Abstract
The mechanical extraction of oils from Brazilian açaí (Euterpe oleracea Mart) produces significant amounts of a byproduct known as “meal”, which is frequently discarded in the environment as waste material. Nevertheless, plant byproducts, especially those from oil extraction, may contain residual polyphenols in their composition and be a rich source of natural bioactive compounds. In this study, the phenolic composition and in vitro biological properties of a hydroethanolic açaí meal extract were elucidated. The major compounds tentatively identified in the extract by high-resolution mass spectrometry were anthocyanins, flavones, and flavonoids. Furthermore, rhamnocitrin is reported in an açaí byproduct for the first time. The extract showed reducing power and was effective in scavenging the ABTS radical cation (820.0 µmol Trolox equivalent∙g−1) and peroxyl radical (975.7 µmol Trolox equivalent∙g−1). NF-κB activation was inhibited at 10 or 100 µg∙mL−1 and TNF-α levels were reduced at 100 µg∙mL−1. However, the antibacterial effects against ESKAPE pathogens was not promising due to the high concentration needed (1250 or 2500 µg∙mL−1). These findings can be related to the diverse polyphenol-rich extract composition. To conclude, the polyphenol-rich extract obtained from açaí meal showed relevant biological activities that may have great applicability in the food and nutraceutical industries.
Keywords: açaí, polyphenols, antioxidant activity, NF-κB, pathogenic bacteria, agroindustrial residues, food by-products
1. Introduction
Agroindustrial activity generates significant amounts of solid waste throughout the processing chain [1], especially during the production of specialty oils from vegetable sources, such as açaí (Euterpe oleracea Mart.). This fruit belongs to the Euterpe gender, which comprises approximately 28 species, including E. precatoria, E. edulis, and E. oleracea. The former is also known as “açaí-do-Pará” and can be found in the Brazilian states of Pará, Tocantins, Maranhão, and Amapá, and in Guiana and Venezuela [2,3].
According to the Production of Plant Extraction and Forestry (PEVS, in Portuguese) published by The Brazilian Institute of Geography and Statistics (IBGE, in Portuguese), the açaí production in Brazil reached more than 227,000 tons, which corresponded to a sum higher than R$ 7700 million (IBGE, 2021) [4]. Açaí fruit is reported to have a high caloric value due to its lipid content (21 to 53%) [5] and to have multiple biological activities due to the presence of polyphenols, which are related to its antioxidant, anti-inflammatory, and antimicrobial properties [3,6].
Açaí oil is extracted from the pulp and has been extensively used for cosmetic and pharmaceutical applications, whereas the pulp is the main value-added commercial product for exportation. However, to extract the pulp, large amounts of residues, mainly composed of seeds and peels, are generated and frequently discarded in the environment. The Brazilian Agricultural Research Corporation (EMBRAPA) indicates that açaí seeds account for 85% of the fruit weight as compared to 15% of the pulp (epicarp and mesocarp), which can be pressed for oil extraction or consumed with other food products [7]. The mechanical processing of açaí pulp for oil extraction generates a fiber-rich byproduct named meal, which is generally discarded in the environment as a residual material. This scenario may imply several environmental damages since the yield of the byproduct is higher than its usage [8], in addition to economic losses [9].
Açaí pulp shows a bioactive potential as a source of phenolic antioxidants and other bioactive molecules [10,11,12,13,14]. Despite the fact that most studies with açaí samples investigate the edible part of the fruit [15], açaí byproducts or residues have been investigated for different purposes, such as the extraction of lignocellulosic byproducts from the residual biomass [16], the composition and antioxidant capacity of açaí seeds [8], the assessment of the antioxidant capacity and characterization of açaí fractions [15], the use of açaí pulp and seed extracts as biosorbents for residual yeasts [17], among others. However, açaí meal remains an unexplored agro-industrial byproduct with bioactive constituents, such as polyphenols, which can have potential applications as an ingredient in the food industry.
Since it is generated from the pulp, açaí meal can be considered an important source of natural antioxidants. It showed a total phenolic content even higher than that of other byproducts, such as those generated during the Fabaceae processing, such as grade B soymilk powder and soy husk powder [18], methanolic and ethanolic extracts from soybean meal, and whole soybean seeds [19]. Phenolic compounds are highly effective antioxidants mainly by scavenging reactive oxygen and nitrogen species (ROS/RNS), chelating metals, and reducing free radicals. During oxidative stress, free radicals can promote deleterious effects in the organism [20,21]. Importantly, polyphenols may inhibit or mitigate oxidative and inflammatory events that are common in individuals with diabetes, obesity, cardiovascular diseases, Alzheimer’s, premature aging, and others [22,23,24,25,26]. Additionally, antioxidant compounds extracted from natural sources, such as winemaking by-products, may show the potential to act as food ingredients, preventing lipid oxidation [27] and also acting as potential replacers of synthetic antioxidants [8]. Therefore, phenolics from açaí meal may also be used as natural additives. However, there is no information available on the antioxidant, anti-inflammatory, and antimicrobial properties of açaí meal extract, which encourages further investigation for the development of novel ingredients in food and nutraceuticals.
To the best of our knowledge, this is the first study reporting a comprehensive phenolic identification and determination of the biological activities of an extract produced from açaí meal. In this study, the phenolic profile of the dry extract of açaí meal was elucidated by high-resolution mass spectrometry (LC-ESI–QTOF-MS/MS). The extract was further tested for its antioxidant, cytotoxic, anti-inflammatory, and antimicrobial properties. This is a pioneering approach reporting the bioactive potential of açaí meal extract.
2. Materials and Methods
2.1. Açaí Byproduct
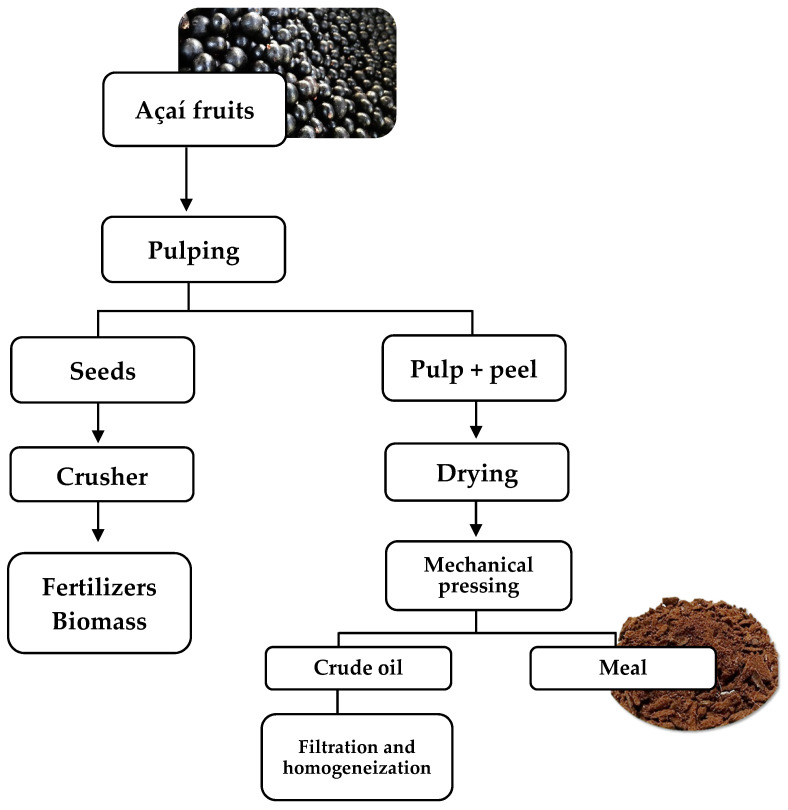
Açaí meal (5 kg) samples were provided by Citróleo Industry and Commerce of Essential Oils, LTDA, Torrinha, São Paulo, Brazil. A diagram illustrating the usual extraction process of oil from açaí and the generation of açaí meal is presented in Figure 1. Notably, the meal was obtained at the end of the oil extraction from açaí pulp, which comprised the following steps: (i) Maceration of the fruits in hot water, followed by (ii) separation of pulp and seeds, (iii) drying of the pulp fraction, (iv) mechanical pressing, which yields the oil and generates the meal, and (vi) oil filtration and homogenization, recovering the açaí meal.
Figure 1.
Flow diagram for the açaí processing.
After the cryogenic milling of the sample, 4 g were mixed with 20 mL of hexane (1:5, w/v) for defatting, followed by stirring, and centrifugation (5000× g, 10 min). This process was performed twice. The precipitate was recovered, and the solvent was evaporated. Then, the dry material was stored at −22 °C until the extract preparation.
2.2. Chemicals and Microbial Strains
All chemicals (reagents, standards, ELISA kit, and culture media) were procured from Sigma-Aldrich (St. Louis, MO, USA), R&D Systems (Minneapolis, MN, USA), and Promega Corporation (Madison, WI, USA). The following standard bacterial and yeast strains were used in the microbiological assays: Methicillin-resistant Staphylococcus aureus (MRSA, ATCC 33591), Staphylococcus aureus (ATCC 25923), Klebsiella pneumoniae (ATCC 27736), Escherichia coli EHEC (ATCC 43895), Pseudomonas aeruginosa (ATCC 27853), Candida albicans (MYA 2876), C. glabrata (ATCC 90030), C. tropicalis (ATCC 750), C. parapsilosis (ATCC 2019), Streptococcus salivarius (ATCC 7073), and S. sanguinis (SK36). The strains were grown in Sabouraud Dextrose agar (SDA, Kasvi), Brain Heart Infusion (BHI), or RPMI culture media.
2.3. LC-ESI-QTOF-MS/MS Phenolic Profile
Interfering substances were removed from the extract by SPE-LC18, as described by de Souza Silva et al. [28].
Briefly, 500 mg of the freeze-dried extract from açaí meal was dissolved in purified acid water (pH 2.0). The mixture was eluted in an SPE-LC18 cartridge with acid water followed by methanol, which recovered the bioactive compounds from the stationary phase. Afterward, methanol was removed under N2 gas flow, and the remaining fraction was suspended in methanol to be injected into the LC-MS/MS system for determining the phenolic profile.
Phenolic compounds were separated on a Phenomenex Luna C18 column (4.6 mm × 250 mm × 5 µm) and a Shimadzu chromatograph (Shimadzu Co, Kyoto, Japan) using the instrumental conditions described in our previous study [28]. The mobile phase was composed of A) water and formic acid (0.25%, v/v) and B) acetonitrile:water:formic acid at 80:19,75:0.25 (%, v/v). The gradient started at 10% B, increasing to 20% B (10 min), 30% B (20 min), 50% B (30 min), 50% B (32 min), 90% B (38 min), and 10% B (45 min), ending the run at 55 min.
