Abstract
Filamentous hemagglutinin (FHA) is a dominant cell surface-associated Bordetella pertussis adhesin. Recognition that this protein is secreted in significant amounts and that bacterial adhesins may have other actvities, prompted an assessment of FHA effects on human macrophages. Incubation of human macrophage-like U937 cells with preparations of FHA resulted in dose-dependent cytotoxicity, with death of 95% of treated cells after 24 h. Based on the use of four independent methods, death of these cells could be largely attributed to apoptosis. FHA-associated apoptosis was also observed in THP-1 macrophage-like cells, fresh human peripheral blood monocyte-derived macrophages (MDM), and BEAS-2B human bronchial epithelial cells. Infection of MDM with wild-type B. pertussis resulted in apoptosis within 6 h, while infection with an FHA-deficient derivative strain was only 50% as effective. FHA-associated cytotoxicity was preceded by host cell secretion of tumor necrosis factor alpha (TNF-α), a potential proapoptotic factor. However, pretreatment of cells with a neutralizing anti-TNF-α monoclonal antibody inhibited only 16% of the FHA-associated apoptosis. On the other hand, a blocking monoclonal antibody directed against TNF-α receptor 1 inhibited FHA-associated apoptosis by 47.7% (P = 0.0001), suggesting that this receptor may play a role in the death pathway activated by FHA. Our in vitro data indicate that secreted and cell-associated FHA elicits proinflammatory and proapoptotic responses in human monocyte-like cells, MDM, and bronchial epithelial cells and suggest a previously unrecognized role for this prominent virulence factor in the B. pertussis-host interaction.
Bordetella pertussis is a gram-negative coccobacillus that infects the human respiratory tract and causes whooping cough (14, 32, 34, 42). This disease is associated with damage to ciliated respiratory epithelial cells, recruitment of inflammatory cells, accumulation of cellular debris and secretions, and a prolonged period of clinical symptoms and signs. B. pertussis expresses and releases a number of potent virulence factors, such as pertussis toxin, adenylate cyclase toxin, and tracheal cytotoxin, to which much of the pathology can be ascribed. A group of B. pertussis protein adhesins mediate colonization of the respiratory tract and attachment to host cells, including filamentous hemagglutinin (FHA), pertactin, tracheal colonization factor, and the pertussis toxin B subunit. FHA plays the dominant role among these attachment factors. FHA is synthesized in a 370-kDa precursor form and is then processed to yield a 220-kDa mature protein that is both anchored to the bacterial surface and secreted in copious amounts into the extracellular milieu (20, 21). FHA binds to ciliated respiratory epithelial cells and other host cells such as local macrophages in a galactose-dependent lectin-like manner, mediated by a carbohydrate recognition domain (31). It serves as a ligand for at least two leukocyte integrins, leukocyte response integrin (LRI) and complement receptor 3 (CR3) (14, 33, 35). An FHA Arg-Gly-Asp (RGD) sequence (residues 1097 to 1099) is recognized by LRI; as a surface-associated ligand, FHA induces upregulated CR3-mediated recognition of itself in an RGD-dependent fashion. FHA also binds sulfated polysaccharides (25) and shares sequence motifs with another CR3 ligand, clotting factor X (35). Given the conditions under which B. pertussis adherence has been examined in vitro (24) and the usual mechanisms of bacterial adherence, the surface-associated form of FHA, rather than the secreted form, is assumed to be responsible for most of these adhesin activities. Nonetheless, accessory adherence roles for secreted FHA in bacterium-host cell and bacterium-bacterium interactions are distinct possibilities (24, 41).
Apoptosis, or programmed cell death, occurs when mammalian cell homeostatic mechanisms are disturbed and cellular inhibitors of apoptosis (cIAPs) are suppressed. The apoptotic innate cellular response is usually regarded as an attempt to eliminate damaged cells or to resolve infection. Cytokine release by apoptotic cells has been suggested as a complementary mechanism for removal of these cells, through recruitment of phagocytes (2, 27). Bacterial pathogens such as Salmonella spp., Shigella flexneri, Yersinia entercolitica, B. pertussis, and many others (47) enhance their survival in the host by inducing apoptosis of immune cells (45, 47). In the setting of bacterial disease, the level of cytokine expression and degree of apoptosis are often correlated (45). Salmonella spp. and S. flexneri, both secrete factors that bind to and activate interleukin-1β (IL-1β) converting enzyme (also known as caspase 1), thereby inducing macrophage apoptosis and increased inflammation (12, 48). Staphylococcus aureus induces apoptosis in epithelial cells primarily through caspase 8 and 3 activation, concomitant with increased tumor necrosis factor (TNF-α) expression (44).
TNF-α is a multifunctional cytokine that is cytotoxic to several cell types but protective to others. By binding the cellular receptors TNF receptor type 1 (TNFR1) or TNFR2, TNF-α can either activate the antiapoptotic NF-κB and JNK–AP-1 transcription factor pathways or induce apoptosis through recruitment of cytoplasmic adapter proteins with death domains (39). For example, neutralization of TNF-α secreted during Chlamydia trachomatis infection of the murine genital tract significantly reduced the percentage of apoptotic cells in the oviduct (29), while other studies have suggested that TNF-α secretion has a protective effect against apoptosis (19, 26).
In this study, we investigated possible proinflammatory activities of FHA in human cell types relevant to infection. We show that this adhesin may induce apoptosis in human monocyte-like cells, macrophages, and bronchial epithelial cells and stimulates secretion of TNF-α. Our data suggest that while TNFR1 partially mediates FHA-associated apoptosis, FHA-associated TNF-α secretion alone may not explain this process. These findings lead to speculation that FHA, in addition to other virulence factors and independent of its well-established role as an adhesin, may interfere with or modify the development of the host immune response to this pathogen and hence the outcome of the host-pathogen interaction. These findings also have implications for the design of acellular pertussis vaccines.
MATERIALS AND METHODS
Bacteria and bacterial products.
B. pertussis 338 is a nalidixic acid-resistant derivative of Tohama I (43). B. pertussis 3586 is a streptomycin-resistant derivative of B. pertussis 338 with a complete deletion of the FHA structural gene, fhaB (3). Cultures were grown at 37°C on Bordet-Gengou agar (Difco, Detroit, Mich.) supplemented with whole sheep blood (13%, vol/vol) and then propagated in supplemented Stainer and Scholte modified broth medium (38) with shaking at 37°C. B. pertussis FHA preparations were provided by Rino Rappuoli and Mariagrazia Pizza (IRIS, Siena, Italy, and Chiron Corporation, Emeryville, Calif.) at concentrations of 200 μg or 1 mg/ml. FHA was purified from culture supernatants using anion exchange chromatography (Matrex Cellufine Sulfate; Millipore Corp., Watford, United Kingdom), filtration with Millipack 100 filters, and other methods used as part of antigen preparation at IRIS-Chiron which exclude adenylate cyclase toxin and bacterial DNA. Enzyme-linked immunosorbent assay (ELISA) did not reveal any pertactin or pertussis toxin in these FHA preparations. The FHA preparations contained 2,000 EU of endotoxin units (EU) per ml. B. pertussis lipopolysaccharide (LPS) was purchased from List Biological (Campbell, Calif.). The endotoxin content of the FHA and LPS preparations was measured with a Limulus amoebocyte lysate assay (QCL-1000 kit; BioWhittaker, Walkersville, Md).
