Abstract
Background:
Metabolic syndrome (MetS), a major contributor to cardiovascular and metabolic diseases, has been linked with exposure to air pollution. However, the relationship between air pollutants and the five components of MetS [abdominal obesity, elevated triglyceride, decreased high-density lipoprotein cholesterol (HDL-C), elevated blood pressure, and elevated fasting blood glucose levels], has not been clearly described.
Objective:
We examined the association between long-term exposure to air pollutants and the occurrence of MetS and its components by using a longitudinal cohort in Taiwan.
Methods:
The MJ Health Research Foundation is a medical institute that conducts regular physical examinations. The development of MetS, based on a health examination and the medical history of an MJ cohort of 93,771 participants who were enrolled between 2006 and 2016 and had two or more examinations, was compared with estimated exposure to air pollutants in the year prior to health examination. The exposure levels to fine particulate matter [PM with an aerodynamic diameter of ()] and nitrogen dioxide () in the participants’ residential areas were estimated using a hybrid Kriging/land-use regression (LUR) model executed using the XGBoost algorithm and a hybrid Kriging/LUR model, respectively. Cox regression with time-dependent covariates was conducted to estimate the effects of annual air pollutant exposure on the risk of MetS and its components.
Results:
During the average follow-up period of 3.4 y, the incidence of MetS was 38.1/1,000 person-years. After mutual adjustment and adjustments for potential covariates, the results indicated that every increase in annual concentration was associated with an increased risk of abdominal obesity [; 95% confidence interval (CI): 1.01, 1.14], hypertriglyceridemia (; 95% CI: 1.11, 1.23), low HDL-C (; 95% CI: 1.02, 1.17), hypertension (; 95% CI: 1.09, 1.21), and elevated fasting blood glucose (; 95% CI: 1.10, 1.20). Furthermore, and may increase the risk of developing MetS among people who already “have” some components of MetS.
Discussion:
Our findings suggest that in apparently healthy adults undergoing physical examination, exposure to and might be associated with the occurrence of MetS and its components. https://doi.org/10.1289/EHP10611
Introduction
In the 2017 Global Burden of Disease Study, cardiovascular disease (CVD) and type 2 diabetes mellitus (T2DM) accounted for 31.8% () and 1.8% (), respectively, of all-cause global deaths for that year.1 Metabolic syndrome (MetS), a cluster of modifiable components—namely abdominal obesity, insulin resistance, dyslipidemia, and elevated blood pressure (BP)—is regarded as an indicator of CVD and T2DM development and as a contributor to all-cause mortality.2,3 Therefore, the identification of MetS can help in the prevention of the onset of the aforementioned diseases.
Traditional risk factors for MetS include older age, the male sex, low socioeconomic status, and poor lifestyle habits.2 In addition, increasing bodies of epidemiological evidence from Asia,4–6 Europe,7–9 and the United States10,11 demonstrate that ambient air pollution may contribute to an increased risk of MetS and its components. A meta-analysis of cohort studies revealed that every annual increment of fine particulate matter [PM with an aerodynamic diameter of ()] was associated with a 4% higher risk of MetS.12 Overall, the population-attributable risk of MetS associated with long-term exposure was estimated to be 12.28%.12
To control air pollution and prevent its destructive effect on health, legislation has been promulgated in several countries, including in the United States,13 the European Union member states,14 China,15 and Taiwan.16 Notably, a quasi-experimental study revealed that the adverse effects of on dyslipidemia was mitigated after the implementation of the Air Pollution Prevention and Control Action Plan in China.17
However, longitudinal studies evaluating the effects of gradually decreasing concentrations of ambient air pollutants are limited. Studies using a time-dependent Cox regression analysis revealed that exposure was associated with a higher risk of MetS and its components.11,18 Similarly, traffic-related air pollutants were reported to be associated with MetS-related outcomes, such as T2DM.19 Therefore, exploring the long-term health effects of nitrogen dioxide (), an indicator of traffic-related emissions,20 is also valuable. Accordingly, the purpose of this study was to execute a time-dependent Cox regression analysis to assess the effects of long-term exposure to and on the incidence of MetS and its components in a cohort selected from the MJ Health Research Foundation.
Methods
Study Population
The study population was a cohort selected from the database of the MJ Health Research Foundation in Taiwan, which has been built up as a cohort to collect individuals’ characteristics, life style, and health status through a health screening program to help researchers investigate the relationships between chronic diseases and modifiable risk factors.21–23 The MJ Health Database is a longitudinal, population-based health research cohort, comprising data (questionnaire, physical examination, and blood tests) from apparently healthy individuals seeking physical examination services at a private health care firm in Taiwan (MJ Health Management Institution).22 Individuals were enrolled beginning in 1994 and data is collected on an ongoing basis with, at present, no termination date. Many participants contributed data from multiple visits. At each visit, behavioral and lifestyle information was obtained via questionnaire, and anthropometric and biological data were obtained via the physical examination. Informed consent was collected before physical examination, and only health data with authorization from the MJ participants were available for research purposes.23 The study protocol was approved by the research ethics committee of the National Taiwan University Hospital (No. 202002093RIND).
Approximately half of the MJ participants paid for themselves or family members to have the health examination, and the other half received the examination as part of their employment benefits. Participants’ records were censored if they did not persistently take the physical examination, or if their companies (for those participating as part of their employment benefits) did not renew the contract with MJ and therefore subsequent data collection was not possible.
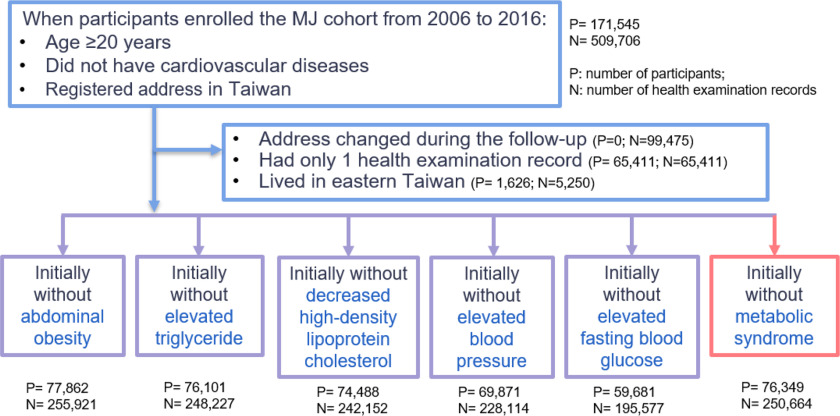
Our selection pool comprised 171,545 participants who provided deidentified health examination records for the period between 2006 and 2016, were of age at the time of enrollment, did not have CVD, and had a registered address in Taiwan. To observe the occurrence of MetS and its components, we excluded 65,411 participants who took only one health examination for inappropriateness to the follow-up study design. Furthermore, because air quality monitoring stations are not as dense in eastern Taiwan as they are in the western regions of the island, 1,626 participants who lived in the east ( of the cohort) were excluded. Only the air pollutants of the participant’s first address were included for the analysis to avoid potential mixing of early and delayed effects. Thus, for participants who changed address, health records before they moved were used for analysis. After excluding records after a changed address, those who had only one health examination record, or those who lived in eastern Taiwan, we categorized the remaining participants into six groups according to their baseline condition as follows: a) without abdominal obesity, b) with normal triglyceride (TG) levels, c) with normal high-density lipoprotein cholesterol (HDL-C) levels, d) with normal BP levels, e) with normal fasting blood glucose (FBG) levels, and f) without MetS. Criteria for each of these groups is described in the section “Definition of MetS and Its Components.” Thus, the final cohort comprised 77,862, 76,101, 74,488, 69,871, 59,681, and 76,349 participants in the above six groups. Such categorization allowed the participants to be included in more than one group (Table S1). Figure 1 illustrates the flowchart of the data selection process.
Figure 1.
Flowchart of data selection from the MJ database from 2006 to 2016. The MJ database is a longitudinal, population-based health research cohort, comprising data (questionnaire, physical examination, and blood tests) from apparently healthy individuals seeking physical examination services at a private health care firm in Taiwan (MJ Health Management Institution).22 Many participants contributed data from multiple visits. At each visit, behavioral and lifestyle information was obtained via questionnaire, and anthropometric and biological data were obtained via the physical examination.23 Only the air pollutants of the participant’s first address were included for the analysis to avoid potential mixing of early and delayed effects. For participants who changed address, only health records before they moved were used for analysis. Therefore, in the first exclusion process, despite 99,475 records being removed, no participant was removed.
