Abstract
Haemophilus somnus causes pneumonia, reproductive failure, infectious myocarditis, thrombotic meningoencephalitis, and other diseases in cattle. Although vasculitis is commonly seen as a result of systemic H. somnus infections, the pathogenesis of vascular damage is poorly characterized. In this study, we demonstrated that H. somnus (pathogenic isolates 649, 2336, and 8025 and asymptomatic carrier isolates 127P and 129Pt) induce apoptosis of bovine endothelial cells in a time- and dose-dependent manner, as determined by Hoechst 33342 staining, terminal deoxynucleotidyl transferase-mediated dUTP-FITC nick end labeling, DNA fragmentation, and transmission electron microscopy. H. somnus induced endothelial cell apoptosis in as little as 1 h of incubation and did not require extracellular growth of the bacteria. Viable H. somnus organisms induced greater endothelial cell apoptosis than heat-killed organisms. Since viable H. somnus cells release membrane fibrils and blebs, which contain lipooligosaccharide (LOS) and immunoglobulin binding proteins, we examined culture filtrates for their ability to induce endothelial cell apoptosis. Culture filtrates induced similar levels of endothelial cell apoptosis, as did viable H. somnus organisms. Heat inactivation of H. somnus culture filtrates partially reduced the apoptotic effect on endothelial cells, which suggested the presence of both heat-labile and heat-stable factors. We found that H. somnus LOS, which is heat stable, induced endothelial cell apoptosis in a time- and dose-dependent manner and was inhibited by the addition of polymyxin B. These data demonstrate that H. somnus and its LOS induce endothelial cell apoptosis, which may play a role in producing vasculitis in vivo.
Haemophilus somnus (24, 26) is a member of the family Pasteurellaceae, which causes bovine pneumonia (3, 16, 17, 23, 32), abortion (9, 58), thrombotic meningoencephalitis (TME) (24, 26, 52), myocarditis (24), infertility (43, 53), and arthritis (26). Vasculitis is a common finding during H. somnus infections (3, 17, 24, 26). H. somnus also can be isolated from the urogenital tracts of male and female cattle in the absence of clinical disease (27, 35, 36). Most of these asymptomatic carrier isolates are sensitive to in vitro killing by bovine serum (8). Conversely, most pathogenic isolates of H. somnus are resistant to in vitro killing by bovine serum (8). Administration of pathogenic strains of H. somnus to cattle reproduces clinical respiratory disease and pulmonary vasculitis (17). In light of the propensity of H. somnus infections to cause septicemia and vasculitis, it is unfortunate that few investigators have examined the in vitro interactions of H. somnus with endothelial cells. Thompson and Little (57) first noted that pathogenic isolates of H. somnus adhered to endothelial cells from carotid arterial explants to a greater extent than Escherichia coli and Salmonella enterica serovar Typhimurium, as viewed with scanning electron microscopy. These authors observed that adherence of H. somnus cells caused cytotoxic changes that included endothelial cell rounding and shrinkage (57). Kwiecien et al. (37) demonstrated that pretreatment of bovine endothelial cells with tumor necrosis factor alpha (TNF-α) increased the adherence of both pathogenic and asymptomatic carrier isolates of H. somnus. These authors also reported that endothelial cells changed their morphology after the addition of H. somnus, which was suggestive of apoptotic changes. Yang et al. (62) demonstrated that H. somnus induced apoptosis in bovine polymorphonuclear leukocytes (PMNs).
The mechanism by which H. somnus induces endothelial cell damage is not clear. Identifying the mechanism is made more difficult because the virulence factors of H. somnus are incompletely characterized. The most studied H. somnus virulence factor is lipooligosaccharide (LOS) which is found in the outer membrane or membrane blebs or is freely released from the organism (29). LOS from pathogenic isolates of H. somnus undergoes phase variation, which may play a role in the evasion of the host immune response (30, 31). Structure analysis of the phase-variable LOS from H. somnus strain 738 has helped to characterize the process of phase variation (11). McQuiston et al. (40) recently demonstrated that the number of 5′ CAAT 3′ nucleotide repeats upstream of lob1a in H. somnus affects the regulation of LOS phase variation. In addition, Wu et al. (60) revealed that transposon mutagenesis of lob2a, a gene encoding an N-acetylglucosamine transferase necessary for the biosynthesis of LOS in pathogenic isolates of H. somnus, reduced the level of bacteremia in a mouse model. These results suggest that LOS phase variation could alter the host response to H. somnus infection. H. somnus also has several immunoglobulin binding proteins (IgBPs), which may protect the organism from phagocytosis and complement-mediated killing (10, 59, 63). IgBPs consist of a network of surface fibrils, a 76-kDa peripheral membrane protein (p76) (7), and the major outer membrane protein (MOMP) (10, 54, 63). The fibrils are found primarily in the culture supernatants, whereas p76 is found principally in the cell pellet (10). LOS, MOMP, IgBPs, and p76 may be found in the membrane blebs. H. somnus has other outer membrane proteins, including a 40-kDa outer membrane protein (18, 54) and lipoproteins LppA and LppB (55, 56), which are similar to lipoproteins from other pathogenic bacteria, such as Pasteurella haemolytica (47) and Neisseria meningitidis (61). Antibodies to a similar, or perhaps identical, 40-kDa outer membrane antigen are protective, which suggests that it may be a virulence factor (18). Perhaps H. somnus lipoproteins activate mammalian cells through the Toll-like family of receptors (i.e., Tlr-2), as has been reported for several gram-positive bacterial pathogens (2, 25). H. somnus organisms have also been shown to release guanosine-like compounds that inhibited the respiratory burst function of bovine peripheral blood PMNs (48). Intracellular survival of H. somnus organisms within bovine phagocytes (12, 38) and modulation of phagocytic effector function (20, 21, 50) have also been described. The role of these and other H. somnus virulence factors in the development of vascular disease in vivo is not clear.
Numerous bacterial pathogens have been shown to induce apoptosis of host cells in vitro, yet it is not clear whether apoptosis plays a role in the pathogenesis of systemic bacterial infections in vivo. Recently, several investigators have demonstrated that Yersenia pseudotuberculosis (44) and S. enterica serovar Typhimurium (45) benefit from inducing apoptosis in vivo. These data suggest that bacterial induction of host cell apoptosis in vitro may be relevant to the pathogenesis of several clinical diseases in vivo.
In this study, we employed several biochemical and morphological methods to determine whether endothelial cell damage induced by H. somnus occurs by apoptosis. The results of these experiments demonstrate that incubation with H. somnus causes morphological and biochemical changes in bovine endothelial cells that are consistent with the process of apoptosis. Furthermore, we provide preliminary evidence that LOS is responsible, in part, for the ability of H. somnus to induce apoptosis in bovine endothelial cells in vitro.
MATERIALS AND METHODS
Chemicals and media.
Staurosporine, paraformaldehyde, amphotericin B, thiamine monophosphate, gentamicin sulfate, polymyxin B, dimethyl sulfoxide, penicillin, streptomycin, Trizma-base, gluteraldehyde, osmium tetroxide, sodium cacodylate, and Dulbeco's modified Eagle's medium (DMEM; containing phenol red, 25 mM HEPES, 4.5 g of dextrose/ml, and 2 mM l-glutamine) were obtained from Sigma Chemical Co. (St. Louis, Mo.). Brain heart infusion (BHI) broth and yeast extract were obtained from Difco (Detroit, Mich.). Anti-factor VIII-fluorescein isothiocyanate (FITC) monoclonal antibody was purchased from Pharmingen (San Diego, Calif.).
Cultivation of pulmonary artery endothelial cells.
Primary bovine pulmonary artery endothelial cells were used because experimental H. somnus pneumonia is characterized by vasculitis of medium to large arteries (17). The cells were isolated by scraping the lumen of dissected vessels from a 15-month-old Hereford-Angus steer, as described previously (49). Isolated cells were seeded into 25-cm2 tissue culture flasks containing DMEM tissue culture medium supplemented with 20% fetal bovine serum (FBS), penicillin-streptomycin (100 U/10 mg/ml), and amphotericin B (2.5 μg/ml). The cells were incubated at 37°C with 5% CO2 until near-confluent monolayers were established. The cells were characterized as endothelial cells by their “cobblestone” morphology and expression of factor VIII (VonWillebrand factor) on their surface, as determined by fluorescence microscopy (49). After verifying the purity of the endothelial cells, the addition of amphotericin B to the medium was discontinued. Endothelial cells were passaged by scraping, rather than by trypsinization. For consistency, endothelial cells used in experiments were passed fewer than 15 times. Frozen stocks of the endothelial cells were stored in a liquid nitrogen tank, with 20% FBS and 5% dimethyl sulfoxide added as cryopreservants.
