Abstract
Background
Halitosis is defined as a foul odour emitted from the oral cavity. Many interventions have been used to control halitosis from mouthwashes to chewing gums. Probiotics have been reported as an alternative method to alleviate halitosis.
Objective
The present study aimed to investigate the effect of probiotics on halitosis from a time perspective.
Design and methods
This is a meta-analysis study performed in indexed databases up to February 2021. Randomised controlled trials that compared the effects of probiotics and placebo on primary outcomes (organoleptic (OLP) scores and volatile sulfur compound (VSC) levels) and secondary outcomes (tongue coating scores (TCS) and plaque index (PI)) were included. Data extraction and quality assessment were conducted independently by two reviewers. Publication bias and leave-one-out analyses were performed.
Results
The standardised mean difference (SMD) and 95% CI were calculated to synthesise data. The data were subgrouped and analysed in the short term (≤4 weeks) and long term (>4 weeks) based on the follow-up time. Seven articles were included in this meta-analysis. The primary outcomes, OLP scores (SMD=−0.58; 95% CI −0.87 to –0.30, p<0.0001) and VSC levels (SMD=−0.26; 95% CI −0.51 to –0.01, p=0.04), both decreased significantly in the probiotics group compared with the placebo group in the short term. However, a significant reduction was observed only in OLP scores (SMD=−0.45; 95% CI −0.85 to –0.04, p=0.03) in the long term. No significant differences were observed in secondary outcomes. There was no evidence of publication bias. The leave-one-out analysis confirmed that the pooled estimate was stable.
Conclusions
According to the results of this work, it seems that probiotics (eg, Lactobacillus salivarius, Lactobacillus reuteri, Streptococcus salivarius and Weissella cibaria) may relieve halitosis in the short term (≤4 weeks). The results of the biased assessment, limited data and heterogeneity of the clinical trials included might reduce the reliability of the conclusions.
Keywords: Microbiology, Infectious diseases & infestations, Public health
Strengths and limitations of this study.
This study included larger randomised controlled trials involved in halitosis and probiotics.
The results were rationally analysed from the follow-up time perspective.
Subgroup analysis was done to identify the sources of heterogeneity based on the component of volatile sulfur compounds.
The included studies had limited patients.
Some studies reported the outcomes with different forms, increasing the heterogeneity of the results.
Introduction
Halitosis, also known as ‘oral malodour’, is typically defined as an unpleasant odour emanating from the oral cavity.1 Halitosis is the third most common disease for patient referral to the dentist, only ranking behind dental caries and periodontal disease.2 According to an epidemiological study, the prevalence of halitosis is approximately 27.5% in the Chinese population.3 People have a higher demand for social interactions and attach more importance to their personal image in today’s society. Halitosis has a significant impact on both patients’ daily work and social activities and may even result in frequent psychological problems such as anxiety, depression and social isolation.4 Clinically, halitosis is categorised into genuine halitosis, pseudo-halitosis and halitophobia.5 The latter two types are related to psychological conditions. Only genuine halitosis is caused by pathological and physiological factors. It includes intraoral halitosis and extraoral halitosis, with the former accounting for 80–90% of the cases.6
The main aetiological factor of genuine halitosis is the volatile sulfur compounds (VSC) produced by oral bacteria via complex microbe–substrate and microbe–microbe interactions and putrefaction of organic substrates in the oral cavity, associated with poor oral hygiene, tongue coating and periodontal disease.7–10 In particular, hydrogen sulfide (H2S), methyl mercaptan (CH3SH) and dimethyl sulfide (C2H6S) are considered significant parameters and markers of halitosis.11 Some microorganisms such as Fusobacterium nucleatum, Porphyromonas gingivalis, Prevotella intermedia, Prevotella nigrescens and Treponema denticola are involved in periodontal diseases and may also facilitate the production of VSC metabolism.12 Some studies using 16S rRNA amplicon sequencing and GC-MS-based metabolite profiling found that the bacterial composition, diversity and metabolites of the halitosis group were different from those of the control group.13 14 Therefore, the anaerobic oral condition might play an important role in the development of halitosis. Consequently, regulating the balance of the oral microbiota to reduce VSC levels is important in the management of halitosis.
The current treatments for halitosis include mechanical cleaning (scaling and tongue scraping) and chemical therapy (antibiotics, mouthwashes and other agents).15 16 However, mechanical therapy is often uncomfortable, even if carried out by the dentist. In addition, although chemical therapy is generally effective for a short time, it is always associated with various side effects including the emergence of dysbacteriosis and staining of the tongue and teeth.17–20 Consequently, new methods with fewer side effects are constantly being suggested to inhibit halitosis.
As live microorganisms, probiotics confer benefits to the host when administered in appropriate amounts.21 Their beneficial effects are primarily related to regulating the local microenvironment through the prevention of adhesion of pathogens and inhibition of growth of pathogens through the production of bacteriocins.22 23 Recently, probiotics such as Lactobacillus reuteri and Bifidobacteria have been widely used in the oral field.24 There is a growing body of evidence that the administration of probiotics might affect the composition of oral biofilms. They have also been investigated in the treatment of periodontal25 26 and peri-implant diseases,27 28 caries,29 oral candidiasis30 31 and oral mucositis induced by chemoradiotherapy.32 Meanwhile, probiotics have also been reported as an alternative strategy to relieve halitosis.33–37 However, a previous systematic review showed that probiotic therapy for halitosis is associated with insufficient evidence for it to be recommended.38 Thus, it is necessary to carry out a focused analysis of the therapeutic effects of probiotics in the treatment of halitosis.
This systematic review and meta-analysis was undertaken to investigate the effect of probiotics in managing halitosis from a time perspective to provide some evidence for the administration of probiotics in this field.
Methods
Patient and public involvement
No patients were involved in the study.
Study design
This systematic review was based on the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and registered in the PROSPERO (CRD42021227504).39 According to the Population, Intervention, Comparison, Outcomes and Study (PICOS) principle, the following focused question was structured: What is the clinical efficacy of probiotics in patients with halitosis when compared with placebo treatment? To answer our research question, we selected clinical trials according to the following study inclusion and exclusion criteria.
