Abstract
Pregnancy-related AKI is a global health problem and is associated with a higher risk of both maternal and fetal morbidity and mortality. Risk factors for developing AKI during pregnancy include older age, history of preeclampsia, and comorbidities like diabetes. Hyperemesis gravidarum is a common cause of AKI during the first trimester, and conditions such as preeclampsia, acute fatty liver disease of pregnancy, thrombotic thrombocytopenic purpura, hemolytic uremic syndrome, and placental abruption are important causes of AKI later in the pregnancy. Diagnosis of pregnancy-related AKI is challenging due to the lack of standard criteria and overlap of clinical manifestations between different etiologies. Timely diagnosis of pregnancy-related AKI is instrumental. Specific treatment includes steroids and immunosuppressive therapy for glomerulonephritis, prompt delivery for severe preeclampsia and acute fatty liver of pregnancy, plasmapheresis for thrombotic thrombocytopenic purpura, and eculizumab for the atypical hemolytic uremic syndrome. Due to the high complexity, management of pregnancy-related AKI should be performed by a multidisciplinary team consisting of the nephrologist, obstetrician, and neonatologist.
Introduction
Acute kidney injury in pregnancy is a public health problem and remains the leading cause of maternal and fetal morbidity and mortality.[1] The incidence of pregnancy-related AKI in the united States has increased in the recent times from 0.04% in 2006 to 0.12% in 2015, with an overall rate of 0.08%.[2] The increasing incidence in pregnancy-related AKI has been attributed to higher rates of detection, higher rate of deliveries in hospital, increase in high-risk pregnancies and higher rates of overall comorbidities due to advanced maternal age.[3] Racial/ethnic differences exist in the incidence of pregnancy-related AKI, and Black and Native American women have a 52% and 45% higher risk respectively of developing AKI in pregnancy as compared to White women.[2] Other risk factors for pregnancy-related AKI include older age, history of preeclampsia, lower socioeconomic status, and history of diabetes. Women with diabetes have a 4.4 fold higher risk of developing AKI during pregnancy as compared to women without diabetes.[2]
Maternal and fetal outcomes with pregnancy-related AKI
Pregnancy-related AKI has shown to be associated with a 14-fold higher likelihood of inpatient mortality and a 10-fold higher risk of cardiovascular events, including higher health care utilization and longer hospital stays. [2] Moreover, pregnancy-related AKI increases the risk of chronic kidney disease and kidney failure. The risk of adverse fetal outcomes of preterm births, low birth weights, neonatal intensive care unit admissions, and perinatal mortality is higher with pregnancy-related AKI. [1] A history of recovered AKI, despite return to normal kidney function before pregnancy, also increases the risk of preeclampsia, fetal growth restriction, and preterm births by 3–5-fold.[4] Animal models that underwent recovery from ischemic-perfusion injury have shown deterioration in kidney function during pregnancy, impairment of fetal growth, and higher rates of pup demise. Additionally, these animal models did not demonstrate the normal increase in creatinine clearance during pregnancy, implying the inability of the recovered kidneys to manifest normal physiologic changes of pregnancy.[5]
Causes of pregnancy-related AKI
The etiology of pregnancy-related AKI can be classified by different stages of pregnancy (Figure 1) or by cause (prerenal, renal, and post-renal). [6, 1, 3] In the first trimester, hyperemesis gravidarum is commonly seen, which can lead to AKI due to volume depletion. De-novo glomerulonephritis, or glomerulonephritis flare such as that of lupus can result in AKI in any of the three trimesters during pregnancy. Preeclampsia is the most common cause of pregnancy-related AKI (15–20%) with onset after 20 weeks of gestation and is defined by blood pressure ≥ 140/90 mm Hg on two occasions 4 hours apart, or ≥ 160/110 mm Hg within a shorter interval, and proteinuria ≥ 300-mg/24-hour urine or spot urine protein creatinine ratio 0.3 (dipstick 1+). Preeclampsia can be diagnosed in the absence of proteinuria if any of the following signs of end-organ dysfunction are present: elevated serum creatinine > 1.1 mg/dl or doubling of serum creatinine compared to previous value, thrombocytopenia (< 100,000/microliter), elevated liver transaminases ≥ two times upper reference range, pulmonary edema, or cerebral/visual symptoms. HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome is a thrombotic microangiopathy with onset in second and third trimester and is complicated by AKI in 3–15% of patients. Acute fatty liver of pregnancy is an infrequent cause of AKI mostly diagnosed after 30 weeks, and many of its laboratory findings like hepatic dysfunction overlap with HELLP syndrome and preeclampsia, which makes the diagnosis challenging. Thrombotic thrombocytopenic purpura usually causes AKI in the late second, and third trimester, due to ADAMTS13 (von Willebrand factor protease) deficiency. AKI in the postpartum period should raise a suspicion for atypical hemolytic uremic syndrome or non-steroidal anti-inflammatory drugs. Obstructive AKI is common in the second and third trimester due to the compression of gravid uterus on the ureter.[6, 1, 7, 3]
Figure 1.
