Abstract
The American Diabetes Association (ADA) “Standards of Care in Diabetes” includes the ADA’s current clinical practice recommendations and is intended to provide the components of diabetes care, general treatment goals and guidelines, and tools to evaluate quality of care. Members of the ADA Professional Practice Committee, a multidisciplinary expert committee, are responsible for updating the Standards of Care annually, or more frequently as warranted. For a detailed description of ADA standards, statements, and reports, as well as the evidence-grading system for ADA’s clinical practice recommendations and a full list of Professional Practice Committee members, please refer to Introduction and Methodology. Readers who wish to comment on the Standards of Care are invited to do so at professional.diabetes.org/SOC.
Recommendations
13.1 Consider the assessment of medical, psychological, functional (self-management abilities), and social domains in older adults to provide a framework to determine targets and therapeutic approaches for diabetes management. B
13.2 Screen for geriatric syndromes (i.e., polypharmacy, cognitive impairment, depression, urinary incontinence, falls, persistent pain, and frailty) in older adults, as they may affect diabetes self-management and diminish quality of life. B
Diabetes is a highly prevalent health condition in the aging population. Over one-quarter of people over the age of 65 years have diabetes, and one-half of older adults have prediabetes (1,2), and the number of older adults living with these conditions is expected to increase rapidly in the coming decades. Diabetes in older adults is also a highly heterogeneous condition. While type 2 diabetes predominates in the older population as much as in the younger population, improvements in insulin delivery, technology, and care over the last few decades have led to increasing numbers of people with childhood and adult-onset type 1 diabetes surviving and thriving into their later decades. Diabetes management in older adults requires regular assessment of medical, psychological, functional, and social domains. When assessing older adults with diabetes, it is important to accurately categorize the type of diabetes as well as other factors, including diabetes duration, the presence of complications, and treatment-related concerns, such as fear of hypoglycemia. Screening for diabetes complications in older adults should be individualized and periodically revisited, as the results of screening tests may impact targets and therapeutic approaches (3–5). Older adults with diabetes have higher rates of premature death, functional disability, accelerated muscle loss, and coexisting illnesses, such as hypertension, coronary heart disease, and stroke, than those without diabetes. At the same time, older adults with diabetes are also at greater risk than other older adults for several common geriatric syndromes, such as polypharmacy, cognitive impairment, depression, urinary incontinence, injurious falls, persistent pain, and frailty (1). These conditions may impact older adults’ diabetes self-management abilities and quality of life if left unaddressed (2,6,7). See Section 4, “Comprehensive Medical Evaluation and Assessment of Comorbidities,” for the full range of issues to consider when caring for older adults with diabetes.
The comprehensive assessment described above may provide a framework to determine targets and therapeutic approaches (8–10), including whether referral for diabetes self-management education is appropriate (when complicating factors arise or when transitions in care occur) or whether the current plan is too complex for the individual’s self-management ability or the caregivers providing care (11). Particular attention should be paid to complications that can develop over short periods of time and/or would significantly impair functional status, such as visual and lower-extremity complications. Please refer to the American Diabetes Association (ADA) consensus report “Diabetes in Older Adults” for details (3).
Neurocognitive Function
Recommendation
13.3 Screening for early detection of mild cognitive impairment or dementia should be performed for adults 65 years of age or older at the initial visit, annually, and as appropriate. B
Older adults with diabetes are at higher risk of cognitive decline and institutionalization (12,13). The presentation of cognitive impairment ranges from subtle executive dysfunction to memory loss and overt dementia. People with diabetes have higher incidences of all-cause dementia, Alzheimer disease, and vascular dementia than people with normal glucose tolerance (14). The effects of hypoglycemia, hyperglycemia, and hyperinsulinemia on the brain are areas of intense research. Poor glycemic control is associated with a decline in cognitive function (15,16), and longer duration of diabetes is associated with worsening cognitive function. There are ongoing studies evaluating whether preventing or delaying diabetes onset may help to maintain cognitive function in older adults. However, studies examining the effects of intensive glycemic and blood pressure control to achieve specific targets have not demonstrated a reduction in brain function decline (17,18).
Clinical trials of specific interventions—including cholinesterase inhibitors and glutamatergic antagonists—have not shown positive therapeutic benefit in maintaining or significantly improving cognitive function or in preventing cognitive decline (19). Pilot studies in individuals with mild cognitive impairment evaluating the potential benefits of intranasal insulin therapy and metformin therapy provide insights for future clinical trials and mechanistic studies (20–23).
Despite the paucity of therapies to prevent or remedy cognitive decline, identifying cognitive impairment early has important implications for diabetes care. The presence of cognitive impairment can make it challenging for clinicians to help their patients reach individualized glycemic, blood pressure, and lipid targets. Cognitive dysfunction makes it difficult for individuals to perform complex self-care tasks (24), such as monitoring glucose and adjusting insulin doses. It also hinders their ability to appropriately maintain the timing of meals and content of the diet. When clinicians are providing care for people with cognitive dysfunction, it is critical to simplify care plans and to facilitate and engage the appropriate support structure to assist individuals in all aspects of care.
Older adults with diabetes should be carefully screened and monitored for cognitive impairment (2). Several simple assessment tools are available to screen for cognitive impairment (24,25), such as the Mini-Mental State Examination (26), Mini-Cog (27), and the Montreal Cognitive Assessment (28), which may help to identify individuals requiring neuropsychological evaluation, particularly those in whom dementia is suspected (i.e., experiencing memory loss and decline in their basic and instrumental activities of daily living). Annual screening is indicated for adults 65 years of age or older for early detection of mild cognitive impairment or dementia (4,29). Screening for cognitive impairment should additionally be considered when an individual presents with a significant decline in clinical status due to increased problems with self-care activities, such as errors in calculating insulin dose, difficulty counting carbohydrates, skipped meals, skipped insulin doses, and difficulty recognizing, preventing, or treating hypoglycemia. People who screen positive for cognitive impairment should receive diagnostic assessment as appropriate, including referral to a behavioral health professional for formal cognitive/neuropsychological evaluation (30).
Hypoglycemia
Recommendations
13.4 Because older adults with diabetes have a greater risk of hypoglycemia than younger adults, episodes of hypoglycemia should be ascertained and addressed at routine visits. B
13.5 For older adults with type 1 diabetes, continuous glucose monitoring is recommended to reduce hypoglycemia. A
13.6 For older adults with type 2 diabetes on multiple daily doses of insulin, continuous glucose monitoring should be considered to improve glycemic outcomes and decrease glucose variability. B
13.7 For older adults with type 1 diabetes, consider the use of automated insulin delivery systems B and other advanced insulin delivery devices such as connected pens E to reduce risk of hypoglycemia, based on individual ability.
Older adults are at higher risk of hypoglycemia for many reasons, including insulin deficiency necessitating insulin therapy and progressive renal insufficiency (31). As described above, older adults have higher rates of unidentified cognitive impairment and dementia, leading to difficulties in adhering to complex self-care activities (e.g., glucose monitoring, insulin dose adjustment). Cognitive decline has been associated with increased risk of hypoglycemia, and conversely, severe hypoglycemia has been linked to increased risk of dementia (32,33). Therefore, as discussed in Recommendation 13.3, it is important to routinely screen older adults for cognitive impairment and dementia and discuss findings with the patients and their caregivers.
People with diabetes and their caregivers should be routinely queried about hypoglycemia (e.g., selected questions from the Diabetes Care Profile) (34) and hypoglycemia unawareness (35). Older adults can also be stratified for future risk for hypoglycemia with validated risk calculators (e.g., Kaiser Hypoglycemia Model) (36). An important step to mitigate hypoglycemia risk is to determine whether the person with diabetes is skipping meals or inadvertently repeating doses of their medications. Glycemic targets and pharmacologic treatments may need to be adjusted to minimize the occurrence of hypoglycemic events (2). This recommendation is supported by results from multiple randomized controlled trials, such as the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study and the Veterans Affairs Diabetes Trial (VADT), which showed that intensive treatment protocols targeting A1C <6.0% with complex drug regimens significantly increased the risk for hypoglycemia requiring assistance compared with standard treatment (37,38). However, these intensive treatment plans included extensive use of insulin and minimal use of glucagon-like peptide 1 (GLP-1) receptor agonists, and they preceded the availability of sodium–glucose cotransporter 2 (SGLT2) inhibitors.
