Abstract
Simple Summary
Proton pump inhibitors (PPIs) are the most commonly used gastric acid suppressants in cancer patients. However, long-term use of PPIs can cause dysbiosis effects disrupting gut microbiota with subsequent impairment of the effectiveness of immune checkpoint inhibitors (ICIs). Our study demonstrates that, in advanced non-small cell lung cancer and urothelial carcinoma, patients receiving ICIs with concomitant PPIs are associated with poorer survival outcomes, when compared not only with those without PPIs but also with patients treated with chemotherapy, implying that PPIs could compromise the effectiveness of ICIs, causing them to be less effective than chemotherapy. As a result, we suggest that clinicians should avoid unnecessary PPI prescription in these patients. Conversely, there is little survival association with PPI in patients with melanoma, renal cell carcinoma, hepatocellular carcinoma, and squamous cell carcinoma of the neck and head. Nevertheless, future high quality prospective studies including more cancer types are warranted.
Abstract
(1) Although emerging evidence suggests that proton pump inhibitor (PPI)-induced dysbiosis negatively alters treatment response to immune checkpoint inhibitors (ICIs) in cancer patients, no study systematically investigates the association between PPIs, ICIs, and chemotherapy; (2) Cochrane Library, Embase, Medline, and PubMed were searched from inception to 20 May 2022, to identify relevant studies involving patients receiving ICIs or chemotherapy and reporting survival outcome between PPI users and non-users. Survival outcomes included overall survival (OS) and progression-free survival (PFS). Network meta-analyses were performed using random-effects models. p-scores, with a value between 0 and 1, were calculated to quantify the treatment ranking, with a higher score suggesting a higher probability of greater effectiveness. We also conducted pairwise meta-analyses of observational studies to complement our network meta-analysis; (3) We identified 62 studies involving 26,484 patients (PPI = 8834; non-PPI = 17,650), including non-small cell lung cancer (NSCLC), urothelial carcinoma (UC), melanoma, renal cell carcinoma (RCC), hepatocellular carcinoma (HCC), and squamous cell carcinoma (SCC) of the neck and head. Eight post-hoc analyses from 18 randomized–controlled trials were included in our network, which demonstrated that, in advanced NSCLC and UC, patients under ICI treatment with concomitant PPI (p-score: 0.2016) are associated with both poorer OS (HR, 1.49; 95% CI, 1.37 to 1.67) and poorer PFS (HR, 1.41; 95% CI, 1.25 to 1.61) than those without PPIs (p-score: 1.000). Patients under ICI treatment with concomitant PPI also had poorer OS (HR, 1.18; 95% CI, 1.07 to 1.31) and poorer PFS (HR, 1.30; 95% CI, 1.14 to 1.48) in comparison with those receiving chemotherapy (p-score: 0.6664), implying that PPIs may compromise ICI’s effectiveness, making it less effective than chemotherapy. Our pairwise meta-analyses also supported this association. Conversely, PPI has little effect on patients with advanced melanoma, RCC, HCC, and SCC of the neck and head who were treated with ICIs; (4) “PPI-induced dysbiosis” serves as a significant modifier of treatment response in both advanced NSCLC and UC that are treated with ICIs, compromising the effectiveness of ICIs to be less than that of chemotherapy. Thus, clinicians should avoid unnecessary PPI prescription in these patients. “PPI-induced dysbiosis”, on the other hand, does not alter the treatment response to ICIs in advanced melanoma, RCC, HCC, and SCC of the head and neck.
Keywords: proton pump inhibitors, immune checkpoint inhibitors, chemotherapy
1. Introduction
Although clinical trials have shown that immune checkpoint inhibitors (ICIs) provide significant efficacy over non-ICIs comparators in many advanced cancers reshaping their therapeutic landscape [1,2,3,4], only a certain proportion of cancer patients have demonstrated a meaningful treatment response to ICIs. Considering metastatic castration-resistant prostate cancer as an example, pembrolizumab, the only FDA approved ICI for prostate cancer, is used predominantly in patients with high microsatellite instability (MSI-H) [5]. Therefore, identifying prognostic predictors and biomarkers for treatment response in patients taking ICIs is crucial, as it helps determine the subgroups of cancer patients who would benefit most from ICIs, improving the precision of therapeutic management. In fact, a variety of clinicopathological features, including age, gender, the Eastern Cooperative Oncology Group (ECOG) performance status, tumor mutation burden, and programmed death ligand 1 (PD-L1) expression, have been discovered as potential modifiers of treatment response in patients treated with ICIs [6,7,8]. Concomitant use of various medications has also been investigated to determine whether they alter the effectiveness of ICIs [9,10]. Notably, PPIs, commonly prescribed gastric acid suppressants for cancer patients, are well-known for their dysbiosis effects that disrupts gut microbiota, which theoretically impairs patients’ response to both ICIs and chemotherapy [11,12].
Six meta-analyses [13,14,15,16,17,18] have investigated the survival impacts of concomitant PPI on the effectiveness of ICI, but their results are contradictory. One [14] demonstrated negligible survival influence of PPI on ICI. Another three [15,17,18] alluded that PPI could negatively affect ICI in advanced cancers, while the remaining two syntheses [13,16] implied melanoma patients may derive survival benefits due to lower disease recurrence. Despite these six meta-analyses, there has been little investigation and discussion on the interaction of PPI against ICI and chemotherapy. Although a subgroup analysis of trials of IMvigor 211 [19] and IMpower150 [20] showed that PPIs significantly compromised the benefit of ICIs over chemotherapy, another exploratory analysis of OAK [21] and POPLAR [22] demonstrated insignificant interaction.
Given the significant increased indications of ICIs due to actively broadening cancer types and the extensive use of PPIs, an updated synthesis is urgently warranted. Thus, our study contains two main objectives; firstly, we aim to investigate the association among PPIs, ICIs, and chemotherapy by conducting a network meta-analysis (NMA); secondly, we will update the comparative survival outcomes of PPI users and non-users in patients receiving ICIs and also in patients taking chemotherapy with a wider range of cancers.
2. Methods
We performed the present systematic review and meta-analysis based on the Cochrane Handbook for Systematic Reviews of Interventions [23] and reported results in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Network Meta-analysis extension statement, and Meta-analysis Of Observational Studies in Epidemiology guidelines (Supplementary Method 1–3). The study was registered on PROSPERO (CRD 42021258800).
2.1. Study Selection
PubMed, Medline, Embase, and Cochrane Library were searched, from inception until 20 May 2022. Three investigators (W.Y.L, Y.C, and Y.C.C) independently identified relevant studies, and discrepancies were addressed by reaching a consensus with senior reviewers (K.Y.C and Y.N.K). Search details are presented in Supplementary Method 4.
2.2. Eligibility Criteria
Three predefined criteria for evidence selection were as follows: (1) randomized–controlled trials (RCTs), prospective, or retrospective cohort studies; (2) studies involving adult patients aged over 18 with cancers receiving ICIs or chemotherapy without concurrent radiotherapy; (3) studies reporting at least one comparative survival outcome between PPI users and non-users, overall survival (OS), or progression-free survival (PFS) (PPI versus non-PPI users), irrespective of indications.
2.3. Data Extraction
Three investigators (H.C.C., E.A, and T.H.W) independently extracted relevant information from eligible articles. Details are available in Supplementary Method 5.
2.4. Quality Assessment
Two investigators (T.H.T and K.Y.C) independently completed a critical appraisal of the included literature by using the Cochrane Risk of Bias tool 2.0 for RCTs, and the Risk of Bias in Non-randomised Studies–of Interventions (ROBINS-I) tool for non-RCTs. Any discrepancy was addressed through discussion with the third investigator (Y.N.K).
2.5. Assessment of Transitivity Assumption
A pivotal concept of NMA is that patients in the network are jointly randomizable. One plausible assessment of transitivity assumption is to place included trials under scrutiny to examine whether important effect modifiers are similarly distributed throughout the network [24]. We pre-specified age, gender, ECOG performance status, and PDL-1 expression status as effect modifiers since these factors are known prognostic factors for cancer patients.
2.6. Main Outcomes and Statistical Analysis
OS and PFS were pooled by obtaining the unadjusted hazard ratio (HR) extracted directly from each reference. When studies did not report the HR but presented Kaplan–Meier survival curves instead, we acquired an estimated HR from the curves through a well-established method [25], i.e., by using a calculation spreadsheet developed by Tierney and colleagues [26]. For baseline effect modifiers, we used weighted mean difference (WMD) and risk ratio (RR) through an inverse variance method to pool continuous and binary characteristics, respectively. When continuous outcomes are not reported as mean with standard difference but instead median with interquartile or range format, which cannot be used for quantitative syntheses, we utilized a well-established and well-validated tool [27] to convert the data to an appropriate format for syntheses. All estimated effects were presented with a 95% confidence interval (CI).
Network and head-to-head meta-analysis were conducted using RStudio with the ‘netmeta’ and ‘meta’ package, respectively (Supplementary Method 5). For the NMA, we produced a network graph to illustrate the structure of evidence followed by league tables for summary of frequentist NMA using a random-effect model. Regarding the league table, interventions were ranked by their p-scores with the netrank function; p-scores were a value between 0 and 1, with a higher score suggesting a higher probability of greater effectiveness. Forest plots of HRs and 95% CIs were generated with “chemotherapy” as the reference. Inconsistency was evaluated through the netsplit function https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2773396, accessed on 6 June 2022—zoi200800r45 and displayed via heat plots with the netheat command. We also performed sensitivity analyses by excluding trials of potential source of inconsistency, unknown PDL-1, and treatment line. Function ‘netmeta::funnel’ was used for depicting the comparison-adjusted funnel plot, which is a common method for depicting publication bias in NMA.
For pairwise meta-analysis, the pooled estimate was based on random-effects with the restricted maximum likelihood method due to inevitable between-trial variance. Heterogeneity was assessed using I-square [28], with values of I2 < 25%, 25% < I2 <50%, and I2 > 50% indicating low, moderate, and high heterogeneity, respectively. Pre-specified sensitivity analyses include subgroup analyses based on cancer types as well as treatment line, and exclusion of studies subject to high risk of bias. Determination of statistical significance in these analyses followed common threshold (p < 0.05). Additionally, function ‘funnel’ and ‘metabias’ are used for examining publication bias when a head-to-head meta-analysis with more than 10 studies.
3. Results
The study identified 21,059 references, with 102 studies for full-text inspection, among which 40 studies did not meet the eligibility criteria (Supplementary Results 1). Eventually, we included a total of 62 studies [9,10,19,20,21,22,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83] for qualitative and quantitative syntheses (Figure 1).
Figure 1.
PRISMA flowchart diagram. We initially extracted a total of 21,059 potential references, including 12,060 from PubMed, 7424 from Embase, 932 from Medline, and 643 from CENTRAL. After a duplicate exclusion, 18,962 studies were identified. Screening the titles and abstracts yielded 102 full-text articles, the eligibility of which was assessed. Forty studies were excluded after reading whole texts owing to reasons elaborated in Supplementary Results 1. Eventually, 62 studies fulfilled the eligibility criteria and were included for qualitative and quantitative syntheses. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
3.1. Characteristics of Included Studies
Table 1 demonstrates there are thirty-six retrospective studies [9,10,29,30,31,34,37,38,40,41,42,47,49,50,52,55,56,57,58,59,60,61,62,64,65,67,68,69,70,72,74,75,76,77,79,80] and eight were post hoc analyses [35,36,44,45,46,53,54,78] from 18 RCTs [19,20,21,22,32,33,39,43,48,51,63,66,71,73,83,84,85,86,87,88,89,90,91], involving 26,484 patients (PPI = 8834; non-PPI = 17,650) enrolled between 2000 and 2022.
