Abstract
Ringworm is a worldwide distributed contagious disease infecting both man and animals that constitute an economic, zoonotic, and health problem concern all over the world. During the last decade, attention has been directed to vaccination as an ideal approach to the control of such diseases. In the present study, non-adjuvanted polyvalent vaccines were prepared from locally isolated hot and virulent dermatophyte species, namely Trichophyton verrucosum (T. verrucosum), Trichophyton mentagrophytes (T. mentagrophytes), and Microsporum canis (M. canis) were immunologically evaluated. The prepared vaccine evaluation was focused on the aspects of immunogenicity and protective efficacy using guinea pigs. Both in its living or inactivated forms, the vaccine-induced significant humoral and cell-mediated immune responses and achieve proper protection of guinea pigs against challenging infections with homologous and heterologous dermatophyte strains. On the other hand, investigations on dermatophyte exo-keratinases showed that it was better produced and more expressed in a mineral-based medium containing pure keratin (3 g/L) than in the same medium with human hair supplementation (2.6 g/L). The maximum dermatophyte productivity of exo-keratinases was found to be between 18 and 21 days post-incubation. Using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), two fractions with molecular weights of 40 kDa (fraction I) and 28 kDa (fraction II) have been identified in the culture filtrate of the three involved dermatophyte species. Both fractions demonstrated keratinolytic activity. The specific activity of the isolated keratinases (number of Keratinase units (KU)/mg protein) was stronger in fraction I, where it reached 18.75, 15.38, and 14 KU/mg protein as compared to 12.9, 8.74, and 12 KU/mg protein in fraction II of T. verrucosum, T. mentagrophytes, and M. canis, respectively. The dermatophyte exo-keratinases proved to be immunogenic as they stimulated high keratinase-specific antibody titers and induced strong delayed skin hypersensitivity reactions in vaccinated animals. Anti-keratinase-specific IgG was detected in sera of guinea pigs immunized with the inactivated or living polyvalent dermatophyte vaccines by a homemade enzyme-linked immunosorbent assay (ELISA) using dermatophyte exo-keratinases as coating antigen. The intradermal injection of dermatophyte exo-keratinases induced specific delayed skin reactions in guinea pigs immunized with the inactivated or the living polyvalent dermatophyte vaccines. The intradermal injection of dermatophyte exo-keratinases in the control non-sensitized guinea pigs was associated with itching, swelling, and bloody scar formation, however, no skin indurations were formed. The development of those post-exo-keratinases injection reactions in the control non-sensitized apparently healthy guinea pigs group, suggests an exo-keratinases possible role in the pathogenesis of dermatophytosis.
Subject terms: Immunology, Microbiology
Introduction
Dermatophytes as a closely related keratinolytic group of fungi with special mentioning of T. mentagrophytes, and M. canis representing the zoophilic groups; T. benhamiae complex and M. canis complex respectively as well as which are considered the most predominantly isolated dermatophyte species from animals infected with superficial mycosis worldwide. Also, T. verrucosum another member of the T. benhamiae complex with less worldwide distribution due to the increased vaccination-based control protocols but still predominantly existed in the Middle East. Dermatophytosis represent an economically important health problem in both productive and pet animals and on the other hand a serious zoonotic threat to human, particularly children and especially nowadays, due to the habitually increased animal-human companionships1–14.
Dermatophytosis is still considered a medical issue due to certain diagnostic complexities appropriate curative treatment selection difficulties, and a suitable case-oriented treatment protocol application period guarantee the overall threat of infection spread either human–human infectious or contagious based or animal-human zoonotic based spreading. Therefore, a proper control technique seems to be an ideal approach to avoiding active dermatophytes cases dealing with obstacles15–18. Several studies have attempted to develop vaccine-based dermatophyte control strategies based on active immunization against dermatophyte infection in animals using killed or living attenuated dermatophyte vaccines15,19–24. In Norway, a vaccine containing an attenuated strain of T. verrucosum is used against cattle ringworm since 1980. It stimulates humoral and cellular immune responses conferring protection against the disease. Vaccination campaigns in densely populated countries have contributed to a substantial decrease in the number of ringworm outbreaks15. Contradictory results, however, have been reported in different countries regarding the efficacy of the dermatophyte vaccines25–27.
The dermatophyte keratinases, on the other hand, seem to play an important role in the pathogenesis and immunity against dermatophytosis28–34. Also, attempts have been made to prepare dermatophyte subunit vaccines based on current knowledge about dermatophytes virulence factors like keratinases and their potential role in disease development, but with limited success so far15. An M. canis recombinant 31.5 kDa keratinase and a crude exo-antigen were evaluated as vaccines in an experimental infection model in guinea pigs. Vaccination induced remarkably high and significant antibody responses and high cell-mediated immune responses towards both antigens. After the challenge, however, scores reflecting the severity of dermatophyte lesions did not differ significantly between vaccinated and control groups at any time after the challenge35.
Despite the availability of effective vaccines against several microbial agents, vaccination against fungal agents, and especially dermatophytosis-causing agents requires improvement and further development in both animals and humans. Therefore, the aim of the current study was the preparation and evaluation of the protective and immunizing efficacy of the newly developed non-adjuvanted polyvalent dermatophyte vaccines, prepared from the most commonly occurring and isolated dermatophyte species. Moreover, to highlight the role of dermatophytes keratinases in the dermatophytic immune-pathogenic cycle.
