Abstract
Thousands of individuals die each year from opioid-related overdoses. While naloxone (Narcan®) is currently the most widely employed treatment to reverse opioid toxicity, high or repeated doses of this antidote often lead to precipitated opioid withdrawal (POW). We hypothesized that a slow linear release of naloxone from a nanoparticle would induce fewer POW symptoms compared to high-dose free naloxone. First, we measured the acute impact of covalent naloxone nanoparticles (Nal-cNPs) on morphine-induced antinociception in the hotplate test. We found that Nal-cNP treatment blocked the antinociceptive effect of morphine within 15 min of administration. Next, we tested the impact of Nal-cNPs on POW symptoms in male morphine-dependent mice. To induce morphine dependence, mice were treated with 5 mg/kg morphine (or saline) twice-daily for six consecutive days. On day 7 mice received 5 mg/kg morphine (or saline) injections 2 hr prior to receiving treatment of either unmodified free naloxone, a high or low dose of Nal-cNP, empty nanoparticle (cNP-empty), or saline. Behavior was analyzed for 0–6 hr followed by 24 and 48 hr time points after treatment. As expected, free naloxone induced a significant increase in POW behavior in morphine-dependent mice compared to saline-treated mice upon free naloxone administration. In comparison, reduced POW behavior was observed with both doses of Nal-cNP. Side effects of Nal-cNP on locomotion and fecal boli production were measured and no significant side-effects were observed. Overall, our data show that sustained release of naloxone from a covalent nanoparticle does not induce severe POW symptoms in morphine-dependent mice.
Keywords: drug delivery system, morphine, naloxone, nanoparticle, withdrawal
1 |. INTRODUCTION
For countless decades, mu opioid receptor (MOR) agonists such as morphine have been utilized to attenuate both acute and chronic pain. While MOR agonists are still commonly used analgesics for chronic pain management, their use is limited, in part, due to high drug abuse potential. In 2002, it was reported that about 11 million US residents abused an opioid medication, 1.5 million of which displayed symptoms of opioid use disorder (OUD) as defined by the DSM-IV((SAMHSA), 2003; Compton & Volkow, 2006). Moreover, in 2017 more than 70,000 people in the United States died from drug overdoses and 68% of those deaths involved a prescription or illicit opioid (CDC/NCHS, 2018). Given such high rates of opioid-related emergency visits, abuse, and deaths, the development of preventive strategies and effective treatments for opioid dependence and overdose remains a clinical need.
In an attempt to reduce opioid-overdose mortality, naloxone (Narcan) is increasingly utilized by both emergency medical personnel and bystanders witnessing an overdose (Wheeler, Jones, Gilbert, & Davidson, 2015). Naloxone is a MOR antagonist that has been shown to reverse respiratory depression brought on by opioid overdose (Dahan, Aarts, & Smith, 2010; Robinson & Wermeling, 2014). While naloxone is relatively safe and virtually free of adverse effects in opioid naïve individuals (Foldes, Duncalf, & Kuwabara, 1969; Mowry, Spyker, Brooks, Zimmerman, & Schauben, 2016), a few factors limit its therapeutic potential, especially in OUD patients experiencing an overdose. First, individuals with OUD often experience renarcotization once treated with naloxone (Dahan et al., 2010). This is primarily due to the relatively short half-life of naloxone (30–81 min; Rzasa Lynn & Galinkin, 2018; Vanky, Hellmundt, Bondesson, Eksborg, & Lundeberg, 2017). The toxic effects of many opioids far outlast the antagonistic effects of a single dose of naloxone, thus individuals can experience a second overdose once naloxone is cleared. To account for the rapid metabolism of naloxone, higher or repeated doses of naloxone are often administered, which leads to the second limitation of naloxone. High circulating levels of naloxone can initiate precipitated opioid withdrawal (POW) symptoms in individuals with prior opioid exposure (Moss & Carlo, 2019; Rzasa Lynn & Galinkin, 2018; Sun, 1998). POW symptoms can include behavioral agitation, pulmonary edema, sweating, nausea, seizures, and craving (Flacke, Flacke, & Williams, 1977; Jain, Singhai, & Swami, 2018; Kanof et al., 1992). The onset of these withdrawal symptoms can increase an individual’s chance of relapse (Clemency et al., 2019). Therefore, a dire need exists to develop new therapeutic treatments for opioid overdose that both effectively reverse opioid toxicity while limiting POW.
We recently demonstrated the feasibility of covalently loaded polylactic acid (PLA) naloxone nanoparticles (Nal-cNP) as an effective drug delivery system (DDS) for the extended linear release of naloxone with MOR antagonism (Kassick et al., 2019). The use of such a next generation DDS is a promising solution to avoid POW symptoms associated with naloxone treatment. Biodegradable nanoparticles have received increasing attention as a valuable tool to effectively deliver drugs in areas such as oncology, diabetes, and infectious diseases (Anand, Tiloke, Naidoo, & Chuturgoon, 2017; Colino, Millan, & Lanao, 2018; Haley & Frenkel, 2008; Mazzucchelli & Corsi, 2017; Soppimath, Aminabhavi, Kulkarni, & Rudzinski, 2001). Their ability to provide controlled release, stabilize drugs and proteins, and deliver small molecules to a specific site of action makes them favorable as therapeutic agents (Kamaly, Yameen, Wu, & Farokhzad, 2016). Despite their benefits, some nanoparticles do possess limitations due to their design, specifically the manner by which drugs are loaded into the matrix. Traditional, non-covalently loaded nanoparticle delivery systems are often associated with burst release which can lead to unwanted side effects (Du & Stenzel, 2014; Huang & Brazel, 2001; Kamaly et al., 2016; Tong & Cheng, 2009; Wightman, Nelson, Lee, Fox, & Smith, 2018). One example of this is Vivitrol®, an extended-release preparation of the MOR antagonist naltrexone that was approved in 2010 to prevent relapse to opioid dependence (Saucier, Wolfe, & Dasgupta, 2018). Non-covalent loading of naltrexone into poly(lactide-co-glycolide) has led to severe POW in a number of cases (Saucier et al., 2018; Wightman et al., 2018). Based on these previous findings, a favorable next generation DDS should avoid burst release of naloxone while still providing an effective antidote to opioid toxicity.
In our previous study with Nal-cNP, nanoparticles possessing a drug loading of approximately 7% w/w blocked the effects of repeated high-dose morphine (10 mg/kg) for up to 98 hr in mice (Kassick et al., 2019). These data with in vitro kinetics suggested that Nal-cNPs can provide a constant release of naloxone for multiple days. However, extended release of naloxone may cause unwanted POW symptoms similar to high-dose free naloxone. The aim of this study was to test the impact of covalent naloxone nanoparticles on POW symptoms in morphine-dependent mice. We hypothesized that in morphine-dependent mice, treatment with Nal-cNP would display reduced POW symptoms compared to mice treated with unmodified free naloxone due to the low linear release from the Nal-cNPs. We also examined possible side effects of acute Nal-cNP treatment by studying the effects of NPs on locomotion, fecal boli (i.e., constipation), and body weight.
2 |. MATERIAL S AND METHODS
2.1 |. Subjects
Adult male C57BL/6J mice (The Jackson Laboratory) weighing approximately 20–31 g were used in these studies (n = 92). Mice were group housed with a controlled temperature and 12 hr light/dark cycle in the Animal Care Facility. Food and water were made available ad libitum except during experimental sessions. Experiments began once the animals had acclimated to Duquesne’s Animal Care Facility for at least 7 days. Behavioral experimentation was performed during the light cycle when mice were 6–8 weeks of age. The experimenter was blinded to treatment until all data were analyzed. Treatments were randomly assigned to mice in experimental groups (high and low doses of Nal-cNP) and control groups (positive control: unmodified free naloxone and negative controls: saline and cNP-empty). Animals were maintained and experiments were approved and conducted in accordance with the guidelines established by the Institutional Animal Care and Use Committee, Duquesne University (Pittsburgh, PA). All efforts were made to minimize animal suffering and to reduce the number of animals used.
2.2 |. Hotplate test
All mice received an acclimation to the hotplate test (one day prior to baseline test and twice before the baseline test), baseline hotplate test (24 hr before treatment), and a post-treatment hotplate test (day of treatment). Mice were acclimated to a metal hotplate maintained at 33 ± 0.5°C (non-noxious temperature) for approximately 2 min per trial. A mobile, transparent, and colorless plexiglass rectangular prism (26 × 10 × 16 cm) was placed on the hot plate to form the observation area. The temperature of the hot plate was monitored at all times. For the baseline and post-treatment tests, the metal hot plate was maintained at 49 ± 0.5°C. Measurements were made by placing one mouse on the hot plate at a time and recording the response latency with a stopwatch to the nearest 0.01 s. A cutoff latency of 30 s was used. Pain-associated behavior responses were characterized by either the licking of the hindpaw or jumping. After each measurement, the plate was wiped clean of all urine and feces. About 24 hr after the baseline hotplate measurement, mice were injected with 10 mg/kg morphine (intraperitoneal) and one of the following treatments: cNP-empty, Nal-cNP (low-dose), or 8 mg/kg free naloxone (subcutaneous). Morphine was administered 30 min before the hotplate test and treatment was administered 15 min before the hotplate test. The maximum possible effect (% MPE) of morphine was calculated using the following formula: % MPE = (treatment latency [s] − baseline latency [s]/30 s − baseline latency [s]). The experimenter was blinded to treatment until all the data were analyzed.
2.3 |. POW experimental design
Due to large sample sizes, the withdrawal experiments were performed in four cohorts of mice. All data were combined for analysis. In each cohort, the withdrawal experiments occurred over the span of 9 days. Morphine dependence was induced using a 6-day morphine-dependency paradigm that has been shown to elicit naloxone-induced opioid withdrawal symptoms in mice (Singh, Sharma, Gupta, & Sharma, 2015; Way, Loh, & Shen, 1969). For six consecutive days, mice received intraperitoneal injections of 5 mg/kg morphine (or saline) twice-daily (Figure 1). Injections were given at 7:00 a.m. (lights on) and 7:00 p.m. (lights off). Mice were in clear plexiglass enclosures (12 × 12 × 20 cm) on a raised glass surface during injection period. Following each daily injection, mice were returned to their group-housed cage. On day 7, mice received their final “lights on” injection at 7:00 a.m. and were left to habituate to the testing room with 60 dB of white noise for 1 hr and 40 min. Next, the mice were individually placed in plexiglass enclosures on a raised glass surface (with black dividers in between) for 20 min. Two hours after receiving their lights on injection, mice received an intraperitoneal injection of one of the five treatments: (a) unmodified free naloxone (free naloxone, 8 mg/kg), (b) high dose of Nal-cNP (Nal-cNP [Hi], 7% w/w; equivalent to 8 mg/kg naloxone), (c) low dose of Nal-cNP (Nal-cNP [Low], 7% w/w; equivalent to 0.7 mg/kg naloxone), (d) covalent nanoparticle not containing naloxone (cNP-empty), or (e) saline. Behavior was video recorded for 6 hr (beginning immediately after the treatment injection). On days 8 and 9 mice were habituated to the testing room with white noise for 1.4 hr and habituated to the plexiglass enclosures for 20 min. Video recording began 24 hr (day 8) and 48 hr (day 9) after the treatment injection (given on day 7). POW responses were determined by observing and scoring the behavior of the animals 6 hr, 24 hr, and 48 hr after the treatment drug was administered. During the initial 6 hr, withdrawal behavior was scored the first 15 min of each hour. Data were analyzed for each hour and cumulatively over the 6 hr testing period. The following behaviors were measured: naloxone-induced jumping, rearing (number of events), forepaw tremors (number of shakes unrelated to grooming), wet dog shakes (full body shakes unrelated to grooming), and forepaw licking (number of non-grooming licking bouts) based on previous characterization of this model (Bhalla, Pais, Tapia, & Gulati, 2015; Rehni & Singh, 2011; Singh et al., 2015). All scoring of videos occurred blinded to treatment of the animals.
