Abstract
A spinal cord injury (SCI) is a destructive event that causes a permanent deficit in neurological function because of poor regenerative potential. Transplantation therapies have attracted attention for restoration of the injured spinal cord, and transplantation of neural precursor cells (NPCs) has been studied worldwide. Several groups have demonstrated functional recovery via this therapeutic intervention due to the multiple beneficial effects of NPC transplantation, such as reconstruction of neuronal circuits, remyelination of axons, and neuroprotection by trophic factors. Our group developed a method to induce NPCs from human induced pluripotent stem cells (hiPSCs) and established a transplantation strategy for SCI. Functional improvement in SCI animals treated with hiPSC-NPCs was observed, and the safety of transplanting these cells was evaluated from multiple perspectives. With selection of a safe cell line and pretreatment of the cells to encourage maturation and differentiation, hiPSC-NPC transplantation therapy is now in the clinical phase of testing for subacute SCI. In addition, a research challenge will be to expand the efficacy of transplantation therapy for chronic SCI. More comprehensive strategies involving combination treatments are required to treat this problematic situation.
Keywords: Spinal cord injury, Transplantation, Neural precursor cells, Induced pluripotent stem cell
INTRODUCTION
Spinal cord injury (SCI) occurs upon sudden high-energy impact such as that in vehicular or contact sports accidents. In recent years, cervical SCI caused by minor traumas among the elderly has been raised as an additional issue for the aging society. In clinical stage, SCI is classified to complete injury, which is defined as no preservation of motor and/or sensory function, and incomplete injury which remains any neurological function below the injured site [1]. More than 70% of patients with complete SCI progress without functional improvement [2]. As a result, 3 million people are estimated to suffer from SCI worldwide, with 180,000 new cases each year [3].
Since Cajal proposed the difficulty of regenerating the central nervous system, numerous researchers have struggled to develop treatments for SCI [4]. However, the effects of conventional treatments, including methylprednisolone sodium succinate infusion, are modest [5,6], and surgical decompression of the injured spinal cord is still the only gold standard therapy for this injury [7,8].
The lack of regenerative capacity in the injured spinal cord is related to many factors, such as characteristics that inhibit axonal regeneration, poor regenerative ability of endogenous neural precursor cells (NPCs), and insufficient support by trophic factors [5]. Particularly in complete transection model, which well reproduces the complete injury in human, there are no spared axons in the injured site and exogenous replacements are essential. Cell transplantation therapies have attracted attention as possible methods to overcome these disadvantages due to their multiple types of therapeutic potentials [3]. We aim to develop a human induced pluripotent stem cell (hiPSC)-derived NPC transplantation therapy to treat this challenging pathology and advance it to clinical application [6,9,10].
In this report, we review various studies on transplantation therapies for SCI, especially NPC transplantation, and described the present stage of clinical translation for our hiPSC-NPC transplantation therapy.
NPC TRANSPLANTATION FOR SCI
Transplantation therapy for SCI has been researched worldwide over a few decades. Several types of cells, such as Schwann cells [11-15], mesenchymal stem cells [16-22], olfactory ensheathing cells [23-25], and NPCs [9,26-31] have been attempted to be used as candidate cell sources. The diverse characteristics of each cell have been found to promote unique beneficial effects for the injured spinal cord and to lead to functional recovery in a SCI animal model [3]. The main concept for NPC transplantation is to replace lost neural cells [32] by compensating for the poor regenerative ability of endogenous NPCs.
Approximately 20 years ago, embryonic cells or tissues were transplanted into SCI animals [33,34]. Transplantation of these heterogeneous cells resulted in functional recovery of the animals along with histological reorganization, and the findings implied the efficacy of cell restoration via transplantation of fetal cells [34]. However, a large number of fetuses are required to obtain enough tissue to apply to humans. Therefore, this treatment has not advanced to clinical use [26]. In addition, advances in biological techniques have led to the routine dissociation and expansion of NPCs from fetal tissues [35]. Collaboration of these culture methods and research to treat SCI have enabled great progress in knowledge to be made in the field of NPC transplantation therapy.
Vacanti et al. [36] transplanted spinal cord progenitor cells isolated from the spinal tissue of adult rats into SCI model animals. Even though engraftment of transplanted cells and functional improvement were observed, the transplanted cells in that study included differentiated neurons. The authors did not fully prove the efficacy of NPCs in transplanting cells. In addition, Ogawa et al. [26] performed transplantation of NPCs derived from the rat embryonic spinal cord. The transplanted cells differentiated into neural cells, and the donor-derived neurons integrated into host tissue. Behavioral improvement was also observed, which was presumed to be caused by the regenerated axons and oligodendrocytes derived from graft cells. Moreover, this study was demonstrated in a contusive injury model animal, which closely mimics the pathology of incomplete injury in clinical study compared with incomplete transection models.
Following these results, the multipotency of NPCs is expected to produce various beneficial effects for the injured spinal cord and attracted researchers wishing to treat SCI.
TRANSPLANTATION OF NPCs IN THE SUBACUTE PHASE
The timing of transplantation is a critical factor in SCI treatment. Treating acute and chronic SCI is considered difficult due to environmental changes, such as the formation of a cavity surrounded by a glial scar [37]. Parr et al. [38] reported a poor survival rate of transplanted cells in the acute and chronic phases compared with the subacute phase. A similar result was revealed from the group of Fehlings [28]. That group observed dead grafted cells in the center area when the cells were transplanted in the chronic phase and failed to treat the injury. Nishimura et al. [39] clarified the underlying difficult conditions of chronic SCI and reported that glial scar formation and inflammation were the most remarkable differences in the injured spinal cord microenvironment between the subacute and chronic phases.
Including the aforementioned reports, subacute phase in rodents are defined as 7-14 days after injury. In contrast, analyzes of gene expression revealed that the inflammatory response is significantly prolonged and the onset of glial scar formation is temporally delayed in nonhuman primates [40]. Thus, the time window for cell transplantation to nonhuman primates are thought to be 14–28 days after injury.
All in all, the subacute phase is considered to be the optimal time for NPC transplantation, and various reports have demonstrated the therapeutic effect of transplanting NPCs at this phase [9,26,28-30,41-43]. To understand the pathology of injured spinal cord is extremely important to achieve sufficient improvement by transplantation therapy. Further research must be conducted to define and evaluate the timing of transplantation to human.
