Abstract
Aim:
To identify patient factors, including gastrointestinal functions that are predictive or associated with weight loss in response to 3mg SQ liraglutide or placebo in obesity.
Methods:
136 obese adults (87% female) were randomized in a placebo-controlled, 16-week trial of liraglutide, escalated to 3mg SQ daily. Gastrointestinal functions were measured at baseline and 16 weeks: gastric emptying of solids (GET1/2); fasting and postprandial gastric volumes; kcal ingested during ad libitum buffet meal and during nutrient drink test. GET1/2 was also measured at 5 weeks. A multiple variable regression model examined variables associated with weight loss >4kg at 16 weeks. A parsimonious model using backward selection identified the final model.
Results:
Weight loss >4kg at 16 weeks occurred in 71% of liraglutide and 16% of placebo-treated patients. In all participants combined, parameters univariately associated with >4kg loss were: GET1/2 at 5 and 16 weeks, weight loss at 5 weeks, and kcal intake during buffet meal at 16 weeks. The final parsimonious model (AUROC 0.832) identified that factors associated with >4kg loss were GET1/2 at 5 weeks (OR=2.505; 95% CI: 1.57–3.997) per 50 minutes and kcal intake during ad libitum meal at 16 weeks (OR=0.721; 95% CI: 0.602–0.864) per 100kcal. Among only the 60 liraglutide-treated subjects, kcal intake at 16 weeks was associated with 4kg loss (AUROC=0.757).
Conclusions:
Slower GET1/2 and weight loss at 5 weeks predicted weight loss >4kg at 16 weeks in all participants. Among liraglutide-treated adults, >4kg loss was associated with ad libitum meal kcal at 16 weeks.
Keywords: energy intake, gastric functions, predictors, regression, genes
INTRODUCTION
The response to non-surgical interventions for obesity such as diet, exercise, and pharmacotherapeutics remains highly variable and is often short lasting.1–3 Liraglutide is a long-acting analog of human glucagon-like peptide-1 (GLP-1) that is approved by the United States Food and Drug Administration at a dosage of 3mg per day administered subcutaneously (SQ) for weight management in adults with BMI ≥30kg/m2, or ≥27kg/m2 with obesity related co-morbidities, and for pediatric population weighing at least 60kg with BMI ≥30kg/m2 aged 12 years and older. It is proven effective in reducing weight in individuals with obesity.4 Systematic reviews have shown that, as a class, GLP-1 agents are the most efficacious medications5,6 and, among the GLP-1 analogs or agonists, liraglutide was the most efficacious medication for inducing weight loss after SQ semaglutide.7
Endogenous GLP-1, GLP-1 analogs, and GLP-1 receptor agonists induce weight loss through several peripheral and central mechanisms including delay of gastric emptying, activation of the ileal brake, increase in satiety, decrease in glucagon secretion, and direct modulation of appetite centers.8–14 While the principal mechanistic driver of weight loss is still unknown, it is established that there is no thermogenic effect of liraglutide, and therefore the dominant mechanism is considered to be related to caloric restriction rather than increased energy expenditure.15 Gastrointestinal functions and postprandial satiation may impact the variable outcomes of obesity therapy. As a pharmacological class, GLP-1 analogs or agonists significantly retard gastric emptying.8 Some GLP-1 agonists are available in short- and long-acting formulations, the latter of which can be administered once weekly. While short-acting exenatide is known to slow gastric emptying, Jones et al. were able to show that the long-acting once weekly exenatide also slowed gastric emptying as compared to placebo.16 Furthermore, Thazhath et al. showed that exenatide increased small bowel transit in both healthy and diabetic volunteers which could be a mechanism by which GLP-1 agonists exert their effects.17 Interestingly, a network meta-analysis that compared multiple GLP-1 agonists’ efficacy to achieve excess weight loss suggests that GLP-1 agents with stronger effects on weight loss, such as liraglutide and semaglutide, have a higher risk of adverse events, particularly nausea and vomiting.7 It has been suggested that this could be a predictor of, or possibly a mechanism for, their weight loss properties.7,18,19 A recently published randomized placebo-controlled trial of liraglutide found that patients who experienced nausea achieved greater absolute weight loss.20 However, the relationship remains unclear as gastrointestinal adverse events generally develop within 4 weeks of starting treatment and may be transient.4,21
In a trial involving 136 participants (initial pilot data and full data published),20,22 3mg liraglutide significantly delayed time to half gastric emptying of solids (GET1/2) at 5 and 16 weeks, with the delay in gastric emptying being significantly correlated with weight loss at 6 weeks.20,22 Given that exogenous GLP-1 is known to increase both fasting and postprandial gastric volumes,23 it is was hypothesized that liraglutide also affects gastric volumes and this may contribute to weight loss by altering appetite.
Energy intake has been used as a clinical measure to assess the effect of liraglutide on weight loss.11,14,24 Weight loss was found to be associated with a decrease in kcal consumed during ad libitum meal in two studies.14,24 A randomized, placebo-controlled trial of 3mg liraglutide reported that calorie intake in a single ad libitum meal correlated with weight loss in nondiabetic patients with obesity.25 However, there was no correlation with gastric emptying measured using plasma acetaminophen levels. The most accepted factors for predicting successful weight loss among patients receiving GLP-1 agonists include dosage and serum levels.7,21,26 However, these same factors are associated with greater risk of adverse events.7,21 Thus, there is a need to identify alternative and patient-level factors that predict or are associated with successful weight loss in patients receiving liraglutide.
Our hypothesis was that measurements at baseline, 5 and 16 weeks of gastric functions such as gastric volumes, gastric emptying, plasma incretin levels, and satiety predict or are associated with weight loss at 16 weeks in response to 3mg liraglutide SQ administered for 16 weeks. Therefore, the objective of this analysis was to identify the best predictors or factors associated with weight loss >4kg among demographic parameters and gastrointestinal functions in obesity in response to 3mg of SQ liraglutide administered for 16 weeks. The >4kg weight loss was selected as a clinically relevant degree of loss over 16 weeks, given that the weighted mean difference in nine clinical trials of liraglutide >1.8mg was 4.49kg (3.72 to 5.26) when administered for mean 42.2 weeks (range 12–160 weeks).
