Abstract
Unlike malaria parasites in humans, non-human primates, rodents, and birds, ungulate malaria parasites and their vectors have received little attention. As a result, understanding of the hosts, vectors, and biology of ungulate malaria parasites has remained limited. In this study, we aimed to identify the vectors of the goat malaria parasite Plasmodium caprae. A total of 1019 anopheline and 133 non-anopheline mosquitoes were collected from goat farms in Thailand, where P. caprae-infected goats were discovered. Anopheline mosquitoes were identified using molecular biological methods that target the cytochrome c oxidase subunit 1 (cox1), the cytochrome c oxidase subunit 2 (cox2) genes, and the internal transcribed spacer 2 (ITS2) region. Pool and individual mosquitoes were tested for P. caprae using the head-thorax parts that contain the salivary glands, with primers targeting three genetic markers including cytochrome b, cytochrome c oxidase subunit 1, and 18S small subunit ribosomal RNA genes. Additionally, goat blood samples were collected concurrently with mosquito surveys and screened to determine the status of malaria infection. This study revealed nine mosquito species belonging to six groups on goat farms, including Hyrcanus, Barbirostris, Subpictus, Funestus, Tessellatus, and Annularis. The DNA of P. caprae was detected in Anopheles subpictus and Anopheles aconitus. This is the first time An. subpictus and An. aconitus have been implicated as probable vectors of P. caprae.
Subject terms: Ecology, Microbiology, Molecular biology, Diseases
Introduction
Humans and various animals are susceptible to malaria, a mosquito-borne disease, so it has been investigated for centuries. The parasites responsible have been researched and understood thoroughly, and the disease has been well-managed to ensure public health. On the other hand, ungulate malaria parasites have undergone a few research studies, primarily based on molecular approaches1–5. Among Plasmodium spp. infecting even-toed ungulates (Order Artiodactyla), Plasmodium cephalophi and P. brucei were first described in duiker antelope in Africa (1913)6. Later, P. bubalis was found in water buffalo (Bovidae: Bubalus bubalis) in India (1919)7 and other Asian countries, including Vietnam (2010 & 2013), Thailand (2014 & 2015), and Nepal (2017)4,8. Several years after the initial detection of P. bubalis, P. caprae was reported in wild goats (Bovidae: Capra aegagrus hircus) in Angola (1923)9, it expanded to domestic goats (Bovidae: Capra aegagrus hircus) in Zambia (2010), Sudan (2014), Thailand (2016), Myanmar (2016), Iran (2017) and Kenya (2017)4,10. Furthermore, P. traguli was detected in mouse deer (Tragulidae: Tragulus javanicus) in Malaysia (1962)11 and P. odocoilei in American white-tailed deer (Cervidae: Odocoileus virginianus) (1967)12,13. Among them, at least three species of malaria parasites are endemic in Southeast Asia, suggesting the presence of mosquito vectors in this region. Although most of these parasites were discovered a long time ago, the descriptions of mosquito vectors have been limited and relied solely on morphology. Thus, a comprehensive picture of malaria parasites and their mosquito vectors remains incomplete.
Thailand is well known as one of the most biodiversity-rich tropical countries in the world. According to Rattanarithikul et al. (2006) and the Walter Reed Biosystematics Unit data repository14,15, there are at least 464 mosquito species, with 83 belonging to the genus Anopheles. Most mosquito vectors for malaria transmission in Southeast Asia belong to cryptic species complexes that are nearly impossible to microscopically identify owing to their overlapping morphological characteristics16. For example, the Barbirostris Complex has six species, five of which exist in Thailand17–19. In contrast, the Minimus complex consists of two sibling species (A and C) and has been incriminated as human malaria vectors in Thailand and Southeast Asia20. Mosquito determination of species complexes based on morphological characteristics is likely to misidentify and mislead vector incrimination as a result21,22.
Despite the limitations and difficulties involved in the identification of mosquito vectors, several previous studies have reported on Plasmodium spp. in ungulates. Wild-caught Anopheles umbrosus and An. letifer in swamp forests were found to carry the Plasmodium sporozoites and oocysts in their salivary glands and midguts. These sporozoites and oocysts resembled those of P. traguli. Therefore, these mosquitoes were incriminated as probable vectors of P. traguli in Malaysian mouse deer23. Several decades later, Plasmodium spp. lineage B were observed in the salivary glands of sylvatic mosquitoes An. gabonensis and An. obscurus in the Gabonese forest, Central Africa2. Cytb sequences from these sporozoites were clustered within the same clade with Plasmodium spp. in the African blue duiker and bay duiker2. Furthermore, parasites resembling the morphology of P. odocoilei were isolated from the salivary glands of An. punctipennis. Phylogenetic analysis showed that the DNA sequences of the parasites found in An. punctipennis were grouped with Plasmodium from white-tailed deer in North America13. Recently, a study of malaria in Murrah dairy buffalo revealed that An. wejchoochotei or An. campestris, and An. peditaeniatus were recognised as potential vectors of P. bubalis type I in Thailand24. However, little is known about the vectors of P. caprae, which is also endemic in many other Asian countries. Although several research studies have been carried out on the prevalence, diagnosis, and evolutionary history of P. caprae thus far, no information about its vectors has been reported10,25. In the present study, we hypothesize that the mosquito vectors of P. caprae are endemic in Thailand and other Southeast Asian countries. Hence, this study aimed to identify anopheline mosquitoes as possible vectors of P. caprae transmission in Thailand.