Mammalian cells and cell culture.
The human monocyte-like cell lines U937 and THP-1, and the human bronchial epithelial cell line BEAS2-B were obtained from the American Type Culture Collection (Manassas, Va.) (#CRL-15932 a, #TIB-202, and #CRL-9606 respectively). The U937 and THP-1 cells were maintained in RPMI 1640 tissue culture medium supplemented with 10% fetal bovine serum. U937 and THP-1 cells were induced to differentiate into macrophage-like cells by treatment with 10 ng of phorbol 12-myristate 13-acetate (Sigma, St. Louis, Mo.) per ml for 48 h and were then washed with phosphate-buffered saline and incubated in RPMI medium without 12-myristate 13-acetate for an additional 24 h prior to use in various assays. Differentiated cells were analyzed by fluorescence-activated cell sorting (FACS) for the presence of the FHA receptors, CR3 (αMβ2), and LRI-IAP with M1-70 and 7G2/B6H12 monoclonal antibodies, respectively, and were found to express these receptors at significantly higher levels than undifferentiated cells (data not shown). BEAS-2B cells were maintained in EMEM medium (BioWhittaker) supplemented with 10% fetal bovine serum and 2 mM l-glutamine. Human peripheral blood monocytes were prepared from the blood of healthy donors as described previously (3). Briefly, mononuclear leukocytes were separated by centrifugation on a Ficoll-Paque (Amersham, Piscataway, N.J.) gradient at 400 × g for 15 min. Monocytes were further purified by a depletion method using magnetic beads (Miltenyi Biotec, Auburn, Calif.). The purified monocytes were allowed to differentiate into macrophage-like cells by incubation in RPMI for 7 to 10 days.
Antibodies and cytokines.
Anti-TNF-α immunoglobulin G1 (IgG1) and anti-TNFR1 IgG2b blocking antibodies were purchased from Alexis Biochemical (San Diego, Calif.), IgG1 and IgG2b isotype control antibodies were purchased from BioSource International (Camarillo, Calif.), and IgG2a isotype control antibody was purchased from PharMingen (San Diego, Calif.). TNF-α was purchased from BioSource International.
Measurement of secreted TNF-α.
TNF-α secretion was measured in tissue culture media after incubating differentiated U937 cells with FHA, LPS, or no additive for various periods of time in 96-well plates (105 cells per well). TNF-α concentrations in tissue culture media were determined with a solid-phase sandwich ELISA (BioSource International). At least three replicates for each condition were analyzed per experiment, and the mean and standard error of the mean were calculated. Each experiment was performed on at least three independent occasions.
Cytotoxicity assay.
Differentiated U937 cells were incubated in 96-well plates at 105 cells per well for various times with various concentrations of purified FHA or LPS. Release of cytosolic lactate dehydrogenase (LDH) was used as a marker of host cell death. LDH was measured with a coupled colorimetric enzymatic assay (CytoTox96 kit; Promega, Madison, Wis.) according to the manufacturer's protocol. The results were expressed as percent viable cells: 100 − [100 (OD of treated cells/OD of fully lysed cells)], where OD is optical density. At least three replicates for each condition were analyzed per experiment, and the mean and standard error of the mean were calculated. Each experiment was performed on at least three independent occasions.
Apoptosis analysis.
Apoptosis was assessed by four different methods.
(iv) TUNEL staining.
After various treatments, U937 cells were differentiated on coverslips and then fixed in 4% paraformaldehyde, permeabilized with 0.1% sodium citrate and 0.1% Triton X-100, and then stained with a terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) procedure (Roche Molecular Biochemical, Indianapolis, Ind.). Labeled apoptotic nuclei were visualized with laser confocal microscopy (MRC1024; Bio-Rad, Hercules, Calif.) at 488 nm and with transmission microscopy.
(ii) Immunoblot analysis of PARP degradation.
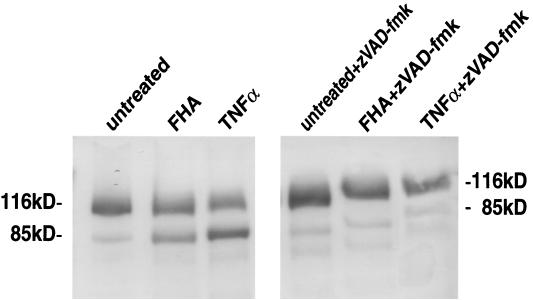
Programmed cell death is accompanied by the proteolytic cleavage of the death substrate poly(ADP-ribose) polymerase (PARP) (116 kDa) into 85- and 25-kDa fragments. The 116- and 85-kDa PARP moieties were detected and visualized by immunoblot analysis using a purified, murine monoclonal anti-PARP antibody, 7D3-6 (PharMingen) and an alkaline phosphatase-conjugated, goat anti-mouse secondary antibody (GIBCO-BRL). Briefly, differentiated U937 cells (106/well) were preincubated in six-well plates for 1 h with or without zVAD-fmk (20 μg/ml), a broad-spectrum inhibitor of caspase proteolytic activity (Enzyme Systems Products, Livermore, Calif.). Cell extracts were prepared by incubating the cells on ice for 30 min in 0.1 ml of buffer containing 20 mM HEPES (pH 7.4), 2 mM EDTA, 250 mM NaCl, 0.1% Nonidet P-40, leupeptin (2 μg/ml), aprotinin (2 μg/ml), 1 mM phenylmethylsulfonyl fluoride, benzamidine (0.5 μg/ml), and 1 mM dithiothreitol. Following centrifugation, 50 μg of cell extract protein was resolved by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis, electro-transferred to a polyvinylide difluoride membrane (Millipore) and probed first with monoclonal antibody 7D3-6 and then with a goat anti-mouse alkaline phosphatase-conjugated antibody (BioSource International).
(iii) Annexin V-FITC staining.
The externalization of membrane phosphatidylserine on apoptotic cells was detected using an Annexin V-FITC staining procedure (Annexin V-FITC Apoptosis Detection kit; PharMingen). Differentiated U937 cells (106) were either treated with FHA or left untreated in six-well plates and then stained for 15 min with both Annexin V-FITC and propidium iodide. Flow cytometric analysis was performed on 105 cells with a FACS Calibur instrument (Becton Dickinson, Mountain View, Calif.).
(iv) ELISA detection of cytoplasmic mono- and oligonucleosomes.