METHODS
Study Design, Participants, and Ethical Approval
This single-center (Mayo Clinic, Rochester, MN, USA), double-blind, placebo-controlled, parallel-group trial of once daily SQ liraglutide 3mg or placebo (1:1) for a total treatment period of 16 weeks has been published elsewhere.20 The current analysis to identify factors that predict or are associated with weight loss was conducted based on the information collected including demographic parameters and gastrointestinal functions as detailed below.
Adults with obesity (BMI >30kg/m2) who were otherwise healthy, 18–65 years of age, and residing within 125 miles of the center were recruited. The study was approved by Mayo Clinic Institutional Review Board (IRB #15-001783). All participants provided written informed consent. This study is registered with ClinicalTrials.gov NCT#03523273 and is closed to participants.
Participants with delayed gastric emptying of solids (>90th percentile according to gender, <87% in males or <81% emptied at 4 hours in females)27 were excluded to ensure participant safety.
Treatment with Liraglutide
The study followed standard FDA recommendations on the use of liraglutide including screening questionnaires for psychiatric symptoms,28 alcohol use disorders,29 eating disorders,30 and intake of medications except for multivitamins within 7 days of the study.
A total of 136 participants were enrolled up to May 1, 2021 and completed the studies by August 31, 2021. Details of randomization and blinding are as in the prior report.20 Liraglutide (SAXENDA® Novo Nordisk, Inc., Plainsboro, NJ, USA) was purchased and stored in Mayo Clinic Research Pharmacy. All liraglutide and saline placebo supplies were dispensed by the Research Pharmacy directly to the study participants; therefore, participants and study staff were unaware of treatment assignment. Liraglutide was escalated as recommended by the FDA: 0.6mg daily for one week and increased by 0.6mg weekly increments until 3.0mg was reached over 4 weeks. Every 4 weeks, participants obtained a new supply of study medication from the Research Pharmacy.
Participants received education and a “Direction for Use” pamphlet provided by the Clinical Research Trials Unit nurses not associated with the research study.
Standardization of Dietetic and Behavioral Advice
Participants in both treatment groups received standardized dietetic and behavioral counseling for weight reduction therapy.20
Procedures
Study protocol and measurements of gastrointestinal functions and satiation
The study protocol is described elsewhere in detail.22 All study participants underwent screening visits, baseline measurements of gastrointestinal, behavioral, and psychological factors, and dose escalation (0.6mg per week for liraglutide, and similar weekly volume increments for placebo). Quantitative traits22 were measured as follows at baseline and after 16 weeks of treatment: gastric emptying of solids (standardized 320-kcal solid-liquid meal27), satiation by ad libitum meal, volume to fullness and maximum tolerated volume of liquid nutrient meal,31 fasting and postprandial gastric volumes (in response to a standard volume of 300mL Ensure®).32 An additional scintigraphic gastric emptying test with the same solid-liquid meal was performed at week 5. On the day of the nutrient drink test, participants had blood samples drawn fasting, and at 15, 45, and 90 minutes during and after nutrient drink ingestion to measure the incretin peptide YY (peptide tyrosine-tyrosine) which was quantified using the Human Peptide YY Double Antibody Radioimmunoassay Kit (Millipore Research, Inc., St. Louis, MO, USA). PYY exists in two or more molecular forms, 1–36 and 3–36, both of which are physiologically active and are detected by the assay.
All participants also had genotyping17 for TCF7L2 rs6923761 (CT/TT vs. CC) and GLP-1R rs7903146 (AG/AA vs. GG) which respectively modify the synthesis of endogenous GLP-1 and the functions of GLP-1 receptors respectively.
Statistical Analysis
A multiple variable regression model was used to examine the likelihood of weight loss >4kg at 16 weeks of the study in all patients and selectively in patients in the liraglutide arm. Sex was not included as a candidate predictor of weight loss outcome in the statistical analysis. A parsimonious model was fit using backward selection to identify the final model. Statistical analyses were performed using SAS Software, version 9.4 (SAS Institute). Odds ratios and corresponding 95% confidence intervals were calculated. All odds ratios for GET1/2 are reported for 10 minutes and 50 minutes of change in Tables 2 and 3 and for 1kg change in the body weight. The rationale for expressing the odds ratio for 50 minutes of change in GET1/2 was based on the observations of the effects of liraglutide in the parent study20 which was a median slowing at 5 weeks of 69.7 minutes (IQR: 32.3–97.1), and a median slowing at 16 weeks of 33.8 minutes (IQR:3.7–63.4). Odds ratios for ad libitum buffet meal are reported per 100 calories of change to better assess the magnitude of the effect. All p-values that were lower than <0.0001 were reported as p<0.0001.
Table 2.
Odds ratios (OR) and 95% confidence intervals from univariate analysis for factors measured at baseline, week 5, and week 16 of the study to achieve weight loss of more than 4 kilograms at 16 weeks, based on all 121 patients (liraglutide and placebo groups). The C-statistic is equal to the area under the receiver operating characteristics curve (AUROC).
