Abstract
Background and hypothesis
Although maintenance treatment with antipsychotics protects against psychotic relapse, high doses may hamper recovery. Therefore, dose reduction or discontinuation may be considered in patients with chronic schizophrenia. Here, we identified risk factors for psychotic relapse when doses are reduced.
Study Design
We systematically searched MEDLINE, EMBASE, and PsycINFO from January 1950 through January 2021 and reviewed randomized controlled trials (RCTs) that reported relapse rates after antipsychotic dose reduction or discontinuation in patients with chronic schizophrenia. We calculated relative risks (RRs) with 95% confidence intervals (CIs) per person-year and sought to identify potential risk factors for relapse. The study is registered with PROSPERO (CRD42017058296).
Study Results
Forty-seven RCTs (54 patient cohorts, 1746 person-years) were included. The RR for psychotic relapse with dose reduction/discontinuation versus maintenance treatment was 2.3 per person-year (95% CI: 1.9 to 2.8). The RR was higher with antipsychotic discontinuation, dose reduction to less than 3–5 mg haloperidol equivalent (HE), or relatively rapid dose reduction (<10 weeks). The RR was lower with long-acting injectable agents versus oral antipsychotic dose reduction. Other factors that increased the risk of psychotic relapse were younger age and short follow-up time.
Conclusions
Clinicians should take several risk factors for psychotic relapse into account when considering dose reduction in patients with chronic schizophrenia. Studies of a relatively fast reduction in antipsychotic dose support a minimum dose of 3–5 mg HE. However, if the dose is tapered more gradually, relapses related to medication withdrawal might be avoided, possibly enabling lower-end doses to be achieved.
Keywords: antipsychotic doses, maintenance treatment, withdrawal, gradual reduction, adverse events, recovery, tapering
Introduction
During the first years after a first psychotic episode, maintenance treatment with antipsychotics is more effective than placebo in preventing relapse.1,2 There is, however, no consensus on the duration of maintenance treatment, and many patients and psychiatrists consider dose reduction or discontinuation in the long term.3 In a large survey, 90% of patients reported antipsychotic-related side effects and more than 50% reported that their quality of life was worse after treatment.4 Moreover, patients have reported that long-term antipsychotic use disturbs their individual efforts and their sense of agency in overcoming psychosis.5 The dose of antipsychotic medication has been found to be positively associated with the severity of side effects.6 Sedation, sexual dysfunction, extrapyramidal symptoms, metabolic syndrome,7–9 diminished cognitive functioning, and loss of motivation and drive10,11 are common during high-dose antipsychotic treatment and hamper recovery, and can also occur at lower doses, at least to some extent.12 Several long-term naturalistic studies suggest that a considerable proportion of first-episode patients who undergo dose reduction or discontinue antipsychotics recover with better global functioning than those prescribed continuous antipsychotic treatment.13–16
Although these findings suggest it is worthwhile to consider dose reduction or discontinuation, schizophrenia tends to be a chronic disorder, with incomplete remission, functional impairment, and social disability.17 Patients whose condition does not go into remission are often prescribed high doses of antipsychotics.18,19 If antipsychotics are stopped or the dose reduced, there is a subsequent higher risk of relapse and rehospitalization.20,21 Recent meta-analyses found discontinuation and dose reduction to less than 4–5 mg haloperidol equivalents (HEs)/day to be associated with relapse, whereas gradual dose reduction reduced the risk of relapse.22,23 However, these studies included cohort studies and a limited number of randomized controlled trials (RCTs), with high heterogeneity and a high risk of confounding and bias.
In the current study, we performed a systematic review and meta-analysis of RCTs, comparing dose reduction or discontinuation of antipsychotics with stable continuous antipsychotic medication use in patients with chronic schizophrenia. We intended to create more homogenous results by comparing the risk of relapse in different subgroups. On the basis of the literature and from a clinical point of view, we distinguished the following subgroups: (1) patients whose dose of antipsychotics is reduced or discontinued,1,24 (2) male/female patients,25,26 (3) inpatients or outpatients,27,28 and (4) patients using long-acting injectable agents (LAI) or oral medication.24,29 We aimed to determine the relative risk (RR) of relapse in patients with chronic schizophrenia and to identify risk factors for psychotic relapse in these subgroups.
Methods
This review was conducted following the PRISMA guidelines.30 A protocol was published in the PROSPERO database under registration number CRD42017058296. We searched PubMed, EMBASE, and PsycINFO for studies of antipsychotic dose reduction from January 1950 through January 2021. This strategy included the following terms: (“Schizophrenia”[Mesh] OR “Schizophrenia”[tw] OR “Schizophrenic”[tw]) AND (“chronic”[tw] OR “clinical”[tw] OR “clinically”[tw]) AND (“Antipsychotic Agents”[Mesh] OR “Antipsychotic”[tw] OR “Antipsychotics”[tw] OR “Tranquilizing Agents”[Mesh] OR “Antipsychotic Agents” [Pharmacological Action] OR “Tranquilizing Agents” [Pharmacological Action] OR “dose reduction”[tiab] OR “dosage reduction”[tiab] OR “Therapeutic window”[tiab]). The reference lists of eligible articles and reviews were hand searched to identify eligible studies not previously identified through the database search (backward and forward tracking of literature).
Study Selection
Two reviewers (JPAMB and GH) independently screened the titles and abstracts of retrieved citations using the following inclusion criteria: (1) Patients were diagnosed with schizophrenia or schizoaffective disorder according to international classification systems (DSM, International Classification of Diseases and Related Health Problems (ICD) Research Criteria for Schizophrenia) or these disorders were explicitly mentioned as a clinical diagnosis, (2) Patients had chronic symptoms, ie, patients had been diagnosed as having chronic schizophrenia or patients lived in long-stay facilities or had received maintenance treatment for at least 5 years, (3) The dose reduction procedure was clearly described and data on relapse rates were provided, and (4) Studies were RCTs comparing dose reduction or discontinuation of antipsychotics with stable continuous use. Full-text articles were excluded if they involved reviews, studies with patients with different diagnoses and which did not provide separate data for schizophrenia patients, or studies involving patients with acute psychotic episodes. Differences in opinion between the reviewers about inclusion were settled in consensus meetings. Special effort was made to screen the reference lists of included articles and reviews to diminish the probability that we would miss a relevant RCT.
Data Extraction
Two authors (JPAMB, GH) extracted data independently and calculated RRs with 95% confidence intervals (95% CI) per person–year. We selected data on the basis of two earlier reviews,22,23 namely: (1) dose reduction/discontinuation characteristics (starting dose before dose reduction, end dose after dose reduction, abrupt, or gradual dose reduction, where abrupt dose reduction was defined as oral medication stopped at once, duration of dose reduction in weeks), (2) patient characteristics (age, gender as a percentage of male subjects, inpatient or outpatient status, duration of illness, method of administration of antipsychotic medication, ie, LAI or oral medication), and (3) study characteristics (follow-up time after dose reduction, blinding, and relapse definition).
Relapse Criteria for Psychosis
The definition of psychotic relapse given in the original articles was used (Supplementary Table 1). Several researchers considered a 20% change in Positive and Negative Syndrome Scale (PANSS) or Brief Psychiatric Rating Scale (BPRS) scores as clinically relevant. If such a rating was not available, clinical judgment, hospitalization, or an increase in antipsychotic dose was considered to reflect psychotic relapse.
Assessment of Risk of Bias
Two authors (GH, NWS) individually assessed all selected studies for risk of bias by using the Cochrane handbook, Risk of Bias 2 tool.31 Any differences in opinion were discussed until a consensus was reached. Six potential sources of bias that could affect the association between exposure and outcome were investigated (Supplementary Table 2).
Statistical Analyses
All statistical analyses were performed using Comprehensive Meta-Analysis software, version 3.0.32 For each study, we calculated the number of patients who underwent dose reduction or drug discontinuation (interventions), or maintenance therapy (control condition), and the number of relapses that occurred during the follow-up period as crude rates per person-years. RR was used as a measure to calculate and pool data on the RR of relapse (intervention vs control). RRs are more intuitive than odds ratios and since the risk of relapse is high, the statistical advantages of using odds ratios are limited.33 Previous studies also used RR and therefore using this measure increased comparability.34,35 Pooled estimates of RRs for continuous and dichotomous outcomes were calculated with two-sided 95% confidence intervals (95% CI) using a DerSimonian-Laird random effects model.32 A 95% CI higher than 1.0 means that the rate of relapse in the intervention condition was significantly higher than in the control condition, and a 95% CI that includes 1.0 means that the relapse rate in the intervention condition was not significantly different from that in the control condition.
The influence of different variables, such as dose reduction, patient, and study characteristics, on the risk of relapse, was investigated by comparing differences between RRs of pools. If a variable was found to be associated with an increased RR of relapse, a subsequent subgroup analysis was conducted to see if other variables could explain the increased risk. As a rule of thumb, at least four groups in at least 2 strata were required to make comparisons meaningful. Since different antipsychotics were used in these studies, we converted doses to HE doses to enable group comparisons (Supplementary Table 3).
Between-study heterogeneity was assessed with Cochran’s I2-statistic. The I² statistic describes the percentage of variation across studies that is due to heterogeneity rather than chance.36,37 According to convention, a chi-squared test <0.05 or I2 statistics >50% indicates high heterogeneity.
RR factor values for continuous variables were not normally distributed but showed clustering of data points. Therefore, meta-regression to identify RR factors was not deemed feasible. We analyzed all variables, dichotomous and continuous, by stratification. Continuous variables were divided into strata using median split. We analyzed all studies, and subgroups of studies based on specific discriminative variables, such as dose reduction or discontinuation,1,24 gender (proportion of male patients),25,26 inpatient or outpatient status,27,28 and LAI or oral medication.24,29 Additional analyses were performed to examine confounding. Publication bias was visually explored with funnel plots (Supplementary figure 1). Two-sided P-values <.05 were considered statistically significant.