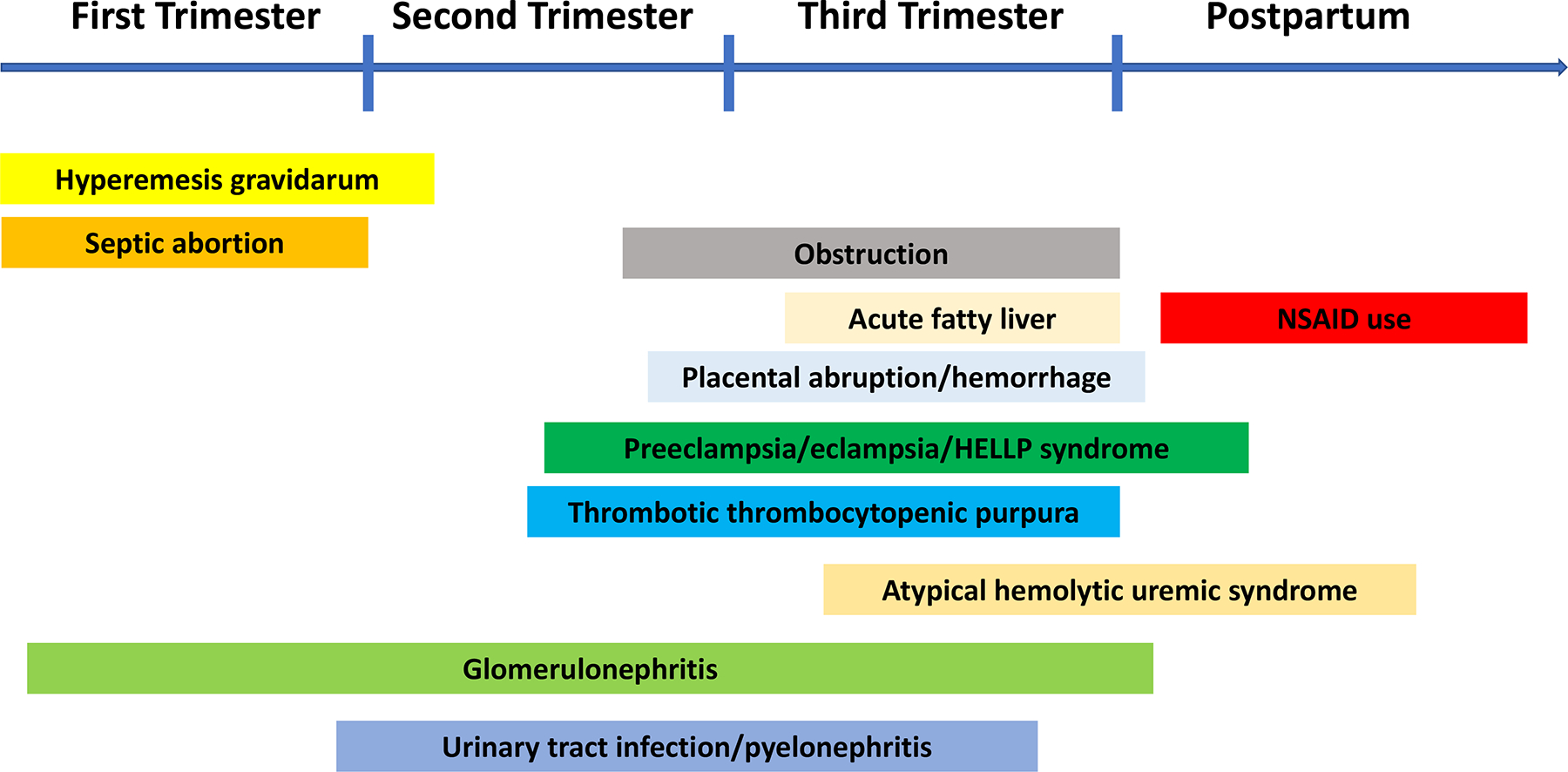
Causes of pregnancy-related acute kidney injury
Diagnosis of pregnancy-related AKI
There are no standard criteria to define pregnancy-related AKI, due to lack of validation of the RIFLE (Risk, Injury, Failure, Loss, End-stage renal disease) criteria, AKIN (Acute Kidney Injury Network), and the KDIGO (Kidney Disease Improving Outcomes) guidelines to diagnose AKI in pregnant women.[8, 9] In a normal pregnancy, there is an increase in glomerular filtration rate (GFR) by about 40–60% due to hyperfiltration, vasodilation, and an increase in effective plasma flow. Due to these normal physiological changes during pregnancy, serum creatinine is usually lower than baseline and decreases below 0.8 mg/dl. [10, 11, 3] Therefore, even a seemingly normal creatinine during pregnancy may in fact be indicative of pregnancy-related AKI. Both the physiological lowering of baseline creatinine and the non-validation of the AKI diagnostic criteria during pregnancy makes the diagnosis of pregnancy-related AKI clinically challenging. AKI markers such as neutrophil gelatinase-associated lipocalin have been studied in the pregnant population, but not yet introduced in clinical practice. [12] Several biomarkers like soluble fms-like tyrosine kinase 1 (s-FLT1) and placenta growth factor (PIGF) have been studied for early diagnosis of preeclampsia, none of them have been approved in the United States due to its low sensitivity.[13] However, till date, serum creatinine remains the most effective and cost-effective marker for diagnosis of AKI during pregnancy. Since screening of kidney function is not done routinely during pregnancy, we recommend getting kidney function test at the diagnosis of pregnancy and during each pregnancy-related hospitalization for the timely detection of AKI.
Urinalysis, urine microscopy, comprehensive metabolic panel, coagulation panel, and appropriate serological workup should be ordered for diagnosis of pregnancy-related AKI. Serum complement levels may be increased in pregnancy due to increased synthesis by the liver and a decline in serum complement (C3 and/or C4) levels is a useful clue in making a diagnosis of lupus nephritis. Kidney ultrasound should be done to rule out pathological hydronephrosis and other obstructive causes of pregnancy-related AKI.
