1. Introduction
Eating Disorders (EDs) have a peak age at onset during key developmental adolescent years (Solmi et al., 2021), and have severe mental and physical health impacts (Voderholzer et al., 2020). The treatment of EDs is complex and typically involves several health care disciplines (Monteleone et al., 2019). However, the efficacy of treatment is modest (Treasure et al., 2020), with remission ranging from 40% to 60% in anorexia nervosa (AN), bulimia nervosa (BN) and binge-eating disorder (BED) (Eddy et al., 2017; Linardon, 2018a; Slade et al., 2018; Steinhausen, 2002; Steinhausen and Weber, 2009; Zipfel et al., 2015). The variability of remission also reflects the heterogeneous definition of remission (Bardone-Cone et al., 2018), which should ideally encompass psychological, cognitive, physical (i.e., body mass index, BMI) and behavioural (i.e., binge eating or purging behaviours) criteria (Lilienfeld, 2019). Relapse frequently occurs in EDs, especially after discharge from inpatient treatment (Gregertsen et al., 2019; Marzola et al., 2021b).
International guidelines (e.g., the National Institute for Health and Care Excellence, NICE, National Guideline Alliance, 2017) recommend psychological interventions as first-line treatment for individuals with AN. Across psychotherapies, manualised family-based therapy (FBT) is suggested for adolescents with AN, while individual psychotherapies (i.e., cognitive-behavioural therapy for eating disorders (CBT-ED), focal psychodynamic psychotherapy (FPT), Maudsley Model of Anorexia Nervosa Treatment for Adults (MANTRA), or Specialist Supportive Clinical Management (SSCM)) are recommended for adults with AN. With the exception of FPT among these therapies, some have been superior to ‘treatment as usual’ conditions, but none has been found to be superior to another manualised specialist therapy (Byrne et al., 2017; Schmidt et al., 2016; van den Berg et al., 2019; Zeeck et al., 2018; Zipfel et al., 2014). International guidelines concur that medications should not be used as the sole or primary treatment for those with AN (Hilbert et al., 2017). For BN, NICE (2017) guidelines recommend individual psychological interventions as first-line treatment, namely FBT for adolescents or CBT-ED (in both the guided self-care and therapist-delivered forms) for adults. This latter intervention is also considered the treatment of choice for BED in adults, yet no evidence-based recommendation is available for adolescents. Antidepressants, and selective serotonin reuptake inhibitors (SSRI) in particular, have been proposed as an adjunctive treatment to psychotherapy for people within binge-eating spectrum disorders although only lisdexamfetamine has been approved in the US for BED (Hilbert et al., 2019; Slade et al., 2018; Treasure et al., 2020). There is currently no approved medication for EDs in Europe except the fluoxetine for bulimia nervosa (Tortorella et al., 2014).
However, notable differences exist among the current guidelines (Hilbert et al., 2017). These inconsistent recommendations reflect methodological limitations. For instance, a network meta-analysis (NMA) by Zeeck et al. (2018) only considered body weight as the primary outcome and found more rapid weight gain in adolescents than in adults and no superiority of a specific psychological approach for adults with AN. A meta-analysis (MA) by Murray et al. (2019) considered both physical and psychological outcomes, and found that specialized psychological treatments outperformed control interventions on weight outcomes, but not on psychological outcomes, and no differences emerged between adolescents and adults with AN. Finally, a recent NMA (Solmi et al., 2021) only based on standalone outpatient treatment of adults with AN concluded that no reliable evidence supports the clear superiority or inferiority of any treatment recommended by the NICE guidelines.
A previous umbrella review summarized meta-analytic evidence on the efficacy and acceptability of interventions for any mental disorders including EDs (Correll et al., 2021). However, that umbrella review only focused on children and adolescents, and only considered a pre-selected range of outcomes. A comprehensive umbrella review is needed to summarize the findings and to grade the quality of previous meta-analyses of RCTs in EDs, without restricting studies to specific age groups or specific outcomes, and providing a synopsis of efficacy, safety, acceptability and tolerability of treatments for ED. Also, currently there is no umbrella review accounting for effect modifiers and confounding factors, and conducting analyses by age group, type of intervention, setting, control condition, ED diagnosis (i.e. AN, BN, BED, EDNOS), and mode of treatment delivery. The present work aims to fill this gap, distilling clinically relevant information from a vast body of data to inform clinical care, clinical guidelines, and research directions.
2. Methods
2.1. Search, inclusion and exclusion criteria
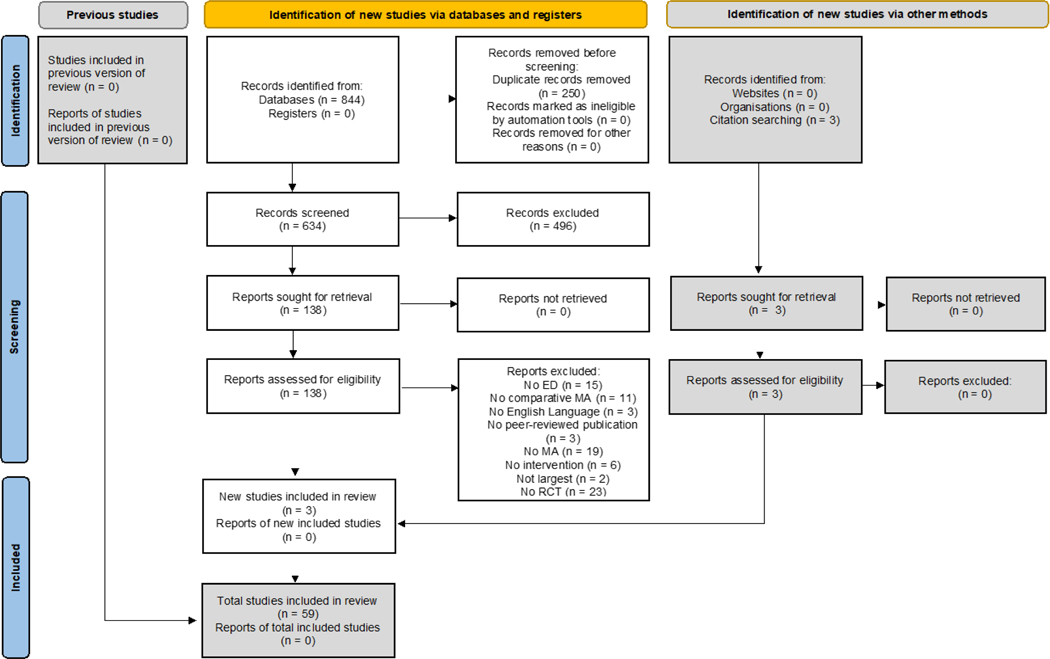
This umbrella review followed an a priori PRISMA 2020-compliant (Page et al., 2021) protocol (https://osf.io/wg5de/?view_only=177c444c41514fadb386ef1d57dee99d) (Figure 1, supplementary Tables 1–2). We conducted a systematic search in PubMed, PsycINFO, and Cochrane database up to December 15th, 2020, using the search key “(anorexia or bulimia or eating disorder) AND (meta-analy*)”. We also manually searched references of included MAs and other relevant reviews. Two independent (FP, MC) authors conducted title/abstract screening, full-text assessment, and data extraction into a pre-defined Excel spreadsheet. A third author (MS) resolved any conflicts.
Fig. 1.
PRISMA 2020 study selection flow diagram.
Included were: a) MAs or NMAs, b) of RCTs testing any intervention in comparison to an active or inactive control condition, c) in any age group and setting, d) in participants with a DSM/ICD diagnosis of an eating disorder (AN, BN, BED, eating disorder not otherwise specified or unspecified feeding or eating disorder (EDNOS or OS/UFED), e) reporting on comparative estimates of efficacy, and discontinuation due to any cause (acceptability) or due to adverse events (tolerability). Exclusion criteria were: a) systematic reviews without MA, b) pooling studies other than RCTs, c) including participants without an ED diagnosis, d) MA reporting on pre-post changes of a given set of interventions.
Whenever two NMAs/MAs reported on the same combination of disorders, interventions, comparisons and/or outcomes, we prioritized NMA over MA, or MA with more RCTs. NMAs have been prioritized over pairwise MAs previously, as they are deemed the highest level of evidence in treatment guidelines (Leucht et al., 2016). To avoid relying on completely indirect estimates, we only extracted effect sizes from NMAs that were based on at least one direct comparison for the outcome of interest.
2.2. Participants, interventions, comparison, outcomes, setting operationalization
Participants were diagnosed with AN, BN, BED, EDNOS/OSFED, or combined ED. Their age group could be adolescents (<18 years only), adults (≥ 18 years old only) or mixed.
We categorized interventions as follows: behavioural therapy (including behavioral weight loss therapy) (BT), CBT-ED, family therapy (FAM) (separating specific family-based therapy, FBT), interpersonal psychotherapy (IPT), Maudsley Model of Anorexia Nervosa Treatment for Adults, mixed or unspecified psychotherapy (PT), multidisciplinary care (including SSCM) (MULTI), psychodynamic-oriented (PSD-O), psychoeducation (EDU), physical exercise, and pharmacological treatments. Pharmacological treatments were subdivided by class (antidepressants, anticonvulsants, antipsychotics, stimulants, anti-obesity). Treatment format was individual (-i), group (-g), self-help (-s), or their combinations.
Comparators were specific drug or psychological intervention (active), treatment as usual (TAU), placebo (PBO), waiting list or no treatment (WL/NT), mixed TAU and WL/NT, mixed active and WL/NT.
Outcomes were ED-specific behaviours (BEHAVIOURAL, i.e. binge-eating and/or purging episodes remission or frequency, compensatory behaviours), neuropsychological functioning (COGNITION, i.e. central coherence, emotion recognition), eating disorder-specific psychopathology (EDP, i.e. body dissatisfaction, drive for thinness, shape/weight concern); functioning and quality of life (F-QOL); general psychiatric symptoms (GENERAL, i.e. depressive/anxiety/obsessive-compulsive); global course of the disease (GLOBAL, i.e. response/remission/relapse/clinical improvement/abstinence from binge eating/abstinence from purging/all outcomes); weight or body mass index (WEIGHT/BODY). Whenever possible, we extracted outcomes both at end of treatment and at follow up (any time point). Acceptability (inferred from drop out due to any cause) and safety (adverse effects of medications) were also extracted.
The setting in which the intervention was delivered was inpatient only, outpatient only, or mixed.
2.3. Quality of evidence
The quality of MAs and NMAs was measured using A Measurement Tool for the Assessment of Multiple Systematic Reviews (AMSTAR-PLUS) (Correll et al., 2017) to quantify both the methodological quality of MAs and NMAs with the first 11 items (AMSTAR) (Shea et al., 2007), and of included RCTs with six additional items (AMSTAR-Content (Correll et al., 2017), for each MA/NMA primary outcome).
Methodological quality was categorized as low (<4), medium (4–7), and high (>7). Content quality was categorized as low (<4), medium (4–6), and high (>6), as done previously (Correll et al., 2021; Solmi et al., 2020). Quality assessment was done by two independent reviewers. Full details on the quality assessment are available elsewhere (Correll et al., 2021; Solmi et al., 2020).
2.4. Statistical analysis
We did not recalculate all NMAs or MAs, and just reported what the original authors had done. However, in order to provide results on the same scale in Figure 2, where possible, we standardized and harmonized effect sizes. For instance, where mean difference was reported, we transformed it into standardized mean difference (SMD). Also, we harmonized outcome reporting, so that SMD>0 and odds ratio/risk ratio (OR/RR) >1 favor intervention for all outcomes. For instance, if a MA reported ED psychopathology improvement as SMD<0, we inverted the mathematical sign and 95% confidence interval. For AN, weight gain was reported as SMD>0, whilst for BED weight loss was reported with SMD>0, so that the statistical direction matched the clinical direction of the finding.
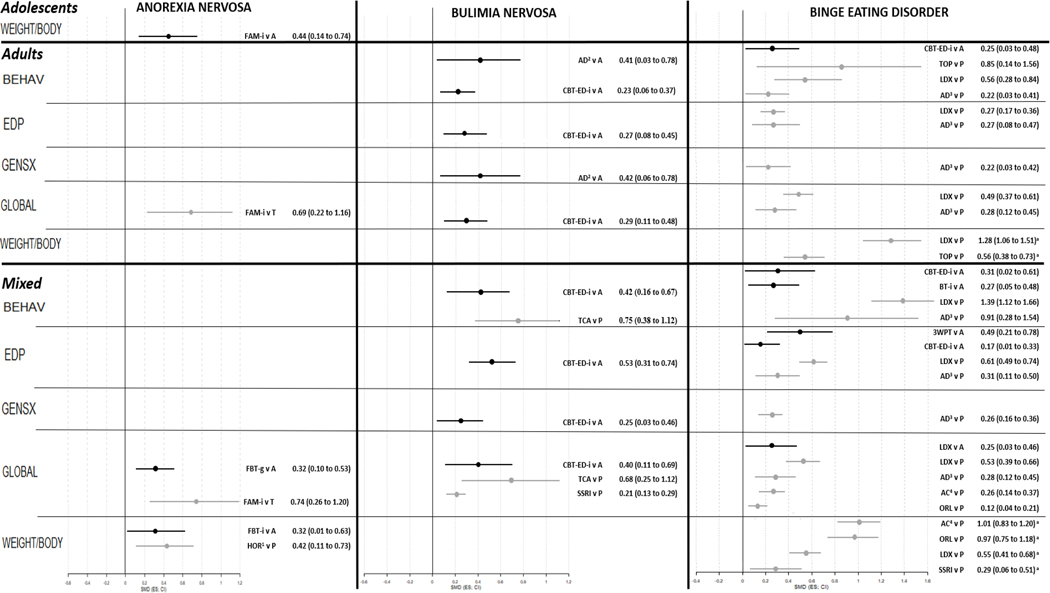
Fig. 2.
Forest plot of interventions with evidence of efficacy in eating disorders.
Results are presented as SMD (ES; CI), ordered by control condition, and by magnitude within the control condition. In forest plot, in black comparisons against Active control, in grey against TAU. Legend: 3WPT, third wave psychotherapies; A, Active control; AC, anticonvulsants; AD, mixed antidepressants; ADU, adults; BEHAV, eating disorder behaviours; BT, behavioural therapy; CBT-ED, cognitive behavioural therapy for eating disorders; CI, confidence interval; EDP, eating disorder psychopathology; ES, effect size; FAM, family therapy; FBT, family-based treatment; g, group therapy; GENSX, general psychiatric symptoms; GLOBAL, response/remission/relapse; HOR, hormones; i, individual therapy; LDX, lisdexamfetamine; MIX, mixed age group; ORL, orlistat; P, placebo; SGAD, second generation antidepressants; SMD, standardized mean difference; SSRI, selective serotonin reuptake inhibitors; T, treatment as usual; TCA, tricyclic antidepressants; TOP, topiramate; WEIGHT/BODY, change in weight or body composition
3. Results
The search flow is reported in Figure 1. Out of 884 articles, 55 MAs and 4 NMAs were included (Albano et al., 2019; Bacaltchuk et al., 1999; Josue Bacaltchuk et al., 2000; Josué Bacaltchuk et al., 2000; Bacaltchuk and Hay, 2003; Barakat et al., 2019; Berkman et al., 2015; Brownley et al., 2016; Cassioli et al., 2020; Claudino et al., 2006; Couturier et al., 2013; Cuijpers et al., 2016; de Vos et al., 2014; Dold et al., 2015; Fisher et al., 2018, 2010; Fornaro et al., 2016; Ghaderi et al., 2018; Ghaderi and Andersson, 1999; Grenon et al., 2018a; Hagan et al., 2020; Hasselbalch et al., 2020; Hay et al., 2019, 2015, 2004, 2001, 2009; Hilbert et al., 2019; Kishi et al., 2012; Lebow et al., 2013; Linardon, 2018b; Linardon et al., 2020, 2019, 2017a, 2017b, 2017c; Loucas et al., 2014; Low et al., 2021; Machado and Ferreira, 2014; Murray et al., 2019; Nakash-Eisikovits et al., 2002; Ng et al., 2013; Nourredine et al., 2020; Palavras et al., 2017; Peat et al., 2017; Perkins et al., 2006; Polnay et al., 2014; Reas and Grilo, 2008; Eric Slade et al., 2018; M Solmi et al., 2021; Stefano et al., 2008, 2006; Svaldi et al., 2019; Swift et al., 2017; Thompson-Brenner et al., 2003; Traviss-Turner et al., 2017; van den Berg et al., 2019; Vocks et al., 2010; Zeeck et al., 2018). Publications excluded after full-text assessment, with reason for exclusion are available in supplementary table 3. MAs and NMAs included in the umbrella review are reported in table 1. Among included (N)MAs, 33 (55.9%) assessed non-pharmacological interventions only, 16 (27.1%) pharmacological interventions only, while 10 (17%) assessed both (AN: 65%/35%/0%; BN: 60%/20%/20%; BED: 47.4%/26.3%/26.3%; combined ED: 94.1%/0%/5.9%). Overall, 33 different non-pharmacological interventions and 19 different (combinations of) medications have been identified. Regarding age groups, 19 (57.6%) (N)MAs reported on adults, five (15.2%) on adolescents, and the remaining nine (27.3%) reported on mixed or unspecified age. Only one MA (1.7%) reported on inpatients, and in AN only; eight (13.6%) reported on outpatients; and the others on any or unspecified settings.
Table 1.
