Abstract
Metastatic involvement of the spine is a common complication of systemic cancer progression. Surgery and external beam radiotherapy are palliative treatment modalities aiming to preserve neurological function, control pain and maintain functional status. More recently, with development of image guidance and stereotactic delivery of high doses of conformal radiation, local tumor control has improved; however recurrent or radiation refractory disease remains a significant clinical problem with limited treatment options. This manuscript represents a narrative overview of novel targeted molecular therapies, chemotherapies, and immunotherapy treatments for patients with breast, lung, melanoma, renal cell, prostate, and thyroid cancers, which resulted in improved responses compared to standard chemotherapy. We present clinical examples of excellent responses in spinal metastatic disease which have not been specifically documented in the literature, as most clinical trials evaluate treatment response based on visceral disease. This review is useful for the spine surgeons treating patients with metastatic disease as knowledge of these responses could help with timing and planning of surgical interventions, as well as promote multidisciplinary discussions, allowing development of an individualized treatment strategy to patients presenting with widespread multifocal progressive disease, where surgery could lead to suboptimal results.
Keywords: Spine metastases, Metastatic cancer, Targeted therapy, Mutation, Chemotherapy, Immunotherapy
INTRODUCTION
Metastatic cancer to spine remains a debilitating consequence of uncontrolled cancer progression with a poor prognosis, that historically portended an overall survival (OS) of less than 6 months from the time of diagnosis [1]. The incidence of spinal metastases is increasing due to a variety of factors including early detection due to improvements in imaging modalities, enhanced response to first-line cancer therapies allowing longer survival and development of distant metastases as a late-stage manifestation of the disease progression, and the inherent poor response of spinal metastases to existing therapies as compared to visceral disease. The incidence of spinal metastases averages approximately 40% depending on the primary cancer and is estimated to exceed 100,000 new patients annually [2-4]. Left unchecked, continued metastatic tumor growth within the spinal column ultimately leads to neurologic compromise, intractable pain, spinal deformity, instability, and significant limitation in the quality of life. The incidence of spine metastatic disease has been estimated to be 16%–74% in patients with lung cancer, 65%–75% in patients with breast cancer, and 65%–90% in prostate cancer patients [5]. Conversely while looking at all spine metastases diagnosed in the United States yearly, 14% are derived from breast, 16.3% from lung, 4.1% from melanoma, 13.1% from renal cell, 6.8% from prostate, and 2.3% from thyroid primary cancer [6]. Mechanistically, metastatic spread to the spine may occur via direct local invasion from neighboring tissues, migration along neural structures, or hematogenous spread of cancer cells from the site of origin into the bone of the spinal column [7,8].
Several frameworks and scoring systems are available to aid with decision making while treating patients with spinal metastatic disease, including the NOMS (neurologic, oncologic, mechanical stability and systemic disease) framework, Tomita score, SINS (spinal instability neoplastic score) score, and Tokuhashi score [9-13]. These various algorithms were created to integrate multidisciplinary assessment, evidence-based medicine, and new technology to optimize patient care. At our institution, the overall philosophy for treating patients with metastatic spine disease includes in depth evaluation of their functional status, systemic disease burden and failure of prior treatments. Surgical interventions are performed to decompress the spinal cord in cases of neurological compromise, to allow clearance for spinal stereotactic radiosurgery and to perform stabilization of symptomatic spinal fractures, however, the magnitude of surgery needs to be adjusted on a case-by-case basis to be minimally disruptive to oncological management as prolonged postsurgical recovery can negatively impact performance status and survival. With the advent of genomic analysis, the identification of targetable mutations in an increasing percentage of patients across various tumor types has changed their oncologic management and outcomes. Examples include non-small cell lung cancer (NSCLC) with ALK rearrangements identified in 4%–5% of patients and epidermal growth factor receptor (EGFR) mutations present in 10%–15% of lung cancer patients; ERBB2, CD340, and human epidermal growth factor receptor 2 (HER2)/Neu alterations in breast cancer samples, with HER2 overexpression detected in 18%–25% patients; and BRAF V600E mutation detectable in 33%-55% melanoma patients. Systemic cancer therapy is rapidly changing with the introduction of antiangiogenic agents, immunotherapy, targeted therapy, and cell cycle inhibitors, although this may not be directly translatable to patients with spine metastases. This manuscript represents a narrative overview of the results of clinical trials. Our intention is to raise awareness of the effectiveness of modern chemotherapy, targeted therapy, and immunotherapy for the treatment of patients with bulky spinal metastases derived from primary lung, breast, melanoma, renal cell, prostate, and thyroid cancers, where systemic treatment can be extremely effective in achieving local control within the spine in combination with surgery and/or radiation therapy.
BREAST CANCER
Patients with breast cancer are typically treated with an alkylating agent (cyclophosphamide) and antimetabolites (methotrexate, 5-fluorouracil), doxorubicin containing combination of agents, or combinatorial regimens including platinum-based compounds (cisplatin) or taxanes (paclitaxel, docetaxel) as firstline therapies (Table 1). According to the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute, pati ents with breast cancer have 6% distant metastases at the time of diagnosis, with 29% 5-year relative survival (SEER; Table 1) [14]. Up to 5% of patients with breast cancer have identifiable bone metastases at the time of diagnosis, with a median survival of less than 2 years [15]. Amongst these patients, over 20,000 patients annually present with epidural compression [16]. Patients with hormone receptor (HR)-positive breast cancer are treated with endocrine therapies including estrogen receptor antagonist tamoxifen irrespective of their HER2 status, which has led to improved disease-free survival with minimal toxicity as shown in several clinical trials HERA (78.6% 4-year disease-free survival vs. 72.2% in the control group), NSABP trial BP-31 (12% improvement in disease-free survival at 3 years with 33% reduction in risk of death) and NCCTG N9831 (37% improvement in OS, with 10 year survival increase from 75.2% to 84%) (Table 1) [17]. Aromatase inhibitors, luteinizing hormone releasing hormone analogs and selective estrogen degraders are other classes of endocrine therapies used in HR+ breast cancer patients with improved progression free survival (PFS) (Table 1) [17,18]. In patients with HER2 amplification, use of humanized monoclonal antibodies including trastuzumab and pertuzumab or lapatinib improves patient outcomes; phase III randomized double blind CLEOPATRA trial initially evaluated a combination of these as first-line therapy with improvement in the median OS of 57.1 months (vs. 40.8 months) and 37% patients alive at 8 years (vs. 23%), with somewhat positive results reported in APHINITY trial, with 7.1% disease recurrence in the trial group (vs. 8.7%) and 94.1% patients invasive disease free at 3 years (vs. 93.2%) (Table 1) [17,19,20]. Anti-vascular endothelial growth factor receptor (VEGFR) inhibitor bevacizumab has been trialed for treatment of patients with breast cancer with improvements in PFS but not OS: E2100 trial reported 11.8 month PFS (vs. 5.9) with median OS of 26.7 months (vs. 25.2), while AVADO trial reported 10.1 month PFS (vs. 8.2) with median OS of 30.2 months (vs. 31.9), and RIBBON2 reported 7.2 month PFS (vs. 5.1) with median OS of 18 months (vs. 16.4) [21]. Heat shock protein 90 inhibitors (including 17-allylamino 17-demethoxygel danamycin) have been trialed in breast carcinoma spine metastases with some success in phase I trials [22].
Table 1.
