Abstract
Anterior uveitis has various causes, but the majority of cases are viral induced. The most common viral anterior uveitis etiology includes double-stranded DNA viruses of the Herpesviridae family, including Alpha herpes virinae (herpes simplex 1 and 2 and varicella zoster virus), Beta herpesvirinae (cytomegalovirus), and less frequently, Gamma herpesvirinae (Epstein-Barr virus). In the last few decades, a growing body of evidence has correlated Fuchs uveitis etiology to the rubella virus from the Matonaviridae family, which has a single-stranded RNA genome. The clinical presentation of each of these uveitis is hypertensive granulomatous anterior uveitis; however, the very slight differences between them, which often overlap, make differential diagnosis sometimes difficult. Therefore, diagnostic laboratory tests such as polymerase chain reaction and antibody index or Goldmann-Witmer coefficient analyses on the aqueous humor help to identify the etiology in doubtful cases and thus to plan targeted treatment.
Keywords: Antibody index, herpes virus, polymerase chain reaction, rubella virus, viral anterior uveitis
INTRODUCTION
Viruses are obligate intracellular parasites, reproducing only within living cells. To date, some 5000 virus species have been described in detail, although there are believed to be millions of them.[1] Viruses are found in almost all ecosystems and represent the most abundant biological entity of all. They can localize in the eye and trigger an immune response that often results in ocular disease. When they affect the anterior part of the eye, they generate viral keratitis or viral anterior uveitis (VAU).
VAU may often be mistaken for noninfectious uveitis and inappropriately treated only with intensive topical steroids, with incomplete healing and relapse when therapy is discontinued.
As different viruses may cause VAU with similar clinical presentations, it is important to identify the causative agent to prescribe the appropriate treatment. The viruses known to cause VAU include herpes simplex virus (HSV), varicellazoster virus (VZV), cytomegalovirus (CMV), Rubella virus (RV), Epstein − Barr virus (EBV), human T-cell leukemia virus type 1 virus, Chikungunya virus, Dengue virus, Ebola virus, and Zika virus. The most commonly implicated viruses in VAU include HSV, VZV, CMV, and RV.[2,3,4] However, the true role of the other viruses remains to be determined, as they may only be bystanders and not true infectious agents.[5]
Herpesviridae are double-stranded DNA viruses; to date, there are 8 that can infect humans: HSV type-1 (HSV-1), HSV type-2 (HSV-2), VZV, CMV, EBV, human herpesvirus type-6 (HHV-6), human herpesvirus type-7 (HHV-7), and human herpesvirus type-8 (HHV-8). These viruses cause common diseases such as herpes labialis (HSV-1), herpes genitalis (HSV-2), chickenpox and herpes zoster (VZV), and mononucleosis (EBV). While all Herpesviridae can cause eye disease, the most common ocular pathogens are HSV-1, HSV-2, VZV, and CMV; they account for 5%–10% of all uveitis cases seen at tertiary referral centers.[6] Although contact with herpes viruses is frequent in the general population, the resulting infection is usually asymptomatic, except for VZV, whose first infection causes the typical vesicular rash (chickenpox). These viruses are transmitted directly through secretions, infected skin, or mucous membranes. Some, especially Alpha herpesvirinae HSV-1, HSV-2, and VZV, are neurotropic viruses: after the first infection, they penetrate into the nerves and into the cell ganglia, where they persist in a latent form, establishing an “indissoluble bond” between the pathogen and the human organism. Providing that the conditions of the host immunity system impairment are favorable, only a small percentage reactivate and spread along the axons at the site of the primary infection, causing clinically manifest disease. The eye is an extension of the central nervous system and is often the primary site of a herpetic infection, where it subsequently reactivates along the richly innervated first branch of the trigeminal nerve. While Gamma herpes virinae EBV is a ubiquitous transforming virus showing tropism for B-lymphocytes and sometimes causes anterior uveitis, Betaherpesvirinae CMV tends to infect corneal endothelial cells as well as neural and myeloid progenitor cells.[7,8,9]
RV is a member of the genus Rubivirus of the Matonaviridae family (“Togaviridae” before 2018). It contains a positive-sense, single-stranded RNA genome. It is a strictly human pathogen transmitted through the respiratory airways and is the causative agent of rubella disease, also called German measles.[10,11]
This review focuses on the main viruses causing VAU, their clinical features, their management, and laboratory tests.
MAIN VIRUSES CAUSING VIRAL ANTERIOR UVEITIS
Herpes simplex virus 1 and 2 anterior uveitis
As most authors do not subtype HSV, there is a lack of data regarding any differences between HSV-1 and HSV-2 in their clinical presentation. However, herpes simplex type 1 is much more common than type 2 due to how it is spread (respiratory versus genital route, respectively).[3,12] Cases of mixed infection with both HSV-1 and HSV-2 have also been reported, with the former more predominant than the latter.[13]
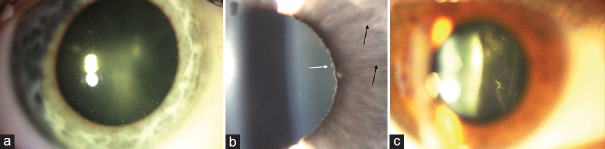
HSV anterior uveitis (HSV-AU) is the most common cause of VAU in Europe, accounting for 15.6%–23.5% of cases.[14,15,16] Involvement is usually unilateral, although rare cases of bilateral involvement have also been described.[17] This is an acute anterior granulomatous uveitis with “mutton fat” keratic precipitates (KPs) in a triangular arrangement (Arlt's triangle) below the horizontal midline, posterior synechiae, sectorial iris atrophy, and ocular hypertension secondary to trabeculitis [Figure 1].[18] On anterior segment examination, an active keratitis or corneal scarring from previous infections and reduced corneal sensitivity can be found. Episcleritis or scleritis can complicate the clinical picture. Herpetic keratouveitis is an autoimmune inflammation following a herpetic infection that involves the corneal stroma or endothelium and the uvea.[19]
Figure 1.
Herpes simplex virus anterior uveitis. (a) Sectoral iris atrophy visible on slit lamp retroillumination. (b) Mutton-fat keratic precipitates typically distributed in a wedge-shaped region on the inferior corneal endothelium, known as Arlt's triangle
It is important to know how to recognize a relapse of uveitis that overlaps keratitis. When present, the latter is a valuable sign of herpetic infection. An acute unilateral anterior uveitis in a subject with a positive history of herpetic keratitis should be considered of herpetic nature unless proven otherwise. However, when keratitis is absent, or there is no history of previous keratitis, this does not exclude a herpetic uveitis origin; moreover, typical signs such as keratitis and iris atrophy are not always present at initial presentation.[18,20] To clarify, HSV-related keratitis may be a rare primary manifestation of viral infection; it can present as superficial punctate keratopathy or corneal vesicles, which can evolve to form microdendritic lesions. Instead, HSV-AU is typically considered to occur during recurrences.
