Abstract
The Helicobacter pylori chromosomal region known as the cytotoxin-gene associated pathogenicity island (cag PAI) is associated with severe disease and encodes proteins that are believed to induce interleukin (IL-8) secretion by cultured epithelial cells. The objective of this study was to evaluate the relationship between the cag PAI, induction of IL-8, and induction of neutrophilic gastric inflammation. Germ-free neonatal piglets and conventional C57BL/6 mice were given wild-type or cag deficient mutant derivatives of H. pylori strain 26695 or SS1. Bacterial colonization was determined by plate count, gastritis and neutrophilic inflammation were quantified, and IL-8 induction in AGS cells was determined by enzyme-linked immunosorbent assay. Deletion of the entire cag region or interruption of the virB10 or virB11 homolog had no effect on bacterial colonization, gastritis, or neutrophilic inflammation. In contrast, these mutations had variable effects on IL-8 induction, depending on the H. pylori strain. In the piglet-adapated strain 26695, which induced IL-8 secretion by AGS cells, deletion of the cag PAI decreased induction. In the mouse-adapted strain SS1, which did not induce IL-8 secretion, deletion of the cagII region or interruption of any of three cag region genes increased IL-8 induction. These results indicate that in mice and piglets (i) neither the cag PAI nor the ability to induce IL-8 in vitro is essential for colonization or neutrophilic inflammation and (ii) there is no direct relationship between the presence of the cag PAI, IL-8 induction, and neutrophilic gastritis.
The gastric bacterium Helicobacter pylori, first cultured in 1983, is now a well-established cause of gastritis and peptic ulcer disease (22, 41). Infection is associated with production of proinflammatory cytokines which lead to chronic or chronic active gastritis. The activity of the gastritis (i.e., the intensity of neutrophil influx) is commonly considered an indicator of severity, and chronic active gastritis is associated with more severe manifestations of disease, such as peptic ulceration and neoplasia (9, 14, 47).
Among the cytokines associated with H. pylori colonization, the CXC family of chemokines may be involved in inducing neutrophilic influx. Two of these CXC chemokines and neutrophil chemoattractants, interleukin-8 (IL-8) and GRO-α, are increased in the gastric mucosa of patients with chronic active gastritis and ulceration (14–16, 18, 47, 53, 54, 56, 61). In addition, coculture of H. pylori with gastric cancer cell lines (15, 32, 52), normal human gastric mucosal cells (44), and immortalized mouse gastric mucus cells (40) results in induction of proinflammatory cytokines IL-8 and IL-6 or, in murine cells, CINC-2β, the rodent equivalent of GRO-α. In vivo, IL-8 secretion is most prominent in the superficial gastric mucosa (18), and it is likely that mucosal epithelial cells are a source of neutrophil-inducing cytokines, although other sources likely exist (10, 26, 34, 62). Thus, it is commonly supposed that severe manifestations of H. pylori-related disease are promoted by H. pylori-induced epithelial secretion of IL-8 and the attendant neutrophilic inflammation.
H. pylori-induced chemokine secretion by gastric epithelial cells appears to be facilitated at least in part by genes in the cag pathogenicity island (PAI). This stretch of approximately 30 open reading frames is present in many but not all strains of H. pylori, and many of the predicted genes are homologous to virulence-associated genes of other bacterial pathogens (2, 11, 12). Homology to the vir genes of Agrobacterium tumefaciens and the ptl genes of Bordetella pertussis suggests that H. pylori cag PAI genes may code for a type IV secretion complex important in delivery of proinflammatory or other pathogenic molecules to host cells (12, 43).
In addition to genetic homologies, there is functional evidence of the role of the cag PAI in virulence. Its presence is associated with increased severity of disease in the human host (9, 47). In addition, induction of IL-8 in cultured cells in vitro appears to depend at least in part on cag genes, at least in some H. pylori strains (2, 11). In spite of genetic, epidemiologic, and in vitro evidence supporting the link between cag genes, IL-8 induction in vitro, and neutrophilic gastric inflammation, however, few animal model studies have been done to investigate the role of cag PAI genes in vivo.
The goal of this study was to use two animal models to evaluate both the cag PAI as a whole and selected cag PAI genes in induction of gastritis in vivo. The germ-free piglet model of infection uses strain 26695, a pig-adapted human isolate (23). Strain 26695 colonizes piglets well and induces gastritis which is largely lymphocytic and lymphofollicular, with a variable neutrophilic component. Strain 26695 induces IL-8 in vitro (2, 38), its genomic DNA sequence is known (57), and its cag PAI is uninterrupted (the cagI and cagII regions are adjacent), allowing deletion of the entire region by simple reverse genetics (2). Mice are poorly colonized by strain 26695 but are well colonized by strain SS1, the other strain used in this study (24, 37).
MATERIALS AND METHODS
Bacterial strains.
Strain 26695 is a human isolate which was adapted to pig colonization by serial passage (3). Strain SS1 is a mouse-adapted human isolate (37). Both strains contain the cag PAI, which is continuous in 26695 (2) but divided into cagI and cagII in SS1 as described for other H. pylori strains (2). Construction of mutant strains by transformation was performed as previously described (2). The mutated genes were HP0524, HP0525, and HP0527 (57). These genes are located in the cagII region of the cag PAI and have previously been referred to as cag10, cag11, and cag13 (2). They are homologous to A. tumefaciens virD4, virB11, and virB10, respectively. In A. tumefaciens, these genes code for proteins which form part of a type IV secretion apparatus, and they are presumed to have a similar function in H. pylori (12, 43). Their presence has been associated with tyrosine phosphorylation of host and bacterial proteins (4, 38, 43, 50, 55) as well as secretion of IL-8 (15) upon contact with cultured cells.
