Abstract
Background
Identifying risk factors for poor outcomes can help with risk stratification and targeting of treatment. Risk factors for mortality and exacerbations have been identified in bronchiectasis but have been almost exclusively studied in European and North American populations. This study investigated the risk factors for poor outcome in a large population of bronchiectasis patients enrolled in India.
Methods
The European Multicentre Bronchiectasis Audit and Research Collaboration (EMBARC) and Respiratory Research Network of India (EMBARC-India) registry is a prospective observational study of adults with computed tomography-confirmed bronchiectasis enrolled at 31 sites across India. Baseline characteristics of patients were used to investigate associations with key clinical outcomes: mortality, severe exacerbations requiring hospital admission, overall exacerbation frequency and decline in forced expiratory volume in 1 s.
Results
1018 patients with at least 12-month follow-up data were enrolled in the follow-up study. Frequent exacerbations (≥3 per year) at baseline were associated with an increased risk of mortality (hazard ratio (HR) 3.23, 95% CI 1.39–7.50), severe exacerbations (HR 2.71, 95% CI 1.92–3.83), future exacerbations (incidence rate ratio (IRR) 3.08, 95% CI 2.36–4.01) and lung function decline. Coexisting COPD, dyspnoea and current cigarette smoking were similarly associated with a worse outcome across all end-points studied. Additional predictors of mortality and severe exacerbations were increasing age and cardiovascular comorbidity. Infection with Gram-negative pathogens (predominantly Klebsiella pneumoniae) was independently associated with increased mortality (HR 3.13, 95% CI 1.62–6.06), while Pseudomonas aeruginosa infection was associated with severe exacerbations (HR 1.41, 95% CI 1.01–1.97) and overall exacerbation rate (IRR 1.47, 95% CI 1.13–1.91).
Conclusions
This study identifies risk factors for morbidity and mortality among bronchiectasis patients in India. Identification of these risk factors may support treatment approaches optimised to an Asian setting.
Short abstract
Long-term follow-up of patients in the Indian Bronchiectasis Registry identifies independent predictors of poor outcome including frequent exacerbations and chronic infection with Gram-negative pathogens such as Klebsiella pneumoniae https://bit.ly/3cWh1u7
Introduction
Bronchiectasis is a global health problem reported to affect up to 566 per 100 000 adults in the UK [1], 701 per 100 000 Medicare recipients in the USA [2] and over 1000 per 100 000 adults aged >40 years in China [3].
Although it can be caused by a wide range of infectious, inflammatory, autoimmune and genetic conditions, severe infections such as tuberculosis (TB) and pneumonia are believed to be the leading causes of bronchiectasis worldwide [4, 5]. The greatest burden of bronchiectasis is therefore likely to be identified in countries with a high incidence of TB and respiratory infections. Despite this, the majority of published data on the epidemiology, clinical features and outcomes of bronchiectasis are derived from Europe and the USA [6, 7]. The Indian Bronchiectasis Registry (EMBARC-India) was established in 2015 in collaboration with the European Multicentre Bronchiectasis Audit and Research Collaboration to provide data on the characteristics and natural history of bronchiectasis in India [4]. The initial report of baseline data from EMBARC-India identified that the characteristics of patients in India were markedly different to those in Europe or the USA. Patients in India were younger, more likely to be male, and had more severe bronchiectasis reflected in both multidimensional severity scores and radiological severity [4]. The most common cause of bronchiectasis was previous pulmonary TB, a cause that was uncommon in Europe.
Bronchiectasis causes long-term impairment of quality of life and high healthcare costs through exacerbations, hospitalisations and premature mortality [8]. Patients experience a highly variable clinical course, and risk factors for severe and progressive disease have been identified in predominantly European cohorts [9–11]. The marked differences in the disease between Europe, USA and India suggest that there is a need for longitudinal data from Asian countries as it is not known if the risk factors for exacerbation and mortality identified in European cohorts are applicable to the Asian setting. It is also unclear if prognostic tools such as the Bronchiectasis Severity Index (BSI) and FACED, which have been studied almost exclusively in European populations, are valid in an Asian setting [9–11]. Identification of outcome predictors in India may allow more targeted follow-up and treatment with results that may then be generalisable to other countries with similar demographics and a high prevalence of pulmonary TB-associated bronchiectasis. Here we report on the clinical outcomes of patients enrolled in EMBARC-India.
Methods
EMBARC-India is a multicentre, prospective, observational cohort study enrolling patients from 31 participating centres across India [4]. The inclusion criteria require a computed tomography (CT) scan of the chest demonstrating bronchiectasis and the clinical syndrome of bronchiectasis defined by cough, sputum production and/or recurrent respiratory infections. Exclusion criteria were inability to provide informed consent, traction bronchiectasis in the context of interstitial lung disease, cystic fibrosis, and previous heart and lung transplantation. As previously reported, the participating centres include both specialised and nonspecialised centres [4].
Data collection and procedures
Standardised data collection was completed through an online web-based case report form at baseline followed by annual review visits [4]. Recorded data included demographics, comorbid illnesses, aetiological testing and past history, severity of disease, exacerbations, microbiology, CT scan information, and management. Lung function testing utilised reference values for South Asian patients as previously described [4]. The Medical Research Council (MRC) dyspnoea scale was used to evaluate breathlessness and CT scans were scored using the modified Reiff score [12]. The aetiology of bronchiectasis was recorded by the managing physician [13]. The study is observational in nature and therefore no specific tests or procedures were mandated by the study protocol.
Severity assessment tools
The two most widely studied bronchiectasis prognostic tools are the BSI and FACED. The BSI is a composite of nine clinical variables with a score from 0 to 24; 0–4 is considered mild, 5–8 moderate and ≥9 severe [11]. The FACED score is a composite of five clinical variables (forced expiratory volume in 1 s (FEV1), age, colonisation by Pseudomonas aeruginosa, radiological extension and dyspnoea) with a score from 0 to 7; 0–2 is considered mild, 3–4 moderate and ≥5 severe [9].
Outcomes
Complete data collection at follow-up included all fields included in the baseline visits plus mortality, cause of death, hospitalisation for severe exacerbations, changes in lung function and exacerbations. Follow-up visits were conducted at 12 months (±3 months) in patients when clinically indicated. Consequently, where patients were discharged from follow-up or failed to attend follow-up visits, no additional data were collected. Key outcomes were exacerbations (defined as an acute respiratory infection resulting in a prescription for antibiotics), severe exacerbations (defined as those requiring hospitalisation) and all-cause mortality. Decline in FEV1 was also modelled.
