Abstract
DNA vaccines encoding the outer surface protein A (OspA) of Borrelia burgdorferi have been shown to induce protective humoral responses capable of preventing but not curing infection in mice. Subsequent studies showed that an established infection or disease could be resolved by passive transfer of antibodies to OspC. In the present study, DNA vaccines encoding either the OspC antigen alone or fused to OspA and under the transcriptional control of the human elongation factor 1α promoter were evaluated for their protective and/or curative potential. In contrast to ospA-containing plasmids, none of the six constructs with ospC alone were immunogenic in vivo, independent of whether they contained promoter or leader sequences from ospA and/or ospC, or alternatively, the signal sequence of the human tissue plasminogen activator. Solely, a DNA vaccine encoding an OspA-OspC fusion product led to expression of the respective polypeptide chain in transfected cells in vitro and to the induction of OspA- and OspC-specific antibodies in vivo. Immune sera raised against the OspA-OspC fusion product conveyed full protection against subsequent infection, most probably via OspA-specific antibodies, but were unable to resolve infection.
Lyme disease, a progressive inflammatory disorder with dermal, cardiac, musculoskeletal, and neurological manifestations, is the most common vector-borne disease in Europe and the United States (22). The multisystem disease is caused by the spirochete Borrelia burgdorferi, which is transmitted by infected Ixodes ticks (1, 3, 23). Because of the high risk of the population in areas of endemicity of acquiring Lyme disease and the multiple problems concerning diagnosis and therapy of this infection, emphasis has been laid on the development of a vaccine. A formulation consisting of recombinant lipidated OspA (rec.OspA) of B. burgdorferi (strain ZS7) was shown to induce protective antibodies (Ab) in mice (21) and humans and constitutes the basis of the first efficacious vaccine against Lyme disease (24).
Aside from conventional whole-cell lysate or protein-based vaccination protocols, a new technology, termed genetic vaccination, is rapidly evolving (25, 32). In this line, we and others have shown that vaccination with ospA-containing plasmids elicited Ab responses able to prevent subsequent infection (12, 20, 35). Similarly, immunization of mice with OspC-encoding DNA vaccines resulted in the production of specific Ab in mice, however, with unknown protective potential (28).
OspA-specific immune serum (IS) failed to treat an established B. burgdorferi infection, since ospA is expressed by spirochetes only in the vector but not in the reservoir hosts (4, 15, 18, 33). In the latter environment, B. burgdorferi express a variety of Osps distinct from OspA and including OspC, OspE, OspF, and pG, which may thus function as targets for Ab-mediated immunoprophylaxis and/or therapy (5, 6, 15, 27, 33). In fact, active immunization of gerbils and mice with rec.OspC conveyed complete protection to subsequent challenge with homologous B. burgdorferi isolates (2, 8, 16, 17, 34). Moreover, passive transfer of OspC-specific Ab was shown to cure established arthritis and carditis in mice and to eradicate spirochetes (33, 34).
In an attempt to develop a combined vaccine that would meet the requirements for prophylactic and therapeutic treatment of Lyme disease, we have now generated DNA vaccines encoding OspC alone or in combination with OspA under the control of various prokaryotic and/or eukaryotic regulatory sequences. The present report describes their expression profiles in vitro and their immunogenicities and protective potentials in vivo.
MATERIALS AND METHODS
Mice and immunization protocols.
BALB/c and C.B-17 SCID mice were bred under specific-pathogen-free conditions at the Max-Planck-Institute for Immunobiology, Freiburg, Germany. For the generation of IS female mice were injected intramuscularly with 50 μg of a plasmid DNA in 100 μl of phosphate-buffered saline. Plasmid DNA was administered repeatedly into the tibialis anterior muscles at various doses at 10-day intervals, and sera were collected 7 to 10 days after the third injection. OspA- and OspC-specific IS of three immunized animals were collected and analyzed.
Cell lines.
The human hepatoma cell line HepG2 cells (ATCC HB-8065) and two BALB/c-derived cell lines, the B-lymphoma K46 (9) and the immortalized dendritic cell D2SC/1 (13), were used for transfection experiments. All cell lines were cultured at 37°C with 7% CO2 in Dulbecco's modified Eagle medium supplemented with 2 mM glutamine, 50 μM β-mercaptoethanol, and 10% fetal calf serum.
Construction and purification of plasmids.
Large-scale purification of expression vectors was conducted using the EndoFree Plasmid Mega Kit (Qiagen GmbH, Hilden, Germany) according to the protocol of the manufacturer. Purified plasmid DNA used in this study (Fig. 1) was adjusted to a final concentration of 1 mg/ml.