The genetic constructs used have been previously described (2). To inactivate individual genes, H. pylori strain SS1 or 26695 was transformed with a DNA construct containing a chloramphenicol resistence cassette (cat) inserted into the coding region of the gene to be interrupted. To delete either cagII or the entire cag region, strain SS1 or 26695 was transformed with a DNA construct containing cag flanking sequences surrounding a chloramphenicol resistance cassette. Gastritis and colonization potential of bacterial mutants were evaluated in germ-free piglets inoculated with 26695 or 26695Δcag and in mice given SS1, SS1virB11::cat, or SS1virB10::cat.
IL-8 assay.
IL-8 induction by H. pylori cocultured with AGS cells was determined by the method of Crabtree et al. (15). Briefly, AGS cells (a gastric carcinoma cell line) were grown in 24-well plates to 60 to 75% confluence in RPMI 1640 supplemented with fetal calf serum. Broth-cultured H. pylori was washed in RPMI 1640 and resuspended to 107/ml, and 1 ml of the suspension was added to AGS cell monolayers. After incubation for 24 h at 37°C, media were collected, bacteria were removed by centrifugation, and media were stored at −70°C until assay. IL-8 was detected with a capture enzyme-linked immunosorbent assay kit (Pharmingen) according to the manufacturer's instructions. Values reported were normalized by subtraction of endogenous IL-8 production (production by unstimulated AGS cells). Specificity of the assay was verified by inhibition with 1 μg of recombinant human IL-8 per ml.
Animal studies.
Germ-free piglets were derived and maintained as previously described (23). They were orally inoculated with 109 CFU of broth-cultured H. pylori at 3 days of age and killed 2, 7, or 14 days after inoculation. Female 6 to 8-week-old helicobacter-free C57BL/6 mice (from Jackson Laboratory) were maintained in microisolator cages and fed sterile lab chow and water ad libitum. They were orally inoculated with 107 CFU of broth-cultured H. pylori and killed 3 or 13 weeks after inoculation. The number of animals in each sacrifice group is given in Table 1.
TABLE 1.
Number of animals examined at each sacrifice interval
| Bacterial strain | No. at each sacrifice interval (time postinfection)
|
||||
|---|---|---|---|---|---|
| 2 days | 3 wk | 7 days | 14 days | 13 wk | |
| Germ-free piglets | |||||
| None | 0 | 4 | 0 | ||
| 26695 | 4 | 6 | 2 | ||
| 26695Δcag | 4 | 6 | 2 | ||
| Mice | |||||
| None | 0 | 6 | |||
| SS1 | 4 | 12 | |||
| SS1virB11::cat | 5 | 5 | |||
| SS1virB10::cat | 3 | 0 | |||
At sacrifice, stomachs were removed and bisected along the greater and lesser curvatures, and bacterial colonization in one half of the stomach was quantified by plate dilution on blood agar plates with and without 20 μg of chloramphenicol per ml. Sections of piglet stomach from cardia, fundus, and antrum were formalin fixed and paraffin embedded for histologic examination. Mouse stomach was sectioned in approximately 1-mm-wide slices and then fixed and embedded as described above.
Immunohistochemistry.
Gastric sections from piglets killed 7 days after inoculation were examined for the presence of cells bearing CD4 and CD8 surface markers. OCT-embedded frozen tissue was cut in 3-μm sections and stained as previously described (25). Primary antibody (mouse monoclonal anti-pig CD4 or CD8; VMRL, Inc., Pullman, Wash.) was diluted 1:100. In control slides, normal mouse serum was substituted for the primary antibody. Sections were scored by enumerating the number of cells per 20× field. For each tissue block, replicate sections stained for CD4 and CD8 were examined to identify fields which were present in both slides. Of these, 6 to 12 fields which contained CD4+ or CD8+ cells were selected and scored. All sections were scored blind without knowledge of their source or antibody stain.
Histopathology.
Hematoxylin-and-eosin stained sections were scored for the extent of gastritis as previously described (24). Briefly, microscopic fields containing gastritis severe enough to displace glands, and fields containing neutrophilic infiltrate were enumerated and expressed as a percentage of the gastric mucosa. All available fields were scored, and slides were examined blind, without knowledge of their source.
Statistics.
Means were compared by t-test or by Mann-Whitney U test. Statistical significance was set at P < 0.05.
RESULTS
Induction of IL-8 in AGS cells.
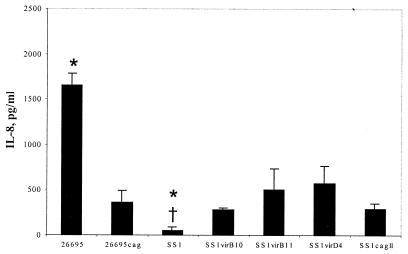
Strain 26695 induced IL-8 secretion when cocultured for 24 h with AGS cells, and deletion of the cag PAI in this strain significantly decreased IL-8 induction to about 20% of the wild-type level (P = 0.02 [Fig. 1]). In contrast to 26695, and despite the presence of the cag PAI, wild-type strain SS1 did not induce IL-8 secretion by AGS cells. IL-8 induction by SS1 was 3% of induction by 26695 (P = 0.02) and not significantly different from background (P = 0.25). Interestingly, however, deletion of the entire cagII region as well as any of the cag genes tested, the virB10, virB11, and virD4 homologues, resulted in slightly increased IL-8 induction by this strain. These differences were consistent among all mutants, and IL-8 induction by all mutant groups combined was significantly greater than induction by wild-type SS1 (P = 0.009 [Fig. 1]).
FIG. 1.
