To the Editor: The emergence of the highly divergent B.1.1.529 (omicron) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) led to concerns about the efficacy of vaccines based on the ancestral spike and to the approval in the United States of bivalent vaccines for coronavirus disease 2019 (Covid-19) that include the ancestral spike and the omicron BA.5 spike proteins.1-3 Since the approval and distribution of these vaccines, additional subvariants containing key mutations that further enhance the ability of the virus to escape from vaccine-elicited antibodies and regulatory-approved monoclonal antibodies have been identified.4 Of particular concern is the R346T mutation, which has arisen in multiple omicron subvariants, including BA.2.75.2, BQ.1.1, and XBB (Fig. S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org). We tested serum samples obtained from participants who had received either one or two monovalent boosters or the bivalent booster to determine the neutralization efficiency of the booster vaccines against wild-type (WA1/2020) virus and primary isolates of omicron subvariants BA.1, BA.5, BA.2.75.2, BQ.1.1, and XBB using an in vitro, live-virus focus reduction neutralization test (FRNT). All the participants provided written informed consent.
We used the FRNT in a VeroE6/TMPRSS2 cell line1 to compare the neutralizing activity in serum samples obtained from participants in three cohorts: the first cohort comprised 12 participants 7 to 28 days after one monovalent booster; the second, 11 participants 6 to 57 days after a second monovalent booster; and the third, 12 participants 16 to 42 days after a bivalent booster. The differences in neutralizing antibody responses among these three cohorts were quantitated by comparing the FRNT50 (the reciprocal dilution of serum that neutralizes 50% of the input virus) geometric mean titers (GMTs) of neutralizing antibodies against the omicron subvariants with that against the ancestral SARS-CoV-2 WA1/2020 strain. Serum samples in which the GMT fell below the limit of detection (1:20) were given an arbitrary FRNT50 value of 10.
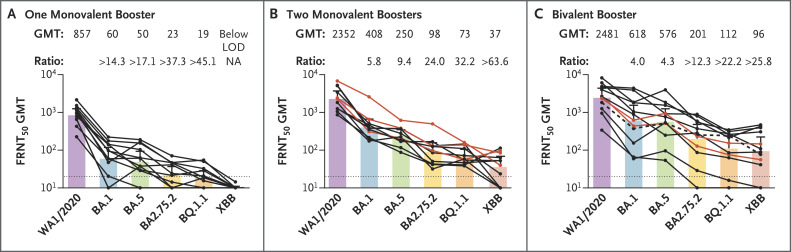
In all three cohorts, neutralization activity was lower against all omicron subvariants than against the WA1/2020 strain; neutralizing activity was lowest against the XBB subvariant (Figure 1 and Fig. S2). In the cohort that received one monovalent booster, the FRNT50 GMTs were 857 against WA1/2020, 60 against BA.1, 50 against BA.5, 23 against BA.2.75.2, 19 against BQ.1.1, and below the limit of detection against XBB. In the cohort that received two monovalent boosters, the FRNT50 GMTs were 2352 against WA1/2020, 408 against BA.1, 250 against BA.5, 98 against BA.2.75.2, 73 against BQ.1.1, and 37 against XBB. The results in both of these cohorts correspond with neutralization titers against BA.1 and BA.5 that were 5 to 9 times as low as that against WA1/2020 and neutralization titers against BA.2.75.2, BQ.1.1, and XBB that were 23 to 63 times as low as that against WA1/2020.
Figure 1. Neutralizing Responses against the WA1/2020 Strain and Omicron Subvariants.
