Abstract
Purpose of review:
To summarize the most recent publications highlighting the trends and disparities among patients diagnosed with high-risk endometrial cancer.
Recent findings:
Endometrial cancer mortality continues to rise, driven by the increasing incidence of high-risk histologic subtypes that accounts for a disproportionate number of endometrial cancer deaths. The lack of progress made in endometrial cancer treatment, particularly of high-risk histologic subtypes, disproportionately affects Black women who are more likely to be diagnosed with these aggressive tumor types. Even when accounting for high-risk histology, various factors across the spectrum of care may influence the survival disparities between Black and White women, including timely access to guideline-concordant care, clinical trial enrollment, and systemic racism that impacts cancer outcomes.
Summary:
In this review, we highlight the disproportionate impact of worsening endometrial cancer mortality and healthcare inequalities contributing to the endometrial cancer survival disparity between Black and White women.
Keywords: endometrial cancer, healthcare disparities, mortality
Introduction
In 2022 there will be an estimated 65,950 new cases of uterine cancer diagnosed in the United States. Uterine cancer remains the most common gynecologic malignancy while ovarian cancer remains the most lethal (1). That paradigm, however, is quickly shifting. While major treatment advances have extended ovarian cancer survival in the past several years, uterine cancer survival has remained relatively stagnant since the mid-1970s (1, 2). Overall trends of uterine and ovarian cancer mortality reflect changes in disease risk, incidence, and treatment. While the mortality of uterine cancer continues to climb, the mortality of ovarian cancer is declining. These oppositional trends in uterine and ovarian cancer mortality have resulted in a near convergence of mortality rates in recent years. Since 1990, ovarian cancer mortality has declined by an average of 2.7% annually, decreasing to 6.0 (per 100,000 women) in 2019. Meanwhile, while uterine cancer mortality declined in the 1990s, mortality has increased nearly 2% annually in the past decade, rising to 5.1 (per 100,000 women) in 2019 (2). While similar patterns are seen across racial and ethnic subgroups, the annual death rate of uterine cancer among Black women has already surpassed the death rate of ovarian cancer (2). As of 2019, the mortality of uterine cancer among Black women is now 1.6-fold higher than ovarian cancer (9.3 vs 5.7 per 100,000) and is nearly two-fold higher compared to White women (3).
The rising incidence of uterine cancer has often been attributed to the rise in obesity, with over 70% of uterine cancer diagnoses attributed to physical inactivity and excess body weight (4). Traditionally, obesity has been associated with estrogen-dependent, low-grade endometrioid tumors that carry a good prognosis. Alternatively, non-endometrioid tumors are generally considered to be estrogen-independent with less significant associations to modifiable risk factors (3). Interestingly, despite an increasing prevalence of obesity, an analysis of the US Cancer Statistics database from 2001 to 2017 demonstrated a stable incidence of endometrioid tumors while the incidence of non-endometrioid tumors increased 3.12% annually. Serous carcinoma, the most frequent of the non-endometrioid histologic subtypes, increased 4.88% annually (5). Thus, while uterine cancer overall is increasing approximately 1% per year, this trend is largely driven by non-endometrioid histologic subtypes, with the sharpest increase observed among Asian, Hispanic, and non-Hispanic Black women (6).
While non-endometrioid tumors account for only 5–10% of uterine cancer diagnoses, they account for nearly 40% of uterine cancer deaths (7). The rising mortality of uterine cancer has been attributed to the rising incidence of aggressive, non-endometrioid histologic subtypes (6). These poor outcomes disproportionately affect Black women who are 2–4 times more likely to present with aggressive, non-endometrioid tumors compared to White women (8, 9). Of women diagnosed with high-risk endometrial cancer (including grade 3 endometrioid adenocarcinoma, carcinosarcoma, clear cell carcinoma and papillary serous carcinoma), serous carcinoma was diagnosed in 26% of Black women compared to 18.6% of Hispanic and 16.6% of non-Hispanic White women (10). Black women are also more likely to present with advanced stage disease and are 21% more likely to die of the disease than both Hispanic and non-Hispanic White women (7). Overall, this reflects the largest Black-White survival disparity of all cancers with Black women experiencing a 63% 5-year relative survival with uterine cancer compared to 84% in White women. While later stage at diagnosis, more aggressive tumor histology, and lower likelihood of timely treatment contribute to this survival disparity, none of these factors completely account for the vast disparities experienced by Black women (6, 11). In this review, we highlight recent publications that have focused on the trends and disparities among women diagnosed with high-risk uterine cancer.
