Abstract
Background
Diabetic kidney disease (DKD) continues to be the leading cause of kidney failure across the world. For decades dietary protein restriction has been proposed for patients with DKD with the aim to retard the progression of chronic kidney disease (CKD) towards kidney failure. However, the relative benefits and harms of dietary protein restriction for slowing the progression of DKD have not been addressed.
Objectives
To determine the efficacy and safety of low protein diets (LPD) (0.6 to 0.8 g/kg/day) in preventing the progression of CKD towards kidney failure and in reducing the incidence of kidney failure and death (any cause) in adult patients with DKD. Moreover, the effect of LPD on adverse events (e.g. malnutrition, hyperglycaemic events, or health‐related quality of life (HRQoL)) and compliance were also evaluated.
Search methods
We searched the Cochrane Kidney and Transplant Register of Studies up to 17 November 2022 through contact with the Information Specialist using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria
We included randomised controlled trials (RCTs) or quasi‐RCTs in which adults with DKD not on dialysis were randomised to receive either a LPD (0.6 to 0.8 g/kg/day) or a usual or unrestricted protein diet (UPD) (≥ 1.0 g/kg/day) for at least 12 months.
Data collection and analysis
Two authors independently selected studies and extracted data. Summary estimates of effect were obtained using a random‐effects model. Results were summarised as risk ratios (RR) with 95% confidence intervals (CI) for dichotomous outcomes and mean difference (MD) or standardised MD (SMD) with 95% CI for continuous outcomes. Confidence in the evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
Main results
We identified eight studies involving 486 participants with DKD. The prescribed protein intake in the intervention groups ranged from 0.6 to 0.8 g/kg/day. The prescribed protein intake in the control groups was ≥ 1.0 g/kg/day, or a calculated protein intake ≥ 1.0 g/kg/day if data on prescribed protein intake were not provided. The mean duration of the interventions was two years (ranging from one to five years). Risks of bias in most of the included studies were high or unclear, most notably for allocation concealment, performance and detection bias. All studies were considered to be at high risk for performance bias due to the nature of the interventions.
Most studies were not designed to examine death or kidney failure. In low certainty evidence, a LPD may have little or no effect on death (5 studies, 358 participants: RR 0.38, 95% CI 0.10 to 1.44; I² = 0%), and the number of participants who reached kidney failure (4 studies, 287 participants: RR 1.16, 95% CI 0.38 to 3.59; I² = 0%). Compared to a usual or unrestricted protein intake, it remains uncertain whether a LPD slows the decline of glomerular filtration rate over time (7 studies, 367 participants: MD ‐0.73 mL/min/1.73 m²/year, 95% CI ‐2.3 to 0.83; I² = 53%; very low certainty evidence).
It is also uncertain whether the restriction of dietary protein intake impacts on the annual decline in creatinine clearance (3 studies, 203 participants: MD ‐2.39 mL/min/year, 95% CI ‐5.87 to 1.08; I² = 53%). There was only one study reporting 24‐hour urinary protein excretion. In very low certainty evidence, a LPD had uncertain effects on the annual change in proteinuria (1 study, 80 participants: MD 0.90 g/24 hours, 95% CI 0.49 to 1.31). There was no evidence of malnutrition in seven studies, while one study noted this condition in the LPD group. Participant compliance with a LPD was unsatisfactory in nearly half of the studies. One study reported LPD had no effect on HRQoL. No studies reported hyperglycaemic events.
Authors' conclusions
Dietary protein restriction has uncertain effects on changes in kidney function over time. However, it may make little difference to the risk of death and kidney failure. Questions remain about protein intake levels and compliance with protein‐restricted diets. There are limited data on HRQoL and adverse effects such as nutritional measures and hyperglycaemic events. Large‐scale pragmatic RCTs with sufficient follow‐up are required for different stages of CKD.
Keywords: Adult; Humans; Diabetes Mellitus; Diabetic Nephropathies; Diet, Protein-Restricted; Diet, Protein-Restricted/adverse effects; Hyperglycemia; Kidney Failure, Chronic; Kidney Failure, Chronic/prevention & control; Malnutrition; Randomized Controlled Trials as Topic; Renal Insufficiency, Chronic
Plain language summary
Low protein diets for adults with diabetic kidney disease
What is the issue?
For people with diabetic kidney disease (DKD) not requiring dialysis, it may be recommended to limit the amount of protein in the diet to slow the progression of chronic kidney disease. However, uncertainty remains about how much protein should be consumed.
What did we do?
We reviewed the evidence about the effect of low protein diets on the progression of kidney disease in adult patients with DKD, not on dialysis. The evidence is current to 17 November 2022. All studies were combined, provided that they compared a low protein diet (0.6 to 0.8 g/kg/day) with a usual or unrestricted protein diet (≥ 1.0 g/kg/day) for 12 months or more.
What did we find?
We identified eight studies enrolling 486 people who had DKD at different stages of chronic kidney disease. Studies included type 1 and type 2 diabetes. The results showed that a low protein diet has uncertain effects on slowing the decline of glomerular filtration rate. Compared with a usual or unrestricted protein diet, a low protein diet may have little or no effect on the number of people who died or progress to kidney failure needing dialysis. The vast majority of studies reported nutritional status, with only one study indicating potential malnutrition in the low protein diet group. There may be little or no difference in health‐related quality of life; however, only one study reported this outcome. Compliance with the low protein diet was unsatisfactory in four of the eight studies
For the most part, the included studies were poorly conducted, and data were often not reported; therefore, the overall certainty of the evidence for our outcomes of interest was either low or very low.
Conclusions
Because there were insufficient data and difficulties in adherence to such a low protein diet, we are uncertain whether a low protein diet slows the progression of kidney disease for people with DKD not on dialysis. More high‐quality studies with large samples and sufficient follow‐up are needed.
Summary of findings
Summary of findings 1. Low protein diet versus usual or unrestricted protein diet for adults with diabetic kidney disease.
| Low protein diet versus usual or unrestricted protein diet for adults with diabetic kidney disease | ||||||
| Patient or population: adults with DKD Settings: all settings Intervention: low protein diet (0.6 to 0.8 (g/kg/day) Comparison: usual or unrestricted protein diet (≥ 1 g/kg/day) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual or unrestricted protein diet | Low protein diet | |||||
| Death (any cause) | 45 per 1000 | 17 per 1000 (4 to 65) | RR 0.38 (0.1 to 1.44) | 358 (5 studies) | ⊕⊕⊝⊝ low1,2 | Eleven participants died in the 5 studies reporting this outcome |
| Kidney failure | 34 per 1000 | 40 per 1000 (13 to 123) | RR 1.16 (0.38 to 3.59) | 287 (4 studies) | ⊕⊕⊝⊝ low1,2 | Eleven participants developed kidney failure in the 4 studies reporting this outcome |
| Change in GFR | The mean decline in GFR with a low protein diet was 0.73 mL/min/1.73 m²/yearlower (2.3 lower to 0.83 higher) than a usual or unrestricted protein diet | ‐ | 367 (7 studies) | ⊕⊝⊝⊝ very low1,3,4 | ‐ | |
| Change in 24‐hour urinary protein excretion | The mean decline in 24‐hour urinary protein excretion with a low protein diet was 0.9 g/24 hours higher (0.49 to 1.31 higher) than a usual or unrestricted protein diet | ‐ | 80 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | ‐ | |
| Malnutrition | See comment | See comment | ‐ | 471 (7 studies) | See comment | Six studies showed no evidence of malnutrition, while one study noted malnutrition in the low protein diet group. The number of participants was not reported |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in the footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). DKD: Diabetic kidney disease; CI: Confidence interval; RR: Risk ratio; GFR: Glomerular filtration rate | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
1 Small studies and wide CIs that include the potential for important benefits and harms 2 Most of the included studies did not contribute to the outcome evaluated 3 Study limitations were due to high or unclear risks of bias 4 Important and unexplained heterogeneity present
Background
Description of the condition
Diabetes is a major public health concern that has reached alarming levels. In 2021, it is estimated that 10% of adults around the world (537 million people) are living with diabetes, and this number is projected to reach 783 million by 2045 (IDF 2021). The increased prevalence of diabetes has led to an increase in patients with diabetic kidney disease (DKD) and kidney failure across the world (Dabelea 2017; IDF 2021). It is estimated that approximately 40% of patients with diabetes will develop chronic kidney disease (CKD) (Parving 2006; Unnikrishnan 2007), an entity referred to as DKD (Tervaert 2010). DKD is a diagnosis that refers to specific pathologic structural and functional changes seen in the kidneys due to diabetes (ADA 2014; KDOQI 2007; KDIGO 2020). These changes lead to a clinical presentation characterised by increased urinary albumin excretion and progressive reductions in kidney function. The natural history of DKD in patients in longitudinally studied populations with type 2 diabetes is similar to that in those with type 1 diabetes. It typically includes several stages, starting with apparent normality in the first few years after diagnosis, followed by incipient nephropathy (known as microalbuminuria), and then by overt nephropathy that will progress to kidney failure requiring dialysis or transplantation, or both (Gross 2005).
DKD continues to be the leading cause of kidney failure in most developed countries (JSDT 2019; UKRR 2020; USRDS 2019) and increases the risk of death, mainly from cardiovascular causes (Sabanayagam 2019). For patients with DKD undergoing dialysis, the healthcare cost increases by an average of 2.8 times/year compared with CKD patients who are not on dialysis (Li 2013). Studies in patients with type 2 diabetes and early stages of kidney disease demonstrated that multifactorial interventions had a long‐term benefit for the development of microvascular and macrovascular complications and death (Gaede 2003; Gaede 2016). Therefore, a target‐driven, long‐term, intensified intervention targeting multiple risk factors, such as lifestyle modification (including dietary counselling, smoking cessation, and adequate physical activity) and pharmacological intervention, may stabilise early‐stage DKD. Furthermore, other diet‐related factors, such as poorer glycaemic control, obesity, and malnutrition, may also impact on severity and progression of DKD (KDIGO 2020; KDOQI 2020).
Description of the intervention
In patients with diabetes and CKD, dietary recommendations change depending on the stage of kidney disease and treatment modality. However, there is uncertainty around dietary recommendations for those with diabetes and non‐dialysis‐dependent CKD as two recent guidelines slightly differ (KDIGO 2020; KDOQI 2020). In adult patients with diabetes with decreased glomerular filtration rate (GFR) (GFR < 60 mL/min/1.73 m²) and not on dialysis, KDOQI 2020 recommended that it was reasonable to prescribe a daily protein intake of 0.6 to 0.8 g/kg/day. According to the recommendation of the World Health Organization on daily protein intake for healthy people, KDIGO 2020 recommended daily protein intake should be maintained at approximately 0.8 g/kg/day for those with diabetes and non‐dialysis‐dependent CKD.