| 95% CI | ||||||
|---|---|---|---|---|---|---|
| Odds ratio | Lower | Upper | P-value | c-statistic | ||
| Baseline Variables | ||||||
| Liraglutide | 12.9 | 5.35 | 31.1 | <0.0001 | 0.78 | |
| rs6923761 genotype (AG/AA vs. GG) | 1.37 | 0.66 | 2.82 | 0.3967 | 0.54 | |
| rs7903146 genotype (CT/TT vs. CC) | 1.02 | 0.50 | 2.11 | 0.9509 | 0.50 | |
| Buffet meal total calories—100 kcal | 0.78 | 0.69 | 0.90 | 0.0003 | 0.70 | |
| Nutrient drink test maximum calories—100 kcal | 0.88 | 0.79 | 0.98 | 0.0251 | 0.62 | |
| Fasting PYY—10 pg/mL | 1.01 | 0.92 | 1.11 | 0.8320 | 0.50 | |
| Mean post prandial PYY—10 pg/mL | 1.00 | 0.92 | 1.09 | 0.9867 | 0.50 | |
| Gastric emptying T1/2 | 10 minutes | 1.11 | 0.96 | 1.28 | 0.1438 | 0.57 |
| 50 minutes | 1.71 | 0.83 | 3.49 | |||
| Week 5 variables | ||||||
| Gastric emptying T1/2 | 10 minutes | 1.23 | 1.13 | 1.35 | <0.0001 | 0.77 |
| 50 minutes | 2.87 | 1.86 | 4.43 | |||
| Change from baseline to week 5 | ||||||
| Weight loss—1 kg body weight | 4.45 | 2.55 | 7.78 | <0.0001 | 0.96 | |
| Gastric emptying T1/2 | 10 minutes | 1.27 | 1.15 | 1.41 | <0.0001 | 0.78 |
| 50 minutes | 3.25 | 2.01 | 5.54 | |||
| Week 16 variables | ||||||
| Fasting PYY—10 pg/mL | 0.99 | 0.87 | 1.13 | 0.8875 | 0.51 | |
| Mean post prandial PYY—10 pg/mL | 1.08 | 0.98 | 1.19 | 0.1280 | 0.57 | |
| Gastric emptying T1/2 | 10 minutes | 1.23 | 1.11 | 1.37 | 0.0001 | 0.72 |
| 50 minutes | 2.86 | 1.66 | 4.91 | |||
| Buffet meal total calories—100 kcal | 0.70 | 0.58 | 0.83 | <0.0001 | 0.76 | |
| Nutrient drink test maximum calories—100 kcal increase | 0.76 | 0.66 | 0.88 | 0.0001 | 0.73 | |
| Change from baseline to week 16 | ||||||
| Gastric emptying T1/2 | 10 minutes | 1.21 | 1.08 | 1.36 | 0.0009 | 0.70 |
| 50 minutes | 2.63 | 1.49 | 4.64 | |||
| Buffet meal total calories—100 kcal increase | 0.89 | 0.73 | 1.08 | 0.2485 | 0.56 | |
| Nutrient drink test maximum calories—100 kcal increase | 0.88 | 0.78 | 1.00 | 0.0434 | 0.61 | |
Table 3.
Odds ratios (OR) and 95% confidence intervals from univariate analysis for factors measured at baseline, week 5, and week 16 of the study to achieve weight loss of more than 4 kilograms at 16 weeks, based on 60 patients in the liraglutide arm alone. The C-statistic is equal to the area under the receiver operating characteristics curve (AUROC).
| 95% CI | ||||||
|---|---|---|---|---|---|---|
| Odds ratio | Lower | Upper | P-value | c-statistic | ||
| Baseline Variable | ||||||
| rs6923761 genotype (AG/AA vs. GG) | 0.98 | 0.32 | 3.02 | 0.9689 | 0.50 | |
| rs7903146 genotype (CT/TT vs. CC) | 0.97 | 0.31 | 3.05 | 0.9604 | 0.50 | |
| Buffet meal total calories—100 kcal | 0.71 | 0.56 | 0.89 | 0.0030 | 0.77 | |
| Nutrient drink test maximum calories—100 kcal | 0.88 | 0.74 | 1.04 | 0.1339 | 0.60 | |
| Gastric emptying T1/2 | 10 minutes | 1.08 | 0.85 | 1.37 | 0.5249 | 0.56 |
| 50 minutes | 1.47 | 0.45 | 4.8 | |||
| Week 5 variable | ||||||
| Gastric emptying T1/2 | 10 minutes | 1.1 | 0.98 | 1.23 | 0.1029 | 0.63 |
| 50 minutes | 1.6 | 0.91 | 2.83 | |||
| Change from baseline to week 5 | ||||||
| Weight loss—1 kg body weight | 4.34 | 1.94 | 9.71 | 0.0004 | 0.92 | |
| Gastric emptying T1/2 | 10 minutes | 1.11 | 0.98 | 1.27 | 0.1072 | 0.64 |
| 50 minutes | 1.71 | 0.89 | 3.28 | |||
| Week 16 variables | ||||||
| Gastric emptying T1/2 | 10 minutes | 1.09 | 0.95 | 1.26 | 0.1944 | 0.64 |
| 50 minutes | 1.57 | 0.78 | 3.12 | |||
| Buffet meal total calories—100 kcal | 0.68 | 0.53 | 0.87 | 0.0019 | 0.76 | |
| Nutrient drink test maximum calories—100 kcal | 0.88 | 0.74 | 1.04 | 0.1339 | 0.60 | |
| Change from baseline to week 16 | ||||||
| Gastric emptying T1/2 | 10 minutes | 1.07 | 0.94 | 1.23 | 0.3189 | 0.61 |
| 50 minutes | 1.41 | 0.72 | 2.80 | |||
| Buffet meal total calories—100 kcal increase | 0.97 | 0.72 | 1.29 | 0.8157 | 0.48 | |
| Nutrient drink test maximum calories—100 kcal increase | 0.97 | 0.82 | 1.15 | 0.7682 | 0.55 | |
Data Availability
Data were collected as part of a previous clinical trial (ClinicalTrials.gov NCT#03523273) in Obesity.20
Role of the Funding Source
The funding source, National Institutes of Health, had no involvement in the study design, in collection, analysis, and interpretation of the data, in writing the report, or in the decision to submit the paper for publication.
All authors had full access to all the data in the study and accept responsibility to submit this work for publication.
RESULTS
Baseline Characteristics
Among the 136 randomized participants, 124 completed the 16-week study (65 placebo and 59 liraglutide). Complete data on the diverse measurements of gastrointestinal functions are available for 121 participants. Baseline characteristics were not significantly different between the two treatment groups (Table 1, which includes age, race, and sex of the participants, adapted from Maselli, et al.20).
Table 1.