The generation of mono- and oligonucleosomes in the cytoplasm of apoptotic cells was measured with a quantitative, photometric sandwich ELISA, involving the use of biotinylated antihistone and peroxidase-conjugated anti-DNA antibodies (Cell Death Detection ELISAPLUS kit; Roche Molecular Biochemical). Cells of various types were allowed to attach to 96-well plates (104 cells/well) for 3 h and then washed and exposed to different stimuli for various periods of time. In some experiments, THP-1 cells were exposed for 20 h to 106 B. pertussis organisms (multiplicity of infection [MOI] = 100) that were heat killed at 56°C for 30 min, or exposed to the culture supernatant of 106 B. pertussis organisms (200 μl). Human monocyte-derived macrophages (MDMs) at 2 × 104 cells/well were incubated in 96-well plates with live bacteria at an MOI of 1:10 for 6 h. (After a 5-min centrifugation at 500 × g, cells were incubated for 6 h and then analyzed for apoptosis.) In other experiments, U937 cells were pretreated for 1 h with anti-TNF-α, anti-TNFR1, or isotype control antibodies (10 μg/ml) (see above) and then exposed to various stimuli. In this ELISA, the results are expressed as a ratio of the absorbance measurement from treated cells to that from untreated cells (enrichment factor [EF]), i.e., as the specific enrichment of mono- and oligonucleosomes in the cytoplasm of treated cells. At least three replicates for each condition were analyzed per experiment, and the mean and standard error of the mean were calculated. Each experiment was performed on at least three independent occasions.
RESULTS
FHA stimulates TNF-α secretion by U937 monocyte-like cells and induces cell death.
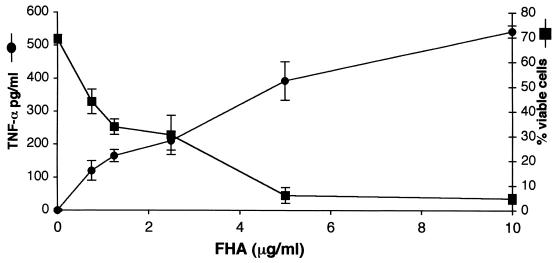
FHA proinflammatory activity was assessed by examining TNF-α secretion by U937 cells following exposure to FHA protein. Differentiated U937 cells were incubated for 20 h with B. pertussis FHA at various concentrations. As shown in Fig. 1, FHA stimulated TNF-α secretion by these cells in a dose-dependent manner, producing TNF-α concentrations of 543 pg/ml after exposure to 10 μg of FHA per ml for 20 h. THP-1 macrophage-like cells secreted 1,052 pg of TNF-α per ml when treated with 10 μg of FHA per ml for 20 h. At this 20-h time point, a near-complete loss of U937 viability was noted, as assessed by LDH leakage. The cytotoxic effect was also FHA dose-dependent, with only 5.4% ± 1.3% cells remaining viable after exposure to FHA at 10 μg/ml (Fig. 1).
FIG. 1.
FHA is associated with proinflammatory and cytotoxic responses in differentiated U937 cells. Cells were incubated for 20 h with various concentrations of FHA, and supernatants were then analyzed for secreted TNF-α (circles) and for the release of LDH (squares). The percentage of viable cells was calculated as described in Materials and Methods. Triplicate measurements were performed for each condition, and the standard error of the mean (bars) was calculated. These are representative data from one of four independent experiments with similar results.
B. pertussis LPS alone is proinflammatory but not cytotoxic.
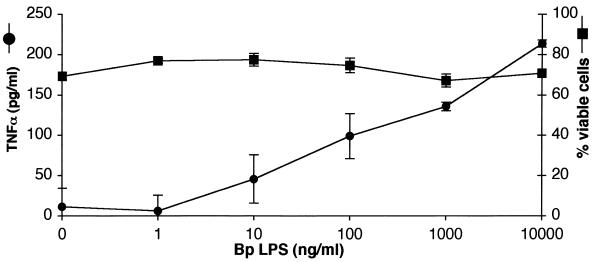
FHA is purified from B. pertussis broth culture supernatants and, like many purified bacterial components, is commonly contaminated with small amounts of LPS. The FHA used in this study contained 2.0 EU of endotoxin/μg of FHA. To rule out the possibility that the observed FHA activity was due to the minute amounts of LPS present in the FHA preparations, U937 cells were incubated with various concentrations of B. pertussis LPS (1 ng/ml-10 μg/ml LPS or 4 to 40,000 EU/ml) for 20 h. Secreted TNF-α and cell viability were measured as described above. As expected, LPS stimulated TNF-α secretion in a dose-dependent fashion (Fig. 2). However, FHA induced far more TNF-α secretion than would have been expected from the known amounts of LPS that contaminated these preparations, based on a comparison of endotoxin activity in FHA and LPS preparations needed to induce secretion of equivalent levels of TNF-α (100 pg/ml): 2.0 EU/ml in the FHA preparation and 400 EU/ml in the LPS preparation. These results indicate that FHA has intrinsic proinflammatory activity or acts synergistically with LPS or both. Furthermore, B. pertussis LPS alone did not affect U937 cell viability (Fig. 2), supporting the proposal that FHA has intrinsic cytotoxic activity.
FIG. 2.
B. pertussis LPS induces a proinflammatory response but not cell death. U937 cells were incubated for 20 h with various concentrations of B. pertussis (Bp) LPS. Supernatants were analyzed for TNF-α (circles) and LDH. The percentage of viable cells was calculated (squares). Triplicate measurements were performed for each condition, and the standard error of the mean (bars) was calculated. These are representative data from one of three independent experiments with similar results.
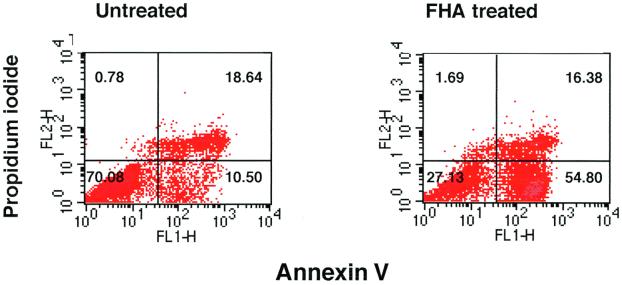
FHA promotes apoptosis in monocyte-like cells.
