the pathophysiology of various brain disorders since glutamate is the principal excitatory neurotransmitter responsible for the execution of primary physiological functions like the acquisition of memory, perception, and motor activity (Benussi et al., 2019). The glutamatergic neurotransmission occurs through the activation of N-methyl-d-aspartate receptors (NMDAR) (Zhou et al., 2020). However, excessive glutamatergic signalling may result in excitotoxicity, and neuronal degeneration, and even neuronal death (Malik and Willnow, 2019, Cabeza et al., 2021). A balanced level of extracellular glutamate is thus needed for normal synaptic function (Halder et al., 2021).
Neuroinflammation causes increased production of proinflammatory cytokines by glial cells (Yang and Zhou, 2019). These proinflammatory cytokines, especially TNF-α, increase glutamate production by enhancing glutaminase and preventing glutamine uptake by inhibiting glutamate reuptake proteins (Iovino et al., 2020). As a result, the extracellular glutamate accumulates in the synapse leading to excitotoxicity and neuronal death (Kang et al., 2021). Therefore, the control of neuroinflammation through balancing the glutamate level in the brain was considered as an ideal therapy for various neurodegenerative diseases (Cunnane et al., 2020). Glutamate regulators like 6-diazo-5-oxo-norleucine (DON) (a glutaminase inhibitor) and ceftriaxone and riluzole (glutamate reuptake protein enhancers) are found to be effective, but are burdened with unknown target specific actions and thus with varying side effects (Clark and Vissel, 2016). Memantine, a medicine currently in practice for the treatment of various neurodegenerative diseases, is an NMDA receptor antagonist drug that has also been found to exhibit some side effects like hypertension, dizziness, headaches, constipation, and somnolence (Blanco-Silvente et al., 2018). Yet another approach in practice is the use of a combination of non-steroidal anti-inflammatory drugs (NSAID) (Ozben and Ozben, 2019). The use of specific anti-TNF biologicals like etanercept has also been shown to be effective, but is not cost-effective (Clark and Vissel, 2016). More importantly, most of these drugs are effective in the correction of only one pathogenic pathway, since they are target specific (Vezzani et al., 2019). Thus, there is tremendous interest in the development of multi-targeted therapeutic agents, which are safe (Zieba et al., 2022). Various natural agents, including the phytonutrients derived from food components, have been shown to possess significant multi-targeted anti-neuroinflammatory and neuroprotective effects without significant side effects Chen et al., 2021, Mohd Sairazi and Sirajudeen, 2020).
Nigella sativa L. belonging to the family of Ranunculaceae, generally referred to as black cumin or black seed is a popular kitchen spice and is generally recognized as safe (GRAS)-listed food component that has been widely used to treat a range of diseases and disorders worldwide (Tavakkoli et al., 2017). The oil fraction of black cumin, comprising both the essential and fixed oils, has been identified as its primary bioactive component (Mazaheri et al., 2019). While linoleic acid forms the major part of the fixed oil, thymoquinone (TQ) was identified as the most abundant molecule in the essential oil fraction (Gawron et al., 2021).Various studies have demonstrated the beneficial effects of natural compounds (Thajudeen et al., 2022, Rajasree et al., 2022, Ilyas et al., 2022). Several studies have linked the pharmacological effects of black cumin oil (antimicrobial, anti-inflammatory, antihypertensive, anti-obese, hypoglycemic, hypolipidemic, hepatoprotective, and neuroprotective) to its TQ content Kooti et al., 2016, Mukhtar et al., 2019).
Ibotenic acid is a potent NMDA receptor agonist resembling the chemical structure of glutamate (an excitatory neurotransmitter). An excitotoxin preferentially binds to NMDA receptors causing prolonged activation and excitotoxicity similar to glutamate. As a result, there occurs an excess influx of chloride and calcium ions and increased water entry in the neurons due to osmotic lysis. Ibotenic acid also induces neuronal loss throughout the nucleus basalis of the Meynert (nbM) complex and destroys the cholinergic cells in the ventral pallidum and substantia innominate complex (Van Dam and De Deyn, 2011). It was demonstrated that the intracerebroventricular administration (ICV) of IBO to rats could induce cortical cholinergic dysfunction with significant neuroinflammation and neurodegeneration (Karthick et al., 2016). Neuroinflammation, glutamate excitotoxicity, and cholinergic dysfunction are known to be the major pathological process that play key role in Alzheimer’s disease.
The present study investigated the pathogenesis of neuroinflammation and receptor modulation by black cumin oil as a function of its TQ content employing ibotenic acid (IBO)-induced neurotoxicity model of rats.
2. Materials and methods
2.1. Animals
Colony inbred strains of adult Wistar rats weighing 200 – 250 g were used in the study. The animals were purchased from M/s Nagarjuna Herbal Concentrates Limited, Cochin, India and were housed at the animal house facility of the Department of Pharmaceutical Sciences, Centre for Professional and Advanced Studies, Kottayam, India, in a properly ventilated polypropylene cage under controlled temperature (22 – 25 ℃), relative humidity (60 – 80 %) and the light–dark cycle of 12 h. The animals were provided with standard rat feed (M/s VRK Nutritional Solutions, Pune, India) and water ad libitum. The animals were acclimatized to the laboratory conditions for a week prior to the experiments. The study was conducted in compliance with the Institutional Animal Ethics Committee guidelines (IAEC) of the Centre for Professional and Advanced Studies, Kottayam, India, with approval no: MGU/DPS/IAEC/2016/PD-5.
2.2. Materials
Ibotenic acid (Cat. No: I2765) was purchased from Sigma-Aldrich, Inc., MA, USA. GAD-65/67 polyclonal antibody (ITT1830), GluR-2 polyclonal antibody (ITT1923) and NMDAε1/2 polyclonal antibody (ITT3149) were purchased from Geno Technology Inc. St Louis MO, USA. A standard black cumin oil containing 2 % of TQ (BCO-2) and a TQ-rich black cumin oil containing 5 % thymoquinone (BCO-5) were obtained from M/s Akay Natural Ingredients, Kerala, India. Sunflower oil was employed as diluent and vehicle control.
2.3. Experimental design
2.3.1. Toxicity study
Detailed toxicity studies [single dose acute (14-day), and sub chronic (90-day) repeated-dose toxicity studies of black cumin oil containing 5 % (w/w) of TQ (BCO-5) were conducted as per OECD 423, 407 & 408 guidelines and reported previously (Kannan et al., 2022).
2.3.2. Ibotenic acid-induced model
Wistar rats of both sex weighing 200 – 250 g were randomly assigned into eight groups, with eight animals per group (Table 1). The dosage was selected based on toxicity studies (Kannan et al., 2022). Ibotenic acid was administered intracerebroventricular (ICV) using the surgical procedures as described by Mahdi et al., and the treatment with TQF1, TQF2, TQF3, TQN1, TQN2 and memantine was continued for 30 days (Mahdi et al., 2019). Behavioral and cognitive parameters were assessed after 24 h of the last dose of treatment. The animals were sacrificed by cervical dislocation under anaesthetic conditions followed by decapitation, and the brains were removed for biochemical assays.
