Abstract
Saliva testing is a vital method for clinical applications, for its noninvasive features, richness in substances, and the huge amount. Due to its direct anatomical connection with oral, digestive, and endocrine systems, clinical usage of saliva testing for these diseases is promising. Furthermore, for other diseases that seeming to have no correlations with saliva, such as neurodegenerative diseases and psychological diseases, researchers also reckon saliva informative. Tremendous papers are being produced in this field. Updated summaries of recent literature give newcomers a shortcut to have a grasp of this topic. Here, we focused on recent research about saliva biomarkers that are derived from humans, not from other organisms. The review mostly addresses the proceedings from 2016 to 2022, to shed light on the promising usage of saliva testing in clinical diagnostics. We recap the recent advances following the category of different types of biomarkers, such as intracellular DNA, RNA, proteins and intercellular exosomes, cell-free DNA, to give a comprehensive impression of saliva biomarker testing.
Subject terms: Diagnostic markers, Molecular medicine
Introduction
High accessibility of saliva makes it the center of biomarker research. Especially during the pandemic, saliva has been regarded as a useful and cost-effective tool for coronavirus disease 2019 (COVID-19).1,2 The detection limit of the viral genome enables it a precise method, with the detection sensitivity ranging from below 100 copies/mL of the virus to 0.38 copies/μL. Coincidentally, human papillomavirus (HPV) genes or somatic variants of head and neck squamous cell carcinomas (HNSCC) were detected more frequently in saliva than that in plasma, indicating that saliva appeared to be a meaningful way to identify HNSCC.3 Accordingly, there are some breakthroughs in saliva testing, such as human immunodeficiency virus (HIV) testing as an indicative factor for acquired immunodeficiency syndrome (AIDS), HPV detection for HNSCC. Besides, myocardial enzyme testing for the monitoring of myocardial infarction and cortisol for stress and inflammation status identification also remind us of the application of saliva in human-derived biomarker discovery. Saliva testing shows its diagnostic value in oral diseases, diabetes, renal diseases, hepatitis, neurodegenerative diseases, and immunodeficiency disease. These breakthroughs mostly rely on the good correlation of the biomarker concentration between saliva and blood. For example, a previous article showed significant correlations of free cortisol levels in saliva and blood, thus enabling the saliva cortisol testing to show psychobiological states.4
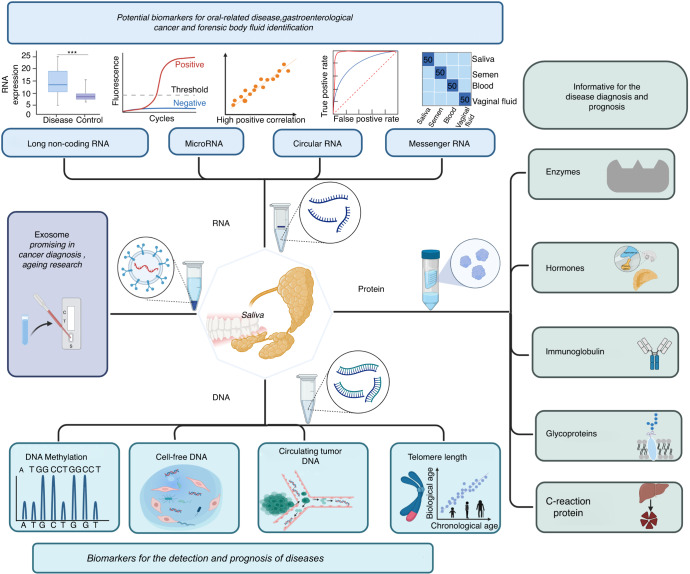
Interestingly, a study showed that people who shared saliva, such as drinking juice with the same straw, had more intimacy with each other.5 On the other hand, women abused by their intimate partner bear detectable cortisol level change in saliva.6 This shows the informativeness of saliva in psychological research. These studies extend our basic knowledge about saliva. Saliva is growing more important to health issues. Salivary gland organoid culture also became possible, which promotes the study of salivary diseases.7 Non-human derived biomarkers, such as oral microbiota, was thoroughly commented in another review.8 The review summarized oral microbes in many situations, such as digestive diseases, cancers, and cardiovascular diseases. It highlights the systematic interaction of oral microbes and humans. In addition, a review published in 2016 has summarized the diagnostic value of saliva in diseases.9 It mainly addresses the proceedings from 2016 to 2022, to shed light on the recent promising usage of saliva testing in clinical diagnostics. Here in this review, we made an update of saliva testing in the framework of major biomarker types, as shown in Fig. 1. Articles whose keywords contain “human”, “saliva” and the corresponding biomarker name were extracted from Web of Science and taken into consideration.
Fig. 1.
The scheme of the review organized by different types of biomarkers. Some categories were listed specifically, such as C-reaction protein, because the research is relatively dominant. The figure is created with BioRender.com
Secreting mechanisms of saliva
Whole saliva is a complex mixed fluid containing saliva, gingival crevicular fluid, epithelial cells, microorganisms, and food debris.10 The fraction of substances in the whole saliva is dynamic and affected by various stimuli. In general, a healthy adult daily produces 500–1 500 mL of the whole saliva at an average speed of 0.3–0.4 mL per min.10,11 Salivary glands are the main producers of saliva, which contain three pairs of major salivary glands (parotid, submandibular and sublingual gland) and 600 to 1 000 minor salivary glands. Acinar cells are the basic units of salivary glands secrete the mixed fluid, which can be categorized into two types: serous and mucous cells. The parotid glands have plasmacytoid acinar cells and secrete thin, watery and amylase-rich saliva. The sublingual and submandibular glands are mixed glands, consisting of serous and mucous cells that secrete a mucus-rich fluid. The mixed fluid produced from acinar cell enters the collecting ducts for reabsorption. During this process, the composition of the secretion changes and eventually leads into the oral cavity along the single large ducts of the salivary glands. The main excretory ducts of parotid glands open onto the oral mucosa near the upper molars. The dominant duct of the submandibular gland and the sublingual gland leads to the sublingual mucosa of the mouth floor. The primary source of the initial fluid secreted by the salivary glands is the intercellular fluid, while the final mixed fluid flowing into the oral cavity is mainly derived from intercellular fluid and blood.12 Thus, various substances in blood also can be detected in saliva. Salivary glands are controlled by autonomic nervous system and regulated by the reflexes. Stimuli such as chewing, taste, smell, sight, and pain can cause salivary secretion.13 Pathologies of the salivary glands, oral tissues and other organs can lead to abnormalities of salivary secretion, causing volume and composition changes. It facilitates research into the diagnosis, treatment and prognosis of diseases using biomarkers in saliva.
The substances in saliva and other common body fluids
There are 99% water, 0.5% organic, inorganic matter, and numerous cellular elements in the whole saliva.14,15 The main prevalent organic substance is composed of enzymes such as amylases, lysozyme, peroxidase, lipase, hormones such as cortisol, cytokines, immunoglobulins, mucins, lactoferrins, etc. While potassium, sodium, chloride, calcium, magnesium, and bicarbonate are the customary inorganic components of saliva.15 Saliva plays a vital role in oral health and general health. It is these salivary substances that maintain the physiological homeostasis of the oral cavity. The major component, water, moistening the surface of the oral cavity, make saliva texture stickiness and perform an oral clearance. Electrolytes can neutralize acid and act as a buffer (bicarbonate) and are responsible for tooth protection by preventing demineralization and enhancing remineralization of enamel (calcium, fluoride, phosphate, and bicarbonate).16 The functions of organic substances include antimicrobial, tissue repair, digestion, and formation of salivary sacs. For instance, lysozyme can hydrolyze the cell wall of Gram-positive bacteria and destroy the structure of bacteria, while immunoglobulin (e.g., sIgA) inhibits microbial adhesion and reduces microbial reproduction. Mucin protects the oropharyngeal mucosa from injury.10 The cellular elements in saliva contained leukocytes, erythrocytes, desquamated oral epithelial cells, microbial cells, and products of cells and microorganisms. The newly identified tumor markers encapsulated in exosomes, have also been found stably exist in saliva.17,18 The composition of and proportion of substances in saliva are dynamic with physiological state. Disease, diet, and mental status can cause changes in saliva composition. The disease-related change of salivary substance facilitates the application of saliva in clinical research, such as the diagnosis, therapy, and prognosis.
Among all the body fluids, sweat is very similar as saliva and is also easily accessed. Whereas, saliva is quite different from sweat, drops secreted through the pores of the skin. It is believed that there are no cells in sweat, while in saliva there are many cells. Instead, trace minerals, lactic acid, and urea appear in sweat.19 Some scholars discussed next-generation digital based biomarkers for tuberculosis diagnosis and therapeutic drug monitoring approach, both in sweat and saliva.20 Recently, studies about sweat distributed in wearable devices or detecting biosensors.21,22 Ray et al. developed a label-free optical detection system to simultaneously examine multiple stress biomarkers.23 They found that cortisol could be detected in sweat, saliva, urine, plasma, and serum; serotonin and dopamine were measurable in plasma and serum; neuropeptide Y was detected in all these four biological fluids. The composition of seven kinds of common body fluids was listed in Table 1.
Table 1.
The composition of different body fluids
| Type | pH | Specific gravity | Major component | Minor component | Volume | Difficulty of accessibility |
|---|---|---|---|---|---|---|
| Saliva | 6.6–7.1 | 1.002–1.008 | >99% H2O | Electrolytes, immunoglobulins, proteins, enzymes, mucins | 0.5–1.5 L | ⭐ |
| Plasma | 7.35–7.45 | 1.025–1.030 | >90% H2O | Ions, proteins, dissolved gases, nutrient molecules, and wastes | 2.75–3.30 L | ⭐⭐⭐ |
| Urine | 4.5–8.0 | 1.010–1.030 | 95% H2O | Urea (2%), creatinine (0.1%), uric acid (0.03%) | 0.8–2.0 L | ⭐⭐ |
| Sweat | 4.0–6.8 | 1.004–1.011 | >99% H2O | NaCl (mostly) and other electrolytes | 0.6–0.7 L (unconscious) | ⭐⭐ |
| Tear fluid | 5.20∼8.35 | 1.008 | 98% H2O | Electrolytes, proteins, lipids, mucins | NA | ⭐⭐ |
| Breast milk | 6.35–7.35 | 1.030 | 87% H2O | 1% protein, 4% lipid, and 7% carbohydrate | 0.75–1.035 L | ⭐⭐⭐ |
| Cerebrospinal fluid | 7.334–7.523 | 1.006–1.008 | 99% H2O | Electrolytes, proteins, glucose | 0.1–0.15 L | ⭐⭐⭐⭐⭐ |
Promising saliva DNA biomarkers
Literature searching results for salivary DNA biomarkers mainly fall into four topics, which are DNA methylation, telomere length, cell-free DNA, and circulating tumor DNA. Among these four topics, DNA methylation studies account for the most part.
DNA methylation
Most epigenetic studies used blood samples for biomarker selection.24 Before 2016, published studies about saliva methylation largely focused on cancer, psychiatry, environmental, and lifestyle related disease.25 In the past five years, saliva methylation studies have mainly focused on the alteration caused by environmental exposure, such as air pollution and smoking. Bakulski et al. investigated the effect of environmental exposure, such as PM2.5 and PM10, on saliva DNA methylation signatures.26 They tested DNA methylation levels in saliva at ages 9 and 15 for association with PM10 exposure at birth. Dawes et al. studied DNA methylation level at cg05575921 using saliva samples from high school students. Their paired serum cotinine (a key nicotine metabolite) values were accessible. The result indicated that saliva DNA methylation held promise for the detection of nascent smoking.27 They did not examine if the marker could predict smoking tendencies. On the other hand, another study indicated that methylation in the tumor suppressor genes p16 and p14 was not detected in saliva from nontobacco users, while partial methylation was detected in 33.3 % samples of smokeless tobacco users.28 Saliva methylation also has the potential in identifying smokers and non-smokers, by detecting methylation status at cg05575921.29 The research showed that smoking induced a demethylation response. Another research also investigated the correlation between smoking and salivary cg05575921 methylation.30
Using saliva DNA can be problematic since it mixes human and bacterial DNA. A high proportion of bacterial DNA may bring possible failure in genetic tests.25,31 Saliva from the same individual at different time points may differ widely regarding cell composition.32 Some scholars constructed a salivary DNA methylation reference panel for individuals aged from 7 to 16 years old, estimating cell-type heterogeneity within the same individual.33 Rounge et al. concluded an association between the saliva methylome and body-mass index (BMI) in adolescence.34 Moccia et al. showed that DNA methylation patterns differed in cord blood and infant saliva,35 leading to distinct birthweight signatures. Another study reported that three obesity-related outcomes were negatively associated with LEP gene methylation in obese boys only.36 A high correlation between blood and saliva methylation levels in respiratory allergy individuals also gave credit to the usefulness of saliva in epigenetic studies.37
In oral diseases, Mimansha et al. detected promoter methylation in tobacco users with and without oral typical premalignant lesions It concluded that promoter methylation levels of various genes were linked to a high probability to precede oral squamous cell carcinomas.38 A significant hypermethylation of p16 appeared in buccal cells and saliva of oral submucous fibrosis patients.39 Saliva cells of smokers with chronic periodontitis tend to have a methylated SOCS1 gene promoter rather than saliva cells from the non-smoking patients, as was reported by Martinez et al.40 Yamaguchi et al. found significant methylation alterations in 2381 CpG sites after orthognathic surgery. It was perhaps due to the response to surgical stress or bone injury.41
In neurodegenerative diseases, Chuang et al. unveiled five significant CpGs.42 Elaheh et al. observed allele-specific methylation in CD4+ cells instead of saliva.43 In autoimmune diseases, altered DNA methylation profiles appear to be present in saliva in Coeliac disease individuals.44 Biomarker research boom in neurodegenerative diseases might be due to the difficulty in the obtaining of the brain for biopsy. In psychiatric disorders, saliva BDNF gene methylation is useful for treatment monitoring of borderline personality disorder (BPD).45 Psychotherapeutic intervention can significantly lower BDNF methylation in BPD patients’ saliva samples. In blood, this tendency did not exist. In attention-deficit/hyperactivity disorder, children under this disorder were tested with altered VIPR2 gene methylation signatures.46 Additionally, there are other applications of salivary epigenetic biomarkers. Hearn et al. showed a high correlation coefficient ( r= 0.92 ~ 0.95, P < 0.001) between saliva and intestinal mucosa.47 This is valuable for the monitoring of intestinal diseases, with the convenience of multiple testing.
In forensic science, saliva methylation is also a heavily watched biomarker for the age estimation of biological samples. Hong et al. used a panel of 7 CpG markers to construct an age prediction panel with high predictability.48 They built a model with a mean absolute deviation (MAD) of 3.13 years. Lee et al. applied the droplet digital PCR (ddPCR) assays to study DNA methylation level, resulting in an MAD of 3.3 years.49 Body fluid discrimination is also a frequently studied question. Using methylation can distinguish different kinds of biological fluids or perform age prediction in sperm, saliva, blood, and menstrual blood.50–56 Age prediction by saliva methylation detection in cigarette butts is also widely used in forensic investigations.57 Interestingly, interethnic methylation differences were observed in Japanese and Indonesian populations.58
Illumina Methylation 450K BeadChip platform was most widely used (Table 2). Additionally, methylated DNA immunoprecipitation coupled to next-generation sequencing (MeDIP-seq) was also explored in detecting saliva methylation.59 These were high throughput detecting assays. Pyrosequencing, methylation-specific polymerase chain reaction and agarose gel electrophoresis and multiplex methylation SNaPshot reactions were preferred in target methylation site testing.
Table 2.