The decision to do kidney biopsy is made based on gestation age, available treatment option, and viability of pregnancy. Kidney biopsy should be performed when diagnosis of AKI can facilitate management to improve maternal and fetal outcomes. It is safe to biopsy in first and second trimester, and there is minimal risk of complications at gestation age less than 25 weeks. As the pregnancy approaches third trimester, due to viability of fetus, clinical effort can be focused on early delivery and a biopsy in the post-partum period if needed.[3]
Management of pregnancy-related AKI
The management of pregnancy-related AKI is challenging due to its associated risk with 2 lives of both mother and baby and should be performed by a multidisciplinary team consisting of a nephrologist, an obstetrician, and a neonatologist. General measures are similar to treatment of AKI in non-pregnant population and includes intravenous hydration in women with volume depletion, use of alkali therapy for metabolic acidosis, blood transfusions for correction of anemia, potassium binders for hyperkalemia, and loop diuretics for volume overload. Specific treatment (Table 1) depends on the specific cause of pregnancy-related AKI. Antibiotics are used to treat urinary tract infection, and analgesics, stent, or nephrostomy may be needed in patients with obstructive AKI. Emergent delivery is the treatment for complications of severe preeclampsia, HELLP syndrome and acute fatty liver of pregnancy. Plasma exchange should be initiated when thrombotic thrombocytopenic purpura is suspected while ADAMTS13 levels are pending. In refractory cases of thrombotic thrombocytopenic purpura, rituximab can be given, but should be used with caution since can cause fetal B cell depletion in the third trimester. Eculizumab is an option for the treatment of atypical hemolytic uremic syndrome. For glomerulonephritis, it is safe to use steroids and immunosuppressants like azathioprine and calcineurin inhibitors (cyclosporine, and tacrolimus) during pregnancy, although risk and benefits should be weighted with each patient. Mycophenolate, sirolimus, and cyclophosphamide are contraindicated in pregnancy due to teratogenicity with mycophenolate and cyclophosphamide and increased fetal mortality in animal studies with sirolimus. Kidney replacement therapy must be initiated when indicated with deterioration of kidney function. Intensive dialysis has shown to improve fetal outcomes with improvement in both birth weights and gestation age in women with kidney failure. The prompt and safe delivery of the fetus should take precedence.[14, 15, 3]
Table 1.