For older people with type 1 diabetes, continuous glucose monitoring (CGM) is a useful approach to predicting and reducing the risk of hypoglycemia (39). In the Wireless Innovation in Seniors with Diabetes Mellitus (WISDM) trial, adults over 60 years of age with type 1 diabetes were randomized to CGM or standard blood glucose monitoring. Over 6 months, use of CGM resulted in a small but statistically significant reduction in time spent with hypoglycemia (glucose level <70 mg/dL) compared with standard blood glucose monitoring (adjusted treatment difference −1.9% [−27 min/day]; 95% CI −2.8% to −1.1% [−40 to −16 min/day]; P < 0.001) (40,41). Among secondary outcomes, glycemic variability was reduced with CGM, as reflected by an 8% (95% CI 6.0–11.5) increase in time spent in range between 70 and 180 mg/dL. A 6-month extension of the trial demonstrated that these benefits were sustained for up to a year (42). These and other short-term trials are supported by observational data from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study indicating that among older adults (mean age 58 years) with long-standing type 1 diabetes, routine CGM and insulin pump use was associated with fewer hypoglycemic events and hyperglycemic excursions and lower A1C levels (43). While the current evidence base for older adults is primarily in type 1 diabetes, the evidence demonstrating the clinical benefits of CGM for people with type 2 diabetes using insulin is growing (44) (see Section 7, “Diabetes Technology”). The DIAMOND (Multiple Daily Injections and Continuous Glucose Monitoring in Diabetes) study demonstrated that in adults ≥60 years of age with either type 1 or type 2 diabetes using multiple daily injections, CGM use was associated with improved A1C and reduced glycemic variability (45). Another population for which CGM may play an increasing role is older adults with physical or cognitive limitations who require monitoring of blood glucose by a surrogate.
The availability of accurate CGM devices that can communicate with insulin pumps through Bluetooth has enabled the development of advanced insulin delivery algorithms for pumps. These algorithms fall into two categories: predictive low-glucose suspend algorithms that automatically shut off insulin delivery if a hypoglycemic event is imminent and hybrid closed-loop algorithms that automatically adjust insulin infusion rates based on feedback from a CGM to keep glucose levels in a target range. Advanced insulin delivery devices have been shown to improve glycemic outcomes in both children and adults with type 1 diabetes. Most trials of these devices have included a broad range of people with type 1 diabetes but relatively few older adults. Recently, two small randomized controlled trials in older adults have been published. The Older Adult Closed Loop (ORACL) trial in 30 older adults (mean age 67 years) with type 1 diabetes found that a hybrid closed-loop insulin delivery strategy was associated with significant improvements in time in range compared with sensor-augmented pump therapy (46). Moreover, they found small but significant decreases in hypoglycemia with the hybrid closed-loop strategy. Boughton et al. (47) reported results of an open-label, crossover design clinical trial in 37 older adults (≥60 years) in which 16 weeks of treatment with a hybrid closed-loop advanced insulin delivery system was compared with sensor-augmented pump therapy. They found that hybrid closed-loop insulin delivery improved the proportion of time glucose was in range largely due to decreases in hyperglycemia. In contrast to the ORACL study, no significant differences in hypoglycemia were observed. Both studies enrolled older individuals whose blood glucose was relatively well managed (mean A1C ∼7.4%), and both used a crossover design comparing hybrid closed-loop insulin delivery to sensor-augmented pump therapy. These trials provide the first evidence that older individuals with long-standing type 1 diabetes can successfully use advanced insulin delivery technologies to improve glycemic outcomes, as has been seen in younger populations. Use of such technologies should be periodically reassessed, as the burden may outweigh the benefits in those with declining cognitive or functional status.
Treatment Goals
Recommendations
13.8 Older adults who are otherwise healthy with few coexisting chronic illnesses and intact cognitive function and functional status should have lower glycemic goals (such as A1C <7.0–7.5% [53–58 mmol/mol]), while those with multiple coexisting chronic illnesses, cognitive impairment, or functional dependence should have less-stringent glycemic goals (such as A1C <8.0% [64 mmol/mol]). C
13.9 Glycemic goals for some older adults might reasonably be relaxed as part of individualized care, but hyperglycemia leading to symptoms or risk of acute hyperglycemia complications should be avoided in all people with diabetes. C
13.10 Screening for diabetes complications should be individualized in older adults. Particular attention should be paid to complications that would lead to functional impairment. C
13.11 Treatment of hypertension to individualized target levels is indicated in most older adults. C
13.12 Treatment of other cardiovascular risk factors should be individualized in older adults considering the time frame of benefit. Lipid-lowering therapy and aspirin therapy may benefit those with life expectancies at least equal to the time frame of primary prevention or secondary intervention trials. E
The care of older adults with diabetes is complicated by their clinical, cognitive, and functional heterogeneity. Some older individuals may have developed diabetes years earlier and have significant complications, others are newly diagnosed and may have had years of undiagnosed diabetes with resultant complications, and still, other older adults may have truly recent-onset disease with few or no complications (48). Some older adults with diabetes have other underlying chronic conditions, substantial diabetes-related comorbidity, limited cognitive or physical functioning, or frailty (49,50). Other older individuals with diabetes have little comorbidity and are active. Life expectancies are highly variable but are often longer than clinicians realize. Multiple prognostic tools for life expectancy for older adults are available (51), including tools specifically designed for older adults with diabetes (52). Older patients also vary in their preferences for the intensity and mode of glucose control (53). Health care professionals caring for older adults with diabetes must take this heterogeneity into consideration when setting and prioritizing treatment goals (9,10) (Table 13.1). In addition, older adults with diabetes should be assessed for disease treatment and self-management knowledge, health literacy, and mathematical literacy (numeracy) at the onset of treatment. See Fig. 6.2 for patient/disease-related factors to consider when determining individualized glycemic targets.
Table 13.1.
Framework for considering treatment goals for glycemia, blood pressure, and dyslipidemia in older adults with diabetes
| Patient characteristics/health status | Rationale | Reasonable A1C goal‡ | Fasting or preprandial glucose | Bedtime glucose | Blood pressure | Lipids |
|---|---|---|---|---|---|---|
| Healthy (few coexisting chronic illnesses, intact cognitive and functional status) | Longer remaining life expectancy | <7.0–7.5% (53–58 mmol/mol) | 80–130 mg/dL (4.4–7.2 mmol/L) | 80–180 mg/dL (4.4–10.0 mmol/L) | <130/80 mmHg | Statin, unless contraindicated or not tolerated |
| Complex/intermediate (multiple coexisting chronic illnesses* or two or more instrumental ADL impairments or mild-to-moderate cognitive impairment) | Intermediate remaining life expectancy, high treatment burden, hypoglycemia vulnerability, fall risk | <8.0% (64 mmol/mol) | 90–150 mg/dL (5.0–8.3 mmol/L) | 100–180 mg/dL (5.6–10.0 mmol/L) | <130/80 mmHg | Statin, unless contraindicated or not tolerated |
| Very complex/poor health (LTC or end-stage chronic illnesses** or moderate-to-severe cognitive impairment or two or more ADL impairments) | Limited remaining life expectancy makes benefit uncertain | Avoid reliance on A1C; glucose control decisions should be based on avoiding hypoglycemia and symptomatic hyperglycemia | 100–180 mg/dL (5.6–10.0 mmol/L) | 110–200 mg/dL (6.1–11.1 mmol/L) | <140/90 mmHg | Consider likelihood of benefit with statin |
This table represents a consensus framework for considering treatment goals for glycemia, blood pressure, and dyslipidemia in older adults with diabetes. The patient characteristic categories are general concepts. Not every patient will clearly fall into a particular category. Consideration of patient and caregiver preferences is an important aspect of treatment individualization. Additionally, a patient’s health status and preferences may change over time. ADL, activities of daily living; LTC, long-term care.
A lower A1C goal may be set for an individual if achievable without recurrent or severe hypoglycemia or undue treatment burden.
Coexisting chronic illnesses are conditions serious enough to require medications or lifestyle management and may include arthritis, cancer, heart failure, depression, emphysema, falls, hypertension, incontinence, stage 3 or worse chronic kidney disease, myocardial infarction, and stroke. “Multiple” means at least three, but many patients may have five or more (66).
The presence of a single end-stage chronic illness, such as stage 3–4 heart failure or oxygen-dependent lung disease, chronic kidney disease requiring dialysis, or uncontrolled metastatic cancer, may cause significant symptoms or impairment of functional status and significantly reduce life expectancy. Adapted from Kirkman et al. (3).
A1C may have limitations in those who have medical conditions that impact red blood cell turnover (see Section 2, “Classification and Diagnosis of Diabetes,” for additional details on the limitations of A1C) (54). Many conditions associated with increased red blood cell turnover, such as hemodialysis, recent blood loss or transfusion, or erythropoietin therapy, are commonly seen in older adults and can falsely increase or decrease A1C. In these instances, plasma blood glucose fingerstick and sensor glucose readings should be used for goal setting (Table 13.1).
Older Adults with Good Functional Status and without Complications
There are few long-term studies in older adults demonstrating the benefits of intensive glycemic, blood pressure, and lipid control. Older adults who can be expected to live long enough to realize the benefits of long-term intensive diabetes management, who have good cognitive and physical function, and who choose to do so via shared decision-making may be treated using therapeutic interventions and goals similar to those for younger adults with diabetes (Table 13.1).
As for all people with diabetes, diabetes self-management education and ongoing diabetes self-management support are vital components of diabetes care for older adults and their caregivers. Self-management knowledge and skills should be reassessed when treatment plan changes are made or an individual’s functional abilities diminish. In addition, declining or impaired ability to perform diabetes self-care behaviors may be an indication that an older person with diabetes needs a referral for cognitive and physical functional assessment, using age-normalized evaluation tools, as well as help establishing a support structure for diabetes care (3,30).
