BACKGROUND:
Awake craniotomy (AC) enables real-time monitoring of cortical and subcortical functions when lesions are in eloquent brain areas. AC patients are exposed to various preoperative, intraoperative, and postoperative stressors, which might affect their mental health.
OBJECTIVE:
To conduct a systematic review to better understand stress, anxiety, and depression in AC patients.
METHODS:
PubMed, Scopus, and Web of Science databases were searched from January 1, 2000, to April 20, 2022, in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guideline.
RESULTS:
Four hundred forty-seven records were identified that fit our inclusion and exclusion criteria for screening. Overall, 24 articles consisting of 1450 patients from 13 countries were included. Sixteen studies (66.7%) were prospective, whereas 8 articles (33.3%) were retrospective. Studies evaluated stress, anxiety, and depression during different phases of AC. Twenty-two studies (91.7%) were conducted on adults, and 2 studies were on pediatrics (8.3 %). Glioma was the most common AC treatment with 615 patients (42.4%). Awake-awake-awake and asleep-awake-asleep were the most common protocols, each used in 4 studies, respectively (16.7%). Anxiety was the most common psychological outcome evaluated in 19 studies (79.2%). The visual analog scale and self-developed questionnaire by the authors (each n = 5, 20.8%) were the most frequently tools used. Twenty-three studies (95.8%) concluded that AC does not increase stress, anxiety, and/or depression in AC patients. One study (4.2%) identified younger age associated with panic attack.
CONCLUSION:
In experienced hands, AC does not cause an increase in stress, anxiety, and depression; however, the psychiatric impact of AC should not be underestimated.
KEY WORDS: Anesthesia, Anxiety, Awake craniotomy, Brain mapping, Psychiatry, Stress, Tumor
ABBREVIATIONS:
- AC
awake craniotomy
- APAIS
Amsterdam preoperative anxiety and information scale
- BDI
Beck depression inventory
- DSM
diagnostic and statistical manual of mental disorders
- GA
general anesthesia
- GAD
generalized anxiety disorder
- HADS
hospital anxiety and depression scale
- HGG
high-grade glioma
- LGG
low-grade glioma
- PASS
pain anxiety symptoms scale
- PCL-C
PTSD checklist civilian
- PCLS
post-traumatic stress disorder checklist scale
- PHQ
patient health questionnaire
- PSS
perceived stress scale
- PTSD
post-traumatic stress disorder
- STAI
state-trait anxiety inventory
- VAS
visual analog scale.
Awake craniotomy (AC) is a safe and well-established neurosurgical technique providing maximal lesion resection in eloquent brain areas while preserving optimal neurological functions.1-9 The eloquent areas of the brain have been defined as the areas associated with critical neurological functions, such as language, sensory, motor, visual, cortical, and subcortical regions.10-12 AC can also be performed for different lesions, ranging from primary tumors13,14 and metastases15,16 to epilepsy17,18 and cavernomas.19-21
AC provides many advantages compared with surgery under general anesthesia (GA) by enabling real-time cortical and subcortical brain mapping under local anesthesia.4,22-28 In the awake-awake-awake approach, the patient is sedated at light, moderate, or deep levels with spontaneous ventilation during craniotomy and closure.6,29,30 Although in the asleep-awake-asleep approach, the patient is under GA with a laryngeal mask airway/endotracheal intubation during craniotomy and closure and awakened and extubated during the mapping and resection step in.6,29,30 Fully awake protocol avoids the contraindications related to airway management and intubation while shortening the waking period.31-33 By contrast, the asleep-awake-asleep procedure can provide patients with more comfort because they are fully asleep during the opening and closure phases of the surgery. Advantages of AC would however need to be balanced against the potential adverse events associated because patients, awake during the entire or part of the operation, are exposed to circumstances which might generate stress, anxiety, and depression. These can include uncertainty about the surgical outcome, anesthesia-associated risks, pain, and feeling uneasy during the procedure.34 Although there are many shared stressors between AC and GA, some additional factors, such as stress and anxiety during mapping and awake surgery, can be specific to the AC procedure.35,36
Preoperative stress and anxiety levels can determine whether patients are suitable candidates for AC.6,37,38 Considering that cognitive performance is an important step in AC, stress and anxiety may impair patients' cognitive abilities and cooperation in the mapping and resection phase of AC, thus reducing the prospects of achieving the desired outcome.39-42 Postoperative stress, anxiety, and depression may also affect the long-term quality of life and prognosis of the patient.43
We aimed to obtain a better understanding of these factors among patients undergoing AC which can help health care providers to better manage the stressors to suit individuals to improve their psychiatric outcomes, thereby providing holistic patient-centered care.44 To the best of our knowledge, this is the first systematic review that accomplishes a synthesis of the published peer-reviewed literature on stress, anxiety, and depression associated with AC.
METHODS
Search Strategy
PubMed, Scopus, and Web of Sciences databases were searched from January 1, 2000, to April 20, 2022, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.45 Details of search terms for each database are presented in Supplementary Table S1, http://links.lww.com/NEU/D453. Three authors (M.M., M.S.M., and S.A.) screened for relevant articles through the reference lists of selected articles.
Inclusion and Exclusion Criteria
Eligibility criteria were (1) original articles published between January 1, 2000, and April 20, 2022; (2) English only; (3) focused on the psychiatric aspects of AC, such as “stress,” “anxiety,” or “depression” and by using psychiatric tools and evaluations; and (4) involved human subjects only. The exclusion criteria were (1) case report articles with ≤4 subjects; (2) qualitative psychiatric studies; (3) studies which inferred stress, anxiety, and depression from nonpsychiatric parameters (ie, heart rate, blood pressure, and blood components); and (4) studies which investigated neurosurgical interventions other than AC, for example, burr holes in deep brain stimulation.46,47
Data Extraction
Extracted data are presented in Tables 1, 2, and 3 and Supplementary Tables S2, http://links.lww.com/NEU/D454; S3, http://links.lww.com/NEU/D455; and S4, http://links.lww.com/NEU/D456. All calculations were performed on Microsoft Excel (version 2016; Microsoft).
TABLE 1.