Search strategy
A critical electronic search was conducted in the bibliographic databases including PubMed, EMBASE, Web of Science and Cochrane Central Register of Controlled Trials up to and including February 2021 to select the published literature. Additionally, grey literature was searched in the database System for Information on Grey literature in European and Google Scholar. The reference lists of the included articles and some related Chinese journals (Chinese Journal of Stomatology, West China Journal of Stomatology, Journal of Oral Science Research, Journal of Practical Stomatology) were also searched manually. There was no language restriction.
An initial search strategy was conducted in PubMed with the combination of Medical Subject Headings (Mesh) terms identified by an asterisk symbol (*) and free-text words as follows: Probiotic OR Probiotic* OR Probiotic therapy OR Probiotic effect OR Probiotic treatment AND halitosis OR halitosis* OR malodor OR oral malodor OR malodour OR bad breath OR fetor oris. The detailed search strategy for each database is shown in online supplemental file 1. Endnote X7 was used for electronic title management. First, primary screening was performed independently by two reviewers (NH and JL) based on the titles and abstracts. The full-text articles were then used to assess the eligibility further. Any disagreement was solved by consulting a third reviewer.
bmjopen-2022-060753supp001.pdf (62.4KB, pdf)
Study inclusion and exclusion criteria
The study population comprised patients diagnosed with halitosis. The intervention was probiotic therapy, representing the experimental group. The control group received placebo treatment. The considered outcomes were halitosis parameters and other indices before and after treatment. During the first stage of the study selection, studies meeting the following conditions were considered eligible for this review: (1) study types: randomised controlled clinical trials (RCTs) or randomised controlled crossover studies; (2) participants: systemically healthy patients diagnosed with halitosis via accepted standards (the organoleptic (OLP) scores and/or the concentrations of VSC); (3) interventions: evaluating the efficacy of probiotics with placebo regardless of the probiotic species and the consumption method; (4) control interventions: placebo treatment; (5) clinical data: the measurement values, including halitosis parameters and other indices before and after treatment. At the second stage of the selection, eligible studies acquired in the first stage were identified according to the following exclusion criteria: (1) in vitro and animal studies, letter to the editor, review articles, interviews and meta-analyses; (2) unclear halitosis identification; (3) studies with no completed data obtained even by contacting the authors; (4) interventions included other measures (eg, studies comparing tongue scraping plus chlorhexidine plus probiotics and tongue scraping plus chlorhexidine).34
Halitosis assessment
The primary outcomes were evaluated for OLP scores and the VSC concentration levels. OLP scores reflecting subjective perception were often treated as the gold standard for diagnosing halitosis clinically and in research.40 41 The OLP scores were estimated by two or three evaluators (with training and experience in calibrating tests). Subjects closed their mouth for 1 min and then exhaled slowly from their mouth into the evaluator’s nose at a distance of 10 cm. The score was evaluated according to a six-point scale of 0–5 (Rosenberg scale).42
Measurement of VSC concentrations is an objective method using the Halimeter or OralChroma, with no significant difference between them.43 Compared with OLP evaluation, measurement of VSC concentrations is a quantitative variable with high sensitivity and reproducibility.44–46 Subjects had to keep their mouth closed and stop talking for 5 min before measurements. For measurement with the Halimeter, a beverage straw (fixed and attached to the device) was inserted into the subject’s mouth, located at the back of the tongue dorsum. Subjects were asked to keep their mouth slightly open and breathe through their nose. For measurement with the OralChroma device, subjects were asked to keep their mouth closed for 30 s with an air-tight syringe. Then, 1 mL of mouth air was extracted from the subject and injected into the OralChroma to measure the VSC concentration.47 The mean of the results given by the evaluators or machines was then used.
Risk of bias
The included studies underwent a quality assessment with the Revised Cochrane risk of bias tool for randomised trials (RoB2).48 This tool assesses the risk of bias in five domain areas including the randomisation process, deviations from intended interventions, missing outcome data, measurement of outcome and selection of the reported result. Each domain assessed bias following several signalling questions. The overall bias was classified as a high risk of bias, some concerns, or a low risk of bias determined by a validated algorithm. After screening the articles, two reviewers (NH and JL) conducted the assessment independently to reach an agreement.
Data extraction
Data were extracted with a researcher-designed data form with the following information: (1) basic information of the included studies (first author’s name and the year of publication); (2) study type (RCT); (3) diagnostic criteria for halitosis; (4) characteristics of the participants (sample volume, age range); (5) treatment (probiotic administration including the type of bacteria, vehicles, doses and frequencies); (6) clinical parameters (including the primary and secondary outcomes of final participants); and (7) significance and follow-up periods.
The follow-up periods referred to the duration of probiotic use. If probiotic treatment ceased during the observation period, only the data before ceasing treatment were included. Concerning clinical parameters, OLP scores and VSC concentrations were considered the primary outcomes, directly associated with halitosis. The secondary outcomes in this review included tongue coating scores (TCS) and plaque index (PI) because they are commonly regarded as causes of halitosis.
