Abstract
Ehrlichia canis and E. chaffeensis are tick-borne obligatory intramonocytic ehrlichiae that cause febrile systemic illness in humans and dogs, respectively. The current study analyzed the pleomorphic multigene family encoding approximately 30-kDa major outer membrane proteins (OMPs) of E. canis and E. chaffeensis. Upstream from secA and downstream of hypothetical transcriptional regulator, 22 paralogs of the omp gene family were found to be tandemly arranged except for one or two genes with opposite orientations in a 28- and a 27-kb locus in the E. canis and E. chaffeensis genomes, respectively. Each locus consisted of three highly repetitive regions with four nonrepetitive intervening regions. E. canis, in addition, had a 6.9-kb locus which contained a repeat of three tandem paralogs in the 28-kb locus. These total 47 paralogous and orthologous genes encoded OMPs of approximately 30 to 35 kDa consisting of several hypervariable regions alternating with conserved regions. In the 5′-end half of the 27-kb locus or the 28-kb locus of each Ehrlichia species, 14 paralogs were linked by short intergenic spaces ranging from −8 bp (overlapped) to 27 bp, and 8 remaining paralogs in the 3′-end half were connected by longer intergenic spaces ranging from 213 to 632 bp. All 22 paralogs, five unknown genes, and secA in the omp cluster in E. canis were transcriptionally active in the monocyte culture, and the paralogs with short intergenic spaces were cotranscribed with their adjacent genes, including the respective intergenic spaces at both the 5′ and the 3′ sides. Although omp genes are diverse, our results suggest that the gene organization of the clusters and the gene locus are conserved between two species of Ehrlichia to maintain a unique transcriptional mechanism for adaptation to environmental changes common to them.
Ehrlichia spp. and related bacteria such as Cowdria and Anaplasma spp. are obligatory intracellular bacteria with a tropism for hematopoietic cells (27). These bacteria have major outer membrane proteins (OMPs) which are encoded by a multigene family that is estimated to occupy ca. 1 to 2% of the genome in some species (20, 21, 22, 26, 30, 33). In the organisms studied thus far these OMPs were shown to be immunoprotective in animals and to induce proinflammatory cytokines by leukocytes in vitro (13, 14, 17, 21, 23, 28). However, the gene locus, organization, expression, and function of these multigene families were not known well.
Canine and human monocytic ehrlichioses are tick-borne zoonoses caused by infection with Ehrlichia canis and E. chaffeensis, respectively. Canine monocytic ehrlichiosis (CME) was first described in 1935 (7) and now occurs worldwide, especially in tropical and subtropical regions (12). Human monocytic ehrlichiosis (HME) was first recognized in the United States in 1987, and it was assumed that E. canis caused this disease because of the positive reaction of the patient's serum to E. canis antigen (15). In 1990, an organism was isolated from a patient with HME (5) and classified as a new species, E. chaffeensis, since the 16S rRNA gene sequence was 1.8% divergent from that of E. canis (1). Since this first report, HME has been increasingly recognized in the United States. Serologic evidence suggests the disease also occurs in Europe, Africa, and Mexico (28). These two Ehrlichia species are transmitted primarily by different species of ticks: E. canis by the brown dog tick, Rhipicephalus sanguineus (10), and E. chaffeensis by the Lone Star tick, Amblyomma americanum (2). The natural reservoirs of these two organisms are also different: canidae are only known reservoirs of E. canis, whereas white-tailed deer (Odocoileus virginianus) is a natural reservoir of E. chaffeensis (8). E. canis and E. chaffeensis cause a febrile systemic illness that is often severe and even be fatal in dogs and humans, respectively. However, E. chaffeensis causes only a mild febrile response with no hematological abnormalities in experimentally infected dogs (6). Conversely, an E. canis-like agent (a new strain of E. canis) causes asymptomatic chronic infection in humans (25).
We originally identified orthologous and paralogous genes encoding immunodominant and immunocross-reactive 30- to 32-kDa major surface proteins in E. chaffeensis (seven genes) and E. canis (three genes) and showed that they belong to a polymorphic multigene family, termed omp-1 for E. chaffeensis and p30 for E. canis (20, 21). In our subsequent review (28) and several meeting presentations, at least 16 omp-1 or 22 p30 genes at a single locus and the tandem gene arrangement were shown. One of the E. chaffeensis genes, p28, was overexpressed in Escherichia coli (21). Immunization with the recombinant P28 protein protected mice from infection with E. chaffeensis, suggesting that P28 is a potential vaccinogen (21). In order to understand the role of the multigene family in the host-specific pathogenesis of the monocytic ehrlichiosis (ME) agents and in the interplay between ehrlichiae and their diverse host environments and in order to explore the most protective OMP protein against ehrlichial infection, it is essential to characterize the OMP multigene family and their gene organization and expression in these two species.
Reddy et al. (26) published five and two members of the multigene families in E. chaffeensis and E. canis, respectively (four genes in E. chaffeensis and one gene in E. canis are the same genes identified by us [20, 21]). McBride et al. (16) published one member of the E. canis multigene family linking four p30 genes previously identified by Ohashi et al. (20) and Reddy et al. (26). Recently, Yu et al. (31) using a rapid adapter primer PCR method, assembled 14 members of the omp family in E. chaffeensis and linked them to seven paralogs which had been previously described by us (21) and Reddy et al. (26). Yu et al. (31) described the locus of the gene cluster to be downstream of a gene (clpx) homologous to an ATP-dependent Clp protease ATP-binding subunit, but the region downstream from an omp gene at the 3′ end of the gene cluster was not described. The relationship of the multigene family between CME and HME agents, however, remains unclear. Furthermore, the transcriptional pattern of these tandemly arranged multigenes is rather unclear. Reddy et al. (26) showed that only one gene at 3′ end of four genes examined by reverse transcription-PCR (RT-PCR) was transcribed by E. chaffeensis in DH82 canine monocytic cell line, whereas McBride et al. (16) found by using RT-PCR that all five members of the multigene family examined were monocistronically transcribed by E. canis in DH82 cells. Yu et al. (31) found that, based on RT-PCR analyses, 6 of 10 tested genes of E. chaffeensis were transcribed in DH82 cells and that none of these genes was cotranscribed. Since DNA template control using the same sets of primers were not shown in any of these three studies, it is not known whether the primer pairs selected produce sufficient intensities of bands for a given amount of template DNA.
In the present study, we were especially careful to avoid sequencing errors due to repetitive elements present among homologous omp genes. We report here for the first time a 28-kb region including 22 paralogs of the multigene family in E. canis (termed the omp cluster) and a 6.9-kb region including 3 paralogs. We also describe a 27-kb omp cluster including 22 paralogs in E. chaffeensis, which was distinct from the previous report by Yu et al. (31) with regard to the gene number and genetic locus. We further examined the transcription and cotranscription of 28 genes including 22 omp paralogs of E. canis using 28 sets of gene-specific primer pairs and another set of primers specific to the adjacent genes flanking 27 intergenic spaces. The DNA template control was included for reactions using every primer pair.
(Part of this study was presented at the 99th and 100th General Meetings of the American Society for Microbiology [Chicago, Ill., 30 May to 1 June 1999, and Los Angeles, Calif., 22 to 24 May 2000].)
MATERIALS AND METHODS
Organisms, culture, and purification.
E. canis Oklahoma and E. chaffeensis Arkansas were propagated in DH82 canine macrophage cell line at 37°C in Dulbecco modified Eagle's medium containing 10% heat-inactivated fetal bovine serum and purified by Percoll density gradient centrifugation (21) or Sephacryl S-1000 column chromatography (29).