Cultivation of bacterial strains.
The pathogenic isolates (2336, 8025, and 649) and asymptomatic carrier isolates (127P and 129Pt) of H. somnus used in these studies are summarized in Table 1. H. somnus strain 8025, originally isolated from a case of TME and used in several published reports, was selected to establish our experimental system (12, 37, 38, 50, 62). We were concerned that strain 8025 might not be representative of other strains of H. somnus for inducing endothelial cell apoptosis. With this in mind, we selected pathogenic and asymptomatic carrier isolates of H. somnus that were used previously in several published studies. Pathogenic isolate 649 was isolated from an aborted fetus and has been used to experimentally reproduce abortion (58). Pathogenic isolate 2336 was isolated from a pneumonic calf and has been used experimentally to reproduce pneumonia with vasculitis and thrombosis (18, 19). Pathogenic isolate 738 was a clonal derivative of isolate 2336 (17). Asymptomatic carrier isolates 127P and 129Pt were isolated from the prepuce of two different bulls during a routine annual fertility examination (8).
TABLE 1.
Strains of H. somnus and LOS used in this study
| Strain | Phenotype | Isolated from: | Surface expression of IgBPs |
|---|---|---|---|
| 649b | Pathogenic | Abortion | Yes |
| 738b | Pathogenic | Pneumonia | Yes |
| 2336a | Pathogenic | Pneumonia | Yes |
| 8025 | Pathogenic | TME | Yes |
| 127Pab | Asymptomatic carrier | Prepuce of bull | Yes |
| 129Ptab | Asymptomatic carrier | Prepuce of bull | No |
Strains were obtained by L. Corbeil (University of California—San Diego, San Diego).
LOS was obtained by T. Inzana (Virginia Tech, Blacksburg).
The cultivation of H. somnus requires a rich and complex medium (28). All H. somnus isolates were incubated without shaking in 25-cm2 tissue culture flasks containing BHI broth supplemented with 0.5% yeast extract and 0.05% thiamine monophosphate (BHI-YT) for 16 h at 37°C with 5% CO2 before adding to endothelial cells. The number of CFU per milliliter was estimated by turbidity (optical density at 600 nm), as extrapolated from a standard curve of H. somnus. To confirm that H. somnus 8025 and other isolates (2336 and 127P) grew in BHI-YT, as well as DMEM supplemented with 20% FBS, growth curves were determined. These experiments were performed using H. somnus 8025, 2336, and 127P cultured overnight in BHI-YT at 37°C with 5% CO2 and subcultured into DMEM or BHI-YT. H. somnus 8025 subcultured into DMEM remained in lag phase for approximately 4 h before entering logarithmic growth. In both media, there was an approximate 1.9 log10 increase in CFU over a 6-h incubation period (data not shown). H. somnus pathogenic isolate 2336 and asymptomatic carrier isolate 127P grew slower than strain 8025 (data not shown). Frozen stock cultures for each isolate of H. somnus were maintained at −70°C in BHI-YT with 10% sterile glycerol. A fresh aliquot was thawed for each experiment. In some experiments, heat-killed H. somnus cells were prepared by incubating the bacteria at 60°C for 1 h. Bacterial death was confirmed by the absence of colonies when plated on chocolate agar plates (Remel, Lenexa, Kans.).
LOS.
LOS was isolated from pathogenic isolates (649 and 738) and asymptomatic carrier isolates (127P and 129Pt) of H. somnus as described previously (29). Briefly, H. somnus was cultured to stationary phase in Columbia broth containing 0.1% Trizma-base and 0.01% thiamine monophosphate. The bacterial cells were pelleted by centrifugation (6,000 × g) and washed with phosphate-buffered saline (PBS), and LOS was extracted from the cells by enzymatic digestion and hot phenol extraction. The pooled aqueous phases were purified by repeated ultracentrifugation until the absorbances of the LOS supernatants were less than 0.02 at 260 and 280 nm. Protein contamination was not detected in the LOS, as analyzed by standard sodium dodecyl sulfate-polyacrylamide gel electrophoresis and silver staining.
Hoechst 33342 staining.
Endothelial cells adherent to glass coverslips were fixed with 4% paraformaldehyde in PBS for 30 min and then stained for 10 min with a solution of Hoechst 33342 (Molecular Probes, Eugene, Org.) diluted to 10 μg/ml in PBS (15). The coverslips were washed in distilled H2O, mounted on glass slides, and viewed by UV fluorescence microscopy with a DAPI (4′,6′-diamidino-2-phenylindole) filter block set at an excitation wavelength of 355 nm and emission wavelength of 465 nm. Untreated and staurosporine (200 nM; 6 h)-treated endothelial cells served as negative and positive controls, respectively. At least 100 cells in five random areas per coverslip were analyzed for apoptosis, based on the presence of nucleic acid condensation. Images were acquired using a digital camera (Photometrics, Tuscon, Ariz.) and were enhanced using the Adobe Photoshop 5.0 software package (Adobe Software Inc., San Jose, Calif.).
In situ TUNEL assay.
Endothelial cells cultured on glass coverslips were fixed with 4% paraformaldehyde for 30 min, and 3′ hydroxyl residues of DNA fragments were stained with a commercial terminal deoxynucleotidyl transferase-mediated dUTP-FITC nick end labeling (TUNEL) kit (Promega, Madison, Wis.) as we have described previously (39). Apoptotic cells were visualized with fluorescence microscopy using a FITC filter block set at excitation and emission wavelengths of 488 and 530 nm, respectively. The coverslips were counterstained with propidium iodide to help rule out TUNEL of necrotic cells. Untreated endothelial cells and cells treated with 200 nM staurosporine were included as negative and positive controls, respectively. At least 100 cells in five random areas per coverslip were analyzed for apoptosis based on the presence of fluorescent nuclei.
Internucleosomal DNA fragmentation.
Agarose gel electrophoresis analysis of internucleosomal DNA fragmentation, following exposure of endothelial cells to H. somnus or its LOS, was performed as described previously (41). DNA was isolated from endothelial cells (106) by using a commercial genomic DNA isolation kit (Wizard genomic DNA isolation kit; Promega) according to the manufacturer's directions. Briefly, endothelial cells were detached from the tissue culture flask via trypsinization (0.05%), centrifuged at 300 × g for 5 min, and washed in ice-cold PBS. Cells were lysed and processed by following the manufacturer's specifications. Isolated DNA was precipitated using standard ethanol and isopropanol techniques and was resuspended in Tris-EDTA buffer (40 mM Tris, 2 mM EDTA). The DNA concentration was quantified spectrophotometrically by measuring absorbance at 260 nm (Smart Spec 3000; Bio-Rad, Hercules, Calif.). Five micrograms of isolated DNA was loaded into a 1.5% agarose gel in 1× TAE buffer (40 mM Tris, 2 mM EDTA, 20 mM acetic acid). A commercially available 200-bp DNA ladder (Promega) was included to confirm the presence of internucleosomal DNA fragments. Following electrophoresis, the gel was stained with ethidium bromide.
Transmission electron microscopy.
Endothelial cells were cultured on no. 2 thickness glass coverslips (Fisher Scientific, Pittsburgh, Pa.) and incubated with H. somnus strains 649, 8025, and 127P. Endothelial cells were fixed for 1 h in Karnovsky's fixative (2.5% glutaraldehyde and 0.1 M sodium cacodylate in PBS, pH 7.4). The coverslips were washed in PBS, stained with 0.5% osmium tetroxide for 10 min, and dehydrated through a graded ethanol series. Endothelial cells and the coverslip were embedded in 100% epoxy resin for 5 h. Following embedding, the coverslip was removed via dry ice and samples were thin sectioned, stained with uranyl acetate and lead citrate, and examined with a Phillips model 410 transmission electron microscope (Phillips Electron Optics, Eindhoven, The Netherlands).
Treatment of endothelial cells with H. somnus.
