Abstract
The most common anesthetic approach in hepato-pancreatic-biliary (HPB) surgery is general anesthesia (GA), but it may result in increased morbidity and mortality and peri-operative risks especially in frail patients. The aim of this study was to assess the safety and effectiveness of neuraxial anesthesia (NA) in HPB in a pilot clinical series. This analysis was conducted on 46 consecutive patients undergoing HPB surgery in an Italian Tertial referral center. Data were prospectively collected and retrospectively analyzed. continuous spinal anesthesia (CSA), combined spino-epidural anesthesia (CSEA) and peridural anesthesia (PA) were used in major and minor hepatectomies and bilio-pancreatic surgery instead of GA. NA was evaluated by analyzing the surgical and anesthesiological short-term outcomes. 46 patients were considered eligible for the study between February 2018 and May 2020. The average age was 69.07 (± 9.95) years. 22 were males and 24 were females. According to the ASA score, 19 (41.30%) patients had ASA II, 22 (47.83%) had ASA III and 5 (10.87%) had ASA IV. 22 (47.83%) patients underwent CSA, 20 (43.48%) CSEA and 4 (8.69%) PA. We performed 8 major and 19 minor hepatectomies, 7 bilio-digestive derivations, 5 Whipple procedures, 4 iatrogenic biliary duct injuries, 2 splenopancreatectomies and 1 hepatic cyst fenestration. Clavien–Dindo ≥ 3 was observed in 3 patients. The conversion rate to endotracheal intubation occurring in 3 of 46 (6.52%) patients. After surgery, no local or pulmonary complications and delirium were reported in our series. The present study demonstrates that NA is a safe and feasible option in selected patients, if performed in referral centers by well-trained anaesthesiologists and surgeons.
Keywords: Neuraxial anesthesia, HPB surgery, Complex surgery, Liver surgery, Pancreatic surgery
Introduction
Nowadays, most malignancies that require surgery are diagnosed in elderly patients [1, 2].
Comorbidities and frailty grant patients an inter-individual variability that needs to be checked before, during and after surgery to best manage both anesthetic and surgical treatment [3–7].
In particular, hepato-pancreatic-biliary (HPB) surgery is one of the most challenging surgical fields because of the multiple metabolic imbalances that may occur during the peri-operative course [8–10].
Patients who undergo HPB surgery are more often old [11, 12] presenting a high anesthetic risk assessed in terms of the American Society of Anesthesiologists (ASA) score [13, 14] and multiple morbidities like chronic obstructive pulmonary disease (COPD) [15], insulin-dependent diabetes mellitus (DM) [16], and previous cardiovascular injuries [2, 17, 18].
Therefore, the best anesthetic techniques are needed to achieve the optimal surgical stress management. Good pain control, early mobilization and rapid recovery have been advocated to reduce the incidence of complications [19].
HPB surgery is usually performed under general anesthesia (GA), due to the long operative time and the aim to better manage hemodynamic parameters during interventions minimizing the blood loss risk [20, 21].
Nevertheless, in many large series, a high rate of complications has been reported for major HPB surgery in high-risk patients [22, 23]. GA may result in increased morbidity and mortality and peri-operative risks especially in frail patients due to invasive ventilation, induced liver stress related to drug metabolism and a higher incidence of post-operative delirium and subsequent loss of cognitive function [21, 24].
To overcome injuries potentially associated with GA, neuraxial anesthesia (NA) is gaining increasing consent and attention as an alternative anesthetic management during abdominal and thoracic surgery [25–27].
At present, the use of NA in COVID-19 patients may consistently help to overcome major intra- and post-operative challenges related to pulmonary impairment and minimize the high infection risk during orotracheal intubation [28–30].
Despite the widespread use of regional anesthesia in several surgical fields, there are no substantial evidences in the literature concerning major HPB surgery with NA.
The only study published so far using epidural anesthesia (EA) in HPB surgery accounts for a preliminary experience of ten cases with a relatively high number of post-operative complications [31].
Later on, a single case report on a left lateral sectionectomy during EA using a laparoscopic approach was published by the same surgical group [32].
In this pilot study, we prospectively investigated the use of NA in HPB patients in a large series of 46 patients.
Therefore, the primary endpoint was to demonstrate the safety and feasibility of NA in HPB surgery.
Secondary endpoints were assessing:
-Hemodynamic stability during surgery;
-Complications related to anesthetic technique: radiculopathies, post-puncture headache and spinal hematoma;
-Rate of post-operative pulmonary complications;
-Rate of post-operative course in intensive care unit (ICU);
-Incidence of post-operative delirium.
Methods
We retrospectively reviewed our prospective database of 46 consecutive patients who underwent HPB surgery with NA between February 2018 and May 2020 at our referral center for HPB surgery at Pineta Grande Hospital, Castel Volturno, Italy.
The patients’ data were analyzed according to Strengthening the Reporting of Observational studies in Epidemiology (STROBE) [33] (Fig. 1).
Fig. 1.
STROBEline flowchart of patients underwent neuraxial anesthesia from February 2018 to May 2020
Research involving human participants and/or animals was conducted acquiring written informed consents from all participants.
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of the University of Molise (protocol number 10/21, approval date: May 12, 2021).
The same team of surgeons trained in HPB surgery carried on all procedures.
The eligibility grade was defined as ASA ≥ 2. Exclusion criteria were: emergency surgery, lack of patient compliance (as neurological/psychiatric ones), lack of patient adherence, presence of coagulation disorders (platelets ≤ 100.000 mcL or International Normalized Ratio (INR) ≥ 1.5), Ejection Fraction (EF) ≤ 30%, moderate or severe aortic stenosis, contraindication to NA as local infection or inflammation in the puncture area and age related spinal deformity (Fig. 1). All cases were discussed in a multi-disciplinary meeting evaluating the surgical indications and an eventual NA approach.
Before surgery, all patients underwent routine blood tests, chest radiography and electrocardiogram (ECG). Spirometry and echocardiography were performed in selected cases. Cirrhotic patients were stratified according to the Model for End-stage Liver Disease (MELD) score [34].