Induction of IL-8 by H. pylori strains 26695, SS1, and cag-deficient mutants. ∗, significantly different from isogenic mutants, P < 0.05; †, significantly different from strain 26695, P < 0.05.
Bacterial colonization.
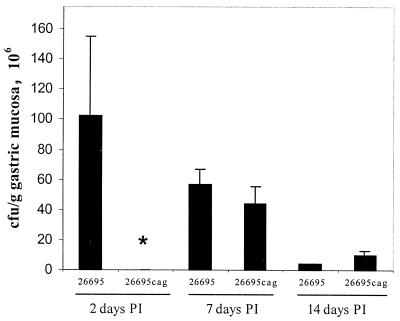
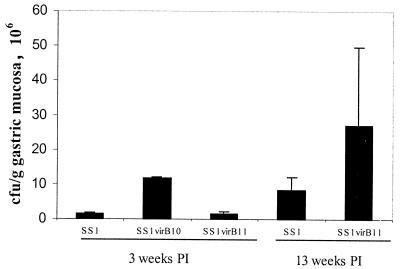
Colonization rates were similar in piglets (Fig. 2) and mice (Fig. 3). In both species, all inoculated animals became colonized with all challenge strains, and the extent of colonization ranged from approximately 105 to 108 CFU/g of gastric mucosa. In piglets, colonization by 26695 decreased over time from greater than 108 CFU/g of gastric mucosa 2 days after inoculation to 4 × 106 CFU/g by 14 days after inoculation. In mice, colonization increased somewhat between 3 and 13 weeks after inoculation. Overall, there was no consistent difference in colonization between wild-type and mutant bacteria. Two days after inoculation, extent of colonization by 26695Δcag was lower than that by 26695, but the difference did not persist to the other sacrifice intervals. Colonization by SS1, SS1 virB11::cat, and SS1virB10::cat differed somewhat from each other, but there were no statistically significant differences.
FIG. 2.
Colonization of piglets by H. pylori strain 26695 and cag-deficient mutants 2, 7, and 14 days postinoculation (PI). Two days after inoculation, colonization by 26695Δcag was significantly less than colonization by 26695 (∗, P < 0.05), but the two strains did not differ at the other sacrifice intervals.
FIG. 3.
Colonization of mice by H. pylori strain SS1, and cag-deficient mutants 3 and 13 weeks postinoculation (PI). Colonization ranged from approximately 106 to 107 CFU/g of gastric mucosa, but there were no significant differences between strains or sacrifice intervals.
Gastritis.
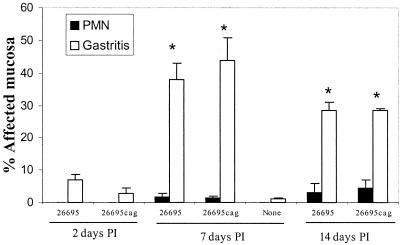
In piglets, gastritis was present 7 and 14 days after inoculation (P = 0.01 and 0.021 compared to uninfected piglets [Fig. 4]). It was characterized primarily by lymphocytic and plasmacytic infiltrate (Fig. 5); neutrophilic infiltrate was variable and mild (Fig. 4). In mice, gastritis was present 13 weeks after inoculation (P = 0.0015, SS1-infected compared to uninfected mice [Fig. 6]) and was characterized by mixed inflammatory infiltrate including lymphocytes, macrophages, plasma cells, and neutrophils as well as multifocal loss of fundic gland morphology with replacement by mucus-type glands (metaplasia) (Fig. 7). Neutrophilic infiltrate was significantly elevated in these mice (P = 0.005, SS1 infected compared to uninfected), and was more prominent than in piglets. In both mice and piglets there was no difference in gastritis or neutrophilic infiltrate between groups infected with wild-type H. pylori and groups infected with cag mutant strains.
FIG. 4.
Gastritis and neutrophilic infiltration (PMN) in piglets colonized by H. pylori strain 26695 and cag-deficient mutants 2, 7, and 14 days postinoculation (PI). Seven and 14 days after inoculation gastritis was significantly greater than in uninfected piglets (∗, P < 0.05), but there were no differences between strains.
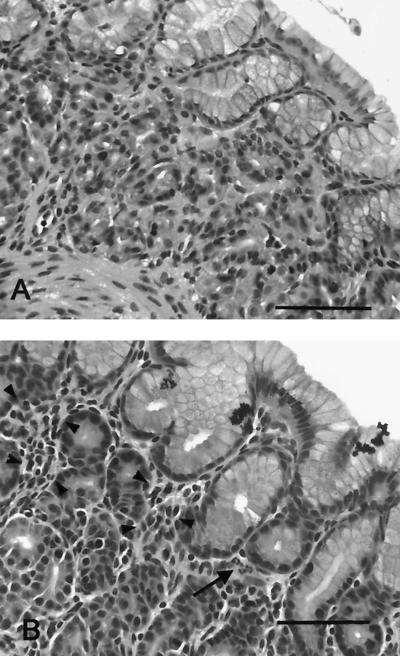
FIG. 5.
Tissue from the gastric cardia of an uninfected gnotobiotic piglet (A) and a piglet infected with H. pylori strain 26695 for 14 days (B). Gastritis is characterized by lymphocytes and plasma cells (arrowheads) with scattered neutrophils (arrow). Hematoxylin and eosin stain. Bar = 75 μm.
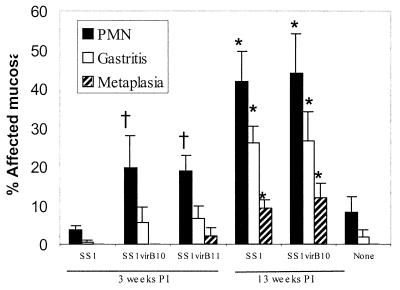
FIG. 6.