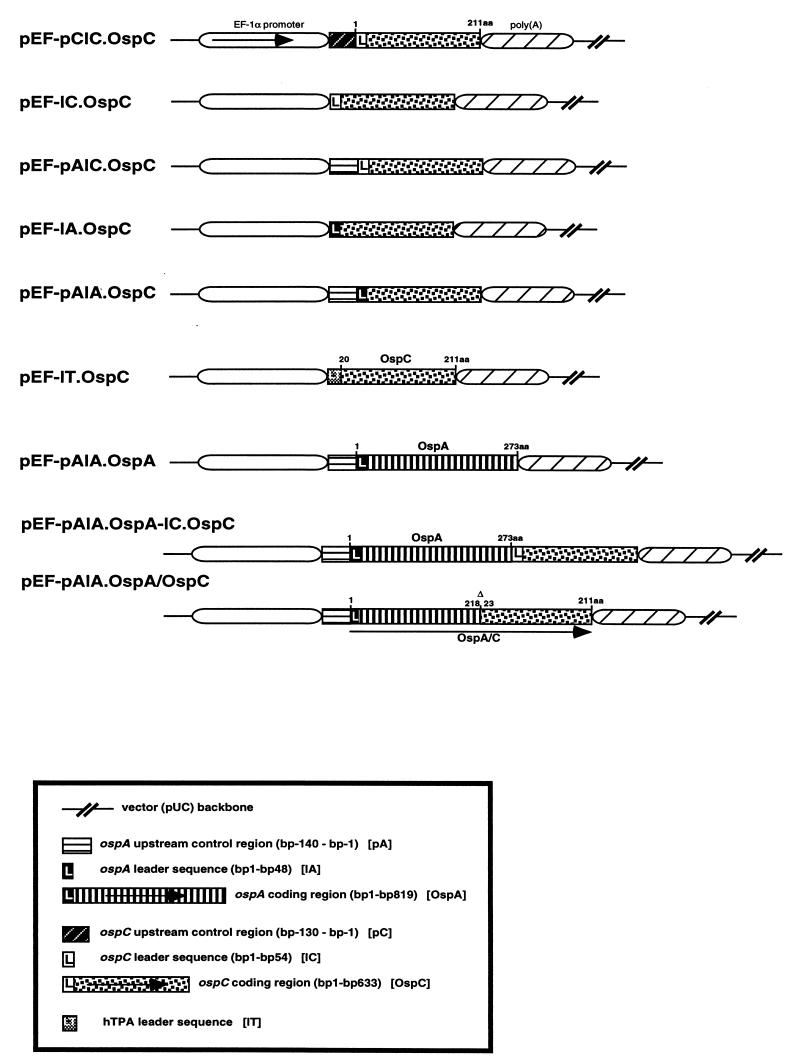
FIG. 1.
DNA vaccine constructs containing ospC and/or ospA used for transfection studies and vaccination of mice. The indicated ospC and ospA regions were inserted into the BstXI site of pEF-Bos vector, which contains the promoter-enhancer region of the human EF-1α chromosomal gene (14).
Plasmid pEF-pCIC.OspC contains ospC from B. burgdorferi strain ZS7, including the OspC leader sequence (lC) and 130 bp of the immediate upstream promoter region (pC) cloned into the BstXI site of pEF-Bos, which contains the promoter-enhancer region of the human elongation factor 1α (EF-1α) gene (14). Plasmid pEF-pAlC.OspC harbors the same ospC with the promoter region replaced by a 140-bp fragment of the ospA promoter region (pA) (20), and in plasmid pEF-lC.OspC the ospC promoter region was deleted. The same ospC fragment was cloned into plasmid pEF-pAlA.OspC and was combined with the ospA leader sequence and promoter region (pAlA). pEF-lT.OspC was generated in a similar way, with the exception that the leader (amino acids [aa] 1 to 24) of the human tissue-type plasminogen activator (hTPA) was fused to ospC. Plasmid pEF-pAlA.OspA-lC.OspC was generated from pEF-pAlA.OspA by cloning ospC, including its natural leader sequence, immediately downstream from ospA. pEF-pAlA.OspA/OspC represents a chimeric construct based on the plasmid pEF-pAlA.OspA in which the ospC coding region (amino acids 23 to 211) was cloned in frame with ospA at aa 218. DNA fragments cloned in pEF-BOS were sequenced in accordance with the manufacturer's recommendation by using a T7 sequencing kit (Pharmacia). Plasmids were propagated in Escherichia coli strain DH5α (GIBCO BRL, Eggenstein, Germany).
Transfection and Northern blot analysis.
Cells were propagated and transfected by electroporation as described (20) except that 25 μg of plasmid DNA was used. In brief, pulse settings were 960 μF and 280 V for K46 and HepG2 cells and 420 V for D2SC/1 cells. After 24 h, cells were solubilized at 4°C by incubation with lysis buffer (10 mM Tris-HCl [pH 7.5], 0.5% Triton X-100, 0.15 M NaCl). The Triton-soluble fraction was separated from the Triton-insoluble fraction by centrifugation (12,000 × g for 5 min at 4°C). Culture supernatants and Triton-soluble and -insoluble fractions of the transfected cells were analyzed for OspA and OspC protein expression.
Total RNA was extracted by lysing the cells in 0.1% NP-40, followed by phenol extraction. Northern blot analysis of RNA from transfected cells was performed by standard procedures using radiolabeled ospC or ospA probes.
Immunoblot analysis.
B. burgdorferi antigens were separated by discontinuous sodium dodecyl sulfate–12.5% polyacrylamide gel electrophoresis and transferred to nitrocellulose or polyvinylidene difluoride membranes. For the detection of Osp-specific Ab in DNA-vaccinated mice, the in situ reaction using 5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium was applied according to published protocols (19).
For the detection of OspA and OspC in transfected eukaryotic cells a highly sensitive alkaline phosphatase-based chemiluminescence method (Phototope Star Western blot detection kit; New England Biolabs, Beverly, Mass.) was applied according the recommendations of the manufacturer. High-titered OspA IS (1:10,000) or OspA-specific monoclonal Ab (MAb) LA-31.1 and OspC-specific rabbit IS (1:10,000) or MAb LA97.1 were employed (10).
Absorption of IS.
