Abstract
In view of their low immunogenicity, biomimetic internal environment, tissue- and organ-like physicochemical properties, and functionalization potential, decellularized extracellular matrix (dECM) materials attract considerable attention and are widely used in tissue engineering. This review describes the composition of extracellular matrices and their role in stem-cell differentiation, discusses the advantages and disadvantages of existing decellularization techniques, and presents methods for the functionalization and characterization of decellularized scaffolds. In addition, we discuss progress in the use of dECMs for cartilage, skin, nerve, and muscle repair and the transplantation or regeneration of different whole organs (e.g., kidneys, liver, uterus, lungs, and heart), summarize the shortcomings of using dECMs for tissue and organ repair after refunctionalization, and examine the corresponding future prospects. Thus, the present review helps to further systematize the application of functionalized dECMs in tissue/organ transplantation and keep researchers up to date on recent progress in dECM usage.
Keywords: Decellularized extracellular matrix, Stem cell differentiation, Decellularized scaffold, Regenerative medicine, Tissue engineering
Graphical abstract
Highlights
-
•
The process of tissue repair using decellularization techniques is described in detail.
-
•
Research advances in different areas of extracellular matrix decellularization are summarized.
-
•
The achievements of extracellular matrix decellularization in tissue repair in recent years are summarized.
1. Introduction
Despite the great social and economic progress, our society still suffers from diseases causing serious tissue and organ damage, which highlights the importance of developing tissue repair technologies. A traditional method of tissue repair is autologous or allogeneic tissue transplantation [1]. However, autologous transplantation causes additional pain and injury to patients and suffers from the limited availability of donor sources [2], while the application of allografts (used to treat patients with donor tissue or organs) is restricted by the associated risks of immune rejection and disease transmission [3]. In view of the above, regenerative medicine, especially tissue engineering, has gained much attention, as it is considered to be the most promising strategy for tissue and organ repair [4].
When employed as matrix scaffolds for regenerative therapy, naturally derived biomaterials are superior to artificial polymers and have been used to construct complex matrices ranging from microtissues consisting of combinations of individual proteins to organ scaffolds generated through the decellularization of entire tissues. Organ/tissue-derived decellularized extracellular matrices (dECMs) feature the properties of a perfect tissue scaffold, namely a unique tissue-specific structure, complex vascular network, and composition, and are therefore well suited for both in vitro and in vivo regenerative medicine [5]. dECMs have been intensively studied since Poel completed the first exploration of their role in 1948 [6]. In the work of Badylak et al. (1995), small tears in the Achilles tendon of dogs were repaired using decellularized submucosa from the porcine small intestine [7], while Ott et al. (2008) first reported the decellularization and recellularization of an entire heart [8]. Macchiarini et al. (2008) extracted and decellularized the trachea of a cadaver and used chondrocytes derived from its epithelial and mesenchymal stem cells to repair the trachea of a patient with airway stenosis [9], while Basonbul and Cohen (2017) realized the endoscopic repair of the tympanic membrane in children using decellularized porcine small intestine submucosa [10]. ECM signaling influences organizational cell adhesion, migration, recruitment, differentiation, and proliferation based on the mechanism of (biological) tissue functioning [11]. These guiding elements allow one to retain at least partial functionality even in the absence of living cell components [12]. For this reason, dECM-based regenerative medicine methods for repairing damaged tissues or organs are being increasingly studied and applied in tissue engineering [13].
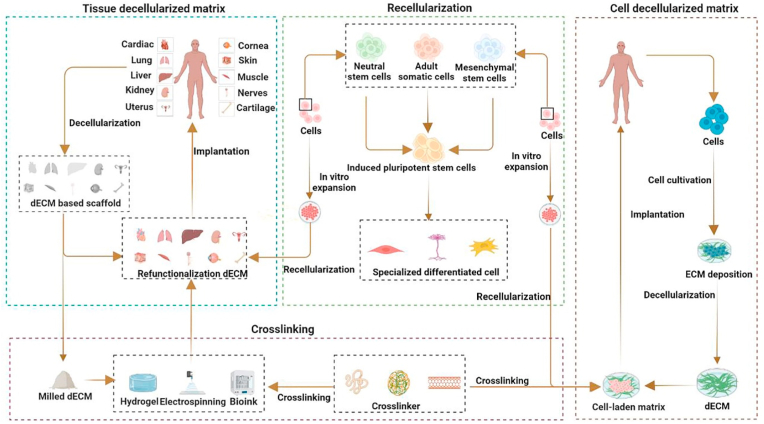
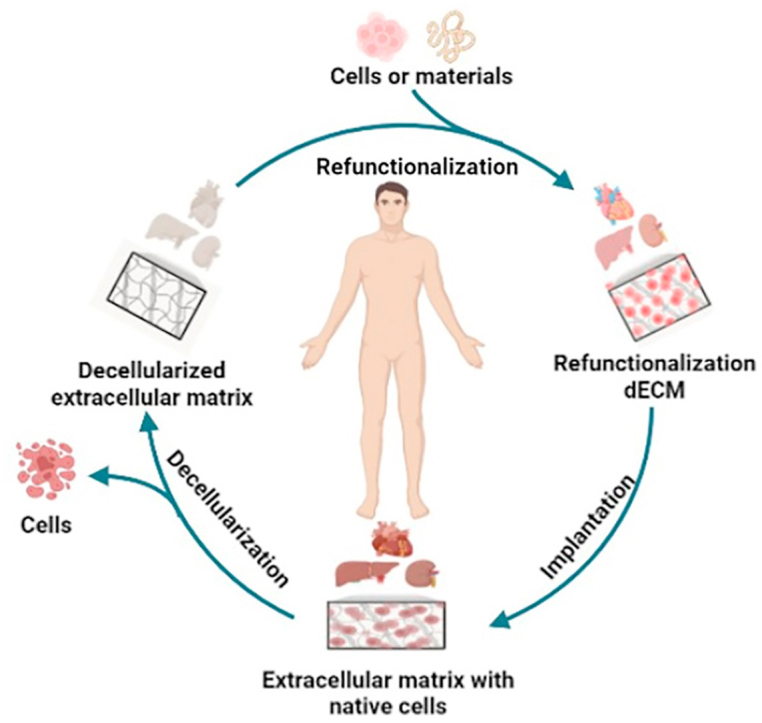
The recent years have witnessed a series of studies in all directions of dECM usage, as exemplified by tissue source expansion, de- and recellularization protocol optimization, the search for more suitable crosslinking materials, and the use of functionalized dECMs for tissue and organ repair. However, progress in the field of functionalized dECM research in different human tissues and organs is inconsistent because of their complexity. For example, several dECM products (e.g., Allderm® and OASIS®) have been successfully translated into clinical applications [14], whereas functionalized renal dECM scaffolds are still unable to perform basic kidney functions in vivo [15]. In this review, we make a review of all the topics concerning ECM, from its composition and characteristics, through protocols for obtaining dECM, as well as its characterization, and finally ending with an extensive description of application examples. This paper can be easily read by researchers, many of whom are newcomers to the subject, and can help them quickly understand the field of dECM. We also present the main differences between cell- and tissue-derived decellular matrices and discuss their functions in stem cell and tissue repair, respectively. Meanwhile, this paper updates some emerging decellularization methods and summarizes their benefits and drawbacks, as well as some progress in the application of dECM in tissue repair, allowing experts in this field of dECM to have quick access to the most recent news. We also outline in the review some of the dECM products that have been commercialized, which are rarely mentioned in other similar articles. Finally, we also summarize some of the issues that still need to be addressed in this area of dECM and deal with future dECM applications in tissue engineering. The application of the functional decellularization of the extracellular matrix to tissue repair is schematically illustrated in Fig. 1.
Fig. 1.
Schematic diagram of a functional decellularized extracellular matrix for individual tissue repair. Adapted reprinted with permission from Ref. [16], based on CC BY-NC-ND License.
2. ECM and dECM overview
2.1. ECM composition and function
The ECM is a distinct tissue-specific three-dimensional (3D) environment made up of structural factors and substances released by residing cells [17]. The cells interact with the ECM, with cellular products such as proteases, growth factors, and cytokines acting as functional cues to control cellular metabolic and secretory activities [13]. ECM proteins, which are broadly classified into fibrin and glycoproteins [18], regulate protein complexes, transmit cellular signals, bind growth factors, aid cell adhesion, and may also have other specific functions depending on the structure [19,20].
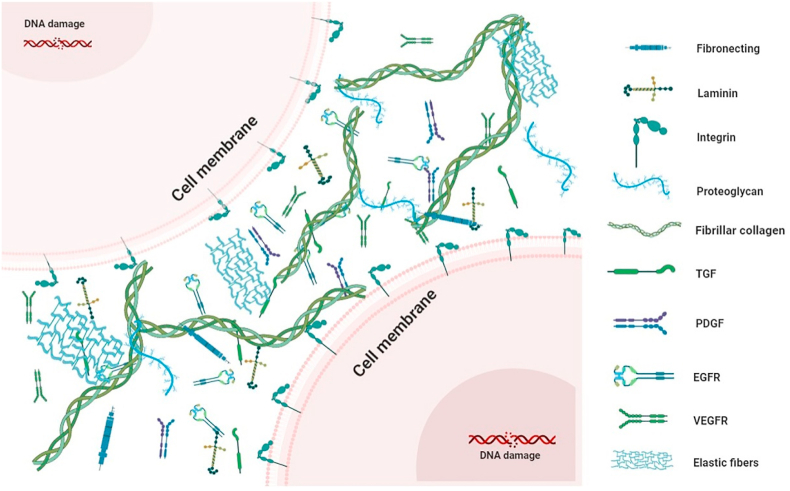
In addition to providing a structural foundation for tissue development, the ECM plays other important roles [21], e.g., is involved in tissue differentiation and organ isolation, establishment, and maintenance by regulating the essential growth factor and receptor hydration levels and the pH of the surrounding microenvironment [20,22], thereby influencing local cell behavior during cell migration, adhesion, differentiation, proliferation, and apoptosis [21]. Moreover, the ECM is involved in mechanical force transmission, signaling, and growth factor release [23]. Thus, the ECM is a highly complex tissue-specific structure and a repository of functional proteins capable of remodeling intrinsic cells and directing cell phenotype, survival, and behavior [21]. The composition of the ECM is illustrated in Fig. 2.
Fig. 2.
The extracellular matrix contains a variety of proteins and growth factors. Proteins are broadly classified into two major groups: fibrin (such as collagen, fibronectin, laminin, fibrillar, and laminin); and glycoproteins (such as proteoglycan). Growth factors, such as transforming growth factor (TGF), platelet-derived growth factor (PDGF), epidermal growth factor receptor (EGFR), Vascular Endothelial Growth Factor Receptor (VEGFR).
2.2. Association between ECM and stem-cell differentiation
Nearly all tissues, including blood and bone marrow, contain stem cells [5]. In vivo physical factors associated with stem-cell direction [24] include cell shape, external mechanical forces, and the ECM. The ECM is known to mechanically and chemically signal stem cells, which, in turn, influence the ECM by releasing growth factors and proteases into the same [25,26]. As a result, stem cells and the ECM are causally related in a reciprocal manner [27,28]. For instance, the dECM derived from adipocytes and bone marrow cells has a unique microenvironment with variable biomolecular structures and mechanical characteristics [29]. More significantly, this tissue-specific milieu affects stem-cell behavior in a variety of ways, e.g., by affecting stem-cell growth, morphology, and susceptibility to adipogenesis or osteogenesis induction [29]. In particular, osteogenesis is favored on harder or more viscous substrates, while adipogenesis is favored on softer substrates [[30], [31], [32]]. Therefore, the ECM can be both an inactive support and significantly impact stem-cell differentiation as a crucial component of stem cell ecology [33]. The relationship between the ECM and stem cell differentiation is shown in Table 1.
Table 1.
Role of ECM in inducing stem-cell fate.
| Role | Mechanism(s) | Function(s) | Ref |
|---|---|---|---|
| structural assistance | cell–matrix communication, mechanical properties, Porosity | Regulating cell proliferation, differentiation, and three-dimensional tissue architecture formation. | [22] |
| biochemical control | Integrins | Controlling cell homing, adhesion, migration, and differentiation. | [[34], [35], [36], [37]]. |
| growth factor control | Sequestration, Gradients,activation, paracrine, reservoir, autocrine | Controlling the dynamic bioavailability of growth factors and preserving stem cell self-renewal, differentiation, and survival. | [[37], [38], [39]]. |
| biological-mechanical control | Elasticity, stiffness, and microstructure of the ECM | modifying cell shape, tissue elongation, interactions between cells and the ECM, and controlling stem-cell destiny. | [37,[40], [41], [42], [43], [44], [45]]. |
Adapted reprinted with permission from Ref. [46], based on CC BY License.
2.3. dECM description and classification
dECM scaffolds are biomaterials formed from human or animal organs/tissues by removing immunogenic cellular components using decellularization techniques [47] and have several advantages over conventional stent materials [11,48,49]. For example, the 3D ECM structure remains intact after the removal of cellular and nuclear materials and preserves the natural ECM components for cell adhesion, proliferation, and differentiation. In addition, dECM stent materials contain cytokines and some signaling molecules in the natural matrix, e.g., Vascular Endothelial Growth Factor, basic fibroblast growth factor, and transforming growth factor-β, which can enhance cell function and promote vascularization. Furthermore, decellularization reduces ECM immunogenicity, allowing the use of tissues and organs of allogeneic origin and expanding the applicability of donated material for transplants. Finally, the dECM is biodegradable and does not induce inflammatory reactions. Similar to the ECM, the dECM mainly contains fibrin and glycosaminoglycan (GAG) [50]. Given that the physicochemical signals and biological properties of the ECM are retained after decellularization, the dECM can be used as a mechanical substrate and 3D biological carrier for subsequent cell inoculation [51,52]. The functionality of the dECM obtained from different tissue types is determined by the various growth factors, adhesion polypeptides, GAG, and collagen contained therein [53,54].
dECM scaffolds are classified as tissue-specific ECM (TS-ECM) and cell-derived ECM (CECM) [46]. TS-ECM is derived from decellularized tissues or organs from homologous or heterologous sources, such as cartilage, nerve, muscle, or heart tissue. CECM is obtained by culturing autologous cells or stem cells under aseptic conditions in vitro. CECM is obtained in a shorter and gentler manner than TS-ESM [55,56]. In addition, CECM scaffolds are more easily fabricated by using different types of cells, whereas with TS-ECM scaffolds, patient-specific cells such as mesenchymal stem cells, osteoblasts, chondrocytes, fibroblasts, and other cell types are required. The CECM scaffold can be prepared using cell-derived ECM particles, substrates, and in vitro scaffold-free live cell sheet culture systems. It can be used as a scaffold to maintain the desired geometry, bioelasticity, porosity, and biomechanical properties to enhance seed cell adhesion, differentiation, and proliferation, as well as to accelerate repair of damaged tissue [[57], [58], [59], [60]]. TS-ECM also has similar biocompatibility. It has been reported that cartilage-derived TS-ECM is more likely to promote chondrocyte differentiation, whereas C-ECM supports chondrocyte/stem cell proliferation and promotes chondrogenic potential, and that these C-ECM, alone or in combination with other factors, have varying abilities to promote chondrogenesis in vitro and in vivo [61]. In experimental animal studies and clinical trials, TS-ECM has been found to have some potential for pathogen transfer, inflammatory or anti-host immune responses, uncontrollable degradation, and other problems [[62], [63], [64]]. In contrast, the CECM stent can largely avoid these disadvantages. It can eliminate pathogen transfer to maintain a sterile matrix while providing the desired geometry and porosity and avoiding the limitations caused by poor cell permeability. In terms of applications, the CECM is often, but not always, used for the coating of biological materials, as exemplified by its application as a two-dimensional (2D) structure to promote wound healing or regeneration in bioengineered tissues [65]. In contrast to the TS-ECM, which is the perfect scaffolding substance for tissue engineering, the CECM is usually a place where primary culture cells or mesenchymal stem cells (MSCs) can be regenerated while maintaining their differentiation and potential for proliferation [61,66,67]. The CECM can regenerate bone marrow-derived MSCs [68,69], neural precursor cells [70], and periodontal ligament stem cells [71] and can enhance the differentiation and proliferation potential of primary cells such as chondrocytes [72,73], myeloid cells [74,75], and hepatocytes [76]. This ability is mainly due to anti-inflammatory and antioxidant effects [73,77], although the underlying mechanisms require further investigation. The TS-ECM exhibits the properties of a perfect tissue scaffold, namely a unique tissue-specific structure and complex vascular network and composition, and has two applications. First, the TS-ECM can be directly transplanted into the recipient, depending on his/her self-repair ability. Second, certain initiating materials can be added upon the implantation of a dECM scaffold to enhance its repair ability [78]. The dECM formed using initiating materials is also denoted as refunctionalized dECM. Currently, several functionalized dECMs such as porcine heart valves [79] and porcine small intestine submucosa have been approved for clinical use by the Food and Drug Administration of the United States [[80], [81], [82], [83]]. Thus, the functionalized dECM is a very promising material for tissue and organ repair.
3. dECM fabrication, characterization, and functionalization
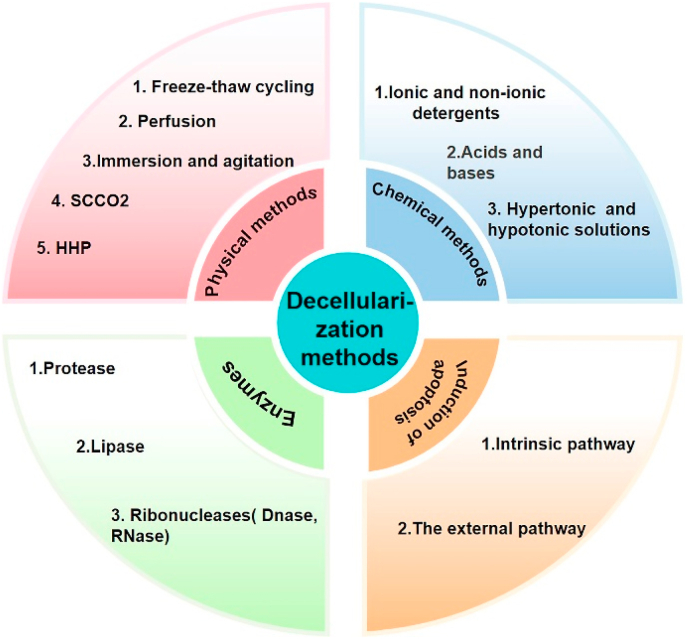
In the past decades of decellularization research, various methods have been developed to obtain the dECM while preserving its biochemical composition, mechanical integrity, 3D structure, and biological activity and minimizing the possibility of immunological resistance [21]. However, there is no perfect decellularization solution, and the employed method heavily depends on the properties of the primary tissue of origin, e.g., age, site, and size [84,85]. Numerous decellularization protocols are available, including traditional strategies of disrupting the outer cell membrane by physical, chemical, and enzymatic methods, all of which remove cellular components but inflict certain structural and compositional damage [86]. Therefore, the combined use of several methods can maximize the emptying of cellular contents while minimizing the adverse effects on the ECM [[87], [88], [89]]. Although new decellularization protocols such as apoptosis [90,91] and vacuum-assisted decellularization [[92], [93], [94]] have been proposed, they are currently not widely used because of their complex mechanisms [86]. A sketch of standard and newly emerged decellularization methods is shown in Fig. 3.
Fig. 3.
Methods of decellularization: chemical methods, physical methods, enzymes, and apoptosis.
3.1. dECM fabrication
3.1.1. Physical methods
Physical decellularization methods rely on the disruption of the cell membrane under the action of force, pressure, or temperature [46], with common physical methods as well as their advantages and disadvantages discussed below.
Effective decellularization can be achieved using freeze–thaw cycling between −80 °C and 37 °C [95]. This method is commonly used for the decellularization of ligaments, tendon tissues, and nerve tissues [96] and has been applied to both the TS-ECM and CECM to improve decellularization efficiency, preserve tissue structure, biomechanical properties, and biochemical composition, and reduce cytotoxicity and chemical residues [97,98]. However, as the resulting membrane and intracellular components are still present after freeze–thaw cycling, subsequent processing is required to eliminate cellular residues [11,96]. Decellularization efficiency usually depends on variables such as cellularity, ECM density, and tissue thickness [21] as well as on the freezing/thawing rate, setting temperature, processing time, and number of cycles [99].
Perfusion is a typical physical decellularization method that involves the cannulation of an organ/tissue followed by the formation of a channel for the flow of a circulating agent through the intrinsic vascular system [100]. For large thick tissues and whole organs, this method minimizes ECM damage inflicted by excessive forces while maximizing decellularization deep inside the organ [5]. The decellularization efficiency of perfusion depends on organ type, perfusion route (arterial or intravenous), mode, and parameters (flow rate, pressure, and temperature), and perfusate composition and kind [87,[101], [102], [103], [104]]. Perfusion-induced decellularization can be highly efficient [[105], [106], [107]] while preserving the rich and complex vascular network [[108], [109], [110]] and has been applied to complex organs such as lungs, liver, kidneys, and heart [111]. However, perfusion is a very complex process requiring additional hardware and sophisticated flow control equipment such as pressure receptors and infusion pumps [[112], [113], [114]].
In another physical method, the tissue is immersed into a decellularization solution under continuous mechanical agitation. The effectiveness of this method depends on parameters such as agitation intensity, decellularizing agent, and tissue size [115,116]. Immersion under agitation is considered the best physical decellularization method [86] because of its high efficiency and short exposure time [113,117] as well as simplicity and ability to preserve the ECM surface structure, collagen structure, mechanical strength, and GAG content [107,[117], [118], [119], [120]].
Newer methods include nonthermal irreversible electroporation, which uses electrical pulses as an alternative to cell lysis and can disrupt cell membranes but is only applicable to small tissues [46]. In addition, supercritical CO2 has been used for bone decellularization [95]. These methods can significantly reduce decellularization time and improve disinfection compared to traditional methods [48]. Effective decellularization requires rapid decompression, and the high hydrostatic pressure used to disrupt cell membranes during decellularization can also kill viruses and thus eliminate the need for further sterilization [95]. Nevertheless, physical methods alone cannot achieve effective decellularization and may require subsequent treatment to eliminate membranous and intracellular residues generated in the tissue [21].
3.1.2. Chemical methods
Chemical agents effectively remove cellular contents by promoting the hydrolytic degradation of biomolecules, disrupting cell membranes, and dissolving the bonds of inter- and extracellular junctions [11,96]. The main chemical decellularization methods use ionic or nonionic decontaminants, acids or bases, and hypertonic or hypotonic solutions [21].
Ionic decontaminants are synthetic organic compounds used primarily to remove genetic material and break down cell nuclei in various tissues and organs (e.g., bone) [95]. Sodium dodecyl sulfate (SDS) has found numerous applications as an ionic decontaminant [121] but may reduce the content of GAG and growth factors in the ECM [95] and is somewhat toxic [122]. Nonionic decontaminants (e.g., Triton X-100) are biodegradable emulsifiers that are used to solubilize proteins and degrade cell membranes [123], cleaving protein–DNA and lipid–protein bonds [124] while maintaining the integrity of protein–protein bonds [46]. The acids commonly used in decellularization include peroxyacetic, hydrochloric, and acetic acids [125], while the corresponding bases include sodium hydroxide, sodium sulfide, and calcium hydroxide [126]. Acidic compounds promote hydrolysis by forming covalent bonds or providing H+ ions [125], while bases function by inducing cellular cleavage and denaturing genetic material [86]. Hyper-/hypotonic solutions are those with solute concentrations higher or lower than intracellular concentrations, respectively, and are used to remove cellular components at the extracellular level. Hypertonic solutions can remove proteins, whereas hypotonic solutions can remove nuclei and DNA [127]. However, neither of these solutions can completely eliminate cellular residues [128].
Even though chemical agents can effectively achieve decellularization, they damage ECM composition and structure. Therefore, chemical decellularization protocols should be optimized for different tissues in combination with other decellularization protocols to minimize ECM damage.
3.1.3. Enzymes
Enzymatic decellularization is highly specific and removes cellular components and unwanted ECM components by disrupting cell–matrix bonds or specific bonds in cells [86], with typical enzymes corresponding to trypsin, collagenase, lipase, dispase, thermophilic proteases, and nucleases [11]. When used in the first step of decellularization, trypsin can completely eliminate nuclei while preserving GAG content [129,130], which is essential for maintaining the biomechanical structure of the ECM [95]. However, studies on the effects of trypsin on collagen and elastin are limited [21]. Nucleases, including ribonuclease (RNase) and deoxyribonuclease (DNase), break down nucleotide sequences during cell lysis [131,132] and are therefore commonly used to remove nucleic acids after physical pressure and chemical decontamination agent–induced cell lysis [133]. However, prolonged nuclease action can lead to the loss of ECM components [118,134,135]. Dispersase can prevent cell aggregation [136]. Lipase is also used in decellularization, as it catalyzes the hydrolysis of lipid macromolecules [11,96]. The effective and relatively rapid removal of lipids from tissues can be achieved using treatment with alcohols such as ethanol and propanol [11,96], which, however, may lead to tissue clouding [11].
Although enzymatic decellularization protocols are highly specific, they may affect the structure and function of the native ECM and are not applicable to thicker tissues.
3.1.4. Apoptosis
Apoptosis, i.e., genetically programmed cell death [137,138], proceeds via extrinsic and intrinsic pathways [12], both of which are driven by extremely complex and incompletely understood molecular processes. During apoptosis, the cell membrane undergoes structural changes resulting in the loss of contact between the cell and the ECM [139]. In addition, as the cell contents are strictly maintained within apoptotic vesicles and the cell membrane [140], their immunogenic material does not leak into the surrounding ECM [[141], [142], [143]]. Based on this, a novel decellularization scheme was developed for the controlled activation of apoptotic pathways via the delivery of appropriate signals [12]. For example, the extrinsic pathway can be activated by the specific ligands of the tumor necrosis factor superfamily death receptor, which alter the environment the cells are exposed to (e.g., temperature, pH, and CO2/O2, NO, and H2O2 levels). The intrinsic pathway can be activated by altering environmental stress or gene editing to make cells naturally undergo apoptosis.
Both approaches induce natural apoptosis by regulating the expression levels or implementing the toxic transgenes of key involved genes. The apoptosis method achieves decellularization with little change in matrix function or structure. The advantages and disadvantages of decellularization methods are summarized in Table 2.
Table 2.