Results
Study Selection
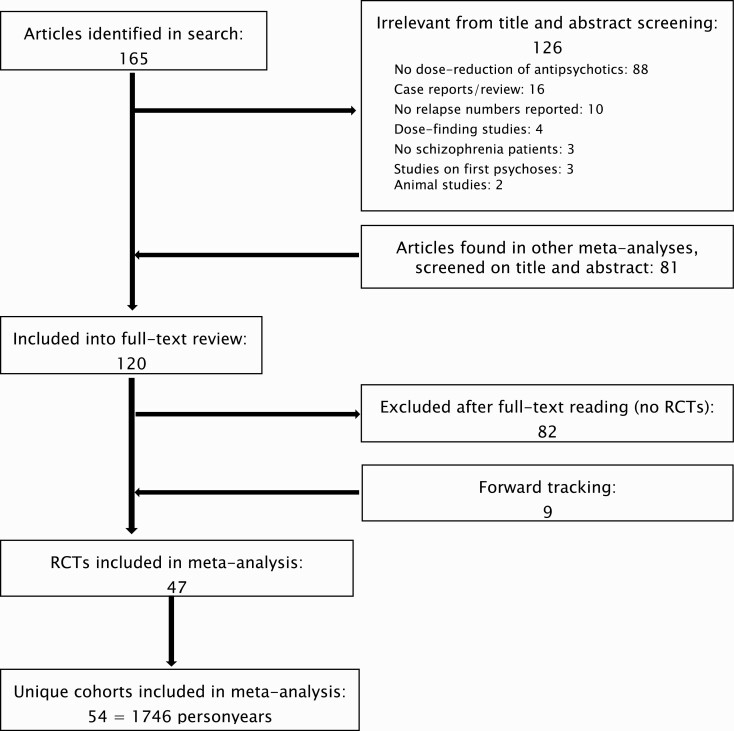
The initial search yielded 165 articles, of which 39 remained after the screening of titles and abstracts. Eighty-one articles were added after reviewing reference lists. After a full-text review of these 120 articles, 82 were discarded, mostly non-RCTs. By forwarding tracking we found another 9 articles, resulting in 47 RCTs, with 54 cohorts of patients (studies). Details of the selection process are shown in a flowchart (figure 1).
Fig. 1.
Flowchart of study selection.
Study and Patient Characteristics
In total, 4571 patients were included, 2366 who underwent dose reduction/discontinuation and 2205 who received maintenance treatment, representing 1746 person-years, with a mean age of 42.2 years (SD 7.9). In total 66% (SD 22) were men, and the mean duration of illness was 14.7 years (SD 6.4). The mean follow-up time was 35 weeks (SD 23). The mean dose reduction was from 20.7 (SD 40.5) to 3.6 (SD 6.2) mg HE/day (Table 1).
Table 1.
Patient and Study Characteristics
| First Author and Cohort | Year | Cohort Size Exp (Participants) | Cohort Size Con (Participants) | Rate Ratio | FU (Weeks) | Blinding | Mean Age (Years) | Mean DOI (Years) | Patient Type | Male Percentage | Reduction Period (Weeks) | Medication Type | Pre Dose (mg) | Post Dose (mg) | Reduction Style | Relapse Definition |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diamond38 | 1960 | 20 | 20 | 3,7 | 26 | Yes | 46,6 | 11,0 | Inpatients | nd | 0 | Oral | 8,5 | 0,0 | Abrupt | Clinical Judgment |
| Blackburn39 | 1961 | 14 | 25 | 4,8 | 16 | No | nd | nd | Inpatients | 100 | 0 | Oral | nd | 0,0 | Abrupt | Rated on scales |
| Freeman40 | 1962 | 46 | 48 | 2,3 | 26 | Yes | 41,1 | 13,0 | Inpatients | nd | 0 | Oral | 4,6 | 0,0 | Abrupt | Clinical Judgment |
| Whittaker 141 | 1963 | 13 | 6 | 2,3 | 10 | No | 50,2 | 16,0 | Outpatients | 100 | 0 | Oral | 6,0 | 0,0 | Abrupt | Clinical Judgment |
| Whittaker 241 | 1963 | 13 | 7 | 2,7 | 10 | Yes | 49,5 | 15,0 | Outpatients | 100 | 0 | Oral | 6,0 | 0,0 | Abrupt | Clinical Judgment |
| Marjerrison42 | 1964 | 171 | 44 | 10,9 | 22 | Yes | 47,0 | 20,0 | Inpatients | 48 | 0 | Oral | nd | 0,0 | Abrupt | Rated on scales |
| Caffey 143 | 1964 | 89 | 44 | 9,9 | 16 | Yes | 40,0 | 10,0 | Inpatients | 100 | 1 | Oral | 7,5 | 0,0 | Gradual | Clinical Judgment |
| Caffey 243 | 1964 | 31 | 26 | 3,2 | 16 | Yes | 40,0 | 10,0 | Inpatients | 100 | 1 | Oral | 7,5 | 4,3 | Gradual | Clinical Judgment |
| Melnyk44 | 1966 | 16 | 9 | 21,0 | 6 | Yes | 42,8 | 12,0 | Inpatients | 65 | 0 | Oral | 7,0 | 0,0 | Abrupt | Clinical Judgment |
| Garfield45 | 1966 | 20 | 20 | 2,8 | 22 | Yes | 42,6 | 11,0 | Inpatients | 0 | 1 | Oral | 12,8 | 0,0 | Gradual | Clinical Judgment |
| Morton46 | 1968 | 20 | 20 | 2,8 | 26 | Yes | nd | nd | nd | 100 | 0 | nd | nd | 0,0 | Abrupt | Clinical Judgment |
| Rassidakis47 | 1970 | 13 | 13 | 1,7 | 39 | No | 49,3 | 24,0 | Inpatients | 43 | 0 | nd | nd | 0,0 | Abrupt | Clinical Judgment |
| Baro48 | 1970 | 43 | 41 | 27,0 | 10 | Yes | nd | nd | Inpatients | 100 | 1 | LAI | 50,1 | 0,0 | Gradual | Clinical Judgment |
| Hershon49 | 1972 | 11 | 11 | 4,5 | 16 | Yes | 55,1 | nd | Inpatients | 52 | 1 | LAI | 11,3 | 0,0 | Gradual | Clinical Judgment |
| Ota50 | 1973 | 49 | 49 | 4,0 | 13 | Yes | 42,9 | nd | Inpatients | 55 | 0 | Oral | 7,5 | 0,0 | Abrupt | Clinical Judgment |
| Andrews51 | 1976 | 17 | 14 | 4,9 | 42 | Yes | 53,8 | nd | Inpatients | 100 | 0 | Oral | 4,6 | 0,0 | Abrupt | Clinical Judgment |
| Lonowski52 | 1978 | 25 | 23 | 0,9 | 15 | Yes | 47,11 | 12,2 | Inpatients | 56 | 15 | LAI | 6,6 | 1,4 | Gradual | Rated on scales |
| Levine 153 | 1980 | 27 | 6 | nd | 15 | No | 31,2 | nd | Inpatients | 46 | nd | Oral | 24,2 | 0,0 | Abrupt | Clinical Judgment |
| Levine 253 | 1980 | 23 | 11 | 3,3 | 15 | No | 32,1 | nd | Inpatients | 52 | nd | LAI | 12,4 | 0,0 | Gradual | Clinical Judgment |
| Lehmann54 | 1980 | 20 | 20 | 2,0 | 24 | Yes | 38,2 | 9,8 | nd | 68 | 2 | LAI | 270,0 | 30,0 | Gradual | Clinical Judgment |
| Cheung55 | 1981 | 13 | 15 | 3,9 | 78 | Yes | 39,6 | 11,0 | Outpatients | 43 | 0 | Oral | 3.3 | 0,0 | Abrupt | Clinical Judgment |
| Odejide56 | 1982 | 27 | 26 | 2,8 | 52 | Yes | nd | nd | Outpatients | nd | 6 | LAI | 20,0 | 0,0 | Gradual | Rated on scales |
| Lehmann57 | 1983 | 60 | 56 | 3,0 | 48 | No | 47,7 | nd | Outpatients | 49 | nd | Oral | 7,3 | 1,5 | Gradual | Clinical Judgment |
| Johnson58 | 1983 | 63 | 62 | 7,6 | 78 | No | 32,0 | nd | Outpatients | 52 | 2 | LAI | nd | 0,0 | Gradual | Rated on scales |
| Kane59 | 1983 | 62 | 32 | 11,9 | 52 | Yes | 29,0 | 6,0 | Inpatients | nd | 3 | LAI | 20,0 | 2,0 | Gradual | Clinical Judgement |
| Wistedt60 | 1983 | 16 | 22 | 1,7 | 24 | Yes | 44,4 | 11,7 | Outpatients | 50 | 3 | LAI | 11,0 | 0,0 | Gradual | Rated on scales |
| Channabasavanna61 | 1987 | 14 | 14 | 13,3 | 12 | Yes | 36,0 | 11,0 | Outpatients | 64 | 0 | Oral | 6,1 | 0,0 | Abrupt | Clinical Judgment |
| Cookson62 | 1987 | 28 | 31 | 3,0 | 44 | Yes | 46,0 | 14,0 | Inpatients | 67 | 2 | LAI | nd | >0 | Gradual | Rated on scales |
| Johnson63 | 1987 | 9 | 9 | 1,5 | 52 | Yes | 40,0 | 10,0 | Outpatients | 46 | 2 | LAI | nd | >0 | Gradual | Rated on scales |
| Hogarthy64 | 1988 | 30 | 25 | 1,3 | 54 | Yes | 28,3 | 7,0 | Outpatients | 57 | 2 | LAI | 25,8 | 4,6 | Gradual | Rated on scales |
| Faraone65 | 1989 | 22 | 7 | 17,0 | 26 | Yes | nd | nd | Outpatients | 100 | 5 | Oral | nd | >0 | Gradual | Clinical Judgment |
| Sampath66 | 1992 | 12 | 12 | 1,9 | 12 | Yes | 57,6 | 31,0 | Inpatients | nd | 2 | LAI | 40,0 | 0,0 | Gradual | Rated on scales |
| Inderbitzin67 | 1994 | 20 | 17 | 1,3 | 30 | Yes | 41,2 | 18,0 | Outpatients | 73 | 22 | LAI | 27,3 | 13,8 | Gradual | Clinical Judgment |
| Hogarthy68 | 1995 | 38 | 41 | 0,2 | 13 | Yes | 33,7 | 9,7 | Outpatients | 66 | 2 | LAI | 22,3 | 12,6 | Gradual | Clinical Judgment |
| Huttunen 69 | 1996 | 9 | 10 | 1,1 | 104 | No | nd | nd | Outpatients | 53 | 4 | Oral | nd | 4,0 | Gradual | Clinical Judgment |
| Hirschowitz 170 | 1997 | 6 | 8 | 2,7 | 52 | Yes | 48,0 | 24,2 | Inpatients | 100 | 2 | Oral | 18,3 | 1,0 | Gradual | Rated on scales |
| Hirschowitz 2 70 | 1997 | 8 | 8 | 0,5 | 52 | Yes | 43,1 | 23,2 | Inpatients | 75 | 2 | Oral | 20,0 | 11,0 | Gradual | Rated on scales |
| Schooler71 | 1997 | 106 | 107 | 1,2 | 104 | Yes | 29,6 | 21,1 | Inpatients | 66 | 2 | LAI | 37,1 | 16,3 | Gradual | Rated on scales |