Patients with Complications and Reduced Functionality
For people with advanced diabetes complications, life-limiting comorbid illnesses, or substantial cognitive or functional impairments, it is reasonable to set less-intensive glycemic goals (Table 13.1). Factors to consider in individualizing glycemic goals are outlined in Fig. 6.2. Based on concepts of competing mortality and time to benefit, people with advanced diabetes complications are less likely to benefit from reducing the risk of microvascular complications (55). In addition, they are more likely to suffer serious adverse effects of therapeutics, such as hypoglycemia (56). However, those with poorly managed diabetes may be subject to acute complications of diabetes, including dehydration, poor wound healing, and hyperglycemic hyperosmolar coma. Glycemic goals should, at a minimum, avoid these consequences.
While Table 13.1 provides overall guidance for identifying complex and very complex patients, there is not yet global consensus on geriatric patient classification. Ongoing empiric research on the classification of older adults with diabetes based on comorbid illness has repeatedly found three major classes of patients: a healthy, a geriatric, and a cardiovascular class (9,57). The geriatric class has the highest prevalence of obesity, hypertension, arthritis, and incontinence, and the cardiovascular class has the highest prevalence of myocardial infarctions, heart failure, and stroke. Compared with the healthy class, the cardiovascular class has the highest risk of frailty and subsequent mortality. Additional research is needed to develop a reproducible classification scheme to distinguish the natural history of disease as well as differential response to glucose control and specific glucose-lowering agents (58).
Vulnerable Patients at the End of Life
For people with diabetes receiving palliative care and end-of-life care, the focus should be to avoid hypoglycemia and symptomatic hyperglycemia while reducing the burdens of glycemic management. Thus, as organ failure develops, several agents will have to be deintensified or discontinued. For a dying person, most agents for type 2 diabetes may be removed (59). There is, however, no consensus for the management of type 1 diabetes in this scenario (60). See the section end-of-life care below for additional information.
Beyond Glycemic Management
Although minimizing hyperglycemia may be important in older individuals with diabetes, greater reductions in morbidity and mortality are likely to result from a clinical focus on comprehensive cardiovascular risk factor modification. There is strong evidence from clinical trials of the value of treating hypertension in older adults (61,62), with treatment of hypertension to individualized target levels indicated in most. There is less evidence for lipid-lowering therapy and aspirin therapy, although the benefits of these interventions for primary and secondary prevention are likely to apply to older adults whose life expectancies equal or exceed the time frames of the clinical trials (63). In the case of statins, the follow-up time of clinical trials ranged from 2 to 6 years. While the time frame of trials can be used to inform treatment decisions, a more specific concept is the time to benefit for a therapy. For statins, a meta-analysis of the previously mentioned trials showed that the time to benefit is 2.5 years (64).
Lifestyle Management
Recommendations
13.13 Optimal nutrition and protein intake is recommended for older adults; regular exercise, including aerobic activity, weight-bearing exercise, and/or resistance training, should be encouraged in all older adults who can safely engage in such activities. B
13.14 For older adults with type 2 diabetes, overweight/obesity, and capacity to safely exercise, an intensive lifestyle intervention focused on dietary changes, physical activity, and modest weight loss (e.g., 5–7%) should be considered for its benefits on quality of life, mobility and physical functioning, and cardiometabolic risk factor control. A
Lifestyle management in older adults should be tailored to frailty status. Diabetes in the aging population is associated with reduced muscle strength, poor muscle quality, and accelerated loss of muscle mass, which may result in sarcopenia and/or osteopenia (65,66). Diabetes is also recognized as an independent risk factor for frailty. Frailty is characterized by decline in physical performance and an increased risk of poor health outcomes due to physiologic vulnerability and functional or psychosocial stressors. Inadequate nutritional intake, particularly inadequate protein intake, can increase the risk of sarcopenia and frailty in older adults. Management of frailty in diabetes includes optimal nutrition with adequate protein intake combined with an exercise program that includes aerobic, weight-bearing, and resistance training. The benefits of a structured exercise program (as in the Lifestyle Interventions and Independence for Elders [LIFE] study) in frail older adults include reducing sedentary time, preventing mobility disability, and reducing frailty (67,68). The goal of these programs is not weight loss but enhanced functional status.
For nonfrail older adults with type 2 diabetes and overweight or obesity, an intensive lifestyle intervention designed to reduce weight is beneficial across multiple outcomes. The Look AHEAD (Action for Health in Diabetes) trial is described in Section 8, “Obesity and Weight Management for the Prevention and Treatment of Type 2 Diabetes.” Look AHEAD specifically excluded individuals with a low functional status. It enrolled people between 45 and 74 years of age and required that they be able to perform a maximal exercise test (69,70). While the Look AHEAD trial did not achieve its primary outcome of reducing cardiovascular events, the intensive lifestyle intervention had multiple clinical benefits that are important to the quality of life of older adults. Benefits included weight loss, improved physical fitness, increased HDL cholesterol, lowered systolic blood pressure, reduced A1C levels, reduced waist circumference, and reduced need for medications (71). Additionally, several subgroups, including participants who lost at least 10% of baseline body weight at year 1, had improved cardiovascular outcomes (72). Risk factor control was improved with reduced utilization of antihypertensive medications, statins, and insulin (73). In age-stratified analyses, older adults in the trial (60 to early 70s) had similar benefits compared with younger people (74,75). In addition, lifestyle intervention produced benefits on aging-relevant outcomes such as reductions in multimorbidity and improvements in physical function and quality of life (76–79).
Pharmacologic Therapy
Recommendations
13.15 In older adults with type 2 diabetes at increased risk of hypoglycemia, medication classes with low risk of hypoglycemia are preferred. B
13.16 Overtreatment of diabetes is common in older adults and should be avoided. B
13.17 Deintensification of treatment goals is recommended to reduce the risk of hypoglycemia if it can be achieved within the individualized A1C target. B
13.18 Simplification of complex treatment plans (especially insulin) is recommended to reduce the risk of hypoglycemia and polypharmacy and decrease the burden of the disease if it can be achieved within the individualized A1C target. B
13.19 Consider costs of care and insurance coverage rules when developing treatment plans in order to reduce risk of cost-related barriers to adherence. B
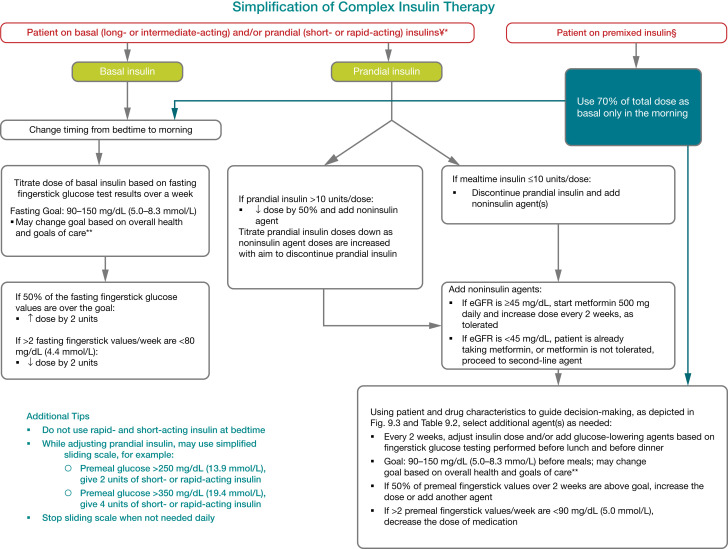
Special care is required in prescribing and monitoring pharmacologic therapies in older adults (80). See Fig. 9.3 for general recommendations regarding glucose-lowering treatment for adults with type 2 diabetes and Table 9.2 for person- and drug-specific factors to consider when selecting glucose-lowering agents. Cost may be an especially important consideration, as older adults tend to be on many medications and live on fixed incomes (81). Accordingly, the costs of care and insurance coverage rules should be considered when developing treatment plans to reduce the risk of cost-related barriers to adherence (82,83). See Table 9.3 and Table 9.4 for median monthly cost in the U.S. of noninsulin glucose-lowering agents and insulin, respectively. It is important to match complexity of the treatment plan to the self-management ability of older adults with diabetes and their available social and medical support. Many older adults with diabetes struggle to maintain the frequent blood glucose monitoring and insulin injection regimens they previously followed, perhaps for many decades, as they develop medical conditions that may impair their ability to follow their treatment plan safely. Individualized glycemic goals should be established (Fig. 6.2) and periodically adjusted based on coexisting chronic illnesses, cognitive function, and functional status (2). Intensive glycemic control with regimens including insulin and sulfonylureas in older adults with complex or very complex medical conditions has been identified as overtreatment and found to be very common in clinical practice (84–88). Ultimately, the determination of whether a person is considered overtreated requires an elicitation of the person’s perceptions of the current medication burden and preferences for treatments. For those seeking to simplify their diabetes regimen, deintensification of regimens in individuals taking noninsulin glucose-lowering medications can be achieved by either lowering the dose or discontinuing some medications, as long as the individualized glycemic targets are maintained (89). When older adults are found to have an insulin regimen with complexity beyond their self-management abilities, lowering the dose of insulin may not be adequate (90). Simplification of the insulin plan to match an individual’s self-management abilities and their available social and medical support in these situations has been shown to reduce hypoglycemia and disease-related distress without worsening glycemic outcomes (91–94). Figure 13.1 depicts an algorithm that can be used to simplify the insulin regimen (93). There are now multiple studies evaluating deintensification protocols in diabetes as well as hypertension, demonstrating that deintensification is safe and possibly beneficial for older adults (89). Table 13.2 provides examples of and rationale for situations where deintensification and/or insulin regimen simplification may be appropriate in older adults.