Summary of Studies Characteristics
| Study | Protocol | Mean AC operation time (min) | Follow-up | Total patient number | Mean age (y) range at the surgery | Lesion type | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| AC | GA | Tumor | Epilepsy (%) | Other (%) | ||||||
| Glioma (HGG/LGG) (%) | Other | |||||||||
| Whittle et al48 | Asleep-awake-asleep or asleep-awake-awake | 62 (range, 10-105; awake phase) | NS | 15 | 45 (25-67) | NA | NS | NS | 0 | 0 |
| Hol et al49 | NS | 275 ± 56 SD | NS | 40 | 44 | 48 | NS | NS | 0 | 0 |
| Klimek et al50 | NS | 275 ± 56 SD | NS | 40 | 48 | 44 | NS | NS | 0 | 0 |
| Goebel et al51 | Awake-awake-awake | NS | Yes—3-7 d | 25 | 46 (23-71) | NA | 0 | 25 (100) | 0 | 0 |
| Santini et al52 | NS | NS | NS | 21 | NS (25-76) | NA | 21 (13/8) (100) | 0 | 0 | 0 |
| Santini et al53 | NS | NS | Yes—3-6 mo | 22 | NS | NA | 22 (8/14) (100) | 0 | 0 | 0 |
| Milian et al54 | Awake-awake-awake | 360.0 ± 108.2 (range, 210-634) | Yes—4 wks for 3 patients | 16 | 38 (28-55) | NA | 15 (8/7) (93.8) | 0 | 1 (6.25) | 0 |
| Beez et al55 | Asleep-awake-asleep and asleep-awake-awake | 76 (20-137; awake phase) | No | 105 | 46 (18-76) | NA | 105 (0/105) (100) | 0 | 0 | 0 |
| Zemmoura et al32 | Hypnosis/asleep-awake-asleep for 6 patients who did not tolerate hypnosis | NS | Yes—NS | 37 | 41 (18-67) | NA | 37 (0/28) (100) | 0 | 0 | 0 |
| Joswig et al56 | Sedated-awake-asleep | 205 (range, 90-300) | Yes—7-25 mo variable | 22 | 50 (17-72)a | NA | 16 (10/6) (72.8) | 0 | 0 | 6 (27.3) |
| Goettel et al57 | Awake-awake-awake | 121 (109-142 IQR) | NS | 48 | 57 (27-88) | NA | 23 (47.9) | 16 (33.3) | 0 | 9 (18.8) |
| Riquin et al58 | Hypnosis/asleep-awake | NS | Yes—3 and 6 mo | 7 | NS (8-16) | NA | NS | NS | NS | NS |
| Wu et al59 | NS | NS | NS | 38 | 40 | NA | NS | NS | NS | NS |
| Ruis et al5 | Awake-awake-awake | NS | No | 70 | 53 (18-81) | NA | 70 (36/34) (100) | 0 | 0 | 0 |
| van Ark et al60 | Asleep-awake-asleep | NS | No | 272 | NS | NS | 80 (29.4) | 161 (59.2) | 0 | 31 (11.4) |
| Hejrati et al61 | Awake-awake-asleep | NS | Yes—3 mo | 20 | 56 (20-72) | NA | NS | NS | NS | NS |
| Cathey et al62 | NS | NS | No | 31 | 60 | NA | 15 (12/3) (28.4) | 12 (38.7) | 0 | 0 |
| Huguet et al63 | Asleep-awake-asleep | NS | Yes—3, 6, and 12 mo | 17 | 15 (9-18) | NA | NS | NS | NS | NS |
| Colgan et al64 | Asleep-awake-asleep | NS | Yes—2 wks | 14 | 44 (27-83) | NA | 10 (7/3) (71.4) | 2 (14.3) | 2 (14.3) | 0 |
| Staub-Bartelt et al65 | NS | NS | No | 54 | 55 (29-81) | 59 (40-83) | AC 31 (57.4) | 4 AC (7.4) | 0 | 0 |
| GA 14 (25.9) | 5 GA (9.26) | |||||||||
| Kamata et al66 | Asleep-awake-?b | NS | No | 405 | PA 34, NPA 40 | NA | NS | NS | NS | NS |
| Bakhshi et al67 | NS | NS | Yes—NS | 96 | 42 | 44 ± 12 | AC 37 (29/8) (38.5) | 0 | 0 | 0 |
| GA 59 (34/25) (61.5) | ||||||||||
| Stalnacke et al68 | Asleep-awake-awake | NS | Yes—3-13 mo | 7 | 51 (39-71) | NA | 7 (100) | 0 | 0 | 0 |
| Rahmani et al43 | Asleep-awake-asleep | NS | Yes—1 mo and 6 mo | 28 | 39 | NA | 28 (28/0) (100) | 0 | 0 | 0 |
AC, awake craniotomy; GA, general anesthesia; HGG, high-grade glioma; LGG, low-grade glioma; NA, not applicable; NS, not specified.
This study reported an age range 17-72 years, which overlaps with the children's range determined in this review; however, because the mean age reported was 50 years, it was considered an adult study.
This study did not specify the full protocol.
TABLE 2.
Details of Psychiatric Evaluations from Studies Reviewed
| References | Psychiatric outcome measured | Psychiatric assessment tool | Preoperative psychiatric assessment | Intraoperative psychiatric assessment | Postoperative psychiatric assessment | Time between surgery and preoperative/postoperative psychiatric assessment | Psychiatric support and coping strategy provided | Intraoperative complications | Other psychiatric assessment |
|---|---|---|---|---|---|---|---|---|---|
| Whittle et al48 | Anxiety | Self-developed questionnaire | No | No | Yes | 4, 5 d | Yes—The neurosurgeon provided counselling twice preoperatively. A speech therapist, an anesthetist, and a theatre nurse provided preoperative counseling too. | None | Fear, discomfort |
| Hol et al49 | Stress and anxiety | VAS | Yes | No | Yes | 1 d | NS | NS | Pain |
| Klimek et al50 | Stress and anxiety | VAS | Yes | No | Yes | 1 d | NS | NS | Pain |
| Goebel et al51 | Acute stress and anxiety | HADS, DSM-IV | Yes | No | Yes | 5 ± 2 d | Yes—Preoperative consultation was provided by neurosurgeons, anesthesiologists, and neuropsychologist a day before the surgery. Postoperative consultation was provided by a neuropsychologist. | Seizure (n = 2). | Distress |
| Santini et al52 | Anxiety and depression | BDI, PASS-20, STAI | Yes | Yes | No | NA | NS | NS | Pain, fear |
| Santini et al53 | Anxiety and depression | BDI, STAI | Yes | No | Yes | NS | NS | NS | Memory, affective state |
| Milian et al54 | PTSD | Self-developed questionnaire | No | No | Yes | 97.3 ± 93.2 wks (mean and SD) | Yes—Postoperative psychiatric support was provided. | NS | Pain, general health |
| Beez et al55 | Anxiety | VAS | No | Yes (beginning, middle, end) | No | NA | Yes—Mainly by the neurosurgeon in a preoperative session. In some centers, specialized nurses or speech therapists joined for additional support. | Seizure (n = 14). | Pain |
| Zemmoura et al32 | Stress | PCLS, PSS, self-developed questionnaire. | No | No | Yes | NS | Yes—Preoperative preparation and training were provided by an anesthesiologist to familiarise the patient with the procedure and gain the patient's approval and confidence in the method. | Nausea (n = 7), vomiting (n = 5), seizure (n = 5). | Hypnosis experience |
| Joswig et al56 | Stress | Self-developed questionnaire | No | No | Yes | NS | Yes—Preoperative preparations and intraoperative support were provided by the neurosurgeon, anesthesiologist and speech therapist. | Failure of AC because of intraoperative seizure or limited cooperation (n = 2). | Fear |
| Goettel et al57 | Anxiety | VAS | No | Yes | Yes | NS | NS | Supraventricular tachycardia (n = 1). Bradycardia and hypotension (n = 1). Intraoperative psychomotor agitation (n = 4). Intraoperative seizures in dexmedetomidine (n = 3). | Pain |
| Riquin et al58 | PTSD and acute stress | DSM IV or DSM V | Yes | No | Yes | 1 wk-3 mo | Yes—Preoperative counseling and support were provided by a child psychiatrist, a neuropsychologist, and a language therapist. A meeting was provided for the child to meet another child who underwent AC to share their experiences. The patient visited the operating room and met the team beforehand to become familiar with the atmosphere. | NS | Pain |
| Wu et al59 | Anxiety | STAI | Yes | No | Yes | 1 d | NS | NS | NS |
| Ruis et al5 | Anxiety and depression | HADS | Yes | No | No | 2 | NS | NS | NS |
| van Ark et al60 | Anxiety | Self-developed questionnaire | Yes | No | Yes | NS | Yes—Preoperative psychological preparation was provided. | Local seizures | Pain, memory |
| Hejrati et al61 | Anxiety and depression | HADS, PHQ | Yes | Yes (fear and pain) | Yes | 3 d and 3 mo | NS | NS | Pain, fear |
| Cathey et al62 | Anxiety | VAS | Yes | Yes | No | NS | Yes—A dedicated operating room nurse monitored the patient during the administration of lavender aromatherapy. | NS | Pain |
| Huguet et al63 | Stress, anxiety, depression | Structured psychological analysis | Yes | No | Yes | Up to 1 y | Yes—A psychologist provided preparation during several meetings with the patient. | Seizure. Speech arrest and paraesthesia (n = 1). | Pain |
| Colgan et al64 | Stress, anxiety, depression | APAIS. GAD-7. PHQ-9. PCL-C | Yes | No | Yes | 2 wks | Yes—A clinical psychologist or a speech-language pathologist counselled the patient 1 to 4 d before the surgery and discussed stress management techniques. | None | Pain, distress |
| Staub-Bartelt et al65 | Anxiety, depression | HADS | Yes | No | No | 1-2 d | Yes—Perioperative psycho-oncological support was provided. A simulation of the awake situation was provided 1 d before surgery, and the entire procedure was practised with the patient. | NS | Distress |
| Kamata et al66 | Anxiety | DSM-V | No | Yes | No | NA | Yes—Intensive preoperative assessment and preparation were provided by the neurosurgeon, anesthesiologist, and nursing staff. A short movie of the entire procedure was shown to provide a virtual experience of the AC. | Seizure (n = 71), nausea and vomiting (n = 130) | Panic attack |
| Bakhshi et al67 | Depression | PHQ-9 | No | No | Yes | NS | NS | NS | NS |
| Stalnacke et al68 | Anxiety and depression | HADS | Yes | No | Yes | Postoperative 5.9 mo (SD, 7.5; range, 2.2–12.9) | NS | NS | Quality of life, mental fatigue |
| Rahmani et al43 | PTSD, anxiety, and depression | HADS, DSM-V | Yes | No | Yes | 1 wk before, 3 and 6 mo after | Yes—The senior neurosurgeon, anesthesiologist, and neuropsychologist provided preoperative counseling and support. | NS | NS |
AC, awake craniotomy; APAIS, Amsterdam Preoperative Anxiety and Information Scale; BDI, Beck depression inventory; DSM, diagnostic and statistical manual of mental disorders; GAD, generalized anxiety disorder; HADS, hospital anxiety and depression scale; NA, not applicable; NS, not specified; PASS, pain anxiety symptoms scale; PCL-C, post-traumatic stress disorder checklist civilian version; PCLS; post-traumatic stress disorder checklist scale; PHQ, patient health questionnaire; PSS, perceived stress scale; PTSD, post-traumatic stress disorder; STAI, state-trait anxiety inventory; VAS, visual analogue scale.