Endothelial cells were washed three times in DMEM without antibiotics or FBS, and antibiotic-free DMEM supplemented with 20% FBS was added to the cells. Viable H. somnus cells were then added to the washed endothelial cells, at multiplicities of infection (MOI) ranging from 0.1:1 to 1,000:1 H. somnus bacteria per endothelial cell. The infected endothelial cells were incubated for 6 h at 37°C with 5% CO2 before detection of apoptosis by Hoechst 33342 staining, TUNEL, internucleosomal DNA fragmentation, and transmission electron microscopy. In some experiments, the role of extracellular H. somnus in the induction of endothelial cell apoptosis was evaluated. To do this, endothelial cells were infected with H. somnus cells for 1 h at an MOI of 10:1 and then gently washed three times in DMEM with 20% FBS and 5 μg of gentamicin/ml. These endothelial cells were incubated in medium containing antibiotics for 5 h, while parallel samples were not washed. Both samples were incubated at 37°C and apoptosis was assessed by Hoechst 33342 staining as described above. Parallel cultures in which extracellular H. somnus cells were not removed and antibiotics were not added were included in these experiments.
Treatment of endothelial cells with H. somnus culture filtrates.
The effect of H. somnus culture filtrates on endothelial cells was examined. Culture filtrates were prepared by passing 5 ml of centrifuged BHI-YT broth (6,000 × g for 10 min) from cultures of H. somnus (approximately 108 CFU) through 0.2-μm-pore-size cellulose acetate filters (Millipore, Bedford, Mass.) as described previously (62). The sterility of the culture filtrates was confirmed by the absence of colonies when plated on chocolate agar plates. Endothelial cells were incubated for 6 h at 37°C with culture filtrates (33% final volume), and apoptosis was assessed by Hoechst 33342 staining as described above.
Treatment of endothelial cells with H. somnus LOS.
Endothelial cells were incubated with a range of LOS (5 to 500 ng/ml) isolated from the various H. somnus strains listed in Table 1 for up to 22 h at 37°C with 5% CO2. Prior to their addition to the tissue culture medium, stock solutions of LOS were heated to 60°C for 5 min to aid in solubility. In some experiments, polymyxin B (10 μg/ml), a lipid A antagonist (46), was added at the same time as the LOS. Apoptosis was assessed by Hoechst 33342 staining and internucleosomal DNA fragmentation as described above.
Statistical analysis.
Data from quantitative evaluations of apoptosis (Hoechst 33342 staining and TUNEL) were analyzed for statistical significance using a repeated-measures analysis of variance (ANOVA), followed by the Tukey-Kramer multiple compassion test, as performed by the Instat software package (GraphPad, San Diego, Calif.). Data are presented as the mean ± standard error of the mean (SEM) of at least three independent experiments. Statistical significance was set at a P value of <0.05.
RESULTS
H. somnus strain 8025 induces endothelial cell apoptosis in a time- and dose-dependent manner.
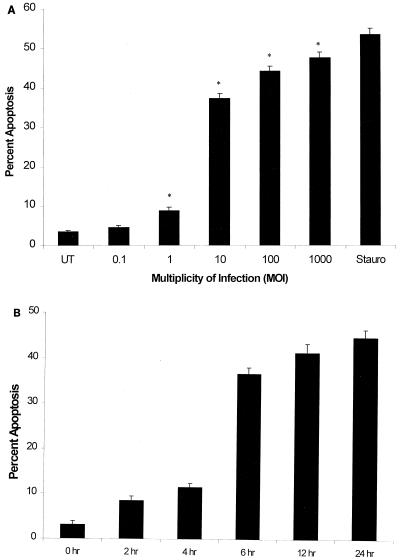
A dose-dependent increase in apoptotic endothelial cells was noted following incubation with H. somnus 8025 organisms for 6 h, as demonstrated by Hoechst 33342 staining (Fig. 1A). Endothelial cells incubated with H. somnus 8025 bacteria at MOI ranging from 1:1 to 1,000:1 induced a significant (P < 0.05) increase in the percent of apoptotic endothelial cells, with maximal effects seen at MOI of 10:1 to 1,000:1. As a positive control, endothelial cells were treated for 6 h with 200 nM staurosporine, a potent inducer of endothelial cell apoptosis (4). A time-dependent induction of apoptosis, when endothelial cells were incubated with H. somnus 8025 cells at an MOI of 10:1, was also demonstrated (Fig. 1B). There was a significant increase in the percentage of apoptotic endothelial cells (P < 0.05) within 2 h of incubation with H. somnus compared to that of untreated endothelial cells. At higher MOI, H. somnus induced endothelial cell apoptosis more rapidly. For example, after incubation with H. somnus for 4 h at an MOI of 100:1, the level of endothelial cell apoptosis detected was similar to that seen following a 6-h incubation with H. somnus at an MOI of 10:1 (data not shown). Based on these results, we selected an MOI of 10:1 and an incubation period of 6 h as our experimental system for evaluating endothelial cell apoptosis in all subsequent H. somnus experiments.
FIG. 1.
H. somnus 8025 induces endothelial cell apoptosis in a dose- and time-dependent manner. (A) Bovine pulmonary artery endothelial cells (5 × 104) were incubated at 37°C with 5% CO2 for 6 h at various MOI. (B) Bovine pulmonary artery endothelial cells (5 × 104) were incubated with H. somnus 8025 organisms at an MOI of 10:1 for 2 to 24 h at 37°C with 5% CO2. Untreated (UT) and staurosporine (200 nM)-treated (Stauro) cells were included as negative and positive controls, respectively. Apoptosis was detected using Hoechst 33342 staining as described previously. These data illustrate the mean ± SEM of three separate experiments. Statistical analysis was performed by ANOVA followed by the Tukey-Kramer significant difference test (∗, P < 0.05).
H. somnus strain 8025 induces ultrastructural changes within endothelial cells consistent with apoptosis.
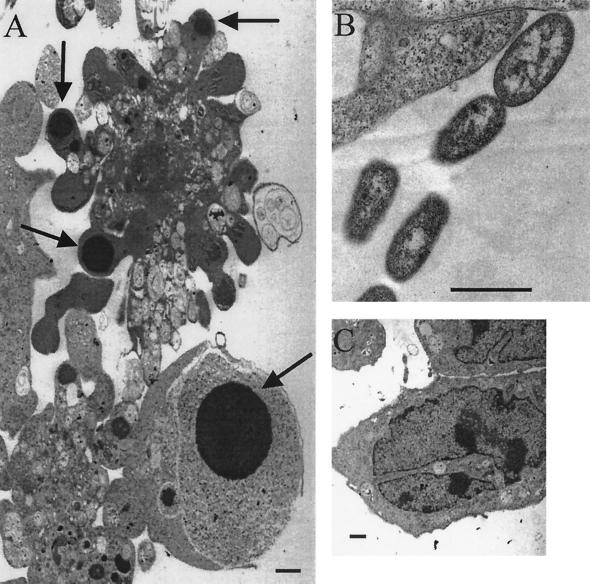
Using transmission electron microscopy, we detected ultrastructural changes in endothelial cells treated with H. somnus 8025 organisms for 6 h that were consistent with apoptosis (Fig. 2A). These included cellular shrinkage, membrane blebbing, and nucleic acid condensation. Similar ultrastructural apoptotic changes were observed in endothelial cells treated with H. somnus isolates 127P and 649 (data not shown). H. somnus 8025 bacteria were seen in close proximity to endothelial cells (Fig. 2B). Control endothelial cells (Fig. 2C) lacked these features. Endothelial cells treated with 200 nM staurosporine (positive control) showed apoptotic changes, including nucleic acid condensation and membrane blebbing (data not shown).
FIG. 2.
H. somnus induces ultrastructural apoptotic changes in endothelial cells. (A) Transmission electron micrograph of endothelial cells infected with H. somnus 8025 organisms for 6 h at an MOI of 10:1 that exhibited nucleic acid condensation (arrows). Magnification, ×8,400. (B) H. somnus 8025 organisms in close proximity to endothelial cells. Magnification, ×29,000. (C) Control endothelial cells with normal nuclear morphology. Magnification, ×10,800. Bar = 1 μm.
Pathogenic and asymptomatic carrier isolates of H. somnus induce bovine endothelial cell apoptosis in vitro.