Demographics and baseline measurements of gastrointestinal functions in two treatment groups. Adapted from Maselli, Atieh et al.20
| Data show median and IQR | Placebo, n=69 | Liraglutide, n=67 |
|---|---|---|
| Age, y | 37.2 (29.3, 45.2) | 42 (32, 51) |
| Sex (% female) | 85.5% | 88.1% |
| Race, % white | 94.2% | 89.6% |
| BMI, kg/m2 | 35.6 (33.1, 39.7) | 35.9 (32.6, 40.2) |
| Baseline weight, kg | 100.0 (92.4, 114.9) | 103.1 (89.1, 111.9) |
| Baseline Gastric emptying T1/2, min | 108.0 (93.1, 128.6) | 117.2 (97.5, 140.0) |
| Baseline gastric fasting volume, mL | 200.8 (179.3, 231.2) | 200.4 (179.3, 231.2) |
| Baseline gastric postprandial vol., mL | 587.0 (525.4, 678.0) | 593.5 (489.3, 648.6) |
| Baseline gastric accommodation vol, mL | 378.5 (322.5, 455.9) | 377.1 (322.6, 445.3) |
| Baseline satiation volume to fullness (VTF), mL | 756 (535.5, 945.0) | 693 (567.0, 871.0) |
| Baseline satiation maximum tolerated (MTV), mL | 1244.3 (995.4, 1493.1) | 1244.3 (995.4, 1244.3) |
| Baseline VAS aggregate score | 206.0 (151.5, 256.5) | 204.0 (156.0. 253.0) |
| Baseline ad libitum meal total calories | 878.6 (708.2, 1151.1) | 829.5 (665.7, 1088.5) |
| Weight at 5 weeks (kg) | 101.4 (90.5 to 114.2) | 100.4 (87.0 to 108.6) |
| Weight at 16 weeks (kg) | 99.0 (90.8 to 114.6) | 97.9 (85.9 to 108.3) |
| Δ Weight at 5 weeks vs. baseline | 0.1 (1.5 to 1.4) | 3.8 (4.8 to 2.5) |
| Δ Weight at 16 weeks vs. baseline | 0.0 (3.1 to 2.1) | 5.8 (8.3 to 3.9) |
| Baseline ad libitum meal total calories | 878.6 (708.2 to 1,151.1) | 829.5 (665.7 to 1,088.5) |
| Ad libitum meal total calories at 16 weeks | 793.7 (624.6 to 1,019.3) | 647.5 (472.4 to 826.4) |
| Δ Ad libitum meal total calories at 16 weeks vs. baseline | 129.2 (197.6 to 23.2) | 184.8 (322.3 to 69.4) |
End-of-study weight loss >4 kg was achieved by 71% of the liraglutide group compared to 16% of placebo group as published previously.20
Univariate Predictors and Factors Associated with Weight Loss in All Patients
Table 2 shows univariate predictors measured at baseline, week 5, and week 16 of the study that were associated with weight loss of >4kg at 16 weeks in all patients. Demographic parameters such as sex, baseline BMI, baseline serum glucose, and age as well as TCF7L2 and GLP1R genotype variation were not significant predictors of weight loss >4kg at 16 weeks.
Baseline variables
As expected, liraglutide treatment was associated with weight loss of >4kg at 16 weeks compared to placebo treatment [OR=12.9 (95% CI: 5.53 to 31.11; P<0.0001)]. At baseline, no other variables were positively associated with weight loss. On the other hand, two variable were significantly associated with a lower odds of weight loss >4kg at 16 weeks: total calories consumed during an ad libitum buffet meal and maximum tolerated calories consumed during nutrient drink test (Table 2). Participants with a 50-minute difference in GET1/2 at baseline were not more likely to achieve weight loss >4kg [OR = 1.71 (95% CI: 0.83 to 3.49; P=0.144)]. Baseline fasting and mean postprandial PYY had no significant utility in prediction of weight loss >4kg at 16 weeks of treatment.
Week 5 variables
GET1/2 was a significant predictor of weight loss >4kg at 16 weeks, with an OR=2.87 (95% CI: 1.86 to 4.43; P<0.0001) for 50 minute difference and had an area under the receiver operator characteristics curve (AUROC) of 0.77. Change in GET1/2 from baseline to week 5 was also a significant predictor with an OR=3.25 (95% CI: 2.01 to 5.54; P<0.0001) for 50 minutes of change and with an AUROC=0.78. As expected, an absolute weight loss of 1kg from baseline to week 5 had an OR=4.45 (95% CI: 2.55 to 7.78; P<0.0001) and AUROC=0.96.
Week 16 associated factors
Total calories consumed during an ad libitum buffet meal at 16 weeks were significantly associated with weight loss >4kg at 16 weeks [OR=0.7 (95% CI: 0.58 to 0.83; P<0.0001)]. Participants with a 50-minute slower GET1/2 where significantly more likely to achieve a weight loss >4kg [OR=2.86 (95% CI: 1.66 to 4.91; P=0.0001)]. Furthermore, those who experienced a 50-minute delay in GET1/2 over the 16-week study period were also significantly more likely to experience clinically meaningful weight loss [OR=2.63 (95% CI: 1.49 to 4.64; P=0.0009)]. An increase in the maximum tolerated calories during the nutrient drink test, though not fasting and mean postprandial peptide YY measurements, was associated with a decreased likelihood of weight loss.
Univariate Predictors and Factors Associated with Weight Loss in Liraglutide Arm
GET1/2 measured at baseline and week 16 were not significant predictors or associated factors with weight loss of >4kg at 16 weeks. GET1/2 at week 5 had an OR=1.6 (95% CI: 0.91 to 2.83; P=0.103) for a 50-minute difference. However, total calories consumed during an ad libitum buffet meal at baseline and 16 weeks were significantly associated with weight loss >4kg at 16 weeks in the liraglutide group, with an OR = 0.71 (95% CI: 0.56 to 0.89; P=0.003) and OR=0.68 (95% CI: 0.53 to 0.87; P=0.0019), respectively. Other variables were not associated with clinically relevant weight loss and are summarized in Table 3.
Multivariable Logistic Regression Analysis
The final multivariable model using baseline variables to predict weight loss >4kg at 16 weeks included liraglutide treatment and total calories consumed during an ad libitum buffet meal at baseline (which was negatively associated with degree of weight loss). This model had an AUROC=0.87 (95% CI: 0.81 to 0.92).
The multivariable model that considered all data collected at 5 and 16 weeks to identify weight loss of >4 kg at 16 weeks among all study subjects revealed two factors that were significant in the final parsimonious model: GET1/2 at 5 weeks (OR=2.5; 95% CI: 1.57 to 3.99) for 50 minutes of change and kcal intake during ad libitum meal at 16 weeks (OR=0.721; 95% CI: 0.602 to 0.864) for 100kcal of change. The AUROC for this multivariable model was 0.832 (Figure 1).
Figure 1.
