Abstract
Objective
Type 2 diabetes is one of the main causes of kidney damage. Recent intervention studies suggest that the progression of type 2 diabetes can be halted, or even brought into remission by lifestyle interventions. In a pragmatic trial, the Reverse Diabetes2 Now programme (RD2N, NL: Keer Diabetes2 Om), a multicomponent lifestyle intervention, reduced the need for bloodglucose lowering medications up to 24 months.
Research design and methods
Here, we retrospectively investigate the effect of RD2N on markers of kidney function in patients selected for impaired kidney function at baseline (eGFR <70 mL/min/1.73 m2 (n=45). Baseline data were retrieved from the intervention database and follow-up data on renal markers were collected from routine medical records. Wilcoxon non-parametric tests were used to assess changes over 6 and 12 months.
Results
After 6 months median eGFR increased significantly from 62.0 (IQR 55.5–65.0) to 69.0 (IQR 55.0–76.5) mL/min/1.73 m2 (p=0.002). Median albumin/creatinine ratio (n=26) remained within the normal range (<3 mg/mmol). The effect on eGFR was similar after exclusion of patients in whom medication was changed (median eGFR 62.0 ((IQR 59.5–66.0) to 69.0 (IQR 60.0–77.0) mL/min/1.73 m2, p=0.006, n=29), suggesting that the effect on eGFR is not related to medication changes. At 12 months, eGFR was not significantly changed (n=22, median eGFR 63.5 mL/min/1.73 m2 (IQR 58.5–71.0), p=0.067).
Conclusions
The retrospective nature of this study and the despite guidelines limited availability of renal markers in routine type 2 diabetes care are limiting. Nevertheless, these data support a favourable effect of RD2N on renal function. Further research, with proper documentation of renal function, urinary protein excretion and dietary intake, is needed to substantiate these results, ideally in a large-scale prospective cohort study.
Keywords: Diabetes mellitus, Weight management, Nutritional treatment, Dietary patterns, Blood pressure lowering
WHAT IS ALREADY KNOWN ON THIS TOPIC.
It is a long held belief that chronic kidney disease (CKD), once established, is irreversible.
Emerging evidence suggests lifestyle intervention might be able to (partially) reverse CKD.
This study retrospectively investigated the effect of a multicomponent, intensive lifestyle intervention (Reverse Diabetes2 Now) on markers of kidney function.
WHAT THIS STUDY ADDS
Type 2 diabetes patients with impaired eGFR at baseline (<70 m/min/1.73 m2) who participated in an intensive lifestyle intervention had a significant increase in eGFR after 6 months.
Results suggest the increase in eGFR is not due to hyperfiltration or changes in medication use during the study period.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Lifestyle factors might be a more important determinant for kidney function than originally thought.
A larger, prospective study including a control or comparison group is warranted to verify our results.
Introduction
Lifestyle is a main driving factor in the development and progression of type 2 diabetes and its complications.1 Unfortunately, lifestyle interventions are considered notoriously difficult, in particular regarding sustained efficacy.2 Yet, intervention studies suggest lifestyle intervention might cause remission of type 2 diabetes.3–10 Two recent pragmatic trials indicate that the multicomponent lifestyle programme Reverse Diabetes2 Now (Keer Diabetes2 Om), developed in the Netherlands, leads to sustained real-life benefits particularly in terms of better glycaemic control with less medication use, reduced body weight and better quality of life (QoL) in type 2 diabetes patients up to 24 months.11 12 Details on the programme have been described elsewhere.11 Briefly, Reverse Diabetes2 Now aims at improving diet quality (restricted carb Mediterranean diet, rcMD), and improving physical activity, relaxation and sleep. The programme has an intervention phase of 6 months, followed by an aftercare phase of 18 months. Participants are guided in groups of approximately 20 by a nurse, coach and dietitian. Multiple healthcare insurers reimburse participation. Medication use could be adjusted per protocol by the patient’s own general practitioner (GP) or nurse practitioner (NP) as appropriate.
Type 2 diabetes is a main cause of chronic kidney disease (CKD) worldwide.13 Based on the beneficial effect of Reverse Diabetes2 Now, it can be hypothesised that the intervention might also be associated with amelioration of renal end organ damage, but assessment of renal effects was not part of the original study. Therefore, here, we retrospectively investigate the effect of Reverse Diabetes2 Now on kidney function in type 2 diabetes patients with impaired baseline eGFR measured as CKD-Epidemiology Collaboration (EPI) as reported.
