Abstract
The American Diabetes Association (ADA) “Standards of Care in Diabetes” includes the ADA’s current clinical practice recommendations and is intended to provide the components of diabetes care, general treatment goals and guidelines, and tools to evaluate quality of care. Members of the ADA Professional Practice Committee, a multidisciplinary expert committee, are responsible for updating the Standards of Care annually, or more frequently as warranted. For a detailed description of ADA standards, statements, and reports, as well as the evidence-grading system for ADA’s clinical practice recommendations and a full list of Professional Practice Committee members, please refer to Introduction and Methodology. Readers who wish to comment on the Standards of Care are invited to do so at professional.diabetes.org/SOC.
For prevention and management of diabetes complications in children and adolescents, please refer to Section 14, “Children and Adolescents.”
Chronic Kidney Disease
Screening
Recommendations
11.1a At least annually, urinary albumin (e.g., spot urinary albumin-to-creatinine ratio) and estimated glomerular filtration rate should be assessed in people with type 1 diabetes with duration of ≥5 years and in all people with type 2 diabetes regardless of treatment. B
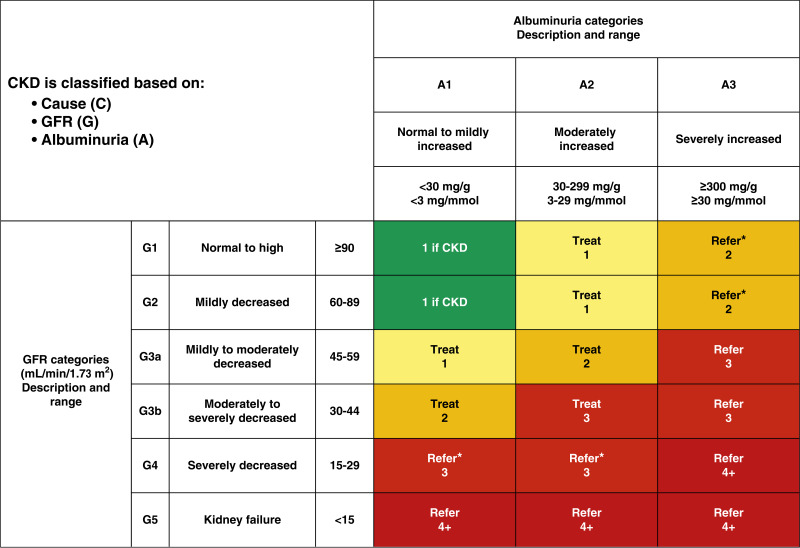
11.1b In people with established diabetic kidney disease, urinary albumin (e.g., spot urinary albumin-to-creatinine ratio) and estimated glomerular filtration rate should be monitored 1–4 times per year depending on the stage of the disease (Fig. 11.1). B
Figure 11.1.
Risk of chronic kidney disease (CKD) progression, frequency of visits, and referral to a nephrologist according to glomerular filtration rate (GFR) and albuminuria. The GFR and albuminuria grid depicts the risk of progression, morbidity, and mortality by color, from best to worst (green, yellow, orange, red, dark red). The numbers in the boxes are a guide to the frequency of visits (number of times per year). Green can reflect CKD with normal estimated GFR and albumin-to-creatinine ratio only in the presence of other markers of kidney damage, such as imaging showing polycystic kidney disease or kidney biopsy abnormalities, with follow-up measurements annually; yellow requires caution and measurements at least once per year; orange requires measurements twice per year; red requires measurements three times per year; and dark red requires measurements four times per year. These are general parameters only, based on expert opinion, and underlying comorbid conditions and disease state, as well as the likelihood of impacting a change in management for any individual patient, must be taken into account. “Refer” indicates that nephrology services are recommended. *Referring clinicians may wish to discuss with their nephrology service, depending on local arrangements regarding treating or referring. Reprinted with permission from Vassalotti et al. (121).
Treatment
Recommendations
11.2 Optimize glucose control to reduce the risk or slow the progression of chronic kidney disease. A
11.3 Optimize blood pressure control and reduce blood pressure variability to reduce the risk or slow the progression of chronic kidney disease. A
11.4a In nonpregnant people with diabetes and hypertension, either an ACE inhibitor or an angiotensin receptor blocker is recommended for those with moderately increased albuminuria (urinary albumin-to-creatinine ratio 30–299 mg/g creatinine) B and is strongly recommended for those with severely increased albuminuria (urinary albumin-to-creatinine ratio ≥300 mg/g creatinine) and/or estimated glomerular filtration rate <60 mL/min/1.73 m2. A
11.4b Periodically monitor serum creatinine and potassium levels for the development of increased creatinine and hyperkalemia when ACE inhibitors, angiotensin receptor blockers, and mineralocorticoid receptor antagonists are used, or hypokalemia when diuretics are used. B
11.4c An ACE inhibitor or an angiotensin receptor blocker is not recommended for the primary prevention of chronic kidney disease in people with diabetes who have normal blood pressure, normal urinary albumin-to-creatinine ratio (<30 mg/g creatinine), and normal estimated glomerular filtration rate. A
11.4d Do not discontinue renin-angiotensin system blockade for increases in serum creatinine (≤30%) in the absence of volume depletion. A
11.5a For people with type 2 diabetes and diabetic kidney disease, use of a sodium–glucose cotransporter 2 inhibitor is recommended to reduce chronic kidney disease progression and cardiovascular events in patients with an estimated glomerular filtration rate ≥20 mL/min/1.73 m2 and urinary albumin ≥200 mg/g creatinine. A
11.5b For people with type 2 diabetes and diabetic kidney disease, use of a sodium–glucose cotransporter 2 inhibitor is recommended to reduce chronic kidney disease progression and cardiovascular events in patients with an estimated glomerular filtration rate ≥20 mL/min/1.73 m2 and urinary albumin ranging from normal to 200 mg/g creatinine. B
11.5c In people with type 2 diabetes and diabetic kidney disease, consider use of sodium–glucose cotransporter 2 inhibitors (if estimated glomerular filtration rate is ≥20 mL/min/1.73 m2), a glucagon-like peptide 1 agonist, or a nonsteroidal mineralocorticoid receptor antagonist (if estimated glomerular filtration rate is ≥25 mL/min/1.73 m2) additionally for cardiovascular risk reduction. A
11.5d In people with chronic kidney disease and albuminuria who are at increased risk for cardiovascular events or chronic kidney disease progression, a nonsteroidal mineralocorticoid receptor antagonist shown to be effective in clinical trials is recommended to reduce chronic kidney disease progression and cardiovascular events. A
11.6 In people with chronic kidney disease who have ≥300 mg/g urinary albumin, a reduction of 30% or greater in mg/g urinary albumin is recommended to slow chronic kidney disease progression. B
11.7 For people with non–dialysis-dependent stage 3 or higher chronic kidney disease, dietary protein intake should be aimed to a target level of 0.8 g/kg body weight per day. A For patients on dialysis, higher levels of dietary protein intake should be considered since protein energy wasting is a major problem in some individuals on dialysis. B
11.8 Patients should be referred for evaluation by a nephrologist if they have continuously increasing urinary albumin levels and/or continuously decreasing estimated glomerular filtration rate and if the estimated glomerular filtration rate is <30 mL/min/1.73 m2. A
11.9 Promptly refer to a nephrologist for uncertainty about the etiology of kidney disease, difficult management issues, and rapidly progressing kidney disease. A
Epidemiology of Diabetes and Chronic Kidney Disease
Chronic kidney disease (CKD) is diagnosed by the persistent elevation of urinary albumin excretion (albuminuria), low estimated glomerular filtration rate (eGFR), or other manifestations of kidney damage (1,2). In this section, the focus is on CKD attributed to diabetes (diabetic kidney disease) in adults, which occurs in 20–40% of people with diabetes (1,3–5). Diabetic kidney disease typically develops after a diabetes duration of 10 years in type 1 diabetes (the most common presentation is 5–15 years after the diagnosis of type 1 diabetes) but may be present at diagnosis of type 2 diabetes. CKD can progress to end-stage renal disease (ESRD) requiring dialysis or kidney transplantation and is the leading cause of ESRD in the U.S. (6). In addition, among people with type 1 or type 2 diabetes, the presence of CKD markedly increases cardiovascular risk and health care costs (7). For details on the management of diabetic kidney disease in children, please see section 14, “Children and Adolescents.”