A Bruker Daltonics mass spectrometer (Bruker Daltonics, Bremen, Germany) equipped with an electrospray ionizer (ESI) operated with nebulizer at 2 bar, dry gas at 8L/min, the temperature at 200 °C, and 4500 V. The resolution ranged from 400 to 30,000 m/z, and data analysis was performed with the software MAXIS 3G–Bruker Daltonics 4.3 (Bruker Daltonics, Billerica, MA, USA). The tentative identification of the compounds was conducted by comparing the accurate masses from parent ion and MS2 spectra with those described in the literature.
2.4. Total Phenolic Compounds, Reducing Power and Free Fadical Scavenging Capacity of Açaí Meal Extract
The freeze-dried extract was tested for its ferric-reducing antioxidant power (FRAP) and scavenging capacity against free radical ABTS and reactive oxygen species (ROS). Photometric measurements were carried out in a microplate reader (SpectraMax® M3, Molecular Devices LLC, Sunnyvale, CA, USA).
2.4.1. Total Phenolic Compounds (TPC) and Reducing Power
The TPC was determined following Singleton, Orthofer, and Lamuela [29], with modifications described elsewhere [28]. Briefly, 20 µL of the açaí meal extract in different concentrations, or gallic acid, were pipetted in a 96 well microplate together 100 µL of a 10% (v/v) Folin-Ciocalteu aqueous solution. The mixture reacted for 5 min in the dark and posteriorly received 75 µL of a 7.5% (v/v) sodium carbonate aqueous solution. After 40 min of reaction at room temperature in the dark, the absorbances were read at 740 nm in a microplate reader, and the results were expressed as milligrams of gallic acid equivalents (GAE) per gram of açaí meal extract (dry weight basis).
The FRAP assay was carried out as previously described by de Souza Silva et al. [28]. Briefly, 20 µL of the samples and standard (ferrous sulfate) at different concentrations were pipetted in a 96-well microplate followed by 30 μL of distilled water and 200 μL of FRAP reagent, which was composed of acetate buffer (0.3 M, pH 3.6), an iron chloride solution (20 mM) and TPTZ solution at 10:1:1 (v/v). The mixture reacted at 37 °C for 8 min and the absorbances were measured at 595 nm. The reducing power was expressed as micromoles of ferrous sulfate per gram of dry extract.
2.4.2. Free Radical Scavenging Capacity
The free radical ABTS scavenging capacity and the oxygen radical absorbance capacity (ORAC) were determined according to de Souza Silva et al. [28] and Melo et al. [30], respectively.
The ABTS radical solution was prepared by mixing the ABTS solution (7 mM) with potassium persulphate solution (140 mM), which reacted for 16 h in the dark at room temperature. The final solution was diluted with potassium phosphate buffer (75 mM, pH 7.4) to an absorbance of 0.7 ± 0.02 at 734 nm. Then, 20 µL of the samples or Trolox and 220 µL of the ABTS radical solution were pipetted in a 96-well microplate and reacted for 6 min in the dark at room temperature. After this, the absorbances were read at 734 nm, and the results were expressed as µmol Trolox equivalents per gram of dry açaí meal extract.
For the ORAC assay, aliquots of 30 µL of the samples or Trolox at different concentrations were pipetted in a 96-well microplate, followed by 60 µL of a fluorescein solution (508.25 nM) in potassium phosphate buffer (75 mM, pH 7.4), and 110 µL of AAPH solution (76 mM). The reaction occurred at 37 °C and the fluorescence was monitored every minute for 2 h. The fluorescence was monitored at 485 nm for excitation and 528 nm for emission, and the results were expressed as µmol of Trolox equivalents per gram of dry açaí meal extract.
The extract was also evaluated for its scavenging capacity towards superoxide radical anion and hypochlorous acid (HOCl) [30]. For the superoxide radical scavenging assay, aliquots of 100 µL of the sample or standard at different concentrations, 50 µL of NBT, 50 µL of PMS, and 100 µL of NADH were sequentially pipetted in a 96-well microplate. The absorbance was monitored at 560 nm every 1 min for 5 min of reaction, and the results were expressed as EC50, that is, the concentration (µg mL−1) of the dry extract capable of quenching 50% of superoxide radicals [30]. For the HOCl scavenging assay, aliquots of 100 µL of potassium phosphate buffer (100 mM, pH 7.4), 100 µL of different concentrations of the sample or standard, 50 µL of dihydrorhodamin 123 (DHR, 7.5 µM), and 50 µL of a HOCl solution (30 µM) were pipetted sequentially in a 96 well microplate. The microplate was incubated at 37 °C and fluorescence was read at 528 ± 20 nm for emission and 485 ± 20 nm for excitation, and the results were also expressed in EC50.
2.5. In Vitro Cytotoxicity and Anti-Inflammatory Activity
2.5.1. Cell Culture
The anti-inflammatory effects of the extract were determined according to our previous study [28]. RAW 264.7 macrophages (ATCC® TIB-71™) transfected with the NF-κBpLUC gene (CQB N° 022/97) were cultured in RPMI media supplemented with fetal bovine serum (10%), penicillin (100 U/mL), and glutamine (2 mM). Cells were grown at 37 °C under 5% CO2.
2.5.2. Cell Viability Assay
The MTT assay was performed to measure cell viability upon exposure to different concentrations of the açaí meal extract. Briefly, macrophages were seeded (2 × 105 cells∙mL−1) onto 96-well plates and incubated at 37 °C in 5% CO2 for 24 h. Next, cells were treated with the extract at 1, 10, or 100 µg∙mL−1 and incubated for 24 h. The supernatant was discarded and an MTT solution (0.3 mg∙mL−1 in RPMI) was added to each well. The plate was incubated for 3 h, then the supernatant was removed, and 200 µL of DMSO was pipetted into the wells. The absorbance of the wells was measured at 540 nm in a microplate reader (SpectraMax M3, Molecular Devices, LLC, Sunnyvale, CA, USA).
2.5.3. NF-κB Activation and TNF-α Quantification
Macrophages were seeded onto a 24-well plate (3 × 105 cells per well) and incubated under the same conditions described earlier. Next, cells were treated with the extract at 1, 10, or 100 µg∙mL−1 for 30 min and then stimulated with LPS (10 ng∙mL−1) for 4 h. The supernatant was recovered and TNF-α levels were quantified by ELISA in a microplate reader following the manufacturer’s instructions. The results were expressed as pg∙mL−1.
To determine the effects of the extract on NF-κB activation, once the supernatant was recovered, macrophages were lysed (TNT lysis buffer) and 10 µL of the suspension was mixed with 25 µL of Luciferase (a reagent containing luciferin). Luminescence was quantified in a microplate reader (SpectraMax® M3, Molecular Devices LLC, Sunnyvale, CA, USA).
2.6. Antimicrobial Activity of the Açaí Meal Extract
2.6.1. Strains and Growth Conditions
Bacteria and yeast strains of medical-dental and/or food interest were maintained as frozen stocks at −80 °C until used. After thawing, they were inoculated in BHI broth and incubated at 37 °C for 24 h. Bacterial and yeast inocula were prepared in sterile saline solution (0.9 %) to 5 × 108 CFU∙mL−1 and 2.5 × 106 CFU∙mL−1, respectively. The suspensions were diluted in Müeller-Hinton (bacteria) or RPMI (yeast) media to obtain an approximate final concentration of 105 CFU∙mL−1 for bacteria and 104 CFU∙mL−1 for yeasts.
2.6.2. Minimum Inhibitory Concentration (MIC)
The MIC of the extract was determined by the microdilution method according to the Clinical and Laboratory Standards Institute M27-S3 [31] and M07-A9 [32]. The açaí meal extract was mixed with sterile saline solution (0.9%) at 30 mg∙mL−1. After solubilization, 100 μL of the samples were added to the wells and serially diluted to obtain concentrations ranging from 9.76 to 5000 μg∙mL−1. Then, aliquots of the microbial suspensions (100 μL) were added to the wells and the microplates were incubated at 37 °C for 24 h under appropriate growth conditions.
After incubation, 50 µL of resazurin (0.01%) was added to each well and the microplate was incubated again at 37 °C for 2 h. The MIC was defined as the lowest concentration of the extract that prevented the growth-induced color change promoted by resazurin. Color change from blue (original resazurin color) to pink indicates the presence of viable cells. Standard antimicrobials were used as positive controls and inoculated culture media served as a negative control. All assays were performed in triplicate of three independent experiments.
2.6.3. Minimum Bactericidal and Fungicidal Concentration (MBC/MFC)
To determine the MBC/MFC, 10 µL of the wells corresponding to the MIC and higher concentrations were subcultured onto BHI agar or SDA plates. The plates were incubated at 37 °C for 24 h. The MBC/MFC was defined as the lowest concentration of açaí meal extract that prevented visible microbial growth on the solid media.
2.7. Statistical Analysis
The assessments were made in triplicate and results were expressed as mean ± standard deviation. Tukey’s post-test was performed for the antioxidant capacity, cytotoxic and anti-inflammatory activities. In all statistical tests, the significance level was considered at p < 0.05.
3. Results and Discussion
3.1. Comprehensive Chemical Characterization of the Extract from Açaí Meal
The açaí meal extract was subjected to LC-ESI-QTOF-MS/MS analysis in the positive mode for the identification of anthocyanins, and in the negative mode for the identification of flavonoids and non-flavonoids (Table 1).
Table 1.