FIGURE 1.
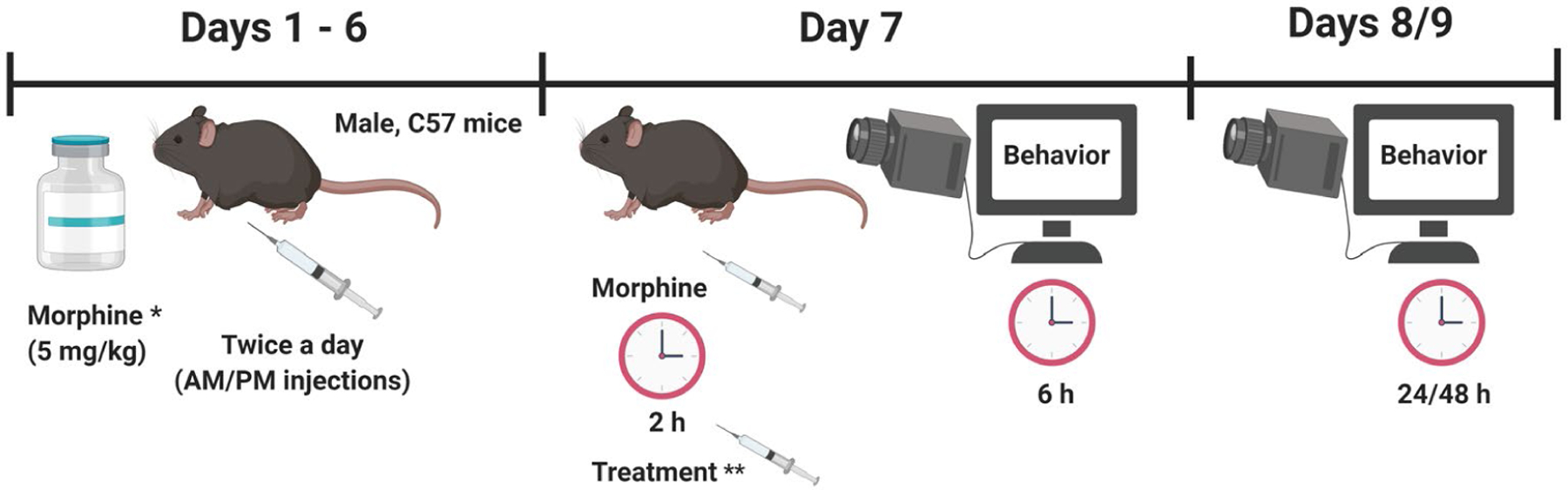
Experimental Design: For 6 consecutive days, mice received intraperitoneal injections of 5 mg/kg morphine (* another group of mice received saline injections instead) twice-daily. On day 7, 2 hr after their AM morphine (or saline) injection, mice received an intraperitoneal injection of one of the five treatments: ** free naloxone (8 mg/kg), Nal-cNP (Hi)—8 mg/kg naloxone, Nal-cNP (Low)—0.7 mg/kg naloxone, cNP-empty, or saline. Behavior was video recorded for 6 hr (beginning immediately after the treatment injection). On days 8 and 9 video recording began 24 hr (day 8) and 48 hr (day 9) after the treatment injection was given (day 7) and behavior was recorded for 15 min. Precipitated opioid withdrawal behavior (naloxone-induced jumping, rearing, forepaw tremors, wet dog shakes, and forepaw licking) was measured the first 15 min of each hr. Figure prepared with BioRender.com software
2.4 |. Overall withdrawal severity (OWS) Z-score calculation
Individual withdrawal behaviors were calculated using an integrated behavioral z-score analysis. Z-scores allow for statistical comparison of related data across studies and indicate how many standard deviations (σ), the mean of an observation (X) is above or below the mean of the control group (μ; Guilloux, Seney, Edgar, & Sibille, 2011). First, individual z-scores were calculated for each withdrawal behavior (naloxone jumps—ZNJ; rearing—ZR; forepaw tremors—ZFT; wet dog shakes—ZWDS; forepaw licking—ZFL) where x = mean of behavior, μ = mean of the control group, and σ = standard deviation of control (see Equation 1). For this study, our positive control group (free naloxone) was the control group by which the individual z-scores were normalized.
| (1) |
Once individual z-scores were calculated for each POW behavior, the z-scores were integrated across behaviors to calculate an OWS score (see Equation 2).
| (2) |
2.5 |. Open field test
Locomotion was measured using the open field test as previously described (Lax et al., 2016; Seibenhener & Wooten, 2015). Briefly, mice were habituated to a dimly lit behavior room for 1 hr with 60 dB white noise. Mice were pretreated with either 7% w/w Nal-cNP (Low) or saline for 10 min before being placed in the Plexiglass open field box (40.6 × 40.6 × 30 cm). The experimenter was blinded to treatment. Total distance traveled (m) and total time spent immobile (s) were measured and recorded for 30 min using an overhead camera and ANY-Maze software (Stoelting Co., version 4.98). The number of fecal boli was counted and recorded after each test.
2.6 |. Drugs
Nal-cNPs were synthesized as previously published (Kassick et al., 2019). Nal-cNP was initially prepared as 10 mg/ml, 7% w/w, 7 mg/kg naloxone/mouse. This concentration was diluted (1:10) to prepare a low dose of Nal-cNP (1 mg/ml, 7% w/w, 0.70 mg/kg naloxone/mouse) where a 30-g mouse received 30 μl of the initial Nal-cNP concentration and 270 μl of 0.9% saline (300 μl total). To prepare the high dose of Nal-cNP (10 mg/ml, 7% w/w, 8 mg/kg naloxone/mouse), a 30-g mouse received 343 μl of the initial Nal-cNP concentration (343 μl total). Naloxone hydrochloride was purchased from Sigma-Aldrich (St. Louis, MO) and dissolved in a vehicle of 0.9% saline. Free naloxone was prepared at a concentration of 0.8 mg/ml (30 g mouse = 300 μl injection, 8 mg/kg naloxone/mouse). Morphine sulfate was purchased from Sigma-Aldrich (St. Louis, MO) and dissolved in 0.9% saline as 0.5 mg/ml (30 g mouse = 300 μl injection, 5 mg/kg morphine/mouse). All drugs were administered either intraperitoneally or subcutaneously.
2.7 |. Statistical analysis
Data are expressed as mean ± SEM or expressed as the percentage of maximal possible effect (% MPE) (Figure 2). Two-way repeated measurements ANOVA followed by a post hoc Bonferroni’s multiple comparison tests were used to determine statistical significance for time and treatment. One-way ANOVA followed by a post hoc Bonferroni’s test was performed to compare cumulative POW behaviors, Z-scores, and % MPE across treatment groups. Unpaired t tests were used to compare locomotor activity, fecal boli, and body weight between treatment groups. p < 0.05 was considered statistically significant for all tests. All graphs and statistical analyses were performed with GraphPad Prism 8.0 software (GraphPad Software, San Diego, CA, USA). Prior to experiments, G* Power 3.1.9.4 was used to conduct a power analysis to determine an appropriate sample size. Using data from a pilot study, the a priori determined an n value of at least 6 as appropriate (power set to 0.90). All data were included and no outliers were removed from this study.
FIGURE 2.