| Carpenter72 | 1999 | 25 | 25 | 1,6 | 54 | Yes | 36,2 | 13,0 | Outpatients | 60 | 6 | LAI | 30 | 10,0 | Gradual | Rated on scales |
| Volavka73 | 2000 | 11 | 12 | 1,1 | 16 | Yes | 40,2 | 20,4 | Inpatients | 87 | 12 | Oral | 32,9 | 19,4 | Gradual | Clinical Judgment |
| Arato74 | 2002 | 71 | 206 | 1,8 | 52 | Yes | 48,8 | 22,0 | Inpatients | 83 | 0 | Oral | nd | 0,0 | Abrupt | Rated on scales |
| Khazaie75 | 2005 | 25 | 25 | 1,2 | 54 | Yes | 34,0 | 12,0 | Outpatients | 72 | 6 | LAI | 20,7 | 10,0 | Gradual | Rated on scales |
| Ushida76 | 2006 | 17 | 17 | nd | 24 | Yes | 40,1 | 15,8 | Outpatients | 53 | 12 | Oral | 14 | 8,0 | Gradual | Clinical Judgment |
| Kramer77 | 2007 | 55 | 56 | 2,1 | 34 | Yes | 40,7 | 12,6 | nd | 51 | 0 | Oral | 10,8 | 0,0 | Abrupt | Rated on scales |
| Rouillon78 | 2008 | 49 | 48 | 2,0 | 26 | No | 39,5 | nd | Outpatients | 65 | nd | Oral | 7,4 | 5,3 | Gradual | Clinical Judgment |
| Hough79 | 2008 | 140 | 133 | 2,7 | 46 | Yes | 39,1 | 11,9 | nd | 54 | 4 | LAI | 6,6 | 0,0 | Gradual | Rated on scales |
| Kane 180 | 2010 | 203 | 205 | 2,3 | 24 | Yes | 37,7 | nd | Outpatients | 60 | 0 | Oral | 2,8 | 0,2 | Abrupt | Rated on scales |
| Kane 280 | 2010 | 144 | 133 | 4,5 | 24 | Yes | 39,5 | nd | Outpatients | 67 | 0 | Oral | 2,8 | 1,4 | Abrupt | Rated on scales |
| Wang81 | 2010 | 245 | 129 | 2,6 | 52 | No | 32,0 | 6,4 | Inpatients | 45 | 8 | Oral | 4,3 | 2,1 | Gradual | Rated on scales |
| Takeuchi82 | 2013 | 31 | 30 | 1,0 | 24 | No | 40,9 | 15,5 | Outpatients | 58 | 4 | Oral | 4,8 | 2,5 | Gradual | Rated on scales |
| Yamanouchi83 | 2014 | 62 | 163 | 2,6 | 52 | No | 60,0 | 32,0 | nd | 57 | 24 | Oral | 20,0 | 15,0 | Gradual | Clinical Judgment |
| Zhou84 | 2018 | 37 | 38 | 0,7 | 52 | Yes | 44,3 | 5,0 | Outpatients | 60 | 16 | Oral | 5,4 | 3,2 | Gradual | Rated on scales |
| Ozawa85 | 2019 | 17 | 18 | 7,4 | 52 | Yes | 64,1 | nd | Inpatients | 37 | 4 | nd | 7,0 | 2,8 | Gradual | Clinical Judgment |
| Huhn86 | 2021 | 10 | 8 | 0,4 | 14 | Yes | 44,7 | 16,8 | Outpatients | 55 | 14 | Oral | 5,8 | 3,5 | Gradual | Rated on scales |
| Total | — | 2366 | 2205 | — | 1872 | — | — | 545 | — | — | — | — | — | — | — | — |
| Mean | — | — | — | 4.3 | 34,7 | — | 42,2 | 14,7 | — | 65,5 | 3,9 | — | 20,7 | 3,6 | — | — |
| SD | — | — | — | 5,2 | 22,5 | — | 7,9 | 6,4 | — | 21,6 | 5,7 | — | 40,5 | 6,2 | — | — |
Note: exp, experimental group = dose reduction or discontinuation condition; con, control group = maintenance condition; FU, follow-up period; DOI, duration of illness; nd, no data.
Analysis of all studies (N = 54)
Overall, patients who underwent dose reduction or discontinuation had an increased risk of psychotic relapse (RR = 2.3 per person-year; 95% CI: 1.9 to 2.8; I2 35%) relative to that of patients on antipsychotic maintenance treatment. Differences in RRs in different strata are given in Table 2. Heterogeneity was low in most analyses.
Table 2.
Relative Risks (RR), Heterogeneity (I2), Number of Studies (n), and P-Values After Stratification of Cohorts for Various Variables
| Variable | Cohort Name: | Total Group of Studies | Dose Reduction Group | Discontinuation (Stop) Group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pools | n | RR (95%CI) | I 2 (%) | P | n | RR (95%CI) | I 2 (%) | P | n | RR (95% CI) | I 2 (%) | P | ||
| Reduction Characteristics | Medication | Discontinued | 25 | 2.63 (2.14 to 3.22) | 5 | .034 | na | na | ||||||
| Dose reduced | 27 | 1.78 (1.32 to 2.39) | 42 | |||||||||||
| End-dose 3mg divide | 0 mg | 25 | 2.63 (2.14 to 3.22) | 5 | .000 | na | na | |||||||
| 0–3 mg | 10 | 2.90 (1.90 to 4.44) | 38 | 10 | 2.90 (1.90 to 4.44) | 38 | .000 | |||||||
| 3 mg+ | 14 | 1.16 (0.90 to 1.50) | 0 | 14 | 1.16 (0.90 to 1.50) | 0 | ||||||||
| End-dose 5mg divide | 0 mg | 25 | 2.63 (2.14 to 3.22) | 5 | .000 | na | na | |||||||
| 0–5 mg | 14 | 2.22 (1.46 to 3.39 | 46 | 14 | 2.22 (1.46 to 3.39) | 46 | .016 | |||||||
| 5 mg+ | 10 | 1.20 (0.91 to 1.58) | 0 | 10 | 1.20 (0.91 to 1.58) | 0 | ||||||||
| Dose reduction style | Abrupt | 17 | 2.42 (1.94 to 3.01) | 0 | .353 | 15 | 2.23 (1.75 to 2.85) | 0 | .093 | |||||
| Gradual | 35 | 2.05 (1.56 to 2.68) | 44 | 11 | 3.27 (2.25 to 4.73) | 15 | ||||||||
| Duration of dose reduction | 0 week | 17 | 2.42 (1.94 to 3.01) | 0 | .022 | 19 | 1.94 (1.33 to 2.84)# | 52 | .066 | 15 | 2.32 (1.75 to 2.85) | 0 | .099 | |
| 1–10 weeks | 26 | 2.28 (1.64 to 3.17) | 52 | 9 | 3.34 (2.21 to 5.05) | 25 | ||||||||
| >10 weeks | 6 | 1.02 (0.58 to 1.81) | 0 | 6 | 1.02 (0.58 to 1.81) | 0 | ||||||||
| Patient Characteristics | Age | 0–42 years | 24 | 2.32 (1.72 to 3.14) | 56 | .303 | 16 | 1.85 (1.28 to 2.67) | 54 | .577 | 8 | 3.37 (2.25 to 5.04) | 28 | .042 |
| >42 years | 22 | 1.90 (1.50 to 2.41) | 0 | 9 | 1.54 (0.90 to 2.61) | 13 | 13 | 2.04 (1.55 to 2.68) | 0 | |||||
| Male percentage | 0–49% | 8 | 2.37 (1.64 to 3.44) | 0 | .016 | 4 | 2.12 (1.20 to 3.74) | 0 | .199 | |||||
| 50–99% | 29 | 1.84 (1.42 to 2.38) | 44 | 10 | 2.75 (1.92 to 3.95) | 37 | ||||||||
| 100% | 10 | 4.26 (2.54 to 7.15) | 0 | 7 | 4.45 (2.45 to 8.07) | 0 | ||||||||
| Patient setting | Inpatients | 25 | 2.58 (1.88 to 3.54) | 41 | .262 | 10 | 2.14 (1.20 to 3.82) | 56 | .401 | 15 | 2.72 (1.93 to 3.84) | 16 | .425 | |
| Outpatients | 22 | 1.98 (1.41 to 2.77) | 41 | 15 | 1.59 (1.08 to 2.35) | 42 | 7 | 3.50 (2.09 to 5.88) | 4 | |||||
| Duration of Illness | 0–10 years | 9 | 2.14 (1.03 to 4.45) | 66 | .199 | 8 | 1.79 (0.85 to 3.75) | 64 | .592 | 11 | 2.79 (2.13 to 3.65)# | 0 | .038 | |
| 11–15 years | 14 | 2.07 (1.53 to 2.81) | 21 | 4 | 1.15 (0.72 to 1.83) | 0 | ||||||||
| 15+ years | 13 | 1.50 (1.20 to 1.88) | 0 | 8 | 1.22 (0.88 to 1.69) | 0 | 5 | 1.80 (1.32 to 2.46) | 0 | |||||
| Method of administration | Oral | 30 | 2.48 (2.01 to 3.07) | 4 | .282 | 15 | 2.39 (1.74 to 3.27) | 7 | .046 | 8 | 3.00 (2.10 to 4.29) | 8 | .647 | |
| LAI | 19 | 1.97 (1.38 to 2.83) | 58 | 11 | 1.39 (0.91 to 2.13) | 47 | 15 | 2.68 (1.96 to 3.67) | 10 | |||||
| Study Characteristics | Follow-up 16 | 0–16 weeks | 16 | 2.80 (1.54 to 5.10) | 46 | .433 | 5 | 0.87 (0.36 to 2.12) | 31 | .090 | 11 | 4.68 (2.76 to 7.94) | 0 | .016 |
| >16 weeks | 36 | 2.18 (1.79 to 2.70) | 31 | 22 | 1.97 (1.45 to 2.67) | 41 | 14 | 2.34 (1.92 to 2.86) | 0 | |||||