Table 1.
Grouping of animals, dose of drugs and route of administration followed in the study.
| Groups | Drug | Dose | Route of administration |
|---|---|---|---|
| Normal control (C1) | Sunflower oil | – | Oral |
| IBO control (C2) | Ibotenic acid (IBO) | 1 µL* | Intracerebroventricular (ICV) |
| Standard (S) | IBO + memantine | 30 mg/kg | Oral |
| TQF1 | IBO + BCO-5 | 10 mg/kg | Oral |
| TQF2 | IBO + BCO-5 | 5 mg/kg | Oral |
| TQF3 | IBO + BCO-5 | 2.5 mg/kg | Oral |
| TQN1 | IBO + BCO-2 | 20 mg/kg | Oral |
| TQN2 | IBO + BCO-2 | 10 mg/kg | Oral |
Ibotenic acid (IBO) was dissolved in sunflower oil (pH 7.2) at a concentration of 5 μg/µL.
The rats were anaesthetized by injecting xylazine (20 mg/kg i.p.) and ketamine hydrochloride (80 mg/kg s.c) and were placed on a warm mat to maintain their body temperature. Sterile PBS drops were applied to both eyes, which prevented the dryness of the cornea during surgical procedures. About 70 % of ethanol was sprayed to the middle forehead of the rat and rubbed with dry cotton swabs. The same area on the forehead was wiped with 2 % chlorhexidine solution and the scrubbings with alcohol and chlorhexidine were repeated thrice. The hair on the forehead was shaved, and chlorhexidine antiseptic solution was applied to avoid contamination. The bregma in the brain was located using the thumb and index finger and the injection point was located using a measuring tape (1.0 ± 0.06 mm posterior to bregma, 1.8 ± 0.1 mm lateral to the sagittal suture and 2.4 mm in depth). The syringe was filled with 1 µL of IBO in sunflower oil (5 µg/µL) and placed at the injection point, perpendicular to the plane of injection. The reflected images of the syringe were aligned in both mirrors, with the lines drawn from a fixed perspective. The needle was inserted with the utmost care until the para film wrapping touches the skin and slowly injected the IBO solution or vehicle for five seconds at the rate of 0.2 µL/s. The syringe was kept steady and perpendicular throughout the procedure and an adequate time of 3 to 5 s were spent before the syringe was removed to avoid diffusion. The IBO injected rat was placed on a warm pad for recovery and its sternal recumbence was maintained. Adequate post-surgical care was maintained until it regained consciousness and were placed into separate cages to avoid contamination and any possible infections. The mobility of the rat in the cage was monitored post-operatively and checked for signs of infection or illness for 5 – 7 days.
Sunflower oil was injected instead of Ibotenic acid (C1) as a sham group and IBO (5 µg/µL) was injected as a control group (C2). Administration of the vehicle without the drug was given to both sham and control groups. Sham and control groups were used as positive and negative control groups, respectively (Glascock et al., 2011).
2.4. Behavioral analysis
Elevated plus maze: The elevated plus maze is a widely used method for evaluating anxiety and memory-related parameters. It consisted of two open arms measuring 50 × 10 cm and two closed arms measuring 50 × 10 × 40 cm with one roof. The two arms were arranged opposite to each other. The experimental animals (rats) were placed in the centre square of the maze facing towards the closed arm and allowed to explore the elevated plus-maze for five minutes freely. Before the next rat was released into the maze, the maze was washed with 20 % ethanol and dried. The number of open and closed arm entries and the amount of time spent on open and closed arms were measured and tabulated. The data from the above-said parameters were analyzed for the total number of entries (open arm) and the percentage of time spent on open arms (Pellow et al., 1985).
Open field test: The behavioral changes were evaluated using the open-field apparatus, a wooden box with dimensions of 60 × 60 × 35 cm. It consisted of four holes of 3.8 cm in diameter equally placed on the floor of the apparatus. The apparatus itself consists of lines; each animal was positioned individually at the centre of the open field and permitted for five minutes to explore freely in the apparatus. The total number of rearing (vertical activity), duration of rearing, grooming (protracted of the coat) and locomotion (number of line crossings) were measured (Pellow et al., 1985).
2.5. Acetylcholinesterase activity
Following the sacrifice of the animals, the brain was carefully removed without damage. The adhering blood particles were removed by washing with an ice-cold buffer solution and the tissues were weighed and homogenized in 0.1 M Phosphate buffer (pH 8). The activity was measured by determining the yellow anion formation from the reaction of the thiocholine generated by enzymatic hydrolysis of ATCh and Ellman’s reagent. The change in absorbance was measured at 412 nm for 3 min at regular intervals of 30 s using a UV–visible spectrophotometer immediately after the enzyme was obtained. Acetylcholinesterase (AChE) inhibition was then evaluated by Ellman's method (Kannan et al., 2018).
2.6. Quantification of NF-κB and TNFα by ELISA
The quantification of cytokines was performed by ELISA kit method following the kit instructions (Cat No: E-EL-R0674; E-EL-R0019; Elabscience, Texas, USA). Optical density was read at 415 nm in an ELISA reader (Erba, Germany, Amable et al., 2013, Kim et al., 2016).
2.7. Immunohistochemical analysis
Four animals per group were processed for immunohistochemical analysis as per Kim et al., 2016. The collected samples were washed with ice-cold normal saline, post-fixed with 4 % paraformaldehyde, were cryoprotected in 30 % sucrose – PBS (0.1 M), and stored at −80 ℃ until processed. The sections were deparaffinised, hydrated and incubated with 3 % hydrogen peroxide for immunohistochemistry. Antigen retrieval was carried out by heating for 10 min in a 10 mM sodium citrate buffer (pH 6.0). Specimens were blocked for 30 min at room temperature using a protein block solution (BSA) and incubated with primary antibodies (N-methyl-d-aspartate receptor (NMDAε1/2) (dilution − 1:250); MPA – Selective glutamate receptor (GluR2) (dilution − 1:200) and glutamic acid decarboxylase 65/67 (GAD 65/67) (dilution − 1:250) at 4 ℃ overnight. Polymer-horseradish peroxidase anti-rabbit was used as a secondary antibody and 3, 3′-diaminobenzidine as the chromogen. The Immune-stained hippocampal coronal sections were scanned with a confocal laser microscope and the immune-stained cells were counted in the cornu Ammonis (CA1) area and dentate gyrus region (AP: Bregma 4.3 to 4.5 mm). Images of five replicate sections were analyzed (Yim et al., 2016).
2.8. Histopathology
For histopathology studies, the brain samples fixed in 10 % formal saline (10 mL of formaldehyde in 90 mL of physiological saline) were used. The paraffin-embedded sections were taken (100 µm thickness) and processed in alcohol - xylene series. The sections were stained with Haematoxylin-Eosin (H&E) dye and examined microscopically.