Statistical analysis
Descriptive statistics are presented as mean or median according to their distribution for continuous variables and number (percentage) for categorical variables. Exacerbation frequency was modelled using generalised linear models with negative binomial errors as recommended by US Food and Drug Administration guidance on modelling exacerbation frequency in bronchiectasis [14]. In view of the different durations of follow-up for each subject, the logarithm of time under observation was included in each model as an offset. Cox proportional hazards regression was used to model time to event for severe exacerbations and all-cause mortality. Decline in lung function was modelled using a mixed model repeated measures approach. For all multivariable models relevant confounders were selected a priori based on their clinical importance and plausibility as confounders of the investigated association as recommended by guidelines from respiratory journal editors [15]. Models incorporate a maximum of one independent variable for each 10 events to limit overfitting [16]. As a sensitivity analysis, all models for variables not included in the BSI were tested after adjustment for severity of disease using the composite BSI. Prognostic tools were evaluated by Cox proportional hazards regression and discrimination was evaluated using the area under the receiver operator characteristic (ROC) curve (AUC) with curves compared using the Delong test [17]. Data are presented as effect estimates with corresponding 95% confidence intervals. No adjustment was made for multiple comparisons, but we do not report p-values except where this aids interpretation of estimates in the linear mixed effects model and for comparisons of the ROC curves. In these analyses statistical significance was set at p<0.05.
Results
Of 2195 patients enrolled in EMBARC-India [4], 1018 patients had at least 12 months of follow-up data for evaluation of longitudinal outcomes. Table 1 shows the characteristics of the patients with follow-up data available compared with the overall cohort. There were no clinically important differences between those with follow-up data and those without.
TABLE 1.
Characteristics of patients included in the follow-up study and patients with baseline data in the EMBARC-India registry
| Follow-up data available | Original cohort baseline data | |
| Patients | 1018 | 2195 |
| Age (years) | 57 (44–66) | 56 (41–66) |
| Age >65 years | 288 (28.3) | 548 (24.9) |
| Female | 466 (45.8) | 946 (43.1) |
| Comorbidities | ||
| Cardiovascular diseases | 188 (18.5) | 355 (16.2) |
| Liver disease | 13 (1.3) | 18 (0.8) |
| Osteoporosis | 82 (8.1) | 130 (5.9) |
| Depression | 38 (3.7) | 92 (4.2) |
| Anxiety | 68 (6.7) | 156 (7.1) |
| Neoplastic disease | 8 (0.8) | 17 (0.8) |
| Diabetes | 145 (14.2) | 315 (14.4) |
| Asthma | 234 (23.0) | 485 (22.1) |
| COPD | 257 (25.2) | 512 (23.3) |
| Smoking | ||
| Never-smoker | 729 (71.6) | 1576 (71.8) |
| Ex-smoker | 241 (23.7) | 506 (23.1) |
| Current smoker | 48 (4.7) | 113 (5.1) |
| BSI score | 6 (3–10) | 7 (3–10) |
| Mild | 330 (32.4) | 728 (33.2) |
| Moderate | 328 (32.2) | 674 (30.7) |
| Severe | 360 (35.4) | 793 (36.1) |
| FACED score | 2 (0–2) | 2 (0–2) |
| Mild | 549 (53.9) | 1228 (55.9) |
| Moderate | 388 (38.1) | 827 (37.7) |
| Severe | 81 (8.0) | 140 (6.4) |
| FEV1 (% pred) | 63.1 (45.2–76.9) | 61.4 (41.9–80.5) |
| BMI (kg·m−2) | 21.6 (18.8–24.5) | 21.5 (18.5–24.5) |
| MRC dyspnoea score | 1.6±1.2 | 1.7±1.2 |
| Exacerbations per year (n) | 1 (0–2) | 1 (0–2) |
| 0 | 394 (38.7) | 746 (34.0) |
| 1 | 205 (20.1) | 445 (20.3) |
| 2 | 232 (22.8) | 477 (21.7) |
| ≥3 | 187 (18.4) | 527 (24.0) |
| Hospitalised for severe exacerbations | 373 (36.6) | 851 (38.8) |
| Bacteriology | ||
| Pseudomonas aeruginosa | 117 (11.5) | 301 (13.7) |
| Haemophilus influenzae | 2 (0.2) | 11 (0.5) |
| Moraxella catarrhalis | 17 (1.7) | 22 (1.0) |
| Streptococcus pneumoniae | 8 (0.8) | 18 (0.8) |
| Staphylococcus aureus | 18 (1.8) | 50 (2.3) |
| Enterobacterales | 94 (9.2) | 215 (9.8) |
| Reiff score | 5 (3–7) | 6 (3–9) |
| Comedications | ||
| ICS | 625 (61.4) | 1387 (63.2) |
| Long-term macrolide | 39 (3.8) | 134 (6.1) |
| Inhaled antibiotic | 42 (4.1) | 79 (3.6) |
| Regular airway clearance | 394 (38.7) | 929 (42.3) |
| Long-term OCS | 7 (0.7) | 36 (1.6) |
| Aetiology | ||
| Tuberculosis | 374 (36.7) | 780 (35.5) |
| Post-infective | 216 (21.2) | 491 (22.4) |
| Idiopathic | 193 (19.0) | 469 (21.4) |
| ABPA | 115 (11.3) | 196 (8.9) |
Data are presented as n, median (interquartile range), n (%) or mean±sd. BSI: Bronchiectasis Severity Index; FEV1: forced expiratory volume in 1 s; BMI: body mass index; MRC: Medical Research Council; ICS: inhaled corticosteroid; OCS: oral corticosteroid; ABPA: allergic bronchopulmonary aspergillosis.
Mortality and hospital admissions
Over a cumulative observation time of 15 479 months (maximum follow-up 4.6 years), there were 51 (2.3%) deaths and 259 (25.4%) patients were hospitalised for severe exacerbations.