| Follow-up 26 | 0–26 weeks | 28 | 2.70 (1.98 to 3.70) | 22 | .152 | 11 | 1.78 (0.99 to 3.19) | 48 | .925 | 17 | 3.39 (2.35 to 4.88) | 0 | .145 | |
| >26 weeks | 24 | 2.02 (1.59 to 2.58) | 42 | 16 | 1.72 (1.23 to 2.41) | 36 | 8 | 2.40 (1.80 to 3.20) | 28 | |||||
| Blind | Yes | 41 | 2.21 (1.74 to 2.79) | 42 | .475 | 21 | 1.67 (1.15 to 2.41) | 51 | .242 | 20 | 2.50 (2.04 to 3.06) | 38 | .462 | |
| No | 11 | 2.56 (1.85 to 3.53) | 0 | 6 | 2.34 (1.52 to 3.63) | 0 | 5 | 3.30 (1.63 to 6.70) | 0 | |||||
| Relapse definition | Clinical judgement | 28 | 2.98 (2.18 to 4.08) | 17 | .030 | 12 | 1.38 (1.31 to 4.33) | 41 | .218 | 16 | 3.11 (2.19 to 4.42) | 0 | .320 | |
| Scales | 24 | 1.93 (1.52 to 2.45) | 43 | 15 | 1.56 (1.17 to 2.14) | 537 | 9 | 2.47 (1.84 to 3.30) | 25 | |||||
Note: Analyses have been done for the total group of studies, and for different subgroups of studies: dose-reduction studies, discontinuation studies, and other subgroups. The latter are presented in Supplementary Table 4. Blank Boxes Indicate an Insufficient Number of Studies (<4).
Note: na, not applicable; # rows combined because of insufficient number of studies in one of the rows (<4).
We found substantial differences in the RR of relapse when we compared the discontinuation group (RR = 2.6; 95% CI: 2.1 to 3.2; I2 5%) with the dose reduction group (RR = 1.8; 95% CI: 1.3 to 2.4; I2 42%) (P = .034). End dose significantly affected the RR of relapse. We found a comparable RR of relapse with dose reduction to less than 3 mg HE/day (RR = 2.9; 95% CI: 1.9 to 4.4; I2 38%) and discontinuation (RR = 2.6; 95% CI: 2.1 to 3.2; I2 5%), but a lower RR with end doses higher than 3 mg HE/day (RR = 1.2; 95% CI: 0.9 to 1.5; I2 0%) (P < .001). The RR for the latter was not significantly different from that for maintenance treatment. The duration of dose reduction influenced the RR of relapse (P = .022): RR = 2.4 (95% CI: 1.9 to 3.0; I2 0%) for dose reduction over 0 weeks or abrupt reduction, RR = 2.3 (95% CI: 1.6 to 3.2; I2 52%) for dose reduction over 1–10 weeks, and RR = 1.0 (95% CI: 0.6 to 1.8; I2 0%) for dose reduction over more than 10 weeks; the latter was not significantly different from that for the maintenance condition.
The RR of relapse was higher in studies involving men only (RR = 4.3; 95% CI: 2.5 to 7.2; I2 0%) than in all other studies (RR = 2.0; 95% CI: 1.6 to 2.4; I2 37%) (P = .006). However, the RR of relapse was not found to be higher in men when data were stratified according to the proportion of men in a study. Nearly all of the studies that included men only investigated abrupt cessation of medication or reduction of medication to doses lower than 3 mg HE/day.
The following characteristics did not significantly affect the overall RR of relapse: starting dose, age, inpatient or outpatient status, duration of illness, LAI or oral drug, follow-up time, blinding, or relapse definition.
Dose Reduction or Discontinuation Studies
In dose-reduction studies (n = 27), there was a more than double RR of relapse when the end dose was lower than 3 mg HE/day (RR = 2.9; 95% CI: 1.9 to 4.4; I2 38%) than when it was higher than 3 mg HE/day (RR = 1.2; 95% CI: 0.9 to 1.5; I2 0%) (P < .001), and an almost 2 times higher RR of relapse when the end dose was lower than 5 mg HE/day (RR = 2.2; 95% CI: 1.5 to 3.4; I2 46%) than when it was higher than 5 mg HE/day (RR = 1.2; 95% CI: 0.9 to 1.6; I2 0%) (P = .016). The risk of relapse was not significantly different between dose reduction to a dose higher than 3–5 mg HE/day and maintenance therapy. There were only 2 cohorts of patients in studies investigating abrupt dose reduction, so comparisons with gradual dose reduction could not be made. The same is true for studies with different durations of dose reduction. In post hoc analyses comparing dose reduction over 0–10 weeks and 10 weeks and longer, the risk of relapse was not significantly different between dose reduction over 10 weeks and longer and maintenance treatment (RR = 1.0; 95% CI: 0.6 to 1.8; I2 0%). LAI seemed to protect against relapse during dose reduction (RR = 1.4; 95% CI: 0.9 to 2.1; I2 47%) compared with oral antipsychotics (RR = 2.4; 95% CI: 1.7 to 3.3; I2 7%)(P = .046). The risk of relapse with dose reduction with LAI was not significantly different from that with maintenance conditions. Starting dose, age, patient setting, duration of illness, follow-up time, blinding or relapse definition did not significantly affect the RR of relapse in dose-reduction studies.
In discontinuation studies (n = 25), abrupt or gradual discontinuation did not significantly affect the RR of relapse. Studies with a duration of dose reduction of 10 weeks or longer were not available. Age 42 years or younger (RR = 3.4; 95% CI: 2.3 to 5.0; I2 28%) was associated with a higher RR of relapse than age 42 years and older (RR = 2.0; 95%CI 1.6–2.7; I2 0%)(P = .042). A shorter duration of illness was associated with a higher RR of relapse (<15 years, RR = 2.8; 95% CI: 2.1 to 3.7; I2 0% vs >15 years RR = 1.8; 95% CI: 1.3 to 2.5; I2 0%) (P = .038). A follow-up of less than 16 weeks was associated with higher RR of relapse (RR = 4.7; 95% CI: 2.8 to 7.9; I2 0%) than a longer follow-up (RR = 2.3; 95% CI: 1.9 to 2.7; I2 0%) (P = .016). Relapse defined on the basis of clinical judgment was also associated with a higher RR of relapse (RR = 3.5; 95% CI: 2.5 to 4.9; I2 0%) than when relapse was assessed with rating scales (RR = 2.2; 95% CI: 2.1 to 3.1; I2 0%) (P = .026). Gender, inpatient or outpatient status, LAI or oral antipsychotics, and blinding did not affect the RR of relapse in discontinuation studies.
Gender, Inpatients, or Outpatients, LAI or Oral Antipsychotics
The different subgroup analyses of males (there was 1 study with females only), inpatients/outpatients, and patients prescribed LAI or oral antipsychotics are presented in Supplementary Table 4. Most results are in line with the results for all studies and those for the dose reduction or discontinuation subgroups. Still, some results are worth mentioning. The end-dose analyses did not find a significant difference in risk of relapse between end doses above 3 mg or 5 mg HE/day and maintenance treatment. The risk of relapse was not significantly different from that of maintenance therapy if doses were reduced over 10 weeks or longer, whereas there was a significant difference when doses were reduced over 10 weeks or less.