Research design and methods
All Reverse Diabetes2 Now participants (2015 until march 2020) were screened for eligibility, based on availability of eGFR at baseline and completion of the programme (see figure 1 for flow chart). Type 2 diabetes patients with eGFR <45 mL/min/1.73 m2 could not participate in Reverse Diabetes2 Now, because of medical safety concerns, unless the medical team decided otherwise. A total of 641 participants were eligible.
Figure 1.
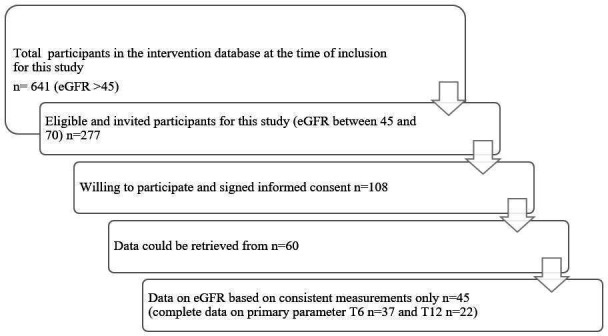
Flow chart of inclusion process.
Considering the biphasic course of kidney function in early renal impairment in type 2 diabetes, and the difficult interpretation of eGFR in the higher range in this population, we aimed to include only participants with established renal function involvement, apparent from suboptimal eGFR (<90) at baseline. Based on a two-sided α=5%, β=20% ca. Fifty-six participants would have to be included to detect a difference in eGFR of 5 mL/min/1.73 m2 with an SD of 13 mL/min/1.73 m2. Assuming that approximately 20% of all eligible participants would be available for the study, we decided on an eGFR cut-off of 70 mL/min/1.73 m2, which made for 277 eligible participants. The more commonly used cut-off of eGFR <60 mL/min/1.73 m2, corresponding to stage III CKD, was not feasible in this primary care population, with only a very small minority of patients with eGFR <60 mL/min/1.73 m2. All 277 patients were invited to participate. One hundred and eight patients were willing to and signed informed consent.
Data on demographics, baseline eGFR, body weight, body mass index (BMI), baseline medication use and HbA1c were available from the intervention database. As the original study design did not include follow-up assessment of kidney parameters (eGFR and albumin/creatinine ratio), blood pressure and complete use medications (apart from the use of blood glucose lowering medications), data had to be retrieved from routine assessment from the patient’s medical record. The researchers contacted the GP, or NP of the participants. Not all general or NPs could be contacted, and in many of the routine medical records the requested data on eGFR and albumin/creatinine ratio were not available. Eventually, follow-up data could be retrieved from 60 individuals. In 15 individuals, however, there were inconsistencies in eGFR reporting, that is, use of MDRD at one point and CKD-EPI at the next (eg, at baseline assessment with MDRD and follow-up with CKD-EPI). These cases were excluded so that this could not affect the results. Most eGFR estimations in this study were based on the CKD Epidemiology Collaboration (CKD-EPI) formula14 (n=30). In 2 individuals eGFR was consistently assessed with MDRD and in 13 individuals it was uncertain whether CKD-EPI or MDRD was used at each time point. Ultimately, data of 45 participants was available for analysis with a variable amount of data per parameter.
Outcomes were checked for normality with the Shapiro Wilk test and visual inspection of QQ-plots. A paired t-test was carried out for normally distributed variables and a Wilcoxon non-parametric test for skewed variables. Normally distributed variables were presented as mean±SD and skewed variables as median (IQR). The difference between variables at different time points was calculated by looking at individual delta’s and subsequently calculating the mean if these delta’s were normally distributed or the median if not. A sensitivity analysis was performed excluding all individuals (n=11) in whom medication for blood pressure and/or kidney function had been changed between baseline and 6 months and individuals for which insufficient data was available (n=5). The following types of medication were considered for this analysis: ACE-inhibitors, angiotensin-II blockers, calcium antagonists, beta blockers and diuretics (thiazides or potassium sparing). A two-sided p<0.05 was interpreted as statistically significant. Due to missing data, statistical analysis was only feasible for a subgroup at T6.