THERAPEUTIC MECHANISMS OF NPC TRANSPLANTATION
Although NPC transplantation improves functional outcomes in SCI animals, the detailed underlying mechanisms of this treatment remain unclear. In recent years, several studies have focused on the mechanisms of recovery mediated by NPC transplantation. Several approaches have been utilized to demonstrate how transplanted cells contribute to the improvement of motor function. Administration of diphtheria toxin to ablate engrafted human cells is frequently used to evaluate the therapeutic potential of the transplanted cells [41,42,44]. Elimination of whole transplanted cells leads to the functional deterioration of host animals, indicating that engrafted NPCs somehow contribute to the recovery of host motor function. Regarding the favorable effects, 3 main mechanisms have been proposed as therapeutic factors for NPC transplantation: reconstruction of the neuronal circuits, remyelination of axons, and neuroprotection by trophic factors (Fig. 1) [45].
Fig. 1.
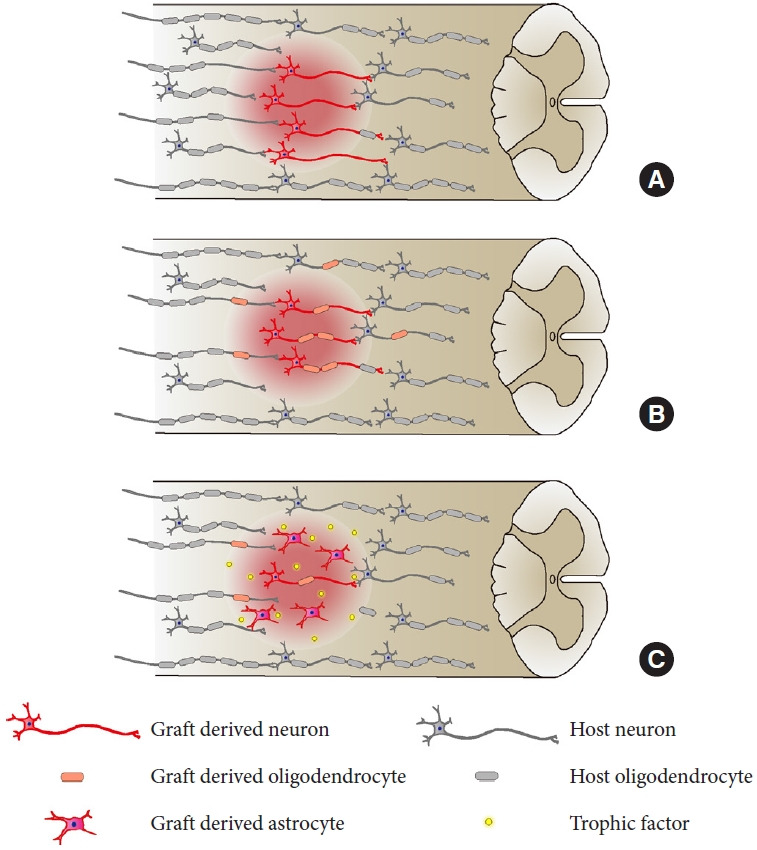
Three main mechanisms have been proposed to repair the injured spinal cord. (A) Reconstruction of the neuronal circuits by relay formation of engrafted cells. (B) Remyelination of graft axons and host axons by graft-derived oligodendrocytes. (C) Neuroprotection by trophic factors secreted from the transplanted cells.
1. Reconstruction of Neuronal Circuits
The strategy for reconstruction of neuronal circuits can be divided into 2 models: (1) regrowth of an injured axon back to the original target and (2) relay formation, in which a new neuron is inserted between the injured axon and the target neuron [46]. The advantage of NPC transplantation is that it can provide neurons that may be capable of extending axons in the injured spinal cord and achieve new neuronal circuits by relay formation.
In the early days, axon tracing techniques or immunohistological staining of neurons was used to annotate the connections between host neurons and graft neurons [29,30,47-49]. Lu et al. [29] demonstrated a large number of grafted axons extending over the injury site even in severe SCI model animals. Immunohistological synaptic markers also show synapse formation between the graft and host neurons, and immunoelectron microscopy analysis has confirmed the detailed formation of each synapse [9,29,30,50,51].
The latest technologies of neuroscience have also attracted attention in this spinal cord area. A previous study by Tuszynski’s group combined the optogenetic stimulation method and a genetically encoded calcium indicator to reveal the relay mechanisms of host neurons and graft neurons. Stimulation of host corticospinal tract axons elicited neuronal network responses throughout the grafted neurons. Moreover, optogenetic stimulation of graft neurons triggered the neuronal activity of host neurons in the caudal area of injury, which implies the existence of an electrophysiological connection in the relay-formed neuronal network [52].
Another strategy is to visualize neuronal activity using a luminescent protein. The AkaBLI system, which is a newly developed redshifted bioluminescence imaging system, has been used to demonstrate in vivo imaging of graft neuronal activity. Upon transduction of the AkaLuc enzyme under the control of a potent neuronal activity-dependent synthetic promoter in transplanted NPCs, engrafted cells show enhanced luminescence upon stimulation of the CST tract. This also indicates the neuronal connections of host neurons and graft neurons [53].
Our group and others have focused on the relationship between graft neurons and host motor functions. Designer Receptor Exclusively Activated by Designer Drugs (DREADD) [54-56], which is a chemogenetically engineered protein that permits control of neuronal activity via administration of the ligand clozapine N-oxide (CNO), has been applied to verify the therapeutic effect on neuronal activity in graft-derived cells. By inhibiting the activity of the graft-derived neurons with inhibitory DREADD receptor, the locomotor function of host animals temporary declined after CNO administration, which directly suggested the contribution of graft neuronal activity to the recovery of locomotor function [31,57]. On top of that, activation of graft neuronal activity by excitatory DREADD results in increased connectivity with host neurons and additional functional recovery [51]. This also shows the connectivity of host and graft neurons and indicates the possibility of improving this therapy by enhancing the neuronal connections.
Accordingly, neurons derived from grafted NPCs have been successfully integrated into host neuronal circuits and play a role in functional recovery with transplantation therapy.
2. Remyelination of Axons
The beneficial effect of remyelination after SCI is still controversial. Duncan et al. [58] inhibited remyelination by deleting Myrf, a gene that plays a crucial role in remyelination. As the deletion of Myrf did not correlate with the motor function of the host animals, the results implied that there is no relationship between functional outcome and remyelination due to endogenous oligodendrocyte precursor cells (OPCs). On the other hand, Yasuda et al. [59] reported the importance of remyelination by graft-derived cells for functional recovery after the transplantation of NPCs. In that report, transplantation of NPCs isolated from myelin-deficient shiverer mutant mice resulted in a lower rate of functional recovery than transplantation of NPCs isolated from wild-type mice.