Systemic, targeted and immunotherapy treatments used to treat patients with breast, melanoma, non-small cell lung, renal cell, prostate, and thyroid cancers
| Histology | Subtype | Incidence of distant metastases at presentation | Early vs. late manifestation | Radiosensitivity | Systemic treatment options | Investigational treatment options |
|---|---|---|---|---|---|---|
| Breast cancer | HR+ HER2+ 10% cases | 37.9% | 3 Months | RS | Endocrine therapy | - |
| ER antagonist tamoxifen | ||||||
| Aromatase inhibitors | ||||||
| LHRH analogs | ||||||
| Estrogen degraders | ||||||
| HR- HER2+ 4% cases | 44.7% | 2 Months | - | Taxanes with | Palbociclib | |
| Humanized MAB: trastuzumab, pertuzumab, lapatinib, tucatinib, neratinib +/- capecitabine | HDACi entinostat, vorinostat | |||||
| PI3Ki buparlisib, pilaralisib | ||||||
| Anti-VEGFR bevacizumab | mTORi everolimus, sirolimus | |||||
| HSP90 inhibitors | Immunotherapy HER2 derived peptide | |||||
| HR+ HER2- 68% cases | 30.6% | 28 Months | - | Endocrine sensitive: | - | |
| Endocrine therapy selective estrogen receptor modulators or downregulators, aromatase inhibitors | ||||||
| CDK4/6 inhibitors: Palbociclib, ribociclib, abemaciclib | ||||||
| PIK3CA mutant: alpelisib + fulvestrant | ||||||
| Endocrine resistant: | ||||||
| Capecitabine | ||||||
| Platinum | ||||||
| Doxorubicin | ||||||
| PARPi talazoparib | ||||||
| HR- HER2- 10% cases | 12.2% | 45.5 Months | - | Capecitabine | PDL1+ pembrolizumab with chemotherapy | |
| Platinum +/- etoposide | PDL1- chemotherapy | |||||
| Doxorubicin | PARPi Olaparib, veliparib, talazoparib | |||||
| Methotrexate, high dose | EGFRi | |||||
| MAB | ||||||
| Sacituzumab govitecan | ||||||
| Melanoma | BRAFV600E | - | - | RR | Dacarbazine | BRAFi dabrafenib, vemurafenib, encorafenib |
| MEKi trametinib, selumetinib, cobimetinib, MEK162 | ||||||
| BRAFi + MEKi | ||||||
| Anti-CTLA4 ipilimumab | ||||||
| PD-1 nivolumab, pembrolizumab | ||||||
| T-VEC + GMCSF | ||||||
| BRAFV600E negative | - | - | - | - | Anti-CTLA4 ipilimumab | |
| PD-1 nivolumab, pembrolizumab | ||||||
| T-VEC + GMCSF | ||||||
| Non-small cell lung cancer | EGFRmut | - | - | RR | Paclitaxel kanglaite | EGFRi afatinib, osimertinib, gefitinib, erlotinib |
| Bevacizumab | ||||||
| EML4-ALK | - | - | - | - | Crizotinib | |
| - | - | - | Fluvastatin | PI3Ki buparlisib | ||
| RANKL MAB denosumab bisphosphonates i.e. | BRAFi dabrafenib for BRAFV600E, MAP2Ki, HER2i | |||||
| MET/VEGFR2i cabozantinib | ||||||
| Zoledronic acid | CTLA4 ipilimumab tremelimumab | |||||
| Platinum based | Anti PD1 nivolumab, pembrolizumab | |||||
| Renal cell | Clear cell VHL/VEGFR, PBRM1, SETD2, BAP1, mTOR | - | - | RR | Bevacizumab/IFN alpha | VEGFR/PDGFRi Axitinib, pazopanib, sorafenib, sunitinib |
| IL2 | Cabozantinib VEGFR/AXL/cMeti | |||||
| Pazopanib, sunitinib or temsirolimus | Lenvatinib FGFR/VEGFRi/everolimus | |||||
| PD-1/PD-L1 Nivolumab, ipilimumab | ||||||
| Everolimus/sorafenib | ||||||
| mTORi everolimus, temsirolimus | ||||||
| Papillary MET NRF2 | - | - | - | - | - | |
| TP53, PTEN, CDKN2A loss, SMARKB1 loss, TFE3-TFEB fusion | - | - | - | - | - | |
| Prostate cancer | - | - | - | RS | Androgen receptor antagonist flutamide, bicalutamide, abiraterone, ketoconazole | |
| LHRH agonist/antagonist leuproline, goserelin, degarelix | ||||||
| Androgen resistant | - | - | - | Doxorubicin | EGFRi gefitinib, erlotinib | |
| Docetaxel | EGFR/HER2i lapatinib | |||||
| Cabazitaxel mitoxantrone | EGFR MAB cetuximab | |||||
| MET/VEGFR2 cabozantinib | ||||||
| PARPi Olaparib | ||||||
| RANKL denosumab | ||||||
| antiCTLA4 ipilimumab | ||||||
| antiPD1/PD-L1 nivolumab, pembrolizumab, atezolizumab | ||||||
| Sipuleucel-T vaccine | ||||||
| Thyroid cancer | Differentiated thyroid carcinoma | - | - | RR | - | VEGFR/Flt3/RET/cKIT/BRAFi Sorafenib |
| VEGFR/FGFR/PDGFR/KIT/RETi Lenvantinib | ||||||
| Anaplastic carcinoma | - | - | - | - | - | |
| Medullary thyroid carcinoma | - | - | - | - | VEGFR/EGFR/RETi Vandetanib | |
| VEGFR2/cMet/AXL/RETi Cabozantinib | ||||||
| - | - | - | - | PI3Ki | ||
| HER2/3-ALK translocations | ||||||
| antiCTLA4 | ||||||
| anti-PD1 |
HR, hormone receptor; HER2, human epidermal growth factor receptor 2; RR, radiation resistant; RS, radiation sensitive; ER, estrogen receptor; LHRH, luteinizing hormone releasing hormone; MAB, monoclonal antibody; HDACi, histone deacetylase inhibitor; PI3Ki, phosphatidylinositol-3-kinase inhibitor; mTORi, mammalian target of rapamycin inhibitor; CDK4/6, cyclin dependent kinases 4/6; PARPi, poly-ADP-ribose polymerase inhibitor; PDL1, programmed death ligand 1; EGFRi, epidermal growth factor receptor inhibitor; BRAFi, v-Raf murine sarcoma viral oncogene homolog B1 inhibitor; MEKi, mitogen activated protein kinase kinase inhibitor; MEK162, mitogen activated protein kinase kinase 162; CTLA4i, cytotoxic T lymphocyte associated protein 4 inhibitor; PD-1, programmed cell death protein 1; T-VEC, talimogene; GMCSF, granulocyte macrophage colony stimulating factor; RANKL, receptor activator of nuclear factor kapppa-B ligand; VHL, von Hippel Lindau; VEGFR, vascular endothelial growth factor receptor; PBRM1, polybromo 1; SETD2, su(var), enhancer of zeste, trithorax - domain containing 2; BAP1, ubiquitin carboxyl-terminal hydrolase 1; FGFR, fibroblast growth factor receptor; VEGFRi, vascular endothelial growth factor inhibitor; TP53, tumor protein p53; PTEN, phosphatase and TENsin homolog deleted on chromosome 10; CDKN2A, cyclin dependent kinase inhibitor 2A; SMARCB1, SWI/SNF related, matrix associated, actin dependent regulator of chromatin; TFE3, transcription factor enhancer 3; TFEB, transcription factor EB; RET, rearranged during transfection; PDGFR, platelet derived growth factor receptor; RETi, rearranged during transfection inhibitor; ALK, anaplastic lymphoma kinase.
Several CDK4/6 inhibitors, including palbociclib, ribociclib and abemaciclib, have been used for treatment of metastatic HR+ HER2- breast cancer patients that develop hormone resistant disease; PALOMA3 trial reported median PFS of 9.5 months (vs. 4.6) with palbociclib use, while MONARCH2 trial reported 16.4 month PFS (vs. 9.3) with abemaciclib [17,23]. Palbociclib has been FDA approved in 2015 [17,23]. Histone deacetylase inhibitors (entinostat, vorinostat), phosphatidylinositol 3 kinase (PI3K) inhibitors (buparlisib, pilaralisib) and mammalian target of rapamycin (mTOR) inhibitors (everolimus, sirolimus) have also shown promising results in patients with hormone resistant and HER2+ metastatic breast as a part of combination therapy (Table 1) [23,24]. NCT00676663 reported 4.3 month PFS (vs. 2.3) with median OS of 28.1 months (vs. 19.8) with entinostat use [23]. Systematic categorization in The Cancer Genome Atlas (TCGA) helped identify other mutations that could be targeted in the future, including fibroblast growth factor receptor (FGFR), PTEN, TP53, AKT1/2, KRAS, and SRC (TCGA). Although breast cancer had historically been considered less immunogenic, several clinical trials of anti-PD1 and anti-PDL1 in patients have been conducted with some success, including vaccinating patient with HER2-derived peptide; phase I/II trial reported 89.7% 5 year PFS (vs 80.2%), with PFS as high as 94.6% 5 year PFS in optimally boosted patients (Tables 2, 3, Supplementary Table 1) [17,25]. Poly-ADP-ribose polymerase (PARP) inhibitors (olaparib, veliparib) with or without immunotherapy, EGFR inhibitors and monoclonal antibodies are being trialed in patients with most difficult to treat triple negative breast cancer; phase II trial reported median PFS of 3.7 months (vs. 1.5 months) and median OS of 12.9 months (vs. 9.4 months) with anti-EGFR inhibitor cetuximab [17]. Combined use of atezolizumab and paclitaxel has been FDA approved for patients with triple negative breast cancer (Tables 1–3; Supplementary Table 1). In our hands, we had notable responses in breast cancer patients treated with CDK4/6 inhibitors in combination with standard systemic treatment. To illustrate bone specific responses, we present in Fig. 1 a case of a 61-year-old patient with a lytic C2 lesion successfully treated using CDK4/6 inhibitor palbociclib in combination with letrozole (Fig. 1). Currently, her OS is 41 months since the time of surgery, and she had been progression free for 31 months while on that regimen.
Table 2.