Results
Description of the study population at baseline
The study population consisted of 45 participants. Slightly more men than women were included. The median age was 68 years (table 1). All participants were overweight (>25 kg/m2) or obese (>30 kg/m2), since this was an inclusion criterium for Reverse Diabetes2 Now. On average participants had the type 2 diabetes diagnosis for 11.7±6.6 years. All participants used blood glucose lowering medications at baseline as this was an inclusion criterion for participation in Reverse Diabetes2 Now as well. 73.3% (33/45) of the participants also used one or more antihypertensives. Medication use remained stable (including stable no use of medication) from baseline up until 6 months in 64.4% (29/45) of participants.
Table 1.
Characteristics of the study population at baseline
| Mean±SD/median (IQR)/n (%) | |
| n | 45 |
| Sex | |
| Male | 25 (55.6) |
| Female | 20 (44.4) |
| Age (years) | 68 (9) |
| Weight (kg) | 96.1±15.2 |
| BMI (kg/m2) | 32.0±4.2 |
| Overweight | 15 (33.3) |
| Obese | 30 (66.7) |
| Years between diagnosis and start intervention* | 11.7±6.6 |
| Use of medication† | |
| Blood glucose lowering | |
| Metformin only | 16 (35.6) |
| Other oral medication (with or without metformin) | 14 (31.1) |
| Insulin (with or without other oral medication) | 15 (33.3) |
| For blood pressure/kidney function† | |
| RAAS-blockage | 27 (60.0) |
| Angiotensin-II blockers | 10 (22.2) |
| ACE-inhibitors | 17 (37.8) |
| Calcium antagonists | 6 (13.3) |
| Bètablockers | 14 (31.1) |
| Diuretics (thiazides or potassium sparing) | 14 (31.1) |
| None | 10 (22.2) |
| No data | 2 (4.4) |
| No or stable use (T0–T6) | 29 (64.4) |
| Changed use (T0–T6) | 11 (24.4) |
| No/insufficient data (T0–T6) | 5 (11.1) |
*Missing data of three participants.
†Participants could be using more than one type or combination drugs. Sum adds up to more than 100%.
BMI, body mass index; IQR, interquartile range; SD, standard deviation.
Kidney function
eGFR data were available for 37 participants after 6 months (table 2). Median kidney function (eGFR) at baseline was 62.0 mL/min/1.73 m2 (IQR 55.5–65.0). After 6 months, median eGFR increased to 69.0 mL/min/1.73 m2 ((IQR 55.0–76.5) (p=0.002), corresponding to a mean increase from baseline of 8.1%±15.1%. See figure 2 for an overview of the individual eGFR data. After 12 months, data of 22 participants was available, with a median eGFR of 63.5 mL/min/1.73 m2 (IQR 58.5–71.0), (p=0.067), corresponding to a mean increase from baseline of 4.8%±12.0%.
Table 2.
Changes in kidney function parameters after 6 months for the total population (n=45) and the group with stable medication use (n=29) and after 12 months for the total population
| Total population (n=45) | T0 | T6 | T12 | ||||
| Mean±SD median (IQR) | Mean±SD median (IQR) | n | P value | Mean±SD median (IQR) | n | P value | |
| eGFR (mL/min/1.73 m2) | 62.0 (55.5–65.0) | 69.0 (55.0–76.5) | 37 | 0.002* | 63.5 (58.5–71.0) | 22 | 0.067 |
| Albumin/creatinine ratio | 1.0 (0.4–1.8) | 0.9 (0.5–3.0) | 26 | 0.262 | 1.0 (0.2–2.7) | 18 | 0.286 |
| HbA1c (%) | 7.3±3.1 | 7.1±3.4 | 43 | 0.137 | 7.4±3.4 | 24 | 0.361 |
| Blood pressure (mmHg) | |||||||
| Systolic | 133.6±14.3 | 129.4±17.2 | 36 | 0.094 | 132.0±13.6 | 27 | 0.826 |
| Diastolic | 76.7±10.3 | 73.8±9.1 | 36 | 0.062 | 72.4±9.3 | 27 | 0.025* |
| MAP† | 95.7±10.2 | 92.3±10.4 | 36 | 0.044* | 92.3±9.3 | 27 | 0.106 |
| BMI (kg/m2) | 32.1±4.2 | 29.3±4.0 | 44 | <0.001* | 29.0±3.7 | 29 | <0.001* |
| Group with stable medication use (n=29) | Mean±SD median (IQR) | Mean±SD median (IQR) | n | P value | N/A | ||
| eGFR (mL/min/1.73 m2) | 62.0 (59.5–66.0) | 69.0 (60.0–77.0) | 25 | 0.006* | |||
| Albumin/creatinine ratio | 1.1 (0.3–3.1) | 0.9 (0.2–4.0) | 26 | 0.249 | |||
| HbA1c (%) | 7.2±3.1 | 7.0±3.5 | 27 | 0.248 | |||
| Blood pressure (mmHg) | |||||||
| Systolic | 134.6±16.3 | 128.9±18.5 | 24 | 0.074 | |||
| Diastolic | 77.7±10.5 | 73.1±10.1 | 24 | 0.016 | |||
| MAP† | 96.7±11.0 | 91.7±11.2 | 24 | 0.015 | |||
| BMI (kg/m2) | 31.5±4.0 | 28.8±4.0 | 29 | <0.001* | |||
*Significant result.