Assessment of Albuminuria and Estimated Glomerular Filtration Rate
Screening for albuminuria can be most easily performed by urinary albumin-to-creatinine ratio (UACR) in a random spot urine collection (1,2). Timed or 24-h collections are more burdensome and add little to prediction or accuracy. Measurement of a spot urine sample for albumin alone (whether by immunoassay or by using a sensitive dipstick test specific for albuminuria) without simultaneously measuring urine creatinine is less expensive but susceptible to false-negative and false-positive determinations as a result of variation in urine concentration due to hydration (8). Thus, to be useful for patient screening, semiquantitative or qualitative (dipstick) screening tests should be >85% positive in those with moderately increased albuminuria (≥30 mg/g) and confirmed by albumin-to-creatinine values in an accredited laboratory (9,10). Hence, it is better to simply collect a spot urine sample for albumin-to-creatinine ratio because it will ultimately need to be done.
Normal albuminuria is defined as <30 mg/g creatinine, moderately elevated albuminuria is defined as ≥30–300 mg/g creatinine, and severely elevated albuminuria is defined as ≥300 mg/g creatinine. However, UACR is a continuous measurement, and differences within the normal and abnormal ranges are associated with renal and cardiovascular outcomes (7,11,12). Furthermore, because of high biological variability of >20% between measurements in urinary albumin excretion, two of three specimens of UACR collected within a 3- to 6-month period should be abnormal before considering a patient to have moderately or severely elevated albuminuria (1,2,13,14). Exercise within 24 h, infection, fever, congestive heart failure, marked hyperglycemia, menstruation, and marked hypertension may elevate UACR independently of kidney damage (15).
Traditionally, eGFR is calculated from serum creatinine using a validated formula (16). The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation is preferred (2). eGFR is routinely reported by laboratories along with serum creatinine, and eGFR calculators are available online at nkdep.nih.gov. An eGFR persistently <60 mL/min/1.73 m2 in concert with a urinary albumin value of >30 mg/g creatinine is considered abnormal, though optimal thresholds for clinical diagnosis are debated in older adults over age 70 years (2,17). Historically, a correction factor for muscle mass was included in a modified equation for African American people; however, race is a social and not a biologic construct, making it problematic to apply race to clinical algorithms, and the need to advance health equity and social justice is clear. Thus, it was decided that the equation should be altered such that it applies to all (16). Hence, a committee was convened, resulting in the recommendation for immediate implementation of the CKD-EPI creatinine equation refit without the race variable in all laboratories in the U.S. Additionally, increased use of cystatin C (another marker of eGFR) is suggested in combination with the serum creatinine because combining filtration markers (creatinine and cystatin C) is more accurate and would support better clinical decisions than either marker alone.
Diagnosis of Diabetic Kidney Disease
Diabetic kidney disease is usually a clinical diagnosis made based on the presence of albuminuria and/or reduced eGFR in the absence of signs or symptoms of other primary causes of kidney damage. The typical presentation of diabetic kidney disease is considered to include a long-standing duration of diabetes, retinopathy, albuminuria without gross hematuria, and gradually progressive loss of eGFR. However, signs of diabetic kidney disease may be present at diagnosis or without retinopathy in type 2 diabetes. Reduced eGFR without albuminuria has been frequently reported in type 1 and type 2 diabetes and is becoming more common over time as the prevalence of diabetes increases in the U.S. (3,4,18,19).
An active urinary sediment (containing red or white blood cells or cellular casts), rapidly increasing albuminuria or total proteinuria, the presence of nephrotic syndrome, rapidly decreasing eGFR, or the absence of retinopathy (in type 1 diabetes) suggests alternative or additional causes of kidney disease. For patients with these features, referral to a nephrologist for further diagnosis, including the possibility of kidney biopsy, should be considered. It is rare for people with type 1 diabetes to develop kidney disease without retinopathy. In type 2 diabetes, retinopathy is only moderately sensitive and specific for CKD caused by diabetes, as confirmed by kidney biopsy (20).
Staging of Chronic Kidney Disease
Stage 1 and stage 2 CKD are defined by evidence of high albuminuria with eGFR ≥60 mL/min/1.73 m2, and stages 3–5 CKD are defined by progressively lower ranges of eGFR (21) (Fig. 11.1). At any eGFR, the degree of albuminuria is associated with risk of cardiovascular disease (CVD), CKD progression, and mortality (7). Therefore, Kidney Disease: Improving Global Outcomes (KDIGO) recommends a more comprehensive CKD staging that incorporates albuminuria at all stages of eGFR; this system is more closely associated with risk but is also more complex and does not translate directly to treatment decisions (2). Thus, based on the current classification system, both eGFR and albuminuria must be quantified to guide treatment decisions. This is also important because eGFR levels are essential for modifications of drug dosages or restrictions of use (Fig. 11.1) (22,23). The degree of albuminuria should influence the choice of antihypertensive medications (see Section 10, “Cardiovascular Disease and Risk Management”) or glucose-lowering medications (see below). Observed history of eGFR loss (which is also associated with risk of CKD progression and other adverse health outcomes) and cause of kidney damage (including possible causes other than diabetes) may also affect these decisions (24).
Acute Kidney Injury
Acute kidney injury (AKI) is diagnosed by a 50% or greater sustained increase in serum creatinine over a short period of time, which is also reflected as a rapid decrease in eGFR (25,26). People with diabetes are at higher risk of AKI than those without diabetes (27). Other risk factors for AKI include preexisting CKD, the use of medications that cause kidney injury (e.g., nonsteroidal anti-inflammatory drugs), and the use of medications that alter renal blood flow and intrarenal hemodynamics. In particular, many antihypertensive medications (e.g., diuretics, ACE inhibitors, and angiotensin receptor blockers [ARBs]) can reduce intravascular volume, renal blood flow, and/or glomerular filtration. There was concern that sodium–glucose cotransporter 2 (SGLT2) inhibitors may promote AKI through volume depletion, particularly when combined with diuretics or other medications that reduce glomerular filtration; however, this has not been found to be true in randomized clinical outcome trials of advanced kidney disease (28) or high CVD risk with normal kidney function (29–31). It is also noteworthy that the nonsteroidal mineralocorticoid receptor antagonists (MRAs) do not increase the risk of AKI when used to slow kidney disease progression (32). Timely identification and treatment of AKI is important because AKI is associated with increased risks of progressive CKD and other poor health outcomes (33).
Elevations in serum creatinine (up to 30% from baseline) with renin-angiotensin system (RAS) blockers (such as ACE inhibitors and ARBs) must not be confused with AKI (34). An analysis of the Action to Control Cardiovascular Risk in Diabetes Blood Pressure (ACCORD BP) trial demonstrates that participants randomized to intensive blood pressure lowering with up to a 30% increase in serum creatinine did not have any increase in mortality or progressive kidney disease (35–38). Moreover, a measure of markers for AKI showed no significant increase of any markers with increased creatinine (37). Accordingly, ACE inhibitors and ARBs should not be discontinued for increases in serum creatinine (<30%) in the absence of volume depletion.
Lastly, it should be noted that ACE inhibitors and ARBs are commonly not dosed at maximum tolerated doses because of fear that serum creatinine will rise. As noted above, this is an error. Note that in all clinical trials demonstrating efficacy of ACE inhibitors and ARBs in slowing kidney disease progression, the maximum tolerated doses were used—not very low doses that do not provide benefit. Moreover, there are now studies demonstrating outcome benefits on both mortality and slowed CKD progression in people with diabetes who have an eGFR <30 mL/min/1.73 m2 (38). Additionally, when increases in serum creatinine reach 30% without associated hyperkalemia, RAS blockade should be continued (36,39).
Surveillance
Both albuminuria and eGFR should be monitored annually to enable timely diagnosis of CKD, monitor progression of CKD, detect superimposed kidney diseases including AKI, assess risk of CKD complications, dose drugs appropriately, and determine whether nephrology referral is needed. Among people with existing kidney disease, albuminuria and eGFR may change due to progression of CKD, development of a separate superimposed cause of kidney disease, AKI, or other effects of medications, as noted above. Serum potassium should also be monitored in patients treated with diuretics because these medications can cause hypokalemia, which is associated with cardiovascular risk and mortality (40–42). Patients with eGFR <60 mL/min/1.73 m2 receiving ACE inhibitors, ARBs, or MRAs should have serum potassium measured periodically. Additionally, people with this lower range of eGFR should have their medication dosing verified, their exposure to nephrotoxins (e.g., nonsteroidal anti-inflammatory drugs and iodinated contrast) should be minimized, and they should be evaluated for potential CKD complications (Table 11.1).
