Abstract
Despite the rapid utilization of immunotherapy, emerging challenges to the current immune checkpoint blockade need to be resolved. Here, we report that elevation of CD73 levels due to its aberrant turnover is correlated with poor prognosis in immune-cold triple-negative breast cancers (TNBCs). We have identified TRIM21 as an E3 ligase that governs CD73 destruction. Disruption of TRIM21 stabilizes CD73 that in turn enhances CD73-catalyzed production of adenosine, resulting in the suppression of CD8+ T cell function. Replacement of lysine 133, 208, 262, and 321 residues by arginine on CD73 attenuated CD73 ubiquitylation and degradation. Diminishing of CD73 ubiquitylation remarkably promotes tumor growth and impedes antitumor immunity. In addition, a TRIM21high/CD73low signature in a subgroup of human breast malignancies was associated with a favorable immune profile. Collectively, our findings uncover a mechanism that governs CD73 proteolysis and point to a new therapeutic strategy by modulating CD73 ubiquitylation.
Regulation of CD73 by TRIM21 orchestrates tumor immune response and tumor growth.
INTRODUCTION
Despite the widespread use of immunotherapy, limitations for current immune checkpoint blockade have drawn substantial attention to identifying new strategies (1, 2). Triple-negative breast cancer (TNBC) is the most aggressive mammary carcinoma subtype lacking few suitable targeted therapies other than sacituzumab govitecan (3, 4). Although a Programmed death-ligand 1 (PD-L1) inhibitor has been applied to treat metastatic PD-L1+ TNBC, more than 80% of patients with TNBC show limited responses due to unclear reasons (5–7). Breast cancer’s “immune-cold” property caused by scarcity of T lymphocyte infiltration and expression of different immune checkpoint proteins has been proposed to be responsible for the decreased anticancer immunity (8). Our endeavor to identify targets for improving anticancer immune responses against TNBC therapy has attracted our attention to CD73.
CD73 is a multifunctional ectoenzyme affecting both tumor cells and immune cells (9–11). Abnormal accumulation of CD73 in tumors has been connected to poor prognosis in various cancers, especially in TNBC (12–14). The essential role of CD73 is to produce extracellular adenosine from adenosine monophosphate (AMP) through functional coordination with CD39 (15, 16). The binding of adenosine to adenosine receptors on the surface of various immune cells initiates a cascading signal that regulates physiological functions for multiple types of immune cells such as regulatory (Foxp3+) T cell, effector T cell, natural killer (NK) cell, myeloid-derived suppressor cell (MDSC), and macrophages and B cell (9). Although mice with CD73 genetic ablation are viable, its conditional knockout leads to the enhanced antitumor effect of adoptive T helper 17 (TH17) cell therapy (17). CD73 expression is tightly modulated in response to hypoxic signaling during malignant tumor progression, where its transcription is principally regulated by signal transducer and activator of transcription 3 (STAT3)/hypoxia-inducible factor–1α (HIF-1α) cascade (18–20) (19). Several cytokines, including transforming growth factor–β (18), interferons (IFNs) (21), tumor necrosis factor (22), interleukin-1β (23), and prostaglandin E2 (24), induce CD73 expression. Furthermore, CD73 expression on tumor cells is altered by Wnt and cyclic AMP (cAMP) signaling (25, 26). While tremendous efforts have been focused on CD73 transcriptional regulation, the pathologically observed accumulation of CD73 protein levels in the malignant tumor is involved in posttranslational modifications (PTMs), and whether PTMs are crucial in modulating tumor immunity remains largely unknown.
Tripartite motif (TRIM) proteins belong to RING-type E3 ubiquitin ligase family and regulate a series of physiological processes, including apoptosis, innate immune responses, autophagy, and tumorigenesis (27–29). TRIMs comprise conserved molecular motifs, including an N-terminal RING domain with E3 ubiquitin ligase activity, one or two B-box domains, a coiled-coil region, and a C-terminal substrate-binding domain (PRY/SPRY) (30). TRIM21 (also known as Ro52 or SSA) was characterized as an antibody-binding protein involved in autoimmune diseases, systemic lupus erythematosus, and Sjögren’s syndrome (31). TRIM21 could bind to internalized antibody-coated viruses, resulting in its destruction (27). Previous works demonstrated that TRIM21 could interact with BECLIN1, B-cell lymphoma 2 (BCL2), and p62 during autophagy and cellular proliferation (27, 32). In addition to its role in inhibiting viral DNA synthesis and steering cellular differentiation (33–35), TRIM21 can negatively regulate innate immune signaling by promoting ubiquitylation of nuclear IFN regulatory factors (36, 37). Knockout of TRIM21 in mice led to severe TH17-mediated contact hypersensitivity responses and enhanced type I IFN responses against DNA viruses (35). Evidence from several lines showed that TRIM21 plays a critical role in suppressing tumorigenesis and tumor stemness by regulating various cell cycle factors and pluripotency factors (38, 39). In addition, recent works demonstrated the involvement of TRIM21 in regulating epithelial-mesenchymal transition in breast cancer and modulating chemo-drug response (40–43). However, both preclinical and clinical relevance of deregulation of TRIM21 that reshapes tumor immunity remains unclear.
In the current study, we unravel a regulatory mechanism by which CD73 is governed by the TRIM21-mediated ubiquitin-proteasomal pathway. Dysregulated CD73 ubiquitylation due to the impaired TRIM21 function remarkably inhibited tumor immunogenicity and promoted tumor progression. Our findings point to a pivotal role of the TRIM21-CD73-adenosine axis in modulating the interaction between tumor and immunity and imply a new therapeutic strategy by manipulating CD73 ubiquitylation.
RESULTS
Elevated expression of CD73 correlates with a poor prognosis of breast cancer
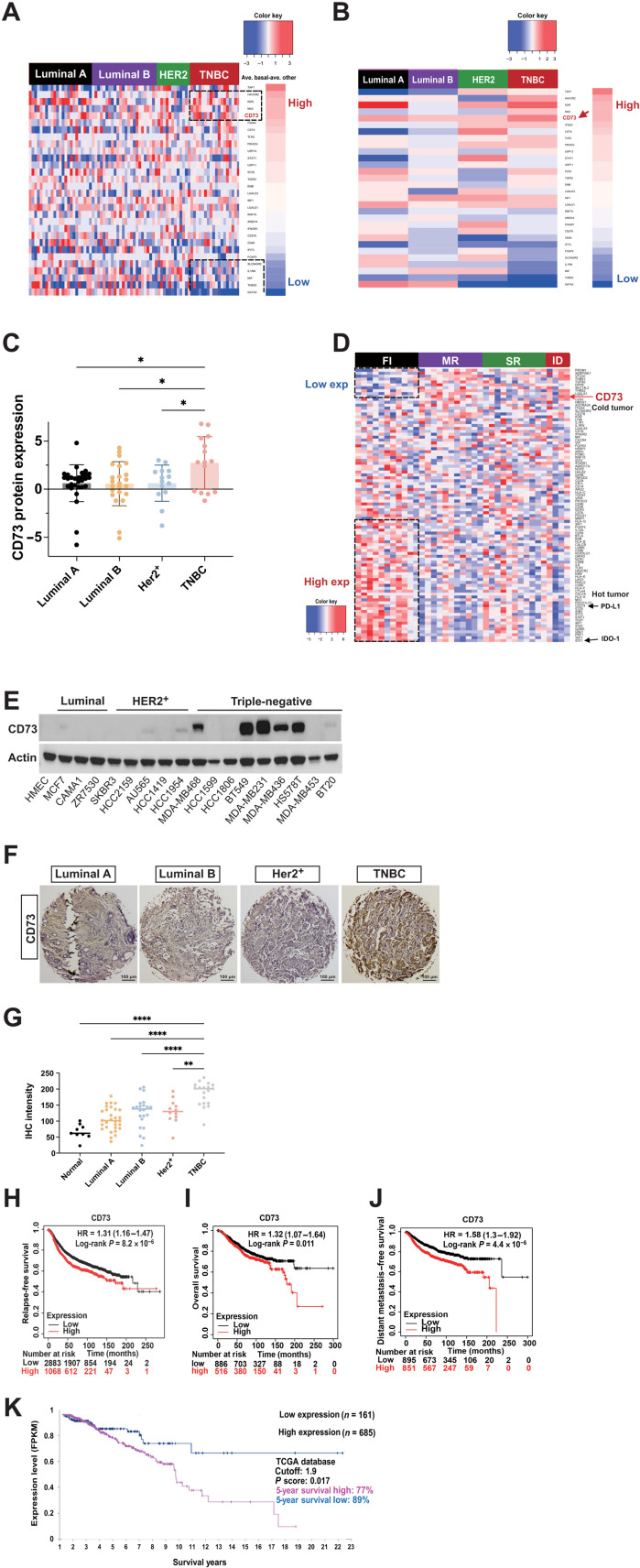
The dysregulated expression of CD73 has been connected to increased tumor progression and metastasis and impaired tumor immunogenicity (19). Genetic ablation of CD73 in mice led to tumor overgrowth and tumor immune evasion (9). Nevertheless, the molecular detail by which deregulation of CD73 contributes to the malignant tumor expansion and immune evasion remains elusive. To determine the impact of CD73 in breast oncogenesis, we have conducted a bioinformatic analysis of proteomic data comprising approximately 100 immune-relevant proteins from 81 patients with breast cancer in The Cancer Genome Atlas (TCGA), including various subtypes of breast cancers such as luminal A, luminal B, Her2+, and TNBC type (44). As shown in Fig. 1 (A to C), compared to the relatively low expression in luminal A breast cancers and moderate expression in luminal B and Her2+ breast cancer types, CD73 protein expression significantly increased in TNBC, which is consistent with the reduced responsiveness to immunotherapy in TNBC observed previously (45). In addition, 38 of TNBC patient specimens from the Gene Expression Omnibus (GEO) database (accession number: GSE88847) were stratified into fully inflamed (FI), margin-restricted (MR), stroma-restricted (SR), and immune-desert (ID) categories. The mRNAs coding for the immune-relevant proteins were quantified, and up-regulation of CD73 mRNA level was associated with the immune-cold phenotype (Fig. 1D), which is in contrast to the high expression of CD274 (best known as PD-L1) and Indoleamine-pyrrole 2,3-dioxygenase 1 (IDO-1) in “hot” tumors (46).
Fig. 1. Abnormal expression of CD73 correlates with a poor prognosis of breast cancer.
(A and B) Proteomic analysis of 81 immune-related proteins in TCGA samples of PAM50-defined intrinsic and hormone receptor subtypes, including 15 triple-negative, 29 luminal A, 23 luminal B, and 14 Her2+ breast tumors, along with 3 normal breast tissue samples. The genes (rows) are sorted by case individually according to the protein expression difference between the average proteomic level in each subtype and the average proteomic level in the other cancer types (A); the mean proteomic differences of each immune-related protein of four breast subtypes were calculated and plotted in (B). (C) Bar graph representing the average CD73 protein expression levels in four subtypes of breast cancer. (D) mRNA analysis of immune-relevant proteins in 38 TNBC patient specimens from the GEO database. The genes (rows) are sorted according to the difference between the average mRNA levels in FI (black), SR (green), MR (purple), and ID (red) breast cancer types. (E) Expression of CD73 in normal human mammary epithelial cells and various subtypes of breast cancer cells detected by immunoblotting using anti-CD73 antibody. (F) Breast cancer specimens TMA stained with anti-CD73 antibody and representative pictures of different breast cancer subtypes are shown. (G) Quantified TMA consisting of luminal A, luminal B, Her2+, and TNBC samples immunostained for CD73. (H to J) Increased accumulation of CD73 levels correlates with poor outcomes in patients with TNBC, including RFS (H), OS (I), and DMFS (J). (K) Increased accumulation of CD73 levels [Fragments Per Kilobase of transcript per Million mapped reads (FPKM)] correlates with a lower OS ratio in patients with advanced breast cancer. **P < 0.01 and ****P < 0.0001. Data (means ± SEM) are representative of at least three independent experiments.
We next systematically measured the mRNA and protein expression levels of CD73 in a panel of 45 breast cancer cell lines (4). As shown in Fig. 1E, CD73 protein expression is relatively low in luminal and Her2+ breast cancer cell lines, where only MCF7, Au565, and HCC1954 breast cancer cells showed a limited expression level of CD73 protein. In contrast, most of the TNBC cells exhibited a significant increase in CD73 protein expression, which is consistent with the results of the TCGA analysis in Fig. 1 (A and B). Similarly, CD73 mRNA levels are also notably higher in TNBC cells compared to those of the other types of breast cancer cell lines (fig. S1A). To confirm the findings in Fig. 1 (A to C), the tissue microarray (TMA) of human breast cancer specimens consisting of 110 different breast samples was assessed using an anti-CD73 antibody, and representative immunohistochemistry (IHC) images for luminal A and B, Her2+, and TNBC tumor samples were shown (Fig. 1F). CD73 staining was significantly increased in TNBC as compared to that in other cancer types. In addition, quantification of staining intensities from different breast cancer types suggests that there may be a subset of TNBC tumors exhibiting significantly higher CD73 protein expression compared to other breast cancer types (Fig. 1G).
