Abstract
PURPOSE:
To identify the safety of niraparib, a PARP inhibitor, in combination with Radium-223 for the treatment of metastatic castrate resistant prostate cancer (mCRPC) in men without known BRCA mutations.
PATIENTS AND METHODS:
Men with progressive mCPRC following ≥1 line of androgen receptor (AR)-targeted therapy and bone metastases but no documented BRCA-1 or BRCA-2 alterations or bulky visceral disease were included. Niraparib dose was escalated in combination with standard dosing of Radium-223 using a time-to-event continual reassessment method. The highest dose level with a DLT probability < 20% was defined as MTD. Secondary endpoints included PSA change and progression free survival. Exploratory analyses included assessing DNA mutations found in ctDNA as well as gene expression changes assessed in whole blood samples.
RESULTS:
Thirty patients were treated with niraparib and radium-223: 13 patients received 100mg, 12 received 200mg and 5 patients received 300mg of niraparib. There were 6 DLT events: 2(13%) for neutropenia, 2(13%) for thrombocytopenia, while fatigue and nausea each occurred once (3%). Anemia (2/13%) and neutropenia (2/13%) were the most common grade 3 adverse events. For patients with prior chemotherapy exposure, the MTD was 100 mg, while the MTD for chemotherapy naïve patients was 200mg. Whole blood gene expression of PAX5 and CD19 were higher in responders and ARG-1, IL-2R and FLT3 expression were higher in non-responders.
CONCLUSIONS:
Combining niraparib with Radium-223 in patients with mCRPC was safe however further studies incorporating biomarkers will better elucidate the role of combinations of PARP inhibitors with DNA damaging and other agents.
Keywords: PARP, Niraparib, Radium-223, Castrate-Resistant, Prostate Cancer
INTRODUCTION:
An estimated 250,000 men were diagnosed with prostate cancer in 2021, which remains the second most common cause of cancer death for men in the United States (1). Prostate cancer cells are dependent on androgen stimulation and the androgen axis for growth and androgen deprivation therapy is the cornerstone of treatment (2). Despite androgen deprivation, progression of disease occurs over time, in part, through mechanisms that re-activate the androgen receptor (AR) independent of androgen stimulation, leading to castrate resistant prostate cancer (3). There are currently several different therapies for mCRPC that are approved which include: taxane chemotherapy, androgen axis inhibitors such has enzalutamide, apalutamide and abiraterone, sipuleucel-T, Radium-223 and in select populations, Poly (ADP-ribose) polymerase (PARP) inhibitors Olaparib or Rucaparib and Pembrolizumab, an anti-PD1 antibody (4–11).
Radium-223 is an alpha particle-emitting, radioactive agent that is FDA approved for treatment of patients with mCRPC and symptomatic bone metastases (12). In comparison to gamma radiation, alpha particle radiation produces higher rates of single strand DNA breaks that are more frequently converted to double strand breaks (13). The agent targets calcium hydroxyapatite in bone and accumulates in regions of osteoblastic activity, emitting alpha particles that have low penetrance depth and high linear energy transfer, making its effect localized to the cortical bone and minimizing bone marrow toxicity (14,15). This localized effect and minimal systemic toxicity creates the potential for Radium-223 to be combined safely with other agents.
Poly (ADP-ribose) polymerase 1 (PARP-1) is a nuclear enzyme that recruits proteins that impact the DNA damage repair (DDR) pathways. Inhibitors of PARP-1 have demonstrated clinical activity in mCRPC when concurrent mutations in genes that code for DNA repair proteins are identified (10,11,16). PARP may also play an important role in prostate cancer through interaction with the AR (17–20). Niraparib is an orally available, highly selective PARP-1/PARP-2 inhibitor that has shown activity in tumors with DNA repair deficiencies and is currently approved, at a dose of 200 to 300 mg daily, for clinical use in ovarian cancer (21–23). Niraparib has also been found to be effective in treating mCRPC with known DNA repair gene defects (24,25). The benefit of PARP inhibitors may also extend to patients with mCRPC without deficiencies in DDR pathways, as evidenced by the phase III results from the PROpel study, which demonstrated an improvement in PFS for patients without mutations in homologous recombination repair (HRR) genes (26).
We hypothesize that for patients with mCRPC, regardless of status of DNA repair mutational status, PARP inhibitors may prove effective when given in combination with agents that stimulate DNA damage. Given that Radium-223 creates high rates of DNA single strand breaks as a mechanism for cell death, and PARP is primarily responsible for repair of such breaks (27) there is the potential for synergy between these agents when treating mCRPC. Additionally, the potential for niraparib to further suppress the AR pathway, suggests another potential mechanism for synergy when combined with Radium-223. We believe these interactions could extend benefit to patients without somatic or germline DNA repair mutations.
We conducted a phase IB study to determine the maximally tolerated dose (MTD) and recommended phase II dosing (RP2D) of niraparib combined with standard dosing of Radium-223 in the treatment of patients with metastatic mCRPC in men with and without prior chemotherapy exposure. Additional correlative studies were conducted to evaluate oncogenic genetic mutations at baseline and gene expression and pathway profile changes induced by therapy.
PATIENTS AND METHODS
Study Design and Participants
This was a multi-center, Phase IB dose escalation trial (ClinicalTrials.gov ID NCT03076203) that was approved by the institutional review boards at all participating institutions. Patients were provided written informed consent prior to starting on trial and enrollment followed international standards of good clinical practice. There were three dose levels of niraparib evaluated along with standard of care dosing of Radium-223 in two cohorts (chemotherapy naïve and prior chemotherapy) of men with mCRPC. The primary objective wacs to determine the MTD and RP2D of niraparib combined with Radium-223. The MTD was defined as the highest dose level at which the probability of a dose limiting toxicity (DLT) was less than 20%.
Since the toxicity of radium-223 is often delayed, a time-to-event continuous reassessment method (TITE-CRM) (28) design was used to identify the maximal tolerated dose (MTD) based on toxicities observed over 12 weeks of treatment (3 cycles of radium 223). A dose limiting toxicity (DLT) was defined as any treatment-related Grade ≥3 non-hematologic clinical that required medical intervention, or persisted for ≥7 days; any grade 4 hematologic toxicity with the exception of neutropenia Grade 4 lasting for <7 days and not associated with fever >38.5 degrees Celsius and/or infection; a dose interruption for a non-DLT laboratory abnormality lasting ≥14 days or a dose interruption for non-hematologic AE leading to <80% of an intended dose of niraparib being administered. Patients were followed from time of enrollment until progression of disease or death. The trial was conducted under the auspices of the Prostate Cancer Clinical Trials Consortium.
Eligible patients had histologic or cytologic diagnosis of adenocarcinoma of the prostate without neuroendocrine or small cell features, bone metastases detected by conventional bone scans and evidence of progressive disease after receiving at least 1 line of AR-targeted therapy in the hormone sensitive or castrate resistant setting. Each patient was required to have serum testosterone levels in the castrate range (less than 50 ng/dL) and this was measured at time of screening. Patients were ineligible if they had: previously received a PARP inhibitor, more than 1 prior line of AR-targeted therapy or more than one chemotherapy agent. Patients who were previously identified to be carriers of a pathogenic germline or somatic mutation in BRCA-1 or BRCA-2 were excluded; however, additional testing to identify BRCA or other DDR pathway mutations was not conducted prior to enrollment. Additionally, patients with bulky visceral disease (defined as > 4cm), brain or leptomeningeal disease or impending spinal cord compression were excluded.