Table 11.1.
Selected complications of chronic kidney disease
| Complication | Physical and laboratory evaluation |
|---|---|
| Blood pressure >130/80 mmHg | Blood pressure, weight |
| Volume overload | History, physical examination, weight |
| Electrolyte abnormalities | Serum electrolytes |
| Metabolic acidosis | Serum electrolytes |
| Anemia | Hemoglobin; iron testing if indicated |
| Metabolic bone disease | Serum calcium, phosphate, PTH, vitamin 25(OH)D |
Complications of chronic kidney disease (CKD) generally become prevalent when estimated glomerular filtration rate falls below 60 mL/min/1.73 m2 (stage 3 CKD or greater) and become more common and severe as CKD progresses. Evaluation of elevated blood pressure and volume overload should occur at every clinical contact possible; laboratory evaluations are generally indicated every 6–12 months for stage 3 CKD, every 3–5 months for stage 4 CKD, and every 1–3 months for stage 5 CKD, or as indicated to evaluate symptoms or changes in therapy. PTH, parathyroid hormone; 25(OH)D, 25-hydroxyvitamin D.
There is a clear need for annual quantitative assessment of urinary albumin excretion. This is especially true after a diagnosis of albuminuria, institution of ACE inhibitors or ARB therapy to maximum tolerated doses, and achievement of blood pressure targets. Early changes in kidney function may be detected by increases in albuminuria before changes in eGFR (43), and this also significantly affects cardiovascular risk. Moreover, an initial reduction of >30% from baseline, subsequently maintained over at least 2 years, is considered a valid surrogate for renal benefit by the Division of Cardiology and Nephrology of the U.S. Food and Drug Administration (FDA) (10). Continued surveillance can assess both response to therapy and disease progression and may aid in assessing participation in ACE inhibitor or ARB therapy. In addition, in clinical trials of ACE inhibitors or ARB therapy in type 2 diabetes, reducing albuminuria to levels <300 mg/g creatinine or by >30% from baseline has been associated with improved renal and cardiovascular outcomes, leading some to suggest that medications should be titrated to maximize reduction in UACR. Data from post hoc analyses demonstrate less benefit on cardiorenal outcomes at half doses of RAS blockade (44). In type 1 diabetes, remission of albuminuria may occur spontaneously, and cohort studies evaluating associations of change in albuminuria with clinical outcomes have reported inconsistent results (45,46).
The prevalence of CKD complications correlates with eGFR (42). When eGFR is <60 mL/min/1.73 m2, screening for complications of CKD is indicated (Table 11.1). Early vaccination against hepatitis B virus is indicated in individuals likely to progress to ESRD (see Section 4, “Comprehensive Medical Evaluation and Assessment of Comorbidities,” for further information on immunization).
Prevention
The only proven primary prevention interventions for CKD are blood glucose and blood pressure control. There is no evidence that renin-angiotensin-aldosterone system (RAAS) inhibitors or any other interventions prevent the development of diabetic kidney disease. Thus, the American Diabetes Association does not recommend routine use of these medications solely for the purpose of prevention of the development of diabetic kidney disease.
Interventions
Nutrition
For people with non-dialysis-dependent CKD, dietary protein intake should be ∼0.8 g/kg body weight per day (the recommended daily allowance) (1). Compared with higher levels of dietary protein intake, this level slowed GFR decline with evidence of a greater effect over time. Higher levels of dietary protein intake (>20% of daily calories from protein or >1.3 g/kg/day) have been associated with increased albuminuria, more rapid kidney function loss, and CVD mortality and therefore should be avoided. Reducing the amount of dietary protein below the recommended daily allowance of 0.8 g/kg/day is not recommended because it does not alter blood glucose levels, cardiovascular risk measures, or the course of GFR decline (47).
Restriction of dietary sodium (to <2,300 mg/day) may be useful to control blood pressure and reduce cardiovascular risk (48,49), and individualization of dietary potassium may be necessary to control serum potassium concentrations (27,40–42). These interventions may be most important for individuals with reduced eGFR, for whom urinary excretion of sodium and potassium may be impaired. For patients on dialysis, higher levels of dietary protein intake should be considered since malnutrition is a major problem for some patients on dialysis (50). Recommendations for dietary sodium and potassium intake should be individualized based on comorbid conditions, medication use, blood pressure, and laboratory data.
Glycemic Targets
Intensive lowering of blood glucose with the goal of achieving near-normoglycemia has been shown in large randomized studies to delay the onset and progression of albuminuria and reduce eGFR in people with type 1 diabetes (51,52) and type 2 diabetes (1,53–58). Insulin alone was used to lower blood glucose in the Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) study of type 1 diabetes, while a variety of agents were used in clinical trials of type 2 diabetes, supporting the conclusion that lowering blood glucose itself helps prevent CKD and its progression. The effects of glucose-lowering therapies on CKD have helped define A1C targets (see Table 6.2).
The presence of CKD affects the risks and benefits of intensive lowering of blood glucose and a number of specific glucose-lowering medications. In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial of type 2 diabetes, adverse effects of intensive management of blood glucose levels (hypoglycemia and mortality) were increased among people with kidney disease at baseline (59,60). Moreover, there is a lag time of at least 2 years in type 2 diabetes to over 10 years in type 1 diabetes for the effects of intensive glucose control to manifest as improved eGFR outcomes (56,60,61). Therefore, in some people with prevalent CKD and substantial comorbidity, target A1C levels may be less intensive (1,62).
Blood Pressure and Use of RAAS Inhibitors
RAAS inhibition remains a mainstay of management for people with diabetic kidney disease with albuminuria and for the treatment of hypertension in people with diabetes (with or without diabetic kidney disease). Indeed, all the trials that evaluated the benefits of SGLT2 inhibition or nonsteroidal mineralocorticoid receptor antagonist effects were done in individuals who were being treated with an ACE inhibitor or ARB, in some trials up to maximum tolerated doses.
Hypertension is a strong risk factor for the development and progression of CKD (63). Antihypertensive therapy reduces the risk of albuminuria (64–67), and among people with type 1 or 2 diabetes with established CKD (eGFR <60 mL/min/1.73 m2 and UACR ≥300 mg/g creatinine), ACE inhibitor or ARB therapy reduces the risk of progression to ESRD (68–70,74–80). Moreover, antihypertensive therapy reduces the risk of cardiovascular events (64).
A blood pressure level <130/80 mmHg is recommended to reduce CVD mortality and slow CKD progression among all people with diabetes. Lower blood pressure targets (e.g., <130/80 mmHg) should be considered for patients based on individual anticipated benefits and risks. People with CKD are at increased risk of CKD progression (particularly those with albuminuria) and CVD; therefore, lower blood pressure targets may be suitable in some cases, especially in individuals with severely elevated albuminuria (≥300 mg/g creatinine).
ACE inhibitors or ARBs are the preferred first-line agents for blood pressure treatment among people with diabetes, hypertension, eGFR <60 mL/min/1.73 m2, and UACR ≥300 mg/g creatinine because of their proven benefits for prevention of CKD progression (68,69,74). ACE inhibitors and ARBs are considered to have similar benefits (75,76) and risks. In the setting of lower levels of albuminuria (30–299 mg/g creatinine), ACE inhibitor or ARB therapy at maximum tolerated doses in trials has reduced progression to more advanced albuminuria (≥300 mg/g creatinine), slowed CKD progression, and reduced cardiovascular events but has not reduced progression to ESRD (74,77). While ACE inhibitors or ARBs are often prescribed for moderately increased albuminuria without hypertension, outcome trials have not been performed in this setting to determine whether they improve renal outcomes. Moreover, two long-term, double-blind studies demonstrated no renoprotective effect of either ACE inhibitors or ARBs in type 1 and type 2 diabetes among those who were normotensive with or without high albuminuria (formerly microalbuminuria) (78,79).