Gastritis, neutrophilic infiltration (PMN), and epithelial metaplasia in mice colonized by H. pylori strain SS1 and cag-deficient mutants 3 and 13 weeks postinoculation (PI). Three weeks after inoculation, neutrophilic inflammation was more prominent in mice colonized by the cag-deficient mutants than in mice colonized by SS1 (†, P < 0.05), but this difference did not persist. Thirteen weeks after inoculation, PMN, gastritis, and metaplasia were all significantly elevated in all mice (∗, P < 0.05), but there were no differences between bacterial strains.
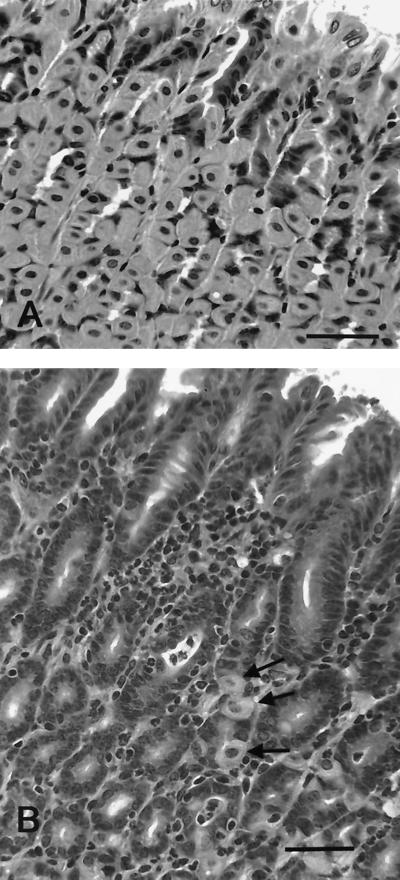
FIG. 7.
Tissue from the gastric fundus of an uninfected mouse (A) and a mouse infected with H. pylori strain SS1 for 13 weeks (B). Gastritis is characterized by a mixed infiltrate of lymphocytes and neutrophils. Note the paucity of parietal and chief cells characteristic of epithelial metaplasia in H. pylori-infected mice (see reference 24). The arrows indicate three remaining parietal cells. Hematoxylin and eosin stain. Bar = 50 μm.
Immunohistochemistry.
Both CD4+ and CD8+ cells were significantly increased in the gastric mucosa of infected piglets compared to uninfected piglets (P < 0.05 [Table 2]). However, both cell types were present in all infected piglets, and there were no differences between piglets given 26695 and those given 26695Δcag. In all piglets, more gastric lamina propria lmphocytes expressed CD8 than CD4 (Table 2).
TABLE 2.
T-cell subsets in germ-free piglets infected with wild-type and cag-deficient H. pylori
| Group | No. of cells/20× microscopic field (mean ± SE)
|
|
|---|---|---|
| CD4+ cells | CD8+ cells | |
| Uninfected | 0.15 ± 0.15 | 2.1 ± 0.5 |
| All infected | 4.5 ± 1.6a | 14.6 ± 2.1a |
| Infected with 26695 | 5.6 ± 2.9 | 13.2 ± 4.0 |
| Infected with 26695Δcag | 3.2 ± 1.2b | 15.9 ± 1.8b |
Significantly greater than uninfected piglets, P < 0.05.
Not significantly different from 26695 group, P > 0.1.
DISCUSSION
The results reported here seem to contradict current dogma in two ways. First, we have shown that in at least one strain of H. pylori, SS1, the ability to induce IL-8 secretion by cultured gastric epithelial cells is not directly correlated with the presence of a cag-PAI. In contrast to strain 26695, strain SS1 did not induce IL-8 secretion despite the presence of the PAI, and, surprisingly, inactivation of several genes in that region actually promoted, rather than inhibited, IL-8 induction. While these finding contrast with many published studies (1, 11, 17, 19, 27, 38, 44, 58), they may actually represent an accurate assessment of the phenotypic diversity of H. pylori. Most published studies used only one or a few well-characterized strains to demonstrate dependence of IL-8 secretion on the cag PAI. Recently published studies, however, suggest that over a broad range of strains, IL-8 induction may not always correlate with presence or absence of the cag PAI. In one study in which 80 clinical isolates were examined, IL-8 induction by cag-positive strains varied widely, and some cag-negative strains were able to induce IL-8 in cultured cells (61). In another study, 153 clinical isolates were examined, and in many cases strains with the cag PAI failed to induce IL-8, while strains without the PAI did induce secretion (7). A third study failed to find either an association between IL-8 induction and cag or an association between in vivo IL-8 levels and in vitro IL-8 induction by the same strain (45). In addition, non-cag-containing bacterial species other than H. pylori have been shown to induce IL-8 in cultured cells in some studies (31). Finally, several H. pylori genes unrelated to cag have been associated with IL-8 induction. In one study cited above, IL-8 induction was partly dependent on oipA, a gene which is unrelated to cag (61). In other studies IL-8 induction has been associated with flagellar morphology (46), heat shock proteins (60), and other H. pylori products (21).
Our demonstration that inactivation of cag genes leads to promotion of IL-8 induction in SS1 has not been previously reported. However, this finding may be explained by diversity in secretory function as reported for other bacterial species. The cag genes examined are presumed to encode part of a type IV secretion system involved in injection of bacterial proteins into the host cell (12, 43, 55), and it is widely assumed that this secretion function is exclusively proinflammatory (1, 27, 51). However, other bacterial species possess secretion systems which can induce either proinflammatory or anti-inflammatory signals to host cells (42, 59). It has been suggested, in fact, that secretion of anti-inflammatory signals may be one way in which nonpathogenic enteric bacteria down-regulate a host immune response. Conceivably, an equivalent mechanism in H. pylori contributes to the virulence of specific strains. That is, the cag-related secretion system may function to deliver anti- as well as proinflammatory signals to target tissues, thereby determining in part the level of the host response.