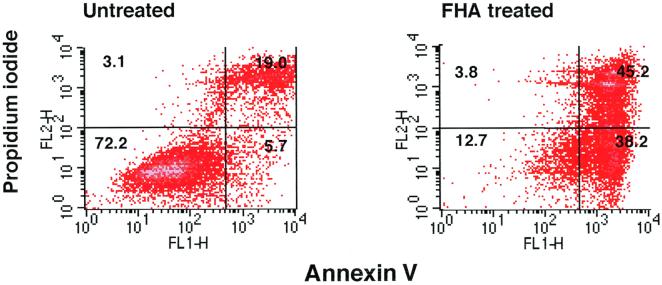
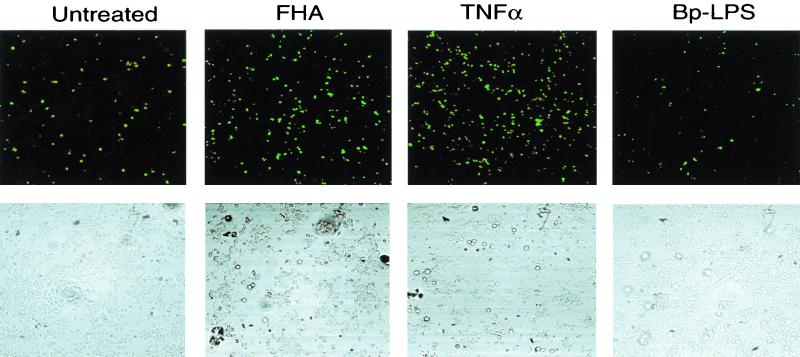
In order to determine whether the cytotoxic effect observed in FHA-treated U937 cells constituted apoptosis, several independent methods were employed. First, differentiated U937 cells were stained with Annexin V-FITC and propidium iodide (PI) after a 20-h incubation in either the presence or absence of 5 μg of FHA per ml. Annexin V binds to cells in both early and late stages of apoptosis, due to its affinity for externalized membrane phosphatidylserine, while PI stains only those cells in the late stages of apoptosis or necrosis. After FHA treatment, 38% of the U937 cells were stained by Annexin V-FITC alone, indicating that these cells were in an early stage of apoptosis (versus 5.7% of the untreated cells) (Fig. 3). Second, FHA-treated U937 cells were labeled by the TUNEL procedure. Apoptotic nuclei were far more common among cells treated with FHA (5 μg/ml) or TNF-α (50 ng/ml) than among untreated cells or cells treated with LPS (1 μg/ml), despite treatment of equal numbers of cells in each experimental condition (Fig. 4). These experiments confirmed that U937 cells respond to TNF-α by undergoing programmed cell death and that LPS is devoid of cytotoxic activity. Third, a PARP cleavage assay was used to determine whether apoptotic pathways had been activated in cells treated with FHA. PARP (a nuclear 116-kDa protein) is cleaved by activated caspase 3 to yield an 85-kDa “death fragment” (40), identified by Western blot analysis. Partial cleavage of the 116-kDa PARP was observed in cells treated for 20 h with FHA (5 μg/ml) or with TNF-α (50 ng/ml) but not in untreated cells (Fig. 5). As expected, cleavage was almost completely abolished when cells were pretreated for 1 h with zVAD-fmk (20 μg/ml), an inhibitor of caspase activity. Taken together, these data strongly suggested that FHA promotes apoptosis, although a partial, contributory role for necrotic pathways could not be excluded.
FIG. 3.
Annexin V-PI staining of U937 cells. Differentiated U937 cells were treated with medium only or with FHA (5 μg/ml) for 20 h and then stained with Annexin V-FITC–PI and analyzed by FACS (see Materials and Methods). These are representative data from one of three experiments with similar results.
FIG. 4.
Assessment of apoptosis in U937 cells using the TUNEL reaction. Cells were either untreated or incubated for 20 h with either FHA (5 μg/ml), TNF-α (50 ng/ml), or B. pertussis LPS (1 μg/ml). Labeled apoptotic nuclei were visualized with laser confocal microscopy at 488 nm (top row). The cells in the same field were also visualized with contrast microscopy (bottom row). These are representative fields from one of three experiments with similar results. Note that fewer cells remain intact after treatment with FHA and TNF-α than after other treatments (bottom row), due to programmed cell death.
FIG. 5.
Assessment of apoptosis by immunoblot analysis of PARP degradation. Differentiated U937 cells were pretreated for 1 h with either medium alone (left panel) or with zVAD-fmk (20 μg/ml) (right panel), and then incubated for 20 h in medium alone, FHA (5 μg/ml), or TNF-α (50 ng/ml). The cells were lysed and analyzed by immunoblotting with anti-PARP monoclonal antibody (see Materials and Methods). These are representative data from one of three experiments with similar results.
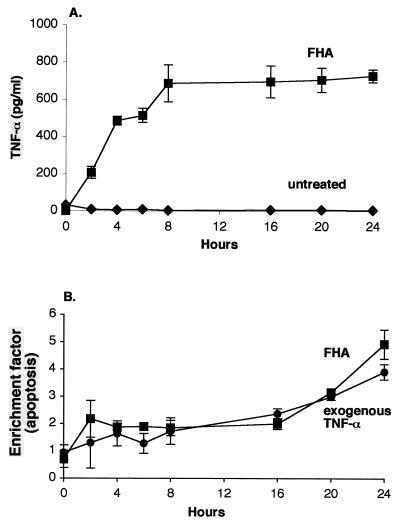
Temporal features of FHA-induced TNF-α secretion and apoptosis.
TNF-α secretion by U937 cells achieved maximum levels after 8 h of incubation using 5 μg of FHA per ml, as shown in Fig. 6A. Significant levels of apoptosis in these cells were detected as early as 2 h into incubation with 5 μg of FHA per ml (EF, 2.2 ± 0.39; P = 0.013), and then continued to increase throughout the 24-h period, achieving levels nearly fivefold higher than those observed in untreated cells (EF, 4.9 ± 0.53; P = 0.0007) (Fig. 6B). These data were collected using an ELISA for cytoplasmic mono- and oligonucleosomes. A similar time course of apoptosis was recorded when these cells were treated instead with 50 ng of exogenous TNF-α per ml.
FIG. 6.
Time course of FHA-associated TNF-α secretion (A) and apoptosis (B) in U937 cells. Differentiated U937 cells were incubated for various periods of time with either medium alone (diamonds [panel A only]), FHA (5 μg/ml) (squares), or purified TNF-α (50 ng/ml) (circles [panel B only]) and then examined for TNF-α secretion (A) and for apoptosis (B) by ELISA methods (see Materials and Methods). The data shown are mean values from the analysis of samples in triplicate and are representative of three separate experiments. Bars indicate the standard error of the mean.
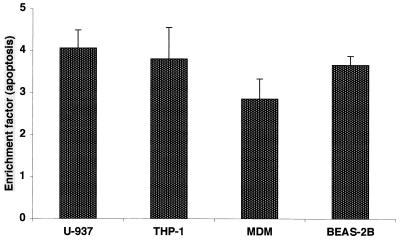
FHA-associated apoptosis is not restricted to U937 cells.