Table 1.
Study characteristics.
| Included Studies | Study Type | Country | Inclusion Period |
Sample Size, n | Therapeutic Modality | Treatment-Naïve, n (%) |
PPI *, n (%) |
PPI Use Window | H2-Blocker, n (%) |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Immune checkpoint inhibitors (n= 36) | CTLA-4, n (%) | PD-1, n (%) | PD-L1, n (%) | ||||||||
| Advanced NSCLC (n = 17) | |||||||||||
| IMpower130 [71] | RCT | 8 countries | 2015–2017 | 483 | 0 | 0 | 483 (100) | 483 (100) | N/A¶ | Following Rx | N/A |
| IMpower131 [51] | RCT | 26 countries | 2015–2017 | 681 | 0 | 0 | 681 (100) | 681 (100) | N/A¶ | Following Rx | N/A |
| IMpower150 [20] | RCT | 26 countries | 2015–2016 | 802 | 0 | 0 | 802 (100) | N/A | 290 (36.2) | 30 d before/after Rx | N/A |
| POPLAR [22] | RCT | USA/Europe | 2013–2014 | 144 | 0 | 0 | 144 (100) | 0 (0) | N/A | 30 d before/after Rx | N/A |
| OAK [21] | RCT | 31 countries | 2014–2015 | 613 | 0 | 0 | 613 (100) | 0 (0) | N/A | 30 d before/after Rx | N/A |
| Baek 2022 [31] | Retrospective | South Korea | 2017–2018 | 1646 | 0 | 1595 (97) | 51 (3) | 0 (0) | 823 (50) | 30 d before Rx | N/A |
| Takada 2022 [67] | Retrospective | Japan | 2016–2019 | 95 | 0 | 85 (89.5) | 10 (10.5) | N/A | 37 (39) | N/A | N/A |
| Alessio 2021 [38] | Retrospective | Italy | 2017–2020 | 950 | 0 | 950 (100) | 0 | 950 (100) | 474 (49.9) | N/A | N/A |
| Balado 2021 [34] | Retrospective | Spain | 2017–2020 | 49 | 0 | 49 (100) | 0 | 49 (100) | 26 (53.1) | 30 d before/after Rx | N/A |
| Kostine 2021 [75] | Retrospective | France | 2015–2017 | 150 | N/A | N/A | N/A | N/A | N/A | 30 d before/after Rx | N/A |
| Muira 2021 [56] | Retrospective | Japan | 2016–2018 | 300 | 0 | 300 (100) | 0 | 40 (13.3) | 163 (54.3) | N/A | N/A |
| Rounis 2021 [61] | Retrospective | Greece | 2017~2019 | 66 | 0 | 66 (1000) | 0 | 0 (0) | 23 (34.8) | 90 d before Rx | N/A |
| Verschueren 2021 [69] | Retrospective | Netherlands | 2015~2019 | 221 | 0 | 214 (97) | 7 (3) | 84 (38) | 96 (43.4) | 30 d before/after Rx | N/A |
| Estevez 2020 [37] | Retrospective | Spain | 2015~2018 | 70 | 0 | 64 (91.4) | 6 (8.6) | 0 (0) | 59 (84.3) | 90 d before Rx | N/A |
| Hossain 2020 [47] | Retrospective | Australia | 2015~2019 | 63 | N/A | N/A | N/A | N/A | 34 (54) | 28 d after Rx | N/A |
| Svaton 2020 [65] | Retrospective | Czech | 2015~2019 | 224 | 0 | 224 (100) | 0 | 9 (4.0) | 64 (28.6) | 30 d before/after Rx | N/A |
| Zhao 2019 [74] | Retrospective | China | 2016–2018 | 109 | 0 | 109 (100) | 0 | 28 (25.7) | 40 (36.7) | 30 d before/after Rx | N/A |
| Advanced UC (n = 8) | |||||||||||
| IMvigor 210 [32] | RCT | USA | 2014~2015 | 429 | 0 | 0 | 429 (100) | 119 (22.5) | 141 (32.9) | 30 d before/after Rx | N/A |
| IMvigor 211 [19] | RCT | USA | 2015~2017 | 467 | 0 | 0 | 467 (100) | 0 (0) | 145 (31.0) | 30 d before/after Rx | N/A |
| Fukuokaya 2022 [79] | Retrospective | Japan | N/A | 227 | N/A | N/A | N/A | N/A | 56 (24.7) | N/A | N/A |
| Kunimitsu 2022 [76] | Retrospective | Japan | 2017~2020 | 79 | 0 | 0 | 79 (100) | 0 (0) | 34 (43.0) | 30 d before/60 d after Rx | N/A |
| Okuyama 2022 [58] | Retrospective | Japan | 2015~2021 | 155 | 0 | 0 | 155 (100) | 0 (0) | 99 (63.9) | 30 d before Rx | N/A |
| Tomisaki 2022 [68] | Retrospective | Japan | 2018–2021 | 40 | 0 | 40 (100) | 0 | 0 (0) | 15 (37.5) | 60 d before/30 d after Rx | N/A |
| Bañobre 2021 [62] | Retrospective | Spain | 2016~2020 | 119 | 0 | 39 (32.7) | 80 (67.3) | 22 (18.5) | 54 (45.4) | N/A | N/A |
| Lida 2021 [80] | Retrospective | Japan | 2018~2021 | 115 | 0 | 0 | 115 (100) | 0 (0) | N/A | 30 d before/after Rx | N/A |
| Advanced melanoma (n = 8) | |||||||||||
| CheckMate 066 [81] | RCT | 80 centers | 2013~2021 | 210 | Nivolumab: 210 (100) | 210 (100) | 49 (23.3) | 30 d before Rx | N/A | ||
| CheckMate 067 [82] | RCT | 21 countries | 2013~now | 945 | Ipilimumab + Nivolumab: 314 (33.2); Ipilimumab: 315 (33.3); Nivolumab: 316 (33.4) |
945 (100) | 161 (17.0) | 30 d before Rx | N/A | ||
| CheckMate 069 [83] | RCT | France/ USA |
2013~2021 | 142 | Ipilimumab + Nivolumab: 95 (67.0); Ipilimumab: 47 (33.1) | 142 (100) | 33 (23.3) | 30 d before Rx | N/A | ||
| Gaucher 2021 [41] | Retrospective | Brazil | 2010~2019 | 110 | 15 (13.6) | 68 (61.8) | 27 (24.6) | 110 (100) | 39 (35.5) | 60 d after Rx | N/A |
| Kostine 2021 [75] | Retrospective | France | 2015~2017 | 293 | N/A | N/A | N/A | N/A | N/A | 30 d before/after Rx | N/A |
| Peng 2021 [59] | Retrospective | USA | 2014~2019 | 233 | 0 | 233 (100) | 0 | 95 (40.8) | 89 (38.2) | 30 d before/after Rx | N/A |
| Afzal 2019 [29] | Retrospective | Lebanon | N/A | 120 | Ipilimumab and/or Pembrolizumab | N/A | 29 (24.2) | N/A | N/A | ||
| Failing 2016 [40] | Retrospective | USA | 2011~2014 | 159 | Ipilimumab:159 (100) | 80 (50) § | 39 (24.5) | N/A | 9 (6) | ||
| Advanced RCC (n = 3) | |||||||||||
| Mollica 2022 [57] | Retrospective | USA | 2010~2021 | 63 | 63 (100) | 0 | 63 (100) | 63 (100) | 25 (39.7) | N/A | N/A |
| Mollica 2022 [57] | Retrospective | USA | 2010~2021 | 156 | 0 | 0 | 156 (100) | 110 (70.5) | 88 (56.4) | N/A | N/A |
| Kostine 2021 [75] | Retrospective | France | 2015~2017 | 83 | N/A | N/A | N/A | N/A | N/A | 30 d before/after Rx | N/A |
| Peng 2021 [59] | Retrospective | USA | 2014~2019 | 233 | 0 | 233 (100) | 0 | 95 (40.8) | 89 (38.2) | 30 d before/after Rx | N/A |
| HCC (n = 2) | |||||||||||
| Jun 2020 [52] | Retrospective | USA | 2017~2019 | 314 | 21 (7) | 293 (93) | 0 | 137 (43.6) | 110 (35.0) | 30 d before Rx | 45 (14.3) |
| Lee 2020 [55] | Retrospective | Taiwan | 2017~2019 | 94 | N/A | N/A | N/A | N/A | 30 (31.9) | 30 d before Rx | N/A |
| Uncategorized cancers † (n = 10) | |||||||||||
| Araujo 2021 [30] | Retrospective | Brazil | N/A | 216 | 35 (16.2) | 130 (60.2) | 27 (12.5) | 0 (0) | 114 (52.8) | 60 d before/after Rx | N/A |
| Buti 2021 [9] | Retrospective | Italy | 2014~2019 | 217 | 13 (6.0) | 186 (85.7) | 18 (8.3) | 45 (20.7) | 104 (47.9) | N/A | N/A |
| Gaucher 2021 [41] | Retrospective | Brazil | 2010~2019 | 370 | 25 (5.4) | 357 (94.6) | 0 | 87 (23.4) | 149 (40.1) | 60 d after Rx | N/A |
| Giordan 2021 [42] | Retrospective | France | 2018~2019 | 154 | 0 | 0 | 154 (100) | 64 (41.6) | 47 (30.5) | 30 d before Rx | N/A |
| Husain 2021 [49] | Retrospective | USA | 2011~2019 | 1091 | 274 (25.1) | 817 (74.9) | N/A | 415 (38.0) | N/A | N/A | |
| Peng 2021 [59] | Retrospective | USA | 2014~2019 | 233 | 0 | 233 (100) | 0 | 95 (40.8) | 89 (38.2) | 30 d before/after Rx | N/A |
| Alessio 2020 [10] | Retrospective | Italy | 2014~2020 | 1012 | 0 | 956 (94.5) | 56 (5.5) | 396 (39.1) | 491 (48.5) | N/A | 56 (5.5) |
| Santamaria 2020 [50] | Retrospective | Spain | 2015~2018 | 102 | 1 (1.0) | 86 (84.3) | 15 (14.7) | 73 (71.6) | 78 (77.2) | 30 d before/after Rx | N/A |
| Ruiz 2020 [60] | Retrospective | Spain | from 2015 | 253 | 31 (12.3) | 222 (87.7) | 0 (0) | 73 (28.9) | 135 (53.4) | 60 d before/30 d after Rx | N/A |
| Kostine 2021 [75] | Retrospective | France | 2015~2017 | 635 | 3 (0.5) | 435 (68.5) | 66 (10) | N/A | 293 (46.1) | 30 d before/after Rx | N/A |
| Chemotherapy (n = 19) | |||||||||||
| Advanced NSCLC (n = 6) | |||||||||||
| Verschueren 2021 [69] | Retrospective | Netherlands | 2015–2019 | 221 | Platinum-based agents | 84 (38) | 101 (45.7) | 30 d before/after Rx | N/A | ||
| Impower 130 [71] | RCT | 8 countries | 2015–2017 | 240 | Platinum-based agents | 240 (100) | N/A¶ | Following Rx | N/A | ||
| IMpower 131 [51] | RCT | 26 countries | 2015–2017 | 340 | Platinum-based agents | 340 (100) | N/A¶ | Following Rx | N/A | ||
| IMpower 150 [20] | RCT | 26 countries | 2015–2016 | 400 | Platinum-based agents | N/A | 151 (37.8) | 30 d before/after Rx | N/A | ||
| POPLAR [22] | RCT | USA/Europe | 2013~2014 | 143 | Docetaxel | 0 (0) | N/A | 30 d before/after Rx | N/A | ||
| OAK [21] | RCT | 31 countries | 2014~2015 | 612 | Docetaxel | 0 (0) | N/A | 30 d before/after Rx | N/A | ||
| Advanced UC (n = 1) | |||||||||||
| IMvigor 211 [19] | RCT | USA | 2015~2017 | 464 | Platinum-based agents | 0 (0) | 185 (39.9) | 30 d before/after Rx | N/A | ||