Phenolic compounds tentatively identified in the optimized açaí meal extract by LC-ESI-QTOF-MS/MS.
| Compounds | Retention Time (min) | Molecular Formula | Exact Mass | Parent Ion (m/z) | Error (ppm) | Fragments (MS2) | |
|---|---|---|---|---|---|---|---|
| Anthocyanins [M+H]+ | Cyanidin 3-O-rutinoside | 11.4 | C27H30O15 | 594.1574 | 595.1652 | −1.346 | 287.0556 |
| Cyanidin 3-O-glucoside | 16.9 | C21H20O11 | 448.1018 | 449.1096 | −13.836 | 287.0548 | |
| Delphinidin 3-rutinoside | 19.0 | C27H30O16 | 610.1528 | 611.1606 | −0.656 | 303.0505 | |
| Pelargonidin 3-O-glucoside | 19.3 | C21H20O10 | 432.1058 | 433.1136 | 1.389 | 271.0607 | |
| Peonidin 3-O-rutinoside | 19.7 | C28H32O15 | 608.1736 | 609.1814 | −0.987 | 301.0725 | |
| Peonidin 3-O-glucoside | 23.1 | C22H22O11 | 462.1165 | 463.1243 | 0.649 | 301.0722 | |
| Malvidin 3-glucoside | 23.1 | C23H24O12 | 492.1268 | 493.1346 | −0.813 | 331.0822 | |
| Phenolic acids [M-H]− |
Caffeoylquinic acid | 12.3 | C16H18O9 | 354.0968 | 353.089 | 5.083 | 191.0562; 179.034 |
| 4-Caffeoylshikimic acid | 15.3 | C16H16O8 | 336.085 | 335.0772 | 2.975 | 179.0347; 161.0319; 135.0464 | |
| Flavones [M-H]− |
6,8-di-C-hexosyl apigenin (vicenin-2) |
14.5 | C27H30O15 | 594.1581 | 593.1503 | 0.168 | 383.0763; 353.0662 |
| 6-C-glycosyl luteolin (isoorientin) | 16.9 | C21H20O11 | 448.1002 | 447.0924 | −1.785 | 447.0924; 357.0610; 327.0505 | |
| 6-C-glycosyl luteolin (isoorientin) | 17.4 | C21H20O11 | 448.1007 | 447.0929 | −0.669 | 447.0929; 357.0617; 327.0499 | |
| 6-C-glycosyl luteolin (isoorientin) | 17.6 | C21H20O11 | 448.0988 | 447.091 | −4.910 | 447.0910; 357.0588; 327.0488 | |
| 6-C-glycosyl apigenin (isovitexin) | 19.0 | C21H20O10 | 432.1053 | 431.0975 | −1.620 | 431.0975; 311.0557; 283.0602; 341.0676 | |
| Rhamnocitrin | 33.2 | C16H12O6 | 300.0633 | 299.0555 | 1.000 | 299; 284; 256; 227 | |
| Flavonoids [M-H]− |
Taxifolin 3-O-glucoside | 15.9 | C21H22O12 | 466.1103 | 465.1025 | −1,716 | 285.0409; 151.0035; 125.0728 |
| Rutin | 19.0 | C27H30O16 | 610.1513 | 609.1435 | −2.786 | 255.0312; 271.0206. 301.0328 | |
| Flavanone [M-H]− | Eriodictyol 7-O-glucoside I | 18.4 | C21H22O11 | 450.1157 | 449.1079 | −0.666 | 269.0441; 259.0616 |
| Flavonols [M-H]− |
Scoparin | 20.1 | C22H22O11 | 462.1160 | 461.1082 | 0.000 | 341.0667; 371.0776; 298.0483 |
| Scoparin | 20.4 | C22H22O11 | 462.1155 | 461.1077 | 0.000 | 341.0667; 371.0895; 298.0483 |
Cyanidin 3-O-glucoside and cyanidin 3-rutinoside showed a fragment at m/z 287, which is also typical of their aglycone forms [33]. Cyanidin 3-O-glucoside was previously identified in grape peels, and when isolated, it was able to inhibit mammal tumor cells [34]. This is one of the many anthocyanin monomers spread in nature [35], which is reported to have anti-inflammatory activity by inhibiting lipopolysaccharide (LPS)-induced activation of the nuclear factor kappa B (NF-κB), among other mechanisms [35,36]. Delphinidin 3-rutinoside exhibited an m/z signal at 611.1606 and was tentatively identified in the açaí meal extract at m/z 303.0505, which is characteristic of a loss of 308 Da (162 + 146). This can be related to the link of a hexose as well as a deoxyhexose at the same position as the aglycone form [37].
Pelargonidin 3-O-glucoside showed a protonated ion at m/z 433.1136 and a fragment at m/z 271.0607, due to the loss of a hexose [M+H-162]+ [38]. According to Zhang et al. [39], pelargonidin 3-glucoside isolated from strawberry extracts showed antiproliferative activity on oral, cervical, prostate, and colon cells. Peonidin 3-O-rutinoside also showed the same behavior as delphinidin 3-rutinoside, losing 308 Da and generating a fragment ion at m/z 301.0725 [37].
Peonidin 3-O-glucoside showed a [M+H]+ at m/z 463.1243 and an MS/MS fragment at m/z 301, which corresponds to the aglycone form of this anthocyanin, as a result of the loss of one hexose [M+H-162]+. Malvidin 3-glucoside (m/z at 493.1346) showed an MS2 fragment at m/z 331.082. The loss of 162 Da corresponds to a neutral loss of a glucoside group [40].
In general, anthocyanins are reported to have multiple biological activities, such as antioxidant and anti-inflammatory [41,42], with potential beneficial health effects, such as in the prevention of cognitive dysfunction and Alzheimer’s disease [43,44]. The antimicrobial potential of anthocyanins, such as cyanidin 3-O-glucoside, against important foodborne pathogens (Escherichia coli and Staphylococcus aureus) has also been investigated. The results indicate that the complex cyanidin 3-O-glucoside plus lauric acid could be adopted mainly as a food preservative or even as a therapeutic ingredient against the aforementioned bacteria strains [45].
Caffeoylquinic acid (deprotonated ion at m/z 353.0890) and 4-caffeoylshikimic acid (deprotonated ion at m/z 335.0722) were the only phenolic acids identified in the extract. Caffeoylquinic acid belongs to the hydroxycinnamic acids group and is an ester of caffeic and quinic acids. This compound is mainly found in coffee beans and beverages [46] and was beneficial in the management of metabolic syndrome, with antidiabetic, anti-inflammatory, and antioxidant potential [47]. The fragmentation of caffeoylquinic acid generated m/z signals at 191.0562, 179.034 [48], and others [49].
The compound 4-caffeoylshikimic acid is an isomer of caffeoylshikimic acid and was previously identified in Euterpe oleracea root extracts [50]. This compound showed MS/MS fragments at m/z 179.0347, 161.0319, and 135.0464, which is in line with the fragmentation pattern reported by Brunschwig et al. [50]. Generally, hydroxycinnamic acids have multiple biological activities, mainly antioxidant, anti-inflammatory, and antimicrobial.
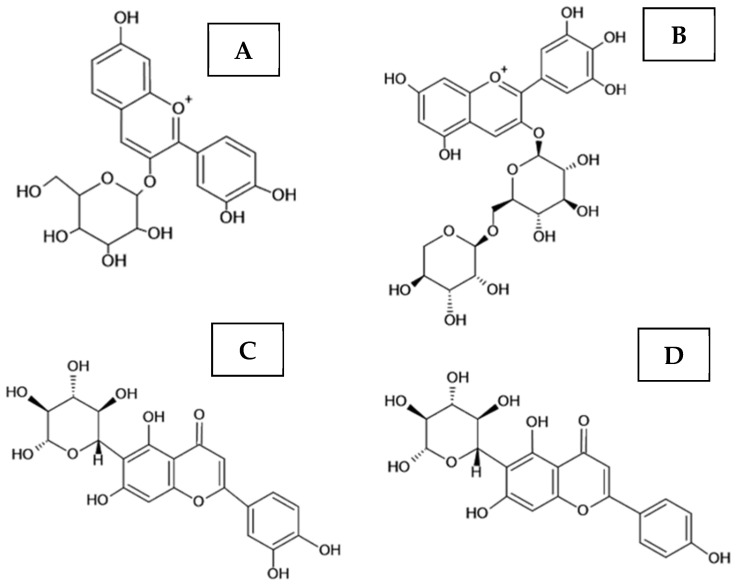
Isoorientin (Figure 2) and vicenin-2 were previously shown to have anti-inflammatory activity.
Figure 2.
The structural formula of the main flavonoids identified in the açaí meal extract (Table 1). (A) = Cyanidin 3-O-glucoside, (B) = Delphinidin 3-rutinoside, (C) = 6-C-glycosyl luteolin (isoorientin), (D) = 6-C-glycosyl apigenin (isovitexin).
Likewise, isoorientin exhibited antibacterial effect against Bacillus subtilis ATCC 11562 [51]. Vicenin-2 (6,8-di-C-hexosyl apigenin), a deprotonated ion at 593.1503, was identified in the açaí meal extract. Vicenin-2 is formed by the presence of aglycone apigenin (270) and 2 units of hexoses (162 + 162), with a final molecular weight of 594 Da [52]. Isovitexin, another apigenin derivative (Figure 2), was also found in the extract. This compound showed a deprotonated ion at m/z 431.0975, followed by m/z 311 and 341, which corresponds to losses of 120 and 90 Da [52].
Isovitexin showed anti-inflammatory activity by selectively inhibiting COX-2 and NO production in LPS-stimulated RAW cells [53] and downregulating the production of pro-inflammatory mediators [54]. Other authors pointed out that luteolin glycosides demonstrate low anti-inflammatory activity in vitro but high activity in vivo since they are transformed into glucuronides that are selectively activated in inflamed sites [55].
Three luteolin derivatives were tentatively identified as isoorientin isomers but they might be orientin isomers, which have the same fragmentation pattern [50,56]. A trend in the fragmentation of the deprotonated ions at m/z 447.09 can be observed, while the losses of 90 Da correspond to 6-C-glucosylation. In addition, the fragments m/z 327 and 357 in the three identified isoorientins are strongly related to glycosylated luteolin derivatives [50,52].
To the best of our knowledge, this is the first time that rhamnocitrin is reported in an açaí product. This compound was previously detected in Rhamnus prinoides with deprotonated ion at m/z 299 [57]. Both its parent ion and MS/MS fragments (m/z 299 and 284) are consistent with the findings of this study (Table 1). Rhamnocitrin is a flavonoid derived from kaempferol with reported strong antioxidant and anti-inflammatory activities, also exerting antimicrobial activity against S. aureus at 50 μg∙mL−1 [58]. Other biological properties have been attributed to this compound. Rhamnocitrin isolated from Bauhinia variegata stem bark presented anticataract activity that is possibly linked to its strong antioxidant capacity and effectiveness against the progression of damages promoted by H2O2 and GSH depletion in lenses [59]. Aglycones and glycoside forms of rhamnocitrin were also identified in green fruits of Rhamnus species [60].
Taxifolin-3-O-glucoside showed a deprotonated ion at 465.1025 and was tentatively identified according to its MS2 fragmentation at m/z 285.0409, 151.0035, and 125.0728 [61]. The fragment signal at m/z 285 indicates the loss of glucose and water (162 + 18) [60].
Rutin ([M-H]− at m/z 609.1435) is a quercetin derivative mainly found in buckwheat, apricots, grapes, and some citric fruits. This glycosylated flavonoid has multiple biological activities, mainly antioxidant and anti-inflammatory [62]. Rutin was reported to inhibit tumor cell proliferation and protect healthy cells from oxidative stress, DNA damage, and inflammatory processes by decreasing ROS production [63]. Rutin fragmentation is characterized by the loss of 308 Da, which is a typical fragment of rutinoside [64].