Material and methods
Dermatophyte strains
Trichophyton verrucosum str. Tv-96-3, T. mentagrophytes str. Tm-96-1, Microsporum canis str. Mc-97-5, and Trichophyton rubrum (T. rubrum) str. Tr-98-1 strains were obtained from the Department of Microbiology, Faculty of Veterinary medicine, Cairo University. These strains were isolated and identified from animal clinical active cases submitted for further confirmed mycological laboratory investigation in the same department as well as they were selected for the vaccine preparation according to the criteria reported by Brandebusemeyer, 199036. According to these criteria, we found that dermatophyte vaccinal strain should be; isolated from badly infected animals, grow rapidly in vitro, forming copious amounts of fungal mats, and be rich in fungal microconidia, which are known to carry the potent immunogenic determinants of the dermatophytes.
Preparation, separation, and lyophilization of dermatophyte cultures
T. mentagrophytes and M. canis were inoculated separately into 0.5L Sabouraud’s dextrose broth (OXOID) and incubated at 25 °C for 4 weeks, while T. verrucosum was inoculated into 0.5L Sabouraud’s dextrose broth supplemented by thiamine (HIMEDIA) and inositol (HIMEDIA) and incubated at 37 °C for 6 weeks. The obtained matt-submerged fungal growth was then separated using sterile gauze. The harvested fungal mats were lyophilized and ground under aseptic conditions to form a fine powder. The number of colony-forming units (CFU)/mg of the lyophilized dermatophyte powder was determined on Sabouraud’s dextrose agar (SDA) plates37.
Preparation of non-adjuvanted polyvalent dermatophyte vaccines
Two dermatophyte vaccine preparations from each species were prepared, a living and an inactivated one. In the living vaccine form, the lyophilized powder from the three selected dermatophyte species was mixed and distributed in 1 ml vials in a dose of 6 × 106 total CFU/vial (2 × 106 CFU from each dermatophyte species. The inactivated vaccine was made in the same way and the inactivation was performed according to Rybnikar, et al. 199638, using Gamma irradiation (400 krad). This killing dose was pre-determined by investigating the effect of varying doses of radio cobalt (100–400 krad) on dermatophyte viability through exposure-post-exposure culturing to confirm the killing efficiency and the minimal dose able to achieve that for the involved dermatophytes strains.
Immunization of guinea pigs
Three groups of adult female guinea pigs were used in this experiment, each group consisted of three animals. The first group was inoculated with the inactivated vaccine, the second with the living vaccine, and the third one was left as unvaccinated control. Moreover, another ten-member based group apparently healthy unvaccinated guinea pigs group was kept with those immunized with the living vaccine (second group) as a contact control. In the first two groups, each animal was injected intramuscularly (I/M) twice, at 2 weeks intervals, with 0.2 ml suspension of the polyvalent vaccine containing 6 × 106 CFU/ml. Two weeks after the second dose, the immunizing and the protective efficacies of the tested vaccine preparations were determined by measurement of the developed immune responses as well as by a challenge test.
Zero-day blood samples were collected from all animals involved in the study before vaccination to exclude ant asymptomatic cases and weekly after the priming vaccination dose, and of course after the challenge infection.
Immune response evaluation testing
The specific antibody production representing humoral immunity was detected using a homemade ELISA39,40, and the cell-mediated immune response was determined using a Trichophytin skin test41.
Homemade dermatophyte ELISA development
An aqueous whole dermatophyte extract antigens prepared from the three dermatophyte species mentioned above as homologous antigens as well as from a Trichophyton rubrum (T. rubrum) strain as heterologous antigen, were used as plate coating antigens in the humoral immunity evaluating mentioned homemade ELISA39.
Post-vaccination challenging infection
All animals were subjected to challenge infection with 0.2 ml of 21 days old culture suspension of the four dermatophytes species on an area of the skin exactly on the following site; caudal thorax, where the hairs were clipped, and the skin surface was gently scratched with sterile sandpaper.
Keratinase production investigation
T. verrucosum, T. mentagrophytes, and M. canis were investigated by inoculating a unified fungal suspension (5 × 106) of each strain separately into a mineral medium enriched with human hairs (2.6 g/L) or keratin (3 g/L) and incubated for 30 days. The keratinolytic activity of the dermatophyte exo-keratinases activity was determined every 3 days according to Siesenop, U. 199842. The characterization of the exo-keratinase was done using SDS-PAGE36,43.
Studies of the immune response to dermatophyte exo-keratinase fractions
The humoral and cell-mediated immune responses developed against dermatophyte exo-keratinases in guinea pigs vaccinated with the living and inactivated dermatophyte vaccines were determined by homemade ELISA and Trichophytin skin tests using exo-keratinase fractions of the 4 dermatophyte species as antigens.
Ethical statement
The current conducted study is reported in accordance with (Animal Research: Reporting of In-Vivo Experiments-ARRIVE) guidelines. All experimental protocols were approved by the Institutional Animal Care and Use Committee-IACUC of the faculty of veterinary medicine, at Cairo University. The guidelines of the (Institutional Animal Care and Use Committee-IACUC of the faculty of veterinary medicine, Cairo University) were completely followed during any procedures involving animal use through the current conducted study. No anesthesia or euthanasia protocols were used with the animal involved during this study as all animal-dependent methodological procedures were considered as no to low pain-causing procedures that ethically can be done on conscious alive animals.
Results
Protective efficacy of the non-adjuvanted polyvalent dermatophyte vaccines
The non-adjuvanted inactivated polyvalent vaccine induced a protection rate of 90.0, 90.9, 66.97, and 41.67% against challenges with virulent strains of T. verrucosum. T. mentagrophytes, M. canis and T. rubrum, respectively. The protective efficacy of the non-adjuvanted living polyvalent vaccine was significantly higher than that of the inactivated one and reached 100, 100, 83.33, and 66.67 against challenge with T. verrucosum. T. mentagrophytes, M. canis and T. rubrum, respectively. Challenge infection of the non-immunized control animals with the same strains induced infection rates of 83.33%, 91.67, 100, and 100%, respectively, (Fig. 1).