Meta-analyses and network meta-analyses of randomized controlled trials of pharmacological and psychological interventions in patients with eating disorders included in the umbrella review
| Author, year | Age group | Setting | Intervention | Controls | Outcomes | A | C |
|---|---|---|---|---|---|---|---|
| Anorexia Nervosa | |||||||
| Albano, 2019(31) | MIX | MIX | EDU-S | MIXED, TAU/WL | ACCEPT, F-QOL, GENSX, WEIGHT/BODY | 8 | 4 |
| Cassioli, 2020(32) | MIX | MIX | PHARMA | PBO | GENSX, WEIGHT/BODY | 8 | 4 |
| Claudino, 2006(33) | MIX | MIX | PHARMA | PBO | ACCEPT, GENSX | 9 | 1 |
| Couturier, 2013(34) | ADO | OUT | FAM-I | ACTIVE | GLOBAL | 9 | 3 |
| de Vos, 2014(35) | MIX | MIX | PHARMA | PBO | WEIGHT/BODY | 9 | 2 |
| Dold, 2015(36) | ADU, MIX | MIX | PHARMA | PBO/NT | ACCEPT, EDP, WEIGHT/BODY | 10 | 4 |
| Fisher, 2010(37) | MIX | MIX | FAM-I | ACTIVE, TAU | EDP, GLOBAL, WEIGHT/BODY | 9 | 2 |
| Fisher, 2018(38) | ADO, ADU, MIX | MIX | FAM-I | ACTIVE, TAU | ACCEPT, EDP, GLOBAL, WEIGHT/BODY | 9 | 3 |
| Hagan, 2020(39) | ADU | MIX | MULTI | MIXED | COG, EDP, GENSX, WEIGHT/BODY | 8 | 4 |
| Hasselbalch, 2020(40) | ADU | MIX | PHARMA | PBO | COG | 9 | 1 |
| Hay, 2015(41) | MIX | OUT | CBT-ED-I, PDO-I | ACTIVE, MIXED, TAU | ACCEPT, EDP, GLOBAL, WEIGHT/BODY | 9 | 3 |
| Hay, 2019(42) | MIX | IN | MULTI | ACTIVE | ACCEPT, GENSX, GLOBAL, WEIGHT/BODY | 9 | 2 |
| Kishi, 2012(43) | MIX | MIX | PHARMA | PBO | ACCEPT, EDP, SAFETY, WEIGHT/BODY | 6 | 4 |
| Lebow, 2013(44) | MIX | MIX | PHARMA | MIXED, PBO | EDP, GENSX | 6 | 2 |
| Linardon, 2017c(45) | MIX | MIX | CBT-ED-I | ACTIVE | EDP | 6 | 3 |
| Murray, 2019(23) | MIX | MIX | MULTI | MIXED | GLOBAL, WEIGHT/BODY | 10 | 3 |
| Ng, 2013(46) | MIX | MIX | EXE-I | PBO | WEIGHT/BODY | 7 | 1 |
| Solmi, 2021(24) | ADU | OUT | CBT-ED-I, FAM-I, MANTRA-I, PDO-I | ACTIVE, TAU | ACCEPT, BEHAV, WEIGHT/BODY | 10 | 2 |
| van den Berg, 2019(16) | ADO, ADU, MIX | MIX, OUT | CBT-ED-I, FAM-I, PDO-I, PT-G, PT-I | ACTIVE, MIXED, PBO | EDP, F-QOL, WEIGHT/BODY | 10 | 4 |
| Zeeck, 2018(17) | ADO, ADU | MIX | CBT-ED-I, FAM-I, IPT-I, MANTRA-I, MULTI, PDO-I, SUP-I | ACTIVE | WEIGHT/BODY | 6 | 2 |
| Bulimia Nervosa | |||||||
| Bacaltchuk, 1999(47) | MIX | OUT | PHARMA | ACTIVE | ACCEPT, BEHAV, GENSX, GLOBAL | 7 | 1 |
| Bacaltchuck, 2000a(48) | MIX | MIX | PHARMA | PBO | GLOBAL | 7 | 6 |
| Bacaltchuk, 2000b(49) | ADU | MIX | PHARMA, PT-I | ACTIVE | ACCEPT, BEHAV, GENSX, GLOBAL | 8 | 2 |
| Bacaltchuk, 2003(50) | MIX | MIX | PHARMA | PBO | ACCEPT, BEHAV, GENSX, GLOBAL | 10 | 3 |
| Couturier, 2013(34) | ADO | OUT | FAM-I | ACTIVE | GLOBAL | 9 | 3 |
| Ghaderi, 1999(51) | ADU | MIX | CBT-ED-I | ACTIVE | BEHAV | 5 | 1 |
| Hay, 2001(52) | MIX | MIX | PHARMA, PT-I | ACTIVE | ACCEPT, BEHAV, GENSX, GLOBAL | 10 | 1 |
| Hay, 2004(53) | ADU | MIX | CBT-ED-I, EDU-I, PT-I | ACTIVE, WL | ACCEPT, BEHAV, F-QOL, GENSX, GLOBAL, WEIGHT/BODY | 5 | 2 |
| Hay, 2009(54) | MIX | MIX | CBT-ED-I, EDU-S, PT-I | ACTIVE, WL, WL/NT | ACCEPT, BEHAV, F-QOL, GENSX, GLOBAL, WEIGHT/BODY | 7 | 1 |
| Linardon, 2017b(55) | MIX | MIX | CBT-ED-I, CBT-ED-I+S, EDU-S, MULTI, PT-I, PT-I+S | ACTIVE, TAU/WL | BEHAV, GENSX | 6 | 3 |
| Linardon, 2017c(45) | ADO, ADU, MIX | MIX | CBT-ED-G, CBT-ED-I, CBT-ED-S | ACTIVE, TAU/WL | BEHAV, EDP, GLOBAL | 6 | 3 |
| Linardon, 2018(56) | MIX | MIX | CBT-ED-I | ACTIVE, TAU/WL | EDP | 8 | 2 |
| Linardon, 2019(57) | MIX | MIX | CBT-ED-I, EDU-S, PT-I | ACTIVE, TAU/WL | GENSX | 8 | 3 |
| Linardon, 2020(58) | MIX | MIX | e-PT | MIXED | BEHAV, EDP | 7 | 2 |
| Loucas, 2014(59) | MIX | MIX | CBT-ED-S | NT, WL | BEHAV, EDP | 10 | 4 |
| Nakash-Eisikovits, 2002(60) | MIX | MIX | PHARMA | PBO | BEHAV | 5 | 3 |
| Polnay, 2014(61) | ADU | OUT | CBT-ED-G | NT | BEHAV, GENSX, GLOBAL | 9 | 3 |
| Slade, 2018(62) | ADU | MIX | BT-G, BT-I, CBT-ED-G, CBT-ED-I, CBT-ED-S, EXE-I, IPT-I, MULTI, PHARMA, PT-I | ACTIVE, WL | GLOBAL | 8 | 3 |
| Svaldi, 2019(63) | MIX | MIX | CBT-ED-I, EDU-S, MULTI, PHARMA, PT-I | TAU/WL | ACCEPT, BEHAV, EDP, GENSX, GLOBAL | 9 | 3 |
| Thompson-Brenner, 2003(64) | MIX | MIX | BT, BT-G, BT-I, CBT-ED, CBT-ED-G, CBT-ED-I, PT-G, PT-I | MIXED | BEHAV | 4 | 1 |
| Binge-Eating Disorder | |||||||
| Berkman, 2015(65) | ADU, MIX | MIX | CBT-ED-I, PHARMA | PBO, WL | BEHAV, GENSX, GLOBAL, SAFETY, WEIGHT/BODY | 11 | 3 |
| Brownley, 2016(66) | ADU | MIX | CBT-ED-I, PHARMA | PBO, WL | BEHAV, EDP, GENSX, GLOBAL, WEIGHT/BODY | 8 | 4 |
| Fornaro, 2016(67) | ADU | MIX | PHARMA | PBO | ACCEPT, BEHAV, GLOBAL, WEIGHT/BODY | 9 | 4 |
| Ghaderi, 2018(68) | MIX | MIX | BT-G, BT-I, CBT-ED-I, CBT-ED-S, IPT-I, PHARMA | ACTIVE, PBO, WL | BEHAV, EDP, GENSX, GLOBAL, WEIGHT/BODY | 9 | 2 |
| Hay, 2009(54) | MIX | MIX | CBT-ED-I | ACTIVE, TAU/NT | ACCEPT, BEHAV, GLOBAL, WEIGHT/BODY | 7 | 1 |
| Hilbert, 2019(22) | MIX | MIX | BT-I, EDU-S, MULTI, PHARMA, PT, PT-I | ACTIVE, TAU/NT | ACCEPT, BEHAV, EDP, GENSX, GLOBAL, WEIGHT/BODY | 9 | 5 |
| Linardon, 2017a(69) | MIX | MIX | PT-I | ACTIVE, WL | BEHAV, EDP, GENSX, GLOBAL | 7 | 0 |
| Linardon, 2017c(45) | MIX | MIX | CBT-ED-G, CBT-ED-I, CBT-ED-S, PT-I | ACTIVE, TAU/WL | BEHAV, EDP, GLOBAL | 6 | 3 |
| Linardon, 2018(56) | MIX | MIX | CBT-ED-I | ACTIVE, TAU/WL | EDP | 8 | 2 |
| Linardon, 2019(57) | MIX | MIX | CBT-ED-I, EDU-S, PT-I | ACTIVE, TAU/WL | GENSX | 8 | 3 |
| Linardon, 2020(58) | MIX | MIX | e-PT | MIXED | BEHAV, EDP | 7 | 2 |
| Loucas, 2014(59) | MIX | MIX | CBT-ED-S | WL | BEHAV, GLOBAL | 10 | 4 |
| Nourredine, 2020(70) | ADU | MIX | PHARMA | PBO | ACCEPT, BEHAV, GENSX, WEIGHT/BODY | na | na |
| Palavras, 2017(71) | ADU | OUT | BT-I | ACTIVE | ACCEPT, BEHAV, WEIGHT/BODY | 9 | 2 |
| Peat, 2017(72) | MIX | MIX | PHARMA | ACTIVE, PBO | BEHAV, EDP, GLOBAL | 6 | 4 |
| Stefano, 2008(73) | MIX | MIX | PHARMA | PBO | BEHAV, GENSX, GLOBAL | 6 | 2 |
| Swift, 2017(74) | ADU | MIX | MULTI, PT-I | ACTIVE, MIXED | ACCEPT | 5 | 0 |
| Reas, 2008(75) | MIX | MIX | PHARMA | PBO | ACCEPT, GLOBAL, WEIGHT/BODY | 8 | 6 |
| Vocks, 2010(76) | MIX | MIX | EDU-S, PHARMA, PT-I | PBO/WL | BEHAV, EDP, WEIGHT/BODY | 7 | 3 |
| Mixed Eating Disorders | |||||||
| Barakat, 2019(77) | MIX | MIX | EDU-S | MIXED | BEHAV, EDP, F-QOL, GENSX, WEIGHT/BODY | 7 | 5 |
| Couturier, 2013(34) | ADO | OUT | FAM-I | ACTIVE | GLOBAL | 9 | 3 |
| Cuijpers, 2016(78) | MIX | MIX | IPT-I | ACTIVE, MIXED | BEHAV, EDP, GLOBAL, WEIGHT/BODY | 10 | 6 |
| Grenon, 2019(79) | MIX | MIX | CBT-ED-G, CBT-ED-I, PT-G, PT-I | ACTIVE, WL | BEHAV, EDP, F-QOL, GENSX | 9 | 4 |
| Hay, 2003(50) | ADU | MIX | CBT-ED-I, CBT-ED-S, EDU-S | ACTIVE, WL | ACCEPT, BEHAV, F-QOL, GENSX, GLOBAL, WEIGHT/BODY | 5 | 2 |
| Hay, 2009(54) | MIX | MIX | CBT-ED-I, CBT-ED-S, EDU-S, PT-I | ACTIVE, WL, WL/NT | ACCEPT, BEHAV, EDP, F-QOL, GENSX, GLOBAL, WEIGHT/BODY | 7 | 1 |
| Linardon, 2017a(69) | MIX | MIX | PT-I | ACTIVE, WL | BEHAV, EDP, GENSX, GLOBAL | 7 | 0 |
| Linardon, 2017c(45) | MIX | MIX | CBT-ED-I | ACTIVE | BEHAV, EDP, GLOBAL | 6 | 3 |
| Linardon, 2018(56) | MIX | MIX | CBT-ED-I, CBT-ED-S | ACTIVE, TAU/WL | BEHAV, EDP | 8 | 2 |
| Linardon, 2020(58) | MIX | MIX | e-PT | ACTIVE, MIXED | BEHAV, EDP | 7 | 2 |
| Loucas, 2014(59) | MIX | MIX | CBT-ED-S | WL | GLOBAL | 10 | 4 |
| Low, 2021(80) | MIX | MIX, OUT | VR-CBT-ED | ACTIVE | BEHAV, EDP, WEIGHT/BODY | 7 | 0 |
| Machado, 2014(81) | MIX | MIX | EXE-I | TAU/WL | BEHAV, F-QOL, WEIGHT/BODY | 7 | 2 |
| Perkins, 2006(82) | MIX | MIX | EDU-S, MULTI | ACTIVE, WL | ACCEPT, BEHAV, EDP, F-QOL, GENSX, WEIGHT/BODY | 9 | 2 |
| Stefano, 2006(83) | MIX | MIX | EDU-S | ACTIVE, WL | ACCEPT, BEHAV | 5 | 0 |
| Swift, 2017(74) | ADU | MIX | MULTI, PHARMA, PT-I | ACTIVE, MIXED | ACCEPT | 5 | 0 |
| Traviss-Turner, 2017(84) | MIX | MIX | EDU-S | MIXED, WL | BEHAV, EDP | 9 | 3 |
A, AMSTAR score; ACCEPT, acceptability, ADO, adolescents; ADU, adults; BEHAV, behavioural symptoms; BT-G, group behavioural therapy; BT-I, individual behavioural therapy; C, AMSTAR-Content score; CBT-ED-G, group cognitive behavioural therapy; CBT-ED-I, individual cognitive behavioural therapy; CBT-ED-S, self-help cognitive behavioural therapy; EDP, eating disorder psychopathology; EDU-I, individual psychoeducation; EDU-S, self-help psychoeducation; EXE-I, individual guided physical exercise; F-QOL, functioning/quality of life; FAM-I, individual family therapy; GENSX, psychiatric general symptoms; GLOBAL, improvement/response/remission/relapse; IN, inpatient; IPT-I, individual interpersonal psychotherapy; MANTRA-I, individual Maudsley Anorexia Treatment for Adults; MIX, mixed or not specified age group or setting; MIXED, active+inactive control; MULTI, mixed active interventions/multiprofessional treatment; PBO. Placebo; PHARMA, pharmacological intervention; PDO-I, individual psychodynamic-oriented psychotherapy; e-PT, digital psychotherapy; PT-I, individual psychotherapy; PT-G, group psychotherapy; OUT, outpatient; VR-CBT-ED, CBT-ED plus virtual reality.
The number of trials for a specific outcome ranged from 2–35 (median=4, interquartile range=2–7).
Quality of included studies is reported in supplementary table 4. Overall AMSTAR/AMSTAR-Content mean scores were 7.9±1.7 and 2.7±1.5. Thirty-five (59.3%) (N)MAs had an AMSTAR >7, one (1.7%) had the maximum score (11), whilst none had an AMSTAR-Content score >6. The main reasons for lower quality rating had to do with the quality of the meta-analyzed RCTs included in (N)MAs, and namely no “double-blind” design in most of the studies (81%), primary outcome result not confirmed in at least one large study with approximately 100 patients per arm (74%), and the presence of publication bias regarding the primary outcome result (78%).
Below, effect sizes versus WL/NT, PBO, TAU and Active are reported by disorder (see tables 2–3). We left results from MAs mixing TAU with active or inactive controls in supplementary tables only, as those comparisons inappropriately mixed different control groups (see supplementary tables 5–6). In the paragraphs below, results are reported with number of RCTs (k), number of subjects (n), effect size with 95% confidence interval, quality score, and quality category (L/low, M/medium, H/high).
Table 2.