To further determine the prognostic value of CD73 expression in patients with TNBC, we analyzed 122 samples of TNBC from the BIG 02-98 adjuvant phase 3 clinical trial (12). The data showed that increased CD73 expression was associated with worse relapse-free survival (RFS; Fig. 1H), overall survival (OS) (Fig. 1I), and distant metastasis–free survival (DMFS) (Fig. 1J) in univariate Cox regression models. Ten-year recurrence-free survival RFS for CD73-Low TNBC (below median) was 75% versus 49% for CD73-High TNBC (above median) [hazard ratio (HR), 2.38; 95% confidence interval (CI), 1.29 to 4.42; P¼0.006], and 10-year OS was 82% for CD73-Low versus 55.7% for CD73-High (HR, 2.51; 95% CI, 1.27 to 4.96; P¼0.008). In addition, we analyzed the data of CD73 mRNA expression from TCGA and observed that up-regulated CD73 expression was associated with poor outcomes in patients with advanced breast cancer (Fig. 1K). Five-year OS was 89% for CD73-Low (below cutoff) versus 77% for CD73-High (above cutoff) (HR, 2.2; 95% CI, 1.25 to 4.75; P¼0.008). Together, analyses from several lines showed a strong correlation between abnormal accumulation of CD73 and poor prognosis of TNBC. Furthermore, luminal A, luminal B, and Her2+ breast cancer patients with high CD73 expression were also associated with worse RFS, OS, and DMFS (fig. S1, B to D).
CD73 is a fast turnover protein whose half-life is regulated by the ubiquitin-proteasome pathway
Although previous studies showed that CD73 was altered at the transcriptional level through several signaling cascades, including STAT3/HIF-1α, Wnt, and cAMP (9), whether the abundance of CD73 protein levels in cancer tissues is attributed to PTMs is unknown. To this end, we performed pulse-chase measurement to examine whether CD73 protein underwent turnover regulation and further determined its turnover half-life. As shown in Fig. 2 (A and B), CD73 protein decreased after cycloheximide treatment in a number of breast cancer cell lines, including MCF7 (luminal), HCC1954 (Her2+), MDA-MB436 (TNBC), MDA-MB468 (TNBC), HS578T (TNBC), and MDA-MB231 (TNBC). While the turnover rate in luminal and Her2+ breast cancer cells was about 2 to 3 hours, the half-life for CD73 protein turnover in TNBC breast cancer cells was much longer (over 4 hours), especially in high basal CD73 expression cells such as HS578T and MDA-MB231 breast cancer cells (Fig. 2, A and B). In HS578T and MDA-MB231 breast cancer cells, no change in CD73 protein level was observed until 8 hours after the cycloheximide treatment. This could be due to the high basal CD73 expression level and the slow protein turnover rate in these TNBC cells (Fig. 1E). These data together with the extended time of CD73 turnover in TNBC cell lines further suggest that there is, at least, a subgroup of TNBCs exhibiting high CD73 expression signature.
Fig. 2. CD73 is a fast turnover protein whose half-life is regulated by the ubiquitin-proteasome pathway.
(A) Different subtypes of breast cancer cell lines (MCF7, HCC1954, MDA-MB436, MDA-MB468, HS578T, and MDA-MB231) were treated with cycloheximide (CHX) (50 μg/ml) followed by collection at indicated time points. CD73 protein levels were determined by immunoblotting using anti-CD73 antibody. (B) CD73 protein turnover curve from (A). The density of CD73 immunoblotting bands was quantified and normalized to the internal control β-actin, and turnover curves were plotted. (C) Different subtypes of breast cancer cell lines were treated with cycloheximide or MG-132 for 8 hours, and CD73 protein levels were determined. (D) Normalized CD73 expression bar graph of (C). (E) CD73 protein was immunoprecipitated (IP) by antibody against CD73 with IgG antibody as a control in MDA-MB468 cells. Poly-ubiquitin (ub) conjugates in association with purified CD73 were determined by immunoblotting (IB) using anti-ubiquitin. (F) MDA-MB468, MDA-MB231, and HS578T breast cancer cells were infected with Lenti-Flag-CD73, and the elevated CD73 protein levels were determined by immunoblotting using anti-CD73 and anti-Flag antibodies. (G) Extracellular adenosine levels in MDA-MB468-CD73, MDA-MB231-CD73, and HS578T-CD73 overexpression stable cells. (H) Immunoblotting of CD73 in MDA-MB468-shCD73, MDA-MB231-CD73, and HS578T-CD73 breast cancer cells. (I) Extracellular adenosine levels in MDA-MB468-shCD73, MDA-MB231-shCD73, and HS578T-shCD73 cells. (J) MDA-MB468 vector control cells and MDA-MB468-CD73WT overexpression cells were cocultured with human peripheral blood mononuclear cells (PBMCs) (CD3-activated T cell), apoptosis of CD8+ T cells analyzed by a cocultured assay using tumor cells with PBMCs (CD3-activated T cell) followed by measuring annexin V using flow cytometry. Schematic diagram of a coculture system (left). *P < 0.05 and ****P < 0.0001. Data (means ± SEM) are representative of at least three independent experiments. MW, molecular weight; ATP, adenosine triphosphate.
We next tested whether CD73 protein turnover was mediated by ubiquitylation. In this regard, MDA-MB468 cells were pretreated with MG-132 and harvested for the preparation of lysates. CD73 protein was immunoprecipitated by antibody against CD73 with immunoglobulin G (IgG) control. The CD73 immune-complex was then resolved by SDS–polyacrylamide gel electrophoresis (SDS-PAGE) followed by immunoblotting using an antibody against ubiquitin (47). As shown in Fig. 2E, CD73 protein was tightly associated with ubiquitin conjugates, further confirming that CD73 is regulated by the ubiquitin-proteasome pathway.
Elevation of CD73 protein in breast cancer cells inhibits T cell accumulation
CD73 is a glycosyl-phosphatidylinositol–linked cell surface enzyme that regulates the proliferation and activity of effector T cells by generation of extracellular adenosine in the tumor (48). To test the effects of CD73 expression changes on adenosine production and T cell function (49), we engineered MDA-MB468, MDA-MB231, and HS578T cells with Flag-tagged CD73 overexpression (Fig. 2F) and determined the extracellular adenosine level using the supernatant of the culture medium (49). As shown in Fig. 2G, overexpression of CD73 in MDA-MB468, MDA-MB231, and HS578T tumor cells significantly increased adenosine production compared with their parental groups. We also generated the stable CD73 knockdown breast cancer cell lines (Fig. 2H). As expected, we found knockdown of CD73 by short hairpin RNA (shRNA) decreased extracellular adenosine production (Fig. 2I) (11).
Furthermore, we measured the effect of elevation of CD73 protein abundance on T cell proliferation using a coculture assay [coculture of breast cancer cells and CD73 antibody activated T cells as described previously (19, 48, 50)]. As shown in fig. S2 (B and C), we observed that T cell proliferation was reduced in response to CD73 overexpression in tumor cells. When MDA-MB468 and MDA-MB231 were treated with 5′-AMP, a substrate of CD73, T cell proliferation was further decreased in both the control group and the CD73 overexpression group. In addition, the apoptosis of activated CD8+ T cells using this coculture system was also determined (Fig. 2J and fig. S2A). We found that CD8+ T cells exhibited greater apoptotic activities when cocultured with CD73-overexpressed MDA-MB231 cells. The results suggest that elevated CD73 expression in breast tumors increases extracellular adenosine production and inhibits T cell activity, confirming the critical role of the CD73-mediated adenosinergic cascade in governing T cell function.
Identification of TRIM21 as a potential ubiquitin E3 ligase governing CD73 proteolysis to regulate T cell function
To identify potential upstream players that regulate CD73 protein abundance, we performed tandem affinity purification coupled with mass spectrometry analysis (47, 51, 52). We initially engineered MDA-MB468 with stable expression of Flag/HA-CD73 and then purified the CD73 complex following a standard Tandem Affinity Purification (TAP) purification protocol (47, 51, 52). Proteins interacting with Flag/HA-CD73 were precipitated with M2–(anti-Flag)–agarose beads from whole-cell extracts, and the purified CD73 complexes were subjected to mass spectrometry analysis. As shown in Fig. 3A, several proteins, including TRIM21, were identified as CD73-binding proteins.
Fig. 3. Identification of TRIM21 as a potential ubiquitin E3 ligase governing CD73 proteolysis.
(A) CD73 complex was purified with a tandem affinity purification protocol followed by mass spectrometry analysis in MDA-MB468-Flag/HA-CD73 cells. Silver staining of the purified CD73 complex was illustrated. TRIM21 was identified as a binding partner of CD73, and the representative spectra were included. (B and C) Validation of biochemical interaction between CD73 and TRIM21 in MDA-MB468 cells by coimmunoprecipitation of endogenous CD73 (B) and by coimmunoprecipitation of endogenous TRIM21 (C). (D) Validation of interaction between TRIM21 with ectopic Flag/HA-CD73 expression in MDA-MB468-Flag/HA-CD73 cells by coimmunoprecipitation of ectopic Flag/HA-CD73. (E) Cellular fractionated protein (cytosol versus membrane) expression of CD73 and TRIM21 was determined by immunoblotting. (F) TRIM21 protein levels and CD73 protein levels of MDA-MB231-TRIM21 and MDA-MB468-TRIM21 stable cells were detected by immunoblotting. (G) Membrane-bound CD73 in MDA-MB468 and MDA-MB468-TRIM21 cells were stained with anti-CD73 antibody and quantified using flow cytometry. (H) TRIM21 protein levels and CD73 protein levels of MDA-MB231-shTRIM21 and MDA-MB468-shTRIM21 cells were measured by immunoblotting. (I and J) MDA-MB468 and MDA-MB468-TRIM21 cells were treated with cycloheximide, and CD73 protein levels were determined. (I) The CD73 protein turnover curve was plotted on (J). (K) Endogenous CD73 was immunoprecipitated in MDA-MB468, MDA-MB468-shTRIM21, and MDA-MB468-TRIM21, and the ubiquitylation of CD73 was determined by immunoblotting. (L) The adenosine levels were determined in MDA-MB468-CD73WT, MDA-MB468-CD73WT-TRIM21, and MDA-MB468-CD73WT-shTRIM21 cells. (M to P) MDA-MB468, MDA-MB468-TRIM21 (M), and MDA-MB468-shTRIM21 (O) were cocultured with human PBMCs with or without 5′-AMP treatment, and CD8+ IFN-γ+ T cell population was measured and quantified using flow cytometry. Representative flow cytometric chart was shown. (N) Bar graph analysis of flow cytometry data of (M). (P) Bar graph analysis of flow cytometry data of (O). ****P < 0.0001. Data (means ± SEM) are representative of at least three independent experiments.
To validate and test whether TRIM21 binding to CD73 has a physiological function, we performed a series of immunoprecipitation assays in the MDA-MB468 cells (52). As shown in Fig. 3 (B and C), we observed endogenous CD73 coimmunoprecipitated with TRIM21 as indicated by CD73 detection on TRIM21
immune-complexes. We further confirmed the interaction between Flag/HA-CD73 and endogenous TRIM21 by coimmunoprecipitation (Fig. 3D). To determine the cellular compartmentation interaction between CD73 and TRIM21, we conducted an immunofluorescence assay (47). As shown in fig. S3A, CD73 (green) was colocalized with TRIM21 (red) primarily in the cytosol, which is consistent with the measurement demonstrated by biochemical cellular fractionation (Fig. 3E), suggesting that TRIM21-guided CD73 ubiquitylation primarily took place before its cellular membrane presentation.