TABLE 3.
Main Outcomes and Conclusions from Each Study Reviewed
| References | Main outcomes | Comment | AC caused an increase in stress/anxiety/depression |
|---|---|---|---|
| Whittle et al48 | A small group of patients reported more than minor anxiety (29%), discomfort (20%), or fear (15%). Most patients can tolerate AC well if they are well informed about the procedure and some simple precautions are taken. Three patients (20%) had little or no memory of the operation. None of the patients was unhappy with the theatre staff numbers. | Single-center study with a small sample size. | No |
| Hol et al49 | AC is physically and emotionally less stressful for patients compared with GA. Both preoperative and postoperative anxiety was lower for AC compared with GA (P < .05). Hospitalization time was 4.53 ± 2.12 for AC and increased significantly for GA to 6.17 ± 1.62 (P = .012). | Patients with surgeries after 11:00 am were excluded because of the effect of the circadian rhythm. Patients with endocrine problems were excluded. | No |
| Klimek et al50 | Postoperative anxiety and stress declined similarly in both the AC and GA groups. AC does not cause a greater emotional challenge compared with GA. Postoperative anxiety (P = .013) and stress (P < .001) decreased in both the AC and GA groups. A significant reduction in mean hospitalization time was seen in AC patients leaving after 4.53 ± 2.12 d and GA patients after 6.17 ± 1.62 d (P = .012). | Patients with surgeries after 11:00 am were excluded because of the effect of the circadian rhythm. Patients with endocrine problems were excluded too. Allocating patients to AC or GA groups cannot be randomised because of ethical reasons. | No |
| Goebel et al51 | Preoperative and postoperative anxiety (P = .17) and depression (P = .35) do not differ in AC. Combining AC and intraoperative MRI is tolerable and reasonable for the patients. | Single-center study with a small sample size. | No |
| Santini et al52 | Warning signs for the minor failure of AC are fear of pain and anxiety. PASS CA (cognitive anxiety) correlated with BDI (P < .05). There was no statistical significance in the psychological questionnaire response of patients who had compliance in AC vs those who did not (P > .05). Minor compliance was defined as the inability to repeat the mapping tasks during AC because of the patient's emotional distress. | Small sample size. Patients were included if they did not have pre-existing psychiatric disorders and had Karnofsky Performance Scale score <70. | No |
| Santini et al53 | Cognitive assessment of patients undergoing AC in addition to language testing before and after the surgery is essential for evaluation. AC resulted in a significant reduction in anxiety. Patients awaiting AC did not have a higher anxiety level compared with those awaiting general anesthesia. Nine (41%) of 22 patients had depressive moods in the preoperative phase. Two patients (9%) improved, and 7 (32%) did not postoperatively. Five patients (24%) had preoperative anxiety, and 2 patients (14%) with postoperative anxiety (P < .05). | Other factors such as personality traits and coping mechanisms were not assessed. | No |
| Patients with long-lasting epilepsy and/or antiepileptic therapy were excluded. | |||
| Milian et al54 | AC is a safe method and does not cause PTSD in patients. Two (12%) of 16 patients reported postoperative PTSD symptoms. One patient had chronic PTSD and the other had resolved symptoms after 3 mo. | The time between surgery and survey varied widely between 1 wk and 284 wks. Self-developed PTSD questionnaire which is not validated. | No |
| Beez et al55 | AC is well-tolerated among low-grade glioma patients. Intraoperative anxiety levels did not change during different phases of the procedure. Female patients had a higher anxiety level compared with male patients (P = .0103). Patients younger than 60 y had higher anxiety (P = .0145). | Different AC protocols were used in each center. | No |
| Zemmoura et al32 | Hypnosis was not superior compared with asleep-awake-asleep craniotomy for resection of low-grade gliomas. Hypnosis can be suggested as an alternative for older patients because of shorter waking periods and no management of airway contradictions. Eight patients (22%) had a pathological score of stress on PSS. | Hypnosis is limited by access to an anesthetic team experienced in both hypnotherapy and neuro-anesthesiology. No control group existed for psychological assessments. | No |
| Joswig et al56 | 29% of patients had transient neurological deficits. AC was not successful in 2 patients (9.1%), which switched to GA. | Single-center study with a small sample size. Retrospective study subject to bias. Patients with good physical, cognitive, and affective states and without language barriers were included. | No |
| Goettel et al57 | The quality of intraoperative brain mapping during AC for supratentorial tumor resection and efficacy of sedation was similar in dexmedetomidine and propofol—remifentanil. VAS for anxiety was not different between different anesthetic groups (P < .05). | The anesthetist was not blind to the medications. The brain mapping duration was short, and results could not be generalized to longer and more complex procedures. Patients with severe cardiovascular or respiratory disease were excluded. | No |
| Riquin et al58 | Two patients had a higher level of preoperative anxiety. Patients experienced little preoperative anxiety. No patient had symptoms of post-traumatic stress disorder or acute stress. | Single-center study with a small sample size. | No |
| Wu et al59 | Listening to music was associated with a decreased level of anxiety and distress among patients after AC. Not listening to music did not increase the anxiety level. Preoperative anxiety in the music vs nonmusic group was not different (P = .311). Postoperative anxiety was higher in the nonmusic group compared with the music group (P < .01). Preoperative and postoperative anxiety was not different in the nonmusic group (P = .097). Preoperative and postoperative anxiety was different in the music group (P < .001). | Single-center study with a small sample size. | No |
| Ruis et al5 | Anxiety of patients awaiting to undergo AC was not increased and was comparable with other surgical procedures. Only 25% of AC patients showed significantly increased anxiety (HADS >7) in the preoperative phase. A significant regression equation (F = 12.3, P < .001) and R2 = 0.153 predicts anxiety based on sex. | Other factors such as personality traits, coping mechanisms, and cognitive functions were not assessed. | No |
| van Ark et al60 | A significant correlation existed between anxiety after the operation and the quantity of memories. Patients undergoing AC experienced less anxiety compared with general anesthesia (P = .02). There was no significant correlation between age and anxiety in preoperative and postoperative phases (P = .417 vs P = .725). Preoperative anxiety was not different between AC and GA groups (P = .096). Preoperative anxiety was lower in AC compared with GA (P = .020). Postoperative anxiety was not different in AC and GA groups (0.564). | Retrospective study; therefore, recall bias is possible. The self-developed questionnaire was not validated. Patients requiring other types of surgeries during the study period were excluded. | No |
| Hejrati et al61 | Mental health was not negatively affected in AC. Preoperative and postoperative anxiety was related to pain intensity. Preoperative and postoperative stress and depression were not related to pain intensity (P < .05). | Treatment effect could not be calculated and selection bias was possible. Heterogeneity of diagnosis was present. Age heterogeneity was present in the sample. The effect of adjuvant therapies, such as chemotherapy and radiotherapy, was not investigated. | No |
| Patients with developmental delays, significant communication barriers, and obesity were excluded. | |||
| Cathey et al62 | Patients were willing to complete lavender aromatherapy, and this can be integrated into the operating room. Intraoperative lavender aromatherapy did not reduce anxiety levels significantly. After lavender aromatherapy, the trend toward reduced anxiety did not reach statistical significance. | A large placebo effect exists in conditions such as anxiety. Potential benefits can be due to a raised sense of control because of lavender self-administration. Patients with a history of asthma, chronic obstructive pulmonary disease, and pregnant patients were excluded. | No |