2.9. Statistical analysis
The statistical study was conducted using GraphPad Prism (Version 7.00) by one-way ANOVA accompanied by Tukey's multiple post-hoc test comparisons. All the values were expressed as Mean ± SEM with P < 0.05 considered as significantly different (a, b, c represents P < 0.001, P < 0.01 and P < 0.05 respectively when compared with control; x, y, z represents P < 0.001, P < 0.01 and P < 0.05 respectively when compared with IBO control; ns – non-significant).
3. Results
3.1. Behavior studies
In the present study, the IVC administration of IBO induced behavioral changes, as evident from the significant decrease (P < 0.001) in the number of open arm entries and the time spent by the animals in the open arm (Group C2; IBO-treated) as compared to vehicle control animals (Group C1). BCO-5 treated group of animals showed significant protective activity at 5 mg/kg b. wt., while BCO-2 treated group showed lesser or no activity even at 20 mg/kg b. wt. (P > 0.05) (Fig. 1a and 1b). Significant improvement in both open arm entries and time spent by the animals in the open arm were observed in both TQF1 and TQF2 groups and were comparable to the memantine treated group (Group S). However, the effect was more pronounced in TQF2. Moreover, this was similar to the normal control group of animals indicating the reversal of the behavior in both TQF2 and memantine treated groups.
Fig. 1.
Behavioural changes after IVC administration of IBO.Fig. 1a and 1b- BCO-5 treated group of animals showed significant protective activity at 5 mg/kg b. wt., while BCO-2 treated group showed lesser or no activity even at 20 mg/kg body weight. Fig. 1c and 1d- Both memantine and BCO-5 at concentrations of 10 and 5 mg/kg body weight (TQF1 and TQF2 respectively) produced a significant increase (P < 0.001) in both the ambulation and rearing behavior.
Ibotenic acid administration also produced a significant decrease (P < 0.001) in the ambulation and rearing behavior in disease control animals (IBO-treated) when compared to that of vehicle control (C1). Both memantine and BCO-5 at concentrations of 10 and 5 mg/kg body weight (TQF1 and TQF2 respectively) produced a significant increase (P < 0.001) in both the ambulation and rearing behavior and were similar to the behavior of the control group of animals (P > 0.05) (Fig. 1c and 1d). However, BCO-2 at both the tested doses of 20 and 10 mg/kg body weight produced only a minimum response, which was not statistically significant to the IBO control group C2.
3.2. Acetylcholinesterase activity
The acetylcholinesterase activity was significantly increased (P < 0.01) in the IBO- treated group (C2) compared with vehicle control demonstrating the neurotoxic effect of IBO. It was observed that both memantine and BCO-2 (10 and 20 mg/kg body weight) produced no significant change (P > 0.05) when compared with the treatment group, C2. BCO-5 on the other hand produced maximum effect at 5 mg/kg body weight itself, as evidenced from the significant decrease (P < 0.01) in AChE activity when compared to IBO control (Fig. 2). However, the AChE activity of BCO-5 at 10 mg/kg was slightly greater than TQF2, but has significantly decreased (P < 0.05) than C2, indicating the effect on AChE (Fig. 2).
Fig. 2.

The acetylcholinesterase activity in treatment and control group. The acetylcholinesterase activity was significantly increased (P < 0.01) in the IBO- treated group (C2) compared with vehicle control demonstrating the neurotoxic effect of IBO.
3.3. Effect of BCO on brain tissue levels of NF-κB and TNF-α
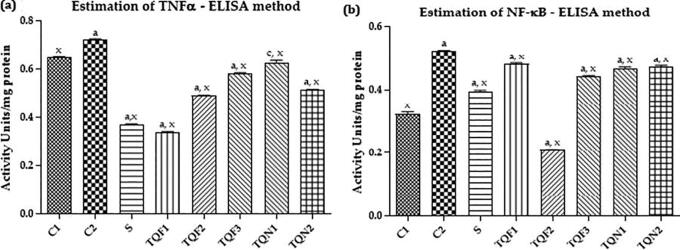
The inflammatory marker TNF-α was significantly increased (P < 0.001) in the IBO- treated group compared with the control group. The treatment with memantine and BCO-5 at all the dosage produced a dose-dependent and significant decrease (P < 0.001) in the activity of TNF-α when compared to that of control. Meanwhile, BCO-2 showed only moderate activity (Fig. 4a).
Fig. 4.
Estimation of NMDA reactive cells, GluR-2 reactive cells and GAD 65/67 reactive cells by immunohistochemical analysis.
The development of excitotoxic lesions in the brain by the administration of IBO was evident from the significant increase (P < 0.001) in the expression of NF-κB activity in IBO treated, compared to the vehicle control group of animals. However, treatment with memantine, BCO-5 (2.5, 5 and 10 mg/kg body weight) and BCO-2 (10 and 20 mg/kg body weight) significantly decreased (P < 0.001) the activity of NF-κB (Fig. 3b). However, BCO-5 at 5 mg/Kg body weight itself showed a more significant effect compared to BCO-2 at 20 mg/kg b. wt.
Fig. 3.
Effect of BCO on brain tissue levels of NF-κB (3a) and TNF-α (3b).
3.4. Estimation of NMDA reactive cells by immunohistochemical analysis
Compared with the vehicle control group of animals, there was an exponential increase in the levels of NMDA positive cells (P < 0.001) in the IBO group (C2). Meanwhile, treatment with BCO-5 (2.5 and 10 mg/kg body weight) and BCO-2 (10 and 20 mg/kg body weight) produced significant alterations (P < 0.001) when compared to both IBO control and vehicle control (Fig. 4a and 4d). In contrast to this, BCO-5 at 5 mg/kg body weight produced a substantial decrease (P < 0.001) in reactive cells, which is comparable to the memantine treated group (S).
3.5. Estimation of GluR-2 reactive cells by immunohistochemical analysis
The quantity of GluR-2 reactive cells was significantly increased (P < 0.001) in IBO group compared to normal control. The results showed (Fig. 4b and 4e) that BCO-5 and BCO-2 had significantly reduced GluR-2 reactive cells (P < 0.001). However, the number of GluR-2 reactive cells in BCO-5 (5 mg/kg) treated group is comparable to normal (C1) rather than between BCO and C1 group.