Statistical analysis
The statistical analysis was performed with Review Manager 5.3 and Stata 12.0. All the data were group-analysed according to the follow-up time. The time ≤4 weeks was considered the short-term period and the time >4 weeks was considered the long-term period. In one study with three observation periods, the values at 4 weeks were analysed in the short term to be consistent with that of the other studies.49 Study heterogeneity was evaluated using Q statistics and the I2 test. A p value <0.10 was treated as the standard test. When I2 was >50% or p<0.10, there was significant heterogeneity between the studies,50–52 so subgroup analysis and sensitivity analysis were then performed to analyse the sources of heterogeneity. The continuous data on the halitosis parameters of the present studies were expressed as the standardised mean difference (SMD) with 95% CI. A random-effect model was used for analysis. Therefore, the mean difference and SD had to be acquired. If the original text did not provide the related data, the mean difference could be calculated and the SD was obtained using the formula: rd=sqrt (r12/n1+r22/n2). Excel sheets in the articles were used to convert the values when provided with median and interquartile range.53 54 Publication bias was performed subjectively by funnel plots and objectively by Egger’s tests. In the Egger’s test, a p value <0.05 indicates the presence of publication bias.55 Sensitivity analysis (leave-one-out method) was conducted to assess the alteration by sequential omission of individual studies.56
Results
Study selection
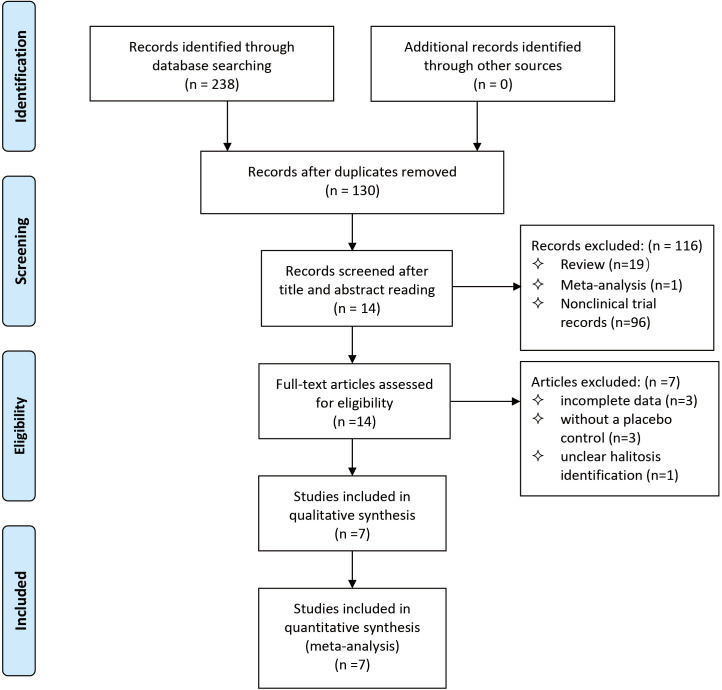
In total, 238 articles were potentially identified by electronic and manual searches. After eliminating the duplicates, 14 articles were included by screening the titles and abstracts. These studies were evaluated by reading the full texts and seven articles met the final inclusion criteria (figure 1).42 49 57–61
Figure 1.
Flow diagram of literature search strategy and inclusion and exclusion criteria.
Study characteristics
Table 1 shows the main characteristics of the included studies. All the studies were RCTs. The number of participants in the studies ranged between 23 and 68, with an age range of 19–70 years. Halitosis was diagnosed with OLP scores and/or VSC concentrations. The probiotics and placebo groups were compared, and the follow-up periods varied from 2 weeks to 12 weeks.
Table 1.
Characteristics of the included studies
| Study | Type | Halitosis criterion | Subject age | Clinical parameters | Probiotics administration (vehicle, strains and frequency) |
Follow-up |
| Mousquer et al61 | RCT Placebo double-masked parallel |
OLP score ≥1 | 29 ≥18 |
OLP VSC TCS |
Gum including 1 billion CFU Lactobacillus salivarius G60 taken twice per day | Baseline 2 weeks |
| Lee et al60 | RCT Placebo double-blind parallel |
VSC ≥1.5 ng/10 mL | 68 20–39 |
OLP VSC (H2S, CH3SH, C2H6S) |
800 mg tablet containing 1.0×108 CFU/g Weissella cibaria taken once per day | Baseline 4 weeks 8 weeks |
| He et al42 | RCT Placebo double-blind parallel |
OLP score ≥2 VSC ≥150 ppb |
28 23–44 |
OLP VSC TCS PI |
Tablet containing 1×109 CFU Streptococcus salivarius K12 taken twice per day | Baseline 4 weeks |
| Keller et al58 | RCT Placebo double-blind crossover |
OLP score >1 | 25 19–25 |
OLP VSC |
Chewing gum containing Lactobacillus reuteri DSM 17938 and Lactobacillus reuteri ATCC PTA 5289, both with a concentration of 1×108 CFU taken twice per day | Baseline 2 weeks |
| Suzuki et al57 | RCT Double-blind placebo-controlled crossover |
OLP score ≥1.5 | 23 22–67 |
OLP VSC (H2S, CH3SH, C2H6S) PI TCS |
Tablet containing 6.7×108 CFU Lactobacillus salivarius WB21 and 280 mg xylitol taken three times per day | Baseline 2 weeks |
| Penala et al59 | RCT Placebo double-blind parallel |
OLP score >2 | 29 25–59 |
OLP PI |
Capsule mixture including Lactobacillus salivarius (2×109 CFU) and Lactobacillus reuteri (2×109 CFU) dissolved in 10 mL distilled water to rinse for 1 min twice daily | Baseline 4 weeks 12 weeks |
| Kim et al49 | RCT Placebo double-blind parallel |
OLP score ≥2 VSC ≥0.15 ng/mL |
58 20–70 |
VSC (H2S, CH3SH, C2H6S) OLP |
Bag of powder mixture including Weissella cibaria CMU (1.0×108 CFU) melted in the mouth once per day | Baseline 2 weeks 4 weeks 8 weeks |
CFU, colony forming units; C2H6S, dimethyl sulfide; CH3SH, methyl mercaptan; H2S, hydrogen sulfide; OLP, organoleptic; PI, plaque index; RCT, randomised controlled trial; TCS, tongue coating score; VSC, volatile sulfur compound.
Risk of bias
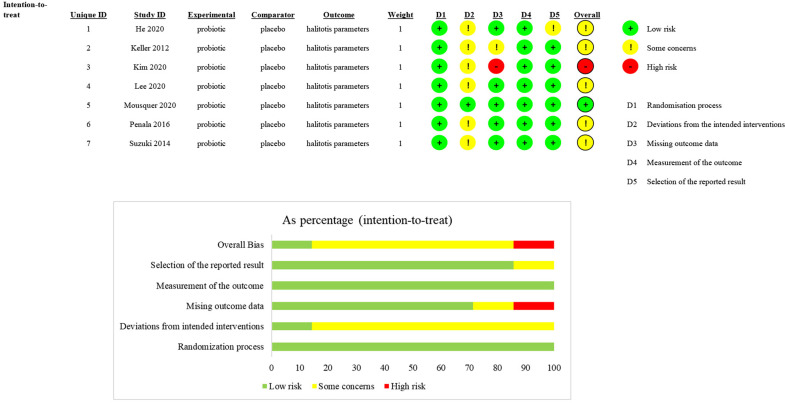
The bias estimation results showed that one study had a low risk of bias, one had a high risk and five showed some concerns. The reason for a high risk of bias was the incomplete outcome data of the OLP scores. Five articles were identified as having some concerns because there were many uncertain factors in their full texts. Figure 2 shows data on the risk of bias.