U.S. Food and Drug Administration approved immunotherapy treatments based on primary cancer
| Cancer type | ImmunoTx | |
|---|---|---|
| Breast | Triple negative breast cancer: | |
| Atezolizumab+paclitaxel protein-bound PD-L1 >1% as first line | ||
| Pembrolizumab, neoadjuvant and adjuvant, in combination with chemotherapy | ||
| Sacituzumab | ||
| HER2+ metastatic breast cancer: | ||
| Margetuximab | ||
| Pertuzumab + trastuzumab | ||
| Melanoma | Adjuvant ipilimumab, nivolumab, or pembrolizumab | |
| First-line therapy | ||
| - Ipilimumab, nivolumab or pembrolizumab | ||
| - Combination nivolumab+ipilimumab | ||
| Tebentafusp | ||
| Atezolizumab | ||
| NSCLC | Unresectable stage III: chemoRT, then durvalumab | |
| First-line therapies | ||
| - Pembrolizumab TPS >50% | ||
| - Squamous NSCLC: pembrolizumab + carboplatin and nab-paclitaxel | ||
| - Nonsquamous NSCLC: pembrolizumab + pemetrexed/platinum vs atezolizumab + bevacizumab, paclitaxel and carboplatin | ||
| Second line therapies | ||
| - Pembrolizumab TPS >1% | ||
| - Atezolizumab or nivolumab | ||
| Amivantamab | ||
| Cemiplimab | ||
| Ramucirumab + erlotinib | ||
| Nivolumab + ipilimumab, combined with platinum doublet | ||
| RCC | Advanced RCC: | |
| First-line therapy nivolumab + ipilimumab | ||
| Second line therapy anti-angiogenic therapy followed by nivolumab | ||
| Pembrolizumab | ||
| Nivolumab +cabozantinib | ||
PD-L1, programmed death-ligand 1; HER2, human epidermal growth factor receptor 2; TPS, tumor proportion score; NSCLC, non-small cell lung cancer; RCC, renal cell carcinoma.
Table 3.
Indications for most common immunotherapy agents
| Immunotherapy agent | Indication |
|---|---|
| CTLA4 inhibitor, ipilimumab, YERVOY | Stage III/IV surgically unresectable malignant melanoma regardless of BRAF status |
| BRAF V600wt unresectable or metastatic | |
| Unresectable or metastatic melanoma, BRAF V600wt or BRAF V600mut, in combination with nivolumab | |
| Cutaneous melanoma, stage IIIABC post resection including LN, adjuvant | |
| First line, metastatic NSCLC PD-L1+, no EGFR/ALK aberrations, with nivolumab | |
| First line, metastatic or recurrent NSCLC, no EGFR/ALK aberrations, with nivolumab and 2 cycles of platinum doublet chemotherapy | |
| Untreated RCC, relapsed and stage IV intermediate and poor risk RCC regardless of PD-L1, combined with nivolumab | |
| Relapsed and stage IV RCC failing TKI, VEGF or mTOR inhibitor use, with nivolumab | |
| PD-1 inhibitor, nivolumab, OPDIVO | First-line failing systemic Tx or metastatic melanoma regardless of BRAF status |
| Unresectable or metastatic melanoma progressive on ipilimumab | |
| Unresectable or metastatic BRAF V600Emut melanoma progressive on BRAF inhibitor | |
| Unresectable or metastatic melanoma, nivolumab with ipilimumab | |
| LN+ or metastatic melanoma post complete resection, adjuvant | |
| First line, metastatic NSCLC PD-L1+, no EGFR/ALK aberrations, with ipilimumab | |
| First line, metastatic or recurrent NSCLC, no EGFR/ALK aberrations, combined with ipilimumab and 2 cycles of platinum doublet chemotherapy | |
| Metastatic NSCLC, progressive on platinum chemotherapy, failed targeted inhibitors for EGFR/ALK aberrations | |
| First line, RCC that is untreated, relapsed or stage IV, intermediate or poor risk RCC regardless PD-L1, combined with ipilimumab | |
| First line, advanced RCC, combined with cabozantinib | |
| RCC progressive on mTOR or VEGFR inhibitors | |
| PD-1 inhibitor, Pembrolizumab, KEYTRUDA | Triple negative breast cancer, early stage, high risk, combined with chemotherapy as neoadjuvant, then single agent adjuvant post resection |
| Triple negative breast cancer, locally recurrent unresectable or metastatic, PD-L1+, in combination with chemotherapy | |
| Metastatic melanoma progressive on ipilimumab | |
| Metastatic melanoma BRAFmut progressive on BRAF inhibitor | |
| Previously untreated melanoma regardless of BRAF status | |
| LN+ melanoma post complete resection | |
| Metastatic melanoma, limited resectability, no residual, adjuvant | |
| Unresectable or metastatic melanoma | |
| First line in metastatic NSCLC with high PD-L1 >50%, no EGFR/ALK mutation | |
| First line w/pemetrexed and carboplatin for metastatic nonsquamous NSCLC, no EGFR/ALK mutation, any PD-L1 status | |
| First line in metastatic squamous NSCLC in combination with carboplatin, paclitaxel or protein-bound paclitaxel, any PD-L1 status | |
| First line in stage III NSCLC patients that are not candidates for surgery, chemo, RT, and metastatic NSCLC patients with PD-L1 >1%, no EGFR/ALK aberrations | |
| Metastatic NSCLC progressive on platinum chemotherapy, having failed targeted inhibitors for EGFR/ALK genomic aberrations, PD-L1+ | |
| First-line metastatic RCC, poor, intermediate, favorable, combined with Axitinib | |
| PD-L1 inhibitor, avelumab, BAVENCIO | First-line advanced RCC, together with Axitinib |
| Alternative to pembrolizumab | |
| PD-L1 inhibitor, durvalumab, IMFINZI | Stage III unresectable NSCLC, not progressing on concurrent platinum-based chemotherapy and radiation therapy |
| PD-L1 inhibitor, atezolizumab, TECENTRIQ | Triple negative breast cancer, unresectable locally advanced or metastatic, PD-L1+ expression, combined with paclitaxel |
| Melanoma, unresectable or metastatic, BRAF V600mut, combined with cobimetinib and vemurafenib | |
| First line, metastatic NSCLC, high PD-L1++, no EGFR/ALK genomic aberrations | |
| First line, metastatic nonsquamous NSCLC, no EGFR/ALK genomic aberrations, in combination with bevacizumab, paclitaxel, and carboplatin | |
| First line, metastatic nonsquamous NSCLC, no EGFR/ALK genomic aberrations, with protein-bound paclitaxel and carboplatin | |
| Stage II-IIIA NSCLC, PD-L1+, post resection and platinum chemotherapy, adjuvant | |
| Metastatic NSCLC progressive on platinum chemotherapy | |
| Metastatic NSCLC, EGFR/ALK genomic aberrations, progressive on targeted therapy |
CTLA4, cytotoxic T-lymphocyte antigen 4; NSCLC, non-small cell lung cancer; PD-L1, programmed death-ligand 1; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; RCC, renal cell carcinoma; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor; mTOR, mammalian target of rapamycin; PD-1, programmed-death 1; TX, treatment; LN, lymph node; VEGFR, vascular endothelial growth factor receptor.
Fig. 1.
Successful use of CDK4/6 inhibitor palbociclib and letrozole in a patient with advanced breast cancer. Patient is a 61-year-old female who presented with 6/10 neck pain, found to have a non-surgical lytic C2 lesion. Per discussion with medical oncology team, the consensus was for systemic treatment and restaging. Sagittal and axial computed tomography cervical and thoracic spine views prior to initiation of treatment (A, B) and 3 months after treatment with letrozole and Palbociclib (C, D), showing resolution of the lesion.
MELANOMA
Survival in patients with metastatic melanoma has historically been poor, with 16% 5-year survival rates and median OS of 6–9 months [26]. Moreover, those patient with spinal metastases fare even worse with a median survival of 4 months [26]. According to SEER, patients with skin melanoma have 4% distant metastases at the time of presentation, with 29.8% 5-year relative survival [14]. BRAF inhibitors, including dabrafenib and vemurafenib, are used in up to 50%–65% of metastatic melanoma patients who carry targetable BRAF mutation. The emergence of these agents had resulted in an improved OS of 13.6 months as compared to systemic dacarbazine, with median PFS of 5.1 months (vs. 2.7 months) when treated with dabrafenib (Table 1, Supplementary Table 1) [27]. In patients with BRAF V600E mutations, the use of MEK inhibitors including trametinib, selumetinib, cobimetinib, and MEK162 have similarly resulted in improved PFS and OS; COLUMBUS trial reported median PFS of 14.9 months versus 7.3 months comparing encorafenib plus binimetinib versus vemurafenib, with the corresponding median OS of 33.6 versus 16.9 months [28].