Mortality increased with increasing age from 0.5% among patients aged 18–40 years to 23.5% in patients aged >80 years. Hospitalisation rates and exacerbation rates also increased with age (table 2). Mortality rates were significantly higher in patients who were current smokers (14.6%) compared with ex-smokers (6.6%) or never-smokers (3.8%). Hospitalisation rates and exacerbation rates were also higher in smokers (table 2). Among the aetiologies, mortality and hospitalisation rates were similar between TB-associated, idiopathic and post-infective bronchiectasis (table 2).
TABLE 2.
Demographics and aetiology and associations with mortality, hospitalisation and mean exacerbations during follow-up
| Patients | Mortality | Hospitalisation | Exacerbations | |
| Age (years) | ||||
| 18–40 | 209 | 1 (0.5) | 30 (14.4) | 0.56±1.2 |
| 41–60 | 397 | 14 (3.5) | 108 (27.2) | 1.08±1.9 |
| 61–80 | 395 | 32 (8.1) | 116 (29.4) | 1.31±2.0 |
| >80 | 17 | 4 (23.5) | 5 (29.4) | 1.64±2.1 |
| Sex | ||||
| Male | 552 | 30 (5.4) | 161 (29.2) | 1.28±1.9 |
| Female | 466 | 21 (4.5) | 98 (21.0) | 0.92±1.6 |
| Comorbidities | ||||
| COPD | 257 | 27 (10.5) | 106 (41.2) | 1.67±2.1 |
| Asthma | 234 | 6 (2.6) | 41 (17.5) | 0.62±1.2 |
| Cardiovascular disease | 188 | 20 (10.6) | 72 (38.3) | 1.38±1.8 |
| Smoking | ||||
| Never-smoker | 729 | 28 (3.8) | 152 (20.9) | 0.88±1.6 |
| Ex-smoker | 241 | 16 (6.6) | 83 (34.4) | 1.49±1.9 |
| Current smoker | 48 | 7 (14.6) | 24 (50.0) | 2.71±2.9 |
| Aetiology | ||||
| Tuberculosis | 374 | 19 (5.1) | 99 (26.5) | 1.42±2.0 |
| Post-infective | 216 | 10 (4.6) | 51 (23.6) | 1.30±2.0 |
| Idiopathic | 193 | 9 (4.7) | 43 (22.3) | 0.74±1.3 |
| ABPA | 115 | 1 (0.9) | 15 (13.0) | 0.40±0.9 |
Data are presented as n, n (%) or mean±sd. ABPA: allergic bronchopulmonary aspergillosis.
Exacerbations at baseline were associated with mortality increasing from 2.3% for patients without a history of exacerbations to 8.6% in patients with ≥3 exacerbations per year. Patients with a prior severe exacerbation requiring hospitalisation were also at increased risk of mortality. Hospitalisations and exacerbations during follow-up were also higher in patients with ≥3 exacerbations per year or a prior hospitalisation (table 3).
TABLE 3.
Exacerbations, infection and functional status and relationships with mortality, hospitalisation and exacerbations
| Patients | Mortality | Hospitalisation | Exacerbations | |
| Baseline exacerbation frequency per year | ||||
| 0 | 394 (38.7) | 9 (2.3) | 57 (14.5) | 0.50±1.0 |
| 1–2 | 437 (42.9) | 26 (5.9) | 120 (27.5) | 1.23±1.5 |
| ≥3 | 187 (18.4) | 16 (8.6) | 82 (43.9) | 2.2±2.5 |
| Prior severe exacerbations | 373 (36.6) | 37 (9.9) | 147 (39.4) | 1.56±2.0 |
| Bacteriology | ||||
| Pseudomonas aeruginosa | 117 (11.5) | 8 (6.8) | 43 (36.8) | 1.62±2.2 |
| Moraxella catarrhalis | 17 (1.7) | 0 (0) | 3 (17.6) | 0.60±1.1 |
| Streptococcus pneumoniae | 8 (0.8) | 1 (12.5) | 2 (25.0) | 1.38±1.1 |
| Staphylococcus aureus | 18 (1.8) | 1 (5.6) | 6 (33.3) | 1.50±1.0 |
| Enterobacterales | 94 (9.2) | 12 (12.8) | 33 (35.1) | 1.51±1.7 |
| MRC dyspnoea score | ||||
| 1 | 194 (19.1) | 0 (0) | 14 (7.2) | 0.40±0.9 |
| 2 | 337 (33.1) | 7 (2.1) | 68 (20.2) | 0.99±1.5 |
| 3 | 241 (23.7) | 18 (7.5) | 88 (36.5) | 1.52±2.1 |
| 4 | 179 (17.6) | 22 (12.3) | 69 (38.5) | 1.63±2.1 |
| 5 | 67 (6.6) | 4 (6.0) | 20 (29.9) | 1.00±1.7 |
Data are presented as n (%) or mean±sd. MRC: Medical Research Council.
The mortality rate was 12.8% in patients isolating Enterobacterales (n=94) and 6.8% in those isolating P. aeruginosa (n=117). Other groups of bacterial pathogens had small numbers. Hospitalisation rates were highest in patients with P. aeruginosa, Enterobacterales and Staphylococcus aureus infections. Functional status measured using MRC dyspnoea score was also associated with increasing mortality and hospitalisation rates (table 3).
FEV1 % predicted was not significantly associated severe exacerbations and only the group with FEV1 30–50% predicted had significantly increased mortality. For mortality, compared with FEV1 >80% predicted (reference group), the corresponding unadjusted hazard ratios (HRs) were 1.68 (95% CI 0.80–3.55) for FEV1 50–80% predicted, 2.40 (95% CI 1.12–5.13) for FEV1 30–49% predicted and 2.20 (95% CI 0.73–6.62) for FEV1 <30% predicted. An important finding of the previous EMBARC-India study was that cystic dilation of the airways was more common in India than in Europe [4]. Cystic dilation, however, was not associated with increased mortality (unadjusted HR 1.30, 95% CI 0.68–2.48), severe exacerbations (unadjusted HR 1.14, 95% CI 0.85–1.54) or exacerbations (unadjusted incidence rate ratio (IRR) 0.85, 95% CI 0.64–1.12).