Being a member of the TRIM family, TRIM21 acts as a ubiquitin E3 ligase governing substrate proteolysis (27–29). To test whether TRIM21 functions as a protein turnover modifier for CD73, we examined the effect of alteration of TRIM21 expression on CD73 protein levels. As shown in Fig. 3 (F to H), overexpression of TRIM21 reduced CD73 protein levels, whereas knockdown of TRIM21 by shRNA up-regulated CD73 expression. To examine the cellular faction of CD73 protein that is affected by TRIM21-guided ubiquitylation, CD73 expression on TRIM21-overpressed MB468 breast cancer cells was analyzed by flow cytometry using an anti-CD73 fluorescent probe. In Fig. 3G, the fluorescence intensity of membrane-bound CD73 in MDA-MB468 TRIM21 overexpression cells is significantly lower than that of the MDA-MB468 control cells. The similar decrease in CD73 protein level was also observed in the cytosol fraction (fig. S3B). These data together suggest that TRIM21-mediated ubiquitylation and degradation of cytosolic CD73 may serve as a threshold bottleneck that fine tunes CD73 protein abundance in cytosol, sequentially modulating membrane-bound CD73 protein levels. We next tested the effect of TRIM21 expression levels on CD73 turnover dynamics by pulse-chase. As shown in Fig. 3 (I and J), overexpression of TRIM21 significantly accelerated CD73 protein turnover. To examine the role of TRIM21 on CD73 degradation through catalyzing CD73 ubiquitylation, we performed a CD73 ubiquitylation assay using MDA-MB468 cells (47). Endogenous CD73 was immunoprecipitated by an anti-CD73 antibody followed by immunoblotting using an antibody against ubiquitin. As shown in Fig. 3K, CD73 underwent ubiquitylation, while knockdown of TRIM21 significantly attenuated CD73 ubiquitin conjugates. We also investigated the effect of TRIM21 overexpression and TRIM21 knockdown on the CD73 gene expression level in MDA-MB468 breast cancer cells. In fig. S3C, the NT5E mRNA (CD73 coding gene) level was unchanged as determined by quantitative polymerase chain reaction (qPCR), suggesting that the decrease in the TRIM21-mediated CD73 protein level is at the posttranslational level instead of the transcriptional level.
We next determined the impact of CD73 ubiquitylation in cancer cells by TRIM21 on T cell function in vitro (53). As shown in Fig. 3L, overexpression of TRIM21 in MDA-MB486 CD73-overexpressed cells significantly reduced adenosine production compared with control, while TRIM21 knockdown increased adenosine production. In a coculture experiment using breast cancer cells and activated T cells (Fig. 3, M to P), T cell proliferation activity and IFN-γ production were increased upon TRIM21 overexpression in tumor cells, while TRIM21 knockdown in tumor cells reduced T cell proliferation activity and IFN-γ production. We have also analyzed the inhibitory effects of 5′-AMP on IFN-γ+ CD8+ T cell population with empty vector and with TRIM21 overexpression MDA-MB468 cells. In fig. S3D, 5′-AMP–activated CD73-induced IFN-γ+ CD8+ T cells inhibition in empty vector cells is significantly lower in TRIM21 overexpression cells, suggesting that the CD73 expression level is decreased, at least partially, through up-regulation of TRIM21-mediated ubiquitylation. Together, the data indicate that TRIM21 is a ubiquitin E3 ligase governing not only CD73 protein abundance but also the subsequent adenosine production and immunosuppression.
Mapping of molecular regions that facilitate the interaction between CD73 and TRIM21
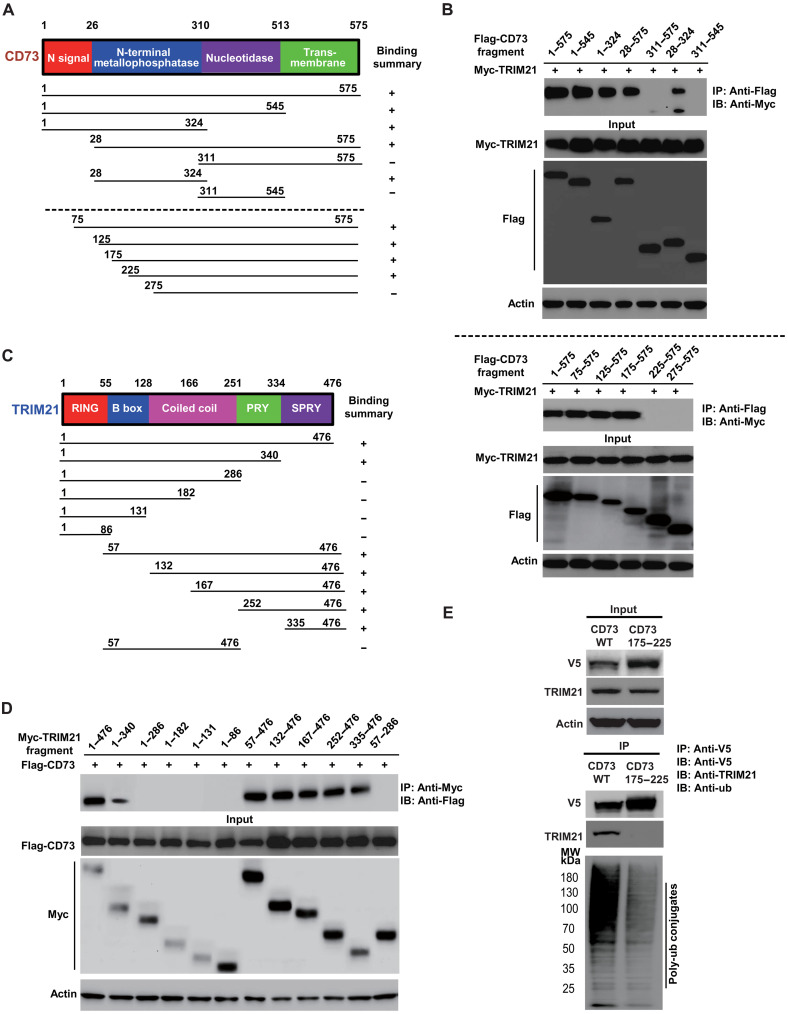
To identify the domains on TRIM21 and CD73 responsible for their interaction, we constructed a series of Flag-tagged CD73 and Myc-tagged TRIM21 truncation mutants (Fig. 4, A to D), cotransfected them into human embryonic kidney (HEK) 293T cells, and performed coimmunoprecipitation experiments. To determine molecular motifs on CD73 that mediate its interaction with TRIM21, we cotransfected a series of Flag-tagged CD73 truncation mutants with Myc-tagged TRIM21, respectively, and then conducted a pull-down assay of individual Flag-tagged CD73 mutant followed by immunoblotting with anti-Myc antibody to detect Myc-TRIM21 (54). As shown in Fig. 4B, the molecular mapping showed amino acid stretches from 176 to 224 on CD73-mediated CD73 interaction with TRIM21. A similar strategy was applied to map the molecular region on TRIM21 that facilitates interaction between TRIM21 and CD73. As shown in Fig. 4D, the amino acid residues from 340 to 476 on TRIM21 (PRY/SPRY domain) were identified as the region mediating the binding between TRIM21 and CD73.
Fig. 4. Mapping of molecular regions that facilitate the interaction between CD73 and TRIM21.
(A) Schematic diagram of human CD73 domains and strategy to engineer a series of CD73 deletion fragments. (B) The interactions between TRIM21 and CD73 fragments were examined by coimmunoprecipitation experiments in HEK-293T cells. Top: Identification of amino acid stretches from 28 to 311 on CD73 mediates the interaction between CD73 and TRIM21, measured by coimmunoprecipitation with a series of Flag-tagged CD73 fragments followed by immunoblotting of coexpressed Myc-TRIM21 antibody against Myc. Bottom: Narrowing down amino acid stretches from 175 to 225 on CD73 facilitates the interaction between CD73 and TRIM21. (C) Schematic diagram of human TRIM21 domains and strategy to engineer a series of TRIM21 deletion mutants. (D) Mapping of the molecular domain on TRIM21 involving in the interaction with CD73. The interactions between CD73 and TRIM21 fragments were examined by coimmunoprecipitation experiments in HEK-293T cells. Amino acid stretches from 340 to 476 on TRIM21 (PRY/SPRY domain) were identified as the region that mediates the interaction between TRIM21 and CD73. (E) Validation of interaction domains between TRIM21 with CD73 by coimmunoprecipitation of ectopic V5-tagged CD73 176 to 225 amino acid deletion mutant in HEK-293T cells. The ubiquitylation status of CD73 and CD73 deletion mutant were also determined using an antibody against ubiquitin.
To confirm the physiological binding between TRIM21 and CD73, we also constructed the V5-tagged CD73 176 to 224 amino acid deletion construct. As shown in Fig. 4E, we further validated that the amino acid stretches from 176 to 224 on CD73-mediated CD73 interaction with endogenous TRIM21 in 293T cells. Moreover, we observed a decreased CD73 ubiquitylation in 293T cells with overexpressed deletion constructs.
Disrupted CD73 ubiquitylation impairs CD73-catalyzed adenosine production and T cell function
Proteomic analyses indicated that the four lysine residues, including K133, K208, K262, and K321, are ubiquitylation sites on CD73. To confirm the importance of these four lysine residues on CD73 turnover control and cascading effect on adenosine production and T cell function, we engineered CD73 ubiquitylation–deficient mutant with the replacement of four lysine residues on CD73 by arginine. In MDA-MB468 cells, while ectopic CD73 expression underwent turnover with a half-life of about 4 hours, the replacement of four lysines on CD73 by arginine significantly attenuated CD73 turnover (Fig. 5, A and B) as well as ubiquitylation (Fig. 5C) after releasing from cycloheximide. Similar results were observed in MDA-MB231 cells (Fig. 5, D to F). To further show that CD734KR mutants also affect the CD8+ T cell proliferation through its enzymatic adenosine production, the extracellular adenosine levels of CD73WT tumor cells and CD734KR tumor cells were determined. In Fig. 5G, MDA-MB468-CD734KR cells exhibited significantly higher adenosine levels compared to MDA-MB468-CD73WT cells. Similar results were also obtained in MDA-MB231 breast cancer cells as indicated in fig. S4A. As shown in Fig. 5 (H and I), disruption of CD73 ubiquitylation by replacement of lysine ubiquitylation sites by arginine inhibited T cell proliferation compared with the control vector. As expected, CD73 inhibitor adenosine 5′-(α,β-methylene) diphosphate (APCP) abolished the inhibition of T cell proliferation by disruption of CD73 enzymatic activity. To further show that TRIM21-guided ubiquitylation acts through CD73 down-regulation and subsequently affects tumor immune response, MDA-MB468 were infected with Lenti-CD73WT, Lenti-CD734KR, and Lenti-TRIM21, and IFN-γ+ CD8+ T cell population was detected using flow cytometry. In Fig. 5J and fig. S4C, it is clear that MDA-MB468-CD734KR ubiquitylation-null cells have lower IFN-γ+ CD8+ T cell population than MDA-MB468-CD73WT cells. In addition, TRIM21 overexpression in MDA-MB468-CD73WT increased the IFN-γ+ CD8+ T cell population in MDA-MB468-CD73WT cells, but the same effect was not observed in MDA-MB468-CD734KR cells. We further validated that the polyfunctionality and polyfunctional strength index of activated human T cells determined by isoPlexis single-cell functional proteomics (Fig. 5, K and L) in vitro was decreased by replacement of lysine ubiquitylation sites in the MDA-MB468 cells compared with the control cells. Likewise, APCP treatment recovered the polyfunctionality and polyfunctional strength. Our results confirm the impact of CD73 ubiquitylation by TRIM21 on the regulation of CD73 protein turnover and T cell function. To further determine whether the four ubiquitylation sites on CD73 are involved in regulating CD73 protein turnover and affect tumor immune response, HEK-293T cells were transiently cotransfected with TRIM21 and different CD73 lysine mutants including CD73K133R, CD73K208R, CD73K262R, CD73K321R, CD73K133R/K208R, and CD73K262R/K321R. These cells were then treated with cycloheximide for 6 hours, and CD73 protein levels were measured by immunoblotting. Results in fig. S4D showed that only CD73K262R/K321R prevented CD73 degradation after cycloheximide treatment. We then established the CD73K133R/K208R and CD73K262R/K321R stable expression MDA-MB231 and MDA-MB468 human breast cancer cells. MDA-MB468-CD73K133R/K208R and MDA-MB468- CD73K262R/K321R were cocultured with human peripheral blood mononuclear cells (PBMCs), and T cell proliferation and function were detected. As shown in fig. S4 (E, F, and I to L), CD73K262R/K321R ubiquitylation site mutant decreased T cell proliferation and significantly altered polyfunctionality and polyfunctional strength index of activated human T cells where effector T cell population markedly diminished in MDA-MB468-CD73K262R/K321R cells but not in MDA-MB468-CD73K133R/K208R (fig. S4, I to L). Similar results were observed in MDA-MB231-CD73K133R/K208R and MDA-MB231-CD73K262R/K321R breast cancer cells (fig. S4, G and H). These results further suggest that amino sites K262 and K321 are critical in mediating CD73 ubiquitylation and its subsequent degradation. Mutating ubiquitylation sites at CD73 K262 and K321 induced T cell immune suppression through CD73-mediated adenosinergic effect.
Fig. 5. Disrupted CD73 ubiquitylation impairs CD73-catalyzed adenosine production and T cell function.