Exposure Assessment
According to the “Air Quality Annual Report of R.O.C. (Taiwan), 2015,”24 yearly distributions of and gradually decreased from 33.5 to and from , respectively, from 2006 to 2016. For , the southwestern region is situated on the leeside of the mountains and under the effect of monsoonal flow, where ambient PM combines with local anthropogenic emissions from industries, and consequently, concentrations increase from the northern to southern part of western Taiwan.25 For , primary emission from vehicles, there were registered motor vehicles in Taiwan, 39.6%, 27.2%, 30.6%, and 2.6% in northern, central, southern, and eastern Taiwan, respectively.26 Compared with similar areas in these four regions, concentrations were higher in northern and southern regions, followed by central and eastern regions (Table S2). The regions correspond to administrative units and are considered to have slight cultural differences. Roughly, the southern region is tropical, including five cities/counties, and the central and northern are subtropical, including five and six cities/counties, respectively. Western Taiwan is essentially the combination of the northern, central, and southern regions (Figure S1).
The average annual concentrations of and for the year prior to the health examination were recorded at the township level according to each participant’s address and used as surrogates for long-term exposure. The original daily concentrations of pollutants were measured continuously and reported hourly— by the attenuation method and tapered element oscillating microbalance technology, and by chemiluminescence24—from 73 fixed air quality monitoring stations, included 68 from western and 5 from eastern Taiwan.27 Given that residents of the eastern coast represented a very small (0.6%) proportion of MJ examinees and that the monitoring stations were not as dense in eastern Taiwan, the decision was made to exclude eastern coast participants. Estimations of and from 2005 to 2015 were modeled from the 68 monitoring stations from the western coast of Taiwan. Modeling was performed as previously described.27,28 Daily concentrations of and at monitoring stations were obtained from the Taiwan Environmental Protection Administration air quality database and were then aggregated into annual averages for modeling. Interpolated and values were generated via a leave-one-out ordinary Kriging model, as explanatory variables in the stepwise land-use regression (LUR).27,28 For , predictors included geospatial variables [i.e., distance to the nearest airport, forest, farmland, and Normalized Difference Vegetation Index (NDVI) within circular buffers], meteorological variables (i.e., temperature, relative humidity, wind speed, wind direction, precipitation, and ultraviolet index), and copollutants [i.e., sulfur dioxide (), ozone (), and ]. At a grid resolution, the hybrid Kriging/LUR with the XGBoost algorithm model showed the adjusted of 10-fold cross-validation was 0.93.27 For , predictors included geospatial variables (i.e., agriculture, forest, transportation, water, building, public facilities, recreation, mining or salt production, industrial parks, incinerator chimneys and powerplants, Chinese restaurants, temples, funeral facilities, crematoria, and NDVI), meteorological variable (i.e., temperature), and copollutants [i.e., , , and PM with an aerodynamic diameter of ()]. The developed hybrid Kriging/LUR model with a grid resolution showed the adjusted of 10-fold cross-validation was 0.88.28
Definition of MetS and Its Components
The definition of MetS applied in this study was based on a joint scientific statement from the International Diabetes Federation and the American Heart Association/National Heart, Lung, and Blood Institute.29 The criteria for the clinical diagnosis of MetS were the presence of three or more of the following components for Asian individuals: a) having a waist circumference of in men and in women, b) having a TG level of or receiving drug treatment for hypertriglyceridemia, c) having an HDL-C level of () in males and () in females or receiving drug treatment for decreased HDL-C, d) having a systolic BP level of or diastolic BP level of or receiving drug treatment for hypertension, and e) having an FBG level of or receiving drug treatment for hyperglycemia.29 The same criteria were used individually to classify change in each component (negative to positive) for the other cohorts as well.
In health examinations by MJ Health Research Foundation, measurements of waist circumference and BP were standardized according to the recommendations by the Health Promotion Administration, Ministry of Health and Welfare, Taiwan. The measurements for FBG, TG, and HDL-C were conducted using a Toshiba C-8000.30
In the present study, time of incident MetS and its components was defined as the first detection of such conditions by examination, which included interview and blood collection. Participants who had moved from their first address, were lost to follow-up (i.e., did not continue to participate in the health examination), or had not developed MetS or its components by 31 December 2016 were regarded as right-censored data in our study.
Covariates
MetS has been associated with age, smoking, heavy carbohydrate intake, and physical inactivity and negatively associated with moderate alcohol intake (women only), education level,2 and marital satisfaction (in women).31 In addition, it has been related to poor sleep quality,32 and either short sleep or long sleep duration.33 We included these variables as covariates for analysis, except for carbohydrate intake which was unavailable in MJ data set. We thus included fried food consumption and processed food consumption as covariates in the present study.
Individual characteristics included as covariates were age, sex, baseline body mass index (BMI), marital status, education level, sleeping time per day, smoking habits, alcohol drinking habits, fried and processed food consumption, and regular exercise. Body weight and height were examined by experienced nurses during examination. BMI was calculated by dividing body weight (in kilograms) by squared height (in meters squared), whereas the other covariates were collected at each health examination via self-administered questionnaire.23 In addition, because of established cultural differences, as well as differences in concentrations of and among northern, central, and southern Taiwan, region of residence was included as a covariate as well.
Marital status was classified as single/divorced/separated/widowed and married/cohabiting; and education level was categorized as junior high school and below, general and vocational high school, college, and master’s degree and above. Sleeping time per day was categorized into the following groups: , 6–8, and . Smoking habits were classified as never smoking/former smoking, secondhand smoke exposure, and frequent smoking/daily smoking; alcohol drinking habits were divided into never drinking/former drinking, occasional drinking, and frequent drinking/daily drinking. Fried and processed food consumption was classified as none, little, or ; 2–3 portions/wk; and . Regular exercise was classified as none, little or weekly; 1–4 h weekly or once per 2–3 d; weekly or daily. Moreover, in sensitivity analyses, age was divided into the following categories: , 45–64, and , and baseline BMI was divided into , 18.5–24, and .
Statistical Analysis
In previous longitudinal studies with lower to higher annual concentrations, the effects of increases in on MetS and its components changed from statistically nonsignificant8,34,35 to significant detrimental.4,36–41 Because the effect of ambient air pollutants on health may have decreased over time as a result of decreasing pollutant concentrations during the follow-up period, we performed a time-dependent Cox regression analysis to estimate the effects of long-term exposure to and on the incidence of abdominal obesity, elevated TG, reduced HDL-C, elevated BP, elevated FBG, and MetS. The terms elevated and reduced refer to values above and below the standard reference range as defined in the “Definition of MetS and Its Components” section. The advantage of a time-dependent Cox regression analysis is that it allows hazard ratios (HRs) to be separated into distinct time windows,42 making it suitable for this study given that concentrations of ambient air pollutants in Taiwan have gradually decreased.24 In the present study, the time-varying average of and are on the yearly scale. Spearman correlation coefficients were used to examine the correlation between and .
The study models were adjusted for the aforementioned covariates, except for baseline BMI, with a time-varying method as well. Missing information on covariates were represented by the previous value available for each participant, contributing an additional of the valid data for analysis.
To assess the robustness of the outcomes, we performed sensitivity analyses, and adjusted for additional covariates. First, we included not only characteristics’ covariates but also baseline waist circumference, TG, HDL-C, BP, and FBG for additional adjustment in each cohort under the consideration that an individual’s baseline status may affect the incidence of MetS and its components. Although some articles included BMI as a covariate in the model,18,43 BMI could be associated with air pollution as well; thus, the model without baseline BMI was examined (Table S3). Second, in previous studies, the effects of noise (which is highly associated with traffic-related nitrogen oxides) on MetS and its components were inconsistent.10,35 In addition, sleeping time has been found to be associated with traffic noise,44 so adjusting for sleeping time with might have been an overadjustment. Considering the lack of traffic noise information, we examined the effects without sleeping time in our study (Table S4). Third, owing to the varied prevalence of MetS across age and sex,2 we performed sensitivity analyses by age and sex stratification. The potential modification effects were examined by adding interaction terms of age, sex, and the air pollutants into the time-dependent Cox regression models.
The area of Taiwan is ,45 with a population of people.46 To illustrate the population density and the distribution of our study participants, Figure 2 was created using the QGIS Desktop software (version 3.22; Open Source Geospatial Foundation). All the statistical analyses were performed using SAS software (version 9.4; SAS Institute Inc.). We considered as indicating statistical significance for a two-tailed test.
Figure 2.