A Makuuchi incision was made in all open liver cases whereas a bi-subcostal incision was used for open pancreatic surgery. A list of diseases and surgical procedures is shown in Table 1.
Table 1.
Histological diagnosis and surgical procedures in patients underwent NA according to the three different anesthetic techniques
| Anesthetic techniques, no. (%) | |||||
|---|---|---|---|---|---|
| CSA | CSEA | PA | TOT | ||
| Histological diagnosis | |||||
| HCC | 10 (76.92) | 3 (23.08) | 0 (0) | 13 | |
| CRLM | 4 (57.14) | 2 (28.57) | 1 (14.29) | 7 | |
| CCA | 3 (50.00) | 2 (33.33) | 1 (16.67) | 6 | |
| Benign | 3 (23.08) | 8 (61.54) | 2 (15.38) | 13 | |
| Pancreatic cancer | 1 (16.67) | 5 (83.33) | 0 (0) | 6 | |
| Gallbladder cancer | 1 (100) | 0 (0) | 0 (0) | 1 | |
| Surgical procedures | |||||
| Open:VLS | 21:1 | 17:3 | 3:1 | 41:5 | |
| Major hepatectomies | 5 (62.50) | 3 (37.50) | 0 (0) | 8 | |
| Mesohepatectomy | 3 (100) | 0 (0) | 0 (0) | 3 | |
| Left hepatectomy | 2 (100) | 0 (0) | 0 (0) | 2 | |
| ALPPS | 0 (0) | 3 (100) | 0 (0) | 3 | |
| Minor hepatectomies | 12 (63.16) | 5 (26.31) | 2 (10.53) | 19 | |
| Wedge resection | 7 (63.63) | 3 (27.27) | 1 (9.10) | 11 | |
| Bisegmentectomy | 3 (60.00) | 2 (40.00) | 0 (0) | 5 | |
| Segmentectomy | 2 (66.67) | 0 (0) | 1 (33.33) | 3 | |
| Iatrogenic biliary duct injury | 2 (50.00) | 1 (25.00) | 1 (25.00) | 4 | |
| Biliodigestive derivation | 2 (28.57) | 5 (71.43) | 0 (0) | 7 | |
| Whipple procedures | 1 (20.00) | 4 (80.00) | 0 (0) | 5 | |
| Splenopancreatectomy | 0 (0) | 1 (50.00) | 1 (50.00) | 2 | |
| Hepatic cyst fenestration | 0 (0) | 1 (100) | 0 (0) | 1 | |
CSA continuous spinal anesthesia, CSEA combined spino-epidural anesthesia, PA peridural anesthesia, HCC hepatocellular carcinoma, CRLM colorectal liver metastases, CCA cholangiocarcinoma, VLS videolaparoscopy, ALPSS associating liver partition and portal vein ligation for staged hepatectomy
Follow-up was performed at three months after surgery to evaluate blood tests and a complete abdominal ultrasound.
Anesthetic management and procedure
In the operating room, two large-caliber venous accesses (16/18-gauge) and a radial artery for invasive blood pressure monitoring were provided. Central Vein Pressure (CVP) was monitored using a central venous catheter (CVC) placed in the internal jugular vein. Then, vital parameters were monitored, and NA was performed.
The anesthesiological protocol provides three different techniques to perform NA: continuous spinal anesthesia (CSA), combined spino-epidural anesthesia (CSEA) and peridural anesthesia (PA).
In the CSA group, a single-needle technique was used. A PAJUNK® Intralong kit SPROTTE® 21-gauge was inserted at T7–T8 level. After free-flow of Cerebrospinal Fluid (CSF) was obtained, a 25-gauge catheter was inserted into the thoracic subarachnoid space.
The CSEA approach combines two different single-needle techniques, epidural anesthesia and subarachnoid anesthesia, to achieve a more effective pain management. The epidural anesthesia was performed using a kit composed of an ORX epidural catheter in Pebax with an Easy-lock fitting and centering device, Tuohy needle (Arrow International, Inc., Redding, PA) with a clear plastic base, and a flat filter 0.22-micron, 1 × 10 ml low-strength syringe. The puncture level was at T7–T8. The Loss of Resistance (LOR) technique was performed to locate the epidural space and a 20-gauge catheter was placed. For subarachnoid anesthesia, a 27/25-gauge Whitacre needle (Arrow International, Inc.) was inserted into the intervertebral space below the epidural catheter placement.
In the PA, an 18-gauge Touhy-shaped needle of the PERIFIX® kit was inserted in the T7–T8 space. The LOR technique was used to locate the peridural space and place the 20-gauge catheter.
In Table 2, the routine drug administration schedule during NA is shown.
Table 2.
Pharmacological protocol
| Continuous spinal anesthesia (CSA) | Combined spino-epidural anesthesia (CSEA) | Peridural anesthesia (PA) |
|---|---|---|
| Start bolus: | Spinal anesthesia: | Start bolus: |
| Bupivacaine (0.5%) 5 mg + magnesium sulfate 50 mg | Ropivacaine (0.3%) 10 mg + magnesium sulfate 50 mg + NaCl solution 0.9% for a total volume of 5 ml | Ropivacaine (0.4%) 7 ml |
| Epidural anesthesia: | ||
| Surgical incision: | Top up of Ropivacaine (0.5%) 10 ml every 90/100 min | |
| Bupivacaine (0.5%) 2.5 mg | Top up: | |
| Post-operative pain therapy: ropivacaine (0.2%) 5/7 ml/h | Ropivacaine (0.35%) 5 ml every 60/90 min | |
| Paracetamol 1 gr as needed (max 3 gr/die) | ||
| Top up: | Post-operative pain therapy: ropivacaine (0.2%) 5/7 ml/h | |
| Bupivacaine (0.5%) 2.5 mg every 50/60 min | Paracetamol 1 gr as needed (max 3 gr/die) | |
| Post-operative pain therapy: | ||
| Ropivacaine (0.22%) in continuous infusion a 0.3/0.5 ml/h |
Pinprick (target: Hollmen score 4 at C4–C5 level) and Ice Tests were performed in all cases to evaluate the extension of the sensory block [35, 36]. Our goal was to extend sensory block up to the T4 dermatome.