IS generated to pEF-pAlA.OspA/OspC were passed over columns with recombinant OspA bound to Sepharose 4B (Pharmacia). Lipidated OspA was conjugated to Sepharose 4B beads as follows. Five milligrams of OspA in 5 ml of 0.1 M NaHCO3 (pH 8.3)–0.5 M NaCl was added to 2.5 ml of CNBr-activated Sepharose 4B that had been washed in 1 mM HCl. The solution was mixed for 2 h at 22°C and then washed with NaHCO3-NaCl buffer. The remaining active groups on the beads were blocked by incubation for 2 h in a solution of 0.2 M glycine (pH 8.0). The final product was then washed three times in alternating cycles of low-pH buffer (0.1 M CH3COONa, 0.5 M NaCl [pH 4.0]) and high-pH buffer (0.1 M Tris-HCl, 0.5 M NaCl [pH 8.0]). After the coupling process, 1 ml of mouse serum was added directly to 500 μl of OspA-conjugated beads and to Sepharose 4B beads that were not coupled to a ligand. The suspensions were incubated for 2 h at 22°C, and the beads were then pelleted at 500 × g and removed. The adsorption was repeated three times.
Determination of Ab titers by ELISA.
OspA- and OspC-specific Ab titers induced in DNA-vaccinated mice were determined by solid-phase enzyme-linked immunosorbent assay employing rec.OspA- or rec.OspC-coated microplates as previously described (34). Absorbance values were converted into Ab titers (micrograms of immunoglobulin per milliliter of serum) using calibration curves with standardized amounts of OspA- and OspC-specific MAb (LA-2 and LA-97.1, respectively) and the four-parameter method.
Passive transfer of IS.
For passive protection, C.B.-17 SCID mice were injected intraperitoneally with polyclonal IS specific for the indicated plasmid DNA 1 h before challenge with 103 B. burgdorferi organisms (ZS7) (34). Control mice received serum from mice immunized with either the control plasmid pEF-Bos or rec.OspC or were left untreated. In one experiment, IS specific for pEF-pAlA.OspA/OspC previously adsorbed on OspA-Sepharose 4B was used. For passive treatment of established infection, C.B.-17 SCID mice were infected subcutaneously followed by repeated injections (four times at 3- to 4-day intervals) of various amounts of the indicated polyclonal IS, starting at day 29 postinfection (p.i.). Animals were monitored for the development of clinical arthritis in the tibiotarsal joints under double-blinded conditions. The severity of arthritis was scored in the right and left tibiotarsal joint as described previously (34). At indicated time points, mice were investigated for the presence of spirochetes by cultivation of ear biopsy specimens, as described previously (34).
RESULTS AND DISCUSSION
DNA vaccines encoding OspC were designed to analyze the effect of various regulatory elements on ospC expression in vitro and on their immunogenicity in vivo (Fig. 1). The eukaryotic expression vector pEF-Bos contained ospC in the presence of the promoter (p) and/or leader (1) sequences of (i) either ospC (pClC; lC) or ospA (pAlA; lA) or combinations thereof (pAlC) or (ii) the leader sequence (1T) derived from the hTPA. Furthermore, pEF-Bos-based plasmids were constructed to carry both ospA and ospC, either fused in frame (pEF-pAlAOspA/OspC) or arranged in tandem (pEF-pAlA.OspA-lC.OspC). For the control, a plasmid DNA containing ospA under the control of its natural signal sequence (pEF-pAlA.OspA) was employed (20).
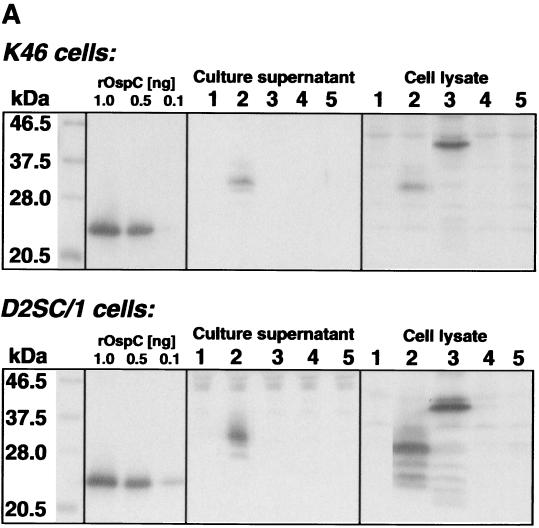
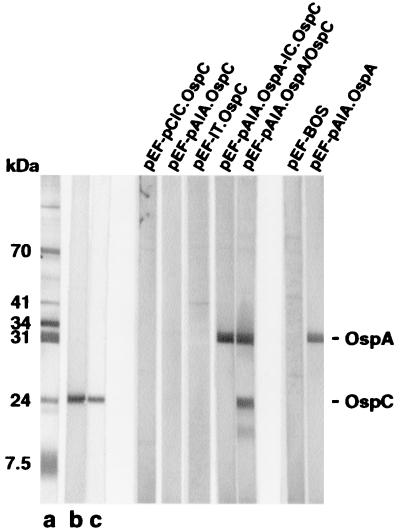
As shown in Table 1, ospC-specific transcripts were undetectable in transfected HepG2 cells, independent of whether ospC was under the control of its own leader sequence (pEF-lC.OspC) or in addition of the 5′ upstream promoter region (130 bp; pEF-pClC.OspC). Since the upstream region of ospA (pAlA) was recently shown to be operative for ospA expression in mammalian cells, even in the absence of strong eukaryotic promoter elements (20), the ospC regulatory sequence (pClC) was replaced by the corresponding ospA sequences (pEF-pAlA.OspC or pEF-lA.OspC). However, none of the two latter constructs led to ospC expression in transfected HepG2 cells as tested by Northern blot analysis (data not shown) or to the production of OspC protein in transfected K46 or D2SC/1 cells. None of the ospC-containing plasmids were immunogenic in mice, as shown by Western blot analysis of IS (Table 1; Fig 2). In contrast, transfection of the same target cells with the control plasmid, pEF-pAlA.OspA, led to expression of both ospA mRNA and OspA protein (Table 1). In addition, immunization of BALB/c mice with pEF-pAlA.OspA resulted in the production of OspA-specific Ab in vivo (Table 1; Fig. 3) (20).