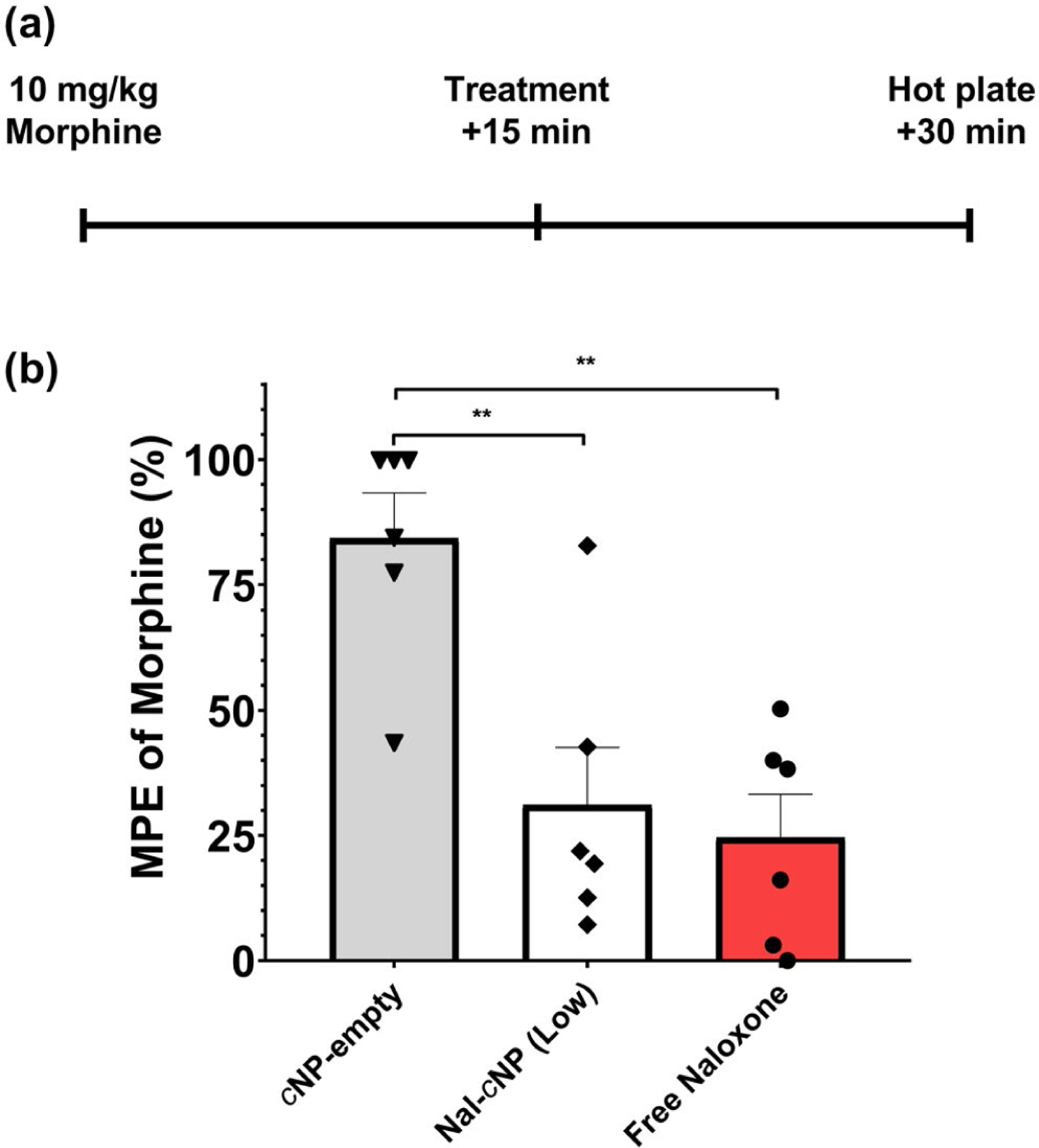
Nal-cNP treatment significantly reduces morphine-induced thermal antinociception in the hotplate test. (a) Morphine (10 mg/kg) was administered 15 min prior to mice receiving free naloxone (8 mg/kg), Nal-cNP (Low), or cNP-empty treatment (n = 6/group). 15 min after treatment, nocifensive behaviors were observed (forepaw licking, forepaw/hindpaw withdrawal, jumping) in the hotplate test. (b) Data from the hotplate test are expressed as the percentage maximum possible effect (%MPE). Bonferroni post hoc tests revealed a significant decrease in % MPE of morphine in mice treated with Nal-cNP and free naloxone compared to cNP-empty (**p < 0.01). There were no significant differences between Nal-cNP and free naloxone treatment (p > 0.9999)
3 |. RESULTS
3.1 |. Nal-cNP treatment blocks morphine-induced thermal antinociception in the hotplate test
In order to better understand the onset action of Nal-cNP we first sought to test the ability of Nal-cNP to reduce the acute thermal antinociceptive effect of morphine in opioid naïve mice (Figure 2). One-way ANOVA was used to determine statistical significance of % MPE of morphine between different treatment groups (cNP-empty (negative control), Nal-cNP (Low), and free naloxone). One-way ANOVA revealed a significant effect of treatment (F (2, 15) = 11.19, n = 18, p = 0.0011). Bonferroni’s multiple comparison test indicated a significant decrease in % MPE of morphine in mice treated with Nal-cNP (p = 0.0048) and free naloxone (p = 0.0019) compared to mice treated with cNP-empty. There were no significant differences between free naloxone and Nal-cNP treatment groups (p > 0.9999).
3.2 |. Nal-cNPs induce reduced POW symptoms compared to free naloxone
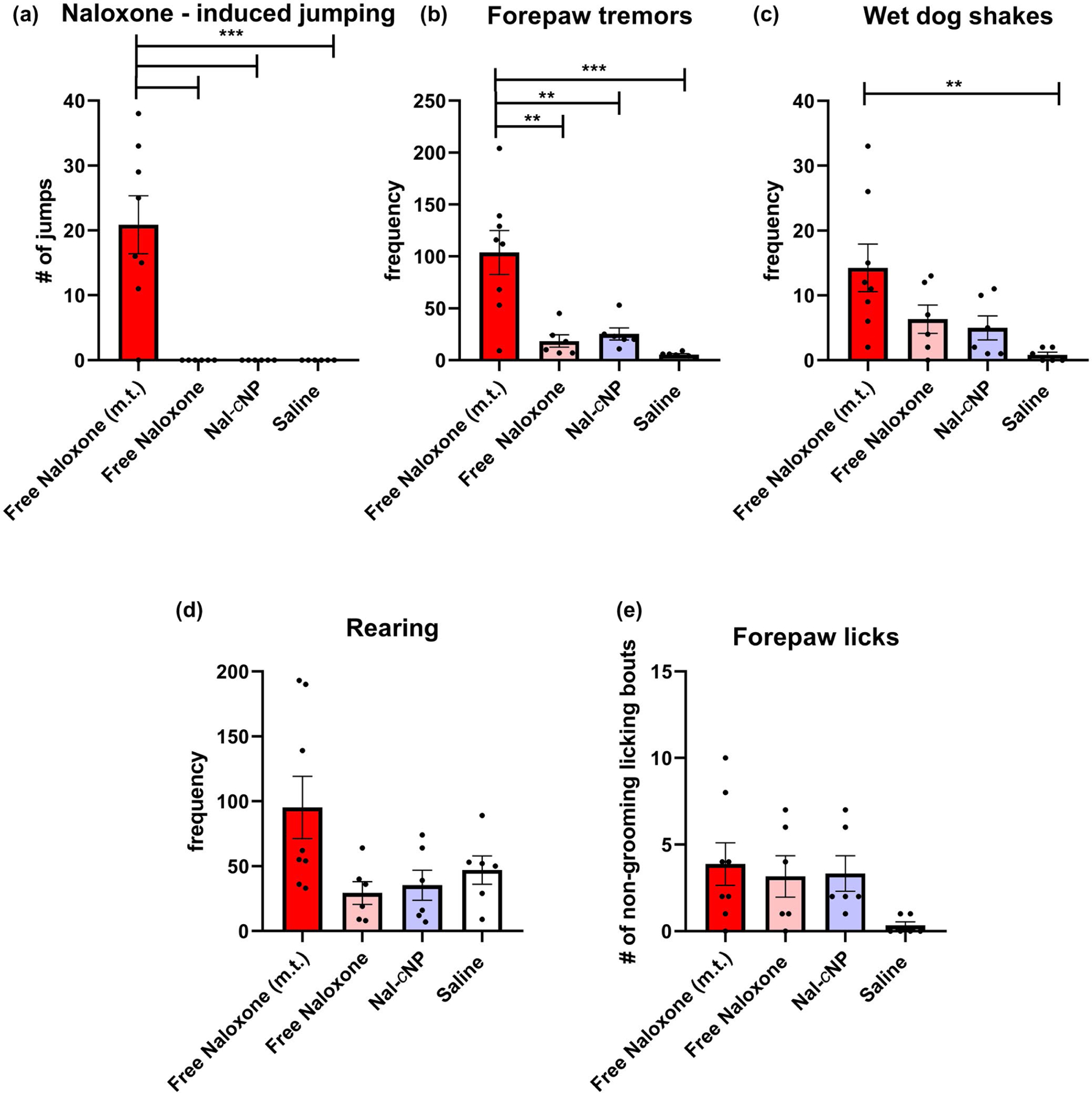
Next, we sought to investigate the time course effects of free naloxone, Nal-cNP (Hi), Nal-cNP (Low), cNP-empty, and saline on withdrawal symptoms in morphine-dependent mice (Figure 3). Two-way repeated measure (RM) ANOVAs were used to determine statistical significance of naloxone-induced jumps, forepaw tremors, wet dog shakes, rearing, and forepaw licking behavior between the different treatment groups 0–6 hr, 24 hr, and 48 hr after treatment injections. Two-way RM ANOVA revealed a significant main effect of time (F (7, 245) = 10.27, n = 40, p < 0.0001) and treatment (F (4, 35) = 27.65, p < 0.001) for naloxone-induced jumps (Figure 3a). Bonferroni’s multiple comparison post hoc test revealed a significant increase in the number of naloxone-induced jumps in the free naloxone group compared to Nal-cNP (Low), Nal-cNP (Hi), cNP-empty, and saline-treated groups (p < 0.0001) within the first hour after treatment. There was also a significant increase in the Nal-cNP (Hi) group compared to Nal-cNP (Low), cNP-empty, and saline-treated groups (p = 0.0014). There were no significant differences between treatment groups at any other time point.
FIGURE 3.