Efficacy of interventions against Placebo/Wait-list/No treatment control.
| Age group | Setting | Outcome | Intervention | I/G/S | Control | N of estimates | % significance | ES type | ES (95% CI) min-max | Source | A / Q |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anorexia Nervosa | |||||||||||
| ADU | MIX | COG | PHARMA (HOR) | I | PBO | 1 | 100% NS | SMD | 0.06 (−0.34 – 0.46) | MA | 9/H |
| MIX | MIX | ACCEPT | PHARMA (AD) | I | PBO | 2 | 100% NS | RR | ns | MA | 9 / H |
| PHARMA (SGA) | I | PBO | 2 | 100% NS | RR | ns | MA | 6 / M | |||
| EDP | PHARMA (SGA) | I | PBO | 4 | 100% NS | SMD | ns | MA | 6 / M | ||
| GENSX | PHARMA (AD) | I | PBO | 2 | 100% NS | SMD | ns | MA | 8.5 / H | ||
| PHARMA (SGA) | I | PBO | 2 | 100% NS | SMD | ns | MA | 8 / H | |||
| SAFETY | PHARMA (SGA) | I | PBO | 2 | 100% DETR | RR | 0.27 (0.10 – 0.75) to 0.09 (0.02 – 0.34) | MA | 6 / M | ||
| WEIGHT/BODY | PHARMA (HOR) | I | PBO | 1 | 100% EFF | d | 0.42 (0.11 – 0.73) | MA | 9 / H | ||
| PHARMA (MIX) | I | PBO | 1 | 100% EFF | d | 0.33 (0.14 – 0.52) | MA | 9 / H | |||
| PHARMA (AD) | I | PBO | 1 | 100% NS | d | ns | MA | 9 / H | |||
| PHARMA (SGA) | I | PBO | 3 | 100% NS | SMD | ns | MA | 8 / H | |||
| Bulimia Nervosa | |||||||||||
| ADU | OUT | BEHAV | CBT-ED | G | WL/NT | 1 | 100% EFF | SMD | 0.56 (0.15 – 0.96) | MA | 9 / H |
| GENSX | CBT-ED | G | WL/NT | 1 | 100% NS | SMD | ns | MA | 9 / H | ||
| GLOBAL | CBT-ED | G | WL/NT | 1 | 100% EFF | RR | 1.29 (1.04 – 1.61) | MA | 9 / H | ||
| MIX | ACCEPT | CBT-ED | I | WL/NT | 1 | 100% NS | RR | ns | MA | 5 / M | |
| PT | I | WL/NT | 1 | 100% NS | RR | ns | MA | 5 / M | |||
| BEHAV | PT | I | WL/NT | 1 | 100% EFF | SMD | 1.22 (0.92 – 1.52) | MA | 5 / M | ||
| CBT-ED | I | WL/NT | 1 | 100% EFF | SMD | 1.01 (0.68 – 1.33) | MA | 5 / M | |||
| GENSX | CBT-ED | I | WL/NT | 1 | 100% EFF | SMD | 0.8 (0.37 – 1.22) | MA | 5 / M | ||
| PT | I | WL/NT | 1 | 100% EFF | SMD | 0.58 (0.18 – 0.98) | MA | 5 / M | |||
| GLOBAL | BT | G | WL/NT | 1 | 100% EFF | OR | 28.7 (3.11 to 455) | NMA | 8 / H | ||
| CBT-ED | G | WL/NT | 1 | 100% EFF | OR | 7.67 (1.51 – 55.7) | NMA | 8 / H | |||
| CBT-ED | S | WL/NT | 2 | 100% EFF | OR | 3.49 (1.20 – 11.2) to 3.81 (1.51 – 10.9) | NMA | 8 / H | |||
| CBT-ED | I | WL/NT | 2 | 100% EFF | OR | 1.49 (1.28 – 1.72) to 3.89 (1.19 – 14.0) | MA; NMA | 6.5 / M | |||
| PT | I | WL/NT | 2 | 50% EFF; 50% NS | OR | ns to 1.54 (1.30 – 1.82) | NMA; MA | 6.5 / M | |||
| BT | I | WL/NT | 1 | 100% NS | OR | ns | NMA | 8 / H | |||
| EDU | I | WL/NT | 1 | 100% NS | OR | ns | NMA | 8 / H | |||
| IPT | I | WL/NT | 1 | 100% NS | OR | ns | NMA | 8 / H | |||
| MULTI | I | WL/NT | 1 | 100% NS | OR | ns | NMA | 8 / H | |||
| PHARMA (AD) | I | PBO | 1 | 100% NS | OR | ns | NMA | 8 / H | |||
| MIX | MIX | ACCEPT | PHARMA (AD) | I | PBO | 10 | 10% EFF; 70% NS; 20% DETR | RR | 0.52 (0.30 – 0.87) to 1.22 (1.01 – 1.47) | MA | 10 / H |
| CBT-ED | I | WL/NT | 1 | 100% NS | RR | ns | MA | 7 / M | |||
| PT | I | WL/NT | 1 | 100% NS | RR | ns | MA | 7 / M | |||
| BEHAV | PT | I | WL/NT | 1 | 100% EFF | SMD | 1.22 (0.92 – 1.52) | MA | 7 / M | ||
| CBT-ED | I | WL/NT | 1 | 100% EFF | SMD | 1.01 (0.68 – 1.33) | MA | 7 / M | |||
| PHARMA (AD) | I | PBO | 9 | 77.8% EFF; 22.2% NS | SMD | ns to 0.75 (0.38 – 1.12) | MA | 4 / M | |||
| CBT-ED | S | WL/NT | 4 | 75% EFF; 25% NS | SMD | ns to 0.44 (0.11 – 0.77) | MA | 10 / H | |||
| BEHAV FU | CBT-ED | S | WL/NT | 1 | 100% NS | RR | ns | MA | 10 / H | ||
| EDP | CBT-ED | S | WL/NT | 4 | 100% NS | SMD | ns | MA | 10 / H | ||
| EDP FU | CBT-ED | S | WL/NT | 1 | 100% NS | SMD | ns | MA | 10 / H | ||
| F-QOL | EDU | S | WL/NT | 1 | 100% NS | SMD | ns | MA | 7 / M | ||
| GENSX | CBT-ED | I | WL/NT | 1 | 100% EFF | SMD | 0.80 (0.37 – 1.22) | MA | 7 / M | ||
| PT | I | WL/NT | 1 | 100% EFF | SMD | 0.58 (0.18 – 0.98) | MA | 7 / M | |||
| EDU | S | WL/NT | 1 | 100% NS | SMD | ns | MA | 7 / M | |||
| PHARMA (AD) | I | PBO | 3 | 100% NS | SMD | ns | MA | 10 / H | |||
| GLOBAL | PT | I | WL/NT | 1 | 100% EFF | RR | 1.54 (1.30 – 1.82) | MA | 7 / M | ||
| CBT-ED | I | WL/NT | 1 | 100% EFF | RR | 1.49 (1.28 – 1.72) | MA | 7 / M | |||
| PHARMA (AD) | I | PBO | 10 | 80% EFF; 20% NS | RR | ns to 3.45 (1.59 – 7.69) | MA | 10 / H | |||
| Binge-Eating Disorder | |||||||||||
| ADU | MIX | ACCEPT | PHARMA (STIM) | I | PBO | 1 | 100% DETR | RR | 0.46 (0.21 – 0.97) | MA | 9 / H |
| PHARMA (MS) | I | PBO | 2 | 50% NS; 50% DETR | RR | 0.52 (0.31 – 0.88) to ns | MA | 5 / M | |||
| BEHAV | PHARMA (STIM) | I | PBO | 1 | 100% EFF | SMD | 0.56 (0.28 – 0.84) | MA | 9 / H | ||
| CBT-ED | I | WL/NT | 1 | 100% EFF | MD | 2.32 (0.09 – 4.56) | MA | 11 / H | |||
| PHARMA (MS) | I | PBO | 2 | 100% EFF | MD | 0.98 (0.15 – 1.80) to 1.31 (0.03 – 2.58) | MA | 5 / M | |||
| PHARMA (AD) | I | PBO | 1 | 100% EFF | MD | 0.67 (0.09 – 1.26) | MA | 8 / H | |||
| EDP | PHARMA (STIM) | I | PBO | 1 | 100% EFF | MD | 6.50 (4.18 – 8.82) | MA | 8 / H | ||
| PHARMA (AD) | I | PBO | 1 | 100% EFF | MD | 3.84 (1.13 – 6.55) | MA | 8 / H | |||
| GENSX | PHARMA (AD) | I | PBO | 1 | 100% EFF | MD | 1.97 (0.28 – 3.67) | MA | 8 / H | ||
| PHARMA (MS) | I | PBO | 1 | 100% NS | SMD | ns | MA | 5 / M | |||
| GLOBAL | CBT-ED | I | WL/NT | 1 | 100% EFF | RR | 4.95 (3.06 – 8.00) | MA | 8 / H | ||
| PHARMA (STIM) | I | PBO | 2 | 100% EFF | RR | 1.58 (1.35 – 1.84) to 2.43 (1.95 – 3.01) | MA | 9 / H | |||
| PHARMA (AD) | I | PBO | 1 | 100% EFF | RR | 1.67 (1.24 – 2.26) | MA | 8 / H | |||
| PHARMA (MS) | I | PBO | 1 | 100% NS | MD | ns | MA | 11 / H | |||
| SAFETY | PHARMA (STIM) | I | PBO | 2 | 100% DETR | RR | 0.61 (0.42 – 0.88) to 0.38 (0.23 – 0.61) | MA | 11 / H | ||
| WEIGHT/BODY | PHARMA (MS) | I | PBO | 1 | 100% EFF | MD | 4.91 (3.41 – 6.42) | MA | 5 / M | ||
| PHARMA (STIM) | I | PBO | 1 | 100% EFF | SMD | 1.28 (1.06 – 1.51) | MA | 9 / H | |||
| PHARMA (AD) | I | PBO | 2 | 100% NS | MD | ns | MA | 8 / H | |||
| MIX | MIX | ACCEPT | PHARMA (AD) | I | PBO | 1 | 100% NS | RR | ns | MA | 8 / H |
| PHARMA (ORL) | I | PBO | 1 | 100% NS | RR | ns | MA | 8 / H | |||
| PHARMA (MIX) | I | PBO | 1 | 100% NS | RR | ns | MA | 8 / H | |||
| PHARMA (MS) | I | PBO | 1 | 100% NS | RR | ns | MA | 8 / H | |||
| BEHAV | PT | I | WL/NT | 1 | 100% EFF | g | 0.89 (0.59 – 1.19) | MA | 7 / M | ||
| CBT-ED | I | WL/NT | 1 | 100% EFF | SMD | 0.83 (0.55 – 1.11) | MA | 9 / H | |||
| PHARMA (STIM) | I | PBO | 1 | 100% EFF | MD | 1.39 (1.12 – 1.66) | NMA | 6 / M | |||
| PHARMA (AD) | I | PBO | 3 | 66.7% EFF; 33.3% NS | MD | ns to 0.91 (0.28 – 1.54) | NMA, MA | 6 / M | |||
| CBT-ED | S | PBO | 2 | 50% EFF; 50% NS | SMD | ns to 0.51 (0.17 – 0.84) | MA | 9.5 / H | |||
| PHARMA (MS) | I | PBO | 1 | 100% NS | SMD | ns | MA | 9 / H | |||
| EDP | PT | I | WL/NT | 4 | 100% EFF | g | 0.77 (0.36 – 1.18) to 1.03 (0.38 – 1.69) | MA | 7 / M | ||
| PHARMA (STIM) | I | PBO | 1 | 100% EFF | MD | 6.51 (5.21 – 7.81) | NMA | 6 / M | |||
| PHARMA (AD) | I | PBO | 1 | 100% EFF | MD | 3.83 (1.43 – 6.24) | NMA | 6 / M | |||
| CBT-ED | S | WL/NT | 1 | 100% EFF | MD | 0.58 (0.17 – 0.98) | MA | 9 / H | |||
| CBT-ED | I | WL/NT | 1 | 100% EFF | MD | 0.50 (0.12 – 0.88) | MA | 9 / H | |||
| EDP FU | PT | I | WL/NT | 1 | 100% EFF | g | 1.34 (0.87 – 1.82) | MA | 7 / M | ||
| GENSX | CBT-ED | I | WL/NT | 1 | 100% EFF | SMD | 0.42 (0.18 – 0.67) | MA | 9 / H | ||
| CBT-ED | S | WL/NT | 1 | 100% EFF | SMD | 0.35 (0.07 – 0.63) | MA | 9 / H | |||
| PHARMA (AD) | I | PBO | 6 | 83.3% EFF; 16.7% NS | MD/SMD | ns to MD 3.84 (1.12 – 6.55) / SMD 0.38 (0.03 – 0.74) | MA | 11 / H | |||
| PT | I | PBO | 2 | 100% NS | g | ns | MA | 7 / M | |||
| GLOBAL | CBT-ED | S | WL/NT | 2 | 100% EFF | RD/RR | RD 0.25 (0.12 – 0.38) / RR 4.58 (1.54 – 13.6) | MA | 9 / H | ||
| PHARMA (STIM) | I | PBO | 1 | 100% EFF | RR | 2.61 (2.04 – 3.33) | NMA | 6 / M | |||
| PHARMA (AD) | I | PBO | 3 | 100% EFF | RR | 1.23 (1.06 – 1.43) to 1.67 (1.24 – 2.26) | NMA, MA | 6 / M | |||
| PHARMA (MIX) | I | PBO | 1 | 100% EFF | RR | 1.35 (1.19 – 1.51) | MA | 8 / H | |||
| PHARMA (ORL) | I | PBO | 1 | 100% EFF | RR | 1.25 (1.06 – 1.45) | MA | 8 / H | |||
| CBT-ED | I | WL/NT | 1 | 100% EFF | RD | 0.4 (0.3 – 0.5) | MA | 9.5 / H | |||
| PHARMA (MS) | I | PBO | 2 | 50% EFF; 50% NS | RR | ns to 1.59 (1.28 – 1.96) | MA | 8.5 / H | |||
| WEIGHT/BODY | PHARMA (STIM) | I | PBO | 1 | 100% EFF | MD | 5.23 (3.94 – 6.52) | MA | 9 / H | ||
| PHARMA (MS) | I | PBO | 1 | 100% EFF | WMD | 4.58 (3.79 – 5.36) | MA | 8 / H | |||
| PHARMA (ORL) | I | PBO | 1 | 100% EFF | WMD | 3.63 (2.87 – 4.40) | MA | 8 / H | |||
| PHARMA (MIX) | I | PBO | 1 | 100% EFF | WMD | 3.42 (2.58 – 4.25) | MA | 8 / H | |||
| PHARMA (AD) | I | PBO | 4 | 25% EFF; 75% NS | WMD | ns to 1.72 (0.37 – 3.06) | MA | 9 / H | |||
| CBT-ED | I | WL/NT | 1 | 100% NS | SMD | ns | MA | 9 / H | |||
| CBT-ED | S | WL/NT | 1 | 100% NS | SMD | ns | MA | 9 / H | |||
| Mixed Eating Disorders | |||||||||||
| ADU | MIX | ACCEPT | CBT-ED | I | WL/NT | 1 | 100% NS | RR | ns | MA | 5 / M |
| EDU | S | WL/NT | 1 | 100% NS | RR | ns | MA | 5 / M | |||
| BEHAV | CBT-ED | I | WL/NT | 1 | 100% EFF | SMD | 0.95 (0.68 – 1.22) | MA | 5 / M | ||
| EDU | S | WL/NT | 1 | 100% NS | RR | ns | MA | 5 / M | |||
| GLOBAL | CBT-ED | I | WL/NT | 1 | 100% EFF | RR | 1.45 (1.25 – 1.69) | MA | 5 / M | ||
| WEIGHT/BODY | CBT-ED | I | WL/NT | 1 | 100% EFF | SMD | 0.33 (0.001 – 0.66) | MA | 5 / M | ||
| MIX | MIX | ACCEPT | CBT-ED | I | WL/NT | 1 | 100% NS | RR | ns | MA | 7 / M |
| EDU | S | WL/NT | 5 | 100% NS | RR | ns | MA | 5 / M | |||
| PT | I | WL/NT | 1 | 100% NS | RR | ns | MA | 7 / M | |||
| BEHAV | PT | I | WL/NT | 4 | 100% EFF | SMD | 0.81 (0.58 – 1.04) to 1.14 (0.89 – 1.39) | MA | 8 / H | ||
| CBT-ED | I | WL/NT | 1 | 100% EFF | SMD | 0.94 (0.70 – 1.18) | MA | 7 / M | |||
| EDU | S | WL/NT | 11 | 54.5% EFF; 45.5% NS | SMD | ns to 0.98 (0.69 – 1.27) | MA | 7 / M | |||
| EDP | EDU | S | WL/NT | 2 | 100% EFF | SMD | 0.58 (0.37 – 0.79) to 0.71 (0.41 – 1.01) | MA | 9 / H | ||
| PT | I | WL/NT | 4 | 75% EFF; 25% NS | SMD | ns to 1.13 (0.61 – 1.66) | MA | 8 / H | |||
| F-QOL | EDU | S | WL/NT | 2 | 50% EFF; 50% NS | SMD | ns to 0.34 (0.02 – 0.67) | MA | 8 / H | ||
| CBT-ED | I | WL/NT | 1 | 100% NS | SMD | ns | MA | 7 / M | |||
| PT | I | WL/NT | 1 | 100% NS | g | ns | MA | 9 / H | |||
| GENSX | PT | I | WL/NT | 5 | 100% EFF | SMD | 0.30 (0.13 – 0.47) to 0.71 (0.29 – 1.14) | MA | 7 / M | ||
| CBT-ED | I | WL/NT | 1 | 100% EFF | SMD | 0.69 (0.30 – 1.08) | MA | 7 / M | |||
| EDU | S | WL/NT | 3 | 33.3% EFF; 66.7% NS | WMD | ns to 0.32 (0.13 – 0.51) | MA | 9 / H | |||
| GLOBAL | PT | I | WL/NT | 1 | 100% EFF | RR | 1.59 (1.20 – 2.08) | MA | 7 / M | ||
| CBT-ED | I | WL/NT | 1 | 100% EFF | RR | 1.45 (1.27 – 1.64) | MA | 7 / M | |||
| CBT-ED | S | WL/NT | 1 | 100% NS | RR | ns | MA | 10 / H | |||
| EDU | S | WL/NT | 1 | 100% NS | RR | ns | MA | 7 / M | |||
| WEIGHT/BODY | CBT-ED | I | WL/NT | 1 | 100% NS | SMD | ns | MA | 7 / M | ||
| EDU | S | WL/NT | 2 | 100% NS | SMD | ns | MA | 8 / H | |||
| PT | I | WL/NT | 1 | 100% NS | SMD | ns | MA | 7 / M | |||
A, quality as per AMSTAR 0–11 score (median if >1 comparison); ACCEPT, acceptability; AD, antidepressants; ADU, adults; BEHAV, behavioural symptoms; BT, behavioural therapy; CBT-ED, cognitive behavioural therapy for eating disorders; CI, confidence interval; EDP, eating disorder psychopathology; EDU, psychoeducation; EFF, statistically significant difference that favours intervention; ES, effect size; F-QOL, functioning/quality of life; FU, at follow up; G, group intervention; g, Hedges’ g; GENSX, psychiatric general symptoms; GLOBAL, improvement/response/remission/relapse; I, individual intervention; IPT, interpersonal psychotherapy; MD, mean difference; MIX, mixed or not specified age group/setting/medications; MULTI, mixed active interventions; NS/ns, no statistically significant difference; OR, odds ratio; OUT, outpatient; PBO, placebo; PHARMA, pharmacological intervention; PT, psychotherapy; Q, quality level (L=low-AMSTAR <4, M=medium-AMSTAR 4 to 7, H=high-AMSTAR >7); RD, risk difference; RR, risk ratio; S, self-help intervention; SMD, standardized mean difference; WEIGHT/BODY, changes in weight/BMI/body composition; WL/NT, wait-list/no treatment; WMD, weighted mean difference.