In patients with advanced metastatic disease, the use of immune checkpoint inhibitors has changed the treatment approach for these patients. These novel agents target immune regulatory molecules including ipilimumab targeting cytotoxic T-lymphocyte antigen 4 (CTLA4), nivolumab and pembrolizumab targeting PD-1 and the combined inhibition of CTLA-4 and PD-1 (Tables 2, 3). With this approach, significant clinical improvements have been achieved in patients with advanced melanoma, with 5 year OS of 18.2% (vs. 8.8%) reported by the NCT00- 324155 trial [29]. Moreover, recent studies showed 82% 1-year survival and 75% 2-year survival in patients receiving a combination of nivolumab and ipilimumab; CheckMate067 reported median OS of 72.1 months versus 36.9 with nivolumab alone versus 19.9 with ipilimumab alone, with 6.5 year OS rates of 57% versus 43% versus 25% with BRAF mutant tumors, and 46% versus 42% versus 22% with BRAF wild type tumors, respectively [30]. Despite these impressive results, immune checkpoint inhibitors can be associated with a significant toxicity profile related to autoimmune manifestations and must be monitored closely [31,32]. In patients without BRAF V600E mutations, immune checkpoint inhibition is considered first-line therapy (Tables 2, 3). Amongst patients with the targetable BRAF V600E mutation, targeted inhibitors are typically given up-front, followed by immune checkpoint inhibition [31,32].
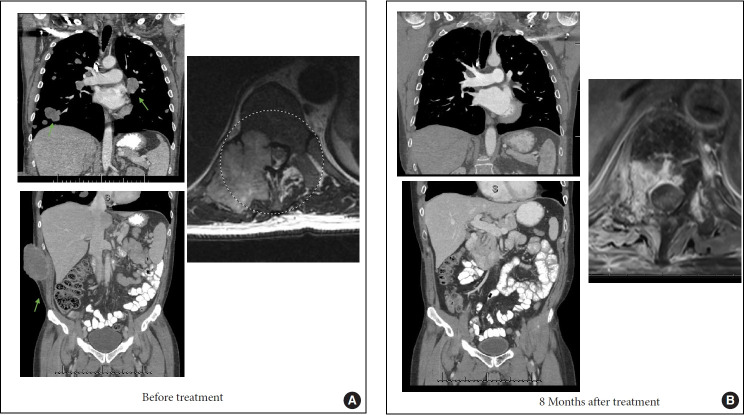
Additional focus has been directed on modulating the immune system towards an antitumor state. For example, recent studies have focused on applications of HSV-1 based oncolytic viruses (e.g., talimogene laherparepvec [T-VEC]) to induce lysis of melanoma cells in patients, with resulting antigen release and immune response when combined with granulocyte-monocyte colony-stimulating factor (GM-CSF) and immunotherapy and median OS of 23.3 months with T-VEC and 18.9 months with GM-CSF, with the corresponding durable response rates of 16.3% versus 2.1% [33]. T-VEC is the first FDA approved oncolytic virus and is additionally being studied in combination with immune checkpoint inhibitors. Other oncolytic viruses and dendritic cell vaccines are being investigated for treating melanoma patients. Several studies are focused on understanding the mechanisms of immune resistance in melanoma patients, exploring prognostic features of response to immunotherapy, and explore ways to reverse immune evasion [34,35]. Fig. 2 demonstrate an example of successful use of immunotherapy in a 61-year-old patient with widely metastatic melanoma refractory to several lines of treatment, who presented complete resolution of bulky metastatic disease and remains disease free for more than 90 months after starting treatment anti-PD-1 inhibitor pembrolizumab.
Fig. 2.
Successful use of pembrolizumab in a patient with widely metastatic melanoma. Patient is 61-year-old with widely metastatic melanoma refractory to several lines of systemic treatment. Per discussion with medical oncology team, the consensus was for systemic treatment. Panel A demonstrates several sites of bulky metastatic disease (arrows) and a large spinal metastasis at T11 (circle) treated with spinal stereotactic radiosurgery 3 months prior to starting anti-programmed-death 1 therapy. Panel B demonstrates complete resolution of the bulky metastatic disease including the epidural spinal cord compression 8 months after treatment with pembrolizumab. The patient currently remains disease free with a follow-up of 90 months.
NON-SMALL CELL LUNG CANCER
NSCLC is likewise associated with poor prognosis with an OS of 8 to 11 months due to rapid lung progression and distant metastatic progression. Skeletal metastasis in common in NSCLC, occurring in 30% of patients and roughly half of skeletal metastases are in the spinal column.36 According to SEER, 56% patients with lung cancer have distant metastases at the time of diagnosis, with 6.3% 5-year relative survival (Table 1) [14]. Paclitaxel and kanglaite are commonly used chemotherapeutic agents in patients with lung cancer that has metastasized to bone [37]. Additionally, up to 80% of patients with squamous cell lung adenocarcinomas and nearly 60% of patients with lung adenocarcinomas contain targetable mutations in membrane growth factor receptors (EGFR, VEGFR) or protein kinases (RAS, RAF, MEK) [38].
Immune checkpoint inhibitor use has been trialed in patients with NSCLC as well (Tables 2, 3; Supplementary Table 1). The CTLA4 inhibitor ipilimumab resulted in marginal improvement in patients with advanced NSCLC in a phase II clinical trial, with no benefit shown in phase III trial, with median OS of 13.4 months (vs. 12.4 months) and median PFS of 5.6 months (vs. 5.6 months) with combined use of chemotherapy with immunotherapy [39]. Another CTLA4 inhibitor tremelimumab has been studied in phase II trial as a maintenance therapy; CCTG BR34 trial reported median OS of 16.6 months (vs. 14.1 months) and median PFS of 7.7 months (vs. 3.2 months) in patients with metastatic NSCLC when combined with durvalumab and platinumbased chemotherapy versus immunotherapy, with no significant improvement [40]. In contrast, anti-PD1 inhibitors nivolumab and pembrolizumab as well as PDL1 inhibitors MEDI4736, MPDL3280A and BMS-936559 show much more promising results; Checkmate017 and Checkmate057 phase III trials show improved survival in NSCLC patients who failed platinum-based chemotherapy of 23% (vs. 8%) 2-year OS in squamous and 29% (vs. 16%) in nonsquamous NSCLC patients when treated with nivolumab vs docetaxel, with pembrolizumab currently approved as first-line treatment in patients with PD-L1 overexpression (Table 3) [41,42].
EGFR mutations are most common in Asian patients, females and patients with NSCLC [43]. EGFR inhibitors including erlotinib and gefitinib have been shown to improve the OS in NSCLC patients with metastatic spine disease for up to 24 to 36 months (Table 1) [40]. Afatinib is another EGFR inhibitor targeting EGFR/HER2/HER4 and has been trialed in lung cancer patients; phase III trial reported with median PFS of 11.1 months (vs. 6.9 months) with afatinib use, with 13.6 month median PFS in patients with exon 19 deletions and L858R EGFR mutations [44]. Significant improvement in OS and PFS has been noted in patients with NSCLC treated with bevacizumab; phase III BEYOND trial reported median PFS of 12.4 months (vs. 7.9 months) in patients with EGFR mutant tumors, median PFS of 8.3 months (vs. 5.6 months) in wild type tumors and OS of 24.3 months (vs. 17.7 months) when treated with bevacizumab in addition to carboplatin and paclitaxel [45,46]. In patients with targetable EGFR mutations including T790M, osimertinib has been shown to prolong survival and is first-line therapy, with median PFS of 10.1 months (vs. 4.4 months) as compared to platinum and pemetrexed [47]. In patients with EML4-ALK fusion commonly present in younger patients and never smokers, a phase II trial with crizotinib has shown promising results; studies report 24.1 month PFS [38]. Buparlisib, which is a PI3K inhibitor, is a potential therapy which may result in antitumor activity by inhibiting osteoclast formation [48]. Other targeted inhibitors including BRAF, MAP2K and HER2 inhibitors are being studied [23]. As an example of effectiveness of targeted therapies, Fig. 3 describes the successful use of erlotinib in a 60-year-old patient with advanced EGFR mutant NSCLC, she had complete response of all her epidural disease without adjuvant radiotherapy for 16 months. Unfortunately, she progressed with brain, lung and spinal metastasis before could be switched to second generation EGFR inhibitors.
Fig. 3.