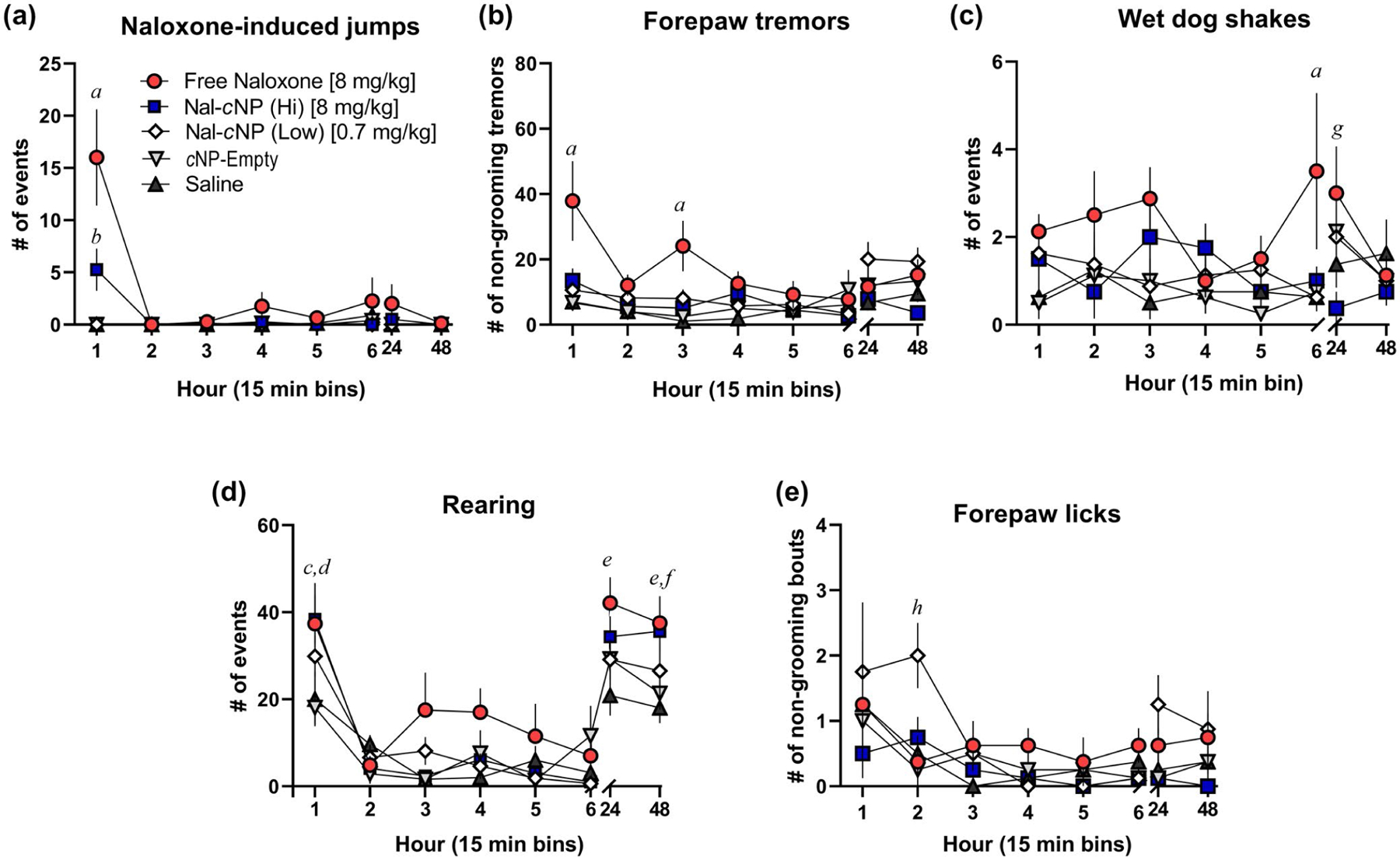
The majority of naloxone-induced POW behaviors were observed within the first hour of treatment. Morphine-dependent mice (n = 8/group) were treated with either free naloxone, Nal-cNP (Hi—8 mg/kg of naloxone), Nal-cNP (Low—0.7 mg/kg), cNP-empty, and saline (n = 8/group) 2 hr after receiving a morphine injection (5 mg/kg). During the first day of testing, behavior was assessed the first 15 min of every hour (6 hr total). Behavior was also assessed 24 hr after treatment and 48 hr after treatment. The data are expressed as mean ± SEM. Bonferroni post hoc tests represents significance between the following groups (p values are reported in results): (a) free naloxone group versus Nal-cNP (Low), Nal-cNP (Hi), cNP-empty, and saline, (b) Nal-cNP (Hi) group versus Nal-cNP (Low), cNP-empty, and saline, (c)free naloxone versus cNP-empty, (d) Nal-cNP (Hi) versus cNP-empty and saline, (e) free naloxone versus saline (f) Nal-cNP (Hi) versus saline, (g) free naloxone versus Nal-cNP (Hi), (h) Nal-cNP (Low) versus cNP-empty, saline, and free naloxone
Two-way RM ANOVA revealed a significant main effect of time (F (7, 245) = 5.100, p < 0.0001) and treatment (F (4, 35) = 4.636, p = 0.0041) for forepaw tremors (Figure 3b). Post hoc analyses revealed a significant increase in forepaw tremors in the free naloxone group compared to the Nal-cNP (Hi) (1 hr p < 0.0001; 3 hr p = 0.0048), Nal-cNP (Low) (1 hr p < 0.0001; 3 hr p = 0.0294), cNP-empty (1 hr p < 0.0001; 3 hr p = 0.0048), saline groups (1 hr p < 0.0001; 3 hr p < 0.001) within the first hour and third hour of treatment. There was a significant increase in forepaw tremors in the Nal-cNP (Low) group compared to the Nal-cNP (Hi) group 48 hr after treatment.
Two-way RM ANOVA failed to reveal a significant effect of time (F (7, 245) = 1.436, p = 0.1912) nor treatment (F (4, 35) = 2.075, p = 0.1050) for wet dog shakes (Figure 3c). There was a significant difference among subjects (F (35, 245) = 4.221, p < 0.0001). Post hoc analyses revealed a significant increase in wet dog shakes in the free naloxone group compared to the Nal-cNP (Hi) (p = 0.0356), Nal-cNP (Low) (p = 0.0083), cNP-empty (p = 0.0137), saline groups (p = 0.0083) within the sixth hour of treatment. Post hoc analyses also revealed a significant increase in wet dog shakes in the free naloxone group compared to the Nal-cNP (Hi) group 24 hr after treatment (p = 0.0023).
Two-way RM ANOVA revealed a significant main effect of time (F (7, 245) = 48.02, p < 0.0001) and treatment (F (4, 35) = 3.826, p = 0.0111) for rearing behavior (Figure 3d). Post hoc analyses revealed a significant increase in rearing behavior in the free naloxone group compared to the cNP-empty group (p = 0.0200), and a significant increase in rearing in the Nal-cNP (Hi) group compared to cNP-empty (p = 0.0116) and saline treatment groups (p = 0.0315) within the first hour of treatment. Additionally, there was a significant increase observed in rearing behavior in the free naloxone group compared to the saline group 24 hr after treatment (p = 0.0066). About 48 hr post-treatment, post hoc analyses revealed a significant increase in rearing in the free naloxone group (p = 0.0175) and the Nal-cNP (Hi) group (p = 0.0460), when compared to the saline group.
Two-way RM ANOVA found a significant main effect of both time (F (7, 245) = 4.585, p < 0.0001) and treatment (F (4, 35) = 2.849, p = 0.0382) for non-grooming bouts of forepaw licking (Figure 3e). Post hoc analyses revealed a significant increase in forepaw licking in the Nal-cNP (Low) group compared to the free naloxone (p = 0.110), cNP-empty (p = 0.0045), and saline (p = 0.0255) treatment groups within the second hour of treatment.
Next, we calculated a cumulative effect of treatment on POW for the first six hours by summing the scores at each of the 1, 2, 3, 4, 5, and 6 hr time points (Figure 4). One-way ANOVA indicated a significant main effect of treatment for jumping behavior (F (4, 35) = 15, n = 40, p < 0.0001). Post hoc analyses revealed that morphine-dependent mice treated with free naloxone displayed significantly more jumping behavior (Figure 4a) (20.88 ± 4.462) compared to Nal-cNP (Hi) (5.500 ± 2.027, p < 0.001), Nal-cNP (Low) (0.1250 ± 0.1250, p < 0.0001), cNP-empty (0.5000 ± 0.2673, p < 0.0001), and saline (1.000 ± 1.000, p < 0.0001). One-way ANOVA did show a significant effect of treatment for forepaw tremors (Figure 4b) (F (4, 35) = 6.403, p < 0.001). Post hoc analyses indicated a significant increase in morphine-dependent mice treated with free naloxone (103.8 ± 21.12) compared to Nal-cNP (Hi) (41.25 ± 6.984, p < 0.001), Nal-cNP (Low) (42.88 ± 10.36, p = 0.0136), cNP-empty (33.00 ± 9.776, p = 0.0027), and saline (25.25 ± 8.163, p < 0.001). For wet dog shakes (Figure 4c), one-way ANOVA revealed a significant effect of treatment (F (4, 35) = 4.200, p = 0.0070) and post hoc analyses showed a significant increase in wet dog shakes in the free naloxone group (14.25 ± 3.663) compared to both cNP-empty (3.125 ± 1.172, p = 0.0075) and saline (4.125 ± 1.172, p = 0.0188) treatment groups. There were no significant effects observed for rearing (Figure 4d) and forepaw licks (Figure 4e) (F (4, 35) = 2.439, p = 0.0652; F (4, 35) = 1.243, p = 0.3107, respectively).
FIGURE 4.
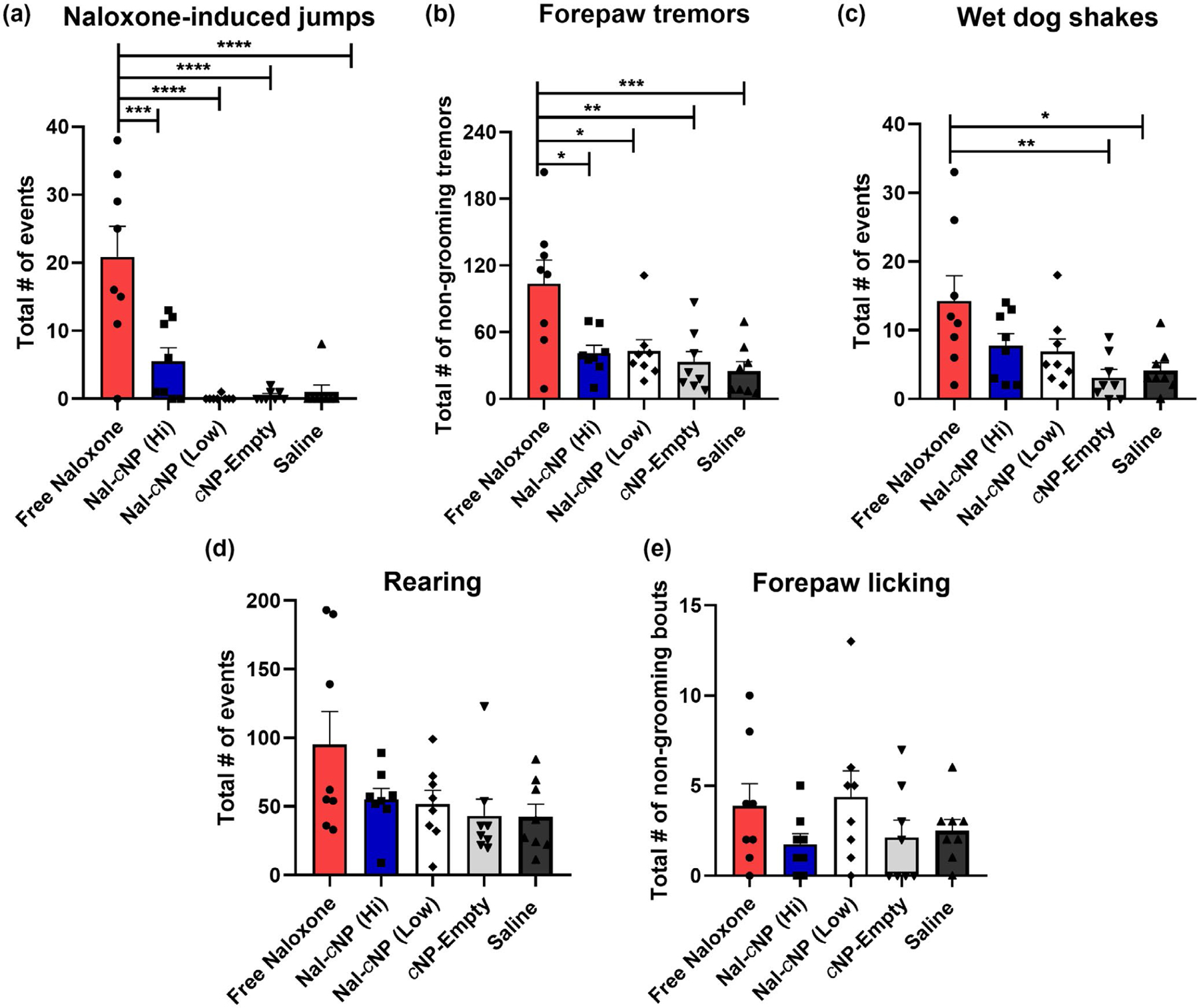
Nal-cNP treatment displayed reduced POW behaviors compared to free naloxone treatment in morphine-dependent mice. These data are expressed as the sum (15 min bins) ± SEM for the first 6 hr of treatment on day 7 (n = 8/group). Bonferroni post hoc tests reveal significant differences in (a) naloxone-induced jumps, (b) forepaw tremors, and (c) wet dog shakes (****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05). No effects were found for (d) rearing or (e) forepaw licking
Next, we calculated the OWS score for each treatment group to better assess the overall effect of Nal-cNPs on POW (Figure 5). In order to calculate the OWS scores, individual z-scores were calculated for each behavior with the free naloxone group as the control meaning the z-score was 0 across behaviors. Within the first 6 hr of treatment, one-way ANOVA revealed a significant main effect of treatment (F (4, 20) = 4.996, n = 25, p = 0.0062) (Figure 5a). The following individual z-scores were calculated for naloxone-induced jumping behavior (free naloxone: z = 0, Nal-cNP (Hi): z = −1.218, Nal-cNP (Low): z = −1.644, cNP-empty: z = −1.614, saline: z = −1.575); rearing behavior (free naloxone: z = 0, Nal-cNP (Hi): z = −0.593, Nal-cNP (Low): z = −0.641, cNP-empty: z = −0.776, saline: z = −0.779); forepaw tremors (free naloxone: z = 0, Nal-cNP (Hi): z = −1.218, Nal-cNP (Low): z = −1.644, cNP-empty: z = −1.614, saline: z = −1.575); rearing behavior (free naloxone: z = 0, Nal-cNP (Hi): z = −1.046, Nal-cNP (Low): z = −1.019, cNP-empty: z = −1.184, saline: z = −1.314); wet dog shakes (free naloxone: z = 0, Nal-cNP (Hi): z = −0.627, Nal-cNP (Low): z = −0.712, cNP-empty: z = −1.074, saline: z = −0.977); forepaw licking (free naloxone: z = 0, Nal-cNP (Hi): z = −0.610, Nal-cNP (Low): z = 0.144, cNP-empty: z = −0.503, saline: z = −0.395). Post hoc analyses determined a significant decrease in OWS scores of both cNP-empty (p = 0.0102) and saline (p = 0.0122) treatment groups when compared to the OWS of the free naloxone group. Although not significant, Nal-cNP (Hi) (p = 0.062) and Nal-cNP (Low) (p = 0.089) trended to show reduced withdrawal behavior in morphine-dependent mice within 6 hr of treatment compared to free naloxone-treated mice. Furthermore, there were no significant differences in OWS scores at 24 hr (F (4, 20) = 2.536, p = 0.0722) (Figure 5b) or 48 hr after treatment (F (4, 20) = 1.509, p = 0.2374) (Figure 5c).