Multivariable models
We investigated the association of individual variables with clinical outcomes after adjusting for relevant confounders. The adjusted effect estimates are shown in table 4 and forest plots displayed in supplementary figure S1. As expected, age was strongly associated with mortality, with effect estimates ranging from HR 4.34 (95% CI 0.56–33.4) for patients aged 41–60 years versus those aged 18–40 years, rising to HR 28.1 (95% CI 3.03–261) for those aged >80 years (table 4). Comparisons on patient characteristics based on age are shown in supplementary table S3. Frequency of exacerbations also significantly increased with increasing age. Sex was not significantly associated with any outcome. A coexisting diagnosis of COPD was significantly associated with increased mortality (HR 2.29, 95% CI 1.20–4.35), severe exacerbations (HR 1.86, 95% CI 1.42–2.43) and overall exacerbations (IRR 1.27, 95% CI 1.02–1.58). Cigarette smoking was also associated with outcomes, with ex-smokers at significantly higher risk of hospitalisation and exacerbations, while current smokers were also at higher risk of mortality (table 4). In contrast to the effects observed with COPD, asthma was associated with a lower risk of mortality, severe exacerbations and exacerbations (table 4). Cardiovascular comorbidity was also independently associated with mortality and severe exacerbations, but not exacerbations. Aetiology was not associated with any outcome except that post-infective bronchiectasis was associated with a higher risk of exacerbation (IRR 1.46, 95% CI 1.08–1.97). Exacerbation frequency was associated with an increase in mortality, hospitalisation risk and future exacerbation frequency, with the greatest risk in those with ≥3 exacerbations per year (table 4). MRC dyspnoea score was also significantly associated with mortality, severe exacerbations and exacerbations (table 4).
TABLE 4.
Multivariable model results
| Mortality | Hospitalisation | Exacerbations | |
| Age (years)# | |||
| 18–40 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 41–60 | 4.34 (0.56–33.4) | 1.60 (1.01–2.52) | 1.48 (1.13–1.96) |
| 61–80 | 7.41 (0.98–55.9) | 1.47 (0.93–2.24) | 1.61 (1.21–2.14) |
| >80 | 28.1 (3.03–261) | 1.92 (0.84–4.37) | 1.98 (1.07–3.67) |
| Sex¶ | |||
| Male | 1.82 (0.89–3.74) | 0.97 (0.72–1.31) | 0.98 (0.80–1.21) |
| Female | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Comorbidities+ | |||
| COPD | 2.29 (1.20–4.35) | 1.86 (1.42–2.43) | 1.27 (1.02–1.58) |
| Asthma | 0.5 (0.24–1.38) | 0.71 (0.51–0.99) | 0.63 (0.49–0.80) |
| Cardiovascular disease | 2.87 (1.59–5.18) | 1.70 (1.28–2.25) | 1.08 (0.86–1.35) |
| Smoking§ | |||
| Never-smoker | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Ex-smoker | 1.52 (0.73–3.15) | 1.51 (1.10–2.07) | 1.34 (1.05–1.71) |
| Current smoker | 4.12 (1.60–10.59) | 2.00 (1.25–3.20) | 2.25 (1.51–3.34) |
| Aetiologyƒ | |||
| Tuberculosis | 1.07 (0.46–2.48) | 1.0 (0.68–1.45) | 1.20 (0.91–1.60) |
| Post-infective | 1.39 (0.54–3.56) | 1.05 (0.69–1.60) | 1.46 (1.08–1.97) |
| Idiopathic | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| ABPA | 0.30 (0.04–2.42) | 0.67 (0.37–1.22) | 0.72 (0.45–1.13) |
| Baseline exacerbation frequency per year## | |||
| 0 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 1 | 2.21 (0.89–5.53) | 1.77 (1.23–2.56) | 2.30 (1.78–2.97) |
| 2 | 2.79 (1.21–6.45) | 1.57 (1.10–2.27) | 1.86 (1.44–2.40) |
| ≥3 | 3.23 (1.39–7.50) | 2.71 (1.92–3.83) | 3.08 (2.36–4.01) |
| Bacteriology¶¶ | |||
| Pseudomonas aeruginosa | 1.24 (0.57–2.69) | 1.41 (1.01–1.97) | 1.47 (1.13–1.91) |
| Enterobacterales | 3.13 (1.62–6.06) | 1.24 (0.86–1.80) | 1.29 (0.96–1.72) |
| Klebsiella pneumoniae | 2.76 (1.17–6.50) | 1.67 (1.01–2.75) | 1.56 (1.05–2.30) |
| MRC dyspnoea score¶¶ | |||
| Per 1-unit increase | 1.65 (1.28–2.14) | 1.30 (1.16–1.45) | 1.15 (1.05–1.26) |
Data are presented as hazard ratio (95% confidence interval) for mortality and hospitalisations or incidence rate ratio (95% confidence interval) for exacerbations. ABPA: allergic bronchopulmonary aspergillosis; MRC: Medical Research Council; FEV1: forced expiratory volume in 1 s. #: adjusted for number of comorbidities, COPD, smoking, MRC dyspnoea score; ¶: adjusted for age, COPD, smoking, MRC dyspnoea score; +: adjusted for smoking, FEV1, exacerbation frequency, age, sex; §: adjusted for COPD, sex, age, number of comorbidities, FEV1; ƒ: adjusted for age, sex, FEV1, smoking, MRC dyspnoea score; ##: adjusted for age, sex, FEV1, smoking, comorbidities; ¶¶: adjusted for age, sex, FEV1, smoking, exacerbations.
Use of inhaled corticosteroids (ICSs) and long-acting bronchodilators was frequent in this population, including in patients without coexisting asthma or COPD (supplementary table S6). ICS was prescribed in 165 (77.5%) patients with asthma, 143 (60.6%) patients with COPD and 295 (53.8%) patients with no coexisting airway disease. Interestingly, ICS use was associated with a reduced frequency of exacerbations (IRR 0.78, 95% CI 0.66–0.93), but not severe exacerbations (HR 0.84, 95% CI 0.67–1.07) or mortality (HR 0.99, 95% CI 0.56–1.73). After adjustment for COPD, asthma, age, sex, smoking and infection status, ICS use was not associated with exacerbation frequency (IRR 0.90, 95% CI 0.75–1.08).
