Abstract
Simple Summary
Probiotics are defined as live microorganisms that, when administered in adequate amounts, confer a health benefit on the host. The effect of a commercial diet containing the probiotic Enterococcus faecium NCIMB 10415 was examined in a crossover study with 11 healthy privately owned dogs. The dogs were fed the same balanced commercial diet, with or without the probiotic, for 16 days, and then fed the alternate diet for an additional 16 days with a 19 days washout period in between. Owners evaluated their dog’s faecal quality daily. Faecal bacterial composition (microbiome) and short-chain fatty acid concentrations as well as serum concentrations of cholesterol, triglycerides, cobalamin and folate were analysed before and after each feeding period. Soft stools were less frequent when fed the diet with probiotic included compared with the diet alone. The probiotic diet also decreased serum cholesterol. Most observed effects were related to the diet itself, irrespective of probiotic inclusion or not. These effects included an increased faecal microbial diversity and content of the short-chain fatty acids butyrate and acetate, as well as a decrease in serum cobalamin concentration. There were indications of a likely prolonged survival (19 days) of the probiotic strain in a few individual dogs, which has not previously been reported for this probiotic strain.
Abstract
In dogs, the use of probiotics for preventive or therapeutic purposes has become increasingly common, however the evidence for beneficial effects are often limited. The aim of this study was to investigate the effects of feeding a diet containing Enterococcus faecium NCIMB 10415 on faecal quality, faecal short-chain fatty acid concentrations, serum concentrations of cholesterol, triglycerides, cobalamin and folate as well as faecal microbiome in adult dogs. Eleven healthy client owned dogs were enrolled in a randomized, double-blinded crossover study. All dogs were fed the same balanced diet with or without incorporation of Enterococcus faecium NCIMB 10415 for 16 days each. Blood and faecal samples were collected at baseline and during the feeding trial and owners recorded daily faecal scores. An Enterococcus spp. ASV, likely representing E. faecium NCIMB 10415 was detected in the faecal microbiome of some dogs 18–19 days after withdrawal of oral supplementation. Inclusion of E. faecium decreased circulating cholesterol (p = 0.008) compared to baseline. There were no differences in cholesterol concentrations between diets. Owners reported 0.6 ± 0.3) days less of loose stools compared to the control diet. Comparing to baseline, both diets significantly increased faecal concentration of acetate and butyrate, decreased serum cobalamin and increased faecal microbial diversity. Decreased serum cobalamin, and increased faecal acetate correlated with decreases in the Fusobacterium, Streptococcus, Blautia, and Peptoclostridium. Except for effects on circulating cholesterol and faecal score, effects were observed regardless of the addition of E. faecium. It is therefore likely that these effects can be contributed to dietary prebiotic effects on the faecal microbiome.
Keywords: probiotics, E. faecium, dog, faecal quality, cobalamin, cholesterol, short chain fatty acids, microbial richness, microbial relative abundance
1. Introduction
In dogs, the use of probiotics for preventive or therapeutic purposes has become increasingly common [1]. Probiotics are defined as live microorganisms that, when administered in adequate amounts, confer a health benefit on the host [2] and a variety of bacterial strains are marketed as probiotics for dogs. Probiotic effects are highly species and strain specific [3,4] and the evidence for their health effect is often limited [4].
Most probiotics alter the gastrointestinal (GI) microbiome composition, favouring lactic acid producing bacteria and creating a more hostile environment for potentially pathogenic Gram-negative bacteria. This is reflected by increased abundance of Lactobacillacea in the faecal microbiome [5,6,7,8,9] and altered faecal SCFA content [5,7,8,9] as a result of supplementing dogs with various strains of probiotics.
Short chain fatty acids support gastrointestinal health as a source of energy and regulator of the microenvironment. Furthermore, studies in dogs indicate that SCFA’s contribute to a probiotic-mediated regulation of circulating cholesterol [10,11,12].
In a recent study, circulating concentrations of cobalamin were below reference range in 44% of dogs 2 weeks after they had been supplemented with a synbiotic containing E. faecium NCIMB 10415, trade name SF68 (5 × 108 CFU/g; Fortiflora®; Proplan Purina) for two weeks [13]. This is concerning, because dogs rely completely on the dietary content and microbial production of the B vitamins: cobalamin and folate [14]. Since cobalamin is essential for several metabolic processes and has been closely linked with gastrointestinal health and disease, this finding could indicate an adverse effect of probiotic use in healthy dogs.
E. faecium NCIMB 10415 (trade name 4b1707) is one of few probiotic strains approved for use in dogs by the European Commission [15]. Currently, a single study, has investigated the effect of E. faecium NCIMB 10415, trade name 4b1707 in dogs. In this study, the probiotic was supplemented as a synbiotic with fructooligosaccharide and acacia (gum arabic), and there was a mildly decreased occurrence of stress related diarrhoea in shelter dogs [16]. Although other studies have reported similar improvements in faecal quality with probiotic supplementations [9,17,18], the overall evidence for a positive clinical effect on the faecal quality is still conflicting [4,19].
The authors are aware, that until there is sufficient evidence to support the registration of bacterial strains included in diet formulations as probiotics, the European Food Safety Authority (EFSA) apply the term “gut flora stabilizer” instead of “probiotic” for feed additives [20]. However, as the term “gut flora stabilizer” may not be familiar to all, the term “probiotic” will be used for the dietary inclusion of E. faecium NCIMB 10415, trade name 4b1707 in the current study.
The current study aimed to investigate the impact of feeding a commercial balanced canine diet with or without E. faecium NCIMB 10415, trade name 4b1707 to privately owned dogs for 16 days, specifically evaluating the effects on faecal quality, faecal microbial diversity and relative abundance, faecal SCFA concentrations as well as serum triglycerides, cholesterol, cobalamin, and folate concentrations.
2. Materials and Methods
2.1. Study Design
The study was designed as a randomized prospective double-blinded crossover study. Prior to initiation, the Danish Animal Experiments Inspectorate (approval number 2018-15-0201-01522), as well as the local administrative and ethical committee at the Department of Veterinary Clinical Sciences, University of Copenhagen, Denmark (approval number 2018-15), ethically approved the study protocol.
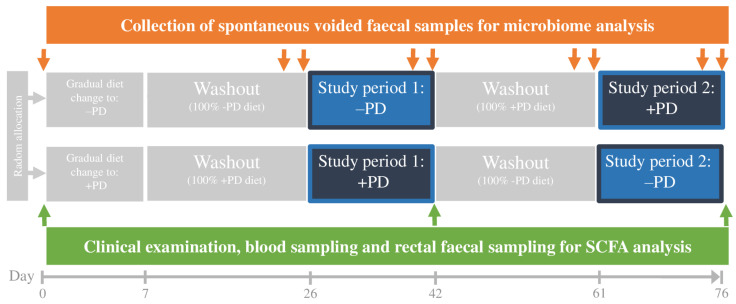
The study consisted of two study periods of 16 days each, with a washout period of 19 days before each study period. Throughout the study, dogs were fed a commercial nutritionally balanced diet. The test diet (+PD) was nutritionally identical to the control diet (−PD), differing only by being manufactured to include E. faecium NCIMB 10415 109 CFU/kg. In each study period, the dogs were exclusively fed the allocated diet and in the preceding washout period they were fed the same diet as allocated for the study period. Figure 1 shows an overview of the study outline.
Figure 1.
Study design. The study was a prospective randomized double-blinded crossover study composed of two study periods of each 16 days, both with a preceding washout period of 19 days. Included dogs (n = 11) were exclusively fed the same nutritionally balanced commercial canine diet with (+PD) or without (−PD) Enterococcus faecium NCIMB 10415 109 CFU/kg incorporated. Orange arrows indicate time points for collection of spontaneously voided faecal sampling for microbiome analysis (day 0, 24, 25, 40, 41, 59, 60, 75 and 76). Green arrows indicate time points for collection of blood samples for serum triglyceride, cholesterol, cobalamin, and folate concentrations as well as rectal faecal sampling for short chain fatty acids (SCFA) analysis (day 0, 42 and 76).
2.2. Animals
Privately owned healthy dogs meeting a set of fixed inclusion criteria were recruited. To be included in the study, the dogs had to be 2–8 years old, have a body weight of at least 10 kg and a body condition score (BCS) of 4/9–6/9 [21]. The dogs had to be clinically healthy based on a physical examination, complete blood cell count (CBC) and biochemistry profile. Chronically ill dogs and dogs receiving medical treatment or having a history of hyporexia or frequent diarrhoea were excluded. Throughout the study, the dogs were housed in their home environment and was cared for by their owners.
2.3. Diets
A canine commercially available dry kibble diet “Probiotic LIVE adult with chicken” (Bacterfield GmBH, Hamburg, Germany) was fed to the dogs during the probiotic period (+PD). The +PD diet was manufactured to contain 109 CFU/kg of E. faecium NCIMB 10415, trade name 4b1707. The diet was based on hydrolysed chicken protein, rice, maize, chicken fat and salmon oil. Dietary fibre consisting of dried beet pulp (3.7% of dry matter (DM)), dried chicory (1.4% DM) and fructooligosaccharides (1.3% DM) as well as brewer’s yeast and mineral supplements were also added. The probiotic was incorporated into the kibbles after extrusion and drying, by a patented technic, in which the probiotic bacteria are suspended in an oil mixture and driven into the kibble pores by altering ambient pressures in a hermetically sealed chamber. The dietary content of E. faecium NCIMB 10415 was controlled and verified by ALS Laboratories (Mirfield, UK) prior to the study. The control diet (−PD) was provided by the same manufacturer and formulated with the same nutritional composition as the +PD, but without the addition of E. faecium. The nutritional compositions of the diets as stated by the manufacturer are provided in Table 1.
Table 1.
Nutritional composition of study diets.
| Content | − PD |
+ PD |
|---|---|---|
| Metabolisable energy a | 17,364 kJ/kg (4150 kcal/kg) | 17,364 kJ/kg (4150 kcal/kg) |
| Crude protein a | 25% | 25% |
| Crude fat a | 15% | 15% |
| Carbohydrate a | 44% | 44% |
| Crude ash a | 7% | 7% |
| Crude fibre a | 2.2% | 2.2% |
|
E. faecium NCIMB 10415 b |
<10 CFU/kg c |
3.8–5.0 × 109 CFU/kg |
Dietary composition of two dry kibble diets used in the present study. −PD; control diet without added probiotic, +PD; probiotic diet, the same diet as the control diet with 3.8–5.0 × 109 CFU/kg Enteroccocus faecium NCIMB 10415 incorporated. CFU: cell forming units. a As stated by the manufacturer of the diet (Bacterfield GmBH, Hamburg, Germany). b Analysis by ALS Laboratories, UK prior to the study. c Below detectable concentration.