| Advanced melanoma (n = 1) | |||||||||||
| CheckMate 066 | RCT | 80 centers | 2013~2021 | 208 | Decarbazine | 0 (0) | 48 (23.1) | 30 d before Rx | N/A | ||
| Uncategorized cancers † (n = 1) | |||||||||||
| Alessio 2021 [38] | Retrospective | Italy | 2017~2020 | 595 | Platinum-based agents | 595 (100) | 321 (53.7) | N/A | N/A | ||
| FOLFOX, n (%) | FOLFIRI/ IFL, n (%) |
Cape-based, n (%) | |||||||||
| Gastroesophageal carcinoma (n = 1) | |||||||||||
| TRIO013/ LOGiC [43] |
RCT | 22 countries | 2008~2012 | 274 | 0 | 0 | 274 (100) | 274 (100) | 119 (43.4) | 20% overlapping Rx | N/A |
| Early-stage colorectal cancer (n = 3) | |||||||||||
| Kitazume 2022 [77] | Retrospective | Japan | 2009~2014 | 606 | 0 | 0 | 606 (100) | 606 (100) | 54 (8.9) | 20% overlapping Rx | N/A |
| Wong 2019 [72] | Retrospective | Canada | 2004~2013 | 389 | 175 (45) | 0 | 214 (55) | 389 (100) | 99 (25.4) | During Rx | N/A |
| Sun 2016 [64] | Retrospective | Canada | 2008~2012 | 298 | 0 | 0 | 298 (100) * | 298 (100) | 77 (26.0) | During Rx | N/A |
| Advanced colorectal cancer (n = 8) | |||||||||||
| Wang 2017 [70] | Retrospective | China | 2010~2014 | 671 | 307 (45.8) | 0 | 364 (54.2) | N/A | 474 (70.6) | During Rx | N/A |
| AXEPT [73] | RCT | Asia | 2013~2015 | 482 | 0 | 243 (50.4) | 239 (49.6) | 0 (0) | 49 (10.2) | 20% overlapping Rx | N/A |
| HORIZON III [92] | RCT | 28 countries | 2006~2009 | 666 | 666 (100) | 0 | 0 | 666 (100) | 87 (13.0) | During Rx | 15 (2.2) |
| N016966 [63] | RCT | N/A | 2004~2005 | 2035 | 1018 (50) | 0 | 1017 (50) | 2035 (100) | 327 (32.1) | During Rx | 115 (5.7) |
| AVF2107g [48] | RCT | 3 countries | 2000~2002 | 780 | 0 | 780 (100) | 0 | 780 (100) | 156 (20.0) | During Rx | 129 (16.5) |
| Carrato 2013 [33] | RCT | N/A | 2007~2010 | 348 | 0 | 348 (100) | 0 | 348 (100) | 39 (11.0) | During Rx | 15 (4.2) |
| VELOUR [39] | RCT | 28 countries | 2007~2010 | 584 | 0 | 584 (100) | 0 | 0 (0) | 105 (18.0) | During Rx | 16 (2.8) |
| RAISE [66] | RCT | 24 countries | 2010~2013 | 946 | 0 | 946 (100) | 0 | 0 (0) | 232 (24.5) | During Rx | 36 (3.8) |
| Post hoc analysis of RCTs (n = 8) | |||||||||||
| Hopkins 2022 [44] | Analysis of 5 trials [20,21,22,51,71] | 4458 | Advanced NSCLC | Immune checkpoint and chemotherapy | N/A | 1225 (27.5)¶ | 30 d before/after Rx | N/A | |||
| Hopkins 2021 [46] | Analysis of IMpower 150 [20] | 1202 | Advanced NSCLC | Immune checkpoint and chemotherapy | N/A | 441 (36.7) | 30 d before/after Rx | N/A | |||
| Homicsko 2022 [78] | Analysis of CheckMate 066 [81]/067 [82]/069 [83] | 1505 | Advanced Melanoma | Immune checkpoint and chemotherapy | 1505 (100) | 291 (19.3) | 30 d before Rx | N/A | |||
| Chalabi 2020 [35] | Analysis of OAK7 and POPLAR [21,22] | 757 | Advanced NSCLC | Immune checkpoint and chemotherapy | 0 (0) | 234 (30.9) | 30 d before/after Rx | N/A | |||
| Hopkins 2020 [45] | Analysis of IMvigor 210 [24] and 211 [19,32] | 1360 | Advanced UC | Immune checkpoint and chemotherapy | 119 (8.75) | 471 (34.6) | 30 d before/after Rx | N/A | |||
| Kim 2021 [54] | Analysis of AXEPT [73] | 482 | Advanced CRC | Chemotherapy | 0 (0) | 49 (10.2) | 20% overlapping Rx | N/A | |||
| Kichenadasse 2021 [53] | Analysis of 6 trials [33,39,48,63,66,92] | 5359 | Advanced CRC | Chemotherapy | 3829 (71.4) | 946 (17.7) | During Rx | N/A | |||
| Chu 2017 [36] | Analysis of TRIO013/LOGiC [43] | 274 | Advanced GEC | Chemotherapy | 274 (100) | 119 (43.4) | 20% overlapping Rx | N/A | |||
Abbreviations: RCT, randomised–controlled trial; PPI, proton pump inhibitor; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; PD-1, programmed cell death protein 1; PDL-1, programmed death ligand 1; Rx, therapy; N/A, non-available; NSCLC, non-small cell lung cancers; RCC, renal cell carcinoma; UC, urothelial carcinoma; GEC, gastroesophageal carcinoma; CRC, colorectal cancer; Cape-based, capecitabine-based; IFL, irinotecan/leucovorin/5-FU; FOLFIRI, 5-FU/folinic acid/irinotecan; and FOLFOX, 5-FU/leucovorin/ oxaliplatin. † The details of cancer type (including the type and treatment line of immune therapy and the PDL-1 expression information) are available in Supplementary Table S2 * Details of PPI are presented in Supplementary Table S3, ¶ Although both IMpower 130 and 131 did report the use of PPI in their study population, they did not separately provide the number of PPI use in patients taking ICI and chemotherapy. Thus, we noted it as N/A but the overall use of PPI was provided in post hoc analysis (Hopkins 2022). § Failing 2016, only used cohort receiving first-line therapy for survival analyses.
Eligibility criteria of 18 RCTs [19,20,21,22,32,33,39,43,48,51,63,66,71,73,81,82,83] are elaborated in Supplementary Table S1; notably, patients with pregnancy, known CNS metastases, clinically significant cardiovascular disease, bleeding events, coagulopathy, and the use of anticoagulants or antiplatelets were excluded.
There were 42 studies [9,10,19,20,21,22,29,30,31,32,34,37,38,40,41,42,47,49,50,51,52,55,56,57,58,59,60,61,62,65,67,68,69,71,74,75,76,77,79,80,81,82,83] which primarily investigated cancer patients receiving ICI, among which 17 studies [20,21,22,31,34,37,38,47,51,56,61,65,67,69,71,74,75], 8 studies [19,32,58,62,68,76,79,80], 7 studies [29,40,41,59,75,81,82,83], 3 studies, 2 studies [52,55], 1 study, and 10 studies [9,10,30,41,42,49,50,59,60,75] reported survival outcomes of advanced non-small cell lung cancer (NSCLC), urothelial carcinoma (UC), melanoma, renal cell carcinoma (RCC), hepatocellular carcinoma (HCC), squamous cell carcinoma (SCC) of the head and neck, and uncategorized cancer cohorts, respectively. Information on uncategorized cancers is listed on Supplementary Table S2. There were 11 studies (retrospective: 4 [64,70,72,77] and post hoc analysis: 7 [33,39,43,48,63,66,73]) which investigated the effect of PPIs on patients with colorectal cancer (CRC) receiving chemotherapy. Prespecified effect modifiers, including age, sex, ECOG, and PDL expression are comparable between PPI users and non-users across the network (Supplementary Table S3). Details of PPI are presented in Supplementary Table S4. The sources of risk of bias arise mostly from bias due to confounding (Supplementary Figure S1). No study was evaluated as critical risk of bias. Supplementary Results 2 provides detailed elaboration of ROBINS-I of each domain.
3.2. Network Meta-Analysis
Eight post hoc analysis [35,36,44,45,46,53,54,78] of 18 RCTs [19,20,21,22,32,33,39,43,48,51,63,66,71,73,81,82,83] enrolling advanced cancers (advanced NSCLC, UC, melanoma, CRC, and gastroesophageal adenocarcinoma) are included in the network. Through the visualization of network plot (Figure 2), patients under ICI treatment with concomitant PPI (red node) are associated with poor OS and PFS, compared not only with those under ICI without PPI, but also with those with chemotherapy. Details of NMA are presented in Supplementary Figures S2 and S3. However, both the netsplit and netheat plots demonstrated significant inconsistency throughout the comparisons (Supplementary Figures S4–S7). After excluding trials of melanoma (Supplementary Results 4), NMA also indicates that patients under ICI treatment with concomitant PPI are associated with poor OS (HR, 1.49; 95% CI, 1.37 to 1.67) and PFS (HR, 1.41; 95% CI, 1.25 to 1.61), compared with those without PPI, and with worse OS (HR, 1.18; 95% CI, 1.07 to 1.31) and PFS (HR, 1.30; 95% CI, 1.14 to 1.48), compared with those with chemotherapy (Supplementary Figures S8–S10), with a substantial decrease in inconsistency (Supplementary Figures S11–S14). According to the net league table with p-scores, ICI without PPI is ranked highest, followed by chemotherapy without PPI, ICI with PPI, and chemotherapy with PPI (Table 2). The funnel plot (Supplementary Figures S15 and S16) demonstrated general symmetry through inspection, which was further supported by the Egger’s test (p = 0.71), indicating no publication bias from small study effects.
Figure 2.
