Abstract
Background
Inspiratory muscle training (IMT) aims to improve respiratory muscle strength and endurance. Clinical trials used various training protocols, devices and respiratory measurements to check the effectiveness of this intervention. The current guidelines reported a possible advantage of IMT, particularly in people with respiratory muscle weakness. However, it remains unclear to what extent IMT is clinically beneficial, especially when associated with pulmonary rehabilitation (PR).
Objectives
To assess the effect of inspiratory muscle training (IMT) on chronic obstructive pulmonary disease (COPD), as a stand‐alone intervention and when combined with pulmonary rehabilitation (PR).
Search methods
We searched the Cochrane Airways trials register, CENTRAL, MEDLINE, Embase, PsycINFO, Cumulative Index to Nursing and Allied Health Literature (CINAHL) EBSCO, Physiotherapy Evidence Database (PEDro) ClinicalTrials.gov, and the World Health Organization International Clinical Trials Registry Platform on 20 October 2022. We also checked reference lists of all primary studies and review articles.
Selection criteria
We included randomized controlled trials (RCTs) that compared IMT in combination with PR versus PR alone and IMT versus control/sham. We included different types of IMT irrespective of the mode of delivery. We excluded trials that used resistive devices without controlling the breathing pattern or a training load of less than 30% of maximal inspiratory pressure (PImax), or both.
Data collection and analysis
We used standard methods recommended by Cochrane including assessment of risk of bias with RoB 2. Our primary outcomes were dyspnea, functional exercise capacity and health‐related quality of life.
Main results
We included 55 RCTs in this review. Both IMT and PR protocols varied significantly across the trials, especially in training duration, loads, devices, number/ frequency of sessions and the PR programs. Only eight trials were at low risk of bias.
PR+IMT versus PR
We included 22 trials (1446 participants) in this comparison. Based on a minimal clinically important difference (MCID) of −1 unit, we did not find an improvement in dyspnea assessed with the Borg scale at submaximal exercise capacity (mean difference (MD) 0.19, 95% confidence interval (CI) −0.42 to 0.79; 2 RCTs, 202 participants; moderate‐certainty evidence).
We also found no improvement in dyspnea assessed with themodified Medical Research Council dyspnea scale (mMRC) according to an MCID between −0.5 and −1 unit (MD −0.12, 95% CI −0.39 to 0.14; 2 RCTs, 204 participants; very low‐certainty evidence).
Pooling evidence for the 6‐minute walk distance (6MWD) showed an increase of 5.95 meters (95% CI −5.73 to 17.63; 12 RCTs, 1199 participants; very low‐certainty evidence) and failed to reach the MCID of 26 meters. In subgroup analysis, we divided the RCTs according to the training duration and mean baseline PImax. The test for subgroup differences was not significant. Trials at low risk of bias (n = 3) demonstrated a larger effect estimate than the overall.
The summary effect of the St George's Respiratory Questionnaire (SGRQ) revealed an overall total score below the MCID of 4 units (MD 0.13, 95% CI −0.93 to 1.20; 7 RCTs, 908 participants; low‐certainty evidence).
The summary effect of COPD Assessment Test (CAT) did not show an improvement in the HRQoL (MD 0.13, 95% CI −0.80 to 1.06; 2 RCTs, 657 participants; very low‐certainty evidence), according to an MCID of −1.6 units.
Pooling the RCTs that reported PImax showed an increase of 11.46 cmH2O (95% CI 7.42 to 15.50; 17 RCTs, 1329 participants; moderate‐certainty evidence) but failed to reach the MCID of 17.2 cmH2O. In subgroup analysis, we did not find a difference between different training durations and between studies judged with and without respiratory muscle weakness.
One abstract reported some adverse effects that were considered "minor and self‐limited".
IMT versus control/sham
Thirty‐seven RCTs with 1021 participants contributed to our second comparison. There was a trend towards an improvement when Borg was calculated at submaximal exercise capacity (MD −0.94, 95% CI −1.36 to −0.51; 6 RCTs, 144 participants; very low‐certainty evidence). Only one trial was at a low risk of bias.
Eight studies (nine arms) used the Baseline Dyspnea Index ‐ Transition Dyspnea Index (BDI‐TDI). Based on an MCID of +1 unit, they showed an improvement only with the 'total score' of the TDI (MD 2.98, 95% CI 2.07 to 3.89; 8 RCTs, 238 participants; very low‐certainty evidence). We did not find a difference between studies classified as with and without respiratory muscle weakness. Only one trial was at low risk of bias.
Four studies reported the mMRC, revealing a possible improvement in dyspnea in the IMT group (MD −0.59, 95% CI −0.76 to −0.43; 4 RCTs, 150 participants; low‐certainty evidence). Two trials were at low risk of bias.
Compared to control/sham, the MD in the 6MWD following IMT was 35.71 (95% CI 25.68 to 45.74; 16 RCTs, 501 participants; moderate‐certainty evidence). Two studies were at low risk of bias. In subgroup analysis, we did not find a difference between different training durations and between studies judged with and without respiratory muscle weakness.
Six studies reported theSGRQ total score, showing a larger effect in the IMT group (MD −3.85, 95% CI −8.18 to 0.48; 6 RCTs, 182 participants; very low‐certainty evidence). The lower limit of the 95% CI exceeded the MCID of −4 units. Only one study was at low risk of bias.
There was an improvement in life quality with CAT (MD −2.97, 95% CI −3.85 to −2.10; 2 RCTs, 86 participants; moderate‐certainty evidence). One trial was at low risk of bias.
Thirty‐two RCTs reported PImax, showing an improvement without reaching the MCID (MD 14.57 cmH2O, 95% CI 9.85 to 19.29; 32 RCTs, 916 participants; low‐certainty evidence). In subgroup analysis, we did not find a difference between different training durations and between studies judged with and without respiratory muscle weakness.
None of the included RCTs reported adverse events.
Authors' conclusions
IMT may not improve dyspnea, functional exercise capacity and life quality when associated with PR. However, IMT is likely to improve these outcomes when provided alone.
For both interventions, a larger effect in participants with respiratory muscle weakness and with longer training durations is still to be confirmed.
Plain language summary
Are exercises for strengthening breathing muscles effective for people with chronic obstructive pulmonary disease?
Key messages
• Exercise combined with specific exercises to strengthen breathing muscles may not improve breathlessness, physical fitness and life quality. Strength of breathing muscles and endurance increased but not enough to make a difference to patients.
• Specific exercises to strengthen breathing muscles compared to no exercise may improve breathlessness, physical fitness and life quality. Strength of breathing muscles and endurance increased, but we don't know if this benefitted patients.
• We don't know whether exercise or specific exercises to strengthen breathing muscles is better for people with weakened breathing muscles who trained for several weeks.
• Future research should focus on people with weakened breathing muscles and studies should include more people.
What is chronic obstructive pulmonary disease (COPD)?
Chronic obstructive pulmonary disease (COPD) is a lung condition characterized by blockages in the airways, which cause shortness of breath and a cough. It appears after the long‐term inhalation of irritating gases like cigarette smoke and chemicals. Training and strengthening the breathing muscles is thought to improve breathing and reduce air obstruction.
What exercise treatments do people with COPD use?
Health professionals use various exercises to help improve people's COPD.
• Some people undertake a program of general exercise and education to help reduce symptoms and improve their exercise capacity and life quality.
• Other people try to improve the strength and endurance of the breathing muscles through a series of breathing exercises using specific devices. This is called 'inspiratory muscle training' (IMT). The devices add resistance to breathing to strengthen the diaphragm and the intercostal muscles between the ribs ‐ the muscles used for breathing. People may then be able to breathe in more air with each breath and be active for longer. The devices are also used by people with healthy lungs to improve their sports performance.
What did we want to find out?
We wanted to find out if exercise combined with IMT compared to exercise alone, and IMT compared to no exercise or sham IMT has a better effect on breathlessness, physical fitness and life quality. (A sham device has no effect on breathing muscles. It allows a fair test of the real devices, because people don't know which they are using.)
We also wanted to check whether IMT was associated with any unwanted effects.
What did we do?
We searched for studies that compared
• exercise combined with IMT with exercise alone; and
• IMT with no exercise or sham IMT.
We compared and summarized the results of the studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
Exercise plus IMT compared with exercise alone We found 22 studies with 1446 participants, which lasted between 2 and 24 weeks. Exercise ranged from training only on a treadmill, with only a cycle, and a combination of exercises (training with a cycle and treadmill, muscle strengthening, stair climbing, and education). The duration and devices of IMT also varied across the studies.
We found that this combination: • probably makes little to no difference to breathlessness (measured with different scales); • has an unknown effect on physical fitness; • may make little to no difference to life quality (measured with different scales); • probably makes little to no difference to strength of breathing muscles. IMT versus no training or sham device We found 37 studies with 1021 participants, which lasted from 2 weeks to a year. IMT varied across the studies regarding devices, resistance, frequency and supervision.
We found out that IMT alone: • may reduce breathlessness measured with one scale, but it is unclear if it has an effect when measured with two other scales; • probably improves physical fitness; • probably improves life quality when measured with one scale, but it is unclear if it has a benefit when measured with another one; • may make little to no difference to strength of breathing muscles.
What are the limitations of the evidence?
The studies used different training durations, resistance, devices, number and frequency of sessions, and physical training programs. This makes it hard to draw firm conclusions. Overall our confidence in the conclusions is reduced because the studies were small, some participants may have been aware of which treatment they were receiving, and generally, there was some diversity in the studies.
How up to date is the evidence?
The evidence is up‐to‐date to 20 October 2022.
Summary of findings
Summary of findings 1. Pulmonary rehabilitation plus inspiratory muscle training compared to pulmonary rehabilitation alone for people with chronic obstructive pulmonary disease.
| Pulmonary rehabilitation plus inspiratory muscle training compared to pulmonary rehabilitation alone for people with chronic obstructive pulmonary disease | ||||||
| Patient or population: people with chronic obstructive pulmonary disease (COPD) Setting: community Intervention: pulmonary rehabilitation (PR) + inspiratory muscle training (IMT) Comparison: PR | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with PR | Risk with PR+IMT | |||||
|
Dyspnea
assessed with Borg scale at submaximal exercise capacity Scale from 0 to 10 (worse) Follow‐up: range 3 months to 4 months |
The mean dyspnea was 4.65 | The mean dyspnea was 0.19 points higher (0.42 lower to 0.79 higher) | ‐ | 202 (2 RCTs) | ⊕⊕⊕⊝ Moderatea | The combination of PR+IMT probably results in little to no difference in dyspnea measured with Borg at submaximal exercise capacity compared to PR alone, considering an MCID of −1 unit |
|
Dyspnea
assessed with mMRC Scale from 0 to 4 (worse) Follow‐up: range 1 month to 2 months |
The mean dyspnea ranged from −0.8 to −0.33 | MD 0.12 lower (0.39 lower to 0.14 higher) | ‐ | 204 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b | The evidence is very uncertain about the effect of the combination of PR+IMT on dyspnea measured with the mMRC compared to PR alone, considering an MCID between −0.5 and −1 unit |
| Functional exercise capacity assessed with 6MWD Follow‐up: range 2 weeks to 6 months | The mean functional exercise capacity was 304.72 metersc | MD 5.95 meters higher (5.73 lower to 17.63 higher) | ‐ | 1199 (12 RCTs) | ⊕⊝⊝⊝ Very lowd,e | The evidence is very uncertain about the effect of the combination of PR+IMT on the 6MWD compared to PR alone, considering an MCID of 26 meters |
|
Health‐related quality of life
assessed with SGRQ total score Scale from 0 to 100 (worse) Follow‐up: range 3 weeks to 6 months |
The mean health‐related quality of life was 14.9c | MD 0.13 higher (0.93 lower to 1.2 higher) | ‐ | 908 (7 RCTs) | ⊕⊕⊝⊝ Lowf | The combination of PR+IMT may result in little to no difference in health‐related quality of life measured with the SGRQ compared to PR alone, considering an MCID of −4 units |
| Health‐related quality of life assessed with CAT Scale from 0 to 40 (worse) Follow‐up: range 3 weeks to 6 months | The mean health‐related quality of life ranged from −3.42 to −3 | MD 0.13 higher (0.8 lower to 1.06 higher) | ‐ | 657 (2 RCTs) | ⊕⊝⊝⊝ Very lowg,h | The evidence is very uncertain about the effect of the combination of PR+IMT on health‐related quality of life measured with the CAT compared to PR alone, considering an MCID of about −1.6 units |
| Inspiratory muscle strength assessed with PImax Follow‐up: range 3 weeks to 6 months | The mean inspiratory muscle strength was 67.37 cmH2Oc | MD 11.46 cmH2O higher (7.42 higher to 15.50 higher) | ‐ | 1329 (17 RCTs) | ⊕⊕⊕⊝ Moderated | The combination of IMT+PR probably slightly increases inspiratory muscle strength (PImax) compared to PR alone, without reaching the MCID of 17.2 cmH2O |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CAT: COPD [chronic obstructive pulmonary disease] Assessment Test; IMT: inspiratory muscle training; MD: mean difference; mMRC: modified Medical Research Council dyspnoea scale; MCID: minimum clinically important difference; PImax: Maximal Inspiratory Pressure; PR: pulmonary rehabilitation; RCT: randomized controlled trial; SGRQ: St George's Respiratory Questionnaire; 6MWD: six‐minute walk distance | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_423482112637765988. | ||||||
aDowngraded by one level for imprecision due to small sample size (rule of thumb: at least 400 participants). bDowngraded by two levels for risk of bias because all the trials are at high risk of bias. cIncluding change and endpoint scores. dDowngraded by one level for risk of bias because most of the evidence is from studies at high risk of bias and with some concern. eDowngraded by two levels for inconsistency due to considerable statistical heterogeneity (I² statistic), confidence intervals not overlapping, and significant variations in the direction of the effects. fDowngraded by two levels for risk of bias due to a considerable bias across the studies in the measurement of the outcome (lack of blinding) and all the trials are at high risk of bias and some concern. gDowngraded by two levels for risk of bias due to considerable bias across the studies in the measurement of the outcome (lack of blinding) and most of the evidence is from studies at high risk of bias and some concern. hDowngraded by one level for inconsistency due to considerable statistical heterogeneity and confidence intervals not overlapping .
Summary of findings 2. Inspiratory muscle training compared to control or sham for people with chronic obstructive pulmonary disease.
| Inspiratory muscle training compared to control or sham for people with chronic obstructive pulmonary disease | ||||||
| Patient or population: people with chronic obstructive pulmonary disease (COPD) Setting: community Intervention: inspiratory muscle training (IMT) Comparison: control or sham | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control or sham | Risk with IMT | |||||
| Dyspnea assessed with Borg scale at submaximal exercise capacity Scale from 1 to 10 (worse) Follow‐up: range 5 weeks to 4 months | The median dyspnea was 1.5 | MD 0.94 lower (1.36 lower to 0.51 lower) | ‐ | 144 (6 RCTs) | ⊕⊝⊝⊝ Very lowa,b | IMT may improve dyspnea measured with Borg scale at submaximal exercise capacity compared to control/sham but the evidence is very uncertain, considering an MCID of −1 unit (only the lower limit of the 95% CI exceeded the MCID) |
| Dyspnea assessed with BDI‐TDI: focal score (TDI) Scale from −9 to +9 (better) follow‐up: range 2 months to 6 months | The median dyspnea was 1.2 | MD 2.98 higher (2.07 higher to 3.89 higher) | ‐ | 238 (8 RCTs) | ⊕⊝⊝⊝ Very lowb,c | IMT may improve dyspnea measured with the BDI‐TDI (Focal score) compared to control/sham but the evidence is very uncertain, considering an MCID of +1 unit |
| Dyspnea assessed with mMRC Scale from 0 to 4 (worse) Follow‐up: range 8 months to 8 months | The median dyspnea was 0.62 | MD 0.59 lower (0.76 lower to 0.43 lower) | ‐ | 150 (4 RCTs) | ⊕⊕⊝⊝ Lowb,d | IMT may improve dyspnea measured with the modified mMRC compared to control/sham, considering an MCID between −0.5 and −1 unit |
| Functional exercise capacity assessed with 6MWD Follow‐up: range 2 weeks to 12 months | The mean functional exercise capacity was 298.4 meters | MD 35.71 meters higher (25.68 higher to 45.74 higher) | ‐ | 501 (16 RCTs) | ⊕⊕⊕⊝ Moderated | IMT probably improves functional exercise capacity measured with the 6MWD compared to control/sham, considering an MCID of 26 meters |
|
Health‐related quality of life
assessed with SGRQ total score Scale from 0 to 100 (worse) Follow‐up: range 2 months to 12 months |
The median health‐related quality of life was 23.61 | MD 3.85 lower (8.18 lower to 0.48 higher) | ‐ | 182 (6 RCTs) | ⊕⊝⊝⊝ Very lowe,f | IMT may improve health‐related quality of life measured with the SGRQ compared to control/sham but the evidence is very uncertain, considering an MCID of −4 units (only the lower limit of the 95% CI exceeded the MCID) |
|
Health‐related quality of life
assessed with CAT Scale from 0 to 40 (worse) Follow‐up: mean 2 months |
The mean health‐related quality of life ranged from −0.5 to 0.3 | MD 2.97 lower (3.85 lower to 2.1 lower) | ‐ | 86 (2 RCTs) | ⊕⊕⊕⊝ Moderateb | IMT probably improves health‐related quality of life measured with CAT compared to control/sham, considering an MCID of about −1.6 units |
| Inspiratory muscle strength assessed with PImax Follow‐up: range 2 weeks to 12 months | The mean inspiratory muscle strength was 51.23 cmH2O | MD 14.57 cmH2O higher (9.85 higher to 19.29 higher) | ‐ | 916 (32 RCTs) | ⊕⊕⊝⊝ Lowd,g,h | IMT may increase inspiratory muscle strength (PImax) slightly compared to control/sham considering the MCID of 17.2 cmH2O |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BDI‐TDI: Baseline Dyspnea Index ‐ Transition Dyspnea Index;CI: confidence interval; CAT: COPD Assessment Test; IMT: inspiratory muscle training; MD: mean difference; mMRC: modified Medical Research Council dyspnoea scale; MCID: minimum clinically important difference; PImax: Maximal Inspiratory Pressure; PR: pulmonary rehabilitation; RCT: randomized controlled trial; SGRQ: St George's Respiratory Questionnaire; 6MWD: six‐minute walk distance | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_423482164894599533. | ||||||
aDowngraded by two levels for risk of bias because most of the evidence is from studies at high risk of bias and some concern, there is an issue with blinding, and high risk of bias studies show different estimates to studies at low risk of bias and some concern. bDowngraded by one level for imprecision due to small sample size (rule of thumb: less than 400). cDowngraded by two levels for risk of bias due to considerable bias across the studies in the measurement of the outcome (lack of blinding) and most of the evidence is from studies at high risk of bias and some concern. dDowngraded by one level for risk of bias because most of the evidence is from studies at high risk of bias and with some concern. eDowngraded by two levels for risk of bias because most of the evidence is from studies at high risk of bias and some concern, and high risk of bias studies show different estimate to studies at low risk of bias and some concern. fDowngraded by two levels for imprecision due to small sample size (rule of thumb: less than 400) and because the 95% CI includes benefit and harm. gWe did not downrate inconsistency although substantial statistical heterogeneity because the studies are on one side of the line of no effect. So we are more confident about the direction of the effect. hDowngraded by one level for publication bias because the funnel plot and the number of studies give rise to serious suspicions about publication bias.
Background
Description of the condition
Chronic obstructive pulmonary disease (COPD) is a respiratory condition that includes bronchitis and emphysema. Chronic bronchitis is defined by the presence of a productive cough for at least three months per year for two consecutive years, during which other causes of cough have been excluded (GOLD 2022). Emphysema is damage to the portion of the lungs responsible for gas transfer called alveoli (Berg 2016). COPD is a significant public health issue, especially in low‐ and middle‐income countries, where nearly 90% of deaths from COPD occur (WHO 2020). COPD was the third leading cause of global deaths in 2016 with about 3 million deaths (WHO 2018), and it is expected to remain in the third ranking until 2030 (WHO 2013).
The main risk factors for COPD are tobacco smoking, second‐hand smoking, air pollution, and exposure to fuel oil fumes (GOLD 2022). COPD is characterized by a non‐reversible airflow obstruction in the lungs. Exposure to irritants stimulates mucus production and damages cilia that clear away mucus and dirt; this causes air to be trapped inside airways, leading to hyperinflation (GOLD 2022; Ramos 2014). Airflow obstruction in emphysema is due to the loss of elastin, which increases lung compliance and decreases elastic recoil (Costanzo 2019). In other words, the lungs lose their ability to return spontaneously to their resting position after inhalation. COPD is a cause of disability as it affects people's ability to breathe normally and has systemic, severe, and long‐term effects (Agustí 2005). Post‐bronchodilator spirometry is the primary test to measure airflow obstruction. It confirms airflow limitation if the ratio between forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) is less than 70% (GOLD 2022).
Clinically, the most common symptoms are dyspnea, chronic cough, wheezing, and sputum production. Dyspnea — also known as shortness of breath or breathlessness — is the most common symptom reported by patients with COPD and is associated with a deterioration in their quality of life and physical activity (Anzueto 2017). Dyspnea results from multiple mechanisms, such as air trapping and dynamic hyperinflation, and it is associated with a significant load on the respiratory muscles (Padula 2006; O'Donnell 2007). The Global Initiative for Chronic Obstructive Lung Disease (GOLD) suggests a therapeutic strategy based on medical history, clinical symptoms, and life quality (ABCD stages) (GOLD 2022).
Description of the intervention
Inspiratory muscle training (IMT), also known as respiratory or ventilatory muscle training, aims to improve inspiratory muscle strength and endurance through a series of breathing exercises. It was developed in the late 1970s (Andersen 1979; Belman 1980; Leith 1976), and it has been used in people with respiratory diseases such as COPD and asthma. IMT focuses on enhancing the performance of respiratory muscles and on improving respiratory symptoms and exercise capacity (Padula 2006).
There are three main categories of IMT devices: threshold loading devices, passive and electronic flow resistive devices, and isocapnic hyperpnea devices (Belman 1994). Other devices exist, and they are reported in Menzes 2018. Most threshold trainers have an adjustable spring‐loaded valve to set the resistance level from 9 cm of water (cmH2O) to 41 cmH2O (or from 7 cmH2O to 41 cmH2O) and allow changes in resistance by 2 cmH2O (Menzes 2018). The threshold pressure is independent of the breathing pattern (Geddes 2005). The passive‐resistive trainer contains holes of different diameters: the biggest hole provides the lowest resistance, whereas the narrowest hole offers the highest resistance (McConnell 2004; Menzes 2018). The respiratory load can be selected by turning the dial towards the chosen hole. However, unlike the threshold device, passive‐resistive trainers depend on the inspiratory flow (Wu 2017). The electronic resistive device is similar to the passive‐resistive trainer, and it has the advantage of dynamically adapting the flow resistance (Menzes 2018). Isocapnic hyperpnea trainers are based on low load and high respiratory flow (60% to 90% of maximal voluntary ventilation (MVV)) so that respiratory muscles contract at a higher speed (for an extended time) (McConnell 2004). That device contains a rebreathing bag to maintain physiological rates of CO2, so patients breathe both fresh air and some of the expired CO2. In addition to multiple devices of IMT, protocols for this therapy differ between teams in terms of frequency, duration, and supervision (Langer 2015).
Various measures are used to evaluate respiratory muscle strength (Laveneziana 2019). Maximal static inspiratory mouth pressure (PImax) is the most commonly used technique to assess the strength of the diaphragm and other inspiratory muscles (Pessoa 2014). It is calculated through a mouthpiece connected to a manometer, either at residual volume or at functional residual capacity (Laveneziana 2019). However, this technique requires the co‐operation of patients. Other approaches to assess inspiratory muscle strength exist, such as sniff nasal inspiratory pressure (SNIP) based on a pressure sensor attached to a catheter placed in the nostril (Maillard 1998), phrenic nerve electric transcutaneous stimulation, and phrenic nerve magnetic stimulation (Caruso 2015).
How the intervention might work
Unlike inspiration, expiration is a passive process. In people with COPD, elastin in the lungs can be reduced, leading to incomplete expiration; this means air is trapped in the airways and leads to hyperinflation (Papandrinopoulou 2012). Static and dynamic distention caused by hyperinflation explains, in part, the pathophysiology of respiratory muscle dysfunction in COPD (O'Donnell 2006). Indeed, inspiratory muscles (the diaphragm, intercostal muscles, and the sternocleidomastoid muscle (SCM)) are exposed to an important load generated from hyperinflation and high airway resistance (Caron 2011). In the early stages of COPD, inspiratory muscles try to adapt to these circumstances. For example, type II muscle fibers of the diaphragm switch into type I, which are highly resistant to fatigue (Clanton 2009). There is also an increase in blood capillaries (Doucet 2004) as well as the oxidative capacity (since type I fibers have high mitochondrial density and enzymes that support the oxidative pathway, so the ability to use oxygen will be increased) (Ottenheijm 2008). At an advanced stage of the disease, oxidative stress, gas exchange abnormalities, and the changes in the chest cavity overcome adaptation mechanisms and the diaphragm will be in a position of impaired mechanical advantage. It loses up to 60% of its muscle tissue and becomes shorter and more horizontal, leading to ineffective mechanical function (Caron 2011; Ottenheijm 2008; Salito 2015). A study showed that IMT induced structural and anatomical changes in external intercostal muscles by changing the distribution of type I fibers and increasing the size of type II fibers (Ramirez Sarmiento 2002). Overall, inspiratory muscle weakness is associated with dyspnea and respiratory failure, despite the ability of the diaphragm to adapt itself to hyperinflation (Bégin 1991; Caron 2011). IMT may improve the strength and endurance of these muscles.
In COPD, it is difficult to work on expiration flow and volume due to mechanical changes. However, it is possible to work on inspiration since it is an active process. In other words, strengthening of the inspiratory muscles increases the inspiratory flow (so there will be an increase in the tidal volume (TV)), decreases the inspiratory time, and improves the expiratory time (Beaumont 2018; Charususin 2016).
Why it is important to do this review
The clinical symptoms most often reported by patients with COPD are dyspnea, a decline in exercise capacity and an impairment in their quality of life (Spruit 2013). It is recommended to start pulmonary rehabilitation (PR) as soon as possible, ideally either during hospitalisation or soon after discharge from the hospital (Spruit 2013). Current guidelines recommend an optimal duration of eight weeks for a PR program (Rochester 2015). Usually, PR consists of physiotherapy, nutritional and psychosocial care, patient therapeutic education, and upper and lower limb training (Beaumont 2015; Spruit 2013).
The American Thoracic Society (ATS) reported that IMT may be beneficial as a stand‐alone intervention and when added to PR in patients with respiratory muscle weakness (Spruit 2013). However, the potential effects of combining IMT and PR are still unclear. Indeed, some recent randomized controlled trials (RCTs) did not find significant improvements in patients with severe COPD (Beaumont 2018; Charususin 2018), and most of the published clinical trials and meta‐analyses recommended further investigation (Beaumont 2018a; Gosselink 2011; Langer 2015; Lötters 2002). Furthermore, there is a lack of certainty in the linear relation between PImax, FEV1, and clinical outcomes. In other words, many RCTs showed that IMT improves PImax, but the extent to which this improvement is clinically significant (i.e. the minimal clinically important difference) has not yet been proved (Beaumont 2018; Schultz 2018). The benefits of unsupervised IMT are also unclear (Langer 2015).
Although many meta‐analyses have been published on different modes and modalities of IMT (Geddes 2005; Geddes 2008; Gosselink 2011; Lötters 2002; O'Brien 2008; Smith 1992), several questions remain unanswered. For example, the optimum duration of a training program has not been established, nor has the effect of IMT on dyspnea and quality of life. There is also a need to investigate its additional effect when added to PR. Moreover, the published meta‐analyses are a few years old, and there are now other studies to be included. A recent clinical trial (Langer 2015), showed that factors other than inspiratory muscle weakness might influence the performance of IMT, such as the variety of protocols, and it is worth exploring these.
Objectives
To assess the effect of inspiratory muscle training (IMT) on chronic obstructive pulmonary disease (COPD) as a stand‐alone intervention and when combined with pulmonary rehabilitation (PR).
Methods
Criteria for considering studies for this review
Types of studies
We included published randomized controlled trials (RCTs), as full‐text articles or abstracts, as well as unpublished RCTs. We included abstracts if they reported at least the number of participants in each group, the duration of the intervention, and the training load. We accepted trials with more than two arms. We excluded observational studies, quasi‐RCTs and cross‐over trials since no washout period for IMT has been established.
Types of participants
We included people with COPD diagnosed according to international standards (GOLD 2022), at any stage of the disease. We placed no restrictions on age, duration, setting, or the kind of pulmonary rehabilitation. We planned to include RCTs with different conditions for the same intervention of interest as long as we could obtain the data of participants with COPD separately.
We classified COPD according to GOLD 2022 stages based on the predicted value of Forced Expiratory Pressure in 1 Second (FEV1):
GOLD 1 ‐ mild: FEV1 ≥ 80% predicted
GOLD 2 ‐ moderate: 50% ≤ FEV1 < 80% predicted
GOLD 3 ‐ severe: 30% ≤ FEV1 < 50% predicted
GOLD 4 ‐ very severe: FEV1 < 30% predicted
Types of interventions
The review consists of two comparisons, as follows.
IMT plus PR versus PR
IMT versus no treatment or sham
First, we included trials that explored the benefit of combining IMT and PR compared to PR only. PR consists of, but is not limited to, exercise training, physiotherapy, therapeutic education, and nutritional and psychosocial care (McCarthy 2015). We included different types of IMT irrespective of the mode of delivery: resistance training (high load, low frequency) or endurance training (low load, high frequency), device (i.e. threshold loading, resistive flow device, isocapnic hyperpnea). We made no restrictions on the duration, supervision (home‐based or in a healthcare setting), or timing (during hospitalization or later) of the intervention. We excluded studies where the training was conducted only once per week (face‐to‐face or distance sessions), regardless of the total duration of the clinical trial. The minimum accepted training load was 30% of PImax or more (Hill 2010). We also excluded trials that used a resistive device without controlling the breathing pattern. If a study conducted an incremental training load that started less than 30% of PImax, we considered only the follow‐up from which the load was equal to our threshold.
According to the proportion of supervised sessions, we defined supervision as:
under 20%: unsupervised;
20% to 70%: partially supervised; and
above 70%: fully supervised.
For the second comparison, we compared IMT with control or sham. We defined sham training as using a resistance of less than 30% of PImax. We accepted control groups if they did not receive any intervention or received an intervention other than exercise training to blind participants (e.g. therapeutic education). We made the exception for breathing exercises if participants in the control group did not receive more than one type of training (e.g. diaphragmatic breathing, pursed lips breathing), and the purpose was not to compare it with IMT.
Types of outcome measures
We analysed the following outcomes in the review, but we did not use them as a basis for including or excluding studies.
Primary outcomes
Dyspnea: the essential scale for our primary analysis is the Borg scale (Borg 1982). We only included the Borg score when it was measured at isotime. We analysed all the scales reported by the trials as long as they were validated, and when possible, we combined them in a meta‐analysis. The other included scales were: Baseline and Transition Dyspnea Indexes (BDI‐TDI) (Mahler 2005), and Modified Medical Research Council (mMRC) (Bestall 1999).
Functional exercise capacity: this can be assessed through multiple tests. We did not exclude studies based on the test used. However, for our analysis, we considered that the most important measurement is the six‐minute walk distance (6MWD) (Holland 2014). Therefore, we included it in the summary of findings table and considered it for subgroup analysis. We included other tests and reported them either in qualitative or quantitative analysis.
Health‐related quality of life: we accepted any scales as long as they were validated. This includes the St. George's Respiratory Questionnaire (SGRQ) (Jones 1992), the chronic respiratory questionnaire (CRQ) (Wijkstra 1994) and the COPD assessment test (CAT) (Jones 2009).
Secondary outcomes
Inspiratory muscle strength: measured by maximal static inspiratory mouth pressure (PImax) (Laveneziana 2019).
Laboratory exercise tests: we were primarily interested in the maximal oxygen uptake (VO2peak), which could be measured through:
incremental cycle ergometer test;
endurance cycle ergometer test;
treadmill test.
Respiratory muscle endurance:
Respiratory muscle endurance pressure (Pthmax): measured by incremental load testing (Laveneziana 2019). It is the maximally tolerated pressure when breathing against a continuously increasing load.
Respiratory muscle endurance time (Tlim): measured by constant load testing (Laveneziana 2019). It is the time an individual can maintain breathing against a fixed load. It can be carried out either through a threshold/resistive or isocapnic hyperpnea device.
Maximal voluntary ventilation (MVV): this is the total volume of expired air between 12 seconds to 15 seconds of deep and fast respiration. MVV is usually compared to predicted MVV (calculated through the forced expiratory volume at 1 second) (Wood 2017).
Respiratory function:
forced expiratory volume at 1 second (FEV1)
residual volume
Adverse events: as defined by the trial authors.
We collected outcomes irrespective of the time frame and summary statistics (change from baseline or final values), with a preference for change score. For each trial, we analysed only the outcomes listed above and not all the outcomes reported in the trial. However, we included all the tests and measurements used for the same outcome.
Search methods for identification of studies
Electronic searches
We searched for all published and unpublished RCTs regarding IMT for COPD, in consultation with the Cochrane Airways Information Specialist. We did not apply restrictions on language or publication status (i.e. published, ongoing, or unpublished).
We searched the following databases for relevant trials in October 2020 and we updated our literature search on 23 August 2021 and on 20 October 2022.
Cochrane Airways Trials Register (Cochrane Airways 2022), via the Cochrane Register of Studies, all years to date (searched 13 October 2020, 23 August 2021 and 20 October 2022)
Cochrane Central Register of Controlled Trials (CENTRAL), via the Cochrane Register of Studies, all years to date (searched 13 October 2020, 23 August 2021 and 20 October 2022)
MEDLINE Ovid SP ALL, 1946 to 12 October 2020 (searched 13 October 2020, 23 August 2021 and 20 October 2022)
Embase Ovid SP, 1974 to week 41 2020 (searched 13 October 2020, 23 August 2021 and 20 October 2022)
PsycINFO Ovid SP, 1967 to October week 1 2020 (searched 13 October 2020, 23 August 2021 and 20 October 2022)
Cumulative Index to Nursing and Allied Health Literature (CINAHL) EBSCO, 1937 to 13 October 2020 (searched 13 October 2020, 23 August 2021 and 20 October 2022)
Physiotherapy Evidence Database (PEDro), 1999 onwards (searched 13 October 2020, 23 August 2021 and 20 October 2022)
We searched the following trials registries.
US National Institutes of Health Ongoing Trials Register, ClinicalTrials.gov(searched 13 October 2020, 23 August 2021 and 20 October 2022)
World Health Organization International Clinical Trials Registry Platform(searched 13 October 2020, 23 August 2021 and 20 October 2022)
The database search strategies are listed in Appendix 1. The search strategy was developed in MEDLINE by the Cochrane Airways Information Specialist in collaboration with the authors and peer‐reviewed by another Cochrane Information Specialist using the PRESS checklist (McGowan 2016). The MEDLINE search strategy was then adapted appropriately for each database.
All databases and trial registries were searched from their inception to the present, with no restriction on language or type of publication. Hand‐searched conference abstracts and grey literature were identified through the Cochrane Airways Trials Register and CENTRAL. When possible, A native‐language speaker translated studies written in a language other than English.
Searching other resources
We checked the reference lists of all primary studies and reviews for additional references. We searched for relevant manufacturers' websites for device information. We searched on PubMed for errata or retractions from included studies published in full‐text and, when possible, reported the date this was done within the review.
Data collection and analysis
Selection of studies
Two review authors from complementary disciplines (OA and WF) independently screened the titles and abstracts of the search results using Covidence, and they coded them as 'include' (eligible or potentially eligible/unclear) or 'exclude'. We retrieved full‐text study reports of all potentially eligible studies, and two review authors (OA and WF) independently screened them for inclusion while also recording the reasons for excluding ineligible studies. We retrieved the full text of potentially relevant reports and removed duplicate records using Covidence.
When appropriate, we contacted the study authors to request further information. We also contacted the manufacturer of the IMT device when we did not understand the concept of training. We resolved disagreements through discussion, without the need for a third review author. We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, is the unit of interest in the review.
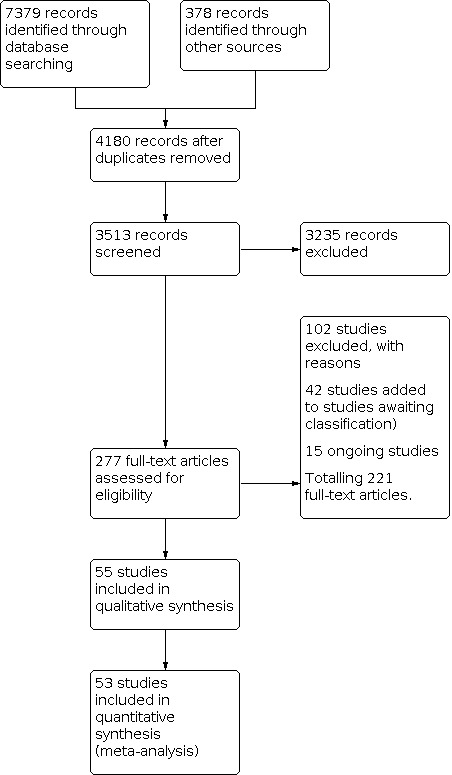
We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Figure 1) and the 'Characteristics of excluded studies' table (Moher 2009).
1.
Data extraction and management
Two review authors (OA and AK) used a data collection form that we piloted on at least one study in the review to extract characteristics from included studies. We extracted the data using Covidence and an Excel spreadsheet. We planned to record any missing information as unclear or not described. Each form included the following information.
General information: study ID, author contact detail, and the person who is completing the form
Methods: aims of the study, study design, total study duration
Participants: inclusion criteria, exclusion criteria, total number randomized, number randomized per group, mean age, age range, sex, COPD stage, clusters (if applicable), number missing, reasons for missing participants, number of participants moved from one group to another, reasons moved, baseline imbalances, and subgroup analysis
Intervention/comparison groups: type of group, type of intervention, type of control, duration, supervision, setting, device, intensity, frequency, type of training (strength/endurance)
Outcomes: primary and secondary outcomes, baseline characteristics, and time points
Notes: funding for studies and notable conflicts of interest of trial authors
Two review authors (OA and AK) independently extracted outcome data from included studies. We noted in the Characteristics of included studies tables if outcome data were not reported in a usable way or if some of the data were missing. We resolved disagreements by reaching a consensus or by involving a third review author (SK). If we identified multiple reports from the same study, we would extract data from all reports directly into a single data collection form. One review author (OA) transferred data into the Review Manager 5 file (Review Manager 2020). We double‐checked that data had been entered correctly by comparing data presented in the systematic review against the study reports. A second review author (WF) spot‐checked study characteristics for accuracy against the study report.
Assessment of risk of bias in included studies
Two review authors (OA and TL) assessed the risk of bias independently for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). We resolved any disagreements by discussion or by involving another review author (SK). We assessed the risk of bias according to the following domains.
Bias arising from the randomization process
Bias due to deviations from intended interventions
Bias due to missing outcome data
Bias in measurement of the outcome
Bias in selection of the reported result
We used RoB 2 to assess risk of bias in randomized studies (Sterne 2019). We used the RoB 2 Excel tool to complete the risk of bias assessment. We used RevMan Web to generate traffic light plots of the domain‐level judgments for each outcome (RevMan Web 2022).
Our effect of interest was the assignment to the intervention at baseline and our main outcomes were those listed in the summary of findings tables. We judged each outcome as being at low risk, some concerns, or high risk according to the RoB 2 algorithm. We provided a quote from the study report, together with a justification for our judgment, in the risk of bias table.
Where information on risk of bias relates to unpublished data or correspondence with a trial author, we noted this in the risk of bias table. To detect reporting bias, we compared the study protocol with the published report, and we contacted the study authors to identify missing or partially reported data. If more than 10 studies were included in the meta‐analysis, we created a funnel plot to explore publication bias. None of the included studies was a cluster‐RCT.
We incorporated the risk of bias assessment in the Results section of the review and it was also part of the GRADE assessment of the certainty of evidence (along with precision, directness, consistency, and publication bias). When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome. Our primary analysis included all the studies without taking the risk of bias judgments into account.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and reported any deviations from it in the 'Differences between protocol and review' section of this systematic review.
Measures of treatment effect
All of our outcomes were continuous data. We calculated a mean difference (MD) with 95% confidence intervals (CIs) where studies used the same scale, and the standardised mean difference (SMD) with 95% CIs where studies used different scales to measure the same concept. We interpreted SMD analysis following the rule of thumb based on Cohen's d effect size (Cohen 1988):
0.2 represents a small effect;
0.5 represents a medium effect;
0.8 represents a large effect.
Depending on how studies reported ordinal data, we analysed the scales as continuous (since all of them were longer than five). We presented all results with a 95% CI.
We undertook a meta‐analysis when it was meaningful. That is to say, it made sense to combine the data, and the populations, interventions and outcomes were similar enough to be pooled in the same forest plot. If both change‐from‐baseline and end‐point scores were available for continuous data, we used change‐from‐baseline, unless there was a low correlation between measurements in individuals.
If adjusted analyses were available (analysis of variance (ANOVA) or analysis of covariance (ANCOVA)), we used these as a preference in our meta‐analyses. If the adjusted MD was reported, we included it in the meta‐analysis using the Generic Inverse Variance method unless adjusted and unadjusted analyses were similar.
Unit of analysis issues
Cluster‐RCTs and dichotomous outcomes: were not included in the review.
Repeated observations on participants: if studies reported outcomes at multiple time points, we chose the longest follow‐up period to keep consistent with the studies. We divided the duration of follow‐up into categories to explore possible differences in the effect estimate. More information is in 'Subgroup analysis and investigation of heterogeneity'.
Studies with more than two groups: we included only the relevant arms.
Two comparisons from the same study within the same meta‐analysis: we combined the active arms or halved the control group to avoid double‐counting.
Dealing with missing data
We requested missing or unclear numerical data from study authors (such as for conference abstracts; randomization, the training load). We did not use imputation because most of the data were available, and it was not possible to receive the participants' data. For studies that reported only the overall effect estimate without providing data for each intervention group, we used the generic inverse variance method to meta‐analyse them.
We used the methods recommended by McGrath 2020 to convert median to mean.
When the data were presented only graphically, and in case we could not get numerical information from the study authors, we used WebPlotDigitizer to extract them from the graphs.
Assessment of heterogeneity
We assessed statistical heterogeneity through visual inspection by detecting overlapping confidence intervals in the forest plot. We used the Chi2 test with a P value of 0.05 to indicate the statistical significance and the I2 test (Higgins 2003), to explore statistical heterogeneity (we considered a value over 50% to represent substantial heterogeneity). We performed subgroup analysis to investigate heterogeneity. We also discussed clinical heterogeneity (e.g. COPD stages) and methodological heterogeneity (e.g. duration of the intervention, number of sessions per week, the total number of sessions, the training load).
Assessment of reporting biases
We created funnel plots to explore possible small‐study and publication biases for functional exercise capacity (6MWD) and respiratory muscle strength (PImax) since they were included in the summary of findings table and more than 10 trials explored these outcomes.
Data synthesis
We expected the included studies to have many variables that could influence the pooled effect estimate. Therefore, we used a random‐effects model for the analysis. We ran a meta‐analysis when it was appropriate; that is, where it made sense to combine different effect estimates and the studies were homogeneous enough to allow reliable interpretation of the analysis.
Ordinal outcomes were meta‐analysed as continuous data following the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2021). Two authors (OA and AK) analysed the data using RevMan 5 (Review Manager 2020), and RevMan Web (RevMan Web 2022).
Subgroup analysis and investigation of heterogeneity
We carried out the following subgroup analyses.
Duration of intervention: short‐term (less than four weeks), medium‐term (between four weeks and seven weeks and six days), long‐term (eight weeks and longer).
Respiratory muscle strength (PImax): studies with participants with respiratory muscle weakness (the mean baseline PImax of the participants was less than or equal to 60 cmH2O) or without respiratory muscle weakness (the mean baseline PImax of the participants was greater than 60 cmH2O).
We used the following outcomes in the subgroup analyses.
Dyspnea: Baseline Dyspnea Index‐Transition Dyspnea Index (BDI‐TDI)
Functional exercise capacity: 6‐minute walk distance (6MWD)
Respiratory muscle strength (PImax)
We included within‐study data when available. We used the formal test for subgroup interactions in RevMan Web (RevMan Web 2022).
Sensitivity analysis
We performed the following sensitivity analyses.
We removed from the primary analysis studies judged to be at high risk of bias and some concerns.
We compared the results of the random‐effects and fixed‐effet models for the BDI‐TDI, the 6MWD, the SGRQ and PImax.
We considered studies to be at high risk of bias overall if we judged at least one of the domains to be high risk.
Summary of findings and assessment of the certainty of the evidence
We created summary of findings tables (Table 1, Table 2) including the following outcomes.
Dyspnea: Borg scale, mMRC and BDI‐TDI
Functional exercise capacity: 6MWD
Health‐related quality of life: SGRQ, COPD Assessment Test (CAT)
Inspiratory muscle strength: PImax
We used the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the body of evidence as it relates to the studies that contribute data for the prespecified outcomes. Our time point was the end of the study. We used the methods and recommendations described in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2021), and the GRADE Handbook, using GRADEpro GDT software. We justified all decisions to downgrade the quality of studies using footnotes and we made comments to aid the reader's understanding of the review where necessary.
Results
Description of studies
Details are available in Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies tables.
Results of the search
We identified from the literature search 7379 records through database searching and 378 from other sources (International Clinical Trials Registry Platform (ICTRP); Epistemonikos).
After removing duplicates and screening titles and abstracts, we checked the eligibility of 277 studies through full‐text review (see Figure 1). Searching the reference lists of past published systematic reviews did not reveal further eligible records. In the end, we included 55 studies in this review, of which six RCTs had more than two arms. Of the 55 studies, 53 contributed data to meta‐analyses.
When contact details were available, we contacted the study authors for clarification. We classified 42 studies as 'awaiting classification' because of insufficient data (although we contacted study authors) or because we couldn't find the abstract (only the title was available in Covidence), and 15 as ongoing studies.
One study in Chinese (ZhouL 2016), and one in Spanish (Bustamante 2007), were translated into English by native speakers (see Acknowledgements). One study in Japanese (Okura 2020) was translated into English using Google Translate.
Included studies
Comparison 1: pulmonary rehabilitation plus inspiratory muscle training versus pulmonary rehabilitation
Population
We included 22 RCTs with 1446 participants in this comparison. We classified the COPD stages according to post‐bronchodilator forced expiratory volume in one second (FEV1) (GOLD 2022). They ranged from mild to moderate (Tout 2013), moderate to severe (Abedi Yekta 2019; Berry 1996; Dekhuijzen 1991; Larson 1999; Magadle 2007; Majewska‐Pulsakowska 2016; Schultz 2018; Wang 2017), mild to very severe (Paneroni 2018), moderate to very severe (Beaumont 2015; Charususin 2018; Fanfa Bordin 2020; Mador 2005; Tounsi 2021), and severe to very severe (Beaumont 2018; Dellweg 2017; Weiner 1992; Weiner 2000). Three studies did not report COPD stage.
The number of participants in the intervention group (PR+IMT) was 742. The mean age ranged from 51.33 to 70.8 years, and the mean body mass index (BMI) ranged from 21.31 to 28.8 kg/m². The number of participants in the control group (PR only) was 704. The mean age ranged from 53.5 to 70.8 years, and the mean BMI ranged from 22.4 to 29.68 Kg/m². In the studies that reported gender, there were around 763 men and 482 women.
Intervention
Pulmonary rehabilitation
The rehabilitation programs varied across the studies. They ranged from training with only a treadmill (Abedi Yekta 2019), with only a cycle ergometer (Larson 1999), and a combination of training protocols in the remaining studies (training with cycle and treadmill, limb muscles strengthening exercises, stair climbing, and therapeutic patient education programs). Five studies monitored training intensity according to heart rate: 50% (Weiner 1992), 60% (Abedi Yekta 2019; Fanfa Bordin 2020), 80% (Dekhuijzen 1991) and 85% (Larson 1999). One study (Tounsi 2021) individualized the training program based on 60% to 80% of the average speed achieved during the six‐minute walk test.
Inspiratory muscle training
Two studies trained their participants for two days a week (Abedi Yekta 2019; Tout 2013), six studies for three days a week (Fanfa Bordin 2020; Mador 2005; Magadle 2007; Wang 2017; Weiner 1992; Weiner 2000), eight studies reported five days a week (Beaumont 2015; Beaumont 2018; De Farias 2019; Dekhuijzen 1991; Dellweg 2017; Larson 1999; Majewska‐Pulsakowska 2016; Paneroni 2018), and four studies trained their participants for the whole week (Berry 1996; Charususin 2018; Schultz 2018; Tounsi 2021). Two studies (Masanga 2011; Sykes 2005) did not provide details about the training. The number of weeks ranged from two weeks (Paneroni 2018) to 24 weeks (Magadle 2007).
The training was unsupervised in one study (Majewska‐Pulsakowska 2016), partially supervised in three studies (De Farias 2019; Larson 1999; Schultz 2018), and fully supervised in the remaining studies.
Three trials performed endurance training, using SpiroTiger (De Farias 2019; Paneroni 2018), and normocapnic hyperpnea (Mador 2005), respectively. The other RCTs conducted strength training with Powerbreathe devices, Threshold IMT device, and Respifit S‐Unit. Five studies did not change the training loads during the study, ranging from 30% to 80% of PImax. In the other RCTs, the training load increased from 15% to 60% of PImax (Weiner 2000), from 30% to 60% of PImax (Abedi Yekta 2019; Larson 1999; Majewska‐Pulsakowska 2016; Schultz 2018; Sykes 2005; Tout 2013), from 50% to 80% of PImax (Berry 1996; Tounsi 2021; Weiner 1992), from 35% to 80% (De Farias 2019), from 50% to 60% (Beaumont 2018), from 50% to 84% of PImax (Charususin 2018), and from 66% to 85% of Maximal Voluntary Ventilation (MVV) (Paneroni 2018).
In Magadle 2007 and Weiner 2000, participants received respectively three months and six months of PR before being allocated to continue with PR alone or to receive IMT.
Comparisons
All the studies focused on our main comparison (PR+IMT vs PR), and there were no indirect comparisons. Three RCTs (Abedi Yekta 2019; Larson 1999; Majewska‐Pulsakowska 2016), had four arms, including both comparisons. One trial had three arms (De Farias 2019). We included the appropriate comparison separately.
Primary outcomes
Dyspnea: two studies explored dyspnea with the Borg scale at isotime and the Modified Medical Research Council (mMRC) scale, one study (Schultz 2018), with BDI‐TDI, and two studies (Beaumont 2015; Beaumont 2018), reported the Multidimensional Dyspnea Profile (MDP).
Functional exercise capacity: 12 studies measured functional exercise capacity with the 6MWD, three studies used the 12‐minute walk distance (12MWD), three studies used exercise time, and five studies used maximal exercise capacity (Wmax). For the latter measurement, Charususin 2018 calculated Wmax by asking participants to cycle at a load of 20 watts (w) and then increasing it by 10 w/min until symptom limitation. Mador 2005 followed a similar protocol starting at no load until the participant could no longer continue cycling for 30 seconds. Dekhuijzen 1991 increased the load by 10% of the predicted Wmax, which was measured through the following formula: "Wmax predicted = 1.7x weight (kg) + 40x FEV,(L)‐25". Wang 2017 chose an incremental load of 5 w/min or 10 w/min.
Health‐related quality of life (HRQoL): seven studies assessed HRQoL with the SGRQ, three studies used the Chronic Respiratory Disease Questionnaire (CRQ), two studies used the COPD Assessment Test (CAT), and one study used the Clinical COPD Questionnaire (CCQ).
Secondary outcomes
Inspiratory muscle strength (PImax): 17 studies reported PImax. Eight of these studies measured it at residual volume.
Laboratory exercise test (VO2peak): five trials measured VO2peak . All studies except for Berry 1996 reported VO2peak in L/min. So we used the mean weight of each group in Berry 1996 to convert from mL/kg/min to L/min.
Respiratory muscle endurance strength (Pthmax): two studies measured Pthmax (Larson 1999; Weiner 1992).
Respiratory muscle endurance time (Tlim) was measured by asking participants to breathe until exhaustion against a load of 30% of PImax (Paneroni 2018), 50% to 60% of PImax (Charususin 2018), 70% of PImax (Dekhuijzen 1991), 70% of MVV (Mador 2005), and 70% to 75% of MVV (Paneroni 2018).
FEV1: six studies reported FEV1 as percentage of predicted and liters. Three studies (Berry 1996; Paneroni 2018; Wang 2017), reported MVV, and one study (Charususin 2018), reported residual volume
Only one abstract (Masanga 2011), reported adverse events.
For both our primary and secondary outcomes, we did not include data from Sykes 2005 in our primary analysis.
Comparison 2: inspiratory muscle training versus control/sham
Population
We included 37 RCTs with 1021 participants in this comparison. As in comparison 1, we classified COPD stages according to post‐bronchodilator FEV1 (GOLD 2022). They ranged from moderate (Leelarungrayub 2017), severe (Lisboa 1997, Weiner 2003; Weiner 2006), mild to severe (Bavarsad 2015), mild to very severe (Dacha 2019), moderate to severe (Abedi Yekta 2019; Belman 1988; Bustamante 2007; Harver 1989; Hsiao 2003; Koppers 2006; Larson 1999; Majewska‐Pulsakowska 2016; Petrovic 2012; Saka 2021; Sanchez Riera 2001; Scherer 2000; Wu 2017; Xu 2018), moderate to very severe (Berton 2015; Chuang 2017; Heijdra 1996; Langer 2018; Nikoletou 2016; Saher 2021), and severe to very severe (Beckerman 2005; Covey 2001; Hill 2006; Hill 2007; Kim 1993; Larson 1988; Preusser 1994; Ramirez Sarmiento 2002; ZhouL 2016).
The number of participants in the IMT group was 526. The mean age ranged from 51.8 to 70.4 years, and the mean BMI ranged from 19.25 to 29 kg/m². For studies that reported gender, there were in total 268 men and 129 women. The number of participants in the control group (control/sham) was 495. The mean age ranged from 54.2 to 71.1 years, and the mean BMI ranged from 18.54 to 28.8 kg/m². For studies that reported gender, there were 269 men and 132 women
Intervention
Inspiratory muscle training
Participants trained from two days a week (Abedi Yekta 2019), to the whole week (Beckerman 2005; Berton 2015; Bustamante 2007; Dacha 2019; Harver 1989; Kim 1993; Koppers 2006; Langer 2018; Larson 1988; Petrovic 2012; Xu 2018). The duration of the intervention ranged from two weeks (Saher 2021) to a year (Beckerman 2005), and the total duration of training ranged from four hours (Abedi Yekta 2019) to 144 hours (Beckerman 2005).
Eight studies conducted training with resistive devices. Three studies (Belman 1988; Harver 1989; Wu 2017), used Pflex (Respironics Inc, Pittsburgh, PA, USA) device, one study (Leelarungrayub 2017), used Portex (Smith Medical ASD), one study (Hsiao 2003), used Respirex (Respirex®2, DHD 22‐1000, Diemolding Healthcare Division, Canastota, NY, USA), two studies (Heijdra 1996; Sanchez Riera 2001), used INSPIRx (Intertech Resources Inc; Ft. Myers, FL; Respirecare Medical Inc., The Hague, the Netherlands), and one study (Bavarsad 2015), used Respivol (Medinet, Milano, Italy).
Participants underwent endurance training with Normocapnic Hyperpnea in two RCTs (Koppers 2006; Scherer 2000), and both endurance and strength training in one RCT (Petrovic 2012), using Respifit S (Mauerbach, Austria). The remaining 25 studies conducted IMT with either Threshold IMT or Powerbreathe devices.
Two trials trained their participants 'as tolerated' (Belman 1988; Bustamante 2007), and one trial (Bavarsad 2015), used an incentive spirometer device at a load equal to or more than the inspiratory volume. The training load increased from 30% to 60% of PImax in six trials (Abedi Yekta 2019; Covey 2001; Larson 1999; Majewska‐Pulsakowska 2016; Nikoletou 2016; Saher 2021), 30% to 45% in one trial (Xu 2018), from 15% to 60% of PImax in two trials (Weiner 2003; Beckerman 2005), from 9% to 100% in one trial (Leelarungrayub 2017), from 50% to 100% of PImax in two trials ( Hill 2006; Langer 2018) and approximately from 50% to 133% in one trial (15 to 40 cmH20) (Chuang 2017) The remaining 23 studies chose a fixed load that ranged from 30% to 80% of PImax.
The training was fully supervised in eight studies, partially supervised in two studies, and unsupervised in 20 studies. Six studies did not report details of supervision ( Harver 1989; Hill 2007; Petrovic 2012; Saher 2021; Weiner 2003; Weiner 2006).
Control/sham
Twenty‐two studies used a sham IMT while 15 studies did not provide any intervention to the control group. One study (Cutrim 2019), provided diaphragmatic breathing at a rate of 15 to 20 breaths/min for both the intervention and the control groups. Participants in the control group underwent therapeutic patient education and pursed lips breathing (Covey 2001), and therapeutic patient education (Larson 1999).
Comparison
All the studies focused on our main comparison (IMT versus control/sham), and there were no indirect comparisons. Two studies (Hsiao 2003; Wu 2017), had three arms, including two intervention groups (each group used a different device or protocol) and a control group. When two arms from the same study were included in a forest plot, we halved the number of participants in the control group.
As for comparison 1, we extracted the appropriate arms from Abedi Yekta 2019, Larson 1999 and Majewska‐Pulsakowska 2016.
Primary outcomes
Dyspnea: six studies measured dyspnea with the Borg scale at isotime. Eight studies assessed dyspnea with BDI‐TDI and four studies with the mMRC.
Functional exercise capacity: 16 studies measured functional exercise capacity with the 6MWD, three studies with 12MWD, seven studies with Wmax, five studies with exercise time, and two studies with the shuttle walk test (SWT). Hill 2006 and Koppers 2006 measured Wmax by increasing the work rate by 10% per minute; Larson 1999 asked the participants to warm up by pedalling for 3 minutes at 10 w followed by 2 minutes at 20 w, and then they started the graded cycle at 30 w; and Lisboa 1997 increased the load by 75 kpm every 2 minutes. Sanchez Riera 2001, and Wu 2017 increased the work rate by 10 w/min after one minute of unloaded pedalling.
To measure exercise time, Berton 2015 asked the participants to cycle at 75% of Wmax; Koppers 2006 set the load at 50% of PImax; Scherer 2000) measured it on a treadmill set to 80% of the incline and to 100% of the speed reached at VO2peak; and Wu 2017 considered it as the time to reach Wmax.
Health‐Related Quality of Life (HRQoL): six studies assessed HRQoL with the SGRQ, five studies used CRQ, two studies used CAT, two studies used SF‐36 (Chuang 2017; Nikoletou 2016), and one study used the CCQ (Leelarungrayub 2017).
Secondary outcomes
Inspiratory muscle strength (PImax): 32 studies reported PImax. Fourteen studies measured it at residual volume, 10 studies at functional residual capacity, one study reported both measurements, and the remaining studies did not report the measurement method.
Laboratory exercise test (VO2peak): 11 studies reported VO2peak.
Respiratory muscle endurance pressure (Pthmax): five studies (Hill 2006; Koppers 2006; Preusser 1994; Ramirez Sarmiento 2002; Weiner 2003), followed the protocol of Nickerson 1982. One study (Larson 1999), started with an initial load of 30% of PImax and increased by 5.7 cmH2O until exhaustion.
Respiratory muscle endurance time (Tlim): 10 studies reported Tlim. Langer 2018, Nikoletou 2016 and Petrovic 2012 asked the participants to breathe as long as possible against 50% to 60% of PImax, and Hill 2006 and Ramirez Sarmiento 2002 asked them to breathe against 80% of PImax. Bustamante 2007 set the load at 66% of PImax, Hsiao 2003 at 70%, and Scherer 2000 at 66% or 75% of MVV.
MVV: two studies measured MVV (Belman 1988; Harver 1989)
Residual volume: two studies measured residual volume (Ramirez Sarmiento 2002; Hill 2006).
Forced expiratory volume at 1 second (FEV1): 10 studies reported FEV1 in %Pred, and 12 studies in litrs.
Adverse events: none of the included studies reported adverse events.
Excluded studies
We excluded 133 studies after the full‐text review. For further details, please refer to Characteristics of excluded studies.
Risk of bias in included studies
We present the risk of bias assessment for each outcome, including all domain judgments and support for judgments, in a spreadsheet (Ammous 2022). We generated traffic light plots in most forest plots of our primary outcomes and for PImax in the secondary outcomes.
Comparison 1. Pulmonary rehabilitation plus inspiratory muscle training versus pulmonary rehabilitation
Dyspnea: one study was at low risk of bias for dyspnea (Borg and mMRC; Charususin 2018), while three others were at high risk of bias. Larson 1999 had issues with intention to treat (ITT) analysis (the number of participants that were not analyzed could impact the results), missing data could depend on its true value and participants were not blinded. Both Beaumont 2018 and Wang 2017 did not blind participants.
Functional exercise capacity: three studies were at low risk of bias for functional exercise capacity (6MWD; Beaumont 2018; Charususin 2018; Dellweg 2017). Most studies that we judged at some concern did not provide sufficient details about allocation concealment, excluding participants from the analysis (less than 5%) and only the journal article was available. We considered one study at high risk of bias because missingness is likely to depend on its true value (Paneroni 2018), and another study because of a lack of details about the randomization process (Tout 2013).
Health‐related quality of life: no study was at low risk of bias for the SGRQ and CAT. The main issue across the studies was the lack of blinding. One study was at low risk of bias for CRQ (Charususin 2018), and the two others were at high risk of bias because of issues with ITT analysis (Larson 1999) and lack of blinding (Mador 2005).
Inspiratory muscle strength: six studies were at low risk of bias (PImax; Beaumont 2018; Charususin 2018; Dellweg 2017; Fanfa Bordin 2020; Schultz 2018; Tounsi 2021). Most studies were at some concern because of a lack of details about allocation concealment and only the journal article was available. Two studies were considered at high risk of bias (Larson 1999; Paneroni 2018), because missingness is likely to depend on its true value and one study because of a lack of details about the randomization process (Tout 2013).
Comparison 2. Inspiratory muscle training versus control/sham
Dyspnea: one study was at low risk of bias for dyspnea (Borg; Langer 2018), two studies were at high risk of bias because participants were not blinded (Larson 1988; Petrovic 2012), and the others were at some concern because lack of details about allocation concealment. Similarly, only Langer 2018 was at low risk of bias for BDI‐TDI. We judged Harver 1989 at high risk of bias because the authors did not mention the reasons behind missing data, Weiner 2003 because the data in the graph were different from the text and Wu 2017 because participants were not blinded. For mMRC, from the four included studies, two studies were at low risk of bias (Langer 2018; Xu 2018), one study at some concern because lack of details about allocation concealment (Saka 2021), and one study at high risk of bias because participants were not blinded (ZhouL 2016).
Functional exercise capacity: two studies were at low risk of bias (6MWD; Cutrim 2019; Xu 2018). Four studies were at high risk of bias because of issues with ITT analysis (Beckerman 2005; Hsiao 2003; Ramirez Sarmiento 2002; Saher 2021), lack of details about missing data (Leelarungrayub 2017), and the data in the graph were different from the text (Weiner 2003). The remaining studies were at some concern because of the lack of details about allocation concealment.
Health‐related quality of life: only Xu 2018 was at low risk of bias for the SGRQ and CAT. Most of the studies that reported the SGRQ were at some concern because of the lack of details about allocation concealment (Berton 2015; Saka 2021), lack of details in the trial register (Saka 2021), and no information about whether adjusted analysis was planned in advance (Berton 2015). All the studies that reported CRQ were at high risk of bias (mainly because participants were not blinded) and some concern (lack of details about allocation concealment and issues with the reported results).
Inspiratory muscle strength: only three studies were at low risk of bias (PImax; Cutrim 2019; Langer 2018; Xu 2018). All the remaining studies did not provide enough details about allocation concealment and studies at high risk of bias had issues with ITT analysis or missingness, or both.
Overall risk of bias
The main issues we found across the studies were the lack of details about allocation concealment (randomization process), lack of blinding (measurement of the outcome, although we considered using a sham IMT equal to blinding participants), and only journal articles were available (selection of the reported results).
Effects of interventions
Comparison 1: pulmonary rehabilitation plus inspiratory muscle training versus pulmonary rehabilitation
See: Table 1
Primary outcomes
Dyspnea
Borg scale
Two studies used the Borg scale at isotime to evaluate dyspnea. Considering a MCID of −1 unit (Ries 2005), there was no improvement in dyspnea with an overall effect estimate (MD 0.19, 95% CI −0.42 to 0.79; I² = 0%; 2 studies, 202 participants; Analysis 1.1). We judged only one study (Charususin 2018), to be at low risk of bias, which revealed a similar effect estimate. One study (Sykes 2005), reported greater improvement in dyspnea at "heavy load" in the IMT+ exercise group but did not report numerical data.
1.1. Analysis.