The methods and marker sets used in saliva methylation studies from 2016 to 2022
| Method used for methylation detection | Marker sets | References |
|---|---|---|
| 1. MALDI-TOF mass spectrometer | 27 CpG sites in the promoter (MT2 region) | 25 |
| 2. Infinium HumanMethylation450K array | 485 000 probes | 26,35,37,41,42,44,46,47,51,301 |
| 3. ddPCR | (1) cg05575921 and DMR16( | 27,29,30 |
| 2) cg14361627, cg14361627, cg08928145 and cg07547549 | 302 | |
| 4. Illumina MethylationEPIC BeadChip | 795,694 probes | 33,54 |
| 5. methylation specific polymerase chain reaction and agarose gel electrophoresis | (1) p16INK4a and p14ARF | 28 |
| (2) SOCS1 | 40 | |
| (3) p16, DAP- K, MGMT, and GSTP1 | 38 | |
| (4) ZNF282 and HPCAL1 | 52 | |
| 6. targeted bisulfite sequencing (TBS) protocol | 3.1 million CpG sites | 34 |
| 7. pyrosequencing | (1) LEP, ICAM-1, CRH, and LINE-1 genes | 36 |
| (2) BDNF | 45 | |
| (3) NMUR2 and UBE2U | 50 | |
| (4) ZC3H12D, BCAS4 and cg06379435 | 53 | |
| (5) FADS1 and FADS2 | 43 | |
| 8. real-time methylation-specific polymerase chain reaction | (1) P16 | 39 |
| (2) VM03 and SM02 | 55 | |
| 9. multiplex methylation SNaPshot reactions | (1) cg00481951, cg19671120, cg14361627, cg08928145, cg12757011, cg18384097 and cg07547549 | 48,51 |
| (2) ELOVL2, FHL2, KLF14, C1orf132/MIR29B2C, and TRIM59 genes | 56 | |
| 10. methylation-sensitive high-resolution melting (MSHRM) | (1) ELOVL2 and EDARADD | 57 |
| (2) EDARADD and FHL2 | 58 | |
| 11. methylated DNA immunoprecipitation coupled to next-generation sequencing (MeDIP-seq) | Genome-wide | 59 |
Telomere length
Matthew et al. investigated telomere length in saliva samples of healthy athletes.60 They showed that samples collected pre-season had shorter telomeres than those collected mid-season. Also, males had longer telomeres than females. More than a decade ago, Lahnert reported an improved method for detecting saliva telomere length for its use in the inference of age.61 It showed that the precision of the measurements in saliva specimens was lower than that in blood. Another research showed that telomere length was longer in saliva than in blood.62
Cell free DNA (cfDNA)
After apoptosis or necrosis, cell debris including nucleotides releases into the interstitial fluid. Thus, individuals with a high rate of cell death tend to have a higher proportion of cfDNA. Interestingly, psychosocial stress exposure can contribute to a higher level of cfDNA release. The correlation between salivary cortisol and cfDNA increase was observed.63 Additionally, saliva parasite-derived cfDNA can also be indicative of the disease.64
Circulating tumor DNA (ctDNA)
While most of cfDNA comes from normal cells of the body, ctDNA is related to tumors. Salivary ctDNA mutations were found to be more frequent in oral cancers than in oropharynx, larynx, and hypopharynx cancers.65 Researchers focused mainly on TP53, CDKN2A, PIK3CA, FAT1, and NOTCH1 genes. It was believed that in liquid biopsy, saliva was superior to plasma in oral cancers, because of the physical location. Besides, salivary ctDNA was investigated in Non-Small Cell Lung Cancer (NSCLC). Li et al. noticed that EGFR mutation length fragments were detected with a size range below 80 bp.66 Epidermal growth factor receptor (EGFR) gene mutations in saliva-derived ctDNA were detected and were consistent with that of plasma ctDNA.67 They could not be applied in the diagnosis of NSCLC. Interestingly, Eun et al. built a mouse model to compare the correlation between the human long interspersed element (hLINE) in mouse saliva or plasma, as an indicator of ctDNA amount with metastasis or recurrence rate in orthotopic head and neck cancer (HNC).68 HPV-associated oropharyngeal cancer (OPC) diagnosis can also benefit from salivary cfDNA detection.69
RNA biomarkers
To give a bigger scope of the focus of studies, we primarily searched the database with keywords “saliva” and “RNA”. A knowledge map was drawn to present the research focus on salivary RNA in the past six years, using R language (https://www.R-project.org/). From Supplementary Figure 1a, five main topics have been considered to study salivary RNA molecules. Understandably, “expression” was the most frequent keyword, as can be seen in Supplementary Figure 1b. Many studies aimed at discovering biomarkers for diagnosis and prognosis, although we did not include the search keyword “biomarkers” for generating this plot. Among all diseases, cancer research ranked at the top of the list. Specifically, squamous cell carcinomas were paid the most attention to. It was consistent with the result of saliva DNA biomarkers. This might be due to the direct relation between epithelial cells in saliva and these cancer types. Scholars from all over the world have been working in this research field, with or without collaborations (Supplementary Fig. 1c). These papers also mentioned serum, possibly for paired studies (Supplementary Fig. 1d, a). In the next paragraph, we discussed the papers searching by the keywords “saliva”, “RNA”, and “biomarkers”.
mRNA
Messenger RNAs (mRNAs) are transcribed from genes to the cytoplasm and guide protein synthesis. As early as in 2004, Li et al. demonstrated that there were approximately 3 000 different mRNAs in cell-free saliva of healthy subjects, and almost 200 of these were present in all subjects.70 Informative cell-free human mRNAs existed in saliva. The salivary transcriptome sequencing has been proposed as a novel clinical approach to salivary diagnostics. Compared with proteomic constituents, salivary transcriptomic biomarkers moved the detection time to an earlier stage of the disease.71 In recent years, published studies about saliva mRNAs markers largely focused on cancers, forensics, Sjögren’s syndrome (SS), and periodontitis, as well as exploration of other diseases.
Accumulated evidence has proven the associations between saliva mRNAs and cancers.72 Li et al. firstly reported seven upregulated mRNAs in the saliva of oral squamous cell carcinoma (OSCC) patients, which have been independently confirmed by multicenter cohorts.73,74 A more recent study demonstrated a good predictive probability of 80% by four biomarkers for patients with OSCC, but not for patients with oral leukoplakia and dysplasia. Moreover, the combination of SAT and IL-8 achieved a high predictive ability of 75.5%, suggesting an important value of the two biomarkers for the early detection of OSCC.75 Recently, researchers have paid attention to the influence of reference gene stability and oral health condition on the performance of saliva mRNA biomarkers for OSCC.76–78 Martin validated the expression stability of five housekeeping genes for use in oral cancer salivary mRNA panels. Six pre-specified OSCC-associated genes were verified to be significantly upregulated in OSCC patients based on delta Cq values.76 Cheng et al. reported chronic periodontitis could apparently change the levels of those potential oral cancer salivary mRNAs except for S100P mRNA.77 Another independent study also suggested that oral inflammatory processes can have influence on the performance of the seven putative salivary mRNA biomarkers.78 Therefore, inflammatory biomarkers such as salivary IL6 mRNA should be utilized in the clinical application of OSCC detection.
Salivary mRNA biomarkers of other cancers have been little explored. Li et al. screened 30 salivary mRNA candidates from the salivary transcription profiles of 63 gastric cancer (GC) and 31 non-GC patients. Nine of the 30 biomarkers were verified in an independent prospective cohort. In addition, the panel of three mRNAs and two microRNAs screened by the LASSO algorithm generated the best area under curves (AUC) value of 0.81.79 Xu et al. subsequently showed that the expression of four mRNA biomarkers in saliva were significantly downregulated in GC patients compared to healthy controls.80 Besides, salivary mRNA biomarkers has been explored in breast cancer,81 ovarian cancer,82 lung cancer,83 and chronic myeloid leukemia.84 Most of those studies indicated that combined analysis of salivary mRNA biomarkers and other known biomarkers could improve the performance of cancer detection. Even so, the clinical utility of those salivary mRNA biomarkers should be further evaluated in more populations.
In forensic science, mRNA profiling for saliva identification have been evaluated by the European DNA Profiling Group (EDNAP) in the last decade. The mRNA profiling could be used for current saliva identification procedures, based on its advantages of higher sensitivity, greater specificity, multiplex detection, and compatibility with current identification method.85,86 Previous studies reported several salivary mRNA biomarkers including HTN3, STATH, MUC7, PRB1, PRB2, and PRB3. The first three biomarkers were evaluated by the EDNAP.85 In recent years, the sensitivity and specificity of those salivary mRNA biomarkers have been investigated. Three of the saliva markers showed high specificity in the discrimination of saliva and other biofluid samples including blood, semen, urine, and vaginal secretions.87 Among them, HTN3 was the most specific saliva biomarker for it was neither detected in nasal mucosa nor trachea samples.88,89 Liu et al. reported that the sensitivity of saliva mRNA using multiplex real-time PCR (RT-PCR) assay.87 In the routine forensic casework with the ParaDNA® Body Fluid ID System, the positive rate of HTN3 mRNA marker in saliva samples ranged from 76.9% to 95%.90,91 Moreover, the stability of the mRNA markers has been investigated recently. Watanabe et al. demonstrated that exposure to blue light (450 nm) had no effect on the stability of the mRNA markers.86
There are also significant changes in the salivary transcriptome of Sjögren’s syndrome patients. Hu et al. successfully identified 3 mRNA biomarkers promising for distinguishing primary SS.92 A recent study showed significantly lower mRNA levels of saliva IL-38 and IL-36R in primary Sjögren’s syndrome (pSS) group. Further experiment showed that IL-38 inhibited pSS possibly through Th17 inflammation.93 In recent years, increased mRNA markers such as NGAL and p16 mRNA levels were reported in salivary glands of pSS, but their expressions in saliva remained unexplored.94,95
In periodontal diseases, several dysregulated immune response related genes have been detected in saliva. Swaminathan et al. found that TLR2 and TLR4 mRNAs were up regulated in epithelial cells of unstimulated whole saliva from chronic periodontitis, while PGRP3 and PGRP4 mRNAs were reduced.96 In their further study, they confirmed that the TLR4 mRNA was significantly higher in the salivary epithelial cells of the gingivitis and the chronic periodontitis cohort as compared to that from the healthy saliva samples. It could be regarded as clinically relevant markers of disease progression from gingivitis to periodontitis.97 A recent study showed that MIP-1α, MMP-2 and MMP-9 mRNA expression was higher in patients with periodontal disease than in healthy individuals. These biomarkers were correlated with the severity of the disease.98 In addition, Detzen et al. reported the expression of CD163 as a potential salivary biomarker of periodontitis.99
Salivary mRNA biomarkers of other diseases have been little explored. Fagin et al. found that BZLF1 mRNA expression correlated with Epstein-Barr virus load in saliva samples, thus indicating active replication of Epstein-Barr virus.100 Angelova and colleagues detected the salivary mRNA levels of IL-6, MMP-8, and GSS in 28 pyelonephritis children, and demonstrated 64, 3.27, and 1.94 times increase, respectively.101 Fan et al. revealed reduced expression of salivary PARK2 in manganese-exposed smelting workers, which may partly explain the manganese-induced Parkinsonian disorder.102 Furthermore, the possibility to use those altered mRNA expressions in saliva as biomarkers for disease diagnosis deserves verification in a larger independent population.
microRNA
MicroRNAs (miRNAs) are a set of small non-coding RNA (sncRNA) molecules that present in saliva stably because of being wrapped in lipoprotein vesicles that protected them from degradation.103 Before 2016, microRNAs in saliva have been studied in a variety of oral-related diseases and gastroenterological cancer. In the past five years, oral-related diseases and gastroenterological cancer have still been the research hotspot for microRNAs in saliva. Many studies have found potential biomarkers for these diseases, as shown in Supplementary table 1.104–111 Otherwise, other diseases and fields have also begun to pay attention to microRNAs in saliva involved in nervous system diseases, immune system diseases, infectious diseases, and forensics.
Table 3.
The influence of saliva collection methods on salivary biomarkers
| Comparative saliva collection methods | Main results | Reference |
|---|---|---|
| Drooling, SOS, Salivette®, Synthetic, and Greiner Bio-One Saliva Collection System (SCS) |
SOS yielded lower concentrations of myoglobin and CRP the Salivette® Cotton and Synthetic swab yielded lower myoglobin and IgE concentrations |
288 |
| Drooling, paraffin gum, and Salivette® |
Saliva volume: paraffin gum > Salivette® > drooling protein concentrations: no significant differences proteome coverage: about 160 proteins of each approach specific proteins depended on the collection approach: observed |
289 |
| Stimulated by Salivette®, Parafilm®, chewing gum, and unstimulated from spit with and without fluid accumulation |
Flow rate: chewing gum > Salivette® = Parafilm® > unstimulated total protein: chewing gum > Parafilm® > others nitrite secretion: chewing gum > others total antioxidant capacity: chewing gum > others alpha-amylase concentration: unstimulated without saliva accumulation < stimulated |
290 |
| Before and after oral hygiene | Oral hygiene decreased salivary flow, reduced the secretion rate of total protein and increased sAA | 290 |
| Salivette® and spiting | Visual similar MS spectra for each individual | 291 |
| RNAPro·SAL™ and standard clinical collection process | Similar protein and exRNA recovery and stability | 292 |
| Passive drooling, Pure·SAL™, and RNAPro·SAL™ | Pure·Sal™ and RNAPro·Sal™ resulted in much clearer protein spots in comparison to passive drooling | 293 |
| Spitting and drooling | No signifcant diferences in bacterial profles | 294 |
| Spitting and drooling | No significant influence on periodontium-associated mRNA expression, DNA methylation levels and epigenetic factors | 293 |
| GeneFiX Saliva DNA Collection Kit | Drinking or eating right before collection does not influence the quantity or quality of the isolated DNA | 296 |
| Salivette® with and without stimulation | Compared with unstimulated saliva, stimulated saliva had decreased glucose levels and increased salivary flow. | 303 |
In neurological diseases, microRNA in saliva has the potential as a biomarker for autism spectrum disorder, alcohol dependence, and concussion. Several researchers had built diagnostic panels for autism spectrum disorder with a sensitivity of 81.0–93.1% and a specificity of 32.0%–87.5%.104–106 Otherwise, microRNA in saliva can be a potential, non-invasive biomarker using for the monitoring of alcohol abuse.107,112 For concussion, a panel of 14 sncRNAs could distinguish concussed subjects immediately after the game (AUC 0.91, 95% confidential interval (CI) 0.81–1) and 36–48 hours later (AUC 0.94, 95% CI 0.86 to 1).108 In immune system diseases, the set of miR-17-5p and let-7i-5p in saliva showed capability for differentiating patients with pSS from controls. It only needed 100 µL saliva using for microRNA extraction and isolation.109 In infectious disease, research focused on the diagnosis of Hand, Foot, and Mouth Disease (HFMD) resulted in 77.08%-92.86% accuracy with the 6-miRNA panel and 68.75%-91.67% with the 4-miRNA panel. sa-miR-221 was found to be consistently and significantly downregulated in HFMD cohorts.110 In forensics, research focuses on microRNA stability in saliva and methodology .113 Tiffany et al. found that saliva samples are more susceptible to environmental factors relative to blood samples.114
Over the years, many studies have investigated microRNAs as disease biomarkers. Research hotspots mainly focus on miR-21, miR-23, miR-1246 and so on. In recent years, some studies have also used the combination of multiple microRNAs as diagnostic markers of diseases, which can improve the diagnostic efficiency to a certain extent.
Circular RNA
Circular RNAs (circRNAs), a type of non-coding endogenous RNA molecules, are highly stable, mostly located in cell membranes, which can modulate gene expression by interacting with proteins or microRNAs. CircRNAs participate in many biological processes. Dysregulated expression of circRNAs leads to abnormal cellular function and growth defects that are associated with disease and cancer.115–117 Bahn et al. validated the existence of salivary circRNAs for the first time in 2015. The circRNAs in saliva may be engaged in intercellular signaling pathways and inflammatory responses.118 The origin of salivary circRNAs may be the products derived from the lysis of oral cells (epithelial cells, leukocytes, and erythrocytes) and oral microorganisms, the secretion of secretory cells, and the exosomes.
CircRNAs have been demonstrated to be responsible for the proceeding of some diseases such as cardiovascular disease, diabetes mellitus, chronic inflammatory diseases, neurological disorders, and cancer.119–121 Zhao et al. found three upregulated circRNAs in the OSCC group. Two of were associated with TNM stage and were the potential biomarkers for diagnosing OSCC.122 A meta-analysis concluded a high accuracy of circRNAs in the diagnosis of OSCC.123 Besides, they found the diagnostic value of circRNAs in saliva was higher than that in tissues. However, this phenomenon may be caused by the unbalanced inclusion of studies. Most articles included tissue samples and only one trial obtained saliva from OSCC patients. According to the meta-analysis, it was found that the clinical application of salivary circRNAs for OSCC was less explored. The association between circRNA and disease has been widely explored. However, they mainly target the tissue and plasma. The simple, rapid, and non-invasive sampling methods and the fact that most substances in peripheral blood also exist in saliva make saliva an ideal biological fluid for disease research. As a result, salivary circRNAs need to be further studied. It is expected that as more circRNAs in saliva are characterized, and the targets are identified, they would be routinely applied to personalized medicine.
Due to tissue-specific and even cell type-specific characteristics,124 salivary circRNAs are also employed as biomarkers in forensic body fluid identification. In forensic identification, mRNAs are widely explored for its potential in distinguishing body fluid types. Given the high stability of circRNAs, they have the potential application to deal with samples of low quality and limited quantity. Song et al. explored microarray expression profiles of circRNAs by which body fluids from semen, venous blood, and saliva could be discriminated. It indicated that circRNAs might be potential biomarkers for forensic body fluid identification.125 The expression of fourteen circRNAs from five human body fluids had been investigated by Liu et al. Incorporating circRNAs into mRNA profiling improved the identification of body fluids and contributed to the establishment of a robust multiplex assay.126 Subsequently, the multiplex assays had been constructed to distinguish forensic body fluids.127 In brief, circRNAs facilitate the identification of biological origin in forensic practice, especially the degraded and aged samples.
lncRNA
Long non-coding RNAs (lncRNAs) are non-coding RNAs more than 200 nucleotides and are important regulators of cellular processes. Detection of lncRNAs in saliva benefit for cancer diagnosis and prognosis. Tang et al. investigated the relative abundance of six well-documented cancer-associated lncRNAs and found a detectable amount of some lncRNAs in saliva. Among them, lncRNA MALAT1 existed in all the involved patients, indicating the potential of salivary lncRNAs as oral cancer biomarkers.128 In pancreatic cancer patients, salivary lncRNA HOTAIR and PVT1 levels were significantly higher than in benign pancreatic tumor patients and healthy controls. Further research found that the expression of salivary HOTAIR and PVT1 was pancreatic cancer-specific and significantly reduced after the curative pancreatectomy.129 Similarly, lnc-PCDH9–13:1 was significantly elevated in cancer tissues, plasma and saliva of hepatocellular carcinoma patients compared with healthy controls. Its abundance was largely reduced after curative hepatectomy but apparently elevated again if recurrence occurred. Possible mechanism lies in that the overexpression of lnc-PCDH9–13:1 could promote cell proliferation and migration in vitro.130 Shieh et al. found that people lacking salivary lncRNA X-inactive specific transcript (XIST) expression had a significantly increased risk of OSCC (AUC = 0.73).131
Although considered to be cancer-specific, lncRNAs are not that specific in noncancerous diseases.132 For instance, two cancer-associated lncRNAs, lncRNAs NEAT1 and MALAT1, were upregulated not only in cancer but also during noncancerous conditions such as SARS-CoV-2 infection.133 Thus, before the usage of salivary lncRNA detection in clinical applications, those biomarkers described above still require more validation studies with larger sample sizes in multi-centers.