FIGURE 5.
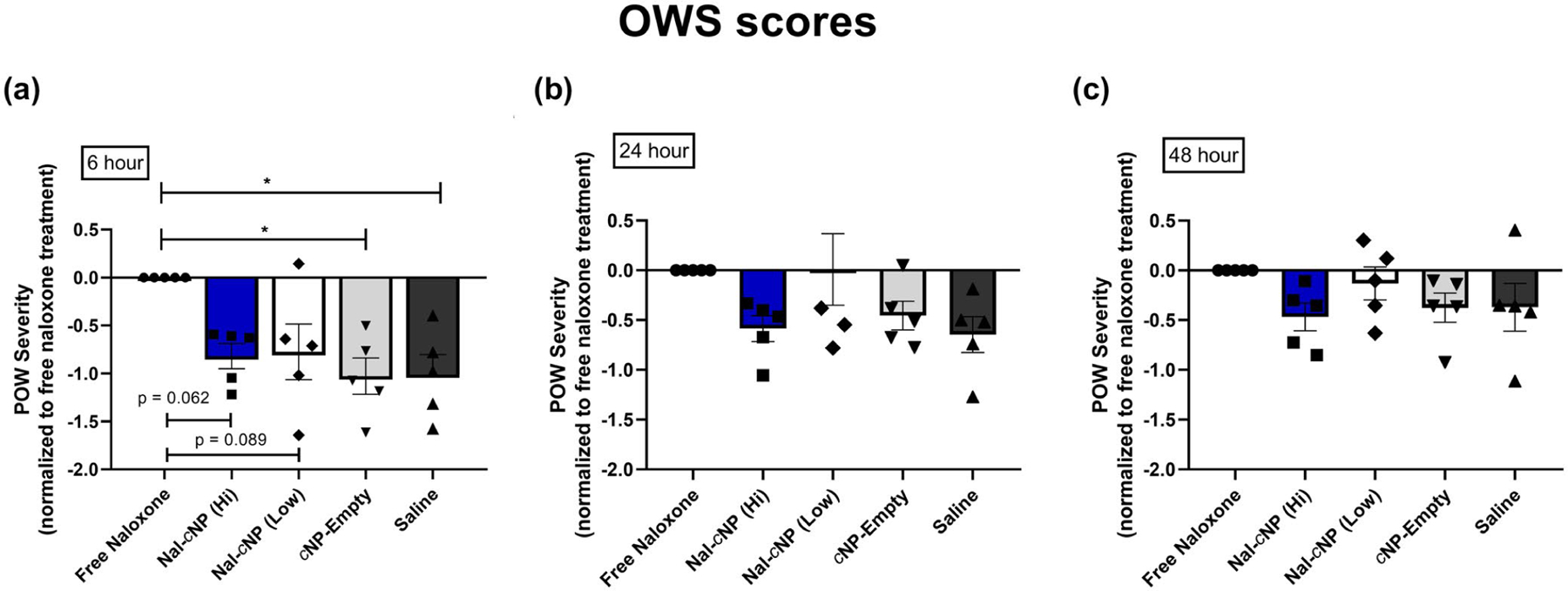
Impact of Nal-cNP (Hi)/(Low) treatment on overall withdrawal severity (OWS) compared to free naloxone treatment. POW severity scores were normalized to free naloxone treatment in morphine-dependent mice (positive control). Symbols represent individual z-scores that were calculated for each behavior. These data are expressed as the average of individual z-scores (a) 6 hr, (b) 24 hr, and (c) 48 hr after treatment (n = 8/group). Post hoc analyses determined a significant decrease in OWS score in the free naloxone group compared to both cNP-empty and saline treatment groups within the first 6 hr of treatment (p < 0.05). Nal-cNP (Hi) (p = 0.062) and Nal-cNP (Low) (p = 0.089) trended to show reduced withdrawal behavior within 6 hr of treatment. Negative values on y-axis indicate reduced POW compared to positive control
3.3 |. Nal-cNP shows minimal withdrawal symptoms in the absence of morphine dependence
To evaluate whether the naloxone-induced POW was specific for morphine-dependent mice, we also compared the total number of withdrawal symptoms in saline control-treated mice (saline twice-daily for 6 consecutive days) within the first 6 hr of treatment (Figure 6). Data from morphine-treated mice in the free naloxone treatment group (from Figure 4) are also shown for comparison. Neither Nal-cNP (low), saline, nor free naloxone-treated mice displayed jumping behavior within the first 6 hr of treatment (0 ± 0) (Figure 6a). One-way ANOVA revealed a significant effect (F (3, 22) = 17.15, n = 26, p < 0.0001) and post hoc Bonferroni’s multiple comparisons test revealed a significant decrease in the saline-treated mice that received Nal-cNP (p < 0.001), free naloxone (p < 0.001), and saline (p < 0.001) compared to morphine-dependent mice treated with free naloxone. One-way ANOVA showed a significant effect in forepaw tremors (F (3, 22) = 11.77, p < 0.0001) (Figure 6b). Post hoc analyses revealed a significant decrease in forepaw tremors in the saline-treated mice that received Nal-cNP (25.33 ± 5.869, p < 0.0061), free naloxone (18.50 ± 5.971, p < 0.0031), and saline (5.333 ± 0.945, p < 0.001) compared to morphine-dependent mice treated with free naloxone (103.8 ± 21.12). One-way ANOVA revealed a significant effect for wet dog shakes (F (3, 22) = 4.927, p = 0.0091) and post hoc analyses revealed a significant decrease (p = 0.0079) in wet dog shakes in saline-treated mice that received saline (0.8333 ± 0.4014) compared to morphine-dependent mice treated with free naloxone (6.333 ± 2.171) (Figure 6c). There were no significant differences in rearing (Figure 6d) and forepaw licking (Figure 6e) behavior between the saline-treated mice and the morphine-dependent mice treated with free naloxone.
FIGURE 6.
Minimal withdrawal behaviors are found in control saline-treated mice compared to morphine-dependent mice treated with free naloxone. Mice received saline (twice-daily) for 6 consecutive days then treated with either free naloxone, Nal-cNP (Low), or saline. These data are expressed as the sum (15 min bins) ± SEM within the first 6 hr of treatment on day 7 (n = 6–8/group). Bonferroni post hoc tests reveal significant differences in (a) naloxone-induced jumps, (b) forepaw tremors, and (c) wet dog shakes (****p < 0.0001; ***p < 0.001; **p < 0.01) compared to morphine-dependent mice treated with free naloxone (free naloxone m.t). No statistically significant differences were found between saline-treated mice suggesting that no POW occurs in the absence of morphine dependency
3.4 |. Nal-cNP does not induce locomotor or acute constipation-related side effects
Lastly, we wanted to investigate the potential side effects of acute Nal-cNP treatment compared to saline-treated mice (Figure 7). In the open field test, unpaired t tests determined that there were no significant differences in total distance traveled (m) (saline: 56.66 ± 5.122; Nal-cNP: 48.43 ± 3.497; F (7, 7) = 2.145, n = 16, p = 0.3353) and total time immobile (s) (saline: 382.1 ± 43.79; Nal-cNP: 429.5 ± 70.38; F (7, 7) = 2.583, p = 0.2338). Similarly, no significant differences were found for the number of fecal boli produced (saline: 1.625 ± 0.5650; Nal-cNP 1.625 ± 0.6529; F (7, 7) = 1.336, p = 0.7122) between saline and Nal-cNP (Low)-treated animals suggesting minimal acute impact of treatment on fecal production. Neither Nal-cNP nor saline significantly altered body weight (g) 3 (saline: 24.60 ± 0.8102, p = 0.6060; Nal-cNP: 24.40 ± 0.5635, p = 0.6932) and 5 days after treatment (saline: 24.95 ± 0.8198, p = 0.4465; Nal-cNP: 24.81 ± 0.5683, p = 0.3968) compared to baseline body weight before treatment (saline: 23.89 ± 1.077; Nal-cNP: 24.05 ± 0.6609).
FIGURE 7.