Daily allowance was initially calculated as Maintenance Energy Requirements (MER [kcal] = 95*BW [kg]0.75) for inactive dogs according to the recommendation by the National Research Council [22]. The dogs were weighed weekly and the daily allowance was adjusted when needed to maintain a stable bodyweight throughout the study. Each dog was randomly allocated to a group being fed either −PD or +PD for the first study period. All dogs were gradually transitioned from their current diet to −PD or +PD (whichever diet they were allocated to for the first study period) over a period of 7 days.
Owners fed their dog according to the calculated daily feeding allowance and dogs had free access to water throughout the study. During the study, no treats, chewing bones or any other feed substances apart from the study diets were allowed. Owners kept formal diaries of their dog’s dietary intake and activity level.
2.4. Faecal Quality
Owners registered consistency for all stools passed during the study periods by using a faecal scoring chart with score 1 being very firm and score 7 being watery diarrhoea [23]. For each study period, faecal quality was evaluated by the number of days on which the owner reported loose stools, defined as faecal score ≥ 4/7 at any point of time throughout the day.
2.5. Faecal SCFA and Blood Analysis
At inclusion and after each study period, a physical examination was performed and baseline blood and rectal faecal samples were collected. Blood was collected by venipuncture (cephalic or jugular vein) using a 23G single-use butterfly needle and vacutainer closed system. A trained veterinary technician performed the blood sampling, with minimal restraining of the dog and in the owner’s presence to reassure the dog. The dogs were fasted (minimum 12 h) for all blood collections.
A standard biochemistry profile (Advia 1800 Chemistry System, Siemens, Ballerup, Denmark) and CBC (Adivia 2120i, Hematology System, Siemens, Ballerup, Denmark) was performed at the Veterinary Diagnostic Laboratory, University of Copenhagen, Denmark. Serum was separated and stored at −80 °C within 30 min of collection and serum cholesterol, triglyceride cobalamin and folate concentrations were analysed at the end of the study period (Idexx, BioResearch Ludwigsburg, Germany).
The faecal samples were stored at −80 °C within 30 min of rectal collection and faecal SCFA concentrations were analysed as described previously [24] by gas chromatography mass spectrometry (Agilent Technologies 7890A GC System, Wilmington, DE, USA) at the Department of Food Science, University of Copenhagen, Denmark.
2.6. Faecal Microbiom Analysis
In addition to rectal faecal samples for SCFA analysis, the owners collected spontaneous voided faeces on day 0, 24, 25, 40, 41, 59, 60, 75 and 76 for microbiome analysis. These samples were frozen at −20 °C immediately after collection and transferred to −80 °C within 17 days for storage until analysis.
DNA extractions were carried out in a dedicated pre-PCR laboratory and PCR was performed at the Danish National High-Throughput Sequencing Center, University of Copenhagen, Denmark, for details see Appendix A.
Raw reads were quality controlled with FastQC/v0.11.8 [25], demultiplexing, adapter removal, as well as low-quality base removal (minquality = 30) using AdapterRemoval/v2.2.4 [26]. Proper read-orientation was ensured using cutadapt/v2.6. DADA2 package was then used to trim the reads (error rate algorithm), clustering of the amplicon sequence variants (ASV) (clustering algorithm), and assignment of taxonomy using Silva/v138 (classification algorithm) was performed. Post clustering the LULU algorithm [27] was used to minimize false positive and Decontam package [28] to remove contaminations. Further analysis was then carried out in R version 4.2.0 (R Core Team, 2022), for further details see Appendix B.
2.7. Statistics
The software system R version 4.2.0 (2022-04-22) (R Core Team, 2012) was also used to analyse blood and faecal SCFA samples. A separate linear mixed model (lme4 and lmerTest package) was applied with serum cobalamin, folate, cholesterol, triglyceride, acetate, propionate and butyrate concentrations as the outcome, individual dog as the random effect, while diet (−PD vs. +PD) and time period were fixed effects in each model.,. Statistical diagnostics were performed for verification regarding distribution assumptions, and detection of observations with undue influence for each model. A generalized mixed modelling using a Poisson distribution (lme4 package) was used to analyse faecal scores, where diet and time period were included as fixed effects and the individual dog as a random effect. For the faecal scores, two models were used. In the first one score 6–7 were grouped together (diarrhoea), score 2–4 were grouped together (not diarrhoea), while score 1 (too hard) was not modified. In the second model score 4–7 were grouped together (too watery), score 2–3 were grouped together (well formed), while score 1 (too hard) was not modified.
For linear and generalized mixed models p < 0.05 was considered significant.
3. Results
3.1. Dogs
Twenty-six dogs were initially recruited. Nine dogs could not be included due to identified CBC and/or biochemistry abnormalities, which was eosinophilia (n = 5), hypermagnesemia (n = 3) and elevated alkaline phosphatase (n = 1). Additionally, 6 dogs were excluded during the course of the study due to owner request to withdraw from the study (n = 3), diarrhoea with a duration >2 days (n = 2) and weight loss despite increasing daily rations (n = 1). For further details on included/excluded dogs, see Table S1.
Eleven dogs completed the full crossover study and were included in the final statistical analysis. They were of mixed breed (n = 2), Border Collie (n = 2), Broholmer (n = 1), Field Trial Cocker Spaniel (n = 1), Labrador Retriever (n = 1), Riesenschnauzer (n = 1), Berger Blanc Suisse (n = 1), Greyhound (n = 1) and West Highland White Terrier (n = 1). The population was composed of four entire males, four neutered males and three entire females. Age range was 3.0–7.3 years (median 4.4 years), body weight 10.9–46.3 kg (median 28.8 kg) and body condition score 4–6/9. All dogs were found to be healthy throughout the study based on clinical examination, CBC, and biochemical profile. Prior to entering the study, the dogs were fed a variety of home cooked and commercially available diets (details available in Table S2).
3.2. Faecal Quality
Overall, diarrhoea (defined as faecal score ≥ 6/7) was infrequent, with a total occurrence of 6 out of a total of 338 days for which a faecal score was available and there was no difference between study periods (p = 0.42). During the +PD period, the number of days where loose stools (defined as faecal score ≥ 4/7) were reported were 2.5/16 days (range 0/16–10/16), while it was 3.3/16 days (range 0/16–14/16) in the −PD period and statistically the dogs had 0.6 ±0.3 less days with loose stools during the +PD feeding period compared to −PD (p = 0.03).
3.3. Faecal SCFA
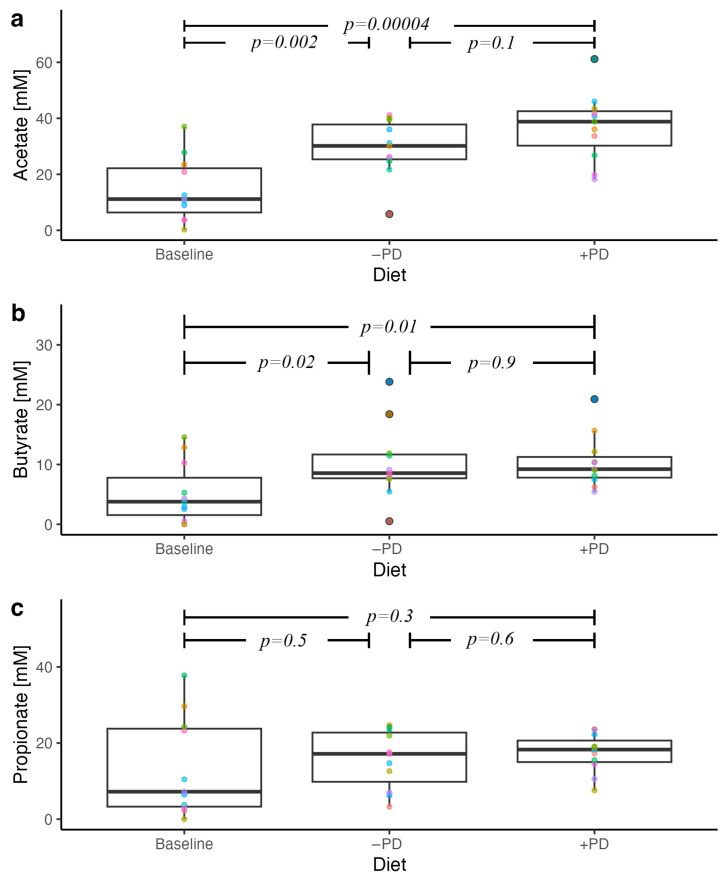
Faecal acetate and butyrate concentrations were significantly increased after feeding either −PD (p = 0.002 and p = 0.02, respectively) or +PD (p < 0.001 and p = 0.01, respectively, Figure 2), compared to baseline, with no difference between +PD and −PD. Faecal propionate concentrations were not affected by either diet compared to baseline (Figure 2).
Figure 2.
Faecal short chain fatty acid concentrations in privately owned healthy adult dogs (n = 11) fed a commercial diet with (+PD) or without (−PD) Enterococcus faecium NCIMB 10415 109 CFU/kg for 16 days each in a crossover study design compared to baseline concentrations. Each dot colour on the plot identifies the individual dog across diets. p values < 0.05 were considered significant.
3.4. Serum Lipids
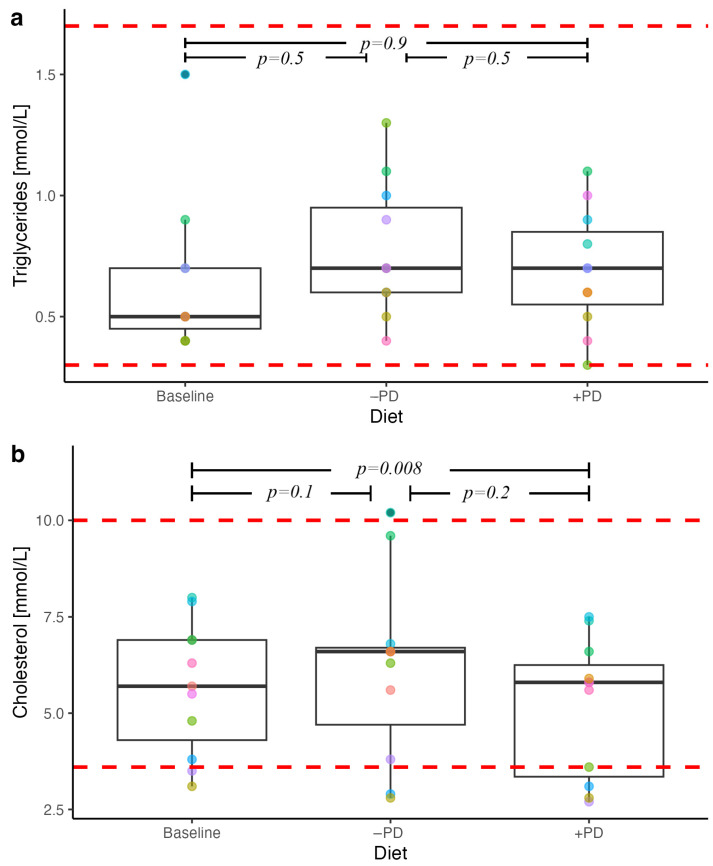
Serum cholesterol concentrations were lower following +PD feeding compared to baseline (p = 0.008, Figure 3). There was no difference between serum cholesterol concentrations following +PD and −PD periods or between −PD and baseline. There were no differences in serum triglyceride concentrations between baseline, +PD or −PD (Figure 3).