Advantages and disadvantages of decellularization methods.
| Methods | Advantages | Disadvantages | Ref | |
|---|---|---|---|---|
| Physical methods | Freeze-thaw cycling | Simple operation low demand for equipment. Cryoprotectant usage can minimize ECM disruption. |
Require more treatments to get rid of the contents of the cells. Ice crystals' disruption of the ECM microstructure. |
[99,[144], [145], [146]] |
| Perfusion | Maximizing the delivery of decellularization deep inside the organ improves decellularization efficiency. | Stringent tissue requirements need for blood vessels. Perfusion is more complex and requires additional hardware and sophisticated flow control equipment. |
[[117], [118], [119],[123], [124], [125], [126]] | |
| Immersion and agitation | The process is simple. Proteins of ECM can be well preserved. |
Need the right mixing strength and time-consuming long. | [119,[129], [130], [131],147] | |
| SCCO2 | Significantly reduce the decellularization time and improve disinfection compared to other methods. | Rapid decompression is necessary. Not widely used. |
[71] | |
| HHP | Eliminating the need for further sterilization. Processes |
Make it challenging for solutions to enter the ECM because of the constant high pressure. | [105,[148], [149], [150], [151], [152]] | |
| Chemical methods | Ionic and non-ionic detergents | Versatile and suitable for a wide range of tissues. | Reduction of active ingredients in ECM Some toxicity |
[105,134] |
| Acids and bases | Cheap and less time-consuming | Damage to the ECM architecture, affecting the contents of the ECM. | [[153], [154], [155]] | |
| Hypertonic and hypotonic solutions | Hypertonic solutions can remove proteins, whereas hypotonic solutions can remove nuclei and DNA. | Unable to completely eliminate cellular residues. | [98,140] | |
| Enzymes | Protease | Less time-consuming | Difficult to intern adequate decellularization. Affecting the contents of the ECM. Not suitable for sensitive tissues |
[141,156] |
| Lipase | Help the process of decellularization by first eliminating the epithelium and endothelium. | Hard to remove all lipids | [[156], [157], [158]] | |
| Ribonucleases (Dnase, RNase) | Effective removal of DNA levels from dECM | Easily residual, difficult to completely eliminate reagents Influencing the structure of ECM |
[127,159,160] | |
| Induction of apoptosis | Intrinsic pathway | The structure and function of dECM remains almost unchanged. | Apoptotic pathways are very complex and not yet fully understood. Not widely used. |
[12] |
| The external pathway | ||||
Abbreviations: ECM: mExtracellular Matrix. dECM: decellularized extracellular matrix. SCCO2:supercritical CO2.
3.2. Characterization of dECM
Any decellularization step can change ECM composition, structural properties, and integrity, thus affecting the biological and mechanical properties of the resulting dECM [21]. In addition, undesirable products may be released during decellularization [161], and residual cellular components may lead to cytocompatibility problems and cause adverse reactions in the host [22]. Therefore, the careful characterization of dECM properties is a task of high importance. The following minimum criteria for competent decellularization quantification were proposed [11]: (1) <50 ng of dsDNA per mg of ECM dry weight; (2) DNA fragment length <200 bp; and (3) lack of visible nucleated material in 4′,6-diamidino-2-phenylindole (DAPI) or hematoxylin and eosin (H&E) -stained tissue sections. These criteria primarily focus on the characterization of DNA removal, as residual DNA is responsible for most adverse host reactions [159,162]. Common characterization techniques include protein composition assays of the dECM, residual assays of cells, observation of general macroscopic structures such as the vascular system and pore size [8,163,164], and biomechanical and structural analyses.
3.2.1. Microscopic techniques
Microstructure assessment methods include those relying on light microscopy, which has been employed for preliminary qualitative research on tissue (e.g., cornea) structure [157]. Phase-contrast microscopy allows the optical path to be easily modified to increase image resolution and contrast [157]. Collagen tissue may be measured quantitatively, and polarized light microscopy can spot any structural alterations in collagen that might take place during decellularization [157]. Detailed in vivo observation is made possible by confocal microscopy [157]. Electron microscopy is mainly used to provide local information on tissue structures [21]. Transmission electron microscopy (TEM) has greater resolving power than light microscopy for ultrastructural feature analysis and is a core technique for gaining insights into natural tissue structures, including the order, diameter, and spacing of collagen fibers [157]. Scanning electron microscopy (SEM) provides lower resolution than TEM and is mainly used to characterize the sample surface and morphology and thus determine the cellular morphology of tissues [157]. Atomic force microscopy (AFM), which uses a piezoelectrically controlled fine tip to scan the tissue surface [157], can measure the interstitial areas between collagenous protofibrils (unlike SEM and TEM) and is used for the nondestructive screening of decellularized tissues and TEM data validation [157]. The second-harmonic imaging of the cornea was reported to provide high spatial resolution and contrast comparable to those achieved by light and electron microscopy imaging [165]. High-frequency ultrasound (50 MHz) was reported to provide higher (up to 30-μm) resolution than traditional ultrasonography methods [166].
3.2.2. Proteomic analysis
The in-depth analysis of dECM proteins is a task of high importance, as they determine the bionomic properties of the dECM [21]. Gel electrophoresis separates proteins according to their molecular weight, thus effectively solving the problem of mixing various protein components in the dECM [167]. The combination of mass spectrometry (MS) and 2D gel electrophoresis is effective for screening proteins and peptides in tissues or organs [21]. MS methods commonly applied to decellularized materials include liquid chromatography tandem mass spectrometry [167], time-of-flight secondary-ion mass spectrometry [168], and matrix-assisted laser desorption/ionization mass spectrometry [169]. Messenger RNA microarrays allow the examination of proteins adsorbed on the dECM surface [170]. However, the available evidence suggests that tissue proteomic analysis based on protein extraction may induce the disruption and dissociation of ECM proteins to varying degrees depending on tissue type and donor age; therefore, advanced strategies are needed to confirm the tissue specificity of the prepared dECM [21].
3.2.3. Cell residue determination
The most common methods used to assess cellular material removal and residuals are histology, DNA staining, and imaging [157]. Routine histological staining and immunofluorescence are used for qualitative verification to prove the efficient removal of nuclear contents and the cytoplasm [21]. The initial step of evaluation often corresponds to H&E staining, in which case hematoxylin is most frequently used to determine the degree of decellularization, while eosin is typically used to evaluate the composition of the nonnuclear ECM [157]. Saffron, Movat's pentachrome, and Masson's trichrome are additional histological stains enabling the qualitative detection of various extracellular and cytoplasmic components [96]. DNA staining for detecting cellular and nuclear components as well as fluorescent DAPI, Hoechst, and PI staining can be used to detect residual DNA [21]. In addition, the terminal Deoxynucleotidyl Transferase mediated dUTP Nick-End Labeling (TUNEL) method is used to assess possible apoptosis during decellularization by detecting the number of DNA fragments based on the fluorescent labeling of nucleic acid ends [157]. DNA staining and imaging are common ways to assess decellularization results and are often used as first-line studies. However, these methods are usually not very accurate and do not give quantitative data. Immunochemical techniques such as toluidine blue, Alcian blue, and Verhoeff-van Gieson staining are frequently employed to identify dECM components [157,171].
3.2.4. Quantification of residual chemicals
Unfavorable residues in decellularized cells can cause severe immunological reactions and hinder recellularization [172]. Therefore, the quantification of residual chemicals after dECM preparation is an essential task. For example, methylene blue binding assays have been used, along with the quantification of residual SDS in decellularized cruciate ligaments by the collagenase-induced digestion of supernatants prepared from scaffolds [173]. Visible-light spectroscopy is another simple method of quantifying SDS in decellularized cells [170]. Residual SDS has also been quantified by gas chromatography, and 6 h was suggested to be the optimal wash time for significantly reducing the content of residual SDS in decellularized liver scaffolds [174]. The presence of residual decellularization chemicals is a major problem, as they can be cytotoxic even at low concentrations [21]. Therefore, it is important to develop suitable decellularization protocols and methods for quantifying residual chemicals to achieve a trade-off between retaining sufficient ultrastructural features and effective proteins of the ECM and simultaneously removing as much cell content as possible and minimizing the amount of residual chemicals.
3.2.5. Biomechanical and structural analyses
The balance between effective decellularization and the sufficient preservation of the ECM structure is a major challenge of organ/tissue decellularization. By correlating the results of mechanical analysis after decellularization with the retention degree of natural ECM structures, one may understand the role of proteins in determining ECM biomechanics [96,175]. Therefore, tissue tensile strength and elasticity can be used to evaluate ECM preservation. Specific tests are used for applications in tissue engineering, as exemplified by (i) swelling tests, in which the entire tissue or film is inflated through a window in the substrate, and its displacement is measured and correlated with mechanical strength [176,177]; (ii) pressure tests, in which the material is compressively deformed between two plates under a known load to determine its resilience during fragmentation [178,179]; and (iii) uniaxial tensile tests used to measure the elastic modulus [171]. Despite the availability of various methods, the mechanical characteristics of biological structures remain challenging to measure, especially under sterile conditions [157]. The test results are difficult to compare because of the wide variation in the technical setup, and the interpretation of the results can lead to discrepancies in data-derived mechanical properties [180].
3.2.6. Disinfection
Disinfection is required to lower the possibility of negative immunological reactions [21]. Standard clinical sterilization methods, including the application of pressurized steam, dry heat, or chemicals, inevitably result in protein denaturation [181]. Other sterilization agents such as electron beam irradiation, gamma irradiation, and ethylene oxide can change the mechanical and ultrastructural characteristics of the dECM [182,183] and impact the functionality of dECM-containing clinical products [184,185]. Disinfection with antibiotics significantly inhibits bacterial growth by disrupting bacterial cell walls and preventing the production of DNA and proteins while having an insignificant impact on decellularized scaffold structures [86]. However, each antibiotic has a limited antibacterial spectrum [186,187]. Compared to other disinfection techniques, treatment with supercritical CO2 can be used as a substitute for sterilization and induces less variation in the mechanical properties of the ECM [188]. Nevertheless, further research is required to confirm whether sterilization can be achieved without destroying the dECM.
3.3. dECM refunctionalization
3.3.1. Crosslinking
Crosslinking, which can be performed physically or chemically, is often used to preserve the 3D structure of the dECM, improve scaffold characteristics [189], and reduce inflammatory potential. Given that the decellularization process usually adversely affects ECM characteristics, the dECM is usually strengthened by crosslinking to obtain modified dECM. For example, tissue decellularization using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) crosslinking combined with chemical extraction can promote the adhesion and differentiation of MSCs [190]. In a recent report, acrylate groups were transferred to a decellularized platform by photocrosslinking to enhance the mechanical properties of the dECM and form hydrogels with high shape stability [191]. In addition, the methylene blue–mediated photo-oxidative crosslinking of a tumor dECM greatly increased the stiffness of the scaffold but hardly changed the amide III band of the peptide and protein secondary structures [192]. The chemical modification of gelatin to afford gelatin methacrylate (GelMA) improved the adherence and homing ability of bone marrow MSCs in the ECM [193]. A GelMA hydrogel with functionalized ECM was used to repair irregular cartilage defects. In addition, dECM crosslinked with hyaluronic acid maintained the natural collagen secondary structure and the microporous structure of the porcine decellularized dermal matrix, exhibited enhanced degradation resistance and moisturizing ability, and promoted wound healing [194]. Methacrylic acid, hyaluronic acid, and gelatin were used to successfully regenerate mature cartilage in vitro and in an autologous goat model, affording a characteristic trap structure and cartilage-specific ECM [195]. The crosslinking effect of glutaraldehyde, oxidized chitosan oligosaccharide, and carbodiimide on the dECM of bass significantly improved the mechanical properties and degradation resistance of the acellular dermal matrix, which indicated that carbodiimide can improve matrix properties and has potential applications in biomaterial engineering [196]. In another study, rat decellularized lung was bend into tannic acid crosslinked tissue (TA-CLT) or EDC/N-hydroxysuccinimide (NHS)-crosslinked tissue (EDC/NHS-CLT) [197]. TA-CLT strongly induced T-cell proliferation and attenuated macrophage proliferation, while EDC/NHS crosslinking provided physical attributes similar to those of natural lung tissue. These studies indicate that the original biological properties of the dECM can be recovered (or even improved) by crosslinking with various reagents, which plays an important role in the reduction of the corresponding inflammatory potential. However, the possible toxic effects of crosslinking agents should not be ignored.
3.3.2. Recellularization
Recellularized scaffolds are those that have had various cell types (e.g., stem cells, chondrocytes, and epithelial cells) implanted on them to impart form and function [198]. For example, tracheal cartilage was successfully regenerated and repaired using photocrosslinked hydrogels combined with chondrocytes [191]. Stem cells are usually classified into pluri- and multipotent ones, among which the former are more capable of differentiation and include induced pluripotent stem cells and embryonic stem cells [86]. MSCs can differentiate into various cell types and show remarkable performance in the field of tissue engineering, as exemplified by tendon repair, bone regeneration, cardiomyogenesis, and skin wound healing [[199], [200], [201], [202], [203], [204], [205], [206], [207]]. In view of its biological and physical characteristics, the dECM is considered a suitable biological scaffold for the application of MSCs in tissue repair, making MSCs differentiate at specific sites and working in concert with cells to heal tissues [207]. In the case of decellularized corneas, recellularization strategies are classified into (1) the ex vivo inoculation of constructs for the downstream transplantation of cellularized grafts and (2) in vivo implantation, which enables the graft to be repopulated with host cells following surgery [157]. Recent studies have dealt with the repair of damaged tissues/organs using cell-free strategies, that is, the activation of cells and their accumulation at damage sites induced by appropriate stimulation and recruitment factors, the recruitment of endogenous stem cells to the damaged sites, and tissue repair [208]. However, these factors require further investigation. Furthermore, the recellularization of decellularized substrates is affected by many factors such as the diversity of cell types, recellularization method, cell density, and culturing conditions [86].
4. Tissue repair using functional dECMs
4.1. Cartilage and bone regeneration
Currently, Bone defects caused by trauma, tumors, or osteoarthritis remain challenging [209,210], two biomaterial types are primary used in cartilage tissue engineering, namely (1) synthetic biomaterials such as polylactic acid [211] and polycaprolactone [212] and (2) natural biomaterials such as fibrin [213], gelatin [214], and collagen [215]. Owing to the complexity of cartilage ECM, natural scaffold or biomaterial-derived ECM holds great promise for cartilage repair [13]. Both macro- and microstructural characteristics are preserved in dECM scaffolds, which greatly improves osteoconductivity [[216], [217], [218]]. Various dECM scaffolds have been used in bone and cartilage repair, including injectable hydrogels and electrospun and 3D printed scaffolds.
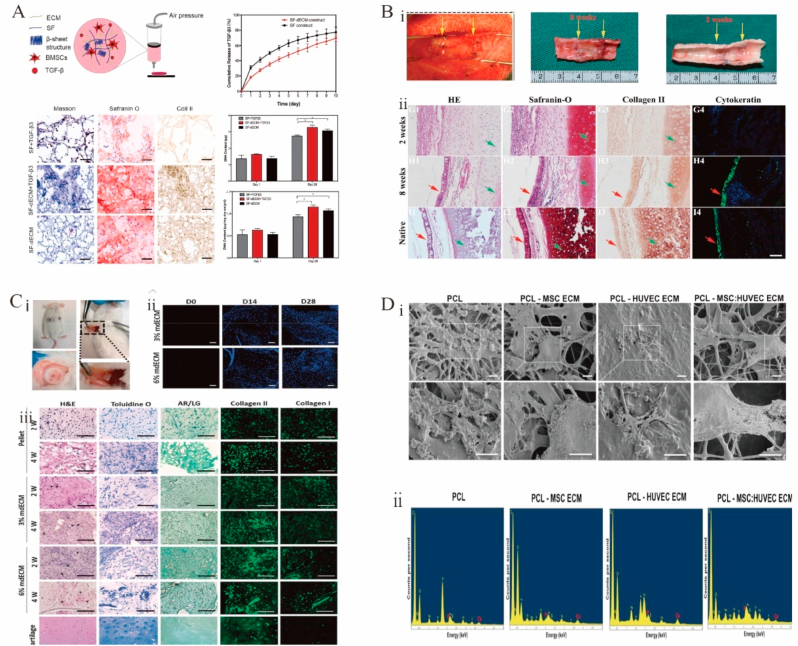
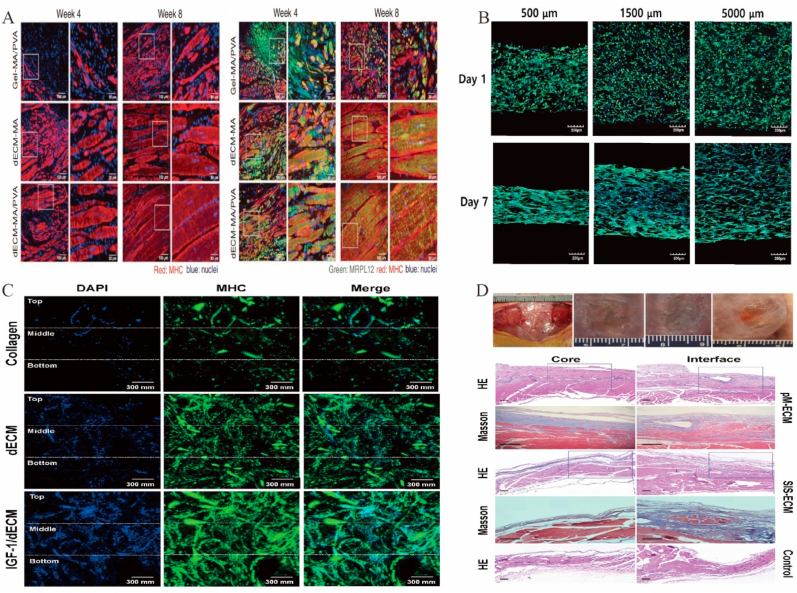
Hydrogels based on dECM have been extensively investigated for cartilage and bone regeneration. Hydrogel has good absorption, satisfactory biocompatibility, and high safety [219]. Bioadhesive hydrogels show great potential for bone regeneration [220]. For example, MSCs were used to prepare well-biocompatible bionic hydrogels inducing chondrogenesis and further hyaline cartilage formation without the addition of induction agents (Fig. 4C) [221]. However, the storage modulus of hydrogels made of bone dECM (∼150 Pa at 6 mg/mL) was lower than that of bone (8–11 GPa) [222], which affected hydrogel mechanical properties. The binding of cartilage ECM particles modified with the affinity peptide sequence PFSSTKT to GelMA hydrogels enhanced the mechanical properties of hydrogels and allowed them to provide a good 3D supported microenvironment [193]. An acrylic anhydride–crosslinked dECM hydrogel facilitated the formation of homogeneous cartilage and the repair of cricoid tracheal injury in a rabbit model (Fig. 4B) [191]. The crosslinking of carbodiimide with GAG in the absence of pepsin improved the mechanical properties and increased the osteogenic capacity of dECM [223,224], which suggests that the use of an enzymatic treatment protocol during matrix decellularization may affect the biomechanics of the subsequent dECM scaffold and the retention of certain matrix factors. Different tissue origins may also be one of the factors influencing the induction of osteogenesis in dECM scaffolds. Some studies showed that dECM scaffolds derived from cartilage or adipose tissue are more osteogenic than those derived from lung or spleen tissue [224].
Fig. 4.
Functional decellularized extracellular matrix in Cartilage and bone repair. (A) These findings demonstrated the ability of the SF-dECM 3BDP scaffolds to encourage chondrogenesis and cartilage regeneration in vivo. Adapted reprinted with permission from Ref. [237](License number:5,442,250,848,007). (B) Application of engineered tracheal cartilage for tracheal reconstruction. (i) Engineered tracheal images at the time of surgery, 2 weeks postoperatively, and 8 weeks postoperatively. (ii) Epithelialization was not evident at postoperative week 2, but by week 8, the epithelial layer was visible and not significantly different compared to that of the natural gas tube. Adapted reprinted with permission from Ref. [191], (License number:5,442,390,855,450). (C) Biological evaluations of mdECM hydrogel in vivo. (i) Pictures taken four weeks after implantation in CD1 mice. (ii) With time, a significant increase in the number of nuclei was seen. (iii) mdECM hydrogel acquired the typical shape of chondrocytes embedded in lacunae after 2 weeks in vivo, which was more evident in the highest concentration. Using toluidine O staining, it was evidenced that the deposition of GAGs had increased. Adapted reprinted with permission from Ref. [221] (License number:5,442,400,012,079). (D) MSCs have differentiated into osteoblasts. (i) After 21 days of osteogenic development, SEM scans showed calcified nodules. (ii) After 21 days in culture, electro spun scaffolds contain calcium and phosphorus. Adapted reprinted with permission from Ref. [229] (License number:5,442,400,497,756). Abbreviation:SF-dECM: silk fibroin and decellularized extracellular matrix, mdECM: from mesenchymal stem cells.
Electrospinning is widely used in tissue engineering [[225], [226], [227], [228]], as it produces fibrous and porous scaffolds with good interconnectivity, high porosity, and elevated surface area. These properties facilitate proliferation, cell attachment, waste exchange, and nutrient uptake [225,227], allowing one to mimic the hierarchically ordered fibrous structure and architecture of the ECM [229]. However, electrospun scaffolds cannot achieve biological functions owing to the lack of cellular activity. In contrast, dECM has good bioactivity, although its mechanical quality is insufficient for regenerating and supporting bone or other hard tissues [230,231]. Thus, the CECM can be combined with electrospun scaffolds to enhance their mechanical quality. CECM-polycaprolactone scaffolds [232] and Ti implants [233] have been used for bone healing with excellent cell proliferation and osteogenic differentiation. Carvalho et al. developed the first ECM–polycaprolactone electrospun scaffold cocultured with the ECM from MSCs/stromal cells and the ECM from umbilical vein endothelial cells, showing that the resulting material had excellent osteogenic properties and a promising future in bone repair (Fig. 4D) [229].
3D bioprinting is an emerging technology in the rapidly advancing area of cartilage tissue engineering and is mainly used to print dECM scaffolds for later cell growth [234]. Recently, 3D printing has been used to create “living” tissue structures by depositing live cells with printable biomaterials (“bioinks”) [235,236]. A bioink prepared using silk fibroin and dECM was mixed with MSCs for the 3D bioprinting of porous structures with high mechanical strength, appropriate degradation rate, and precise shape capable of supporting BMSC proliferation and promoting cartilage differentiation (Fig. 4A) [237]. However, the difficulty of preparing suitable bioinks and the toxicity of most chemical crosslinking procedures used for bioink fabrication hinder the widespread adoption of this technology.
CECM is used as a cell culture matrix in bone tissue engineering and regenerative medicine because of its good biocompatibility and biodegradability. CECM is rich in collagen and proteoglycans, which facilitate the proliferation and osteogenic differentiation of MSCs and maintain their multidirectional differentiation potential while having lower reactive oxygen species levels and higher bone morphogenetic protein-2 (BMP-2) sensitivity [238]. In addition, in vivo transplantation assays have shown that MSCs still have the ability to form large amounts of bone tissue after the expansion of additional generations on an ECM-based culture platform [239,240]. Sun et al. reported that CECM is more biased to support chondrocyte/stem cell proliferation and promote chondrogenic potential than dECM of cartilage tissue origin due to a number of microstructural (mean pore size and fiber diameter), micromechanical, insoluble (e.g., collagen and GAG), and soluble factors [61]. Some researchers, too, have developed hybrid scaffolds consisting of CECM and various types of inorganic materials. For example, Antebi et al. combined stromal CECM into a collagen/hydroxyapatite (COL/HA) scaffold and found that MSCs cultured on the cECM-Col/HA scaffold proliferated significantly faster than those cultured on the Col/HA scaffold, and the expression levels of the osteogenic markers alkaline phosphatase (ALP), bone bridging protein, and Runx2 were also higher than those of cells cultured on the Col/HA scaffold alone [241]. The construction of a hybrid scaffold using human lung fibroblast-derived decellularized ECM as a carrier and inoculation with human placenta-derived MSCs was found to induce more new bone formation and a more complete repair of bone defects [242]. This shows that CECM tends to promote the proliferation and differentiation potential of MSCs, improving their quantity and quality.
4.2. Skin repair
The skin consists of three layers, namely a compound squamous epithelium mainly composed of keratinocytes, a subcutaneous tissue containing fat, and a dense dermis with fibroblasts rich in ECM and containing extensive blood vessels, hair follicles, and sweat glands [243]. The loss of skin integrity may lead to severe physiological imbalance and subsequent injury [244]. Although skin damage can heal spontaneously [245], this self-healing capacity may be exceeded in cases of major trauma such as large or severe burns and skin wounds caused by chronic diseases such as diabetes. Under these conditions, autografting remains the standard of care [246,247] but is not suitable for large burns because of the limited availability of skin grafts and is less effective for treating diabetic wounds [248]. Diabetic wounds are one of the most common diabetic complications, and they are chronic and difficult to heal [249]. Growth factors are essential for regulating the cellular response to the wound healing process [250,251]. A variety of biomaterials and bioactive compounds have now been shown to be effective in wound healing [252]. A novel angiogenic 3D bioprinted peptide patch to improve skin wound healing [253]. Luckily, dECM-based biomaterials can improve the healing of diabetic wounds and increase the survival of third-degree-burn patients [243,254].
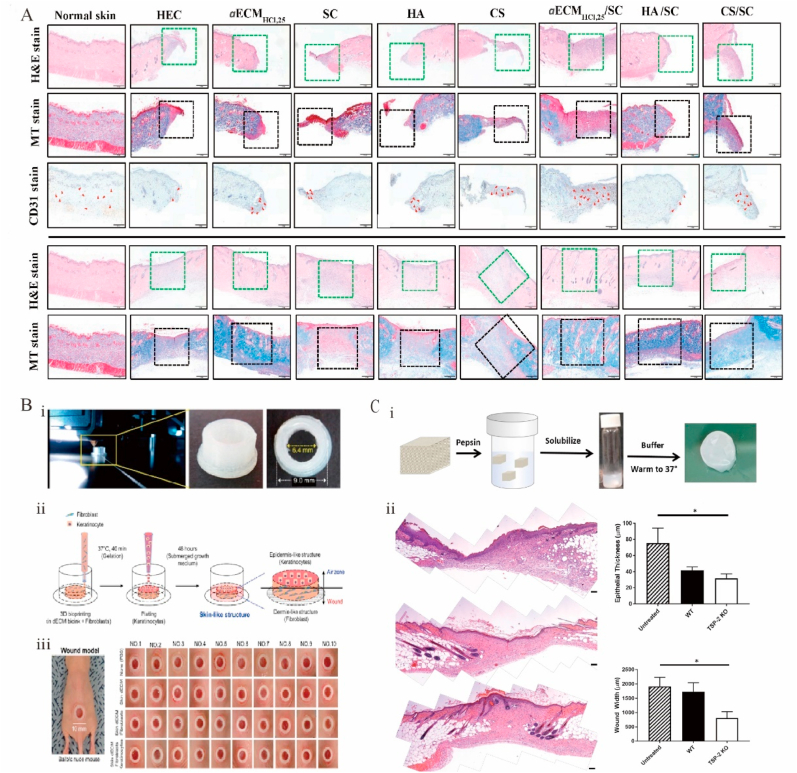
In skin-repair applications, dECM-based hydrogels have been found to facilitate diabetic wound healing [248]. In one study, during the healing process, histological H&E staining (Fig. 5A) was performed on hydrogel dressings, and the results were compared with those obtained for normal skin on days 8 and 14. The wounds were closed on day 14 with no significant inflammation and a moderate number of fibroblasts occupying the dermis, which was considered skin wound healing [255]. A TSP-2 KO dECM hydrogel accelerated wound healing in diabetic mice by promoting wound angiogenesis and remodeling (Fig. 5C) [256].. Previous studies have mainly demonstrated the occurrence of revascularization and epithelialization during skin repair. A new composite hydrogel dressing containing a glycophorin and decellularized pepsin–formic acid–soluble ECM was shown to synergistically promote diabetic wound healing and help regenerate hair follicles and sweat glands [255]. Placenta-derived dECM hydrogels containing sulfated GAG were shown to effectively promote wound healing, which implies a combined biological function for ECMs containing sulfated GAG [257]. Several dECM products (e.g., Allderm® and OASIS®) have been successfully translated into clinical applications [14] but do not rebuild the hair follicle or sweat gland structure, which is the key factor for assessing skin repair. Some reports suggest that human placenta–derived dECM can regulate hair follicle formation [258,259].