Protein and hormone biomarkers
Human saliva harbors a diverse repertoire of proteins and peptides that can be informative for disease diagnosis and prognosis. To date, more than 3 000 different proteins have been identified in human saliva using proteomic approaches.134 The identified protein species are varying from high molecular weight proteins such as glycoproteins, to small molecular weight proteins or peptides. Saliva is identified as a functional equivalent to serum. For example, one proteomic study found 1939 proteins in saliva, with 597 proteins that have been observed in plasma.135 The proteins and peptides in the saliva not only play important roles in maintaining oral health but may also be used as biological markers to survey health and other disease status. Chu et al. identified three proteins as potential biomarkers of oral cancer.136 Studies found that the saliva of patients with Parkinson’s disease (PD) was different in composition from that of healthy controls.137,138 Besides, human salivary glands harbor many peptides and protein-structured hormones. Salivary hormones used in clinical research have recently drawn much attention because it offers a non-invasive alternative to the test of plasma or serum hormones. This section was structured by different types of proteins and hormones. It is noteworthy that a few non-protein hormones were also included in this section.
Enzymes
Eating is triggered by the release of saliva. Saliva is important for food digestion and energy obtaining for its multiple enzymes. Enzymes in saliva include salivary amylase (also known as ptyalin),139,140 lysozyme, lingual lipase, carbonic anhydrase, maltase, phosphatase, carbonic anhydrase, kallikrein, and peroxidases.141–143 Many of the enzymes have been utilized as diagnostic markers. Approaches based on salivary enzymatic methodologies are widely used for the diagnosis of diseases, including cardiovascular diseases,144 Alzheimer’s disease,145 oral diseases,146,147 and cancer.148 Two major focuses of salivary enzymes were pepsin and amylase.
Pepsin is the proteolytic enzyme of pepsinogen and is activated by hydrochloric acid, which has a hydrolytic effect on proteins. It is released solely by gastric chief cells and participate in the development of reflux-related disorders. It can be detected in saliva when gastroesophageal reflux or laryngopharyngeal reflux occurs. For example, some studies have shown that pepsin in saliva is a sensitive biomarker for the diagnosis of Gastroesophageal reflux disease (GERD) and laryngopharyngeal reflux (LPR).149–152 But the reported value of salivary pepsin, such as sensitivity and specificity, is highly variable among the research groups.153 Meta-analysis has investigated the diagnostic value of saliva pepsin in saliva for GERD [sensitivity: 0.60 (95% CI 0.41–0.76), specificity:0.71 (95% CI 0.51–0.86)] and LPR [sensitivity: 64% [95% CI 43–80%], specificity: 68% (95% CI 55–78%)].149,154 It may not be as helpful as previously believed. Differences in study design and enrollment criteria might be the source of heterogeneity. In addition, choosing a protocol for optimal saliva collection promotes the goal of using salivary pepsin as a biomarker for reflux in future studies.
Salivary amylase, including α-amylase and β-amylase, is the primary enzyme in saliva. It breaks down the large macromolecules, like sugars and amylopectin, and changes them into simpler components. Decreased salivary amylase is associated with increased obesity and inflammatory markers in children, such as MCP-1, TNF-α, IL-6, and CRP.155 Furthermore, decreased salivary amylase has been found to be strongly associated with OSCC and premalignant lesions, suggesting salivary amylase as a potential biomarker for the early detection of oral cancer.156 Besides, quantification of salivary amylase can be used as an evaluation index to assess salivary gland function in patients with oral cancer undergoing radiotherapy.157 Even a larger sample size is needed, Shah et al. have confirmed a considerable increase in salivary amylase levels in patients with diabetes, which means that salivary amylase can be a potential biomarker for the diagnosis and monitoring of diabetes.158–160
Other minor enzymes in salvia such as phosphatase and lysozymes help to catalyze the chemical reactions in the mouth, or act as the first defense of our body by performing their anti-bacterial, anti-viral, and anti-fungal properties, as well as killing other foreign agents.161 Lysozyme, derived from monocytes and macrophages, is widely presented in human tissues and secretions, including saliva, tears, and plasma. Currently, salivary lysozyme is expected to be a biomarker of stress. High pressure from examination or occupation can lead to lower concentrations of salivary lysozyme in students, nurses, or line workers.162–164 The same conclusion was obtained in more and more studies which demonstrated that salivary lysozyme was inversely associated with stress and suggested that stress influenced non-specific immunity. For oral diseases, active MMP-8 has been studied for point-of-care assay.165,166 It was strongly predictive for Stage IV periodontitis with a sensitivity of 89.7%, a specificity of 73.6%, and an AUC of 0.856.165 Besides, saliva levels of aspartate aminotransferase (AST) and proteinases are significantly associated with the intensity and extent of periodontal inflammation.167,168
All in all, saliva is rich in enzymes, which not only play an important role in the digestion of food, but also can be used as markers to diagnose or detect the occurrence and development of diseases. As a non-invasive detection method, salivary enzymes have wide application prospects.
Immunoglobulin
Saliva contains a variety of immunoglobulins, including immunoglobulin A (IgA), IgG, IgM and IgE and so on. They play a significant role in the body and oral immunity. IgA, mainly secretory IgA (sIgA) produced by the plasma cells in salivary glands, is the most important and abundant antibody. Salivary IgA acts as the first line of defense against pathogenic bacteria. For example, numerous studies have indicated the protective role of salivary IgA against dental caries in both children and adults, by preventing the adhesion of bacteria to the tooth surface.169–171 Meanwhile, the levels of IgA in saliva are correlated with the risk of upper-respiratory tract infections (URTIs). Low salivary IgA levels can increase the frequency and duration of URTIs. Tsukinoki et al. that salivary IgA may help prevent SARS-CoV-2 infection.172 Calheira et al. standardized an immunoassay to detect salivary IgA antibody specific to Porphyromonas gingivalis antigens, to discriminate between individuals with and without leprosy.173 Leicht et al. considered that IgA in saliva might predict a change in lung infection status in patients with cystic fibrosis.174 In addition to salivary IgA, Riis et al. reported that the salivary Human Cytomegalovirus (HCMV) IgG test had 51% sensitivity and 97% specificity.175 With further technical development, HCMV IgG oral fluid testing might replace serum HCMV IgG measurement. Additionally, Kaplan et al. concluded that Immunoglobulin free light chains in saliva acted as a potential marker with the sensitivity of 89% and specificity of 80% for disease activity in multiple sclerosis.176 Although the contents of the Ig in saliva were lower than that in serum, the level of salivary Ig is sufficient for immunological diagnosis in several diseases.
Glycoproteins
Glycoproteins are an important part of the salivary proteome and play a fundamental role in many biological processes.177 Accumulated evidence displayed that the aberrant glycoproteins in saliva were associated with tumor growth and progression as well as other diseases, making salivary glycoproteins promising diagnostic biomarkers.
In the study of diagnosis or prognosis in tumors, scholars have paved the way for salivary biomarkers application. Ragusa et al. have hydrolyzed the glycoproteins in saliva and quantified glycosylation levels. They found fucose levels higher in both cancer samples than in healthy controls, providing preliminary assessment for early diagnosis of tumor.178 Yang et al. have validated the differential expression level of galactosylated N/O-linked glycans in saliva in patients with breast cancer compared with healthy controls, promoting the study for early-stage diagnosis.179 Similarly, they also found alterations of salivary glycoprotein N-glycan profiles could be potential biomarkers distinguishing breast cancer or benign breast diseases and healthy subjects. This provided information on N-glycans during the development of breast cancer.180 It has also been reported that fucosylated N-/O-linked glycans in salivary proteins exhibited significantly differential expression levels in gastric cancer, facilitating the discovery of biomarkers for gastric cancer diagnosis.181 Zanotti et al. have observed that concentrations of saliva EGFR, a type of glycoprotein, were higher in oral cancer, leading to worse prognosis as well.182
In other diseases, glycoproteins in saliva can also exhibit potential as biomarkers while validation is needed in further clinical trials. A mucin-like glycoprotein called endogenous proteoglycan 4 (PRG4) in saliva samples was observed at elevated levels in SS patients, improving the understanding of the disease growth and treatment.183 Grant et al. have found that the best performing panels, including MMP9, S100A8, alpha-1-acid glycoprotein, and pyruvate kinase, could distinguish between periodontal health and disease states.184 In Alzheimer’s disease (AD), whether salivary lactoferrin could be the candidate biomarker reached inconsistent conclusions. A systematic review found that lactoferrin had the potential as future salivary biomarker for AD.185 However, Gleerup et al. have observed that salivary lactoferrin could not differentiate between neurodegenerative dementias.186 These findings indicated that heterogeneity of cohorts or medications leading to altered saliva production might impact the efficacy of potential biomarkers.
Overall, multiple glycoproteins in saliva have been investigated and may be candidates for future biomarkers in different diseases, while further validation should be carried out in larger and representative clinical cohorts.
C-reaction protein (CRP)
CRP is a well-known sensitive inflammatory biomarker, usually detected in serum samples. As saliva sampling is non-invasive, simple, and patient-friendly, salivary CRP may be an alternative biomarker for clinical diagnosis.
In infectious diseases, CRP in saliva has been investigated to reflect the serum level. Jacobs et al. have reported that markers including salivary CRP may be indicative in the diagnosis of tuberculosis (TB), with a decent sensitivity of 78.1% and specificity of 83.3%.187 The salivary CRP level was reported much higher in patients (aged 2 to 17 years) with pneumonia than in healthy children (P < 0.001). Besides, saliva and serum CRP level was highly correlated in pediatric patients with pneumonia (r = 0.679; P < 0.001).188 Similarly, Lin et al. observed a correlation of CRP levels between serum and saliva samples over time in septic neonates.189 A higher salivary concentration of CRP was also observed in adult patients with sepsis compared to patients without sepsis, though without statistical difference (P > 0.05).190 The capacity of salivary CRP as a biomarker for sepsis both in neonates and adults exhibited differences, which needs further validation and mechanisms exploitation. Other than inflammation and infectious diseases, salivary CRP may also be potential biomarkers for other diseases such as acne vulgaris191 and Huntington’s disease.192 It indicated that inflammatory markers might also function in the physiological process of non-inflammatory diseases.
Salivary CRP is broadly detected via the enzyme-linked immunosorbent assay (ELISA) technique, which can be cumbersome and time-consuming. Therefore, many scholars have devoted themselves to developing efficient detection methods for clinical translation applications. For example, a photothermal biosensor for the measurement of CRP in saliva has been developed with rapid, simple, and minimally invasive characteristics.193 Many methods have also been investigated to improve analysis performance of detecting CRP in human saliva, such as the silicon nanowire luminescent sensor,194 a label-free and highly sensitive imaging sensor based on a plasmonic nano cup array,195 a label-free biosensor based on an electrolyte-gated organic thin-film transistor (EGOTFT),196 electrochemical methods,197 etc. However, there’s still a long way to go for the clinical application of these novel methods due to sample heterogeneity and small sample size.
Hormones
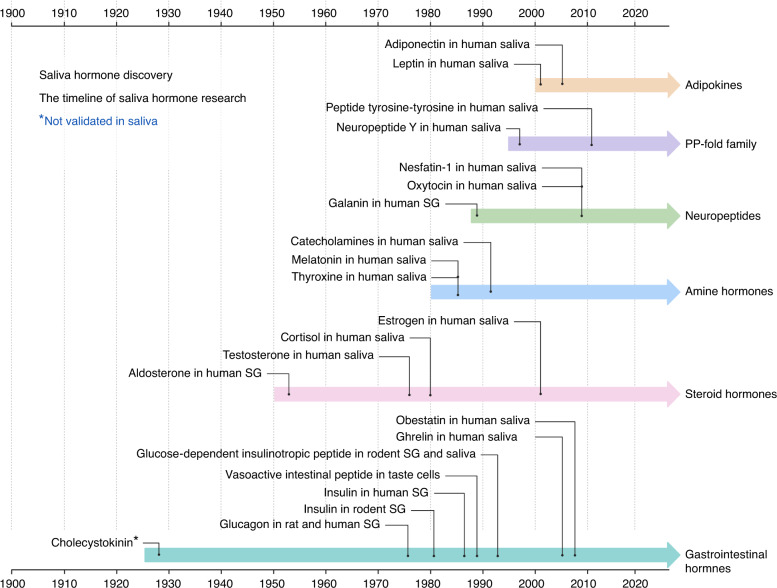
Steroids, non-steroids, peptides, and protein hormones can be detected in saliva. Nevertheless, some hormones are of little use for saliva testing, such as dehydroepiandrosterone sulfate, thyroxine, and pituitary hormones.12 The research timeline of valuable salivary hormones was extracted from published papers,12,198–212 displayed in Fig. 2.
Fig. 2.
The timeline of salivary hormones. The time was determined by the publishing year of the oldest and most relevant papers. Not all hormones were included since there was no evidence showing the existence of them in saliva or the poor correlation of the concentration between saliva and target organ, such as follicle-stimulating hormone (FSH). The figure is created with BioRender.com
Cortisol is a hormone secreted by the adrenal glands that can regulate the metabolism of sugar and is distributed in various body fluids, including saliva, urine, and serum. Cortisol has been studied as a biomarker of diverse diseases. Gozansky et al. have found that salivary cortisol is better than serum total cortisol for evaluation of dynamic hypothalamic-pituitary-adrenal (HPA) axis activity, because more physiologically relevant data can be obtained from salivary cortisol.213 The HPA axis is the main neuroendocrine axis that regulates mammalian stress responses.214 Dysfunction of the HPA axis is associated with mental and functional problems, such as anxiety, insomnia, depression, bipolar disorder, affective disorder, cognition impairment, fibromyalgia (FM), allergic bowel syndrome, and alcoholism. HPA axis reacts to the stressor by secreting cortisol. Salivary cortisol, as a stress marker, has become a hot spot for research in recent years.164,215 A close relationship was found in patients with FM between salivary cortisol levels and the disease process. Compared with healthy individuals, increased salivary cortisol levels were detected in initial stages of FM, while decreased levels were detected in those with a longer duration of disease.216 Cortisol was reported to be a useful salivary biomarker of non-motor symptoms in patients with PD, since approximately higher levels of cortisol were identified in PD compared to the healthy controls.138 Furthermore, salivary cortisol can be used for differential diagnosing of Cushing’s syndrome,217,218 monitoring the state of oxidative stress,219 and recognizing inflammatory periodontal diseases (IPD).220 Cortisol testing often needs to be conducted at midnight, which is not always possible. More importantly, venous blood collection would cause the fluctuation of cortisol levels, which decrease the accuracy of evaluation. Saliva collection by the patients themselves is convenient and accurate. It has been utilized in clinical diagnosis.
Oxytocin, a neuro-hormone produced by the hypothalamus and finally released from the posterior hypophysis,221 is distributed in the periphery and central nervous system.222 Over the past decades, oxytocin has been extensively studied due to its important function in reproductive physiology, affective processes, and social behaviors, including stimulating the milk production in mammary glands, promoting the uterine smooth muscle contraction during labor, regulating parental nurturing behaviors and empathy-related behaviors.221,223,224 In addition, oxytocin was reported to be associated with pain relief in aging.222 A negative correlation was also found between oxytocin levels and depression/anxiety.225 Though saliva as an easy-access and low-invasive sample can be a good choice for oxytocin analysis, salivary oxytocin levels represent only 5%-10% of blood oxytocin levels, making the measurement of salivary oxytocin very difficult.226 The application of salivary oxytocin is limited by the means of detection. With the rapid advancement of science and technology, salivary oxytocin holds a great prospect for research and applications.