A frequent finding is an irregular pupil, caused by iris atrophy, typically sectoral in HSV, which is determined by ischemic necrosis of iris stroma.[21]
Unilateral involvement, granulomatous KPs, sectoral iris atrophy, pupillary distortion, and acute intraocular pressure (IOP) spikes at recurrence are highly suggestive features for a clinical diagnosis of herpetic AU. It is not yet clear whether the inflammation of the uvea is due to a cytotoxic effect of the virus or an immunopathological mechanism.[22]
Despite concerns of renal toxicity and drug resistance, systemic formulations of the guanosine analog of acyclovir and its prodrug valacyclovir are reasonably well tolerated and remain the mainstay of therapy in the management of herpetic eye diseases, both for the acute disease and for long-term prophylaxis to prevent recurrences.[23,24,25,26] Valacyclovir is preferred because of its greater bioavailability and because of the simpler dosing schedule; in fact, valacyclovir 1000 mg three times daily is as effective in acute herpetic uveitis as acyclovir 800 mg five times daily.[27,28] In our practice, patients are kept on a daily dose of prophylactic oral valacyclovir (500 or 1000 mg) for a minimum of 2 years after the first episode of uveitis, with a tendency toward life-long treatment.[29] Systemic antiviral therapy is generally accompanied by topical steroid therapy to counteract the inflammatory response.
Varicella zoster virus anterior uveitis
About 40%–60% of immunocompetent patients with herpes zoster ophthalmicus (HZO) can develop anterior uveitis, which can be present for many months and which can be observed during chicken pox only rarely. However, the diagnosis of VZV anterior uveitis (VZV-AU) can be very difficult in the absence of dermatitis along the first trigeminal branch (zoster sine herpete).
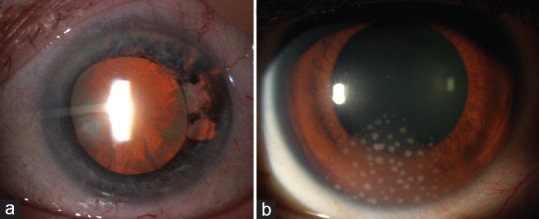
Granulomatous KPs, posterior synechiae, pupillary distortion, and elevated IOP are also characteristics of this type of uveitis.[30] Although it is impossible to determine exactly whether the virus responsible for herpetic uveitis is HSV or VZV without laboratory tests, some clinical features can lead to suspecting one or the other infectious agent. In fact, in VZV-AU, iris atrophy is much more extended and defined than it is in HSV, which has a circular segmental appearance [Figure 2]. The viral load of VZV in the aqueous humor (AH) correlates with the intensity of iris atrophy and pupil distortion.[31] In addition, the abolished corneal sensitivity is related to its cytolytic action on the nerve cells. Furthermore, episcleritis or scleritis are more frequent complications of VZV-AU than of HSV-AU.[32,33]
Figure 2.
Varicellazoster virus anterior uveitis. Sectoral iris atrophy visible on slit lamp retroillumination (more extended and defined than herpes simplex virus uveitis)
Although uveitis is typically unilateral, bilateral involvement has been reported in up to 13% of cases, especially in patients with underlying immunosuppression or severe atopy.[34]
In the course of the disease, the eyelids, conjunctiva, cornea, sclera, optic nerve, and orbital structures may become involved early due to direct damage from the viral infection; their later involvement is the result of the vasculitis and the immune reaction to the viral antigens.[30]
While anterior uveitis may develop during the acute phase of HZO, it is more commonly seen 2−4 weeks after the onset of HZO and may even develop many years after the initial episode. Involvement of the tip of the nose is a predictor of ocular inflammation (Hutchinson's sign).[35]
Treatment of VZV-AU is the same as that of HSV-AU (see above).
Cytomegalovirus anterior uveitis
CMV anterior uveitis (CMV-AU) is the most common ocular manifestation of CMV disease in immunocompetent individuals. It is thought to be due to a local reactivation of latent CMV, which has a specific tropism for the corneal endothelium.[9] Although CMV-AU has been seen all over the world, most reports come from Asia.[36,37,38,39] The prevalence of CMV infection in the Asian population with VAU is higher than that in the West, possibly because of its apparently higher seroprevalence in Asian countries (approximately 69.1%–98.6%) than in the West (approximately 41.9%–57%). Differing genetic susceptibilities or pathogenic strains of the virus may give rise to this geographic disparity.[40,41]
CMV has a spectrum of ocular manifestations. As well as usually being unilateral, it may manifest as an acute relapsing hypertensive anterior uveitis, a self-limiting iritis with sector iris atrophy, or a chronic anterior uveitis with mild inflammation. Corneal endotheliitis may be associated with the anterior uveitis or may be an isolated manifestation.[42]
Acute recurrent hypertensive CMV-AU determines what was previously called Posner-Schlossman syndrome (PSS).[43] Although a few studies have reported that HSV can cause PSS, most cases are caused by CMV, to the extent that we believe it is correct to speak of acute CMV-AU rather than of PSS.[44,45] It typically presents in patients aged 30 − 60 years, with a male: female ratio of 2:1. An acute onset of unilateral blurring of vision may be associated with halos and ipsilateral headache. The eye has minimally ciliary injection, and corneal epithelial edema may be present. A few medium-to-large gray-white coin-shaped granulomatous KPs located centrally or at the periphery of the cornea are present [Figure 3]. The presence of coin-shaped KPs has a positive predictive value of 90.9% for CMV.[46]
Figure 3.
Cytomegalovirus anterior uveitis. (a) Coin-shaped keratic precipitates, (b) Same precipitates at higher magnification
If there is stromal iris atrophy taking on a moth-eaten appearance which is either sectoral or diffuse, heterochromia may be present. This iris atrophy is postulated to be due to ischemic necrosis of the iris stroma as a result of direct viral invasion or vasculitis, which is consistent with the findings of CMV in the iris smooth muscle cells.[47]
Chronic CMV-AU tends to present in patients aged 50–80 years, at a mean age of 65 years. Interestingly, Asian and European patients differ in terms of clinical presentation. Chronic CMV-AU in the eyes of Asian patients resembles Fuchs uveitis (FU) (see below), while European patients have fewer brown KPs, they are located inferiorly and are characteristically coin-shaped.[40]
In every CMV-AU, the pupil remains round, and posterior synechiae are absent.[21,48] Vitreous inflammation is mild or absent.[49] IOP increases during the course of the disease, with maximum IOP of CMV-AU generally higher than that in HSV- or VZV-AU.[50] In acute CMV-AU, IOP often exceeds 50 mmHg in the presence of subtle subepithelial edema.[40] Secondary glaucoma requiring surgery is the most common complication of CMV-AU, followed by posterior subcapsular cataract due to chronic uveitis or to the use of topical corticosteroids to reduce intraocular inflammation.[38,51]
The corneal endothelial cell count is significantly low in CMV-positive eyes, and the degree of corneal endothelial cell loss correlates significantly with the viral load in the AH.[52,53,54] In some eyes, the uveitis may be complicated by corneal endotheliitis, in which the endothelial cells are the primary target of CMV infection. Immune ring formation may be seen in CMV endotheliitis.[55]
Management of CMV-AU involves the use of topical or systemic antivirals, topical steroids, and topical anti-glaucoma medications. Our approach to antiviral therapy is to offer ganciclovir gel as the first treatment option in combination with a topical steroid. For patients who fail to respond or who show frequent recurrence while on topical treatment and have progressive visual loss, we then offer a 3-month course of oral valganciclovir. If these patients continue to relapse, have progressive visual loss, and have no contraindications, they are offered a much longer course of oral valganciclovir.[56]
Rubella virus anterior uveitis
The importance of RV in uveitis is due to its established association with FU. Ernst Fuchs first described the clinical syndrome in 1906.[57] Its etiology remained unknown for about a century, although several theories were proposed to explain its pathogenesis, including immune dysregulation, defective sympathetic innervation, and infectious etiologies.[58]
In the early 2000s, a tenacious association between RV and FU was demonstrated thanks to improvements in the ability to identify anterior chamber infections using polymerase chain reaction (PCR) and to quantitative antibody studies. In 2004, Quentin and Reiber first showed that RV-specific antibodies were detected in the anterior chamber in 87% of the eyes affected by FU, preceding other authors.[59,60,61,62] RV anterior uveitis is difficult to diagnose by RV RNA detection alone because positive PCR is not reliable. Indeed, several studies showed that 10%–20% of suspected cases were PCR-positive, whereas 87%–100% of AH samples were RV-IgG-positive.[59,60,63]
Furthermore, Birnbaum et al. showed that FU was less common in patients born after the introduction of the live-attenuated obligatory rubella vaccine in the US in 1969. Specifically, the percentages of patients with FU born in 1919–1958 (no population vaccination), in 1958–1969 (partial population vaccination), and after 1969 (complete population vaccination) were 4.58%, 2.97%, and 1.18%, respectively.[64]
However, although the primary role of RV in FU pathogenesis has largely been demonstrated, uncertainties remain concerning all cases around the world.[65] Currently, CMV infection accounts for 16%–42% of cases of FU in Asia; in Western countries, FU is predominantly associated with RV.[65,66,67] This is due to the different seroprevalence of CMV and rubella infections in the various geographic areas of the world.