3.6. Estimation of GAD 65/67 reactive cells by immunohistochemical analysis
The GAD levels in the brains of rats were decreased when administered with IBO as evidenced by the significant reduction (P < 0.001) in the number of GAD reactive cells in IBO treated group compared to that of control. Meanwhile, treatment with memantine and BCO-5 (10 and 5 mg/kg) significantly increased (P < 0.001) the number of GAD positive cells when compared to IBO control. BCO-5 at 2.5 mg/kg and BCO-2 at 20 mg/kg produced lesser activity (P < 0.05 and P < 0.01, respectively). BCO-2 at 10 mg/kg body weight showed no activity (P > 0.05) when compared to IBO control (Fig. 4c and 4f). On the whole, the order of activity of the black cumin oil with varying levels of TQ content was found to be in the order BCO-5 at 5 mg/kg body weight (TQF2) > BCO-5 at 10 mg/kg body weight (TQF1) > BCO-5 at 2.5 mg/kg body weight (TQF3) > BCO-2 at 20 mg/kg body weight (TQN1) > BCO-2 at 10 mg/kg body weight (TQN2).
3.7. Histopathology
The intracerebroventricular injection of IBO produced lesions in the brain of animals, as evidenced by the presence of necrosis and hyperplastic astrocytes in the IBO treated group. The treatment with memantine and BCO-5 at concentrations of 10 and 5 mg/kg body weight showed normal astrocytes and glial cells in all groups, except BCO-5 at 2.5 mg/kg body weight and BCO-2 at 20 mg/kg body weight where hyperplastic astrocytes were observed. The cerebellum of memantine, BCO-5 (10, 5 and 2.5 mg/kg body weight) and BCO- 2 at 20 mg/kg body weight treated animals were found to be normal. However, BCO-2 at 10 mg/kg body weight showed necrosis (Fig. 5).
Fig. 5.
Histopathological leisions in the brain of animals in response to different treatments.
4. Discussion
Over the past two decades, there has been a significant rise in the use of herbal medicines (Naseef et al., 2021, Ilyas et al., 2021). A number of botanical extracts and phytochemicals have also found scientific research-based applications as phytonutrients and are widely available as 'nutraceuticals' or 'dietary supplements' or as 'functional foods' (Rajasree et al., 2021, Laj et al., 2022). Black cumin seed is one such culinary spice possessing wide range of medicinal effects, especially on brain functions (Oskouei et al., 2018). Both the black cumin oil and its major component TQ have shown to exhibit beneficial neuropharmacological effects suitable for the management and treatment of Alzheimer’s disease, Parkinson’s disease, anxiety, depression, encephalomyelitis, epilepsy, traumatic brain injury, ischemia and other neurodegenerative conditions (Samarghandian et al., 2018). However, although TQ has been regarded as the main bioactive molecule of black cumin oil, systematic investigations on the role of TQ content on neuroprotective effect has not been investigated yet (Tavakkoli et al., 2017). Thus, the present study attempted to evaluate the anti-neuroinflammatory effect and hence the neuroprotective activity of standardized black cumin oil as a function of its TQ content, using ICV administered IBO-induced neuroinflammatory model of rats.
Ibotenic acid has been shown to affect significant loss of nerve cells at various parts of the brain, including the striatum, hippocampus, substantia nigra, and cortex and produces vigorous gliosis in the region of neuronal loss (Zong et al., 2016). The non-neuronal inflammatory cells thus produced have the phenotype of macrophages and can damage the healthy axons and contribute to the increase in vascular permeability at the site of the lesion (Gaudet and Fonken 2018). Thus, ICV-administration of IBO has been shown to affect both the resident microglia and the protoplasmic astrocytes leading to inflammation and neuronal cell death. It is also validated as a model for anxiety-related behavioral changes and excitotoxic lesions resembling Alzheimer's disease (Tan and Kuner, 2021, Yang et al., 2020).
Significant behavioral changes, as observed in elevated plus-maze and open field study, clearly indicate the development of significant neurotoxicity upon administration of IBO as observed by Afees et al. (2022). The elevated plus-maze open field is a widely used behavioral experiment to assess the anxiolytic effects of pharmacologically active compounds (Walf and Frye, 2007, Sestakova et al., 2013). In the elevated plus-maze experiment, both the number of entries and the time spent in the open arms were significantly increased upon treatment with BCO-5 compared to the IBO-treated disease control group, indicating the anxiolytic effects of BCO-5. The behavioral patterns were also reversed among BCO-5 treated animals, as evident from the significant increase in the ambulation and rearing frequencies compared to IBO group. However, the fact that BCO-2 showed no significant improvement in the behaviour indicates a definite role of TQ in the anxiolytic effect of black cumin oil. In a separate clinical study involving 15 healthy persons, we have shown that BCO-5 alleviated anxiety and stress levels. The anxiolytic effect of TQ due to its effects on the GABA-ergic pathways and on the NO-cGMP levels have already been reported (Gilhotra and Dhingra, 2011). The anxiolytic effect has great importance to reduce the psychiatric burden among the Alzheimer’s patients (Becker et al., 2018).
Obesity is one of the major causes of the occurrence and development of cardiovascular events, thus the body weight gain was considered as one factor for cardiovascular risk (Powell-Wiley et al., 2021). It is noted that hyperlipidemia is closely associated with cardiovascular disease and high blood pressure is an important risk factor for CVD occurrence (Powell-Wiley et al., 2021). It has been demonstrated that the acetylcholine and cholinergic neurotransmission pathway play a vital role in learning, memory and spontaneous alertness, cognition, emotional behavior, wakefulness and attention (Lee et al., 2021, Luchicchi et al., 2014). Acetylcholinesterase (AChE) is an enzyme that catalyzes the breakdown and hence increased AChE causes the depletion of acetylcholine (Colovic et al., 2013). So, one of the therapeutic strategies for may ailments including Alzheimer’s disease is the use of AChE inhibitors (Khazdair, 2015). In the present study, BCO-5 at 10 and 5 mg/kg body weight dosage produced significant inhibition of AChE activity and produced significantly high levels of acetylcholine in the brain tissues of animals as compared to IBO-treated disease control groups, which caused a significant reduction in acetylcholine levels compared to the vehicle control group. However, the BCO-2 group produced no significant effect, indicating the positive role of TQ in AChE inhibition and hence in the neuroprotective effect of black cumin oil. Previous research has shown the AChE inhibitory effects for other molecules found in black cumin oil, such as thymol, carvacrol, and thymohydroquinone (Silva et al., 2018).
Tumor necrosis factor (TNF-α) is an important pro-inflammatory cytokine and NF-κB is a transcription factor found in excessive levels in the brain tissues of Alzheimer's patients (Mohan et al., 2021, McCaulley and Grush, 2015). The TNF-α/NF-κB signalling pathway closely links neuroinflammation and neurodegeneration in the Alzheimer's pathogenesis (Zhou et al., 2014). NF- κB is a crucial mediator of neuronal inflammation and can regulate pro-inflammatory markers such as cytokines (Mohan et al., 2021). We observed a significant decrease in both TNF-α and NF-κB levels when treated with both BCO-5 and BCO-2, with a more significant effect for BCO-5 in a dose-dependent manner, further supporting the role of TQ in ameliorating the neuroinflammation. Various other phytochemicals, including curcumin, resveratrol, and green tea catechins have also been reported to modulate the brain tissue levels of TNF-α and NF-κB; but at higher doses (Jit et al., 2021). The significant activity of BCO-5 at relatively lower doses of 5 to 10 mg/kg body weight indicate the strong anti- neuroinflammatory effect, probably due to the better bioavailability of TQ. Previous reports have shown that TQ inhibits NF-kB mediated neural inflammation by activating Nrf2/ARE signalling pathway and prevents PI3k and Akt phosphorylation in BV2 microglia (Wang et al., 2015).