Treatment
Patients were assigned to one of the three different treatment arms based using TITE-CRM (28) design which allowed for three different dose levels of niraparib to be tested simultaneously with Radium-223. Subjects were given niraparib at doses of 100mg, 200mg or 300mg oral daily in combination with standard dosing of Radium-223, 55kBq per kg body weight, given at 4 week intervals for a total of 6 injections. Following 6 cycles, subjects were to continue on niraparib alone until objective progression, intolerance of therapy or withdrawal of consent. Enrollment was stratified by prior chemotherapy use and we aimed to enroll up to 30 patients total, with no more than 15 patients in each arm.
The TITE-CRM model was specifically chosen to improve the time to completion of the study, given that toxicities to Radium-223 are often delayed by several weeks. In the TITE-CRM, dose levels were assigned to each newly enrolled subject based on DLT data from subjects already enrolled on trial, which were weighted to account for the proportion of the observation period that each enrolled subject had been observed. Dose interruptions and reductions of niraparib were allowed for management of AEs per study protocol. The dose of radium-223 was not adjusted, both agents were held in the event of a DLT and radium-223 was not continued as a single agent in the event of niraparib discontinuation. Patients were removed from the study if they had an AE on the lowest dose of niraparib (100 mg) or if the subject required a dose interruption of more than 28 days. The study treatment also stopped for any patient who had clinical disease progression, intercurrent illness that made further treatment unsafe, developed myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML), or withdrew of the consent.
Endpoints and Clinical Assessments
The primary outcome of this phase IB study was determination of the MTD and RP2D of niraparib combined with standard dosing of Radium-223 in the treatment of patients with mCRPC. Secondary endpoints included proportion of subjects with 50% prostate specific antigen (PSA) reduction at 12 weeks, radiographic progression free survival (rPFS) at 6 months and long-term safety and tolerability of treatment combination. AEs were defined by the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 4.0. Subjects were re-assessed for any AEs weekly for the first 4 weeks, then bi-weekly for the next 8 weeks and finally monthly until disease progression or death. Any individual who was withdrawn from the study due to an AE was followed until event had stabilized or resolved. Disease status (response or progression) was assessed every 12 weeks and were determined using a combination of the revised Response Evaluation Criteria in Solid Tumors (RECIST 1.1) criteria and the guideline for prostate cancer endpoints developed by the Prostate Cancer Clinical Trials Working Group 3 (29,30).
Statistical Analysis
The TITE-CRM statistical design was used, assuming prior probabilities of toxicity for the three doses of 0.04, 0.08, and 0.12, with a target probability of toxicity for the optimal dose of 0.20, and a DLT evaluation time of 84 days. Calculation of the next dose to be assigned and the final toxicity probability estimates were performed using the SAS macro developed by the University of Michigan Comprehensive Cancer Center Biostatistics Unit (31). Patients were stratified within group by prior chemotherapy exposure and analyses were performed separately by strata. rPFS was estimated using the Kaplan-Meier method. Summary statistics were used to assess PSA change and to describe translational results. Time was calculated from day of first treatment until incidence of toxicity, progression or death.
Exploratory Correlative Endpoints
Gene expression changes in whole blood samples at baseline and during treatment (cycle 1, day 15 and cycle 3, day 15) were evaluated in 18 of 30 patients. RNA from whole blood, collected from participants, was isolated using the Qiagen miRNA isolation kit (32) and was analyzed using the PanCancer Immune Pathways and PanCancer Driver Pathways panels, as previously described (33). The PanCaner Driver and Immune Panels included 770 genes each from 24 different driver pathways and 13 cancer associated canonical pathways, respectively.
In addition, plasma samples were collected at baseline in 15 patients and analyzed for circulating tumor DNA (ctDNA) using the Guardant 360 Liquid Biopsy platform (34). Each sample was assessed for different somatic and germline mutations including single nucleotide variants (SNVs), insertions, deletions, gene fusions and copy number variants (CNVs). For the 15 other participants, there were not viable samples to run such analysis.
Data Availability Statement
Data from this study is available through the Prostate Cancer Clinical Trials Consortium by emailing PCCTC@mskcc.org.
RESULTS
Patients
From May 2018 to January 2020 30 patients were enrolled at 6 different clinical sites in the United States and patient demographics are included in Table 1. Two patients were withdrawn from the study early. One patient withdrew shortly after randomization and never received a dose of either trial medication. One patient in the prior chemotherapy cohort at dose level 2 (Niraparib 200 mg) was removed from the study due prolonged thrombocytopenia. The remainder of the patients continued therapy until progression of disease or death. All patients were included in treatment analysis. The median age of patients was 69 years. Twenty-five patients were Caucasian, five patients identified as black or African American. For patients who had previously received chemotherapy, the median time from completion of chemotherapy to treatment start date was 86 weeks (range 21 to 193 weeks).
Table 1:
Patient, Tumor and Treatment Characteristics
| Study Arm | 100mg (N=13) | 200mg (N=12) | 300mg (N=5) |
|---|---|---|---|
| Patient Characteristics | |||
| Age (median, years) | 71 | 66 | 73 |
| ECOG Performance status 0 1 |
6 (46%) 7 (54%) |
8 (67%) 4 (33%) |
3 (60%) 2 (40%) |
| Race Caucasian African American |
11 (85%) 2 (15%) |
10 (83%) 2 (17%) |
4 (80%) 1 (20%) |
| Baseline Hemoglobin (median, g/dL) | 11.7 | 12.4 | 13.1 |
| Baseline Platelet Count (median, B/L) | 212 | 217 | 244 |
| Disease Characteristics | |||
| Total Gleason Score at Diagnosis 6 7 8–10 |
0 3 (23%) 10 (76.9%) |
3 (25%) 1 (8.3%) 8 (66.7%) |
1 (20%) 1 (20%) 3 (60%) |
| Sites of Metastases Bone Only Bone and Lymph Node Only Visceral Metastases |
6 (46%) 3 (23%) 2 (15%) |
6 (50%) 3 (25%) 2 (16%) |
2 (40%) 3 (60%) 0 |
| Treatment Characteristics | |||
| Prior Prostatectomy | 1 (8%) | 2 (17%) | 2 (40%) |
| Prior Definitive Radiation Therapy | 5 (38%) | 3 (25%) | 1 (20%) |
| Prior AR Signaling Inhibitors Abiraterone Enzalutamide |
7 (54%) 6 (46%) |
8 (67%) 4 (33%) |
2 (40%) 2 (40%) |
| Prior Chemotherapy Docetaxel Cabazitaxel |
10 (77%) 9 (69%) 1 (8%) |
5 (42%) 5 (42%) 0 |
0 0 0 |
Maximum Tolerated Dose
DLTs were evaluated in all three treatment groups and stratified by prior chemotherapy exposure (Table 2). There were no DLT events for patients who received the 100 mg dose of niraparib and no patients were removed from the study due to a treatment related adverse event. In the cohort that had never received chemotherapy, 7 patients received a 200 mg dose of niraparib and there was 1 DLT event, leading to an estimated probability of DLT at this dose level of 0.156 with a 95% confidence interval of 0.042 to 0.385. There were 2 DLT events in the 5 patients who received a 300 mg dose of niraparib, leading to an estimated probability of DLT for this cohort of 0.214. Since the probability of DLT was greater than 0.2 in the 300 mg cohort, the 200 mg dose of niraparib was confirmed as the MTD and RP2D in chemotherapy naïve patients. There were 3 DLT events in 5 patients who had previously received chemotherapy for the 200 mg treatment group, leading to an unacceptable DLT probability and a recommendation of 100 mg niraparib as the MTD and RP2D for patients with prior chemotherapy exposure.