(A and B) MDA-MB468-TRIM21-CD73WT and MDA-MB468-TRIM21-CD734KR cells were generated by infecting MDA-MB468-TRIM21 cells with lenti-V5-CD73 WT and lenti-V5-CD734KR (replacement of lysine 133, 208, 262, and 321 residues with arginine). Established stable cells were treated with cycloheximide or MG-132, and CD73 protein levels were determined at different time points (A). (B) Normalized CD73 expression bar graph of (A). (C) V5-CD73 were immunoprecipitated in MDA-MB468-TRIM21-CD73WT and MDA-MB468-TRIM21-CD734KR cells, and ubiquitinated CD73 was determined. (D and E) MDA-MB231-TRIM21-CD73WT and MDA-MB231-TRIM21-CD734KR cells were treated with cycloheximide or MG-132, and CD73 protein levels were determined. (E) Normalized CD73 expression bar graph of (D). (F) V5-CD73 were immunoprecipitated in MDA-MB231-TRIM21-CD73WT and MDA-MB231-TRIM21-CD734KR cells, and ubiquitinated CD73 was determined. (G) The adenosine level in MDA-MB468-CD73WT and MDA-MB468-CD734KR cells was determined. (H) MDA-MB468-CD73WT and MDA-MB468-CD734KR cells were cocultured with human PBMCs and treated with/without 100 μM CD73 inhibitor (APCP). T cell proliferation was determined by Ki67 staining. Representative flow cytometric chart was shown. (I) Bar graph analysis of flow cytometry data of (H). (J) MDA-MB468-CD73WT, MDA-MB468-TRIM21-CD73WT, MDA-MB468-CD734KR, and MDA-MB468-TRIM21-CD734KR cells were cocultured with human PBMCs. CD8+ IFN-γ+ T cell population was measured and quantified using flow cytometry. (K and L) Human PBMCs were cocultured with MDA-MB468-CD73WT and MDA-MB468-CD734KR breast cancer cells. The polyfunctionality and polyfunctional strength index (K) indicated by relative abundance and composition of secreted cytokines (L) of human T cells at the single-cell level were measured by IsoPlexis/IsoLight assays using Isoplexis Human Adaptive Immune Chips.
***P < 0.001, ****P < 0.0001. Data (means ± SEM) are representative of at least three independent experiments. ns, not significant; TNF-α, tumor necrosis factor–α; GM-CSF, granulocyte-macrophage colony-stimulating factor; TGF-β1, transforming growth factor–β1;MIP-1α, Macrophage Inflammatory Protein-1 alpha; CCL-11, Eotaxin; IP-10, Interferon γ–Inducible Protein-10; MIP-1b, Macrophage Inflammatory Protein-1 beta; MCP-1, Monocyte Chemoattractant Protein-1.
IFN-γ secreted by T cell modulates reciprocal expression of TRIM21 and CD73 through a feedback loop
IFN-γ has been reported to modulate expression of TRIM family members including TRIM21 (55–57). In this regard, we stimulated MDA-MB468 tumor cells with IFN-γ at various time points (0, 0.5, 1, 3, 6, 9, and 24 hours) and measured the expression levels of TRIM21 as well as CD73 using immunoblotting. As shown in Fig. 6 (A and B), TRIM21 protein levels were markedly up-regulated in response to IFN-γ within half-hour upon the stimulation. In contrast, CD73 was drastically down-regulated 24 hours after the IFN-γ treatment. In a coculture system, IFN-γ released by T cells increased TRIM21 protein expression and led to a decreased CD73 protein level as compared to the negative control. In contrast, administration of IFN-γ–neutralizing antibodies, which previously were known to counteract IFN-γ activity, led to reduced TRIM21 protein levels and reversed the CD73 expression level in MDA-MB468 tumor cells (Fig. 6C), suggesting a role of IFN-γ produced from activated T cells in regulating reciprocal expression of TRIM21 and CD73. To further test the importance of TRIM21-mediated ubiquitylation, we examined the effect of the disruption of CD73 ubiquitylation sites on CD73 by comparing the IFN-γ–induced protein dynamic pattern between CD73WT and CD734KR (ubiquitylation-deficient mutant). As shown in Fig. 6 (D to G), disruption of CD73 ubiquitylation sites (K133, K208, K262, and K321) diminished the IFN-γ–mediated down-regulation of CD73 in both MDA-MB468 and MDA-MB231 cells. Moreover, the IFN-γ–mediated degradation of CD73 was blocked by MG-132 (Fig. 6, D to G). Our results suggest an IFN-γ–TRIM21–CD73 feedback regulatory loop between tumor cells and effector T cells in orchestrating tumor immunogenicity.
Fig. 6. IFN-γ secreted by T cell modulates reciprocal expression of TRIM21 and CD73 through a feedback loop.
(A) MDA-MB468 cells were treated with IFN-γ (100 μg/ml), and cell lysates were collected at indicated time point. TRIM21 and CD73 protein levels were determined by immunoblotting. (B) Normalized TRIM21 and CD73 expression bar graph of (A). (C) MDA-MB468 cells were cocultured with or without human PBMCs and treated with IFN-γ–neutralizing antibody (Ab; 1 μg/ml), and TRIM21 and CD73 protein levels were determined by immunoblotting. Feedback regulation of CD73 by secreted IFN-γ from T cells. Activated T cell suppresses the CD73 expression level in tumor cells compared to control, which could be reversed by IFN-γ antibody (right). (D) MDA-MB468-CD73WT and MDA-MB468-CD734KR cells were treated with IFN-γ and MG-132. Cell lysates were collected at indicated time points and followed by measuring CD73 protein expression (D). (E) Normalized CD73 expression bar graph of (D) suggesting replacement of four lysine residues on CD73 by arginine significantly blocks the IFN-γ–induced down-regulation of CD73 in MDA-MB468 cells. (F and G) Replacement of four lysine residues on CD73 by arginine significantly blocks the IFN-γ–induced down-regulation of CD73 in MDA-MB231 cells.
**P < 0.01, ***P < 0.001, ****P < 0.0001. Data (means ± SEM) are representative of at least three independent experiments.
The TRIM21-CD73 axis orchestrates tumor immune evasion in vivo
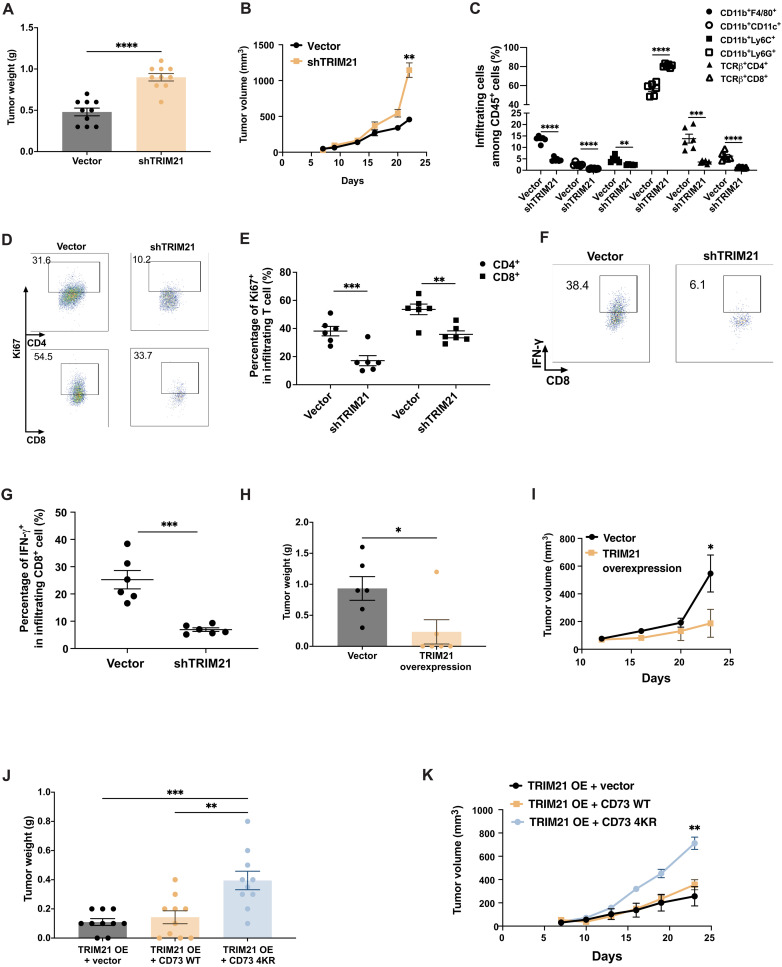
To determine the impact of TRIM21 in regulating the tumor growth in vivo, we constructed murine TRIM21 knockdown cell lines 4T1-TRIM21-sh#1 and 4T1-TRIM21-sh#2 (blank vector as control) (fig. S5, A and B). As shown in Fig. 7 (A and B), engineered 4T1 control and 4T1 shTRIM21 breast cancer cells were injected subcutaneously into the mammary fat pad of female BALB/C mice. The mammary tumors volume was also obtained by caliper measurements and calculated using the formula: V = (W2 × L)/2, where V is the tumor volume, W is the tumor width, and L is the tumor length. TRIM21 knockdown increased tumor burden 21 days after tumor cell injection, suggesting that knockdown of TRIM21 with concomitant up-regulation of CD73 in tumor cells facilitated tumor progression. To further determine whether TRIM21 knockdown that induced increased tumor growth in vivo was due to the increased membrane-bound CD73 protein level, we examined the tumor membrane surface CD73 fluorescence intensity using anti-CD73 antibodies and found that the knockdown of TRIM21 in 4T1 cells led to an increased membrane-bound CD73 expression (fig. S5C). Furthermore, we examined the tumor immune infiltrates by flow cytometry. Frequencies of macrophage (CD11b+F4/80+), dendritic cells (CD11b+CD11c+), and CD4+ and CD8+ T cells among CD45+ tumor infiltrates in TRIM21 knockdown tumor-bearing mice were markedly decreased compared to that in the control mice. In contrast, the frequency of immunosuppressive MDSCs, especially granulocytic MDSC (CD11b+Ly6G+), was increased (Fig. 7C). We also found that TRIM21 knockdown in tumor cells inhibited the proliferation capacity of tumor-infiltrating CD4+ and CD8+ T cells as indicated by the expression levels of Ki67 (Fig. 7, D and E). Notably, there was a significant reduction in IFN-γ secretion by infiltrating CD8+ T cells in TRIM21 knockdown tumor-bearing mice (Fig. 7, F and G). On the other hand, overexpression of TRIM21 (OE) impaired the tumor development compared to the control cell line (vector) (Fig. 7, H and I). To determine whether the role of TRIM21 in tumor growth is through CD73 regulation, we overexpressed both TRIM21 and CD73WT [OE + wild type (WT)] or both TRIM21 and CD734KR with mutated ubiquitylation sites (OE + 4KR mutant) in E0771 murine TNBC cell lines. E0771-TRIM21, E0771-TRIM21-CD73WT, and E0771-TRIM21-CD734KR breast cancer cells were injected subcutaneously into the mammary fat pad of female C57BL/6 mice. It is noted that there is no significant difference between E0771-TRIM21-CD73 WT tumor growth and E0771-TRIM21 tumor growth. However, tumor development was promoted in E0771-TRIM21-CD734KR tumor–bearing mice (Fig. 7, J and K), confirming the importance of TRIM21-mediated ubiquitylation of CD73 in tumor growth. To further confirm whether there is also a negative correlation between TRIM21 and CD73 on CD8+ T cells, multi-IHC staining has been done using anti-TRIM21 and anti-CD73 antibodies. While TRIM21-CD73 cascade plays an important role in tumor cells, no significant correlation between TRIM21 and CD73 at the protein level was observed in CD8+ T cells, suggesting that CD73 protein levels in T cell can be modulated potentially by other mechanisms. In this regard, the specific biological, biochemical, and/or physiological significance of TRIM21-mediated ubiquitylation and the underlying molecular mechanism regulating CD73 immunological activity in T cells remain to be fully determined.
Fig. 7. TRIM21-mediated ubiquitylation of CD73 orchestrates tumor immune evasion in vivo.
(A and B) 4T1 control and 4T1-shTRIM21 breast cancer cells were orthotopically injected into the right fourth mammary gland of the BALB/c wild-type (WT) mice. Phosphate-buffered saline (PBS) was used in control mouse group. Tumor weight was measured at the end point (A), and tumor growth curve was plotted (B) (n = 10). (C) 4T1 control and 4T1-shTRIM21 tumors were harvested 21 days after tumor challenge and analyzed by flow cytometry for accumulation of infiltrating main immune cell subsets as indicated by total CD45+ tumor infiltrates. (D) 4T1 control and 4T1-shTRIM21 tumors were harvested 21 days after tumor challenge and analyzed. T cell proliferation was determined by Ki67 staining using flow cytometry. (E) Percentage of Ki67+ among tumor-infiltrating CD4+ or CD8+ T cells in 4T1 control and 4T1-shTRIM21 tumors. (F) Tumor-infiltrating CD8+ IFN-γ+ T cells in 4T1 control and 4T1-shTRIM21 tumors were determined by flow cytometry. (G) Percentage of IFN-γ+ in tumor-infiltrating CD8+ T cells in 4T1 control and 4T1-shTRIM21 tumors was shown. (H and I) E0771 control and E0771-TRIM21 breast cancer cells were orthotopically injected into the right fourth mammary gland of the C57BL/6 WT mice. PBS was used in control mouse group. Tumor weight was measured at the end point (H), and tumor growth curve was generated (I) (n = 6). (J and K) E0771-TRIM21, E0771-TRIM21-CD73WT, and E0771-TRIM21-CD734KR breast cancer cells were orthotopically injected into the right fourth mammary gland of the C57BL/6 WT mice. PBS was used in control mouse group. Tumor weight was measured at the end point (J), and tumor growth curve was plotted (K) (n = 10). *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. Data (means ± SEM) are representative of at least two independent experiments with three to five independently analyzed mice per group.