| Huguet et al63 | Professional psychological preparation and support from families were the key elements for the successful completion of AC in children. Only 1 patient showed persistent depressive thoughts postoperatively in long-term follow-up. | Single-center study with a small sample size. | No |
| Colgan et al64 | General anxiety was reduced after AC. In the preoperative phase, 75% of patients had high anxiety, 33% had moderate to high generalized anxiety, and 33% had moderate to severe depression. In the postoperative phase, 20% reported moderate to high general anxiety and 20% had moderate to severe depression. | Single-center study with a small sample size. High-grade tumors are associated with specific clinical features. The questionnaire was not specific to AC. The interviewer for some was the same as the person who interacted during the AC, so answers might be influenced. Pregnant patients were excluded. | No |
| Staub-Bartelt et al65 | The prevalence of distress, anxiety, or depression was not significantly different in awake vs nonawake surgery. AC did not affect anxiety and depression scores. Six patients (17%) in AC reported increased anxiety compared with 6 patients in the GA group (32%). Five patients in the AC group reported depression (14%) compared with 3 patients (16%) in the GA group. The prevalence of anxiety (P = .223) and depression (P = .882) did not differ in AC and GA groups. | A small sample size because of strict inclusion criteria. Only patients with full data set of psycho-oncological testing were included. The size of the 2 groups differed, which may cause analysis bias. 74.65% of patients were excluded because of missing data due to the retrospective design of the study. | No |
| Kamata et al66 | Sixteen of 405 patients had a panic attack during AC. Intraoperative anxiety (P = .0002) and age younger than 39 y (P = .0328) are associated with a panic attack during AC. | This study was conducted single-center and retrospectively. | No |
| Bakhshi et al67 | Resection of tumors under AC did not increase postoperative depression compared with GA. Twelve patients (12%) in AC and 29 patients (30%) in GA groups had postoperative depression. The median depression scale between AC and GA groups was not different (P = .06). | The sample size in the AC group was smaller. Some patients underwent other postoperative treatments such as chemotherapy and radiotherapy, which could affect stress, anxiety, and depression. Patients with a confirmed diagnosis of depression 1 y before the brain diagnosis were excluded. | No |
| Stalnacke et al68 | Preoperative and postoperative anxiety and depression did not change significantly. However, the postoperative anxiety trend increased to above threshold level for 4 patients, although this was not significant. Surgeries in eloquent areas are safe and can be tolerated by patients. | Women were over-represented in the sample. The sample was underpowered for identifying minor or medium changes. | No |
| Rahmani et al43 | There were no statistically significant changes in preoperative and postoperative stress and anxiety. Patients with speech disturbances, female patients, and those suspected of glioblastoma had higher preoperative anxiety. Female patients had significantly higher postoperative anxiety than male patients (P = .001). | No control group of patients with similar lesions operated under GA. Postoperative pharmacological and psychological treatments could bias the findings during the follow-up. Patients with previous craniotomy and/or cranioplasty were excluded. | No |
AC, awake craniotomy; BDI, Beck depression inventory; GA, general anesthesia; HADS, hospital anxiety and depression scale; PASS, pain anxiety symptoms scale; PSS, perceived stress scale; PTSD, post-traumatic stress disorder; VAS, visual analog scale.
RESULTS
Our search yielded 440 results: 55 from PubMed, 283 from Scopus, and 102 from Web of Science. After screening reference lists, 7 records were added. Duplicate records (n = 157) were removed, the remaining studies (n = 290) were screened based on their titles and abstracts, and nonrelevant studies (n = 151) were removed. The remaining articles (n = 139) were then fully read for eligibility. Overall, 24 studies met the eligibility criteria for the final review (Figure).
FIGURE.
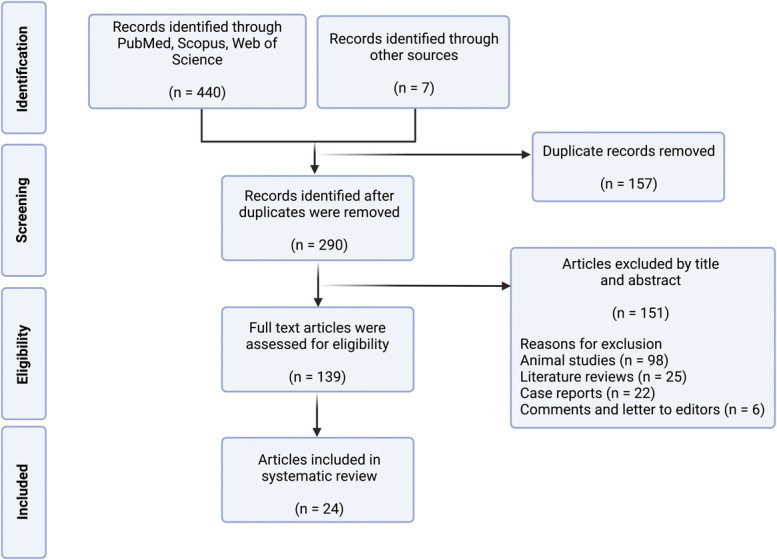
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart demonstrating search, screen, inclusion, and exclusion process for this study.
Literature Search Results Overview
An overview of studies included is presented in Supplementary Table S2, http://links.lww.com/NEU/D454.5,32,43,48-68 Of 24 studies included, 17 (70.8%) were from Europe,5,32,48-56,58,60,61,63,65,68 4 (16.7%) from Asia,43,59,66,67 and 3 (12.5%) from North America.57,62,64 Sixteen studies (66.7%) were conducted prospectively, and 8 studies (33.3%) were retrospective. Twenty-one articles (87.5%) focused on brain tumors only,5,32,43,48-53,55,57-68 whereas the other 3 studies (12.5%) had a mixture of lesions.54,56,64
Anesthesia Protocols, Operation Time, and Follow-up
Although AC is a well-established surgical approach, considerable differences exist in surgery, anesthesia, and stimulation techniques used in different centers depending on multiple factors, such as team preference, lesion type and location, patient characteristics, and comorbidities.6,69 The most common protocols were the awake-awake-awake5,51,54,57 and the asleep-awake-asleep protocols,43,60,63,64 each reported in 4 studies (16.7%; Table 1). Studies which used the awake-awake-awake approach measured acute stress and anxiety,51 post-traumatic stress disorder (PTSD), anxiety alone,57 and anxiety and depression.5 By contrast, studies which used asleep-awake-asleep measured anxiety48,55,60; combination of stress, anxiety, and depression63,64; or combination of PTSD, anxiety, and depression.43 Eight studies (33.3%) did not specify the anesthetic protocol used.49,50,52,53,59,62,65,67
The shortest follow-up was 3 to 7 days,51 and the longest follow-up was 13 months.68 Postoperative anxiety was not significantly different at the 1-month (P = .99) and 6-month (P = .26) follow-up compared with preoperative anxiety.43 Similarly, postoperative depression at the 1-month (P = .79) and 6-month follow-up (P = .95) was not statistically different compared with preoperative depression levels.43 Eight studies (33.3%) did not specify the length of their follow-up.32,48-50,52,57,59,67
Awake Craniotomy in Adults and Pediatrics
One thousand four hundred fifty patients were included in this systematic review (Table 1). Kamata et al66 contained the largest sample size with 405 patients (27.9%), followed by van Ark et al60 with 272 patients (18.8%). The smallest sample size was in Riquin et al58 and Stalnacke et al,68 each with 7 patients (0.483%). Apart from 2 studies (8.3%) which were conducted on pediatrics,58,63 all other articles (91.7%) had adult patient populations (Supplementary Table S3, http://links.lww.com/NEU/D455). In total, 1426 patients (98.3%) were adults, and 24 patients (1.7%) were pediatrics. Multiple factors, including concerns about tolerance, case complexity, a higher risk of postoperative neurocognitive deficits, and a lower frequency of lesions in eloquent areas, make AC less widely used in children.70-73
Awake Craniotomy and General Anesthesia
Five studies (20.8%) compared stress, anxiety, and depression among patients undergoing AC and GA (Supplementary Table S3, http://links.lww.com/NEU/D455).49,50,60,65,67 Hol et al49 reported that both preoperative and 12 hours postoperative anxiety levels were higher in the GA group compared with AC (P < .05). Supporting these findings, another retrospective study comparing AC with GA found that preoperative anxiety was significantly lower in the AC group compared with GA (P = .020).60 By contrast, Staub-Bartelt et al65 did not find significantly different preoperative anxiety levels in AC compared with GA patients (P = .630). In another article, both the GA and AC groups had significantly reduced postoperative stress (P < .001) and anxiety (P = .013) compared with corresponding preoperative levels.50 In the same study, the AC group had a significantly lower 12 hours postoperative anxiety when compared with the GA group (P = .009). Van Ark et al60 showed that a significant reduction in postoperative anxiety compared with the preoperative level was only seen in the GA group (P < .001) and not in the AC group (P = .612). By comparing 37 AC patients (38.5%) with 59 GA patients (61.4%), Bakhshi et al67 did not find a statistically significant difference in mean postoperative depression levels between groups (P = .06), concluding that AC tumor resection does not increase postoperative depression compared with tumor resection under GA.