Inequalities Across the Spectrum of Care
From diagnosis to treatment, disparities exist throughout the spectrum of care, particularly regarding Black women with high-risk histologic subtypes. Aggressive, non-endometrioid uterine cancer remains a diagnosis primarily made in the postmenopausal period with approximately 85% of women experiencing postmenopausal bleeding (PMB), making this symptom an important indicator of further diagnostic testing (8, 12). For women presenting with PMB, the American College of Obstetrics and Gynecology (ACOG) recommends evaluation with endometrial biopsy, dilation and curettage, or transvaginal ultrasound (TVUS). Published guidelines recommend using TVUS to avoid unnecessary invasive procedures, using an endometrial thickness (ET) threshold of 4mm to prompt tissue sampling (12). Using this approach, a negative predictive value of 99–100% has been reported. However, a simulated cohort study by Doll et al demonstrated that this approach may be insufficient in Black women due to a higher prevalence of uterine fibroids and non-endometrioid histology. The presence of uterine fibroids limits visualization of the endometrium by distorting the endometrial cavity, and a thin endometrial lining may not exclude the presence of a non-endometrioid cancer given that these are often focal lesions. Thus, an ET threshold of 4mm may prompt a biopsy in less than half of uterine cancer cases among Black women, thereby missing 5 times more cases of uterine cancer among Black women compared to White women (12).
Once the diagnosis is confirmed, delays in treatment further exacerbate racial disparities. The primary treatment for high-risk endometrial cancer remains a surgical approach with hysterectomy, bilateral salpingo-oophorectomy and surgical staging followed by adjuvant treatment if indicated based on pathology findings. Huang et al established five evidence-based quality metrics related to endometrial cancer treatment as determined by current literature and guidelines, including surgical treatment within 6 weeks of diagnosis, use of a minimally invasive surgical approach, nodal assessment, adjuvant radiation and systemic chemotherapy (13). When evaluating these quality metrics among 310,208 cases identified in the National Cancer Database, compared to White women, Black women were less likely to undergo surgery within 6 weeks of diagnosis (65.8 vs 75.6%), less likely to undergo a minimally invasive approach (58.5 vs 72.9%), less likely to receive nodal evaluation (71.3 vs 74.2%), and less likely to receive adjuvant chemotherapy (72.7 vs 73.2%) (p<0.05 for all) (13). Among a Medicare population with high-risk endometrial cancer, Corey et al found that Black women were 36% more likely to not receive adjuvant treatment. For those who did receive adjuvant treatment, 9% of Black women experienced a treatment delay, which contributed to poorer overall survival (14). Additional studies however have found that Medicaid expansion may provide some benefit, with the greatest benefit occurring for women diagnosed at 53–57 years of age. Among this group, women in states with Medicaid expansion were diagnosed at an earlier stage and demonstrated improved survival (15, 16). Thus, Medicaid expansion may reduce some barriers to care resulting in earlier stage at diagnosis, more rapid intervention, and improved survival.
Several studies have suggested that while improved access to care may reduce outcome disparities, survival disparities would likely not be eliminated. Park et al evaluated survival differences between Black and White women with endometrial cancer in the Military Health System which provides equal access to beneficiaries. Among 144 Black and 1439 White women diagnosed with endometrial cancer between 1988 and 2013, survival disparities persisted even after adjusting for age, diagnosis period, stage, histology/grade, and adjuvant treatment. Multivariable analysis found that racial disparities were confined to women with low-risk features (stage I/II disease or low-grade) or no adjuvant treatment, suggesting that receipt of guideline-concordant care reduces disparities in outcomes (17). For women with non-endometrioid uterine cancer from 2004 to 2014 in the National Cancer Database (NCB), Dholkia et al found that only 43.8% of patients received guideline-concordant care. Interestingly, there was no difference by race. Furthermore, when guideline-concordant care was received, survival improved to a similar degree in White, Hispanic, and non-Hispanic Black women (18). These findings were in opposition to findings among ovarian, cervical, vulvar and low-risk endometrioid uterine cancer where non-White women were less likely to receive guideline-concordant care (19–22). Among a Medicare-eligible population with uterine cancer, Saris et al examined cancer-specific survival by racial/ethnic groups in patients who underwent primary surgical staging after controlling for comorbidities, age, stage, and histology. Most outcome disparities were again noted in stage I disease, with adjustment for treatment-specific factors ameliorating survival differences in higher stage disease. The authors suggest that these findings likely reflect differences in recurrence rates of early-stage disease and treatment for recurrent disease, which often includes clinical trials (23). Inequal access to clinical trials is described below. Finally, studies among other cancer types have suggested that treatment refusal may be responsible for inequal receipt of adjuvant treatment. However, Barrington et al found that chemotherapy refusal by race is responsible for only 2 months of the overall 3.2-year survival disparity between Black and White patients with endometrial cancer (15). Overall, while inequitable surgery rates, stage at diagnosis, and timely access to guideline-concordant care contribute to the survival gap between Black and White women, several studies have suggested that none of these factors completely explain the poorer prognosis among Black women, particularly for early-stage disease (24).