Figure 1.

Protective efficacy of the inactivated and the living non-adjuvanted polyvalent dermatophyte vaccines against challenge with virulent dermatophyte species.
Among guinea pigs immunized with the living polyvalent vaccine, only two animals developed ringworm lesions at the site of immunization. M. canis was isolated from both cases. On the other hand, contact non-immunized animals that were kept in the same cages remained apparently healthy during the observation period, which extended to 8 weeks post-challenge.
Immune responses to the non-adjuvanted polyvalent dermatophyte vaccine
Humoral immune response
Guinea pigs immunized with the non-adjuvanted inactivated or living polyvalent dermatophyte vaccines developed anti-dermatophyte specific IgG that was measured by ELISA. In guinea pigs immunized with the inactivated vaccine, the Geometric mean titers (GMT) of the IgG-specific antibody titers against T. verrucosum, T. mentagrophytes, M. canis, and T. rubrum were equal to 1810, 905. 640 and 40 ELISA units/ml, respectively, when measured 2 weeks post-second dose, a significant rise in the antibody titers were measured 2 weeks post-challenge with the virulent dermatophyte strains. The antibody titers reached 5120 units/ml against T. verrucosum, T. mentagrophytes, M. canis, and 905 units/ml against T. rubrum (Fig. 2A).
Figure 2.

(A) ELISA GMT of anti-T. verrucosum, T. mentagrophytes, M. canis, and T. rubrum -specific IgG in sera of Guinea pigs immunized with the non- adjuvanted inactivated polyvalent dermatophyte vaccines; (B) ELISA GMT of anti-T. verrucosum, T. mentagrophytes, M. canis, and T. rubrum IgG in sera of Guinea pigs immunized with the non- adjuvanted living polyvalent dermatophyte vaccines.
Significantly higher antibody titers were measured in guinea pigs immunized with the non-adjuvanted living polyvalent vaccine (Fig. 2B). The specific GMT of IgG measured 2 weeks after the second vaccinal dose reached 2560, 1810, 5120 and 40 ELISA units/ml against T. verrucosum, T. mentagrophytes, M. canis and T. rubrum, respectively. Further rise in the antibody titers was recorded 2 weeks post-challenge the GMT reached 7241 units/ml against T. verrucosum and 10,240 units/ml against T. mentagrophytes.
Cell-mediated immune response
Using the Trichophytin skin test, strong delayed hypersensitivity reactions were recorded against the homologous and heterologous dermatophyte Trichophytin in the immunized guinea pigs. The skin reactivity was more pronounced in those immunized with the living vaccine (Figs. 3 and 4).
Figure 3.

Illustrative chart of a single dermatophyte species based-Trichophytin skin test in Guinea pigs vaccinated with the living, inactivated non-adjuvanted polyvalent dermatophyte vaccines, and the control unimmunized group. Tuberculin test was done using Mycobacterium bovis purified protein derivatives (PPD) as non-specific antigen and NaCl 0.9% was used as negative control.
Figure 4.

Trichophytin skin test in Guinea pig sensitized with non-adjuvanted polyvalent dermatophyte vaccine, typical delayed hypersensitivity skin reaction is illustrated.
Isolation and characterization of dermatophyte exo-keratinases
Dermatophyte exo-keratinases were better produced in a mineral medium containing pure keratin (3 g/L) than in the same medium with human hair (2.6 g/L). The maximum production of exo-keratinases was found to be between 18 and 21 days post incubation (Fig. 5). Similar, if not identical fractionation patterns have been demonstrated with culture filtrates from the three dermatophyte species. The exo-keratinase fractions separated by the gel chromatography and monitored by SDS-PAGE revealed two bands in the culture filtrate of each dermatophyte. The first band corresponding to a molecular weight of about 40 kDa and the second fraction had a molecular weight of 28 kDa (Fig. 6), (Supplementary Figure S1).
Figure 5.
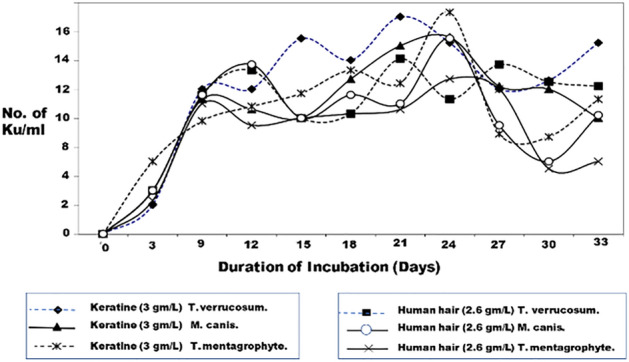
Correlation curve illustrates the effect of keratin source and incubation time on in vitro production of exo-keratinases (keratin unit/ml) by M. canis, T. verrucosum, and T. mentagrophytes.
Figure 6.

SDS-PAGE of culture supernatant of dermatophyte species grown on mineral medium containing 3 g/L keratin for identification of dermatophyte exo-keratinase based on the molecular weight. Lanes 1, 2, 5, and 6 showed 28 kDa bands (Fraction II) while 9 and 10 showed 42.5 kDa for the same fraction, and Lanes 3, 4, 7, and 8 showed 41 kDa bands (Fraction I), while 11 and 12 showed 27 kDa bands for the same fraction. (M) Indicating for the molecular weight protein ladder/marker (6500–180,000, Sigma-Aldrich). The molecular weight was estimated using a GELPRO3 analyzer.