Patients were always awake, a Venturi Mask (FiO2 40–50%) was placed and sedation was achieved with a continuous infusion of propofol (2–4 mg/kg/h) with a Richmond Agitation–Sedation Scale (RASS) [37] score of ≥ -2. All patients were continuously monitored using pulse oximetry, ECG, serial hemogasanalysis and invasive arterial blood pressure measurements. Depth of anesthesia was routinely checked using the Bispectral Index (BIS) system [38]. Hypotension due to the spinal anesthesia was managed with a continuous infusion of noradrenaline titrated, at an average dosage of 0.12 ± 0.06 mcg/kg/min, to maintain a Mean Arterial Pressure (MAP) above 65 mmHg. At the end of surgery, the vasopressor infusion was stopped in all patients and ondansetron (4 mg intravenous) was administered as prophylaxis for post-operative nausea and vomiting (PONV) [39].
No additional distractions were used during the surgical procedures.
Transfer to the post-operative ICU was decided depending on the overall vital parameters, intra- and post-operative hemogasanalysis samples, respiratory performance of the patient, and eventual further needs for continuous infusion of inotropic/vasopressors could be given.
To state the benefits of our procedures, we subsequently evaluated:
Presence/absence of delirium (hypo or hyperkinetic) through the Confusion Assessment Method-Intensive Care Unit (CAM-ICU) at 15 min and 8–24 h from the end of surgery [40];
Presence of post-operative pain through Numeric Rating Scale (NRS) [41];
Presence of PONV [39];
Level of motor block through the Bromage scale [42].
Post-operative complication was assessed according to the Clavien–Dindo classification and Comprehensive Complication Index [43, 44].
Statistical analysis
The IBM Statistical Package for the Social Sciences (IBM SPSS®) was used to analyze data.
Group analysis was performed to define the homogeneity between them. To better define the impact of NA on liver function, we performed a statistical analysis of the blood tests values before surgery and before discharge. Creatinine, estimated glomerular filtration rate (eGFR), INR and hemoglobin (Hb) were evaluated, each one stratified for the anesthesiological technique. The eGFR was calculated according to the CDK-EPI formula [45]. Statistical significance was defined as a two-tailed p value < 0.05. The Shapiro–Wilk test was applied to the quantitative elements to test their distributions. Quantitative independent data were analyzed by analysis of variance (ANOVA) or Kruskal–Wallis test in cases of non-normal distribution. The differences between time points were assessed by paired t test, if they were normally distributed, and by Wilcoxon signed-rank test or Kolmogorov–Smirnov test, if they were not normally distributed.
Quantitative data were expressed as mean ± standard deviation (SD). Categorical variables were analyzed using the Chi-ssquare test (χ2).
Results
A total of 46 patients (22 males and 24 females) were included in our study. 41 (89.13%) patients were approached open, and 5 (10.87%) patients underwent laparoscopic surgery for minor liver resections.
Mean age was 69.07 years ± 9.95 with an average body mass index (BMI) of 25.48 ± 2.48.
The ASA score of 19 (41.30%) patients was ASA II, 22 (47.83%) were ASA III and 5 (10.87%) were ASA IV.
22 (47.83%) patients underwent CSA, 20 (43.48%) underwent CSEA and 4 (8.69%) underwent PA.
The most frequent comorbidities were hypertension (65.22%), pulmonary disease (34.78%), and diabetes mellitus (30.43%). 4 (8.70%) patients had a MELD score ≥ 13.
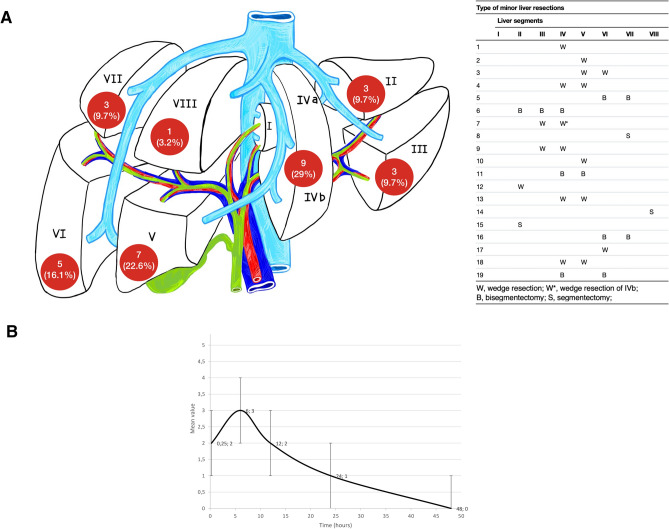
We performed 8 (17.39%) major liver resections defined as resection of three or more segments as stated by the Brisbane Classification [46], 19 (41.30%) minor liver resections, 7 (15.22%) bilio-digestive derivation, 5 (10.87%) Whipple procedures, and 7 (15.22%) other procedures (Table 1 and Fig. 2). In two cases of minor resections, a simultaneous right hemicolectomy and left hemicolectomy were associated.
Fig. 2.
A Type of minor liver resections, B Trend of mean pain value (± SD) according to Numeric Rating Scale (NRS) measured at 15 min and 6, 12, 24 and 48 h after surgery
The baseline characteristics of the study population and surgical procedures according to the NA technique are described in Tables 1 and 3. Statistical analysis of the three groups of NA techniques is also shown in Table 3.
Table 3.