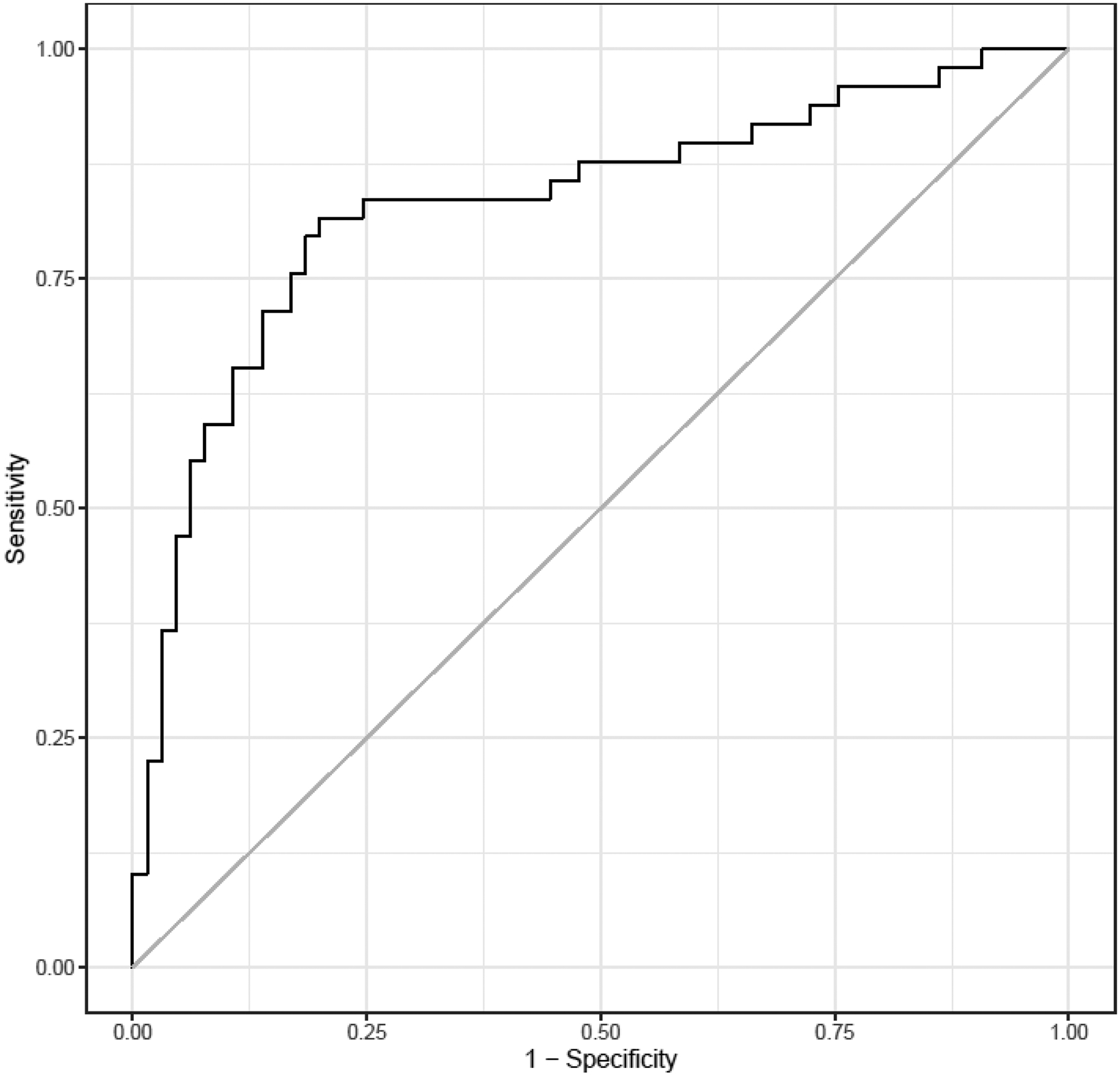
ROC curve evaluating variables included in the parsimonious model that are associated with weight loss >4 kilograms at 16 weeks in all patients. Area under the curve=0.832
When limiting the analysis to the liraglutide group only, the ROC curve evaluating weight loss of >4kg at 16 week showed an AUROC of 0.814 when including baseline and week 5 GET1/2, and kcal intake during ad libitum meal at 16 weeks (Figure 2). When applying the parsimonious model the only individual parameter associated with weight loss >4 kg was kcal intake during ad labium meal at 16 weeks, with an AUROC=0.757.
Figure 2.
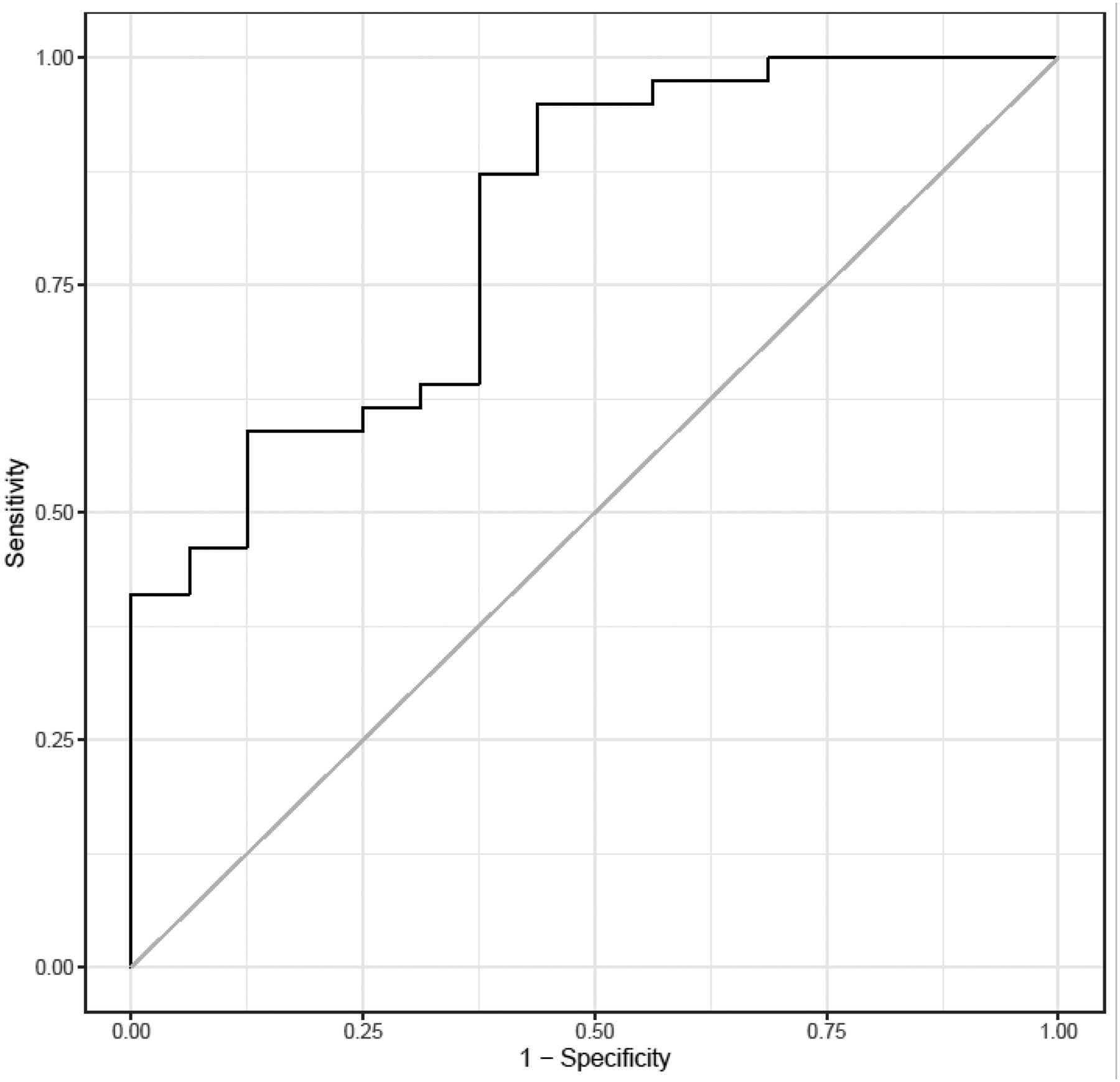
ROC curve evaluating weight loss of > 4kg at 16 weeks in the liraglutide group only using baseline GES T1/2, week 5 GES T1/2, and meal total kcal at 16 weeks. Area under the curve=0.814. GES T1/2: gastric emptying of solids time to half emptying.
DISCUSSION
This analysis showed that, among patients with obesity attempting weight loss with either liraglutide or placebo treatment, a delay in gastric emptying at 5 weeks predicted weight loss >4kg at 16 weeks, as evident on univariate analysis and in the parsimonious model. The total calories consumed at baseline during an ad libitum buffet meal and maximum tolerated calories consumed during nutrient drink test were both significant negative predictors of weight loss of >4kg at 16 weeks. This suggests that obese patients with higher kcal intake at baseline were less likely to lose >4kg when assigned to treatment with liraglutide or placebo.
When considering only the patients treated with liraglutide, the two factors associated with weight loss >4kg at 16 weeks were weight loss >1kg in the first 5 weeks (AUROC=0.96) and reduction in kcal intake during ad libitum meal at 16 weeks (AUROC=0.76). Among these parameters, the observations at 5 weeks could be regarded as predictors of weight loss >4kg, whereas kcal intake at the 16-week ad libitum meal is an associated factor.