Comparison 1: PR+IMT vs PR, Outcome 1: Dyspnea: Borg (at submaximal exercise: 50% to 80% of Wmax)
Using GRADE, we downgraded the certainty of the evidence for Borg by 1 point due to serious concerns regarding imprecision.
mMRC scale
We did not find any improvement in dyspnea using the mMRC scale (MD −0.12, 95% CI −0.39 to 0.14; I² = 0%; 2 studies, 204 participants; Analysis 1.2). The MCID is estimated to be between −0.5 to −1 unit (Araújo 2017; Cazzola 2015).
1.2. Analysis.

Comparison 1: PR+IMT vs PR, Outcome 2: Dyspnea: Modified Medical Research Council (mMRC)
We downgraded the certainty of evidence to very low due to very serious concerns with risk of bias and serious concerns with imprecision.
BDI‐TDI and MDP
There was no significant difference between the two arms in the studies that reported the BDI‐TDI (Schultz 2018), and the MDP (Beaumont 2015; Beaumont 2018).
Functional exercise capacity
6MWD
We pooled the 12 studies that reported the 6MWD test in a meta‐analysis that showed no evidence of a difference between groups (MD 5.95, 95% CI −5.73 to 17.63; I² = 61%; 12 studies, 1199 participants; Analysis 1.3). The mean and upper bounds of the 95% CI were lower than the MCID of 26 meters indicating that the mean change was not clinically relevant (Puhan 2011). Sykes 2005 narratively reported a better 6MWD in the intervention group.
1.3. Analysis.

Comparison 1: PR+IMT vs PR, Outcome 3: Functional exercise capacity: 6‐minute walk distance (6MWD) (meters)
We had very low confidence in the results due to serious concerns with risk of bias and very serious concerns with inconsistency.
We conducted a subgroup analysis according to the duration of the intervention (short, medium and long‐term interventions). The test for subgroup differences was not significant (Chi² = 0.30, df = 2 (P = 0.86), I² = 0%, Analysis 1.4).
1.4. Analysis.

Comparison 1: PR+IMT vs PR, Outcome 4: Functional exercise capacity: 6‐minute walk distance (6MWD) (meters) (subgroup analysis: duration of the intervention)
In the following subgroup analysis, we divided the studies according to their baseline PImax and the test for subgroup differences was not significant (Chi² = 1.94, df = 1 (P = 0.16), I² = 48.3%, Analysis 1.5).
1.5. Analysis.

Comparison 1: PR+IMT vs PR, Outcome 5: Functional exercise capacity: 6‐minute walk distance (6MWD) (meters) (subgroup analysis: with or without respiratory muscle weakness)
One study (Wang 2017), reported a subgroup analysis for intervention group participants with or without respiratory muscle weakness, with greater improvement in 6MWD for the weakened respiratory muscle group.
In sensitivity analysis, keeping just the studies at low risk of bias (Beaumont 2018; Charususin 2018; Dellweg 2017), increased the mean difference without exceeding the MCID (MD 8.90, 95% CI −11.86 to 29.65; I² = 80%; 4 studies, 379 participants). However, it decreased when switching to the fixed‐effect model (MD 0.73, 95% CI ‐4.80 to 6.26, I² = 61%).
12MWD
Three studies reported the 12MWD and showed a larger effect in the intervention group (MD 155.77 meters, 95% CI −84.53 to 396.08; I² = 79%; 80 participants; Analysis 1.6)
1.6. Analysis.

Comparison 1: PR+IMT vs PR, Outcome 6: Functional exercise capacity: 12‐minute walk distance (12MWD) (meters)
Wmax
Five studies reported Wmax and showed no difference between the groups (MD −1.01 watts, 95% CI −6.96 to 4.94; I² = 25%; 326 participants; Analysis 1.7).
1.7. Analysis.

Comparison 1: PR+IMT vs PR, Outcome 7: Functional exercise capacity: Wmax (watt)
Exercise time (seconds)
Four studies reported exercise time (seconds) and also showed a larger effect in the intervention group (MD 58.62 seconds, 95% CI −25.09 to 142.32; I² = 0%; 192 participants; Analysis 1.8). We did not include Berry 1996 in the meta‐analysis because they did not specify the level of exercise at which they measured exercise time. However, it is unclear to what extent these changes are clinically relevant.
1.8. Analysis.

Comparison 1: PR+IMT vs PR, Outcome 8: Functional exercise capacity: exercise time (seconds)
Health‐related quality of life (HRQoL)
SGRQ
We pooled seven trials that explored the effect of the intervention on HRQoL using the SGRQ, which showed no difference in total scores (MD 0.13, 95% CI −0.93 to 1.20; I² = 0%; 908 participants; Analysis 1.9). This mean difference did not exceed the MCID threshold of −4 units (Welling 2015).
1.9. Analysis.

Comparison 1: PR+IMT vs PR, Outcome 9: Health‐related quality of life (HRQoL): St George's Respiratory Questionnaire (SGRQ)
We downgraded the certainty of evidence by two levels due to very serious concerns with risk of bias.
Two studies that reported the three SGRQ domains showed no differences in symptoms (MD −2.33, 95% CI −6.28 to 1.62; Analysis 1.9), activity (MD 0.28, 95% CI −1.65 to 2.20) or impact (MD −1.63, 95% CI −5.38 to 2.11) scores.
In sensitivity analysis, effect estimates remained unchanged with a fixed‐effect model. We could not explore the summary effect of low risk of bias studies because all the studies were at high risk of bias and some concerns.
CRQ
There were no differences in CRQ domain scores (Analysis 1.10): three RCTs reported 'Dyspnea' (MD −0.30, 95% CI −1.90 to 1.29) and 'Fatigue' (MD 0.28, 95% CI −0.76 to 1.31). Two RCTs reported 'Emotion' (MD −0.63, 95% CI −2.53 to 1.26) and 'Mastery' (MD −0.05, 95% CI −1.18 to 1.08). We used the generic inverse variance method to pool the results because Charususin 2018 showed a different adjusted mean difference compared to non‐adjusted analysis. None of the differences exceeded the MCID threshold of +0.5 units (Alma 2018). One abstract (Sykes 2005), narratively reported an improved CRQ score in the intervention group.
1.10. Analysis.

Comparison 1: PR+IMT vs PR, Outcome 10: Health‐related quality of life (HRQoL): Chronic Respiratory Disease Questionnaire (CRQ)
CAT
Two studies that reported the CAT scale showed no difference between groups (MD 0.13, 95% CI −0.80 to 1.06; I² = 50%; 657 participants; Analysis 1.11) and the difference did not exceed the threshold of clinical significance, MCID of −1.6 units (American Thoracic Society). We considered the certainty of evidence to be very low due to very serious concerns with risk of bias and serious concerns with inconsistency.
1.11. Analysis.

Comparison 1: PR+IMT vs PR, Outcome 11: Health‐related quality of life (HRQoL): COPD Assessment Test (CAT)
Secondary outcomes
Inspiratory muscle strength: PImax (cmH2O)
Aggregated data from 17 studies showed higher inspiratory muscle strength in the intervention group (MD 11.46, 95% CI 7.42 to 15.15; I² = 84%; 1329 participants; Analysis 1.12), but this did not exceed the MCID of 17.2 (Iwakura 2020). The statistical heterogeneity is due to the studies that reported change from baseline and had narrow confidence intervals.
1.12. Analysis.

Comparison 1: PR+IMT vs PR, Outcome 12: Inspiratory muscle strength: PImax (cmH20)
We downgraded the certainty of evidence by one level due to serious concerns with risk of bias.
We performed a subgroup analysis according to the duration of intervention, which did not reveal a difference between subgroups (Chi² = 0.05, df = 2 (P = 0.98), I² = 0%; Analysis 1.13).
1.13. Analysis.

Comparison 1: PR+IMT vs PR, Outcome 13: Inspiratory muscle strength: PImax (cmH2O) (subgroup analysis: duration of the intervention)
We carried out subgroup analysis between studies with participants with and without respiratory muscle weakness. The test for subgroup differences was not significant (Chi² = 1.72, df = 1 (P = 0.19), I² = 41.8%; Analysis 1.14).
1.14. Analysis.

Comparison 1: PR+IMT vs PR, Outcome 14: Inspiratory muscle strength: PImax (cmH2O) (subgroup analysis: with or without respiratory muscle weakness)
Wang 2017 divided the participants of the intervention group according to the state of their respiratory muscles, and they reported a larger change in PImax with the normal respiratory muscle group. Sykes 2005 reported a higher PImax narratively in the intervention group.
Sensitivity analysis showed minimal impact of fixed‐effect models on the synthesised results (MD 10.53, 95% CI 9.25 to 11.81, I² = 84%). Retaining studies at low risk of bias (Beaumont 2018; Charususin 2018; Dellweg 2017; Fanfa Bordin 2020; Schultz 2018; Tounsi 2021), also had minimal impact (MD 13.43, 95% CI 11.81 to 15.04; I² = 90%; 1008 participants).
Laboratory exercise test: VO2peak
We combined five studies showing no additional effect of the intervention on VO2peak (MD −0.01 L/min, 95% CI −0.05 to 0.03; I² = 0%; 313 participants; Analysis 1.15). Sykes 2005 reported no difference between the two groups, but without numerical data.
1.15. Analysis.

Comparison 1: PR+IMT vs PR, Outcome 15: Laboratory exercise test: VO2peak (L/min)
Respiratory muscle endurance pressure (Pthmax) (cmH2O)
Two studies reported Pthmax, and we pooled them in an SMD meta‐analysis because they used different techniques to measure the outcome. We got an overall effect estimate (SMD 1.22 cmH2O, 95% CI −0.18 to 2.66; I² = 80%; 52 participants; Analysis 1.16), which suggests a large effect according to Cohen's d effect size.
1.16. Analysis.

Comparison 1: PR+IMT vs PR, Outcome 16: Respiratory muscle endurance: respiratory muscle endurance pressure (Pthmax) (cmH2O)
Respiratory muscle endurance time: Tlim (seconds)
We performed separate analyses according to the nature of the endurance test. We pooled three studies that measured the outcome through sustained ventilation according to %PImax (MD 84.62, 95% CI −50.77 to 220.02; 236 participants; Analysis 1.17), and two studies that measured it according to %MVV (MD 477.69, 95% CI 215.43 to 739.94; 51 participants; Analysis 1.18). For both methods of measurement, we found a larger effect in the intervention group.
1.17. Analysis.

Comparison 1: PR+IMT vs PR, Outcome 17: Respiratory muscle endurance time: Tlim (seconds) (sustained ventilation according to PImax)
1.18. Analysis.

Comparison 1: PR+IMT vs PR, Outcome 18: Respiratory muscle endurance time: Tlim (seconds) (sustained ventilation according to MVV)
Maximal voluntary ventilation (MVV)
We pooled in an SMD meta−analysis two studies that reported MVV in L/min (Berry 1996; Wang 2017), and one study in %Pred (Paneroni 2018). We got an overall effect estimate (SMD 0.40, 95% CI −0.02 to 0.83; I² = 4%; 93 participants; Analysis 1.19), which suggests a moderate effect according to Cohen's d effect size.
1.19. Analysis.

Comparison 1: PR+IMT vs PR, Outcome 19: Maximal voluntary ventilation (MVV)
Respiratory function: forced expiratory volume at 1 second (FEV1)
We combined the studies according to whether they reported FEV1 in %Pred and in liters. We did not find a better effect of PR+IMT, showing an overall effect estimate (MD 0.77, 95% CI −1.72 to 3.26; I² = 0%; 6 studies, 173 participants; Analysis 1.20) and (MD 0.04, 95% CI −0.04 to 0.13; I² = 56%; 6 studies, 889 participants; Analysis 1.21) respectively.
1.20. Analysis.

Comparison 1: PR+IMT vs PR, Outcome 20: Respiratory function: forced expiratory volume at 1 second (FEV1) (%Pred)
1.21. Analysis.

Comparison 1: PR+IMT vs PR, Outcome 21: Respiratory function: forced expiratory volume at 1 second (FEV1) (Liters)
Respiratory function: residual volume
One study reported this outcome (Charususin 2018) and did not show a difference between the two groups.
Adverse events
One abstract (Masanga 2011) reported some adverse effects that were considered "minor and self‐limited": headache (six participants), jaw pain (six participants), neck pain (six participants), back pain (four participants), abdominal pain (two participants), cough (one participant), blood‐streaked sputum (one participant), shoulder pain (one participant) and chest pain (one participant).
Comparison 2: inspiratory muscle training versus control/sham
See: Table 2
Primary outcomes
Dyspnea
Borg scale
Six studies used the Borg scale to assess dyspnea at isotime, and we entered them into a meta‐analysis. Breathlessness was lower with the intervention and the mean difference was close to the MCID of −1 unit (the lower limit of the 95% CI exceeded it), though results are imprecise (MD −0.94, 95% CI −1.36 to −0.51; I² = 0%; 6 studies, 144 participants; Analysis 2.1). Only one study was at low risk of bias.
2.1. Analysis.

Comparison 2: IMT vs control/sham, Outcome 1: Dyspnea: Borg (at submaximal exercise capacity)
We downgraded the certainty by three levels due to very serious concerns with risk of bias and serious concerns with imprecision.
BDI‐TDI
Eight studies (nine arms) used BDI‐TDI to measure dyspnea (Analysis 2.2). Three studies (four arms) reported the TDI 'Change in Functional impairment' (MD 0.88, 95% CI 0.51 to 1.25), TDI 'Change in Magnitude of task' (MD 0.73, 95% CI 0.35 to 1.12), and TDI 'Change in Magnitude of effort' (MD 0.86, 95% CI 0.42 to 1.30), showing no potential improvement in dyspnea, according to an MCID of +1 unit (Mahler 2005). Eight studies (nine arms) reported the TDI 'Focal score' and revealed a greater effect with IMT (MD 2.98, 95% CI 2.07 to 3.89; I² = 65%; 238 participants).
2.2. Analysis.

Comparison 2: IMT vs control/sham, Outcome 2: Dyspnea: Baseline and Transition Dyspnea Indexes (BDI‐TDI)
We created a subgroup analysis of the TDI 'Focal score' according to studies judged with or without respiratory muscle weakness. The test for subgroup differences was not significant (Chi² = 2.55, df = 1 (P = 0.11), I² = 60.8%; Analysis 2.3).
2.3. Analysis.

Comparison 2: IMT vs control/sham, Outcome 3: Dyspnea: Transition Dyspnea Index (TDI): Focal score (subgroup analysis: with or without respiratory muscle weakness)
In sensitivity analysis, the overall effect estimates remained unchanged when switching to the fixed‐effect model in the first three subgroups, while it increased to (MD 4.04, 95% CI 3.70 to 4.39, I² = 65%) with 'Focal score'. This effect mainly resulted from (Sanchez Riera 2001), which got the greatest weight.
Only one study was at low risk of bias (Langer 2018), and we considered the certainty of evidence to be very low due to very serious concerns with risk of bias and serious concerns with imprecision.
mMRC
Four studies reported the mMRC score, and the overall effect estimate revealed a possible improvement in dyspnea in the IMT group (MD −0.59, 95% CI −0.76 to −0.43; I² = 17%; 150 participants; Analysis 2.4), considering an MCID between −0.5 and −1 unit. We considered the certainty of evidence to be low due to serious concerns with risk of bias and imprecision.
2.4. Analysis.

Comparison 2: IMT vs control/sham, Outcome 4: Dyspnea: Modified Medical Research Council (mMRC)
Functional exercise capacity
6MWD
We combined 16 studies (17 arms) that reported the 6MWD, showing a better effect with the IMT compared to control/sham (MD 35.71, 95% CI 25.68 to 45.74; I² = 16%; 501 participants; Analysis 2.5).
2.5. Analysis.

Comparison 2: IMT vs control/sham, Outcome 5: Functional exercise capacity: 6‐minute walk distance (6MWD) (meters)
We judged the certainty of evidence to be moderate due to serious concerns with risk of bias.
In the first subgroup analysis, we divided the studies according to the duration of the intervention. Only one study (Saher 2021), had a follow‐up of fewer than four weeks. The test for subgroup differences was not significant (Chi² = 0.37, df = 2 (P = 0.83), I² = 0%; Analysis 2.6).
2.6. Analysis.

Comparison 2: IMT vs control/sham, Outcome 6: Functional exercise capacity: 6‐minute walk distance (6MWD) (meters) (subgroup analysis: duration of the intervention)
In the following subgroup analysis, we divided the studies according to the mean baseline PImax. The test for subgroup differences was not significant (Chi² = 0.17, df = 1 (P = 0.68), I² = 0%; Analysis 2.7). One trial (Xu 2018), performed a subgroup analysis within study data using the same cut‐off.
2.7. Analysis.

Comparison 2: IMT vs control/sham, Outcome 7: Functional exercise capacity: 6‐minute walk distance (6MWD) (meters) (subgroup analysis: with or without respiratory muscle weakness)
In sensitivity analysis, keeping just the studies at low risk of bias (Cutrim 2019; Xu 2018), revealed a larger effect, standing at around twice our MCID (MD 49.13, 95% CI −27.62 to 125.88, I² = 83%). However, we should note that its confidence interval exceeded the line of no effect. The overall effect estimate remained nearly the same when switching to the fixed‐effect model.
12MWD and Wmax
We did not find an additional effect of IMT when we pooled the studies that used the 12MWD and Wmax, revealing, respectively, an overall effect estimate (MD −33.31, 95% CI −158.10 to 91.48; I² = 59%; 3 studies, 101 participants = 101; Analysis 2.8) and (MD 0.66, 95% CI −6.44 to 7.76; I² = 42%; 7 studies, 206 participants; Analysis 2.9). However, for the 12MWD, we noticed large differences in baseline data between the two groups of Preusser 1994, which appeared significant when testing it with the RevMan calculator (P = 0.03). We removed that study in a sensitivity analysis, and we got a positive overall effect (MD 12.76, 95% CI −65.71 to 91.23, I² = 0%).
2.8. Analysis.

Comparison 2: IMT vs control/sham, Outcome 8: Functional exercise capacity: 12‐minute walk distance (12MWD) (meters)
2.9. Analysis.

Comparison 2: IMT vs control/sham, Outcome 9: Functional exercise capacity: Wmax (watt)
Exercise time
Five studies (six arms) reported exercise time, but we did not pool the studies because they used different measurement methods (Analysis 2.10). Globally, their results were consistent and showed a trend of a greater effect in the IMT group compared to the control/sham group.
2.10. Analysis.

Comparison 2: IMT vs control/sham, Outcome 10: Functional exercise capacity: exercise time (seconds)
SWT
Three studies used the SWT, and we included two trials in our primary analysis. We did not find an additional effect in the IMT group (MD −7.45 meters, 95% CI −92.74 to 77.83; Analysis 2.11).
2.11. Analysis.

Comparison 2: IMT vs control/sham, Outcome 11: Functional exercise capacity: shuttle walk test (SWT) (meters)
Health‐related quality of life (HRQoL)
SGRQ
Two studies (Berton 2015; Saka 2021), reported the items 'Symptoms' (MD −2.10, 95% CI −3.50 to −0.71), 'Activity' (MD −9.86, 95% CI −15.08 to −4.63) and 'Impact' (MD −6.06, 95% CI −13.76 to 1.65). Six studies reported the 'total score' of the SGRQ, showing a larger effect in the IMT group (MD −3.85, 95% CI −8.18 to 0.48; I² = 66%; 182 participants; Analysis 2.12). The lower limit of the 95% CI exceeded the MCID of −4 units. The statistical heterogeneity is due to the effect of Saka 2021. Only one study was at low risk of bias (Xu 2018), and it has a similar effect estimate to the overall. The overall effect estimate increased to MD −5.11 (95% CI −6.81 to −3.40, I² = 66%), when switching to the fixed‐effect model.
2.12. Analysis.

Comparison 2: IMT vs control/sham, Outcome 12: Health‐related quality of life (HRQoL): St George Respiratory Questionnaire (SGRQ)
We judged the certainty of the evidence as 'very low' due to very serious concerns with risk of bias and imprecision.
CRQ
Four studies (5 arms) reported the four items of the CRQ, while one trial (Larson 1999), reported only dyspnea and fatigue. We divided the items into subgroups. The RCTs showed consistent results across the items, and there was an improvement (considering an MCID of +0.5 unit) in 'Dyspnea' (MD 1.63, 95% CI 0.23 to 3.03; Analysis 2.13), 'Fatigue' (MD 1.32, 95% CI 0.08 to 2.55), 'Emotion' (MD 2.64, 95% CI 0.82 to 4.46), and 'Mastery' (MD 1.57, 95% CI 0.07 to 3.06). None of the included studies was at low risk of bias.
2.13. Analysis.

Comparison 2: IMT vs control/sham, Outcome 13: Health‐related quality of life (HRQoL): Chronic Respiratory Disease Questionnaire (CRQ)
CAT
Two trials reported CAT, revealing an overall effect estimate (MD −2.97, 95% CI −3.85 to −2.10; participants = 86; I² = 0%; Analysis 2.14). We downgraded the certainty of evidence by one level due to serious concerns with imprecision.
2.14. Analysis.

Comparison 2: IMT vs control/sham, Outcome 14: Health‐related quality of life (HRQoL): COPD Assessment Test (CAT)
SF‐36 and CCQ
In the studies that used SF‐36 (Chuang 2017; Nikoletou 2016), and CCQ (Leelarungrayub 2017), there was a better effect in the IMT group in some domains of the scales.
Secondary outcomes
Inspiratory muscle strength: PImax (cmH2O)
PImax
32 RCTs (34 arms) measured PImax. The upper limit of the 95% CI of summary effect between IMT and control/sham exceeded the MCID of 17.2 cmH2O (MD 14.57 cmH2O, 95% CI 9.85 to 19.29; I² = 89%; 916 participants; Analysis 2.15).
2.15. Analysis.

Comparison 2: IMT vs control/sham, Outcome 15: Inspiratory muscle strength: PImax (cmH2O)
We downgraded the certainty of evidence by two levels due to serious concerns with risk of bias and a strongly suspected publication bias.
We divided the studies according to the training duration (Analysis 2.16). The test for subgroup differences was not significant (Chi² = 0.67, df = 2 (P = 0.72), I² = 0%).
2.16. Analysis.

Comparison 2: IMT vs control/sham, Outcome 16: Inspiratory muscle strength: PImax (cmH2O) (subgroup analysis: duration of the intervention)
We carried out subgroup analysis between studies with participants with and without respiratory muscle weakness. The test for subgroup differences was not significant (Chi² = 0.34, df = 1 (P = 0.56), I² = 0%; Analysis 2.17).
2.17. Analysis.

Comparison 2: IMT vs control/sham, Outcome 17: Inspiratory muscle strength: PImax (cmH2O) (subgroup analysis: with or without respiratory muscle weakness)
We also conducted a subgroup analysis of the measurement method. The test for subgroup differences was not significant (Chi² = 0.93, df = 2 (P = 0.63), I² = 0%; Analysis 2.18) between studies that measured PImax at residual volume, at functional residual capacity, and the studies that did not report the level of measurement.
2.18. Analysis.

Comparison 2: IMT vs control/sham, Outcome 18: Inspiratory muscle strength: PImax (cmH2O) (subgroup analysis: method of measurement)
In sensitivity analysis, keeping just the studies at low risk of bias (Cutrim 2019; Langer 2018; Xu 2018), showed a similar result (MD 12.79 cmH2O, 95% CI 3.63 to 21.95, I² = 55%). Similarly, the overall effect estimate of the random‐effects model did not differ from the fixed‐effect model.
Laboratory exercise test (VO2peak)
We combined 11 studies (12 arms) in an SMD meta‐analysis, since they were reported in different units (L/min, mL/min, and mL/kg/min). We got an overall effect estimate suggesting a low to moderate effect according to Cohen's rule of thumb (SMD 0.31, 95% CI 0.05 to 0.57; I² = 14%; 286 participants; Analysis 2.19).
2.19. Analysis.

Comparison 2: IMT vs control/sham, Outcome 19: Laboratory exercise test: VO2peak
Respiratory muscle endurance pressure (Pthmax) (cmH2O)
Eight RCTs used Pthmax in their respiratory endurance assessment, and they showed a larger overall effect in the IMT group (MD 9.71, 95% CI 4.93 to 14.50; I² = 53%; 179 participants; Analysis 2.20).
2.20. Analysis.

Comparison 2: IMT vs control/sham, Outcome 20: Respiratory muscle endurance: respiratory muscle endurance pressure (Pthmax) (cmH2O)
Respiratory muscle endurance time Tlim (seconds)
We pooled 10 studies (11 arms) that reported Tlim, and they showed a better improvement in the outcome in the IMT group (MD 270.57, 95% CI 182.44 to 358.71; I² = 63%; 260 participants; Analysis 2.21). One trial (Heijdra 1996) considered it as the time to reach Pthmax (not included in the analysis).
2.21. Analysis.

Comparison 2: IMT vs control/sham, Outcome 21: Respiratory muscle endurance time: Tlim (seconds)
Maximal voluntary ventilation (MVV)
Two studies reported MVV. We pooled them in an SMD meta‐analysis because Belman 1988 did not report the unit. We got a summary effect suggesting a large effect according to Cohen's d effect size (SMD 0.99, 95% CI 0.28 to 1.69; I² = 0%; 36 participants; Analysis 2.22).
2.22. Analysis.

Comparison 2: IMT vs control/sham, Outcome 22: Maximal voluntary ventilation (MVV)
Respiratory function: forced expiratory volume at 1 second
We ran two analyses according to FEV1 unit without finding a significant difference between IMT and control/sham in either unit. Ten studies (11 arms) reported FEV1 in %Pred showing a difference of (MD 2.62 %predicted, 95% CI 0.20 to 5.04 Analysis 2.23), while 12 studies (13 arms) reported it in liters (MD 0.04 L, 95% CI −0.06 to 0.14 Analysis 2.24).
2.23. Analysis.

Comparison 2: IMT vs control/sham, Outcome 23: Respiratory function: forced expiratory volume at 1 second (FEV1) (%pred)
2.24. Analysis.

Comparison 2: IMT vs control/sham, Outcome 24: Respiratory function: forced expiratory volume at 1 second (FEV1) (Liters)
Respiratory function: residual volume
Hill 2006 reported residual volume in %Pred and liters, showing a better improvement in the IMT group, while Ramirez Sarmiento 2002 reported it in liters and did not show a difference.
Adverse events
None of the included studies reported a side effect of the intervention.
Discussion
This review has summarised the available evidence of the effect of inspiratory muscle training, used alone or in combination with pulmonary rehabilitation, in people with COPD. We combined the results of the studies in a logical way, so that no data were lost, taking into account the variety of scales, respiratory measurements and training protocols.
Summary of main results
We included 55 RCTs in this review (including trials with more than two arms). Twenty‐two studies (1446 participants) investigated the effect of PR with IMT compared to PR, while 37 studies (1021 participants) focused on the effect of IMT compared to control/sham. Three trials (Abedi Yekta 2019; Larson 1999; Majewska‐Pulsakowska 2016), explored both comparisons (four arms).
Comparison 1: pulmonary rehabilitation plus inspiratory muscle training versus pulmonary rehabilitation
In our first comparison (PR+IMT vs PR), we did not find a significant improvement in the intervention group in dyspnea measured with the Borg scale at isotime, referring to an MCID of −1 unit (moderate‐certainty evidence). We also did not find a potential effect with the mMRC scale, based on an MCID between −0.5 to −1 unit (very low‐certainty evidence.
Studies assessed functional exercise capacity with four measurements: 6MWD, 12MWD, Wmax, and exercise time. We did not find an additional effect of combining PR and IMT with the 6MWD, which we considered our main measurement for this outcome. The overall effect estimate did not reach the MCID of 26 meters (low‐certainty evidence). In subgroup analysis, we divided the studies according to the training duration and mean baseline PImax. The test for subgroup differences was not significant. In sensitivity analysis, three studies at low risk of bias showed a relatively greater treatment effect.
The overall effect estimate of the 12MWD and exercise time showed a larger effect in the intervention group. However, it remains unclear to what extent this difference is clinically relevant because we did not find an MCID for this outcome. The studies that measured Wmax did not reveal a difference between the two groups.
Seven studies investigated the effect of the intervention on HRQoL through the SGRQ. The overall effect estimate showed a positive effect in two domains ('Symptoms' and 'Impact'), without exceeding the MCID of −4 units. There was no difference between the two groups with 'Impact' and 'Total score' (low‐certainty evidence).
In the CRQ, we only found a clinically relevant difference (−0.5 units) in the 'Emotion' domain. The other items ('Dyspnea', 'Fatigue' and 'Mastery') did not show a difference, noting that only three studies reported this scale.
Two studies reported the CAT scale, and the overall effect estimate failed to reveal a benefit based on an MCID of about −1.6 units (very low‐certainty evidence).
The combination of PR and IMT might have a greater effect on inspiratory muscle strength (PImax) than PR alone, but our treatment effect failed to reach the MCID (moderate‐certainty evidence). In subgroup analysis, we did not find a difference between different training durations nor between studies with participants with or without respiratory muscle weakness.
The overall effect estimate of VO2peak, MVV, FEV1 (%Pred and L), and residual volume did not reveal a difference between the two interventions. For respiratory muscle endurance tests (Tlim and Pthmax), we discovered a more significant effect in the PR plus IMT. Nonetheless, the clinical relevance of these estimations remained doubtful since we did not find an MCID.
Comparison 2: inspiratory muscle training versus control/sham
In our second comparison, IMT versus control/sham, taking account of the Borg MCID (−1 unit), we found a trend of an improvement in dyspnea in the IMT group (only the lower limit of the 95% CI exceeded the MCID) when the scale was measured at submaximal exercise capacity (very low‐certainty evidence).
The studies that used BDI−TDI did not reveal a clinically meaningful change (MCID +1 unit) after IMT in the three items of the scale (Functional impairment, Magnitude of task, Magnitude of effort). On the other hand, we found a larger effect with 'Focal score' of the TDI (very low‐certainty evidence). The difference between the items might be explained by the fact that more studies reported Focal score (N = 8) than the other items (N = 3). In subgroup analysis, we did not find a difference between studies with participants judged with and without respiratory muscle weakness.
The overall effect estimate of the mMRC showed a possible effect with IMT that exceeded the MCID of −0.5 units (low‐certainty evidence).
Turning to functional exercise capacity, we combined 16 studies (17 arms) that used the 6MWD, showing a larger effect exceeding the MCID: 26 meters (moderate‐certainty evidence). Following the same subgroup analysis of our first comparison, we did not find a difference between different training durations and between studies with participants judged with and without respiratory muscle weakness. In sensitivity analysis, studies at low risk of bias (N = 2) showed a greater treatment effect.
Studies also used other measurements to assess exercise capacity, and the overall estimation of the 12MWD and Wmax did not show an advantageous effect of IMT. We did not combine the studies that reported exercise time because the methods of measurement were inconsistent. Still, all the effect estimates were on the right‐hand side of the line of no effect with different degrees of positive effect.
We pooled separately three scales (SGRQ, CRQ and CAT) that assessed the HRQoL in a meta‐analysis. All the scales revealed a better improvement in life quality with IMT, but unlike CRQ and CAT, only the lower limit of the 95% CI of SGRQ exceeded the MCID (−4 units; very low‐certainty evidence). We noticed a larger effect estimate favouring the intervention in some items when other scales were used (CAT, SF‐36, CCQ).
PImax was the main secondary outcome in our second comparison. There was a trend to a greater effect with IMT, considering an MCID of 17.2 cmH2O (low‐certainty evidence). We did not find a difference between medium‐term and long‐term training nor between studies with participants with or without respiratory muscle weakness. Measuring the outcome at residual volume or functional residual capacity showed similar results. We got similar results with studies at low risk of bias (N = 3).
We combined the studies that reported VO2peak in an SMD meta‐analysis. According to Cohen's rule of thumb, we considered the overall effect estimate between low to moderate.
The studies that reported respiratory muscle endurance tests (Pthmax, Tlim, MVV) showed a beneficial effect favouring IMT. However, we could not judge the clinical relevance of this effect since we did not find the MCID of these outcomes. The overall effect estimate of FEV1 (%Pred, L) did not reveal a potential improvement with IMT compared to control/sham.
Overall completeness and applicability of evidence
This review was conducted on patients with stable COPD, aged 44 years and over. It explored a wide range of participants and included all RCTs regardless of COPD stages and training protocols (duration, load, supervision, devices, follow‐up, measurements). Most studies, except Ahmad 2013 excluded participants who were free from exacerbation for a couple of weeks preceding the trial and were not hospitalised. Therefore, the results of this review may not be applicable to IMT conducted just after an acute exacerbation. We also noticed a shift over the years from using resistive devices to threshold devices. We excluded RCTs that used resistive trainers without controlling the breathing pattern because participants could adapt a non‐fatiguing respiratory pattern without exposing the respiratory muscles to the workload. Currently, with technological development, there has been increasing use of electronic devices that allow remote monitoring and accurate adjustment of the training load.
Studies that looked at the combined effect of PR plus IMT included people with mild to very severe COPD. One study (Dellweg 2017), performed the intervention on hypercapnic patients who remained dependent on non‐invasive ventilation after prolonged weaning. Two studies (Beaumont 2015; Charususin 2018), specifically trained participants with reduced PImax, and two trials did subgroup analysis within study data according to the state of respiratory muscles (PImax ≤ or > 60 cmH2O) (Beaumont 2018; Wang 2017). Participants in Magadle 2007 and Weiner 2000 received respectively three months and six weeks of PR before the start of IMT, while most of the other studies excluded this category of patients. These two studies had no impact on the overall effect size when we removed them in a sensitivity analysis. Apart from De Farias 2019, Mador 2005 and Paneroni 2018, which conducted endurance training, all the studies performed strength training with various loads and different training durations.
Furthermore, there was a wide range of PR protocols, starting from just breathing exercises and respiratory drainage to a mix of interventions (e.g. cycling, treadmill, muscle strengthening, therapeutic patient education). To the best of our knowledge, the current guidelines have not yet established a consensus for an optimal duration or components of a rehabilitation program. This might be explained by the multiple factors that could interfere with it, such as participants' motivation and financial and logistic resources.
In our second comparison, we were also exposed to a diversity of training protocols in the studies that compared IMT with control/sham. More than half of the included RCTs used a sham IMT, and only two (Bavarsad 2015; Dacha 2019), had some participants with mild COPD. One trial (Saher 2021), focused on patients receiving non‐invasive ventilation as part of COPD management. Almost all the trials excluded participants who had exacerbations before starting the trial, and two RCTs worked on patients with limited functional performance (Covey 2001; ZhouL 2016). Two studies had a long intervention duration (Beckerman 2005; Kim 1993), equal to one year and six months respectively; two studies trained their participants with a load up to 100% of their PImax (Chuang 2017; Hill 2006); two trials conducted endurance IMT (Koppers 2006; Scherer 2000), and one trial performed both endurance and strength exercises (Petrovic 2012). The remaining studies had close characteristics (i.e. strength training, similar loads).
This review did not compare threshold IMT with normocapnic hyperpnea training (endurance IMT) nor compare training loads. We thought it would be best to work on these objectives in a separate review.
Overall, this review included all kinds of participants and interventions without preferences. However, our results might not be applicable for hospitalised patients following an exacerbation or patients with severe COPD who require long‐term oxygen therapy.
Quality of the evidence
Using the GRADE approach, we assessed the certainty of the evidence of five outcomes: dyspnea (Borg, mMRC, BDI‐TDI), functional exercise capacity (6MWD), HRQoL (SGRQ, CAT), and PImax. When the number of RCTs exceeded 10, we created a funnel plot to investigate publication bias.
For both comparisons, we considered the certainty of evidence to be from very low to moderate across all the outcomes (see Table 1; Table 2).
Comparison 1: pulmonary rehabilitation plus inspiratory muscle training versus pulmonary rehabilitation
In our first comparison (PR+IMT versus PR), we downgraded the certainty of evidence by one level for the Borg scale measured at submaximal exercise capacity because of serious concerns about risk of bias related to blinding participants.
We downgraded the certainty of evidence of the mMRC to very low due to very serious concerns with risk of bias (all the trials are at high risk of bias) and serious concerns with imprecision (the sample size is less than 400, rule of thumb).
We considered the certainty of evidence of the 6MWD to be very low due to serious concerns with risk of bias (studies at low risk of bias had different effect estimate from the overall effect estimate) and very serious concerns with inconsistency, although we explained part of the heterogeneity by the difference in training durations. Indeed, there was substantial statistical heterogeneity, and the effect estimates were wide on both sides of the line of no effect, making the benefit of the intervention doubtful. The funnel plot was symmetrical (Figure 2), so publication bias was improbable.
2.

Funnel plot of comparison 7, PR+IMT vs PR, outcome: 7.3 functional exercise capacity: 6‐minute walk distance (6MWD)
We judged the quality of evidence of the SGRQ (total score) as low because of very serious concerns with risk of bias. In fact, none of the studies was at low risk of bias; the main issue was the lack of blinding of participants (outcome measurement).
We considered the certainty of evidence of CAT to be very low due to very serious concerns with risk of bias (most of the evidence is from studies at high risk of bias and some concerns, lack of blinding) and serious concerns with inconsistency (considerable statistical heterogeneity, and confidence intervals do not overlap).
We downgraded the certainty of evidence of PImax by one level due to serious concerns with risk of bias (most of the evidence is from studies at high risk of bias and some concerns). We did not consider statistical heterogeneity because all the effect estimates were on one side showing a benefit). The funnel plot did not raise suspicions of publication bias (Figure 3). As a result, we considered the certainty of the evidence as moderate.
3.

Funnel plot of comparison 7, PR+IMT vs PR, outcome: 7.12 respiratory muscle strength: PImax
Comparison 2: inspiratory muscle training versus control/sham
In our second comparison (IMT versus control/sham), we downgraded the certainty of evidence of Borg measured at submaximal exercise capacity to very low due to very serious concerns with risk of bias (most of the studies were at high risk or some concerns, with different effect estimates compared to low risk of bias studies, issues with blinding) and serious concerns with imprecision (small sample size less than 400 participants, rule of thumb).
For the same reasons explained above, we downgraded the certainty of evidence for dyspnea assessed with BDI‐TDI by three levels.
We considered the certainty of evidence of mMRC to be low due to serious concerns with risk of bias (most of the evidence is from studies at high risk of bias and some concerns) and imprecision (sample size less than 400 participants, rule of thumb).
We judged the quality of evidence of the 6MWD as moderate due to serious concerns with risk of bias (most of the evidence is from studies at high risk of bias and with some concerns). We did not downgrade inconsistency, despite the substantial statistical heterogeneity, because the effect estimates were on one side of the line of no effect. So we were more confident about the direction of the effect. The funnel plot raised some concerns about publication bias (Figure 4), without downgrading the evidence.
4.

Funnel plot of comparison 8, IMT vs control/sham, outcome: 8.8 functional exercise capacity: 6‐minute walk distance (6MWD)
We downgraded the certainty of evidence of the SGRQ by three levels because of very serious concerns with risk of bias (studies with greater weight were at high risk of bias and some concerns, showing different effect estimates from studies at low risk of bias) and very serious concerns with imprecision (sample size less than 400 participants, rule of thumb; the 95% CI includes benefit and harm).
We considered the certainty of evidence of CAT to be moderate due to serious concerns with imprecision (sample size less than 400, rule of thumb).
We judged the certainty of evidence of PImax as low due to serious concerns with risk of bias (most of the evidence is from studies at high risk of bias and with some concerns) and a strongly suspected publication bias (Figure 5).
5.