The flavanone eriodictyol-7-O-glucoside I, [M-H]− at m/z 449.1079, was identified in the açaí meal extract and is characterized by the loss of a hexose [M-H-162]−. This flavonoid was already identified in açaí juice [65]. Eriodictyol showed antimicrobial [66] and anti-inflammatory properties by inhibiting the synthesis of proinflammatory cytokines [67].
Lastly, the flavonol scoparin was tentatively identified with a deprotonated ion at 461.10 and MS2 fragments at m/z 341 and 371, which corresponds to losses of 120 and 90 Da. This compound was previously found in an açaí dietary supplement [68].
Collectively, the LC-ESI-QTOF-MS data and previous literature reports suggest that the phenolic compounds tentatively identified in the açaí meal extract are likely to scavenge free radicals and possess anti-inflammatory and antimicrobial activity. Açaí meal still contains several residual phenolic compounds that may present these properties, and upon recovery, could be used in the food and health industry.
3.2. Antioxidant Capacity: TPC, Reducing Power and Free Radical Scavenging
Several studies indicate that total phenolic compounds (TPC) recovered from plant materials can be correlated with antiradical activity, reducing power and the protective effect against ROS-induced DNA damage [69,70,71,72]. The TPC and scavenging effects of the açaí meal extract on the synthetic radical ABTS radical cation, ion reduction (FRAP), and ROS deactivation (peroxyl radical, superoxide anion, and hypochlorous acid) are shown in Table 2.
Table 2.
The antioxidant capacity of the optimized açaí meal extract as determined by the total phenolic content (TPC), ferric-reducing antioxidant power (FRAP), and scavenging of ABTS radical cation (ABTS•+), and ROS assays [peroxyl radical (ROO•), superoxide anion (O2•−), and hypochlorous acid (HOCl)].
| Antioxidant Activity | |||||
|---|---|---|---|---|---|
| TPC (mg GAE∙g−1) |
FRAP (µmol FS∙g−1) |
ABTS•+ (µmol TE∙g−1) |
ROO∙
(µmol TE∙g−1) |
O2− (EC50 µg∙mL−1) |
HOCl (EC50 µg∙mL−1) |
| 88.4 ± 0.4 | 986.0 ± 22.0 | 820.0 ± 36.4 | 975.7 ± 69.0 | 37.1 ± 1.9 | 4.2 ± 0.7 |
GAE—gallic acid equivalents; FS—ferrous sulfate; TE—Trolox equivalent. Data were expressed on a dry basis (mass of lyophilized açaí meal extract). The final values are the averages of the triplicates ± standard deviation.
Regarding the TPC, the result of this work (Table 2) was higher than that determined by Kang et al. (2012) [73] for E. oleracea pulp extract (31.2 mg GAE∙g−1), for methanolic extracts of Colombian açaí analyzed by Garzón et al. (2017) (47.86 mg GAE∙g−1) [33], and for açaí seeds extract analyzed by Melo et al. (2021) (64.58 ± 1.89 mg GA∙g−1) [8]. Açaí meal extract also presented higher TPC than the açaí freeze-dried pulp analyzed by Batista et al. (2016) [74], which found 55.20 mg GAE∙g−1 for the extract obtained without supercritical extraction, and values ranging from 54.57 a 75.65 mg GAE∙g−1 when the supercritical extraction was applied. Additionally, açaí meal extract showed superior reducing power assessed by TPC assay than different waste from wine and cider industries, namely grape marc (11.40 ± 0.23 mg GAE∙g−1), grape stalks (15.37 ± 0.31 mg GAE∙g−1) and apple pomace (6.5 mg GAE∙g−1) [75].
The phenolic profile and antioxidant properties of methanol extracts from Colombian açaí were investigated by Garzón et al. [33]. The samples scavenged the ABTS•+ radical at 210.49 ± 30.71 µmol Trolox∙g−1, while the açaí meal extract tested in this study showed a four-fold higher activity (820.0 ± 36.4 µmol Trolox∙g−1) (Table 2). The extraction method and sample concentration can significantly influence the sample effectiveness [76]. The açaí meal extract also showed an ABTS radical scavenging capacity superior to three byproducts of wine grapes cultivars: Chenin Blanc (218 µmol Trolox∙g−1), Petit Verdot (626 µmol Trolox∙g−1), and Syrah (653 µmol Trolox∙g−1) [30].
Likewise, the açaí meal extract exhibited greater ABTS radical cation scavenging capacity than the extract obtained from açaí seeds (763.09 ± 17.27 µmol Trolox∙g−1) [8]. The açaí meal extract also showed higher ABTS radical cation scavenging activity than ethanolic extracts obtained from avocado by-products (cultivars Hass and Fuerte), exhibiting 1.2- and 1.4-times higher antiradical activity than Hass and Fuerte seeds extracts, respectively [77].
When compared to optimized ethanolic extract from açaí fruit [78], açaí meal extract also showed a superior capacity to scavenge ABTS radical cation, showing a result about 54 times higher. The extract from açaí meal also showed a higher capacity of scavenging against ABTS radical cation when compared to optimized extracts from açaí juçara fruits (Euterpe edulis Mart.) [79]. Extracts of açaí juçara pulp obtained with water, ethanol 99.9%, and ethanol 70% showed antioxidant activity of 16.53 ± 0.20, 15.96 ± 0.07, and 17.52 ± 0.01 µmol Trolox∙g−1 against ABTS radical cation, lower values compared to the açaí meal extract. However, the behavior presented by Silva et al. [79] is in accordance with the trend observed in this study regarding the polarity of the extractor solvent, indicating that a moderate-polar solvent can be more efficient than pure ethanol for the antioxidant activity. As shown in Table 2, the extract showed reducing power at 986.0 ± 22.0 µmol FS∙g−1. These values are higher than those of other residues from the pulp, raw peel, seeds, lyophilized peel, and oven-dried peel of different fruits, such as avocado, pineapple, banana, and watermelon [80].
Since the presence of phenolic compounds in a sample can be correlated to its antioxidant activity [81], a correlation analysis was performed for TPC and antioxidant activity assessed by FRAP and ABTS radical scavenging assays of the sample tested in this study. TPC and FRAP showed a positive and strong correlation with R = 0.8531, and TPC and ABTS radical scavenging assay showed a positive and very strong correlation with an R = 0.9730. Hence, this statistical analysis indicate that açaí meal extract is a material with high antiradical activity which is correlated to the phenolic content, showing a diverse profile in polyphenols as presented in Table 1. Additionally, considering that the processes of free radical generation are involved in the activation of NF-κB, it would be possible to suggest that the anti-inflammatory activity is due to, at least in part, the antioxidant properties of the phenolic compounds present in the extract.
The antioxidant activity of pulps from Euterpe precatoria Mart., known as açaí-do-Amazonas, and E. oleracea Mart., açaí-do-Pará, were evaluated by Kang et al. [73]. The same authors reported that the peroxyl radical scavenging activity of açaí-do-Amazonas (1828.4 µmol Trolox∙g−1 of dry extract) was stronger than that of açaí-do-Pará (1014.0 µmol Trolox∙g−1 of the dry extract [73]. This is consistent with the ORAC data showing that the extract from açaí-do-Pará meal scavenged the peroxyl radical at 975.7 ± 69.0 µmol Trolox∙g−1 of dry extract (Table 2). These findings suggest that the meal extract had a peroxyl radical scavenging activity similar to that of the pulp extract.
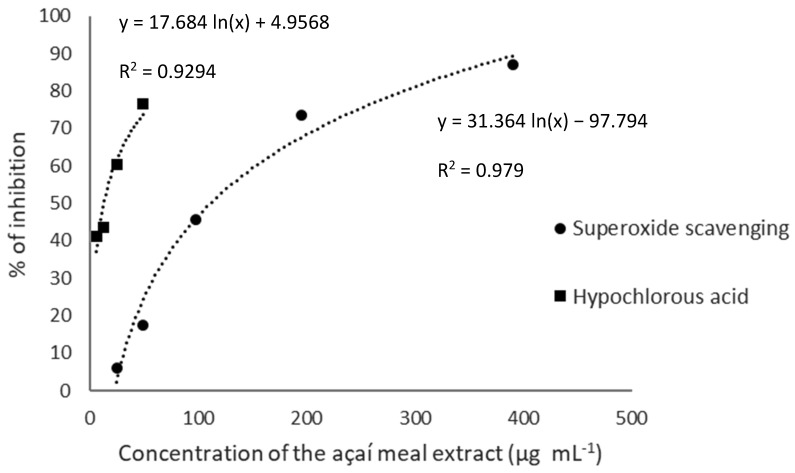
The açaí meal extract showed a stronger peroxyl scavenging capacity (Figure 3) than that of Brazilian native plants studied by Infante et al. [82], that is, 4- and 1.5-fold higher than that of Eugenia neonitida Sobral extract and its phenolic-rich fraction, respectively [83].
Figure 3.
EC50 graphics showing the scavenging of superoxide and hypochlorous acid of the optimized açaí meal extract. The figure indicates the scavenging percentage (%) as a function of the extract concentration (µg∙mL−1).
Superoxide anion (O2•−) production by the electron transport chain occurs spontaneously and may increase during the inflammatory process. Superoxide anion can be converted into hydrogen peroxide by the superoxide dismutase enzyme, which can interact with transition metals and produce hydroxyl radical—the starter of the lipid peroxidation chain and a precursor of peroxyl radical [21]. The açaí meal extract showed a higher capacity to scavenge superoxide anion when compared to extracts from the seeds of Eugenia brasiliensis, E. involucrate, and E. myrcianthes [83].
Hypochlorous acid (HOCl) is a highly oxidative species generated inside neutrophils in response to microbial attacks. HOCl is generated during the oxidation of chloride ions by hydrogen peroxide through the action of myeloperoxidase. When it reaches high concentrations in the organism, HOCl may be sharply damaging [20]. The açaí meal extract was found to effectively scavenge HOCl, with an EC50 of 4.2 ± 0.7 µg∙mL−1 (Figure 3). Ethanolic extracts obtained from avocado by-products (peels and seeds) were capable to quench 50% of HOCl at concentrations ranging from 5.2 to 8.6 µg∙mL−1 [77]. The EC50 concentrations of extracts produced from rachis and pomaces of different grape cultivars ranged from 17 to 128 µg∙mL−1 [30], being the açaí meal extract also 1.18 to 7.4-times higher in the quench of HOCl than some extracts obtained from Brazilian native fruits [84], and 8.3 times higher even when compared to white açaí juice [85]. Additionally, the açaí byproduct extract showed higher activity in quenching HOCl when compared to Trolox (134. µg∙mL−1) [86]. Therefore, the present results indicate that the açaí meal extract can be more effective in quenching hypochlorous acid rather than extracts obtained from other plant byproducts or fruits.