Significant results are presented in bold. Results are ordered as significant ones first, ordered by ES magnitude and % significant comparisons, and then non-significant ones in alphabetical order.
Table 3.
Efficacy of interventions against Treatment As Usual/Active control.
| Age group | Setting | Outcome | Intervention | I/G/S | Control | N of estimates | % significance | ES type | ES (95% CI) | Source | A / Q |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anorexia Nervosa | |||||||||||
| ADO | OUT | GLOBAL FU | FBT | I | ACTIVE | 1 | 100% EFF | OR | 2.08 (1.07 – 4.03) | MA | 9 / H |
| MIX | ACCEPT | FAM | I | TAU | 1 | 100% NS | RR | ns | MA | 9 / H | |
| FAM | I | ACTIVE | 1 | 100% NS | RR | ns | MA | 9 / H | |||
| EDP | PT | I | TAU | 1 | 100% NS | g | ns | MA | 10 / H | ||
| FAM | I | ACTIVE | 1 | 100% NS | SMD | ns | MA | 9 / H | |||
| EDP FU | FAM | I | ACTIVE | 1 | 100% NS | SMD | ns | MA | 9 / H | ||
| GLOBAL | FAM | I | ACTIVE | 1 | 100% NS | RR | ns | MA | 9 / H | ||
| GLOBAL FU | FAM | I | ACTIVE | 1 | 100% NS | RR | ns | MA | 9 / H | ||
| WEIGHT/BODY | FAM | I | ACTIVE | 3 | 33.3% EFF; 66.7% NS | SMD | ns to 0.44 (0.14 – 0.74) | NMA, MA | 6 / M | ||
| FBT | I | ACTIVE | 2 | 100% NS | SMD | ns | NMA | 6 / M | |||
| PT | I | TAU | 1 | 100% NS | g | ns | MA | 10 / H | |||
| MULTI | I | ACTIVE | 4 | 100% NS | SMD | ns | NMA | 6 / M | |||
| PDO | I | ACTIVE | 1 | 100% NS | SMD | ns | NMA | 6 / M | |||
| WEIGHT/BODY FU | FAM | I | ACTIVE | 1 | 100% NS | SMD | ns | MA | 9 / H | ||
| ADU | OUT | ACCEPT | CBT-ED | I | TAU | 1 | 100% NS | RR | ns | NMA | 10 / H |
| FBT | I | TAU | 1 | 100% NS | RR | ns | NMA | 10 / H | |||
| MANTRA | I | TAU | 1 | 100% NS | RR | ns | NMA | 10 / H | |||
| PDO | I | TAU | 1 | 100% NS | RR | ns | NMA | 10 / H | |||
| CBT-ED | I | ACTIVE | 1 | 100% NS | RR | ns | NMA | 10 / H | |||
| FBT | I | ACTIVE | 1 | 100% NS | RR | ns | NMA | 10 / H | |||
| BEHAV | CBT-ED | I | TAU | 1 | 100% NS | SMD | ns | NMA | 10 / H | ||
| FBT | I | TAU | 1 | 100% NS | SMD | ns | NMA | 10 / H | |||
| MANTRA | I | TAU | 1 | 100% NS | SMD | ns | NMA | 10 / H | |||
| PDO | I | TAU | 1 | 100% NS | SMD | ns | NMA | 10 / H | |||
| CBT-ED | I | ACTIVE | 1 | 100% NS | SMD | ns | NMA | 10 / H | |||
| FBT | I | ACTIVE | 1 | 100% NS | SMD | ns | NMA | 10 / H | |||
| BEHAV FU | CBT-ED | I | TAU | 1 | 100% NS | SMD | ns | NMA | 10 / H | ||
| FBT | I | TAU | 1 | 100% NS | SMD | ns | NMA | 10 / H | |||
| MANTRA | I | TAU | 1 | 100% NS | SMD | ns | NMA | 10 / H | |||
| PDO | I | TAU | 1 | 100% NS | SMD | ns | NMA | 10 / H | |||
| CBT-ED | I | ACTIVE | 1 | 100% NS | SMD | ns | NMA | 10 / H | |||
| FBT | I | ACTIVE | 1 | 100% NS | SMD | ns | NMA | 10 / H | |||
| WEIGHT/BODY | CBT-ED | I | TAU | 1 | 100% NS | SMD | ns | NMA | 10 / H | ||
| FBT | I | TAU | 1 | 100% NS | SMD | ns | NMA | 10 / H | |||
| MANTRA | I | TAU | 1 | 100% NS | SMD | ns | NMA | 10 / H | |||
| PDO | I | TAU | 1 | 100% NS | SMD | ns | NMA | 10 / H | |||
| CBT-ED | I | ACTIVE | 1 | 100% NS | SMD | ns | NMA | 10 / H | |||
| FBT | I | ACTIVE | 1 | 100% NS | SMD | ns | NMA | 10 / H | |||
| WEIGHT/BODY FU | CBT-ED | I | TAU | 1 | 100% NS | SMD | ns | NMA | 10 / H | ||
| FBT | I | TAU | 1 | 100% NS | SMD | ns | NMA | 10 / H | |||
| MANTRA | I | TAU | 1 | 100% NS | SMD | ns | NMA | 10 / H | |||
| PDO | I | TAU | 1 | 100% NS | SMD | ns | NMA | 10 / H | |||
| CBT-ED | I | ACTIVE | 1 | 100% NS | SMD | ns | NMA | 10 / H | |||
| FBT | I | ACTIVE | 1 | 100% NS | SMD | ns | NMA | 10 / H | |||
| MIX | ACCEPT | FAM | I | ACTIVE | 1 | 100% NS | RR | ns | MA | 9 / H | |
| EDP | FAM | I | ACTIVE | 1 | 100% NS | SMD | ns | MA | 9 / H | ||
| PT | I | TAU | 1 | 100% NS | g | ns | MA | 10 / H | |||
| EDP FU | FAM | I | ACTIVE | 1 | 100% NS | SMD | ns | MA | 9 / H | ||
| GLOBAL | FAM | I | TAU | 1 | 100% EFF | RR | 3.50 (1.49 – 8.23) | MA | 9 / H | ||
| FAM | I | ACTIVE | 2 | 100% NS | RR | ns | MA | 9 / H | |||
| GLOBAL FU | FAM | I | ACTIVE | 1 | 100% NS | RR | ns | MA | 9 / H | ||
| WEIGHT/BODY | MULTI | I | TAU | 1 | 100% NS | SMD | ns | NMA | 6 / M | ||
| CBT-ED | I | ACTIVE | 3 | 100% NS | SMD | ns | NMA | 6 / M | |||
| FAM | I | ACTIVE | 1 | 100% NS | SMD | ns | MA | 9 / H | |||
| FBT | I | ACTIVE | 1 | 100% NS | SMD | ns | NMA | 6 / M | |||
| IPT | I | ACTIVE | 1 | 100% NS | SMD | ns | NMA | 6 / M | |||
| MANTRA | I | ACTIVE | 1 | 100% NS | SMD | ns | NMA | 6 / M | |||
| PDO | I | ACTIVE | 2 | 100% NS | SMD | ns | NMA | 6 / M | |||
| WEIGHT/BODY FU | FAM | I | ACTIVE | 1 | 100% NS | SMD | ns | MA | 9 / H | ||
| MIX | IN | ACCEPT | MULTI | NA | ACTIVE | 1 | 100% DETR | RR | 0.75 (0.64 – 0.88) | MA | 9 / H |
| GENSX | MULTI | NA | ACTIVE | 2 | 100% NS | SMD | ns | MA | 9 / H | ||
| GLOBAL | MULTI | NA | ACTIVE | 1 | 100% NS | RR | ns | MA | 9 / H | ||
| WEIGHT/BODY | MULTI | NA | ACTIVE | 1 | 100% NS | SMD | ns | MA | 9 / H | ||
| OUT | ACCEPT | CBT-ED | I | ACTIVE | 1 | 100% NS | RR | ns | MA | 9 / H | |
| PDO | I | TAU | 1 | 100% NS | RR | ns | MA | 9 / H | |||
| EDP | CBT-ED | I | ACTIVE | 1 | 100% NS | SMD | ns | MA | 9 / H | ||
| PT | I | TAU | 1 | 100% NS | g | ns | MA | 10 / H | |||
| EDP FU | CBT-ED | I | ACTIVE | 1 | 100% NS | SMD | ns | MA | 9 / H | ||
| F-QOL | PT | I | TAU | 1 | 100% NS | g | ns | MA | 10 / H | ||
| GLOBAL | CBT-ED | I | ACTIVE | 1 | 100% NS | RR | ns | MA | 9 / H | ||
| PDO | I | TAU | 1 | 100% NS | RR | ns | MA | 9 / H | |||
| WEIGHT/BODY | CBT-ED | I | ACTIVE | 1 | 100% NS | MD | ns | MA | 9 / H | ||
| PT | I | TAU | 1 | 100% NS | g | ns | MA | 10 / H | |||
| WEIGHT/BODY FU | CBT-ED | I | ACTIVE | 1 | 100% NS | MD | ns | MA | 9 / H | ||
| MIX | ACCEPT | FBT | I | ACTIVE | 1 | 100% NS | RR | ns | MA | 9 / H | |
| FAM | I | TAU | 1 | 100% NS | RR | ns | MA | 9 / H | |||
| EDP | CBT-ED | I | TAU | 1 | 100% NS | g | ns | MA | 10 / H | ||
| FAM | I | TAU | 2 | 100% NS | SMD | ns | MA | 9.5 / H | |||
| PDO | I | TAU | 1 | 100% NS | g | ns | MA | 10 / H | |||
| PT | I | TAU | 2 | 100% NS | g | ns | MA | 10 / H | |||
| PT | G | TAU | 1 | 100% NS | g | ns | MA | 10 / H | |||
| CBT-ED | I | ACTIVE | 1 | 100% NS | g | ns | MA | 6 / M | |||
| FAM | I | ACTIVE | 2 | 100% NS | SMD | ns | MA | 9 / H | |||
| FBT | G | ACTIVE | 1 | 100% NS | MD | ns | MA | 9 / H | |||
| EDP FU | CBT-ED | I | ACTIVE | 1 | 100% NS | g | ns | MA | 6 / M | ||
| FBT | I | ACTIVE | 2 | 100% NS | SMD | ns | MA | 9 / H | |||
| F-QOL | CBT-ED | I | TAU | 1 | 100% NS | g | ns | MA | 10 / H | ||
| PDO | I | TAU | 1 | 100% NS | g | ns | MA | 10 / H | |||
| PT | I | TAU | 1 | 100% NS | g | ns | MA | 10 / H | |||
| PT | I | ACTIVE | 1 | 100% NS | g | ns | MA | 10 / H | |||
| GLOBAL | FBT | G | ACTIVE | 1 | 100% EFF | RR | 1.79 (1.20 – 2.63) | MA | 9 / H | ||
| FAM | I | TAU | 2 | 50% EFF; 50% NS | RR | ns to 3.83 (1.60 – 9.13) | MA | 9 / H | |||
| FAM | I | ACTIVE | 3 | 100% NS | RR | ns | MA | 9 / H | |||
| GLOBAL FU | FBT | I | ACTIVE | 3 | 100% NS | RR | ns | MA | 9 / H | ||
| WEIGHT/BODY | FBT | I | ACTIVE | 5 | 33.3% EFF; 66.7% NS | SMD | ns to 0.32 (0.01 – 0.63) | MA | 9 / H | ||
| CBT-ED | I | TAU | 1 | 100% NS | g | ns | MA | 10 / H | |||
| EXE | I | TAU | 1 | 100% NS | g | ns | MA | 7 / M | |||
| FAM | I | TAU | 1 | 100% NS | g | ns | MA | 10 / H | |||
| PDO | I | TAU | 1 | 100% NS | g | ns | MA | 10 / H | |||
| PT | I | TAU | 3 | 100% NS | g | ns | MA | 10 / H | |||
| PT | G | TAU | 1 | 100% NS | g | ns | MA | 10 / H | |||
| PT | I | ACTIVE | 1 | 100% NS | g | ns | MA | 10 / H | |||
| WEIGHT/BODY FU | FBT | I | ACTIVE | 2 | 100% NS | SMD | ns | MA | 9 / H | ||
| Bulimia Nervosa | |||||||||||
| ADO | OUT | GLOBAL FU | FBT | I | ACTIVE | 1 | 100% EFF | OR | 2.22 (1.02 – 4.82) | MA | 9 / H |
| MIX | BEHAV | CBT-ED | I | ACTIVE | 1 | 100% NS | g | ns | MA | 6 / M | |
| EDP | CBT-ED | I | ACTIVE | 1 | 100% NS | g | ns | MA | 6 / M | ||
| ADU | MIX | ACCEPT | PT | I | ACTIVE | 2 | 50% EFF; 50% NS | RR | ns to 1.75 (1.14 – 2.63) | MA | 8 / H |
| CBT-ED | I | ACTIVE | 2 | 100% NS | RR | ns | MA | 5 / M | |||
| EDU | I | ACTIVE | 1 | 100% NS | RR | ns | MA | 5 / M | |||
| PHARMA (AD) | I | ACTIVE | 1 | 100% NS | RR | ns | MA | 8 / H | |||
| BEHAV | PHARMA (AD) | I | ACTIVE | 1 | 100% EFF | RR | 2.08 (1.09 – 25.0) | MA | 8 / H | ||
| CBT-ED | I | ACTIVE | 4 | 25% EFF; 25% NS; 50% NA* | g | ns to 0.23 (0.06 – 0.37) | MA | 5 / M | |||
| EDU | I | ACTIVE | 1 | 100% NS | SMD | ns | MA | 5 / M | |||
| PT | I | ACTIVE | 2 | 100% NS | SMD | ns | MA | 6.5 / M | |||
| EDP | CBT-ED | I | ACTIVE | 1 | 100% EFF | g | 0.27 (0.08 – 0.45) | MA | 6 / M | ||
| F-QOL | CBT-ED | I | TAU | 1 | 100% NS | SMD | ns | MA | 5 / M | ||
| GENSX | PHARMA (AD) | I | ACTIVE | 1 | 100% EFF | RR | 2.13 (1.09 – 100) | MA | 8 / H | ||
| CBT-ED | I | ACTIVE | 2 | 100% NS | SMD | ns | MA | 5 / M | |||
| EDU | I | ACTIVE | 1 | 100% NS | SMD | ns | MA | 5 / M | |||
| PT | I | ACTIVE | 1 | 100% NS | SMD | ns | MA | 8 / H | |||
| GENSX FU | EDU | I | ACTIVE | 1 | 100% NS | SMD | ns | MA | 5 / M | ||
| GLOBAL | CBT-ED | I | ACTIVE | 2 | 100% EFF | OR | 1.20 (1.03 – 1.41) to 1.70 (1.21 – 2.38) | MA | 5.5 / M | ||
| PT | I | ACTIVE | 3 | 66.7% NS; 33.3% DETR | RR | 0.83 (0.69 – 0.98) to ns | NMA, MA | 8 / H | |||
| BT | I | ACTIVE | 1 | 100% NS | OR | ns | NMA | 8 / H | |||
| BT | G | ACTIVE | 1 | 100% NS | OR | ns | NMA | 8 / H | |||
| CBT-ED | G | ACTIVE | 1 | 100% NS | OR | ns | NMA | 8 / H | |||
| CBT-ED | S | ACTIVE | 2 | 100% NS | OR | ns | NMA | 8 / H | |||
| EXE | I | ACTIVE | 1 | 100% NS | OR | ns | NMA | 8 / H | |||
| IPT | I | ACTIVE | 1 | 100% NS | OR | ns | NMA | 8 / H | |||
| MULTI | I | ACTIVE | 1 | 100% NS | OR | ns | NMA | 8 / H | |||
| PHARMA (AD) | I | ACTIVE | 2 | 100% NS | OR | ns | NMA, MA | 8 / H | |||
| WEIGHT/BODY | CBT-ED | I | ACTIVE | 1 | 100% NS | SMD | ns | MA | 5 / M | ||
| MIX | OUT | ACCEPT | PHARMA (AD) | I | ACTIVE | 1 | 100% DETR | RR | 0.46 (0.23 – 0.92) | MA | 7 / M |
| BEHAV | PHARMA (AD) | I | ACTIVE | 1 | 100% NS | SMD | ns | MA | 7 / M | ||
| GENSX | PHARMA (AD) | I | ACTIVE | 1 | 100% NS | RR | ns | MA | 7 / M | ||
| GLOBAL | PHARMA (AD) | I | ACTIVE | 1 | 100% NS | RR | ns | MA | 7 / M | ||
| MIX | ACCEPT | PT | I | ACTIVE | 3 | 66.7% EFF; 33.3% NS | RR | ns to 1.82 (1.11 – 3.03) | MA | 10 / H | |
| CBT-ED | I | ACTIVE | 2 | 100% NS | RR | ns | MA | 7 / M | |||
| EDU | I | ACTIVE | 1 | 100% NS | RR | ns | MA | 7 / M | |||
| PHARMA (AD) | I | ACTIVE | 1 | 100% NS | RR | ns | MA | 10 / H | |||
| BEHAV | CBT-ED | I | ACTIVE | 12 | 41.7% EFF; 58.3% NS | g | ns to 0.42 (0.16 – 0.67) | MA | 6 / M | ||
| CBT-ED | I+S | ACTIVE | 2 | 50% EFF; 50% NS | g | ns to 0.30 (0.07 – 0.53) | MA | 6 / M | |||
| CBT-ED | G | ACTIVE | 1 | 100% NS | g | ns | MA | 6 / M | |||
| EDU | S | ACTIVE | 1 | 100% NS | SMD | ns | MA | 7 / M | |||
| PHARMA (AD) | I | ACTIVE | 1 | 100% NS | SMD | ns | MA | 10 / H | |||
| PT | I | ACTIVE | 2 | 100% NS | SMD | ns | MA | 8.5 / H | |||
| PT | I+S | ACTIVE | 2 | 100% NS | g | ns | MA | 6 / M | |||
| BEHAV FU | CBT-ED | I | ACTIVE | 1 | 100% EFF | g | 0.31 (0.10 – 0.52) | MA | 6 / M | ||