Table 2:
Probabilities of Dose Limiting Toxicities
| Chemotherapy Naïve | |||
|---|---|---|---|
| Dose Level | Patients Treated | DLTs | DLT Probability |
| 100 mg | 3 | 0 | 0.089 (0.019 – 0.281) |
| 200 mg | 7 | 1 | 0.156 (0.042 – 0.385) |
| 300 mg | 5 | 2 | 0.214 (0.068 – 0.455) |
| Prior Chemotherapy Exposure | |||
|---|---|---|---|
| Dose | Patients Treated | DLTS | DLT Probability |
| 100 mg | 10 | 0 | 0.127 ( 0.029 – 0.352) |
| 200 mg | 5 | 3 | 0.207 (0.061 – 0.455) |
| 300mg | 0 | 0 | 0.272 (0.095 – 0.521) |
Safety
There were 297 recorded adverse events that occurred during the trial, 153 of which were deemed attributable to study medications (Table 3). Most commonly, low grade disturbances to the gastrointestinal system occurred: with nausea (60%), diarrhea (30%) and constipation (27%) observed most frequently. Constitutional complaints such as fatigue (53%) and decreased appetite (27%) were also frequently observed. Significant hematologic toxicities were encountered infrequently, with only three patients with grade 3 or higher neutropenia. The average time to neutrophil recovery was 5.5 days, while the average time to platelet recovery was 18 days. There were no deaths considered related to study drug. One patient died secondary to widespread progression of disease and this event was not considered to be related to either study medication.
Table 3:
Most Frequently Observed Treatment Related Adverse Events
| Prior Chemotherapy Exposure | Chemotherapy Naïve | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Dose Level | 100mg | 200mg | 100mg | 200mg | 300mg |
| Patients | 10 | 5 | 3 | 7 | 5 |
| Max Grade 2 TRAE | |||||
| Hematologic | |||||
| Anemia | 1 (10%) | 1 (20%) | - | 1 (14%) | 1 (20%) |
| Thrombocytopenia | 2 (20%) | 1 (20%) | - | 2 (29%) | - |
| Neutropenia | 2 (20%) | 1 (20%) | - | - | - |
| Gastrointestinal | |||||
| Diarrhea | 2 (20%) | 1 (20%) | - | 3 (43%) | 3 (60%) |
| Constipation | 3 (30%) | - | - | 2 (29%) | 3 (60%) |
| Dry Mouth | 1 (10%) | - | - | 2 (29%) | 1 (20%) |
| Nausea | 4 (40%) | 4 (80%) | 1 (33%) | 5 (71%) | 3 (60%) |
| Vomiting | - | 1 (20%) | 2 (29%) | 1 (20%) | |
| Other | |||||
| Hypertension | 2 (20%) | - | 1 (33%) | - | - |
| Fatigue | 4 (40%) | 3 (60%) | - | 3 (43%) | 5 (100%) |
| Decrease Appetite | 3 (30%) | 1 (20%) | 2 ( 67%) | 2 (29%) | - |
|
| |||||
| Max Grade 4 TRAE | |||||
| Hematologic | |||||
| Anemia | - | 1 (20%) | - | 1 (14%) | 1 (20%) |
| Thrombocytopenia | - | 1 (20%) | - | 1 (14%) | - |
| Neutropenia | - | 2 (40%) | - | - | 1 (20%) |
| Gastrointestinal | |||||
| Nausea | - | - | - | - | 1 (20%) |
| Other | |||||
| Hypertension | 1 (10%) | 1 (20%) | 1 (33%) | - | 1 (20%) |
Clinical Outcome
PSA data was recorded for 28/30 patients. Of these, 9 patients had a PSA decline, of whom 3 had a PSA decline of > 50% (Supplemental Figure 1). For 5 participants, the PSA assessment was made prior to the 12th week, given that progressive disease had already occurred.
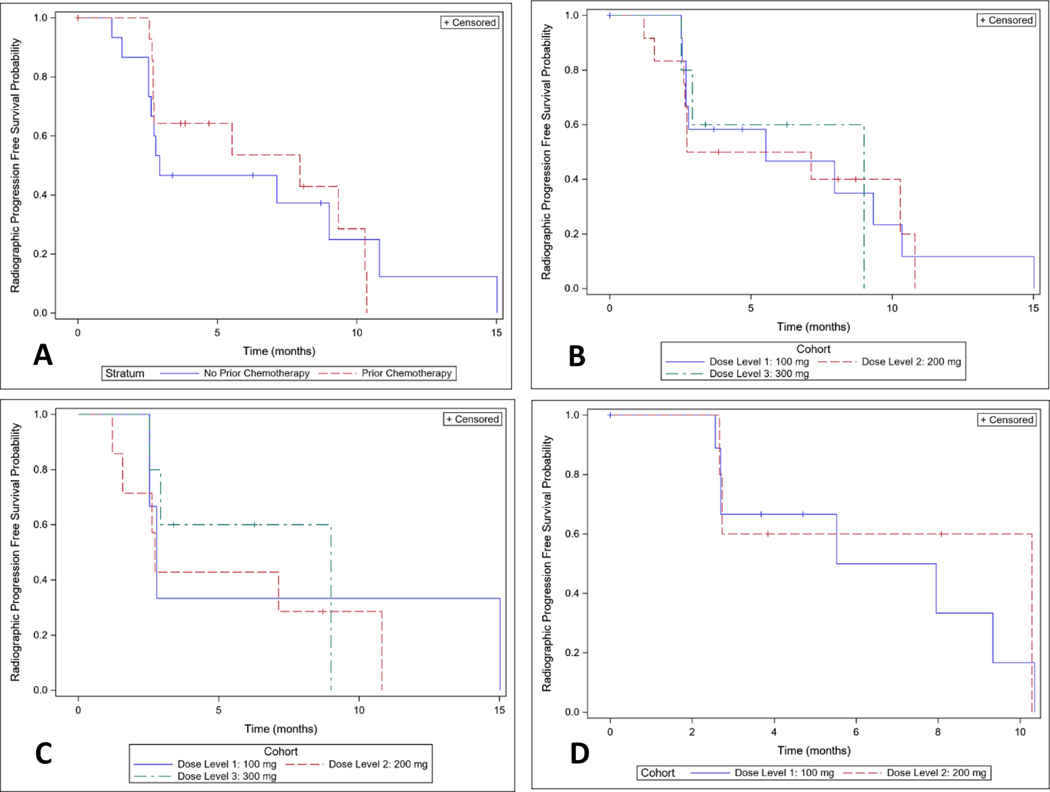
The median rPFS for all patients included in analysis was 7.1 months (C.I. 2.7–9.3 months) with an estimated 6-month rPFS of 51% (C.I. 31–67%). For patients who were chemotherapy naïve (Figure 1A), the median rPFS was 2.9 months (C.I. 2.5–10.8 months) and for patients with prior chemotherapy exposure the median rPFS was 8.0 months (C.I. 2.7–10.3 months). The estimated 6 month rPFS for these groups was 47% (C.I. 21–69%) and 54% (C.I. 23–77%). Radiographic progression free survival distributions were similar by dose overall (Figure 1B) and in chemotherapy naïve (Figure 1C) and prior chemotherapy cohorts (Figure 1D).
Figure 1: Radiographic Progression Free Survival (rPFS):
A) Overall rPFS stratified by prior chemotherapy exposure; B) Overall rPFS stratified by dose cohort; C) rPFS of the chemotherapy naive cohort, stratified by dose cohort; D) rPFS of the prior chemotherapy exposed cohort stratified by dose cohort.