FU accounts for 0.5%–16.8% of uveitis; its prevalence has been estimated to be between 1.8% and 22.7% in developed countries, but only 0%–5.6% in developing countries.[68] Although FU may begin in early childhood, the mean age at diagnosis varies between the third and the fifth decade of life, with no difference in sex.[69,70,71] Although FU is most commonly unilateral, bilateral involvement is possible (10%), detected almost always at baseline rather than during follow-up.[72] This chronic disease is characterized by low-grade inflammation involving the anterior uvea and vitreous.[60]
The diagnosis of FU is only clinical; however, because it can have various clinical forms and its characteristic findings may not be present at the onset of disease, it is frequently misdiagnosed, resulting in the diagnosis being delayed for years.[73] Patients with the disease are often asymptomatic for years, the diagnosis being made only during a routine eye examination, when patients may complain of floaters or blurry vision.[65]
Unlike HSV-AU and VZV-AU, the absence of ciliary injection is characteristic. One of the most distinctive findings is the presence of white small-to-medium-sized stellate KPs that are distributed diffusely over the endothelium, unlike other types of uveitis, which have an inferior location [Figure 4].[74] Slit-lamp examination reveals mild anterior chamber reaction that might be persistent.
Figure 4.
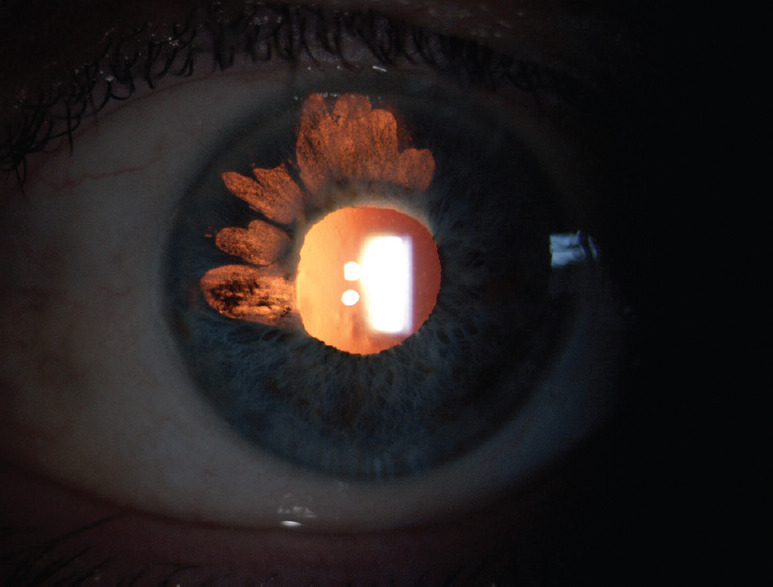
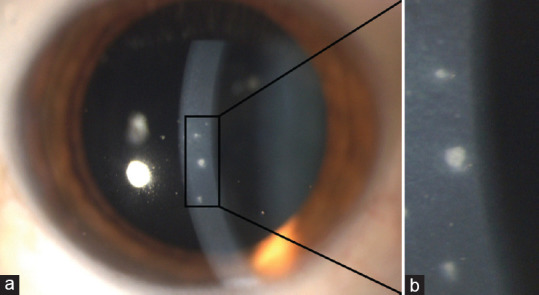
Fuchs uveitis. (a) Stellate keratic precipitates throughout the corneal area, (b) Koeppe nodules localized at the pupillary margin (white arrow) and normal radial iris vessels that become visible because of iris atrophy (black arrows), (c) Anterior vitreous cells
The major iris finding in FU patients is heterochromia, generally subtle or absent in dark or brown irises, whereas prominent in light colored ones [Figure 5]. The affected eye is hypochromic because of pigment deprivation. Actually, iris heterochromia maybe present in <40% of patients.[72] In addition, in light-colored irises, the progressive atrophy of the anterior stroma exposes the pigmented epithelium of the iris, resulting in a darker iris (inverse heterochromia).[65] Iris changes often precede heterochromia and are a more sensitive and reliable sign of FU; they include iris stromal smoothing and the consequent loss of the normal corrugated texture as well as reduced iris thickness, as demonstrated by anterior segment optical coherence tomography.[75] Diffuse iris atrophy with fine transillumination defects mainly at pupillary margin is commonly detected. Iris nodules in FU have been observed in about 20%−30% of cases in several series; they can be observed at iris surface (Busacca nodules) or at the pupillary margin Koeppe nodules, Figure 4].[70,72,76] Another iris feature is the absence of posterior synechiae.[77]
Figure 5.
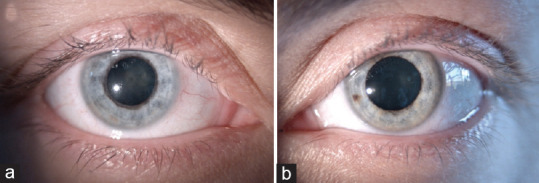
Fuchs uveitis heterochromia. (a) The affected eye is a hypochromic light-blue color, (b) Unaffected eye
Due to diffuse iris atrophy, normal radial vessels on the iris surface and fine rubeotic-like bridging vessels on the iridocorneal angle are seen on slit-lamp examination and gonioscopy. Amsler et al. first noted hyphema after paracentesis in patients affected with FU, attributing this phenomenon to the presence of these abnormal blood vessels.[78] Nevertheless, Amsler's sign is not specific for FU.[79] Because low-to-moderate vitreitis is seen in the vast majority of FU patients, it should be considered a major diagnostic element [Figure 4].[80,81]
To clarify, the FU cases associated with CMV in the literature often present with features that differ from those of RV-associated cases, including different KP morphology or absence of vitritis.[40] In addition, CMV-associated FU appears due to active viral infection in the anterior chamber, as shown by the PCR data and by response to antiviral therapy, whereas RV-associated FU seems to have a stronger relationship with anterior chamber antibody production – so with a previous RV infection – and does not require specific therapy.[82] Thus, although some reports in the literature support the hypothesis that FU could be related to various microbial agents, we believe that in most cases, the clinical signs of FU are well defined and attributable to a previous rubella infection.[83,84]
In addition to the clinical picture, other instruments such as fluorescein angiography (FA) and enhanced depth imaging OCT (EDI-OCT) have been used to better characterize the disease. FA almost always shows disc hyperfluorescence, less often retinal vasculitis of the small peripheral retinal vessels (13.6%).[85] In any case, cystoid macular edema is typically absent, as all other anterior uveitis are by definition not associated with macular edema.[2] Recently, Cerquaglia et al. found a diffuse full-thickness choroidal thinning in EDI-OCT, suggesting an inflammatory condition involving the whole uveal tunic.[86]
Gradual progression of the disease is associated with cataract formation and glaucoma.