Absent kidney disease, ACE inhibitors or ARBs are useful to manage blood pressure but have not proven superior to alternative classes of antihypertensive therapy, including thiazide-like diuretics and dihydropyridine calcium channel blockers (80). In a trial of people with type 2 diabetes and normal urinary albumin excretion, an ARB reduced or suppressed the development of albuminuria but increased the rate of cardiovascular events (81). In a trial of people with type 1 diabetes exhibiting neither albuminuria nor hypertension, ACE inhibitors or ARBs did not prevent the development of diabetic glomerulopathy assessed by kidney biopsy (78). This was further supported by a similar trial in people with type 2 diabetes (79).
Two clinical trials studied the combinations of ACE inhibitors and ARBs and found no benefits on CVD or CKD, and the drug combination had higher adverse event rates (hyperkalemia and/or AKI) (82,83). Therefore, the combined use of ACE inhibitors and ARBs should be avoided.
Direct Renal Effects of Glucose-Lowering Medications
Some glucose-lowering medications also have effects on the kidney that are direct, i.e., not mediated through glycemia. For example, SGLT2 inhibitors reduce renal tubular glucose reabsorption, weight, systemic blood pressure, intraglomerular pressure, and albuminuria and slow GFR loss through mechanisms that appear independent of glycemia (30,84–87). Moreover, recent data support the notion that SGLT2 inhibitors reduce oxidative stress in the kidney by >50% and blunt increases in angiotensinogen as well as reduce NLRP3 inflammasome activity (88–90). Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) also have direct effects on the kidney and have been reported to improve renal outcomes compared with placebo (91–95). Renal effects should be considered when selecting antihyperglycemia agents (see Section 9, “Pharmacologic Approaches to Glycemic Treatment”).
Selection of Glucose-Lowering Medications for People with Chronic Kidney Disease
For people with type 2 diabetes and established CKD, special considerations for the selection of glucose-lowering medications include limitations to available medications when eGFR is diminished and a desire to mitigate risks of CKD progression, CVD, and hypoglycemia (96,97). Drug dosing may require modification with eGFR <60 mL/min/1.73 m2 (1).
The FDA revised its guidance for the use of metformin in CKD in 2016 (98), recommending use of eGFR instead of serum creatinine to guide treatment and expanding the pool of people with kidney disease for whom metformin treatment should be considered. The revised FDA guidance states that 1) metformin is contraindicated in patients with an eGFR <30 mL/min/1.73 m2, 2) eGFR should be monitored while taking metformin, 3) the benefits and risks of continuing treatment should be reassessed when eGFR falls to <45 mL/min/1.73 m2 (99,100), 4) metformin should not be initiated for patients with an eGFR <45 mL/min/1.73 m2, and 5) metformin should be temporarily discontinued at the time of or before iodinated contrast imaging procedures in patients with eGFR 30–60 mL/min/1.73 m2.
A number of recent studies have shown cardiovascular protection from SGLT2 inhibitors and GLP-1 RAs as well as renal protection from SGLT2 inhibitors and possibly from GLP-1 RAs. Selection of which glucose-lowering medications to use should be based on the usual criteria of an individual patient’s risks (cardiovascular and renal in addition to glucose control) as well as convenience and cost.
SGLT2 inhibitors are recommended for people with stage 3 CKD or higher and type 2 diabetes, as they slow CKD progression and reduce heart failure risk independent of glucose management (101). GLP-1 RAs are suggested for cardiovascular risk reduction if such risk is a predominant problem, as they reduce risks of CVD events and hypoglycemia and appear to possibly slow CKD progression (102–105).
A number of large cardiovascular outcomes trials in people with type 2 diabetes at high risk for CVD or with existing CVD examined kidney effects as secondary outcomes. These trials include EMPA-REG OUTCOME [BI 10773 (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients], CANVAS (Canagliflozin Cardiovascular Assessment Study), LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results), and SUSTAIN-6 (Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes) (71,86,91,94,102). Specifically, compared with placebo, empagliflozin reduced the risk of incident or worsening nephropathy (a composite of progression to UACR >300 mg/g creatinine, doubling of serum creatinine, ESRD, or death from ESRD) by 39% and the risk of doubling of serum creatinine accompanied by eGFR ≤45 mL/min/1.73 m2 by 44%; canagliflozin reduced the risk of progression of albuminuria by 27% and the risk of reduction in eGFR, ESRD, or death from ESRD by 40%; liraglutide reduced the risk of new or worsening nephropathy (a composite of persistent macroalbuminuria, doubling of serum creatinine, ESRD, or death from ESRD) by 22%; and semaglutide reduced the risk of new or worsening nephropathy (a composite of persistent UACR >300 mg/g creatinine, doubling of serum creatinine, or ESRD) by 36% (each P < 0.01). These analyses were limited by evaluation of study populations not selected primarily for CKD and examination of renal effects as secondary outcomes.
Some large clinical trials of SGLT2 inhibitors have focused on people with advanced CKD, and assessment of primary renal outcomes is either completed or ongoing. Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE), a placebo-controlled trial of canagliflozin among 4,401 adults with type 2 diabetes, UACR ≥300–5,000 mg/g creatinine, and eGFR range 30–90 mL/min/1.73 m2 (mean eGFR 56 mL/min/1.73 m2 with a mean albuminuria level of >900 mg/day), had a primary composite end point of ESRD, doubling of serum creatinine, or renal or cardiovascular death (28,72). It was stopped early due to positive efficacy and showed a 32% risk reduction for development of ESRD over control (28). Additionally, the development of the primary end point, which included chronic dialysis for ≥30 days, kidney transplantation or eGFR <15 mL/min/1.73 m2 sustained for ≥30 days by central laboratory assessment, doubling from the baseline serum creatinine average sustained for ≥30 days by central laboratory assessment, or renal death or cardiovascular death, was reduced by 30%. This benefit was on background ACE inhibitor or ARB therapy in >99% of the patients (28). Moreover, in this advanced CKD group, there were clear benefits on cardiovascular outcomes demonstrating a 31% reduction in cardiovascular death or heart failure hospitalization and a 20% reduction in cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke (28,73,105).
A second trial in advanced diabetic kidney disease was the Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) study (106). This trial examined a cohort similar to that in CREDENCE except 67.5% of the participants had type 2 diabetes and CKD (the other one-third had CKD without type 2 diabetes), and the end points were slightly different. The primary outcome was time to the first occurrence of any of the components of the composite, including ≥50% sustained decline in eGFR or reaching ESRD or cardiovascular death, or renal death. Secondary outcome measures included time to the first occurrence of any of the components of the composite kidney outcome (≥50% sustained decline in eGFR or reaching ESRD or renal death), time to the first occurrence of either of the components of the cardiovascular composite (cardiovascular death or hospitalization for heart failure), and time to death from any cause. The trial had 4,304 participants with a mean eGFR at baseline of 43.1 ± 12.4 mL/min/1.73 m2 (range 25–75 mL/min/1.73 m2) and a median UACR of 949 mg/g (range 200–5,000 mg/g). There was a significant benefit by dapagliflozin for the primary end point (hazard ratio [HR] 0.61 [95% CI 0.51–0.72]; P < 0.001) (106).
The HR for the kidney composite of a sustained decline in eGFR of ≥50%, ESRD, or death from renal causes was 0.56 (95% CI 0.45–0.68; P < 0.001). The HR for the composite of death from cardiovascular causes or hospitalization for heart failure was 0.71 (95% CI 0.55–0.92; P = 0.009). Finally, all-cause mortality was decreased in the dapagliflozin group compared with the placebo group (P < 0.004).
In addition to renal effects, while SGLT2 inhibitors demonstrated reduced risk of heart failure hospitalizations, some also demonstrated cardiovascular risk reduction. GLP-1 RAs clearly demonstrated cardiovascular benefits. Namely, in the EMPA-REG OUTCOME, CANVAS, Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58 (DECLARE-TIMI 58), LEADER, and SUSTAIN-6 trials, empagliflozin, canagliflozin, dapagliflozin, liraglutide, and semaglutide, respectively, each reduced cardiovascular events, evaluated as primary outcomes, compared with placebo (see Section 10, “Cardiovascular Disease and Risk Management,” for further discussion). While the glucose-lowering effects of SGLT2 inhibitors are blunted with eGFR <45 mL/min/1.73 m2, the renal and cardiovascular benefits were still seen at eGFR levels of 25 mL/min/1.73 m2 with no significant change in glucose (28,30,51,62,71,94,106,107). Most participants with CKD in these trials also had diagnosed atherosclerotic cardiovascular disease (ASCVD) at baseline, although ∼28% of CANVAS participants with CKD did not have diagnosed ASCVD (31).