Biological activities of exo-keratinases
The specific keratinolytic activity of the purified dermatophyte exo-keratinase (the number of KU/mg protein) was determined. It was stronger in fraction I, where it reached 18.75, 15.38, and 14 KU/mg protein, as compared to 12.9, 8.74 and 12 KU/mg protein in fraction II of T. verrucosum, T. mentagrophytes and M. canis, respectively.
Immunological activities of dermatophyte exo-keratinases
Humoral immune response
Using the heat-inactivated exo-keratinases as coating antigens in the ELISA test, anti-keratinase specific IgG was measured in sera of guinea pigs immunized with the non-adjuvanted inactivated or living polyvalent dermatophyte vaccines (Fig. 7). The anti-keratinase IgG antibodies increased slowly following vaccination with the inactivated dermatophyte vaccine and sharply two weeks post-challenge reaching to a maximum level of 1810 ELISA units/ml. The antibody titers were significantly higher in the sera of animals immunized with the living vaccine, where they reached a level of 2560 ELISA units/ ml, 2 weeks post challenge.
Figure 7.
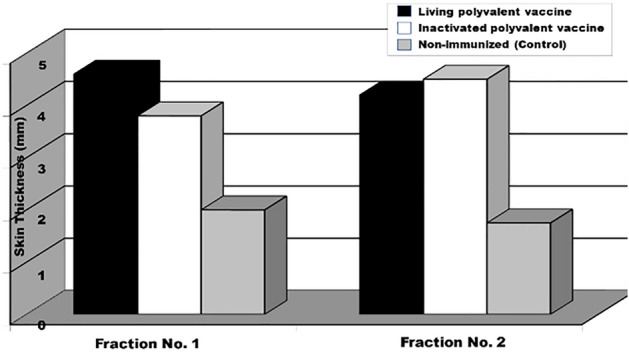
Delayed Skin reaction to dermatophyte exo-keratinase antigens (Fraction I and II) in guinea pigs immunized with the living or the inactivated non-adjuvanted polyvalent dermatophyte vaccines.
Cell-mediated immune response
The intradermal injection of dermatophyte exo-keratinases (pooled fraction I or pooled fraction II) induced specific delayed skin reaction in guinea pigs immunized with the non-adjuvanted inactivated or the living polyvalent dermatophyte vaccines (Fig. 7). The reaction was associated with the development of strong cellular reaction of delayed nature at sites of injection. This reaction involved itching, induration, and bloody scar formation. It had been observed also that the injection of the dermatophyte keratinases in the control non-sensitized guinea pigs induced inflammatory reaction associated with erythema, itching, and bloody crust formation, but without skin induration.
Discussion
In the history of veterinary clinical dermatology, it has been observed that clinical dermatophyte infection is most often seen in young animals and following recovery or clearance of the original dermatophyte infection, re-infection is rare whether by the original dermatophyte species or by a different one44. These observation stands behind the repeated trials to develop active immunization against dermatophytosis in animals.
The aim of the present work was to develop a broad-spectrum polyvalent dermatophyte vaccine against animal ringworm, therefore, the most frequently isolated dermatophyte species from cattle and pet animals according to the frequency of their isolation in previous literature, namely, T. verrucosum, T. mentagrophytes, and M. canis were selected as a candidate for this vaccine.
Two types of non-adjuvanted polyvalent dermatophyte vaccines were prepared and their immunizing and protective efficacies were evaluated in a guinea pig model. The first vaccine was inactivated by gamma radiation38,45 where a dose of 400 k rad induced complete inactivation of the three dermatophyte species. The non-adjuvanted inactivated polyvalent dermatophyte vaccine, prepared from the three above-mentioned dermatophyte species, protected guinea pigs against challenge infection with virulent homologous strains (66.67–100%). The obtained results agreed with what has been reported by several previously conducted studies in the same field21–24,46–49.
It is worth to mention that a protection rate of 41.67% has been recorded when vaccinated guinea pigs were challenged with a heterologous dermatophyte species, namely, the T. rubrum strain. This dermatophyte species induced a 100% infection rate among the control non-immunized animals. The recorded cross-protection might be attributed to the cross-antigenic relationship between different dermatophyte species44,50,51. The living polyvalent vaccine was significantly more protective than the inactivated one, a result, which is comparable with those reported by other researchers15,20.
In contrast to the inactivated dermatophyte vaccine, which did not induce any adverse side effects on the immunized guinea pigs, the use of the living vaccine was, however, associated with certain disadvantages, as two of the immunized animals developed clinical manifestations of ringworm. The recovered dermatophyte species in these cases was M. canis. The failure of M .canis as a protective antigen has also been reported previously by DeBoer et al. 200225. The adverse side effects of the living vaccine and the possibility of inducing infection certainly detract from its protective value. However, the lesions associated with the use of the living vaccine, if occurred, are mild and the infected animals undergo rapid recovery. The process of lypholization of the dermatophyte fungal mass during the vaccine preparation together with the intramuscular route of injection of the living dermatophyte vaccine significantly reduces the viability and virulence of the dermatophyte species52. The use of the inactivated dermatophyte vaccines in the control of dermatophytosis is recommended by Westhoff et al., 201021,22 because of its proven safety.