We have also studied the activity of black cumin oil on various receptors and their subtypes by using immunohistochemical analysis to gather better information on their pathway or mode of action. The NMDA receptor-mediated glutamatergic neurotransmission has been shown to play a crucial role in the synaptic functions and the plasticity of neurons. Excessive glutamatergic neurotransmission and neurotransmitter glutamate play a vital role in the pathogenesis of anxiety and neurodegenerative disorders, specifically in Alzheimer's (Schwartz et al., 2012, Celli et al., 2019). Strong activation of NMDA receptors leads to enhanced synaptic strength, leading to neuroinflammation and nerve cell death (Wang and Reddy, 2017). Besides, previous studies have shown that continuous activation of NMDA receptors can increase β amyloid (Aβ) levels in the brain (Fouad et al., 2018). The significant decrease in the NMDA-positive immunoreactive cells observed upon the immunohistochemical analysis in the present study may be due to the NMDAR antagonistic property of the BCO-5 in a dose-dependent manner.
We also studied the activity of BCO-5 on AMPA receptor GluR-2 subunits since they mediate fast excitatory neurotransmission along with specific synaptic responses related to cognition. The GluR-2 receptors present in the hippocampal cornu Ammonis (CA1) region of the brain play a significant role in dementia and behavioral changes (Gao et al., 2021, Li et al., 2020). Administration of IBO leads to the up-regulation of GluR-2 expression and the GluR- 2 positive immunoreactive cells were found to be significantly increased as reported previously (Karthick et al., 2016). However, the treatment with BCO-5 significantly reduced the level of GluR-2 immunoreactive cells, indicating its specific affinity on AMPA receptor subtypes. Thus, it could be concluded that BCO-5 acts by both NMDA and AMPA subtypes of glutamate receptors.
Apart from glutamate receptor studies, we also studied the effect of BCO-5 on GABA-ergic system by immunohistochemical analysis of glutamic acid decarboxylase (GAD), which is an enzyme involved in the biosynthesis of GABA. GAD 65 and 67 subtypes are present in the brain and had effects on cognitive performances, autism, Alzheimer's and neuropathic pain (Rozycka and Liguz‐Lecznar, 2017). Ibotenic acid is proved to be an inhibitor of GAD (Maruca et al., 2020). The present study showed a dose-dependent increase in GAD when treated with BCO-5 as evidenced by the increased number of immunoreactive cells. The increase in the production of GAD can result in the conversion of the excess extracellular glutamate to GABA thereby decreasing the glutamate excitotoxicity (Gilhotra and Dhingra, 2011),. The observed results are also in line with the previou study of Gilhotra and Dhingra (2011), where TQ modulated the level of GABA in stressed mouse.
It is known that ICV injection of IBO leads to the direct exposure of the toxin to the nerve cells resulting in severe neuronal lesions in rats. The histopathological investigation from the study indicated the occurrence of inflammatory lesions as evident by hyperplastic astrocytes and necrosis in the cerebellum of the IBO- treated animals. The present study showed that the protection offered by BCO-5 at 5 mg/kg body weight was at par with memantine treatment. Both BCO-5 group and memantine treated group had normal cerebellum with normal astrocytes and glial cells. However, in the BCO-2 treated group, even at a higher dose, there were necrotic lesions. Thus, BCO-5 treatment has been shown to reverse the brain damage caused by IBO as compared to BCO-2.
Thus, the present study has shown that black cumin oil containing 5 % TQ (BCO-5) was highly effective in ameliorating neurotoxic effects induced by IBO, as compared to the common black cumin oil with around 2 % TQ (BCO-2). The low TQ containing BCO-2 even at a 4-fold higher dosage failed to show significant changes in majority of the parameters examined in the current study as compared to BCO-5. The anxiolytic effects of BCO-5 were evident from the increase in ambulation and rearing behavior. BCO-5 has shown protective effects on the various important pathways involved in the pathogenesis of neurodegenerative disease such as Alzheimer’s disease. BCO-5 acted on the acetylcholine pathway (via AChE inhibition), glutamate pathway (via NMDA and AMPA subtypes of glutamate receptors, and GAD) and neuroinflammation (via NF-κB signalling pathway), improving associated anxiety- related behavior and protected the brain cells from necrosis. Thus, BCO-5 may be considered as a potent lead as compared to normal BCO-2 for improvement of various neurodegenerative conditions, especially to manage stress and anxiety, which very often makes significant psychological burden to the Alzheimer’s disease patients.
5. Conclusions
In the present study, it was found that black cumin oil exhibits significant anti-neuroinflammatory and neuroprotective effect as a function of its TQ content. Black cumin oil with 5 % TQ (BCO-5) was found to offer a significant neuroprotective effect at 5 mg/kg body weight and even reversed the behavioral changes induced by the ICV-administration of IBO. Black cumin oil with 2 % TQ (BCO-2) on the other hand showed either poor protective effect or no effect even at 4-fold higher dosage. Moreover, BCO-5 significantly reduced the key mediators of neuroinflammation-TNF-α and NF-κB. Immunohistochemical analysis further revealed the significant ability of BCO-5 to decrease the NMDA and GluR-2 positive immune-reactive cells and increase the number of GAD. Histopathological results showed that treatment with BCO-5 could effectively protect from the neuronal damage caused by ICV-administration of IBO. Significant neuroinflammation and damage to the neuronal cells with the presence of hyperplastic astrocytes and necrosis were observed among IBO-treated rats. These changes in the biochemical markers together with the improvement in behavioral changes indicate the potential therapeutic effect of BCO-5 in counteracting the anxiety/stress-related neurological disorders, especially for Alzheimer's disease.
6. Institutional review board statement
The animal study protocol was approved by the institutional review board of Centre for Professional and Advanced Studies, Kottayam, India (approval number MGU/DPS/IAEC/2016PhD-05 dated 15–05-2016).
CRediT authorship contribution statement
Sibi P Ittiyavirah: Funding acquisition, Project administration, Validation, Conceptualization, Methodology, Software, Formal analysis, Investigation, Resources, Data curation, Writing – review & editing. Kannan Ramalingam: Funding acquisition, Project administration, Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – review & editing. Arathy Sathyan: Project administration, Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – review & editing. R.S. Rajasree: Project administration, Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Visualization, Supervision. Mohamed Saheer Kuruniyan: Funding acquisition, Project administration, Data curation, Writing – review & editing, Visualization. Muhammed Elayadeth-Meethal: Funding acquisition, Project administration, Data curation, Writing – review & editing, Visualization. Punnoth Poonkuzhi Naseef: Funding acquisition, Data curation, Writing – review & editing, Visualization, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University, Saudi Arabia for funding this work through the Research Group Program under Grant No: RGP 2/218/43.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Sibi P Ittiyavirah, Email: sibitho@gmail.com.