3.3. In Vitro Cytotoxicity and Anti-Inflammatory Activity
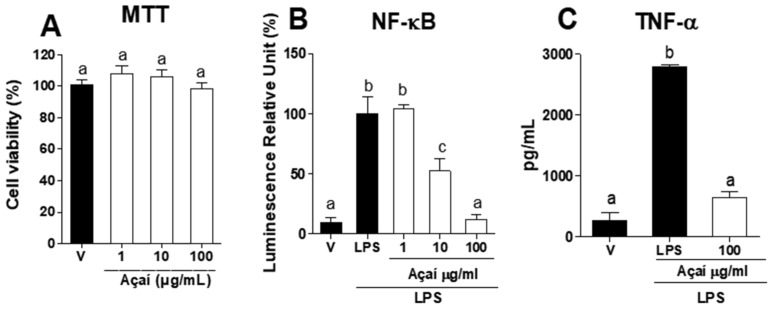
The açaí meal extract was further tested for its cytotoxic effects on macrophages and anti-inflammatory activity in vitro. Cells treated with the extract at 1, 10, or 100 µg∙mL−1 showed 100% viability, with no significant difference compared to the vehicle control (p > 0.05) (Figure 4A).
Figure 4.
The inhibitory activity of the optimized açaí meal extract on NF-κB activation and TNF-α levels. (A) Viability of macrophages exposed to the extract at 1, 10, or 100 μg∙mL−1. (B) NF-κB activation in cells pretreated with the extract at 1, 10, or 100 μg∙mL−1 after stimulation with lipopolysaccharide (LPS) (10 ng∙mL−1) for 4 h. (C) TNF-α levels detected in the supernatant of cultures pretreated with the extract at 100 μg∙mL−1 after stimulation with LPS (10 ng∙mL−1) for 4 h. The data were expressed as mean ± SEM, n = 4. V—vehicle. Different letters indicate significant statistical differences (p < 0.05).
Sprenger et al. [87] found that the hydroethanolic extracts of açaí leaves, pulp, and seeds were nontoxic to treated cells, with LC50 of 2033, 1053, and 1310 µg∙mL−1, respectively. Dias-Souza et al. [88] evaluated the cytotoxicity of an açaí pulp extract produced with 80% (v/v) methanol against liver carcinoma cells. The authors reported that treatment with the extract at 15.62 µg∙mL−1 decreased tumor cell proliferation and did not affect the viability of cells used as controls, which is consistent with the viability data of the present study.
The inflammatory process is a natural response mechanism against the invasion of microorganisms and other antigens into the body tissues or bloodstream, as well as in cell death, tissue damage, and diseases such as cancer [89,90]. In this study, the açaí meal extract was tested for its ability to modulate the activation of the NF-κB transcription factor (Figure 4B) and TNF-α release (Figure 4C) in LPS-stimulated cells at nontoxic concentrations. The data demonstrated that treatment with the extract at 10 or 100 μg∙mL−1 significantly reduced NF-κB activation compared to the LPS-control group (p < 0.05). Interestingly, NF-κB activation levels between cells treated with the extract at 100 μg∙mL−1 and the control (V) were not statistically different, highlighting the potency of the phenolics present in the extract. Additionally, the group treated with the extract (100 μg∙mL−1) showed significantly lower TNF-α levels compared to the LPS control (p < 0.05).
Ethyl acetate extracts of the açaí species E. precatoria and E. oleracea were tested for their anti-inflammatory properties by Kang et al. [73]. The authors reported that E. precatoria extract at 20 µg∙mL−1 inhibited LPS-induced NF-κB activation by 23%, whereas E. oleracea extracts did not show any activity. A strong inhibition can be observed regarding the NF-κB factor and TNF-α levels in cells treated with the meal extract obtained from E. oleracea processing.
Previous studies indicated that the extracts from the pulp of açaí and the juice can inhibit NF-κB activation and TNF-α synthesis. Taken altogether, these findings suggest that different parts of açaí could be used to prevent the onset and/or aggravation of inflammatory conditions such as atherosclerosis [6,91,92]. More importantly, açaí byproducts may be a new source of compounds with strong anti-inflammatory potential to be further explored for the development of drugs, cosmetic formulations, and/or even to be incorporated into functional foods.
3.4. Antimicrobial Activity
As shown in Table 3, the açaí meal extract showed antimicrobial activity against S. aureus, MRSA and P. aeruginosa, with MIC/MBC values (µg∙mL−1) of 1250/1250; 2500/2500, and 2500/2500, respectively.
Table 3.
The Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of the lyophilized optimized açaí meal extract.
| Strain | Gram Staining | MIC/MBC (µg∙mL−1) |
|---|---|---|
| Staphylococcus aureus (ATCC 25923) | + | 1250/1250 |
| S. aureus (MRSA, ATCC 33591) | + | 2500/2500 |
| Pseudomonas aeruginosa (ATCC 27853) | − | 2500/2500 |
Other relevant pathogenic strains [Escherichia coli EHEC (ATCC 43895), Candida albicans (MYA 2876), C. glabrata (ATCC 90030), C. tropicalis (ATCC 750), C. parapsilosis (ATCC 2019), Streptococcus salivarius (ATCC 7073), S. sanguinis (SK36) and Klebsiella pneumoniae (ATCC 27736)] were also tested, however the extract did not show any significant antimicrobial activity against them.
Phenolic compounds such as flavan-3-ols, flavonols, and anthocyanins are known to have antimicrobial activity stronger than that of other polyphenols. Compounds belonging to these groups were identified by LC-MS in the optimized açaí meal extract. Their mechanisms o.f action are diverse, including alterations in cell membrane permeability, inhibition of biofilm formation, neutralization of bacterial toxins, and, importantly, the ability to interact synergistically with antibiotics and/or other natural antimicrobial substances [93,94].
The antimicrobial property of phenolic compounds can be influenced by their solubility and affinity of functional groups with the microbial cell membrane. For instance, the hydroxyl group can interact with components of the membrane and promote its disruption with consequent extravasation of the intracellular content [95]. Moreover, the position of the hydroxyl group in the molecules can also influence their biological effects by affecting the charge gradient and potential difference of the membrane. Through this mechanism, the adenosine triphosphate pool is drastically reduced leading to cell death [96].
According to Newman and Cragg (2020) [97], 64% of new drugs discovered and approved by the FDA (Food and Drug Administration) were natural products or derivatives thereof. These data not only strengthen scientific research in this sector, but also increase the challenges for researchers to identify new molecules, elucidate the control of action and propose therapeutic use. However, the MIC/MBC values (µg/mL) for S. aureus, S. aureus MRSA and P. aeruginosa were, respectively, 1.250/1.250, 2.500/2.500 and 2.500/2.500. Studies carried out by Freires et al. (2015) [98] demonstrated that MIC at concentrations from 1000 to 2000 have weak antibacterial activity and above this value is considered without activity. Thus, the authors decided not to invest in other more complex tests. However, the reporting of these results is relevant since this is the first study exploring the antimicrobial activity of an açaí meal extract.
4. Conclusions
The findings of this study demonstrated that the açaí meal byproduct is a rich source of phenolics compounds with multiple in vitro biological activities, mainly antioxidant and anti-inflammatory. The chemical characterization of the açaí meal extract showed a diverse phenolic composition. A positive and strong correlation was found between total phenolic content and antiradical activity for FRAP and ABTS radical scavenging assays. This is the first time that the anti-inflammatory potential of açaí meal is assessed, with important results regarding the inhibitory effects of NF-κB activation and TNF-α release. The açaí meal did not show a promising potential for antimicrobial activity against bacteria belonging to the ESKAPE group.
Therefore, açaí meal could be considered a new natural source of antioxidant compounds with related biological activities for potential industrial applications. This work opens new possibilities for studies to investigate the effects of the açaí meal extract on lipid matrices as a natural antioxidant. By assessing the biological potential of açaí meal and determining its phenolic composition, the present study may impact the açaí production chain. Açaí meal is mostly discarded in the environment, but as shown here, it has great potential to become a value-added product. In summary, açaí meal could have great applicability in the food, pharmaceutical, and cosmetic industries. Future studies, should consider new lots and possible variations due to crop year/seasonality. Likewise, the findings observed in our study require validation in food systems or animal/human trials depending on the final application.
Acknowledgments
The authors gratefully acknowledge the funding agencies listed in the Funding section for providing the financial support for developing and publishing this paper, as well as the support of Gisandro Reis de Carvalho in contributing to the formatting of this paper.
Author Contributions
Conceptualization: methodology, investigation, formal analysis, data analysis, writing—original draft preparation, A.P.d.S.S.; writing—review and editing, A.C.d.C.; methodology, writing—original draft preparation, data analysis, review, J.G.L.; methodology, data analysis, writing— original draft, M.F.; methodology, data analysis, writing—original draft, review, J.d.C.O.S.; writing—review and editing, P.L.R.; writing—review and editing, resources, funding acquisition, supervision, S.M.d.A. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data that support the finding of this study are available from the corresponding authors upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico/CNPq (grant number 130777/2016-1); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brazil (CAPES)-Finance Code 001; São Paulo Research Foundation/FAPESP (process 2019/14358-0); and Citróleo Industry and Commerce of Essential Oils, LTDA, Torrinha, São Paulo, Brazil.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Leal C., Gouvinhas I., Santos R.A., Rosa E., Silva A.M., Saavedra M.J., Barros A.I. Potential application of grape (Vitis vinifera L.) stem extracts in the cosmetic and pharmaceutical industries: Valorization of a by-product. Ind. Crop. Prod. 2020;154:112675. doi: 10.1016/j.indcrop.2020.112675. [DOI] [Google Scholar]
- 2.Chang S.K., Alasalvar C., Shahidi F. Superfruits: Phytochemicals, antioxidant efficacies, and health effects—A comprehensive review. Crit. Rev. Food Sci. Nutr. 2018;59:1580–1604. doi: 10.1080/10408398.2017.1422111. [DOI] [PubMed] [Google Scholar]
- 3.Yamaguchi K.K.D.L., Pereira L.F.R., Lamarão C.V., Lima E.S., da Veiga-Junior V.F. Amazon acai: Chemistry and biological activities: A review. Food Chem. 2015;179:137–151. doi: 10.1016/j.foodchem.2015.01.055. [DOI] [PubMed] [Google Scholar]
- 4.IBGE The Brazilian Institute of Geography and Statistics. Production of Plant Extraction and Forestry. Vegetable Extraction: Table 1—Amount Produced and the Production Value of Brazil, Great Regions and Federal Units, According to the Extractive Products. [(accessed on 10 October 2022)];2021 Available online: https://www.ibge.gov.br/estatisticas/economicas/agricultura-e-pecuaria/9105-producao-da-extracao-vegetal-e-da-silvicultura.html?=&t=resultados.