In addition to its protective efficacy, the tested dermatophyte vaccine induced significant humoral and cellular immune responses. Several authors have documented the production of humoral and cellular immune responses in animals following vaccination or infection by dermatophytes15,20,39,40,51,53,54. The role of the immune responses in the clearance of an active infection or resistance to upcoming dermatophyte infections has been reported by several studies20,47,51,55–60.
The challenge of the immunized animals with different virulent dermatophytes was associated with a significant rise in antibody titers39. This increase was also significant when the challenge was made by a heterologous dermatophyte, T. rubrum. This is of particular importance, as it documents the strong cross-antigenic relationship between dermatophytes and the possible broad-spectrum protective value of the prepared vaccine against a long list of dermatophytes species rather than those actually used in vaccine preparation50.
According to Selvam et al. 201261 there are several important biotechnological applications of microbial keratinase, and dermatophyte keratinases are considered as a possible promising candidate for prophylactic and therapeutic application against dermatophytosis. The dermatophyte keratinases have been identified and studied by several investigators31,32,34,35,37,62–67. In the current work, the dermatophyte exo-keratinases produced by T. verrucosum, T. mentagrophytes, and M. canis proved to be highly immunogenic as indicated by the induction of high antibody titers and strong delayed skin hypersensitivity reaction in vaccinated animals. Comparable results have been recorded in the following studies27,35,68–70.
The development of inflammatory reaction, itching, and bloody scar formation in the apparently healthy non-immunized animals injected with dermatophyte exo-keratinases explains its possible role in the inflammatory reaction and the itching associated with dermatophytosis. This reaction differs, however, from that recorded in the immunized animals, which manifested skin induration typical to the specific delayed hypersensitivity reaction. In the Trichophytin skin test, all Trichophytin preparations from homologous or heterologous dermatophyte species induced delayed hypersensitivity reaction in vaccinated guinea pigs. This finding indicated the possibility of presence of dermatophyte group- specific antigen(s) on which those cross-reactivity reaction were occurred.
To conclude, developing a polyvalent dermatophyte vaccine showed a promising protective prophylactic choice that is able to stand against dermatophytosis, with no or minimal post-vaccination reaction in the case of inactivated and living vaccines, respectively (Supplementary Information).
Supplementary Information
Author contributions
All authors are contributed through the all stages of the conducted study as follows; H.A.-E., R.S., F.E.-S., H.H., and H.A.-E. were involved in the establishment of a proper research methodology and outcome result, investigation and analysis, Also, H.A.-E., R.S., and H.A.-E. were responsible for primary draft writing, final writing, reviewing, and editing process.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This study was self-funded.
Data availability
The datasets used and/or analyzed related to the animal cases tested during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-26567-3.
References
- 1.Weber A. Mycozoonoses with special regard to ringworm of cattle. Mycoses. 2000;43:20–22. [PubMed] [Google Scholar]
- 2.Kumar R, Khurana R. Prevalence of dermatophytosis in cattle farms at Hisar. Haryana Vet. 2005;44:39–42. [Google Scholar]
- 3.Bond R. Superficial veterinary mycoses. Clin. Dermatol. 2010;28(2):226–236. doi: 10.1016/j.clindermatol.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Aghamirian MR, Ghiasian SA. Dermatophytes as a cause of epizoonoses in dairy cattle and humans in Iran: Epidemiological and clinical aspects. Mycoses. 2011;54(4):52–56. doi: 10.1111/j.1439-0507.2009.01832.x. [DOI] [PubMed] [Google Scholar]
- 5.Kano R. Cutaneous mycoses in Japan originating from animals. Med. Mycol. J. 2012;53(1):19–23. doi: 10.3314/mmj.53.19. [DOI] [PubMed] [Google Scholar]
- 6.Nweze EI. Dermatophytosis in domesticated animals. Rev. Inst. Med. Trop. Sao Paulo. 2012;53(2):94–99. doi: 10.1590/S0036-46652011000200007. [DOI] [PubMed] [Google Scholar]
- 7.Moretti A, Agnetti F, Mancianti F, Nardoni S, Righi C, Moretta I, Morganti G, Papini M. Dermatophytosis in animals: Epidemiological, clinical and zoonotic aspects. G Ital. Dermatol. Venereol. 2013;148(6):563–572. [PubMed] [Google Scholar]