TABLE 1.
In vitro transfection and genetic vaccination with ospA- and ospC- containing plasmid DNA
| Plasmid constructa | Northern blot analysis of HepG2 cellsd
|
Immunoblot analysisb of:
|
ELISA analysis of mouse serac
|
|||||
|---|---|---|---|---|---|---|---|---|
| K46 cells
|
D2SC/1 cells
|
OspA (concn [μg/ml]) | OspC (concn [μg/ml]) | |||||
| ospA | ospC | OspA | OspC | OspA | OspC | |||
| pEF-BOS | − | − | − | − | − | − | − (<0.2) | − (<0.2) |
| pEF-pClC.OspC | − | − | − | − | ||||
| pEF-lC.OspC | − | − | − | − | ||||
| pEF-pAlC.OspC | − | − | − | − | ||||
| pEF-lA.OspC | − | − | − | − | ||||
| pEF-pAlA.OspC | − | − | − | − | ||||
| pEF-lT.OspC | + | ++ | ++ | − | ||||
| pEF-pAlA.OspA | + | + | + | + (485) | ||||
| pEF-pAlA.OspA-lC.OspC | + | − | − | − | − | − | + (23) | − (<0.2) |
| pEF-pAlA.OspA/OspC | + | + | + | + | + | + | + (123) | + (5.5) |
pEF-, cloning vector pEF-BOS; pA, ospA-specific promoter sequence (140 bp); lA, OspA leader sequence; pC, OspC-specific promoter sequence (130 bp); lC, OspC leader sequence.
Scoring of protein expression in 106 K46 or 4 × 105 D2SC/1 transfected cells: ++, high (>1 ng); +, medium (1 to 0.1 ng); −, no expression or below detection limit (<0.1 ng).
Ab production after three more intramuscular injections of 50 μg of plasmid DNA/mouse (serum pool of three individual mice): +, concentration >0.2; −, concentration <0.2.
Scoring of mRNA expression in 106 HepG2 cells: +, specific transcripts detected; −, no expression or below detection limit.
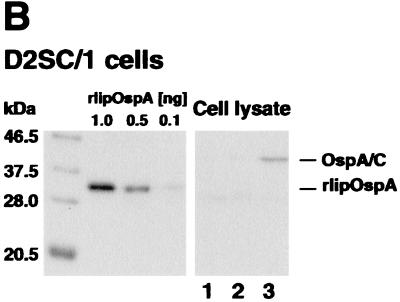
FIG. 2.
Immunoblot analysis of cell lysates and cell supernatants from K46 and D2SC/1 cells transfected with the following DNA vaccines: pEF-Bos (lane 1), pEF-IT.OspC (lane 2), pEF-pAlA.OspA/OspC (lane 3), pEF-pClC.OspC (lane 4), and pEF-pAlA.OspC (lane 5). (A) Immunoblots were probed with OspC-specific MAb LA97.1 or (B) with OspA-specific MAb LA31.1. As controls, different amounts of either rOspC (A) or rlipOspA (B) were applied.
FIG. 3.
Immunoblot analysis of B. burgdorferi ZS7 lysates probed with IS from DNA-vaccinated mice as indicated above each lane. Lane a was probed with a mixture of MAb specific for p100 (93 kDa), Hsp70 (70 kDa), Hsp60 (60 kDa), flagellin (41 kDa), OspB (34 kDa), OspA (31 kDa), OspC (24 kDa), LA7 (20 kDa), and p7.5 (7.5 kDa). Lane b was probed with a polyclonal OspC-GST IS, and lane c was probe with the OspC-specific MAb LA97.1.
The meaning of differences observed between in vitro and in vivo properties of the various ospC- and ospA-containing plasmids is unclear at present. However, the absence of detectable ospC-specific mRNA in the transfection experiments in vitro suggests a block in transcription or, alternatively, immediate degradation of the ospC-specific mRNA in the eukaryotic environment. Alternatively, it is possible that ospC transcripts are transiently produced but escape detection and that the inability to find protein is due to (i) rapid intracellular degradation of nascent OspC polypeptide chains resulting in insufficient amounts of antigen, (ii) inefficient processing of OspC resulting in inappropiate protein folding, or (iii) impaired secretion of native OspC protein.