- 5.Oliveira M.S.P., Schwartz G. Exotic Fruits. Academic Press; Cambridge, MA, USA: 2018. oleraceae; pp. 1–5. [Google Scholar]
- 6.Kang J., Xie C.H., Li Z.M., Nagarajan S., Schauss A.G., Wu T., Wu X.L. Flavonoids from acai (Euterpe oleraceae Mart.) pulp and their antioxidant and anti-inflammatory activities. Food Chem. 2011;128:152–157. doi: 10.1016/j.foodchem.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Embrapa . Empresa Brasileira de Pesquisa Agropecuária, Sistemas de Produção 4—Açaí. Embrapa Amazônia Oriental; Belém, PA, USA: 2005. p. 137. [Google Scholar]
- 8.Melo P.S., Selani M.M., Gonçalves R.H., Paulino J.d.O., Massarioli A.P., de Alencar S.M. Açaí seeds: An unexplored agro-industrial residue as a potential source of lipids, fibers, and antioxidant phenolic compounds. Ind. Crop. Prod. 2021;161:113204. doi: 10.1016/j.indcrop.2020.113204. [DOI] [Google Scholar]
- 9.Yi C., Shi J., Kramer J., Xue S., Jiang Y., Zhang M., Ma Y., Pohorly J. Fatty acid composition and phenolic antioxidants of winemaking pomace powder. Food Chem. 2009;114:570–576. doi: 10.1016/j.foodchem.2008.09.103. [DOI] [Google Scholar]
- 10.Hogan S., Chung H., Zhang L., Li J., Lee Y., Dai Y., Zhou K. Antiproliferative and antioxidant properties of anthocyanin-rich extract from açai. Food Chem. 2010;118:208–214. doi: 10.1016/j.foodchem.2009.04.099. [DOI] [Google Scholar]
- 11.Schreckinger M.E., Lotton J., Lila M.A., De Mejia E.G. Berries from South America: A Comprehensive Review on Chemistry, Health Potential, and Commercialization. J. Med. Food. 2010;13:233–246. doi: 10.1089/jmf.2009.0233. [DOI] [PubMed] [Google Scholar]
- 12.Rufino M.D.S.M., Pérez-Jiménez J., Arranz S., Alves R.E., de Brito E.S., Oliveira M.S., Saura-Calixto F. Açaí (Euterpe oleraceaee) ‘BRS Pará’: A tropical fruit source of antioxidant dietary fiber and high antioxidant capacity oil. Food Res. Int. 2011;44:2100–2106. doi: 10.1016/j.foodres.2010.09.011. [DOI] [Google Scholar]
- 13.Wong D.Y.S., Musgrave I.F., Harvey B.S., Smid S.D. Açaí (Euterpe oleraceaee Mart.) berry extract exerts neuroprotective effects against β-amyloid exposure in vitro. Neurosci. Lett. 2013;556:221–226. doi: 10.1016/j.neulet.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 14.Bonomo L.D.F., Silva D.N., Boasquivis P.F., Paiva F.A., Guerra J.F.D.C., Martins T.A.F., Torres G.D.J., de Paula I.T.B.R., Caneschi W.L., Jacolot P., et al. Açaí (Euterpe oleraceae Mart.) Modulates Oxidative Stress Resistance in Caenorhabditis elegans by Direct and Indirect Mechanisms. PLoS ONE. 2014;9:e89933. doi: 10.1371/journal.pone.0089933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferreira D.S., Gomes A.L., Da Silva M.G., Alves A.B., Agnol W.H.D., Ferrari R.A., Carvalho P.R.N., Pacheco M.T.B. Antioxidant Capacity and Chemical Characterization of Açaí (Euterpe oleraceae Mart.) Fruit Fractions. Food Sci. Technol. 2016;4:95–102. doi: 10.13189/fst.2016.040502. [DOI] [Google Scholar]
- 16.Linan L.Z., Cidreira A.C.M., da Rocha C.Q., de Menezes F.F., de Moraes Rocha G.J., Paiva A.E.M. Utilization of Acai Berry Residual Biomass for Extraction of Lignocellulosic Byproducts. J. Bioresour. Bioprod. 2021;6:323–337. doi: 10.1016/j.jobab.2021.04.007. [DOI] [Google Scholar]
- 17.Rossetto R., Maciel G.M., Bortolini D.G., Ribeiro V.R., Haminiuk C.W.I. Acai pulp and seeds as emerging sources of phenolic compounds for enrichment of residual yeasts (Saccharomyces cerevisiae) through biosorption process. LWT. 2020;128:109447. doi: 10.1016/j.lwt.2020.109447. [DOI] [Google Scholar]
- 18.Tyug T.S., Prasad K.N., Ismail A. Antioxidant capacity, phenolics and isoflavones in soybean by-products. Food Chem. 2010;123:583–589. doi: 10.1016/j.foodchem.2010.04.074. [DOI] [Google Scholar]
- 19.Zamindar N., Bashash M., Khorshidi F., Serjouie A., Shirvani M.A., Abbasi H., Sedaghatdoost A. Antioxidant efficacy of soybean cake extracts in soy oil protection. J. Food Sci. Technol. 2017;54:2077–2084. doi: 10.1007/s13197-017-2646-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halliwell B., Aeschbach R., Löliger J., Aruoma O. The characterization of antioxidants. Food Chem. Toxicol. 1995;33:601–617. doi: 10.1016/0278-6915(95)00024-V. [DOI] [PubMed] [Google Scholar]
- 21.Gomes A., Fernandes E., Silva A.M., Santos C.M., Pinto D.C., Cavaleiro J.A., Lima J.L. 2-Styrylchromones: Novel strong scavengers of reactive oxygen and nitrogen species. Bioorganic Med. Chem. 2007;15:6027–6036. doi: 10.1016/j.bmc.2007.06.046. [DOI] [PubMed] [Google Scholar]
- 22.Li H., Christman L.M., Li R., Gu L. Synergic interactions between polyphenols and gut microbiota in mitigating inflammatory bowel diseases. Food Funct. 2020;11:4878–4891. doi: 10.1039/D0FO00713G. [DOI] [PubMed] [Google Scholar]
- 23.Romão M.H., de Bem G., Santos I.B., Soares R.D.A., Ognibene D., de Moura R.S., da Costa C.A., Resende C. Açaí (Euterpe oleraceae Mart.) seed extract protects against hepatic steatosis and fibrosis in high-fat diet-fed mice: Role of local renin-angiotensin system, oxidative stress and inflammation. J. Funct. Foods. 2019;65:103726. doi: 10.1016/j.jff.2019.103726. [DOI] [Google Scholar]
- 24.Martinez R.M., Guimarães D.D.A.B., Berniz C.R., de Abreu J.P., da Rocha A.P.M., de Moura R.S., Resende A.C., Teodoro A.J. Açai (Euterpe oleraceae Mart.) Seed Extract Induces Cell Cycle Arrest and Apoptosis in Human Lung Carcinoma Cells. Foods. 2018;7:178. doi: 10.3390/foods7110178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wedick N.M., Pan A., Cassidy A., Rimm E.B., Sampson L., Rosner B., Willett W., Hu F.B., Sun Q., van Dam R.M. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am. J. Clin. Nutr. 2012;95:925–933. doi: 10.3945/ajcn.111.028894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Circu M.L., Aw T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010;48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carpes S.T., Pereira D., De Moura C., Dos Reis A.S., Da Silva L.D., Oldoni T.L.C., Almeida J.F., Plata-Oviedo M.V.S. Lyophilized and microencapsulated extracts of grape pomace from winemaking industry to prevent lipid oxidation in chicken pâté. Braz. J. Food Technol. 2020;23:e2019112. doi: 10.1590/1981-6723.11219. [DOI] [Google Scholar]
- 28.Silva A.P.D.S., Rosalen P.L., de Camargo A.C., Lazarini J.G., Rocha G., Shahidi F., Franchin M., de Alencar S.M. Inajá oil processing by-product: A novel source of bioactive catechins and procyanidins from a Brazilian native fruit. Food Res. Int. 2021;144:110353. doi: 10.1016/j.foodres.2021.110353. [DOI] [PubMed] [Google Scholar]
- 29.Singleton V.L., Orthofer R., Lamuela-Raventós R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth. Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- 30.Melo P.S., Massarioli A.P., Denny C., dos Santos L.F., Franchin M., Pereira G.E., Vieira T.M.F.D.S., Rosalen P.L., de Alencar S.M. Winery by-products: Extraction optimization, phenolic composition and cytotoxic evaluation to act as a new source of scavenging of reactive oxygen species. Food Chem. 2015;181:160–169. doi: 10.1016/j.foodchem.2015.02.087. [DOI] [PubMed] [Google Scholar]
- 31.Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. CLSI; Wayne, PA, USA: 2008. [Google Scholar]
- 32.Reference Method for Broth Dilution antibacterial Susceptibility Testing of Bacterial. CLSI; Wayne, PA, USA: 2012. [Google Scholar]
- 33.Garzón G.A., Narváez-Cuenca C.-E., Vincken J.-P., Gruppen H. Polyphenolic composition and antioxidant activity of açai (Euterpe oleraceae Mart.) from Colombia. Food Chem. 2017;217:364–372. doi: 10.1016/j.foodchem.2016.08.107. [DOI] [PubMed] [Google Scholar]
- 34.Fernandes I., Faria A., Azevedo J., Soares S., Calhau C., De Freitas V., Mateus N. Influence of Anthocyanins, Derivative Pigments and Other Catechol and Pyrogallol-Type Phenolics on Breast Cancer Cell Proliferation. J. Agric. Food Chem. 2010;58:3785–3792. doi: 10.1021/jf903714z. [DOI] [PubMed] [Google Scholar]
- 35.Tan C., Kong Y., Tong Y., Deng H., Wang M., Zhao Y., Wan M., Lin S., Liu X., Meng X., et al. Anti-apoptotic effects of high hydrostatic pressure treated cyanidin-3-glucoside and blueberry pectin complexes on lipopolysaccharide-induced inflammation in Caco-2 cells. J. Funct. Foods. 2021;86:104709. doi: 10.1016/j.jff.2021.104709. [DOI] [Google Scholar]
- 36.Ma M.-M., Li Y., Liu X.-Y., Zhu W.-W., Ren X., Kong G.-Q., Huang X., Wang L.-P., Luo L.-Q., Wang X.-Z. Cyanidin-3-O-glucoside Ameliorates Lipopolysaccharide-Induced Injury Both In Vivo and In Vitro Suppression of NF-κB and MAPK Pathways. Inflammation. 2015;38:1669–1682. doi: 10.1007/s10753-015-0144-y. [DOI] [PubMed] [Google Scholar]
- 37.Li Z.-H., Guo H., Xu W.-B., Ge J., Li X., Alimu M., He D.-J. Rapid Identification of Flavonoid Constituents Directly from PTP1B Inhibitive Extract of Raspberry (Rubus idaeus L.) Leaves by HPLC–ESI–QTOF–MS-MS. J. Chromatogr. Sci. 2016;54:805–810. doi: 10.1093/chromsci/bmw016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Rosso V., Hillebrand S., Montilla E.C., Bobbio F.O., Winterhalter P., Mercadante A.Z. Determination of anthocyanins from acerola (Malpighia emarginata DC.) and açai (Euterpe oleraceae Mart.) by HPLC–PDA–MS/MS. J. Food Compos. Anal. 2008;21:291–299. doi: 10.1016/j.jfca.2008.01.001. [DOI] [Google Scholar]
