Abstract
Antioxidants are being explored as novel therapeutics for the treatment of neurodegenerative diseases such as Alzheimer’s disease (AD) through strategies such as chemically linking antioxidants to synthesize novel co-drugs. The main objective of this study was to assess the cytoprotective effects of the novel antioxidant compound VANL-100 in a cellular model of beta-amyloid (Aβ)-induced toxicity. The cytotoxic effects of Aβ in the presence and absence of all antioxidant compounds were measured using the 3-(4,5-dimethylthiazol-2-yl)2-5-diphenyl-2H-tetrazolium bromide (MTT) assay in SH-SY5Y cells in both pre-treatment and co-treatment experiments. In pre-treatment experiments, VANL-100, or one of its parent compounds, naringenin (NAR), alpha-lipoic acid (ALA), or naringenin + alpha-lipoic acid (NAR + ALA), was administrated 24 h prior to an additional 24-h incubation with 20 μM non-fibril or fibril Aβ25–35. Co-treatment experiments consisted of simultaneous treatment with Aβ and antioxidants. Pre-treatment and co-treatment with VANL-100 significantly attenuated Aβ-induced cell death. There were no significant differences between the protective effects of VANL-100, NAR, ALA, and NAR + ALA with either form of Aβ, or in the effect of VANL-100 between 24-h pre-treatment and co-treatment. These results demonstrate that the novel co-drug VANL-100 is capable of eliciting cytoprotective effects against Aβ-induced toxicity.
Keywords: Alzheimer’s disease, beta-amyloid, antioxidants, naringenin, alpha lipoic acid
1. Introduction
Alzheimer’s disease (AD) is currently the leading cause of dementia and is projected to affect over 130 million people globally by the year 2050 [1,2,3,4,5]. Despite advancements in research and biomedical technology over the last two decades, its prevalence and incidence rates continue to rise, and the currently available treatment options do not prevent, delay the progression of, or cure AD [6,7,8,9]. In an effort to uncover novel therapies for AD, researchers have begun exploring compounds that target mechanisms implicated in the development and progression of AD. Based on promising pre-clinical findings, human clinical trials began assessing the effectiveness of individual antioxidants or antioxidant combinations that included antioxidant compounds solely or antioxidants combined with drugs currently used to treat AD [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. Antioxidant compounds can be administered together but unbound (combination) or chemically linked (conjugated). The application of antioxidant conjugate therapy (ACT) particularly for AD is based on the role of oxidative stress during the onset and progression of AD. This is outlined in the oxidative stress hypothesis of AD, which postulates the potential mechanisms by which oxidative stress contributes to AD pathology [33,34,35,36,37].
Oxidative stress can be defined as an imbalance between the production and accumulation of toxic reactive oxygen species (ROS) and endogenous antioxidants within the body, resulting in an insufficient or dysfunctional antioxidant defense system [38,39,40,41]. During oxidative stress, ROS target and damage essential cellular structures and biomolecules such as DNA, proteins, and lipids via oxidation [38,39,40,41]. ROS have also been implicated with other pathological hallmarks of AD such as the abnormal aggregation of tau and beta-amyloid (Aβ), further inducing cellular toxicity [42,43,44,45,46,47,48].
Two notable antioxidants that have been explored for their neuroprotective effects in models of neurodegeneration are naringenin (NAR), a naturally occurring polyphenol found in plants and citrus fruit, and alpha-lipoic acid (ALA), an endogenously produced compound that is also found in food such as red meat, broccoli, and spinach [49,50,51,52,53,54]. The individual effects of NAR and ALA in models of AD are well explored in the literature [50,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79] and presented as potential therapeutic modalities for targeting and treating AD, as well as functioning as potential chemical constituents for ACT.
A previous study conducted by Saleh et al. (2017) explored the neuroprotective effects of NAR, ALA, and the novel co-drug VANL-100 (Figure 1), which is the product of the covalent linkage of NAR and ALA. The findings demonstrated the heightened neuroprotective effects of VANL-100, both in vitro by increasing antioxidant capacity and in vivo by reducing infarct volume in a rodent model of an ischemic stroke [80]. Additionally, the novel co-drug VAN-100 was 100 times more potent than the parent compounds, eliciting neuroprotection at lower doses. However, the potential benefits of VANL-100 in other models of neurodegeneration such as AD have not been explored.
Figure 1.
Chemical structures of alpha-lipoic acid (ALA), naringenin (NAR), and VANL-100.
In this study, we evaluated the cytoprotective effects of the novel co-drug VANL-100 against Aβ-induced cytotoxicity. Additionally, we compared the effects of VANL-100 to its parent compounds, NAR and ALA, alone and together (NAR + ALA) in a mixture but not covalently bound. This is the first report to explore the effects of NAR and ALA as conjugate therapy in a cellular model of AD. For the purposes of this study, a co-drug is defined as the conjugated drug product from the covalent linkage of two or more chemical entities.
2. Results
2.1. Non-Fibril and Fibril Aβ25–35 Induced Dose-Dependent Cell Death
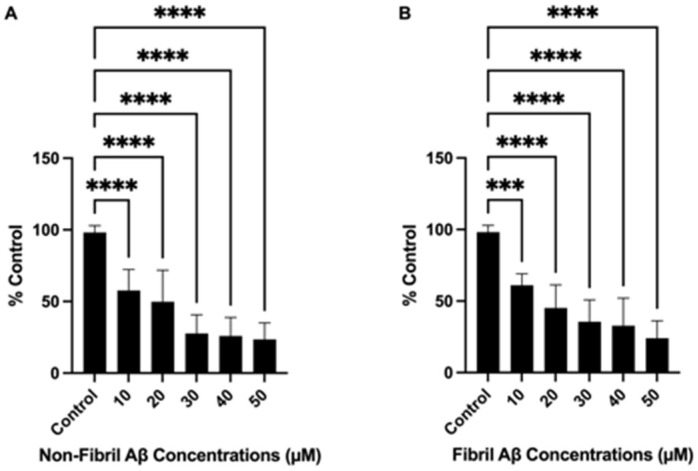
Prior to testing the neuroprotective effects of the antioxidant compounds, the toxicities of non-fibril and fibril Aβ25–35 were individually tested in SH-SY5Y cells (Figure 2A,B). Considering that the toxicity profile of Aβ differs across various formulations [81,82], we explored the effect of Aβ in its non-fibril and fibrillated states to assess and compare the effects of different compositions on toxicity. Additionally, we utilized the 25–35 protein fragment of Aβ due to its characteristic high toxicity and proven ability to exert neurotoxic effects in AD models [83,84,85]. Since the Aβ25–35 peptide is a well-characterized biologically active fragment that retains the toxic properties of Aβ1–42, we chose this fragment to assess the potential protective effects of our novel antioxidant and its parent compounds in vitro. Cell viability was evaluated using the 3-(4,5-dimethylthiazol-2-yl)2-5-diphenyl-2H-tetrazolium bromide (MTT) assay after 24 h of Aβ25–35 exposure. There were no significant differences in the toxicity levels of non-fibril compared to fibril Aβ25–35 in the SH-SY5Y cells in this study. Cell viability was significantly decreased as Aβ concentrations increased compared to untreated controls (*** p = 0.0001; **** p < 0.0001). We selected 20 μM for both non-fibril and fibril Aβ25–35 as the effective dose to carry out subsequent experiments with the antioxidant compounds. This is in line with the literature on effective doses for Aβ treatment to induce cell death in this cell line without completely eradicating all cells [63,86,87,88,89,90,91,92].
Figure 2.
Effects of beta-amyloid (Aβ)25–35 on cell viability. Cell viability, represented as % control, is shown on the y-axis, and Aβ25–35 concentrations are displayed on the x-axis. The bars represent the mean ± standard error of the mean (SEM) of six independent experiments. Cells were incubated with increasing concentrations (10, 20, 30, 40, and 50 μM) of non-fibril (A) or fibril (B) Aβ25–35. Non-fibril and fibril Aβ25–35 reduced cell viability in a dose-dependent manner. Statistical significance vs. controls was assessed using one-way analysis of variance (ANOVA). *** p = 0.0001; **** p < 0.0001.
2.2. Antioxidant Compounds Did Not Induce Cell Loss or Reduce Cell Viability
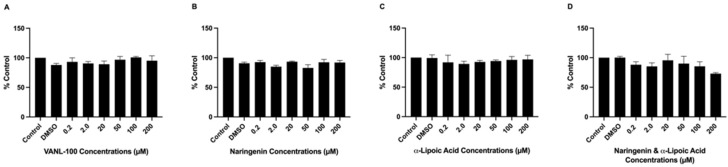
Figure 3 depicts the effects of VANL-100, NAR, ALA, and NAR + ALA on SH-SY5Y cell viability. None of the antioxidant compounds or combinations resulted in cell loss at 0.2, 2.0, 20, 50, 100, or 200 μM (p > 0.05). Cell viability was evaluated using the MTT assay 24 h after exposure to the antioxidant compounds. This assessment was necessary to confirm that the antioxidant compounds were not exerting toxic effects on the cells alone, prior to treatment with Aβ.
Figure 3.
Effects of VANL-100, NAR, ALA, and NAR + ALA on cell viability. Cell viability, represented as % control, is shown on the y-axis, and antioxidant concentrations are displayed on the x-axis. The bars represent the mean ± SEM of three independent experiments. Cells were incubated with different concentrations of each antioxidant and combination (0.2, 2.0, 20, 50, 100 and 200 μM). (A) VANL-100; (B) NAR alone; (C) ALA alone; (D) NAR + ALA. None of the antioxidant compounds at any dose significantly induced cell loss or reduced cell viability. Statistical significance vs. controls was assessed using one-way ANOVA. p < 0.05.
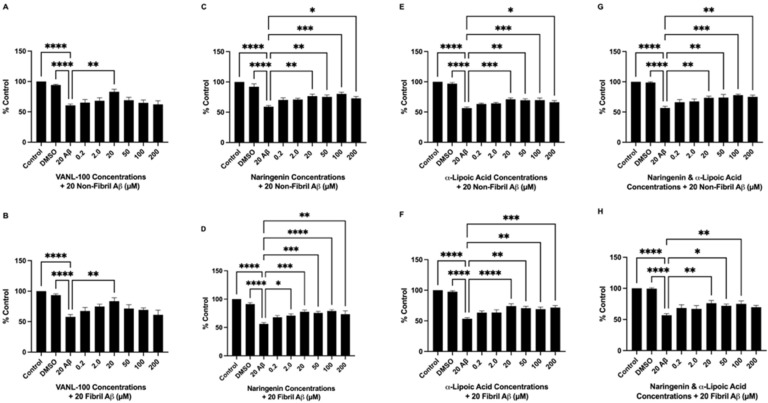
2.3. Effect of Antioxidant Compounds on Aβ-Induced Cell Death
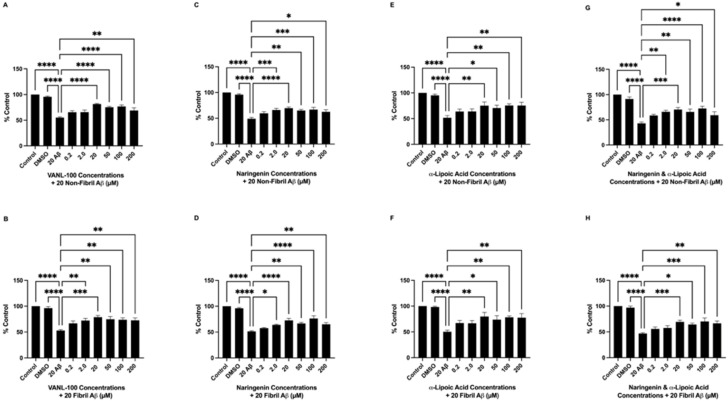
In 24-h pre-treatment and co-treatment experiments, the effect of VANL-100, NAR, ALA, and NAR + ALA were assessed against non-fibril and fibril Aβ25–35 in SH-SY5Y cells. During pre-treatment, cells were treated with 0.2, 2.0, 20, 50, 100, or 200 μM of each antioxidant 24 h prior to Aβ25–35 addition. After 24 h, cells were co-incubated with the same doses of the antioxidants in addition to 20 μM non-fibril or fibril Aβ25–35, which was followed by cell viability measurement via MTT assay 24 h later. In co-treatment, cells received 0.2, 2.0, 20, 50, 100, or 200 μM of each antioxidant simultaneously with 20 μM non-fibril and fibril Aβ25–35, and cell viability was measured 24 h later via MTT assay. Results from pre-treatment experiments (Figure 4A–H) revealed significant improvements in cell viability for all antioxidant compounds when combined with Aβ25–35 compared to treatment with Aβ25–35 alone. VANL-100 significantly increased cell viability compared to non-fibril Aβ25–35 alone at doses of 20, 50, 100 (p < 0.0001), and 200 μM (p = 0.0066) and fibril Aβ25–35 at doses of 2.0 (p = 0.0051), 20 (p = 0.0001), 50 (p = 0.0013), 100 (p = 0.0022) and 200 μM (p = 0.0041), as shown in Figure 4A,B, respectively. NAR significantly increased cell viability compared to non-fibril Aβ25–35 at 2.0 (p = 0.0008), 20 (p < 0.0001), 50 (p = 0017), 100 (p = 0.0004) and 200 μM (p = 0.0104), and fibril Aβ25–35 at doss of 2.0 (p = 0.0139), 20 (p < 0.0001), 50 (p = 0018), 100 (p < 0.0001), 200 μM (p = 0.0061), as shown in Figure 4C,D, respectively. ALA significantly attenuated the reduction in cell viability induced by non-fibril Aβ25–35 at doses of 20 (p = 0.0070), 50 (p = 0.0451), 100 (p = 0.0058), 200 μM (p = 0.0063), and fibril Aβ25–35 at doses of 20 (p = 0.0025), 50 (p = 0.0230), 100 (p = 0.0045), 200 μM (p = 0.0059) as shown in Figure 4E,F, respectively. The combination of NAR + ALA also significantly increased cell viability compared to non-fibril Aβ25–35 alone at doses of 2.0 (p = 0.0018), 20 (p = 0.0002), 50 (p = 0020), 100 (p = 0.0001), 200 μM (p = 0.0423), and fibril Aβ25–35 at doses of 20 (p = 0.0008), 50 (p = 0129), 100 (p = 0.0005), 200 μM (p = 0.0044), as shown in Figure 4G,H, respectively.