To rule out the possibility of a cell line-specific phenomenon, we examined the ability of FHA to promote apoptosis in two additional monocyte- or macrophage-like cells (THP-1 cells and fresh human peripheral MDMs) and a human bronchial epithelial cell line (BEAS2-B). After exposure to FHA (5 μg/ml), all of these cell types were found to undergo programmed cell death to a significantly higher degree (three- to fourfold higher) than untreated cells (Fig. 7). Apoptosis associated with FHA in the human peripheral blood MDMs was dose dependent (data not shown). After 20 h of incubation with FHA (5 μg/ml) MDMs appeared to be in an earlier phase of apoptosis (55% in early phase versus 16% in late phase) (Fig. 8) than FHA-treated U937 cells (38% versus 45%) (Fig. 3). These data suggest that at least two cell types with relevance during the course of B. pertussis infection are susceptible to FHA-associated programmed cell death.
FIG. 7.
FHA-associated apoptosis in various human cell types. Differentiated U937 and THP-1 monocyte/macrophages, fresh peripheral blood MDMs, and BEAS-2B bronchial epithelial cells were incubated either with medium alone or FHA (5 μg/ml) for 20 h. Cells were then analyzed for apoptosis by an ELISA method. The data shown are mean values from the analysis of samples in triplicate and are representative of three separate experiments. Error bars indicate the standard error of the mean.
FIG. 8.
Annexin V-PI staining of fresh human MDMs. Cells were treated with medium only or with FHA (5 μg/ml) for 20 h and stained with Annexin V-FITC-PI and analyzed by FACS (see Materials and Methods). These are representative data from one of three experiments with similar results.
B. pertussis cells induce apoptosis.
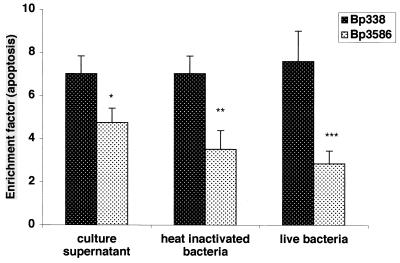
Despite the fact that FHA is secreted by B. pertussis in significant amounts, some protein also remains cell associated and is presented to host cells in the context of other bacterial structures. Wild-type B. pertussis 338 and an isogenic FHA-deficient strain (B. pertussis 3586) were studied for their ability to induce apoptosis. Although both strains were capable of inducing apoptosis in fresh human MDMs at levels significantly greater than those seen in uninfected cells, the FHA-deficient derivative strain was significantly less competent than its wild-type parent after 6 h of incubation at an MOI 1:10 (EF, 7.6 ± 1.4 versus 2.85 ± 0.6; P<0.01) (Fig. 9). Heat-killed bacteria displayed similar FHA-dependent proapoptotic activity after 20 h of incubation with THP-1 macrophage-like cells at an MOI of 100 (EF, 7.0 ± 0.82 versus 3.5 ± 0.87, P = 0.019). We verified that the FHA preparation does not lose its ability to induce apoptosis when subjected to the conditions used for heat killing: 30 min at 56°C (data not shown). We also examined the proapoptotic effect of culture supernatants from the two strains, which were prepared from equal numbers of cells in log phase. Both supernatants exhibited significant activity, although the supernatant from FHA-deficient cells was less active (EF, 7.0 ± 0.83 versus 4.8 ± 0.66, P = 0.04). These results support our observations of FHA-associated proapoptotic activity and suggest that this activity is relevant in the context of the intact and live bacterium. These data support the concept that other secreted B. pertussis components share this activity (8, 16, 17).
FIG. 9.
Apoptosis induced in macrophages by B. pertussis strains. Differentiated THP-1 monocytes were exposed for 20 h to culture supernatant from equal numbers of either B. pertussis BP 338 or BP 3586, or heat-inactivated bacteria at an MOI of 1:100. MDMs were exposed to live BP 338 or BP 3586 at an MOI of 1:10 for 6 h. Apoptosis was assessed by an ELISA method. The data shown are mean values from the analysis of samples in triplicate and are representative of three separate experiments. Error bars indicate the standard error of the mean. Statistical significance is indicated for comparisons between the two strains as analyzed by t test (two-sample test, assuming equal variances): P = 0.019 (∗), P = 0.04 (∗∗), and P = 0.01 (∗∗∗).
TNF-α secretion is insufficient to explain FHA-induced apoptosis, although TNFR1 is a possible mediator.
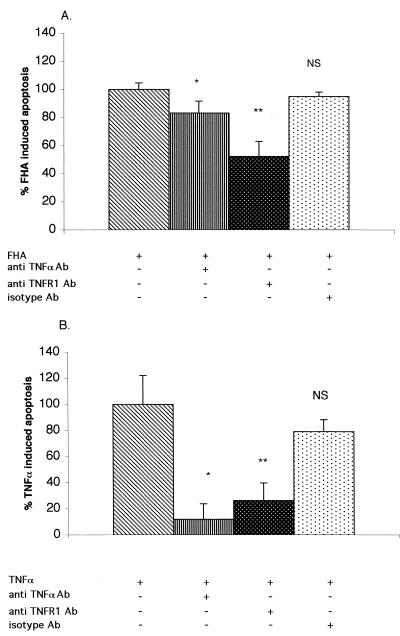
Given that TNF-α alone is capable of promoting apoptosis in U937 cells, we hypothesized that the TNF-α released upon FHA treatment acts as an autocrine signal and is responsible for the proapoptotic effect of FHA. U937 cells were pretreated for 1 h with either anti-TNF-α or anti-TNFR1 blocking antibodies or appropriate isotype antibody controls, and then treated for 20 h with either FHA (5 μg/ml) or TNF-α (50 ng/ml). Apoptosis was measured with an ELISA method as described above. The results obtained with antibody pretreatments were expressed relative to the results obtained with FHA or TNF-α alone. As shown in Fig. 10A, a neutralizing anti-TNF-α antibody had only a minor inhibitory effect on FHA-associated apoptosis (16.1% inhibition), although it blocked 88.2% of the apoptosis induced by exogenous TNF-α (Fig. 10B), suggesting that secreted TNF-α does not play a major role in FHA-induced apoptosis. On the other hand, a blocking anti-TNFR1 antibody reduced apoptosis to 52% ± 11% of the levels observed without antibody pretreatment (P = 0.0001) (Fig. 10A) and 26% of the levels in TNF-α-induced cells (P = 0.044) (Fig. 10B). Thus, anti-TNFR1 antibody had a greater inhibitory effect on FHA-associated apoptosis than did anti-TNF-α antibody. These findings argue that TNFR1 plays a role in mediating FHA-associated apoptosis.
FIG. 10.