Figure 13.1.
Algorithm to simplify insulin regimen for older adults with type 2 diabetes. eGFR, estimated glomerular filtration rate. *Basal insulins: glargine U-100 and U-300, detemir, degludec, and human NPH. **See Table 13.1. ¥Prandial insulins: short-acting (regular human insulin) or rapid-acting (lispro, aspart, and glulisine). §Premixed insulins: 70/30, 75/25, and 50/50 products. Adapted with permission from Munshi et al. (93).
Table 13.2.
Considerations for treatment regimen simplification and deintensification/deprescribing in older adults with diabetes (93,128)
| Patient characteristics/health status | Reasonable A1C/treatment goal | Rationale/considerations | When may regimen simplification be required? | When may treatment deintensification/deprescribing be required? |
|---|---|---|---|---|
| Healthy (few coexisting chronic illnesses, intact cognitive and functional status) | <7.0–7.5% (53–58 mmol/mol) | • Patients can generally perform complex tasks to maintain good glycemic control when health is stable • During acute illness, patients may be more at risk for administration or dosing errors that can result in hypoglycemia, falls, fractures, etc. |
• If severe or recurrent hypoglycemia occurs in patients on insulin therapy (regardless of A1C) • If wide glucose excursions are observed • If cognitive or functional decline occurs following acute illness |
• If severe or recurrent hypoglycemia occurs in patients on noninsulin therapies with high risk of hypoglycemia (regardless of A1C) • If wide glucose excursions are observed • In the presence of polypharmacy |
| Complex/intermediate (multiple coexisting chronic illnesses or two or more instrumental ADL impairments or mild-to-moderate cognitive impairment) | <8.0% (64 mmol/mol) | • Comorbidities may affect self-management abilities and capacity to avoid hypoglycemia • Long-acting medication formulations may decrease pill burden and complexity of medication regimen |
• If severe or recurrent hypoglycemia occurs in patients on insulin therapy (even if A1C is appropriate) • If unable to manage complexity of an insulin regimen • If there is a significant change in social circumstances, such as loss of caregiver, change in living situation, or financial difficulties |
• If severe or recurrent hypoglycemia occurs in patients on noninsulin therapies with high risk of hypoglycemia (even if A1C is appropriate) • If wide glucose excursions are observed • In the presence of polypharmacy |
| Community-dwelling patients receiving care in a skilled nursing facility for short-term rehabilitation | Avoid reliance on A1C, glucose target 100–200 mg/dL (5.55–11.1 mmol/L) | • Glycemic control is important for recovery, wound healing, hydration, and avoidance of infections • Patients recovering from illness may not have returned to baseline cognitive function at the time of discharge • Consider the type of support the patient will receive at home |
• If treatment regimen increased in complexity during hospitalization, it is reasonable, in many cases, to reinstate the prehospitalization medication regimen during the rehabilitation | • If the hospitalization for acute illness resulted in weight loss, anorexia, short-term cognitive decline, and/or loss of physical functioning |
| Very complex/poor health (LTC or end-stage chronic illnesses or moderate-to-severe cognitive impairment or two or more ADL impairments) | Avoid reliance on A1C and avoid hypoglycemia and symptomatic hyperglycemia | • No benefits of tight glycemic control in this population • Hypoglycemia should be avoided • Most important outcomes are maintenance of cognitive and functional status |
• If on an insulin regimen and the patient would like to decrease the number of injections and fingerstick blood glucose monitoring events each day • If the patient has an inconsistent eating pattern |
• If on noninsulin agents with a high hypoglycemia risk in the context of cognitive dysfunction, depression, anorexia, or inconsistent eating pattern • If taking any medications without clear benefits |
| At the end of life | Avoid hypoglycemia and symptomatic hyperglycemia | • Goal is to provide comfort and avoid tasks or interventions that cause pain or discomfort • Caregivers are important in providing medical care and maintaining quality of life |
• If there is pain or discomfort caused by treatment (e.g., injections or finger sticks) • If there is excessive caregiver stress due to treatment complexity |
• If taking any medications without clear benefits in improving symptoms and/or comfort |
Treatment regimen simplification refers to changing strategy to decrease the complexity of a medication regimen (e.g., fewer administration times, fewer blood glucose checks) and decreasing the need for calculations (such as sliding-scale insulin calculations or insulin-carbohydrate ratio calculations). Deintensification/deprescribing refers to decreasing the dose or frequency of administration of a treatment or discontinuing a treatment altogether. ADL, activities of daily living; LTC, long-term care.
Metformin
Metformin is the first-line agent for older adults with type 2 diabetes. Recent studies have indicated that it may be used safely in individuals with estimated glomerular filtration rate ≥30 mL/min/1.73 m2 (95). However, it is contraindicated in those with advanced renal insufficiency and should be used with caution in those with impaired hepatic function or heart failure because of the increased risk of lactic acidosis. Metformin may be temporarily discontinued before procedures, during hospitalizations, and when acute illness may compromise renal or liver function. Additionally, metformin can cause gastrointestinal side effects and a reduction in appetite that can be problematic for some older adults. Reduction or elimination of metformin may be necessary for those experiencing persistent gastrointestinal side effects. For those taking metformin long-term, monitoring for vitamin B12 deficiency should be considered (96).
Thiazolidinediones
Thiazolidinediones, if used at all, should be used very cautiously in older adults on insulin therapy as well as in those with or at risk for heart failure, osteoporosis, falls or fractures, and/or macular edema (97,98). Lower doses of a thiazolidinedione in combination therapy may mitigate these side effects.
Insulin Secretagogues
Sulfonylureas and other insulin secretagogues are associated with hypoglycemia and should be used with caution. If used, sulfonylureas with a shorter duration of action, such as glipizide, are preferred. Glyburide is a longer-acting sulfonylurea and should be avoided in older adults (99).
Incretin-Based Therapies
Oral dipeptidyl peptidase 4 (DPP-4) inhibitors have few side effects and minimal risk of hypoglycemia, but their cost may be a barrier to some older adults. DPP-4 inhibitors do not reduce or increase major adverse cardiovascular outcomes (100). Across the trials of this drug class, there appears to be no interaction by age-group (101–103). A challenge of interpreting the age-stratified analyses of this drug class and other cardiovascular outcomes trials is that while most of these analyses were prespecified, they were not powered to detect differences.
GLP-1 receptor agonists have demonstrated cardiovascular benefits among people with diabetes and established atherosclerotic cardiovascular disease (ASCVD) and those at higher ASCVD risk, and newer trials are expanding our understanding of their benefits in other populations (100). See Section 9, “Pharmacologic Approaches to Glycemic Treatment,” and Section 10, “Cardiovascular Disease and Risk Management,” for a more extensive discussion regarding the specific indications for this class of agents. In a systematic review and meta-analysis of GLP-1 receptor agonist trials, these agents have been found to reduce major adverse cardiovascular events, cardiovascular deaths, stroke, and myocardial infarction to the same degree for people over and under 65 years of age (104). While the evidence for this class of agents for older adults continues to grow, there are a number of practical issues that should be considered specifically for older people. These drugs are injectable agents (with the exception of oral semaglutide) (105), which require visual, motor, and cognitive skills for appropriate administration. Agents with a weekly dosing schedule may reduce the burden of administration. GLP-1 receptor agonists may also be associated with nausea, vomiting, and diarrhea. Given the gastrointestinal side effects of this class, GLP-1 receptor agonists may not be preferred in older adults who are experiencing unexplained weight loss.
Sodium–Glucose Cotransporter 2 Inhibitors
SGLT2 inhibitors are administered orally, which may be convenient for older adults with diabetes. In those with established ASCVD, these agents have shown cardiovascular benefits (100). This class of agents has also been found to be beneficial for people with heart failure and to slow the progression of chronic kidney disease. See Section 9, “Pharmacologic Approaches to Glycemic Treatment,” and Section 10, “Cardiovascular Disease and Risk Management,” for a more extensive discussion regarding the indications for this class of agents. The stratified analyses of the trials of this drug class indicate that older adults have similar or greater benefits than younger people (106–108). While understanding of the clinical benefits of this class is evolving, side effects such as volume depletion, urinary tract infections, and worsening urinary incontinence may be more common among older people.
Insulin Therapy
The use of insulin therapy requires that individuals or their caregivers have good visual and motor skills and cognitive ability. Insulin therapy relies on the ability of the older person with diabetes to administer insulin on their own or with the assistance of a caregiver. Insulin doses should be titrated to meet individualized glycemic targets and to avoid hypoglycemia.