Genomic Southern blot analysis.
Genomic DNAs were extracted from purified ehrlichiae and digested with restriction enzymes. DNA probes (A to F in Fig. 1) was labeled with [α-32P]dATP by the random primer method with a kit (Amersham Pharmacia Biotech, Piscataway, N.J.). Southern hybridization procedure was as described elsewhere (21), and a hybridization temperature of 65°C (high-stringency condition) was used to avoid the binding of the probes to other homologous genes. The membrane was exposed to Hyperfilm (Amersham Pharmacia Biotech).
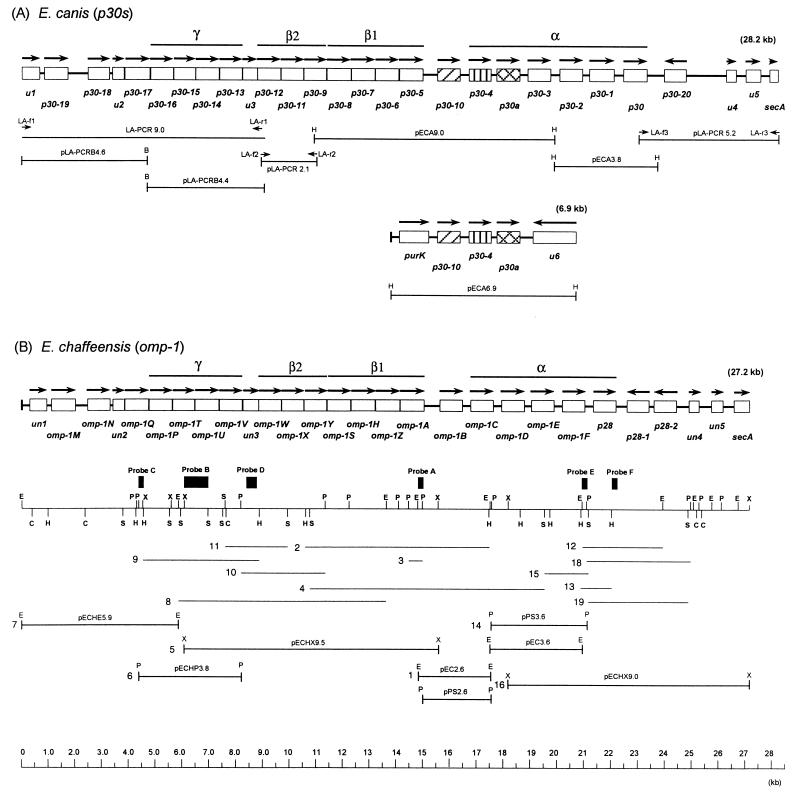
FIG. 1.
Schematic representation of gene organization of the p30 and omp-1 multigene families of ME agents. Genes are represented as open boxes, with arrows indicating their orientation. (A) E. canis omp cluster (28.2 kb) and another locus (6.9 kb). The LA-PCR amplicon or the recombinant clones are shown below in a diagram of gene organization. (B) E. chaffeensis omp cluster and its restriction map. Fragments hybridized by genomic Southern blot analysis with probes A to E in Fig. 2 and the cloned fragments are shown by lines with identification numbers under the map. Bars indicate the repetitive regions α, β (subregions β1 and β2), or γ, that were identified by the dot plot analysis in Fig. 3. E, EcoRI; H, HindIII; C, ClaI; P, PstI; S, SpeI; X, XbaI.
Cloning and sequencing of overlapping DNA fragments of ehrlichiae.
The DNA fragments, which were detected by genomic Southern blot analysis, were inserted into pBluescript II KS(+) vector or pMW119 vector. The recombinant plasmids were introduced into E. coli DH5α. The ehrlichial DNA fragments were isolated by the colony hybridization method with the labeled probes used in the genomic Southern blotting. For the amplification of large DNAs (∼9.0 kb), we used a Long and Accurate PCR kit (LA-PCR; Takara Biomedical, Ohtsu, Japan) using primer pairs of 36 nucleotides (nt) with BamHI sites. The PCR condition were 35 cycles consisting of 20 s of denaturation at 98°C, 10 s of annealing at 56°C, and 15 min of extension at 68°C. The amplicons obtained were digested with BamHI, and they were cloned by using the same plasmid vectors as those described above. For sequencing, multiple small fragments (∼2 kb) were generated and subcloned from these large fragments based on the restriction digestion analysis. The overlapping areas were further confirmed by PCR using primers flanking the junctions of the fragments. Sequencing was performed with universal or suitable synthetic primers by the dideoxy chain termination method.
Preparation of cDNA.
Total RNA was prepared from 5 × 106 E. canis-infected DH82 cells using the RNeasy Mini Kit according to the manufacturer's instruction (Qiagen, Valencia, Calif.). A 5-μg RNA sample was treated with 10 U of RNase-free DNase I (Epicentre, Madison, Wis.) in Tris-HCl buffer (pH 7.5) containing 10 mM MgCl2 and 1 U of RNase inhibitor for 30 min at 37°C. The RNA was repurified by using the same kit to remove the DNase I. For cDNA synthesis, the isolated RNA (2.5 μg) was reverse transcribed in 20 μl of the reaction mixture using 200 ng of random hexamer primers and Superscript II (Life Technologies, Inc., Gaithersburg, Md.) at 42°C for 50 min.
DNA-PCR and RT-PCR.
Twenty-eight primer pairs for the amplification of 28 genes were designed based on the gene-specific region and the target size. Twenty-nine primer pairs for the amplification of 27 sets of two adjacent genes connected by their intergenic spaces were designed based on other gene-specific regions and target size. One long space was examined with three pairs of primers which amplify three overlapping fragments covering the intergenic space and the flanking genes. The nucleotide positions and the sizes of each amplicon are shown in Table 1. A PCR reaction mixture (50 μl) contained 5 ng of genomic DNA from purified E. canis for DNA-PCR or 0.5 μl of the cDNA products for reverse transcription-PCR (RT-PCR), 10 pmol each of gene-specific primer, a 0.2 mM concentration of deoxynucleoside triphosphate mixture, 2.5 U of Taq polymerase, and 1.5 mM MgCl2. The PCR conditions were 1 cycle of 3 min of denaturation at 94°C, followed by 28 cycles consisting of 1 min of denaturation at 94°C, 1 min of annealing at 54°C, and 1 min of extension at 72°C. RT-PCR without reverse transcriptase was carried out using respective primer pairs to rule out the contamination of DNA in the RNA preparation (negative control). The amplicons were electrophoresed and visualized by ethidium bromide staining.
TABLE 1.