Molecular and Genomic Alterations and Targeted Therapies
While high-risk endometrial cancer has traditionally been defined by certain histologic subtypes, this aggressive behavior is often driven by genetic and molecular markers. For example, inactivating mutations in the tumor suppression gene TP53 is associated with more aggressive tumor behavior and worse prognosis and is present in over 90% of serous tumors (25). Recent studies also demonstrate that serous tumors are molecularly distinct from endometrioid tumors with higher prevalence of mutations in PIK3CA, PPP2R1A, ERBB2 and FBXW7 among others (26, 27). Serous tumors are also less likely to present with microsatellite instability (MSI) or high total mutational burden (TMB-H) compared to endometrioid tumors (26, 27). Given that racial disparities in endometrial cancer prognosis cannot be explained by socioeconomic or treatment factors alone, it has been hypothesized that molecular or genetic markers may contribute to poor outcomes among Black women (27, 28). However, when stratified by histology, few significant differences are found between non-endometrioid tumors of Black and White women (26, 27). While certain molecular markers often signify more aggressive behavior, these differences in tumor biology may be actionable and allow for alternative treatment paradigms.
Historically, few treatment options have been available beyond traditional systemic and radiation therapies, particularly in the setting of advanced or recurrent endometrial cancer which often includes high-risk histologic subtypes. Trastuzumab is one example of the ways molecular markers can be exploited to identify targeted therapies. HER2-neu is a tyrosine kinase receptor that is responsible for cell growth, survival, and proliferation through ErbB signaling (29). ERBB2 amplification and Her2neu overexpression has been identified in approximately 30% of uterine serous carcinomas and may be more frequent in Black women (27, 30). Mutations in HER2-neu leading to over-expression of the receptor can be identified through immunohistochemistry testing and its presence has been associated with worse survival, similar to findings in patients with breast cancer (29). Trastuzumab is a HER2-neu monoclonal antibody which has been shown to extend progression free survival in advanced and recurrent HER2 positive uterine cancer (31). Challenges exist, however, in defining HER2/neu positivity and no studies have been identified to assess whether there is a race-based discrepancy of how trastuzumab is offered or administered among women with HER2 positive uterine cancer (32).
Furthermore, when tumor biology limits the use of adjuvant treatments, new combination therapies may be considered. The immune checkpoint inhibitor pembrolizumab has shown compelling results among patients with microsatellite instability-high (MSI-H) or mismatch repair-deficient (MMRd) tumors, however high-risk endometrial cancers are less likely to carry these molecular characteristics. Lenvatinib, a tyrosine kinase inhibitor, also showed limited efficacy for recurrent endometrial cancer. However, the recent findings of KEYNOTE 775 by Makker et al demonstrated benefit in progression free survival and overall survival with the combination of pembrolizumab and lenvatinib regardless of mismatch repair status, a major advance for the treatment of aggressive, non-endometrioid tumors (33). More research is needed to understand these aggressive tumor types on a molecular and genomic level and to determine how tumor biology might guide the identification and utilization of targeted therapies.