Cataract is the most common complication and usually presents in its posterior subcapsular form. Hence, FU diagnosis should be excluded in any young patient with unilateral lens opacification and no history of trauma or steroid use.[79]
Elevations in IOP are initially intermittent but can later become chronic as a result of corticosteroid-induced ocular hypertension, abnormal angle vessels, trabecular scarring, or peripheral anterior synechiae formation.[58] Secondary glaucoma has been reported in 6.5%–59% of FU patients.[87]
As concerns treatment, topical anti-inflammatory drugs are not indicated because inflammation is low grade. Indeed, these drugs may speed up cataract and glaucoma formation. Elevated IOP should be treated with anti-glaucoma medications and/or laser or surgical procedures.[65]
LABORATORY TESTS
To date, molecular analysis is the VAU diagnostic standard, relegating to a secondary role the serological investigations aimed at defining the specific antibody response.[12,88] In fact, with molecular diagnostics, we can obtain better performance both in terms of sensitivity and specificity. The limitations of this approach are the scarce quantity of the humor sample that can be collected and the moment when the sample is taken with regard to the replication phase of the pathogen.[15,60] In fact, during the acute phase with active viral replication, the probability of finding pathogen DNA or RNA with PCR will be greater when antibody production is still zero or scarce, while for a sample taken during the chronic phase, the possibility of finding specific antibodies will be greater than for genetic material.[89] For this reason, it may be useful to study the specific humoral immune response in AH to indirectly increase sensitivity by demonstrating the presence of the pathogen.[7,90]
The diagnosis of central nervous system infectious diseases is supported by the demonstrated formation of pathogen-specific antibodies in the cerebrospinal fluid. Similarly, this diagnostic approach has also been adopted in the case of suspected VAU.[91,92] However, the presence of specific IgG antibodies in the humor alone is not enough to demonstrate an intraocular synthesis of antibodies, as passive diffusion of antibodies is possible from the serum. To distinguish between intraocular IgG and that due to plasma filtration, the specific IgG antibody concentrations against the suspected pathogen measured in the AH and serum are linked in index form with the respective total IgG concentration in humor and serum.
The quantification of total IgG and specific IgG levels in serum and AH makes it possible to determine the Goldmann-Witmer coefficient (GWC) specific for the suspected pathogen and to evaluate whether the IgG measured in the humor are of intraocular synthesis or are present in the humor but derive from the blood by passive diffusion at the level of the blood-ocular barrier.[93] Thus, the specific GWC for the pathogen is expressed by the ratio between the quotient of the concentrations of specific antibodies in AH and serum (QIgG spec) and the quotient of the concentration of total IgG in the AH and serum (QIgG tot) according to the mathematical ratio:
GWC = QIgG spec/QIgG tot
If GWC exceedes 3.0, i.e., the concentration of specific IgG measured in humor is three times higher than that measured in serum, then IgG can only be considered ocular synthesis. GWC has a limitation in the event that there is moderate or severe damage to the blood-ocular barrier. In these cases, GWC can yield false positives. This can be overcome by using the specific antibody index (IA) or modified Golmann-Witmer, which represents an evolution inthe GWC as it takes into account the degree of permeability of the blood-ocular barrier.[59] Barrier damage is a function of the ratio of albumin concentration in serum and AH. In these cases, albumin serves as a reference protein for the integrity of the blood-ocular barrier as it is found in ocular fluids only if inflammation alters the barrier and allows the passage of albumin from the circulating blood. Barrier damage is a function of the ratio of albumin concentration in serum and AH:
Qalb (evaluation parameter of the barrier function) = Albhumor/Albserum
To determine AI, it is necessary to calculate the Qlim (upper limit in the Reiber quotient diagram), which represents the discrimination line defined as 0 mg/L of local synthesis of antibodies, i.e., the maximum value of passively filtered immunoglobulins from the serum in those certain conditions of barrier state (in general, the cases without local synthesis of IgG in the humor are below this line).[94,95]
Intraocular antibody production has been considered when the specific AI exceeded 1.7.[96] Further studies are required to confirm this cutoff.
AI turns out to be more sensitive than GWC, but to calculate it, a larger volume of the starting sample is required to determine the humor albumin concentration.
When the clinical features of uveitis are distinctive and make it possible to define a targeted therapy, aqueous tap is not done. This test is, however, important in doubtful cases. In fact, the analysis of AH appears to be decisive in the diagnosis of similar, mystifying VAU, as the serum antibody analysis is only informative to rule out a type of uveitis if immunological memory towards a particular virus is absent [Figure 6].[14,97,98] Molecular diagnostics and analysis of intraocular antibody production can considerably increase VAU diagnostic sensitivity and confirm the importance of accurately defining the virus to be analyzed basedon the clinical characteristics of uveitis to make the most of the sample taken.[96] In extreme cases, when the clinical diagnosis does not correlate with the specific prognosis, anterior chamber paracentesis could clarify the etiological agent.[83]
Figure 6.
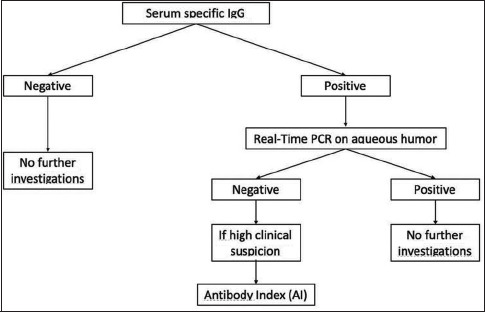
Diagnostic algorithm for doubtful cases of viral anterior uveitis
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank Jacqueline Costa for proofreading the manuscript.