Effect of Age and Sex in Psychiatric Outcome of Awake Craniotomy Patients
The lowest reported mean age among AC patients was 15 years,63 and the highest was 60 years (Table 1).62 Patients younger than 50 years had a higher level of preoperative anxiety compared with older patients (P = .037).5 Another study reported that intraoperative anxiety was higher in patients younger than 60 years (P = .0145).55 By contrast, Hejrati et al61 did not find any differences in stress, anxiety, and depression levels of young (younger than 35 years) and old (50 years or older) patients. Van Ark et al60 also did not find any significant correlation between anxiety and age before and after surgery. Four studies (16.7%) did not specify the mean age.52,53,58,60 A caveat was that the definition of young age varied between different studies. For example, Beez et al55 considered younger than 60 years as young, whereas Ruis et al5 considered younger than 55 years as young based on the median of their sample. Moreover, patients younger than 39 years were considered young by Kamata et al,66 whereas Milian et al54 did not define the young age cohort.
Ruis et al5 reported that female patients had more preoperative anxiety compared with male patients (P = .005). Similarly, Rahmani et al43 reported that female patients had significantly higher preoperative anxiety (P = .001) and depression (P = .001). In addition, Beez et al55 found female patients had significantly higher intraoperative anxiety compared with male patients (P = .0103). Van Ark et al60 found that only preoperative anxiety (P = .032), and not postoperative anxiety (P = .87), was significantly elevated in female patients. Three studies (12.5) did not specify sex of patients (Supplementary Table S3, http://links.lww.com/NEU/D455).58,60,62
Lesion Characteristics
Glioma was the most represented lesion type, corresponding to 615 patients (42.4%; Table 1). Eight studies (33.3%) did not specify the lesion type.48-50,58,59,61,63,66 Rahmani et al43 reported that patients with glioblastoma had a higher preoperative anxiety level compared with patients with anaplastic astrocytoma (P = .017).
Details of Psychiatric Assessments
Nineteen studies (79.2%) evaluated anxiety as one of their psychiatric outcomes,5,43,48-53,55,57,59-66,68 and 10 studies (41.7%) measured stress or PTSD (Table 2).32,43,49-51,54,56,58,63,64 Four studies (16.7%) assessed depression as part of their psychiatric evaluations.43,63,64,67 Only 3 studies (12.5%) evaluated PTSD.43,54,58
The visual analog scale74 and questionnaires developed by investigators were the most common tools used in the articles reviewed (Table 2). Five studies (20.8%) used visual analog scale,49,50,55,57,62 and another 5 studies (20.8%) used a self-developed questionnaire.32,48,54,56,60 Among those who used a self-developed questionnaire, Whittle et al48 developed a 10-item questionnaire asking patients about their memories of operation, sensation experience, level of fear, relaxation, comfort, adequacy of preoperative explanation, awake phase length, their views on the number of staff present, and recommendations for change. Milian et al54 developed a questionnaire inspired by DSM-IV to ask patients about core aspects of PTSD during the initial phase of the surgery and the AC phase. A questionnaire containing 11 items was designed with the help of a psychiatrist to complement other assessment tools.32 Their questionnaire asked patients about their sensations, memories, feeling, sense of time, pleasant, and unpleasant experiences. The 19-item questionnaire by Joswig et al56 asked patients about their comfort, experience, instructions received, and memories after AC. The questionnaire by Van Ark et al60 asked patients about the quality and quantity of their memories and experience.
Sixteen studies (66.7%) conducted psychiatric evaluations during the preoperative phase,5,43,49-53,58-65,68 and 18 studies (75.0%) performed postoperative psychiatric evaluation (Table 2).32,43,48-51,53,54,56-59,61,63,64,67,68 Only 5 studies (20.8%) used intraoperative psychiatric evaluation.52,55,57,62,66 Eleven studies (45.8%) did psychiatric evaluations at preoperative and postoperative phases of AC.43,49-51,53,58-61,63,68 Not all studies investigated stress, anxiety, and depression in patients undergoing AC in follow-up. Hejrati et al61 demonstrated that AC does not affect stress, anxiety, and depression at the 3-month follow-up. Similarly, Rahmani et al43 found that stress and anxiety did not significantly change at the 6-month follow-up, although the mean anxiety had a decreasing trend.
Fourteen studies (58.3%) specified psychiatric support and coping strategies provided for AC patients (Table 2).5,32,43,48,51,54-66 Neurosurgeons and anesthetists were among the team involved in counseling and psychiatric support for patients in 6 studies (25.0%) each, respectively,32,43,48,51,55,56,66 followed by psychiatrists/psychologists/neuropsychologists in 5 studies (20.8%).43,51,58,63,64 Psychiatric support was provided by nurses in 4 studies (16.7%).48,55,62,66 Some coping mechanisms provided were mentioned. For example, Riquin et al58 offered meetings with previous pediatric patients to share their experience of AC procedures. They also arranged a preoperative visit to the operating room and meetings with the entire team to familiarize patients with the environment. A day before the surgery, a simulation of the awake situation was provided, and the entire procedure was practiced with the patient.65 Kamata et al66 reported that intensive preoperative preparations were provided, and patients were shown a short movie of the entire procedure to familiarize them with AC. Staub-Bartelt et al65 reported that all patients were offered preoperative psycho-oncological support at hospitalization, and 15 patients (27.8%) accepted the offer. Another study reported that stress management techniques were offered by a psychologist or a speech-language pathologist.64 In 1 study, 22 patients (88.0%) reported the care and support received by the staff as the most positive aspect of their experience during AC.51 Ten studies (41.7%) did not specify the psychiatric support provided.5,49,50,52,53,57,59,61,67,68
Intraoperative Complications
Eight studies (33.3%) reported intraoperative seizure as the most common complication (Table 2).32,51,55-57,60,63,66 An intraoperative seizure occurred in 13.6% of patients reported by Beez et al55 with a mean duration of 12 seconds (range, 2-30 seconds), mainly in patients with a history of epilepsy; however, all the surgeries were successfully continued as planned. In another study, 5 patients (12.0%) experienced intraoperative focal seizures, which were resolved after irrigating the brain with cold saline, while 7 patients (16.0%) had nausea and 5 patients (12.0%) experienced vomiting.32 However, all their procedures continued. Fourteen studies (58.3%) did not specify intraoperative complications.5,43,49,50,52-54,58,59,61,62,65,67,68
DISCUSSION
AC is a well-established surgical intervention for pathologies in eloquent brain areas that maximize patient outcomes and minimize neurological deficits. Several studies have shown evidence for positive patient perception and tolerance of AC.33,75-78 AC requires multidisciplinary teamwork including, but not limited to, neurosurgeons, neurologists, psychiatrists, psychologists, neurophysiologists, speech and physical therapists, and specialized nurses.79 Multiple biopsychosocial factors can influence mental health,80 and psychiatric outcomes after neurosurgery have been proposed to affect the quality of life and even the 5-year survival rate.81 The studies reviewed here showed that AC can be well-tolerated by patients and does not result in elevated stress, anxiety, and depression (Table 3).
There is no assessment tool designed specifically for evaluating psychiatric outcomes among AC patients. Therefore, different centers used different psychiatric assessment tools, including questionnaires developed by different groups. Such heterogeneity can affect the findings. Therefore, a robust consensus psychiatric assessment tool needs to be established to specifically examine stress, anxiety, and depression in patients undergoing AC.
Different studies assessed stress, anxiety, and depression at various phases of AC, namely preoperative, intraoperative, and postoperative, with some studies focusing on a single phase and others assessing multiple phases. Patients can be exposed to multiple factors at different phases of AC. Some studies showed that postoperative stress and anxiety of patients undergoing AC could be significantly different compared with the GA group. Anticipating surgery can be a stress factor for patients,58 and some findings indicate that preoperative anxiety is lower among AC patients.