Addressing Systemic Racism
As we advance our understanding of the molecular and genomic characteristics of aggressive, high-risk endometrial cancer and develop new targeted therapies, attention must be drawn to the structural racism affecting research, opportunity, and health equity of racially marginalized groups. For example, participation in clinical trials is known to improve survival, and balanced accrual by race and ethnicity allows for improved generalizability of clinical trial results. However, non-White racial/ethnic groups are frequently under-represented in clinical trials despite willingness to participate (34, 35). The consortium for enhancing minority participation in clinical trials (EMPaCT) was established in 2009 and involves five NCI-designated comprehensive cancer centers. Semi-structured interviews conducted within the EMPaCT consortium identified significant institutional barriers to the recruitment of underrepresented populations, including the need for improved access to clinical trials and the need for care navigation to simplify recruitment and access to care at academic medical centers (35). Participation in clinical trials often requires time, travel, and resources with limited reimbursement that may influence a patient’s decision to enroll, whereas that burden is often less for those receiving standard of care (36). In another study, Arend et al utilized Next Generation Sequencing within a personalized medicine workflow to identify molecular targets in patients with advanced or recurrent endometrial cancer with the aim of reducing treatment disparities. Despite a carefully crafted integrated clinical workflow, racial disparities were found in the number of Black versus non-Black patients initiated on targeted therapies (28.2% vs 38.2%, respectively) and enrolled in clinical trial (15% vs 22.6%, respectively) (37). These studies demonstrate that unequal economic and social opportunities are often associated with race, and efforts should be made to reduce these inequalities in clinical trial enrollment and access to targeted therapies (38).
Conclusion
In this review, we highlight several recent publications addressing trends, disparities, and breakthroughs affecting women with high-risk endometrial cancer. The rising rate of endometrial cancer mortality, particularly among Black women, is alarming and is likely driven by the increasing incidence of aggressive, high-risk histologic subtypes which impact Black women 2–4 times more frequently (5, 6, 8). While care inequalities such as surgery rates, treatment delays, and receipt of guideline-concordant care and adjuvant therapies may contribute to the racial disparities in endometrial cancer survival, no single factor is solely responsible for the survival disparity between Black and White women (Figure 1). These findings are not surprising given that race is a social construct, rather than a biologic one, with several overlapping and intertwining factors contributing to the systemic racism that drives disease and differential outcomes (39). As such, we recognize that while disparities in high-risk uterine cancer outcomes are most notable among Black women, disparities in uterine cancer incidence and outcomes are also notable for other racial/ethnic groups. A detailed review of these disparities are beyond the scope of this review, but have been addressed in more detail elsewhere (39).