- 39.Zhang Y., Seeram N.P., Lee R., Feng L., Heber D. Isolation and Identification of Strawberry Phenolics with Antioxidant and Human Cancer Cell Antiproliferative Properties. J. Agric. Food Chem. 2008;56:670–675. doi: 10.1021/jf071989c. [DOI] [PubMed] [Google Scholar]
- 40.Yoshimura Y., Zaima N., Moriyama T., Kawamura Y. Different Localization Patterns of Anthocyanin Species in the Pericarp of Black Rice Revealed by Imaging Mass Spectrometry. PLoS ONE. 2012;7:e31285. doi: 10.1371/journal.pone.0031285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen S., Zhou H., Zhang G., Meng J., Deng K., Zhou W., Wang H., Wang Z., Hu N., Suo Y. Anthocyanins from Lycium ruthenicum Murr. Ameliorated d-galactose-inducedmemory impairment, oxidative stress, and neuroinfammation in adult rats. J. Agric. Food Chem. 2019;67:3140–3149. doi: 10.1021/acs.jafc.8b06402. [DOI] [PubMed] [Google Scholar]
- 42.Jugran A.K., Rawat S., Devkota H.P., Bhatt I.D., Rawal R.S. Diabetes andplant-derived natural products: From ethnopharmacological approaches to theirpotential for modern drug discovery and development. Phytoth. Res. 2022;35:223–245. doi: 10.1002/ptr.6821. [DOI] [PubMed] [Google Scholar]
- 43.Wei J., Zhang G., Zhang X., Xu D., Gao J., Fan J., Zhou Z. Anthocyanins from Black Chokeberry (Aroniamelanocarpa Elliot) Delayed Aging-Related Degenerative Changes of Brain. J. Agric. Food Chem. 2017;65:5973–5984. doi: 10.1021/acs.jafc.7b02136. [DOI] [PubMed] [Google Scholar]
- 44.Liu F., Zhao F., Wang W., Sang J., Jia L., Li L., Lu F. Cyanidin-3-O-glucoside inhibits Aβ40 fibrillogenesis, disintegrates preformed fibrils, and reduces amyloid cytotoxicity. Food Funct. 2020;11:2573–2587. doi: 10.1039/C9FO00316A. [DOI] [PubMed] [Google Scholar]
- 45.Li L., Zhou P., Wang Y., Pan Y., Chen M., Tian Y., Zhou H., Yang B., Meng H., Zheng J. Antimicrobial activity of cyanidin-3-O-glucoside–lauric acid ester against Staphylococcus aureus and Escherichia coli. Food Chem. 2022;383:132410. doi: 10.1016/j.foodchem.2022.132410. [DOI] [PubMed] [Google Scholar]
- 46.Soares M.J., Sampaio G.R., Guizellini G.M., Figueira M.S., Pinaffi A.C.D.C., Freitas R.A.M.S., Shahidi F., de Camargo A.C., Torres E.A.F.D.S. Regular and decaffeinated espresso coffee capsules: Unravelling the bioaccessibility of phenolic compounds and their antioxidant properties in milk model system upon in vitro digestion. LWT. 2020;135:110255. doi: 10.1016/j.lwt.2020.110255. [DOI] [Google Scholar]
- 47.Naveed M., Hejazi V., Abbas M., Kamboh A.A., Khan G.J., Shumzaid M., Ahmad F., Babazadeh D., Xia F.F., Modarresi-Ghazani F., et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018;97:67–74. doi: 10.1016/j.biopha.2017.10.064. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y., Xiong H., Xu X., Xue X., Liu M., Xu S., Liu H., Gao Y., Zhang H., Li X. Compounds Identification in Semen Cuscutae by Ultra-High-Performance Liquid Chromatography (UPLCs) Coupled to Electrospray Ionization Mass Spectrometry. Molecules. 2018;23:1199. doi: 10.3390/molecules23051199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kolniak-Ostek J., Oszmiański J. Characterization of phenolic compounds in different anatomical pear (Pyrus communis L.) parts by ultra-performance liquid chromatography photodiode detector-quadrupole/time of flight-mass spectrometry (UPLC-PDA-Q/TOF-MS) Int. J. Mass Spectrom. 2015;392:154–163. doi: 10.1016/j.ijms.2015.10.004. [DOI] [Google Scholar]
- 50.Brunschwig C., Leba L.-J., Saout M., Martial K., Bereau D., Robinson J.-C. Chemical Composition and Antioxidant Activity of Euterpe oleraceae Roots and Leaflets. Int. J. Mol. Sci. 2016;18:61. doi: 10.3390/ijms18010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhong L., Lin Y., Wang C., Niu B., Xu Y., Zhao G., Zhao J. Chemical Profile, Antimicrobial and Antioxidant Activity Assessment of the Crude Extract and Its Main Flavonoids from Tartary Buckwheat Sprouts. Molecules. 2022;27:374. doi: 10.3390/molecules27020374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferreres F., Silva B.M., Andrade P.B., Seabra R.M., Ferreira M.A. Approach to the study of C-glycosyl flavones by ion trap HPLC-PAD-ESI/MS/MS: Application to seeds of quince (Cydonia oblonga) Phytochem. Anal. 2003;14:352–359. doi: 10.1002/pca.727. [DOI] [PubMed] [Google Scholar]
- 53.Kim M.K., Yun K.J., Lim D.H., Kim J., Jang Y.P. Anti-Inflammatory Properties of Flavone di-C-Glycosides as Active Principles of Camellia Mistletoe, Korthalsella japonica. Biomol. Ther. 2016;24:630–637. doi: 10.4062/biomolther.2016.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Odontuya G., Hoult J.R.S., Houghton P.J. Structure-activity relationship for antiinflammatory effect of luteolin and its derived glycosides. Phytother. Res. 2005;19:782–786. doi: 10.1002/ptr.1723. [DOI] [PubMed] [Google Scholar]
- 55.Francisco V., Figueirinha A., Costa G., Liberal J., Lopes M.C., García-Rodríguez C., Geraldes C.F., Cruz M.T., Batista M.T. Chemical characterization and anti-inflammatory activity of luteolin glycosides isolated from lemongrass. J. Funct. Foods. 2014;10:436–443. doi: 10.1016/j.jff.2014.07.003. [DOI] [Google Scholar]
- 56.Jang D., Jung Y.S., Kim M.-S., Oh S.E., Nam T.G., Kim D.-O. Developing and Validating a Method for Separating Flavonoid Isomers in Common Buckwheat Sprouts Using HPLC-PDA. Foods. 2019;8:549. doi: 10.3390/foods8110549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen G.-L., Mutie F.M., Xu Y.-B., Saleri F.D., Hu G.-W., Guo M.-Q. Antioxidant, Anti-inflammatory Activities and Polyphenol Profile of Rhamnus prinoides. Pharmaceuticals. 2020;13:55. doi: 10.3390/ph13040055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martini N., Katerere D., Eloff J. Biological activity of five antibacterial flavonoids from Combretum erythrophyllum (Combretaceae) J. Ethnopharmacol. 2004;93:207–212. doi: 10.1016/j.jep.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 59.Bodakhe S.H., Ram A., Verma S., Pandey D.P. Anticataract activity of rhamnocitrin isolated from Bauhinia variegata stem bark. Orient. Pharm. Exp. Med. 2012;12:227–232. doi: 10.1007/s13596-012-0059-1. [DOI] [Google Scholar]
- 60.Cuoco G., Mathe C., Vieillescazes C. Liquid chromatographic analysis of flavonol compounds in green fruits of three Rhamnus species used in Stil de grain. Microchem. J. 2014;115:130–137. doi: 10.1016/j.microc.2014.03.006. [DOI] [Google Scholar]
- 61.Kim B.-R., Paudel S.B., Han A.-R., Park J., Kil Y.-S., Choi H., Jeon Y.G., Park K.Y., Kang S.-Y., Jin C.H., et al. Metabolite Profiling and Dipeptidyl Peptidase IV Inhibitory Activity of Coreopsis Cultivars in Different Mutations. Plants. 2021;10:1661. doi: 10.3390/plants10081661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen M., Zhang X., Wang H., Lin B., Wang S., Hu G. Determination of Rutin in Rat Plasma by Ultra Performance Liquid Chromatography Tandem Mass Spectrometry and Application to Pharmacokinetic Study. J. Chromatogr. Sci. 2015;53:519–525. doi: 10.1093/chromsci/bmu078. [DOI] [PubMed] [Google Scholar]
- 63.Ben Sghaier M., Pagano A., Mousslim M., Ammari Y., Kovacic H., Luis J. Rutin inhibits proliferation, attenuates superoxide production and decreases adhesion and migration of human cancerous cells. Biomed. Pharmacother. 2016;84:1972–1978. doi: 10.1016/j.biopha.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 64.Zhou C., Xie Z., Lei Z., Huang Y., Wei G. Simultaneous identification and determination of flavonoids in Dendrobium officinale. Chem. Central J. 2018;12:40. doi: 10.1186/s13065-018-0403-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dias A.L.S., Rozet E., Larondelle Y., Hubert P., Rogez H., Quetin-Leclercq J. Development and validation of an UHPLC-LTQ-Orbitrap MS method for non-anthocyanin flavonoids quantification in Euterpe oleraceae juice. Anal. Bioanal. Chem. 2013;405:9235–9249. doi: 10.1007/s00216-013-7325-z. [DOI] [PubMed] [Google Scholar]
- 66.Ho K.-V., Lei Z., Sumner L.W., Coggeshall M.V., Hsieh H.-Y., Stewart G.C., Lin C.-H. Identifying Antibacterial Compounds in Black Walnuts (Juglans nigra) Using a Metabolomics Approach. Metabolites. 2018;8:58. doi: 10.3390/metabo8040058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee J.K. Anti-inflammatory effects of eriodictyol in lipopolysaccharidestimulated raw 264.7 murine macrophages. Arch. Pharmacal Res. 2011;34:671–679. doi: 10.1007/s12272-011-0418-3. [DOI] [PubMed] [Google Scholar]
- 68.Mulabagal V., Calderón A.I. Liquid chromatography/mass spectrometry based fingerprinting analysis and mass profiling of Euterpe oleraceae (açaí) dietary supplement raw materials. Food Chem. 2012;134:1156–1164. doi: 10.1016/j.foodchem.2012.02.123. [DOI] [PubMed] [Google Scholar]