Female sex and younger age were risk factors for a high level of anxiety in AC in some studies, consistent with observations from other neurosurgical procedures.82-84 Multiple aspects of the operation, such as the length of the operation, the extent of the blood loss, lesion size and localization, craniotomy size, and the duration of hospitalization, can impact stress, anxiety, and depression. Future studies are required to address the importance of such variables in stress, anxiety, and depression in AC.
Psychological support at follow-up can improve stress, anxiety, and depression in patients who undergo AC. Such support can be tailored to individual patients by identifying risk factors at the preoperative stage that can make patients susceptible to elevated stress, anxiety, and depression.
Although no randomized data exist, it is likely that such support delivered by teams experienced in performing AC is critical in reducing psychiatric morbidity in patients. Such programs, in general, are more commonly integrated within AC protocols than GA craniotomy routines and might explain the lower psychiatric burden reported with the former in some comparative studies. These AC paradigms translated to GA surgeries might therefore directly benefit patients undergoing craniotomy under GA.
Limitations
Our review was limited to articles published in English, and there was heterogeneity in the articles reviewed. Eight studies with a total of 830 patients (57.2%) were retrospective, which can affect the level of the evidence available and the strength of analyses. Furthermore, the number of patients in each study and data collection period varied widely. No study evaluated stress, anxiety, and depression at all preoperative, intraoperative, and postoperative phases of AC. Therefore, future studies are required to evaluate psychiatric outcomes during all AC phases. Other factors such as personality traits, coping strategies provided, and cognitive functions can also influence the level of stress and anxiety, making their comparison more challenging. In addition, the studies reviewed had different inclusion and exclusion criteria, which can introduce selection bias. Some studies had a long and varied interval between the time of surgery and survey, with potential recall biases. Future studies are required to compare the overall survival and progression-free survival of patients undergoing AC with different anesthetic approaches. Despite such limitations, the current review can be a useful addition to help understand mental health in neurosurgical procedures.
CONCLUSION
Based on the current report, AC can be regarded as a safe neurosurgical approach which does not cause an increase in the stress, anxiety, and depression of patients. The benefits of AC outweigh its risks, and potential psychiatric challenges are manageable by experienced teams with good psychiatric support for patients. Comprehensive psychiatric assessments should be performed during preoperative, intraoperative, and postoperative phases of AC, using specific tools designed to improve patient outcomes. Future large-scale, multicenter studies with a long-term follow-up are required to address some of the outstanding questions.
Supplementary Material
Footnotes
Supplemental digital content is available for this article at neurosurgery-online.com.
Contributor Information
Mohammad Sadegh Mashayekhi, Email: sadegh@student.ubc.ca.
Saman Arfaie, Email: saman.arfaie@mail.mcgill.ca.
Yimin Chen, Email: drymchen@126.com.
Kasra Hendi, Email: kasra.hendi@gmail.com.
Angela Tian Hui Kwan, Email: angela.kwan@mail.utoronto.ca.
Faraz Honarvar, Email: faraz.honarvar@queensu.ca.
Arad Solgi, Email: aradsolgi93@gmail.com.
Xuxing Liao, Email: drliao210409@163.com.
Keyoumars Ashkan, Email: k.ashkan@nhs.net.
Funding
This study did not receive any funding or financial support.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplemental Digital Content
Supplementary Table S1. Search Terms Used for Each Database.
Supplementary Table S2. A Summary of Studies Characteristics. AC, awake craniotomy; GA, general anesthesia; NS, not specified; UK, United Kingdom.
Supplementary Table S3. Prevalence and Demographics of Patients from Included Studies. AC, awake craniotomy; GA, general anesthesia; NA, not applicable; NS, not specified. *This study reported an age range of 17 to 72 years, which overlaps with the children's range determined in this review; however, because the mean age reported was 50 years, it was considered an adult study.
Supplementary Table S4. A Summary of Lesion Characteristics from Studies Reviewed. AC, awake craniotomy; GA, general anesthesia; NA, not applicable; NS, not specified. *The numbers in each category do not add up to the total number reported.
Moroccan doorway, by Maria Orlova, from pexels.com.
REFERENCES
- 1.Duffau H, Lopes M, Arthuis F, et al. Contribution of intraoperative electrical stimulations in surgery of low grade gliomas: a comparative study between two series without (1985-96) and with (1996-2003) functional mapping in the same institution. J Neurol Neurosurg Psychiatry. 2005;76(6):845-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bello L, Gallucci M, Fava M, et al. Intraoperative subcortical language tract mapping guides surgical removal of gliomas involving speech areas. Neurosurgery. 2007;60(1):67-80; discussion 80-82. [DOI] [PubMed] [Google Scholar]
- 3.Kim SS, McCutcheon IE, Suki D, et al. Awake craniotomy for brain tumors near eloquent cortex: correlation of intraoperative cortical mapping with neurological outcomes in 309 consecutive patients. Neurosurgery. 2009;64(5):836-845; discussion 345-346. [DOI] [PubMed] [Google Scholar]
- 4.De Witt Hamer PC, Robles SG, Zwinderman AH, Duffau H, Berger MS. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J Clin Oncol. 2012;30(20):2559-2565. [DOI] [PubMed] [Google Scholar]
- 5.Ruis C. Monitoring cognition during awake brain surgery in adults: a systematic review. J Clin Exp Neuropsychol. 2018;40(10):1081-1104. [DOI] [PubMed] [Google Scholar]
- 6.Zhang K, Gelb AW. Awake craniotomy: indications, benefits, and techniques. Colomb J Anesthesiol. 2018;46(2S):46-51. [Google Scholar]
- 7.Ferguson SD, McCutcheon IE. Surgical management of gliomas in eloquent cortex. Prog Neurol Surg. 2018;30:159-172. [DOI] [PubMed] [Google Scholar]
- 8.Whiting BB, Lee BS, Mahadev V, et al. Combined use of minimal access craniotomy, intraoperative magnetic resonance imaging, and awake functional mapping for the resection of gliomas in 61 patients. J Neurosurg. 2019;132(1):159-167. [DOI] [PubMed] [Google Scholar]
- 9.Hall S, Kabwama S, Sadek A-R, et al. Awake craniotomy for tumour resection: the safety and feasibility of a simple technique. Interdiscip Neurosurg. 2021;24:101070. [Google Scholar]
- 10.Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65(4):476-483. [DOI] [PubMed] [Google Scholar]
- 11.Desmurget M, Bonnetblanc F, Duffau H. Contrasting acute and slow-growing lesions: a new door to brain plasticity. Brain. 2007;130(pt 4):898-914. [DOI] [PubMed] [Google Scholar]
- 12.Chang EF, Clark A, Smith JS, et al. Functional mapping-guided resection of low-grade gliomas in eloquent areas of the brain: improvement of long-term survival. Clinical article. J Neurosurg. 2011;114(3):566-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonen T, Sela G, Yanakee R, Ram Z, Grossman R. Surgery-independent language function decline in patients undergoing awake craniotomy. World Neurosurg. 2017;99:674-679. [DOI] [PubMed] [Google Scholar]
- 14.Fukutomi Y, Yoshimitsu K, Tamura M, Masamune K, Muragaki Y. Quantitative evaluation of efficacy of intraoperative examination monitor for awake surgery. World Neurosurg. 2019;126:e432-e438. [DOI] [PubMed] [Google Scholar]
- 15.Sills AK. Current treatment approaches to surgery for brain metastases. Neurosurgery. 2005;57(5 suppl):S24-S32; discusssion S1-S4. [DOI] [PubMed] [Google Scholar]
- 16.Chua TH, See AAQ, Ang BT, King NKK. Awake craniotomy for resection of brain metastases: a systematic review. World Neurosurg. 2018;120:e1128-e1135. [DOI] [PubMed] [Google Scholar]