Figure 3.
Serum lipid concentrations in privately owned healthy adult dogs (n = 11) fed a commercial diet with (+PD) or without (−PD) Enterococcus faecium NCIMB 10415 109 CFU/kg for 16 days each in a crossover study design compared to baseline concentrations. Each dot colour on the plot identifies the individual dog across diets. Red dotted lines represent laboratory reference ranges. p values < 0.05 were considered significant.
3.5. B Vitamins
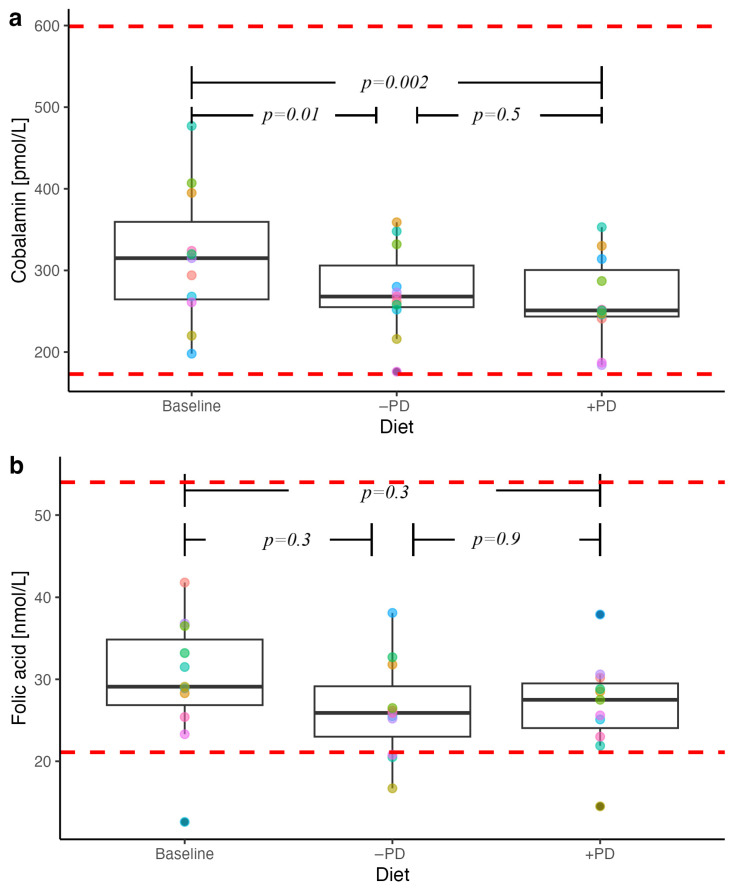
Serum cobalamin concentrations were within reference ranges throughout the study, but was significantly lower after feeding both +PD (p = 0.002) and −PD (p = 0.01) compared to baseline, with no intergroup difference (Figure 4). Serum folate concentrations did not differ between baseline, +PD or −PD feeding (Figure 4).
Figure 4.
Serum B-vitamin concentrations in privately owned healthy adult dogs (n = 11) fed a commercial diet with (+PD) or without (−PD) Enterococcus faecium NCIMB 10415 109 CFU/kg for 16 days each in a crossover study design compared to baseline concentrations at inclusion. Each dot colour on the plot identifies the individual dog across diets. Red dotted lines represent laboratory reference ranges. p values < 0.05 were considered significant.
3.6. Microbiome
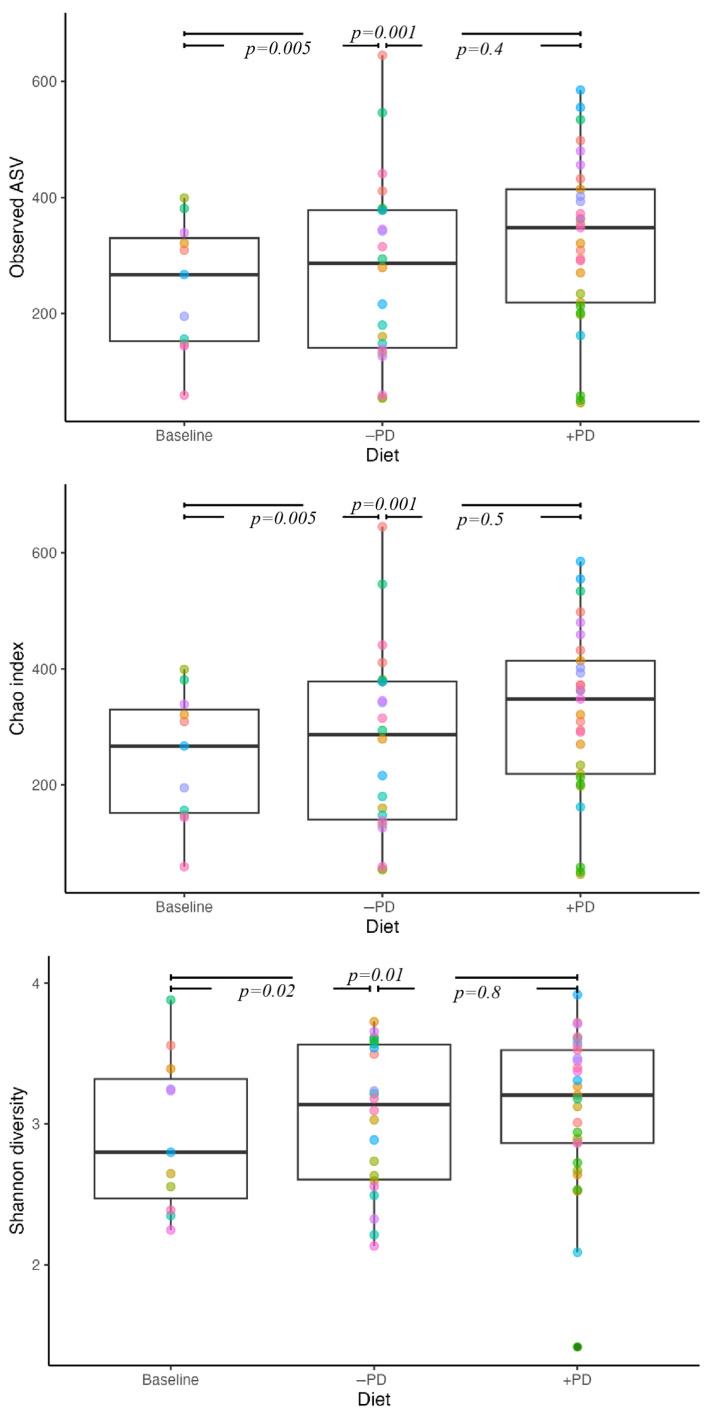
Observed faecal microbial richness was increased for +PD and −PD when compared to baseline (estimate 241.5, p = 0.001 and estimate 216.8, p = 0.005, respectively, Figure 5), with no intergroup difference and no effect of feeding +PD prior or after −PD. The Chao1 and Shannon index findings were similar to the observed faecal microbial richness.
Figure 5.
Faecal microbiome alpha diversity in privately owned healthy adult dogs (n = 11) fed a commercial diet with (+PD) or without (−PD) Enterococcus faecium NCIMB 10415 109 CFU/kg for 16 days each, in a crossover study design. Boxplots showing the observed richness (Observed ASV), Chao1 and Shannon index, of the faecal microbiome when the study population was fed a diet manufactured with (+PD) or without (−PD) Enterococcus faecium NCIMB 10415 as compared to baseline diversity at inclusion. Each number on the plot identifies the individual dog across diets.
For the beta diversity, no separation of faecal microbial communities was detected relating to the diet or diet order using Bray–Curtis (R2 = 0.014, p = 0.3 and R2 = 0.04, p = 0.1, respectively), unweighted UniFrac (R2 = 0.02, p = 0.2 and R2 = 0.03, p = 0.1, respectively) or weighted UniFrac (R2 = 0.002, p = 0.4 and R2 = 0.002, p = 0.4, respectively). For details on beta diversity, please refer to Figure S1.
Several differences in the proportions of the relative abundance of various ASVs were observed when comparing the effect of feeding +PD to baseline, −PD to baseline, and +PD to −PD. For +PD compared to baseline, Enterococcus (ASV313) increased in relative abundance, while Enterococcus (ASV335) decreased. The relative abundance of Enterococcus (ASV313) also increased with +PD feeding compared to −PD feeding. A relative decrease in the abundance of Lachnoclostridium (ASV32) and of Prevotella 9 (ASV82) was associated with the +PD and −PD compared to baseline. While Blautia (ASV41) only increased with +PD feeding compared to baseline, several Bacteroides (ASV63, ASV102, ASV149, and ASV153) increased with −PD feeding compared to baseline. Three of these Bacteroides (ASV63, ASV149, and ASV153) decreased with +PD compared to −PD feeding, while three Blautia (ASV24, ASV52, and ASV101) increased. In addition, Bacteroides (ASV75), Lachnospira (ASV16), Lachnoclostridium (ASV116), and an unknown genus of Eggerthellaceae decreased with +PD compared to −PD feeding (for further details see Table 2).
Table 2.
Effect of diets on relative bacterial abundance in faecal microbiota.
| Genus | ASV | +PD Compared to Baseline | −PD Compared to Baseline | +PD Compared to −PD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimated Effect | Std. Error | FDR | Estimated Effect | Std. Error | FDR | Estimated Effect | Std. Error | FDR | ||
| Lachnoclostridium | ASV32 | −3.5 | 0.67 | 2.8 × 10−4 | −3.2 | 0.78 | 0.007 | |||
| Prevotella_9 | ASV82 | −3.3 | 0.61 | 2.8 × 10−4 | −2.8 | 0.64 | 0.006 | |||
| Blautia | ASV41 | 4.2 | 1.1 | 0.019 | ||||||
| Enterococcus | ASV313 | 2.9 | 0.81 | 0.031 | 2.5 | 0.45 | 0.03 | |||
| Enterococcus | ASV335 | −6.2 | 1.8 | 0.043 | −8.4 | 2.3 | 0.017 | |||
| Bacteroides | ASV110 | 3.0 | 0.64 | 3.4 × 10−4 | −1.0 | 0.26 | 0.0098 | |||
| Lachnospiraceae_UCG−010 | ASV332 | 4.3 | 1.1 | 0.007 | ||||||
| Bacteroides | ASV153 | 4.7 | 1.3 | 0.023 | −2.5 | 0.50 | 8.5 × 10−4 | |||
| Bacteroides | ASV63 | 2.0 | 0.58 | 0.028 | −1.4 | 0.25 | 1.3 × 10−4 | |||
| Bacteroides | ASV102 | 3.0 | 0.90 | 0.028 | ||||||
| Bacteroides | ASV149 | −3.7 | 0.83 | 0.0030 | ||||||
| Ruminiclostridium_9 | ASV436 | −2.7 | 0.62 | 0.0032 | ||||||
| Holdemania | ASV356 | −1.9 | 0.48 | 0.0067 | ||||||
| Blautia | ASV52 | 0.92 | 0.24 | 0.0079 | ||||||
| Blautia | ASV24 | 0.71 | 0.20 | 0.0098 | ||||||
| Bacteroides | ASV75 | −1.8 | 0.50 | 0.0098 | ||||||
| Unknown Eggerthellaceae | ASV84 | −1.3 | 0.34 | 0.0098 | ||||||
| Blautia | ASV101 | 1.5 | 0.43 | 0.0098 | ||||||
| Lachnospira | ASV16 | −1.5 | 0.41 | 0.010 | ||||||
| Lachnoclostridium | ASV116 | −1.0 | 0.33 | 0.027 | ||||||
The effect of a commercial diet with (+PD) or without (−PD) Enterococcus faecium NCIMB 10415 109 CFU/kg, for 16 days each on, relative bacterial abundance in faecal microbiota in client owned healthy dogs (n = 11). False discovery rate (FDR) < 0.05 was considered significant. ASV; Amplicon Sequence Variant.