A second surprising finding of this study was the lack of congruence between IL-8 induction in vitro and neutrophilic gastritis in vivo in mice. It is widely assumed that IL-8 induction in gastric epithelial cells is the mechanism whereby neutrophilic gastritis is induced by H. pylori. However, this hypothesis has not been tested in animal models. In humans, IL-8 and neutrophils are both present in gastric mucosa colonized by H. pylori (18, 47). The presumption of causation is based on two types of studies, the in vitro induction of IL-8 by H. pylori and the epidemiologic association between cag-containing strains and disease.
The relationship between in vivo studies in cultured cells and in vivo pathogenesis must be interpreted with caution. Gastric cancer cell lines used for in vitro assays differ from gastric surface mucus cells in many ways, including surface markers and receptors, lack of polarity, and synthetic capability. Thus, their interactions with bacteria are likely to differ. Even freshly isolated gastric epithelial cells may not reflect in vivo conditions, since these cells are mixtures of parietal cells, chief cells, neck cells, and surface mucus cells. Only a minority of cells may be of the same phenotype and response to H. pylori as the surface mucus cells that encounter H. pylori in vivo. Another critical difference between in vivo and in vitro interactions between H. pylori and gastric epithelial cells is the multiplicity of infection. Cell culture studies typically use ratios of 50 to 100 bacteria per gastric epithelial cell, several orders of magnitude greater than the ratio found in vivo (105 to 106 CFU/biopsy or more than 10 epithelial cells to one bacterium) (6). Further, bacteria in vitro surround and closely adhere to the gastric epithelial cells in the absence of intervening mucus. In vivo, bacteria are mostly within the mucus, a minority of bacteria actually adhere to the epithelial cells, and those only adhere to the luminal surface. Thus, interactions between bacteria and epithelial cells in vivo and in vitro are likely to differ markedly, and induction of IL-8 synthesis by bacterial contact in vitro does not necessarily indicate that such induction occurs in vivo or is of sufficient magnitude to have a primary effect.
Epidemiologic studies provide stronger evidence of an association between cagA or the cag PAI and increased severity of disease. Many such studies are published (for recent reviews, see references 5 and 13), and most indicate that the cag PAI has a role in the pathogenicity of H. pylori. However, the epidemiologic association between cag and disease is not absolute, suggesting that the relationship is likely not direct. Not all published studies have shown a relationship between the cag PAI and increased severity of disease (8, 28, 29, 35, 36, 39, 48, 63), and no study has shown an absolute relationship. Even when there is an overall statistical association between infection by strains which express cag and increased severity of disease, many patients examined have severe disease but were colonized by cag-negative strains (28, 30, 33). Thus, although the cag PAI clearly contributes to disease due to H. pylori, cag-negative strains can also be pathogenic.
Taken together, the occurrence of cag-negative disease in humans and the absence of an effect of cag in animal models are most easily explained by the role of the host response in disease outcome. Data are accumulating that in both humans and experimental animals, the outcome of H. pylori-related disease is strongly dependent on host immune response (20, 49). Different hosts may differ in their response to H. pylori antigens, thus leading to different outcomes in individuals infected with strains of similar antigenicity. Greater antigenicity of cag-dependent antigens in some individual hosts, host strains, or host species could explain the tendency toward greater pathogenicity of these strains. Clearly, however, cag-independent antigens can induce disease, at least in animals, and cag-negative strains are not necessarily innocuous.
ACKNOWLEDGMENTS
This work was supported in part by PHS grants NIH R01 AI43643, R29 DK-45340, and R01 CA67498-01 from the NIH (Eaton) and by PHS grants NIH R01 AI43643, AI38166, R29 DK-45340, DK53727, P30 DK52574, and R01 CA67498-01 from the NIH (Berg).