Baseline characteristics of patients according to type of NA
| No. (%) and/or mean ± SD | ||||||
|---|---|---|---|---|---|---|
| CSA | CSEA | PA | TOT | p value | ||
| No. of patients | 22 (47.83) | 20 (43.48) | 4 (8.69) | 46 | ||
| Sex (male:female) | 11:11 | 8:12 | 3:1 | 22:24 | 0.414 | |
| Age (years) | 69 ± 9.78 | 69.95 ± 10.84 | 65 ± 6.48 | 69.07 ± 9.95 | 0.671 | |
| BMI (Kg/m2) | 25.50 ± 2.72 | 25.40 ± 2.19 | 25.75 ± 3.20 | 25.48 ± 2.48 | 0.810 | |
| ASA | II | 8 (42.10) | 9 (47.37) | 2 (10.53) | 19 | 0.743 |
| III | 12 (54.55) | 8 (36.36) | 2 (9.09) | 22 | ||
| IV | 2 (40.00) | 3 (60.00) | 0 (0) | 5 | ||
| MELD score | 1–10 |
21 (53.85) 7.00 ± 0.89 |
16 (41.02) 7.44 ± 1.36 |
2 (5.13) 6.50 ± 0.71 |
39 (84.78) 7.15 ± 1.11 |
|
| 11–20 |
1 (14.29) 16 ± 0 |
4 (57.14) 14.25 ± 3.86 |
2 (28.57) 15.50 ± 4.95 |
7 (15.22) 14.86 ± 3.48 |
0.270 | |
| General | 7.40 ± 2.10 | 8.80 ± 3.41 | 11.00 ± 5.94 | 8.32 ± 3.23 | ||
| Risk factors | Smokers | 5 (55.56) | 2 (22.22) | 2 (22.22) | 9 | 0.162 |
| Ex-smokers | 5 (33.33) | 9 (60.00) | 1 (6.67) | 15 | ||
| Alcohol | 3 (37.50) | 4 (50.00) | 1 (12.50) | 8 | 0.792 | |
| Comorbidities | Hypertension | 19 (63.33) | 8 (26.67) | 3 (10.00) | 30 | |
| Pulmonary disease | 9 (56.25) | 6 (37.50) | 1 (6.25) | 16 | ||
| DM | 4 (28.57) | 8 (57.14) | 2 (14.29) | 14 | ||
| MI | 2 (28.57) | 4 (57.14) | 1 (14.29) | 7 | ||
| AF | 0 (0) | 4 (100) | 0 (0) | 4 | ||
| HCV | 2 (66.67) | 1 (33.33) | 0 (0) | 3 | ||
| HBV | 1 (50.00) | 1 (50.00) | 0 (0) | 2 | ||
| CKD | 1 (50.00) | 1 (50.00) | 0 (0) | 2 | ||
| AAA | 1 (50.00) | 1 (50.00) | 0 (0) | 2 | ||
| Neurological disease | 0 (0) | 2 (100) | 0 (0) | 2 | ||
SD standard deviation, CSA continuous spinal anesthesia, CSEA combined spino-epidural anesthesia, PA peridural anesthesia, BMI body mass index (Kg/m2), ASA American Society of Anesthesiologist physical status, MELD model for end-stage liver disease, DM diabetes mellitus, MI myocardial infarction, AF atrial fibrillation, HCV hepatitis C virus, HBV hepatitis B virus, CKD chronic kidney disease, AAA abdominal aortic aneurysm
Types of minor resections are listed in Fig. 2.
Peri-operative course and complications
NA was well tolerated in 43 out of the 46 patients. 3 of 22 (13.64%) patients in the CSA group were converted to endotracheal intubation (ETI) in one case due to hemodynamic instability during mesohepatectomy and in two due to subjective intolerance to the procedure (Table 4).
Table 4.
Intraoperative, post-operative course and blood samples analysis according to type of NA
| No. (%) or mean ± SD | ||||||
|---|---|---|---|---|---|---|
| CSA | CSEA | PA | Total | p value | ||
| Intraoperative course | ||||||
| Pringle maneuver | 12 (75.00) | 2 (12.50) | 2 (12.50) | 16 | 0.005 | |
| Intraoperative blood transfusion |
4 (50.00) - 2 Mesoepatectomy - 1 Left Hepatectomy - 1 Segmentectomy IIs |
4 (50.00) - 3 Whipple - 1 ALPPS |
0 (0) | 8 | 0.443 | |
| Conversion to ETI |
3 (100) - 1 Mesoepatectomy - 1 Wedge Resection IIIs - 1 Segmentectomy VIIIs |
0 (0) | 0 (0) | 3 | ||
| Transfer to ICU | 5 (50.00) | 4 (40.00) | 1 (10.00) | 10 | 0.964 | |
| Operative time, (min) | 303.55 ± 97.72 | 264.00 ± 124.30 | 262.50 ± 35.71 | 282.78 ± 107.10 | 0.462 | |
| Post-operative course | ||||||
| Clavien–Dindo classification | I | 12 (52.17) | 9 (39.13) | 2 (8.70) | 23 | |
| II | 8 (40.00) | 11 (55.00) | 1 (5.00) | 20 | ||
| III | 0 (0) | 0 (0) | 1 (100) | 1 | ||
| IV | 0 (0) | 0 (0) | 0 (0) | 0 | ||
| V | 2 (100) | 0 (0) | 0 (0) | 2 | ||
| Comprehensive complication index | I | 12 (52.17) | 9 (39.13) | 2 (8.70) | 23 | |
| II | 8 (50.00) | 6 (37.50) | 2 (12.50) | 16 | ||
| IIIa | 0 (0) | 3 (100) | 0 (0) | 3 | ||
| IIIb | 0 (0) | 2 (100) | 0 (0) | 2 | ||
| IVa | 0 (0) | 0 (0) | 0 (0) | 0 | ||
| IVb | 0 (0) | 0 (0) | 0 (0) | 0 | ||
| V | 2 (100) | 0 (0) | 0 (0) | 2 | ||
| Post-operative blood transfusion | 4 (44.44) | 5 (55.56) | 0 (0) | 9 | 0.346 | |
| TPN | 5 (31.25) | 9 (56.25) | 2 (12.50) | 16 | 0.248 | |
| PONV | 0 | 3 (100) | 0 | 3 | ||
| Length of stay, (days) | 9.73 ± 5.69 | 14.75 ± 12.36 | 9.5 ± 4.79 | 11.89 ± 9.36 | 0.404 | |
| Blood samples | ||||||
| Creatinine, mg/dL | Pre-OP | 0.86 ± 0.17 | 0.86 ± 0.27 | 0.94 ± 0.31 | ||
| Post-OP | 0.85 ± 0.27 | 0.76 ± 0.27 | 0.70 ± 0.20 | |||
| p value | 0.520 | 0.051 | 0.144 | |||
| eGFR, ml/min/1.73 m2 | Pre-OP | 81.01 ± 15.48 | 78.31 ± 21.53 | 81.18 ± 18.53 | ||
| Post-OP | 81.80 ± 18.43 | 84.85 ± 24.30 | 95.96 ± 15.65 | |||
| p value | 0.543 | 0.112 | 0.348 | |||
| INR | Pre-OP | 0.98 ± 0.08 | 1.01 ± 0.12 | 1.05 ± 0.07 | ||
| Post-OP | 1.06 ± 0.10 | 1.04 ± 0.08 | 1.09 ± 0.03 | |||
| p value | 0.109 | 0.251 | 0.066 | |||
| Hb, g/dL | Pre-OP | 13.31 ± 1.27 | 12.53 ± 1.56 | 12.85 ± 2.22 | ||
| Post-OP | 10.99 ± 2.20 | 10.88 ± 1.47 | 10.62 ± 2.14 | |||
| p value | < 0.001 | < 0.001 | 0.002 | |||
SD standard deviation, CSA continuous spinal anesthesia, CSEA combined spino-epidural anesthesia, PA peridural anesthesia, ALPSS associating liver partition and portal vein ligation for staged hepatectomy, ETI endotracheal intubation, ICU intensive care unit, TPN total parental nutrition, PONV post-operative nausea and vomiting, eGFR estimated glomerular filtration rate, INR international normalized ratio, Hb hemoglobin, PO post-operative