Figure 1.
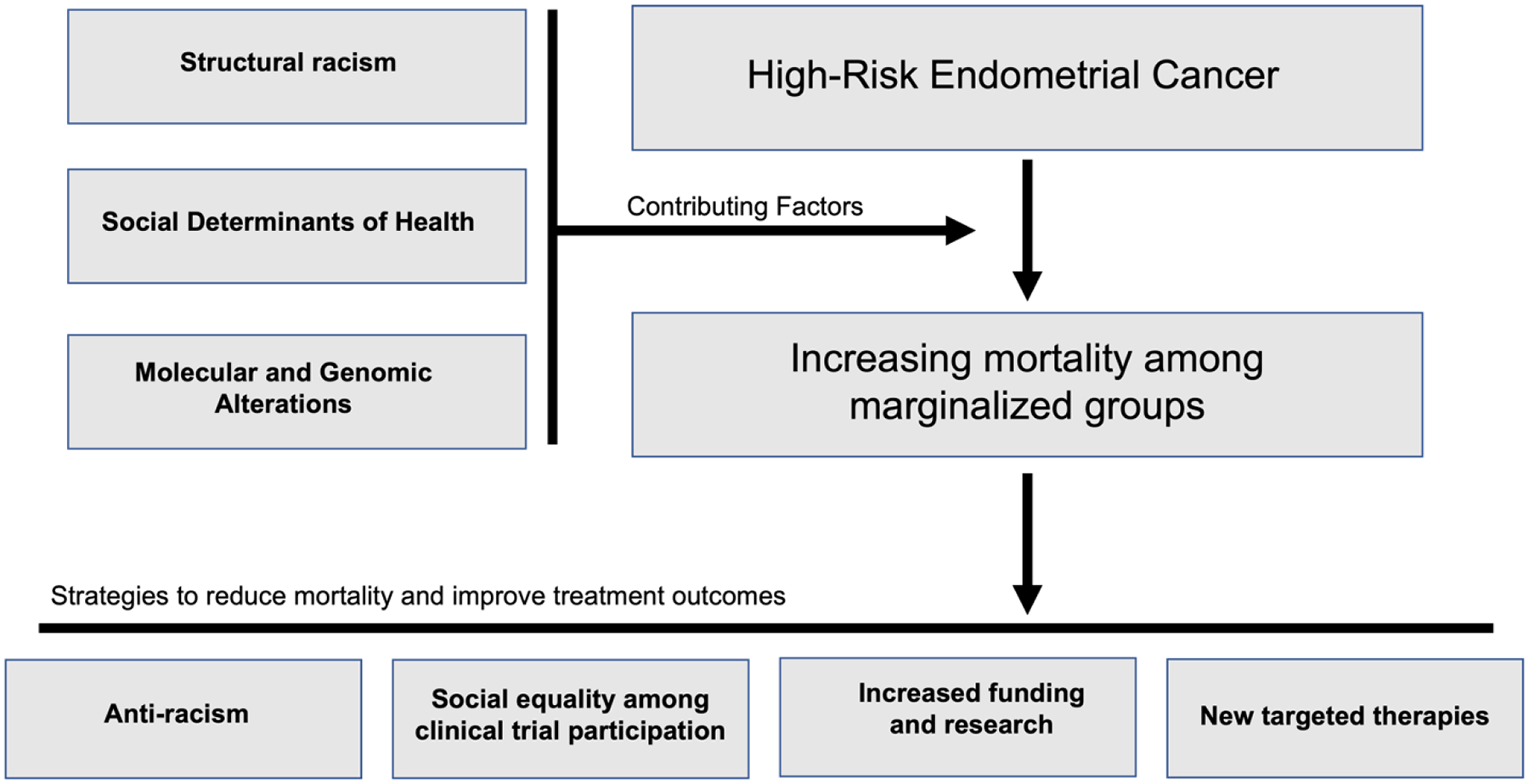
Contributing factors and potential strategies to reduce increasing mortality of high-risk endometrial cancer among marginalized groups
Future research must address the molecular and genomic drivers of these high-risk histologic types to better understand potentially modifiable risk factors and define the most effective approach to treatment, including the utilization of targeted therapies. For example, ovarian cancer research has demonstrated decreased risk with opportunistic salpingectomy, prophylactic bilateral salpingo-oophorectomy for patients with predisposing BRCA1/2 mutations and improvements in survival with the utilization of targeted therapeutics such as PARP inhibitors (2). In contrast, uterine cancer lacks the degree of research funding seen in ovarian cancer despite comparable number of deaths and profound racial disparities (2). Between 2007 and 2018, funding for uterine cancer research by the National Cancer Institute (NCI) increased by less than a million dollars while ovarian cancer research increased by more than $23 million. By 2018, NCI funding for uterine cancer research was one seventh that for ovarian cancer (40). In order to improve progress in uterine cancer mortality and reduce survival disparities, concerted efforts must be focused on improving research, access to clinical trials, and reducing the systemic racism that drives the healthcare inequalities of racially marginalized groups.
Key Points.
Endometrial cancer mortality continues to rise with a disproportionate impact on Black women
Healthcare disparities influence endometrial cancer survival, although no individual factor accounts for the vast survival differences between Black and White women
Further research is needed to understand the molecular and genomic characteristics of high-risk endometrial cancer to discern potentially modifiable risk factors
Different treatment approaches, including the utilization of targeted therapies, are needed to impact the increasing mortality in endometrial cancer
Financial Support and Sponsorship
This work was supported by the UCLA Patient-Centered Outcomes Research Training in Urologic and Gynecologic Cancers (PCORT UroGynCan, National Institutes of Health Award Number 5T32CA251072).
Footnotes
Conflicts of Interest
None
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. [DOI] [PubMed] [Google Scholar]
- 2*.Giaquinto AN, Broaddus RR, Jemal A, Siegel RL. The Changing Landscape of Gynecologic Cancer Mortality in the United States. Obstet Gynecol. 2022;139(3):440–2. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first study reporting the opposing mortality trends of uterine and ovarian cancer by racial/ethnic group, highlighting the disproportiate impact of rising uterine cancer mortality trends on Black women.