Results
Detection of P. caprae in goat blood samples
A total of 423 goat blood samples were collected from 18 goat farms in three provinces of Thailand at different times, including Kanchanaburi in 2020 (three farms), 2021 (three farms), and 2022 (two farms), Nan in 2020 (five farms) and 2021 (three farms), and Phetchaburi in 2021 (two farms); among which 401 samples were collected simultaneously with mosquito samples from the same farm. Furthermore, 22 blood samples collected in Ratchaburi in 2018 were included in this study. Nested PCR amplification targeting three gene loci, cytb, cox1, and 18S rRNA, identified six P. caprae positive samples of 423 blood samples; 2 samples were from Ratchaburi in 2018, 3 samples were from Nan in 2020, and 1 sample was from Phetchaburi in 2021. The PCR products of two P. caprae positive samples collected in Ratchaburi in 2018 were further confirmed by Sanger DNA sequencing. BLASTN searches of cytb sequences showed 100% identity with P. caprae (accession nos. LC326032 & LC090215), 97%–98.0% identity with P. odocoilei (accession nos. MH177860 & MK502145), and 96.2% identity with P. bubalis (accession nos. LC090213 & LC090214) in the GenBank database. Furthermore, the cox1 sequences showed 99.6% identity with P. caprae (accession nos. LC326032 & LC090215), 98.8% identity with P. odocoilei (accession no. OL999536), and 98.0% with P. bubalis (accession no. LC090214). The 18S rRNA sequences revealed a similarity of 92.6% with P. bubalis (accession nos. OL624705–OL624709) and 92.2% similarity with P. falciparum (accession no. LR131366). The 18S rRNA sequences from other ungulate malaria parasites are not available in the database. This result confirmed the endemicity of goat malaria in Thailand.
Species composition of mosquitoes collected from goat farms by morphology
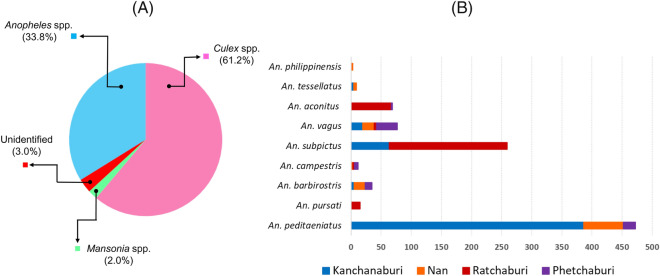
Using CDC light traps, a total of 201 female mosquitoes were collected from four goat farms in Kanchanaburi and Phetchaburi, while 951 female anopheline mosquitoes were manually collected by mouth aspirators from eight goat farms in Kanchanaburi, Nan, Ratchaburi, and Phetchaburi provinces during 2020–2021. Morphological examinations of the mosquitoes collected by CDC light traps revealed that 123 (61.2%) were Culex spp., 68 (33.8%) were Anopheles spp., and 4 (2.0%) were Mansonia spp. Six mosquito species (3.0%) remained unidentified due to damage to their wings and legs (Fig. 1A). Among 951 anopheline mosquitoes collected by mouth aspirators, nine mosquito species belonging to six groups/subgroups were identified consisting of Anopheles peditaeniatus and An. pursati (Hyrcanus), An. barbirostris and An. campestris (Barbirostris), An. subpictus and An. vagus (Subpictus), An. aconitus (Funestus), An. tessellatus (Tessellatus) and An. philippinensis (Annularis). Details concerning the species and the number of mosquitoes in each province are illustrated in Fig. 1B.
Figure 1.
Charts illustrating the composition of mosquitoes determined by morphology. (A) The percentages of each genus of mosquitoes collected by CDC light traps in this study. (B) The number of mosquitoes of Anopheles spp. collected by mouth aspirators in four provinces, including Kanchanaburi, Nan, Ratchaburi and Phetchaburi, as indicated in light green, blue, yellow and dark green, respectively.
Identification of P. caprae in anopheline and non-anopheline mosquitoes
Three hundred and twenty-two anopheline mosquitoes were dissected to separate their head and thorax parts containing the salivary glands from the abdomen parts containing the midguts. The salivary glands and midguts were then stained with 0.1% mercurochrome dye, and the presence of oocysts or sporozoites was examined under a microscope. However, no parasites were found. Subsequently, the head and thorax parts containing salivary glands were pooled from one to three samples, depending on the groups and species, to generate 358 pools for DNA extraction. The number of each pool was as follows: Hyrcanus (n = 508, 175 pools), Barbirostris (n = 48, 19 pools), Subpictus (n = 379, 130 pools), Aconitus (n = 72, 27 pools) Tessellatus (n = 9, 5 pools), and Annularis (n = 3, 2 pools). PCR screening targeting Plasmodium cytb, cox1, and 18S rRNA genes and following sequencing analysis revealed three pools (0.84%) out of 358 pools were positive for P. caprae. These mosquito samples were the Aconitus group (ID THMosGoat21-02_P11) and the Subpictus group (IDs THMosGoat21-01_P18 and THMosGoat21-01_P38) (Table 1). It should be noted that three mosquito pools that were PCR positive to P. caprae consisted of two pools of unfed mosquitoes (one pool each of An. subpictus and An. aconitus) and one pool of blood-fed mosquitoes (An. subpictus). Minimum infection rates (MIR) were 1.4% (95% CI 0.25–7.46) in the Aconitus group mosquito and 0.9% (0.25–3.24) in the Subpictus group mosquito (Table 2).
Table 1.