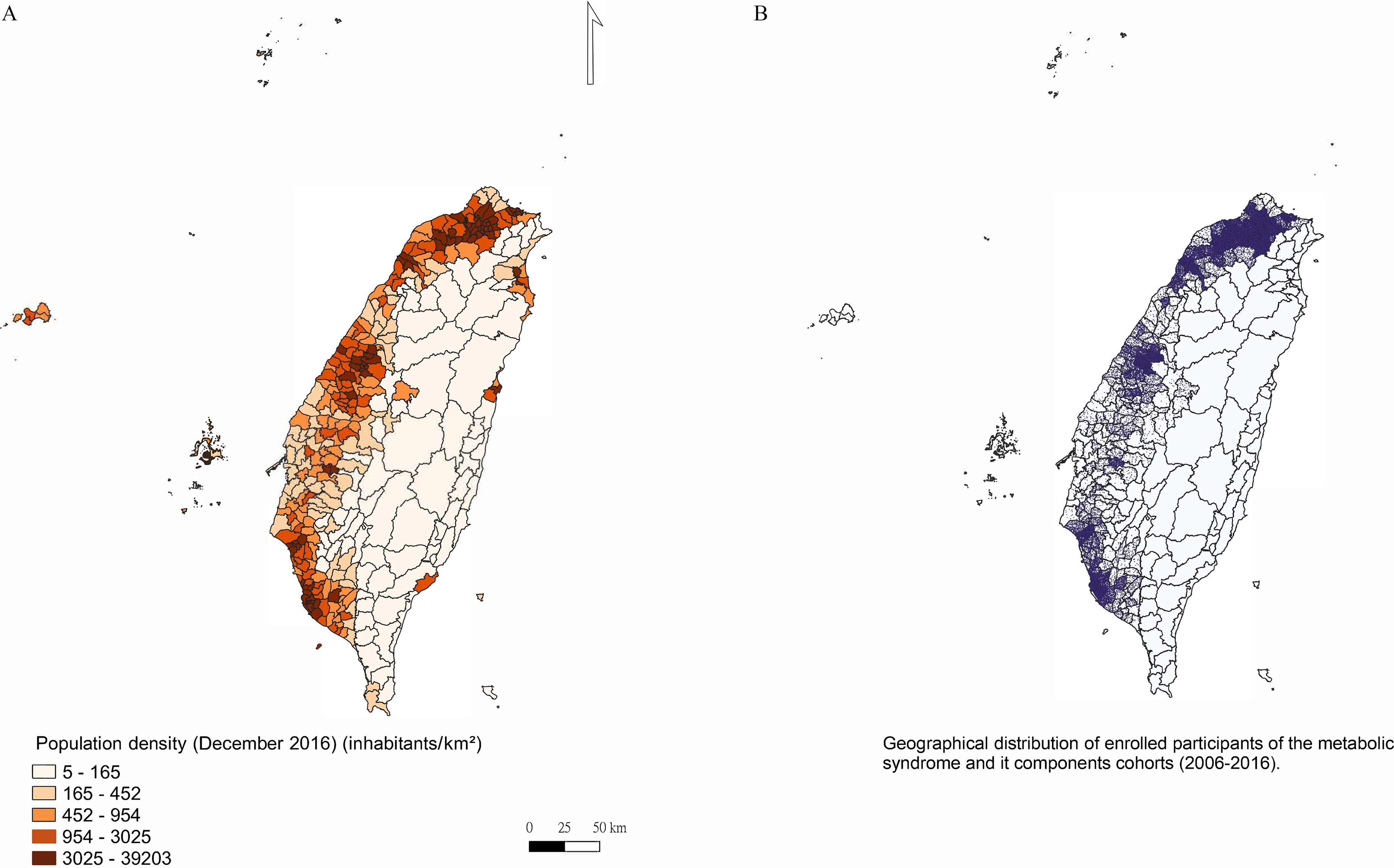
Geographical distributions of population density in Taiwan (), and the participants of the metabolic syndrome and its components cohorts (). The area of Taiwan is ,44 with a population of people.45 (A) Population density. (B) General distribution of enrolled participants; each dot represents one participant. The small, outlined areas represent townships. This figure was created using the QGIS Desktop software (version 3.22; Open Source Geospatial Foundation).
Results
Figure 2 shows the geographical distributions of the overall study participants (), which could be similar to the population density in Taiwan (). Table 1 presents the baseline characteristics of the study population, which was divided into six cohorts: without abdominal obesity, with normal TG, with normal HDL-C, with normal BP, with normal FBG, and without MetS. Most of the participants (66.1%–74.9%) were of age at the time of enrollment, and the male and female distributions in the population were similar. In terms of BMI, 58.0%–65.9% of the participants were within the normal range (). More than 60% of the participants were married or cohabiting and had a college-level education or higher. Approximately 80% of the participants were never smokers or former smokers, and 84.3%–87.1% of them were never alcohol drinkers or former drinkers. Furthermore, of the participants slept 6–8 h/d, and nearly half did no, little or weekly regular exercise. Regarding fried and processed food consumption, and 60% of the participants consumed none and little or portion per week, respectively. Most of the participants (68.7%–71.9%) lived in northern Taiwan. Overall, there were 93,771 participants enrolled in the six cohorts: 5.1% () in only one cohort, and 36.9% () in all six cohorts (Table S1). The annual concentration of was revealed to be mildly correlated with in the six cohorts in whole Taiwan, with Spearman correlation coefficients ranging from 0.249 to 0.291 (all ; Table S5).
Table 1.
Baseline characteristics [ (%) or ] of the MJ Health Database study population between 2006 and 2016, Taiwan.
| Variable | Cohort without abdominal obesity () | Cohort with normal TG () | Cohort with normal HDL-C () |
Cohort with normal BP () | Cohort with normal FBG () | Cohort without MetS () |
|---|---|---|---|---|---|---|
| Already diagnosed with MetS | ||||||
| No | 69,970 (93.6) | 68,633 (93.5) | 67,163 (90.2) | 63,061 (94.1) | 54,903 (96.0) | 76,349 (100) |
| Yes | 4,765 (6.4) | 4,771 (6.5) | 7,266 (9.8) | 3,930 (5.9) | 2,287 (4.0) | 0 (0) |
| Missing | 3,127 | 2,697 | 59 | 2,880 | 2,490 | 0 |
| Enrolled age (y) | ||||||
| 53,821 (69.1) | 52,354 (68.8) | 49,209 (66.1) | 51,388 (73.5) | 44,694 (74.9) | 53,006 (69.4) | |
| 45–64 | 21,206 (27.2) | 20,488 (26.9) | 21,821 (29.3) | 16,963 (24.3) | 13,324 (22.3) | 20,594 (27.0) |
| 2,835 (3.6) | 3,259 (4.3) | 3,458 (4.6) | 1,520 (2.2) | 1,663 (2.8) | 2,749 (3.6) | |
| Sex | ||||||
| Male | 37,055 (47.6) | 33,623 (44.2) | 37,469 (50.3) | 31,064 (44.5) | 25,145 (42.1) | 35,573 (46.6) |
| Female | 40,807 (52.4) | 42,478 (55.8) | 37,019 (49.7) | 38,807 (55.5) | 34,536 (57.9) | 40,766 (53.4) |
| Body mass index (kg/m2) | ||||||
| 6,891 (8.9) | 6,773 (8.9) | 6,130 (8.2) | 6,461 (9.2) | 6,033 (10.1) | 6,518 (8.5) | |
| 18.5–24 | 51,311 (65.9) | 46,206 (60.7) | 43,187 (58.0) | 42,900 (61.4) | 37,041 (62.1) | 47,820 (62.6) |
| 19,643 (25.2) | 23,103 (30.4) | 25,152 (33.8) | 20,504 (29.3) | 16,593 (27.8) | 22,004 (28.8) | |
| Missinga | 17 | 19 | 19 | 6 | 14 | 7 |
| Marital status | ||||||
| Single/divorced/separated/widowed | 26,155 (33.8) | 26,324 (34.8) | 24,659 (33.3) | 24,031 (34.6) | 22,003 (37.1) | 25,670 (33.8) |
| Married/cohabitating | 51,227 (66.2) | 49,307 (65.2) | 49,457 (66.7) | 45,411 (65.4) | 37,315 (62.9) | 50,325 (66.2) |
| Missinga | 480 | 470 | 372 | 429 | 363 | 354 |
| Education level | ||||||
| Junior high school and below | 8,748 (11.3) | 9,373 (12.4) | 9,616 (12.9) | 6,405 (9.2) | 5,736 (9.6) | 8,696 (11.4) |
| General and vocational high school | 14,822 (19.1) | 14,237 (18.8) | 13,809 (18.6) | 13,816 (18.9) | 11,057 (18.6) | 14,312 (18.8) |
| College | 43,004 (55.4) | 41,637 (54.9) | 40,188 (54.1) | 39,950 (57.4) | 34,220 (57.5) | 42,185 (55.4) |
| Master’s degree and above | 11,001 (14.2) | 10,572 (14.0) | 10,682 (14.4) | 10,063 (14.5) | 8,450 (14.2) | 10,975 (14.4) |
| Missinga | 287 | 282 | 193 | 267 | 218 | 181 |
| Smoking habits | ||||||
| Never smoking/former smoking | 60,882 (78.4) | 60,732 (80.1) | 58,107 (78.2) | 54,083 (77.6) | 46,807 (78.7) | 60,056 (78.8) |
| Secondhand smoke exposure | 3,290 (4.2) | 3,411 (4.5) | 3,196 (4.3) | 3,022 (4.3) | 2,623 (4.4) | 3,294 (4.3) |
| Frequent smoking/daily smoking | 13,447 (17.3) | 11,722 (15.5) | 13,035 (17.5) | 12,545 (18.0) | 10,060 (16.9) | 12,857 (16.9) |
| Missinga | 243 | 236 | 150 | 221 | 191 | 142 |
| Alcohol drinking habitsb | ||||||
| Never drinking/former drinking | 66,801 (86.3) | 66,154 (87.4) | 62,822 (84.7) | 60,452 (87.0) | 51,990 (87.6) | 65,718 (86.4) |
| Occasional drinking | 7,316 (9.4) | 6,750 (8.9) | 7,667 (10.3) | 6,484 (9.3) | 5,231 (8.8) | 7,213 (9.5) |
| Frequent drinking/daily drinking | 3,322 (4.3) | 2,774 (3.7) | 3,677 (5.0) | 2,553 (3.7) | 2,148 (3.6) | 3,108 (4.1) |
| Missinga | 423 | 423 | 322 | 382 | 312 | 310 |
| Sleeping time per day (h) | ||||||
| 16,751 (21.6) | 16,946 (22.3) | 16,706 (22.5) | 15,027 (21.6) | 12,939 (21.8) | 16,713 (21.9) | |