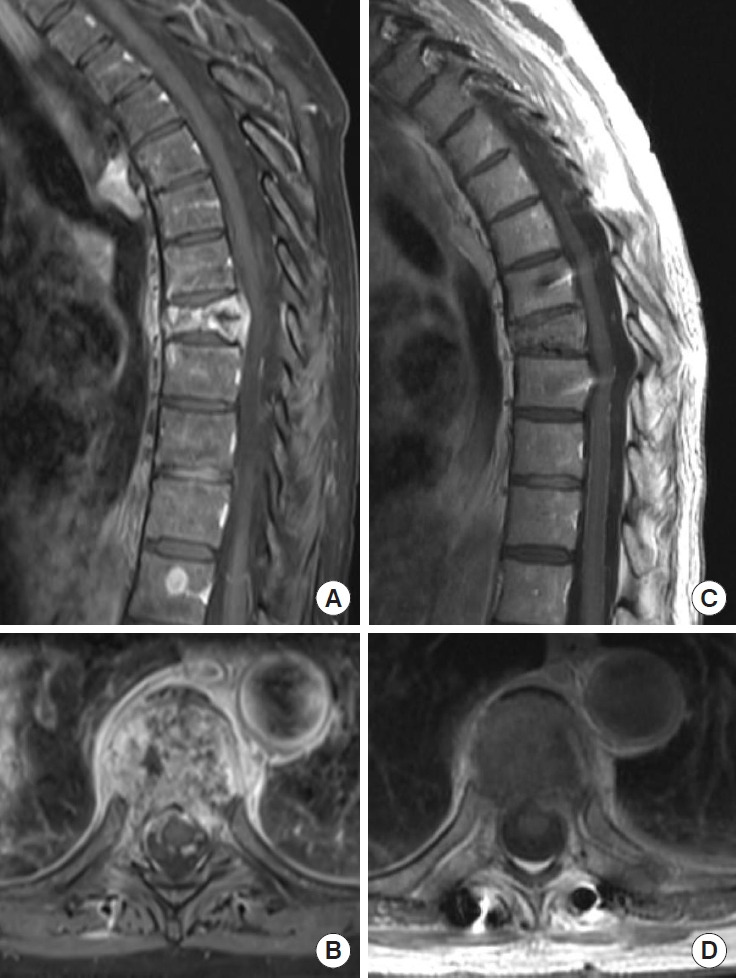
Successful use of epidermal growth factor receptor (EGFR) inhibitor osimertinib in a patient with advanced untreated EGFR mutated non-small cell lung cancer. Patient is a 60-yearold female who presented with thoracic pain, found to have a midthoracic pathologic compression fracture. Per discussion with medical oncology team, the consensus was for systemic treatment after a percutaneous spine fusion. Sagittal and axial magnetic resonance imaging cervical and thoracic spine prior to the initiation of treatment (A, B) and 4 months after treatment with osimertinib (C, D) showing resolution of the lesion.
RENAL CELL CARCINOMA
Renal cell carcinoma (RCC) is diagnosed in approximately 75,000 people yearly, with approximately 30% of patients developing bone metastases. According to SEER, 16% patients with RCC present with distant metastatic disease, with 5-year relative survival of 13.9% [14]. RCC is poorly responsive to hormonal and cytotoxic chemotherapies, with anti-VEGFR tyrosine kinase inhibitors (TKIs), immune checkpoint inhibitors, and other TKIs being the mainstay of treatment (Table 1, Supplementary Table 1). Multiple histologic and molecular RCC subtypes have been described with the most frequent subtype being clear cell, a subtype seen in approximately 70% of patients that is associated with VHL mutation and resulting downstream activation of angiogenesis via VEGFR, with additional mutations in PBRM1, SETD2, and BAP1 described by TCGA, as well as alterations of the mTOR pathway components. In contrast, papillary RCC has been associated with alterations in Met and NRF2. Other RCC subtypes harbor mutations in TP53 and PTEN, show loss of CDKN2A and SMARKB1, as well as TFE3-TFEB fusions. Miller et al. [49] studied 100 advanced RCC patients and reported improved OS with combined stereotactic radiosurgery (SRS)/TKI as compared to SRS alone, reporting lower levels of local failure; at 12 months, local failure occurred in 4% patients treated with first-line TKI, as compared to 19%–27% in therapy naïve patients or patients undergoing SRS w/wo TKI post failing firstline therapy. After nephrectomy with or without resection of metastatic disease, RCC patients usually undergo treatment with first-line systemic therapy including bevacizumab/IFN alpha, high dose IL2, pazopanib, sunitinib or temsirolimus. Second line therapies include axitinib, cabozantinib, lenvatinib/everolimus and nivolumab; other options include everolimus and sorafenib (Tables 1-3).
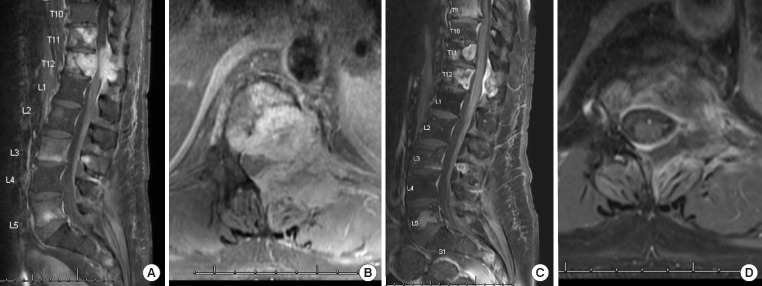
Some of the commonly used targeted inhibitors include VEGFR inhibitors and TKI, including bevacizumab targeting VEGFR, lenvatinib targeting FGFR/VEGFR and Cabozantinib targeting VEGFR/AXL/cMet, with their benefits documented in several phase III clinical trials including CABOSUN, which reported improved PFS of 8.2 months (vs. 5.6 months) as compared to sunitinib with 34% reduction in rate of progression or death [50,51]. In our hands, cabozantinib has resulted in robust response in bulky spinal involvement as illustrated in Fig. 4 where complete resolution of severe spinal cord compression is demonstrated.
Fig. 4.
Successful use of a multitargeted tyrosine kinase inhibitor cabozantinib in a patient with advanced clear cell renal cell cancer. Patient is a 51-year-old male with progressive clear cell renal cell carcinoma. Per discussion with medical oncology team, the consensus was for systemic treatment and restaging, as patient was not a candidate for surgery. Sagittal and axial magnetic resonance imaging of thoracic and lumbar spine prior to initiation of treatment showing significant compression of the thecal sac (A,B) and 3 months after treatment with cabozantinib (C, D) showing resolution of the lesion and significant improvement in cord compression and disease burden.
Axitinib, pazopanib, sorafenib, sunitinib targeting VEGFR/PDGFR had likewise shown promising results in patients with metastatic RCC; median PFS was reported as high as 15.7 months with median OS of 29.9 months, with median PFS of 7.4 months and median OS of 13.6 months in sorafenib refractory patients [52]. PD-1/PD-L1 (nivolumab, ipilimumab) and mTOR inhibitors (everolimus, temsirolimus) have additionally been trialed for treatment of metastatic RCC with promising results (Tables 1-3). Multiple phase 3 clinical trials are ongoing including CLEAR (pembrolizumab-lenvatinib vs. everolimus-lenvatinib vs. sunitinib with PFS of 23.9 months vs. 9.2 months), CheckMate214 (nivolumab-ipilimimab vs. sunitinib with median OS not reached vs. 38.4 months), IMmotion151 (atezolizumab-bevacizumab vs. sunitinib with final OS 36.1 months vs. 38.7 months), JAVELIN Renal 101 (avelumab-axitinib vs. sunitinib with median PFS of 13.3 months and 5.6 months), KEYNOTE-426 (pembrolizumab-axitinib vs. sunitinib with median PFS of 20.6 months vs. 11.3 months), and ADAPT (DC immunotherapy/sunitinib vs. sunitinib with median OS of 27.7 months vs. 32.4 months, and PFS of 6 months vs. 7.83 months) (cinicaltrials.org) for patients with metastatic RCC (Supplementary Table 1).
PROSTATE CANCER
Prostate cancer will commonly metastasize to bone and OS in patients with spine metastases is roughly 2.5 years [53]. According to SEER, 7% patients diagnosed with prostate cancer have distant metastases at presentation, with 30.6% 5-year relative survival [14]. Androgen receptor antagonists including flutamide, bicalutamide and abiraterone are commonly used as first-line androgen deprivation agents resulting in improvement in patient outcomes; as reported by STAMPEDE trial median OS was not reached with addition of zoledronic acid, 81 months with addition of docetaxel, 76 months with addition of both, and 71 months with standard of care (Table 1) [54]. Other androgen deprivation therapies include ketoconazole or abiraterone that inhibit CYP17 with resulting androgen synthesis blockade, and use of LHRH agonists/antagonists including leuproline, goserelin, degarelix with resulting downregulation in LHRH receptor signaling, with degarelix inducing and maintaining androgen deprivation for up to 1 year (Table 1) [55,56].
In patients with androgen resistant prostate cancer, a variety of other therapies have been utilized (Table 1). Small TKIs gefitinib, erlotinib targeting EGFR and lapatinib targeting EGFR/HER2 had shown some success with improvement in prostatespecific antigen (PSA) levels; phase II trial of lapatinib resulted in no radiologic evidence of metastatic disease in 7 of twenty nine patients [57,58]. Monoclonal EGFR antibody cetuximab has been used alone and in combination with various chemotherapy agents including doxorubicin, docetaxel, and mitoxantrone with some improvement in PSA levels and/or median survival; combination of cetuximab with doxorubicin resulted in stable disease in 65% patients with castration resistant prostate cancer with bone disease (Table 1) [59,60]. MET/VEGFR2 inhibitor cabozantinib has likewise been trialed in patients with metastatic prostate cancer in several phase III trials including COMET-1/2, with no significant improvement in OS, with 15% (vs. 17%) responders and median OS of 11 months (vs. 9.8 months) [61]. PARP inhibitors including olaparib have been used in patients with androgen resistant prostate cancer, where phase II TOPARP-A trial showed an overall 33% response rate, especially in patients with underlying mutations in DNA damage repair pathways or BRCA1/2, while TOPARP-B trial reported 54.3% composite response, with radiographic response in 24.2% and PSA response in 37% [62].