Potential biases in the review process
The review is based on a published protocol (Ammous 2020). We discuss deviations from the protocol in the Differences between protocol and review section.
One of the main issues with the included studies in this review is the risk of bias, using RoB 2, which is outcome‐dependent. Only six out of 22 studies were at low risk of bias for the first comparison, while only three out of 37 studies were at low risk for the second comparison. Across all the outcomes, the major problems were lack of detail about allocation concealment, some issues with missing data, participants being aware of their intervention (for the participant‐reported outcome that involves judgments), and not publishing a protocol or listing the study in a trial register before launching the studies. Sometimes studies at high risk of bias tended to show a larger effect than the others, which reduced the confidence in our results.
One trial provided IMT for a short period of time (two weeks; Saher 2021), and participants received non‐invasive ventilation throughout the trial.
From all our included studies, only one abstract (Masanga 2011), reported adverse events. Although it is unlikely that IMT may be associated with harm, we have some concerns due to the variety of side effects reported by Masanga 2011, and because the included studies did not discuss them. That is to say, one of the reasons for discontinuing the trial was the inability of participants to continue the intervention. But the studies did not discuss the reasons that made patients take that decision.
Moreover, when dividing the studies according to mean baseline PImax to classify participants with or without respiratory muscle weakness, we assumed that the proportion of participants not belonging to their assigned subgroup was balanced across the studies. In other words, getting individual data and conducting a subgroup analysis within study data was impossible. So, we assumed this variation was balanced between the studies, considering that PImax follows a normal distribution with fewer outliers.
Agreements and disagreements with other studies or reviews
Ten published reviews that explored the effect of IMT on COPD have been published over the years. The first one was published in the 90s (Smith 1992). This review included 17 trials in which the participants had chronic airflow limitation without specifying the types of the diseases. They summarised the effect estimate with SMD meta‐analysis and interpreted it according to Cohen’s d effect size. They found a small to moderate effect favouring IMT for MVV, PImax and laboratory exercise tests. However, this review included trials that run resistance training without controlling the breathing pattern.
Ten years later, another systematic review was published (Lötters 2002), including 15 RCTs. Unlike our review, this study pooled altogether in an SMD meta‐analysis RCTs that looked at the effect of IMT as a stand‐alone intervention and when associated with PR. Then, they conducted a subgroup analysis to investigate the additional effect of PR. They found that IMT alone might improve dyspnea and respiratory muscle strength and endurance. When combined with PR, a beneficial effect was seen only in participants with respiratory muscle weakness.
The following review (16 studies) was conducted by Crowe 2005, followed by an update with 18 studies (O'Brien 2008), in which they compared IMT separately with different types of interventions (exercises, breathing techniques, education, PR). The authors concluded, based on a study‐level analysis, that IMT might improve respiratory muscle strength. However, there was less confidence with dyspnea and HRQoL. In our review, we considered the participants who underwent therapeutic patient education or one type of breathing exercises as control, because the aim was not to compare IMT with another intervention but to create a psychological effect for patients not receiving IMT. We excluded the studies that focused on comparing IMT with breathing exercises. Similarly, the same authors worked on another systematic review (Geddes 2005), and then they updated it (Geddes 2008). These reviews compared IMT with no intervention, low versus high IMT, and two different modes of IMT, which is not the purpose of our review.
A narrative review (Shoemakher 2009), reported that IMT, in comparison with sham IMT or no intervention, might improve dyspnea, HRQoL and PImax. Following that, Gosselink 2011 showed similar results to Lötters 2002. The authors included 32 RCTs, and found a possible effect of IMT in improving dyspnea, functional exercise capacity, HRQoL, PImax and respiratory muscle endurance. In subgroup analysis, they reported a benefit of IMT when associated with PR only in participants with respiratory muscle weakness. They also found a larger effect of strength training compared to endurance training.
In a subsequent study, Beaumont 2018a included 37 trials in a meta‐analysis showing an improvement in dyspnea, HRQoL, functional exercise capacity and PImax after threshold IMT. However, they did not find a difference when combining IMT with PR. The last meta‐analysis (Figueiredo 2020), compared IMT alone or associated with other interventions with control, sham, or other interventions. The authors did not find an improvement in dyspnea, HRQoL, or a larger effect in participants with respiratory muscle weakness. They showed a trend of a larger effect with higher training loads.
Our systematic review is the first to have excluded trials that used resistive trainers without controlling the breathing pattern. We are also the first that have excluded the trials that did not measure Borg at isotime. Generally, our results were consistent with past systematic reviews regarding no effect when adding IMT to PR and a possible benefit with IMT as a stand‐alone intervention. However, we could not conclude that there is a better effect in participants with respiratory muscle weakness. A possible explanation for this finding includes the fact that most studies worked on participants without respiratory muscle weakness.
Authors' conclusions
Implications for practice.
When associated with pulmonary rehabilitation (PR), inspiratory muscle training (IMT) may not have an additional benefit on dyspnea, functional exercise capacity and health‐related quality of life. There was an increase in inspiratory muscle strength and endurance, but this difference was not clinically meaningful.
IMT may improve dyspnea, functional exercise capacity (mainly the 6MWD) and health‐related quality of life, compared to sham or no intervention. There was also an increase in inspiratory muscle strength and endurance, but judging the clinical significance of these outcomes was challenging due to a non‐standardised minimal clinically important difference.
For both interventions, we could not conclude that there was a possible larger effect in participants with respiratory muscle weakness and with longer durations of training.
Implications for research.
Overall, we did not detect any potential advantage of combining PR and IMT, which was consistent with past systematic reviews. However, it is still unclear if this intervention is more beneficial in participants with respiratory muscle weakness, and future research may focus more on this group of participants.
We think there is enough evidence for the effect of IMT alone since our results are consistent with past systematic reviews. However, all published trials had a small sample size. So we believe future trials should increase the number of participants, investigate the possible larger effect on participants with respiratory muscle weakness and compare different IMT protocols.
IMT may be a starter intervention for patients unable to undergo PR (e.g. severe COPD, logistical or financial issues). Still, this should be explored further in clinical trials.
For both comparisons, we highly suggest providing a sham IMT for the control group. Although a sham IMT might not influence the results of an observer‐reported outcome such as the 6‐minute walk distance, we strongly believe that participants who know they are undergoing an intervention may overestimate the effect measured with participant‐reported outcomes that involve judgments. Furthermore, future research may consider training with higher inspiratory flow rate and training at high lung volume (closer to total lung capacity) to improve performance in hyperinflated COPD patients.
What's new
| Date | Event | Description |
|---|---|---|
| 9 January 2023 | Amended | Search date corrected in Abstract and PLS to be 20 October 2022 |
History
Protocol first published: Issue 11, 2020 Review first published: Issue 1, 2023
Risk of bias
Risk of bias for analysis 1.1 Dyspnea: Borg (at submaximal exercise: 50% to 80% of Wmax).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Charususin 2018 | Low risk of bias | The allocation sequence was random, concealed, and baseline characteristics were similar. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | There is evidence that missingness did not affect the outcome. | Low risk of bias | Participants were blinded. | Low risk of bias | No differences between the protocol and the journal article. | Low risk of bias | No detected issue with the five domains. |
| Larson 1999 | Some concerns | Randomisation was reported only as a sentence. | High risk of bias | ITT was not conducted with a substantial impact on the results. | High risk of bias | Missingness in the outcome is likely to depend on its true value. | High risk of bias | Participants were not blinded and the outcome is a participant‐reported outcome that involves judgement. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, and only the trial register is available. ITT analysis was not implemented, missingness is likely to be dependant on its value and participants were not blinded. |
Risk of bias for analysis 1.2 Dyspnea: Modified Medical Research Council (mMRC).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Beaumont 2018 | Low risk of bias | Allocation sequence was random, concealed, and baseline characteristics were similar. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Only the data from two participants out of 149 were missing. | High risk of bias | Participants were not blinded. | Low risk of bias | No differences between the trial register, the conference abstracts and the journal article. | High risk of bias | Participant were not blinded (no sham) and the outcome is a participant‐reported outcome. |
| Wang 2017 | Low risk of bias | The allocation sequence was random, concealed, and baseline characteristics were similar between the groups. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Only 7% of the data were missing, we considered LOCF imputation a valid method for this kind of intervention. | High risk of bias | Participants were not blinded. | Some concerns | Only the journal article is available. | High risk of bias | Participants were not blinded and only the journal article is available. |
Risk of bias for analysis 1.3 Functional exercise capacity: 6‐minute walk distance (6MWD) (meters).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Beaumont 2015 | Some concerns | No information about allocation concealment. | Low risk of bias | All participants were analysed according to their allocation. | Low risk of bias | No loss of follow up or exclusion. | Low risk of bias | Observer‐reported outcome that do not involve judgements. | Low risk of bias | A trial register is available, and both adjustet and unadjusted analysis were reported. | Some concerns | Lack of information about the randomisation process. |
| Beaumont 2018 | Low risk of bias | Allocation sequence was random, concealed, and baseline characteristics were similar. | Low risk of bias | "All analyses were performed on an intention‐to‐treat basis". | Low risk of bias | The data from two participants out of 149 were missing. | Low risk of bias | "All the data were collected by a research nurse blinded to treatment allocation". | Low risk of bias | A trial register is available and there is only one way to measure the outcome. | Low risk of bias | No detected issues with the five domains. |
| Charususin 2018 | Low risk of bias | The allocation sequence was random, concealed, and baseline characteristics were similar. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | There is evidence that missingness did not bias the results. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | A trial protocol is available with no differences with the journal article. | Low risk of bias | No detected issues with the five domains. |
| De Farias 2019 | Some concerns | The allocation sequence was random, lack of details about allocation concealment, and no baseline differences. | Some concerns | 2 participants out of 33 were not analysed. | Low risk of bias | The data of 2 participants out of 33 were missing (6%). | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the abstract and the protocol are available. | Some concerns | Lack of details about the randomisation process, reason for missingness, and only the abstract et the protocol are available. |
| De Farias 2019 | Some concerns | The allocation sequence was random, lack of details about allocation concealment, and no baseline differences. | Some concerns | 2 participants out of 33 were not analysed. | Low risk of bias | The data of 2 participants out of 33 were missing (6%). | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the abstract and the protocol are available. | Some concerns | Lack of details about the randomisation process, reason for missingness, and only the abstract et the protocol are available. |
| Dellweg 2017 | Low risk of bias | The sequence was random, concealed, and baseline characteristics were similar between the groups. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | A trial register is available, and no differences between the methods and the results sections. | Low risk of bias | No detected issues with the five domains. |
| Mador 2005 | Some concerns | Randomisation was reported only as a sentence. | Some concerns | The reasons for exclusion were not reported, and it is unlikely that a per‐protocol analysis was conducted. | Some concerns | The data of 9 participants out of 38 were missing, and the proportion of missingness was balanced between the two groups. | Low risk of bias | Observer‐reported outcome that do not involve judgements | Some concerns | Only the journal is available, and no adjusted analysis were conducted. | Some concerns | Lack of details about the randomisation process, reasons for exclusion and/or loss of follow up were not explained, and only the journal article is available. |
| Magadle 2007 | Some concerns | No information about allocation concealment. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | the data of 2 participants out of 31 were missing (6%). | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available, and there is only one way to measure the outcome. | Some concerns | Lack of details about allocation concealement and only the trial register is available. |
| Paneroni 2018 | Low risk of bias | Allocation sequence was concealed. | Low risk of bias | No participants were excluded. | High risk of bias | 11% of the participants discontinued the intervention. The reasons were: intolerance to the intervention and low compliance. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | A trial register is available, with no differences with the journal article. No adusted analysis were conducted. | High risk of bias | Missingness is likely to be dependant on its true value. |
| Schultz 2018 | Some concerns | No information about allocation concealment. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Missingness was balanced between the groups. | Low risk of bias | The outcome was adequately measured, and it is an observer reported outcome that do not involve judgements. | Low risk of bias | No differences between the trial register, the abstracts, the methods and the results sections. | Some concerns | Lack of details about allocation concealment, and no issues with the other domains. |
| Tounsi 2021 | Low risk of bias | The allocation sequence was random, concealed, and baseline characteristics were similar. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Missingness is independent on its true value. | Low risk of bias | The outcome was measured according to the guidelines and it is an observer‐reported outcome that does not involve judgement. | Low risk of bias | No differences between the journal article and the trial register. | Low risk of bias | No detected issues with the five domains. |
| Tout 2013 | High risk of bias | No details about randomisation in the trial (we only discovered that it was a RCT in a personal communication). | Low risk of bias | No deviations were reported and all participants were analysed according to their initial allocation. | Low risk of bias | All the data are available. | Low risk of bias | Outcome measurement was appropriate and it is an observer reported outcome that do not involve judgements | Low risk of bias | Only the journal article is available, and no differences between the methods and the results sections. | High risk of bias | Lack of details about the randomisation process and only the journal article is available. |
| Wang 2017 | Low risk of bias | The allocation sequence was random, concealed, and baseline characteristics were similar between the groups. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Taking account that only 7% of the data are missing, we considered LOCF imputation a valid method for this kind on intervention. | Low risk of bias | Outcome measurement was appropriate and outcome assessors were blinded. | Some concerns | Only the journal article is available, both adjusted and unadjusted analysis were reported. | Some concerns | Only the journal article is available. |
Risk of bias for analysis 1.4 Functional exercise capacity: 6‐minute walk distance (6MWD) (meters) (subgroup analysis: duration of the intervention).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.4.1 Short‐term (<4 weeks) | ||||||||||||
| Beaumont 2015 | Some concerns | No information about allocation concealment. | Low risk of bias | All participants were analysed according to their allocation. | Low risk of bias | No loss of follow up or exclusion. | Low risk of bias | Observer‐reported outcome that do not involve judgements. | Low risk of bias | A trial register is available, and both adjustet and unadjusted analysis were reported. | Some concerns | Lack of information about the randomisation process. |
| Dellweg 2017 | Low risk of bias | The sequence was random, concealed, and baseline characteristics were similar between the groups. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | A trial register is available, and no differences between the methods and the results sections. | Low risk of bias | No detected issues with the five domains. |
| Paneroni 2018 | Low risk of bias | Allocation sequence was concealed. | Low risk of bias | No participants were excluded. | High risk of bias | 11% of the participants discontinued the intervention. The reasons were: intolerance to the intervention and low compliance. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | A trial register is available, with no differences with the journal article. No adusted analysis were conducted. | High risk of bias | Missingness is likely to be dependant on its true value. |
| Schultz 2018 | Some concerns | No information about allocation concealment. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Missingness was balanced between the groups. | Low risk of bias | The outcome was adequately measured, and it is an observer reported outcome that do not involve judgements. | Low risk of bias | No differences between the trial register, the abstracts, the methods and the results sections. | Some concerns | Lack of details about allocation concealment, and no issues with the other domains. |
| Subgroup 1.4.2 Medium‐term ( ≥4 weeks and <8 weeks) | ||||||||||||
| Beaumont 2018 | Low risk of bias | Allocation sequence was random, concealed, and baseline characteristics were similar. | Low risk of bias | "All analyses were performed on an intention‐to‐treat basis". | Low risk of bias | The data from two participants out of 149 were missing. | Low risk of bias | "All the data were collected by a research nurse blinded to treatment allocation". | Low risk of bias | A trial register is available and there is only one way to measure the outcome. | Low risk of bias | No detected issues with the five domains. |
| Dellweg 2017 | Low risk of bias | The sequence was random, concealed, and baseline characteristics were similar between the groups. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | A trial register is available, and no differences between the methods and the results sections. | Low risk of bias | No detected issues with the five domains. |
| Subgroup 1.4.3 Long‐term ( ≥8 weeks) | ||||||||||||
| Charususin 2018 | Low risk of bias | The allocation sequence was random, concealed, and baseline characteristics were similar. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | There is evidence that missingness did not bias the results. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | A trial protocol is available with no differences with the journal article. | Low risk of bias | No detected issues with the five domains. |
| De Farias 2019 | Some concerns | The allocation sequence was random, lack of details about allocation concealment, and no baseline differences. | Some concerns | 2 participants out of 33 were not analysed. | Low risk of bias | 2 participants out of 33 were not analysed (6%). | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the abstract and the trial protocol are available. | Some concerns | Lack of details about the randomisation process, reason for missingness, and only the abstract et the protocol are available. |
| De Farias 2019 | Some concerns | The allocation sequence was random, lack of details about allocation concealment, and no baseline differences. | Some concerns | 2 participants out of 33 were not analysed. | Low risk of bias | 2 participants out of 33 were not analysed (6%). | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the abstract and the trial protocol are available. | Some concerns | Lack of details about the randomisation process, reason for missingness, and only the abstract et the protocol are available. |
| Mador 2005 | Some concerns | Randomisation was reported only as a sentence. | Some concerns | The reasons for exclusion were not reported, and it is unlikely that a per‐protocol analysis was conducted. | Some concerns | The data of 9 participants out of 38 were missing, and the proportion of missingness was balanced between the two groups. | Low risk of bias | Observer‐reported outcome that do not involve judgements | Some concerns | Only the journal is available, and no adjusted analysis were conducted. | Some concerns | Lack of details about the randomisation process, reasons for exclusion and/or loss of follow up were not explained, and only the journal article is available. |
| Magadle 2007 | Some concerns | No information about allocation concealment. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | the data of 2 participants out of 31 were missing (6%). | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available, and there is only one way to measure the outcome. | Some concerns | Lack of details about allocation concealement and only the trial register is available. |
| Tounsi 2021 | Low risk of bias | The allocation sequence was random, concealed, and baseline characteristics were similar. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Missingness is independent on its true value. | Low risk of bias | The outcome was measured according to the guidelines and it is an observer‐reported outcome that does not involve judgement. | Low risk of bias | No differences between the journal article and the trial register. | Low risk of bias | No detected issues with the five domains. |
| Tout 2013 | High risk of bias | No details about randomisation in the trial (we only discovered that it was a RCT in a personal communication). | Low risk of bias | No deviations were reported and all participants were analysed according to their initial allocation. | Low risk of bias | All the data are available. | Low risk of bias | Outcome measurement was appropriate and it is an observer reported outcome that do not involve judgements | Low risk of bias | Only the journal article is available, and no differences between the methods and the results sections. | High risk of bias | Lack of details about the randomisation process and only the journal article is available. |
| Wang 2017 | Low risk of bias | The allocation sequence was random, concealed, and baseline characteristics were similar between the groups. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Taking account that only 7% of the data are missing, we considered LOCF imputation a valid method for this kind on intervention. | Low risk of bias | Outcome measurement was appropriate and outcome assessors were blinded. | Some concerns | Only the journal article is available, both adjusted and unadjusted analysis were reported. | Some concerns | Only the journal article is available. |
Risk of bias for analysis 1.5 Functional exercise capacity: 6‐minute walk distance (6MWD) (meters) (subgroup analysis: with or without respiratory muscle weakness).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.5.1 With respiratory muscle weakness | ||||||||||||
| Charususin 2018 | Low risk of bias | The allocation sequence was random, concealed, and baseline characteristics were similar. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | There is evidence that missingness did not bias the results. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | A trial protocol is available with no differences with the journal article. | Low risk of bias | No detected issues with the five domains. |
| Dellweg 2017 | Low risk of bias | The sequence was random, concealed, and baseline characteristics were similar between the groups. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | A trial register is available, and no differences between the methods and the results sections. | Low risk of bias | No detected issues with the five domains. |
| Tout 2013 | High risk of bias | No details about randomisation in the trial (we only discovered that it was a RCT in a personal communication). | Low risk of bias | No deviations were reported and all participants were analysed according to their initial allocation. | Low risk of bias | All the data are available. | Low risk of bias | Outcome measurement was appropriate and it is an observer reported outcome that do not involve judgements | Low risk of bias | Only the journal article is available, and no differences between the methods and the results sections. | High risk of bias | Lack of details about the randomisation process and only the journal article is available. |
| Subgroup 1.5.2 Without respiratory muscle weakness | ||||||||||||
| Beaumont 2015 | Some concerns | No information about allocation concealment. | Low risk of bias | All participants were analysed according to their allocation. | Low risk of bias | No loss of follow up or exclusion. | Low risk of bias | Observer‐reported outcome that do not involve judgements. | Low risk of bias | A trial register is available, and both adjustet and unadjusted analysis were reported. | Some concerns | Lack of information about the randomisation process. |
| Beaumont 2018 | Low risk of bias | Allocation sequence was random, concealed, and baseline characteristics were similar. | Low risk of bias | "All analyses were performed on an intention‐to‐treat basis". | Low risk of bias | The data from two participants out of 149 were missing. | Low risk of bias | "All the data were collected by a research nurse blinded to treatment allocation". | Low risk of bias | A trial register is available and there is only one way to measure the outcome. | Low risk of bias | No detected issues with the five domains. |
| De Farias 2019 | Some concerns | The allocation sequence was random, lack of details about allocation concealment, and no baseline differences. | Some concerns | 2 participants out of 33 were not analysed | Low risk of bias | The data of 2 participants out of 33 are missing (6%) | Low risk of bias | Outcome assessors were blinded | Some concerns | Only the abstract and the trial protocol are available | Some concerns | Lack of details about the randomisation process, reason for missingness, and only the abstract et the protocol are available. |
| De Farias 2019 | Some concerns | The allocation sequence was random, lack of details about allocation concealment, and no baseline differences. | Some concerns | 2 participants out of 33 were not analysed | Low risk of bias | The data of 2 participants out of 33 are missing (6%) | Low risk of bias | Outcome assessors were blinded | Some concerns | Only the abstract and the trial protocol are available | Some concerns | Lack of details about the randomisation process, reason for missingness, and only the abstract et the protocol are available. |
| Mador 2005 | Some concerns | Randomisation was reported only as a sentence. | Some concerns | The reasons for exclusion were not reported, and it is unlikely that a per‐protocol analysis was conducted. | Some concerns | The data of 9 participants out of 38 were missing, and the proportion of missingness was balanced between the two groups. | Low risk of bias | Observer‐reported outcome that do not involve judgements | Some concerns | Only the journal is available, and no adjusted analysis were conducted. | Some concerns | Lack of details about the randomisation process, reasons for exclusion and/or loss of follow up were not explained, and only the journal article is available. |
| Magadle 2007 | Some concerns | No information about allocation concealment. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | the data of 2 participants out of 31 were missing (6%). | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available, and there is only one way to measure the outcome. | Some concerns | Lack of details about allocation concealement and only the trial register is available. |
| Paneroni 2018 | Low risk of bias | Allocation sequence was concealed. | Low risk of bias | No participants were excluded. | High risk of bias | 11% of the participants discontinued the intervention. The reasons were: intolerance to the intervention and low compliance. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | A trial register is available, with no differences with the journal article. No adusted analysis were conducted. | High risk of bias | Missingness is likely to be dependant on its true value. |
| Schultz 2018 | Some concerns | No information about allocation concealment. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Missingness was balanced between the groups. | Low risk of bias | The outcome was adequately measured, and it is an observer reported outcome that do not involve judgements. | Low risk of bias | No differences between the trial register, the abstracts, the methods and the results sections. | Some concerns | Lack of details about allocation concealment, and no issues with the other domains. |
| Tounsi 2021 | Low risk of bias | The allocation sequence was random, concealed, and baseline characteristics were similar. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Missingness was independent on its true value. | Low risk of bias | The outcome was measured according to the guidelines and it is an observer‐reported outcome that does not involve judgement. | Low risk of bias | No differences between the journal article and the trial register. | Low risk of bias | No detected issues with the five domains. |
| Wang 2017 | Low risk of bias | The allocation sequence was random, concealed, and baseline characteristics were similar between the groups. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Taking account that only 7% of the data are missing, we considered LOCF imputation a valid method for this kind on intervention. | Low risk of bias | Outcome measurement was appropriate and outcome assessors were blinded. | Some concerns | Only the journal article is available, both adjusted and unadjusted analysis were reported. | Some concerns | Only the journal article is available. |
Risk of bias for analysis 1.7 Functional exercise capacity: Wmax (watt).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Charususin 2018 | Low risk of bias | The allocation sequence was random, concealed, and baseline characteristics were similar. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | There is evidence that the results was not biased by missing data. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | No differences between the trial register, the protocol, and the journal article. | Low risk of bias | No detected issues with the five domains. |
| Dekhuijzen 1991 | Some concerns | Only a statements about randomisation was reported, with no information about allocation concealment. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Participants were blinded. | High risk of bias | The data of 4 week were not reported. | High risk of bias | Lack of details about the randomisation process, allocation concealment, and only the journal article is available. Data of the 4th week were not reported. |
| Larson 1999 | Some concerns | Randomisation was reported only as a sentence. | High risk of bias | 59% of the participants were excluded. | High risk of bias | Missingness is likely to be dependant on its true value. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, and only the trial register is available. ITT analysis was not conducted and missingness is likely to be dependant on the outcome. |
| Mador 2005 | Some concerns | Randomisation was reported only as a sentence. | Some concerns | Some concern with ITT analysis. | Some concerns | The reasons of missingness were not reported, but its proportion was balanced between the groups. | Low risk of bias | Observer‐reported outcome that do not involve judgements. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, reasons for exclusion and/or loss of follow up, and only the journal article is available. |
| Wang 2017 | Low risk of bias | The allocation sequence was random, concealed, and baseline characteristics were similar between the groups. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Considering only 6% of the data was missing, we consider LOCF imputation valid for this kind of intervention. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available. | Some concerns | Only the journal article is available. |
Risk of bias for analysis 1.9 Health‐related quality of life (HRQoL): St George's Respiratory Questionnaire (SGRQ).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.9.1 Symptoms | ||||||||||||
| Beaumont 2018 | Low risk of bias | Allocation sequence was random, concealed, and baseline characteristics were similar. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Only the data of two participants out of 149 were missing. | High risk of bias | Participant‐reported outcome and no sham was used. | Low risk of bias | No differences between the trial register, the conference abstracts, data were reported correctly according to their distribution with no differences with the methods section. | High risk of bias | HRQoF is a participants‐reported outcome and there are no information about blinding or the use of a sham training. |
| Tout 2013 | High risk of bias | No details about randomisation in the trial (we only discovered that it was a RCT in a personal communication). | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | High risk of bias | Participant reported outcome that involves judgements, and participants were not blinded. | Some concerns | Only the trial register is available. | High risk of bias | Allocation was probably not concealed, participants were not blinded and only the journal article is available. |
| Subgroup 1.9.2 Activity | ||||||||||||
| Beaumont 2018 | Low risk of bias | Allocation sequence was random, concealed, and baseline characteristics were similar. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Only the data of two participants out of 149 were missing. | High risk of bias | Participant‐reported outcome and no sham was used. | Low risk of bias | No differences between the trial register, the conference abstracts, data were reported correctly according to their distribution with no differences with the methods section. | High risk of bias | HRQoF is a participants‐reported outcome and there are no information about blinding or the use of a sham training. |
| Tout 2013 | High risk of bias | No details about randomisation in the trial (we only discovered that it was a RCT in a personal communication). | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | High risk of bias | Participant reported outcome that involves judgements, and participants were not blinded. | Some concerns | Only the trial register is available. | High risk of bias | Allocation was probably not concealed, participants were not blinded and only the journal article is available. |
| Subgroup 1.9.3 Impact | ||||||||||||
| Beaumont 2018 | Low risk of bias | Allocation sequence was random, concealed, and baseline characteristics were similar. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Only the data of two participants out of 149 were missing. | High risk of bias | Participant‐reported outcome and no sham was used. | Low risk of bias | No differences between the trial register, the conference abstracts, data were reported correctly according to their distribution with no differences with the methods section. | High risk of bias | HRQoF is a participants‐reported outcome and there are no information about blinding or the use of a sham training. |
| Tout 2013 | High risk of bias | No details about randomisation in the trial (we only discovered that it was a RCT in a personal communication). | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | High risk of bias | Participant reported outcome that involves judgements, and participants were not blinded. | Some concerns | Only the trial register is available. | High risk of bias | Allocation was probably not concealed, participants were not blinded and only the journal article is available. |
| Subgroup 1.9.4 Total | ||||||||||||
| Abedi Yekta 2019 | Some concerns | No information about allocation concealment. | Low risk of bias | All participants were analysed according to the initial allocation. | Low risk of bias | Missing data is balanced between the the four groups. | High risk of bias | HRQoF is a participant‐reported outcome that involves judgements. | Low risk of bias | A trial register is available, and no adjusted analysis were conducted. | High risk of bias | Lack of details about the randomisation process and participants were not blinded. |
| Beaumont 2018 | Low risk of bias | Allocation sequence was random, concealed, and baseline characteristics were similar. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Only the data of two participants out of 149 were missing. | High risk of bias | Participant‐reported outcome and no sham was used. | Low risk of bias | No differences between the trial register, the conference abstracts, data were reported correctly according to their distribution with no differences with the methods section. | High risk of bias | HRQoF is a participants‐reported outcome and there are no information about blinding or the use of a sham training. |
| Magadle 2007 | Some concerns | No information about allocation concealment. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | The data of 2 participants out of 31 were missing. | Low risk of bias | Participants were blinded. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about allocation concealment and only the trial register is available. |
| Majewska‐Pulsakowska 2016 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | High risk of bias | The scale is validated, participants were not blinded, and it is a participant reported‐outcome that do not involve judgements. | Some concerns | Only the journal article is available | High risk of bias | Lack of details about the randomisation process, only the journal article is available and participants were not blinded. |
| Schultz 2018 | Some concerns | No information about allocation concealment. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Missingness was balanced between the two groups. | Low risk of bias | Participant‐reported outcome that involves judgements, and a sham was used. | Low risk of bias | No differences between the trial register, the methods and the results sections. | Some concerns | Lack of details about allocation concealment. |
| Tout 2013 | High risk of bias | No details about randomisation in the trial (we only discovered that it was a RCT in a personal communication). | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | High risk of bias | Participant reported outcome that involves judgements, and participants were not blinded. | Some concerns | Only the trial register is available. | High risk of bias | Allocation was probably not concealed, participants were not blinded and only the journal article is available. |
| Wang 2017 | Low risk of bias | The allocation sequence was random, concealed, and baseline characteristics were similar between the groups. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Taking account only 7% of the data were missing, we considered LOCF a valid imputation method for this kind of intervention. | High risk of bias | Participants were not blinded, and it is an observer reported outcome that involves judgements. | Some concerns | Only the journal article is available, and both adjusted and unadjusted analysis were reported. | High risk of bias | Participants were not blinded and only the journal article is available. |
Risk of bias for analysis 1.10 Health‐related quality of life (HRQoL): Chronic Respiratory Disease Questionnaire (CRQ).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.10.1 Dyspnea | ||||||||||||
| Charususin 2018 | Low risk of bias | The allocation sequence was random, concealed, and baseline characteristics were similar. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | There is evidence that the results were not biased by missing data. | Low risk of bias | Participants were blinded. | Low risk of bias | No differences between the trial register, the protocol, and the journal article. | Low risk of bias | No detected issues with the five domains. |
| Larson 1999 | Some concerns | Randomisation was reported only as a sentence. | High risk of bias | 59% of the participants were excluded. | High risk of bias | Misssingness is likely to be dependant on its true value. | Some concerns | Issues with blinding. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, and there are still issues with blinding. There is some concern with ITT, missingness is likely to be dependant on the outcome and only the journal article is available. |
| Mador 2005 | Some concerns | Randomisation was reported only as a sentence. | Low risk of bias | Sone concern with ITT analysis. | Some concerns | The reasons of missingness were not reported, but its proportion was balanced between the groups. | High risk of bias | No sham was used. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, reasons for exclusion and/or loss of follow up, participants were not blinded, and only the journal article is available. |
| Subgroup 1.10.2 Fatigue | ||||||||||||
| Charususin 2018 | Low risk of bias | The allocation sequence was random, concealed, and baseline characteristics were similar. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | There is evidence that the results were not biased by missing data. | Low risk of bias | Participants were blinded. | Low risk of bias | No differences between the trial register, the protocol, and the journal article. | Low risk of bias | No detected issues with the five domains. |
| Larson 1999 | Some concerns | Randomisation was reported only as a sentence. | High risk of bias | 59% of the participants were excluded. | High risk of bias | Misssingness is likely to be dependant on its true value. | Some concerns | Issues with blinding. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, and there are still issues with blinding. There is some concern with ITT, missingness is likely to be dependant on the outcome and only the journal article is available. |
| Mador 2005 | Some concerns | Randomisation was reported only as a sentence. | Low risk of bias | Sone concern with ITT analysis. | Some concerns | The reasons of missingness were not reported, but its proportion was balanced between the groups. | High risk of bias | No sham was used. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, reasons for exclusion and/or loss of follow up, participants were not blinded, and only the journal article is available. |
| Subgroup 1.10.3 Emotion | ||||||||||||
| Charususin 2018 | Low risk of bias | The allocation sequence was random, concealed, and baseline characteristics were similar. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | There is evidence that the results were not biased by missing data. | Low risk of bias | Participants were blinded. | Low risk of bias | No differences between the trial register, the protocol, and the journal article. | Low risk of bias | No detected issues with the five domains. |
| Mador 2005 | Some concerns | Randomisation was reported only as a sentence. | Low risk of bias | Sone concern with ITT analysis. | Some concerns | The reasons of missingness were not reported, but its proportion was balanced between the groups. | High risk of bias | No sham was used. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, reasons for exclusion and/or loss of follow up, participants were not blinded, and only the journal article is available. |
| Subgroup 1.10.4 Mastery | ||||||||||||
| Charususin 2018 | Low risk of bias | The allocation sequence was random, concealed, and baseline characteristics were similar. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | There is evidence that the results were not biased by missing data. | Low risk of bias | Participants were blinded. | Low risk of bias | No differences between the trial register, the protocol, and the journal article. | Low risk of bias | No detected issues with the five domains. |
| Mador 2005 | Some concerns | Randomisation was reported only as a sentence. | Low risk of bias | Sone concern with ITT analysis. | Some concerns | The reasons of missingness were not reported, but its proportion was balanced between the groups. | High risk of bias | No sham was used. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, reasons for exclusion and/or loss of follow up, participants were not blinded, and only the journal article is available. |
Risk of bias for analysis 1.11 Health‐related quality of life (HRQoL): COPD Assessment Test (CAT).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Schultz 2018 | Some concerns | No information about allocation concealment. | Low risk of bias | ITT was balanced between the groups | Low risk of bias | Missingness was balanced between the groups | Low risk of bias | Participant‐reported outcome that involves judgements, and a sham was used | Low risk of bias | No differences between the trial register, the methods and the results sections. | Some concerns | Lack of details about allocation concealment. |
| Wang 2017 | Low risk of bias | The allocation sequence was random, concealed, and baseline characteristics were similar between the groups. | Low risk of bias | ITT analysis was conducted | Low risk of bias | Taking account only 7% of the data were missing, we considered LOCF a valid imputation method for this kind of intervention. | High risk of bias | Participants were not blinded, and it is an observer reported outcome that involves judgements. | Some concerns | Only the journal article is available, and both adjusted and unadjusted analysis were reported. | High risk of bias | Participants were not blinded and only the journal article is available. |
Risk of bias for analysis 1.12 Inspiratory muscle strength: PImax (cmH20).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Beaumont 2015 | Some concerns | No information about allocation concealment. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | The method of measurement was appropriate and it is an observer‐reported outcome that do not involve judgement. | Low risk of bias | A trial register is available, and both adjusted and unadjusted analysis were reported. | Some concerns | No information about allocation concealment. |
| Beaumont 2018 | Low risk of bias | Allocation sequence was random, concealed, and baseline characteristics were similar. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Only the data of two participants out of 149 were missing. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | No differences between the trial register, the conferences abstract and the methods and the results sections. | Low risk of bias | No detected issues with the five domains. |
| Berry 1996 | Some concerns | Only a statement about randomisation was reported. Baseline data were not reported. | Low risk of bias | No deviations were reported, and only one participant was excluded because of exacerbation (non related to the intervention). | Low risk of bias | One participant was missing out of 16 (6%) | Low risk of bias | Observer‐reported outcome that do not involves judgements. | Some concerns | Only the journal article and adjusted endpoint scores are available. | Some concerns | No information about allocation concealment, only the journal article is available and only endpoint scores were reported |
| Charususin 2018 | Low risk of bias | The allocation sequence was random, concealed, and baseline characteristics were similar. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | There is evidence that missing data did not affect the results. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | A protocol is available. | Low risk of bias | No detected issues with the five domains. |
| De Farias 2019 | Some concerns | The allocation sequence was random, lack of details about allocation concealment, and no baseline differences. | Some concerns | 2 participants out of 33 were not analysed | Low risk of bias | The data of 2 participants out of 33 are missing (6%) | Low risk of bias | Outcome assessors were blinded | Some concerns | Only the abstract and the trial protocol are available | Some concerns | Lack of details about the randomisation process, reason for missingness, and only the abstract et the protocol are available. |
| De Farias 2019 | Some concerns | The allocation sequence was random, lack of details about allocation concealment, and no baseline differences. | Some concerns | 2 participants out of 33 were not analysed | Low risk of bias | The data of 2 participants out of 33 are missing (6%) | Low risk of bias | Outcome assessors were blinded | Some concerns | Only the abstract and the trial protocol are available | Some concerns | Lack of details about the randomisation process, reason for missingness, and only the abstract et the protocol are available. |
| Dekhuijzen 1991 | Some concerns | Only a statements about randomisation was reported, with no information about allocation concealment. | Low risk of bias | "All participants were analysed according to their initial allocation" | Low risk of bias | No missing data. | Low risk of bias | The method of measurement is appropriate and it is an observer reported outcome that do not involve judgement | Some concerns | Only the journal article is available, and no mention of adjusted analysis. | Some concerns | Lack of details about the randomisation process, allocation concealment, and only the journal article is available. |
| Dellweg 2017 | Low risk of bias | The sequence was random, concealed, and baseline characteristics were similar between the groups. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | A trial register is available, and all‐time points were reported. No differences between the trial register, the methods and results sections. | Low risk of bias | No detected issues with the five domains. |
| Fanfa Bordin 2020 | Low risk of bias | The sequence was random, concealed, and baseline characteristics were similar between the groups. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | Missingness in the outcome did not depend on its true value. | Low risk of bias | The method of measurement is appropriate, and outcome assessors were blinded. | Low risk of bias | No differences between the trial register and the journal article. | Low risk of bias | No detected issues with the five domains. |
| Larson 1999 | Some concerns | Randomisation was reported only as a sentence. | Some concerns | It is unclear if some excluded participant could have been included. | High risk of bias | Missingness is likely to be dependant on its true value. | Low risk of bias | Observer reported outcome that do not involve judgement | Some concerns | Only the journal article is available and only significance was reported | High risk of bias | Lack of details about the randomisation process, and only the trial register is available. There is some concern with ITT and missingness is likely to be dependant on the outcome. |
| Mador 2005 | Some concerns | Randomisation was reported only as a sentence. | Some concerns | No deviations were reported, and the reason for exclusion were not explained. | Some concerns | The reasons of missingness were not reported, but its proportion was balanced between the groups. | Low risk of bias | Observer‐reported outcome that do not involves judgements. | Some concerns | Only the journal article is available, no differences between the methods and the results section, and no adjusted analysis were conducted. | Some concerns | Lack of details about the randomisation process, reasons for exclusion and/or loss of follow up, and only the journal article is available. |
| Magadle 2007 | Some concerns | No information about allocation concealment. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | Only the data of 2 participants out of 31 were missing (6%). | Low risk of bias | The method of measuring the outcome is appropriate, and it is an Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about allocation concealment and only the trial register is available. |
| Paneroni 2018 | Low risk of bias | Allocation sequence was concealed. | Low risk of bias | No participants were excluded. | High risk of bias | Missingness in the outcome is likely to be dependant on its true value. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | No differences between the trial register, the methods and the results sections. | High risk of bias | Missingness is likely to be dependant on its true value |
| Schultz 2018 | Some concerns | No information about allocation concealment. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Missingness was balnced between the groups. | Low risk of bias | The method of measurement is adequate, and it is an observer reported outcome that do not involve judgements. | Low risk of bias | No differences between the trial register, the methods and the results sections. | Low risk of bias | Lack of details about allocation concealement. |
| Tounsi 2021 | Low risk of bias | The allocation sequence was random, concealed, and baseline characteristics were similar. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Missingness is independent on its true value | Low risk of bias | The outcome was measured according to the guidelines and it is an observer‐reported outcome that does not involve judgement. | Low risk of bias | No differences between the journal article and the trial register. | Low risk of bias | No detected issues with the five domains. |
| Wang 2017 | Low risk of bias | The allocation sequence was random, concealed, and baseline characteristics were similar between the groups. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Taking account only 7% of the data were missing, we consider LOCF imputation a valid technique for this kind of intervention. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available, and both adjusted and unadjusted analysis were reported. | Some concerns | Only the journal article is available. |
| Weiner 1992 | Some concerns | Randomisation was reported only as a sentence. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | The method of measurement is valid, and the outcome is observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process and only the journal article is available. |
| Weiner 2000 | High risk of bias | Clear imbalance in the group sizes. | Low risk of bias | No deviations or exclusions. | Low risk of bias | The drop out is balanced between the two groups. | Low risk of bias | The method of measured is valid, and it is observer‐reported outcome that do not involves judgements. | Some concerns | Only the journal article is available, and no adjusted analysis were reported. | High risk of bias | Substantial differences between group sizes and only the journal article is available. |
Risk of bias for analysis 1.13 Inspiratory muscle strength: PImax (cmH2O) (subgroup analysis: duration of the intervention).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.13.1 Short‐term (<4 weeks) | ||||||||||||
| Beaumont 2015 | Some concerns | No information about allocation concealment. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | The method of measurement was appropriate and it is an observer‐reported outcome that do not involve judgement. | Low risk of bias | A trial register is available, and both adjusted and unadjusted analysis were reported. | Some concerns | No information about allocation concealment. |
| Dellweg 2017 | Low risk of bias | The sequence was random, concealed, and baseline characteristics were similar between the groups. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | A trial register is available, and all‐time points were reported. No differences between the trial register, the methods and results sections. | Low risk of bias | No detected issues with the five domains. |
| Paneroni 2018 | Low risk of bias | Allocation sequence was concealed. | Low risk of bias | No participants were excluded. | High risk of bias | Missingness in the outcome is likely to be dependant on its true value. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | No differences between the trial register, the methods and the results sections. | High risk of bias | Missingness is likely to be dependant on its true value |
| Schultz 2018 | Some concerns | No information about allocation concealment. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Missingness was balnced between the groups. | Low risk of bias | The method of measurement is adequate, and it is an observer reported outcome that do not involve judgements. | Low risk of bias | No differences between the trial register, the methods and the results sections. | Low risk of bias | Lack of details about allocation concealement. |
| Subgroup 1.13.2 Medium‐term ( ≥ 4 weeks and <8 weeks) | ||||||||||||
| Beaumont 2018 | Low risk of bias | Allocation sequence was random, concealed, and baseline characteristics were similar. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Only the data of two participants out of 149 were missing. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | No differences between the trial register, the conferences abstract and the methods and the results sections. | Low risk of bias | No detected issues with the five domains. |
| Dekhuijzen 1991 | Some concerns | Only a statements about randomisation was reported, with no information about allocation concealment. | Low risk of bias | "All participants were analysed according to their initial allocation" | Low risk of bias | No missing data. | Low risk of bias | The method of measurement is appropriate and it is an observer reported outcome that do not involve judgement | Some concerns | Only the journal article is available, and no mention of adjusted analysis. | Some concerns | Lack of details about the randomisation process, allocation concealment, and only the journal article is available. |
| Dellweg 2017 | Low risk of bias | The sequence was random, concealed, and baseline characteristics were similar between the groups. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | A trial register is available, and all‐time points were reported. No differences between the trial register, the methods and results sections. | Low risk of bias | No detected issues with the five domains. |
| Weiner 2000 | High risk of bias | Clear imbalance in the group sizes. | Low risk of bias | No deviations or exclusions. | Low risk of bias | The drop out is balanced between the two groups. | Low risk of bias | The method of measured is valid, and it is observer‐reported outcome that do not involves judgements. | Some concerns | Only the journal article is available, and no adjusted analysis were reported. | High risk of bias | Substantial differences between group sizes and only the journal article is available. |
| Subgroup 1.13.3 Long‐term ( ≥ 8 weeks) | ||||||||||||
| Berry 1996 | Some concerns | Only a statement about randomisation was reported. Baseline data were not reported. | Low risk of bias | No deviations were reported, and only one participant was excluded because of exacerbation (non related to the intervention). | Low risk of bias | One participant was missing out of 16 (6%) | Low risk of bias | Observer‐reported outcome that do not involves judgements. | Some concerns | Only the journal article and adjusted endpoint scores are available. | Some concerns | No information about allocation concealment, only the journal article is available and only endpoint scores were reported. |
| Charususin 2018 | Low risk of bias | The allocation sequence was random, concealed, and baseline characteristics were similar. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | There is evidence that missing data did not affect the results. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | A protocol is available. | Low risk of bias | No detected issues with the five domains. |
| De Farias 2019 | Some concerns | The allocation sequence was random, lack of details about allocation concealment, and no baseline differences. | Some concerns | 2 participants out of 33 were not analysed | Low risk of bias | The data of 2 participants out of 33 are missing (6%) | Low risk of bias | Outcome assessors were blinded | Some concerns | Only the abstract and the trial protcol are available | Some concerns | Lack of details about the randomisation process, reason for missingness, and only the abstract et the protocol are available. |
| De Farias 2019 | Some concerns | The allocation sequence was random, lack of details about allocation concealment, and no baseline differences. | Some concerns | 2 participants out of 33 were not analysed | Low risk of bias | The data of 2 participants out of 33 are missing (6%) | Low risk of bias | Outcome assessors were blinded | Some concerns | Only the abstract and the trial protcol are available | Some concerns | Lack of details about the randomisation process, reason for missingness, and only the abstract et the protocol are available. |
| Dekhuijzen 1991 | Some concerns | Only a statements about randomisation was reported, with no information about allocation concealment. | Low risk of bias | "All participants were analysed according to their initial allocation" | Low risk of bias | No missing data. | Low risk of bias | The method of measurement is appropriate and it is an observer reported outcome that do not involve judgement | Some concerns | Only the journal article is available, and no mention of adjusted analysis. | Some concerns | Lack of details about the randomisation process, allocation concealment, and only the journal article is available. |
| Fanfa Bordin 2020 | Low risk of bias | The sequence was random, concealed, and baseline characteristics were similar between the groups. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | Missingness in the outcome did not depend on its true value. | Low risk of bias | The method of measurement is appropriate, and outcome assessors were blinded. | Low risk of bias | No differences between the trial register and the journal article. | Low risk of bias | No detected issues with the five domains. |
| Larson 1999 | Some concerns | Randomisation was reported only as a sentence. | Some concerns | It is unclear if some excluded participant could have been included. | High risk of bias | Missingness is likely to be dependant on its true value. | Low risk of bias | Observer reported outcome that do not involve judgement | Some concerns | Only the journal article is available and only significance was reported | High risk of bias | Lack of details about the randomisation process, and only the trial register is available. There is some concern with ITT and missingness is likely to be dependant on the outcome. |
| Mador 2005 | Some concerns | Randomisation was reported only as a sentence. | Some concerns | No deviations were reported, and the reason for exclusion were not explained. | Some concerns | The reasons of missingness were not reported, but its proportion was balanced between the groups. | Low risk of bias | Observer‐reported outcome that do not involves judgements. | Some concerns | Only the journal article is available, no differences between the methods and the results section, and no adjusted analysis were conducted. | Some concerns | Lack of details about the randomisation process, reasons for exclusion and/or loss of follow up, and only the journal article is available. |
| Magadle 2007 | Some concerns | No information about allocation concealment. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | Only the data of 2 participants out of 31 were missing (6%). | Low risk of bias | The method of measuring the outcome is appropriate, and it is an Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about allocation concealment and only the trial register is available. |
| Tounsi 2021 | Low risk of bias | The allocation sequence was random, concealed, and baseline characteristics were similar. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Missingness is independent on its true value. | Low risk of bias | The outcome was measured according to the guidelines and it is an observer‐reported outcome that does not involve judgement. | Low risk of bias | No differences between the journal article and the trial register. | Low risk of bias | No detected issues with the five domains. |
| Wang 2017 | Low risk of bias | The allocation sequence was random, concealed, and baseline characteristics were similar between the groups. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Taking account only 7% of the data were missing, we consider LOCF imputation a valid technique for this kind of intervention. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available, and both adjusted and unadjusted analysis were reported. | Some concerns | Only the journal article is available. |
| Weiner 1992 | Some concerns | Randomisation was reported only as a sentence. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | The method of measurement is valid, and the outcome is observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process and only the journal article is available. |