Fig. 5.
Functional decellularized extracellular matrix in the skin repair. (A) Histological analysis of normal skin and wounds treated with different dressings showed that the aECMHCl, 25/SC dressing promotes the healing of diabetic wounds. Adapted reprinted with permission from Ref. [255], based on CC BY License. (B) Skin substitutes and generation of chimney structures through 3D printing. (i, ii) Constructing chimney structures using a 3D printer and creating skin substitutes using 3D cell-printing technology. (iii) Validating uniform chimney model production in all experimental groups. Adapted reprinted with permission from Ref. [262], based on CC BY License. (C) Genetic manipulation allows for tissue-derived hydrogel repair of diabetic skin wounds. (i) A schematic diagram of the hydrogel preparation and example macroscopic images of the hydrogel (ii) Representative suture images of untreated, WT gel treated, or thrombospin-2 knockout gel-treated. Adapted reprinted with permission from Ref. [256]. Copyright © 2018 American Chemical Society.
CECM also offers great advantages in skin repair. An electrostatically spun fibrous membrane of l-propylene-co-caprolactone (PLCL) and human fibroblast-derived ECM (hFDM) was found to proliferate faster and exhibit a more elongated capillary-like morphology when human umbilical vein endothelial cells (HUVECs) were inoculated on hFDM-PLCL [260]. Tang et al. discovered that incorporating the extracellular matrix secreted by human adipose-derived stem cells into electrostatically spun poly nanofiber dressings improved wound healing in a surgically created total skin excision mouse model [261].
3D bioprinted dECMs have also been used for skin repair and regeneration. A skin-derived dECM bioink mimicking the microstructure and bioactivity of skin more closely than previously developed homogeneous bioinks was reported [243]. Porcine-derived dECM has been used as a bioink to produce artificial skin tissue by 3D printing. Synthetic skin was 3D printed using bioinks based on porcine skin, human dermal fibroblasts, and keratin-forming cells with dECM and evaluated for skin wound healing using a mouse wound model (Fig. 5B) [262]. The artificial skin was revealed to be a suitable skin substitute for the treatment of burns and autologous skin grafts. The application of dECM-based 3D bioprinting provides a new strategy for printing full skin layers, including structures (e.g., gland and hair follicles).
4.3. Cardiac repair
Heart disease can cause serious harm to patients [263]. dECM stents mimicking the natural heart environment hold great promise for cardiac therapy [264,265]. The dECM is used in various forms for cardiac therapy and is generally used in the form of a solid scaffold for natural vascular system structures or as a soluble material that can be formed into injectable hydrogels for tissue repair [5,266].
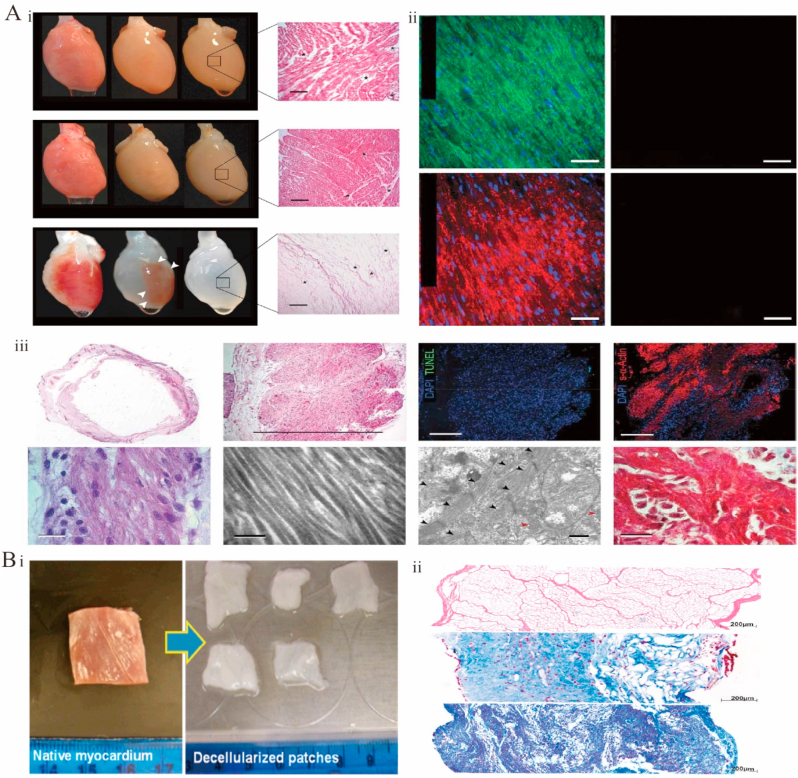
Solid dECM scaffolds are directly used after decellularization without any further microstructure disintegration. This approach preserves specific dECM components, the natural tissue structure, and the vascular system [267]. Solid stents are classified according to their application as tissue-engineered dECM patches or whole hearts [267]. Perfusion-decellularized whole-heart stents preserve the 3D structure of the original heart, including the vascular system [[268], [269], [270]], chamber geometry, and valve function, which is crucial for the subsequent synchronized heart beating [8,271,272]. In 2008, Ott et al. reported the first-time decellularization and recellularization of an entire heart [8]. Specifically, a whole rat heart was decellularized by perfusion through coronary arteries using 1% Triton X-100 and SDS (Fig. 6A–i). The heart preserved the complex ECM composition, chamber geometry, and vascular structure (Fig. 6A–ii). In the following years, the method was extended to larger cardiac organs from pigs and humans to enable the development of human-sized heart grafts [163,205,[273], [274], [275]]. For example, a porcine decellularized whole heart was obtained by retrograde coronary perfusion [276]. The first decellularized scaffold of a whole human heart was prepared using perfusion [163], which preserved the 3D structure, heart chamber geometry, vascular system, and mechanical properties. In addition, after recellularization, the myocardial genes of the heart expressed electrical binding properties. Cardiac patches were shown to be implantable [267]. In a rat model, the ventricular function was similar to baseline values after decellularization from porcine patch treatment [277]. Cardiac dECM patches were developed (Fig. 6B–i), and the physical characteristics of the heart tissue were recovered by reinoculating the cells, with the resulting tissue showing angiogenic potential and cardiac regeneration functions (Fig. 6B–ii) [278].
Fig. 6.
Functional decellularized extracellular matrix in the Cardiac repair. (A) Whole heart perfusion decellularization experiment in rats. (i) Gross view of whole heart decellularization in rats, H&E staining, and immunofluorescence images. (ii)Sections of decellularized rat hearts stained with immunofluorescence did not detect nuclei or contractile proteins. (iii) Analysis of the histology and electron micrographs of recellularized rat heart constructs. Adapted reprinted with permission from Ref. [8] (License number:5,442,460,220,568) (B) Natural myocardial and decellularized myocardial scaffolds. (i)Morphology of natural myocardium and decellularized myocardial scaffolds. (ii)H&E staining of the stent after decellularization and the H&E staining of the stent after two weeks and four weeks of recellularization. Adapted reprinted with permission from Ref. [278] (License number:5,442,470,287,789).
Soluble dECM has a broad scope of application in cardiac repair, as it is suitable for preparing 3D and 2D hydrogels in vivo or in vitro and can be directly applied to the myocardium or added to other materials or cells to make cell-containing biologically active injectable gels or cardiac patches [279]. Some limitations of soluble heart dECM include the poor retention of heart structure and mechanical properties. Many research groups have tried to solve these problems and tune dECM properties by crosslinking or adding different materials. The results indicate that the mechanical properties, gelation, and degradation of the soluble dECM in the heart can be regulated using crosslinking agents, polymers, and matrix metalloproteinase–inhibiting drugs [[280], [281], [282], [283]]. In addition, 2D coating or hydrogel models are excellent platforms for assessing the cellular response to different dECM [267]. Soluble dECM niches have been used to induce cell–matrix binding, improve infarct environments, model bioecological niches and matrix rigidity, and evaluate the way dECM regulates stem-cell phenotype and matrix binding [267]. Soluble dECM is a flexible system for the development of composite biomaterial scaffolds that can be loaded with stem cells. The development of multimaterial scaffolds, 3D printed structures, and electrospun materials may promote the further application of dECM in cardiac repair.
4.4. Nervous system
The nervous system includes the peripheral and central nervous systems, with the central nervous system composed of the spinal cord and the brain [284] working together to produce and transmit signals. Neurons themselves have relatively low regenerative capacity, especially in the central nervous system. Currently, spinal-cord injury or stroke damage cannot be repaired using surgical methods [248]. Neural dECM biomaterials have received considerable attention due to their ability to guide the growth of neurons and axons, stimulate myelin regeneration in Schwann cell axons, and promote the differentiation of stem cells into neurons [[285], [286], [287]]. dECM stents feature an intact native structure that can guide cell migration and direct axonal trajectories [288,289]. In addition, preserved ECM substances such as GAG regulate synapse formation and affect stem-cell proliferation [290,291].
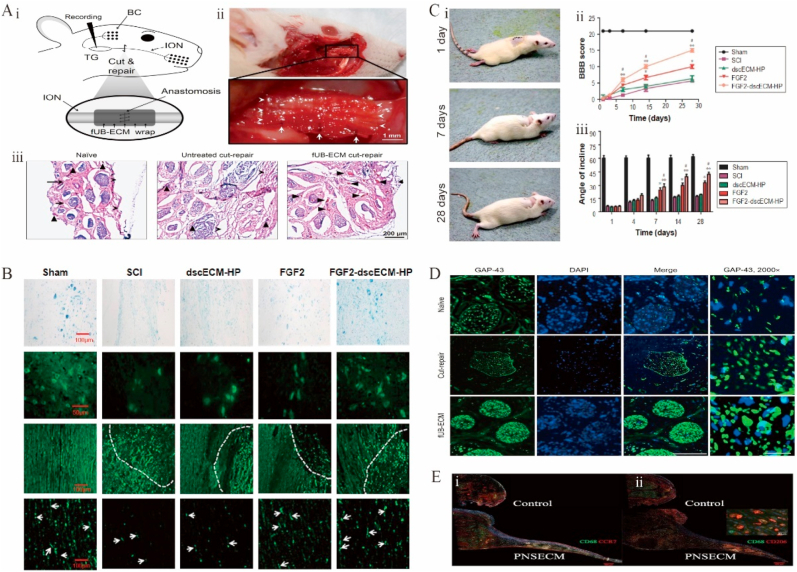
Despite the certain regeneration ability of peripheral nerves, severe injuries usually require intervention [292]. In another study, fetal porcine-bladder ECM wraps were used to repair the infraorbital nerve transection of the trigeminal nerve in rats (Fig. 7A–i). This treatment significantly healed the outer and inner nerve tissue (Fig. 7A–ii) and increased the expression of growth-associated protein-43 and neovascularization (Fig. 7A–iii), as observed 28 days after surgery (Fig. 7D) [279]. However, whisker-evoked response properties and ionic axon remyelination were observed, and the number of neurofilament-positive axons remained unchanged, which suggested that improvements in tissue remodeling do not necessarily imply axonal regeneration or the recovery of mechanoreceptor cortical signals. Compared to controls, repair sites obtained using hydrogels with a peripheral nerve–specific ECM saw a tendency for macrophages to be distributed at the edges of regenerating bridges (Fig. 7E) [293]. CECM has also made progress as a cell culture substrate in neural tissue engineering. Gu et al. cultured dorsal root ganglion neurons from Sprague-Dawley rats at embryonic day 18 in Sherwan CECM and found that the axons of the neurons grew faster and were more divergent. In a follow-up study, they went on to combine Schwann CECM with chitosan catheters and filament fibers to form a hybrid scaffold that was implanted into the sciatic nerve interstitial site in rats, again showing enhanced regeneration of the sciatic nerve [294].
Fig. 7.
Functional decellularized extracellular matrix in nervous system repair. (A) Positive modulating effect of FUB-ECM on tissue remodeling after ION dissection repair. (i) Schematic of the ION cut and repair model showing the anastomosis and FUB-ECM nerve wrap. (ii) After 28 days, fUB-ECM nerve wraps remained sutured around the ION. (iii) H&E staining showed that in fUB-ECM cut-repair IONs, the organization resembled naïve IONs.Adapted reprinted with permission from Ref. [279], based on CC BY License. (B) ScI rat locomotor function assessments. (i) Movement of scI rats' hind limbs after FgF2-dscecM-hP hydrogel treatment, (ii) The Basso, Beattie, and Bresnahan scores, (iii) After various treatments, the scI rats' inclination angle [298]. (C) After 28 days of therapy, spinal cord-injured rats had nerve fiber healing and axonal regrowth. Adapted reprinted with permission from Ref. [298], based on CC BY License. (D) Growth-associated protein-43 expression is increased by FUB-ECM nerve wrapping during ION cut and repair. Adapted reprinted with permission from Ref. [279], based on CC BY License. (E) Insert shows surface CD206 labeling and intracellular CD68 labeling. In controlled portions, no further expansion of the proximal stump was seen. Adapted reprinted with permission from Ref. [293] (License number:5,442,480,428,926) Abbreviations:ION:infraorbital nerve. FUB-ECM: fetal porcine urinary bladder extracellular matrix. SCI: Spinal cord injury. FgF2:factor-2. DscecM: spinal cord extracellular matrix. hP:heparin-poloxamer.
The ability of the central nervous system to self-heal after structural damage is not as well developed as that of the peripheral nervous system. Therefore, dECM stents for the central nervous system have received little attention [295,296]. Stents for the repair of spinal-cord injuries are currently derived from the spinal cord, sciatic nerve, paravertebral muscle, brain, optic nerve, and bladder matrices [284]. The MS analysis of dECMs derived from the sciatic nerve and spinal cord revealed that proteins associated with axonal growth and myelin regeneration, e.g., cohesin, are unique to the spinal cord, whereas some proteins such as Col IV α1 and α2 are unique to the sciatic nerve [297]. Sciatic dECM hydrogels were hypothesized to better promote axonal myelin regeneration in the spinal cord, whereas spinal dECM hydrogels were thought to better promote synapse formation. However, the rats did not show full recovery of hind paw mobility after the use of dECM hydrogels in a spinal-cord injury rat model. To investigate this issue, exogenous nerve regenerative growth factors (Fig. 7B), FGF-2, and a heparin poloxamer (Fig. 7C) were added to the spinal dECM hydrogel, and the rats regained movement to the extent of uninjured controls after treatment [298]. Nevertheless, effective methods for spinal-cord decellularization are not yet available, and existing ones rely on a combination of chemical, physical, enzymatic, and detergent techniques.
dECM-based therapies for brain injury have received little attention, as research has mainly focused on tissues from brain or urinary bladder matrices decellularized for use at injured brain sites [284]. A bioink based on a porcine brain dECM was used to develop patient-specific glioblastoma microarrays [299]. The brain dECM prevents neurological deficits caused by traumatic brain damage by reducing proinflammatory microglial cell responses, lowering glial scar formation, and improving the neurobehavioral function [300].
Although neuronal dECM biomaterials are partially successful in treating peripheral nerve injuries, no discernible effects have been observed for the treatment of central nervous system injuries [301]. Although nervous system repair has been extensively researched, there is still an urgent need to develop improved bionic scaffolds.
4.5. Respiratory organs
Although lung transplantation is currently the only treatment option for patients with advanced chronic obstructive pulmonary disease [248], its application is limited by a severe shortage of donor lungs [302]. Pulmonary tissue engineering focuses on two main methods, namely vehicle carriers for distal lung delivery and drug and whole-lung substitutes [303,304].
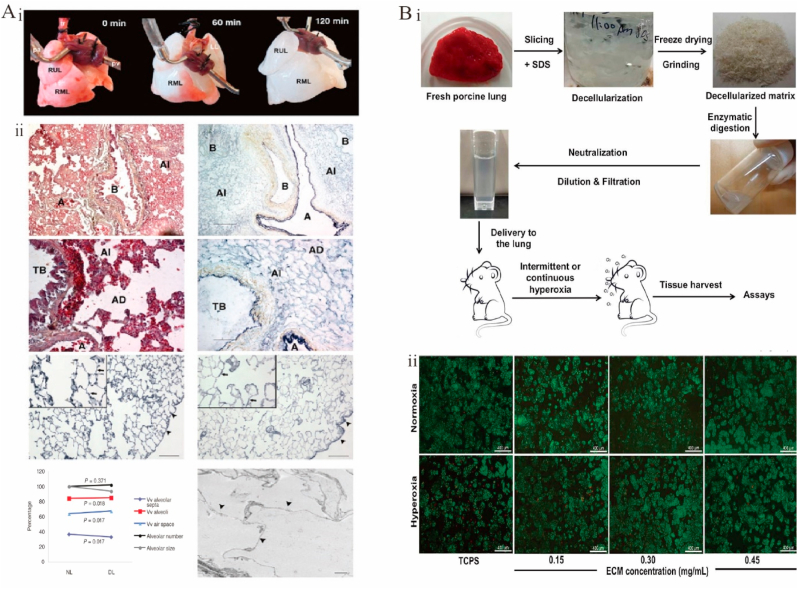
In 2010, Ott et al. used decellularization to produce a bioartificial lung (Fig. 8A–i), providing cell-free blood vessels, airways, and alveoli while preserving alveolar septa, alveolar surface area, and extracellular matrix proteins (Fig. 8A–ii) and enabling gas exchange both in vivo and in vitro [305]. In 2011, respiratory function was partially restored in rats with in situ transplanted recellularized lungs [306,307]. In 2017, transplanted artificial lungs were shown to facilitate gas exchange in a porcine model [308]. Recently, lung dECM–based 3D printing has been used to simulate lungs structure and function. For example, 3D printing was used to mimic the vascular and subsegmental bronchial structures of human lungs in an alginate-dECM lung composite [309]. The composite retained biological functions at multiple stages of tissue maturation, including the tissue-specific differentiation of primary human stem cells, regulation of in vivo immune responses, and vascularization after transplantation. The possibility of employing decellularized lungs as a natural 3D bioengineering matrix was established using a bioreactor system with a decellularized mouse lung matrix [310], and a model was provided for studying lung regrowth from stem cells and developing a rapid, controlled, and automated lung decellularization protocol [311]. Human CECM-active scaffolds with lung epithelial cells and primary fibroblasts from patients with non-chronic lung disease were used to create a unique cellular microenvironment for lung tissue engineering that demonstrated excellent bioactivity [312].
Fig. 8.
Functional decellularized extracellular matrix in respiratory organs repair. (A) Perfusion decellularization of whole rat lungs. (i) Photographs of a rat lung, mounted on a decellularization apparatus allowing antegrade pulmonary arterial perfusion. (ii) Corresponding Movat pentachrome and Verhoeff's elastic-tissue staining of thin sections from parenchyma of native (left) and decellularized (right) rat lung. Adapted reprinted with permission from Ref. [305] (License number:5,450,760,527,174). (B) Manufacture and evaluation of lung ECM solutions. (i) Lung ECM solution preparation and delivery. (ii) Live/dead staining images of Human lung epithelial cells on tissue culture polystyrene in mediums without lung ECM and with 0.15, 0.3and 0.4 mg/mL lung ECM under normoxia and hyperoxia. Adapted reprinted with permission from Ref. [316], based on CC BY License.
Another approach in lung tissue engineering is the use of drug carriers for distal lung drug delivery. Both dripping and nebulization are effective methods to deliver drugs to lungs [[313], [314], [315]]. Although coarse particles can be dripped through the tracheal tube, their distal lung distribution is inconsistent [314]. Nebulization encourages the distal lung ECM to be distributed uniformly, although the amount of ECM in the solution and the size of ECM microparticles are limited [316]. However, dripping and nebulization can reduce alveolar exudation and septal thickening. Decellularized ECM powder from bladder mucosa was demonstrated to alleviate bleomycin-induced pulmonary fibrosis when dripped into the lungs [317]. Lung-specific dECM microparticle suspensions/solutions for nebulization showed efficacy in minimizing lung oxidative injury. The nebulized lung dECM can act as both a mediator and a drug delivery vehicle of organ restoration. Ideally, the remodeled lung tissue should restore the mechanical properties of the lung (Fig. 8B) [316]. Despite significant advances in the fabrication of biomaterials for the lung dECM, this technology is still in its infancy. The functionalized lung dECM shows great promise for applications in the development of lung regrowth stimulation and lung disease models.
4.6. Digestive system
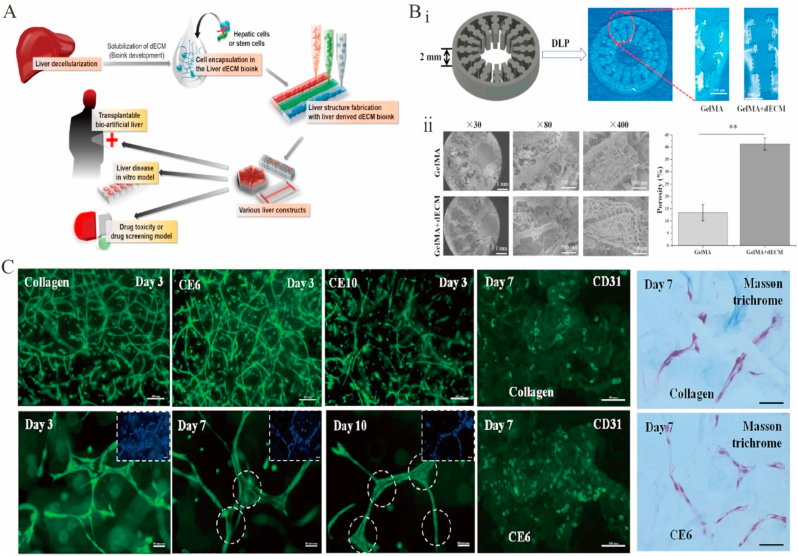
Liver diseases have attracted increasing attention. In 2010, Uygun et al. reported a new method of transplantable liver graft production using the effective recellularization of a decellularized liver matrix with human hepatocytes [318]. In 2011, a natural ECM scaffold for in vitro liver regeneration was developed by perfusing an entire liver with a natural liver vascular network detergent to delaminate cells [319]. In 2015, a human liver was decellularized and evaluated in terms of biocompatibility and quality [320]. In 2017, a liver dECM bioink inducing stem-cell differentiation and enhancing human hepatocellular carcinoma (HepG2) cell function was developed (Fig. 9A) [321]. In 2018, a hydrogel made of decellularized goat liver was shown to be effective in 2D/3D human hepatocyte and vascular endothelial cell culturing, featuring an increased potential to develop prevascularized liver structures for tissue-engineering applications (Fig. 9C) [322]. In another study on liver tissue engineering, extrusion printing was used to develop a thermally crosslinked liver dECM bioink in which HepG2 cells were evaluated [321]. However, the use of HepG2 cells in liver tissue engineering is limited by the fact that they do not fully reflect the normal function of hepatocytes. Researchers have also developed a dECM bioink as a platform for hepatocellular carcinoma progression studies using digital light processing methods [323]. Mao et al. prepared a liver-specific bioink suitable for digital light processing by combining GelMA with liver dECM, which was wrapped in hiHep cells to form a cell-loaded bioink (Fig. 9B) [324]. Research on dECM-based liver tissue engineering is rapidly developing, and a number of milestones have been achieved. Therefore, new therapies for the treatment of liver diseases in clinical settings are expected in the future.
Fig. 9.
Functional decellularized extracellular matrix in digestive system repair. (A) Diagram of liver dECM bioink synthesis and use in liver tissue engineering via 3D cell printing. Adapted reprinted with permission from Ref. [321]. Copyright © 2017 American Chemical Society. (B) Macroscopic (i) and microscopic (ii) images of digital light processing printed (methacrylated gelatin) GelMA/dECM and GelMA scaffolds. Adapted reprinted with permission from Ref. [324](License number:5,450,770,224,374). (C) Decellularized Caprine liver ECM hydrogels for vasculogenesis assay. Adapted reprinted with permission from Ref. [322] (License number:5,450,771,384,453).
4.7. Urinary system
Kidneys, which are responsible for maintaining the water balance of the body and excreting waste products, contain glomeruli for tubular devices and ultrafiltration for reabsorption [325,326]. Kidney transplantation is regarded as the most effective treatment for patients with end-stage renal disease [327]. Currently, dECM stents provide a treatment for patients with kidney disease, retaining both the structure and composition of the kidney ECM and its unique functions such as reabsorption, secretion, and filtration [[328], [329], [330]].
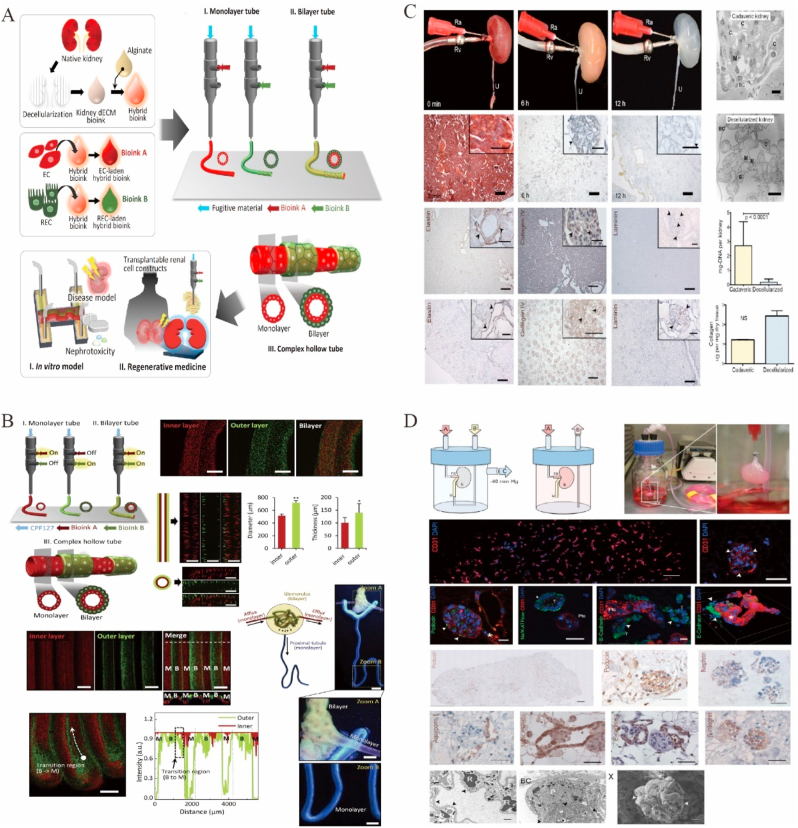
Kidneys can be decellularized to <10% residual DNA content using enzymatic digestion [331] or 1% SDS [332]. Since the first in situ bioengineered kidney transplant in rodents was reported in 2013 (Fig. 10C and D) [332], a growing number of studies have focused on maintaining kidney-specific growth factors, increasing vascular integrity, and reducing decellularization time [110,327,333]. Renal dECM with preserved glomerular, tubular, and vascular structures has been used as a platform for renal regenerative therapies, as exemplified by embryonic stem cells grown on renal dECM (Fig. 11A) [334] and generated from adipose tissue stem cells (Fig. 11B–D) [335]. Abhigyan et al. developed a new platform by decellularizing fibroblasts grown on surfaces with macromolecular crowding to mimic the natural kidney microenvironment, and human immortalized foot cells cultured on this platform showed superior viability and metabolic activity for up to 28 days [336]. To address complex renal tubular structures such as bilayer glomeruli or proximal renal tubules positioned side-by-side with blood vessels, a 3D microfluidic renal tubular tissue chip was designed for drug screening and regenerative medicine applications (Fig. 10A and B) [337]. Although a kidney-resembling structure has been reconstructed in vitro, its function has not been demonstrated in vivo [15]. Further exploration is needed before functionalized decellularized matrices can be used for clinical kidney repair.