- 8.Ndiaye D, Ndiaye M, Badiane A, Seck MC, Faye B, Ndiaye JL, Tine R, Ndir O. Dermatophytosis diagnosed at the laboratory of parasitology and mycology of Le Dantec Hospital in Dakar between 2007 and 2011. J. Mycol. Med. 2013;23(4):219–224. doi: 10.1016/j.mycmed.2013.07.054. [DOI] [PubMed] [Google Scholar]
- 9.Nweze EI, Eke IE. Dermatophytes and dermatophytosis in the eastern and southern parts of Africa. Med. Mycol. 2018;56:13–28. doi: 10.1093/mmy/myx025. [DOI] [PubMed] [Google Scholar]
- 10.Paryuni AD, Indarjulianto S, Widyarini S. Dermatophytosis in companion animals: A review. Vet. World. 2020;13(6):1174–1181. doi: 10.14202/vetworld.2020.1174-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassan AH, Hamed R, Elyazeed HA. Cross-sectional study on dermatological affections of companion animals caused by dermatophytes and other keratinophilic fungi in Greater Cairo Area, Egypt. Adv. Anim. Vet. Sci. 2021;9:615–622. [Google Scholar]
- 12.Segal E, Elad D. Human and zoonotic dermatophytoses: Epidemiological aspects. Front. Microbiol. 2021;12:713532. doi: 10.3389/fmicb.2021.713532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang C-C, Wechtaisong W, Chen S-Y, Cheng M-C, Chung C-S, Lin L-S, Lien Y-Y, Tsai Y-L. Prevalence and risk factors of zoonotic dermatophyte infection in pet rabbits in northern Taiwan. J. Fungi. 2022;8:627. doi: 10.3390/jof8060627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Čmoková A, Kolařík M, Dobiáš R, et al. Resolving the taxonomy of emerging zoonotic pathogens in the Trichophyton benhamiae complex. Fungal Divers. 2020;104:333–387. doi: 10.1007/s13225-020-00465-3. [DOI] [Google Scholar]
- 15.Lund A, Bratberg AM, Næss B, Gudding R. Control of bovine ringworm by vaccination in Norway. Vet. Immunol. Immunopathol. 2013;158(1–2):37–45. doi: 10.1016/j.vetimm.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Nardoni S, Mugnaini L, Papini R, Fiaschi M, Mancianti F. Canine and feline dermatophytosis due to Microsporum gypseum: A retrospective study of clinical data and therapy outcome with griseofulvin. J. Mycol. Med. 2013;23(3):164–167. doi: 10.1016/j.mycmed.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Hassan AH, Hamed R, Elyazeed HA. Recent trends in rapid diagnostic techniques for dermatophytosis. Int. J. Vet. Sci. Med. 2020;17:115–123. doi: 10.1080/23144599.2020.1850204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Khikani FHO, Ayit AS. Major challenges in dermatophytosis treatment: Current options and future visions. Egypt. J. Dermatol. Venereo. 2021;41(1):1–9. doi: 10.4103/ejdv.ejdv_23_20. [DOI] [Google Scholar]
- 19.Kurtdede A, Ural K, Gazyagci S, Cingi CÇ. Usage of inactivated Microsporum canis vaccine in cats naturally infected with M. canis. Mikol. Lek. 2007;14:19–21. [Google Scholar]
- 20.Lund A, Deboer DJ. Immunoprophylaxis of dermatophytosis in animals. Mycopathologia. 2008;166(5–6):407–424. doi: 10.1007/s11046-008-9111-6. [DOI] [PubMed] [Google Scholar]
- 21.Westhoff DK, Orveillon FX, Farnow D, Klös MC, Elbers K. Safety of a non- adjuvanted therapeutic vaccine for the treatment of feline dermatophytosis. Vet Rec. 2010;167(23):899–903. doi: 10.1136/vr.c4140. [DOI] [PubMed] [Google Scholar]
- 22.Westhoff DK, Kloes MC, Orveillon FX, Farnow D, Elbers K, Mueller RS. Treatment of feline dermatophytosis with an inactivated fungal vaccine. Open Mycol. J. 2010;4(10–17):18. [Google Scholar]
- 23.Cambier L, Băguţ ET, Heinen MP, Tabart J, Antoine N, Mignon B. Assessment of immunogenicity and protective efficacy of Microsporum canis secreted components coupled to monophosphoryl lipid—A adjuvant in a vaccine study using guinea pigs. Vet. Microbiol. 2015;175(2–4):304–311. doi: 10.1016/j.vetmic.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 24.Hussein AA, Al-Janabi S, Al-Khikani FHO. Prophylaxis and therapeutic ability of inactivated dermatophytic vaccine against dermatophytosis in the rabbits as an animal model. Turk. J. Pharm. Sci. 2021;18(3):326–331. doi: 10.4274/tjps.galenos.2020.81226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeBoer DJ, Moriello KA, Blum JL, Volk LM, Bredahl LK. Safety and immunologic effects after inoculation of inactivated and combined live-inactivated dermatophytosis vaccines in cats. Am. J. Vet. Res. 2002;63(11):1532–1537. doi: 10.2460/ajvr.2002.63.1532. [DOI] [PubMed] [Google Scholar]
- 26.Milan R, Alois R, Josef C, Jana B, Evzen W. Recombinant protein and DNA vaccines derived from hsp60 Trichophyton mentagrophytes control the clinical course of trichophytosis in bovine species and guinea-pigs. Mycoses. 2004;47(9–10):407–417. doi: 10.1111/j.1439-0507.2004.01028.x. [DOI] [PubMed] [Google Scholar]