Ten (21.74%) patients were transferred to the ICU after surgery for a maximum stay of 48 h. We reported 3 out of 20 (15%) cases of PONV in the CSEA group. No local complications related to the anesthetic procedure were observed. The mean pain value is shown in Fig. 2. No severe pulmonary post-operative complications, morbidity due to CVC placement and delirium occurred in our series.
Clavien–Dindo complications, Comprehensive Complication Index and all characteristics of intra- and post-operative course are listed in Table 4.
No intra-operative mortalities occurred. 30-day mortality was observed in 2 (4.35%) patients, both of whom underwent mesohepatectomy under the CSA anesthetic technique. In one case, the mortality was due to cardiac shock on post-operative day (POD) 1, and the second patient died of liver failure 10 days after surgery.
All patients were mobilized during the first POD.
Considering our protocol (Table 2), no patient required additional post-operative intravenous rescue analgesic drugs.
The epidural/spinal catheter was removed on POD 4 as scheduled by our protocol.
No reoperations were needed.
All patients had R0 resection margins.
Discussion
Our study showed that NA is a safe and feasible technique for complex HPB surgery.
There were no major technical drawbacks and NA was performed in all 46 cases using different anesthetic techniques such as CSA, CSEA and PA.
Our study included a large majority of elderly patients, mean age 69.07 yrs. ± 9.95, which is comparable to many series in the literature [47–49]. The mean operative time of 282 ± 107 min was within the range of reported data elsewhere and it was not hampered by the absence of curarizations [50, 51]. Furthermore, the NA technique did not affect the risk of bleeding and hemodynamic stability.
The intra-operative and post-operative blood transfusion rate were similar to those in other recent series reported by referral centers for HPB surgery [48, 52–54]. Despite some limitations due to the patient’s breathing, no major troubles were recorded during the most challenging surgical steps, such as the handling of major vessels or lymphadenectomies.
Transfer to the ICU was generally performed as a precaution due to the prolonged surgery only in less than 25% of patients according to data reported in the literature [55].
Major concerns performing NA in HPB patients may be represented by the theoretical risk of coagulopathy at the site of local anesthesia rather than during surgery [56].
Radiculopathies, post-puncture headaches or spinal hematoma did not occur in any patient.
In all cases, a good pain control was achieved with early mobilization and oral intake. PONV occurred in only three patients undergoing CSEA. The complications rate was low (Table 4) as reported in a large series [57–59].
Two patients died of non-anesthetic-related causes after major surgery such as mesohepatectomy.
No significant variation was observed in the post-operative INR, creatinine and eGFR. However, the risks of coagulation disorders and renal impairment is frequently associated to NA [56].
The conversion rate was low occurring in 3 of 46 patients and was generally due to a better handling of technical procedures or patient’s intolerance. All three conversions were easily performed without any difficulty neither for surgeons or patients.
The hospital stay was longer than in other series [48, 60, 61]. This is mainly because of the pivotal character of the study and, at the same time, to the wide geographical referral of patients making a safe and early discharge of patients.
The minimally invasive approach was used in only five cases, but the good results achieved may allow a wider use of NA also in the laparoscopic HPB surgery.
No statistically significant differences were found among the three different NA techniques. Our personal experience suggests a special pledge for the CSA technique.
Limitations
The heterogeneity of the patients’ sample and of the performed procedures represents the major limitation of this series, but it opens the road to a larger use of NA. Further multicenter studies could confirm our data and better define the indications and drawbacks of this anesthetic approach for HPB surgery.
Conclusions
Despite a general concern for hemodynamic instability and potential renal impairment limited using NA in complex procedures, this pilot series shows that NA is safe and feasible, allowing minor/major liver resections and pancreatic surgery also with a minimally invasive approach if surgery is performed in referral centers by well-trained anesthesiologists and surgeons.
In patients with comorbidities associated with a higher risk of post-operative delirium/pulmonary complications, NA may be considered a valid option, thereby reducing the need for ICU stay after surgery.
The complete absence of post-operative delirium, a major complication after GA [62, 63], reinforces the potential role option of NA in elderly and frail patients.
Our data may open new perspective in the management of these complex patients.
Funding
This research received no specific grants from any funding agency in the public, commercial, or not-for-profit sectors.