Figure 4.
Viability of SH-SY5Y cells pre-treated with VANL-100, NAR, ALA, or NAR + ALA for 24 h followed by 24-h treatment with Aβ25–35. Cell viability, represented as % control, is shown on the y-axis, and antioxidant concentrations are displayed on the x-axis. The bars represent the mean ± SEM of six independent experiments. (A,B) VANL-100 + non-fibril and fibril Aβ25–35, respectively. (C,D) NAR + non-fibril and fibril Aβ25–35, respectively. (E,F) ALA + non-fibril and fibril Aβ25–35, respectively. (G,H) NAR + ALA + non-fibril and fibril Aβ25–35, respectively. Statistical significance vs. Aβ was assessed using one-way ANOVA. * p <0.05; ** p< 0.01; *** p < 0.001; **** p < 0.0001.
Similarly, results from co-treatment experiments (Figure 5A–H) indicated significant improvement in viability when cells were treated with Aβ25–35 in combination with each of the antioxidant compounds compared to treatment with Aβ25–35 alone. Co-treatment with VANL-100 significantly improved cell viability compared to non-fibril Aβ25–35 at 20 μM (p = 0.0017) and fibril Aβ25–35 at 20 μM (p = 0.0019), as shown in Figure 5A,B, respectively. NAR co-treatment significantly increased cell viability compared to non-fibril Aβ25–35 at 20 (p = 0.0014), 50 (p = 0038), 100 (p = 0.0001), and 200 μM (p = 0.0161), and fibril Aβ25–35 at 2.0 (p = 0.0115), 20 (p = 0.0001), 50 (p = 0.0005), 100 (p < 0.0001), and 200 μM (p = 0.0023), as shown in Figure 5C,D, respectively. ALA co-treatment significantly increased cell viability compared to non-fibril Aβ25–35 at 20 (p = 0.0003), 50 (p = 0.0011), 100 (p = 0.0010), and 200 μM (p = 0.0207), and fibril Aβ25–35 at 20 (p < 0.0001), 50 (p = 0.0011), 100 (p = 0.0031), and 200 μM (p = 0.0005), as shown in Figure 5E,F, respectively. Co-treatment with the combination of NAR + ALA significantly improved cell viability compared to non-fibril Aβ25–35 at 20 (p = 0.034), 50 (p = 0.0026), 100 (p = 0.0002), 200 μM (p = 0.0012), and fibril Aβ25–35 at 20 (p = 0.0036), 50 (p = 0.0338), and 100 (p = 0.0066), as shown in Figure 5G,H, respectively. A summary of these results is shown in Table 1.
Figure 5.
Viability of SH-SY5Y cells co-treated with VANL-100, NAR, ALA, or NAR + ALA and Aβ25–35 for 24 h. Cell viability, represented as % control, is shown on the y-axis, and antioxidant concentrations are displayed on the x-axis. The bars represent the mean ± SEM of six independent experiments. (A,B) VANL-100 + non-fibril and fibril Aβ25–35, respectively. (C,D) NAR + non-fibril and fibril Aβ25–35, respectively. (E,F) ALA + non-fibril and fibril Aβ25–35, respectively. (G,H) NAR + ALA + non-fibril and fibril Aβ25–35, respectively. Statistical significance vs. Aβ was assessed using one-way ANOVA. * p <0.05; ** p< 0.01; *** p < 0.001; **** p < 0.0001.
Table 1.
Summary of results. NAR: Naringenin; ALA: Alpha-lipoic acid; NAR + ALA; Naringenin + Alpha-lipoic acid; NF: Non-fibril; F: Fibril.
| Timepoint | Antioxidant Compound(s) | Treatment | Result (p-Value) |
Figure |
|---|---|---|---|---|
| 24-h pre-treatment |
VANL-100 | 0.2 μM + 20 μM NF Aβ 0.2 μM + 20 μM F Aβ |
p > 0.05 p > 0.05 |
Figure 4A,B |
| 2.0 μM + 20 μM NF Aβ 2.0 μM + 20 μM F Aβ |
p > 0.05 p = 0.0051 |
|||
| 20 μM + 20 μM NF Aβ 20 μM + 20 μM F Aβ |
p < 0.0001 p = 0.0001 |
|||
| 50 μM + 20 μM NF Aβ 50 μM + 20 μM F Aβ |
p < 0.0001 p = 0.0013 |
|||
| 100 μM + 20 μM NF Aβ 100 μM + 20 μM F Aβ |
p < 0.0001 p = 0.0022 |
|||
| 200 μM + 20 μM NF Aβ 200 μM + 20 μM F Aβ |
p = 0.0066 p = 0.0041 |
|||
| NAR | 0.2 μM + 20 μM NF Aβ 0.2 μM + 20 μM F Aβ |
p > 0.05 p > 0.05 |
Figure 4C,D | |
| 2.0 μM + 20 μM NF Aβ 2.0 μM + 20 μM F Aβ |
p = 0.0008 p = 0.0139 |
|||
| 20 μM + 20 μM NF Aβ 20 μM + 20 μM F Aβ |
p < 0.0001 p < 0.0001 |
|||
| 50 μM + 20 μM NF Aβ 50 μM + 20 μM F Aβ |
p = 0.017 p = 0.0018 |
|||
| 100 μM + 20 μM NF Aβ 100 μM + 20 μM F Aβ |
p = 0.0004 p < 0.0001 |
|||
| 200 μM + 20 μM NF Aβ 200 μM + 20 μM F Aβ |
p = 0.0104 p = 0.0061 |
|||
| ALA | 0.2 μM + 20 μM NF Aβ 0.2 μM + 20 μM F Aβ |
p > 0.05 p > 0.05 |
Figure 4E,F | |
| 2.0 μM + 20 μM NF Aβ 2.0 μM + 20 μM F Aβ |
p > 0.05 p > 0.05 |
|||
| 20 μM + 20 μM NF Aβ 20 μM + 20 μM F Aβ |
p = 0.0070 p = 0.0025 |
|||
| 50 μM + 20 μM NF Aβ 50 μM + 20 μM F Aβ |
p = 0.0451 p = 0.0230 |
|||
| 100 μM + 20 μM NF Aβ 100 μM + 20 μM F Aβ |
p = 0.0058 p = 0.0045 |
|||
| 200 μM + 20 μM NF Aβ 200 μM + 20 μM F Aβ |
p = 0.0063 p = 0.0059 |
|||
| NAR + ALA | 0.2 μM + 20 μM NF Aβ 0.2 μM + 20 μM F Aβ |
p > 0.05 p > 0.05 |
Figure 4G,H | |
| 2.0 μM + 20 μM NF Aβ 2.0 μM + 20 μM F Aβ |
p = 0.0018 p > 0.05 |
|||
| 20 μM + 20 μM NF Aβ 20 μM + 20 μM F Aβ |
p = 0.0002 p = 0.0008 |
|||
| 50 μM + 20 μM NF Aβ 50 μM + 20 μM F Aβ |
p = 0.0020 p = 0.0129 |
|||
| 100 μM + 20 μM NF Aβ 100 μM + 20 μM F Aβ |
p = 0.0001 p = 0.0005 |
|||
| 200 μM + 20 μM NF Aβ 200 μM + 20 μM F Aβ |
p = 0.0423 p = 0.0044 |
|||
| Co-treatment | VANL-100 | 0.2 μM + 20 μM NF Aβ 0.2 μM + 20 μM F Aβ |
p > 0.05 p > 0.05 |
Figure 5A,B |
| 2.0 μM + 20 μM NF Aβ 2.0 μM + 20 μM F Aβ |
p > 0.05 p > 0.05 |
|||
| 20 μM + 20 μM NF Aβ 20 μM + 20 μM F Aβ |
p = 0.0017 p = 0.0019 |
|||
| 50 μM + 20 μM NF Aβ 50 μM + 20 μM F Aβ |
p > 0.05 p > 0.05 |
|||
| 100 μM + 20 μM NF Aβ 100 μM + 20 μM F Aβ |
p > 0.05 p > 0.05 |
|||
| 200 μM + 20 μM NF Aβ 200 μM + 20 μM F Aβ |
p > 0.05 p > 0.05 |
|||
| NAR | 0.2 μM + 20 μM NF Aβ 0.2 μM + 20 μM F Aβ |
p > 0.05 p > 0.05 |
Figure 5C,D | |
| 2.0 μM + 20 μM NF Aβ 2.0 μM + 20 μM F Aβ |
p > 0.05 p = 0.0115 |
|||
| 20 μM + 20 μM NF Aβ 20 μM + 20 μM F Aβ |
p = 0.0014 p = 0.0001 |
|||
| 50 μM + 20 μM NF Aβ 50 μM + 20 μM F Aβ |
p = 0.0038 p = 0.0005 |
|||
| 100 μM + 20 μM NF Aβ 100 μM + 20 μM F Aβ |
p < 0.0001 p = 0.0001 |
|||
| 200 μM + 20 μM NF Aβ 200 μM + 20 μM F Aβ |
p = 0.0161 p = 0.0023 |
|||
| ALA | 0.2 μM + 20 μM NF Aβ 0.2 μM + 20 μM F Aβ |
p > 0.05 p > 0.05 |
Figure 5E,F | |
| 2.0 μM + 20 μM NF Aβ 2.0 μM + 20 μM F Aβ |
p > 0.05 p > 0.05 |
|||
| 20 μM + 20 μM NF Aβ 20 μM + 20 μM F Aβ |
p = 0.0003 p < 0.0001 |
|||
| 50 μM + 20 μM NF Aβ 50 μM + 20 μM F Aβ |
p = 0.0011 p = 0.0011 |
|||
| 100 μM + 20 μM NF Aβ 100 μM + 20 μM F Aβ |
p = 0.0010 p = 0.0031 |
|||
| 200 μM + 20 μM NF Aβ 200 μM + 20 μM F Aβ |
p = 0.0207 p = 0.0005 |
|||
| NAR + ALA | 0.2 μM + 20 μM NF Aβ 0.2 μM + 20 μM F Aβ |
p > 0.05 p > 0.05 |
Figure 5G,H | |
| 2.0 μM + 20 μM NF Aβ 2.0 μM + 20 μM F Aβ |
p > 0.05 p > 0.05 |
|||
| 20 μM + 20 μM NF Aβ 20 μM + 20 μM F Aβ |
p = 0.034 p = 0.0036 |
|||
| 50 μM + 20 μM NF Aβ 50 μM + 20 μM F Aβ |
p = 0.0026 p = 0.0338 |
|||
| 100 μM + 20 μM NF Aβ 100 μM + 20 μM F Aβ |
p = 0.0002 p = 0.0066 |
|||
| 200 μM + 20 μM NF Aβ 200 μM + 20 μM F Aβ |
p = 0.0012 p > 0.05 |
2.4. Cytoprotective Effects of VANL-100 Are Not Significantly Different to Parent Compounds
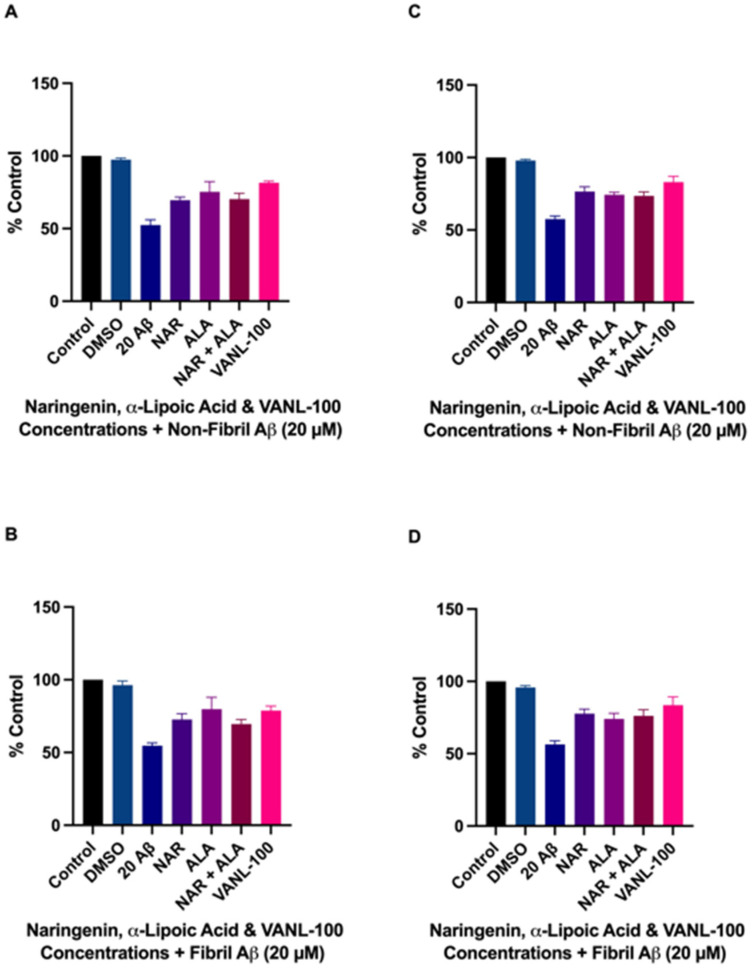
Of the three doses (20, 50, and 100 μM) that elicited the greatest neuroprotective effects on cell survival, we selected the 20 μM dose to compare the protective effects of VANL-100 to NAR, ALA, and NAR + ALA to determine if VANL-100 elicits greater protective effects than its parent compounds. Comparisons were made for both pre-treated and co-treated cells in combination with either 20 μM non-fibril or fibril Aβ25–35. For both non-fibril and fibril Aβ25–35 (Figure 6A,B, respectively), the cytoprotective effects of cells pre-treated with VANL-100 were not significantly different from those observed in cells pre-treated with 20 μM NAR, ALA, or NAR + ALA. Similarly, following co-treatment with non-fibril and fibril Aβ25–35 (Figure 6C,D, respectively), the cytoprotective effects of VANL-100 were not significantly different (p > 0.05) from its parent compounds alone or when combined but not covalently linked.