In inpatients a short duration of illness affected the RR of relapse (0–10 years: RR = 5.1; 95% CI: 2.2 to 11.9; I2 54%; 11–15 years: RR = 2.2; 95% CI: 1.1 to 4.3; I2 23%; more than 15 years: RR = 1.5; 95% CI: 1.2 to 1.9; I2 0%) (P = .015). In patients on oral antipsychotics, a follow-up of 26 weeks or less was associated with a higher risk of relapse (RR = 3.3; 95% CI: 2.4 to 4.6; I2 0%) than a follow-up longer than 26 weeks (RR = 2.0; 95% CI: 1.5 to 2.6; I2 0%) (P = .011). In patients on LAI antipsychotics, the risk of relapse was by and large not different from that of maintenance treatment.
Discussion
The current study is an extensive meta-analysis of RR factors for psychotic relapse following dose reduction or discontinuation of antipsychotics in patients with chronic schizophrenia. We found that the discontinuation of antipsychotics and certain dose reduction characteristics substantially influenced the risk of psychotic relapse. Discontinuation of antipsychotic medication almost doubled the risk of relapse compared with dose reduction. This is in line with register studies and meta-analyses of dose reduction and discontinuation studies.22,23,87–89 Previous uncontrolled cohort studies did not find an increased risk of relapse after antipsychotics were discontinued, which might be explained by the inclusion of relatively stable patients who would be less likely to relapse.90
End Dose
We found evidence to suggest that there is a threshold dose of antipsychotics above which patients are better protected against relapse. This dose is 3–5 mg HE/day for most patients, although there are interindividual differences. The greatest difference in RR was seen with doses lower and higher than 3 mg HE/day. Dose reduction to an end dose higher than 3 mg HE/day resulted in a substantially lower RR of relapse than with a lower end dose. In fact, the risk of relapse with a higher end dose (>3 mg HE/day) was comparable to that of maintenance treatment. This is in line with earlier studies.23,34,89 Our previous meta-analysis of cohort studies found a threshold around 5 mg HE/day.22 In a recent, methodologically different, meta-analysis of relapse rates in dose-response studies, prevention of relapse increased up to 5 mg/day risperidone equivalent, which is comparable with 5 mg HE/day.34 Higher doses conferred little further advantage in reducing the relative relapse rate, while lower doses increased the relative relapse rate in a hyperbolic way.34 In their meta-analysis of patients with schizophrenia in stable remission, Leucht et al. found that risperidone in doses higher than 2.5 mg provided better protection against relapse than in lower doses.34 This suggests that the condition of patients with an optimal response to antipsychotics can remain stable on a relatively low dose. For first-generation antipsychotics, this effect could be seen with doses of 3 mg HE/day or higher.34 Most of the studies included in our analyses concerned first-generation antipsychotics (90%). Another recent meta-analysis of RCTs of different maintenance doses compared standard, low, and very low doses. The risk of relapse was higher (+44%) with low daily doses (4–8 mg HE) and very low doses (<4 mg HE; +72%) than with a standard daily dose (8 mg HE).35 The most recent network meta-analysis comparing continuing, reducing, switching, and discontinuing antipsychotics in patients with chronic psychosis found that fewer relapses occurred with continuing or switching at standard doses and more relapses when doses were lower than the standard dose or the drug was discontinued.91 Taken together, findings suggest that an optimal maintenance dose of antipsychotics is 3–5 mg HE/day in patients with chronic schizophrenia. Lower doses, or no antipsychotics, are associated with an increased risk of relapse. Nevertheless, it should be borne in mind that the finding of a threshold might be an artifact due to the manner in which the drug dose was reduced or stopped in these studies. Other authors have already advocated the strategy of gradual dose reduction, pointing out that gradual dose reduction can prevent withdrawal-related relapse.92 The idea that it is the process of reducing or discontinuing antipsychotics, rather than the actual dose used, that increases the risk of relapse, has gained traction in recent years. In this respect, it is worth mentioning that the dose-related pattern of relapse risk is hyperbolic, matching that for antipsychotic receptor occupancy, which strongly suggests that the risk of relapse corresponds to rate of change in receptor occupancy.34 This implies that the risk of relapse is not only associated with the underlying health problem but also with the effect of drug withdrawal, which complicates the interpretation of findings from dose reduction or discontinuation trials.93 Fast reduction of the dose of antipsychotics can cause withdrawal symptoms, such as anxiety, agitation, and insomnia, symptoms which may be mistaken for those of relapse of the underlying condition. Moreover, the experience of withdrawal effects might itself provoke psychotic relapse, especially when withdrawal is not gradual enough.93,94 Somatic and psychiatric adverse events emerged 4 weeks after antipsychotic withdrawal, particularly after longer duration of treatment.95 Furthermore, there is evidence that antipsychotic withdrawal in itself may be psychotogenic, as suggested by the effect of stopping antipsychotic-like drugs in formerly nonpsychotic patients or when clozapine is discontinued abruptly.93
The abovementioned mechanisms can be caused by rebound effects, which is consistent with the concept that neuro-adaptation occurs in various receptor systems during treatment; adaptation may take months or even years to resolve.94 Dose reduction should therefore be gradual enough to negate the upregulation of density and sensitivity of receptors.94–96 In other words, the process of dose reduction may have a larger impact on the risk of relapse than the end dose of antipsychotics.
Duration of Dose Reduction
Continuation of medication and gradual reduction both played a role in preventing relapse. The observation that a gradual withdrawal of medication was associated with a lower risk of relapse was mainly due to the effect of reducing doses over a period longer than 10 weeks. This is consistent with an additional analysis of Leucht et al., in which doses were gradually tapered to the lowest effective dose.97 Gradual dose reduction may enable lower-end doses to be achieved. In this respect, it is worth mentioning that, with dose reduction, LAI antipsychotics were protective against relapse when compared with oral antipsychotics. This is consistent with earlier findings, in which relapse occurred earlier and more often with reduction of oral (short half-life) versus reduction of LAI antipsychotics. The same was true for different LAI antipsychotics, comparing a once-monthly LAI, with a relatively short half-life, with a once-every-3-months LAI.98 This suggests that gradual reduction, which is inherent to LAI medication dose reduction, and a long reduction time protect against relapse.
We included only 6 studies in which the dose of antipsychotics was reduced over 10 weeks or longer, which limits the power to draw firm conclusions. None of these studies were discontinuation studies, and in a further survey the end dose in these studies turned out to be relatively high (>5 mg HE/day).
Other Outcomes
The risk of relapse appeared to be higher when reducing the dose of oral antipsychotics than when reducing the dose of LAI antipsychotics. This is probably because of the inherently gradual and slower reduction with LAI reduction. At the same time, the lower risk of relapse with LAI may also indicate better adherence to treatment with these drugs.34,98
The finding that the RR of relapse was significantly higher in young patients and in patients with a short duration of illness underlines the need to take these factors into account when clinicians consider antipsychotic dose reduction or discontinuation.
While end dose and duration of dose reduction were risk factors for relapse overall and in dose-reduction studies, various factors were associated with the risk of relapse in studies in which antipsychotic medication was stopped. We speculate that potential risk factors become stronger if antipsychotics are discontinued, because discontinuation causes greater disruption of a stable equilibrium in receptor occupancy after years of antipsychotic therapy than dose reduction, and might give rise to greater withdrawal effects. This is in line with our finding that the RR of relapse was higher in discontinuation studies than in dose reduction studies.
The risk of relapse was high in discontinuation studies with a short follow-up (less than 16 weeks), which suggests that most relapses occur relatively soon after medication discontinuation and that relapses are associated with withdrawal symptoms. Young patients and patients with a short duration of illness who stopped their medication were at the highest risk of relapse, which is in line with previous findings.22,23 A possible explanation for the lower risk of relapse in older patients is the decline in hyperdopaminergic functioning with age, with a decrease in dopaminergic receptor availability.99,100 In studies in which medication was stopped, clinical judgment rather than objective testing was associated with the highest risk of relapse. This might be explained by expectation bias on the part of clinicians.
Adverse Events and Relapse Risk
In this meta-analysis, we found evidence that withdrawal effects might play a role in relapse and therefore might have influenced the results. First, a short follow-up was associated with a higher RR of relapse, suggesting that relapse occurs more often shortly after dose reduction, while there is no reason to expect this because relapses normally occur divided over time.101 Second, there were fewer relapses when the antipsychotic dose was reduced slowly. However, it should be borne in mind there were no studies with a long duration of reduction and only 6 with a reduction duration longer than 10 weeks, and these studies had a high-end dose. Still, it is possible that a more gradual dose reduction causes fewer relapses, even when the dose is reduced to less than 3–5 mg HE/day. Third, that drug withdrawal may play a role in relapses is supported by the finding that reducing the dose of LAI antipsychotics, it itself gradual, was associated with fewer relapses than reducing the dose of oral antipsychotics. A study has been started to investigate the effect of tapering antipsychotic medication over a long period of time, possibly even more than 1 year, in patients with schizophrenia (not first episode).102 Fourth, the risk of relapse is higher with drug discontinuation than with dose reduction, which can be explained by discontinuation causing a greater disruption of receptor equilibrium, what may indicate withdrawal. This is in line with other studies where relapse rates with (very) low doses increase steeply,34 similar to the inverse hyperbolic pattern of dopamine receptor occupancy with lowest doses (below 2.5–5 mg HE/day).103
Strength and Limitations
We carried out an extensive database search covering several decennia to produce a comprehensive overview of RCTs of risk factors for psychotic relapse in chronic schizophrenia and supplemented the search by screening reference lists from other publications and adding relevant publications, resulting in a relatively large number of RCTs. We controlled for confounding and heterogeneity by carrying out a number of sensitivity analyses. Overall heterogeneity was low in most analyses, which makes findings more reliable and robust. Funnel plots showed a scattered distribution of rate ratios, indicating that the likelihood of publication bias was low. The differences in risk of relapse we found between studies of dose reduction and dose discontinuation, studies with male patients only, studies with male and female patients, and studies of oral medication and LAI medication support our initial choice to analyze these groups of studies separately.