REFERENCES
- 1.Radosavljevic A, Agarwal M, Chee SP, Zierhut M. Epidemiology of viral induced anterior uveitis. Ocul Immunol Inflamm. 2022;30:297–309. doi: 10.1080/09273948.2020.1853177. [DOI] [PubMed] [Google Scholar]
- 2.Relvas LJ, Caspers L, Chee SP, Zierhut M, Willermain F. Differential diagnosis of viral-induced anterior uveitis. Ocul Immunol Inflamm. 2018;26:726–31. doi: 10.1080/09273948.2018.1468470. [DOI] [PubMed] [Google Scholar]
- 3.Pleyer U, Chee SP. Current aspects on the management of viral uveitis in immunocompetent individuals. Clin Ophthalmol. 2015;9:1017–28. doi: 10.2147/OPTH.S60394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambert N, Strebel P, Orenstein W, Icenogle J, Poland GA. Rubella. Lancet. 2015;385:2297–307. doi: 10.1016/S0140-6736(14)60539-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Groot-Mijnes JD, de Visser L, Zuurveen S, Martinus RA, Völker R, ten Dam-van Loon NH, et al. Identification of new pathogens in the intraocular fluid of patients with uveitis. Am J Ophthalmol. 2010;150:628–36. doi: 10.1016/j.ajo.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nalcacioglu-Yüksekkaya P, Ozdal PC, Teke MY, Kara C, Ozturk F. Presumed herpetic anterior uveitis: A study with retrospective analysis of 79 cases. Eur J Ophthalmol. 2014;24:14–20. doi: 10.5301/ejo.5000331. [DOI] [PubMed] [Google Scholar]
- 7.Smit D, Meyer D, Maritz J, de Groot-Mijnes JD. Polymerase chain reaction and goldmann-witmer coefficient to examine the role of Epstein-Barr virus in uveitis. Ocul Immunol Inflamm. 2019;27:108–13. doi: 10.1080/09273948.2017.1370653. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham ET, Zierhut M. Epstein-Barr virus and the eye. Ocul Immunol Inflamm. 2020;28:533–7. doi: 10.1080/09273948.2020.1760549. [DOI] [PubMed] [Google Scholar]
- 9.Hosogai M, Shima N, Nakatani Y, Inoue T, Iso T, Yokoo H, et al. Analysis of human cytomegalovirus replication in primary cultured human corneal endothelial cells. Br J Ophthalmol. 2015;99:1583–90. doi: 10.1136/bjophthalmol-2014-306486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung AK, Hon KL, Leong KF. Rubella (German measles) revisited. Hong Kong Med J. 2019;25:134–41. doi: 10.12809/hkmj187785. [DOI] [PubMed] [Google Scholar]
- 11.Bennett AJ, Paskey AC, Ebinger A, Pfaff F, Priemer G, Höper D, et al. Relatives of rubella virus in diverse mammals. Nature. 2020;586:424–8. doi: 10.1038/s41586-020-2812-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugita S, Shimizu N, Watanabe K, Mizukami M, Morio T, Sugamoto Y, et al. Use of multiplex PCR and real-time PCR to detect human herpes virus genome in ocular fluids of patients with uveitis. Br J Ophthalmol. 2008;92:928–32. doi: 10.1136/bjo.2007.133967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaneko H, Kawana T, Ishioka K, Ohno S, Aoki K, Suzutani T. Evaluation of mixed infection cases with both herpes simplex virus types 1 and 2. J Med Virol. 2008;80:883–7. doi: 10.1002/jmv.21154. [DOI] [PubMed] [Google Scholar]
- 14.Errera MH, Goldschmidt P, Batellier L, Degorge S, Héron E, Laroche L, et al. Findings in detection of herpesviridae by polymerase chain reaction and intraocular antibody production in a case series of anterior uveitis. Ocul Immunol Inflamm. 2013;21:61–8. doi: 10.3109/09273948.2012.730653. [DOI] [PubMed] [Google Scholar]
- 15.Chronopoulos A, Roquelaure D, Souteyrand G, Seebach JD, Schutz JS, Thumann G. Aqueous humor polymerase chain reaction in uveitis – Utility and safety. BMC Ophthalmol. 2016;16:189. doi: 10.1186/s12886-016-0369-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cimino L, Aldigeri R, Marchi S, Mastrofilippo V, Viscogliosi F, Coassin M, et al. Changes in patterns of uveitis at a tertiary referral center in Northern Italy: Analysis of 990 consecutive cases. Int Ophthalmol. 2018;38:1–10. doi: 10.1007/s10792-017-0466-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de-la-Torre A, Valdes-Camacho J, Foster CS. Bilateral herpes simplex uveitis: Review of the literature and own reports. Ocul Immunol Inflamm. 2017;25:497–502. doi: 10.3109/09273948.2016.1142572. [DOI] [PubMed] [Google Scholar]
- 18.Wensing B, Mochizuki M, De Boer JH. Clinical characteristics of herpes simplex virus associated anterior uveitis. Ocul Immunol Inflamm. 2018;26:333–7. doi: 10.1080/09273948.2017.1420806. [DOI] [PubMed] [Google Scholar]
- 19.Bagga B, Kate A, Joseph J, Dave VP. Herpes simplex infection of the eye: An introduction. Community Eye Health. 2020;33:68–70. [PMC free article] [PubMed] [Google Scholar]
- 20.Van der Lelij A, Ooijman FM, Kijlstra A, Rothova A. Anterior uveitis with sectoral iris atrophy in the absence of keratitis: A distinct clinical entity among herpetic eye diseases. Ophthalmology. 2000;107:1164–70. doi: 10.1016/s0161-6420(00)00115-9. [DOI] [PubMed] [Google Scholar]
- 21.Takase H, Kubono R, Terada Y, Imai A, Fukuda S, Tomita M, et al. Comparison of the ocular characteristics of anterior uveitis caused by herpes simplex virus, varicella-zoster virus, and cytomegalovirus. Jpn J Ophthalmol. 2014;58:473–82. doi: 10.1007/s10384-014-0340-6. [DOI] [PubMed] [Google Scholar]
- 22.Kardeş E, Bozkurt K, Sezgin Akçay Bİ, Ünlü C, Aydoğan Gezginaslan T, Ergin A. Clinical features and prognosis of herpetic anterior uveitis. Turk J Ophthalmol. 2016;46:109–13. doi: 10.4274/tjo.92053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilhelmus KR, Dawson CR, Barron BA, Bacchetti P, Gee L, Jones DB, et al. Risk factors for herpes simplex virus epithelial keratitis recurring during treatment of stromal keratitis or iridocyclitis. Herpetic Eye Disease Study Group. Br J Ophthalmol. 1996;80:969–72. doi: 10.1136/bjo.80.11.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lairson DR, Begley CE, Reynolds TF, Wilhelmus KR. Prevention of herpes simplex virus eye disease: A cost-effectiveness analysis. Arch Ophthalmol. 2003;121:108–12. doi: 10.1001/archopht.121.1.108. [DOI] [PubMed] [Google Scholar]
- 25.Wilhelmus KR, Beck RW, Moke PS, Dawson CR, Barron BA, Jones DB, et al. Acyclovir for the prevention of recurrent herpes simplex virus eye disease. N Engl J Med. 1998;339:300–6. doi: 10.1056/NEJM199807303390503. [DOI] [PubMed] [Google Scholar]