Based on evidence from the CREDENCE and DAPA-CKD trials, as well as secondary analyses of cardiovascular outcomes trials with SGLT2 inhibitors, cardiovascular and renal events are reduced with SGLT2 inhibitor use in patients with an eGFR of 20 mL/min/1.73 m2, independent of glucose-lowering effects (73,105).
While there is clear cardiovascular risk reduction associated with GLP-1 RA use in people with type 2 diabetes and CKD, the proof of benefit on renal outcomes will come with the results of the ongoing FLOW (A Research Study to See How Semaglutide Works Compared with Placebo in People With Type 2 Diabetes and Chronic Kidney Disease) trial with injectable semaglutide (108). As noted above, published data address a limited group of people with CKD, mostly with coexisting ASCVD. Renal events, however, have been examined as both primary and secondary outcomes in large published trials. Adverse event profiles of these agents also must be considered. Please refer to Table 9.2 for drug-specific factors, including adverse event information, for these agents. Additional clinical trials focusing on CKD and cardiovascular outcomes in people with CKD are ongoing and will be reported in the next few years.
For people with type 2 diabetes and CKD, the selection of specific agents may depend on comorbidity and CKD stage. SGLT2 inhibitors may be more useful for individuals at high risk of CKD progression (i.e., with albuminuria or a history of documented eGFR loss) (Fig. 9.3) due to an apparent large beneficial effect on CKD incidence. However, for people with type 2 diabetes and diabetic kidney disease, use of an SGLT2 inhibitor in individuals with eGFR ≥20 mL/min/1.73 m2 and UACR ≥200 mg/g creatinine is recommended to reduce CKD progression and cardiovascular events. This is a change in eGFR from previous recommendations that suggested an eGFR level >25 mL/min/1.73 m2. The reason for the lower limit of eGFR is as follows. The major clinical trials for SGLT2 inhibitors that showed benefit for people with diabetic kidney disease are CREDENCE and DAPA-CKD (28,105). CREDENCE enrollment criteria included an eGFR >30 mL/min/1.73 m2 and UACR >300 mg/g (28,105). DAPA-CKD enrolled individuals with eGFR >25 mL/min/1.73 m2 and UACR >200 mg/g. Subgroup analyses from DAPA-CKD (109) and analyses from the EMPEROR heart failure trials suggest that SGLT2 inhibitors are safe and effective at eGFR levels of >20 mL/min/1.73 m2. The Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction (EMPEROR-Preserved) enrolled 5,998 participants (110), and the Empagliflozin Outcome Trial in Patients With Chronic Heart Failure and a Reduced Ejection Fraction (EMPEROR-Reduced) enrolled 3,730 participants (111); enrollment criteria included eGFR >60 mL/min/1.73 m2, but efficacy was seen at eGFR >20 mL/min/1.73 m2 in people with heart failure. Hence, the new recommendation is to use SGLT2 inhibitors in individuals with eGFR as low as 20 mL/min/1.73 m2. In addition, the DECLARE-TIMI 58 trial suggested effectiveness in participants with normal urinary albumin levels (112). In sum, for people with type 2 diabetes and diabetic kidney disease, use of an SGLT2 inhibitor is recommended to reduce CKD progression and cardiovascular events in people with an eGFR ≥20 mL/min/1.73 m2.
Of note, GLP-1 RAs may also be used at low eGFR for cardiovascular protection but may require dose adjustment (113).
Renal and Cardiovascular Outcomes of Mineralocorticoid Receptor Antagonists in Chronic Kidney Disease
MRAs historically have not been well studied in diabetic kidney disease because of the risk of hyperkalemia (114,115). However, data that do exist suggest sustained benefit on albuminuria reduction. There are two different classes of MRAs, steroidal and nonsteroidal, with one group not extrapolatable to the other (116). Late in 2020, the results of the first of two trials, the Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-DKD) trial, which examined the renal effects of finerenone, demonstrated a significant reduction in diabetic kidney disease progression and cardiovascular events in people with advanced diabetic kidney disease (32,117). This trial had a primary end point of time to first occurrence of the composite end point of onset of kidney failure, a sustained decrease of eGFR >40% from baseline over at least 4 weeks, or renal death. A prespecified secondary outcome was time to first occurrence of the composite end point cardiovascular death or nonfatal cardiovascular events (myocardial infarction, stroke, or hospitalization for heart failure). Other secondary outcomes included all-cause mortality, time to all-cause hospitalizations, and change in UACR from baseline to month 4, and time to first occurrence of the following composite end point: onset of kidney failure, a sustained decrease in eGFR of ≥57% from baseline over at least 4 weeks, or renal death.
The double-blind, placebo-controlled trial randomized 5,734 people with CKD and type 2 diabetes to receive finerenone, a novel nonsteroidal MRA, or placebo. Eligible participants had a UACR of 30 to <300 mg/g, an eGFR of 25 to <60 mL/min/1.73 m2, and diabetic retinopathy, or a UACR of 300–5,000 mg/g and an eGFR of 25 to <75 mL/min/1.73 m2. The mean age of participants was 65.6 years, and 30% were female. The mean eGFR was 44.3 mL/min/1.73 m2, and the mean albuminuria was 852 mg/g (interquartile range 446–1,634 mg/g). The primary end point was reduced with finerenone compared with placebo (HR 0.82 [95% CI 0.73–0.93]; P = 0.001), as was the key secondary composite of cardiovascular outcome (HR 0.86 [95% CI 0.75–0.99]; P = 0.03). Hyperkalemia resulted in 2.3% discontinuation in the study group compared with 0.9% in the placebo group. However, the study was completed, and there were no deaths related to hyperkalemia. Of note, 4.5% of the total group were being treated with SGLT2 inhibitors.
The Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease (FIGARO-DKD) trial assessed the safety and efficacy of finerenone in reducing cardiovascular events among people with type 2 diabetes and CKD with elevated UACR (30 to <300 mg/g creatinine) and eGFR 25–90 mL/min/1.73 m2 (118). The study randomized eligible subjects to either finerenone (n = 3,686) or placebo (n = 3,666). Participants with an eGFR of 25–60 mL/min/1.73 m2 at the screening visit received an initial dose at baseline of 10 mg once daily, and if eGFR at screening was ≥60 mL/min/1.73 m2, the initial dose was 20 mg once daily. An increase in the dose from 10 to 20 mg once daily was encouraged after 1 month, provided the serum potassium level was ≤4.8 mmol/L and eGFR was stable. The mean age of participants was 64.1 years (31% were female), and the median follow-up duration was 3.4 years. The median A1C was 7.7%, the mean systolic blood pressure was 136 mmHg, and the mean GFR was 67.8 mL/min/1.73 m2. People with heart failure with a reduced ejection fraction and uncontrolled hypertension were excluded.
The primary composite outcome was cardiovascular death, myocardial infarction, stroke, and hospitalization for heart failure. The finerenone group showed a 13% reduction in the primary end point compared with the placebo group (12.4% vs. 14.2%; HR 0.87 [95% CI 0.76–0.98]; P = 0.03). This benefit was primarily driven by a reduction in heart failure hospitalizations: 3.2% vs. 4.4% in the placebo group (HR 0.71 [95% CI 0.56–0.90]).
Of the secondary outcomes, the most noteworthy was a 36% reduction in end-stage kidney disease: 0.9% vs. 1.3% in the placebo group (HR 0.64 [95% CI 0.41–0.995]). There was a higher incidence of hyperkalemia in the finerenone group, 10.8% vs. 5.3%, although only 1.2% of the 3,686 individuals on finerenone stopped the study due to hyperkalemia (0.6% vs. 0.4% of the placebo group).
The FIDELITY prespecified pooled efficacy and safety analysis incorporated individuals from both the FIGARO-DKD and FIDELIO-DKD trials (N = 13,171) to allow for evaluation across the spectrum of severity of CKD, since the populations were different (with a slight overlap) and the study designs were similar (119). The analysis showed a 14% reduction in composite cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, and hospitalization for heart failure for finerenone vs. placebo (12.7% vs. 14.4%; HR 0.86 [95% CI 0.78–0.95]; P = 0.0018).