Several limitations should be considered when interpreting the results. This is a meta-analysis of trials that were not set up to identify risk factors for relapse or to find the best way to perform dose reduction. This limitation should be kept in mind when interpreting our findings. Although comparisons were statistically significant, some comparisons between RRs had overlapping 95% CIs, which is acceptable but limits the power of comparisons.
Bias by indication can affect RRs. The selection of patients for dose reduction trials may be affected by the stability of their mental condition, and stabilized patients may be included more often than unstable patients. In addition, caregivers and patients might refrain from participating in a dose reduction trial, because they do not want to change the patient’s stable condition. The use of a dose reduction protocol with more intense monitoring of patients may have induced performance bias (more close monitoring and expectation to find relapse may influence relapse rates).
Conventional antipsychotics were prescribed in most studies, whereas clozapine was not used in any of the studies, which limits the generalizability of findings. Multiple subgroup analyses might result in false negative and false positive significance tests that increase in likelihood as more subgroup analyses are performed. We used the criteria for relapse used by the authors of studies, but there are several definitions in use, which limits study comparison and the drawing of practical implications of findings.104 Residual confounding cannot be ruled out. In conclusion, we identified a number of RR factors for psychotic relapse when doses were reduced or discontinued in patients with schizophrenia with a long duration of illness and treated with antipsychotics for several years. We found the end dose to be an important factor in relapse occurrence. Stopping the medication or reducing the dose to less than 3–5 mg HE/day increased the risk of relapse. However, there are many, not mutually exclusive, explanations for this finding. That said, a relatively fast reduction in antipsychotic dose, which was the case in most of the included studies, might have had a pronounced influence by inducing relapse or relapse-like symptoms that can be ascribed to the effect of antipsychotic withdrawal rather than to the underlying psychosis vulnerability of the patient. Other factors that increased the risk of relapse were younger age, shorter duration of illness, and short follow-up time. Reducing the dose of LAI antipsychotics was associated with a lower relapse rate than reducing the dose of oral medication, such that the relapse rate of the former was often not significantly different from that of maintenance treatment.
Patients tend to be ambivalent about using antipsychotics long-term. We need to discuss with patients or their caregivers the potential negative effects of long-term antipsychotic use, balancing these effects against the increased risk of relapse when doses of antipsychotic medication are reduced too quickly or by too much. In this way, shared treatment plans can be made as safe as possible.
Implications for Practice
Our data suggest that the dose of antipsychotic medication should not be reduced to less than 3–5 mg HE/day in patients with chronic schizophrenia, although it might be possible to lower the dose further while avoiding relapse if the dose is tapered carefully and (very) slowly, preferably over more than 10 weeks, and probably longer, considering the hyperbolic pharmacology of antipsychotics. Arguments not to reduce doses to below 3–5 mg HE may be outweighed by other considerations, such as side effects or patient preference. Gradual tapering might prevent withdrawal symptoms, which might be perceived as a psychotic relapse.94,96 Patients should be closely monitored after every dose reduction step.
Supplementary Material
Contributor Information
Jan P A M Bogers, High Care Clinics and Rivierduinen Academy, Mental Health Services Rivierduinen, Leiden, The Netherlands.
George Hambarian, Mental Health Services Rivierduinen, Leiden, The Netherlands.
Niels Walburgh Schmidt, Mental Health Services Rivierduinen, Leiden, The Netherlands.
Jentien M Vermeulen, Department of Psychiatry, Amsterdam University Medical Center, Amsterdam, The Netherlands.
Lieuwe de Haan, Department of Psychiatry, Amsterdam University Medical Center, Amsterdam, The Netherlands.
Conflict of Interest
The authors report no financial interests or conflicts of interest.
References
- 1. Leucht S, Tardy M, Komossa K, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379: 2063–2071. [DOI] [PubMed] [Google Scholar]
- 2. Schneider-Thoma J, Chalkou K, Dörries C, et al. Comparative efficacy and tolerability of 32 oral and long-acting injectable antipsychotics for the maintenance treatment of adults with schizophrenia: a systematic review and network meta-analysis. Lancet. 2022;399:824–836. [DOI] [PubMed] [Google Scholar]
- 3. Shimomura Y, Kikuchi Y, Suzuki T, Uchida H, Mimura M, Takeuchi H.. Antipsychotic treatment in the maintenance phase of schizophrenia: an updated systematic review of the guidelines and algorithms. Schizophr Res. 2020;215:8–16. [DOI] [PubMed] [Google Scholar]
- 4. Read J, Williams JR.. Positive and negative effects of antipsychotic medication: an international online survey of 832 recipients. Curr Drug Saf. 2019;14:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bjornestad J, Lavik KO, Davidson L, Hjeltnes A, Moltu C, Veseth M.. Antipsychotic treatment—a systematic literature review and meta-analysis of qualitative studies. J Ment Health. 2020;29:513–523. [DOI] [PubMed] [Google Scholar]
- 6. Yoshida K, Takeuchi H.. Dose-dependent effects of antipsychotics on efficacy and adverse effects in schizophrenia. Behav Brain Res. 2021;402:113098. doi: 10.1016/j.bbr.2020.113098. [DOI] [PubMed] [Google Scholar]
- 7. Ray WA, Chung CP, Murray KT, Hall K, Stein CM.. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360:225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Andreasen NC, Liu D, Ziebell S, Vora A, Ho BC.. Relapse duration, treatment intensity, and brain tissue loss in schizophrenia: a prospective longitudinal MRI study. Am J Psychiatry. 2013;170:609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gallego JA, Nielsen J, De Hert M, Kane JM, Correll CU.. Safety and tolerability of antipsychotic polypharmacy. Expert Opin Drug Saf. 2012;11:527–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Longdon E, Read J.. “People with problems, not patients with illnesses”: using psychosocial frameworks to reduce the stigma of psychosis. Isr J Psychiatry Relat Sci 2017;54: 24–28. [PubMed] [Google Scholar]
- 11. Wang Y, Chan RCK, Shum DHK.. Schizophrenia and prospective memory impairments: a review. Clin Neuropsychol. 2018;32:836–857. [DOI] [PubMed] [Google Scholar]
- 12. Stroup TS, Gray N.. Management of common adverse effects of antipsychotic medications. World Psychiatry. 2018;17: 341–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harrow M, Jobe TH, Faull RN.. Do all schizophrenia patients need antipsychotic treatment continuously throughout their lifetime? A 20-year longitudinal study. Psychol Med. 2012;42:2145–2155. [DOI] [PubMed] [Google Scholar]
- 14. Moilanen J, Haapea M, Miettunen J, et al. Characteristics of subjects with schizophrenia spectrum disorder with and without antipsychotic medication. A 10-year follow-up of the Northern Finland 1966 Birth Cohort Study. Eur Psychiatry. 2013;28:53–58. [DOI] [PubMed] [Google Scholar]
- 15. Wunderink L, Nieboer RM, Wiersma D, Sytema S, Nienhuis FJ.. Recovery in remitted first-episode psychosis at 7 years of follow-up of an early dose reduction/discontinuation or maintenance treatment strategy: long-term follow-up of a 2-year randomized clinical trial. JAMA Psychiatry. 2013;70: 913–920. [DOI] [PubMed] [Google Scholar]
- 16. Harrow M, Jobe TH, Faull RN.. Does treatment of schizophrenia with antipsychotic medications eliminate or reduce psychosis? A 20-year multi-follow-up study. Psychol Med. 2014;44:3007–3016. [DOI] [PubMed] [Google Scholar]
- 17. Tandon R, Nasrallah HA, Keshavan MS.. Schizophrenia, “just the facts” 4. Clinical features and conceptualization. Schizophr Res. 2009;110:1–23. [DOI] [PubMed] [Google Scholar]
- 18. Millan MJ, Andrieux A, Bartzokis G, et al. Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov. 2016;15:485–515. [DOI] [PubMed] [Google Scholar]
- 19. Fusar-Poli P, McGorry PD, Kane JM.. Improving outcomes of first-episode psychosis: an overview. World Psychiatry. 2017;16:251–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gaebel W, Riesbeck M.. Are there clinically useful predictors and early warning signs for pending relapse? Schizophr Res. 2014;152:469–477. [DOI] [PubMed] [Google Scholar]
- 21. Taipale H, Tanskanen A, Correll CU, Tiihonen J.. Real world effectiveness of antipsychotic doses for relapse prevention in patients with first-episode schizophrenia in Finland: a nationwide, register-based cohort study. Lancet Psychiatry. 2022;9:271–279. [DOI] [PubMed] [Google Scholar]