Kannan Ramalingam, Email: kannanramalingam.g@gmail.com.
Arathy Sathyan, Email: arathysathyan28@gmail.com.
R.S. Rajasree, Email: rajasreejkrishnan@gmail.com.
Mohamed Saheer Kuruniyan, Email: mkurunian@kku.edu.sa.
Muhammed Elayadeth-Meethal, Email: muhammed@kvasu.ac.in.
Punnoth Poonkuzhi Naseef, Email: drnaseefpp@gmail.com.
References
- Afees O.J., Ayodele B.R., Gbenga A., Taiye A.S., Yinka O.S., Dayo O., Sunday F.O., Leviticus A., Esther O.O., Igbo E.J., Dorcas T.O. Localised streptozotocin-induced structural and cognitive changes in the hippocampal cornu ammonis 1 (CA-1) neurons and mitigating effects of Zingiber officinale. Phytomed. Plus. 2022;2 [Google Scholar]
- Amable, P.R., Carias, R.B.V., Teixeira, M.V.T., da Cruz Pacheco, Í., Corrêa do Amaral, R.J.F., Granjeiro, J.M., Borojevic, R., 2013. Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell. Res. Ther. 4, 1-13. [DOI] [PMC free article] [PubMed]
- Becker E., Rios C.L.O., Lahmann C., Ruecker G., Bauer J., Boeker M. Anxiety as a risk factor of Alzheimer's disease and vascular dementia. Br. J. Psychiatry. 2018;213:654–660. doi: 10.1192/bjp.2018.173. [DOI] [PubMed] [Google Scholar]
- Benussi A., Alberici A., Buratti E., Ghidoni R., Gardoni F., Di Luca M., Padovani A., Borroni B. Toward a glutamate hypothesis of frontotemporal dementia. Front. Neurosci. 2019;13:1–9. doi: 10.3389/fnins.2019.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Silvente L., Capella D., Garre-Olmo J., Vilalta-Franch J., Castells X. Predictors of discontinuation, efficacy, and safety of memantine treatment for Alzheimer’s disease: meta-analysis and meta-regression of 18 randomized clinical trials involving 5004 patients. BMC Geriatr. 2018;18:160–168. doi: 10.1186/s12877-018-0857-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza O.A., Zhang Z., Khoury E.S., Sheldon R.A., Sharma A., Zhang F., Slusher B.S., Kannan R.M., Kannan S., Ferriero D.M. Neuroprotective effects of a dendrimer-based glutamate carboxypeptidase inhibitor on superoxide dismutase transgenic mice after neonatal hypoxic-ischemic brain injury. Neurobiol. Dis. 2021;148 doi: 10.1016/j.nbd.2020.105201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celli R., Santolini I., Van Luijtelaar G., Ngomba R.T., Bruno V., Nicoletti F. Targeting metabotropic glutamate receptors in the treatment of epilepsy: rationale and current status. Expert Opin. Ther. Targets. 2019;23:341–351. doi: 10.1080/14728222.2019.1586885. [DOI] [PubMed] [Google Scholar]
- Chen X., Drew J., Berney W., Lei W. Neuroprotective natural products for Alzheimer’s disease. Cells. 2021;10:6–7. doi: 10.3390/cells10061309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I., Vissel B. Excess cerebral TNF causing glutamate excitotoxicity rationalizes treatment of neurodegenerative diseases and neurogenic pain by anti-TNF agents. J. Neuroinflamm. 2016;13:1–16. doi: 10.1186/s12974-016-0708-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colovic M., Krstic D., Lazarevic-Pasti T., Bondzic A., Vasic V. Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr. Neuropharmacol. 2013;11:315. doi: 10.2174/1570159X11311030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnane S.C., Trushina E., Morland C., Prigione A., Casadesus G., Andrews Z.B., Beal M.F., Bergersen L.H., Brinton R.D., de la Monte S., Eckert A. Brain energy rescue: an emerging therapeutic concept for neurodegenerative disorders of ageing. Nat. Rev. Drug Discov. 2020;19:609–633. doi: 10.1038/s41573-020-0072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Sabato D., Quan N., Godbout J. Neuroinflammation: the devil is in the details. J. Neurochem. 2016;139:136–153. doi: 10.1111/jnc.13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad I., Sharaf N., Abdelghany R., El Sayed N. Neuromodulatory effect of thymoquinone in attenuating glutamate-mediated neurotoxicity targeting the amyloidogenic and apoptotic pathways. Front. Neurol. 2018;236:1–12. doi: 10.3389/fneur.2018.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T.H., Ni R.J., Liu S., Tian Y., Wei J., Zhao L., Wang Q., Ni P., Ma X., Li T. Chronic lithium exposure attenuates ketamine-induced mania-like behavior and c-Fos expression in the forebrain of mice. Pharmacol. Biochem. Behavior. 2021;202 doi: 10.1016/j.pbb.2021.173108. [DOI] [PubMed] [Google Scholar]
- Gaudet A.D., Fonken L.K. Glial cells shape pathology and repair after spinal cord injury. Neurotherapeutics. 2018;15:554–577. doi: 10.1007/s13311-018-0630-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawron G., Krzyczkowski W., Lyzen R., Kadzinski L., Banecki B. Influence of supercritical carbon dioxide extraction conditions on extraction yield and composition of Nigella sativa L. seed oil—modelling, optimization and extraction kinetics regarding fatty acid and thymoquinone content. Molecules. 2021;26:6419. doi: 10.3390/molecules26216419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilhotra N., Dhingra D. Thymoquinone produced antianxiety-like effects in mice through modulation of GABA and NO levels. Pharmacol Rep. 2011;63:660–669. doi: 10.1016/s1734-1140(11)70577-1. [DOI] [PubMed] [Google Scholar]
- Glascock J., Osman E., Coady T., Rose F., Shababi M., Lorson C. Delivery of therapeutic agents through intracerebroventricular (ICV) and intravenous (IV) injection in mice. J. Vis. Exp. 2011;56:2968. doi: 10.3791/2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder S., Anand U., Nandy S., Oleksak P., Qusti S., Alshammari E.M., Batiha G.E.S., Koshy E.P., Dey A. Herbal drugs and natural bioactive products as potential therapeutics: A review on pro-cognitives and brain boosters perspectives. Saudi Pharm J. 2021;29:879–907. doi: 10.1016/j.jsps.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilyas U.K., Elayadeth-Meethal M., Kuruniyan M.S., Quadri S.A., Rajasree R.S., Naseef P.P. Densitometric quantification and optimization of polyphenols in Phyllanthus maderaspatensis by HPTLC. Saudi J. Biol. Sci. 2022;29(3):1521–1529. doi: 10.1016/j.sjbs.2021.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilyas U., Katare D.P., Naseef P.P., Kuruniyan M.S., Elayadeth-Meethal M., Aeri V. Immunomodulatory activity of Phyllanthus maderaspatensis in LPS-stimulated mouse macrophage RAW 264.7 cells. Separations. 2021;8(9):129. [Google Scholar]
- Iovino L., Tremblay M.E., Civiero L. Glutamate-induced excitotoxicity in Parkinson's disease: the role of glial cells. J. Pharmacol. Sci. 2020;144:151–164. doi: 10.1016/j.jphs.2020.07.011. [DOI] [PubMed] [Google Scholar]
- Jit B.P., Pradhan B., Dash R., Bhuyan P.P., Behera C., Behera R.K., Sharma A., Alcaraz M., Jena M. Phytochemicals: potential therapeutic modulators of radiation induced signaling pathways. Antioxidants. 2021;11:49. doi: 10.3390/antiox11010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y.P., Mockabee-Macias A., Jiang C., Falzone A., Prieto-Farigua N., Stone E., Harris I.S., DeNicola G.M. Non-canonical glutamate-cysteine ligase activity protects against ferroptosis. Cell Metab. 2021;33:174–189. doi: 10.1016/j.cmet.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan, R., George, S., PS, B.C., Maliakel, B., Ittiyavirah, S. and Krishnakumar, I.M., 2022. Thymoquinone-rich black cumin oil improves sleep quality, alleviates anxiety/stress on healthy subjects with sleep disturbances–A pilot polysomnography study. J. Herb. Med. 32, 100507.