Correlative Endpoints
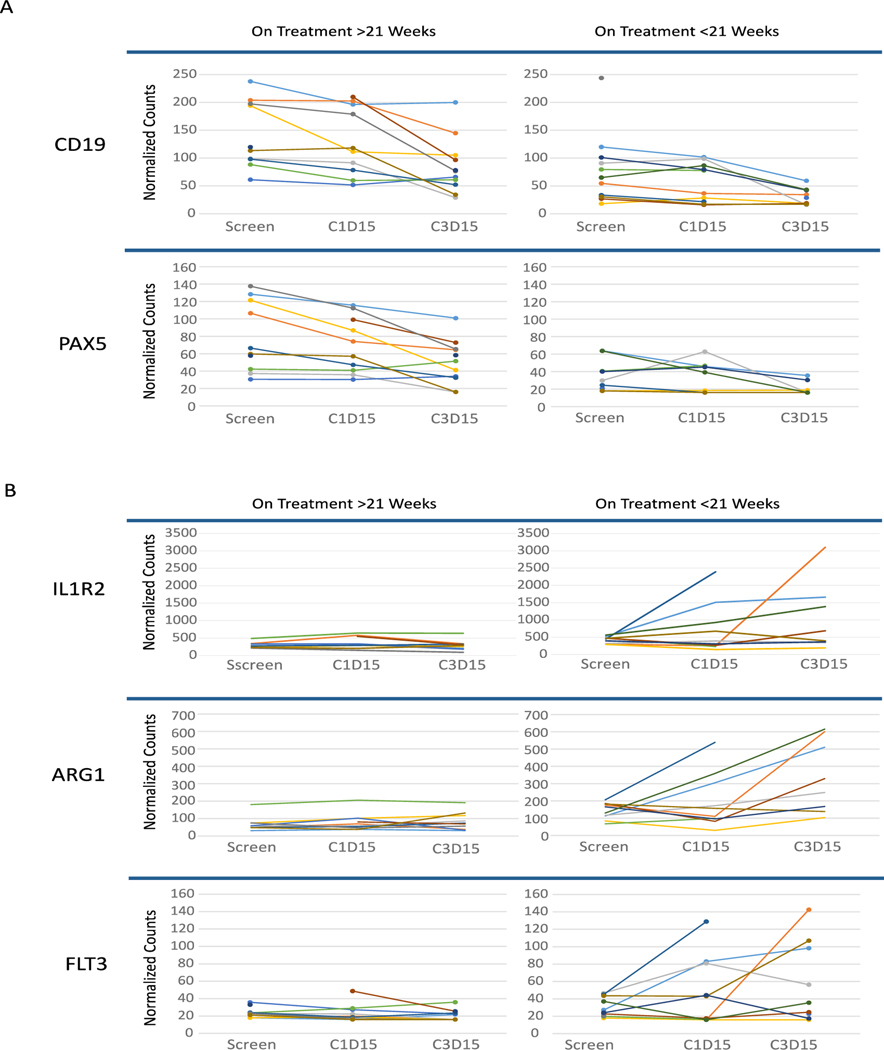
We performed gene expression profiling in total RNA from blood obtained from patients at the stated time points, with the hypothesis that potential differences in gene expression would be observed between patients experiencing longer duration on treatment as a surrogate marker for response. Indeed, several intriguing changes in gene expression profiles between patients based on treatment duration (median 21 weeks duration for the 18 patients that had viable RNA available for analysis) were detected. At baseline, paired box protein 5 (PAX5) and CD-19 expression were higher in the group that was on treatment for longer than 21 weeks in comparison to patients that stayed on treatment for less than 21 weeks (Figure 2A). By cycle 3 day 15, expression of both PAX5 (linear fold change = −5.08) and CD19 (linear fold change = −3.82) was more attenuated in the longer treatment duration group in comparison to the shorter treatment duration group.
Figure 2. Gene expression differences in blood from enrolled patients with treatment duration greater or less than median 21 weeks.
A) CD19 and PAX5 expression appears to be higher at baseline and decrease with treatment in patients treated longer than median 21 weeks compared to patients treated less than 21 weeks. B) IL1R2, ARG1, and FLT3 expression increases over time in patients with duration of treatment shorter than median 21 weeks but does not in patients with treatment of duration than median 21 weeks.
In contrast, gene expression patterns for Interleukin-1 receptor type 2 (IL-1R2), Arginase 1 (ARG1), and FMS like tyrosine kinase 3 (FLT3) increased in the group treated for less than 21 weeks in comparison to the group treated for longer than 21 weeks (Figure 2B). Specifically, by cycle 3 day 15, ARG1 (linear fold change = 3.32), FLT3 (linear fold change = 3.32) and IL-1R2 (linear fold change = 2.80) had all markedly increased in patients who experienced a relatively faster time to progression. These changes in gene expression are intriguing and need further investigation in future studies.
ctDNA was detected in 14/15 samples collected at baseline. Of these, 7 had AR amplification and 4 instances of point mutations within the gene were detected (Table 4). Somatic mutations in TP53 and PIK3CA were detected in 7 and 4 patients respectively as well. Deletions in RB1 and BRCA2 were also detected via copy number variations in 7 and 4 patients respectively. There were 6 mutations found in BRCA2, two of which were germline SNVs, and one mutation found in ATM and CDK12 genes, respectively. Notably, the median time to progression for 7 patients with HRR variants was only 21 weeks; however, the median time to progression for the two patients with germline BRCA2 mutations was 43 weeks, significantly longer than the median duration for the entire population.
Table 4:
Baseline ctDNA results for samples with Relevant Co-occurring Alterations
| Patient Number | SNV/InDel | CNV | Dose and Cohort | Time to Progression (weeks) |
|---|---|---|---|---|
| 1 | CDK12 K480 | 100 mg - C | 19 | |
| CDK12 L867P | ||||
| AR L702H | ||||
|
| ||||
| 2 | AR amplification | 200 mg - C | 30 | |
| RB1 homozygous del | ||||
|
| ||||
| 3 | PIK3CA N345K | BRCA2 LOH deletion | 200 mg - N | 13 |
| ATM M2405V | RB1 deletion | |||
| AR L702H | AR amplification | |||
|
| ||||
| 4 | AR amplification | 200 mg - N | 31 | |
| BRCA2 homozygous del | ||||
| RB1 LOH deletion | ||||
|
| ||||
| 5 | PIK3CA N345K | PIK3CA amplification | 100 mg - N | 15 |
| AR amplification | ||||
| RB1 homozygous del | ||||
|
| ||||
| 6 | AR W742C | RB1 homozygous del | 100 mg - N | 32 |
| EGFR D837N | EGFR amplification | |||
|
| ||||
| 7 | AR F877L | ATM LOH deletion | 100 mg - N | 21 |
| PIK3CA H1047R | BRCA2 LOH deletion | |||
| RB1 deletion | ||||
|
| ||||
| 8 | BRAF K601E | 300 mg - N | 38 | |
| AR T878A | ||||
| BRCA2 H1003fs (g) | ||||
| BRCA2 Q2859fs | ||||
| RB1 L572fs | ||||
|
| ||||
| 9 | BRCA2 deletion | 200 mg - C | 12 | |
| RB1 LOH deletion | ||||
| PIK3CA amplification | ||||
| AR amplification | ||||
|
| ||||
| 10 | BRCA2 c682–1G>A | 100 mg - C | 49 | |
| BRCA2 K2849fs (g) | ||||
|
| ||||
| 11 | PIK3CA H1047R | AR amplification | 100 mg - C | 18 |
Legend: ctDNA = circulating tumor DNA, C = prior chemotherapy, N = chemotherapy naïve, CNV=copy number variant, SNV= single nucleotide variant, InDel= insertion/deletion variant, LOH = loss of heterozygosity, del = deletion, fs = frameshift, (g) = germline
DISCUSSION:
This multi-center phase IB trial was the first to assess the combination of a PARP inhibitor with Radium-223 in patients with mCRPC without known BRCA mutations. The combination of 200 mg of niraparib with standard dosing of radium-223 was found to be tolerable in a subset of patients who were chemotherapy naïve, while a dose of 100 mg niraparib was tolerable for patients who had experienced prior treatment with taxane therapy. Currently, the combination of olaparib with radium-223 is under investigation and investigators used a 3+3 trial design to evaluate 12 patients at two different dose levels of olaparib, determining 200 mg twice daily as their R2PD (35). Importantly, our study demonstrates the feasibility and utility of the TITE-CRM statistical design when investigating therapeutics that have a delayed toxicity profile like radio-ligands. We were able to safely evaluate 30 patients over three different dose levels simultaneously, which accelerated trial accrual without exposing participants to excess risk. This trial can serve as a framework for the development of other combined modality therapies using radio-ligands, which may prove pivotal with the recent approval of Lu 177 vipivotide tetraxetan for patients with mCRPC (36).