| EDP | CBT-ED | I | ACTIVE | 9 | 87.8% EFF; 22.2% NS | g | ns to 0.53 (0.31 – 0.74) | MA | 6 / M | ||
| EDP FU | CBT-ED | I | ACTIVE | 1 | 100% NS | g | ns | MA | 6 / M | ||
| F-QOL | CBT-ED | I | ACTIVE | 1 | 100% NS | SMD | ns | MA | 7 / M | ||
| GENSX | CBT-ED | I | ACTIVE | 4 | 25% EFF; 75% NS | g | ns to 0.25 (0.03 – 0.46) | MA | 6.5 / M | ||
| CBT-ED | I+S | ACTIVE | 1 | 100% NS | g | ns | MA | 6 / M | |||
| PHARMA (AD) | I | ACTIVE | 1 | 100% NS | SMD | ns | MA | 10 / H | |||
| PT | I+S | ACTIVE | 1 | 100% NS | g | ns | MA | 6 / M | |||
| GENSX FU | CBT-ED | I+S | ACTIVE | 1 | 100% NS | g | ns | MA | 6 / M | ||
| GLOBAL | CBT-ED | I | ACTIVE | 7 | 42.9% EFF; 57.1% NS | OR | ns to 2.08 (1.23 – 3.53) | MA | 6 / M | ||
| PT | I | ACTIVE | 4 | 75% NS; 25% DETR | RR | 0.82 (0.69 – 0.98) to ns | MA | 10 / H | |||
| PHARMA (AD) | I | ACTIVE | 1 | 100% NS | RR | ns | MA | 10 / H | |||
| GLOBAL FU | CBT-ED | I | ACTIVE | 1 | 100% NS | OR | ns | MA | 6 / M | ||
| WEIGHT/BODY | CBT-ED | I | ACTIVE | 1 | 100% NS | SMD | ns | MA | 7 / M | ||
| Binge Eating Disorder | |||||||||||
| ADU | OUT | ACCEPT | BT | I | ACTIVE | 1 | 100% NS | RR | ns | MA | 9 / H |
| ACCEPT FU | BT | I | ACTIVE | 1 | 100% NS | RR | ns | MA | 9 / H | ||
| BEHAV | CBT-ED | I | ACTIVE | 1 | 100% EFF | MD | 2.04 (0.35 – 3.73) | MA | 9 / H | ||
| BEHAV FU | BT | I | ACTIVE | 1 | 100% NS | MD | ns | MA | 9 / H | ||
| WEIGHT/BODY | BT | I | ACTIVE | 1 | 100% NS | MD | ns | MA | 9 / H | ||
| WEIGHT/BODY FU | BT | I | ACTIVE | 1 | 100% NS | MD | ns | MA | 9 / H | ||
| MIX | ACCEPT | PT | I | ACTIVE | 1 | 100% NS | OR | ns | MA | 5 / M | |
| MIX | MIX | ACCEPT | PT | I | ACTIVE | 1 | 100% EFF | OR | 5.00 (2.50 – 10.0) | MA | 9 / H |
| CBT-ED | I | ACTIVE | 1 | 100% NS | RR | ns | MA | 7 / M | |||
| BEHAV | BT | I | ACTIVE | 1 | 100% EFF | SMD | 0.27 (0.05 – 0.48) | MA | 9 / H | ||
| CBT-ED | I | ACTIVE | 4 | 50% EFF; 50% NS | SMD | ns to 0.31 (0.02 – 0.61) | MA | 6 / M | |||
| CBT-ED | G | ACTIVE | 1 | 100% NS | g | ns | MA | 6 / M | |||
| CBT-ED | S | ACTIVE | 1 | 100% NS | g | ns | MA | 6 / M | |||
| IPT | I | ACTIVE | 1 | 100% NS | SMD | ns | MA | 9 / H | |||
| PHARMA (STIM) | I | ACTIVE | 1 | 100% NS | SMD | ns | NMA | 6 / M | |||
| PT | I | ACTIVE | 3 | 100% NS | g | ns | MA | 7 / M | |||
| BEHAV FU | BT | I | ACTIVE | 1 | 100% DETR | SMD | −0.24 (−0.46 – −0.01) | MA | 9 / H | ||
| CBT-ED | I | ACTIVE | 1 | 100% NS | g | ns | MA | 6 / M | |||
| IPT | I | ACTIVE | 1 | 100% NS | SMD | ns | MA | 9 / H | |||
| PT | I | ACTIVE | 1 | 100% NS | SMD | ns | MA | 9 / H | |||
| EDP | PT | I | ACTIVE | 3 | 66.7% EFF; 33.3% NS | g | ns to 0.49 (0.21 – 0.78) | MA | 7 / M | ||
| CBT-ED | I | ACTIVE | 6 | 16.7% EFF; 83.3% NS | g | ns to 0.17 (0.01 – 0.33) | MA | 7 / M | |||
| CBT-ED | G | ACTIVE | 1 | 100% NS | g | ns | MA | 6 / M | |||
| CBT-ED | S | ACTIVE | 1 | 100% NS | g | ns | MA | 6 / M | |||
| PHARMA (STIM) | I | ACTIVE | 1 | 100% NS | SMD | ns | NMA | 6 / M | |||
| EDP FU | PT | I | ACTIVE | 2 | 50% EFF; 50% NS | g | ns to 0.51 (0.19 – 0.83) | MA | 8 / H | ||
| CBT-ED | I | ACTIVE | 1 | 100% NS | g | ns | MA | 6 / M | |||
| GENSX | BT | I | ACTIVE | 1 | 100% NS | MD | ns | MA | 9 / H | ||
| CBT-ED | I | ACTIVE | 1 | 100% NS | g | ns | MA | 8 / H | |||
| PT | I | ACTIVE | 3 | 100% NS | g | ns | MA | 7 / M | |||
| GENSX FU | BT | I | ACTIVE | 1 | 100% NS | MD | ns | MA | 9 / H | ||
| PT | I | ACTIVE | 3 | 100% NS | g | ns | MA | 7 / M | |||
| GLOBAL | PHARMA (STIM) | I | ACTIVE | 1 | 100% EFF | RR | 1.56 (1.06 – 2.29) | NMA | 6 / M | ||
| BT | G | ACTIVE | 1 | 100% NS | RD | ns | MA | 9 / H | |||
| CBT-ED | I | ACTIVE | 3 | 100% NS | OR | ns | MA | 6 / M | |||
| CBT-ED | G | ACTIVE | 1 | 100% NS | OR | ns | MA | 6 / M | |||
| CBT-ED | S | ACTIVE | 1 | 100% NS | OR | ns | MA | 6 / M | |||
| IPT | I | ACTIVE | 1 | 100% NS | RD | ns | MA | 9 / H | |||
| PT | I | ACTIVE | 3 | 100% NS | OR | ns | MA | 7 / M | |||
| GLOBAL FU | BT | G | ACTIVE | 1 | 100% DETR | RD | −0.13 (−0.25 – −0.02) | MA | 9 / H | ||
| CBT-ED | I | ACTIVE | 1 | 100% NS | OR | ns | MA | 6 / M | |||
| IPT | I | ACTIVE | 1 | 100% NS | RD | ns | MA | 9 / H | |||
| PT | I | ACTIVE | 1 | 100% NS | OR | ns | MA | 9 / H | |||
| WEIGHT/BODY | PT | I | ACTIVE | 2 | 50% NS; 50% DETR | MD | −0.46 (−0.94 – −0.07) to ns | MA | 9 / H | ||
| BT | I | ACTIVE | 1 | 100% NS | SMD | ns | MA | 9 / H | |||
| CBT-ED | I | ACTIVE | 1 | 100% NS | SMD | ns | MA | 7 / M | |||
| IPT | I | ACTIVE | 1 | 100% NS | MD | ns | MA | 9 / H | |||
| WEIGHT/BODY FU | BT | I | ACTIVE | 1 | 100% NS | SMD | ns | MA | 9 / H | ||
| IPT | I | ACTIVE | 1 | 100% NS | MD | ns | MA | 9 / H | |||
| PT | I | ACTIVE | 1 | 100% NS | SMD | ns | MA | 9 / H | |||
| Mixed Eating Disorders | |||||||||||
| ADO | OUT | GLOBAL | FBT | I | ACTIVE | 3 | 33.3% EFF; 66.7% NS | OR | ns to 2.14 (1.29 – 3.53) | MA | 9 / H |
| GLOBAL FU | FBT | I | ACTIVE | 1 | 100% EFF | OR | 2.35 (1.33 – 4.14) | MA | 9 / H | ||
| ADU | MIX | ACCEPT | CBT-ED | I | ACTIVE | 1 | 100% NS | RR | ns | MA | 5 / M |
| CBT-ED | S | ACTIVE | 1 | 100% NS | RR | ns | MA | 5 / M | |||
| PHARMA (MIX) | I | ACTIVE | 1 | 100% NS | OR | ns | MA | 5 / M | |||
| PT | I | ACTIVE | 1 | 100% NS | OR | ns | MA | 5 / M | |||
| BEHAV | CBT-ED | S | ACTIVE | 1 | 100% EFF | SMD | 0.42 (0.09 – 0.76) | MA | 5 / M | ||
| CBT-ED | I | ACTIVE | 2 | 50% EFF; 50% NS | SMD | ns to 0.19 (0.05 – 0.33) | MA | 5 / M | |||
| F-QOL | CBT-ED | I | ACTIVE | 1 | 100% NS | SMD | ns | MA | 5 / M | ||
| GENSX | CBT-ED | I | ACTIVE | 2 | 50% EFF; 50% NS | SMD | ns to 0.49 (0.01 – 0.97) | MA | 5 / M | ||
| CBT-ED | S | ACTIVE | 2 | 100% NS | SMD | ns | MA | 5 / M | |||
| GLOBAL | CBT-ED | I | ACTIVE | 1 | 100% EFF | RR | 1.23 (1.09 – 1.39) | MA | 5 / M | ||
| CBT-ED | S | ACTIVE | 1 | 100% NS | RR | ns | MA | 5 / M | |||
| WEIGHT/BODY | CBT-ED | I | ACTIVE | 2 | 100% NS | SMD | ns | MA | 5 / M | ||
| CBT-ED | S | ACTIVE | 1 | 100% NS | SMD | ns | MA | 5 / M | |||
| MIX | OUT | BEHAV | VR-CBT-ED | I | ACTIVE | 2 | 50% EFF; 50% NS | MD | ns to 0.29 (0.01 – 0.57) | MA | 7 / M |
| MIX | ACCEPT | CBT-ED | I | ACTIVE | 1 | 100% NS | RR | ns | MA | 7 / M | |
| CBT-ED | S | ACTIVE | 1 | 100% NS | RR | ns | MA | 7 / M | |||
| EDU | S | ACTIVE | 5 | 100% NS | RR | ns | MA | 9 / H | |||
| MULTI | S | ACTIVE | 2 | 100% NS | RR | ns | MA | 9 / H | |||
| BEHAV | CBT-ED | S | ACTIVE | 1 | 100% EFF | SMD | 0.42 (0.09 – 0.76) | MA | 7 / M | ||
| e-PT | I | ACTIVE | 1 | 100% EFF | g | 0.28 (0.07 – 0.51) | MA | 7 / M | |||
| CBT-ED | I | ACTIVE | 4 | 50% EFF; 50% NS | g | ns to 0.29 (0.09 – 0.49) | MA | 8.5 / H | |||
| PT | I | ACTIVE | 5 | 20% EFF; 80% NS | SMD | ns to 0.19 (0.05 – 0.33) | MA | 9 / H | |||
| IPT | I | ACTIVE | 1 | 100% DETR | g | −0.22 (−0.39 – −0.05) | MA | 10 / H | |||
| EDU | S | ACTIVE | 9 | 100% NS | RR | ns | MA | 9 / H | |||
| PT | G | ACTIVE | 2 | 100% NS | g | ns | MA | 9 / H | |||
| BEHAV FU | CBT-ED | I | ACTIVE | 3 | 100% NS | RR | ns | MA | 9 / H | ||
| CBT-ED | G | ACTIVE | 1 | 100% NS | RR | ns | MA | 9 / H | |||
| EDU | S | ACTIVE | 2 | 100% NS | RR | ns | MA | 9 / H | |||
| PT | I | ACTIVE | 3 | 100% NS | RR | ns | MA | 9 / H | |||
| EDP | CBT-ED | I | ACTIVE | 9 | 100% EFF | g | 0.18 (0.01 – 0.35) to 0.37 (0.18 – 0.56) | MA | 8 / H | ||
| MULTI | I | ACTIVE | 2 | 50% EFF; 50% NS | SMD | ns to 0.72 (0.25 – 1.20) | MA | 7 / M | |||
| IPT | I | ACTIVE | 4 | 50% EFF; 50% NS | g | ns to 0.56 (0.27 – 0.85) | MA | 10 / H | |||
| CBT-ED | S | ACTIVE | 3 | 66.7% EFF; 33.3% NS | g | ns to 0.39 (0.14 – 0.65) | MA | 8 / H | |||
| PT | I | ACTIVE | 11 | 9.1% EFF; 91.9% NS | g | ns to 0.17 (0.03 – 0.32) | MA | 7 / M | |||
| CBT-ED | G | ACTIVE | 1 | 100% NS | g | ns | MA | 9 / H | |||
| EDU | S | ACTIVE | 2 | 100% NS | SMD | ns | MA | 9 / H | |||
| PT | G | ACTIVE | 1 | 100% NS | g | ns | MA | 9 / H | |||
| EDP FU | PT | I | ACTIVE | 6 | 16.7% EFF; 83.3% NS | g | ns to 0.92 (0.71 – 1.21) | MA | 7 / M | ||
| CBT-ED | I | ACTIVE | 3 | 66.7% EFF; 33.3% NS | g | ns to 0.33 (0.05 – 0.62) | MA | 9 / H | |||
| CBT-ED | G | ACTIVE | 1 | 100% NS | g | ns | MA | 9 / H | |||
| EDU | S | ACTIVE | 1 | 100% NS | MD | ns | MA | 9 / H | |||
| F-QOL | CBT-ED | I | ACTIVE | 2 | 100% NS | SMD | ns | MA | 8 / H | ||
| F-QOL FU | CBT-ED | I | ACTIVE | 1 | 100% NS | g | ns | MA | 9 / H | ||
| GENSX | CBT-ED | I | ACTIVE | 4 | 100% NS | SMD | ns | MA | 8 / H | ||
| CBT-ED | S | ACTIVE | 2 | 100% NS | SMD | ns | MA | 7 / M | |||
| EDU | S | ACTIVE | 5 | 100% NS | SMD | ns | MA | 9 / H | |||
| PT | I | ACTIVE | 4 | 100% NS | g | ns | MA | 8 / H | |||
| GENSX FU | CBT-ED | I | ACTIVE | 2 | 100% NS | g | ns | MA | 9 / H | ||
| EDU | S | ACTIVE | 2 | 100% NS | SMD | ns | MA | 8 / H | |||
| PT | I | ACTIVE | 5 | 100% NS | g | ns | MA | 7 / M | |||
| GLOBAL | CBT-ED | I | ACTIVE | 1 | 100% NS | RR | ns | MA | 7 / M | ||
| CBT-ED | S | ACTIVE | 1 | 100% NS | RR | ns | MA | 7 / M | |||
| IPT | I | ACTIVE | 1 | 100% NS | g | ns | MA | 10 / H | |||
| PT | I | ACTIVE | 1 | 100% NS | RR | ns | MA | 7 / M | |||
| GLOBAL FU | CBT-ED | I | ACTIVE | 1 | 100% NS | OR | ns | MA | 6 / M | ||
| PT | I | ACTIVE | 1 | 100% NS | RR | ns | MA | 6 / M | |||
| WEIGHT/BODY | CBT-ED | I | ACTIVE | 1 | 100% EFF | SMD | 0.18 (0.01 – 0.34) | MA | 7 / M | ||
| CBT-ED | S | ACTIVE | 1 | 100% NS | SMD | ns | MA | 7 / M | |||
| EDU | S | ACTIVE | 1 | 100% NS | WMD | ns | MA | 9 / H | |||
| IPT | I | ACTIVE | 1 | 100% NS | g | ns | MA | 10 / H | |||
| MULTI | I | ACTIVE | 1 | 100% NS | MD | ns | MA | 10 / H | |||
unclear from the MA if the effect size was significant or not;
A, quality as per AMSTAR 0–11 score (median if >1 comparison); ACCEPT, acceptability; AD, antidepressants; ADU, adults; BEHAV, behavioural symptoms; BT, behavioural therapy; CBT-ED, cognitive behavioural therapy for eating disorders; eCBT-ED, electronic/virtual CBT-ED; CI, confidence interval; DETR, statistically significant difference that favours control; EDP, eating disorder psychopathology; EDU, psychoeducation; EFF, statistically significant difference that favours intervention; ES, effect size; EXE, guided physical exercise; F-QOL, functioning/quality of life; FAM, mixed family interventions; FBT, family-based therapy; FU, at follow up; G, group intervention; g, Hedges’ g; GENSX, psychiatric general symptoms; GLOBAL, improvement/response/remission/relapse; I, individual intervention; IPT, interpersonal psychotherapy; MANTRA, Maudsley Anorexia Treatment for Adults; MD, mean difference; MIX, mixed or not specified age group/setting/medications; MULTI, mixed active interventions; NA, not assessed; NS/ns, no statistically significant difference; Q, quality level (L=low-AMSTAR <4, M=medium-AMSTAR 4 to 7, H=high-AMSTAR >7); OR, odds ratio; OUT, outpatient; PDO, psychodynamic-oriented psychotherapy; PHARMA, pharmacological intervention; PT, psychotherapy; RD, risk difference; RR, risk ratio; S, self-help intervention; SMD, standardized mean difference; STIM, psychostimulants; VR-CBT-ED, CBT-ED plus virtual reality; TAU, treatment as usual; WEIGHT/BODY, changes in weight/BMI/body composition; WMD, weighted mean difference.