- 3.Clarke MA, Devesa SS, Harvey SV, Wentzensen N. Hysterectomy-Corrected Uterine Corpus Cancer Incidence Trends and Differences in Relative Survival Reveal Racial Disparities and Rising Rates of Nonendometrioid Cancers. J Clin Oncol. 2019;37(22):1895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Islami F, Goding Sauer A, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68(1):31–54. [DOI] [PubMed] [Google Scholar]
- 5.Eakin CM, Liao CI, Salani R, et al. The Association of Obesity and Type I Uterine Cancer - Is This an Oversimplification? Am J Obstet Gynecol. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke MA, Devesa SS, Hammer A, Wentzensen N. Racial and Ethnic Differences in Hysterectomy-Corrected Uterine Corpus Cancer Mortality by Stage and Histologic Subtype. JAMA Oncol. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferriss JS, Erickson BK, Shih IM, Fader AN. Uterine serous carcinoma: key advances and novel treatment approaches. Int J Gynecol Cancer. 2021;31(8):1165–74. [DOI] [PubMed] [Google Scholar]
- 8.Abel MK, Liao CI, Chan C, et al. Racial disparities in high-risk uterine cancer histologic subtypes: A United States Cancer Statistics study. Gynecol Oncol. 2021;161(2):470–6. [DOI] [PubMed] [Google Scholar]
- 9.Johnson AL, Medina HN, Schlumbrecht MP, et al. The role of histology on endometrial cancer survival disparities in diverse Florida. PLoS One. 2020;15(7):e0236402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bregar AJ, Alejandro Rauh-Hain J, Spencer R, et al. Disparities in receipt of care for high-grade endometrial cancer: A National Cancer Data Base analysis. Gynecol Oncol. 2017;145(1):114–21. [DOI] [PubMed] [Google Scholar]
- 11.Giaquinto AN, Miller KD, Tossas KY, et al. Cancer statistics for African American/Black People 2022. CA Cancer J Clin. 2022;72(3):202–29. [DOI] [PubMed] [Google Scholar]
- 12*.Doll KM, Romano SS, Marsh EE, Robinson WR. Estimated Performance of Transvaginal Ultrasonography for Evaluation of Postmenopausal Bleeding in a Simulated Cohort of Black and White Women in the US. JAMA Oncol. 2021;7(8):1158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]; Doll et al. provides the first simulated cohort evaluating the performance of transvaginal ultrasound endometrial thickness thresholds as a screening measure to prompt endometrial biopsy by race among patients reporting postmenopausal bleeding. Their findings suggest that current screening methods exacerbate racial disparities in endometrial cancer stage at diagnosis given that screening missed 5 times more cases among Black women in this simulated cohort.
- 13.Huang AB, Huang Y, Hur C, et al. Impact of quality of care on racial disparities in survival for endometrial cancer. Am J Obstet Gynecol. 2020;223(3):396 e1–e13. [DOI] [PubMed] [Google Scholar]
- 14.Corey L, Cote ML, Ruterbusch JJ, et al. Disparities in adjuvant treatment of high-grade endometrial cancer in the Medicare population. Am J Obstet Gynecol. 2022;226(4):541 e1–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrington DA, Sinnott JA, Nixon D, et al. More than treatment refusal: a National Cancer Database analysis of adjuvant treatment refusal and racial survival disparities among women with endometrial cancer. Am J Obstet Gynecol. 2022;227(2):244 e1–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albright BB, Nasioudis D, Craig S, et al. Impact of Medicaid expansion on women with gynecologic cancer: a difference-in-difference analysis. Am J Obstet Gynecol. 2021;224(2):195 e1–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park AB, Darcy KM, Tian C, et al. Racial disparities in survival among women with endometrial cancer in an equal access system. Gynecol Oncol. 2021;163(1):125–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dholakia J, Llamocca E, Quick A, et al. Guideline-concordant treatment is associated with improved survival among women with non-endometrioid endometrial cancer. Gynecol Oncol. 2020;157(3):716–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaspers M, Llamocca E, Quick A, et al. Black and Hispanic women are less likely than white women to receive guideline-concordant endometrial cancer treatment. Am J Obstet Gynecol. 2020;223(3):398 e1–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rauh-Hain JA, Melamed A, Schaps D, et al. Racial and ethnic disparities over time in the treatment and mortality of women with gynecological malignancies. Gynecol Oncol. 2018;149(1):4–11. [DOI] [PubMed] [Google Scholar]
- 21.Bristow RE, Chang J, Ziogas A, Anton-Culver H. Adherence to treatment guidelines for ovarian cancer as a measure of quality care. Obstet Gynecol. 2013;121(6):1226–34. [DOI] [PubMed] [Google Scholar]