Fig. 10.
Functional decellularized extracellular matrix in the urinary system repair. (A) Diagram of kidney dECM bioink research and functional validation [337]. (B) Monolayer and bilayer structures are printed coaxially in intricate hollow tubes. Adapted reprinted with permission from Ref. [337] (License number:5,450,780,535,429). (C) Gross views and immunohistochemical staining images of rats before and after whole kidney perfusion decellularization. Adapted reprinted with permission from Ref. [332] (License number:5,450,781,044,751). (D) Apparatus and histological images of recellularization and culture of decellularized rat kidney Adapted reprinted with permission from Ref. [332] (License number:5,450,781,044,751).
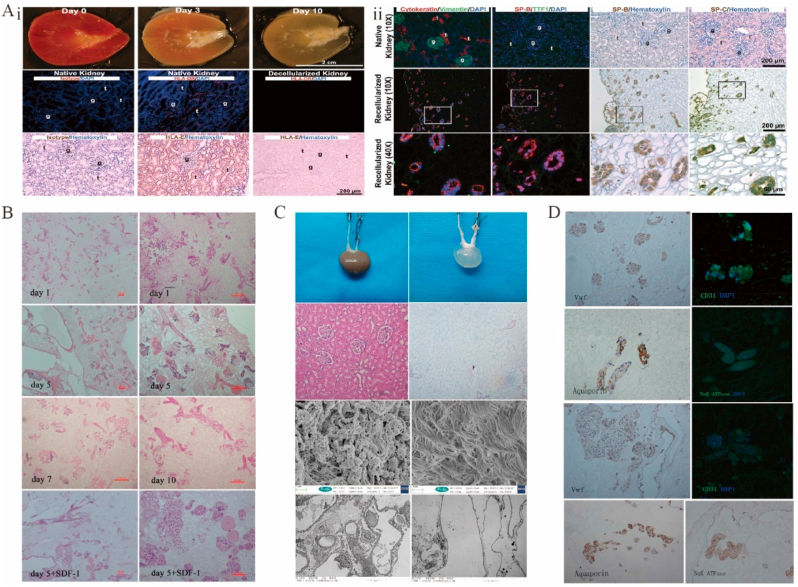
Fig. 11.
Functional decellularized extracellular matrix in the urinary system repair. (A) Decellularization and in-cellularitytion of the rhesus monkey kidney. (i) Decellularization of rhesus monkey kidney sections. (ii) Immunohistochemistry of decellularized kidney scaffolds recellularized with undifferentiated human embryonic stem cells. Adapted reprinted with permission from Ref. [334], based on CC BY License. (B) H&E staining after kidney recellularization [335]. (C) Perfusion decellularization and histology of whole rat kidneys [335]. (D) Day 5: recellularized kidney immunohistochemistry and immunofluorescence stains following SDS decellularization and whole organ culturing. Adapted reprinted with permission from Ref. [335] (License number:5,450,790,149,095).
4.8. Reproductive system
Despite the numerous advances in the treatment of female infertility, endometriosis, endometrial cancer, and serious uterine adhesions still require hysterectomy, which may lead to uterine dysfunction or infertility [338]. Infertility is still a widespread problem [339]. Uterus transplantation, which has shown great potential in infertility treatment, has become an effective option for these patients [340,341]. Induction of angiogenesis is a clinically effective strategy for the treatment of thin endometrium [342]. As a tissue scaffold, functionalized decellularized tissue may provide follicular growth support for the development of new infertility treatments [343,344], including the treatment of uterine disease–caused infertility [345]. Recent studies have confirmed that biomaterials with tissue-specific ECM characteristics can promote uterine regeneration and support pregnancy in severely damaged uterine models [346]. Functionalized decellularized tissue has also been used to repair endometrial loss, and the current dECM selection for repairing endometrial damage is focused on three tissue sources, namely endometrial tissue–derived dECM (most specific) (Fig. 12A) [347], amniotic membrane–derived dECM from fetal placenta (most widely used) [348], and urinary tissue–derived dECM (structurally similar to uterine endometrial tissue–derived dECM) (Fig. 12B) [349]. Although urinary tissue–derived dECM has the lowest DNA content and maintains the bioactive components to a considerable extent, the tissue obtained after decellularization does not maintain the tubular structure and is less compact. Both in vitro and in vivo, urinary tissue-derived dECM showed a significantly low tensile strength and modulus of elasticity while featuring a rapid degradation rate, which led to the failure of uterine regeneration due to insufficient support strength (Fig. 12C and D) [350]. After a month, the dECM uterus had completely broken down, but the place where the uterus had been cut had not healed. Poor mechanical properties result in the deformation of the transplanted uterus and prevent the maintenance of macroscopic (especially fine tissue) structures, while rapid stent degradation prevents tissue regeneration and reconstruction as well as endometrial cell growth and extension. The mechanical properties of urinary tissue–derived dECM can be significantly enhanced by crosslinking natural genistein and proanthocyanidins, which also allows one to decrease the rate of enzymatic degradation and achieve significant cell permeability, low immunoreactivity, and excellent biocompatibility [350]. In rats with an annulated uterus, the uterus was completely regenerated after 90 days, whereas a decellularized rabbit uterine matrix was mostly degraded. However, the most suitable concentration for crosslinking has further drawbacks.
Fig. 12.
Functional decellularized extracellular matrix in reproductive system repair. (A) Rabbit uterine decellularization. (i) Rabbit uterus before decellularization, and its tissue H&E, Masson's trichrome, and DAPI stain. (ii) After decellularization of the rabbit uterus and its tissue H&E, Masson's trichrome, and DAPI stain, it can be seen that the cells have been largely removed. Adapted reprinted with permission from Ref. [347], based on CC BY NC ND License. (B) UBM repairs the uterine horn in rats. (i) Appearance and structure of UBM. (ii) Appearance of the uterine horns in the experimental groups; (iii) Complete degradation of UBM material in the experimental groups after 4 weeks. Adapted reprinted with permission from Ref. [349], based on CC BY License. (C) Non-cross-linked and cross-linked dUECM subcutaneous embedding experiments were performed 14 days later for (i) histological observation (ii) and HE staining. Adapted reprinted with permission from Ref. [350], based on CC BY License.(D) Morphological observation of cross-linked dECM in the reconstructed uterus of rats in vivo. Adapted reprinted with permission from Ref. [350], based on CC BY License. Abbreviations: UBM: Urinary bladder matrix. dUECM:decellularized rabbit uterus matrix.
4.9. Functional dECM in other systems
4.9.1. Muscle
According to the World Health Organization, 1.71 billion people worldwide were affected by skeletal muscle disease in 2021. Regenerative medicine offers a new way of thinking about this class of diseases. The culturing and differentiation of C2C12 myogenic cells on dECM scaffolds resulted in the formation of myofibers with large diameters, high fusion indices, and great metabolic capacity (Fig. 13D) [351]. Compared to native skeletal muscle, decellularized rabbit skeletal muscle retained approximately 50% of collagen, 74% of elastin, and 83% of GAG (Fig. 13C) [352]. The morphology of the dECM scaffold usually affects the alignment of skeletal muscle cells. Myogenic cells from C2C12 mice were inoculated on directed and nondirected dECM scaffolds, and myogenic cell arrangement, myotube formation, and myogenic gene expression were found to be increased in the former case [353]. A polyvinyl alcohol–dECM bioink produced by photocrosslinking was used to 3D print skeletal muscle structures. Well-aligned printed skeletal muscle structures significantly expressed MHC+, reduced muscle fibers, and increased myofiber formation, producing greater tonicity (Fig. 13A) [354]. These studies demonstrate the regulatory role of the topographical arrangement of the dECM in directing myogenesis, which allows for targeted cell growth and enhanced muscle production in vitro and muscle regeneration in vivo.
Fig. 13.
Functional decellularized extracellular matrix in Muscle repair. (A) MHC-stained images of TA muscle taken at 4- and 8-weeks post-implantation (red), demonstrating new muscle fiber formation. Dual immunofluorescence imaging of MRPL12 (green) and MHC (red), indicating that hMPC is differentiated. Adapted reprinted with permission from Ref. [354] (License number: 5,450,791,471,088). (B) Confocal fluorescence images of muscle structures at 500, 1500, and 5000 m line width on days 1 and 7; green indicates F-actin (oncoprotein) and blue indicates the nucleus (Dpi). Adapted reprinted with permission from Ref. [357](License number: 5,450,800,377,648). (C) After seven days, collagen, dECM, and IGF-1/dECM scaffolds were stained for MHC (blue = nuclei; green = MHC). IGF-1/dECM had higher MHC expression than other groups, indicating more myotubes. Adapted reprinted with permission from Ref. [352] (License number: 5,450,800,774,816). (D) Rat abdominal wall repair: gross and morphological observations Left: surgical repair using perfusion skeletal muscle ECM (pM-ECM); right: control. In pM-ECM-healed defects, samples without seroma had neo-muscle fibres along the margin and in the middle. Adapted reprinted with permission from Ref. [351] (License number: 5,450,801,213,501)..
The morphology of skeletal muscle–derived dECM can be regulated at the micron or nanometer level to guide cell behavior and muscle differentiation. However, the morphology and scale that are most effective in achieving optimal cellular responses remain unclear [355]. Microscale cell-loaded dECM muscle structures were 3D printed with linewidths of 300–1000 μm [356] to afford a structure supporting the formation of densely arranged myotubes and featuring a high cell survival rate. Such muscle structures were implanted into defects in rat tibial anterior volumetric muscle loss injuries and achieved the regeneration of new muscle and minimal fibrosis compared with unpatterned controls. C2C12-loaded scaffolds with 500–5000-μm fibers were fabricated by 3D bioprinting, with cells cultured in the 500-μm patterned scaffolds showing the greatest orientation extent, elongation, and mechanical strength (Fig. 13B) [357].. In summary, the abovementioned studies highlight the importance of porosity in the fabrication of skeletal muscle–derived dECM. Excessive porosity leads to reduced structural integrity, while inadequate porosity increases the risks of hypoxia and cell death [358]. In addition, mechanical properties are a key factor to consider in skeletal muscle tissue engineering [359]. To mimic muscle structure, researchers used a dECM bioink to 3D print skeletal muscle scaffolds that exhibited a stiffness of 12 ± 3 kPa in vitro and supported myogenic differentiation, myogenic cell proliferation, and myotube formation [357]. Porous/fibrous scaffolds were fabricated by hot stretching and had a stiffness (12.4 ± 3.5 kPa) close to that of natural muscle (11.5 ± 1.3 kPa) [360]. Despite the numerous advances in the 3D printing of skeletal muscle–derived dECM, the commercialization of dECM using rigorous bioprinting techniques has not yet been achieved [360]. Currently, substantial research and development are required before such methods become standard clinical practice.
4.9.2. Stroma in cornea
The cornea is the most important refractive element in enabling vision, with cornea damage or infection often resulting in stroma scarring and thinning, which may impair vision and even cause blindness [361]. In such cases, the most common treatment is to partly or fully replace the cornea with human donor tissues [362]. However, as with all transplants, the donor sources are highly restricted, with less than 5% of patients undergoing corneal transplantation [363]. Consequently, the replacement of diseased corneas with synthetic ones has been actively studied as an alternative to conventional corneal tissue transplantation [[364], [365], [366]]. Decellularized cryomilled corneal powder has been used for corneal repair and regeneration [367,368]. Human cornea–derived dECM particles dispersed in a human fibrin sealant were used for anterior corneal stromal reconstruction [369]. The results showed that the incorporation of corneal tissue–derived ECM particles into fibrin hydrogels does not pose toxicity or safety risks and shows great promise as a minimally invasive treatment for superficial corneal epithelial trauma and anterior stromal injury. Decellularized bovine and porcine corneas have been shown to be biocompatible and maintain the optical transparency, mechanical properties, and structural integrity of the original cornea [370,371]. Decellularized porcine corneas have been clinically used as tissue-engineered scaffolds for allogeneic corneal transplants (Fig. 14D) [372]. The derived hydrogels allowed rapid corneal epithelialization [373], although the achieved transparency and mechanical strength were low. Crosslinking is an effective way to increase mechanical strength, e.g., the N-cyclohexyl-N’-(2-morpholinoethyl) carbodiimide metho-p-toluene sulfonate/N-hydroxysuccinimide (CMC/NHS) crosslinker increases the toughness of decellularized porcine cornea–derived hydrogels without affecting their biological activity [374,375]. In addition, a high secretion of collagenase and metalloproteinases was observed during stromal repair [376], which may prevent the long-term retention of decellularized corneal hydrogels on corneal defects. A hybrid hydrogel composed of hyaluronic acid methacrylate (HAMA) and porcine decellularized corneal stromal matrix (pDCSM) was produced by crosslinking and used to directly fill corneal defects of various shapes, showing excellent adhesion properties and cell penetration performance owing to its microporous 3D network structure [377]. This system also exhibited low swelling, slow degradation, enhanced physical characteristics, and cornea-matched transparency, and was capable of withstanding ultrahigh intraocular pressure. Histological analysis showed that the corneal stroma filled with the pDSCM pregel solution and the 2% (w/v) HAMA pregel solution mixed in a 3:1 vol ratio underwent ordered alignment (Fig. 14A and B) [377]. The corneal model was used with CMC/NHS crosslinked with pDCSM-G, which promoted epithelial recovery and stromal regeneration (Fig. 14C) [378]. Although cornea-derived dECM hydrogels have been used for corneal repair, the design of cells containing functional corneal structures and maintaining transparency remains difficult [21].
Fig. 14.
Functional decellularized extracellular matrix in the cornea. (A) Postoperative observation of the hydrogel treated corneas (i) Typical images of fluorescein and cobalt blue stains in rabbit experiment eyes. (ii) Slit lamp and anterior segment optical coherence to-mography images. (iii) Confocal micrographs [377]. (B) Eight weeks after surgery, histological examination of the hydrogel-treated rabbit corneas. (i) H&E staining results. (ii) Images of immunofluorescence stained using biomarkers α-SMA (green) and DAPI (blue). Adapted reprinted with permission from Ref. [377], based on CC BY License. (C) Application of CMC/NHS cross-linked decellularized porcine corneal hydrogels. (i) Postoperative observation and (ii) histological analysis of CMC/NHS cross-linked decellularized porcine cornea hydrogels. Adapted reprinted with permission from Ref. [378](License number: 5,450,810,577,129). (D) Comparison of corneas before and after surgery. (i) In vivo confocal microscopic and ultrasound biomicroscopic images of patients. (ii) Corneal slit lamp micrograph before and after surgery. Adapted reprinted with permission from Ref. [372](License number:5,450,810,989,732). Abbreviations: CMC/NHS:N-cyclohexyl-N′-(2-morpholinethyl) carbodiimide metho-p-toluene sulfonate/N-hydroxy succinimide. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
5. Conclusion and outlook
With the advent of numerous decellularization techniques, dECM has evolved from simple tissue scaffolds to whole-organ scaffolds, while the recent emergence of 3D printing technologies exploiting dECM-based bioinks enables the fabrication of more complex whole-organ scaffolds. In this review, we described the composition and role of the ECM in stem-cell differentiation and summarized the advantages and disadvantages of existing decellularization techniques and methods for the refunctionalization of decellularized scaffolds. The applications, development progress, and challenges faced by functionalized dECM scaffolds in different tissues and organs were discussed, as exemplified by the repair of cartilage, skin, nerve, and muscle injuries and the transplantation or regeneration of whole organs such as liver, heart, uterus, kidneys, and lungs. Some of these materials have been successfully used in both animal models and clinical applications, as exemplified by human dermal dECM–based products for treating ligament and tendon injuries, namely Allopatch HD™ (MTF sports drug) and GraftJacket® (Wright Medical), and the cell-free pericardium-based heart valve product CardioCel® (Admetus IHS Inc.) (Table 3).
Table 3.
Commercialized products of decellularized extracellular matrix.
| Commercial options | Materials | Applications |
|---|---|---|
| Allderm® | Human allogeneic dermal decellularized matrix | Repair of skin wounds |
| OASIS® | Decellularized stroma of the porcine small intestine submucosa (SIS) | Repair of skin wounds |
| Prima™ Plus | Decellularized heart valves of the porcine | Repair of heart valves |
| CardioCel® | Decellularized heart valves of cattle | Repair of heart valves |
| GraftJacket® | Human allogeneic dermal decellularized matrix | Repair of tendon and ligament injuries |
| Allopatch HD™ | Human allogeneic dermal decellularized matrix | Repair of tendon and ligament injuries |
| AxoGuard® | Decellularized stroma of the porcine small intestine submucosa | Used as a nerve connector |
| Human Acellular Vessel | Decellularized matrix of human allogeneic blood vessels | Repair of blood vessels(Phase 3 clinical trial) |
Although the dECM technology has achieved long-term advances in the field of tissue engineering, several persistent challenges remain, e.g., (1) the need for (i) standardized decellularization protocols and characterization methods to better preserve the biological and mechanical properties of the ECM and (ii) more sensitive testing of residual cellular components and toxic products. (2) Moreover, better sterilization methods, especially those for virus eradication, should be developed while minimizing their impact on the mechanical and biological characteristics of tissue materials. (3) The optimization of the dECM functionalization protocol should be continued. The crosslinking of loading factors, bioactive molecules, and/or mechanical modifications contribute to tissue repair and regeneration. Recellularization processes must consider clinically relevant sources of regenerable cells, inoculation methods (at this stage, the most common delivery techniques are cell infusion, cell injection, or simple surface application [5]), and bioreactors with physiologically relevant organ culture conditions. (4) The diversity and complexity of tissues and organs need to be considered, as different tissues and organs have different structures, which complicates the production of optimal bionic materials derived from the dECM. dECM-derived bioinks offer a viable approach to 3D printing, although further in-depth studies are required. (5) The optimization of hematologic reconstitution and thrombosis and inflammatory response reduction are essential for successful tissue and organ regeneration. Currently, thrombosis remains a challenge when whole-heart dECMs are used in vivo [5]. Moreover, DNA with length of <200 bp does not cause significant inflammatory responses [379], which should be taken into account in future studies. (6) Although the ideal decellularization protocol removes all immunogenicity from porcine tissues, the risk of using animal sources in humans still exists [21]. The assessment of immunogenicity and biocompatibility is critical for final health-authority approval, commercial viability, and clinical application.
Despite the numerous challenges associated with the clinical use of refunctionalized dECMs for the repair of various organs and tissues in the human body, these materials are considered to be low immunogenic scaffolds for tissue/organ repair providing an environment for stem-cell differentiation and growth and featuring properties that can be optimized by crosslinking various natural tissue-engineering materials. However, these materials should be further evaluated in vivo animal experiments while considering the differences between animal models and humans. In conclusion, dECM refunctionalization is a promising solution for regenerative medicine and tissue engineering.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Natural Science Foundation of Hunan Province (No. 2020JJ4791), Changsha Science and Technology Project (No. kq1901121).
Contributor Information
Lanjie Lei, Email: leilanjie1988@163.com.
Shisheng Li, Email: lissdoctor@csu.edu.cn.
Data availability
No data was used for the research described in the article.