†MAP calculated as: (SBP+2(DBP))/3.
BMI, body mass index; DBP, diastolic blood pressure; IQR, interquartile range; MAP, mean arterial pressure; NA, not available; SBP, systolic blood pressure; SD, standard deviation.
Figure 2.
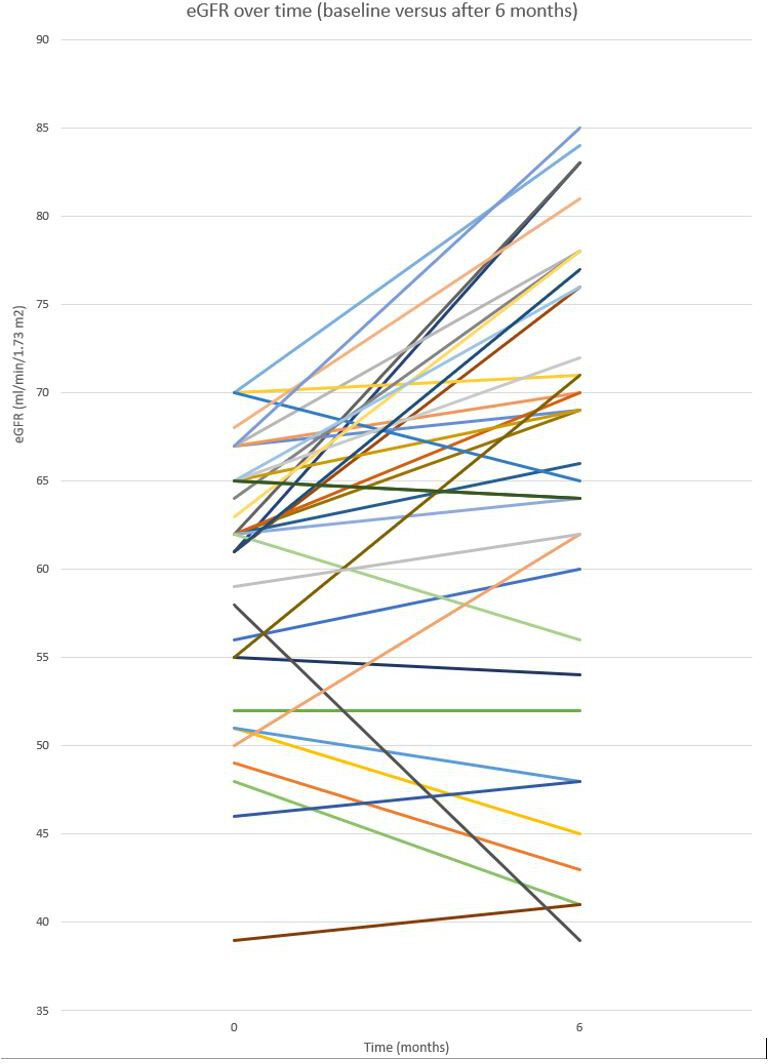
Overview of individual eGFR values at baseline (0) and at T6 (n=37)
Data on albumin/creatinine ratio after 6 months were available in 26 participants. Median albumin/creatinine was within the normal range (<3 mg/mmol) at baseline (1.0 mg/mmol (IQR 0.4–1.8)) and this did not change after 6 months (median 0.9 mg/mmol (IQR 0.5–3.0), p=0.262). After 12 months, data of 18 participants was available. Median albumin/creatinine ratio was still 1.0 ((IQR 0.2–2.7), p=0.286).