Three ASVs (ASV313, ASV335, and ASV796) belonging to the Enterococcus genus were detected throughout the study (Table 3). ASV313 was detected in 18/42 faecal samples from the +PD period regardless of order of the diets but was not present in any baseline samples. For dogs that were fed −PD after +PD, Enterococcus ASV313 was present in 3/22 samples from 3 different dogs at the end of the washout period (18–19 days after +PD was no longer provided), but was not present at the end of the −PD period. ASV335 and ASV796 were detected in a few baseline samples as well as a few, +PD and −PD samples when +PD was fed prior to −PD
Table 3.
Faecal Enterococcus spp.
| Order of Diet | - | +PD Followed by −PD | −PD Followed by +PD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Time of Sampling | Baseline | Washout Baseline | +PD Period | Washout +PD | −PD Period | Washout Baseline | −PD Period | Washout −PD |
+PD Period |
| Enterococcus ASV313 | 0/11 | 3/23 | 9/23 | 3/22 | 0/22 | 0/19 | 0/17 | 0/18 | 9/19 |
| Enterococcus ASV335 | 1/11 | 0/23 | 0/23 | 0/22 | 1/22 | 0/19 | 0/17 | 0/18 | 0/19 |
| Enterococcus ASV796 | 1/11 | 2/23 | 2/23 | 0/22 | 0/22 | 0/19 | 0/17 | 0/18 | 0/19 |
Number of dogs that had the three Enterococcus genera ASV313, ASV335, and ASV796 present in their faecal microbiome at various point of time during the study and depending on whether they were fed a diet without probiotic (−PD) prior to or after being fed at diet containing 109/kg E. faecium NCIMB 10415 (+PD).
3.7. Correlation between Faecal Microbial Relative Abundance, Fecal Short Chain Fatty Acids and Serum Biomarker Concentrations
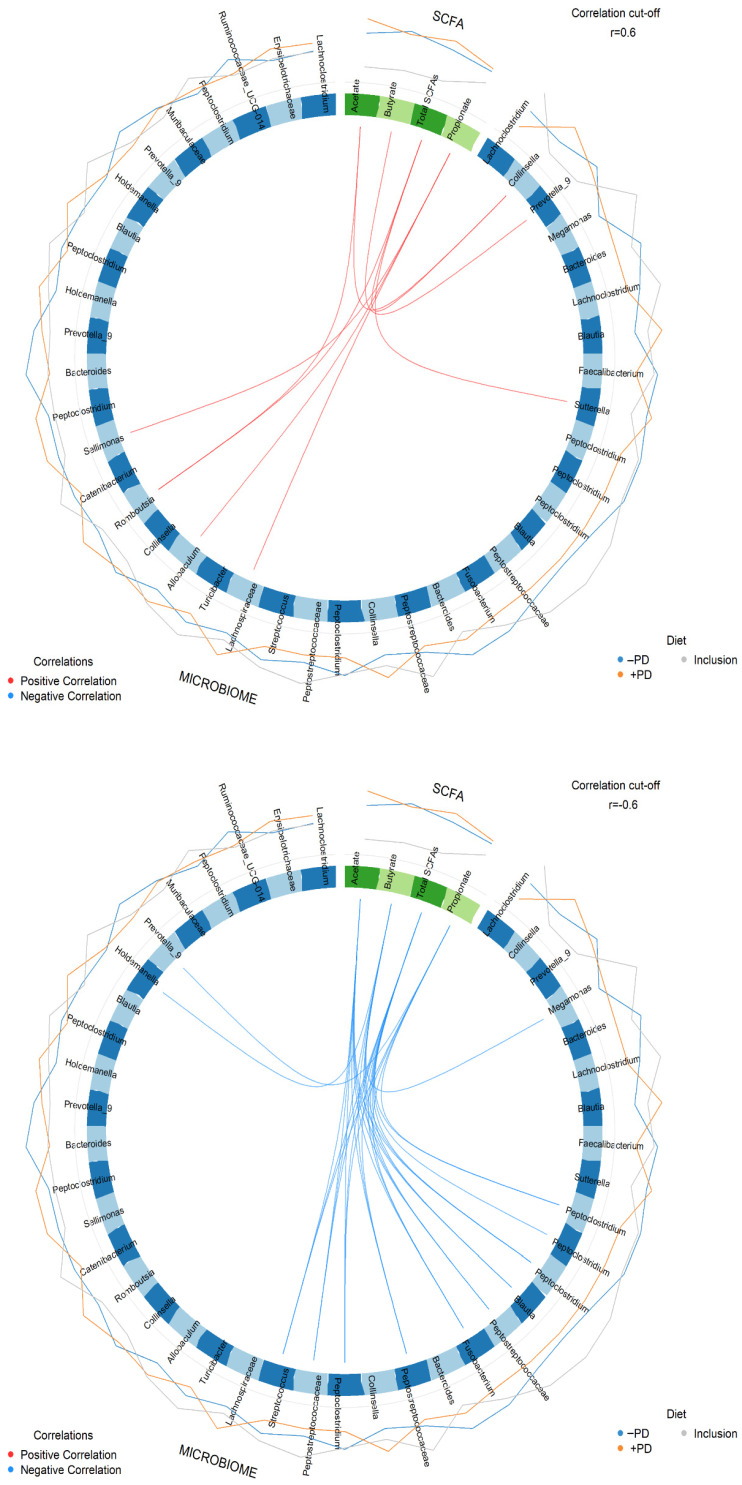
Several ASVs positively correlated with the abundance of faecal SCFA concentrations (Figure 6). For acetate a positive correlation with Romboutsia (ASV13, r = 0.65) and Sutterella (ASV109, r = 0.6) was found. For propionate, positive correlations with Sellimonas (ASV37, r = 0.61), Allobaculum (ASV39, r = 0.62) and unknown genus of Lachnospiraceae (ASV68, r = 0.62) were observed. For butyrate, a positive correlation with Prevotella_9 (ASV30, r = 0.66) was observed. While for the sum of all SCFAs a positive correlation was observed with Romboutsia (ASV13, r = 0.68).
Figure 6.
Correlation between microbial abundance and faecal acetate, propionate, butyrate and total short chain fatty acids concentration (SCFA). Circos plot illustrating positive correlations (r ≥ 0.6, connecting red lines) and negative correlations (r ≤ −0.6, connecting blue lines) performed using multiblock sparse partial least squares discriminant analysis of faecal acetate, propionate, butyrate and total SCFAs, and relative abundance of faecal microbes at study inclusion (baseline samples, circumferential grey line), feeding a diet without (−PD, circumferential blue line) or with 109/kg Enterococcus faecium NCIMB 10415 (+PD, circumferential orange line).
Several negative correlations were observed between the SCFAs and the relative abundance of ASVs. Acetate concentration correlated negatively with Fusobacterium (ASV64, r = −0.64), Streptococcus (ASV161, r = −0.65), Peptoclostridium (ASV217, r = −0.69 and ASV295 r = −0.75), Blautia (ASV221 r = −0.70) and three unknown genera of the Peptostreptococcaceae (ASV222, r = −0.73, ASV307, r = −0.77, and ASV363, r = −0.72). For propionate a negative correlation was observed with Fusobacterium (ASV64, r = −0.72), three ASVs of the Peptoclostridium (ASV104, r = −0.60, ASV165, r = −0.60, and ASV217, r = −0.69), Prevotella_9 (ASV112, r = −0.62) and Streptococcus (ASV161, r = −0.63). While for butyrate, a negative correlation was observed with Megamonas (ASV8, r = −0.74), Lachnoclostridium (ASV113, r = −0.6), two Peptoclostridium (ASV132, r = −0.60 and ASV295, r = −0.79), Catenibacterium (ASV167, r = −0.60), Blautia (ASV221, r = −0.76), three ASVs of unknown genera of the Peptostreptococcaceae (ASV222, r = −0.76, ASV307, r = −0.82 and ASV363, r = −0.81) and Holdemanella (ASV224, r = −0.64). No negative correlation was observed with the total sum of SCFAs.