| 6–8 | 56,114 (72.3) | 54,328 (71.6) | 53,112 (71.5) | 50,300 (72.2) | 42,810 (72.0) | 54,801 (71.9) |
| 4,731 (6.1) | 4,560 (6.0) | 4,491 (6.0) | 4,298 (6.2) | 3,733 (6.3) | 4,647 (6.1) | |
| Missinga | 266 | 267 | 179 | 246 | 199 | 161 |
| Regular exercise | ||||||
| None, little or weekly | 35,252 (49.6) | 34,312 (49.7) | 33,498 (49.3) | 33,239 (52.1) | 28,042 (51.4) | 34,779 (49.9) |
| 1–4 h weekly or once per 2–3 d | 26,581 (37.4) | 25,678 (37.2) | 24,962 (36.8) | 23,412 (36.7) | 20,249 (37.1) | 26,001 (37.3) |
| weekly or daily | 9,195 (12.9) | 9,047 (13.1) | 9,428 (13.9) | 7,126 (11.2) | 6,219 (11.4) | 8,985 (12.9) |
| Missinga | 6,834 | 7,064 | 6,600 | 6,094 | 5,171 | 6,584 |
| Fried food consumption per week (portions) | ||||||
| None, little or | 22,525 (29.0) | 21,849 (28.8) | 21,370 (28.8) | 19,233 (27.6) | 16,376 (27.5) | 21,904 (28.8) |
| 2–3 | 40,811 (52.6) | 39,706 (52.4) | 38,849 (52.3) | 36,876 (53.0) | 31,493 (52.9) | 40,015 (52.5) |
| 14,252 (18.4) | 14,267 (18.8) | 14,072 (18.9) | 13,504 (19.4) | 11,610 (19.5) | 14,251 (18.7) | |
| Missinga | 274 | 279 | 197 | 258 | 202 | 179 |
| Processed food consumption per week (portions) | ||||||
| None, little or | 48,818 (62.9) | 47,402 (62.5) | 45,996 (61.9) | 43,233 (62.1) | 36,854 (62.0) | 47,669 (62.6) |
| 2–3 | 25,588 (33.0) | 25,255 (33.3) | 25,064 (33.7) | 23,411 (33.6) | 20,093 (33.8) | 25,364 (33.3) |
| 3,175 (4.1) | 3,161 (4.2) | 3,226 (4.3) | 2,964 (4.3) | 2,529 (4.3) | 3,150 (4.1) | |
| Missinga | 281 | 283 | 202 | 263 | 205 | 184 |
| Region | ||||||
| Northc | 54,142 (69.5) | 54,215 (71.2) | 51,167 (68.7) | 50,234 (71.9) | 41,235 (69.1) | 53,446 (70.0) |
| Centrald | 10,178 (13.1) | 9,553 (12.6) | 10,352 (13.9) | 8,868 (12.7) | 8,722 (14.6) | 10,153 (13.3) |
| Southe | 13,542 (17.4) | 12,333 (16.2) | 12,969 (17.4) | 10,769 (15.4) | 9,724 (16.3) | 12,750 (16.7) |
Note: The criteria for the clinical diagnosis of MetS were the presence of any three or more of the following components for Asian individuals a) having a waist circumference of in men and in women, b) having a TG level of or receiving drug treatment for hypertriglyceridemia, c) having an HDL-C level of () in males and () in females or receiving drug treatment for decreased HDL-C, d) having a systolic BP level of or diastolic BP level of or receiving drug treatment for hypertension, and e) having an FBG level of or receiving drug treatment for hyperglycemia.29 The same criteria were used individually to classify change in each component (event to happen) for the other cohorts as well. BP, blood pressure; FBG, fasting blood glucose; HDL-C, high-density lipoprotein cholesterol; MetS, metabolic syndrome; SD, standard deviation; TG, triglyceride.
Missing information on covariates were initially represented by the previous value available of each participant. Participants without available value for representation were not eligible for data analysis depending on the covariates included in the models in Table 3 and Tables S3 and S4.
Never drinking/former drinking: teetotaler, abstainer, or drank less than once weekly; occasional drinking: drank once or twice weekly; frequent drinking/daily drinking: drank more than three times weekly.
The northern region included Taipei, New Taipei, Keelung, Hsinchu, and Taoyuan Cities and Hsinchu County.
The central region included Taichung City and Miaoli, Changhua, Nantou, and Yunlin Counties.
The southern region included Kaohsiung, Tainan, and Chiayi Cities and Chiayi and Pingtung Counties.
Table 2 presents the results obtained for the three regions (northern, central, and southern Taiwan). Participants living in the southern region had the highest annual exposure concentration of , and those in the northern and southern regions had the highest exposure concentration of . During the exposure period of 2005 to 2015, the data shows a gradual decreasing trend, with fluctuation in the annual average concentrations of and (Figure S2; the complete data set is included as Excel Table S1). Over the period of 2005–2015, based on the residences of the participants in the cohorts of MetS and its components, decreased by and decreased by , respectively. For our longitudinal analysis (Table 2), we included 77,862 participants who were initially free of abdominal obesity; the average incidence of abdominal obesity was 31.95/1,000 person-years over a follow-up period of , and the incidence was lowest in southern Taiwan (23.11/1,000 person-years). Among the participants with normal TG at baseline, the average incidence of elevated TG was 50.01/1,000 person-years, ranging from 49.46 (south) to 52.16 (central). For those with initially normal HDL-C, the average incidence of reduced HDL-C was 28.89/1,000 person-years, ranging from 23.22 (north) to 44.32 (south). The average incidence of elevated BP in the normal BP cohort was 56.81, ranging from 55.25 (south) to 63.31 (central). The average incidence of elevated FBG in the normal FBG cohort was 112.77/1,000 person-years, with the lowest rate being in central Taiwan (88.09) and the highest being in the north (123.12). Among the cohort without MetS, MetS occurred after of follow-up, with an average incidence of 38.07/1,000 person-years and the lowest rate occurring in the southern region (36.39) and the highest rate occurring in the central region (40.73). A total of 84.0% () participants did not have incident any component of MetS by the end of follow-up, whereas 0.1% () had incident all the five components (Table S1).
Table 2.