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, with undue reservation.
Declarations
Conflict of interest
None reported.
Research involving human participants and/or animals and Informed consent
Research involving human participants and/or animals was conducted acquiring written informed consents from all participants.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Takagi K, Umeda Y, Yoshida R, Nobuoka D, Kuise T, Fushimi T, et al. The outcome of complex hepato-pancreato-biliary surgery for elderly patients: a propensity score matching analysis. Dig Surg. 2019;36(4):323–330. doi: 10.1159/000489826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dedinská I, Laca L, Miklušica J, Palkoci B, Skálová P, Lauková S, et al. Complications of liver resection in geriatric patients. Ann Hepatol. 2017;16(1):149–156. doi: 10.5604/16652681.1226934. [DOI] [PubMed] [Google Scholar]
- 3.Ceccarelli G, Andolfi E, Fontani A, Calise F, Rocca A, Giuliani A. Robot-assisted liver surgery in a general surgery unit with a “Referral Centre Hub&Spoke Learning Program”. Early outcomes after our first 70 consecutive patients. Minerva Chir. 2018;73(5):460–8. doi: 10.23736/S0026-4733.18.07651-4. [DOI] [PubMed] [Google Scholar]
- 4.Gani F, Cerullo M, Amini N, Buettner S, Margonis GA, Sasaki K, et al. Frailty as a risk predictor of morbidity and mortality following liver surgery. J Gastrointest Surg. 2017;21(5):822–830. doi: 10.1007/s11605-017-3373-6. [DOI] [PubMed] [Google Scholar]
- 5.Komici K, Cappuccio M, Scacchi A, Vaschetti R, Delli Carpini G, Picerno V, et al. The prevalence and the impact of frailty in hepato-biliary pancreatic cancers: a systematic review and meta-analysis. J Clin Med. 2022;11(4):1116. doi: 10.3390/jcm11041116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marte G, Scuderi F, Rocca A, Surfaro G, Migliaccio C, Ceriello A. Laparoscopic splenectomy: a single center experience. Unusual cases and expanded inclusion criteria for laparoscopic approach. Updates Surg. 2013;Jun(2):115. doi: 10.1007/s13304-013-0197-0. [DOI] [PubMed] [Google Scholar]
- 7.Rocca A, Brunese MC, Cappuccio M, Scacchi A, Martucci G, Buondonno A, et al. Impact of physical activity on disability risk in elderly patients hospitalized for mild acute diverticulitis and diverticular bleeding undergone conservative management. Medicina (Kaunas). 2021;57(4):360. doi: 10.3390/medicina57040360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gasteiger L, Eschertzhuber S, Tiefenthaler W. Perioperative management of liver surgery-review on pathophysiology of liver disease and liver failure. Eur Surg. 2018;50(3):81–86. doi: 10.1007/s10353-018-0522-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gholson CF, Provenza JM, Bacon BR. Hepatologic considerations in patients with parenchymal liver disease undergoing surgery. Am J Gastroenterol. 1990;85(5):487–496. [PubMed] [Google Scholar]
- 10.Mohamedahmed AYY, Zaman S, Albendary M, Wright J, Abdalla H, Patel K, et al. Laparoscopic versus open hepatectomy for malignant liver tumours in the elderly: systematic review and meta-analysis. Updates Surg. 2021;73(5):1623–1641. doi: 10.1007/s13304-021-01091-7. [DOI] [PubMed] [Google Scholar]
- 11.Turrentine FE, Wang H, Simpson VB, Jones RS. Surgical risk factors, morbidity, and mortality in elderly patients. J Am Coll Surg. 2006;203(6):865–877. doi: 10.1016/j.jamcollsurg.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 12.Ripamonti L, De Carlis R, Lauterio A, Mangoni I, Frassoni S, Bagnardi V, et al. Major hepatectomy for perihilar cholangiocarcinoma in elderly patients: is it reasonable? Updates Surg. 2022;74(1):203–211. doi: 10.1007/s13304-021-01111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aiolfi A, Lombardo F, Bonitta G, Danelli P, Bona D. Systematic review and updated network meta-analysis comparing open, laparoscopic, and robotic pancreaticoduodenectomy. Updates Surg. 2021;73(3):909–922. doi: 10.1007/s13304-020-00916-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peltrini R, Imperatore N, Carannante F, Cuccurullo D, Capolupo GT, Bracale U, et al. Age and comorbidities do not affect short-term outcomes after laparoscopic rectal cancer resection in elderly patients. A multi-institutional cohort study in 287 patients. Updates Surg. 2021;73(2):527–37. doi: 10.1007/s13304-021-00990-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casadei R, Zanini N, Morselli-Labate AM, Calculli L, Pezzilli R, Potì O, et al. Prognostic factors in periampullary and pancreatic tumor resection in elderly patients. World J Surg. 2006;30(11):1992–2001. doi: 10.1007/s00268-006-0122-5. [DOI] [PubMed] [Google Scholar]
- 16.Sreedharan R, Abdelmalak B. Diabetes mellitus: preoperative concerns and evaluation. Anesthesiol Clin. 2018;36(4):581–597. doi: 10.1016/j.anclin.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Komici K, Vitale DF, Leosco D, Mancini A, Corbi G, Bencivenga L, et al. Pressure injuries in elderly with acute myocardial infarction. Clin Interv Aging. 2017;12:1495–1501. doi: 10.2147/CIA.S135676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amato B, Compagna R, Rocca A, Bianco T, Milone M, Sivero L, et al. Fondaparinux vs warfarin for the treatment of unsuspected pulmonary embolism in cancer patients. Drug Des Devel Ther. 2016;10:2041–2046. doi: 10.2147/DDDT.S106153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rocca A, Cipriani F, Belli G, Berti S, Boggi U, Bottino V, et al. The Italian consensus on minimally invasive simultaneous resections for synchronous liver metastasis and primary colorectal cancer: a delphi methodology. Updates Surg. 2021;73:1247–1265. doi: 10.1007/s13304-021-01100-9. [DOI] [PubMed] [Google Scholar]