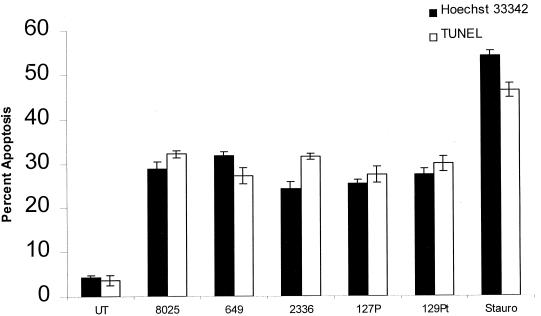
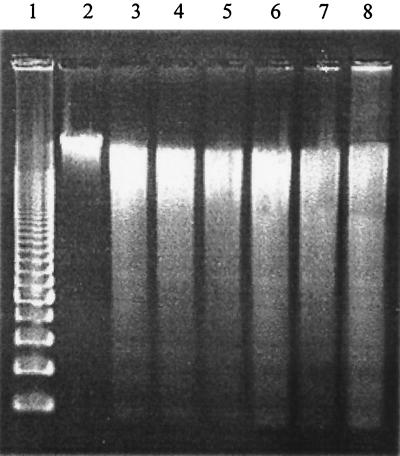
Because pathogenic isolates of H. somnus are associated with vascular disease in vivo, we hypothesized that they would induce endothelial cell apoptosis to a greater extent than asymptomatic carrier isolates. To test this hypothesis, endothelial cells were incubated with several pathogenic isolates (649, 2336, and 8025) and asymptomatic carrier isolates (127P and 129Pt) of H. somnus. Pathogenic and asymptomatic carrier isolates of H. somnus induced significant and generally comparable levels of endothelial cell apoptosis (P < 0.05), as assessed by Hoechst 33342 staining and TUNEL (Fig. 3). Because there was no significant difference between the levels of TUNEL- and Hoechst 33342-positive cells (except for a small difference for pathogenic isolate 2336), we conclude that necrosis was not a significant contributor to endothelial cell death in our experimental system. Internucleosomal DNA fragmentation studies of endothelial cells treated with pathogenic and asymptomatic carrier isolates of H. somnus showed comparable levels of apoptosis (Fig. 4).
FIG. 3.
Pathogenic and asymptomatic carrier isolates of H. somnus induce bovine endothelial cell apoptosis, as detected by Hoechst 33342 staining and TUNEL. Volumes containing 5 × 104 endothelial cells were adhered to glass coverslips and incubated with pathogenic H. somnus isolates 8025, 649, and 2336 and asymptomatic carrier isolates 127P and 129Pt at an MOI of 10:1 for 6 h at 37°C with 5% CO2. Apoptosis was detected by Hoechst 33342 staining and TUNEL on separate coverslips. Controls consisted of untreated cells (UT) and 200 nM staurosporine (Stauro)-treated endothelial cells. These data represent the mean ± SEM of three independent experiments. All isolates of H. somnus used in this experiment induced significant levels of endothelial cell apoptosis compared to those of untreated endothelial cells (P < 0.05; Tukey-Kramer significant difference test).
FIG. 4.
Pathogenic and asymptomatic carrier isolates of H. somnus induce internucleosomal DNA fragmentation in bovine endothelial cells. Bovine pulmonary artery endothelial cells (5 × 105) were incubated for 6 h at 37°C (5% CO2) with H. somnus isolates (649, 8025, 2336, 127P, and 129Pt) at an MOI of 10:1 DNA (5 μg) was loaded into a 1.5% agarose gel as described in Materials and Methods. Lane 1, 200-bp DNA molecular weight marker; lane 2, DNA from untreated endothelial cells; lane 3, DNA from endothelial cells treated with 200 nM staurosporine (positive control); lanes 4 to 8, DNA from endothelial cells incubated with H. somnus isolates 649, 8025, 2336, 127P, and 129Pt, respectively. Note the presence of a DNA ladder in all of the H. somnus-treated samples and in the staurosporine control. These data represent one of three independent experiments that were performed.
Extracellular growth of H. somnus is not required for bovine endothelial cell apoptosis.
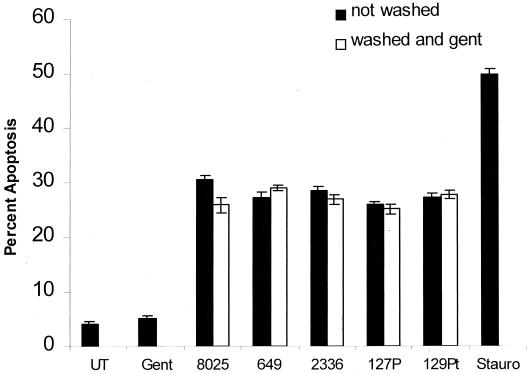
We observed an approximate 1.9 log10 increase in viable CFU per milliliter when H. somnus strain 8025, and slightly less for strains 2336 and 127P, organisms were incubated at 37°C for 6 h in DMEM supplemented with 20% FBS (data not shown). Because extracellular multiplication of H. somnus will substantially increase the MOI to which the endothelial cells are exposed, we examined whether endothelial cell apoptosis is reduced in the absence of extracellular H. somnus. To address this point, extracellular H. somnus organisms were removed by washing (described in Materials and Methods) after a 1-h incubation with endothelial cells, and residual extracellular H. somnus organisms were killed by the addition of gentamicin (5 μg/ml) to the tissue culture medium. We determined that the MIC of gentamicin for all of the H. somnus isolates listed in Table 1 was less than 2.5 μg/ml (data not shown). There was no significant difference in the level of endothelial cell apoptosis when H. somnus was not removed or when H. somnus was removed and gentamicin was added (Fig. 5). The exception was a small difference noted for H. somnus 8025. The addition of gentamicin alone had no direct effect on endothelial cell apoptosis (Fig. 5).
FIG. 5.
Elimination of extracellular H. somnus does not alter endothelial cell apoptosis. Volumes containing 5 × 104 endothelial cells were adhered to glass coverslips and incubated with H. sonnus pathogenic isolates 8025, 649, and 2336 and asymptomatic carrier isolates 127P and 129Pt at an MOI of 10:1 for 1 h at 37°C with 5% CO2. After 1 h, each experimental group was further divided into two subgroups. For the first subgroup (■), H. somnus organisms were not removed from the endothelial cells. For the second subgroup (□), nonadherent H. somnus organisms were washed away and gentamicin was added (5 μg/ml) to kill residual extracellular bacteria. The two groups were incubated for an additional 5 h at 37°C with 5% CO2, and apoptosis was detected by Hoechst 33342 staining. Controls consisted of untreated cells (UT) and staurosporine (200 nM; Stauro)-treated endothelial cells as a positive control. These data illustrate the mean ± SEM of three separate experiments. Statistical analysis was performed by ANOVA followed by the Tukey-Kramer significant difference test. Significant levels of endothelial cell apoptosis were detected whether H. somnus was removed (P < 0.05) compared to untreated endothelial cells.
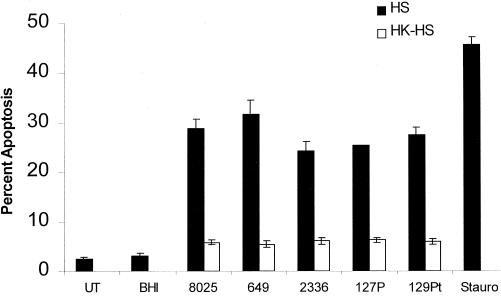
Heat-killed H. somnus organisms have a decreased ability to cause apoptosis in bovine endothelial cells.
Heat-killed H. somnus organisms (60°C for 60 min) induced significantly less endothelial cell apoptosis (P < 0.05) than viable H. somnus (Fig. 6). However, heat-killed H. somnus organisms still elicited a significant level of apoptosis (P < 0.05) compared to that of untreated endothelial cells. No viable H. somnus CFU were detected after heating at 60°C for 60 min.
FIG. 6.
Heat-killed H. somnus organisms induce less endothelial cell apoptosis than viable H. somnus organisms. Volumes containing 5 × 104 adherent endothelial cells were incubated with 108 heat-killed (60°C for 60 min) (□) or viable (■) H. somnus organisms at an MOI of 10:1 for 6 h at 37°C with 5% CO2. Apoptosis was assessed by Hoechst 33342 staining. Controls consisted of untreated (UT) and staurosporine (200 nM; Stauro)-treated endothelial cells. The data illustrate the mean ± SEM of three separate experiments. Statistical analysis was performed by ANOVA followed by the Tukey-Kramer significant difference test.