- Kannan R., Ittiyavirah S., Harindran J. Acetylcholinesterase and growth inhibitory effects – various grades of N. Sativa oils. Int. J. Pharm. Sci. Res. 2018;10:245–250. [Google Scholar]
- Karthick C., Periyasamy S., Jayachandran K., Anusuyadevi M. Intrahippocampal administration of ibotenic acid induced cholinergic dysfunction via NR2A/NR2B expression: implications of resveratrol against Alzheimer disease pathophysiology. Front. Mol. Neurosci. 2016;9:28. doi: 10.3389/fnmol.2016.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazdair M.R. The protective effects of Nigella sativa and its constituents on induced neurotoxicity. J. Toxicol. 2015;15:1–7. doi: 10.1155/2015/841823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Roh J., Park C. Immunohistochemistry for pathologists: protocols, pitfalls, and tips. J. Pathol. Transl. Med. 2016;50:411. doi: 10.4132/jptm.2016.08.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooti W., Hasanzadeh-Noohi Z., Sharafi-Ahvazi N., Asadi-Samani M., Ashtary- Larky D. Phytochemistry, pharmacology, and therapeutic uses of black seed (Nigella sativa) Chin. J. Nat. Med. 2016;14:732–745. doi: 10.1016/S1875-5364(16)30088-7. [DOI] [PubMed] [Google Scholar]
- Laj N., Elayadeth-Meethal M., Huxley V.A.J., Hussain R.R., Kuruniyan M.S., Naseef P.P. Quorum-sensing molecules: sampling, identification and characterization of N-acyl-homoserine lactone in Vibrio sp. Saudi J. Biol. Sci. 2022;29(4):2733–2737. doi: 10.1016/j.sjbs.2021.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.J., Choi T.I., Kim Y.M., Lee S., Han B., Bak I.S., Moon S., Yu D.Y., Shin K.S., Kwon Y.K., Moon C. Regulation of habenular G-protein gamma 8 on learning and memory via modulation of the central acetylcholine system. Mol. Psychiatry. 2021;26:3737–3750. doi: 10.1038/s41380-020-00893-2. [DOI] [PubMed] [Google Scholar]
- Li W., Kutas M., Gray J.A., Hagerman R.H., Olichney J.M. The role of glutamate in language and language disorders-evidence from ERP and pharmacologic studies. Neurosci. Biobehav. Rev. 2020;119:217–241. doi: 10.1016/j.neubiorev.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchicchi A., Bloem B., Viaña J., Mansvelder H., Role L. Illuminating the role of cholinergic signalling in circuits of attention and emotionally salient behaviors. Front. Synaptic Neurosci. 2014;6:24. doi: 10.3389/fnsyn.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdi O., Baharuldin M.T.H., Nor N.H.M., Chiroma S.M., Jagadeesan S., Moklas M.A.M. Chemicals used for the induction of Alzheimer’s disease-like cognitive dysfunctions in rodents. Biomed. Res. Ther. 2019;6:3460–3484. [Google Scholar]
- Malik A.R., Willnow T.E. Excitatory amino acid transporters in physiology and disorders of the central nervous system. Int. J. Mol. Sci. 2019;20:5671. doi: 10.3390/ijms20225671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruca A., Rocca R., Catalano R., Mesiti F., Costa G., Lanzillotta D., Salatino A., Ortuso F., Trapasso F., Alcaro S., Artese A. Natural products extracted from fungal species as new potential anti-cancer drugs: a structure-based drug repurposing approach targeting HDAC7. Molecules. 2020;25:5524. doi: 10.3390/molecules25235524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaheri Y., Torbati M., Azadmard-Damirchi S., Savage G.P. A comprehensive review of the physicochemical, quality and nutritional properties of Nigella sativa oil. Food Rev. Int. 2019;35:342–362. [Google Scholar]
- McCaulley M., Grush K. Alzheimer’s Disease: Exploring the role of inflammation and implications for treatment. Int. J. Alzheimers Dis. 2015;15:1–10. doi: 10.1155/2015/515248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan M., Abhimannue A., Kumar B. Modulation of proinflammatory cytokines and enzymes by polyherbal formulation Guggulutiktaka ghritam. J. Ayurveda Integr. Med. 2021;12:13–19. doi: 10.1016/j.jaim.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohd Sairazi N., Sirajudeen K. Natural products and their bioactive compounds: neuroprotective potentials against neurodegenerative diseases. Evid. Based Complem. Altern. Med. 2020;1–30 doi: 10.1155/2020/6565396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhtar H., Qureshi A.S., Anwar F., Mumtaz M.W., Marcu M. Nigella sativa L. seed and seed oil: Potential sources of high-value components for development of functional foods and nutraceuticals/pharmaceuticals. J. Essent. Oil Res. 2019;31:171–183. [Google Scholar]
- Naseef P.P., Elayadeth-Meethal M., Anjana A., Muhas C., Kuruniyan M.S. Therapeutic potential of induced iron depletion using iron chelators in COVID-19. Saudi J. Biol. Sci. 2021 doi: 10.1016/j.sjbs.2021.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskouei Z., Akaberi M., Hosseinzadeh H. A glance at black cumin (Nigella sativa) and its active constituent, thymoquinone, in ischemia: a review. Iran. J. Basic Med. Sci. 2018;21:1200–1206. doi: 10.22038/ijbms.2018.31703.7630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozben T., Ozben S. Neuro-inflammation and anti-inflammatory treatment options for Alzheimer’s disease. Clin. Biochem. 2019;72:87–89. doi: 10.1016/j.clinbiochem.2019.04.001. [DOI] [PubMed] [Google Scholar]