Summary statistics of concentrations of air pollutants (2005–2015) and incidence of metabolic syndrome and its components in the MJ Health Research cohort by region between 2006 and 2016, Taiwan.
| Statistics | Cohort without abdominal obesity () | Cohort with normal TG () | Cohort with normal HDL-C () | Cohort with normal BP () | Cohort with normal FBG () | Cohort without MetS () |
|---|---|---|---|---|---|---|
| 1-y average concentration for the year before health check-up () | ||||||
| () | ||||||
| North | ||||||
| Central | ||||||
| South | ||||||
| Total | ||||||
| (ppb) | ||||||
| North | ||||||
| Central | ||||||
| South | ||||||
| Total | ||||||
| Participants with event [ (%)]a | 8,632 (11.1%) | 12,643 (16.6%) | 7,266 (9.8%) | 13,055 (18.7%) | 20,541 (34.5%) | 9,898 (13.0%) |
| Follow-up period (y) | ||||||
| Incidence rate (per 1,000 person-years)b | ||||||
| North | 34.23 | 49.74 | 23.22 | 55.93 | 123.12 | 37.94 |
| Central | 32.28 | 52.16 | 36.16 | 63.31 | 88.09 | 40.73 |
| South | 23.11 | 49.46 | 44.32 | 55.25 | 95.31 | 36.39 |
| Total | 31.95 | 50.01 | 28.89 | 56.81 | 112.77 | 38.07 |
Note: BP, blood pressure; FBG, fasting blood glucose; HDL-C, high-density lipoprotein cholesterol; MetS, metabolic syndrome; , nitrogen dioxide; , fine particulate matter (PM with an aerodynamic diameter of ); SD, standard deviation; TG, triglyceride.
Event referred to occurrence of MetS and components. For cohort without abdominal obesity, event meant having a waist circumference of in men and in women. For cohort with normal TG, event meant having a TG level of or receiving drug treatment for hypertriglyceridemia. For cohort with normal HDL-C, event meant having an HDL-C level of () in males and () in females or receiving drug treatment for decreased HDL-C. For cohort with normal BP, event meant having a systolic BP level of or diastolic BP level of or receiving drug treatment for hypertension. For, cohort with normal FBG, event meant having an FBG level of or receiving drug treatment for hyperglycemia. For cohort without MetS, event meant the presence of any three or more of the above components.
Incidence rate was calculated by dividing the number of events by total per 1,000 person-years for each cohort.
Table 3 presents the results of our time-dependent Cox regression analysis performed to assess the effects of two pollutants simultaneously on the HRs of developing abdominal obesity, elevated TG, reduced HDL-C, elevated BP, elevated FBG, and MetS. In Model 1—with adjustments for age, sex, marital status, education level, sleeping time per day, smoking habits, alcohol drinking habits, and fried and processed food consumption—every increase in concentration in the year prior to the health examination significantly enhanced the HRs for all five components of MetS (i.e., abdominal obesity; TG, BP, or FBG above reference range; and HDL-C below reference range), and increase in concentration enhanced the HR for FBG above reference range. In Model 2, which included an additional adjustment for regular exercise, the results were similar to Model 1. In Model 3, which additionally adjusted for baseline status (waist circumference, TG, HDL-C, systolic and diastolic BP, and FBG for each cohort) and baseline BMI, and the association of remained significant; however, the association of with FBG became statistically nonsignificant. Every increase in was associated with an increased risk for abdominal obesity [; 95% confidence interval (CI): 1.01, 1.14], elevated TG (; 95% CI: 1.11, 1.23), reduced HDL-C (; 95% CI: 1.02, 1.17), elevated BP (; 95% CI: 1.09, 1.21), and elevated FBG (; 95% CI: 1.10, 1.20). As for the incidence of MetS, we present the results of participants who had none or some (1–2) components of MetS at baseline. For the participants initially without any component of MetS, we found no association between exposure to higher (; 95% CI: 0.89, 1.12) and (; 95% CI: 0.76, 1.03) and increased risk of MetS incidence. For those with one component of MetS, a increase in was associated with a 12% greater risk of MetS (; 95% CI: 1.04, 1.20). For those had two components of MetS, every increase in and every increase in was associated with a 14% (; 95% CI: 1.07, 1.22) and a 10% (; 95% CI: 1.03, 1.18) increased risk for MetS incidence, respectively.
Table 3.
Associations [aHR (95% CI)] of and with metabolic syndrome and its components among participants of the MJ Health Research cohort in Taiwan between 2006 and 2016.
| MetS and its components | Model 1a | Model 2b | Model 3c | |||
|---|---|---|---|---|---|---|
| Development of abdominal obesity in the no abdominal obesity cohort | (; ) | (; ) | (; ) | |||
| 1.15 (1.01, 1.12) | 1.06 (0.99, 1.12) | 1.08 (1.02, 1.15) | 1.01 (0.95, 1.07) | 1.07 (1.01, 1.14) | 0.97 (0.92, 1.03) | |
| Development of reduced HDL-C in the normal HDL-C cohort | (; ) | (; ) | (; ) | |||
| 1.16 (1.10, 1.22) | 1.03 (0.99, 1.08) | 1.17 (1.11, 1.23) | 1.02 (0.97, 1.07) | 1.17 (1.11, 1.23) | 0.99 (0.94, 1.03) | |
| Development of elevated TG in the normal TG cohort | (; ) | (; ) | (; ) | |||
| 1.10 (1.02, 1.18) | 1.01 (0.95, 1.07) | 1.12 (1.04, 1.20) | 1.00 (0.94, 1.07) | 1.09 (1.02, 1.17) | 0.94 (0.88, 1.01) | |
| Development of elevated BP in the normal BP cohort | (; ) | (; ) | (; ) | |||
| 1.18 (1.12, 1.24) | 1.03 (0.98, 1.08) | 1.18 (1.12, 1.24) | 1.01 (0.97, 1.06) | 1.15 (1.09, 1.21) | 1.04 (0.99, 1.10) | |
| Development of elevated FBG in the normal FBG cohort | (; ) | (; ) | (; ) | |||
| 1.11 (1.06, 1.15) | 1.07 (1.03, 1.11) | 1.11 (1.06, 1.16) | 1.06 (1.02, 1.10) | 1.15 (1.10, 1.20) | 1.03 (0.99, 1.07) | |
| Development of MetS based on the number of MetS components exhibited at baseline | (; ) | (; ) | (; ) | |||
| 0 | (; ) | (; ) | (; ) | |||
| 0.99 (0.89, 1.11) | 0.87 (0.75, 1.01) | 0.99 (0.89, 1.11) | 0.87 (0.75, 1.01) | 0.99 (0.89, 1.12) | 0.88 (0.76, 1.03) | |
| 1 | (; ) | (; ) | (; ) | |||
| 1.12 (1.04, 1.20) | 1.02 (0.94, 1.11) | 1.12 (1.04, 1.21) | 1.02 (0.94, 1.11) | 1.12 (1.04, 1.20) | 1.01 (0.93, 1.10) | |
| 2 | (; ) | (; ) | (; ) | |||
| 1.12 (1.05, 1.19) | 1.13 (1.06, 1.21) | 1.12 (1.05, 1.19) | 1.14 (1.06, 1.21) | 1.14 (1.07, 1.22) | 1.10 (1.03, 1.18) | |
Note: All estimates were calculated for every increase in and every increase in in the annual average concentrations, two-pollutant model, determined using time-dependent Cox regression. Missing information on covariates were initially represented by the previous value available of each participant. Participants without available value for representation were not eligible for data analysis depending on the covariates in the models, leading to different eligible numbers of participants in different models. The terms elevated and reduced refer to above and below normal reference range, respectively. aHR, adjusted hazard ratio; CI, confidence interval; BP, blood pressure; , number of participants with the incident outcome; FBG, fasting blood glucose; HDL-C, high-density lipoprotein cholesterol; MetS, metabolic syndrome; , number of participants without missing variables in each model; , nitrogen dioxide; , fine particulate matter (PM with an aerodynamic diameter of ); TG, triglyceride.
Adjusted by age, sex, marital status (single/divorced/separation/widowed, married/cohabitating), education level (junior high school and below, general and vocational high school, college, master’s degree and above), smoking habits (never smoking/former smoking, secondhand smoke exposure, frequent smoking/daily smoking), alcohol drinking habits (never drinking/former drinking, occasional drinking, frequent drinking/daily drinking), sleeping time per day (, 6–8, ), fried food consumption (none, little or portion weekly, 2–3 portions weekly, portions weekly), processed food consumption (none, little or portion weekly, 2–3 portions weekly, portions weekly), and region (north, central, south).
Additionally adjusted for the covariates from Model 1 and exercise (none, little or weekly, 1–4 h weekly or once per 2–3 d; weekly or daily).
Additionally adjusted for the covariates from Model 2, baseline body mass index (, 18.5–24, ), and the initial status—baseline waist circumference for abdominal obesity cohort, baseline TG for elevated TG cohort, baseline HDL-C for reduced HDL-C cohort, baseline systolic BP and baseline diastolic BP for elevated BP cohort, baseline FBG for elevated FBG cohort.