In addition, proteomic data from Clinical Proteomic Tumor Analysis Consortium (CPTAC) further identified a subgroup of patients exhibiting a dysregulated TRIM21-CD73 axis with impaired immune cell tumor infiltration. Data in Fig. 8A suggested a negative correlation between TRIM21 and CD73 expression in patients with TNBC. Patients with high TRIM21 showed high stromal and immune scores and low tumor purity (Fig. 8B and fig. S6A). When these patients were stratified on the basis of the TRIM21 expression level (Fig. 8C and fig. S6B), a favorable immune profile was significantly observed in the TRIM21-high expression group, including increased infiltration of CD8+ T cells, cytotoxic T lymphocyte, TH1 with enhanced T cell costimulation, and type I IFN response. We have also analyzed TMA slides from patients with breast cancer containing different subtypes of breast malignancies. The TMA was stained using anti-TRIM21, anti-CD73, anti-CD3, anti-PanCK, and anti-Ki67 antibodies. Staining intensity was subsequently quantified using automated HistoQuest software, revealing that the TRIM21 expression level was positively correlated with CD3+ T cell density and proliferation in breast cancer samples (Fig. 8, D to F). In addition, stratification of CD73 and TRIM21 staining intensities from normal, G2, G3, and medullary TMAs into tumor Ki67 high and Ki67 low showed a reciprocal expression signature of TRIM21 and CD73 correlated with tumor cell proliferation, particularly in patients with high-grade breast cancer (fig. S6, C to E). Overall, our data demonstrate that TRIM21 plays a critical role in orchestrating tumor immune evasion by regulating CD73 protein expression through ubiquitin-mediated proteasomal degradation (Fig. 8G).
Fig. 8. The signature of TRIM21low/CD73high is associated with unfavorable immune response.
(A) Proteomic data from CPTAC identified that the expression of CD73 is negatively correlated with TRIM21 expression in TNBC tumor samples. (B) The correlation heat map visualized of overall immune profile (tumor mutational burden, MSI, stromal score, immune score, ESTIMATE score, and tumor purity) with TRIM21 expression in TNBC. The correlation coefficient is labeled from blue to red. Blue represents negative correlation, and red represents positive correlation. (C) Correlation analysis of tumor-infiltrating immune cells in TRIM21-high expression and TRIM21-low TNBC expression breast cancer samples. Rows represented different types of tumor-infiltrating immune cells, and columns represented breast cancer samples. The correlation coefficient is labeled from blue to red. Blue represents negative correlation, and red represents positive correlation. (D to F) Nine hundred eighty-five human breast cancer TMAs were stained with anti-TRIM21, anti-CD73, anti-CD3, anti-Ki67, and anti-PanCK antibodies. Tumor TRIM21 expression level was positively related to total CD3+ T cell density (D), T cell proliferation in tumor nests (E), and total compartments analyzed (F). (G) The proposed mechanistic model: Tumor TRIM21 constitutively governs CD73 protein stability through the ubiquitin-proteasomal pathway. Induction of tumor TRIM21 by IFN-γ enables effector T cell accumulation and functionality, thereby counteracting tumor immune evasion. TCR, T cell receptor.
DISCUSSION
Most breast cancers are cold-immune responsive, while a limited number of patients could benefit from immune checkpoint inhibitors. The current clinical urgency is to explore the in-depth mechanism of immune responsiveness for breast tumors and identify novel strategies to turn “cold” breast tumors hot. Recent pathological and therapeutic studies have drawn close attention to CD73, a negative immunomodulatory protein whose abnormal elevation promotes tumor progression and impairs antitumor immunity. In addition to the ongoing clinical immunotherapy trials showing safe and promising antitumor activity of the anti-CD73 monoclonal antibodies, our present discovery of CD73 turnover regulation by the ubiquitin-proteasome system implies an avenue to block the elevation of CD73 protein in breast cancer by developing small-molecule inhibitors that destabilizes CD73 for degradation. To fully uncover the mystery of CD73 in immune-cold breast cancer, we have raised several questions, including how CD73 protein abundance is controlled and how the deregulated CD73 proteolysis contributes to the immune escape.
In the past decade, a series of studies identified the tumor-promoting effects of CD73 in various types of solid tumors, including breast cancer (58–63). Results from TCGA on CD73 overexpression analysis indicated that CD73 transcription levels significantly increased in many cancer types (58–63). It has been demonstrated that the increased expression of CD73 in tumors is positively correlated with poor patient survival time (58–63). In both triple-negative and hormonal receptor–positive types of breast cancers, elevated expression of CD73 results in increased cancer cell proliferation and cell invasion (14, 64). Also, breast cancer patients with low tumor CD73 expression tend to have better RFS and OS as compared to the patient with high CD73 expression levels (12). In our present work, results from the proteomic analysis together with an array of patient tissue cohort specimen further confirmed that the aberrant accumulation of CD73 protein is coupled with unfavorable immune profiles particularly in TNBC. The evidence from our study highlights the importance of TRIM21-mediated ubiquitylation of CD73 as an immune escape mechanism and a potential therapeutic target in TNBC treatment.
CD73 is a membrane surface ecto-5′-nucleotidase, the enzymatic function is to convert extracellular AMP to adenosine, which has been demonstrated to modulate immune cells such as regulatory T cell, effector T cell, NK cell, and MDSC mainly through A2AR and A2BR receptors to create an immune suppressive tumor microenvironment (9). In addition, up-regulated CD73 expression and activity confer tumor resistance to therapies (9, 12, 65). In this regard, targeting CD73 has been considered as an effective approach in combination with immune checkpoint blockade, adoptive T cell therapy, agonistic immunotherapy, chemotherapy, and radiation therapy. The prevailing strategy to achieve this purpose has been exclusively using anti-CD73 antibody to block the surface CD73, while the clinical efficacy for the current CD73 antibodies needs further improvement (9, 12, 65). Previous studies have strongly implied the turnover regulation on multiple immune checkpoint proteins and its potential in drug development (66, 67). Thus, it is logical to postulate that the high renewal or replacement of CD73 by the newly synthesized CD73 protein could lead to reduced turnover rate, causing difficulty for CD73 antibody to efficiently neutralize the membrane surface CD73. Here, we demonstrate that the intracellular CD73 protein levels are regulated by the ubiquitin-proteasome pathway, where abnormal accumulation of CD73 protein due to the deregulated ubiquitin-proteasome system leads to unexpected excess production of extracellular adenosine resulting in decreased T cell function. Therefore, dysregulated CD73 turnover due to its aberrant ubiquitylation could be a pivotal factor that enables tumor immune escape and potentially contributes to the immune therapy resistance.
To comprehensively understand how CD73 is regulated by the ubiquitin-proteasome pathway and subsequently modulates tumor immunogenicity, we have shown that (i) increased CD73 plasma presentation by inhibiting the proteasomal pathway impairs T cell survival and function using a breast cancer cell PBMC coculture system; (ii) TRIM21, a E3 ligase, identified from a mass spectrometry screening assay, interacts with CD73 through amino acid residues 175 to 225 of CD73 to dictate its cellular quantity maintenance; (iii) knocking down TRIM21 or overexpression of ubiquitylation-deficient mutant CD73 in breast cancer cells significantly disrupts the antitumor T cell immunity and promotes tumor development; (iv) reciprocal expression of TRIM21 and CD73 is regulated by IFN-γ secreted from activated T cells; and (v) a TRIM21high/CD73low signature in a subgroup of human breast malignancies is associated with a favorable immune profile, warranting further investigations to define the functional and prognostic significance of a TRIM21-CD73 axis in breast cancer. Collectively, both coculture and in vivo mouse xenograft studies offer clues into the modulation of tumor CD73 at the protein level, paving the way for potential therapeutic targets to treat the breast cancer.
Although studies have suggested that targeting the ubiquitylation of immune checkpoint may serve as a therapeutic rationale in many human malignancies (67), breast cancer, normally considered an immune-cold human malignancy, receives little attention in this regard. Unlike hormonal receptor–positive breast cancer, TNBC lacks effective therapeutic interventions. Most patients with TNBC who received anti-Programmed cell death protein 1 (anti–PD-1)/anti-PD-L1 immunotherapy show limited response. These challenges raise an important question: Does breast cancer have prominent and significant alternative pathways to modulate the tumor immune response? In this study, the TRIM21-CD73-adenosine axis dictates the essential steps of the tumor immunity cycle between tumor cells and immune cells. Moreover, IFN-γ produced by effector T cells is capable of activating TRIM21-CD73 ubiquitylation to potentially amplify antitumor T cell activity, suggesting that a favorable feedback mechanism might occur whenever tumor TRIM21 is activated. Nevertheless, further work is needed to elucidate the exact mechanism of the mode of action of the TRIM21-CD73 ubiquitylation during tumor development, particularly in response to immunotherapy.
MATERIALS AND METHODS
Cell lines and chemical reagents
HEK-293T, 45 human breast cancer cell lines [American Type Culture Collection (ATCC) Breast Cancer Cell Panel (ATCC 30-4500 K)], 4T1, and E0771 were obtained from the ATCC (Manassas, VA). HEK-293T and MDA-MB231 cells were cultured in Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum [FBS; streptomycin (100 U/ml) and penicillin (100 U/ml)] at 37°C with 95% humidity. MDA-MB468 cells were cultured in L-15 medium supplemented with 10% FBS [streptomycin (100 U/ml) and penicillin (100 U/ml)] at 37°C with 95% humidity. Other breast cancer cell lines were maintained as per the manufacturer’s protocol. For IFN-γ stimulation, breast cancer cell lines MDA-MB231 and MDA-MB468 were treated with IFN-γ (100 ng/ml) for different time points. All chemical reagents and antibodies used in this study are listed in Table 1.
Table 1. Chemicals and antibodies used in this study.
| List | Antibodies/ chemical |
Dilution | Vendors | Catalog no. | Fluorophore |
|---|---|---|---|---|---|
| Chemical | Cycloheximide | 50 μg/ml | Cayman | 14126 | |
| MG-132 | 10 μM | Cayman | 13697 | ||
| Antibody | Ubiquitin | 1:1000 | Cell Signaling | 58395 | |
| CD73 | 1:1000 | Cell Signaling | 13160 | ||
| TRIM21 | 1:1000 | Abcam | Ab91423 | ||
| β-Actin | 1:1000 | Cell Signaling | 4970 | ||
| TRIM21 | 1:100 | ProteinTech | 12108-1-AP | Opal 690 | |
| CD73 | 1:100 | Cell Signaling | 13160 s | Opal 620 | |
| CD3 | No | Viocare Medical | PP215AA | Opal 650 | |
| CD8 | 1:200 | Cell Signaling | 70306S | Opal 520 | |
| Ki67 | 1:500 | Abcam | ab15580 | Opal 570 | |
| PanCK | 1:200 | Abcam | ab7753 | Opal 540 |
Plasmids and transfection
The full-length and deletion mutant constructs of CD73 were generated by PCR amplification of the full-length or partial coding sequence of human CD73 and subsequently cloned into mammalian expression vectors with Flag or hemagglutinin (HA) tag. TRIM21 full-length and deletion mutant constructs were generated by PCR amplification and then cloned into mammalian expression vectors with Myc-tag. The CD73 ubiquitylation–deficient mutant constructs were generated using the site-directed mutagenesis kit (Agilent) as described previously (54) and cloned into mammalian expression vectors with V5-tag. CD73 constructs with Lys-133, Lys-208, Lys-262, and Lys-321 site mutations were constructed as follows: A398G, 5′-GACTGATCGAGCCACTCCTCAGAGA CCAAAT-3′ (forward) and 5′-ATTTGGCCTCTCTGAGGAGTGGCTCGATCGGAG TC-3′ (reverse); A623G, 5′-CTGCATTACAACCTGAAGTAGATAAGTTAAGAACTCTA AATGTGAACAAAATT-3′ (forward) and 5′-AATTTTGTTCACATTTAGAGTTCTTA AC TTA TCTACTTCAGGTTGTAATGCAG-3′ (reverse); A785G, 5′-GAGGTGCCTG CTGGGA GGTACCCATTCATAGTC-3′ (forward) and 5′-GACTATGAATGGGTA CCTCCC AGCAGGCACCTC-3′ (reverse); A962G, 5′-CAGCATTCCTGAAGATCCAAG CATAAGAGCAGACATTAACAA-3′ (forward) and 5′-TTGTTAATGTCTGCTCTTA TGCTTGGATCTTCAGGAATGCTG-3′ (reverse). shRNAs of TRIM21 were purchased from Sigma-Aldrich and listed as follows: for human: pLKO.1-sh-hTRIM21-1# (TRCN0000002787) and pLKO.1-sh-hTRIM21-2# (TRCN00004768); for mouse: pLKO.1-sh-mTRIM21-1# (TRCN0000040691) and pLKO.1-sh-mTRIM21-2# (TRCN0000238076). TRIM21-Flag plasmid was purchased from ABM Company. TRIM21 lentiviral (mouse, LV441179) and blank vector (LV590) were purchased from ABM Company. HEK-293T, MDA-MB231, and MDA-MB468 cells were transfected with plasmids at a confluence of 50 to 70% using Lipofectamine 2000 (Invitrogen).