Once-daily basal insulin injection therapy is associated with minimal side effects and may be a reasonable option in many older adults (109). When choosing a basal insulin, long-acting insulin analogs have been found to be associated with a lower risk of hypoglycemia compared with NPH insulin in the Medicare population. Multiple daily injections of insulin may be too complex for an older person with advanced diabetes complications, life-limiting coexisting chronic illnesses, or limited functional status. Figure 13.1 provides a potential approach to insulin regimen simplification.
Other Factors to Consider
The needs of older adults with diabetes and their caregivers should be evaluated to construct a tailored care plan. Impaired social functioning may reduce these individuals’ quality of life and increase the risk of functional dependency (7). The person’s living situation must be considered as it may affect diabetes management and support needs. Social and instrumental support networks (e.g., adult children, caretakers) that provide instrumental or emotional support for older adults with diabetes should be included in diabetes management discussions and shared decision-making.
The need for ongoing support of older adults becomes even greater when transitions to acute care and long-term care (LTC) become necessary. Unfortunately, these transitions can lead to discontinuity in goals of care, errors in dosing, and changes in nutrition and activity (110). Older adults in assisted living facilities may not have support to administer their own medications, whereas those living in a nursing home (community living centers) may rely completely on the care plan and nursing support. Those receiving palliative care (with or without hospice) may require an approach that emphasizes comfort and symptom management while de-emphasizing strict metabolic and blood pressure control.
Special Considerations for Older Adults with Type 1 Diabetes
Due in part to the success of modern diabetes management, people with type 1 diabetes are living longer, and the population of these people over 65 years of age is growing (111–113). Many of the recommendations in this section regarding a comprehensive geriatric assessment and personalization of goals and treatments are directly applicable to older adults with type 1 diabetes; however, this population has unique challenges and requires distinct treatment considerations (114). Insulin is an essential life-preserving therapy for people with type 1 diabetes, unlike for those with type 2 diabetes. To avoid diabetic ketoacidosis, older adults with type 1 diabetes need some form of basal insulin even when they are unable to ingest meals. Insulin may be delivered through an insulin pump or injections. CGM is approved for use by Medicare and can play a critical role in improving A1C, reducing glycemic variability, and reducing risk of hypoglycemia (45) (see Section 7, “Diabetes Technology,” and Section 9, “Pharmacologic Approaches to Glycemic Treatment”). In older people with type 1 diabetes, administration of insulin may become more difficult as complications, cognitive impairment, and functional impairment arise. This increases the importance of caregivers in the lives of these individuals. Many older people with type 1 diabetes require placement in LTC settings (i.e., nursing homes and skilled nursing facilities) and unfortunately can encounter staff that are less familiar with insulin pumps or CGM. Some staff may be less knowledgeable about the differences between type 1 and type 2 diabetes. In these instances, the individual or the person’s family may be more familiar with their diabetes management plan than the staff or health care professionals. Education of relevant support staff and health care professionals in rehabilitation and LTC settings regarding insulin dosing and use of pumps and CGM is recommended as part of general diabetes education (see Recommendations 13.20 and 13.21).
Treatment in Skilled Nursing Facilities and Nursing Homes
Recommendations
13.20 Consider diabetes education for the staff of long-term care and rehabilitation facilities to improve the management of older adults with diabetes. E
13.21 People with diabetes residing in long-term care facilities need careful assessment to establish individualized glycemic goals and to make appropriate choices of glucose-lowering agents based on their clinical and functional status. E
13.22 Consider use of continuous glucose monitoring to assess risk for hypoglycemia in older adults treated with sulfonylureas or insulin. E
Management of diabetes in the LTC setting is unique. Individualization of health care is important for all people with diabetes; however, practical guidance is needed for health care professionals as well as the LTC staff and caregivers (115). Training should include diabetes detection and institutional quality assessment. LTC facilities should develop their own policies and procedures for prevention and management of hypoglycemia. With the increased longevity of populations, the care of people with diabetes and its complications in LTC is an area that warrants greater study.
Resources
Staff of LTC facilities should receive appropriate diabetes education to improve the management of older adults with diabetes. Treatments for each patient should be individualized. Special management considerations include the need to avoid both hypoglycemia and the complications of hyperglycemia (2,116). For more information, see the ADA position statement “Management of Diabetes in Long-term Care and Skilled Nursing Facilities” (115).
Nutritional Considerations
An older adult residing in an LTC facility may have irregular and unpredictable meal consumption, undernutrition, anorexia, and impaired swallowing. Furthermore, therapeutic diets may inadvertently lead to decreased food intake and contribute to unintentional weight loss and undernutrition. Meals tailored to a person’s culture, preferences, and personal goals may increase quality of life, satisfaction with meals, and nutrition status (117). It may be helpful to give insulin after meals to ensure that the dose is appropriate for the amount of carbohydrate the individual consumed in the meal.
Hypoglycemia
Older adults with diabetes in LTC are especially vulnerable to hypoglycemia. They have a disproportionately high number of clinical complications and comorbidities that can increase hypoglycemia risk: impaired cognitive and renal function, slowed hormonal regulation and counterregulation, suboptimal hydration, variable appetite and nutritional intake, polypharmacy, and slowed intestinal absorption (118). Oral agents may achieve glycemic outcomes similar to basal insulin in LTC populations (84,119). CGM may be a useful approach to monitoring for hypoglycemia among individuals treated with insulin in LTC, but the data are limited.
Another consideration for the LTC setting is that unlike in the hospital setting, health care professionals are not required to evaluate patients daily. According to federal guidelines, assessments should be done at least every 30 days for the first 90 days after admission and then at least once every 60 days. Although in practice patients may actually be seen more frequently, the concern is that these individuals may have uncontrolled glucose levels or wide excursions without the practitioner being notified. Health care professionals may adjust treatment plans by telephone, fax, or in person directly at the LTC facilities, provided they are given timely notification of blood glucose management issues from a standardized alert system.
The following alert strategy could be considered:
Call health care professional immediately in cases of low blood glucose levels (<70 mg/dL [3.9 mmol/L]).
- Call as soon as possible when
- glucose values are 70–100 mg/dL (3.9–5.6 mmol/L) (treatment plan may need to be adjusted),
- glucose values are consistently >250 mg/dL (13.9 mmol/L) within a 24-h period,
- glucose values are consistently >300 mg/dL (16.7 mmol/L) over 2 consecutive days,
- any reading is too high for the glucose monitoring device, or
- the person is sick, with vomiting, symptomatic hyperglycemia, or poor oral intake.
End-of-Life Care
Recommendations
13.23 When palliative care is needed in older adults with diabetes, health care professionals should initiate conversations regarding the goals and intensity of care. Strict glucose and blood pressure control are not necessary E, and simplification of regimens can be considered. Similarly, the intensity of lipid management can be relaxed, and withdrawal of lipid-lowering therapy may be appropriate. A
13.24 Overall comfort, prevention of distressing symptoms, and preservation of quality of life and dignity are primary goals for diabetes management at the end of life. C
The management of the older adult at the end of life receiving palliative medicine or hospice care is a unique situation. Overall, palliative medicine promotes comfort, symptom control and prevention (pain, hypoglycemia, hyperglycemia, and dehydration), and preservation of dignity and quality of life in older adults with limited life expectancy (116,120). In the setting of palliative care, health care professionals should initiate conversations regarding the goals and intensity of diabetes care; strict glucose and blood pressure control may not be consistent with achieving comfort and quality of life. Avoidance of severe hypertension and hyperglycemia aligns with the goals of palliative care. In a multicenter trial, withdrawal of statins among people with diabetes in palliative care was found to improve quality of life (121–123). The evidence for the safety and efficacy of deintensification protocols in older adults is growing for both glucose and blood pressure control (88,124) and is clearly relevant for palliative care. An individual has the right to refuse testing and treatment, whereas health care professionals may consider withdrawing treatment and limiting diagnostic testing, including a reduction in the frequency of blood glucose monitoring (125,126). Glucose targets should aim to prevent hypoglycemia and hyperglycemia. Treatment interventions need to be mindful of quality of life. Careful monitoring of oral intake is warranted. The decision process may need to involve the individual, family, and caregivers, leading to a care plan that is both convenient and effective for the goals of care (127). The pharmacologic therapy may include oral agents as first line, followed by a simplified insulin regimen. If needed, basal insulin can be implemented, accompanied by oral agents and without rapid-acting insulin. Agents that can cause gastrointestinal symptoms such as nausea or excess weight loss may not be good choices in this setting. As symptoms progress, some agents may be slowly tapered and discontinued.
Different patient categories have been proposed for diabetes management in those with advanced disease (59).
A stable patient: Continue with the person’s previous regimen, with a focus on 1) the prevention of hypoglycemia and 2) the management of hyperglycemia using blood glucose testing, keeping levels below the renal threshold of glucose, and hyperglycemia-mediated dehydration. There is no role for A1C monitoring.