When considering both placebo- and liraglutide-treated patients, the AUROC curve for the parsimonious model that incorporated both GET1/2 at 5 weeks and kcal intake during ad libitum meal at 16 weeks was 0.832 compared to 0.77 for GET1/2 at 5 weeks on univariate analysis. Therefore, retardation of GET1/2 at 5 weeks remains a relevant predictor of weight loss at 16 weeks among all study participants even without consideration of the kcal ingested at ad libitum meal at 16 weeks. On the other hand, the reduced energy intake at ad libitum meal at 16 weeks may reflect the effects of liraglutide on appetite and would likely not serve pragmatic and prognostic utility.
The parsimonious model using backward selection for the liraglutide group identified only one significant variable associated with weight loss of >4kg at 16 weeks, which was the kcal intake at ad libitum meal at 16 weeks, and this confirms a prior reports.25 However, greater delay of GET1/2 at baseline and 5 weeks trended towards greater weight loss at week 16, but this failed to meet statistical significance. Our study suggests that further research is necessary to characterize the associations of liraglutide treatment of obesity with central mechanisms in addition to satiation mediated peripherally by satiation-associated hormones or gastric functions. Future studies evaluating the mechanisms of GLP-1 receptor agonists may benefit from exploring the potential role of central appetite suppression by concomitant measurement of subjective appetite, such as with visual analog scales, as this may explain that only total energy intake at ad libitum meal, rather than GET1/2, was associated with clinically meaningful weight loss. Although most subjects that achieved weight loss >4kg were in the liraglutide arm, the assessment of predictive value confined to the liraglutide group and dichotomizing continuous variables (>4kg or not) reduced statistical power to identify predictors of weight loss in the liraglutide group alone.33 We noted that the odds ratio of clinically meaningful weight loss among the liraglutide arm was 1.6 (95% CI: 0.91 to 2.83; P=0.1029) when comparing participants with a 50-minute difference in GET1/2 at 5 weeks; our findings were likely underpowered to demonstrate this association at the significance level of p<0.05. Otherwise, demographic parameters such as sex, baseline BMI, baseline serum glucose, and age were of no predictive utility, suggesting they may not need to be considered when initiating GLP-1 agents.