Figure 6.
Effects of pre-treatment and co-treatment with VANL-100 compared to parent compounds. Cell viability, represented as % control, is shown on the y-axis, and antioxidant concentrations are displayed on the x-axis. The bars represent the mean ± SEM of six independent experiments. (A,B) Pre-treatment with VANL-100 compared to parent compounds against non-fibril and fibril Aβ25–35, respectively. (C,D) Co-treatment with VANL-100 compared to parent compounds against non-fibril and fibril Aβ25–35, respectively. Statistical significance vs. VANL-100 was assessed using one-way ANOVA. p < 0.05.
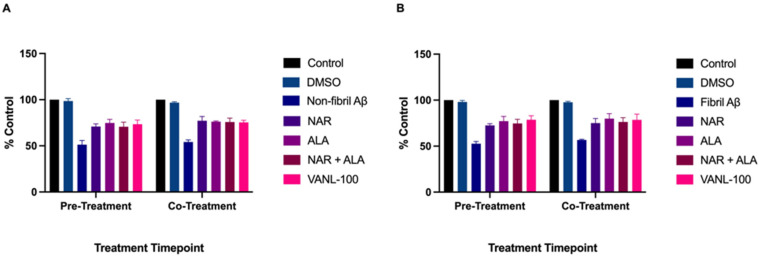
2.5. Effect of Treatment Timepoint on Cell Survival
Finally, we evaluated the overall protective effects of 24-h pre-treatment compared to co-treatment with 20 μM VANL-100, NAR, ALA, or NAR + ALA against 20 μM non-fibril and fibril Aβ25–35 (Figure 7). Although all antioxidant compounds elicited cytoprotection and increased cell survival in non-fibril and fibril Aβ25–35 treated cells, there was no significant difference in cell survival between the overall effect of 24-h pre-treatment and co-treatment (p > 0.05) for VANL-100, NAR, ALA, or NAR + ALA.
Figure 7.
Effect of antioxidant treatment timepoint on overall cell survival. Cell viability, represented as % control, is shown on the y-axis, and antioxidant treatment time is displayed on the x-axis. The bars represent the mean ± SEM of three independent experiments. (A) Comparison of pre-treatment and co-treatment of antioxidants in combination with non-fibril Aβ25–35. (B) Comparison of pre-treatment and co-treatment of antioxidants in combination with fibril Aβ25–35. Statistical significance of pre-treatment vs. co-treatment was assessed using two-way ANOVA. p < 0.05.
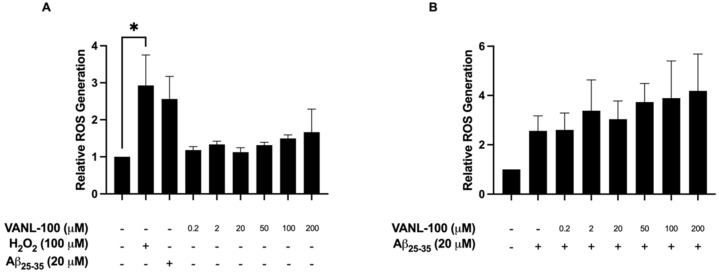
2.6. Effect of VANL-100 on ROS Levels in SH-SY5Y Cells
To determine the generation of intracellular ROS induced by Aβ25–35, we utilized the 2′,7′-dichllorodihydrofluorescein diacetate (H2DCFDA) fluorescence assay. SH-SY5Y cells were treated with 20 μM fibril Aβ25–35, as well as increasing doses of VANL-100 either alone or co-treated with 20 μM fibril Aβ25–35. Cells were treated with 100 μM hydrogen peroxide (H2O2) as a positive control, and the H2DCFDA assay results showed that treatment with H2O2 significantly increased ROS generation (p = 0.0149; Figure 8A). Although relative ROS levels were consistently higher in cells treated with Aβ25–35 alone (Figure 8A), this increase was not statistically different from control ROS levels. Treatment with increasing doses of VANL-100 alone (0.2, 2, 20, 50, 100, and 200 μM) did not significantly increase ROS production (p > 0.05; Figure 8A), supporting our viability results that VANL-100 does not induce cellular toxicity when administered alone. The level of ROS following co-treatment with VANL-100 and 20 μM fibril Aβ25–35 was not significantly different from Aβ25–35 exposure alone (Figure 8B); however, values were also not statistically different from those detected in control cells. These results indicate that VANL-100 alone does not induce ROS production, but its ability to scavenge ROS may be impaired by the presence of Aβ25–35.
Figure 8.
Effect of VANL-100 treatment on ROS generation in SH-SY5Y cells. Relative ROS generation is shown on the y-axis, and individual treatments are displayed on the x-axis. The bars represent the mean ± SEM of four independent experiments. (A) Effect of H2O2, Aβ25–35, and VANL-100 alone on ROS production. (B) Effect of Aβ25–35 alone and VANL-100 with Aβ25–35 on ROS production. Statistical significance was assessed using one-way ANOVA. * p < 0.05.
3. Discussion
The objectives of this study were to assess the neuroprotective capability of the novel antioxidant co-drug VANL-100 in an in vitro model of AD and to determine whether its cytoprotective effects were greater than its parent compounds, NAR and ALA, either alone or combined but not covalently linked (NAR + ALA). Additionally, we aimed to explore the applicability of VANL-100 as a form of ACT for the prevention (pre-treatment) or treatment of neurotoxicity associated with AD pathology. We first assessed the neurotoxicity induced by various doses of both non-fibril and fibril Aβ25–35. The results indicated a dose-dependent decrease in SH-SY5Y cell viability as Aβ concentrations increased. We then selected the 20 μM dose as the treatment of non-fibril and fibril Aβ25–35 to be applied in subsequent antioxidant experiments, as this dose induced cell death but did not eradicate all cells. This is in line with the current literature, which identifies this as an appropriate dose for assessing Aβ25–35-induced cell death, particularly in the SH-SY5Y cell line [63,86,87,88,89,90,91,92]. We then assessed whether the antioxidant compounds VANL-100, NAR, ALA, or NAR + ALA exerted any toxic effects on the cells. Results indicated that these compounds did not reduce cell viability or induce cell death. Overall, our results indicate that the novel conjugate drug VANL-100 can provide cytoprotection against Aβ-induced cell loss. This protection was observed at various doses (20, 50, 100, and 200 μM) and both when cells were pre-treated 24 h prior to Aβ exposure and when co-treated with Aβ. Additionally, both parent compounds, NAR and ALA, when alone and when combined but not covalently linked (NAR + ALA), also attenuated the reduction in cell viability induced by Aβ at similar concentrations (2.0, 20, 50, 100, and 200 μM), during both pre-treatment and co-treatment. For all treatment conditions, there were no significant differences in the neuroprotective effects of VANL-100, NAR, ALA, or NAR + ALA and no significant difference in the cytoprotective effects elicited by the antioxidant compounds following either 24-h pre-treatment or co-treatment.
The involvement of oxidative stress in AD has been validated, and our findings provide early evidence of the potential use of antioxidants, alone and in combined or conjugated forms, to address Aβ toxicity. Since the currently available treatment options for AD such as donepezil, rivastigmine, and memantine [7,93,94] are ineffective in preventing, reversing, or slowing the progression of the disease and are primarily indicated for symptom management, it is necessary to explore alternative methods for preventing and treating AD. More recently, researchers have explored disease-modifying candidate compounds and drugs [8,95]. However, they have proven ineffective, likely due to an insufficient understanding of the intricate pathophysiology of AD and an inappropriate selection of treatment targets and dosages. Addressing the presence of oxidative stress in AD pathology via antioxidants and ACT serves as a promising approach to mitigating oxidative damage and targeting the interaction between ROS and Aβ that contributes to the onset and development of AD.
Researchers have reported the role of Aβ-induced oxidative stress through mechanisms that include the presence of extracellular senile plaques/fibrils comprised of aggregated Aβ peptides with the metal ions iron, copper, and zinc [96,97,98]. These redox-active metals can catalyze the production of ROS when bound to Aβ [96,97,98]. Subsequently, newly generated ROS can oxidize both Aβ peptides as well as surrounding biomolecules. The oxidation of biomolecules such as lipids within neuronal membranes obstructs membrane integrity [99]. Due to its oxidation by ROS and redox-active metals, Aβ clearance is impaired and significantly decreased in those with AD [42,100]. In turn, Aβ exerts toxic effects through the dysregulation of calcium homeostasis and membrane potential depletion, disrupting the cytoskeleton and synaptic function which stimulates neuronal apoptosis [101,102,103,104,105,106]. This has led researchers to explore antioxidant-based targets to address the involvement of oxidative stress in AD pathology.
The improvement in cellular metabolism observed in cells treated with Aβ25–35 and the antioxidant compounds (VANL-100, NAR, or ALA) compared to cells treated with Aβ25–35 alone can be attributed to various factors including the pathways proposed to be involved in Aβ-induced cell death and the ability of the current antioxidant compounds to attenuate the activation of these pathways. It is reported in the literature that Aβ induces neuronal death via apoptotic mechanisms such as c-Jun N-terminal kinase (JNK) activation, caspase-3 activation, and p38 mitogen-activated protein kinase (MAPK) activation, including neuroinflammatory pathways such as nuclear factor kappa B (NFκB) [107,108,109,110,111,112,113]. These pathways have also been implicated in oxidative stress-induced cell death, and the utilization of compounds that stimulate the production of antioxidant and detoxifying enzymes has been shown to inhibit apoptosis, resulting in reduced cell death [114,115,116]. Our current results are in line with the literature and support the neuroprotective role of antioxidants NAR and ALA against Aβ-induced toxicity.