It also demonstrated a 23% reduction in the composite kidney outcome, consisting of sustained ≥57% decrease in eGFR from baseline over ≥4 weeks, or renal death, for finerenone vs. placebo (5.5% vs. 7.1%; HR 0.77 [95% CI 0.67–0.88]; P = 0.0002).
The pooled FIDELITY trial analysis confirms and strengthens the positive cardiovascular and renal outcomes with finerenone across the spectrum of CKD, irrespective of baseline ASCVD history (with the exclusion of those with heart failure with reduced ejection fraction).
Referral to a Nephrologist
Health care professionals should consider referral to a nephrologist if the patient has continuously rising UACR levels and/or continuously declining eGFR, if there is uncertainty about the etiology of kidney disease, for difficult management issues (anemia, secondary hyperparathyroidism, significant increases in albuminuria in spite of good blood pressure management, metabolic bone disease, resistant hypertension, or electrolyte disturbances), or when there is advanced kidney disease (eGFR <30 mL/min/1.73 m2) requiring discussion of renal replacement therapy for ESRD (2). The threshold for referral may vary depending on the frequency with which a health care professional encounters people with diabetes and kidney disease. Consultation with a nephrologist when stage 4 CKD develops (eGFR <30 mL/min/1.73 m2) has been found to reduce cost, improve quality of care, and delay dialysis (120). However, other specialists and health care professionals should also educate their patients about the progressive nature of CKD, the kidney preservation benefits of proactive treatment of blood pressure and blood glucose, and the potential need for renal replacement therapy.
Footnotes
Disclosure information for each author is available at https://doi.org/10.2337/dc23-SDIS.
Suggested citation: ElSayed NA, Aleppo G, Aroda VR, et al., American Diabetes Association. 11. Chronic kidney disease and risk management: Standards of Care in Diabetes—2023. Diabetes Care 2023;46(Suppl. 1):S191–S202
References
- 1. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care 2014;37:2864–2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National Kidney Foundation . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1–150 [DOI] [PubMed] [Google Scholar]
- 3. Afkarian M, Zelnick LR, Hall YN, et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA 2016;316:602–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011;305:2532–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Boer IH; DCCT/EDIC Research Group . Kidney disease and related findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care 2014;37:24–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johansen KL, Chertow GM, Foley RN, et al. US Renal Data System 2020 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis 2021;77(Suppl. 1):A7–A8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fox CS, Matsushita K, Woodward M, et al.; Chronic Kidney Disease Prognosis Consortium . Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet 2012;380:1662–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yarnoff BO, Hoerger TJ, Simpson SK, et al.; Centers for Disease Control and Prevention CKD Initiative . The cost-effectiveness of using chronic kidney disease risk scores to screen for early-stage chronic kidney disease. BMC Nephrol 2017;18:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coresh J, Heerspink HJL, Sang Y, et al.; Chronic Kidney Disease Prognosis Consortium and Chronic Kidney Disease Epidemiology Collaboration . Change in albuminuria and subsequent risk of end-stage kidney disease: an individual participant-level consortium meta-analysis of observational studies. Lancet Diabetes Endocrinol 2019;7:115–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levey AS, Gansevoort RT, Coresh J, et al. Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: a scientific workshop sponsored by the National Kidney Foundation in collaboration with the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis 2020;75:84–104 [DOI] [PubMed] [Google Scholar]
- 11. Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol 2013;24:302–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Groop P-H, Thomas MC, Moran JL, et al.; FinnDiane Study Group . The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes 2009;58:1651–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gomes MB, Gonçalves MF. Is there a physiological variability for albumin excretion rate? Study in patients with diabetes type 1 and non-diabetic individuals. Clin Chim Acta 2001;304:117–123 [DOI] [PubMed] [Google Scholar]
- 14. Naresh CN, Hayen A, Weening A, Craig JC, Chadban SJ. Day-to-day variability in spot urine albumin-creatinine ratio. Am J Kidney Dis 2013;62:1095–1101 [DOI] [PubMed] [Google Scholar]
- 15. Tankeu AT, Kaze FF, Noubiap JJ, Chelo D, Dehayem MY, Sobngwi E. Exercise-induced albuminuria and circadian blood pressure abnormalities in type 2 diabetes. World J Nephrol 2017;6:209–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Delanaye P, Glassock RJ, Pottel H, Rule AD. An age-calibrated definition of chronic kidney disease: rationale and benefits. Clin Biochem Rev 2016;37:17–26 [PMC free article] [PubMed] [Google Scholar]
- 17. Kramer HJ, Nguyen QD, Curhan G, Hsu C-Y. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA 2003;289:3273–3277 [DOI] [PubMed] [Google Scholar]
- 18. Molitch ME, Steffes M, Sun W, et al.; Epidemiology of Diabetes Interventions and Complications Study Group . Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the Diabetes Control and Complications Trial and the Epidemiology of Diabetes Interventions and Complications study. Diabetes Care 2010;33:1536–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. He F, Xia X, Wu XF, Yu XQ, Huang FX. Diabetic retinopathy in predicting diabetic nephropathy in patients with type 2 diabetes and renal disease: a meta-analysis. Diabetologia 2013;56:457–466 [DOI] [PubMed] [Google Scholar]
- 20. Levey AS, Coresh J, Balk E, et al.; National Kidney Foundation . National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003;139:137–147 [DOI] [PubMed] [Google Scholar]
- 21. Flynn C, Bakris GL. Noninsulin glucose-lowering agents for the treatment of patients on dialysis. Nat Rev Nephrol 2013;9:147–153 [DOI] [PubMed] [Google Scholar]
- 22. Matzke GR, Aronoff GR, Atkinson AJ Jr, et al. Drug dosing consideration in patients with acute and chronic kidney disease—a clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2011;80:1122–1137 [DOI] [PubMed] [Google Scholar]
- 23. Coresh J, Turin TC, Matsushita K, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 2014;311:2518–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vassalotti JA, Centor R, Turner BJ, Greer RC, Choi M; National Kidney Foundation Kidney Disease Outcomes Quality Initiative . Practical approach to detection and management of chronic kidney disease for the primary care clinician. Am J Med 2016;129:153–162.e7 [DOI] [PubMed] [Google Scholar]
- 25. Zhou J, Liu Y, Tang Y, et al. A comparison of RIFLE, AKIN, KDIGO, and Cys-C criteria for the definition of acute kidney injury in critically ill patients. Int Urol Nephrol 2016;48:125–132 [DOI] [PubMed] [Google Scholar]
- 26. Hoste EAJ, Kellum JA, Selby NM, et al. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol 2018;14:607–625 [DOI] [PubMed] [Google Scholar]
- 27. James MT, Grams ME, Woodward M, et al.; CKD Prognosis Consortium . A meta-analysis of the association of estimated GFR, albuminuria, diabetes mellitus, and hypertension with acute kidney injury. Am J Kidney Dis 2015;66:602–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380:2295–2306 [DOI] [PubMed] [Google Scholar]
- 29. Nadkarni GN, Ferrandino R, Chang A, et al. Acute kidney injury in patients on SGLT2 inhibitors: a propensity-matched analysis. Diabetes Care 2017;40:1479–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wanner C, Inzucchi SE, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:323–334 [DOI] [PubMed] [Google Scholar]
- 31. Neuen BL, Ohkuma T, Neal B, et al. Cardiovascular and renal outcomes with canagliflozin according to baseline kidney function: data from the CANVAS Program. Circulation 2018;138:1537–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bakris GL, Agarwal R, Anker SD, et al.; FIDELIO-DKD Investigators . Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020;383:2219–2229 [DOI] [PubMed] [Google Scholar]
- 33. Thakar CV, Christianson A, Himmelfarb J, Leonard AC. Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin J Am Soc Nephrol 2011;6:2567–2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med 2000;160:685–693 [DOI] [PubMed] [Google Scholar]