Network plot for comparative association among PPI, ICI, and chemotherapy in terms of (A) overall survival and (B) progression-free survival. The thickness of the connecting line corresponds to the number of trials among comparators. We specifically highlight the arm ICI with baseline PPI as red node, the red arrow as its significant comparison with ICI, and chemotherapy without baseline PPI to reiterate our main findings. Blue arrows also indicate significant survival association between two nodes. Conversely, gray arrows suggest little association between two arms. PPI, proton pump inhibitor; ICI, immune checkpoint inhibitor; and HR, hazard ratio.
Table 2.
League table of pairwise comparisons in the network for the hazard ratio (with 95% CIs) of OS (A) and PFS (B).
| (A) Overall Survival | |||
| Immune check point inhibitors (p-score, 1.0000) |
0.76 (0.67–0.85) | 0.70 (0.62–0.78) | 0.64 (0.53–0.78) |
| 0.79 (0.72–0.86) | Chemotherapy (p-score, 0.6664) |
0.85 (0.70–1.04) | 0.82 (0.76–0.89) |
| 0.67 (0.60–0.73) | 0.85 (0.76–0.94) | Immune check point inhibitors and PPI (p-score, 0.2016) |
1.07 (0.92–1.24) |
| 0.66 (0.60–0.72) | 0.84 (0.78–0.90) | 0.99 (0.89–1.09) | Chemotherapy and PPI (p-score, 0.1319) |
| (B) Progression-Free Survival | |||
| Immune check point inhibitors (p-score, 0.9706) |
0.84 (0.70–1.00) | 0.90 (0.71–1.13) | 0.72 (0.61–0.85) |
| 0.92 (0.81–1.04) | Chemotherapy (p-score, 0.6958) |
0.84 (0.77–0.92) | 0.81 (0.64–1.03) |
| 0.80 (0.70–0.91) | 0.87 (0.80–0.94) | Chemotherapy and PPI (p-score, 0.3217) |
0.83 (0.68–1.02) |
| 0.71 (0.62–0.80) | 0.77 (0.67–0.88) | 0.89 (0.78–1.01) | Immune check point inhibitors and PPI (p-score, 0.0119) |
Note: Treatments are ranked by their p-score of overall survival with the top left representing the best, whereas the bottom right represents the worst. Estimates in the upper right triangle are direct comparisons, while those in the lower left are from the network meta-analysis.
3.3. Prespecified Sensitivity Analyses (Supplementary Results 5 and 6)
We excluded data of IMpower 130, IMpower 131 [51], IMvigor 210, TRIO-013/LOGiC [43], AVF2107g, N016966, and Carrato 2013 [33] because these trials enrolled treatment-naïve patients (Supplementary Figures S17–S23). Regarding the PDL-1 expression, we excluded data of Chu 2017 [36] and Kichenadasse 2021 [53] because of unavailable PDL-1 information (Supplementary Figures S24–S30). After the sensitivity analyses, the association among PPI, ICI, and chemotherapy remains the same, with no significant inconsistency.
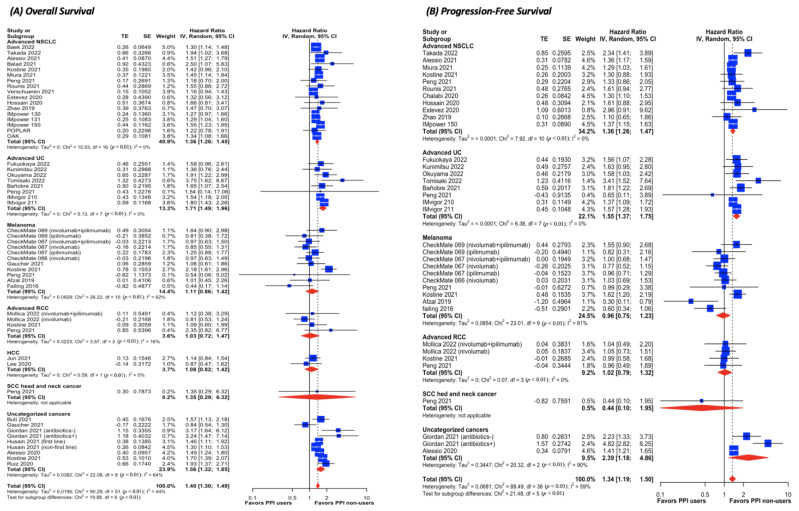
3.4. Pairwise Meta-Analysis for ICI Cohorts (Figure 3 and Supplementary Results 7)
Figure 3.
Forest plot of comparative (A) overall survival and (B) progression-free survival in cancer patients treated with ICI between PPI users and non-users. The size of squares is proportional to the weight of each study. Horizontal lines indicate the 95% CI of each study; diamond, the pooled estimate with 95%; CI, confidential interval; HCC, hepatocellular carcinoma; HR, hazard ratio; NSCLC, non-small cell lung cancer; PPI, proton pump inhibitor; RCC, renal cell carcinoma; SCC, squamous cell carcinoma; and UC, urothelial carcinoma.
In patients with NSCLC, the use of PPIs poses a 36% higher risk of death (HR, 1.36; 95% CI, 1.28 to 1.45; I2 = 0%) and disease progression (HR, 1.36; 95% CI, 1.26 to 1.47; I2 = 0%) than those without PPIs. The same trend is observed in patients with UC regarding OS (HR, 1.71; 95% CI, 1.49 to 1.96; I2 = 0%) and PFS (HR, 1.55; 95% CI, 1.37 to 1.75; I2 = 0%). There is no significant difference in either of the survival outcomes with respect to advanced melanoma, RCC, HCC, and SCC of the head and neck. Scatters in the funnel plot with Egger’s test suggest no publication bias in either OS or PFS (Supplementary Figures S31 and S32). Sensitivity analysis of excluding studies subject to high risk of bias yields the same association with the significant decrease in the statistical heterogeneity (Supplementary Figures S33 and S34).
3.5. Pairwise Meta-Analysis for Chemotherapy Cohorts (Supplementary Results 9)
The use of PPIs is associated with a significant 12% higher all-cause mortality in patients with advanced NSCLC receiving chemotherapy, with a marginally significant higher progression rate (Supplementary Figures S35 and S36). For GI cancers, using a PPI also confers a significantly higher risk of death and disease progression rate (Figures S35 and S36) but with substantial heterogeneity.
When CRC cancer patients are stratified based on chemotherapeutic regimens, concomitant PPI use negatively affects the effectiveness of these regimens in patients with FU-based regimens (Supplementary Figures S37 and S38). Conversely, survival outcomes are similar between PPI users and non-users in patients receiving capecitabine-based regimens (Supplementary Figures S37 and S38).
3.6. Meta-Analysis Using Adjusted HR (Supplementary Results 10)
Supplementary Table S5 provides adjusted variables for adjusted outcomes and Supplementary Figures S39–S44 provide the results of the meta-analysis using adjusted HR. No significant discrepancy is noted between unadjusted and adjusted outcomes. We finally summarize the meta-analyses findings with both unadjusted and adjusted results in Supplementary Table S6.
4. Discussion
Although various meta-analyses have investigated the influence of PPIs on the effectiveness of ICIs, no synthesis, to date, has explored the interaction among PPIs, ICIs, and chemotherapy. Our NMA demonstrated that baseline PPI use not only has a negative prognostic influence on advanced cancer patients treated with ICIs but is shown to compromise the effectiveness of ICI, causing it to be even worse than chemotherapy. It is also noteworthy that trials in the network not only included advanced cancer patients with ECOG PS < 2 but explicitly excluded patients with medical conditions warranting long-term antiplatelets or anticoagulants that may require baseline PPI to prevent GI bleeding, such as coronary artery disease and thromboembolism, which reflect high burdens of co-morbidities and which can obviously confound PPI’s survival impact. Therefore, there is a low risk of unmeasured confounding bias for our network, although included trials contain no pertinent information on the indications of PPI. However, through the visualization of netheat plots (Supplementary Figures S6 and S7), significant inconsistency exists in the network, with melanoma deemed to be the source of the inconsistency, which decreased substantially after the removal of melanoma trials. This, together with the head-to-head meta-analyses, provides a plausible reason as to why melanoma contributes to the source of inconsistency, as melanoma was shown to be neutrally affected by PPI. Conversely, only advanced NSCLC and UC patients treated with ICIs are negatively affected by PPI. Although effect modifiers were comparably distributed and no significant inconsistency was detected in our NMA after the removal of melanoma trials, conceptual heterogeneity was inevitable as different cancers encompass distinct histopathological features, clinical behaviors, and responses to therapy. Ideally, for the most precise synthesis, every cancer type should possess their own network. However, the number of trials to date are too limited for us to construct an individual network for respective cancer types. We acknowledge this as our major limitation, and the findings of our NMA should be interpreted together with pairwise meta-analyses supplemented by real-world evidence.
On the basis of our syntheses, “PPI-induced dysbiosis” serves as a significant modifier of treatment response to ICIs in both advanced NSCLC and UC, and various basic science studies have already laid a solid foundation for this clinical observation. The higher diversity of gut microbiota correlates with a higher response to ICIs owing to its positive correlation with T-cell numbers and activity [11]. Notably, PPI users were found to have lower diversity, regardless of indications, compared with non-users, with Firmicutes being the most strongly affected species [84]. Moreover, Routy et al. [11] indicated that NSCLC patients responded well to anti-PD1 agents that had an over-presentation of Firmicutes and higher memory T-helper cell reactivity against commensals in the peripheral blood. Another possible explanation for the negative association between the effectiveness of PPIs and ICIs may be related to H. pylori infection. Oster and colleagues [85] unveiled that H. pylori infection negatively altered the response to ICI in a pre-clinical setting. They also demonstrated that NSCLC patients with seropositive H. pylori were associated with significantly lower OS and PFS when compared with those with H. pylori seronegativity. Consequently, PPI could be a potential surrogate marker for H. pylori infection causing the negative association observed in our study. Another study also implied that dysbiosis effects were minimal when regimens were administered in a later-line context [45], due to the development of immunosurveillance evasion in advanced cancers [86]. However, we demonstrate that the detrimental effects of PPIs may be independent of the treatment line on the basis of the prespecified sensitivity analyses.