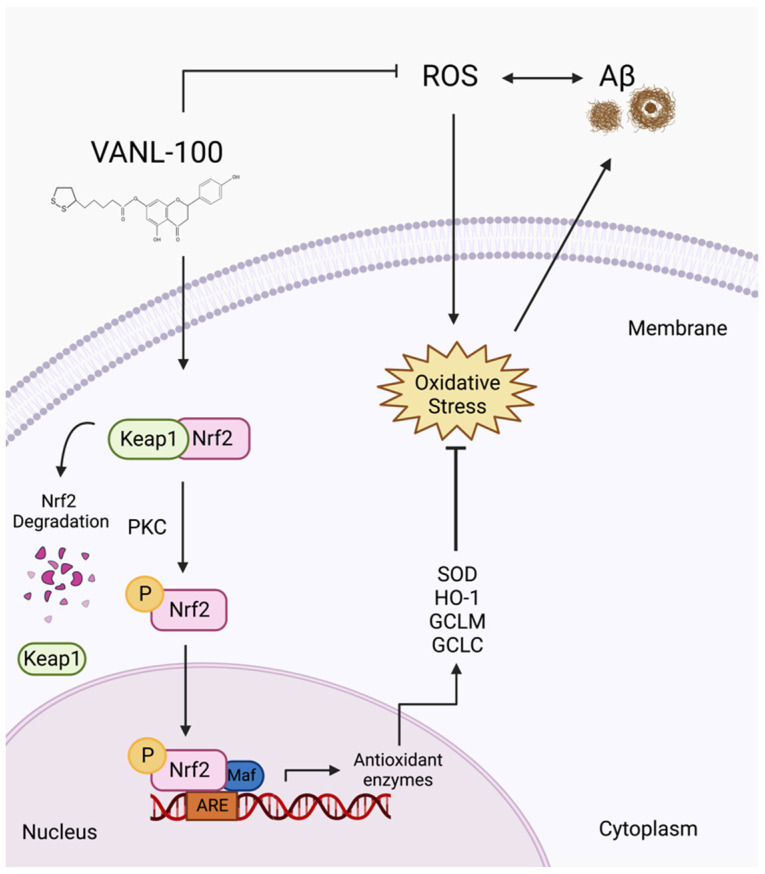
Interestingly, both NAR and ALA have been reported to elicit their neuroprotective antioxidant effects through similar mechanisms and using similar intracellular antioxidant signaling pathways, including through the activation of nuclear factor erythroid 2-related factor 2 (Nrf2) [117,118,119,120,121,122,123,124,125,126,127,128]. Nrf2 is the primary transcription factor responsible for the activity of the antioxidant defense system; it regulates the expression of antioxidant and detoxifying genes and the subsequent production of antioxidant and detoxifying enzymes [129,130,131,132]. Nrf2 may serve as a potential mechanism of protection for VANL-100 (Figure 9). We propose that VANL-100 activates Nrf2 by stimulating the cytoplasmic dissociation of Nrf2 from the inhibitory protein Kelch-like ECH-associated protein 1 (Keap1) via phosphorylation by kinases such as PKC. Activation of Nrf2 leads to the nuclear translocation of phosphorylated Nrf2, where Nrf2 binds to small Maf proteins on the antioxidant response element (ARE) in the promoter region of antioxidant genes such as superoxide dismutase (SOD), heme-oxygenase 1 (HO-1), glutamate-cysteine ligase modifiers and catalytic subunits (GCLM and GCLC, respectively). This stimulates the transcription of antioxidant and detoxifying genes that code for enzymes that inhibit oxidative stress and the subsequent production of Aβ plaques.
Figure 9.
Schematic of the proposed mechanism of action of VANL-100. VANL-100 may act as an Nrf2 activator to stimulate the production of antioxidant and detoxifying enzymes that inhibit oxidative stress and its contribution to the production and accumulation of Aβ. Created using BioRender.com.
Our results demonstrated the cytoprotective effects of all antioxidant compounds studied, but in contrast to the findings of Saleh et al. (2017), we did not see enhanced protection from VANL-100 compared to NAR and ALA when alone or in combination but not covalently bound (NAR + ALA). There are several factors that may have contributed to these findings. It is important to recognize that Saleh et al. (2017) tested the effects of VANL-100 and its parent compounds in a rodent model of an ischemic stroke. Since the pathophysiologies of an ischemic stroke and AD are significantly different, and the complexity of in vivo neurotoxicity is not always captured in vitro, it is expected that discrepancies in our findings may exist when utilizing two different models of neurodegeneration. Additionally, Saleh et al. (2017) used oxygen and glucose deprivation in mixed neocortical primary cultures for their in vitro experiments, whereas we used SH-SY5Y cells treated with Aβ in our cellular model. It is plausible that, compared to its parent compounds, the effects of this conjugated drug, VANL-100, may have a distinctive impact based on the mechanisms of neurotoxicity and/or in neuronal cells compared to the neuroblastoma cell line. Taken together, these factors likely contributed to the observation that the neuroprotective effects of VANL-100 in the present study were not significantly different from those of the parent compounds.
Researchers have reported increased ROS production following Aβ exposure in various cell lines including SH-SY5Y cells [133,134,135,136,137,138,139,140]. Additionally, antioxidant compounds such as NAR and ALA have been shown to attenuate cell stress due to elevated ROS production induced by Aβ exposure and other mediators of oxidative stress [64,68,76,118,141,142]. Since the neuroprotective effects of the parent compounds NAR and ALA against ROS generation have already been described in the literature, it was necessary to also assess whether the novel compound VANL-100 exerts similar effects on ROS generation in vitro. H2O2 was used as a positive control and significantly increased ROS generation. When administered alone, VANL-100 did not significantly increase ROS production, which supports results from cell viability experiments that indicate that VANL-100 does not exert toxic effects on SH-SY5Y cells. Although not statistically significant, Aβ25–35 treatment alone consistently resulted in higher ROS levels which remained elevated when cells were co-treated with Aβ25–35 and VANL-100. It is possible that Aβ25–35 may be inducing an antioxidant response that masks some of the effects of Aβ25–35 on ROS levels. Future studies should examine ROS target molecules to gain a better understanding of the mechanism of Aβ25–35-induced cell death in SH-SY5Y cells. The results also suggest that VANL-100 is not protecting SH-SY5Y cells via the inhibition of ROS generation. While this is possible, additional experiments would be needed to better understand the mechanisms involved. It is possible that the toxic effects of Aβ25–35-induced ROS generation may interfere with the capacity of VANL-100 to scavenge ROS. Additionally, the variability in the data, along with the lack of significant increase in ROS following Aβ25–35 treatment, indicates that additional optimization of the assay or alternative methods for examining ROS should be carried out. Although significant improvements in viability were observed following 24 h of co-treatment with Aβ25–35 and VANL-100, this timepoint may not be optimal for assessing ROS levels. Reproducing these experiments across various time points may provide evidence for the impact of VANL-100 on ROS levels. Additional experiments, including the examination of ROS targets, are necessary to discern the neuroprotective effects VANL-100 exerts on reducing intracellular ROS production in this cell line.
The SH-SY5Y cell line is widely utilized in studies requiring a reliable neuron-like cell culture model, which supports our use of SH-SY5Y in this study. However, the utilization of this neuroblastoma cell line is also the main limitation of our study. It is arguable that the cell line may not be the most appropriate model of AD since it is a cancer cell line. The SH-SY5Y cell line can be further manipulated using agents such as retinoic acid to stimulate differentiation and morphological characteristics comparable to neuronal cells [143,144]. Additionally, an alternative method for modelling AD could include the utilization of primary neurons with genetic modifications that mimic AD. These include, but are not limited to, neurons derived from single, double, and triple transgenic AD mouse models, which are frequently utilized in preclinical studies [145]. Utilizing animal models that meticulously recapitulate some of the clinical pathologies of AD are necessary next steps for discerning molecular mechanisms and advancing preclinical investigations of ACTs.
Future directions include exploring the mechanisms by which VANL-100 elicits neuroprotective effects in the SH-SY5Y cellular model of AD. As previously mentioned, the parent compounds of VANL-100, NAR, and ALA, possess antioxidant neuroprotective effects through their mechanism of action as activators of Nrf2, the primary transcription factor involved in the body’s endogenous antioxidant response to oxidative stress [117,118,119,120,121,122,123,124,125,126,127,128]. It is plausible that VANL-100 may provide neuroprotection by acting through similar mechanisms as NAR and ALA to activate Nrf2 signaling pathways and stimulate the production of antioxidant and detoxifying enzymes that target perpetrators of oxidative stress such as ROS. Additionally, it is necessary to assess the effects of VANL-100 in other models of AD, including, but not limited to, primary neurons, including those obtained from transgenic AD mice and in vivo explorations, since researchers have reported the neuroprotective effects of its parent compounds, NAR and ALA, in transgenic and non-transgenic AD rodent models [58,66,72,73,146,147,148,149]. Although both parent compounds of VANL-100 have been shown to provide neuroprotective effects in various models of AD, our work is the first to explore the role of VANL-100 as a form of ACT in an early cellular model of AD.
4. Materials and Methods
4.1. Materials
VANL-100 was synthesized following the synthesis and proton NMR validation protocol previously described [80]. NAR was obtained from Sigma-Aldrich (Cat. No. N5893). ALA was obtained from EMD Millipore (Cat. No. 437692). VANL-100, NAR, and ALA were dissolved in DMSO (Sigma Life Science, Cat. No. D2650). Aβ25–35 was purchased from Cedarlane (product No. A15416-25) and prepared in sterile distilled water at a concentration of 1 mM. Aliquots were stored at −20 °C until treatment. For experiments examining the effects of soluble Aβ25–35, aliquots were used immediately after thawing, to limit aggregation of the peptide. Aggregates were prepared as described previously, with aliquots incubated at 37 °C for four days prior to treatment [150]. MTT was purchased from EMD Millipore (Cat. No. 475989) and dissolved in phosphate buffer saline (PBS). H2DCFDA was purchased from Invitrogen by Thermo Fisher Scientific (Cat. No. C6827) and dissolved in DMSO.
4.2. Cell Culture
The human neuroblastoma SH-SY5Y cell line was obtained from ATCC (CRL-2266) and maintained in Dulbecco’s Modified Eagle Medium (DMEM; Gibco by Life Technologies) supplemented with 10% fetal bovine serum (FBS; Gibco by Life Technologies) and 5% penicillin-streptomycin (Gibco by Life Technologies). The cells were grown and maintained at 37 °C in a humidified environment of 5% CO2 and 95% air.
4.3. Antioxidant Treatments
SH-SY5Y cells were seeded in 96-well plates and treated with antioxidants once they reached 70–80% confluence. Pre-treatment experiments consisted of 24-h treatment with 0.2, 2.0, 20, 50, 100, and 200 μM of VANL-100, NAR alone, ALA alone, or NAR + ALA, followed by co-incubation with 20 μM non-fibril or fibril Aβ25–35 for an additional 24 h. Co-treatment experiments consisted of simultaneous co-treatment with 0.2, 2.0, 20, 50, 100, and 200 μM of each antioxidant compound with 20 μM non-fibril or fibril Aβ25–35. Twenty-four hours after Aβ25–35 administration, cell viability was assessed using the MTT assay. DMSO was used to dissolve the antioxidants and was therefore used as the vehicle in all experiments. The final concentration of DMSO in all wells was 0.1%.
4.4. Cell Viability and Toxicity Assay
After 24 h of incubation, the media was aspirated and 100 μL of the MTT reagent (0.75 mg/mL in PBS) was added to each well and incubated for 3 h. Post incubation, the MTT reagent was removed and 100 μL of DMSO was added to each well. Following incubation for 15 min, absorbance was read at 600 nm. The cell viability and toxicity of all antioxidant compounds and Aβ25–35 were individually established in addition to the protective effects of VANL-100 and its parent compounds against non-fibril or fibril Aβ25–35-treated cells.
4.5. Reactive Oxygen Species Determination
ROS levels were detected by the H2DCFDA fluorescence assay [151]. Cells were seeded in black clear-bottom 96-well plates in phenol-red free media and incubated overnight. On the day of the experiment, 20 μM H2DCFDA was prepared in phenol-red free media, 100 μL was added to each well, and the plates were incubated for 30 min. The media was aspirated, and the cells were fed with fresh phenol-red free media. Cells were then treated with antioxidant compounds and Aβ25–35. After 24 h of incubation, ROS generation was measured with fluorescence plate spectroscopy at an excitation of 485 nm and an emission of 520 nm. Cells treated with 100 μM H2O2 served as a positive control. Because cell viability was reduced following 24-h treatment with Aβ25–35, MTT assay measurements from the same wells were used to normalize the ROS values.
4.6. Statistical Analysis
All data are represented as a mean ± standard error of the mean (SEM). Statistical analyses were performed and interpreted using GraphPad Prism 9. One-way analysis of variance (ANOVA) was conducted to compare control groups and treatment groups, followed by Dunnett’s post-hoc test. Two-way ANOVA was conducted to compare the effects of pre-treatment and co-treatment time points, followed by Sidak’s post-hoc test. Statistical significance was defined by p < 0.05.
5. Conclusions
Our results demonstrate for the first time the neuroprotective effects of the novel co-drug VANL-100 in an Aβ-induced cellular model of AD. These findings support the use of antioxidant combinations or conjugate compounds as a potential novel therapeutic strategy for combating oxidative stress and the impact of abnormal protein aggregation during the onset and progression of AD. Additionally, our results support the role of oxidative stress in the pathogenesis of AD and the necessary presence of antioxidants to maintain optimal cellular conditions and overall cell health. Future studies exploring the mechanisms by which VANL-100 elicits neuroprotection are necessary to elucidate the underlying molecular pathways involved in its protection of and applicability to other models/conditions of neurodegeneration. This would further elucidate the efficacy and appropriateness of VANL-100 and other antioxidant-based co-drugs as potential drug candidates for the prevention and/or treatment of neurodegenerative diseases that are characterized by oxidative stress and/or abnormal protein aggregation.