Summary of the screening of P. caprae in anopheline mosquitoes collected from goat farms.
| Group | No. collected | No. of pools | No. of positive pools | No. of pools sequenced for determination of mosquito species | ||
|---|---|---|---|---|---|---|
| cytb | cox1 | 18S rRNA | ||||
| Hyrcanus | 508 | 175 | 0 | 0 | 0 | 37 (An. peditaeniatus), 3 (An. pursati) |
| Barbirostris | 48 | 19 | 0 | 0 | 0 | 10 (An. barbirostris), 5 (An. campestris) |
| Subpictus | 379 | 130 | 2 | 2 | 2 | 32 (An. subpictus), 20 (An. vagus) |
| Aconitus | 72 | 27 | 1 | 1 | 1 | 12 (An. aconitus) |
| Tessellatus | 9 | 5 | 0 | 0 | 0 | 5 (An. tessellatus) |
| Annularis | 3 | 2 | 0 | 0 | 0 | 2 (An. philippinensis) |
| Total | 1,019 | 358 | 3 | 3 | 3 | 126 |
A total of 3 pools of anopheline mosquitoes were positive using PCR assays targeting the cytb, cox1 and 18S rRNA genes. The number of pools used for species identification was also indicated.
Table 2.
Minimum infection rates (MIR) of P. caprae among collected mosquitoes.
| Species | Total no. of mosquitoes | Pool size (range) | No. of tested | No. of positive pools | MIR (%) (95% CI) |
|---|---|---|---|---|---|
| An. aconitus | 72 | 1–3 | 72 | 1 | 1.4 (0.25–7.46) |
| An. subpictus | 296 | 1–3 | 221 | 2 | 0.9 (0.25–3.24) |
From 133 non-anopheline mosquito samples, 8 pools of 25 Culex spp. samples and 3 pools from 4 Mansonia spp. samples were subjected to PCR examination, as described above. However, none were positive for Plasmodium spp.
Phylogenetic analyses of P. caprae in anopheline mosquitoes
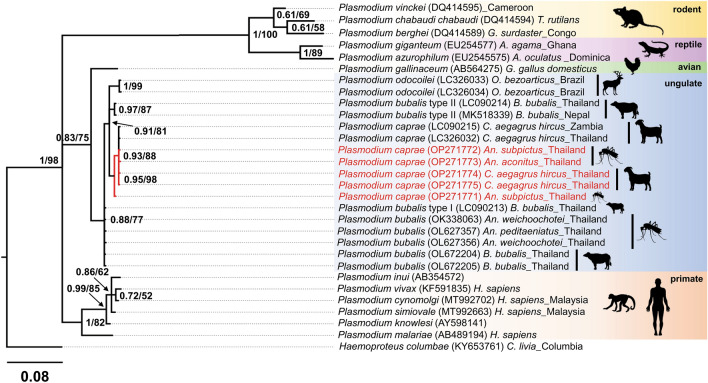
BLASTN similarity searches using cytb sequences obtained from two pools of An. subpictus mosquitoes (THMosGoat21-01_P18 & THMosGoat21-01_P38) and one pool of An. aconitus (THMosGoat21-02_P11) revealed 100% similarity to P. caprae (accession nos. LC090215 & LC326032) isolated from goats in Zambia and Thailand, 97–98% similarity to P. odocoilei (accession nos. MH177860 & LC326035), and 96.2% similarity to P. bubalis (accession nos. LC090213 & LC090214). The phylogenetic tree inferred from the cytb sequences showed that Plasmodium sequences derived from An. subpictus (group Subpictus) and An. aconitus (Funestus group) were clustered together with P. caprae from Thailand and Zambia (accession nos. LC326032 & LC090215) with high Bayesian posterior probability (BPP = 0.96) and bootstrap value (BV = 94) (Fig. 2).
Figure 2.
The phylogenetic position of P. caprae detected from Anopheles mosquitoes and goat blood in this study. The phylogenetic tree was inferred by Bayesian inference using partial cytb sequences (632 bp) with the Haemoproteus columbae sequence as a root. The posterior probabilities are given by Bayesian inference, and the bootstrap values by Maximum likelihood (≥ 0.68/58) are given in the nodes. Sequences obtained in this study are highlighted in red, and reference sequences retrieved from the GenBank database are highlighted in black. The length of substitutions/site (0.03) is indicated.
BLASTN searches of the partial Plasmodium cox1 sequences of An. subpictus and An. aconitus showed 100% similarity to the P. caprae sequences (accession nos. LC326032 & LC090215), 98–98.8% similarity to P. odocoilei (accession nos. LC326034 & OL999536), and 98.2–98.7% similarity to P. bubalis (accession nos. LC090214 & LC090213). Similar to the phylogenetic tree inferred from the cytb sequences, the Plasmodium cox1 sequences detected from An. subpictus and An. aconitus mosquitoes were clustered together with the P. caprae cox1 sequences from goats in Zambia and Thailand with a BPP value of 0.95 and a BV of 98 (Fig. 3).
Figure 3.
The phylogenetic position of P. caprae obtained in this study inferred by Bayesian inference using a partial cox1 gene (231 bp). All sequences were rooted with Haemoproteus columbae. Bayesian posterior probabilities (BPP ≥ 0.61) and bootstrap values (BV ≥ 52) are given at the nodes. The length of the substitutions/site (0.08) is shown.
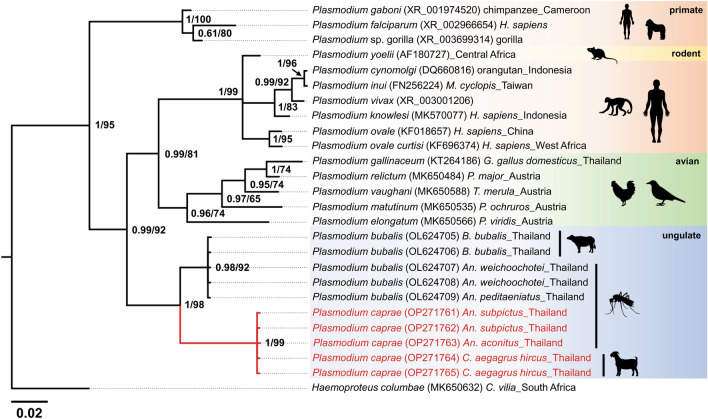
The Plasmodium 18S rRNA sequences obtained from three mosquito pools were identical to the sequences obtained from goats. The phylogenetic tree revealed that all five sequences for 18S rRNA in this study were clustered in the same clade with P. bubalis sequences from water buffaloes and mosquitoes with high support for posterior probabilities of 1 and a bootstrap value of 99 (Fig. 4).