In an earlier study, gastric emptying was not significantly associated with weight loss in patients on liraglutide.25 In fact, other studies have suggested that liraglutide was not associated with delayed gastric emptying.14,34,35 However, gastric emptying in those studies was measured using a suboptimal methodology that utilizes acetaminophen absorption, which is an indirect way of assessing liquid emptying rather than solid emptying.36
One of the strengths of our current studies is the use of gastric emptying scintigraphy based on a 320kcal egg meal, given the different rates of emptying between liquids and solids.37 Other strengths of our studies include a much larger sample size of 121 patients analyzed compared to 61 patients25, and the longer treatment span of 16 weeks compared to 6 weeks in the most recent study that tried to identify a short-term biomarker for the effectiveness of liraglutide.25
The main limitation of this study is that it is a post hoc analysis of prospectively acquired data. Thus, although the gastric emptying prolongation at 5 weeks did not reach statistical significance in the liraglutide treatment group alone (P=0.1029), it is worth noting that this observation may have been impacted by the relatively small sample size of 60 patients and that, in this cohort, there were few patients with significantly accelerated gastric emptying at baseline. Interestingly, when analyzing the entire study sample, several predictors of weight loss >4kg emerged that were not also present in the liraglutide arm alone, such as slower GET1/2 at week 5 and greater change in GET1/2 between baseline and week 5. The post hoc nature of the study, relatively small sample treated with liraglutide, and the lack of consistent predictors of weight loss >4kg when analyzing the liraglutide arm alone require cautious interpretation. It argues for further studies to identify early predictors of long-term weight management success with liraglutide and other anti-obesity medications targeting GLP-1 receptors as well as GIP and glucagon receptors that could also affect gastric motor functions in addition to their effects on appetite. Furthermore, we did not assess the role of central appetite suppression which may have helped to elucidate the impact this may play on energy intake or successful weight loss. Other limitations include the lack of frequent assessment of caloric intake using ad libitum meal or nutrient drink tests at points other than the beginning and end of the study, the absence of direct measurements of central (e,g., hypothalamic) functions, lack of measurements of serum liraglutide concentrations to assess pharmacokinetic predictors, not correcting for multiple comparisons, and a predominantly female sample which may limit generalizability.
However, we are encouraged by the facts that this study reports the largest sample size with the most reliable method of measuring gastric emptying and it provided the finding that gastric emptying prolongation at 5 weeks of treatment is a significant predictor of greater than 4kg weight loss at the end of the 16 weeks of treatment with liraglutide. It will be important to emphasize standardized evaluations of physiological effects of anti-obesity medications, including more recent agents such as tirzepatide, to better understand the mechanisms and predictors of weight loss. Multiple reasons have been elucidated in other studies on why there is interindividual variability in response to liraglutide treatment including serum levels of liraglutide and genetic factors. In our study, the drug levels were not assessed, and the genetic factors did not appear to predict successful weight loss.
We conclude that our study has important clinical implications, specifically that weight loss of >1kg at 5 weeks and gastric emptying (GET1/2) retardation at 5 weeks can serve as useful and valid predictors of weight loss >4kg over 16 weeks. This information may facilitate assessment of the benefit-to-cost ratio of this relatively expensive treatment that requires daily subcutaneous injection. Further research is needed to more precisely understand the likely multifactorial mechanisms of liraglutide of producing weight loss, including gastric emptying, satiation, satiety, and energy expenditure.
Acknowledgement:
The authors thank Mrs. Cindy Stanislav for secretarial assistance.
Funding support:
Grant R01-DK67071 from National Institutes of Health to Michael Camilleri.
Footnotes
Trial registry: ClinicalTrials.gov NCT#03523273 - Data were collected as part of this previous clinical trial which has been published in Obesity 2022;30(8):1608–1620. Maselli D*, Atieh J*, Clark MM, Eckert D, Taylor A, Carlson P, Burton DD, Busciglio I, Harmsen WS, Vella A, Acosta A, Camilleri M (*joint first authors). Effects of liraglutide on gastrointestinal functions and weight in obesity: a randomized clinical and pharmacogenomic trial.
Conflicts of interest:
A. Acosta is a stockholder in Gila Therapeutics, Phenomix Sciences and Lipiquester; he serves as a consultant for Rhythm Pharmaceuticals, General Mills, Gila Therapeutics.
M. Camilleri is a stockholder in Phenomix Sciences and serves as a consultant to Kallyope (with consulting fee paid to his employer, Mayo Clinic).
The other authors have no conflicts of interest.
REFERENCES
- 1.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. The lancet. 2014;384(9945):766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. New England Journal of Medicine. 2017;376(3):254–266. [DOI] [PubMed] [Google Scholar]
- 3.Webb VL, Wadden TA. Intensive lifestyle intervention for obesity: principles, practices, and results. Gastroenterology. 2017;152(7):1752–1764. [DOI] [PubMed] [Google Scholar]
- 4.Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. New England Journal of Medicine. 2015;373(1):11–22. [DOI] [PubMed] [Google Scholar]
- 5.Khera R, Murad MH, Chandar AK, et al. Association of pharmacological treatments for obesity with weight loss and adverse events: a systematic review and meta-analysis. Jama. 2016;315(22):2424–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khera R, Pandey A, Chandar AK, et al. Effects of weight-loss medications on cardiometabolic risk profiles: a systematic review and network meta-analysis. Gastroenterology. 2018;154(5):1309–1319. e1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vosoughi K, Atieh J, Khanna L, et al. Association of glucagon-like peptide 1 analogs and agonists administered for obesity with weight loss and adverse events: a systematic review and network meta-analysis. EClinicalMedicine. 2021;42:101213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maselli DB, Camilleri M. Effects of GLP-1 and its analogs on gastric physiology in diabetes mellitus and obesity. Adv Exp Med Biol. 2020;1307:171–192. [DOI] [PubMed] [Google Scholar]