Microbiology results showed that P. aeruginosa was associated with an increased risk of severe exacerbation (HR 1.41, 95% CI 1.01–1.97) and total exacerbations (IRR 1.47, 95% CI 1.13–1.91), while infection with Enterobacterales was associated with increased mortality (HR 3.13, 95% CI 1.62–6.06). This population includes a number of different Gram-negative organisms, but predominantly Klebsiella pneumoniae (61.9%), followed by Escherichia coli (12.4%). An analysis of K. pneumoniae alone showed that isolation of this pathogen was associated with increased mortality, hospitalisation and exacerbations. The results of all models are shown in table 4 and supplementary figure S1.
In view of the strong relationship between K. pneumoniae and outcome, patients with this pathogen and patients without K. pneumoniae were compared (supplementary table S4). Patients infected with K. pneumoniae were younger, more likely to be male, had more severe bronchiectasis, were more likely to have underlying COPD and to be a current smoker, and had worse symptoms and quality of life. Two-thirds of patients had a history of hospitalisation in the previous year. One-third of patients infected with K. pneumoniae had associated diabetes. These data suggest patients with K. pneumoniae had a particularly severe phenotype.
Results for those parameters not included in the BSI were validated by adjusting for the composite BSI. These results are shown in supplementary table S2. A potential bias in the microbiology analysis is that not all patients had sputum samples sent for microbiological testing [4]. In an analysis limited to those who had at least one sample sent for culture, both P. aeruginosa (HR 2.51, 95% CI 1.64–3.84) and Enterobacterales (HR 1.62, 95% CI 1.01–2.61) were associated with increased severe exacerbations, while Enterobacterales remained associated with mortality (HR 2.78, 95% CI 1.17–6.60). The HR for P. aeruginosa and mortality was 2.46 (95% CI 0.97–6.26).
Decline in forced expiratory volume in 1 s
The mean FEV1 decline across the study population as a whole was estimated as 23.9 (95% CI −3.5 to 51.4) mL per year. In a model incorporating all of the variables in tables 2 and 3, significant predictors of FEV1 decline were baseline FEV1 (−162 (95% CI −112 to −213) mL per year per 1 L increase in FEV1; p<0.0001 indicating greater lung function decline in patients with higher baseline lung function), ≥2 exacerbations per year (−79 (95% CI −0.5 to −154) mL per year; p=0.037), COPD-associated bronchiectasis −83 (95% CI −10 to −157) mL per year; p=0.027) and increasing age (−30 (95% CI −10 to −50) mL per year per 10 years; p=0.010). P. aeruginosa infection was not independently associated with FEV1 decline (−11 (95% CI −99 to 78 mL); p=0.8). Supplementary table S5 shows overall model results.
Performance of severity assessment tools
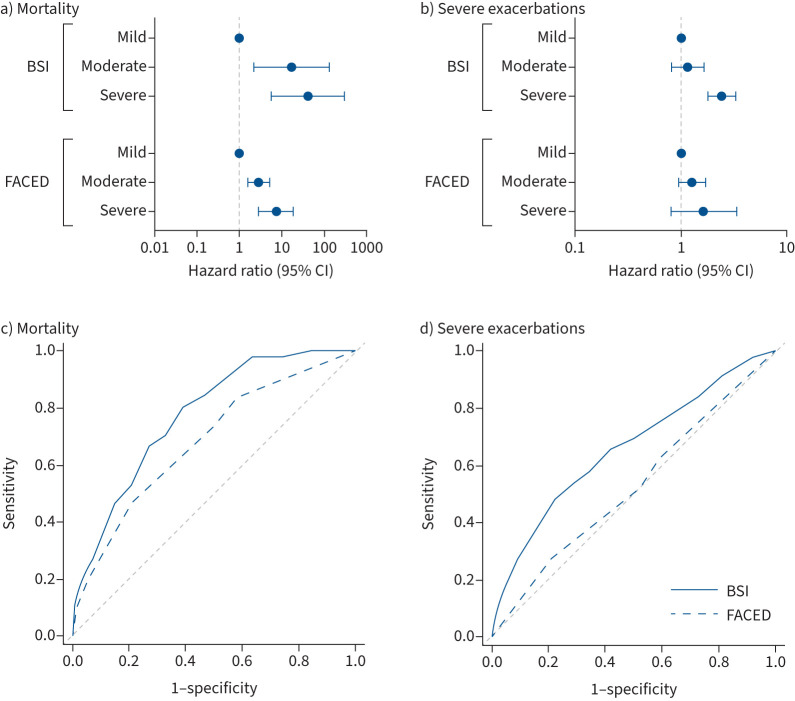
The BSI and FACED tools were evaluated. Patients were evenly distributed into mild, moderate and severe groups using the BSI (mild n=330 (32.4%), moderate n=328 (32.2%) and severe n=360 (35.4%)), but groups were highly skewed towards mild bronchiectasis using the FACED tool (mild n=549 (53.9%), moderate n=388 (38.1%) and severe n=81 (8.0%)). For both tools, mortality and hospital admissions increased with increasing scores. The HRs for mortality compared with the mild BSI group were 17.1 (95% CI 2.26–130.4) for moderate BSI scores and 41.1 (95% CI 5.6–299.8) for severe BSI scores. For FACED the corresponding HRs were 2.86 (95% CI 1.59–5.15) for moderate and 7.16 (95% CI 2.76–18.6) for severe disease (figure 1a).
FIGURE 1.
Performance of severity assessment tools (Bronchiectasis Severity Index (BSI) and FACED) for predicting clinical outcomes. a, b) Cox proportional hazards regression. Each panel displays the hazard ratios for a) mortality and b) severe exacerbations for moderate and severe patients compared with the mild group. c, d) Receiver operator characteristic curves for predicting c) mortality and d) severe exacerbations compared with the reference line (area under the curve 0.5).
There was no significant difference in hospitalisation risk when comparing mild and moderate patients according to the BSI (HR 1.15, 95% CI 0.81–1.65), but a clearly increased risk in severe patients (HR 2.41, 95% CI 1.78–3.26). FACED was not significantly associated with severe exacerbations for moderate and severe groups (HR 1.27 (95% CI 0.95–1.69) and HR 1.64 (95% CI 0.81–3.33), respectively) (figure 1b).
There was increasing risk of exacerbation with increasing BSI score (IRR 1.28 (95% CI 1.03–1.60) for moderate and IRR 2.07 (95% CI 1.68–2.54) for severe). In contrast FACED was not significantly associated with exacerbation risk (IRR 1.18 (95% CI 0.96–1.45) for moderate and IRR 1.67 (95% CI 0.99–2.85) for severe).