REFERENCES
- 1.Aihara M, Tsuchimoto D, Takizawa H, Azuma A, Wakebe H, Ohmoto Y, Imagawa K, Kikuchi M, Mukaida N, Matsushima K. Mechanisms involved in Helicobacter pylori-induced interleukin-8 production by a gastric cancer cell line, MKN45. Infect Immun. 1997;65:3218–3224. doi: 10.1128/iai.65.8.3218-3224.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akopyants N S, Clifton S W, Kersulyte D, Crabtree J E, Youree B E, Reece C A, Bukanov N O, Drazek E S, Roe B A, Berg D E. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol Microbiol. 1998;28:37–53. doi: 10.1046/j.1365-2958.1998.00770.x. [DOI] [PubMed] [Google Scholar]
- 3.Akopyants N S, Eaton K A, Berg D E. Adaptive mutation and cocolonization during Helicobacter pylori infection of gnotobiotic piglets. Infect Immun. 1995;63:116–121. doi: 10.1128/iai.63.1.116-121.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asahi M, Azuma T, Ito S, Ito Y, Suto H, Nagai Y, Tsubokawa M, Tohyama Y, Maeda S, Omata M, Suzuki T, Sasakawa C. Helicobacter pylori CagA protein can be tyrosine phosphorylated in gastric epithelial cells. J Exp Med. 2000;191:593–602. doi: 10.1084/jem.191.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atherton J C. CagA, the cag pathogenicity island and Helicobacter pylori virulence. Gut. 1999;44:307–308. doi: 10.1136/gut.44.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atherton J C, Tham K T, Peek R M, Cover T L, Blaser M J. Density of Helicobacter pylori infection in vivo as assessed by quantitative culture and histology. J Infect Dis. 1996;174:552–556. doi: 10.1093/infdis/174.3.552. [DOI] [PubMed] [Google Scholar]
- 7.Audibert C, Burucoa B, Janvier B, Fauchere J L. Structure of the cag pathogenicity island of 153 French isolates of Helicobacter pylori and correlation with the IL-8 in vitro induction. Gut. 2000;47:A30. . (Abstract.) [Google Scholar]
- 8.Bach S, Makristathis A, Pinto A, Quina M, Rotter M, Hirschl A M. Helicobacter pylori type I strains among Austrian and Portuguese patients with gastritis, peptic ulcer or gastric cancer. Eur J Clin Microbiol Infect Dis. 1999;18:807–810. doi: 10.1007/s100960050405. [DOI] [PubMed] [Google Scholar]
- 9.Blaser M J, Perezperez G I, Kleanthous H, Cover T L, Peek R M, Chyou P H, Stemmermann G N, Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–2115. [PubMed] [Google Scholar]
- 10.Bliss C M, Golenbock D T, Keates S, Linevsky J K, Kelly C P. Helicobacter pylori lipopolysaccharide binds to CD14 and stimulates release of interleukin-8, epithelial neutrophil-activating peptide 78, and monocyte chemotactic protein 1 by human monocytes. Infect Immun. 1998;66:5357–5363. doi: 10.1128/iai.66.11.5357-5363.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Censini S, Lange C, Xiang Z, Crabtree J E, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type-I specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14654. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Covacci A, Telford J, Del G G, Parsonnet J, Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. 1999;284:1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- 13.Cover T L, Blaser M J. Helicobacter pylori factors associated with disease. Gastroenterology. 1999;117:257–260. doi: 10.1016/s0016-5085(99)70575-5. [DOI] [PubMed] [Google Scholar]
- 14.Crabtree J E. Role of cytokines in pathogenesis of Helicobacter pylori-induced mucosal damage. Dig Dis Sci. 1998;43:S46–S55. [PubMed] [Google Scholar]
- 15.Crabtree J E, Covacci A, Farmery S M, Xiang Z, Tompkins D S, Perry S, Lindley I J D, Rappuoli R. Helicobacter pylori induced interleukin-8 expression in gastric epithelial cells is associated with CagA positive phenotype. J Clin Pathol. 1995;48:41–45. doi: 10.1136/jcp.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crabtree J E, Farmery S M. Helicobacter pylori and gastric mucosal cytokines: evidence that CagA-positive strains are more virulent. Lab Investig. 1995;73:742–745. [PubMed] [Google Scholar]
- 17.Crabtree J E, Kersulyte D, Li S D D, Lindley I J D, Berg D E. Modulation of Helicobacter pylori induced interleukin-8 synthesis in gastric epithelial cells mediated by cag PAI encoded VirD4 homologue. J Clin Pathol. 1999;52:653–657. doi: 10.1136/jcp.52.9.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crabtree J E, Wyatt J I, Trejdosiewicz L K, Peichl P, Nichols P H, Ramsay N, Primrose J N, Lindley I J. Interleukin-8 expression in Helicobacter pylori infected, normal, and neoplastic gastroduodenal mucosa. J Clin Pathol. 1994;47:61–66. doi: 10.1136/jcp.47.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crabtree J E, Xiang Z, Lindley I J, Tompkins D S, Rappuoli R, Covacci A. Induction of interleukin-8 secretion from gastric epithelial cells by a cagA negative isogenic mutant of Helicobacter pylori. J Clin Pathol. 1995;48:967–969. doi: 10.1136/jcp.48.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D'Elios M M, Manghetti M, Almerigogna F, Amedei A, Costa F, Burroni D, Baldari C T, Romagnani S, Telford J L, Delprete G. Different cytokine profile and antigen-specificity repertoire in Helicobacter pylori-specific T cell clones from the antrum of chronic gastritis patients with or without peptic ulcer. Eur J Immunol. 1997;27:1751–1755. doi: 10.1002/eji.1830270723. [DOI] [PubMed] [Google Scholar]
- 21.Ding S Z, Cho C H, Lam S K. Helicobacter pylori induces interleukin-8 expression in endothelial cells and the signal pathway is protein tyrosine kinase dependent. Biochem Biophys Res Commun. 1997;240:561–565. doi: 10.1006/bbrc.1997.7699. [DOI] [PubMed] [Google Scholar]