Figure 4.
The phylogenetic position of P. caprae obtained in this study using a partial 18S rRNA gene (335 bp). All sequences were rooted with Haemoproteus columbae. Posterior probabilities and bootstrap values (≥ 0.61/65) are given in the nodes. The length of the substitutions/site (0.02) is shown.
Molecular identification of anopheline mosquitoes
To identify anopheline mosquito species collected from goat farms using a molecular approach, the partial sequences of cox1, cox2, and ITS2 were employed for DNA amplification and sequencing in three Plasmodium-positive mosquito pools and 123 negative pools in this study. The sequences obtained were evaluated for BLASTN searches against the GenBank and BOLD databases. A similarity of ≥ 97% for each sequence deposited in the GenBank or BOLD databases was considered the same species. The results were generally consistent among three genetic markers, except for some cox1 sequences from five species. The results of the cox1 BLASTN search showed the closely related mosquito species in the same group, while the cox2 and ITS2 searches presented the same species among three markers.
Regarding An. aconitus, the BLASTN search results were in complete agreement among cox1, cox2 and ITS2. The per cent identity of the An. aconitus in this study with reference sequences in GenBank ranged from 97.0 to 99.4% with HQ877378 (cox1 sequences) and 99.5–100% with JX070686 (cox2 sequences), while An. aconitus-ITS2 sequences showed 100% with MF535233. However, the BLASTN searches for An. subpictus had discordant results between cox2, ITS2 and cox1. In detail, the An. subpictus-cox2 sequences showed 93.6–96.4% similarity to An. subpictus in India (KX669656), while the ITS2 sequences showed 99.2%–100% identity to An. subpictus in Vietnam (GQ870330). In contrast, cox1 sequences revealed different species, ranging from 91.9 to 93.3% similarity to An. epiroticus (KT382821). However, previous studies on evolutionary divergence suggested that the threshold for intraspecific variation should be 2–3%26–28. Therefore, the final identification for this mosquito species was An. subpictus, supported by the higher % similarity of the cox2 and ITS2 sequences.
Discussion
This study aimed to identify the possible vectors of P. caprae in Thailand. We collected mosquitoes from goat farms in four provinces comprising Kanchanaburi, Nan, Ratchaburi, and Phetchaburi in 2020 and 2021. Unfortunately, goat blood collection in Ratchaburi province was not permitted on this trip in June 2021, where we found P. caprae-positive mosquitoes. Therefore, 22 goat blood samples previously collected from the same farm in 2018 were rescreened and the sequences of three marker genes were obtained. Five mosquito species were present on the goat farms where P. caprae-positive goats were detected, including An. subpictus, An. aconitus, An. pursati, An. vagus, and An. campestris. These mosquitoes are prevalent not only in Thailand but throughout Asian countries14; An. subpictus and An. vagus exist in Thailand, Vietnam, Cambodia, Indonesia, Malaysia, India and Sri Lanka, whereas An. aconitus and An. campestris can be found in Thailand, Vietnam, and Cambodia29,30. Among them, An. subpictus, An. vagus, and An. aconitus were recently incriminated for transmitting human malaria parasites31.
This study found the DNA of P. caprae in two dominant mosquito species of An. subpictus and An. aconitus of Ratchaburi in 2021. Therefore, these mosquito species are probable vectors of goat malaria parasites. However, no sporozoites were observed in the salivary glands of these mosquitoes. This is most likely due to the low prevalence (2–5%) and very low parasitemia of P. caprae in goats4,10,25, resulting in a low infection rate and parasite burden in mosquitoes. The results of this study agreed with the findings of previous studies that showed anopheline mosquitoes could be vectors of ungulate malaria parasites. For example, the sporozoites and oocysts of the mouse deer malaria parasite P. traguli were found in the salivary glands of wild-caught An. umbrosus and An. letifer23. Additionally, An. umbrosus was also found in malaria high-risk areas of Ranong province in southern Thailand32. However, none of these species was found in the present study. In another study, the sporozoites of the white-tailed deer malaria parasite P. odocoilei were isolated from An. punctipennis in North America13. Elsewhere, haemosporidian parasites of antelopes and other vertebrates (family Plasmodiidae) were observed in sylvatic anopheline mosquitoes in Gabon, Central Africa2. The most recent study reported on the potential vectors of An. wejchoochotei or An. campestris and An. peditaeniatus for the transmission of P. bubalis in water buffalo24. Therefore, our results provide supporting information that anopheline mosquitoes could be vectors for ungulate malaria transmission in general. However, more concrete evidence is required to conclude the vectors of P. caprae. According to Makanga et al., microscopic observation of Plasmodium sporozoites in salivary glands and oocysts in the midgut are prerequisites for malaria vector conclusion33. Thus, it is suggested that infected mosquitoes be allowed to feed on naive vertebrate animals and observe the blood-stage parasites23.
Phylogenetic analysis based on Bayesian inference and Maximum likelihood confirmed that the Plasmodium parasites detected from An. subpictus and An. aconitus in this study were genetically very close and were clustered within the same clade with P. caprae previously isolated from goat blood in Thailand and Zambia4,10, suggesting that these mosquito species are highly likely vectors for P. caprae.
Conclusion
In this study, An. subpictus and An. aconitus collected from goat farms were PCR-positive for P. caprae. Although it could not be concluded that two mosquito species were natural vectors for goat malaria due to the lack of morphological observation of sporozoites in the salivary glands or oocysts in the midgut, the findings of this study suggest a potential role of An. subpictus and An. aconitus mosquitoes in the transmission of P. caprae.