In addition, the optimal diet for diabetes from the perspective of diabetologists (ADA 2019) is low‐energy and low‐carbohydrate because carbohydrate is a readily available source of energy and the primary dietary influence on postprandial blood glucose, which may be applied in a variety of eating patterns that meet individualized dietary plans. Therefore, restricting the amount of dietary protein below 0.8 g/kg/day in a person with diabetes, who may have also been counselled to restrict carbohydrates and fat, may significantly decrease the caloric content of the diet. This severely restricted diet, if followed, may lead to a decline in the quality of life (QoL) and an increase in the risk of malnutrition (Pan 2008; Robertson 2007). However, in CKD with reduced GFR, a high protein intake is associated with the development of increased intra‐glomerular pressure and glomerular hyperfiltration, which in turn contributes to accelerating the decline in kidney function (Hostetter 1986). Thus, nephrologists (KDOQI 2020) state a recommendation of normal/high energy (25 to 35 kcal/kg/day) and low protein diets (LPD) (0.6 to 0.8 g/kg/day) as an option in patients with DKD not on dialysis. Because of the reduced energy amount resulting from the lower protein intake, a protein restriction diet needs more carbohydrates to meet energy requirements, whilst high carbohydrates may worsen glycaemic control in diabetes. These are two competing views. Thus, the impact of protein/diet restrictions on DKD should balance the benefits and harms.
Most adults, especially those in developed countries, have a usual protein intake of more than 1.0 g/kg/day. Traditionally, a LPD is rigid and restrictive, which is prescribed using an exchange system to include both high and low‐biological value proteins. In general, a focus on vegetables, fruits, whole grains, fibre, legumes, right plant‐based proteins, unsaturated fats and less meat may help DKD patients achieve this protein restriction (KDIGO 2020). Compliance with a LPD is typically assessed by regular measurement of 24‐hour urinary excretion of urea nitrogen using the Maroni formula (Maroni 1985). Considering the uncertainty around recommendations for optimal dietary protein intake in people with DKD not on dialysis, whether limiting protein intake to less than 0.8 g/kg/day (i.e. 0.6 to 0.8 g/kg/day) provides more benefits to patients with DKD than a usual or unrestricted protein diet (UPD) (≥ 1.0 g/kg/day) needs to be further explored.
How the intervention might work
In the 1980s, it was hypothesised that lower dietary protein intake could reduce glomerular hyperfiltration and delay the progression of chronic renal insufficiency in experimental animal models (Klahr 1983). Since then, clinical trials have compared different levels of protein intake in CKD patients with or without diabetes and demonstrated the beneficial role of LPD in delaying the gradual loss of kidney function (Barsotti 1988; Ciavarella 1987; Giordano 2014; Jungers 1987). Other long‐term studies in rats have demonstrated that LPD ameliorates diabetes‐induced kidney injuries, including the accumulation of abnormal mitochondria, tubular cell damage and inflammation, which is related to autophagy restoration and inhibition of the mTORC1 pathway (Kitada 2016). In 2007, a Cochrane review (Robertson 2007) was published with the aim of assessing the role of LPD in DKD patients. Robertson 2007 reported that a LPD appeared to slow the progression of DKD, although in a non‐significant way. Another Cochrane review (Hahn 2020) evaluated the efficacy of LPD in patients with non‐diabetic CKD. Hahn 2020 found that a LPD probably reduced the number of people with CKD stage 4 or 5 (moderate certainty evidence). Therefore, such a diet may have the potential to protect the kidneys. This is partly because limiting protein intake contributes to a significant decrease in urinary urea nitrogen output and a concomitant decrease in kidney workload. It is suggested that dietary protein restriction also favours the correction of metabolic acidosis and CKD‐metabolic bone disease (Garneata 2016; Williams 1991). Additionally, a low protein intake may ameliorate oxidative stress and insulin resistance (Chauveau 2011; Gao 2010; Kim 2010), which are the common features of accelerated atherosclerosis in patients with DKD. These experimental and clinical data suggest that dietary protein restriction in people with DKD may prevent the natural progression of CKD towards kidney failure.
Why it is important to do this review
This is an update of the Cochrane review, which was first published in 1997 (Waugh 1997) and updated in 2007 (Robertson 2007) with the aim to explore whether dietary protein restriction benefits adults with DKD by delaying the onset of kidney failure and/or slowing the rate of decline in GFR. The 2007 update analysed a total of seven RCTs with 222 patients and reported that limiting protein intake may slow the decline of GFR, but more studies were needed (Robertson 2007). However, few events (e.g. death and kidney failure) were observed with LPD compared with those occurring with UPD. Overall there remains considerable controversy as to whether dietary protein restriction does retard the rate of decline in kidney function in people with DKD at different stages of CKD. During the latest decade, several new RCTs assessing dietary protein restriction in DKD have been published. Thus, in this review, we aimed to update Robertson 2007 to determine whether the addition of further RCTs could modify previous findings.
Objectives
To determine the efficacy of LPD (0.6 to 0.8 g/kg/day) in preventing the progression of CKD towards kidney failure and in reducing the incidence of kidney failure and death (any causes) in adult patients with DKD. Moreover, the effect of LPD on adverse events ( e.g. malnutrition, hyperglycaemic events, or health‐related (HR) QoL) and compliance was also evaluated.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) or quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods). Interventions should last a minimum of 12 months. Cross‐over studies were excluded for primary outcomes but were considered for secondary outcomes if the starting period of the intervention was randomly allocated and each intervention was in place for at least 12 months.
Types of participants
Inclusion criteria
Adults with type 1 or type 2 diabetes as defined by authors in each study. In studies that included a minority of non‐diabetic patients, analyses were restricted to patients with diabetes only.
Clinically and/or pathologically diagnosed DKD. Clinical diagnosis of DKD was based on the presence of albuminuria and/or reduced GFR in the absence of signs or symptoms of other causes of kidney disease and should adhere to the standard guidelines (ADA 1999; ADA 2008; ADA 2010; ADA 2018; KDOQI 2007). If necessary, we used the authors’ definition of DKD. Information on glycaemic control and antihyperglycaemic treatment should be stated.
Exclusion criteria
Design or analysis flawed, for example, if anti‐hypertensive treatment was started or increased at the same time as the diet was changed. However, studies with antihyperglycaemic treatment being started or increased at the same point in time were not excluded because a restricted protein intake may not provide sufficient energy, and as a result, needs more carbohydrates to reach the energy request and, in turn, worsens the control of diabetes.
Because of the difficulty of controlling confounding factors and the major concern for LPD in DKD, studies including pregnant women or malnourished patients were excluded.
Studies were excluded if insufficient details of protein diet were given or if the intervention was carried out immediately in pre‐dialysis patients.
Types of interventions
Studies comparing a reduced or modified protein diet (0.6 to 0.8 g/kg/day) with a UPD (≥ 1.0 g/kg/day) for at least 12 months
Studies in which people received supplements of essential amino acids, keto‐analogues, or both, were included, provided that the total nitrogen intake differed between the experimental and the control groups
Studies in which people received different types of protein (animal or plant) were also included, provided that the daily protein intake met the request of the treatment groups.
Types of outcome measures
Primary outcomes
Kidney failure: defined by the need to initiate chronic dialysis or to receive a kidney transplant during follow‐up
Change in GFR: defined by the mean annual change in GFR from baseline to end of follow‐up. In this analysis, estimated (e) GFR was used interchangeably with measured GFR
Death (any cause).
Secondary outcomes
Change in creatinine clearance (CrCl) as defined by the mean annual change from baseline to end of follow‐up
Change in 24‐hour urinary protein excretion as defined by the mean annual change from baseline to end of follow‐up
End of study body weight
End of study body mass index (BMI)
Adverse effects, e.g. development of malnutrition as defined by the study authors, hyperglycaemic events, or other adverse events during follow‐up reported in included studies
Measures of compliance with LPD, assessed by urinary urea, dietary interview, or other useful methods
HRQoL measured using validated scales, e.g. the short‐form 36 Health Status Questionnaire (SF‐36) (Ware 1992), the EuroQol‐5 Dimension (EQ‐5D) (EuroQol 1990), or similar.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Register of Studies (up to 17 November 2022) through contact with the Information Specialist using search terms relevant to this review. The Register contains studies identified from the following sources:
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Searches of kidney and transplant journals and the proceedings and abstracts from major kidney and transplant conferences
Searching the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of search strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available on the Cochrane Kidney and Transplant website under CKT Register of Studies.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies, and clinical practice guidelines.
Contacting relevant individuals/organisations seeking information about unpublished or incomplete studies.
Grey literature sources (e.g. abstracts, dissertations, and theses), in addition to those already included in the Cochrane Kidney and Transplant Register of Studies, were not searched.
Data collection and analysis
Selection of studies
The initial version of this review was undertaken in 1997 (Waugh 1997) and updated in 2007 (Robertson 2007).
This new review was undertaken by three authors. The search strategy described was used to obtain titles and abstracts of studies that were relevant to the review. The titles and abstracts were screened independently by two authors, who discarded studies that were not applicable; however, studies and reviews that might include relevant data or information on trials were retained initially. Two authors independently assessed retrieved abstracts and, if necessary, the full text of these studies to determine which studies satisfied the inclusion criteria. Disagreements were resolved in consultation with a third author.
Data extraction and management
Data extraction was carried out independently by two authors using standard data extraction forms. Studies reported in non‐English language journals were translated before assessment. Where more than one report of a study was identified, data from the most complete report were extracted, but the remaining reports were checked for additional information. We also contacted principal investigators for missing data whenever necessary. Any discrepancy between published versions was evaluated and highlighted.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2021) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at risk of bias?
Measures of treatment effect
For dichotomous outcomes (death, kidney failure and adverse effects), results were expressed as risk ratios (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment (GFR, CrCl, urinary protein excretion, body weight, and BMI), the mean difference (MD) was used, or the standardised mean difference (SMD) if different scales had been used. Where standard deviations (SD) for continuous outcomes were missing and not available from triallists, these were imputed (Higgins 2021). Any standard errors (SE) and CIs were transformed into SDs where appropriate.
Unit of analysis issues
Data from cross‐over studies were planned to be analysed for secondary outcomes, provided that separate data for the first part of the study were available. No eligible cross‐over studies were identified.
Dealing with missing data
Any further information required from the original author was requested by emailing the corresponding author/s, and any relevant information obtained in this manner was included in the review. Evaluation of important numerical data such as screened, randomised patients, as well as intention‐to‐treat, as‐treated and per‐protocol population, was carefully performed. Attrition rates, for example, drop‐outs, losses to follow‐up and withdrawals, were investigated. Issues of missing data and imputation methods (for example, last‐observation‐carried‐forward) were critically appraised (Higgins 2021).
Assessment of heterogeneity
We assessed the heterogeneity by visual inspection of the forest plot. We quantified statistical heterogeneity using the I² statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than sampling error (Higgins 2003). A guide to the interpretation of I² values was as follows:
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity.
The importance of the observed value of I² depends on the magnitude and direction of treatment effects and the strength of evidence for heterogeneity (e.g. P value from the Chi² test or a CI for I²) (Higgins 2021).