Acknowledgments
The authors would like to acknowledge the Department of Biomedical Sciences and the Ontario Veterinary College at the University of Guelph for supporting this work. A.E.C. is the recipient of an Ontario Veterinary College Graduate Scholarship.
Author Contributions
Conceptualization, A.E.C.; methodology, A.E.C.; validation, A.E.C. and B.E.K.; formal analysis, A.E.C.; investigation, A.E.C.; resources, T.M.S. and B.E.K.; data curation, A.E.C.; writing—original draft preparation, A.E.C.; writing—review and editing, T.M.S. and B.E.K.; visualization, A.E.C.; supervision, T.M.S. and B.E.K.; project administration, A.E.C., T.M.S., and B.E.K.; funding acquisition, A.E.C. and T.M.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Government of Ontario’s Ontario Graduate Scholarship and the Atlantic Canada Opportunities Agency, grant number 12213. The APC was funded by T.M.S. and B.E.K.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.World Alzheimer Report 2022—Life after Diagnosis: Navigating Treatment, Care and Support. 416. [(accessed on 12 October 2022)]. Available online: https://www.alzint.org/u/World-Alzheimer-Report-2022.pdf.
- 2.Mayeux R., Stern Y. Epidemiology of Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2012;2:a006239. doi: 10.1101/cshperspect.a006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheltens P., Strooper B.D., Kivipelto M., Holstege H., Chételat G., Teunissen C.E., Cummings J., van der Flier W.M. Alzheimer’s Disease. Lancet. 2021;397:1577–1590. doi: 10.1016/S0140-6736(20)32205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reitz C., Mayeux R. Alzheimer Disease: Epidemiology, Diagnostic Criteria, Risk Factors and Biomarkers. Biochem. Pharmacol. 2014;88:640–651. doi: 10.1016/j.bcp.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodríguez-Gómez O., Palacio-Lacambra M.E., Palasí A., Ruiz-Laza A., Boada-Rovira M. Prevention of Alzheimer’s Disease: A Global Challenge for next Generation Neuroscientists. J. Alzheimers Dis. 2014;42((Suppl. 4)):S515–S523. doi: 10.3233/JAD-141479. [DOI] [PubMed] [Google Scholar]
- 6.Srivastava S., Ahmad R., Khare S.K. Alzheimer’s Disease and Its Treatment by Different Approaches: A Review. Eur. J. Med. Chem. 2021;216:113320. doi: 10.1016/j.ejmech.2021.113320. [DOI] [PubMed] [Google Scholar]
- 7.Breijyeh Z., Karaman R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules. 2020;25:5789. doi: 10.3390/molecules25245789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yiannopoulou K.G., Anastasiou A.I., Zachariou V., Pelidou S.-H. Reasons for Failed Trials of Disease-Modifying Treatments for Alzheimer Disease and Their Contribution in Recent Research. Biomedicines. 2019;7:97. doi: 10.3390/biomedicines7040097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Se Thoe E., Fauzi A., Tang Y.Q., Chamyuang S., Chia A.Y.Y. A Review on Advances of Treatment Modalities for Alzheimer’s Disease. Life Sci. 2021;276:119129. doi: 10.1016/j.lfs.2021.119129. [DOI] [PubMed] [Google Scholar]
- 10.National Institute on Aging (NIA) Evaluation of the Safety, Tolerability and Impact on Biomarkers of Anti-Oxidant Treatment of Mild to Moderate Alzheimer’s Disease. National Institute on Aging; Bethesda, MD, USA: 2009. [Google Scholar]
- 11.Goktas Z. The Effect of Black Mulberry (Morus Nigra) Consumption on Cognitive Functions and Antioxidant Capacity in Individuals Diagnosed with Dementia. [(accessed on 12 October 2022)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT05406648.
- 12.Park S.-Y. Effects of Mitochondrial-Targeted Antioxidant on Carotid Artery Endothelial Function and Brain Blood Flow in Mild Cognitive Impairment (MCI) Patients. [(accessed on 12 October 2022)];2022 Available online: https://www.clinicaltrials.gov/ct2/show/NCT03514875.
- 13.Shinto L. Fish Oil and Alpha Lipoic Acid in Mild Alzheimer’s Disease. [(accessed on 12 October 2022)];2017 Available online: https://www.clinicaltrials.gov/ct2/show/NCT00090402.
- 14.US Department of Veterans Affairs . A Single Center, Multi-Site, Randomized, Double-Blind, Placebo-Controlled Trial of Resveratrol With Glucose and Malate (RGM) to Slow the Progression of Alzheimer’s Disease. U.S. Department of Veterans Affairs; Washington, DC, USA: 2012. [Google Scholar]
- 15.Oregon Health and Science University . Lutein and Oxidative Stress in Alzheimer’s Disease—A Pilot Study. Oregon Health & Science University; Portland, OR, USA: 2019. [Google Scholar]
- 16.National Institute on Aging (NIA) A Randomized, Double-Blind, Placebo-Controlled Trial of Vitamin E and Donepezil HCL (Aricept) to Delay Clinical Progression from Mild Cognitive Impairment (MCI) to Alzheimer’s Disease (AD) National Institute on Aging; Bethesda, MD, USA: 2009. [Google Scholar]
- 17.Paul F. Sunphenon EGCg (Epigallocatechin-Gallate) in the Early Stage of Alzheimer’s Disease. [(accessed on 12 October 2022)];2021 Available online: https://clinicaltrials.gov/ct2/show/NCT00951834.
- 18.Vina J. Effect of Activation of the Receptor PPARg/RXR as a Possible Treatment for Alzheimer’s Disease. Role of Genistein. [(accessed on 12 October 2022)];2021 Available online: https://clinicaltrials.gov/ct2/show/NCT01982578.
- 19.Taipei City Psychiatric Center, Taiwan . The Effects of Omega-3 Fatty Acids Monotherapy in Alzheimer’s Disease and Mild Cognitive Impairment: A Preliminary Randomized Double-Blind Placebo-Controlled Study. Taipei City Psychiatric Center; Taipei, Taiwan: 2008. [DOI] [PubMed] [Google Scholar]
- 20.John Douglas French Foundation . A Phase II, Double-Blind, Placebo-Controlled Study of the Safety and Tolerability of Two Doses of Curcumin C3 Complex Versus Placebo in Patients with Mild to Moderate Alzheimer’s Disease. John Douglas French Foundation; Pasadena, CA, USA: 2009. [Google Scholar]
- 21.Alzheimer’s Disease Cooperative Study (ADCS) Phase II Study to Evaluate the Impact on Biomarkers of Resveratrol Treatment in Patients with Mild to Moderate Alzheimer’s Disease. 2016. [(accessed on 12 October 2022)]. Available online: https://scholars.uky.edu/en/projects/phase-ii-study-to-evaluate-the-impact-on-biomarkers-of-resveratro-3.
- 22.Schmitt F. Prevention of Alzheimer’s Disease by Vitamin E and Selenium (PREADVISE) [(accessed on 12 October 2022)];2018 Available online: https://clinicaltrials.gov/ct2/show/NCT00040378.
- 23.Medical College of Wisconsin . Randomized, Placebo-Controlled Clinical Trial of Resveratrol Supplement Effects on Cognition, Function and Behavior in Patients with Mild-to-Moderate Alzheimer’s Disease. Medical College of Wisconsin; Milwaukee, WI, USA: 2015. [Google Scholar]
- 24.Shinto L. Lipoic Acid and Omega-3 Fatty Acids in Alzheimer’s Disease. [(accessed on 12 October 2022)];2017 Available online: https://www.clinicaltrials.gov/ct2/show/NCT01058941.
- 25.The University of Texas Health Science Center at San Antonio Pilot Study to Investigate the Safety and Feasibility of Senolytic Therapy to Modulate Progression of Alzheimer’s Disease (SToMP-AD) 2022. [(accessed on 12 October 2022)]. Available online: https://www.clinicalconnection.com/clinical-trials-from-other-databases/full-listing-from-other-databases/508404/58195737/pilot-study-to-investigate-the-safety-and-feasibility-of-senolytic-therapy-to-modulate-progression-of-alzheimer-s-disease-stomp-ad.
- 26.US Department of Veterans Affairs CSP #546—A Randomized, Clinical Trial of Vitamin E and Memantine in Alzheimer’s Disease (TEAM-AD) [(accessed on 12 October 2022)];2014 Available online: https://www.clinicaltrials.gov/ct2/show/NCT00235716.
- 27.Life Extension Foundation Inc Open Label, Crossover, Pilot Study to Assess the Efficacy & Safety of Perispinal Admin.of Etanercept(Enbrel®) in Comb.w/Nutritional Supplements vs. Nutritional Supplements Alone in Subj. w/Mild to Mod. Alzheimer’s Disease Receiving Std. Care. 2016. [(accessed on 12 October 2022)]. Available online: https://faseb.onlinelibrary.wiley.com/doi/abs/10.1096/fasebj.30.1_supplement.lb296.
- 28.Wake Forest University Health Sciences Phase II Clinical Trial to Evaluate the Safety and Feasibility of Senolytic Therapy in Alzheimer’s Disease. [(accessed on 12 October 2022)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT04685590.
- 29.Kirkland J.L. ALSENLITE: An Open-Label Pilot Study of Senolytics for Alzheimer’s Disease. [(accessed on 12 October 2022)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT04785300.
- 30.Lipsitz L. Senolytics to Alleviate Mobility Issues and Neurological Impairment in Aging. 2022. [(accessed on 12 October 2022)]. Available online: https://www.marcusinstituteforaging.org/current-studies/senolytics-alleviate-mobility-issues-and-neurological-impairment-aging-stamina-study.
- 31.de la Torre R. Prevention of Cognitive Decline in ApoE4 Carriers with Subjective Cognitive Decline After EGCG and a Multimodal Intervention. [(accessed on 12 October 2022)];2020 Available online: https://clinicaltrials.gov/ct2/show/NCT03978052.
- 32.Shinto L. Pilot Study: Lipoic Acid and Omega-3 Fatty Acid for Alzheimer’s Disease Prevention. [(accessed on 12 October 2022)];2017 Available online: https://clinicaltrials.gov/ct2/show/NCT01780974.