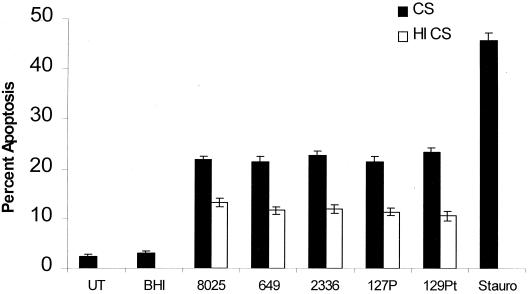
H. somnus culture filtrates induce endothelial cell apoptosis.
To evaluate the release of an H. somnus virulence factor in endothelial cell apoptosis, culture filtrates of H. somnus were added to endothelial cells. There was a significant increase (P < 0.05) in endothelial cell apoptosis (Fig. 7) following incubation with H. somnus culture filtrates (33% final volume). H. somnus culture filtrates at final volumes of less than 33% induced less apoptosis in endothelial cells (data not shown). Heat inactivation (60°C for 60 min) of the culture filtrates significantly decreased (P < 0.05) the level of endothelial cell apoptosis, although it still remained elevated (P < 0.05) compared to that of BHI-YT (33% final volume)-treated endothelial cells.
FIG. 7.
H. somnus culture filtrates induce endothelial cell apoptosis. Volumes containing 5 × 104 adherent endothelial cells were treated for 6 h with culture filtrates prepared from H. somnus organisms cultured in BHI-YT (■) (33% final volume; prepared with approximately 108 CFU) at 37°C with 5% CO2, using the same isolates described in Fig. 5. Heat-inactivated (□) (60°C for 60 min) culture filtrates were tested in the same experiments. Controls consisted of untreated cells (UT), cells treated with BHI-YT (33% final volume), and staurosporine (200 nM; Stauro)-treated endothelial cells. The data illustrate the mean ± SEM of three separate experiments. Statistical analysis was performed by ANOVA followed by the Tukey-Kramer significant difference test. Significant levels of endothelial cell apoptosis (P < 0.05) were detected when endothelial cells were incubated with H. somnus culture filtrates or heat-inactivated filtrates and were compared to control (untreated) endothelial cells. Heat inactivation of culture filtrates significantly reduced the level of endothelial cell apoptosis (P < 0.05) compared to that of culture filtrates.
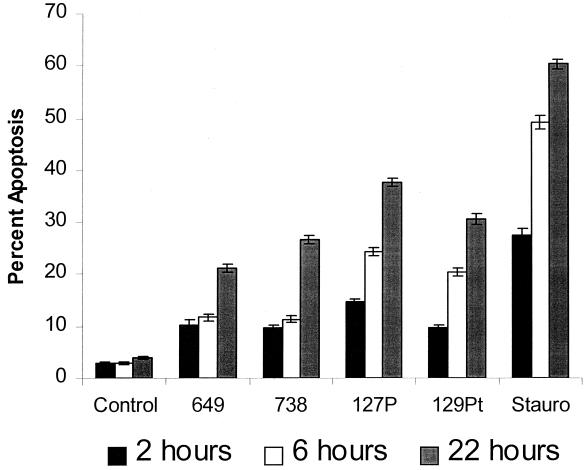
LOS from H. somnus induces apoptosis of bovine endothelial cells.
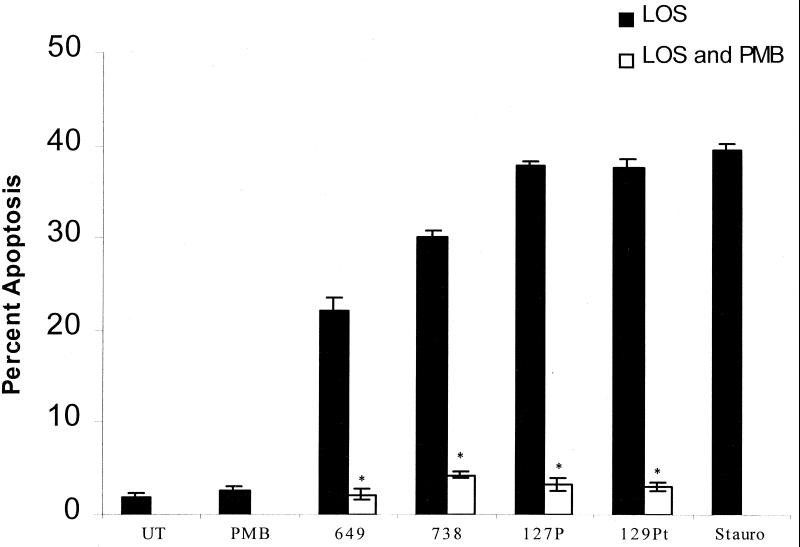
The data in Fig. 6 suggest that endothelial cell apoptosis is induced by both heat-stable and heat-labile components released by H. somnus. LOS is a candidate for the heat-stable factor that mediates endothelial cell apoptosis. To examine this possibility, LOS isolated from several pathogenic isolates (649 and 738) and asymptomatic carrier isolates (127P and 129Pt) of H. somnus were tested for their apoptotic effects on bovine endothelial cells. LOS (500 ng/ml) isolated from different isolates of H. somnus induced apoptosis in bovine endothelial cells in a time-dependent manner (Fig. 8). Endothelial cells treated with H. somnus with LOS from strains 649, 738, 127P, and 129Pt at concentrations ranging from 5 to 500 ng/ml induced various levels of endothelial cell apoptosis (data not shown). Significant levels of endothelial cell apoptosis (P < 0.05) were observed as early as 2 h of incubation. The preincubation of LOS with polymyxin B (10 μg/ml) (Fig. 9) resulted in a significant decrease in endothelial cell apoptosis (P < 0.005). The abilities of LOS to induce endothelial cell apoptosis and polymyxin B to inhibit this process were confirmed by performing internucleosomal DNA fragmentation assays (data not shown).
FIG. 8.
H. LOS induces bovine endothelial cell apoptosis. Bovine pulmonary artery endothelial cells (5 × 104) were incubated with 500 ng of LOS/ml isolated from pathogenic isolates (649 and 738) and asymptomatic carrier isolates (127P and 129Pt) of H. somnus for 2, 6, and 22 h at 37°C with 5% CO2. Endothelial cells incubated in medium alone (control) or with staurosporine (200 nM; Stauro) were used as negative and positive controls, respectively. Apoptosis was detected by Hoechst 33342 staining. Five random areas per coverslip were sampled, and 100 random cells were analyzed for apoptosis. The data illustrate the mean ± SEM of three separate experiments. Statistical analysis was performed by ANOVA followed by the Tukey-Kramer significant difference test. Asymptomatic carrier and pathogenic isolate LOS used in these experiments induced significant (P < 0.05) levels of endothelial cell apoptosis at each time point, compared to those of untreated (Control) cells.
FIG. 9.
Polymyxin B inhibits LOS-mediated apoptosis of bovine endothelial cells. Bovine pulmonary artery endothelial cells (5 × 104) were incubated with 500 ng of H. somnus LOS/ml with (□) or without (■) 10 μg polymyxin B (PMB)/ml for 22 h at 37°C with 5% CO2. Controls consisted of endothelial cells incubated in medium alone (UT), 10 μg of polymyxin B/ml, or staurosporine (200 nM; Stauro). Apoptosis was detected by Hoechst 33342 staining. The data illustrate the mean ± SEM of three separate experiments. Statistical analysis was performed by ANOVA followed by the Tukey-Kramer significant difference test. There was a significant reduction in the level of apoptosis (∗, P < 0.005) when endothelial cells were treated with polymyxin B and LOS compared to that of LOS-treated endothelial cells.