Melatonin, a neurohormone produced by the pineal gland, can regulate circadian rhythms (sleep-wake rhythm, neuroendocrine rhythms, and body temperature cycles).227 Melatonin is presented in various body fluids, including blood, saliva, and cerebrospinal fluid. The level of melatonin in the saliva is about 30% of that in blood, recognized to present the free, unbound plasma melatonin levels.228 Like blood melatonin, salivary melatonin is an indicator of the circadian phase.228 Besides, the detection and quantification of daytime salivary melatonin content can positively reflect the levels of some depression-related inflammatory markers, such as CCL2/MCP-1, CCL3/MPI-1α, and VEGF-A.229 Yvan et al. has found a positive correlation between salivary melatonin levels and urinary excretion of 6-sulphatoxymelaton in children. This suggested salivary melatonin a potential marker for the diagnosis of chronobiological disorders in prepubertal children.230 Further, melatonin has protective, oncostatic, and antioxidant properties.231 Salivary melatonin levels in patients with lung cancer and prostate cancer were lower compared to those in controls.232,233
Sex hormones can also be detected in saliva. Mitova et al. have proved that saliva is an appropriate medium for studying the quantities of sex hormones.234 It has been reported that there was a high linear correlation between salivary and plasma for 17a-hydroxyprogesterone (17-OHP), progesterone (P), androstenedione (A4), and cortisol (F).235 In addition, salivary steroids stand for only the free, bioactive fraction of steroids circulating in blood.236 The detection of salivary cortisol at midnight is routinely used to screen for Cushing’s syndrome.237 Also, salivary testosterone was correlated significantly with free serum testosterone in adult men and women by an improved liquid chromatography/tandem mass spectrometry or mass spectrometry (LC-MS/MS) method.238 Saliva testosterone may be a reliable alternative to serum testosterone in the diagnosis of androgen disorders and monitoring the disease status in clinical research. The amount of asprosin hormone, reported for the first time by Ugur et al., was higher in saliva than in blood. The increased level of asprosin in saliva correlates with increased BMI.239 The properties have made salivary hormone measurement beneficial for various disciplines like psychology, endocrinology, and pediatrics to assess endocrine sex-related hormone levels.236
Multiple platforms have been established to measure the concentration of the hormones in the saliva. Arevalo et al. employed an electrochemical bioplatform for simultaneously determining four fertility-related target hormones, such as estradiol (E2), progesterone (P4), luteinizing hormone (LH), and prolactin (PRL).240 Xu et al. described a method of LC/MS for the simultaneous quantification of seven trace steroid hormones in human saliva and showed excellent specificity and sensitivity.241 With the rapid development of technology, the detection of salivary hormones has a wide application prospect in the diagnosis of diseases and in monitoring the disease status.
Metabolomic biomarkers
Saliva metabolome has been largely understudied compared to genome, transcriptome, and proteome. Researchers analyzed the metabolomes of 16 healthy adults.242 They were able to quantify or identify 308 salivary metabolites in human saliva. Based on the identified metabolites, researchers also explored the potential use of them in distinguishing disease status. Zhou et al. found that metabolomic biomarkers could assist in diagnosis and monitoring of orthodontically induced external apical root resorption (OIEARR).243 Some scholars also agreed with the point that saliva metabolome was meaningful for oral diseases. They found that saliva represented as metabolomic fingerprints for patients with periodontitis.244 For more severe oral disease, such as OSCC, saliva metabolome also showed its promising result. In a Japanese cohort, 25 metabolites were revealed as potential biomarkers to discriminate OSCC patients and healthy controls.245 Similar as the interests about epigenetic change brought by environmental factors, a study also paid attention to metabolomic profile alteration under different environment.246 They concluded that the metabolomic features could be used in identification and measurement of exposures to mobile source air toxics.
Exosome biomarkers
Saliva has less complicated components than blood, and the salivary exosome biomarkers are enriched 1000-fold lower than that of biomarkers in blood.247 Exosomes contain abundant proteins, mRNA, microRNAs, cytokines, and transcription factor receptors,248 which can be promising in cancer diagnosis, aging research,249 and many other diseases.250–252 Using saliva exosomes avoids potential contaminants or confusing substances, such as food debris, thus benefiting biomarker discovery. Some differences in substances were only present in salivary exosomes, not whole saliva.111 For instance, saliva membrane-based markers CD9 and CD81 were expressed differently in oral disease patients and healthy individuals.253,254 It is reported that microRNAs detected in saliva and serum are mostly enriched in exosomes.255 Saliva exosomes were mainly secreted by salivary glands, with part of them from oral colonization flora. By shotgun mass spectroscopy analysis, novel biomarkers of salivary exosomes in patients with inflammatory bowel disease (IBD) and healthy controls for IBD were revealed.256 The study found that proteasome subunit alpha type 7 (PSMA7) showed apparent differences between patients with IBD and healthy controls. In IBD, salivary exosomal PSMA7 plays a role in identifying IBD with healthy controls.256 Sun et al. confirmed the existence of BPIFA1, CRNN, MUC5B, and IQGAP either in salivary microvesicles or in exosomes between normal subjects and lung cancer patients.257 Additionally, neurodegenerative diseases were given much attention for saliva exosome biomarker discovery.258
There have been reviews summarizing salivary exosomal biomarkers in cancer,259–263 neurodegenerative diseases,264 mental diseases,265 systematic disease,266 wound healing267,268 and isolation methods.269,270 Research articles emphasize protein biomarkers250,257,271–275 and RNA markers.276–279 The methodology of salivary exosomes mainly focuses on isolation, quantification, and characterization.280,281 Some point-of-care testing kits were also invented. Detecting exosomal miRNA in saliva was useful for the diagnosis of lung cancer.282
A few studies aimed at resolving the regulating mechanism of salivary exosomes. Salivary exosomes from mice with pancreatic ductal adenocarcinoma (PDAC) suppress the tumor-killing capacity of nature killing (NK) cells.275 CD9 and CD81 participate in the functional regulation of cell adhesion and migration. Decreased expression of these two markers benefits the metastasis of tumor cells.254 On the contrary, overexpressed miR-24–3p promotes the propagation of tumor cells by interacting directly with the PER1 gene.277 Programmed death-ligand 1 (PD-L1) mRNA in salivary exosomes inhibits the damage to the inflammatory system.283
Saliva collection and preservation
There are two types of whole saliva distinguished by the method of collection, unstimulated whole saliva (USWS) and stimulated whole saliva (SWS). Passive drooling and spitting method are primarily used in collecting USWS. For the collection of SWS, chewing different things (like natural gum) have been used. Recent developments in standardized saliva collection devices allow safe, simple, and convenient collection of samples from patients. For a more detailed overview of saliva collection methods and devices, the reader is referred to previous reviews on this subject.284–287 Briefly, the Super·Sal™ (Oasis Diagnostic) and Versi·Sal™ (Oasis Diagnostic) devices sample unstimulated whole saliva by passive drooling, while the Salivette® (Sarstedt) and SalivaBio Oral Swab (SOS, Salimetrics) can sample unstimulated saliva from specific regions or stimulated saliva by simply moving it in the oral cavity.285 With the availability of these new technologies, researchers have focused on the effect of saliva collection methods and devices on different salivary biomarkers. We reviewed those advances to contribute insight into saliva sampling for further study design and clinical application.
The influence of saliva collection methods and devices on different types of salivary biomarkers are summarized in Table 3. Although different saliva collection methods yielded similar proteome coverage, the specific protein types and concentrations observed depended on the collection approach, because of different salivary flow rates and protein secretion rates.288–293 Rigid criteria for saliva collection may not be required in salivary nucleic acid analysis. Several studies have revealed that the method of saliva collection has a minimal impact on nucleic acid analysis, including exRNA recovery, mRNA expression, gDNA profiles, DNA quality and quantity, DNA methylation levels and epigenetic factors.292,294–296
In addition to the standardized collection method, an appropriate storage condition is probably another key factor for the successful analysis of saliva constituents. Human salivary exosomes remained undestroyed without the existence of protease inhibitor, under different storage temperatures.297 However, owing to the interference of complicated saliva components, in particular bacteria and enzymes, proper saliva storage is mandatory. Generally, the saliva collection tube should be properly sealed and placed upright.298 The saliva sample should be kept between 2 and 8 °C in cold packs or containers with ice, and transported to the processing laboratory as soon as possible within 24 h to a maximum of 48 h. After arrival at the laboratory, the saliva sample should be processed immediately, otherwise directly stored at -80 °C.299,300 Alternatively, when using commercial collection kits, transportation and storage requirements may differ, so it’s important to ensure that all instructions for manufacturers are followed.298 Moreover, there are also differences in the recommended preservation condition and time for specifically interested biomarkers (Table 4).
Table 4.
Stabilities of salivary biomarkers on different preservation conditions
| Biomarkers | Collection methods | Preservatives | Temperature | Stable Time | Reference |
|---|---|---|---|---|---|
| Total protein | Sublingual cotton roll | None | RT | ≥ 24 hours | 304 |
| SAA, UA, total protein and low-molecular-weight antioxidants | Saliva Collection System | None | -20 °C | ≥ 2 weeks | 305 |
| IgA and lysozyme | unstimulated saliva | None | -30 °C | ≥ 3 months | 306 |
| Cortisol | Salivette® | NaN3 | 18°C | ≥ 3 weeks | 307 |
| None | 5°C | ≥ 3 months | 308 | ||
| -20°C/-80°C | ≥ 1 year | ||||
| Total RNA | Drooling | RNAlater | RT | ≥ 7 days | 309 |
| -80°C | ≥ 4 weeks | ||||
| Metabolomics | Drooling | None | RT/4°C | ≥ 6 hours | 310 |
| -20°C | ≥ 4 weeks | ||||
| NaN3 | 25°C | ≥ 8 hours | |||
| 4°C | ≥ 48 hours |
Conclusion
In this review, the updated research about salivary biomarkers was discussed. RNA biomarkers were most widely studied in all kinds of diseases. However, in our opinion, hormones were the most promising markers in clinical diagnostics. Future studies need to be conducted to overcome the fluctuation caused by the circadian rhythm and convenient wearable or portable devices for monitoring the dynamic changes in patients. This will benefit a lot for patients who need to be tested multiple times and are hard to obtain blood or other types of body fluids.
Supplementary information
Acknowledgements
The current research was funded by the National Natural Science Foundation of China (U20A20394, 81870754, 82272416, 82202614), State Key Laboratory of Oral Diseases (2022KXK0402), Clinical and Translational Medicine Research Fund of Chinese Academy of Medical Sciences (2020-I2M-C&T-B-097). Thanks Chunying Zhang, Shuangshuang Wang (Department of Forensic Genetics, West China School of Basic Medical Sciences & Forensic Medicine, Sichuan University, Chengdu, Sichuan Province, China), Jiajia Song, Jin Li and Zhaodan, Xin for manuscript revision and figure production.
Author contributions
B.Y. and X.Z. contributed to the conception of the study; M.S. and H.B. wrote the manuscript. B.Y., X.Z., and P.Z. revised the manuscript. All authors approved the final manuscript.
Competing interests
: The authors declare no competing interests.
Footnotes
These authors contributed equally: Mengyuan Song, Hao Bai.
Supplementary information
The online version contains supplementary material available at 10.1038/s41368-022-00209-w.
References
- 1.Chen Y, Liu F, Lee LP. Quantitative and ultrasensitive in situ immunoassay technology for SARS-CoV-2 detection in saliva. Sci. Adv. 2022;8:eabn3481. doi: 10.1126/sciadv.abn3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ning B, et al. A smartphone-read ultrasensitive and quantitative saliva test for COVID-19. Sci. Adv. 2021;7:19–23. doi: 10.1126/sciadv.abe3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, et al. Detection of somatic mutations and HPV in the saliva and plasma of patients with head and neck squamous cell carcinomas. Sci. Transl. Med. 2015;7:1–8. doi: 10.1126/scitranslmed.aaa8507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang B, et al. Wearable aptamer-field-effect transistor sensing system for noninvasive cortisol monitoring. Sci. Adv. 2022;8:1–16. doi: 10.1126/sciadv.abk0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas AJ, Woo B, Nettle D, Spelke E, Saxe R. Early concepts of intimacy: young humans use saliva sharing to infer close relationships. Science (1979) 2022;375:311–315. doi: 10.1126/science.abh1054. [DOI] [PubMed] [Google Scholar]
- 6.Pico-Alfonso MA, Garcia-Linares MI, Celda-Navarro N, Herbert J, Martinez M. Changes in cortisol and dehydroepiandrosterone in women victims of physical and psychological intimate partner violence. Biol. Psychiatry. 2004;56:233–240. doi: 10.1016/j.biopsych.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Yoon Y-J, et al. Salivary gland organoid culture maintains distinct glandular properties of murine and human major salivary glands. Nat. Commun. 2022;13:1–16. doi: 10.1038/s41467-022-30934-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng X, et al. Oral microbiota in human systematic diseases. Int J. Oral. Sci. 2022;14:1–11. doi: 10.1038/s41368-022-00163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang CZ, et al. Saliva in the diagnosis of diseases. Int J. Oral. Sci. 2016;8:133–137. doi: 10.1038/ijos.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pedersen AML, Sorensen CE, Proctor GB, Carpenter GH, Ekstrom J. Salivary secretion in health and disease. J. Oral. Rehabil. 2018;45:730–746. doi: 10.1111/joor.12664. [DOI] [PubMed] [Google Scholar]
- 11.Mese H, Matsuo R. Salivary secretion, taste and hyposalivation. J. Oral. Rehabil. 2007;34:711–723. doi: 10.1111/j.1365-2842.2007.01794.x. [DOI] [PubMed] [Google Scholar]
- 12.Vining RF, McGinley RA. The measurement of hormones in saliva: Possibilities and pitfalls. J. Steroid Biochem. 1987;27:81–94. doi: 10.1016/0022-4731(87)90297-4. [DOI] [PubMed] [Google Scholar]
- 13.Lee VM, Linden RW. An olfactory-submandibular salivary reflex in humans. Exp. Physiol. 1992;77:221–224. doi: 10.1113/expphysiol.1992.sp003578. [DOI] [PubMed] [Google Scholar]
- 14.Tiwari M. Science behind human saliva. J. Nat. Sci. Biol. Med. 2011;2:53–58. doi: 10.4103/0976-9668.82322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaczor-Urbanowicz KE, et al. Clinical validity of saliva and novel technology for cancer detection. Biochim Biophys. Acta Rev. Cancer. 1872;49–59:2019. doi: 10.1016/j.bbcan.2019.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao X, Jiang S, Koh D, Hsu C-YS. Salivary biomarkers for dental caries. Periodontol 2000. 2016;70:128–141. doi: 10.1111/prd.12100. [DOI] [PubMed] [Google Scholar]
- 17.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogawa Y, et al. Proteomic analysis of two types of exosomes in human whole saliva. Biol. Pharm. Bull. 2011;34:13–23. doi: 10.1248/bpb.34.13. [DOI] [PubMed] [Google Scholar]
- 19.Crook M. Differential diagnosis by laboratory medicine. J. Clin. Pathol. 2004;57:112–112. doi: 10.1136/jcp.57.1.112. [DOI] [Google Scholar]
- 20.Brasier N, Osthoff M, de Ieso F, Eckstein J. Next-Generation digital biomarkers for tuberculosis and antibiotic stewardship: Perspective on novel molecular digital biomarkers in sweat, saliva, and exhaled breath. J. Med Internet Res. 2021;23:e25907. doi: 10.2196/25907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang X, et al. Garment embedded sweat-activated batteries in wearable electronics for continuous sweat monitoring. npj Flex. Electron. 2022;6:10. doi: 10.1038/s41528-022-00144-0. [DOI] [Google Scholar]
- 22.Zhong B, Jiang K, Wang L, Shen G. Wearable sweat loss measuring devices: from the role of sweat loss to advanced mechanisms and designs. Adv. Sci. 2022;9:2103257. doi: 10.1002/advs.202103257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ray P, Steckl AJ. Label-free optical detection of multiple biomarkers in sweat, plasma, urine, and saliva. ACS Sens. 2019;4:1346–1357. doi: 10.1021/acssensors.9b00301. [DOI] [PubMed] [Google Scholar]
- 24.Langie SAS, et al. Salivary DNA methylation profiling: aspects to consider for biomarker identification. Basic Clin. Pharm. Toxicol. 2017;121:93–101. doi: 10.1111/bcpt.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishitani S, Parets SE, Haas BW, Smith AK. DNA methylation analysis from saliva samples for epidemiological studies. Epigenetics. 2018;13:352–362. doi: 10.1080/15592294.2018.1461295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakulski KM, et al. Prenatal particulate matter exposure is associated with saliva dna methylation at age 15: applying cumulative dna methylation scores as an exposure biomarker. Toxics. 2021;9:262. doi: 10.3390/toxics9100262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dawes K, et al. Saliva DNA methylation detects nascent smoking in adolescents. J. Child Adolesc. Psychopharmacol. 2019;29:535–544. doi: 10.1089/cap.2018.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramakrishnan P, S S, Kundu P. Methylation status of p16INK4a and p14ARF gene in saliva of smokeless tobacco users: a pilot descriptive study. J. Oral. Maxillofac. Surg. Med Pathol. 2021;33:221–226. doi: 10.1016/j.ajoms.2020.10.001. [DOI] [Google Scholar]
- 29.Philibert R, Dogan M, Beach SRH, Mills JA, Long JD. AHRR methylation predicts smoking status and smoking intensity in both saliva and blood DNA. Am. J. Med. Genet., Part B: Neuropsychiatr. Genet. 2020;183:51–60. doi: 10.1002/ajmg.b.32760. [DOI] [PubMed] [Google Scholar]
- 30.Dawes K, et al. The relationship of smoking to cg05575921 methylation in blood and saliva DNA samples from several studies. Sci. Rep. 2021;11:1–12. doi: 10.1038/s41598-021-01088-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abraham JE, et al. Saliva samples are a viable alternative to blood samples as a source of DNA for high throughput genotyping. BMC Med Genomics. 2012;5:19. doi: 10.1186/1755-8794-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bearer EL, Mulligan BS. Epigenetic Changes Associated with Early Life Experiences: Saliva, A Biospecimen for DNA Methylation Signatures. Curr. Genomics. 2018;19:676–698. doi: 10.2174/1389202919666180307150508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Middleton LYM, et al. Saliva cell type DNA methylation reference panel for epidemiological studies in children. Epigenetics. 2022;17:161–177. doi: 10.1080/15592294.2021.1890874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rounge TB, et al. Genome-wide DNA methylation in saliva and body size of adolescent girls. Epigenomics. 2016;8:1495–1505. doi: 10.2217/epi-2016-0045. [DOI] [PubMed] [Google Scholar]
- 35.Moccia C, et al. Birthweight DNA methylation signatures in infant saliva. Clin. Epigenetics. 2021;13:1–13. doi: 10.1186/s13148-021-01053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dunstan J, et al. Associations of LEP, CRH, ICAM-1, and LINE-1 methylation, measured in saliva, with waist circumference, body mass index, and percent body fat in mid-childhood. Clin. Epigenetics. 2017;9:29. doi: 10.1186/s13148-017-0327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murata Y, et al. Evaluation of the usefulness of saliva for DNA methylation analysis in cohort studies. Neuropsychopharmacol. Rep. 2019;39:301–305. doi: 10.1002/npr2.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel, M., Gawande, M. N., Chaudhary, M. S. & Hande, A. H. DNA methylation patterns in saliva of tobacco users with high-risk oral potentially malignant disorders. J Pharm Res Int 129–137 10.9734/jpri/2021/v33i32b31754 (2021).