References
- 1.Carvalho M.S., Poundarik A.A., Cabral J.M.S., da Silva C.L., Vashishth D. Biomimetic matrices for rapidly forming mineralized bone tissue based on stem cell-mediated osteogenesis. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-32794-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu P., Chen H., Jin R., Weng T., Ho J.K., You C., Zhang L., Wang X., Han C. Non-viral gene delivery systems for tissue repair and regeneration. J. Transl. Med. 2018;16:29. doi: 10.1186/s12967-018-1402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D'Eredità R. Porcine small intestinal submucosa (SIS) myringoplasty in children: a randomized controlled study. Int. J. Pediatr. Otorhinolaryngol. 2015;79:1085–1089. doi: 10.1016/j.ijporl.2015.04.037. [DOI] [PubMed] [Google Scholar]
- 4.Ivanov A.A., Kuznetsova A.V., Popova O.P., Danilova T.I., Yanushevich O.O. Modern approaches to acellular therapy in bone and dental regeneration. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms222413454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor D.A., Sampaio L.C., Ferdous Z., Gobin A.S., Taite L.J. Decellularized matrices in regenerative medicine. Acta Biomater. 2018;74:74–89. doi: 10.1016/j.actbio.2018.04.044. [DOI] [PubMed] [Google Scholar]
- 6.Poel W.E. Preparation of acellular homogenates from muscle samples. Science. 1948;108:390–391. doi: 10.1126/science.108.2806.390-a. [DOI] [PubMed] [Google Scholar]
- 7.Badylak S.F., Tullius R., Kokini K., Shelbourne K.D., Klootwyk T., Voytik S.L., Kraine M.R., Simmons C. The use of xenogeneic small intestinal submucosa as a biomaterial for Achilles tendon repair in a dog model. J. Biomed. Mater. Res. 1995;29:977–985. doi: 10.1002/jbm.820290809. [DOI] [PubMed] [Google Scholar]
- 8.Ott H.C., Matthiesen T.S., Goh S.K., Black L.D., Kren S.M., Netoff T.I., Taylor D.A. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat. Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 9.Macchiarini P., Jungebluth P., Go T., Asnaghi M.A., Rees L.E., Cogan T.A., Dodson A., Martorell J., Bellini S., Parnigotto P.P., Dickinson S.C., Hollander A.P., Mantero S., Conconi M.T., Birchall M.A. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–2030. doi: 10.1016/s0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 10.Basonbul R.A., Cohen Use of porcine small intestinal submucosa for pediatric endoscopic tym panic membrane repair. World.J.Otorhinolaryngol.Head.Neck.Surg. 2017;3:142–147. doi: 10.1016/j.wjorl.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crapo P.M., Gilbert T.W., Badylak S.F. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bourgine P.E., Pippenger B.E., Todorov A., Jr., Tchang L., Martin I. Tissue decellularization by activation of programmed cell death. Biomaterials. 2013;34:6099–6108. doi: 10.1016/j.biomaterials.2013.04.058. [DOI] [PubMed] [Google Scholar]
- 13.Benders K.E., van Weeren P.R., Badylak S.F., Saris D.B., Dhert W.J., Malda J. Extracellular matrix scaffolds for cartilage and bone regeneration. Trends Biotechnol. 2013;31:169–176. doi: 10.1016/j.tibtech.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Parmaksiz M., Dogan A., Odabas S., Elçin A.E., Elçin Y.M. Clinical applications of decellularized extracellular matrices for tissue engineering and regenerative medicine. Biomed. Mater. 2016;11 doi: 10.1088/1748-6041/11/2/022003. [DOI] [PubMed] [Google Scholar]
- 15.Mandrycky C., Phong K., Zheng Y. Tissue engineering toward organ-specific regeneration and disease modeling. MRS.Commun. 2017;7:332–347. doi: 10.1557/mrc.2017.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X., Chen X., Hong H., Hu R., Liu J., Liu C. Decellularized extracellular matrix scaffolds: Recent trends and emerg ing strategies in tissue engineering. Bioact. Mater. 2022;10:15–31. doi: 10.1016/j.bioactmat.2021.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Retraction-Engineered whole organs and complex tissues. Lancet. 2018;392:11. doi: 10.1016/s0140-6736(18)31560-5. [DOI] [PubMed] [Google Scholar]
- 18.Williams C., Black L.D. Biomaterials for Cardiac Regeneration. 2015. The role of extracellular matrix in cardiac development; pp. 1–35. [Google Scholar]
- 19.Murphy S.V., Atala A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014;32:773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 20.Mouw J.K., Ou G., Weaver V.M. Extracellular matrix assembly: a multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 2014;15:771–785. doi: 10.1038/nrm3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim B.S., Das S., Jang J., Cho D.W. Decellularized extracellular matrix-based bioinks for engineering tissue- and organ-specific microenvironments. Chem. Rev. 2020;120:10608–10661. doi: 10.1021/acs.chemrev.9b00808. [DOI] [PubMed] [Google Scholar]
- 22.Choudhury D., Tun H.W., Wang T., Naing M.W. Organ-derived decellularized extracellular matrix: a game changer for bioink manufacturing? Trends Biotechnol. 2018;36:787–805. doi: 10.1016/j.tibtech.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Brown B.N., Badylak S.F. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl. Res. 2014;163:268–285. doi: 10.1016/j.trsl.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jhala D., Vasita R. A review on extracellular matrix mimicking strategies for an artificial stem cell niche. Polym. Rev. 2015;55:561–595. doi: 10.1080/15583724.2015.1040552. [DOI] [Google Scholar]
- 25.Gattazzo F., Urciuolo A., Bonaldo P. Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim. Biophys. Acta. 2014;1840:2506–2519. doi: 10.1016/j.bbagen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones D.L., Wagers A.J. No place like home: anatomy and function of the stem cell niche. Nat. Rev. Mol. Cell Biol. 2008;9:11–21. doi: 10.1038/nrm2319. [DOI] [PubMed] [Google Scholar]
- 27.Thorne J.T., Segal T.R., Chang S., Jorge S., Segars J.H., Leppert P.C. Dynamic reciprocity between cells and their microenvironment in reproduction. Biol. Reprod. 2015;92:25. doi: 10.1095/biolreprod.114.121368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaul H., Ventikos Y. Dynamic reciprocity revisited. J. Theor. Biol. 2015;370:205–208. doi: 10.1016/j.jtbi.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Marinkovic M., Block T.J., Rakian R., Li Q., Wang E., Reilly M.A., Dean D.D., Chen X.D. One size does not fit all: developing a cell-specific niche for in vitro study of cell behavior, Matrix. Biol. 2016;52–54:426–441. doi: 10.1016/j.matbio.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park J.S., Chu J.S., Tsou A.D., Diop R., Tang Z., Wang A., Li S. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-β. Biomaterials. 2011;32:3921–3930. doi: 10.1016/j.biomaterials.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vertelov G., Gutierrez E., Lee S.A., Ronan E., Groisman A., Tkachenko E. Rigidity of silicone substrates controls cell spreading and stem cell differentiation. Sci. Rep. 2016;6 doi: 10.1038/srep33411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lv H., Li L., Sun M., Zhang Y., Chen L., Rong Y., Li Y. Mechanism of regulation of stem cell differentiation by matrix stiffness. Stem Cell Res. Ther. 2015;6:103. doi: 10.1186/s13287-015-0083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y.K., Chen C.S. Cell adhesion and mechanical stimulation in the regulation of mesenchymal stem cell differentiation. J. Cell Mol. Med. 2013;17:823–832. doi: 10.1111/jcmm.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Legate K.R., Wickström S.A., Fässler R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 2009;23:397–418. doi: 10.1101/gad.1758709. [DOI] [PubMed] [Google Scholar]
- 35.Li Y., Gautam A., Yang J., Qiu L., Melkoumian Z., Weber J., Telukuntla L., Srivastava R., Whiteley E.M., Brandenberger R. Differentiation of oligodendrocyte progenitor cells from human embryonic stem cells on vitronectin-derived synthetic peptide acrylate surface. Stem Cell. Dev. 2013;22:1497–1505. doi: 10.1089/scd.2012.0508. [DOI] [PubMed] [Google Scholar]
- 36.Brafman D.A., Phung C., Kumar N., Willert K. Regulation of endodermal differentiation of human embryonic stem cells through integrin-ECM interactions. Cell Death Differ. 2013;20:369–381. doi: 10.1038/cdd.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo H., Deng N., Dou L., Ding H., Criswell T., Atala A., Furdui C.M., Zhang Y. 3-D human renal tubular organoids generated from urine-derived stem cells for nephrotoxicity screening. ACS Biomater. Sci. Eng. 2020;6:6701–6709. doi: 10.1021/acsbiomaterials.0c01468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morrison S.J., Spradling A.C. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cells. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fuchs E., Tumbar T., Guasch G. Socializing with the neighbors: stem cells and their niche. Cells. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 40.Giamblanco N., Martines E., Marletta G. Laminin adsorption on nanostructures: switching the molecular orientation by local curvature changes. Langmuir. 2013;29:8335–8342. doi: 10.1021/la304644z. [DOI] [PubMed] [Google Scholar]
- 41.González-García C., Sousa S.R., Moratal D., Rico P., Salmerón-Sánchez M. Effect of nanoscale topography on fibronectin adsorption, focal adhesion size and matrix organisation. Colloids Surf. B.Biointerfaces. 2010;77:181–190. doi: 10.1016/j.colsurfb.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 42.Engler A.J., Sen S., Sweeney H.L., Discher D.E. Matrix elasticity directs stem cell lineage specification. Cells. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 43.Gilbert P.M., Havenstrite K.L., Magnusson K.E., Sacco A., Leonardi N.A., Kraft P., Nguyen N.K., Thrun S., Lutolf M.P., Blau H.M. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirata M., Yamaoka T. Effect of stem cell niche elasticity/ECM protein on the self-beating cardiomyocyte differentiation of induced pluripotent stem (iPS) cells at different stages. Acta Biomater. 2018;65:44–52. doi: 10.1016/j.actbio.2017.10.032. [DOI] [PubMed] [Google Scholar]
- 45.Muncie J.M., Ayad N.M.E., Lakins J.N., Xue X., Fu J., Weaver V.M. Mechanical tension promotes formation of gastrulation-like nodes and patterns mesoderm specification in human embryonic stem cells. Dev. Cell. 2020;55:679–694. doi: 10.1016/j.devcel.2020.10.015. e611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.viLiu C., Pei M., Li Q., Zhang Y. Decellularized extracellular matrix mediates tissue construction and regeneration. Front. Med. 2022;16:56–82. doi: 10.1007/s11684-021-0900-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hinderer S., Layland S.L., Schenke-Layland K. ECM and ECM-like materials - biomaterials for applications in regenerative medicine and cancer therapy. Adv. Drug Deliv. Rev. 2016;97:260–269. doi: 10.1016/j.addr.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 48.Traverse J.H. Using biomaterials to improve the efficacy of cell therapy following acute myocardial infarction. J.Cardiovasc.Transl.Res. 2012;5:67–72. doi: 10.1007/s12265-011-9330-y. [DOI] [PubMed] [Google Scholar]
- 49.Arenas-Herrera J.E., Ko I.K., Atala, Yoo J.J. Decellularization for whole organ bioengineering. Biomed. Mater. 2013;8 doi: 10.1088/1748-6041/8/1/014106. [DOI] [PubMed] [Google Scholar]
- 50.Dutta R.C., Dutta A.K. Comprehension of ECM-cell dynamics: a prerequisite for tissue regeneration. Biotechnol. Adv. 2010;28:764–769. doi: 10.1016/j.biotechadv.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 51.Hoshiba T. Decellularized extracellular matrix for cancer research. Materials. 2019;12 doi: 10.3390/ma12081311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dhandayuthapani B., Yoshida Y., Maekawa T., Kumar D.S. Polymeric scaffolds in tissue engineering application: a review. Int.J.Polym.Sci. 2011;2011:1–19. doi: 10.1155/2011/290602. [DOI] [Google Scholar]
- 53.Saldin L.T., Cramer M.C., Velankar S.S., White L.J., Badylak S.F. Extracellular matrix hydrogels from decellularized tissues: structure and function. Acta Biomater. 2017;49:1–15. doi: 10.1016/j.actbio.2016.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He N., Zhang L., Cui J., Li Z. Bone marrow vascular niche: home for hematopoietic stem cells. Bone Marrow Res. 2014 doi: 10.1155/2014/128436. (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu H., Hoshiba T., Kawazoe N., Chen G. Comparison of decellularization techniques for preparation of extracellular matrix scaffolds derived from three-dimensional cell culture. J. Biomed. Mater. Res. 2012;100:2507–2516. doi: 10.1002/jbm.a.34150. [DOI] [PubMed] [Google Scholar]
- 56.Ingrassia D., Sladkova M., Palmer M., Xia W., Engqvist H., de Peppo G.M. Stem cell-mediated functionalization of titanium implants. J. Mater. Sci. Mater. Med. 2017;28:133. doi: 10.1007/s10856-017-5944-1. [DOI] [PubMed] [Google Scholar]
- 57.Lu H., Hoshiba T., Kawazoe N., Koda I., Song M., Chen G. Cultured cell-derived extracellular matrix scaffolds for tissue engineering. Biomaterials. 2011;32:9658–9666. doi: 10.1016/j.biomaterials.2011.08.091. [DOI] [PubMed] [Google Scholar]
- 58.Narayanan K., Leck K.J., Gao S., Wan A.C. Three-dimensional reconstituted extracellular matrix scaffolds for tissue engineering. Biomaterials. 2009;30:4309–4317. doi: 10.1016/j.biomaterials.2009.04.049. [DOI] [PubMed] [Google Scholar]
- 59.Volpato F.Z., Führmann T., Migliaresi C., Hutmacher D.W., Dalton P.D. Using extracellular matrix for regenerative medicine in the spinal cord. Biomaterials. 2013;34:4945–4955. doi: 10.1016/j.biomaterials.2013.03.057. [DOI] [PubMed] [Google Scholar]
- 60.Michel G., Tonon T., Scornet D., Cock J.M., Kloareg B. The cell wall polysaccharide metabolism of the brown alga Ectocarpus siliculosus. Insights into the evolution of extracellular matrix polysaccharides in Eukaryotes. New Phytol. 2010;188:82–97. doi: 10.1111/j.1469-8137.2010.03374.x. [DOI] [PubMed] [Google Scholar]
- 61.Sun Y., Yan L., Chen S., Pei M. Functionality of decellularized matrix in cartilage regeneration: a comparison of tissue versus cell sources. Acta Biomater. 2018;74:56–73. doi: 10.1016/j.actbio.2018.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Skóra J., Pupka A., Dorobisz A., Barć P., Korta K., Dawiskiba T. Evaluation of the humoral and cellular immune responses after implantation of a PTFE vascular prosthesis. Postepy Hig. Med. Dosw. 2012;66:469–474. doi: 10.5604/17322693.1002205. [DOI] [PubMed] [Google Scholar]
- 63.Liao J., Guo X., Grande-Allen K.J., Kasper F.K., Mikos A.G. Bioactive polymer/extracellular matrix scaffolds fabricated with a flow perfusion bioreactor for cartilage tissue engineering. Biomaterials. 2010;31:8911–8920. doi: 10.1016/j.biomaterials.2010.07.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheng N.C., Estes B.T., Awad H.A., Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells by a porous scaffold derived from native articular cartilage extracellular matrix. Tissue Eng. 2009;15:231–241. doi: 10.1089/ten.tea.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Assunção M., Dehghan-Baniani D., Yiu C.H.K., Später T., Beyer S., Blocki A. Cell-derived extracellular matrix for tissue engineering and regenerative medicine. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.602009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pei M., Li J.T., Shoukry M., Zhang Y. A review of decellularized stem cell matrix: a novel cell expansion system for cartilage tissue engineering. Eur. Cell. Mater. 2011;22:333–343. doi: 10.22203/ecm.v022a25. ; discussion 343. [DOI] [PubMed] [Google Scholar]
- 67.Pei M. Environmental preconditioning rejuvenates adult stem cells’ proliferation and chondrogenic potential. Biomaterials. 2017;117:10–23. doi: 10.1016/j.biomaterials.2016.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.He F., Liu X., Xiong K., Chen S., Zhou L., Cui W., Pan G., Luo Z.P., Pei M., Gong Y. Extracellular matrix modulates the biological effects of melatonin in mesenchymal stem cells. J. Endocrinol. 2014;223:167–180. doi: 10.1530/joe-14-0430. [DOI] [PubMed] [Google Scholar]
- 69.Pei M., Li J., Zhang Y., Liu G., Wei L., Zhang Y. Expansion on a matrix deposited by nonchondrogenic urine stem cells strengthens the chondrogenic capacity of repeated-passage bone marrow stromal cells. Cell Tissue Res. 2014;356:391–403. doi: 10.1007/s00441-014-1801-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoshiba T., Sugano Y., Yokoyama N. Murine neural stem cell (NSC) line, MEB5-derived decellularized matrix as an in vitro extracellular matrix model in NSC niche. Chem. Lett. 2018;47:1498–1501. doi: 10.1246/cl.180788. [DOI] [Google Scholar]
- 71.Xiong X., Yang X., Dai H., Feng G., Zhang Y., Zhou J., Zhou W. Extracellular matrix derived from human urine-derived stem cells enhances the expansion, adhesion, spreading, and differentiation of human periodontal ligament stem cells. Stem Cell Res. Ther. 2019;10:396. doi: 10.1186/s13287-019-1483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pei M., He F. Extracellular matrix deposited by synovium-derived stem cells delays replicative senescent chondrocyte dedifferentiation and enhances redifferentiation. J. Cell. Physiol. 2012;227:2163–2174. doi: 10.1002/jcp.22950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yan J., Chen X., Pu C., Zhao Y., Liu X., Liu T., Pan G., Lin J., Pei M., Yang H., He F. Synovium stem cell-derived matrix enhances anti-inflammatory properties of rabbit articular chondrocytes via the SIRT1 pathway. Mater.Sci.Eng.C.Mater.Biol.Appl. 2020;106 doi: 10.1016/j.msec.2019.110286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.He F., Pei M. Rejuvenation of nucleus pulposus cells using extracellular matrix deposited by synovium-derived stem cells. Spine (Phila.Pa.1976) 2012;37:459–469. doi: 10.1097/BRS.0b013e31821fcc64. [DOI] [PubMed] [Google Scholar]
- 75.Pei M., Shoukry M., Li J., Daffner S.D., France J.C., Emery S.E. Modulation of in vitro microenvironment facilitates synovium-derived stem cell-based nucleus pulposus tissue regeneration. Spine (Phila.Pa.1976) 2012;37:1538–1547. doi: 10.1097/BRS.0b013e31825150bf. [DOI] [PubMed] [Google Scholar]
- 76.Kanninen L.K., Porola P., Niklander J., Malinen M.M., Corlu A., Guguen-Guillouzo C., Urtti A., Yliperttula M.L., Lou Y.R. Hepatic differentiation of human pluripotent stem cells on human liver progenitor HepaRG-derived acellular matrix. Exp. Cell Res. 2016;341:207–217. doi: 10.1016/j.yexcr.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Y., Pizzute T., Li J., He F., Pei M. sb203580 preconditioning recharges matrix-expanded human adult stem cells for chondrogenesis in an inflammatory environment - a feasible approach for autologous stem cell based osteoarthritic cartilage repair. Biomaterials. 2015;64:88–97. doi: 10.1016/j.biomaterials.2015.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jeon J., Lee M.S., Yang H.S. Differentiated osteoblasts derived decellularized extracellular matrix to promote osteogenic differentiation. Biomater. Res. 2018;22:4. doi: 10.1186/s40824-018-0115-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gerson C.J., Elkins R.C., Goldstein S., Heacox A.E. Structural integrity of collagen and elastin in SynerGraft® decellularized-cryopreserved human heart valves. Cryobiology. 2012;64:33–42. doi: 10.1016/j.cryobiol.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 80.Beale E.W., Hoxworth R.E., Livingston E.H., Trussler A.P. The role of biologic mesh in abdominal wall reconstruction: a systematic review of the current literature. Am. J. Surg. 2012;204:510–517. doi: 10.1016/j.amjsurg.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 81.Beres A., Christison-Lagay E.R., Romao R.L., Langer J.C. Evaluation of Surgisis for patch repair of abdominal wall defects in children. J. Pediatr. Surg. 2012;47:917–919. doi: 10.1016/j.jpedsurg.2012.01.046. [DOI] [PubMed] [Google Scholar]
- 82.Cosentino M., Kanashiro A., Vives A., Sanchez J., Peraza M.F., Moreno D., Perona J., De Marco V., Ruiz-Castañe E., Sarquella J. Surgical treatment of Peyronie's disease with small intestinal submucosa graft patch. Int. J. Impot. Res. 2016;28:106–109. doi: 10.1038/ijir.2016.10. [DOI] [PubMed] [Google Scholar]
- 83.Darrien J.H., Kasem H. Successful closure of gastrocutaneous fistulas using the Surgisis(®) anal fistula plug. Ann. R. Coll. Surg. Engl. 2014;96:271–274. doi: 10.1308/003588414x13814021677755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Philips C., Campos F., Roosens A., Sánchez-Quevedo M.D.C., Declercq H., Carriel V. Qualitative and quantitative evaluation of a novel detergent-based method for decellularization of peripheral nerves. Ann. Biomed. Eng. 2018;46:1921–1937. doi: 10.1007/s10439-018-2082-y. [DOI] [PubMed] [Google Scholar]
- 85.Mendibil U., Ruiz-Hernandez R., Retegi-Carrion S., Garcia-Urquia N., Olalde-Graells B., Abarrategi A. Tissue-specific decellularization methods: rationale and strategies to achieve regenerative compounds. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21155447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang X., Chen X., Hong H., Hu R., Liu J., Liu C. Decellularized extracellular matrix scaffolds: recent trends and emerging strategies in tissue engineering. Bioact. Mater. 2022;10:15–31. doi: 10.1016/j.bioactmat.2021.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Willemse J., Verstegen M.M.A., Vermeulen A., Schurink I.J., Roest H.P., van der Laan L.J.W., de Jonge J. Fast, robust and effective decellularization of whole human livers using mild detergents and pressure controlled perfusion. Mater.Sci.Eng.C.Mater.Biol.Appl. 2020;108 doi: 10.1016/j.msec.2019.110200. [DOI] [PubMed] [Google Scholar]
- 88.Nieto-Nicolau N., López-Chicón P., Fariñas O., Bolívar S., Udina E., Navarro X., Casaroli-Marano R.P., Vilarrodona A. Effective decellularization of human nerve matrix for regenerative medicine with a novel protocol. Cell Tissue Res. 2021;384:167–177. doi: 10.1007/s00441-020-03317-3. [DOI] [PubMed] [Google Scholar]
- 89.Wu L.C., Kuo Y.J., Sun F.W., Chen C.H., Chiang C.J., Weng P.W., Tsuang Y.H., Huang Y.Y. Optimized decellularization protocol including α-Gal epitope reduction for fabrication of an acellular porcine annulus fibrosus scaffold. Cell Tissue Bank. 2017;18:383–396. doi: 10.1007/s10561-017-9619-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Song Y.H., Maynes M.A., Hlavac N., Visosevic D., Daramola K.O., Porvasnik S.L., Schmidt C.E. Development of novel apoptosis-assisted lung tissue decellularization methods. Biomater. Sci. 2021;9:3485–3498. doi: 10.1039/d1bm00032b. [DOI] [PubMed] [Google Scholar]
- 91.Novoseletskaya E., Grigorieva O., Nimiritsky P., Basalova N., Eremichev R., Milovskaya I., Kulebyakin K., Kulebyakina M., Rodionov S., Omelyanenko N., Efimenko A. Mesenchymal stromal cell-produced components of extracellular matrix potentiate multipotent stem cell response to differentiation stimuli. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.555378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shi Q., Chen Y., Li M., Zhang T., Ding S., Xu Y., Hu J., Chen C., Lu H. Designing a novel vacuum aspiration system to decellularized large-size enthesis with preservation of physicochemical and biological properties. Ann. Transl. Med. 2020;8:1364. doi: 10.21037/atm-20-3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang Z., Sun F., Lu Y., Zhang B., Zhang G., Shi H. Rapid preparation method for preparing tracheal decellularized scaffolds: vacuum assistance and optimization of DNase I. ACS.Omega. 2021;6:10637–10644. doi: 10.1021/acsomega.0c06247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rabbani M., Zakian N., Alimoradi N. Contribution of physical methods in decellularization of animal tissues. J.Med.Signals.Sens. 2021;11:1–11. doi: 10.4103/jmss.JMSS_2_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Amirazad H., Dadashpour M., Zarghami N. Application of decellularized bone matrix as a bioscaffold in bone tissue engineering. J. Biol. Eng. 2022;16:1. doi: 10.1186/s13036-021-00282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gilbert T.W., Sellaro T.L., Badylak S.F. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 97.Liu X., Cai Y., Xia C., Wu H., Li Q., Xu Z., Lu F. An innovative method to obtain porous porcine aorta scaffolds for tissue engineering. Artif. Organs. 2019;43:1162–1169. doi: 10.1111/aor.13519. [DOI] [PubMed] [Google Scholar]
- 98.Aeberhard P.A., Grognuz A., Peneveyre C., McCallin S., Hirt-Burri N., Antons J., Pioletti D., Raffoul W., Applegate L.A. Efficient decellularization of equine tendon with preserved biomechanical properties and cytocompatibility for human tendon surgery indications. Artif. Organs. 2020;44:E161–e171. doi: 10.1111/aor.13581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Roth S.P., Glauche S.M., Plenge A., Erbe I., Heller S., Burk J. Automated freeze-thaw cycles for decellularization of tendon tissue - a pilot study. BMC Biotechnol. 2017;17:13. doi: 10.1186/s12896-017-0329-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Goh S.-K., Bertera S., Vaidya V., Dumpe S., Barner S., Mathew S., Banerjee I. Development of perfusion bioreactor for whole organ engineering — a culture system that enhances cellular engraftment, survival and phenotype of repopulated pancreas. Technology. 2018;6:118–134. doi: 10.1142/s2339547818500085. [DOI] [Google Scholar]
- 101.Wang Z., Wang Z., Yu Q., Xi H., Weng J., Du X., Chen D., Ma J., Mei J., Chen C. Comparative study of two perfusion routes with different flow in decellularization to harvest an optimal pulmonary scaffold for recellularization. J.Biomed.Mater.Res.A. 2016;104:2567–2575. doi: 10.1002/jbm.a.35794. [DOI] [PubMed] [Google Scholar]
- 102.Struecker B., Butter A., Hillebrandt K., Polenz D., Reutzel-Selke A., Tang P., Lippert S., Leder A., Rohn S., Geisel D., Denecke T., Aliyev K., Jöhrens K., Raschzok N., Neuhaus P., Pratschke J., Sauer I.M. Improved rat liver decellularization by arterial perfusion under oscillating pressure conditions. J.Tissue.Eng.Regen.Med. 2017;11:531–541. doi: 10.1002/term.1948. [DOI] [PubMed] [Google Scholar]