Risk of bias for analysis 1.14 Inspiratory muscle strength: PImax (cmH2O) (subgroup analysis: with or without respiratory muscle weakness).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.14.1 With respiratory muscle weakness | ||||||||||||
| Charususin 2018 | Low risk of bias | The allocation sequence was random, concealed, and baseline characteristics were similar. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | There is evidence that missing data did not affect the results. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | A protocol is available. | Low risk of bias | No detected issues with the five domains. |
| Dekhuijzen 1991 | Some concerns | Only a statements about randomisation was reported, with no information about allocation concealment. | Low risk of bias | "All participants were analysed according to their initial allocation" | Low risk of bias | No missing data. | Low risk of bias | The method of measurement is appropriate and it is an observer reported outcome that do not involve judgement | Some concerns | Only the journal article is available, and no mention of adjusted analysis. | Some concerns | Lack of details about the randomisation process, allocation concealment, and only the journal article is available. |
| Dellweg 2017 | Low risk of bias | The sequence was random, concealed, and baseline characteristics were similar between the groups. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | A trial register is available, and all‐time points were reported. No differences between the trial register, the methods and results sections. | Low risk of bias | No detected issues with the five domains. |
| Weiner 1992 | Some concerns | Randomisation was reported only as a sentence. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | The method of measurement is valid, and the outcome is observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process and only the journal article is available. |
| Weiner 2000 | High risk of bias | Clear imbalance in the group sizes. | Low risk of bias | No deviations or exclusions. | Low risk of bias | The drop out is balanced between the two groups. | Low risk of bias | The method of measured is valid, and it is observer‐reported outcome that do not involves judgements. | Some concerns | Only the journal article is available, and no adjusted analysis were reported. | High risk of bias | Substantial differences between group sizes and only the journal article is available. |
| Subgroup 1.14.2 Without respiratory muscle weakness | ||||||||||||
| Beaumont 2015 | Some concerns | No information about allocation concealment. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | The method of measurement was appropriate and it is an observer‐reported outcome that do not involve judgement. | Low risk of bias | A trial register is available, and both adjusted and unadjusted analysis were reported. | Some concerns | No information about allocation concealment. |
| Beaumont 2018 | Low risk of bias | Allocation sequence was random, concealed, and baseline characteristics were similar. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Only the data of two participants out of 149 were missing. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | No differences between the trial register, the conferences abstract and the methods and the results sections. | Low risk of bias | No detected issues with the five domains. |
| De Farias 2019 | Some concerns | The allocation sequence was random, lack of details about allocation concealment, and no baseline differences. | Some concerns | 2 participants out of 33 were not analysed | Some concerns | The data of 2 participants out of 33 are missing | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the abstract and the trial protocol are available. | Some concerns | Lack of details about the randomisation process, reason for missingness, and only the abstract et the protocol are available. |
| De Farias 2019 | Some concerns | The allocation sequence was random, lack of details about allocation concealment, and no baseline differences. | Some concerns | 2 participants out of 33 were not analysed | Some concerns | The data of 2 participants out of 33 are missing | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the abstract and the trial protocol are available. | Some concerns | Lack of details about the randomisation process, reason for missingness, and only the abstract et the protocol are available. |
| Fanfa Bordin 2020 | Low risk of bias | The sequence was random, concealed, and baseline characteristics were similar between the groups. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | Missingness in the outcome did not depend on its true value. | Low risk of bias | The method of measurement is appropriate, and outcome assessors were blinded. | Low risk of bias | No differences between the trial register and the journal article. | Low risk of bias | No detected issues with the five domains. |
| Larson 1999 | Some concerns | Randomisation was reported only as a sentence. | Some concerns | It is unclear if some excluded participant could have been included. | High risk of bias | Missingness is likely to be dependant on its true value. | Low risk of bias | Observer reported outcome that do not involve judgement | Some concerns | Only the journal article is available and only significance was reported | High risk of bias | Lack of details about the randomisation process, and only the trial register is available. There is some concern with ITT and missingness is likely to be dependant on the outcome. |
| Mador 2005 | Some concerns | Randomisation was reported only as a sentence. | Some concerns | No deviations were reported, and the reason for exclusion were not explained. | Some concerns | The reasons of missingness were not reported, but its proportion was balanced between the groups. | Low risk of bias | Observer‐reported outcome that do not involves judgements. | Some concerns | Only the journal article is available, no differences between the methods and the results section, and no adjusted analysis were conducted. | Some concerns | Lack of details about the randomisation process, reasons for exclusion and/or loss of follow up, and only the journal article is available. |
| Magadle 2007 | Some concerns | No information about allocation concealment. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | Only the data of 2 participants out of 31 were missing (6%). | Low risk of bias | The method of measuring the outcome is appropriate, and it is an Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about allocation concealment and only the trial register is available. |
| Paneroni 2018 | Low risk of bias | Allocation sequence was concealed. | Low risk of bias | No participants were excluded. | High risk of bias | Missingness in the outcome is likely to be dependant on its true value. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | No differences between the trial register, the methods and the results sections. | High risk of bias | Missingness is likely to be dependant on its true value |
| Schultz 2018 | Some concerns | No information about allocation concealment. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Missingness was balnced between the groups. | Low risk of bias | The method of measurement is adequate, and it is an observer reported outcome that do not involve judgements. | Low risk of bias | No differences between the trial register, the methods and the results sections. | Low risk of bias | Lack of details about allocation concealement. |
| Tounsi 2021 | Low risk of bias | The allocation sequence was random, concealed, and baseline characteristics were similar. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Missingness is independent on its true value. | Low risk of bias | The outcome was measured according to the guidelines and it is an observer‐reported outcome that does not involve judgement. | Low risk of bias | No differences between the journal article and the trial register. | Low risk of bias | No detected issues with the five domains. |
| Wang 2017 | Low risk of bias | The allocation sequence was random, concealed, and baseline characteristics were similar between the groups. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Taking account only 7% of the data were missing, we consider LOCF imputation a valid technique for this kind of intervention. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available, and both adjusted and unadjusted analysis were reported. | Some concerns | Only the journal article is available. |
Risk of bias for analysis 2.1 Dyspnea: Borg (at submaximal exercise capacity).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Dacha 2019 | Low risk of bias | From personal communication with the trialist, the suequence was random, concealed, and the groups had similar baseline characteristics. | Low risk of bias | Form personal communication with the trialist, ITT was conducted. | Low risk of bias | No missing data. | Low risk of bias | Participants were blinded, and it is a participant‐reported outcome that involves judgement. | Some concerns | Only the abstract and the trial register are available. | Some concerns | Only the abstract and the trial register are available. |
| Hill 2006 | Some concerns | No information about allocation concealment. | Low risk of bias | No deviations were detected, and all participants were analysed according to their initial allocation. | Low risk of bias | The data of only two participants (5%) were missing. | Low risk of bias | The scale is validated and participants were blinded. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about allocation concealment, and only the journal article is available. |
| Koppers 2006 | Some concerns | Randomisation was reported only as a sentence, the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | 7% of the data are missing (3 participants out of 39) | Low risk of bias | The scale is validated, and a sham was used. | Some concerns | No differences between the methods and the results sections, and only the journal article is available. | Some concerns | Lack of details about the randomisation process and only the journal article is available. |
| Langer 2018 | Low risk of bias | Allocation was random, concealed, and baseline characteristics were similar between the groups. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Participants were blinded. | Low risk of bias | A trial register is available, with no differences with the journal article. | Low risk of bias | No detected issues with the five domains. |
| Larson 1999 | Some concerns | Randomisation was reported only as a sentence. | Some concerns | 77 participants were disqualified from participation (out of 130), and it is unclear if the reasons might have an impact on the outcome. | High risk of bias | Around half of the data were missing, and missingness is likely to be dependant on its true value. | High risk of bias | Participants were not blinded, and it is a participant‐reported outcome that involves judgements. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, and only journal article is available. There is some concern with ITT analysis, missingness is likely to be dependant on its value and participants were not blinded. |
| Petrovic 2012 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | No missing data. | High risk of bias | No mention of blinding, and it is a participant‐reported outcome that involves judgements. | Some concerns | The trial register did not provide precise information. | High risk of bias | Lack of details about the randomisation process, participants were not blinded, and the trial register does not provide supplementary information. |
Risk of bias for analysis 2.2 Dyspnea: Baseline and Transition Dyspnea Indexes (BDI‐TDI).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 2.2.1 Functional impairment | ||||||||||||
| Harver 1989 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | High risk of bias | missingness in each group was not mentioned. | Low risk of bias | A sham was used. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, missingness, and only the journal article is available. |
| Weiner 2003 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Participants were blinded. | High risk of bias | data in the graph are different from what was reported in the text. | High risk of bias | Lack of details about the randomisation process, only the journal article is available and data in the graphs are different from the text. |
| Wu 2017 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | High risk of bias | Participants were not blinded. | Some concerns | There are some differences between the trial register and the journal article. | High risk of bias | Lack of details about the randomisation process,probably participant were not blinded and there are differences in reporting blinding between the trial register and journal article. |
| Wu 2017 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | High risk of bias | Participants were not blinded. | Some concerns | There are some differences between the trial register and the journal article. | High risk of bias | Lack of details about the randomisation process,probably participant were not blinded and there are differences in reporting blinding between the trial register and journal article. |
| Subgroup 2.2.2 Magnitude of task | ||||||||||||
| Harver 1989 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | High risk of bias | missingness in each group was not mentioned. | Low risk of bias | A sham was used. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, missingness, and only the journal article is available. |
| Weiner 2003 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Participants were blinded. | High risk of bias | data in the graph are different from what was reported in the text. | High risk of bias | Lack of details about the randomisation process, only the journal article is available and data in the graphs are different from the text. |
| Wu 2017 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | High risk of bias | Participants were not blinded. | Some concerns | There are some differences between the trial register and the journal article. | High risk of bias | Lack of details about the randomisation process,probably participant were not blinded and there are differences in reporting blinding between the trial register and journal article. |
| Wu 2017 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | High risk of bias | Participants were not blinded. | Some concerns | There are some differences between the trial register and the journal article. | High risk of bias | Lack of details about the randomisation process,probably participant were not blinded and there are differences in reporting blinding between the trial register and journal article. |
| Subgroup 2.2.3 Magnitude of effort | ||||||||||||
| Harver 1989 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | High risk of bias | missingness in each group was not mentioned. | Low risk of bias | A sham was used. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, missingness, and only the journal article is available. |
| Weiner 2003 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Participants were blinded. | High risk of bias | data in the graph are different from what was reported in the text. | High risk of bias | Lack of details about the randomisation process, only the journal article is available and data in the graphs are different from the text. |
| Wu 2017 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | High risk of bias | Participants were not blinded. | Some concerns | There are some differences between the trial register and the journal article. | High risk of bias | Lack of details about the randomisation process,probably participant were not blinded and there are differences in reporting blinding between the trial register and journal article. |
| Wu 2017 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | High risk of bias | Participants were not blinded. | Some concerns | There are some differences between the trial register and the journal article. | High risk of bias | Lack of details about the randomisation process,probably participant were not blinded and there are differences in reporting blinding between the trial register and journal article. |
| Subgroup 2.2.4 Focal score | ||||||||||||
| Chuang 2017 | Some concerns | Only a statement about randomisation was reported, and the two groups had similar baseline characteristics. | Low risk of bias | No participants were excluded based on the intervention received. | High risk of bias | Missingness is likely to be dependant on its true value. | High risk of bias | Participants were not blinded. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, participants were not blinded and only the journal article is available. |
| Harver 1989 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | High risk of bias | missingness in each group was not mentioned. | Low risk of bias | A sham was used. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, missingness, and only the journal article is available. |
| Langer 2018 | Low risk of bias | Allocation was random, concealed, and baseline characteristics were similar between the groups. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Participants were blinded. | Low risk of bias | No differences between the trial register, the conference abstracts and the journal article. | Low risk of bias | No detected issues with the five domains. |
| Lisboa 1997 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Participants were blinded. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about allocation concealment and only the journal article is available. |
| Sanchez Riera 2001 | Some concerns | Randomisation was reported only as a sentence, and baseline characteristics were similar between the groups. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Participants were blinded. | High risk of bias | Adjusted analysis were conducted only for the training group. | High risk of bias | Lack of details about the randomisation process and adjusted analysis were conducted only for the training group. |
| Scherer 2000 | Some concerns | No information about allocation concealment, and both groups had similar baseline characteristics. | Low risk of bias | One participant excluded due to rip fracture. | Low risk of bias | missingness was balanced between the two groups. | Low risk of bias | Participants were blinded. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, only the journal article is available and missingness could be dependent on its true value. |
| Weiner 2003 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Participants were blinded. | High risk of bias | data in the graph are different from what was reported in the text. | High risk of bias | Lack of details about the randomisation process, only the journal article is available and data in the graphs are different from the text. |
| Wu 2017 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | High risk of bias | Participants were not blinded. | Some concerns | There are some differences between the trial register and the journal article. | High risk of bias | Lack of details about the randomisation process,probably participant were not blinded and there are differences in reporting blinding between the trial register and journal article. |
| Wu 2017 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | High risk of bias | Participants were not blinded. | Some concerns | There are some differences between the trial register and the journal article. | High risk of bias | Lack of details about the randomisation process,probably participant were not blinded and there are differences in reporting blinding between the trial register and journal article. |
Risk of bias for analysis 2.3 Dyspnea: Transition Dyspnea Index (TDI): Focal score (subgroup analysis: with or without respiratory muscle weakness).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 2.3.1 With respiratory muscle weakness | ||||||||||||
| Chuang 2017 | Some concerns | Only a statement about randomisation was reported, and the two groups had similar baseline characteristics. | Low risk of bias | No participants were excluded based on the intervention received. | High risk of bias | Missingness is likely to be dependant on its true value. | High risk of bias | Participants were not blinded. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, participants were not blinded and only the journal article is available. |
| Harver 1989 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | High risk of bias | missingness in each group was not mentioned. | Low risk of bias | A sham was used. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, missingness, and only the journal article is available. |
| Sanchez Riera 2001 | Some concerns | Randomisation was reported only as a sentence, and baseline characteristics were similar between the groups. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Participants were blinded. | High risk of bias | Adjusted analysis were conducted only for the training group. | Some concerns | Lack of details about the randomisation process and adjusted analysis were conducted only for the training group. |
| Wu 2017 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | High risk of bias | serious concern with blinding | Some concerns | There are some differences between the trial register and the journal article. | High risk of bias | Lack of details about the randomisation process,probably participant were not blinded and there are differences in reporting blinding between the trial register and journal article. |
| Wu 2017 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | High risk of bias | serious concern with blinding | Some concerns | There are some differences between the trial register and the journal article. | High risk of bias | Lack of details about the randomisation process,probably participant were not blinded and there are differences in reporting blinding between the trial register and journal article. |
| Subgroup 2.3.2 Without respiratory weakness | ||||||||||||
| Langer 2018 | Low risk of bias | Allocation was random, concealed, and baseline characteristics were similar between the groups. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Participants were blinded. | Low risk of bias | No differences between the trial register, the conference abstracts and the journal article. | Low risk of bias | No detected issues with the five domains. |
| Lisboa 1997 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Participants were blinded. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about allocation concealment and only the journal article is available. |
| Scherer 2000 | Some concerns | No information about allocation concealment, and both groups had similar baseline characteristics. | Low risk of bias | One participant excluded due to rip fracture. | Low risk of bias | missingness was balanced between the two groups. | Low risk of bias | Participants were blinded. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, only the journal article is available and missingness could be dependent on its true value. |
Risk of bias for analysis 2.4 Dyspnea: Modified Medical Research Council (mMRC).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Langer 2018 | Low risk of bias | Allocation was random, concealed, and baseline characteristics were similar between the groups. | Low risk of bias | ITT analysis was conducted | Low risk of bias | No missing data | Low risk of bias | Participants were blinded | Low risk of bias | No differences between the trial register, the abstract, and the article | Low risk of bias | No issues with the five domains |
| Saka 2021 | Some concerns | Lack of details about allocation concealment | Low risk of bias | All participants were analysed according to their initial allocation | Low risk of bias | Missingness is unlikely to be dependent on its true value | Low risk of bias | A sham IMT was used | Some concerns | Lack of details in the trial register | Some concerns | Lack of details about the randomisation process, and the trial register did not provide sufficient information. |
| Xu 2018 | Low risk of bias | The sequence was random, concealed, and the two groups had similar baseline characteristics. | Low risk of bias | ITT analysis was conducted | Low risk of bias | Missingness is independent on ites true value | Low risk of bias | A sham IMT was used | Low risk of bias | No differences between the abstracts, the trial register and the article | Low risk of bias | No issues with the five domains |
| ZhouL 2016 | Low risk of bias | Allocation was concealed. | Some concerns | Three participants were excluded (6%) | Some concerns | No details of the reasons of missingness, but its proportion is small (6%) | High risk of bias | Participants were not blinded, and it is a participant reported outcome that involve judgements. | Some concerns | Only the journal article is available | High risk of bias | Some issues with ITT analysis, missingness could be dependant on its true value, participants were not blinded and only the journal article is available. |
Risk of bias for analysis 2.5 Functional exercise capacity: 6‐minute walk distance (6MWD) (meters).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Bavarsad 2015 | Some concerns | No information about allocation concealment, and the two groups had similar baseline characteristics. | Some concerns | Only two participants out of 40 were excluded (no potential impact on the results). | Low risk of bias | Missingness is independent on its true value. | Low risk of bias | Observer‐reported outcome that do not involve judgement, and the method of measurement was appropriate. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation technique, allocation concealment, and only the journal article is available. |
| Beckerman 2005 | Some concerns | No information about allocation concealment, and the two groups had similar baseline characteristics. | High risk of bias | No reasons for drop out were reported for five participants (11%). | High risk of bias | 11 participants dropped out (6 died). However, No reasons for drop out were reported for the remaining participants. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available. | High risk of bias | No information about allocation concealment and only the journal article is available. 26% of the participants dropped out of the study with no reason reported for 11%. |
| Chuang 2017 | Some concerns | Only a statement about randomisation was reported, and the two groups had similar baseline characteristics. | Low risk of bias | Participants were analysed according to their initial allocation. | Low risk of bias | Missingness is unlikely to be dependant on its true value. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, and only the journal articile is available. |
| Cutrim 2019 | Low risk of bias | The sequence was random, concealed, and the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | The method of measurement was valid, and it is observer reported outcome that do not involve judgements. | Low risk of bias | A trial register is available, and no differences between the methods and the results sections. | Low risk of bias | No detected issue with the five domains. |
| Hill 2006 | Some concerns | No information about allocation concealment. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | The data of only two participants (5%) are missing. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about allocation concealment, and only the journal article is available. |
| Hsiao 2003 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | High risk of bias | 10% of participants were excluded (per protocol analysis). | Some concerns | Missingness is balanced between the two groups. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process and only the journal article is available. ITT was not conducted and missingness could be dependant on its true value. |
| Hsiao 2003 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | High risk of bias | 10% of participants were excluded (per protocol analysis). | Some concerns | Missingness is balanced between the two groups. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process and only the journal article is available. ITT was not conducted and missingness could be dependant on its true value. |
| Koppers 2006 | Some concerns | Randomisation was reported only as a sentence, the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | 7% of the data are missing (3 participants out of 39). | Low risk of bias | The method of measurement was valid and it is an observer reported outcome that do not involve judgements. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process and only the journal article is available. |
| Leelarungrayub 2017 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | No participants were excluded. | High risk of bias | 6 participants discontinued training, but there is no mention of the proportion of missingness in each group. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process and only the journal article is available. Missingness may be dependant on its true value. |
| Lisboa 1997 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Lack of details about allocation concealment and only the journal article is available. | Some concerns | Lack of details about allocation concealment and only the journal article is available. |
| Ramirez Sarmiento 2002 | Some concerns | Randomisation was reported only as a sentence, and no differences between the groups at visual inspection. | High risk of bias | Two participants were excluded (12%). | Low risk of bias | Missingness did not depend on its true value. | Low risk of bias | Observer reported outcome that do not involve judgement, and the method of measurement was valid. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, only the journal article is available and ITT analysis was not conduted. |
| Saher 2021 | Some concerns | Randomisation was reported only as a sentence. Authors used concealed envelopes without mentioning if they were opaque | High risk of bias | ITT analysis was not applied | Some concerns | Missingness could depend on its true value | Low risk of bias | Oberserver reported outcome that do not involve judgement | Some concerns | Only the journal article is available | High risk of bias | Lack of details about the randomisation process, only the journal article is available, missingness could depend on its true value, and ITT analysis was not conducted. |
| Saka 2021 | Some concerns | Lack of details about allocation concealment | Low risk of bias | All participants were analysed according to their initial allocation | Low risk of bias | Missingness is independent on its true value | Low risk of bias | Observer reported outcome that do not involve judgement | Some concerns | Lack of details in the trial register | Some concerns | Lack of details about the randomisation process, and the trial register did not provide sufficient information |
| Scherer 2000 | Some concerns | No information about allocation concealment, and both groups had similar baseline characteristics. | Low risk of bias | One participant excluded due to rip fracture. | Some concerns | Missingness was balanced between the two groups. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, only the journal article is available and missingness could be depandant on its true value. |
| Weiner 2003 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | High risk of bias | data in the graph are different from what was reported in the text. | High risk of bias | Lack of details about the randomisation process, only the journal article is available and data in the graphs are different from the text. |
| Xu 2018 | Low risk of bias | The sequence was random, concealed, and the two groups had similar baseline characteristics. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | No differences between the protocol, trial register, and the journal article. | Low risk of bias | No detected issues with the five domains. |
| ZhouL 2016 | Low risk of bias | Allocation was concealed. | Some concerns | 3 participants were excluded. | Some concerns | No details of the reasons of missingness, but its proportion is small (6%). | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available. | Some concerns | No ITT analysis was conducted, missingness could be dependant on its true value, and only the journal article is available. |
Risk of bias for analysis 2.6 Functional exercise capacity: 6‐minute walk distance (6MWD) (meters) (subgroup analysis: duration of the intervention).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 2.6.1 Short‐term (<4 weeks) | ||||||||||||
| Saher 2021 | Some concerns | Randomisation was reported only as a sentence. Authors used concealed envelopes without mentioning if they were opaque | High risk of bias | ITT analysis was not applied | Some concerns | Missingness could depend on its true value | Low risk of bias | Observer reported outcome that do not involve judgement | Some concerns | Only the journal article is available | High risk of bias | Lack of details about the randomisation process, only the journal article is available, missingness could depend on its true value, and ITT analysis was not conducted. |
| Subgroup 2.6.2 Medium‐term (≥4 weeks and <8 weeks) | ||||||||||||
| Chuang 2017 | Some concerns | Only a statement about randomisation was reported, and the two groups had similar baseline characteristics. | Low risk of bias | Participants were analysed according to their initial allocation. | Low risk of bias | Missingness is unlikely to be dependant on its true value. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, and only the journal articile is available. |
| Koppers 2006 | Some concerns | Randomisation was reported only as a sentence, the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | 7% of the data are missing (3 participants out of 39). | Low risk of bias | The method of measurement was valid and it is an observer reported outcome that do not involve judgements. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process and only the journal article is available. |
| Leelarungrayub 2017 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | No participants were excluded. | High risk of bias | 6 participants discontinued training, but there is no mention of the proportion of missingness in each group. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process and only the journal article is available. Missingness may be dependant on its true value. |
| Lisboa 1997 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Lack of details about allocation concealment and only the journal article is available. | Some concerns | Lack of details about allocation concealment and only the journal article is available. |
| Subgroup 2.6.3 Long‐term (≥8 weeks) | ||||||||||||
| Bavarsad 2015 | Some concerns | No information about allocation concealment, and the two groups had similar baseline characteristics. | Some concerns | Only two participants out of 40 were excluded (no potential impact on the results). | Low risk of bias | Missingness is independent on its true value. | Low risk of bias | Observer‐reported outcome that do not involve judgement, and the method of measurement was appropriate. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation technique, allocation concealment, and only the journal article is available. |
| Beckerman 2005 | Some concerns | No information about allocation concealment, and the two groups had similar baseline characteristics. | High risk of bias | No reasons for drop out were reported for five participants (11%). | High risk of bias | 11 participants dropped out (6 died). However, No reasons for drop out were reported for the remaining participants. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available. | Some concerns | No information about allocation concealment and only the journal article is available. 26% of the participants dropped out of the study with no reason reported for 11%. |
| Cutrim 2019 | Low risk of bias | The sequence was random, concealed, and the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | The method of measurement was valid, and it is observer reported outcome that do not involve judgements. | Low risk of bias | A trial register is available, and no differences between the methods and the results sections. | Low risk of bias | No detected issue with the five domains. |
| Hill 2006 | Some concerns | No information about allocation concealment. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | The data of only two participants (5%) are missing. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about allocation concealment, and only the journal article is available. |
| Hsiao 2003 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | High risk of bias | 10% of participants were excluded (per protocol analysis). | Some concerns | Missingness is balanced between the two groups. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process and only the journal article is available. ITT was not conducted and missingness could be dependant on its true value. |
| Hsiao 2003 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | High risk of bias | 10% of participants were excluded (per protocol analysis). | Some concerns | Missingness is balanced between the two groups. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process and only the journal article is available. ITT was not conducted and missingness could be dependant on its true value. |
| Ramirez Sarmiento 2002 | Some concerns | Randomisation was reported only as a sentence, and no differences between the groups at visual inspection. | High risk of bias | Two participants were excluded (12%). | Low risk of bias | Missingness did not depend on its true value. | Low risk of bias | Observer reported outcome that do not involve judgement, and the method of measurement was valid. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, only the journal article is available and ITT analysis was not conduted. |
| Saka 2021 | Some concerns | Lack of details about allocation concealment | Low risk of bias | All participants were analysed according to their initial allocation | Low risk of bias | Missingness is independent on its true value | Low risk of bias | Observer reported outcome that do not invovle judgement | Some concerns | Lack of details in the trial register | Some concerns | Lack of details about the randomisation process, and the trial register did not provide sufficient information. |
| Scherer 2000 | Some concerns | No information about allocation concealment, and both groups had similar baseline characteristics. | Low risk of bias | One participant excluded due to rip fracture. | Some concerns | Missingness was balanced between the two groups. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, only the journal article is available and missingness could be depandant on its true value. |
| Weiner 2003 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | High risk of bias | data in the graph are different from what was reported in the text. | High risk of bias | Lack of details about the randomisation process, only the journal article is available and data in the graphs are different from the text. |
| Xu 2018 | Low risk of bias | The sequence was random, concealed, and the two groups had similar baseline characteristics. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | No differences between the protocol, trial register, and the journal article. | Low risk of bias | No detected issues with the five domains. |
| ZhouL 2016 | Low risk of bias | Allocation was concealed. | Some concerns | 3 participants were excluded. | Some concerns | No details of the reasons of missingness, but its proportion is small (6%). | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available. | Some concerns | No ITT analysis was conducted, missingness could be dependant on its true value, and only the journal article is available. |
Risk of bias for analysis 2.7 Functional exercise capacity: 6‐minute walk distance (6MWD) (meters) (subgroup analysis: with or without respiratory muscle weakness).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 2.7.1 With respiratory muscle weakness | ||||||||||||
| Chuang 2017 | Some concerns | Only a statement about randomisation was reported, and the two groups had similar baseline characteristics. | Low risk of bias | Participants were analysed according to their initial allocation. | Low risk of bias | Missingness is unlikely to be dependant on its true value. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, and only the journal articile is available. |
| Leelarungrayub 2017 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | No participants were excluded. | High risk of bias | 6 participants discontinued training, but there is no mention of the proportion of missingness in each group. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process and only the journal article is available. Missingness may be dependant on its true value. |
| Saher 2021 | Some concerns | Randomisation was reported only as a sentence. Authors used concealed envelopes without mentioning if they were opaque | High risk of bias | ITT analysis was not applied | Some concerns | Missingness could depend on its true value | Low risk of bias | Observer reported outcome that do not involve judgement | Some concerns | Only the journal article is available | High risk of bias | Lack of details about the randomisation process, only the journal article is available, missingness could depend on its true value, and ITT analysis was not conducted. |
| Xu 2018 | Low risk of bias | The sequence was random, concealed, and the two groups had similar baseline characteristics. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | No differences between the protocol, trial register, and the journal article. | Low risk of bias | No detected issues with the five domains. |
| ZhouL 2016 | Low risk of bias | Allocation was concealed. | Some concerns | 3 participants were excluded. | Some concerns | No details of the reasons of missingness, but its proportion is small (6%). | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available. | Some concerns | No ITT analysis was conducted, missingness could be dependant on its true value, and only the journal article is available. |
| Subgroup 2.7.2 Without respiratory muscle weakness | ||||||||||||
| Bavarsad 2015 | Some concerns | No information about allocation concealment, and the two groups had similar baseline characteristics. | Some concerns | Only two participants out of 40 were excluded (no potential impact on the results). | Low risk of bias | Missingness is independent on its true value. | Low risk of bias | Observer‐reported outcome that do not involve judgement, and the method of measurement was appropriate. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation technique, allocation concealment, and only the journal article is available. |
| Beckerman 2005 | Some concerns | No information about allocation concealment, and the two groups had similar baseline characteristics. | High risk of bias | No reasons for drop out were reported for five participants (11%). | High risk of bias | 11 participants dropped out (6 died). However, No reasons for drop out were reported for the remaining participants. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available. | Some concerns | No information about allocation concealment and only the journal article is available. 26% of the participants dropped out of the study with no reason reported for 11%. |
| Hill 2006 | Some concerns | No information about allocation concealment. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | The data of only two participants (5%) are missing. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about allocation concealment, and only the journal article is available. |
| Hsiao 2003 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | High risk of bias | 10% of participants were excluded (per protocol analysis). | Some concerns | Missingness is balanced between the two groups. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process and only the journal article is available. ITT was not conducted and missingness could be dependant on its true value. |
| Koppers 2006 | Some concerns | Randomisation was reported only as a sentence, the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | 7% of the data are missing (3 participants out of 39). | Low risk of bias | The method of measurement was valid and it is an observer reported outcome that do not involve judgements. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process and only the journal article is available. |
| Lisboa 1997 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Lack of details about allocation concealment and only the journal article is available. | Some concerns | Lack of details about allocation concealment and only the journal article is available. |
| Ramirez Sarmiento 2002 | Some concerns | Randomisation was reported only as a sentence, and no differences between the groups at visual inspection. | High risk of bias | Two participants were excluded (12%). | Low risk of bias | Missingness did not depend on its true value. | Low risk of bias | Observer reported outcome that do not involve judgement, and the method of measurement was valid. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, only the journal article is available and ITT analysis was not conduted. |
| Saka 2021 | Some concerns | Lack of details about allocation concealment | Low risk of bias | All participants were analysed according to their initial allocation | Low risk of bias | Missingness is independent on its true value | Low risk of bias | Observer reported outcome that do not invovle judgement | Some concerns | Lack of details in the trial register | Some concerns | Lack of details about the randomisation process, and the trial register did not provide sufficient information. |
| Scherer 2000 | Some concerns | No information about allocation concealment, and both groups had similar baseline characteristics. | Low risk of bias | One participant excluded due to rip fracture. | Some concerns | Missingness was balanced between the two groups. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, only the journal article is available and missingness could be depandant on its true value. |
| Weiner 2003 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | High risk of bias | data in the graph are different from what was reported in the text. | High risk of bias | Lack of details about the randomisation process, only the journal article is available and data in the graphs are different from the text. |
| Xu 2018 | Low risk of bias | The sequence was random, concealed, and the two groups had similar baseline characteristics. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | No differences between the protocol, trial register, and the journal article. | Low risk of bias | No detected issues with the five domains. |
Risk of bias for analysis 2.9 Functional exercise capacity: Wmax (watt).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Hill 2006 | Some concerns | No information about allocation concealment. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | The data of only two participants (5%) were missing. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | Only the journal article is avaible. | Some concerns | Lack of details about allocation concealment, and only the journal article is available. |
| Koppers 2006 | Some concerns | Randomisation was reported only as a sentence, the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | 7% of the data are missing (3 participants out of 39). | Low risk of bias | Observer reported outcome that do not involve judgements. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process and only the journal article is available. |
| Larson 1999 | Some concerns | Randomisation was reported only as a sentence. | High risk of bias | 59% of the participants were excluded. | High risk of bias | Missingness is likely to be dependent on its true value. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, and only the trial register is available. There is some concern with ITT and missingness is likely to be dependant on the outcome. |
| Lisboa 1997 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about allocation concealment and only the journal article is available. |
| Ramirez Sarmiento 2002 | Some concerns | Randomisation was reported only as a sentence, and no differences between the groups at visual inspection. | High risk of bias | serious concern with ITT analysis. | Low risk of bias | Missingness is independent on its true value. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, only the journal article is available and ITT analysis was not conducted appropriately. |
| Sanchez Riera 2001 | Some concerns | Randomisation was reported only as a sentence, and baseline characteristics were similar between the groups. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process and only the journal article is available. |
| Wu 2017 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Observer‐reported outcome that do not involves judgements. | Some concerns | Some differences between the trial register and the journal article in blinding. | Some concerns | Lack of details about the randomisation process and there are differences in reporting blinding between the trial register and journal article. |
| Wu 2017 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Observer‐reported outcome that do not involves judgements. | Some concerns | Some differences between the trial register and the journal article in blinding. | Some concerns | Lack of details about the randomisation process and there are differences in reporting blinding between the trial register and journal article. |
Risk of bias for analysis 2.12 Health‐related quality of life (HRQoL): St George Respiratory Questionnaire (SGRQ) .
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 2.12.1 Symptoms | ||||||||||||
| Berton 2015 | Some concerns | No information about allocation concealment. | Some concerns | One excluded participant (4%) would unlikely affect the results. | Some concerns | The proportion of missingness was balanced between the intervention and the control group | Low risk of bias | The scale is validated and a sham was used. | Some concerns | No information if adjusted analysis were planned in advance. | Some concerns | No information about allocation concealment, one participant was excluded because of non adherence, missingness maybe dependant on its true value, and no details if adjusted analysis were planned in advance. |
| Saka 2021 | Some concerns | Lack of details about allocation concealment | Low risk of bias | All participants were analysed according to their initial allocation | Low risk of bias | Missingness is independant on its true value | Low risk of bias | A sham IMT was used | Some concerns | Lack of details in the trial register | Some concerns | Lack of details about the randomisation process, and the trial register did not provide sufficient information. |
| Subgroup 2.12.2 Activity | ||||||||||||
| Berton 2015 | Some concerns | No information about allocation concealment. | Some concerns | One excluded participant (4%) would unlikely affect the results. | Some concerns | The proportion of missingness was balanced between the intervention and the control group | Low risk of bias | The scale is validated and a sham was used. | Some concerns | No information if adjusted analysis were planned in advance. | Some concerns | No information about allocation concealment, one participant was excluded because of non adherence, missingness maybe dependant on its true value, and no details if adjusted analysis were planned in advance. |
| Saka 2021 | Some concerns | Lack of details about allocation concealment | Low risk of bias | All participants were analysed according to their initial allocation | Low risk of bias | Missingness is independant on its true value | Low risk of bias | A sham IMT was used | Some concerns | Lack of details in the trial register | Some concerns | Lack of details about the randomisation process, and the trial register did not provide sufficient information. |
| Subgroup 2.12.3 Impact | ||||||||||||
| Berton 2015 | Some concerns | No information about allocation concealment. | Some concerns | One excluded participant (4%) would unlikely affect the results. | Some concerns | The proportion of missingness was balanced between the intervention and the control group | Low risk of bias | The scale is validated and a sham was used. | Some concerns | No information if adjusted analysis were planned in advance. | Some concerns | No information about allocation concealment, one participant was excluded because of non adherence, missingness maybe dependant on its true value, and no details if adjusted analysis were planned in advance. |
| Saka 2021 | Some concerns | Lack of details about allocation concealment | Low risk of bias | All participants were analysed according to their initial allocation | Low risk of bias | Missingness is independant on its true value | Low risk of bias | A sham IMT was used | Some concerns | Lack of details in the trial register | Some concerns | Lack of details about the randomisation process, and the trial register did not provide sufficient information. |
| Subgroup 2.12.4 Total | ||||||||||||
| Abedi Yekta 2019 | Some concerns | No information about allocation concealment. | Low risk of bias | All participants were analysed according to the initial allocation. | Some concerns | Missing data is balanced between the the four groups. | High risk of bias | Participants were not blinded, and it is a participant‐reported outcome that involve judgements. | Low risk of bias | No differences between the trial register and the journal article. | High risk of bias | Lack of details about the randomisation process, missingness could be dependant on its true value, and participants were not blinded. |
| Beckerman 2005 | Some concerns | No information about allocation concealment, and the two groups had similar baseline characteristics. | High risk of bias | No reason for drop out were reported for five participants (11%). | High risk of bias | 11 participants dropped out (6 died). However, No reasons for drop out were reported for the remaining participants. | Low risk of bias | Participants were blinded. | Some concerns | Only the journal article is available. | High risk of bias | No information about allocation concealment and only the journal article is available. 26% of the participants dropped out of the study with no reason reported for 11%. |
| Berton 2015 | Some concerns | No information about allocation concealment. | Some concerns | One excluded participant (4%) would unlikely affect the results. | Some concerns | The proportion of missingness was balanced between the intervention and the control group | Low risk of bias | The scale is validated and a sham was used. | Some concerns | No information if adjusted analysis were planned in advance. | Some concerns | No information about allocation concealment, one participant was excluded because of non adherence, missingness maybe dependant on its true value, and no details if adjusted analysis were planned in advance. |
| Majewska‐Pulsakowska 2016 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | High risk of bias | Participant reported outcome that involves judgements and participants were not blinded. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, only the journal article is available and participants were not blinded. |
| Saka 2021 | Some concerns | Lack of details about allocation concealment | Low risk of bias | All participants were analysed according to their initial allocation | Low risk of bias | Missingness is independant on its true value | Low risk of bias | A sham IMT was used | Some concerns | Lack of details in the trial register | Some concerns | Lack of details about the randomisation process, and the trial register did not provide sufficient information. |
| Xu 2018 | Low risk of bias | The sequence was random, concealed, and the two groups had similar baseline characteristics. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Missingness did not depend on its true value. | Low risk of bias | A sham was used, and the scale is validated. | Low risk of bias | No differences between the protocol, the trial register and the journal article. | Low risk of bias | No detected issues with the five domains. |
Risk of bias for analysis 2.13 Health‐related quality of life (HRQoL): Chronic Respiratory Disease Questionnaire (CRQ).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 2.13.1 Dyspnea | ||||||||||||
| Bustamante 2007 | Some concerns | Only a statement about randomisation was reported, the two groups had similar baseline characteristics. | Low risk of bias | Participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | A sham IMT was used, and the scale is validated. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, and only the journal article is available. |
| Hill 2006 | Some concerns | No information about allocation concealment. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | Only 5% of the data were missing. | Low risk of bias | Participants were blinded, and it is a participant reported outcome that involves judgements. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about allocation concealment, and only the journal article is available. |
| Larson 1999 | Some concerns | Randomisation was reported only as a sentence. | High risk of bias | No ITT analysis | High risk of bias | Missingness is likely to be dependant on its true value. | Some concerns | Some concern with blinding. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, and there are still issues with blinding. There is some concern with ITT, missingness is likely to be dependant on the outcome and only the journal article is available. |
| Nikoletou 2016 | Some concerns | No information about allocation concealment, and the two groups had similar baseline characteristics. | Some concerns | Some of the participants were excluded, but still less than 5%. | Some concerns | Missingness was balanced between the two groups. | Low risk of bias | Participants were blinded. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, some issues with ITT analysis, important amount of missing data and only the journal article is available. |
| Wu 2017 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | High risk of bias | Participants were not blinded. | Some concerns | Insufficient details in the trial register. | High risk of bias | Lack of details about the randomisation process,probably participant were not blinded and there are differences in reporting blinding between the trial register and journal article |
| Wu 2017 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | High risk of bias | Participants were not blinded. | Some concerns | Insufficient details in the trial register. | High risk of bias | Lack of details about the randomisation process,probably participant were not blinded and there are differences in reporting blinding between the trial register and journal article |
| Subgroup 2.13.2 Fatigue | ||||||||||||
| Bustamante 2007 | Some concerns | Only a statement about randomisation was reported, the two groups had similar baseline characteristics. | Low risk of bias | Participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | A sham IMT was used, and the scale is validated. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, and only the journal article is available. |
| Hill 2006 | Some concerns | No information about allocation concealment. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | Only 5% of the data were missing. | Low risk of bias | Participants were blinded, and it is a participant reported outcome that involves judgements. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about allocation concealment, and only the journal article is available. |
| Larson 1999 | Some concerns | Randomisation was reported only as a sentence. | High risk of bias | No ITT analysis | High risk of bias | Missingness is likely to be dependant on its true value. | Some concerns | Some concern with blinding. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, and there are still issues with blinding. There is some concern with ITT, missingness is likely to be dependant on the outcome and only the journal article is available. |
| Nikoletou 2016 | Some concerns | No information about allocation concealment, and the two groups had similar baseline characteristics. | Some concerns | Some of the participants were excluded, but still less than 5%. | Some concerns | Missingness was balanced between the two groups. | Low risk of bias | Participants were blinded. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, some issues with ITT analysis, important amount of missing data and only the journal article is available. |
| Wu 2017 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | High risk of bias | Participants were not blinded. | Some concerns | Insufficient details in the trial register. | High risk of bias | Lack of details about the randomisation process,probably participant were not blinded and there are differences in reporting blinding between the trial register and journal article |
| Wu 2017 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | High risk of bias | Participants were not blinded. | Some concerns | Insufficient details in the trial register. | High risk of bias | Lack of details about the randomisation process,probably participant were not blinded and there are differences in reporting blinding between the trial register and journal article |
| Subgroup 2.13.3 Emotion | ||||||||||||
| Bustamante 2007 | Some concerns | Only a statement about randomisation was reported, the two groups had similar baseline characteristics. | Low risk of bias | Participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | A sham IMT was used, and the scale is validated. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, and only the journal article is available. |
| Hill 2006 | Some concerns | No information about allocation concealment. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | Only 5% of the data were missing. | Low risk of bias | Participants were blinded, and it is a participant reported outcome that involves judgements. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about allocation concealment, and only the journal article is available. |
| Nikoletou 2016 | Some concerns | No information about allocation concealment, and the two groups had similar baseline characteristics. | Some concerns | Some of the participants were excluded, but still less than 5%. | Some concerns | Missingness was balanced between the two groups. | Low risk of bias | Participants were blinded. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, some issues with ITT analysis, important amount of missing data and only the journal article is available. |
| Wu 2017 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | High risk of bias | Participants were not blinded. | Some concerns | Insufficient details in the trial register. | High risk of bias | Lack of details about the randomisation process,probably participant were not blinded and there are differences in reporting blinding between the trial register and journal article |
| Wu 2017 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | High risk of bias | Participants were not blinded. | Some concerns | Insufficient details in the trial register. | High risk of bias | Lack of details about the randomisation process,probably participant were not blinded and there are differences in reporting blinding between the trial register and journal article |
| Subgroup 2.13.4 Mastery | ||||||||||||
| Bustamante 2007 | Some concerns | Only a statement about randomisation was reported, the two groups had similar baseline characteristics. | Low risk of bias | Participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | A sham IMT was used, and the scale is validated. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, and only the journal article is available. |
| Hill 2006 | Some concerns | No information about allocation concealment. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | Only 5% of the data were missing. | Low risk of bias | Participants were blinded, and it is a participant reported outcome that involves judgements. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about allocation concealment, and only the journal article is available. |
| Nikoletou 2016 | Some concerns | No information about allocation concealment, and the two groups had similar baseline characteristics. | Some concerns | Some of the participants were excluded, but still less than 5%. | Some concerns | Missingness was balanced between the two groups. | Low risk of bias | Participants were blinded. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, some issues with ITT analysis, important amount of missing data and only the journal article is available. |
| Wu 2017 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | High risk of bias | Participants were not blinded. | Some concerns | Insufficient details in the trial register. | High risk of bias | Lack of details about the randomisation process,probably participant were not blinded and there are differences in reporting blinding between the trial register and journal article |
| Wu 2017 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | High risk of bias | Participants were not blinded. | Some concerns | Insufficient details in the trial register. | High risk of bias | Lack of details about the randomisation process,probably participant were not blinded and there are differences in reporting blinding between the trial register and journal article |
Risk of bias for analysis 2.14 Health‐related quality of life (HRQoL): COPD Assessment Test (CAT).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Saka 2021 | Some concerns | Lack of details about allocation concealment | Low risk of bias | ITT analysis was conducted | Low risk of bias | Missingness was unlikely to be dependent on its true value | Low risk of bias | A sham IMT was used | Some concerns | Lack of details in the trial register | Some concerns | Lack of details about the randomisation process, and the trial register did not provide sufficient information. |
| Xu 2018 | Low risk of bias | The sequence was random, concealed, and the two groups had similar baseline characteristics. | Low risk of bias | ITT analysis was conducted | Low risk of bias | The reasons for missingness are independent on the intervention | Low risk of bias | A sham IMT was used | Low risk of bias | No differences between the protocol, the register and the journal article | Low risk of bias | No detected issues in the five domains |