In addition, anti-CTLA4 immunotherapy including ipilimumab has been trialed in patients with prostate cancer in phase III trials with improvement in PFS of 5.6 months (vs. 3.8 months) and measured PSA levels (Tables 2, 3) [63]. Some success was noted with use of PD1/PD-L1 inhibitors, including nivolumab, pembrolizumab, and atezolizumab, especially in patients with mismatch repair impairment, hypermutated prostate cancer lesions and those with microsatellite instability; in CheckMate 9KD trial, combination of nivolumab with docetaxel resulted in 9-month radiographic PFS and OS of 18.2 months (Tables 2, 3). 64 Several tumor vaccines including an autologous Sipuleucel-T vaccine comprised of antigen presenting cells co-cultured with PA2024 prostatic acid phosphatase linked to GM-CSF have been successfully used to treat prostate cancer, with significant improvement in the reported OS in patients of up to 13 months in several phase III trials including IMPACT trial [65]. Another area of investigation focuses on the development of chimeric antigen receptor T cells (CAR-T cells) for treating patients with metastatic prostate cancer, which had been previously tested and successfully applied for treatment of patients with hematologic malignancies [66,67].
THYROID CANCER
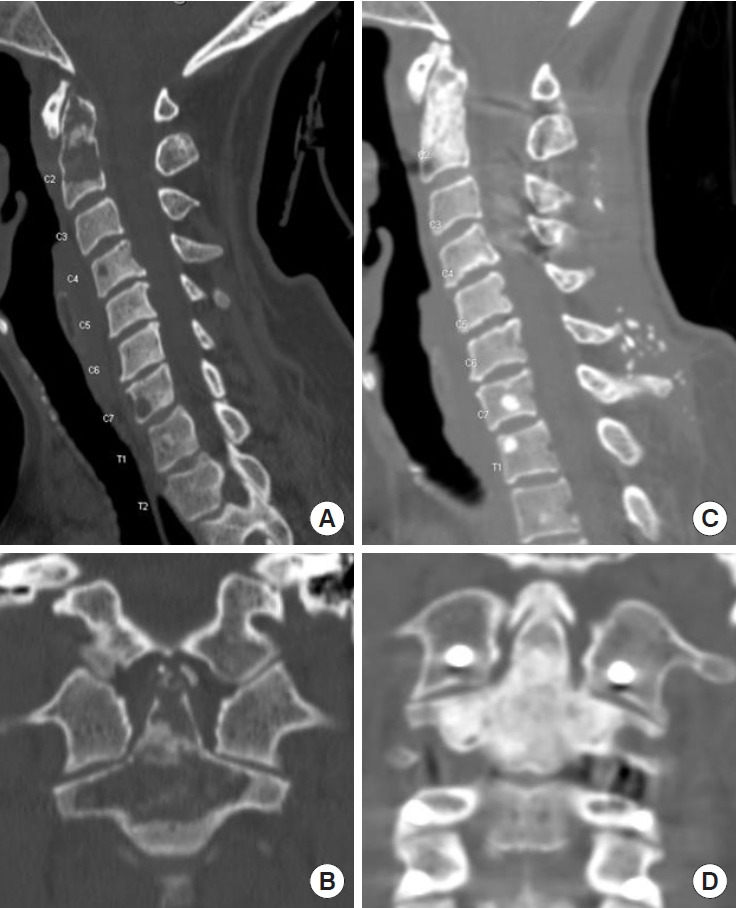
Although the percentage of patients with spine metastases arising from primary thyroid cancer, including differentiated thyroid carcinoma, anaplastic carcinoma and medullary thyroid carcinoma is not high, it is a common endocrine malignancy, and up to 30% patients will develop resistance to standard therapy and progress to metastatic disease (Table 1). According to SEER, 3% patients with thyroid cancer have distant metastases at the time of diagnosis, with 53.3% 5-year relative survival [14]. Targeted inhibitors for the mitogen-activated protein kinase pathway have been trialed in patients with metastatic thyroid cancer. Sorafenib targeting VEGFR/Flt3/RET/cKIT/BRAF and lenvatinib targeting VEGFR/FGFR/PDGFR/KIT/RET have been trialed in patients with differentiated thyroid carcinoma with improvement in PFS and OS, respectively, by several phase II trials, as well as DECISION and SELECT phase III trials, the former of which reported median PFS of 10.8 months (vs. 5.8 months) with sorafenib use in radioactive iodine-refractory locally advanced or metastatic differentiated thyroid cancer, while the latter reported median OS of 52.2 months in lenvatinib responders veruss 19 months in nonresponders (Table 1; Supplementary Table 1) [68,69]. We have seen robust results using lenvatinib in patients with bulky metastasis of follicular thyroid carcinoma in the spine. We would like to present a 70-year-old patient who had rapid recurrence of multilevel cervical spine metastasis after treatment with radioactive iodine and maximal doses of targeted radiation but had remained progression free for 36 months since starting lenvatinib (Fig. 5). Vandetanib, targeting VEGFR/EGFR/RET, and cabozantinib, targeting VEGFR2/cMet/AXL/RET, have been trialed in patients with medullary thyroid carcinoma with improvement in PFS in phase II and phase III trials, with 30.5 months (vs. 19.3 months) median OS with vandetanib use, with 26.6 (vs. 21.1) median OS with cabozantinib use, including 44.3 months (vs. 18.9 months) median OS in patients with RET M918T positive disease [70,71]. More recent studies focus on targeting PI3K pathway, ALK translocations, as well as HER2/3 receptors (Supplementary Table 1) [72]. Panebianco et al reported ELM4-ALK and STRN-ALK fusions in patients with papillary and poorly differentiated thyroid carcinomas [73]. Other studies are investigating the effects of immunotherapy including anti-CTLA4 and anti-PD1, and vaccine use (Table 3; Supplementary Table 1) [74,75].
Fig. 5.
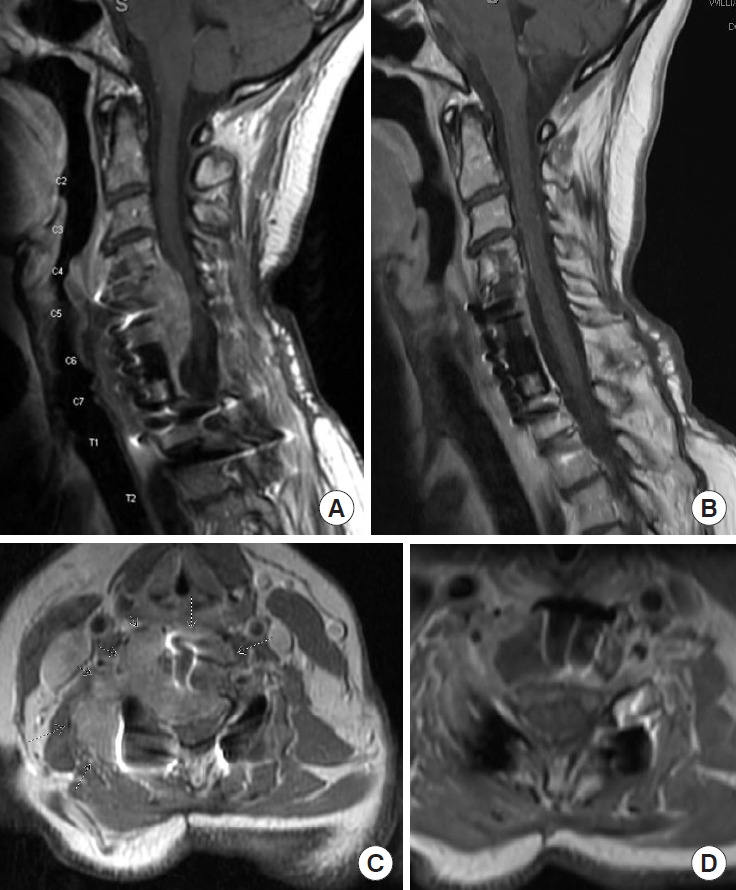
Successful use of multitargeted tyrosine kinase inhibitor Lenvatinib in a patient with recurrent follicular thyroid carcinoma. Patient is a 70-year-old female who had undergone prior corpectomy with cage placement and posterior spinal fusion, followed by treatment with iodine, external beam radiation therapy and spine stereotactic radiosurgery, with significant progression of her disease shortly thereafter. Sagittal and axial magnetic resonance imaging of cervical and thoracic spine prior to initiation of treatment showing significant anterior cord compression C4–7 (A, B) and 3 months after treatment with Lenvatinib (C, D), showing resolution of the lesion and significant improvement in cord compression.