On the other hand, “PPI-induced dysbiosis” does not seem to not alter the treatment response to ICIs in patients with advanced melanoma, RCC, HCC, and SCC of the head and neck. This result contradicts previous syntheses [13,16] that demonstrated the potential benefit of PPIs for melanoma patients treated with ICIs. There are two feasible explanations for such a distinctive observation. Firstly, only two studies were included in previous syntheses, which underlies the convincing mechanism, as a small body of evidence can underpower the result, giving rise to a false positive association. Secondly, the quality assessment tool used in their syntheses was the Newcastle–Ottawa Scale, which is now considered outdated and unreliable due to the advent of ROBINS-I, which is the gold-standard tool in current evidence-based medicine. When ROBINS-I was used in the appraisal of these two studies, it was discovered that the study [40] contributing to the positive effect of PPI on melanoma was subject to high risk of bias. Note that after excluding this study [40], statistical heterogeneity decreased substantially, as we demonstrated in Supplementary Results 8. In fact, pre-clinical studies have shed much light on potential dysbiosis effects on melanoma. For those treated with ICI, responders were found to have a significantly higher alpha diversity of gut microbiota than non-responders [87,88], and using antibiotics during the treatment window was associated with a shorter PFS [89,90]. However, PPIs were shown to exert a pro-apoptotic effect on melanoma cells through the inhibition of vacuolar ATPase, an ATP-dependent proton pump [91,93], which disturbs pH gradient across melanoma cells, resulting in caspase-dependent cell death [94]. We assumed that these two aforementioned mechanisms counterbalance each other and contribute to neutral survival impacts of PPI on melanoma. For patients with advanced RCC, our result resonates with a large pooled analysis [95] of individual data from clinical trials illustrating that PPIs conferred to negligible survival influence on RCC patients treated with tyrosine kinase inhibitors (TKIs), presumably due to insignificant effects on bioavailability of TKIs. The same neutral association is observed in patients with HCC, and SCC of the head and neck as well. However, such association in these cancers currently lacks a plausible mechanism, and clinicians should keep in mind that it exhibits a false negative due to small number of studies and the small sample size. We anticipate more RCTs or large observational studies of these cancers for larger pooled analysis in the future.
Regarding the chemotherapeutic modalities, they varied in CRC among included trials. We believe that the high variance in therapeutic modalities among CRC trials accounts for the main origins of heterogeneity, not only in pairwise analysis but also in NMA. Nonetheless, our study shows that patients treated with capecitabine-based therapy are not affected by baseline PPIs. Capecitabine is almost completely metabolized to fluorouracil after being absorbed through the GI walls, and it was believed that PPI hampers the solubility, absorption, and distribution of capecitabine by increasing the intragastric pH level [96]. However, in vitro data appear not to support this interaction, as capecitabine does not become ionized until it reaches a pH level of 8.8, when it is poorly absorbed, which is obviously higher than that induced by PPI [97,98]. In contrast, fluorouracil-based agents are negatively affected by concomitant PPI, although with considerable heterogeneity. This appears to arise from complex combinations with other chemotherapeutic agents and target therapy. Unfortunately, to date, scarce pharmacokinetic studies explore the effect of PPIs on oxaliplatin, irinotecan, and target therapy, and our findings suggest that interactions between PPIs and these modalities should be addressed in the future, warranting further clinical and pharmacokinetic investigations.
One caveat of incorporating CRC into the network is that ICIs currently have limited roles in the CRC treatment field. Although a recent trial, KEYNOTE-177 [99], has established the antitumor efficacy of ICIs in treatment-naïve advanced CRC, only patients harboring deficient-MMR/MS-instable tumors are eligible for using ICIs, with the rationale being that a high mutational burden from deficient-MMR/MS-instability results in overexpression of immunogenic antigens and upregulation of immune checkpoint proteins [100]. On the other hand, the role of ICI in advanced CRC with preserved-MMR/MS-stable diseases is still under exploration, with multiple active recruiting trials investigating the combination of ICI and traditional regimens, which is hypothesized to evoke immunogenic responses.
Another limitation to our network is in regard to the PDL-1 status. Although PDL-1 expression is well-balanced across our network, and sensitivity analyses demonstrated no significant impact of PDL-1 status on our NMA, readers should bear in mind that they suffer from three limitations. Firstly, different cancers contain their own exclusive immune profile and present distinctive responses to different ICIs with a varying correlation to the PDL-1 expression. For instance, in POPLAR and OAK trials, advanced NSCLC patients with higher PDL-1 expression treated with atezolizumab derived higher survival benefits over chemotherapy [21,22] Conversely, in advanced UC, the issue of whether ICI has survival superiority over chemotherapy varied among different agents. Pembrolizumab was shown to result in better survival benefits than chemotherapy, [101,102] whereas atezolizumab did not [19]. The role of PDL-1 expression remains undetermined in UC to date, with nivolumab (CheckMate 275) [103], durvalumab [104], and pembrolizumab (KeyNote 045) [101,102] predicting a better survival response rate using PDL-1, unlike atezolizumab (IMvigor 211) which does not. Furthermore, for CRC patients, Checkmate 142 [105] suggested the PDL-1status is not a predictive biomarker, but a mismatch repair/DNA microsatellite instability (MMR/MS) is. Secondly, PDL-1 expression can be evaluated by different methodologies. There are four commonly used immunohistochemistry assays, including 22C3, 28-8, SP142, and SP263, to stain tumors, and these scoring systems were not directly comparable metrics of PDL-1 expression level. Thirdly, it should be noted that studies of unknown PDL-1 status correspond exactly to CRC studies, which are also a source of heterogeneity. Thus, it implies our sensitivity analysis could be potentially biased.
In summary, the present study contains following novelties. Firstly, we investigated the interaction of PPIs against ICIs versus chemotherapy, which has allowed us to gain further insight into the detrimental magnitude of PPIs in affected cancers. Secondly, only NSCLC and UC are negatively affected by PPI-induced dysbiosis and melanoma, whereas RCC, HCC, and SCC do not seem to be affected. Therefore, when cancer patients have indications of using gastric acid suppressants, such as antiemesis for ICI and chemotherapy, peptic ulcers, and reflux esophagitis, H2-blockers may be considered given PPI’s potential detrimental effects on ICI in certain cancers and on specific chemotherapeutic regimens. On the other hand, several limitations should be addressed in the future. Firstly, constructing respective networks for different cancers would provide greater knowledge of the interaction between PPIs and therapeutic modalities. Secondly, the included patients were mostly ECOG-PS 0-2, which hinders the generalizability to other cancer patients. Thirdly, information on PDL-1 expression is scarce, so we are unable to look further into the magnitude of PPIs’ effects on ICI-treated patients with different PDL-1 expressions. Last but not least, there is still inadequate data for probing the impact of PPIs on other types of malignancy, such as prostate cancer, CRC, and HCC, as ICIs are still looking for their niche in these cancers.
5. Conclusions
“PPI-induced dysbiosis” serves as a significant modifier of treatment response in both advanced NSCLC and UC that are treated with ICIs, compromising the effectiveness of ICIs to be less than that of chemotherapy. Thus, clinicians should avoid unnecessary PPI prescription in these patients. “PPI-induced dysbiosis”, on the other hand, does not alter the treatment response to ICIs in advanced melanoma, RCC, HCC, and SCC of the head and neck. Future high quality prospective studies including more cancer types, and more detailed PDL-1 status are warranted.
Acknowledgments
Our team would like to thank Tim Stubbings for manuscript proofreading.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15010284/s1, Supplementary Method 1. PRISMA NMA Checklist of Items to Include When Reporting A Systematic Review Involving a Network Meta-analysis. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; NMA, network meta-analysis; Supplementary Method 2. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 checklist; Supplementary Method 3. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) checklist; Supplementary Method 4. Search strategy; Supplementary Method 5. Data extraction and data synthesis; Supplementary Results 1. Details of full-text inspection; Supplementary Results 2. Details of risk of bias assessment for survival outcome (Figure S1); Supplementary Results 3. Details of NMA of overall survival and progression-free survival (Figures S2–S7); Supplementary Results 4. Network meta-analysis after excluding melanoma trials (Figures S8–S16); Supplementary Results 5. Sensitivity analyses of NMA, excluding first line therapy (Figures S17–S23); Supplementary Results 6. Sensitivity analyses of NMA, excluding unknown PDL-1 status (Figures S24–S30); Supplementary Results 7. Assessment of publication bias in pairwise meta-analysis of ICI cohorts (Figures S31 and S32); Supplementary Results 8. Sensitivity analysis of excluding studies of high risk of bias (Figures S33 and S34); Supplementary Results 9. Pairwise meta-analyses of chemotherapy cohorts (Figures S35–S38); Supplementary Results 10. Pairwise meta-analyses using adjusted hazard ratios (HR) (Figures S39–S44); Table S1. Eligibility criteria of trial patients included in NMA; Table S2. Details of uncategorized cancers; Table S3. Effect modifiers across the network; Table S4. Details of proton pump inhibitors; Table S5. Covariates of studies reporting adjusted estimates; Table S6. Summary of findings of our meta-analysis.