Similarly, a study by Salewski et al. [60] demonstrated a practical difference upon transplantation of iPSC-NPCs derived from shiverer mutant mice and wild-type mice, which also implies the importance of remyelination due to transplanted cells. Despite these controversial results, myelinating injured axons is recognized as a critical process after SCI. Several studies have aimed to increase the myelinating potential of transplanting cells. The use of OPC-enriched NPCs for transplantation is a representative example of such a strategy [61-64]. Kawabata et al. [61] and Kamata et al. [64] transplanted gliogenic NPCs generated by a culture protocol modified from one previously reported. Remyelination by graft-derived oligodendrocytes was confirmed by immunoelectron microscopy analysis after OPC-enriched NPCs were transplanted into SCI animals. Similarly, Fehlings’ group observed remyelination of axons and functional recovery after transplanting oligodendrogenic NPCs directly reprogrammed from somatic bone marrow cells [62]. Overall, animals treated with OPC-enriched NPC exhibit functional improvement. Thus, remyelination by transplanted cells is considered to exert a beneficial effect on the injured spinal cord [61-64].
3. Neuroprotection by Trophic Factors
After the primary mechanical trauma to the spinal cord, secondary injury, which causes progressive damage through complex biochemical factors, begins and continues for weeks or months [65]. Neuroprotection by trophic factors secreted from transplanted NPCs is presumed to mediate secondary injury and support the regeneration of neural cells. Hawryluk et al. [66] reported elevated expression levels of glial-derived neurotrophic factor (GDNF), leukemia inhibitory factor, and basic fibroblast growth factor (bFGF). Another previous study has identified 49 neurotrophic factors expressed by transplanted cells, including insulin-like growth factor 1 (IGF-1), brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT3), and transforming growth factor 1 [67]. Similarly, we have previously demonstrated the secretion of nerve growth factor, BDNF, vascular endothelial growth factor (VEGF), NT3, NT4, ciliary neurotrophic factor (CNTF), and hepatocyte growth factor (HGF) from hiPSC-NPCs, and these factors exerted favorable effects on behavior soon after transplantation [9,43,68]. NT3, NT4, and CNTF encourage axonal sparing after injury, and VEGF enhances angiogenesis at the lesion site by promoting cell survival pathways [43]. HGF is known to promote NPC proliferation and neuronal differentiation, thereby playing a key role in the enhancement of functional recovery [68]. Because of these favorable effects of trophic factors, delivering growth factors is thought to promote therapeutic effects. Karimi-Abdolrezaee et al. [28] combined NPC transplantation with the delivery of growth factors such as platelet-derived growth factor (PDGF-AA), bFGF, and epidermal growth factor (EGF) to promote cell survival in the environment of the injured spinal cord. Lu et al. [29] achieved long-distance growth of grafted neurons by supporting graft survival with BDNF, NT-3, PDGF-AA, IGF-1, EGF, bFGF, acidic FGF, GDNF, HGF, and a calpain inhibitor.
In recent years, microRNA (miRNA), such as miRNA-210 and miRNA 126, has also been reported to mediate the environment of injured spinal cord via angiogenesis or attenuation of inflammation [69,70]. In case of NPC transplantation, Yang et al. [71] showed upregulation of miRNA-375-3p and miRNA-1-3p, and downregulation of miRNA-363-3p, miRNA-449a-5p, and miRNA-3074 in mice treated with oligodendrogenic NPCs. As bioinformatics analysis of these miRNA indicates the relation with cell proliferation and neuronal differentiation, these results suggest the possibility that miRNA promoted functional recovery in oligodendrogenic NPC transplantation.
Despite this evidence, the detailed effects of each factor are still being investigated, and further studies are needed.
CELL SOURCES FOR GENERATING NPCs
In basic research, NPCs are generated from several cell sources. In the early era, somatic stem cells were used to prepare NPCs, as described above [26,36]. Similarly, Lu et al. [29] demonstrated the neuronal connections of host and graft cells by transplanting NPCs dissected from the embryonic rat spinal cord in a recent study. In the same way, NPCs have been generated from fetal spinal cells of rodents or humans in several studies [27,28,31,72]. Reprogramming from somatic cells has also been performed in recent studies. Fehling's group transplanted OPC-enriched NPCs, which were directly reprogrammed from bone marrow somatic cells and demonstrated functional recovery [62,63].
In the past few decades, methods to induce NPCs from pluripotent cells have been investigated [73]. Kumagai et al. [74] promoted functional recovery by transplanting NPCs generated from embryonic stem cells (ESCs). However, using somatic stem cells or ESCs for the clinical phase is associated with ethical and immunological concerns and therefore casts a shadow over the advancement of this transplantation therapy. iPSCs were invented by Yamanaka’s group, and the use of these new pluripotent stem cells as a source of NPCs resolves these problems [75]. In addition to this ethical problem, iPSCs raised a possibility of autologous transplantation. This was an obvious advantage to allogenic transplantations, such as NPCs derived from somatic stem cells or ESCs, which requires immunosuppression when transplanting to human spinal cord. Tsuji et al. [76] transplanted NPCs derived from murine iPSC, followed by a report from Nori et al. [9] which transplanted human iPSC. Lu et al. [30] transplanted NPCs derived from iPSCs that were harvested from a healthy 86-year-old male and induced to differentiate. Salewski et al. [60] transplanted NPCs derived from iPSCs generated by a nonviral piggyBac transposon approach.
According to these reports from several groups, iPSC-NPC transplantation shows favorable results. Moreover, using hiPSCs as a cell source for NPCs enables the transplanted cells to successfully survive the injured spinal cord and differentiate into neural cells [9,43]. Similar to NPCs derived from other cell sources, neurons differentiated from hiPSC-NPCs integrate into host neuronal circuits and alter the motor activity of host animals [9,49,57]. hiPSC-NPCs also secrete nerve growth factor, BDNF, and HGF, which exert favorable effects on behavior soon after transplantation [9]. Additionally, remyelination of axons by graft oligodendrocytes has been observed after enrichment of OPCs in transplanted cells [61,64]. Overall, the multiple beneficial factors clearly improve the locomotor function of host animals, and hiPSC-NPCs have become a leading candidate cell type for transplantation for SCI treatment.
SAFETY OF hiPSC-NPC TRANSPLANTATION
Although hiPSC-NPC transplantation leads to a favorable result for SCI animals, transplantation of immature cells has a risk of tumorigenicity. The potency of tumorigenic change differs by hiPS cell line [77,78]. Even if safe iPS cell line-derived NPCs are transplanted, tumor-like growth occasionally occurs [79]. For clinical application, establishing a safe transplantation therapy is indispensable, and we have handled this problem from multiple perspectives.