Figure 2.
Quality assessment of the selected studies (the Revised Cochrane risk of bias tool for randomised trials (RoB2)). Green represents a low risk of bias, yellow represents some concerns and red represents a high risk of bias.
Study outcomes
Primary outcomes
With regard to OLP, the studies by Keller et al and Penala et al showed a significant decrease in the probiotic group compared with the placebo group after treatment (p<0.05).58 59 In the study by Lee et al, which involved different follow-up periods, OLP scores decreased significantly in the test groups at 4 weeks (p=0.002) but not at 8 weeks (p=0.188) compared with the baseline.60 The results of the other four studies indicated that the OLP scores did not differ between the two groups.
With regard to VSC, six articles determined VSC concentrations, with three studies detecting the p values of VSC and subgroups (H2S, CH3SH and C2H6S).49 57 60 According to the results, only two studies57 60 reported a significant improvement in VSC levels in the experimental groups compared with the placebo groups.
Secondary outcomes
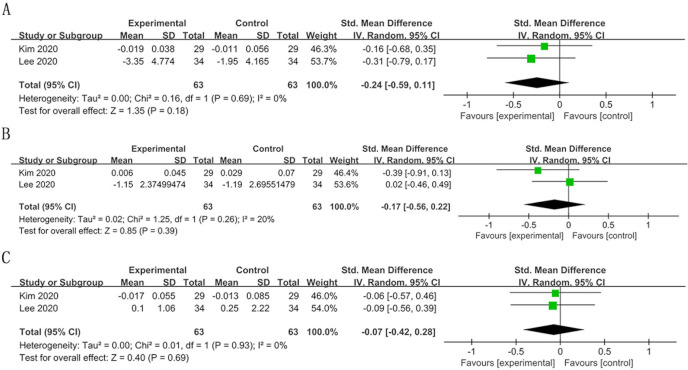
With regard to TCS, three studies evaluated the changes between the probiotic and placebo groups at 4 weeks.42 57 61 Although a reduced tendency was observed after treatment compared with baseline p values, there was no significant difference between the two groups.
With regard to PI, in the three studies involved,42 57 59 only one study showed a significant reduction in PI in the experimental group compared with the control group at 12 weeks.59
Quantitative synthesis
A meta-analysis was performed including studies with similar clinical parameters of OLP, VSC, TCS and PI, according to the follow-up time. Although the detection methods of VSC were different, both of the devices exhibited similar sensitivity and specificity in the detection of halitosis.43 Therefore, we analysed these values together. Considering the limitations of the included studies and follow-up time, the pooled estimations of TCS and PI were only performed in the short term.
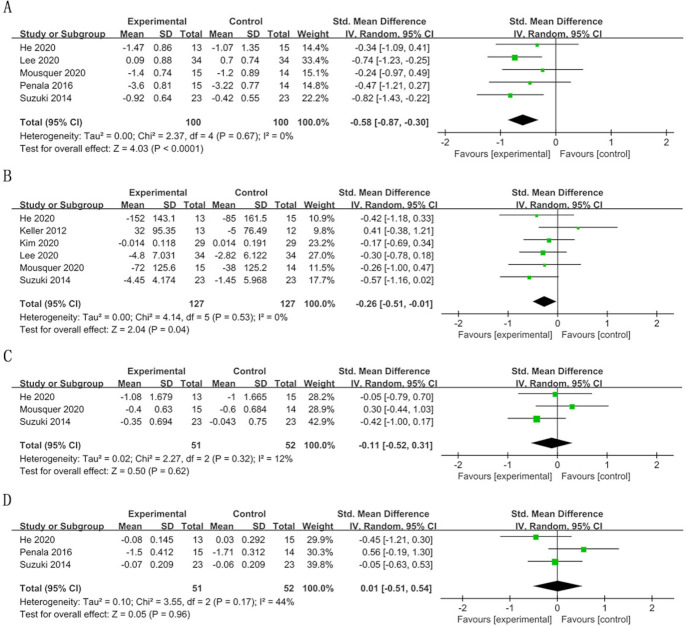
In the short term, the OLP scores significantly decreased in the probiotic group compared with the control group (SMD=−0.58; 95% CI −0.87 to –0.30, p<0.0001) (figure 3). A similar result was observed for VSC (SMD=−0.26; 95% CI −0.51 to –0.01, p=0.04) and H2S levels (SMD=−0.73; 95% CI −1.36 to –0.10, p=0.02). Other items (TCS, PI, CH3SH and C2H6S) were not significantly different between the experimental and control groups. The heterogeneity of each outcome was low (I2 <50%), except for H2S levels (I2=75%) (figures 3 and 4).
Figure 3.
Forest plot of halitosis parameters in the short term (≤4 weeks): (A) organoleptic (OLP) score; (B) volatile sulfur compound (VSC) concentration; (C) tongue coating score (TCS); (D) plaque index (PI).
Figure 4.
Forest plot of volatile sulfur compound (VSC) subgroups in the short term (≤4 weeks): (A) hydrogen sulfide (H2S); (B) methyl mercaptan (CH3SH); (C) dimethyl sulfide (C2H6S).