- 103.Elebring E., Kuna V.K., Kvarnström N., Sumitran-Holgersson S. Cold-perfusion decellularization of whole-organ porcine pancreas supports human fetal pancreatic cell attachment and expression of endocrine and exocrine markers. J. Tissue Eng. 2017;8 doi: 10.1177/2041731417738145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Meșină M., Mîndrilă I., Mesina C., Obleagă C., Istrătoaie O. A perfusion decellularization heart model - an interesting tool for ce ll-matrix interaction studies. JMMS. 2019;6:137–142. doi: 10.22543/7674.61.p137142. [DOI] [Google Scholar]
- 105.Seetapun D., Ross J.J. Eliminating the organ transplant waiting list: the future with perfusion decellularized organs. Surgery. 2017;161:1474–1478. doi: 10.1016/j.surg.2016.09.041. [DOI] [PubMed] [Google Scholar]
- 106.Park S.M., Yang S., Rye S.M., Choi S.W. Effect of pulsatile flow perfusion on decellularization. Biomed. Eng. Online. 2018;17:15. doi: 10.1186/s12938-018-0445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Simsa R., Vila X.M., Salzer E., Teuschl A., Jenndahl L., Bergh N., Fogelstrand P. Effect of fluid dynamics on decellularization efficacy and mechanical properties of blood vessels. PLoS One. 2019;14 doi: 10.1371/journal.pone.0220743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Duisit J., Amiel H., Wüthrich T., Taddeo A., Dedriche A., Destoop V., Pardoen T., Bouzin C., Joris V., Magee D., Vögelin E., Harriman D., Dessy C., Orlando G., Behets C., Rieben R., Gianello P., Lengelé B. Perfusion-decellularization of human ear grafts enables ECM-based scaffolds for auricular vascularized composite tissue engineering. Acta Biomater. 2018;73:339–354. doi: 10.1016/j.actbio.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 109.Verstegen M.M.A., Willemse J., van den Hoek S., Kremers G.J., Luider T.M., van Huizen N.A., Willemssen F., Metselaar H.J., Jnm I.J., van der Laan L.J.W., de Jonge J. Decellularization of whole human liver grafts using controlled perfusion for transplantable organ bioscaffolds. Stem Cell. Dev. 2017;26:1304–1315. doi: 10.1089/scd.2017.0095. [DOI] [PubMed] [Google Scholar]
- 110.Kajbafzadeh A.M., Khorramirouz R., Nabavizadeh B., Ladi Seyedian S.S., Akbarzadeh A., Heidari R., Masoumi A., Azizi B., Seyed Hossein Beigi R. Whole organ sheep kidney tissue engineering and in vivo transplantation: effects of perfusion-based decellularization on vascular integrity. Mater.Sci.Eng.C.Mater.Biol.Appl. 2019;98:392–400. doi: 10.1016/j.msec.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 111.Sohn S., Buskirk M.V., Buckenmeyer M.J., Londono R., Faulk D. Whole organ engineering: approaches, challenges, and future directions. Appl. Sci. 2020;10:4277. [Google Scholar]
- 112.Schilling B.K., Lamberti K.K., Snowden M.J., Baker J.S., Byrd K., Komatsu C., Solari M.G., Marra K.G. Design and fabrication of an automatable, 3D printed perfusion device for tissue infusion and perfusion engineering, tissue. Eng.Part A. 2020;26:253–264. doi: 10.1089/ten.tea.2019.0209. [DOI] [PubMed] [Google Scholar]
- 113.Matuska A.M., McFetridge P.S. Laser micro-ablation of fibrocartilage tissue: effects of tissue processing on porosity modification and mechanics. J. Biomed. Mater. Res. B Appl. Biomater. 2018;106:1858–1868. doi: 10.1002/jbm.b.33997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Farag A., Hashimi S.M., Vaquette C., Volpato F.Z., Hutmacher D.W., Ivanovski S. Assessment of static and perfusion methods for decellularization of PCL membrane-supported periodontal ligament cell sheet constructs. Arch. Oral Biol. 2018;88:67–76. doi: 10.1016/j.archoralbio.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 115.Duisit J., Amiel H., Orlando G., Dedriche A., Behets C., Gianello P., Lengelé B. Face graft scaffold production in a rat model. Plast. Reconstr. Surg. 2018;141:95–103. doi: 10.1097/prs.0000000000003910. [DOI] [PubMed] [Google Scholar]
- 116.Nguyen M.T.N., Tran H.L.B. Effect of modified bovine pericardium on human gingival fibroblasts in vitro. Cells Tissues Organs. 2018;206:296–307. doi: 10.1159/000501807. [DOI] [PubMed] [Google Scholar]
- 117.Tchoukalova Y.D., Hintze J.M., Hayden R.E., Lott D.G. Tracheal decellularization using a combination of chemical, physical and bioreactor methods. Int. J. Artif. Organs. 2017 doi: 10.5301/ijao.5000648. [DOI] [PubMed] [Google Scholar]
- 118.Wilson S.L., Sidney L.E., Dunphy S.E., Dua H.S., Hopkinson A. Corneal decellularization: a method of recycling unsuitable donor tissue for clinical translation? Curr. Eye Res. 2016;41:769–782. doi: 10.3109/02713683.2015.1062114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Monibi F.A., Bozynski C.C., Kuroki K., Stoker A.M., Pfeiffer F.M., Sherman S.L., Cook J.L. Development of a micronized meniscus extracellular matrix scaffold for potential augmentation of meniscal repair and regeneration, tissue. Eng.Part C Methods. 2016;22:1059–1070. doi: 10.1089/ten.TEC.2016.0276. [DOI] [PubMed] [Google Scholar]
- 120.Qu J., Van Hogezand R.M., Zhao C., Kuo B.J., Carlsen B.T. Decellularization of a fasciocutaneous flap for use as a perfusable scaffold. Ann. Plast. Surg. 2015;75:112–116. doi: 10.1097/sap.0000000000000157. [DOI] [PubMed] [Google Scholar]
- 121.Nakamura N., Kimura T., Nam K., Fujisato T., Iwata H., Tsuji T., Kishida A. Induction of in vivo ectopic hematopoiesis by a three-dimensional structured extracellular matrix derived from decellularized cancellous bone. ACS Biomater. Sci. Eng. 2019;5:5669–5680. doi: 10.1021/acsbiomaterials.8b01491. [DOI] [PubMed] [Google Scholar]
- 122.Huang Z., Godkin O., Schulze-Tanzil G. The challenge in using mesenchymal stromal cells for recellularization of decellularized cartilage. Stem Cell Rev. Rep. 2017;13:50–67. doi: 10.1007/s12015-016-9699-8. [DOI] [PubMed] [Google Scholar]
- 123.Cui H., Chai Y., Yu Y. Progress in developing decellularized bioscaffolds for enhancing skin construction. J.Biomed.Mater.Res.A. 2019;107:1849–1859. doi: 10.1002/jbm.a.36688. [DOI] [PubMed] [Google Scholar]
- 124.Xia C., Mei S., Gu C., Zheng L., Fang C., Shi Y., Wu K., Lu T., Jin Y., Lin X., Chen P. Decellularized cartilage as a prospective scaffold for cartilage repair. Mater.Sci.Eng.C.Mater.Biol.Appl. 2019;101:588–595. doi: 10.1016/j.msec.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 125.Paulo Zambon J., Atala A., Yoo J.J. Methods to generate tissue-derived constructs for regenerative medicine applications. Methods. 2020;171:3–10. doi: 10.1016/j.ymeth.2019.09.016. [DOI] [PubMed] [Google Scholar]
- 126.Akbari Zahmati A.H., Alipoor R., Rezaei Shahmirzadi A., Khori V., Abolhasani M.M. Chemical Decellularization Methods and Its Effects on Extracellular Ma trix. IMMINV. 2017;2:76. doi: 10.24200/imminv.v2i3.63. [DOI] [Google Scholar]
- 127.Cornelison R.C., Wellman S.M., Park J.H., Porvasnik S.L., Song Y.H., Wachs R.A., Schmidt C.E. Development of an apoptosis-assisted decellularization method for maximal preservation of nerve tissue structure. Acta Biomater. 2018;77:116–126. doi: 10.1016/j.actbio.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kim J.K., Koh Y.D., Kim J.O., Seo D.H. Development of a decellularization method to produce nerve allografts using less invasive detergents and hyper/hypotonic solutions. J.Plast.Reconstr.Aesthet.Surg. 2016;69:1690–1696. doi: 10.1016/j.bjps.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 129.Yang B., Zhang Y., Zhou L., Sun Z., Zheng J., Chen Y., Dai Y. Development of a porcine bladder acellular matrix with well-preserved extracellular bioactive factors for tissue engineering. Tissue Eng. C.Methods. 2010;16:1201–1211. doi: 10.1089/ten.TEC.2009.0311. [DOI] [PubMed] [Google Scholar]
- 130.Rieder E., Kasimir M.T., Silberhumer G., Seebacher G., Wolner E., Simon P., Weigel G. Decellularization protocols of porcine heart valves differ importantly in efficiency of cell removal and susceptibility of the matrix to recellularization with human vascular cells. J. Thorac. Cardiovasc. Surg. 2004;127:399–405. doi: 10.1016/j.jtcvs.2003.06.017. [DOI] [PubMed] [Google Scholar]
- 131.Matuska A.M., McFetridge P.S. The effect of terminal sterilization on structural and biophysical properties of a decellularized collagen-based scaffold; implications for stem cell adhesion. J. Biomed. Mater. Res. B Appl. Biomater. 2015;103:397–406. doi: 10.1002/jbm.b.33213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Keane T.J., Badylak S.F. The host response to allogeneic and xenogeneic biological scaffold materials. J.Tissue.Eng.Regen.Med. 2015;9:504–511. doi: 10.1002/term.1874. [DOI] [PubMed] [Google Scholar]
- 133.Petersen T.H., Calle E.A., Zhao L., Lee E.J., Gui L., Raredon M.B., Gavrilov K., Yi T., Zhuang Z.W., Breuer C., Herzog E., Niklason L.E. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McCrary M.W., Vaughn N.E., Hlavac N., Song Y.H., Wachs R.A., Schmidt C.E. Novel sodium deoxycholate-based chemical decellularization method for peripheral nerve, tissue. Eng.Part C Methods. 2020;26:23–36. doi: 10.1089/ten.TEC.2019.0135. [DOI] [PubMed] [Google Scholar]
- 135.Simsa R., Padma A.M., Heher P., Hellström M., Teuschl A., Jenndahl L., Bergh N., Fogelstrand P. Systematic in vitro comparison of decellularization protocols for blood vessels. PLoS One. 2018;13 doi: 10.1371/journal.pone.0209269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang F., Wang Z., Sun X., Wang F., Xu X., Zhang X. Safety and efficacy of dispase and plasmin in pharmacologic vitreolysis. Invest. Ophthalmol. Vis. Sci. 2004;45:3286–3290. doi: 10.1167/iovs.04-0026. [DOI] [PubMed] [Google Scholar]
- 137.Derradji H., Baatout S. Apoptosis: a mechanism of cell suicide. Vivo. 2003;17:185–192. [PubMed] [Google Scholar]
- 138.Elmore S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.van Engeland M., Kuijpers H.J., Ramaekers F.C., Reutelingsperger C.P., Schutte B. Plasma membrane alterations and cytoskeletal changes in apoptosis. Exp. Cell Res. 1997;235:421–430. doi: 10.1006/excr.1997.3738. [DOI] [PubMed] [Google Scholar]
- 140.Raff M. Cell suicide for beginners. Nature. 1998;396:119–122. doi: 10.1038/24055. [DOI] [PubMed] [Google Scholar]
- 141.Cascalho M., Ogle B.M., Platt J.L. The future of organ transplantation. Ann. Transplant. 2006;11:44–47. [PubMed] [Google Scholar]
- 142.Savill J., Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–788. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 143.Kurosaka K., Takahashi M., Watanabe N., Kobayashi Y. Silent cleanup of very early apoptotic cells by macrophages. J. Immunol. 2003;171:4672–4679. doi: 10.4049/jimmunol.171.9.4672. [DOI] [PubMed] [Google Scholar]
- 144.Song M., Liu Y., Hui L. Preparation and characterization of acellular adipose tissue matrix using a combination of physical and chemical treatments. Mol. Med. Rep. 2018;17:138–146. doi: 10.3892/mmr.2017.7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ferdowsi Khosroshahi A., Soleimani Rad J., Kheirjou R., Roshangar B., Rashtbar M., Salehi R., Ranjkesh M.R., Roshangar L. Adipose tissue-derived stem cells upon decellularized ovine small intestine submucosa for tissue regeneration: an optimization and comparison method. J. Cell. Physiol. 2020;235:1556–1567. doi: 10.1002/jcp.29074. [DOI] [PubMed] [Google Scholar]
- 146.Rahman S., Griffin M., Naik A., Szarko M., Butler P.E.M. Optimising the decellularization of human elastic cartilage with trypsin for future use in ear reconstruction. Sci. Rep. 2018;8:3097. doi: 10.1038/s41598-018-20592-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Guimaraes A.B., Correia A.T., Alves B.P., Da Silva R.S., Martins J.K., Pêgo-Fernandes P.M., Xavier N.S., Dolhnikoff M., Cardoso P.F.G. Evaluation of a physical-chemical protocol for porcine tracheal decellularization. Transplant. Proc. 2019;51:1611–1613. doi: 10.1016/j.transproceed.2019.01.042. [DOI] [PubMed] [Google Scholar]
- 148.Buse E.E., Hilbert S.L., Hopkins R.A., Converse G.L. Pulse duplicator hydrodynamic testing of bioengineered biological heart valves. Cardiovasc.Eng.Technol. 2016;7:352–362. doi: 10.1007/s13239-016-0275-9. [DOI] [PubMed] [Google Scholar]
- 149.Park C.S., Kim Y.J., Lee J.R., Lim H.G., Chang J.E., Jeong S., Kwon N. Anticalcification effect of a combination of decellularization, organic solvents and amino acid detoxification on glutaraldehyde-fixed xenopericardial heart valves in a large-animal long-term circulatory model. Interact. Cardiovasc. Thorac. Surg. 2017;25:391–399. doi: 10.1093/icvts/ivx131. [DOI] [PubMed] [Google Scholar]
- 150.Taylor D.A., Sampaio L.C., Cabello R., Elgalad A., Parikh R., Wood R.P., Myer K.A., Yeh A.T., Lee P.F. Decellularization of whole human heart inside a pressurized pouch in an inverted orientation. J.Vis.Exp. 2018;141:58–123. doi: 10.3791/58123. [DOI] [PubMed] [Google Scholar]
- 151.Reimer J.M., Syedain Z.H., Haynie B.H., Tranquillo R.T. Pediatric tubular pulmonary heart valve from decellularized engineered tissue tubes. Biomaterials. 2015;62:88–94. doi: 10.1016/j.biomaterials.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sierad L.N., Shaw E.L., Bina A., Brazile B., Rierson N., Patnaik S.S., Kennamer A., Odum R., Cotoi O., Terezia P., Branzaniuc K., Smallwood H., Deac R., Egyed I., Pavai Z., Szanto A., Harceaga L., Suciu H., Raicea V., Olah P., Simionescu A., Liao J., Movileanu I., Harpa M., Simionescu D.T. Functional heart valve scaffolds obtained by complete decellularization of porcine aortic roots in a novel differential pressure gradient perfusion system. Tissue Eng. C Methods. 2015;21:1284–1296. doi: 10.1089/ten.TEC.2015.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pan J., Yan S., Gao J.J., Wang Y.Y., Lu Z.J., Cui C.W., Zhang Y.H., Wang Y., Meng X.Q., Zhou L., Xie H.Y., Zheng J., Zheng M.H., Zheng S.S. In-vivo organ engineering: perfusion of hepatocytes in a single liver lobe scaffold of living rats. Int. J. Biochem. Cell Biol. 2016;80:124–131. doi: 10.1016/j.biocel.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 154.Abedin E., Lari R., Mahdavi Shahri N., Fereidoni M. Development of a demineralized and decellularized human epiphyseal bone scaffold for tissue engineering: a histological study. Tissue Cell. 2018;55:46–52. doi: 10.1016/j.tice.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 155.Schneider C., Lehmann J., van Osch G.J., Hildner F., Teuschl A., Monforte X., Miosga D., Heimel P., Priglinger E., Redl H., Wolbank S., Nürnberger S. Systematic comparison of protocols for the preparation of human articular cartilage for use as scaffold material in cartilage tissue engineering, tissue. Eng.Part.C.Methods. 2016;22:1095–1107. doi: 10.1089/ten.TEC.2016.0380. [DOI] [PubMed] [Google Scholar]
- 156.Heath, D. E., A Review of Decellularized Extracellular Matrix Biomaterials for Regen erative Engineering Applications, Regen. Eng. Transl. Med. 5 155-166. 10.1007/s40883-018-0080-0. [DOI]
- 157.Wilson S.L., Sidney L.E., Dunphy S.E., Rose J.B., Hopkinson A. Keeping an eye on decellularized corneas: a review of methods, characterization and applications. J. Funct. Biomater. 2013;4:114–161. doi: 10.3390/jfb4030114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kuljanin M., Brown C.F.C., Raleigh M.J., Lajoie G.A., Flynn L.E. Collagenase treatment enhances proteomic coverage of low-abundance proteins in decellularized matrix bioscaffolds. Biomaterials. 2017;144:130–143. doi: 10.1016/j.biomaterials.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 159.Zheng M.H., Chen J., Kirilak Y., Willers C., Xu J., Wood D. Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA: possible implications in human implantation. J. Biomed. Mater. Res. B Appl. Biomater. 2005;73:61–67. doi: 10.1002/jbm.b.30170. [DOI] [PubMed] [Google Scholar]
- 160.Yam G.H., Yusoff N.Z., Goh T.W., Setiawan M., Lee X.W., Liu Y.C., Mehta J.S. Decellularization of human stromal refractive lenticules for corneal tissue engineering. Sci. Rep. 2016;6 doi: 10.1038/srep26339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Badylak S.F. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl. Immunol. 2004;12:367–377. doi: 10.1016/j.trim.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 162.Nagata S., Hanayama R., Kawane K. Autoimmunity and the clearance of dead cells. Cells. 2010;140:619–630. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 163.Sánchez P.L., Fernández-Santos M.E., Costanza S., Climent A.M., Moscoso I., Gonzalez-Nicolas M.A., Sanz-Ruiz R., Rodríguez H., Kren S.M., Garrido G., Escalante J.L., Bermejo J., Elizaga J., Menarguez J., Yotti R., Pérez del Villar C., Espinosa M.A., Guillem M.S., Willerson J.T., Bernad A., Matesanz R., Taylor D.A., Fernández-Avilés F. Acellular human heart matrix: a critical step toward whole heart grafts. Biomaterials. 2015;61:279–289. doi: 10.1016/j.biomaterials.2015.04.056. [DOI] [PubMed] [Google Scholar]
- 164.Wang B., Tedder M.E., Perez C.E., Wang G., de Jongh Curry A.L., To F., Elder S.H., Williams L.N., Simionescu D.T., Liao J. Structural and biomechanical characterizations of porcine myocardial extracellular matrix. J. Mater. Sci. Mater. Med. 2012;23:1835–1847. doi: 10.1007/s10856-012-4660-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Morishige N., Wahlert A.J., Kenney M.C., Brown D.J., Kawamoto K., Chikama T., Nishida T., Jester J.V. Second-harmonic imaging microscopy of normal human and keratoconus cornea. Invest. Ophthalmol. Vis. Sci. 2007;48:1087–1094. doi: 10.1167/iovs.06-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Aslanides I.M., Reinstein D.Z., Silverman R.H., Lazzaro D.R., Rondeau M.J., Rodriguez H.S., Coleman D.J. High-frequency ultrasound spectral parameter imaging of anterior corneal scars. Clao.j. 1995;21:268–272. [PubMed] [Google Scholar]
- 167.Welham N.V., Chang Z., Smith L.M., Frey B.L. Proteomic analysis of a decellularized human vocal fold mucosa scaffold using 2D electrophoresis and high-resolution mass spectrometry. Biomaterials. 2013;34:669–676. doi: 10.1016/j.biomaterials.2012.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Griffiths L.G., Choe L.H., Reardon K.F., Dow S.W., Christopher Orton E. Immunoproteomic identification of bovine pericardium xenoantigens. Biomaterials. 2008;29:3514–3520. doi: 10.1016/j.biomaterials.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hernandez P., Müller M., Appel R.D. Automated protein identification by tandem mass spectrometry: issues and strategies. Mass Spectrom. Rev. 2006;25:235–254. doi: 10.1002/mas.20068. [DOI] [PubMed] [Google Scholar]
- 170.Hussein K.H., Park K.M., Kang K.S., Woo H.M. Biocompatibility evaluation of tissue-engineered decellularized scaffolds for biomedical application. Mater.Sci.Eng.C.Mater.Biol.Appl. 2016;67:766–778. doi: 10.1016/j.msec.2016.05.068. [DOI] [PubMed] [Google Scholar]
- 171.Luo Y., Lou D., Ma L., Gao C. Optimizing detergent concentration and processing time to balance the decellularization efficiency and properties of bioprosthetic heart valves. J.Biomed.Mater.Res.A. 2019;107:2235–2243. doi: 10.1002/jbm.a.36732. [DOI] [PubMed] [Google Scholar]
- 172.Oh J.Y., Kim M.K., Lee H.J., Ko J.H., Wee W.R., Lee J.H. Processing porcine cornea for biomedical applications, Tissue. Eng.Part C Methods. 2009;15:635–645. doi: 10.1089/ten.TEC.2009.0022. [DOI] [PubMed] [Google Scholar]
- 173.Woods T., Gratzer P.F. Effectiveness of three extraction techniques in the development of a decellularized bone-anterior cruciate ligament-bone graft. Biomaterials. 2005;26:7339–7349. doi: 10.1016/j.biomaterials.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 174.Park K.M., Park S.M., Yang S.R., Hong S.H., Woo H.M. Preparation of immunogen-reduced and biocompatible extracellular matrices from porcine liver. J. Biosci. Bioeng. 2013;115:207–215. doi: 10.1016/j.jbiosc.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 175.Das S., Gao G., Lee J.Y., Jang J., Cho D.-W. Biofabrication and 3D Tissue Modeling. The Royal Society of Chemistry; 2019. Chapter 7 Decellularized Tissue Matrix-based 3D Tissue Modeling; pp. 148–170. [Google Scholar]
- 176.Tsakalakos T. The bulge test: A comparison of the theory and experiment for isotropi c and anisotropic films. Thin Solid Films. 1981;75:293–305. doi: 10.1016/0040-6090(81)90407-7. [DOI] [Google Scholar]
- 177.Glass D.H., Roberts C.J., Litsky A.S., Weber P.A. A viscoelastic biomechanical model of the cornea describing the effect of viscosity and elasticity on hysteresis. Invest Ophthalmol.Vis.Sci. 2008;49:3919–3926. doi: 10.1167/iovs.07-1321. [DOI] [PubMed] [Google Scholar]
- 178.Krupa I., Nedelčev T., Račko D., Lacík I. Mechanical properties of silica hydrogels prepared and aged at physiol ogical conditions: testing in the compression mode. J. Sol. Gel Sci. Technol. 2009;53:107–114. doi: 10.1007/s10971-009-2064-5. [DOI] [Google Scholar]
- 179.Stammen J.A., Williams S., Ku D.N., Guldberg R.E. Mechanical properties of a novel PVA hydrogel in shear and unconfined compression. Biomaterials. 2001;22:799–806. doi: 10.1016/s0142-9612(00)00242-8. [DOI] [PubMed] [Google Scholar]
- 180.Ahearne M., Siamantouras E., Yang Y., Liu K.K. Mechanical characterization of biomimetic membranes by micro-shaft poking. J. R. Soc. Interface. 2009;6:471–478. doi: 10.1098/rsif.2008.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Song J.J., Ott H.C. Organ engineering based on decellularized matrix scaffolds. Trends Mol. Med. 2011;17:424–432. doi: 10.1016/j.molmed.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 182.Freytes D.O., Stoner R.M., Badylak S.F. Uniaxial and biaxial properties of terminally sterilized porcine urinary bladder matrix scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 2008;84:408–414. doi: 10.1002/jbm.b.30885. [DOI] [PubMed] [Google Scholar]
- 183.Sun W.Q., Leung P. Calorimetric study of extracellular tissue matrix degradation and instability after gamma irradiation. Acta Biomater. 2008;4:817–826. doi: 10.1016/j.actbio.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 184.Moreau M.F., Gallois Y., Baslé M.F., Chappard D. Gamma irradiation of human bone allografts alters medullary lipids and releases toxic compounds for osteoblast-like cells. Biomaterials. 2000;21:369–376. doi: 10.1016/s0142-9612(99)00193-3. [DOI] [PubMed] [Google Scholar]
- 185.Gouk S.S., Lim T.M., Teoh S.H., Sun W.Q. Alterations of human acellular tissue matrix by gamma irradiation: histology, biomechanical property, stability, in vitro cell repopulation, and remodeling. J. Biomed. Mater. Res. B Appl. Biomater. 2008;84:205–217. doi: 10.1002/jbm.b.30862. [DOI] [PubMed] [Google Scholar]
- 186.Wang W., Itoh S., Takakuda K. Comparative study of the efficacy of decellularization treatment of allogenic and xenogeneic nerves as nerve conduits. J.Biomed.Mater.Res.A. 2016;104:445–454. doi: 10.1002/jbm.a.35589. [DOI] [PubMed] [Google Scholar]
- 187.Bombelli S., Meregalli C., Scalia C., Bovo G., Torsello B., De Marco S., Cadamuro M., Viganò P., Strada G., Cattoretti G., Bianchi C., Perego R.A. Nephrosphere-derived cells are induced to multilineage differentiation when cultured on human decellularized kidney scaffolds. Am. J. Pathol. 2018;188:184–195. doi: 10.1016/j.ajpath.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 188.Qiu Q.Q., Leamy P., Brittingham J., Pomerleau J., Kabaria N., Connor J. Inactivation of bacterial spores and viruses in biological material using supercritical carbon dioxide with sterilant. J. Biomed. Mater. Res. B Appl. Biomater. 2009;91:572–578. doi: 10.1002/jbm.b.31431. [DOI] [PubMed] [Google Scholar]
- 189.Rowland C.R., Lennon D.P., Caplan A.I., Guilak F. The effects of crosslinking of scaffolds engineered from cartilage ECM on the chondrogenic differentiation of MSCs. Biomaterials. 2013;34:5802–5812. doi: 10.1016/j.biomaterials.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Xing H., Yin H., Sun C., Ren X., Tian Y., Yu M., Jiang T. Preparation of an acellular spinal cord scaffold to improve its biological properties. Mol. Med. Rep. 2019;20:1075–1084. doi: 10.3892/mmr.2019.10364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sun W., Yang Y., Wang L., Tang H., Zhang L., She Y., Xiao X., Hu X., Feng Q., Chen C. Utilization of an acellular cartilage matrix-based photocrosslinking hydrogel for tracheal cartilage regeneration and circumferential tracheal repair. Adv. Funct. Mater. 2022;32 doi: 10.1002/adfm.202201257. [DOI] [Google Scholar]
- 192.Lü W.-D., Sun R.-F., Hu Y.-R., Lu J.-R., Gu L., Liu Z.-G., Lei G.-Y., Qiang Z., Cai L. Photooxidatively crosslinked acellular tumor extracellular matrices as potential tumor engineering scaffolds. Acta Biomater. 2018;71:460–473. doi: 10.1016/j.actbio.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 193.Huang B., Li P., Chen M., Peng L., Luo X., Tian G., Wang H., Wu L., Tian Q., Li H., Yang Y., Jiang S., Yang Z., Zha K., Sui X., Liu S., Guo Q. Hydrogel composite scaffolds achieve recruitment and chondrogenesis in cartilage tissue engineering applications. J.Nanobiotechnology. 2022;20:25. doi: 10.1186/s12951-021-01230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ding Z., Dan N., Chen Y. Study of a Hydrophilic Healing-Promoting Porcine Acellular Dermal Matr ix. Processes. 2022;10:916. doi: 10.3390/pr10050916. [DOI] [Google Scholar]
- 195.Xia H., Zhao D., Zhu H., Hua Y., Xiao K., Xu Y., Liu Y., Chen W., Liu Y., Zhang W., Liu W., Tang S., Cao Y., Wang X., Chen H.H., Zhou G. Lyophilized scaffolds fabricated from 3D-printed photocurable natural hydrogel for cartilage regeneration. ACS Appl. Mater. Interfaces. 2018;10:31704–31715. doi: 10.1021/acsami.8b10926. [DOI] [PubMed] [Google Scholar]
- 196.Ma, P., Wang, Y., Li, B., Hou, H., Cross-linking effects of carbodiimide, oxidized chitosan oligosacchari de and glutaraldehyde on acellular dermal matrix of basa fish (Pangasi us bocourti), Int. J. Biol. Macromol. 164 677-686. 10.1016/j.ijbiomac.2020.07.019. [DOI] [PubMed]
- 197.Shirani A., Ganji F., Golmohammadi M., Hashemi S.M., Mozafari M., Amoabediny G., Karkuki Osguei N., Samadikuchaksaraei A. Cross-linked acellular lung for application in tissue engineering: Effects on biocompatibility, mechanical properties and immunological resp onses. Mater. Sci. Eng. C, Mater. Biol. Appl. 2021;122:111938. doi: 10.1016/j.msec.2021.111938. [DOI] [PubMed] [Google Scholar]