Assessment of reporting biases
There were insufficient data to generate funnel plots to assess for the potential existence of small study bias for the outcomes of kidney failure, death (any cause), change in GFR and change in proteinuria. Where there were multiple publications from the same trial, the primary publications and additional reports were reviewed to identify all outcomes to reduce the risk of selective outcome reporting bias.
Data synthesis
Data were pooled using the random‐effects model; however, the fixed‐effects model was also used to ensure the robustness of the model chosen and susceptibility to outliers when appropriate.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were planned to assess differences in results possibly related to participant groups, different ways of measuring the change in GFR, dietary compliance, study quality and disease severity. There were too few studies in each analysis to allow meaningful subgroup analyses.
Sensitivity analysis
There were insufficient extractable data to perform the following sensitivity analyses in order to explore the influence of the following factors on effect size:
Repeating the analysis, excluding unpublished studies
Repeating the analysis taking account of the risk of bias, as specified
Repeating the analysis, excluding any very long or large studies, to establish how much they dominated the results.
Summary of findings and assessment of the certainty of the evidence
We presented the main results of the review in a 'Summary of findings' table. The table presents key information concerning the certainty of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schunemann 2021a). The 'Summary of findings' table also includes an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008; GRADE 2011). The GRADE approach defines the certainty of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. This was assessed by two authors. The certainty of a body of evidence involves consideration of the within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, the precision of effect estimates and risk of publication bias (Schunemann 2021b). We presented the following outcomes in the 'Summary of findings' table.
Death (any cause)
Kidney failure
Change in GFR
Change in 24‐hour urinary protein excretion
Adverse effects: nutritional status.
Results
Description of studies
Results of the search
After searching the Specialised Register, a total of 56 records were identified. After screening titles and abstracts and full‐text review, eight studies (20 records) were included, and 30 studies (36 records) were excluded. No ongoing studies were identified (Figure 1).
1.
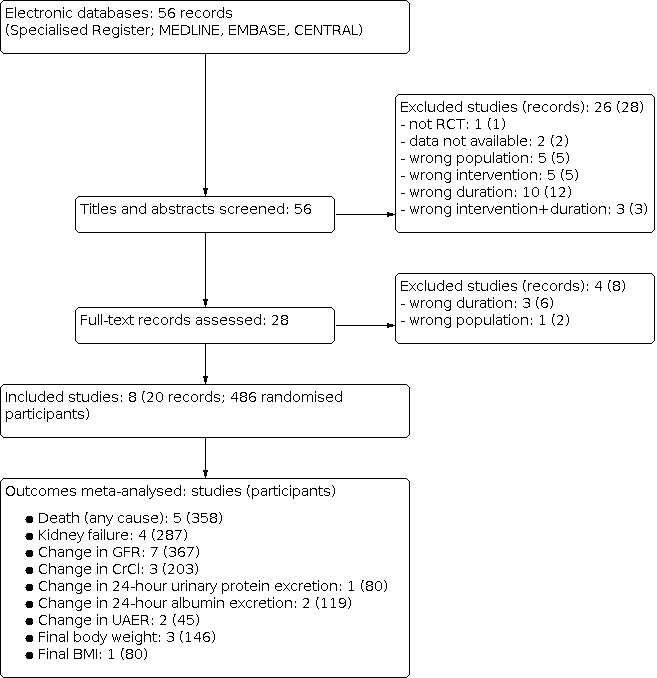
Flow diagram of study selection
Included studies
See Characteristics of included studies; Table 2.
1. Summary of included studies at time of randomisation.
| Study | No. of participants | Mean age ± SD (years) | Men % | Type of diabetes | CKD stage | Mean GFR ± SD (mL/min/1.73 m²) | Follow‐up (years) |
| Brouhard 1990 | 15 | 33 ± 13 | 60 | Type 1 | 1‐3 | 81.1 ± 32.4 | 1 |
| Dullaart 1993 | 30 | 41 ± 13 | 90 | Type 1 | 1 | 126 ± 30 | 2 |
| Dussol 2004 | 63 | 58 ± 12 | 83 | Mixed | 1‐3 | 86 ± 24 | 2 |
| Hansen 2002 | 82 | 40.5 ± 8.5 | 65 | Type 1 | 1‐3 | 68 ± 31 | 4 |
| Koya 2009 | 112 | 56.9 ± 8.2 | 59 | Type 2 | 1‐3 | 62.3 ± 25.2 | 5 |
| Meloni 2002 | 69 | 54.4 ± 15.3 | 55 | Mixed | 3 | 44.4 ± 4.9 | 1 |
| Meloni 2004 | 80 | 54.5 ± 15.6 | 49 | Mixed | 3 | 44.5 ± 4.9 | 1 |
| Zeller 1991 | 35 | 34 ± 8 | 60 | Type 1 | 2‐4 | 47.4 ± 23.8 | 2.8 |
CKD: chronic kidney disease; GFR: glomerular filtration rate
Eight studies (486 participants) were included (Brouhard 1990; Dullaart 1993; Dussol 2004; Hansen 2002; Koya 2009; Meloni 2002; Meloni 2004; Zeller 1991). Four studies (Brouhard 1990; Dullaart 1993; Hansen 2002; Zeller 1991) were carried out in patients with type 1 diabetes, one study (Koya 2009) in patients with type 2 diabetes, and three studies (Dussol 2004; Meloni 2002; Meloni 2004) in patients with type 1 and type 2 diabetes. Sample sizes ranged from 15 to 112 patients. The mean duration of the interventions was two years (ranging from one to five years).
In four studies (Brouhard 1990; Dussol 2004; Hansen 2002; Koya 2009), most participants with DKD had CKD stages 1 to 3 (KDIGO 2020). One study (Dullaart 1993) only included participants with CKD stage 1.
In two studies (Meloni 2002; Meloni 2004), the majority of participants had CKD stage 3. Many of the patients appear to overlap as the biochemical data were similar or identical for both the baseline and post‐intervention data in both the LPD and the UPD groups. Therefore, data from Meloni 2004 were extracted, and Meloni 2002 was checked for additional information. Zeller 1991 included participants with CKD stages 2 to 4.
In all but two studies, participants in both groups were balanced in terms of baseline characteristics. In Brouhard 1990, the unrestricted group had slightly worse factors at baseline but none significant, while in Dullaart 1993, the baseline BMI was significantly higher in the LPD group.
In all studies, the intervention group was a LPD containing prescribed protein intake from 0.6 to 0.8 g/kg/day. Five studies (Brouhard 1990; Dullaart 1993; Dussol 2004; Koya 2009; Zeller 1991) prescribed a UPD (≥ 1.0 g/kg/day) as the comparison intervention. In the other three studies (Hansen 2002; Meloni 2002; Meloni 2004), the comparison intervention was a free protein diet without data on the specific level of prescribed protein intake, and the actual calculated protein intake was higher than 1.0 g/kg/day.
Excluded studies
See Characteristics of excluded studies.
Thirty studies (36 records) were excluded.
One study was not randomised (Garcia Garcia 1990)
Six studies enrolled participants with diabetes but without kidney disease (Pecis 1994; Pedersen 1989; Pijls 1999; Rudberg 1988; Stephenson 2005; Wiseman 1987)
Eight studies investigated interventions not relevant to this review (Azadbakht 2003; Barsotti 1993; Chen 2012h; Ciarambino 2012; Facchini 2003; Qiu 2012a; Suratkal 2012; Wheeler 2002)
Thirteen studies followed up for less than one year (Bending 1988; Ciavarella 1987; Cohen 1987; Effendi 2000; Gross 2002; Hansen 1999; Parillo 1988; Pinto 1991; Raal 1994; Rosenberg 1987; Velazquez Lopez 2008; Zeller 1986; Zucchelli 1988b).
Two studies did not report the exact number of participants with DKD in each group (Aoyagi 2006; Silan 2003).
Risk of bias in included studies
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Random sequence generation
Five studies (Dullaart 1993; Dussol 2004; Hansen 2002; Koya 2009; Meloni 2004) specified appropriate methods for random sequence generation and were considered to be at low risk of bias. The risk of bias from random sequence generation methods was unclear in the remaining three studies.
Allocation concealment
Two studies (Dullaart 1993; Koya 2009) were judged to have adequate allocation concealment and were judged to be at low risk of bias. The risk from allocation concealment was unclear in the remaining six studies.
Blinding
Performance bias
Participants underwent different diet procedures that could not be blinded. Therefore, all eight studies were open‐label and judged to be at high risk of performance bias.
Detection bias
Detection bias (blinding of outcome assessment) was recorded separately for GFR and kidney failure. Since GFR was measured or calculated based on laboratory data and unlikely to be influenced by a lack of blinding, all studies reported this outcome and were considered to be at low risk of detection bias. For kidney failure (the need to initiate chronic dialysis), only Dussol 2004 was rated with a low risk of detection bias, as the authors provided information to indicate that GFR < 15 mL/min/1.73 m² was the criteria for the onset of kidney failure, which was unlikely to be affected by investigators knowledge of the dietary assignment. The remaining seven studies did not provide any information on how to assess the onset of kidney failure or information on whether the onset of kidney failure was determined by personnel independent of the study investigators.
Incomplete outcome data
Attrition bias was assessed to be at low risk in seven studies (Brouhard 1990; Dullaart 1993; Dussol 2004; Hansen 2002; Koya 2009; Meloni 2002; Meloni 2004). Zeller 1991 was considered to be at high risk of attrition bias as 25% of participants were lost to follow‐up or discontinued diet, and two patients in the LPD group whose mean protein intake exceeded 0.8 g/kg/day were excluded from the general analysis.
Selective reporting
Studies were considered to be at high risk if data were provided in a format which could not be entered into the meta‐analyses or if the study did not provide data on death, GFR, the requirement for dialysis, urinary protein/albumin excretion, or the nutritional status of the participants. Selective reporting was considered to be at low risk in three studies (Dullaart 1993; Dussol 2004; Hansen 2002), and the remaining five studies were judged to be at high risk of bias (Brouhard 1990; Koya 2009; Meloni 2002; Meloni 2004; Zeller 1991).
Other potential sources of bias
Five studies (Brouhard 1990; Dullaart 1993; Dussol 2004; Koya 2009; Zeller 1991) were considered to be at low risk for other potential biases as they were funded by education or government organisations. In the remaining three studies, it was unclear as there was insufficient information to permit judgement regarding funding sources.
Effects of interventions
See: Table 1
See Table 1.
Death (any cause)
Five studies (Brouhard 1990; Hansen 2002; Koya 2009; Meloni 2002; Meloni 2004) reported the number of deaths. Of these, three studies (Brouhard 1990; Meloni 2002; Meloni 2004) did not specifically report deaths, but there appeared to be no deaths, which was inferred from part of the information provided about participant recruitment or attrition during the study follow‐up. The certainty of the evidence was considered low (Table 1) because of imprecision and potential publication bias. A LPD may have little or no effect on deaths compared with a UPD (Analysis 1.1 (5 studies, 358 participants): RR 0.38, 95% CI 0.10 to 1.44; I² = 0%).