Disease specific treatment of acute kidney injury in pregnancy
| Causes of AKI during pregnancy | Treatment |
|---|---|
| Hyperemesis gravidarum/prerenal causes | Hydration |
| Septic abortion/urinary tract infection | Antibiotics |
| Preeclampsia/HELLP/acute fatty liver of pregnancy | Delivery |
| Thrombotic thrombocytopenic purpura | Plasma exchange, rituximab |
| Atypical hemolytic uremic syndrome | Eculizumab |
| Obstructive uropathy | Analgesics, stent, nephrostomy |
| Placental abruption and hemorrhage | Control bleeding, delivery |
| Glomerulonephritis | Steroids, immunosuppression |
HELLP; elevated liver enzymes, low platelet count
In conclusion, there remains a need to increase the awareness of pregnancy-related AKI. Management of pregnancy-related AKI requires a multidisciplinary approach in a tertiary care center, and should include a nephrologist, maternal fetal specialist, and a neonatologist. The implementation of specific interventions for the prevention, diagnosis, and management of AKI in pregnant women will help improve clinical care and outcomes of this high-risk condition.
Funding
Silvi Shah is supported by the National Institutes of Health (NIH) K23 career development award, under Award Number 1K23HL151816-01A1, and the intramural funds from the Division of Nephrology, University of Cincinnati. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders of the study had no role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Footnotes
Competing interests
All the authors have no disclosures and competing interests. The results presented in this paper have not been published previously in whole or part, except in abstract format.
References
- 1.Jim B, Garovic VD. Acute Kidney Injury in Pregnancy. Semin Nephrol. 2017. Jul;37(4):378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah S, Meganathan K, Christianson AL, Harrison K, Leonard AC, Thakar CV. Pregnancy-Related Acute Kidney Injury in the United States: Clinical Outcomes and Health Care Utilization. Am J Nephrol. 2020;51(3):216–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taber-Hight E, Shah S. Acute Kidney Injury in Pregnancy. Adv Chronic Kidney Dis. 2020. Nov;27(6):455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tangren JS, Powe CE, Ankers E, Ecker J, Bramham K, Hladunewich MA, et al. Pregnancy Outcomes after Clinical Recovery from AKI. J Am Soc Nephrol. 2017. May;28(5):1566–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillis EE, Brands MW, Sullivan JC. Adverse Maternal and Fetal Outcomes in a Novel Experimental Model of Pregnancy after Recovery from Renal Ischemia-Reperfusion Injury. J Am Soc Nephrol. 2021. Feb;32(2):375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yadav A, Salas MAP, Coscia L, Basu A, Rossi AP, Sawinski D, et al. Acute kidney injury during pregnancy in kidney transplant recipients. Clin Transplant. 2022;36(5):e14668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah S, Gupta A. Hypertensive Disorders of Pregnancy. Cardiol Clin. 2019. Aug;37(3):345–54. [DOI] [PubMed] [Google Scholar]
- 8.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004. Aug;8(4):R204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vijayan M, Avendano M, Chinchilla KA, Jim B. Acute kidney injury in pregnancy. Curr Opin Crit Care. 2019. Dec;25(6):580–90. [DOI] [PubMed] [Google Scholar]
- 11.Wiles K, Bramham K, Seed PT, Nelson-Piercy C, Lightstone L, Chappell LC. Serum Creatinine in Pregnancy: A Systematic Review. Kidney Int Rep. 2019. Mar;4(3):408–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel M, Sachan R, Gangwar R, Sachan P, Natu S. Correlation of serum neutrophil gelatinase-associated lipocalin with acute kidney injury in hypertensive disorders of pregnancy. Int J Nephrol Renovasc Dis. 2013;6:181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leaños-Miranda A, Graciela Nolasco-Leaños A, Ismael Carrillo-Juárez R, José Molina-Pérez C, Janet Sillas-Pardo L, Manuel Jiménez-Trejo L, et al. Usefulness of the sFlt-1/PlGF (Soluble fms-Like Tyrosine Kinase-1/Placental Growth Factor) Ratio in Diagnosis or Misdiagnosis in Women With Clinical Diagnosis of Preeclampsia. Hypertension. 2020. Sep;76(3):892–900. [DOI] [PubMed] [Google Scholar]
- 14.Hladunewich MA, Hou S, Odutayo A, Cornelis T, Pierratos A, Goldstein M, et al. Intensive Hemodialysis Associates with Improved Pregnancy Outcomes: A Canadian and United States Cohort Comparison. J Am Soc Nephrol. 2014;25(5):1103–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah S, Verma P. Overview of Pregnancy in Renal Transplant Patients. Int J Nephrol. 2016;2016:4539342. [DOI] [PMC free article] [PubMed] [Google Scholar]