- 39.Kaliyaperumal S, Sankarapandian S. Evaluation of p16 hypermethylation in oral submucous fibrosis: A quantitative and comparative analysis in buccal cells and saliva using real-time methylation-specific polymerase chain reaction. South Asian J. Cancer. 2016;05:073–079. doi: 10.4103/2278-330X.181645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Martinez JHC, et al. Effect of smoking on the DNA methylation pattern of the SOCS1 promoter in epithelial cells from the saliva of patients with chronic periodontitis. J. Periodontol. 2019;90:1279–1286. doi: 10.1002/JPER.18-0692. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi T, et al. Orthognathic surgery induces genomewide changes longitudinally in DNA methylation in saliva. Oral. Dis. 2019;25:508–514. doi: 10.1111/odi.12998. [DOI] [PubMed] [Google Scholar]
- 42.Chuang YH, et al. Parkinson’s disease is associated with DNA methylation levels in human blood and saliva. Genome Med. 2017;9:76. doi: 10.1186/s13073-017-0466-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rahbar E, et al. Allele-specific methylation in the FADS genomic region in DNA from human saliva, CD4+ cells, and total leukocytes. Clin. Epigenetics. 2018;10:46. doi: 10.1186/s13148-018-0480-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hearn NL, Chiu CL, Lind JM. Comparison of DNA methylation profiles from saliva in Coeliac disease and non-coeliac disease individuals. BMC Med Genomics. 2020;13:16. doi: 10.1186/s12920-020-0670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas M, et al. Increased BDNF methylation in saliva, but not blood, of patients with borderline personality disorder. Clin. Epigenetics. 2018;10:109. doi: 10.1186/s13148-018-0544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilmot B, et al. Methylomic analysis of salivary DNA in childhood ADHD identifies altered DNA methylation in VIPR2. J. Child Psychol. Psychiatry. 2016;57:152–160. doi: 10.1111/jcpp.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hearn NL, Coleman AS, Ho V, Chiu CL, Lind JM. Comparing DNA methylation profiles in saliva and intestinal mucosa. BMC Genomics. 2019;20:1–9. doi: 10.1186/s12864-019-5553-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hong SR, et al. DNA methylation-based age prediction from saliva: High age predictability by combination of 7 CpG markers. Forensic Sci. Int Genet. 2017;29:118–125. doi: 10.1016/j.fsigen.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 49.Ho Lee M, et al. Application of droplet digital PCR method for DNA methylation-based age prediction from saliva. Leg. Med. 2022;54:101992. doi: 10.1016/j.legalmed.2021.101992. [DOI] [PubMed] [Google Scholar]
- 50.Alghanim H, Balamurugan K, McCord B. Development of DNA methylation markers for sperm, saliva and blood identification using pyrosequencing and qPCR/HRM. Anal. Biochem. 2020;611:113933. doi: 10.1016/j.ab.2020.113933. [DOI] [PubMed] [Google Scholar]
- 51.Lee HY, Jung SE, Lee EH, Yang WI, Shin KJ. DNA methylation profiling for a confirmatory test for blood, saliva, semen, vaginal fluid and menstrual blood. Forensic Sci. Int Genet. 2016;24:75–82. doi: 10.1016/j.fsigen.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 52.Ghai M, Naidoo N, Evans DL, Kader F. Identification of novel semen and saliva specific methylation markers and its potential application in forensic analysis. Forensic Sci. Int Genet. 2020;49:102392. doi: 10.1016/j.fsigen.2020.102392. [DOI] [PubMed] [Google Scholar]
- 53.Silva DSBS, et al. Developmental validation studies of epigenetic DNA methylation markers for the detection of blood, semen and saliva samples. Forensic Sci. Int Genet. 2016;23:55–63. doi: 10.1016/j.fsigen.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 54.Watanabe K, Taniguchi K, Toyomane K, Akutsu T. A new approach for forensic analysis of saliva-containing body fluid mixtures based on SNPs and methylation patterns of nearby CpGs. Forensic Sci. Int Genet. 2022;56:102624. doi: 10.1016/j.fsigen.2021.102624. [DOI] [PubMed] [Google Scholar]
- 55.Senst A, Dressler J, Edelmann J, Kohl M. Development of a qPCR assay for detection of secretion types: Identification of semen, vaginal secretion and saliva using tissue-specific methylation patterns. Rechtsmedizin. 2019;29:94–100. doi: 10.1007/s00194-018-0294-y. [DOI] [Google Scholar]
- 56.Jung SE, et al. DNA methylation of the ELOVL2, FHL2, KLF14, C1orf132/MIR29B2C, and TRIM59 genes for age prediction from blood, saliva, and buccal swab samples. Forensic Sci. Int Genet. 2019;38:1–8. doi: 10.1016/j.fsigen.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 57.Hamano Y, Manabe S, Morimoto C, Fujimoto S, Tamaki K. Forensic age prediction for saliva samples using methylation-sensitive high resolution melting: Exploratory application for cigarette butts. Sci. Rep. 2017;7:10444. doi: 10.1038/s41598-017-10752-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oka H, Dwi Ariani M, Akazaki T, Miyauchi M, Kitagawa M. Some tips on age estimation using DNA methylation in saliva samples as an index across the Japanese and Indonesian ethnicities. Leg. Med. 2022;56:102042. doi: 10.1016/j.legalmed.2022.102042. [DOI] [PubMed] [Google Scholar]
- 59.Staunstrup NH, et al. Saliva as a blood alternative for genome-wide dna methylation profiling by methylated dna immunoprecipitation (Medip) sequencing. Epigenomes. 2017;1:14. doi: 10.3390/epigenomes1030014. [DOI] [Google Scholar]
- 60.Machan M, et al. The impact of concussion, sport, and time in season on saliva telomere length in healthy athletes. Front Sports Act. Living. 2022;4:816607. doi: 10.3389/fspor.2022.816607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lahnert P. An improved method for determining telomere length and its use in assessing age in blood and saliva. Gerontology. 2005;51:352–356. doi: 10.1159/000086374. [DOI] [PubMed] [Google Scholar]
- 62.Geronimus AT, et al. Coming up short: Comparing venous blood, dried blood spots & saliva samples for measuring telomere length in health equity research. PLoS One. 2021;16:e0255237. doi: 10.1371/journal.pone.0255237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hummel EM, et al. Cell-free DNA release under psychosocial and physical stress conditions. Transl. Psychiatry. 2018;8:236. doi: 10.1038/s41398-018-0264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weerakoon KG, et al. Droplet digital PCR diagnosis of human schistosomiasis: parasite cell-free DNA detection in diverse clinical samples. J. Infect. Dis. 2017;216:1611–1622. doi: 10.1093/infdis/jix521. [DOI] [PubMed] [Google Scholar]
- 65.Xi Y, et al. The implications of gene mutations in salivary DNA for noninvasive diagnosis of head and neck cancer with a focus on oral cancer. Oral. Oncol. 2022;130:105924. doi: 10.1016/j.oraloncology.2022.105924. [DOI] [PubMed] [Google Scholar]
- 66.Li F, et al. Ultra-short circulating tumor dna (UsctDNA) in plasma and saliva of non-small cell lung cancer (NSCLC) patients. Cancers (Basel) 2020;12:1–15. doi: 10.3390/cancers12082041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ding S, et al. Saliva-derived cfDNA is applicable for EGFR mutation detection but not for quantitation analysis in non-small cell lung cancer. Thorac. Cancer. 2019;10:1973–1983. doi: 10.1111/1759-7714.13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eun YG, Yoon YJ, Won KY, Lee YC. Circulating tumor DNA in saliva in an orthotopic head and neck cancer mouse model. Anticancer Res. 2020;40:191–199. doi: 10.21873/anticanres.13940. [DOI] [PubMed] [Google Scholar]
- 69.Hanna GJ, et al. Salivary HPV DNA informs locoregional disease status in advanced HPV-associated oropharyngeal cancer. Oral. Oncol. 2019;95:120–126. doi: 10.1016/j.oraloncology.2019.06.019. [DOI] [PubMed] [Google Scholar]
- 70.Li Y, et al. RNA profiling of cell-free saliva using microarray technology. J. Dent. Res. 2004;83:199–203. doi: 10.1177/154405910408300303. [DOI] [PubMed] [Google Scholar]
- 71.Zimmermann BG, Noh JP, Wong DT. Genomic targets in saliva. in. Ann. N. Y. Acad. Sci. 2007;1098:184–191. doi: 10.1196/annals.1384.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arantes LMRB, et al. Serum, plasma and saliva biomarkers for head and neck cancer. Expert Rev. Mol. Diagn. 2018;18:85–112. doi: 10.1080/14737159.2017.1404906. [DOI] [PubMed] [Google Scholar]
- 73.Elashoff D, et al. Prevalidation of salivary biomarkers for oral cancer detection. Cancer Epidemiol. Biomark. Prev. 2012;21:664–672. doi: 10.1158/1055-9965.EPI-11-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Y, et al. Salivary transcriptome diagnostics for oral cancer detection. Clin. Cancer Res. 2004;10:8442–8450. doi: 10.1158/1078-0432.CCR-04-1167. [DOI] [PubMed] [Google Scholar]
- 75.Michailidou E, et al. Salivary mRNA markers having the potential to detect oral squamous cell carcinoma segregated from oral leukoplakia with dysplasia. Cancer Epidemiol. 2016;43:112–118. doi: 10.1016/j.canep.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 76.Martin JL. Validation of reference genes for oral cancer detection panels in a prospective blinded cohort. PLoS One. 2016;11:e0158462. doi: 10.1371/journal.pone.0158462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheng YSL, et al. Chronic periodontitis can affect the levels of potential oral cancer salivary mRNA biomarkers. J. Periodontal Res. 2017;52:428–437. doi: 10.1111/jre.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Horváth J, et al. Oral health may affect the performance of mRNA-based saliva biomarkers for oral squamous cell cancer. Pathol. Oncol. Res. 2018;24:833–842. doi: 10.1007/s12253-017-0296-1. [DOI] [PubMed] [Google Scholar]
- 79.Li F, et al. Discovery and validation of salivary extracellular RNA biomarkers for noninvasive detection of gastric cancer. Clin. Chem. 2018;64:1513–1521. doi: 10.1373/clinchem.2018.290569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu F, Jiang M. Evaluation of predictive role of carcinoembryonic antigen and salivary mRNA biomarkers in gastric cancer detection. Medicine. 2020;99:e20419. doi: 10.1097/MD.0000000000020419. [DOI] [PubMed] [Google Scholar]
- 81.Bentata M, et al. Splicing factor transcript abundance in saliva as a diagnostic tool for breast cancer. Genes (Basel) 2020;11:1–12. doi: 10.3390/genes11080880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang J, Xiang C, Liu J. Clinical significance of combining salivary mRNAs and carcinoembryonic antigen for ovarian cancer detection. Scand. J. Clin. Lab Invest. 2021;81:39–45. doi: 10.1080/00365513.2020.1852478. [DOI] [PubMed] [Google Scholar]
- 83.Gu X, He J, Ji G, Shen L. Combined use of circulating tumor cells and salivary mRNA to detect non-small-cell lung cancer. Med. (U. S.) 2020;99:e19097. doi: 10.1097/MD.0000000000019097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Uzoma IC, Taiwo IA, Nna EO, Durosinmi MA, Ukaejiofo EO. Detection of BCR-ABL1 fusion gene transcripts in the saliva of Nigerian patients with chronic myeloid leukemia. Niger. J. Clin. Pr. 2019;22:51–55. doi: 10.4103/njcp.njcp_225_18. [DOI] [PubMed] [Google Scholar]
- 85.Wang Z, Zhang SH, Zhou D, Zhao SM, Li CT. Messenger RNA profiling for forensic body fluid identification: research and applications. J. Forensic Med. 2013;29:368–374. [PubMed] [Google Scholar]
- 86.Watanabe K, Akutsu T, Takamura A, Sakurada K. Practical evaluation of an RNA-based saliva identification method. Sci. Justice. 2017;57:404–408. doi: 10.1016/j.scijus.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 87.Liu, B. et al. Development of a multiplex system for the identification of forensically relevant body fluids. Forensic Sci Int Genet47, (2020). [DOI] [PubMed]
- 88.Hanson E, Ingold S, Haas C, Ballantyne J. Messenger RNA biomarker signatures for forensic body fluid identification revealed by targeted RNA sequencing. Forensic Sci. Int Genet. 2018;34:206–221. doi: 10.1016/j.fsigen.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 89.Ypma RJF, et al. Calculating LRs for presence of body fluids from mRNA assay data in mixtures. Forensic Sci. Int Genet. 2021;52:102455. doi: 10.1016/j.fsigen.2020.102455. [DOI] [PubMed] [Google Scholar]
- 90.Blackman S, et al. Developmental validation of the ParaDNA® Body Fluid ID System—A rapid multiplex mRNA-profiling system for the forensic identification of body fluids. Forensic Sci. Int Genet. 2018;37:151–161. doi: 10.1016/j.fsigen.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 91.Kulstein G, Pably P, Fürst A, Wiegand P, Hadrys T. “The acid test”—validation of the ParaDNA® Body Fluid ID Test for routine forensic casework. Int J. Leg. Med. 2019;133:751–757. doi: 10.1007/s00414-018-1971-9. [DOI] [PubMed] [Google Scholar]
- 92.Hu S, et al. Preclinical validation of salivary biomarkers for primary Sjögren’s syndrome. Arthritis Care Res (Hoboken) 2010;62:1633–1638. doi: 10.1002/acr.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Luo D, Chen Y, Zhou N, Li T, Wang H. Blockade of Th17 response by IL-38 in primary Sjögren’s syndrome. Mol. Immunol. 2020;127:107–111. doi: 10.1016/j.molimm.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 94.Aqrawi, L. A., Jensen, J. L., Fromreide, S., Galtung, H. K. & Skarstein, K. Expression of NGAL-specific cells and mRNA levels correlate with inflammation in the salivary gland, and its overexpression in the saliva, of patients with primary Sjögren’s syndrome. Autoimmunity 333–343 10.1080/08916934.2020.1795140 (2020). [DOI] [PubMed]
- 95.Wang X, et al. Progenitor cell niche senescence reflects pathology of the parotid salivary gland in primary Sjögren’s syndrome. Rheumatol. (U. Kingd.) 2020;59:3003–3013. doi: 10.1093/rheumatology/keaa012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Swaminathan V, Prakasam S, Puri V, Srinivasan M. Role of salivary epithelial toll-like receptors 2 and 4 in modulating innate immune responses in chronic periodontitis. J. Periodontal Res. 2013;48:757–765. doi: 10.1111/jre.12066. [DOI] [PubMed] [Google Scholar]
- 97.AlQallaf H, et al. Differential profiles of soluble and cellular toll like receptor (TLR)-2 and 4 in chronic periodontitis. PLoS One. 2018;13:e0200231. doi: 10.1371/journal.pone.0200231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kluknavská J, et al. Expression of selected inflammatory proteins and metalloproteinases in periodontitis. Eur. Rev. Med Pharm. Sci. 2022;26:1825–1831. doi: 10.26355/eurrev_202203_28326. [DOI] [PubMed] [Google Scholar]
- 99.Detzen L, Chen SCY, Cheng B, Papapanou PN, Lalla E. Increased levels of soluble CD163 in periodontitis patients. J. Clin. Periodontol. 2017;44:585–590. doi: 10.1111/jcpe.12731. [DOI] [PubMed] [Google Scholar]