Significant results are presented in bold. Results are ordered as significant ones first, ordered by ES magnitude and % significant comparisons, and then non- significant ones in alphabetical order.
3.1. Anorexia nervosa
AN was investigated in two NMAs and 18 MAs; four (20.0%) reported data on adolescents, seven (35.0%) on adult patients.
In adolescent outpatients, individual family-based therapy proved efficacious versus active control for global course of the disease at follow up (k=3, n=183, OR=2.08, 95%CI=1.07–4.03, AMSTAR/Quality=9/H), and individual family therapy in mixed settings for change in weight or body composition (k=4, n=178, SMD=0.44, 95%CI=0.14–0.74, AMSTAR/Quality 9/H) (40.0% of interventions).
In adults, overall, 25.0% of the interventions had evidence of efficacy, but none in the outpatient setting, 33.3% in mixed settings, and only compared to TAU; specifically, individual family therapy proved beneficial for global course of the disease (k=2, n=81, RR=3.50, 95%CI=1.49–8.23, AMSTAR/Quality=9/H).
Results on mixed age groups are reported in eResults section.
3.2.1. Bulimia nervosa efficacy
BN was investigated in one NMA and 19 MAs; two (10.0%) reported data on adolescents, six (30.0%) on adults.
For adolescents, compared to active control, in outpatients individual family-based therapy improved global course of the disease at follow-up (k=2, n=37, OR=2.22, 95%CI=1.02–4.82, AMSTAR/Quality=9/H), whereas in Mixed settings cognitive behavioural therapy for eating disorders did not differ from active control.
In adults, 42.9% of the interventions proved beneficial and 7.1% had both beneficial and detrimental effects. In outpatients, only group cognitive behavioural therapy for eating disorders was covered, compared to wait list-no treatment, with efficacy regarding eating disorder behaviours (k=4, n=51, SMD=0.56, 95%CI=0.15–0.96, AMSTAR/Quality=9/H) and global course of the disease (k=2, n=31, RR=1.29, 95%CI=1.04–1.61, AMSTAR/Quality=9/H). In mixed settings, compared to wait list-no treatment, individual cognitive behavioural therapy for eating disorders and individual psychotherapy were both beneficial for eating disorder behaviours (k=9, n=323, SMD=1.01, 95%CI=0.68–1.33, AMSTAR/Quality=5/M; k=5, n=206, SMD=1.22, 95%CI=0.92–1.52, AMSTAR/Quality=5/M), general psychiatric symptoms (k=6, n=223, SMD=0.80, 95%CI=0.37–1.22, AMSTAR/Quality=5/M; k=3, n=101, SMD=0.58, 95%CI=0.18–0.98, AMSTAR/Quality=5/M) and global course of the disease (k=5, n=204, OR=1.49, 95%CI=1.28–1.72, AMSTAR/Quality=6.5/M; k=na, n=554, OR=3.89, 95%CI=1.19–14.0, AMSTAR/Quality=6.5/M; k=4, n=162, OR=1.54, 95%CI=1.30–1.82, AMSTAR/Quality=6.5/M). Global course of the disease was ameliorated also by group behavioural therapy (k=na, n=207, OR=28.7, 95%CI=3.11–455, AMSTAR/Quality=8/H), group cognitive behavioural therapy for eating disorders (k=na, n=245, OR=7.67, 95%CI=1.51–55.7, AMSTAR/Quality=8/H) and self-help cognitive behavioural therapy for eating disorders (k=na, n=302, OR=3.49, 95%CI=1.20–11.2, AMSTAR/Quality=8/H; k=na, n=392, OR=3.81, 95%CI=1.51–10.9, AMSTAR/Quality=8/H). Compared to Active control, individual cognitive behavioural therapy for eating disorders had the broadest efficacy, namely for eating disorder behaviours (k=25, n=na, g=0.23, 95%CI=0.06–0.37, AMSTAR/Quality=5/M), EDP (k=16, n=na, g=0.27, 95%CI=0.08–0.45, AMSTAR/Quality=6/M), and global course of the disease (k=7, n=484, OR=1.20, 95%CI=1.03–1.41, AMSTAR/Quality=5/M; k=14, n=na, OR=1.70, 95%CI=1.21–2.38, AMSTAR/Quality=6/M). Individual psychotherapy was worse than control for global course of the disease (k=6, n=257, RR=0.83, 95%CI=0.69–0.98, AMSTAR/Quality=8/H). Antidepressants were beneficial for eating disorder behaviours (k=3, n=89, RR=2.08, 95%CI=1.09–25.0, AMSTAR/Quality=8/H) and general psychiatric symptoms (k=3, n=117, RR=2.13, 95%CI=1.09–100, AMSTAR/Quality=8/H).
Results on mixed age groups are reported in eResults section.
3.2.2. Bulimia Nervosa acceptability
Individual psychotherapy was more acceptable than active control (k=6, n=295, RR=1.75, 95%CI=1.14–2.63, AMSTAR/Quality=8/H).
3.3.1. Binge-Eating Disorder efficacy
Data for BED were extracted from one NMA and 18 MAs; none provided information on adolescents, six (31.6%) specifically on adults.
In adult outpatients, individual cognitive behavioural therapy for eating disorders outperformed active control improving eating disorder behaviours (k=4, n=323, MD=2.04, 95%CI=0.35–3.73, AMSTAR/Quality=9/H). In mixed settings, 57.1% of the interventions proved beneficial, and 28.6% had both favourable and unfavourable outcomes. Compared to wait list-no treatment, individual cognitive behavioural therapy for eating disorders proved beneficial for eating disorder behaviours (k=3, n=208, MD=2.32, 95%CI=0.09–4.56, AMSTAR/Quality=11/H) and global course of the disease (k=4, n=401, RR=4.95, 95%CI=3.06–8.00, AMSTAR/Quality=8/H). Compared to placebo, antidepressants were superior for eating disorder behaviours (k=7, n=na, MD=0.67, 95%CI=0.09–1.26, AMSTAR/Quality=8/H), eating disorder psychopathology (k=3, n=na, MD=3.84, 95%CI=1.13–6.55, AMSTAR/Quality=8/H), general psychiatric symptoms (k=3, n=na, MD=1.97, 95%CI=0.28–3.67, AMSTAR/Quality=8/H) and global course of the disease (k=8, n=548, RR=1.67, 95%CI=1.24–2.26, AMSTAR/Quality=8/H). Also, stimulants improved eating disorder behaviours (k=3, n=1033, SMD=0.56, 95%CI=0.28–0.84, AMSTAR/Quality=9/H), eating disorder psychopathology (k=3, n=na, MD=6.50, 95%CI=4.18–8.82, AMSTAR/Quality=8/H), global course of the disease (k=3, n=1033, RR=1.58, 95%CI=1.35–1.84, AMSTAR/Quality=9/H; k=3, n=1033, RR=2.43, 95%CI=1.95–3.01, AMSTAR/Quality=9/H) and change in weight or body composition (k=3, n=1033, SMD=1.28, 95%CI=1.06–1.51, AMSTAR/Quality=9/H). Anticonvulsants improved eating disorder behaviours (k=na, n=na, MD=0.98, 95%CI 0.15–1.80, AMSTAR/Quality=5/M; k=3, n=528, MD=1.31, 95%CI=0.03–2.58, AMSTAR/Quality=5/M) and change in weight or body composition (k=3, n=528, MD=4.91, 95%CI=3.41–6.42, AMSTAR/Quality=5/M). No intervention outperformed active control.
Results on mixed age groups are reported in eResults section.
3.3.2. Binge-Eating Disorder acceptability
Antidepressants induced more adverse events (k=3, n=938, RR=0.61, 95%CI=0.42–0.88, AMSTAR/Quality=11/H; k=3, n=938, RR=0.38, 95%CI=0.23–0.61, AMSTAR/Quality=11/H) and had lower acceptability (k=3, n=1032, RR=0.46, 95%CI=0.21–0.97, AMSTAR/Quality=9/H) than placebo.
Anticonvulsivants had lower acceptability (k=3, n=528, RR=0.52, 95%CI=0.31–0.88, AMSTAR/Quality=5/M) than placebo.
4. Discussion
To our knowledge this is the first umbrella review to systematically assess efficacy and acceptability of interventions specifically for EDs from (N)MAs of RCTs, and both the quality of the (N)MAs and of the meta-analysed RCTs. This umbrella review also follows recent recommendations regarding the need to consider a wide range of outcomes in EDs (Murray et al., 2018) including treatment acceptability (Grenon et al., 2018b). Pooling data from 55 MAs and 4 NMAs, this work advances the field by filtering the enormous body of meta-analytic evidence on interventions in EDs (1,154 effect sizes), rating quality, and providing clinicians, guideline developers and researchers with an informative synopsis of what works better than treatment as usual and/or active control, for which symptoms, at which time-points, and based on what quality of evidence.
For AN, family-based treatment was the only intervention supported by some evidence against other active treatments for both remission and weight gain. In BN, individual cognitive behavioural therapy for ED outperformed other active treatments for ED psychopathology and behaviours, as well as remission. Among pharmacological interventions, tricyclic antidepressants (TCAs) and SSRIs were the most efficacious for ED behaviours and remission. In BED, general psychotherapy (i.e., any manualised psychological therapy) was more efficacious versus active controls for ED psychopathology and drop-out, but less effective for weight reduction; while behavioural treatments (including behavioural weight loss treatment) and cognitive behavioural therapy for ED were more efficacious than active treatments for ED psychopathology and behaviours, respectively. Lisdexamfetamine and antidepressants were more efficacious than placebo for ED psychopathology, ED behaviours, and remission. Antidepressants were also more effective than placebo for general psychiatric symptoms, while lisdexamfetamine as well as anticonvulsants and sibutramine were more effective than placebo for weight reduction. All these findings refer to status at the end of treatment.
Regarding data on follow-up, a smaller body of evidence was available. In adolescent outpatients with AN and with BN, family-based therapy was more effective than active control on remission. In BN, cognitive behavioural therapy for ED outperformed other active treatments regarding ED behaviours. In BED, behavioural treatment was less effective than active control for ED behaviours and remission, whereas psychotherapy was more effective for ED psychopathology.
This distilled evidence calls for a critical analysis of current treatment guidelines for EDs. For AN, this umbrella review supports the practice guidelines that each recommend family-based therapy for younger patients (Hilbert et al., 2017), although a recent Cochrane review (Fisher et al., 2019) commented that there is a limited amount of low-quality evidence to suggest that family therapy approaches may be more effective compared to treatment as usual. In young adults (mean age: 15.2–26.2) with AN, family therapy (not family-based treatment) was more effective than treatment as usual regarding global remission at the end of treatment (medium-large effect). This finding is novel and seems to have been neglected by guidelines, which recommend individual psychotherapy for adults with AN, especially cognitive behavioural therapy for ED (Hilbert et al., 2017). A recent NMA (M Solmi et al., 2021) found no superiority of the most recommended individual psychotherapies (e.g. CBT-ED, MANTRA, SSCM, psychodynamic therapies) versus treatment as usual in adult outpatients with AN. However, that work focused on symptoms, and not global remission as the outcome. Thus, the current findings suggest that interventions involving family might be considered in young adults with AN, although research should aim to develop good alternatives, which does not essentially shift the treatment responsibility to families, as not all adults with AN have families involved in their care. No medication was found to be effective in reducing ED behaviours/psychopathology or general symptoms or in improving weight outcome, supporting previous evidence that the use of medications cannot be recommended to improve body weight or ED-specific psychopathology in AN (Treasure et al., 2020; Zipfel et al., 2015), unless psychiatric comorbidity is present.
In BN, the most robust evidence exists for individual cognitive behavioural therapy for ED, which was more effective in reducing specific and, possibly, general symptoms compared with active treatments at the end of treatment. The positive effect on ED behaviours persisted at follow-up. Compared to wait list-no treatment, not only cognitive behavioural therapy for ED but also general psychotherapy (i.e., any manualised psychological therapy) was more efficacious. The evidence for adolescents was different, here family-based therapy outperformed other active interventions on remission at follow-up. These findings support international clinical guidelines (Hilbert et al., 2017) which recommend individual cognitive behavioural therapy for ED as the first line treatment for adults with BN and family-based therapies for younger patients. The NICE guidelines (2017) suggest the use of guided self-help cognitive behavioural therapy for ED as the first choice: however, this recommendation is not corroborated by our findings that confirm the efficacy of self-help as well as group-delivered cognitive behavioural therapy for ED only in comparison to wait list-no treatment, where expectation effects are not controlled for. NICE includes a cost-effectiveness outcome that was not considered in the present work, which might contribute to the highlighted discrepancy. Furthermore, antidepressants (both SSRIs and the TCAs) outperformed active treatments for ED behaviours and general symptoms and were more effective than placebo for ED behaviours and remission. However, antidepressants were less acceptable than placebo, when contrasted with psychotherapy. These findings are in line with the recommendations from most guidelines (Hilbert et al., 2017), suggesting antidepressants (i.e., the SSRIs) and psychotherapy for people with BN, although there was no evidence relating to the combination of these two treatments over monotherapy.
In BED, the largest evidence existed for lisdexamfetamine and antidepressants. Across psychotherapies, general psychotherapy (i.e., any manualised psychological therapy) and behavioural treatment outperformed active treatments for ED psychopathology as did cognitive behavioural therapy for ED for ED behaviours at the end of treatment. Although general psychotherapy was less efficacious than active treatments for weight reduction, it was considered more acceptable and its effect on ED psychopathology persisted at follow-up in comparison to both active treatments and wait list-no treatment. In contrast, behavioural treatment was less efficacious than active treatments at follow-up. Our findings only partially concur with guidelines (Hilbert et al., 2017) that consistently recommend the use of cognitive behavioural therapy for ED as the specific psychological intervention for BED. Indeed, other (non-CBT-ED) psychotherapies including third wave psychotherapies were efficacious when pooled with cognitive behavioural therapy for ED. However, cognitive behavioural therapy remains the psychological treatment of choice for BED with evidence for a broad set of efficacy outcomes, relatively low attrition and some evidence on low side effects, and cognitive behavioural therapy was evaluated in the largest number of RCTs in comparison with wait-list. Regarding pharmacological interventions, antidepressants (SSRIs), anticonvulsants (namely topiramate) and anti-obesity drugs (orlistat and sibutramine) are recommended in some but not all the guidelines. However, lisdexamfetamine is the only medication indicated for BED by the US Food and Drug Administration (Fornaro et al., 2016). Nevertheless, for consideration of a range of treatment options, the present findings suggest that all guidelines may consider pharmacological treatments for BED, given the benefits on a wide range of outcomes at the end of treatment. However, there is no evidence regarding the effectiveness of medications at follow-up in people with BED, direct comparisons with psychological interventions are lacking and, compared to placebo, lisdexamfetamine was associated with more adverse events, and topiramate was considered less acceptable. This lack of evidence on sustainability at follow-up may explain why the European Medications Agency (EMA) has not approved any medication for BED, and medications can only be recommended off-label in clinical guidelines.
Remarkably, three findings emerged as transdiagnostic features of treatments for people with EDs and are worth outlining. First, except for long-term effects of family-based treatment, no treatment showed a significant and positive impact for psychopathology and/or behaviours of adolescents with AN or with BN, while for those with BED studies of family-based treatment are missing. Future studies are encouraged to assess treatment effectiveness in adolescents given that most of EDs, except BED, have onset during adolescence (Volpe et al., 2016). Second, long-term treatment effects were found only for psychotherapy interventions, while medications displayed significant effects only at the end of treatment. This finding suggests that maintenance treatment studies with medications are needed. Third, some medications showed either less acceptability or safety than placebo. This finding suggests that clinicians and patients need to be aware of and monitor if/to what degree pharmacological or psychotherapeutic interventions potentially do harm. Overall, these findings may help clinicians with decision making and suggest possible areas for future research.