A patient with organ failure: Preventing hypoglycemia is of greatest significance. Dehydration must be prevented and treated. In people with type 1 diabetes, insulin administration may be reduced as the oral intake of food decreases but should not be stopped. For those with type 2 diabetes, agents that may cause hypoglycemia should be reduced in dose. The main goal is to avoid hypoglycemia, allowing for glucose values in the upper level of the desired target range.
A dying patient: For people with type 2 diabetes, the discontinuation of all medications may be a reasonable approach, as these individuals are unlikely to have any oral intake. In people with type 1 diabetes, there is no consensus, but a small amount of basal insulin may maintain glucose levels and prevent acute hyperglycemic complications.
Footnotes
Disclosure information for each author is available at https://doi.org/10.2337/dc23-SDIS.
Suggested citation: ElSayed NA, Aleppo G, Aroda VR, et al., American Diabetes Association. 13. Older adults: Standards of Care in Diabetes—2023. Diabetes Care 2023;46(Suppl. 1):S216–S229
References
- 1. Laiteerapong N, Huang ES. Diabetes in older adults. In Diabetes in America. 3rd ed. Cowie CC, Casagrande SS, Menke A, et al., Eds. Bethesda, MD, National Institute of Diabetes and Digestive and Kidney Diseases (US), 2018. Accessed 19 October 2022. Available from https://www.ncbi.nlm.nih.gov/books/NBK567980/ [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention . National Diabetes Statistics Report, 2020: Estimates of Diabetes and its Burden in the United States. Accessed 19 October 2022. Available from https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
- 3. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care 2012;35:2650–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Young-Hyman D, de Groot M, Hill-Briggs F, Gonzalez JS, Hood K, Peyrot M. Psychosocial care for people with diabetes: a position statement of the American Diabetes Association. Diabetes Care 2016;39:2126–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Institute of Medicine of the National Academies . Cognitive Aging: Progress in Understanding and Opportunities for Action. Accessed 22 October 2022. Available from https://nationalacademies.org/hmd/Reports/2015/Cognitive-Aging.aspx [PubMed]
- 6. Sudore RL, Karter AJ, Huang ES, et al. Symptom burden of adults with type 2 diabetes across the disease course: diabetes & aging study. J Gen Intern Med 2012;27:1674–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Laiteerapong N, Karter AJ, Liu JY, et al. Correlates of quality of life in older adults with diabetes: the diabetes & aging study. Diabetes Care 2011;34:1749–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McClintock MK, Dale W, Laumann EO, Waite L. Empirical redefinition of comprehensive health and well-being in the older adults of the United States. Proc Natl Acad Sci U S A 2016;113:E3071–E3080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Laiteerapong N, Iveniuk J, John PM, Laumann EO, Huang ES. Classification of older adults who have diabetes by comorbid conditions, United States, 2005-2006. Prev Chronic Dis 2012;9:E100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blaum C, Cigolle CT, Boyd C, et al. Clinical complexity in middle-aged and older adults with diabetes: the Health and Retirement Study. Med Care 2010;48:327–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tinetti ME, Costello DM, Naik AD, et al. Outcome goals and health care preferences of older adults with multiple chronic conditions. JAMA Netw Open 2021;4:e211271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes--systematic overview of prospective observational studies. Diabetologia 2005;48:2460–2469 [DOI] [PubMed] [Google Scholar]
- 13. Roberts RO, Knopman DS, Przybelski SA, et al. Association of type 2 diabetes with brain atrophy and cognitive impairment. Neurology 2014;82:1132–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu WL, von Strauss E, Qiu CX, Winblad B, Fratiglioni L. Uncontrolled diabetes increases the risk of Alzheimer’s disease: a population-based cohort study. Diabetologia 2009;52:1031–1039 [DOI] [PubMed] [Google Scholar]
- 15. Yaffe K, Falvey C, Hamilton N, et al. Diabetes, glucose control, and 9-year cognitive decline among older adults without dementia. Arch Neurol 2012;69:1170–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rawlings AM, Sharrett AR, Schneider ALC, et al. Diabetes in midlife and cognitive change over 20 years: a cohort study. Ann Intern Med 2014;161:785–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Launer LJ, Miller ME, Williamson JD, et al.; ACCORD MIND investigators . Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet Neurol 2011;10:969–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murray AM, Hsu FC, Williamson JD, et al.; Action to Control Cardiovascular Risk in Diabetes Follow-On Memory in Diabetes (ACCORDION MIND) Investigators . ACCORDION MIND: results of the observational extension of the ACCORD MIND randomised trial. Diabetologia 2017;60:69–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ghezzi L, Scarpini E, Galimberti D. Disease-modifying drugs in Alzheimer’s disease. Drug Des Devel Ther 2013;7:1471–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Craft S, Baker LD, Montine TJ, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol 2012;69:29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Freiherr J, Hallschmid M, Frey WH 2nd, et al. Intranasal insulin as a treatment for Alzheimer’s disease: a review of basic research and clinical evidence. CNS Drugs 2013;27:505–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alagiakrishnan K, Sankaralingam S, Ghosh M, Mereu L, Senior P. Antidiabetic drugs and their potential role in treating mild cognitive impairment and Alzheimer’s disease. Discov Med 2013;16:277–286 [PubMed] [Google Scholar]
- 23. Tomlin A, Sinclair A. The influence of cognition on self-management of type 2 diabetes in older people. Psychol Res Behav Manag 2016;9:7–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. National Institute on Aging . Assessing Cognitive Impairment in Older Patients. Accessed 19 October 2022. Available from https://www.nia.nih.gov/health/assessing-cognitive-impairment-older-patients
- 25. Alzheimer’s Association . Cognitive Assessment. Accessed 19 October 2022. Available from https://alz.org/professionals/healthcare-professionals/cognitive-assessment
- 26. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 27. Borson S, Scanlan JM, Chen P, Ganguli M. The Mini-Cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc 2003;51:1451–1454 [DOI] [PubMed] [Google Scholar]
- 28. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699 [DOI] [PubMed] [Google Scholar]
- 29. Moreno G, Mangione CM, Kimbro L; American Geriatrics Society Expert Panel on Care of Older Adults with Diabetes Mellitus . Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 update. J Am Geriatr Soc 2013;61:2020–2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. American Psychological Association . Guidelines for the Evaluation of Dementia and Age-Related Cognitive Change, 2021. Accessed 22 October 2022. Available from https://www.apa.org/practice/guidelines/dementia.aspx [DOI] [PubMed]
- 31. Lee AK, Lee CJ, Huang ES, Sharrett AR, Coresh J, Selvin E. Risk factors for severe hypoglycemia in black and white adults with diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Diabetes Care 2017;40:1661–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Feinkohl I, Aung PP, Keller M, et al.; Edinburgh Type 2 Diabetes Study (ET2DS) Investigators . Severe hypoglycemia and cognitive decline in older people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes Care 2014;37:507–515 [DOI] [PubMed] [Google Scholar]
- 33. Lee AK, Rawlings AM, Lee CJ, et al. Severe hypoglycaemia, mild cognitive impairment, dementia and brain volumes in older adults with type 2 diabetes: the Atherosclerosis Risk in Communities (ARIC) cohort study. Diabetologia 2018;61:1956–1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fitzgerald JT, Davis WK, Connell CM, Hess GE, Funnell MM, Hiss RG. Development and validation of the Diabetes Care Profile. Eval Health Prof 1996;19:208–230 [DOI] [PubMed] [Google Scholar]
- 35. Clarke WL, Cox DJ, Gonder-Frederick LA, Julian D, Schlundt D, Polonsky W. Reduced awareness of hypoglycemia in adults with IDDM: a prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care 1995;18:517–522 [DOI] [PubMed] [Google Scholar]