The corresponding AUCs for the BSI were 0.77 (95% CI 0.72–0.83) for mortality and 0.66 (95% CI 0.62–0.70) for severe exacerbations, and for FACED were 0.68 (95% CI 0.61–0.76) for mortality and 0.52 (95% CI 0.48–0.56) for severe exacerbations (figure 1c and d). Comparisons of the curves between the BSI and FACED for both outcomes were statistically significant (p<0.001) using the Delong test.
Discussion
To the best of our knowledge, this is the largest prospective study of bronchiectasis outcomes in an Asian country and one of the largest studies of long-term outcomes in bronchiectasis conducted to date. The results identify important predictors of poor outcomes in bronchiectasis, some of which represent targets for treatment with implications beyond India [18]. Specifically, mortality was independently associated with increasing age, comorbidities such as COPD and cardiovascular disease, dyspnoea, cigarette smoking, frequent exacerbations, and infection with Gram-negative organisms such as K. pneumoniae. Severe and frequent exacerbations were associated with having COPD, more dyspnoea, frequent exacerbations in the past and P. aeruginosa infection. Exacerbations and a diagnosis of COPD were also independently associated with lung function decline. K. pneumoniae was identified as an important pathogen associated with severe disease and poor outcomes.
These data are of particular interest because the majority of these risk factors for poor outcome identified in India are treatable or preventable. Frequent exacerbations can be prevented through airway clearance, appropriate treatment of underlying causes and prophylactic antibiotic treatment such as macrolides [19, 20]. Smoking cessation may be expected to reduce or prevent poor outcomes since most outcomes were not significantly worse in ex-smokers. Dyspnoea and functional status can also be improved with interventions such as pulmonary rehabilitation in bronchiectasis, as well as bronchodilators in the presence of airflow obstruction [21]. Ischaemic heart disease also has evidence-based treatment options, and the independent relationship between comorbidities and outcomes is a reminder that management of chronic respiratory diseases must include assessment of pathology outside the lungs [22]. Chronic airway infection is well recognised as a driver of morbidity and mortality in European cohorts of bronchiectasis. P. aeruginosa has previously been shown to increase the risk of death by 3-fold compared with uninfected controls [23]. Surprisingly in this study we found that Gram-negative organisms from the order Enterobacterales (formerly known as Enterobacteriaceae), predominantly K. pneumoniae, were more strongly associated with mortality than P. aeruginosa. It is well recognised that K. pneumoniae is a challenging and highly virulent pathogen in other contexts in India, and multidrug resistance (including carbapenem resistance) has been reported [24]. To the best of our knowledge, however, this is the first report of the prognostic significance of K. pneumoniae chronic infection in bronchiectasis patients. It is notable that although there were multiple causes of bronchiectasis in India, aetiology was not a major predictor of outcome.
The finding of worse outcomes with the Enterobacterales group has wider importance beyond India. Although K. pneumoniae was found to be uncommon in the US Bronchiectasis Research Registry [7], a review of global microbiology found this to be a prevalent pathogen in Asia [5]. K. pneumoniae accounted for 14% of patients in Thailand and 22.4% of patients in South Korea, being second only to P. aeruginosa in both studies [25, 26]. In Europe the Enterobacterales group of organisms may have been underestimated as a cause of infection in bronchiectasis. In the original BSI study, Enterobacteriaceae (as they were classified at the time) had a similar prevalence to the classical bronchiectasis pathogens S. aureus and Streptococcus pneumoniae, but were associated with a higher mortality, higher hospitalisation rate, more frequent exacerbations, and a mean St George's Respiratory Questionnaire score of 55.2 compared with 43.7 for S. aureus, 45.1 for Haemophilus influenzae and 49.3 for S. pneumoniae [11].
An important finding of this study is that all clinical outcomes worsened in patients with a co-diagnosis of COPD [27, 28]. These relationships persisted despite adjustment for parameters associated with COPD such as smoking, lung function, breathlessness (MRC dyspnoea score) and comorbid cardiovascular disease. The mechanism by which coexisting COPD worsens clinical outcomes is therefore unclear. In cohorts outside India is has been well established that coexisting COPD increases mortality risk in bronchiectasis and vice versa. Emphysema, pulmonary hypertension, enhanced bacterial infection, and airway inflammation and systemic inflammation are all increased in combined COPD and bronchiectasis, and associated with worse outcomes in these diseases [28–31]. Our data support the view that combined COPD and bronchiectasis should be considered a disease subtype at increased risk requiring intensified monitoring and treatment.
In the setting of scarce healthcare resources, it is important to prioritise follow-up and treatment towards patients most at risk of poor outcomes. The prognostic variables identified in this study will therefore be important to evaluate risk in the Indian setting. There are several variables in common with those identified in studies such as the BSI derivation and validation studies [11], but also predictors such as Enterobacterales and smoking that are unique to the Indian setting. The BSI and FACED tools were developed to risk stratify patients with bronchiectasis, and are now recommended by several guidelines for use in clinical practice [20, 32], but were almost exclusively studied in European populations. Since both the patient population and outcomes are so different between Europe and India it is important to validate these tools in an Indian setting. We found that the BSI adequately predicted mortality in India with an AUC similar to that observed in European studies. It was, however, less discriminating for the prediction of severe exacerbations requiring hospital admission in India than observed in European studies. In contrast, the FACED score did not adequately predict either mortality or hospital admissions in India. The FACED score awards 2 out of 7 points for having an age >70 years [9] and consequently in the Indian population, which is much younger than European populations, it is much less common to see patients classified as severe. This demonstrates that FACED is not an adequate tool and may be misleading in populations with a high proportion of younger patients. This is likely to have broad generalisability to other populations with a lower average age, such as in Asia, but also specific aetiologies, such as primary ciliary dyskinesia.
There are currently no formal bronchiectasis guidelines in India or for the majority of Asian countries. The prognostic data presented here represent an important first step in identifying key priorities for treatment in such guidelines. Prevention of exacerbations, smoking cessation, exercise, treatment of chronic infection and treatment of comorbidities represent important treatment objectives or treatable traits, and should be the focus of future research and implementation strategies in Asia. This is particularly important as our prior study using the EMBARC-India registry found a low rate of adherence to European Respiratory Society (ERS) guideline recommendations [20]. For example, only 3.1% received testing for immunoglobulins and allergic bronchopulmonary aspergillosis as per ERS guidelines, and the majority of patients with frequent exacerbations (≥3 per year) had not been offered prophylactic antibiotic treatment [4].