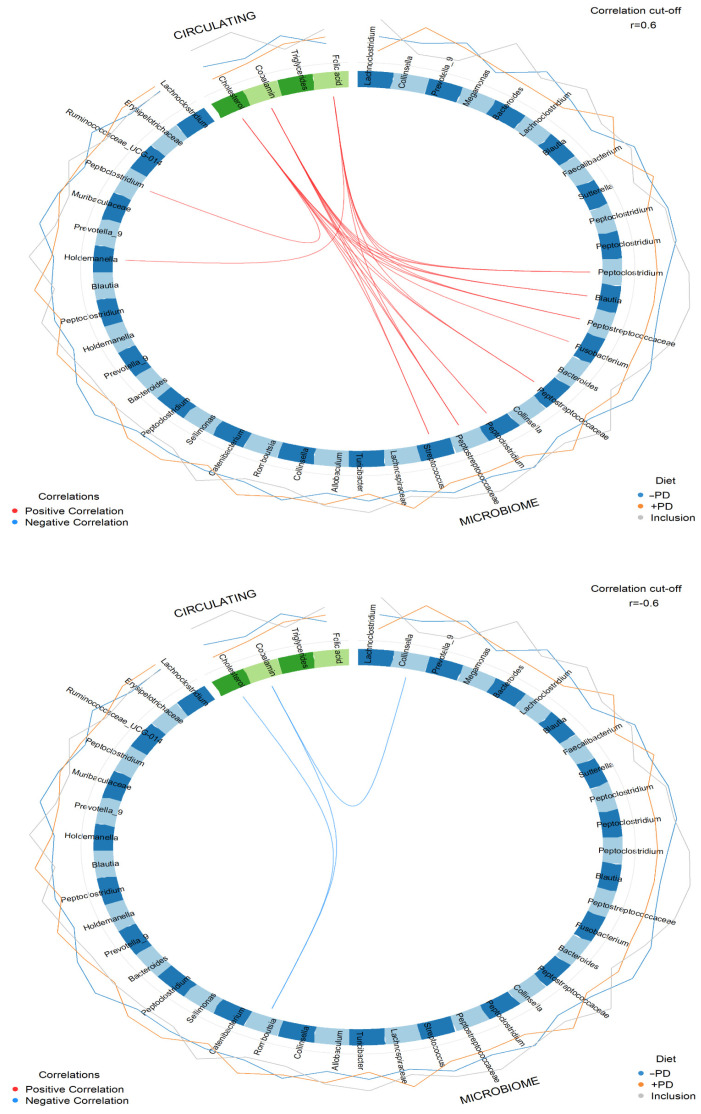
When analysing for possible correlations between serum biomarkers and faecal microbial relative abundance, it should be noted that the faecal microbial ASVs correlating with changes in serum cobalamin and cholesterol levels, reflected by the decrease in serum cobalamin concentration during feeding of the −PD and +PD and the decrease in serum cholesterol concentration during +PD feeding (Figure 7). Positive correlations (r ≥ 0.6) between cobalamin and ASVs belonging to the following genera was found: Fusobacterium (ASV64, r = 0.63), Streptococcus (ASV161, r = 0.66), two ASVs belonging to the Peptoclostridium (ASV217, r = 0.70 and ASV295, r = 0.76), Blautia (ASV221 r = 0.71), two of unknown genera of the Peptostreptococcaceae family (ASV222, r = 0.74 and ASV307, r = 0.78), and Peptostreptococcus (ASV363, r = 0.73). Positive correlations (r ≥ 0.6) between serum cholesterol and one Streptococcus (ASV161, r = 0.61), three unknown genera of the Pepstreptococcaceae (ASV363, r = 0.75, ASV307, r = 0.79, and ASV222, r = 0.74), two of the Peptoclostridium genus (ASV217, r = 0.64 and ASV295, r = 0.78), and one Blautia (ASV221, r = 0.72). A negative correlation was observed between Romboutsia (ASV13) and serum cobalamin (r = −0.67) and cholesterol (r = −0.63), and between Collinsella (ASV53, r = −0.6) and serum cobalamin.
Figure 7.
Correlation between faecal microbial abundance and serum cobalamin, folate, cholesterol, and triglycerides. Circos plot illustrating positive correlations (r ≥ 0.6, connecting red lines) and negative correlations (r ≤ −0.6, connecting blue lines) performed using multiblock sparse partial least squares discriminant analysis of the serum concentrations of cobalamin, folate, cholesterol and triglycerides, and relative abundance of faecal microbes at study inclusion (baseline concentrations, circumferential grey line), when feeding a diet without (−PD, circumferential blue line) or with 109/kg Enterococcus faecium NCIMB 10415 (+PD, circumferential orange line).
4. Discussion
This study investigated faecal SCFAs and microbiome changes as well as selected circulating metabolic markers in relation to feeding healthy client-owned adult dogs a nutritionally balanced dry diet with or without a probiotic incorporated at the level of manufacturing. In addition, a possible interaction between faecal SCFA and microbial changes with circulating concentrations of cobalamin, folic acid, cholesterol, and triglycerides was investigated. Incorporation of E. faecium NCIMB 10415 had a cholesterol lowering effect compared with baseline concentrations and slightly improved faecal quality compared to when the dogs were fed the same diet without addition of E. faecium. The diets, both with and without E. faecium NCIMB 10415, increased the faecal microbiome diversity, changed the specific bacterial relative abundances, and increased faecal butyrate and acetate content. Furthermore, serum cobalamin concentrations decreased with the feeding of the study diets irrespective of E. faecium NCIMB 10415 incorporation but remained within reference ranges.
Overall, the diets seemed well tolerated with few reported days of diarrhoea (faecal score ≥ 6/7) for dogs which completed the study. The owner-perceived faecal quality was slightly, but significantly, improved when feeding the diet containing E. faecium NCIMB 10415109 cfu/kg compared to without, however, the clinical relevance seems limited. During the study, two dogs were withdrawn due to diarrhoea >2 days. The dogs were privately owned, and suboptimal owner compliance made reintroduction of the diets impossible. Therefore, it is unknown whether it was the diet or other unrelated causes that resulted in diarrhoea in these two dogs.
The small positive effect on faecal quality is in line with a previous study where the proportion of days with diarrhoea was slightly, but significantly lower in dogs entering a kennel supplemented with a symbiotic containing E. faecium NCIMB 10415 (2%) compared with the placebo group (3.2%).
In the current study, faecal microbial analysis revealed increases in Enterococcus ASV313 to be associated with +PD feeding compared to baseline as well as compared to −PD feeding. Two other ASVs belonging to the Enterococcus genus (ASV335/ASV796) were also found. However, ASV335 decreased with +PD feeding and none of the diets were significantly associated with ASV796. Enterococcus ASV313 is therefore most likely to be E. faecium NCIMB 10415 (trade name 4b1707). However, this was not confirmed by for example qPCR with primers specific for the E. faecium NCIMB 10415. In a subset of samples from dogs that were allocated to be fed +PD in the first period, ASV313 was detected in the faecal microbiome at the end of the washout period 18–19 days after +PD had been discontinued. This might represent a prolonged survival of E. faecium NCIMB 10415 in these individual dogs. However, none of the dogs that had faecal presence of ASV313 by the end of the washout between +PD and −PD had ASV313 present at the end of the −PD study period and it is unknown for exactly how long into the −PD period the ASV313 persisted.
A prolonged survival of E. faecium NCIMB 10415 incorporated into the +PD was not suspected during the planning of the current study as survival beyond the supplementation period, has not previously been described for any E. faecium NCIMB 10415 probiotic in dogs (or other species). Based on culturing of rifampin resistant bacteria, prolonged survival has however been reported for E. faecium EE3, that could be identified for up to 3 months after cessation of the probiotic [10] and for L. fermentum AD1-CCM7421 that persisted in lower counts for at least 5 weeks after withdrawal of oral supplementation [5]. Despite a possible prolonged survival in a few dogs, where ASV313 was detected during the post +PD washout in the current study, the duration of the 19-day washout period was probably adequate, since ASV313 could not be identified in any of the dogs fed −PD as the second diet at the end of the 16 day −PD study period. A recent review recommended the use of a minimum 4-week washout period for diet interventions with faecal microbial composition as the outcome [29], while 4 weeks would have been ideal, a longer duration might have impacted the willingness of the dog owners to adhere to study protocol.
Recent studies suggest that breed [30,31] and neutering [32,33] might affect the faecal microbiome in dogs. The dogs included in the current study were heterogeneous in terms of breed, with only one breed (Border collie, n = 2) represented with more than one dog. At the same time the dogs acted as their own control in the cross-over design. It is therefore less likely that breed or neutering status was a major confounder in the faecal microbiota analyses in the current study.
Several differences observed between baseline and study periods were similar irrespective of feeding the +PD or −PD diet. This indicates an effect of the dietary composition rather than the inclusion of E. Faecium. The dietary composition of the diets fed to the dogs prior to inclusion was not controlled. All dogs were fed commercially available diets, varying in fibre content and types which possibly resulted in differing prebiotic effects. The dietary fibres included in the current study diet mainly consisted of beet pulp, which is a fermentable fibre with a prebiotic effect that has previously been shown to affect faecal SCFA concentrations [34,35,36] and faecal microbiome composition [34,36]. The diets also contained fructooligosaccharides which has been proposed to have similar prebiotic effects as beet pulp [35]. It is therefore likely that the dietary fibre composition played a significant role in the observed effects.
In previous studies Blautia spp. correlated positively with increased faecal content of butyrate in dogs [37,38] and decreased inflammation in the gastrointestinal tract of dogs [38,39,40,41] and humans [42]. Blautia spp. might also have the ability to produce bactericins inhibiting the growth of opportunistic pathogens [43,44], such as C. perfringens, and in humans the genus has been attributed with the ability to promote the metabolism of unbound lipids and glucose, hereby decreasing possible obesity-associated inflammation [45]. In the current study none of the identified ASVs belonging to Blautia spp. correlated with faecal butyrate concentration. However, several Blautia spp. increased in relative abundance with E. faecium NCIMB 10415 incorporation into the diet, compared to the study diet without E. faecium NCIMB 10415 and compared to baseline.
The Prevotella_9 (ASV30) was correlated with faecal butyrate concentration. Prevotella spp. are recognized as fibre fermenters and producers of SCFAs [46,47,48,49] and are known to increase in abundance in high fibre diets [50]. Like butyrate, faecal acetate was increased after feeding the diet, irrespective of probiotic inclusion, and the acetate concentration were observed to positively correlate with ASVs belonging to the Romboutsia and Suterella genera. The Romboutsia genus is a diverse genus within the Peptostreptococcaceae and at least some gut microbial strains in mice and humans are known producers of acetate, as well as formate and lactate [51,52].
Interestingly, several of the ASVs correlating with decreasing concentrations of faecal acetate are also correlating with increasing concentrations of cobalamin, namely Fusobacterium (ASV64), Streptococcus (ASV161), Peptoclostridium (ASV217 and ASV295), Blautia (ASV221), two unknown genera of the Peptostreptococcaceae family (ASV222 and ASV307), and Peptostreptococcus (ASV363). Previous studies have highlighted the importance of appropriate pH for Fusobacterium, Streptococcus, Blautia, and Peptoclostridium species [53,54,55,56], and a decreasing pH level has been shown to decrease their relative abundance. Furthermore, the genera Fusobacterium, Streptococcus, Blautia, and Peptoclostridium contain species with the ability to either produce or play an important role in gut microbial cobalamin production [57]. It is therefore possible, that the diet, with or without E. faecium NCIMB 10415, caused an increase in the relative abundance of acetate producing microbes, which have decreased the relative abundance of pH sensitive producers of cobalamin.. This could explain how, although within reference concentrations, serum cobalamin concentrations were significantly decreased after both feeding periods. It is therefore possible that the observed cobalamin lowering effect is related to changes in the dietary prebiotic/fibre content instead of the E. faecium NCIMB 10415 incorporation. However, due to a detectable prolonged survival during the washout period of the dogs fed the +PD diet first, it is not possible to completely exclude a possible cobalamin lowering effect of E. faecium NCIMB 10415 in the current study. In a study investigating the effect of supplementing a symbiotic containing E. faecium SF68, psyllium and brewer’s yeast to healthy adult dogs, circulating cobalamin and folate, 8/18 dogs were classified with hypocobalaminenia 14 days after withdrawal of the supplement [13]. The authors proposed that the microbiome consumption of cobalamin is increased either by E. faecium itself or other microbiome bacteria, for which the abundance has been positively affected by E. faecium symbiotic supplementation [13]. Since the cobalamin lowering effect observed in the current study was associated with diet irrespective of probiotic inclusion or order of study periods, it is possible that the change in diet alone may disrupt the balance of the gut microbiome enough to inadvertently decrease relative abundance of cobalamin producing microbes.