Author Contributions
Conceptualization, Y.C. and K.-Y.C.; methodology, Y.-N.K. and K.-Y.C.; formal analysis, W.-Y.L., Y.-C.C. and K.-Y.C.; investigation, W.-Y.L., Y.-C.C. and C.-H.H.; data curation, C.-H.H., E.A.-L., T.-H.W. and T.-H.T.; writing—original draft preparation, W.-Y.L. and C.-H.H.; writing—review and editing, Y.C., Y.-C.C., H.-E.T., Y.-N.K. and K.-Y.C.; supervision, Y.-N.K. and K.-Y.C. All authors have read and agreed to the published version of the manuscript. Please refer to the CRediT taxonomy for the term explanation.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Tang J., Yu J.X., Hubbard-Lucey V.M., Neftelinov S.T., Hodge J.P., Lin Y. Trial watch: The clinical trial landscape for PD1/PDL1 immune checkpoint inhibitors. Nat. Rev. Drug. Discov. 2018;17:854–855. doi: 10.1038/nrd.2018.210. [DOI] [PubMed] [Google Scholar]
- 2.Ribas A., Wolchok J.D. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motzer R.J., Tannir N.M., McDermott D.F., Aren Frontera O., Melichar B., Choueiri T.K., Plimack E.R., Barthélémy P., Porta C., George S., et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feld E., Mitchell T.C. Immunotherapy in melanoma. Immunotherapy. 2018;10:987–998. doi: 10.2217/imt-2017-0143. [DOI] [PubMed] [Google Scholar]
- 5.Rizzo A., Mollica V., Cimadamore A., Santoni M., Scarpelli M., Giunchi F., Cheng L., Lopez-Beltran A., Fiorentino M., Montironi R., et al. Is There a Role for Immunotherapy in Prostate Cancer? Cells. 2020;9:2051. doi: 10.3390/cells9092051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rizzo A., Mollica V., Santoni M., Ricci A.D., Rosellini M., Marchetti A., Montironi R., Ardizzoni A., Massari F. Impact of Clinicopathological Features on Survival in Patients Treated with First-line Immune Checkpoint Inhibitors Plus Tyrosine Kinase Inhibitors for Renal Cell Carcinoma: A Meta-analysis of Randomized Clinical Trials. Eur. Urol. Focus. 2021;8:514–521. doi: 10.1016/j.euf.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Conforti F., Pala L., Bagnardi V., De Pas T., Martinetti M., Viale G., Gelber R.D., Goldhirsch A. Cancer immunotherapy efficacy and patients’ sex: A systematic review and meta-analysis. Lancet Oncol. 2018;19:737–746. doi: 10.1016/S1470-2045(18)30261-4. [DOI] [PubMed] [Google Scholar]
- 8.Califano R., Gomes F., Ackermann C.J., Rafee S., Tsakonas G., Ekman S. Immune checkpoint blockade for non-small cell lung cancer: What is the role in the special populations? Eur. J. Cancer. 2020;125:1–11. doi: 10.1016/j.ejca.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Buti S., Bersanelli M., Perrone F., Tiseo M., Tucci M., Adamo V., Stucci L.S., Russo A., Tanda E.T., Spagnolo F., et al. Effect of concomitant medications with immune-modulatory properties on the outcomes of patients with advanced cancer treated with immune checkpoint inhibitors: Development and validation of a novel prognostic index. Eur. J. Cancer. 2020;142:18–28. doi: 10.1016/j.ejca.2020.09.033. [DOI] [PubMed] [Google Scholar]
- 10.Cortellini A., Tucci M., Adamo V., Stucci L.S., Russo A., Tanda E.T., Spagnolo F., Rastelli F., Bisonni R., Santini D., et al. Integrated analysis of concomitant medications and oncological outcomes from PD-1/PD-L1 checkpoint inhibitors in clinical practice. J. Immunother. Cancer. 2020;8:11. doi: 10.1136/jitc-2020-001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Routy B., le Chatelier E., DeRosa L., Duong C.P.M., Alou M.T., Daillère R., Fluckiger A., Messaoudene M., Rauber C., Roberti M.P., et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 12.Iida N., Dzutsev A., Stewart C.A., Smith L., Bouladoux N., Weingarten R.A., Molina D.A., Salcedo R., Back T., Cramer S., et al. Commensal Bacteria Control Cancer Response to Therapy by Modulating the Tumor Microenvironment. Science. 2019;342:967–970. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li M., Zeng C., Yao J., Ge Y., An G. The association between proton pump inhibitors use and clinical outcome of patients receiving immune checkpoint inhibitors therapy. Int. Immunopharmacol. 2020;88:3052–3063. doi: 10.1016/j.intimp.2020.106972. [DOI] [PubMed] [Google Scholar]
- 14.Li C., Xia Z., Li A., Meng J. The effect of proton pump inhibitor uses on outcomes for cancer patients treated with immune checkpoint inhibitors: A meta-analysis. Ann. Transl. Med. 2020;8:1655. doi: 10.21037/atm-20-7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin B.-D., Jiao X.-D., Zhou X.-C., Shi B., Wang J., Liu K., Wu Y., Ling Y., Zang Y.-S. Effects of concomitant proton pump inhibitor use on immune checkpoint inhibitor efficacy among patients with advanced cancer. Oncoimmunology. 2021;10:1929727. doi: 10.1080/2162402X.2021.1929727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu C., Guo H., Mao H., Tong J., Yang M., Yan X. An Up-To-Date Investigation Into the Correlation Between Proton Pump Inhibitor Use and the Clinical Efficacy of Immune Checkpoint Inhibitors in Advanced Solid Cancers: A Systematic Review and Meta-Analysis. Front. Oncol. 2022;12:753234. doi: 10.3389/fonc.2022.753234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rizzo A., Cusmai A., Giovannelli F., Acquafredda S., Rinaldi L., Misino A., Montagna E.S., Ungaro V., Lorusso M., Palmiotti G. Impact of Proton Pump Inhibitors and Histamine-2-Receptor Antagonists on Non-Small Cell Lung Cancer Immunotherapy: A Systematic Review and Meta-Analysis. Cancers. 2022;14:1404. doi: 10.3390/cancers14061404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dar S., Merza N., Rahim M., Qatani A., Varughese T., Mohammad A., Masood F., Reza F., Shucenwan, Almas T., et al. Impact of proton-pump inhibitors on the efficacy of immune checkpoint inhibitors in non-small cell lung cancer: A systematic review and meta-analysis. Ann. Med. Surg. 2022;78:103752. doi: 10.1016/j.amsu.2022.103752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powles T., Durán I., Van Der Heijden M.S., Loriot Y., Vogelzang N.J., De Giorgi U., Oudard S., Retz M.M., Castellano D., Bamias A., et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018;391:748–757. doi: 10.1016/S0140-6736(17)33297-X. [DOI] [PubMed] [Google Scholar]
- 20.Socinski M.A., Jotte R.M., Cappuzzo F., Orlandi F., Stroyakovskiy D., Nogami N., Rodríguez-Abreu D., Moro-Sibilot D., Thomas C.A., Barlesi F., et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018;378:2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 21.Rittmeyer A., Barlesi F., Waterkamp D., Park K., Ciardiello F., von Pawel J., Gadgeel S.M., Hida T., Kowalski D.M., Dols M.C., et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. Erratum in Lancet 2017, 389, e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fehrenbacher L., Spira A., Ballinger M., Kowanetz M., Vansteenkiste J., Mazieres J., Park K., Smith D., Artal-Cortes A., Lewanski C., et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JPT T.J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021); Cochrane: 2021. [(accessed on 14 March 2021)]. Available online: https://training.cochrane.org/handbook.
- 24.Efthimiou O., Debray T.P.A., van Valkenhoef G., Trelle S., Panayidou K., Moons K.G.M., Reitsma J.B., Shang A., Salanti G., on behalf of GetReal Methods Review Group GetReal in network meta-analysis: A review of the methodology. Res. Synth. Methods. 2016;7:236–263. doi: 10.1002/jrsm.1195. [DOI] [PubMed] [Google Scholar]
- 25.Parmar M.K., Torri V., Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat. Med. 1998;17:2815–2834. doi: 10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 26.Tierney J.F., Stewart L.A., Ghersi D., Burdett S., Sydes M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Afzal M.Z., Shirai K. What impact do the proton pump inhibitors have on the efficacy of immune check point inhibitors in metastatic malignant melanoma? J. Clin. Oncol. 2019;37((Suppl. S15)):e21040. doi: 10.1200/JCO.2019.37.15_suppl.e21040. [DOI] [Google Scholar]
- 30.Araujo H.A., Moniz C.M.V., Braghiroli O.F.M., Mak M.P., Uratani L.F., Tiecher R.D., Moraes P.M., Barbosa I., De Camargo V.P., Braghiroli M.I.F.M., et al. Proton pump inhibitors and antibiotics impact on toxicities and clinical outcomes in cancer patients treated with immunotherapy. J. Clin. Oncol. 2021;39:2652. doi: 10.1200/JCO.2021.39.15_suppl.2652. [DOI] [Google Scholar]
- 31.Baek Y.H., Kang E.J., Hong S., Park S., Kim J.H., Shin J.Y. Survival outcomes of patients with nonsmall cell lung cancer concomitantly receiving proton pump inhibitors and immune checkpoint inhibitors. Int. J. Cancer. 2022;150:1291–1300. doi: 10.1002/ijc.33892. [DOI] [PubMed] [Google Scholar]
- 32.Balar A.V., Galsky M.D., Rosenberg J.E., Powles T., Petrylak D.P., Bellmunt J., Loriot Y., Necchi A., Hoffman-Censits J., Perez-Gracia J.L., et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet. 2017;389:67–76. doi: 10.1016/S0140-6736(16)32455-2. Erratum in Lancet 2017, 390, 848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carrato A., Swieboda-Sadlej A., Staszewska-Skurczynska M. Fluorouracil, leucovorin, and irinotecan plus either sunitinib or placebo in metastatic colorectal cancer: A randomized, phase III trial. J. Clin. Oncol. 2013;31:1341–1347. doi: 10.1200/JCO.2012.45.1930. [DOI] [PubMed] [Google Scholar]
- 34.Balado A.C., Lores M.T., Sánchez R., Ferrán B.B., Montero E.L., Torre A.M., Del Molino B.M.P., Ferro I.Z. 4CPS-301 Association of antibiotics and proton pump inhibitors on clinical activity of firstline pembrolizumab for non-small cell lung cancer: 2 years of real world data. BJM. 2021;28:A65. doi: 10.1136/ejhpharm-2021-eahpconf.133. [DOI] [Google Scholar]
- 35.Chalabi M., Cardona A., Nagarkar D., Scala A.D., Gandara D., Rittmeyer A., Albert M., Powles T., Kok M., Herrera F. Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: Pooled post hoc analyses of the OAK and POPLAR trials. Ann. Oncol. 2020;31:525–531. doi: 10.1016/j.annonc.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Chu M.P., Hecht J.R., Slamon D., Wainberg Z.A., Bang Y.J., Hoff P.M., Sobrero A., Qin S., Afenjar K., Houe V., et al. Association of Proton Pump Inhibitors and Capecitabine Efficacy in Advanced Gastroesophageal Cancer: Secondary Analysis of the TRIO-013/LOGiC Randomized Clinical Trial. JAMA Oncol. 2017;3:767–773. doi: 10.1001/jamaoncol.2016.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conde-Estévez D., Monge-Escartín I., Ríos-Hoyo A., Monzonis X., Echeverría-Esnal D., Moliner L., Duran-Jordà X., Taus Á., Arriola E. Prognostic factors and effect on survival of immune-related adverse events in patients with non-small-cell lung cancer treated with immune checkpoint blockage. J. Chemother. 2020;33:32–39. doi: 10.1080/1120009X.2020.1849488. [DOI] [PubMed] [Google Scholar]