This study has important limitations, the most important of which is incomplete follow-up of all subjects. This is inevitable in a real-life registry-based study and particularly in a healthcare system where routine follow-up of all patients with bronchiectasis would not be practical. This has the potential to introduce bias, but we observed no meaningful differences in the characteristics of patients who were and were not followed-up. India is a very diverse country. Different climatic conditions, pollution levels, access to healthcare and lifestyle factors among other variables will be different in different regions of the country. Severe exacerbations were defined as requirement for hospitalisation, but indications for hospitalisation vary and therefore this is likely to represent a mix of different reasons for admission, including severity, comorbidity, requirement for intravenous antibiotics and other reasons. While we have identified a number of potential risk factors for poor outcome it cannot be proven beyond doubt that such relationships are causal and it is primarily intervention studies that will be required to establish the causal nature [33].
In summary, this study of long-term outcomes of over 1000 patients with bronchiectasis in India identifies key risk factors for mortality, severe exacerbations, exacerbations and FEV1 decline, providing a framework for risk stratification and improvement of disease outcomes in India.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00611-2022.Supplement (320.4KB, pdf)
Shareable PDF
Footnotes
Conflict of interest: M. Loebinger reports grants from AstraZeneca, Insmed and Grifols, outside the submitted work. F. Blasi reports grants from AstraZeneca, Bayer, Chiesi, GlaxoSmithKline, Menarini and Pfizer; consulting fees from Novartis, Pfizer, Zambon, Vertex, Viatris, AstraZeneca, Bayer, Chiesi, GlaxoSmithKline, Grifols, Guidotti, Insmed and Menarini, outside the submitted work. A. Shoemark reports grants from AstraZeneca, outside the submitted work. E. Polverino reports grants from Chiesi, Zambon, Shionogi, Teva, CSL Boehring, Insmed and Grifols, outside the submitted work. T. Welte reports grants from AstraZeneca and GlaxoSmithKline, outside the submitted work. M. Shteinberg reports grants from Trudell, GlaxoSmithKline, Novartis, Boehringer Ingelheim, AstraZeneca, Kamada, Vertex, Teva, Actelion and Rafa, outside the submitted work. S. Aliberti reports grants from Insmed, Chiesi and Fisher & Paykel; consulting fees from McGraw Hill, Insmed, Zambon, AstraZeneca, CSL Behring, Grifols, Fondazione Charta, Boehringer Ingelheim, Chiesi, Zcube, Menarini and GlaxoSmithKline, outside the submitted work. S. Limaye reports grants from Glenmark, supporting the present manuscript. J.D. Chalmers reports grants from AstraZeneca, Novartis, Boehringer Ingelheim, Insmed, GlaxoSmithKline and Gilead Sciences; consulting fees from AstraZeneca, Insmed, Boehringer Ingelheim, Janssen, Chiesi, Novartis, GlaxoSmithKline, Pfizer and Zambon, outside the submitted work. All other authors have nothing to disclose.
This article has an editorial commentary: https://doi.org/10.1183/13993003.01977-2022
Support statement: Supported by the Innovative Medicines Initiative and European Federation of Pharmaceutical Industries and Associations (EFPIA) companies under the European Commission-funded Horizon 2020 Framework Program and Inhaled Antibiotics for Bronchiectasis and Cystic Fibrosis (iABC; grant 115721), and by the European Respiratory Society through the EMBARC2 (European Multicentre Bronchiectasis Audit and Research Collaboration) consortium. EMBARC2 is supported by project partners AstraZeneca, Chiesi, Grifols, Janssen, Insmed, Novartis and Zambon. J.D. Chalmers is supported by the GlaxoSmithKline/Asthma and Lung UK Chair of Respiratory Research. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Quint JK, Millett ERC, Joshi M, et al. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: a population-based cohort study. Eur Respir J 2016; 47: 186–193. doi: 10.1183/13993003.01033-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henkle E, Chan B, Curtis JR, et al. Characteristics and health-care utilization history of patients with bronchiectasis in US Medicare enrollees with prescription drug plans, 2006 to 2014. Chest 2018; 154: 1311–1320. doi: 10.1016/j.chest.2018.07.014 [DOI] [PubMed] [Google Scholar]
- 3.Zhou Y, Wang C, Yao W, et al. [The prevalence and risk factors of bronchiectasis in residents aged 40 years old and above in seven cities in China]. Zhonghua nei ke za zhi 2013; 52: 379–382. [PubMed] [Google Scholar]
- 4.Dhar R, Singh S, Talwar D, et al. Bronchiectasis in India: results from the European Multicentre Bronchiectasis Audit and Research Collaboration (EMBARC) and Respiratory Research Network of India registry. Lancet Glob Heal 2019; 7: e1269–e1279. doi: 10.1016/S2214-109X(19)30327-4 [DOI] [PubMed] [Google Scholar]
- 5.Chandrasekaran R, Mac Aogáin M, Chalmers JD, et al. Geographic variation in the aetiology, epidemiology and microbiology of bronchiectasis. BMC Pulm Med 2018; 18: 83. doi: 10.1186/s12890-018-0638-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chalmers JD, Aliberti S, Polverino E, et al. The EMBARC European bronchiectasis registry: protocol for an international observational study. ERJ Open Res 2016; 2: 00081-2015. doi: 10.1183/23120541.00081-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aksamit TR, O'Donnell AE, Barker A, et al. Adult patients with bronchiectasis: a first look at the US bronchiectasis research registry. Chest 2017; 151: 982–992. doi: 10.1016/j.chest.2016.10.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diel R, Chalmers JD, Rabe KF, et al. Economic burden of bronchiectasis in Germany. Eur Respir J 2019; 53: 1802033. doi: 10.1183/13993003.02033-2018 [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Garcia MA, de Gracia J, Vendrell Relat M, et al. Multidimensional approach to non-cystic fibrosis bronchiectasis: the FACED score. Eur Respir J 2014; 43: 1357–1367. doi: 10.1183/09031936.00026313 [DOI] [PubMed] [Google Scholar]
- 10.McDonnell MJ, Aliberti S, Goeminne PC, et al. Multidimensional severity assessment in bronchiectasis: an analysis of seven European cohorts. Thorax 2016; 71: 1110–1118. doi: 10.1136/thoraxjnl-2016-208481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chalmers JD, Goeminne P, Aliberti S, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med 2014; 189: 576–585. doi: 10.1164/rccm.201309-1575OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reiff DB, Wells AU, Carr DH, et al. CT findings in bronchiectasis: limited value in distinguishing between idiopathic and specific types. AJR Am J Roentgenol 1995; 165: 261–267. doi: 10.2214/ajr.165.2.7618537 [DOI] [PubMed] [Google Scholar]
- 13.Araujo D, Shteinberg M, Aliberti S, et al. Standardised classification of the aetiology of bronchiectasis using an objective algorithm. Eur Respir J 2017; 50: 1701289. doi: 10.1183/13993003.01289-2017 [DOI] [PubMed] [Google Scholar]
- 14.Tracey LA. Case study on developing an inhalational therapy for non-cystic fibrosis bronchiectasis (NCFB). Part 2: endpoint considerations. 2018. www.fda.gov/media/114392/download Date last accessed: 7 September 2022.