- 198.Hillebrandt K.H., Everwien H., Haep N., Keshi E., Pratschke J., Sauer I.M. Strategies based on organ decellularization and recellularization. Transpl. Int. 2019;32:571–585. doi: 10.1111/tri.13462. [DOI] [PubMed] [Google Scholar]
- 199.Kenry, Lee W.C., Loh K.P., Lim C.T. When stem cells meet graphene: opportunities and challenges in regenerative medicine. Biomaterials. 2018;155:236–250. doi: 10.1016/j.biomaterials.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 200.Bhumiratana S., Bernhard J.C., Alfi D.M., Yeager K., Eton R.E., Bova J., Shah F., Gimble J.M., Lopez M.J., Eisig S.B., Vunjak-Novakovic G. Tissue-engineered autologous grafts for facial bone reconstruction. Sci. Transl. Med. 2016;8 doi: 10.1126/scitranslmed.aad5904. 343ra383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Paduano F., Marrelli M., Alom N., Amer M., White L.J., Shakesheff K.M., Tatullo M. Decellularized bone extracellular matrix and human dental pulp stem cells as a construct for bone regeneration. J. Biomater. Sci. Polym. Ed. 2017;28:730–748. doi: 10.1080/09205063.2017.1301770. [DOI] [PubMed] [Google Scholar]
- 202.Milan P.B., Lotfibakhshaiesh N., Joghataie M.T., Ai J., Pazouki A., Kaplan D.L., Kargozar S., Amini N., Hamblin M.R., Mozafari M., Samadikuchaksaraei A. Accelerated wound healing in a diabetic rat model using decellularized dermal matrix and human umbilical cord perivascular cells. Acta Biomater. 2016;45:234–246. doi: 10.1016/j.actbio.2016.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gholipourmalekabadi M., Sameni M., Radenkovic D., Mozafari M., Mossahebi-Mohammadi M., Seifalian A. Decellularized human amniotic membrane: how viable is it as a delivery system for human adipose tissue-derived stromal cells? Cell Prolif. 2016;49:115–121. doi: 10.1111/cpr.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lovati A.B., Bottagisio M., Moretti M. Decellularized and engineered tendons as biological substitutes: a critical review. Stem Cell. Int. 2016;(2016) doi: 10.1155/2016/7276150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kitahara H., Yagi H., Tajima K., Okamoto K., Yoshitake A., Aeba R., Kudo M., Kashima I., Kawaguchi S., Hirano A., Kasai M., Akamatsu Y., Oka H., Kitagawa Y., Shimizu H. Heterotopic transplantation of a decellularized and recellularized whole porcine heart. Interact. Cardiovasc. Thorac. Surg. 2016;22:571–579. doi: 10.1093/icvts/ivw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yuan X., Wei Y., Villasante A., Ng J.J.D., Arkonac D.E., Chao P.G., Vunjak-Novakovic G. Stem cell delivery in tissue-specific hydrogel enabled meniscal repair in an orthotopic rat model. Biomaterials. 2017;132:59–71. doi: 10.1016/j.biomaterials.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Song H., Yin Z., Wu T., Li Y., Luo X., Xu M., Duan L., Li J. Enhanced effect of tendon stem/progenitor cells combined with tendon-derived decellularized extracellular matrix on tendon regeneration. Cell Transplant. 2018;27:1634–1643. doi: 10.1177/0963689718805383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wang X., Wang G., Zingales S., Zhao B. Biomaterials Enabled Cell-Free Strategies for Endogenous Bone Regenera tion. Tissue Eng. B Rev. 2018;24:463–481. doi: 10.1089/ten.TEB.2018.0012. [DOI] [PubMed] [Google Scholar]
- 209.Yang L., Liu Y., Sun L., Zhao C., Chen G., Zhao Y. Biomass Microcapsules with Stem Cell Encapsulation for Bone Repair. Nanomicro.Lett. 2021;14:4. doi: 10.1007/s40820-021-00747-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lei L., Ma B., Xu C., Liu H. Emerging tumor-on-chips with electrochemical biosensors. TrAC, Trends Anal. Chem. 2022;153:116640. doi: 10.1016/j.trac.2022.116640. [DOI] [Google Scholar]
- 211.Oshima Y., Harwood F.L., Coutts R.D., Kubo T., Amiel D. Variation of mesenchymal cells in polylactic acid scaffold in an osteochondral repair model, Tissue. Eng.Part C Methods. 2009;15:595–604. doi: 10.1089/ten.TEC.2008.0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Jeong C.G., Zhang H., Hollister S.J. Three-dimensional polycaprolactone scaffold-conjugated bone morphogenetic protein-2 promotes cartilage regeneration from primary chondrocytes in vitro and in vivo without accelerated endochondral ossification. J.Biomed.Mater.Res.A. 2012;100:2088–2096. doi: 10.1002/jbm.a.33249. [DOI] [PubMed] [Google Scholar]
- 213.Ahmed T.A., Giulivi A., Griffith M., Hincke M. Fibrin glues in combination with mesenchymal stem cells to develop a tissue-engineered cartilage substitute, Tissue. Eng.Part A. 2011;17:323–335. doi: 10.1089/ten.TEA.2009.0773. [DOI] [PubMed] [Google Scholar]
- 214.Shin H., Olsen B.D., Khademhosseini A. The mechanical properties and cytotoxicity of cell-laden double-networ k hydrogels based on photocrosslinkable gelatin and gellan gum biomacr omolecules. Biomaterials. 2012;33:3143–3152. doi: 10.1016/j.biomaterials.2011.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tohyama H., Yasuda K., Minami A., Majima T., Iwasaki N., Muneta T., Sekiya I., Yagishita K., Takahashi S., Kurokouchi K., Uchio Y., Iwasa J., Deie M., Adachi N., Sugawara K., Ochi M. Atelocollagen-associated autologous chondrocyte implantation for the repair of chondral defects of the knee: a prospective multicenter clinical trial in Japan. J. Orthop. Sci. 2009;14:579–588. doi: 10.1007/s00776-009-1384-1. [DOI] [PubMed] [Google Scholar]
- 216.He J., Li Z., Yu T., Wang W., Tao M., Ma Y., Wang S., Fan J., Tian X., Wang X., Lin Y., Ao Q. Preparation and evaluation of acellular sheep periostea for guided bone regeneration. J.Biomed.Mater.Res.A. 2020;108:19–29. doi: 10.1002/jbm.a.36787. [DOI] [PubMed] [Google Scholar]
- 217.Wei W., Li J., Chen S., Chen M., Xie Q., Sun H., Ruan J., Zhou H., Bi X., Zhuang A., You Z., Gu P., Fan X. In vitro osteogenic induction of bone marrow mesenchymal stem cells with a decellularized matrix derived from human adipose stem cells and in vivo implantation for bone regeneration. J.Mater.Chem.B. 2017;5:2468–2482. doi: 10.1039/c6tb03150a. [DOI] [PubMed] [Google Scholar]
- 218.Kara A., Tamburaci S., Tihminlioglu F., Havitcioglu H. Bioactive fish scale incorporated chitosan biocomposite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2019;130:266–279. doi: 10.1016/j.ijbiomac.2019.02.067. [DOI] [PubMed] [Google Scholar]
- 219.Lei L., Bai Y., Qin X., Liu J., Huang W., Lv Q. Current Understanding of Hydrogel for Drug Release and Tissue Engineering. Gels. 2022;8:301. doi: 10.3390/gels8050301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Yang L., Wang X., Yu Y., Shang L., Xu W., Zhao Y. Bio-inspired dual-adhesive particles from microfluidic electrospray fo r bone regeneration. Nano Res. 2022 doi: 10.1007/s12274-022-5202-9. [DOI] [Google Scholar]
- 221.Antich C., Jiménez G., de Vicente J., López-Ruiz E., Chocarro-Wrona C., Griñán-Lisón C., Carrillo E., Montañez E., Marchal J.A. Development of a biomimetic hydrogel based on predifferentiated mesenchymal stem-cell-derived ECM for cartilage tissue engineering. Adv.Healthc.Mater. 2021;10 doi: 10.1002/adhm.202001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sawkins M.J., Bowen W., Dhadda P., Markides H., Sidney L.E., Taylor A.J., Rose F.R., Badylak S.F., Shakesheff K.M., White L.J. Hydrogels derived from demineralized and decellularized bone extracellular matrix. Acta Biomater. 2013;9:7865–7873. doi: 10.1016/j.actbio.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kusuma G.D., Yang M.C., Brennecke S.P., O'Connor A.J., Kalionis B., Heath D.E. Transferable matrixes produced from decellularized extracellular matrix promote proliferation and osteogenic differentiation of mesenchymal stem cells and facilitate scale-up. ACS Biomater. Sci. Eng. 2018;4:1760–1769. doi: 10.1021/acsbiomaterials.7b00747. [DOI] [PubMed] [Google Scholar]
- 224.Beachley V., Ma G., Papadimitriou C., Gibson M., Corvelli M., Elisseeff J. Extracellular matrix particle-glycosaminoglycan composite hydrogels for regenerative medicine applications. J.Biomed.Mater.Res.A. 2018;106:147–159. doi: 10.1002/jbm.a.36218. [DOI] [PubMed] [Google Scholar]
- 225.Li W.J., Laurencin C.T., Caterson E.J., Tuan R.S., Ko F.K. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J. Biomed. Mater. Res. 2002;60:613–621. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 226.Bhardwaj N., Kundu S.C. Electrospinning: a fascinating fiber fabrication technique. Biotechnol. Adv. 2010;28:325–347. doi: 10.1016/j.biotechadv.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 227.Yoshimoto H., Shin Y.M., Terai H., Vacanti J.P. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003;24:2077–2082. doi: 10.1016/s0142-9612(02)00635-x. [DOI] [PubMed] [Google Scholar]
- 228.Sill T.J., von Recum H.A. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials. 2008;29:1989–2006. doi: 10.1016/j.biomaterials.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 229.Carvalho M.S., Silva J.C., Udangawa R.N., Cabral J.M.S., Ferreira F.C., da Silva C.L., Linhardt R.J., Vashishth D. Co-culture cell-derived extracellular matrix loaded electrospun microfibrous scaffolds for bone tissue engineering. Mater.Sci.Eng.C.Mater.Biol.Appl. 2019;99:479–490. doi: 10.1016/j.msec.2019.01.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hong Y., Huber A., Takanari K., Amoroso N.J., Hashizume R., Badylak S.F., Wagner W.R. Mechanical properties and in vivo behavior of a biodegradable synthetic polymer microfiber-extracellular matrix hydrogel biohybrid scaffold. Biomaterials. 2011;32:3387–3394. doi: 10.1016/j.biomaterials.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bracaglia L.G., Fisher J.P. Extracellular matrix-based biohybrid materials for engineering compliant, matrix-dense tissues. Adv.Healthc.Mater. 2015;4:2475–2487. doi: 10.1002/adhm.201500236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Thibault R.A., Scott Baggett L., Mikos A.G., Kasper F.K. Osteogenic differentiation of mesenchymal stem cells on pregenerated extracellular matrix scaffolds in the absence of osteogenic cell culture supplements. Tissue Eng. 2010;16:431–440. doi: 10.1089/ten.TEA.2009.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Datta N., Holtorf H.L., Sikavitsas V.I., Jansen J.A., Mikos A.G. Effect of bone extracellular matrix synthesized in vitro on the osteoblastic differentiation of marrow stromal cells. Biomaterials. 2005;26:971–977. doi: 10.1016/j.biomaterials.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 234.Hollister S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005;4:518–524. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 235.Markstedt K., Mantas A., Tournier I., Martínez Ávila H., Hägg D., Gatenholm P. 3D bioprinting human chondrocytes with nanocellulose-alginate bioink for cartilage tissue engineering applications. Biomacromolecules. 2015;16:1489–1496. doi: 10.1021/acs.biomac.5b00188. [DOI] [PubMed] [Google Scholar]
- 236.Jang J., Kim T.G., Kim B.S., Kim S.W., Kwon S.M., Cho D.W. Tailoring mechanical properties of decellularized extracellular matrix bioink by vitamin B2-induced photo-crosslinking. Acta Biomater. 2016;33:88–95. doi: 10.1016/j.actbio.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 237.Zhang X., Liu Y., Luo C., Zhai C., Li Z., Zhang Y., Yuan T., Dong S., Zhang J., Fan W. Crosslinker-free silk/decellularized extracellular matrix porous bioink for 3D bioprinting-based cartilage tissue engineering. Mater.Sci.Eng.C.Mater.Biol.Appl. 2021;118 doi: 10.1016/j.msec.2020.111388. [DOI] [PubMed] [Google Scholar]
- 238.Zhang W., Zhu Y., Li J., Guo Q., Peng J., Liu S., Yang J., Wang Y. Cell-derived extracellular matrix: basic characteristics and current applications in orthopedic tissue engineering. Tissue Eng. B Rev. 2016;22:193–207. doi: 10.1089/ten.TEB.2015.0290. [DOI] [PubMed] [Google Scholar]
- 239.Lai Y., Sun Y., Skinner C.M., Son E.L., Lu Z., Tuan R.S., Jilka R.L., Ling J., Chen X.D. Reconstitution of marrow-derived extracellular matrix ex vivo: a robust culture system for expanding large-scale highly functional human mesenchymal stem cells. Stem Cell. Dev. 2010;19:1095–1107. doi: 10.1089/scd.2009.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Chen X.D., Dusevich V., Feng J.Q., Manolagas S.C., Jilka R.L. Extracellular matrix made by bone marrow cells facilitates expansion of marrow-derived mesenchymal progenitor cells and prevents their differentiation into osteoblasts. J. Bone Miner. Res. 2007;22:1943–1956. doi: 10.1359/jbmr.070725. [DOI] [PubMed] [Google Scholar]
- 241.Antebi B., Zhang Z., Wang Y., Lu Z., Chen X.D., Ling J. Stromal-cell-derived extracellular matrix promotes the proliferation and retains the osteogenic differentiation capacity of mesenchymal stem cells on three-dimensional scaffolds. Tissue Eng. C Methods. 2015;21:171–181. doi: 10.1089/ten.TEC.2014.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kim I.G., Hwang M.P., Du P., Ko J., Ha C.W., Do S.H., Park K. Bioactive cell-derived matrices combined with polymer mesh scaffold for osteogenesis and bone healing. Biomaterials. 2015;50:75–86. doi: 10.1016/j.biomaterials.2015.01.054. [DOI] [PubMed] [Google Scholar]
- 243.Kim B.S., Kwon Y.W., Kong J.S., Park G.T., Gao G., Han W., Kim M.B., Lee H., Kim J.H., Cho D.W. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: a step towards advanced skin tissue engineering. Biomaterials. 2018;168:38–53. doi: 10.1016/j.biomaterials.2018.03.040. [DOI] [PubMed] [Google Scholar]
- 244.Clark R.A., Ghosh K., Tonnesen M.G. Tissue engineering for cutaneous wounds. J. Invest. Dermatol. 2007;127:1018–1029. doi: 10.1038/sj.jid.5700715. [DOI] [PubMed] [Google Scholar]
- 245.Gurtner G.C., Werner S., Barrandon Y., Longaker M.T. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 246.Kuna V.K., Padma A.M., Håkansson J., Nygren J., Sjöback R., Petronis S., Sumitran-Holgersson S. Significantly accelerated wound healing of full-thickness skin using a novel composite gel of porcine acellular dermal matrix and human peripheral blood cells. Cell Transplant. 2017;26:293–307. doi: 10.3727/096368916x692690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.He P., Zhao J., Zhang J., Li B., Gou Z., Gou M., Li X. Bioprinting of skin constructs for wound healing. Burns.Trauma. 2018;6:5. doi: 10.1186/s41038-017-0104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Brown M., Li J., Moraes C., Tabrizian M., Li-Jessen N.Y.K. Decellularized extracellular matrix: new promising and challenging biomaterials for regenerative medicine. Biomaterials. 2022;289 doi: 10.1016/j.biomaterials.2022.121786. [DOI] [PubMed] [Google Scholar]
- 249.Guan G., Zhang Q., Jiang Z., Liu J., Wan J., Jin P., Lv Q. Multifunctional Silk Fibroin Methacryloyl Microneedle for Diabetic Wou nd Healing. Small. 2022;18 doi: 10.1002/smll.20220306. [DOI] [PubMed] [Google Scholar]
- 250.Lei L., Wang X., Zhu Y., Su W., Lv Q., Li D. Antimicrobial hydrogel microspheres for protein capture and wound healing. Mater. Des. 2022;215:110478. doi: 10.1016/j.matdes.2022.110478. [DOI] [Google Scholar]
- 251.Yang L., Sun L., Zhang H., Bian F., Zhao Y. Ice-Inspired Lubricated Drug Delivery Particles from Microfluidic Elec trospray for Osteoarthritis Treatment. ACS. Nano. 2021;15:20600–20606. doi: 10.1021/acsnano.1c09325. [DOI] [PubMed] [Google Scholar]
- 252.Lei L., Zhu Y., Qin X., Chai S., Liu G., Su W., Lv Q., Li D. Magnetic biohybrid microspheres for protein purification and chronic w ound healing in diabetic mice. Chem. Eng. J. 2021;425:130671. doi: 10.1016/j.cej.2021.130671. [DOI] [Google Scholar]
- 253.Guan G., Qizhuang L., Liu S., Jiang Z., Zhou C., Liao W. 3D-bioprinted peptide coupling patches for wound healing. Mater. Today Biol. 2022;13:100188. doi: 10.1016/j.mtbio.2021.100188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.García-Gareta E. Introduction to biomaterials for skin repair and regeneration. Biomater. Skin Repair Regen. Woodhead Publishing. 2019:8–27. doi: 10.1016/B978-0-08-102546-8.02001-X. [DOI] [Google Scholar]
- 255.Hsieh C.M., Wang W., Chen Y.H., Wei P.S., Liu Y.H., Sheu M.T., Ho H.O. A novel composite hydrogel composed of formic acid-decellularized pepsin-soluble extracellular matrix hydrogel and sacchachitin hydrogel as wound dressing to synergistically accelerate diabetic wound healing. Pharmaceutics. 2020;12 doi: 10.3390/pharmaceutics12060538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Morris A.H., Lee H., Xing H., Stamer D.K., Tan M., Kyriakides T.R. Tunable hydrogels derived from genetically engineered extracellular matrix accelerate diabetic wound healing. ACS Appl. Mater. Interfaces. 2018;10:41892–41901. doi: 10.1021/acsami.8b08920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wang C., Li G., Cui K., Chai Z., Huang Z., Liu Y., Chen S., Huang H., Zhang K., Han Z., Li Y., Yu G., Han Z.C., Liu N., Li Z. Sulfated glycosaminoglycans in decellularized placenta matrix as critical regulators for cutaneous wound healing. Acta Biomater. 2021;122:199–210. doi: 10.1016/j.actbio.2020.12.055. [DOI] [PubMed] [Google Scholar]
- 258.Choi J.S., Kim J.D., Yoon H.S., Cho Y.W. Full-thickness skin wound healing using human placenta-derived extracellular matrix containing bioactive molecules. Tissue Eng. 2013;19:329–339. doi: 10.1089/ten.TEA.2011.0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Su Z., Ma H., Wu Z., Zeng H., Li Z., Wang Y., Liu G., Xu B., Lin Y., Zhang P., Wei X. Enhancement of skin wound healing with decellularized scaffolds loaded with hyaluronic acid and epidermal growth factor. Mater.Sci.Eng.C.Mater.Biol.Appl. 2014;44:440–448. doi: 10.1016/j.msec.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 260.Du P., Casavitri C., Suhaeri M., Wang P.Y., Lee J.H., Koh W.G., Park K. A fibrous hybrid patch couples cell-derived matrix and poly(l-lactide-co-caprolactone) for endothelial cells delivery and skin wound repair. ACS Biomater. Sci. Eng. 2019;5:900–910. doi: 10.1021/acsbiomaterials.8b01118. [DOI] [PubMed] [Google Scholar]
- 261.Tang K.C., Yang K.C., Lin C.W., Chen Y.K., Lu T.Y., Chen H.Y., Cheng N.C., Yu J. Human adipose-derived stem cell secreted extracellular matrix incorporated into electrospun poly(lactic-co-glycolic acid) nanofibrous dressing for enhancing wound healing. Polymers. 2019;11 doi: 10.3390/polym11101609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Jang K.S., Park S.J., Choi J.J., Kim H.N., Shim K.M., Kim M.J., Jang I.H., Jin S.W., Kang S.S., Kim S.E., Moon S.H. Therapeutic efficacy of artificial skin produced by 3D bioprinting. Materials. 2021;14:5177. doi: 10.3390/ma14185177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Laflamme M.A., Murry C.E. Heart regeneration. Nature. 2011;473:326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Wang Z., Long D.W., Huang Y., Chen W.C.W., Kim K., Wang Y. Decellularized neonatal cardiac extracellular matrix prevents widespread ventricular remodeling in adult mammals after myocardial infarction. Acta Biomater. 2019;87:140–151. doi: 10.1016/j.actbio.2019.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Boroumand S., Asadpour S., Akbarzadeh A., Faridi-Majidi R., Ghanbari H. Heart valve tissue engineering: an overview of heart valve decellularization processes. Regen. Med. 2018;13:41–54. doi: 10.2217/rme-2017-0061. [DOI] [PubMed] [Google Scholar]
- 266.Spang M.T., Christman K.L. Extracellular matrix hydrogel therapies: in vivo applications and development. Acta Biomater. 2018;68:1–14. doi: 10.1016/j.actbio.2017.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Bejleri D., Davis M.E. Decellularized extracellular matrix materials for cardiac repair and regeneration. Adv.Healthc.Mater. 2019;8 doi: 10.1002/adhm.201801217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Nguyen D.T., O'Hara M., Graneli C., Hicks R., Miliotis T., Nyström A.C., Hansson S., Davidsson P., Gan L.M., Magnone M.C., Althage M., Heydarkhan-Hagvall S. Humanizing miniature hearts through 4-flow cannulation perfusion decellularization and recellularization. Sci. Rep. 2018;8:7458. doi: 10.1038/s41598-018-25883-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Bäcker H., Polgár L., Soós P., Lajkó E., Láng O., Merkely B., Szabó G., Dohmen P.M., Weymann A., Kőhidai L. Impedimetric analysis of the effect of decellularized porcine heart scaffold on human fibrosarcoma, endothelial, and cardiomyocyte cell lines. Med.Sci.Monit. 2017;23:2232–2240. doi: 10.12659/msm.901527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Bai R., Tian L., Li Y., Zhang J., Wei Y., Jin Z., Liu Z., Liu H. Combining ECM hydrogels of cardiac bioactivity with stem cells of high cardiomyogenic potential for myocardial repair. Stem Cell. Int. 2019;(2019) doi: 10.1155/2019/6708435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Rajabi S., Pahlavan S., Ashtiani M.K., Ansari H., Abbasalizadeh S., Sayahpour F.A., Varzideh F., Kostin S., Aghdami N., Braun T., Baharvand H. Human embryonic stem cell-derived cardiovascular progenitor cells efficiently colonize in bFGF-tethered natural matrix to construct contracting humanized rat hearts. Biomaterials. 2018;154:99–112. doi: 10.1016/j.biomaterials.2017.10.054. [DOI] [PubMed] [Google Scholar]
- 272.Kc P., Shah M., Liao J., Zhang G. Prevascularization of decellularized porcine myocardial slice for cardiac tissue engineering. ACS Appl. Mater. Interfaces. 2017;9:2196–2204. doi: 10.1021/acsami.6b15291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Methe K., Bäckdahl H., Johansson B.R., Nayakawde N., Dellgren G., Sumitran-Holgersson S. An alternative approach to decellularized whole porcine heart. Biores.Open.Access. 2014;3:327–338. doi: 10.1089/biores.2014.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Remlinger N.T., Wearden P.D., Gilbert T.W. J.Vis.Exp.; 2012. Procedure for Decellularization of Porcine Heart by Retrograde Coronary Perfusion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Lee P.F., Chau E., Cabello R., Yeh A.T., Sampaio L.C., Gobin A.S., Taylor D.A. Inverted orientation improves decellularization of whole porcine hearts. Acta Biomater. 2017;49:181–191. doi: 10.1016/j.actbio.2016.11.047. [DOI] [PubMed] [Google Scholar]
- 276.Wainwright J.M., Czajka C.A., Patel U.B., Freytes D.O., Tobita K., Gilbert T.W., Badylak S.F. Preparation of cardiac extracellular matrix from an intact porcine heart. Tissue Eng. C Methods. 2010;16:525–532. doi: 10.1089/ten.TEC.2009.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Wainwright J.M., Hashizume R., Fujimoto K.L., Remlinger N.T., Pesyna C., Wagner W.R., Tobita K., Gilbert T.W., Badylak S.F. Right ventricular outflow tract repair with a cardiac biologic scaffold. Cells Tissues Organs. 2012;195:159–170. doi: 10.1159/000331400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Wang B., Borazjani A., Tahai M., Curry A.L., Simionescu D.T., Guan J., To F., Elder S.H., Liao J. Fabrication of cardiac patch with decellularized porcine myocardial scaffold and bone marrow mononuclear cells. J.Biomed.Mater.Res.A. 2010;94:1100–1110. doi: 10.1002/jbm.a.32781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Ren T., Faust A., van der Merwe Y., Xiao B., Johnson S., Kandakatla A., Gorantla V.S., Badylak S.F., Washington K.M., Steketee M.B. Fetal extracellular matrix nerve wraps locally improve peripheral nerve remodeling after complete transection and direct repair in rat. Sci. Rep. 2018;8:4474. doi: 10.1038/s41598-018-22628-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Johnson T.D., Lin S.Y., Christman K.L. Tailoring material properties of a nanofibrous extracellular matrix derived hydrogel. Nanotechnology. 2011;22 doi: 10.1088/0957-4484/22/49/494015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Wassenaar J.W., Braden R.L., Osborn K.G., Christman K.L. Modulating in vivo degradation rate of injectable extracellular matrix hydrogels. J.Mater.Chem.B. 2016;4:2794–2802. doi: 10.1039/c5tb02564h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Singelyn J.M., Christman K.L. Modulation of material properties of a decellularized myocardial matrix scaffold. Macromol. Biosci. 2011;11:731–738. doi: 10.1002/mabi.201000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Grover G.N., Rao N., Christman K.L. Myocardial matrix-polyethylene glycol hybrid hydrogels for tissue engineering. Nanotechnology. 2014;25 doi: 10.1088/0957-4484/25/1/014011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Buckenmeyer M.J., Meder T.J., Prest T.A., Brown B.N. Decellularization techniques and their applications for the repair and regeneration of the nervous system. Methods. 2020;171:41–61. doi: 10.1016/j.ymeth.2019.07.023. [DOI] [PubMed] [Google Scholar]
- 285.Chen S., Du Z., Zou J., Qiu S., Rao Z., Liu S., Sun X., Xu Y., Zhu Q., Liu X., Mao H.Q., Bai Y., Quan D. Promoting neurite growth and Schwann cell migration by the harnessing decellularized nerve matrix onto nanofibrous guidance. ACS Appl. Mater. Interfaces. 2019;11:17167–17176. doi: 10.1021/acsami.9b01066. [DOI] [PubMed] [Google Scholar]
- 286.Viswanath A., Vanacker J., Germain L., Leprince J.G., Diogenes A., Shakesheff K.M., White L.J., des Rieux A. Extracellular matrix-derived hydrogels for dental stem cell delivery. J.Biomed.Mater.Res.A. 2017;105:319–328. doi: 10.1002/jbm.a.35901. [DOI] [PubMed] [Google Scholar]
- 287.Kočí Z., Výborný K., Dubišová J., Vacková I., Jäger A., Lunov O., Jiráková K., Kubinová Š. Extracellular matrix hydrogel derived from human umbilical cord as a scaffold for neural tissue repair and its comparison with extracellular matrix from porcine tissues. Tissue Eng. C Methods. 2017;23:333–345. doi: 10.1089/ten.TEC.2017.0089. [DOI] [PubMed] [Google Scholar]
- 288.Harris G.M., Madigan N.N., Lancaster K.Z., Enquist L.W., Windebank A.J., Schwartz J., Schwarzbauer J.E. Nerve guidance by a decellularized fibroblast extracellular matrix. Matrix Biol. 2017;60–61:176–189. doi: 10.1016/j.matbio.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Zhao B., Sun X., Li X., Yang Q., Li Y., Zhang Y., Li B., Ma X. Improved preparation of acellular nerve scaffold and application of PKH26 fluorescent labeling combined with in vivo fluorescent imaging system in nerve tissue engineering. Neurosci. Lett. 2013;556:52–57. doi: 10.1016/j.neulet.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 290.Heng B.C., Gong T., Wang S., Lim L.W., Wu W., Zhang C. Decellularized matrix derived from neural differentiation of embryonic stem cells enhances the neurogenic potential of dental follicle stem cells. J. Endod. 2017;43:409–416. doi: 10.1016/j.joen.2016.10.033. [DOI] [PubMed] [Google Scholar]