Methods
Sampling site description and mosquito collection
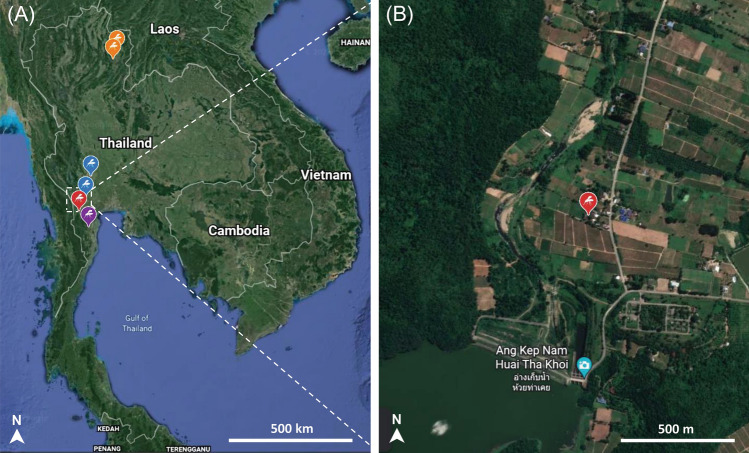
Anopheline and non-anopheline mosquitoes were collected from goat farms in six districts across four provinces of northern and western Thailand during the rainy seasons from June 2020 to November 2021. One or two areas were chosen from each district, including Lao Khwan District (14°28′33.4ʺN 99°48′25.0ʺE and 14°28′51.1ʺN 99°48′11.7ʺE) in Kanchanaburi (2 nights in June 2020), Wiang Sa District (18°31′56.9ʺN 100°37′50.2ʺE) and Mueang Nan District (18°49′07.0ʺN 100°46′36.9ʺE) in Nan (2 nights in August 2020), Ban Kha District (13°17′32.3ʺN 99°25′06.8ʺE) in Ratchaburi (2 nights in June 2021), Kaeng Krachan District (12°53′45.7ʺN 99°42′45.4ʺE) in Phetchaburi (1 night in October 2021), and Ban Mai District in Kanchanaburi (13°53′27.3ʺN99°37′16.2ʺE and 13°54′40.8ʺN 99°37′52.4ʺE) (1 night in November 2021) (Fig. 5A). It should be noted that goat malaria parasites were previously found in Nan, Kanchanaburi, and Phetchaburi10,25. Nan is in a high mountainous area that is traversed by the Nan River, while the other three Western provinces are surrounded by dense rainforests (Fig. 5B).
Figure 5.
(A) Map illustrating the sampling sites in four provinces of Thailand. The orange, blue, red, and purple mosquito silhouettes represent sampling sites in Nan, Kanchanaburi, Ratchaburi, and Phetchaburi provinces. (B) A magnified satellite view of mosquito and blood sampling sites in Ratchaburi, where P. caprae-positive blood and mosquitoes were found. The images were obtained and modified from Google Earth Pro version 7.3.4.8248 (https://google-earth-pro.updatestar.com/en).
The mosquitoes were collected using CDC light traps and mouth aspirators. For the first method, CDC light traps (John W. Hock Co., Gainesville, FL, USA) were placed approximately 1.5 m above the ground near the corner of the goat stables. These traps were kept overnight from 6:00 PM to 6:00 AM the following day. Regarding the second method, a mosquito net was hung and covered around the tables where the goats are raised. Anopheline mosquitoes were collected using mouth aspirators (10 mm in diameter × 200 mm in length), and then temporarily stored in paper cups. Mosquito collection by mouth aspirator was from 7:00 PM to midnight34.
Morphological identification, salivary gland dissection, DNA extraction, and amplification of ITS2, cox1, and cox2 genes of mosquitoes
The collected anopheline mosquitoes were identified by groups or species under a stereomicroscope based on the pictorial identification key14. For some groups that have a species complex, the mosquitoes were identified up to the group level. The gonotrophic status of the mosquitoes, including unfed, blood-fed, half-gravid, and gravid, was determined. The unfed mosquitoes were observed to have no blood in the abdomen, while those that were fed blood were partially or fully engorged with red blood. The dark red colour of blood covering 3–4 segments and the ovaries/eggs covering the rest of the mosquito abdomen were assigned as half-gravid, while the gravid mosquitoes were blood-free and the ovaries/eggs covered almost all of the abdomen35. Anopheline mosquitoes were dissected using a 26G and ½ inch-long sterile needle to separate salivary glands (head and thorax parts) from the midgut (body part). Additionally, 0.1% mercurochrome dye was used to stain sporozoites in salivary glands, after which samples were examined under a microscope at 1,000 times magnification.
Previous studies have revealed that infection can be more easily detected when mosquito samples are pooled36,37. Therefore, the anopheline mosquitoes in this study were grouped into pools according to species/group, sampling site, and gonotrophic status. Based on the sample availability of each captured species, a pool contained one to three mosquitoes of the same species/group, sampling site and gonotrophic status. Genomic DNA was extracted from the head and thorax parts of the mosquito that had salivary glands. Non-anopheline mosquitoes were pooled according to genus without dissection. DNA extraction was carried out using NucleoSpin Tissue kit (Macherey–Nagel, Germany) following the manufacturer’s protocol with minor modifications. In the last step, genomic DNA was eluted twice with an elution buffer. The first elution solution was used for the screening of P. caprae, while the second was employed for the confirmation of mosquito species. The mosquito species was subsequently identified by a molecular method targeting cox1, cox2 genes, and the ITS2 region. Primers targeting the ITS2 region were described in a previous publication38, while primers targeting the cox1 and cox2 genes were from a related study24. The primers and thermocycling conditions for the identification of mosquito species are described in detail in Table S1.