Properties of the 28-kb omp locus and the 6.9-kb locus of E. canis and the 27-kb omp locus of E. chaffeensis
| E. canis genes or intergenic spaces | ORF (bp) | Space (bp) | Amino acid no.a | Molecular mass (Da) | Coding region (nt position) | Regions for RT-PCR (nt position and size [bp]) | E. chaffeensis genes or intergenic spaces | ORF (bp) | Space (bp) | Amino acid no.a | Molecular mass (Da) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (28.2 kb) | (27.2 kb) | ||||||||||
| u1 (partial) | 612 | 204 | NDd | 1–612 | 187–454 (268) | un1 | 693 | 213 | 24,477 | ||
| u1-p30-19 | 230 | 546–904 (359) | un1-omp-1M | 182 | |||||||
| p30-19 | 888 | 296 | 32,760 | 843–1730 | 963–1342 (380) | omp-1M | 894 | 298 | 33,291 | ||
| p30-19-p30-18 | 760 | 1689–2523 (835) | omp-1M-omp-1N | 468 | |||||||
| p30-18 | 825 | 275 | 31,030 | 2491–3315 | 2902–3250 (349) | omp-1N | 852 | 284 | 32,093 | ||
| p30-18-u2 | 108 | 3135–3665 (531) | omp-1N-un2 | 109 | |||||||
| u2 | 444 | 148 | 17,570 | 3425–3868 | 3454–3816 (363) | un2 | 444 | 148 | 17,583 | ||
| u2-p30-17 | 13 | 3796–4039 (244) | un2-omp-1Q | 12 | |||||||
| p30-17 | 948 | 316 | 35,241 | 3882–4829 | 4053–4434 (382) | omp-1Q | 891 | 297 | 33,376 | ||
| p30-17-p30-16 | 18 | 4719–4961 (243) | omp-1Q-omp-1P | 19 | |||||||
| p30-16 | 849 | 283 | 32,495 | 4848–5696 | 5244–5620 (377) | omp-1P | 855 | 285 | 32,576 | ||
| p30-16-p30-15 | 19 | 5602–5854 (253) | omp-1P-omp-1T | 15 | |||||||
| p30-15 | 792 | 264 | 30,624 | 5716–6508 | 6214–6461 (248) | omp-1T | 816 | 272 | 31,232 | ||
| p30-15-p30-14 | 17 | 6442–6668 (227) | omp-1T-omp-1U | 18 | |||||||
| p30-14 | 867 | 289 | 32,887 | 6525–7391 | 6748–6994 (247) | omp-1U | 885 | 295 | 33,614 | ||
| p30-14-p30-13 | 27 | 7320–7567 (248) | omp-1U-omp-1V | 24 | |||||||
| p30-13 | 834 | 278 | 30,828 | 7419–8252 | 7600–8085 (486) | omp-1V | 837 | 279 | 30,841 | ||
| p30-13-u3 | 13 | 8064–8286 (223) | omp-1V-un3 | 26 | |||||||
| u3 | 555 | 185 | 21,967 | 8266–8820 | 8268–8748 (481) | un3 | 588 | 196 | 23,120 | ||
| u3-p30-12 | 5b | 8729–9022 (294) | un3-omp-1W | 8b | |||||||
| p30-12 | 867 | 289 | 32,459 | 8816–9682 | 9057–9401 (345) | omp-1W | 849 | 283 | 31,661 | ||
| p30-12-p30-11 | 11 | 9612–9879 (268) | omp-1W-omp-1X | 11 | |||||||
| p30-11 | 837 | 279 | 31,378 | 9694–10530 | 9859–10150 (292) | omp-1X | 825 | 275 | 30,475 | ||
| p30-11-p30-9 | 12 | 10376–10630 (255) | omp-1X-omp-1Y | 12 | |||||||
| p30-9 | 843 | 281 | 31,523 | 10543–11385 | 10935–11312 (378) | omp-1Y | 855 | 285 | 31,710 | ||
| p30-9-p30-8 | 25 | 11148–11665 (518) | omp-1Y-omp-1S | 27 | |||||||
| p30-8 | 897 | 299 | 33,084 | 11411–12307 | 11899–12152 (254) | omp-1S | 873 | 291 | 31,973 | ||
| p30-8-p30-7 | 14 | 12119–12663 (545) | omp-1S-omp-1H | 28 | |||||||
| p30-7 | 888 | 296 | 33,279 | 12322–13209 | 12641–12842 (202) | omp-1H | 894 | 298 | 33,025 | ||
| p30-7-p30-6 | 15 | 12825–13273 (449) | omp-1H-omp-1Z | 27 | |||||||
| p30-6 | 882 | 294 | 32,867 | 13225–14106 | 13253–13712 (460) | omp-1Z | 900 | 300 | 33,042 | ||
| p30-6-p30-5 | 26 | 13691–14247 (557) | omp-1Z-omp-1A | 15 | |||||||
| p30-5 | 879 | 293 | 32,231 | 14133–15011 | 14368–14616 (249) | omp-1A | 891 | 297 | 32,961 | ||
| p30-5-p30-10 | 550 | 14916–15670 (755) | omp-1A-omp-1B | 583 | |||||||
| p30-10 | 840 | 280 | 30,960 | 15562–16401 | 15827–16118 (292) | omp-1B | 849 | 283 | 31,015 | ||
| p30-10-p30-4 | 330 | 16306–16797 (492) | omp-1B-omp-1C | 305 | |||||||
| p30-4 | 828 | 276 | 30,658 | 16732–17559 | 17172–17479 (308) | omp-1C | 840 | 280 | 30,320 | ||
| p30-4-p30a | 213 | 17477–17873 (397) | omp-1C-omp-1D | 306 | |||||||
| p30a | 861 | 287 | 32,013 | 17773–18633 | 18010–18256 (247) | omp-1D | 858 | 286 | 31,508 | ||
| p30a-p30-3 | 299 | 18514–19177 (664) | omp-1D-omp-1E | 264 | |||||||
| p30-3 | 849 | 283 | 31,624 | 18933–19781 | 19158–19494 (337) | omp-1E | 834 | 278 | 30,542 | ||
| p30-3-p30-2 | 345 | 19698–20231 (534) | omp-1E-omp-1F | 312 | |||||||
| p30-2 | 840 | 280 | 30,801 | 20127–20966 | 20213–20592 (380) | omp-1F | 840 | 280 | 30,729 | ||
| p30-2-p30-1 | 256 | 20879–21560 (682) | omp-1F-p28 | 311 | |||||||
| p30-1 | 921 | 307 | 34,106 | 21223–22143 | 21540–22064 (525) | p28 | 843 | 281 | 30,342 | ||
| p30-1-p30 | 355 | 22045–22532 (488) | p28-p28-1 | 407 | |||||||
| p30 | 864 | 288 | 31,589 | 22499–23362 | 22746–22991 (246) | p28-1 | 813 | 271 | 30,048 | ||
| p30-p30-20 | 632 | 23263–24081 (819) | p28-1-p28-2 | 185 | |||||||
| p30-20 | 825 | 275 | 30,842 | 24818–23992 | 24104–24432 (329) | p28-2 | 834 | 278 | 31,850 | ||
| p30-20-u4 | 1,497 | p28-2-un4 | 1,295 | ||||||||
| p30-20-u4 (A)c | 24765–25251 (487) | ||||||||||
| p30-20-u4 (B)c | 25067–25824 (758) | ||||||||||
| p30-20-u4 (C)c | 25578–26365 (788) | ||||||||||
| u4 | 360 | 120 | 13,814 | 26316–26675 | 26345–26652 (308) | un4 | 378 | 126 | 14,270 | ||
| u4-u5 | 374 | 26629–27173 (545) | un4-un5 | 464 | |||||||
| u5 | 546 | 182 | 19,749 | 27050–27595 | 27156–27542 (387) | un5 | 426 | 142 | 15,246 | ||
| u5-secA | 341 | 27525–27981 (457) | un5-secA | 408 | |||||||
| secA (partial) | 318 | 106 | ND | 27937–28254 | 27963–28219 (257) | secA (partial) | 582 | 194 | ND | ||
| (6.9 kb) | |||||||||||
| upstream of purk | 328 | ND | |||||||||
| purk | 1,089 | 363 | 40,742 | 329–1417 | ND | ||||||
| purk-p30-10 | 338 | ND | |||||||||
| p30-10 | 840 | 280 | 30,960 | 1756–2595 | 2021–2312 (292) | ||||||
| p30-10-p30-4 | 330 | ND | |||||||||
| p30-4 | 828 | 276 | 30,658 | 2926–3753 | 3366–3673 (308) | ||||||
| p30-4-p30a | 213 | ND | |||||||||
| p30a | 861 | 287 | 32,013 | 3967–4827 | 4204–4450 (247) | ||||||
| p30a-u6 | 484 | ND | |||||||||
| u6 (partial) | 1,602 | 534 | ND | 6913–5309 | ND |
Amino acid numbers including signal peptides for P30s and OMP-1s.