- 15.Lederer DJ, Bell SC, Branson RD, et al. Control of confounding and reporting of results in causal inference studies. Guidance for authors from editors of respiratory, sleep, and critical care journals. Ann Am Thorac Soc 2019; 16: 22–28. doi: 10.1513/AnnalsATS.201808-564PS [DOI] [PubMed] [Google Scholar]
- 16.Peduzzi P, Concato J, Kemper E, et al. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996; 49: 1373–1379. doi: 10.1016/s0895-4356(96)00236-3 [DOI] [PubMed] [Google Scholar]
- 17.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44: 837–845. [PubMed] [Google Scholar]
- 18.Boaventura R, Sibila O, Agusti A, et al. Treatable traits in bronchiectasis. Eur Respir J 2018; 52: 1801269. doi: 10.1183/13993003.01269-2018 [DOI] [PubMed] [Google Scholar]
- 19.Chalmers JD, Boersma W, Lonergan M, et al. Long-term macrolide antibiotics for the treatment of bronchiectasis in adults: an individual participant data meta-analysis. Lancet Respir Med 2019; 7: 845–854. doi: 10.1016/S2213-2600(19)30191-2 [DOI] [PubMed] [Google Scholar]
- 20.Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 2017; 50: 1700629. doi: 10.1183/13993003.00629-2017 [DOI] [PubMed] [Google Scholar]
- 21.Patel S, Cole AD, Nolan CM, et al. Pulmonary rehabilitation in bronchiectasis: a propensity-matched study. Eur Respir J 2019; 53: 1801264. doi: 10.1183/13993003.01264-2018 [DOI] [PubMed] [Google Scholar]
- 22.McDonnell MJ, Aliberti S, Goeminne PC, et al. Comorbidities and the risk of mortality in patients with bronchiectasis: an international multicentre cohort study. Lancet Respir Med 2016; 4: 969–979. doi: 10.1016/S2213-2600(16)30320-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finch S, McDonnell MJ, Abo-Leyah H, et al. A comprehensive analysis of the impact of Pseudomonas aeruginosa colonization on prognosis in adult bronchiectasis. Ann Am Thorac Soc 2015; 12: 1602–1611. doi: 10.1513/AnnalsATS.201506-333OC [DOI] [PubMed] [Google Scholar]
- 24.Kumarasamy KK, Toleman MA, Walsh TR, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis 2010; 10: 597–602. doi: 10.1016/S1473-3099(10)70143-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palwatwichai A, Chaoprasong C, Vattanathum A, et al. Clinical, laboratory findings and microbiologic characterization of bronchiectasis in Thai patients. Respirology 2002; 7: 63–66. doi: 10.1046/j.1440-1843.2002.00367.x [DOI] [PubMed] [Google Scholar]
- 26.Park J, Kim S, Lee YJ, et al. Factors associated with radiologic progression of non-cystic fibrosis bronchiectasis during long-term follow-up. Respirology 2016; 21: 1049–1054. doi: 10.1111/resp.12768 [DOI] [PubMed] [Google Scholar]
- 27.De Soyza A, McDonnell MJ, Goeminne PC, et al. Bronchiectasis rheumatoid overlap syndrome is an independent risk factor for mortality in patients with bronchiectasis: a multicenter cohort study. Chest 2017; 151: 1247–1254. doi: 10.1016/j.chest.2016.12.024 [DOI] [PubMed] [Google Scholar]
- 28.Polverino E, Dimakou K, Hurst J, et al. The overlap between bronchiectasis and chronic airway diseases: state of the art and future directions. Eur Respir J 2018; 52: 1800328. doi: 10.1183/13993003.00328-2018 [DOI] [PubMed] [Google Scholar]
- 29.Patel IS, Vlahos I, Wilkinson TMA, et al. Bronchiectasis, exacerbation indices, and inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004; 170: 400–407. doi: 10.1164/rccm.200305-648OC [DOI] [PubMed] [Google Scholar]
- 30.Parr DG, Guest PG, Reynolds JH, et al. Prevalence and impact of bronchiectasis in α1-antitrypsin deficiency. Am J Respir Crit Care Med 2007; 176: 1215–1221. doi: 10.1164/rccm.200703-489OC [DOI] [PubMed] [Google Scholar]
- 31.Diaz AA, Young TP, Maselli DJ, et al. Bronchoarterial ratio in never-smokers adults: implications for bronchial dilation definition. Respirology 2017; 22: 108–113. doi: 10.1111/resp.12875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Jahdali H, Alshimemeri A, Mobeireek A, et al. The Saudi Thoracic Society guidelines for diagnosis and management of noncystic fibrosis bronchiectasis. Ann Thorac Med 2017; 12: 135–161. doi: 10.4103/atm.ATM_171_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crichton ML, Aliberti S, Chalmers JD. A systematic review of pharmacotherapeutic clinical trial end-points for bronchiectasis in adults. Eur Respir Rev 2019; 28: 180108. doi: 10.1183/16000617.0108-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00611-2022.Supplement (320.4KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-00611-2022.Shareable (846KB, pdf)