Recent studies indicated that expression of ospA is enhanced by the eukaryotic TPA signal sequence (12) and that the humoral immune response to plasmid DNA encoding OspC is improved upon fusion of ospC to TPA (28). Consequently, the natural ospC leader sequences (pClC) were replaced by the TPA signal sequence to generate plasmid pEF-lT.OspC (Fig. 1). Transfection of either human HepG2 or mouse K46 and D2SC/1 cells with this construct resulted in production of both ospC-specific mRNA and OspC protein (Table 1; Fig. 2). The apparent molecular mass of the secreted OspC was higher (31 kDa) than expected from the molecular mass of OspC (23 kDa) (31). This finding parallels similar studies of Luke et al. using ospA under the control of TPA (12) and indicates posttranslational modification of Osps caused by the mammalian cell. The total amount of OspC synthesized by 106 D2SC/1 cells reached 0.4 μg, indicative of a highly effective secretion process. However, DNA immunization employing TPA-OspC fusion constructs failed to elicit OspC-specific humoral immune responses, even after repeated challenge and application of up to 200 μg of plasmid DNA/injection (data not shown). This is in contrast to findings of Weiss et al. (28). In their study, T- and B-cell responses in mice were greatly improved with ospC-containing plasmids in which the OspC regulatory sequences were replaced by the TPA leader sequence. The discrepancy could be due to the different transcriptional control elements used (cytomegalovirus [CMV] versus EF-1α, here) (20, 28), the different routes of plasmid inoculation (i.d. versus i.m., here) or minor amino acid differences at the TPA leader-OspC junction (VSASDICNN versus VSPSOGSNN, here). It is also possible that the differential responsiveness against the two constructs expressing OspC is due to diffential folding of OspC and/or rapid degradation of the secreted protein.
To analyze whether the natural leader-promoter sequences of ospA are able to drive the expression of ospC in addition to ospA (20), plasmid constructs containing ospA and ospC were generated (Fig. 1). Transfection of HepG2 cells with plasmid DNA pEF-pAlA.OspA-lC.OspC, harboring ospA and ospC, including pAlA and lC, in tandem array, led to ospA- but not ospC-specific transcripts (Table 1; Fig. 1). Neither OspA nor OspC protein was detectable in transfected K46 or D2SC/1 cells (Fig. 2), and only small amounts (23 μg/ml) of OspA- but not OspC-specific IS were elicited in mice injected with this plasmid DNA (Table 1). The fact that the related plasmid construct lacking ospC, pAlA.OspA, is readily expressed in vitro and induced high amounts of OspA-specific IS (485 μg/ml) (20) suggests that leader sequence of OspC may contain inhibitory elements controlling the expression of B. burgdorferi genes. This is supported by the finding that HepG2 cells transfected with plasmid pEF-pAlA. OspA/OspC, encoding a chimeric product consisting of a truncated version of OspA (aa 1 to 218) and the mature OspC protein (aa 23 to 211), resulted in the production of high amounts of ospA-ospC-specific transcripts in vitro (Table 1). Moreover, a fusion protein with the expected molecular mass (41 kDa) was expressed in K46 and D2SC/1 cells (Table 1) and reacted with both OspA- and OspC-specific MAb (Fig. 2). Immunization of BALB/c mice with the plasmid DNA construct pEF-pAlA.OspA/OspC led to the induction of IS with specificities for both OspA (123μg/ml) and OspC (5.5 μg/ml) (Fig. 3). As shown in Table 2, passive transfer of IS specific for pEF-pAlA. OspA/OspC into C.B-17 SCID mice 1 h before challenge with B. burgdorferi (ZS7) led to nearly complete protection against disease (few intermittent signs of mild arthritis [three of eight mice]) and infection (in eight of nine mice) in a dose-dependent manner. Partial protection (two of three mice) was observed in mice that received IS containing less than 1 μg of OspA-specific and 0.1 μg of OspC-specific Ab. In light of the fact that IS passively transferred to OspC are able to resolve established B. burgdorferi infection in mice (33, 34) we analyzed the protective potential of the pEF-pAlA. OspA/OspC-specific IS to resolve an established infection. For this purpose C.B-17 SCID mice were infected intraperitoneally with spirochetes (103/mouse) and administered repeatedly with the above IS, starting at day 29 p.i., a time point at which arthritis was fully developed. However, no effect of the IS on the course of infection was observed (data not shown).
TABLE 2.
Prevention of experimental B. burgdorferi infection in C.B-17 SCID mice by passive transfer of IS to pEF-pAlA.OspA/OspCa
| IS concn (μl/mouse) | Mouse no. | Development of arthritis (day p.i.)b
|
||||
|---|---|---|---|---|---|---|
| 12 | 21 | 35 | 55 | 122 | ||
| 0 | 1 | (±)/(±) | +/+ | ++/++ | Δ | |
| 2 | (±)/(±) | +/(+) | ++/++ | Δ | ||
| 3 | (±)/(±) | +/± | ++/+ | Δ | ||
| 250∗ | 1 | − | − | − | − | − |
| 2 | (±)/− | (±)/(±) | − | (±)/− | − | |
| 3 | − | −/(±) | − | − | − | |
| 50∗∗ | 1 | − | − | − | − | − |
| 2 | − | − | − | − | − | |
| 3 | − | − | − | − | − | |
| 5∗∗∗ | 1 | (±)/− | − | − | − | − |
| 2 | − | +/± | ++/++ | Δ | ||
| 3 | − | − | − | ±/(±) | − | |
Each mouse was challenged with 103 spirochetes.
Scoring of arthritis in the tibiotarsal joints (right/left) was as follows: ++, severe; +, prominent; ±, mild; (±), reddening without significant swelling; −, no signs of arthritis; Δ, mouse was sacrificed. Bispecific IS against OspA and OspC passively transferred at doses as indicated: ∗, 30 μg of OspA- and 1.4 μg of OspC-specific antibodies; ∗∗, 6 μg of OspA- and 0.3 μg of OspC-specific antibodies; ∗∗∗, 0.6 μg of OspA- and 0.03 μg of OspC-specific antibodies.