- 26.Uchoa UB, Rezende RA, Carrasco MA, Rapuano CJ, Laibson PR, Cohen EJ. Long-term acyclovir use to prevent recurrent ocular herpes simplex virus infection. Arch Ophthalmol. 2003;121:1702–4. doi: 10.1001/archopht.121.12.1702. [DOI] [PubMed] [Google Scholar]
- 27.Colin J, Prisant O, Cochener B, Lescale O, Rolland B, Hoang-Xuan T. Comparison of the efficacy and safety of valaciclovir and acyclovir for the treatment of herpes zoster ophthalmicus. Ophthalmology. 2000;107:1507–11. doi: 10.1016/s0161-6420(00)00222-0. [DOI] [PubMed] [Google Scholar]
- 28.Beutner KR, Friedman DJ, Forszpaniak C, Andersen PL, Wood MJ. Valaciclovir compared with acyclovir for improved therapy for herpes zoster in immunocompetent adults. Antimicrob Agents Chemother. 1995;39:1546–53. doi: 10.1128/aac.39.7.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miserocchi E, Waheed NK, Dios E, Christen W, Merayo J, Roque M, et al. Visual outcome in herpes simplex virus and varicella zoster virus uveitis: A clinical evaluation and comparison. Ophthalmology. 2002;109:1532–7. doi: 10.1016/s0161-6420(02)01113-2. [DOI] [PubMed] [Google Scholar]
- 30.Tugal-Tutkun I, Cimino L, Akova YA. Review for disease of the year: Varicella zoster virus-induced anterior uveitis. Ocul Immunol Inflamm. 2018;26:171–7. doi: 10.1080/09273948.2017.1383447. [DOI] [PubMed] [Google Scholar]
- 31.Kido S, Sugita S, Horie S, Miyanaga M, Miyata K, Shimizu N, et al. Association of varicella zoster virus load in the aqueous humor with clinical manifestations of anterior uveitis in herpes zoster ophthalmicus and zoster sine herpete. Br J Ophthalmol. 2008;92:505–8. doi: 10.1136/bjo.2007.125773. [DOI] [PubMed] [Google Scholar]
- 32.Jabs DA, Mudun A, Dunn JP, Marsh MJ. Episcleritis and scleritis: Clinical features and treatment results. Am J Ophthalmol. 2000;130:469–76. doi: 10.1016/s0002-9394(00)00710-8. [DOI] [PubMed] [Google Scholar]
- 33.Okhravi N, Odufuwa B, McCluskey P, Lightman S. Scleritis. Surv Ophthalmol. 2005;50:351–63. doi: 10.1016/j.survophthal.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Kahloun R, Attia S, Jelliti B, Attia AZ, Khochtali S, Ben Yahia S, et al. Ocular involvement and visual outcome of herpes zoster ophthalmicus: Review of 45 patients from Tunisia, North Africa. J Ophthalmic Inflamm Infect. 2014;4:25. doi: 10.1186/s12348-014-0025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zaal MJ, Völker-Dieben HJ, D’Amaro J. Prognostic value of Hutchinson's sign in acute herpes zoster ophthalmicus. Graefe's Arch. Clin Exp Ophthalmol. 2003;241:187–91. doi: 10.1007/s00417-002-0609-1. [DOI] [PubMed] [Google Scholar]
- 36.De Schryver I, Rozenberg F, Cassoux N, Michelson S, Kestelyn P, Lehoang P, et al. Diagnosis and treatment of cytomegalovirus iridocyclitis without retinal necrosis. Br J Ophthalmol. 2006;90:852–55. doi: 10.1136/bjo.2005.086546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Markomichelakis NN, Canakis C, Zafirakis P, Marakis T, Mallias I, Theodossiadis G. Cytomegalovirus as a cause of anterior uveitis with sectoral iris atrophy. Ophthalmology. 2002;109:879–82. doi: 10.1016/s0161-6420(02)00961-2. [DOI] [PubMed] [Google Scholar]
- 38.Accorinti M, Gilardi M, Pirraglia MP, Amorelli GM, Nardella C, Abicca I, et al. Cytomegalovirus anterior uveitis: Long-term follow-up of immunocompetent patients. Graefes Arch Clin Exp Ophthalmol. 2014;252:1817–24. doi: 10.1007/s00417-014-2782-4. [DOI] [PubMed] [Google Scholar]
- 39.Woo JH, Lim WK, Ho SL, Teoh SC. Characteristics of cytomegalovirus uveitis in immunocompetent patients. Ocul Immunol Inflamm. 2015;23:378–83. doi: 10.3109/09273948.2014.950384. [DOI] [PubMed] [Google Scholar]
- 40.Chan NS, Chee SP, Caspers L, Bodaghi B. Clinical features of CMV-Associated anterior uveitis. Ocul Immunol Inflamm. 2018;26:107–15. doi: 10.1080/09273948.2017.1394471. [DOI] [PubMed] [Google Scholar]
- 41.La Distia Nora R, Putera I, Mayasari YD, Hikmahwati W, Pertiwi AM, Ridwan AS, et al. Clinical characteristics and treatment outcomes of cytomegalovirus anterior uveitis and endotheliitis: A systematic review and meta-analysis. Surv Ophthalmol. 2022;67:1014–30. doi: 10.1016/j.survophthal.2021.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Chee SP, Bacsal K, Jap A, Se-Thoe SY, Cheng CL, Tan BH. Clinical features of cytomegalovirus anterior uveitis in immunocompetent patients. Am J Ophthalmol. 2008;145:834–40. doi: 10.1016/j.ajo.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 43.Posner A, Schlossman A. Syndrome of unilateral recurrent attacks of glaucoma with cyclitic symptoms. Arch Ophthal. 1948;39:517–35. doi: 10.1001/archopht.1948.00900020525007. [DOI] [PubMed] [Google Scholar]
- 44.Megaw R, Agarwal PK. Posner-Schlossman syndrome. Surv Ophthalmol. 2017;62:277–85. doi: 10.1016/j.survophthal.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Murata K, Ishida K, Ozawa K, Sawada A, Mochizuki K, Yamamoto T. The characteristics of Posner-Schlossman syndrome: A comparison in the surgical outcome between cytomegalovirus-positive and cytomegalovirus-negative patients. Medicine (Baltimore) 2019;98:e18123. doi: 10.1097/MD.0000000000018123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hwang YS, Shen CR, Chang SHL, Lai CC, Liu CL, Chen KJ, et al. The validity of clinical feature profiles for cytomegaloviral anterior segment infection. Graefes Arch Clin Exp Ophthalmol. 2011;249:103–10. doi: 10.1007/s00417-010-1510-y. [DOI] [PubMed] [Google Scholar]
- 47.Daicker B. Cytomegalovirus panuveitis with infection of corneo-trabecular endothelium in AIDS. Ophthalmologica. 1988;197:169–75. doi: 10.1159/000309939. [DOI] [PubMed] [Google Scholar]
- 48.Jap A, Chee SP. Viral anterior uveitis. Curr Opin Ophthalmol. 2011;22:483–8. doi: 10.1097/ICU.0b013e32834be021. [DOI] [PubMed] [Google Scholar]
- 49.Antoun J, Willermain F, Makhoul D, Motulsky E, Caspers L, Relvas LJ. Topical ganciclovir in cytomegalovirus anterior uveitis. J Ocul Pharmacol Ther. 2017;33:313–8. doi: 10.1089/jop.2016.0138. [DOI] [PubMed] [Google Scholar]