After 6 months, average BMI had decreased from 32.1±4.2 kg/m2 to 29.3±4.0 kg/m2 (p<0.001). MAP had decreased as well by 3.4 mm Hg to 92.3±10.4 mm Hg (p=0.044). After 6 months, HbA1c was on average 7.1±3.4%, which was non-significant change (p=0.137).
After 12 months, average BMI decreased further to 29.0±3.7 kg/m2 (p<0.001). The change in MAP (to 92.3±9.3 mm Hg) was non-significant at that time (p=0.106). Average HbA1c was 7.4±3.4%, the change in HbA1c was non-significant (p=0.361).
Twenty-nine out of 45 participants remained stable with respect to their use of medication (for blood pressure or kidney function) between baseline and 6 months. After exclusion of the 11 individuals with unstable use, and 5 individuals with insufficient data on medication use at T6, results on renal markers remained similar. In this subgroup, median eGFR increased from 62.0 mL/min/1.73 m2 (IQR 59.5–66.0) to 69.0 mL/min/1.73 m2 (IQR 60.0–77.0), which equals an average increase of 7.7%±12.3% (p=0.006). See table 2 for results on secondary parameters.
Conclusions
In this retrospective study we found that type 2 diabetes patients with baseline eGFR below 70 mL/min/1.73 m2 who participated in an intensive lifestyle intervention (Reverse Diabetes2 Now) had a significant increase in eGFR after 6 months. The available data at 12 months might support the assumption that the effect is sustained, but require further substantiation. Average albumin/creatinine ratio remained stable within the normal range (<3 mg/mmol), suggesting that the rise in eGFR is not due to induction of hyperfiltration. In line, the factors that can elicit hyperfiltration, namely high BMI and poor glycaemic control (reflected by HbA1c), both improved, although borderline for glycaemic control. The sensitivity analysis showed that the effects are probably not due to changes in medication use. The favourable effect on kidney function might be considered remarkable in view of the common assumption that CKD, once established, is irreversible, yet supports other recent data on (partial) reversal of CKD.15–17
It is not the first time that an increase in eGFR is reported in relation to lifestyle interventions. Several dietary intervention studies, including adults with CKD, overweight adults and elderly men and women with high risk of cardiovascular disease with and without type 2 diabetes, found an average increase in eGFR between 2.5% and 6.7%.15–17 In these studies the average albumin/creatinine ratio also stayed within the normal range. In a systematic review from 2017 including 17 (non) randomised controlled trials with more than 1600 adults with CKD (including end stage kidney disease) it was also concluded that dietary interventions were associated with increased QoL, decreased blood pressure and increased eGFR.15 In this review, the authors called for pragmatic trials for answering research questions related to this theme. In a recent practice-based cohort study in the UK including 143 type 2 diabetes patients with normal renal function or mild CKD adhering to a low carb diet it was found that over an average of 30 months serum creatinine improved significantly with a mean of 4.7 (14.9) μmol/L.18
The effects of Reverse Diabetes2 Now have been investigated in the setting of a pragmatic trial, which inherently has advantages and disadvantages. Data quality and collection can be limiting, since researchers are dependent on the data collection through regular care. This limitation also applies to the current study. The extent of missing data is remarkable, however, since routine collection of renal data is part of the guidelines for diabetes management in general practice in The Netherlands. Improvements can be made in current medical practices, which would also benefit the organisation and possibilities for carrying out similar scientific (pragmatic) studies. In this small study population, the missing data limited the interpretation of results, especially after 12 months. It must also be mentioned that the eGFR cut-off of 70 mL/min/1.73 m2 does not correspond to the more commonly used eGFR cut-off <60 mL/min/1.73 m2 indicating stage III kidney disease. Nevertheless, the cut-off of 70 mL/min/1.73 m2 ensures the presence of established kidney function impairment and is considerably below the range of renal function where the biphasic renal function changes of early diabetic renal involvement complicate the interpretation of renal function data.
The pragmatic setting, however, is a plus in view of the fact that the other studies on lifestyle and kidney disease mentioned before have mostly been carried out in a controlled environment, less well reflecting real life conditions.