Inhibition of FHA-associated (A) and TNF-α-associated (B) apoptosis by anti-TNF-α and anti-TNFR1 blocking antibodies. Differentiated U937 cells were pretreated for 1 h with medium alone, anti-TNF-α antibody (10 μg/ml), anti-TNFR1 antibody (10 μg/ml), or an isotype-control antibody (10 μg/ml). Cells were then treated for 20 h with medium alone, FHA (5 μg/ml) (A), or TNF-α (50 ng/ml) (B). Cells were then analyzed for apoptosis by an ELISA method. The amount of apoptosis from cells without pretreatment was defined as 100%. The data shown are mean values from the analysis of samples in triplicate and are representative of three separate experiments. Bars indicate the standard error of the mean. Statistical significance was analyzed by t test as indicated for the comparisons between cells with and without pretreatment (two-sample test, assuming equal variances). (A) P = 0.044 (∗) and P = 0.0001 (∗∗); (B) P = 0.01 (∗) and P = 0.02 (∗∗). NS, not significant.
DISCUSSION
The investigation of the roles played by B. pertussis FHA in the pathogenesis of pertussis has focused on its activities as an adherence factor and immunogen (13, 20, 32, 34). However, as this is one of the most abundant secreted, as well as cell surface-associated B. pertussis proteins and a protein with multiple putative functional domains and eukaryotic motifs, the possibility of other important biological activities is a distinct and relevant issue.
The results of this study show that exposure to FHA preparations promotes apoptosis in multiple cell types: at least two types of human transformed macrophage-like cells, in fresh human peripheral blood monocyte-derived macrophages, and in transformed human bronchial epithelial cells. FHA-associated apoptosis in human macrophage-like cells is preceded by release of TNF-α. These responses to FHA are dose dependent, and are also observed after infection by intact B. pertussis in an FHA-dependent manner. While these activities have not yet been specifically examined in vivo, the consistency of the effects observed in different cell types and with purified FHA and with whole bacteria, its dose dependency, the prior observations of apoptosis during animal infection by Bordetella species (10), and the relevance of in vitro cell culture models in studying bacterial pathogen-induced apoptosis (6, 12, 16, 17, 26, 45, 47) all support the meaningfulness of these data. The difficulty in establishing a relevant model of disease for this human-adapted pathogen and the known dependence of B. pertussis on FHA for initial colonization of an animal host (4) will pose significant challenges in assessing FHA-associated apoptosis in vivo.
The concept of synergy between multiple bacterial cell surface or secreted factors in exerting a proinflammatory effect is well established (5). This concept and the known activities of LPS guided our comparison of FHA with LPS in the assessment of these host cell responses. Like FHA, LPS stimulated dose-dependent TNF-α secretion by monocytes/macrophages. However, the FHA preparations had a far greater proinflammatory effect when FHA and LPS were compared using amounts with equal endotoxin activity. Furthermore, unlike FHA, B. pertussis LPS had no apparent cytotoxic or proapoptotic effect. Similar results were previously described for the effect of Escherichia coli LPS on cell viability and TNF-α release in human monocytes (28). The results of our experiments indicate that FHA-associated TNF-α secretion and apoptosis cannot be ascribed to LPS alone and may depend upon a biological activity that is intrinsic to FHA. We cannot, however, rule out a contributory role for Bordetella LPS or other minor contaminants, nor can we rule out independent participation of pathways leading to necrosis.
Specific B. pertussis virulence factors were previously described as inducing death signals and damage in host cells. B. pertussis adenylate cyclase toxin has been shown to trigger apoptosis in murine macrophages both in vitro and in vivo, and has been suggested as a mechanism of deleting immune cells (8, 16, 17). B. pertussis tracheal cytotoxin (TCT) has been previously associated with respiratory epithelial cytopathology, mediated in part by release of IL-1α (11). TCT may act synergistically with LPS, leading to up-regulation of cytokine-inducible nitric oxide synthase (5). Both B. pertussis and B. bronchiseptica were recently shown to induce apoptosis in the J774 murine macrophage cell line in vitro and in mouse lung cells (10) in a mouse model of infection, although the latter species was more potent. Phase-reversed FHA expression by B. bronchiseptica in the Bvg-repressed phase did not lead to apoptosis in this study. These findings raise three issues: (i) the possible differences in the biological activity of FHA produced by these two species and differential host species susceptibility, (ii) the possible importance of additional bacterial products and structures in determining FHA effect, and (iii) the possible importance of differential tissue and cell localization by Bordetella species in vivo in explaining local pathology. The possibility of differential proinflammatory and proapoptotic activities by FHA from these two Bordetella species has not been addressed, nor has a more precise mapping of these activities in the cell-associated and secreted FHA protein been performed (15). Factors other than TCT and adenylate cyclase toxin may be important. In one study, B. bronchiseptica pertactin mutants were significantly less toxic to primary porcine alveolar macrophages than were wild-type strains, suggesting a role for pertactin in B. bronchiseptica induced necrosis and apoptosis (6).
In a recent report, purified FHA was shown to stimulate the release of IL-10, IL-6, and to a lesser degree TNF-α and to suppress IL-10-mediated IL-12 secretion in J774 murine macrophages (23). These results suggested that FHA may facilitate B. pertussis persistence in the respiratory tract by delaying the development of cell-mediated immunity (23). Our data support this concept and further suggest that FHA may interfere with immune responses by promoting apoptosis of some immune defense cell subsets.
The mechanisms of FHA-associated apoptosis are not yet entirely clear. TNF-α is recognized by two cellular receptors, TNFR1 and TNFR2 (39). In most cells, TNFR1 is expressed in greater numbers than TNFR2. When engaged by TNF-α both TNFR1 and TNFR2 can activate NFκB transcription-dependent pathways by recruiting cellular proteins such as cIAP1, cIAP2, TNFR-associated factor-1, and TNFR-associated factor-2 (37). This response may confer on TNF-α both a protective effect against cell damage and a proapoptotic effect in some models (19). LPS effects signaling through NFκB-dependent pathways (7, 18, 22, 46), which might explain the protective nature of LPS in our study and in others. It has been shown that TNFR1 and TNFR2 can interact with death domains of cytoplasmic proteins such as TNFR-associated death domain and Fas-associated death domain, thereby activating apoptosis (9, 30) through caspase 8 and other downstream effector caspases (1). Thus, an intriguing question raised by our study was whether TNF-α secreted in response to FHA might serve as an autocrine death signal for apoptosis in these human macrophage-like cells.
We have shown that FHA-associated apoptosis is less well inhibited by a specific anti-TNF-α neutralizing antibody than is TNF-α induced apoptosis, suggesting that FHA-associated proapoptosis involves factors other than secreted TNF-α. In contrast, blocking anti-TNFR1 antibody significantly inhibited cell death, suggesting that this receptor has an important role in mediating the FHA-associated process. The possibility that FHA induces the release of other TNF family ligands that might interact with TNFR1 or that FHA can directly interact with TNFR1 to activate a death pathway should be further investigated. Furthermore, the possibility that FHA may also promote apoptosis in macrophages by suppressing the synthesis of apoptosis inhibitory proteins (such as cIAP1 and cIAP2) should be addressed.