- 100.Fagin U, Nerbas L, Vogl B, Jabs WJ. Analysis of BZLF1 mRNA detection in saliva as a marker for active replication of Epstein-Barr virus. J. Virol. Methods. 2017;244:11–16. doi: 10.1016/j.jviromet.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 101.Angelova S, Salim A, Kiselova-Kaneva Y, Ivanova D, Peev S. Association of mRNA Levels of IL6, MMP-8, GSS in saliva and pyelonephritis in children. Molecules. 2020;25:85. doi: 10.3390/molecules25010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fan X, Luo Y, Fan Q, Zheng W. Reduced expression of PARK2 in manganese-exposed smelting workers. Neurotoxicology. 2017;62:258–264. doi: 10.1016/j.neuro.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen X, Liang H, Zhang J, Zen K, Zhang CY. Horizontal transfer of microRNAs: molecular mechanisms and clinical applications. Protein Cell. 2012;3:28–37. doi: 10.1007/s13238-012-2003-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hicks SD, Ignacio C, Gentile K, Middleton FA. Salivary miRNA profiles identify children with autism spectrum disorder, correlate with adaptive behavior, and implicate ASD candidate genes involved in neurodevelopment. BMC Pediatr. 2016;16:52. doi: 10.1186/s12887-016-0586-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hicks SD, et al. Saliva MicroRNA differentiates children with autism from peers with typical and atypical development. J. Am. Acad. Child Adolesc. Psychiatry. 2020;59:296–308. doi: 10.1016/j.jaac.2019.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sehovic E, et al. Identification of developmental disorders including autism spectrum disorder using salivary miRNAs in children from Bosnia and Herzegovina. PLoS One. 2020;15:e0232351. doi: 10.1371/journal.pone.0232351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rosato AJ, et al. Salivary microRNAs identified by small RNA sequencing and machine learning as potential biomarkers of alcohol dependence. Epigenomics. 2019;11:739–749. doi: 10.2217/epi-2018-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Di Pietro V, et al. Unique diagnostic signatures of concussion in the saliva of male athletes: the Study of Concussion in Rugby Union through MicroRNAs (SCRUM) Br. J. Sports Med. 2021;55:1395–1404. doi: 10.1136/bjsports-2020-103274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sembler-Møller ML, Belstrøm D, Locht H, Pedersen AML. Distinct microRNA expression profiles in saliva and salivary gland tissue differentiate patients with primary Sjögren’s syndrome from non-Sjögren’s sicca patients. J. Oral. Pathol. Med. 2020;49:1044–1052. doi: 10.1111/jop.13099. [DOI] [PubMed] [Google Scholar]
- 110.Min N, et al. Circulating salivary miRNA hsa-miR-221 as clinically validated diagnostic marker for hand, foot, and mouth disease in pediatric patients. EBioMedicine. 2018;31:299–306. doi: 10.1016/j.ebiom.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Han P, Bartold PM, Salomon C, Ivanovski S. Salivary small extracellular vesicles associated miRNAs in periodontal status—A pilot study. Int J. Mol. Sci. 2020;21:2809. doi: 10.3390/ijms21082809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mead EA, et al. Non-Invasive microRNA profiling in saliva can serve as a biomarker of alcohol exposure and its effects in humans. Front Genet. 2021;12:804222. doi: 10.3389/fgene.2021.804222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang Z, et al. Characterization of microRNA expression profiles in blood and saliva using the Ion Personal Genome Machine(®) System (Ion PGMTM System) Forensic Sci. Int Genet. 2016;20:140–146. doi: 10.1016/j.fsigen.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 114.Layne TR, et al. microRNA detection in blood, urine, semen, and saliva stains after compromising treatments. J. Forensic Sci. 2019;64:1831–1837. doi: 10.1111/1556-4029.14113. [DOI] [PubMed] [Google Scholar]
- 115.Barrett SP, Salzman J. Circular RNAs: analysis, expression and potential functions. Development. 2016;143:1838–1847. doi: 10.1242/dev.128074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ren X, Du Y, You L, Zhao Y. Potential functions and implications of circular RNA in gastrointestinal cancer. Oncol. Lett. 2017;14:7016–7020. doi: 10.3892/ol.2017.7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee ECS, et al. The roles of circular RNAs in human development and diseases. Biomed. Pharmacother. 2019;111:198–208. doi: 10.1016/j.biopha.2018.12.052. [DOI] [PubMed] [Google Scholar]
- 118.Bahn JH, et al. The landscape of MicroRNA, piwi-interacting RNA, and circular RNA in human saliva. Clin. Chem. 2015;61:221–230. doi: 10.1373/clinchem.2014.230433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Saaoud F, et al. Circular RNAs are a novel type of non-coding RNAs in ROS regulation, cardiovascular metabolic inflammations and cancers. Pharm. Ther. 2021;220:107715. doi: 10.1016/j.pharmthera.2020.107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kristensen LS, et al. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019;20:675–691. doi: 10.1038/s41576-019-0158-7. [DOI] [PubMed] [Google Scholar]
- 121.Wang W, et al. Circular RNAs as potential biomarkers and therapeutics for cardiovascular disease. PeerJ. 2019;7:e6831. doi: 10.7717/peerj.6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhao S-Y, Wang J, Ouyang S-B, Huang Z-K, Liao L. Salivary circular RNAs Hsa_Circ_0001874 and Hsa_Circ_0001971 as novel biomarkers for the diagnosis of oral squamous cell carcinoma. Cell Physiol. Biochem. 2018;47:2511–2521. doi: 10.1159/000491624. [DOI] [PubMed] [Google Scholar]
- 123.Wang M, et al. Diagnostic value of CircRNAs as Potential Biomarkers in Oral Squamous Cell Carcinoma: a Meta-Analysis. Front Oncol. 2021;11:693284. doi: 10.3389/fonc.2021.693284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kristensen LS, Jakobsen T, Hager H, Kjems J. The emerging roles of circRNAs in cancer and oncology. Nat. Rev. Clin. Oncol. 2022;19:188–206. doi: 10.1038/s41571-021-00585-y. [DOI] [PubMed] [Google Scholar]
- 125.Song F, Luo H, Xie M, Zhu H, Hou Y. Microarray expression profile of circular RNAs in human body fluids. Forensic Sci. Int Genet Suppl. Ser. 2017;6:e55–e56. doi: 10.1016/j.fsigss.2017.09.005. [DOI] [Google Scholar]
- 126.Liu B, et al. Characterization of tissue-specific biomarkers with the expression of circRNAs in forensically relevant body fluids. Int J. Leg. Med. 2019;133:1321–1331. doi: 10.1007/s00414-019-02027-y. [DOI] [PubMed] [Google Scholar]
- 127.Liu B, et al. Development of a multiplex system for the identification of forensically relevant body fluids. Forensic Sci. Int Genet. 2020;47:102312. doi: 10.1016/j.fsigen.2020.102312. [DOI] [PubMed] [Google Scholar]
- 128.Tang H, Wu Z, Zhang J, Su B. Salivary lncRNA as a potential marker for Oral squamous cell carcinoma diagnosis. Mol. Med Rep. 2013;7:761–766. doi: 10.3892/mmr.2012.1254. [DOI] [PubMed] [Google Scholar]
- 129.Xie Z, et al. Salivary HOTAIR and PVT1 as novel biomarkers for early pancreatic cancer. Oncotarget. 2016;7:25408–25419. doi: 10.18632/oncotarget.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xie Z, et al. Lnc-PCDH9-13:1 Is a Hypersensitive and Specific Biomarker for Early Hepatocellular Carcinoma. EBioMedicine. 2018;33:57–67. doi: 10.1016/j.ebiom.2018.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shieh TM, et al. Lack of salivary long non‐coding rna xist expression is associated with increased risk of oral squamous cell carcinoma: A cross‐sectional study. J. Clin. Med. 2021;10:4622. doi: 10.3390/jcm10194622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chandra Gupta S, Nandan Tripathi Y. Potential of long non-coding RNAs in cancer patients: From biomarkers to therapeutic targets. Int. J. Cancer. 2017;140:1955–1967. doi: 10.1002/ijc.30546. [DOI] [PubMed] [Google Scholar]
- 133.Rodrigues AC, et al. NEAT1 and MALAT1 are highly expressed in saliva and nasopharyngeal swab samples of COVID-19 patients. Mol. Oral. Microbiol. 2021;36:291–294. doi: 10.1111/omi.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Amado FML, Ferreira RP, Vitorino R. One decade of salivary proteomics: Current approaches and outstanding challenges. Clin. Biochem. 2013;46:506–517. doi: 10.1016/j.clinbiochem.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 135.Yan W, et al. Systematic comparison of the human saliva and plasma proteomes. Proteom. Clin. Appl. 2009;3:116–134. doi: 10.1002/prca.200800140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chu HW, et al. Identification of salivary biomarkers for oral cancer detection with untargeted and targeted quantitative proteomics approaches. Mol. Cell Proteom. 2019;18:1796–1806. doi: 10.1074/mcp.RA119.001530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Figura M, et al. Proteomic Profile of Saliva in Parkinson’s Disease Patients: A Proof of Concept Study. Brain Sci. 2021;11:661. doi: 10.3390/brainsci11050661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Figura MA-O, Friedman AA-O. & Neurol Neurochir, P. In search of Parkinson’s disease biomarkers - is the answer in our mouths? A systematic review of the literature on salivary biomarkers of Parkinson’s disease. Neurol. Neurochir. Pol. 2020;54:14–20. doi: 10.5603/PJNNS.a2020.0011. [DOI] [PubMed] [Google Scholar]
- 139.Loo JA, Yan W, Ramachandran P, Wong DT. Comparative human salivary and plasma proteomes. J. Dent. Res. 2010;89:1016–1023. doi: 10.1177/0022034510380414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Peyrot Des Gachons C, Breslin PA. Salivary amylase: digestion and metabolic syndrome. Curr. Diab Rep. 2016;16:102. doi: 10.1007/s11892-016-0794-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pigman W, Reid AJ. The organic compounds and enzymes of human saliva. J. Am. Dent. Assoc. 1952;45:326–338. doi: 10.14219/jada.archive.1952.0197. [DOI] [PubMed] [Google Scholar]
- 142.Raus FJ, Tarbet WJ, Miklos FL. Salivary enzymes and calculus formation. J. Periodontal Res. 1968;3:232–235. doi: 10.1111/j.1600-0765.1968.tb01925.x. [DOI] [PubMed] [Google Scholar]
- 143.Humphrey SP, Williamson RT. A review of saliva: normal composition, flow, and function. J. Prosthet. Dent. 2001;85:162–169. doi: 10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]
- 144.Tripoliti EE, et al. Point-of-care testing devices for heart failure analyzing blood and saliva samples. IEEE Rev. Biomed. Eng. 2020;13:17–31. doi: 10.1109/RBME.2019.2905730. [DOI] [PubMed] [Google Scholar]
- 145.Bermejo-Pareja F, Antequera D, Vargas T, Molina JA, Carro E. Saliva levels of Abeta1-42 as potential biomarker of Alzheimer’s disease: a pilot study. BMC Neurol. 2010;10:108. doi: 10.1186/1471-2377-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kaczor-Urbanowicz, K. E. et al. Identification of salivary protein biomarkers for orthodontically induced inflammatory root resorption. Proteomics Clin Appl11, (2017). [DOI] [PubMed]
- 147.Li F, et al. Characterization of human salivary extracellular RNA by next-generation sequencing. Clin. Chem. 2018;64:1085–1095. doi: 10.1373/clinchem.2017.285072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kumar S, et al. Biofunctionalized nanostructured zirconia for biomedical application: a smart approach for oral cancer detection. Adv. Sci. (Weinh.) 2015;2:1500048. doi: 10.1002/advs.201500048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang J, Zhao Y, Ren JJ, Xu Y. Pepsin in saliva as a diagnostic biomarker in laryngopharyngeal reflux: a meta-analysis. Eur. Arch. Oto-Rhino-Laryngol. 2018;275:671–678. doi: 10.1007/s00405-017-4845-8. [DOI] [PubMed] [Google Scholar]
- 150.Wang CP, et al. Saliva pepsin detection and proton pump inhibitor response in suspected laryngopharyngeal reflux. Laryngoscope. 2019;129:709–714. doi: 10.1002/lary.27502. [DOI] [PubMed] [Google Scholar]
- 151.Hayat JO, et al. Pepsin in saliva for the diagnosis of gastro-oesophageal reflux disease. Gut. 2015;64:373–380. doi: 10.1136/gutjnl-2014-307049. [DOI] [PubMed] [Google Scholar]
- 152.Wang YJ, et al. Salivary pepsin as an intrinsic marker for diagnosis of sub-types of gastroesophageal reflux disease and gastroesophageal reflux disease-related disorders. J. Neurogastroenterol. Motil. 2020;26:74–84. doi: 10.5056/jnm19032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dy F, Amirault J, Mitchell PD, Rosen R. Salivary pepsin lacks sensitivity as a diagnostic tool to evaluate extraesophageal reflux disease. J. Pediatr. 2016;177:53–58. doi: 10.1016/j.jpeds.2016.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Guo ZH, Wu H, Jiang JL, Zhang C. Pepsin in saliva as a diagnostic marker for gastroesophageal reflux disease: a meta-analysis. Med. Sci. Monit. 2018;24:9509–9516. doi: 10.12659/MSM.913978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Selvaraju V, Venkatapoorna CMK, Babu JR, Geetha T. Salivary amylase gene copy number is associated with the obesity and inflammatory markers in children. Diabetes Metab. Syndr. Obes. 2020;13:1695–1701. doi: 10.2147/DMSO.S251359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Awasthi N. Role of salivary biomarkers in early detection of oral squamous cell carcinoma. Indian J. Pathol. Microbiol. 2017;60:464–468. doi: 10.4103/IJPM.IJPM_140_16. [DOI] [PubMed] [Google Scholar]
- 157.Sommerland H, Ullman M, Jennische E, Skottner A, Oldfors A. Muscle regeneration. The effect of hypophysectomy on cell proliferation and expression of insulin-like growth factor-I. Acta Neuropathol. 1989;78:264–269. doi: 10.1007/BF00687756. [DOI] [PubMed] [Google Scholar]
- 158.Shah VS, Pareikh D, Manjunatha BS. Salivary alpha-amylase-biomarker for monitoring type II diabetes. J. Oral. Maxillofac. Pathol. 2021;25:441–445. doi: 10.4103/jomfp.jomfp_84_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tiongco REG, Arceo ES, Rivera NS, Flake CCD, Policarpio AR. Estimation of salivary glucose, amylase, calcium, and phosphorus among non-diabetics and diabetics: Potential identification of non-invasive diagnostic markers. Diabetes Metab. Syndrome: Clin. Res. Rev. 2019;13:2601–2605. doi: 10.1016/j.dsx.2019.07.037. [DOI] [PubMed] [Google Scholar]
- 160.Kheirmand Parizi M, Akbari H, Malek-Mohamadi M, Kheirmand Parizi M, Kakoei S. Association of salivary levels of immunoglobulin-a and amylase with oral-dental manifestations in patients with controlled and non-controlled type 2 diabetes. BMC Oral. Health. 2019;19:175. doi: 10.1186/s12903-019-0868-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dowd FJ. Saliva and dental caries. Dent. Clin. North Am. 1999;43:579–597. doi: 10.1016/S0011-8532(22)00815-1. [DOI] [PubMed] [Google Scholar]
- 162.Perera S, Uddin M, Hayes JA. Salivary lysozyme: a noninvasive marker for the study of the effects of stress of natural immunity. Int J. Behav. Med. 1997;4:170–178. doi: 10.1207/s15327558ijbm0402_5. [DOI] [PubMed] [Google Scholar]
- 163.Yang Y, et al. Self perceived work related stress and the relation with salivary IgA and lysozyme among emergency department nurses. Occup. Environ. Med. 2002;59:836–841. doi: 10.1136/oem.59.12.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chojnowska S, Ptaszynska-Sarosiek I, Kepka A, Knas M, Waszkiewicz N. Salivary Biomarkers of Stress, Anxiety and Depression. J. Clin. Med. 2021;10:517. doi: 10.3390/jcm10030517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Deng K, Pelekos G, Jin L, Tonetti MS. Diagnostic accuracy of a point-of-care aMMP-8 test in the discrimination of periodontal health and disease. J. Clin. Periodontol. 2021;48:1051–1065. doi: 10.1111/jcpe.13485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lähteenmäki, H. et al. Ammp-8 point-of-care/chairside oral fluid technology as a rapid, non-invasive tool for periodontitis and peri-implantitis screening in a medical care setting. Diagnostics10, (2020). [DOI] [PMC free article] [PubMed]
- 167.Podzimek S, Vondrackova L, Duskova J, Janatova T, Broukal Z. Salivary markers for periodontal and general diseases. Dis. Markers. 2016;2016:9179632. doi: 10.1155/2016/9179632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chambers DA, et al. A longitudinal study of aspartate aminotransferase in human gingival crevicular fluid. J. Periodontal Res. 1991;26:65–74. doi: 10.1111/j.1600-0765.1991.tb01628.x. [DOI] [PubMed] [Google Scholar]
- 169.Ranadheer E, et al. The relationship between salivary IgA levels and dental caries in children. J. Indian Soc. Pedod. Prev. Dent. 2011;29:106–112. doi: 10.4103/0970-4388.84681. [DOI] [PubMed] [Google Scholar]
- 170.Mousavizadeh A et al. The relationship between salivary and serum IgA and IgG levels and dental caries in adults. Clin Lab67, 10.7754/Clin.Lab.2020.201209 (2021). [DOI] [PubMed]