Risk of bias for analysis 2.15 Inspiratory muscle strength: PImax (cmH2O).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Beckerman 2005 | Some concerns | No information about allocation concealment, and the two groups had similar baseline characteristics. | High risk of bias | No reason for drop out were reported for five participants (11%). | High risk of bias | 11 participants dropped out (6 died). However, No reasons for drop out were reported for the remaining participants. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available. | High risk of bias | No information about allocation concealment or a trial register. 26% of the participants dropped out of the study with no reason reported for 11%. |
| Belman 1988 | Some concerns | Randomisation was reported only as a sentence. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | missing data are due to intercurrent illnessess and pneumothorax. | Low risk of bias | Observed‐reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about allocation concealment and only the journal article is available. |
| Berton 2015 | Some concerns | No information about allocation concealment. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | Missingness is independent on its true value. | Low risk of bias | Outcome assessors were blinded. | Some concerns | No information if adjusted analysis were planned in advance. | Some concerns | No information about allocation concealment and if adjusted analysis were planned in advance. |
| Bustamante 2007 | Some concerns | Only a statement about randomisation was reported, the two groups had similar baseline characteristics. | Low risk of bias | No participants were excluded. | Low risk of bias | No missing data. | Low risk of bias | Observer‐reported outcome that do not involve judgements, and the method of measurement was valid. | Some concerns | Only the journal article is available. | Some concerns | No details about allocation concealment, randomisation and only the journal article is available. |
| Chuang 2017 | Some concerns | Only a statement about randomisation was reported, and the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | The reason of missingness was loss of contact. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, and only the journal article is available. |
| Covey 2001 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Some concerns | No ITT analysis were conducted, with no impact on the results. | Some concerns | Missingness is balanced between the two groups | Low risk of bias | Observer reported outcome that do not involve judgements, and the method of measurement was valid. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, no ITT was conducted, missingness could depend on its true value, and only the journal article is available. |
| Cutrim 2019 | Low risk of bias | The sequence was random, concealed, and the two groups had similar baseline characteristics. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | No missing data. | Low risk of bias | Observer‐reported outcome that do not involves judgements, and the measurement was valid. | Low risk of bias | No differences between the trial register and the journal article | Low risk of bias | No detected issues with the five domains. |
| Dacha 2019 | Low risk of bias | From personal communication with the trialist, the suequence was random, concealed, and the groups had similar baseline characteristics. | Low risk of bias | From personal communication, ITT analysis was conducted. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the abstract and the trial register are available (study ongoing) | Some concerns | Only the abstract and the trial register are available (ongoing study). |
| Harver 1989 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | High risk of bias | Missingness in each group was not explained. | Low risk of bias | Observer reported outcome that do not involve judgement, and the method of measurement was valid. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, missingness, and only the journal article is available. |
| Heijdra 1996 | Some concerns | Randomisation was reported only as a sentence. | Low risk of bias | Participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | The method of measurement was correct, and it is an observer reported outcome that do not involve judgements. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randmisation process, and only the journal article is available. |
| Hill 2006 | Some concerns | No information about allocation concealment. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | The data of only two participants (5%) are missing. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about allocation concealment, and only the journal article is available. |
| Hsiao 2003 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | High risk of bias | 10% of participants were excluded (per protocol analysis). | Some concerns | Missingness is unlikely to be dependant on its true value. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process and only the journal article is available. There are serious issues with ITT and missingness is likely to be dependant on the outcome. |
| Hsiao 2003 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | High risk of bias | 10% of participants were excluded (per protocol analysis). | Some concerns | Missingness is unlikely to be dependant on its true value. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process and only the journal article is available. There are serious issues with ITT and missingness is likely to be dependant on the outcome. |
| Kim 1993 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | Participants were analysed according to their initial allocation. | Some concerns | Missingness is balanced between the two groups. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, only the journal article is available and missingness could depend on the outcome. |
| Koppers 2006 | Some concerns | Randomisation was reported only as a sentence, the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | 7% of the data are missing (3 participants out of 39). | Low risk of bias | Observer reported outcome that do not involve judgements. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process and only the journal article is available. |
| Langer 2018 | Low risk of bias | Allocation was random, concealed, and baseline characteristics were similar between the groups. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded, and the method of measurement was valid. | Low risk of bias | No differences between the trial register, the abstracts, and the journal article. | Low risk of bias | No detected issues with the five domains. |
| Larson 1988 | Low risk of bias | Allocation sequence was concealed, and both groups had similar baseline characteristics. | High risk of bias | 4 participants out of 45 were discarded (around 8%), and also two participants were not reported although they completed the training. | Some concerns | Reasons for drop out were not reported, but the proportion of missing data is balanced between the two groups. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Some participants were excluded with no provided reasons, no ITT was conducted, missingness is unlikely to affect the outcome, and only the trial register is available. |
| Larson 1999 | Some concerns | Randomisation was reported only as a sentence. | Some concerns | It is unclear if the reasons of exclusion might have an impact on the results. | High risk of bias | some of the reasons of missingness are: lack of interest, inability to perform training, poor adherence. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, and only the trial register is available. There is some concern with ITT and missingness is likely to be dependant on the outcome. |
| Leelarungrayub 2017 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | No participants were excluded. | High risk of bias | 6 participants discontinued training, but there is no mention of the proportion of missingness in each group. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process and only the journal article is available. Missingness may be dependant on its true value. |
| Lisboa 1997 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about allocation concealment and only the journal article is available. |
| Nikoletou 2016 | Some concerns | No information about allocation concealment, and the two groups had similar baseline characteristics. | Some concerns | 3 participants (5%) were excluded due to poor adherence. | Some concerns | Missingness is balanced between the two groups. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Lack of details about the randomisation process, no ITT analysis, important amount of missing data and only the journal article is available. | Some concerns | Lack of details about the randomisation process, some issues with ITT analysis, important amount of missing data and only the journal article is available. |
| Petrovic 2012 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Observer reported outcome that do not involve judgements, and the method of measurement is valid. | Some concerns | No additional information were provided in the trial register. | Some concerns | Lack of details about the randomisation process and the trial register does not provide supplementary information. |
| Preusser 1994 | Some concerns | No information about allocation concealment, and both groups had similar baseline characteristics. | Low risk of bias | No participants were excluded. | Low risk of bias | Missingness is independent on its true value. | Low risk of bias | Observer reported outcome that do not involve judgement | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process and only the journal article is available. |
| Ramirez Sarmiento 2002 | Some concerns | Randomisation was reported only as a sentence, and no differences between the groups at visual inspection. | High risk of bias | 12% of the participants were excluded. | Low risk of bias | Missingness is independent on its true value. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, only the journal article is available and ITT analysis was not conduted appropriately. |
| Saher 2021 | Some concerns | Randomisation was reported only as a sentence. Authors used concealed envelopes without mentioning if they were opaque | High risk of bias | ITT analysis was not applied | Some concerns | Missingness could depend on its true value | Low risk of bias | Observer reported outcome that do not involve judgement | Some concerns | Only the journal article is available | High risk of bias | Lack of details about the randomisation process, only the journal article is available, missingness could depend on its true value, and ITT analysis was not conducted. |
| Saka 2021 | Some concerns | Lack of details about allocation concealment | Low risk of bias | All participants were analysed according to their initial allocation | Low risk of bias | Missingness is independent on its true value | Low risk of bias | The outcome was measured according to international guidelines | Some concerns | Lack of details in the trial register | Some concerns | Lack of details about the randomisation process, and the trial register did not provide sufficient information. |
| Sanchez Riera 2001 | Some concerns | Randomisation was reported only as a sentence, and baseline characteristics were similar between the groups. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process and only the journal article is available. |
| Scherer 2000 | Some concerns | No information about allocation concealment, and both groups had similar baseline characteristics. | Low risk of bias | One participant excluded due to rip fracture. | Some concerns | Missingness was balanced between the two groups | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, only the journal article is available and missingness could be dependent on its true value. |
| Weiner 2003 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | High risk of bias | data in the graph are different from what was reported in the text. | High risk of bias | Lack of details about the randomisation process, only the journal article is available and data in the graphs are different from the text. |
| Weiner 2006 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar PImax level. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process and only the journal article is available. |
| Wu 2017 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Observer reported outcome that do not involve judgements, and the method of measurement was valid. | Some concerns | There were some differences between the trial register and the journal article, without biasing the results. | Some concerns | Lack of details about the randomisation process and there are differences in reporting blinding between the trial register and journal article. |
| Wu 2017 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Observer reported outcome that do not involve judgements, and the method of measurement was valid. | Some concerns | There were some differences between the trial register and the journal article, without biasing the results. | Some concerns | Lack of details about the randomisation process and there are differences in reporting blinding between the trial register and journal article. |
| Xu 2018 | Low risk of bias | The sequence was random, concealed, and the two groups had similar baseline characteristics. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Missingness is independent on its true value. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | No differences between the trial register, the conference abstracts and the journal article. | Low risk of bias | No detected issue with the five domains. |
| ZhouL 2016 | Low risk of bias | Allocation was concealed. | Some concerns | 3 participants were excluded (6%) | Some concerns | No details of the reasons of missingness, but its proportion is small (6%). | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available. | Some concerns | Some issues with ITT analysis, missingness could be dependant on its true value, and only the journal article is available. |
Risk of bias for analysis 2.16 Inspiratory muscle strength: PImax (cmH2O) (subgroup analysis: duration of the intervention).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 2.16.1 Short‐term (<4 weeks) | ||||||||||||
| Saher 2021 | Some concerns | Randomisation was reported only as a sentence. Authors used concealed envelopes without mentioning if they were opaque | High risk of bias | ITT analysis was not applied | Some concerns | Missingness could depend on its true value | Low risk of bias | Observer reported outcome that do not invovle judgement | Some concerns | Only the journal article is available | High risk of bias | Lack of details about the randomisation process, only the journal article is available, missingness could depend on its true value, and ITT analysis was not conducted. |
| Subgroup 2.16.2 Medium‐term (≥4 weeks and <8 weeks) | ||||||||||||
| Belman 1988 | Some concerns | Randomisation was reported only as a sentence. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | missing data are due to intercurrent illnessess and pneumothorax. | Low risk of bias | Observed‐reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about allocation concealment and only the journal article is available. |
| Bustamante 2007 | Some concerns | Only a statement about randomisation was reported, the two groups had similar baseline characteristics. | Low risk of bias | No participants were excluded. | Low risk of bias | No missing data. | Low risk of bias | Observer‐reported outcome that do not involve judgements, and the method of measurement was valid. | Some concerns | Only the journal article is available. | Some concerns | No details about allocation concealment, randomisation and only the journal article is available. |
| Chuang 2017 | Some concerns | Only a statement about randomisation was reported, and the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | The reason of missingness was loss of contact. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, and only the journal article is available. |
| Koppers 2006 | Some concerns | Randomisation was reported only as a sentence, the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | 7% of the data are missing (3 participants out of 39). | Low risk of bias | Observer reported outcome that do not involve judgements. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process and only the journal article is available. |
| Larson 1988 | Low risk of bias | Allocation sequence was concealed, and both groups had similar baseline characteristics. | High risk of bias | No ITT analysis. | Some concerns | Reasons for drop out were not reported, but the proportion of missing data is balanced between the two groups. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Some participants were excluded with no reasons provided, no ITT was conducted missingness is unlikely to affect the outcome, and only the trial register is available. |
| Leelarungrayub 2017 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | No participants were excluded. | High risk of bias | 6 participants discontinued training, but there is no mention of the proportion of missingness in each group. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process and only the journal article is available. Missingness may be dependant on its true value. |
| Nikoletou 2016 | Some concerns | No information about allocation concealment, and the two groups had similar baseline characteristics. | Some concerns | 3 participants (5%) were excluded due to poor adherence. | Some concerns | Missingness is balanced between the two groups. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Lack of details about the randomisation process, no ITT analysis, important amount of missing data and only the journal article is available. | Some concerns | Lack of details about the randomisation process, some issues with ITT analysis, important amount of missing data and only the journal article is available. |
| Ramirez Sarmiento 2002 | Some concerns | Randomisation was reported only as a sentence, and no differences between the groups at visual inspection. | High risk of bias | 12% of the participants were excluded. | Low risk of bias | Missingness is independent on its true value. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, only the journal article is available and ITT analysis was not conduted appropriately. |
| Subgroup 2.16.3 Long‐term (≥8 weeks) | ||||||||||||
| Beckerman 2005 | Some concerns | No information about allocation concealment, and the two groups had similar baseline characteristics. | High risk of bias | No reason for drop out were reported for five participants (11%). | High risk of bias | 11 participants dropped out (6 died). However, No reasons for drop out were reported for the remaining participants. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available. | High risk of bias | No information about allocation concealment or a trial register. 26% of the participants dropped out of the study with no reason reported for 11%. |
| Berton 2015 | Some concerns | No information about allocation concealment. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | Missingness is independent on its true value. | Low risk of bias | Outcome assessors were blinded. | Some concerns | No information if adjusted analysis were planned in advance. | Some concerns | No information about allocation concealment and if adjusted analysis were planned in advance. |
| Chuang 2017 | Some concerns | Only a statement about randomisation was reported, and the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | The reason of missingness was loss of contact. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, and only the journal article is available. |
| Covey 2001 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Some concerns | No ITT analysis were conducted, with no impact on the results. | Some concerns | Missingness is balanced between the two groups | Low risk of bias | Observer reported outcome that do not involve judgements, and the method of measurement was valid. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, no ITT was conducted, missingness could depend on its true value, and only the journal article is available. |
| Cutrim 2019 | Low risk of bias | The sequence was random, concealed, and the two groups had similar baseline characteristics. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | No missing data. | Low risk of bias | Observer‐reported outcome that do not involves judgements, and the measurement was valid. | Low risk of bias | No differences between the trial register and the journal article | Low risk of bias | No detected issues with the five domains. |
| Dacha 2019 | Low risk of bias | From personal communication with the trialist, the suequence was random, concealed, and the groups had similar baseline characteristics. | Low risk of bias | From personal communication with the trialist, ITT was conducted. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the abstract and the trial register are available (study ongoing) | Some concerns | Only the abstract and the trial register are available (ongoing study). |
| Harver 1989 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | High risk of bias | Missingness in each group was not explained. | Low risk of bias | Observer reported outcome that do not involve judgement, and the method of measurement was valid. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, missingness, and only the journal article is available. |
| Heijdra 1996 | Some concerns | Randomisation was reported only as a sentence. | Low risk of bias | Participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | The method of measurement was correct and it is an observer‐reported outcome that do not involve judgements. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, and only the journal article is available. |
| Hill 2006 | Some concerns | No information about allocation concealment. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | The data of only two participants (5%) are missing. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about allocation concealment, and only the journal article is available. |
| Hsiao 2003 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | High risk of bias | 10% of participants were excluded (per protocol analysis). | Some concerns | Missingness is unlikely to be dependant on its true value. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process and only the journal article is available. There are serious issues with ITT and missingness is likely to be dependant on the outcome. |
| Hsiao 2003 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | High risk of bias | 10% of participants were excluded (per protocol analysis). | Some concerns | Missingness is unlikely to be dependant on its true value. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process and only the journal article is available. There are serious issues with ITT and missingness is likely to be dependant on the outcome. |
| Kim 1993 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | Participants were analysed according to their initial allocation. | Some concerns | Missingness is balanced between the two groups. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, only the journal article is available and missingness could depend on the outcome. |
| Langer 2018 | Low risk of bias | Allocation was random, concealed, and baseline characteristics were similar between the groups. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded, and the method of measurement was valid. | Low risk of bias | No differences between the trial register, the abstracts, and the journal article. | Low risk of bias | No detected issues with the five domains. |
| Larson 1988 | Low risk of bias | Allocation sequence was concealed, and both groups had similar baseline characteristics. | High risk of bias | No ITT analysis. | Some concerns | Reasons for drop out were not reported, but the proportion of missing data is balanced between the two groups. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Some participants were excluded with no reasons provided, no ITT was conducted missingness is unlikely to affect the outcome, and only the trial register is available. |
| Larson 1999 | Some concerns | Randomisation was reported only as a sentence. | Some concerns | It is unclear if the reasons of exclusion might have an impact on the results. | High risk of bias | some of the reasons of missingness are: lack of interest, inability to perform training, poor adherence. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, and only the trial register is available. There is some concern with ITT and missingness is likely to be dependant on the outcome. |
| Lisboa 1997 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about allocation concealment and only the journal article is available. |
| Petrovic 2012 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Observer reported outcome that do not involve judgements, and the method of measurement is valid. | Some concerns | No additional information were provided in the trial register. | Some concerns | Lack of details about the randomisation process and the trial register does not provide supplementary information. |
| Preusser 1994 | Some concerns | No information about allocation concealment, and both groups had similar baseline characteristics. | Low risk of bias | No participants were excluded. | Low risk of bias | Missingness is independent on its true value. | Low risk of bias | Observer reported outcome that do not involve judgement | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process and only the journal article is available. |
| Ramirez Sarmiento 2002 | Some concerns | Randomisation was reported only as a sentence, and no differences between the groups at visual inspection. | High risk of bias | 12% of the participants were excluded. | Low risk of bias | Missingness is independent on its true value. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, only the journal article is available and ITT analysis was not conduted appropriately. |
| Saka 2021 | Some concerns | Lack of details about allocation concealment | Low risk of bias | All participants were analysed according to their initial allocation | Low risk of bias | Missingness is independant on its true value | Low risk of bias | The outcome was measured according to international guidelines | Some concerns | The trial register did not provide sufficient information | Some concerns | Lack of details about the randomisation process, and the trial register did not provide sufficient information. |
| Sanchez Riera 2001 | Some concerns | Randomisation was reported only as a sentence, and baseline characteristics were similar between the groups. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process and only the journal article is available. |
| Scherer 2000 | Some concerns | No information about allocation concealment, and both groups had similar baseline characteristics. | Low risk of bias | One participant excluded due to rip fracture. | Some concerns | Missingness was balanced between the two groups | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, only the journal article is available and missingness could be dependent on its true value. |
| Weiner 2003 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | High risk of bias | data in the graph are different from what was reported in the text. | High risk of bias | Lack of details about the randomisation process, only the journal article is available and data in the graphs are different from the text. |
| Weiner 2006 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar PImax level. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process and only the journal article is available. |
| Wu 2017 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Observer reported outcome that do not involve judgements, and the method of measurement was valid. | Some concerns | There were some differences between the trial register and the journal article, without biasing the results. | Some concerns | Lack of details about the randomisation process and there are differences in reporting blinding between the trial register and journal article. |
| Wu 2017 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Observer reported outcome that do not involve judgements, and the method of measurement was valid. | Some concerns | There were some differences between the trial register and the journal article, without biasing the results. | Some concerns | Lack of details about the randomisation process and there are differences in reporting blinding between the trial register and journal article. |
| Xu 2018 | Low risk of bias | The sequence was random, concealed, and the two groups had similar baseline characteristics. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Missingness is independent on its true value. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | No differences between the trial register, the conference abstracts and the journal article. | Low risk of bias | No detected issue with the five domains. |
| ZhouL 2016 | Low risk of bias | Allocation was concealed. | Some concerns | 3 participants were excluded (6%) | Some concerns | No details of the reasons of missingness, but its proportion is small (6%). | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available. | Some concerns | Some issues with ITT analysis, missingness could be dependant on its true value, and only the journal article is available. |
Risk of bias for analysis 2.17 Inspiratory muscle strength: PImax (cmH2O) (subgroup analysis: with or without respiratory muscle weakness).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 2.17.1 With respiratory muscle weakness | ||||||||||||
| Chuang 2017 | Some concerns | Only a statement about randomisation was reported, and the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | The reason of missingness was loss of contact. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, and only the journal article is available. |
| Harver 1989 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | High risk of bias | Missingness in each group was not explained. | Low risk of bias | Observer reported outcome that do not involve judgement, and the method of measurement was valid. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, missingness, and only the journal article is available. |
| Leelarungrayub 2017 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | No participants were excluded. | High risk of bias | 6 participants discontinued training, but there is no mention of the proportion of missingness in each group. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process and only the journal article is available. Missingness may be dependant on its true value. |
| Preusser 1994 | Some concerns | No information about allocation concealment, and both groups had similar baseline characteristics. | Low risk of bias | No participants were excluded. | Low risk of bias | Missingness is independent on its true value. | Low risk of bias | Observer reported outcome that do not involve judgement | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process and only the journal article is available. |
| Saher 2021 | Some concerns | Randomisation was reported only as a sentence. Authors used concealed envelopes without mentioning if they were opaque | High risk of bias | ITT analysis was not applied | Some concerns | Missingness could depend on its true value | Low risk of bias | Observer reported outcome that do not involve judgement | Some concerns | Only the journal article is available | High risk of bias | Lack of details about the randomisation process, only the journal article is available, missingness could depend on its true value, and ITT analysis was not conducted. |
| Sanchez Riera 2001 | Some concerns | Randomisation was reported only as a sentence, and baseline characteristics were similar between the groups. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process and only the journal article is available. |
| Weiner 2006 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar PImax level. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process and only the journal article is available. |
| Wu 2017 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Observer reported outcome that do not involve judgements, and the method of measurement was valid. | Some concerns | There were some differences between the trial register and the journal article, without biasing the results. | Some concerns | Lack of details about the randomisation process and there are differences in reporting blinding between the trial register and journal article. |
| Wu 2017 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Observer reported outcome that do not involve judgements, and the method of measurement was valid. | Some concerns | There were some differences between the trial register and the journal article, without biasing the results. | Some concerns | Lack of details about the randomisation process and there are differences in reporting blinding between the trial register and journal article. |
| Xu 2018 | Low risk of bias | The sequence was random, concealed, and the two groups had similar baseline characteristics. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Missingness is independent on its true value. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | No differences between the trial register, the conference abstracts and the journal article. | Low risk of bias | No detected issue with the five domains. |
| ZhouL 2016 | Low risk of bias | Allocation was concealed. | Some concerns | 3 participants were excluded (6%) | Some concerns | No details of the reasons of missingness, but its proportion is small (6%). | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available. | Some concerns | Some issues with ITT analysis, missingness could be dependant on its true value, and only the journal article is available. |
| Subgroup 2.17.2 Without respiratory muscle weakness | ||||||||||||
| Beckerman 2005 | Some concerns | No information about allocation concealment, and the two groups had similar baseline characteristics. | High risk of bias | No reason for drop out were reported for five participants (11%). | High risk of bias | 11 participants dropped out (6 died). However, No reasons for drop out were reported for the remaining participants. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available. | High risk of bias | No information about allocation concealment or a trial register. 26% of the participants dropped out of the study with no reason reported for 11%. |
| Belman 1988 | Some concerns | Randomisation was reported only as a sentence. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | missing data are due to intercurrent illnessess and pneumothorax. | Low risk of bias | Observed‐reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about allocation concealment and only the journal article is available. |
| Berton 2015 | Some concerns | No information about allocation concealment. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | Missingness is independent on its true value. | Low risk of bias | Outcome assessors were blinded. | Some concerns | No information if adjusted analysis were planned in advance. | Some concerns | No information about allocation concealment and if adjusted analysis were planned in advance. |
| Bustamante 2007 | Some concerns | Only a statement about randomisation was reported, the two groups had similar baseline characteristics. | Low risk of bias | No participants were excluded. | Low risk of bias | No missing data. | Low risk of bias | Observer‐reported outcome that do not involve judgements, and the method of measurement was valid. | Some concerns | Only the journal article is available. | Some concerns | No details about allocation concealment, randomisation and only the journal article is available. |
| Covey 2001 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Some concerns | No ITT analysis were conducted, with no impact on the results. | Some concerns | Missingness is balanced between the two groups | Low risk of bias | Observer reported outcome that do not involve judgements, and the method of measurement was valid. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, no ITT was conducted, missingness could depend on its true value, and only the journal article is available. |
| Dacha 2019 | Low risk of bias | From personal communication with the trialist, the suequence was random, concealed, and the groups had similar baseline characteristics. | Low risk of bias | From personal communication with the trialist, ITT was conducted. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the abstract and the trial register are available (study ongoing) | Some concerns | Only the abstract and the trial register are available (ongoing study). |
| Hill 2006 | Some concerns | No information about allocation concealment. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | The data of only two participants (5%) are missing. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about allocation concealment, and only the journal article is available. |
| Hsiao 2003 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | High risk of bias | 10% of participants were excluded (per protocol analysis). | Some concerns | Missingness is unlikely to be dependant on its true value. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process and only the journal article is available. There are serious issues with ITT and missingness is likely to be dependant on the outcome. |
| Kim 1993 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | Participants were analysed according to their initial allocation. | Some concerns | Missingness is balanced between the two groups. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, only the journal article is available and missingness could depend on the outcome. |
| Koppers 2006 | Some concerns | Randomisation was reported only as a sentence, the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | 7% of the data are missing (3 participants out of 39). | Low risk of bias | Observer reported outcome that do not involve judgements. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process and only the journal article is available. |
| Langer 2018 | Low risk of bias | Allocation was random, concealed, and baseline characteristics were similar between the groups. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded, and the method of measurement was valid. | Low risk of bias | No differences between the trial register, the abstracts, and the journal article. | Low risk of bias | No detected issues with the five domains. |
| Larson 1999 | Some concerns | Randomisation was reported only as a sentence. | Some concerns | It is unclear if the reasons of exclusion might have an impact on the results. | High risk of bias | some of the reasons of missingness are: lack of interest, inability to perform training, poor adherence. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, and only the trial register is available. There is some concern with ITT and missingness is likely to be dependant on the outcome. |
| Lisboa 1997 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about allocation concealment and only the journal article is available. |
| Nikoletou 2016 | Some concerns | No information about allocation concealment, and the two groups had similar baseline characteristics. | Some concerns | 3 participants (5%) were excluded due to poor adherence. | Some concerns | Missingness is balanced between the two groups. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Lack of details about the randomisation process, no ITT analysis, important amount of missing data and only the journal article is available. | Some concerns | Lack of details about the randomisation process, some issues with ITT analysis, important amount of missing data and only the journal article is available. |
| Petrovic 2012 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Observer reported outcome that do not involve judgements, and the method of measurement is valid. | Some concerns | No additional information were provided in the trial register. | Some concerns | Lack of details about the randomisation process and the trial register does not provide supplementary information. |
| Ramirez Sarmiento 2002 | Some concerns | Randomisation was reported only as a sentence, and no differences between the groups at visual inspection. | High risk of bias | 12% of the participants were excluded. | Low risk of bias | Missingness is independent on its true value. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, only the journal article is available and ITT analysis was not conduted appropriately. |
| Saka 2021 | Some concerns | Lack of details about allocation concealment | Low risk of bias | All participants were analysed according to their initial allocation | Low risk of bias | Missingness is independent on its true value | Low risk of bias | The outcome was measured according to international guidelines | Some concerns | Lack of details in the trial register | Some concerns | Lack of details about the randomisation process, and the trial register did not provide sufficient information. |
| Scherer 2000 | Some concerns | No information about allocation concealment, and both groups had similar baseline characteristics. | Low risk of bias | One participant excluded due to rip fracture. | Some concerns | Missingness was balanced between the two groups | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, only the journal article is available and missingness could be dependent on its true value. |
| Xu 2018 | Low risk of bias | The sequence was random, concealed, and the two groups had similar baseline characteristics. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Missingness is independent on its true value. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | No differences between the trial register, the conference abstracts and the journal article. | Low risk of bias | No detected issue with the five domains. |
Risk of bias for analysis 2.18 Inspiratory muscle strength: PImax (cmH2O) (subgroup analysis: method of measurement).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 2.18.1 Residual Volume (RV) | ||||||||||||
| Beckerman 2005 | Some concerns | No information about allocation concealment, and the two groups had similar baseline characteristics. | High risk of bias | No reason for drop out were reported for five participants (11%). | High risk of bias | 11 participants dropped out (6 died). However, No reasons for drop out were reported for the remaining participants. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available. | High risk of bias | No information about allocation concealment or a trial register. 26% of the participants dropped out of the study with no reason reported for 11%. |
| Covey 2001 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Some concerns | No ITT analysis were conducted, with no impact on the results. | Some concerns | Missingness is balanced between the two groups | Low risk of bias | Observer reported outcome that do not involve judgements, and the method of measurement was valid. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, no ITT was conducted, missingness could depend on its true value, and only the journal article is available. |
| Cutrim 2019 | Low risk of bias | The sequence was random, concealed, and the two groups had similar baseline characteristics. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | No missing data. | Low risk of bias | Observer‐reported outcome that do not involves judgements, and the measurement was valid. | Low risk of bias | No differences between the trial register and the journal article | Low risk of bias | No detected issues with the five domains. |
| Heijdra 1996 | Some concerns | Randomisation was reported only as a sentence. | Low risk of bias | Participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | The method of measurement was correct, and it is an observer reported outcome that do not involve judgements. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randmisation process, and only the journal article is available. |
| Hsiao 2003 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | High risk of bias | 10% of participants were excluded (per protocol analysis). | Some concerns | Missingness is unlikely to be dependant on its true value. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process and only the journal article is available. There are serious issues with ITT and missingness is likely to be dependant on the outcome. |
| Hsiao 2003 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | High risk of bias | 10% of participants were excluded (per protocol analysis). | Some concerns | Missingness is unlikely to be dependant on its true value. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process and only the journal article is available. There are serious issues with ITT and missingness is likely to be dependant on the outcome. |
| Kim 1993 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | Participants were analysed according to their initial allocation. | Some concerns | Missingness is balanced between the two groups. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, only the journal article is available and missingness could depend on the outcome. |
| Koppers 2006 | Some concerns | Randomisation was reported only as a sentence, the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | 7% of the data are missing (3 participants out of 39). | Low risk of bias | Observer reported outcome that do not involve judgements. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process and only the journal article is available. |
| Larson 1988 | Low risk of bias | Allocation sequence was concealed, and both groups had similar baseline characteristics. | High risk of bias | 4 participants out of 45 were discarded (around 8%), and also two participants were not reported although they completed the training. | Some concerns | Reasons for drop out were not reported, but the proportion of missing data is balanced between the two groups. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Some participants were excluded with no provided reasons, no ITT was conducted, missingness is unlikely to affect the outcome, and only the trial register is available. |
| Larson 1999 | Some concerns | Randomisation was reported only as a sentence. | Some concerns | It is unclear if the reasons of exclusion might have an impact on the results. | High risk of bias | some of the reasons of missingness are: lack of interest, inability to perform training, poor adherence. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, and only the trial register is available. There is some concern with ITT and missingness is likely to be dependant on the outcome. |
| Leelarungrayub 2017 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | No participants were excluded. | High risk of bias | 6 participants discontinued training, but there is no mention of the proportion of missingness in each group. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process and only the journal article is available. Missingness may be dependant on its true value. |
| Saher 2021 | Some concerns | Randomisation was reported only as a sentence. Authors used concealed envelopes without mentioning if they were opaque | High risk of bias | ITT analysis was not applied | Some concerns | Missingness could depend on its true value | Low risk of bias | Observer reported outcome that do not involve judgement | Some concerns | Only the journal article is available | High risk of bias | Lack of details about the randomisation process, only the journal article is available, missingness could depend on its true value, and ITT analysis was not conducted. |
| Scherer 2000 | Some concerns | No information about allocation concealment, and both groups had similar baseline characteristics. | Low risk of bias | One participant excluded due to rip fracture. | Some concerns | Missingness was balanced between the two groups | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, only the journal article is available and missingness could be dependent on its true value. |
| Weiner 2003 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | High risk of bias | data in the graph are different from what was reported in the text. | High risk of bias | Lack of details about the randomisation process, only the journal article is available and data in the graphs are different from the text. |
| Weiner 2006 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar PImax level. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process and only the journal article is available. |
| Wu 2017 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Observer reported outcome that do not involve judgements, and the method of measurement was valid. | Some concerns | There were some differences between the trial register and the journal article, without biasing the results. | Some concerns | Lack of details about the randomisation process and there are differences in reporting blinding between the trial register and journal article. |
| Wu 2017 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Observer reported outcome that do not involve judgements, and the method of measurement was valid. | Some concerns | There were some differences between the trial register and the journal article, without biasing the results. | Some concerns | Lack of details about the randomisation process and there are differences in reporting blinding between the trial register and journal article. |
| Subgroup 2.18.2 Functional Residual Capacity (FRC) | ||||||||||||
| Belman 1988 | Some concerns | Randomisation was reported only as a sentence. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | missing data are due to intercurrent illnessess and pneumothorax. | Low risk of bias | Observed‐reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about allocation concealment and only the journal article is available. |
| Harver 1989 | Some concerns | Randomisation was reported only as a sentence, and both groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | High risk of bias | Missingness in each group was not explained. | Low risk of bias | Observer reported outcome that do not involve judgement, and the method of measurement was valid. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, missingness, and only the journal article is available. |
| Hill 2006 | Some concerns | No information about allocation concealment. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | The data of only two participants (5%) are missing. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about allocation concealment, and only the journal article is available. |
| Langer 2018 | Low risk of bias | Allocation was random, concealed, and baseline characteristics were similar between the groups. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded, and the method of measurement was valid. | Low risk of bias | No differences between the trial register, the abstracts, and the journal article. | Low risk of bias | No detected issues with the five domains. |
| Lisboa 1997 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about allocation concealment and only the journal article is available. |
| Nikoletou 2016 | Some concerns | No information about allocation concealment, and the two groups had similar baseline characteristics. | Some concerns | 3 participants (5%) were excluded due to poor adherence. | Some concerns | Missingness is balanced between the two groups. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Lack of details about the randomisation process, no ITT analysis, important amount of missing data and only the journal article is available. | Some concerns | Lack of details about the randomisation process, some issues with ITT analysis, important amount of missing data and only the journal article is available. |
| Petrovic 2012 | Some concerns | Randomisation was reported only as a sentence, and the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Observer reported outcome that do not involve judgements, and the method of measurement is valid. | Some concerns | No additional information were provided in the trial register. | Some concerns | Lack of details about the randomisation process and the trial register does not provide supplementary information. |
| Preusser 1994 | Some concerns | No information about allocation concealment, and both groups had similar baseline characteristics. | Low risk of bias | No participants were excluded. | Low risk of bias | Missingness is independent on its true value. | Low risk of bias | Observer reported outcome that do not involve judgement | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process and only the journal article is available. |
| Sanchez Riera 2001 | Some concerns | Randomisation was reported only as a sentence, and baseline characteristics were similar between the groups. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process and only the journal article is available. |
| Subgroup 2.18.3 Not reported | ||||||||||||
| Berton 2015 | Some concerns | No information about allocation concealment. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | Missingness is independent on its true value. | Low risk of bias | Outcome assessors were blinded. | Some concerns | No information if adjusted analysis were planned in advance. | Some concerns | No information about allocation concealment and if adjusted analysis were planned in advance. |
| Bustamante 2007 | Some concerns | Only a statement about randomisation was reported, the two groups had similar baseline characteristics. | Low risk of bias | No participants were excluded. | Low risk of bias | No missing data. | Low risk of bias | Observer‐reported outcome that do not involve judgements, and the method of measurement was valid. | Some concerns | Only the journal article is available. | Some concerns | No details about allocation concealment, randomisation and only the journal article is available. |
| Chuang 2017 | Some concerns | Only a statement about randomisation was reported, and the two groups had similar baseline characteristics. | Low risk of bias | All participants were analysed according to their initial allocation. | Low risk of bias | The reason of missingness was loss of contact. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | Some concerns | Lack of details about the randomisation process, and only the journal article is available. |
| Dacha 2019 | Low risk of bias | From personal communication with the trialist, the suequence was random, concealed, and the groups had similar baseline characteristics. | Low risk of bias | From personal communication, ITT analysis was conducted. | Low risk of bias | No missing data. | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the abstract and the trial register are available (study ongoing) | Some concerns | Only the abstract and the trial register are available (ongoing study). |
| Ramirez Sarmiento 2002 | Some concerns | Randomisation was reported only as a sentence, and no differences between the groups at visual inspection. | High risk of bias | 12% of the participants were excluded. | Low risk of bias | Missingness is independent on its true value. | Low risk of bias | Observer reported outcome that do not involve judgement. | Some concerns | Only the journal article is available. | High risk of bias | Lack of details about the randomisation process, only the journal article is available and ITT analysis was not conduted appropriately. |
| Saka 2021 | Some concerns | Lack of details about allocation concealment | Low risk of bias | All participants were analysed according to their intial allocation | Low risk of bias | Missingness is independent on its true value | Low risk of bias | The outcome was measured according to international guidelines | Some concerns | Lack of details in the trial register | Some concerns | Lack of details about the randomisation process, and the trial register did not provide sufficient information. |
| Xu 2018 | Low risk of bias | The sequence was random, concealed, and the two groups had similar baseline characteristics. | Low risk of bias | ITT analysis was conducted. | Low risk of bias | Missingness is independent on its true value. | Low risk of bias | Outcome assessors were blinded. | Low risk of bias | No differences between the trial register, the conference abstracts and the journal article. | Low risk of bias | No detected issue with the five domains. |
| ZhouL 2016 | Low risk of bias | Allocation was concealed. | Some concerns | 3 participants were excluded (6%) | Some concerns | No details of the reasons of missingness, but its proportion is small (6%). | Low risk of bias | Outcome assessors were blinded. | Some concerns | Only the journal article is available. | Some concerns | Some issues with ITT analysis, missingness could be dependant on its true value, and only the journal article is available. |
Acknowledgements
We acknowledge Dr. Rujan Shrestha, Medical Officer, Nepal and Dr Karla Elizabeth Duque Jacome, Ecuador for their translating support.
We thank the staff of Cochrane Airways, especially Emma Jackson (Managing Editor), Emma Dennett (Deputy Co‐ordinating Editor) and Elizabeth Stovold (Information Specialist) for their support.
The authors and Cochrane Airways Editorial Team are grateful to the following peer and consumer reviewers for their time and comments: Julia Robertson (Australia), Lissa M Spencer (Australia) and Elena Gimeno‐Santos (Spain).
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways.
This project was supported by the National Institute for Health and Care Research (NIHR), via Cochrane Infrastructure funding to Cochrane Airways. The views and opinions expressed here are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health and Social Care.
Appendices
Appendix 1. Database search strategies
| Database/search platform/date of last search | Search strategy | Results |
|
Airways Register (via Cochrane Register of Studies)
Date of most recent search: 19 October 2022 |
1 MeSH DESCRIPTOR Pulmonary Disease, Chronic Obstructive Explode All AND INSEGMENT 2 MeSH DESCRIPTOR Bronchitis, Chronic AND INSEGMENT 3 (obstruct*) near3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*) AND INSEGMENT 4 COPD:MISC1 AND INSEGME 5 (COPD OR AECOPD):TI,AB,KW AND INSEGMENT 6 #1 OR #2 OR #3 OR #4 OR #5 AND INSEGMENT 7 MESH DESCRIPTOR Breathing Exercises AND INSEGMENT 8 MESH DESCRIPTOR Respiratory Muscles EXPLODE ALL AND INSEGMENT 9 MESH DESCRIPTOR Exercise Tolerance AND INSEGMENT 10 (IMT or RMT):ti,ab AND INSEGMENT 11 ((inspiratory or ventilat* or respiratory) NEAR3 (muscle or resistance) NEAR3 (train* or strength* or endur*)) AND INSEGMENT 12 (threshold NEAR2 (load or device*)) AND INSEGMENT 13 (resist* NEAR2 device*) AND INSEGMENT 14 isocapnic hyperpnea AND INSEGMENT 15 #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #14 OR #13 16 #6 AND #15 17 INREGISTER 18 #16 AND #17 |
Oct 2020=937 Aug 2021=44 Oct 2022=265 ( together with CENTRAL) |
|
CENTRAL (via Cochrane Register of Studies)
Date of most recent search: 19 October 2022 |
1 MeSH DESCRIPTOR Pulmonary Disease, Chronic Obstructive Explode All AND CENTRAL:TARGET 2 MeSH DESCRIPTOR Bronchitis, Chronic AND CENTRAL:TARGET 3 (obstruct*) near3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*) AND CENTRAL:TARGET 4 COPD:MISC1 AND CENTRAL:TARGET 5 (COPD OR AECOPD):TI,AB,KW AND CENTRAL:TARGET 6 #1 OR #2 OR #3 OR #4 OR #5 AND CENTRAL:TARGET 7 MESH DESCRIPTOR Breathing Exercises AND CENTRAL:TARGET 8 MESH DESCRIPTOR Respiratory Muscles EXPLODE ALL AND CENTRAL:TARGET 9 MESH DESCRIPTOR Exercise Tolerance AND CENTRAL:TARGET 10 (IMT or RMT):ti,ab AND CENTRAL:TARGET 11 ((inspiratory or ventilat* or respiratory) NEAR3 (muscle or resistance) NEAR3 (train* or strength* or endur*)) AND CENTRAL:TARGET 12 (threshold NEAR2 (load or device*)) AND CENTRAL:TARGET 13 (resist* NEAR2 device*) AND CENTRAL:TARGET 14 isocapnic hyperpnea AND CENTRAL:TARGET 15 #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #14 OR #13 AND CENTRAL:TARGET 16 #6 AND #15 AND CENTRAL:TARGET 17 CENTRAL:TARGET 18 #16 AND #17 AND CENTRAL:TARGET | Oct 2020=1239 Aug 2021=69 Oct 2022=265 ( together with the Airways Register) |
|
MEDLINE (Ovid) ALL
Date of most recent search: 20 October 2022 |
1 Lung Diseases, Obstructive/ 2 exp Pulmonary Disease, Chronic Obstructive/ 3 emphysema$.tw. 4 (chronic$ adj3 bronchiti$).tw. 5 (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).tw. 6 (COPD or AECOPD or AECB).ti,ab. 7 or/1‐6 8 Breathing Exercises/ 9 exp Respiratory Muscles/ 10 Exercise Tolerance/ 11 Muscle Strength/ 12 (IMT or RMT).ti,ab. 13 ((inspiratory or ventilat$ or respiratory) adj3 (muscle or resistance) adj3 (train$ or strength$ or endur$)).tw. 14 (threshold adj2 (load or device$)).tw. 15 (resist$ adj2 device$).tw. 16 isocapnic hyperpnea.tw. 17 or/8‐16 18 (controlled clinical trial or randomized controlled trial).pt. 19 (randomized or randomised).ab,ti. 20 placebo.ab,ti. 21 randomly.ab,ti. 22 trial.ab,ti. 23 groups.ab,ti. 24 or/18‐23 25 Animals/ 26 Humans/ 27 25 not (25 and 26) 28 24 not 27 29 7 and 17 and 28 | Oct 2020=1609 Aug 2021=86 Oct 2022=172 |
|
Embase (Ovid)
Date of most recent search: 20 October 2022 |
1 Chronic Obstructive Lung Disease/ 2 Obstructive Airway Disease/ 3 Chronic Bronchitis/ 4 Lung Emphysema/ 5 emphysema$.tw. 6 (chronic$ adj3 bronchiti$).tw. 7 (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).tw. 8 (COPD or AECOPD or AECB).ti,ab. 9 or/1‐8 10 breathing exercise/ 11 breathing muscle/ 12 exercise tolerance/ 13 muscle strength/ 14 (IMT or RMT).ti,ab. 15 ((inspiratory or ventilat$ or respiratory) adj3 (muscle or resistance) adj3 (train$ or strength$ or endur$)).tw. 16 (threshold adj2 (load or device$)).tw. 17 (resist$ adj2 device$).tw. 18 isocapnic hyperpnea.tw. 19 or/10‐18 20 Randomized Controlled Trial/ 21 randomization/ 22 controlled clinical trial/ 23 Double Blind Procedure/ 24 Single Blind Procedure/ 25 Crossover Procedure/ 26 (clinica$ adj3 trial$).tw. 27 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (mask$ or blind$ or method$)).tw. 28 exp Placebo/ 29 placebo$.ti,ab. 30 random$.ti,ab. 31 ((control$ or prospectiv$) adj3 (trial$ or method$ or stud$)).tw. 32 (crossover$ or cross‐over$).ti,ab. 33 or/20‐32 34 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 35 human/ or normal human/ or human cell/ 36 34 and 35 37 34 not 36 38 33 not 37 39 9 and 19 and 38 | Oct 2020=2093 Aug 2021=146 Oct 2022=251 |
| CINHAL (EBSCO) Date of most recent search: 19 October 2022 | S41 S7 AND S17 AND S40 S40 S39 NOT S38 S39 S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 S38 S36 NOT S37 S37 (MH "Human") S36 S33 OR S34 OR S35 S35 TI (animal model*) S34 (MH "Animal Studies") S33 (MH "Animals+") S32 AB (cluster W3 RCT) S31 MH (crossover design) OR MH (comparative studies) S30 AB (control W5 group) S29 PT (Randomized Controlled Trial) S28 (MH "Placebos") S27 MH ("sample size") AND AB (assigned OR allocated OR control) S26 TI (trial) S25 AB (random*) S24 TI (randomised OR randomized) S23 (MH "Cluster Sample") S22 (MH "Pretest‐Posttest Design") S21 (MH "Random Assignment") S20 (MH "Single‐Blind Studies") S19 (MH "Double‐Blind Studies") S18 (MH "Randomized Controlled Trials") S17 S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 S16 isocapnic hyperpnea S15 resist* N2 device* S14 threshold* N2 (load or device*) S13 (inspiratory or ventilat* or respiratory) N3 (muscle or resistance) N3 (train* or strength* or endur*) S12 AB (IMT or RMT) or TI (IMT or RMT) S11 (MH "Muscle Strength") S10 (MH "Exercise Tolerance" S9 (MH "Respiratory Muscles+") S8 (MH "Breathing Exercises") S7 S1 or S2 or S3 or S4 or S5 or S6 S6 COPD or COAD or COBD or AECB S5 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*) and (obstruct*) and (disease*) S4 chronic bronchitis S3 "emphysema*" S2 (MH "Lung Diseases, Obstructive") S1 (MH "Pulmonary Disease, Chronic Obstructive+") | Oct 2020=182
Aug 2021=22 Oct 2022=76 |
|
PsycINFO (Ovid) Date of most recent search: 19 October 2022 |
1 exp Chronic Obstructive Pulmonary Disease/ 2 emphysema$.tw. 3 (chronic$ adj3 bronchiti$).tw. 4 (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).tw. 5 (COPD or AECOPD or AECB).ti,ab. 6 or/1‐5 7 ((inspiratory or ventilat$ or respiratory) adj3 (muscle or resistance) adj3 (train$ or strength$ or endur$)).tw. 8 (IMT or RMT).ti,ab. 9 (threshold adj2 (load or device$)).tw. 10 (resist$ adj2 device$).tw. 11 isocapnic hyperpnea.tw. 12 or/7‐11 13 random$.tw. 14 (clinical adj5 trial$).tw. 15 (control$ adj5 trial$).tw. 16 ((clinical or control$ or comparativ$) adj5 (study or studies)).tw. 17 placebo$.tw. 18 (single blind$ or single‐blind$).tw. 19 (double blind$ or double‐blind$).tw. 20 (triple blind$ or triple‐blind$).tw. 21 or/13‐20 22 6 and 12 and 21 |
Oct 2020=10
August 2021=1 Oct 2022=3 |
| PEDro Date of most recent search: 20 October 2022 | Abstract & title: inspiratory muscle copd random* Topic: chronic respiratory disease Method: Clinical trial When searching: Match all search terms (AND) |
Oct 2020=81
Aug 2021=1 Oct 2022=5 |
| ClinicalTrials.gov Date of most recent search: 20 October 2022 |
Study type: Interventional
Condition: COPD Intervention: inspiratory muscle training OR threshold load OR threshold device OR resistive device |
Oct 2020=58 Aug 2021=6 Oct 2022=12 |
|
WHO trials portal
Date of most recent search: 20 October 2022 |
Condition: COPD Intervention: inspiratory muscle training OR threshold load OR threshold device OR resistive device |
Oct 2020=347 Aug 2021=2 Oct 2022=9 |
Data and analyses
Comparison 1. PR+IMT vs PR.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Dyspnea: Borg (at submaximal exercise: 50% to 80% of Wmax) | 2 | 202 | Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.42, 0.79] |
| 1.2 Dyspnea: Modified Medical Research Council (mMRC) | 2 | 204 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.39, 0.14] |
| 1.3 Functional exercise capacity: 6‐minute walk distance (6MWD) (meters) | 12 | 1199 | Mean Difference (IV, Random, 95% CI) | 5.95 [‐5.73, 17.63] |
| 1.4 Functional exercise capacity: 6‐minute walk distance (6MWD) (meters) (subgroup analysis: duration of the intervention) | 12 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.4.1 Short‐term (<4 weeks) | 4 | 687 | Mean Difference (IV, Random, 95% CI) | 10.25 [‐24.98, 45.49] |
| 1.4.2 Medium‐term ( ≥4 weeks and <8 weeks) | 2 | 178 | Mean Difference (IV, Random, 95% CI) | 36.72 [‐67.67, 141.11] |
| 1.4.3 Long‐term ( ≥8 weeks) | 7 | 363 | Mean Difference (IV, Random, 95% CI) | 7.82 [‐6.90, 22.54] |
| 1.5 Functional exercise capacity: 6‐minute walk distance (6MWD) (meters) (subgroup analysis: with or without respiratory muscle weakness) | 12 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.5.1 With respiratory muscle weakness | 3 | 219 | Mean Difference (IV, Random, 95% CI) | 31.17 [‐10.50, 72.84] |
| 1.5.2 Without respiratory muscle weakness | 9 | 981 | Mean Difference (IV, Random, 95% CI) | 0.38 [‐11.65, 12.42] |
| 1.6 Functional exercise capacity: 12‐minute walk distance (12MWD) (meters) | 3 | 80 | Mean Difference (IV, Random, 95% CI) | 155.77 [‐84.53, 396.08] |
| 1.7 Functional exercise capacity: Wmax (watt) | 5 | 326 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐6.96, 4.94] |
| 1.8 Functional exercise capacity: exercise time (seconds) | 3 | 192 | Mean Difference (IV, Random, 95% CI) | 58.62 [‐25.09, 142.32] |