The consequences and ramifications of our findings for pertussis pathogenesis remain to be explored. FHA, in addition to its role in adherence and colonization, may contribute to a local proinflammatory and proapoptotic response in bronchial epithelial cells and local macrophages. Subsequent recruitment or modification of immune effector cells may produce a more favorable local environment for bacterial adherence and persistence. From an alternative perspective, these events might benefit the host through loss of preferred target cells for bacterial attachment or through activation of antibacterial defenses. Our findings are supported by histopathologic data in natural infection and suggest a number of important future lines of investigation. Among these are the implications of these findings for possible adverse effects associated with FHA in pertussis vaccines.
ACKNOWLEDGMENTS
We thank Rino Rappuoli and Mariagrazia Pizza (IRIS) for FHA preparations.
This work was supported in part by a grant from the National Institute of Allergy and Infectious Diseases, National Institutes of Health: R01 AI39587 (to D.A.R.).
REFERENCES
- 1.Ashkenazi A, Dixit V M. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 2.Beutler B, Cerami A. Tumor necrosis, cachexia, shock, and inflammation: a common mediator. Annu Rev Biochem. 1988;57:505–518. doi: 10.1146/annurev.bi.57.070188.002445. [DOI] [PubMed] [Google Scholar]
- 3.Boschwitz J S, Batanghari J W, Kedem H, Relman D A. Bordetella pertussis infection of human monocytes inhibits antigen-dependent CD4 T cell proliferation. J Infect Dis. 1997;176:678–686. doi: 10.1086/514090. [DOI] [PubMed] [Google Scholar]
- 4.Cotter P A, Yuk M H, Mattoo S, Akerley B J, Boschwitz J, Relman D A, Miller J F. Filamentous hemagglutinin of Bordetella bronchiseptica is required for efficient establishment of tracheal colonization. Infect Immun. 1998;66:5921–5929. doi: 10.1128/iai.66.12.5921-5929.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flak T A, Goldman W E. Signaling and cellular specificity of airway nitric oxide production in pertussis. Cell Microbiol. 1999;1:51–60. doi: 10.1046/j.1462-5822.1999.00004.x. [DOI] [PubMed] [Google Scholar]
- 6.Forde C B, Shi X, Li J, Roberts M. Bordetella bronchiseptica-mediated cytotoxicity to macrophages is dependent on bvg-regulated factors, including pertactin. Infect Immun. 1999;67:5972–5978. doi: 10.1128/iai.67.11.5972-5978.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghosh S, May M J, Kopp E B. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 8.Gueirard P, Druilhe A, Pretolani M, Guiso N. Role of adenylate cyclase-hemolysin in alveolar macrophage apoptosis during Bordetella pertussis infection in vivo. Infect Immun. 1998;66:1718–1725. doi: 10.1128/iai.66.4.1718-1725.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haridas V, Darnay B G, Natarajan K, Heller R, Aggarwal B B. Overexpression of the p80 TNF receptor leads to TNF-dependent apoptosis, nuclear factor-kappa B activation, and c-Jun kinase activation. J Immunol. 1998;160:3152–3162. [PubMed] [Google Scholar]
- 10.Harvill E T, Cotter P A, Miller J F. Pregenomic comparative analysis between Bordetella bronchiseptica RB50 and Bordetella pertussis Tohama I in murine models of respiratory tract infection. Infect Immun. 1999;67:6109–6118. doi: 10.1128/iai.67.11.6109-6118.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heiss L N, Moser S A, Unanue E R, Goldman W E. Interleukin-1 is linked to the respiratory epithelial cytopathology of pertussis. Infect Immun. 1993;61:3123–3128. doi: 10.1128/iai.61.8.3123-3128.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hersh D, Monack D M, Smith M R, Ghori N, Falkow S, Zychlinsky A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci USA. 1999;96:2396–2401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hewlett E L. Pertussis: current concepts of pathogenesis and prevention. Pediatr Infect Dis J. 1997;16:S78–S84. doi: 10.1097/00006454-199704001-00002. [DOI] [PubMed] [Google Scholar]
- 14.Ishibashi Y, Claus S, Relman D A. Bordetella pertussis filamentous hemagglutinin interacts with a leukocyte signal transduction complex and stimulates bacterial adherence to monocyte CR3 (CD11b/CD18) J Exp Med. 1994;180:1225–1233. doi: 10.1084/jem.180.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacob-Dubuisson F, Kehoe B, Willery E, Reveneau N, Locht C, Relman D A. Molecular characterization of Bordetella bronchiseptica filamentous hemagglutinin and its secretion machinery. Microbiol. 2000;146:1211–1221. doi: 10.1099/00221287-146-5-1211. [DOI] [PubMed] [Google Scholar]
- 16.Khelef N, Guiso N. Induction of macrophage apoptosis by Bordetella pertussis adenylate cyclase-hemolysin. FEMS Microbiol Lett. 1995;134:27–32. doi: 10.1111/j.1574-6968.1995.tb07909.x. [DOI] [PubMed] [Google Scholar]
- 17.Khelef N, Zychlinsky A, Guiso N. Bordetella pertussis induces apoptosis in macrophages: role of adenylate cyclase-hemolysin. Infect Immun. 1993;61:4064–4071. doi: 10.1128/iai.61.10.4064-4071.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirschning C J, Wesche H, Merrill Ayres T, Rothe M. Human toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J Exp Med. 1998;188:2091–2097. doi: 10.1084/jem.188.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurrelmeyer K M, Michael L H, Baumgarten G, Taffet G E, Peschon J J, Sivasubramanian N, Entman M L, Mann D L. Endogenous tumor necrosis factor protects the adult cardiac myocyte against ischemic-induced apoptosis in a murine model of acute myocardial infarction. Proc Natl Acad Sci USA. 2000;97:5456–5461. doi: 10.1073/pnas.070036297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Locht C, Bertin P, Menozzi F D, Renauld G. The filamentous haemagglutinin, a multifaceted adhesion produced by virulent Bordetella spp. Mol Microbiol. 1993;9:653–660. doi: 10.1111/j.1365-2958.1993.tb01725.x. [DOI] [PubMed] [Google Scholar]
- 21.Makhov A M, Hannah J H, Brennan M J, Trus B L, Kocsis E, Conway J F, Wingfield P T, Simon M N, Steven A C. Filamentous hemagglutinin of Bordetella pertussis. A bacterial adhesin formed as a 50-nm monomeric rigid rod based on a 19-residue repeat motif rich in beta strands and turns. J Mol Biol. 1994;241:110–124. doi: 10.1006/jmbi.1994.1478. [DOI] [PubMed] [Google Scholar]
- 22.Manna S K, Aggarwal B B. Lipopolysaccharide inhibits TNF-induced apoptosis: role of nuclear factor-kappaB activation and reactive oxygen intermediates. J Immunol. 1999;162:1510–1518. [PubMed] [Google Scholar]