- Pellow S., Chopin P., File S., Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Powell-Wiley T.M., Poirier P., Burke L.E., Despres J.P., Gordon-Larsen P., Lavie C.J., Lear S.A., Ndumele C.E., Neeland I.J., Sanders P., St-Onge M.P. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143(21):e984–e1010. doi: 10.1161/CIR.0000000000000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasree R.S., Ittiyavirah S.P., Naseef P.P., Kuruniyan M.S., Anisree G.S., Elayadeth-Meethal M. An evaluation of the antioxidant activity of a methanolic extract of Cucumis melo L. Fruit (F1 hybrid) Separations. 2021;8(8):123. [Google Scholar]
- Rajasree R.S., Ittiyavirah S.P., Naseef P.P., Kuruniyan M.S., Elayadeth-Meethal M., Sankar S. The anti-inflammatory properties of the methanolic extract of Cucumis melo Linn. against prostate enlargement in Wistar rats. Saudi J. Biol. Sci. 2022;29(9) doi: 10.1016/j.sjbs.2022.103396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozycka A., Liguz-Lecznar M. The space where aging acts: Focus on the GABA ergic synapse. Aging Cell. 2017;16(4):634–643. doi: 10.1111/acel.12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarghandian S., Farkhondeh T., Samini F. A review on possible therapeutic effect of Nigella sativa and thymoquinone in neurodegenerative diseases. CNS Neurol. Disord. Drug Targets. 2018;17:412–420. doi: 10.2174/1871527317666180702101455. [DOI] [PubMed] [Google Scholar]
- Schwartz T., Siddiqui U., Raza S. Memantine as an augmentation therapy for anxiety disorders. Case Rep. Psychiatry. 2012;12:1–3. doi: 10.1155/2012/749796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestakova N., Puzserova A., Kluknavsky M., Bernatova I. Determination of motor activity and anxiety-related behavior in rodents: methodological aspects and role of nitric oxide. Interdiscip. Toxicol. 2013;6:126–135. doi: 10.2478/intox-2013-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva E.R., de Carvalho F.O., Teixeira L.G., Santos N.G.L., Felipe F.A., Santana H.S.R., Shanmugam S., Quintans Júnior L.J., de Souza Araújo A.A., Nunes P.S. Pharmacological effects of carvacrol in in vitro studies: a review. Curr. Pharm. Des. 2018;24:3454–3465. doi: 10.2174/1381612824666181003123400. [DOI] [PubMed] [Google Scholar]
- Tan L.L., Kuner R. Neocortical circuits in pain and pain relief. Nat. Rev. Neurosci. 2021;22:458–471. doi: 10.1038/s41583-021-00468-2. [DOI] [PubMed] [Google Scholar]
- Tavakkoli A., Ahmadi A., Razavi B., Hosseinzadeh H. Black seed (Nigella Sativa) and its constituent thymoquinone as an antidote or a protective agent against natural or chemical toxicities. Iran. J. Pharm. Res. 2017;16:2–23. [PMC free article] [PubMed] [Google Scholar]
- Thajudeen K.Y., Alsayari A., Najib Ullah S.N.M., Salam S., Elayadeth-Meethal M., Uoorakkottil I. Validation, Optimization and Hepatoprotective Effects of Boeravinone B and Caffeic Acid Compounds from Boerhavia diffusa Linn. Separations. 2022;9(7):177. [Google Scholar]
- Van Dam D., De Deyn P.P. Animal models in the drug discovery pipeline for Alzheimer's disease. Br. J. Pharmacol. 2011;2011(164):1285–1300. doi: 10.1111/j.1476-5381.2011.01299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A., Balosso S., Ravizza T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat. Rev. Neurol. 2019;15:459–472. doi: 10.1038/s41582-019-0217-x. [DOI] [PubMed] [Google Scholar]
- Walf A., Frye C. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Gao H., Zhang W., Zhang W., Fang L. Thymoquinone inhibits lipopolysaccharide-induced inflammatory mediators in BV2 microglial cells. Int. Immunopharmacol. 2015;26:169–173. doi: 10.1016/j.intimp.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Wang R., Reddy P. Role of glutamate and NMDA receptors in Alzheimer’s disease. J. Alzheimer's Dis. 2017;57:1041–1048. doi: 10.3233/JAD-160763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Ji J., Liu C., Zhou M., Li H., Ye S., Hu Q. HJ22, a Novel derivative of piperine, Attenuates ibotenic acid-induced cognitive impairment, oxidativestress, apoptosis and inflammation via inhibiting the protein-protein interaction of Keap1-Nrf2. Int. Immunopharmacol. 2020;83 doi: 10.1016/j.intimp.2020.106383. [DOI] [PubMed] [Google Scholar]
- Yang Q.Q., Zhou J.W. Neuroinflammation in the central nervous system: symphony of glial cells. Glia. 2019;67:1017–1035. doi: 10.1002/glia.23571. [DOI] [PubMed] [Google Scholar]
- Yim N., Ryu S.W., Choi K., Lee K.R., Lee S., Choi H., Kim J., Shaker M.R., Sun W., Park J.H., Kim D. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein–protein interaction module. Nat. Commun. 2016;7(1):1–9. doi: 10.1038/ncomms12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Liang J., Wang J., Fei Z., Qin G., Zhang D., Zhou J., Chen L. Up-regulation of astrocyte excitatory amino acid transporter 2 alleviates central sensitization in a rat model of chronic migraine. J. Neurochem. 2020;155:370–389. doi: 10.1111/jnc.14944. [DOI] [PubMed] [Google Scholar]
- Zhou M., Yuan M., Zhao M., Hou M., Med C., Ma M., Yu M. Genistein antagonizes inflammatory damage induced by β-amyloid peptide in microglia through TLR4 and NF-κB. Nutrition. 2014;30:90–95. doi: 10.1016/j.nut.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Zieba A., Stępnicki P., Matosiuk D., Kaczor A.A. What are the challenges with multi-targeted drug design for complex diseases? Expert. Opin. Drug Discov. 2022:1–11. doi: 10.1080/17460441.2022.2072827. [DOI] [PubMed] [Google Scholar]
- Zong N., Li F., Deng Y., Shi J., Jin F., Gong Q. Icariin, a major constituent from Epimedium brevicornum, attenuates ibotenic acid-induced excitotoxicity in rat hippocampus. Behav. Brain Res. 2016;313:111–119. doi: 10.1016/j.bbr.2016.06.055. [DOI] [PubMed] [Google Scholar]