- 33.Markesbery W.R. Oxidative Stress Hypothesis in Alzheimer’s Disease. Free Radic. Biol. Med. 1997;23:134–147. doi: 10.1016/S0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- 34.Rosini M., Simoni E., Milelli A., Minarini A., Melchiorre C. Oxidative Stress in Alzheimer’s Disease: Are We Connecting the Dots? J. Med. Chem. 2014;57:2821–2831. doi: 10.1021/jm400970m. [DOI] [PubMed] [Google Scholar]
- 35.Ionescu-Tucker A., Cotman C.W. Emerging Roles of Oxidative Stress in Brain Aging and Alzheimer’s Disease. Neurobiol Aging. 2021;107:86–95. doi: 10.1016/j.neurobiolaging.2021.07.014. [DOI] [PubMed] [Google Scholar]
- 36.Wang X., Wang W., Li L., Perry G., Lee H., Zhu X. Oxidative Stress and Mitochondrial Dysfunction in Alzheimer’s Disease. Biochim. Biophys. Acta. 2014;1842:1240–1247. doi: 10.1016/j.bbadis.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cioffi F., Adam R.H.I., Broersen K. Molecular Mechanisms and Genetics of Oxidative Stress in Alzheimer’s Disease. J. Alzheimers Dis. 2019;72:981–1017. doi: 10.3233/JAD-190863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Z., Zhong C. Oxidative Stress in Alzheimer’s Disease. Neurosci. Bull. 2014;30:271–281. doi: 10.1007/s12264-013-1423-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh A., Kukreti R., Saso L., Kukreti S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules. 2019;24:1583. doi: 10.3390/molecules24081583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cenini G., Lloret A., Cascella R. Oxidative Stress in Neurodegenerative Diseases: From a Mitochondrial Point of View. Oxid. Med. Cell. Longev. 2019;2019:2105607. doi: 10.1155/2019/2105607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teleanu D.M., Niculescu A.-G., Lungu I.I., Radu C.I., Vladâcenco O., Roza E., Costăchescu B., Grumezescu A.M., Teleanu R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022;23:5938. doi: 10.3390/ijms23115938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheignon C., Tomas M., Bonnefont-Rousselot D., Faller P., Hureau C., Collin F. Oxidative Stress and the Amyloid Beta Peptide in Alzheimer’s Disease. Redox Biol. 2017;14:450–464. doi: 10.1016/j.redox.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hensley K., Butterfieldld D.A., Hall N., Cole P., Subramaniam R., Mark R., Mattson M.P., Markesbery W.R., Harris M.E., Aksenov M., et al. Reactive Oxygen Species as Causal Agents in the Neurotoxicity of the Alzheimer’s Disease-Associated Amyloid Beta Peptidea. Ann. N. Y. Acad. Sci. 1996;786:120–134. doi: 10.1111/j.1749-6632.1996.tb39057.x. [DOI] [PubMed] [Google Scholar]
- 44.Giovanna C., Cecchi C., Pensalfini A., Bonini S.A., Ferrari-Toninelli G., Liguri G., Memo M., Uberti D. Generation of Reactive Oxygen Species by Beta Amyloid Fibrils and Oligomers Involves Different Intra/Extracellular Pathways. Amino Acids. 2010;38:1101–1106. doi: 10.1007/s00726-009-0319-7. [DOI] [PubMed] [Google Scholar]
- 45.Tamagno E., Guglielmotto M., Vasciaveo V., Tabaton M. Oxidative Stress and Beta Amyloid in Alzheimer’s Disease. Which Comes First: The Chicken or the Egg? Antioxidants. 2021;10:1479. doi: 10.3390/antiox10091479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guglielmotto M., Giliberto L., Tamagno E., Tabaton M. Oxidative Stress Mediates the Pathogenic Effect of Different Alzheimer’s Disease Risk Factors. Front. Aging Neurosci. 2010;2:3. doi: 10.3389/neuro.24.003.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Butterfield D.A., Swomley A.M., Sultana R. Amyloid β-Peptide (1–42)-Induced Oxidative Stress in Alzheimer Disease: Importance in Disease Pathogenesis and Progression. Antioxid. Redox Signal. 2013;19:823–835. doi: 10.1089/ars.2012.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cai Z., Zhao B., Ratka A. Oxidative Stress and β-Amyloid Protein in Alzheimer’s Disease. Neuromol. Med. 2011;13:223–250. doi: 10.1007/s12017-011-8155-9. [DOI] [PubMed] [Google Scholar]
- 49.Nouri Z., Fakhri S., El-Senduny F.F., Sanadgol N., Abd-ElGhani G.E., Farzaei M.H., Chen J.-T. On the Neuroprotective Effects of Naringenin: Pharmacological Targets, Signaling Pathways, Molecular Mechanisms, and Clinical Perspective. Biomolecules. 2019;9:690. doi: 10.3390/biom9110690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goyal A., Verma A., Dubey N., Raghav J., Agrawal A. Naringenin: A Prospective Therapeutic Agent for Alzheimer’s and Parkinson’s Disease. J. Food Biochem. 2022. p. e14415. Epub ahead of print . [DOI] [PubMed]
- 51.Heo H.J., Kim D.-O., Shin S.C., Kim M.J., Kim B.G., Shin D.-H. Effect of Antioxidant Flavanone, Naringenin, from Citrus junos on Neuroprotection. J. Agric. Food Chem. 2004;52:1520–1525. doi: 10.1021/jf035079g. [DOI] [PubMed] [Google Scholar]
- 52.Molz P., Schröder N. Potential Therapeutic Effects of Lipoic Acid on Memory Deficits Related to Aging and Neurodegeneration. Front. Pharmacol. 2017;8:849. doi: 10.3389/fphar.2017.00849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pei X., Hu F., Luo F., Huang X., Li X., Xing S., Long D. The Neuroprotective Effects of Alpha-Lipoic Acid on an Experimental Model of Alzheimer’s Disease in PC12 Cells. J. Appl. Toxicol. 2022;42:285–294. doi: 10.1002/jat.4213. [DOI] [PubMed] [Google Scholar]
- 54.Kaur D., Behl T., Sehgal A., Singh S., Sharma N., Chigurupati S., Alhowail A., Abdeen A., Ibrahim S.F., Vargas-De-La-Cruz C., et al. Decrypting the Potential Role of α-Lipoic Acid in Alzheimer’s Disease. Life Sci. 2021;284:119899. doi: 10.1016/j.lfs.2021.119899. [DOI] [PubMed] [Google Scholar]
- 55.Wu J., Kou X., Ju H., Zhang H., Yang A., Shen R. Design, Synthesis and Biological Evaluation of Naringenin Carbamate Derivatives as Potential Multifunctional Agents for the Treatment of Alzheimer’s Disease. Bioorg. Med. Chem. Lett. 2021;49:128316. doi: 10.1016/j.bmcl.2021.128316. [DOI] [PubMed] [Google Scholar]
- 56.Ghofrani S., Joghataei M.-T., Mohseni S., Baluchnejadmojarad T., Bagheri M., Khamse S., Roghani M. Naringenin Improves Learning and Memory in an Alzheimer’s Disease Rat Model: Insights into the Underlying Mechanisms. Eur. J. Pharmacol. 2015;764:195–201. doi: 10.1016/j.ejphar.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 57.Lawal M., Olotu F.A., Soliman M.E.S. Across the Blood-Brain Barrier: Neurotherapeutic Screening and Characterization of Naringenin as a Novel CRMP-2 Inhibitor in the Treatment of Alzheimer’s Disease Using Bioinformatics and Computational Tools. Comput. Biol. Med. 2018;98:168–177. doi: 10.1016/j.compbiomed.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 58.Zhou T., Liu L., Wang Q., Gao Y. Naringenin Alleviates Cognition Deficits in High-Fat Diet-Fed SAMP8 Mice. J. Food Biochem. 2020;44:e13375. doi: 10.1111/jfbc.13375. [DOI] [PubMed] [Google Scholar]
- 59.Sang Z., Wang K., Shi J., Liu W., Cheng X., Zhu G., Wang Y., Zhao Y., Qiao Z., Wu A., et al. The Development of Advanced Structural Framework as Multi-Target-Directed Ligands for the Treatment of Alzheimer’s Disease. Eur. J. Med. Chem. 2020;192:112180. doi: 10.1016/j.ejmech.2020.112180. [DOI] [PubMed] [Google Scholar]
- 60.Ma J., Yang W.-Q., Zha H., Yu H.-R. Effect of naringenin on learning and memory ability on model rats with Alzheimer disease. Zhong Yao Cai. 2013;36:271–276. [PubMed] [Google Scholar]
- 61.Yang Z., Kuboyama T., Tohda C. Naringenin Promotes Microglial M2 Polarization and Aβ Degradation Enzyme Expression. Phytother. Res. 2019;33:1114–1121. doi: 10.1002/ptr.6305. [DOI] [PubMed] [Google Scholar]
- 62.Ahsan A.U., Sharma V.L., Wani A., Chopra M. Naringenin Upregulates AMPK-Mediated Autophagy to Rescue Neuronal Cells From β-Amyloid (1–42) Evoked Neurotoxicity. Mol. Neurobiol. 2020;57:3589–3602. doi: 10.1007/s12035-020-01969-4. [DOI] [PubMed] [Google Scholar]
- 63.Zhang N., Hu Z., Zhang Z., Liu G., Wang Y., Ren Y., Wu X., Geng F. Protective Role of Naringenin Against Aβ25–35-Caused Damage via ER and PI3K/Akt-Mediated Pathways. Cell Mol. Neurobiol. 2018;38:549–557. doi: 10.1007/s10571-017-0519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Md S., Gan S.Y., Haw Y.H., Ho C.L., Wong S., Choudhury H. In Vitro Neuroprotective Effects of Naringenin Nanoemulsion against β-Amyloid Toxicity through the Regulation of Amyloidogenesis and Tau Phosphorylation. Int. J. Biol. Macromol. 2018;118:1211–1219. doi: 10.1016/j.ijbiomac.2018.06.190. [DOI] [PubMed] [Google Scholar]
- 65.Shinto L., Quinn J., Montine T., Dodge H.H., Woodward W., Baldauf-Wagner S., Waichunas D., Bumgarner L., Bourdette D., Silbert L., et al. A Randomized Placebo-Controlled Pilot Trial of Omega-3 Fatty Acids and Alpha Lipoic Acid in Alzheimer’s Disease. J. Alzheimers Dis. 2014;38:111–120. doi: 10.3233/JAD-130722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y.-H., Wang D.-W., Xu S.-F., Zhang S., Fan Y.-G., Yang Y.-Y., Guo S.-Q., Wang S., Guo T., Wang Z.-Y., et al. α-Lipoic Acid Improves Abnormal Behavior by Mitigation of Oxidative Stress, Inflammation, Ferroptosis, and Tauopathy in P301S Tau Transgenic Mice. Redox Biol. 2018;14:535–548. doi: 10.1016/j.redox.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Sousa C.N.S., da Silva Leite C.M.G., da Silva Medeiros I., Vasconcelos L.C., Cabral L.M., Patrocínio C.F.V., Patrocínio M.L.V., Mouaffak F., Kebir O., Macedo D., et al. Alpha-Lipoic Acid in the Treatment of Psychiatric and Neurological Disorders: A Systematic Review. Metab. Brain Dis. 2019;34:39–52. doi: 10.1007/s11011-018-0344-x. [DOI] [PubMed] [Google Scholar]
- 68.Metsla K., Kirss S., Laks K., Sildnik G., Palgi M., Palumaa T., Tõugu V., Palumaa P. α-Lipoic Acid Has the Potential to Normalize Copper Metabolism, Which Is Dysregulated in Alzheimer’s Disease. J. Alzheimers Dis. 2022;85:715–728. doi: 10.3233/JAD-215026. [DOI] [PubMed] [Google Scholar]
- 69.Zarini-Gakiye E., Vaezi G., Parivar K., Sanadgol N. Age and Dose-Dependent Effects of Alpha-Lipoic Acid on Human Microtubule- Associated Protein Tau-Induced Endoplasmic Reticulum Unfolded Protein Response: Implications for Alzheimer’s Disease. CNS Neurol. Disord. Drug Targets. 2021;20:451–464. doi: 10.2174/1871527320666210126114442. [DOI] [PubMed] [Google Scholar]
- 70.Fava A., Pirritano D., Plastino M., Cristiano D., Puccio G., Colica C., Ermio C., De Bartolo M., Mauro G., Bosco D. The Effect of Lipoic Acid Therapy on Cognitive Functioning in Patients with Alzheimer’s Disease. J. Neurodegener. Dis. 2013;2013:454253. doi: 10.1155/2013/454253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Memudu A.E., Adewumi A.E. Alpha Lipoic Acid Ameliorates Scopolamine Induced Memory Deficit and Neurodegeneration in the Cerebello-Hippocampal Cortex. Metab. Brain Dis. 2021;36:1729–1745. doi: 10.1007/s11011-021-00720-9. [DOI] [PubMed] [Google Scholar]
- 72.Sancheti H., Akopian G., Yin F., Brinton R.D., Walsh J.P., Cadenas E. Age-Dependent Modulation of Synaptic Plasticity and Insulin Mimetic Effect of Lipoic Acid on a Mouse Model of Alzheimer’s Disease. PLoS ONE. 2013;8:e69830. doi: 10.1371/journal.pone.0069830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sancheti H., Kanamori K., Patil I., Díaz Brinton R., Ross B.D., Cadenas E. Reversal of Metabolic Deficits by Lipoic Acid in a Triple Transgenic Mouse Model of Alzheimer’s Disease: A 13C NMR Study. J. Cereb. Blood Flow Metab. 2014;34:288–296. doi: 10.1038/jcbfm.2013.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Erdoğan M.E., Aydın S., Yanar K., Mengi M., Kansu A.D., Cebe T., Belce A., Çelikten M., Çakatay U. The Effects of Lipoic Acid on Redox Status in Brain Regions and Systemic Circulation in Streptozotocin-Induced Sporadic Alzheimer’s Disease Model. Metab. Brain Dis. 2017;32:1017–1031. doi: 10.1007/s11011-017-9983-6. [DOI] [PubMed] [Google Scholar]