- 38.Cortellini A., Di Maio M., Nigro O., Leonetti A., Cortinovis D.L., Aerts J.G., Guaitoli G., Barbieri F., Giusti R., Ferrara M.G., et al. Differential influence of antibiotic therapy and other medications on oncological outcomes of patients with non-small cell lung cancer treated with first-line pembrolizumab versus cytotoxic chemotherapy. J. Immunother. Cancer. 2021;9:e002421. doi: 10.1136/jitc-2021-002421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cutsem E.V., Tabernero J., Lakomy R. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J. Clin. Oncol. 2012;30:3499–3506. doi: 10.1200/JCO.2012.42.8201. [DOI] [PubMed] [Google Scholar]
- 40.Failing J., Finnes H.D., Kottschade L.A., Allred J.B., Markovic S. Effects of commonly used chronic medications on the outcomes of ipilimumab therapy in patients with metastatic melanoma. J. Clin. Oncol. 2016;34:609–615. doi: 10.1200/JCO.2016.34.15_suppl.e21038. [DOI] [PubMed] [Google Scholar]
- 41.Gaucher L., Adda L., Séjourné A., Joachim C., Guillaume C., Poulet C., Liabeuf S., Gras-Champel V., Masmoudi K., Houessinon A., et al. Associations between dysbiosis-inducing drugs, overall survival and tumor response in patients treated with immune checkpoint inhibitors. Ther. Adv. Med Oncol. 2021;13:17588359211000591. doi: 10.1177/17588359211000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giordan Q., Salleron J., Vallance C., Moriana C., Clement-Duchene C. Impact of Antibiotics and Proton Pump Inhibitors on Efficacy and Tolerance of Anti-PD-1 Immune Checkpoint Inhibitors. Front. Immunol. 2021;12:716317. doi: 10.3389/fimmu.2021.716317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hecht J.R., Bang Y.J., Qin S.K., Chung H.C., Xu J.M., Park J.O., Jeziorski K., Shparyk Y., Hoff P.M., Sobrero A., et al. Lapatinib in Combination With Capecitabine Plus Oxaliplatin in Human Epidermal Growth Factor Receptor 2-Positive Advanced or Metastatic Gastric, Esophageal, or Gastroesophageal Adenocarcinoma: TRIO-013/LOGiC--A Randomized Phase III Trial. J. Clin. Oncol. 2016;34:443–451. doi: 10.1200/JCO.2015.62.6598. [DOI] [PubMed] [Google Scholar]
- 44.Hopkins A.M., Badaoui S., Kichenadasse G., Karapetis C.S., McKinnon R.A., Rowland A., Sorich M.J. Efficacy of Atezolizumab in Patients With Advanced NSCLC Receiving Concomitant Antibiotic or Proton Pump Inhibitor Treatment: Pooled Analysis of Five Randomized Control Trials. J. Thorac. Oncol. 2022;17:758–767. doi: 10.1016/j.jtho.2022.02.003. [DOI] [PubMed] [Google Scholar]
- 45.Hopkins A.M., Kichenadasse G., Karapetis C.S., Rowland A., Sorich M.J. Concomitant proton pump inhibitor use and survival in urothelial carcinoma treated with atezolizumab. Clin. Cancer Res. 2020;26:5487–5493. doi: 10.1158/1078-0432.CCR-20-1876. [DOI] [PubMed] [Google Scholar]
- 46.Hopkins A.M., Kichenadasse G., McKinnon R.A., Abuhelwa A.Y., Logan J.M., Badaoui S., Karapetis C.S., Rowland A., Sorich M.J. Efficacy of first-line atezolizumab combination therapy in patients with non-small cell lung cancer receiving proton pump inhibitors: Post hoc analysis of IMpower150. Br. J. Cancer. 2021;126:42–47. doi: 10.1038/s41416-021-01606-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hossain T., Htut S., Pathmanathan S., Fletcher J., Pandey R., Lui A., Lyle M., Rainey N. Immunotherapy efficacy and concomitant proton pump inhibitor use in non-small cell lung cancer. Asia-Pac. J. Clin. Oncol. 2020;16((Suppl. S2)):37. [Google Scholar]
- 48.Hurwitz H., Fehrenbacher L., Novotny W. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 49.Husain M., Xu M., Patel S., Johns A., Grogan M., Li M., Lopez G., Miah A., Hoyd R., Liu Y., et al. Proton pump inhibitor use (PPI) in patients treated with immune checkpoint inhibitors (ICI) for advanced cancer: Survival and prior therapy. J. Clin. Oncol. 2021;39:2633. doi: 10.1200/JCO.2021.39.15_suppl.2633. [DOI] [Google Scholar]
- 50.Iglesias-Santamaría A. Impact of antibiotic use and other concomitant medications on the efficacy of immune checkpoint inhibitors in patients with advanced cancer. Clin. Transl. Oncol. 2020;22:1481–1490. doi: 10.1007/s12094-019-02282-w. [DOI] [PubMed] [Google Scholar]
- 51.Jotte R., Cappuzzo F., Vynnychenko I., Stroyakovskiy D., Rodríguez-Abreu D., Hussein M., Soo R., Conter H.J., Kozuki T., Huang K.-C., et al. Atezolizumab in Combination With Carboplatin and Nab-Paclitaxel in Advanced Squamous NSCLC (IMpower131): Results From a Randomized Phase III Trial. J. Thorac. Oncol. 2020;15:1351–1360. doi: 10.1016/j.jtho.2020.03.028. [DOI] [PubMed] [Google Scholar]
- 52.Jun T., Dharmapuri S., Marron T.U., Sung M.W., Ang C. Effect of baseline medications on response to immunotherapy in hepatocellular carcinoma. J. Clin. Oncol. 2020;38:484. doi: 10.1200/JCO.2020.38.4_suppl.484. [DOI] [Google Scholar]
- 53.Kichenadasse G., Miners J.O., Mangoni A.A., Karapetis C.S., Hopkins A.M., Sorich M.J. Proton Pump Inhibitors and Survival in Patients With Colorectal Cancer Receiving Fluoropyrimidine-Based Chemotherapy. J. Natl. Compr. Cancer Netw. JNCCN. 2021;19:1037–1044. doi: 10.6004/jnccn.2020.7670. [DOI] [PubMed] [Google Scholar]
- 54.Kim S.Y., Lee J.S., Kang J., Morita S., Park Y.S., Sakamoto J., Muro K., Xu R.-H., Kim T.W. Proton Pump Inhibitor Use and the Efficacy of Chemotherapy in Metastatic Colorectal Cancer: A Post Hoc Analysis of a Randomized Phase III Trial (AXEPT) Oncol. 2021;26:e954–e962. doi: 10.1002/onco.13735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee P.C., Chao Y., Lee C., Hou M.C., Huang Y.H. Effect of antibiotics, proton pump inhibitors and steroids on survival and response to immune-checkpoint inhibitors in patients with hepatocellular carcinoma. J. Hepatol. 2020;73:S912. doi: 10.1016/S0168-8278(20)32252-2. [DOI] [Google Scholar]
- 56.Miura K., Sano Y., Niho S., Kawasumi K., Mochizuki N., Yoh K., Matsumoto S., Zenke Y., Ikeda T., Nosaki K., et al. Impact of concomitant medication on clinical outcomes in patients with advanced non-small cell lung cancer treated with immune checkpoint inhibitors: A retrospective study. Thorac. Cancer. 2021;12:1983–1994. doi: 10.1111/1759-7714.14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mollica V., Santoni M., Matrana M.R., Basso U., De Giorgi U., Rizzo A., Maruzzo M., Marchetti A., Rosellini M., Bleve S., et al. Concomitant Proton Pump Inhibitors and Outcome of Patients Treated with Nivolumab Alone or Plus Ipilimumab for Advanced Renal Cell Carcinoma. Target. Oncol. 2021;17:61–68. doi: 10.1007/s11523-021-00861-y. [DOI] [PubMed] [Google Scholar]
- 58.Okuyama Y., Hatakeyama S., Numakura K., Narita T., Tanaka T., Miura Y., Sasaki D., Noro D., Tokui N., Okamoto T., et al. Prognostic impact of proton pump inhibitors for immunotherapy in advanced urothelial carcinoma. BJUI Compass. 2021;3:154–161. doi: 10.1002/bco2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peng K., Chen K., Teply B.A., Yee G.C., Farazi P.A., Lyden E.R. Impact of Proton Pump Inhibitor Use on the Effectiveness of Immune Checkpoint Inhibitors in Advanced Cancer Patients. Ann. Pharmacother. 2021;56:377–386. doi: 10.1177/10600280211033938. [DOI] [PubMed] [Google Scholar]
- 60.Pérez-Ruiz E., Jiménez-Castro J., Berciano-Guerrero M.-A., Valdivia J., Estalella-Mendoza S., Toscano F., Artacho M.R.D.L.B., Garrido-Siles M., Martínez-Bautista M.J., Roldan R.V., et al. Impact of intestinal dysbiosis-related drugs on the efficacy of immune checkpoint inhibitors in clinical practice. Clin. Transl. Oncol. 2020;22:1778–1785. doi: 10.1007/s12094-020-02315-9. [DOI] [PubMed] [Google Scholar]
- 61.Rounis K., Makrakis D., Papadaki C., Monastirioti A., Vamvakas L., Kalbakis K., Gourlia K., Xanthopoulos I., Tsamardinos I., Mavroudis D., et al. Prediction of outcome in patients with non-small cell lung cancer treated with second line PD-1/PDL-1 inhibitors based on clinical parameters: Results from a prospective, single institution study. PLoS One. 2021;16:e0252537. doi: 10.1371/journal.pone.0252537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ruiz-Bañobre J., Molina-Díaz A., Fernández-Calvo O., Fernández-Núñez N., Medina-Colmenero A., Santomé L., Lázaro-Quintela M., Mateos-González M., García-Cid N., López-López R., et al. Rethinking prognostic factors in locally advanced or metastatic urothelial carcinoma in the immune checkpoint blockade era: A multicenter retrospective study. ESMO Open. 2021;6:100090. doi: 10.1016/j.esmoop.2021.100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saltz L.B., Clarke S., Díaz-Rubio E. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J. Clin. Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 64.Sun J., Ilich A.I., Kim C.A., Chu M.P., Wong G.G., Ghosh S., Danilak M., Mulder K.E., Spratlin J.L., Chambers C.R., et al. Concomitant Administration of Proton Pump Inhibitors and Capecitabine is Associated With Increased Recurrence Risk in Early Stage Colorectal Cancer Patients. Clin. Color. Cancer. 2015;15:257–263. doi: 10.1016/j.clcc.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 65.Svaton M., Zemanova M., Zemanova P., Kultan J., Fischer O., Skrickova J., Jakubikova L., Cernovska M., Hrnciarik M., Jirousek M., et al. Impact of Concomitant Medication Administered at the Time of Initiation of Nivolumab Therapy on Outcome in Non-small Cell Lung Cancer. Anticancer. Res. 2020;40:2209–2217. doi: 10.21873/anticanres.14182. [DOI] [PubMed] [Google Scholar]
- 66.Tabernero J., Yoshino T., Cohn A.L. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): A randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16:499–508. doi: 10.1016/S1470-2045(15)70127-0. [DOI] [PubMed] [Google Scholar]
- 67.Takada K., Shimokawa M., Takamori S., Shimamatsu S., Hirai F., Ono Y., Tagawa T., Okamoto T., Hamatake M., Okamoto I., et al. The clinical impact of concomitant medication use on the outcome of postoperative recurrent non-small-cell lung cancer in patients receiving immune checkpoint inhibitors. PloS ONE. 2022;17:e0263247. doi: 10.1371/journal.pone.0263247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tomisaki I., Harada M., Minato A., Nagata Y., Kimuro R., Higashijima K., Harada K., Fujimoto N. Impact of the Use of Proton Pump Inhibitors on Pembrolizumab Effectiveness for Advanced Urothelial Carcinoma. Anticancer. Res. 2022;42:1629–1634. doi: 10.21873/anticanres.15638. [DOI] [PubMed] [Google Scholar]