Goat blood collection, microscopic examination, and DNA extraction
A total of 401 blood samples were collected from the jugular veins of goats in three provinces of Thailand, including Nan, Kanchanaburi, and Phetchaburi, between 2020 and 2021. Due to the owner’s opposition, goat blood samples in Ratchaburi were not collected concurrently with the time the mosquitoes were captured (June 2021). Therefore, 22 goat blood samples collected from the same goat farm in June 2018 were used for parasite screening. The goat was restrained, then 8 mL of blood was withdrawn, kept in BD Vacutainer® containing 1.5 mL of anticoagulant acid citrate dextrose solution (BD Franklin Lakes, NJ, USA), and brought to the laboratory at the Faculty of Veterinary Science, Chulalongkorn University. Genomic DNA was extracted from 1.5 mL of whole blood using the NucleoSpin® Blood extraction kit (Macherry-Nagel, Germany) following the manufacturer’s protocol.
PCR detection of Plasmodium’s cytb, 18S rRNA, and cox1 genes in mosquito and goat blood
Mosquitoes and goat blood samples were screened for the presence of Plasmodium spp. using three sets of primers targeting cytb, cox1 and 18S rRNA genes. The primers used for the detection of P. caprae in goat blood and mosquito samples were described in previous studies4,24,39,40. Conventional PCR was applied for confirmation of mosquito species, but nested PCR was required for P. caprae detection because parasitemia in the goat blood was extremely low, according to previous results10. Each PCR reaction was carried out in a volume of 12.5 µL consisting of 6.25 μL of 2 × buffer KOD FX Neo, 2.5 μL of dNTPs (0.4 mM each), 0.375 μL of each primer (10 pmol/μL), 0.25 μL of KOD FX Neo DNA polymerase (Toyobo, Japan), one μL of the extracted DNA template and 1.75 μL of sterile distilled water. The primers and thermocycling conditions used for Plasmodium detection are described in detail in Table S2. P. caprae from a previous study was used as a positive control25 and sterile distilled water as a no template negative control. All PCR amplifications were conducted in an Axygen® MaxyGene Thermal Cycler (Life science, USA). Gel electrophoresis was set at 100 V, 400 mA, and run for 40 min on 1.5% agarose gel stained with ethidium bromide. The result was evaluated under a UV transilluminator. Regarding the detection of P. caprae, the PCR products of positive mosquitoes and blood samples were increased to 50 µL for gel purification and sequencing. Gel purification was performed using NucleoSpin® Gel and PCR clean-up (Macherey–Nagel, Düren, Germany) according to the manufacturer's protocol. The purified samples were bidirectionally sequenced employing the primers used in the second amplification.
DNA sequences and statistical analyses
The chromatograms of all target genes were carefully checked and then the sequences were manually trimmed and edited using BioEdit v.7.2.541. Low-quality and ambiguous chromatograms were excluded from further analysis. The ClustalW implemented in BioEdit was used to align all obtained sequences then the parasite was identified based on a BLASTN search against the NCBI GenBank database. All consensus sequences were combined with reference sequences retrieved from GenBank. MrBayes v3.2.7 was utilised to construct phylogenetic trees using the Bayesian Inference (BI) and Markov Chain Monte Carlo methods42. BI phylogenetic analysis was run for 10,000,000 generations using two independent runs of four chains each. Tracer v1.751 was used to evaluate the mixing and convergence of runs and effective sample sizes (EES > 200)43. Furthermore, IQ-TREE was also used for phylogenetic tree reconstruction based on Maximum Likelihood (ML), as previously described44. The final phylogenetic trees were visualised and decorated in FigTree v1.4.4 (available online at http://tree.bio.ed.ac.uk/software/figtree/).
The minimum infection rate (MIR) was determined for each mosquito species, in which Plasmodium DNA was found to assess the infection rate of positive mosquitoes. It was assumed that a mosquito pool had at least one infected mosquito if Plasmodium DNA was found. As a result, MIR was calculated using the formula mentioned: (number of positive pools/total number of mosquitoes studied) × 10045. The MIR was estimated using the Wilson confidence interval method for binomial proportions with a 95% confidence interval (CI).
Ethical statement and biosafety
This study has been approved by the Institutional Animal Care and Use Committee of the Faculty of Veterinary Science, Chulalongkorn University (IACUC No. 2031083). All experiments were carried out according to the Institutional Biosafety Committee (IBC No. 2031037) and university policies and regulations. Goat blood and mosquito samples were collected with the farm owners’ consent. This study was carried out in accordance with ARRIVE guidelines (https://arriveguidelines.org).
Supplementary Information
Acknowledgements
We would like to thank all faculty members at School of Agricultural Resources, Chulalongkorn University for their support in collecting the goat blood and mosquito samples in Nan Province.
Author contributions
A.H.L.N. contributed to the sample collection, mosquito identification, investigation, methodology, data analysis, and writing—original draft. S.P. contributed to writing—reviewing & editing. W.K. contributed to methodology and resources. O.K. and M.A. contributed to conceptualization, funding acquisition, and writing—reviewing & editing. M.K. contributed to conceptualization, data curation, funding acquisition, methodology, project administration, resources, supervision, validation, and writing—reviewing & editing. All authors read and reviewed the manuscript.
Funding
This research project was supported by the Second Century Fund (C2F), Chulalongkorn University and the 90th Anniversary of Chulalongkorn University Scholarship under the Ratchadapisek Somphot Endowment Fund (GCUGR1125652082D) to A.H.L.N. M. A. and M. K. were financially supported in part by the National Research Center for Protozoan Diseases—Obihiro University of Agriculture and Veterinary Medicine (NRCPD-OUAVM) joint research in the fiscal year 2022–2023. This Research is funded by Thailand Science Research and Innovation Fund Chulalongkorn University (FOOD66310010) and the National Research Council of Thailand (NRCT5-RSA63001-10) to MK.