- 75.Park E., Gim J., Kim D.K., Kim C.-S., Chun H.S. Protective Effects of Alpha-Lipoic Acid on Glutamate-Induced Cytotoxicity in C6 Glioma Cells. Biol. Pharm. Bull. 2019;42:94–102. doi: 10.1248/bpb.b18-00603. [DOI] [PubMed] [Google Scholar]
- 76.Dieter F., Esselun C., Eckert G.P. Redox Active α-Lipoic Acid Differentially Improves Mitochondrial Dysfunction in a Cellular Model of Alzheimer and Its Control Cells. Int. J. Mol. Sci. 2022;23:9186. doi: 10.3390/ijms23169186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kamarudin M.N.A., Mohd Raflee N.A., Hussein S.S.S., Lo J.Y., Supriady H., Abdul Kadir H. (R)-(+)-α-Lipoic Acid Protected NG108-15 Cells against H₂O₂-Induced Cell Death through PI3K-Akt/GSK-3β Pathway and Suppression of NF-Κβ-Cytokines. Drug Des. Devel. Ther. 2014;8:1765–1780. doi: 10.2147/DDDT.S67980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Staykov H., Lazarova M., Hassanova Y., Stefanova M., Tancheva L., Nikolov R. Neuromodulatory Mechanisms of a Memory Loss-Preventive Effect of Alpha-Lipoic Acid in an Experimental Rat Model of Dementia. J. Mol. Neurosci. 2022;72:1018–1025. doi: 10.1007/s12031-022-01979-y. [DOI] [PubMed] [Google Scholar]
- 79.Koriyama Y., Nakayama Y., Matsugo S., Kato S. Protective Effect of Lipoic Acid against Oxidative Stress Is Mediated by Keap1/Nrf2-Dependent Heme Oxygenase-1 Induction in the RGC-5 Cellline. Brain Res. 2013;1499:145–157. doi: 10.1016/j.brainres.2012.12.041. [DOI] [PubMed] [Google Scholar]
- 80.Saleh T.M., Saleh M.C., Connell B.J., Song Y.-H. A Co-Drug Conjugate of Naringenin and Lipoic Acid Mediates Neuroprotection in a Rat Model of Oxidative Stress. Clin. Exp. Pharmacol. Physiol. 2017;44:1008–1016. doi: 10.1111/1440-1681.12799. [DOI] [PubMed] [Google Scholar]
- 81.Vander Zanden C.M., Wampler L., Bowers I., Watkins E.B., Majewski J., Chi E.Y. Fibrillar and Nonfibrillar Amyloid Beta Structures Drive Two Modes of Membrane-Mediated Toxicity. Langmuir. 2019;35:16024–16036. doi: 10.1021/acs.langmuir.9b02484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kawai R., Chiba S., Okuwaki K., Kanada R., Doi H., Ono M., Mochizuki Y., Okuno Y. Stabilization Mechanism for a Nonfibrillar Amyloid β Oligomer Based on Formation of a Hydrophobic Core Determined by Dissipative Particle Dynamics. ACS Chem. Neurosci. 2020;11:385–394. doi: 10.1021/acschemneuro.9b00602. [DOI] [PubMed] [Google Scholar]
- 83.Kandel N., Matos J.O., Tatulian S.A. Structure of Amyloid Β25–35 in Lipid Environment and Cholesterol-Dependent Membrane Pore Formation. Sci. Rep. 2019;9:2689. doi: 10.1038/s41598-019-38749-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Naldi M., Fiori J., Pistolozzi M., Drake A.F., Bertucci C., Wu R., Mlynarczyk K., Filipek S., De Simone A., Andrisano V. Amyloid β-Peptide 25–35 Self-Assembly and Its Inhibition: A Model Undecapeptide System to Gain Atomistic and Secondary Structure Details of the Alzheimer’s Disease Process and Treatment. ACS Chem. Neurosci. 2012;3:952–962. doi: 10.1021/cn3000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Clementi M.E., Marini S., Coletta M., Orsini F., Giardina B., Misiti F. Aβ(31–35) and Aβ(25–35) Fragments of Amyloid Beta-Protein Induce Cellular Death through Apoptotic Signals: Role of the Redox State of Methionine-35. FEBS Lett. 2005;579:2913–2918. doi: 10.1016/j.febslet.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 86.Ali M.Y., Jannat S., Edraki N., Das S., Chang W.K., Kim H.C., Park S.K., Chang M.S. Flavanone Glycosides Inhibit β-Site Amyloid Precursor Protein Cleaving Enzyme 1 and Cholinesterase and Reduce Aβ Aggregation in the Amyloidogenic Pathway. Chem. Biol. Interact. 2019;309:108707. doi: 10.1016/j.cbi.2019.06.020. [DOI] [PubMed] [Google Scholar]
- 87.Sarkar B., Dhiman M., Mittal S., Mantha A.K. Curcumin Revitalizes Amyloid Beta (25–35)-Induced and Organophosphate Pesticides Pestered Neurotoxicity in SH-SY5Y and IMR-32 Cells via Activation of APE1 and Nrf2. Metab. Brain Dis. 2017;32:2045–2061. doi: 10.1007/s11011-017-0093-2. [DOI] [PubMed] [Google Scholar]
- 88.Zhi Z., Tang X., Wang Y., Chen R., Ji H. Sinensetin Attenuates Amyloid Beta25-35-Induced Oxidative Stress, Inflammation, and Apoptosis in SH-SY5Y Cells Through the TLR4/NF-ΚB Signaling Pathway. Neurochem. Res. 2021;46:3012–3024. doi: 10.1007/s11064-021-03406-x. [DOI] [PubMed] [Google Scholar]
- 89.Liu X.-Y., Zhang L.-J., Chen Z., Liu L.-B. The PTEN Inhibitor BpV(Pic) Promotes Neuroprotection against Amyloid β-Peptide (25–35)-Induced Oxidative Stress and Neurotoxicity. Neurol. Res. 2017;39:758–765. doi: 10.1080/01616412.2017.1317916. [DOI] [PubMed] [Google Scholar]
- 90.Zheng X., Xie Z., Zhu Z., Liu Z., Wang Y., Wei L., Yang H., Yang H., Liu Y., Bi J. Methyllycaconitine Alleviates Amyloid-β Peptides-Induced Cytotoxicity in SH-SY5Y Cells. PLoS ONE. 2014;9:e111536. doi: 10.1371/journal.pone.0111536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gao L., Li X., Meng S., Ma T., Wan L., Xu S. Chlorogenic Acid Alleviates Aβ25-35-Induced Autophagy and Cognitive Impairment via the MTOR/TFEB Signaling Pathway. Drug Des. Devel. Ther. 2020;14:1705–1716. doi: 10.2147/DDDT.S235969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Adewusi E.A., Fouche G., Steenkamp V. Effect of Four Medicinal Plants on Amyloid-β Induced Neurotoxicity in SH-SY5Y Cells. Afr. J. Tradit. Complement. Altern. Med. 2013;10:6–11. [PMC free article] [PubMed] [Google Scholar]
- 93.How Is Alzheimer’s Disease Treated? [(accessed on 18 October 2022)]; Available online: https://www.nia.nih.gov/health/how-alzheimers-disease-treated.
- 94.Amenta F., Parnetti L., Gallai V., Wallin A. Treatment of Cognitive Dysfunction Associated with Alzheimer’s Disease with Cholinergic Precursors. Ineffective Treatments or Inappropriate Approaches? Mech. Ageing Dev. 2001;122:2025–2040. doi: 10.1016/S0047-6374(01)00310-4. [DOI] [PubMed] [Google Scholar]
- 95.Salomone S., Caraci F., Leggio G.M., Fedotova J., Drago F. New Pharmacological Strategies for Treatment of Alzheimer’s Disease: Focus on Disease Modifying Drugs. Br. J. Clin. Pharmacol. 2012;73:504–517. doi: 10.1111/j.1365-2125.2011.04134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pithadia A.S., Lim M.H. Metal-Associated Amyloid-β Species in Alzheimer’s Disease. Curr. Opin. Chem. Biol. 2012;16:67–73. doi: 10.1016/j.cbpa.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 97.Greenough M.A., Camakaris J., Bush A.I. Metal Dyshomeostasis and Oxidative Stress in Alzheimer’s Disease. Neurochem. Int. 2013;62:540–555. doi: 10.1016/j.neuint.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 98.Das N., Raymick J., Sarkar S. Role of Metals in Alzheimer’s Disease. Metab. Brain Dis. 2021;36:1627–1639. doi: 10.1007/s11011-021-00765-w. [DOI] [PubMed] [Google Scholar]
- 99.Sadžak A., Mravljak J., Maltar-Strmečki N., Arsov Z., Baranović G., Erceg I., Kriechbaum M., Strasser V., Přibyl J., Šegota S. The Structural Integrity of the Model Lipid Membrane during Induced Lipid Peroxidation: The Role of Flavonols in the Inhibition of Lipid Peroxidation. Antioxidants. 2020;9:430. doi: 10.3390/antiox9050430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jeynes B., Provias J. Evidence for Altered LRP/RAGE Expression in Alzheimer Lesion Pathogenesis. Curr. Alzheimer Res. 2008;5:432–437. doi: 10.2174/156720508785908937. [DOI] [PubMed] [Google Scholar]
- 101.Reiss A.B., Arain H.A., Stecker M.M., Siegart N.M., Kasselman L.J. Amyloid Toxicity in Alzheimer’s Disease. Rev. Neurosci. 2018;29:613–627. doi: 10.1515/revneuro-2017-0063. [DOI] [PubMed] [Google Scholar]
- 102.Marambaud P., Dreses-Werringloer U., Vingtdeux V. Calcium Signaling in Neurodegeneration. Mol. Neurodegener. 2009;4:20. doi: 10.1186/1750-1326-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fernandez-Perez E.J., Peters C., Aguayo L.G. Membrane Damage Induced by Amyloid Beta and a Potential Link with Neuroinflammation. Curr. Pharm. Des. 2016;22:1295–1304. doi: 10.2174/138161282210160304111702. [DOI] [PubMed] [Google Scholar]
- 104.Kommaddi R.P., Das D., Karunakaran S., Nanguneri S., Bapat D., Ray A., Shaw E., Bennett D.A., Nair D., Ravindranath V. Aβ Mediates F-Actin Disassembly in Dendritic Spines Leading to Cognitive Deficits in Alzheimer’s Disease. J. Neurosci. 2018;38:1085–1099. doi: 10.1523/JNEUROSCI.2127-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Morishima Y., Gotoh Y., Zieg J., Barrett T., Takano H., Flavell R., Davis R.J., Shirasaki Y., Greenberg M.E. β-Amyloid Induces Neuronal Apoptosis Via a Mechanism That Involves the c-Jun N-Terminal Kinase Pathway and the Induction of Fas Ligand. J. Neurosci. 2001;21:7551–7560. doi: 10.1523/JNEUROSCI.21-19-07551.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Takada E., Okubo K., Yano Y., Iida K., Someda M., Hirasawa A., Yonehara S., Matsuzaki K. Molecular Mechanism of Apoptosis by Amyloid β-Protein Fibrils Formed on Neuronal Cells. ACS Chem. Neurosci. 2020;11:796–805. doi: 10.1021/acschemneuro.0c00011. [DOI] [PubMed] [Google Scholar]
- 107.Yao M., Nguyen T.-V.V., Pike C.J. β-Amyloid-Induced Neuronal Apoptosis Involves c-Jun N-Terminal Kinase-Dependent Downregulation of Bcl-w. J. Neurosci. 2005;25:1149–1158. doi: 10.1523/JNEUROSCI.4736-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen G., Xu T., Yan Y., Zhou Y., Jiang Y., Melcher K., Xu H.E. Amyloid Beta: Structure, Biology and Structure-Based Therapeutic Development. Acta Pharmacol. Sin. 2017;38:1205–1235. doi: 10.1038/aps.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tare M., Modi R.M., Nainaparampil J.J., Puli O.R., Bedi S., Fernandez-Funez P., Kango-Singh M., Singh A. Activation of JNK Signaling Mediates Amyloid-ß-Dependent Cell Death. PLoS ONE. 2011;6:e24361. doi: 10.1371/journal.pone.0024361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Marín N., Romero B., Bosch-Morell F., Llansola M., Felipo V., Romá J., Romero F.J. β-Amyloid-Induced Activation of Caspase-3 in Primary Cultures of Rat Neurons. Mech. Ageing Dev. 2000;119:63–67. doi: 10.1016/S0047-6374(00)00172-X. [DOI] [PubMed] [Google Scholar]
- 111.Ghasemi R., Zarifkar A., Rastegar K., Maghsoudi N., Moosavi M. Repeated Intra-Hippocampal Injection of Beta-Amyloid 25–35 Induces a Reproducible Impairment of Learning and Memory: Considering Caspase-3 and MAPKs Activity. Eur. J. Pharmacol. 2014;726:33–40. doi: 10.1016/j.ejphar.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 112.Zhang G., Yao L., Du Y., Zhang R., Bu N., Liu J., Yuan H., Wu H. [Expression of p38MAPK in the hippocampal CA1 region of rats with Abeta25-35-induced Alzheimer disease] Nan Fang Yi Ke Da Xue Xue Bao. 2008;28:1176–1179. [PubMed] [Google Scholar]
- 113.Jin Y., Wang H. Naringenin Inhibit the Hydrogen Peroxide-Induced SH-SY5Y Cells Injury Through Nrf2/HO-1 Pathway. Neurotox Res. 2019;36:796–805. doi: 10.1007/s12640-019-00046-6. [DOI] [PubMed] [Google Scholar]