- 17.Sitnikov AR, Grigoryan YA, Mishnyakova LP. Awake craniotomy without sedation in treatment of patients with lesional epilepsy. Surg Neurol Int. 2018;9:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minkin K, Gabrovski K, Karazapryanov P, et al. Awake epilepsy surgery in patients with focal cortical dysplasia. World Neurosurg. 2021;151:e257-e264. [DOI] [PubMed] [Google Scholar]
- 19.Matsuda R, Coello AF, De Benedictis A, Martinoni M, Duffau H. Awake mapping for resection of cavernous angioma and surrounding gliosis in the left dominant hemisphere: surgical technique and functional results: clinical article. J Neurosurg. 2012;117(6):1076-1081. [DOI] [PubMed] [Google Scholar]
- 20.Domingo RA, Vivas-Buitrago T, Sabsevitz DS, Middlebrooks EH, Quinones-Hinojosa A. Awake craniotomy with cortical and subcortical speech mapping for supramarginal cavernoma resection. World Neurosurg. 2020;141:260. [DOI] [PubMed] [Google Scholar]
- 21.Wang AT, Pillai P, Guran E, et al. Anesthetic management of awake craniotomy for resection of the language and motor cortex vascular malformations. World Neurosurg. 2020;143:e136-e148. [DOI] [PubMed] [Google Scholar]
- 22.Szelényi A, Bello L, Duffau H, et al. Intraoperative electrical stimulation in awake craniotomy: methodological aspects of current practice. Neurosurg Focus. 2010;28(2):E7. [DOI] [PubMed] [Google Scholar]
- 23.Jung J, Lavrador JP, Patel S, et al. First United Kingdom experience of navigated transcranial magnetic stimulation in preoperative mapping of brain tumors. World Neurosurg. 2019;122:e1578-e1587. [DOI] [PubMed] [Google Scholar]
- 24.Bukhari SS, Shamim MS. Can awake glioma surgery be the new standard of care in developing countries? Surg Neurol Int. 2020;11:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lavrador JP, Gioti I, Hoppe S, et al. Altered motor excitability in patients with diffuse gliomas involving motor eloquent areas: the impact of tumor grading. Neurosurgery. 2020;88(1):183-192. [DOI] [PubMed] [Google Scholar]
- 26.Ghimire P, Lavrador JP, Baig Mirza A, et al. Intraoperative mapping of pre-central motor cortex and subcortex: a proposal for supplemental cortical and novel subcortical maps to Penfield's motor homunculus. Brain Struct Funct. 2021;226(5):1601-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavrador JP, Ghimire P, Gullan R, Ashkan K, Vergani F, Bhangoo R. Preoperative and intraoperative anatomical-functional mapping in insular glioma surgery: integrated model to improve surgical outcome. J Neurosurg Sci. 2022;66(1):74-75. [DOI] [PubMed] [Google Scholar]
- 28.Lavrador JP, Oviedova A, Pereira N, et al. Minimally invasive approach to a deep-seated motor eloquent brain tumour: a technical note. J Surg Case Rep. 2022;2022(1):rjab611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eseonu CI, ReFaey K, Garcia O, John A, Quiñones-Hinojosa A, Tripathi P. Awake craniotomy anesthesia: a comparison of the monitored anesthesia care and asleep-awake-asleep techniques. World Neurosurg. 2017;104:679-686. [DOI] [PubMed] [Google Scholar]
- 30.Frati A, Pesce A, Palmieri M, et al. Hypnosis-aided awake surgery for the management of intrinsic brain tumors versus standard awake-asleep-awake protocol: a preliminary, promising experience. World Neurosurg. 2019;121:e882-e891. [DOI] [PubMed] [Google Scholar]
- 31.Deras P, Moulinié G, Maldonado IL, Moritz-Gasser S, Duffau H, Bertram L. Intermittent general anesthesia with controlled ventilation for asleep-awake-asleep brain surgery: a prospective series of 140 gliomas in eloquent areas. Neurosurgery. 2012;71(4):764-771. [DOI] [PubMed] [Google Scholar]
- 32.Zemmoura I, Fournier E, El-Hage W, Jolly V, Destrieux C, Velut S. Hypnosis for awake surgery of low-grade gliomas: description of the method and psychological assessment. Neurosurgery. 2016;78(1):53-61. [DOI] [PubMed] [Google Scholar]
- 33.Wrede KH, Stieglitz LH, Fiferna A, et al. Patient acceptance of awake craniotomy. Clin Neurol Neurosurg. 2011;113(10):880-884. [DOI] [PubMed] [Google Scholar]
- 34.Kelm A, Sollmann N, Ille S, Meyer B, Ringel F, Krieg SM. Resection of gliomas with and without neuropsychological support during awake craniotomy—effects on surgery and clinical outcome. Original Research. Front Oncol. 2017;7:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serletis D, Bernstein M. Prospective study of awake craniotomy used routinely and nonselectively for supratentorial tumors. J Neurosurg. 2007;107(1):1-6. [DOI] [PubMed] [Google Scholar]
- 36.Ghazanwy M, Chakrabarti R, Tewari A, Sinha A. Awake craniotomy: a qualitative review and future challenges. Saudi J Anaesth. 2014;8(4):529-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Potters JW, Klimek M. Awake craniotomy: improving the patient's experience. Curr Opin Anaesthesiol. 2015;28(5):511-516. [DOI] [PubMed] [Google Scholar]
- 38.Oteri V, Martinelli A, Crivellaro E, Gigli F. The impact of preoperative anxiety on patients undergoing brain surgery: a systematic review. Neurosurg Rev. 2021;44(6):3047-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Derakshan N, Eysenck MW. Anxiety, processing efficiency, and cognitive performance: new developments from attentional control theory. Eur Psychol. 2009;14(2):168-176. [Google Scholar]
- 40.Wahab SS, Grundy PL, Weidmann C. Patient experience and satisfaction with awake craniotomy for brain tumours. Br J Neurosurg. 2011;25(5):606-613. [DOI] [PubMed] [Google Scholar]
- 41.Rossi M, Nibali MC, Torregrossa F, Bello L, Grasso G. Innovation in neurosurgery: the concept of cognitive mapping. World Neurosurg. 2019;131:364-370. [DOI] [PubMed] [Google Scholar]
- 42.Hendi K, Rahmani M, Larijani A, et al. Changes in cognitive functioning after surgical resection of language-related, eloquent-area, high-grade gliomas under awake craniotomy. Cogn Behav Neurol. 2022;35(2):130-139. [DOI] [PubMed] [Google Scholar]
- 43.Rahmani M, Hendi K, Ajam H, et al. Alteration of anxiety and depression after awake craniotomy: a prospective study on patients with language eloquent high-grade glioma. J Neurosurg Sci. Published online ahead of print May 3, 2021. DOI: 10.23736/S0390-5616.21.05323-6. [DOI] [PubMed] [Google Scholar]
- 44.Honeybul S, Gillett GR, Ho K. Futility in neurosurgery: a patient-centered approach. Neurosurgery. 2013;73(6):917-922. [DOI] [PubMed] [Google Scholar]
- 45.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hasegawa H, Samuel M, Douiri A, Ashkan K. Patients' expectations in subthalamic nucleus deep brain stimulation surgery for Parkinson disease. World Neurosurg. 2014;82(6):1295-1299.e2. [DOI] [PubMed] [Google Scholar]
- 47.Lin HY, Hasegawa H, Mundil N, Samuel M, Ashkan K. Patients' expectations and satisfaction in subthalamic nucleus deep brain stimulation for Parkinson disease: 6-year follow-up. World Neurosurg. 2019;121:e654-e660. [DOI] [PubMed] [Google Scholar]
- 48.Whittle IR, Midgley S, Georges H, Pringle AM, Taylor R. Patient perceptions of “awake” brain tumour surgery. Acta Neurochir (Wien). 2005;147(3):275-277; discussion 277. [DOI] [PubMed] [Google Scholar]
- 49.Hol JW, Klimek M, van der Heide-Mulder M, et al. Awake craniotomy induces fewer changes in the plasma amino acid profile than craniotomy under general anesthesia. J Neurosurg Anesthesiol. 2009;21(2):98-107. [DOI] [PubMed] [Google Scholar]
- 50.Klimek M, Hol JW, Wens S, et al. Inflammatory profile of awake function-controlled craniotomy and craniotomy under general anesthesia. Mediators Inflamm. 2009;2009:670480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goebel S, Nabavi A, Schubert S, Mehdorn HM. Patient perception of combined awake brain tumor surgery and intraoperative 1.5-T magnetic resonance imaging: the Kiel experience. Neurosurgery. 2010;67(3):594-600; discussion 600. [DOI] [PubMed] [Google Scholar]
- 52.Santini B, Talacchi A, Casagrande F, et al. Eligibility criteria and psychological profiles in patient candidates for awake craniotomy: a pilot study. J Neurosurg Anesthesiol. 2012;24(3):209-216. [DOI] [PubMed] [Google Scholar]