- 20.Owens WD, Felts JA, Spitznagel EL., Jr ASA physical status classifications: a study of consistency of ratings. Anesthesiology. 1978;49(4):239–243. doi: 10.1097/00000542-197810000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Snowden C, Prentis J. Anesthesia for hepatobiliary surgery. Anesthesiol Clin. 2015;33(1):125–141. doi: 10.1016/j.anclin.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Slakey DP, Simms E, Drew B, Yazdi F, Roberts B. Complications of liver resection: laparoscopic versus open procedures. Jsls. 2013;17(1):46–55. doi: 10.4293/108680812X13517013317716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dezfouli SA, Ünal UK, Ghamarnejad O, Khajeh E, Ali-Hasan-Al-Saegh S, Ramouz A, et al. Systematic review and meta-analysis of the efficacy of prophylactic abdominal drainage in major liver resections. Sci Rep. 2021;11:1–12. doi: 10.1038/s41598-021-82333-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strøm C, Rasmussen LS, Sieber FE. Should general anaesthesia be avoided in the elderly? Anaesthesia. 2014;69(Suppl 1):35–44. doi: 10.1111/anae.12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellakany MH. Thoracic spinal anesthesia is safe for patients undergoing abdominal cancer surgery. Anesth Essays Res. 2014;8(2):223–228. doi: 10.4103/0259-1162.134516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castellani D, Starnari R, Faloia L, Stronati M, Venezia A, Gasparri L, et al. Radical cystectomy in frail octogenarians in thoracic continuous spinal anesthesia and analgesia: a pilot study. Ther Adv Urol. 2018;10:343–9. doi: 10.1177/1756287218795427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gramatica L, Jr, Brasesco OE, Mercado Luna A, Martinessi V, Panebianco G, Labaque F, et al. Laparoscopic cholecystectomy performed under regional anesthesia in patients with chronic obstructive pulmonary disease. Surg Endosc. 2002;16(3):472–475. doi: 10.1007/s00464-001-8148-0. [DOI] [PubMed] [Google Scholar]
- 28.Hotta K. Regional anesthesia in the time of COVID-19: a minireview. J Anesth. 2021;35(3):341–344. doi: 10.1007/s00540-020-02834-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macfarlane AJR, Harrop-Griffiths W, Pawa A. Regional anaesthesia and COVID-19: first choice at last? Br J Anaesth. 2020;125(3):243–247. doi: 10.1016/j.bja.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aldrighetti L, Boggi U, Falconi M, Giuliante F, Cipriani F, Ratti F, et al. Perspectives from Italy during the COVID-19 pandemic: nationwide survey-based focus on minimally invasive HPB surgery. Updates Surg. 2020;72(2):241–247. doi: 10.1007/s13304-020-00815-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamoto K, Fukumori D, Yamamoto F, Yamamoto M, Igimi H, Yamashita Y. First report of hepatectomy without endotracheal general anesthesia. J Am Coll Surg. 2013;216(5):908–914. doi: 10.1016/j.jamcollsurg.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto K, Ishizawa T, Kokudo N. Laparoscopic hepatectomy under epidural anesthesia. Ann Surg. 2014;260:e1. doi: 10.1097/SLA.0000000000000816. [DOI] [PubMed] [Google Scholar]
- 33.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12(12):1500–1524. doi: 10.1016/j.ijsu.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 34.Kamath PS, Kim WR. The model for end-stage liver disease (MELD) Hepatology. 2007;45(3):797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 35.Büttner J, Klose R. Alkalinization of mepivacaine for axillary plexus anesthesia using a catheter. Reg Anaesth. 1991;14(1):17–24. [PubMed] [Google Scholar]
- 36.Jankowska A, Veillette Y. Comparison of differential blockade during spinal anesthesia using isobaric vs. hyperbaric lidocaine 2% Can J Anaesth. 2000;47(2):137–42. doi: 10.1007/BF03018849. [DOI] [PubMed] [Google Scholar]
- 37.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O'Neal PV, Keane KA, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 38.Kissin I. Depth of anesthesia and bispectral index monitoring. Anesth Analg. 2000;90(5):1114–1117. doi: 10.1097/00000539-200005000-00021. [DOI] [PubMed] [Google Scholar]
- 39.Gan TJ, Diemunsch P, Habib AS, Kovac A, Kranke P, Meyer TA, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014;118(1):85–113. doi: 10.1213/ANE.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 40.Gusmao-Flores D, Salluh JI, Chalhub R, Quarantini LC. The confusion assessment method for the intensive care unit (CAM-ICU) and intensive care delirium screening checklist (ICDSC) for the diagnosis of delirium: a systematic review and meta-analysis of clinical studies. Crit Care. 2012;16(4):R115. doi: 10.1186/cc11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiarotto A, Maxwell LJ, Ostelo RW, Boers M, Tugwell P, Terwee CB. Measurement Properties of Visual Analogue Scale, Numeric Rating Scale, and Pain Severity Subscale of the Brief Pain Inventory in Patients With Low Back Pain: A Systematic Review. J Pain. 2019;20(3):245–263. doi: 10.1016/j.jpain.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 42.Bromage PR (1978) Epidural analgesia: WB Saunders Company
- 43.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. 2013;258(1):1–7. doi: 10.1097/SLA.0b013e318296c732. [DOI] [PubMed] [Google Scholar]