- 35. Beddhu S, Greene T, Boucher R, et al. Intensive systolic blood pressure control and incident chronic kidney disease in people with and without diabetes mellitus: secondary analyses of two randomised controlled trials. Lancet Diabetes Endocrinol 2018;6:555–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Collard D, Brouwer TF, Peters RJG, Vogt L, van den Born BH. Creatinine rise during blood pressure therapy and the risk of adverse clinical outcomes in patients with type 2 diabetes mellitus. Hypertension 2018;72:1337–1344 [DOI] [PubMed] [Google Scholar]
- 37. Malhotra R, Craven T, Ambrosius WT, et al.; SPRINT Research Group . Effects of intensive blood pressure lowering on kidney tubule injury in CKD: a longitudinal subgroup analysis in SPRINT. Am J Kidney Dis 2019;73:21–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Qiao Y, Shin J-I, Chen TK, et al. Association between renin-angiotensin system blockade discontinuation and all-cause mortality among persons with low estimated glomerular filtration rate. JAMA Intern Med 2020;180:718–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ohkuma T, Jun M, Rodgers A, et al.; ADVANCE Collaborative Group . Acute increases in serum creatinine after starting angiotensin-converting enzyme inhibitor-based therapy and effects of its continuation on major clinical outcomes in type 2 diabetes mellitus. Hypertension 2019;73:84–91 [DOI] [PubMed] [Google Scholar]
- 40. Hughes-Austin JM, Rifkin DE, Beben T, et al. The relation of serum potassium concentration with cardiovascular events and mortality in community-living individuals. Clin J Am Soc Nephrol 2017;12:245–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bandak G, Sang Y, Gasparini A, et al. Hyperkalemia after initiating renin-angiotensin system blockade: the Stockholm Creatinine Measurements (SCREAM) project. J Am Heart Assoc 2017;6:e005428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nilsson E, Gasparini A, Ärnlöv J, et al. Incidence and determinants of hyperkalemia and hypokalemia in a large healthcare system. Int J Cardiol 2017;245:277–284 [DOI] [PubMed] [Google Scholar]
- 43. Zelniker TA, Raz I, Mosenzon O, et al. Effect of dapagliflozin on cardiovascular outcomes according to baseline kidney function and albuminuria status in patients with type 2 diabetes: a prespecified secondary analysis of a randomized clinical trial. JAMA Cardiol 2021;6:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Epstein M, Reaven NL, Funk SE, McGaughey KJ, Oestreicher N, Knispel J. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care 2015;21(Suppl.):S212–S220 [PubMed] [Google Scholar]
- 45. de Boer IH, Gao X, Cleary PA, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group . Albuminuria changes and cardiovascular and renal outcomes in type 1 diabetes: the DCCT/EDIC study. Clin J Am Soc Nephrol 2016;11:1969–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sumida K, Molnar MZ, Potukuchi PK, et al. Changes in albuminuria and subsequent risk of incident kidney disease. Clin J Am Soc Nephrol 2017;12:1941–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Klahr S, Levey AS, Beck GJ, et al.; Modification of Diet in Renal Disease Study Group . The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med 1994;330:877–884 [DOI] [PubMed] [Google Scholar]
- 48. Mills KT, Chen J, Yang W, et al.; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators . Sodium excretion and the risk of cardiovascular disease in patients with chronic kidney disease. JAMA 2016;315:2200–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018;71:1269–1324 [DOI] [PubMed] [Google Scholar]
- 50. Murray DP, Young L, Waller J, et al. Is dietary protein intake predictive of 1-year mortality in dialysis patients? Am J Med Sci 2018;356:234–243 [DOI] [PubMed] [Google Scholar]
- 51. DCCT/EDIC Research Group . Effect of intensive diabetes treatment on albuminuria in type 1 diabetes: long-term follow-up of the Diabetes Control and Complications Trial and Epidemiology of Diabetes Interventions and Complications study. Lancet Diabetes Endocrinol 2014;2:793–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. de Boer IH, Sun W, Cleary PA, et al.; DCCT/EDIC Research Group . Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med 2011;365:2366–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 54. Patel A, MacMahon S, Chalmers J, et al.; ADVANCE Collaborative Group . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 55. Ismail-Beigi F, Craven T, Banerji MA, et al.; ACCORD trial group . Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010;376:419–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zoungas S, Chalmers J, Neal B, et al.; ADVANCE-ON Collaborative Group . Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med 2014;371:1392–1406 [DOI] [PubMed] [Google Scholar]
- 57. Zoungas S, Arima H, Gerstein HC, et al.; Collaborators on Trials of Lowering Glucose (CONTROL) group . Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta-analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol 2017;5:431–437 [DOI] [PubMed] [Google Scholar]
- 58. Agrawal L, Azad N, Bahn GD, et al.; VADT Study Group . Long-term follow-up of intensive glycaemic control on renal outcomes in the Veterans Affairs Diabetes Trial (VADT). Diabetologia 2018;61:295–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Papademetriou V, Lovato L, Doumas M, et al.; ACCORD Study Group . Chronic kidney disease and intensive glycemic control increase cardiovascular risk in patients with type 2 diabetes. Kidney Int 2015;87:649–659 [DOI] [PubMed] [Google Scholar]
- 60. Perkovic V, Heerspink HL, Chalmers J, et al.; ADVANCE Collaborative Group . Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int 2013;83:517–523 [DOI] [PubMed] [Google Scholar]
- 61. Wong MG, Perkovic V, Chalmers J, et al.; ADVANCE-ON Collaborative Group . Long-term benefits of intensive glucose control for preventing end-stage kidney disease: ADVANCE-ON. Diabetes Care 2016;39:694–700 [DOI] [PubMed] [Google Scholar]
- 62. National Kidney Foundation . KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis 2012;60:850–886 [DOI] [PubMed] [Google Scholar]
- 63. Leehey DJ, Zhang JH, Emanuele NV, et al.; VA NEPHRON-D Study Group . BP and renal outcomes in diabetic kidney disease: the Veterans Affairs Nephropathy in Diabetes Trial. Clin J Am Soc Nephrol 2015;10:2159–2169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA 2015;313:603–615 [DOI] [PubMed] [Google Scholar]
- 65. Cushman WC, Evans GW, Byington RP, et al.; ACCORD Study Group . Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. UK Prospective Diabetes Study Group . Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998;317:703–713 [PMC free article] [PubMed] [Google Scholar]
- 67. de Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care 2017;40:1273–1284 [DOI] [PubMed] [Google Scholar]
- 68. Brenner BM, Cooper ME, de Zeeuw D, et al.; RENAAL Study Investigators . Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345:861–869 [DOI] [PubMed] [Google Scholar]
- 69. Lewis EJ, Hunsicker LG, Bain RP; The Collaborative Study Group . The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med 1993;329:1456–1462 [DOI] [PubMed] [Google Scholar]
- 70. Lewis EJ, Hunsicker LG, Clarke WR, et al.; Collaborative Study Group . Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001;345:851–860 [DOI] [PubMed] [Google Scholar]
- 71. Zinman B, Wanner C, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 72. Jardine MJ, Mahaffey KW, Neal B, et al.; CREDENCE study investigators . The Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) study rationale, design, and baseline characteristics. Am J Nephrol 2017;46:462–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mahaffey KW, Jardine MJ, Bompoint S, et al. Canagliflozin and cardiovascular and renal outcomes in type 2 diabetes mellitus and chronic kidney disease in primary and secondary cardiovascular prevention groups. Circulation 2019;140:739–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Heart Outcomes Prevention Evaluation Study Investigators . Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet 2000;355:253–259 [PubMed] [Google Scholar]
- 75. Barnett AH, Bain SC, Bouter P, et al.; Diabetics Exposed to Telmisartan and Enalapril Study Group . Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med 2004;351:1952–1961 [DOI] [PubMed] [Google Scholar]
- 76. Wu HY, Peng CL, Chen PC, et al. Comparative effectiveness of angiotensin-converting enzyme inhibitors versus angiotensin II receptor blockers for major renal outcomes in patients with diabetes: a 15-year cohort study. PLoS One 2017;12:e0177654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Parving HH, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S; Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group . The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 2001;345:870–878 [DOI] [PubMed] [Google Scholar]