Overlapping (bp).
Three overlapping regions (A, B, and C) of 1,497-bp space designed for analysis of RT-PCR.
ND, not determined.
Sequence analysis and GenBank accession numbers.
Binary comparison and dot plot analysis of the entire DNA sequences of the gene cluster were performed by using the GAP program from the Genetics Computer Group (Madison, Wis.) and Omiga 2.0 (Oxford Molecular Group Inc., Hunt Valley, Md.). Amino acid sequence alignments were performed by using CLUSTAL V. The phylogenetic analysis was carried out using PHYLIP software version 3.5.7 (9). The phylogram was constructed using neighbor-joining method with the Kimura formula, and 1,000 bootstrap replications were conducted to evaluate the reliability of the tree. The DNA sequences reported here have been assigned the GenBank accession numbers U72291 for the E. chaffeensis 27-kb region, AF078553 for the E. canis 28-kb region, and AF324792 for the E. canis 6.9-kb region.
RESULTS
Assembly and confirmation of the omp gene clusters of E. canis and E. chaffeensis by cloning overlapping fragments.
The cloning strategy is shown in Fig. 1. We previously cloned two HindIII DNA fragments of E. canis in pECA9.0 and pECA3.8 and three genes of the p30 multigene family (p30, p30-1, and p30a) were identified within these fragments by partial sequencing (Fig. 1A) (21). In the current study these two DNA fragments were completely sequenced and linked by a PCR using the genomic DNA as a template. Three additional overlapping DNAs of 9.0, 2.1, and 5.2 kb were obtained by LA-PCR with three primer pairs (Fig. 1A) designed based on DNA sequence of the E. chaffeensis omp cluster as described below. The BamHI digestion of the 9.0-kb amplicon generated two DNA fragments of 4.6 and 4.4 kb (Fig. 1A). All fragments except for the 5.2-kb DNA were cloned into the pBluescript vector. The 5.2-kb fragment could be cloned only when the pMW119 vector, a low-copy-number plasmid, was used. These recombinant plasmids were designated pLA-PCRB4.6, pLA-PCRB4.4, pLA-PCR2.1, and pLA-PCR5.2. An additional HindIII fragment of 6.9 kb hybridized to the p30a gene probe in genomic Southern blot analysis (20) was also cloned from E. canis genomic DNA by the colony hybridization method, and the clone was designated pECA6.9. As previously shown (20), since the band intensities of two loci hybridized with the p30a probe were almost identical, these two loci may be located in a single E. canis genome.
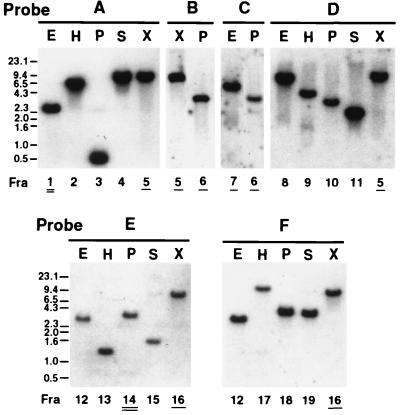
In E. chaffeensis, we had previously identified a 6.3-kb locus, including six homologous genes of omp-1 multigene family, by cloning four overlapping fragments in pPS2.6, pEC2.6, pPS3.6, and pEC3.6 (Fig. 1B) (21). In this study, 9.5-, 3.8-, and 5.9-kb overlapping DNA fragments upstream from the 6.3-kb locus were cloned from genomic DNA by combination of genomic Southern blotting and colony hybridization with probes A (a PstI-EcoRI fragment), B (XbaI-SpeI), and C (PstI-XbaI) that were prepared from pEC2.6, pECHX9.5, and pECHP3.8, respectively (Fig. 1B and 2). A 9.0-kb fragment overlapping with the 6.3-kb locus could be cloned in the pMW119 plasmid vector (but not in pBluescript) and identified by colony hybridization with probe E (EcoRI-PstI) from pPS3.6. The recombinant clones were designated pECHX9.5, pECH3.8, pECH5.9, and pECHX9.0. The genomic Southern blotting result is shown to verify the accuracy of the sequence assembly of E. chaffeensis omp cluster in Fig. 2. In addition to probes A, B, C, and E, probes D and F were used as representatives to verify the sequences in the 5′-end half and 3′-end half of the gene cluster, respectively. The restriction map based on the sequence of the omp cluster completely matched with the results of the blotting (Fig. 1B and 2). The genomic Southern blot analysis revealed that at least seven genes (omp-1Q, omp-1T, omp-1U, un3, omp-1A, omp-1F, and p28) were a single copy.
FIG. 2.
Genomic Southern blot analysis of E. chaffeensis omp cluster. The number under each lane represents the hybridized fragment shown in Fig. 1B. The numbers with a double or single underline show the fragments cloned in our previous studies (20, 21) and in the present study, respectively. The locations of probes A to F are shown on the restriction map in Fig. 1B.
Comparative analysis of the 28- and 6.9-kb omp loci of E. canis and the 27-kb omp locus of E. chaffeensis.
The omp locus of 28,254 bp in E. canis had 28 protein-coding genes (Fig. 1A and Table 1), and the G+C content was 29.36% (AT-rich). By sequence identity analysis, 22 of the 28 genes were found to belong to the p30 multigene family. These 22 paralogs were homologous, but not identical, and were tandemly arranged except for one gene with an opposite orientation. One of six remaining genes had a high similarity with secA, which is known to be required for OMP transport (3), and another gene (u1 in Fig. 1A) was similar to hypothetical transcriptional regulators of Sinorhizobium meliloti ORF3 and R. prowazekii RP497. No gene homologous to the four remaining genes (u2 to u5 in Fig. 1A) was found by database search. Another locus of 6,913 bp had five protein-coding genes, and the G+C content was 29.46%. A 3.1-kb DNA consisting of a set of three identical genes (p30-10, p30-4, and p30a) and two intergenic spaces, identical to those in the 28-kb locus, was repeated within this locus (Fig. 1A). Two other genes in this locus were purK encoding a phosphoribosylaminoimidazole carboxylase and u6 of unknown function.
In E. chaffeensis, the omp cluster of 27,190 bp also had 28 protein-coding genes, including 22 paralogs in the omp-1 multigene family, and the G+C content was 30.95% (Fig. 1B). The gene organization in E. chaffeensis was similar to that of E. canis. However, two genes were in the opposite orientation in E. chaffeensis (p28-1 and p28-2) instead of the one gene (p30-20) in E. canis. None of the genes were identical between E. canis and E. chaffeensis. The genetic locus of omp clusters were identical between E. chaffeensis and E. canis. Namely, omp-1 genes were located downstream from the hypothetical transcription regulator (un1 in Fig. 1B) and upstream from secA in the genome. The identity of the entire DNA sequences between these two omp clusters is 64.3%. Universal start codons were found in all 44 omp genes. Our results on E. chaffeensis were clearly in contrast to those of Yu et al. (31), as discussed in detail in the Discussion.