- 50.Terada Y, Kaburaki T, Takase H, Goto H, Nakano S, Inoue Y, et al. Distinguishing features of anterior uveitis caused by herpes simplex virus, varicella-zoster virus, and cytomegalovirus. Am J Ophthalmol. 2021;227:191–200. doi: 10.1016/j.ajo.2021.03.020. [DOI] [PubMed] [Google Scholar]
- 51.Shirahama S, Kaburaki T, Takada S, Nakahara H, Tanaka R, Komae K, et al. Comparison of visual field defect progression in secondary Glaucoma due to anterior uveitis caused by three types of herpes viruses. Graefes Arch Clin Exp Ophthalmol. 2020;258:639–45. doi: 10.1007/s00417-019-04559-w. [DOI] [PubMed] [Google Scholar]
- 52.Miyanaga M, Sugita S, Shimizu N, Morio T, Miyata K, Maruyama K, et al. A significant association of viral loads with corneal endothelial cell damage in cytomegalovirus anterior uveitis. Br J Ophthalmol. 2010;94:336–40. doi: 10.1136/bjo.2008.156422. [DOI] [PubMed] [Google Scholar]
- 53.Kandori M, Miyazaki D, Yakura K, Komatsu N, Touge C, Ishikura R, et al. Relationship between the number of cytomegalovirus in anterior chamber and severity of anterior segment inflammation. Jpn J Ophthalmol. 2013;57:497–502. doi: 10.1007/s10384-013-0268-2. [DOI] [PubMed] [Google Scholar]
- 54.Choi JA, Kim KS, Jung Y, Park HY, Park CK. Cytomegalovirus as a cause of hypertensive anterior uveitis in immunocompetent patients. J Ophthalmic Inflamm Infect. 2016;6:32. doi: 10.1186/s12348-016-0100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chee SP, Bacsal K, Jap A, Se-Thoe SY, Cheng CL, Tan BH. Corneal endotheliitis associated with evidence of cytomegalovirus infection. Ophthalmology. 2007;114:798–803. doi: 10.1016/j.ophtha.2006.07.057. [DOI] [PubMed] [Google Scholar]
- 56.Chee SP, Jap A. Cytomegalovirus anterior uveitis: Outcome of treatment. Br J Ophthalmol. 2010;94:1648–52. doi: 10.1136/bjo.2009.167767. [DOI] [PubMed] [Google Scholar]
- 57.Fuchs E. Komplicationen der Heterochromie. Zeitschrift Augenheilkd. 1906;1906:191–212. [Google Scholar]
- 58.Liu Y, Takusagawa HL, Chen TC, Pasquale LR. Fuchs heterochromic iridocyclitis and the rubella virus. Int Ophthalmol Clin. 2011;51:1–12. doi: 10.1097/IIO.0b013e31822d6923. [DOI] [PubMed] [Google Scholar]
- 59.Quentin CD, Reiber H. Fuchs heterochromic cyclitis: Rubella virus antibodies and genome in aqueous humor. Am J Ophthalmol. 2004;138:46–54. doi: 10.1016/j.ajo.2004.02.055. [DOI] [PubMed] [Google Scholar]
- 60.De Groot-Mijnes JD, De Visser L, Rothova A, Schuller M, Van Loon AM, Weersink AJ. Rubella virus is associated with Fuchs heterochromic iridocyclitis. Am J Ophthalmol. 2006;141:212–5. doi: 10.1016/j.ajo.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 61.Stunf S, Petrovec M, Žigon N, Hawlina M, Kraut A, de Groot-Mijnes JD, et al. High concordance of intraocular antibody synthesis against the rubella virus and Fuchs heterochromic uveitis syndrome in Slovenia. Mol Vis. 2012;18:2909–14. [PMC free article] [PubMed] [Google Scholar]
- 62.Suzuki J, Goto H, Komase K, Abo H, Fujii K, Otsuki N, et al. Rubella virus as a possible etiological agent of Fuchs heterochromic iridocyclitis. Graefes Arch Clin Exp Ophthalmol. 2010;248:1487–91. doi: 10.1007/s00417-010-1434-6. [DOI] [PubMed] [Google Scholar]
- 63.Ruokonen PC, Metzner S, Ücer A, Torun N, Hofmann J, Pleyer U. Intraocular antibody synthesis against rubella virus and other microorganisms in Fuchs’ heterochromic cyclitis. Graefes Arch Clin Exp Ophthalmol. 2010;248:565–71. doi: 10.1007/s00417-009-1239-7. [DOI] [PubMed] [Google Scholar]
- 64.Birnbaum AD, Tessler HH, Schultz KL, Farber MD, Gao W, Lin P, et al. Epidemiologic relationship between fuchs heterochromic iridocyclitis and the United States rubella vaccination program. Am J Ophthalmol. 2007;144:424–8. doi: 10.1016/j.ajo.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 65.Sun Y, Ji Y. A literature review on Fuchs uveitis syndrome: An update. Surv Ophthalmol. 2020;65:133–43. doi: 10.1016/j.survophthal.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 66.Cimino L, Aldigeri R, Salvarani C, Zotti CA, Boiardi L, Parmeggiani M, et al. The causes of uveitis in a referral centre of Northern Italy. Int Ophthalmol. 2010;30:521–9. doi: 10.1007/s10792-010-9359-y. [DOI] [PubMed] [Google Scholar]
- 67.Wakabayashi T, Morimura Y, Miyamoto Y, Okada AA. Changing patterns of intraocular inflammatory disease in Japan. Ocul Immunol Inflamm. 2003;11:277–86. doi: 10.1076/ocii.11.4.277.18260. [DOI] [PubMed] [Google Scholar]
- 68.Tsirouki T, Dastiridou A, Symeonidis C, Tounakaki O, Brazitikou I, Kalogeropoulos C, et al. A focus on the epidemiology of uveitis. Ocul Immunol Inflamm. 2018;26:2–16. doi: 10.1080/09273948.2016.1196713. [DOI] [PubMed] [Google Scholar]
- 69.Chee SP, Jap A. Presumed fuchs heterochromic iridocyclitis and Posner-Schlossman syndrome: comparison of cytomegalovirus-positive and negative eyes. Am J Ophthalmol. 2008;146:883–9.e1. doi: 10.1016/j.ajo.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 70.Tugal-Tutkun I, Güney-Tefekli E, Kamaci-Duman F, Corum I. A cross-sectional and longitudinal study of fuchs uveitis syndrome in Turkish patients. Am J Ophthalmol. 2009;148:510–5.e1. doi: 10.1016/j.ajo.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 71.Yang P, Fang W, Jin H, Li B, Chen X, Kijlstra A. Clinical features of Chinese patients with Fuchs’ syndrome. Ophthalmology. 2006;113:473–80. doi: 10.1016/j.ophtha.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 72.Accorinti M, Spinucci G, Pirraglia MP, Bruschi S, Pesci FR, Iannetti L. Fuchs’ heterochromic iridocyclitis in an Italian tertiary referral centre: Epidemiology, clinical features, and prognosis. J Ophthalmol 2016. 2016 doi: 10.1155/2016/1458624. 1458624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Norrsell K, Sjödell L. Fuchs’ heterochromic uveitis: A longitudinal clinical study. Acta Ophthalmol. 2008;86:58–64. doi: 10.1111/j.1600-0420.2007.00990.x. [DOI] [PubMed] [Google Scholar]
- 74.Chan NS, Chee SP. Demystifying viral anterior uveitis: A review. Clin Exp Ophthalmol. 2019;47:320–33. doi: 10.1111/ceo.13417. [DOI] [PubMed] [Google Scholar]