1.1. Analysis.

Comparison 1: Low protein diet versus usual or unrestricted protein diet, Outcome 1: Death (any cause)
Kidney failure
Four studies (Dullaart 1993; Dussol 2004; Hansen 2002; Koya 2009) reported the number of participants reaching kidney failure. The certainty of the evidence was considered low (Table 1) because of imprecision and potential publication bias. A LPD may make little or no difference to the number of participants who progressed to kidney failure compared with a UPD (Analysis 1.2 (4 studies, 287 participants): RR 1.16, 95% CI 0.38 to 3.59; I² = 0%).
1.2. Analysis.

Comparison 1: Low protein diet versus usual or unrestricted protein diet, Outcome 2: Kidney failure
Change in glomerular filtration rate
All eight studies reported the annual change in GFR. Since the data from Meloni 2002 and Meloni 2004 were almost the same at baseline and at the end of the study, we considered these two studies overlapped; therefore, we only used the data from Meloni 2004 in our meta‐analysis. Studies used different methods to evaluate the change in GFR. Because of study limitations (high or unclear risks of bias), moderate heterogeneity (imprecision), and few participants (potential publication bias), the certainty of the evidence was considered to be very low (Table 1). It is uncertain whether a LPD impacts the annual change in GFR when compared with a UPD (Analysis 1.3 (7 studies, 367 participants): MD ‐0.73 mL/min/1.73 m²/year, 95% CI ‐2.3 to 0.83; I² = 53%). Heterogeneity was reduced by the removal of Brouhard 1990 and Zeller 1991, but it was unclear why the data provided by these studies differed from the other studies.
1.3. Analysis.

Comparison 1: Low protein diet versus usual or unrestricted protein diet, Outcome 3: Change in GFR [mL/min/1.73 m²/year]
Change in creatinine clearance
Three studies (Koya 2009; Meloni 2004; Zeller 1991) reported the change in CrCl and were included in the meta‐analysis. Because of moderate heterogeneity, imprecision, and a high or unclear risk of bias in most studies, the certainty of the evidence was considered to be very low. It is uncertain whether a LPD influences the annual decline in CrCl when compared with a UPD (Analysis 1.4 (3 studies, 203 participants): MD ‐2.39 mL/min/year, 95% CI ‐5.87 to 1.08; I² = 53%). The removal of Zeller 1991 reduced this heterogeneity to 12%. There were attrition bias and unclear selection bias in Zeller 1991, so it is possible that an increased risk of bias in these domains might contribute to the heterogeneity.
1.4. Analysis.

Comparison 1: Low protein diet versus usual or unrestricted protein diet, Outcome 4: Change in CrCl [mL/min/year]
Change in 24‐hour urinary protein excretion
Meloni 2004 reported 24‐hour urinary protein excretion at baseline and at follow‐up. Because of small numbers, a high risk of bias and imprecision, the certainty of the evidence was considered to be very low (Table 1). Therefore, it is uncertain whether a LPD reduces the 24‐hour urinary protein excretion (Analysis 1.5 (1 study, 80 participants): MD 0.90 g/24 hours, 95% CI 0.49 to 1.31).
1.5. Analysis.

Comparison 1: Low protein diet versus usual or unrestricted protein diet, Outcome 5: Change in 24‐hour urinary protein excretion
Two studies (Dussol 2004; Hansen 2002) reported data on 24‐hour urinary albumin excretion, and two studies (Brouhard 1990; Dullaart 1993) reported urinary albumin excretion rate (UAER). Because a surrogate outcome of change rate (%) in UAER was used in Dullaart 1993 (not the change value), we estimated SMD for UAER. Compared with the UPD, LPD has uncertain effects on the decline in 24‐hour urinary albumin excretion (Analysis 1.6 (2 studies, 119 participants): MD 0.00 g/24 hours, 95% CI ‐0.07 to 0.07; I² = 0%; very low certainty evidence), but may decrease UAER in patients treated with LPD (Analysis 1.7 (2 studies, 45 participants): SMD 0.68, 95% CI 0.08 to 1.29; I² = 0%).
1.6. Analysis.

Comparison 1: Low protein diet versus usual or unrestricted protein diet, Outcome 6: Change in 24‐hour urinary albumin excretion
1.7. Analysis.

Comparison 1: Low protein diet versus usual or unrestricted protein diet, Outcome 7: Change in UAER
Adverse effects and nutritional status
No studies reported hyperglycaemic events or other adverse events. Seven studies (Dullaart 1993; Dussol 2004; Hansen 2002; Koya 2009; Meloni 2002; Meloni 2004; Zeller 1991) reported nutritional status. Six studies reported no evidence of malnutrition, while Meloni 2002 reported malnutrition in the LPD group (as measured by serum levels of albumin and prealbumin and anthropometric parameters). The definition of malnutrition and the number of participants involved in Meloni 2002 were not given, but serum prealbumin and serum albumin decreased in the LPD group.
Three studies (Dullaart 1993; Dussol 2004; Meloni 2002) reported final body weight. Because of imprecision and potential publication bias, the certainty of the evidence was considered to be low. LPD may make little or no difference to the final body weight (Analysis 2.1 (3 studies, 146 participants): MD 1.03 kg, 95% CI ‐3.04 to 5.09; I² = 23%).
2.1. Analysis.

Comparison 2: Nutritional measures, Outcome 1: Final body weight
Meloni 2004 reported final BMI. The certainty of the evidence was rated very low because of a high or unclear risk of bias, imprecision and small participants. Thus a LPD had uncertain effects on final BMI (Analysis 2.2 (1 study, 80 participants): MD ‐1.20 kg/m², 95% CI ‐2.60 to 0.20; very low certainty evidence).
2.2. Analysis.

Comparison 2: Nutritional measures, Outcome 2: Final body mass index
Compliance
All eight studies assessed dietary compliance and measured at one to six monthly intervals or regularly, either by an independent measurement of urinary urea excretion (Brouhard 1990; Dullaart 1993; Meloni 2004) or by a combination of urinary urea excretion and food record (Dussol 2004; Hansen 2002; Koya 2009; Meloni 2002; Zeller 1991). This was facilitated by dietitians. The prescribed protein intake across the included studies in intervention groups ranged from 0.6 to 0.8 g/kg/day, but the calculated protein intake ranged from 0.68 to 1.0 g/kg/day. Brouhard 1990 did not report the calculated protein intake, and in four studies (Dussol 2004; Hansen 2002; Koya 2009; Meloni 2004), the calculated protein intake exceeded 0.8 g/kg/day in the intervention groups, indicating that participant compliance to a restricted protein diet in nearly half of the studies was unsatisfactory.
Health‐related quality of life
Koya 2009 assessed the QoL using the SF‐36 questionnaire (physical function, social function, physical role, emotional role, mental health, energy, pain and general health perceptions). There were no significant differences in HRQoL between the LPD and UPD groups during the study period.
Discussion
Summary of main results
This review summarises eight small RCTs involving 486 people with DKD, which compared a LPD (0.6 to 0.8 g/kg/day) with a UPD (≥ 1.0 g/kg/day). Dietary protein restriction was evaluated with the mean duration of interventions ranging from one to five years. The studies included people with CKD stages 1 to 4. The risks of bias in the included studies were often high or unclear, and these risks, combined with the imprecision in effect estimates, resulted in low or very low confidence in the results.
For the primary outcomes, a LPD may make little or no difference to the number of participants who died regardless of cause (low certainty evidence) or those who progressed to kidney failure (low certainty evidence). It remains uncertain whether a LPD impacts the annual decline in GFR compared to a UPD (very low certainty evidence).
Many studies were not designed to assess the effect of dietary protein restriction on the change in proteinuria or albuminuria. As a result, there was uncertainty about the effect of protein restriction on this outcome.
Seven studies assessed nutritional status. Six studies had no participants with malnutrition, while one study noted malnutrition in the LPD group on a prescribed intake of 0.6 g/kg/day, as measured by serum levels of prealbumin and albumin. No study formally assessed hyperglycaemic events in the participants. Only one study reported that QoL was assessed and that the LPD regimen did not affect HRQoL. Nearly half of the studies reported that compliance with a LPD of 0.6 to 0.8 g/kg/day was unsatisfactory.
Overall, these data suggest that current evidence for dietary protein restriction in the setting of DKD is of low or very low certainty and insufficient as a guide for clinical practice.
Overall completeness and applicability of evidence
One major limitation was the relatively small number of included studies, with only one study enrolling more than 100 participants (Koya 2009). All the included studies were published before 2009.
Many confounding variables, such as type of diabetes, different ways of measuring the change in GFR, dietary compliance, and different stages of CKD (CKD 1‐5, not on dialysis), were not reported in detail. Thus subgroup analysis allowing for variations in these aspects could not be properly carried out due to the paucity of studies. Many studies were of low methodological quality or did not present adequate data. For the primary outcomes, such as death and kidney failure, there were too few studies of sufficient size or duration to examine these outcomes, and an adequate follow‐up interval should be at least three to five years. Numerical data on weight loss were provided in a few studies though nearly all the studies reported that participants’ body weight was measured.
Quality of the evidence
The quality of study evidence was assessed using the Cochrane risk of bias tool together with GRADE methodology. Most studies had unclear risk methods for allocation concealment and were rated at high risk of bias in the selective reporting domain. None of the participants or study investigators were considered to be masked to treatment allocation as all studies were open‐label. We assessed detection bias for GFR and kidney failure separately. Because GFR measurement was a laboratory outcome, all studies were considered at low risk of detection bias. For the outcome assessment of kidney failure, most studies were judged to be at an unclear risk of detection bias, as information on how to assess the onset of kidney failure was not provided.
We downgraded for the possibility of publication bias due to the very low numbers of data observations for each outcome or the small number of included studies. Confidence in evidence for death (any cause), kidney failure, change in GFR or 24‐hour urinary protein excretion, and weight loss was low or very low, meaning future researches are likely to offer different results.
Potential biases in the review process
This review adhered as closely as possible to our published protocol (Jiang 2021). A comprehensive search of the Cochrane Kidney and Transplant’s Specialised Register was performed by the Information Specialist, which reduced the likelihood of potential studies being omitted from the review. As with most systematic reviews, there remains the possibility that unpublished studies with positive or negative results may not have been identified. We are aware of the potential for publication bias due to the small number of studies in the review. At least two authors independently evaluated all the identified studies in an effort to address any bias or errors in study selection, data extraction and risk of bias assessment.
Agreements and disagreements with other studies or reviews
In 1997, a published Cochrane review evaluated protein restriction among adults with DKD who followed a LPD for at least four months, but there was no evidence from RCTs that explored the impact of dietary protein on kidney failure or death (Waugh 1997). The 2007 update (Robertson 2007) identified one RCT exploring the number of participants who died or developed kidney failure (Hansen 2002), and pooled data from seven RCTs in patients with type 1 diabetes showed a non‐significant reduction of GFR decline in the LPD group. In this review, with interventions lasting for a minimum of 12 months, it seems to be more powerful than in the 2007 version. However, there was also very limited data available to assess the impact of protein restriction on death or kidney failure, although we were able to report on these outcomes separately.