| 1.9 Health‐related quality of life (HRQoL): St George's Respiratory Questionnaire (SGRQ) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.9.1 Symptoms | 2 | 169 | Mean Difference (IV, Random, 95% CI) | ‐2.33 [‐6.28, 1.62] |
| 1.9.2 Activity | 2 | 169 | Mean Difference (IV, Random, 95% CI) | 0.28 [‐1.65, 2.20] |
| 1.9.3 Impact | 2 | 169 | Mean Difference (IV, Random, 95% CI) | ‐1.63 [‐5.38, 2.11] |
| 1.9.4 Total | 7 | 908 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.93, 1.20] |
| 1.10 Health‐related quality of life (HRQoL): Chronic Respiratory Disease Questionnaire (CRQ) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.10.1 Dyspnea | 3 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐1.90, 1.29] | |
| 1.10.2 Fatigue | 3 | Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.76, 1.31] | |
| 1.10.3 Emotion | 2 | Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐2.53, 1.26] | |
| 1.10.4 Mastery | 2 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐1.18, 1.08] | |
| 1.11 Health‐related quality of life (HRQoL): COPD Assessment Test (CAT) | 2 | 657 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.80, 1.06] |
| 1.12 Inspiratory muscle strength: PImax (cmH20) | 17 | 1329 | Mean Difference (IV, Random, 95% CI) | 11.46 [7.42, 15.50] |
| 1.13 Inspiratory muscle strength: PImax (cmH2O) (subgroup analysis: duration of the intervention) | 17 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.13.1 Short‐term (<4 weeks) | 4 | 687 | Mean Difference (IV, Random, 95% CI) | 12.63 [4.14, 21.11] |
| 1.13.2 Medium‐term ( ≥ 4 weeks and <8 weeks) | 4 | 233 | Mean Difference (IV, Random, 95% CI) | 12.27 [3.75, 20.79] |
| 1.13.3 Long‐term ( ≥ 8 weeks) | 11 | 478 | Mean Difference (IV, Random, 95% CI) | 11.52 [5.50, 17.53] |
| 1.14 Inspiratory muscle strength: PImax (cmH2O) (subgroup analysis: with or without respiratory muscle weakness) | 16 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.14.1 With respiratory muscle weakness | 5 | 282 | Mean Difference (IV, Random, 95% CI) | 14.84 [11.35, 18.34] |
| 1.14.2 Without respiratory muscle weakness | 11 | 1031 | Mean Difference (IV, Random, 95% CI) | 10.57 [5.23, 15.91] |
| 1.15 Laboratory exercise test: VO2peak (L/min) | 5 | 313 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.05, 0.03] |
| 1.16 Respiratory muscle endurance: respiratory muscle endurance pressure (Pthmax) (cmH2O) | 2 | 52 | Std. Mean Difference (IV, Random, 95% CI) | 1.22 [‐0.18, 2.62] |
| 1.17 Respiratory muscle endurance time: Tlim (seconds) (sustained ventilation according to PImax) | 3 | 236 | Mean Difference (IV, Random, 95% CI) | 84.63 [‐50.77, 220.02] |
| 1.18 Respiratory muscle endurance time: Tlim (seconds) (sustained ventilation according to MVV) | 2 | 51 | Mean Difference (IV, Random, 95% CI) | 477.69 [215.43, 739.94] |
| 1.19 Maximal voluntary ventilation (MVV) | 3 | 93 | Std. Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.02, 0.83] |
| 1.20 Respiratory function: forced expiratory volume at 1 second (FEV1) (%Pred) | 6 | 173 | Mean Difference (IV, Random, 95% CI) | 0.77 [‐1.72, 3.26] |
| 1.21 Respiratory function: forced expiratory volume at 1 second (FEV1) (Liters) | 6 | 889 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.04, 0.13] |
Comparison 2. IMT vs control/sham.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Dyspnea: Borg (at submaximal exercise capacity) | 6 | 144 | Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐1.36, ‐0.51] |
| 2.2 Dyspnea: Baseline and Transition Dyspnea Indexes (BDI‐TDI) | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.2.1 Functional impairment | 3 | 95 | Mean Difference (IV, Random, 95% CI) | 0.88 [0.51, 1.25] |
| 2.2.2 Magnitude of task | 3 | 95 | Mean Difference (IV, Random, 95% CI) | 0.73 [0.35, 1.12] |
| 2.2.3 Magnitude of effort | 3 | 95 | Mean Difference (IV, Random, 95% CI) | 0.86 [0.42, 1.30] |
| 2.2.4 Focal score | 8 | 238 | Mean Difference (IV, Random, 95% CI) | 2.98 [2.07, 3.89] |
| 2.3 Dyspnea: Transition Dyspnea Index (TDI): Focal score (subgroup analysis: with or without respiratory muscle weakness) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.3.1 With respiratory muscle weakness | 4 | 152 | Mean Difference (IV, Random, 95% CI) | 3.52 [2.55, 4.49] |
| 2.3.2 Without respiratory weakness | 3 | 70 | Mean Difference (IV, Random, 95% CI) | 2.28 [1.10, 3.46] |
| 2.4 Dyspnea: Modified Medical Research Council (mMRC) | 4 | 150 | Mean Difference (IV, Fixed, 95% CI) | ‐0.59 [‐0.76, ‐0.43] |
| 2.5 Functional exercise capacity: 6‐minute walk distance (6MWD) (meters) | 16 | 501 | Mean Difference (IV, Random, 95% CI) | 35.71 [25.68, 45.74] |
| 2.6 Functional exercise capacity: 6‐minute walk distance (6MWD) (meters) (subgroup analysis: duration of the intervention) | 16 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.6.1 Short‐term (<4 weeks) | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 33.06 [23.05, 43.07] |
| 2.6.2 Medium‐term (≥4 weeks and <8 weeks) | 4 | 131 | Mean Difference (IV, Random, 95% CI) | 31.15 [1.50, 60.81] |
| 2.6.3 Long‐term (≥8 weeks) | 11 | 336 | Mean Difference (IV, Random, 95% CI) | 38.47 [22.75, 54.20] |
| 2.7 Functional exercise capacity: 6‐minute walk distance (6MWD) (meters) (subgroup analysis: with or without respiratory muscle weakness) | 15 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.7.1 With respiratory muscle weakness | 5 | 178 | Mean Difference (IV, Random, 95% CI) | 33.74 [25.08, 42.40] |
| 2.7.2 Without respiratory muscle weakness | 11 | 291 | Mean Difference (IV, Random, 95% CI) | 29.80 [12.86, 46.73] |
| 2.8 Functional exercise capacity: 12‐minute walk distance (12MWD) (meters) | 3 | 101 | Mean Difference (IV, Random, 95% CI) | ‐33.31 [‐158.10, 91.48] |
| 2.9 Functional exercise capacity: Wmax (watt) | 7 | 206 | Mean Difference (IV, Random, 95% CI) | 0.66 [‐6.44, 7.76] |
| 2.10 Functional exercise capacity: exercise time (seconds) | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.11 Functional exercise capacity: shuttle walk test (SWT) (meters) | 2 | 57 | Mean Difference (IV, Random, 95% CI) | ‐7.45 [‐92.74, 77.83] |
| 2.12 Health‐related quality of life (HRQoL): St George Respiratory Questionnaire (SGRQ) | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.12.1 Symptoms | 2 | 53 | Mean Difference (IV, Random, 95% CI) | ‐2.10 [‐3.50, ‐0.71] |
| 2.12.2 Activity | 2 | 53 | Mean Difference (IV, Random, 95% CI) | ‐9.86 [‐15.08, ‐4.63] |
| 2.12.3 Impact | 2 | 53 | Mean Difference (IV, Random, 95% CI) | ‐6.06 [‐13.76, 1.65] |
| 2.12.4 Total | 6 | 182 | Mean Difference (IV, Random, 95% CI) | ‐3.85 [‐8.18, 0.48] |
| 2.13 Health‐related quality of life (HRQoL): Chronic Respiratory Disease Questionnaire (CRQ) | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.13.1 Dyspnea | 5 | 178 | Mean Difference (IV, Random, 95% CI) | 1.63 [0.23, 3.03] |
| 2.13.2 Fatigue | 5 | 178 | Mean Difference (IV, Random, 95% CI) | 1.32 [0.08, 2.55] |
| 2.13.3 Emotion | 4 | 163 | Mean Difference (IV, Random, 95% CI) | 2.64 [0.82, 4.46] |
| 2.13.4 Mastery | 4 | 154 | Mean Difference (IV, Random, 95% CI) | 1.57 [0.07, 3.06] |
| 2.14 Health‐related quality of life (HRQoL): COPD Assessment Test (CAT) | 2 | 86 | Mean Difference (IV, Random, 95% CI) | ‐2.97 [‐3.85, ‐2.10] |
| 2.15 Inspiratory muscle strength: PImax (cmH2O) | 32 | 916 | Mean Difference (IV, Random, 95% CI) | 14.57 [9.85, 19.29] |
| 2.16 Inspiratory muscle strength: PImax (cmH2O) (subgroup analysis: duration of the intervention) | 32 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.16.1 Short‐term (<4 weeks) | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 12.60 [6.94, 18.26] |
| 2.16.2 Medium‐term (≥4 weeks and <8 weeks) | 8 | 223 | Mean Difference (IV, Random, 95% CI) | 11.89 [6.76, 17.02] |
| 2.16.3 Long‐term (≥8 weeks) | 26 | 748 | Mean Difference (IV, Random, 95% CI) | 15.02 [9.26, 20.78] |
| 2.17 Inspiratory muscle strength: PImax (cmH2O) (subgroup analysis: with or without respiratory muscle weakness) | 28 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.17.1 With respiratory muscle weakness | 10 | 323 | Mean Difference (IV, Random, 95% CI) | 11.08 [7.51, 14.64] |
| 2.17.2 Without respiratory muscle weakness | 19 | 499 | Mean Difference (IV, Random, 95% CI) | 13.82 [5.36, 22.29] |
| 2.18 Inspiratory muscle strength: PImax (cmH2O) (subgroup analysis: method of measurement) | 32 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.18.1 Residual Volume (RV) | 15 | 466 | Mean Difference (IV, Random, 95% CI) | 12.63 [8.46, 16.81] |
| 2.18.2 Functional Residual Capacity (FRC) | 9 | 206 | Mean Difference (IV, Random, 95% CI) | 11.87 [7.33, 16.41] |
| 2.18.3 Not reported | 8 | 244 | Mean Difference (IV, Random, 95% CI) | 19.52 [4.63, 34.41] |
| 2.19 Laboratory exercise test: VO2peak | 11 | 286 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [0.05, 0.57] |
| 2.20 Respiratory muscle endurance: respiratory muscle endurance pressure (Pthmax) (cmH2O) | 8 | 179 | Mean Difference (IV, Random, 95% CI) | 9.71 [4.93, 14.50] |
| 2.21 Respiratory muscle endurance time: Tlim (seconds) | 10 | 260 | Mean Difference (IV, Random, 95% CI) | 270.57 [182.44, 358.71] |
| 2.22 Maximal voluntary ventilation (MVV) | 2 | 36 | Std. Mean Difference (IV, Random, 95% CI) | 0.99 [0.28, 1.69] |
| 2.23 Respiratory function: forced expiratory volume at 1 second (FEV1) (%pred) | 10 | 314 | Mean Difference (IV, Random, 95% CI) | 2.62 [0.20, 5.04] |
| 2.24 Respiratory function: forced expiratory volume at 1 second (FEV1) (Liters) | 12 | 362 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.06, 0.14] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abedi Yekta 2019.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics PR+IMT
PR
IMT
Control/sham
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics PR: consisted of aerobic exercise performed on a treadmill ergometer, at 40%‐60% of the heart rate, 2 d/week, for 8 weeks and 40 min/session. IMT: conducted 2 d/week for 8 weeks, 15 min/session with Powerbreathe KH4. Each session lasted 15 min, all sessions were supervised, and the training load was set at 40%‐60% of S‐Index. PR+IMT: consisted of a combination of PR and IMT protocols. Control: no intervention received by this group. |
|
| Outcomes | HRQoL: SGRQ (Total) | |
| Identification |
Sponsorship source: Shahid Beheshti University of Medical Sciences Country: Iran Setting: Hospital Hussein Department of Physiotherapy Author's name: Saeed Rezaei Institution: Department of Sports Medicine, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran Email: saeedrezaee1394@yahoo.com Clinical trial register: https://www.irct.ir/trial/29724 |
|
| Notes | A change of 4 units in SGRQ score was considered significant. | |
Bavarsad 2015.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics IMT
Control/Sham
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics IMT The training consisted of unsupervised sessions, 15 min/d, 6 d/week for 8 weeks. The device used was an incentive spirometer (Respivol), at a load equal to or more than the initial inspiratory volume. The researchers were informed of the training sessions through phone calls during the 8 weeks. A checklist, which was designed to be completed on weekdays, was prepared for the participants so that they could mark the relevant day after a training session Control No intervention was received by this group. |
|
| Outcomes | Dyspnea: Borg
Functional exercise capacity: 6MWD Respiratory function: FEV1 (%pred) Respiratory function: FEV1 (L) |
|
| Identification |
Sponsorship source: The Deputy of Research Affairs at the Ahvaz Jundishapur University of Medical Sciences Country: Iran Setting: Specialized Pulmonary Clinic of Ahvaz Author's name: Esmaeil Eidani Institution: Pulmonary Unit, Department of Medicine, Ahvaz Jundishapur University of Medical Sciences Email: esmaileidani@gmail.com Address: Ahzav, Iran |
|
| Notes | ||
Beaumont 2015.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group Subgroup analysis: FEV1 > or ≤ 50% of the predicted value |
|
| Participants |
Baseline characteristics PR+IMT
PR
Overall
Included criteria COPD diagnosed according to American Thoracic Society/European Respiratory Society criteria; PImax > 60 cmH2O at admission. Excluded criteria
Pretreatment: FEV 1 was lower in the IMT group; the Borg scale was higher in the control group. |
|
| Interventions |
Intervention characteristics PR+IMT
PR Participants in this group received only the standardised PR program. |
|
| Outcomes | Dyspnea: Borg Dyspnea: MDP
Functional exercise capacity: 6MWD Respiratory muscle strength: PImax |
|
| Identification |
Sponsorship source: EA3878 (G.E.T.B.O.), CIC INSERM 0502, University Hospital of Brest Country: France Setting: Pulmonary Rehabilitation Unit, Morlaix Hospital Centre, European University of Occidental Brittany Author's name: Marc Beaumont Institution: Pulmonary Rehabilitation Unit, Morlaix HospitalCentre, European University of Occidental Brittany Email: marc.beaumont@univ‐brest.fr Address: Morlaix29672, Cedex, France Clinical trial register: NCT01545011 |
|
| Notes | P‐value of the change from baseline is from the adjusted analysis. | |
Beaumont 2018.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group Subgroup analysis: PImax (> or ≤ 60 cmH2O) |
|
| Participants |
Baseline characteristics PR+IMT
PR
Overall
Included criteria
Excluded criteria
Pretreatment: 6 patients with lobectomy or pneumonectomy in the control group were included. |
|
| Interventions |
Intervention characteristics PR+IMT
PR: participants in this group received only the standardised PR program. |
|
| Outcomes | Dyspnea: Borg Dyspnea: mMRC Dyspnea: MDP:
Functional exercise capacity: 6MWD HRQoL: SGRQ
Respiratory muscle strength: PImax |
|
| Identification |
Country: France Setting: Rehabilitation programme unit of Centre Hospitalier des Pays de Morlaix Author's name: Marc Beaumont Institution: Pulmonary Rehabilitation Unit, Morlaix HospitalCentre, European University of Occidental Brittany Email: marc.beaumont@univ‐brest.fr Address: 29672 Morlaix CEDEX, France Clinical trial register: NCT02074813 |
|
| Notes | Borg and MDP scales were conducted at the end of 6MWD. Subgroup analysis according to PImax (> or ≤ 60 cmH2O) was conducted. See supplementary table. | |
Beckerman 2005.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics IMT
Control/sham
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics IMT: participants trained daily in 2 sessions of 15 min each, 6 times/week for 12 months. The training was performed using a threshold inspiratory muscle trainer (POWERbreathe; Gaiam Ltd; Southam, Warwickshire, UK). The participants started breathing at a resistance that required generation of 15% of Pimax for 1 week. The load was then increased incrementally, 5% to 10% each session, to reach 60% of Pimax at the end of the first month. IMT was then continued at 60% of the Pimax adjusted monthly to the new Pimax achieved. The training was conducted in a rehabilitation center for 1 month under the supervision of a respiratory therapist followed by home training, verified by a respiratory therapist daily by phone and once weekly by a personal visit, for the next 11 months. Control/sham: this group trained with the same protocol at a load equal to 7 cm H2O. |
|
| Outcomes | Dyspnea: Borg Functional exercise capacity: 6MWD HRQoL: SGRQ Respiratory muscle strength: PImax |
|
| Identification |
Country: Israel Setting: Home Author's name: Paltiel Weiner Institution: Department of Medicine A, Hillel Yaffe Medical Center Email: weiner@hillel‐yaffe.health.gov.il Address: Hadera, Israel 38100 |
|
| Notes | The study reported data as mean and SE, we computed SD for the baseline characteristics. The SE reported in the text of the trial is less than shown in the graph. | |
Belman 1988.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics IMT
Control/sham
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics IMT: the training protocol consisted of unsupervised sessions, 2 sessions/d, 7/week for 6 weeks. Each session lasted 15 min, using a Pflex device (the breathing pattern was controlled), and the training load was as tolerated. Control/sham: the control group received similar training at a load of around 7.5‐10 cmH2O. |
|
| Outcomes | Respiratory muscle strength: PImax (FRC) Respiratory muscle endurance:
Respiratory function: FEV1 (L) |
|
| Identification |
Country: USA Setting: hospital Author's name: Michael J Belman Institution: Division of Pulmonary Medicine, Cedars‐Sinai Medical Center, and The University of California Address: Los Angeles, California 90048 |
|
| Notes | ||
Berry 1996.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics PR+IMT
PR
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics PR+IMT
PR + sham IMT: this group underwent PR as described above, and IMT at 15% of PImax for the duration of the 12‐week study period. |
|
| Outcomes | Dyspnea: Borg Functional exercise capacity:
Respiratory muscle strength: PImax Laboratory exercise test: VO2peak (mL/kg/min) Respiratory muscle endurance: MVV Respiratory function:
|
|
| Identification |
Sponsorship source: Country: USA Setting: Wake Forest University Author's name: MichaelJ.Berry Institution: Department of Health and Sport Science Address: P.O. Box 7234, Wake ForestUniversity, Winston‐Salem, NC 27109 |
|
| Notes | All values are adjusted means ± SEM. Values were adjusted using pre‐intervention scores as the covariate. | |
Berton 2015.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics IMT
Control/sham
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics IMT: the training was unsupervised, 30 min/d, 7 d/week, for 8 weeks. The device used was Powerbreathe(Southam, UK) set at 30% of PImax. Control/sham: this group underwent similar training at no load. |
|
| Outcomes | Dyspnea: Borg Functional exercise capacity: exercise time HRQoL: SGRQ
Inspiratory muscle strength (PImax) |
|
| Identification |
Country: Brazil Author's name: Danilo C. Berton Institution: Graduation Program in Pulmonology, Federal University of Rio Grande do Sul (UFRGS), Brazil Email: dberton@hcpa.edu.br Address: Rua Ramiro Barcelos, 2350, Room 2050. Postal Code: 90035‐003, Porto Alegre, RS, Brazil Clinical trial register: NCT 01945398 |
|
| Notes | Participants were instructed to maintain diaphragmatic breathing, with a breathing rate of 15‐20 breaths/min. | |
Bustamante 2007.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics IMT
Control/sham
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics IMT: the training was unsupervised, conducted twice a day, 15 min/session, 7 d/week, for 6 weeks. Threshold IMT device was used at the maximum tolerated load. Control/sham: this group underwent a similar training at a load equal to 7cmH20. |
|
| Outcomes | HRQoL: CRQ
Respiratory muscle strength: PImax Respiratory muscle endurance time: Tlim (Threshold device) Notes: sustained time with a threshold of 66% of PImax |
|
| Identification |
Country: Spain Author's name: Víctor Bustamante Madariaga Institution: Servicio de Neumología. Hospital de Basurto. Osakidetza. Vizcaya. España Email: VICTOR.BUSTAMANTEMADARIAGA@hbas.osakidetza.net |
|
| Notes | ||
Charususin 2018.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics PR+IMT
PR
Overall
Included criteria
Excluded criteria
Pretreatment: "One of the centres offering a 36 session program (32% of total inclusions) consistently exceeded between‐group differences in the centre offering 20 sessions (36% of total inclusions). In the other centres offering 36 sessions (combined 32% of total inclusions), between‐group differences in these outcomes were consistently smaller than in the centre offering a lower training volume (see online supplementary table E4)" |
|
| Interventions |
Intervention characteristics PR+IMT
PR +sham IMT: this group underwent the same training as described above with IMT load at 10% of PImax. The load was not modified throughout the intervention period. |
|
| Outcomes | Dyspnea: Borg
Functional exercise capacity:
HRQoL: CRQ
Respiratory muscle strength: PImax Laboratory exercise test: VO2peak Respiratory muscle endurance: MVV Respiratory muscle endurance time: Tlim Respiratory function:
|
|
| Identification |
Sponsorship source: DL and HD are postdoctoral fellows of Research Foundation Flanders. HaB International (Southam, UK) and McRoberts (The Hague, The Netherlands) provided equipment for testing and training in this study on loan. This study was further supported by local funds throughout the participating centers. The following specific funding sources were reported: University Hospital Leuven, Belgium (FWO grant GOA4516N en KU Leuven grant C22/15/035); Ghent University Hospital, Belgium (UZ Gent grant FS/LGZ/994); Institut Universitaire de Cardiologie et de Pneumologie de Québec, Université Laval, Quebec, Canada (Ordre professionnel de la physiothérapie du Québec). Country: Belgium, The Netherlands, Germany, Canada Setting: multicenter Author's name: Daniel Langer Institution: Department of Rehabilitation Sciences, KU Leuven Email: daniel. langer@ kuleuven. be Address: Leuven 3001, Belgium Clinical trial register: NCT01397396 |
|
| Notes | Adjusted difference (95% CI) at post‐training with its P value were reported. | |
Chuang 2017.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics IMT
Control/sham
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics IMT: the training was conducted 5 d/week, for 8 weeks. Each session lasted for 21–30 min. It consisted of a cycle of 2 min inspiratory training with a pressure threshold loading device and 1 min of rest, and then repeated 7 cycles. The training load ranged from 15 cmH20 to 40 cmH2O. Each participant was followed up once a day by phone from Monday–Friday, and visits were made by research assistants every 2 weeks to increase the pressure threshold loading progressively after assessing the participant’s condition. Control: no intervention was received by this group. |
|
| Outcomes | Dyspnea: BDI Functional exercise capacity: 6MWD HRQoL: SF‐36 Questionnaire
Respiratory muscle strength: PImax |
|
| Identification |
Country: Taiwan Author's name: Hsiao‐Yun Chang Institution: Department of Nursing, Fooyin University, Kaohsiung City Email: chang369@gmail.com Address: Taiwan |
|
| Notes | ||
Covey 2001.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants | IMT
Control/sham
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics IMT: participants performed IMT at home 5 d/week, 30 min/d for 16 weeks (for a total of 80 training sessions) using Threshold IMT device. Starting training loads were 30% of PImax as tolerated. Participants were visited weekly at home by a nurse who supervised training, evaluated training loads, and progressively increased the training load as tolerated with a goal of achieving 60% of PImax. Home visits generally lasted approximately 30 min. An interval training protocol was used with participants performing 6 work sets of 5 mins’ duration separated by rest intervals lasting 1‐3 min. Control/sham: participants were visited by a nurse every 2 weeks for 16 weeks for a structured program of health education. Each home‐based session lasted approximately 1‐1.5 h and covered such topics as nutrition, relaxation techniques, pursed lip breathing, respiratory medications, respiratory infection, energy conservation techniques, oxygen therapy, and smoking cessation. |
|
| Outcomes | Dyspnea: Borg HRQoL: CRQ Respiratory muscle strength (PImax) Respiratory muscle endurance pressure: Pthmax |
|
| Identification |
Sponsorship: This study was supported by a grant from the National Institutes of Nursing Research, grant number NRO1428; and was conducted at the University of Illinois at Chicago, College of Nursing, Chicago, Ill and Hines VA Hospital, Section of Critical Care and Pulmonary Medicine, Hines, Ill. Country: USA Setting: home‐based Author's name: Margaret K. Covey Institution: University of Illinois at Chicago, College of Nursing Email: mkcovey@uic.edu Address: 845 South Damen Avenue, Chicago, IL 60612 |
|
| Notes | ||
Cutrim 2019.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics IMT
Control/sham
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics IMT: the session of IMT consisted of 30 min (in a clinical setting) 3 times/week, using the Threshold Inspiratory Muscle Training device (POWERbreathe Medic+Plus, NCS, Barueri, SP, Brazil). The inspiratory load was set at 30% of PImax, for 12 weeks. During exercise, participants were instructed to maintain diaphragmatic breathing at a rate of 15–20 breaths/min. Control/sham: no intervention received by this group (except diaphragmatic breathing). |
|
| Outcomes | Functional exercise capacity: 6MWD Respiratory muscle strength: PImax |
|
| Identification |
Sponsorship source: Fundação de Amparo à Pesquisa do Estado do Maranhão (FAPEMA); Hospital Universitário Presidente Dutra; Cristiano Mostarda received grants from CNPq (Universal 442374/2014‐3) and FAPEMA (Bolsa Produtividade and Universal 00358/15). Country: Brazil Setting: Hospital Universitário Presidente Dutra Author's name: Cristiano Teixeira Mostardaa Institution: Universidade Federal do Maranhão, São Luís, Brazil Email: cristiano.mostarda@gmail.com Address: Av. dos Portugueses, 1966, Cidade Universitária Dom Delgado, São Luís, MA, Brazil Clinical trial register: RBR‐4mz6w9 |
|
| Notes | Participants were instructed to maintain diaphragmatic breathing at a rate of 15–20 breaths/min. Adjusted analysis for age, weight and baseline were reported. | |
Dacha 2019.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics IMT
Control/sham
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics IMT: the training was conducted twice a day, 7 d/week, for 8 weeks. All the sessions were supervised, 5 min each, using Powerbreathe device set at a 50% of PImax. Control/sham: participants in this group received a similar protocol with a training load at 10% of PImax. |
|
| Outcomes | Dyspnea: Borg Respiratory muscle strength: PImax Laboratory exercise test: VO2peak |
|
| Identification |
Sponsorship source: KU Leuven Country: Belgium Author's name: Sauwaluk Dacha Institution: Faculty of Kinesiology and Rehabilitation Sciences, Department of Rehabilitation Sciences, Research Group for Rehabilitation in Internal Disorders Email: sauwaluk.dacha@kuleuven.be Address: KU Leuven, Leuven, Belgium Clinical trial register: NCT03240640 |
|
| Notes | This study is still ongoing. | |
De Farias 2019.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants | Overall
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention characteristics PR+ sham IMT
PR+ threshold IMT
PR + isocapnic hyperpnea
|
|
| Outcomes | Dyspnea: mMRC Functional exercise capacity: 6MWD Respiratory muscle strength: PImax |
|
| Identification |
Sponsorship source: no funding Country: Brazil Author's name: Guilherme Augusto de Freitas Institution: PneumoCardioVascular Lab/HUOL, Empresa Brasileira de Serviços Hospitalares ‐ EBSERH), Universidade Federal do Rio Grande do Norte (UFRN), Email: fregonezi.guilherme@gmail.com Address: Natal, Rio Grande do Norte, Brazil |
|
| Notes | ||
Dekhuijzen 1991.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics PR+IMT
PR
Overall
Included criteria: patients with functional limitations due to COPD Excluded criteria: not reported |
|
| Interventions |
Intervention characteristics PR+IMT
PR: this group received only the PR described above. |
|
| Outcomes | HRQoL: ADL Functional exercise capacity:
Respiratory muscle strength: PImax Laboratory exercise test: VO2peak Respiratory muscle endurance time: Tlim |
|
| Identification |
Country: The Netherlands Setting: Medical Centre Dekkerswald (outpatient clinic) Author's name: P. N. Richard Dekhuijzen Institution: the University of Nijmegen, Department of Pulmonary Diseases, Medical Centre Dekkerswald Address: Groesheek, the Netherlands |
|
| Notes | We used convertunits.com to convert from Kpa to cmH2O. | |
Dellweg 2017.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics PR+IMT
PR
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics PR+IMT:
PR+ (sham IMT): participants in this group received a similar protocol as described above with IMT training load fixed at 5 cm H2O, and using Threshold IMT (Philips‐Respironics, Pittsburgh, PA, USA). |
|
| Outcomes | Functional exercise capacity: 6MWD Respiratory muscle strength: PImax (RV) Respiratory function: FEV1 |
|
| Identification |
Sponsorship source: no funding Country: Germany Author's name: Dominic Dellweg Institution: Department for Pulmonology, Intensive Care and Rehabilitation Email: d.dellweg@fkkg.de Address: 1, 57392 Schmallenberg, Germany Clinical trial register: NCT00291460 |
|
| Notes | ||
Fanfa Bordin 2020.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics PR+IMT
PR
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics PR+IMT
PR: this group received only the PR described above. |
|
| Outcomes | Respiratory muscle strength: PImax | |
| Identification |
Country: Brazil Setting: Santa Cruz do Sul Hospital, Brazil Author's name: Diogo Fanfa Bordin Institution: Universidade de Santa Cruz do Sul (Unisc) – Santa Cruz do Sul (RS), Brazil Email: diogo.fanfa@hotmail.com Clinical trial register: NCT02014155 |
|
| Notes | ||
Harver 1989.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics IMT
Control/sham
Overall
Included criteria
Excluded criteria: not reported |
|
| Interventions |
Intervention characteristics IMT: participants trained twice a day, 15 min each session, 7 d/week, for 8 weeks. The training device was Pflex (with a controlled breathing pattern), and the training load ranged from 5 cmH2O to 35 cmH2O (≈30% PImax FRC) Control/sham: this group received a similar IMT protocol with a training load set at 5cmH2O |
|
| Outcomes | Dyspnea: BDI‐TDI:
Respiratory muscle strength: PImax
Respiratory function: FEV1
Respiratory muscle endurance: MVV |
|
| Identification |
Sponsorship source: The American Lung Association of New Hampshire and by grants HL07449 and HL29068 from the National Heart, Lung, and Blood Institute Country: USA Setting: Outpatient pulmonary clinic and pulmonary function laboratory Author's name: Andrew Harver Institution: Department of Psychology, SUNY Stony Brook Address: Stony Brook, NY 11794 |
|
| Notes | ||
Heijdra 1996.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics IMT
Control/sham
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics IMT: participants trained at home, daily, 2 sessions of 15 min/day for 10 weeks. They used incentive flowmeter (INSPIRx; Resprecare Medical Inc., the Hague, the Netherlands) set at 60% of PImax Control/sham: participants received a similar training protocol and the resistance was set at 10% of PImax |
|
| Outcomes | Inspiratory muscle strength (PImax) Respiratory muscle endurance time (Tlim) |
|
| Identification |
Sponsorship source: Dutch Asthma Foundation (No. 90‐27) Country: The Netherlands Author's name: Yvonne F. Heijdra, Department of Pulmonary Diseases Institution: University of Nijmegen, Medical Centre Dekkerswald Address: P.O. Box 9001, 6560 GB Groesbeek, the Netherlands |
|
| Notes | ||
Hill 2006.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics IMT
Control/sham
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics IMT: participants attended supervised training sessions 3 times/week for 8 weeks. Each session lasted 21 min and comprised 7 cycles of 2 min of breathing on an inspiratory threshold loading device (Threshold IMT; Respironics, Cedar Grove, NJ, USA) followed by 1 minute of rest. The training load was set at a range of around 45%‐101% of PImax Control/sham: this group received a similar IMT protocol with a training load set at 10% of PImax |
|
| Outcomes | Dyspnea: Borg
Functional exercise capacity:
HRQoL: CRQ
Respiratory muscle strength (PImax) Respiratory muscle endurance: Respiratory muscle endurance pressure (Pthmax) Laboratory exercise test: VO2peak (mL/kg/min) Respiratory function: FEV1
Respiratory function: RV
|
|
| Identification |
Sponsorship source: The National Health and Medica Research Council (Canberra, Australia) grant number 212016 Country: Australia Author's name: P.R. Eastwood Institution: Dept of Pulmonary Physiology, Sir Charles Gairdner Hospital Email: peter.eastwood@health.wa.gov.au Address: Hospital Avenue Nedlands Western Australia, Australia 6009 |
|
| Notes | ||
Hill 2007.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics IMT
Control/sham
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics IMT: participants attended supervised training sessions 3 times/week for 8 weeks. Each session lasted 21 min and comprised 7 cycles of 2 min of breathing on an inspiratory threshold loading device (Threshold IMT; Respironics, Cedar Grove, NJ, USA) followed by 1 minute of rest. The training load was set at a range of around 45%‐101% of PImax Control/sham: this group received a similar IMT protocol with a training load set at 10% of PImax |
|
| Outcomes | Respiratory muscle endurance time: Tlim Respiratory muscle endurance pressure (Pthmax)
|
|
| Identification |
Sponsorship source: The National Health and Medica Research Council (Canberra, Australia) grant number 212016 Country: Australia Author's name: P.R. Eastwood Institution: Dept of Pulmonary Physiology, Sir Charles Gairdner Hospital Email: peter.eastwood@health.wa.gov.au Address: Hospital Avenue Nedlands Western Australia, Australia 6009 |
|
| Notes | ||
Hsiao 2003.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics IMT (Threshold device)
IMT (Incentive spirometer)
Control/sham
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics IMT: participants trained twice a day, 15 min/session, 5days/week for 8 weeks. The sessions were unsupervised. Participants were divided into two groups using either Threshold IMT or Respirex, which were set at 50% of PImax Control/sham: this group did not receive any intervention |
|
| Outcomes | Functional exercise capacity: 6MWD Respiratory muscle strength: PImax (RV) Respiratory muscle endurance time: Tlim (Threshold device)
|
|
| Identification |
Country: Taiwan Setting: Pulmonary clinic of 1 university hospital Author's name: Ying Tai Wu Institution: School and graduate institute of physical therapy, college of medicine, National Taiwan University Hospital Address: 7 Chung Shan South Road, Taipei 100, Taiwan |
|
| Notes | ||
Kim 1993.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics IMT
Control/sham
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics IMT: participants trained 7 days/week, 30 min/session, for 6 months. Training sessions were unsupervised, and the device used was a Threshold IMT device set at 30% of PImax Control/sham: this group received IMT at no load |
|
| Outcomes | Functional exercise capacity: 12MWD Respiratory muscle endurance time: Tlim
Respiratory muscle strength: PImax (RV) |
|
| Identification |
Sponsorship source: supported by a grant from the National Center for Nursing Research, grant number NRO1428 Country: USA Author's name: Mija Kim Institution: College of Nursing, University of Illinois at Chicago |
|
| Notes | ||
Koppers 2006.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group Subgroup analysis: PImax < 75%pred |
|
| Participants | IMT
Control/sham
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics IMT: respiratory muscle endurance training was performed by means of tube breathing. A tube (internal diameter, 3 cm) connected to a mouthpiece was added to the respiratory system to rebreathe exhaled carbon dioxide (normocapnic hyperpnea). The maximum ventilatory capacity that can be sustained for 15 min is approximately 60% of MVV. Therefore, the aimed level of ventilation during training was set at 60% of MVV, which was calculated from 35 times FEV1 (60% MVV = 0.6 * 35 * FEV1). The dead space was adjusted to 60% of the participant's IVC plus the resting tidal volume. Participants trained twice daily, for 15 min, 7 d/week for 5 weeks. Control/sham: participant breathed 6‐7 times/min through an incentive flowmeter (Inspirx; Resprecare Medical; the Hague, the Netherlands). Airflow resistance was set at 5% of PImax. |
|
| Outcomes | Dyspnea: Borg
Functional exercise capacity:
HRQoL: CRQ (Total) Respiratory muscle strength: PImax (RV) Laboratory exercise test: VO2peak (mL/kg/min) Respiratory muscle endurance: Respiratory muscle endurance pressure (Pthmax) Respiratory function: FEV1 (L) |
|
| Identification |
Sponsorship source: Country: The Netherlands Setting: Department of Pulmonology Dekkerswald, University Medical Center Nijmegen Author's name: Ralph J. H. Koppers Institution: MedicalCenter Leeuwarden, Leeuwarden; and Department of Pulmonology (Drs. Vos, Boot, and Folgering), Dekkerswald, University Medical Center Nijmegen Email: R.J.H.Koppers@ZNB.nl Address: Nijmegen, the Netherlands |
|
| Notes | ||
Langer 2018.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics IMT
Control/sham
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics IMT: the training was performed with a home‐based protocol using an electronic device: Powerbreathe KH2 (HAB International, Southam, UK). Participants trained 2‐3 daily sessions of 30 breaths (4‐5 min/session) performed 7 days/week for 8 weeks. The training load started at around 40% of PImax and it was increased weekly until the highest tolerable intensity Control/sham: this group performed IMT at a load of < 10% of PImax |
|
| Outcomes | Dyspnea: Borg
Dyspnea: BDI‐TDI: Total Dyspnea: mMRC Functional exercise capacity: Exercise time (constant cycle ergometer test) Respiratory muscle strength: PImax
Respiratory muscle endurance time (Tlim)
Laboratory exercise test: VO2peak (Constant cycle ergometer test) (L/min) Respiratory function: FEV1 (L) |
|
| Identification |
Sponsorship source: this work was supported by the Ontario Thoracic Society, Spear/StartEndowment Fund, Queen’s University. D. Langer received a postdoctoral fellowship and travel grant from the Research Foundation Flanders; thePowerBreathe devices used in the study were provided by HaB International Country: Canada Setting: Queen’s University Health Sciences and Affiliated Teaching Hospitals Author's name: D. E. O’Donnell Institution: Respiratory Investigation Unit, Queen’s University and Kingston Health Sciences Centre Email: odonnell@queensu.ca Address: 102 Stuart St., Kingston, ON, Canada, K7L 2V6 Clinical trial register: NCT01900873 |
|
| Notes | webplotdigitizer used to extract IMT training load intensity. Adjusted analysis were provided. | |
Larson 1988.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics IMT
Control/sham
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics IMT: participants trained 7 d/week for 8 weeks using the Threshold IMT device at 30% of PImax. They initiated the training for 15 min/d during the first week and gradually increased the duration to 30 min/day for the remaining 7 weeks. Control/sham: this group received the same IMT protocol with the training load set at 15% of PImax |
|
| Outcomes | Respiratory muscle strength: PImax (RV) Functional exercise capacity: 12MWD Respiratory muscle endurance time: Tlim
|
|
| Identification |
Sponsorship source: supported in part by Grant No. HL‐31558 from the National Institutes of Health and by a grant from Sigma Theta Tau, Psi Chapter Country: USA Author's name: Janet L. Larson Institution: College of Nursing, University of Illinois at Chicago Address: 845 S.Damen Avenue, Chicago,IL 60612 |
|
| Notes | ||
Larson 1999.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics PR+IMT
PR
IMT
Control/sham
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics PR: participants performed cycle ergometer training at home on a calibrated stationary cycle ergometer (BodyGuard 990; BodyGuard, Sandnes, Norway), 20 min/d, 5 d/week for 2 months. An interval training protocol was used with participants performing 4 work sets, 5 min in duration, separated by rest intervals (2–4 min) of unloaded cycling. The training was initiated at 50% of the peak work rate, taken from the best baseline graded exercise test, and evaluated weekly with progressive increases as tolerated. Patients were instructed to pedal at a rate of 60 revolutions/minute (rpm), and they were encouraged to push themselves to the limits of their dyspnea, without exceeding a heart rate equal to 85% of the predicted maximal heart rate. IMT: the participants trained with Threshold IMT (HealthScan, Cedar Grove, NJ), 30 min/d, 5 d/week for 2 months. the training was initiated at 30% of PImax with progressive increases up to 60% of PImax PR+IMT: participants received both PR and IMT as described above. Control/sham: this group participated in a structured program of health education |
|
| Outcomes | Dyspnea: Borg
HRQoL: CRQ
Respiratory muscle strength: PImax (RV) Respiratory muscle endurance: respiratory muscle endurance pressure (Pthmax) Functional exercise capacity: Wmax Laboratory exercise test: VO2peak (L/min) |
|
| Identification |
Sponsorship source: funded by a research grant from the National Institute of Nursing Research, National Institutes of Health, RO1‐NR01428 Country: USA Author's name: Janet L. Larson Institution: University of Illinois at Chicago Email: LLarson@uic.ed Address: 845 S. Damen, Chicago, IL60612 |
|
| Notes | ||
Leelarungrayub 2017.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics IMT
Control/sham
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics IMT: participants trained with Portex (Smith Medical ASD), 20‐30 min/session, 7 d/week for 6 weeks. They started breathing through a 6 mm hole once daily for the first 2 weeks, before changing to 4 mm and 2 mm holes in the 2nd and 4th week, respectively. 30 slowly repeated inspirations passed through the device, with a 3‐min interval of rest in each of 4 training sessions. Control: this group did not receive any intervention |
|
| Outcomes | Functional exercise capacity: 6MWD HRQoL: CCQ
Respiratory muscle strength: PImax (RV) Respiratory function: FEV1
|
|
| Identification |
Sponsorship source: Country: Thailand Setting: Sansai hospital, Sansai, Chiang Mai Province, Thailand Author's name: Jirakrit leelarungrayub Institution: Department of Physical Therapy, Faculty of associated Medical sciences, Chiang Mai University Email: donrawee.leela@cmu.ac.th Address: Intawaroroj road, Sripoom, Chiang Mai 50200, Thailand |
|
| Notes | We calculated the SD manually from the SE (SD = SE*√N). This study also used a prototype that was excluded from our analysis because it hasn't been validated yet. We contacted the Portex manufacturer to ask for further information about the device. | |
Lisboa 1997.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics IMT
Control/Sham
Overall
Included criteria
Excluded criteria: not reported |
|
| Interventions |
Intervention characteristics IMT: participants trained at home using a Threshold IMT device (HealthScan Products Inc., NJ, USA), for 30 min/d, 6 d/week for 10 weeks at 30% of PImax Control/sham: this group received a similar IMT protocol and trained at 10% of PImax |
|
| Outcomes | Dyspnea: BDI‐TDI (Total) Dyspnea: Borg
Functional exercise capacity: 6MWD Respiratory muscle strength: PImax (FRC) Respiratory function: FEV1 (L) Functional exercise capacity: Wmax
Laboratory exercise test: VO2peak (mL/min) |
|
| Identification |
Country: Chile Author's name: C. Lisboa Institution: Dept of respiratory disease, Catholic University of Chile Address: Santiago, Chile |
|
| Notes | The authors mentioned COPD in the discussion section. | |
Mador 2005.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics PR+IMT
PR
Overall
Included criteria
|
|
| Interventions |
Intervention characteristics PR+IMT
PR: this group received only the PR program described above |
|
| Outcomes | Functional exercise capacity:
HRQoL: CRQ Respiratory muscle strength: PImax Respiratory muscle endurance time: Tlim |
|
| Identification |
Country: USA Author's name: M. Jeffery Mador Institution: Division of Pulmonary, Critical Care, and Sleep Medicine, Section 111S Email: mador@acsu.buffalo.edu Address: State University of New York at Buffalo, Veterans Administration Medical Center, 3495 Bailey Ave, Buffalo, NY 14215 |
|
| Notes | ||
Magadle 2007.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics PR+IMT
PR
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics PR+IMT
PR (+sham IMT): this group received a similar training protocol and participants performed IMT at no load. |
|
| Outcomes | Functional exercise capacity: 6MWD HRQoL: SGRQ (Total) Respiratory muscle strength: PImax (RV) Respiratory function: FEV1 (%pred) |
|
| Identification |
Country: Israel Setting: community‐based rehabilitation center Author's name: Paltiel Weiner Institution: Department of Medicine A, Hillel Yaffe Medical Center Email: weiner@hillel‐yaffe.health.gov.il Address: Hadera 38100, Israel |
|
| Notes | Participants were enrolled in a 1st phase that consisted of 36 sessions of PR for 3 months (90 min/session). | |
Majewska‐Pulsakowska 2016.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics PR+IMT
PR
IMT
Control/sham
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics PR: consisted of training on a cycle ergometer, 3 times/week for 8 weeks in an ambulatory setting under the supervision of a cardiologist. Each training session began (warm‐up) and finished (relaxation) with a pedalling load of 10 W for 3 min. The duration of a training session was initially 23 min, and then it was gradually increased up to 45 min. IMT: consisted of home‐based training using Threshold IMT device (Respironics; Philips Healthcare, DA Best, The Netherlands), twice a day (5‐15 min), 5 d/week for 8 weeks. The training load ranged from 30%‐60% of PImax PR+IMT: this group received both interventions described above. Control/sham: this group did not receive any intervention. |
|
| Outcomes | HRQoL: SGRQ Respiratory function: FEV1
|
|
| Identification |
Country: Poland Author's name: K. Wytrychowski Institution: Department of Internal Diseases, Geriatry andAllergology, Wroclaw Medical University Email: Polande‐mail:anhw@op.plK Address: 66 Sklodowskiej‐Curie St., 50‐369 Wroclaw, Poland |
|
| Notes | ||
Masanga 2011.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics PR+IMT
PR
Overall
Included criteria: stable patients with moderate to severe COPD Excluded criteria: not reported |
|
| Interventions |
Intervention characteristics PR+IMT:
PR: Participants received only the PR program described above. |
|
| Outcomes | This abstract reported only adverse events: headache (6), jaw pain (6), neck pain (6), back pain (4), abdominal pain (2), cough (1), blood‐streaked sputum (1), shoulder pain (1) and chest pain (1) | |
| Identification |
Country: Philippines Author's name: L. Masanga |
|
| Notes | ||
Nikoletou 2016.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics IMT
Control/sham
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics IMT: 2 sessions/d, 6 d/week for 7 weeks, home‐based, using the Powerbreathe inspiratory muscle trainer (HaB International, Southam, Warwickshire, UK). Participants started training at 30% of their baseline PImax and increased the intensity once a week as tolerated. The average weekly increase in the intensity of training was 5% and the mean (SD) intensity at the end of the program was 62% (SD: 11.7) of the baseline PImax Control/sham: participants received a similar IMT program and trained at 15% of PImax |
|
| Outcomes | Functional exercise capacity: incremental SWT Dyspnea: Borg
HRQoL: CRQ
HRQoL: SF‐36 Questionnaire
Respiratory muscle strength: PImax (FRC) Respiratory muscle endurance time: Tlim |
|
| Identification |
Country: UK Setting: respiratory outpatient clinics at King’s College Hospital, GP practices and British Lung Foundation Breathe Easy groups Author's name: Dimitra Nikoletou Institution: School of Rehabilitation Sciences, Faculty of Health, Social care and Education, Kingston University and St George’s University of London Email: D.Nikoletou@sgul.kingston.ac.uk Address: Grosvenor Wing, Cranmer Terrace, London SW17 0RE, UK |
|
| Notes | ||
Paneroni 2018.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics PR+IMT
PR
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics PR+IMT:
PR (+sham IMT): participants in this group received a similar training PR program, and they performed IMT using Threshold IMT device at no resistance |
|
| Outcomes | Functional exercise capacity: 6MWD Respiratory muscle strength: PImax Respiratory muscle endurance time: Tlim (normocapnic hyperpnea)
Respiratory muscle endurance time: Tlim (Threshold device)
Respiratory muscle endurance: MVV |
|
| Identification |
Country: Italy Setting: Respiratory Rehabilitation Divisions of the Lumezzane and Pavia Institutes of the Istituti Clinici Scientifici Maugeri, Italy Author's name: Mara Paneroni Institution: Istituti Clinici Scientifici Maugeri, IRCCS Address: Salvatore Maugeri 2, 27100 Pavia, Italy Clinical trial register: NCT01556139 |
|
| Notes | ||
Petrovic 2012.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics IMT
Control/sham
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics IMT: performed once/d, 7 d/week, for 8 weeks using Respifit Sdevice (Mauerbach, Australia). The training consisted of strength training at 80% of PImax (≃12 min each session) and endurance training at 60% of PImax (≃ 13.5 min each session) Control/sham: this group did not receive any intervention |
|
| Outcomes | Dyspnea: Borg
Respiratory muscle strength: PImax Respiratory muscle endurance time: Tlim
Laboratory exercise test: VO2peak (mL/min)
|
|
| Identification |
Country: Austria Setting: outpatient clinic Author's name: Milos Petrovic Institution: Pulmonary Department and Karl Landsteiner Institute for Clinical and Experimental Pulmology Email: milos.petrovic@wienkav.at Address: Hietzing Hospital, Wolkensbergenstrasse 1, 1130 Vienna, Austria Clinical trial register: NCT00469313 |
|
| Notes | ||
Preusser 1994.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics IMT
Control/sham
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics IMT: consisted of supervised sessions, 3 days/week for 12 weeks, using Threshold IMT device at 52% of PImax. The training duration ranged from 5 min in week 1 to 18 min in week 12 Control/sham: this group received a similar IMT protocol and trained at 22% of PImax |
|
| Outcomes | Functional exercise capacity: 12MWD Respiratory muscle strength: PImax (FRC) Respiratory muscle endurance: Respiratory muscle endurance pressure (Pthmax)
|
|
| Identification |
Sponsorship source: supported by grant F31 NR06378, National Center for Nursing Research; a grant from the American Nurses Foundation; and the Epsilon Chapter of Sigma Theta Tau Country: USA Author's name: Barbara A. Preusser Institution: College of Nursing, The Ohio State University, and the Department of Pulmonary Medicine, The Ohio State University Hospitals, Columbus Address: University of Utah College of Nursing, 25 South Medical Drive, Salt Lake City 84112 |
|
| Notes | ||
Ramirez Sarmiento 2002.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics IMT
Control/sham
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics IMT: participants trained 30 min/day, 5 d/week for 5 weeks. The sessions were supervised and using Threshold IMT device set at 50% of PImax Control/sham: participants received a similar IMT protocol and trained at no load |
|
| Outcomes | Functional exercise capacity:
Respiratory muscle strength: PImax Respiratory muscle endurance: Respiratory muscle endurance pressure (Pthmax)
Respiratory muscle endurance time: Tlim
Laboratory exercise test: VO2peak (mL/kg/min)
Respiratory function: FEV1 (%pred) Respiratory function: RV (%pred) |
|
| Identification |
Sponsorship source: supported, in part, by grants BIOMED (BMH‐4‐CT98‐3406), FIS, SEPAR, and SIBEL Country: Spain Author's name: Mauricio Orozco‐Levi Institution: Servei Grup de Recera de Pneumologica, Hospital del MarIMIM Email: morozco@imim.es Address: Passeig Maritim 25, E‐080003, Barcelona (Catalonia), Spain |
|
| Notes | ||
Saher 2021.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics IMT
Control
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics IMT: participants performed IMT 15 min/session, 2 session/day, for 10 days. They used an IMT threshold device (Powerbreath, Gaiam Ltd., Southam, UK). The training load started at 30% of PImax, and it was increased by 5%‐10% daily until 60% Control: this group did not receive any training Note: both groups received non‐invasive ventilation (NIV) as part of COPD management. |
|
| Outcomes | Functional exercise capacity: 6MWD Inspiratory muscle strength (PImax) |
|
| Identification |
Country: India Setting: Metro Center for Respiratory Disease, Metro Hospital Author's name: T. Saher Institution: Center for Physiotherapy and Rehabilitation Sciences Email: jmoiz@jmi.ac.in Address: Jamia Millia Islamia, New Delhi, 110025, India |
|
| Notes | ||
Saka 2021.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics IMT
Sham IMT
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics IMT: the training was performed 15 min twice a day, 5 d/week, for 8 weeks. Home‐based training with 1 session supervised by a physiotherapist. Participants used Threshold IMT device (Threshold IMT® Philips Respironics, UK) at a load of 30% of PImax. Sham IMT: participants received the same protocol with a training load set at 15% of PImax. |
|
| Outcomes | Dyspnea: mMRC Functional exercise capacity: 6MWD HRQoL: SGRQ
HRQoL: CAT Inspiratory muscle strength (PImax) Respiratory function: FEV1
|
|
| Identification |
Sponsorship: Scientific Research Projects Unit of Bezmialem Vakıf University, project number 9.2017/31 Country: Turkey Setting: Bezmialem Vakif University Division of Physiotherapy and Rehabilitation, Cardiopulmonary Physiotherapy and Rehabilitation Department. Author's name: Seda Saka Institution: Cardiopulmonary Physiotherapy Rehabilitation Department, Institute of Health Sciences, Bezmialem Vakif University, Istanbul, Turkey Email: fztsedasaka@gmail.com Address: Division of Physiotherapy and Rehabilitation, Faculty of Health Sciences, Bezmialem Vakif University, Silahtaraga St. No:189, Alibeykoy, 34060, Istanbul, Turkey Clinical trial register: NCT03517839 |
|
| Notes | ||
Sanchez Riera 2001.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics IMT
Control/Sham
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics IMT: participants trained at home 30 min/d, 6 d/week for 6 months using incentive flowmeter device (INSPIRx; Intertech Resources Inc; Ft. Myers, FL) set at 30% of PImax. The breathing pattern was controlled during the exercise. Control/sham: participants received a similar IMT protocol and trained at no load. |
|
| Outcomes | Dyspnea: Borg (Constant cycle ergometer test) Functional exercise capacity: SWT
Functional exercise capacity: Wmax
Respiratory muscle strength: PImax (FRC) Laboratory exercise test: VO2peak (L/min) Respiratory muscle endurance: Respiratory muscle endurance pressure (Pthmax) |
|
| Identification |
Sponsorship source: Supported by “Junta de Andalucia” grant No. 94//535–119 Country: Spain Setting: home‐based training Author's name: Hildegard Sanchez Riera Institution: Pneumology Service, Virgen Del Rocio University Hospital Email: ablucil@mx2.redestb.es Address: “La Motilla,” C/Rayo 4, 41700 Dos Hermanas, Sevilla, Spain |
|
| Notes | CRQ was not included because study did not fully report control group data | |
Scherer 2000.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics IMT
Control/sham
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics IMT: respiratory muscle endurance training was conducted twice daily, 5 d/week for 8 weeks. The device was developed and consisted of tubing (I.D. 5 19 mm) connecting a rebreathing bag with a mouthpiece at a 90° angle. The breathing frequency was 60% of MVV. Control/sham: participants trained following the same pattern of the description above, at no load using incentive spirometer (COACH 2 Volumetric Incentive Spirometer; DHD Healthcare, Canastota, NY). The respiratory rate was 6‐8 breaths/min, and the target inspiration was set at 70% of each participant’s ventilatory capacity. |
|
| Outcomes | Dyspnea: BDI‐TDI (Total) Functional exercise capacity:
Respiratory muscle strength: PImax (RV) Laboratory exercise test: VO2peak (mL/kg/min) Respiratory muscle endurance time: Tlim
Respiratory function: FEV1 (%pred) |
|
| Identification |
Sponsorship source: supported by grants from Astra Pharmaceutica, Dietikon, and Merck Sharpe and Dohme‐Chibret, and Rhône‐Poulenc Rorer Country: Switzerland Setting: outpatient clinic of the Pulmonary Division of the Triemli Hospital Author's name: Thomas A. Scherer Institution: LungenZentrum Hirslanden Email: thsche@swissonline.ch Address: Witellikerstrasse 36, 8008 Zurich, Switzerland |
|
| Notes | ||
Schultz 2018.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics PR+IMT
PR
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics PR+IMT:
PR (+ sham IMT): this group received a similar training protocol as described above and the IMT was conducted at no load. |
|
| Outcomes | Dyspnea: BDI‐TDI (Total) Functional exercise capacity: 6MWD HRQoL
Respiratory muscle strength: PImax Respiratory function: FEV1 (L) |
|
| Identification |
Sponsorship source: this study was supported by Deutsche Rentenversicherung Bayern Süd. Funding information for this article has been deposited with the Crossref Funder Registry Country: Germany Setting: Bad Reichenhall Clinic Author's name: Konrad Schultz Institution: Center for Rehabilitation, Pulmonology and Orthopedics, Klinik Bad Reichenhall Email: konrad.schultz@klinik‐bad‐reichenhall.de Address: Salzburger Strasse 8–11, 83435 Bad Reichenhall, Germany Clinical trial register: DRKS00004609 |
|
| Notes | Adjusted mean differences with 95% CI were reported | |
Sykes 2005.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics PR+IMT
PR
Overall
Included criteria: not reported Excluded criteria: not reported |
|
| Interventions |
Intervention characteristics PR+IMT: participants received exercise training and IMT with Threshold IMT at a load ranged from 30%‐60% of PImax PR: this group received only exercise training |
|
| Outcomes | ||
| Identification |
Country: China Setting: Tai Po Hospital Author's name: Sykes |
|
| Notes | The systematic review (Gosselink 2011) reported data from this trial. | |
Tounsi 2021.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics PR+IMT
PR
Overall
Included criteria
Exclusion criteria
|
|
| Interventions |
Intervention characteristics PR+IMT:
PR: this group received a similar training protocol as described above without IMT. |
|
| Outcomes | Functional exercise capaticy: 6MWD Respiratory muscle strength: PImax |
|
| Identification |
Sponsorship source: the authors received no funding for this work Country: Tunisia Setting: Farhat Hached Hospital of Sousse Author's name: Bilel Tounsi Institution: 1/ Laboratory of Exercise Physiology and Rehabilitation (APERE, UR‐EA 3300), Sport Sciences Department, Picardie Jules Verne University, Amiens, France, 2/ Research Laboratory of Exercise Physiology and Pathophysiology: From Integral to Molecular Biology, Medicine and Health (LR19ES09), Faculty of Medicine of Sousse, University of Sousse, Sousse, Tunisia Email: bilel.tounsi@u‐picardie.fr Address: Department of sports sciences, Picardie Jules Verne University, Amiens, France Clinical trial register: NCT04084405 |
|
| Notes | ||
Tout 2013.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics PR+IMT
PR
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics PR+IMT:
PR: this group received only the PR protocol described above. |
|
| Outcomes | Functional exercise capacity: 6MWD HRQoL: SGRQ
Respiratory function: FEV1 (L) |
|
| Identification |
Country: Lebanon Author's name: Rola Tout Institution: Institut de Physiothérapie, université Saint‐Joseph Email: rolatout@yahoo.com Address: URAF, rue de Damas, Beyrouth, Lebanon |
|
| Notes | ||
Wang 2017.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group Subgroup analysis: PImax less or more than 60 cmH2O |
|
| Participants |
Baseline characteristics PR+IMT
PR
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics PR+IMT:
PR: this group received only the PR protocol described above |
|
| Outcomes | Dyspnea: Borg
Dyspnea: mMRC Functional exercise capacity:
HRQoL: CAT HRQoL: SGRQ (Total) Respiratory muscle strength: PImax Respiratory muscle endurance: MVV Laboratory exercise test: VO2peak
Respiratory function: FEV1
|
|
| Identification |
Sponsorship source: this work was supported by Guangzhou Municipal Science and Technology Project (201507020033), Medical Scientific Research Foundation of Guangdong Province (A2016399), Open Project of State Key Laboratory of Respiratory Disease (SKLRD2016OP019), and Clinical Research training program of Southern Medical University (LC2016PY032). Country: China Setting: Zhujiang Hospital affiliated to Southern Medical University Author's name: Xin Chen Institution: Department of Respiratory Medicine, Zhujiang Hosptial, Southern Medical University Email: chen_xin1020@163.com Address: 253 Gongye Road, Guangzhou 510282, China Clinical trial register: NCT02285400 |
|
| Notes | Adjusted P values were reported. Authors reported a subgroup analysis for intervention group participants with or without respiratory muscle weakness. |
|
Weiner 1992.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics PR+IMT
PR
Overall
Included criteria
Excluded criteria: not reported |
|
| Interventions |
Intervention characteristics PR+IMT:
PR+ (sham IMT): this group received the same training protocol as described above, and IMT was conducted at no load. |
|
| Outcomes | Functional exercise capacity:
Respiratory muscle strength: PImax (RV) Respiratory muscle endurance: respiratory muscle endurance pressure (Pthmax)
Respiratory function: FEV1 (%pred) |
|
| Identification |
Country: Israel Author's name: Paltiel Weiner Institution: Department of Medicine, Hillel‐Yaffe Medical Center, and the Institute for Respiratory Disease Address: Hadera, Israel |
|
| Notes | ||
Weiner 2000.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics PR+IMT
PR
Overall
Included criteria
Excluded criteria: not reported |
|
| Interventions |
Intervention characteristics PR+IMT: the training program is the same as described in (Weiner 1992). Participants trained 3 times/week and each session consisted of 1 h of supervised training. When only exercise training was performed, participants trained the whole hour, and when IMT was added, exercise training was cut to 30 min. PR+ SHAM: participants in this group received the same rehabilitation protocol and IMT was conducted at no load |
|
| Outcomes | Respiratory muscle strength: PImax (RV) | |
| Identification |
Country: Israel Author's name: Paltiel Weiner Institution: Department of Medicine A, Hillel‐Yaffe Medical Center Address: Hadera, Israel |
|
| Notes | ||
Weiner 2003.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics IMT
Control/sham
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics IMT: participants trained daily, 1 h/d, 6 times/week for 3 months using Threshold IMT device. They started breathing at a resistance equal to 15% of their PImax or PEmax for 1 week. The resistance then was increased incrementally 5% to 10% each session, to reach 60% of their PImax or PEmax at the end of the first month of training. Control/sham: participants trained at 7 cmH2O throughout the trial |
|
| Outcomes | Dyspnea: BDI‐TDI:
Functional exercise capacity: 6MWD Respiratory muscle strength: PImax (RV) Respiratory muscle endurance: respiratory muscle endurance pressure (Pthmax) |
|
| Identification |
Country: Israel Author's name: Paltiel Weiner Institution: Department of Medicine A, Hillel Yaffe Medical Center Email: weiner@hillel‐yaffe.health.gov.il Address: Hadera, Israel 38100 |
|
| Notes | Because only the IMT group data were reported in the results, and there were differences between the graphs and what was reported numerically, we included both groups' data from the graphs to guarantee consistency. | |
Weiner 2006.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics IMT
Control/sham
Overall
Included criteria
Excluded criteria: not reported |
|
| Interventions |
Intervention characteristics IMT: participants trained daily, 1 h/d, 6 d/week for 8 weeks using Powerbreathe (Southam, UK). They started breathing at a resistance equal to 15% of their PImax for 1 week. The resistance was then increased incrementally (5%–10% each session), to reach 60% of their PImax at the end of the 1st month. IMT was then continued at 60% of their PImax adjusted weekly to the new PImax achieved Control/sham: participants trained with a resistance of 7 cmH20 following the same protocol described above |
|
| Outcomes | Respiratory muscle strength: PImax (RV) | |
| Identification |
Country: Israel Author's name: Paltiel Weiner Institution: Department of Medicine A, Hillel Yaffe Medical Center Email: weiner@hillel‐yaffe.health.gov.il Address: Hadera 38100 Israel |
|
| Notes | ||
Wu 2017.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics IMT (Threshold device)
IMT (resistive device)
Control/sham
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics IMT: participants trained twice a day, 15 min/session for 8 weeks using either Threshold IMT (Respironics Inc; Pittsburgh, PA, USA) or Pflex (Respironics Inc, Pittsburgh, PA, USA) devices set at 60% of PImax Control/sham: no intervention received by this group |
|
| Outcomes | Dyspnea: BDI‐TDI
Functional exercise capacity:
HRQoL: CRQ
Respiratory muscle strength: PImax (RV) Laboratory exercise test: VO2peak (L/min) (Incremental cycle ergometer test) Respiratory function: FEV1
|
|
| Identification |
Sponsorship source: the study was supported by the Science and Technology Project of Guangdong Province (2017A020211018) and the Guangzhou Healthcare collaborative innovation major project (201604040012) and State's Key Project of Research and Development Plan(2017YFSF11078). Country: China Setting: Guangzhou Institute of Respiratory Disease Author's name: Rongchang Chen Institution: Guangzhou Institute of Respiratory Disease, State Key Laboratory of Respiratory Disease Email: chenrc_vip@163.com Address: Guangzhou, China Clinical trial register: NCT03101774 |
|
| Notes | ||
Xu 2018.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group Subgroup analysis: PImax: < or > 60 cmH2O |
|
| Participants |
Baseline characteristics IMT
Control/sham
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics IMT: participants trained daily, at home, 48 min/d for 8 weeks using Threshold IMT (Respironics, USA). The training load ranged from 30%‐45% of PImax Control/sham: participants received the same protocol and trained at no load. |
|
| Outcomes | Dyspnea: mMRC Functional exercise capacity: 6MWD HRQoL:
Respiratory muscle strength: PImax Respiratory function: FEV1
|
|
| Identification |
Sponsorship source: this work was funded unconditionally by Clinical Research training program of Southern Medical University (LC2016PY032), National Key R&D Program of China (2017YFC1310601), The Guangzhou Healthcare Collaborative Innovation Major Project (201604020012), Guangzhou Innovation and Entrepreneurship Education Project of Universities (201709T26), Special Fundsfor the Cultivation of Guangdong College Students’ Scientific and Technological Innovation (PDJHB0101). The sponsors have no any role in design, conduct, data interpretation of the study, and preparation, review or approval of this manuscript Country: China Setting: Zhujiang Hospital of Southern Medical University Author's name: Xin Chen Institution: Department of Respiratory Medicine, Zhujiang Hosptial, Southern Medical University Email: chen_xin1020@163.com Address: 253 Gongye Road, Guangzhou 510282, China Clinical trial register: NCT02326181 |
|
| Notes | ||
ZhouL 2016.
| Study characteristics | ||
| Methods |
Study design: RCT Study grouping: parallel‐group |
|
| Participants |
Baseline characteristics IMT
Control/sham
Overall
Included criteria