It is possible that part of the observed effect is in reality due to biological variability in repeated measurements (4%–7%) or measurement error (2%).19 However, the increase in eGFR after 6 months exceeds this range, suggesting this, at least in part, reflects a true improvement. The sensitivity analysis suggested the effects on kidney function are not due to changes in medication use, but this subgroup was relatively small. Because no control group was included, no causal link can be established.
Current clinical dietary guidelines for CKD patients focus on specific dietary components including salt, potassium and protein and there is less focus on total dietary quality (including those specific components).
In the literature, it suggested that the MD, consisting of ample amounts of vegetables, fruits, extra virgin olive oil, legumes, nuts and seeds, might be the diet of choice for kidney patients.20 Our data seem to support this statement. Possibly, the increase in eGFR is a consequence of increased protein intake, which could be a consequence of adhering to the rcMD (as a consequence of relatively fewer carbohydrates). For future research on the effects of Reverse Diabetes2 Now, it is advisable to document actual dietary intake and 24-hour urine in at least a subgroup of the study population to quantify protein intake and determine whether or not this is responsible for an effect on eGFR. High proteins diets, for example, a ketogenic diet, are popular among type 2 diabetes patients, because this can impact glycaemic control. There have been health safety concerns with respect to high (animal)protein diets for people with impaired kidney function, since this might cause intraglomerular hypertension and consequent kidney hyperfiltration, glomerular injury and proteinuria.21 Long-term high protein intake may even lead to de novo CKD. This is, however, not applicable to Reverse Diabetes2 Now, since this is not a high (animal)protein diet but a rcMD focusing on diet quality and it does not eliminate a specific macronutrient group. A recent observational study on dietary intake in a small group of Reverse Diabetes2 Now participants found that protein intake remained fairly similar over time.22 Lastly, the focus of Reverse Diabetes2 Now was not on losing weight, but on achieving a healthy diet and lifestyle in general, also aimed at improving sleep, physical activity and relaxation. For future studies, it is also interesting to investigate possible underlying mechanisms.
Taken together, this retrospective analysis suggests that in type 2 diabetes patients with suboptimal eGFR Reverse Diabetes2 Now might improve kidney function. Lifestyle intervention might be a more important determinant for kidney function than originally thought. A larger, prospective study including a control group is warranted.
Footnotes
Contributors: NW retrieved the data and in cooperation with GN composed the concept manuscript. WG and HP critically revised the manuscript several times and added to the discussion. HP took on the role of guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: No, there are no competing interests.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants but this retrospective study is part of the study on Reverse Diabetes2 Now of which the results have been published last year (2020) in The British Medical Journal, Nutrition, Prevention & Health. The Medical Ethical Reviewing Committee of Wageningen University (NL) reviewed the study protocol of this original study and is of the opinion that it does not fall within the remit of the Dutch ‘Medical Research Involving Human Subjects Act’ (17 January 2019). Participants gave informed consent to participate in the study before taking part.