Two recently published systematic reviews (Li 2019; Zhu 2018) evaluated the effects of protein restriction in participants with DKD, both of which evaluated change in GFR or proteinuria but did not evaluate the number of participants dying or reaching kidney failure. Zhu 2018 evaluated eleven RCTs (687 participants) that had a mean length of follow‐up of more than two months. In this review, a LPD (0.6 to 0.8 g/kg/day) compared with a UPD did not seem to slow down the decline in GFR and the increase in urinary protein level. Similarly, 20 RCTs with at least three months of follow‐up, including 1,372 participants with DKD, were reviewed by Li 2019. The authors noted the dietary protein restriction was significantly effective for decreasing proteinuria and that the final GFR in the LPD group seemed to be higher than in the UPD group, although in a non‐significant way. In this review, pooling of the seven RCTs with at least one‐year follow‐up resulted in a non‐significant improvement in GFR of 0.73 mL/min/1.73 m²/year in the LPD group. Variation among participants also needs to be taken into account. Studies did not give sufficient details to quantify this, or a small sample size could not provide great statistical power to observe differences between treatment groups. Thus a small average benefit may conceal larger benefits in some patients.
Authors' conclusions
Implications for practice.
Overall, available data from RCTs outlined in this review suggest that current evidence of dietary protein restriction in the setting of DKD is of low or very low certainty and insufficient to guide clinical practice. There were also very limited data available on adverse effects (e.g. weight loss and hyperglycaemia events) and participants’ QoL, which could be affected by difficulties in maintaining dietary compliance. In practice, the optimum level of dietary protein intake would probably be a compromise between efficacy and compliance. Although a dietary protein intake of 0.6 to 0.8 g/kg/day was recommended by the KDOQI Clinical Practice Guideline 2020 as a dietary intervention for diabetic adults with CKD 3‐5, not on dialysis (KDOQI 2020), clinicians should inform their patients of the lack of high‐quality evidence for these benefits as well as the well‐recognised adverse effects of this intervention. It remains uncertain whether a dietary protein intake of 0.6 to 0.8 g/kg/day should be indicated in adults with DKD at high risk of progression to kidney failure. We still cannot determine what level of protein intake is most effective at different stages of CKD in such a population.
Implications for research.
Given that the sample sizes of the existing studies are generally small and insufficient to determine the effect of LPD on death and kidney failure, there is a need for more large‐scale pragmatic RCTs with an emphasis on different stages of CKD as well as adequate follow‐up. In addition, clinical trials comparing different levels of protein intake or altering protein type in those with DKD are also necessary. Outcome measures should include not only albuminuria, proteinuria, and change of GFR but also the incidents of kidney failure and death. QoL and other adverse events, including weight loss, incidents of malnutrition, and hyperglycaemic events, should be of greater concern. Further information on the role of compliance with restricted protein intake on definite outcomes is also required. With satisfactory adherence to the diet and without compromising the QoL, whether patients with DKD should be offered the option of a reduced protein intake in order to retard the progression of kidney failure needs to be further evaluated (Piccoli 2016).
History
Protocol first published: Issue 7, 2021
Acknowledgements
We thank Professor Norman Waugh and Professor Lynn M Robertson, who contributed to the earlier versions of this review, contributing to the design, quality assessment, data collection, entry, analysis and interpretation, and writing.
We are grateful to the following peer reviewers for their time and comments: Helen L MacLaughlin (Queensland University of Technology, School of Exercise and Nutrition Sciences and Royal Brisbane and Women’s Hospital, Australia), and Vincenzo Bellizzi, MD, PhD (Division of Nephrology, Dialysis & Renal Transplantation, University Hospital "San Giovanni di Dio e Ruggi d'Aragona" in Salerno – Italy).
We also thank Ms Ruth Mitchell, the Information Specialist who provides us with the Cochrane Library search strategy and relevant information, and the staff of Cochrane Kidney and Transplant (Ms Tess Cooper and Ms Narelle Willis) for their assistance with this review.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. sub‐scales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Low protein diet versus usual or unrestricted protein diet.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Death (any cause) | 5 | 358 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.10, 1.44] |
| 1.2 Kidney failure | 4 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.38, 3.59] |
| 1.3 Change in GFR [mL/min/1.73 m²/year] | 7 | 367 | Mean Difference (IV, Random, 95% CI) | ‐0.73 [‐2.30, 0.83] |
| 1.4 Change in CrCl [mL/min/year] | 3 | 203 | Mean Difference (IV, Random, 95% CI) | ‐2.39 [‐5.87, 1.08] |
| 1.5 Change in 24‐hour urinary protein excretion | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 0.90 [0.49, 1.31] |
| 1.6 Change in 24‐hour urinary albumin excretion | 2 | 119 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.07, 0.07] |
| 1.7 Change in UAER | 2 | 45 | Std. Mean Difference (IV, Random, 95% CI) | 0.68 [0.08, 1.29] |
Comparison 2. Nutritional measures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Final body weight | 3 | 146 | Mean Difference (IV, Random, 95% CI) | 1.03 [‐3.04, 5.09] |
| 2.2 Final body mass index | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐2.60, 0.20] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brouhard 1990.
| Study characteristics | ||
| Methods |
|
|
| Participants | General information
Baseline characteristics
Comorbidities/other information
|
|
| Interventions | LPD group
UPD group
Co‐interventions
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated but no information on the method used is available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Masking of patients or study investigators was not reported; participants underwent different diet procedures that could not be blinded |
| Blinding of outcome assessment (detection bias) Change in GFR | Low risk | Laboratory measurement of GFR was unlikely to be influenced by a lack of blinding |
| Blinding of outcome assessment (detection bias) Need to start dialysis | Unclear risk | No information was provided; need to start dialysis was not recorded as a study outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants appeared to have completed follow‐up |
| Selective reporting (reporting bias) | High risk | Only GFR and UAER were reported, but chronic dialysis and nutritional status were not reported. Deaths were also not reported, but there appeared to be no deaths |
| Other bias | Low risk | Grants from Texas Methodist Foundation, and Juvenile Diabetes Foundation International, and Grant RR73 from General Clinical Research Centre Program of the Division of Research Resources |
Dullaart 1993.
| Study characteristics | ||
| Methods |
|
|
| Participants | General information
Baseline characteristics
Comorbidities/other information
|
|
| Interventions | LPD group
UPD group
Co‐interventions
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Masking of patients or study investigators was not reported. Actually, participants underwent different diet procedures that could not be blinded |
| Blinding of outcome assessment (detection bias) Change in GFR | Low risk | GFR was measured by 125I iothalamate and unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Need to start dialysis | Unclear risk | No information was provided. Need to start dialysis was not recorded as a study outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient from the LPD group after randomisation decided not to participate further. Data were available for all the remaining participants |
| Selective reporting (reporting bias) | Low risk | Reported GFR, UAER and body weight. Data for death and the need to start dialysis were not reported but there appeared to be no deaths and no patients needing dialysis |
| Other bias | Low risk | Grant from the Dutch Diabetes Research Fund |
Dussol 2004.
| Study characteristics | ||
| Methods |
|
|
| Participants | General information
Baseline characteristics
Comorbidities/other information
|
|
| Interventions | LPD group
UPD group
Co‐interventions
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was stratified according to the type of diabetes (type 1 or type 2) and performed using random numbers |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated but no information on method used is available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) Change in GFR | Low risk | GFR was measured using 99mTc‐DTPA and unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Need to start dialysis | Low risk | End‐stage kidney disease (GFR < 15 ml/min/1.73m²) was the criteria for starting dialysis |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups (24% in the LPD group; 27% in the UPD group), with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | Reported dialysis, GFR and nutritional status. Deaths were not reported but there appeared to be no deaths |
| Other bias | Low risk | Grant from Programme Hospitalier de Recherche Clinique 1998 |
Hansen 2002.
| Study characteristics | ||
| Methods |
|
|
| Participants | General information
Baseline characteristics
Comorbidities/other information
|
|
| Interventions | LPD group
UPD group
Co‐interventions
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | With concealed randomisation the patients were in blocks of two according to the level of GFR |
| Allocation concealment (selection bias) | Unclear risk | Concealed randomisation stated but no information on method used is available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) Change in GFR | Low risk | GFR was measured using 51Cr‐EDTA and unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Need to start dialysis | Unclear risk | No information provided on the criteria used to commence dialysis |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study, and no patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All expected outcomes (death, commencement of dialysis, GFR, nutritional status) were reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Koya 2009.
| Study characteristics | ||
| Methods |
|
|
| Participants | General information
Baseline characteristics
Comorbidities/other information
|
|
| Interventions | LPD group
UPD group
Co‐interventions
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimization |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not masked |
| Blinding of outcome assessment (detection bias) Change in GFR | Low risk | GFR was calculated using modified MDRD formula and unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Need to start dialysis | Unclear risk | No information provided on the criteria used to commence dialysis |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 21% lost to follow‐up/discontinued diet but all participants included in analysis |
| Selective reporting (reporting bias) | High risk | The data of proteinuria/albuminuria provided in a format that could not be extracted to calculate the change over time |
| Other bias | Low risk | Grant from the Ministry of Health, Labour and Welfare of Japan |
Meloni 2002.
| Study characteristics | ||
| Methods |
|
|
| Participants | General information
Baseline characteristics
Comorbidities/other information
|
|
| Interventions | LPD group
UPD group
Co‐interventions
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated but no information on method used is available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Masking of patients or study investigators was not reported. Actually, participants underwent different diet procedures that could not be blinded |
| Blinding of outcome assessment (detection bias) Change in GFR | Low risk | GFR was measured using 51Cr‐EDTA and unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Need to start dialysis | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants appeared to have completed follow‐up |
| Selective reporting (reporting bias) | High risk | Reported GFR and nutritional status. Deaths were not reported, but there appeared to be no deaths. Patients needing to start dialysis during follow‐up were not reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Meloni 2004.
| Study characteristics | ||
| Methods |
|
|
| Participants | General information
Baseline characteristics
Comorbidities/other information
|
|
| Interventions | LPD group
UPD group
Co‐interventions
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Simple randomisation using dedicated software generating casual numbers to assign participants to treatment groups and remaining participants were placed in control group" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Masking of patients or study investigators was not reported. Actually, participants underwent different diet procedures that could not be blinded |
| Blinding of outcome assessment (detection bias) Change in GFR | Low risk | GFR was measured using 51Cr‐EDTA and unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Need to start dialysis | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants appeared to have completed follow‐up |
| Selective reporting (reporting bias) | High risk | Reported GFR and nutritional status. Deaths were not reported, but there appeared to be no deaths. Patients needing to start dialysis during follow‐up did not report |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Zeller 1991.