By down regulating several DNA repair pathways, PARP inhibitors have gained interest as radiosensitizers in many different cancer models and recent studies have demonstrated safety of PARP inhibitors with other radiation modalities (37–39). Our study adds to these findings as we were able to show tolerability of 200 mg of niraparib with standard of care dosing of Radium-223 in patients without prior chemotherapy exposure. The majority of adverse events related to therapy were either grade 1 or 2, with nausea, diarrhea or constipation and fatigue reported as the most common symptoms. Despite potential overlapping toxicities to the bone marrow, we did not observe significant issues with impaired hematopoiesis and there were few grade 3 events of cytopenia. In all but 1 instance, the cytopenia resolved after holding niraparib. There were no instances of grade 3 infections and there were no deaths attributable to study medications.
Currently niraparib is approved as a monotherapy for treatment of ovarian cancer at 300 mg daily and studies are ongoing in biomarker selected metastatic prostate cancer patients at 200 mg daily in combination with abiraterone acetate 1000 mg. In regards to combination with radiation therapy, studies evaluating Olaparib in lung and glioblastoma have concluded that, when combined with radiation, the MTD of Olaparib needs to be reduced from the recommend monotherapy dose in order to limit hematologic toxicity (37,40). Additionally trials are currently enrolling that are testing reduced doses, 100 mg or 200 mg, of Niraparib in combination with radiation therapy for high risk early stage prostate cancer (41,42). Recently de Haan and colleagues were able to show that a 25 mg dose of Olaparib was able to effectively reduce baseline poly ADP-ribose (PAR) levels along with radiation induced PARylation in lung tumor tissue and peripheral blood mononuclear cells, concluding that a reduced dose of a PARP inhibitor still has biologic activity in combination with radiation therapy (37). Taken together, our findings that using either 100 mg or 200 mg doses of Niraparib in combination with radiation therapy are not likely to drastically reduce the clinical activity of the PARP inhibitor.
Our translational studies demonstrate that using the NanoString platform to perform gene expression profiling on whole blood samples was feasible. Although one limitation to testing whole blood is the uncertainty of the source of RNA, whether it is tumor-derived vs. from normal cells (43).
We observed differences in PAX5 and CD19 expression when comparing patients who had a response to therapy to those without a response. At baseline, RNA transcripts were higher for both of these targets in responders compared to non-responders and a statistically significant reduction in mRNA level was observed with treatment in those who remained on study for longer than 21 weeks. PAX5 is an oncogene that encodes a potent transcription factor leading to B-cell development and aberrant expression has been observed frequently with development of aggressive B-cell malignancies (44). CD19, a cell surface marker expressed on all B lymphocytes, is critical for cell function and CD19 expression is in part regulated by PAX5 (45). Additionally, significant PAX5 expression has also been demonstrated in non-lymphoid malignancies, including breast, small cell lung cancer and pediatric tumors and is believed to play a role in cell survival and metastatic potential (44). One down-stream target of PAX5 is c-MET, a tyrosine kinase receptor for which mutations and aberrant expression are known promote oncogenesis (46).
B-cells in the tumor microenvironment can promote tumorigenesis and increase metastatic potential by causing antibody mediated T-cell suppression, promoting lymphangiogenesis and producing immunomodulatory cytokines that suppress the anti-tumor immune response (47). In prostate cancer, it has been observed that B-cell infiltration is higher in high grade tumors and in tumors that eventually recurred or progressed in comparison to low or intermediate grade tumors (48). Additionally, infiltrating B cells were shown to support castrate resistant cell growth by secreting lymphotoxin which lead to cell survival (49).
ARG1 expression was similar at baseline when comparing patients who responded to therapy to those who did not; however, in the patients who did not respond to therapy, by cycle 3 day 15, ARG1 expression was nearly 4 times higher. This suggests that expression of ARG1 could possibly serve as a protective mechanism, limiting the effect of treatment. ARG1 expression by tumor associated macrophages (TAMs) has been demonstrated as a mechanism to suppress the effect of infiltrating T cells by limiting the amount of L-arginine available for T cell function (50). Interestingly we observed expansion of FLT3 transcripts mirrored ARG1 in patients who had relatively faster time to progression. FLT3 critically drives dendritic cell maturation and activity in the tumor microenvironment. This may either lead to an amplified immune response, through increased antigen presentation to infiltrating cytotoxic T cells, or diminished local immune response, by expansion of T regulatory cells (Treg) (51).
With these hypothesis-generating observations in whole blood RNA expression, a speculative mechanism for the effect of niraparib with Radium-223 may be through the immune response within the tumor microenvironment. In patients who stayed on treatment for longer than 21 weeks we observed changes indicative of decreased B-cell function which may allow for increased immune activity within the microenvironment. Conversely, resistance to therapy may be mediated through decreased local T-cell activity, by increased ARG1 expression by TAMs and FLT3 expression driving Treg expansion. Further investigation is needed to explore this phenomenon, but if the mechanism is valid, one could speculate that adding therapies to improve T-cell mediated immune response could augment this therapeutic approach. Notably, recent phase III trials have reported improved outcomes in mCRPC when combining PARP inhibitors with abiraterone acetate plus prednisone in those with germline/somatic homologous recombination defects, with potentially a less robust benefit in unselected patients (25,52). Trials are also evaluating the combination of PARP inhibitors with other androgen inhibitors, immune checkpoint inhibitors and Lu-PSMA-617 (53–55). Thus, the optimal combination partner remains to be determined.
Our exploratory analysis of baseline ctDNA discovered 4 patients with somatic BRCA variants and two patients that had germline BRCA2 mutations. These two patients were in the 300 mg and 100 mg cohorts and had dramatically longer times to progression of 38 and 49 weeks, respectively. Additionally, the patient in the 100 mg cohort had previously received chemotherapy. These outcomes are concordant with prior observations of favorable responses to Radium-223 in patients with known BRCA mutations (56–58), in addition to the apparent effect of PARP inhibitors in this population. Given the known efficacy of PARP inhibitors in patients with germline/somatic BRCA mutations, we intentionally excluded patients with prior record of these alterations at trial enrollment. However, this expected finding highlights the impact of ctDNA analysis and further demonstrates the importance of screening patients for molecular biomarkers before choosing therapies.
This study was not powered nor designed to evaluated efficacy, but we were able to collect data on PSA change and rPFS to help describe trends. PSA increased in the majority of patients on study; however, rapid PSA response is not a hallmark of Radium-223 therapy, as Radium-223 can cause an early PSA flare in patients who are responding to therapy (59,60). Nearly 30% of patients had a PSA decline, however only 10% had a PSA decline of > 50% at week 12. Three of the 9 patients with a PSA decline were found to have BRCA mutations on ctDNA; 5 of the remaining 6 patients did not have blood samples evaluated for ctDNA. In regards to rPFS, there did not appear to be a statistically significant difference between patients who had prior chemotherapy exposure and those who were chemotherapy naïve, with wide overlapping confidence intervals ranging from 2.5 to 10.8 months.