References
- 1. Zhang Y, Pan X-F, Chen J, et al. Combined lifestyle factors and risk of incident type 2 diabetes and prognosis among individuals with type 2 diabetes: a systematic review and meta-analysis of prospective cohort studies. Diabetologia 2020;63:21–33. 10.1007/s00125-019-04985-9 [DOI] [PubMed] [Google Scholar]
- 2. Haw JS, Galaviz KI, Straus AN, et al. Long-Term sustainability of diabetes prevention approaches: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med 2017;177:1808–17. 10.1001/jamainternmed.2017.6040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403. 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andrews RC, Cooper AR, Montgomery AA, et al. Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the early ACTID randomised controlled trial. The Lancet 2011;378:129–39. 10.1016/S0140-6736(11)60442-X [DOI] [PubMed] [Google Scholar]
- 5. Gregg EW, Chen H, Wagenknecht LE, et al. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA 2012;308:2489–96. 10.1001/jama.2012.67929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mottalib A, Sakr M, Shehabeldin M, et al. Diabetes remission after nonsurgical intensive lifestyle intervention in obese patients with type 2 diabetes. J Diabetes Res 2015;2015:1–4. 10.1155/2015/468704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hallberg SJ, McKenzie AL, Williams PT, et al. Effectiveness and Safety of a Novel Care Model for the Management of Type 2 Diabetes at 1 Year: An Open-Label, Non-Randomized, Controlled Study. Diabetes Ther 2018;9:583–612. 10.1007/s13300-018-0373-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (direct): an open-label, cluster-randomised trial. Lancet 2018;391:541–51. 10.1016/S0140-6736(17)33102-1 [DOI] [PubMed] [Google Scholar]
- 9. Lean MEJ, Leslie WS, Barnes AC, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the direct open-label, cluster-randomised trial. Lancet Diabetes Endocrinol 2019;7:344–55. 10.1016/S2213-8587(19)30068-3 [DOI] [PubMed] [Google Scholar]
- 10. Athinarayanan SJ, Adams RN, Hallberg SJ, et al. Long-Term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year Non-randomized clinical trial. Front Endocrinol 2019;10:348. 10.3389/fendo.2019.00348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pot GK, Battjes-Fries MC, Patijn ON, et al. Nutrition and lifestyle intervention in type 2 diabetes: pilot study in the Netherlands showing improved glucose control and reduction in glucose lowering medication. BMJ Nutr Prev Health 2019;2:bmjnph-2018:43–50. 10.1136/bmjnph-2018-000012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pot GK, Battjes-Fries MC, Patijn ON, et al. Lifestyle medicine for type 2 diabetes: practice-based evidence for long-term efficacy of a multicomponent lifestyle intervention (reverse Diabetes2 now). BMJ Nutr Prev Health 2020;3:bmjnph-2020:188–95. 10.1136/bmjnph-2020-000081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dunkler D, Kohl M, Heinze G, et al. Modifiable lifestyle and social factors affect chronic kidney disease in high-risk individuals with type 2 diabetes mellitus. Kidney Int 2015;87:784–91. 10.1038/ki.2014.370 [DOI] [PubMed] [Google Scholar]
- 14. Inal BB, Oguz O, Emre T, et al. Evaluation of MDRD, Cockcroft-Gault, and CKD-EPI formulas in the estimated glomerular filtration rate. Clin Lab 2014;60:1685. 10.7754/clin.lab.2014.131110 [DOI] [PubMed] [Google Scholar]
- 15. Palmer SC, Maggo JK, Campbell KL, et al. Dietary interventions for adults with chronic kidney disease. Cochrane Database Syst Rev 2017;4:CD011998. 10.1002/14651858.CD011998.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tirosh A, Golan R, Harman-Boehm I, et al. Renal function following three distinct weight loss dietary strategies during 2 years of a randomized controlled trial. Diabetes Care 2013;36:2225–32. 10.2337/dc12-1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Díaz-López A, Bulló M, Martínez-González Miguel Ángel, et al. Effects of Mediterranean diets on kidney function: a report from the PREDIMED trial. Am J Kidney Dis 2012;60:380–9. 10.1053/j.ajkd.2012.02.334 [DOI] [PubMed] [Google Scholar]
- 18. Unwin D, Unwin J, Crocombe D, et al. Renal function in patients following a low carbohydrate diet for type 2 diabetes: a review of the literature and analysis of routine clinical data from a primary care service over 7 years. Curr Opin Endocrinol Diabetes Obes 2021;28:469–79. 10.1097/MED.0000000000000658 [DOI] [PubMed] [Google Scholar]
- 19. Fraser CG. Inherent biological variation and reference values. Clin Chem Lab Med 2004;42:758–64. 10.1515/CCLM.2004.128 [DOI] [PubMed] [Google Scholar]
- 20. Chauveau P, Aparicio M, Bellizzi V, et al. Mediterranean diet as the diet of choice for patients with chronic kidney disease. Nephrol Dial Transplant 2018;33:725–35. 10.1093/ndt/gfx085 [DOI] [PubMed] [Google Scholar]
- 21. Ko G-J, Rhee CM, Kalantar-Zadeh K, et al. The effects of high-protein diets on kidney health and longevity. J Am Soc Nephrol 2020;31:1667–79. 10.1681/ASN.2020010028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pot GK, de Jong HBT, Battjes-Fries MCE, et al. Observational study on dietary changes of participants following a multicomponent lifestyle program (reverse Diabetes2 now). J Hum Nutr Diet 2022;35:791–803. 10.1111/jhn.12976 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.