Excluded criteria
|
|
| Interventions |
Intervention characteristics IMT: participants trained twice a day, without supervision, 30 min/d, 6 d/week for 8 weeks using Threshold IMT device set at 60% of PImax. This group also received non‐invasive positive pressure ventilation Control: this group received long‐term oxygen therapy |
|
| Outcomes | Dyspnea: mMRC Functional exercise capacity: 6MWD Respiratory muscle strength: PImax |
|
| Identification |
Sponsorship source: National Natural Science Foundation of China (81361128004); Public welfare research and Capacity building special fund project (2014A020215033); Guangzhou Medical University Scientific Research Fund (2014c22) Country: China Setting: Guangzhou Institute of Respiratory diseases Comments: Author's name: Chen Rongchang Institution: Frist Affiliated Hospital of Guangzhou Medical University Email: member@wc.rf.org Address: Guangzhou 510120, China Clinical trial register: 0192675 |
|
| Notes | ||
6MWD: six‐minute walk distance; 12MWD: 12‐minute walk distance; ADL: activities of daily living; ATS: American Thoracic Society; BDI: Baseline Dyspnea Index; BMI: body mass index; CAT: Chronic Obstructive Pulmonary Disease Assessment Test; CCQ: Clinical COPD Questionnaire; CI: confidence interval; COPD: chronic obstructive pulmonary disease; CPET: cardiopulmonary exercise testing; CRQ: Chronic Respiratory Disease Questionnaire; ERS: European Respiratory Society; FEV1: forced expiratory volume at 1 second; FRC: functional residual capacity; FVC: forced vital capacity; GOLD: Global Initiative for Chronic Obstructive Lung Disease; HRQoL: health‐related quality of life; IMT: inspiratory muscle training; ISWT: incremental shuttle walk test; IVC: inspiratory vital capacity; MDP: Multidimensional Dyspnea Profile; MIP: maximal inspiratory pressure; mMRC: Modified Medical Research Council; MVV: maximal voluntary ventilation; PEmax: maximal expiratory pressure; PFT: pulmonary function test; PImax: maximal inspiratory pressure; PR: pulmonary rehabilitation; Pthmax: respiratory muscle endurance pressure; RCT: randomized controlled trial; RV: residual volume; SD: standard deviation; SE: standard error; SEM: standard error of the mean; SGRQ: St George's Respiratory Questionnaire; SpO2: Peripheral oxygen saturation; SWT: shuttle walk test; TDI: Transition Dyspnea Index; VO2max: maximal oxygen consumption; Wmax: maximum exercise workload
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahmad 2013 | Ineligible intervention |
| Aldrich 1985 | Ineligible study design |
| Anand 2013 | Ineligible intervention |
| Baines 2005 | Ineligible comparator |
| Basso Vanelli 2016 | Ineligible comparator |
| Battaglia 2009 | Ineligible intervention |
| Belman 1994 | Ineligible comparator |
| Bgin 1991 | Ineligible study design |
| Bissett 2016 | Ineligible patient population |
| Bjerre Jepsen 1981 | Ineligible intervention |
| Cader 2010 | Ineligible patient population |
| Chen 1985 | Ineligible intervention |
| Daynes 2018 | Ineligible study design |
| de Andrade 2005 | Ineligible study design |
| de Lucas Ramos 1998 | Ineligible study design |
| Di Mambro 2007 | Ineligible study design |
| DRKS00005637 | Ineligible comparator |
| DRKS00006021 | Cancelled Clinical trial |
| Elbouhy 2014 | Ineligible patient population |
| Elmorsi 2016 | Ineligible study design |
| Enright 2005 | Ineligible comparator |
| Garcia 2008 | Ineligible study design |
| Goldstein 1989 | Ineligible intervention |
| Gregg 1989 | Ineligible study design |
| Guyatt 1992 | Ineligible intervention |
| Hart 2000 | Ineligible patient population |
| Heydari 2015 | Ineligible comparator |
| Hopp 1996 | Ineligible study design |
| Ibakordor 2013 | Ineligible comparator |
| Ionescu 2005 | Non‐RCT |
| Izumizaki 2008 | Ineligible study design |
| Johnson 1996 | Ineligible study design |
| Kivastik 2015 | Ineligible study design |
| Koch 2020 | Ineligible study design |
| Kolesnikova 2016 | Ineligible intervention |
| Levine 1986 | Ineligible comparator |
| Liao 2015 | Ineligible intervention |
| Lin 2012 | Ineligible intervention |
| Lisboa 1994 | Ineligible patient population |
| Lisboa 1995a | Ineligible study design |
| Lisboa 1995b | Ineligible patient population |
| Lisboa 1998 | Ineligible study design |
| Madariaga 2007 | Ineligible comparison |
| Madsen 1985 | Ineligible comparator |
| Martin 2006 | Ineligible intervention |
| McKeon 1986 | Ineligible patient population |
| Meshcheriakova 2006 | Ineligible intervention |
| Minoguchi 2002 | Ineligible study design |
| NCT01218295 | Ineligible study design |
| NCT01556139 | Ineligible patient population |
| NCT01747694 | Ineligible intervention |
| NCT01945398 | Ineligible study design |
| NCT01956565 | Ineligible study design |
| NCT02186340 | Ineligible study design |
| NCT02278523 | Ineligible comparator |
| NCT02579200 | Ineligible patient population |
| NCT02914093 | Ineligible comparator |
| NCT02935166 | Ineligible study design |
| NCT03186092 | Ineligible patient population |
| NCT03500042 | Ineligible outcomes |
| NCT03739879 | Ineligible study design |
| NCT03844711 | Ineligible comparator |
| NCT03880630 | Ineligible study design |
| NCT04084405 | Ineligible comparator |
| NCT04117399 | Ineligible outcomes |
| NCT04460261 | Ineligible study design |
| Neves 2014a | Ineligible intervention |
| Neves 2014b | Ineligible study design |
| Nield 2007 | Ineligible intervention |
| Noseda 1987 | Ineligible comparator |
| O'Connor 2019 | Ineligible study design |
| Okura 2019 | Ineligible study design |
| Okura 2020 | Ineligible study design |
| PACTR201703002095224 | Ineligible comparator |
| Padula 2001 | Ineligible patient population |
| Perez 2010 | Ineligible intervention |
| Pescaru 2016 | Ineligible study design |
| Quintero 1999 | Ineligible study design |
| Richardson 1989 | Ineligible study design |
| Rocha 2015 | Ineligible intervention |
| Sassoon 1992 | Ineligible intervention |
| Serón 2005 | Ineligible patient population |
| Shahin 2008 | Ineligible study design |
| Shioya 2007 | Ineligible intervention |
| Similowski 1994 | Ineligible study design |
| Sivashanmugam 2019 | Ineligible comparator |
| Soicher 1998 | Ineligible study design |
| Sonne 1982 | Ineligible study design |
| Sudo 1997 | Ineligible intervention |
| Sugiyama 2010 | Ineligible study design |
| Sun 2003 | Ineligible intervention |
| TCTR20191009004 | Ineligible intervention |
| UMIN000030937 | Ineligible intervention |
| Van't Hul 2006 | Ineligible intervention |
| Villafranca 1998 | Ineligible study design |
| Wada 2016 | Ineligible intervention |
| Wu 2006 | Ineligible intervention |
| Xi 2015 | Ineligible intervention |
| Yamaguti 2012 | Ineligible intervention |
| Yan 1996 | Ineligible intervention |
| Yang 2005 | Ineligible intervention |
| Zhang 2008 | Ineligible study design |
Characteristics of studies awaiting classification [ordered by study ID]
Barter 1987.
| Methods | Unfound |
| Participants | Unfound |
| Interventions | Unfound |
| Outcomes | Unfound |
| Notes |
Bustamante 1997.
| Methods | Unfound |
| Participants | Unfound |
| Interventions | Unfound |
| Outcomes | Unfound |
| Notes |
Cassidy 2009.
| Methods |
Study design: RCT Study grouping: parallel‐group |
| Participants |
Baseline characteristics IMT
Control/sham
Overall
Included criteria: patients with COPD, following an acute exacerbation Excluded criteria: not reported |
| Interventions |
Intervention characteristics IMT: participants underwent 8 weeks of unsupervised IMT using a Threshold IMT device Control/sham: participants received sham IMT |
| Outcomes | Respiratory muscle strength: PImax Functional exercise capacity: 6MWD HRQoL: CRQ |
| Notes |
Country: Ireland Setting: home‐based training Author's name: C.Cassidy Institution: Respiratory Assessment Unit, CREST Directorate Address: St. James’s Hospital, Dublin 8, Ireland |
Cejudo 1998.
| Methods | Unfound |
| Participants | Unfound |
| Interventions | Unfound |
| Outcomes | Unfound |
| Notes |
Chen 2017.
| Methods |
Study design: RCT Study grouping: parallel‐group Clinical trial register: NCT02200549 |
| Participants |
Baseline characteristics PR+IMT
PR
Overall
Included criteria: not reported Excluded criteria: not reported |
| Interventions |
Intervention characteristics PR+IMT:
PR: training with a cycle ergometer for 8 weeks |
| Outcomes | Dyspnea Functional exercise capacity HRQoL: CRQ Respiratory muscle strength |
| Notes |
Country: China Author's name: X.Chen Institution: Zhujiang Hospital‐ Southern Medical University, Department of Respiratory Medicine Address: Guangzhou, China |
Croitoru 2013.
| Methods |
Study design: RCT Study grouping: parallel‐group |
| Participants |
Baseline characteristics PR+IMT
PR
Overall
Included criteria: not reported Excluded criteria: not reported |
| Interventions |
Intervention characteristics PR+IMT:
PR: participants received the same PR protocol described above. |
| Outcomes | Functional exercise capacity: 6MWD HRQoL: SGRQ Respiratory muscle strength: PImax |
| Notes |
Del Castillo Otero 1998.
| Methods | Unfound |
| Participants | Unfound |
| Interventions | Unfound |
| Outcomes | Unfound |
| Notes |
Di Marzo 2000.
| Methods | Unfound |
| Participants | Unfound |
| Interventions | Unfound |
| Outcomes | Unfound |
| Notes |
Di Marzo 2002.
| Methods | Unfound |
| Participants | Unfound |
| Interventions | Unfound |
| Outcomes | Unfound |
| Notes |
Downes Vogel 2002.
| Methods | Unfound |
| Participants | Unfound |
| Interventions | Unfound |
| Outcomes | Unfound |
| Notes |
Eastwood 2005.
| Methods | Unfound |
| Participants | Unfound |
| Interventions | Unfound |
| Outcomes | Unfound |
| Notes |
Gething 2001.
| Methods | Unfound |
| Participants | Unfound |
| Interventions | Unfound |
| Outcomes | Unfound |
| Notes |
Göhl 2006.
| Methods | Unfound |
| Participants | Unfound |
| Interventions | Unfound |
| Outcomes | Unfound |
| Notes |
IRCT201104266299N1.
| Methods |
Study design: RCT Study grouping: parallel‐group |
| Participants |
Baseline characteristics Overall
Included criteria
Exclusion criteria
|
| Interventions |
Intervention characteristics IMT: participants trained at home daily for 21 min, 6 d/week for 8 weeks Control/sham: no intervention |
| Outcomes | Dyspnea HRQoL: SGRQ |
| Notes |
IRCT20180205038633N1.
| Methods |
Study design: RCT Study grouping: parallel‐group |
| Participants |
Baseline characteristics PR+IMT
PR
IMT
Control/sham:
Overall
Inclusion/exclusion criteria
|
| Interventions |
Intervention characteristics IMT: the intensity of training was 40%‐60% (S‐Index 40%‐60%), 2 d/week, and 5 sessions of dermal muscle training with repeat 50 (5 Repeat 10) for about 15 min/session. PR: aerobic exercise method: with a treadmill and foot pedometer, 2 times a week, with 40%‐60% heart rate reserve for 40 min/session PR+IMT: in each session, breathing exercises were performed first and then aerobic exercises were performed on the lower extremities Control: this group did not take any special intervention other than the usual treatments (control group) |
| Outcomes | Dyspnea: mMRC Respiratory muscle strength: PImax Functional exercise capacity: 6MWD Respiratory function: FEV1 |
| Notes |
ISRCTN19258620.
| Methods |
Study design: RCT Study grouping: parallel‐group |
| Participants |
Baseline characteristics Inclusion criteria
Exclusion criteria
|
| Interventions | IMT: using Powerbreathe device |
| Outcomes | Dyspnea: Borg Functional exercise capacity: SWT Respiratory muscle strength: PImax Respiratory muscle endurance pressure: Pthmax Respiratory muscle endurance time: Tlim |
| Notes |
Jones 1985.
| Methods |
Study design: RCT Study grouping: parallel‐group |
| Participants |
Baseline characteristics Overall
Inclusion criteria: FEV1 < 1.2 L |
| Interventions |
Intervention characteristics IMT: training with resistive device for 10 weeks Control/sham: placebo training group |
| Outcomes | Functional exercise capacity: Wmax Functional exercise capacity: 12MWD |
| Notes |
Koppers 2004.
| Methods | Unfound |
| Participants | Unfound |
| Interventions | Unfound |
| Outcomes | Unfound |
| Notes |
Liu 1989.
| Methods | Unfound |
| Participants | Unfound |
| Interventions | Unfound |
| Outcomes | Unfound |
| Notes |
Manuel Vargas 1995.
| Methods |
Study design: RCT Study grouping: parallel‐group |
| Participants |
Baseline characteristics Overall
|
| Interventions |
Intervention characteristics IMT: participants trained with a Threshold device at 30% of PImax Control/sham: no training was provided |
| Outcomes | Functional exercise capacity |
| Notes |
Mendoza 2007.
| Methods | Unfound |
| Participants | Unfound |
| Interventions | Unfound |
| Outcomes | Unfound |
| Notes | Unfound |
Meshcherykova 2018.
| Methods |
Study design: RCT Study grouping: parallel‐group |
| Participants |
Baseline characteristics IMT
Control/sham
Overall
Included criteria: patients with severe COPD |
| Interventions |
Intervention characteristics IMT: participants received IMT for 3 months Control/sham: training with no load |
| Outcomes | Dyspnea: BDI‐TDI Functional exercise capacity: 6MWD Respiratory function: FEV1 Respiratory function: RV |
| Notes |
NCT01056081.
| Methods |
Study design: RCT Study grouping: parallel‐group |
| Participants |
Baseline characteristics Overall:
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention characteristics PR+IMT
PR: this group received only PR |
| Outcomes | Dyspnea Functional exercise capacity: exercise time Respiratory muscle strength: PImax |
| Notes |
NCT01903772.
| Methods |
Study design: RCT Study grouping: parallel‐group |
| Participants |
Inclusion Criteria:
Exclusion Criteria:
|
| Interventions |
Intervention characteristics IMT: Inspiratory Muscle Training Three times daily inspiratory muscle training (2x30 breaths) at an intensity of >50% PImax Sham IMT: Twice daily inspiratory muscle training (3x30 breaths) at an intensity of 5 centimeters of water (H2O) |
| Outcomes | Dyspnea Functional exercise capacity Respiratory muscle strength: PImax Inspiratory muscle endurance capacity |
| Notes | It is unclear whether the study was completed, and no contact details were found. |
NCT02392715.
| Methods |
Study design: RCT Study grouping: parallel‐group |
| Participants |
Baseline characteristics Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention characteristics PR+IMT:
PR: (+sham IMT): this group received general exercise training with a sham IMT set at 5 cmH2O. |
| Outcomes | Functional exercise capacity: 6MWD HRQoL: SGRQ Inspiratory muscle strength (PImax) |
| Notes |
NCT02673242.
| Methods |
Study design: RCT Study grouping: parallel‐group |
| Participants |
Baseline characteristics Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention characteristics IMT: using a Threshold device for 6 weeks Control/sham: participants received sham training or another intervention |
| Outcomes | Dyspnea: mMRC Functional exercise capacity: 6MWD HRQoL: CAT |
| Notes |
NCT03080662.
| Methods |
Study design: RCT Study grouping: parallel‐group |
| Participants |
Baseline characteristics Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention characteristics IMT: training with ORYGEN DUAL Sham Valve for 5 weeks Control/sham: training with the same protocol with no load |
| Outcomes | |
| Notes |
NCT03438019.
| Methods |
Study design: RCT Study grouping: parallel‐group |
| Participants |
Baseline characteristics Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention characteristics The TIRE IMT group: will receive a tablet with the TIRE software installed and a PrO2 device through which they will train. Training consists of 6 levels (A‐F) with 6 inspirations at each level for a total of 36 breaths. Recovery times between breaths range from 40‐5 seconds as the participant advances each level. TIRE data will be stored in the tablet for subsequent interrogation and data retrieval. The standard IMT group: will receive a Threshold Inspiratory Muscle Trainer. This device incorporates a flow‐independent one‐way valve to ensure consistent resistance and features an adjustable specific pressure setting to be set based on MIP values of each participant. Participants will be instructed to perform up to 36 breaths daily. To compare with TIRE training, we will ask participants to perform this within a 30‐min session. The sham IMT group: will also receive a Threshold device and undergo the exact protocol of group 2 but with minimal resistance applied (7 cm H2O, the lowest in the device). |
| Outcomes | Dyspnea: mMRC Functional exercise capacity: 6MWD HRQoL:
Respiratory muscle strength: PImax |
| Notes |
NCT03790410.
| Methods |
Study design: RCT Study grouping: parallel‐group |
| Participants |
Baseline characteristics Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention characteristics PR+IMT: PR consists of breathing exercises, bicycle ergometer conditionings, relaxations, and strength and endurance training. IMT consists of strengthening exercises on the diaphragm muscle. PR: This group undertook only PR. |
| Outcomes | Respiratory muscle strength: PImax |
| Notes |
Newall 1998.
| Methods | Unfound |
| Participants | Unfound |
| Interventions | Unfound |
| Outcomes | Unfound |
| Notes |
Newall 2000.
| Methods | Unfound |
| Participants | Unfound |
| Interventions | Unfound |
| Outcomes | Unfound |
| Notes | Unfound |
NTR2990.
| Methods | Unfound |
| Participants | Unfound |
| Interventions | Unfound |
| Outcomes | Unfound |
| Notes |
Pertuze 1994.
| Methods | Unfound |
| Participants | Unfound |
| Interventions | Unfound |
| Outcomes | Unfound |
| Notes |
Ramirez Sarmiento 2000.
| Methods | Unfound |
| Participants | Unfound |
| Interventions | Unfound |
| Outcomes | Unfound |
| Notes |
Reidi 2005.
| Methods |
Study design: RCT Study grouping: parallel‐group |
| Participants |
Baseline characteristics Overall
Inclusion criteria: FEV1 < 60% |
| Interventions |
Intervention characteristics IMT: the training was performed through the Threshold set at 30 of PImax. The program consisted of 4 weeks, with 3 weekly sessions where both groups were reassessed weekly Control/sham: the training consisted of performing the threshold with or without the natural resistance of the equipment < 7 cmH2O |
| Outcomes | Respiratory muscle strength: PImax |
| Notes |
Valderramas 2009.
| Methods | Unfound |
| Participants | Unfound |
| Interventions | Unfound |
| Outcomes | Unfound |
| Notes |
Vargas 1995.
| Methods |
Study design: RCT Study grouping: parallel‐group |
| Participants | |
| Interventions |
Intervention characteristics IMT: participants used an inexpensive pressure threshold load valve constructed according to the Appropriate Technology principles of the WHO, adjusted at 30% of MIP for 3 months Control/sham: this group did not receive any intervention. |
| Outcomes | Respiratory muscle strength: PImax |
| Notes |
Vargas 1998.
| Methods | Unfound |
| Participants | Unfound |
| Interventions | Unfound |
| Outcomes | Unfound |
| Notes |
Wang 2004.
| Methods |
Study design: RCT Study grouping: parallel‐group |
| Participants |
Baseline characteristics Overall
|
| Interventions |
Intervention characteristics IMT: twice a day for 6 months Control/sham: no intervention was received |
| Outcomes | Respiratory function: FEV1 (%pred) |
| Notes |
Wanke 1994.
| Methods |
Study design: RCT Study grouping: parallel‐group |
| Participants |
Baseline characteristics PR+IMT
PR
|
| Interventions |
Intervention characteristics PR+IMT:
PR: cycle ergometer training alone |
| Outcomes | Functional exercise capacity |
| Notes |
Weiner 2006a.
| Methods | Unfound |
| Participants | Unfound |
| Interventions | Unfound |
| Outcomes | Unfound |
| Notes |
Wolstenholme 1998.
| Methods | Unfound |
| Participants | Unfound |
| Interventions | Unfound |
| Outcomes | Unfound |
| Notes |
6MWD: six‐minute walk distance; 12MWD: 12‐minute walk distance; ATS: American Thoracic Society; BDI: Baseline Dyspnea Index; BMI: body mass index; CAT: COPD Assessment Test ; COPD: chronic obstructive pulmonary disease; CRQ: Chronic Respiratory Disease Questionnaire; ERS: European Respiratory Society; FEV1: forced expiratory volume at 1 second; GOLD: Global Initiative for Chronic Obstructive Lung Disease; HRQoL: health‐related quality of life; IMT: inspiratory muscle training; MIP: maximal inspiratory pressure; mMRC: Modified Medical Research Council; PImax: maximal inspiratory pressure; PR: pulmonary rehabilitation; RCT: randomized controlled trial; RV: residual volume; SGRQ: St George's Respiratory Questionnaire; SWT: shuttle walk test; TDI: Transition Dyspnea Index; TIRE: test of incremental respiratory endurance; WHO: World Health Organization; Wmax: maximum exercise workload
Characteristics of ongoing studies [ordered by study ID]
CTRI/2020/11/029226.
| Study name | Effects of inspiratory muscle training vs autogenic drainage in hospitalised COPD patients |
| Methods |
Study design: RCT Study grouping: parallel‐group |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
IMT: conducted for 30 min with a total of 10 sessions Control: this group did not receive any intervention |
| Outcomes | HRQoL: SGRQ Pulmonary function tests |
| Starting date | 20 November 2020 |
| Contact information |
Sponsorship source: KLE Academy of Higher Education and Research
Country: India Author's name: Varun Naik Institution: Physiotherapy Department Nehru Nagar Belgaum Belgaum Email: drvarunnaik@gmail.com Address: KARNATAKA 590010 Clinical trial register: CTRI/2020/11/029226 |
| Notes |
CTRI/2021/05/033469.
| Study name | To test the efficacy of inspiratory muscle training device Airofit in reducing breathlessness in COPD patients |
| Methods |
Study design: RCT Study grouping: parallel‐group |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention characteristics IMT (Airofit device group): the Airofit is a new IMT device, which combines a calibrated inspiratory flow resistance with a pressure transducer and mobile device app. As the user inhales through the device, a pressure load is created; the magnitude of the load is proportional to the rate of air flow, as well as to the flow resistance properties of the load setting. The former has been identified as a limitation of flow‐resistive IMT. To overcome the flow‐dependency of the training load, the Airofit uses a pressure transducer to communicate with bespoke software, in real time. The measurement of pressure provides the user with instantaneous visual feedback of the pressure created by their inspiratory muscles, i.e. their training effort. The software also provides the user with a personalized visual training intensity target (50% of MIP). A total of 19 patients with a spirometry‐proven stable COPD will be enrolled in the experimental arm. IMT (Powerbreathe device group): 19 participants will train at a load of 50% of PImax. Sham IMT: the participants will use control device ‘Breathing pacer’ |
| Outcomes | Inspiratory muscle strength (PImax) Adverse events |
| Starting date | 1 June 2021 |
| Contact information |
Sponsorship source: Airofit AS Copenhagen, Denmark Country: India Author's name: Atul Deshmukh Institution: Padmashree Dr. D Y Patil University Navi Mumbai Email: atul.deshmukh@dypatil.edu Address: Centre for Interdisciplinary Research, Ground floor, Central Research Facility, Near Simulation Laboratory Padmashree Dr. D Y Patil Univerisity Campus Sector 7 Nerul Navi Mumbai, MAHARASHTRA, 400706, India |
| Notes |
CTRI201712010952.
| Study name | Effect of breathing exercises to improve the strength of respiratory muscles in COPD population |
| Methods |
Study design: RCT Study grouping: parallel‐group |
| Participants |
Inclusion criteria: diagnosed COPD patients Exclusion criteria: women with COPD, those unable to comprehend |
| Interventions |
Intervention : IMT+PR Control: PR |
| Outcomes | 6MWD Respiratory muscle strength (PImax) CRQ |
| Starting date | 10 April 2017 |
| Contact information | veenakiran_nambiar@yahoo.co.in |
| Notes |
De Souza 2019.
| Study name | Does inspiratory muscle training (IMT) reduce depression in patients with COPD? |
| Methods |
Study design: RCT Study grouping: parallel‐group |
| Participants | Patients with COPD presenting with inspiratory muscle weakness |
| Interventions |
Intervention characteristics IMT: participants trained at 50% of PImax, 2 sessions/d of 30 breaths for 8 weeks, with a weekly face‐to‐face session Control/sham: participants trained at 10% of PImax with a similar protocol |
| Outcomes | Dyspnea: mMRC Functional exercise capacity: 6MWD Respiratory muscle strength: PImax |
| Starting date | |
| Contact information | souzayr@gmail.com |
| Notes |
Formiga 2020.
| Study name | Novel versus traditional inspiratory muscle training regimens as home‐based, stand‐alone therapies in COPD: protocol for a randomized controlled trial |
| Methods |
Study design: RCT Study grouping: parallel‐group |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention characteristics All groups will train once a day for 8 weeks TIRE: participants will use an on‐screen training template set at 50% of their MIP and SMIP. The training will consist of 6 levels (A‐F) with 6 inspirations per level for up to 36 efforts per session. Pre‐set recovery times between breaths: 60 seconds at level A to 50, 40, 30, 20 and 10 seconds at levels B to F, respectively Threshold IMT: participants will train using a one‐way spring‐loaded valve set at 50% of their MIP. The training will consist of 36 inspirations performed using the device within a 30‐min period. Sham‐IMT: participants will use a one‐way spring‐loaded valve set to its minimal resistance (−9 cmH2O). The training will consist of 36 inspirations performed using the device within a 30‐min period. |
| Outcomes | Dyspnea: mMRC Respiratory muscle strength: PImax HRQoL: CAT Functional exercise capacity: 6MWD Respiratory muscle strength (PImax) Pulmonary function test |
| Starting date | 1 May 2021 |
| Contact information | dosbaba.filip@fnbrno.cz |
| Notes | Clinical tiral register: NCT04415788 |
JPRN‐UMIN000039893.
| Study name | The effectiveness of inspiratory muscle training in patients with chronic obstructive pulmonary disease |
| Methods |
Study design: RCT Study grouping: parallel‐group |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention characteristics PR+IMT:
PR +sham IMT: this group will undergo the same rehabilitation program with an IMT load set at 10% of PImax |
| Outcomes | Functional exercise capacity: 6MWD HRQoL: CAT Inspiratory muscle strength (PImax) |
| Starting date | 01/11/2016 |
| Contact information |
Sponsorship source: Akita University Graduate School of Health Sciences Country: Japan Author's name: Takanobu Shioya Institution: Akita University Graduate School of Health Sciences Department of Physical Therapy Email: shioya@hos.akita‐u.ac.jp Address: 1‐1‐1, Hondo, Akita Japan Clinical trial register: JPRN‐UMIN000039893 |
| Notes |
JPRN‐UMIN000043099.
| Study name | Effect of inspiratory muscle training on diaphragm and exercise tolerance in patients with COPD |
| Methods |
Study design: RCT Study grouping: parallel‐group |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention characteristics IMT: IMT will be performed for 12 weeks Control: this group will not receive any intervention |
| Outcomes | Exercise capacity |
| Starting date | 22 January 2021 |
| Contact information |
Sponsorship source: Kindai University Hospital Country: Japan Author's name: Masashi Shiraishi Institution: Kindai University Hospital Department of Rehabilitation Email: masashi‐shiraishi@med.kindai.ac.jp Address: 377‐2 Onohigashi, Osakasayama‐city 589‐8511 Japan Clinical trial register: JPRN‐UMIN000043099 |
| Notes |
NCT04120142.
| Study name | Effect of inspiratory muscle training during PR on dyspnoea and exercise tolerance in COPD patients |
| Methods |
Study design: RCT Study grouping: parallel‐group |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention characteristics PR+IMT: IMT + aerobic exercise PR: aerobic exercise alone |
| Outcomes | Dyspnea Respiratory muscle strength (PImax) |
| Starting date | 1 February 2019 |
| Contact information | |
| Notes |
NCT04201522.
| Study name | The effect of respiratory training on exercise tolerance in COPD (ERTET) |
| Methods |
Study design: RCT Study grouping: parallel‐group |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention characteristics IMT: participants will train for 6 weeks, 15 min twice daily, 5 d/week at 60% of the peak of minute ventilation, at home by means of a respiratory device (SpiroTiger, Idiag, Fehraltorf, CH) Control/sham: participants will undergo the training protocol at rest's minute ventilation |
| Outcomes | Respiratory muscle strength (PImax) |
| Starting date | 14 March 2017 |
| Contact information | ferid.oueslati@criucpq.ulaval.ca |
| Notes |
NCT04387318.
| Study name | Inspiratory muscle training and neuromuscular electrical stimulation in chronic obstructive pulmonary disease |
| Methods |
Study design: RCT Study grouping: parallel‐group |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
PR+IMT:
PR: this group will undergo only the PR protocol described above |
| Outcomes | HRQoL: SGRQ Respiratory muscle strength (PImax) Respiratory muscle endurance |
| Starting date | 1 October 2019 |
| Contact information | albuisa@gmail.com |
| Notes |
NCT04802096.
| Study name | Effects of inspiratory muscle training in addition to pulmonary rehabilitation in patients with moderate to severe COPD exacerbation |
| Methods |
Study design: RCT Study grouping:parallel‐group |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
PR+IMT:
PR: this group will undergo the same PR program as the intervention group |
| Outcomes | Functional exercise capacity: 6MWD HRQoL: SGRQ HRQoL: CAT |
| Starting date | 15 March 2021 |
| Contact information |
Sponsorship source: National Taiwan University Hospital Country: Taiwan Author's name: Wei‐Yu Huang Institution: School and Graduate Institute of Physical Therapy of National Taiwan University Email: r08428013@ntu.edu.tw Address: Taipei, Zhongzheng Dist, Taiwan, 100 Clinical trial register: NCT04802096 |
| Notes |
RBR‐10nyzcqc.
| Study name | Effects of Inspiratory Muscle Training on Breathlesness, Exercise Capacity and Postural Control in Patients with COPD |
| Methods |
Study design: RCT Study grouping:parallel‐group |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
IMT: 22 patients will train at around 50% of their PImax. The training consists of 30 breaths in a row, with deep and strong inspiration (lasting 4‐5 minutes), twice a day, every day for 8 weeks. Each week, the patient will return to our center to perform a new manovacuometry and update the load value, maintaining the ~ 50% of the PImax value, and after this update, one of the sessions of the day will be held in person. Sham IMT: 16 patients will train at around 10% of their PImax. The training consists of 30 breaths in a row, with deep and strong inspiration (lasting 4‐5 minutes), twice a day, every day for 8 weeks. Every week, the patient will return to our center to perform a new manovacuometry, the data will be recorded, however the load will be kept at around 10% of the initial PImax value, after this registration, one of the sessions of the day will be held in person. This value will not make any changes during the study period. After the eight‐week protocol, patients will return to perform the initial evaluations and tests again, in the same order they did the first time, and will receive a new medical order for serum vitamin D and calcium measurement for comparison with the first. The CG participants after the study period will undergo the same protocol applied to the IG as treatment. |
| Outcomes | Dyspnea: mMRC Functional exercise capacity: 6MWD |
| Starting date | |
| Contact information | yves.souza@uva.br |
| Notes |
RBR 42rmqy.
| Study name | Inspiratory muscle training in chronic obstructive pulmonary disease oxygen‐dependent patients: a randomized controlled trial |
| Methods |
Study design: RCT Study grouping: parallel‐group |
| Participants |
IMT
Control/sham
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention characteristics IMT: the experimental group will have 30 individuals that will receive home‐based IMT, 7 d/week, over 4 weeks. The daily training will be split into 2 daily sessions (i.e. morning and afternoon). Each daily session will be comprised of four 4‐min sets of respiratory training with 1‐min rest intervals between each set. The resistance will be provided by the Power Breathe Classic, which allows individuals to exercise the inspiratory muscles during the training session. Training will be individually tailored for each participant. The initial training load for each participant will be set at 50% of his/her PImax. The participants will be trained and instructed to do the exercise program on their own with no supervision. Once a week, during the physical therapist’s home visit, the clinician will determine the new values for maximal inspiratory strength, and will adjust the load to 50% of the new values. The device will be covered with opaque material, so that participants will be blinded to the training load. Control/sham: the control group with 30 individuals will receive a sham intervention. Sham respiratory muscle training will be delivered using a Power Breathe with no resistance (0 cmH2O) or progression, over the 4‐week period, 40 min/d, 7 d/week |
| Outcomes | HRQoL: ADL Respiratory muscle strength (PImax) Laboratory exercise test: VO2peak |
| Starting date | 30 October 2019 |
| Contact information | viniciusmaldaner@gmail.com |
| Notes |
TCTR20210604001.
| Study name | Is single bout of inspiratory muscle training alter blood pressure and cardio autonomics modulation in COPD patients? : a pilot study |
| Methods |
Study design: RCT Study grouping: parallel‐group |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention characteristics IMT: participants will perform single session of IMT exercise with 60% of MIP. They will complete 6 breaths/set for 5 sets with 1‐min rest between each set Sham IMT: participants will undergo the same program described above at no load |
| Outcomes | |
| Starting date | 11 January 2021 |
| Contact information |
Sponsorship source: Khon Kaen University Khon Kaen, Thailand Country: Thailand Author's name: EAKARACH WONGSAYA Institution: School of Physical Therapy, Faculty of Associated Medical Sciences, Khon Kaen University Email: eakarach.wo@up.ac.th Address: 123 School of Physical Therapy, Faculty of Associated Medical Sciences, Khon Kaen University University 40002 Khon Kaen Thailand Clinical trial register: TCTR20210604001 |
| Notes |
Tctr 2022.
| Study name | Effect of inspiratory muscle training on cardiovascular autonomic functions in chronic obstructive pulmonary disease patients |
| Methods |
Study design: RCT Study grouping: parallel‐group |
| Participants | Patients with COPD |
| Interventions |
IMT Home‐based IMT will be performed daily using PowerBreathe device. Total eight week of IMT will be provided. The IMT group will start training at 40% of their initial PImax. The new PImax value will be measured every week by the researcher. The training load in each week will be increased continuously over time by adjusting to at least 50% of PImax or gradually increase to the highest tolerable intensity during each of the supervised sessions. The highest tolerable intensity means that the rates of perceived inspiratory effort using a modified Borg dyspnea scale will be the range 4 to 6 of 10 (moderate to very severe). Daily training will consist of 2 sessions of 30 breaths.,The participants will receive only the standard care treatment from the physicians such as pharmacological treatment. They will meet the physician at week 1 and week 8. Control This group will not receive any intervention |
| Outcomes | Dyspnea:
Functional exercise capacity: 6MWD Respiratory muscle strength: PImax Spirometry measurements |
| Starting date | 2022 |
| Contact information | |
| Notes |
6MWD: six‐minute walk distance; ADL: activities of daily living; BMI: body mass index; CAT: Chronic Obstructive Pulmonary Disease Assessment Test; COPD: chronic obstructive pulmonary disease; CRQ: Chronic respiratory disease questionnaire; DBP: diastolic blood pressure; FEV1: forced expiratory volume at 1 second; FVC: forced vital capacity; GOLD: Global Initiative of Chronic Obstructive Lung Disease; HRQoL: health‐related quality of life; IMT: inspiratory muscle training; MIP: maximal inspiratory pressure; mMRC: Modified Medical Research Council; PImax: maximal inspiratory pressure; PR: pulmonary rehabilitation; RCT: randomized controlled trial; SBP: systolic blood pressure; SGRQ: St George's Respiratory Questionnaire; SMIP: sustained maximal inspiratory pressure; TIRE: test of incremental respiratory endurance
Differences between protocol and review
Because few studies used the Borg scale and reported it at different levels of effort, we decided to replace it with PImax for subgroup analysis. The 6MWD and PImax are respectively clinical and physiological outcomes, and we believed that analysing both outcomes together would present an opportunity to check for the possible correlation between them.
Because many RCTs were included, we conducted a sensitivity analysis by keeping just the studies at an overall low risk of bias.
We conducted a sensitivity analysis by removing (Preusser 1994) because we noticed large differences in baseline data between the two groups.
We did not use Robvis to generate weighted bar plots because it was possible to create them with Revman Web, and finally we kept just the PRISMA flow diagram and funnel plots.
Contributions of authors
Conception of the review: OA/WF Co‐ordination of the review: OA Search and collection of studies for inclusion in the review: OA/WF Data extraction: OA/AK Assessment of the risk of bias in the included studies: OA/TL Analysis of data: OA/AK Assessment of certainty in the body of evidence: OA/TL Interpretation of data: WF/SK Writing the review: OA Clinical and statistical comments: RG/AR
Contributions of editorial team
Sally Spencer (Co‐ordinating Editor) edited the review; advised on methodology, interpretation and content; and approved the review prior to publication. Rebecca Fortescue (Co‐ordinating Editor) checked the data in the review. Anne Holland (Contact Editor): edited the review; and advised on methodology, interpretation and content. Emma Dennett (Deputy Co‐ordinating Editor): advised on methodology, interpretation and content; and edited the review. Emma Jackson (Managing Editor): co‐ordinated the editorial process; conducted peer review; obtained translations; and edited the plain language summary and reference sections of the review. Elizabeth Stovold (Information Specialist): designed the search strategy; ran the searches; and edited the search methods section.
Vittoria Lutje (Freelance Information Specialist): ran the updated search.
Kayleigh Kew (Freelance Editor): edited the review and advised on methodology.
Sources of support
Internal sources
-
University of Sfax, Faculty of Medicine, Tunisia
Omar Ammous is supported by the University of Sfax, Faculty of Medicine, to conduct this systematic review as his medical thesis.
External sources
-
National Institute for Health and Care Research (NIHR), UK
Provision of Cochrane Infrastructure funding to Cochrane Airways
Declarations of interest
OA: none known RG: none known AK: none known WF: none known TL: none known AR: none known SK: none known
Edited (no change to conclusions)
References
References to studies included in this review
Abedi Yekta 2019 {published data only}
- Abedi Yekta AH, Poursaeid Esfahani M, Salehi S, Hassabi M, Khosravi S, Kharabian S, et al. Assessment of the effects of inspiratory muscle training (IMT) and aerobic training on the quality of life of patients with chronic obstructive pulmonary disease. Tanaffus 2019;18(3):223-9. [PMC free article] [PubMed] [Google Scholar]
Bavarsad 2015 {published data only}
- Bavarsad MB, Shariati A, Eidani E, Latifi M. The effect of home-based inspiratory muscle training on exercise capacity,exertional dyspnea and pulmonary function in COPD patients. Iranian Journal of Nursing and Midwifery Research 2015;20(5):613-18. [DOI: 10.4103/1735-9066.164588] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beaumont 2015 {published and unpublished data}
- Beaumont M, Mialon P, Le Ber-Moy C, Lochon C, Peran L, Pichon R, et al. Inspiratory muscle training during pulmonary rehabilitation in chronic obstructive pulmonary disease: a randomized trial. Chronic Respiratory Disease 2015;12(4):305-12. [DOI: 10.1177/1479972315594625] [DOI] [PubMed] [Google Scholar]
- NCT01545011. Effects of inspiratory muscle training on dyspnea in subjects with chronic obstructive pulmonary disease (IMT) [Effects of inspiratory muscle training combined with a pulmonary rehabilitation program versus a program of pulmonary rehabilitation alone on dyspnea: a randomized trial]. clinicaltrials.gov/show/nct01545011 (first received 6 March 2012).
Beaumont 2018 {published data only}
- Beaumont M, Mialon P, Le Ber C, Le Mevel P, Peran L, Meurisse O, et al. Effects of inspiratory muscle training on dyspnoea in severe COPD patients during pulmonary rehabilitation: controlled randomised trial. European Respiratory Journal 2018;51(1):01. [DOI: 10.1183/13993003.01107-2017] [DOI] [PubMed] [Google Scholar]
- Beaumont M, Mialon P, Le Ber C, Le Mevel P, Peran L, Meurisse O, et al. Effects of inspiratory muscle training on dyspnea in severe COPD patients during pulmonary rehabilitation: controlled randomized trial. European Respiratory Journal 2017;50(suppl 61):OA2924. [DOI: 10.1183/1393003.congress-2017.OA2924] [DOI] [PubMed] [Google Scholar]
- NCT02074813. Inspiratory muscle training during pulmonary rehabilitation in COPD (EMI II) [Effects of inspiratory muscle training (IMT) on dyspnea in COPD during pulmonary rehabilitation: randomized controlled trial]. clinicaltrials.gov/show/NCT02074813 (first received 28 February 2014).
Beckerman 2005 {published data only}
- Beckerman M, Magadle R, Weiner M, Weiner P. The effects of 1 year of specific inspiratory muscle training in patients with COPD. Chest 2005;128(5):3177-82. [DOI: 10.1378/chest.128.5.3177] [DOI] [PubMed] [Google Scholar]
- Beckermann M, Weiner M, Weiner P. The effects of one year specific inspiratory muscle training in patients with COPD. In: European Respiratory Journal. Vol. 26 Suppl 49. 2005:Abstract No. 539.
- Weiner P, Beckerman M, Magadle R, Berar-Yanay N. Maintenance of inspiratory muscle training in COPD patients: one year follow up [Abstract]. In: European Respiratory Journal. Vol. 22. 2003:P3438. [DOI] [PubMed]
- Weiner P, Magadle R, Beckerman M, Weiner M, Berar-Yanay N. Maintenance of inspiratory muscle training in COPD patients: one year follow-up. European Respiratory Journal 2004;23(1):61-5. [DOI: 10.1183/09031936.03.00059503] [DOI] [PubMed] [Google Scholar]
Belman 1988 {published data only}
- Belman MJ, Shadmehr R. Targeted resistive ventilatory muscle training in chronic obstructive pulmonary disease. Journal of Applied Physiology 1988;65(6):2726-35. [DOI: 10.1152/jappl.1988.65.6.2726] [DOI] [PubMed] [Google Scholar]
Berry 1996 {published data only}
- Berry MJ, Adair NE, Sevensky KS, Quinby A, Lever, HM. Inspiratory muscle training and whole-body reconditioning in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1996;153(6):1812-16. [DOI: 10.1164/ajrccm.153.6.8665039] [DOI] [PubMed] [Google Scholar]
Berton 2015 {published data only}
- Axmann de Castro M. Efeito do treinamento muscular inspiratório sobre a dispneia, tolerância ao exercício e metaborreflexo da musculatura ventilatória em pacientes com doença pulmonar obstrutiva crônica [Masters thesis]. Porto Alegre: Federal University of Rio Grande do Sul (UFRGS), 2015. [https://www.lume.ufrgs.br/bitstream/handle/10183/119402/000969567.pdf?sequence=1] [Google Scholar]
- Berton DC, Castro M, Frohlich LF, Castilho M, Dorneles R, Chiappa G, et al. Effects of inspiratory muscle training on leg blood flow and exercise tolerance in COPD. European Respiratory Journal 2015;46 Suppl 59:PA2250. [Google Scholar]
- Castro MA, Fröhlich LF, Chiappa GR, Knorst MM, Neder JA, Berton DC, et al. Improvement in exercise capacity after inspiratory muscle training is related to increased calf blood flow during inspiratory load in COPD. SM Journal of Pulmonary Medicine 2016;2(1):1013. [Google Scholar]
Bustamante 2007 {published data only}
- Bustamante V, Gáldiz Iturri JB, Gorostiza Manterola A, Camino Buey J, Talayero Sebastián N, Peña Víctor S. Comparison of 2 methods for inspiratory muscle training in patients with chronic obstructive pulmonary disease [Comparación de 2 métodos de entrenamiento muscular inspiratorio en pacientes con EPOC]. Archivos de Bronconeumología 2007;43(8):431-8. [DOI: 10.1157/13108782] [DOI] [PubMed] [Google Scholar]
Charususin 2018 {published and unpublished data}
- Charususin N, Gosselink R, Decramer M, Demeyer H, McConnell A, Saey D, et al. Randomised controlled trial of adjunctive inspiratory muscle training for patients with COPD. Thorax 2018;73(10):942-50. [DOI: 10.1136/thoraxjnl-2017-211417] [DOI] [PubMed] [Google Scholar]
- Charususin N, Gosselink R, Decramer M, Demeyer H, McConnell A, Saey D, et al. Relation between training quality, improvements in inspiratory muscle function, and changes in exercise capacity following an inspiratory muscle training intervention (IMTCO study). European Respiratory Journal 2017;50 Suppl 61:PA3278. [Google Scholar]
- Charususin N, Gosselink R, Decramer M, McConnell A, Demeyer H, Saey D, et al. A multicentre randomised controlled trial of inspiratory muscle training for patients with chronic obstructive pulmonary disease (IMTCO study). European Respiratory Journal 2017;50 Suppl 61:OA2925. [DOI: 10.1183/1393003.congress-2017.OA2925] [DOI] [Google Scholar]
- Charususin N, Gosselink R, Decramer M, McConnell A, Saey D, Maltais F, et al. Inspiratory muscle training protocol for patients with chronic obstructive pulmonary disease (IMTCO study): a multicentre randomised controlled trial. BMJ Open 2013;3(8):e003101. [DOI: 10.1136/bmjopen-2013-003101] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charususin NA, Gosselink R, Decramer M, McConnell A, Saey D, Maltais F, et al. Inspiratory muscle training for patients with chronic obstructive pulmonary disease (IMTCO study): a multicentre randomised controlled trial. European Respiratory Journal 2016;48 Suppl 60:OA1519. [DOI: 10.1183/13993003.congress-2016.OA1519] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloeckl R, Schneeberger T, Jarosch I, Charususin N, Langer D, Kenn K, et al. Complementary inspiratory muscle training during pulmonary rehabilitation in COPD patients with inspiratory muscle weakness – a subgroup analysis of a randomized, controlled trial (IMTCO study). European Respiratory Journal 2017;50 Suppl 61:PA3277. [DOI: 10.1183/1393003.congress-2017.PA3277] [DOI] [Google Scholar]
- NCT01397396. Effects of inspiratory muscle training in COPD (IMTCO) [Effects of inspiratory muscle training in patients with chronic obstructive pulmonary disease - a randomized controlled trial]. clinicaltrials.gov/show/NCT01397396 (first received 19 July 2011).
Chuang 2017 {published data only}
- Chuang H-Y, Chang H-Y, Fang Y-Y, Guo S-E. The effects of threshold inspiratory muscle training in patients with chronic obstructive pulmonary disease: a randomised experimental study. Journal of Clinical Nursing 2017;26(23-4):4830-8. [DOI: 10.1111/jocn.13841] [DOI] [PubMed] [Google Scholar]
Covey 2001 {published data only}
- Covey MK, Larson JL, Wirtz SE, Berry JK, Pogue NJ, Alex CG, et al. High-intensity inspiratory muscle training in patients with chronic obstructive pulmonary disease and severely reduced function. Journal of Cardiopulmonary Rehabilitation 2001;21(4):231-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cutrim 2019 {published data only}
- Cutrim AL, Duarte AA, Silva-Filho AC, Dias CJ, Urtado CB, Ribeiro RM, et al. Inspiratory muscle training improves autonomic modulation and exercise tolerance in chronic obstructive pulmonary disease subjects: a randomized-controlled trial. Respiratory Physiology and Neurobiology 2019;263:31-7. [DOI: 10.1016/j.resp.2019.03.003] [DOI] [PubMed] [Google Scholar]
Dacha 2019 {published data only}
- Dacha S, Rodrigues A, Louvaris Z, Janssens L, Janssens W, Gosselink R, et al. Effects of inspiratory muscle training (IMT) on dyspnea, respiratory muscle function and respiratory muscle activation in patients with COPD during endurance cycling. European Respiratory Journal 2019;54 Suppl 63:PA2199. [DOI: 10.1183/13993003.congress-2019.PA2199] [DOI] [Google Scholar]
De Farias 2019 {published and unpublished data}
- Farias CA, Gualdi LP, da Silva SB, Parreira VF, Montemezzo D, Resqueti VR, et al. Effects of different modalities of inspiratory muscle training as an add-on to conventional treatment of patients with chronic obstructive pulmonary disease (COPD): study protocol for a randomized controlled trial. Trials 2019;20(1):231. [DOI: 10.1186/s13063-019-3271-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias C, Fregonezi G, Batista IP, Castro L, Lopes N, Gualdi L, et al. Effects of respiratory muscle training with different modalities in COPD patients: a randomized controlled trial. European Respiratory Journal 2019;54 Suppl 63:PA2198. [DOI: 10.1183/13993003.congress-2019.PA2198] [DOI] [Google Scholar]
- RBR-94v6kd. Effects of exercise of the respiratory muscles with different types of devices in people with chronic obstructive pulmonary disease. https://ensaiosclinicos.gov.br/rg/RBR-94v6kd/ (first received 23 November 2016).
Dekhuijzen 1991 {published data only}
- Dekhuijzen PN, Beek MM, Folgering HT, Van Herwaarden CL. Psychological changes during pulmonary rehabilitation and target-flow inspiratory muscle training in COPD patients with a ventilatory limitation during exercise. International Journal of Rehabilitation Research 1990;13:109-17. [DOI] [PubMed] [Google Scholar]
- Dekhuijzen PN, Folgering HT, Van Herwaarden CL. Target-flow inspiratory muscle training during pulmonary rehabilitation in patients with COPD. Chest 1991;99(1):128-33. [DOI: 10.1378/chest.99.1.128] [DOI] [PubMed] [Google Scholar]
- Dekhuijzen PNR, Van Herwaarden CLA, Folgering H. Target-flow inspiratory muscle training (IMT) increases inspiratory muscle strength and endurance. European Respiratory Journal 1989;2:389. [Google Scholar]
Dellweg 2017 {published data only}
- Dellweg D, Reissig K, Hoehn E, Siemon K, Haidl P. Inspiratory muscle training during rehabilitation in successfully weaned hypercapnic patients with COPD. Respiratory Medicine 2017;123:116-23. [DOI: 10.1016/j.rmed.2016.12.006] [DOI] [PubMed] [Google Scholar]
- NCT00291460. Inspiratory muscle training in hypercapnic COPD [Randomized controlled trial of inspiratory muscle training in patients with stable hypercapnic respiratory failure due to COPD]. clinicaltrials.gov/show/NCT00291460 (first received 14 February 2006).
Fanfa Bordin 2020 {published data only}
- Fanfa Bordin D, Cardoso DM, Wagner LE, Beckenkamp PR, Goncalves da Silva AL, Nunes Paiva D. Sternocleidomastoid muscle activation following inspiratory muscle training in patients with chronic obstructive pulmonary disease: a randomized clinical trial. Fisioterapia e Pesquisa 2020;27(2):133-9. [DOI: 10.1590/1809-2950/19009727022020] [DOI] [Google Scholar]
- NCT02014155. Effects of inspiratory muscle training on respiratory electromyographic activity in patients with COPD participants and non-participants of a pulmonary rehabilitation program. clinicaltrials.gov/show/nct02014155 (first received 18 December 2013).
Harver 1989 {published data only}
- Harver, AA, Mahler D, Daubenspeck JA. Targeted inspiratory muscle training improves respiratory muscle function and reduces dyspnea in patients with chronic obstructive pulmonary disease. Annals of Internal Medicine 2020;111(2):117-24. [DOI] [PubMed] [Google Scholar]
- Harver A, Mahler DA, Daubenspeck JA. Targeted inspiratory muscle training improves respiratory muscle function and reduces dyspnea in patients with chronic obstructive pulmonary disease. Annals of Internal Medicine 1989;111(2):117-24. [DOI: 10.7326/0003-4819-111-2-117] [DOI] [PubMed] [Google Scholar]
Heijdra 1996 {published data only}
- Heijdra YF, Dekhuijzen PN, Van Herwaarden CL, Folgering HT. Nocturnal saturation improves by target-flow inspiratory muscle training in patients with COPD. American Journal Respiratory Critical Care Medicine 1996;153(1):260-5. [DOI] [PubMed] [Google Scholar]
- Heijdra YF, Dekhuijzen PN, Van Herwaarden CL, Folgering HT. Target flow inspiratory muscle training (IMT) improves the nocturnal saturation in patients with COPD. European Respiratory Journal 1994;7 Suppl 18:237s. [DOI] [PubMed] [Google Scholar]
Hill 2006 {published data only}
- Hill K, Jenkins SC, Philippe DL, Cecins N, Shepherd KL, Green DJ, et al. High-intensity inspiratory muscle training in COPD. European Respiratory Journal 2006;27(6):1119-28. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hill 2007 {published data only}
- Hill K, Jenkins SC, Philippe DL, Shepherd KL, Hillman DR, Eastwood PR. Comparison of incremental and constant load tests of inspiratory muscle endurance in COPD. European Respiratory Journal 2007;30(3):479-86. [DOI: 10.1183/09031936.00095406] [DOI] [PubMed] [Google Scholar]
Hsiao 2003 {published data only}
- Hsiao SF, Wu YT, Wu HD, Wang TG, Wang TG. Comparison of effectiveness of pressure threshold and targeted resistance devices for inspiratory muscle training in patients with chronic obstructive pulmonary disease. Journal of the Formosan Medical Association / Taiwan yi zhi 2003;102(4):240-5. [PubMed] [Google Scholar]
Kim 1993 {published data only}
- Kim MJ, Larson JL, Covey MK, Vitalo CA, Alex CG, Patel M. Inspiratory muscle training in patients with chronic obstructive pulmonary disease. Nursing Research 1993;42(6):356-62. [PubMed] [Google Scholar]
Koppers 2006 {published data only}
- Koppers RJ, Vos PJ, Boot CR, Folgering HT. Exercise performance improves in patients with COPD due to respiratory muscle endurance training. Chest 2006;129(4):886-92. [DOI: 10.1378/chest.129.4.886] [DOI] [PubMed] [Google Scholar]
Langer 2018 {published data only}
- Dacha S, Langer D, Ciavaglia C, Webb K, Preston M, O'Donnell DE. Effect of inspiratory muscle training (IMT) on static and dynamic respiratory muscle function in patients with COPD. European Respiratory Journal 2017;50 Suppl 61:OA2923. [DOI: 10.1183/1393003.congress-2017.OA2923] [DOI] [Google Scholar]
- Dacha S, Langer D, Ciavaglia C, Webb K, Preston M, O'Donnell DE. Effect of inspiratory muscle training on respiratory muscle function and diaphragm activation in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2017;195(Meeting Abstracts):A2860. [DOI: 10.1164/ajrccm-conference.2017.A109] [DOI] [Google Scholar]
- Langer D, Charususin N, Jacome C, Hoffman M, McConnell A, Decramer M, et al. Efficacy of a novel method for inspiratory muscle training in people with chronic obstructive pulmonary disease. Physical Therapy 2015;95(9):1264-73. [DOI: 10.2522/ptj.20140245] [DOI] [PubMed] [Google Scholar]
- Langer D, Ciavaglia C, Faisal A, Webb KA, Neder JA, Gosselink R, et al. Inspiratory muscle training reduces diaphragm activation and dyspnea during exercise in COPD. Journal of Applied Physiology 2018;125(2):381-92. [DOI: 10.1152/japplphysiol.01078.2017] [DOI] [PubMed] [Google Scholar]
- Langer D, Ciavaglia C, Webb K, Preston M, Neder JA, Gosselink R, et al. Inspiratory muscle training reduces respiratory neural drive (RND) during exercise in patients with COPD. European Respiratory Journal 2014;44 Suppl 58:1912. [Google Scholar]
- NCT01900873. Effects of inspiratory muscle training on dyspnea perception during exercise in patients with COPD (IMTCOCOPD). clinicaltrials.gov/show/NCT01900873 (first received 17 July 2013).
Larson 1988 {published data only}
- Larson JL, Kim MJ, Sharp JT, Larson DA. Inspiratory muscle training with a pressure threshold breathing device in patients with chronic obstructive pulmonary disease. American Review of Respiratory Disease 1988;138(3):689-96. [DOI: 10.1164/ajrccm/138.3.689] [DOI] [PubMed] [Google Scholar]
Larson 1999 {published data only}
- Larson JL, Covey MK, Wirtz SE, Berry JK, Alex CG, Langbein WE, et al. Cycle ergometer and inspiratory muscle training in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1999;160(2):500-7. [DOI: 10.1164/ajrccm.160.2.9804067] [DOI] [PubMed] [Google Scholar]
Leelarungrayub 2017 {published data only}
- Leelarungrayub J, Pinkaew D, Puntumetakul R, Klaphajone J. REffects of a simple prototype respiratory muscle trainer on respiratory muscle strength, quality of life and dyspnea, and oxidative stress in COPD patients: a preliminary study. International Journal of Chronic Obstructive Pulmonary Disease 2017;12:1415-25. [DOI: 10.2147/COPD.S131062] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lisboa 1997 {published data only}
- Lisboa C, Villafranca C, Leiva A, Cruz E, Pertuze J, Borzone G. Inspiratory muscle training in chronic airflow limitation: effect on exercise performance. European Respiratory Journal 1997;10(3):537-42. [PubMed] [Google Scholar]
Mador 2005 {published data only}
- Mador MJ, Deniz O, Aggarwal A, Shaffer M, Kufel TJ, Spengler CM, et al. Effect of respiratory muscle endurance training in patients with COPD undergoing pulmonary rehabilitation. Chest 2005;128(3):1216-24. [DOI: 10.1378/chest.128.3.1216] [DOI] [PubMed] [Google Scholar]
- Mador MJ, Deniz O, Aggarwal A, Shaffer M, Kufel TJ, Spengler CM. Effect of respiratory muscle endurance training in patients with COPD undergoing pulmonary rehabilitation. In: American Thoracic Society 99th International Conference; 2003 May 16-21; Seattle. 2003:B046 Poster D8. [DOI] [PubMed]
Magadle 2007 {published data only}
- Magadle R, McConnell AK, Beckerman M, Weiner P. Inspiratory muscle training in pulmonary rehabilitation program in COPD patients. Respiratory Medicine 2007;101(7):1500-5. [DOI: 10.1016/j.rmed.2007.01.010] [DOI] [PubMed] [Google Scholar]
Majewska‐Pulsakowska 2016 {published data only}
- Majewska-Pulsakowska M, Wytrychowski K, Rozek-Piechura K. The role of inspiratory muscle training in the process of rehabilitation of patients with chronic obstructive pulmonary disease. Advances in Experimental Medicine and Biology 2016;885:47-51. [DOI: 10.1007/5584_2015_194] [DOI] [PubMed] [Google Scholar]
Masanga 2011 {published data only}
- Masanga L, Fernandez L. Additive effects of high-intensity inspiratory muscle training in pulmonary rehabilitation among COPD patients in the Philippines. Respirology 2011;16 Suppl 2:119 [1130]. [DOI: 10.1111/j.1400-1843.2011.02071.x] [DOI] [Google Scholar]
Nikoletou 2016 {published data only}
- Nikoletou D, Backley JA, Gearing J, Man WD, Mustafa N, Johnson LC, et al. Inspiratory muscle strength and endurance training in moderate to severe COPD patients [Abstract]. In: American Thoracic Society 100th International Conference; 2004 May 21-26; Orlando. 2004:D96 Poster 115.
- Nikoletou D, Backley JA, Gearing J, Man WD, Mustfa N, Johnson LC, et al. A double-blind, randomised controlled trial of inspiratory muscle training in COPD patients [Abstract]. Thorax 2003;58 Suppl 3:iii77. [Google Scholar]
- Nikoletou D, Man WD-C, Mustfa N, Moore J, Rafferty G, Grant RL, et al. Evaluation of the effectiveness of a home-based inspiratory muscle training programme in patients with chronic obstructive pulmonary disease using multiple inspiratory muscle tests. Disability and Rehabilitation 2016;38(3):250-9. [DOI: 10.3109/09638288.2015.1036171] [DOI] [PubMed] [Google Scholar]
Paneroni 2018 {published data only}
- Paneroni M, Simonelli C, Saleri M, Trainini D, Fokom G, Speltoni I, et al. Short-term effects of normocapnic hyperpnea and exercise training in patients with chronic obstructive pulmonary disease: a pilot study. American Journal of Physical Medicine and Rehabilitation 2018;97(12):866-72. [DOI: 10.1097/PHM.0000000000000988] [DOI] [PubMed] [Google Scholar]
Petrovic 2012 {published data only}
- NCT00469313. Efficacy of inspiratory muscle training on inspiratory capacity in patients with COPD. clinicaltrials.gov/show/NCT00469313 (first received 4 May 2007).
- Petrovic M, Reiter M, Zipko H, Pohl W, Wanke T. Effects of inspiratory muscle training on dynamichyperinflation in patients with COPD. International Journal of Chronic Obstructive Pulmonary Disease 2012;7:797-805. [DOI: 10.2147/COPD.S23784] [DOI] [PMC free article] [PubMed] [Google Scholar]
Preusser 1994 {published data only}
- Preusser BA, Winningham ML, Clanton TL. The effects of high versus low-intensity inspiratory muscle interval training in patients with COPD. Chest 1994;106(1):110-17. [DOI] [PubMed] [Google Scholar]
- Preusser BA, Winningham ML, Clanton TL. High- vs low-intensity inspiratory muscle interval training in patients with COPD. Chest 1994;106(1):110-7. [DOI: 10.1378/chest.106.1.110] [DOI] [PubMed] [Google Scholar]
Ramirez Sarmiento 2002 {published data only}
- Ramirez-Sarmiento A, Orozco-Levi M, Guell R, Barreiro E, Hernandez N, Mota S, et al. Inspiratory muscle training in patients with chronic obstructive pulmonary disease: structural adaptation and physiologic outcomes. American Journal of Respiratory and Critical Care Medicine 2002;166(11):1491-7. [DOI: 10.1164/rccm.200202-075OC] [DOI] [PubMed] [Google Scholar]
Saher 2021 {published data only}
- Saher T, Moiz JA, Bhati P, Ali MS, Talwar D. Effect of inspiratory muscle training in hypercapnic chronic obstructive pulmonary disease patients during acute care: a randomised clinical trial. Comparative Exercise Physiology 2021;17(1):55-63. [Google Scholar]
Saka 2021 {published data only}
- NCT03517839. Assessment of impact of inspiratory muscle training on movement fear due to dyspnea in chronic obstructive pulmonary disease. clinicaltrials.gov/show/nct03517839 (first received 7 May 2018).
- Saka S, Gurses HN, Bayram M. Effect of inspiratory muscle training on dyspnea-related kinesiophobia in chronic obstructive pulmonary disease: a randomized controlled trial. Complementary Therapies in Clinical Practice 2021;44:101418. [DOI] [PubMed] [Google Scholar]
Sanchez Riera 2001 {published data only}
- Sanchez Riera H, Montemayor Rubio T, Ortega Ruiz F, Cejudo Ramos P, Del Castillo Otero D, Elias Hernandez T, et al. Inspiratory muscle training in patients with COPD: effect on dyspnea, exercise performance, and quality of life. Chest 2001;120(3):748-56. [DOI: 10.1378/chest.120.3.748] [DOI] [PubMed] [Google Scholar]
Scherer 2000 {published data only}
- Scherer TA, Spengler CM, Owassapian D, Imhof E, Boutellier U. Respiratory muscle endurance training in chronic obstructive pulmonary disease: impact on exercise capacity, dyspnea, and quality of life. American Journal of Respiratory and Critical Care Medicine 2000;162(5):1709-14. [DOI: 10.1164/ajrccm.162.5.9912026] [DOI] [PubMed] [Google Scholar]
Schultz 2018 {published data only}
- Schultz K, Jelusic D, Wittmann M, Kramer B, Huber V, Fuchs S, et al. Inspiratory muscle training does not improve clinical outcomes in 3-week COPD rehabilitation: results from a randomised controlled trial. European Respiratory Journal 2018;51(1):1702000. [DOI: 10.1183/13993003.02000-2017] [DOI] [PubMed] [Google Scholar]
- Schultz K, Kramer B, Fuchs S, Wingart S, Lehbert N, Huber V, et al. Effects of routine inspiratory muscle training (IMT) as add-on to pulmonary rehabilitation (PR) in COPD. European Respiratory Journal 2015;46 Suppl 59:PA544. [DOI: 10.1183/13993003.congress-2015.PA544] [DOI] [Google Scholar]
Sykes 2005 {published data only}
- Sykes K, Hang HW. Inspiratory muscle training in the treatment of chronic obstructive pulmonary disease: randomized controlled trial. American Journal of Recreation Therapy 2005;4(2):39-48. [Google Scholar]
Tounsi 2021 {published data only}
- Tounsi B, Acheche A, Lelard T, Tabka Z, Trabelsi Y, Ahmaidi S. Effects of specific inspiratory muscle training combined with whole-body endurance training program on balance in COPD patients: randomized controlled trial. PLOS ONE 23 September 2023;16(9):e0257595. [DOI: 10.1371/journal.pone.0257595] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tout 2013 {published data only}
- Tout R, Tayara L, Halimi M. The effects of respiratory muscle training on improvement of the internal and external thoraco-pulmonary respiratory mechanism in COPD patients. Annals of Physical and Rehabilitation Medicine 2013;56(3):193-211. [DOI: 10.1016/j.rehab.2013.01.008] [DOI] [PubMed] [Google Scholar]
Wang 2017 {published data only}
- Wang K, Zeng G-Q, Li R, Luo Y-W, Wang M, Hu Y-H, et al. Cycle ergometer and inspiratory muscle training offer modest benefit compared with cycle ergometer alone: a comprehensive assessment in stable COPD patients. International Journal of Chronic Obstructive Pulmonary Disease 2017;12:2655-68. [DOI: 10.2147/COPD.S140093] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuwen L, Peng L, Yun L, Yitai C, Yuxia H, Kai W, et al. Effects of combined cycle ergometer and inspiratory muscle training in patients with stable chronic obstructive pulmonary disease. Respirology 2016;21 Suppl 3:176 (APSR6-0158). [DOI: 10.1111/resp.12939-15] [DOI] [Google Scholar]
Weiner 1992 {published data only}
- Weiner P, Azgad Y, Ganam R, Weiner M. Inspiratory muscle training combined with general exercise reconditioning in patients with COPD. Chest 1992;102(5):1351-6. [DOI: 10.1378/chest.102.5.1351] [DOI] [PubMed] [Google Scholar]
- Weiner P, Azgad Y, Weiner M, Ganem R. Inspiratory muscle training combined with general exercise reconditioning in chronic obstructive pulmonary disease. Harefuah 1993;124(7):396-400, 456. [PubMed] [Google Scholar]
Weiner 2000 {published data only}
- Weiner P, Magadle R, Berar-Yanay N, Davidovich A, Weiner M. The cumulative effect of long-acting bronchodilators, exercise, and inspiratory muscle training on the perception of dyspnea in patients with advanced COPD. Chest 2000;118(3):672-8. [DOI: 10.1378/chest.118.3.672] [DOI] [PubMed] [Google Scholar]
Weiner 2003 {published data only}
- Weiner P, Magadle R, Beckerman M, Weiner M, Berar-Yanay N. Comparison of specific expiratory, inspiratory, and combined muscle training programs in COPD. Chest 2003;124(4):1357-64. [DOI: 10.1378/chest.124.4.1357] [DOI] [PubMed] [Google Scholar]
Weiner 2006 {published data only}
- Weiner P, Weiner M. Inspiratory muscle training may increase peak inspiratory flow in chronic obstructive pulmonary disease. Respiration 2006;73(2):151-6. [DOI: 10.1159/000088095] [DOI] [PubMed] [Google Scholar]
Wu 2017 {published data only}
- NCT03101774. Inspiratory muscle training on respiratory muscle function, quality of life and exercise capacity in stable COPD [Effects of respiratory physiology-oriented inspiratory muscle training on respiratory muscle function, quality of life and exercise capacity in stable COPD with inspiratory muscle weakness: a randomised controlled trial]. clinicaltrials.gov/show/nct03101774 (first received 5 April 2017).
- Wu W, Guan L, Zhang X, Li X, Yang Y, Guo B, et al. Effects of two types of equal-intensity inspiratory muscle training in stable patients with chronic obstructive pulmonary disease: a randomised controlled trial. Respiratory Medicine 2017;132:84-91. [DOI: 10.1016/j.rmed.2017.10.001] [DOI] [PubMed] [Google Scholar]
Xu 2018 {published data only}
- Xu W, Li R, Guan L, Wang K, Hu Y, Xu L, et al. Combination of inspiratory and expiratory muscle training in same respiratory cycle versus different cycles in COPD patients: a randomized trial. Respiratory Research 2018;19(1):225. [DOI: 10.1186/s12931-018-0917-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
ZhouL 2016 {published data only}