A unique characteristic of the gene organization in the omp clusters conserved between two Ehrlichia spp. is the diversity of intergenic spaces (−8 to 1,497 bp). In 5′-end half of each omp cluster, 14 genes (u2 to P30-5 in E. canis and un2 to omp-1A in E. chaffeensis) were linked by short intergenic spaces ranging from −8 to 28 bp (Table 1). A set of two genes (u3 and p30-12 in E. canis and un3 and omp-1W in E. chaffeensis) was overlapped by 5 or 8 bp. Eight genes in the 3′-end half (p30-10 to p30-20 in E. canis and omp-1B to p28-2 in E. chaffeensis) were connected by longer intergenic spaces ranging from 213 to 632 bp.
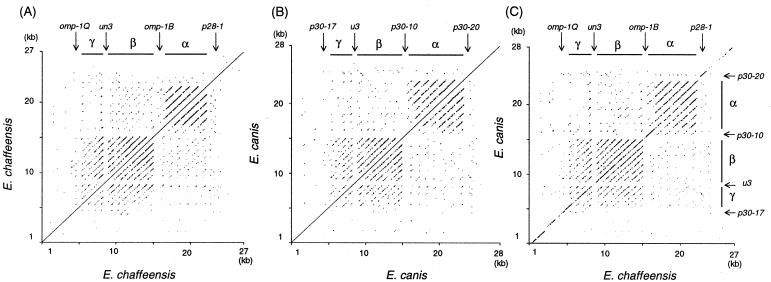
The dot plot analysis of the omp cluster in a single species and between two species revealed three large repetitive regions (α, β, and γ in Fig. 3) consisting of multiple homologous DNA segments (>30 bp) which span 3 to 6.4 kb in each cluster. The α region was shorter in E. chaffeensis than in E. canis (p28-1 is in the nonrepetitive region). Alternating with these repetitive regions were four nonrepetitive areas (upstream of p30-17, u3, and p30-10 and downstream of p30-20 in E. canis; upstream of omp-1Q, un3, omp-1B, and downstream of p28-1 in E. chaffeensis) (Fig. 3). Repetitive elements are expected to involve in genome fluidity and antigenic variation (24). Thus, the gene organization and the repetitive regions of the omp clusters were conserved between these two monocytic Ehrlichia spp.
FIG. 3.
Dot plot analysis of the omp cluster in E. canis (A), E. chaffeensis (B), and between the two species (C). The repetitive regions consisting of multiple homologous DNA segments were analyzed using the dot plot program with Omiga 2.0 software. The window cutoff was set to 30 bp and at an 80% minimum percentile score. The bars show three repetitive regions (α, β, and γ).
Properties and relationships of proteins encoded by the multigene families.
A total of 44 distinct genes in the p30 and omp-1 multigene families encoded 264- to 316-amino-acid proteins with molecular masses of 30,624 to 35,241 Da (Table 1). The putative signal peptides consisting of 25- to 31-amino-acid residues, one of which was determined based on the N-terminal amino acid sequence analysis of the native and mature protein (21), were also found in all proteins at the N termini. The molecular masses of the predicted mature proteins were 27,304 to 32,940 Da in 44 proteins. The estimated isoelectric points of proteins predicted from open reading frames were 4.59 to 9.43, and the 5′ half of each omp cluster had basic proteins, and the 3′ half had acidic proteins (Table 2). The protein sequence identities of all paralogs of the omp genes in E. chaffeensis and E. canis were 19.1 to 82.7% and 19.3 to 71.8%, respectively. We defined orthologs based on gene locations and protein sequence identities due to nonsegregation of omp genes into two Ehrlichia spp. in α and β1 regions (Fig. 4). The identities of all orthologs of omp genes between the two species were in the range of 45.5 to 79.3% (Table 2). The omp cluster of E. canis appears to lack a protein closely related to P28-2 of E. chaffeensis, because the sequence identities of P28-2 with 22 E. canis proteins were uniformly low (21.5 to 29.4%). The identities of the partial sequence of two orthologs (U1-UN1 and SecA) at both ends of the cluster were high: 83.1 and 94.3%, respectively (Table 2), whereas four orthologs (U2-UN2, U3-UN3, U4-UN4, and U5-UN5) of unknown function had the low sequence identities of 21.6 to 45.5%.
TABLE 2.
pIs and identities of OMP orthologs between E. canis and E. chaffeensis
|
E. canis
|
E. chaffeensis
|
Orthologa (% identity) | ||
|---|---|---|---|---|
| Paralog | pI | Paralog | pI | |
| U1 | NDb | UN1 | 9.64 | 83.1c |
| P30-19 | 8.31 | OMP-1M | 8.31 | 73.6 |
| P30-18 | 9.29 | OMP-1N | 9.43 | 63.3 |
| U2 | 9.66 | UN2 | 10.1 | 21.6 |
| P30-17 | 8.15 | OMP-1Q | 7.81 | 46.0 |
| P30-16 | 8.09 | OMP-1P | 9.34 | 65.7 |
| P30-15 | 9.33 | OMP-1T | 8.67 | 45.5 |
| P30-14 | 9.17 | OMP-1U | 9.01 | 61.6 |
| P30-13 | 9.22 | OMP-1V | 8.51 | 62.2 |
| U3 | 9.65 | UN3 | 9.07 | 23.2 |
| P30-12 | 6.61 | OMP-1W | 9.04 | 61.1 |
| P30-11 | 5.86 | OMP-1X | 5.54 | 71.3 |
| P30-9 | 7.72 | OMP-1Y | 6.43 | 73.3 |
| P30-8 | 6.34 | OMP-1S | 6.08 | 60.1 |
| P30-7 | 8.17 | OMP-1H | 8.64 | 59.5 |
| P30-6 | 8.83 | OMP-1Z | 5.58 | 52.4 |
| P30-5 | 8.62 | OMP-1A | 6.61 | 64.2 |
| P30-10 | 5.61 | OMP-1B | 5.01 | 79.3 |
| P30-4 | 7.72 | OMP-1C | 5.77 | 57.0 |
| P30a | 8.56 | OMP-1D | 4.59 | 57.0 |
| P30-3 | 5.70 | OMP-1E | 5.96 | 55.5 |
| P30-2 | 5.88 | OMP-1F | 6.17 | 74.2 |
| P30-1 | 6.20 | P28 | 5.42 | 64.8 |
| P30 | 5.97 | (P28)d | 68.3 | |
| P30-20 | 5.95 | P28-1 | 5.30 | 78.7 |
| NIe | P28-2 | 5.60 | ||
| U4 | 4.35 | UN4 | 4.33 | 26.7 |
| U5 | 4.18 | UN5 | 3.46 | 45.5 |
| SecA | ND | SecA | ND | 94.3f |
Protein sequence identities between orthologs.
ND, not determined.
Identity between 202-amino-acid partial sequences at the positions from 11 to 213 residues of UN1.
Both P30 a P30-1 are orthologs of P28.
No ortholog corresponding to P28-2 was identified.
Identity between 106-amino-acid partial sequences from the start codons.
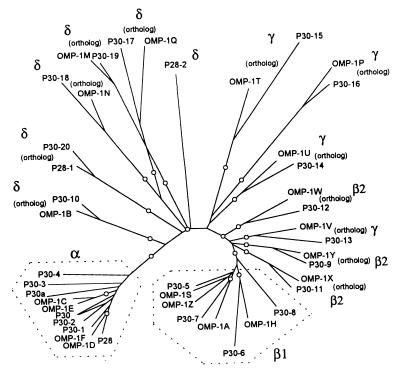
FIG. 4.