Limitations of this umbrella review need to be acknowledged. First, we selected network over pairwise meta-analyses, and chose the largest (rather than the highest quality) of the meta-analyses regarding the same population, intervention, control, and outcome. Individual RCTs not pooled in previous MAs were not included. Second, we combined different active control conditions, leading to a loss of granularity regarding potential differences in the efficacy between different active treatment conditions. However, we separated TAU from active control treatments. Third, the number of studies and participants varied across interventions, comparisons, and outcomes. However, for transparency, we also reported how large the body of evidence was for each result. Fourth, fewer data were available for long-term outcomes, which this is a limitation of treatment literature. Fifth, apart from few MAs (M Solmi et al., 2021), many MAs, and most of those with significant findings considered mixed settings of care and did not yield specific recommendations for the inpatient and outpatient levels of care. Sixth, feasibility and acceptability were poorly reported in the included (N)MAs. Seventh, the duration of follow-up data cannot be gleaned from the present findings. Finally, individual patient data meta/analyses are needed to generate fine grained insight on specific clinical subpopulations. Nevertheless, despite these limitations, to our knowledge, this is the most comprehensive and inclusive umbrella review of interventions for people with EDs that can inform clinicians, patients and their families, guideline developers and researchers.
Based in these findings, many gaps remain in the literature. For example, long-term effects and the effectiveness of combined interventions (e.g., the combination of psychotherapy and pharmacotherapy, which is an effective strategy in common mental disorders (Cuijpers et al., 2020)), has been poorly studied or there are, in people with BED (Hilbert et al., 2019), many different combination treatment arms, which prevent detailed comparison. Similarly, second order treatments for those individuals for whom first line treatments fail have been neglected. Moreover, no meta-analytic evidence was found for treatments in people with OSFED. Finally, the cost-effectiveness outcome, required by NICE, has not been largely addressed in ED treatment studies and has thus not been reported in (N)MAs, but this outcome needs to be considered in future studies.
In conclusion, this umbrella review supports the recommendations from most guidelines relating to BN, and extends support of multidimensional short-term treatment efficacy, especially for cognitive behavioural therapy for ED. Our results also confirm the absence of a differential effect across most specialized individual psychological interventions for AN, apart from family-based therapy in adolescents, and interventions involving families show some efficacy in young adults. Importantly, such interventions outperformed active control on a limited number of outcomes. Our findings are partially consistent with current guidelines for BED, supporting the efficacy of psychotherapy (including cognitive behavioural therapy for ED). In addition, short-term efficacy of medications (especially SSRIs and the lisdexamfetamine) may be considered and long-term effects require further study.
Despite the importance of the transdiagnostic perspective in EDs (Fairburn et al., 2003) and in other mental disorders (Fusar-Poli et al., 2019), these findings highlight the importance of not viewing EDs as a monolithic diagnostic entity. Differences across the main ED diagnoses in terms of treatment response need to be considered by researchers and clinicians, while acknowledging some transdiagnostic general psychopathology (Monteleone and Cascino, 2021; Solmi et al., 2018), and risk factors (Marzola et al., 2021a). Future meta-analyses should avoid clustering different populations (namely intervention studies should have more descriptors related to the clinical presentation such as weight status or illness duration), interventions, controls, and outcomes, and novel treatments should be tested in particular for individuals with AN of all ages and for adolescents with all specific EDs.
Supplementary Material
Acknowledgments
JT and US receive salary support from the National Institute of Health Research (NIHR) Mental Health Biomedical Research Centre (BRC) at South London and Maudsley NHS Foundation Trust (SLaM) and King’s College London (KCL). US is also supported by an NIHR Senior Investigator Award. The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the NIHR, or the UK Department of Health. CMB is supported by NIMH (R01MH120170; R01MH124871; R01MH119084; R01MH118278; R01 MH124871); Brain and Behavior Research Foundation Distinguished Investigator Grant; Swedish Research Council (Vetenskapsrådet, award: 538-2013-8864); Lundbeck Foundation (Grant no. R276-2018-4581). FFA is supported by ISCIII (PI17/01167, PI20/132 and CIBERobn is an initiative from ISCIII) and FEDER, by Generalitat de Catalunya-CERCA program and by EU Grants (PRIME 847879, Eat2benice 728018 and COST 19115).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CMB reports: Shire (grant recipient, Scientific Advisory Board member); Idorsia (consultant); Lundbeckfonden (grant recipient); Pearson (author, royalty recipient); Equip Health Inc. (clinical advisory board).
PH receives/has received sessional fees and lecture fees from the Australian Medical Council, Therapeutic Guidelines publication, and New South Wales Institute of Psychiatry and royalties/honoraria from Hogrefe and Huber, McGraw Hill Education, and Blackwell Scientific Publications, Biomed Central and PlosMedicine and she has received research grants from the NHMRC and ARC. She is Chair of the National Eating Disorders Collaboration Steering Committee in Australia (2019-) and was Member of the ICD-11 Working Group for Eating Disorders (2012–2018) and was Chair Clinical Practice Guidelines Project Working Group (Eating Disorders) of RANZCP (2012–2015). She has prepared a report under contract for Takeda (formerly Shire) Pharmaceuticals in regards to Binge Eating Disorder (July 2017) and is a consultant to Takeda Pharmacueticals. All views in this paper are her own.
SZ is involved in editorial duties by Elsevier, Wiley, Thieme and Frontiers; he received several grants from the German Federal Ministry of Education and Research (e.g. 01GV0624)
FFA received consultancy honoraria from Novo Nordisk and editorial honoraria as EIC from Wiley, and he received several national and international Grants (FIS-ISCIII and EU-H2020).The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
AH received: royalties for books on the treatment of binge-eating disorder and obesity with Hogrefe; honoraria for workshops and lectures on binge-eating disorder and obesity and their treatment; honoraria as associate editor of the International Journal of Eating Disorders and Psychotherapeut; honoraria as a reviewer from Mercator Research Center Ruhr, Oxford University Press, and the German Society for Nutrition; and honoraria as a consultant for WeightWatchers and Zweites Deutsches Fernsehen.
CUC has been a consultant and/or advisor to or has received honoraria from AbbVie, Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, Gerson Lehrman Group, IntraCellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, Medscape, Mylan, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Sequirus Sumitomo Dainippon, Sunovion, Supernus, Takeda, Teva and Viatris. He has provided expert testimony for Janssen and Otsuka. He served on a Data Safety Monitoring Board for Lundbeck, Rovi, Supernus, and Teva. He received royalties from UpToDate and grant support from Janssen and Takeda. He is also a stock option holder of LB Pharma.
MS has received honoraria/has been a consultant for Angelini, Lundbeck, unrelated to this work.
Footnotes
Conflicts of interest
Other authors have nothing to declare and report no financial relationships with commercial interests.
References
- Albano G, Hodsoll J, Kan C, Lo Coco G, Cardi V, 2019. Task-sharing interventions for patients with anorexia nervosa or their carers: a systematic evaluation of the literature and meta-analysis of outcomes. Int. Rev. Psychiatry 10.1080/09540261.2019.1588711 [DOI] [PubMed]
- Bacaltchuk Josue, Hay P, Mari JJ, 2000. Antidepressants versus placebo for the treatment of bulimia nervosa: A systematic review, in: Australian and New Zealand Journal of Psychiatry. 10.1046/j.1440-1614.2000.00709.x [DOI] [PubMed] [Google Scholar]
- Bacaltchuk J, Hay PP, 2003. Antidepressants versus placebo for people with bulimia nervosa. Cochrane Database Syst. Rev. 10.1002/14651858.cd003391 [DOI] [PubMed]
- Bacaltchuk J, Trefiglio RP, De Oliveira IR, Lima MS, Mari JJ, 1999. Antidepressants versus psychotherapy for bulimia nervosa: A systematic review. J. Clin. Pharm. Ther 24. 10.1046/j.1365-2710.1999.00192.x [DOI] [PubMed]
- Bacaltchuk Josué, Trefiglio RP, Oliveira IR, Hay P, Lima MS, Mari JJ, 2000. Combination of antidepressants and psychological treatments for bulimia nervosa: A systematic review. Acta Psychiatr. Scand 10.1034/j.1600-0447.2000.101004256.x [DOI] [PubMed]
- Barakat S, Maguire S, Smith KE, Mason TB, Crosby RD, Touyz S, 2019. Evaluating the role of digital intervention design in treatment outcomes and adherence to eTherapy programs for eating disorders: A systematic review and meta-analysis. Int. J. Eat. Disord 10.1002/eat.23131 [DOI] [PubMed]
- Bardone-Cone A, Hunt R, Watson H, 2018. An Overview of Conceptualizations of Eating Disorder Recovery, Recent Findings, and Future Directions. Curr. Psychiatry Rep 20. 10.1007/S11920-018-0932-9 [DOI] [PubMed] [Google Scholar]
- Berkman ND, Brownley KA, Peat CM, Lohr KN, Cullen KE, Morgan LC, Bann CM, Wallace IF, Bulik CM, 2015. Management and outcomes of binge-eating disorder. Comp. Eff. Rev. 160. [PubMed] [Google Scholar]
- Brownley KA, Berkman ND, Peat CM, Lohr KN, Cullen KE, Bann CM, Bulik CM, 2016. Binge-eating disorder in adults a systematic review and meta-analysis. Ann. Intern. Med 10.7326/M15-2455 [DOI] [PMC free article] [PubMed]
- Byrne S, Wade T, Hay P, Touyz S, Fairburn CG, Treasure J, Schmidt U, McIntosh V, Allen K, Fursland A, Crosby RD, 2017. A randomised controlled trial of three psychological treatments for anorexia nervosa. Psychol. Med 47, 2823–2833. 10.1017/S0033291717001349 [DOI] [PubMed] [Google Scholar]
- Cassioli E, Sensi C, Mannucci E, Ricca V, Rotella F, 2020. Pharmacological treatment of acute-phase anorexia nervosa: Evidence from randomized controlled trials. J. Psychopharmacol 34, 864–873. 10.1177/0269881120920453 [DOI] [PubMed] [Google Scholar]
- Claudino AM, Silva de Lima M, Hay PP, Bacaltchuk J, Schmidt UU, Treasure J, 2006. Antidepressants for anorexia nervosa. Cochrane Database Syst. Rev. 10.1002/14651858.cd004365.pub2 [DOI] [PubMed]
- Correll CU, Cortese S, Croatto G, Monaco F, Krinitski D, Arrondo G, Ostinelli EG, Zangani C, Fornaro M, Estradé A, Fusar-Poli P, Carvalho AF, Solmi M, 2021. Efficacy and acceptability of pharmacological, psychosocial, and brain stimulation interventions in children and adolescents with mental disorders: an umbrella review. World Psychiatry 20, 244–275. 10.1002/wps.20881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CU, Rubio JM, Inczedy-Farkas G, Birnbaum ML, Kane JM, Leucht S, 2017. Efficacy of 42 pharmacologic cotreatment strategies added to antipsychotic monotherapy in schizophrenia: Systematic overview and quality appraisal of the meta-analytic evidence. JAMA Psychiatry 74, 675–684. 10.1001/jamapsychiatry.2017.0624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couturier J, Kimber M, Szatmari P, 2013. Efficacy of family-based treatment for adolescents with eating disorders: a systematic review and meta-analysis. Int. J. Eat. Disord 46, 3–11. 10.1002/eat.22042 [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Donker T, Weissman MM, Ravitz P, Cristea IA, 2016. Interpersonal psychotherapy for mental health problems: A comprehensive meta-analysis. Am. J. Psychiatry 173. 10.1176/appi.ajp.2015.15091141 [DOI] [PubMed]
- Cuijpers P, Noma H, Karyotaki E, Vinkers CH, Cipriani A, Furukawa TA, 2020. A network meta-analysis of the effects of psychotherapies, pharmacotherapies and their combination in the treatment of adult depression. World Psychiatry 19, 92–107. 10.1002/WPS.20701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos J, Houtzager L, Katsaragaki G, van de Berg E, Cuijpers P, Dekker J, 2014. Meta analysis on the efficacy of pharmacotherapy versus placebo on anorexia nervosa. J. Eat. Disord 2, 27. 10.1186/s40337-014-0027-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dold M, Aigner M, Klabunde M, Treasure J, Kasper S, 2015. Second-generation antipsychotic drugs in anorexia nervosa: A meta-analysis of randomized controlled trials. Psychother. Psychosom 84. 10.1159/000369978 [DOI] [PubMed] [Google Scholar]
- Eddy KT, Tabri N, Thomas JJ, Murray HB, Keshaviah A, Hastings E, Edkins K, Krishna M, Herzog DB, Keel PK, Franko DL, 2017. Recovery From Anorexia Nervosa and Bulimia Nervosa at 22-Year Follow-Up. J. Clin. Psychiatry 78, 184–189. 10.4088/JCP.15M10393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairburn CG, Cooper Z, Shafran R, 2003. Cognitive behaviour therapy for eating disorders: a “transdiagnostic” theory and treatment. Behav. Res. Ther 41, 509–528. 10.1016/S0005-7967(02)00088-8 [DOI] [PubMed] [Google Scholar]
- Fisher CA, Hetrick SE, Rushford N, 2010. Family therapy for anorexia nervosa. Cochrane Database Syst. Rev. 10.1002/14651858.CD004780.pub2 [DOI] [PubMed]
- Fisher CA, Skocic S, Rutherford KA, Hetrick SE, 2019. Family therapy approaches for anorexia nervosa. Cochrane database Syst. Rev 5, CD004780. 10.1002/14651858.CD004780.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CA, Skocic S, Rutherford KA, Hetrick SE, 2018. Family therapy approaches for anorexia nervosa. Cochrane Database Syst. Rev 10.1002/14651858.CD004780.pub3 [DOI] [PMC free article] [PubMed]
- Fornaro M, Solmi M, Perna G, De Berardis D, Veronese N, Orsolini L, Ganança L, Stubbs B, 2016. Lisdexamfetamine in the treatment of moderate-to-severe binge eating disorder in adults: Systematic review and exploratory meta-analysis of publicly available placebo-controlled, randomized clinical trials. Neuropsychiatr. Dis. Treat 12. 10.2147/NDT.S109637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Solmi M, Brondino N, Davies C, Chae C, Politi P, Borgwardt S, Lawrie SM, Parnas J, McGuire P, 2019. Transdiagnostic psychiatry: a systematic review. World Psychiatry 18, 192–207. 10.1002/WPS.20631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaderi A, Andersson G, 1999. Meta-analysis of CBT for Bulimia Nervosa: Investigating the Effects Using DSM-III-R and DSM-IV Criteria. Scand. J. Behav. Ther 28. 10.1080/028457199440034 [DOI] [Google Scholar]
- Ghaderi A, Odeberg J, Gustafsson S, Råstam M, Brolund A, Pettersson A, Parling T, 2018. Psychological, pharmacological, and combined treatments for binge eating disorder: a systematic review and meta-analysis. PeerJ 6, e5113. 10.7717/peerj.5113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregertsen E, Mandy W, Kanakam N, Armstrong S, Serpell L, 2019. Pre-treatment patient characteristics as predictors of drop-out and treatment outcome in individual and family therapy for adolescents and adults with anorexia nervosa: A systematic review and meta-analysis. Psychiatry Res. 271, 484–501. 10.1016/J.PSYCHRES.2018.11.068 [DOI] [PubMed] [Google Scholar]
- Grenon R, Carlucci S, Brugnera A, Schwartze D, Hammond N, Ivanova I, Mcquaid N, Proulx G, Tasca GA, 2018a. Psychotherapy for eating disorders: A meta-analysis of direct comparisons. Psychother. Res 1–13. 10.1080/10503307.2018.1489162 [DOI] [PubMed] [Google Scholar]
- Grenon R, Carlucci S, Brugnera A, Schwartze D, Hammond N, Ivanova I, Mcquaid N, Proulx G, Tasca GA, 2018b. Psychotherapy for eating disorders: A meta-analysis of direct comparisons. Psychother. Res 1–13. 10.1080/10503307.2018.1489162 [DOI] [PubMed] [Google Scholar]