In contrast to the effect on serum cobalamin, only feeding with the diet containing E. faecium NCIMB 10415 had a serum cholesterol lowering effect. Similar to the current study, supplementation with Lactobacillus johnsonii CPN23 (0.1 mL/kg BW, 108 CFU/mL) or Lactobacullus acidophilus NCDC15 (0.1 mL/kg BW, 108 CFU/mL) for 9 weeks, resulted in lower circulating cholesterol and triglyceride concentrations compared with controls [11]. Additionally, a study investigating the effect of E. faecium SF68 on hepatic biomarkers in 18 healthy adult dogs, observed a lowering effect on circulating cholesterol that remained within the reference range 14 days after cessation of supplementation [58]. Another strain of E. faecium (E. faecium EE3) has been reported to normalise both high and low serum cholesterol in clinically unaffected dogs after supplementation for 1 week [10]. In contrast, supplementation with Lactobacillus fermentum AD1 (3 × 109 CFU/mL) and Bifidobacterium animalis B/12 (1.04 × 109 CFU/mL) did not affect circulating cholesterol concentrations of healthy dogs [8,12]. These findings illustrate an inherent difference in specific effect between probiotic species and strains, with formulation and dosage being another possible contributing factor.
Some microbes are known to enhance bile acid conjugation within the intestinal tract, which could explain the serum cholesterol lowering effect of certain probiotics. This leads to impaired reabsorption of bile acid into the entero-hepatic circulation and thus increased de novo host production of bile acid for which cholesterol is a precursor [59,60]. Incorporating or binding of cholesterol to the microbiome cell membrane could also play a role [61]. In hamsters fed a high cholesterol diet, the presence of butyrate, propionate and acetate in the gastrointestinal tract decreased circulating cholesterol concentration by enhancing faecal bile acid secretion and upregulating bile acid synthesis [62]. Studies in different mammalian species further suggest that propionate [63,64] and acetate [65] are capable of decreasing cholesterol production through hepatic enzymatic inhibition. In the present study, the increased faecal concentration of acetate and butyrate in +PD fed dogs compared to baseline could suggest that increased acetate and butyrate might also play a role in the cholesterol lowering effect. However, faecal acetate and butyrate was also significantly increased in the −PD fed dogs without significantly decreasing cholesterol concentrations compared to baseline.
5. Conclusions
This study investigated metabolic and faecal microbial effects of a commercially available diet containing the probiotic E. faecium NCIMB 10415 109 CFU/kg for adult dogs by comparing baseline at study start and nutritionally similar diets manufactured with or without incorporation of the probiotic. Results suggest that in some dogs, E. faecium NCIMB 10415 can possibly survive in the canine gut microbiome for 19 days after withdrawal of oral supplementation. Analyses of the microbial diversity and changes in relative abundance of some specific microbes indicate that E. faecium NCIMB 10415 has a mild positive effect on the microbiome and owners also reported mildly improved faecal quality when comparing the diet with or without E. faecium NCIMB 10415. In addition, the probiotic exerted a mild cholesterol lowering effect, which might be favourable in conditions associated with hypercholesterolemia.
Despite a small possibility of prolonged survival of E. faecium NCIMB 10415 in the gut, beyond the 19 days of washout, the observed increase of faecal butyrate and acetate, and decreased circulating cobalamin, in both feeding periods, are most likely attributable to properties of the study diet alone. The effect on cobalamin was seen with both the diet with and without E. faecium NCIMB 10415, and the effect was mild and no hypocobalaminemia developed as described in previous probiotic studies.
Acknowledgments
We would like to acknowledge veterinary nurse Mette Rasmussen, Department of Veterinary Clinical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Denmark for her always keen and professional handling of sample collections from participating dogs. Tom Gilbert, Section for Hologenomics, Faculty of Health and Medical Sciences, University of Copenhagen, Denmark is also acknowledged for his valuable help with access to laboratory facilities and advises on overcoming challenges encountered along the way. Finally, we would like to express a great gratitude towards all dog owners who together with their dogs were recruited for the study.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani13010144/s1, Figure S1: Microbiome beta diversity; Table S1: All recruited dogs; Table S2: Diets and supplements fed to the dogs prior to study enrolment.
Appendix A. DNA Extraction, V3-V4 Bacterial 16S rRNA Metabarcoding
For each faecal sample, the surface layer was removed and only faeces from the core was used, to avoid contamination from the environment. Approximately 300 mg of faecal sample was used for DNA extraction using the Macherey Nagel Nucleospin Soil DNA extraction kit, according to manufacturer instructions (Macherey-Nagel GmbH & Co KG, Düren, Germany). After extraction, the DNA concentration was estimated using a Qubit™ 3.0 fluorometer (Thermo Fisher Scientific Inc., Waltham, MA, USA). The faecal bacterial microbiota composition was determined through metabarcoding targeting the V3–V4 region of the 16S rRNA gene using 341 F (5′-CCTAYGGGRBGCASCAG-3′) and 806 R (5′-GGACTACNNGGGTATCTAAT-3′) primers.
Prior to PCR, quantitative PCR (qPCR) was performed to screen for PCR inhibition and determine optimal number of cycles for PCR amplification on a dilution series (2:1, 1:1, 1:5, 1:10) of a small subset consisting of 16 samples of DNA extracts [66]. The qPCR was performed in 25 μL reactions containing 1 μL DNA template (except for the 2:1 dilution were 2 μL was used), 2.5 μL of Gold PCR buffer, 2.5 μL MgCl2, 14 μL ddH20, 0.2 μL DNTP, 0.15 μL Taq gold, 0.63 μL BSA, 0.6 μM 16S forward primer, 0.6 μM 16S-reverse primer and 1 μL of SYBR Green/ROX solution (Invitrogen, Waltham, MA, USA). The qPCR amplifications were performed on an Mx3005 qPCR machine (Agilent Technologies, Santa Clara, CA, USA) with the following cycling conditions: 95 °C for 10 min, followed by 45 cycles of 95 °C for 15 s, 53 °C for 20 s, and 72 °C for 40 s.
PCR was performed using the same reaction composition used for qPCR with the exclusion of the SYBR green dye, under the following conditions; denaturation at 95 °C for 10 min followed by 30 cycles of denaturation at 95 °C for 15 s, annealing at 53 °C for 20 s and extension at 72 °C for 40 s. Tagged primers were used in different combinations to allow multiplexing of samples during sequencing. According to the qPCR results, 30 PCR cycles of amplification using a 1:2 dilution was optimal to avoid PCR inhibition. Using ultrapure water, PCR blanks were included in all PCR reactions. To minimize the risk of contamination, pre- and post-PCR products were handled in two different laboratories designated for each process, respectively, while PCR master mix solutions were prepared in a designated DNA template-free laboratory. PCR products were visualized using 2% agarose gel electrophoresis (GE) to check the amplification products’ quality and amount, where after PCR products were equimolarly pooled (to avoid bias introduced by differential amplification) according to their tag. Extraction and PCR blanks were included in the pools for downstream quality filtering, in a non-equimolar fashion to avoid excessive dilution. Pools were then purified using SPRI bead purification [67], with a beads-to-sample ratio of 1:1, including two washing steps in 0.5 mL of ethanol 80% and elution in 35 μL of Elution Buffer (10 mM Tris-HCl). DNA concentration was measured using Qubit 3.0 (Thermo-Fisher Scientific, Waltham, MA, USA), following the manufacturer’s recommendations. The Tagsteady PCR-free Illumina library preparation protocol [27] was then used to 223 generate sequencing libraries. The Tagsteady library quantification was indexed using 224 NEBNext® Library Quant Kit for Illumina® (NEB, New England Biolabs, Ipswich, MA, 225 USA), according to manufacturer’s recommendations. Sequencing of 300 bp paired-end 226 reads was performed at the Danish National High-Throughput Sequencing Center, Uni-227 versity of Copenhagen, Denmark, using an Illumina MiSeq platform with reagent kit v3, 228,600 cycles.
Appendix B. Bacterial 16S rRNA Gene Taxonomy and Statistical Analysis
Composition analyses was performed using the phyloseq package, where alpha diversity (ASV richness, Chao1, and Shannon index) was calculated. Differences in alpha diversity were statistically tested using a linear mixed model test (lme4 and lmerTest package), with individual dog included as a random effect, while diet and diet order were included as fixed effects. The beta diversity was analysed and plotted using Non-metric multidimensional scaling and based on Bray–Curtis, unweighted and weighted UniFrac dissimilarities, and tested using analysis of similarity (ANOSIM) (vegan package) testing effect of diet and the diet-order. Compositional differences in ASV abundance were tested in a negative binomial mixed model (NBZIMM package) with individual dog and sequencing depth included as random effects, while diet, diet-order, and time were included as fixed effects. p-values were adjusted using false discovery rate (FDR) and were considered significant when FDR < 0.05. Multiblock sparse partial least squares discriminant analysis (Multiblock sPLS-DA) was performed using the mixomics package to asses, which ASVs were correlated with circulating concentrations of cobalamin, folate, cholesterol, triglycerides, and SCFAs, controlling for repeated measurements from the same individual dog.
Author Contributions
Conceptualization, C.R.B., I.N.K., S.N. and T.G.H.; Methodology, C.R.B., I.N.K. and T.G.H.; Software, I.N.K.; Validation, I.N.K.; Formal analysis, I.N.K., B.K., P.B.H. and K.K.; Investigation, S.N., I.N.K., B.K., P.B.H., K.K. and K.S.; Resources, C.R.B., T.G.H., S.N., I.N.K., P.B.H., K.K. and B.K.; Data Curation, S.N., I.N.K., K.S., P.B.H. and K.K.; Writing—original draft preparation, S.N. and I.N.K.; Writing—review and editing, C.R.B. and K.S.; Visualisation, S.N., I.N.K., P.B.H. and K.K.; Supervision, C.R.B. and I.N.K.; Project administration, C.R.B., S.N. and I.N.K.; Funding acquisition, T.G.H., J.B. and C.R.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The animal study protocol was approved by the Local Administrative and Ethical Committee at the Department of Veterinary Clinical Sciences, University of Copenhagen, Denmark (approval number 2018-15, date: 22 August 2018) as well as the Danish Animal Experiments Inspectorate (approval number 2018-15-0201-01522, date: 10 September 2018).
Informed Consent Statement
Informed consent was obtained from the owner of all dogs involved in the study.
Data Availability Statement
The datasets used and/or analyzed during the current study are available at either UCPH ERDA data (https://sid.erda.dk/sharelink/eZ0NFSju9q) or at the EMBL Nucleotide Sequence Database (ENA), with the study accession number PRJEB55896.