In conclusion, niraparib in combination with Radium-223 was demonstrated to be safe in combination for the treatment of mCRPC for patients who have previously progressed on AR therapies. We intend to continue investigation with a prospective phase II trial that will better evaluate efficacy endpoints and assess potential long term toxicities and will serve as a companion to the ongoing trial of olaparib with Radium223 in unselected patients with mCRPC (35).
Supplementary Material
TRANSLATIONAL RELEVANCE:
Poly (ADP-ribose) polymerase (PARP) inhibitors have demonstrated to be effective in treating mCRPC that harbor mutations in DNA damage repair (DDR) pathways. However, their effectiveness outside this population is limited and investigation into their role in combination with other therapies is prudent. Additionally, advances in radiopharmaceuticals in metastatic castrate resistant prostate cancer (mCRPC) has created a need for trials evaluating their safety with other approved agents. This novel designed Phase 1b study evaluated the safety and tolerability of niraparib combined with Radium-223 in treatment of men with mCRPC. We were able to demonstrate the utility of a time-to-event continuous reassessment method of enrollment and the combination of therapies proved to be manageable with a 200 mg dose of niraparib. While the combination was safe no increase in clinical activity was found. We demonstrated that gene expression profiling on whole blood was feasible and observed different patterns in expression of immunomodulatory genes between responders and non-responders to therapy.
Acknowledgments:
The authors would like to thank Ms. Deborah Della Manna and Dr. Jianqing Zhang for performing the Nanostring experiments. Additionally, we thank Jake Vinson, Garrett Abrams, Sarah Wise and other members of the Prostate Cancer Clinical Trials Consortium for their ongoing support.
Grant support:
Department of Defense (DOD) Congressionally Directed Medical Research Programs (CDMRP) Clinical Consortium Award W81XWH-22-2-0020; P30 CA056036; R01CA217329
Financial Support:
Janssen Pharmaceutical, Bayer, DOD-Prostate Cancer Clinical Trials Consortium
Footnotes
Conflicts of interest:
Zachary Quinn
• The author declares no potential conflicts of interest
Benjamin Leiby
• Consultant for Bayer Healthcare Pharmaceuticals in 2017
Guru Sonpavde:
• My conflicts and Disclosures in the past 36 months are as follows (relevant or not relevant):
• Advisory Board: BMS, Genentech, EMD Serono, Merck, Sanofi, Seattle Genetics/Astellas, Astrazeneca, Exelixis, Janssen, Bicycle Therapeutics, Pfizer, Gilead, Scholar Rock, G1 Therapeutics, Eli Lilly/Loxo Oncology, Infinity Pharmaceuticals, Lucence Health, IMV, Vial
• Consultant/Scientific Advisory Board (SAB): Suba Therapeutics
• Research Support: Sanofi (iaward), Astrazeneca, Gilead, Helsinn, Lucence, Predicine, BMS, EMD Serono, Jazz Therapeutics, Genecentric
• Steering committee of studies: BMS, Bavarian Nordic, Seattle Genetics, QED, G1 Therapeutics (all unpaid), and Astrazeneca, EMD Serono, Debiopharm (paid).
• Data safety monitoring committee: Mereo
• Employment: Spouse employed by Myriad
Atish Choudhury
• Honoraria: OncLive, Bayer, Targeted Oncology, Aptitude Health, Journal of Clinical Pathways, Cancer Network, Clinical Care Options, Great Debates & Updates, Pfizer, Springer Healthcare;
• Consulting: Blackstone; Advisory Board: Clovis, Dendreon, Bayer, Eli Lilly, AstraZeneca, Astellas, Blue Earth, Janssen, Tolmar;
• Research Funding: Bayer
Christopher Sweeney
• Dr. Sweeney reports personal fees and other from Sanofi, personal fees and other from Janssen, personal fees and other from Astellas, personal fees from Leuchemix, personal fees from Genentech, personal fees and other from Bayer, personal fees from Lilly, other from Dendreon, outside the submitted work;
• In addition, Dr. Sweeney has a patent Combination of abiraterone and cabozantinib for prostate cancer pending to Exelixis, a patent Parthenolide as a treatment for cancer issued to Indiana University, and a patent Dimethylaminoparthenolide as a treatment for cancer issued to Leuchemix.
David J. Einstein:
• Research funding to institution: Bristol-Myers Squibb, Cardiff Oncology, MiNK Therapeutics, Puma Biotechnology
• Discounted research sequencing from Foundation Medicine
Russel Szmulewitz
• Honoraria: Astellas Pharma, Pfizer/Astellas
• Consulting: AstraZeneca, Abbvie, Exelixis, Merck, Amgen, Janssen Oncology, Sanofi, Astellas Pharma, Pfizer
• Research Funding: Abbvie, Astellas Pharma, Macrogenics, Janssen oncology, Plexxikon, Harpoon therapeutics, Merck, Novartis,
• Patents: Patent licensed by University of Chicago of which I am Co-inventor to corcept Therapeutics for combination AR/GR inhibition in prostate cancer
Oliver Sartor
• Consultant: Advanced Accelerator Applications (AAA), Astellas, AstraZeneca, Bayer, Blue Earth Diagnostics, Inc., Bavarian Nordic, Bristol Myers Squibb, Clarity Pharmaceuticals, Clovis, Constellation, Dendreon, EMD Serono, Fusion, Isotopen Technologien Meunchen, Janssen, Myovant, Myriad, Noria Therapeutics, Inc., Novartis, Noxopharm, Progenics, POINT Biopharma, Pfizer, Sanofi, Tenebio, Telix, Theragnostics
• Grant/Research Support: Advanced Accelerator Applications, Amgen, AstraZeneca, Bayer, Constellation, Endocyte, Invitae, Janssen, Lantheus, Merck, Progenics, Tenebio
Karen Knudsen, PhD, MBA
• Consulting\Advisory Board: CellCentric, Janssen, Sanofi
Eddy Yang
• Relevant COI: research funding support from PCCTC
• Other COI: Consultant AstraZeneca; Advisory Board Bayer, Clovis
• Research funding: Eli Lilly, PUMA; Clinical Trial Funding ASCO TAPUR, Elevation Oncology, PUMA, Clovis, Bayer Eli Lilly
William Kevin Kelly:
My conflicts and Disclosures in the past 36 months are as follows (relevant or not relevant):
• Consultant/Scientific Advisory Board (SAB): Janssen (non-compensated), Bayer (non-compensated), Merck-Sharpe Dome
• Speaker’s Bureau: none
• Research Support: Sanofi (institution); Novartis (institution); Janssen Oncology (institution); Bayer (institution); Exelixis (institution); Seattle Genetics (institution); Amgen (institution); Regeneron (institution); Endocyte (institution); BioClin Therapeutics (institution);Sarah Cannon Research Institute (institution); Roche (institution)
• Steering committee of studies: none
• Data safety monitoring committee: T-Immunity
• Employment: none
Clinical trial support: Bayer and Janssen Pharmaceuticals
REFERENCES:
- 1.Cancer Stat Facts: Prostate Cancer. National Cancer Institute: Surveillance, Epidemiology, and End Results Program; 2021. [Google Scholar]