Shown is the neutralization activity against the WA1/2020 strain of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the omicron subvariants BA.1, BA.5, BA.2.75.2, BQ.1.1, and XBB in 12 participants who received one monovalent booster (Panel A), in 11 participants who received two monovalent boosters (Panel B), and in 12 participants who received the updated bivalent booster (Panel C). The focus reduction neutralization test (FRNT50 [the reciprocal dilution of serum that neutralizes 50% of the input virus]) geometric mean titer (GMT) of neutralizing antibodies against the WA1/2020 strain and each omicron subvariant is shown at the top of each panel, along with the ratio of the neutralization GMT against the WA1/2020 strain to that against each omicron subvariant. The connecting lines between the variants represent matched serum samples. The horizontal dotted lines represent the limit of detection of the assay (FRNT50 GMT 20). The red lines in Panels B and C indicate the participants who reported previous SARS-CoV-2 infection, and the dashed line in Panel C indicates one participant who received two monovalent boosters before the bivalent booster. The colored bars represent the FRNT50 GMT among the participants in the cohort, and the 𝙸 bars indicate 95% confidence intervals, which were not adjusted for multiplicity and may not be used for hypothesis testing. LOD denotes limit of detection, and NA not applicable.
In the cohort that received the BA.5-containing bivalent booster, the neutralizing activity against all the omicron subvariants as compared with that against WA1/2020 was better than in the other two cohorts (Figure 1C). The FRNT50 GMTs were 2481 against WA1/2020, 618 against BA.1, 576 against BA.5, 201 against BA.2.75.2, 112 against BQ.1.1, and 96 against XBB. The results in this cohort correspond with neutralization titers against BA.1 and BA.5 that were 4 times as low as that against WA1/2020 and neutralization titers against BA.2.75.2, BQ.1.1, and XBB that were 12 to 26 times as low as that against WA1/2020.
Persons who received either one or two monovalent Covid-19 vaccine boosters had much lower neutralization activity against omicron subvariants (especially against BA.2.75.2, BQ.1.1, and XBB, which contain the predicted escape mutation R346T) than that against the WA1/2020 strain. Persons who received the BA.5-containing bivalent booster had better neutralizing activity against all omicron subvariants (especially against BA.2.75.2, BQ.1.1, and XBB) than those who received either one or two monovalent boosters, even though the neutralization GMT against WA1/2020 was similar in the cohort that received the two monovalent boosters and the cohort that received the bivalent booster. These responses are consistent with recent observations in persons with breakthrough omicron infection showing broadened neutralizing activity against omicron subvariants.5 Limitations of this study include the small cohort size, differences in age among the cohorts, the unknown effect of previous exposure to SARS-CoV-2, and comparison of the vaccines at a single time point. These serologic data show an overall neutralization benefit with bivalent booster immunizations.
Supplementary Appendix
Disclosure Forms
This letter was published on December 21, 2022, at NEJM.org.
Footnotes
Supported in part by grants from the National Institute of Allergy and Infectious Diseases (NIAID) and the National Institutes of Health (NIH) (NIH P51OD011132, 3U19AI057266-17S1, 1U54CA260563, HHSN272201400004C, NIH/NIAID Centers of Excellence for Influenza Research and Response [CEIRR] under contract 75N93021C00017 [to Emory University]), an Emory Executive Vice President for Health Affairs Synergy Fund award, funding from the Pediatric Research Alliance Center for Childhood Infections and Vaccines and Children’s Healthcare of Atlanta, COVID-Catalyst-I3 Funds from the Woodruff Health Sciences Center and Emory School of Medicine, and a Woodruff Health Sciences Center 2020 COVID-19 CURE Award.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Edara VV, Manning KE, Ellis M, et al. mRNA-1273 and BNT162b2 mRNA vaccines have reduced neutralizing activity against the SARS-CoV-2 omicron variant. Cell Rep Med 2022;3:100529-100529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyke KE, Atmar RL, Islas CD, et al. Rapid decline in vaccine-boosted neutralizing antibodies against SARS-CoV-2 Omicron variant. Cell Rep Med 2022;3:100679-100679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalkias S, Harper C, Vrbicky K, et al. A bivalent omicron-containing booster vaccine against Covid-19. N Engl J Med 2022;387:1279-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheward DJ, Kim C, Fischbach J, et al. Omicron sublineage BA.2.75.2 exhibits extensive escape from neutralising antibodies. Lancet Infect Dis 2022;22:1538-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linderman SL, Lai L, Bocangel Gamarra EL, et al. Neutralizing antibody responses in patients hospitalized with SARS-CoV-2 Delta or Omicron infection. J Clin Invest 2022. October 18 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.