| Study characteristics | ||
| Methods |
|
|
| Participants | General information
Baseline characteristics
Comorbidities/other information
|
|
| Interventions | LPD group
UPD group
Co‐interventions
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated but no information on method used is available |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Masking of patients or study investigators was not reported. Actually, participants underwent different diet procedures that could not be blinded |
| Blinding of outcome assessment (detection bias) Change in GFR | Low risk | GFR was measured using125I‐labeled iothalamate clearance and unlikely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Need to start dialysis | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 25% lost to follow‐up/discontinued diet. Two patients in the LPD group whose mean protein intake exceeded 0.8 g/kg/day were excluded from the general analysis |
| Selective reporting (reporting bias) | High risk | Reported deaths, GFR and nutritional status. Patients needing to start dialysis during follow‐up did not report. Change in proteinuria could not be extracted |
| Other bias | Low risk | Grants from the Juvenile Diabetes Foundation and the Texas Kidney Foundation and a General Clinical Research Center grant (MOI‐RR00633) |
ACEIs: angiotensin‐converting enzyme inhibitors; ARBs: angiotensin receptor blockers; BMI: body mass index; BP: blood pressure; CCBs: calcium channel blockers; CrCl: creatinine clearance; DBP: diastolic blood pressure; DKD: diabetic kidney disease; GFR: glomerular filtration rate; Hb: haemoglobin; HRQoL: health‐related quality of life; LPD: low protein diet; MDRD: Modification of Diet in Renal Disease; RCT: randomised controlled trial; SBP: systolic blood pressure; SCr: serum creatinine; SD: standard deviation; SF‐36: short‐form 36 Health Status Questionnaire; UAER: urinary albumin excretion ratio; UPD: usual or unrestricted protein diet; UTI: urinary tract infection; 51Cr‐EDTA: chromium‐51 ethylenediamine tetra‐acetic acid; 99mTc‐DTPA: technetium‐99m‐diethylene triamine penta‐acetic acid
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aoyagi 2006 | RCT comparing regular protein restriction diet (0.6 g/kg/day) with mild protein restriction diet (1.1 g/kg/day) in type 2 diabetic patients with nephropathy under angiotensin II receptor blockade; the number of patients in each group could not be determined |
| Azadbakht 2003 | Wrong intervention and duration: cross‐over design comparing a diet containing 0.8 g/kg protein (70% animal and 30% vegetable protein) with a similar diet containing 0.8 g/kg protein (35% soy protein, 30% vegetable protein and 35% animal protein); the starting period of the intervention was only 7 weeks |
| Barsotti 1993 | Wrong intervention: low protein intake (0.7 g/kg/day) versus very low protein intake (0.3 g/kg/day); it was unclear whether randomisation was used |
| Bending 1988 | Wrong duration: cross‐over study with patients only studied for 3 weeks in each phase |
| Chen 2012h | Wrong intervention: keto‐amino acids (100 mg/kg/day) versus no addition in participants on low protein intake (0.8 g/kg/day) |
| Ciarambino 2012 | Wrong intervention and duration: low protein intake (0.8 g/kg/day, 7 days a week) versus a protein diet (0.8 g/kg/day, 6 days a week) in participants with a usual protein intake (1.2 g/kg/day); follow‐up only 4 weeks |
| Ciavarella 1987 | Wrong duration: 3 to 6 months duration only |
| Cohen 1987 | Wrong duration: cross‐over study with participants only studied for 3 weeks in each phase |
| Effendi 2000 | Wrong duration: standard protein diet (> 1 g/kg/day) versus a LPD (0.8 g/kg/day) but duration of follow‐up is only 4 weeks |
| Facchini 2003 | Wrong intervention: carbohydrate‐restricted, low‐iron‐available, polyphenol‐enriched diet (not an unrestricted diet) versus conventional standard‐of‐care dietary treatment |
| Garcia Garcia 1990 | Not randomised |
| Gross 2002 | Wrong duration: cross‐over study with participants only studied for 4 weeks in each phase |
| Hansen 1999 | Wrong duration: low protein intake versus UPD but duration only 8 weeks |
| Parillo 1988 | Wrong duration: 10 days duration only |
| Pecis 1994 | Wrong population and duration: diabetic participants with normoalbuminuria; duration only 3 weeks |
| Pedersen 1989 | Wrong population and duration: cross‐over study comparing an UPD with limited protein intake in diabetic patients with normoalbuminuria; each intervention for 4 weeks only |
| Pijls 1999 | Wong population: more than 60% diabetic participants with normoalbuminuria |
| Pinto 1991 | Wrong duration: cross‐over study comparing low protein intake with a UPD; each intervention for 3 weeks only |
| Qiu 2012a | Wrong intervention: LPD (0.6 g/kg/day) + keto‐analogues versus dietary protein intake of 0.8 g/kg/day (< 1.0 g/kg/day) |
| Raal 1994 | Wrong duration: 6 months duration only |
| Rosenberg 1987 | Wrong duration: cross‐over study comparing low protein intake with a high protein diet; each intervention for 11 days only |
| Rudberg 1988 | Wrong population and duration: cross‐over study including diabetic patients with normoalbuminuria; only studied for 10 days in each phase |
| Silan 2003 | RCT comparing a LPD (0.6 g/kg/day) with a UPD in type 1 diabetic patients with progressive DKD; the number of patients in each group could not be determined |
| Stephenson 2005 | Wrong population and duration: cross‐over study including diabetic patients with normoalbuminuria; only studied for 8 weeks in each phase |
| Suratkal 2012 | Wrong intervention: keto‐analogues versus no supplements in participants on the same LPDs |
| Velazquez Lopez 2008 | Wrong duration: 4 months duration only |
| Wheeler 2002 | Wrong intervention and duration: cross‐over study comparing plant‐based protein intake with predominantly animal‐based protein diet in participants on macronutrients equivalent between the two diets; each intervention for 6 weeks only |
| Wiseman 1987 | Wrong population and duration: cross‐over study in diabetic patients with normoalbuminuria comparing low protein intake with UPD; each intervention for 3 weeks only |
| Zeller 1986 | Wrong duration: patients followed for only a mean of 6.7 months |
| Zucchelli 1988b | Wrong duration: 4 weeks only |
LPD: low protein diet; RCT: randomised controlled trial; UPD: usual or unrestricted protein diet
Differences between protocol and review
None
Contributions of authors
Draft the protocol: SJ
Study selection: SJ, JF
Extract data from studies: SJ, JF
Enter data into RevMan: SJ
Carry out the analysis: SJ
Draft the final review: SJ, WL
Disagreement resolution: WL
Update the review: SJ, WL
Sources of support
Internal sources
Department of Nephrology, China‐Japan Friendship Hospital, No. 2 East Yinghuayuan Street, Chaoyang District, Beijing 100029, China
External sources
-
Beijing Natural Science Foundation (7202179) and Science and Technology Project of Beijing (D171100002817003), China
The sponsors have no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Declarations of interest
Shimin Jiang: no relevant interests were disclosed
Jinying Fang: no relevant interests were disclosed
Wenge Li: no relevant interests were disclosed
New
References
References to studies included in this review
Brouhard 1990 {published data only}
- Brouhard BH, LaGrone L. Effect of dietary protein restriction on functional renal reserve in diabetic nephropathy. American Journal of Medicine 1990;89(4):427-31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Brouhard BH. Functional renal reserve increases after protein restriction in diabetic nephropathy [abstract]. Kidney International 1988;33(1):184. [CENTRAL: CN-00583871] [Google Scholar]
Dullaart 1993 {published data only}
- Dullaart RP, Beusekamp BJ, Meijer S, Doormaal JJ, Sluiter WJ. Long-term effects of protein-restricted diet on albuminuria and renal function in IDDM patients without clinical nephropathy and hypertension. Diabetes Care 1993;16(2):483-92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dussol 2004 {published data only}
- Dussol B, Iovanna C, Raccah D, Darmon P, Morange S, Vague P, et al. A randomized trial of low-protein diet in type 1 and in type 2 diabetes mellitus patients with incipient and overt nephropathy. Journal of Renal Nutrition 2005;15(4):398-406. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dussol B, Morange S, Iovanna C, Raccah D, Vialettes B, Vague P, et al. A randomized trial of low-protein diet in type I and type 2 diabetes mellitus patients with incipient and overt nephropathy [abstract no: PUB095]. Journal of the American Society of Nephrology 2004;15:782A. [CENTRAL: CN-00583507] [DOI] [PubMed] [Google Scholar]
Hansen 2002 {published data only}
- Andresdottir G, Bakker SJ, Hansen HP, Parving HH, Rossing P. Urinary sulphate excretion and progression of diabetic nephropathy in Type 1 diabetes. Diabetic Medicine 2013;30(5):563-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Andresdottir G, Bakker SJ, Hansen HP, Parving HH, Rossing P. Urinary sulphate excretion is a predictor for progression of diabetic nephropathy [abstract no: TH-PO498]. Journal of the American Society of Nephrology 2011;22(Abstract Suppl):227A. [Google Scholar]
- Hansen HP, Tauber-Lassen E, Jensen BR, Parving HH. Effect of dietary protein restriction on prognosis in patients with diabetic nephropathy. Kidney International 2002;62(1):220-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hansen HP, Tauber-Lassen E, Jensen BR, Parving HH. Effect of protein restriction on prognosis in type 1 diabetic patients with diabetic nephropathy [abstract no: A0770]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):147A. [CENTRAL: CN-00445638] [Google Scholar]
- Nielsen SE, Hansen HP, Jensen BR, Parving HH, Rossing P. Urinary (U) neutrophil gelatinase-associated lipocalin (NGAL) and progression of nephropathy in type 1 diabetic patients [abstract no: PUB114]. Journal of the American Society of Nephrology 2009;20(Abstract Suppl):853A. [Google Scholar]
- Nielsen SE, Hansen HP, Jensen BR, Parving HH, Rossing P. Urinary neutrophil gelatinase-associated lipocalin and progression of diabetic nephropathy in type 1 diabetic patients in a four-year follow-up study. Nephron 2011;118(2):c130-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pilemann-lyberg S, Lindhardt M, Persson FI, Hansen HP, Rossing P. Effect of dietary protein restriction and uric acid on progression of diabetic nephropathy [abstract no: FR-PO784]. Journal of the American Society of Nephrology 2014;25(Abstract Suppl):548A. [Google Scholar]
Koya 2009 {published data only}
- Koya D, Haneda M, Inomata S, Suzuki Y, Suzuki D, Makino H, et al. Long-term effect of modification of dietary protein intake on the progression of diabetic nephropathy: a randomised controlled trial. Diabetologia 2009;52(10):2037-45. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meloni 2002 {published data only}
- Meloni C, Morosetti M, Suraci C, Pennafina MG, Tozzo C, Taccone-Gallucci M, et al. Severe dietary protein restriction in overt diabetic nephropathy: benefits or risks? Journal of Renal Nutrition 2002;12(2):96-101. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Meloni C, Morosetti M, Taccone-Gallucci M, Palombo G, Latorre PC, Casciani CU. Dietary protein restriction and progression of chronic renal failure in diabetic nephropathy: risk or benefit? [abstract no: A0581]. Journal of the American Society of Nephrology 1996;7(9):1362. [CENTRAL: CN-00446731] [Google Scholar]