- 75.Invernizzi A, Cigada M, Savoldi L, Cavuto S, Fontana L, Cimino L. In vivo analysis of the iris thickness by spectral domain optical coherence tomography. Br J Ophthalmol. 2014;98:1245–9. doi: 10.1136/bjophthalmol-2013-304481. [DOI] [PubMed] [Google Scholar]
- 76.Rothova A, La Hey E, Baarsma GS, Breebaart AC. Iris nodules in Fuchs’ heterochromic uveitis. Am J Ophthalmol. 1994;118:338–42. doi: 10.1016/s0002-9394(14)72958-7. [DOI] [PubMed] [Google Scholar]
- 77.Callear AB, Murray PI, Reynolds A, Harry J. Iris crystals in chronic uveitis. Investig Ophthalmol Vis Sci. 1997;38:703–6. doi: 10.1136/bjo.83.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Amsler M, Verrey E. Heterochromie de Fuchs et fragilitè vasculaire. Ophthalmologica. 1946;111:177–83. doi: 10.1159/000300320. [DOI] [PubMed] [Google Scholar]
- 79.Mohamed Q, Zamir E. Update on Fuchs’ uveitis syndrome. Curr Opin Ophthalmol. 2005;16:356–63. doi: 10.1097/01.icu.0000187056.29563.8d. [DOI] [PubMed] [Google Scholar]
- 80.Özdamar Erol Y, İnanç M, Özdal P. Fuchs’ uveitis: Is it different from what we know? Ocul Immunol Inflamm. 2022;30:62–7. doi: 10.1080/09273948.2020.1795207. [DOI] [PubMed] [Google Scholar]
- 81.Groen-Hakan F, van de Laar S, van der Eijk-Baltissen AA, ten Dam-van Loon N, de Boer J, Rothova A. Clinical manifestations, prognosis, and vaccination status of patients with rubella virus-associated uveitis. Am J Ophthalmol. 2019;202:37–46. doi: 10.1016/j.ajo.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 82.Standardization of Uveitis Nomenclature (SUN) Working Group. Classification criteria for fuchs uveitis syndrome. Am J Ophthalmol. 2021;228:262–7. doi: 10.1016/j.ajo.2021.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cimino L, Aldigeri R, Parmeggiani M, Belloni L, Zotti CA, Fontana L, et al. Searching for viral antibodies and genome in intraocular fluids of patients with Fuchs uveitis and non-infectious uveitis. Graefes Arch Clin Exp Ophthalmol. 2013;251:1607–12. doi: 10.1007/s00417-013-2287-6. [DOI] [PubMed] [Google Scholar]
- 84.Schwab IR. The epidemiologic association of Fuchs’ heterochromic iridocyclitis and ocular toxoplasmosis. Am J Ophthalmol. 1991;111:356–62. doi: 10.1016/s0002-9394(14)72322-0. [DOI] [PubMed] [Google Scholar]
- 85.Bouchenaki N, Herbort CP. Fluorescein angiographic findings and clinical features in Fuchs’ uveitis. Int Ophthalmol. 2010;30:511–9. doi: 10.1007/s10792-010-9366-z. [DOI] [PubMed] [Google Scholar]
- 86.Cerquaglia A, Iaccheri B, Fiore T, Lupidi M, Torroni G, Fruttini D, et al. Full-thickness choroidal thinning as a feature of Fuchs Uveitis Syndrome: Quantitative evaluation of the choroid by Enhanced Depth Imaging Optical Coherence Tomography in a cohort of consecutive patients. Graefes Arch Clin Exp Ophthalmol. 2016;254:2025–31. doi: 10.1007/s00417-016-3475-y. [DOI] [PubMed] [Google Scholar]
- 87.Nilforushan N, Yadgari M, Alemzadeh SA. Surgical management of glaucoma in Fuchs uveitis syndrome: Trabeculectomy or Ahmed glaucoma valve. J Curr Ophthalmol. 2019;31:24–30. doi: 10.1016/j.joco.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sugita S, Ogawa M, Shimizu N, Morio T, Ohguro N, Nakai K, et al. Use of a comprehensive polymerase chain reaction system for diagnosis of ocular infectious diseases. Ophthalmology. 2013;120:1761–8. doi: 10.1016/j.ophtha.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 89.Anwar Z, Galor A, Albini TA, Miller D, Perez V, Davis JL. The diagnostic utility of anterior chamber paracentesis with polymerase chain reaction in anterior uveitis. Am J Ophthalmol. 2013;155:781–6. doi: 10.1016/j.ajo.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 90.De Groot-Mijnes JD, Rothova A, Van Loon AM, Schuller M, Ten Dam-Van Loon NH, De Boer JH, et al. Polymerase chain reaction and goldmann-witmer coefficient analysis are complimentary for the diagnosis of infectious uveitis. Am J Ophthalmol. 2006;141:313–8. doi: 10.1016/j.ajo.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 91.Reiber H, Kruse-Sauter H, Quentin CD. Antibody patterns vary arbitrarily between cerebrospinal fluid and aqueous humor of the individual multiple sclerosis patient: Specificity-independent pathological B cell function. J Neuroimmunol. 2015;278:247–54. doi: 10.1016/j.jneuroim.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 92.Liekfeld A, Schweig F, Jaeckel C, Wernecke KD, Hartmann C, Pleyer U. Intraocular antibody production in intraocular inflammation. Graefes Arch Clin Exp Ophthalmol. 2000;238:222–7. doi: 10.1007/s004170050347. [DOI] [PubMed] [Google Scholar]
- 93.Groen-Hakan F, Babu K, Tugal-Tutkun I, Pathanapithoon K, de Boer JH, Smith JR, et al. Challenges of diagnosing viral anterior uveitis. Ocul Immunol Inflamm. 2017;25:710–20. doi: 10.1080/09273948.2017.1353105. [DOI] [PubMed] [Google Scholar]
- 94.Reiber H. Proteins in cerebrospinal fluid and blood: Barriers, CSF flow rate and source-related dynamics. Restor Neurol Neurosci. 2003;21:79–96. [PubMed] [Google Scholar]
- 95.Asgari M, de Zélicourt DA, Kurtcuoglu V. Barrier dysfunction or drainage reduction: Differentiating causes of CSF protein increase. Fluids Barriers CNS. 2017;14:14. doi: 10.1186/s12987-017-0063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.De Simone L, Belloni L, Aldigeri R, Zerbini A, Mastrofilippo V, Sangiovanni A, et al. Aqueous tap and rapid diagnosis of cytomegalovirus anterior uveitis: The Reggio Emilia experience. Graefes Arch Clin Exp Ophthalmol. 2019;257:181–6. doi: 10.1007/s00417-018-4180-9. [DOI] [PubMed] [Google Scholar]
- 97.Errera MH, Goldschmidt P, Batellier L, Degorge S, Héron E, Laroche L, et al. Real-time polymerase chain reaction and intraocular antibody production for the diagnosis of viral versus toxoplasmic infectious posterior uveitis. Graefes Arch Clin Exp Ophthalmol. 2011;249:1837–46. doi: 10.1007/s00417-011-1724-7. [DOI] [PubMed] [Google Scholar]
- 98.Westeneng AC, Rothova A, de Boer JH, de Groot-Mijnes JD. Infectious uveitis in immunocompromised patients and the diagnostic value of polymerase chain reaction and goldmann-witmer coefficient in aqueous analysis. Am J Ophthalmol. 2007;144:781–5. doi: 10.1016/j.ajo.2007.06.034. [DOI] [PubMed] [Google Scholar]