Lentiviral infection
pHage-CD73-Flag/HA, pLKO.1, pLKO.1-sh-hTRIM21-1#, pLKO.1-sh-hTRIM21-2#, pLKO.1-sh-mTRIM21-1#, pLKO.1-sh-mTRIM21-2#, pLenti6/V5 vector, pLenti6/V5-CD73-wildtype (pLenti6/V5-CD73-WT), and pLenti6/V5-CD73 with K133R/K208R/K262R/K321R mutation (pLenti6/V5-CD73-4KR) were cotransfected with pVSV-G, pRRE, and pRSV-REV into HEK-293T using Lipofectamine 2000. TRIM21 lentiviral and blank vector were cotransfected with 2nd Generation Packaging Mix & Lentifectin Combo Pack (ABM Company, LV003-G074). The supernatant containing packaged lentiviral particles was collected, centrifuged, mixed with polybrene, and added into MDA-MB231, MDA-MB468, HCC1937, 4T1, or E0771 target cells separately. The stable cell lines were generated by culturing the cells in the medium containing antibiotic blasticidin (10 μg/ml) or puromycin (2 μg/ml). The stable cell lines 4T1 and E0771 with CD73-WT or CD73-4KR were generated by sequential infection with TRIM21 lentiviral vector and pLenti6/V5-CD73-WT or pLenti6/V5-CD73-4KR separately, selected by both of blastidicin and puromycin subsequently.
Immunoblotting and immunoprecipitation assay
Cells were harvested and lysed in radioimmune precipitation assay lysis buffer (Upstate Biotechnology) containing protease inhibitor mixture (Sigma-Aldrich) or 1× SDS loading buffer as described before (68). The protein concentration was determined using Bio-Rad protein assay reagent. Western blotting was performed using anti-CD73, anti-TRIM21, anti-Flag, anti-Myc, anti-V5, anti–β-actin, and horseradish peroxidase (HRP)–conjugated goat anti-mouse or anti-rabbit secondary antibody (Promega). Signals were detected with enhanced chemiluminesence reagents (Bio-Rad). Semiquantification of data was performed using ImageJ. For immunoprecipitation assay, cell lysate was incubated with anti-Flag M2 gel overnight at 4°C on a rotator or preincubated anti-CD73 or anti-TRIM21 antibody for 1 hour at 4°C on a rotator, followed by the addition of protein A/G plus agarose (Pierce) to the reaction overnight at 4°C. After five washes with immune precipitation assay lysis buffer supplemented with protease inhibitor mixture, complexes were released from the anti-Flag M2 gel by boiling for 5 min in 2× SDS-PAGE loading buffer.
Ubiquitylation assay
Cell pellets were lysed in 2% SDS and 5 mM dithiothreitol and diluted into 1% NP-40 buffer. The final concentrations in the lysate used for anti-CD73 immunoprecipitation were 0.2% SDS, 0.5 mM dithiothreitol, 1% NP-40, 50 mM tris (pH 8), 150 mM NaCl, 10 mM MgCl2, and protease inhibitor cocktail. After five washes with radioimmune precipitation assay lysis buffer supplemented with protease inhibitor mixture, complexes were released from the protein A/G plus agarose by boiling for 5 min in 2× SDS-PAGE loading buffer. The eluted samples were run through 8% SDS-PAGE gel, transferred to 0.2 μM nitrocellulose membrane, and then blotted with an anti-ubiquitin antibody.
Purification of CD73 complex and mass spectrometry
MDA-MB468 cells that stably expressed Flag/HA-tagged CD73 were washed twice with phosphate-buffered saline (PBS) and lysed by NP-40 buffer [1% NP-40, 10% glycerol, 25 mM tris-HCl (pH 7.9), and protease inhibitor cocktails]. CD73-interacting proteins were purified by immunopurification with Flag-M2 beads and washing four times with TBST buffer [137 mM NaCl, 20 mM tris-HCl (pH 7.6), and 0.1% Tween 20]. The complex was eluted with 3× Flag peptide in tris-buffered saline buffer. The eluted pellet was then separated on SDS-PAGE followed by Silver staining as per the manufacturer’s protocol. The interest bands were cut out for mass spectrum analysis (54).
Indirect cell immunofluorescence
A standard immunostaining procedure was used to analyze the localization of CD73 and TRIM21 (68). Cells were seeded and grew on thick slides until they reached 50 to 70% confluence, then washed with PBS, fixed by immersion at room temperature with 4% polyformaldehyde for 20 min, and permeabilized with 0.1% Triton X-100 in PBS at 4°C for 10 min. Slides were then washed with PBS and blocked with blocking buffer consisting of 4% bovine serum albumin (BSA) in PBS for 30 min at room temperature. The cells were then incubated with primary anti-CD73 (diluted 1:25) and anti-TRIM21 antibodies (diluted 1:25) in blocking buffer overnight at 4°C, followed by incubation with the secondary anti-rabbit Alexa Fluor–labeled antibody (diluted 1:100) or anti-mouse fluorescein isothiocyanate–labeled antibody (diluted 1:100) in blocking buffer at room temperature for 1 hour. Subsequently, cells were stained with 4′,6-diamidino-2-phenylindole (DAPI; 5 μg/ml) for 2 min and washed with PBS. Stained cells were captured and quantified by the Lionheart FX Automated Microscope (BioTek).
Measurement of CD73 activity
The supernatant of cell culture media was collected and centrifuged at 10,000 rpm for 5 min to remove insoluble particles. The concentration of adenosine in culture media was detected as per the manufacturer’s protocol (Cell Biolabs, San Diego, USA). Briefly, the supernatant was mixed with reaction mix in which adenosine was converted into inosine by adenosine deaminase. Then, inosine is converted into hypoxanthine by purine nucleoside phosphorylase. Last, hypoxanthine is converted to xanthine and hydrogen peroxide by xanthine oxidase. The resulting hydrogen peroxide is then detected with a highly specific fluorometric probe. HRP catalyzes the reaction between the probe and hydrogen peroxide, which bind in a 1:1 ratio. Samples are compared to a known concentration of adenosine standard within the 96-well microtiter plate format. Samples and standards are incubated for 15 min and then read with a standard 96-well fluorometric plate reader.
T cell suppression assay
Human PBMCs were obtained from donors and incubated at ratios of 1:10 and 1:20 with MDA-MB231, MDA-MB468, MDA-MB231-CD73-Flag, MDA-MB468-CD73-Flag, MDA-MB231-TRIM21-Flag, MDA-MB468-TRIM21-Flag, MDA-MB231-shTRIM21, and MDA-MB468-shTRIM21 breast cancer cells separately to construct a coculture system. For the coculture, we seeded breast cancer cells 1 day in advance and then allowed interaction with PBMCs for 3 days. T cells were stimulated with anti-CD3 (30 ng/ml; Miltenyi Biotech), supplemented with or without 2 μM 5′-AMP. After 72 hours, T cell proliferation was measured by prolifer A510 staining using flow cytometry (BioLegend, USA).
Analysis of cells by flow cytometry
All samples were initially incubated with 2.4G2 to block antibody binding to Fc receptors. Single-cell suspensions were stained with 1 μg of relevant monoclonal antibodies and then washed twice with cold PBS. For cell apoptosis analysis, cells were rinsed with 1× binding buffer and then stained with annexin V and 7-Aminoactinomycin D (7-AAD) along with other surface antibodies in binding buffer at room temperature in the dark for 10 min and immediately run on a flow cytometer. Ki67 staining was performed according to the manufacturer’s instructions (eBioscience). Surface and intracellular CD73 staining was performed using an anti-CD73 staining kit according to the manufacturer’s instruction (BD Biosciences). Intracellular IFN-γ staining under stimulation with phorbol 12-myristate 13-acetate (50 ng/ml), ionomycin (5 μg/ml), and brefeldin A (10 μg/ml) in the presence of the relevant tumor antigen peptides was performed according to the manufacturer’s instruction (BD Biosciences). For surface marker staining, cells were stained with CD4/CD8/CD25/CD73/Ki-67/tcrb and fixed in 4% formaldehyde for 10 min at room temperature. Cells were washed with ice-cold PBS containing 2% BSA, followed by the second wash step with ice-cold PBS. Cells were resuspended in 80% methanol and incubated for 30 min at −20°C. Samples were harvested on LSRII, and data were analyzed with FlowJo software.
In vitro T cell assay
Human PBMCs were obtained from donors and cocultured at ratios of 1:10 and 1:20 with MDA-MB231, MDA-MB468, MDA-MB231-CD73, MDA-MB468-CD73, MDA-MB231-TRIM21, MDA-MB468-TRIM21, MDA-MB231-shTRIM21, and MDA-MB468-shTRIM21 breast cancer cells separately or incubated with the tumor conditional medium (TCM) from MDA-MB468-CD73WT or MDA-MB468-CD734KR cells. Splenocytes were obtained from WT or Pmel mice and incubated with 4T1-TRIM21, 4T1-TRIM21-CD73, 4T1TRIM21-CD734KR, E0771TRIM21, E0771TRIM21-CD73, and E0771TRIM21-CD734KR breast cancer cells separately. For the coculture, we seeded breast cancer cells 1 day in advance and then allowed interaction with PBMCs or splenocytes for 3 days. PBMCs and splenocytes were stimulated with anti-CD3 (1 μg/ml) or gp100 (0.5 μg/ml), supplemented with or without 5 μM 5′-AMP. For the TCM incubation, TCM was obtained from tumor cells treated with or without 50 μM APCP for 24 hours and added in PBMCs in the presence of anti-CD3 (1 μg/ml). After 72 hours, cells were analyzed for T cell apoptosis, cytokine production, and proliferation by LSRII. The polyfunctionality and polyfunctional strength index indicated by relative abundance and composition of secreted cytokines at the single-cell level was measured by IsoPlexis IsoLight assays using Isoplexis Human Adaptive Immune Chips in enriched CD8+ T cells from TCM incubation assay.
In vivo tumor challenge
BALB/C and C57BL/6 mice (8-week-old female) were purchased from Charles River Laboratories. Engineered 4T1 control and 4T1 shTRIM21 breast cancer cells (2 × 106) were injected subcutaneously into the mammary fat pad of female BALB/C mice on day 0. E0771 TRIM21, E0771 TRIM21-CD73, and E0771 TRIM21-CD73-4KR breast cancer cells (2 × 106) were injected subcutaneously into the mammary fat pad of female C57BL/6 mice on day 0. Tumor volumes were measured along three orthogonal axes (a, b, and c) and calculated as abc/2 every 2 to 4 days. The tumor weight was determined at end point. All animal experiments were approved by the Institutional Animal Care and Use Committee of Northwestern University.
RNA extraction and real-time qPCR
Total RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. The complementary DNA synthesis was performed using SuperScript One-Step RT-PCR (Invitrogen). Real-Time Quantitative Reverse Transcription PCR was used to quantify genes by SYBR Green (Bio-Rad), and relative abundance of each mRNA was normalized to glyceraldehyde-3-phosphate dehydrogenase mRNA. The primers used for
Real-Time Quantitative Reverse Transcription PCR are as follows: CD73, 5′-CTGGGGCACTCTGGTTTTGA-3′ (forward) and 5′-TCCCCGCAGGCACTTCTTTG-3′ (reverse).