Meloni 2004 {published data only}
- Meloni C, Tatangelo P, Cipriani S, Rossi V, Suraci C, Tozzo C, et al. Adequate protein dietary restriction in diabetic and nondiabetic patients with chronic renal failure. Journal of Renal Nutrition 2004;14(4):208-13. [MEDLINE: ] [PubMed] [Google Scholar]
Zeller 1991 {published data only}
- Parving HH. Protein restriction and renal failure in diabetes mellitus. New England Journal of Medicine 1991;324(24):1743-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zeller K, Whittaker E, Sullivan L, Raskin P, Jacobson HR. Effect of restricting dietary protein on the progression of renal failure in patients with insulin-dependent diabetes mellitus. New England Journal of Medicine 1991;324(2):78-84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zeller KR, Jacobson H, Raskin P. The effect of dietary protein and phosphorus restriction on renal function in diabetic nephropathy - results of a 5 year study [abstract]. Kidney International 1990;37(1):246. [CENTRAL: CN-00644207] [Google Scholar]
- Zeller KR, Jacobson H, Raskin P. The effect of dietary protein modification on renal function in diabetic nephropathy-preliminary report of an ongoing study [abstract]. Kidney International 1987;31(1):225. [CENTRAL: CN-00644226] [Google Scholar]
References to studies excluded from this review
Aoyagi 2006 {published data only}
- Aoyagi M, Maeda Y, Shiigai T, Mori H, Funayama I, Tabei K. Effect of dietary protein restriction diet among type 2 diabetic patients under angiotensin II receptor blockade [abstract no: TH-PO249]. Journal of the American Society of Nephrology 2006;17(Abstracts):159A. [Google Scholar]
Azadbakht 2003 {published data only}
- Azadbakht L, Shakerhosseini R, Atabak S, Jamshidian M, Mehrabi Y, Esmaill-Zadeh A. Beneficiary effect of dietary soy protein on lowering plasma levels of lipid and improving kidney function in type II diabetes with nephropathy. European Journal of Clinical Nutrition 2003;57(10):1292-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Barsotti 1993 {published data only}
- Barsotti G, Cupisti A, Morelli E, Meola M, Meriggioli M, Giovannetti S. Implications of dietary treatment in diabetic nephropathy [abstract]. In: 12th International Congress of Nephrology; 1993 Jun 13-18; Jerusalem, Israel. 1993:440. [CENTRAL: CN-00740508]
Bending 1988 {published data only}
- Bending JJ, Dodds RA, Keen H, Viberti GC. Renal response to restricted protein intake in diabetic nephropathy. Diabetes 1988;37(12):1641-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chen 2012h {published data only}
- Chen N, Jin Y, Ren H, Xu J, Shen P, Huang X. Anti-inflammatory effects of low protein diet supplemented with keto-amino acid in the treatment of type 2 diabetic nephropathy [abstract no: 34]. Kidney Research & Clinical Practice 2012;31(2):A24. [EMBASE: 70814743] [Google Scholar]
Ciarambino 2012 {published data only}
- Ciarambino T, Ferrara N, Castellino P, Paolisso G, Coppola L, Giordano M. Effects of six days a week low protein dietary intervention on depressive symptoms in elderly diabetic subjects [Effetti della dieta ipoproteica 6 giorni la settimana sui sintomi depressivi in anziani diabetici tipo 2]. Giornale di Gerontologia 2012;60(1):8-13. [EMBASE: 364932097] [Google Scholar]
Ciavarella 1987 {published data only}
- Ciavarella A, Di Mizio G, Stefoni S, Borgnino LC, Vannini P. Reduced albuminuria after dietary protein restriction in insulin-dependent diabetic patients with clinical nephropathy. Diabetes Care 1987;10(4):407-13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cohen 1987 {published data only}
- Cohen D, Dodds R, Viberti G. Effect of protein restriction in insulin dependent diabetics at risk of nephropathy. British Medical Journal Clinical Research Ed 1987;294(6575):795-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak A, Rowe D. Effect of protein restriction in insulin dependent diabetics at risk of nephropathy. British Medical Journal Clinical Research Ed 1987;294(6583):1351-2. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Effendi 2000 {published data only}
- Effendi I, Bardiman S, Ali Z. The role of protein intake restriction on microalbuminuria in type 2 DM (NIDDM) patients [abstract no: Fp.4.4]. In: 13th Asian Colloquium in Nephrology; 2000 Nov 23-25; Bali, Indonesia. 2000:97. [CENTRAL: CN-00460684]
Facchini 2003 {published data only}
- Facchini FS, Saylor KL. A low-iron-available, polyphenol-enriched, carbohydrate-restricted diet to slow progression of diabetic nephropathy. Diabetes 2003;52(5):1204-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Garcia Garcia 1990 {published data only}
- Garcia Garcia M, Esmatjes ME, Ferrer J, Revert L. Protein restriction on the progression of renal insufficiency in diabetic nephropathy. Nephrology Dialysis Transplantation 1990;5:672. [CENTRAL: CN-00260516] [Google Scholar]
Gross 2002 {published data only}
- Gross JL, Zelmanovitz T, Moulin CC, De Mello V, Perassolo M, Leitao C, et al. Effect of a chicken-based diet on renal function and lipid profile in patients with type 2 diabetes: a randomized crossover trial. Diabetes Care 2002;25(4):645-51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hansen 1999 {published data only}
- Hansen HP, Christensen PK, Tauber-Lassen E, Klausen A, Jensen BR, Parving HH. Low-protein diet and kidney function in insulin-dependent diabetic patients with diabetic nephropathy. Kidney International 1999;55(2):621-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hansen HP, Christensen PK, Tauber-Lassen E, Klausen A, Parving HH. Low protein diet and kidney function in insulin dependent diabetic patients with diabetic nephropathy [abstract no: P1215]. Nephrology 1997;3(Suppl 1):S377. [CENTRAL: CN-00460895] [DOI] [PubMed] [Google Scholar]
Parillo 1988 {published data only}
- Parillo M, Riccardi G, Pacioni D, Iovine C, Contaldo F, Isernia C, et al. Metabolic consequences of feeding a high-carbohydrate, high-fiber diet to diabetic patients with chronic kidney failure. American Journal of Clinical Nutrition 1988;48(2):255-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pecis 1994 {published data only}
- Pecis M, Azevedo MJ, Gross JL. Chicken and fish diet reduces glomerular hyperfiltration in IDDM patients. Diabetes Care 1994;17(7):665-72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pedersen 1989 {published data only}
- Pedersen MM, Mogensen CE, Jorgensen FS, Moller B, Lykke G, Pedersen O. Renal effects from limitation of high dietary protein in normoalbuminuric diabetic patients. Kidney International - Supplement 1989;36(27):S115-21. [MEDLINE: ] [PubMed] [Google Scholar]
Pijls 1999 {published data only}
- Pijls LT, Vries H, Donker AJ, Eijk JT. The effect of protein restriction on albuminuria in patients with type 2 diabetes mellitus: a randomized trial. Nephrology Dialysis Transplantation 1999;14(6):1445-53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pijls LT, Vries H, Eijk JT, Donker AJ. Protein restriction, glomerular filtration rate and albuminuria in patients with type 2 diabetes mellitus: a randomized trial. European Journal of Clinical Nutrition 2002;56(12):1200-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pinto 1991 {published data only}
- Pinto JR, Bending JJ, Dodds RA, Viberti GC. Effect of low protein diet on the renal response to meat ingestion in diabetic nephropathy. European Journal of Clinical Investigation 1991;21(2):175-83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Qiu 2012a {published data only}
- Qiu HY, Liu F, Zhao LJ, Huang SM, Zuo C, Zhong H, et al. Comparison of the effects of alpha-keto/ amino acid supplemented low protein diet and diabetes diet in patients with diabetic nephropathy. Sichuan da Xue Xue Bao. Yi Xue Ban/Journal of Sichuan University. Medical Science Edition 2012;43(3):425-8. [MEDLINE: ] [PubMed] [Google Scholar]
Raal 1994 {published data only}
- Raal FJ, Kalk WJ, Lawson M, Esser JD, Buys R, Fourie L, et al. Effect of moderate dietary protein restriction on the progression of overt diabetic nephropathy: a 6-mo prospective study. American Journal of Clinical Nutrition 1994;60(4):579-85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rosenberg 1987 {published data only}
- Rosenberg ME, Howe RB, Zanjani ED, Hostetter TH. The response of erythropoietin to dietary protein in human renal disease. Journal of Laboratory & Clinical Medicine 1989;113(6):735-42. [MEDLINE: ] [PubMed] [Google Scholar]
- Rosenberg ME, Swanson JE, Thomas BL, Hostetter TH. Glomerular and hormonal responses to dietary protein intake in human renal disease. American Journal of Physiology 1987;253(6 Pt 2):F1083-90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rosenberg ME, Thomas BL, Swanson JE, Hostetter TH. Effect of protein intake on glomerular function in human renal disease [abstract no: 61]. Kidney International 1987;29(1):325. [Google Scholar]
- Rosenberg ME, Thomas BL, Swanson JE, Hostetter TH. Hormonal and glomerular responses to dietary protein intake in human renal disease [abstract]. Kidney International 1987;31(1):215. [CENTRAL: CN-00644163] [DOI] [PubMed] [Google Scholar]
Rudberg 1988 {published data only}
- Rudberg S, Dahlquist G, Aperia A, Persson B. Reduction of protein intake decreases glomerular filtration rate in young type 1 (insulin-dependent) diabetic patients mainly in hyperfiltering patients. Diabetologia 1988;31(12):878-83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Silan 2003 {published data only}
- Silan L, Cerna K, Urbanova E, Kovac G, Kremery S, Hutar R. Favourable effect of dietary protein restriction on prognosis in type 1 diabetic patients with diabetic nephropathy [abstract no: M332]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):104. [CENTRAL: CN-00447735] [Google Scholar]
Stephenson 2005 {published data only}
- Stephenson TJ, Setchell KDR, Kendall CWC, Jenkins DJA, Anderson JW, Fanti P. Effect of soy protein-rich diet on renal function in young adults with insulin-dependent diabetes mellitus. Clinical Nephrology 2005;64(1):1-11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Suratkal 2012 {published data only}
- Suratkal LH, Khandekar A. The study of effect of ketoanalogues in delaying progression of chronic kidney disease in patients with diabetic nephropathy [abstract no: 310]. Kidney Research & Clinical Practice 2012;31(2):A95. [EMBASE: 70815019] [Google Scholar]
Velazquez Lopez 2008 {published data only}
- Velazquez Lopez L, Sil Acosta MJ, Goycochea Robles MV, Torres Tamayo M, Castaneda Limones R. Effect of protein restriction diet on renal function and metabolic control in patients with type 2 diabetes: a randomized clinical trial. Nutricion Hospitalaria 2008;23(2):141-7. [MEDLINE: ] [PubMed] [Google Scholar]
Wheeler 2002 {published data only}
- Wheeler ML, Fineberg SE, Fineberg NS, Gibson RG, Hackward LL. Animal versus plant protein meals in individuals with type 2 diabetes and microalbuminuria: effects on renal, glycemic, and lipid parameters. Diabetes Care 2002;25(8):1277-82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wiseman 1987 {published data only}
- Wiseman MJ, Bognetti E, Dodds R, Keen H, Viberti GC. Changes in renal function in response to protein restricted diet in type 1 (insulin-dependent) diabetic patients. Diabetologia 1987;30(3):154-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zeller 1986 {published data only}