- 22. Bogers JPAM, Hambarian G, Michiels M, Vermeulen J, de Haan L.. Risk factors for psychotic relapse after dose reduction or discontinuation of antipsychotics in patients with chronic schizophrenia: a systematic review and meta-analysis. Schizophr Bull Open. 2020;1(1). doi: 10.1093/schizbullopen/sgaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tani H, Takasu S, Uchida H, Suzuki T, Mimura M, Takeuchi H.. Factors associated with successful antipsychotics dose reduction in schizophrenia: a systematic review of prospective clinical trials and meta-analysis of randomized controlled trials. Neuropsychopharmacology. 2020;45:887–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lally J, MacCabe JH.. Antipsychotic medication in schizophrenia: a review. Br Med Bull. 2015;114:169–179. [DOI] [PubMed] [Google Scholar]
- 25. Häfner H. Gender differences in schizophrenia. Psychoneuroendocrinology. 2003;28(suppl2):17–54. [DOI] [PubMed] [Google Scholar]
- 26. Seeman MV. Does gender influence outcome in schizophrenia? Psychiatr Q. 2019;90:173–184. [DOI] [PubMed] [Google Scholar]
- 27. Kasckowa JW, Twamleyc E, Mulchahey JJ, et al. Health-related quality of well-being in chronically hospitalized patients with schizophrenia: comparison with matched outpatients. Psychiatr Res. 2001;103:69–78. [DOI] [PubMed] [Google Scholar]
- 28. Sugai T, Suzuki Y, Yamazaki M, et al. Difference in prevalence of metabolic syndrome between Japanese outpatients and inpatients with schizophrenia: a nationwide survey. Schizophr Res. 2016;171:68–73. [DOI] [PubMed] [Google Scholar]
- 29. Nasrallah HA. The case for long-acting antipsychotic agents in the post-CATIE era. Acta Psychiatr Scand. 2007;115: 260–267. [DOI] [PubMed] [Google Scholar]
- 30. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P.. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Higgins JPT, Savović J, Page MJ, et al. Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (online handbook; updated February 2021). [Google Scholar]
- 32. Borenstein M, Hedges LV, Higgins JPT, et al. Introduction to Meta-Analysis. Chichester, UK: John Wiley & Sons Ltd; 2009. [Google Scholar]
- 33. Cook A, Sheikh A.. Hypothesis testing. Prim Care Respir J. 2000;8:16–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leucht S, Bauer S, Med C, et al. Examination of dosing of antipsychotic drugs for relapse prevention in patients with stable schizophrenia: a meta-analysis. JAMA Psychiatry. 2021;78:1238–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Højlund M, Kemp AF, Haddad PM, Neill JC, Correll CU.. Standard versus reduced dose of antipsychotics for relapse prevention in multi-episode schizophrenia: a systematic review and meta-analysis of randomised controlled trials. Lancet Psychiatry. 2021;8:471–486. [DOI] [PubMed] [Google Scholar]
- 36. Higgins JP, Thompson SG.. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 37. Higgins JP, Thompson SG, Deeks JJ, Altman DG.. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Diamond LS, Marks JB.. Discontinuance of tranquilizers among chronic schizophrenic patients receiving maintenance dosage. J Nerv Ment Dis. 1960;131:247–251. [DOI] [PubMed] [Google Scholar]
- 39. Blackburn HL, Allen JL.. Behavioral effects of interrupting and resuming tranquilizing medication among schizophrenics. J Nerv Ment Dis. 1961;133:303–308. [DOI] [PubMed] [Google Scholar]
- 40. Freeman LS, Alson E.. Prolonged withdrawal of chlorpromazine in chronic patients. Dis Nerv Syst. 1962;23:522–525. [PubMed] [Google Scholar]
- 41. Whittaker CB, Hoy RM.. Withdrawal of perphenazine in chronic schizophrenia. Br J Psychiatry. 1963;109:422–427. [DOI] [PubMed] [Google Scholar]
- 42. Marjerrison G, Irvine D, Stewart CN, Williams R, Matheu H, Demay M.. Withdrawal of long-term phenothiazines from chronically hospitalized psychiatric patients. Can Psychiatr Assoc J. 1964;9:290–298. [DOI] [PubMed] [Google Scholar]
- 43. Caffey EM, Jr., Diamond LS, Frank TV, et al. Discontinuation or reduction of chemotherapy in chronic schizophrenics. J Chronic Dis. 1964;17:347–358. [DOI] [PubMed] [Google Scholar]
- 44. Melnyk WT, Worthington AG, Laverty SG.. Abrupt withdrawal of chlorpromazine and thioridazine from schizophrenic inpatients. Can Psychiatr Assoc J. 1966;11:410–413. [Google Scholar]
- 45. Garfield SL, Gershon S, Sletten I, Neubauer H, Ferrel E.. Withdrawal of ataractic medication in schizophrenic patients. Dis Nerv Syst. 1966;27:321–325. [PubMed] [Google Scholar]
- 46. Morton MA. A study of the withdrawal of chlorpromazine or trifluoperazine in chronic schizophrenia. Am J Psychiatry. 1968;124:1585–1588. [DOI] [PubMed] [Google Scholar]
- 47. Rassidakis NC, Kondakis X, Papanastassiou A, Michalakeas A.. Withdrawal of antipsychotic drugs from chronic psychiatric patients. Bull Menninger Clin. 1970;34:216–222. [PubMed] [Google Scholar]
- 48. Baro F, Brugmans J, Dom R, Van Lommel R.. Maintenance therapy of chronic psychotic patients with a weekly oral dose of R 16341. A controlled double-blind study. J Clin Pharmacol. 1970;10:330–341. [DOI] [PubMed] [Google Scholar]
- 49. Hershon HI, Kennedy PF, McGuire RJ.. Persistence of extra-pyramidal disorders and psychiatric relapse after withdrawal of long-term phenothiazine therapy. Br J Psychiatry. 1972;120:41–50. [DOI] [PubMed] [Google Scholar]
- 50. Ota KY, Kurland AA.. A double-blind comparison of haloperidol oral concentrate, haloperidol solutabs and placebo in the treatment of chronic schizophrenia. J Clin Pharmacol. 1973;13:99–110. [DOI] [PubMed] [Google Scholar]
- 51. Andrews P, Hall JN, Snaith RP.. A controlled trial of phenothiazine withdrawal in chronic schizophrenic patients. Br J Psychiatry. 1976;128:451–455. [DOI] [PubMed] [Google Scholar]
- 52. Lonowski DJ, Sterling FE, Kennedy JC.. Gradual reduction of neuroleptic drugs among chronic schizophrenics. A double-blind controlled study. Acta Psychiatr Scand. 1978;57:97–102. [DOI] [PubMed] [Google Scholar]
- 53. Levine J, Schooler NR, Severe J, et al. Discontinuation of oral and depot fluphenazine in schizophrenic patients after one year of continuous medication: a controlled study. Adv Biochem Psychopharmacol. 1980;24:483–493. [PubMed] [Google Scholar]
- 54. Lehmann E, Quadbeck H, Tegeler J, Fararuni M, Heinrich K.. Drug-response differences of high and standard dosage of fluphenazine-decanoate in relation to schizophrenic symptoms. Pharmakopsychiatr Neuropsychopharmakol. 1980;13: 117–129. [DOI] [PubMed] [Google Scholar]
- 55. Cheung HK. Schizophrenics fully remitted on neuroleptics for 3-5 years - to stop or continue drugs? Br J Psychiatry. 1981;138:490–494. [DOI] [PubMed] [Google Scholar]
- 56. Odejide OA, Aderounmu AF.. Double-blind placebo substitution: withdrawal of fluphenazine decanoate in schizophrenic patients. J Clin Psychiatry. 1982;43:195–196. [PubMed] [Google Scholar]
- 57. Lehmann HE, Wilson WH, Deutsch M.. Minimal maintenance medication: effects of three dose schedules on relapse rates and symptoms in chronic schizophrenic outpatients. Compr Psychiatry. 1983;24:293–303. [DOI] [PubMed] [Google Scholar]
- 58. Johnson DA, Pasterski G, Ludlow JM, Street K, Taylor RD.. The discontinuance of maintenance neuroleptic therapy in chronic schizophrenic patients: drug and social consequences. Acta Psychiatr Scand. 1983;67:339–352. [DOI] [PubMed] [Google Scholar]
- 59. Kane JM. Low dose medication strategies in the maintenance treatment of schizophrenia. Schizophr Bull. 1983;9: 528–532. [DOI] [PubMed] [Google Scholar]
- 60. Wistedt B, Ranta J.. Comparative double-blind study of flupenthixol decanoate and fluphenazine decanoate in the treatment of patients relapsing in a schizophrenic symptomatology. Acta Psychiatr Scand. 1983;67:378–388. [DOI] [PubMed] [Google Scholar]
- 61. Channabasavanna SM, Michael A.. Penfluridol maintenance therapy in schizophrenia: a controlled study. Indian J Psychiatry. 1987;29:333–336. [PMC free article] [PubMed] [Google Scholar]
- 62. Cookson IB. The effects of a 50% reduction of cis(z)-flupenthixol decanoate in chronic schizophrenic patients maintained on a high dose regime. Int Clin Psychopharmacol. 1987;2:141–149. [DOI] [PubMed] [Google Scholar]
- 63. Johnson DA, Ludlow JM, Street K, Taylor RD.. Double-blind comparison of half-dose and standard-dose flupenthixol decanoate in the maintenance treatment of stabilised out-patients with schizophrenia. Br J Psychiatry. 1987;151: 634–638. [DOI] [PubMed] [Google Scholar]
- 64. Hogarty GE, McEvoy JP, Munetz M, et al. Dose of fluphenazine, familial expressed emotion, and outcome in schizophrenia. Results of a two-year controlled study. Arch Gen Psychiatry. 1988;45:797–805. [DOI] [PubMed] [Google Scholar]
- 65. Faraone SV, Green AI, Brown W, Yin P, Tsuang MT.. Neuroleptic dose reduction in persistently psychotic patients. Hosp Community Psychiatry. 1989;40:1193–1195. [DOI] [PubMed] [Google Scholar]
- 66. Sampath G, Shah A, Krska J, Soni SD.. Neuroleptic discontinuation in the very stable schizophrenic patient: relapse rates and serum neuroleptic levels. Hum Psychopharmacol. 1992;7:355–364. [Google Scholar]