- 69.Verschueren M.V., van der Welle C.M.C., Tonn M., Schramel F., Peters B.J.M., van de Garde E.M.W. The association between gut microbiome affecting concomitant medication and the effectiveness of immunotherapy in patients with stage IV NSCLC. Sci. Rep. 2021;11:23331. doi: 10.1038/s41598-021-02598-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang X., Liu C., Wang J., Fan Y., Wang Z., Wang Y. Proton pump inhibitors increase the chemosensitivity of patients with advanced colorectal cancer. Oncotarget. 2017;8:58801–58808. doi: 10.18632/oncotarget.18522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.West H., McCleod M., Hussein M., Morabito A., Rittmeyer A., Conter H.J., Kopp H.-G., Daniel D., McCune S., Mekhail T., et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:924–937. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 72.Wong G.G., Ha V., Chu M.P., Dersch-Mills D., Ghosh S., Chambers C.R., Sawyer M.B. Effects of Proton Pump Inhibitors on FOLFOX and CapeOx Regimens in Colorectal Cancer. Clin. Color. Cancer. 2018;18:72–79. doi: 10.1016/j.clcc.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 73.Xu R.-H., Muro K., Morita S., Iwasa S., Han S.W., Wang W., Kotaka M., Nakamura M., Ahn J.B., Deng Y.-H., et al. Modified XELIRI (capecitabine plus irinotecan) versus FOLFIRI (leucovorin, fluorouracil, and irinotecan), both either with or without bevacizumab, as second-line therapy for metastatic colorectal cancer (AXEPT): A multicentre, open-label, randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2018;19:660–671. doi: 10.1016/s1470-2045(18)30140-2. [DOI] [PubMed] [Google Scholar]
- 74.Zhao S., Gao G., Li W., Li X., Zhao C., Jiang T., Jia Y., He Y., Li A., Su C., et al. Antibiotics are associated with attenuated efficacy of anti-PD-1/PD-L1 therapies in Chinese patients with advanced non-small cell lung cancer. Lung Cancer. 2019;130:10–17. doi: 10.1016/j.lungcan.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 75.Kostine M., Mauric E., Tison A., Barnetche T., Barre A., Nikolski M., Rouxel L., Dutriaux C., Dousset L., Prey S., et al. Baseline co-medications may alter the anti-tumoural effect of checkpoint inhibitors as well as the risk of immune-related adverse events. Eur. J. Cancer. 2021;157:474–484. doi: 10.1016/j.ejca.2021.08.036. [DOI] [PubMed] [Google Scholar]
- 76.Kunimitsu Y., Morio K., Hirata S., Yamamoto K., Omura T., Hara T., Harada K., Fujisawa M., Yano I. Effects of Proton Pump Inhibitors on Survival Outcomes in Patients with Metastatic or Unresectable Urothelial Carcinoma Treated with Pembrolizumab. Biol. Pharm. Bull. 2022;45:590–595. doi: 10.1248/bpb.b21-00939. [DOI] [PubMed] [Google Scholar]
- 77.Kitazume Y., Kawazoe H., Uozumi R., Yoshizawa T., Iihara H., Fujii H., Takahashi M., Arai T., Murachi Y., Sato Y., et al. Proton pump inhibitors affect capecitabine efficacy in patients with stage II–III colorectal cancer: A multicenter retrospective study. Sci. Rep. 2022;12:1–10. doi: 10.1038/s41598-022-10008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Homicsko K., Dummer R., Hoeller C., Wolchok J.D., Hodi F.S., Larkin J., Ascierto P.A., Atkinson V., Robert C., Postow M.A., et al. Proton Pump Inhibitor Use and Efficacy of Nivolumab and Ipilimumab in Advanced Melanoma. Cancers. 2022;14:2300. doi: 10.3390/cancers14092300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fukuokaya W., Kimura T., Komura K., Uchimoto T., Nishimura K., Oyama Y., Abe H., Azuma H., Miki J., Egawa S. MP03-12 association of use of potassium competitive acid blockers and effectiveness of pembrolizumab in patients with metastatic urothelial carcinoma. J. Urol. 2022;207:e24. doi: 10.1097/JU.0000000000002515.12. [DOI] [Google Scholar]
- 80.Iida K., Nagai T., Nozaki S., Etani T., Naiki T., Nakane A., Akita H., Kubota H., Kamiya H., Kawai N., et al. MP41-15 proton pump inhibitor use is a negative prognostic factor for metastatic urothelial carcinoma progression in pembrolizumab-treated patients. J. Urol. 2021;206:e766–e767. doi: 10.1097/JU.0000000000002062.15. [DOI] [Google Scholar]
- 81.Ascierto P.A., Long G.V., Robert C., Brady B., Dutriaux C., Di Giacomo A.M., Mortier L., Hassel J.C., Rutkowski P., McNeil C., et al. Survival Outcomes in Patients With Previously Untreated BRAF Wild-Type Advanced Melanoma Treated With Nivolumab Therapy: Three-Year Follow-up of a Randomized Phase 3 Trial. JAMA Oncol. 2019;5:187–194. doi: 10.1001/jamaoncol.2018.4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hodi F.S., Chiarion-Sileni V., Gonzalez R., Grob J.-J., Rutkowski P., Cowey C.L., Lao C.D., Schadendorf D., Wagstaff J., Dummer R., et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1480–1492. doi: 10.1016/S1470-2045(18)30700-9. [DOI] [PubMed] [Google Scholar]
- 83.Hodi F.S., Chesney J., Pavlick A.C., Robert C., Grossmann K.F., McDermott D.F., Linette G.P., Meyer N., Giguere J.K., Agarwala S.S., et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016;17:1558–1568. doi: 10.1016/S1470-2045(16)30366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jackson M.A., Goodrich J.K., Maxan M.-E., Freedberg D.E., Abrams J.A., Poole A.C., Sutter J.L., Welter D., Ley R.E., Bell J.T., et al. Proton pump inhibitors alter the composition of the gut microbiota. [(accessed on 19 January 2019)];Gut. 2016 65:749–756. doi: 10.1136/gutjnl-2015-310861. Available online: http://www.ncbi.nlm.nih.gov/pubmed/26719299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oster P., Vaillant L., Riva E., McMillan B., Begka C., Truntzer C., Richard C., Leblond M.M., Messaoudene M., Machremi E., et al. Helicobacter pylori infection has a detrimental impact on the efficacy of cancer immunotherapies. Gut. 2021;71:457–466. doi: 10.1136/gutjnl-2020-323392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thorsson V., Gibbs D.L., Brown S.D., Wolf D., Bortone D.S., Ou Yang T.-H., Porta-Pardo E., Gao G.F., Plaisier C.L., Eddy J.A., et al. The Immune Landscape of Cancer. Immunity. 2018;48:812–830.e14. doi: 10.1016/j.immuni.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Matson V., Fessler J., Bao R., Chongsuwat T., Zha Y., Alegre M.-L., Luke J.J., Gajewski T.F. The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104–108. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gopalakrishnan V., Spencer C.N., Nezi L., Reuben A., Andrews M.C., Karpinets T.V., Prieto P.A., Vicente D., Hoffman K., Wei S.C., et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ueda K., Yonekura S., Ogasawara N., Matsunaga Y., Hoshino R., Kurose H., Chikui K., Uemura K., Nakiri M., Nishihara K., et al. The Impact of Antibiotics on Prognosis of Metastatic Renal Cell Carcinoma in Japanese Patients Treated With Immune Checkpoint Inhibitors. Anticancer. Res. 2019;39:6265–6271. doi: 10.21873/anticanres.13836. [DOI] [PubMed] [Google Scholar]
- 90.Elkrief A., El Raichani L., Richard C., Messaoudene M., Belkaid W., Malo J., Belanger K., Miller W., Jamal R., Letarte N., et al. Antibiotics are associated with decreased progression-free survival of advanced melanoma patients treated with immune checkpoint inhibitors. Oncoimmunology. 2019;8:e1568812. doi: 10.1080/2162402X.2019.1568812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.De Milito A., Fais S. Tumor acidity, chemoresistance and proton pump inhibitors. Future Oncol. 2005;1:779–786. doi: 10.2217/14796694.1.6.779. [DOI] [PubMed] [Google Scholar]
- 92.Schmoll H.-J., Cunningham D., Sobrero A., Karapetis C.S., Rougier P., Koski S.L., Kocakova I., Bondarenko I., Bodoky G., Mainwaring P., et al. Cediranib With mFOLFOX6 Versus Bevacizumab With mFOLFOX6 As First-Line Treatment for Patients With Advanced Colorectal Cancer: A Double-Blind, Randomized Phase III Study (HORIZON III) J. Clin. Oncol. 2012;30:3588–3595. doi: 10.1200/JCO.2012.42.5355. [DOI] [PubMed] [Google Scholar]
- 93.Nishi T., Forgac M. The vacuolar (H+)-ATPases--nature’s most versatile proton pumps. Nat. Rev. Mol. Cell Biol. 2002;3:94–103. doi: 10.1038/nrm729. [DOI] [PubMed] [Google Scholar]
- 94.Marino M.L., Fais S., Djavaheri-Mergny M., Villa A., Meschini S., Lozupone F., Venturi G., Della Mina P., Pattingre S., Rivoltini L., et al. Proton pump inhibition induces autophagy as a survival mechanism following oxidative stress in human melanoma cells. Cell Death Dis. 2010;1:e87. doi: 10.1038/cddis.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lalani A.A., McKay R.R., Lin X., Simantov R., Kaymakcalan M.D., Choueiri T.K. Proton Pump Inhibitors and Survival Outcomes in Patients With Metastatic Renal Cell Carcinoma. Clin. Genitourin. Cancer. 2017;15:724–732. doi: 10.1016/j.clgc.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 96.Cheng V., Lemos M., Hunter N., Badry N., Lemos J. Concomitant use of capecitabine and proton pump inhibitors—Is it safe? J. Oncol. Pharm. Pract. 2019;25:1705–1711. doi: 10.1177/1078155219846952. [DOI] [PubMed] [Google Scholar]
- 97.Röhss K., Lind T., Wilder-Smith C. Esomeprazole 40 mg provides more effective intragastric acid control than lansoprazole 30 mg, omeprazole 20 mg, pantoprazole 40 mg and rabeprazole 20 mg in patients with gastro-oesophageal reflux symptoms. Eur. J. Clin. Pharmacol. 2004;60:531–539. doi: 10.1007/s00228-004-0804-6. [DOI] [PubMed] [Google Scholar]
- 98.Wishart D.S., Feunang Y.D., Guo A.C., Lo E.J., Marcu A., Grant J.R., Sajed T., Johnson D., Li C., Sayeeda Z., et al. DrugBank 5.0: A Major Update to the DrugBank Database for 2018. Nucleic Acids Res. 2018;46:D1074–D1082. doi: 10.1093/nar/gkx1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Diaz L.A., Shiu K.-K., Jr., Kim T.-W., Jensen B.V., Jensen L.H., Punt C., Smith D., Garcia-Carbonero R., Benavides M., Gibbs P., et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): Final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022;23:659–670. doi: 10.1016/S1470-2045(22)00197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Le D.T., Uram J.N., Wang H., Bartlett B.R., Kemberling H., Eyring A.D., Skora A.D., Luber B.S., Azad N.S., Laheru D., et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bellmunt J., De Wit R., Vaughn D.J., Fradet Y., Lee J.-L., Fong L., Vogelzang N.J., Climent M.A., Petrylak D.P., Choueiri T.K., et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017;376:1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fradet Y., Bellmunt J., Vaughn D.J., Lee J.L., Fong L., Vogelzang N.J., Climent M.A., Petrylak D.P., Choueiri T.K., Necchi A., et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: Results of >2 years of follow-up. Ann. Oncol. 2019;30:970–976. doi: 10.1093/annonc/mdz127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sharma P., Retz M., Siefker-Radtke A., Baron A., Necchi A., Bedke J., Plimack E.R., Vaena D., Grimm M.-O., Bracarda S., et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18:312–322. doi: 10.1016/S1470-2045(17)30065-7. [DOI] [PubMed] [Google Scholar]
- 104.Powles T., O’Donnell P.H., Massard C., Arkenau H.T., Friedlander T.W., Hoimes C.J., Lee J.L., Ong M., Sridhar S.S., Vogelzang N.J., et al. Efficacy and Safety of Durvalumab in Locally Advanced or Metastatic Urothelial Carcinoma: Updated Results From a Phase 1/2 Open-label Study. JAMA Oncol. 2017;3:e172411. doi: 10.1001/jamaoncol.2017.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Overman M.J., McDermott R., Leach J.L., Lonardi S., Lenz H.-J., Morse M.A., Desai J., Hill A., Axelson M., Moss R.A., et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.