- 114.Zeng H., Chen Q., Zhao B. Genistein Ameliorates Beta-Amyloid Peptide (25–35)-Induced Hippocampal Neuronal Apoptosis. Free Radic. Biol. Med. 2004;36:180–188. doi: 10.1016/j.freeradbiomed.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 115.Lee S.Y., Lee J.W., Lee H., Yoo H.S., Yun Y.P., Oh K.W., Ha T.Y., Hong J.T. Inhibitory Effect of Green Tea Extract on β-Amyloid-Induced PC12 Cell Death by Inhibition of the Activation of NF-ΚB and ERK/P38 MAP Kinase Pathway through Antioxidant Mechanisms. Mol. Brain Res. 2005;140:45–54. doi: 10.1016/j.molbrainres.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 116.Martorana F., Foti M., Virtuoso A., Gaglio D., Aprea F., Latronico T., Rossano R., Riccio P., Papa M., Alberghina L., et al. Differential Modulation of NF-κB in Neurons and Astrocytes Underlies Neuroprotection and Antigliosis Activity of Natural Antioxidant Molecules. Oxidative Med. Cell Longev. 2019;2019:e8056904. doi: 10.1155/2019/8056904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang K., Chen Z., Huang L., Meng B., Zhou X., Wen X., Ren D. Naringenin Reduces Oxidative Stress and Improves Mitochondrial Dysfunction via Activation of the Nrf2/ARE Signaling Pathway in Neurons. Int. J. Mol. Med. 2017;40:1582–1590. doi: 10.3892/ijmm.2017.3134. [DOI] [PubMed] [Google Scholar]
- 118.Lou H., Jing X., Wei X., Shi H., Ren D., Zhang X. Naringenin Protects against 6-OHDA-Induced Neurotoxicity via Activation of the Nrf2/ARE Signaling Pathway. Neuropharmacology. 2014;79:380–388. doi: 10.1016/j.neuropharm.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 119.de Oliveira M.R., Brasil F.B., Andrade C.M.B. Naringenin Attenuates H2O2-Induced Mitochondrial Dysfunction by an Nrf2-Dependent Mechanism in SH-SY5Y Cells. Neurochem. Res. 2017;42:3341–3350. doi: 10.1007/s11064-017-2376-8. [DOI] [PubMed] [Google Scholar]
- 120.de Oliveira M.R., Andrade C.M.B., Fürstenau C.R. Naringenin Exerts Anti-Inflammatory Effects in Paraquat-Treated SH-SY5Y Cells Through a Mechanism Associated with the Nrf2/HO-1 Axis. Neurochem. Res. 2018;43:894–903. doi: 10.1007/s11064-018-2495-x. [DOI] [PubMed] [Google Scholar]
- 121.Wang K., Chen Z., Huang J., Huang L., Luo N., Liang X., Liang M., Xie W. Naringenin Prevents Ischaemic Stroke Damage via Anti-Apoptotic and Anti-Oxidant Effects. Clin. Exp. Pharmacol. Physiol. 2017;44:862–871. doi: 10.1111/1440-1681.12775. [DOI] [PubMed] [Google Scholar]
- 122.Zhang Y., Liu B., Chen X., Zhang N., Li G., Zhang L.-H., Tan L.-Y. Naringenin Ameliorates Behavioral Dysfunction and Neurological Deficits in a D-Galactose-Induced Aging Mouse Model Through Activation of PI3K/Akt/Nrf2 Pathway. Rejuvenation Res. 2017;20:462–472. doi: 10.1089/rej.2017.1960. [DOI] [PubMed] [Google Scholar]
- 123.Petersen Shay K., Moreau R.F., Smith E.J., Hagen T.M. Is α-Lipoic Acid a Scavenger of Reactive Oxygen Species in Vivo? Evidence for Its Initiation of Stress Signaling Pathways That Promote Endogenous Antioxidant Capacity. IUBMB Life. 2008;60:362–367. doi: 10.1002/iub.40. [DOI] [PubMed] [Google Scholar]
- 124.Lee J., Jung S.-Y., Yang K.-J., Kim Y., Lee D., Lee M.H., Kim D.-K. α-Lipoic Acid Prevents against Cisplatin Cytotoxicity via Activation of the NRF2/HO-1 Antioxidant Pathway. PLoS ONE. 2019;14:e0226769. doi: 10.1371/journal.pone.0226769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu L., Yang S., Wang H. α-Lipoic Acid Alleviates Ferroptosis in the MPP+-Induced PC12 Cells via Activating the PI3K/Akt/Nrf2 Pathway. Cell Biol. Int. 2021;45:422–431. doi: 10.1002/cbin.11505. [DOI] [PubMed] [Google Scholar]
- 126.Xia D., Zhai X., Wang H., Chen Z., Fu C., Zhu M. Alpha Lipoic Acid Inhibits Oxidative Stress-induced Apoptosis by Modulating of Nrf2 Signalling Pathway after Traumatic Brain Injury. J. Cell. Mol. Med. 2019;23:4088–4096. doi: 10.1111/jcmm.14296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sun H., Guo X., Wang Z., Wang P., Zhang Z., Dong J., Zhuang R., Zhou Y., Ma G., Cai W. Alphalipoic Acid Prevents Oxidative Stress and Peripheral Neuropathy in Nab-Paclitaxel-Treated Rats through the Nrf2 Signalling Pathway. Oxidative Med. Cell Longev. 2019;2019:3142732. doi: 10.1155/2019/3142732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang J., Zhou X., Wu W., Wang J., Xie H., Wu Z. Regeneration of Glutathione by α-Lipoic Acid via Nrf2/ARE Signaling Pathway Alleviates Cadmium-Induced HepG2 Cell Toxicity. Environ. Toxicol. Pharmacol. 2017;51:30–37. doi: 10.1016/j.etap.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 129.Zgorzynska E., Dziedzic B., Walczewska A. An Overview of the Nrf2/ARE Pathway and Its Role in Neurodegenerative Diseases. Int. J. Mol. Sci. 2021;22:9592. doi: 10.3390/ijms22179592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ma Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang H., Davies K.J.A., Forman H.J. Oxidative Stress Response and Nrf2 Signaling in Aging. Free Radic. Biol. Med. 2015;88:314–336. doi: 10.1016/j.freeradbiomed.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Huang Y., Li W., Su Z., Kong A.-N.T. The Complexity of the Nrf2 Pathway: Beyond the Antioxidant Response. J. Nutr. Biochem. 2015;26:1401–1413. doi: 10.1016/j.jnutbio.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang H., Ma J., Tan Y., Wang Z., Sheng C., Chen S., Ding J. Amyloid-β 1–42 Induces Reactive Oxygen Species-Mediated Autophagic Cell Death in U87 and SH-SY5Y Cells. J. Alzheimer’s Dis. 2010;21:597–610. doi: 10.3233/JAD-2010-091207. [DOI] [PubMed] [Google Scholar]
- 134.Qu M., Zhou Z., Xu S., Chen C., Yu Z., Wang D. Mortalin Overexpression Attenuates Beta-Amyloid-Induced Neurotoxicity in SH-SY5Y Cells. Brain Res. 2011;1368:336–345. doi: 10.1016/j.brainres.2010.10.068. [DOI] [PubMed] [Google Scholar]
- 135.Shi C., Zhao L., Zhu B., Li Q., Yew D.T., Yao Z., Xu J. Protective Effects of Ginkgo Biloba Extract (EGb761) and Its Constituents Quercetin and Ginkgolide B against β-Amyloid Peptide-Induced Toxicity in SH-SY5Y Cells. Chem.-Biol. Interact. 2009;181:115–123. doi: 10.1016/j.cbi.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 136.Zhang L., Yu H., Zhao X., Lin X., Tan C., Cao G., Wang Z. Neuroprotective Effects of Salidroside against Beta-Amyloid-Induced Oxidative Stress in SH-SY5Y Human Neuroblastoma Cells. Neurochem. Int. 2010;57:547–555. doi: 10.1016/j.neuint.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 137.Sheehan J.P., Swerdlow R.H., Miller S.W., Davis R.E., Parks J.K., Parker W.D., Tuttle J.B. Calcium Homeostasis and Reactive Oxygen Species Production in Cells Transformed by Mitochondria from Individuals with Sporadic Alzheimer’s Disease. J. Neurosci. 1997;17:4612–4622. doi: 10.1523/JNEUROSCI.17-12-04612.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen W.-T., Kuo Y.-Y., Lin G.-B., Lu C.-H., Hsu H.-P., Sun Y.-K., Chao C.-Y. Thermal Cycling Protects SH-SY5Y Cells against Hydrogen Peroxide and β-Amyloid-Induced Cell Injury through Stress Response Mechanisms Involving Akt Pathway. PLoS ONE. 2020;15:e0240022. doi: 10.1371/journal.pone.0240022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wiatrak B., Jawień P., Matuszewska A., Szeląg A., Kubis-Kubiak A. Effect of Amyloid-β on the Redox System Activity in SH-SY5Y Cells Preincubated with Lipopolysaccharide or Co-Cultured with Microglia Cells. Biomed. Pharmacother. 2022;149:112880. doi: 10.1016/j.biopha.2022.112880. [DOI] [PubMed] [Google Scholar]
- 140.Wang H., Xu Y., Yan J., Zhao X., Sun X., Zhang Y., Guo J., Zhu C. Acteoside Protects Human Neuroblastoma SH-SY5Y Cells against β-Amyloid-Induced Cell Injury. Brain Res. 2009;1283:139–147. doi: 10.1016/j.brainres.2009.05.101. [DOI] [PubMed] [Google Scholar]
- 141.Mani S., Sekar S., Chidambaram S.B., Sevanan M. Naringenin Protects against 1-Methyl-4-Phenylpyridinium- Induced Neuroinflammation and Resulting Reactive Oxygen Species Production in SH-SY5Y Cell Line: An in Vitro Model of Parkinson’s Disease. Pharmacogn. Mag. 2018;14:458. doi: 10.4103/pm.pm_23_18. [DOI] [Google Scholar]
- 142.Cores Á., Michalska P., Pérez J.M., Crisman E., Gómez C., Villacampa M., Menéndez J.C., León R. Enantioselective Synthesis and Pharmacological Evaluation of Aza-CGP37157–Lipoic Acid Hybrids for the Treatment of Alzheimer’s Disease. Antioxidants. 2022;11:112. doi: 10.3390/antiox11010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Korecka J.A., van Kesteren R.E., Blaas E., Spitzer S.O., Kamstra J.H., Smit A.B., Swaab D.F., Verhaagen J., Bossers K. Phenotypic Characterization of Retinoic Acid Differentiated SH-SY5Y Cells by Transcriptional Profiling. PLoS ONE. 2013;8:e63862. doi: 10.1371/journal.pone.0063862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Encinas M., Iglesias M., Liu Y., Wang H., Muhaisen A., Ceña V., Gallego C., Comella J.X. Sequential Treatment of SH-SY5Y Cells with Retinoic Acid and Brain-Derived Neurotrophic Factor Gives Rise to Fully Differentiated, Neurotrophic Factor-Dependent, Human Neuron-like Cells. J. Neurochem. 2000;75:991–1003. doi: 10.1046/j.1471-4159.2000.0750991.x. [DOI] [PubMed] [Google Scholar]
- 145.Sasaguri H., Nilsson P., Hashimoto S., Nagata K., Saito T., De Strooper B., Hardy J., Vassar R., Winblad B., Saido T.C. APP Mouse Models for Alzheimer’s Disease Preclinical Studies. EMBO J. 2017;36:2473–2487. doi: 10.15252/embj.201797397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Haider S., Liaquat L., Ahmad S., Batool Z., Siddiqui R.A., Tabassum S., Shahzad S., Rafiq S., Naz N. Naringenin Protects AlCl3/D-Galactose Induced Neurotoxicity in Rat Model of AD via Attenuation of Acetylcholinesterase Levels and Inhibition of Oxidative Stress. PLoS ONE. 2020;15:e0227631. doi: 10.1371/journal.pone.0227631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Khan M.B., Khan M.M., Khan A., Ahmed M.E., Ishrat T., Tabassum R., Vaibhav K., Ahmad A., Islam F. Naringenin Ameliorates Alzheimer’s Disease (AD)-Type Neurodegeneration with Cognitive Impairment (AD-TNDCI) Caused by the Intracerebroventricular-Streptozotocin in Rat Model. Neurochem. Int. 2012;61:1081–1093. doi: 10.1016/j.neuint.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 148.Farr S.A., Price T.O., Banks W.A., Ercal N., Morley J.E. Effect of Alpha-Lipoic Acid on Memory, Oxidation, and Lifespan in SAMP8 Mice. J. Alzheimers Dis. 2012;32:447–455. doi: 10.3233/JAD-2012-120130. [DOI] [PubMed] [Google Scholar]
- 149.Sharman M.J., Gyengesi E., Liang H., Chatterjee P., Karl T., Li Q.-X., Wenk M.R., Halliwell B., Martins R.N., Münch G. Assessment of Diets Containing Curcumin, Epigallocatechin-3-Gallate, Docosahexaenoic Acid and α-Lipoic Acid on Amyloid Load and Inflammation in a Male Transgenic Mouse Model of Alzheimer’s Disease: Are Combinations More Effective? Neurobiol. Dis. 2019;124:505–519. doi: 10.1016/j.nbd.2018.11.026. [DOI] [PubMed] [Google Scholar]
- 150.Garcia N., Santafé M.M., Tomàs M., Lanuza M.A., Tomàs J. Short-Term Effects of β-Amyloid25-35 Peptide Aggregates on Transmitter Release in Neuromuscular Synapses. J. Neuropathol. Exp. Neurol. 2008;67:250–259. doi: 10.1097/NEN.0b013e318165e300. [DOI] [PubMed] [Google Scholar]
- 151.Ng N.S., Ooi L. A Simple Microplate Assay for Reactive Oxygen Species Generation and Rapid Cellular Protein Normalization. Bio Protoc. 2021;11:e3877. doi: 10.21769/BioProtoc.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.