- 36. Karter AJ, Warton EM, Lipska KJ, et al. Development and validation of a tool to identify patients with type 2 diabetes at high risk of hypoglycemia-related emergency department or hospital use. JAMA Intern Med 2017;177:1461–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gerstein HC, Miller ME, Byington RP, et al.; Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Duckworth W, Abraira C, Moritz T, et al.; VADT Investigators . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 39. Toschi E, Slyne C, Sifre K, et al. The relationship between cgm-derived metrics, A1C, and risk of hypoglycemia in older adults with type 1 diabetes. Diabetes Care 2020;43:2349–2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carlson AL, Kanapka LG, Miller KM, et al. Hypoglycemia and glycemic control in older adults with type 1 diabetes: baseline results from the WISDM Study. J Diabetes Sci Technol 2021;15:582–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pratley RE, Kanapka LG, Rickels MR, Ahmann A, Aleppo G, Beck R, et al.; Wireless Innovation for Seniors With Diabetes Mellitus (WISDM) Study Group . Effect of continuous glucose monitoring on hypoglycemia in older adults with type 1 diabetes: a randomized clinical trial. JAMA 2020;323:2397–2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miller KM, Kanapka LG, Rickels MR, et al. Benefit of continuous glucose monitoring in reducing hypoglycemia is sustained through 12 months of use among older adults with type 1 diabetes. Diabetes Technol Ther 2022;24:424–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gubitosi-Klug RA, Braffett BH, Bebu I, et al. Continuous glucose monitoring in adults with type 1 diabetes with 35 years duration from the DCCT/EDIC Study. Diabetes Care 2022;45:659–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Karter AJ, Parker MM, Moffet HH, Gilliam LK, Dlott R. Association of real-time continuous glucose monitoring with glycemic control and acute metabolic events among patients with insulin-treated diabetes. JAMA 2021;325:2273–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ruedy KJ, Parkin CG, Riddlesworth TD; DIAMOND Study Group . Continuous glucose monitoring in older adults with type 1 and type 2 diabetes using multiple daily injections of insulin: results from the DIAMOND trial. J Diabetes Sci Technol 2017;11:1138–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McAuley SA, Trawley S, Vogrin S, et al. Closed-loop insulin delivery versus sensor-augmented pump therapy in older adults with type 1 diabetes (ORACL): a randomized, crossover trial. Diabetes Care 2022;45:381–390 [DOI] [PubMed] [Google Scholar]
- 47. Boughton CK, Hartnell S, Thabit H, et al. Hybrid closed-loop glucose control compared with sensor augmented pump therapy in older adults with type 1 diabetes: an open-label multicentre, multinational, randomised, crossover study. Lancet Healthy Longev 2022;3:e135–e142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Selvin E, Coresh J, Brancati FL. The burden and treatment of diabetes in elderly individuals in the U.S. Diabetes Care 2006;29:2415–2419 [DOI] [PubMed] [Google Scholar]
- 49. Bandeen-Roche K, Seplaki CL, Huang J, et al. Frailty in older adults: a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci 2015;70:1427–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kalyani RR, Tian J, Xue QL, et al. Hyperglycemia and incidence of frailty and lower extremity mobility limitations in older women. J Am Geriatr Soc 2012;60:1701–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pilla SJ, Schoenborn NL, Maruthur NM, Huang ES. Approaches to risk assessment among older patients with diabetes. Curr Diab Rep. 2019;19:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Griffith KN, Prentice JC, Mohr DC, Conlin PR. Predicting 5- and 10-year mortality risk in older adults with diabetes. Diabetes Care 2020;43:1724–1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Brown SES, Meltzer DO, Chin MH, Huang ES. Perceptions of quality-of-life effects of treatments for diabetes mellitus in vulnerable and nonvulnerable older patients. J Am Geriatr Soc 2008;56:1183–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. NGSP . Factors that Interfere with HbA1c Test Results. Accessed 19 October 2022. Available from http://www.ngsp.org/factors.asp
- 55. Huang ES, Zhang Q, Gandra N, Chin MH, Meltzer DO. The effect of comorbid illness and functional status on the expected benefits of intensive glucose control in older patients with type 2 diabetes: a decision analysis. Ann Intern Med 2008;149:11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Huang ES, Laiteerapong N, Liu JY, John PM, Moffet HH, Karter AJ. Rates of complications and mortality in older patients with diabetes mellitus: the diabetes and aging study. JAMA Intern Med 2014;174:251–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Leung V, Wroblewski K, Schumm LP, Huisingh-Scheetz M, Huang ES. Re-examining the classification of older adults with diabetes by comorbidities and relationship with frailty, disability, and 5-year mortality. J Gerontol A Biol Sci Med Sci 2021;76:2071–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rooney MR, Tang O, Echouffo Tcheugui JB, et al. American Diabetes Association framework for glycemic control in older adults: implications for risk of hospitalization and mortality. Diabetes Care 2021;44:1524–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sinclair A, Dunning T, Colagiuri S. IDF Global Guideline For Managing Older People With Type 2 Diabetes. International Diabetes Federation, 2013 [Google Scholar]
- 60. Angelo M, Ruchalski C, Sproge BJ. An approach to diabetes mellitus in hospice and palliative medicine. J Palliat Med 2011;14:83–87 [DOI] [PubMed] [Google Scholar]
- 61. Beckett NS, Peters R, Fletcher AE, et al.; HYVET Study Group . Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008;358:1887–1898 [DOI] [PubMed] [Google Scholar]
- 62. de Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care 2017;40:1273–1284 [DOI] [PubMed] [Google Scholar]
- 63. Gencer B, Marston NA, Im K, et al. Efficacy and safety of lowering LDL cholesterol in older patients: a systematic review and meta-analysis of randomised controlled trials. Lancet 2020;396:1637–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yourman LC, Cenzer IS, Boscardin WJ, et al. Evaluation of time to benefit of statins for the primary prevention of cardiovascular events in adults aged 50 to 75 years: a meta-analysis. JAMA Intern Med 2021;181:179–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Park SW, Goodpaster BH, Strotmeyer ES, et al. Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes 2006;55:1813–1818 [DOI] [PubMed] [Google Scholar]
- 66. Park SW, Goodpaster BH, Strotmeyer ES, et al.; Health, Aging, and Body Composition Study . Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care 2007;30:1507–1512 [DOI] [PubMed] [Google Scholar]
- 67. Pahor M, Guralnik JM, Ambrosius WT, et al.; LIFE study investigators . Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA 2014;311:2387–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gill TM, Baker DI, Gottschalk M, Peduzzi PN, Allore H, Byers A. A program to prevent functional decline in physically frail, elderly persons who live at home. N Engl J Med 2002;347:1068–1074 [DOI] [PubMed] [Google Scholar]
- 69. Curtis JM, Horton ES, Bahnson J, et al.; Look AHEAD Research Group . Prevalence and predictors of abnormal cardiovascular responses to exercise testing among individuals with type 2 diabetes: the Look AHEAD (Action for Health in Diabetes) study. Diabetes Care 2010;33:901–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bray G, Gregg E, Haffner S, et al.; Look Ahead Research Group . Baseline characteristics of the randomised cohort from the Look AHEAD (Action for Health in Diabetes) study. Diab Vasc Dis Res 2006;3:202–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wing RR, Bolin P, Brancati FL, et al.; Look AHEAD Research Group . Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gregg EW, Jakicic JM, Blackburn G, et al.; Look AHEAD Research Group . Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol 2016;4:913–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gregg EW, Chen H, Wagenknecht LE, et al.; Look AHEAD Research Group . Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA 2012;308:2489–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rejeski WJ, Bray GA, Chen SH, et al.; Look AHEAD Research Group . Aging and physical function in type 2 diabetes: 8 years of an intensive lifestyle intervention. J Gerontol A Biol Sci Med Sci 2015;70:345–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Espeland MA, Rejeski WJ, West DS, et al.; Action for Health in Diabetes Research Group . Intensive weight loss intervention in older individuals: results from the Action for Health in Diabetes Type 2 diabetes mellitus trial. J Am Geriatr Soc 2013;61:912–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Houston DK, Neiberg RH, Miller ME, et al. Physical function following a long-term lifestyle intervention among middle aged and older adults with type 2 diabetes: the Look AHEAD Study. J Gerontol A Biol Sci Med Sci 2018;73:1552–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Simpson FR, Pajewski NM, Nicklas B, et al.; Indices for Accelerated Aging in Obesity and Diabetes Ancillary Study of the Action for Health in Diabetes (Look AHEAD) Trial . Impact of multidomain lifestyle intervention on frailty through the lens of deficit accumulation in adults with type 2 diabetes mellitus. J Gerontol A Biol Sci Med Sci 2020;75:1921–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Espeland MA, Gaussoin SA, Bahnson J, et al. Impact of an 8-year intensive lifestyle intervention on an index of multimorbidity. J Am Geriatr Soc 2020;68:2249–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gregg EW, Lin J, Bardenheier B, et al.; Look AHEAD Study Group . Impact of intensive lifestyle intervention on disability-free life expectancy: the Look AHEAD Study. Diabetes Care 2018;41:1040–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Valencia WM, Florez H. Pharmacological treatment of diabetes in older people. Diabetes Obes Metab 2014;16:1192–1203 [DOI] [PubMed] [Google Scholar]
- 81. Zhang JX, Bhaumik D, Huang ES, Meltzer DO. Change in insurance status and cost-related medication non-adherence among older U.S. adults with diabetes from 2010 to 2014. J Health Med Econ 2018;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Schmittdiel JA, Steers N, Duru OK, et al. Patient-provider communication regarding drug costs in Medicare Part D beneficiaries with diabetes: a TRIAD Study. BMC Health Serv Res 2010;10:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Patel MR, Resnicow K, Lang I, Kraus K, Heisler M. Solutions to address diabetes-related financial burden and cost-related nonadherence: results from a pilot study. Health Educ Behav 2018;45:101–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Arnold SV, Lipska KJ, Wang J, Seman L, Mehta SN, Kosiborod M. Use of intensive glycemic management in older adults with diabetes mellitus. J Am Geriatr Soc 2018;66:1190–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Andreassen LM, Sandberg S, Kristensen GBB, Sølvik UØ, Kjome RLS. Nursing home patients with diabetes: prevalence, drug treatment and glycemic control. Diabetes Res Clin Pract 2014;105:102–109 [DOI] [PubMed] [Google Scholar]