Conflicts of Interest
The study was performed in collaboration with Bacterfield GmbH, Hamburg, Germany. The company supplied the test diets and financed laboratory tests. Co-author Therese G. Hosbjerg and Jørgen Baymler were employed by Bacterfield GmbH, Hamburg, Germany and Therese G. Hosbjerg took part in the formulating the study design, but other than that did the funders not have any role in in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. No other authors received any specific funding for this work.
Funding Statement
This research was funded by Bacterfield GmbH, 20354 Hamburg, Germany.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Hosbjerg T.G. The Role of Probiotics in Veterinary Medicine—Prophylactic Use and in Conjunction with Disease. [(accessed on 22 December 2022)]. Available online: https://www.bacterfield.com/co/articles/the-role-of-probiotics-in-veterinary-medicine---prophylactic-use-and-in-conjunction-with-disease.html.
- 2.Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 3.Azaïs-Braesco V., Bresson J.L., Guarner F., Corthier G. Not All Lactic Acid Bacteria Are Probiotics, …but Some Are. Br. J. Nutr. 2010;103:1079–1081. doi: 10.1017/S0007114510000723. [DOI] [PubMed] [Google Scholar]
- 4.Jugan M.C., Rudinsky A.J., Parker V.J., Gilor C. Use of Probiotics in Small Animal Veterinary Medicine. J. Am. Vet. Med. Assoc. 2017;250:519–528. doi: 10.2460/javma.250.5.519. [DOI] [PubMed] [Google Scholar]
- 5.Strompfová V., Lauková A., Gancarčíková S. Effectivity of Freeze-Dried Form of Lactobacillus Fermentum AD1-CCM7421 in Dogs. Folia Microbiol. 2012;57:347–350. doi: 10.1007/s12223-012-0139-0. [DOI] [PubMed] [Google Scholar]
- 6.Baillon M.-L.A., Marshall-Jones Z.V., Butterwick R.F. Effects of Probiotic Lactobacillus Acidophilus Strain DSM13241 in Healthy Adult Dogs. Am. J. Vet. Res. 2004;65:338–343. doi: 10.2460/ajvr.2004.65.338. [DOI] [PubMed] [Google Scholar]
- 7.Kumar S., Pattanaik A.K., Sharma S., Jadhav S.E., Dutta N., Kumar A. Probiotic Potential of a Lactobacillus Bacterium of Canine Faecal-Origin and Its Impact on Select Gut Health Indices and Immune Response of Dogs. Probiotics Antimicro. Prot. 2017;9:262–277. doi: 10.1007/s12602-017-9256-z. [DOI] [PubMed] [Google Scholar]
- 8.Strompfová V., Pogány Simonová M., Gancarčíková S., Mudroňová D., Farbáková J., Mad’ari A., Lauková A. Effect of Bifidobacterium Animalis B/12 Administration in Healthy Dogs. Anaerobe. 2014;28:37–43. doi: 10.1016/j.anaerobe.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Gagné J.W., Wakshlag J.J., Simpson K.W., Dowd S.E., Latchman S., Brown D.A., Brown K., Swanson K.S., Fahey G.C. Effects of a Synbiotic on Fecal Quality, Short-Chain Fatty Acid Concentrations, and the Microbiome of Healthy Sled Dogs. BMC Vet. Res. 2013;9:246. doi: 10.1186/1746-6148-9-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcináková M., Simonová M., Strompfová V., Lauková A. Oral Application of Enterococcus Faecium Strain EE3 in Healthy Dogs. Folia Microbiol. 2006;51:239–242. doi: 10.1007/BF02932129. [DOI] [PubMed] [Google Scholar]
- 11.Kumar S., Pattanaik A., Sharma S., Jadhav S. Species-Specific Probiotic Lactobacillus Johnsonii CPN23 Supplementation Modulates Blood Biochemical Profile and Erythrocytic Antioxidant Indices in Labrador Dogs. Indian J. Anim. Sci. 2016;86:918–924. [Google Scholar]
- 12.Strompfová V., Marciňáková M., Simonová M., Bogovič-Matijašić B., Lauková A. Application of Potential Probiotic Lactobacillus Fermentum AD1 Strain in Healthy Dogs. Anaerobe. 2006;12:75–79. doi: 10.1016/j.anaerobe.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Lucena R., Olmedilla A.B., Blanco B., Novales M., Ginel P.J. Effect of Enterococcus Faecium SF68 on Serum Cobalamin and Folate Concentrations in Healthy Dogs. J. Small Anim. Pract. 2018;59:438–443. doi: 10.1111/jsap.12845. [DOI] [PubMed] [Google Scholar]
- 14.Kook P.H., Hersberger M. Daily Oral Cyanocobalamin Supplementation in Beagles with Hereditary Cobalamin Malabsorption (Imerslund-Gräsbeck Syndrome) Maintains Normal Clinical and Cellular Cobalamin Status. J. Vet. Intern. Med. 2019;33:751–757. doi: 10.1111/jvim.15380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Union Register of Feed Additives Pursuant to Regulation (EC) No 1831/2003. European Commission; Brussels, Belgium: 2019. Appendixes 1. [Google Scholar]
- 16.Rose L., Rose J., Gosling S., Holmes M. Efficacy of a Probiotic-Prebiotic Supplement on Incidence of Diarrhea in a Dog Shelter: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Vet. Intern. Med. 2017;31:377–382. doi: 10.1111/jvim.14666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gómez-Gallego C., Junnila J., Männikkö S., Hämeenoja P., Valtonen E., Salminen S., Beasley S. A Canine-Specific Probiotic Product in Treating Acute or Intermittent Diarrhea in Dogs: A Double-Blind Placebo-Controlled Efficacy Study. Vet. Microbiol. 2016;197:122–128. doi: 10.1016/j.vetmic.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 18.Fenimore A., Martin L., Lappin M.R. Evaluation of Metronidazole With and Without Enterococcus Faecium SF68 in Shelter Dogs with Diarrhea. Top. Companion Anim. Med. 2017;32:100–103. doi: 10.1053/j.tcam.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Jensen A.P., Bjørnvad C.R. Clinical Effect of Probiotics in Prevention or Treatment of Gastrointestinal Disease in Dogs: A Systematic Review. J. Vet. Intern. Med. 2019;33:1849–1864. doi: 10.1111/jvim.15554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.feednavigator.com. Will the Revised EU Feed Additives Legislation Be Fit for Purpose? [(accessed on 5 September 2022)]. Available online: https://www.feednavigator.com/Article/2019/04/17/Will-the-revised-EU-feed-additives-legislation-be-fit-for-purpose.
- 21.Laflamme D.P. Development and Validation of a Body Condition Score System for Dogs.: A Clinical Tool. Canine Pract. 1997;22:10–15. [Google Scholar]
- 22.National Research Council . Nutrient Requirements of Dogs and Cats. National Academies Press; Washington, DC, USA: 2006. (Animal nutrition series). [Google Scholar]
- 23.Nestlé Purina® Company Purina Fecal Score Chart. [(accessed on 22 December 2022)]. Available online: https://www.purinainstitute.com/centresquare/nutritional-and-clinical-assessment/purina-fecal-scoring-chart.
- 24.Wiese M., Khakimov B., Nielsen S., Sørensen H., van den Berg F., Nielsen D.S. CoMiniGut-a Small Volume in Vitro Colon Model for the Screening of Gut Microbial Fermentation Processes. PeerJ. 2018;6:e4268. doi: 10.7717/peerj.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrews S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Babraham Bioinformatics, Babraham Institute; Cambridge, UK: 2010. [Google Scholar]
- 26.Schubert M., Lindgreen S., Orlando L. AdapterRemoval v2: Rapid Adapter Trimming, Identification, and Read Merging. BMC Res. Notes. 2016;9:88. doi: 10.1186/s13104-016-1900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frøslev T.G., Kjøller R., Bruun H.H., Ejrnæs R., Brunbjerg A.K., Pietroni C., Hansen A.J. Algorithm for Post-Clustering Curation of DNA Amplicon Data Yields Reliable Biodiversity Estimates. Nat. Commun. 2017;8:1188. doi: 10.1038/s41467-017-01312-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Callahan B., Davis N.M., Ernst F.G.M. Decontam: Identify Contaminants in Marker-Gene and Metagenomics Sequencing Data. [(accessed on 29 December 2022)]. Available online: https://bioc.ism.ac.jp/packages/release/bioc/manuals/decontam/man/decontam.pdf.