Previous studies have shown that Ab to OspC prevent subsequent infection in laboratory animals (2, 8, 16, 17, 34). We therefore tested whether IS generated by immunization with pAlA.OspA/OspC still conveyed protection following removal of OspA-specific Ab. As depicted in Table 3, only the untreated IS but not the adsorbed IS prevented infection in C.B-17 SCID mice in a dose-dependent way. These data suggest that the protective activity of pAlA.OspA/OspC-induced IS is mainly associated with OspA-specific Ab and that OspC-specific Ab are not protective at all or not present in sufficient amounts to resolve infection. This is supported by the fact that IS generated against rec.OspC was able to convey full protection to C.B-17 SCID mice, even at concentrations of 1 μg/mouse (Table 3) (33). The lack of protective OspC-specific Ab in IS to pAlA.OspA/OspC is reminiscent of a related study showing that IS raised to distinct preparations of rec.OspC have differential capacities to prevent infection (2, 33). This emphasizes the need for proper functional controls of Ab responses elicited by newly designed vaccines.
TABLE 3.
Prevention of experimental B. burgdorferi infection in C.B-17 SCID mice by passive transfer of unabsorbed or absorbed (OspA) IS to pEF-pAlA.OspA/OspC
| IS (concn [μl/mouse])d | Adsorbeda | Mouse no. | Development of arthritisb (days p.i.)
|
Ear culturec | ||||
|---|---|---|---|---|---|---|---|---|
| 11 | 21 | 36 | 52 | 63 | ||||
| pEF-BOS (250) | 1 | − | +/± | ++/++ | ++/++ | ++/++ | + | |
| 2 | − | +/± | (+)/++ | ++/++ | ++/++ | + | ||
| 3 | − | ++/+ | ++/++ | ++/++ | ++/++ | + | ||
| pEF-pAlA.OspA/OspC (250 1∗) | 1 | − | ±/(±) | ++/++ | ++/++ | ++/++ | Cont. | |
| 2 | − | − | − | − | − | − | ||
| 3 | − | − | − | − | − | Cont. | ||
| pEF-pAlA.OspA/OspC (50 1∗∗) | 1 | − | −/(±) | − | − | − | − | |
| 2 | − | − | − | − | − | − | ||
| 3 | − | ±/(+) | +/+ | +/+ | +/+ | + | ||
| pEF-pAlA.OspA/OspC (5 1∗∗∗) | 1 | − | (±)/(±) | +/+ | +/+ | † | ND | |
| 2 | − | (±)/− | +/± | +/+ | † | ND | ||
| 3 | − | (+)/± | ±/+ | (±)/± | +/++ | ND | ||
| pEF-pAlA.OspA/OspC (250 2∗) | OspA | 1 | − | (±)/± | (+)/(+) | † | ND | |
| OspA | 2 | − | (±)/(±) | − | ±/+ | ++/++ | ND | |
| OspA | 3 | − | +/+ | +/+ | ++/++ | +/± | ND | |
| pEF-pAlA.OspA/OspC (50 2∗∗) | OspA | 1 | − | (±)/(±) | (±)/+ | +/++ | ++/++ | ND |
| OspA | 2 | − | (±)/± | +/++ | ++/++ | ++/++ | ND | |
| OspA | 3 | − | (+)/+ | +/+ | ++/++ | ++/++ | ND | |
| pEF-pAlA.OspA/OspC (5 2∗∗∗) | OspA | 1 | − | −/(±) | ±/± | +/+ | † | ND |
| OspA | 2 | − | +/+ | ++/++ | ++/++ | ++/++ | ND | |
| OspA | 3 | − | +/± | +/+ | +/+ | † | ND | |
| Recombinant OspC° | 1 | − | − | − | − | − | − | |
| 2 | − | − | − | − | − | − | ||
| 3 | − | −/(±) | − | − | − | Cont. | ||
IS were adsorbed with OspA or left unadsorbed as indicated.
The scoring system for clinical arthritis is explained in footnotes a and b of Table 2. †, mouse died.
Ear biopsy specimens were examined at day 66 p.i. +, presence of spirochetes; −, absence of spirochetes; Cont., culture contaminated; ND, not done.
Bispecific IS against OspA and OspC passively transferred at doses as indicated: 1∗, 1∗∗, 1∗∗∗, 30, 6, and 0.6 μg of OspA- and 1.4, 0.3, and 0.03 μg of OspC-specific antibodies, respectively; 2∗, 2∗∗, and 2∗∗∗, 0.6, 0.1, and 0.01 μg of OspA- and 7.5, 1.5, and 0.15 μg of OspC-specific antibodies, respectively; ○, 10 μg of OspC-specific antibodies.
The finding that the plasmid DNA containing a truncated version of ospA encoding aa 1 to 218 still induced protective Ab needs some comments. Recent studies with MAb to OspA, including MAb 184.1 and LA-2 (10, 11), indicated that the protective epitopes are mainly associated with an exposed variable region on the C-terminal domain of OspA. The fact that most of the neutralizing Ab recognize an epitope that includes the strictly conserved tryptophan residue (Trp-216), which is also encoded by the pAlA. OspA/OspC plasmid construct, suggests that the relevant protective OspA epitope is preserved in the generated fusion protein.