- 9.Cifuentes L, Camilleri M, Acosta A. Gastric Sensory and Motor Functions and Energy Intake in Health and Obesity—Therapeutic Implications. Nutrients. 2021;13(4):1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Umapathysivam MM, Lee MY, Jones KL, et al. Comparative effects of prolonged and intermittent stimulation of the glucagon-like peptide 1 receptor on gastric emptying and glycemia. Diabetes. 2014;63(2):785–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horowitz M, Flint A, Jones KL, et al. Effect of the once-daily human GLP-1 analogue liraglutide on appetite, energy intake, energy expenditure and gastric emptying in type 2 diabetes. Diabetes research and clinical practice. 2012;97(2):258–266. [DOI] [PubMed] [Google Scholar]
- 12.Farr OM, Sofopoulos M, Tsoukas MA, et al. GLP-1 receptors exist in the parietal cortex, hypothalamus and medulla of human brains and the GLP-1 analogue liraglutide alters brain activity related to highly desirable food cues in individuals with diabetes: a crossover, randomised, placebo-controlled trial. Diabetologia. 2016;59(5):954–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ten Kulve JS, Veltman DJ, van Bloemendaal L, et al. Endogenous GLP1 and GLP1 analogue alter CNS responses to palatable food consumption. Journal of Endocrinology. 2016;229(1):1–12. [DOI] [PubMed] [Google Scholar]
- 14.Van Can J, Sloth B, Jensen C, Flint A, Blaak E, Saris W. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. International journal of obesity. 2014;38(6):784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harder H, Nielsen L, Thi TD, Astrup A. The effect of liraglutide, a long-acting glucagon-like peptide 1 derivative, on glycemic control, body composition, and 24-h energy expenditure in patients with type 2 diabetes. Diabetes care. 2004;27(8):1915–1921. [DOI] [PubMed] [Google Scholar]
- 16.Jones KL, Huynh LQ, Hatzinikolas S, et al. Exenatide once weekly slows gastric emptying of solids and liquids in healthy, overweight people at steady-state concentrations. Diabetes Obes Metab. 2020;22(5):788–797. [DOI] [PubMed] [Google Scholar]
- 17.Thazhath SS, Marathe CS, Wu T, et al. The Glucagon-Like Peptide 1 Receptor Agonist Exenatide Inhibits Small Intestinal Motility, Flow, Transit, and Absorption of Glucose in Healthy Subjects and Patients With Type 2 Diabetes: A Randomized Controlled Trial. Diabetes. 2016;65(1):269–275. [DOI] [PubMed] [Google Scholar]
- 18.Horowitz M, Aroda VR, Han J, Hardy E, Rayner CK. Upper and/or lower gastrointestinal adverse events with glucagon-like peptide-1 receptor agonists: Incidence and consequences. Diabetes Obes Metab. 2017;19(5):672–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trautmann ME, Han J, Ruggles J. Early Pharmacodynamic Effects of Exenatide Once Weekly in Type 2 Diabetes Are Independent of Weight Loss: A Pooled Analysis of Patient-level Data. Clin Ther. 2016;38(6):1464–1473. [DOI] [PubMed] [Google Scholar]
- 20.Maselli D, Atieh J, Clark MM, et al. Effects of liraglutide on gastrointestinal functions and weight in obesity: A randomized clinical and pharmacogenomic trial. Obesity (Silver Spring). 2022;30(8):1608–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Astrup A, Rössner S, Van Gaal L, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. The Lancet. 2009;374(9701):1606–1616. [DOI] [PubMed] [Google Scholar]
- 22.Halawi H, Khemani D, Eckert D, et al. Effects of liraglutide on weight, satiation, and gastric functions in obesity: a randomised, placebo-controlled pilot trial. The Lancet Gastroenterology & Hepatology. 2017;2(12):890–899. [DOI] [PubMed] [Google Scholar]
- 23.Delgado-Aros S, Kim D-Y, Burton DD, et al. Effect of GLP-1 on gastric volume, emptying, maximum volume ingested, and postprandial symptoms in humans. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2002;282(3):G424–G431. [DOI] [PubMed] [Google Scholar]
- 24.Flint A, Kapitza C, Zdravkovic M. The once‐daily human GLP‐1 analogue liraglutide impacts appetite and energy intake in patients with type 2 diabetes after short‐term treatment. Diabetes, Obesity and Metabolism. 2013;15(10):958–962. [DOI] [PubMed] [Google Scholar]
- 25.Saxena AR, Banerjee A, Corbin KD, Parsons SA, Smith SR. Energy intake as a short‐term biomarker for weight loss in adults with obesity receiving liraglutide: A randomized trial. Obesity science & practice. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SH, Abbasi F, Nachmanoff C, et al. Effect of the glucagon-like peptide-1 analogue liraglutide versus placebo treatment on circulating proglucagon-derived peptides that mediate improvements in body weight, insulin secretion and action: A randomized controlled trial. Diabetes Obes Metab. 2021;23(2):489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camilleri M, Iturrino J, Bharucha AE, et al. Performance characteristics of scintigraphic measurement of gastric emptying of solids in healthy participants. Neurogastroenterology & Motility. 2012;24(12):1076–e1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta psychiatrica scandinavica. 1983;67(6):361–370. [DOI] [PubMed] [Google Scholar]
- 29.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA, Project ACQI. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Archives of internal medicine. 1998;158(16):1789–1795. [DOI] [PubMed] [Google Scholar]
- 30.Yanovski SZ, Marcus MD, Wadden TA, Walsh BT. The questionnaire on eating and weight patterns-5 (QEWP-5): An updated screening instrument for binge eating disorder. The International journal of eating disorders. 2015;48(3):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chial H, Camilleri C, Delgado‐Aros S, et al. A nutrient drink test to assess maximum tolerated volume and postprandial symptoms: effects of gender, body mass index and age in health. Neurogastroenterology & Motility. 2002;14(3):249–253. [DOI] [PubMed] [Google Scholar]
- 32.Bouras E, Delgado-Aros S, Camilleri M, et al. SPECT imaging of the stomach: comparison with barostat, and effects of sex, age, body mass index, and fundoplication. Gut. 2002;51(6):781–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Statistics in medicine. 2006;25(1):127–141. [DOI] [PubMed] [Google Scholar]
- 34.Frandsen CS, Dejgaard TF, Andersen HU, et al. Liraglutide as adjunct to insulin treatment in type 1 diabetes does not interfere with glycaemic recovery or gastric emptying rate during hypoglycaemia: A randomized, placebo‐controlled, double‐blind, parallel‐group study. Diabetes, Obesity and Metabolism. 2017;19(6):773–782. [DOI] [PubMed] [Google Scholar]
- 35.Jelsing J, Vrang N, Hansen G, Raun K, Tang‐Christensen M, Bjerre Knudsen L. Liraglutide: short‐lived effect on gastric emptying—long lasting effects on body weight. Diabetes, Obesity and Metabolism. 2012;14(6):531–538. [DOI] [PubMed] [Google Scholar]
- 36.Kim D-Y, Myung S-J, Camilleri M Novel testing of human gastric motor and sensory functions: rationale, methods, and potential applications in clinical practice. The American journal of gastroenterology. 2000;95(12):3365–3373. [DOI] [PubMed] [Google Scholar]
- 37.Abell TL, Camilleri M, Donohoe K, et al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Journal of nuclear medicine technology. 2008;36(1):44–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data were collected as part of a previous clinical trial (ClinicalTrials.gov NCT#03523273) in Obesity.20