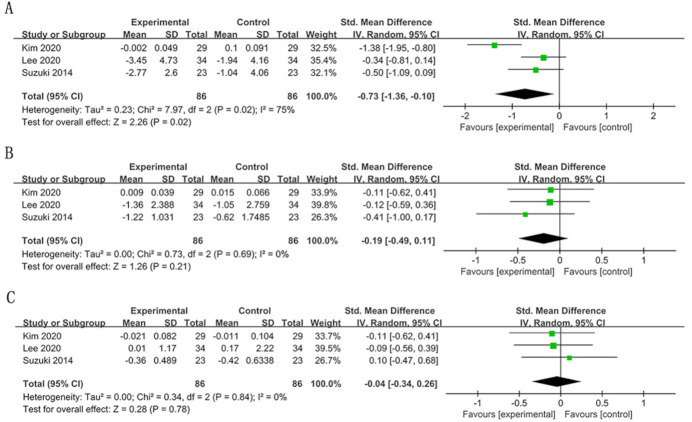
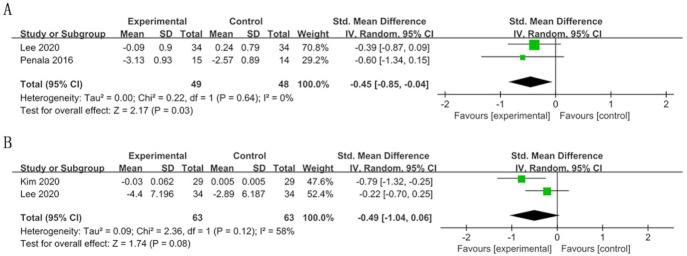
In the long term there was a significant improvement in OLP scores in the experimental group (SMD=−0.45; 95% CI −0.85 to –0.04, p=0.03) (figure 5). The results failed to show a significant difference in VSC concentrations and their subgroup levels (figures 5 and 6). The heterogeneity of VSC concentrations was substantial (I2=58%).
Figure 5.
Forest plot of halitosis parameters in the long term (>4 weeks): (A) organoleptic (OLP) score; (B) volatile sulfur compound (VSC) concentration.
Figure 6.
Forest plot of volatile sulfur compound (VSC) subgroups in the long term (>4 weeks): (A) hydrogen sulfide (H2S); (B) methyl mercaptan (CH3SH); (C) dimethyl sulfide (C2H6S).
Publication bias
In this systematic review and meta-analysis we found no evidence of publication bias according to the results of the funnel plots and Egger’s tests (p>0.05) (see online supplemental figures S1–S5).
bmjopen-2022-060753supp002.pdf (767.7KB, pdf)
Sensitivity analysis
Sensitivity analysis (leave-one-out method) showed no significant change in the pooled estimation when excluding any individual study (see online supplemental figures S6–S9).
Discussion
Summary of the findings
This meta-analysis showed that probiotics significantly reduced the OLP scores compared with the placebo group regardless of the duration of observation, confirming the benefits of probiotics for halitosis treatment. The probiotics group showed a significant reduction in VSC concentrations in the short term (≤4 weeks), with no noticeable difference in the long term (>4 weeks). Meta-analyses were also performed in the H2S, CH3SH and C2H6S subgroups to assess the concrete difference in VSC levels. The results showed that only H2S levels reduced noticeably in the short term when the probiotic treatment was administered. For TCS and PI, the results showed no significant differences between the experimental and placebo groups in the short term. There was no evidence of publication bias. The sensitivity analysis confirmed that the pooled estimate was stable.
Comparison of outcomes and possible mechanisms
Concerning the primary outcomes, the pooled estimation of OLP scores and VSC concentrations in the included articles were in favour of probiotic therapy rather than placebo in the short term.42 49 57–59 61 The biological mechanisms may be related to the interaction between probiotics and oral microbiota. According to the present studies, probiotic therapy reduces odorous compound levels by inhibiting the decomposition of amino acids and proteins by anaerobic bacteria.7 62 The significantly lower VSC levels with probiotic treatment in the short term might indicate a decrease in anaerobic bacteria activity. In contrast to our findings, a previous study indicated that it could not confirm the effect of probiotics on reducing VSC in the short term.38 The number of included articles may explain this difference. However, with regard to the results in the long term, only OLP scores showed a significant reduction. Oral microbiota contain VSC-producing bacteria and also other bacteria capable of producing other oral malodorous compounds (eg, indoles, skatole, pyridine, picolines and polyamines).63 The underlying mechanisms of the difference may result from the variation and abundance of the microbiota over time, which in turn affects the efficacy of probiotics, especially VSC concentration levels.35 49 61 Therefore, no significant effect on VSC concentrations in the long term may be due to the inhibitory effect of probiotics on those other bacteria. Therefore, the data on the changes in microorganisms in different periods are significant for the evaluation of probiotic effects. However, from the present studies, insufficient data in the included studies, differences in detection methods, bacterial species and heterogeneity of clinical trials limited the microorganism statistical analysis in this review.
Meanwhile, we found that the short-term outcome of H2S concentration change other than CH3SH and C2H6S was consistent with the total VSC levels. This might be related to differences in the function of probiotics and in the number and species of bacteria associated with each VSC reduction.12 35 64 Additionally, the regular VSC measurement device was reported to be more sensitive towards H2S than CH3SH and C2H6S,46 which may also account for the above result.
Regarding the secondary outcomes, based on the present meta-analysis there was no significant difference between the experimental and placebo groups in secondary outcomes during the observation time. One possible reason was the short observation time in the included studies, as one study included in the analysis showed a significant improvement in PI at 12 weeks.59 Tongue coating and periodontitis are often regarded as the leading causes of halitosis.42 65 However, in an original article the TCS and PI showed a pronounced decline after using probiotics compared with the baseline, with no decrease in the placebo group.61 This phenomenon might be related to the type of probiotics, some of which were reported to boost salivary flow by interacting with the oral microbiota.66
From the current studies, there are two main types of studies on the effect of probiotics on halitosis: one is to observe the effect during continuous use of probiotics and the other is to observe the effect at follow-up after stopping the use of probiotics. A recently published study indicated that no significant effect of probiotic use was found, which is different from our finding. The reason for the difference may be that this study analysed the collected follow-up data after stopping using probiotics for at least 2 weeks.67 In addition, OLP, as the gold standard, demonstrated the efficacy of probiotics in managing halitosis. However, the results of VSC concentration and subgroup analysis in the long term undermined this effect. These results with various different outcomes showed the inconsistency in this study. According to Bradford-Hill criteria, there would be less persuasive evidence for causation between the management of halitosis and probiotics.68 Therefore, more clinical and systematic studies are needed to explore and verify the probiotic effect on the management of halitosis in future research.