Multiplex IHC
The breast cancer TMA (US Biomax Inc.), including invasive ductal carcinoma cases (grade 2, n = 28; grade 3, n = 25) and medullary carcinoma cases (n = 9), was used for multiplex IHC staining of TRIM21, CD73, CD3, CD8, Ki67, and PanCK by using the Opal multiple color IHC kit (Akoya Biosciences) as described previously (53). Briefly, the deparaffinized and rehydrolyzed TMA slide was implemented antigen retrieval in AR9 retrieval buffer (Akoya Biosciences), followed by 6 cycles of staining procedures including blocking and binding of primary antibodies and second HRP-linked antibodies and visualized with the corresponding Opal fluorophores. Each staining cycle was finished up with heating in AR6 retrieval buffer (Akoya Biosciences) to release the bounded primary and second antibodies but did not disturb the resident fluorophores. After six-round staining procedures, the slide was counterstained with DAPI. The single-marker staining with individual opal fluorophore was used as the reference for the “spectral unmixing process.” The antibodies and corresponding fluorophores are listed in table S1.
Acquirement of multispectral images and data analysis
The Opal fluorophore signals on the finished staining TMA slide were captured with the Vectra 3 Automated Quantitative Pathology Imaging System (PerkinElmer) at ×200 magnifications and proceeded with spectral unmixing into four individual fluorophores based on the unique emitting spectrum of each single fluorophore using InForm Advanced Image Analysis software (Akoya Biosciences). Subsequently, the spectral unmixed images underwent cell segmentation based on DAPI and cell phenotyping based on specific cellular markers through the trained algorithm of Inform. The exported data containing composite images, cell segmentation, and cell phenotyping from InForm were further carried out quantitative analyses of cellular densities and protein intensities using R-based phenoptrReports and phenoptr (Akoya Biosciences).
Kaplan-Meier analysis
This analysis was performed with the online Kaplan-Meier Plotter tool (https://kmplot.com) for breast cancer (69). Patients were divided according to best cutoff value. Two end points were analyzed for RFS, OS, and DMFS. Postprogression survival was calculated and tested by the log-rank test.
Estimating of immune cell infiltration and sample clustering
The TRIM21 protein data in patients with breast cancer were obtained from The Cancer Proteome Atlas (TCPA) database. To estimate the role of TRIM21 protein on immune cell infiltration (ICI), the R packages of “ESTIMATE” (version 1.0.13) and “CIBERSORT” (70) were adopted to estimate the immune score, stromal score, and other immune cell types of ICI. The comprehensive immune and stromal score were evaluated by ESTIMATE score (71). In addition, the microsatellite instability (MSI) testing and tumor mutational burden (TMB) are genomic biomarkers used to identify patients who are likely to benefit from immune checkpoint inhibitors (72, 73). The TMB score and MSI score in each breast cancer sample were obtained from the TCGA database. The Spearman analysis was carried out for the correlation assay between TRIM21 expression, ICI, TMB, and MSI. The TRIM21 and CD73 protein data in TNBC cell lines were obtained from Cancer Cell Line Encyclopedia (https://portals.broadinstitute.org/ccle/home). The coefficient of RIM21 and CD73 protein was calculated by Spearman correlation analysis.
Statistical analysis
Unless specified, results were expressed as means ± SEM. Experiments were performed at least twice unless otherwise specified. Statistical analyses were performed using SPSS (SPSS, Chicago, IL, USA), GraphPad Prism (version 8, GraphPad Software, San Diego, CA), or R software (version 3.3.3). The significance of the differences in the assays was analyzed by Student’s t test or one or two-way analysis of variance (ANOVA), followed by Tukey’s multiple comparisons test. Comparison of survival curves or tumor-free curve was performed using log-rank (Mantel-Cox) test. Pearson correlation or Spearman correlation was used to study the correlation between two molecules. A value of P < 0.05 was considered significant.
Acknowledgments
We thank the proteomic core at the Feinberg School of Medicine at Northwestern University for mass spectrometry analysis and postdata analysis (NCI CCSG P30 CA060553 and P41 GM108569). We thank all members of the Wan and Zhang laboratories for helpful discussion. This work was also supported by the RHLCCC Flow Cytometry Facility, Immunotherapy Assessment Core, Image CORE, Animal Resources Facility, and a Cancer Center Support Grant (NCI CA060553).
Funding: This work was supported by NIH R01CA258857, NIH R01CA258765, NIH R01CA250110, and NIH R01CA202948. This work was also partially supported by Northwestern University Friends of Prentice Award SP0052611.
Author contributions: Y.W., B.Z., Z.F., S.C., and Y.Z. conceived the project. Y.W. and B.Z. supervised research. Z.F., S.C., Y.Z., D.Z., P.X., Q.J., J.C., S.X., and Y.X. performed research. Y.W., B.Z., Z.F., S.C., and Y.Z. analyzed and interpreted data. D.Z., Y.X., X.L., and X.S. performed bioinformatic and computational analyses. Z.F., Y.Z., S.C., D.Z., and P.X. produced figures with input from Y.W., B.Z., and X.L. M.C., W.J.G., K.K., and Y.Y. oversaw clinical bioinformatic analyses. Y.Z., Z.F., S.C., Z.B., and Y.W. wrote the manuscript with input from all authors. All authors discussed the results and commented on the manuscript.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Figs. S1 to S6
REFERENCES AND NOTES
- 1.Galon J., Bruni D., Tumor immunology and tumor evolution: Intertwined histories. Immunity 52, 55–81 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Hegde P. S., Chen D. S., Top 10 Challenges in Cancer Immunotherapy. Immunity 52, 17–35 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Aysola K., Desai A., Welch C., Xu J., Qin Y., Reddy V., Matthews R., Owens C., Okoli J., Beech D. J., Piyathilake C. J., Reddy S. P., Rao V. N., Triple negative breast cancer - An overview. Hereditary Genet. 2013, 001 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peppercorn J., Perou C. M., Carey L. A., Molecular subtypes in breast cancer evaluation and management: Divide and conquer. Cancer Invest. 26, 1–10 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Vikas P., Borcherding N., Zhang W., The clinical promise of immunotherapy in triple-negative breast cancer. Cancer Manag. Res. 10, 6823–6833 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marra A., Viale G., Curigliano G., Recent advances in triple negative breast cancer: The immunotherapy era. BMC Med. 17, 90 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sceneay J., Goreczny G. J., Wilson K., Morrow S., DeCristo M. J., Ubellacker J. M., Qin Y., Laszewski T., Stover D. G., Barrera V., Hutchinson J. N., Freedman R. A., Mittendorf E. A., McAllister S. S., Interferon signaling is diminished with age and is associated with immune checkpoint blockade efficacy in triple-negative breast cancer. Cancer Discov. 9, 1208–1227 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonaventura P., Shekarian T., Alcazer V., Valladeau-Guilemond J., Valsesia-Wittmann S., Amigorena S., Caux C., Depil S., Cold tumors: A therapeutic challenge for immunotherapy. Front. Immunol. 10, 168 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S., Wainwright D. A., Wu J. D., Wan Y., Matei D. E., Zhang Y., Zhang B., CD73: An emerging checkpoint for cancer immunotherapy. Immunotherapy 11, 983–997 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neo S. Y., Yang Y., Record J., Ma R., Chen X., Chen Z., Tobin N. P., Blake E., Seitz C., Thomas R., Wagner A. K., Andersson J., de Boniface J., Bergh J., Murray S., Alici E., Childs R., Johansson M., Westerberg L. S., Haglund F., Hartman J., Lundqvist A., CD73 immune checkpoint defines regulatory NK cells within the tumor microenvironment. J. Clin. Investig. 130, 1185–1198 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goswami S., Walle T., Cornish A. E., Basu S., Anandhan S., Fernandez I., Vence L., Blando J., Zhao H., Yadav S. S., Ott M., Kong L. Y., Heimberger A. B., de Groot J., Sepesi B., Overman M., Kopetz S., Allison J. P., Pe’er D., Sharma P., Immune profiling of human tumors identifies CD73 as a combinatorial target in glioblastoma. Nat. Med. 26, 39–46 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buisseret L., Pommey S., Allard B., Garaud S., Bergeron M., Cousineau I., Ameye L., Bareche Y., Paesmans M., Crown J. P. A., di Leo A., Loi S., Piccart-Gebhart M., Willard-Gallo K., Sotiriou C., Stagg J., Clinical significance of CD73 in triple-negative breast cancer: Multiplex analysis of a phase III clinical trial. Ann. Oncol. 29, 1056–1062 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu J., Wang X., Lu Q., Wang J., Li L., Liao X., Zhu W., Lv L., Zhi X., Yu J., Jin Y., Zou Q., Ou Z., Liu X., Zhou P., Extracellular 5′-nucleotidase (CD73) promotes human breast cancer cells growth through AKT/GSK-3β/β-catenin/cyclinD1 signaling pathway. Int. J. Cancer 142, 959–967 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Loi S., Pommey S., Haibe-Kains B., Beavis P. A., Darcy P. K., Smyth M. J., Stagg J., CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc. Natl. Acad. Sci. U.S.A. 110, 11091–11096 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Resta R., Yamashita Y., Thompson L. F., Ecto-enzyme and signaling functions of lymphocyte CD73. Immunol. Rev. 161, 95–109 (1998). [DOI] [PubMed] [Google Scholar]
- 16.Colgan S. P., Eltzschig H. K., Eckle T., Thompson L. F., Physiological roles for ecto-5′-nucleotidase (CD73). Purinergic Signal. 2, 351–360 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chatterjee S., Thyagarajan K., Kesarwani P., Song J. H., Soloshchenko M., Fu J., Bailey S. R., Vasu C., Kraft A. S., Paulos C. M., Yu X. Z., Mehrotra S., Reducing CD73 expression by IL1β-programmed Th17 cells improves immunotherapeutic control of tumors. Cancer Res. 74, 6048–6059 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J., Wang L., Chen X., Li L., Li Y., Ping Y., Huang L., Yue D., Zhang Z., Wang F., Li F., Yang L., Huang J., Yang S., Li H., Zhao X., Dong W., Yan Y., Zhao S., Huang B., Zhang B., Zhang Y., CD39/CD73 upregulation on myeloid-derived suppressor cells via TGF-β-mTOR-HIF-1 signaling in patients with non-small cell lung cancer. Onco. Targets. Ther. 6, e1320011 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen S., Fan J., Zhang M., Qin L., Dominguez D., Long A., Wang G., Ma R., Li H., Zhang Y., Fang D., Sosman J., Zhang B., CD73 expression on effector T cells sustained by TGF-β facilitates tumor resistance to anti-4-1BB/CD137 therapy. Nat. Commun. 10, 150 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Synnestvedt K., Furuta G. T., Comerford K. M., Louis N., Karhausen J., Eltzschig H. K., Hansen K. R., Thompson L. F., Colgan S. P., Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J. Clin. Investig. 110, 993–1002 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiss J., Yegutkin G. . G., Koskinen K., Savunen T., Jalkanen S., Salmi M., IFN-beta protects from vascular leakage via up-regulation of CD73. Eur. J. Immunol. 37, 3334–3338 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Antonioli L., Pacher P., Vizi E. S., Hasko G., CD39 and CD73 in immunity and inflammation. Trends Mol. Med. 19, 355–367 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brisevac D., Bjelobaba I., Bajic A., Clarner T., Stojiljkovic M., Beyer C., Andjus P., Kipp M., Nedeljkovic N., Regulation of ecto-5′-nucleotidase (CD73) in cultured cortical astrocytes by different inflammatory factors. Neurochem. Int. 61, 681–688 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Al-Taei S., Salimu J., Spary L. K., Clayton A., Lester J. F., Tabi Z., Prostaglandin E2-mediated adenosinergic effects on CD14+ cells: Self-amplifying immunosuppression in cancer. Onco. Targets. Ther. 6, e1268308 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spychala J., Zimmermann A. G., Mitchell B. S., Tissue-specific regulation of the ecto-5′-nucleotidase promoter. Role of the camp response element site in mediating repression by the upstream regulatory region. J. Biol. Chem. 274, 22705–22712 (1999). [DOI] [PubMed] [Google Scholar]