- 171.Haeri-Araghi H, Zarabadipour M, Safarzadeh-Khosroshahi S, Mirzadeh M. Evaluating the relationship between dental caries number and salivary level of IgA in adults. J. Clin. Exp. Dent. 2018;10:e66–e69. doi: 10.4317/jced.54271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tsukinoki K, et al. Detection of cross-reactive immunoglobulin A against the severe acute respiratory syndrome-coronavirus-2 spike 1 subunit in saliva. PLoS One. 2021;16:10. doi: 10.1371/journal.pone.0249979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Calheira MC, et al. Immunoassay standardization for the detection of immunoglobulin A (IgA) against Porphyromonas gingivalis antigens in saliva of individuals with and without leprosy. AMB Express. 2021;11:7. doi: 10.1186/s13568-021-01312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Leicht CA, Goosey-Tolfrey VL, Bishop NC. Exercise intensity and its impact on relationships between salivary immunoglobulin A, saliva flow rate and plasma cortisol concentration. Eur. J. Appl Physiol. 2018;118:1179–1187. doi: 10.1007/s00421-018-3847-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Riis JL, et al. Correspondence between cytomegalovirus immunoglobulin-G levels measured in saliva and serum. Front Immunol. 2020;11:11. doi: 10.3389/fimmu.2020.02095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kaplan B, et al. Immunoglobulin free light chains in saliva: a potential marker for disease activity in multiple sclerosis. Clin. Exp. Immunol. 2018;192:7–17. doi: 10.1111/cei.13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gao Z, et al. Abnormal sialylation and fucosylation of saliva glycoproteins: Characteristics of lung cancer-specific biomarkers. Curr. Res. Pharmacol. Drug Discov. 2022;3:100079. doi: 10.1016/j.crphar.2021.100079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ragusa A, et al. Differential glycosylation levels in saliva from patients with lung or breast cancer: a preliminary assessment for early diagnostic purposes. Metabolites. 2021;11:566. doi: 10.3390/metabo11090566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yang J, et al. Abnormal Galactosylated-Glycans recognized by Bandeiraea Simplicifolia Lectin I in saliva of patients with breast Cancer. Glycoconj. J. 2020;37:373–394. doi: 10.1007/s10719-020-09910-6. [DOI] [PubMed] [Google Scholar]
- 180.Yang J, et al. Alternations of N-glycans recognized by Phaseolus vulgaris leucoagglutinin in the saliva of patients with breast cancer. Neoplasma. 2021;68:994–1004. doi: 10.4149/neo_2021_210301N264. [DOI] [PubMed] [Google Scholar]
- 181.Shu J, et al. Identification of N- and O-linked glycans recognized by AAL in saliva of patients with atrophic gastritis and gastric cancer. Cancer Biomark. 2018;22:669–681. doi: 10.3233/CBM-171087. [DOI] [PubMed] [Google Scholar]
- 182.Zanotti L, et al. Epidermal growth factor receptor detection in serum and saliva as a diagnostic and prognostic tool in oral cancer. Laryngoscope. 2017;127:E408–e414. doi: 10.1002/lary.26797. [DOI] [PubMed] [Google Scholar]
- 183.Das N, et al. Proteomics analysis of tears and saliva from Sjogren’s syndrome patients. Front Pharm. 2021;12:787193. doi: 10.3389/fphar.2021.787193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Grant MM, et al. Discovery, validation, and diagnostic ability of multiple protein-based biomarkers in saliva and gingival crevicular fluid to distinguish between health and periodontal diseases. J. Clin. Periodontol. 2022 doi: 10.1111/jcpe.13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gleerup HS, Hasselbalch SG, Simonsen AH. Biomarkers for Alzheimer’s disease in saliva: a systematic review. Dis. Markers. 2019;2019:4761054. doi: 10.1155/2019/4761054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gleerup HS, Jensen CS, Høgh P, Hasselbalch SG, Simonsen AH. Lactoferrin in cerebrospinal fluid and saliva is not a diagnostic biomarker for Alzheimer’s disease in a mixed memory clinic population. EBioMedicine. 2021;67:103361. doi: 10.1016/j.ebiom.2021.103361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jacobs R, et al. Host biomarkers detected in saliva show promise as markers for the diagnosis of pulmonary tuberculosis disease and monitoring of the response to tuberculosis treatment. Cytokine. 2016;81:50–56. doi: 10.1016/j.cyto.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 188.Tsai CM, et al. Use of saliva sample to detect C-reactive protein in children with pneumonia. Pediatr. Pulmonol. 2020;55:2457–2462. doi: 10.1002/ppul.24947. [DOI] [PubMed] [Google Scholar]
- 189.Lin GC, et al. Directed Transport of CRP Across In Vitro Models of the Blood-Saliva Barrier Strengthens the Feasibility of Salivary CRP as Biomarker for Neonatal Sepsis. Pharmaceutics. 2021;13:256. doi: 10.3390/pharmaceutics13020256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Galhardo LF, et al. Inflammatory markers in saliva for diagnosis of sepsis of hospitalizes patients. Eur. J. Clin. Invest. 2020;50:e13219. doi: 10.1111/eci.13219. [DOI] [PubMed] [Google Scholar]
- 191.Monib KME, El-Fallah AA, Salem RM. Inflammatory markers in acne vulgaris: Saliva as a novel diagnostic fluid. J. Cosmet. Dermatol. 2022;21:1280–1285. doi: 10.1111/jocd.14236. [DOI] [PubMed] [Google Scholar]
- 192.Corey-Bloom J, et al. Levels of Interleukin-6 in saliva, but not plasma, correlate with clinical metrics in Huntington’s disease patients and healthy control subjects. Int J. Mol. Sci. 2020;21:6363. doi: 10.3390/ijms21176363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lee SH, et al. A photothermal biosensor for detection of C-reactive protein in human saliva. Sens Actuators B Chem. 2017;246:471–476. doi: 10.1016/j.snb.2017.01.188. [DOI] [Google Scholar]
- 194.Leonardi AA, et al. Silicon nanowire luminescent sensor for cardiovascular risk in saliva. J. Mater. Sci.: Mater. Electron. 2020;31:10–17. [Google Scholar]
- 195.Hu W, et al. C-reaction protein detection in human saliva by nanoplasmonic color imaging. J. Biomed. Nanotechnol. 2019;15:1724–1733. doi: 10.1166/jbn.2019.2769. [DOI] [PubMed] [Google Scholar]
- 196.Macchia E, et al. Selective single-molecule analytical detection of C-reactive protein in saliva with an organic transistor. Anal. Bioanal. Chem. 2019;411:4899–4908. doi: 10.1007/s00216-019-01778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chen X, et al. Electrochemical methods for detection of biomarkers of Chronic Obstructive Pulmonary Disease in serum and saliva. Biosens. Bioelectron. 2019;142:111453. doi: 10.1016/j.bios.2019.111453. [DOI] [PubMed] [Google Scholar]
- 198.Aydin S, et al. Nesfatin-1 and ghrelin levels in serum and saliva of epileptic patients: hormonal changes can have a major effect on seizure disorders. Mol. Cell Biochem. 2009;328:49–56. doi: 10.1007/s11010-009-0073-x. [DOI] [PubMed] [Google Scholar]
- 199.Bauer FE, Ghatei MA, Zintel A, Bloom SR. Galanin: hydrokinetic action on salivary glands in man. Aliment Pharm. Ther. 1989;3:591–596. doi: 10.1111/j.1365-2036.1989.tb00252.x. [DOI] [PubMed] [Google Scholar]
- 200.Calvi JL, et al. Measurement of cortisol in saliva: a comparison of measurement error within and between international academic-research laboratories. BMC Res Notes. 2017;10:1–6. doi: 10.1186/s13104-017-2805-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Dawidson I, Blom M, Lundeberg T, Theodorsson E, Angmar-Månsson B. Neuropeptides in the saliva of healthy subjects. Life Sci. 1996;60:269–278. doi: 10.1016/S0024-3205(96)00627-3. [DOI] [PubMed] [Google Scholar]
- 202.Gann PH, Giovanazzi S. Van Horn, L., Branning, A. & Chatterton, J. Saliva as a medium for investigating intra- and interindividual differences in sex hormone levels in premenopausal women. Cancer Epidemiol. Biomark. Prev. 2001;10:59–64. [PubMed] [Google Scholar]
- 203.Gröschl M, et al. Identification of leptin in human saliva. J. Clin. Endocrinol. Metab. 2001;86:5234–5239. doi: 10.1210/jcem.86.11.7998. [DOI] [PubMed] [Google Scholar]
- 204.Gröschl M, et al. Identification of ghrelin in human saliva: production by the salivary glands and potential role in proliferation of oral keratinocytes. Clin. Chem. 2005;51:997–1006. doi: 10.1373/clinchem.2004.040667. [DOI] [PubMed] [Google Scholar]
- 205.Hurtado MD, et al. Salivary peptide tyrosine-tyrosine 3-36 modulates ingestive behavior without inducing taste aversion. J. Neurosci. 2013;33:18368–18380. doi: 10.1523/JNEUROSCI.1064-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Landman AD, Sanford LM, Howland BE, Dawes C, Pritchard ET. Testosterone in human saliva. Experientia. 1976;32:940–941. doi: 10.1007/BF02003781. [DOI] [PubMed] [Google Scholar]
- 207.Ozbay Y, et al. Obestatin is present in saliva: Alterations in obestatin and ghrelin levels of saliva and serum in ischemic heart disease. J. Biochem Mol. Biol. 2008;41:55–61. doi: 10.5483/bmbrep.2008.41.1.055. [DOI] [PubMed] [Google Scholar]
- 208.VAKKURI O. Diurnal rhythm of melatonin in human saliva. Acta Physiol. Scand. 1985;124:409–412. doi: 10.1111/j.1748-1716.1985.tb07676.x. [DOI] [PubMed] [Google Scholar]
- 209.Wang C, Wakelin K, White J, Wood PJ. Salivary androgens in hirsutism: Are they of use in routine evaluation? Ann. Clin. Biochem. 1986;23:590–595. doi: 10.1177/000456328602300517. [DOI] [PubMed] [Google Scholar]
- 210.White-Traut R, et al. Detection of salivary oxytocin levels in lactating women. Dev. Psychobiol. 2009;51:367–373. doi: 10.1002/dev.20376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Williams JS, Williams GH. 50Th Anniversary of Aldosterone. J. Clin. Endocrinol. Metab. 2003;88:2364–2372. doi: 10.1210/jc.2003-030490. [DOI] [PubMed] [Google Scholar]
- 212.Zolotukhin S. Metabolic hormones in saliva: origins and functions. Oral. Dis. 2013;19:219–229. doi: 10.1111/odi.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Gozansky WS, Lynn JS, Laudenslager ML, Kohrt WM. Salivary cortisol determined by enzyme immunoassay is preferable to serum total cortisol for assessment of dynamic hypothalamic–pituitary–adrenal axis activity. Clin. Endocrinol. (Oxf.) 2005;63:336–341. doi: 10.1111/j.1365-2265.2005.02349.x. [DOI] [PubMed] [Google Scholar]
- 214.Rosenfeld D, et al. Transgene-free remote magnetothermal regulation of adrenal hormones. Sci. Adv. 2020;6:eaaz3734. doi: 10.1126/sciadv.aaz3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Noushad S, et al. Physiological biomarkers of chronic stress: a systematic review. Int J. Health Sci. 2021;15:45–59. [PMC free article] [PubMed] [Google Scholar]
- 216.Illescas-Montes R, et al. Application of salivary biomarkers in the diagnosis of fibromyalgia. Diagnostics (Basel) 2021;11:63. doi: 10.3390/diagnostics11010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bastin P, Maiter D, Gruson D. [Salivary cortisol testing: preanalytic and analytic aspects] Ann. Biol. Clin. (Paris) 2018;76:393–405. doi: 10.1684/abc.2018.1355. [DOI] [PubMed] [Google Scholar]
- 218.Backlund N, et al. Reference intervals of salivary cortisol and cortisone and their diagnostic accuracy in Cushing’s syndrome. Eur. J. Endocrinol. 2020;182:569–582. doi: 10.1530/EJE-19-0872. [DOI] [PubMed] [Google Scholar]
- 219.Vrbanovic E, Lapic I, Rogic D, Alajbeg IZ. Changes in salivary oxidative status, salivary cortisol, and clinical symptoms in female patients with temporomandibular disorders during occlusal splint therapy: a 3-month follow up. BMC Oral. Health. 2019;19:100. doi: 10.1186/s12903-019-0791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Neupane SP, Virtej A, Myhren LE, Bull VH. Biomarkers common for inflammatory periodontal disease and depression: a systematic review. Brain Behav. Immun. Health. 2022;21:100450. doi: 10.1016/j.bbih.2022.100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Caicedo Mera JC, Cardenas Molano MA, Garcia Lopez CC, Acevedo Triana C, Martinez Cotrina J. Discussions and perspectives regarding oxytocin as a biomarker in human investigations. Heliyon. 2021;7:e08289. doi: 10.1016/j.heliyon.2021.e08289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lussier D, Cruz-Almeida Y, Ebner NC. Musculoskeletal pain and brain morphology: oxytocin’s potential as a treatment for chronic pain in aging. Front Aging Neurosci. 2019;11:338. doi: 10.3389/fnagi.2019.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Walum H, Young LJ. The neural mechanisms and circuitry of the pair bond. Nat. Rev. Neurosci. 2018;19:643–654. doi: 10.1038/s41583-018-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Carter CS, et al. Is oxytocin ‘nature’s medicine’? Pharm. Rev. 2020;72:829–861. doi: 10.1124/pr.120.019398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Thomas SJ, Larkin T. Cognitive distortions in relation to plasma cortisol and oxytocin levels in major depressive disorder. Front Psychiatry. 2019;10:971. doi: 10.3389/fpsyt.2019.00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ziegler TE. Measuring peripheral oxytocin and vasopressin in nonhuman primates. Am. J. Primatol. 2018;80:e22871. doi: 10.1002/ajp.22871. [DOI] [PubMed] [Google Scholar]
- 227.Nous A, Engelborghs S. & Smolders, I. Melatonin levels in the Alzheimer’s disease continuum: a systematic review. Alzheimers Res Ther. 2021;13:52. doi: 10.1186/s13195-021-00788-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kennaway DJ. A critical review of melatonin assays: Past and present. J. Pineal Res. 2019;67:e12572. doi: 10.1111/jpi.12572. [DOI] [PubMed] [Google Scholar]
- 229.Sundberg I, et al. Daytime melatonin levels in saliva are associated with inflammatory markers and anxiety disorders. Psychoneuroendocrinology. 2020;112:104514. doi: 10.1016/j.psyneuen.2019.104514. [DOI] [PubMed] [Google Scholar]
- 230.Touitou Y, Auzeby A, Camus F, Djeridane Y. Daily profiles of salivary and urinary melatonin and steroids in healthy prepubertal boys. J. Pediatr. Endocrinol. Metab. 2009;22:1009–1015. doi: 10.1515/JPEM.2009.22.11.1009. [DOI] [PubMed] [Google Scholar]
- 231.Nijakowski K, Surdacki M, Sobieszczanska M. Salivary melatonin changes in oncological patients: a systematic review. Metabolites. 2022;12:439. doi: 10.3390/metabo12050439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Chang WP, Lin CC. Relationships of salivary cortisol and melatonin rhythms to sleep quality, emotion, and fatigue levels in patients with newly diagnosed lung cancer. Eur. J. Oncol. Nurs. 2017;29:79–84. doi: 10.1016/j.ejon.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 233.Farahani H, Alaee M, Amri J, Baghinia MR, Rafiee M. Serum and saliva concentrations of biochemical parameters in men with prostate cancer and benign prostate hyperplasia. Lab Med. 2020;51:243–251. doi: 10.1093/labmed/lmz053. [DOI] [PubMed] [Google Scholar]
- 234.Mitova N, Rashkova M. Sex hormones in the saliva and periodontal health of children in puberty. J. Imab. 2019;25:2817–2821. doi: 10.5272/jimab.2019254.2817. [DOI] [Google Scholar]
- 235.Liu J, et al. Quantification of 10 steroid hormones in human saliva from Chinese adult volunteers. J. Int. Med. Res. 2018;46:1414–1427. doi: 10.1177/0300060517752733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kaczor-Urbanowicz KE, et al. Saliva diagnostics – Current views and directions. Exp. Biol. Med. 2016;242:459–472. doi: 10.1177/1535370216681550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ceccato F, Boscaro M. Cushing’s syndrome: screening and diagnosis. High. Blood Press Cardiovasc Prev. 2016;23:209–215. doi: 10.1007/s40292-016-0153-4. [DOI] [PubMed] [Google Scholar]
- 238.Keevil BG, et al. Salivary testosterone measurement by liquid chromatography tandem mass spectrometry in adult males and females. Ann. Clin. Biochem. 2014;51:368–378. doi: 10.1177/0004563213506412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ugur K, Aydin S. Saliva and blood asprosin hormone concentration associated with obesity. Int J. Endocrinol. 2019;2019:8. doi: 10.1155/2019/2521096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Arevalo B, et al. Simultaneous determination of four fertility-related hormones in saliva using disposable multiplexed immunoplatforms coupled to a custom-designed and field-portable potentiostat. Anal. Methods. 2021;13:3471–3478. doi: 10.1039/D1AY01074C. [DOI] [PubMed] [Google Scholar]