- 2.Dai C, Heemers H, Sharifi N. Androgen Signaling in Prostate Cancer. Cold Spring Harb Perspect Med 2017;7(9) doi 10.1101/cshperspect.a030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol 2005;23(32):8253–61 doi 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 4.Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol 2008;26(2):242–5 doi 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- 5.Eisenberger M, Hardy-Bessard AC, Kim CS, Geczi L, Ford D, Mourey L, et al. Phase III Study Comparing a Reduced Dose of Cabazitaxel (20 mg/m(2)) and the Currently Approved Dose (25 mg/m(2)) in Postdocetaxel Patients With Metastatic Castration-Resistant Prostate Cancer-PROSELICA. J Clin Oncol 2017;35(28):3198–206 doi 10.1200/JCO.2016.72.1076. [DOI] [PubMed] [Google Scholar]
- 6.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013;368(2):138–48 doi 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beer TM, Armstrong AJ, Rathkopf D, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in Men with Chemotherapy-naive Metastatic Castration-resistant Prostate Cancer: Extended Analysis of the Phase 3 PREVAIL Study. Eur Urol 2017;71(2):151–4 doi 10.1016/j.eururo.2016.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010;363(5):411–22 doi 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 9.Hansen AR, Massard C, Ott PA, Haas NB, Lopez JS, Ejadi S, et al. Pembrolizumab for advanced prostate adenocarcinoma: findings of the KEYNOTE-028 study. Ann Oncol 2018;29(8):1807–13 doi 10.1093/annonc/mdy232. [DOI] [PubMed] [Google Scholar]
- 10.de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med 2020;382(22):2091–102 doi 10.1056/NEJMoa1911440. [DOI] [PubMed] [Google Scholar]
- 11.Abida W, Patnaik A, Campbell D, Shapiro J, Bryce AH, McDermott R, et al. Rucaparib in Men With Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J Clin Oncol 2020;38(32):3763–72 doi 10.1200/JCO.20.01035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fossa SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369(3):213–23 doi 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 13.Hodgkins PS, O’Neil P, Stevens D, Fairman MP. The severity of alpha-particle-induced DNA damage is revealed by exposure to cell-free extracts. Radiat Res 1996;146(6):660–7. [PubMed] [Google Scholar]
- 14.Morris MJ, Corey E, Guise TA, Gulley JL, Kevin Kelly W, Quinn DI, et al. Radium-223 mechanism of action: implications for use in treatment combinations. Nature Reviews Urology 2019;16(12):745–56 doi 10.1038/s41585-019-0251-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nilsson S. Alpha-Emitter Radium-223 in the Management of Solid Tumors: Current Status and Future Directions. American Society of Clinical Oncology Educational Book 2014(34):e132–e9 doi 10.14694/EdBook_AM.2014.34.e132. [DOI] [PubMed] [Google Scholar]
- 16.Rose M, Burgess JT, O’Byrne K, Richard DJ, Bolderson E. PARP Inhibitors: Clinical Relevance, Mechanisms of Action and Tumor Resistance. Front Cell Dev Biol 2020;8:564601 doi 10.3389/fcell.2020.564601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masson M, Niedergang C, Schreiber V, Muller S, Menissier-de Murcia J, de Murcia G. XRCC1 is specifically associated with poly(ADP-ribose) polymerase and negatively regulates its activity following DNA damage. Mol Cell Biol 1998;18(6):3563–71 doi 10.1128/mcb.18.6.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polkinghorn WR, Parker JS, Lee MX, Kass EM, Spratt DE, Iaquinta PJ, et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov 2013;3(11):1245–53 doi 10.1158/2159-8290.CD-13-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Chang W, Yang G, Ren C, Park S, Karantanos T, et al. Targeting poly(ADP-ribose) polymerase and the c-Myb-regulated DNA damage response pathway in castration-resistant prostate cancer. Sci Signal 2014;7(326):ra47 doi 10.1126/scisignal.2005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodwin JF, Schiewer MJ, Dean JL, Schrecengost RS, de Leeuw R, Han S, et al. A hormone-DNA repair circuit governs the response to genotoxic insult. Cancer Discov 2013;3(11):1254–71 doi 10.1158/2159-8290.CD-13-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones P, Altamura S, Boueres J, Ferrigno F, Fonsi M, Giomini C, et al. Discovery of 2-{4-[(3S)-piperidin-3-yl]phenyl}−2H-indazole-7-carboxamide (MK-4827): a novel oral poly(ADP-ribose)polymerase (PARP) inhibitor efficacious in BRCA-1 and −2 mutant tumors. J Med Chem 2009;52(22):7170–85 doi 10.1021/jm901188v. [DOI] [PubMed] [Google Scholar]
- 22.Moore KN, Secord AA, Geller MA, Miller DS, Cloven N, Fleming GF, et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol 2019;20(5):636–48 doi 10.1016/S1470-2045(19)30029-4. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Martin A, Pothuri B, Vergote I, DePont Christensen R, Graybill W, Mirza MR, et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med 2019;381(25):2391–402 doi 10.1056/NEJMoa1910962. [DOI] [PubMed] [Google Scholar]
- 24.Smith MR, Scher HI, Sandhu S, Efstathiou E, Lara PN Jr., Yu EY, et al. Niraparib in patients with metastatic castration-resistant prostate cancer and DNA repair gene defects (GALAHAD): a multicentre, open-label, phase 2 trial. Lancet Oncol 2022;23(3):362–73 doi 10.1016/S1470-2045(21)00757-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chi KN, Rathkopf DE, Smith MR, Efstathiou E, Attard G, Olmos D, et al. Phase 3 MAGNITUDE study: First results of niraparib (NIRA) with abiraterone acetate and prednisone (AAP) as first-line therapy in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) with and without homologous recombination repair (HRR) gene alterations. Journal of Clinical Oncology 2022;40(6_suppl):12- doi 10.1200/JCO.2022.40.6_suppl.012.34752147 [DOI] [Google Scholar]
- 26.Clarke NW, Armstrong AJ, Thiery-Vuillemin A, Oya M, Shore N, Loredo E, et al. Abiraterone and Olaparib for Metastatic Castration-Resistant Prostate Cancer. NEJM Evidence;0(0):EVIDoa2200043 doi doi: 10.1056/EVIDoa2200043. [DOI] [PubMed] [Google Scholar]
- 27.Zheng F, Zhang Y, Chen S, Weng X, Rao Y, Fang H. Mechanism and current progress of Poly ADP-ribose polymerase (PARP) inhibitors in the treatment of ovarian cancer. Biomed Pharmacother 2020;123:109661 doi 10.1016/j.biopha.2019.109661. [DOI] [PubMed] [Google Scholar]
- 28.Normolle D, Lawrence T. Designing dose-escalation trials with late-onset toxicities using the time-to-event continual reassessment method. J Clin Oncol 2006;24(27):4426–33 doi 10.1200/JCO.2005.04.3844. [DOI] [PubMed] [Google Scholar]
- 29.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45(2):228–47 doi 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 30.Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations From the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol 2016;34(12):1402–18 doi 10.1200/JCO.2015.64.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biostatistics TCFC. 2009. TITE-CRM RESOURCES. University of Michigan School of Public Health <https://sph.umich.edu/ccb/tite-resources.html> [Google Scholar]
- 32.Qiagen. 2022. PAXgene Blood miRNA Kit. <https://www.qiagen.com/us/products/discovery-and-translational-research/dna-rna-purification/rna-purification/total-rna/paxgene-blood-mirna-kit/>.