- 22.Bristow RE, Powell MA, Al-Hammadi N, et al. Disparities in ovarian cancer care quality and survival according to race and socioeconomic status. J Natl Cancer Inst. 2013;105(11):823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saris DH, Smith AJB, Brensinger C, et al. Disparities in cancer-specific and overall survival in black women with endometrial cancer: A Medicare-SEER study. Gynecol Oncol Rep. 2022;40:100922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doll KM, Snyder CR, Ford CL. Endometrial cancer disparities: a race-conscious critique of the literature. Am J Obstet Gynecol. 2018;218(5):474–82 e2. [DOI] [PubMed] [Google Scholar]
- 25.Javadian P, Washington C, Mukasa S, Benbrook DM. Histopathologic, Genetic and Molecular Characterization of Endometrial Cancer Racial Disparity. Cancers (Basel). 2021;13(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilhite AM, Baca Y, Xiu J, et al. Molecular profiles of endometrial cancer tumors among Black patients. Gynecol Oncol. 2022;166(1):108–16. [DOI] [PubMed] [Google Scholar]
- 27.Lin DI, Fine A, Danziger NA, et al. Molecular analysis of endometrial serous carcinoma reveals distinct clinicopathologic and genomic subgroups. Gynecol Oncol. 2022;164(3):558–65. [DOI] [PubMed] [Google Scholar]
- 28.Asare A, Yao H, Lara OD, et al. Race-associated molecular changes in gynecologic malignancies. Cancer Res Commun. 2022;2(2):99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tymon-Rosario J, Siegel ER, Bellone S, et al. Trastuzumab tolerability in the treatment of advanced (stage III-IV) or recurrent uterine serous carcinomas that overexpress HER2/neu. Gynecol Oncol. 2021;163(1):93–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Towner M, Kim JJ, Simon MA, et al. Disparities in gynecologic cancer incidence, treatment, and survival: a narrative review of outcomes among black and white women in the United States. Int J Gynecol Cancer. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fader AN, Roque DM, Siegel E, et al. Randomized Phase II Trial of Carboplatin-Paclitaxel Versus Carboplatin-Paclitaxel-Trastuzumab in Uterine Serous Carcinomas That Overexpress Human Epidermal Growth Factor Receptor 2/neu. J Clin Oncol. 2018;36(20):2044–51. [DOI] [PubMed] [Google Scholar]
- 32.Buza N HER2 Testing in Endometrial Serous Carcinoma: Time for Standardized Pathology Practice to Meet the Clinical Demand. Arch Pathol Lab Med. 2021;145(6):687–91. [DOI] [PubMed] [Google Scholar]
- 33*.Makker V, Colombo N, Casado Herraez A, et al. Lenvatinib plus Pembrolizumab for Advanced Endometrial Cancer. N Engl J Med. 2022;386(5):437–48. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this phase III randomized clinical trial, Makker et al. demonstrated that the combination of lenvatinib plus pembrolizumab provided an improved median progression free survival and overall survival in endometrial cancer patients who have previously failed at least one platinum-based chemotherapy regimen, including those patients with mismatch repair-proficient disease.
- 34.Clair K, Bristow RE. Looking at cancer health disparities in gynecologic oncology in 2020. Curr Opin Obstet Gynecol. 2021;33(4):355–9. [DOI] [PubMed] [Google Scholar]
- 35.Niranjan SJ, Wenzel JA, Martin MY, et al. Perceived Institutional Barriers Among Clinical and Research Professionals: Minority Participation in Oncology Clinical Trials. JCO Oncol Pract. 2021;17(5):e666–e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fitzpatrick OM, Murphy C, Duignan E, et al. The cost of cancer care: how far would you go for a trial? Ir J Med Sci. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arend RC, Goel N, Roane BM, et al. Systematic Next Generation Sequencing is feasible in clinical practice and identifies opportunities for targeted therapy in women with uterine cancer: Results from a prospective cohort study. Gynecol Oncol. 2021;163(1):85–92. [DOI] [PubMed] [Google Scholar]
- 38.Brown CE, Curtis JR, Doll KM. A Race-Conscious Approach Toward Research on Racial Inequities in Palliative Care. J Pain Symptom Manage. 2022;63(5):e465–e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whetstone S, Burke W, Sheth SS, et al. Health Disparities in Uterine Cancer: Report From the Uterine Cancer Evidence Review Conference. Obstet Gynecol. 2022;139(4):645–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.National Cancer Institute: Cancer Funding Statistics 2022. [Available from: https://fundedresearch.cancer.gov/nciportfolio/stats.jsp.