Data availability
The nucleotide sequences obtained in this study were deposited in the GenBank™ database (https://www.ncbi.nlm.nih.gov/nuccore) under the following accession numbers: OP271761–OP271765 (P. caprae’s 18S rRNA), OP271766–OP271770 (P. caprae’s cytb), and OP271771–OP271775 (P. caprae’s cox1). Nucleotide sequences under accession numbers OP271761–OP271763, OP271766–OP271768, and OP271771–OP271773 were derived from P. caprae positive mosquitoes, while the remaining nucleotide sequences originated from goats.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Masahito Asada, Email: masada@obihiro.ac.jp.
Morakot Kaewthamasorn, Email: morakot.k@chula.ac.th.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-26833-4.
References
- 1.Asada M, et al. Close relationship of Plasmodium sequences detected from South American pampas deer (Ozotoceros bezoarticus) to Plasmodium spp. in North American white-tailed deer. Int. J. Parasitol. 2018;7:44–47. doi: 10.1016/j.ijppaw.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boundenga L, et al. Haemosporidian parasites of antelopes and other vertebrates from Gabon, Central Africa. PLoS ONE. 2016;11:e0148958. doi: 10.1371/journal.pone.0148958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinsen ES, Perkins SL, Schall JJ. A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): Evolution of life-history traits and host switches. Mol. Phylogen. Evol. 2008;47:261–273. doi: 10.1016/j.ympev.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Templeton TJ, et al. Ungulate malaria parasites. Sci. Rep. 2016;6:23230. doi: 10.1038/srep23230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Templeton TJ, Martinsen E, Kaewthamasorn M, Kaneko O. The rediscovery of malaria parasites of ungulates. Parasitology. 2016;143:1501–1508. doi: 10.1017/s0031182016001141. [DOI] [PubMed] [Google Scholar]
- 6.Bruce D, Harvey D, Hamerton AE, Bruce L. Plasmodium cephalophi, sp. nov. Proc. R. Soc. B. 1913;87:45–47. [Google Scholar]
- 7.Sheather AL. A malarial parasite in the blood of a buffalo. J. Comp. Pathol. 1919;32:223–229. doi: 10.1016/S0368-1742(19)80026-7. [DOI] [Google Scholar]
- 8.Kandel RC, et al. First report of malaria parasites in water buffalo in Nepal. Vet. Parasitol. Reg. Stud. Rep. 2019;18:100348. doi: 10.1016/j.vprsr.2019.100348. [DOI] [PubMed] [Google Scholar]
- 9.de Mello F, Paes S. Sur une plasmodiae du sang des chèvres. C. R. Séanc. Soc. Biol. 1923;88:829–830. [Google Scholar]
- 10.Kaewthamasorn M, et al. Genetic homogeneity of goat malaria parasites in Asia and Africa suggests their expansion with domestic goat host. Sci. Rep. 2018;8:5827. doi: 10.1038/s41598-018-24048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garnham PC, Edeson JF. Two new malaria parasites of the Malayan mousedeer. Riv. Malariol. 1962;41:1–8. [PubMed] [Google Scholar]
- 12.Garnham PC, Kuttler KL. A malaria parasite of the white-tailed deer (Odocoileus virginianus) and its relation with known species of Plasmodium in other ungulates. Proc. R. Soc. Lond. B. 1980;206:395–402. doi: 10.1098/rspb.1980.0003. [DOI] [PubMed] [Google Scholar]
- 13.Martinsen E, et al. Hidden in plain sight: Cryptic and endemic malaria parasites in North American white-tailed deer (Odocoileus virginianus) Sci. Adv. 2016;2:e1501486. doi: 10.1126/sciadv.1501486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rattanarithikul R, et al. Illustrated keys to the mosquitoes of Thailand. IV. Anopheles. Southeast Asian. Trop. Med. Public Health. 2006;37:1–128. [PubMed] [Google Scholar]
- 15.Walter Reed Biosystematics Unit. Systematic catalogue of Culicidae. http://mosquitocatalog.org (2021).