- 78. Mauer M, Zinman B, Gardiner R, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med 2009;361:40–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Weil EJ, Fufaa G, Jones LI, et al. Effect of losartan on prevention and progression of early diabetic nephropathy in American Indians with type 2 diabetes. Diabetes 2013;62:3224–3231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bangalore S, Fakheri R, Toklu B, Messerli FH. Diabetes mellitus as a compelling indication for use of renin angiotensin system blockers: systematic review and meta-analysis of randomized trials. BMJ 2016;352:i438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Haller H, Ito S, Izzo JL Jr, et al.; ROADMAP Trial Investigators . Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med 2011;364:907–917 [DOI] [PubMed] [Google Scholar]
- 82. Yusuf S, Teo KK, Pogue J, et al.; ONTARGET Investigators . Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008;358:1547–1559 [DOI] [PubMed] [Google Scholar]
- 83. Fried LF, Emanuele N, Zhang JH, et al.; VA NEPHRON-D Investigators . Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013;369:1892–1903 [DOI] [PubMed] [Google Scholar]
- 84. Cherney DZI, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014;129:587–597 [DOI] [PubMed] [Google Scholar]
- 85. Heerspink HJL, Desai M, Jardine M, Balis D, Meininger G, Perkovic V. Canagliflozin slows progression of renal function decline independently of glycemic effects. J Am Soc Nephrol 2017;28:368–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Neal B, Perkovic V, Mahaffey KW, et al.; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657 [DOI] [PubMed] [Google Scholar]
- 87. Zelniker TA, Braunwald E. Cardiac and renal effects of sodium-glucose co-transporter 2 inhibitors in diabetes: JACC state-of-the-art review. J Am Coll Cardiol 2018;72:1845–1855 [DOI] [PubMed] [Google Scholar]
- 88. Woods TC, Satou R, Miyata K, et al. Canagliflozin prevents intrarenal angiotensinogen augmentation and mitigates kidney injury and hypertension in mouse model of type 2 diabetes mellitus. Am J Nephrol 2019;49:331–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Heerspink HJL, Perco P, Mulder S, et al. Canagliflozin reduces inflammation and fibrosis biomarkers: a potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia 2019;62:1154–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yaribeygi H, Butler AE, Atkin SL, Katsiki N, Sahebkar A. Sodium-glucose cotransporter 2 inhibitors and inflammation in chronic kidney disease: possible molecular pathways. J Cell Physiol 2018;234:223–230 [DOI] [PubMed] [Google Scholar]
- 91. Marso SP, Daniels GH, Brown-Frandsen K, et al.; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cooper ME, Perkovic V, McGill JB, et al. Kidney disease end points in a pooled analysis of individual patient-level data from a large clinical trials program of the dipeptidyl peptidase 4 inhibitor linagliptin in type 2 diabetes. Am J Kidney Dis 2015;66:441–449 [DOI] [PubMed] [Google Scholar]
- 93. Mann JFE, Ørsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377:839–848 [DOI] [PubMed] [Google Scholar]
- 94. Marso SP, Bain SC, Consoli A, et al.; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–1844 [DOI] [PubMed] [Google Scholar]
- 95. Shaman AM, Bain SC, Bakris GL, et al. Effect of the glucagon-like peptide-1 receptor agonists semaglutide and liraglutide on kidney outcomes in patients with type 2 diabetes: pooled analysis of SUSTAIN 6 and LEADER. Circulation 2022;145:575–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Karter AJ, Warton EM, Lipska KJ, et al. Development and validation of a tool to identify patients with type 2 diabetes at high risk of hypoglycemia-related emergency department or hospital use. JAMA Intern Med 2017;177:1461–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Moen MF, Zhan M, Hsu VD, et al. Frequency of hypoglycemia and its significance in chronic kidney disease. Clin J Am Soc Nephrol 2009;4:1121–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. U.S. Food and Drug Administration . FDA drug safety communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function, 2017. Accessed 20 October 2022. Available from https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-warnings-regarding-use-diabetes-medicine-metformin-certain
- 99. Lalau JD, Kajbaf F, Bennis Y, Hurtel-Lemaire AS, Belpaire F, De Broe ME. Metformin treatment in patients with type 2 diabetes and chronic kidney disease stages 3A, 3B, or 4. Diabetes Care 2018;41:547–553 [DOI] [PubMed] [Google Scholar]
- 100. Chu PY, Hackstadt AJ, Chipman J, et al. Hospitalization for lactic acidosis among patients with reduced kidney function treated with metformin or sulfonylureas. Diabetes Care 2020;43:1462–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. McGuire DK, Shih WJ, Cosentino F, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol 2021;6:148–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation 2019;139:2022–2031 [DOI] [PubMed] [Google Scholar]
- 103. Mann JFE, Hansen T, Idorn T, et al. Effects of once-weekly subcutaneous semaglutide on kidney function and safety in patients with type 2 diabetes: a post-hoc analysis of the SUSTAIN 1-7 randomised controlled trials. Lancet Diabetes Endocrinol 2020;8:880–893 [DOI] [PubMed] [Google Scholar]
- 104. Mann JFE, Muskiet MHA. Incretin-based drugs and the kidney in type 2 diabetes: choosing between DPP-4 inhibitors and GLP-1 receptor agonists. Kidney Int 2021;99:314–318 [DOI] [PubMed] [Google Scholar]
- 105. Bakris GL. Major advancements in slowing diabetic kidney disease progression: focus on SGLT2 inhibitors. Am J Kidney Dis 2019;74:573–575 [DOI] [PubMed] [Google Scholar]
- 106. Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al.; DAPA-CKD Trial Committees and Investigators . Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–1446 [DOI] [PubMed] [Google Scholar]
- 107. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357 [DOI] [PubMed] [Google Scholar]
- 108. Novo Nordisk A/S . A research study to see how semaglutide works compared to placebo in people with type 2 diabetes and chronic kidney disease (FLOW). In: ClinicalTrials.gov. Bethesda, MD, National Library of Medicine, 2019. Accessed 20 October 2022. Available from https://clinicaltrials.gov/ct2/show/NCT03819153
- 109. Chertow GM, Vart P, Jongs N, et al.; DAPA-CKD Trial Committees and Investigators . Effects of dapagliflozin in stage 4 chronic kidney disease. J Am Soc Nephrol 2021;32:2352–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Anker SD, Butler J, Filippatos G, et al.; EMPEROR-Preserved Trial Investigators . Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451–1461 [DOI] [PubMed] [Google Scholar]
- 111. Packer M, Anker SD, Butler J, et al.; EMPEROR-Reduced Trial Investigators . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424 [DOI] [PubMed] [Google Scholar]
- 112. Mosenzon O, Wiviott SD, Heerspink HJL, et al. The effect of dapagliflozin on albuminuria in DECLARE-TIMI 58. Diabetes Care 2021;44:1805–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Romera I, Cebri´n-Cuenca A, Álvarez-Guisasola F, Gomez-Peralta F, Reviriego J. A review of practical issues on the use of glucagon-like peptide-1 receptor agonists for the management of type 2 diabetes. Diabetes Ther 2019;10:5–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Bomback AS, Kshirsagar AV, Amamoo MA, Klemmer PJ. Change in proteinuria after adding aldosterone blockers to ACE inhibitors or angiotensin receptor blockers in CKD: a systematic review. Am J Kidney Dis 2008;51:199–211 [DOI] [PubMed] [Google Scholar]
- 115. Sarafidis P, Papadopoulos CE, Kamperidis V, Giannakoulas G, Doumas M. Cardiovascular protection with sodium-glucose cotransporter-2 inhibitors and mineralocorticoid receptor antagonists in chronic kidney disease: a milestone achieved. Hypertension 2021;77:1442–1455 [DOI] [PubMed] [Google Scholar]
- 116. Agarwal R, Kolkhof P, Bakris G, et al. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J 2021;42:152–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Filippatos G, Anker SD, Agarwal R, et al.; FIDELIO-DKD Investigators . Finerenone and cardiovascular outcomes in patients with chronic kidney disease and type 2 diabetes. Circulation 2021;143:540–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Pitt B, Filippatos G, Agarwal R, et al.; FIGARO-DKD Investigators . Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med 2021;385:2252–2263 [DOI] [PubMed] [Google Scholar]
- 119. Agarwal R, Filippatos G, Pitt B, et al.; FIDELIO-DKD and FIGARO-DKD investigators . Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J 2022;43:474–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Smart NA, Dieberg G, Ladhani M, Titus T. Early referral to specialist nephrology services for preventing the progression to end-stage kidney disease. Cochrane Database Syst Rev 2014;6:CD007333. [DOI] [PubMed] [Google Scholar]
- 121. Vassalotti JA, Centor R, Turner BJ, Greer RC, Choi M; National Kidney Foundation Kidney Disease Outcomes Quality Initiative . Practical approach to detection and management of chronic kidney disease for the primary care clinician. Am J Med 2016;129:153–162.e7 [DOI] [PubMed] [Google Scholar]