- 291.Baiguera S., Del Gaudio C., Lucatelli E., Kuevda E., Boieri M., Mazzanti B., Bianco A., Macchiarini P. Electrospun gelatin scaffolds incorporating rat decellularized brain extracellular matrix for neural tissue engineering. Biomaterials. 2014;35:1205–1214. doi: 10.1016/j.biomaterials.2013.10.060. [DOI] [PubMed] [Google Scholar]
- 292.Chen C.C., Yu J., Ng H.Y., Lee A.K., Chen C.C., Chen Y.S., Shie M.Y. The physicochemical properties of decellularized extracellular matrix-coated 3D printed poly(ε-caprolactone) nerve conduits for promoting Schwann cells proliferation and differentiation. Materials. 2018;11:1665. doi: 10.3390/ma11091665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Prest T.A., Yeager E., LoPresti S.T., Zygelyte E., Martin M.J., Dong L., Gibson A., Olutoye O.O., Brown B.N., Cheetham J. Nerve-specific, xenogeneic extracellular matrix hydrogel promotes recovery following peripheral nerve injury. J.Biomed.Mater.Res.A. 2018;106:450–459. doi: 10.1002/jbm.a.36235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Gu Y., Zhu J., Xue C., Li Z., Ding F., Yang Y., Gu X. Chitosan/silk fibroin-based, Schwann cell-derived extracellular matrix-modified scaffolds for bridging rat sciatic nerve gaps. Biomaterials. 2014;35:2253–2263. doi: 10.1016/j.biomaterials.2013.11.087. [DOI] [PubMed] [Google Scholar]
- 295.Guo S.Z., Ren X.J., Wu B., Jiang T. Preparation of the acellular scaffold of the spinal cord and the study of biocompatibility. Spinal Cord. 2010;48:576–581. doi: 10.1038/sc.2009.170. [DOI] [PubMed] [Google Scholar]
- 296.Ribatti D., Conconi M.T., Nico B., Baiguera S., Corsi P., Parnigotto P.P., Nussdorfer G.G. Angiogenic response induced by acellular brain scaffolds grafted onto the chick embryo chorioallantoic membrane. Brain Res. 2003;989:9–15. doi: 10.1016/s0006-8993(03)03225-6. [DOI] [PubMed] [Google Scholar]
- 297.Zou J.-L., Liu S., Sun J.-H., Yang W.-H., Xu Y.-W., Rao Z.-L., Jiang B., Zhu Q.-T., Liu X.-L., Wu J.-L., Chang C., Mao H.-Q., Ling E.-A., Quan D.-P., Zeng Y.-S. Peripheral Nerve-Derived Matrix Hydrogel Promotes Remyelination and In hibits Synapse Formation. Adv. Funct. Mater. 2018;28:1705739. doi: 10.1002/adfm.201705739. [DOI] [Google Scholar]
- 298.Xu H.L., Tian F.R., Xiao J., Chen P.P., Xu J., Fan Z.L., Yang J.J., Lu C.T., Zhao Y.Z. Sustained-release of FGF-2 from a hybrid hydrogel of heparin-poloxamer and decellular matrix promotes the neuroprotective effects of proteins after spinal injury. Int.J.Nanomedicine. 2018;13:681–694. doi: 10.2147/ijn.S152246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Yi H.G., Jeong Y.H., Kim Y., Choi Y.J., Moon H.E., Park S.H., Kang K.S., Bae M., Jang J., Youn H., Paek S.H., Cho D.W. A bioprinted human-glioblastoma-on-a-chip for the identification of patient-specific responses to chemoradiotherapy. Nat.Biomed.Eng. 2019;3:509–519. doi: 10.1038/s41551-019-0363-x. [DOI] [PubMed] [Google Scholar]
- 300.Wu Y., Wang J., Shi Y., Pu H., Leak R.K., Liou A.K.F., Badylak S.F., Liu Z., Zhang J., Chen J., Chen L. Implantation of brain-derived extracellular matrix enhances neurological recovery after traumatic brain injury. Cell Transplant. 2017;26:1224–1234. doi: 10.1177/0963689717714090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Madhusudanan P., Raju G., Shankarappa S. Hydrogel systems and their role in neural tissue engineering. J. R. Soc. Interface. 2020;17 doi: 10.1098/rsif.2019.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.De Santis M.M., Bölükbas D.A., Lindstedt S., Wagner D.E. How to build a lung: latest advances and emerging themes in lung bioengineering. Eur. Respir. J. 2018;52 doi: 10.1183/13993003.01355-2016. [DOI] [PubMed] [Google Scholar]
- 303.Skolasinski S.D., Panoskaltsis-Mortari A. Lung tissue bioengineering for chronic obstructive pulmonary disease: overcoming the need for lung transplantation from human donors. Expet Rev. Respir. Med. 2019;13:665–678. doi: 10.1080/17476348.2019.1624163. [DOI] [PubMed] [Google Scholar]
- 304.Moztarzadeh S., Mottaghy K., Sefat F., Samadikuchaksaraei A., Mozafari M. Chapter 13 - Nanoeng. Biomater. Lung Regen. 2019:305–323. doi: 10.1016/B978-0-12-813355-2.00013-2. [DOI] [Google Scholar]
- 305.Ott H.C., Clippinger B., Conrad C., Schuetz C., Pomerantseva I., Ikonomou L., Kotton D., Vacanti J.P. Regeneration and orthotopic transplantation of a bioartificial lung. Nat. Med. 2010;16:927–933. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 306.Song J.J., Kim S.S., Liu Z., Madsen J.C., Mathisen D.J., Vacanti J.P., Ott H.C. Enhanced in vivo function of bioartificial lungs in rats. Ann. Thorac. Surg. 2011;92:998–1005. doi: 10.1016/j.athoracsur.2011.05.018. ; discussion 1005-1006. [DOI] [PubMed] [Google Scholar]
- 307.Petersen T.H., Calle E.A., Colehour M.B., Niklason L.E. Bioreactor for the long-term culture of lung tissue. Cell Transplant. 2011;20:1117–1126. doi: 10.3727/096368910x544933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Zhou H., Kitano K., Ren X., Rajab T.K., Wu M., Gilpin S.E., Wu T., Baugh L., Black L.D., Mathisen D.J., Ott H.C. Bioengineering human lung grafts on porcine matrix. Ann. Surg. 2018;267:590–598. doi: 10.1097/sla.0000000000002129. [DOI] [PubMed] [Google Scholar]
- 309.De Santis M.M., Alsafadi H.N., Tas S., Bölükbas D.A., Prithiviraj S., Da Silva I.A.N., Mittendorfer M., Ota C., Stegmayr J., Daoud F., Königshoff M., Swärd K., Wood J.A., Tassieri M., Bourgine P.E., Lindstedt S., Mohlin S., Wagner D.E. Extracellular-matrix-reinforced bioinks for 3D bioprinting human tissue. Adv. Mater. 2021;33 doi: 10.1002/adma.202005476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Price A.P., England K.A., Matson A.M., Blazar B.R., Panoskaltsis-Mortari A. Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded, Tissue. Eng.Part A. 2010;16:2581–2591. doi: 10.1089/ten.TEA.2009.0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Price A.P., Godin L.M., Domek A., Cotter T., D'Cunha J., Taylor D.A., Panoskaltsis-Mortari A. Automated decellularization of intact, human-sized lungs for tissue engineering, Tissue. Eng.Part C Methods. 2015;21:94–103. doi: 10.1089/ten.TEC.2013.0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Doryab A., Schmid O. Bioactive cell-derived ECM scaffold forms a unique cellular microenvironment for lung tissue engineering. Biomedicines. 2022;10 doi: 10.3390/biomedicines10081791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Okamoto T., Tang X., Janocha A., Farver C.F., Gladwin M.T., McCurry K.R. Nebulized nitrite protects rat lung grafts from ischemia reperfusion injury. J. Thorac. Cardiovasc. Surg. 2013;145:1108–1116. doi: 10.1016/j.jtcvs.2012.04.006. e1101. [DOI] [PubMed] [Google Scholar]
- 314.Liu F., Li W., Pauluhn J., Trübel H., Wang C. Lipopolysaccharide-induced acute lung injury in rats: comparative assessment of intratracheal instillation and aerosol inhalation. Toxicology. 2013;304:158–166. doi: 10.1016/j.tox.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 315.Strüber M., Fischer S., Niedermeyer J., Warnecke G., Gohrbandt B., Görler A., Simon A.R., Haverich A., Hohlfeld J.M. Effects of exogenous surfactant instillation in clinical lung transplantation: a prospective, randomized trial. J. Thorac. Cardiovasc. Surg. 2007;133:1620–1625. doi: 10.1016/j.jtcvs.2006.12.057. [DOI] [PubMed] [Google Scholar]
- 316.Wu J., Ravikumar P., Nguyen K.T., Hsia C.C., Hong Y. Lung protection by inhalation of exogenous solubilized extracellular matrix. PLoS One. 2017;12 doi: 10.1371/journal.pone.0171165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Manni M.L., Czajka C.A., Oury T.D., Gilbert T.W. Extracellular matrix powder protects against bleomycin-induced pulmonary fibrosis. Tissue Eng. 2011;17:2795–2804. doi: 10.1089/ten.tea.2011.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Uygun B.E., Soto-Gutierrez A., Yagi H., Izamis M.L., Guzzardi M.A., Shulman C., Milwid J., Kobayashi N., Tilles A., Berthiaume F., Hertl M., Nahmias Y., Yarmush M.L., Uygun K. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat. Med. 2010;16:814–820. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Baptista P.M., Siddiqui M.M., Lozier G., Rodriguez S.R., Atala A., Soker S. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology. 2011;53:604–617. doi: 10.1002/hep.24067. [DOI] [PubMed] [Google Scholar]
- 320.Mazza G., Rombouts K., Rennie Hall A., Urbani L., Vinh Luong T., Al-Akkad W., Longato L., Brown D., Maghsoudlou P., Dhillon A.P., Fuller B., Davidson B., Moore K., Dhar D., De Coppi P., Malago M., Pinzani M. Decellularized human liver as a natural 3D-scaffold for liver bioengineering and transplantation. Sci. Rep. 2015;5 doi: 10.1038/srep13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Lee H., Han W., Kim H., Ha D.H., Jang J., Kim B.S., Cho D.W. Development of liver decellularized extracellular matrix bioink for three-dimensional cell printing-based liver tissue engineering. Biomacromolecules. 2017;18:1229–1237. doi: 10.1021/acs.biomac.6b01908. [DOI] [PubMed] [Google Scholar]
- 322.Agarwal T., Narayan R., Maji S., Ghosh S.K., Maiti T.K. Decellularized caprine liver extracellular matrix as a 2D substrate co ating and 3D hydrogel platform for vascularized liver tissue engineering. J. Tissue Eng. Regen. Med. 2017;12:e1678–e1690. doi: 10.1002/term.2594. [DOI] [PubMed] [Google Scholar]
- 323.Ma X., Yu C., Wang P., Xu W., Wan X., Lai C.S.E., Liu J., Koroleva-Maharajh A., Chen S. Rapid 3D bioprinting of decellularized extracellular matrix with regionally varied mechanical properties and biomimetic microarchitecture. Biomaterials. 2018;185:310–321. doi: 10.1016/j.biomaterials.2018.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Mao Q., Wang Y., Li Y., Juengpanich S., Li W., Chen M., Yin J., Fu J., Cai X. Fabrication of liver microtissue with liver decellularized extracellular matrix (dECM) bioink by digital light processing (DLP) bioprinting. Mater.Sci.Eng.C.Mater.Biol.Appl. 2020;109 doi: 10.1016/j.msec.2020.110625. [DOI] [PubMed] [Google Scholar]
- 325.Montserrat N., Garreta E., Izpisua Belmonte J.C. Regenerative strategies for kidney engineering. FEBS J. 2016;283:3303–3324. doi: 10.1111/febs.13704. [DOI] [PubMed] [Google Scholar]
- 326.Petrosyan A., Zanusso I., Lavarreda-Pearce M., Leslie S., Sedrakyan S., De Filippo R.E., Orlando G., Da Sacco S., Perin L. Decellularized renal matrix and regenerative medicine of the kidney: a different point of view. Tissue Eng. B Rev. 2016;22:183–192. doi: 10.1089/ten.TEB.2015.0368. [DOI] [PubMed] [Google Scholar]
- 327.Zambon J.P., Ko I.K., Abolbashari M., Huling J., Clouse C., Kim T.H., Smith C., Atala A., Yoo J.J. Comparative analysis of two porcine kidney decellularization methods for maintenance of functional vascular architectures. Acta Biomater. 2018;75:226–234. doi: 10.1016/j.actbio.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 328.Zheng C.X., Sui B.D., Hu C.H., Qiu X.Y., Zhao P., Jin Y. Reconstruction of structure and function in tissue engineering of solid organs: toward simulation of natural development based on decellularization. J.Tissue.Eng.Regen.Med. 2018;12:1432–1447. doi: 10.1002/term.2676. [DOI] [PubMed] [Google Scholar]
- 329.Hussein K.H., Saleh T., Ahmed E., Kwak H.H., Park K.M., Yang S.R., Kang B.J., Choi K.Y., Kang K.S., Woo H.M. Biocompatibility and hemocompatibility of efficiently decellularized whole porcine kidney for tissue engineering. J.Biomed.Mater.Res.A. 2018;106:2034–2047. doi: 10.1002/jbm.a.36407. [DOI] [PubMed] [Google Scholar]
- 330.He M., Callanan A., Lagaras K., Steele J.A.M., Stevens M.M. Optimization of SDS exposure on preservation of ECM characteristics in whole organ decellularization of rat kidneys. J. Biomed. Mater. Res. B Appl. Biomater. 2017;105:1352–1360. doi: 10.1002/jbm.b.33668. [DOI] [PubMed] [Google Scholar]
- 331.Ross E.A., Abrahamson D.R., St John P., Clapp W.L., Williams M.J., Terada N., Hamazaki T., Ellison G.W., Batich C.D. Mouse stem cells seeded into decellularized rat kidney scaffolds endothelialize and remodel basement membranes. Organogenesis. 2012;8:49–55. doi: 10.4161/org.20209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Song J.J., Guyette J.P., Gilpin S.E., Gonzalez G., Vacanti J.P., Ott H.C. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat. Med. 2013;19:646–651. doi: 10.1038/nm.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Hu D., Zhang D., Liu B., Zhou Y., Yu Y., Shen L., Long C., Liu X., Lin T., He D., Wei G. [Optimization of preparation of rat kidney decellularized scaffold by combining freeze-thawing with perfusion] Sheng Wu Gong Cheng Xue Bao. 2019;35:307–318. doi: 10.13345/j.cjb.180272. [DOI] [PubMed] [Google Scholar]
- 334.Nakayama K.H., Lee C.C., Batchelder C.A., Tarantal A.F. Tissue specificity of decellularized rhesus monkey kidney and lung scaffolds. PLoS One. 2013;8 doi: 10.1371/journal.pone.0064134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Xue A., Niu G., Chen Y., Li K., Xiao Z., Luan Y., Sun C., Xie X., Zhang D., Du X., Kong F., Guo Y., Zhang H., Cheng G., Xin Q., Guan Y., Zhao S. Recellularization of well-preserved decellularized kidney scaffold using adipose tissue-derived stem cells. J.Biomed.Mater.Res.A. 2018;106:805–814. doi: 10.1002/jbm.a.36279. [DOI] [PubMed] [Google Scholar]
- 336.Satyam A., Tsokos M.G., Tresback J.S., Zeugolis D.I., Tsokos G.C. Cell derived extracellular matrix-rich biomimetic substrate supports podocyte proliferation, differentiation and maintenance of native phenotype. Adv. Funct. Mater. 2020;30 doi: 10.1002/adfm.201908752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Singh N.K., Han W., Nam S.A., Kim J.W., Kim J.Y., Kim Y.K., Cho D.W. Three-dimensional cell-printing of advanced renal tubular tissue analogue. Biomaterials. 2020;232 doi: 10.1016/j.biomaterials.2019.119734. [DOI] [PubMed] [Google Scholar]
- 338.Gan L., Duan H., Xu Q., Tang Y.Q., Li J.J., Sun F.Q., Wang S. Human amniotic mesenchymal stromal cell transplantation improves endometrial regeneration in rodent models of intrauterine adhesions. Cytotherapy. 2017;19:603–616. doi: 10.1016/j.jcyt.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 339.Wang X., Wu D., Li W., Yang L. Emerging biomaterials for reproductive medicine. Eng. Regen. 2021;2:230–245. doi: 10.1016/j.engreg.2021.11.006. [DOI] [Google Scholar]
- 340.Wall A., Testa G. Living donation, listing, and prioritization in uterus transplantation. Am. J. Bioeth. 2018;18:20–22. doi: 10.1080/15265161.2018.1478026. [DOI] [PubMed] [Google Scholar]
- 341.Brännström M. Current status and future direction of uterus transplantation. Curr. Opin. Organ Transplant. 2018;23:592–597. doi: 10.1097/mot.0000000000000568. [DOI] [PubMed] [Google Scholar]
- 342.Lei L., Lv Q., Jin Y., An H., Shi Z., Hu G., Yang Y., Wang X., Yang L. Angiogenic Microspheres for the Treatment of a Thin Endometrium. ACS Biomater. Sci. Eng. 2021;7:4914–4920. doi: 10.1021/acsbiomaterials.1c00615. [DOI] [PubMed] [Google Scholar]
- 343.Alshaikh A.B., Padma A.M., Dehlin M., Akouri R., Song M.J., Brännström M., Hellström M. Decellularization and recellularization of the ovary for bioengineering applications; studies in the mouse. Reprod. Biol. Endocrinol. 2020;18:75. doi: 10.1186/s12958-020-00630-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Pors S.E., Ramløse M., Nikiforov D., Lundsgaard K., Cheng J., Andersen C.Y., Kristensen S.G. Initial steps in reconstruction of the human ovary: survival of pre-antral stage follicles in a decellularized human ovarian scaffold. Hum.Reprod. 2019;34:1523–1535. doi: 10.1093/humrep/dez077. [DOI] [PubMed] [Google Scholar]
- 345.Hellström M., Bandstein S., Brännström M. Uterine tissue engineering and the future of uterus transplantation. Ann. Biomed. Eng. 2017;45:1718–1730. doi: 10.1007/s10439-016-1776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Campo H., López-Martínez S., Cervelló I. Decellularization methods of uterus in tissue engineering. Adv. Exp. Med. Biol. 2021;1345:141–152. doi: 10.1007/978-3-030-82735-9_12. [DOI] [PubMed] [Google Scholar]
- 347.Campo H., García-Domínguez X., López-Martínez S., Faus A., Vicente Antón J.S., Marco-Jiménez F., Cervelló I. Tissue-specific decellularized endometrial substratum mimicking different physiological conditions influences in vitro embryo development in a rabbit model. Acta Biomater. 2019;89:126–138. doi: 10.1016/j.actbio.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 348.Chen X., Zhou Y., Sun Y., Ji T., Dai H. Transplantation of decellularized and lyophilized amniotic membrane inhibits endometrial fibrosis by regulating connective tissue growth factor and tissue inhibitor of matrix metalloproteinase-2. Exp. Ther. Med. 2021;22:968. doi: 10.3892/etm.2021.10400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Zhang H., Zhang Q., Zhang J., Sheng F., Wu S., Yang F., Li W. Urinary bladder matrix scaffolds improve endometrial regeneration in a rat model of intrauterine adhesions. Biomater. Sci. 2020;8:988–996. doi: 10.1039/c9bm00651f. [DOI] [PubMed] [Google Scholar]
- 350.Yao, Q., Zheng, Y.-W., Lin, H.-L., Lan, Q.-H., Huang, Z.-W., Wang, L.-F., Chen, R., Xiao, J., Kou, L., Xu, H.-L., Zhao, Y.-Z., Exploiting crosslinked decellularized matrix to achieve uterus regener ation and construction, Artif. Cell Nanomed. Biotechnol. 48 218-229. 10.1080/21691401.2019.1699828. [DOI] [PubMed]
- 351.Zhang J., Hu Z.Q., Turner N.J., Teng S.F., Cheng W.Y., Zhou H.Y., Zhang L., Hu H.W., Wang Q., Badylak S.F. Perfusion-decellularized skeletal muscle as a three-dimensional scaffold with a vascular network template. Biomaterials. 2016;89:114–126. doi: 10.1016/j.biomaterials.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 352.Lee H., Ju Y.M., Kim I., Elsangeedy E., Lee J.H., Yoo J.J., Atala A., Lee S.J. A novel decellularized skeletal muscle-derived ECM scaffolding system for in situ muscle regeneration. Methods. 2020;171:77–85. doi: 10.1016/j.ymeth.2019.06.027. [DOI] [PubMed] [Google Scholar]
- 353.Kim W., Lee H., Lee J., Atala A., Yoo J.J., Lee S.J., Kim G.H. Efficient myotube formation in 3D bioprinted tissue construct by biochemical and topographical cues. Biomaterials. 2020;230 doi: 10.1016/j.biomaterials.2019.119632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Lee H., Kim W., Lee J., Park K.S., Yoo J.J., Atala A., Kim G.H., Lee S.J. Self-aligned myofibers in 3D bioprinted extracellular matrix-based construct accelerate skeletal muscle function restoration. Appl. Phys. Rev. 2021;8 doi: 10.1063/5.0039639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Skoog Shelby, Kumar A., Girish Narayan, Roger J., Goering Peter. Biological responses to immobilized microscale and nanoscale surface topographies, Pharmacology and Therapeutics. The Journal of the International Encyclopedia of Pharmacology and Therapeutics. 2018;182:33–55. doi: 10.1016/j.pharmthera.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 356.Choi Y.J., Jun Y.J., Kim D.Y., Yi H.G., Chae S.H., Kang J., Lee J., Gao G., Kong J.S., Jang J., Chung W.K., Rhie J.W., Cho D.W. A 3D cell printed muscle construct with tissue-derived bioink for the treatment of volumetric muscle loss. Biomaterials. 2019;206:160–169. doi: 10.1016/j.biomaterials.2019.03.036. [DOI] [PubMed] [Google Scholar]
- 357.Choi Y.J., Kim T.G., Jeong J., Yi H.G., Park J.W., Hwang W., Cho D.W. 3D cell printing of functional skeletal muscle constructs using skeletal muscle-derived bioink. Adv.Healthc.Mater. 2016;5:2636–2645. doi: 10.1002/adhm.201600483. [DOI] [PubMed] [Google Scholar]
- 358.Jin Y., Jeon E.J., Jeong S., Min S., Choi Y.S., Kim S.H., Lee J.S., Shin J., Yu J.H., Ahn D.H., Kim Y.G., Yang H.S., Kang T.J., Cho S.R., Choi N., Cho S.W. Reconstruction of Muscle Fascicle-Like Tissues by Anisotropic 3D Patte rning. Adv. Funct. Mater. 2021;31 doi: 10.1002/adfm.202006227. [DOI] [Google Scholar]
- 359.Lacraz G., Rouleau A.J., Couture V., Söllrald T., Drouin G., Veillette N., Grandbois M., Grenier G. Increased stiffness in aged skeletal muscle impairs muscle progenitor cell proliferative activity. PLoS One. 2015;10 doi: 10.1371/journal.pone.0136217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Jin Y., Shahriari D., Jeon E.J., Park S., Choi Y.S., Back J., Lee H., Anikeeva P., Cho S.W. Functional skeletal muscle regeneration with thermally drawn porous fibers and reprogrammed muscle progenitors for volumetric muscle injury. Adv. Mater. 2021;33 doi: 10.1002/adma.202007946. [DOI] [PubMed] [Google Scholar]
- 361.Ljubimov A.V., Saghizadeh M. Progress in corneal wound healing. Prog. Retin. Eye Res. 2015;49:17–45. doi: 10.1016/j.preteyeres.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Tan D.T., Dart J.K., Holland E.J., Kinoshita S. Corneal transplantation. Lancet. 2012;379:1749–1761. doi: 10.1016/s0140-6736(12)60437-1. [DOI] [PubMed] [Google Scholar]
- 363.Gain P., Jullienne R., He Z., Aldossary M., Acquart S., Cognasse F., Thuret G. Global survey of corneal transplantation and eye banking. JAMA.Ophthalmol. 2016;134:167–173. doi: 10.1001/jamaophthalmol.2015.4776. [DOI] [PubMed] [Google Scholar]
- 364.Islam M.M., Buznyk O., Reddy J.C., Pasyechnikova N., Alarcon E.I., Hayes S., Lewis P., Fagerholm P., He C., Iakymenko S., Liu W., Meek K.M., Sangwan V.S., Griffith M. Biomaterials-enabled cornea regeneration in patients at high risk for rejection of donor tissue transplantation. NPJ.Regen.Med. 2018;3:2. doi: 10.1038/s41536-017-0038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Jirásková N., Rozsival P., Burova M., Kalfertova M. AlphaCor artificial cornea: clinical outcome. Eye. 2011;25:1138–1146. doi: 10.1038/eye.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Fagerholm P., Lagali N.S., Ong J.A., Merrett K., Jackson W.B., Polarek J.W., Suuronen E.J., Liu Y., Brunette I., Griffith M. Stable corneal regeneration four years after implantation of a cell-free recombinant human collagen scaffold. Biomaterials. 2014;35:2420–2427. doi: 10.1016/j.biomaterials.2013.11.079. [DOI] [PubMed] [Google Scholar]
- 367.Yin H., Lu Q., Wang X., Majumdar S., Jun A.S., Stark W.J., Grant M.P., Elisseeff J.H. Tissue-derived microparticles reduce inflammation and fibrosis in cornea wounds. Acta Biomater. 2019;85:192–202. doi: 10.1016/j.actbio.2018.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Kim H., Park M.N., Kim J., Jang J., Kim H.K., Cho D.W. Characterization of cornea-specific bioink: high transparency, improved in vivo safety. J. Tissue Eng. 2019;10 doi: 10.1177/2041731418823382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Chandru A., Agrawal P., Ojha S.K., Selvakumar K., Shiva V.K., Gharat T., Selvam S., Thomas M.B., Damala M., Prasad D., Basu S., Bhowmick T., Sangwan V.S., Singh V. Human cadaveric donor cornea derived extra cellular matrix microparticles for minimally invasive healing/regeneration of corneal wounds. Biomolecules. 2021;11 doi: 10.3390/biom11040532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Du L., Wu X., Pang K., Yang Y. Histological evaluation and biomechanical characterisation of an acellular porcine cornea scaffold. Br. J. Ophthalmol. 2011;95:410–414. doi: 10.1136/bjo.2008.142539. [DOI] [PubMed] [Google Scholar]
- 371.Ponce Márquez S., Martínez V.S., McIntosh Ambrose W., Wang J., Gantxegui N.G., Schein O., Elisseeff J. Decellularization of bovine corneas for tissue engineering applications. Acta Biomater. 2009;5:1839–1847. doi: 10.1016/j.actbio.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 372.Zhang M.C., Liu X., Jin Y., Jiang D.L., Wei X.S., Xie H.T. Lamellar keratoplasty treatment of fungal corneal ulcers with acellular porcine corneal stroma. Am. J. Transplant. 2015;15:1068–1075. doi: 10.1111/ajt.13096. [DOI] [PubMed] [Google Scholar]
- 373.Zhou Q., Guaiquil V.H., Wong M., Escobar A., Ivakhnitskaia E., Yazdanpanah G., Jing H., Sun M., Sarkar J., Luo Y., Rosenblatt M.I. Hydrogels derived from acellular porcine corneal stroma enhance corneal wound healing. Acta Biomater. 2021;134:177–189. doi: 10.1016/j.actbio.2021.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Ahn J.I., Kuffova L., Merrett K., Mitra D., Forrester J.V., Li F., Griffith M. Crosslinked collagen hydrogels as corneal implants: effects of sterically bulky vs. non-bulky carbodiimides as crosslinkers. Acta Biomater. 2013;9:7796–7805. doi: 10.1016/j.actbio.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 375.Mölzer C., Shankar S.P., Masalski V., Griffith M., Kuffová L., Forrester J.V. TGF-β1-activated type 2 dendritic cells promote wound healing and induce fibroblasts to express tenascin c following corneal full-thickness hydrogel transplantation. J.Tissue.Eng.Regen.Med. 2019;13:1507–1517. doi: 10.1002/term.2853. [DOI] [PubMed] [Google Scholar]
- 376.West-Mays J.A., Dwivedi D.J. The keratocyte: corneal stromal cell with variable repair phenotypes. Int. J. Biochem. Cell Biol. 2006;38:1625–1631. doi: 10.1016/j.biocel.2006.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Shen, X., Li, S., Zhao, X., Han, J., Chen, J., Rao, Z., Zhang, K., Quan, D., Yuan, J., Bai, Y., Dual-crosslinked regenerative hydrogel for sutureless long-term repair of corneal defect, Bioact. Mater. 20 (2023) 434-448. 10.1016/j.bioactmat.2022.06.006. [DOI] [PMC free article] [PubMed]
- 378.Wang F., Shi W., Li H., Wang H., Sun D., Zhao L., Yang L., Liu T., Zhou Q., Xie L. Decellularized porcine cornea-derived hydrogels for the regeneration of epithelium and stroma in focal corneal defects. Ocul. Surf. 2020;18:748–760. doi: 10.1016/j.jtos.2020.07.020. [DOI] [PubMed] [Google Scholar]
- 379.Gilbert T.W., Freund J.M., Badylak S.F. Quantification of DNA in biologic scaffold materials. J. Surg. Res. 2009;152:135–139. doi: 10.1016/j.jss.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.