DISCUSSION
There have been few reports characterizing the interaction of H. somnus organisms and bovine endothelial cells, which is surprising since vascular disease and septicemia are commonly observed in H. somnus infections (26). Thompson and Little (57) were the first to demonstrate that H. somnus adhered to endothelial cells from arterial explants. Adherence was associated with cytotoxic changes, including rounding and shrinkage of the endothelial cells (57). The focus of the aforementioned study was to compare the adherence of H. somnus, E. coli, and S. enterica serovar Typhimurium organisms to vascular endothelial cells. Although these findings suggest that apoptosis might be occurring, the mechanism of cellular cytotoxicity was not addressed. However, the authors suggested that it might be linked to H. somnus endotoxin. Kwiecien et al. (37) demonstrated that treatment of bovine endothelial cells with TNF-α increased the adherence of H. somnus. These findings were accompanied by morphological changes in the endothelial cells (i.e., membrane blebbing, rounding, and cellular shrinkage) that are characteristic of an apoptotic process. However, it was not clear in this study whether the morphological changes were induced by TNF-α, H. somnus, or a combination thereof. These findings led us to hypothesize that H. somnus damages the endothelial cell in vitro via apoptosis.
Several bacterial pathogens (Staphylococcus aureus [41] Rickettsia rickettsii [5], Orientia tsutsugamushi [33, 34], and others) have been shown to induce endothelial cell apoptosis in vitro. This process may contribute to the pathogenesis of the diseases they cause in vivo by impairing immune surveillance and promoting bacterial survival. In the present study, we found a similar ability of pathogenic and asymptomatic carrier isolates of H. somnus to cause endothelial cell apoptosis in vitro (Fig. 3 and 4). These results were surprising, since asymptomatic carrier isolates of H. somnus are typically not associated with septicemia and vasculitis in vivo. However, they were similar to the H. somnus adherence results reported by Kwiecien et al. (37). In the aforementioned study, pathogenic and asymptomatic carrier isolates of H. somnus adhered to endothelial cells at comparable levels and appeared to induce similar morphological changes (37). The results of our study, together with those of Kwiecien et al. suggest that pathogenic and asymptomatic carrier isolates of H. somnus both possess the virulence factor(s) necessary to induce endothelial cell apoptosis. However, it is likely that asymptomatic carrier isolates of H. somnus do not reach vascular endothelial cells in vivo. Perhaps sensitivity to serum killing (8), killing by phagocytic cells, or other factors prevent hematogenous dissemination of asymptomatic carrier isolates of H. somnus.
Our data demonstrate that H. somnus rapidly delivers its apoptotic signal to endothelial cells within 1 h of treatment and that extracellular growth by H. somnus is not necessary for endothelial cell apoptosis (Fig. 5). H. somnus strain 8025 could be an exception to this observation, since its growth rate and release of apoptotic activity into its culture filtrates appeared to be enhanced compared to those of other strains of H. somnus. Although internalized H. somnus organisms were rarely observed during our transmission electron microscopy studies, we cannot exclude the possibility that they are partially responsible for the apoptotic effect.
Previously, our group and other investigators have examined the interaction of H. somnus organisms with phagocytic cells (12, 20, 21, 38, 50, 62). In an earlier study, we demonstrated that H. somnus culture filtrates had no effect on chromatin condensation in bovine PMNs (62), which differs from what we found for endothelial cells. In that study, heat-killed H. somnus organisms induced less chromatin condensation in bovine PMNs than did viable H. somnus, which is consistent with the findings in the present report. Perhaps longer incubation periods with H. somnus culture filtrates would have produced chromatin condensation in bovine PMNs. Alternatively, perhaps bovine PMNs lack the receptor(s) necessary to transduce the H. somnus released virulence factor(s) present in culture filtrates.
Our data suggest that both heat-labile and heat-stable factors released from H. somnus cause endothelial cell apoptosis (Fig. 7). One likely candidate for such a virulence factor is LOS, which is a heat-stable constituent of the outer membrane of certain nonenteric gram-negative bacteria (29). The related molecule, lipopolysaccharide (LPS), has been shown to cause apoptosis of bovine endothelial cells in vitro (14, 42) and vasculitis in vivo (1, 64). In the present study, LOS isolated from both pathogenic and asymptomatic carrier isolates of H. somnus induced endothelial cell apoptosis in a time- and dose-dependent manner (Fig. 8 and 9). Somewhat surprisingly, LOS from asymptomatic carrier isolates of H. somnus generally induced slightly higher levels of endothelial cell apoptosis than LOS from pathogenic isolates. This may be explained by the lack of phase variation in H. somnus asymptomatic carrier isolate LOS. As a result, these might contain more lipid A and less carbohydrate, on a weight basis, than LOS from pathogenic isolates (29, 30). It is possible that the membrane blebs produced by viable H. somnus organisms also contain heat-labile components (i.e., lipoproteins and IgBPs), which may facilitate LOS delivery to and amplify the biological effects on the endothelial cell. IgBPs, which are heat labile, do not appear to play a major role in endothelial apoptosis. This is demonstrated by H. somnus strain 129Pt, which lacks IgBPs and surface fibrils (7, 10) yet induced levels of endothelial cell apoptosis comparable to those of H. somnus strains that contained IgBPs (i.e., 649, 2336, 8028, and 127P). Perhaps heat-labile factors mediate apoptosis independently of LOS or other heat-stable virulence factors. Results similar to our own were noted when human endothelial cells were treated with LOS from N. meningitidis (13), which is structurally similar to that of H. somnus. N. meningitidis-mediated cytotoxicity of human endothelial cells in vitro was influenced by the expression of pili and capsule (13, 51). However, the majority of clinical disease in meningococcal meningitis appears to be due to high serum levels of LOS and subsequent cytokine release (P. Brandtzaeg, R. Ovstebo, and P. Kierulf, abstr. S21, J. Endotoxin Res. 1[Suppl. 1]:7). Overall, these data suggest that LOS from several pathogens may be a key virulence factor in the pathogenesis of vascular disease.
We have obtained additional data supporting the relevance of the in vitro investigations reported in this paper. We have detected apoptotic inflammatory cells in fixed lung tissue from cattle experimentally infected with H. somnus and apoptotic endothelial cells in spontaneous cases of TME (unpublished data). These data suggest that endothelial cell apoptosis is not an artifact of our in vitro experimental conditions. Because a relationship between apoptotic endothelial cells and thrombosis has been reported (4, 22), we believe that our results indicate a possible mechanism by which H. somnus could induce thrombosis in vivo.
In summary, we have demonstrated that H. somnus induces apoptosis of bovine endothelial cells in vitro. Viable bacteria elicited higher levels of endothelial cell apoptosis than did killed H. somnus bacteria. The release of heat-labile and heat-stable virulence factors by H. somnus also mediated endothelial cell apoptosis. Exposure of endothelial cells to H. somnus LOS resulted in apoptosis, suggesting that LOS may be a key virulence factor capable of mediating endothelial cell damage, and ultimately vasculitis.
ACKNOWLEDGMENTS
The work was supported by funding from the University of Wisconsin School of Veterinary Medicine, the United States Department of Agriculture National Research Initiative (00-35204-9212 to C.J.C., 99-35204-7670 to T.J.I., and 98-35204-6733 to L.B.C.), and the University-Industry Research fund from the University of Wisconsin.
We thank Jim Vann for assistance in endothelial cell preparation, Renate Bromberg for assistance with electron microscopy, and Mike Howard for preparation of H. somnus LOS.