Tables S3 and S4 present models without baseline BMI and without sleeping time, respectively. The associations between , , MetS, and its components were similar to the findings in Table 3.
Figure S3 presents the potential modification effects of age and sex by adding the interaction terms in stratified analyses. Such findings were similar to our major results in Table 3. For sex, positive associations between , elevated TG, reduced HDL-C, elevated BP, elevated FBG, and from initially having one or more components of MetS were found in both male and female participants. In addition, the association between and abdominal obesity was significant in male (; 95% CI: 1.07, 1.23) but not in female (; 95% CI: 0.91, 1.06, ); the association of with elevated BP was more prominent in female (; 95% CI: 1.14, 1.30) than male (; 95% CI: 1.06, 1.18, ). For age, positive associations between , elevated TG, elevated BP, and elevated FBG were found in all age subgroups (, 45–64, of age). In the abdominal obesity cohort, participants of age had higher risk (; 95% CI: 1.03, 1.18) when exposed to increased than participants of age (; 95% CI: 0.80, 1.07, ). No significant modification effect of age or sex subgroup in the associations between , MetS, and its components was observed. The complete data set is included as Excel Table S2.
Discussion
Primary Findings
By using a time-dependent Cox regression analysis to consider the gradually changing concentrations of and in cohorts of 59,681–77,862 participants from the MJ Health Database with a follow-up period of 3.0–3.5 y, we observed associations between long-term and exposure and incidence of abdominal obesity, elevated TG, reduced HDL-C, elevated BP, elevated FBG, and MetS. An additional exposure to of annually was associated with an increased risk of abdominal obesity (7%), hypertriglyceridemia (17%), reduced HDL-C (9%), hypertension (15%), and elevated FBG (15%). The associations between and incident MetS and its components remained robust when additionally adjusting for either baseline BMI (Table S3) or sleeping time (Table S4). In the sensitivity analyses, the association between and abdominal obesity was significant in male and participants, whereas the association of with elevated BP was more prominent in female participants. For age subgroups (, 45–64, ), no interactions were observed between , elevated TG, elevated BP, and elevated FBG (Figure S3). In addition, the effects of long-term and exposure were particularly pronounced in participants who had some components of MetS at baseline (abdominal obesity, high TG levels, low HDL-C levels, high BP levels, and high FBG levels). For participants who had had one or two components of MetS, every increase in was associated with 12% and 14% risk of MetS incidence, respectively. For those who already had two components of MetS, every increase in was associated with a 10% risk of MetS incidence.
Related Literature
In 14 May 2012, Taiwan tightened the air quality standards of 24-h and yearly average concentrations to 35 and , respectively, as well as 24-h and yearly average concentrations to 100 and , respectively.16 In the present study, which included exposure data for the period from 2005 to 2015, although the annual average concentration of (based on each participant’s address) was , the concentration decreased gradually over time, as did that of . Therefore, the changes in air pollutant concentrations must be carefully considered when examining their effects on MetS and its components, and time-dependent Cox regression analysis may be appropriate for the present study.
In other countries with comparatively lower annual concentrations (), longitudinal studies using linear regression, logistic regression, or generalized estimating equations have revealed that the effects of increases in exposure on MetS and its components were statistically nonsignificant, namely, in Germany8,35 and Southern California.34 However, when exposure was used as a time-dependent variable, an annual median value of was demonstrated to be associated with higher risks of abdominal obesity, hypertriglyceridemia, reduced HDL-C, hypertension, hyperglycemia, and MetS from the National Health Insurance Service-National Health Screening Cohort in Korea,18 which is consistent with our findings. Moreover, people living in regions with high annual concentrations of (median ) in China have been reported to be at higher risk of abdominal obesity from 31 China provinces,4 reduced HDL-C,36 and MetS41 from the Henan Rural Cohort study, elevated BP among rural and urban regions,37–39 and elevated FBG among an elderly population.40
In comparatively lower annual concentrations () of (an indicator of traffic-related emissions), exposure was reported to be associated with elevated total cholesterol and low-density lipoprotein but not with reduced HDL-C, elevated FBG among young adults from the Southern California Children’s Health Study,34 or with MetS from the population-based survey in Augsburg, Germany.35 In studies where annual average concentrations were higher (20–), exposure was associated with increased BP among older adults in Taiwan5 but not with elevated FBG among Taiwanese5 and Mexican Americans.47 When annual average concentration was , an increased risk of MetS was reported in Germany8 and China.41 However, association between concentrations and hypertension was inconsistent.38,39 In our study, the effect of on elevated FBG remained significant (Table 3, Model 1 and 2) before baseline status were added for additional adjustment. Therefore, we could not exclude the possibility of an existing impact of on baseline BMI and FBG before the study enrollment. The inconsistency in findings between previous studies and the present study could be partly attributable to the differences in concentrations, the study methodology, and analysis.
Possible Mechanisms
Exposure to PM was associated with DNA hypomethylation, and PM-induced reactive oxygen species and elevated cytokine levels has been reported to elicit systemic inflammation in murine and human studies.48 In healthy young adults, high exposure to was found to relate to changes in DNA methylation in genes involved in glucose and lipid metabolism, inflammation, oxidative stress, platelet activation, and cell survival and apoptosis.49 In a study with nonsmoking participants, exposure was found to be associated with autonomic nervous system imbalance, as well as with impaired endothelial function 24 h after exposure. Both were regarded to be relevant to hypertension.50 As for the potential mechanisms of the observed effects of , inhaled oxidized antioxidants within the epithelial lining fluid and triggered extracellular damage and oxidative stress,51 which can inhibit glucose metabolism in rodents.52 Korean6 and German53 populations exposed to ambient concentrations were found to have impaired glucose metabolism, an important component of MetS. These mechanisms may support our findings.
Limitations and Strengths
Although we used a prospective cohort and rigorous exposure assessment of air pollutants, our study has some limitations. First, in this study, exposure assessment was based on the estimated concentration in the township the participant gave as their residence in the questionnaire. Personal habits, work exposure, commute, indoor/outdoor differences, and microenvironments render potential deviations in these measurements from the true exposure. However, these deviations tend to be in a random misclassification manner, thus causing the observed relationship between outcomes and exposure to be biased toward the null hypothesis. On the other hand, the outcomes measurements are also subject to measurement error. Given that those health care workers performing the health examination were unaware of the study hypothesis, bias is unlikely. Despite the efforts of standardization in questionnaire, equipment, and measurements,30,54 misclassification could not totally be avoided. Again, these misclassifications likely weaken the observed association. Therefore, the observed relationship likely underestimates the true effects. Second, the participants who changed address during the follow-up were excluded because their exposure could not be attributed to an exact level within the time period. This potentially induced selection bias to the study. However, moving has not been reported as a risk or protective factor for MetS. Third, the surrounding vegetation (greenness) in the participants’ residential areas was not directly included in the study models, neither was participants’ occupation. Some researchers have reported a negative association between MetS-related components and residential surrounding greenness,55–57 but the protective effect was inconsistent with that in urban regions.35,58 Exposure to annual concentrations of and was estimated at the township level, and green space was regarded as an explanatory predictor in the modeling procedure.27,28 In addition, data on real exposure to greenness for urban residents were unavailable. Accordingly, greenness was not adjusted for in this study. On the other hand, although some occupations have been reported to be associated with incident MetS,59 the MJ health questionnaire changed options of the occupational items during our research period, and the categories could not objectively present workers’ intensity of labor. Therefore, we did not include occupation as a covariate. Fourth, the incidence was based on the month of the participants took the health examination. Therefore, a time lag between the actual incidence of MetS and its components and the health examination existed, and the short-term effects of and exposure on MetS and its components were not achievable.
Regardless of these limitations, in Table 3, our study used time-dependent analysis to examine the health effects of and exposure, and found that exposure to was associated with the occurrence the components of MetS. The sensitivity analyses by age and sex stratifications showed that the association between and abdominal obesity was significant in the male and groups, and the association between and elevated BP was more prominent in the female group. Furthermore, the results of incident MetS indicates people who already had components of MetS could be vulnerable to the development of MetS when exposed to increased and concentrations. Our results generally support the hypothesis that long-term exposure to and are associated with increased risk of MetS and its components. Strategies aimed at improving air quality and using personal protective equipment might reduce the risks, especially for people who already have some components of MetS.
Conclusions
Our findings suggest that exposure to high levels of ( above Taiwan’s current air quality standards)16 was associated with increased risk of abdominal obesity, hypertriglyceridemia, reduced HDL-C, elevated BP, and elevated FBG. Moreover, we also observed that exposure to and were associated with the risk of developing MetS among people who already had some components of MetS. Additional studies are required to confirm the consistency of the effects of and exposure at different exposure ranges.