1. Prediction and Detection of Tumorigenic Change
The ideal strategy to avoid tumorigenicity after transplantation is to predict the risk of tumorigenic change before transplantation and to select safe cell lines. To achieve selection accuracy, we compared the gene expression profiles of tumorigenic and nontumorigenic hiPSC-NPCs by comprehensive DNA methylation analysis [80]. The genomic regions surrounding the transcriptional start sites of the tumor suppressor genes were hypermethylated in tumorigenic NPCs but not in nontumorigenic NPCs. Interestingly, the aberrant DNA methylation profile was more pronounced when the number of passages increased, even for the cell lines that were initially nontumorigenic. The methylation profiles and the passage number limits should be included in the criteria for clinical settings.
Detecting tumorigenic formation at the early stage will also be required to guarantee the safety of this therapy. In tumorigenic hiPSC-NPCs, the differentiation-resistant properties of abnormal cells cause the continuous growth of transplanted cells. Tanimoto et al. [78] reported a method to detect these proliferating cells using positron emission tomography (PET). The immature NPCs showed a high expression level of the 19 kFa translocator protein (TSPO), also known as the peripheral-type benzodiazepine receptor. PET with [18F] FEDAC (a TSPO radioligand) succeeded in visualizing the remaining undifferentiated hiPSC-NPC derived cells, which were TSPO+ and Nestin+ cells, by histological analysis. This technique could also play a key role in the clinical stage.
2. Prevention of Tumorigenic Overgrowth by Pretreatment With a Notch Signaling Inhibitor
Despite the careful selection of cell lines for transplantation, the infrequent occurrence of tumorigenic changes remains a concern for this therapy. To address this problem, we investigated pretreatment with a gamma secretase inhibitor (GSI), which inhibits Notch signaling that controls the induction of NPCs [50,81]. Pretreatment of GSI promoted neuronal differentiation and maturation of hiPSC-NPCs in vitro. By this pretreatment, transplantation of tumorigenic hiPSC-NPCs resulted without any tumor formation. Thus, this study indicates the effectiveness of GSI pretreatment in preventing cell overgrowth. Moreover, pretreatment with GSI before transplantation of nontumorigenic hiPSC-NPCs leads to further functional recovery of host animals according to the maturation and neuronal differentiation induced by Notch signal inhibition [50]. Therefore, GSI pretreatment is considered a critical process in hiPSC-NPC transplantation treatment to prevent tumorigenic changes and enhance therapeutic efficacy.
3. Usage of Suicide Genes to Eliminate Tumorigenic hiPSC-NPCs
Even though selection of cell lines and pretreatment to prevent overgrowth decreases the risk of tumorigenesis, measures should be taken to eliminate proliferating cells. In order to solve this issue, we have proposed several methods to ablate tumorigenic transplanted cells by using a suicide gene system, which is also applied to transplantation therapy in other disease [77,82,83]. In the first method, induced caspase-9, which is a member of the caspase family of cysteine proteases that have been implicated in apoptosis and cytokine processing, was transduced into tumorigenic hiPSC-NPCs and transplanted into SCI animals [77]. As expected, the transplanted cells formed tumor-like growths. When the apoptosis inducer was injected, the transplanted cells were completely ablated, indicating this system’s effectiveness in salvaging from undesired overgrowth. However, this system ablated all transplanted cells, including the differentiated cells that were contributing to the functional recovery. Eventually, the improved motor function achieved by the transplantation deteriorated after the suicide gene system was triggered.
To overcome this disadvantage, we next chose the herpes simplex virus type 1 thymidine kinase (HSVtk) gene as a candidate suicide gene [82]. Via HSVtk, ganciclovir (GCV), the prodrug, can be converted to cytotoxic GCV-triphosphate. Thus, HSVtk-expressing cells can be eliminated by GCV induction. This system kills the cell by causing a delay in the S and G2 phases, resulting in apoptosis of only proliferating cells. Upon transplanting HSVtk-transduced tumorigenic hiPSC-NPCs and stimulating the suicide by GCV, only proliferative cells were ablated, and the improved locomotor function was sustained. Therefore, this new approach enables treatment of tumorigenesis without sacrifice of the improved motor function.
CLINICAL APPLICATION OF hiPSC-NPC TRANSPLANTATION
Given the considerable evidence from basic and preclinical studies revealing the effectiveness and safety of hiPSC-NPC transplantation, our transplantation therapy for SCI has reached the clinical application stage [6].
Similar to other transplantation therapies for SCI, the treatments in our previous studies have mainly treated SCI at the subacute phase, which is considered an optimal time for treatment due to the neural plasticity and reactivity at this stage [10].
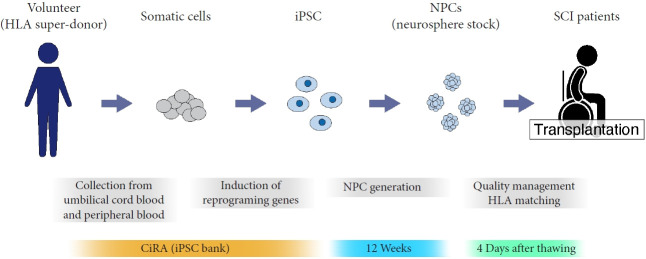
Since autologous transplantation is unrealistic for the subacute phase because of the lack of a generation period for transplanted iPSCs, allograft transplantation of NPCs derived from an hiPSC stock at the Center for iPS Cell Research and Application (CiRA) is being applied in this clinical trial. The hiPSC stocks were generated from human leukocyte antigen (HLA) superdonors who are homozygous at the 3 major HLA gene loci to maintain a pool of immunologically safe iPSC clones corresponding to various HLA types. The quality of the generated cells has been thoroughly checked based on general characteristics, marker expression of differentiated cells, and genomic factors to ensure safety after transplantation (Fig. 2) [10].
Fig. 2.
Schematic illustration of iPSC-NPC transplantation therapy in the clinical trial. iPSC, induced pluripotent stem cell; NPC, neural precursor cell; HLA, human leukocyte antigen; SCI, spinal cord injury; CiRA, Center for iPS Cell Research and Application.
Beginning in 2021, we began recruiting complete SCI patients (American Spinal Cord Injury Association Impairment Scale A) within 14–28 days of injury. hiPSC-NPCs pretreated with GSI are being transplanted, and patients will be followed up for one year with proper neurologic evaluations.