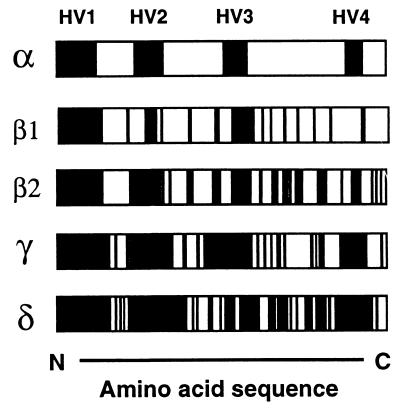
Phylogram of OMPs of E. canis and E. chaffeensis. A total of 24 OMPs were segregated between two species (marked as “ortholog”), but 20 remaining proteins were not. The tree was constructed using the neighbor-joining (NEIGHBOR program from PHYLIP) method based on the alignment generated with CLUSTAL V, and 1,000 bootstrap replications were performed. The nodes supported by bootstrap values of >75% are indicated with an open circle symbol. The OMPs encoded by three repetitive regions in Fig. 3 are indicated by α, β1, β2, and γ. The OMPs encoded by nonrepetitive regions were marked with a “δ.”
To further characterize the relationship between the two sets of 22 paralogs, a phylogram was constructed based on the sequence identities of 44 different proteins in the p30 and omp-1 multigene families (Fig. 4). Overall, 24 proteins were segregated between two species, but 20 remaining proteins were not. Of these 20 proteins, 19 (all except P28-2) were segregated into two groups consisting of 11 proteins encoded by genes that belong to the region α in Fig. 1 and 3 (sequence identities of 50 to 82.7%) and 8 proteins encoded by genes that belong to the region β1 in Fig. 1 and 3 (sequence identities of 47.6 to 68.7%). Amino acid sequence alignments of the proteins of the multigene families revealed that substitutions or deletions of several contiguous amino acid residues were present throughout the molecules, especially as represented by the significant differences among the sequences in the regions defined as hypervariable (HV). The HV regions among proteins encoded by genes within each of the repetitive regions α, β1, β2, and γ and among the proteins encoded by genes within no repetitive regions (designated δ) are shown schematically in Fig. 5. Among the proteins in α, four HV regions were clearly distinct from the intervening conserved regions, but among the proteins in δ the substitutions were dispersed throughout the molecules. In each region the levels of amino acid substitutions of the protein molecules were greater in the order of: δ > γ > β > α.
FIG. 5.
Schematic diagram of diversity of the protein structure of OMPs. The symbols “α” to “δ” represent proteins grouped in Fig. 3 and 4. The black and white areas show the HV and conserved sequences, respectively, among proteins in each group.
Transcription of the multigene family in E. canis.
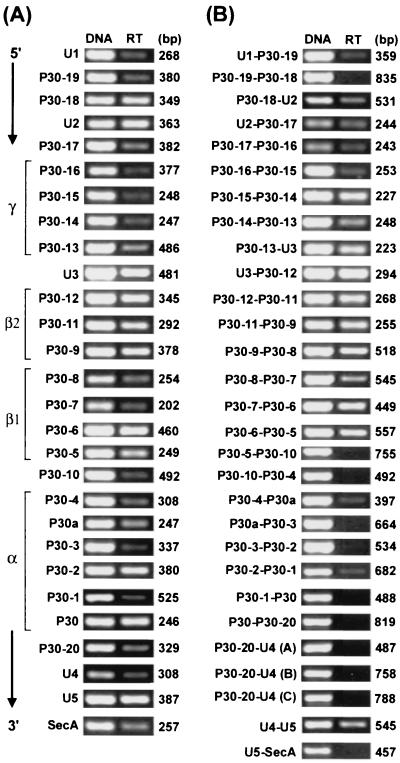
The RT-PCR was performed with 28 cycles of the linear range amplification using primer pairs specific to each of all 28 genes within the 28-kb locus in E. canis cultured in DH82 cells. Primer positions and amplicon sizes are shown in Table 1. DNA template controls are shown for each primer pair to demonstrate the ability of the primer to amplify the target sequence (Fig. 6A). Without reverse transcriptase, no amplicon was detected in RT-PCR analyses using any of primer pairs, indicating the absence of contamination of genomic DNA in the RNA preparation (data not shown). RT-PCR products for all 22 genes in the OMP locus were detected, indicating that all of these genes were transcriptionally active (Fig. 6A).
FIG. 6.
(A) RT-PCR analysis of 28 genes in the E. canis omp cluster. (B) RT-PCR analysis of 27 intergenic spaces and flanking genes on both sides in the E. canis omp cluster. The transcripts of the longest intergenic space and the flanking P30-20 and U4 were analyzed by three overlapping RT-PCRs (A, B, and C). DNA (5 ng of genomic DNA from purified E. canis) template control shows the intensity of the band detected with each pair of primers. Each amplicon with each primer pair was detected as a single band on the agarose gel stained with ethidium bromide. RT, RT-PCR. Amplified regions are shown in Table 1. The numbers on the right indicate the respective amplicon sizes.
To investigate the cotranscription of genes in the omp cluster, the presence of transcripts of 27 sets of two adjacent genes, including their intergenic spaces, was analyzed by RT-PCR using 29 specific primer pairs. The longest intergenic space (1.4 kb) between P30-20 and U4 was examined as three overlapping segments using three pairs of primers to examine whether the entire space and both flanking genes were cotranscribed and to prevent the PCR efficiency from being reduced due to the long sequence. Amplicon positions and their sizes are shown in Table 1.
The transcript of two adjacent genes (u1 and p30-19) connected by the short intergenic space at the 5′ end of the E. canis omp cluster was detected at a low level in comparison to the DNA control using the same primer pair. The transcript of P30-19–P30-18 connected by the long space was undetectable. The transcript of the following 14 sets of two adjacent genes connected by short intergenic spaces (P30-8–U2 to P30-6–P30-5) was clearly detected. The transcript of the remaining nine sets of two adjacent genes connected by long intergenic spaces (P30-5–P30-10 to P30-20–U4) in the 3′-end half of the cluster was undetectable or detectable only at low levels (P30-4–P30a and P30-2–P30-1) relative to the DNA control using the same primer pairs (Fig. 6B). The transcript of two adjacent genes (u4 and u5) with their intergenic space was detected. These results suggest that the transcription at the 5′-end half region of the omp cluster is primarily polycistronic while that at the 3′-end half is primarily monocistronic. We were unable to determine whether p30-10 is transcribed at both loci or either one of the loci. The transcript of two adjacent genes (purK and p30-10) and its intergenic space in the 6.9-kb locus was not detectable (data not shown). Therefore, if p30-10 in this locus is transcriptionally active, this is also monocistronic.