Limitations
There were several limitations in the present study throughout the whole review process. First, although both electronic and hand searches were conducted in four primary databases, it was not possible to retrieve all the relevant studies. Second, this study lacked persuasive evidence for causation between the management of halitosis and probiotics due to the inconsistency of the pooled results. Third, all included interventions differed in the species of probiotics, the doses and frequencies used and administration periods. A subgroup analysis was necessary to evaluate the source of efficacy concerning the probiotic species, but the small size of the included articles prevented further analysis. All these factors would inevitably affect the accuracy of the outcomes. Fourth, the detection methods of VSC were different. Although there is no significant difference between them, the combined analysis might still affect the reliability of the results. Fifth, in some included studies the primary outcomes were presented in different forms, such as percentages or interquartile range. Finally, some important parameters, including the microorganism species and changes, were not presented completely in some articles. The absence of partial original data or the differences caused by data conversion equally impaired the final results, although many methods were tried to reduce the bias.
Conclusion
This systematic review and meta-analysis indicates that probiotics (eg, Lactobacillus salivarius, Lactobacillus reuteri, Streptococcus salivarius and Weissella cibaria) may ease halitosis by reducing the VSC concentration levels in the short term, but there is no significant effect on the major cause of halitosis such as plaque and tongue coating. Considering the heterogeneity of the clinical trials included and the small sample size, more high-quality random clinical trials are required in the future to verify the results and to provide evidence for the efficacy of probiotics in the management of halitosis.
Supplementary Material
Footnotes
NH and JL contributed equally.
Contributors: NH and JL collected and analysed the data and drafted the manuscript. NH and XQ helped with the literature searching and statistical analysis. YW and CW provided help with the literature searching and figure revises. XQ and YL critically reviewed the manuscript. LL designed the experiment and critically reviewed the manuscript. NH and JL contributed equally to this paper. LL accepted full responsibility for the work of the study, had access to the data, and controlled the decision to publish as the guarantor. All authors have read and approved the final version of the manuscript.
Funding: This work was supported by the National Natural Science Foundation of China, China (Grant No. 81972538).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Porter SR, Scully C. Oral malodour (halitosis). BMJ 2006;333:632–5. 10.1136/bmj.38954.631968.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rayman S, Almas K. Halitosis among racially diverse populations: an update. Int J Dent Hyg 2008;6:2–7. 10.1111/j.1601-5037.2007.00274.x [DOI] [PubMed] [Google Scholar]
- 3.Liu XN, Shinada K, Chen XC, et al. Oral malodor-related parameters in the Chinese general population. J Clin Periodontol 2006;33:31–6. 10.1111/j.1600-051X.2005.00862.x [DOI] [PubMed] [Google Scholar]
- 4.Sanz M, Roldán S, Herrera D. Fundamentals of breath malodour. J Contemp Dent Pract 2001;2:1–17. [PubMed] [Google Scholar]
- 5.Yaegaki K, Coil JM. Examination, classification, and treatment of halitosis; clinical perspectives. J Can Dent Assoc 2000;66:257–61. [PubMed] [Google Scholar]
- 6.Madhushankari GS, Yamunadevi A, Selvamani M, et al. Halitosis – an overview. Part I: Classification, etiology, and pathophysiology of halitosis. J Pharm Bioallied Sci 2015;7:S339–43. 10.4103/0975-7406.163441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scully C, Porter S, Greenman J. What to do about halitosis. BMJ 1994;308:217–8. 10.1136/bmj.308.6923.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silva MF, Cademartori MG, Leite FRM, et al. Is periodontitis associated with halitosis? A systematic review and meta-regression analysis. J Clin Periodontol 2017;44:1003–9. 10.1111/jcpe.12786 [DOI] [PubMed] [Google Scholar]
- 9.Quirynen M, Dadamio J, Van den Velde S, et al. Characteristics of 2000 patients who visited a halitosis clinic. J Clin Periodontol 2009;36:970–5. 10.1111/j.1600-051X.2009.01478.x [DOI] [PubMed] [Google Scholar]
- 10.Stephen AS, Dhadwal N, Nagala V, et al. Interdental and subgingival microbiota may affect the tongue microbial ecology and oral malodour in health, gingivitis and periodontitis. J Periodontal Res 2021;56:1174–84. 10.1111/jre.12931 [DOI] [PubMed] [Google Scholar]
- 11.Tonzetich J. Production and origin of oral malodor: a review of mechanisms and methods of analysis. J Periodontol 1977;48:13–20. 10.1902/jop.1977.48.1.13 [DOI] [PubMed] [Google Scholar]
- 12.Foo LH, Balan P, Pang LM, et al. Role of the oral microbiome, metabolic pathways, and novel diagnostic tools in intra-oral halitosis: a comprehensive update. Crit Rev Microbiol 2021;47:359–75. 10.1080/1040841X.2021.1888867 [DOI] [PubMed] [Google Scholar]
- 13.Ye W, Zhang Y, He M, et al. Relationship of tongue coating microbiome on volatile sulfur compounds in healthy and halitosis adults. J Breath Res 2019;14:016005. 10.1088/1752-7163/ab47b4 [DOI] [PubMed] [Google Scholar]
- 14.Jo J-K, Seo S-H, Park S-E, et al. Identification of salivary microorganisms and metabolites associated with halitosis. Metabolites 2021;11:362. 10.3390/metabo11060362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loesche WJ, Kazor C. Microbiology and treatment of halitosis. Periodontol 2000 2002;28:256–79. 10.1034/j.1600-0757.2002.280111.x [DOI] [PubMed] [Google Scholar]