Supposing that the application of newly designed plasmid DNA constructs containing opsA and ospC will finally lead to the induction of protective anti-OspA and anti-OspC antibodies, another inherent problem in vaccine development against Lyme disease is the heterogeneity of OspA (29, 30) and the even greater heterogeneity of OspC, especially in Europe (26, 31). However, as for the multivalent rec.OspA protein vaccine (7), this obstacle may be overcome by the generation of DNA constructs encompassing the existing polymorphism of individual genes.
In conclusion, our data show that immunization with a plasmid DNA vaccine encoding a B. burgdorferi OspA-OspC fusion protein, led to the production of Ab with specificities for both proteins. However, exclusively Ab to OspA, but not to OspC express protective activity in vivo. Only the analysis of further related plasmid DNA constructs containing ospA and ospC, including their genetic variants, and probably other osp genes, could lead to the elucidation of the optimal requirements that are essential for the generation of suitable prophylactic and therapeutic plasmid DNA vaccines against Lyme disease.
ACKNOWLEDGMENT
This study was supported in part by SmithKline Beecham.
REFERENCES
- 1.Benach J L, Bosler E M, Hanrahan J P, Coleman J L, Habicht G S, Bast T F, Cameron D J, Ziegler J L, Barbour A G, Burgdorfer W, Edelman R, Kaslow R A. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med. 1983;308:740–742. doi: 10.1056/NEJM198303313081302. [DOI] [PubMed] [Google Scholar]
- 2.Bockenstedt L K, Hodzic E, Feng S L, Bourrel K W, Desilva A, Montgomery R R, Fikrig E, Radolf J D, Barthold S W. Borrelia burgdorferi strain-specific ospC-mediated immunity in mice. Infect Immun. 1997;65:4661–4667. doi: 10.1128/iai.65.11.4661-4667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burgdorfer W, Barbour A G, Hayes S F, Benach J L, Grunwaldt E, Davis J P. Lyme disease - a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 4.de Silva A M, Telford S R, Brunet L R, Barthold S W, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–275. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Silva A M, Fikrig E. Arthropod-specific and host-specific gene-expression by Borrelia burgdorferi. J Clin Investig. 1997;99:377–379. doi: 10.1172/JCI119169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fikrig E, Barthold S W, Sun W, Feng W, Telford S R, 3rd, Flavell R A. Borrelia burgdorferi P35 and P37 proteins, expressed in vivo, elicit protective immunity. Immunity. 1997;6:531–539. doi: 10.1016/s1074-7613(00)80341-6. . (Erratum, 9:0, 1998.) [DOI] [PubMed] [Google Scholar]
- 7.Gern L, Rais O, Capiau C, Hauser P, Lobet Y, Simoen E, Voet P, Pêtre J. Immunization of mice by recombinant OspA preparations and protection against Borrelia burgdorferi infection induced by Ixodes ricinus tick bites. Immunol Lett. 1994;39:249–258. doi: 10.1016/0165-2478(94)90166-x. [DOI] [PubMed] [Google Scholar]
- 8.Gilmore R D, Jr, Kappel K J, Dolan M C, Burkot T R, Johnson B J B. Outer surface protein C (OspC), but not P39, is a protective immunogen against a tick-transmitted Borrelia burgdorferi challenge: evidence for a conformational protective epitope in OspC. Infect Immun. 1996;64:2234–2239. doi: 10.1128/iai.64.6.2234-2239.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim K J, Kanellopoulos-Langevin C, Merwin R M, Sachs D H, Asofsky R. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J Immunol. 1979;122:549–554. [PubMed] [Google Scholar]
- 10.Kramer M D, Schaible U E, Wallich R, Moter S E, Petzoldt D, Simon M M. Characterization of Borrelia burgdorferi associated antigens by monoclonal antibodies. Immunobiology. 1990;181:357–366. doi: 10.1016/S0171-2985(11)80504-8. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Dunn J J, Luft B J, Lawson C L. Crystal structure of Lyme disease antigen outer surface protein A complexed with an Fab. Proc Natl Acad Sci USA. 1997;94:3584–3589. doi: 10.1073/pnas.94.8.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luke C J, Carner K, Liang X, Barbour A G. An OspA-based DNA vaccine protects mice against infection with Borrelia burgdorferi. J Infect Dis. 1997;175:91–97. doi: 10.1093/infdis/175.1.91. [DOI] [PubMed] [Google Scholar]
- 13.Lutz M B, Granucci F, Winzler C, Marconi G, Paglia P, Foti M, Aßmann C U, Cairns L, Rescigno M, Ricciardi-Castagnoli P. Retroviral immortalization of phagocytic and dendritic cell clones as a tool to investigate functional heterogeneity. J Immunol Methods. 1994;174:269–279. doi: 10.1016/0022-1759(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 14.Mizushima S, Nagata S. pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res. 1990;18:5322. doi: 10.1093/nar/18.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montgomery R R, Malawista S E, Feen K J M, Bockenstedt L K. Direct demonstration of antigenic substitution of Borrelia burgdorferi ex vivo: exploration of the paradox of the early immune response to outer surface proteins A and C in Lyme disease. J Exp Med. 1996;183:261–269. doi: 10.1084/jem.183.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Preac-Mursic V, Wilske B, Patsouris E, Jauris S, Will G, Soutschek E, Rainhardt S, Lehnert G, Klockmann U, Mehraein P. Active immunization with pC protein of Borrelia burgdorferi protects gerbils against B. burgdorferi infection. Infection. 1992;20:342–349. doi: 10.1007/BF01710681. [DOI] [PubMed] [Google Scholar]