CONCLUSION
The number of patients with metastatic spine disease continues to rise. Moreover, even the most extensive surgical resection and aggressive radiation therapies are frequently insufficient to control the disease without adjunct medical therapy, which can successfully address residual microscopic disease and prevent recurrence, specifically within the bone environment. Historically, the ultimate demise in patients with metastatic spine disease is due to a combination of their extensive systemic disease burden and aggressive spine disease resulting in neurologic compromise. In the recent years, development of individualized, targeted therapies and novel treatment protocols had heavily depended on the identification of novel mutations and improved understanding of the biology of many cancers, giving hope to patients with spinal metastases to prevent disease progression, avert neurologic deficit and improve their quality of life. However, there remains a large void with developing therapeutic agents that can specifically target cancer within the unique bone milieu. Current literature is lacking in safety, efficacy, and estimates of overall response rates from the use of many of the new treatment agents when administered to patients with spine metastatic disease, however robust response can be achieved in select cases as described in this manuscript. Identification of predictors of favorable response to targeted inhibition, chemotherapy or immunotherapy of spine metastases derived from various primary cancers is a necessary next step.
Footnotes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Conceptualization: EF, CT; Data curation: EF, CT; Formal analysis: EF; Funding acquisition: CT; Methodology: EF, CT; Project administration: CT; Visualization: EF; Writing - original draft: EF; Writing - review & editing: EF, JB, CAB, LR, CT.
SUPPLEMENTARY MATERIALS
Supplementary Table 1 can be found via https://doi.org/10.14245/ns.2244290.145.
Targeted therapies and immunotherapies approved by the U.S. Food and Drug Administration in the last 2 years
REFERENCES
- 1.Perrin RG, Laxton AW. Metastatic spine disease: epidemiology, pathophysiology, and evaluation of patients. Neurosurg Clin N Am. 2004;15:365–73. doi: 10.1016/j.nec.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 3.Hernandez RK, Adhia A, Wade SW, et al. Prevalence of bone metastases and bone-targeting agent use among solid tumor patients in the United States. Clin Epidemiol. 2015;7:335–45. doi: 10.2147/CLEP.S85496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.North RB, LaRocca VR, Schwartz J, et al. Surgical management of spinal metastases: analysis of prognostic factors during a 10-year experience. J Neurosurg Spine. 2005;2:564–73. doi: 10.3171/spi.2005.2.5.0564. [DOI] [PubMed] [Google Scholar]
- 5.D’Oronzo S, Coleman R, Brown J, et al. Metastatic bone disease: pathogenesis and therapeutic options: up-date on bone metastasis management. J Bone Oncol. 2019;15:004. doi: 10.1016/j.jbo.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright E, Ricciardi F, Arts M, et al. Metastatic spine tumor epidemiology: comparison of trends in surgery across two decades and three continents. World Neurosurg. 2018;114:e809–17. doi: 10.1016/j.wneu.2018.03.091. [DOI] [PubMed] [Google Scholar]
- 7.Wu G, Broniscer A, McEachron TA, et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. 2012;44:251–3. doi: 10.1038/ng.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Attalla K, Duzgol C, McLaughlin L, et al. The spinal distribution of metastatic renal cell carcinoma: support for locoregional rather than arterial hematogenous mode of early bony dissemination. Urol Oncol. 2021;39:196.e9–196.e14. doi: 10.1016/j.urolonc.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laufer I, Rubin DG, Lis E, et al. The NOMS framework: approach to the treatment of spinal metastatic tumors. Oncologist. 2013;18:744–51. doi: 10.1634/theoncologist.2012-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomita K, Kawahara N, Kobayashi T, et al. Surgical strategy for spinal metastases. Spine (Phila Pa 1976) 2001;26:298–306. doi: 10.1097/00007632-200102010-00016. [DOI] [PubMed] [Google Scholar]
- 11.Fourney DR, Frangou EM, Ryken TC, et al. Spinal instability neoplastic score: an analysis of reliability and validity from the spine oncology study group. J Clin Oncol. 2011;29:3072–7. doi: 10.1200/JCO.2010.34.3897. [DOI] [PubMed] [Google Scholar]
- 12.Tokuhashi Y, Matsuzaki H, Toriyama S, et al. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 1990;15:1110–3. doi: 10.1097/00007632-199011010-00005. [DOI] [PubMed] [Google Scholar]
- 13.Tokuhashi Y, Matsuzaki H, Oda H, et al. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 2005;30:2186–91. doi: 10.1097/01.brs.0000180401.06919.a5. [DOI] [PubMed] [Google Scholar]
- 14. Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER Research Data, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2021, based on the November 2020 submission. Accessed February 27, 2022.
- 15.Chan-Seng E, Charissoux M, Larbi A, et al. Spinal metastases in breast cancer: single center experience. World Neurosurg. 2014;82:1344–50. doi: 10.1016/j.wneu.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Groenen KHJ, van der Linden YM, Brouwer T, et al. The Dutch national guideline on metastases and hematological malignancies localized within the spine; a multidisciplinary collaboration towards timely and proactive management. Cancer Treat Rev. 2018;69:29–38. doi: 10.1016/j.ctrv.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Pondé N, Aftimos P, Piccart M. Antibody-drug conjugates in breast cancer: a comprehensive review. Curr Treat Options Oncol. 2019;20:37. doi: 10.1007/s11864-019-0633-6. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto Y, Nishimura R, Tanigawa T, et al. Efficacy and safety of TS-1 monotherapy for advanced/metastatic breast cancer - an observational study by the Kumamoto Breast Cancer Cooperative Group (KBCCG) Gan To Kagaku Ryoho. 2014;41:1221–5. [PubMed] [Google Scholar]
- 19.Swain SM, Miles D, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:519–30. doi: 10.1016/S1470-2045(19)30863-0. [DOI] [PubMed] [Google Scholar]
- 20.von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377:122–31. doi: 10.1056/NEJMoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kümler I, Christiansen OG, Nielsen DL. A systematic review of bevacizumab efficacy in breast cancer. Cancer Treat Rev. 2014;40:960–73. doi: 10.1016/j.ctrv.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Yan W, Xiao J, Liu T, et al. The effects of Hsp90 expression alteration on spinal metastases of breast carcinoma. Tumour Biol. 2013;34:1391–7. doi: 10.1007/s13277-012-0584-z. [DOI] [PubMed] [Google Scholar]
- 23.Cofano F, Monticelli M, Ajello M, et al. The targeted therapies era beyond the surgical point of view: what spine surgeons should know before approaching spinal metastases. Cancer Control. 2019;26:1073274819870549. doi: 10.1177/1073274819870549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munster PN, Thurn KT, Thomas S, et al. A phase II study of the histone deacetylase inhibitor vorinostat combined with tamoxifen for the treatment of patients with hormone therapy-resistant breast cancer. Br J Cancer. 2011;104:1828–35. doi: 10.1038/bjc.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mittendorf EA, Clifton GT, Holmes JP, et al. Final report of the phase I/II clinical trial of the E75 (nelipepimut-S) vaccine with booster inoculations to prevent disease recurrence in high-risk breast cancer patients. Ann Oncol. 2014;25:1735–42. doi: 10.1093/annonc/mdu211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodwin CR, Sankey EW, Liu A, et al. A systematic review of clinical outcomes for patients diagnosed with skin cancer spinal metastases. J Neurosurg Spine. 2016;24:837–49. doi: 10.3171/2015.4.SPINE15239. [DOI] [PubMed] [Google Scholar]
- 27.Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, openlabel, phase 3 randomised controlled trial. Lancet. 2012;380:358–65. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 28.Dummer R, Ascierto PA, Gogas HJ, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19:603–15. doi: 10.1016/S1470-2045(18)30142-6. [DOI] [PubMed] [Google Scholar]
- 29.Maio M, Grob JJ, Aamdal S, et al. Five-year survival rates for treatment-naive patients with advanced melanoma who received ipilimumab plus dacarbazine in a phase III trial. J Clin Oncol. 2015;33:1191–6. doi: 10.1200/JCO.2014.56.6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolchok JD, Rollin L, Larkin J. Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2017;377:2503–4. doi: 10.1056/NEJMc1714339. [DOI] [PubMed] [Google Scholar]
- 31.Day D, Hansen AR. Immune-related adverse events associated with immune checkpoint inhibitors. BioDrugs. 2016;30:571–84. doi: 10.1007/s40259-016-0204-3. [DOI] [PubMed] [Google Scholar]