- 86. Lipska KJ, Ross JS, Miao Y, Shah ND, Lee SJ, Steinman MA. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med 2015;175:356–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Thorpe CT, Gellad WF, Good CB, et al. Tight glycemic control and use of hypoglycemic medications in older veterans with type 2 diabetes and comorbid dementia. Diabetes Care 2015;38:588–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. McAlister FA, Youngson E, Eurich DT. Treatment deintensification is uncommon in adults with type 2 diabetes mellitus: a retrospective cohort study. Circ Cardiovasc Qual Outcomes 2017;10:e003514. [DOI] [PubMed] [Google Scholar]
- 89. Seidu S, Kunutsor SK, Topsever P, Hambling CE, Cos FX, Khunti K. Deintensification in older patients with type 2 diabetes: a systematic review of approaches, rates and outcomes. Diabetes Obes Metab 2019;21:1668–1679 [DOI] [PubMed] [Google Scholar]
- 90. Weiner JZ, Gopalan A, Mishra P, et al. Use and discontinuation of insulin treatment among adults aged 75 to 79 years with type 2 diabetes. JAMA Intern Med 2019;179:1633–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Abdelhafiz AH, Sinclair AJ. Deintensification of hypoglycaemic medications-use of a systematic review approach to highlight safety concerns in older people with type 2 diabetes. J Diabetes Complications 2018;32:444–450 [DOI] [PubMed] [Google Scholar]
- 92. Sussman JB, Kerr EA, Saini SD, et al. Rates of deintensification of blood pressure and glycemic medication treatment based on levels of control and life expectancy in older patients with diabetes mellitus. JAMA Intern Med 2015;175:1942–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Munshi MN, Slyne C, Segal AR, Saul N, Lyons C, Weinger K. Simplification of insulin regimen in older adults and risk of hypoglycemia. JAMA Intern Med 2016;176:1023–1025 [DOI] [PubMed] [Google Scholar]
- 94. Jude EB, Malecki MT, Gomez Huelgas R, et al. Expert panel guidance and narrative review of treatment simplification of complex insulin regimens to improve outcomes in type 2 diabetes. Diabetes Ther 2022;13:619–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA 2014;312:2668–2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Aroda VR, Edelstein SL, Goldberg RB, et al.; Diabetes Prevention Program Research Group . Long-term metformin use and vitamin B12 deficiency in the Diabetes Prevention Program Outcomes Study. J Clin Endocrinol Metab 2016;101:1754–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Schwartz AV, Chen H, Ambrosius WT, et al. Effects of TZD use and discontinuation on fracture rates in ACCORD Bone Study. J Clin Endocrinol Metab 2015;100:4059–4066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Billington EO, Grey A, Bolland MJ. The effect of thiazolidinediones on bone mineral density and bone turnover: systematic review and meta-analysis. Diabetologia 2015;58:2238–2246 [DOI] [PubMed] [Google Scholar]
- 99. American Geriatrics Society 2015 Beers Criteria Update Expert Panel . American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015;63:2227–2246 [DOI] [PubMed] [Google Scholar]
- 100. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018: a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018;41:2669–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Leiter LA, Teoh H, Braunwald E, et al.; SAVOR-TIMI 53 Steering Committee and Investigators . Efficacy and safety of saxagliptin in older participants in the SAVOR-TIMI 53 trial. Diabetes Care 2015;38:1145–1153 [DOI] [PubMed] [Google Scholar]
- 102. Green JB, Bethel MA, Armstrong PW, et al.; TECOS Study Group . Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;373:232–242 [DOI] [PubMed] [Google Scholar]
- 103. White WB, Cannon CP, Heller SR, et al.; EXAMINE Investigators . Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327–1335 [DOI] [PubMed] [Google Scholar]
- 104. Karagiannis T, Tsapas A, Athanasiadou E, et al. GLP-1 receptor agonists and SGLT2 inhibitors for older people with type 2 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract 2021;174:108737. [DOI] [PubMed] [Google Scholar]
- 105. Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2019;381:841–851 [DOI] [PubMed] [Google Scholar]
- 106. Zinman B, Wanner C, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 107. Neal B, Perkovic V, Mahaffey KW, et al.; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657 [DOI] [PubMed] [Google Scholar]
- 108. Wiviott SD, Raz I, Bonaca MP, et al.; DECLARE–TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357 [DOI] [PubMed] [Google Scholar]
- 109. Bradley MC, Chillarige Y, Lee H, et al. Severe hypoglycemia risk with long-acting insulin analogs vs neutral protamine Hagedorn insulin. JAMA Intern Med 2021;181:598–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Pandya N, Hames E, Sandhu S. Challenges and strategies for managing diabetes in the elderly in long-term care settings. Diabetes Spectr 2020;33:236–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Livingstone SJ, Levin D, Looker HC, et al.; Scottish Diabetes Research Network Epidemiology Group; Scottish Renal Registry . Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008-2010. JAMA 2015;313:37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Miller RG, Secrest AM, Sharma RK, Songer TJ, Orchard TJ. Improvements in the life expectancy of type 1 diabetes: the Pittsburgh Epidemiology of Diabetes Complications study cohort. Diabetes 2012;61:2987–2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Bullard KM, Cowie CC, Lessem SE, et al. Prevalence of diagnosed diabetes in adults by diabetes type—United States, 2016. MMWR Morb Mortal Wkly Rep 2018;67:359–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Heise T, Nosek L, Rønn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes 2004;53:1614–1620 [DOI] [PubMed] [Google Scholar]
- 115. Munshi MN, Florez H, Huang ES, et al. Management of diabetes in long-term care and skilled nursing facilities: a position statement of the American Diabetes Association. Diabetes Care 2016;39:308–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Sinclair A, Morley JE, Rodriguez-Mañas L, et al. Diabetes mellitus in older people: position statement on behalf of the International Association of Gerontology and Geriatrics (IAGG), the European Diabetes Working Party for Older People (EDWPOP), and the International Task Force of Experts in Diabetes. J Am Med Dir Assoc 2012;13:497–502 [DOI] [PubMed] [Google Scholar]
- 117. Dorner B, Friedrich EK, Posthauer ME. Practice paper of the American Dietetic Association: individualized nutrition approaches for older adults in health care communities. J Am Diet Assoc 2010;110:1554–1563 [DOI] [PubMed] [Google Scholar]
- 118. Migdal A, Yarandi SS, Smiley D, Umpierrez GE. Update on diabetes in the elderly and in nursing home residents. J Am Med Dir Assoc 2011;12:627–632.e2 [DOI] [PubMed] [Google Scholar]
- 119. Pasquel FJ, Powell W, Peng L, et al. A randomized controlled trial comparing treatment with oral agents and basal insulin in elderly patients with type 2 diabetes in long-term care facilities. BMJ Open Diabetes Res Care 2015;3:e000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Quinn K, Hudson P, Dunning T. Diabetes management in patients receiving palliative care. J Pain Symptom Manage 2006;32:275–286 [DOI] [PubMed] [Google Scholar]
- 121. Kutner JS, Blatchford PJ, Taylor DH Jr, et al. Safety and benefit of discontinuing statin therapy in the setting of advanced, life-limiting illness: a randomized clinical trial. JAMA Intern Med 2015;175:691–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Dunning T, Martin P. Palliative and end of life care of people with diabetes: issues, challenges and strategies. Diabetes Res Clin Pract 2018;143:454–463 [DOI] [PubMed] [Google Scholar]
- 123. Bouça-Machado R, Rosário M, Alarcão J, Correia-Guedes L, Abreu D, Ferreira JJ. Clinical trials in palliative care: a systematic review of their methodological characteristics and of the quality of their reporting. BMC Palliat Care 2017;16:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Sheppard JP, Burt J, Lown M, et al.; OPTIMISE Investigators . Effect of antihypertensive medication reduction vs usual care on short-term blood pressure control in patients with hypertension aged 80 years and older: the OPTIMISE randomized clinical trial. JAMA 2020;323:2039–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ford-Dunn S, Smith A, Quin J. Management of diabetes during the last days of life: attitudes of consultant diabetologists and consultant palliative care physicians in the UK. Palliat Med 2006;20:197–203 [DOI] [PubMed] [Google Scholar]
- 126. Petrillo LA, Gan S, Jing B, Lang-Brown S, Boscardin WJ, Lee SJ. Hypoglycemia in hospice patients with type 2 diabetes in a national sample of nursing homes. JAMA Intern Med 2018;178:713–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Mallery LH, Ransom T, Steeves B, Cook B, Dunbar P, Moorhouse P. Evidence-informed guidelines for treating frail older adults with type 2 diabetes: from the Diabetes Care Program of Nova Scotia (DCPNS) and the Palliative and Therapeutic Harmonization (PATH) program. J Am Med Dir Assoc 2013;14:801–808 [DOI] [PubMed] [Google Scholar]
- 128. Munshi MN, Slyne C, Segal AR, Saul N, Lyons C, Weinger K. Liberating A1C goals in older adults may not protect against the risk of hypoglycemia. J Diabetes Complications 2017;31:1197–1199 [DOI] [PubMed] [Google Scholar]