- 27.Mignon B, Tabart J, Baldo A, Mathy A, Losson B, Vermout S. Immunization and dermatophytes. Curr. Opin. Infect. Dis. 2008;21(2):134–140. doi: 10.1097/QCO.0b013e3282f55de6. [DOI] [PubMed] [Google Scholar]
- 28.Viani FC, Dos Santos JI, Paula CR, Larson CE, Gambale W. Production of extracellular enzymes by Microsporum canis and their role in its virulence. Med. Mycol. 2001;39(5):463–468. doi: 10.1080/mmy.39.5.463.468. [DOI] [PubMed] [Google Scholar]
- 29.Muhsin TM, Hadi RB. Degradation of keratin substrates by fungi isolated from sewage sludge. Mycopathologia. 2002;154(4):185–189. doi: 10.1023/A:1016335623534. [DOI] [PubMed] [Google Scholar]
- 30.Viani FC, Cazares Viani PR, Gutierrez Rivera IN, Gonçalves da Silva E, Rodrigues Paula C, Gambale W. Extracellular proteolytic activity and molecular analysis of Microsporum canis strains isolated from symptomatic and asymptomatic cats. Rev. Iberoam. Micol. 2007;24(1):19–23. doi: 10.1016/S1130-1406(07)70004-9. [DOI] [PubMed] [Google Scholar]
- 31.Essien JP, Umoh AA, Akpan EJ, Eduok SI, Umoiyoho A. Growth, keratinolytic proteinase activity and thermo-tolerance of dermatophytes associated with alopecia in Uyo, Nigeria. Acta Microbiol. Immunol. Hung. 2009;56(1):61–69. doi: 10.1556/AMicr.56.2009.1.4. [DOI] [PubMed] [Google Scholar]
- 32.Giudice MC, Reis-Menezes AA, Rittner GM, Mota AJ, Gambale W. Isolation of Microsporum gypseum in soil samples from different geographical regions of Brazil, evaluation of the extracellular proteolytic enzymes activities (keratinase and elastase) and molecular sequencing of selected strains. Braz. J. Microbiol. 2012;43(3):895–902. doi: 10.1590/S1517-83822012000300007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nenoff P, Handrick W, Krüger C, Vissiennon T, Wichmann K, Gräser Y, Tchernev G. Dermatomycoses due to pets and farm animals: Neglected infections? Hautarzt. 2012;63(11):848–858. doi: 10.1007/s00105-012-2379-y. [DOI] [PubMed] [Google Scholar]
- 34.Mercer DK, Stewart CS. Keratin hydrolysis by dermatophytes. Med. Mycol. 2019;57:13–22. doi: 10.1093/mmy/myx160. [DOI] [PubMed] [Google Scholar]
- 35.Descamps FF, Brouta F, Vermout SM, Willame C, Losson BJ, Mignon BR. A recombinant 31.5 kDa keratinase and a crude exo-antigen from Microsporum canis fail to protect against a homologous experimental infection in guinea pigs. Vet. Dermatol. 2003;14(6):305–312. doi: 10.1111/j.1365-3164.2003.00342.x. [DOI] [PubMed] [Google Scholar]
- 36.Brandebusemeyer, E. Untersuchungen der Virulenz, Verträglichkeit und Wirksarnkeit einer Vakzine gegen die Trichophytie des Rindes an Rind und Meerschweinchen. Hannover, Tierärztl. Hochsch. Diss. (1990).
- 37.Gebhardt, T. Isolierung und Darstellung von Keratinasen der Derrnatophyten Trichophyton equinum und Microsporum canis. Hannover, Tierärztl. Hochsch. Diss. (1996).
- 38.Rybnikar A, Vrzal V, Chumela HM, Weigh E. Vaccination of cattle against trichophytosis using Czech dermatophyte vaccine. Veterinarstui. 1996;39:435–436. [Google Scholar]
- 39.DeBoer DJ, Moriello KA. Humoral and cellular immune responses to Microsporum canis in naturally occurring feline dermatophytosis. J. Med. Vet. Mycol. 1993;31:121–132. doi: 10.1080/02681219380000141. [DOI] [PubMed] [Google Scholar]
- 40.Sparkes AH, Stakes CR, Gruffyd-Johnes TJ. SDS-PAGE separation of dermatophyte antigens and western immunoblotting in feline dermatophytosis. Mycopathologia. 1994;128:91–98. doi: 10.1007/BF01103015. [DOI] [PubMed] [Google Scholar]
- 41.Balogh E, Meszaros C, Halmy K. Die Anwendung des Lymphocyten transformations testes bei der Untersuchung der mykotischen Sensibilisation. Mykosen. 1971;14:207–211. doi: 10.1111/j.1439-0507.1971.tb03038.x. [DOI] [PubMed] [Google Scholar]
- 42.Siesenop, U. Isolierung und Darstellung von Keratinasen Verschiedener Trichophyton mentagrophytes-Stämme. Hannover, Tierärztl. Hochsch. Diss. (1993)
- 43.Laemmli UK. Cleavage of structural protein during the assembly of the head bacteriophage T'-4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 44.Grappel SF, Bishop CT, Blank F. Immunology of dermatophytes and dermatophytosis. Bacteriol. Rev. 1974;38(2):222–250. doi: 10.1128/br.38.2.222-250.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rybnikar A, Chumela J, Vrzal V. Development of immunity after vaccination of cattle against trichophytosis. Vet. Med. 1989;34:97–100. [PubMed] [Google Scholar]
- 46.Gudding R, Lund A. Immunoprophylaxis of bovine dermatophytosis. Can. Vet. J. 1995;36:302–306. [PMC free article] [PubMed] [Google Scholar]
- 47.Mikaili, A., Chalabi, M., Ghashghaie, A. & Mostafaie, A. Immunization against bovine dermatophytosis with live Trichophyton verrucosum. Afr. J. Microbiol. Res.6(23), 4950–4953. http://www.academicjournals.org/AJMR. 10.5897/AJMR11.947. ISSN 1996-0808 (2012).