- 32.Linardou H, Gogas H. Toxicity management of immunotherapy for patients with metastatic melanoma. Ann Transl Med. 2016;4:272. doi: 10.21037/atm.2016.07.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andtbacka RHI, Kaufman HL, Collichio F, et al. Talimogene Laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33:2780–8. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 34.Carreno BM, Magrini V, Becker-Hapak M, et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science. 2015;348:803–8. doi: 10.1126/science.aaa3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uyttenhove C, Pilotte L, Théate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–74. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 36.Goodwin CR, Khattab MH, Sankey EW, et al. Factors associated with life expectancy in patients with metastatic spine disease from adenocarcinoma of the lung. Global Spine J. 2015;5:417–24. doi: 10.1055/s-0035-1554778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao L, Long L, Hu C. Efficacy of paclitaxel combined with kanglaite injection in treatment of bone metastases of lung cancer. Iran J Public Health. 2019;48:1445–51. [PMC free article] [PubMed] [Google Scholar]
- 38.Shroff GS, de Groot PM, Papadimitrakopoulou VA, et al. Targeted therapy and immunotherapy in the treatment of non-small cell lung cancer. Radiol Clin North Am. 2018;56:485–95. doi: 10.1016/j.rcl.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 39.Govindan R, Szczesna A, Ahn MJ, et al. Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non-small-cell lung cancer. J Clin Oncol. 2017;35:3449–57. doi: 10.1200/JCO.2016.71.7629. [DOI] [PubMed] [Google Scholar]
- 40.Helissey C, Champiat S, Soria JC. Immune checkpoint inhibitors in advanced nonsmall cell lung cancer. Curr Opin Oncol. 2015;27:108–17. doi: 10.1097/CCO.0000000000000167. [DOI] [PubMed] [Google Scholar]
- 41.Horn L, Spigel DR, Vokes EE, et al. Nivolumab versus docetaxel in previously treated patients with advanced non-smallcell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057) J Clin Oncol. 2017;35:3924–33. doi: 10.1200/JCO.2017.74.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reck M, Taylor F, Penrod JR, et al. Impact of nivolumab versus docetaxel on health-related quality of life and symptoms in patients with advanced squamous non-small cell lung cancer: results from the CheckMate 017 Study. J Thorac Oncol. 2018;13:194–204. doi: 10.1016/j.jtho.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 43.Savas P, Hughes B, Solomon B. Targeted therapy in lung cancer: IPASS and beyond, keeping abreast of the explosion of targeted therapies for lung cancer. J Thorac Dis. 2013;5 Suppl 5(Suppl 5):S579–92. doi: 10.3978/j.issn.2072-1439.2013.08.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sequist LV, Yang JCH, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–34. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 45.Lauro S, Onesti CE, Righini R, et al. The use of bevacizumab in non-small cell lung cancer: an update. Anticancer Res. 2014;34:1537–45. [PubMed] [Google Scholar]
- 46.Raparia K, Villa C, DeCamp MM, et al. Molecular profiling in non-small cell lung cancer: a step toward personalized medicine. Arch Pathol Lab Med. 2013;137:481–91. doi: 10.5858/arpa.2012-0287-RA. [DOI] [PubMed] [Google Scholar]
- 47.Wang S, Li J. Second-generation EGFR and ErbB tyrosine kinase inhibitors as first-line treatments for non-small cell lung cancer. Onco Targets Ther. 2019;12:6535–48. doi: 10.2147/OTT.S198945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang S, Niu X, Bao X, et al. The PI3K inhibitor buparlisib suppresses osteoclast formation and tumour cell growth in bone metastasis of lung cancer, as evidenced by multimodality molecular imaging. Oncol Rep. 2019;41:2636–46. doi: 10.3892/or.2019.7080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller JA, Balagamwala EH, Angelov L, et al. Spine stereotactic radiosurgery with concurrent tyrosine kinase inhibitors for metastatic renal cell carcinoma. J Neurosurg Spine. 2016;25:766–74. doi: 10.3171/2016.4.SPINE16229. [DOI] [PubMed] [Google Scholar]
- 50.Bergerot P, Burns K, Prajapati D, et al. Advances in the treatment of metastatic renal cell carcinoma. Cancer Treat Res. 2018;175:127–37. doi: 10.1007/978-3-319-93339-9_6. [DOI] [PubMed] [Google Scholar]
- 51.Choueiri TK, Halabi S, Sanford BL, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the alliance A031203 CABOSUN Trial. J Clin Oncol. 2017;35:591–7. doi: 10.1200/JCO.2016.70.7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Escudier B, Gore M. Axitinib for the management of metastatic renal cell carcinoma. Drugs R D. 2011;11:113–26. doi: 10.2165/11591240-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amelot A, Terrier LM, Le Nail LR, et al. Spine metastasis in patients with prostate cancer: survival prognosis assessment. Prostate. 2021;81:91–101. doi: 10.1002/pros.24084. [DOI] [PubMed] [Google Scholar]
- 54.James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–77. doi: 10.1016/S0140-6736(15)01037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klotz L, Boccon-Gibod L, Shore ND, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008;102:1531–8. doi: 10.1111/j.1464-410X.2008.08183.x. [DOI] [PubMed] [Google Scholar]
- 56.Attard G, Reid AHM, A’Hern R, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27:3742–8. doi: 10.1200/JCO.2008.20.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goodwin CR, Abu-Bonsrah N, Rhines LD, et al. Molecular markers and targeted therapeutics in metastatic tumors of the spine: changing the treatment paradigms. Spine (Phila Pa 1976) 2016;41 Suppl 20:S218–23. doi: 10.1097/BRS.0000000000001833. [DOI] [PubMed] [Google Scholar]
- 58.Whang YE, Armstrong AJ, Rathmell WK, et al. A phase II study of lapatinib, a dual EGFR and HER-2 tyrosine kinase inhibitor, in patients with castration-resistant prostate cancer. Urol Oncol. 2013;31:82–6. doi: 10.1016/j.urolonc.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 59.Slovin SF, Kelly WK, Wilton A, et al. Anti-epidermal growth factor receptor monoclonal antibody cetuximab plus Doxorubicin in the treatment of metastatic castration-resistant prostate cancer. Clin Genitourin Cancer. 2009;7:E77–82. doi: 10.3816/CGC.2009.n.028. [DOI] [PubMed] [Google Scholar]
- 60.Fleming MT, Sonpavde G, Kolodziej M, et al. Association of rash with outcomes in a randomized phase II trial evaluating cetuximab in combination with mitoxantrone plus prednisone after docetaxel for metastatic castration-resistant prostate cancer. Clin Genitourin Cancer. 2012;10:6–14. doi: 10.1016/j.clgc.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 61.Sonpavde GP, Pond GR, Fizazi K, et al. Cabozantinib for Progressive metastatic castration-resistant prostate cancer following docetaxel: combined analysis of two phase 3 trials. Eur Urol Oncol. 2020;3:540–3. doi: 10.1016/j.euo.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mateo J, Carreira S, Sandhu S, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373:1697–708. doi: 10.1056/NEJMoa1506859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beer TM, Kwon ED, Drake CG, et al. Randomized, doubleblind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol. 2017;35:40–7. doi: 10.1200/JCO.2016.69.1584. [DOI] [PubMed] [Google Scholar]
- 64.Pritchard CC, Morrissey C, Kumar A, et al. Complex MSH2 and MSH6 mutations in hypermutated microsatellite unstable advanced prostate cancer. Nat Commun. 2014;5:4988. doi: 10.1038/ncomms5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schellhammer PF, Chodak G, Whitmore JB, et al. Lower baseline prostate-specific antigen is associated with a greater overall survival benefit from sipuleucel-T in the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) trial. Urology. 2013;81:1297–302. doi: 10.1016/j.urology.2013.01.061. [DOI] [PubMed] [Google Scholar]
- 66.Priceman SJ, Gerdts EA, Tilakawardane D, et al. Co-stimulatory signaling determines tumor antigen sensitivity and persistence of CAR T cells targeting PSCA+ metastatic prostate cancer. Oncoimmunology. 2018;7:e1380764. doi: 10.1080/2162402X.2017.1380764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Junghans RP, Ma Q, Rathore R, et al. Phase I trial of anti-PSMA designer CAR-T cells in prostate cancer: possible role for interacting interleukin 2-T cell pharmacodynamics as a determinant of clinical response. Prostate. 2016;76:1257–70. doi: 10.1002/pros.23214. [DOI] [PubMed] [Google Scholar]
- 68.Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372:621–30. doi: 10.1056/NEJMoa1406470. [DOI] [PubMed] [Google Scholar]
- 69.Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384:319–28. doi: 10.1016/S0140-6736(14)60421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wells SA, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30:134–41. doi: 10.1200/JCO.2011.35.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Elisei R, Schlumberger MJ, Müller SP, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31:3639–46. doi: 10.1200/JCO.2012.48.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Naoum GE, Morkos M, Kim B, et al. Novel targeted therapies and immunotherapy for advanced thyroid cancers. Mol Cancer. 2018;17:51. doi: 10.1186/s12943-018-0786-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Panebianco F, Nikitski AV, Nikiforova MN, et al. Characterization of thyroid cancer driven by known and novel ALK fusions. Endocr Relat Cancer. 2019;26:803–14. doi: 10.1530/ERC-19-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Capdevila J, Wirth LJ, Ernst T, et al. PD-1 blockade in anaplastic thyroid carcinoma. J Clin Oncol. 2020;38:2620–7. doi: 10.1200/JCO.19.02727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ma M, Lin B, Wang M, et al. Immunotherapy in anaplastic thyroid cancer. Am J Transl Res. 2020;12:974–88. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Targeted therapies and immunotherapies approved by the U.S. Food and Drug Administration in the last 2 years