- Zeller KR, Raskin P, Rosenstock J, Jacobson H. The effect of dietary protein restriction in diabetic nephropathy: reduction in proteinuria [abstract no: 15]. Kidney International 1986;29:209. [Google Scholar]
Zucchelli 1988b {published data only}
- Zucchelli P, Zuccala A, Sturani A. Glomerular dysfunction in diabetic nephropathy. Postgraduate Medical Journal 1988;64 Suppl 3:22-30. [MEDLINE: ] [PubMed] [Google Scholar]
Additional references
ADA 1999
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1999;22 Suppl 1:S5-20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ADA 2008
- American Diabetes Association. Standards of medical care in diabetes – 2008. Diabetes 2008;31 Suppl 1:S12-54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ADA 2010
- American Diabetes Association. Standards of medical care in diabetes – 2010 [Erratum in: Diabetes Care. 2010 Mar;33(3):692]. Diabetes Care 2010;33 Suppl 1:S11-61. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
ADA 2014
- Tuttle KR, Bakris GL, Bilous RW, Chiang JL, Boer IH, Goldstein-Fuchs J, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. American Journal of Kidney Diseases 2014;64(4):510-33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ADA 2018
- American Diabetes Association. 10. Microvascular complications and foot care: standards of medical care in diabetes - 2018. Diabetes Care 2018;41(Suppl 1):S105-18. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ADA 2019
- Evert AB, Dennison M, Gardner CD, Garvey WT, Lau KH, MacLeod J, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care 2019;42(5):731-54. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barsotti 1988
- Barsotti G, Ciardella F, Morelli E, Cupisti A, Mantovanelli A, Giovannetti S. Nutritional treatment of renal failure in type 1 diabetic nephropathy. Clinical Nephrology 1988;29(6):280-7. [MEDLINE: ] [PubMed] [Google Scholar]
Chauveau 2011
- Chauveau P, Aparicio M. Benefits in nutritional interventions in patients with CKD stage 3-4. Journal of Renal Nutrition 2011;21(1):20-2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dabelea 2017
- Dabelea D, Stafford JM, Mayer-Davis EJ, D'Agostino R Jr, Dolan L, Imperatore G, et al. Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA 2017;317(8):825-35. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
EuroQol 1990
- EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990;16(3):199-208. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gaede 2003
- Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. New England Journal of Medicine 2003;348(5):383-93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gaede 2016
- Gaede P, Oellgaard J, Carstensen B, Rossing P, Lund-Andersen H, Parving HH, et al. Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: 21 years follow-up on the Steno-2 randomised trial. Diabetologia 2016;59(11):2298-307. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gao 2010
- Gao X, Wu J, Dong Z, Hua C, Hu H, Mei C. A low-protein diet supplemented with ketoacids plays a more protective role against oxidative stress of rat kidney tissue with 5/6 nephrectomy than a low-protein diet alone. British Journal of Nutrition 2010;103(4):608-16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Garneata 2016
- Garneata L, Stancu A, Dragomir D, Stefan G, Mircescu G. Ketoanalogue-supplemented vegetarian very low-protein diet and CKD progression. Journal of the American Society of Nephrology 2016;27(7):2164-76. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Giordano 2014
- Giordano M, Ciarambino T, Castellino P, Cataliotti A, Malatino L, Ferrara N, et al. Long-term effects of moderate protein diet on renal function and low-grade inflammation in older adults with type 2 diabetes and chronic kidney disease. Nutrition 2014;30(9):1045-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gross 2005
- Gross JL, Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care 2005;28(1):164-76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hahn 2020
- Hahn D, Hodson EM, Fouque D. Low protein diets for non-diabetic adults with chronic kidney disease. Cochrane Database of Systematic Reviews 2020, Issue 10. Art. No: CD001892. [DOI: 10.1002/14651858.CD001892.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated September 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Hostetter 1986
- Hostetter TH, Meyer TW, Rennke HG, Brenner BM. Chronic effects of dietary protein in the rat with intact and reduced renal mass. Kidney International 1986;30(4):509-17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
IDF 2021
- International Diabetes Federation. IDF Diabetes Atlas, 10th edition 2021. www.diabetesatlas.org (accessed 18 November 2022).
JSDT 2019
- Nitta K, Masakane I, Hanafusa N, Taniguchi M, Hasegawa T, Nakai S, et al. Annual dialysis data report 2017, JSDT Renal Data Registry. Renal Replacement Therapy 2019;5:53. [DOI: 10.1186/s41100-019-0248-1] [DOI] [Google Scholar]
Jungers 1987
- Jungers P, Chauveau P, Ployard F, Lebkiri B, Ciancioni C, Man NK. Comparison of ketoacids and low protein diet on advanced chronic renal failure progression. Kidney International - Supplement 1987;22:S67-71. [MEDLINE: ] [PubMed] [Google Scholar]
KDIGO 2020
- Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney International 2020;98(4S):S1-115. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
KDOQI 2007
- KDOQI. KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. American Journal of Kidney Diseases 2007;49(2 Suppl 2):S12-154. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
KDOQI 2020
- Ikizler TA, Burrowes JD, Byham-Gray LD, Campbell KL, Carrero JJ, Chan W, et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update [Erratum in: Am J Kidney Dis. 2021 Feb;77(2):308]. American Journal of Kidney Diseases 2020;76(3 Suppl 1):S1-107. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kim 2010
- Kim HJ, Vaziri ND, Norris K, An WS, Quiroz Y, Rodriguez-Iturbe B. High-calorie diet with moderate protein restriction prevents cachexia and ameliorates oxidative stress, inflammation and proteinuria in experimental chronic kidney disease. Clinical & Experimental Nephrology 2010;14(6):536-47. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kitada 2016
- Kitada M, Ogura Y, Suzuki T, Sen S, Lee SM, Kanasaki K, et al. A very-low-protein diet ameliorates advanced diabetic nephropathy through autophagy induction by suppression of the mTORC1 pathway in Wistar fatty rats, an animal model of type 2 diabetes and obesity. Diabetologia 2016;59(6):1307-17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Klahr 1983
- Klahr S, Buerkert J, Purkerson ML. Role of dietary factors in the progression of chronic renal disease. Kidney International 1983;24(5):579-87. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Li 2013
- Li R, Bilik D, Brown MB, Zhang P, Ettner SL, Ackermann RT, et al. Medical costs associated with type 2 diabetes complications and comorbidities. American Journal of Managed Care 2013;19(5):421-30. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Li 2019
- Li XF, Xu J, Liu LJ, Wang F, He SL, Su Y, et al. Efficacy of low-protein diet in diabetic nephropathy: a meta-analysis of randomized controlled trials. Lipids in Health & Disease 2019;18(1):82. [MEDLINE: ] [DOI] [PMC free article] [PubMed]
Maroni 1985
- Maroni BJ, Steinman TI, Mitch WE. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney International 1985;27(1):58-65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pan 2008
- Pan Y, Guo LL, Jin HM. Low-protein diet for diabetic nephropathy: a meta-analysis of randomized controlled trials. American Journal of Clinical Nutrition 2008;88(3):660-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Parving 2006
- Parving HH, Lewis JB, Ravid M, Remuzzi G, Hunsicker LG, DEMAND investigators. Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney International 2006;69(11):2057-63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Piccoli 2016
- Piccoli GB, Ventrella F, Capizzi I, Vigotti FN, Mongilardi E, Grassi G, et al. Low-protein diets in diabetic chronic kidney disease (CKD) patients: are they feasible and worth the effort? Nutrients 2016;8(10):649. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sabanayagam 2019
- Sabanayagam C, Chee ML, Banu R, Cheng CY, Lim SC, Tai ES, et al. Association of diabetic retinopathy and diabetic kidney disease with all-cause and cardiovascular mortality in a multiethnic Asian population. JAMA Network Open 2019;2(3):e191540. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schunemann 2021a
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated September 2021). Cochrane, 2021. Available from www.cochrane-handbook.org.
Schunemann 2021b
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated September 2021). Cochrane, 2021. Available from www.cochrane-handbook.org.
Tervaert 2010
- Tervaert TW, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB, et al. Pathologic classification of diabetic nephropathy. Journal of the American Society of Nephrology 2010;21(4):556-63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
UKRR 2020
- Pyart R, Evans KM, Steenkamp R, Casula A, Wong E, Magadi W, et al. The 21st UK Renal Registry annual report: a summary of analyses of adult data in 2017. Nephron 2020;144(2):59-66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Unnikrishnan 2007
- Unnikrishnan RI, Rema M, Pradeepa R, Deepa M, Shanthirani CS, Deepa R, et al. Prevalence and risk factors of diabetic nephropathy in an urban South Indian population: the Chennai Urban Rural Epidemiology Study (CURES 45). Diabetes Care 2007;30(8):2019-24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
USRDS 2019
- Saran R, Robinson B, Abbott KC, Bragg-Gresham J, Chen X, Gipson D, et al. US Renal Data System 2019 annual data report: epidemiology of kidney disease in the United States. American Journal of Kidney Diseases 2019;75(1 Suppl 1):A6-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473-83. [MEDLINE: ] [PubMed] [Google Scholar]
Williams 1991
- Williams B, Hattersley J, Layward E, Walls J. Metabolic acidosis and skeletal muscle adaptation to low protein diets in chronic uremia. Kidney International 1991;40(4):779-86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zhu 2018
- Zhu HG, Jiang ZS, Gong PY, Zhang DM, Zou ZW, Qian-Zhang, et al. Efficacy of low-protein diet for diabetic nephropathy: a systematic review of randomized controlled trials. Lipids in Health and Disease 2018;17(1):141. [MEDLINE: ] [DOI] [PMC free article] [PubMed]
References to other published versions of this review
Jiang 2021
- Jiang S, Fang J, Li W. Protein restriction for diabetic kidney disease. Cochrane Database of Systematic Reviews 2021, Issue 7. Art. No: CD014906. [DOI: 10.1002/14651858.CD014906] [DOI] [PMC free article] [PubMed] [Google Scholar]
Robertson 2007
- Robertson L, Waugh N, Robertson A. Protein restriction for diabetic renal disease. Cochrane Database of Systematic Reviews 2007, Issue 4. Art. No: CD002181. [DOI: 10.1002/14651858.CD002181.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Waugh 1997
- Waugh NR, Robertson AM. Protein restriction for diabetic renal disease. Cochrane Database of Systematic Reviews 1997, Issue 4. Art. No: CD002181. [DOI: 10.1002/14651858.CD002181] [DOI] [PMC free article] [PubMed] [Google Scholar]