- 22.Dunn B E, Cohen H, Blaser M J. Helicobacter pylori. Clin Microbiol Rev. 1997;10:720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eaton K A, Morgan D R, Krakowka S. Campylobacter pylori virulence factors in gnotobiotic piglets. Infect Immun. 1989;57:1119–1125. doi: 10.1128/iai.57.4.1119-1125.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eaton K A, Ringler S R, Danon S J. Murine splenocytes induce severe gastritis and delayed-type hypersensitivity and suppress bacterial colonization in Helicobacter pylori-infected SCID mice. Infect Immun. 1999;67:4594–4602. doi: 10.1128/iai.67.9.4594-4602.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eaton K A, Ringler S S, Krakowka S. Vaccination of gnotobiotic piglets against Helicobacter pylori. J Infect Dis. 1998;178:1399–1405. doi: 10.1086/314463. [DOI] [PubMed] [Google Scholar]
- 26.Evans D J, Evans D G, Takemura T, Nakano H, Lampert H C, Graham D Y, Granger D N, Kvietys P R. Characterization of a Helicobacter pylori neutrophil-activating protein. Infect Immun. 1995;63:2213–2220. doi: 10.1128/iai.63.6.2213-2220.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glocker E, Lange C, Covacci A, Bereswill S, Kist M, Pahl H L. Proteins encoded by the cag pathogenicity island of Helicobacter pylori are required for NF-κ B activation. Infect Immun. 1998;66:2346–2348. doi: 10.1128/iai.66.5.2346-2348.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Go M F, Graham D Y. Presence of the cagA gene in the majority of Helicobacter pylori strains is independent of whether the individual has duodenal ulcer or asymptomatic gastritis. Helicobacter. 1996;1:107–111. doi: 10.1111/j.1523-5378.1996.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 29.Graham D Y, Genta R M, Graham D P, Crabtree J E. Serum CagA antibodies in asymptomatic subjects and patients with peptic ulcer: lack of correlation of IgG antibody in patients with peptic ulcer or asymptomatic Helicobacter pylori gastritis. J Clin Pathol. 1996;49:829–832. doi: 10.1136/jcp.49.10.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heikkinen M, Janatuinen E, Mayo K, Megraud F, Julkunen R, Pikkarainen P. Usefulness of anti-Helicobacter pylori and anti-CagA antibodies in the selection of patients for gastroscopy. Am J Gastroenterol. 1997;92:2225–2229. [PubMed] [Google Scholar]
- 31.Hickey T E, Baqar S, Bourgeois A L, Ewing C P, Guerry P. Campylobacter jejuni-stimulated secretion of interleukin-8 by INT407 cells. Infect Immun. 1999;67:88–93. doi: 10.1128/iai.67.1.88-93.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang J Z, Otoole P W, Doig P, Trust T J. Stimulation of interleukin-8 production in epithelial cell lines by Helicobacter pylori. Infect Immun. 1995;63:1732–1738. doi: 10.1128/iai.63.5.1732-1738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jenks P J, Megraud F, Labigne A. Clinical outcome after infection with Helicobacter pylori does not appear to be reliably predicted by the presence of any of the genes of the cag pathogenicity island. Gut. 1998;43:752–758. doi: 10.1136/gut.43.6.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim J S, Jung H C, Kim J M, Song I S, Kim C Y. Interleukin-8 expression by human neutrophils activated by Helicobacter pylori soluble proteins. Scand J Gastroenterol. 1998;33:1249–1255. doi: 10.1080/00365529850172322. [DOI] [PubMed] [Google Scholar]
- 35.Kodama K, Ito A, Nishizono A, Fujioka T, Nasu M, Yahiro K, Hirayama T, Uemura N. Divergence of virulence factors of Helicobacter pylori among clinical isolates does not correlate with disease specificity. J Gastroenterol. 1999;34(Suppl. 11):6–9. [PubMed] [Google Scholar]
- 36.Kurihara N, Kamiya S, Yamaguchi H, Osaki T, Shinohara H, Kitahora T, Ishida H, Ozawa A, Otani Y, Kubota T, Kumai K, Kitajima M. Characteristics of Helicobacter pylori strains isolated from patients with different gastric diseases. J Gastroenterol. 1998;33(Suppl. 10):10–13. [PubMed] [Google Scholar]
- 37.Lee A, Orourke J, Deungria M C, Robertson B, Daskalopoulos G, Dixon M F. A standardized mouse model of Helicobacter pylori infection: Introducing the Sydney strain. Gastroenterology. 1997;112:1386–1397. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 38.Li S, Kersulyte D, Lindley I, Neelam B, Berg D, Crabtree J. Multiple genes in the left half of the cag pathogenicity island of Helicobacter pylori are required for tyrosine kinase-dependent transcription of interleukin-8 in gastric epithelial cells. Infect Immun. 1999;67:3893–3899. doi: 10.1128/iai.67.8.3893-3899.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maeda S, Kanai F, Ogura K, Yoshida H, Ikenoue T, Takahashi M, Kawabe T, Shiratori Y, Omata M. High seropositivity of anti-CagA antibody in Helicobacter pylori-infected patients irrelevant to peptic ulcers and normal mucosa in Japan. Dig Dis Sci. 1997;42:1841–1847. doi: 10.1023/a:1018846723379. [DOI] [PubMed] [Google Scholar]
- 40.Maekawa T, Kinoshita Y, Matsushima Y, Okada A, Fukui H, Waki S, Kishi K, Kawanami C, Nakata H, Hassan S, Wakatsuki Y, Ota H, Amano K, Nakao M, Chiba T. Helicobacter pylori induces proinflammatory cytokines and major histocompatibility complex class II antigen in mouse gastric epithelial cells. J Lab Clin Med. 1997;130:442–449. doi: 10.1016/s0022-2143(97)90045-7. [DOI] [PubMed] [Google Scholar]
- 41.Marshall B J, Warren J R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;i:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 42.Neish A S, Gewirtz A T, Zeng H, Young A N, Hobert M E, Karmali V, Rao A S, Madara J L. Prokaryotic regulation of epithelial responses by inhibition of IkappaB-alpha ubiquitination. Science. 2000;289:1560–1563. doi: 10.1126/science.289.5484.1560. [DOI] [PubMed] [Google Scholar]