DISCUSSION
This study for the first time demonstrated: (i) the conservation of the omp multigene cluster structure and the genetic locus between CME and HME agents, (ii) the presence of three repetitive regions (α, β, and γ) within the omp cluster and of phylogenetically promiscuous relationships among orthologs and paralogs within the repetitive regions, and (iii) cotranscription of intergenic spaces with flanking genes on both sides at 5′-end half and other regions of the omp cluster. There were several significant differences in the clustered genes of E. chaffeensis between the study by Yu et al. (31) and the current study: (i) 12 omp paralogs were identified upstream of omp-1A by Yu et al., but 13 paralogs were identified in the corresponding region in the present study; (ii) the clustered genes were found to be located downstream from the clpx gene at the 5′ end by Yu et al., whereas in the present study these genes were arranged downstream from the un1 gene homologous to the hypothetical transcription regulator gene at 5′ end and upstream from secA at the 3′ end (the latter region was not identified by Yu et al.) (iii) a DNA probe corresponding to 3′-end omp-1W to 5′-end omp-1Y hybridized to 17.6- and 5.3-kb ClaI genomic DNA fragments according to Yu et al., but the probe can hybridize with only 17.6-kb ClaI-DNA in the present study. It is unlikely that these differences were generated by mutations within the omp cluster in E. chaffeensis (not strain differences, since the same strain Arkansas was used) maintained in two different laboratories, since the locus or gene numbers were conserved between the two different species: E. canis and E. chaffeensis. The cloning strategy used here was completely different from that of Yu et al. (31). Yu et al. assembled the sequence of the DNA fragments by using a rapid adapter primer PCR method. We used a combination of genomic Southern blot analysis, colony hybridization, and/or LA-PCR to avoid errors of sequence assembly due to the presence of repetitive sequences between homologous genes. If we had not used this strategy, for example, we might have misassembled the DNAs between the two (28- and 6.9-kb) loci in the E. canis genome. Although by the current genomic Southern blot analysis of E. chaffeensis, at least seven omp-1 genes were confirmed to be a single copy, for the remaining genes it is not known whether additional gene copies such as the E. canis 6.9-kb segment exist.
Multigene families encoding a major surface protein responsible for pathogenesis, such as antigenic variation, have been well documented in several bacteria and protozoa. For example, for the human pathogens Neisseria gonorrhoeae and N. meningitidis, pil has a gene organization similar to that of the multigene families of monocytic ehrlichiae (11, 24). PilE is the main structural pilin component and is expressed from the intact pilE gene. Several silent pilin genes (pilS) are tandemly arranged and contain only the 3′ region of the gene without a start codon or transcriptional signals. These silent regions can be moved into the expression site by a recombination event, generating phenotypic changes by the expression of different PilE sequences. However, in the case of monocytic ehrlichiae, all genes of the multigene families had universal start codons and all were functionally active. The gene expressions appear to be regulated primarily by polycistronic event(s) on the 5′-end half of the omp cluster and monocistronic events on the 3′-end half. Therefore, it seems to be important to retain the unique gene organization for specific regulation of the expression of paralogs within the omp cluster rather than to modulate directly the gene arrangement of multigenes by rapid recombination events. Indeed, there is no direct evidence of the recombinational gene exchange responsible for such rapid antigenic variation in Ehrlichia spp. to date. E. canis often causes persistent infection in dogs, which may lead to severe chronic illness or death (27). Probably, E. canis in persistent infections escapes from the host immune surveillance by modulating the expression levels of individual genes in the multigene family.
The current transcriptional analysis provide more complete and different results from previous results, which examined 4 to 10 paralogs (16, 26, 31). Using RT-PCR, Yu et al. showed that six genes of E. chaffeensis, including genes in the region with the shortest intergenic spaces, namely, omp-1S, omp-1H, and omp-1Z, are monocistronically transcribed (31). In the present study, the orthologous genes of E. canis (p30-8, p30-7, and p30-6) were cotranscribed with the adjacent genes on both sides. In contrast to Reddy et al. (26), who reported only a single omp transcription in 4 genes of E. chaffeensis, in the current study all of the 28 genes were transcribed by E. canis in DH82 cells. The transcriptional initiation site of each omp gene has not been determined experimentally, but we previously identified putative promoters in the long intergenic spaces between the genes at the 3′ end of the cluster (21). Thus, these genes may be independently regulated by their own promoter. Although repetitive sequences and the likely presence of long transcripts make it difficult, determination of the transcript size and a quantitative transcriptional analysis will be needed to further analyze the transcription mechanisms of these genes.
Recently, we reported the features of the p44 multigene family (orthologs of p30s and omp-1) of the human granulocytic ehrlichiosis (HGE) agent (32). The 16S rRNA sequence analysis showed 7.5 to 7.8% divergence between the HGE agent and ME agents, suggesting that they shared a common ancestor approximately 390 million years ago, while E. canis and E. chaffeensis were estimated to have diverged approximately 90 million years ago based on 1.8% divergence of the 16S rRNA gene sequences (1, 4, 18, 19). Our previous studies showed that the multiple p44 paralogs are dispersed widely throughout the HGE agent genome (rather than making a large gene cluster) (32) and that at least total 20 paralogs of the HGE agent were transcriptionally active under different conditions (unpublished data). Consequently, the following process may be inferred in the evolution of the HGE agent and ME agent. (i) The common ancestor of the HGE agent and ME agents gained (an) original major surface antigen gene(s). (ii) Thereafter, it began to produce the multiple paralogs. The HGE and ME agent lineages independently developed respective gene organizations between 90 to 390 million years ago. The HGE agent acquired the mainly trans-regulation system for the gene expression of genome-distributed paralogs. The ME agents developed the mainly cis-regulation system for gene expression of the clustered paralogs during this time. (iii) Eventually, the ancient omp cluster was vertically inherited by ME agent lineages (e.g., E. canis and E. chaffeensis). The mutations within respective omp clusters have been accumulated after divergence into the lineages of these two species, but the gene organization and the genetic locus have been retained with minor modification in each ME agent, probably for the preservation of the unique gene expression mechanism. It is important to take the genetic divergence and promiscuous relationships among OMP paralogs and orthologs (e.g., P30-6 is more closely related to OMP-1H rather than to OMP-1Z) into consideration in designing PCR primers and in future phylogenic analyses of the omp cluster among ME agents. The present results provide the essential information upon which to build functional and immunologic studies on OMPs in vitro and in animal models of ehrlichiosis.
ACKNOWLEDGMENTS
We thank Ning Zhi and Haibin Hung for valuable discussions and help in DNA and protein sequence analysis.
This work was supported by the National Institutes of Health grants R01-AI40934 and R01-AI47407.