TRANSPLANTATION THERAPY FOR THE CHRONIC PHASE
The next mission for this treatment is to expand toward the chronic phase after injury. More than 90% of patients still suffer from their impairments and disabilities in the chronic phase; therefore, this phase is the most significant stage for related research [10]. However, as Nishimura et al. proved the difficulty of treating chronic SCI by only NPC transplantation, several studies have failed to recover motor functions in the chronic phase, and only a few reports have shed light on this challenging situation [84-86]. Additionally, the timing of chronic phase in the studies of rodent model lay from 28 days to 91 days after injury [28,37,38,87]. The underlying environment in chronic phase will not be in a homogeneous pathology following the progression after injury, thus, the wide range of this phase results in confusion and difficulty for treating.
Since NPC transplantation alone is insufficient for treating chronic SCI, combination with other therapeutic factors is required to overcome this difficult situation. Okubo et al. [37] reported functional recovery upon transplantation of GSI-pretreated hiPSC-NPCs into chronic SCI model mice. Notch signaling inhibition promoted the maturation of transplanted cells and resulted in significantly enhanced axonal regrowth, remyelination, and inhibitory synapse formation with host neurons. Additionally, GSI pretreatment caused phosphorylation of p38 mitogen-activated protein kinases, which are also key molecules required to promote axonal regeneration. Through these favorable factors, we succeeded in improving locomotor function in the chronic phase.
Another promising therapy involves concomitant rehabilitation. Rehabilitation is known to be an effective treatment for SCI, even in the clinical stage [88-92]. In our previous report, combining rehabilitative treatment with transplantation of NPCs harvested from embryonic mouse spinal cords enhanced the independent therapeutic effects of each single therapy [93]. The synergistic effects of this combination therapy facilitated neuronal differentiation of transplanted cells and maturation of the central pattern generator. Since hiPSC-derived cells were not used in this study, the synergistic effects of hiPSC-NPCs and rehabilitation need to be confirmed in the near future.
The use of chondroitinase ABC (ChABC) to degrade chondroitin sulfate proteoglycans, which form a potent barrier for axon regeneration, is also a promising strategy to treat chronic SCI [94]. Upon delivery of ChABC, transplanted iPSC-NPCs can survive and differentiate into neural cells. Furthermore, the differentiated neurons form functional synapses and improve the motor function of host animals.
Finally, the Marsala group transplanted human spinal cord-derived neural stem cells into 4 humans with chronic SCI [95]. By using a cell line authorized by U.S. Food and Drug Administration, the safety of the transplantation was implied, although it did not have the statistical power to evaluate functional changes.
CONCLUSION
Due to the numerous efforts and evidence accumulated to date, hiPSC-NPC transplantation therapy has advanced to the clinical application stage. Nevertheless, several aspects remain to be investigated, such as the detailed therapeutic mechanisms and strategies to enhance the therapeutic effects. Although numerous studies have significantly succeeded to improve functional outcomes, no one has fully recovered the injured spinal cord and behaviors, especially in sever injury models. The pathophysiological knowledge of injured spinal cord and mechanisms to treat this injury must lead to an optimal strategy for complete improvement from SCI. Additionally, the permanent effect of transplantation has not been proven in animal models because of the short lifespan compared with human. To secure safety and confirm the persistent functional enhancement, long term studies using primate models are required. Lastly, to establish and enable this transplantation therapy to proceed to the treatment of chronic SCI, further basic researches are required.
Footnotes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant-in- Aid for Scientific Research (B) (22H03205) to NN.
Author Contribution
Conceptualization: NN; Data curation: TK; Formal analyssi: TK; Methodology: TK; Project administration: NN; Visualization: NN; Writing - original draft: TK; Writing - review & editing: NN, HO, MN.
REFERENCES
- 1.Lukovic D, Moreno-Manzano V, Lopez-Mocholi E, et al. Complete rat spinal cord transection as a faithful model of spinal cord injury for translational cell transplantation. Sci Rep. 2015;5:9640. doi: 10.1038/srep09640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirshblum S, Snider B, Eren F, et al. Characterizing natural recovery after traumatic spinal cord injury. J Neurotrauma. 2021;38:1267–84. doi: 10.1089/neu.2020.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assinck P, Duncan GJ, Hilton BJ, et al. Cell transplantation therapy for spinal cord injury. Nat Neurosci. 2017;20:637–47. doi: 10.1038/nn.4541. [DOI] [PubMed] [Google Scholar]
- 4.Illis LS. Central nervous system regeneration does not occur. Spinal Cord. 2012;50:259–63. doi: 10.1038/sc.2011.132. [DOI] [PubMed] [Google Scholar]
- 5.Okano H, Kaneko S, Okada S, et al. Regeneration-based therapies for spinal cord injuries. Neurochem Int. 2007;51:68–73. doi: 10.1016/j.neuint.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Sugai K, Sumida M, Shofuda T, et al. First-in-human clinical trial of transplantation of iPSC-derived NS/PCs in subacute complete spinal cord injury: study protocol. Regen Ther. 2021;18:321–33. doi: 10.1016/j.reth.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hachem LD, Ahuja CS, Fehlings MG. Assessment and management of acute spinal cord injury: from point of injury to rehabilitation. J Spinal Cord Med. 2017;40:665–75. doi: 10.1080/10790268.2017.1329076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fehlings MG, Vaccaro A, Wilson JR, et al. Early versus delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS) PLoS One. 2012;7:e32037. doi: 10.1371/journal.pone.0032037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nori S, Okada Y, Yasuda A, et al. Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc Natl Acad Sci U S A. 2011;108:16825–30. doi: 10.1073/pnas.1108077108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagoshi N, Okano H, Nakamura M. Regenerative therapy for spinal cord injury using iPSC technology. Inflamm Regen. 2020;40:40. doi: 10.1186/s41232-020-00149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu XM, Guenard V, Kleitman N, et al. Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult rat spinal cord. J Comp Neurol. 1995;351:145–60. doi: 10.1002/cne.903510113. [DOI] [PubMed] [Google Scholar]