- 33.Yang ES, Nassar AH, Adib E, Jegede OA, Alaiwi SA, Manna DLD, et al. Gene Expression Signature Correlates with Outcomes in Metastatic Renal Cell Carcinoma Patients Treated with Everolimus Alone or with a Vascular Disrupting Agent. Mol Cancer Ther 2021;20(8):1454–61 doi 10.1158/1535-7163.MCT-20-1091. [DOI] [PubMed] [Google Scholar]
- 34.Inc GH. Guardant360 CDx Gene List. 2021. [Google Scholar]
- 35.McKay RR, Xie W, Ajmera A, Saraiya B, Parikh M, Folefac E, et al. A phase 1/2 study of olaparib and radium-223 in men with metastatic castration-resistant prostate cancer (mCRPC) with bone metastases (COMRADE): Results of the phase 1 study. Journal of Clinical Oncology 2021;39(15_suppl):e17020-e doi 10.1200/JCO.2021.39.15_suppl.e17020. [DOI] [Google Scholar]
- 36.2022. Novartis Pluvicto™ approved by FDA as first targeted radioligand therapy for treatment of progressive, PSMA positive metastatic castration-resistant prostate cancer. <https://www.novartis.com/news/media-releases/novartis-pluvictotm-approved-fda-first-targeted-radioligand-therapy-treatment-progressive-psma-positive-metastatic-castration-resistant-prostate-cancer>. [Google Scholar]
- 37.de Haan R, van den Heuvel MM, van Diessen J, Peulen HMU, van Werkhoven E, de Langen AJ, et al. Phase I and Pharmacologic Study of Olaparib in Combination with High-dose Radiotherapy with and without Concurrent Cisplatin for Non-Small Cell Lung Cancer. Clin Cancer Res 2021;27(5):1256–66 doi 10.1158/1078-0432.CCR-20-2551. [DOI] [PubMed] [Google Scholar]
- 38.Karam SD, Reddy K, Blatchford PJ, Waxweiler T, DeLouize AM, Oweida A, et al. Final Report of a Phase I Trial of Olaparib with Cetuximab and Radiation for Heavy Smoker Patients with Locally Advanced Head and Neck Cancer. Clin Cancer Res 2018;24(20):4949–59 doi 10.1158/1078-0432.CCR-18-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chabot P, Hsia TC, Ryu JS, Gorbunova V, Belda-Iniesta C, Ball D, et al. Veliparib in combination with whole-brain radiation therapy for patients with brain metastases from non-small cell lung cancer: results of a randomized, global, placebo-controlled study. J Neurooncol 2017;131(1):105–15 doi 10.1007/s11060-016-2275-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chalmers AJ, Short S, Watts C, Herbert C, Morris A, Stobo J, et al. Phase I clinical trials evaluating olaparib in combination with radiotherapy (RT) and/or temozolomide (TMZ) in glioblastoma patients: Results of OPARATIC and PARADIGM phase I and early results of PARADIGM-2. Journal of Clinical Oncology 2018;36(15_suppl):2018- doi 10.1200/JCO.2018.36.15_suppl.2018. [DOI] [Google Scholar]
- 41.A Multi-Center Trial of Androgen Suppression With Abiraterone Acetate, Leuprolide, PARP Inhibition and Stereotactic Body Radiotherapy in Prostate Cancer. https://ClinicalTrials.gov/show/NCT04194554. [Google Scholar]
- 42.Niraparib With Standard Combination Radiation Therapy and Androgen Deprivation Therapy in Treating Patients With High Risk Prostate Cancer. https://ClinicalTrials.gov/show/NCT04037254. [Google Scholar]
- 43.Jensen K, Konnick EQ, Schweizer MT, Sokolova AO, Grivas P, Cheng HH, et al. Association of Clonal Hematopoiesis in DNA Repair Genes With Prostate Cancer Plasma Cell-free DNA Testing Interference. JAMA Oncol 2021;7(1):107–10 doi 10.1001/jamaoncol.2020.5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Brien P, Morin P Jr., Ouellette RJ, Robichaud GA The Pax-5 gene: a pluripotent regulator of B-cell differentiation and cancer disease. Cancer Res 2011;71(24):7345–50 doi 10.1158/0008-5472.CAN-11-1874. [DOI] [PubMed] [Google Scholar]
- 45.Chung EY, Psathas JN, Yu D, Li Y, Weiss MJ, Thomas-Tikhonenko A. CD19 is a major B cell receptor–independent activator of MYC-driven B-lymphomagenesis. The Journal of Clinical Investigation 2012;122(6):2257–66 doi 10.1172/JCI45851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kanteti R, Nallasura V, Loganathan S, Tretiakova M, Kroll T, Krishnaswamy S, et al. PAX5 is expressed in small-cell lung cancer and positively regulates c-Met transcription. Lab Invest 2009;89(3):301–14 doi 10.1038/labinvest.2008.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuen GJ, Demissie E, Pillai S. B lymphocytes and cancer: a love-hate relationship. Trends Cancer 2016;2(12):747–57 doi 10.1016/j.trecan.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woo JR, Liss MA, Muldong MT, Palazzi K, Strasner A, Ammirante M, et al. Tumor infiltrating B-cells are increased in prostate cancer tissue. J Transl Med 2014;12:30 doi 10.1186/1479-5876-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature 2010;464(7286):302–5 doi 10.1038/nature08782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Korrer MJ, Zhang Y, Routes JM. Possible role of arginase-1 in concomitant tumor immunity. PLoS One 2014;9(3):e91370 doi 10.1371/journal.pone.0091370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson KR, Villadangos JA, Mintern JD. Dendritic cell Flt3 - regulation, roles and repercussions for immunotherapy. Immunol Cell Biol 2021;99(9):962–71 doi 10.1111/imcb.12484. [DOI] [PubMed] [Google Scholar]
- 52.Saad F, Armstrong AJ, Thiery-Vuillemin A, Oya M, Loredo E, Procopio G, et al. PROpel: Phase III trial of olaparib (ola) and abiraterone (abi) versus placebo (pbo) and abi as first-line (1L) therapy for patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). Journal of Clinical Oncology 2022;40(6_suppl):11- doi 10.1200/JCO.2022.40.6_suppl.011. [DOI] [Google Scholar]
- 53.A Study of Olaparib and Durvalumab in Prostate Cancer. https://ClinicalTrials.gov/show/NCT03810105. [Google Scholar]
- 54.Study of Pembrolizumab (MK-3475) Plus Olaparib Versus Abiraterone Acetate or Enzalutamide in Metastatic Castration-resistant Prostate Cancer (mCRPC) (MK-7339–010/KEYLYNK-010). https://ClinicalTrials.gov/show/NCT03834519. [Google Scholar]
- 55.177Lu-PSMA-617 Therapy and Olaparib in Patients With Metastatic Castration Resistant Prostate Cancer. https://ClinicalTrials.gov/show/NCT03874884. [Google Scholar]
- 56.Isaacsson Velho P, Qazi F, Hassan S, Carducci MA, Denmeade SR, Markowski MC, et al. Efficacy of Radium-223 in Bone-metastatic Castration-resistant Prostate Cancer with and Without Homologous Repair Gene Defects. Eur Urol 2019;76(2):170–6 doi 10.1016/j.eururo.2018.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steinberger AE, Cotogno P, Ledet EM, Lewis B, Sartor O. Exceptional Duration of Radium-223 in Prostate Cancer With a BRCA2 Mutation. Clin Genitourin Cancer 2017;15(1):e69–e71 doi 10.1016/j.clgc.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 58.van der Doelen MJ, Isaacsson Velho P, Slootbeek PHJ, Pamidimarri Naga S, Bormann M, van Helvert S, et al. Impact of DNA damage repair defects on response to radium-223 and overall survival in metastatic castration-resistant prostate cancer. Eur J Cancer 2020;136:16–24 doi 10.1016/j.ejca.2020.05.001. [DOI] [PubMed] [Google Scholar]
- 59.Alva A, Nordquist L, Daignault S, George S, Ramos J, Albany C, et al. Clinical Correlates of Benefit From Radium-223 Therapy in Metastatic Castration Resistant Prostate Cancer. Prostate 2017;77(5):479–88 doi 10.1002/pros.23286. [DOI] [PubMed] [Google Scholar]
- 60.Castello A, Macapinlac HA, Lopci E, Santos EB. Prostate-specific antigen flare induced by (223)RaCl2 in patients with metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging 2018;45(13):2256–63 doi 10.1007/s00259-018-4051-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from this study is available through the Prostate Cancer Clinical Trials Consortium by emailing PCCTC@mskcc.org.