- Hagan KE, Christensen KA, Forbush KT, 2020. A preliminary systematic review and meta-analysis of randomized-controlled trials of cognitive remediation therapy for anorexia nervosa. Eat. Behav 10.1016/j.eatbeh.2020.101391 [DOI] [PMC free article] [PubMed]
- Hasselbalch KC, Lanng KR, Birkeland M, Sjögren M, 2020. Potential shortcomings in current studies on the effect of intranasal oxytocin in Anorexia Nervosa and healthy controls - A systematic review and meta-analysis. Psychopharmacology (Berl). 237. 10.1007/s00213-020-05626-5 [DOI] [PubMed] [Google Scholar]
- Hay PJ, Claudino AM, Touyz S, Abd Elbaky G, 2015. Individual psychological therapy in the outpatient treatment of adults with anorexia nervosa. Cochrane Database Syst. Rev 10.1002/14651858.CD003909.pub2 [DOI] [PMC free article] [PubMed]
- Hay PJ, Touyz S, Claudino AM, Lujic S, Smith CA, Madden S, 2019. Inpatient versus outpatient care, partial hospitalisation and waiting list for people with eating disorders. Cochrane Database Syst. Rev 10.1002/14651858.CD010827.pub2 [DOI] [PMC free article] [PubMed]
- Hay PP, Bacaltchuk J, Stefano S, 2004. Psychotherapy for bulimia nervosa and binging, in: Cochrane Database of Systematic Reviews. 10.1002/14651858.cd000562.pub2 [DOI] [PubMed]
- Hay PP, Claudino AM, Kaio MH, 2001. Antidepressants versus psychological treatments and their combination for bulimia nervosa. Cochrane Database Syst. Rev 10.1002/14651858.cd003385 [DOI] [PMC free article] [PubMed]
- Hay PPJ, Bacaltchuk J, Stefano S, Kashyap P, 2009. Psychological treatments for bulimia nervosa and binging. Cochrane Database Syst. Rev 10.1002/14651858.CD000562.pub3 [DOI] [PMC free article] [PubMed]
- Hilbert A, Hoek H, Schmidt R, 2017. Evidence-based clinical guidelines for eating disorders: international comparison. Curr. Opin. Psychiatry 30, 423–437. 10.1097/YCO.0000000000000360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbert A, Petroff D, Herpertz S, Pietrowsky R, Tuschen-Caffier B, Vocks S, Schmidt R, 2019. Meta-Analysis of the Efficacy of Psychological and Medical Treatments for Binge-Eating Disorder. J. Consult. Clin. Psychol 87. 10.1037/ccp0000358 [DOI] [PubMed] [Google Scholar]
- Kishi T, Kafantaris V, Sunday S, Sheridan EM, Correll CU, 2012. Are antipsychotics effective for the treatment of anorexia nervosa? Results from a systematic review and meta-analysis. J. Clin. Psychiatry 73. 10.4088/JCP.12r07691 [DOI] [PubMed] [Google Scholar]
- Lebow J, Sim LA, Erwin PJ, Murad MH, 2013. The effect of atypical antipsychotic medications in individuals with anorexia nervosa: A systematic review and meta-analysis. Int. J. Eat. Disord 10.1002/eat.22059 [DOI] [PubMed]
- Leucht S, Chaimani A, Cipriani A, Davis J, Furukawa TA, Salanti G, 2016. Network meta-analyses should be the highest level of evidence in treatment guidelines. Eur. Arch. Psychiatry Clin. Neurosci 266, 477–480. 10.1007/S00406-016-0715-4 [DOI] [PubMed] [Google Scholar]
- Lilienfeld SO, 2019. What is “evidence” in psychotherapies? World Psychiatry 18, 245–246. 10.1002/wps.20654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linardon J, 2018a. Rates of abstinence following psychological or behavioral treatments for binge-eating disorder: Meta-analysis. Int. J. Eat. Disord 51, 785–797. 10.1002/EAT.22897 [DOI] [PubMed] [Google Scholar]
- Linardon J, 2018b. Meta-analysis of the effects of cognitive-behavioral therapy on the core eating disorder maintaining mechanisms: implications for mechanisms of therapeutic change. Cogn. Behav. Ther 10.1080/16506073.2018.1427785 [DOI] [PubMed]
- Linardon J, Fairburn CG, Fitzsimmons-Craft EE, Wilfley DE, Brennan L, 2017a. The empirical status of the third-wave behaviour therapies for the treatment of eating disorders: A systematic review. Clin. Psychol. Rev 58, 125–140. 10.1016/J.CPR.2017.10.005 [DOI] [PubMed] [Google Scholar]
- Linardon J, Kothe EJ, Fuller-Tyszkiewicz M, 2019. Efficacy of psychotherapy for bulimia nervosa and binge-eating disorder on self-esteem improvement: Meta-analysis. Eur. Eat. Disord. Rev 10.1002/erv.2662 [DOI] [PubMed]
- Linardon J, Shatte A, Messer M, Firth J, Fuller-Tyszkiewicz M, 2020. E-mental health interventions for the treatment and prevention of eating disorders: An updated systematic review and meta-analysis. J. Consult. Clin. Psychol 88. 10.1037/ccp0000575 [DOI] [PubMed] [Google Scholar]
- Linardon J, Wade T, de la Piedad Garcia X, Brennan L, 2017b. Psychotherapy for bulimia nervosa on symptoms of depression: A meta-analysis of randomized controlled trials. Int. J. Eat. Disord 10.1002/eat.22763 [DOI] [PubMed]
- Linardon J, Wade TD, de la Piedad Garcia X, Brennan L, 2017c. The efficacy of cognitive-behavioral therapy for eating disorders: A systematic review and meta-analysis. J. Consult. Clin. Psychol 85, 1080–1094. 10.1037/ccp0000245 [DOI] [PubMed] [Google Scholar]
- Loucas CE, Fairburn CG, Whittington C, Pennant ME, Stockton S, Kendall T, 2014. E-therapy in the treatment and prevention of eating disorders: A systematic review and meta-analysis. Behav. Res. Ther 63. 10.1016/j.brat.2014.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low TL, Ho R, Ho C, Tam W, 2021. The efficacy of virtual reality in the treatment of binge-purging eating disorders: A meta-analysis. Eur. Eat. Disord. Rev 29. 10.1002/erv.2804 [DOI] [PubMed] [Google Scholar]
- Machado GC, Ferreira ML, 2014. Physiotherapy improves eating disorders and quality of life in bulimia and anorexia nervosa. Br. J. Sports Med 10.1136/bjsports-2014-094088 [DOI] [PubMed]
- Marzola E, Cavallo F, Panero M, Porliod A, Amodeo L, Abbate-Daga G, 2021a. The role of prenatal and perinatal factors in eating disorders: a systematic review. Arch. Womens. Ment. Health 24, 185–204. 10.1007/s00737-020-01057-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzola E, Longo P, Sardella F, Delsedime N, Abbate-Daga G, 2021b. Rehospitalization and “Revolving Door” in Anorexia Nervosa: Are There Any Predictors of Time to Readmission? Front. Psychiatry 12, 1088. 10.3389/fpsyt.2021.694223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone AM, Cascino G, 2021. A systematic review of network analysis studies in eating disorders: Is time to broaden the core psychopathology to non specific symptoms. Eur. Eat. Disord. Rev 29, 531–547. 10.1002/ERV.2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone AM, Fernandez-Aranda F, Voderholzer U, 2019. Evidence and perspectives in eating disorders: a paradigm for a multidisciplinary approach. World Psychiatry 18, 369–370. 10.1002/WPS.20687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray SB, Loeb KL, Le Grange D, 2018. Treatment outcome reporting in anorexia nervosa: Time for a paradigm shift? J. Eat. Disord 10.1186/s40337-018-0195-1 [DOI] [PMC free article] [PubMed]
- Murray SB, Quintana DS, Loeb KL, Griffiths S, Le Grange D, 2019. Treatment outcomes for anorexia nervosa: A systematic review and meta-analysis of randomized controlled trials. Psychol. Med 10.1017/S0033291718002088 [DOI] [PubMed]
- Nakash-Eisikovits O, Dierberger A, Westen D, 2002. A multidimensional meta-analysis of pharmacotherapy for bulimia nervosa: Summarizing the range of outcomes in controlled clinical trials. Harv. Rev. Psychiatry 10.1080/10673220216226 [DOI] [PubMed]
- National Guideline Alliance U, 2017. Eating disorders: recognition and treatment, Eating disorders: recognition and treatment. London. [Google Scholar]
- Ng LWC, Ng DP, Wong WP, 2013. Is supervised exercise training safe in patients with anorexia nervosa? A meta-analysis. Physiother. (United Kingdom). 10.1016/j.physio.2012.05.006 [DOI] [PubMed]
- Nourredine M, Jurek L, Auffret M, Iceta S, Grenet G, Kassai B, Cucherat M, Rolland B, 2020. Efficency and safety of topiramate in binge eating disorder: A systematic review and meta-analysis. CNS Spectr. 10.1017/S1092852920001613 [DOI] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D, 2021. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed]
- Palavras MA, Hay P, Filho CA dos S, Claudino A, 2017. The efficacy of psychological therapies in reducing weight and binge eating in people with bulimia nervosa and binge eating disorder who are overweight or obese—A critical synthesis and meta‐analyses. Nutrients. 10.3390/nu9030299 [DOI] [PMC free article] [PubMed]
- Peat CM, Berkman ND, Lohr KN, Brownley KA, Bann CM, Cullen K, Quattlebaum MJ, Bulik CM, 2017. Comparative Effectiveness of Treatments for Binge-Eating Disorder: Systematic Review and Network Meta-Analysis. Eur. Eat. Disord. Rev 10.1002/erv.2517 [DOI] [PubMed]
- Perkins SSJ, Murphy RR, Schmidt UU, Williams C, 2006. Self-help and guided self-help for eating disorders. Cochrane Database Syst. Rev 10.1002/14651858.cd004191.pub2 [DOI] [PubMed]
- Polnay A, James VAW, Hodges L, Murray GD, Munro C, Lawrie SM, 2014. Group therapy for people with bulimia nervosa: Systematic review and meta-analysis. Psychol. Med 10.1017/S0033291713002791 [DOI] [PubMed]
- Reas DL, Grilo CM, 2008. Review and meta-analysis of pharmacotherapy for binge-eating disorder. Obesity. 10.1038/oby.2008.333 [DOI] [PMC free article] [PubMed]
- Schmidt U, Ryan E, Bartholdy S, Renwick B, Keyes A, O’Hara C, McClelland J, Lose A, Kenyon M, Dejong H, Broadbent H, Loomes R, Serpell L, Richards L, Johnson-Sabine E, Boughton N, Whitehead L, Bonin E, Beecham J, Landau S, Treasure J, 2016. Two-year follow-up of the MOSAIC trial: A multicenter randomized controlled trial comparing two psychological treatments in adult outpatients with broadly defined anorexia nervosa. Int. J. Eat. Disord 49, 793–800. 10.1002/EAT.22523 [DOI] [PubMed] [Google Scholar]
- Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, Porter AC, Tugwell P, Moher D, Bouter LM, 2007. Development of AMSTAR: A measurement tool to assess the methodological quality of systematic reviews. BMC Med. Res. Methodol 7. 10.1186/1471-2288-7-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade E, Keeney E, Mavranezouli I, Dias S, Fou L, Stockton S, Saxon L, Waller G, Turner H, Serpell L, Fairburn CG, Kendall T, 2018. Treatments for bulimia nervosa: a network meta-analysis. Psychol. Med 48, 2629–2636. 10.1017/S0033291718001071 [DOI] [PubMed] [Google Scholar]
- Slade Eric, Keeney E, Mavranezouli I, Dias S, Fou L, Stockton S, Saxon L, Waller G, Turner H, Serpell L, Fairburn CG, Kendall T, 2018. Treatments for bulimia nervosa: a network meta-analysis. Psychol. Med 48, 2629–2636. https://doi.org/DOI: 10.1017/S0033291718001071 [DOI] [PubMed] [Google Scholar]
- Solmi M, Collantoni E, Meneguzzo P, Degortes D, Tenconi E, Favaro A, 2018. Network analysis of specific psychopathology and psychiatric symptoms in patients with eating disorders. Int. J. Eat. Disord 51, 680–692. 10.1002/eat.22884 [DOI] [PubMed] [Google Scholar]
- Solmi M, Fornaro M, Ostinelli EG, Zangani C, Croatto G, Monaco F, Krinitski D, Fusar‐Poli P, Correll CU, 2020. Safety of 80 antidepressants, antipsychotics, anti‐attention‐deficit/hyperactivity medications and mood stabilizers in children and adolescents with psychiatric disorders: a large scale systematic meta‐review of 78 adverse effects. World Psychiatry 19, 214–232. 10.1002/wps.20765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solmi Marco, Radua J, Olivola M, Croce E, Soardo L, Salazar de Pablo G, Il Shin J, Kirkbride JB, Jones P, Kim JH, Kim JY, Carvalho AF, Seeman MV, Correll CU, Fusar-Poli P, 2021. Age at onset of mental disorders worldwide: large-scale meta-analysis of 192 epidemiological studies. Mol. Psychiatry 10.1038/s41380-021-01161-7 [DOI] [PMC free article] [PubMed]
- Solmi M, Wade TD, Byrne S, Giovane C. Del Fairburn CG, Ostinelli, Crescenzo F, De Johnson C, Schmidt U, Treasure J, Favaro A, Zipfel S, Cipriani A, 2021. Comparative efficacy and acceptability of psychological interventions for the treatment of adult outpatients with anorexia nervosa: a systematic review and network meta-analysis. The Lancet Psychiatry 8, 215–224. 10.1016/S2215-0366(20)30566-6 [DOI] [PubMed] [Google Scholar]
- Stefano SC, Bacaltchuk J, Blay SL, Appolinário JC, 2008. Antidepressants in short-term treatment of binge eating disorder: Systematic review and meta-analysis. Eat. Behav 10.1016/j.eatbeh.2007.03.006 [DOI] [PubMed]
- Stefano SC, Bacaltchuk J, Blay SL, Hay P, 2006. Self-help treatments for disorders of recurrent binge eating: A systematic review. Acta Psychiatr. Scand 10.1111/j.1600-0447.2005.00735.x [DOI] [PubMed]
- Steinhausen H-C, 2002. The Outcome of Anorexia Nervosa in the 20th Century. Am. J. Psychiatry 159, 1284–1293. 10.1176/appi.ajp.159.8.1284 [DOI] [PubMed] [Google Scholar]
- Steinhausen HC, Weber S, 2009. The outcome of bulimia nervosa: Findings from one-quarter century of research. Am. J. Psychiatry 10.1176/appi.ajp.2009.09040582 [DOI] [PubMed]
- Svaldi J, Schmitz F, Baur J, Hartmann AS, Legenbauer T, Thaler C, Von Wietersheim J, De Zwaan M, Tuschen-Caffier B, 2019. Efficacy of psychotherapies and pharmacotherapies for Bulimia nervosa. Psychol. Med 10.1017/S0033291718003525 [DOI] [PubMed]
- Swift JK, Greenberg RP, Tompkins KA, Parkin SR, 2017. Treatment refusal and premature termination in psychotherapy, pharmacotherapy, and their combination: A meta-analysis of head-to-head comparisons. Psychotherapy 54. 10.1037/pst0000104 [DOI] [PubMed] [Google Scholar]
- Thompson-Brenner H, Glass S, Westen D, 2003. A multidimensional meta-analysis of psychotherapy for bulimia nervosa. Clin. Psychol. Sci. Pract 10. 10.1093/clipsy/bpg024 [DOI] [Google Scholar]
- Tortorella A, Fabrazzo M, Monteleone AM, Steardo L, Monteleone P, 2014. The role of drug therapies in the treatment of anorexia and bulimia nervosa: A review of the literature. J. Psychopathol 20. [Google Scholar]
- Traviss-Turner GD, West RM, Hill AJ, 2017. Guided Self-help for Eating Disorders: A Systematic Review and Metaregression. Eur. Eat. Disord. Rev 25, 148–164. 10.1002/erv.2507 [DOI] [PubMed] [Google Scholar]
- Treasure J, Duarte TA, Schmidt U, 2020. Eating disorders. Lancet. 10.1016/S0140-6736(20)30059-3 [DOI] [PubMed]
- van den Berg E, Houtzager L, de Vos J, Daemen I, Katsaragaki G, Karyotaki E, Cuijpers P, Dekker J, 2019. Meta-analysis on the efficacy of psychological treatments for anorexia nervosa. Eur. Eat. Disord. Rev 27, 331–351. 10.1002/erv.2683 [DOI] [PubMed] [Google Scholar]
- Vocks S, Tuschen-Caffier B, Pietrowsky R, Rustenbach SJ, Kersting A, Herpertz S, 2010. Meta-analysis of the effectiveness of psychological and pharmacological treatments for binge eating disorder. Int. J. Eat. Disord 10.1002/eat.20696 [DOI] [PubMed]
- Voderholzer U, Haas V, Correll C, Körner T, 2020. Medical management of eating disorders: an update. Curr. Opin. Psychiatry 33, 542–553. 10.1097/YCO.0000000000000653 [DOI] [PubMed] [Google Scholar]
- Volpe U, Tortorella A, Manchia M, Monteleone AM, Albert U, Monteleone P, 2016. Eating disorders: What age at onset? Psychiatry Res. 238. 10.1016/j.psychres.2016.02.048 [DOI] [PubMed] [Google Scholar]
- Zeeck A, Herpertz-Dahlmann B, Friederich HC, Brockmeyer T, Resmark G, Hagenah U, Ehrlich S, Cuntz U, Zipfel S, Hartmann A, 2018. Psychotherapeutic treatment for anorexia nervosa: A systematic review and network meta-analysis. Front. Psychiatry 10.3389/fpsyt.2018.00158 [DOI] [PMC free article] [PubMed]
- Zipfel S, Giel KE, Bulik CM, Hay P, Schmidt U, 2015. Anorexia nervosa: aetiology, assessment, and treatment. The Lancet Psychiatry 2, 1099–1111. 10.1016/S2215-0366(15)00356-9 [DOI] [PubMed] [Google Scholar]
- Zipfel S, Wild B, Groß G, Friederich H-C, Teufel M, Schellberg D, Giel KE, de Zwaan M, Dinkel A, Herpertz S, Burgmer M, Löwe B, Tagay S, von Wietersheim J, Zeeck A, Schade-Brittinger C, Schauenburg H, Herzog W, ANTOP study group, 2014. Focal psychodynamic therapy, cognitive behaviour therapy, and optimised treatment as usual in outpatients with anorexia nervosa (ANTOP study): randomised controlled trial. Lancet 383, 127–137. 10.1016/S0140-6736(13)61746-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.