- 45.Stevens LA, Schmid CH, Greene T, Zhang YL, Beck GJ, Froissart M, et al. Comparative performance of the CKD epidemiology collaboration (CKD-EPI) and the modification of diet in renal disease (MDRD) study equations for estimating GFR levels above 60 mL/min/17.3 m2. Am J Kidney Dis. 2010;56(3):486–95. doi: 10.1053/j.ajkd.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strasberg SM, Belghiti J, Clavien PA, Gadzijev E, Garden JO, Lau WY, et al. The Brisbane 2000 terminology of liver anatomy and resections. HPB. 2000;2(3):333–339. doi: 10.1016/S1365-182X(17)30755-4. [DOI] [Google Scholar]
- 47.Fretland ÅA, Dagenborg VJ, Bjørnelv GMW, Kazaryan AM, Kristiansen R, Fagerland MW, et al. Laparoscopic versus open resection for colorectal liver metastases: the OSLO-COMET randomized controlled trial. Ann Surg. 2018;267(2):199–207. doi: 10.1097/SLA.0000000000002353. [DOI] [PubMed] [Google Scholar]
- 48.Robles-Campos R, Lopez-Lopez V, Brusadin R, Lopez-Conesa A, Gil-Vazquez PJ, Navarro-Barrios Á, et al. Open versus minimally invasive liver surgery for colorectal liver metastases (LapOpHuva): a prospective randomized controlled trial. Surg Endosc. 2019;33(12):3926–3936. doi: 10.1007/s00464-019-06679-0. [DOI] [PubMed] [Google Scholar]
- 49.Rocca A, Scacchi A, Cappuccio M, Avella P, Bugiantella W, De Rosa M, et al. Robotic surgery for colorectal liver metastases resection: a systematic review. Int J Med Robot. 2021;17(6):e2330. doi: 10.1002/rcs.2330. [DOI] [PubMed] [Google Scholar]
- 50.Beppu T, Wakabayashi G, Hasegawa K, Gotohda N, Mizuguchi T, Takahashi Y, et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for colorectal liver metastases with propensity score matching: a multi-institutional Japanese study. J Hepatobiliary Pancreat Sci. 2015;22(10):711–720. doi: 10.1002/jhbp.261. [DOI] [PubMed] [Google Scholar]
- 51.Seiler CA, Wagner M, Bachmann T, Redaelli CA, Schmied B, Uhl W, et al. Randomized clinical trial of pylorus-preserving duodenopancreatectomy versus classical Whipple resection-long term results. Br J Surg. 2005;92(5):547–556. doi: 10.1002/bjs.4881. [DOI] [PubMed] [Google Scholar]
- 52.Ratti F, Cipriani F, Reineke R, Catena M, Paganelli M, Comotti L, et al. Intraoperative monitoring of stroke volume variation versus central venous pressure in laparoscopic liver surgery: a randomized prospective comparative trial. HPB (Oxford) 2016;18(2):136–144. doi: 10.1016/j.hpb.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aldrighetti L, Guzzetti E, Pulitanò C, Cipriani F, Catena M, Paganelli M, et al. Case-matched analysis of totally laparoscopic versus open liver resection for HCC: short and middle term results. J Surg Oncol. 2010;102(1):82–86. doi: 10.1002/jso.21541. [DOI] [PubMed] [Google Scholar]
- 54.Ercolani G, Solaini L, D'Acapito F, Isopi C, Pacilio CA, Moretti C, et al. Implementation of a patient blood management in an Italian City hospital: is it effective in reducing the use of red blood cells? Updates Surg. 2022 doi: 10.1007/s13304-022-01409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Merath K, Cerullo M, Farooq A, Canner JK, He J, Tsilimigras DI, et al. Routine intensive care unit admission following liver resection: what is the value proposition? J Gastrointest Surg. 2020;24(11):2491–2499. doi: 10.1007/s11605-019-04408-5. [DOI] [PubMed] [Google Scholar]
- 56.Buell JF. Laparoscopic hepatectomy under epidural anesthesia without general endotracheal anesthesia: feasible but applicable? Ann Surg. 2014;260:e2. doi: 10.1097/SLA.0000000000000815. [DOI] [PubMed] [Google Scholar]
- 57.Haney CM, Studier-Fischer A, Probst P, Fan C, Müller PC, Golriz M, et al. A systematic review and meta-analysis of randomized controlled trials comparing laparoscopic and open liver resection. HPB (Oxford). 2021;23:1467–1481. doi: 10.1016/j.hpb.2021.03.006. [DOI] [PubMed] [Google Scholar]
- 58.Ciria R, Ocaña S, Gomez-Luque I, Cipriani F, Halls M, Fretland ÅA, et al. A systematic review and meta-analysis comparing the short- and long-term outcomes for laparoscopic and open liver resections for liver metastases from colorectal cancer. Surg Endosc. 2020;34(1):349–360. doi: 10.1007/s00464-019-06774-2. [DOI] [PubMed] [Google Scholar]
- 59.Ratti F, Catena M, Di Palo S, Staudacher C, Aldrighetti L. Laparoscopic approach for primary colorectal cancer improves outcome of patients undergoing combined open hepatic resection for liver metastases. World J Surg. 2015;39(10):2573–2582. doi: 10.1007/s00268-015-3127-0. [DOI] [PubMed] [Google Scholar]
- 60.Cannon RM, Scoggins CR, Callender GG, McMasters KM, Martin RC., 2nd Laparoscopic versus open resection of hepatic colorectal metastases. Surgery. 2012;152(4):567–73. doi: 10.1016/j.surg.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 61.Martínez-Cecilia D, Cipriani F, Shelat V, Ratti F, Tranchart H, Barkhatov L, et al. Laparoscopic versus open liver resection for colorectal metastases in elderly and octogenarian patients: a multicenter propensity score based analysis of short- and long-term outcomes. Ann Surg. 2017;265(6):1192–1200. doi: 10.1097/SLA.0000000000002147. [DOI] [PubMed] [Google Scholar]
- 62.Tanaka S, Ueno M, Iida H, Kaibori M, Nomi T, Hirokawa F, et al. Preoperative assessment of frailty predicts age-related events after hepatic resection: a prospective multicenter study. J Hepatobiliary Pancreat Sci. 2018;25(8):377–387. doi: 10.1002/jhbp.568. [DOI] [PubMed] [Google Scholar]
- 63.Ishihara A, Tanaka S, Ueno M, Iida H, Kaibori M, Nomi T, et al. Preoperative risk assessment for delirium after hepatic resection in the elderly: a prospective multicenter study. J Gastrointest Surg. 2021;25(1):134–144. doi: 10.1007/s11605-020-04562-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, with undue reservation.