Supplementary Material
Acknowledgments
Part of the data used in this research was authorized by and received from the MJ Health Research Foundation (authorization code: MJHRF2018011A). Any interpretation or conclusion described in this paper does not represent the views of the MJ Health Research Foundation.
This study was supported by the National Health Research Institutes (NHRI-110-EMGP09).
References
- 1.GBD 2017 Causes of Death Collaborators. 2018. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392(10159):1736–1788, PMID: , 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, et al. 2008. The metabolic syndrome. Endocr Rev 29(7):777–822, PMID: , 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isomaa B, Almgren P, Tuomi T, Forsén B, Lahti K, Nissén M, et al. 2001. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 24(4):683–689, PMID: , 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 4.Cao S, Guo Q, Xue T, Wang B, Wang L, Duan X, et al. 2021. Long-term exposure to ambient PM2.5 increase obesity risk in Chinese adults: a cross-sectional study based on a nationwide survey in China. Sci Total Environ 778:145812, PMID: , 10.1016/j.scitotenv.2021.145812. [DOI] [PubMed] [Google Scholar]
- 5.Chuang KJ, Yan YH, Chiu SY, Cheng TJ. 2011. Long-term air pollution exposure and risk factors for cardiovascular diseases among the elderly in Taiwan. Occup Environ Med 68(1):64–68, PMID: , 10.1136/oem.2009.052704. [DOI] [PubMed] [Google Scholar]
- 6.Hwang MJ, Kim JH, Koo YS, Yun HY, Cheong HK. 2020. Impacts of ambient air pollution on glucose metabolism in Korean adults: a Korea National Health and Nutrition Examination Survey study. Environ Health 19(1):70, PMID: , 10.1186/s12940-020-00623-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai Y, Hansell AL, Blangiardo M, Burton PR, BioSHaRE, de Hoogh K, et al. 2017. Long-term exposure to road traffic noise, ambient air pollution, and cardiovascular risk factors in the HUNT and lifelines cohorts. Eur Heart J 38(29):2290–2296, PMID: , 10.1093/eurheartj/ehx263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matthiessen C, Lucht S, Hennig F, Ohlwein S, Jakobs H, Jöckel KH, et al. 2018. Long-term exposure to airborne particulate matter and NO2 and prevalent and incident metabolic syndrome—results from the Heinz Nixdorf Recall Study. Environ Int 116:74–82, PMID: , 10.1016/j.envint.2018.02.035. [DOI] [PubMed] [Google Scholar]
- 9.Zhang S, Mwiberi S, Pickford R, Breitner S, Huth C, Koenig W, et al. 2021. Longitudinal associations between ambient air pollution and insulin sensitivity: results from the KORA cohort study. Lancet Planet Health 5(1):e39–e49, PMID: , 10.1016/S2542-5196(20)30275-8. [DOI] [PubMed] [Google Scholar]
- 10.Yu Y, Paul K, Arah OA, Mayeda ER, Wu J, Lee E, et al. 2020. Air pollution, noise exposure, and metabolic syndrome—a cohort study in elderly Mexican-Americans in Sacramento area. Environ Int 134:105269, PMID: , 10.1016/j.envint.2019.105269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallwork RS, Colicino E, Zhong J, Kloog I, Coull BA, Vokonas P, et al. 2017. Ambient fine particulate matter, outdoor temperature, and risk of metabolic syndrome. Am J Epidemiol 185(1):30–39, PMID: , 10.1093/aje/kww157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ning J, Zhang Y, Hu H, Hu W, Li L, Pang Y, et al. 2021. Association between ambient particulate matter exposure and metabolic syndrome risk: a systematic review and meta-analysis. Sci Total Environ 782:146855, PMID: , 10.1016/j.scitotenv.2021.146855. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Environmental Protection Agency. 2021. Evolution of the Clean Air Act. https://www.epa.gov/clean-air-act-overview/evolution-clean-air-act#intro [accessed 30 July 2021].
- 14.European Union. 2008. Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on ambient air quality and cleaner air for Europe. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02008L0050-20150918 [accessed 30 July 2021].
- 15.Huang J, Pan X, Guo X, Li G. 2018. Health impact of China’s Air Pollution Prevention and Control Action Plan: an analysis of national air quality monitoring and mortality data. Lancet Planet Health 2(7):e313–e323, PMID: , 10.1016/S2542-5196(18)30141-4. [DOI] [PubMed] [Google Scholar]
- 16.Taiwan Air Quality Monitoring Network. 2020. Air quality standards. https://airtw.epa.gov.tw/ENG/Information/Standard/Rules.aspx [accessed 30 July 2021].
- 17.Li J, Yao Y, Xie W, Wang B, Guan T, Han Y, et al. 2021. Association of long-term exposure to PM2.5 with blood lipids in the Chinese population: findings from a longitudinal quasi-experiment. Environ Int 151:106454, PMID: , 10.1016/j.envint.2021.106454. [DOI] [PubMed] [Google Scholar]
- 18.Lee S, Park H, Kim S, Lee EK, Lee J, Hong YS, et al. 2019. Fine particulate matter and incidence of metabolic syndrome in non-CVD patients: a nationwide population-based cohort study. Int J Hyg Environ Health 222(3):533–540, PMID: , 10.1016/j.ijheh.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Xu L, Shan Z, Teng W, Han C. 2019. Association between air pollution and type 2 diabetes: an updated review of the literature. Ther Adv Endocrinol Metab 10:2042018819897046, PMID: , 10.1177/2042018819897046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. 2003. Health Aspects of Air Pollution with Particulate Matter, Ozone and Nitrogen Dioxide: Report on a WHO Working Group, Bonn, Germany 13–15 January 2003. https://apps.who.int/iris/handle/10665/107478 [accessed 30 July 2021].
- 21.MJ Health Research Foundation. 2015. MJ Health Survey Database, MJ BioData [Data file, in Chinese]. http://www.mjhrf.org [accessed 31 July 2020].
- 22.Wu X, Tsai SP, Tsao CK, Chiu ML, Tsai MK, Lu PJ, et al. 2017. Cohort profile: the Taiwan MJ Cohort: half a million Chinese with repeated health surveillance data. Int J Epidemiol 46(6):1744–1744g, PMID: , 10.1093/ije/dyw282. [DOI] [PubMed] [Google Scholar]
- 23.MJ Health Research Foundation. 2016. The Introduction of MJ Health Database. Technical Report No. MJHRF-TR-01. http://www.mjhrf.org/file/file/report/MJHRF-TR-01%20MJ%20Health%20Database.pdf [accessed 30 March 2022].
- 24.Environmental Protection Administration, Executive Yuan, R.O.C. (Taiwan). Air Quality Annual Report of R.O.C. (Taiwan), 2015 [in Chinese]. https://www.epa.gov.tw/DisplayFile.aspx?FileID=920B8BD6AA7922CA [accessed 30 March 2022].
- 25.Cheng FY, Hsu CH. 2019. Long-term variations in PM2.5 concentrations under changing meteorological conditions in Taiwan. Sci Rep 9(1):6635, PMID: , 10.1038/s41598-019-43104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Directorate General of Highways, R.O.C. (Taiwan). 2019. Annual statistics of registered motor vehicles in 2017. https://www.thb.gov.tw/sites/ch/modules/StatisticsSummary/StatisticsSummary-List?node=47df19cb-4615-4f2e-b322-6bcceef70406&y=2017&id=cdb43826-93b2-41ff-a68d-a5d41573b747 [accessed 30 March 2022].
- 27.Wong PY, Lee HY, Chen YC, Zeng YT, Chern YR, Chen NT, et al. 2021. Using a land use regression model with machine learning to estimate ground level PM2.5. Environ Pollut 277:116846, PMID: , 10.1016/j.envpol.2021.116846. [DOI] [PubMed] [Google Scholar]
- 28.Chen TH, Hsu YC, Zeng YT, Lung SCC, Su HJ, Chao HJ, et al. 2020. A hybrid kriging/land-use regression model with Asian culture-specific sources to assess NO2 spatial-temporal variations. Environ Pollut 259:113875, PMID: , 10.1016/j.envpol.2019.113875. [DOI] [PubMed] [Google Scholar]
- 29.Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. 2009. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120(16):1640–1645, PMID: , 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 30.Wang ML. 2016. MJ Health Screening Equipment Use and Replacement Records. Technical Report No. MJHRF-TR-06. http://www.mjhrf.org/file/file/report/MJHRF-TR-06%20Screening%20Equipment.pdf [accessed 30 March 2022].