- 23.McGuirk P, Mills K H. Direct anti-inflammatory effect of a bacterial virulence factor: IL-10-dependent suppression of IL-12 production by filamentous hemagglutinin from Bordetella pertussis. Eur J Immunol. 2000;30:415–422. doi: 10.1002/1521-4141(200002)30:2<415::AID-IMMU415>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 24.Menozzi F D, Boucher P E, Riveau G, Gantiez C, Locht C. Surface-associated filamentous hemagglutinin induces autoagglutination of Bordetella pertussis. Infect Immun. 1994;62:4261–4269. doi: 10.1128/iai.62.10.4261-4269.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menozzi F D, Mutombo R, Renauld G, Gantiez C, Hannah J H, Leininger E, Brennan M J, Locht C. Heparin-inhibitable lectin activity of the filamentous hemagglutinin adhesin of Bordetella pertussis. Infect Immun. 1994;62:769–778. doi: 10.1128/iai.62.3.769-778.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monack D M, Mecsas J, Bouley D, Falkow S. Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection of mice. J Exp Med. 1998;188:2127–2137. doi: 10.1084/jem.188.11.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muhl H, Nold M, Chang J H, Frank S, Eberhardt W, Pfeilschifter J. Expression and release of chemokines associated with apoptotic cell death in human promonocytic U937 cells and peripheral blood mononuclear cells. Eur J Immunol. 1999;29:3225–3235. doi: 10.1002/(SICI)1521-4141(199910)29:10<3225::AID-IMMU3225>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 28.Njamkepo E, Pinot F, Francois D, Guiso N, Polla B S, Bachelet M. Adaptive responses of human monocytes infected by Bordetella pertussis: the role of adenylate cyclase hemolysin. J Cell Physiol. 2000;183:91–99. doi: 10.1002/(SICI)1097-4652(200004)183:1<91::AID-JCP11>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 29.Perfettini J L, Darville T, Gachelin G, Souque P, Huerre M, Dautry-Varsat A, Ojcius D M. Effect of Chlamydia trachomatis infection and subsequent tumor necrosis factor alpha secretion on apoptosis in the murine genital tract. Infect Immun. 2000;68:2237–2244. doi: 10.1128/iai.68.4.2237-2244.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pimentel-Muinos F X, Seed B. Regulated commitment of TNF receptor signaling: a molecular switch for death or activation. Immunity. 1999;11:783–793. doi: 10.1016/s1074-7613(00)80152-1. [DOI] [PubMed] [Google Scholar]
- 31.Prasad S M, Yin Y, Rodzinski E, Tuomanen E I, Masure H R. Identification of a carbohydrate recognition domain in filamentous hemagglutinin from Bordetella pertussis. Infect Immun. 1993;61:2780–2785. doi: 10.1128/iai.61.7.2780-2785.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rappuoli R. Pathogenicity mechanisms of Bordetella. Curr Top Microbiol Immunol. 1994;192:319–336. doi: 10.1007/978-3-642-78624-2_14. [DOI] [PubMed] [Google Scholar]
- 33.Relman D, Tuomanen E, Falkow S, Golenbock D T, Saukkonen K, Wright S D. Recognition of a bacterial adhesin by an integrin: macrophage CR3 (alphaMbeta2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell. 1990;61:1375–1382. doi: 10.1016/0092-8674(90)90701-f. [DOI] [PubMed] [Google Scholar]
- 34.Relman D A. Bordetella pertussis: determinants of virulence. In: Moss J, Iglewski B, Vaughan M, Tu A T, editors. Handbook of natural toxins. Vol. 8. New York, N.Y: Marcel Dekker; 1995. pp. 367–405. [Google Scholar]
- 35.Rozdzinski E, Sandros J, vanderFlier M, Young A, Spellerberg B, Bhattacharyya C, Straub J, Musso G, Putney S, Starzyk R, Tuomanen E. Inhibition of leukocyte-endothelial cell interactions and inflammation by peptides from a bacterial adhesin which mimic coagulation factor X. J Clin Investig. 1995;95:1078–1085. doi: 10.1172/JCI117754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saukkonen K, Cabellos C, Burroughs M, Prasad S, Tuomanen E. Integrin-mediated localization of Bordetella pertussis within macrophages: role in pulmonary colonization. J Exp Med. 1991;173:1143–1149. doi: 10.1084/jem.173.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shu H B, Takeuchi M, Goeddel D V. The tumor necrosis factor receptor 2 signal transducers TRAF2 and c-IAP1 are components of the tumor necrosis factor receptor 1 signaling complex. Proc Natl Acad Sci USA. 1996;93:13973–13978. doi: 10.1073/pnas.93.24.13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stainer D W, Scholte M J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970;63:211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- 39.Tartaglia L A, Goeddel D V. Two TNF receptors. Immunol Today. 1992;13:151–153. doi: 10.1016/0167-5699(92)90116-O. [DOI] [PubMed] [Google Scholar]
- 40.Tewari M, Quan L T, O'Rourke K, Desnoyers S, Zeng Z, Beidler D R, Poirier G G, Salvesen G S, Dixit V M. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- 41.Tuomanen E. Piracy of adhesins: attachment of superinfecting pathogens to respiratory cilia by secreted adhesins of Bordetella pertussis. Infect Immun. 1986;54:905–908. doi: 10.1128/iai.54.3.905-908.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weiss A A, Hewlett E L. Virulence factors of Bordetella pertussis. Annu Rev Microbiol. 1986;40:661–686. doi: 10.1146/annurev.mi.40.100186.003305. [DOI] [PubMed] [Google Scholar]
- 43.Weiss A A, Hewlett E L, Myers G A, Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983;42:33–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wesson C A, Deringer J, Liou L E, Bayles K W, Bohach G A, Trumble W R. Apoptosis induced by Staphylococcus aureus in epithelial cells utilizes a mechanism involving caspases 8 and 3. Infect Immun. 2000;68:2998–3001. doi: 10.1128/iai.68.5.2998-3001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson M, Seymour R, Henderson B. Bacterial perturbation of cytokine networks. Infect Immun. 1998;66:2401–2409. doi: 10.1128/iai.66.6.2401-2409.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang F X, Kirschning C J, Mancinelli R, Xu X P, Jin Y, Faure E, Mantovani A, Rothe M, Muzio M, Arditi M. Bacterial lipopolysaccharide activates nuclear factor-kappaB through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J Biol Chem. 1999;274:7611–7614. doi: 10.1074/jbc.274.12.7611. [DOI] [PubMed] [Google Scholar]
- 47.Zychlinsky A, Sansonetti P. Perspectives series: host/pathogen interactions. Apoptosis in bacterial pathogenesis. J Clin Investig. 1997;100:493–495. doi: 10.1172/JCI119557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zychlinsky A, Thirumalai K, Arondel J, Cantey J R, Aliprantis A O, Sansonetti P J. In vivo apoptosis in Shigella flexneri infections. Infect Immun. 1996;64:5357–5365. doi: 10.1128/iai.64.12.5357-5365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]