- 12.Takami T, Oudega M, Bates ML, et al. Schwann cell but not olfactory ensheathing glia transplants improve hindlimb locomotor performance in the moderately contused adult rat thoracic spinal cord. J Neurosci. 2002;22:6670–81. doi: 10.1523/JNEUROSCI.22-15-06670.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearse DD, Pereira FC, Marcillo AE, et al. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10:610–6. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- 14.Hill CE, Moon LD, Wood PM, et al. Labeled Schwann cell transplantation: cell loss, host Schwann cell replacement, and strategies to enhance survival. Glia. 2006;53:338–43. doi: 10.1002/glia.20287. [DOI] [PubMed] [Google Scholar]
- 15.Sparling JS, Bretzner F, Biernaskie J, et al. Schwann cells generated from neonatal skin-derived precursors or neonatal peripheral nerve improve functional recovery after acute transplantation into the partially injured cervical spinal cord of the rat. J Neurosci. 2015;35:6714–30. doi: 10.1523/JNEUROSCI.1070-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osaka M, Honmou O, Murakami T, et al. Intravenous administration of mesenchymal stem cells derived from bone marrow after contusive spinal cord injury improves functional outcome. Brain Res. 2010;1343:226–35. doi: 10.1016/j.brainres.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Honmou O, Onodera R, Sasaki M, et al. Mesenchymal stem cells: therapeutic outlook for stroke. Trends Mol Med. 2012;18:292–7. doi: 10.1016/j.molmed.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Nakajima H, Uchida K, Guerrero AR, et al. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J Neurotrauma. 2012;29:1614–25. doi: 10.1089/neu.2011.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quertainmont R, Cantinieaux D, Botman O, et al. Mesenchymal stem cell graft improves recovery after spinal cord injury in adult rats through neurotrophic and pro-angiogenic actions. PLoS One. 2012;7:e39500. doi: 10.1371/journal.pone.0039500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsushita T, Lankford KL, Arroyo EJ, et al. Diffuse and persistent blood-spinal cord barrier disruption after contusive spinal cord injury rapidly recovers following intravenous infusion of bone marrow mesenchymal stem cells. Exp Neurol. 2015;267:152–64. doi: 10.1016/j.expneurol.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Morita T, Sasaki M, Kataoka-Sasaki Y, et al. Intravenous infusion of mesenchymal stem cells promotes functional recovery in a model of chronic spinal cord injury. Neuroscience. 2016;335:221–31. doi: 10.1016/j.neuroscience.2016.08.037. [DOI] [PubMed] [Google Scholar]
- 22.Honmou O, Yamashita T, Morita T, et al. Intravenous infusion of auto serum-expanded autologous mesenchymal stem cells in spinal cord injury patients: 13 case series. Clin Neurol Neurosurg. 2021;203:106565. doi: 10.1016/j.clineuro.2021.106565. [DOI] [PubMed] [Google Scholar]
- 23.Fouad K, Schnell L, Bunge MB, et al. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J Neurosci. 2005;25:1169–78. doi: 10.1523/JNEUROSCI.3562-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richter MW, Fletcher PA, Liu J, et al. Lamina propria and olfactory bulb ensheathing cells exhibit differential integration and migration and promote differential axon sprouting in the lesioned spinal cord. J Neurosci. 2005;25:10700–11. doi: 10.1523/JNEUROSCI.3632-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbour HR, Plant CD, Harvey AR, et al. Tissue sparing, behavioral recovery, supraspinal axonal sparing/regeneration following sub-acute glial transplantation in a model of spinal cord contusion. BMC Neurosci. 2013;14:106. doi: 10.1186/1471-2202-14-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogawa Y, Sawamoto K, Miyata T, et al. Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J Neurosci Res. 2002;69:925–33. doi: 10.1002/jnr.10341. [DOI] [PubMed] [Google Scholar]
- 27.Iwanami A, Kaneko S, Nakamura M, et al. Transplantation of human neural stem cells for spinal cord injury in primates. J Neurosci Res. 2005;80:182–90. doi: 10.1002/jnr.20436. [DOI] [PubMed] [Google Scholar]
- 28.Karimi-Abdolrezaee S, Eftekharpour E, Wang J, et al. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci. 2006;26:3377–89. doi: 10.1523/JNEUROSCI.4184-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu P, Wang Y, Graham L, et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150:1264–73. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu P, Woodruff G, Wang Y, et al. Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron. 2014;83:789–96. doi: 10.1016/j.neuron.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dell’Anno MT, Wang X, Onorati M, et al. Human neuroepithelial stem cell regional specificity enables spinal cord repair through a relay circuit. Nat Commun. 2018;9:3419. doi: 10.1038/s41467-018-05844-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bjorklund A, Lindvall O. Cell replacement therapies for central nervous system disorders. Nat Neurosci. 2000;3:537–44. doi: 10.1038/75705. [DOI] [PubMed] [Google Scholar]
- 33.Anderson DK, Howland DR, Reier PJ. Fetal neural grafts and repair of the injured spinal cord. Brain Pathol. 1995;5:451–57. doi: 10.1111/j.1750-3639.1995.tb00624.x. [DOI] [PubMed] [Google Scholar]
- 34.Diener PS, Bregman BS. Fetal spinal cord transplants support the development of target reaching and coordinated postural adjustments after neonatal cervical spinal cord injury. J Neurosci. 1998;18:763–78. doi: 10.1523/JNEUROSCI.18-02-00763.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–10. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 36.Vacanti MP, Leonard JL, Dore B, et al. Tissue-engineered spinal cord. Transplant Proc. 2001;33:592–8. doi: 10.1016/s0041-1345(00)02158-8. [DOI] [PubMed] [Google Scholar]
- 37.Okubo T, Nagoshi N, Kohyama J, et al. Treatment with a gamma-secretase inhibitor promotes functional recovery in human iPSC- derived transplants for chronic spinal cord injury. Stem Cell Reports. 2018;11:1416–32. doi: 10.1016/j.stemcr.2018.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parr AM, Kulbatski I, Tator CH. Transplantation of adult rat spinal cord stem/progenitor cells for spinal cord injury. J Neurotrauma. 2007;24:835–45. doi: 10.1089/neu.2006.3771. [DOI] [PubMed] [Google Scholar]