- 241.Xu B, et al. Simultaneous quantitative analysis of seven steroid hormones in human saliva: a novel method based on O-ethylhydroxylamine hydrochloride as derivatization reagent. Rapid Commun. Mass Spectrom. 2022;36:10. doi: 10.1002/rcm.9242. [DOI] [PubMed] [Google Scholar]
- 242.Dame ZT, et al. The human saliva metabolome. Metabolomics. 2015;11:1864–1883. doi: 10.1007/s11306-015-0840-5. [DOI] [Google Scholar]
- 243.Zhou J, Hu H, Huang R. A pilot study of the metabolomic profiles of saliva from female orthodontic patients with external apical root resorption. Clin. Chim. Acta. 2018;478:188–193. doi: 10.1016/j.cca.2017.12.046. [DOI] [PubMed] [Google Scholar]
- 244.Rzeznik M, et al. Identification of a discriminative metabolomic fingerprint of potential clinical relevance in saliva of patients with periodontitis using 1H nuclear magnetic resonance (NMR) spectroscopy. PLoS One. 2017;12:e0182767. doi: 10.1371/journal.pone.0182767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ohshima M, Sugahara K, Kasahara K, Katakura A. Metabolomic analysis of the saliva of Japanese patients with oral squamous cell carcinoma. Oncol. Rep. 2017;37:2727–2734. doi: 10.3892/or.2017.5561. [DOI] [PubMed] [Google Scholar]
- 246.Ladva CN, et al. Metabolomic profiles of plasma, exhaled breath condensate, and saliva are correlated with potential for air toxics detection. J. Breath. Res. 2018;12:016008. doi: 10.1088/1752-7163/aa863c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hyun KA, Gwak H, Lee J, Kwak B, Jung HIl. Salivary exosome and cell-free DNA for cancer detection. Micromachines. 2018;9:340. doi: 10.3390/mi9070340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Michael A, et al. Exosomes from human saliva as a source of microRNA biomarkers. Oral. Dis. 2010;16:34–38. doi: 10.1111/j.1601-0825.2009.01604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Machida T, et al. MicroRNAs in salivary exosome as potential biomarkers of aging. Int J. Mol. Sci. 2015;16:21294–21309. doi: 10.3390/ijms160921294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Rani K, et al. A novel approach to correlate the salivary exosomes and their protein cargo in the progression of cognitive impairment into Alzheimer’s disease. J. Neurosci. Methods. 2021;347:108980. doi: 10.1016/j.jneumeth.2020.108980. [DOI] [PubMed] [Google Scholar]
- 251.Rani K, et al. Neuronal exosomes in saliva of Parkinson’s disease patients: a pilot study. Parkinsonism Relat. Disord. 2019;67:21–23. doi: 10.1016/j.parkreldis.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 252.Cao Z, et al. Synuclein in salivary extracellular vesicles as a potential biomarker of Parkinson’s disease. Neurosci. Lett. 2019;696:114–120. doi: 10.1016/j.neulet.2018.12.030. [DOI] [PubMed] [Google Scholar]
- 253.Zlotogorski-Hurvitz A, Dayan D, Chaushu G, Salo T, Vered M. Morphological and molecular features of oral fluid-derived exosomes: oral cancer patients versus healthy individuals. J. Cancer Res Clin. Oncol. 2016;142:101–110. doi: 10.1007/s00432-015-2005-3. [DOI] [PubMed] [Google Scholar]
- 254.Tobón-Arroyave SI, Celis-Mejía N, Córdoba-Hidalgo MP, Isaza-Guzmán DM. Decreased salivary concentration of CD9 and CD81 exosome-related tetraspanins may be associated with the periodontal clinical status. J. Clin. Periodontol. 2019;46:470–480. doi: 10.1111/jcpe.13099. [DOI] [PubMed] [Google Scholar]
- 255.Gallo A, Tandon M, Alevizos I, Illei GG. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS One. 2012;7:1–5. doi: 10.1371/journal.pone.0030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zheng X, et al. Salivary exosomal PSMA7: a promising biomarker of inflammatory bowel disease. Protein Cell. 2017;8:686–695. doi: 10.1007/s13238-017-0413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Sun Y, et al. Comparative proteomic analysis of exosomes and microvesicles in human saliva for lung cancer. J. Proteome Res. 2018;17:1101–1107. doi: 10.1021/acs.jproteome.7b00770. [DOI] [PubMed] [Google Scholar]
- 258.Rani K, et al. Neuronal exosomes in saliva of Parkinson’s disease patients: a pilot study. Parkinsonism Relat. Disord. 2019;67:21–23. doi: 10.1016/j.parkreldis.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 259.Nair S, Tang KD, Kenny L, Punyadeera C. Salivary exosomes as potential biomarkers in cancer. Oral. Oncol. 2018;84:31–40. doi: 10.1016/j.oraloncology.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 260.Faur CI, et al. Salivary exosomal microRNAs as biomarkers for head and neck cancer detection—a literature review. Maxillofac. Plast. Reconstr. Surg. 2021;43:19. doi: 10.1186/s40902-021-00303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Zhan C, Yang X, Yin X, Hou J. Exosomes and other extracellular vesicles in oral and salivary gland cancers. Oral. Dis. 2020;26:865–875. doi: 10.1111/odi.13172. [DOI] [PubMed] [Google Scholar]
- 262.Deng Y, Cao Y, Wang L, Ye D. The role and application of salivary exosomes in Malignant Neoplasms. Cancer Manag Res. 2021;13:5813–5820. doi: 10.2147/CMAR.S321225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Hoshino I. The usefulness of microRNA in urine and saliva as a biomarker of gastroenterological cancer. Int J. Clin. Oncol. 2021;26:1431–1440. doi: 10.1007/s10147-021-01911-1. [DOI] [PubMed] [Google Scholar]
- 264.Rastogi S, et al. The evolving landscape of exosomes in neurodegenerative diseases: Exosomes characteristics and a promising role in early diagnosis. Int J. Mol. Sci. 2021;22:1–31. doi: 10.3390/ijms22010440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Cohn W, et al. Integrated multiomics analysis of salivary exosomes to identify biomarkers associated with changes in mood states and fatigue. Int J. Mol. Sci. 2022;23:5257. doi: 10.3390/ijms23095257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Han Y, Jia L, Zheng Y, Li W. Salivary exosomes: Emerging roles in systemic disease. Int J. Biol. Sci. 2018;14:633–643. doi: 10.7150/ijbs.25018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Escandon P, et al. Unravelling novel roles of salivary exosomes in the regulation of human corneal stromal cell migration and wound healing. Int J. Mol. Sci. 2022;23:4330. doi: 10.3390/ijms23084330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Zhou W, et al. Discovery of exosomes from tick saliva and salivary glands reveals therapeutic roles for cxcl12 and il-8 in wound healing at the tick–human skin interface. Front Cell Dev. Biol. 2020;8:1–22. doi: 10.3389/fcell.2020.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Zlotogorski-Hurvitz A, et al. Human saliva-derived exosomes: comparing methods of isolation. J. Histochemistry Cytochemistry. 2015;63:181–189. doi: 10.1369/0022155414564219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Cheshmi B, Cheshomi H. Salivary exosomes: properties, medical applications, and isolation methods. Mol. Biol. Rep. 2020;47:6295–6307. doi: 10.1007/s11033-020-05659-1. [DOI] [PubMed] [Google Scholar]
- 271.Sun Y, et al. Systematic comparison of exosomal proteomes from human saliva and serum for the detection of lung cancer. Anal. Chim. Acta. 2017;982:84–95. doi: 10.1016/j.aca.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 272.Nakamichi E, et al. Detection of serum/salivary exosomal Alix in patients with oral squamous cell carcinoma. Oral. Dis. 2021;27:439–447. doi: 10.1111/odi.13565. [DOI] [PubMed] [Google Scholar]
- 273.Huang X, Hu X, Zhao M, Zhang Q. Analysis of salivary exosomal proteins in young adults with severe periodontitis. Oral. Dis. 2020;26:173–181. doi: 10.1111/odi.13217. [DOI] [PubMed] [Google Scholar]
- 274.Wang WC, et al. Salivary exosome proteomics and bioinformatics analysis in 7,12-Dimethylbenz[a]anthracene-induced oral cancer with radiation therapy—A Syrian Golden Hamster Model. Diagnostics. 2022;12:65. doi: 10.3390/diagnostics12010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Imai A, et al. Comprehensive analysis and comparison of proteins in salivary exosomes of climacteric and adolescent females. Odontology. 2021;109:82–102. doi: 10.1007/s10266-020-00538-4. [DOI] [PubMed] [Google Scholar]
- 276.Ogawa Y, Taketomi Y, Murakami M, Tsujimoto M, Yanoshita R. Small RNA transcriptomes of two types of exosomes in human whole saliva determined by next generation sequencing. Biol. Pharm. Bull. 2013;36:66–75. doi: 10.1248/bpb.b12-00607. [DOI] [PubMed] [Google Scholar]
- 277.He L, et al. Salivary exosomal miR-24-3p serves as a potential detective biomarker for oral squamous cell carcinoma screening. Biomedicine Pharmacother. 2020;121:109553. doi: 10.1016/j.biopha.2019.109553. [DOI] [PubMed] [Google Scholar]
- 278.Lin Y, et al. Evaluation of salivary exosomal chimeric GOLM1-NAA35 RNA as a potential biomarker in esophageal carcinoma. Clin. Cancer Res. 2019;25:3035–3045. doi: 10.1158/1078-0432.CCR-18-3169. [DOI] [PubMed] [Google Scholar]
- 279.Mi B, et al. Saliva exosomes-derived UBE2O mRNA promotes angiogenesis in cutaneous wounds by targeting SMAD6. J. Nanobiotechnology. 2020;18:1–14. doi: 10.1186/s12951-020-00624-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Zlotogorski-Hurvitz A, Dekel BZ, Malonek D, Yahalom R, Vered M. FTIR-based spectrum of salivary exosomes coupled with computational-aided discriminating analysis in the diagnosis of oral cancer. J. Cancer Res Clin. Oncol. 2019;145:685–694. doi: 10.1007/s00432-018-02827-6. [DOI] [PubMed] [Google Scholar]
- 281.Wu M, et al. One-step quantification of salivary exosomes based on combined aptamer recognition and quantum dot signal amplification. Biosens. Bioelectron. 2021;171:112733. doi: 10.1016/j.bios.2020.112733. [DOI] [PubMed] [Google Scholar]
- 282.Zhou P, et al. A portable point-of-care testing system to diagnose lung cancer through the detection of exosomal miRNA in urine and saliva. Chem. Commun. 2020;56:8968–8971. doi: 10.1039/D0CC03180A. [DOI] [PubMed] [Google Scholar]
- 283.Yu J, et al. Detection of exosomal PD-L1 RNA in saliva of patients with periodontitis. Front Genet. 2019;10:1–9. doi: 10.3389/fgene.2019.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Khurshid Z, et al. Human saliva collection devices for proteomics: An update. Int. J. Mol. Sci. 2016;17:846. doi: 10.3390/ijms17060846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Bellagambi FG, et al. Saliva sampling: Methods and devices. An overview. TrAC - Trends Anal. Chem. 2020;124:115781. doi: 10.1016/j.trac.2019.115781. [DOI] [Google Scholar]
- 286.Gröschl M. Saliva: A reliable sample matrix in bioanalytics. Bioanalysis. 2017;9:655–668. doi: 10.4155/bio-2017-0010. [DOI] [PubMed] [Google Scholar]
- 287.Amado F, Calheiros-Lobo MJ, Ferreira R, Vitorino R. Sample treatment for saliva proteomics. in. Adv. Exp. Med. Biol. 2019;1073:23–56. doi: 10.1007/978-3-030-12298-0_2. [DOI] [PubMed] [Google Scholar]
- 288.Topkas E, Keith P, Dimeski G, Cooper-White J, Punyadeera C. Evaluation of saliva collection devices for the analysis of proteins. Clin. Chim. Acta. 2012;413:1066–1070. doi: 10.1016/j.cca.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 289.Golatowski C, et al. Comparative evaluation of saliva collection methods for proteome analysis. Clin. Chim. Acta. 2013;419:42–46. doi: 10.1016/j.cca.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 290.Justino AB, Teixeira RR, Peixoto LG, Jaramillo OLB, Espindola FS. Effect of saliva collection methods and oral hygiene on salivary biomarkers. Scand. J. Clin. Lab Invest. 2017;77:415–422. doi: 10.1080/00365513.2017.1334261. [DOI] [PubMed] [Google Scholar]
- 291.Costa MM, et al. Optimization and standardization of human saliva collection for MALDI-TOF MS. Diagnostics. 2021;11:1304. doi: 10.3390/diagnostics11081304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Chiang SH, et al. RNAPro•SAL: A device for rapid and standardized collection of saliva RNA and proteins. Biotechniques. 2015;58:69–76. doi: 10.2144/000114254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Khurshid Z, et al. Human salivary protein extraction from RNAPro·SALTM, Pure·SALTM, and passive drooling method. Eur. J. Dent. 2017;11:385–389. doi: 10.4103/ejd.ejd_183_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Lim Y, Totsika M, Morrison M, Punyadeera C. The saliva microbiome profiles are minimally affected by collection method or DNA extraction protocols. Sci. Rep. 2017;7:8523. doi: 10.1038/s41598-017-07885-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Han P, Ivanovski S. Effect of saliva collection methods on the detection of periodontium-related genetic and epigenetic biomarkers-a pilot study. Int J. Mol. Sci. 2019;20:4729. doi: 10.3390/ijms20194729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Hughes SR, Chapleau RR. Comparing DNA quantity and quality using saliva collection following food and beverage consumption. BMC Res Notes. 2019;12:165. doi: 10.1186/s13104-019-4211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Tuan L, Tsm K, Wns S, Nazatul W, Shahidan S. Establishment of the collection, storage and preservation methods and their influence on stability of human salivary exosome. J. Biomed. Clin. Sci. 2018;3:44–52. [Google Scholar]
- 298.Woo JS, Lu DY. Procurement, transportation, and storage of saliva, buccal swab, and oral wash specimens. in. Methods Mol. Biol. 2019;1897:99–105. doi: 10.1007/978-1-4939-8935-5_10. [DOI] [PubMed] [Google Scholar]
- 299.Pramanik R, et al. Effects of the UK biobank collection protocol on potential biomarkers in saliva. Int J. Epidemiol. 2012;41:1786–1797. doi: 10.1093/ije/dys166. [DOI] [PubMed] [Google Scholar]
- 300.Guerin JS, et al. Molecular Medicine Ireland guidelines for standardized biobanking. Biopreservation Biobanking. 2010;8:3–63. doi: 10.1089/bio.2010.8101. [DOI] [PubMed] [Google Scholar]
- 301.Oelsner KT, Guo Y, To SBC, Non AL, Barkin SL. Maternal BMI as a predictor of methylation of obesity-related genes in saliva samples from preschool-age Hispanic children at-risk for obesity. BMC Genomics. 2017;18:57. doi: 10.1186/s12864-016-3473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Ho Lee M, et al. Application of droplet digital PCR method for DNA methylation-based age prediction from saliva. Leg. Med. 2022;54:101992. doi: 10.1016/j.legalmed.2021.101992. [DOI] [PubMed] [Google Scholar]
- 303.Cui Y, et al. Correlations of salivary and blood glucose levels among six saliva collection methods. Int J. Environ. Res Public Health. 2022;19:4122. doi: 10.3390/ijerph19074122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Rosa N, et al. Protein quality assessment on saliva samples for biobanking purposes. in. Biopreservation Biobanking. 2016;14:289–297. doi: 10.1089/bio.2015.0054. [DOI] [PubMed] [Google Scholar]
- 305.Nunes LAS, Brenzikofer R, Macedo DV. Reference intervals for saliva analytes collected by a standardized method in a physically active population. Clin. Biochem. 2011;44:1440–1444. doi: 10.1016/j.clinbiochem.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 306.Ng V, Koh D, Fu Q, Chia SE. Effects of storage time on stability of salivary immunoglobulin A and lysozyme. Clin. Chim. Acta. 2003;338:131–134. doi: 10.1016/j.cccn.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 307.Gröschl M, Wagner R, Rauh M, Dörr HG. Stability of salivary steroids: The influences of storage, food and dental care. Steroids. 2001;66:737–741. doi: 10.1016/S0039-128X(01)00111-8. [DOI] [PubMed] [Google Scholar]
- 308.Garde AH, Hansen ÅM. Long-term stability of salivary cortisol. Scand. J. Clin. Lab Invest. 2005;65:433–436. doi: 10.1080/00365510510025773. [DOI] [PubMed] [Google Scholar]
- 309.Sullivan R, et al. An optimised saliva collection method to produce high-yield, high-quality RNA for translational research. PLoS One. 2020;15:e0229791. doi: 10.1371/journal.pone.0229791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Duarte D, et al. Evaluation of saliva stability for nmr metabolomics: Collection and handling protocols. Metabolites. 2020;10:1–15. doi: 10.3390/metabo10120515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.