REFERENCES
- 1.Aguillon J C, Ferreira V, Nunez E, Paredes L, Molina M C, Colombo A, Hermosilla T, Ferreira A. Immunomodulation of LPS ability to induce the local Shwartzman reaction. Scand J Immunol. 1996;44:551–555. doi: 10.1046/j.1365-3083.1996.d01-345.x. [DOI] [PubMed] [Google Scholar]
- 2.Aliprantis A O, Yang R B, Mark M R, Suggett S, Devaux B, Radolf J D, Klimpel G R, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–739. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 3.Andrews J J, Anderson T D, Slife L N, Stevenson G W. Microscopic lesions associated with the isolation of Haemophilus somnus from pneumonic bovine lungs. Vet Pathol. 1985;22:131–136. doi: 10.1177/030098588502200206. [DOI] [PubMed] [Google Scholar]
- 4.Bombeli T, Karsan A, Tait J F, Harlan J M. Apoptotic vascular endothelial cells become procoagulant. Blood. 1997;89:2429–2442. [PubMed] [Google Scholar]
- 5.Clifton D R, Goss R A, Sahni S K, van Antwerp D, Baggs R B, Marder V J, Silverman D J, Sporn L A. NF-kappa B-dependent inhibition of apoptosis is essential for host cell survival during Rickettsia rickettsii infection. Proc Natl Acad Sci USA. 1998;95:4646–4651. doi: 10.1073/pnas.95.8.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole S P, Guiney D G, Corbeil L B. Two linked genes for outer membrane proteins are absent in four non-disease strains of Haemophilus somnus. Mol Microbiol. 1992;6:1895–1902. doi: 10.1111/j.1365-2958.1992.tb01362.x. [DOI] [PubMed] [Google Scholar]
- 7.Cole S P, Guiney D G, Corbeil L B. Molecular analysis of a gene encoding a serum-resistance-associated 76 kDa surface antigen of Haemophilus somnus. J Gen Microbiol. 1993;139:2135–2143. doi: 10.1099/00221287-139-9-2135. [DOI] [PubMed] [Google Scholar]
- 8.Corbeil L B, Blau K, Prieur D J, Ward A C. Serum susceptibility of Haemophilus somnus from bovine clinical cases and carriers. J Clin Microbiol. 1985;22:192–198. doi: 10.1128/jcm.22.2.192-198.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corbeil L B, Arthur J E, Widders P R, Smith J W, Barbet A F. Antigenic specificity of convalescent serum from cattle with Haemophilus somnus-induced experimental abortion. Infect Immun. 1987;55:1381–1386. doi: 10.1128/iai.55.6.1381-1386.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corbeil L B, Bastida-Corcuera F D, Beveridge T J. Haemophilus somnus immunoglobulin binding proteins and surface fibrils. Infect Immun. 1997;65:4250–4257. doi: 10.1128/iai.65.10.4250-4257.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox A D, Howard M D, Brisson J R, van der Zwan M, Thibault P, Perry M B, Inzana T J. Structural analysis of the phase-variable lipooligosaccharide from Haemophilus somnus strain 738. Eur J Biochem. 1998;253:507–516. doi: 10.1046/j.1432-1327.1998.2530507.x. [DOI] [PubMed] [Google Scholar]
- 12.Czuprynski C J, Hamilton H L. Bovine neutrophils ingest but do not kill Haemophilus somnus. Infect Immun. 1985;50:431–436. doi: 10.1128/iai.50.2.431-436.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn K L, Virji M, Moxon E R. Investigations into the molecular basis of meningococcal toxicity for human endothelial and epithelial cells: the synergistic effect of LPS and pili. Microb Pathog. 1995;18:81–96. doi: 10.1016/s0882-4010(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 14.Frey E A, Finlay B B. Lipopolysaccharide induces apoptosis in a bovine endothelial cell line via a soluble CD14 dependent pathway. Microb Pathog. 1998;24:101–109. doi: 10.1006/mpat.1997.0178. [DOI] [PubMed] [Google Scholar]
- 15.Fuse T, Yoon K W, Kato T, Yamada K. Heat-induced apoptosis in human glioblastoma cell line A172. Neurosurgery. 1998;42:843–849. doi: 10.1097/00006123-199804000-00092. [DOI] [PubMed] [Google Scholar]
- 16.Gogolewski R P, Kania S A, Inzana T J, Widders P R, Liggitt H D, Corbeil L B. Protective ability and specificity of convalescent serum from Haemophilus somnus pneumonia. Infect Immun. 1987;55:1403–1411. doi: 10.1128/iai.55.6.1403-1411.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gogolewski R P, Leathers C W, Liggitt H D, Corbeil L B. Experimental Haemophilus somnus pneumonia in calves and immunoperoxidase localization of bacteria. Vet Pathol. 1987;24:250–256. doi: 10.1177/030098588702400309. [DOI] [PubMed] [Google Scholar]
- 18.Gogolewski R P, Kania S A, Liggitt H D, Corbeil L B. Protective ability of antibodies against 28- and 40-kilodalton outer membrane antigens of Haemophilus somnus. Infect Immun. 1988;56:2307–2316. doi: 10.1128/iai.56.9.2307-2316.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gogolewski R P, Schaefer D C, Wassan S K, Corbeil R R, Corbeil L B. Pulmonary persistence of Haemophilus somnus in the presence of specific antibody. J Clin Microbiol. 1989;27:1767–1774. doi: 10.1128/jcm.27.8.1767-1774.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomis S M, Godson D L, Beskorwayne T, Wobeser G A, Potter A A. Modulation of phagocytic function of bovine mononuclear phagocytes by Haemophilus somnus. Microb Pathog. 1997;22:13–21. doi: 10.1006/mpat.1996.0086. [DOI] [PubMed] [Google Scholar]
- 21.Gomis S M, Godson D L, Wobeser G A, Potter A A. Intracellular survival of Haemophilus somnus in bovine blood monocytes and alveolar macrophages. Microb Pathog. 1998;25:227–235. doi: 10.1006/mpat.1998.0228. [DOI] [PubMed] [Google Scholar]
- 22.Greeno E W, Bach R R, Moldow C F. Apoptosis is associated with increased cell surface tissue factor procoagulant activity. Lab Investig. 1996;75:281–289. [PubMed] [Google Scholar]
- 23.Groom S, Little P, Rosendal S. Virulence differences among three strains of Haemophilus somnus following intratracheal challenge of calves. Can J Vet Res. 1988;52:349–354. [PMC free article] [PubMed] [Google Scholar]
- 24.Harris F W, Janzen E D. The Haemophilus somnus disease complex (haemophilosis): a review. Can J Vet Res. 1989;30:816–822. [PMC free article] [PubMed] [Google Scholar]
- 25.Hirschfeld M, Kirschning C J, Schwandner R, Wesche H, Weis J H, Wooten R M, Weis J J. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J Immunol. 1999;163:2382–2386. [PubMed] [Google Scholar]
- 26.Humphrey J D, Stephens L. Haemophilus somnus: a review. Vet Bull. 1983;53:987–1004. [Google Scholar]
- 27.Humphrey J D, Little P B, Stephens L R, Barnum D A, Doig P A, Thorsen J. Prevalence and distribution of Haemophilus somnus in the male bovine reproductive tract. Am J Vet Res. 1982;43:792–795. [PubMed] [Google Scholar]
- 28.Inzana T J, Corbeil L B. Development of a defined medium for Haemophilus somnus isolated from cattle. Am J Vet Res. 1987;48:366–369. [PubMed] [Google Scholar]
- 29.Inzana T J, Iritani B, Gogolewski R P, Kania S A, Corbeil L B. Purification and characterization of lipooligosaccharides from four strains of Haemophilus somnus. Infect Immun. 1988;56:2830–2837. doi: 10.1128/iai.56.11.2830-2837.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inzana T J, Gogolewski R P, Corbeil L B. Phenotypic phase variation in Haemophilus somnus lipooligosaccharide during bovine pneumonia and after in vitro passage. Infect Immun. 1992;60:2943–2951. doi: 10.1128/iai.60.7.2943-2951.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inzana T J, Hensley J, McQuiston J, Lesse A J, Campagnari A A, Boyle S M, Apicella M A. Phase variation and conservation of lipooligosaccharide epitopes in Haemophilus somnus. Infect Immun. 1997;65:4675–4681. doi: 10.1128/iai.65.11.4675-4681.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson J A, Andrews J J, Hargis J W. Experimental Haemophilus somnus pneumonia in calves. Vet Pathol. 1987;24:129–134. doi: 10.1177/030098588702400205. [DOI] [PubMed] [Google Scholar]
- 33.Kee S H, Cho K A, Kim M K, Lim B U, Chang W H, Kang J S. Disassemply of focal adhesions during apoptosis of endothelial cell line ECV304 infected with Orientia tsutsugamushi. Microb Pathog. 1999;27:265–271. doi: 10.1006/mpat.1999.0304. [DOI] [PubMed] [Google Scholar]