- 39.Nishimura S, Yasuda A, Iwai H, et al. Time-dependent changes in the microenvironment of injured spinal cord affects the therapeutic potential of neural stem cell transplantation for spinal cord injury. Mol Brain. 2013;6:3. doi: 10.1186/1756-6606-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishimura S, Sasaki T, Shimizu A, et al. Global gene expression analysis following spinal cord injury in non-human primates. Exp Neurol. 2014;261:171–9. doi: 10.1016/j.expneurol.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 41.Cummings BJ, Uchida N, Tamaki SJ, et al. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci U S A. 2005;102:14069–74. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abematsu M, Tsujimura K, Yamano M, et al. Neurons derived from transplanted neural stem cells restore disrupted neuronal circuitry in a mouse model of spinal cord injury. J Clin Invest. 2010;120:3255–66. doi: 10.1172/JCI42957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kobayashi Y, Okada Y, Itakura G, et al. Pre-evaluated safe human iPSC-derived neural stem cells promote functional recovery after spinal cord injury in common marmoset without tumorigenicity. PLoS One. 2012;7:e52787. doi: 10.1371/journal.pone.0052787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uezono N, Zhu Y, Fujimoto Y, et al. Prior treatment with anti-high mobility group Box-1 antibody boosts human neural stem cell transplantation-mediated functional recovery after spinal cord injury. Stem Cells. 2018;36:737–50. doi: 10.1002/stem.2802. [DOI] [PubMed] [Google Scholar]
- 45.Nori S, Nakamura M, Okano H. Plasticity and regeneration in the injured spinal cord after cell transplantation therapy. Prog Brain Res. 2017;231:33–56. doi: 10.1016/bs.pbr.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 46.Bonner JF, Steward O. Repair of spinal cord injury with neuronal relays: from fetal grafts to neural stem cells. Brain Res. 2015;1619:115–23. doi: 10.1016/j.brainres.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kadoya K, Lu P, Nguyen K, et al. Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration. Nat Med. 2016;22:479–87. doi: 10.1038/nm.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu P, Gomes-Leal W, Anil S, et al. Origins of neural progenitor cell-derived axons projecting caudally after spinal cord injury. Stem Cell Reports. 2019;13:105–14. doi: 10.1016/j.stemcr.2019.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kajikawa K, Imaizumi K, Shinozaki M, et al. Cell therapy for spinal cord injury by using human iPSC-derived region-specific neural progenitor cells. Mol Brain. 2020;13:120. doi: 10.1186/s13041-020-00662-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okubo T, Iwanami A, Kohyama J, et al. Pretreatment with a gamma-secretase inhibitor prevents tumor-like overgrowth in human iPSC-derived transplants for spinal cord injury. Stem Cell Reports. 2016;7:649–63. doi: 10.1016/j.stemcr.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kawai M, Imaizumi K, Ishikawa M, et al. Long-term selective stimulation of transplanted neural stem/progenitor cells for spinal cord injury improves locomotor function. Cell Rep. 2021;37:110019. doi: 10.1016/j.celrep.2021.110019. [DOI] [PubMed] [Google Scholar]
- 52.Ceto S, Sekiguchi KJ, Takashima Y, et al. Neural stem cell grafts form extensive synaptic networks that integrate with host circuits after spinal cord injury. Cell Stem Cell. 2020;27:430–40.e5. doi: 10.1016/j.stem.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ago K, Nagoshi N, Imaizumi K, et al. A non-invasive system to monitor in vivo neural graft activity after spinal cord injury. Commun Biol. 2022;5:803. doi: 10.1038/s42003-022-03736-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nichols CD, Roth BL. Engineered G-protein coupled receptors are powerful tools to investigate biological processes and behaviors. Front Mol Neurosci. 2009;2:16. doi: 10.3389/neuro.02.016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roth BL. DREADDs for neuroscientists. Neuron. 2016;89:683–94. doi: 10.1016/j.neuron.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campbell EJ, Marchant NJ. The use of chemogenetics in behavioural neuroscience: receptor variants, targeting approaches and caveats. Br J Pharmacol. 2018;175:994–1003. doi: 10.1111/bph.14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kitagawa T, Nagoshi N, Kamata Y, et al. Modulation by DREADD reveals the therapeutic effect of human iPSC-derived neuronal activity on functional recovery after spinal cord injury. Stem Cell Reports. 2022;17:127–42. doi: 10.1016/j.stemcr.2021.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duncan GJ, Manesh SB, Hilton BJ, et al. Locomotor recovery following contusive spinal cord injury does not require oligodendrocyte remyelination. Nat Commun. 2018;9:3066. doi: 10.1038/s41467-018-05473-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yasuda A, Tsuji O, Shibata S, et al. Significance of remyelination by neural stem/progenitor cells transplanted into the injured spinal cord. Stem Cells. 2011;29:1983–94. doi: 10.1002/stem.767. [DOI] [PubMed] [Google Scholar]
- 60.Salewski RP, Mitchell RA, Li L, et al. Transplantation of induced pluripotent stem cell-derived neural stem cells mediate functional recovery following thoracic spinal cord injury through remyelination of axons. Stem Cells Transl Med. 2015;4:743–54. doi: 10.5966/sctm.2014-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kawabata S, Takano M, Numasawa-Kuroiwa Y, et al. Grafted human iPS cell-derived oligodendrocyte precursor cells contribute to robust remyelination of demyelinated axons after spinal cord injury. Stem Cell Reports. 2016;6:1–8. doi: 10.1016/j.stemcr.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nagoshi N, Khazaei M, Ahlfors JE, et al. Human spinal oligodendrogenic neural progenitor cells promote functional recovery after spinal cord injury by axonal remyelination and tissue sparing. Stem Cells Transl Med. 2018;7:806–18. doi: 10.1002/sctm.17-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nori S, Khazaei M, Ahuja CS, et al. Human oligodendrogenic neural progenitor cells delivered with chondroitinase ABC facilitate functional repair of chronic spinal cord injury. Stem Cell Reports. 2018;11:1433–48. doi: 10.1016/j.stemcr.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kamata Y, Isoda M, Sanosaka T, et al. A robust culture system to generate neural progenitors with gliogenic competence from clinically relevant induced pluripotent stem cells for treatment of spinal cord injury. Stem Cells Transl Med. 2021;10:398–413. doi: 10.1002/sctm.20-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alizadeh A, Dyck SM, Karimi-Abdolrezaee S. Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Front Neurol. 2019;10:282. doi: 10.3389/fneur.2019.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hawryluk GW, Mothe A, Wang J, et al. An in vivo characterization of trophic factor production following neural precursor cell or bone marrow stromal cell transplantation for spinal cord injury. Stem Cells Dev. 2012;21:2222–38. doi: 10.1089/scd.2011.0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang YW, Denham J, Thies RS. Oligodendrocyte progenitor cells derived from human embryonic stem cells express neurotrophic factors. Stem Cells Dev. 2006;15:943–52. doi: 10.1089/scd.2006.15.943. [DOI] [PubMed] [Google Scholar]