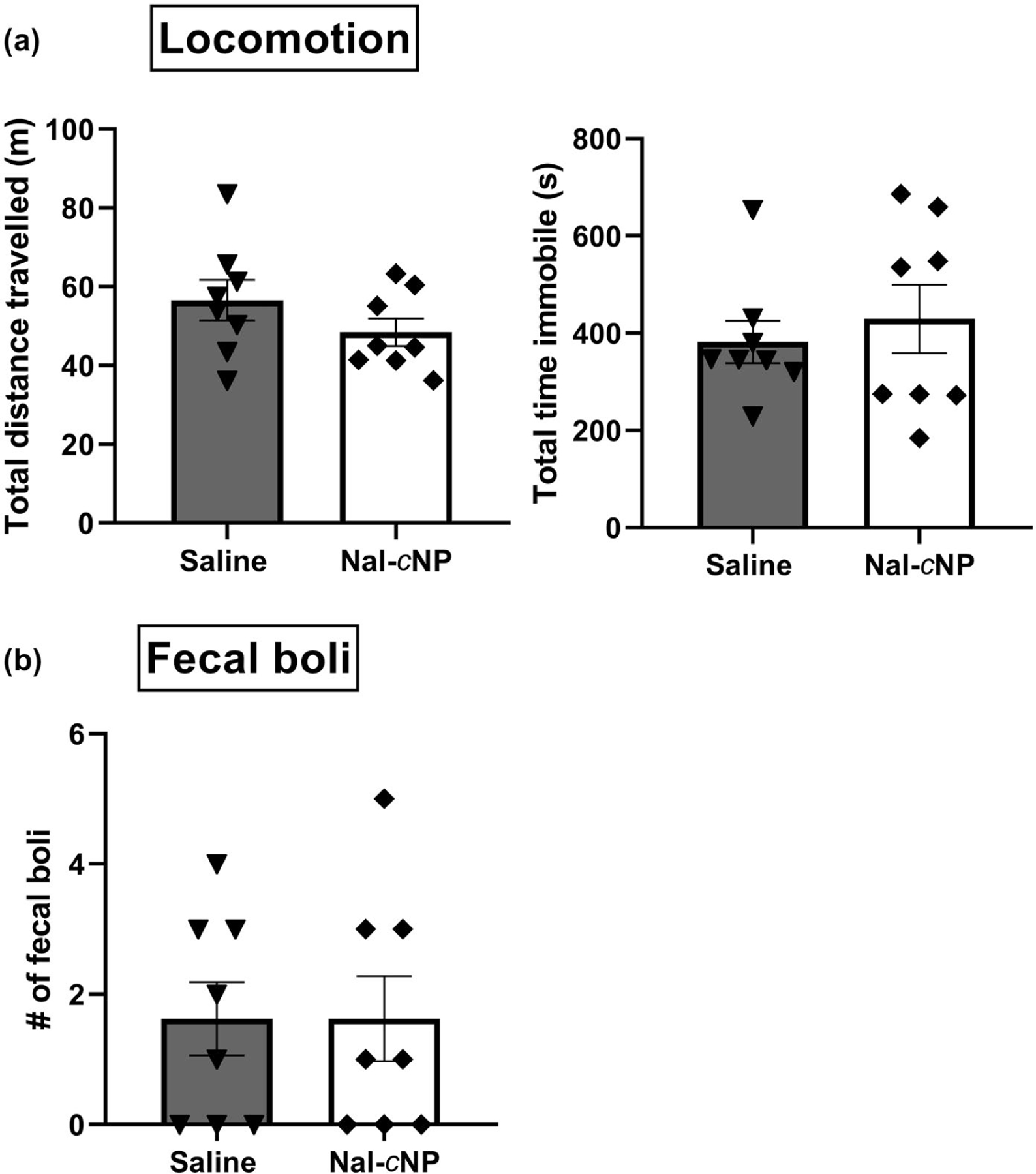
Nal-cNP treatment produced negligible side effects in naïve mice. (a) Locomotion and (b) fecal boli production were observed to assess the side effect profile of Nal-cNP. Unpaired t tests determined there were no significant differences in total distance traveled (m), total time immobile (s), and the number of fecal boli between saline and Nal-cNP (Low)-treated animals (n = 8/group) in the open field test. Data are expressed as mean ± SEM
4 |. DISCUSSION
This study sought to test the impact of covalently loaded naloxone nanoparticles on POW symptoms in a mouse model of opioid dependence. We found that the Nal-cNP particles reduced thermal antinociceptive effects of morphine in the hotplate test, demonstrating that acute treatment of Nal-cNP can produce MOR antagonism. Additionally, we demonstrated that the 6-day morphine-dependency paradigm used for this study was effective as evidenced by the rapid onset of POW behaviors observed when free naloxone was administered. The Nal-cNP particles attenuated the majority of POW behaviors in comparison to free naloxone treatment, principally naloxone-induced jumps, forepaw tremors, and wet dog shakes, within the first 6 hr of treatment. As a result, Nal-cNP treatment produced a lower withdrawal compared to free naloxone treatment. It is worth mentioning, mice treated with Nal-cNP particles displayed a similar frequency of withdrawal behavior as negative control treatment groups cNP-empty and saline, suggesting that phenotype was driven by spontaneous withdrawal rather than POW. Lastly, in the open field test there were no significant differences between saline and Nal-cNP treatment groups in locomotor activity and production of fecal boli, suggesting negligible side effects are associated with acute Nal-cNP use.
To ensure acute MOR antagonism of Nal-cNPs, we first sought to study the impact of Nal-cNPs on morphine-induced thermal antinociception compared to free naloxone. The hotplate test has been used for decades to assess acute thermal nociception (Fennessy & Lee, 1970; Holck, Kimura, & Kimura, 1950; Pleuvry & Tobias, 1971). Consistent with our previous findings (Kassick et al., 2019), we found that free naloxone treatment displayed a significant decrease in % MPE of morphine compared to the negative control cNP-empty. These data are consistent with other hotplate tests in that naloxone treatment decreases the antinociceptive effects of morphine and other MOR agonists (O’Callaghan & Holtzman, 1975; Smith, 1976; Szekely, Dunai-Kovacs, Miglecz, Ronai, & Bajusz, 1978). In the current study, we found that Nal-cNP (Low) significantly reduced the % MPE of morphine compared to the negative control cNP-empty in the hotplate test. Additionally, there were no significant differences between Nal-cNP (Low) and free naloxone treatment groups. Despite the slow-sustained release of naloxone of Nal-cNP, the covalent nanoparticle displays similar MOR antagonism within the same time frame as unmodified naloxone (within 15 min of treatment). This acute action of Nal-cNP is clinically relevant in that in order to reverse an opioid overdose, a fast MOR antagonistic effect would be required to reverse opioid toxicity. However, it is possible that the MOR antagonistic effect is dependent on the behavioral endpoint. While the antinociceptive effects of morphine is blocked within 15 min of treatment, a higher dose and/or different time-frame may exist for Nal-cNP to reverse opioid toxicity. Therefore, additional experiments with Nal-cNP in an opioid overdose model are necessary to test whether Nal-cNP can reverse symptoms associated with opioid toxicity such as respiratory depression.
In order to examine the ability of Nal-cNPs to prevent POW in opioid-dependent mice, we first had to select an appropriate mouse model of opioid dependence. Individuals with OUD are likely to develop severe POW when given high doses of MOR antagonists like naloxone (Gangahar, 2015; Kim & Nelson, 2015). POW symptoms include agitation, anxiety, gastrointestinal distress, sweating, and tachycardia (Himmelsbach, 1941; Sun, 1998; Wesson & Ling, 2003). Hospital cases of patients treated with the MOR antagonist naltrexone have displayed POW symptoms lasting up to 36 hr (Iovcheva, Zlateva, & Asparuhova, 2007). The major difference between Vivitrol and our Nal-cNP (beyond naltrexone vs. naloxone differences) is the burst release seen in Vivitrol compared to the linear release seen in our formulation. The reduced POW seen with Nal-cNP suggests that covalent loading of nanoparticles may be a critical factor in reducing these symptoms.
Previous mouse models of morphine dependence have demonstrated that mice exhibit POW symptoms such as jumping, circling, wet dog shakes, rearing, forepaw tremors, and forepaw licking when treated with naloxone (Enquist, Ferwerda, Milan-Lobo, & Whistler, 2012; Gao et al., 2016; Singh et al., 2015; Way et al., 1969; Zhang et al., 2016). Based on these findings we utilized a 6-day morphine-dependency paradigm. We found this paradigm to be effective in that free naloxone treatment (8 mg/kg) induced POW behaviors such as naloxone-induced jumps, rearing, forepaw tremors, and wet dog shakes. The majority of the naloxone-induced POW behaviors were observed within the first 15 min of treatment (Figure 1). However, a few behaviors were also displayed within 3 hr (forepaw tremors), 6 hr (wet dog shakes), 24 hr (wet dog shakes, rearing), and 48 hr (rearing) after treatment. Since free naloxone is rapidly metabolized, we were surprised to see POW behaviors exhibited 24 hr and 48 hr after treatment. The free naloxone behavior exhibited 24 hr and 48 hr is most likely due to spontaneous withdrawal rather than naloxone-induced POW. The naloxone-induced POW behaviors we observed in our present study are consistent with previous reports. For example, in a previous study, morphine-dependent mice treated with naloxone (8 mg/kg) displayed a mean value of 21 jumps (±1.26 SD) and 15 wet dog shakes (1.18 SD; Singh et al., 2015). In our study, the same dose of naloxone produced a mean value of 20.88 jumps (±4.462 SEM) and 14.25 wet dog shakes (±3.663 SEM). However, we did not see differences in rearing behavior or forepaw licking as previously mentioned (Singh et al., 2015). One reason for this could be strain differences in that the previous study was done in swiss albino mice and the present study was conducted in C57 mice. In fact, some studies have reported substantial strain differences in naloxone-induced POW, primarily due to the differences in the development of morphine dependence (Kest et al., 2002; Metten, Crabbe, & Belknap, 2009). More importantly, within the first 6 hr, Nal-cNPs reduced the majority of POW behaviors compared to free naloxone treatment in morphine-dependent mice (Figure 4). The free naloxone-treated group had significantly more naloxone-induced jumps and forepaw tremors than both Nal-cNP (Hi) and Nal-cNP (Low) treatment groups. Although not significant, Nal-cNP (Hi) and Nal-cNP (Low) treatment groups displayed reduced wet dog shakes when compared to the free naloxone group. Rearing and forepaw licking were relatively similar across treatment groups. This finding suggests that the ability of Nal-cNPs to reduce POW behavior is partially dependent on the particular withdrawal behavior observed.