- 17.Probert W S, LeFebvre R B. Protection of C3H/HeN mice from challenge with Borrelia burgdorferi through active immunization with OspA, OspB, or OspC, but not with OspD or the 83-kilodalton antigen. Infect Immun. 1994;62:1920–1926. doi: 10.1128/iai.62.5.1920-1926.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siebers A, Zhong W, Wallich R, Simon M M. Loss of pathogenic potential after cloning of the low-passage Borrelia burgdorferi ZS7 tick isolate: a cautionary note. Med Microbiol Immunol. 1999;188:125–130. doi: 10.1007/s004300050114. [DOI] [PubMed] [Google Scholar]
- 20.Simon M M, Gern L, Hauser P, Zhong W, Nielsen P J, Kramer M D, Brenner C, Wallich R. Protective immunization with plasmid DNA containing the outer surface lipoprotein A gene of Borrelia burgdorferi is independent of an eukaryotic promoter. Eur J Immunol. 1996;26:2831–2840. doi: 10.1002/eji.1830261206. [DOI] [PubMed] [Google Scholar]
- 21.Simon M M, Schaible U E, Kramer M D, Müller-Hermelink H K, Wallich R. Recombinant outer surface protein A of Borrelia burgdorferi induces antibodies protective against spirochetal infection in mice. J Infect Dis. 1991;164:123–132. doi: 10.1093/infdis/164.1.123. [DOI] [PubMed] [Google Scholar]
- 22.Steere A C. Lyme disease. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 23.Steere A C, Grodzicki R L, Kornblatt A N, Craft J E, Barbour A G, Burgdorfer W, Schmid G P, Johnson E, Malawista S E. The spirochetal etiology of Lyme disease. N Engl J Med. 1983;308:733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- 24.Steere A C, Sikand V K, Meurice F, Parenti D L, Fikrig E, Schoen R T, Nowakowski J, Schmid C H, Laukamp S, Buscarino C, Krause D S. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. N Engl J Med. 1998;339:209–215. doi: 10.1056/NEJM199807233390401. [DOI] [PubMed] [Google Scholar]
- 25.Tang D-C, DeVit M, Johnston S A. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356:152–154. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- 26.Theisen M, Frederiksen B, Lebech A M, Vuust J, Hansen K. Polymorphism in ospC gene of Borrelia burgdorferi and immunoreactivity of OspC protein: implications for taxonomy and for use of OspC protein as a diagnostic antigen. J Clin Microbiol. 1993;31:2570–2576. doi: 10.1128/jcm.31.10.2570-2576.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallich R, Brenner C, Kramer M D, Simon M M. Molecular cloning and immunological characterization of a novel linear-plasmid-encoded gene, pG, of Borrelia burgdorferi expressed only in vivo. Infect Immun. 1995;63:3327–3335. doi: 10.1128/iai.63.9.3327-3335.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiss R, Durnberger J, Mostbock S, Scheiblhofer S, Hartl A, Breitenbach M, Strasser P, Dorner F, Livey I, Crowe B, Thalhamer J. Improvement of the immune response against plasmid DNA encoding OspC of Borrelia by an ER-targeting leader sequence. Vaccine. 1999;18:815–824. doi: 10.1016/s0264-410x(99)00338-2. [DOI] [PubMed] [Google Scholar]
- 29.Wilske B, Busch U, Fingerle V, Jauris-Heipke S, Mursic V P, Rössler D, Will G. Immunological and molecular variability of OspA and OspC. Implications for Borrelia vaccine development. Infection. 1996;24:208–212. doi: 10.1007/BF01713341. [DOI] [PubMed] [Google Scholar]
- 30.Wilske B, Preac-Mursic V, Göbel U B, Graf B, Jauris S, Soutschek E, Schwab E, Zumstein G. An OspA serotyping system for Borrelia burgdorferi based on reactivity with monoclonal antibodies and OspA sequence analysis. J Clin Microbiol. 1993;31:340–350. doi: 10.1128/jcm.31.2.340-350.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilske B, Preac-Mursic V, Jauris S, Hofmann A, Pradel I, Soutschek E, Schwab E, Will G, Wanner G. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect Immun. 1993;61:2182–2191. doi: 10.1128/iai.61.5.2182-2191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolff J A, Malone R W, Williams P, Chong W, Acsadi G, Jani A, Felgner P L. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 33.Zhong W, Gern L, Stehle T, Museteanu C, Kramer M, Wallich R, Simon M M. Resolution of experimental and tick-borne Borrelia burgdorferi infection in mice by passive, but not active immunization using recombinant OspC. Eur J Immunol. 1999;29:946–957. doi: 10.1002/(SICI)1521-4141(199903)29:03<946::AID-IMMU946>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 34.Zhong W, Stehle T, Museteanu C, Siebers A, Gern L, Kramer M, Wallich R, Simon M M. Therapeutic passive vaccination against chronic Lyme disease in mice. Proc Natl Acad Sci USA. 1997;94:12533–12538. doi: 10.1073/pnas.94.23.12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhong W, Wiesmüller K-H, Kramer M D, Wallich R, Simon M M. Plasmid DNA and protein vaccination of mice to the outer surface protein A of Borrelia burgdorferi leads to induction of T helper cells with specificity for a major epitope and augmentation of protective IgG antibodies in vivo. Eur J Immunol. 1996;26:2749–2757. doi: 10.1002/eji.1830261130. [DOI] [PubMed] [Google Scholar]