- 53.Santini B, Talacchi A, Squintani G, Casagrande F, Capasso R, Miceli G. Cognitive outcome after awake surgery for tumors in language areas. J Neurooncol. 2012;108(2):319-326. [DOI] [PubMed] [Google Scholar]
- 54.Milian M, Luerding R, Ploppa A, et al. “Imagine your neighbor mows the lawn”: a pilot study of psychological sequelae due to awake craniotomy: clinical article. J Neurosurg. 2013;118(6):1288-1295. [DOI] [PubMed] [Google Scholar]
- 55.Beez T, Boge K, Wager M, et al. Tolerance of awake surgery for glioma: a prospective European Low Grade Glioma Network multicenter study. Acta Neurochir (Wien). 2013;155(7):1301-1308. [DOI] [PubMed] [Google Scholar]
- 56.Joswig H, Bratelj D, Brunner T, Jacomet A, Hildebrandt G, Surbeck W. Awake craniotomy: first-year experiences and patient perception. World Neurosurg. 2016;90:588-596.e2. [DOI] [PubMed] [Google Scholar]
- 57.Goettel N, Bharadwaj S, Venkatraghavan L, Mehta J, Bernstein M, Manninen PH. Dexmedetomidine vs propofol-remifentanil conscious sedation for awake craniotomy: a prospective randomized controlled trial. Br J Anaesth. 2016;116(6):811-821. [DOI] [PubMed] [Google Scholar]
- 58.Riquin E, Dinomais M, Malka J, et al. Psychiatric and psychologic impact of surgery while awake in children for resection of brain tumors. World Neurosurg. 2017;102:400-405. [DOI] [PubMed] [Google Scholar]
- 59.Wu PY, Huang ML, Lee WP, Wang C, Shih WM. Effects of music listening on anxiety and physiological responses in patients undergoing awake craniotomy. Complement Ther Med. 2017;32:56-60. [DOI] [PubMed] [Google Scholar]
- 60.van Ark TJ, Klimek M, de Smalen P, Vincent A, Stolker RJ. Anxiety, memories and coping in patients undergoing intracranial tumor surgery. Clin Neurol Neurosurg. 2018;170:132-139. [DOI] [PubMed] [Google Scholar]
- 61.Hejrati N, Spieler D, Samuel R, Regli L, Weyerbrock A, Surbeck W. Conscious experience and psychological consequences of awake craniotomy. World Neurosurg. 2019;129:e381-e386. [DOI] [PubMed] [Google Scholar]
- 62.Cathey K, Gunyon N, Chung N, et al. A feasibility study of lavender aromatherapy in an awake craniotomy environment. J Patient Cent Res Rev. 2020;7(1):19-30. [PMC free article] [PubMed] [Google Scholar]
- 63.Huguet L, Lohkamp LN, Beuriat PA, et al. Psychological aspects of awake brain surgery in children-interests and risks. Childs Nerv Syst. 2020;36(2):273-279. [DOI] [PubMed] [Google Scholar]
- 64.Colgan DD, Eddy A, Aulet-Leon M, et al. Compassion, communication, and the perception of control: a mixed methods study to investigate patients' perspectives on clinical practices for alleviating distress and promoting empowerment during awake craniotomies. Br J Neurosurg. Published online ahead of print December 1, 2021. DOI: 10.1080/02688697.2021.2005773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Staub-Bartelt F, Radtke O, Hänggi D, Sabel M, Rapp M. Impact of anticipated awake surgery on psychooncological distress in brain tumor patients. Front Oncol. 2021;11:795247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kamata K, Maruyama T, Komatsu R, Ozaki M. Intraoperative panic attack in patients undergoing awake craniotomy: a retrospective analysis of risk factors. J Anesth. 2021;35(6):854-861. [DOI] [PubMed] [Google Scholar]
- 67.Bakhshi SK, Pidani AS, Khalil M, Shamim MS. Is there a higher frequency of postoperative depression in patients undergoing awake craniotomy for brain tumors?: a prospective study. Cureus. 2021;13(11):e19877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stålnacke M, Bergenheim T, Sjöberg RL. Neuropsychological function and quality of life after resection of suspected lower-grade glioma in the face primary motor area. J Clin Med. 2021;10(4):580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leal RTM, Barcellos BM, Landeiro JA. Technical aspects of awake craniotomy with mapping for brain tumors in a limited resource setting. World Neurosurg. 2018;113:67-72. [DOI] [PubMed] [Google Scholar]
- 70.Grill J, Viguier D, Kieffer V, et al. Critical risk factors for intellectual impairment in children with posterior fossa tumors: the role of cerebellar damage. J Neurosurg. 2004;101(2 suppl):152-158. [DOI] [PubMed] [Google Scholar]
- 71.Nazemi KJ, Butler RW. Neuropsychological rehabilitation for survivors of childhood and adolescent brain tumors: a view of the past and a vision for a promising future. J Pediatr Rehabil Med. 2011;4(1):37-46. [DOI] [PubMed] [Google Scholar]
- 72.Vago C, Bulgheroni S, Usilla A, et al. Adaptive functioning in children in the first six months after surgery for brain tumours. Disabil Rehabil. 2011;33(11):953-960. [DOI] [PubMed] [Google Scholar]
- 73.Delion M, Terminassian A, Lehousse T, et al. Specificities of awake craniotomy and brain mapping in children for resection of supratentorial tumors in the language area. World Neurosurg. 2015;84(6):1645-1652. [DOI] [PubMed] [Google Scholar]
- 74.Williams VSL, Morlock RJ, Feltner D. Psychometric evaluation of a visual analog scale for the assessment of anxiety. Health Qual Life Outcomes. 2010;8(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pringle AM, Taylor R, Whittle IR. Anxiety and depression in patients with an intracranial neoplasm before and after tumour surgery. Br J Neurosurg. 1999;13(1):46-51. [DOI] [PubMed] [Google Scholar]
- 76.Khu KJ, Doglietto F, Radovanovic I, et al. Patients' perceptions of awake and outpatient craniotomy for brain tumor: a qualitative study. J Neurosurg. 2010;112(5):1056-1060. [DOI] [PubMed] [Google Scholar]
- 77.Sarubbo S, Latini F, Panajia A, et al. Awake surgery in low-grade gliomas harboring eloquent areas: 3-year mean follow-up. Neurol Sci. 2011;32(5):801-810. [DOI] [PubMed] [Google Scholar]
- 78.Howie E, Bambrough J, Karabatsou K, Fox JR. Patient experiences of awake craniotomy: an interpretative phenomenological analysis. J Health Psychol. 2016;21(11):2612-2623. [DOI] [PubMed] [Google Scholar]
- 79.Spena G, Schucht P, Seidel K, et al. Brain tumors in eloquent areas: a European multicenter survey of intraoperative mapping techniques, intraoperative seizures occurrence, and antiepileptic drug prophylaxis. Neurosurg Rev. 2017;40(2):287-298. [DOI] [PubMed] [Google Scholar]
- 80.Mofatteh M. Risk factors associated with stress, anxiety, and depression among university undergraduate students. AIMS Public Health. 2020;8(1):36-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jiang C, Wang J. Post-traumatic stress disorders in patients with low-grade glioma and its association with survival. J Neurooncol. 2019;142(2):385-392. [DOI] [PubMed] [Google Scholar]
- 82.Caumo W, Schmidt AP, Schneider CN, et al. Risk factors for preoperative anxiety in adults. Acta Anaesthesiol Scand. 2001;45(3):298-307. [DOI] [PubMed] [Google Scholar]
- 83.Janda M, Steginga S, Langbecker D, Dunn J, Walker D, Eakin E. Quality of life among patients with a brain tumor and their carers. J Psychosomatic Res. 2007;63(6):617-623. [DOI] [PubMed] [Google Scholar]
- 84.Perks A, Chakravarti S, Manninen P. Preoperative anxiety in neurosurgical patients. J Neurosurg Anesthesiol. 2009;21(2):127-130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Search Terms Used for Each Database.
Supplementary Table S2. A Summary of Studies Characteristics. AC, awake craniotomy; GA, general anesthesia; NS, not specified; UK, United Kingdom.
Supplementary Table S3. Prevalence and Demographics of Patients from Included Studies. AC, awake craniotomy; GA, general anesthesia; NA, not applicable; NS, not specified. *This study reported an age range of 17 to 72 years, which overlaps with the children's range determined in this review; however, because the mean age reported was 50 years, it was considered an adult study.
Supplementary Table S4. A Summary of Lesion Characteristics from Studies Reviewed. AC, awake craniotomy; GA, general anesthesia; NA, not applicable; NS, not specified. *The numbers in each category do not add up to the total number reported.