- Zhou L-Q, Li X-Y, Li Y, Guo B-P, Guan L-L, Chen X, et al. Inspiratory muscle training followed by non-invasive positive pressure ventilation in patients with severe chronic obstructive pulmonary disease: a randomized controlled trial. Nan fang yi ke da xue xue bao [Journal of Southern Medical University] 2016;36(8):1069-74. [PubMed] [Google Scholar]
References to studies excluded from this review
Ahmad 2013 {published data only}
- Ahmad H, Justine M, Othman Z, Mohan V, Mirza FT. The outcomes of short term inspiratory muscle training (IMT) combined with chest physiotherapy in hospitalized COPD patients. Bangladesh Journal of Medical Science 2013;12(4):398-404. [DOI: 10.3329/bjms.v12i4.13302] [DOI] [Google Scholar]
Aldrich 1985 {published data only}
- Aldrich TK. The application of muscle endurance training techniques to the respiratory muscles in COPD. Lung 1985;163(1):15-22. [DOI: 10.1007/BF02713802] [DOI] [PubMed] [Google Scholar]
Anand 2013 {published data only}
- Anand A, Narwal R, Sindhwani G. Accessory inspiratory muscles energy technique effect on pulmonary function in COPD subjects. Indian Journal of Physiotherapy and Occupational Therapy 2013;7(3):192-7. [DOI: 10.5958/j.0973-5674.7.3.091] [DOI] [Google Scholar]
Baines 2005 {published data only}
- Baines SJ, Enright SJ. Does a threshold loading device maintain functional improvements following a programme of high intensity inspiratory muscle training in patients with chronic obstructive pulmonary disease? [Abstract]. European Respiratory Journal 2005;26 Suppl 49:4583. [Google Scholar]
Basso Vanelli 2016 {published data only}
- Basso-Vanelli R, Pantoni C, Borghi-Silva A, Labadessa I, Regueiro E, Di Lorenzo V, et al. Inspiratory muscle training improves thoracoabdominal asynchronism during unsupported upper limb exercise in COPD patients-pilot study. European Respiratory Journal 2016;48 Suppl 60:PA1373. [DOI: 10.1183/13993003.congress-2016.PA1373] [DOI] [Google Scholar]
- Basso-Vanelli RP, Pires Di Lorenzo VA, Labadessa IG, Regueiro Eloisa MG, Jamami Mauricio. Effects of inspiratory muscle training and calisthenics-and-breathing exercises in COPD with and without respiratory muscle weakness. Respiratory Care 2016;61(1):50-60. [DOI: 10.4187/respcare.03947] [DOI] [PubMed] [Google Scholar]
- NCT01510041. Effects of respiratory muscle training and respiratory exercise in exercise tolerance, performing daily life activities and quality of life of patients with chronic obstructive pulmonary disease. clinicaltrials.gov/show/NCT01510041 (first received 13 January 2012).
Battaglia 2009 {published data only}
- Battaglia E, Fulgenzi A, Ferrero ME. Rationale of the combined use of inspiratory and expiratory devices in improving maximal inspiratory pressure and maximal expiratory pressure of patients with chronic obstructive pulmonary disease. Archives of Physical Medicine and Rehabilitation 2009;90(6):913-18. [DOI: 10.1016/j.apmr.2008.12.019] [DOI] [PubMed] [Google Scholar]
Belman 1994 {published data only}
- Belman MJ, Botnick WC, Nathan SD, Chon KH. Ventilatory load characteristics during ventilatory muscle training. Journal of Cardiopulmonary Rehabilitation and Prevention 1994;14(6):409. [DOI] [PubMed] [Google Scholar]
Bgin 1991 {published data only}
- Bégin P, Grassino A. Inspiratory muscle dysfunction and chronic hypercapnia in chronic obstructive pulmonary disease. American Review of Respiratory Disease 1991;143(5 Pt 1):905-12. [DOI: 10.1164/ajrccm/143.5_Pt_1.905] [DOI] [PubMed] [Google Scholar]
Bissett 2016 {published data only}
- Bissett B, Leditschke IA, Neeman T, Boots R, Paratz J. Inspiratory muscle training to enhance recovery from prolonged mechanical ventilation: a randomized trial. American Journal of Respiratory and Critical Care Medicine 2016;193(Meeting Abstracts):A2613. [Google Scholar]
Bjerre Jepsen 1981 {published data only}
- Bjerre-Jepsen K, Secher NH, Kok-Jensen A. Inspiratory resistance training in severe chronic obstructive pulmonary disease. European Journal of Respiratory Diseases 1981;62(6):405-11. [PubMed] [Google Scholar]
Cader 2010 {published data only}
- Cader SA, Vale RG, Castro JC, Bacelar SC, Biehl C, Gomes MC, et al. Inspiratory muscle training improves maximal inspiratory pressure and may assist weaning in older intubated patients: a randomised trial. Journal of Physiotherapy 2010;56(3):171-7. [DOI: 10.1016/s1836-9553(10)70022-9] [DOI] [PubMed] [Google Scholar]
Chen 1985 {published data only}
- Chen H, Dukes R, Martin BJ. Inspiratory muscle training in patients with chronic obstructive pulmonary disease. American Review of Respiratory Disease 1985;131(2):251-5. [DOI: 10.1164/arrd.1985.131.2.251] [DOI] [PubMed] [Google Scholar]
Daynes 2018 {published data only}
- Daynes E, Greening NJ, Harvey-Dunstan TC, Singh SJ. High-frequency airway oscillating device for respiratory muscle training in subjects with COPD. Respiratory Care 2018;63(5):584-90. [DOI: 10.4187/respcare.05837] [DOI] [PubMed] [Google Scholar]
de Andrade 2005 {published data only}
- Andrade AD, Silva TN, Vasconcelos H, Marcelino M, Rodrigues-Machado MG, Filho VC, et al. Inspiratory muscular activation during threshold therapy in elderly healthy and patients with COPD. Journal of Electromyography and Kinesiology 2005;15(6):631-9. [DOI: 10.1016/j.jelekin.2005.06.002] [DOI] [PubMed] [Google Scholar]
de Lucas Ramos 1998 {published data only}
- Lucas Ramos P, Rodriguez Gonzalez-Moro JM, Garcia de Pedro J, Santacruz Siminiani A, Tatay Marti E, Cubillo Marcos JM. Training of inspiratory muscles in chronic obstructive lung disease. Its impact on functional changes and exercise tolerance. Archivos de Bronconeumologia 1998;34(2):64-70. [PubMed] [Google Scholar]
Di Mambro 2007 {published data only}
- Di Mambro TR, Figueiredo PH, Wanderley TR, Kristki AL, Guimaraes FS. Inspiratory muscle training in chronic obstructive pulmonary disease: impact on quality of life, exercise intolerance, and dyspnea. Fisioterapia e Pesquisa 2007;14(2):65-71. [Google Scholar]
DRKS00005637 {published data only}
- DRKS00005637. Respiratory muscle activation by respiratory muscle training in patients with advanced COPD [Respiratory muscle activation by respiratory muscle training in patients with advanced COPD - EMG-Train COPD]. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=DRKS00005637 (first received 4 February 2014).
DRKS00006021 {published data only}
- DRKS00006021. Influence of a controlled inspiratory muscle training on the course of COPD after acute exacerbation (AECOPD): a controlled study. www.drks.de/DRKS00006021 (first received 9 May 2014).
Elbouhy 2014 {published data only}
- Elbouhy MS, AbdelHalim HA, Hashem AM. Effect of respiratory muscles training in weaning of mechanically ventilated COPD patients. Egyptian Journal of Chest Diseases and Tuberculosis 2014;63(3):679-87. [DOI: ] [Google Scholar]
Elmorsi 2016 {published data only}
- Elmorsi AS, Eldesoky ME, Mona AA, Shalaby NM, Abdalla Dina A, et al. Effect of inspiratory muscle training on exercise performance and quality of life in patients with chronic obstructive pulmonary disease. Egyptian Journal of Chest Diseases and Tuberculosis 2016;65(1):41-6. [DOI: 10.1016/j.ejcdt.2015.10.006] [DOI] [Google Scholar]
Enright 2005 {published data only}
- Enright SJ, Baines SJ. Evaluation of a new computerised method of high intensity inspiratory muscle training in patients with chronic airways obstruction [Abstract]. European Respiratory Journal 2005;26 Suppl 49:Abstract No. 4584. [Google Scholar]
Garcia 2008 {published data only}
- Garcia S, Rocha M, Pinto P, Lopes AM, Bárbara C. Treino de músculos inspiratórios em doentes com DPOC. Revista Portuguesa de Pneumologia 2008;14(2):177-94. [PubMed] [Google Scholar]
- Garcia S, Rocha M, Pinto P, Lopes AM, Barbara C. Inspiratory muscle training in COPD patients. Revista Portuguesa de Pneumologia 2008;14(2):177-94. [PubMed] [Google Scholar]
Goldstein 1989 {published data only}
- Goldstein R, De Rosie J, Long S, Dolmage T, Avendano MA. Applicability of a threshold loading device for inspiratory muscle testing and training in patients with COPD. Chest 1989;96(3):564-71. [DOI] [PubMed] [Google Scholar]
Gregg 1989 {published data only}
- Gregg BL. Inspiratory muscle training with a weighted incentive spirometer in subjects with chronic airways obstruction. Respiratory Care 1989;34(10):860-7. [Google Scholar]
Guyatt 1992 {published data only}
- Guyatt G, Keller J, Singer J, Halcrow S, Newhouse M. Controlled trial of respiratory muscle training in chronic airflow limitation. Thorax 1992;47(8):598-602. [DOI: 10.1136/thx.47.8.598] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hart 2000 {published data only}
- Hart N, Cramer D, Sylvester K, Nickol AH, Ward SP, Moxham J, et al. Effect of using powerbreathe™ on inspiratory muscle strength. Thorax 2000;55 Suppl 3:A51. [Google Scholar]
Heydari 2015 {published data only}
- Heydari A, Farzad M, Ahmadi Hosseini SH. Comparing inspiratory resistive muscle training with incentive spirometry on rehabilitation of COPD patients. Rehabilitation Nursing 2015;40(4):243-8. [DOI: 10.1002/rnj.136] [DOI] [PubMed] [Google Scholar]
Hopp 1996 {published data only}
- Hopp LJ, Kim MJ, Larson JL, Sharp JT. Incremental threshold loading in patients with chronic obstructive pulmonary disease. Nursing Research 1996;45(4):196–202. [DOI] [PubMed] [Google Scholar]
- Hopp LJ. Incremental threshold loading in chronic obstructive pulmonary disease patients. Nursing Research 1992;45:147p. [DOI] [PubMed] [Google Scholar]
Ibakordor 2013 {published data only}
- Ibakordor S, Dhillon DK, Saravanan S. A safe inspiratory threshold load in chronic obstructive pulmonary disease patients. Indian Journal of Physiotherapy and Occupational Therapy 2013;7(2):48-52. [DOI: 10.5958/j.0973-5674.7.2.011] [DOI] [Google Scholar]
Ionescu 2005 {published data only}
- Ionescu AA, Mickleborough TD, Bolton CE, Lindley MR, Chatham K, Nixon LS, et al. Limb and inspiratory muscle dynamic strength in patients with moderate chronic obstructive pulmonary disease [Abstract]. Thorax 2005;2 Suppl II:ii50. [Google Scholar]
Izumizaki 2008 {published data only}
- Izumizaki M, Satake M, Takahashi H, Sugawara K, Shioya T, Homma I. Effects of inspiratory muscle thixotropy on the 6-min walk distance in COPD. Respiratory Medicine 2008;102(7):970-7. [DOI: 10.1016/j.rmed.2008.02.007] [DOI] [PubMed] [Google Scholar]
Johnson 1996 {published data only}
- Johnson PH, Cowley AJ, Kinnear WJ. Evaluation of the Threshold trainer for inspiratory muscle endurance training: comparison with the weighted plunger method. European Respiratory Journal 1996;9(12):2681-4. [DOI: 10.1183/09031936.96.09122681] [DOI] [PubMed] [Google Scholar]
Kivastik 2015 {published data only}
- Kivastik J, Arend M, Maestu J. Comparison of different inspiratory muscle warm-up protocols. European Respiratory journal 2015;46 Suppl 59:PA950. [Google Scholar]
Koch 2020 {published data only}
- Koch R, Augusto TR, Ramos AG, Muller PT. Inspiratory muscle training potentiates the beneficial effects of proportional assisted ventilation on exertional dyspnea and exercise tolerance in COPD: a proof-of-concept randomized and controlled trial. COPD 2020;17(4):1-8. [DOI: 10.1080/15412555.2020.1789085] [DOI] [PubMed] [Google Scholar]
Kolesnikova 2016 {published data only}
- Kolesnikova E, Arutyunov G, Kostyukevich O, Rylova A. Respiratory muscles trainings are effective in lowering the pneumonia frequency in patients with heart failure and chronic obstructive pulmonary disease. European Respiratory Journal 2016;48 Suppl 60:PA3773. [DOI: 10.1183/13993003.congress-2016.PA3773] [DOI] [Google Scholar]
Levine 1986 {published data only}
- Levine S, Weiser P, Gillen J. Evaluation of a ventilatory muscle endurance training program in the rehabilitation of patients with chronic obstructive pulmonary disease. American Review of Respiratory Disease 1986;133(3):400-6. [DOI: 10.1164/arrd.1986.133.3.400] [DOI] [PubMed] [Google Scholar]
Liao 2015 {published data only}
- Liao LY, Chen KM, Chung WS, Chien JY. Efficacy of a respiratory rehabilitation exercise training package in hospitalized elderly patients with acute exacerbation of COPD: a randomized control trial. International Journal of Chronic Obstructive Pulmonary Disease 2015;10(1):1703-9. [DOI: 10.2147/COPD.S90673] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2012 {published data only}
- Lin W-C, Yuan S-C, Chien J-Y, Weng S-C, Chou M-C, Kuo H-W. The effects of respiratory training for chronic obstructive pulmonary disease patients: a randomised clinical trial. Journal of Clinical Nursing 2012;21:2870-8. [DOI: 10.1111/j.1365-2702.2012.04124.x] [DOI] [PubMed] [Google Scholar]
Lisboa 1994 {published data only}
- Lisboa C, Munoz V, Beroiza T, Leiva A, Cruz E. Inspiratory muscle training in chronic airflow limitation: comparison of two different training loads with a threshold device. European Respiratory Journal 1994;7(7):1266-74. [DOI] [PubMed] [Google Scholar]
Lisboa 1995a {published data only}
- Lisboa C, Villafranca C, Pertuze J, Leiva A, Repetto P. Clinical effects of inspiratory muscle training in patients with chronic airflow limitation. Revista Medica de Chile 1995;123(9):1108-15. [PubMed] [Google Scholar]
Lisboa 1995b {published data only}
- Lisboa C, Villafranca C, Leiva A, Repetto L, Cruz E, Pertuze R. Inspiratory muscle training in patients with chronic bronchial obstruction: factors related to the improvement of dyspnoea. Enfermedades respir. Cir. Torac 1995;11(1):7-15. [Google Scholar]
Lisboa 1998 {published data only}
- Lisboa C, Borzone G, Cruz E. Inspiratory muscle training in patients with chronic obstructive pulmonary disease. Revista Medica de Chile 1998;126(5):563-8. [PubMed] [Google Scholar]
Madariaga 2007 {published data only}
- Madariaga VB, Iturri JB, Manterola AG, Buey JC, Sebastian NT, Pena VS. Comparison of 2 methods for inspiratory muscle training in patients with chronic obstructive pulmonary disease. Archivos de Bronconeumologia 2007;43(8):431-8. [DOI: 10.1157/13108782] [DOI] [PubMed] [Google Scholar]
Madsen 1985 {published data only}
- Madsen F, Secher NH, Kay L, Rube N. Inspiratory resistance versus general physical training in patients with chronic obstructive pulmonary disease. European Journal of Respiratory Diseases 1985;67(3):167-76. [PubMed] [Google Scholar]
Martin 2006 {published data only}
- Martin D, Davenport W, Gonzalez-Rothi J, Baz M, Banner J, Caruso L, et al. Inspiratory muscle strength training improves outcome in failure to wean patients [Abstract]. European Respiratory Journal 2006;28 Suppl 50:369s [E2204]. [DOI] [PMC free article] [PubMed] [Google Scholar]
McKeon 1986 {published data only}
- McKeon JL, Turner J, Kelly C, Dent A, Zimmerman PV. The effect of inspiratory resistive training on exercise capacity in optimally treated patients with severe chronic airflow limitation. Australian and New Zealand Journal of Medicine 1986;16(5):648-52. [DOI: 10.1111/j.1445-5994.1986.tb00005.x] [DOI] [PubMed] [Google Scholar]
Meshcheriakova 2006 {published data only}
- Meshcheriakova NN, Belevskiy AS, Cherniak AV, Nekludova GV, Appaeva AA. Threshold PEP and IMT devices (PID) for COPD patient respiratory training [Abstract]. European Respiratory Journal 2006;28 Suppl 50:553s [P3187]. [Google Scholar]
Minoguchi 2002 {published data only}
- Minoguchi H, Hibuya M, Miyagawa T, Kokubu F, Yamada M, Tanaka H, et al. Cross-over comparison between respiratory muscle stretch gymnastics and inspiratory muscle training. Internal Medicine 2002;41(10):805-12. [DOI: 10.2169/internalmedicine.41.805] [DOI] [PubMed] [Google Scholar]
NCT01218295 {published data only}
- NCT01218295. Inspiratory muscle training with normocapnic hyperpnea in chronic obstructive pulmonary disease (COPD) [Efficacy of inspiratory muscle training by means of Spirotiger® in COPD patients]. clinicaltrials.gov/show/nct01218295 (first received 11 October 2010).
NCT01556139 {published data only}
- NCT01556139. Effectiveness of respiratory muscle training by Spirotiger in chronic patients [Evaluation of the effectiveness of respiratory muscle training by the technique of hypocapnia hyperpnea (Spirotiger) in COPD and CHF patients]. clinicaltrials.gov/show/NCT01556139 (first received 16 March 2012).
NCT01747694 {published data only}
- NCT01747694. Respiratory muscle exercise training in COPD patients. clinicaltrials.gov/show/nct01747694 (first received 12 December 2012).
NCT01945398 {published data only}
- NCT01945398. IMT in ventilatory muscle metaboreflex in COPD [Effect of inspiratory muscle training in the ventilatory muscle metaboreflex in chronic obstructive pulmonary disease patients]. clinicaltrials.gov/show/nct01945398 (first received 18 September 2013).
NCT01956565 {published data only}
- NCT01956565. Feasibility of inspiratory muscle training in people with chronic obstructive pulmonary disease (COPD) who decline pulmonary rehabilitation. clinicaltrials.gov/show/NCT01956565 (first received 8 October 2013).
NCT02186340 {published data only}
- NCT02186340. Effects of inspiratory muscle training on breathing pattern in patients with chronic obstructive pulmonary disease. clinicaltrials.gov/show/NCT02186340 (first received 10 July 2014).
NCT02278523 {published data only}
- NCT02278523. The respiratory physiology variation of COPD patients in inspiratory muscle training. clinicaltrials.gov/show/nct02278523 (first received 30 October 2014).
NCT02579200 {published data only}
- NCT02579200. Inspiratory muscle training for dyspneic patients with COPD-HF overlap: a multicenter, randomized controlled trial. clinicaltrials.gov/show/nct02579200 (first received 19 October 2015).
NCT02914093 {published data only}
- NCT02914093. IMT in hypercapnic patients with COPD (THYPISK-f) [Feasibility of inspiratory muscle training in patients with chronic hypercapnia and severe chronic obstructive pulmonary disease]. ClinicalTrials.gov/show/NCT02914093 (first received 26 September 2016).
NCT02935166 {published data only}
- NCT02935166. Innovations in respiratory muscles training in patients with chronic obstructive pulmonary disease (INNOTORIO) [INNOTORIO: Innovations in the training of the inspiratory muscles on patients with chronic obstructive pulmonary disease: design of a digital dual valve and evaluation of a new high-intensity training scheme of short duration]. clinicaltrials.gov/show/nct02935166 (first received 17 October 2016).
NCT03186092 {published data only}
- NCT03186092. Effects of respiratory muscle training on respiratory muscle strength, functional capacity and quality of life in pulmonary hypertension. clinicaltrials.gov/show/nct03186092 (first received 14 June 2017).
NCT03500042 {published data only}
- NCT03500042. Effects of different modes of respiratory muscle training on respiratory mechanics and NRD in patient with stable COPD [Effects of different modes of respiratory muscular threshold loads training on respiratory mechanics and neural respiratory drive (NRD) in patient with stable chronic obstructive pulmonary disease (COPD)]. clinicaltrials.gov/show/NCT03500042 (first received 17 April 2018).
NCT03739879 {published data only}
- NCT03739879. Effectivity of inspiratory muscle trainer in the chronic obstructive pulmonary disease rehabilitation program. clinicaltrials.gov/show/nct03739879 (first received 14 November 2018).
NCT03844711 {published data only}
- NCT03844711. Transcutaneous electrical diaphragmatic stimulation and inspiratory muscle training in patients with COPD exacerbated. clinicaltrials.gov/show/nct03844711 (first received 18 February 2019).
NCT03880630 {published data only}
- Inspiratory muscle activation pattern analysis in assisting precision in inspiratory muscle training in patients with chronic respiratory disease. clinicaltrials.gov/ct2/show/NCT03880630 (first received 19 March 2019).
NCT04084405 {published data only}
- NCT04084405. Effect of inspiratory muscle training on balance in chronic obstructive pulmonary disease patients. clinicaltrials.gov/show/nct04084405 (first received 10 September 2019).
NCT04117399 {published data only}
- NCT04117399. Effect of inspiratory muscle training on posture in chronic obstructive pulmonary disease patients. clinicaltrials.gov/show/nct04117399 (first received 7 October 2019).
NCT04460261 {published data only}
- NCT04460261. The effects of 'functional' inspiratory muscle training [The effects of a new integrated exercise program called 'functional' inspiratory muscle training in geriatric individuals with and without chronic obstructive pulmonary disease]. ClinicalTrials.gov/show/NCT04460261 (first received 7 July 2020).
Neves 2014a {published data only}
- Neves LF, Reis MH, Plentz RD, Matte DL, Coronel CC, Sbruzzi G. Expiratory and expiratory plus inspiratory muscle training improves respiratory muscle strength in subjects with COPD: systematic review. Respiratory Care 2014;59(9):1381-8. [DOI: 10.4187/respcare.02793] [DOI] [PubMed] [Google Scholar]
Neves 2014b {published data only}
- Neves L, Chiappa A, Da Silva V, Vieira P, Cipriano G Jr, Arena R, et al. Comparative effects of inspiratory muscle training and resistance training on respiratory and skeletal muscle strength in COPD: responses of the a pulmonary rehabilitation program. European Respiratory Journal 2014;44:P598. [Google Scholar]
Nield 2007 {published data only}
- Nield MA, Soo Hoo GW, Roper JM, Santiago S. Efficacy of pursed-lips breathing: a breathing pattern retraining strategy for dyspnea reduction. Journal of Cardiopulmonary Rehabilitation and Prevention 2007;27(4):237-44. [DOI: 10.1097/01.HCR.0000281770.82652.cb] [DOI] [PubMed] [Google Scholar]
Noseda 1987 {published data only}
- Noseda A, Carpiaux JP, Vandeput W, Prigogine T, Schmerber J. Resistive inspiratory muscle training and exercise performance in COPD patients. A comparative study with conventional breathing retraining. Bulletin Europeen de Physiopathologie Respiratoire 1987;23(5):457-63. [PubMed] [Google Scholar]
O'Connor 2019 {published data only}
- O'Connor C, Lawson R, Waterhouse J, Mills GH. Is inspiratory muscle training (IMT) an acceptable treatment option for people with chronic obstructive pulmonary disease (COPD) who have declined pulmonary rehabilitation (PR) and can IMT enhance PR uptake? A single-group prepost feasibility study in a home-based setting. BMJ Open 2019;9(8):e028507. [DOI: 10.1136/bmjopen-2018-028507] [DOI] [PMC free article] [PubMed] [Google Scholar]
Okura 2019 {published data only}
- Okura K, Kawagoshi A, Iwakura M, Shibata K, Furukawa Y, Sugawara K, et al. The effectiveness of inspiratory muscle training in patients with COPD. Journal of the Japan Society for Respiratory Care and Rehabilitation 2019;28(2):274-8. [DOI: 10.15032/jsrcr.28.2_274] [DOI] [Google Scholar]
Okura 2020 {published data only}
- Okura K, Takahashi H, Shiotani T, Iida Y, Inagaki T, Ogawa T, et al. Inspiratory muscle training for patients with chronic obstructive pulmonary disease Effect on physical activity ─ Multicenter, randomized controlled trial ─ [─多施設による無作為化比較対照試験─]. 理学療法学 2020;47(6):551-9. [Google Scholar]
PACTR201703002095224 {published data only}
- PACTR201703002095224. Respiratory muscle training in chronic obstructive pulmonary disease [Comparative study between two different respiratory training protocols in patients with chronic obstructive pulmonary disease]. www.who.int/trialsearch/Trial2.aspx?TrialID=PACTR201703002095224 (first received 12 March 2017).
Padula 2001 {published data only}
- Padula CA, Yeaw E. Inspiratory muscle training: an exploration of a home-based intervention. Journal of Applied Research 2001;1(2):85-94. [Google Scholar]
Perez 2010 {published data only}
- Perez ME, Guirao L, Martinez A, Ortega P, Pleguezuelos E, Samitier B. Effect of a specific respiratory exercise program on respiratory muscle strength. In: 71st Annual Assembly of the American Academy of Physical Medicine and Rehabilitation; 2010 Nov 4-7; Seattle. Vol. 2. 2010:S25.
Pescaru 2016 {published data only}
- Pescaru C, Oancea C, Tudorache E. New device used in COPD rehabilitation. TrainAir system. European Respiratory Journal 2016;48:PA695. [DOI: 10.1183/13993003.congress-2016.PA695] [DOI] [Google Scholar]
Quintero 1999 {published data only}
- Quintero JI, Borzone G, Leiva A, Villafranca C, Lisboa C. Effects of inspiratory muscle training on the oxygen cost of breathing in patients with chronic obstructive pulmonary disease. Revista Medica De Chile 1999;127(4):421-8. [PubMed] [Google Scholar]
Richardson 1989 {published data only}
- Richardson J, Dunn L, Pardy R. Inspiratory resistive endurance training in patients with chronic obstructive pulmonary disease: a pilot study. Physiotherapie Canada 1989;41(2):85-92. [Google Scholar]
Rocha 2015 {published data only}
- Rocha T, Souza H, Brandao DC, Rattes C, Ribeiro L, Campos SL, et al. The manual diaphragm release technique improves diaphragmatic mobility, inspiratory capacity and exercise capacity in people with chronic obstructive pulmonary disease: a randomised trial. Journal of Physiotherapy 2015;61(4):182-9. [DOI: 10.1016/j.jphys.2015.08.009] [DOI] [PubMed] [Google Scholar]
Sassoon 1992 {published data only}
- Sassoon CS, Lodia R, Rheeman CH, Kuei JH, Light RW, Mahutte CK. Inspiratory muscle work of breathing during flow-by, demand-flow, and continuous-flow systems in patients with chronic obstructive pulmonary disease. American Review of Respiratory Disease 1992;145(5):1219-22. [DOI: 10.1164/ajrccm/145.5.1219] [DOI] [PubMed] [Google Scholar]
Serón 2005 {published data only}
- Serón P, Riedemann P, Muñoz S, Doussoulin A, Villarroel P, Cea X. Effect of inspiratory muscle training on muscle strength and quality of life in patients with chronic airflow limitation: a randomized controlled trial [Efecto del entrenamiento muscular inspiratorio sobre la fuerza muscular y la calidad de vida en pacientes con limitación crónica del flujo aéreo. Ensayo clínico aleatorizado]. Archivos de Bronconeumología 2005;41(11):601-6. [DOI: 10.1157/13081248] [DOI] [PubMed] [Google Scholar]
Shahin 2008 {published data only}
- Shahin B, Germain M, Kazem A, Annat G. Benefits of short inspiratory muscle training on exercise capacity, dyspnea, and inspiratory fraction in COPD patients. International Journal of Chronic Obstructive Pulmonary Disease 2008;3(3):423-7. [DOI: 10.2147/copd.s1822] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shioya 2007 {published data only}
- Shioya T, Satake M, Takahashi H, Sugawara K, Kasai C, Kiyokawa N, et al. Combination of chest wall mobilization and respiratory muscle training in comprehensive outpatient pulmonary rehabilitation improves pulmonary function in patients with COPD [Abstract]. In: Respirology. Vol. 14 Suppl 3. 2009:A191 [PD 05-05].
- Shioya T, Satake M, Takahashi H, Sugawara K, Ksai C, Kiyokawa N, et al. Combination of chest wall mobilization and respiratory muscle training in comprehensive outpatient pulmonary rehabilitation improves pulmonary function in patients with COPD [Abstract]. In: European Respiratory Journal. Vol. 30 Suppl 51. 2007:188s [P1160].
Similowski 1994 {published data only}
- Similowski T, Derenne JP. Inspiratory muscle testing in stable COPD patients. European Respiratory Journal 1994;7(10):1871-6. [DOI] [PubMed] [Google Scholar]
Sivashanmugam 2019 {published data only}
- Sivashanmugam M, Vinod Kumar V, Sridhar R, Kumar R, Stanley G. Comparison of short term inspiratory muscle training and peripheral muscle training on lung function and quality of life in chronic obstructive pulmonary disease patients - a prospective observational study. Lung India 2019;9 Suppl:S107-8. [DOI: 10.4103/0970-2113.271103] [DOI] [Google Scholar]
Soicher 1998 {published data only}
- Soicher J, Dechman G. Inspiratory muscle function in chronic obstructive pulmonary disease (COPD). Physical Therapy Reviews 1998;3(1):31-9. [DOI: 10.1179/ptr.1998.3.1.31] [DOI] [Google Scholar]
Sonne 1982 {published data only}
- Sonne LJ, Davis JA. Increased exercise performance in patients with severe COPD following inspiratory resistive training. Chest 1982;81(4):436-9. [DOI: 10.1378/chest.81.4.436] [DOI] [PubMed] [Google Scholar]
Sudo 1997 {published data only}
- Sudo E, Ohga E, Matsuse T, Teramoto S, Nagase T, Katayama H, et al. The effects of pulmonary rehabilitation combined with inspiratory muscle training on pulmonary function and inspiratory muscle strength in elderly patients with chronic obstructive pulmonary disease. Nihon Ronen Igakkai zasshi [Japanese Journal of Geriatrics] 1997;34(11):929-34. [DOI: 10.3143/geriatrics.34.929] [DOI] [PubMed] [Google Scholar]
Sugiyama 2010 {published data only}
- Sugiyama N, Yokoyama T, Abe T, Kono Y, Sugiyama S, Setoguchi Y. Combined effects of short-term lower and upper extremity training plus inspiratory muscle training in patients with chronic obstructive pulmonary disease. Journal of Tokyo Medical University 2010;68(3):322-9. [Google Scholar]
Sun 2003 {published data only}
- Sun JX, Yin MX, Shao H, Li ZS, Li SW. Effect of respiratory muscle gymnastics on lung function and quality of life in the old patients with chronic obstructive pulmonary disease. Zhonghua Linchuang Kangfu Zazhi 2003;7(27):3698-9. [Google Scholar]
TCTR20191009004 {published data only}
- TCTR20191009004. Physiological change in lung function and respiratory muscle strength in chronic obstructive pulmonary disease patients; cause by pursed-lip breathing exercise using windmill toy model. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=TCTR20191009004 (first received 9 October 2019).
UMIN000030937 {published data only}
- UMIN000030937. Efficacy and applicability of respiratory muscle training on the balance of inspiratory-to-expiratory muscle strength and breathing timing in patients with chronic obstructive pulmonary disease: a preparatory research study. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=JPRN-UMIN000030937 1 February 2018.
Van't Hul 2006 {published data only}
- Van 't Hul A, Gosselink R, Hollander P, Postmus P, Kwakkel G. Training with inspiratory pressure support in patients with severe COPD. European Respiratory Journal 2006;27(1):65-72. [DOI: 10.1183/09031936.06.00036505] [DOI] [PubMed] [Google Scholar]
Villafranca 1998 {published data only}
- Villafranca C, Borzone G, Leiva A, Lisboa C. Effect of inspiratory muscle training with an intermediate load on inspiratory power output in COPD. European Respiratory Journal 1998;11(1):28-33. [DOI: 10.1183/09031936.98.11010028] [DOI] [PubMed] [Google Scholar]
Wada 2016 {published data only}
- Wada JT, Borges-Santos E, Porras DC, Paisani DM, Cukier A, Lunardi AC, et al. Effects of aerobic training combined with respiratory muscle stretching on the functional exercise capacity and thoracoabdominal kinematics in patients with COPD: a randomized and controlled trial. International Journal of Chronic Obstructive Pulmonary Disease 2016;11:2691-700. [DOI: 10.2147/COPD.S114548] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2006 {published data only}
- Wu X, Hou L, Bai W. Effects of breathing training on quality of life and activities of daily living in elderly patients with stable severe chronic obstructive pulmonary disease. Chinese Journal of Rehabilitation Medicine 2006;21(3-4):307-10. [Google Scholar]
Xi 2015 {published data only}
- Xi F, Wang Z, Qi Y, Brightwell R, Roberts P, Stewart A, et al. Long-term effect of respiratory training for chronic obstructive pulmonary disease patients at an outpatient clinic: a randomised controlled trial. Clinical and Translational Medicine 2015;4(1):31. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamaguti 2012 {published data only}
- Yamaguti WP, Claudino RC, Neto AP, Chammas MC, Gomes AC, Salge JM, et al. Diaphragmatic breathing training program improves abdominal motion during natural breathing in patients with chronic obstructive pulmonary disease: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2012;93(4):571-7. [DOI: 10.1016/j.apmr.2011.11.026] [DOI] [PubMed] [Google Scholar]
Yan 1996 {published data only}
- Yan Q, Sun Y. Quantitative research for improving respiratory muscle contraction by breathing exercise. Chinese Medical Journal 1996;109(10):771-5. [PubMed] [Google Scholar]
Yang 2005 {published data only}
- Yang S-Y, Feng E-Z, Shen J-L, Zhang Y, Zhao L-H, Wu X-M, et al. Effect of suitable electrostimulation on phrenic nerve in ameliorating diaphragmatic fatigue and pulmonary function in patients with stable chronic cor pulmonale at high altitude area. Chinese Journal of Clinical Rehabilitation 2005;9(23):28-30. [Google Scholar]
Zhang 2008 {published data only}
- Zhang Z-Q, Chen R-C, Yang Q-K, Li P, Wang C-Z, Zhang Z-H, et al. Effects of respiratory training in relation to respiratory pathophysiology on respiratory muscle function and exercise tolerance in chronic obstructive pulmonary disease patients. [Chinese]. Journal of Clinical Rehabilitative Tissue Engineering Research 2008;12(20):3966-71. [Google Scholar]
References to studies awaiting assessment
Barter 1987 {published data only}
- Barter C, Nosworthy J, Flynn M, Pretto JE. Effect of leg and inspiratory muscle training on exercise performance and minute ventilation in chronic airflow obstruction. [Abstract]. Australian and New Zealand Journal of Medicine 1987;17:511. [Google Scholar]
Bustamante 1997 {published data only}
- Bustamante V, Galdiz JB, Ruiz L, Cabriada V, Serrano L, Sobradillo V. Benefits evolution of inspiratory muscle training after 6 months discontinuation [Abstract]. European Respiratory Journal 1997;10 Suppl 25:7S. [Google Scholar]
Cassidy 2009 {published data only}
- Cassidy C. A study investigating the effects of inspiratory muscle training (IMT) in subjects with chronic obstructive pulmonary disease (COPD) following an acute exacerbation [Abstract]. Irish Journal of Medical Science 2009;178 Suppl 11:S435-6. [Google Scholar]
Cejudo 1998 {published data only}
- Cejudo P, Sanchez H, Ortega F, Toral J, Villagomez R, Montemayor T. Home ventilatory muscle training in patients with chronic obstructive pulmonary disease (COPD). European Respiratory Journal 1998;12 Suppl 28:3S. [DOI] [PubMed] [Google Scholar]
Chen 2017 {published data only}
- Chen X, Luo P, Chen Y, Huang Y, Wang K, Hu Y, et al. Effects of combined cycle ergometer and inspiratory muscle training in patients with stable chronic obstructive pulmonary disease. In: American Journal of Respiratory and Critical Care Medicine. Vol. 195. 2017:A2861. [DOI: 10.1164/ajrccm-conference.2017.A109] [DOI]
- Chen X, Xu W-H. Combined use of inspiratory and expiratory threshold pressure training with data monitor in COPD. In: European Respiratory Journal. Vol. 52 Suppl 62. 2018:PA4046.
- NCT02200549. Effects of combined cycle training and inspiratory muscle training in patients with COPD [Effects of combined cycle training and inspiratory muscle training on exercise performance, health-related quality, dyspnoea, body composition, depressive symptomatology in patients with chronic obstructive pulmonary disease (COPD)]. clinicaltrials.gov/show/nct02200549 (first received 25 July 2014).
Croitoru 2013 {published data only}
- Croitoru A, Ionita D, Pele I, Marinescu L, Gologanu D, Dumitrescu A, et al. Inspiratory muscle training with threshold loading in a rehabilitation program of COPD patients. European Respiratory Journal 2013;42 Suppl 57:774s [P3732]. [Google Scholar]
Del Castillo Otero 1998 {published data only}
- Del Castillo-Otero D, Sanchez-Riera H, Ortega-Ruiz F, Cejudo-Ramos P, Toral-Marin J, Elias-Hernandez T, et al. Home respiratory muscle training in COPD. Archivos de Bronconeumologia 1998;34 Suppl 1:33. [Google Scholar]
Di Marzo 2000 {published data only}
- Di Marzo A, Torrice M, Ciappi G. Inspiratory muscle training and relaxation therapy in advanced COPD patients. European Respiratory Journal 2000;16 Suppl 31:46s. [Google Scholar]
Di Marzo 2002 {published data only}
- Di Marzo A, Mancuso A, Bevignani G. Breathing reeducation with a pressure threshold device in advanced COPD patients. American Journal of Respiratory and Critical Care Medicine 2002;165 Suppl 8:A506. [Google Scholar]
Downes Vogel 2002 {published data only}
- Downes Vogel PJ. Effect of adding inspiratory muscle training to a pulmonary rehabilitation program for patients with COPD which includes upper extremity exercises. American Journal of Respiratory and Critical Care Medicine 2002;165 Suppl 8:A737. [Google Scholar]
Eastwood 2005 {published data only}
- Eastwood P, Hill K, Jenkins S, Philippe D, Shepherd K, Cecins N, et al. High intensity inspiratory muscle training (HIMT) improves dyspnea and health-related quality of life (QoL) in COPD [Abstract]. In: American Thoracic Society International Conference; 2005 May 20-25; San Diego. 2005:[C63] [Poster: H96].
Gething 2001 {published data only}
- Gething AD, Davies B, Williams EM. Improvements in cycling endurance following nine weeks of inspiratory muscle training. Thorax 2001;56 Suppl 3:iii50. [Google Scholar]
Göhl 2006 {published data only}
- Gohl O, Schacher C, Grensemann S, Worth H. Effects of inspiratory muscle training with the RESPIFIT S in addition to an outpatient exercise training program for patients with COPD [Abstract]. European Respiratory Journal 2006;28 Suppl 50:556s [P3202]. [Google Scholar]
IRCT201104266299N1 {published data only}
- IRCT201104266299N1. The effect of inspiratory muscle training in COPD patients. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=IRCT201104266299N1 (first received 22 September 2011).
IRCT20180205038633N1 {published data only}
- IRCT20180205038633N1. The assessment of effectiveness of respiratory muscle training (IMT)and aerobic training in patients with pulmonary disease [The assessment of effectiveness of respiratory muscle training (IMT)and aerobic training on quality of life in patients with chronic obstructive pulmonary disease (COPD)]. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=IRCT20180205038633N1 (first received 21 March 2017).
ISRCTN19258620 {published data only}
- ISRCTN19258620. Inspiratory muscle training in patients with chronic obstructive pulmonary disease (COPD). ictrptest.azurewebsites.net/Trial2.aspx?TrialID=ISRCTN19258620 1 March 2000.
Jones 1985 {published data only}
- Jones DT, Thomson RJ, Sears MR. Physical exercise and resistive breathing training in severe chronic airways obstruction--are they effective? European Journal of Respiratory Diseases 1985;67(3):159-66. [PubMed] [Google Scholar]
Koppers 2004 {published data only}
- Koppers R, Vos P, Folgering H. Endurance respiratory muscle training improves outcomes of pulmonary rehabilitation in COPD-patients [Abstract]. In: American Thoracic Society 100th International Conference; 2004 May 21-26; Orlando. 2004:B60 Poster K8.
Liu 1989 {published data only}
- Liu Y, Zhao ZS, Yang DC. Inspiratory muscle loading training compared with the breathing gymnastics in patients with COPD. Chinese Journal of Geriatrics 1989;8(2):112-14. [Google Scholar]
Manuel Vargas 1995 {published data only}
- Manuel Vargas D, Alonso Puig X, Pia de la Maza CM, Morales XP, Vargas BD, Bunout BD, et al. Effects of an inspiratory muscle training program and nutritional support in patients with chronic obstructive lung disease. Revista Medica de Chile 1995;123:1225-34. [PubMed] [Google Scholar]
Mendoza 2007 {published data only}
- Mendoza L, Levia A, Jover E, Cavada G, Lisboa C. Effect of inspiratory muscle training on lung function, dyspnea, exercise tolerance, and quality of life in COPD patients [Abstract]. European Respiratory Journal 2007;30 Suppl 51:514s [E3083]. [Google Scholar]
Meshcherykova 2018 {published data only}
- Meshcherykova N, Belevsky A, Cherniak A. The effect of inspiratory muscle training on lung hyperinflation in COPD patients. European Respiratory Journal 2018;52 Suppl 62:PA4139. [DOI: 10.1183/13993003.congress-2018.PA4139] [DOI] [Google Scholar]
NCT01056081 {published data only}
- NCT01056081. Inspiratory muscle training in chronic obstructive pulmonary disease [I]. clinicaltrials.gov/show/nct01056081 26 January 2010.
NCT01903772 {published data only}
- NCT01903772. Effects of inspiratory muscle training in chronic obstructive pulmonary disease (COPD) (IMTCO). clinicaltrials.gov/show/NCT01903772 19 July 2013.
NCT02392715 {published data only}
- NCT02392715. Inspiratory muscle training combined with general exercise training in COPD (IMTGET) [Inspiratory muscle training combined with general exercise training, compared to general exercise training alone in patients with COPD: randomized controlled trial]. clinicaltrials.gov/show/nct02392715 19 March 2015.
NCT02673242 {published data only}
- NCT02673242. Inspiratory muscle training for patients with chronic obstructive pulmonary disorder. clinicaltrials.gov/show/NCT02673242 3 February 2016.
NCT03080662 {published data only}
- NCT03080662. Impact of inspiratory muscle training on daily physical activity (INAF). clinicaltrials.gov/show/nct03080662 15 March 2017.
NCT03438019 {published data only}
- NCT03438019. Inspiratory muscle training in COPD [Inspiratory muscle strength and endurance training in veterans with COPD]. clinicaltrials.gov/show/nct03438019 19 February 2018.
NCT03790410 {published data only}
- NCT03790410. The effect of inspiratory muscle training on postural control in chronic obstructive pulmonary disease. clinicaltrials.gov/show/nct03790410 31 December 2018.
Newall 1998 {published data only}
- Newall C, Caine MP, Stockley RA, McConnel AK, Hill SL. Inspiratory muscle training using a pressure threshold device in patients with COPD. European Respiratory Journal 1998;12 Suppl 28:267S. [Google Scholar]
Newall 2000 {published data only}
- Newall C, Richardson B, McConnell AK, Stockley RA, Hill SL. Inspiratory muscle training (IMT) as an adjunct to a pulmonary rehabilitation programme in COPD. American Journal of Respiratory and Critical Care Medicine 2000;161(3 Suppl):A753. [Google Scholar]
NTR2990 {published data only}
- NTR2990. Effects of inspiratory muscle training in patients with chronic obstructive pulmonary disease. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=NTR2990 (first received 18 July 2011).
Pertuze 1994 {published data only}
- Pertuze J, Villafranca C, Leiva A, Reppeto P, Cruz E, Lisboa C. Improved quality of life of patients with chronic bronchial obstruction with inspiratory muscle training. Enfermedades Respir. Cir. Torac 1994;10(4):299. [Google Scholar]
Ramirez Sarmiento 2000 {published data only}
- Ramirez Sarmiento A, Orozo Levi M, Belalcazar V, Mendez R, Barreiro E, Guell R, et al. Structural basis of improved muscle function after inspiratory training in severe COPD patients. European Respiratory Journal 2000;16 Suppl 31:37s. [Google Scholar]
Reidi 2005 {published data only}
- Reidi C, Toledo A, Ribeiro KP, Silva MI, Costa D. Effects of a respiratory muscle training with or without load in patients with COPD. Rehabilitar 2005;7(27):4-10. [Google Scholar]
Valderramas 2009 {published data only}
- Valderramas S, Souza MC. Inspiratory muscle training in patients with chronic obstructive pulmonary disease submitted to a pulmonary rehabilitation program [Abstract]. In: European Respiratory Society Annual Congress; 2009 Sep 12-16; Vienna. 2009:[E4293].
Vargas 1995 {published data only}
- Vargas M, Puig A, la Maza M, Morales P, Vargas D, Bunout B, et al. Patients with chronic airflow limitation: effects of the inspiratory muscle training with threshold load valve, built with appropriate technology, associated to nutritional support. Revista Medica de Chile 1995;123(10):1225-34. [PubMed] [Google Scholar]
Vargas 1998 {published data only}
- Vargas R, Sanchez H, Del Castillo D, Cejudo P, Ortega F, Montemayor T. Impact of ventilatory muscle training on dysnea, exercise and quality of life in COPD. Neumosur 1998;10(1):17. [Google Scholar]
Wang 2004 {published data only}
- Wang H, Wan S-Y. Effect of respiratory muscle training on pulmonary function and quality of life in patients with chronic obstructive pulmonary diseases in remission period. Chinese Journal of Clinical Rehabilitation 2004;8(18):3436-7. [Google Scholar]
Wanke 1994 {published data only}
- Wanke T, Formanek D, Lahrmann H, Brath H, Wild M, Wagner C, et al. Effects of combined inspiratory muscle and cycle ergometer training on exercise performance in patients with COPD. European Respiratory Journal 1994;7(12):2205-11. [DOI: 10.1183/09031936.94.07122205] [DOI] [PubMed] [Google Scholar]
Weiner 2006a {published data only}
- Weiner P, McConnell AK, Magadle R, Beckerman M. The addition of inspiratory muscle training to pulmonary rehabilitation program in patients with significant COPD [Abstract]. European Respiratory Journal 2006;28 Suppl 50:554s [P3194]. [Google Scholar]
Wolstenholme 1998 {published data only}
- Wolstenholme RJ, Thomas E, Enright S. An evaluation of the clinical effectiveness of two respiratory muscle training devices in the rehabilitation of patients with chronic airflow obstruction. [Abstract]. European Respiratory Journal. Supplement 1998;12 Suppl 28:353S. [Google Scholar]
References to ongoing studies
CTRI/2020/11/029226 {published data only}
- CTRI/2020/11/029226. Effects of inspiratory muscle training v/s autogenic drainage in hospitalised COPD patient. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2020/11/029226 19 November 2020.
CTRI/2021/05/033469 {published data only}
- CTRI/2021/05/033469. To test the efficacy of inspiratory muscle training device Airofit in reducing breathlessness in COPD patients. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=54407 1 June 2021.
CTRI201712010952 {published data only}
- CTRI201712010952. Effect of breathing exercises to improve the strength of respiratory muscles in COPD population. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2017/12/010952 21 December 2017.
De Souza 2019 {published data only}
- De Souza Y, Medeiros S, Da Silva KM, Macedo J, Condesso D, Figueira B, et al. Does inspiratory muscle training (IMT) reduce depression in patients with COPD? European Respiratory Journal 2019;54 Suppl 63:PA2197. [DOI: 10.1183/13993003.congress-2019.PA2197] [DOI] [Google Scholar]
Formiga 2020 {published data only}
- Formiga MF, Dosbaba F, Hartman M, Batalik L, Plutinsky M, Brat K, et al. Novel versus traditional inspiratory muscle training regimens as home-based, stand-alone therapies in COPD: protocol for a randomized controlled trial. International Journal of Chronic Obstructive Pulmonary Disease 2020;15:2147-55. [DOI: 10.2147/COPD.S266234] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04415788. Inspiratory muscle training and COPD [Novel versus traditional inspiratory muscle training regimens as home-based, stand-alone therapies in COPD]. clinicaltrials.gov/show/NCT04415788 4 June 2020. [DOI] [PMC free article] [PubMed]
JPRN‐UMIN000039893 {published data only}
- JPRN-UMIN000039893. The effectiveness of inspiratory muscle training in patients with chronic obstructive pulmonary disease. trialsearch.who.int/Trial2.aspx?TrialID=JPRN-UMIN000039893 (first received 22 March 2020).
JPRN‐UMIN000043099 {published data only}
- JPRN-UMIN000043099. Effect of inspiratory muscle Training on diaphragm and exercise tolerance in patients with COPD. trialsearch.who.int/Trial2.aspx?TrialID=JPRN-UMIN000043099 (first received 22 January 2021).
NCT04120142 {published data only}
- NCT04120142. Effect of inspiratory muscle training on dyspnoea and exercise tolerance in COPD patients [Effect of inspiratory muscle training during PR on dyspnoea and exercise tolerance in chronic obstructive (COPD)]. clinicaltrials.gov/show/nct04120142 (first received 9 October 2019).
NCT04201522 {published data only}
- NCT04201522. The effect of respiratory training on exercise tolerance in COPD (ERTET) [The effect of respiratory training with normocapnic hyperpnea on exercise tolerance in COPD]. clinicaltrials.gov/show/NCT04201522 17 December 2019.
NCT04387318 {published data only}
- NCT04387318. Inspiratory muscle training and neuromuscular electrical stimulation in chronic obstructive pulmonary disease [Effects of inspiratory muscle training and neuromuscular electrical stimulation in patients with chronic obstructive pulmonary disease]. clinicaltrials.gov/show/NCT04387318 13 May 2020.
NCT04802096 {published data only}
- NCT04802096. Effects of inspiratory muscle training in addition to pulmonary rehabilitation in patients with moderate to severe COPD exacerbation. clinicaltrials.gov/ct2/show/NCT04802096 17 March 2021.
RBR‐10nyzcqc {published data only}
- Effects of Inspiratory Muscle Training on Breathlesness, Exercise Capacity and Postural Control in Patients with COPD.. https://ensaiosclinicos.gov.br/rg/RBR-10nyzcqc 2021.
RBR 42rmqy {published data only}
- RBR 42rmqy. Inspiratory muscle training in chronic obstructive pulmonary disease oxygen-dependent patients: a randomized controlled trial [Effectiveness of a respiratory muscle training program in the ability to exercise exercise capacide , subjective perception of dyspnea, respiratory and peripheral muscle strength and quality of life in patients participating in the home oxygen therapy program of the health state department of the Distrito Federal -: inspiratory muscle training]. ictrptest.azurewebsites.net/Trial2.aspx?TrialID=RBR-42rmqy 29 September 2017.
TCTR20210604001 {published data only}
- TCTR20210604001. Is single bout of inspiratory muscle training alter blood pressure and cardio autonomics modulation in COPD patients? : a pilot study. trialsearch.who.int/Trial2.aspx?TrialID=TCTR20210604001 11 January 2021.
Tctr 2022 {published data only}
- Effect of inspiratory muscle training on cardiovascular autonomic functions in chronic obstructive pulmonary disease patients. https://trialsearch.who.int/Trial2.aspx?TrialID=TCTR20220513001 2022.
Additional references
Agustí 2005
- Agustí G, Alvar N. Systemic effects of chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society 2005;2(4):367-70. [DOI] [PubMed] [Google Scholar]
Alma 2018
- Alma H, De Jong C, Tsiligianni I, Sanderman R, Kocks J, Van Der Molen T. Clinically relevant differences in COPD health status: systematic review and triangulation. European Respiratory Journal 2018;52 Suppl 62:PA1168. [DOI] [PubMed] [Google Scholar]
American Thoracic Society
- COPD Assessment Test (CAT). https://www.thoracic.org/members/assemblies/assemblies/srn/questionaires/copd.php.
Ammous 2022
- Ammous O, Feki W, Lotfi T, Khamis AM, Gosselink R, Rebai A, et al. ROB2_IRPG_beta_v7_IMT_COP.xlsm. 10.6084/m9.figshare.20066087 (accessed 14 June 2022) 15 November 2022. [DOI: ]
Andersen 1979
- Andersen JB, Dragsted L, Kann T, Johansen SH, Nielsen KB, Karbo E, et al. Resistive breathing training in severe chronic obstructive pulmonary disease. A pilot study. Scandinavian Journal of Respiratory Diseases 1979;60(3):151-6. [PubMed] [Google Scholar]
Anzueto 2017
- Anzueto A, Miravitlles M. Pathophysiology of dyspnea in COPD. Postgraduate Medicine 2017;129(3):366-74. [DOI] [PubMed] [Google Scholar]
Araújo 2017
- Araújo Oliveira AL Andrade L, Marques A. Minimal clinically important difference and predictive validity of the mMRC and mBorg in acute exacerbations of COPD. European Respiratory Journal 2017/09/01;50 Suppl 61:PA4705. [Google Scholar]
Beaumont 2015
- Beaumont M, Mialon P, Le Ber-Moy C, Lochon C, Péran L, Pichon R, et al. Inspiratory muscle training during pulmonary rehabilitation in chronic obstructive pulmonary disease: a randomized trial. Chronic Respiratory Disease 2015;12(4):305-12. [DOI] [PubMed] [Google Scholar]
Beaumont 2018a
- Beaumont M, Forget P, Couturaud F, Reychler G. Effects of inspiratory muscle training in COPD patients: a systematic review and meta-analysis. Clinical Respiratory Journal 2018;12(7):2178-88. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bégin 1991
- Bégin P, Grassino A. Inspiratory muscle dysfunction and chronic hypercapnia in chronic obstructive pulmonary disease. American Review of Respiratory Disease 1991;143(5 Pt 1):905-12. [DOI] [PubMed] [Google Scholar]
Belman 1980
- Belman M-J, Mittman C. Ventilatory muscle training improves exercise capacity in chronic obstructive pulmonary disease patients. American Review of Respiratory Disease 1980;121(2):273-80. [DOI] [PubMed] [Google Scholar]
Belman 1994
- Belman MJ, Botnick WC, Nathan SD, Chon KH. Ventilatory load characteristics during ventilatory muscle training. American Journal of Respiratory and Critical Care Medicine 1994;149(4 Pt 1):925-9. [DOI] [PubMed] [Google Scholar]
Berg 2016
- Berg K, Wright JL. The pathology of chronic obstructive pulmonary disease: progress in the 20th and 21st centuries. Archives of Pathology and Laboratory Medicine 2016;140(12):1423-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bestall 1999
- Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999;54(7):581-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boles 2007
- Boles J-M, Bion J, Connors A, Herridge M, Marsh B, Melot C, et al. Weaning from mechanical ventilation. European Respiratory Journal 2007;29(5):1033. [DOI] [PubMed] [Google Scholar]
Borg 1982
- Borg Gunnar aV. Psychophysical bases of perceived exertion. Medicine & Science in Sports & Exercise 1982;14(5):377-381. [PubMed] [Google Scholar]
Caron 2011
- Caron MA, Debigaré R, Dekhuijzen PN, Maltais F. Diaphragm and skeletal muscle dysfunction in COPD [L'atteinte du diaphragme et du quadriceps dans la BPCO: une manifestation systémique de cette maladie?]. Revue des Maladies Respiratoires 2011;28(10):1250-64. [DOI] [PubMed] [Google Scholar]
Caruso 2015
- Caruso P, Albuquerque AL, Santana PV, Cardenas LZ, Ferreira JG, Prina E, et al. Diagnostic methods to assess inspiratory and expiratory muscle strength. Jornal Brasileiro de Pneumologia 2015;41(2):110-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cazzola 2015
- Cazzolla M, Hanania NA, MacNee W, Rudell K, Hackford C, Tamimi N. A review of the most common patient-reported outcomes in COPD--revisiting current knowledge and estimating future challenges. International Journal of Chronic Obstructive Pulmonary Disease 2015;10:725-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Charususin 2016
- Charususin N, Gosselink R, McConnell A, Demeyer H, Topalovic M, Decramer M, et al. Inspiratory muscle training improves breathing pattern during exercise in COPD patients. European Respiratory Journal 2016;47(4):1261-4. [DOI] [PubMed] [Google Scholar]
Clanton 2009
- Clanton TL, Levine S. Respiratory muscle fiber remodeling in chronic hyperinflation: dysfunction or adaptation? Journal of Applied Physiology 2009;107(1):324-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clark 1996
- Clark CJ, Cochrane L, Mackay E. Low intensity peripheral muscle conditioning improves exercise tolerance and breathlessness in COPD. European Respiratory Journal 1996;9(12):2590. [DOI] [PubMed] [Google Scholar]
Cochrane Airways 2022
- Cochrane Airways Trials Register. airways.cochrane.org/trials-register (accessed 19 October 2022).
Cohen 1988
- Jacob Cohen. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum Associates, 1988. [Google Scholar]
Costanzo 2019
- Costanzo LS. Physiology. 7th edition. Wolters Kluwer, 2019. [LCCN 2017054852 | ISBN 9781496367617] [Google Scholar]
Covidence [Computer program]
- Covidence. Melbourne, Australia: Veritas Health Innovation, accessed 19 October 2020. Available at covidence.org.
Crowe 2005
- Crowe J, Reid WD, Geddes EL, O'Brien K, Brooks D. Inspiratory muscle training compared with other rehabilitation interventions in adults with chronic obstructive pulmonary disease: a systematic literature review and meta-analysis. COPD 2005;3:319-29. [DOI] [PubMed] [Google Scholar]
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Doucet 2004
- Doucet M, Debigaré R, Joanisse DR, Côté C, Leblanc P, Grégoire J, et al. Adaptation of the diaphragm and the vastus lateralis in mild-to-moderate COPD. European Respiratory Journal 2004;24(6):971-9. [DOI] [PubMed] [Google Scholar]
Figueiredo 2020
- Figueiredo RI, Azambuja AM, Cureau FV, Sbruzzi G. Inspiratory muscle training in COPD. Respiratory Care 2020;65(8):1189. [DOI] [PubMed] [Google Scholar]
Geddes 2005
- Geddes EL, Reid DW, Crowe J, O'Brien K, Brooks D. Inspiratory muscle training in adults with chronic obstructive pulmonary disease: a systematic review. Respiratory Medicine 2005;99(11):1440-58. [DOI] [PubMed] [Google Scholar]
Geddes 2008
- Geddes EL, O'Brien K, Reid WD, Brooks D, Crowe J. Inspiratory muscle training in adults with chronic obstructive pulmonary disease: an update of a systematic review. Respiratory Medicine 2008;102(12):1715-29. [DOI] [PubMed] [Google Scholar]
GOLD 2022
- Global Initiative for Chronic Obstructive Lung Disease. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2022 report). https://goldcopd.org/2022-gold-reports-2/ (accessed prior to 1 November 2022).
Gosselink 2011
- Gosselink R, De Vos J, Van den Heuvel SP, Segers J, Decramer M, Kwakkel G. Impact of inspiratory muscle training in patients with COPD: what is the evidence? European Respiratory Journal 2011;37(2):416-25. [DOI] [PubMed] [Google Scholar]
GRADE Handbook
- Schünemann H, Brożek J, GGuyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
GRADEpro GDT [Computer program]
- GRADEpro Guideline Development Tool [Software]. Version accessed October 2022. Hamilton (ON): McMaster University and Evidence Prime, 2022. Available at gradepro.org.
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2021
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Hill 2010
- Hill K, Cecins NM, Eastwood PR, Jenkins SC. Inspiratory muscle training for patients with chronic obstructive pulmonary disease: a practical guide for clinicians. Archives of Physical Medicine and Rehabilitation 2010;91(9):1466-70. [PMID: ] [DOI] [PubMed] [Google Scholar]
Holland 2014
- Holland Anne E, Spruit Martijn A, Troosters Thierry, Puhan Milo A, Pepin Véronique, Saey Didier, McCormack Meredith C, Carlin Brian W, Sciurba Frank C, Pitta Fabio, Wanger Jack, MacIntyre Neil, Kaminsky David A, Culver Bruce H, Revill Susan M, Hernandes Nidia A, Andrianopoulos Vasileios, Camillo Carlos Augusto, Mitchell Katy E, Lee Annemarie L, Hill Catherine J, Singh Sally J. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J 2014/12/01;44(6):1428. [DOI] [PubMed] [Google Scholar]
Iwakura 2020
- Iwakura M, Okura K, Kubota M, Sugawara K, Kawagoshi A, Takahashi H, et al. Estimation of minimal clinically important difference for quadriceps and inspiratory muscle strength in older outpatients with chronic obstructive pulmonary disease:a prospective cohort study. Physical Therapy Research 2020;24(1):35-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 1992
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. The American Review of Respiratory Disease 1992;145(6):1321-1327. [DOI] [PubMed] [Google Scholar]
Jones 2009
- Jones PW, Harding G, Berry P, Wiklund I, Chen W-H, Kline Leidy N. Development and first validation of the COPD Assessment Test. The European Respiratory Journal 2009;34(3):648-654. [DOI] [PubMed] [Google Scholar]
Langer 2015
- Langer D, Charususin N, Jácome C, Hoffman M, McConnell A, Decramer M, et al. Efficacy of a novel method for inspiratory muscle training in people with chronic obstructive pulmonary disease. Physical Therapy 2015;95(9):1264-73. [DOI] [PubMed] [Google Scholar]
Laveneziana 2019
- Laveneziana P, Albuquerque A, Aliverti A, Babb T, Barreiro E, Dres M, et al. ERS statement on respiratory muscle testing at rest and during exercise. European Respiratory Journal 2019;53(6):1801214. [DOI] [PubMed] [Google Scholar]
Leith 1976
- Leith DE, Bradley M. Ventilatory muscle strength and endurance training. Journal of Applied Physiology 1976;41(4):508-16. [DOI] [PubMed] [Google Scholar]
Lötters 2002
- Lötters F, Van Tol B, Kwakkel G, Gosselink R. Effects of controlled inspiratory muscle training in patients with COPD: a meta-analysis. European Respiratory Journal 2002;20(3):570-7. [DOI] [PubMed] [Google Scholar]
Mahler 2005
- Mahler DA, Witek TJ Jr. The MCID of the transition dyspnea index is a total score of one unit. COPD 2005;2(1):99-103. [DOI] [PubMed] [Google Scholar]
Maillard 1998
- Maillard JO, Burdet L, Van Melle G, Fitting JW. Reproducibility of twitch mouth pressure, sniff nasal inspiratory pressure, and maximal inspiratory pressure. European Respiratory Journal 1998;11(4):901-5. [DOI] [PubMed] [Google Scholar]
Maltais 1997
- Maltais F, LeBlanc P, Jobin J, Bėrubė C, Bruneau J, Carrier L. Intensity of training and physiologic adaptation in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1997;155(2):555-61. [DOI] [PubMed] [Google Scholar]
McCarthy 2015
- McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2015, Issue 2. Art. No: CD003793. [DOI: 10.1002/14651858.CD003793.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
McConnell 2004
- McConnell AK, Romer LM. Respiratory muscle training in healthy humans: resolving the controversy. International Journal of Sports Medicine 2004;25(04):284-93. [DOI] [PubMed] [Google Scholar]
McGowan 2016
- McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. Journal of Clinical Epidemiology 2016;75:40-6. [DOI: 10.1016/j.jclinepi.2016.01.021] [DOI] [PubMed] [Google Scholar]
McGrath 2020
- McGrath S, Zhao X-F, Steele R, Thombs BD, Benedetti A, DEPRESsion Screening Data (DEPRESSD) Collaboration. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Statistical Methods in Medical Research 2020;29(9):2520-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Menzes 2018
- Menzes KK, Nascimento LR, Avelino PR, Polese JC, Salmela LF. A review on respiratory muscle training devices. Journal of Pulmonary and Respiratory Medicine 2018;08(02):451. [Google Scholar]
Meyer 2013
- Meyer FJ, Borst MM, Buschmann HC, Ewert R, Friedmann-BetteB, Ochmann U, et al. Exercise testing in respiratory medicine. Pneumologie 2013;67:16-34. [DOI: 10.1055/s-0032-1325901] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nickerson 1982
- Nickerson BG, Keens TG. Measuring ventilatory muscle endurance in humans as sustainable inspiratory pressure. Journal of Applied Physiology 1982;52(3):768-72. [DOI: 10.1152/jappl.1982.52.3.768] [DOI] [PubMed] [Google Scholar]
O'Brien 2008
- O'Brien K, Geddes E, Reid W, Brooks D, Crowe J. Inspiratory muscle training compared with other rehabilitation interventions in chronic obstructive pulmonary disease: a systematic review update. Journal of Cardiopulmonary Rehabilitation and Prevention 2008;28(2):128-41. [DOI] [PubMed] [Google Scholar]
O'Donnell 2006
- O'Donnell DE. Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society 2006;3(2):180-4. [DOI] [PubMed] [Google Scholar]
O'Donnell 2007
- O'Donnell DE, Banzett RB, Carrieri-Kohlman V, Casaburi R, Davenport PW, Gandevia SC, et al. Pathophysiology of dyspnea in chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society 2007;4(2):145-68. [DOI] [PubMed] [Google Scholar]
Ottenheijm 2008
- Ottenheijm CA, Heunks LM, Dekhuijzen RP. Diaphragm adaptations in patients with COPD. Respiratory Research 2008;9(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Padula 2006
- Padula CA, Yeaw E. Inspiratory muscle training: integrative review. Research and Theory for Nursing Practice 2006;20(4):291-304. [DOI] [PubMed] [Google Scholar]
Papandrinopoulou 2012
- Papandrinopoulou D, Tzouda V, Tsoukalas G. Lung compliance and chronic obstructive pulmonary disease. Pulmonary Medicine 2012;2012:6. [Article ID 542769] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pessoa 2014
- Sclauser Pessoa IM, Franco Parreira V, Fregonezi GA, Sheel AW, Chung F, Reid WD. Reference values for maximal inspiratory pressure: a systematic review. Canadian Respiratory Journal 2014;21:43-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Puhan 2011
- Puhan MA, Chandra D, Mosenifar Z, Ries A, Make B, Hansel NN, et al. The minimal important difference of exercise tests in severe COPD. European Respiratory Journal 2011;37(4):784. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramos 2014
- Ramos FL, Krahnke JS, Kim V. Clinical issues of mucus accumulation in COPD. International Journal of Chronic Obstructive Pulmonary Disease 2014;9:139-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.16.2. Copenhagen: The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Ries 2005
- Ries AL. Minimally clinically important difference for the UCSD shortness of breath questionnaire, Borg Scale, and visual analog scale. COPD 2005;2(1):105-10. [DOI] [PubMed] [Google Scholar]
Rochester 2015
- Rochester CL, Vogiatzis I, Holland AE, Lareau SC, Marciniuk DD, Puhan MA, et al. An official American Thoracic Society/European Respiratory Society policy statement: enhancing implementation, use, and delivery of pulmonary rehabilitation. American Journal of Respiratory and Critical Care Medicine 2015;192(11):1373-86. [PMID: ] [DOI] [PubMed] [Google Scholar]
Salito 2015
Schultz 2018
- Schultz K, Jelusic D, Wittmann M, Krämer B, Huber V, Fuchs S, et al. Inspiratory muscle training does not improve clinical outcomes in 3-week COPD rehabilitation: results from a randomised controlled trial. European Respiratory Journal 2018;51(1):1702000. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2021
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Shoemakher 2009
- Shoemaker MJ, Donker S, Lapoe A. Inspiratory muscle training in patients with chronic obstructive pulmonary disease: the state of the evidence. Cardiopulmonary Physical Therapy Journal 2009;20(3):5-15. [PMC free article] [PubMed] [Google Scholar]
Smith 1992
- Smith K, Cook D, Guyatt GH, Madhavan J, Oxman AD. Respiratory muscle training in chronic airflow limitation: a meta-analysis. American Review of Respiratory Disease 1992;145(3):533-9. [DOI] [PubMed] [Google Scholar]
Spruit 2013
- Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. American Journal of Respiratory and Critical Care Medicine 2013;188(8):e13-64. [DOI] [PubMed] [Google Scholar]
Sterne 2019
- Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [PMID: ] [DOI] [PubMed] [Google Scholar]
WebPlotDigitizer [Computer program]
- WebPlotDigitizer version 4.4. automeris.io/WebPlotDigitizer, 2010. [EMAIL ADRESS: ankitrohatgi@hotmail.com]
Welling 2015
- Welling Jorrit BA, Hartman Jorine E, Ten Hacken NH T, Klooster K, Slebos DJ. The minimal important difference for the St George's Respiratory Questionnaire in patients with severe COPD. European Respiratory Journal 2015;46(6):1598. [DOI] [PubMed] [Google Scholar]
WHO 2013
- World Health Organization. Projections of mortality and causes of death, 2015 and 2030. www.who.int/healthinfo/global_burden_disease/projections2015_2030/en/ (accessed prior to 14 May 2021).
WHO 2018
- World Health Organization. The top 10 causes of death. www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed prior to 14 May 2021).
WHO 2020
- World Health Organization. WHO burden of COPD. www.who.int/respiratory/copd/burden/en/ (accessed 8 March 2020). [https://www.who.int/respiratory/copd/burden/en/]
Wijkstra 1994
- Wijkstra PJ, TenVergert EM, Van Altena R, Otten V, Postma DS, Kraan J, Koëter GH. Reliability and validity of the chronic respiratory questionnaire (CRQ).. Thorax 1994;49(5):465-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2017
- Wood KL. Tests of respiratory muscle function - pulmonary disorders. www.msdmanuals.com/professional/pulmonary-disorders/tests-of-pulmonary-function-pft/tests-of-respiratory-muscle-function (accessed prior to 6 September 2020).
Wu 2017
- Wu W, Zhang X, Lin L, Ou Y, Li X, Guan Li, et al. Transdiaphragmatic pressure and neural respiratory drive measured during inspiratory muscle training in stable patients with chronic obstructive pulmonary disease. International Journal of Chronic Obstructive Pulmonary Disease 2017;12:773-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Ammous 2020
- Ammous O, Feki W, Lotfi T, Khamis AM, Rebai A, Kammoun S. Inspiratory muscle training, with or without concomitant pulmonary rehabilitation, for chronic obstructive pulmonary disease (COPD). Cochrane Database of Systematic Reviews 2020, Issue 11. Art. No: CD013778. [DOI: 10.1002/14651858.CD013778] [DOI] [PMC free article] [PubMed] [Google Scholar]