- 68.Takano M, Kawabata S, Shibata S, et al. Enhanced functional recovery from spinal cord injury in aged mice after stem cell transplantation through HGF induction. Stem Cell Reports. 2017;8:509–18. doi: 10.1016/j.stemcr.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu J, Zeng L, Huang J, et al. miR-126 promotes angiogenesis and attenuates inflammation after contusion spinal cord injury in rats. Brain Res. 2015;1608:191–202. doi: 10.1016/j.brainres.2015.02.036. [DOI] [PubMed] [Google Scholar]
- 70.Dai J, Yu GY, Sun HL, et al. MicroRNA-210 promotes spinal cord injury recovery by inhibiting inflammation via the JAKSTAT pathway. Eur Rev Med Pharmacol Sci. 2018;22:6609–15. doi: 10.26355/eurrev_201810_16135. [DOI] [PubMed] [Google Scholar]
- 71.Yang J, Xiong LL, Wang YC, et al. Oligodendrocyte precursor cell transplantation promotes functional recovery following contusive spinal cord injury in rats and is associated with altered microRNA expression. Mol Med Rep. 2018;17:771–82. doi: 10.3892/mmr.2017.7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Watanabe K, Nakamura M, Iwanami A, et al. Comparison between fetal spinal-cord- and forebrain-derived neural stem/progenitor cells as a source of transplantation for spinal cord injury. Dev Neurosci. 2004;26:275–87. doi: 10.1159/000082144. [DOI] [PubMed] [Google Scholar]
- 73.Okada Y, Matsumoto A, Shimazaki T, et al. Spatiotemporal recapitulation of central nervous system development by murine embryonic stem cell-derived neural stem/progenitor cells. Stem Cells. 2008;26:3086–98. doi: 10.1634/stemcells.2008-0293. [DOI] [PubMed] [Google Scholar]
- 74.Kumagai G, Okada Y, Yamane J, et al. Roles of ES cell-derived gliogenic neural stem/progenitor cells in functional recovery after spinal cord injury. PLoS One. 2009;4:e7706. doi: 10.1371/journal.pone.0007706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 76.Tsuji O, Miura K, Okada Y, et al. Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc Natl Acad Sci U S A. 2010;107:12704–9. doi: 10.1073/pnas.0910106107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Itakura G, Kawabata S, Ando M, et al. Fail-safe system against potential tumorigenicity after transplantation of iPSC derivatives. Stem Cell Reports. 2017;8:673–84. doi: 10.1016/j.stemcr.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tanimoto Y, Yamasaki T, Nagoshi N, et al. In vivo monitoring of remnant undifferentiated neural cells following human induced pluripotent stem cell-derived neural stem/progenitor cells transplantation. Stem Cells Transl Med. 2020;9:465–77. doi: 10.1002/sctm.19-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nori S, Okada Y, Nishimura S, et al. Long-term safety issues of iPSC-based cell therapy in a spinal cord injury model: oncogenic transformation with epithelial-mesenchymal transition. Stem Cell Reports. 2015;4:360–73. doi: 10.1016/j.stemcr.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Iida T, Iwanami A, Sanosaka T, et al. Whole-genome DNA methylation analyses revealed epigenetic instability in tumorigenic human iPS cell-derived neural stem/progenitor cells. Stem Cells. 2017;35:1316–27. doi: 10.1002/stem.2581. [DOI] [PubMed] [Google Scholar]
- 81.Crawford TQ, Roelink H. The notch response inhibitor DAPT enhances neuronal differentiation in embryonic stem cellderived embryoid bodies independently of sonic hedgehog signaling. Dev Dyn. 2007;236:886–92. doi: 10.1002/dvdy.21083. [DOI] [PubMed] [Google Scholar]
- 82.Kojima K, Miyoshi H, Nagoshi N, et al. Selective ablation of tumorigenic cells following human induced pluripotent stem cell-derived neural stem/progenitor cell transplantation in spinal cord injury. Stem Cells Transl Med. 2019;8:260–70. doi: 10.1002/sctm.18-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.de Luzy IR, Law KCL, Moriarty N, et al. Human stem cells harboring a suicide gene improve the safety and standardisation of neural transplants in Parkinsonian rats. Nat Commun. 2021;12:3275. doi: 10.1038/s41467-021-23125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Keirstead HS, Nistor G, Bernal G, et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Karimi-Abdolrezaee S, Eftekharpour E, Wang J, et al. Synergistic effects of transplanted adult neural stem/progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord. J Neurosci. 2010;30:1657–76. doi: 10.1523/JNEUROSCI.3111-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kumamaru H, Saiwai H, Kubota K, et al. Therapeutic activities of engrafted neural stem/precursor cells are not dormant in the chronically injured spinal cord. Stem Cells. 2013;31:1535–47. doi: 10.1002/stem.1404. [DOI] [PubMed] [Google Scholar]
- 87.Jin Y, Bouyer J, Shumsky JS, et al. Transplantation of neural progenitor cells in chronic spinal cord injury. Neuroscience. 2016;320:69–82. doi: 10.1016/j.neuroscience.2016.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wernig A, Nanassy A, Muller S. Maintenance of locomotor abilities following Laufband (treadmill) therapy in para- and tetraplegic persons: follow-up studies. Spinal Cord. 1998;36:744–9. doi: 10.1038/sj.sc.3100670. [DOI] [PubMed] [Google Scholar]
- 89.Winchester P, McColl R, Querry R, et al. Changes in supraspinal activation patterns following robotic locomotor therapy in motor-incomplete spinal cord injury. Neurorehabil Neural Repair. 2005;19:313–24. doi: 10.1177/1545968305281515. [DOI] [PubMed] [Google Scholar]
- 90.Barriere G, Leblond H, Provencher J, et al. Prominent role of the spinal central pattern generator in the recovery of locomotion after partial spinal cord injuries. J Neurosci. 2008;28:3976–87. doi: 10.1523/JNEUROSCI.5692-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Battistuzzo CR, Callister RJ, Callister R, et al. A systematic review of exercise training to promote locomotor recovery in animal models of spinal cord injury. J Neurotrauma. 2012;29:1600–13. doi: 10.1089/neu.2011.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shibata T, Tashiro S, Shinozaki M, et al. Treadmill training based on the overload principle promotes locomotor recovery in a mouse model of chronic spinal cord injury. Exp Neurol. 2021;345:113834. doi: 10.1016/j.expneurol.2021.113834. [DOI] [PubMed] [Google Scholar]
- 93.Tashiro S, Nishimura S, Iwai H, et al. Functional recovery from neural stem/progenitor cell transplantation combined with treadmill training in mice with chronic spinal cord injury. Sci Rep. 2016;6:30898. doi: 10.1038/srep30898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Suzuki H, Ahuja CS, Salewski RP, et al. Neural stem cell mediated recovery is enhanced by Chondroitinase ABC pretreatment in chronic cervical spinal cord injury. PLoS One. 2017;12:e0182339. doi: 10.1371/journal.pone.0182339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Curtis E, Martin JR, Gabel B, et al. A first-in-human, phase I study of neural stem cell transplantation for chronic spinal cord injury. Cell Stem Cell. 2018;22:941–50.e6. doi: 10.1016/j.stem.2018.05.014. [DOI] [PubMed] [Google Scholar]