- 69.Prior R.L., Cao G., Martin A., Sofic E., McEwen J., O’Brien C., Lischner N., Ehlenfeldt M., Kalt W., Krewer A.G., et al. Antioxidant Capacity As Influenced by Total Phenolic and Anthocyanin Content, Maturity, and Variety of Vaccinium Species. J. Agric. Food Chem. 1998;46:2686–2693. doi: 10.1021/jf980145d. [DOI] [Google Scholar]
- 70.de Camargo A.C., Regitano-D’Arce M.A.B., Biasoto A.C.T., Shahidi F. Low Molecular Weight Phenolics of Grape Juice and Winemaking Byproducts: Antioxidant Activities and Inhibition of Oxidation of Human Low-Density Lipoprotein Cholesterol and DNA Strand Breakage. J. Agric. Food Chem. 2014;62:12159–12171. doi: 10.1021/jf504185s. [DOI] [PubMed] [Google Scholar]
- 71.Ayoub M., de Camargo A.C., Shahidi F. Antioxidants and bioactivities of free, esterified and insoluble-bound phenolics from berry seed meals. Food Chem. 2016;197:221–232. doi: 10.1016/j.foodchem.2015.10.107. [DOI] [PubMed] [Google Scholar]
- 72.Butkevičiūtė A., Urbštaitė R., Liaudanskas M., Obelevičius K., Janulis V. Phenolic Content and Antioxidant Activity in Fruit of the Genus Rosa L. Antioxidants. 2022;11:912. doi: 10.3390/antiox11050912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kang J., Thakali K.M., Xie C., Kondo M., Tong Y., Ou B., Jensen G., Medina M.B., Schauss A.G., Wu X. Bioactivities of açaí (Euterpe precatoria Mart.) fruit pulp, superior antioxidant and anti-inflammatory properties to Euterpe oleraceae Mart. Food Chem. 2012;133:671–677. doi: 10.1016/j.foodchem.2012.01.048. [DOI] [Google Scholar]
- 74.Batista C.D.C.R., de Oliveira M.S., Araújo M.E., Rodrigues A.M.C., Botelho J.R.S., da Silva Souza Filho A.P., Machado N., Carvalho Junior R.N. Supercritical CO 2 extraction of açaí (Euterpe oleraceae) berry oil: Global yield, fatty acids, allelopathic activities, and determination of phenolic and anthocyanins total compounds in the residual pulp. J. Supercrit. Fluids. 2016;107:364–369. doi: 10.1016/j.supflu.2015.10.006. [DOI] [Google Scholar]
- 75.Sette P., Fernandez A., Soria J., Rodriguez R., Salvatori D., Mazza G. Integral valorization of fruit waste from wine and cider industries. J. Clean. Prod. 2020;242:118486. doi: 10.1016/j.jclepro.2019.118486. [DOI] [Google Scholar]
- 76.Kumar M., Dahuja A., Tiwari S., Punia S., Tak Y., Amarowicz R., Bhoite A.G., Singh S., Joshi S., Panesar P.S., et al. Recent trends in extraction of plant bioactives using green technologies: A review. Food Chem. 2021;353:129431. doi: 10.1016/j.foodchem.2021.129431. [DOI] [PubMed] [Google Scholar]
- 77.Tremocoldi M.A., Rosalen P.L., Franchin M., Massarioli A.P., Denny C., Daiuto E.R., Paschoal J.A.R., Melo P.S., de Alencar S.M. Exploration of avocado by-products as natural sources of bioactive compounds. PLoS ONE. 2018;13:e0192577. doi: 10.1371/journal.pone.0192577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de Moura C., dos Reis A.S., da Silva L.D., de Lima V.A., Oldoni T.L.C., Pereira C., Carpes S.T. Optimization of phenolic compounds extraction with antioxidant activity from acai, blueberry and goji berry using response surface methodology. Emir. J. Food Agric. 2018:180–189. doi: 10.9755/ejfa.2018.v30.i3.1639. [DOI] [Google Scholar]
- 79.Silva F., De Miranda D., Carnier M., Maza P., Boldarine V., Rischiteli A.S., Avila F., Pontes L., Hachul A., Neto N., et al. Low dose of Juçara pulp (Euterpe edulis Mart.) minimizes the colon inflammatory milieu promoted by hypercaloric and hyperlipidic diet in mice. J. Funct. Foods. 2021;77:104343. doi: 10.1016/j.jff.2020.104343. [DOI] [Google Scholar]
- 80.Morais D.R., Rotta E.M., Sargi S.C., Schmidt E.M., Bonafe E.G., Eberlin M.N., Sawaya A.C., Visentainer J.V. Antioxidant activity, phenolics and UPLC–ESI(–)–MS of extracts from different tropical fruits parts and processed peels. Food Res. Int. 2015;77:392–399. doi: 10.1016/j.foodres.2015.08.036. [DOI] [Google Scholar]
- 81.Tiveron A.P., Melo P.S., Bergamaschi K.B., Vieira T.M.F.D.S., Regitano-D’Arce M.A.B., De Alencar S.M. Antioxidant Activity of Brazilian Vegetables and Its Relation with Phenolic Composition. Int. J. Mol. Sci. 2012;13:8943–8957. doi: 10.3390/ijms13078943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Infante J., Rosalen P.L., Lazarini J.G., Franchin M., De Alencar S.M. Antioxidant and Anti-Inflammatory Activities of Unexplored Brazilian Native Fruits. PLoS ONE. 2016;11:e0152974. doi: 10.1371/journal.pone.0152974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lazarini J.G., Franchin M., Soares J.C., Nani B.D., Massarioli A.P., De Alencar S.M., Rosalen P.L. Anti-inflammatory and antioxidant potential, in vivo toxicity, and polyphenolic composition of Eugenia selloi B.D.Jacks. (pitangatuba), a Brazilian native fruit. PLoS ONE. 2020;15:e0234157. doi: 10.1371/journal.pone.0234157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Soares J.C., Rosalen P.L., Lazarini J.G., Massarioli A.P., da Silva C.F., Nani B.D., Franchin M., de Alencar S.M. Comprehensive characterization of bioactive phenols from new Brazilian superfruits by LC-ESI-QTOF-MS, and their ROS and RNS scavenging effects and anti-inflammatory activity. Food Chem. 2019;281:178–188. doi: 10.1016/j.foodchem.2018.12.106. [DOI] [PubMed] [Google Scholar]
- 85.da Silveira T.F.F., de Souza T.C.L., Carvalho A.V., Ribeiro A.B., Kuhnle G.G., Godoy H.T. White açaí juice (Euterpe oleraceae): Phenolic composition by LC-ESI-MS/MS, antioxidant capacity and inhibition effect on the formation of colorectal cancer related compounds. J. Funct. Foods. 2017;36:215–223. doi: 10.1016/j.jff.2017.07.001. [DOI] [Google Scholar]
- 86.Rodrigues E., Mariutti L.R.B., Mercadante A.Z. Carotenoids and Phenolic Compounds from Solanum sessiliflorum, an Unexploited Amazonian Fruit, and Their Scavenging Capacities against Reactive Oxygen and Nitrogen Species. J. Agric. Food Chem. 2013;61:3022–3029. doi: 10.1021/jf3054214. [DOI] [PubMed] [Google Scholar]
- 87.Sprenger L.K., Giese E.G., Dos Santos J.N., Molento M.B. In vitro antibacterial effect of Euterpe oleraceae Mart. and Theobroma grandiflorum hydroalcoholic extracts. Arch. Vet. Sci. 2016;21:21–32. doi: 10.5380/avs.v21i2.43627. [DOI] [Google Scholar]
- 88.Dias-Souza M.V., dos Santos R.M., Cerávolo I.P., Cosenza G., Marçal P.H.F., Figueiredo F.J.B. Euterpe oleraceae pulp extract: Chemical analyses, antibiofilm activity against Staphylococcus aureus, cytotoxicity and interference on the activity of antimicrobial drugs. Microb. Pathog. 2018;114:29–35. doi: 10.1016/j.micpath.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 89.Golovinskaia O., Wang C.-K. Review of Functional and Pharmacological Activities of Berries. Molecules. 2021;26:3904. doi: 10.3390/molecules26133904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang H., Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016;8:33–42. doi: 10.1016/j.cofs.2016.02.002. [DOI] [Google Scholar]
- 91.Xie C., Kang J., Li Z., Schauss A., Badger T.M., Nagarajan S., Wu T., Wu X. The açaí flavonoid velutin is a potent anti-inflammatory agent: Blockade of LPS-mediated TNF-α and IL-6 production through inhibiting NF-κB activation and MAPK pathway. J. Nutr. Biochem. 2012;23:1184–1191. doi: 10.1016/j.jnutbio.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 92.Brito C., Stavroullakis A., Oliveira T., Prakki A. Cytotoxicity and potential anti-inflammatory activity of velutin on RAW 264.7 cell line differentiation: Implications in periodontal bone loss. Arch. Oral Biol. 2017;83:348–356. doi: 10.1016/j.archoralbio.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 93.Daglia M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012;23:174–181. doi: 10.1016/j.copbio.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 94.Nile S.H., Park S.W. Edible berries: Bioactive components and their effect on human health. Nutrition. 2014;30:134–144. doi: 10.1016/j.nut.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 95.Xue J., Davidson P.M., Zhong Q. Thymol Nanoemulsified by Whey Protein-Maltodextrin Conjugates: The Enhanced Emulsifying Capacity and Antilisterial Properties in Milk by Propylene Glycol. J. Agric. Food Chem. 2013;61:12720–12726. doi: 10.1021/jf4043437. [DOI] [PubMed] [Google Scholar]
- 96.Ultee A., Bennik M.H.J., Moezelaar R. The Phenolic Hydroxyl Group of Carvacrol Is Essential for Action against the Food-Borne Pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002;68:1561–1568. doi: 10.1128/AEM.68.4.1561-1568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020;83:770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- 98.Freires I.A., Denny C., Benso B., De Alencar S.M., Rosalen P.L. Antibacterial Activity of Essential Oils and Their Isolated Constituents against Cariogenic Bacteria: A Systematic Review. Molecules. 2015;20:7329–7358. doi: 10.3390/molecules20047329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the finding of this study are available from the corresponding authors upon reasonable request.