- 31.Troxel WM, Matthews KA, Gallo LC, Kuller LH. 2005. Marital quality and occurrence of the metabolic syndrome in women. Arch Intern Med 165(9):1022–1027, PMID: , 10.1001/archinte.165.9.1022. [DOI] [PubMed] [Google Scholar]
- 32.Lian Y, Yuan Q, Wang G, Tang F. 2019. Association between sleep quality and metabolic syndrome: a systematic review and meta-analysis. Psychiatry Res 274:66–74, PMID: , 10.1016/j.psychres.2019.01.096. [DOI] [PubMed] [Google Scholar]
- 33.Che T, Yan C, Tian D, Zhang X, Liu X, Wu Z. 2021. The association between sleep and metabolic syndrome: a systematic review and meta-analysis. Front Endocrinol (Lausanne) 12:773646, PMID: , 10.3389/fendo.2021.773646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JS, Chen Z, Alderete TL, Toledo-Corral C, Lurmann F, Berhane K, et al. 2019. Associations of air pollution, obesity and cardiometabolic health in young adults: the Meta-AIR study. Environ Int 133(pt A):105180, PMID: , 10.1016/j.envint.2019.105180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voss S, Schneider A, Huth C, Wolf K, Markevych I, Schwettmann L, et al. 2021. Long-term exposure to air pollution, road traffic noise, residential greenness, and prevalent and incident metabolic syndrome: results from the population-based KORA F4/FF4 cohort in Augsburg, Germany. Environ Int 147:106364, PMID: , 10.1016/j.envint.2020.106364. [DOI] [PubMed] [Google Scholar]
- 36.Mao S, Chen G, Liu F, Li N, Wang C, Liu Y, et al. 2020. Long-term effects of ambient air pollutants to blood lipids and dyslipidemias in a Chinese rural population. Environ Pollut 256:113403, PMID: , 10.1016/j.envpol.2019.113403. [DOI] [PubMed] [Google Scholar]
- 37.Cao H, Li B, Liu K, Pan L, Cui Z, Zhao W, et al. 2021. Association of long-term exposure to ambient particulate pollution with stage 1 hypertension defined by the 2017 ACC/AHA Hypertension Guideline and cardiovascular disease: the CHCN-BTH cohort study. Environ Res 199:111356, PMID: , 10.1016/j.envres.2021.111356. [DOI] [PubMed] [Google Scholar]
- 38.Du J, Shao B, Gao Y, Wei Z, Zhang Y, Li H, et al. 2021. Associations of long-term exposure to air pollution with blood pressure and homocysteine among adults in beijing, China: a cross-sectional study. Environ Res 197:111202, PMID: , 10.1016/j.envres.2021.111202. [DOI] [PubMed] [Google Scholar]
- 39.Li N, Chen G, Liu F, Mao S, Liu Y, Liu S, et al. 2020. Associations between long-term exposure to air pollution and blood pressure and effect modifications by behavioral factors. Environ Res 182:109109, PMID: , 10.1016/j.envres.2019.109109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Li T, Ma R, Yin Z, Wang J, He MZ, et al. 2020. Long-term exposure to ambient fine particulate matter and fasting blood glucose level in a Chinese elderly cohort. Sci Total Environ 717:137191, PMID: , 10.1016/j.scitotenv.2020.137191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hou J, Liu X, Tu R, Dong X, Zhai Z, Mao Z, et al. 2020. Long-term exposure to ambient air pollution attenuated the association of physical activity with metabolic syndrome in rural Chinese adults: a cross-sectional study. Environ Int 136:105459, PMID: , 10.1016/j.envint.2020.105459. [DOI] [PubMed] [Google Scholar]
- 42.Dekker FW, de Mutsert R, van Dijk PC, Zoccali C, Jager KJ. 2008. Survival analysis: time-dependent effects and time-varying risk factors. Kidney Int 74(8):994–997, PMID: , 10.1038/ki.2008.328. [DOI] [PubMed] [Google Scholar]
- 43.Park SK, Auchincloss AH, O’Neill MS, Prineas R, Correa JC, Keeler J, et al. 2010. Particulate air pollution, metabolic syndrome, and heart rate variability: the Multi-Ethnic Study of Atherosclerosis (MESA). Environ Health Perspect 118(10):1406–1411, PMID: , 10.1289/ehp.0901778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evandt J, Oftedal B, Hjertager Krog N, Nafstad P, Schwarze PE, Marit Aasvang G. 2017. A population-based study on nighttime road traffic noise and insomnia. Sleep 40(2), PMID: . [DOI] [PubMed] [Google Scholar]
- 45.Ministry of the Interior, R.O.C. (Taiwan). 2017. https://ws.moi.gov.tw/001/Upload/400/relfile/0/4405/48349492-6f8c-453ba9d1-4a8f0593b979/year_en.html [accessed 19 December 2022].
- 46.Ministry of Digital Affairs, R.O.C. (Taiwan). 2022. Population density at the township level of Taiwan in 2016. https://data.gov.tw/dataset/8410 [accessed 19 December 2022].
- 47.Chen Z, Salam MT, Toledo-Corral C, Watanabe RM, Xiang AH, Buchanan TA, et al. 2016. Ambient air pollutants have adverse effects on insulin and glucose homeostasis in Mexican Americans. Diabetes Care 39(4):547–554, PMID: , 10.2337/dc15-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clementi E, Talusan A, Vaidyanathan S, Veerappan A, Mikhail M, Ostrofsky D, et al. 2019. Metabolic syndrome and air pollution: a narrative review of their cardiopulmonary effects. Toxics 7(1):6, PMID: , 10.3390/toxics7010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li H, Chen R, Cai J, Cui X, Huang N, Kan H. 2018. Short-term exposure to fine particulate air pollution and genome-wide DNA methylation: a randomized, double-blind, crossover trial. Environ Int 120:130–136, PMID: , 10.1016/j.envint.2018.07.041. [DOI] [PubMed] [Google Scholar]
- 50.Brook RD, Urch B, Dvonch JT, Bard RL, Speck M, Keeler G, et al. 2009. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension 54(3):659–667, PMID: , 10.1161/HYPERTENSIONAHA.109.130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petit PC, Fine DH, Vásquez GB, Gamero L, Slaughter MS, Dasse KA. 2017. The pathophysiology of nitrogen dioxide during inhaled nitric oxide therapy. ASAIO J 63(1):7–13, PMID: , 10.1097/MAT.0000000000000425. [DOI] [PubMed] [Google Scholar]
- 52.Salmon AB. 2012. Oxidative stress in the etiology of age-associated decline in glucose metabolism. Longev Healthspan 1(1):7, PMID: , 10.1186/2046-2395-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teichert T, Vossoughi M, Vierkötter A, Sugiri D, Schikowski T, Schulte T, et al. 2013. Association between traffic-related air pollution, subclinical inflammation and impaired glucose metabolism: results from the SALIA study. PLoS One 8(12):e83042, PMID: , 10.1371/journal.pone.0083042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chuang YC. 2016. MJ Health data-cleaning procedure. MJ Health Research Foundation Technical report, MJHRF-TR-04. http://www.mjhrf.org/main/page/resource/en/#resource08 [accessed 30 March 2022].
- 55.de Keijzer C, Basagaña X, Tonne C, Valentín A, Alonso J, Antó JM, et al. 2019. Long-term exposure to greenspace and metabolic syndrome: a Whitehall II study. Environ Pollut 255(pt 2):113231, PMID: , 10.1016/j.envpol.2019.113231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Twohig-Bennett C, Jones A. 2018. The health benefits of the great outdoors: a systematic review and meta-analysis of greenspace exposure and health outcomes. Environ Res 166:628–637, PMID: , 10.1016/j.envres.2018.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang BY, Liu KK, Markevych I, Knibbs LD, Bloom MS, Dharmage SC, et al. 2020. Association between residential greenness and metabolic syndrome in Chinese adults. Environ Int 135:105388, PMID: , 10.1016/j.envint.2019.105388. [DOI] [PubMed] [Google Scholar]
- 58.Huang B, Xiao T, Grekousis G, Zhao H, He J, Dong G, et al. 2021. Greenness-air pollution-physical activity-hypertension association among middle-aged and older adults: evidence from urban and rural China. Environ Res 195:110836, PMID: , 10.1016/j.envres.2021.110836. [DOI] [PubMed] [Google Scholar]
- 59.van Zon SKR, Amick BC III, de Jong T, Brouwer S, Bültmann U. 2020. Occupational distribution of metabolic syndrome prevalence and incidence differs by sex and is not explained by age and health behavior: results from 75 000 Dutch workers from 40 occupational groups. BMJ Open Diabetes Res Care 8(1):e001436, PMID: , 10.1136/bmjdrc-2020-001436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.