- 34.Kim M K, Kee S H, Cho K A, Chung M H, Lim B U, Chang W H, Kang J S. Apoptosis of endothelial cell line ECV304 persistently infected with Orientia tsutsugamushi. Microbiol Immunol. 1999;43:751–757. doi: 10.1111/j.1348-0421.1999.tb02466.x. [DOI] [PubMed] [Google Scholar]
- 35.Kwiecien J M, Little P B. Isolation of pathogenic strains of Haemophilus somnus from the female bovine reproductive tract. Can J Vet Res. 1992;56:127–134. [PMC free article] [PubMed] [Google Scholar]
- 36.Kwiecien J M, Little P B. Haemophilus somnus and reproductive disease in the cow. Can J Vet Res. 1991;32:595–601. [PMC free article] [PubMed] [Google Scholar]
- 37.Kwiecien J M, Little P B, Hayes M A. Adherence of Haemophilus somnus to tumor necrosis factor-alpha-stimulated bovine endothelial cells in culture. Can J Vet Res. 1994;58:211–219. [PMC free article] [PubMed] [Google Scholar]
- 38.Lederer J A, Brown J F, Czuprynski C J. “Haemophilus somnus”, a facultative intracellular pathogen of bovine mononuclear phagocytes. Infect Immun. 1997;55:381–387. doi: 10.1128/iai.55.2.381-387.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leite F, Malazdrewich C, Yoo H S, Maheswaran S K, Czuprynski C J. Use of TUNEL staining to detect apoptotic cells in the lungs of cattle experimentally infected with Pasteurella haemolytica. Microb Pathog. 1999;27:179–185. doi: 10.1006/mpat.1999.0295. [DOI] [PubMed] [Google Scholar]
- 40.McQuiston J H, Cox A D, Wu Y, Boyle S M, Inzana T J. Characterization of a DNA region containing 5′-(CAAT)(n)-3′ DNA sequences involved in lipooligosaccharide biosynthesis in Haemophilus somnus. Microb Pathog. 2000;28:301–312. doi: 10.1006/mpat.1999.0351. [DOI] [PubMed] [Google Scholar]
- 41.Menzies B E, Kourteva I. Internalization of Staphylococcus aureus by endothelial cells induces apoptosis. Infect Immun. 1998;66:5994–5998. doi: 10.1128/iai.66.12.5994-5998.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Messmer U K, Briner V A, Pfeilschifter J. Tumor necrosis factor-alpha and lipopolysaccharide induce apoptotic cell death in bovine glomerular endothelial cells. Kidney Int. 1999;55:2322–2337. doi: 10.1046/j.1523-1755.1999.00473.x. [DOI] [PubMed] [Google Scholar]
- 43.Miller R B, Lein D H, McEntee K E, Hall C E, Shaw S. Haemophilus somnus infection of the reproductive tract of cattle: a review. J Am Vet Med Assoc. 1983;182:1390–1392. [PubMed] [Google Scholar]
- 44.Monack D M, Mescas J, Bouley D, Falkow S. Yersenia-induced apoptosis in vivo aids in the establishment of a systemic infection in mice. J Exp Med. 1998;188:2127–2137. doi: 10.1084/jem.188.11.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monack D M, Hersh D, Ghori N, Bouley D, Zychlinski A, Falkow S. Salmonella exploits caspase-1 to colonize peyer's patches in a murine typhoid model. J Exp Med. 2000;192:249–258. doi: 10.1084/jem.192.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morrison D C, Jacobs D M. Binding of polymyxin B to the lipid A portion of bacterial LPS. Immunocytochemistry. 1976;13:813–818. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- 47.Nardini P M, Mellors A, Lo R Y. Characterization of a fourth lipoprotein from Pasteurella haemolytica A1 and its homology to the OmpA family of outer membrane proteins. FEMS Microbiol Lett. 1998;165:71–77. doi: 10.1111/j.1574-6968.1998.tb13129.x. [DOI] [PubMed] [Google Scholar]
- 48.Pfeifer C G, Campos M, Beskorwayne T, Babiuk L A, Potter A A. Effect of Haemophilus somnus on phagocytosis and hydrogen peroxide production by bovine polymorphonuclear leukocytes. Microb Pathog. 1992;13:191–202. doi: 10.1016/0882-4010(92)90020-o. [DOI] [PubMed] [Google Scholar]
- 49.Ryan U S, Clements E, Halliston D, Ryan J W. Isolation and culture of pulmonary artery endothelial cells. Tissue Cell. 1978;10:535–554. doi: 10.1016/s0040-8166(16)30347-0. [DOI] [PubMed] [Google Scholar]
- 50.Sample A K, Czuprynski C J. Elimination of hydrogen peroxide by Haemophilus somnus, a catalase-negative pathogen of cattle. Infect Immun. 1991;59:2239–2244. doi: 10.1128/iai.59.7.2239-2244.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scheuerpflug I, Rudel T, Ryll R, Pandit J, Meyer T F. Roles of PilC and PilE proteins in pilus-mediated adherence of Neisseria gonorrhoeae and Neisseria meningitidis to human erythrocytes and endothelial and epithelial cells. Infect Immun. 1999;67:834–843. doi: 10.1128/iai.67.2.834-843.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stephens L R, Little P B, Wilkie N, Barnum D A. Infectious thromboembolic meningoencephalitis in cattle: a review. J Am Vet Med Assoc. 1981;178:378–384. [PubMed] [Google Scholar]
- 53.Stephens L R, Little P B, Slee P, Poullton P, Lancombe M, Kosior E. Investigation of purulent vaginal discharge in cows, with particular reference to Haemophilus somnus. Aust Vet J. 1986;63:182–185. doi: 10.1111/j.1751-0813.1986.tb02969.x. [DOI] [PubMed] [Google Scholar]
- 54.Tagawa Y, Ishikawa H, Yuasa N. Purification and partial characterization of the major outer membrane protein of of Haemophilus somnus. Infect Immun. 1993;61:91–96. doi: 10.1128/iai.61.1.91-96.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Theisen M, Rioux C R, Potter A A. Molecular cloning, nucleotide sequence, and characterization of a 40,000-molecular-weight lipoprotein of Haemophilus somnus. Infect Immun. 1992;60:826–831. doi: 10.1128/iai.60.3.826-831.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Theisen M, Rioux C R, Potter A A. Molecular cloning, nucleotide sequence, and characterization of lppB, encoding an antigenic 40-kilodalton lipoprotein of Haemophilus somnus. Infect Immun. 1993;61:1793–1798. doi: 10.1128/iai.61.5.1793-1798.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thompson K G, Little P B. Effect of Haemophilus somnus on bovine endothelial cell in organ culture. Am J Vet Res. 1981;42:748–754. [PubMed] [Google Scholar]
- 58.Widders P R, Paisley L G, Gogolewski R P, Evermann J F, Smith J W, Corbeil L B. Experimental abortion and the systemic immune response to “Haemophilus somnus” in cattle. Infect Immun. 1986;54:555–560. doi: 10.1128/iai.54.2.555-560.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Widders P R, Paisley L A, Dorrance M, Yarnall M, Corbeil L B. Immunoglobulin-binding activity among pathogenic and carrier isolates of Haemophilus somnus. Infect Immun. 1989;57:639–642. doi: 10.1128/iai.57.2.639-642.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu Y, McQuiston J H, Cox A, Pack T D. Molecular cloning and mutagenesis of a DNA locus involved in lipooligosaccharide biosynthesis in Haemophilus somnus. Infect Immun. 2000;68:310–319. doi: 10.1128/iai.68.1.310-319.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang Q L, Tinsley C R, Gotschlich E C. Novel lipoprotein expressed by Neisseria meningitidis but not by Neisseria gonorrhoeae. Infect Immun. 1995;63:1631–1636. doi: 10.1128/iai.63.5.1631-1636.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang Y F, Sylte M J, Czuprynski C J. Apoptosis: a possible tactic of Haemophilus somnus for evasion of killing by bovine neutrophils? Microb Pathog. 1998;24:351–359. doi: 10.1006/mpat.1998.0205. [DOI] [PubMed] [Google Scholar]
- 63.Yarnell M, Widders P R, Corbeil L B. Isolation and characterization of Fc receptors from Haemophilus somnus. Scand J Immunol. 1988;28:129–137. doi: 10.1111/j.1365-3083.1988.tb02424.x. [DOI] [PubMed] [Google Scholar]
- 64.Ziesche R, Petkov V, Williams J, Zakeri S M, Mosgoller W, Knofler M, Block L H. Lipopolysaccharide and interleukin-1 augment the effects of hypoxia and inflammation in human pulmonary arterial tissue. Proc Natl Acad Sci USA. 1996;93:12478–12483. doi: 10.1073/pnas.93.22.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]