- 48.Chansiripornchai P, Suanpairintr N. Treatment of Microsporum canis infection in a cat using a fungal vaccine. Thai J. Vet. Med. 2015;45:645–649. [Google Scholar]
- 49.Portuondo DL, Ferreira LS, Urbaczek AC, Batista-Duharte A, Carlos IZ. Adjuvants and delivery systems for antifungal vaccines: Current state and future developments. Med Mycol. 2015;53:69–89. doi: 10.1093/mmy/myu045. [DOI] [PubMed] [Google Scholar]
- 50.Shukla NP, Agarwal GP. Antigenic relationship between downy and granular forms of Trichophyton mentagrophytes and Trichophyton rubrum. Microbiol. Immunol. 1983;27(4):311–314. doi: 10.1111/j.1348-0421.1983.tb00590.x. [DOI] [PubMed] [Google Scholar]
- 51.Pier AC, Hodges AB, Lauze JM, Riasbeck M. Experimental immunity to Microsporum canis and cross reactions with other dermatophytes of veterinary importance. J. Med. Vet. Mycol. 1995;33:93–97. doi: 10.1080/02681219580000211. [DOI] [PubMed] [Google Scholar]
- 52.Rybnikár A, Schmied J, Strossa J. Lyophilization of dermatophytes. Vet. Med. (Praha) 1987;32(8):497–508. [PubMed] [Google Scholar]
- 53.Okafor JI, Ada N. Keratinolytic activity of five human isolates of the dermatophytes. J. Commun. Dis. 2000;32(4):300–305. [PubMed] [Google Scholar]
- 54.Burstein VL, Beccacece I, Guasconi L, Mena CJ, Cervi L, Chiapello LS. Skin immunity to dermatophytes: From experimental infection models to human disease. Front. Immunol. 2020;11:605644. doi: 10.3389/fimmu.2020.605644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seebacher C. Epidemiology, clinic and treatment of dermatomycoses caused by zoophilic dermatophytes. Mycoses. 2000;43(1):4–7. [PubMed] [Google Scholar]
- 56.DeBoer DJ, Moriello KA. Investigations of a killed dermatophyte cell-wall vaccine against infection with Microsporum canis in cats. Res. Vet. Sci. 1995;59(2):110–113. doi: 10.1016/0034-5288(95)90042-X. [DOI] [PubMed] [Google Scholar]
- 57.Almeida SR. Immunology of dermatophytosis. Mycopathol. Sao-Paulo. 2008;166:277–283. doi: 10.1007/s11046-008-9103-6. [DOI] [PubMed] [Google Scholar]
- 58.Bagut ET, Cambier L, Heinen MP, Cozma V, Monod M, et al. Development of an enzyme linked immunosorbent assay for serodiagnosis of ringworm infection in cattle. Clin. Vaccine Immunol. 2013;20:1150–1154. doi: 10.1128/CVI.00243-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zahran, R.N., & Abdeen, E.A. Evaluation of the immune response to T. verrucosum vaccines. Am. J. Immunol.9(4), 139–147. 10.3844/ajisp.2013.139.147. http://www.thescipub.com/aji.toc (2013).
- 60.Abera, D. Review on immunity to fungal infections in animals. Anim. Vet. Sci.10(2), 15–20. http://www.sciencepublishinggroup.com/j/avs. 10.11648/j.avs.20221002.11 ISSN: 2328-5842 (print); ISSN: 2328-5850 (online) (2022).
- 61.Selvam K, Vishnupriya B. Biochemical and molecular characterization of microbial keratinase and its remarkable applications. Int. J. Pharma Bio Archiv. 2012;3(2):267–275. [Google Scholar]
- 62.Siesenop U, Böhm HK. Comparative studies on keratinase production of Trichophyton mentagrophytes strains of animal origin. Mycoses. 1995;38(5–6):205–209. doi: 10.1111/j.1439-0507.1995.tb00050.x. [DOI] [PubMed] [Google Scholar]
- 63.Muhsin TM, Aubaid AH. Partial purification and some biochemical characteristics of exocellular keratinase from Trichophyton mentagrophytes var. erinacei. Mycopathologia. 2001;150(3):121–125. doi: 10.1023/A:1010900403141. [DOI] [PubMed] [Google Scholar]
- 64.Sharma A, Chandra S, Sharma M. Difference in keratinase activity of dermatophytes at different environmental conditions is an attribute of adaptation to parasitism. Mycoses. 2012;55(5):410–415. doi: 10.1111/j.1439-0507.2011.02133x. [DOI] [PubMed] [Google Scholar]
- 65.Grumbt M, Monod M, Yamada T, Hertweck C, Kunert J, Staib P. Keratin degradation by dermatophytes relies on cysteine dioxygenase and a sulfite efflux pump. J. Invest. Dermatol. 2013;133:1550–1555. doi: 10.1038/jid.2013.41. [DOI] [PubMed] [Google Scholar]
- 66.Raheem Ademola RR, OmoladeOlabowale A, FolorunsoJamiu B, Oluwadun A, Onilude AA. Comparative study of keratinolytic activities of dermatophytes in various keratin substrates. Virol. Mycol. 2013;2:3. doi: 10.4172/2161-0517.1000117. [DOI] [Google Scholar]
- 67.Elavarashi E, Kindo AJ, Kumar P. Optimization of keratinase production by dermatophytes using poultry feathers in-vitro. Asian J. Microbiol. Biotechnol. Environ. Exp. Sci. 2018;20(4):1195–1199. [Google Scholar]
- 68.Mignon BR, Leclipteux T, Focant C, Nikkels AJ, Piérard GE, Losson BJ. Humoral and cellular immune response to a crude exo-antigen and purified keratinase of Microsporum canis in experimentally infected guinea pigs. Med. Mycol. 1999;37(2):123–129. doi: 10.1080/02681219980000191. [DOI] [PubMed] [Google Scholar]
- 69.Brouta F, Descamps F, Vermout S, Monod M, Losson B, Mignon B. Humoral and cellular immune response to a Microsporum canis recombinant keratinolytic metalloprotease (r-MEP3) in experimentally infected guinea pigs. Med. Mycol. 2003;41(6):495–501. doi: 10.1080/13693780310001615385. [DOI] [PubMed] [Google Scholar]
- 70.Mignon B. New studies on the characterization of virulence factors in Microsporum canis. Bull. Mem. Acad. R Med. Belg. 2005;160(5–6):270–275. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed related to the animal cases tested during the current study are available from the corresponding author on reasonable request.