- 67. Inderbitzin LB, Lewine RR, Scheller-Gilkey G, et al. A double-blind dose-reduction trial of fluphenazine decanoate for chronic, unstable schizophrenic patients. Am J Psychiatry. 1994;151:1753–1759. [DOI] [PubMed] [Google Scholar]
- 68. Hogarty GE, McEvoy JP, Ulrich RF, et al. Pharmacotherapy of impaired affect in recovering schizophrenic patients. Arch Gen Psychiatry. 1995;52:29. [DOI] [PubMed] [Google Scholar]
- 69. Huttunen MO, Tuhkanen H, Haavisto E, et al. Low- and standard-dose depot haloperidol combined with targeted oral neuroleptics. Psychiatr Serv. 1996;47:83–85. [DOI] [PubMed] [Google Scholar]
- 70. Hirschowitz J, Hitzemann R, Piscani K, et al. The dose reduction in schizophrenia (DORIS) study: a final report. Schizophr Res. 1997;23:31–43. [DOI] [PubMed] [Google Scholar]
- 71. Schooler NR, Keith SJ, Severe JB, et al. Relapse and rehospitalization during maintenance treatment of schizophrenia. The effects of dose reduction and family treatment. Arch Gen Psychiatry. 1997;54:453–463. [DOI] [PubMed] [Google Scholar]
- 72. Carpenter WT, Jr., Buchanan RW, Kirkpatrick B, Lann HD, Breier AF, Summerfelt AT.. Comparative effectiveness of fluphenazine decanoate injections every 2 weeks versus every 6 weeks. Am J Psychiatry. 1999;156:412–418. [DOI] [PubMed] [Google Scholar]
- 73. Volavka J, Cooper TB, Czobor P, et al. High-dose treatment with haloperidol: the effect of dose reduction. J Clin Psychopharmacol. 2000;20:252–256. [DOI] [PubMed] [Google Scholar]
- 74. Arato M, O’Connor R, Meltzer HY, Group ZS.. A 1-year, double-blind, placebo-controlled trial of ziprasidone 40, 80 and 160 mg/day in chronic schizophrenia: the ziprasidone extended use in schizophrenia (ZEUS) study. Int Clin Psychopharmacol. 2002;17:207–215. [DOI] [PubMed] [Google Scholar]
- 75. Khazaie H, Shakeri J.. Comparative efficacy of every 2 weeks versus every 6 weeks injections of fluphenazine decanoate. Arch Iranian Med. 2005;8:109–114. [Google Scholar]
- 76. Uchida H, Suzuki T, Yamazawa R, et al. Reducing the dose of antipsychotic agents ameliorates visual hypersensitivity attack. An ideal treatment option in terms of the adverse effect. J Clin Psychopharmacol. 2006;26:50–55. [DOI] [PubMed] [Google Scholar]
- 77. Kramer M, Simpson G, Maciulis V, et al. Paliperidone extended-release tablets for prevention of symptom recurrence in patients with schizophrenia: a randomised, double-blind, placebocontrolled study. J Clin Psychopharmacol. 2007;27: 6–14. [DOI] [PubMed] [Google Scholar]
- 78. Rouillon F, Chartier F, Gasquet I.. Strategies of treatment with olanzapine in schizophrenic patients during stable phase: results of a pilot study. Eur Neuropsychopharmacol. 2008;18:646–652. [DOI] [PubMed] [Google Scholar]
- 79. Hough D, Gopal S, Vijapukar U, Lim P, Morozova M, Eerdekens M.. Paliperidone palmitate in prevention of symptom recurrence in patients with schizophrenia: a randomised, double-blind, placebo-controlled study. Proceedings of the 161st Annual Meeting of the American Psychiatric Association; 2008 May 3–8, Washington DC, USA.
- 80. Kane JM, Detke HC, Naber D, et al. Olanzapine long-acting injection: a 24-week, randomized, double-blind trial of maintenance treatment in patients with schizophrenia. Am J Psychiatry. 2010;167:181–189. [DOI] [PubMed] [Google Scholar]
- 81. Wang CY, Xiang YT, Cai ZJ, et al. Risperidone maintenance treatment in schizophrenia: a randomized, controlled trial. Am J Psychiatry. 2010;167:676–685. [DOI] [PubMed] [Google Scholar]
- 82. Takeuchi H, Suzuki T, Remington G, et al. Effects of risperidone and olanzapine dose reduction on cognitive function in stable patients with schizophrenia: an open-label, randomized, controlled, pilot study. Schizophr Bull. 2013;39:993–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yamanouchi Y, Sukegawa T, Inagaki A, et al. Evaluation of the individual safe correction of antipsychotic agent polypharmacy in Japanese patients with chronic schizophrenia: validation of safe corrections for antipsychotic polypharmacy and the high-dose method. Int J Neuropsychopharmacol. 2014;18:pyu016. doi: 10.1093/ijnp/pyu016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhou Y, Li G, Li D, Cui H, Ning Y.. Dose reduction of risperidone and olanzapine can improve cognitive function and negative symptoms in stable schizophrenic patients: a single-blinded, 52-week, randomized controlled study. J Psychopharmacol. 2018;32:524–532. [DOI] [PubMed] [Google Scholar]
- 85. Ozawa C, Bies RR, Pillai N, Suzuki T, Mimura M, Uchida H.. Model-guided antipsychotic dose reduction in schizophrenia: a pilot, single-blind randomized controlled trial. J Clin Psychopharmacol. 2019;39:329–335. [DOI] [PubMed] [Google Scholar]
- 86. Huhn M, Leucht C, Rothe P, et al. Reducing antipsychotic drugs in stable patients with chronic schizophrenia or schizoaffective disorder: a randomized controlled pilot trial. Eur Arch Psychiatry Clin Neurosci. 2021;271:293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tiihonen J, Mittendorfer-Rutz E, Torniainen M, Alexanderson K, Tanskanen A.. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am J Psychiatry. 2016;173:600–606. [DOI] [PubMed] [Google Scholar]
- 88. Tiihonen J, Mittendorfer-Rutz E, Majak M, et al. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29823 patients with schizophrenia. JAMA Psychiatry. 2017;74:686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. De Hert M, Sermon J, Geerts P, Vansteelandt K, Peuskens J, Detraux J.. The use of continuous treatment versus placebo or intermittent treatment strategies in stabilized patients with schizophrenia: a systematic review and meta-analysis of randomized controlled trials with first- and second-generation antipsychotics. CNS Drugs. 2015;29:637–658. [DOI] [PubMed] [Google Scholar]
- 90. Correll CU, Rubio JM, Kane JM.. What is the risk-benefit ratio of long-term antipsychotic treatment in people with schizophrenia? World Psychiatry. 2018;17:149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ostuzzi G, Vita G, Bertolini F, et al. Continuing, reducing, switching, or stopping antipsychotics in individuals with schizophrenia-spectrum disorders who are clinically stable: a systematic review and network meta-analysis. Lancet Psychiatry. 2022;9:614–624. doi: 10.1016/S2215-0366(22)00158-4. [DOI] [PubMed] [Google Scholar]
- 92. Horowitz MA, Murray RM, Taylor D.. Confounding of antipsychotic discontinuation studies by withdrawal-related relapse. Schizophr Bull. 2022;48:294–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Moncrieff J. Antipsychotic maintenance treatment: time to rethink? PLoS Med. 2015;12:e1001861. doi: 10.1371/journal.pmed.10011861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Horowitz MA, Jauhar S, Natesan S, Murray RM, Taylor D.. A method for tapering antipsychotic treatment that may minimize the risk of relapse. Schizophr Bull. 2021;47:1116–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Brandt L, Schneider-Thoma J, Siafis S, et al. Adverse events after antipsychotic discontinuation: an individual participant data meta-analysis. Lancet Psychiatry. 2022;9:232–242. [DOI] [PubMed] [Google Scholar]
- 96. Horowitz MA, Moncrieff J, De Haan L, et al. Tapering antipsychotic medication: practical considerations. Psychol Med. 2021. doi: 10.1017/S0033291721003299. [DOI] [PubMed] [Google Scholar]
- 97. Leucht S, Siafis S, Davis JM.. Comment & Response to Højlund and colleagues. JAMA Psychiatry. 2021;177:e1–e2. [Google Scholar]
- 98. Weiden PJ, Kim E, Bermak J, Turkoz I, Gopal S, Berwaerts J.. Does half-life matter after antipsychotic discontinuation? J Clin Psychiatry. 2017;78(7):e813–e820. doi: 10.4088/JCP.16m11308. [DOI] [PubMed] [Google Scholar]
- 99. Matuskey D, Worhunksy P, Correa E, et al. Age-related changes in binding of the D2/3 receptor radioligand [11C](+)PHNO in healthy volunteers. Neuroimage. 2016;130: 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Seamen KL, Smith CT, Juarez EJ, et al. Differential regional decline in dopamine receptor availability across adulthood: linear and nonlinear effects of age. Hum Brain Mapp. 2019;40:3125–3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Johnstone EC, Geddes J.. How high is the relapse rate in schizophrenia? Acta Psychiatr Scand Suppl. 1994;382:6–10. [DOI] [PubMed] [Google Scholar]
- 102. Moncrieff J, Lewis G, Freemantle N, et al. Randomised controlled trial of gradual antipsychotic reduction and discontinuation in people with schizophrenia and related disorders: the RADAR trial (Research into Antipsychotic Discontinuation and Reduction). BMJ Open. 2019;9:e030912. doi: 10.1136/bmjopen-2019-030912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Horowitz MA, Macaulay A, Taylor D.. Limitations in research on maintenance treatment for individuals with schizophrenia. JAMA Psychiatry. 2022;79:83–85. [DOI] [PubMed] [Google Scholar]
- 104. Moncrieff J, Crellin NE, Long MA, Cooper RE, Stockmann T.. Definitions of relapse in trials comparing antipsychotic maintenance with discontinuation or reduction for schizophrenia spectrum disorders: a systematic review. Schizophr Res. 2020;225:47–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.