- 16.Manguin S, Garros C, Dusfour I, Harbach RE, Coosemans M. Bionomics, taxonomy, and distribution of the major malaria vector taxa of Anopheles subgenus Cellia in Southeast Asia: An updated review. Infect. Genet. Evol. 2008;8:489–503. doi: 10.1016/j.meegid.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Brosseau L, et al. A multiplex PCR assay for the identification of five species of the Anopheles barbirostris complex in Thailand. Parasit. Vectors. 2019;12:223. doi: 10.1186/s13071-019-3494-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paredes-Esquivel C, Donnelly MJ, Harbach RE, Townson H. A molecular phylogeny of mosquitoes in the Anopheles barbirostris Subgroup reveals cryptic species: implications for identification of disease vectors. Mol. Phylogen. Evol. 2009;50:141–151. doi: 10.1016/j.ympev.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Taai K, Harbach RE. Systematics of the Anopheles barbirostris species complex (Diptera: Culicidae: Anophelinae) in Thailand. Zool. J. Linn. Soc. 2015;174:244–264. doi: 10.1111/zoj.12236. [DOI] [Google Scholar]
- 20.Garros C, Van Bortel W, Trung HD, Coosemans M, Manguin S. Review of the Minimus Complex of Anopheles, main malaria vector in Southeast Asia: From taxonomic issues to vector control strategies. Trop. Med. Int. Health. 2006;11:102–114. doi: 10.1111/j.1365-3156.2005.01536.x. [DOI] [PubMed] [Google Scholar]
- 21.Dahan-Moss Y, et al. Member species of the Anopheles gambiae complex can be misidentified as Anopheles leesoni. Malar. J. 2020;19:89. doi: 10.1186/s12936-020-03168-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Bortel W, et al. Confirmation of Anopheles varuna in Vietnam, previously misidentified and mistargeted as the malaria vector Anopheles minimus. Am. J. Trop. Med. Hyg. 2001;65:729–732. doi: 10.4269/ajtmh.2001.65.729. [DOI] [PubMed] [Google Scholar]
- 23.Wharton RH, Eyles DE, Warren M, Moorhouse DE, Sandosham AA. Investigations leading to the identification of members of the Anopheles umbrosus group as the probable vectors of mouse deer malaria. Bull. 1963;29:357–374. [PMC free article] [PubMed] [Google Scholar]
- 24.Nugraheni YR, et al. Myzorhynchus series of Anopheles mosquitoes as potential vectors of Plasmodium bubalis in Thailand. Sci. Rep. 2022;12:5747. doi: 10.1038/s41598-022-09686-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tu HLC, et al. Development of a novel multiplex PCR assay for the detection and differentiation of Plasmodium caprae from Theileria luwenshuni and Babesia spp. in goats. Acta Trop. 2021;220:105957. doi: 10.1016/j.actatropica.2021.105957. [DOI] [PubMed] [Google Scholar]
- 26.Cywinska A, Hunter FF, Hebert PD. Identifying Canadian mosquito species through DNA barcodes. Med. Vet. Entomol. 2006;20:413–424. doi: 10.1111/j.1365-2915.2006.00653.x. [DOI] [PubMed] [Google Scholar]
- 27.Hebert PD, Cywinska A, Ball SL, de Waard JR. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. B. 2003;270:313–321. doi: 10.1098/rspb.2002.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogola EO, Chepkorir E, Sang R, Tchouassi DP. A previously unreported potential malaria vector in a dry ecology of Kenya. Parasit. Vectors. 2019;12:80. doi: 10.1186/s13071-019-3332-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maquart PO, Fontenille D, Rahola N, Yean S, Boyer S. Checklist of the mosquito fauna (Diptera, Culicidae) of Cambodia. Parasite. 2021;28:60. doi: 10.1051/parasite/2021056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tainchum K, et al. Diversity of Anopheles species and trophic behavior of putative malaria vectors in two malaria endemic areas of northwestern Thailand. J. Vector. Ecol. 2014;39:424–436. doi: 10.1111/jvec.12118. [DOI] [PubMed] [Google Scholar]
- 31.Vantaux A, et al. Anopheles ecology, genetics and malaria transmission in northern Cambodia. Sci. Rep. 2021;11:6458. doi: 10.1038/s41598-021-85628-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chookaew S, et al. Anopheles species composition in malaria high-risk areas in Ranong Province. Dis. Control J. 2020;46:483–493. doi: 10.14456/dcj.2020.45. [DOI] [Google Scholar]
- 33.Makanga B, et al. Ape malaria transmission and potential for ape-to-human transfers in Africa. Proc. Natl. Acad. Sci. USA. 2016;113:5329–5334. doi: 10.1073/pnas.1603008113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ariey F, Gay F, Ménard R. Malaria Control and Elimination. Springer; 2020. [Google Scholar]
- 35.Williams J, Pinto J. Training Manual on Malaria Entomology. Springer; 2012. [Google Scholar]
- 36.Rigg CA, Hurtado LA, Calzada JE, Chaves LF. Malaria infection rates in Anopheles albimanus (Diptera: Culicidae) at Ipetí-Guna, a village within a region targeted for malaria elimination in Panamá. Infect. Genet. Evol. 2019;69:216–223. doi: 10.1016/j.meegid.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Torres-Cosme R, et al. Natural malaria infection in anophelines vectors and their incrimination in local malaria transmission in Darién Panama. PLoS ONE. 2021;16:e0250059. doi: 10.1371/journal.pone.0250059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beebe NW, Saul A. Discrimination of all members of the Anopheles punctulatus complex by polymerase chain reaction-restriction fragment length polymorphism analysis. Am. J. Trop. Med. Hyg. 1995;53:478–481. doi: 10.4269/ajtmh.1995.53.478. [DOI] [PubMed] [Google Scholar]
- 39.Perkins SL, Schall JJ. A molecular phylogeny of malarial parasites recovered from cytochrome b gene sequences. J. Parasitol. 2002;88:972–978. doi: 10.1645/0022-3395(2002)088[0972:AMPOMP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 40.Snounou G, et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol. Biochem. Parasitol. 1993;61:315–320. doi: 10.1016/0166-6851(93)90077-B. [DOI] [PubMed] [Google Scholar]
- 41.Hall TA. BioEdit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic. Acids. Symp. Ser. 1999;41:95–98. [Google Scholar]
- 42.Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 43.Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA. Posterior summarization in Bayesian phylogenetics using tracer 1.7. Syst. Biol. 2018;67:901–904. doi: 10.1093/sysbio/syy032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ventim R, et al. Avian malaria infections in western European mosquitoes. Parasitol. Res. 2012;111:637–645. doi: 10.1007/s00436-012-2880-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The nucleotide sequences obtained in this study were deposited in the GenBank™ database (https://www.ncbi.nlm.nih.gov/nuccore) under the following accession numbers: OP271761–OP271765 (P. caprae’s 18S rRNA), OP271766–OP271770 (P. caprae’s cytb), and OP271771–OP271775 (P. caprae’s cox1). Nucleotide sequences under accession numbers OP271761–OP271763, OP271766–OP271768, and OP271771–OP271773 were derived from P. caprae positive mosquitoes, while the remaining nucleotide sequences originated from goats.