- 29.Jarett J.K., Kingsbury D.D., Dahlhausen K.E., Ganz H.H. Best Practices for Microbiome Study Design in Companion Animal Research. Front. Vet. Sci. 2021;8:644836. doi: 10.3389/fvets.2021.644836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.You I., Kim M.J. Comparison of Gut Microbiota of 96 Healthy Dogs by Individual Traits: Breed, Age, and Body Condition Score. Animals. 2021;11:2432. doi: 10.3390/ani11082432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reddy K.E., Kim H.-R., Jeong J.Y., So K.-M., Lee S., Ji S.Y., Kim M., Lee H.-J., Lee S., Kim K.-H., et al. Impact of Breed on the Fecal Microbiome of Dogs under the Same Dietary Condition. J. Microbiol. Biotechnol. 2019;29:1947–1956. doi: 10.4014/jmb.1906.06048. [DOI] [PubMed] [Google Scholar]
- 32.Coelho L.P., Kultima J.R., Costea P.I., Fournier C., Pan Y., Czarnecki-Maulden G., Hayward M.R., Forslund S.K., Schmidt T.S.B., Descombes P., et al. Similarity of the Dog and Human Gut Microbiomes in Gene Content and Response to Diet. Microbiome. 2018;6:72. doi: 10.1186/s40168-018-0450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scarsella E., Stefanon B., Cintio M., Licastro D., Sgorlon S., Dal Monego S., Sandri M. Learning Machine Approach Reveals Microbial Signatures of Diet and Sex in Dog. PLoS ONE. 2020;15:e0237874. doi: 10.1371/journal.pone.0237874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kröger S., Vahjen W., Zentek J. Influence of Lignocellulose and Low or High Levels of Sugar Beet Pulp on Nutrient Digestibility and the Fecal Microbiota in Dogs. J. Anim. Sci. 2017;95:1598–1605. doi: 10.2527/jas.2016.0873. [DOI] [PubMed] [Google Scholar]
- 35.Middelbos I.S., Fastinger N.D., Fahey G.C., Jr. Evaluation of Fermentable Oligosaccharides in Diets Fed to Dogs in Comparison to Fiber Standards. J. Anim. Sci. 2007;85:3033–3044. doi: 10.2527/jas.2007-0080. [DOI] [PubMed] [Google Scholar]
- 36.Finet S., He F., Clark L.V., de Godoy M.R.C. Functional Properties of Miscanthus Fiber and Prebiotic Blends in Extruded Canine Diets. J. Anim. Sci. 2022;100:skac078. doi: 10.1093/jas/skac078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandri M., Dal Monego S., Conte G., Sgorlon S., Stefanon B. Raw Meat Based Diet Influences Faecal Microbiome and End Products of Fermentation in Healthy Dogs. BMC Vet. Res. 2016;13:65. doi: 10.1186/s12917-017-0981-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Brito C.B.M., Menezes Souza C.M., Bastos T.S., Mesa D., Oliveira S.G., Félix A.P. Effect of Dietary Inclusion of Dried Apple Pomace on Faecal Butyrate Concentration and Modulation of Gut Microbiota in Dogs. Arch. Anim. Nutr. 2021;75:48–63. doi: 10.1080/1745039X.2020.1867463. [DOI] [PubMed] [Google Scholar]
- 39.Suchodolski J.S., Markel M.E., Garcia-Mazcorro J.F., Unterer S., Heilmann R.M., Dowd S.E., Kachroo P., Ivanov I., Minamoto Y., Dillman E.M., et al. The Fecal Microbiome in Dogs with Acute Diarrhea and Idiopathic Inflammatory Bowel Disease. PLoS ONE. 2012;7:e51907. doi: 10.1371/journal.pone.0051907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minamoto Y., Minamoto T., Isaiah A., Sattasathuchana P., Buono A., Rangachari V.R., McNeely I.H., Lidbury J., Steiner J.M., Suchodolski J.S. Fecal Short-chain Fatty Acid Concentrations and Dysbiosis in Dogs with Chronic Enteropathy. J. Vet. Intern. Med. 2019;33:1608–1618. doi: 10.1111/jvim.15520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.AlShawaqfeh M., Wajid B., Minamoto Y., Markel M., Lidbury J., Steiner J., Serpedin E., Suchodolski J. A Dysbiosis Index to Assess Microbial Changes in Fecal Samples of Dogs with Chronic Inflammatory Enteropathy. FEMS Microbiol. Ecol. 2017;93:fix136. doi: 10.1093/femsec/fix136. [DOI] [PubMed] [Google Scholar]
- 42.Chen L., Wang W., Zhou R., Ng S.C., Li J., Huang M., Zhou F., Wang X., Shen B., Kamm M.A., et al. Characteristics of Fecal and Mucosa-Associated Microbiota in Chinese Patients with Inflammatory Bowel Disease. Medicine. 2014;93:e51. doi: 10.1097/MD.0000000000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Azevedo A.C., Bento C.B.P., Ruiz J.C., Queiroz M.V., Mantovani H.C. Distribution and Genetic Diversity of Bacteriocin Gene Clusters in Rumen Microbial Genomes. Appl. Environ. Microbiol. 2015;81:7290–7304. doi: 10.1128/AEM.01223-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hatziioanou D., Gherghisan-Filip C., Saalbach G., Horn N., Wegmann U., Duncan S.H., Flint H.J., Mayer M.J., Narbad A. Discovery of a Novel Lantibiotic Nisin O from Blautia Obeum A2-162, Isolated from the Human Gastrointestinal Tract. Microbiology. 2017;163:1292–1305. doi: 10.1099/mic.0.000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kimura I., Ozawa K., Inoue D., Imamura T., Kimura K., Maeda T., Terasawa K., Kashihara D., Hirano K., Tani T., et al. The Gut Microbiota Suppresses Insulin-Mediated Fat Accumulation via the Short-Chain Fatty Acid Receptor GPR43. Nat. Commun. 2013;4:1829. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee A.H., Vidal S., Oba P.M., Wyss R., Miao Y., Adesokan Y., Swanson K.S. Evaluation of a Novel Animal Milk Oligosaccharide Biosimilar: Macronutrient Digestibility and Gastrointestinal Tolerance, Fecal Metabolites, and Fecal Microbiota of Healthy Adult Dogs and in Vitro Genotoxicity Assays. J. Anim. Sci. 2021;99:skab014. doi: 10.1093/jas/skab014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pilla R., Suchodolski J.S. The Role of the Canine Gut Microbiome and Metabolome in Health and Gastrointestinal Disease. Front. Vet. Sci. 2020;6:498. doi: 10.3389/fvets.2019.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phungviwatnikul T., Alexander C., Do S., He F., Suchodolski J.S., de Godoy M.R.C., Swanson K.S. Effects of Dietary Macronutrient Profile on Apparent Total Tract Macronutrient Digestibility and Fecal Microbiota, Fermentative Metabolites, and Bile Acids of Female Dogs after Spay Surgery. J. Anim. Sci. 2021;99:skab225. doi: 10.1093/jas/skab225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Myint H., Iwahashi Y., Koike S., Kobayashi Y. Effect of Soybean Husk Supplementation on the Fecal Fermentation Metabolites and Microbiota of Dogs. Anim. Sci. J. 2017;88:1730–1736. doi: 10.1111/asj.12817. [DOI] [PubMed] [Google Scholar]
- 50.Li Q., Lauber C.L., Czarnecki-Maulden G., Pan Y., Hannah S.S. Effects of the Dietary Protein and Carbohydrate Ratio on Gut Microbiomes in Dogs of Different Body Conditions. mBio. 2017;8:e01703-16. doi: 10.1128/mBio.01703-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gerritsen J., Hornung B., Renckens B., van Hijum S.A., Dos Santos V.A.M., Rijkers G.T., Schaap P.J., de Vos W.M., Smidt H. Genomic and Functional Analysis of Romboutsia Ilealis CRIBT Reveals Adaptation to the Small Intestine. PeerJ. 2017;5:e3698. doi: 10.7717/peerj.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gerritsen J., Umanets A., Staneva I., Hornung B., Ritari J., Paulin L., Rijkers G.T., de Vos W.M., Smidt H. Romboutsia Hominis Sp. Nov., the First Human Gut-Derived Representative of the Genus Romboutsia, Isolated from Ileostoma Effluent. Int. J. Syst. Evol. Microbiol. 2018;68:3479–3486. doi: 10.1099/ijsem.0.003012. [DOI] [PubMed] [Google Scholar]
- 53.Firrman J., Liu L., Mahalak K., Tanes C., Bittinger K., Tu V., Bobokalonov J., Mattei L., Zhang H., Van den Abbeele P. The Impact of Environmental PH on the Gut Microbiota Community Structure and Short Chain Fatty Acid Production. FEMS Microbiol. Ecol. 2022;98:fiac038. doi: 10.1093/femsec/fiac038. [DOI] [PubMed] [Google Scholar]
- 54.Ilhan Z.E., Marcus A.K., Kang D.-W., Rittmann B.E., Krajmalnik-Brown R. PH-Mediated Microbial and Metabolic Interactions in Fecal Enrichment Cultures. mSphere. 2017;2:e00047-17. doi: 10.1128/mSphere.00047-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu X., Mao B., Gu J., Wu J., Cui S., Wang G., Zhao J., Zhang H., Chen W. Blautia—A New Functional Genus with Potential Probiotic Properties? Gut Microbes. 2021;13:1875796. doi: 10.1080/19490976.2021.1875796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pereira F.L., Oliveira Júnior C.A., Silva R.O.S., Dorella F.A., Carvalho A.F., Almeida G.M.F., Leal C.A.G., Lobato F.C.F., Figueiredo H.C.P. Complete Genome Sequence of Peptoclostridium Difficile Strain Z31. Gut Pathog. 2016;8:11. doi: 10.1186/s13099-016-0095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodionov D.A., Arzamasov A.A., Khoroshkin M.S., Iablokov S.N., Leyn S.A., Peterson S.N., Novichkov P.S., Osterman A.L. Micronutrient Requirements and Sharing Capabilities of the Human Gut Microbiome. Front. Microbiol. 2019;10:1316. doi: 10.3389/fmicb.2019.01316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lucena R., Novales M., Blanco B., Hernández E., Ginel P.J. Effect of Probiotic Enterococcus Faecium SF68 on Liver Function in Healthy Dogs. J. Vet. Intern. Med. 2019;33:2628–2634. doi: 10.1111/jvim.15625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singhal N., Maurya A.K., Mohanty S., Kumar M., Virdi J.S. Evaluation of Bile Salt Hydrolases, Cholesterol-Lowering Capabilities, and Probiotic Potential of Enterococcus Faecium Isolated From Rhizosphere. Front. Microbiol. 2019;10:1567. doi: 10.3389/fmicb.2019.01567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hlivak P., Odraska J., Ferencik M., Ebringer L., Jahnova E., Mikes Z. One-Year Application of Probiotic Strain Enterococcus Faecium M-74 Decreases Serum Cholesterol Levels. Bratisl. Lek. Listy. 2005;106:67–72. [PubMed] [Google Scholar]
- 61.Lye H.-S., Rusul G., Liong M.-T. Removal of Cholesterol by Lactobacilli via Incorporation and Conversion to Coprostanol. J. Dairy Sci. 2010;93:1383–1392. doi: 10.3168/jds.2009-2574. [DOI] [PubMed] [Google Scholar]
- 62.Zhao Y., Liu J., Hao W., Zhu H., Liang N., He Z., Ma K.Y., Chen Z.-Y. Structure-Specific Effects of Short-Chain Fatty Acids on Plasma Cholesterol Concentration in Male Syrian Hamsters. J. Agric. Food Chem. 2017;65:10984–10992. doi: 10.1021/acs.jafc.7b04666. [DOI] [PubMed] [Google Scholar]
- 63.Wong J.M.W., de Souza R., Kendall C.W.C., Emam A., Jenkins D.J.A. Colonic Health: Fermentation and Short Chain Fatty Acids. J. Clin. Gastroenterol. 2006;40:235. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 64.Pereira D.I.A., Gibson G.R. Effects of Consumption of Probiotics and Prebiotics on Serum Lipid Levels in Humans. Crit. Rev. Biochem. Mol. Biol. 2002;37:259–281. doi: 10.1080/10409230290771519. [DOI] [PubMed] [Google Scholar]
- 65.Fushimi T., Suruga K., Oshima Y., Fukiharu M., Tsukamoto Y., Goda T. Dietary Acetic Acid Reduces Serum Cholesterol and Triacylglycerols in Rats Fed a Cholesterol-Rich Diet. Br. J. Nutr. 2006;95:916–924. doi: 10.1079/BJN20061740. [DOI] [PubMed] [Google Scholar]
- 66.Murray D.C., Coghlan M.L., Bunce M. From Benchtop to Desktop: Important Considerations When Designing Amplicon Sequencing Workflows. PLoS ONE. 2015;10:e0124671. doi: 10.1371/journal.pone.0124671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.DeAngelis M.M., Wang D.G., Hawkins T.L. Solid-Phase Reversible Immobilization for the Isolation of PCR Products. Nucleic Acids Res. 1995;23:4742–4743. doi: 10.1093/nar/23.22.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available at either UCPH ERDA data (https://sid.erda.dk/sharelink/eZ0NFSju9q) or at the EMBL Nucleotide Sequence Database (ENA), with the study accession number PRJEB55896.