- 43.Odenbreit S, Puls J, Sedlmaier B, Gerland E, Fischer W, Haas R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497–1500. doi: 10.1126/science.287.5457.1497. [DOI] [PubMed] [Google Scholar]
- 44.Ogura K, Takahashi M, Maeda S, Ikenoue T, Kanai F, Yoshida H, Shiratori Y, Mori K, Mafune K I, Omata M. Interleukin-8 production in primary cultures of human gastric epithelial cells induced by Helicobacter pylori. Dig Dis Sci. 1998;43:2738–2743. doi: 10.1023/a:1026671815512. [DOI] [PubMed] [Google Scholar]
- 45.Ohsuga M, Kusugami K, Ina K, Ando T, Yamaguchi H, Imada A, Nishio Y, Shimada M, Tsuzuki T, Noshiro M, Konagaya T, Kaneko H. Comparison between in vivo and in vitro chemokine production in Helicobacter pylori infection. Aliment Pharmacol Ther. 2000;14(Suppl. 1)1:205–215. doi: 10.1046/j.1365-2036.2000.014s1205.x. [DOI] [PubMed] [Google Scholar]
- 46.Ohta-Tada U, Takagi A, Koga Y, Kamiya S, Miwa T. Flagellin gene diversity among Helicobacter pylori strains and IL-8 secretion from gastric epithelial cells. Scand J Gastroenterol. 1997;32:455–459. doi: 10.3109/00365529709025080. [DOI] [PubMed] [Google Scholar]
- 47.Peek R M, Miller G G, Tham K T, Perezperez G I, Zhao X M, Atherton J C, Blaser M J. Heightened inflammatory response and cytokine expression in vivo to cagA(+) Helicobacter pylori strains. Lab Investis. 1995;73:760–770. [PubMed] [Google Scholar]
- 48.Sadakane Y, Kusaba K, Nagasawa Z, Tanabe I, Kuroki S, Tadano J. Prevalence and genetic diversity of cagD, cagE, and vacA in Helicobacter pylori strains isolated from Japanese patients. Scand J Gastroenterol. 1999;34:981–986. doi: 10.1080/003655299750025075. [DOI] [PubMed] [Google Scholar]
- 49.Sakagami T, Dixon M, O'Rourke J, Howlett R, Alderuccio F, Vella J, Shimoyama T, Lee A. Atrophic gastric changes in both Helicobacter felis and Helicobacter pylori infected mice are host dependent and separate from antral gastritis. Gut. 1996;39:639–648. doi: 10.1136/gut.39.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Segal E D, Cha J, Lo J, Falkow S, Tompkins L S. Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc Natl Acad Sci USA. 1999;96:14559–14564. doi: 10.1073/pnas.96.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharma S A, Tummuru M K R, Blaser M J, Kerr L D. Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-kappa B in gastric epithelial cells. J Immunol. 1998;160:2401–2407. [PubMed] [Google Scholar]
- 52.Sharma S A, Tummuru M K R, Miller G G, Blaser M J. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect Immun. 1995;63:1681–1687. doi: 10.1128/iai.63.5.1681-1687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shimoyama T, Crabtree J E. Mucosal chemokines in Helicobacter pylori infection. J Physiol Pharmacol. 1997;48:315–323. [PubMed] [Google Scholar]
- 54.Shimoyama T, Everett S M, Dixon M F, Axon A T R, Crabtree J E. Chemokine mRNA expression in gastric mucosa is associated with Helicobacter pylori cagA positivity and severity of gastritis. J Clin Pathol. 1998;51:765–770. doi: 10.1136/jcp.51.10.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stein M, Rappuoli R, Covacci A. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc Natl Acad Sci USA. 2000;97:1263–1268. doi: 10.1073/pnas.97.3.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suzuki H, Mori M, Sakaguchi A A, Suzuki M, Miura S, Ishii H. Enhanced levels of C-X-C chemokine, human GRO alpha, in Helicobacter pylori-associated gastric disease. J Gastroenterol Hepatol. 1998;13:516–520. doi: 10.1111/j.1440-1746.1998.tb00679.x. [DOI] [PubMed] [Google Scholar]
- 57.Tomb J F, White O, Kerlavage A R, Clayton R A, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 58.Tummuru M K R, Sharma S A, Blaser M J. Helicobacter pylori picB, a homologue of the Bordetella pertussis toxin secretion protein, is required for induction of IL-8 in gastric epithelial cells. Mol Microbiol. 1995;18:867–876. doi: 10.1111/j.1365-2958.1995.18050867.x. [DOI] [PubMed] [Google Scholar]
- 59.Xavier R J, Podolsky D K, Neish A S, Gewirtz A T, Zeng H, Young A N, Hobert M E, Karmali V, Rao A S, Madara J L. Microbiology. How to get along—friendly microbes in a hostile world. Science. 2000;289:1483–1484. doi: 10.1126/science.289.5484.1483. [DOI] [PubMed] [Google Scholar]
- 60.Yamaguchi H, Osaki T, Kurihara N, Kitajima M, Kai M, Takahashi M, Taguchi H, Kamiya S. Induction of secretion of interleukin-8 from human gastric epithelial cells by heat-shock protein 60 homologue of Helicobacter pylori. J Med Microbiol. 1999;48:927–933. doi: 10.1099/00222615-48-10-927. [DOI] [PubMed] [Google Scholar]
- 61.Yamaoka Y, Kita M, Kodama T, Sawai N, Imanishi J. Helicobacter pylori cagA gene and expression of cytokine messenger RNA in gastric mucosa. Gastroenterology. 1996;110:1744–1752. doi: 10.1053/gast.1996.v110.pm8964399. [DOI] [PubMed] [Google Scholar]
- 62.Yoshida M, Wakatsuki Y, Kobayashi Y, Itoh T, Murakami K, Mizoguchi A, Usui T, Chiba T, Kita T. Cloning and characterization of a novel membrane-associated antigenic protein of Helicobacter pylori. Infect Immun. 1999;67:286–293. doi: 10.1128/iai.67.1.286-293.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zheng P Y, Hua J, Yeoh K G, Ho B. Jul. Association of peptic ulcer with increased expression of Lewis antigens but not cagA, iceA, and vacA in Helicobacter pylori isolates in an Asian population. Gut. 2000;47:18–22. doi: 10.1136/gut.47.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]