To ensure that the naloxone-induced opioid withdrawal we observed was not an artifact, we used the same experimental design as before (Figure 1) except instead of receiving morphine injections twice-daily, another group of mice were treated with saline twice-daily (Figure 6). Saline-treated mice were treated with either free naloxone, Nal-cNP (Low), or saline. We found that saline-treated mice displayed significantly reduced withdrawal behaviors than morphine-dependent mice. In saline-treated mice, free naloxone failed to produce naloxone-induced jumps. Additionally, morphine-dependent mice treated with free naloxone exhibited significantly more forepaw tremors than all of the saline-treated animals regardless of treatment. Although not significant, there was noticeably less wet dog shakes and rearing observed in the saline-treated animals than the morphine-dependent mice treated with free naloxone. Additionally, there were no significant differences between the Nal-cNP treatment group and the saline treatment group. Taken together, these data show that free naloxone alone did not produce withdrawal symptoms in naïve (morphine free) mice. These data are consistent with an abundance of data showing that MOR inhibition in the absence of significant MOR stimulation induces few noticeable effects in rodents (Singh et al., 2015) or humans (Borras et al., 2004; Grevert & Goldstein, 1978).
The clinical opiate/opioid withdrawal scale (COWS) is a scale used by clinicians to assess the degree of opioid withdrawal within a patient in order for clinicians to make inferences about their patient’s level of opioid dependence (Tompkins et al., 2009; Wesson & Ling, 2003). Similarly, in this study we sought to assess the magnitude of opioid withdrawal in morphine-dependent mice by calculating an OWS score. Using an integrated behavioral z-score analysis, each individual behavior was compiled into a normalized z-score and then compared across treatment groups (Figure 5). It has been reported that the pooling of cohorts and behavioral tests can strengthen the reliability of effects and reduce test-to-test variability (Guilloux et al., 2011). POW severity scores were normalized to free naloxone treatment in morphine-dependent mice. There was a significant decrease in the OWS scores of both cNP-empty and saline treatment groups when compared to the OWS of the free naloxone group. Although not significant, the OWS scores of Nal-cNP (Hi) and Nal-cNP (Low) treatment groups are markedly reduced compared the OWS score of the free naloxone group within 6 hr of treatment. The fact that the OWS scores of both Nal-cNP doses were not significantly different from our negative control treatment groups—cNP-empty and saline are equally important. Our previous study reported that 7% w/w Nal-cNP blocked the analgesic properties of 10 mg/kg morphine for up to 98 hr in a mouse model of neuropathic pain (Kassick et al., 2019). Therefore, we predicted that our Nal-cNPs might produce higher OWS scores than those of cNP-empty and saline due to the sustained release of naloxone for up to 98 hr. Although this finding was unexpected, it would be a clinical benefit to have a sustained delivery of naloxone devoid of withdrawal symptoms. Furthermore, the Nal-cNP (Low) dose used in the present study is the same dose we previously reported to block the analgesic effect of morphine (0.7 mg/kg naloxone release). Therefore, in the case a much higher dose of morphine or fentanyl is administered, a dose ~10-fold higher (Nal-cNP-Hi) would still display reduced POW compared to Narcan. Thus, Nal-cNPs have the potential to be administered to OUD patients with a wide range of drug accumulation and opioid toxicity without the risk of POW.
Lastly, we set out to begin investigating the side effect profile of Nal-cNP by testing the acute effects of Nal-cNP on locomotion and fecal boli in the open field test (Figure 7). The open field test has been used to assess general locomotor activity, behavioral disruption, anxiety-like behavior, and exploratory drive (Bailey & Crawley, 2009; Seibenhener & Wooten, 2015). The number of fecal boli produced has also been used to measure anxiety-like behavior and stress (Crumeyrolle-Arias et al., 2014) as well as an opioid-specific side effect (Thorpe, 2001). There were no significant differences detected between Nal-cNP treatment and saline in total distance traveled, total time spent immobile, and fecal boli production during open field. These data suggest that acute treatment of Nal-cNP produces negligible locomotor (total distance) or anxiety-like (fecal boli and time immobile) behavior. Further research would need to be done to investigate potential side effects associated with repeated use of Nal-cNPs.
Future studies will be performed in order to further evaluate the therapeutic potential and side effect profile of Nal-cNP. Firstly, studies with female mice will be carried out to study potential sex-based differences in Nal-cNP activity both on MOR antagonism and POW. This study was limited in that we demonstrated constant linear release of naloxone via Nal-cNP allowed for a lower OWS in only male morphine-dependent mice. Additionally, while the present study evaluated side effects of acute Nal-cNP, future studies will assess the side effects associated with repeat dosing of Nal-cNP. It is critical to know if multiple Nal-cNP injections are relatively safe and equally as effective as the first exposure. Lastly, it is still unknown whether Nal-cNP can reverse opioid-overdose toxicity and prevent renarcotization, another phenomenon limiting the use of naloxone. Therefore, future studies will determine the ability of Nal-cNP to prevent renarcotization as well as respiratory depression using an overdose model with long-lasting synthetic opioids. Overall, the findings from this study and our previous research (Kassick et al., 2019) support the theoretical framework of sustained naloxone delivery as a viable solution for opioid dependence with reduced side effects (e.g., withdrawal symptoms) compared to high-dose naloxone.
Supplementary Material
Significance.
The opioid epidemic remains a major problem worldwide. Currently, opioid overdoses are relieved with high doses of naloxone to reverse opioid-induced respiratory depression. While acutely effective, repeated or high doses of naloxone can lead to precipitated opioid withdrawal (POW), especially in patients with opioid use disorder. Therefore, there is a vital need for novel effective methods to reverse opioid overdose that do not induce POW. In this study, we show that covalent naloxone nanoparticles (Nal-cNPs) induce lower POW symptoms in morphine-dependent mice compared to high-dose free naloxone.
ACKNOWLEDGMENTS
We would like to dedicate this manuscript and research to the memory of Dr. Eric Simon, a pioneer in opioid biochemistry and biology.
Footnotes
DECLARATION OF TRANSPARENCY
The authors, reviewers and editors affirm that in accordance to the policies set by the Journal of Neuroscience Research, this manuscript presents an accurate and transparent account of the study being reported and that all critical details describing the methods and results are present.
CONFLICT OF INTEREST
There are no conflict of interest to declare.
OPEN RESEARCH BADGES
This article has earned Open Data and Open Materials badges. Data and materials are available at https://dsc.duq.edu/.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the Supporting Information section.
Transparent Peer Review Report
Transparent Science Questionnaire for Authors
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon request.
REFERENCES
- (SAMHSA), S. A. a. M. H. S. A. (2003). Overview of findings from the 2002 National Survey on Drug Use and Health Rockville, MD: S. A. a. M. H. S. Administration. Office of Applied Studies. [Google Scholar]
- Anand K, Tiloke C, Naidoo P, & Chuturgoon AA (2017). Phytonanotherapy for management of diabetes using green synthesis nanoparticles. Journal of Photochemistry and Photobiology B: Biology, 173, 626–639. [DOI] [PubMed] [Google Scholar]
- Bailey K, & Crawley JN (2009). Anxiety-related behaviors in mice. In Buccafusco J (Ed.), Methods of behavior analysis in neuroscience (pp. 79–82). Boca Raton, FL: CRC Press/Taylor & Francis. [Google Scholar]
- Bhalla S, Pais G, Tapia M, & Gulati A (2015). Endothelin ETA receptor antagonist reverses naloxone-precipitated opioid withdrawal in mice. Canadian Journal of Physiology and Pharmacology, 93(11), 935–944. [DOI] [PubMed] [Google Scholar]
- Borras MC, Becerra L, Ploghaus A, Gostic JM, DaSilva A, Gonzalez RG, & Borsook D (2004). fMRI measurement of CNS responses to naloxone infusion and subsequent mild noxious thermal stimuli in healthy volunteers. Journal of Neurophysiology, 91(6), 2723–2733. 10.1152/jn.00249.2003 [DOI] [PubMed] [Google Scholar]
- CDC/NCHS. (2018). National vital statistics system, mortality Retrieved from https://wonder.cdc.gov
- Clemency BM, Eggleston W, Shaw EW, Cheung M, Pokoj NS, Manka MA, … Hostler D (2019). Hospital observation upon reversal (HOUR) with naloxone: A prospective clinical prediction rule validation study. Academic Emergency Medicine, 26(1), 7–15. 10.1111/acem.13567 [DOI] [PubMed] [Google Scholar]
- Colino CI, Millan CG, & Lanao JM (2018). Nanoparticles for signaling in biodiagnosis and treatment of infectious diseases. International Journal of Molecular Sciences, 19(6), 1627. 10.3390/ijms19061627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton WM, & Volkow ND (2006). Major increases in opioid analgesic abuse in the United States: Concerns and strategies. Drug and Alcohol Dependence, 81(2), 103–107. 10.1016/j.drugalcdep.2005.05.009 [DOI] [PubMed] [Google Scholar]
- Crumeyrolle-Arias M, Jaglin M, Bruneau A, Vancassel S, Cardona A, Dauge V, … Rabot S (2014). Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology, 42, 207–217. [DOI] [PubMed] [Google Scholar]
- Dahan A, Aarts L, & Smith TW (2010). Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology, 112(1), 226–238. 10.1097/ALN.0b013e3181c38c25 [DOI] [PubMed] [Google Scholar]
- Du AW, & Stenzel MH (2014). Drug carriers for the delivery of therapeutic peptides. Biomacromolecules, 15(4), 1097–1114. 10.1021/bm500169p [DOI] [PubMed] [Google Scholar]
- Enquist J, Ferwerda M, Milan-Lobo L, & Whistler JL (2012). Chronic methadone treatment shows a better cost/benefit ratio than chronic morphine in mice. Journal of Pharmacology and Experimental Therapeutics, 340(2), 386–392. 10.1124/jpet.111.187583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennessy MR, & Lee JR (1970). Modification of morphine analgesia by drugs affecting adrenergic and tryptaminergic mechanisms. Journal of Pharmacy and Pharmacology, 22(12), 930–935. 10.1111/j.2042-7158.1970.tb08475.x [DOI] [PubMed] [Google Scholar]