REFERENCES
- 1.Anderson B E, Dawson J E, Jones D C, Wilson K H. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J Clin Microbiol. 1991;29:2838–2842. doi: 10.1128/jcm.29.12.2838-2842.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson B E, Sims K G, Olson J G, Childs J E, Piesman J F, Happ C M, Maupin G O, Johnson B J. Amblyoma americanum: a potential vector of human ehrlichiosis. Am J Trop Med Hyg. 1993;49:239–244. doi: 10.4269/ajtmh.1993.49.239. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein H D. The biogenesis and assembly of bacterial membrane proteins. Curr Opin Microbiol. 2000;3:203–209. doi: 10.1016/s1369-5274(00)00076-x. [DOI] [PubMed] [Google Scholar]
- 4.Chen S-M, Dumler J S, Bakken J S, Walker D H. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–595. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dawson J E, Anderson B E, Fishbein D B, Sanchez J L, Goldsmith C S, Wilson K H, Duntley C W. Isolation and characterization of an Ehrlichia sp. from a patient diagnosed with human ehrlichiosis. J Clin Microbiol. 1991;29:2741–2745. doi: 10.1128/jcm.29.12.2741-2745.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawson J E, Biggie K L, Warner C K, Cookson K, Jenkins S, Levine J F, Olson J G. Polymerase chain reaction evidence of Ehrlichia chaffeensis an etiologic agent of human ehrlichiosis in dogs from southeast Virginia. Am J Vet Res. 1996;57:1175–1179. [PubMed] [Google Scholar]
- 7.Dnatien A, Lestoquard F. Existence and algerie d'une rickettsia du chien. Bull Soc Pathol Exot. 1935;28:418–419. [Google Scholar]
- 8.Ewing S A, Dawson J E, Kokan A A, Barker R W, Warner C K, Panciera R J, Fox C J, Kocan K M, Blouin E F. Experimental transmission of Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) among white-tailed deer by Amblyoma americanum (Acari: Ixodidae) J Med Entomol. 1995;32:368–374. doi: 10.1093/jmedent/32.3.368. [DOI] [PubMed] [Google Scholar]
- 9.Felsenstein J. PHYLIP (phylogeny inference package), version 3.5.7. Seattle: University of Washington; 1995. [Google Scholar]
- 10.Groves M G, Dennis G L, Amyx H L, Huxsoll D L. Transmission of Ehrlichia canis to dogs by ticks (Rhipicephalus sanguineus) Am J Vet Res. 1975;36:937–940. [PubMed] [Google Scholar]
- 11.Haas R, Meyer T F. The repertoire of silent pilus genes in Neisseria gonorrhoeae: evidence for gene conversion. Cell. 1986;44:107–115. doi: 10.1016/0092-8674(86)90489-7. [DOI] [PubMed] [Google Scholar]
- 12.Keefe T J, Holland C J, Salyer P E, Ristic M. Distribution of Ehrlichia canis among military working dogs in the world and selected civilian dogs in the United State. J Am Vet Med Assoc. 1982;181:236–238. [PubMed] [Google Scholar]
- 13.Kim H-Y, Rikihisa Y. Characterization of monoclonal antibodies to the 44-kilodalton major outer membrane protein of the human granulocytic ehrlichiosis. J Clin Microbiol. 1998;36:3278–3284. doi: 10.1128/jcm.36.11.3278-3284.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim H-Y, Rikihisa Y. Expression of interleukin-1β, tumor necrosis factor alpha, and interleukin-6 in human peripheral blood leukocytes exposed to human granulocytic ehrlichiosis agent or recombinant major surface protein P44. Infect Immun. 2000;68:3394–3402. doi: 10.1128/iai.68.6.3394-3402.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maeda K, Markowitz N, Hawley R C, Ristic M, Cox D, McDade J E. Human infection with Ehrlichia canis, a leukocytic rickettsia. N Engl J Med. 1987;316:853–856. doi: 10.1056/NEJM198704023161406. [DOI] [PubMed] [Google Scholar]
- 16.McBride J W, Yu X-J, Walker D H. A conserved, transcriptionally active p28 multigene locus of Ehrlichia canis. Gene. 2000;254:245–252. doi: 10.1016/s0378-1119(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 17.Mwangi D M, McKeever D J, Nyanjui J K, Barbet A F, Mahan S M. Major antigenic proteins 1 and 2 of Cowdria ruminantium are targets for T-lymphocyte responses of immune cattle. Ann N Y Acad Sci. 1998;849:372–374. doi: 10.1111/j.1749-6632.1998.tb11073.x. [DOI] [PubMed] [Google Scholar]
- 18.Ochman H, Elwyn S, Moran A. Calibrating bacterial evolution. Proc Natl Acad Sci USA. 1999;96:12638–12643. doi: 10.1073/pnas.96.22.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ochman H, Willson A C. Evolution in bacteria: evidence for a universal substitution rate in cellular genomes. J Med Evol. 1987;26:74–86. doi: 10.1007/BF02111283. [DOI] [PubMed] [Google Scholar]
- 20.Ohashi N, Unver A, Zhi N, Rikihisa Y. Cloning and expression of immunodominant 30-kDa major outer membrane protein of Ehrlichia canis and application of the recombinant protein for serodiagnosis. J Clin Microbiol. 1998;36:2671–2680. doi: 10.1128/jcm.36.9.2671-2680.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohashi N, Zhi N, Zhang Y, Rikihisa Y. Immunodominant major outer membrane proteins of Ehrlichia chaffeensis are encoded by a polymorphic multigene family. Infect Immun. 1998;66:132–139. doi: 10.1128/iai.66.1.132-139.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmer G H, Eid G, Barbet A F, McGuire T C, McElwain T F. The immunoprotective Anaplasma marginale major surface protein 2 is encoded by a polymorphic multigene family. Infect Immun. 1994;62:3808–3816. doi: 10.1128/iai.62.9.3808-3816.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmer G H, Oberle S M, Barbet A F, Goff W L, Davis W C, McGuire T C. Immunization of cattle with a 36-kilodalton surface protein induces protection against homologous and heterologous Anaplasma marginale challenge. Infect Immun. 1988;56:1526–1531. doi: 10.1128/iai.56.6.1526-1531.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parkhill J, Achtman M, James K D, Bentley S D, et al. Complete DNA sequence of a serogroup A strain of Neisseria meningitidis Z2491. Nature. 2000;404:502–506. doi: 10.1038/35006655. [DOI] [PubMed] [Google Scholar]
- 25.Perez M, Rikihisa Y, Wen B. Antigenic and genetic characterization of an Ehrlichia canis-like agent isolated from a human in Venezuela. J Clin Microbiol. 1996;34:2133–2139. doi: 10.1128/jcm.34.9.2133-2139.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddy G R, Sulsona C R, Barbet A F, Mahan S M, Burridge M J, Alleman A R. Molecular characterization of a 28 kDa surface antigen gene family of the tribe Ehrlichieae. Biochem Biophys Res Commun. 1998;247:636–643. doi: 10.1006/bbrc.1998.8844. [DOI] [PubMed] [Google Scholar]
- 27.Rikihisa Y. The tribe Ehrlichieae and ehrlichial disease. Clin Microbiol Rev. 1991;4:286–308. doi: 10.1128/cmr.4.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rikihisa Y. Clinical and biological aspects of infection caused by Ehrlichia chaffeensis. Microbes Infect. 1999;1:367–376. doi: 10.1016/s1286-4579(99)80053-7. [DOI] [PubMed] [Google Scholar]
- 29.Rikihisa Y, Ewing S A, Fox J C, Siregar A G, Pasaribu F H, Malole M B. Enzyme-linked immunosorbent assay and Western immunoblot analysis of Ehrlichia canis and canine granulocytic Ehrlichia. J Clin Microbiol. 1992;30:143–148. doi: 10.1128/jcm.30.1.143-148.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sulsona C R, Mahan S M, Barbet A F. The map1 gene of Cowdria ruminantium is a member of a multigene family containing both conserved and variable gene. Biochem Biophys Res Commun. 1999;257:300–305. doi: 10.1006/bbrc.1999.0459. [DOI] [PubMed] [Google Scholar]
- 31.Yu X-J, McBride J W, Zhang X-F, Walker D H. Characterization of the complete transcriptionally active Ehrlichia chaffeensis 28 kDa outer membrane protein multigene family. Gene. 2000;248:59–68. doi: 10.1016/s0378-1119(00)00147-5. [DOI] [PubMed] [Google Scholar]
- 32.Zhi N, Ohashi N, Rikihisa Y. Multiple p44 genes encoding major outer membrane proteins are expressed in the human granulocytic ehrlichiosis agent. J Biol Chem. 1999;274:17828–17836. doi: 10.1074/jbc.274.25.17828. [DOI] [PubMed] [Google Scholar]
- 33.Zhi N, Ohashi N, Rikihisa Y, Horowitz H W, Wormer G P, Hechemy K. Cloning and expression of the 44-kilodalton major outer membrane protein gene of the human granulocytic ehrlichiosis agent and application of the recombinant protein to serodiagnosis. J Clin Microbiol. 1998;36:1666–1673. doi: 10.1128/jcm.36.6.1666-1673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]