- 16.Pham TAV, Ueno M, Zaitsu T, et al. Clinical trial of oral malodor treatment in patients with periodontal diseases. J Periodontal Res 2011;46:722–9. 10.1111/j.1600-0765.2011.01395.x [DOI] [PubMed] [Google Scholar]
- 17.Costalonga M, Herzberg MC. The oral microbiome and the immunobiology of periodontal disease and caries. Immunol Lett 2014;162:22–38. 10.1016/j.imlet.2014.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fedorowicz Z, Aljufairi H, Nasser M, et al. Mouthrinses for the treatment of halitosis. Cochrane Database Syst Rev 2008;4:Cd006701. 10.1002/14651858.CD006701.pub2 [DOI] [PubMed] [Google Scholar]
- 19.Scully C, Greenman J, Halitology GJ. Halitology (breath odour: aetiopathogenesis and management). Oral Dis 2012;18:333–45. 10.1111/j.1601-0825.2011.01890.x [DOI] [PubMed] [Google Scholar]
- 20.van Steenberghe D, Avontroodt P, Peeters W, et al. Effect of different mouthrinses on morning breath. J Periodontol 2001;72:1183–91. 10.1902/jop.2000.72.9.1183 [DOI] [PubMed] [Google Scholar]
- 21.Guarner F, Perdigon G, Corthier G, et al. Should yoghurt cultures be considered probiotic? Br J Nutr 2005;93:783–6. 10.1079/bjn20051428 [DOI] [PubMed] [Google Scholar]
- 22.Cosseau C, Devine DA, Dullaghan E, et al. The commensal Streptococcus salivarius K12 downregulates the innate immune responses of human epithelial cells and promotes host-microbe homeostasis. Infect Immun 2008;76:4163–75. 10.1128/IAI.00188-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devine DA, Marsh PD. Prospects for the development of probiotics and prebiotics for oral applications. J Oral Microbiol 2009;1. 10.3402/jom.v1i0.1949. [Epub ahead of print: 01 05 2009]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gungor OE, Kirzioglu Z, Kivanc M. Probiotics: can they be used to improve oral health? Benef Microbes 2015;6:647–56. 10.3920/BM2014.0167 [DOI] [PubMed] [Google Scholar]
- 25.Vicario M, Santos A, Violant D, et al. Clinical changes in periodontal subjects with the probiotic Lactobacillus reuteri Prodentis: a preliminary randomized clinical trial. Acta Odontol Scand 2013;71:813–9. 10.3109/00016357.2012.734404 [DOI] [PubMed] [Google Scholar]
- 26.Vivekananda MR, Vandana KL, Bhat KG. Effect of the probiotic Lactobacilli reuteri (Prodentis) in the management of periodontal disease: a preliminary randomized clinical trial. J Oral Microbiol 2010;2. 10.3402/jom.v2i0.5344. [Epub ahead of print: 02 Nov 2010]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flichy-Fernández AJ, Ata-Ali J, Alegre-Domingo T, et al. The effect of orally administered probiotic Lactobacillus reuteri-containing tablets in peri-implant mucositis: a double-blind randomized controlled trial. J Periodontal Res 2015;50:775–85. 10.1111/jre.12264 [DOI] [PubMed] [Google Scholar]
- 28.Alqahtani F, Alshaikh M, Mehmood A, et al. Efficacy of antibiotic versus probiotics as adjuncts to mechanical debridement for the treatment of peri-implant mucositis. J Oral Implantol 2022;48:99–104. 10.1563/aaid-joi-D-20-00259 [DOI] [PubMed] [Google Scholar]
- 29.Laleman I, Detailleur V, Slot DE, et al. Probiotics reduce mutans streptococci counts in humans: a systematic review and meta-analysis. Clin Oral Investig 2014;18:1539–52. 10.1007/s00784-014-1228-z [DOI] [PubMed] [Google Scholar]
- 30.Mendonça FHBP, dos Santos SSF, da Silva de Faria I, et al. Effects of probiotic bacteria on Candida presence and IgA anti-Candida in the oral cavity of elderly. Braz Dent J 2012;23:534–8. 10.1590/s0103-64402012000500011 [DOI] [PubMed] [Google Scholar]
- 31.Li D, Li Q, Liu C, et al. Efficacy and safety of probiotics in the treatment of Candida-associated stomatitis. Mycoses 2014;57:141–6. 10.1111/myc.12116 [DOI] [PubMed] [Google Scholar]
- 32.Xia C, Jiang C, Li W, et al. A phase II randomized clinical trial and mechanistic studies using improved probiotics to prevent oral mucositis induced by concurrent radiotherapy and chemotherapy in nasopharyngeal carcinoma. Front Immunol 2021;12:618150. 10.3389/fimmu.2021.618150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burton JP, Chilcott CN, Moore CJ, et al. A preliminary study of the effect of probiotic Streptococcus salivarius K12 on oral malodour parameters. J Appl Microbiol 2006;100:754–64. 10.1111/j.1365-2672.2006.02837.x [DOI] [PubMed] [Google Scholar]
- 34.Jamali Z, Aminabadi NA, Samiei M, et al. Impact of chlorhexidine pretreatment followed by probiotic Streptococcus salivarius strain K12 on halitosis in children: a randomised controlled clinical trial. Oral Health Prev Dent 2016;14:305–13. 10.3290/j.ohpd.a36521 [DOI] [PubMed] [Google Scholar]
- 35.Benic GZ, Farella M, Morgan XC, et al. Oral probiotics reduce halitosis in patients wearing orthodontic braces: a randomized, triple-blind, placebo-controlled trial. J Breath Res 2019;13:036010. 10.1088/1752-7163/ab1c81 [DOI] [PubMed] [Google Scholar]
- 36.Gurpinar B, Yildirim G, Kumral TL, et al. A simple method to reduce halitosis; tongue scraping with probiotics. J Breath Res 2019;14:016008. 10.1088/1752-7163/ab503e [DOI] [PubMed] [Google Scholar]
- 37.Yoo H-J, Jwa S-K, Kim D-H, et al. Inhibitory effect of Streptococcus salivarius K12 and M18 on halitosis in vitro. Clin Exp Dent Res 2020;6:207–14. 10.1002/cre2.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoo J-I, Shin I-S, Jeon J-G, et al. The effect of probiotics on halitosis: a systematic review and meta-analysis. Probiotics Antimicrob Proteins 2019;11:150–7. 10.1007/s12602-017-9351-1 [DOI] [PubMed] [Google Scholar]