- Flacke JW, Flacke WE, & Williams GD (1977). Acute pulmonary edema following naloxone reversal of high-dose morphine anesthesia. Anesthesiology, 47(4), 376–377. 10.1097/00000542-197710000-00009 [DOI] [PubMed] [Google Scholar]
- Foldes FF, Duncalf D, & Kuwabara S (1969). The respiratory, circulatory, and narcotic antagonistic effects of nalorphine, levallorphan, and naloxone in anaesthetized subjects. Canadian Anaesthetists’ Society Journal, 16(2), 151–161. 10.1007/BF03005795 [DOI] [PubMed] [Google Scholar]
- Gangahar D (2015). A case of rhabdomyolysis associated with severe opioid withdrawal. American Journal on Addictions, 24(5), 400–402. 10.1111/ajad.12255 [DOI] [PubMed] [Google Scholar]
- Gao S, Gao H, Fan Y, Zhang G, Sun F, Zhao J, … Wang K (2016). Yiguanjian cataplasm attenuates opioid dependence in a mouse model of naloxone-induced opioid withdrawal syndrome. Journal of Traditional Chinese Medicine, 36(4), 464–470. 10.1016/S0254-6272(16)30063-2 [DOI] [PubMed] [Google Scholar]
- Grevert P, & Goldstein A (1978). Endorphins: Naloxone fails to alter experimental pain or mood in humans. Science, 199(4333), 1093–1095. 10.1126/science.343250 [DOI] [PubMed] [Google Scholar]
- Guilloux JP, Seney M, Edgar N, & Sibille E (2011). Integrated behavioral z-scoring increases the sensitivity and reliability of behavioral phenotyping in mice: Relevance to emotionality and sex. Journal of Neuroscience Methods, 197(1), 21–31. 10.1016/j.jneumeth.2011.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley B, & Frenkel E (2008). Nanoparticles for drug delivery in cancer treatment. Urologic Oncology, 26(1), 57–64. 10.1016/j.urolonc.2007.03.015 [DOI] [PubMed] [Google Scholar]
- Himmelsbach CK (1941). The morphine abstinence syndrome, its nature and treatment. Annals of Internal Medicine, 15(5), 829–839. [Google Scholar]
- Holck HG, Kimura KK, & Kimura TE (1950). Comparison of analgetic activity of 67 arylalkamines with that of morphine and meperidine by the mouse hot plate method. Journal of the American Pharmaceutical Association, 39(6), 354–359. 10.1002/jps.3030390616 [DOI] [PubMed] [Google Scholar]
- Huang X, & Brazel CS (2001). On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. Journal of Controlled Release, 73(2), 121–136. 10.1016/S0168-3659(01)00248-6 [DOI] [PubMed] [Google Scholar]
- Iovcheva M, Zlateva S, & Asparuhova M (2007). Precipitated withdrawal reaction to opiates in cases of improper use of naltrexone. Journal of IMAB—Annual Proceeding (Scientific Papers), 13, 79–81. [Google Scholar]
- Jain S, Singhai K, & Swami M (2018). Seizure as a primary presentation in opioid withdrawal. Psychiatry and Clinical Neurosciences, 72(10), 802–803. 10.1111/pcn.12770 [DOI] [PubMed] [Google Scholar]
- Kamaly N, Yameen B, Wu J, & Farokhzad OC (2016). Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chemical Reviews, 116(4), 2602–2663. 10.1021/acs.chemrev.5b00346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanof PD, Handelsman L, Aronson MJ, Ness R, Cochrane KJ, & Rubinstein KJ (1992). Clinical characteristics of naloxone-precipitated withdrawal in human opioid-dependent subjects. Journal of Pharmacology and Experimental Therapeutics, 260(1), 355–363. [PubMed] [Google Scholar]
- Kassick AJ, Allen HN, Yerneni SS, Pary F, Kovaliov M, Cheng C, … Averick S (2019). Covalent poly(lactic acid) nanoparticles for the sustained delivery of naloxone. ACS Applied Bio Materials, 2(8), 3418–3428. 10.1021/acsabm.9b00380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kest B, Palmese CA, Hopkins E, Adler M, Juni A, & Mogil JS (2002). Naloxone-precipitated withdrawal jumping in 11 inbred mouse strains: Evidence for common genetic mechanisms in acute and chronic morphine physical dependence. Neuroscience, 115(2), 463–469. 10.1016/S0306-4522(02)00458-X [DOI] [PubMed] [Google Scholar]
- Kim HK, & Nelson LS (2015). Reducing the harm of opioid overdose with the safe use of naloxone : A pharmacologic review. Expert Opinion on Drug Safety, 14(7), 1137–1146. 10.1517/14740338.2015.1037274 [DOI] [PubMed] [Google Scholar]
- Lax NC, Ahmed KT, Ignatz CM, Spadafora C, Kolber BJ, & Tidgewell KJ(2016). Marine cyanobacteria-derived serotonin receptor 2C active fraction induces psychoactive behavioral effects in mice. Pharmaceutical Biology, 54(11), 2723–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzucchelli S, & Corsi F (2017). Diabetes management strategies: Can nanoparticles be used to therapeutically deliver insulin? Therapeutic Delivery, 8(2), 49–51. 10.4155/tde-2016-0081 [DOI] [PubMed] [Google Scholar]
- Metten P, Crabbe JC, & Belknap JK (2009). Genetic correlates of morphine withdrawal in 14 inbred mouse strains. Drug and Alcohol Dependence, 99(1–3), 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss RB, & Carlo DJ (2019). Higher doses of naloxone are needed in the synthetic opioid era. Substance Abuse Treatment, Prevention, and Policy, 14(1), 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowry JB, Spyker DA, Brooks DE, Zimmerman A, & Schauben JL (2016). 2015 annual report of the American association of poison control centers’ national poison data system (NPDS): 33rd annual report. Clinical Toxicology, 54(10), 924–1109. 10.1080/15563650.2016.1245421 [DOI] [PubMed] [Google Scholar]
- O’Callaghan JP, & Holtzman SG (1975). Quantification of the analgesic activity of narcotic antagonists by a modified hot-plate procedure. Journal of Pharmacology and Experimental Therapeutics, 192(3), 497–505. [PubMed] [Google Scholar]
- Pleuvry BJ, & Tobias MA (1971). Comparison of the antinociceptive activities of physostigmine, oxotremorine and morphine in the mouse. British Journal of Pharmacology, 43(4), 706–714. 10.1111/j.1476-5381.1971.tb07205.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehni AK, & Singh N (2011). Modulation of src-kinase attenuates naloxone-precipitated opioid withdrawal syndrome in mice. Behavioural Pharmacology, 22(2), 182–190. 10.1097/FBP.0b013e328343d7a0 [DOI] [PubMed] [Google Scholar]
- Robinson A, & Wermeling DP (2014). Intranasal naloxone administration for treatment of opioid overdose. American Journal of Health System Pharmacy, 71(24), 2129–2135. 10.2146/ajhp130798 [DOI] [PubMed] [Google Scholar]
- Rzasa Lynn R, & Galinkin JL (2018). Naloxone dosage for opioid reversal: Current evidence and clinical implications. Therapeutic Advances in Drug Safety, 9(1), 63–88. 10.1177/2042098617744161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucier R, Wolfe D, & Dasgupta N (2018). Review of case narratives from fatal overdoses associated with injectable naltrexone for opioid dependence. Drug Safety, 41(10), 981–988. 10.1007/s40264-018-0653-3 [DOI] [PubMed] [Google Scholar]
- Seibenhener ML, & Wooten MC (2015). Use of the open field maze to measure locomotor and anxiety-like behavior in mice. Journal of Visualized Experiments, (96), e52434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Sharma B, Gupta S, & Sharma BM (2015). In vivo and in vitro attenuation of naloxone-precipitated experimental opioid withdrawal syndrome by insulin and selective KATP channel modulator. Psychopharmacology (Berl), 232(2), 465–475. 10.1007/s00213-014-3680-5 [DOI] [PubMed] [Google Scholar]
- Smith WD (1976). A comparison in mice of naloxone and nalorphine as antagonists to neuroleptanalgesic drugs. British Journal of Anaesthesia, 48(11), 1039–1044. 10.1093/bja/48.11.1039 [DOI] [PubMed] [Google Scholar]
- Soppimath KS, Aminabhavi TM, Kulkarni AR, & Rudzinski WE (2001). Biodegradable polymeric nanoparticles as drug delivery devices. Journal of Controlled Release, 70(1), 1–20. 10.1016/S0168-3659(00)00339-4 [DOI] [PubMed] [Google Scholar]
- Sun HL (1998). Naloxone-precipitated acute opioid withdrawal syndrome after epidural morphine. Anesthesia & Analgesia, 86(3), 544–545. 10.1213/00000539-199803000-00019 [DOI] [PubMed] [Google Scholar]
- Szekely JI, Dunai-Kovacs Z, Miglecz E, Ronai AZ, & Bajusz S (1978). In vivo antagonism by naloxone of morphine, beta-endorphin and a synthetic enkephalin analog. Journal of Pharmacology and Experimental Therapeutics, 207(3), 878–883. [PubMed] [Google Scholar]
- Thorpe DM (2001). Management of opioid-induced constipation. Current Pain and Headache Reports, 5(3), 237–240. 10.1007/s11916-001-0037-7 [DOI] [PubMed] [Google Scholar]
- Tompkins DA, Bigelow GE, Harrison JA, Johnson RE, Fudala PJ, & Strain EC (2009). Concurrent validation of the clinical opiate withdrawal scale (COWS) and single-item indices against the clinical institute narcotic assessment (CINA) opioid withdrawal instrument. Drug and Alcohol Dependence, 105(1–2), 154–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong R, & Cheng J (2009). Ring-opening polymerization-mediated controlled formulation of polylactide-drug nanoparticles. Journal of the American Chemical Society, 131(13), 4744–4754. 10.1021/ja8084675 [DOI] [PubMed] [Google Scholar]
- Vanky E, Hellmundt L, Bondesson U, Eksborg S, & Lundeberg S (2017). Pharmacokinetics after a single dose of naloxone administered as a nasal spray in healthy volunteers. Acta Anaesthesiologica Scandinavica, 61(6), 636–640. 10.1111/aas.12898 [DOI] [PubMed] [Google Scholar]
- Way EL, Loh HH, & Shen FH (1969). Simultaneous quantitative assessment of morphine tolerance and physical dependence. Journal of Pharmacology and Experimental Therapeutics, 167(1), 1–8. [PubMed] [Google Scholar]
- Wesson DR, & Ling W (2003). The clinical opiate withdrawal scale (COWS). Journal of Psychoactive Drugs, 35(2), 253–259. 10.1080/02791072.2003.10400007 [DOI] [PubMed] [Google Scholar]
- Wheeler E, Jones TS, Gilbert MK, & Davidson PJ (2015). Opioid overdose prevention programs providing naloxone to laypersons—United States, 2014. MMWR. Morbidity and Mortality Weekly Report, 64(23), 631–635. [PMC free article] [PubMed] [Google Scholar]
- Wightman RS, Nelson LS, Lee JD, Fox LM, & Smith SW (2018). Severe opioid withdrawal precipitated by Vivitrol®. American Journal of Emergency Medicine, 36(6), 1128.e1–1128.e2. [DOI] [PubMed] [Google Scholar]
- Zhang G, Wu X, Zhang YM, Liu H, Jiang Q, Pang G, … Stackman RW Jr. (2016). Activation of serotonin 5-HT(2C) receptor suppresses behavioral sensitization and naloxone-precipitated withdrawal symptoms in morphine-dependent mice. Neuropharmacology, 101, 246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.