- 26.Spychala J., Kitajewski J., Wnt and beta-catenin signaling target the expression of ecto-5′-nucleotidase and increase extracellular adenosine generation. Exp. Cell Res. 296, 99–108 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Hatakeyama S., TRIM family proteins: Roles in autophagy, immunity, and carcinogenesis. Trends Biochem. Sci. 42, 297–311 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Vunjak M., Versteeg G. A., TRIM proteins. Curr. Biol. 29, R42–R44 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Valletti A., Marzano F., Pesole G., Sbisa E., Tullo A., Targeting chemoresistant tumors: Could TRIM proteins-p53 axis be a possible answer? Int. J. Mol. Sci. 20, 1776 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozato K., Shin D. M., Chang T. H., Morse H. C. III, TRIM family proteins and their emerging roles in innate immunity. Nat. Rev. Immunol. 8, 849–860 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukuda-Kamitani T., Kamitani T., Ubiquitination of Ro52 autoantigen. Biochem. Biophys. Res. Commun. 295, 774–778 (2002). [DOI] [PubMed] [Google Scholar]
- 32.Pan J. A., Sun Y., Jiang Y. P., Bott A. J., Jaber N., Dou Z., Yang B., Chen J. S., Catanzaro J. M., du C., Ding W. X., Diaz-Meco M. T., Moscat J., Ozato K., Lin R. Z., Zong W. X., TRIM21 ubiquitylates SQSTM1/p62 and suppresses protein sequestration to regulate redox homeostasis. Mol. Cell 61, 720–733 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang B., Wang J., Sun B., Trim21: A novel negative regulator in DNA sensor signaling. Cell. Mol. Immunol. 10, 190–192 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Z., Bao M., Lu N., Weng L., Yuan B., Liu Y. J., The E3 ubiquitin ligase TRIM21 negatively regulates the innate immune response to intracellular double-stranded DNA. Nat. Immunology 14, 172–178 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou G., Wu W., Yu L., Yu T., Yang W., Wang P., Zhang X., Cong Y., Liu Z., Tripartite motif-containing (TRIM) 21 negatively regulates intestinal mucosal inflammation through inhibiting TH1/TH17 cell differentiation in patients with inflammatory bowel diseases. J. Allergy Clin. Immunol. 142, 1218–1228.e12 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Stacey K. B., Breen E., Jefferies C. A., Tyrosine phosphorylation of the E3 ubiquitin ligase TRIM21 positively regulates interaction with IRF3 and hence TRIM21 activity. PLOS ONE 7, e34041 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu H., Li M., Song Y., Xu W., TRIM21 restricts coxsackievirus B3 replication, cardiac and pancreatic injury via interacting with MAVS and positively regulating IRF3-mediated type-I interferon production. Front. Immunol. 9, 2479 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding Q., He D., He K., Zhang Q., Tang M., Dai J., Lv H., Wang X., Xiang G., Yu H., Downregulation of TRIM21 contributes to hepatocellular carcinoma carcinogenesis and indicates poor prognosis of cancers. Tumour Biol. 36, 8761–8772 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Du L., Li Y.-J., Fakih M., Wiatrek R. L., Duldulao M., Chen Z., Chu P., Garcia-Aguilar J., Chen Y., Role of SUMO activating enzyme in cancer stem cell maintenance and self-renewal. Nat. Commun. 7, 12326 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Itou J., Li W., Ito S., Tanaka S., Matsumoto Y., Sato F., Toi M., Sal-like 4 protein levels in breast cancer cells are post-translationally down-regulated by tripartite motif-containing 21. J. Biol. Chem. 293, 6556–6564 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muller J., Maurer V., Reimers K., Vogt P. M., Bucan V., TRIM21, a negative modulator of LFG in breast carcinoma MDA-MB-231 cells in vitro. Int. J. Oncol. 47, 1634–1646 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin Y., Zhang Y., Li B., Zhang J., Dong Z., Hu X., Wan Y., TRIM21 mediates ubiquitination of Snail and modulates epithelial to mesenchymal transition in breast cancer cells. Int. J. Biol. Macromol. 124, 846–853 (2019). [DOI] [PubMed] [Google Scholar]
- 43.Jin Y., Zhao X., Zhang Q., Zhang Y., Fu X., Hu X., Wan Y., Cancer-associated mutation abolishes the impact of TRIM21 on the invasion of breast cancer cells. Int. J. Biol. Macromol. 142, 782–789 (2020). [DOI] [PubMed] [Google Scholar]
- 44.Mertins P., Mani D. R., Ruggles K. V., Gillette M. A., Clauser K. R., Wang P., Wang X., Qiao J. W., Cao S., Petralia F., Kawaler E., Mundt F., Krug K., Tu Z., Lei J. T., Gatza M. L., Wilkerson M., Perou C. M., Yellapantula V., Huang K.-L., Lin C., McLellan M. D., Yan P., Davies S. R., Townsend R. R., Skates S. J., Wang J., Zhang B., Kinsinger C. R., Mesri M., Rodriguez H., Ding L., Paulovich A. G., Fenyö D., Ellis M. J., Carr S. A.; NCI CPTAC , Proteogenomics connects somatic mutations to signalling in breast cancer. Nature 534, 55–62 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garrido-Castro A. C., Lin N. U., Polyak K., Insights into molecular classifications of triple-negative breast cancer: Improving patient selection for treatment. Cancer Discov. 9, 176–198 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gruosso T., Gigoux M., Manem V. S. K., Bertos N., Zuo D., Perlitch I., Saleh S. M. I., Zhao H., Souleimanova M., Johnson R. M., Monette A., Ramos V. M., Hallett M. T., Stagg J., Lapointe R., Omeroglu A., Meterissian S., Buisseret L., van den Eyden G., Salgado R., Guiot M. C., Haibe-Kains B., Park M., Spatially distinct tumor immune microenvironments stratify triple-negative breast cancers. J. Clin. Invest. 129, 1785–1800 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou Z., Zhou H., Ponzoni L., Luo A., Zhu R., He M., Huang Y., Guan K.-L., Bahar I., Liu Z., Wan Y., EIF3H orchestrates Hippo pathway-mediated oncogenesis via catalytic control of YAP stability. Cancer Res. , (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jin D., Fan J., Wang L., Thompson L. F., Liu A., Daniel B. J., Shin T., Curiel T. J., Zhang B., CD73 on tumor cells impairs antitumor T-cell responses: A novel mechanism of tumor-induced immune suppression. Cancer Res. 70, 2245–2255 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thompson L. F., Boss G. R., Spiegelberg H. L., Jansen I. V., O’Connor R. D., Waldmann T. A., Hamburger R. N., Seegmiller J. E., Ecto-5′-nucleotidase activity in T and B lymphocytes from normal subjects and patients with congenital X-linked agammaglobulinemia. J. Immunol. 123, 2475–2478 (1979). [PubMed] [Google Scholar]
- 50.Neubert N. J., Soneson C., Barras D., Baumgaertner P., Rimoldi D., Delorenzi M., Marraco S. A. F., Speiser D. E., A well-controlled experimental system to study interactions of cytotoxic t lymphocytes with tumor cells. Front. Immunol. 7, 326 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gamper A. M., Qiao X., Kim J., Zhang L., DeSimone M. C., Rathmell W. K., Wan Y., Regulation of KLF4 turnover reveals an unexpected tissue-specific role of pVHL in tumorigenesis. Mol. Cell 45, 233–243 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu D., Zhou Z., Davidson N. E., Huang Y., Wan Y., Novel insight into KLF4 proteolytic regulation in estrogen receptor signaling and breast carcinogenesis. J. Biol. Chem. 287, 13584–13597 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poropatich K., Dominguez D., Chan W. C., Andrade J., Zha Y., Wray B., Miska J., Qin L., Cole L., Coates S., Patel U., Samant S., Zhang B., OX40+ plasmacytoid dendritic cells in the tumor microenvironment promote antitumor immunity. J. Clin. Invest. 130, 3528–3542 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu D., Gur M., Zhou Z., Gamper A., Hung M. C., Fujita N., Lan L., Bahar I., Wan Y., Interplay between arginine methylation and ubiquitylation regulates KLF4-mediated genome stability and carcinogenesis. Nat. Commun. 6, 8419 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao B., Wang Y., Xu W., Li S., Li Q., Xiong S., Inhibition of histone deacetylase activity suppresses IFN-γ induction of tripartite motif 22 via CHIP-mediated proteasomal degradation of IRF-1. J. Immunol. 191, 464–471 (2013). [DOI] [PubMed] [Google Scholar]
- 56.Gao B., Wang Y., Xu W., Duan Z., Xiong S., A 5′ extended IFN-stimulating response element is crucial for IFN-gamma-induced tripartite motif 22 expression via interaction with IFN regulatory factor-1. J. Immunol. 185, 2314–2323 (2010). [DOI] [PubMed] [Google Scholar]
- 57.Sjöstrand M., Ambrosi A., Brauner S., Sullivan J., Malin S., Kuchroo V. K., Espinosa A., Wahren-Herlenius M., Expression of the immune regulator tripartite-motif 21 is controlled by IFN regulatory factors. J. Immunol. 191, 3753–3763 (2013). [DOI] [PubMed] [Google Scholar]
- 58.Monteiro I., Vigano S., Faouzi M., Treilleux I., Michielin O., Ménétrier-Caux C., Caux C., Romero P., de Leval L., CD73 expression and clinical significance in human metastatic melanoma. Oncotarget 9, 26659–26669 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang X., Zhang T., Song Z., Li L., Zhang X., Liu J., Liu X., Qiu L., Qian Z., Zhou S., Feng L., Hu G., Meng B., Zhai Q., Ren X., Fu K., Li L., Wang P., Zhang H., Tumor CD73/A2aR adenosine immunosuppressive axis and tumor-infiltrating lymphocytes in diffuse large B-cell lymphoma: Correlations with clinicopathological characteristics and clinical outcome. Int. J. Cancer 145, 1414–1422 (2019). [DOI] [PubMed] [Google Scholar]
- 60.Jiang T., Xu X., Qiao M., Li X., Zhao C., Zhou F., Gao G., Wu F., Chen X., Su C., Ren S., Zhai C., Zhou C., Comprehensive evaluation of NT5E/CD73 expression and its prognostic significance in distinct types of cancers. BMC Cancer 18, 267 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mandapathil M., Boduc M., Netzer C., Güldner C., Roessler M., Wallicek-Dworschak U., Jahns E., Stuck B., CD73 expression in lymph node metastases in patients with head and neck cancer. Acta Otolaryngol. 138, 180–184 (2018). [DOI] [PubMed] [Google Scholar]
- 62.Chen Q., Pu N., Yin H., Zhang J., Zhao G., Lou W., Wu W., CD73 acts as a prognostic biomarker and promotes progression and immune escape in pancreatic cancer. J. Cell. Mol. Med. 24, 8674–8686 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Turiello R., Pinto A., Morello S., CD73: A promising biomarker in cancer patients. Front. Pharmacol. 11, 609931 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou P., Zhi X., Zhou T., Chen S., Li X., Wang L., Yin L., Shao Z., Ou Z., Overexpression of Ecto-5′-nucleotidase (CD73) promotes T-47D human breast cancer cells invasion and adhesion to extracellular matrix. Cancer Biol. Ther. 6, 426–431 (2007). [DOI] [PubMed] [Google Scholar]
- 65.Roh M., Wainwright D. A., Wu J. D., Wan Y., Zhang B., Targeting CD73 to augment cancer immunotherapy. Curr. Opin. Pharmacol. 53, 66–76 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bekes M., Langley D. R., Crews C. M., PROTAC targeted protein degraders: The past is prologue. Nat. Rev. Drug Discov. 21, 181–200 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu J., Cheng Y., Zheng M., Yuan B., Wang Z., Li X., Yin J., Ye M., Song Y., Targeting the ubiquitination/deubiquitination process to regulate immune checkpoint pathways. Signal Transduct. Target. Ther. 6, 28 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou Z., Song X., Chi J. J., Gius D. R., Huang Y., Cristofanilli M., Wan Y., Regulation of KLF4 by posttranslational modification circuitry in endocrine resistance. Cell. Signal. 70, 109574 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou Z., Luo A., Shrivastava I., He M., Huang Y., Bahar I., Liu Z., Wan Y., Regulation of XIAP turnover reveals a role for USP11 in promotion of tumorigenesis. EBioMedicine 15, 48–61 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Newman A. M., Liu C. L., Green M. R., Gentles A. J., Feng W., Xu Y., Hoang C. D., Diehn M., Alizadeh A. A., Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 12, 453–457 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoshihara K., Shahmoradgoli M., Martínez E., Vegesna R., Kim H., Torres-Garcia W., Treviño V., Shen H., Laird P. W., Levine D. A., Carter S. L., Getz G., Stemke-Hale K., Mills G. B., Verhaak R. G. W., Inferring tumour purity and stromal and immune cell admixture from expression data. Nat. Commun. 4, 2612 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chalmers Z. R., Connelly C. F., Fabrizio D., Gay L., Ali S. M., Ennis R., Schrock A., Campbell B., Shlien A., Chmielecki J., Huang F., He Y., Sun J., Tabori U., Kennedy M., Lieber D. S., Roels S., White J., Otto G. A., Ross J. S., Garraway L., Miller V. A., Stephens P. J., Frampton G. M., Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 9, 34 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singhi A. D., George B., Greenbowe J. R., Chung J., Suh J., Maitra A., Klempner S. J., Hendifar A., Milind J. M., Golan T., Brand R. E., Zureikat A. H., Roy S., Schrock A. B., Miller V. A., Ross J. S., Ali S. M., Bahary N., Real-time targeted genome profile analysis of pancreatic ductal adenocarcinomas identifies genetic alterations that might be targeted with existing drugs or used as biomarkers. Gastroenterology 156, 2242–2253.e4 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S6