- 39.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bollen CML, Beikler T. Halitosis: the multidisciplinary approach. Int J Oral Sci 2012;4:55–63. 10.1038/ijos.2012.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erovic Ademovski S, Lingström P, Winkel E, et al. Comparison of different treatment modalities for oral halitosis. Acta Odontol Scand 2012;70:224–33. 10.3109/00016357.2011.635601 [DOI] [PubMed] [Google Scholar]
- 42.He L, Yang H, Chen Z, et al. The effect of Streptococcus salivarius K12 on halitosis: a double-blind, randomized, placebo-controlled trial. Probiotics Antimicrob Proteins 2020;12:1321–9. 10.1007/s12602-020-09646-7 [DOI] [PubMed] [Google Scholar]
- 43.Vandekerckhove B, Van den Velde S, De Smit M, et al. Clinical reliability of non-organoleptic oral malodour measurements. J Clin Periodontol 2009;36:964–9. 10.1111/j.1600-051X.2009.01473.x [DOI] [PubMed] [Google Scholar]
- 44.Rosenberg M, Kulkarni GV, Bosy A, et al. Reproducibility and sensitivity of oral malodor measurements with a portable sulphide monitor. J Dent Res 1991;70:1436–40. 10.1177/00220345910700110801 [DOI] [PubMed] [Google Scholar]
- 45.Seemann R, Duarte da Conceicao M, Filippi A, et al. [Halitosis management by the general dental practitioner- results of an International Consensus Workshop*]. Swiss Dent J 2014;124:1205–11. [DOI] [PubMed] [Google Scholar]
- 46.Rosenberg M, Septon I, Eli I, et al. Halitosis measurement by an industrial sulphide monitor. J Periodontol 1991;62:487–9. 10.1902/jop.1991.62.8.487 [DOI] [PubMed] [Google Scholar]
- 47.Laleman I, Dadamio J, De Geest S, et al. Instrumental assessment of halitosis for the general dental practitioner. J Breath Res 2014;8:017103. 10.1088/1752-7155/8/1/017103 [DOI] [PubMed] [Google Scholar]
- 48.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 49.Kim D-H, Kang M-S, Yeu J-E, et al. Inhibitory effect of the probiotic bacteria, Weissella cibaria CMU on halitosis: a randomized placebo-controlled study. J Korean Acad Oral Health 2020;44:246–52. 10.11149/jkaoh.2020.44.4.246 [DOI] [Google Scholar]
- 50.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 51.Hedges LV, Vevea JL. Fixed- and random-effects models in meta-analysis. Psychol Methods 1998;3:486–504. 10.1037/1082-989X.3.4.486 [DOI] [Google Scholar]
- 52.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 53.Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luo D, Wan X, Liu J, et al. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 2018;27:1785–805. 10.1177/0962280216669183 [DOI] [PubMed] [Google Scholar]
- 55.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patsopoulos NA, Evangelou E, Ioannidis JPA. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol 2008;37:1148–57. 10.1093/ije/dyn065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suzuki N, Yoneda M, Tanabe K, et al. Lactobacillus salivarius WB21-containing tablets for the treatment of oral malodor: a double-blind, randomized, placebo-controlled crossover trial. Oral Surg Oral Med Oral Pathol Oral Radiol 2014;117:462–70. 10.1016/j.oooo.2013.12.400 [DOI] [PubMed] [Google Scholar]
- 58.Keller MK, Bardow A, Jensdottir T, et al. Effect of chewing gums containing the probiotic bacterium Lactobacillus reuteri on oral malodour. Acta Odontol Scand 2012;70:246–50. 10.3109/00016357.2011.640281 [DOI] [PubMed] [Google Scholar]
- 59.Penala S, Kalakonda B, Pathakota KR, et al. Efficacy of local use of probiotics as an adjunct to scaling and root planing in chronic periodontitis and halitosis: a randomized controlled trial. J Res Pharm Pract 2016;5:86–93. 10.4103/2279-042X.179568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee D-S, Lee S-A, Kim M, et al. Reduction of halitosis by a tablet containing Weissella cibaria CMU: a randomized, double-blind, placebo-controlled study. J Med Food 2020;23:649–57. 10.1089/jmf.2019.4603 [DOI] [PubMed] [Google Scholar]
- 61.Mousquer CR, Della Bona A, Milani DC, et al. Are Lactobacillus salivarius G60 and inulin more efficacious to treat patients with oral halitosis and tongue coating than the probiotic alone and placebo? A randomized clinical trial. J Periodontol 2020;91:775–83. 10.1002/JPER.19-0089 [DOI] [PubMed] [Google Scholar]
- 62.Suzuki N, Yoneda M, Takeshita T, et al. Induction and inhibition of oral malodor. Mol Oral Microbiol 2019;34:85–96. 10.1111/omi.12259 [DOI] [PubMed] [Google Scholar]
- 63.Takeshita T, Suzuki N, Nakano Y, et al. Discrimination of the oral microbiota associated with high hydrogen sulfide and methyl mercaptan production. Sci Rep 2012;2:215. 10.1038/srep00215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kleinberg I, Westbay G. Oral malodor. Crit Rev Oral Biol Med 1990;1:247–59. 10.1177/10454411900010040401 [DOI] [PubMed] [Google Scholar]
- 65.Iatropoulos A, Panis V, Mela E, et al. Changes of volatile sulphur compounds during therapy of a case series of patients with chronic periodontitis and halitosis. J Clin Periodontol 2016;43:359–65. 10.1111/jcpe.12521 [DOI] [PubMed] [Google Scholar]
- 66.Ferrer MD, López-López A, Nicolescu T, et al. Topic application of the probiotic Streptococcus dentisani improves clinical and microbiological parameters associated with oral health. Front Cell Infect Microbiol 2020;10:465. 10.3389/fcimb.2020.00465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.López-Valverde N, López-Valverde A, Macedo de Sousa B, et al. Role of probiotics in halitosis of oral origin: a systematic review and meta-analysis of randomized clinical studies. Front Nutr 2021;8:787908. 10.3389/fnut.2021.787908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hill AB. The environment and disease: association or causation? Proc R Soc Med 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-060753supp001.pdf (62.4KB, pdf)
bmjopen-2022-060753supp002.pdf (767.7KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The data supporting the findings of this study are available from the corresponding author upon reasonable request.